Note: Descriptions are shown in the official language in which they were submitted.
CA 02323488 2000-09-12
WO 99146318 PGT/US98104950
1
PRO-FRAGRANCE SILICONE -POLYMER AND
COMPOSITIONS THEREOF
TECHNICAL FIELD
The present invention relates to a pro-fragrance silicone polymer and
compositions thereof, more specifically a pro-fragrance silicone polymer
derived
~o from an aminoalkyi polysiloxane and a fragrance carbonyl compound.
BACKGROUND
Most consumers have come to expect pleasingly scented laundry
products, as well as such products imparting the pleasing scent to laundered
~5 fabrics. Consumers also desire that laundered fabrics retain a pleasant
fragrance over time. Perfume additives have been employed to impart an
aesthetically pleasing scent io laundry products such as laundry detergents
and
fabric conditioners. In some case, these perfumes also impart their fragrance
to
fabrics treated with the perfumed laundry product. Consumers have also come
Zo to expect laundry products which provide a soft andlor smooth tactile
sensation
to the laundered products. Additionally, consumers have come to expect hair
products which produce a pleasing scent and providing softness to the hair.
Certain odoriferous acetals and ketats capable of producing additional
desirable odor upon hydrolysis, have long been known in pertumery. See
z5 Steffen Arctander, "Perfume and Flavor Chemicals," Arctander, N.J., 19fi9,
and
U_S. patent 5,37$,468, Suffis et al., issued January 3, 1995. Certain classes
of
acetals and ketals, formulated into detergent compositions can produce a
delayed release of fragrance. Such pro-fragrance acetals and ketals, once
deposited onto fabrics during washing process, tend to produce pleasing
3o fragrances upon hydrolysis of the acetals and ketals by humidity and sweat
during wearing of the laundered fabrics. See, PTCIUS96I04060, Mao et al.
_ Silicone polymers are widely known and have been used for providing
softness and smoothness to a surface, e.g., of skin, textile fibers, tissues,
and
hair. t7epending on structures and properties of silicone polymer, there are
two
s5 main areas for the use of such silicone polymer. One area is for surface
treating
CA 02323488 2000-09-12
WO 99/4631$ PG?t11S98/04950
2
to provide softness to materials such as fibers, tissues, or clothes as pre-
treating
agent. Conventional silicone polymers are also used in various products, e.g.,
cleaning or laundry detergents, fabric softeners, shampoos, heir conditioner,
which are applied later during process of washing, for maintaining softness of
- .
materials such as fabrics and hair. In addition, such products containing
silicone
polymers tend to be used on a daily basis to maintain softness or provide
further
softness to fabrics and/or hair.
Aminoalicy polysiloxanes are one type of conventional silicone polymer
employed as a softening agent in applications for washing; for example,
~o detergent, fabric softener, and the like. Such amino polysiloxanes can be
formulated alone or in combination with any materials commonly used in laundry
products, e.g., surtactants such as anionic, cationic, and nonionic
surfactants
and polyurethane in compositions. See Japan publication (KOKAI) H8-32x952,
issued December 10, 1996, and Japan patent publication (KQKAI) H8-246354,
~s issued September 24, 1996, and U.S. Patent 5,591,880 to Anthony J.
O'Lenick,
Jr, issued January 7, 1997. One class of amino polysiloxanes having an
alkylene
moiety is disclosed to provide improved dispersion in addition to softness to
fabrics. See U.S. Patent 5,378,7$7 to Vrckovnik et al., issued January 3,
1995.
Due to deposition property of silicone polymers to maintain softness of fabric
and
zo the hair, these silicone polymers are formulated into applications such as
detergents and softeners for daily washing in addition to pre-treatment.
However, neither of these approaches successfully provide a pro-
fragrance silicone polymer having improved deposition onto fabric andlor hair,
providing softness, andlor releasing a pleasant fragrance by hydrolysis.
2s Based on the foregoing, there is a need for providing a pro-fragrance
silicone polymer that can provide a fabric andlor hair softening effect,
andlor
desirable post-deposition release of a pleasant odor.
SUMMARY
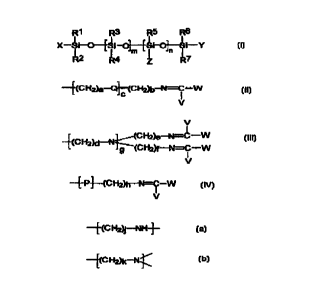
so A pro-fragrance silicone polymer comprising the following structure:
R5 R6
X-Si--O--~Si-O~--~Si-O~-Si-Y
RZ R4 m Z n R7
(I).
CA 02323488 2000-09-12
WO 99146318 PCTIU$9$/04950
3
X, Y, and Z are independently the following:
- _"~(C H2)a--Q~-(C HZ)b'~N=C-W
V (II):
V
(CHZ)d~~ (CH2)e-N=C-W
~(CHZ)f--N=C--W
I ,
ii. V (III};
--~P~--(CH2)h-'N=C-W
iii. U
(IV}; or
IV. a hydrocarbons of 1 to about 10 carbons or phenyl,
wherein at least one of X, Y, and Z are formula {II}, (1II), or (IV}. P has a
first and
a second randomly repeating monomer unit, wherein the first randomly repeating
monomer un'tt is
--~-{C HZ)j-NN-~-
~o and the second randomly repeating monomer unit is
---~(C H2lk-.-n1~
Q is oxygen, sulfur, or -NH-. V and W are independently hydrogen; or
straight, branched, or cyclic, saturated or unsaturated hydrocarbons of 1 to
about
30 carbons. R1, R2, R3, R4, Rb, Rs, and R7 are independently hydrocarbons of
~5 1 to about 10 carbons or a phenyl; m is an integer of 0 to about 500; n is
an
integer of 0 to about 100; the sum of m and n is an integer of at least 1; a
is an
integer of 1 to about 20; b is an integer of 1 to about 20; c is an integer of
0 to
about 100; d is an integer of 1 to about 20; a is an integer of 1 to about 20;
f is an
integer of 1 to about 20; g is an integer of 1 to about 100; h is an integer
of 1 to
2o about 20; j is an integer of 1 to about 20; and k is an integer of 1 to
about 20.
These and other features, aspects, and advantages of the present
invention will become better understood from a reading of the following
description, and appended maims.
CA 02323488 2000-09-12
WO 99146318 PGT/US98/04950
4
DETAILED DESCRIPTION
While the specification concludes with claims particularly pointing out and -
distinctly claiming the invention, it is believed that the present invention
will be
better understood from the following description. . ,
All percentages and ratios used hereinafter are by weight of tot2~l
composition, unless otherwise indicated.
Ali measurements referred to herein are made at 25°C unless
otherwise
specified.
All percentages, ratios, and levels of ingredients referred to herein are
based on the actual amount of the ingredient, and do not include solvents,
fillers,
or other materials with which the ingredient may be combined as a commercially
available product, unless otherwise indicated. _
All publications, patent applications, and issur~d patents mentioned herein
are hereby incorporated in their entirety by reference. Citation of any
reference
is is not an admission regarding any determination as to its availability as
prior art
to the claimed invention.
Herein, "comprising" means that other steps and other components which
do not afFect the end result can be added. This term encompasses the terms
"consisting oT' and "consisting essentially of."
za Herein, "pro-fragrance silicone polymer" means a component that is
converted info an active fragrance for providing desirablE odor by chemical
breakdown of the component; e.g., by. hydrolysis.
Herein, "schiff base" refers to a class of unit obtained by chemical reaction
(e.g., condensation, dehydration) from an aldehyde or a ketone with primary
2s . amines. The general formula is R(R')C=NR".
Herein, "silicone polymer" means a polymer which contains - Si(R)2-O-
repeating units in the backbone. The silicone polymer includes a component
called "polysiloxane" and "siloxane."
Herein, "aminoalkyl polysiloxane," is a polymer which contains -Si(R)2-O-
3o repeating units and at least one aminoalkyl substituent of -
-R-NHZ in terminal or branched position.
Herein, "carbonyl compound" means a compound which has at least one -
carbonyl unit of -CQ-, including aidehydes, ketones, and carboxylic acids
having
-COOR unit; preferably, aldehydes or ketones_
CA 02323488 2000-09-12
WO 99/46318 pCTIUS98104950
Herein, "hydrocarbon" means an organic compound consisting of carbon
and hydrogen, and is straight, branched, or cyclic, saturated or unsaturated;
in
certain embodiments the hydrocarbon may also include one or more oxygen
atoms_
s Herein, "alkyl" means a carbon-containing chain, which may be straight,
branched, or cyclic; saturated or unsaturated; and substituted (mono- or poly-
) or
unsubstituted.
Herein, "alkoxy!" means ..O-R, wherein R is alkyl, for example, methoxy,
ethoxy, propoxy, butoxy, pentoxy.
A. Pro-fragrance Silicone Polymer
The present invention relates to a pro-fragrance Silicone polymer
comprising the following structure:
R1 R3 R5 R6
X-Si-O--~Si--O~--~Si-O~--~-S i-Y
m n
R2 R4 Z R7
(1).
X, Y, and Z are independently the follouving:
--~(CHz)ay(CH2)IJ I~C--~W
~5 V (II)~
V
(CH2)d---N (CH2)e N C W
~(CHZ)f-N=C--W
i
ii. V (III);
-~-P~--(CH2)h-N=C-W
iii. V (IV); or
iv. a hydrocarbons of 1 to about 10 carbons or a phenyl,
wherein at least one of X, Y, and Z are formula (II), (III), or (IV). P has a
first and
2o a second randomly repeating monomer unit (hereinafter "RRMU"), wherein the
first RRMU is
-~-(CHZ)j---NH~-
and the second RRMU is
CA 02323488 2000-09-12
WO 99146318 r~. r maya~uyyw
6
--.~(C H~) k'-'N~
Q is oxygen, sulfur, or -NH-. V and W are independently hydrogen; or
straight, branched, or cyclic, saturated or unsaturated hydrocarbons of 1 to
about
30 carbons. R1, R2, R3, R4, R5, R6, and R~ are hydrocarbons of 1 to about 10
carbons or a phenyl; m is an integer of 0 to about 500; n is an integer of 0
to
about 1 Q0; the sum of m and n is an integer of at least 1; a is an integer of
1 to
about 20; b is an integer of 1 to about 20; c is an integer of 0 to about 100;
d is
an integer of 1 to about 20; a is an integer of 1 to about 20; f is an integer
of 1 to
about 20; g is an integer of 1 to about 100; h is an integer of 1 to about 20;
j is an
io integer of 1 to about 20: and k is an integer of 1 to about 20.
In the above structure, X, Y, and Z are the same or different from one
another seieGted from the group consisting of the following formula:
---~(C H2)a~~'(C H2)b
i- V (II);
V
{C ~Z)d-N~{C HZ)e~N=C-"W
~''(CHz~--N=C-W
1
ii. V (IIl);
.-.~-P~--(CHZ)h N=C-W
iii. V
~s (l~: or
iv. a hydrocarbons of 1 to about 10 carbons or a phenyl,
wherein at least one of X, Y, and Z are formula (II), (III), or (IV),
P has a first and a second RRMU, wherein the first RRMU is
--~(C H~)~ NH~--
zo and the second RRMU is
--~{C H2)k-N
Preferably, a is an integer of 1 to about 6; b is an integer of 1 to about 10;
c is an integer of 0 to about 10; d is an integer of 1 to about fi; a is an
integer of 1
to about 10; f is an integer of 1 to about 10; g is an integer of 1 to about
20; h is
CA 02323488 2000-09-12
WO 99146318 PCT/1JS98I04950
7
an integer of '~ to about 10; j is an integer of 1 to about 6; and k is an
integer of 1
to about 6_
Preferably, R1, R2, R3, R4, R~. Rs, and R7 of the above structure (I) are
_ the same and are an alkyl of 1 to about 10 carbons or a phenyl. Mare
preferably,
s R1, R2, R3, R4, Rb, Rs, and R7 are methyl or phenyl.
In the above structure, X and Y are the same or different and are formula
(II), {III). (IV), or hydrocarbons of 1 to about 10 carbons or phenyl.
Preferably,
when X and Y are the same, X and Y are formula (II) or a hydrocarbon of 1 to
about 10 carbons or a phenyl. When X and Y are different, at least one of X
and
io Y are preferably formula (II), (III), or (IV)_ When one of X and Y are
formula (II),
{III), or (IV), Z and the remainder of X and Y are preferably a hydrocarbon of
1 to
about 10 carbons or a phenyl.
In the above structure, preferably, Z is formula (II), (III}, or (IV) when X
and Y are hydrocarbons of 1 to about 10 carbons or phenyl; more preferably X
~s and Y are the same. In another embodiment, Z can be formula (II), when X
and.
Y are the same, preferably fomnula (II).
There are any combinations of formula (II), (I11), and (IV) for X, Y and Z.
depending on size and structure of formula (e.g_, configuration, and numbers
of
repeating unit such as -(CH2)- of formula (II) and (III) or the first and
second
2o RRMU of formula (IV).) Such size and balk of structure generally cause
steric
effects to the structure (I), thereby formuta (II), (III}, and (IV) leading to
suitable
positions of X, Y, and Z.
' The formula {I1), (III), and (IV) herein include a schlff base unit having a
general formula -N=C(V)W which originates in fragrance carbonyl compounds
25 such as aldehydes or ketones. V and W of (II), (III), and (IV) can be
independently hydrogen; or straight, branched, or cyclic, saturated or
unsaturated hydrocarbons of 1 to about 30 carbons.
V and W of (II), (III), and (IV) herein are preferably derived from a
fragrance carbonyl compound, preferably an aldehyde of a ketone. When V and
_ 3o W are derived from aldehydes, one of W and V are hydrogen and the
remainder
is a hydrocarbon of 1 to about 30 carbons that can include alkyl, alkenyl, or
aryl
moieties. When V and W are derived from ketones, both V and W are preferably
hydrocarbons of 1 to about 30 carbons.
CA 02323488 2000-09-12
W O 99146318 PCTIUS98/04950
8
In one embodiment, V and W can be bonded together to form a ring_
When V and W form a ring, the fragrance carbonyl compound is a cyclic carbonyl
. -
compound. Preferably, the sum of V and W contains 1 to about 50 carbons.
In another embodiment, the pro-fragrance silicone polymer can include
two of formula (II) substituents. In this embodiment, X and Y are suitable
pos~ions (terminal position) to substitute formula (II). Z is methyl or
phenyl. A
preferred embodiment is the following {structure (V)):
R1 R3 R5 R6
W -C=N-(CH2)b~Q-(CH2)a~-Si-p-~S i--O~--~S i-p~-~;-f (CH~B-Q~---(GHxjb---N=C--
W
V R2 R4 ~ R7 c V
wherein V and W are hydrogen or straight, branched, or cyclic, saturated or
~o unsaturated hydrocarbons of 1 ~to about 30 carbons; m is an integer of 0 to
about
500; and n is an integer of 1 to about 100.
Some specific examples of the present pro-fragrance silicone polymer
having structure (~ useful herein include, but are not limited to;
cH.,
v~a
~s which is derived from 2-methyl-3-(4-t butylphenyl)-propanal (lily aldehyde)
and aminopropyl polysiloxane,
H
~~-o-~~-.cue V-b
9 f
l.r.!
which is derived from 2-hexyl-3-phenyl-2-propenal (a.-hexyl cinnamic aldehyde}
and aminopropyl polysiloxane, and '
CA 02323488 2000-09-12
WO 991~i6318 pCT/US98/04950
9
CH3 CH3
H
H N(CH3~--~~i~ ~ i-(CH3y~N (V-c), and
H3 CH3
H CHs CW3
I
N(CH3?s-f ~i-O~Si-(CH3)sN (V-c')
CH3 CH3 H
which are derived from 2,4-dimethyl-3-cyclohexyl carboxyaldehyde and
aminopropyi poiysiloxane.
s Another preferred embodiment of the pro-fragrance silicone polymer
having formula (II) at position Z of structure (I) is the following (structure
V)<):
R~ R3 R~ R6
X s2 p--f-~i p~~i O n S~ Y V1
R ~4 ( ( )
~(CH~~r-~-,jCHz)b--N=C-W
c
V
wherein X and Y are the same or different methyl or phenyl; V and W are
hydrogen or straight, branched, or cyclic, saturated or unsaturated
hydrocarbons
~o of 9 to about 30 carbons; m is an integer of 0 to about 500; and n is an
integer of
1 to about 100.
Specifrc preferred pro-fragrance silicone polymers having the structure
(VI) are nonlimitingly illustrated by the following-
CHg CHg CHg CHg (V1-~)
1"I~C-~i-O--ESi-Q~--~Si--O~-Si---CH3
CH3 GH3 m ~ n L H
C 3 H
C3HgNHG2Ha
~s wherein m is integer from about 26 to about 30; n is integer from 2 to
about 6.
CA 02323488 2000-09-12
PCTIUS98J04950
When the above structure (VI) include formula (IV) instead of formula (II),
the pro-fragrance silicone polymer is a highly branched compound having the
following structure:
RI R3 R5 R6
V X-~i_~Si_~Si_~~i-Y V
W-G=N--(CH~)h.~ R2 ~ ~~ CH _N--.,~'(CH2)h-N=~-W
rN-(CH2)k, (CHz)k-N ( 2)k ~(CH2)h-N =C-W
W-C=N-(CI-!~ )h V
V N---(CH2)k-N-(CH2)k V
V ~H /(CHp)k-N~(CH )~N C W VI-b
( 2)k-N ( 2)h--N=C~W (
W-~ N-(CHZ)h~ N-(CM~)k V
W-i =N-(CH2)h V
V (CHZ)h-NoC-W
(CH2)k-N~
(CHZ)h-N= IC-..-W
V
s Other preferred pro-fragrance silicone polymers useful herein is the
following (structure VII):
Rt
W-C-N-(~b'~'Q_~CH~a~~.0-.~ ;_ ~~~~t~a- tab-f~G-W
v ~ ~~ ~ v (VII)
a-~---(fib-N=C w
1
v
wherein V and W are hydrogen or straight, branched, ar cyclic, saturated or
unsaturated hydrocarbons of 1 to about 30 carbons; m is an integer of 0 to
about
:0 500; and n is an integer of 1 to about 700.
In another preferred embodiment, the pro-fragrance silicone polymer of
the present invention includes the following (structure VIII):
R~ R3 R5 R6
X-~ i-p-~Si-O~--ESi-O~Si--~(CH~a,C~(CH~-N=C-W
R2 R4 Z R~ V
CA 02323488 2000-09-12
WO 99146318 PCT/US98/04950
91
wherein V and W are hydrogen or straight, branched, or cyclic, saturated or
unsaturated hydrocarbons of 1 to about 30 carbons; m is an integer of 0 to
about
aOQ; and n is integer of b to about 100.
The pro-fragrance silicone polymer of the present invention is derived from
s an aminoalkyt polysiloxane and a fragrance carbonyl compound by condensation
which produces a schiff base unit. Such fragrance sources of the pro-fragrance
silicone polymer produce a pleasant fragrance. Without being bound by theory,
it
is believed that due to less volatility, a silicone polymer (e.g., aminoalkyl
polysiloxane) generally produces little smell so that the pro-fragrance
silicone
o polymer derived herein in itself tends to be smelled weakly d wring
depositing onto
the surface, e.g., of fabric or hair. Most of the pleasing smell is produced
by
hydrolysis of schiff base moiety of the pro-fragrance silicone polymer rather
than
provide good fragrance in a non-hydrolyzed state. Consequently, the pro-
fragrance silicone polymer herein releases a pleasant fragrance by hydrolysis.
~s Hydrolysis is a chemical reaction against condensation or dehydration.
The mechanism of hydrolysis generally proceeds against steps of condensation
on a reactive center. Such hydrolysis, herein, is caused on the schiff base of
structure (I) which is prepared by an aminoalkyl polysiloxane and a fragrance
carbonyl compound. The schiff base of the pro-fragrance silicone polymer tends
2o to be hydrolyzed by humidity and sweat when the pro-fragrance when
depositing
to the fabric and the hair. The hydrolysis of the pro-fragrance silicone
polymer
herein can be characterized by a half life less than fi0 minutes when measured
at
pH 0 by the Hydrolysis Half life (t-112) Test as described herein.
The pro-fragrance silicone polymers of the present invention are stable
under pH conditions encountered in the formulation and storage of products
containing the pro-fragrance silicone (e.g., laundry detergents, fabric
softeners,
shampoos, hair conditioners) which have a pH of from about 7.1 to about 13,
and
during solution-use of such products. Due to hydrophilicity and high degree of
heteroatom incorporation, these pro-fragrance compounds give reasonably good
3o deposition from e.g., a taundering solution onto fabrics (or lathering
solution to
hair.) Because the pro-fragrance silicone polymers are subject to hydrolysis
when the pH is reduced, they are hydrolyzed to release fragrance carbonyl
compound when the fabrics (or other surtace) upon which they have been
deposited are exposed even to reduced pH such as is present in rinse water and
ss humidity. Such a reduction in pH is preferably at least about 0.1, more
preferably
CA 02323488 2000-09-12
WO 99146318 PCTNS98/04950
12
at least about 0.5 units. Preferably the pH is reduced by at least about 0.5
units
to a pH of about 7.5 or less, more preferably about 6.9 or less_ Preferably,
the
solution in which the fabric (or other surtace) is washed is alkaline_
The pro-fragrance silicone polymer of the present invention may be further - _
designed to readily disperse in aqueous solution and thereby result in
reasonably
good deposition, e_g_, from laundering solution onto fabric.
B. Arninoafk~ Pol~rsiloxane
The pro-fragrance silicone polymer of the present invention can be derived
from an aminoalkyl polysiloxane and a fragrance carbonyl compound by
o condensation_
The arninoalkyl polysiloxane of the present invention can include any
component which provides softening efficacy to, e.g., fabric or hair. The
aminoalkyl polysiloxane useful herein also can provide durability, wrinkle
control,
and water dispersability, along w'tth softening to the fabrics and the hair.
For
~s example, laundry detergents for fabrics; softeners far fabrics or textile
fibers,
cleaning products for the hair or the skin are useful.
Such known aminoalkyl polysiloxane that can provide the pro-fragrance
silicone polymer of the present invention are commercially available as an
amino-
denatured silicone poiymer_ Such amino-denatured pro-fr2~grance silicone
ao polymers include tradename DMS-A11, DMS-A12, DMS-A15, DMS-A21, pMS-
A32, AMS-132, AMS-152, AMS-162, AMS-233, ATM-1192, and ATM-1322,
supplied by Gelest, (nc. (US); tradename X-22-161AS, X-22-1B1A, X-22-1618,
KF-8012, KF-393, KF-859, KF-860, KF-861, KF-867, KF-869, KF-8$0, KF-8002,
KF-8004, KF-8005, KF-858, KF-864. KF-865, KF-868, and KF-8003, supplied by
zs ShinEtsu Chemical Industrial Co. Ltd.; tradename TSF4700, TSF4701,
TSF4702, TSF4703, TSF4704, TSF4705, TEX150, TEX151, TEX154, TSF4706,
TSL9346, TSL938f>, TSL9306, SF1921, SF1925, SF1708-D1, SM2658, SF1708,
SF1921, SF1925 and SM2658, supplied by Toshiba Silicones, KK; tradename
SM8702, SM8702C, SM8709, SF8411, SF8417, BY16-828, BY16-849, BY16-
so 850, BY16-853, BY16-853B, BY16-872, BY22-007, BY22-812, BY22-816, BY22-
819, and BY22-823, supplied by Toray Dow Corning Silicone, KK.
C. Fra rance Carbonv! Compound ,
Fragrance carbonyl compounds useful herein derives the pro-fragrance
silicone polymer of the present invention.
CA 02323488 2000-09-12
WO 99146318 PGTIUS98J04950
!3
The fragrance carbonyl compound can be saturated, unsaturated, linear.
. branched, or cyclic, preferably C7 or higher unsaturated. The formulae of
the
fragrance carbonyl compound can include alkyl, alkenyl, or aryl moieties. The
carbonyl compound can further include those having additional functional
groups
s such as alcohols, esters, or ethers as well.
Preferably, the fragrance carbonyl compound include a fragrance
aldehyde or a fragrance ketone, conventionally used for Condensation, for
example, a pro~fragrance schiff base formation.
Preferred fragrance aldehyde useful herein include an aliphatic aldehyde,
!o a terpenic aidehyde, and an aromatic aldehyde_
Nonlimiting examples of the aliphatic aldehyde include hexyl aldehyde
(caproaldehyde), heptyl aldehyde, octyl aldehyde (caprylaldehyde), nonyl
aldehyde (pelargonaldehyde), decyl aldehyde (capraldehyde), undecys aldehyde,
dodecyl aldehyde (lauric aldehyde), tridecyl aldehyde, 3,5,5-trimethylhexanal,
2-
t5 methyldecanal (methyioctylacetasdehyde), 2-methylundecanas
(methylnonylacetasdehyde), trans-2-hexenal (leaf aldehyde), cis-4-heptenal,
traps-2-cis-6-nonadienal (cucumber aldehyde), cts-4-decenal, trams--4-decenal,
1t)-undecen-1-at (undecenvic aldehyde), traps-2-dodecenal, 2,8,10-trimethyl-9-
undecenal, 2,6,10-trimethyl-5,9-undecadienal, 3,7-dimethyl-2,6-octadienal
20 (citral), 3,7-dimethyl-6~octen-1-al (citronellas), 7-
hydroxy.3,7..dimethyloctan-1-al
(hydroxy citronella!), p-mentha-1,8-dien-7-al (perilla aldehyde).
Examples of the terpenic aldehyde useful for the pro-fragrance silicone
polymer herein include 3,7-dimethyl-7-rnethoxyoctan-1-al
(methoxydihydrocitronellal), crtronellyioxy acetaldehyde, 2,4-diemthyl-3-
z5 cyclohexenys carboxyaldehyde, 2,4,f>~trimethyi-3-cyClohexene-1-
carboxyaldehyde (isocyclocitrat), 5-methoxy-octahydro-4,7-menthano-lH-indene-
2-carboxyaldehyde (scentenal), 4-{4-methyl-3-pentenyl)-3-cyclohexen-1-
carboxyaldehyde (myrac asdehyde), 4-(4-hydroxy-4-methyl-pentylj-3-cyclohexen-
1-carboxyaldehyde (lyral), 1-methyl-4-(4-methyl-pentyl)-3-~cyclohexen-
. 3o carboxyaldehyde {vernaldehyde), 4-(tricyclo[5.2.1.02,6]decylsden-8)-
butenal
(dupical), 7-formyl-5-isopropyl-2-methyl-bicyclo~[2_2.2]oct-2-ene (maceal), 2-
methyl-ø(2,6,6-trimethyl-1-cyclahexen-1-yl)-2-butenaf {boronal), 2-methyl-4-
(2,6,6-trimethyl-2-cyclohexen-1-yl)-butanal (cetvnal).
Examples of the aromatic aldehyde useful for the pro-fragrance silicone
~s polymer of the present invention inGude benzaldehyde, phenylacetaldehyde
CA 02323488 2000-09-12
WU 9914631 tS PCT/US98/04950
Z4
- (hyacinth aldehyde), 3-phenylpropanal (phenylpropylaldehyde), 3-pheny-2-
propenal (cinnamic aldehyde), 2-pentyl-3-phenyl-2-propenat (a-amyl cinnamic .
..
aldehyde), 2-hexyl-3-phenyl-2-propenal (a-hexyl cinnamic aldehyde), 2-
phenylpropanal (hydratropic aldehyde), 4-methoxybenzaldehyde (anis aldehyde),
s p-methylphenylacetaldehyde (p-tolyl acetaldehyde), 4-isopropylbenzaidehyde
(cumin aldehyde), 2-methyl-3-(4-isopropyiphenyl)-propanal (cyclamen aldehyde),
3-(p-t butylphenyl)-propanal, 3-(p-ethylphenyl)-2,2-dimethylpropanal (p-ethyl
2,2-
dimethylhydrocinnamic aldehyde), 2-methyl-3-(p-methoxyphenyl)-propanal, 2-
methyl-3-(4-t butylphenyl)-propanal (a-t butyl-a-methylhydrocinnamic aldehyde,
~o lily aldehyde), 2-hydroxybenzaldehyde (salicylic aldehyde), 3,4-
methylenedioxy
benzaldehyde {heliotropine), 2-methyl-3-(3,4-methylenedioxy-phenyl)-propanal
(helional), 4-hydroxy-3-methoxybenzaldehyde (vanillin), 3-ethoxy-4
hydroxybenzaldehyde {ethyl vanillin), 3,4-dimethyoxy-benzaldehyde fr~iethyl
vanillin): aldehydes having low volatility by virtue of incorporation of
bulky~~ polar
~s moieties.
The preferred fragrance ketone useful herein include an aliphatic ketone,
a terpenic and sesquiterpenic ketone, a cyclic ketone, or an aromatic ketone.
The ketones can be saturated, unsaturated, linear, branched, or cyclic,
preferably
including alkyl, alkenyl, or aryl moieties. The ketanes can include other
2o functional groups such as ethers or esters.
Nonlimiting examples of the aliphatic ketone includes 3-hydroxy-2
butanone (acetoine), 2,3-butanedione (diacetyl), 2-heptanone (methyl amyl
ketone), 3-octanone (ethyl amyl ketone), 2-octanone (methyl hexyl ketone), 2
undecanone (methyl nonyl ketone), 6-methyl-5-hepten-2-one, acetyl
2s diisoamylene (koavone).
Examples of the terpenic and sesquiterpenic ketone includes 1,7,7-
trimethyl bicyclo[2.2.1]heptan-2-one (camphor), 7,8 p-menthadien-6-one
(carvone), p-menthan-3-one {menthone), dp..menth-4(8)..en-3-one (dpulegone),
p-menth-1-en-3-one (piperitone), 1,3,3-trimethyi-bicyclo(2.2.1)heptan-2-one
30 {fenchone), 6,10,-dimethyl-5,9-undecadiene-2-one (geranyl acetone), acetyl
_
cedrene (cedryl methyl ketone), 5,6-dimethyl-8-isopropenylbicyclo-(4.4_pJ-'I-
decen-3-one (nootkatone), 4-(2,2,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-one
(a
-ionone), 4-(2,2,6-trimethyl-1-cyclohexen~1-yl)-3-buten-2-one (/3-ionone), 5
(2,2,6-trirnethyl-2-cyclohexen-1-yt)-4-pentan-3-one (a-methyl ionone), 5-
(2,2,6
ss trimethyt-1-eyclohexen-l..yl)-4-pentan-3-one (~-methyl ionone), 5-12,2,6-
trimethyt
CA 02323488 2000-09-12
WO 99r46318 PCTIUS98r0a~50
2-cyclohexen-1-yi)-3-methyl-3-buten-2-one {y-methyl ionone), 5-(2,2,6-
trimethyl..
1-cyclohexen~1-yl)-3-methyl-3-buten-2-one (S-methyl ionone), 1-(2,Z,6-
trimethyl-
2-cyclohexen-1-yl)-1,6-heptadien-3--one (ally) ionone), a-irane, p-irons, y-
irons, 1-
(2.2,6-trimethyl-2-cyclohexen-1-yl)-2-buten-1-one (a-damascone), 1-(2,2,6-
s trimethyi-1-cyclohexen-1-y!)-2-buten-1-one (~i-damascone), 1-(2,2,6-
trimethyl-3-
cyclohexen-1-yl)-2-buten-1-one (fi-damascone), 1-(3,3-dimethyl~-6-cyclohexen-1-
yl)-yenta-4-en-1-one (a-dynascone), 1-(3,3-dimethyl-1-cyclQhexen-'I-yl)-yenta-
4-
en-1-one (p-dynascone).
Examples of the cyclic ketone include 3-hydroxy-2-methyl--4H pyran-4-one
~o (maltol), 2-ethyl-3-hydroxy-4H pyran-4-one (ethyt maltol), 2,5~iemthy4-4-
hydroxy
2H furan-3-one, 4,5-dimethyl-3-hydroxy-5H-furan-2-one (sugar lactone), p-f
butylcyclohexanone, 2-amylGyclopentanone. 2-heptylcyclopentanone, 3-methyl
2-pentyl-2-cyciopenten-1-one (dihydrojasmone), 3-methyl-2-(2-cis-penten-1..y1)-
.2
cyalapenten-1-ane (cis jasrnone). 6 (or 7)-ethytidene-octahydro-5.8-methano-2H
~s benzopyrane (florex), 7-methyl- actahydro-1,4-methanonaphthalen-6(2f-~-one
{pIicatone), 4-cyclohexyl-4-methyl-2-pentanone, 1-(p-rnenthen-6(2)-yl)-1-
propanone, 2.2,5-trimethyl-5-pentylcyclopentanone, 4-{1-ethoxyvinyl)-3,3,5,5-
tetramethyl-cyclohexanone, 6,7-dihydro-'1,1,2.3,3-pentamethyl-~l(51-n-
indanone,
7-acetyl-1.2,3,4,5.8,7.8-octahydro-1,1,6,7-tetramethylnaphthalene (lso E
Super),
2o methyl 2,6,10-trimethyl-2,5,9,cyclododecatrien-1-yl ketone (trimofix "O").
Examples of the aromatic ketone includes, acetophenone {methyl phenyl
ketone), p-methyl acetophenone (p-tolyl methyl ketone), benzyl acetone, 7-
methyl-3,4-dihydro-(2I~-1,5-benzodipxepin-3-pne {calone), 4-(4-hydroxyphenyl)-
2-butanone (raspberry ketone), p-methoxyphenylbutanone (anisyl acetone), 4-(4-
zs hydroxy-3-methoxyphenyl)-2-butanone (Zingerone), 2-acetonaphthone (methyl
[3-
naphthyl ketone}, 4-phenyl-4-methyl-2-pentanone, and benzophenone (diphenyl
ketone). Other exemplary ketones include Ethyl ketopropinate (ethyl pyruvate),
isoamyl ketopropionate {isoamyl pyruvate), ethyl acetoacetate, ethyl Y-
ketovalerate (ethyl levulinate), methyl jasmonate, and methyl
dihydrojasmonate_
3o D. Method ef Makin Pro-fra rance Silicon P I m r
The pro-fragrance silicone polymer of the present invention can be
prepared by any method used for achieving a conventional condensation
reaction, e.g., Dean-Stark method with the acid-catalyzed reaction of an
aldehyde and an amine, preferably the primary amine. For making the pro-
CA 02323488 2000-09-12
WO 99/46318 PCT/L1~98104950
16
fragrance silicone polymer, preferably an aminoalkyl pofysiloxane is prepared
as
the primary amine. ,
The reaction can generally proceed under a catalyst and solvent. The .
preferred catalyst is a Lewis acid. Exemplary acidic catalysts are p--toluene
s sulfonic acid, methane sutfonic acid, sulfuric acid, hydrochloric acid,
suifosalicylic
acid, and mixtures thereof or supported sulfonic acid catalysts, e.g.,
AMB~RLYST 15T"". lNhen the catalysis is sensitive to strong acid conditions
and
can undergo undesirable side reactions, acid catalysts with pKa's among 3 and
4
are the most desirable to minimize unnecESSary side-reaction. Non-acidic
1o catalysts are useful in the present invention. Examples are Girder KSF,
boron
trifluoride etherate, potassium hydrogen sulfate, copper sulfate, and ion
exchanger.
The solvent useful herein can be any solvent used in the conventional
condensation or dehydration by Dean-Stark apparatus, which has sufficient
1s stability during the process. "SuffiClent stability" herein means a solvent
which
does not cause decomposition or degradation as a side-reaction during the
heating step. The preferred solvent is hexane, benzene, or toluene. See
Meskens. F., Synthesis, (7) 501 (1981) and Meskens, F., Jannsen Chim Acta (1 )
{1983), Bunton, C.A. et al, J. Org. Chem. (44), 3238, (197$), and Cort, O., et
2o al, J. Org. Chem. (51), 1310 (1986, Meskens, F., Synthesis, (7), 501,
(1981) and
Lu, T.-J, et al. J. Org. Chem_ (fi0), 2931, (1995).
A preferred method of making the pro-fragrance silicone polymer of the
present invention includes the steps of (a) adding the aminoaikyl polysiloxane
and the fragrance carbonyl compound to a environment wherein the carbonyl
zs compound is selected from the group consisting of an aldehyde, a ketone,
and
mixtures thereof; and (b) pressurizing the mixture of step (a), wherein the
pressurization causes the fragrance carbonyl compound and the aminoalkyl
polysiloxane to react and form the pro-fragrance silicone polymer. Herein,
"pressurizing" means changing any condition of the reaction for deriving the
pro-
3o fragrance silicone polymer from the aminoalky) polysiloxane and the
fragrance - _
carbonyl compound, by increasing the temperature. Pressurizing herein includes
the conditions produced by physically decreasing the volume by pressure,
filling
the environment with a gas stream which provides high pressure, and heating;
preferably the pressurization herein is achieved by heating. Preferably, step
(a)
ss further comprises adding a catalyst, solvent, a dehydrating agent, or
mixtures
CA 02323488 2000-09-12
WO 9914631$ PGT/U598/04950
17
thereof. The preferred solvent is hexane, benzene, or toluene. The catalyst
useful herein includes an acidic catalysts (e.g., p-toluene sulfonic acid,
methane
sulfonic acid, sulfuric acid, hydrochloric acid, and sulfosalicyiic acid
preferably)
_ and non-acidic catalysts (e.g., Girder KSF, bore~n trifluoride etherate,
potassium
s hydrogen sulfate, copper sulfate, and ion excharger), preferably a Lewis
acid.
E. Test Methods for Determining H~rdrolyrsis Half life (t-1121
Hydrolysis half life is the measurement used to determine the ease with
which the pro-fragrance silicone polymer undergoes acid hydrolysis and thereby
releases fragrance components) upon exposure to acid conditions. The pro-
~o fragrance silicone polymer of the present invention preferably has a half-
life of
less than f0 minutes, under the described hydrolysis conditions at pH 0; more
preferably a half-life at pH 2 of less than f0 minutes.
When the pro-fragrance silicone polymer is used in granular detergents,
the more reactive pro-fragrance silicone polymer, that is, those with half-
life at pH
~s 2 of less than one minute, are most suitable, although those having a half-
fife of
less than 60 minutes at pH 0 are also useful. For liquid detergent
applications,
pro-fragrance silicone polymers having a half life of less than 60 minutes at
pH 0,
and half life greater than one minute at pH 2 should preferably be used.
Hydrolysis half life is determined by UVIV spectroscopy in a 90110
2o dioxanelwater system at 30°C by following the appearance of the
carbonyl
absorbance. Because of the hydrophobicity of the pro-fragrance silicone
polymer
herein, a high dioxanelwater ratio is needed to ensure solubility of the pro
fragrance silicone polymer. The pH of the water used is achieved by using
aqueous HCI. The concentration of the pro-fragrance silicone polymer in the
25 dioxanelwater system can be adjusted to achieve convenient, measurable
absorbance changes.
All measurements are carried out using a Hewlett Packard 8452 A Diode
Array Spectrophotometer using quartz 1 cm path length cuvette cells. Materials
used include 1,4-dioxane HPLC Grade 99.9°/a (Sigma-Aldrich), 1 N HCI
volumetric
. 3o solution {J.T. Baker), deipnized water filtered with MiIIiQPIus
(Millipore) at
resistivity of '18.2 M Ohm cm_ The pH's are measured using an 4rion 230 A
_ standardized with pH 4 and pH 7 buffers. The 1 N HCI standard is used
directly
for pH 0 conditions. For pH 2 conditions, 1 N HCI is diluted with deionized
water.
Pro-fragrance silicone polymer is weighed out in a l0ml volumetric flask
35 using an analytical balance {Mettler AE 200) Precision is 1/10 mg. The
weighed
CA 02323488 2000-09-12
WO 99146318 PCTNS98IOA950
t8
material is dissolved in about 8ml dioxane. Both the dioxane solution of pro-
fragrance silicone polymer and aqueous acid solution prepared as described
supra are pre-heated in their separate containers to a temperature of 3p _+
0.25°C by means of a water-bath. 1.OOOmI of aqueous acid solution is
added to -
s the pro-fragrance solution by means of an Eppendorf pipetter. This is
followed
by diluting to the l0rnl mark with dioxane- Hydrolysis time is measured,
starting
upon addition of the acid. The pro-fragrance solution is mixed for 30 seconds
by
shaking, and the solution is transferred to a quartz cuvette. The absorbance
of
the pro-fragrance solution (A~ is followed at a regular series of time
intervals,
~o and the cuvette is kept in the water-bath at the above-indicated
temperature
between measurements. Initial absorbance (Ao) measurements are carried out
using an equal concentration of pro-fragrance in a 90110 v/v dioxane -
deioniaed
water solution, and final absorbance (Af) measurements are taken using the
hydrolyzed pro-fragrance solution after the hydrolysis is complete- ; The
~s wavelength at which the hydrolysis is followed is chosen at the wavelength
of the
absorbance maximum of the starting fragrance carbonyl compound such as
aldehydes or ketones_
Reaction half lifes are determined using conventional procedures. The
observed first-order rate constant (kobs) is determined by slope of the fine
2o provided by plotting the following function vs time (min):
Ln [(Ao - Af)I (At - Af)l
wherein the function is the natural log of the ratio between the absorbance
difference at initial time (Ao) and final time (Af) over the absorbance
difference at
time t (A~ and final time (Af).
zs Half life as defined herein is the time required for half of the pro-
fragrance
silicone polymer to be hydrolyzed, and is determined from the observed rate
constant (kobs) bY the following function:
Ln ( 1/2) - -kobs t 112
F. Method of Use
3° I. Acceotabl Carrier
The pro-fragrance silicone polymer of the present invention can be used in
a variety of the products in different industries. For example, the products
which
desire the release of fragrances and providing softness to the hair and
fabrics.
In another aspect of the present invention, a composition has an effective
se amount of the pro-fragrance silicone polymer and an acceptable carrier.
Herein,
CA 02323488 2000-09-12
WO 99/46318 PC'jYU598/04950
19
- "acceptable carrier' means one yr more compatible solid or liquid filler
diluent or
. encapsulating substances which do not substantially reduce the efficacy of
the
polymer. The specific carrier will depend upon the final form of the
composition.
For example, when the composition is a laundry detergent composition, the
s acceptable carrier would preferably be carriers which are acceptable during
washing and drying, e.g., detersive surfactants, builders, soil releasing
agents,
and the like.
Detergent Comr~osition
The pro-fragrance silicone polymer can be used in 'a detergent
1o composition. Preferably, the pro-fragrance silicone polymer formulated in a
detergent composition is at levels of from about 0.0001 % to about 20%; more
preferably from about 0.01 % to 10%.
The pro-fragrance silicone polymer can be used as the sole fragrance
compound of the detergent composition, or in combination with other pro
1s fragrances andlor in combination with other fragrance materials, extenders,
fixatives, diluents and the like. For example, incorporation of the pro-
fragrance
silicone polymer into a waxy substance, such as a fatty triglyceride, may
further
improve storage stability of the present pro-fragrance silicone polymer in
granular
laundry detergents, especially those comprising bleaches. In liquid or gel
forms
zo of detergent compositions, hydrophobic liquid extenders, diluents or
fixatives can
be used to form an emulsion wherein the pro-fragrance silicone polymer is
further
stabilized by separating it from the aqueous phase.
Nonlimiting examples of such stabilizing materials include dipropylene
glycol, diethyl phthalate and acetyl triethyl citrate. Just as there exist
2s - hydrophobic perfumery ingredients which can be used to stabilize the pro
fragrance silicone polymer, there also exist detergency ingredients which also
have a perfume stabilizing effect and can be formulated with the pro-fragrance
silicone polymer. Such ingredients include fatty acid amines, low foaming waxy
nonionic materials commonly used in automatic dishwashing detergents, and the
- 30 like. In general, where pro-fragrance silicone polymers are used along
with other
fragrance materials in detergent compositions herein, it is preferred that the
pro-
fragrance silicone polymer be added separately from the other fragrance
materials.
CA 02323488 2000-09-12
w0 9914b318 PCT/US98I04950
2Q
- a. Detersive Surfactants
The detergent composition incorporating a detersive surfactant, preferably _
a synthetic detergent surfactants, have a detergent level of from about 0.5%
to
about 50%, by weight. The detergent compositions containing soap preferably
include from about 10% to about 90°i6 soap_
Many detergent surfactants which are conventional for detergent
surfactants can be used. Mixtures of anionic and nonionic surfactants are
especially useful. Other conventional useful surtactants are listed in
standard
texts. See also U.S. Patent 3,664,961, issued May 23, 1972.
1o The detergent compositions herein, preferably, have a pH of from about
7.1 to about 13, more typically from about 7.a tQ about 9.5 for liquid
detergents
and from about 8 to about 12 for granular detergents when measured at 1
concentration of the distilled water at 20°C _
b. Additional Detergent Ingredients
~ s In addition to the pro-fragrance silicone polymer, detergent compositions
may further include one or more additionai detergent ingredients, commonly
used
in detergent products, such as materials for assisting or enhancing cleaning
performance, treatment of the substrate to be cleaned, or to modify the
aesthetics of the detergent composition (e.g., conventional perfumes,
colorants,
2o dyes, etc.). Such additional ingredients are known to those skilled in the
art. The
following are illustrative examples of other detergent ingredients.
(i) Builders - Detergent builders can optionally be included in the
compositions herein to assist in controlling mineral hardness and in the
remaval
of particulate soils. Suitable builders include those of U_S. Patent
3,308,067,
2s issued Mar. 7, 1967; 4,144,2.26, issued Mar. 13, 1979 and 4.246,495, issued
Mar. 27, 1979. Inorganic as well as organic builders can be used.
The level of builder can vary widely depending upon the end use of the
composition and its desired physical form. When present, the compositions will
typically comprise at least about 1 % builder. Preferably, liquid formulations
3o typically comprise from about 5% to about 50%, and granular formulations _
typically comprise from about 10% to about $0%. Lower or higher levels of
builder, however, are not meant to be excluded_
ii Soil Release Agents - Soil Release agents are desirably used in
laundry detergents of the instant invention. Suitable soil release agents
include
3s those of U.S. Patent 4,968,451, issued Nov. 6, 1990; the nonionic end-
capped
CA 02323488 2000-09-12
WO 99/46318 PGTIUS9810495a
21
1,2-propylene/polyoxyethylene terephthalate polyesters of U.S. Patent
4,711,730, Dec. 8, 1987; the partly- and fully- anionic-end-capped oligomeric
esters of U.S. Patent 4,721,580, issued Jan. 26, 1988; the nonionic-Capped
block
polyester oligomeric compounds of U.S. Patent 4,702,857, issued Oct. 27, 1987;
and the anionic, especially sulfoaroyl, end-capped terephthalate esters of
U.S.
Patent 4,877,89fi, issued Oct. 31, 1989. Another preferred soil release agent
is
a suffonated end-capped type described in U.S. Patent 5,415,807.
c. Other Ingiredients
The compositions herein can contain other ingredients such as enzymes,
~o bleaches, fabric softening agents, dye transfer inhibitors, suds
suppressors, and
chelating agents, all well known within the art.
d. Formulations With or Without Conventional PerFumer~r Materials
While the pro-fragrance silicone polymer of the present invention can be
used alone and simply mixed with essential detergent ingredient, most notably
~s surfactant, they can also be desirably combined into three-part
formulations
which combine (a) a non-fragrance detergent base containing one or more
synthetic detergents. (b) one or more pro-fragrance silicone polymers in
accordance with the invention and (c) a fully-formulated fragrance_ The latter
provides desirable in-package and in-use (wash-time) fragrance, while the pro-
zo fragrance provides a long-term fragrance to the laundered textile fabrics.
It is
preferred that the pro-fragrance silicone polymer be added separately from the
conventional fragrances to the detergent compositions.
e. Formulations with other Special-Purpose Fragrance Delivering
Compounds
25 laetergent compositions in accordance with the present invention may
further, optionally, contain other known compounds having the capability to
substantively enhance a fragrance_ Such compounds include, but are not limited
to, the aluminum alkoxides such as isobutylaluminium diferanylate as disclosed
in U.S. Patent 4,055,634, issued Oct. 25, 1977; or the known ~titanate and
3a zirconate esters or oligoesters of fragrant materials such as those
disclosed in
U.S. Patent 3,947,574, issued March 30, 1976 and U.S. Patent 3,779,932,
issued Dec. 18, 1973. When using such organoaluminium, organotitanium or
organozinc derivatives, they may be incorporated into the detergent
compositions
of the present invention described herein at their art-known levels.
CA 02323488 2000-09-12
WO 99/46318 PCT/US98I04950
22
EXAMPLES
The pro-fragrance silicone polymer of the present invention may be .
prepared by the following examples. _ The following examples further describe
and demonstrate embodiments within the scope of the present invention. The
examples are given solely fpr the purpose of illustration and are not to be
construed as limitations of the present invention, as many variations thereof
are
possible without departing from the spirit and scope of the invention.
Example 1
This example describes the preparation of pro-fragrance silicone polymer
~o of structure V-a.
A 50 ml portion of benzene, 4D mmol of 2-methyl-3-(4-t butylphenyl)-
propanat (lily aldehyde), 20 mmol of aminopropyl polysiloxane, and 0_031 mot
of
p-toluenesulfonic acid are stirred in a round-bottomed flask with Dean-Stark
attachment under reflex for 24 hours. The reaction mixture is washed several
times with aqueous Na2C03 solution and is extracted as an organic solution.
The organic solution is then dried over Na2C03 and Na2SO4. The organic
solution is evaporated in vacuo after filtration. A 95.3% yield of the
compound
(V-a) is obtained.
An embodiment of this example is shown in the following scheme:
o '
CH3 CH3
!i
H~N(CH2)3-~Si-O~---Si-(CHZ)3NHz
CH3 CH3 p-TsOhi in benzen
a
N(C~ZYs-~
i ~ (V-a)
Example 2
This example describes a the preparation of pro-.fragrance silicone ' ,
polymer having structure V-b_
2s A 50 mf portion of benzene, 20 mmol of 2-hexyl-3-phenyl-2-propenal (a-
hexyl cinnamic aldehyde), '10 mmol of aminopropyl polysiloxane, and 0.016 mol
CA 02323488 2000-09-12
WO 99146318 PCTIUS98104950
23
of p-toluenesulfonic acid are stirred in 2~ round-bottomed flask with Dean-
Stark
attachment under reflex for 24 hours. The reaction mixture is washed several
times with aqueous Na2C03 solution and is extracted as an organic solution.
The organic solution is then dried over Na2COg and Na2S0~. The organic
solution is evaporated in vacuo after filtration. A 97.5% yield of the
compound
(V-b) is obtained.
Example 3
This example describes the preparation of a pro-fragrance silicone
polymer having structure V-c.
~o A ~0 ml portion of benzene, 20 mmol of 2,4-dimethyl-3-cychexyl
carboxyaidehyde, 7 0 mmol of aminopropyi polysiloxane, and 0.016 mol of p-
toluenesulfoniG acid are stirred in a round-bottomed flask with Dean-Stark
attachment under reflex for 24 hours. The reaction mixture is washed several
times with aqueous Na2CO3 solution and is extracted as an organic solution.
~s The organic solution is then dried over Na2C03 and Na2SQ4- The organic
solution is evaporated in vacuo after filtration. A 99.2% yield of the
compound
(v-c and c') is obtained.
CA 02323488 2000-09-12
WO 99/46318 PCT/US98I04950
24
- Example 4
This example describes a granular laundry composition having the pro-
fragrance silicone polymer of Example 1,
,_Pro=fragrance Silicone Polymer_af ~_p
Example 1
.
. C11 _C13 21
Dodecyl Benzene Sulfonate 0
_
_ _
________ :
____ __.
-_
C12-C13
Alkyl Ethoxylate EO 1-8
- 1.2
_
-_____-__
-_._ ...
..... __~.0___
Sodium_Tripolyphpsphate_._________________
..zeolite Na 4A_..____....
...._._.._.._......_..___ __ .___ ____
_ Sodium Silicate 2_0 ratio_____________2.0
____._._
-__.
__..____ __23.4-__
_ Sodium Carbonate..__.____.________._________
__Enzyme_(SavinaseTMandlor LipolaseT'~frorn1
Novo 4
)
_ ..__
_ _
Carbo meth I Cellulose ._.
~x
.. 0.3
._
_._ ..y.._ _._.________
-__
_____ . __ ..- 0
. Anionic_SoiLRelease Agent *~ 3 _.
_._......_ .
Bd htener ____.__________._ .._-0
___
.. .2
_..___._._.______._._________________
_
_ _ ____
. Silicone Su _ _
s Suppressor (Dow Coming Corp) 0_2 .
I
d ~:3
_
Perfume~'2 ______________________ ________.__._........
_______ __.
. Sodium Sulfate.~~____.___________________________._____...
0.5
___ __ up to
Moisture balance ' 100
;1 See U.S. 4,968,451
'2 Perfume composition of the following formula:
_ Benzyl_sali late _ 20
___...._____ ___.._
_ Ethylene brassylate.________________ _..._._.....
_ Galaxolide (50°~ soln._in benzyl benzoate) 20
Hexyi cinnarnic aldeh~de__._.____ ___.._.____.._
Tetrah dro linalool 20
..x ___________________________.__.__.__
toil .__________ .__1~0-__ .
The embodiments disclosed and represented by the previous examples
have many advantages. For example, the pro-fragrance silicone polymer
~o obtained has at feast one schiff base moiety, thereby the compound can
provide
a fabric softening effect and desirable odor including release of fragrance by
hydrolysis of the compound.
CA 02323488 2000-09-12
WO 9914638 PCT/US98I04950
It is understood that examples and embodiments described herein are for
illustrative purpose only and that various modifications or changes in right
thereof
will be suggested to one skill the art and are to be included in the spirit
and
purview of this application and scope of the appended claims.
5