Note: Descriptions are shown in the official language in which they were submitted.
CA 02323764 2000-11-15
1
REFRACTORY LINING SYSTEM FOR HIGH WEAR
ARRA nF HIGH TEMPERATURE REACTION VESSEL
The present invention relates generally to
refractory lining systems for high temperature reaction
vessels. More particularly, the present invention
relates to a novel refractory lining system for a high
temperature reaction vessel comprising a refractory
ring lining composed of high thermal conductive
refractory brick and a dimensionally stabilizing heat
transfer medium. The system has particular utility in
high temperature reaction processes such as smelting
and direct ironmaking or steelmaking, but may be used
in other systems and high temperature reaction methods
as well.
CA 02323764 2000-11-15
2
Background Of The Invention
Direct ironmaking and steelmaking processes are
known. One such process involves in-bath smelting,
where coal, partially reduced iron ore, and oxygen are
reacted in an iron bath. The overall process generally
includes the three constituent processes of (1)
carburization of the metal bath, (2) decarburization of
the bath, and (3) heat transfer to the bath. Chemical
reduction reactions primarily take place in the bath,
while oxidation reactions primarily take place above
the bath, e.g., in a foamy slag layer. The reaction
process produces CO and H2 gases, which are
postcombusted with oxygen in and above the foamy slag
to generate heat for driving the process. This
postcombustion produces very high temperatures in the
reaction process zone and the postcombustion zone of
the smelting vessel.
Control of the reaction process in a direct
steelmaking process is important for process efficiency
and safety. The reaction process primarily is
controlled by regulating the feed rates of the raw
materials, which include ore pellets, coal, oxygen, and
flux. The oxygen feed rate, coal feed rate, and ore
feed rate generally are set according to the desired
steel production rate, but may be modified to adjust
the operating temperature or char volume in the
reaction process. The char volume is controlled to
achieve a stable foamy slag, and the slag basicity,
CA 02323764 2000-11-15
3
which affects the stability of the foam, is controlled
by the rate of flux addition.
These raw materials are introduced to the reaction
process through tuyeres and lances. The gas blowing
practice, including tuyere or lance position, hard or
soft blow, primary-to-secondary oxygen ratio, and
nitrogen (inert gas) stirring energy, has a lesser, but
still significant, effect on the reaction process. In
particular, the degree of postcombustion is largely
controlled by lance position and by the primary-to-
secondary oxygen ratio for a given lance design.
Primary oxygen is oxygen that is blown well into the
slag (a "hard blow") to convert char into carbon
monoxide and generate local heat of combustion for the
reaction process. Secondary oxygen is oxygen that is
blown into the slag and/or free space (head space)
above the slag (a "soft blow") to convert rising carbon
monoxide into carbon dioxide (a process known as "post
combustion") and to generate additional heat of
combustion for driving the reaction process.
The reaction vessel in a direct steelmaking
process is exposed to extreme, and sometimes critical,
conditions. For example, the operating parameters in a
direct steelmaking process of the present invention may
include molten slag and gas temperatures of up to
1800°C, and the molten slag composition may have a
basicity value in the range of about 1.0 to 1.5, where
the basicity value represents the ratio of basic oxides
to acid oxides. For example, in the direct steelmaking
CA 02323764 2000-11-15
4
process of the present invention, the basicity value
represents the wt.% ratio of lime to silica.
Accordingly, successful operation of the reaction
process requires mechanical, chemical, and thermal
contairunent .
It is known to use a carbon bosh refractory lining
in a high temperature blast furnace, and to provide
stave coolers placed inside the bosh shell in such a
manner that they cover the entire inner surface of the
bosh shell. These linings generally include refractory
bricks. However, conventional blast furnaces do not
operate under the above-described extreme conditions of
a direct steelmaking process. Rather, blast furnaces
generally operate with slag temperatures of about
2800°F or below, whereas a direct steelmaking process
according to the present invention may operate with
slag temperatures in the range of about 3000 to 3200°F.
Refractory bricks in the postcombustion zone of a
steelmaking process of the present invention thus are
subject to high wear conditions from a number of
factors, including mechanical wear from the abrasive
washing action of molten slag, chemical wear from the
slag constituents, and thermal wear from exposure to
high temperatures in the postcombustion zone.
Replacement of refractory lining is costly, both for
material and labor, and in terms of system down time.
CA 02323764 2000-11-15
Summary Of The Invention
It is therefore an object of the present invention
to provide an improved high temperature reaction vessel
and refractory lining system for high temperature
reaction processes, such as direct ironmaking and
steelmaking.
Another object of the present invention is to
extend the life of refractories in the most severe wear
areas of a high temperature reaction vessel, such as
the postcombustion zone of a smelting vessel.
Yet another object of the present invention is to
provide a refractory-lined high temperature vessel
having a stable refractory lining thickness during high
temperature operation.
Still another object of the present invention is
to provide a cost efficient refractory-lined high
temperature reaction vessel for a direct ironmaking or
steelmaking process.
In one aspect, the present invention provides a
refractory-lined high temperature reaction vessel
comprising a refractory ring lining with a surface
exposed to the interior of the vessel, the lining being
constructed of refractory brick, a hollow cooler
exterior to the refactory ring lining, and a heat
transfer medium disposed between the refractory ring
lining and the cooler. The heat transfer medium fills
the space between the refractory brick and the cooler,
and is composed of a material that accommodates
CA 02323764 2000-11-15
6
relative movement between the refractory brick and the
cooler.
In another aspect, the present invention provides
a refractory brick comprising magnesia (Mg0) and
graphite. The brick is manufactured such that the
graphite particles have an orientation providing a
relatively high thermal conductivity in one direction
through the brick, that is, a thermal conductivity in
one direction that is higher than the thermal
conductivity in directions perpendicular to that
direction. The graphite preferably is flake graphite,
and preferably is present in an amount within the range
of about 10 to 20%, based on the weight of the brick.
The graphite also preferably has a particle size
distribution selected to provide maximum brick density.
In yet another aspect, the present invention
relates to a method of manufacturing the refractory
brick. The method generally includes the steps of
mixing magnesia (Mg0) and graphite in an amount
sufficient to provide a predetermined thermal
conductivity and refractory stability, and pressing the
magnesia - graphite mixture to form a refractory brick
having a graphite particle orientation that is
favorable to high thermal conductivity in one desired
direction through the brick.
In still another aspect, the present invention
relates to a smelting method for use in a vessel that
includes a hollow cooler surrounding a refractory
lining having a surface that faces the interior of the
CA 02323764 2000-11-15
7
vessel. The cooler includes at least one cooler panel
having a first surface in thermal contact with the
refractory lining ("hot surface", also called "cooling
surface"), a second surface in contact with a coolant
passing through the cooler panel (coolant heat transfer
surface), and a thermal conductivity bridge between the
first and second surfaces, so that heat may be
withdrawn from the refractory lining and exhausted from
the vessel by the coolant. In one embodiment, the
first and second surfaces merely are opposite sides of
the radially inner wall of a hollow cooler panel made,
for example, of copper, in which case the thermal
conductivity bridge is the copper that extends between
the first and second surfaces, i.e., the thickness of
the wall. The method comprises the steps of forming a
layer of slag on a melt in the vessel, forming a
protective frozen layer of slag on the interior-facing
surface of the refractory lining in a zone where the
surface contacts the layer of slag, and maintaining the
protective frozen layer at or about the softening point
of the slag.
These and other objects, aspects and features of
the present invention readily will be apparent from the
following detailed description of the preferred
embodiments, considered together with the accompanying
drawings.
CA 02323764 2000-11-15
8
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a cross-sectional view of a refractory-
lined high temperature reaction vessel according to a
first embodiment of the present invention.
Fig. 2 is a horizontal cross-sectional view of a
high wear area of the refractory lining of the vessel
of Fig. 1.
Fig. 3 is a schematic cross-sectional view of the
refractory-lined vessel of Fig. 1 in the high wear area
of the vessel.
Fig. 4 is a perspective view of a Mgo-graphite
brick of the present invention, and a method of making
such brick.
Fig. 5 is a graph illustrating the calculated
mechanical stability thickness of a Mg0-graphite
refractory brick having a 10 wt.% graphite content.
Fig. 6 is a graph illustrating the calculated
mechanical stability thickness of a Mg0-graphite
refractory brick having a 20 wt.% graphite content.
Fig. 7 is a cross-sectional view of a refractory-
lined high temperature reaction vessel according to a
second embodiment of the present invention.
Fig. 8 is a cross-sectional view of a refractory-
lined high temperature reaction vessel according to a
third embodiment of the present invention.
Fig. 9A is a cross-sectional view of a refractory-
lined high temperature reaction vessel according to a
fourth embodiment of the present invention.
CA 02323764 2000-11-15
9
Fig. 9B is an enlarged detail view of an
alternative embodiment of the reaction vessel wall of
Fig. 9A, including a cooler having an L-shaped cross-
section.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Referring now to the drawings, wherein reference
numerals ending in the same two digits designate like
or similar elements in the different embodiments,
preferred embodiments of a refractory-lined high
temperature reaction vessel of the present inventian
are illustrated in connection with a direct steelmaking
process.
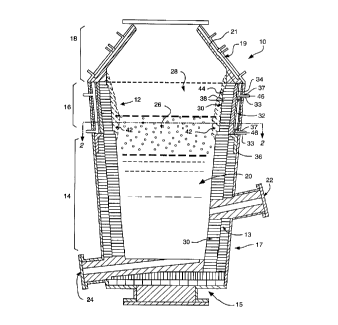
Fig. 1 illustrates in cross-section a high
temperature reaction vessel l0 including a refractary
lining system 12 of the present invention. The vessel
10 generally includes a lower portion 14, a central
portion 16, and an upper portion 18.
The lower vessel portion 14, which includes the
hearth 15 and lower bosh 17 of the vessel 10, may be
conventional. The lower vessel portion 14 holds the
hot liquid metal (e.g., iron or steel) 20 during the
reaction process, and generally is composed of
conventional materials including refractory brick.
Specifically, as shown in Fig. 1, the lower vessel
portion may include a first refractory brick lining 30,
e.g., composed of a refractory material compatible with
the reaction process materials (i.e., chemically
CA 02323764 2000-11-15
compatible with metal and slag) and a second refractory
brick lining (safety lining) 13 composed of a different
refractory brick having, e.g., a high thermal
insulation characteristic and high strength. The lower
vessel portion 14 also may include various submerged
tuyeres or taps, as is known in the art. For example,
in the embodiment of Fig. 1, the vessel 10 includes a
charge hole 22 and a drain hole 24, shown in
longitudinal cross-section.
10 The upper vessel portion 18 also may be
conventional, and may include various lances and vents.
As shown, the upper vessel portion 18 preferably
includes a truncated conical cap formed by hollow
cooling panels 19. Panels 19 are made of copper and
are water-cooled. The surface of each cooling panel
19 that faces the interior of the vessel 10 optionally
may have a refractory material coating 21 thereon.
In the direct steelmaking process of the present
invention, the various reaction elements, e.g., coal,
iron ore, flux, oxygen and inert gases (e. g.,
nitrogen), are introduced or blown into the vessel
through lances or tuyeres, and off-gases are vented to
an off-gas system through vents, as is known in the
art. The upper vessel portion 18 of the present
invention is configured to enclose and seal the
reaction vessel 10, to maintain the reaction process at
a superatmospheric pressure. The pressure within the
vessel 10 is controlled by regulating the amount of gas
allowed to flow from the reaction vessel 10 to an off-
CA 02323764 2000-11-15
11
gas system (not shown). As noted above, control of the
pressure in the reaction vessel 10 directly affects the
efficiency of the reaction process. For example, the
pressure in the vessel 10 directly affects the
formation of a slag layer 26, which defines in part a
high wear area of the reaction vessel 10, and the size
and dynamic characteristics of the slag layer 26
directly affect the efficiency of the reaction process,
as is known in the art. The present system
successfully has been operated at a pressure in the
range of about 10 to 12 psi, and it is anticipated that
the reaction process of the present invention will be
operated at greater pressures, for example, up to about
50 psi. Those skilled in the art readily will be able
to determine the optimal pressure or range of pressures
suitable for operation of various reaction processes in
accordance with the present invention.
The central vessel portion 16 generally spans at
least a portion of the layer of foamy slag 26 and at
least a portion of the postcombustion area 28
(generally defined by respective bold dashed lines in
Fig. 1), which together constitute a high wear area of
the vessel 10 when the vessel is used in a direct
steelmaking process of the present invention. As
shown, the central portion 16 preferably includes a
refractory lining system 12 composed of refractory
brick lining 30, hollow cooler panels 32, and a ram
mixture 34 disposed between the brick lining 30 and the
cooler panels 32.
CA 02323764 2000-11-15
12
The system 12 also preferably includes a metallic
outer shell 36 to provide additional structural support
to the vessel 10. In particular, nipples 33 of cooler
panels 32 may be inserted through holes 37 in the outer
shell 36 and fixed thereto, e.g., by welding, to
securely fix and support cooler panels 32 in the
refractory lining system 12. The shell 36 preferably
is made of high strength carbon steel, such as ASTM
A633 grade C. However, those skilled in the art
readily will be able to select a material suitable for
any application.
Fig. 2 is an enlarged horizontal cross-sectional
view of the central vessel portion 16 taken along
sectional line 2-2 of Fig. 1. As shown in Figs. 1 and
2, the refractory brick lining 30 includes refractory
bricks 38 which generally are assembled as a wall of
concentric rings or courses, using a conventional key-
up construction. In the present embodiment, these
rings are assembled in a key-up construction with a
horizontal expansion allowance in the range of about
0.3 to 0.7%, preferably about 0.3%, such that expansion
of the bricks 38 upon heating of the reaction vessel 10
to an operative temperature causes contact between
adjacent bricks. Of course, it will be appreciated
that the assembly and expansion allowance may vary in
accordance with the desired application, and those
skilled in the art readily will be able to modify the
assembly accordingly.
CA 02323764 2000-11-15
13
Fig. 3 is an enlarged schematic cross-sectional
drawing of a portion of the refractory lining 12 of the
high temperature reaction vessel 10 of the present
invention in the high wear slag layer and
postcombustion zone during stable operation in a direct
steelmaking process. As shown therein, during the
direct steelmaking process of the present invention,
the refractory brick lining 30 generally forms the
interior surface of the vessel 10 adjacent the layer of
foamy slag 26. The cooler panels 32 are located
radially outside the refractory brick lining 30, and
the ram mixture 34 is disposed between the refractory
brick lining 30 and the cooler panels 32. Shell 36
surrounds cooler panels 32 and provides additional
support to the refractory lining 12.
In one aspect, the direct steelmaking method of
the present invention includes the step of continuously
extracting an amount of heat from the layer of foamy
molten slag 26 sufficient to form and maintain a
protective layer of "frozen" slag 42 on the interior-
facing surface ("hot face") 44 of the refractory brick
lining 30 in at least a portion of a high temperature
reaction zone (high wear zone) of the reaction vessel
10. This dynamic frozen slag layer 42 is illustrated
in Figs. 1 to 3 by dashed cross-hatching. As shown,
the frozen slag layer 42 is formed between the foamy
molten slag 26 and the refractory brick lining 30.
Moreover, since the reaction process is a violent,
dynamic process, the foamy slag splashes and spatters
CA 02323764 2000-11-15
14
on the interior face 44 of the reaction vessel
throughout the postcombustion area 28 and head space.
Therefore, it will be appreciated that the protective
frozen slag layer 42 effectively prevents excessive
wear or failure of the refractory brick lining 30 in
the high wear areas during the reaction process, by
preventing the constantly moving molten foamy slag 26
from contacting the refractory bricks 38 of the
refractory brick lining 30, which contact gradually
would erode the bricks by mechanical and chemical wear.
It also will be appreciated that the frozen slag layer
42 forms a thermal insulative layer that effectively
reduces thermal wear or failure of the refractory
lining system 12 in the high wear area due to the high
temperatures generated by the postcombustion reaction.
Referring to Figs. 1 to 3, the protective frozen
slag layer 42 is formed and maintained by providing a
refractory cooling system 12 capable of conducting
sufficient heat from the interior face 44 (e. g.,
adjacent the layer of molten foamy slag 26) to the
cooler panels 32, to maintain a thin protective frozen
slag layer 42 at or about the softening or melting
point of the slag. Thus, the refractory cooling system
12 must be designed and installed in such a manner as
to obtain a high degree of consistent heat transfer
from the slag layer 26 and postcombustion area 28
through the refractory brick lining 30 and ram mixture
34 to a coolant 46 circulating through the cooler
panels 32.
v
CA 02323764 2002-04-03
Fig: 4 schematically illustrates a preferred
embodiment of a refractory brick 38 of the present
invention. Generally, the refractory brick 38 is
composed of Mg0-graphite. Magnesia (Mg0) is the
primary constituent of the refractory brick because of
its high refractory characteristics and its
compatibility with smelting process stags (it is
preferred to also include magnesia in the layer of
slag, in an amount sufficient to promote formation of
a slag coating). Graphite 48 is added to increase the
thermal conductivity of the brick. The refractory
brick 38 also includes an effective amount of resin
binder, preferably a liquid phenolic resin, in an
amount in the range of about 2 to 6 wt.%, preferably
about 3 to 3.5 wt.%. In the present embodiment,
commercially available Quaker Oats UP-44O resin or
Borden RL-9S2 resin was used, in an amount in the
range of about 3 to 3.5 wt.%. The refractory brick 38
also may include a number of other constituents,
including antioxidants such as elemental Mg, A1, or
Si, which protect the brick from oxidation during
initial heat-up and during the reaction process, by
forming metal carbides at the hot-face 44. in the
preferred embodiment, about 2 to 6 wt.% of A1 or Al-Mg
alloy is included, preferably about 3 to 4 wt.%. In
the present embodiment, an Al-Mg alloy comprising
about 50 wt.% Al was used.
The overall size and composition of the
refractory brick 38 is determined based on a balancing
of various considerations. On the one hand, it will be
appreciated that a greater radial thickness of the
refractory brick lining 30 in the high wear region
CA 02323764 2000-11-15
16
(i.e., the area where the slag layer 26 is maintained
and the postcombustion area 28) will result in greater
overall insulation and lower overall heat loss from the
reaction process. Also, the thicker the lining, the
greater the mechanical stability in a large vessel;
that is, it will be better able to withstand brief
process upsets, heating processes, and cooling
shutdowns, while remaining firmly in place. On the
other hand, it will be appreciated that extraction of
an amount of heat through the refractory brick
sufficient to form and maintain a frozen layer of slag
on the interior face of the brick (hot face) requires a
high rate of thermal transfer through the refractory
brick lining 30. Accordingly, the size and constituent
elements of the refractory brick 38 are selected to
achieve an optimum balance of mechanical stability
(brick density), slag corrosion resistance, and thermal
conductivity.
The particle size and purity of the magnesia (Mg0)
generally are selected to provide desired brick
strength and density. In the present embodiment, the
magnesia (Mg0) preferably is fused or sintered
magnesia, and preferably has a high purity, most
preferably at least about 99% pure. A particle size
distribution that will maximize packing density and
brick strength preferably is used. In the present
embodiment, the majority (and most preferably at least
about 75%) of the magnesia (weight basis) preferably
will pass 4 mesh and be held on 200 mesh (USA STD/ASTM
CA 02323764 2000-11-15
17
E 11-87 Seive Series). For example, the particle size
distribution may be as follows: -4+14: 50%; -14+48:30%;
-48+200:5% and -200:15%. Of course, those skilled in
the art readily will be able to select a size and
purity distribution of the magnesia to achieve the
desired density and strength for a particular
application.
In the present embodiment, the thermal
conductivity and density of the refractory brick 38 are
optimized by making the brick 38 using a coarse flake
graphite 48. Examples of suitable graphite flakes
commercially available include graphite flakes grades
1040, 475, or 499 distributed by the Brazilian National
Graphite Co. The refractory brick 38 is made by mixing
the Mg0 particles with coarse flake graphite particles
48 and pressing the mixture such that the orientation
of the flake graphite particles 48 is favorable to high
thermal conductivity in a desired direction of the
brick 38. Of course, as noted above, the brick also
comprises a binder, and the brick is baked or fired at
a temperature and for a time sufficient to form a
finished brick, as is known in the art.
As shown in phantom in Fig. 4, the brick may be
formed in a mold by pressing the mixture with a force
Fp from the top, e.g., with a Crossley hydraulic press.
The present inventors have discovered that, when such a
refractory brick 38 is formed and pressed in this
manner, the volume of material in the mold generally is
reduced by approximately 10%. The density of a 10 wt.%
CA 02323764 2000-11-15
18
graphite brick thereby will be increased from a density
of about 100 lbs/ft3 to about 185 lbs/ft3, and the
graphite flake particles 48 generally will align in an
orientation perpendicular to the direction from which
force is being applied. Similarly, the density of a 20
wt.% graphite brick may be increased from a density of
about 75 lbs/ft3 to about 185 lbs/ft3. If, as is
preferred, the cross-section of the brick mold (on a
plane perpendicular to the direction of force) has a
long dimension (as shown in Fig. 4), then the graphite
particles will tend to align themselves with that long
dimension of the press mold. Moreover, it will be
appreciated that the relative dimensions thus affect
the degree of orientation achieved. In this manner, a
resultant refractory brick 38 can be formed having a
thermal conductivity in one direction that is greater
than its thermal conductivity in directions
perpendicular to that direction, while maximizing the
brick density. As schematically illustrated in Figs. 3
and 4, bricks 38 include flake graphite particles 48
oriented in a longitudinal direction of the brick 38.
In this manner, the refractory brick can be set in the
vessel in a key-up construction so as to provide a
consistent high thermal conductivity in a direction
from the slag 26 to the ram mixture 34 and cooler panels
32. When so set in the vessel, the refractory brick is
arranged such that the direction of higher thermal
conductivity is generally perpendicular to the interior
facing surface ("hot face") 94 exposed to the interior
of the vessel 10 and perpendicular to cooler panels 32.
x
CA 02323764 2002-04-03
- 19 -
The size and purity of the graphite particles is
selected to provide optimal packing density and heat '
conductivity characteristics. Generally, there is a
trade-off between size and purity. Smaller particles
generally provide greater purity larger particles .
generally provide greater heat conductivity. In the
present embodiment, the graphite has a high purity,
preferably at least about 9.7 wt.% carbon, e.g., about
97 to 99%, and most preferably about 99 wt.% carbon.
The graphite preferably is sized predominantly from 20
to 80 mesh (USA STD/ASTM E 11-87 Seive Series), with no
more than i0 wt.% finer than 80 mesh, and with at least
40 wt.% (e. g., about half of the graphite sized from
to 4O mesh. Preferably, the graphite has a size
distribution wherein about 90 to 100 wt.% of the
graphite has a~particle size distribution between about
180 and 850 microns in the longest direction, and at
least 50 wt.% is between about 450 and 850 microns in
the longest direction.
As noted above, one object of the present
invention is to provide a refractory brick 38 having a
relatively high thermal conductivity in one direction.
20 Table 1 illustrates the thermal conductivity in the
longitudinal direction at 2500°F for seven MgO bricks
having a graphite content within the range of 0 to 40
wt.%. All of the bricks were prepared using the
procedure depicted in Figure 4, employing a Crossley
hydraulic press. The conductivity was measured by a
standard ASTM laser technique (ASTM E-1461, 1992).
CA 02323764 2001-10-29
TABLE 1
Thermal Conductivitv of Various M O-Graphite Bricks
Approximate Thermal Conductivity
Brick Graphite Content (wt.$) W/m°K at 1370°C
A 0 5.3
B 5 7.0
10 C 10 10.6
D 15 10.5
E 20 15.4
F 30 16.8
G 40 37.8
It will be appreciated that the thermal
conductivity for any given brick will vary with the
temperature thereof. In the direct steelmaking process
of the present invention, the temperature of the brick
20 varies in the range of about 204°C (at the cold face)
to about 1760°C (at the hot face); the value of 1370°C
has been chosen for purposes of example only, because
during direct reduction of iron ore a large portion of
the brick is at or about 1370°C. In any event, as
shown in Table 1, the thermal conductivity of Mg0-
graphite bricks varies directly with the graphite
content. That is, the higher the graphite content, the
higher the thermal conductivity.
In the direct steelmaking process of the present
invention, however, since oxygen is injected into the
postcombustion area 28 to oxidize Hi or C0, the oxygen
also may oxidize graphite particles in the refractory
CA 02323764 2000-11-15
21
brick 38, causing excessive chemical wear of the
refractory lining. Moreover, the present inventors
have recognized that brick having a lower carbon
content has a much greater oxidation resistance
because: (1) slag forming the frozen protective layer
42 adheres better when the brick 38 has a lower
graphite content; (2) antioxidants, (e. g., elemental
Mg, A1, or Si) added to the brick 38 are more effective
at lower graphite content; and (3) microstructures
resulting from the use of less graphite offer inherent
protection to the graphite from oxidation. Further,
the inherent lower hardness of brick made with a
relatively higher graphite content makes the brick more
susceptible to mechanical erosion by moving fluids and
solids, i.e., by molten foamy slag 26.
Through theoretical calculations, therefore, the
present inventors have determined that, although higher
refractory thickness stability would be calculated with
brick containing a greater graphite content, e.g.,
about 30 to 40 wt.$ graphite, other considerations
limit the preferred range to about 10 to 20 wt.%
graphite. In making this determination, the present
inventors began with the premise that there is a
sufficient temperature drop across the thin frozen
layer of slag 42 to maintain the protective layer of
slag 42 at or near its melting or softening point. of
course, since the composition of the layer of slag 26
is nonhomogeneous and dynamic, it does not have a sharp
melting point, but softens and melts over a range of
CA 02323764 2001-10-29
22
temperatures. Nevertheless, it will be apparent that,
by controlling the reaction process conditions,
including slag composition, temperature, pressure, and
the like, the thermal conductivity of the layer of slag
26 may be maintained within a range sufficiently
defined to satisfy the desired criteria.
Table 2 lists various selected parameters of the
frozen slag layer used for calculating the mechanical
stability of a Mg0-graphite brick.
TABLE 2
Thickness Slag Conductivity
(Centimetres) (W/m°K)
4 0.635 1.8
0 0.635 3.5
0.305 1.8
x 0.305 3.5
Fig. 5 is a graph of the calculated mechanical
stability thickness of a 10 wt.% graphite content
refractory brick over a typical reaction process
temperature range (e.g., temperature of molten slag) of
1500 to 1800°C using the parameters of Table 2.
Fig. 6 is a graph of the calculated mechanical
stability thickness of a 20 wt.% graphite content
refractory brick over a typical reaction process
temperature range of 1500 to 1800°C using the
parameters of Table 2.
CA 02323764 2000-11-15
23
In the present embodiment, the initial preheat
thickness of the refractory brick lining is 18 inches,
and the minimum thickness (T~,,) for providing
mechanical stability is assumed to be about 4 inches.
Therefore, the calculated mechanical stability
represents the thickness of the brick at process
equilibrium, and is determined based on the slag having
a melting point of about 1400°C, a thickness of either
0.25 or 0.5 inches (i.e., thickness of protective
frozen slag layer 42), and a thermal conductivity of
either 12.5 or 25.0 BTU-in/ft2-°F-hr.
As shown in Figs. 5 and 6, the calculated
mechanical stability (thickness) varies depending on
the assumed thickness of the frozen slag layer and the
thermal conductivity of the slag, but in all cases it
decreases as the difference between the slag melting
point and the reaction process temperature increases.
Thus, to maximize cooling efficiency the reaction
process preferably should be operated with a
predictable slag composition and the minimum process
temperature compatible with production requirements.
As noted above, the brick also may include one or
more antioxidants, to improve the anticorrosion
(chemical wear) characteristics of the brick. These
antioxidants preferably are selected from the group of
metals consisting of A1, Mg, Si, and alloys of two or
more of these metals. In the present embodiment, the
antioxidants preferably are supplied as particles
having a size predominantly (weight basis) in the range
CA 02323764 2000-11-15
24
of about 4 to 40 mesh (USA STD/ASTM E 11-87 Sieve
Series). However, those skilled in the art readily
will be able to determine the optimal type, amount, and
size of any antioxidants suitable for the desired
application.
Referring again to Fig. 3, the cooler panels 32
generally include a cooler panel wall 50 with a cooling
panel hot face 52 adjacent the ram mixture 34, and a
cooler panel cold face 53 adjacent the coolant 46. The
design criteria for the cooler panels 32 in a smelting
reaction process generally are twofold. First, the
cooler panels 32 must provide highly effective heat
transfer from the outer surface (hot face) 52 of the
cooler panel 32 through the cooler panel wall 50 and
the inner surface 53 (cold face) to the contained
circulating coolant 46. Second, the cooler panels 32
must be capable of withstanding the extreme conditions
prevailing in a smelting reaction process, where
aggressive liquid slag is in contact with the lining at
high temperatures and where intensive bath turbulence
under a pressurized reaction process occurs. The
cooler panels 32 must have sufficient mechanical
strength and stability to withstand abrasion against
the sliding solid burden material. In particular, the
cooler panels 32 must be able to withstand the above-
described extreme or emergency situations in the
reaction process. The cooler panels 32 also must not
distort to an extent that the pressurized coolant 46
CA 02323764 2000-11-15
can enter the reaction process through any cracks,
thereby risking an explosion.
Three basic design criteria for the cooler panels
32 are (1) the ratio of the effective surface area of
the hot face (i.e., surface 52) to the effective
surface area of the cold face (i.e., surface 53), (2)
the rate of flow of coolant through the cooler panels,
and (3) the thickness of the cooler panel wall 50
across which the heat must be conducted. With respect
10 to the coolant flow, the design criteria include the
specific heat value and the boiling point of the
coolant 46. In particular, the coolant flow rate
should be sufficient to maintain the coolant 46 at a
temperature below its boiling point, and to prevent
nucleate boiling. With respect to the heat transfer
wall thickness, the design criteria include the
specific heat conductivity of the cooler panel
material.
In the present embodiment, the cooler panels 32
20 are designed and sized for manufacturing capability and
ease of installation. The cooler panels 32 generally
are cast, and are installed by insertion through the
opened top end of the vessel. The cooler panels 32
preferably are equally sized. In the present
embodiment, a ring of 10 equally sized cooler panels 32
is installed.
In the present embodiment, each cooler panel 32 is
composed of cast copper plate material, and the coolant
46 is water. The maximum cooler panel plate thickness
CA 02323764 2000-11-15
26
at the hot face (i.e., the distance between the hot
face and the nearest coolant contact surface) is 20mm
and the minimum specific heat conductivity of the plate
material is 320 kcal/m/h/°C (at 0°C). The maximum
ratio of cooler panel hot face to coolant contact
surface projected onto the hot face is 1.3. The
minimum coolant flow velocity to suppress nucleate
(isolated) boiling in the water coolant is 2.4 m/s.
Of course, other cooler panel plate materials and
coolants may be used in the refractory lining system of
the present invention. For example, alternative cooler
panel plate materials include copper-bronze and
aluminum, and the water coolant optionally may include
antifreeze. Those skilled in the art readily will be
able to determine the design criteria for these and
other alternative cooler panel plate materials and
coolants suitable for a particular application.
The ram mixture 34 satisfies a number of design
criteria features of the refractory system of the
present invention. First, the ram mixture 34 is a heat
transfer material designed to effectively conduct and
transfer heat from the refractory brick lining 30 to
the cooler 32. Therefore, the ram mixture 34
preferably has a highly uniform conductivity throughout
its thickness, and most preferably in the range of
about 80 to 160 BTU-in/ft2-hr-°F.
The ram mixture preferably is a mixture of free
carbon in the amount of about 10 to 30 wt.%, SiC in the
amount of about 50 to 80 wt.%, Si02 in the amount of
CA 02323764 2001-10-29
27
about 5 to 18 wt.%, and A1203 in the amount of about 1
to 10 wt.%.
The vessel 10 of the present embodiment is
made using a ram mixture 34 including the constituent
elements and characteristics set forth below in Table
3.
TABLE 3
RAM MIX COMPOSITION
Material Amount (wt.%Z
Free Carbon 17
SiC 68
SiO2 1O
A1203 3
Other* 5
OTHER CHARACTERISTICS
Density-fired, 108
pcf
Effective conductivity 11.9 W/m°K
(*Includes about 3 wt.% Sodium-silicate type binders and
other oxides such as iron-oxide.)
Of course, those skilled in the art readily will be
able to select alternative ram mixtures suitable for
any particular application.
It also will be appreciated that the ram mixture
34 provides dimensional stability. The refractory
brick lining 30 and the cooler panels 32 generally have
different thermal expansion rates. This tends to cause
CA 02323764 2000-11-15
28
formation of air gaps between the refractory bricks 38
and the cooler panels 32. These air gaps could greatly
reduce the efficiency of heat conductivity.
Accordingly, in the present embodiment the ram mixture
34 preferably is installed in such a manner that
intimate contact is made and maintained between the ram
mixture 34 and each of the refractory bricks 38 and the
cooler panels 32, to maximize heat conductivity and
transfer. In the present embodiment, this installation
is achieved by a pneumatic injection/ramming of the ram
mixture 34, to substantially eliminate formation of any
air gaps.
It further will be appreciated that the high
temperature steelmaking reaction process of the present
embodiment is a dynamic reaction process. Moreover,
this process requires a start-up process following
preheat. In the initial heat and in reheats, the
refractory brick lining 30 generally is directly
exposed to the molten slag layer 26. Thus, as noted
above, the molten layer of slag 26 gradually will erode
the interior face 44 of the refractory brick 38, until
the reaction process reaches an equilibrium state and a
protective frozen slag layer 42 is formed and
maintained on the interior surface 44 of the refractory
brick 38. In order to control and minimize the erasion
of the refractory brick 38, the present inventors have
determined that initial oxidation and erosion may be
substantially reduced by coating the Mg0-graphite
refractory brick 38 with a glaze former, such as
CA 02323764 2000-11-15
29
potassium silicate, sodium silicate, or mixtures
thereof with clay. For example, a suitable clay is
high purity calcined kaolin (mineral) clay. In the
present embodiment, the glaze former is an air-setting
mortar, e.g., Seneca Chief,M manufactured by North
American Refractory Company. Alternative glaze formers
include borate glasses, which are low temperature glaze
formers. Of course, those skilled in the art readily
will be able to identify alternative glaze formers
suitable for a particular application.
It will therefore be appreciated that the high
temperature reaction vessel of the present embodiment
achieves all of the above-listed objects and advantages
of the present invention.
Fig. 7 schematically illustrates another
0
embodiment of a high temperature reaction vessel 110
according to the present invention. In the embodiment
of Fig. 7, the construction of the reaction vessel 110
is substantially similar to the embodiment of Fig. 1.
For example, the reaction vessel 110 includes a lower
portion 114 for holding the liquid metal 120, a central
portion 116, including a refractory lining 112, and an
upper portion 118, including cooling panels 119 and a
refractory material 121. However, in the embodiment of
Fig. 7, the refractory lining 112 includes only a
refractory brick lining 130 and cooler panels 132; the
refractory lining 112 does not include any ram mixture.
CA 02323764 2000-11-15
The utilization of the reaction vessel 110 and
refractory lining 112 of the embodiment of Fig. 7 is
substantially the same as the reaction vessel and
refractory lining of the embodiment of Fig. 1. In this
regard, although it is believed that the refractory
lining 112 of Fig. 7 will not operate as efficiently as
the refractory lining 12 of Fig. 1, because it does not
include a heat transfer material (ram mixture 34) to
enhance the vessel s thermal conductivity and
10 dimensional stability, it may provide satisfactory
utility in certain applications.
Fig. 8 schematically illustrates a third
embodiment of a high temperature reaction vessel 210
according to the present invention. In the embodiment
of Fig. 8, the construction of the reaction vessel 210
is substantially similar to the embodiment of Fig. 1.
For example, as shown, the reaction vessel 210 includes
a lower portion 214 for holding the liquid metal 220, a
central portion 216, including a refractory lining 212,
20 and an upper portion 218, including hollow cooling
panels 219 and a refractory material 221. The
refractory lining 212 includes a refractory brick
lining 230, hollow cooler panels 232 and a ram mixture
234 disposed therebetween. However, in the embodiment
of Fig. 8, the relative vertical length of central
portion 216 is extended. Specifically, in this
embodiment, two rings of 10 cooler panels 232 are
provided. Fig. 8 also illustrates three tuyeres 225,
e.g., for blowing in nitrogen gas to stir the reaction
CA 02323764 2000-11-15
31
process, and a tap hole 223, for tapping liquid metal
220 from the reaction vessel 210. The embodiment of
Fig. 8 provides an advantage over the embodiment of
Fig. 1, in that the vessel 210 has a relatively larger
volume for slag formation and post combustion in the
reaction process, including a larger free space 228 for
accommodating the foamy slag layer 226.
Fig. 9A schematically illustrates a fourth
embodiment of a high temperature reaction vessel
according to the present invention. In the embodiment
of Fig. 9A, the construction of the reaction vessel 310
is substantially similar to the embodiment of Fig. 8.
For example, as shown, the reaction vessel 310 includes
a lower portion 314, included a tap hole 323, a central
portion 316, including a refractory lining 312, and an
upper portion 318, including cooling panels 319 and a
refractory material 321. The refractory lining 312
includes a refractory brick lining 330, cooler panels
332 and a ram mixture 334 disposed therebetween.
However, in the embodiment of Fig. 9A, the vessel 310
also has a relatively broad and thick base portion 314,
for accommodating one or more tap holes 323. This also
provides a greater relative tap volume (illustrated as
the region between a first (pretap) metal level (L,,~,)
and a second (post tap) metal level (L~,,~)). Further, as
discussed in greater detail above, in the direct
steelmaking process of the present invention, the
refractory brick lining is believed to eventually
achieve a stable or equilibrium state at a thickness of
CA 02323764 2000-11-15
32
about 4 to 6 inches. Accordingly, in the embodiment of
Fig. 9A, the refractory brick lining is designed to
have an initial preheat thickness of about 6 inches.
This reduces the cost of manufacturing the vessel 310,
minimizes transition time to equilibrium, and minimizes
the amount of refractory material absorbed into the
slag during initial preheat and reheat processes, while
maintaining desired refractory lining stability. Also,
as in the embodiment of Fig. 8, the vessel 310 in Fig.
9A is advantageous in that it provides a central
portion 316 having a relatively large volume, including
a relatively large free space 328. Thus, this
embodiment can easily accommodate a greater relative
fluctuation in the slag volume (illustrated as the
region between a first slag level (LS1) and a second
slag level (L$2)) associated with the tapping of the
liquid metal, and permits greater flexibility in
designing and locating a lance 353 for introducing raw
materials to the reaction process.
Finally, Fig. 9H is an enlarged sectional view of
a portion of the wall of the reaction vessel in Fig.
9A, illustrating an alternative embodiment for cooler
panels 332. Specifically, in the embodiment of Fig.
9B, each cooler panel 332 has an L-shaped cross-section
forming an annular ledge or base portion 350L extending
radially inward. The refractory bricks 338 then are
assembled in refractory rings and stacked on the
annular ledge 350L. In this manner, the embodiment of
Fig. 9B provides a significant advantage, in that
CA 02323764 2000-11-15
33
refractory bricks located in the lower vessel portion
314 below the refractory lining 312, which are
unprotected by the present refractory lining 312 and
subject to relatively higher wear over time, may be
removed and replaced without having to also remove and
replace the refractory bricks 338 of the refractory
lining 312 (i.e., disassemble and reassemble the
refractory rings).
Although the present invention has been described
with reference to specific embodiments thereof, it is
not limited to such specific embodiments. Those
skilled in the art readily will appreciate numerous
equivalent embodiments and modifications of the present
invention, including alternative structures and
elements, without departing from the spirit of the
invention, which is defined in the following claims.