Note: Descriptions are shown in the official language in which they were submitted.
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
CONFOCAL MICROSCOPY IMAGING SYSTEM
This is a continuation-in-part of United States Patent Application Serial No.
09/042,527, filed March 16,1998, which is incorporated by reference herein in
its entirety.
Field of the Invention
The present invention relates to methods and apparatus for identifying
pharmacological agents useful for the diagnosis and treatment of disease by
performing a
variety of assays on cell extracts, cells or tissues where the measurement of
biological
activity involves the use of various embodiments of a line-scan confocal
imaging system
and associated data processing routines.
Background of the Invention
There is currently a need in drug discovery and development and in general
biological research for methods and apparatus for accurately performing cell-
based assays.
Cell-based assays are advantageously employed for assessing the biological
activity of
chemical compounds and the mechanism-of action of new biological targets. In a
cell-
based assay, the activity of interest is measured in the presence of both
competing and
complementary processes. As pertains to chemical compound screening,
information is
available as to the specific activity of the compound. For example, it is
possible to assess
not only whether a compound binds the target of the assay, but also whether it
is an agonist
or an antagonist of the normal activity of the target. Frequently, the target
is a cell-surface
receptor. In some signaling pathways, the member of the pathway of greatest
potential
therapeutic value is not the receptor but an intracellular signaling protein
associated with the
receptor. It is, therefore, desirable to develop methods to assay activity
throughout the
pathway, preferably in the cellular milieu.
In addition, there is a need to quickly and inexpensively screen large
numbers of chemical compounds. This need has arisen in the pharmaceutical
industry
where it is common to test chemical compounds for activity against a variety
of
biochemical targets, for example, receptors, enzymes and nucleic acids. These
chemical
compounds are collected in large libraries, sometimes exceeding one million
distinct
compounds. The use of the term chemical compound is intended to be interpreted
broadly
so as to include, but not be limited to, simple organic and inorganic
molecules, proteins,
peptides, nucleic acids and oligonucleotides, carbohydrates, lipids, or any
chemical
structure of biological interest.
-1-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99105589
In the field of compound screening, cell-based assays are run on collections
of cells. The measured response is usually an average over the cell
population. For
example, a popular instrument used for ion channel assays is disclosed in U.S.
Patent No.
5,355,215. A typical assay consists of measuring the time-dependence of the
fluorescence
of an ion-sensitive dye, the fluorescence being a measure of the intra-
cellular concentration
of the ion of interest which changes as a consequence of the addition of a
chemical
compound. The dye is loaded into the population of cells disposed on the
bottom of the
well of a multiwell plate at a time prior to the measurement. In general, the
response of the
cells is heterogeneous in both magnitude and time. This variability may
obscure or prevent
the observation of biological activity important to compound screening. The
heterogeneity
may arise from experimental sources, but more importantly, heterogeneity is
fundamental in
any population of cells. Among others, the origin of the variability may be a
consequence
of the life-cycle divergence among the population, or the result of the
evolutionary
divergence of the number of active target molecules. A method that mitigates,
compensates
for, or even utilizes the variations would enhance the value of cell-based
assays in the
characterization of the pharmacological activity of chemical compounds.
Quantification of the response of individual cells circumvents the problems
posed by the non-uniformity of that response of a population of cells.
Consider the case
where a minor fraction of the population responds to the stimulus. A device
that measures
the average response will have less sensitivity than one determining
individual cellular
response. The latter method generates a statistical characterization of the
response profile
permitting one to select the subset of active cells. Additional
characterization of the
population will enhance the interpretation of the response profile.
Various measurement devices have been used in the prior art in an attempt to
address this need. Flow-cytometer-based assays are widely practiced and
measure cell
properties one at a time by passing cells through a focused laser beam.
Several
disadvantages accompany this method. Most important to the pharmaceutical
industry is
that assays can not readily be performed on compounds disposed in microtiter
plates. In
addition, the throughput is poor, typically 10-100 seconds per sample, the
observation time
of each cell is <1 ms, prohibiting kinetic assays, and finally, only the cell-
averaged signal
can be determined.
In addition, many assays require determination of the relative locations of
the fluorescence signals. Devices called scanning cytometers, as disclosed in
U.S. Patent
No. 5,107,422 and U.S. Patent No. 5,547,849, are widely used for imaging
single cells. In
order to gain acceptable speed, these devices operate at low (~5-l0~cm)
resolution. Thus,
-2-
CA 02324262 2000-09-15
WO 99/47963 PCTNS99/05589
these devices offer little advantage over flow cytometers for assays requiring
spatial
information on the distribution of the fluorescence signals.
An additional alternative technology is the fast-camera, full-field
microscope. These devices have the ability to obtain images at a resolution
and speed
$ comparable to the present invention, on certain samples. However, they are
not confocal
and are consequently susceptible to fluorescence background and cannot be used
to
optically section the sample. In addition, simultaneous, multi-parameter data
is not readily
obtained.
In contrast to the prior art, the present invention can be used to perform
mufti-parameter fluorescence imaging on single cells and cell populations in a
manner that
is sufficiently rapid and versatile for use in compound screening. Methods and
apparatus
are provided for obtaining and analyzing both the primary response of
individual cells and
additional measures of the heterogeneity of the sample population. In
addition, the
locations of these multiple fluorophores can be determined with sub-cellular
resolution.
Finally, the present invention can be used to image rapidly changing events at
video-rates.
Together these capabilities enable new areas of research into the mechanism-of
action of
drug candidates.
The present invention may also be employed in an inventive fluorescence-
based biochemical assay, somewhat analogous to the surface scintillation assay
("SSA")
which is among the more widely used methods for screening chemical compounds.
Figs. 1 (a) - 1 (f) depict the steps of a receptor-binding SSA. In Fig. 1 (a),
soluble membranes 10 with chosen receptors 12 are added to a well 20
containing a liquid
30. These membranes are isolated from cells expressing the receptors. In Fig.
1 (h), radio-
labeled ligands 14 are added to the well. The ligand is known to have a high
binding
affinity for the membrane receptors. The most common radio labels are 3H, 355,
'ZSI, sap and
32P. In Fig. 1(c), beads 16 are added to the well. The beads are coated with a
material, such
as wheat germ agglutinin, to which the membranes strongly adhere. The beads
have a
diameter of 3-8 pm and are made of plastic doped with a scintillant.
Alternatively, the
order of the operations depicted in Figs. 1 (b) and 1 (c) may be interchanged.
The radiolabels decay by emitting high energy electrons, or beta particles,
which travel approximately 1-100 pm before stopping, depending on the radio-
isotope. If
the radiolabels are bound to the membranes attached to the beads, the beta
particles may
travel into the beads and cause bursts of luminescence. If the radio-labels
are dispersed
throughout the liquid, the emitted beta particles will not generally excite
luminescence in
the beads. In Fig. 1 (d), the luminescence of the beads caused by decay of the
radio labels is
detected. In Fig. 1 (e), a test compound 18 is added to the well. The purpose
of the assay is
-3-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
to determine the extent to which this compound will displace the radio-labeled
ligands. If
radio-labeled ligands are displaced and diffuse into the liquid, the
luminescence of the beads
will be reduced. In Fig. 1 (f), the luminescence of the beads is again
detected. By
measuring the reduction in luminescence, the activity of the test compound can
be
determined.
Figs. 2(a) - 2(f) depict an alternative embodiment of a receptor-binding SSA.
This embodiment is essentially the same as that described in Figs. 1 (a) - 1
(f) except that
instead of using beads, the embodiment shown in Figs. 2(a) - 2(f) uses a well
bottom 22
made of plastic doped with scintillant and coated with a material to which the
membranes
adhere. Consequently, instead of detecting the luminescence of the beads, the
embodiment
shown in Figs. 2(a) - 2(f) detects the luminescence of the well bottom.
Figs. 3(a) - 3(d) depict the steps of an embodiment of an enzyme SSA. In
Fig. 3(a), scintillant-doped beads 40 with radio-labeled peptides 42 attached
thereto are
added to a well 50 containing a liquid 60. In Fig. 3(b), a test compound 44 is
added to the
well. In Fig. 3(c), enzymes 46 are added to the well. If not inhibited,
enzymes 46 will
cleave radio-labeled peptides 42 from beads 40. As a result, the radio label
will diffuse into
the solution, and radio-label decay will not produce luminescence in beads 40.
If, on the
other hand, test compound 44 inhibits enzymes 46, typically by blocking the
enzyme active
site, enzymes 46 will not cleave the radio label and the decay of the radio
label will produce
luminescence in the beads. In Fig. 3(d), the luminescence of the beads is
measured and the
activity of the test compound can be determined.
Figs. 4(a) - 4(d) depict an alternative embodiment of an enzyme SSA. In
Fig. 4(a), radio-labeled peptides 42 are attached to a scintillant-doped well
bottom 52. In
Fig. 4(b), the test compound 44 is added to the well. In Fig. 4(c), enzymes 46
are added to
the well. In Fig. 4(d), the luminescence of the well bottom is measured to
determine the
activity of the test compound.
The above examples illustrate the general principle of the SSA, namely that
the activity of interest is assayed by a change in the number of radio labels
within a radio-
decay length of the scintillant. One of the attractions of SSAs is that the
radio labels not
attached to the scintillant need not be removed from the well in a wash step.
That is SSAs
are homogeneous assays.
A radioimmunoassay (RIA) is a specific form of a receptor binding assay in
which the receptor is an antibody and the ligand is most often a natural or
synthetic peptide,
protein, carboydrate or small organic molecule. RIAs are an indirect method
for measuring
the concentration of ligand in any prepared sample, most often a biological
sample such as
plasma, cerebrospinal fluid, urine, or cellular extract. In a standard RIA,
the antibody has a
-4-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
specific affinity for the ligand and the assay contains the antibody, a fixed
concentration of
radiolabeled ligand and an unknown concentration of non-labelled ligand. The
concentration of the unlabelled ligand is determined by the degree to which it
binds to the
antibody and thereby blocks binding of the labelled ligand. RIAs are most
often performed
as heterogenous assays that require the separation of bound ligand from
unbound ligand
with a wash step. RIAs have also been developed using an. SSA configuration in
which the
antibody receptor is attached to a scindllant filled bead and the wash step is
eliminated.
SSAs and RIAs, however, suffer from a number of disadvantages. First,
these assays require handling radioactive material, which is both expensive
and time
consuming. Second, these assays are only effective in large wells. The rate of
luminescence emission from the beads or well bottoms is proportional to the
beta particle
emission rate. A typical 3H assay yields less than one detected photon per 3H
decay. To
increase the speed of the assay, the quantity of radio-labeled ligand must be
increased, and
correspondingly the quantities of membranes, beads and test compound. In order
to
perform a tritium SSA in 10-60 seconds, 10' beads must be used. This quantity
of beads
requires a well of approximately 150 pL. SSAs are not effective in the pL-
volume wells
desirable for screening large numbers of compounds.
As described below, the present invention, inter alia, replaces the radio-
labeled ligands of the SSA and the RIA with fluorescent-labeled ligands. In so
doing, it
introduces a homogenous format for the RIA and it advantageously retains the
homogeneous format of the SSA. This is particularly important in p,L-volume
wells, for
which surface tension renders washing impractical. However, in a homogeneous
format,
fluorescence can be a problem as can be illustrated with the receptor-binding
assay. When
the test compound is added, some fluorescent-labeled ligands are displaced and
diffuse
freely throughout the volume of the well, while others remain attached to the
membranes. It
is the fluorescence of the fluorescent-labeled ligands attached to the
membranes that is used
to determine the activity of the test compound. If the fluorescence is
detected from the
entire well, however, the emission from the fluorescent -labeled ligands in
the volume of the
well will obscure the emission from the fluorescent-labeled ligands attached
to the
membranes.
One method addressing this problem is described in U.S. Patent No.
5,355,215 to Schroeder et al. and shown in Figs. 5(a) and 5(b). According to
the Schroeder
et al. method, the samples are illuminated by a beam 134 of light that is
directed at the
bottom of the well at an oblique angle, shown as A in Fig. 5, so that it does
not illuminate
the entire well. In addition, while the beam illuminates area 114',
fluorescence is detected
-S-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
only from area 114a which is under the well volume which receives the least
amount of
illumination.
The Schroeder et al. method, however, suffers from a number of
disadvantages. First, because it detects only a small portion of the well
bottom, the
$ Schroeder et al. method can only be performed with a sufficient degree of
accuracy on fairly
large wells. It is not suitable to image.samples disposed in the approximately
1-mm
diameter wells of a 1536-well plate. Second, the geometric constraints of the
angled
illumination preclude the use of high numerical aperture collection optics,
necessary to
achieve sufficient sensitivity and resolution to image micron-sized objects,
such as
individual cells, at the bottom of the well.
Another approach to this problem uses a point-scan microscope. For
example, in U.S. Patent No. 5,547,849 to Baer et al., the use of a point-scan
confocal
system is taught. Baer et al. teach a method to increase the slow speed of
image acquisition,
inherent in point-scan confocal techniques, by sacrificing spatial resolution.
If, for example,
1 S one expands the diameter of the illumination beam on the sample by a
factor of 10, then the
illumination area is increased 100-fold, permitting one to scan 100-times
faster, under
certain conditions. The speed increase is achieved, however, at the expense of
resolution.
Further, the detection devices appropriate to said scanning method, as
disclosed in the '849
patent, are inferior, principally in terms of sensitivity, to those
advantageously used in the
present invention. Finally, the degree of background rejection is diminished
along with the
resolution. Thus the device disclosed in the '849 patent has lesser
sensitivity, higher
background and lower resolution than the present invention, all of which are
important in
the present application.
The present invention includes novel embodiments of a line-scan confocal
~croscope. Line-scan confocal microscopes are known in art. Two representative
embodiments are the system disclosed by White et al. in U.S. Patent No.
5,452,125 and that
published by Brakenhoff and Visscher in J. Microscopy 171 I7-26 (1993), shown
in Fig. 7.
Both use a scanning mirror to sweep the illumination across the sample. The
same mirror
de-scans the fluorescence radiation. After spatial filtering with a slit, the
fluorescence is
rescanned for viewing by eye. The use of the oscillating mirror enables these
microscopes
to rapidly scan a field-of view. Line illumination is advantageous principally
in
applications requiring rapid imaging. The potential speed increase inherent in
the
parallelism of line illumination as compared to point illumination is,
however, only realized
if the imaging system is capable of detecting the light emitted from each
point of the sample
along the illumination line, simultaneously. An essential feature of the
disclosed apparatus
-6-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
is the use of a detection device having manifold, independent detection
elements in a plane
conjugate to the object plane.
According to the present invention, the sample must lie in a "plane", where
the depth-of field of the imaging system determines the precision of
"planarity". In a
preferred embodiment, the imaged area is 1 mmZ and the depth- of field is 10
pm. Thus, if
the entire field is to be in focus simultaneously, the sample must be flat to
1 part in 100.
This is true of many sample substrates (e.g. microtiter plates) over a local
area (such as the
central area of the well bottom). It is not practical, however, to require
that the sample
substrate be flat over its entire surface. For a microtiter plate having an
extent of 100 mm,
planarity of 1 part in 10,000 would be necessary.
The present invention provides for an optical autofocus system which
maintains in "focus" the portion of the sample substrate being imaged. An
optical autofocus
mechanism has the advantage of being fast and being operational with non-
conducting
substrates such as plastic microtiter plates and microscope slides.
Advantageously, this
focus mechanism operates with negligible delay, that is, the response time of
the focusing
mechanism is short relative to the image acquisition-time, preferably a
fraction of a second.
Optically-based autofocus mechanisms suitable for the present application are
known. For
example, an astigmatic-lens-based system for the generation of a position
error signal
suitable for servo control is disclosed in Applied Optics 23 565-570 (1984),
and a focus
error detection system utilizing a "skew beam" is disclosed in SPIE 200 73-78
(1979). In a
preferred embodiment of the present invention, the sample substrate is a
microtiter plate. In
this case, the preferred means of accomplishing the focusing depends further
on the
properties of the plate. If the thickness of the plate bottom were uniform to
within a fraction
of the depth-of focus, then a focusing mechanism that maintained the plate
bottom at a
constant offset from the object plane would be adequate. Presently, commonly
used
microtiter plates are not sufficiently uniform. Thus, the focusing mechanism
must track the
surface on which the sample resides, which is typically the inside of the
microtiter plate
well. An aspect of the present invention is a novel autofocus mechanism for
rapidly
focusing on a discontinuous surface, such as the well bottom of a microtiter
plate.
There is, therefore, a need for a method and apparatus for screening large
numbers of chemical compounds accurately, quickly and inexpensively, in a
homogeneous
format. In addition, there is a need for a methods and apparatus that can
perform multi-
parameter fluorescence imaging with sufficient resolution to image individual
cells and sub-
cellular events. There is also a need for an imaging system that can
additionally monitor a
statistically significant population of cells at video-rates.
_7_
CA 02324262 2000-09-15
WO 99/47963 PCTNS99/05589
Summary of the Invention
The present invention relates to a line-scan confocal microscope and the use
of a line-scan confocal imaging (LCI) system to assay biological activity.
In a prefer ed embodiment, the line-scan confocal imaging system employs
laser light sources of multiple wavelengths for illuminating the sample and
exciting
fluorophores to emit electromagnetic energy. These wavelengths include the
ultraviolet
spectrum as well as the visible.
The present invention is able to conduct a rapid series of assays on micro-
well plates by use of an autofocus capability which allows the LCI system to
rapidly move
from one well to another but not lose the advantage of the confocal
microscope's inherent
ability to resolve thin optical sections.
In various embodiments of the present invention the sample is moved to
effect a scan of the line of illumination over the sample. In other
embodiments, an
oscillating minor is used to produce a rapidly moving line of illumination
effecting a scan
of a sample which remains at a fixed position. By way of example, images can
be obtained
at a rate of up to 50 frames per second.
The present invention preferably provides for integrated dispensing allowing
the addition of substances to initiate rapidly changing biological events,
such as the
propagation of an action potential in nerve or muscle cells.
The present invention preferably makes use of a multi-element solid state
detection device such as a charged coupled device (CCD). This device is
preferably read
continuously. In a preferred embodiment, the present invention uses a
rectangular CCD
which avoids the need for a full two dimensional detector and allows higher
read speeds. In
addition, a larger effective field-of view is achievable in the stage-scanning
embodiment.
The present invention also provides in a preferred embodiment, a capability
to conduct specialized data analysis simultaneously with data acquisition to
allow it to
operate in a high-throughput screening mode.
This invention provides methods of performing a wide variety of biological
assays utilizing fluorescence. In one embodiment the target of interest may be
in a fixed or
live cell or in a subcellular organelle or on the cell membrane. These assays
involve the
determination of one or more parameters which requires the excitation of one
or more
fluorescent labels which are, in general, sensitive to different wavelengths
of incident light.
In addition these assays require the simultaneous and precise imaging of the
emitted light at
one or more wavelengths from which the location in two or three dimensions and
the
intensity of the fluorescently labeled species and their correlations are
determined.
_g_
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
In addition, this invention provides methods to perform assays which require
either a single imaging of a response, by means of fluorescent emission, or
rapidly repeated
imaging of the same area or cell. In various embodiments, imaging is performed
at rates as
high as SO frames per second. This ability to image rapidly, in multiple
wavelengths and
with high spatial resolution allows the present invention to perform assays
that could not
previously be performed or to perform them in a superior manner.
The present invention relates to several methods for screening chemical
compounds and for performing many types of assays involving the use of
fluorophores or
fluorescent probes. In general these assays and screening procedures involve
the use of a
test compound and reagents some or all of which are intrinsically fluorescent,
tagged with
fluorescent labels or are metabolized into fluorescent product. The test
compound and the
reagents may be combined in a variety of ways.
In one embodiment, the reagents are added to a well containing a liquid.
This may be a single well or one of many wells on a multiwell plate. The
biological activity
of interest is determined by the presence or absence of fluorophores disposed
on the bottom
of the well or on the surface of beads disposed on the bottom of the well as
measured with a
line-scan confocal microscope. This embodiment has in common with the SSA
format the
determination of activity from the localization of the detected species. In
the case of the
SSA, the localization is proximal to the scintillant. In the present method,
the localization is
to a region of the well, preferably the bottom. In the case of the SSA,
sensitivity to the
proximal species is determined by the decay length of the beta particles. In
the present
method, sensitivity to the localized fluorophore is determined by the optical-
sectioning
depth of the confocal microscope.
In addition, the present invention can perform high throughput assays
requiring scanning multiple samples in a rapid and automatic manner. These
samples may
be individual micro-wells and may involve wells containing a liquid and live
or fixed cells
or components of cells. The present invention also provides environmental
controls
required to retain liquid samples or sustain live cells during the analysis.
Brief Description of the Drawings
These and other objects, features and advantages of the invention will be
more readily apparent from the following detailed description in which:
Figs. 1(a) - 1(f) illustrate a first receptor-binding SSA.
Figs. 2(a) - 2(f) illustrate a second receptor-binding SSA.
Figs. 3(a) - 3(d) illustrate a first enzyme SSA.
Figs. 4(a) - 4(d) illustrate a second enzyme SSA.
-9-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
Fig. 5(a) and 5(b) are schematic views of a prior art apparatus for imaging
samples disposed on the bottom of a well.
Fig. 6 is a schematic view of a first embodiment of a line-scan confocal
microscope used to image samples according to the present invention.
Fig. 7 is a schematic view of a prior art microscope..
Figs. 8(a) and 8(b) are, respectively, a top view and a side view of the ray
path of a multicolor embodiment of the present invention, without a scanning
minor. Fig.
8(c) is a top view of the ray path of a single beam autofocus.
Figs. 9(a) and 9(b) are, respectively, a top view and a side view of the ray
path of the multicolor embodiment of the present invention with the scanning
minor. Fig.
9(c) is a top view of the ray path of the single beam autofocus.
Fig. 10 is a side view of the two beam autofocus system.
Fig. 11(a)-11(c) illustrates the rectangular CCD camera and readout register,
Figs. 12(a) and 12(b) are cross-sectional views of ray paths formed by the
line-scan confocal microscope in the present invention employing conventional
dark-field
imaging.
Figs. 13(a) and 13(b) are cross-sectional views of ray paths formed by the
line-scan confocal microscope in the present invention using inverse dark-
field imaging.
Fig. 14 is a cross-sectional view of ray paths fonmed by the line-scan
confocal microscope in the present invention using inverse dark-field imaging,
where an
area larger than the diffraction-limited area of the sample plane is
illuminated.
Figs. 15(a) - 15(f) illustrate a first embodiment of a receptor-binding assay
according to the present invention.
Figs. 16(a) - 16(f) illustrates a second embodiment of a receptor-binding
assay according to the present invention.
Figs. 17(a) - 17(d) illustrate a first embodiment of an enzyme assay
according to the present invention.
Figs. 18(a) - 18(d) illustrate a second embodiment of an enzyme assay
according to the present invention.
Figs. 19(a) - 19(d) shows a transcription factor translocation assay.
Figs. 20(a) - 20(d) shows a translocation assay data analysis.
Figs. 21 (a) - 21 (e) shows another data analysis.
Fig. 22 shows neuroblastoma cell calcium response to Carbachol.
Figs. 23(a) - 23(h) shows neuroblastoma cell calcium response to 50 mM
KCI.
Figs. 24(a) - 24(c) shows homogeneous live cell receptor binding assay.
- 10-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
Figs. 25(a) - 25(c) shows homogeneous live cell receptor binding assay.
Figs. 26(a) - 26(d) shows homogeneous live cell receptor binding assay with
Cy3 labeled ligand.
Figs. 27(a) - 27(d) shows 4,um diameter silica beads with varying numbers of
$ Cy5labels.
Figs. 28(a) - 28(d) shows the data from the translocation assay, ion channel
assay and cell surface receptor binding as graphs.
Detailed Description of the Invention
All patent applications, publications, and other references that are listed
herein are hereby incorporated by reference in their entireties.
The present invention if useful for identifying pharmacological agents for the
treatment of disease. It provides a high throughput method for conducting a
wide variety of
biological assays where one or more fluorescent reagents are employed to
measure a
biological response. Such assays can be conducted on chemical compounds or any
molecule of biological interest, included but not limited to drug candidates,
such as those
found in combinatorial libraries. In addition, this invention provides a
method for the
diagnosis of pathological states from cell and tissue samples. This invention
also provides a
method for profiling multiple biological responses of drug candidates on whole
cells using
fluorescent reagents.
The techniques of the present invention may be used in assays in which data
is acquired on individual cells, on a cellular or sub-cellular level,
sufficiently rapidly so as
to permit the acquisition of such data on a sufficient number of cells to
constitute a
statistically meaningful sample of the cell population. The present invention
is able to make
simultaneous measurements on multiple parameters and is also able to correlate
multiple
signals from individual cells. It may therefore be employed to assay
heterogeneous cellular
responses and to assay responses confined to a small subset of cells.
In addition, the present invention can image the simultaneous activation of
multiple signal pathways and can correlate multiple signals simultaneously and
over time.
This capability is vital when the temporal response of individual cells or a
comparison of
the temporal response of individual cells is required for the specific assay.
In addition, the present invention can image fluorescent signals from the
confocal plane of cells in the presence of unbound fluorophore or in the
presence of
intrinsically fluorescent chemical compounds, including potential drug
candidates.
These assays may make use of any known fluorophore or fluorescent label
including but not limited to fluorescein, rhodamine, Texas Red, Amersham Corp.
stains
-11-
CA 02324262 2000-09-15
WO 99/47963 PCTNS99/05589
Cy3, CyS, Cy5.5 and Cy7, Hoechst's nuclear stains and Coumarin stains. (See
Haugland
R.P. Handbook of Fluorescent Probes and Research Chemicals 6'" Ed., 1996,
Molecular
Probes, Inc., Eugene, Oregon.)
These assays include but are not limited to receptor-binding assays, assays of
S infra-cellular electric potential or pH, assays of ion concentrations,
enzyme activity assays,
trafficking assays, kinetic imaging assays and assays of rare cellular events.
Receptor-binding and enzyme activity assays may be bead-based or cell-
based assays. Some examples of bead-based assays are described in WO 98/55866.
However, the method described therein makes use of point scan confocal
technology and
the present linescan confocal imaging system would have a significant
advantage in terms
of rate of data acquisition.
OPTICAL CONFIGURATION
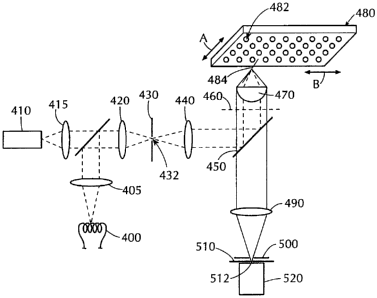
Fig. 6 shows a first embodiment of the present invention. The microscope
comprises a source 400 or 410 of electromagnetic radiation for example, in the
optical
range, 350-750nm, a cylindrical lens 420, a first slit mask 430, a first relay
lens 440, a
dichroic minor 450, an objective lens 470, a microtiter plate 480 containing a
two-
dimensional array of sample wells 482, a tube lens 490, a filter 500, a second
slit mask 510
and a detector 520. These elements are arranged along optical axis OA with
slit apertures
432, 512 in masks 430, 510 extending perpendicular to the plane of Fig. 6. The
focal
lengths of lenses 440, 470 and 490 and the spacings between these lenses as
well as the
spacings between mask 430 and lens 440, between objective lens 470 and
microtiter plate
480 and between lens 490 and mask 510 are such as to provide a confocal
microscope. In
this embodiment, electromagnetic radiation from a lamp 400 or a laser 410 is
focused to a
line using a cylindrical lens 420. The shape of the line is optimized by a
first slit mask 430.
The slit mask 430 is depicted in an image plane of the optical system, that is
in a plane
conjugate to the object plane. The illumination stripe formed by the aperture
432 in the slit
mask 430 is relayed by lens 440, dichroic mirror 450 and objective lens 470
onto a
microtiter plate 480 which contains a two-dimensional array of sample wells
482. For
convenience of illustration, the optical elements of Fig. 6 are depicted in
cross-section and
the well plate in perspective. The projection of the line of illumination onto
well plate 480
is depicted by line 484 and is also understood to be perpendicular to the
plane of Fig. 6. As
indicated by arrows A and B, well plate 480 may be moved in two dimensions (X,
Y)
parallel to the dimensions of the array by means not shown.
In an alternative embodiment, the slit mask 430 resides in a Fourier plane of
the optical system, that is in a plane conjugate to the objective back focal
plane (BFP) 460.
-I2-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
In this case the aperture 432 lies in the plane of the figure, the lens 440
relays the
illumination stripe formed by the aperture 432 onto the back focal plane 460
of the
objective 470 which transforms it into a line 484 in the object plane
perpendicular to the
plane of Fig. 6.
In an additional alternative embodiment the slit mask 430 is removed
entirely. According to this embodiment, the illumination source is the laser
410, the light
from which is focused into the hack focal plane 460 of the objective 470. This
can be
accomplished by the combination of the cylindrical lens 420 and the spherical
lens 440 as
shown in Fig. 6, or the illumination can be focused directly into the plane
460 by the
cylindrical lens 420.
An image of the sample area, for example a sample in a sample well 482, is
obtained by projecting the line of illumination onto a plane within the
sample, imaging the
fluorescence emission therefrom onto a detector 520 and moving the plate 480
in a direction
perpendicular to the line of illumination, synchronously with the reading of
the detector
520. In the embodiment depicted in Fig. 6, the fluorescence emission is
collected by the
objective lens 470, projected through the dichroic beamsplitter 450, and
imaged by lens 490
through filters 500 and a second slit mask 510 onto a detector 520, such as is
appropriate to
a confocal imaging system having an infinity-corrected objective lens 470. The
dichroic
beamsplitter 450 and filter 500 preferentially block light at the illumination
wavelength.
2p The detector 520 illustratively is a camera and may be either one
dimensional or two
dimensional. If a one dimensional detector is used, slit mask 510 is not
needed. The
illumination, detection and translation procedures are continued until the
prescribed area has
been imaged. Mechanical motion is simplified if the sample is translated at a
continuous
rate. Continuous motion is most useful if the camera read-time is small
compared to the
exposure-time. In a preferred embodiment, the camera is read continuously. The
displacement d of the sample during the combined exposure-time and read-time
may be
greater than or less than the width of the illumination line W, exemplarily
O.SW s d s SW.
All of the wells of a multiwell plate can be imaged in a similar manner.
Alternatively, the microscope can be configured to focus a line of
illumination across a number of adjacent wells, limited primarily by the field-
of view of the
optical system. Finally, more than one microscope can be used simultaneously.
The size and shape of the illumination stripe 484 is determined by the width
and length of the Fourier transform stripe in the objective lens back focal
plane 460. For
example, the length of the line 484 is determined by the width of the line in
460 and
conversely the width in 484 is determined by the length in 460. For
diffraction-limited
performance, the length of the illumination stripe at 460 is chosen to
overfill the objective
-13-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
back aperture. It will be evident to one skilled in the art that the size and
shape of the
illumination stripe 484 can be controlled by the combination of the focal
length of the
cylindrical lens 420 and the beam size at 420, that is by the effective
numerical aperture in
each dimension, within the restrictions imposed by aberrations in the
objective, and the
objective field of view.
The dimensions of the line of illumination 484 are chosen to optimize the
signal to noise ratio. Consequently, they are sample dependent. Depending on
the assay,
the resolution may be varied between diffraction-limited, i.e., less than 0.5
um, and
approximately 5 Itm. The beam length is preferably determined by the objective
field of
view, exemplarily between .5 and 1.5 mm. A Nikon ELWD, 0.6 NA, 40X objective,
for
example, has a field of view of approximately 0.75 mm. The diffraction-limited
resolution
for 633 nm radiation with this objective is approximately 0.6 p.m or
approximately 1100
resolution elements.
The effective depth resolution is determined principally by the width of
aperture 512 in slit mask 510 or the width of the one dimensional detector and
the image
magnification created by the combination of the objective lens 470 and lens
490. The best
depth resolution of a confocal microscope approaches 1 um. In the present
application, a
depth resolution of 5-10 pro may be sufficient or even advantageous.
For example, when the sample of interest, such as a live cell, contains
insufficient fluorophores in a diffraction-limited volume to permit an
adequate signal-to-
noise image in a sufficiently brief image-acquisition time, it is advantageous
to illuminate
and collect the emission from a larger than diffraction-limited volume. A
similar situation
prevails in the case of video-rate kinetics studies of transient events such
as ion-channel
openings. Practically, this is accomplished by underfilling the back aperture
of the
objective lens, which is equivalent to increasing the diameter of the
illumination aperture.
The effective numerical aperture ("NA") of the illumination is less than the
NA of the
objective. The fluorescence emission is, however, collected with the full NA
of the
objective lens. The width of aperture 512 must be increased so as to detect
emission from
the larger illumination volume. At an aperture width a few times larger than
the diffraction
limit, geometrical optics provides an adequate approximation for the size of
the detection-
volume element:
Lateral Width: ad = ddMi,
Axial Width: zd = ~2a~/tana,
where M is the magnification, dd is the width of aperture 512 and a is the
half angle
subtended by the objective 470. It is an important part of the present
invention that the
-14-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
illumination aperture 432 or its equivalent in the embodiment having no
aperture and the
detection aperture 512 be independently controllable.
MULTI-WAVELENGTH CONFIGURATION
An embodiment enabling mufti-wavelength fluorescence imaging is
preferred for certain types of assays. It is generally advantageous and often
necessary that
two or more measurements be made simultaneously since one important parameter
in a
biological response is time.
The number of independent wavelengths or colors will depend on the
specific assay being performed. In one embodiment three illumination
wavelengths are
used. Figs. 8(a) and 8(b) depict the ray paths in a three-color line-scan
confocal imaging
system, from a top view and a side view respectively. In general, the system
comprises
several sources S" of electromagnetic radiation, collimating lenses L", and
mirrors M" for
producing a collimated beam that is focused by cylindrical lines CL into an
elongated beam
at first spatial filter SF,, a confocal microscope between first spatial
filter SF" and second
spatial filter SFZ and an imaging lens IL, beamsplitters DM, and DMZ and
detectors D" for
separating and detecting the different wavelength components of fluorescent
radiation from
the sample. Spatial filters SF, and SF~ and SFZ preferably are slit masks.
In particular, Fig. 8(a) depicts sources, S,, SZ and S3, for colors ~,,, ~,Z
and ~,3,
and lenses L,, LZ and L3 that collimate the light from the respective sources.
Lenses L,, LZ
and L3, preferably are adjusted to compensate for any chromaticity of the
other lenses in the
system. Mirrors M,, MZ and M3 are used to combine the illumination colors from
sources
S". The mirrors MZ and M, are partially transmitting, partially reflecting and
preferentially
dichroic. MZ, for example, should preferentially transmit ~,3, and
preferentially reflect ~,Z. It
is thus preferential that ~,3 be greater than .12.
Operation of the microscope in a confocal mode requires that the combined
excitation beams from sources S" be focused to a "line", or an highly
eccentric ellipse, in
the object plane OP. As discussed in connection to Fig. 6 above, a variety of
configurations
may be used to accomplish this. In the embodiment depicted in Fig. 8, the
combined
illumination beams are focused by cylindrical lens CL into an elongated
ellipse that is
coincident with the slit in the spatial filter SF,. As drawn in Figs. 8a and
8b, the slit mask
SF~ resides in an image plane of the system, aligned perpendicular to the
propagation of the
illumination light and with its long axis in the plane of the page of Fig. 8a.
The lenses TL
and OL relay the illumination line from the plane containing SF, to the object
plane OP. A
Wing minor, TM, is for convenience. In another embodiment, DM3 is between TL
and
-15-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
OL and CL focuses the illumination light directly into the BFP. Other
embodiments will be
evident to one skilled in the art.
Referring to Fig. 8(b), the light emitted by the sample and collected by the
objective lens, OL, is imaged by the tube lens, TL, onto the spatial filter,
SFZ. SFZ is
preferentially a slit aligned so as to extend perpendicular to the plane of
the page. Thus, the
light passed by filter SFZ is substantially a line of illumination. SFZ may be
placed in the
primary image plane or any plane conjugate thereto. DM3 is partially
reflecting, partially
transmitting and preferably "multichroic". Multi-wavelength "dichroic"
mirrors, or
"multichroic" mirrors can be obtained that preferentially reflect certain
wavelength bands
and preferentially transmit others.
8~,, will be defined to be the fluorescence emission excited by ~,,, This
will,
in general, be a distribution of wavelengths somewhat longer than ~.,. 8~.2
and 8~.3 are
defined analogously. DM3 preferentially reflects ~,", and preferentially
transmits 8~.",
n=1,2,3. The light transmitted by SFZ is imaged onto the detection devices,
which reside in
planes conjugate to the primary image plane. In Fig. 8(a), an image of the
spatial filter SFZ
is created by lens IL on all three detectors, D". This embodiment is preferred
in applications
requiring near-perfect registry between the images generated by the respective
detectors. In
another embodiment, individual lenses IL" are associated with the detection
devices, the
lens pairs IL and IL" serving to relay the image of the spatial filter SFZ
onto the respective
detectors D". The light is split among the detectors by mirrors DM, and DMZ.
The mirrors
are partially transmitting, partially reflecting, and preferentially dichroic.
DM,
preferentially reflects 8~,, and preferentially transmits 8~,2 and 8~.3. The
blocking filter, BF,,
preferentially transmits 8~,, effectively blocking all other wavelengths
present. DMZ
preferentially reflects 8~,2 and preferentially transmits 8~,3. The blocking
filters, BFZ and
BF3, preferentially transmit 8~,2 and 8~,3 respectively, effectively blocking
all other
wavelengths present.
SCANNING MIRROR CONFIGURATION
In some embodiments of this invention, rapid data acquisition requires
framing images at video rates. Video-rate imaging generally refers to 30 or 60
frames per
second. In the present use, it is intended to connote frame rates with an
order-of magnitude
of 30 Hz. In a preferred embodiment, video-rate imaging is achieved by
illuminating along
one dimension of the sample plane and scanning the illumination beam in the
direction
perpendicular thereto so as to effect a relative translation of the
illumination and sample.
The scanning stage is generally massive. Consequently, it cannot be moved
sufficiently
rapidly.
- 16-
Image
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
Fig. 9 depicts an embodiment of the invention utilizing a scanning mirror,
SM. The mirror is advantageously placed in a plane conjugate to the objective
back focal
plane (BFP):.A rotation in the BFP (or a plane conjugate thereto) effects a
translation in the
object plane (OP) and its conjugate planes. The full scan range of SM need
only be a few
degrees for typical values of the focal lengths of the lenses RL, and RLz. As
shown in Fig.
9, this lens pair images the BFP onto the SM at a magnification of one, but a
variety of
magnifications can be advantageously used. The limiting factors to the image
acquisition
rate are the camera read-rate and the signal strength. In the imaging mode
described above,
data can be acquired continuously at the camera read-rate, exemplarily 1 MHz.
With a
scanning mirror, it is preferable to acquire data uni-directionally. The
idealized scanning
motion allowing one to acquire data continuously is the sawtooth. In practice,
the
combination of turn-around and return scan times will constitute ~1/3-2/3 of
the scan
period. Assuming 50% dead-time, a minor oscillation frequency of 50 Hz and a
pixel
acquisition rate of 1 MHz, 10,000 pixels would be acquired per fi~ame at 50
fi~ames per
s~ond, which is sufficient to define and track individual objects, such as
cells, from frame
to frame. 104 pixels per image is, however, 102-times fewer than was generally
considered
above. Depending on the application, it is advantageous to acquire relatively
smaller
images at high resolution, e.g. 50-pm X 50-~tm at 0.5-~m X 0.5-pm pixelation,
or relatively
larger images at lower resolution, e.g. 200-pm X 200-pm at 2-pm pixelation.
AUTOFOCUS
According to the present invention, the sample must lie in the object plane of
an imaging system. Accordingly, the invention provides an autofocus mechanism
that
maintains the portion of the sample in the field-of view of the imaging system
within the
object plane of that system. The precision of planarity is determined by the
depth-of field
of the system. In a preferred embodiment, the depth-of field is approximately
10 pm and
the field-of view is approximately 1 mm2.
The disclosed autofocus system operates with negligible delay, that is, the
response time is short relative to the image acquisition-time, exemplarily
0.01-0.1 s. In
addition, the autofocus light source is independent of the illumination light
sources and the
sample properties. Among other advantages, this configuration permits the
position of the
sample carrier along the optical axis of the imaging system to be determined
independent of
the position of the object plane.
One embodiment of a single-beam autofocus is provided in Figs. 8 and 9,
where a separate light source, S4 of wavelength .14, and detector D4 are
shown. The
wavelength ~.4 is necessarily distinct from the sample fluorescence, and
preferentially a
-17-
CA 02324262 2000-09-15
WO 99/47963 PC'T/US99/05589
wavelength that cannot excite appreciable fluorescence in the sample. Thus,
~.4 is
preferentially in the near infrared, exemplarily 800-1000 nm. The partially
transmitting,
partially reflecting mirror, DM4, is preferentially dichroic, reflecting ~.4
and transmitting ~,n
and 8~,", n=1,2,3. Optically-based autofocus mechanisms suitable for the
present
$ application are known. For example, an astigmatic-lens-based system for the
generation of
a position error signal suitable for servo control is disclosed in Applied
Optics 23 565-570
(1984). A focus error detection system utilizing a "skew beam" is disclosed in
SPIE 200
73-78 (1979). The latter approach is readily implemented according to Figs. 8
and 9, where
D4 is a split detector.
For use with a microtiter plate having a sample residing on the well bottom,
the servo loop must, however, be broken to move between wells. This can result
in
substantial time delays because of the need to refocus each time the
illumination is moved
to another well.
Continuous closed-loop control of the relative position of the sample plane
and the object plane is provided in a preferred embodiment of the present
invention,
depicted in Fig. 10. This system utilizes two independent beams of
electromagnetic
radiation. One, originating from S5, is focused on the continuous surface,
exemplarily the
bottom of a microtiter plate. The other, originating from S4, is focused on
the discontinuous
surface, exemplarily the well bottom of a microtiter plate. In one embodiment,
the beams
originating from S 4 and SS have wavelengths ~,4 and ~.s, respectively. ~.4 is
collimated by
L4, apertured by iris I4, and focused onto the discontinuous surface by the
objective lens OL.
~,5 is collimated by Ls, apertured by iris I5, and focused onto the continuous
surface by the
lens CFL in conjunction with the objective lens OL. The reflected light is
focused onto the
detectors D4 and Ds by the lenses IL4 and ILS, respectively. The partially
transmitting,
p~~ly reflecting mirror, DM4, is preferentially dichroic, reflecting .14 and
~,s and
transmitting ~," and 8Jl", n=1,2,3. The mirrors, M4, Ms and M6, are partially
transmitting,
partially reflecting. In the case that ~.4 and ~.5 are distinct, M6 is
preferentially dichroic.
According to the embodiment wherein the sample resides in a microtiter
plate, ~,4 is focused onto the~well bottom. The object plane can be offset
from the well
bottom by a variable distance. This is accomplished by adjusting L4 or
alternatively by an
offset adjustment in the servo control loop. For convenience of description,
it will be
assumed that ~,4 focuses in the object plane.
The operation of the autofocus system is as follows. If the bottom of the
sample well is not in the focal plane of objective lens OL, detector D4
generates an error
signal that is supplied through switch SW to the Z control. The Z control
controls a motor
(not shown) for moving the microtiter plate toward or away from the objective
lens.
-18-
CA 02324262 2000-09-15
WO 99/47963 PCTNS99/05589
Alternatively, the Z control could move the objective lens. If the bottom PB
of the
microtiter plate is not at the focal plane of the combination of the lens CFL
and the
objective lens OL, detector Ds generates an error signal that is applied
through switch SW
to the Z control. An XY control controls a motor (not shown) for moving the
microtiter
S plate in the object plane OP of lens OL.
As indicated, the entire scan is under computer control. An exemplary scan
follows: At the completion of an image in a particular well, the computer
operates SW to
switch control of the servo mechanism from the error signal generated by D4 to
that
generated by Ds; the computer then directs the XY control to move the plate to
the next
well, after which the servo is switched back to D4.
The "coarse" focusing mechanism utilizing the signal from the bottom of the
plate is used to maintain the position of the sample plane to within the well-
to-well
variations in the thickness of the plate bottom, so that the range over which
the "fine"
mechanism is required to search is minimized. If, for example, the diameter of
the iris Is is
2 rnrn and ILS is 100 mm, then the image size on the detector will be ~ 100
pm. Similarly,
if the diameter of the iris I4 is 0.5 mm and IL4 is 100 mm, then the image
size on the
detector will be ~ 400 pm. The latter is chosen to be less sensitive so as to
function as a
"coarse" focus.
As with the single-beam embodiment described above, the wavelengths ~,4
~d ~s ~'e necessarily distinct from the sample fluorescence, and
preferentially wavelengths
that cannot excite appreciable fluorescence in the sample. Thus, ~.4 and ~,s
are preferentially
in the near infrared, such as 800-1000 nm. In addition, the two wavelengths
are preferably
distinct, for example ~.4 = 830 nm, ~,s = 980 nm.
In an alternative embodiment of two-beam autofocus, ~.4 = JLs and the two
beams may originate from the same source. Preferentially, the two beams are
polarized
perpendicular to one another and M6 is a polarizing beamsplitter.
Pseudo-closed loop control is provided in the preferred embodiment of
single-beam autofocus which operates as follows. At the end of a scan the
computer
operates SW to switch control to a sample-and-hold device which maintains the
Z control
output at a constant level while the plate is moved on to the next well after
which SW is
switched back to D4
DETECTION DEVICES
An essential feature of the disclosed apparatus is the use of a detection
device having manifold, independent detection elements in a plane conjugate to
the object
plane. As discussed above, line illumination is advantageous principally in
applications
-19-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
requiring rapid imaging. The potential speed increase inherent in the
parallelism of line
illumination as compared to point illumination is, however, only realized if
the imaging
system is capable of detecting the light emitted from each point of the sample
along the
illumination line, simultaneously.
It is possible to place a charge-coupled device (CCD), or other camera, at the
output of the prior art imaging systems described above (White et al., US
5,452,125 and
Brakenhoff and Visscher, J. Microscopy 171 17-26 (1993)). The resulting
apparatus has
three significant disadvantages compared to the present invention. One is the
requirement
of rescanning the image onto the two-dimensional detector, which adds
unnecessary
complexity to the apparatus. Another is the requirement of a full two-
dimensional detector
having sufficient quality over the 1000 pixel x 1000 pixel array that
typically constitutes the
camera. The third disadvantage is the additional time required to read the
full image from
the two-dimensional device.
The present invention is designed to avoid these disadvantages and optimize
not only imaging speed, within the constraints of high-sensitivity and low-
noise detection,
but also throughput. One embodiment uses a continuous-read line-camera, and in
a
preferred embodiment a rectangular CCD is used as a line-camera. Both
embodiments have
no dead-time between lines within an image or between images. An additional
advantage
of the present invention is that a larger effective field-of view is
achievable in the stage-
scanning embodiment, discussed below.
The properties required of the detection device can be further clarified by
considering the following preferred embodiment. The resolution limit of the
objective lens
is < 1 pm, typically -r0.5 p.m, and the detector comprises an array of 1000
independent
elements. Resolution, field-of view (FOV) and image acquisition-rate are not
independent
variables, necessitating compromise among these performance parameters. In
general, the
magnification of the optical system is set so as to image as large a FOV as
possible without
sacrificing resolution. For example, a ~1 mm field-of view could be imaged
onto a 1000-
element array at 1-um pixelation. If the detection elements are 20-pm square,
then the
system magnification would be set to 20X. Note that this will not result in 1-
pm resolution.
Pixelation is not equivalent to resolution. If, for example, the inherent
resolution limit of
the objective lens is 0.5 pm and each 0.5 pm X 0.5 um region in the object
plane is mapped
onto a pixel, the true resolution of the resulting digital image is not 0.5
pm. To achieve true
0.5-um resolution, the pixelation would need to correspond to a region ~0.2 ~m
X 0.2 pm
in the object plane. In one preferred embodiment, the magnification of the
imaging system
is set to achieve the true resolution of the optics.
-20-
CA 02324262 2000-09-15
WO 99/47963 PCTNS99/05589
Presently, the highest detection efficiency, lowest noise detection devices
having sufficient read-out speed for the present applications are CCD cameras.
In Figure
11, a rectangular CCD camera is depicted having an m x n array of detector
elements where
m is substantially less than n. The image of the fluorescence emission covers
one row that
is preferably proximate to the read register. This minimizes transfer time and
avoids
accumulating spurious counts into the signal from the rows between the
illuminated row
and the read-register.
In principle, one could set the magnification of the optical system so that
the
height of the image of the slit SFZ on the CCD camera is one pixel, as
depicted in Figure 11.
~ practice, it is difficult to maintain perfect alignment between the
illumination line and the
camera row-axis, and even more difficult to maintain alignment among three
cameras and
the illumination in the mufti-wavelength embodiment as exemplified in Figs. 8
and 9. By
binning together a few of the detector elements, exemplarily two to five, in
each column of
the camera the alignment condition can be relaxed while suffering a minimal
penalty in
read-noise or read-time.
An additional advantage of the preferred embodiment having one or more
rectangular CCD cameras as detection devices in conjunction with a variable-
width
detection spatial filter, SFZ in Figs. 8 and 9 and 510 in Fig. 6, each
disposed in a plane
conjugate to the object plane, is elucidated by the following. As discussed
above, in one
embodiment of the present invention the detection spatial filter is omitted
and a line-camera
is used as a combined detection spatial filter and detection device. But as
was also
discussed above, a variable-width detection spatial filter permits the
optimization of the
detection volume so as to optimize the sample-dependent signal-to-noise ratio.
The
following preferred embodiment retains the advantage of a line-camera, namely
speed, and
the flexibility of a variable detection volume. The magnification is set so as
to image a
diffraction-limited line of height h onto one row of the camera. The width of
the detection
spatial filter d is preferably variable h s d s l Oh. The detectors in the
illuminated columns
of the camera are binned, prior to reading, which is an operation that
requires a negligible
time compared to the exposure- and read-times.
In one preferred embodiment, the cameras are Princeton Instruments
NT'E/CCD-1340/100-EMD. The read-rate in a preferred embodiment is 1 MHz at a
few
electrons of read-noise. The pixel format is 1340x100, and the camera can be
wired to shift
the majority of the rows (80%) away from the region of interest, making the
camera
effectively 1340x20.
In addition to the above mentioned advantage of a continuous read camera,
namely the absence of dead-time between successive acquisitions, an additional
advantage
-21 -
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
is that it permits the acquisition of rectangular images having a length
limited only by the
extent of the sample. The length is determined by the lesser of the camera
width and the
extent of the line illumination. In a preferred embodiment the sample is
disposed on the
bottom of a well in a 96-well microtiter plate, the diameter of which is 7 mm.
A strip 1 ~m
X 1 mm is illuminated and the radiation emitted from the illuminated area is
imaged onto
the detection device. The optical train is designed such that the field-of
view is ~lmm2.
According to the present invention, an image of the well-bottom can be
generated at 1-pm
pixelation over a 1 X 7-mm field.
ENVIRONMENTAL CONTROL
In an embodiment of the present invention, assays are performed on live
cells. Live-cell assays frequently require a reasonable approximation to
physiological
conditions to run properly. Among the important parameters is temperature. It
is desirable
to incorporate a means to raise and lower the temperature, in particular, to
maintain the
temperature of the sample at 37C. In another embodiment, control over relative
humidity,
and/or COZ and/or OZ is necessary to maintain the viability of live cells. In
addition,
controlling humidity to minimize evaporation is important for small sample
volumes.
Three embodiments providing a microtiter plate at an elevated temperature,
preferably 37C, compatible with the LCI system follow.
The imaging system preferably resides within a light-proof enclosure. In a
first embodiment, the sample plate is maintained at the desired temperature by
maintaining
the entire interior of the enclosure at that temperature. At 37C, however,
unless elevated
humidity is purposefully maintained, evaporation cooling will reduce the
sample volume
limiting the assay duration.
A second embodiment provides a heated cover for the microwell plate which
allows the plate to move under the stationary cover. The cover has a single
opening above
the well aligned with the optical axis of the microscope. This opening permits
dispensing
into the active well while maintaining heating and limited circulation to the
remainder of
the plate. A space between the heated cover plate and microwell plate of
approximately 0.5
rni'n allows free movement of the microwell plate and minimizes evaporation.
As the
contents of the interrogated well are exposed to ambient conditions though the
dispenser
opening for at most a few seconds, said contents suffer no significant
temperature change
during the measurement.
In a third embodiment, a thin, heated sapphire window is used as a plate
bottom enclosure. A pattern of resistive heaters along the well separators
maintain the
window temperature at the desired level.
-22-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
In additional embodiments, the three disclosed methods can be variously
combined.
INTEGRATED DISPENSER
One embodiment of the video-rate configuration of the imaging system is
further configured to initiate kinetic assays, in particular ion-channel
assays, with a timed
reagent dispense. Initiation of channel opening is accomplished by dispensing
a solution
into the micro well. For example, voltage-gated channels can be opened by
addition of a
solution of KC 1 to depolarize the plasma membrane. The time-dependence of the
channel
opening and subsequent closing and the corresponding change in intracellular
concentration
is often sufficiently rapid to require video-rate imaging. The intrinsic speed
of the imaging
system is irrelevant, however, unless the channel response can be initiated
rapidly.
One embodiment of the present invention provides an integrated dispenser.
For assays run in 96- or 384-well plates, addition volumes in this range 20-
100 uL are
desirable. A single head dispenser, as is appropriate, for example, to the
addition of an
agonist of ion-channel activity, is the IVEK Dispense 2000. Comparable units
are available
from CAVRO. More generally, it is desirable to be able to dispense a unique
compound
into each well. One embodiment provides a single head dispenser on a robotic
motion
device that shuttles the dispense head between the analysis station, the
source plate
containing the unique compounds and the tip cleansing station. The latter is a
wash station
for a fixed tip dispenser and a tip changing station for a disposable tip
dispenser. This
system provides the desired functionality relatively inexpensively, but it is
low throughput,
requiring approximately 30 seconds per compound aspiration-dispense-cleanse
cycle. An
alternative embodiment is provided by integrating a mufti-head dispenser such
as the
Hamilton Microlab MPH-96 into the disclosed LCI system. The MPH-96 consists of
96
independent fixed tip dispensers mounted to a robotic motion device capable of
executing
the aspirate-dispense-wash cycle described above.
In an additional preferred embodiment of the invention, employed in
automated screening assays, the imaging system is integrated with plate-
handling robots,
such as the Zymark Twister.
DARK FIELD CONFOCAL CONFIGURATION
In the case that the desired lateral resolution is less than the diffraction
limit,
the background fluorescence due to the supernatant liquid can be decreased by
an inventive
application of the dark-field imaging technique. Figs. 12(a) and 12(b) depict
the ray paths
in conventional dark-field. In Fig. 12(a), a sample 600 is illuminated by a
hollow cone of
-23-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
light 610 from an objective lens 620. This cone of light is created, for
example, by placing
an opaque bar 630 at lens 440 in Fig. 10(a). In Fig. 12(b), the fluorescent
emission from
sample 600 is then collected through the center of the objective lens 620.
Because of the
differing angles of illumination and collection, the only plane which is both
illuminated and
detected is the plane containing sample 600.
Figs. 13(a) and 13(b) depict the ray paths in inverted dark-field. In Fig:
13(a), a sample 700 is illuminated with a beam of light 710 that passes
through the center of
an objective lens 720. In Fig. 13(b), fluorescent emissions are then collected
only from
around the outside of objective lens 720. Collection from around the outside
of the
objective may be achieved by placing, for example, an opaque bar 730 at lens
490 in Fig.
10(a). Like conventional dark-field, inverted dark-field involves illumination
at one angle
and collection at a different angle so that only the sample plane is both
illuminated and
detected.
Fig. 14 depicts the focal region in the case described above where it is
advantageous to illuminate a larger than diffraction-limited area of the
sample plane. The
illumination and collection rays are the same as those in the inverted dark-
field geometry of
Fig. 13. If a stop is placed in a plane conjugate to the objective back focal
plane having a
width matched to the illumination beam, the dark-field configuration is
achieved. That this
configuration confers a decrease in the out-of plane fluorescence impinging on
the detector
can be understood from Fig. 14. The fluorescence from the shaded regions above
and
below the object plane is not passed by the stop. In point-scan confocal,
fluorescence from
these out-of plane regions is rejected efficiently by the detection aperture.
In line-scan
confocal, the out-of plane fluorescence from one lateral position along the
line contributes
to the background signal at other points along the line: this is the origin of
the degradation
~ signal-to-background in line-scan relative to point-scan confocal. The
inverse dark-field
configuration of line-scan confocal recovers a significant fraction of the
background
rejection attributes of point-scan confocal while retaining the speed
advantage of the line-
scan configuration.
REAL-TIME DATA ANALYSIS
The present invention is capable of generating megabytes of data per second,
continuously. In one embodiment, the system is integrated with a fast high-
density, high-
volume storage device to which the data can be spooled in real time for
subsequent analysis.
In a preferred embodiment, data analysis is run essentially simultaneously
with data
acquisition. Thus, the data is processed prior to storage. In general, only
the results of the
analysis are archived, but it is advantageous to archive selected raw data, as
well.
-24-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
Examples of real time analysis routines are provided below in conjunction
with each of the assay groups. In all cases, procedures are used to optimize
the software
code for operation on the hardware platform of interest. In a presently
preferred
embodiment, the computer is a 32-bit processor such as the Pentium II. In this
case, all data
S is accessed in 32-bit parcels.
In general the acquisition and analysis of the data comprises a number of
discrete steps. First, the fluorescence is converted into one or more digital
images in which
the digital values are proportional to the intensity of the fluorescent
radiation incident on
each pixel of the detection device. Within this step a correction is made for
the non-
l0 uniform response of the imaging system across the field of view wherein the
background
subtracted data are divided by a so-called flat-field file. Second, a binary
bitmap is
generated from one of the digital images in which all values meeting certain
criteria are
replaced by one, all values failing to meet the criteria are replaced by zero.
In one
embodiment, the criteria include a threshold value determined from the image
itself. Third,
15 the bitmap is searched for groups of contiguous value-one pixels. In one
embodiment the
groups are further tested against minimum- and/or maximum-size criteria.
Fourth, for the
qualified groups, the values of the corresponding pixels in the same image or
in another
image are summed and recorded, and the average and other statistical
properties of the sums
determined and recorded. Additions to and variations on this basic procedure
appropriate to
20 the various assays are disclosed below.
ASSAYS
Numerous variations of the assay methods described below can be practiced
in accordance with the invention. In general, a characteristic spatial and/or
temporal
25 distribution of one or more fluorescently-labeled species is used to
quantify the assay.
Advantageously, the fluorescence is observed from an essentially planar
surface using a
line-scan confocal microscope. This section is organized by assay-type
according generally
to increasing degree of complexity in the associated data analysis routine.
The organization
is not strict, however, because the analysis algorithms are often applicable
to more than one
30 assay-type.
BINDING ASSAYS
A first assay-type that can be advantageously performed according to the
methods of the present invention is a binding assay. In general, the degree of
binding of a
35 fluorescently-labeled ligand to the target of interest is quantified from
the analysis of one or
more fluorescence images of a sample containing at least the target and the
labeled ligand
-25-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
and obtained with the disclosed line-scan confocal imaging system. The ligands
utilized
include, but are not limited to, fluorophore conjugated natural and synthetic
peptides and
proteins, sugars, lipids, nucleic acid sequences, viral particles,
bacteriophage particles,
natural and synthetic toxins, known pharmaceutical agents, small organic
molecules or
synthetic analogues of neuro-transmitters or intrinsically fluorescent small
molecules,
peptides or proteins, synthetic compounds from combinatorial libraries, random
peptides,
proteins from cDNA expression libraries, and peptidomimetics. (See Haugland
R.P.
Handbook of Fluorescent Probes and Research Chemicals 6'~ Ed. Chap. 18.) The
targets
include, but are not limited to cellular extracts or purified preparations of
receptors, ligand-
gated and ion-gated channel proteins, enzymes, transcription factors,
cytoskeletal proteins,
and antibodies and can be derived from viruses, bacteria, bacteriophages,
invertebrate and
vertebrate cells. Exemplary receptors include but are not limited to
acetylcholine,
adrenergic (a and (3), muscarinic, dopamine, glycine, glutamine, serotonin,
aspartate,
gamma-amino butyric acid (GAGA), purinergic, histamine, norepinephrine,
Substance P,
Neuropeptide Y, enkephaline, neurotensin, cholecystokinin (CCK), endorphin
(opiod),
melanocrotin/ACTH, somatostatin, parathyroid hormone, growth hormone,
thyrotropin,
thyroxin, cytokine, chemokine, insulin, insulin-like growth factor (IGF), stem
cell factor,
Luteinizing hormone-releasing hormone, gonadotropin, angiotensin, endothelin,
neurotensin, interferon, bradykinin, vasopressin, oxytocin, vasoactive
intestinal polypeptide
(~), corticotropin releasing-hormone, neurotrophin, erythropoetin,
prostaglandin,
leukotriene, thromboxane A2, calcitonin, T-cell, LDL/HDL, Epidermal growth
factor
(EGF), Estrogen, and Galainan.
BEAD-BASED BINDING
Figs. 15(a) - 15(f) depict the steps of an embodiment of a receptor-binding
assay that can be performed according to the present invention. In Fig. 15(a),
membranes
210 prepared from cells or tissues and containing the receptor target 212 are
added to a well
220 containing a liquid 230. In Fig. 15(b), fluorescent-labeled ligands 214
are added to
well 220; these ligands bind to the membrane receptors 212. In Fig. 15(c),
beads 224 are
added to the well 220. Alternatively, the order of 15(b) and 15(c) may be
interchanged, and
in a preferred embodiment, the membrane-coated beads are prepared separately,
prior to
addition to the well. Beads 224 have a diameter in the range of approximately
1-20 um and
are coated with a material, such as wheat germ agglutinin, to which the
membranes 210
adhere or have a surface that allows for the direct covalent or non-covalent
binding of
membranes.
-26-
CA 02324262 2000-09-15
WO 99/4?963 PCT/US99/05589
The foregoing steps are the same as those of the corresponding steps in the
prior art SSA depicted in Figs. 1(a) - 1(f) except that the labels are
fluorescent rather than
radioactive. _ However, in the present invention, beads 224 are not
luminescent and they
have a density such that they sink to, or can be spun down to, the bottom of
the well or are
S magnetic so that they can be moved to the bottom of the well using an
external magnet. In
Fig. 15(d), the fluorescent labels are imaged using, for example, a line-scan
confocal
microscope schematically depicted as element 240. In Fig. 15(e), a test
compound 218 is
added to the well. As in the prior art assays, the purpose of the present
assay is to determine
the extent to which the test compound displaces the fluorescently-labeled
ligands 214 from
the membrane receptors 212. In Fig. 15(f), the fluorescent labels still bound
to the
membranes 210 are imaged. By comparing the two fluorescent images, the
activity of the
test compound can be determined.
In an alternative embodiment of the assay depicted in Figs. 1 S(a) - 15(f),
the
imaging step depicted in Fig. 15(d) can be eliminated and the activity of the
test compound
can be determined by comparing the image obtained in Fig. 1 S(f) to the image
of a control
well or the image expected from the known quantity of the fluorescent-labeled
ligands
added to the well and their known affinity to the receptors.
In a specific embodiment of the assay depicted in Figs. 15 (a) - 15 (f), the
receptor is an antibody that recognizes the ligand, and the fluorescently-
labeled ligand is
added to the reaction along with a sample containing an unknown amount of
unlabelled
ligand. As in prior art radioimmunoassays, the purpose of the present assay is
to determine
the concentration of unlabelled ligand in the sample by measuring extent to
which it
displaces the fluorescently-labeled ligands 214 from the antibody receptor.
SURFACE BINDING
Figs. 16(a) - 16(f) depict the steps of a second embodiment of a receptor-
binding assay according to the present invention. In Fig. 16(a), membranes 250
prepared
from cells or tissues and containing the receptor target 252 are added to a
well 260
containing a liquid 270. The well bottom 262 is coated with a material such as
wheat germ
agglutinin, to which the membranes adhere. In Fig. 16(b), membranes 250 are
shown
bound to this material. In Fig. 16(c), fluorescently-labeled ligands 254 are
added to well
260 and bind to the membrane receptors 252. Alternatively, the order of Figs.
16(b) and
16(c) may be interchanged.
In Fig. 16(d), the fluorescence of the fluorescent labels is imaged using, for
example, a line-scan confocal microscope schematically depicted by element
280. In Fig.
16(e), a test compound 258 is added to well 260. In Fig. 16(f), the
fluorescent labels still
-27-
CA 02324262 2000-09-15
WO 99/47963 PGT/US99/05589
attached to the membranes 250 are imaged and compared to the first image to
determine the
activity of test compound 258.
. In an alternative embodiment of the assay depicted in Figs. 16(a) - 16(f),
the
imaging in Fig. 16(d) can be eliminated and the activity of the test compound
can be
determined by comparing the image obtained in Fig. 16(f) to the image of a
control well or
the image expected from the known quantity of the fluorescent-labeled ligands
added to the
well and their known affinity to the receptors.
CELL-BASED BINDING
In an alternative embodiment, ligand-target binding is advantageously
assayed on collections of cells expressing the target. In general, there are a
number of
advantages to cell-based assays for screening chemical compounds. In
particular, the
activity of interest is measured in the presence of both competing and
complementary
cellular processes affecting the biological activity of the compound. In
cellular assays,
cells prepared from cell lines or tissues are placed in tissue culture wells
or on microscope
slides. The cells can be live and intact or penmeabilized with reagents such
as digoxigenin,
or, alternatively, fixed with reagents such as formaldehyde. One or more
fluorescent-
labelled ligands are added to the cells along with any non-fluorescent
reagents required for
the assay; the fluorescent-labelled Iigands bind to one or more components of
the cells. A
test compound is then added to the cells. Alternatively, the order of addition
of fluorescent
ligands and chemical compounds may be interchanged. The fluorescent labels are
imaged
using, for example, a line-scan confocal microscope schematically depicted as
element 240.
The purpose of the present assay is to determine the extent to which the test
compound
displaces the fluorescently-labeled ligands from the receptors. The
fluorescent labels still
bound to the cells are imaged in the presence and the absence of test
compound. By
comparing the two fluorescent images, the activity of the test compound can be
determined.
In an alternative embodiment of a cell-based receptor binding assay, the
imaging step in the absence of compound can be eliminated and the activity of
the test
compound can be determined by comparing the image obtained in the presence of
compound to the image of a control well or the image expected from the known
quantity of
the fluorescent-labeled ligands added to the well and their known affinity to
the receptors.
ADVANTAGES OF LINESCAN CONFOCAL IMAGING IN BINDING ASSAYS
In a first embodiment, ligand-target binding is performed with one excitation
wavelength and one emission wavelength. Data are provided in Fig. 27
exemplifying the
speed and sensitivity of the present invention. A detailed analysis of its
performance
-28-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
relative to the prior art wherein ligands are radiolabeled to allow for their
detection, follows.
The prior art for receptor-ligand assays includes SSA formats as well as
formats in which
bound and unbound ligand are physically separated and the amount of ligand
bound to the
receptor is measured by the addition of liquid scintillant.
S First, the present invention can be used in small-volume wells, exemplarily
1
pL. In a receptor-ligand binding assay employing radiolabeled ligand, each
radio label, 3H
for example, can decay only once, producing at most 90 photons per decay, at a
decay rate
of less than 10-g per second. A single fluorescent molecule, will produce
10° - 10' photons
in total, and it will emit between 10' and 106 photons per second. Thus, the
count-rate for a
fluorescent label is approximately 10" relative to 3H. The present invention,
therefore,
requires immensely fewer labels, membranes and beads per well. For example,
while a
tritium SSA requires 10' beads per well, the present invention requires less
than 103 beads
per well. As a result, the present invention can be performed in pL-volume
wells and in far
less time. In addition, in an SSA it is difficult to alter the imaging time,
because radio labels
decay at a fixed rate. In contrast, the excitation rate of fluorescent labels
can be increased so
as to increase the photon emission rate, thereby reducing the required imaging
time. The
excitation rate cannot, however, be increased without limit. In fact, it is
the existence of the
so-called saturation limit of the fluorophore emission rate that underlies the
substantial
advantage of the line-scan confocal over the point-scan confocal in the
present application.
Second, the present invention does not require the time and expense of
handling
radioactivity. Third, because the present invention can be performed in small-
volume wells,
the compound and reagent consumption is much lower than for SSAs resulting in
further
cost reductions. Finally, the present invention does not require scintillant-
doped beads or
well bottoms, reducing costs even further.
The present invention uses a line-scan confocal microscope to image the
fluorescence of the sample in the well. The confocal aspect of the microscope
allows for
optical sectioning, i.e., detection of fluorescence from the plane in which
the sample is
located while minimizing the detection of fluorescence from the bulk of the
solution. This
eliminates the need for wash steps to remove unbound fluorescent-labeled
ligand; this step,
while it is not required in an SSA, is still required in any receptor-ligand
binding assay,
including RIA, in which scintillant containing beads are not used. The
confocal aspect of the
microscope also eliminates any interference that may originate from intrinsic
fluorescent test
compounds. The line-scan aspect allows the sample to be imaged more rapidly
than in
traditional point-scanning without losing appreciable background rejection.
The speed
~~'ease depends on the fluorophore density, the lateral resolution, the field
of view, and
parameters of the hardware including the objective NA, the detection
sensitivity and camera
-29-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
read-rate. Theoretically, the speed increase can approach the number of pixels
per line,
which is 1000 in a preferred embodiment of the present invention. Practically,
the increase
is approximately 100X.
In order to quantify these advantages, an exemplary sample will be described.
The assay is cell-based, wherein the location of the fluorescence is to be
resolved to a
precision of 1 pm. Thus the image of a 1-mm diameter sample area will consist
of 103
lines of 103 pixels. The fluorescence signal of interest might originate from
ligands on the
cell surface or from a localized source within the cell, such as a receptor in
the nucleus. In
either case, the local concentration of the fluorophore is the important
parameter. For an
engineered cell line expressing 105 receptors per cell, the cell-averaged
concentration is ~l
pM. A few thousand receptors localized in the nucleus results in a comparable
local
concentration. Consistent with the desired lateral resolution of ~1 pm, there
are ~2 x 103
fluorophores per pixel. It is assumed that the intrinsic cellular background
fluorescence is
less than, but on the order of, the label fluorescence, and that the desired
signal-to-noise
ratio is minimally 10. Then, the number of detected photons needs to be nearly
10', taking
into account the shot noise of the signal and background and the read noise of
a high quality
solid state detector. The collection and detection efficiency of the present
device, using an
approximately 0.7 NA objective, blocking filters, and a solid state camera is
~1%, requiring
that 105 photons be emitted per pixel, or 102 photons per molecule. It is
desirable that the
image be acquired in less than 1 second, preferably in a fraction of a second.
If the pixels
are acquired in a serial fashion, then the pixel dwell-time must be less than
1 ~.s, requiring a
photon emission rate of greater than 108 per second per molecule. This is
beyond the
saturation value of most fluorophores, which is typically 106. Importantly,
the flux required
to achieve saturation, 1 OS - 1 O6 W/cm2, is sufficient to drive non-linear
photo-induced
bleaching of the fluorophores, as well. Finally, the highest efficiency
detection devices
cannot be used at the data rates required in serial scanning. By contrast, the
emission rate
per fluorophore need only be 105 if 103 pixels are illuminated simultaneously.
The
increased rejection of background fluorescence of point-scan confocal does not
warrant the
disadvantage of dramatically decreased scan speed.
The exemplary data of Fig. 27 demonstrate that the disclosed system has
sufficient sensitivity to quantify tens of fluorophores per bead, while
clearly resolving
hundreds of individual beads in less than 1 second. Comparable data can be
acquired in
cell-based binding experiments, as will be exemplified below.
DATA ANALYSIS
-30-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
The data analysis routines are closely related whether the binding be cell-
based or bead-based and are presented together, below. The data can be
analyzed by the
following routines, the simplest of which is the Threshold Image Analysis
algorithm. The
purpose of the routine is to determine the amount of a fluorescently-labeled
species that is
localized in a contiguous or punctate manner so as to exceed a minimum
fluorescence
intensity, and optionally so as to not exceed a maximum fluorescence
intensity. In one
embodiment the analysis is used to assay the activity of a chemical compound.
The steps of the algorithm are as follows:
1. Acquire a digitized image of the labeled species.
2. Open file row-by-row and
Subtract camera offset value from image,
ii. Multiply each row in the image by the inverse of the corresponding row in
the
flat-field image file.
3. Optionally, histogram the image to determine the background level.
4. Establish selection criteria including a minimum value and optionally a
maximum
value. The values are determined, for example, as a fixed multiple of the mean
background level, as a fixed number of counts above the mean background level,
by
statistical analysis on the background histogram peak width or by using a pre-
determined value.
5. Compare each pixel in the image to the selection criteria. For each pixel
in the
image meeting the criteria, add the value to a running sum. The total number
of
qualified pixels and the average intensity are reported.
This routine is used advantageously to process data similar to that in Fig.
27, in which the
~dividual beads are clearly distinguishable from the background and the
artefacts due to
clumped beads or cells are small. Such a routine is appropriate for the assay-
type having
membranes bound to the well bottom, as well.
A second routine applicable to analyzing binding data is the Localization
Analysis algorithm which entails an additional shape analysis protocol. As
with the
Threshold routine, the purpose is to determine the amount of a fluorescently-
labeled species
that is localized in a contiguous or punctate manner. In one embodiment the
analysis is used
to assay the activity of a chemical compound.
The steps of the algorithm are as follows:
1. Acquire image of the labeled species.
2. Open file row-by-row and
i. Subtract camera offset value from the image,
-31 -
CA 02324262 2000-09-15
WO 99/47963 PCT/US991055$9
ii. Multiply each row in the image by the inverse of the corresponding row in
the
flat-field image file.
3. Optionally, histogram and sum the pixel values of the image.
4. Establish selection criteria including a minimum value and optionally a
maximum
value. The values are determined, for example, as a fixed multiple of the mean
background level, as a fixed number of counts above the mean background level,
by
statistical analysis on the background histogram peak width or by using a pre-
determined value.
5. Compare each pixel in the image to the selection criteria. All qualified
pixels are
assigned a value of 1 and all others are assigned a value of 0, thereby
effecting a 16-
to 1-bit compression.
6. "Clean" the edge of the image by setting to 0 all 1-valued contiguous
pixels in the
binary mask having an edge-touching member.
7. Search the bitmap for objects, defined as groups of contiguous value-1
pixels, by:
i. Searching the image in a line-by-line pattern to find a pixel of value 1.
ii. Determining all value-1 pixels contiguous to the pixel identified in i).
iii. Optionally, applying a minimum and maximum size filter to the object, the
sizes having been previously determined.
iv. If the object qualifies, proceed to step 8, otherwise change all 1-valued
pixels
in the object to 0 and continue searching for next object.
v. If the end of the bitmap is reached, proceed to step 9.
8. For each object passing the filter criteria:
i. Optionally, create a new rectangular bitmap with extended borders that
contains the object plus n extra 0 pixels in each direction from the edge of
the
object. n is the number of dilation steps to be performed below and has been
previously determined.
ii. If step 8.i. was implemented, then dilate the object by applying a
dilation step
n times in which pixels of value 0 that touch 1-valued pixels are set to value
1.
iii. For each collection of 1-valued pixels in either the dilated bitmap, or
in the
original bitmap if step 8.i. was not implemented, sum and average the
corresponding pixel values from the image to calculate the average pixel
intensities under the mask.
iv. Change to 0 all pixels of the object in the original bitmap image and
return to
step 7 to search for more objects.
-32-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
9. After all objects have been counted, the average intensity of the
fluorescently-labeled
species per object and optionally the fraction of the total intensity of the
species
localized is calculated for all objects in the image and reported together
with
statistical information such as the standard deviation.
The distinguishing operation in this routine, shared by all the following
algorithms, is the creation of the binary mask in steps 4-6. Mask generation
is depicted in
Fig. 20. The selection criteria of objects for the mask can optionally include
minimum and
maximum values, size and shape. For example, in one embodiment, the analysis
routine for
the bead-based assays include a roundness filter in step 7.iii.
In a second embodiment, the emission of two or more fluorescently-labeled
species is detected simultaneously, excited by one or more illumination
wavelengths. As
applied in a binding assay, the first fluorescently-labeled species is used to
identify the
object to which the second fluorescently-labeled species binds. Two examples
of two-color
cell-based binding assays are provided in Figs. 24 and 26. An exemplary
procedure that can
be used to analyze such images is the Co-localization Analysis routine which
is designed to
determine the amount of a first fluorescently-labeled species localized with
respect to a
second fluorescently-labeled species. In one embodiment the analysis is used
to assay the
activity of a chemical compound, for example, where activity depends on a
subcellular
localization of interest.
The steps of the algorithm are as follows:
1. Acquire digitized images of the first and second labeled species
respectively.
2. Open files row-by-row and
i. Subtract respective camera offset values from each image,
ii. Multiply each row in each image by the inverse of the corresponding row in
its
respective flat field image file.
3. Optionally, histogram the image of the f rst species to determine the
background
level and sum the intensity of the image of the second species.
4. Establish selection criteria including a minimum value and optionally a
maximum
value. These values are determined, for example, as a fixed multiple of the
mean
background level, as a fixed number of counts above the mean background level,
by
statistical analysis on the background histogram peak width or by using a pre-
determined value.
5. Compare each pixel in the image of the first species to the selection
criteria. All
qualified pixels are assigned a value of 1 and all others are assigned a value
of 0,
thereby effecting a 16- to 1-bit compression.
-33-
CA 02324262 2000-09-15
WO 99/47963 PCTNS99/05589
6. "Clean" the edge of the image by setting to 0 all I-valued contiguous
pixels in the
binary mask having an edge-touching member.
7. Search the bitmap for objects, defined as groups of contiguous value-1
pixels, by:
i. Searching the image in a line-by-line pattern to find a pixel of value 1.
ii. Determining all value-1 pixels contiguous to the pixel identified in i).
iii. Optionally, applying a minimum and maximum size filter to the object, the
sizes having been previously determined.
iv. If the object qualifies, proceed to step 8, otherwise change all 1-valued
pixels
in the object to 0 and continue searching for next object.
v. If the end of the bitmap is reached, proceed to step 9.
8. For each object passing the filter criteria:
i. Optionally, create a new rectangular bitmap with extended borders that
contains the object plus n extra 0 pixels in each direction from the edge of
the
object. n is the number of dilation steps to be perfonmed below and has been
previously determined.
ii. If step 8.i. was implemented, then dilate the object by applying a
dilation step
n times in which pixels of value 0 that touch 1-valued pixels are set to value
1.
iii. For each collection of 1-valued pixels in either the dilated bitmap, or
the
original bitmap if step 8.i. was not executed, sum and average the
corresponding pixel values from the image of the second species to calculate
the average pixel intensities under the mask.
iv. Change to 0 all pixels of the object in the original bitmap image and
return to
step 7 to search for more objects.
9. After all objects have been counted, the average intensity of the second
fluorescently-labeled species per object and optionally the fraction of the
total
intensity of the second species co-localized with the first species is
calculated for all
objects in the image and reported together with statistical information such
as the
standard deviation.
The advantage of this more elaborate routine is that the object, whether it be
a cell or
a bead, can be independently identified. As exemplified in Fig. 24, not all
cells respond.
The independent identification of cells, enables, for example, the ratio of
responding to non-
responding cells to be tabulated along with the degree of response among those
that respond.
~s algorithm, despite its additional complexity, can be implemented so as to
analyze 1-
Megapixel images in under 1 second on a Pentium II platform.
-34-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
TRANSLOCATION ASSAYS
An additional assay-type that can be performed advantageously according to
the second embodiment, that is where the emission of two or more fluorescently-
labeled
species is detected simultaneously, excited by one or more illumination
wavelengths, is the
translocation assay. In these assays, the translocation of interest is of one
or more species,
which may be proteins, lipids or other molecular complexes or sub-cellular
structures such
as vesicles, from one well-defined region of a cell to another. These include
but are not
limited to: synaptin (vesicle membrane protein), transcription factors (NF-xB,
NFAT, AP-
1 ), hormone receptors, LDL/HDL receptors, T-cell receptors, and PTH
receptors.
The prototypical translocation assay is a special case of the co-localization
measurement. Exemplarily, the co-localization of the first and second species
is quantified
by the fraction of the second species co-localized with respect to the first,
or the ratio of the
second species co-localized with the first and that resident elsewhere in the
cell. An
expanded analysis routine preferentially used to process translocation image
data is provided
below.
Exemplary translocation images and analysis procedures are provided in
Figs. 19-21. The labeled location is the cell nucleus, the label being a
fluorophore specific
for DNA, such as Hoechst 33342. Other nucleic acid specific stains are known
in the art
(e.g., see Haugland, R.P. Handbook of Fluorescent Probes and Research
Chemicals, 6'" Ed.
Chapter 8). The second species is a transcription factor whose migration from
the
cytoplasm to the nucleus is the subject of the assay. This protein can be
labeled by a variety
of methods, including expression as a fusion with GFP, and contacting the
sample with a
fluorescently-labeled antibody specific to the transcription factor protein.
The following Translocation Data Analysis routine can be used to determine
~e amount of a first fluorescently-labeled species that is distributed in a
correlated or anti-
correlated manner with respect to a second fluorescently-labeled species. In
one
embodiment the analysis is used to assay the activity of a chemical compound.
The steps of the algorithm are as follows:
1. Acquire images of the first and second labeled species respectively.
2. Open files row-by-row and
i. Subtract respective camera offset values from each image,
ii. Multiply each row in each image by the inverse of the corresponding row in
its
respective flat-field image file.
3. Optionally, histogram the image of the first species to determine the
background
level and sum the intensity of the image of the second species.
-35-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
4. Establish selection criteria including a minimum value and optionally a
maximum
value. These values are determined, for example, as a fixed multiple of the
mean
background level, as a fixed number of counts above the mean background level,
by
statistical analysis on the background histogram peak width or by using a pre-
y determined value.
5. Compare each pixel in the image of the first species to the selection
criteria. All
qualified pixels are assigned a value of 1 and all others are assigned a value
of 0,
thereby effecting a 16- to 1-bit compression.
6. "Clean" the edge of the image by setting to 0 all 1-valued contiguous
pixels in the
binary mask having an edge-touching member.
7. Search the bitmap for objects, defined as groups of contiguous value-1
pixels, by:
i. Searching the image in a line-by-line pattern to find a pixel of value 1.
ii. Determining all value-1 pixels contiguous to the pixel identified in i).
iii. Optionally, applying a minimum and maximum size filter to the object, the
size having been previously determined.
iv. If the object qualifies, proceed to step 8, otherwise change all 1-valued
pixels
in the object to 0 and continue searching for next object.
v. If the end of the bitmap is reached, proceed to step 9.
8. For each object passing the filter criteria:
i. Create a new rectangular bitmap with extended borders that contains the
object plus n extra 0 pixels in each direction from the edge of the object. n
is
the number of dilation steps to be performed below and has been previously
determined.
ii. Dilate the object by applying a dilation step n times in which pixels of
value
0 that touch 1-valued pixels are set to value 1.
iii. Compare the dilated bitmap with the original full size bitmap. Set to 0
all
pixels in the dilated bitmap that are 1-valued in the corresponding region of
the original bitmap. This produces an annular mask and ensures only one
object is captured when the bitmap borders were increased during dilation.
iv. Create another bitmap from the original object, erode it m times by
setting to
0 value-1 pixels touching value-0 pixels. m is typically equal to n and
determined previously.
v. For each collection of 1-valued pixels in the annular and eroded bitmaps,
average the corresponding pixel values from the image of the second species
to calculate the average pixel intensities under the eroded and annular masks.
-36-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99I05589
vi. Calculate the ratio of eroded to annular intensities for each object and
save in
a table.
vii. _ Change to 0 all pixels of the object in the original bitmap image and
return to
step 7 to search for more objects.
9. After all objects have been counted, the average intensity ratio of all
objects in the
image is calculated along with statistical information such as the standard
deviation.
The new feature of this routine over those disclosed above is the creation in
Step 8 of two daughter masks, one an annular extension of the primary mask,
and one an
eroded version of the primary mask. The latter is used to quantify the co-
localization of
species-two with species-one, the transcription factor and the cell nucleus
(actually, DNA),
respectively, in the present example. The former mask is used to quantify
species-two not
co-localized. In the present example, the ratio of these two quantities is
formed on an cell-
by-cell basis and the results tabulated.
According to the methods of the present invention, the data acquisition and
analysis can be performed in approximately one second. For comparison, two
prior art
examples are cited. In Ding et al. (J. Biol. Chem, 273, 28897-28905 {1998)), a
comparable
two-color translocation assay was performed. The advantages of the present
invention
include: 1) approximately SOX faster image acquisition per data channel, 2)
simultaneous
two-color image acquisition, 3) superior sensitivity of approximately lOX,
permitting lower
staining levels, 4) confocal detection, allowing elimination of a rinse step,
5) focus-time of
approximately 0.1 s compared to approximately 30 s, 6) data analysis time of
approximately
0.2 s/frame compared to 3-6 s/frame, and 7) continuous image acquisition. The
second
example of prior art is Deptala et al. (Cytometry, 33, 376-382, (1998)). The
present
invention provides 1) higher spatial resolution, approximately 4X, 2)
approximately 16X
higher pixel acquisition rates, 3) faster data analysis, 4) autofocus operable
in microtiter
plates, and 5) data analysis time of approximately 0.2 s/frame compared to 3-6
s/frame.
ENDOCYTOSIS, EXOCYTOSIS AND RECEPTOR SEQUESTRATION
Endocytosis and exocytosis, generally, and receptor sequestration and
recycling, specifically, are additional processes that can be assayed
according to the first or
second embodiments and the associated image analysis protocols disclosed
above.
Fluorescence labeling can be accomplished according to a variety of known
methods. For
example, an elegant experiment comprising the labeling of both the receptor
and ligand is
disclosed by Tarasova et al. (J. Biol. Chem., 272, 14817-14824 (1997)). The
present
imaging system is approximately SOX faster per data channel and acquires the
two images
-37-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
simultaneously. In addition, the present analysis protocols, the Co-
localization algorithm
for example, can be used to process sequestration image data in real-time. No
such
examples are_ known in the prior art.
Many other assays requiring similar imaging and analysis capabilities are
known in the art. For example, assays involving phagocytosis and related
cellular events,
(e.g., J. Immunology, (1983) 130, 1910; J. Leukocyte Biol. (1988) 43, 304);
additional
assays involving both receptor-mediated and non-receptor-mediated endocytosis
and
exocytosis (e.g. Neuron 14, 983 (1995); J. Physiol. 460, 287 (1993) and
Science 255, 200
(1992), including receptor-mediated endocytosis of Low-Density Lipoprotein
Complexes
(see J. Cell Biol. I21, 1257 (1993) and the delivery of Transferin to
vertebrate cells (see Cell
49, 423 (1994)); imaging the endocytosis and lateral mobility of fluorescently-
labeled
epidermal growth factor (see Proc. Natl. Acad. Sci. USA 75, 2135 (1975); J.
Cell Biol. 109,
2105 ( 1989)); monitoring the uptake and internal processing of exogenous
materials by
endocytosis of fluorescent dextrans (see J. Biol. Chem. 269, 12918 (1994)),
and the imaging
of the endocytosis-mediated recycling of synaptic vesicles in actively firing
neurons by use
of hydrophilic dyes (see Nature 314, 357 (1985)). In addition, the genetic
engineering of
cell lines expressing green fluorescent protein (GFP)fused to proteins that
localize to
exocytotic and secretory vesicles (such as chromogranin B, a secretory granule
protein (see
J. Cell Sci. 110,1453 (1997) or tPA which is localized to growth cones in
differentiated
neuronal cells (see Mol. Biol. Cell 9: 2463 (1998)) allow for the monitoring
of exocytosis. A
wide variety of fluorescent labels are available for such assays (See Haugland
R.P.
Handbook of Fluorescent Probes and Research chemicals, 6'" Ed. Chap. 17).
ION CHANNELS
A third embodiment of the present invention, one version of which is
depicted in Fig. 9, can be used to image the time-dependent response of one or
more
fluorescently-labeled species at a rate of approximately 30 frames per second.
This permits
the capture of transient phenomenon, such as the opening and closing of ion
channels.
Exemplary ion channels include but are not limited to: K+-gated voltage, Na+-
gated voltage,
Ca+'-gated voltage, C1-, Na+/K+ ATPase, and P-glycoproteins.
The following Kinetic Imaging Data Analysis algorithm defines and tracks
individual cells from frame to fi~ame, enabling simultaneous kinetic analysis
on a sufficient
number of cells to obtain statistically meaningful data.The steps of the
algorithm are as
follows:
1. Acquire one (indicator only), two (marker and indicator or two indicators)
or more
digitized images as a fimction of time.
-38-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
2. Open files row-by-row and
i. Subtract respective camera offset values from each image,
ii. Multiply each row in each image by the inverse of the corresponding row in
its
respective flat-field image file. Subtract respective camera offset values
from each
image.
3. Optionally, histogram the image of the first species to determine the
background
level.
4. Establish selection criteria including a minimum value and optionally a
maximum
value. The values are determined, for example, as a fixed multiple of the mean
background level, as a fixed number of counts above the mean background level,
by
statistical analysis on the background histogram peak width or by using a pre-
determined value.
5. Compare each pixel in the image of the first species to the selection
criteria. All
qualified pixels are assigned a value of 1 and all others are assigned a value
of 0,
thereby effecting a 16- to 1-bit compression.
6. "Clean" the edge of the image by setting to 0 all 1-valued contiguous
pixels in the
binary mask having an edge-touching member.
7. Search the bitmap for objects, defined as groups of contiguous value-1
pixels, by:
i Searching the image in a line-by-line pattern to find a pixel of value 1.
ii Determining all value-1 pixels contiguous to the pixel identified in i.
iii. Optionally, applying a minimum and maximum size filter to the object, the
size having been previously determined.
iv. If the object qualifies, proceed to step 8, otherwise change to 0 all 1-
valued
pixels in the object and continue searching for next object.
v. If the end of the bitmap is reached, proceed to step 9.
8. For each object passing the filter criteria: average the corresponding
pixels from each
of the images in the time series. If a single indicator is used, record the
intensities.
If ratiometric indicators are used, divide the value of one image by the other
for each
image in the time series and record the results.
9. After all objects have been analyzed, the results of the analysis of step 8
are reported
for each object. Kinetic parameters, including the rise time, fall time and
amplitude
are reported for each object as are statistical information derived from the
set of
kinetic analyses and from the set of all objects at fixed times.
Two examples of the use of the present invention to image and analyze
transient events associated with ion channels are provided in Figs. 22 and 23.
These assays
-39-
CA 02324262 2000-09-15
WO 99/47963 PCTNS99/05589
used the Ca~"-sensitive dye, Fluo-3 to indicate the changes in infra-cellular
Ca''''
concentration. In the first of the experiments, the change was caused by a
Ca'~' second
signal initiated by the activation of acetylcholine receptors, and in the
second experiment the
change was due to activation of voltage-gated Ca+' channels.
Ion channels have been an area of intense research activity in recent years.
The advantages of the present invention over the prior art will be made clear
by the
following comparisons.
In compound screening applications, a prior art standard, cited in the
Background Section, is disclosed in U.S. Patent No. 5,355,21 S. This device,
used primarily
for detecting induced changes in intracellular Ca2~, includes a dispenser to
initiate transient
events. The principal advantages of the present invention over this prior art
are the
following: 1) imaging and analysis permitting the determination of individual
cellular
responses as compared to a response averaged over the well, 2) increased
sensitivity,
requiring lower reagent loading and lower illumination intensity, and enabling
smaller
sample volumes, and 3) the acquisition of images at video rates compared to a
maximum
rate of 1 point per second.
In research applications, the system of Tsien and co-workers disclosed in the
Handbook of Biological Confocal Microscopy, J. B. Pawley, ed., Plenum Press,
New York,
1995, pp. 459-478, serves as a standard. It has a demonstrated capability to
image at rates
beyond the present invention. This cannot be accomplished, however, on samples
presently
of interest. The prior art requires 102-103 greater fluorophores per pixel to
achieve rates
comparable to the present invention at a comparable signal-to-noise ratio. In
addition, the
present invention can acquire images at 12- or 16-bit resolution, giving it a
4-16X greater
dynamic range.
A second example of a research system is disclosed in Sun et al., J.
Physiology, 509, 67-80, 1998. According to Sun, data is generated at rates up
to 650 Hz per
600-pixel line with 5 microsecond per pixel integration time, using a
conventional spot
scanning confocal microscope. Only one-dimensional "imaging" is performed.
Transients
can be monitored for objects lying along the scanned line. In addition, this
rate could only
be achieved with 1-ps pixel integration time, requiring a 102-103 greater
concentration of
fluorophores to achieve image quality comparable to the present invention.
The capabilities of the present invention to image and analyze changes in
infra-cellular ion concentrations in response to external stimuli has multiple
applications in
compound screening and in general biological research applications. {See e.g.
J. Cell Biol.
3$ 137(3), 633-648 (1997); J. Biol. Chem. 271(9), 4999-5006 (1996); Science
280, 69-76
(1998); Biochem, J., 324, 645-651 (1997)). A wide variety of fluorescent
indicators are
-40-
CA 02324262 2000-09-15
WO 99/47963 PCT/EJS99/05589
available sensitive to specific ions (see Haugland R.P. Handbook of
Fluorescent Probes and
Research Chemicals, 6'" Ed. Chaps 18, 22 and 24). These indicators allow
measurement of
concentrations of Mg2+, Zn2+, Ca2+, Na', Fe2+ Hgz+, Pbz+, Cdz+, Nii+, Co2+
A1'', Gaz+, Eu3+,
Tb3+, Tb3+, Sm3+, and Dy'+. In addition, assays for Na+ and K+ can be
performed even in the
presence of physiological concentrations of other monovalent cations (see J.
Biol. Chem.
264, 19449 ( 1989)), including assays of Na' levels or Na+ efflux in a variety
of cells such as
blood, brain and muscle cells (see J. biol. Chem. 268, 18640 (1993); J.
Neurosci. 14, 2464
(1994); Am J. Physiol. 267, H568 (1994)), and changes in K+ in sperm cells,
nerve terminals
synaptosomes and lymphocytes. In addition, the present invention can be used
to assay CI-
concentrations in vesicles, liposomes and live cells (see Am. J. Physiol. 259,
c375 (1990).
In addition, the present invention can be used to assay changes in membrane
potential in cells and sub-cellular organelles. The ability to rapidly image
changes in
membrane potential is vital to assays for cell and organelle viability, nerve-
impulse
generation, muscle contraction, cell signaling and ion-channel gating (see
Biophys J. 67, 208
(1994); Neuron 13, 1187 (1994); J. Membrane Biol. 130,1 (1992)). Fluorescent
indicators
are available that respond to fast (millisecond) potential changes in
excitable cells such as
neurons, cardiac cells and intact brain cells. (See Haugland R.P. Handbook of
Fluorescent
probes and Research Chemicals, 6'" Ed. Chap. 25). The fluorescent probes that
respond to
fast transmembrane potential changes typically show only a 2-10% change in
fluorescence
per 100mv. The plasma membrane of a cell has a transmembrane potential of
approximately
-70mv and some organelles such as mitochondria maintain transmembrane
potentials of -
150mV. Thus, assays involving such rapid changes require the high sensitivity,
rapid data
acquisition ability common to the various embodiments of the present
invention.
FRET-BASED MEASUREMENTS
The present invention can be advantageously used to perform assays which
involve fluorescence resonance energy transfer (FRET). FRET occurs when one
fluorophore, the donor, absorbs a photon and transfers the absorbed energy non-
radiatively
to another fluorophore, the acceptor. The acceptor then emits the energy at
its characteristic
wavelength. The donor and acceptor molecules must be in close proximity, less
than
approximately 10 nm, for efficient energy transfer to occur (see Methods
Enzymol. 211, 353
- 388 (1992); Methods Enzymol. 246, 300-334 (1995)). The proximity requirement
can be
used to construct assays sensitive to small separations between the donor-
acceptor pair.
FRET typically requires a single excitation wavelength and two emission
wavelengths, and
an analysis consisting of the ratio of the donor and acceptor emission
intensities. FRET
donor acceptor pairs can be constructed for both bead-based assays and cell-
based assays.
-41 -
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
Several green fluorescent protein (GFP) mutants displaying enhanced
fluorescence and
altered emission wavelengths can be paired for FRET cell-based assays by
fusing the GFP
FRET donor to one protein and the GFP FRET acceptor to either the same protein
or to
another protein expressed within the same cell. Such FRET pairing can be used
to measure
intramolecular changes, such as Caz+-calmodulin binding of CaZ+ or
intermolecular
interactions, such as receptor dimerization. The Kinetic Imaging algorithm
disclosed above
can be preferentially used.
TRANSIENT TRANSFECTION
Among the significant advantages of an image-based measurement is the
opportunity both to observe rare events, lost within the average, and to
normalize the
primary response on an object-by-object basis to a secondary, response. Both
features can
be important in assays using a cell line having a transiently transfected
target. Gene
expression and subsequent protein production following transfection is often
inefficient and
transient (see BioTechniques 24:478-482 (1998)). Methods to monitor the
transfection
efficiency that can be advantageously used with the present invention are
known in the art.
For example, the gene of interest can be transfected together with the gene
for green
fluorescent protein (GFP), so that the two proteins will be expressed either
as a fusion or as
separate entities. The present invention can be used to measure the amount of
indicator
present at one wavelength and the response associated with the target at
another. The
former signal can be used to normalize the response of the latter for the
amount of target
present. This allows the present invention to perform assays on targets too
unstable to be
used in currently available screening and to monitor transfection efficiencies
of only a few
percent. The Kinetic Imaging algorithm disclosed above can be used to analyze
such data,
where only one image frame is required. Viral infection of cells can be
monitored, either
directly through expression of viral proteins, or indirectly by acquisition of
a new
phenotype, even if only a few percent of cells are infected. Finally, this
invention provides a
method for detecting a rare event, such as the acquisition of a new phenotype
by an
individual cell or group of cells due to the transfection of a specific cDNA
as a result of the
transfection of the entire cell population with a library of diverse cDNAs.
ENZYME ASSAYS
The present invention can also be used to conduct general assays of enzyme
activity. Exemplary intracellular enzymes include but are not limited to:
carbonic
~ydrase, guanine nucleotide-binding proteins (G proteins), adenyl cyclase,
calmodulin, PI,
PIP and PIP2 kinases, CAMP kinase and cAMP hydrolase, cytochrome P-450,
-42-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
serine/threonine protein kinases, tyrosine protein kinases, protein
phosphatases, ~i-lactamase,
(3-galactosidase, dihydrofolate reductase, phosphodiesterases, caspases,
proteosome
proteases, nitric oxide synthase, thymidine kinase, nucleoside deaminase,
glutathione-S-
transferase, lipoxygenases, and phospholipases.
Figs. 17(a) - 17(d) depict the steps of a first embodiment of an enzyme assay
according to the present invention. In Fig. 17(a), beads 310 with a known
quantity of
fluorescent-labeled peptides 312 attached thereto are added to a well 320
containing a liquid
330. Beads 310 have a density such that they sink to the bottom of the well.
In Fig. 17(b), a
test compound 314 is added to the well. In Fig. 17(c), enzymes 316 are added
to the well.
The order of the steps depicted in Figs. 17(a), 17(b) and 17(c) is
interchangeable except that
at no time should the well contain the peptides and enzymes without the test
compound. If
not inhibited, enzymes 316 will cleave peptides 312, and the fluorescent
labels will diffuse
into the liquid. If, on the other hand, test compound 314 inhibits enzymes
316, typically by
blocking the enzyme active sites, enzymes 316 will not cleave the fluorescent
labels. In Fig.
17(d), the fluorescent labels still attached to the beads are imaged using,
for example, a line-
scan confocal microscope schematically depicted as element 340. From this
image, the
activity of test compound 314 can be determined.
In an alternative embodiment of the assay depicted in Figs. 17(a) - 17(d), the
activity of the test compound can be determined by comparing the image
obtained in Fig.
17(d) to the image obtained by imaging the fluorescence of the fluorescent
labels in Figs.
17(a) or 17(b) or the image of a control well.
Figs. 18(a) - 18(d) depict the steps of a second embodiment of an enzyme
assay according to the present invention. In Fig. 18(a), a known quantity of
fluorescent-
labeled peptides 352 are attached to the bottom 362 of a well 360. In Fig.
18(b), a test
compound 354 is added to the well. In Fig. 18(c), enzymes 356 are added to the
well. In
Fig. 18(d), the fluorescent labels still attached to the bottom of the well
are imaged using,
for example, a line-scan confocal microscope schematically depicted as element
380 to
determine the activity of test compound 354.
In an alternative embodiment of the assay depicted in Figs. 18(a) - 18(d), the
~h~ty of the test compound can be determined by comparing the image obtained
in Fig.
18(d) to the image obtained by imaging the fluorescence of the fluorescent
labels in Figs.
18(a) or 18(b) or the image of a control well.
Another example of an assay that may be performed according to the present
invention is a tyrosine kinase assay. Tyrosine kinases phosphorylate tyrosine
residues of
substrate peptides. The substrate peptide has both a tyrosine residue and a
fluorescent tag.
In this assay, an antibody that at one end is selective for phosphorylated
tyrosine is bound at
- 43 -
CA 02324262 2000-09-15
WO 99/47963 PC'T/US99/05589
the other end to a surface such as a bead or the bottom of a well. Tyrosine
kinase and a
fluorescent-tagged peptide with a tyrosine residue are added to the well. If
the tyrosine
kinase phosphorylates the peptide, the phosphorylated tyrosine will bind to
the antibody,
thereby localizing the fluorescent tag on the surface to which the antibody is
attached. If the
tyrosine kinase does not phosphorylate the peptide, the fluorescent tags on
the peptides will
be dispersed throughout the well. The extent of phosphorylation of the peptide
can be
determined by measuring the fluorescence adjacent to the surface. Such an
assay can also be
conducted where an antibody is used that is specific to the fluorescent
product produced by
the action of the enzyme upon the fluorescent substrate.
In addition, live-cell enzyme assays can be performed according to the
present invention. A number of techniques for investigating enzymatic activity
in live cells
are known in the art (See Biochem. Histochem 70,243 (1995), J. Fluorescence 3,
119
(1993)) as are substrates that yield fluorescent products when acted on by
enzymes (See
Haugland R.P. Handbook of Fluorescent Probes and Research Chemical 6'~ Ed.
Chap. 10).
~ general, these assays use probes that passively enter the cell and are
subsequently
processed by intracellular enzymes to generate products retained within the
cell. Other
substrates yield insoluble fluorescent products that precipitate at the site
of enzymatic
activity. The present invention can assay the degree of enzymatic activity and
determine the
precise spatial localization of the enzymatic activity using such probes.
Probes are available
for assaying a wide variety of enzymes using the present invention including
but not limited
to phosphatases, ATPases, S'-nucleotidase, DNA and RNA polymerases,
peptidases,
proteases, esterases and peroxidase.
Enzyme activity assays can be performed according either the first or second
experimental embodiments and the associated image analysis protocols disclosed
above.
MORPHOLOGY
The methods of the present invention can also be used to perform assays that
require a determination of cellular or sub-cellular morphology, including but
not limited to
axons and organelles. To perform such assays, a fluorescent probe is
introduced into the
structure of interest, such as a cell or organelle, by direct nucro-injection
or by contacting
cells with cell-permeant reagents that are metabolized or otherwise altered so
as to be
retained in the structure of interest. If it is to be used with live cells,
the fluorescent label
must be non-toxic and biologically inert. Many appropriate dyes are available
commercially
(See Haugland R.P. Handbook of Fluorescent Probes and Research Chemicals 6'"
Ed. Chap.
15) for use in assays, for example, involving flow in capillaries, neuronal
cell connectivity,
translocation of dye through gap junctions, cell division and cell lysis and
liposome fusion.
_,
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
In addition, these tracers can be used to track movement of labeled cells in
culture, tissues or
intact organisms. Many techniques employing fluorescent tracers to assay cell
or sub-
cellular morphology or movement are known in the art and may involve use of
membrane
tracers, biotinylated dextran conjugators, fluorescent microspheres or
proteins and protein
conjugates (See Meth. Cell Biol. 29, I53 (1989); Cytometry 21. 230 (1995);
Cell 84, 381
(1996); Biochem. Biophys. Acta 988, 319 (1989); Cytometry 14, 747 (1993). The
various
embodiments of the present invention have significant advantages when used in
these types
of assays. The present invention allows rapid imaging of multiple parameters
with very fine
spatial resolution.
NUCLEIC ACIDS
The present invention can also be used to conduct assays of nucleic acids. A
specific DNA assay that would benefit from the spatial resolution and mufti-
wavelength
imaging capability of the present invention is fluorescence-in-situ
hybridization (FISH).
FISH is an important technique for localizing and determining the relative
abundance of
specific nucleic acid sequences in cells, tissue, interphase nuclei and
metaphase
chromosomes and is used in clinical diagnostics and gene mapping (see Histo-
chem J. 27, 4
(1995); Science 247, 64 (1990); Trends Genet. 9, 71 (1993) and Science 250,
559 (1990)).
A variety of fluorescent hybridization probes are available for multicolor
fluorescent DNA
~d RNA hybridization techniques (see Haugland R.P. Handbook of Fluorescent
Probes and
Research Chemicals, 6'" Ed. Chap. 8.4). An additional technique determines
chromosome
banding by the use of an AT or GC selective DNA-dyes with a nucleic acid
counter stain.
This technique is widely used for karotype analysis and chromosome structure
studies (see
Human Genet. 57,1 ( 1981 )).
REACTIVE OXYGEN SPECIES
The present invention can also be used to assay levels of various reactive
oxygen species such as singlet oxygen, superoxides and nitric oxide. The
importance of
these reactive oxygen species has only recently been realized (See Biochem
Pharmacol
47,373 (1994), J. Cell Biol. 126, 901 (1994)). It is now known that singlet
oxygen is
responsible for much of the physiological damage caused by reactive oxygen
species (See J.
Photochem. Photobiol. 11,241 ( 1991 )). Nitric Oxide (NO), in particular, is
now known to
play a critical role as a molecular mediator in a variety of physiological
processes including
neurotransmission and blood-pressure regulation (See Current Biology 2,437
(1995), J.
Med. Chem. 38,4343 (1995), Cell 78, 919 (1994)). Techniques are known in the
art to
perform assays to measure NO indirectly. For example, under physiological
conditions, NO
-45-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
is oxidized to nitrite and this can be detected by monitoring absorbance at
548 nm or by use
of a probe which reacts with nitrite to form an identifiable fluorescent
product. (See
Haugland R. P., Handbook of Fluorescent Probes and Research Chemicals 6'~ Ed.
Chap. 21).
pH
The present invention can also be used to perform assays involving
measurements of pH changes within cells or in cell-free media. The importance
of the role
of intracellular pH has been recognized in many diverse physiological and
pathological
processes including cell proliferation, apoptosis, fertilization, malignancy,
multi-drug
resistance, ion transport, lysosomal storage disorders and Alzheimer's
disease. (See Cell
Physiol. Biochem. 2, 159 (1992); J. Biol. Chem. 270, 6235 (1995); Biophys. J.
68, 739
(1995); J. Biol. Chem. 270, 19599 (1995); Cancer Res, 54, 5670 (1994)).
Fluorescent
probes useful for assays of pH in the physiological range are available
commercially (See
Haugland R.P., Handbook of Fluorescent Probes and Research Chemicals 6'~ Ed.
Chap. 23).
EXAMPLES
The invention described and claimed herein can be further appreciated by one
skilled in the art through reference to the examples which follow. These
examples are
provided merely to illustrate several aspects of the invention and shall not
be construed to
limit the invention in any way.
Transcription Factor Translocation
Cells were grown in 96-well plates, fixed, incubated with Texas-Red-labeled
antibody to the transcription factor protein, rinsed, and then stained with 5
pM Hoechst
33342 in buffer.
The images in Fig. 19 are 0.5 x 0.5 mm~ square with 1.08 x 1.08 pmz
pixelation. Texas Red emission was excited at 568 nm and detected with a 600-
nm long
pass filter. Hoechst emission was excited at 364 nm and detected with a 420-
480-nm
bandpass filter. Image acquisition time was 0.9 sec. There are 150 cells per
image
Fig. 19 a) is an image of a field of cells which were not activated before
fixing. The Texas Red intensity in the nucleus is low compared to the
cytoplasm. Fig. 19 b)
is the composite of the images in Fig. 19 a) and that due to the Hoechst 33342
emission.
Fig. 19 c) is an image of a field of cells which were activated prior to
fixing.
Due to color scaling, the cytoplasm is difficult to see without saturating the
nucleus. Fig. 19
-46-
CA 02324262 2000-09-15
WO 99/47963 PCTNS99/05589
d) is the composite of the images in Fig. 19 c) and that due to the Hoechst
33342 emission
from the same sample.
The data analysis was performed according to the following method. The
area highlighted in Fig. 19 b) is reproduced in Fig. 20 which depicts the mask
generation
steps. A binary representation of the Hoechst image was generated by applying
an
appropriate threshold, those values greater than the threshold were set to
one, those less than
the threshold were set to zero. This served as the primary mask. Two daughter
masks were
then generated, one by eroding the primary mask, the other by dilating the
primary mask and
subtracting the original mask to form an annular mask. The Texas-Red-emission
image was
multiplied by the eroded binary mask, as depicted in Fig. 21, and the pixels
summed as a
measure of the quantity of labeled transcription factor in the nucleus.
Similarly, the Texas-
Red-emission image was multiplied by the annular binary mask, as depicted in
Fig. 21, and
the pixels summed as a measure of the quantity of labeled transcription factor
in the
cytoplasm. The degree of activation is assessed using the ratio of nuclear to
cytoplasmic
intensity.
This ratio is represented in the bar graph in Fig. 28a for cells with and
without activation.
Transient Ca Imaging of Muscarinic Receptor and Voltage-gated Channel
Stimulation
The cells in Figs. 22 and 23 were from a neuroblastoma line. They were
grown and imaged in standard media. These cells express a muscarinic
acetylcholine
receptor that can be stimulated with Carbachol generating a large infra-
cellular Ca release as
a second signal. In addition the cells express a voltage-gated "L" Ca channel
which can be
stimulated by depolarizing the cell membrane with a large change in the
external K+
concentration and which can be inhibited with Verapamil.
In general, the image sequences were initiated by rapidly adding 100 pL of
reagent in growth media to cells in 100 pL of growth media in a 96-well plate.
The
turbulence caused by the added volume generates a small distortion in cell
shape. This
distortion is visible as a transient alteration of the Ca fluorescence
assigned to each cell in
the first image frame after addition.
In Fig. 22 a movie with 1.2 seconds between frames is displayed. The image
sequence was initiated by the rapid addition of 100 pM Carbachol. The final
image is a
binary mask, used to identify and enumerate fluorescent objects in the image,
generated
from the pre-injection frame. Even though the pre-injection image appears dim,
it is quite
bright. The mask is applied to each image in the series, and for each object,
the integrated
intensity, normalized to the pre-injection image, is plotted vs. time, as
displayed in Fig. 28b.
-47-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
The mask was not processed for overlapping cells. For example, object 1 is
likely more than
one cell, but showed no response. Object 7 may be 2 overlapping cells, with
one showing a
delayed response.
Fig. 23a-h are selected frames of a movie showing the response of the
neuroblastoma cells to a depolarization event initiated by the addition of 50
mM KCI which
opens the voltage-gated "L" channels. The analysis procedure was as is
described above in
connection to Fig. 28b. The results are displayed in Fig. 28c. Note the
increased sensitivity
obtained by using the "cell average" rather than the "image average".
Live-celt G-Protein Coupled Receptor Binding
The images displayed in Figs. 24a-c to 25a-c were obtained on live cells in
96-well plates. The cells had been transfected with a G-protein coupled
receptor, for which
the natural peptide ligand is known. Prior to imaging, the cells were
incubated with the
native unlabeled ligand in normal growth media containing 10% serum for 20
minutes at
37°C, followed by 20 minutes with 20 nM fluorescein-labeled ligand and
100 nM LDS 751,
also at 37°C. Samples were not rinsed.
These images are (0.5 x 0.5) mmz are with (1.08 x 1.08) pmt pixelation.
Fluorescein emission was excited at 488 nm and detected with a 45-nm bandpass
filter
centered at 535 nm. LDS 751 emission also excited at 488 nm and was detected
with a 40-
nm bandpass filter, centered at 690 nm. Image acquisition time was 0.9 sec.
These cells
have 100,000 receptors/cell or about 25 receptors/um2 of membrane surface.
Fig. 24a is an image of the cells after incubation with the labeled ligand. No
wash step was preformed prior to imaging. The substantial variation in
reception activity is
evident. Some cells bind so little ligand that they appear as depressions in
the background.
A cell-by-cell analysis of the binding activity is facilitated by making a
mask from an image
of LDS 751 emission, a non-specific nucleic acid stain, shown in Fig. 25b. The
staining is
not entirely uniform, but the vast majority of cell volume is revealed. The
overlay in Fig.
25c of the binary mask generated from thresholding the data in Fig. 25b with
the receptor
binding image yields a pseudo-color map of receptor activity. High activity is
represented
as yellow, while low activity is shown as orange-red.
In Fig. 25 three images are displayed corresponding to points on the titration
curve of the 20-nM labeled ligand with the unlabeled ligand. The curve is
displayed in Fig.
28d. A K; = 3t1 x 101° M for the unlabeled ligand is calculated.
Images illustrating receptor binding on a different mammalian cell line are
shown in Fig. 26a-d. Fig. 26a is an image of the cells incubated with a 256 nM
Cy3-labeled
iigand. A range of binding activity is visible. Fig. 26b shows an overlay of
the Cy3 data
-48-
CA 02324262 2000-09-15
WO 99/47963 PCT/US99/05589
with a simultaneously acquired image of the 1-pM Hoechst 33342 stained nuclei.
The latter
serves as a reliable identifier of the individual cells. In Fig. 26c, the
image is of the cells
incubated with 256 nM Cy3-labeled ligand in the presence of 10 uM unlabeled
ligand, and
in Fig. 26d, this data is displayed with the image of the I-pM Hoechst 33342
stained nuclei
overlaid. The effect of displaced fluid by unlabeled cells is evident in Fig.
26c. In the high
correlation between Figs. 26c and d exemplifies the effectiveness of
identifying cells by
their excluded volume.
Simulated Bead-Based Receptor-Binding
In Fig. 27a-d images of Cy5-labeled silica beads are presented. The
experiment is a simulation of a receptor-binding assay in which fluorescently-
labeled
ligands bind to membrane-bound receptors supported on microspheres.
Silica microspheres, 4 pm in diameter, were coated with polyethylenimine and
biotinylated with a biotin NHS-ester. The activity of the beads was assayed
with a
fluorimeter by quantifying the amount of Cy5-labeled streptavidin removed from
solution
by adsorption onto a known quantity of beads. Each bead was found to hold 1.3
x 106
streptavidin molecules. Beads were loaded with a known quantity of Cy5
molecules by pre-
mixing an appropriate ratio of Cy5-labeled and non-labeled streptavidin and
incubating with
~e beads. The loadings were equivalent to 0.16, 1.6 and 16 fmole/200 pg of
polystyrene
beads. Each bead had an average of 17, 170, or 1700 labels, respectively. The
samples were
placed in Costar 96-well plates for imaging. Cy5 was excited with 647nm laser
light and the
emitted fluorescence was detected through a 40-nm bandpass f lter centered at
690 nm. The
scanned images were acquired at 1-pm pixelation in approximately 0.7 seconds.
Beads loaded with 170 and 1700 molecules were readily detectable and the 17-
fluor
beads are discernable in images constituting Fig. 27. Beads loaded only with
non-labeled
streptavidin did not produce appreciable intensities.
35
-49-