Note: Descriptions are shown in the official language in which they were submitted.
CA 02324347 2000-09-19
WO 99/48495 PCTIUS99/06220
BENZOATES DERIVATIVES FOR INHIBITING ANGIOGENESIS
FIELD OF THE INVENTION
The present invention relates to methods for effectively inhibiting
unwanted angiogenesis. More particularly, this invention relates to methods of
treating
diseases associated with unwanted angiogenesis and to delivering anti-
angiogenic activity
to mammals having such diseases.
BACKGROUND OF THE INVENTION
Angiogenesis is the development of new blood vessels from existing
microvessels. The process of generating new blood vessels plays an important
role in
embryonic development, in the inflammatory response, in the development of
metastases
(tumor induced angiogenesis or TIA), in diabetic retinopathy, in the formation
of the
arthritic panus and in psoriasis. Under normal physiological conditions,
humans or
animals only undergo angiogenesis in very specific, restricted situations. For
example,
angiogenesis is normally observed in wound healing, in fetal and embryonal
development
and in the formation of the corpus luteum, endometrium and placenta. The
control of
angiogenesis is a highly regulated system involving angiogenic stimulators and
inhibitors.
The control of angiogenesis has been found to be altered in certain disease
states and, in
many cases, the pathological damage associated with the disease is related to
the
uncontrolled angiogenesis.
In tumor angiogenesis, for example, capillary sprouts are formed, their
formation being induced by a group of tumor cells. However, compared with
blood
vessels produced in normal angiogenic microenvironments, tumor microvessels
are
morphologically and functionally unique. Their vascular networks typically
show
disorganized or aberrant architecture, luminal sizes vary and blood flow can
fluctuate
chaotically. There are two principal types of tumor angiogenesis in terms of
the events
CA 02324347 2000-09-19
WO 99/48495 PCTIUS99/06220
2
which follow implantation of metastatic seedlings on surfaces and in organs.
The first or
primary angiogenesis is the initial vascularization of the mass of multiplying
tumor cells
and is regarded as an essential prerequisite for the survival and further
growth of a
metastatic deposit. The second is a continuing or secondary angiogenesis and
is the
phenomenon which occurs in waves at the periphery of a growing tumor mass.
This
second angiogenesis is essential for the accretion of new microcirculatory
territories into
the service of the expanding and infiltrating tumor.
Persistent, unregulated angiogenesis occurs in a multiplicity of disease
states, tumor metastasis and abnormal growth by endothelial cells and supports
the
pathological damage seen in these conditions. The diverse pathological states
created due
to unregulated angiogenesis have been grouped together as angiogenic dependent
or
angiogenic associated diseases. Therapies directed to the control of the
angiogenic
processes could lead to the abrogation or mitigation of these diseases.
One example of a disease mediated by angiogenesis is ocular neovascular
disease. This disease is characterized by invasion of new blood vessels into
the structures
of the eye such as the retina or cornea. It is the most common cause of
blindness and is
involved in approximately twenty eye diseases. In age related macular
degeneration, the
associated visual problems are caused by an ingrowth of choroidal capillaries
through
defects in Bruch's membrane with proliferation of fibrovascular tissue beneath
the retinal
pigment epithelium. Angiogenic damage is also associated with diabetic
retinopathy,
retinopathy of prematurity, corneal graft rejection, neovascular glaucoma and
retrolental
fibroplasia. Diseases associated with retinal/choroidal neovascularization
include, but are
not limited to, diabetic retinopathy, macular degeneration, sickle cell
anemia, sarcoid,
syphilis, pseudoxanthoma elasticum and Pagets disease.
Another disease in which angiogenesis is believed to be involved is
rheumatoid arthritis. The blood vessels in the synovial lining of the joints
undergo
angiogenesis. In addition to forming new vascular networks, the endothelial
cells release
factors and reactive oxygen species that lead to pannus growth and cartilage
destruction.
An important area of current research in therapeutic oncology is focused
on the discovery and development of anti-angiogenic agents which target tumor
vasculature by inhibiting or suppressing new blood vessel growth. Several
kinds of
compounds have been used to prevent angiogenesis. For instance, Taylor, et al.
have
used protamine to inhibit angiogenesis (see, Taylor, et al., Nature 297:307
(1982)).
CA 02324347 2000-09-19
WO 99148495 PC"TNS99/06220
3
However, the toxicity of protamine limits its practical use as a therapeutic.
In addition,
Folkman, et al. have disclosed the use of heparin and steroids to control
angiogenesis
(see, Folkman, et al., Science 221:719 (1983) and U.S. Pat. Nos. 5,001,116 and
4,994,443). Steroids, such as tetrahydrocortisol, which lack gluco- and
mineral-corticoid
activity, have been found to be angiogenic inhibitors. In addition,
angiostatin proteins
have been shown to reversibly inhibit proliferation of endothelial cells.
Angiostatin is
capable of inhibiting angiogenesis-related diseases and modulating angiogenic
processes
(see, e.g., WO 95/292420).
In view of the foregoing, it is apparent that there remains a need in the art
for methods and compounds for inhibiting angiogenesis, either by competitively
inhibiting an angiogenesis factor or by some other mechanism. Such methods and
compounds would have an adverse effect on the growth of tumors and, in
addition, could
be used to treat many of the other diseases set forth above. The methods of
the present
invention fulfill this and other needs.
SUMMARY OF THE INVENTION
In one aspect, this invention relates to a method of inhibiting the
vascularization of endothelial cells, the method comprising contacting a cell,
tissue or
organ which has endothelial cells with an anti-angiogenic amount of a compound
of
Formula I. Compounds of Formula I have the following general formula:
R
I
In Formula I, Rl is a functional group including, but not limited to, C~-C6-
alkyl.
In Formula I, R2, R3, R4 and RS are each independently selected and are
functional groups including, but not limited to, hydrogen, C~-C6-alkyl, C2-C6-
alkenyl, C2-
C6-alkynyl, aryl, hydroxyl, C,-C6-alkoxy, halogen, N02 and NH2.
In Formula I, R6, R7, R8 and R9 are each independently selected and are
functional groups including, but not limited to, hydrogen, C~-C6-alkyl, C2-C6-
alkenyl, C2-
C6-alkynyl, aryl, hydroxyl, C,-C6-alkoxy, halogen, N02 and NH2.
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
4
In Formula I, X, if present, is a functional group including, but not limited
to, the following: oxygen, sulfur, -CH2-, or carboxy.
In Formula I, Y is a hetervatom including oxygen or sulfur.
In a preferred embodiment of the invention, the compound of Formula I is
methyl 3,5-diiodo-4.-(4'-methoxyphenoxy)benzoate ("BTO-956").
In another aspect, this invention relates to a method for effectively
inhibiting unwanted angiogenesis in a tissue or organ, the method comprising
contacting
the cell with a compound of Formula I, or a pharmaceutical composition
thereof, in an
amount Buff cient to inhibit angiogenesis. In a presently preferred
embodiment, the cell is
in a mammalian subject.
In yet another aspect, this invention relates to a method of treating
mammalian diseases mediated by or associated with undesired and uncontrolled
angiogenesis, the method comprising administering to a mammal an anti-
angiogenic
compound of Formula I in a dosage sufficient to inhibit angiogenesis. These
methods are
useful for ameliorating the effects of conditions that are characterized by
abnormal or
undesirable angiogenesis or endothelial cell proliferation.
In another aspect, this invention relates to methods that reduce the level of
tumor necrosis factor a (TNF-a) produced by a cell.
In still yet another aspect, this invention relates to methods of using such
compounds to reduce TNF-a production and to treat inflammatory diseases.
Other features, objects and advantages of the invention and its preferred
embodiments will become apparent from the detailed description which follows.
BRIEF DESCRIPTION OF THE DRAWINGS
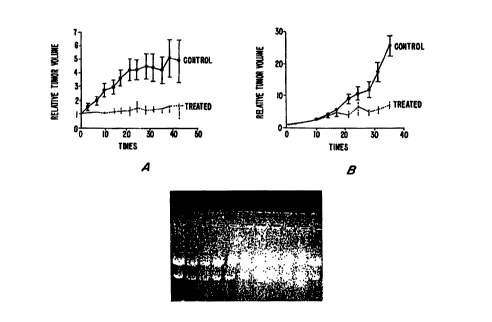
FIG. 1 illustrates the mean growth response as relative tumor volume of
MDA MB-231 (A) and OVCAR3 (B) tumors in nude mice exposed to BTO-956.
FIG. 2 illustrates that BTO-956 induces a prometaphase arrest in MCF-7
breast carcinoma cells.
FIG. 3 illustrates the inhibition of in vitro microtubules assembly by BTO-
956.
FIG. 4 illustrates the disruption of microtubule networks by BTO-956.
FIG. 5 illustrates that BTO-956 competes with colchicine for binding to
tubulin in vitro.
FIG. 6 illustrates that BTO-956 inhibits the proliferation of human
vascular endothelial cells.
FIG. 7 illustrates that BTO-956 reduces TNF-a mRNA accumulation.
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
FIG. 8 illustrates the effect of different concentrations of BTO-956 on
TNF-a gene expressions.
FIG. 9 illustrates that total RNA was intact in cells treated with different
concentrations of BTO-956 and colchicine.
DETAILED DESCRIPTION OF THE
INVENTION AND PREFERRED EMBODIMENTS
A. DEFINITIONS
The term "angiogenesis" refers to he generation of new blood vessels into
cells, tissue, organs or tumors.
The term "metastasis" refers to the process by which tumor cells are spread
to distant parts of the body. The term is also used herein to refer to a tumor
that develops
through the metastatic process.
The term "independently selected" is used herein to indicate that the R
groups, e.g., R', R2, R3 and R4, can be identical or different (e.g., R~, R2,
R3 and R4 may
all be hydrogens or Rl and R4 may be hydrogen and R2 and R3 may be halogen,
etc. ).
The term "alkyl" is used herein to refer to a branched or unbranched,
saturated or unsaturated, monovalent hydrocarbon radical having from 1-12
carbons and
preferably, from 1-6 carbons. When the alkyl group has from 1-6 carbon atoms,
it is
referred to as a "lower alkyl." Suitable alkyl radicals include, for example,
methyl, ethyl,
n-propyl, i-propyl, 2-propenyl (or allyl), n-butyl, t-butyl, i-butyl (or 2-
methylpropyl), etc.
As used herein, the term encompasses "substituted alkyls."
"Substituted alkyl" refers to alkyl as just described including one or more
functional groups such as lower alkyl, aryl, acyl, halogen (i.e:, alkylhalos,
e.g., CF3),
hydroxy, amino, alkoxy, alkylamine, acylamino, acyloxy, aryloxy, aryloxyalkyl,
mercapto, both saturated and unsaturated cyclic hydrocarbons, heterocycles and
the like.
These groups may be attached to any carbon of the alkyl moiety.
The term "S-alkyl" is used herein to refer to the group -SR, where R is
lower alkyl or substituted lower alkyl as def ned herein.
The term "aryl" is used herein to refer to an aromatic substituent which
may be a single aromatic ring or multiple aromatic rings which are fused
together, linked
covalently, or linked to a common group such as a methylene or ethylene
moiety. The
common linking group may also be a carbonyl as in benzophenone. The aromatic
rings)
may include phenyl, naphthyl, biphenyl, diphenylmethyl and benzophenone among
3 S others. The term "aryl" encompasses "arylalkyl."
CA 02324347 2000-09-19
WO 99/48495 PCTIUS99/06220
6
"Substituted aryl" refers to aryl as just described including one or more
functional groups such as lower alkyl, aryl, halogen, alkylhalos (e.g. CF3),
hydroxy,
amino, alkoxy, alkylamine, acylamino, acyloxy, mercapto and both saturated and
unsaturated cyclic hydrocarbons which are fused to the aromatic ring(s),
linked covalently
or linked to a common group such as a methylene or ethylene moiety. The
linking group
may also be a carbonyl such as in cyclohexyl phenyl ketone. The term
"substituted aryl"
encompasses "substituted arylalkyl."
The term "halogen" is used herein to refer to fluorine, bromine, chlorine
and iodine atoms.
The term "hydroxy" is used herein to refer to the group -OH.
The term "amino" is used to refer to the group NRR', where R and R'
may independently be hydrogen, alkyl, substituted alkyl, aryl, substituted
aryl or acyl.
The term "nitro" is used herein to refer to the group N02.
The term "alkoxy" is used herein to refer to the -OR group, where R is a
lower alkyl, substituted lower alkyl, aryl, substituted aryl, arylalkyl or
substituted
arylalkyl wherein the alkyl, aryl, substituted aryl, arylalkyl and substituted
arylalkyl
groups are as described herein. Suitable alkoxy radicals include, for example,
methoxy,
ethoxy, phenoxy, substituted phenoxy, benzyloxy, phenethyloxy, t-butoxy, etc.
The term "alkenyl" is used herein to refer to an unsaturated branched,
straight chain or cyclic monovalent hydrocarbon radical having at least one
carbon-
carbon double bonds. The radical can be in either the cis or trans
conformation about the
double bond(s). Suitable alkenyl radicals include, for example, ethenyl,
propenyl,
isopropenyl, cyclopropenyl, butenyl, isobutenyl, cyclobutenyl, tert-butenyl,
pentenyl,
hexenyl, etc.
The term "aikynyl" is used herein to refer to an unsaturated branched,
straight chain or cyclic monovalent hydrocarbon radical having at least one
carbon-
carbon triple bond. Suitable alkynyl radicals include, for example, ethynyl,
propynyl,
butynyl, isobutynyl, pentynyl, hexynyl, etc.
The term "contacting" is used herein interchangeably with the following:
combined with, added to, mixed with, passed over, incubated with, flowed over,
etc.
Moreover, the compounds of present invention can be "administered" by any
conventional method such as, for example, parenteral, oral, topical and
inhalation routes
as described herein.
The term "pharmaceutically acceptable salt" refers to those salts of
compounds which retain the biological effectiveness and properties of the free
bases and
which are obtained by reaction with inorganic acids such as hydrochloric acid,
hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, methanesulfonic
acid,
CA 02324347 2000-09-19
WO 99/48495 PCT1US99/06220
7
ethanesulfonic acid, ptoluenesulfonic acid, salicylic acid and the like.
Pharmaceutically
acceptable salts include, for example, alkali metal salts, such as sodium and
potassium,
alkaline earth salts and ammonium salts.
"An amount sufficient," "an effective amount," "therapeutically effective
S amount" or "anti-angeogenic" amount refer to an amount of a compound or
composition
effective to depress, suppress or inhibit angiogenesis or result in
amelioration of
symptoms associated with an angiogenic disease. The desired result can be
either a
subjective relief of a symptorn(s) or an objectively identifiable improvement
in the
recipient of the dosage, a decrease in the vascularization of endothelial
cells or a decrease
in the rate of angiogenesis as noted by a clinician or other qualified
observer.
The terms "treating cancer," "therapy," and the like refer generally to any
improvement in the mammal having the cancer wherein the improvement can be
ascribed
to treatment with the compounds of the present invention. The improvement can
be
either subjective or objective. For example, if the mammal is human, the
patient may
IS note improved vigor or vitality or decreased pain as subjective symptoms of
improvement
or response to therapy. Alternatively, the clinician may notice decrease in
tumor size or
tumor burden based on physical exam, laboratory parameters, tumor markers or
radiographic findings. Some laboratory signs that the clinician may observe
for response
to therapy include normalization of tests such as white blood cell count, red
blood cell
count, platelet count, erythrocyte sedimentation rate, and various enzyme
levels.
Additionally, the clinician may observe a decrease in a detectable tumor
marker.
Alternatively, other tests can be used to evaluate objective improvement such
as
sonograms, nuclear magnetic resonance testing and positron emissions testing.
"Inhibiting the growth of tumor cells" can be evaluated by any accepted
method of measuring whether growth of the tumor cells has been slowed or
diminished.
This includes direct observation and indirect evaluation such as subjective
symptoms or
objective signs as discussed above.
CA 02324347 2000-09-19
WO 99148495 PCT/US99/06220
B. THE COMPOUNDS
The present invention relates to the discovery that compounds of Formula
I are useful for inhibiting angiogenesis and/or for treating angiogenic
diseases.
Compounds of Formula I have the following general formula:
R
I
wherein R', R2, R3, R4 R5, R6, R7, Rg, R9, X and Y are as defined above. In a
preferred
embodiment of the invention, the compound of Formula I is methyl 3,5-diiodo-4-
(4'-
methoxyphenoxy)benzoate ("BTO-956").
The compounds used in the methods of the current invention can be made
in accordance with the procedure outlined in Borrows, et al., J. Chem. Soc.
1949, 5185-
190 and WO 97/46228, the teachings of which are incorporated herein by
reference. In
general, the process is accomplished in a series of steps. For instance,
starting with the
case of BTO-956, a substituted phenol and a substituted benzoate are reacted
to yield
methyl 3,5-dinitro-4-(4-methoxyphenoxy)benzoate. The vitro groups of the
benzoate are
then reduced to amines and subsequently replaced by iodine. This method for
preparing
BTO-956 is essentially as described in: Masuda, K., Imashiro, Y., and Okada,
Y.
"Synthesis of Triiodothyroformic Acid and its Derivatives," J. Takeda Res.
Lab., 1970,
29, 545-552, the teachings of which are incorporated herein by reference.
Further
synthetic details are set forth in Example 1.
Compounds suitable for use in the methods of the present invention can
readily be identified using in vitro and in vivo screening assays. Such assays
may screen
for the ability of a particular compound to inhibit angiogenesis or the
vascularization of
endothelial cells in vitro and in vivo. For instance, the chick embryo
chorioallantoic
membrane (CAM) assay, which is described in more detail below, can be used to
screen a
given compound for its ability to inhibit vascularization. In the
chorioallantoic membrane
assay, fertilized chick embryos are removed from their shell on day 3 or 4,
and a
methylcellulose disc containing a compound of Formula I is implanted on the
chorioallantoic membrane. The embryos are examined 48 hours later and, if a
clear
avascular zone appears around the methylcellulose disc, the diameter of that
zone is
measured. This assay can be used to assess the anti-angeogenic properties of
the
compounds of Formula I.
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
9
Another useful screening assay to assess the efficacy of compounds of
Formula I is the corneal micropocket angiogenesis assay (CMA). The rat corneal
micropocket assay can be used to assess the ability of compounds of Formula I
to inhibit
corneal angiogenesis (see, "Quantitative Angiogenesis Assays: Progress and
Problems,"
Nat. Med., 3: 1203-1208, 1997) and "Inhibition of Tumor Angiogenesis Using a
Soluble
Receptor Establishes a Role for Tie2 in Pathologic Vascular Growth," J. Clin.
Invest.,
100: 2072-2078, 1997). In this assay, the compound of Formula I is mixed with
a
polymer (e.g., Hydron solution; Interferon Sciences, New Brunswick, N~ and
implanted
in a small pocket surgically created in the superficial layers of the cornea
of a rat. Under
normal circumstances, this wound stimulates an angiogenic response which is
readily
visible as the appearance of neovessels on the normally avascular cornea. If
the
compound of Formula I is effective, specifically as an anti-angiogenic agent,
it inhibits or
block this response. In one experimental design, a group of five animals
(including a
control group with only polymer implants) is tested over a range of drug doses
which can
induce tumor growth delay. Three doses are tested in the assay. Assessment of
an anti-
angiogenic response by this method is categorical. In other words, a treated
eye is either
positive or negative for corneal angiogenesis. This assay determines whether a
compound of Formula I is directly anti-angiogenic in an in vivo mammalian
model of
angiogenesis.
In addition, the human microvascular endothelial cell assay (HMVEC) can
be used to assess the efficacy of compounds of Formula I. HMVEC are seeded
into a 96-
well plate at a x concentration of 5 x 103 cellslwell in a volume of 100
pl/well of
Endothelial Growth Medium. Plates are then incubated at 37°C in 5% C02
for 24 h and
then aliquots of the compound of Formula I are added to the HMVEC preparations
and
plates are then incubated at 37°C in 5% C02 for 3 days. The relative
number of cells is
determined by adding 20 ~l/ml of Alamar Blue for 3-6 h at 37°C and
measuring color
changes indicating metabolic activity by using a Fluorescence Measurement
System. In
this assay, the intensity of the fluorophore signal is directly proportional
to cell number.
The HMVEC assay can also be carried out using human umbilical vein
microvascular endothelial cells (HUMVEC). This assay is carried out similarly
to the
above assay, but HiJMVEC cells are used. In addition, other assays known to
those of
skill in the art can readily be used to screen the compounds of the present
invention for
anti-angiogenic properties.
It will be readily apparent to those skilled in the art that the compounds of
Formula I can be administered alone, in the form of a pharmaceutically
acceptable salt
and/or in the form of a pharmaceutical composition.
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
C. USES FOR THE COMPOUNDS OF THE PRESENT INVENTION
As explained above, the present invention relates to the discovery that the
compounds of Formula I are useful for inhibiting angiogenesis and, in turn,
for treating
diseases associated with unwanted angiogenesis. As such, in one embodiment,
the
5 present invention provides a method of inhibiting unwanted angiogenesis in a
cell, the
method comprising contacting the cell with an effective amount, i.e., an anti-
angiogenic
amount, of a compound of Formula I. In another embodiment, the present
invention
provides a method of inhibiting the vascularization of endothelial cells, the
method
comprising contacting a cell, tissue or organ containing the endothelial cells
with an
10 effective amount of a compound of Formula I. In a presently preferred
embodiment, the
cells are in a mammalian subject.
This invention relates to a method of treating mammalian diseases
associated with undesired and uncontrolled angiogenesis, the method comprising
administering to a mammal an anti-angiogenic compound of Formula I in an
amount, i.e.,
a dosage, sufficient to inhibit angiogenesis. The particular dosage of a
compound of
Formula I required to inhibit angiogenesis and/or angiogenic diseases will
depend upon
the severity of the condition, the route of administration, and related
factors that will be
decided by the attending physician. Generally, accepted and effective daily
doses will be
the amount sufficient to effectively inhibit angiogenesis and/or angiogenic
diseases.
The methods of treatment provided by this invention are practiced by
administering to a mammal in need thereof a dose of a compound of Formula I
(or a
pharmaceutically acceptable salt or solvate thereof) that is effective to
inhibit
angiogenesis and/or angiogenic diseases. The term "inhibit" is used herein to
include its
generally accepted meaning which includes prophylactically treating a human
subject to
incurring angiogenesis and/or angiogenic diseases, and holding in check and/or
treating
existing angiogenesis and/or angiogenic diseases. As such, the present
invention includes
both medical therapeutic and/or prophylactic treatment, as appropriate.
The methods of the present invention can be used to treat a wide variety of
diseases. Diseases associated with corneal neovascularization that can be
treated using
the methods of the present invention include, but are not limited to, diabetic
retinopathy,
retinopathy of prematurity, corneal graft rejection, neovascular glaucoma and
retrolental
fibroplasia, epidemic keratoconjunctivitis, vitamin A deficiency, contact lens
overwear,
atopic keratitis, superior limbic keratitis, pterygium keratitis sicca,
sjogrens, acne rosacea,
phylectenulosis, syphilis, Mycobacteria infections, lipid degeneration,
chemical burns,
bacterial ulcers, fungal ulcers, Herpes simplex infections, Herpes zvster
infections,
protozoan infections, Kaposi sarcoma, Mooren ulcer, Terrien's marginal
degeneration,
marginal keratolysis, trauma, rheumatoid arthritis, systemic lupus,
polyarteritis, Wegeners
CA 02324347 2000-09-19
WO 99/48495 . PCTIUS99106220
11
sarcoidosis, Scleritis, Steven's Johnson disease, periphigoid radial
keratotomy, and
corneal graph rejection.
Diseases associated with retinal/choroidal neovascularization that can be
treated using the methods of the present invention include, but are not
limited to, diabetic
retinopathy, macular degeneration, sickle cell anemia, sarcoid, syphilis,
pseudoxanthoma
elasticum, Pagets disease, vein occlusion, artery occlusion, carotid
obstructive disease,
chronic uveitis/vitritis, mycobacterial infections, Lyme's disease, systemic
lupus
erythematosis, retinopathy of prematurity, Eales disease, Bechets disease,
infections
causing a retinitis or choroiditis, presumed ocular histoplasmosis, Bests
disease, myopia,
optic pits, Stargarts disease, pars planitis, chronic retinal detachment,
hyperviscosity
syndromes, toxoplasmosis, trauma and post-laser complications. Other diseases
include,
but are not limited to, diseases associated with nzbeosis (neovasculariation
of the angle)
and diseases caused by the abnormal proliferation of fibrovascular or fibrous
tissue,
including all forms of proliferative vitreoretinopathy, whether or not
associated with
diabetes.
Diseases associated with chronic inflammation can also be treated using
the methods of the present invention. Diseases with symptoms of chronic
inflammation
include, but are not limited to, inflammatory bowel diseases, such as Crohn's
disease and
ulcerative colitis, psoriasis, sarcoidosis and rheumatoid arthritis. Unwanted
or
uncontrolled angiogenesis is a key element that these chronic inflammatory
diseases all
have in common. The chronic inflammation depends on continuous formation of
capillary sprouts to maintain an influx of inflammatory cells. The influx and
presence of
the inflammatory cells produce granulomas and, thus, maintain the chronic
inflammatory
state. Inhibition of angiogenesis using the compositions and methods of the
present
invention prevents the formation of the granulomas, thereby alleviating the
disease.
As mentioned above, the methods of the present invention can be used to
treat patients with inflammatory bowel diseases, such as Crohn's disease and
ulcerative
colitis. Crohn's disease occurs as a chronic transmural inflammatory disease
that most
commonly affects the distal ileum and colon, but may also occur in any part of
the
gastrointestinal tract from the mouth to the anus and perianal area. Patients
with Crohn's
disease generally have chronic diarrhea associated with abdominal pain, fever,
anorexia,
weight loss and abdominal swelling. Prevention of angiogenesis by the
compositions and
methods of the present invention inhibits the formation of the sprouts and
prevents the
formation of granulomas. Ulcerative colitis is also a chronic, nonspecific,
inflammatory
and ulcerative disease arising in the colonic mucosa and is characterized by
the presence
of bloody diarrhea.
CA 02324347 2000-09-19
WO 99148495 PCTIUS99106220
12
The inflammatory bowel diseases also exhibit extra intestinal
manifestations, such as skin lesions. Such lesions are characterized by
inflammation and
angiogenesis and can occur at many sites other than in the gastrointestinal
tract. The
compositions and methods of the present invention can also be used to treat
these lesions
by preventing the angiogenesis, thus reducing the influx of inflammatory cells
and the
lesion formation.
Sarcoidosis is another chronic inflammatory disease that is characterized
as a multisystem granulomatous disorder. The granulomas of this disease can
form
anywhere in the body and, thus, the symptoms depend on the site of the
granulomas and
whether the disease active. The granulomas are created by the angiogenic
capillary
sprouts providing a constant supply of inflammatory cells. The compounds and
method
of this invention can be used to treat sarcoidosis.
The methods of the present invention can also be used to treat the chronic
inflammatory conditions associated with psoriasis. Psoriasis, a skin disease,
is another
chronic and recurrent disease that is characterized by papules and plaques of
various
sizes. Prevention of the formation of the new blood vessels necessary to
maintain the
characteristic lesions leads to relief from the symptoms.
Another disease which can be treated using the methods of the present
invention is rheumatoid arthritis. Rheumatoid arthritis is a chronic
inflammatory disease
characterized by nonspecific inflammation of the peripheral joints. It is
thought that the
blood vessels in the synovial lining of the joints undergo angiogenesis. In
addition to
forming new vascular networks, the endothelial cells release factors and
reactive oxygen
species that lead to pannus growth and cartilage destruction. The factors
involved in
angiogenesis may actively contribute to, and help maintain, the chronically
inflamed state
of rheumatoid arthritis.
Other diseases that can be treated using the methods of the present
invention are hemangiomas, Osler-Weber-Rendu disease, or hereditary
hemorrhagic
telangiectasia, solid or blood borne tumors and acquired immune deficiency
syndrome.
The methods of this invention are also effective in inhibiting angiogenesis
associated with malignant tumor growth. This includes cancerous tumor growth
on cells
tissues and organs. The methods of the present invention are useful in
treating the
growth of a number of tumor cells and for treating a wide variety of cancers.
Such tumor
cells include, by way of example and not limitation, lung, colon, breast,
ovarian, prostate
and hepatic tumor cells as well as squamous cell carcinomas. Such cancers
include, by
way of example and not limitation, carcinomas such as pharynx, colon, rectal,
pancreatic,
stomach, liver, lung, breast, skin, prostate, ovary, cervical, uterine and
bladder cancers;
leukemias; lymphomas; gliomas; retinoblastomas; and sarcomas.
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
13
In a particularly preferred embodiment, the present invention relates to
methods of administering compounds of Formula I in combination with active
immunotherapy (e.g., tumor vaccination). Because the compounds of Formula I
are not
immunotoxic, the immune system is not significantly suppressed and, thus,
active
immunotherapy can advantageously be carried out in combination with the
chemotherapy. When used in conjunction with immunotherapy, the compound of
Formula I can be administered prior to and/or during administration of the
immunotherapeutic agent (e.g., a tumor vaccine).
In still another embodiment, the present invention provides a method for
reducing the level of TNF-a produced by a cell. TNF-a and its various modes of
action
are generally described by Abbas, et al., Cellular and Molecular Immunology,
Abbas, et al., 2nd Ed., W.B. Saunders Company, 1994, pp. 244-249, the
teachings of
which are incorporated herein by reference. TNF-a plays an integral role in
destroying
tumors, mediating responses to tissue injury and protecting hosts from
infections by
various microorganisms. However, its activity appears to be excessive in some
disease
states and inflammatory reactions such as rheumatoid arthritis, cachexia and
septic shock.
The excess TNF-a results in an exaggerated immune response exemplified by over
stimulation of interleukin-6 and granulocyte/macrophage-colony stimulating
factor (GM-
CSF) secretion, enhanced cytotoxicity of polymorphonuclear neutrophils and
prolonged
expression of cellular adhesion molecules, all of which can have detrimental
effects.
Contacting cells with the compounds of Formula I results in decreased
levels of TNF-a. Reduced levels of TNF-a can result from any of several
mechanisms
including, for example, downregulation of expression of a gene that encodes
TNF-a, a
reduction in TNF-a mRNA stability or translation efficiency, decreased
stability of the
TNF-a polypeptide, and reduced secretion of TNF-a from a cell. Reduced levels
of TNF-
a can be measured in a cell, biological sample or the blood stream. As a
result of their
ability to inhibit TNF-a, the compounds of Formula I can be used to treat
inflammatory
diseases. Such diseases include, but are not limited to, the inflammatory
diseases set
forth above (e.g., chronic inflammation, chronic disease, inflammatory bowel
disease,
sarcoidosis, psoriasis, rheumatoid arthritis, and the like). Using the assay
set forth in
Example VIII, compounds of Formula I can readily be screened for their ability
to reduce
T1VF-a levels.
TNF-a is noted for its pro-inflammatory actions which result in tissue
injury, such as induction of procoagulant activity on vascular endothelial
cells, increased
adherence of neutrophils and lymphocytes, and stimulation of the release of
platelet
activating factor from macrophages, neutrophils and vascular endothelial
cells. As such,
targeting moieties which are directed to these cells and which are conjugated
to liposomes
CA 02324347 2000-09-19
WO 99/48495 PCT/US99106220
14
or other drug delivery systems comprising the compounds of Formula I are
preferred
embodiments of this invention. For instance, in a preferred embodiment,
monoclonal
antibodies to TNF-a (Tracey, et al., Nature 1987, 330, 662-664; Silva, et al.,
J. Infect. Sis.
1990, 162, 421-427; and Williams, et al., Proc. Natl. Acad Sci. 1992, 89, 9784-
9788) are
S conjugated to liposomes comprising compounds of Formula I.
Moreover, in accordance with the above methods, mammalian subjects
include, but are not limited to, humans, laboratory animals, domestic pets and
farm
animals.
D. PHARMACEUTICAL FORMULATIONS/R.OUTES OF
ADMINISTRATION
In the methods of the present invention, the compounds of Formula I can
be delivered or administered to a mammal, e.g., a human patient, alone, in the
form of a
pharmaceutically acceptable salt, or in the form of a pharmaceutical
composition where
the compound is mixed with suitable carriers or excipient(s) in a
therapeutically effective
amount, e.g., at doses effective to depress, suppress or inhibit angiogenesis
or result in
amelioration of symptoms associated with angiogenic diseases.
The compounds of Formula I, which are used in the methods of the present
invention, can be incorporated into a variety of formulations for therapeutic
administration. More particularly, compounds of Formula I can be formulated
into
pharmaceutical compositions by combination with appropriate, pharmaceutically
acceptable carriers or diluents, and may be formulated into preparations in
solid, semi-
solid, liquid or gaseous forms, such as tablets, capsules, pills, powders,
granules, dragees,
gels, slurnes, ointments, solutions, suppositories, injections, inhalants and
aerosols. As
such, administration of the compounds can be achieved in various ways,
including oral,
buccal, rectal, parenteral, intraperitoneal, intradermal, transdermal,
intracheal, etc.,
administration. Moreover, the compound can be administered in a local rather
than
systemic manner, for example via injection of the compound directly into a
solid tumor,
often in a depot or sustained release formulation. In addition, the compounds
can be
administered in a targeted drug delivery system, for example, in a liposome
coated with
tumor-specific antibody. Such liposomes will be targeted to and taken up
selectively by
the tumor.
In addition, the compounds of Formula I can be formulated with common
excipients, diluents or Garners, and compressed into tablets, or formulated as
elixirs or
solutions for convenient oral administration, or administered by the
intramuscular or
intravenous routes. The compounds can be administered transdermally, and may
be
formulated as sustained release dosage forms and the like.
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
Compounds of Formula I can be administered alone, in combination with
each other, or they can be used in combination with other known compounds
(e.g., other
anti-cancer drugs or ether drugs, such as AZT, anti-inflammatories,
antibiotics,
corticosteroids, vitamins, etc.). For instance, the compound of Formula I can
be used in
5 conjunctive therapy with other known anti-angiogenic chemotherapeutic or
antineoplastic
agents (e.g., vinca alkaloids, antibiotics, antimetabolites, platinum
coordination
complexes, etc.). For instance, the compounds of Formula I can be used in
conjunctive
therapy with a vinca alkaloid compound, such as vinblastine, vincristine,
taxol, etc.; an
antibiotic, such as adriamycin (doxorubicin), dactinomycin (actinomycin D),
10 daunorubicin (daunomycin, rubidomycin), bleomycin, plicamycin (mithramycin)
and
mitomycin (mitomycin C), etc.; an antimetabolite, such as methotrexate,
cytarabine
(AraC), azauridine, azaribine, fluorodeoxyuridine, deoxycoformycin,
mercaptopurine,
etc.; or a platinum coordination complex, such as cisplatin (cis-DDP),
carboplatin, etc. In
addition, those of skill in the art will appreciate that the compounds of the
present
15 invention can be used in conjunctive therapy with other known anti-
angiogenic
chemotherapeutic or antineoplastic compounds. In pharmaceutical dosage forms,
the
compounds may be administered in the form of their pharmaceutically acceptable
salts, or
they may also be used alone or in appropriate association, as well as in
combination with
other pharmaceutically active compounds.
Suitable formulations for use in the present invention are found in
Remington's Pharmaceutical Sciences (Mack Publishing Company, Philadelphia,
PA,
17th ed. (1985)), which is incorporated herein by reference. Moreover, for a
brief review
of methods for drug delivery, see, Larger, Science 249:1527-1533 (1990), which
is
incorporated herein by reference. The pharmaceutical compositions described
herein can
be manufactured in a manner that is known to those of skill in the art, i.e.,
by means of
conventional mixing, dissolving, granulating, dragee-making, levigating,
emulsifying,
encapsulating, entrapping or lyophilizing processes. The following methods and
excipients are merely exemplary and are in no way limiting.
For injection, the compounds can be formulated into preparations by
dissolving, suspending or emulsifying them in an aqueous or nonaqueous
solvent, such as
vegetable or other similar oils, synthetic aliphatic acid glycerides, esters
of higher
aliphatic acids or propylene glycol; and if desired, with conventional
additives such as
solubilizers, isotonic agents, suspending agents, emulsifying agents,
stabilizers and
preservatives. Preferably, the compounds of the invention may be formulated in
aqueous
solutions, preferably in physiologically compatible buffers such as Hanks's
solution,
Ringer's solution, or physiological saline buffer. For transmucosal
administration,
CA 02324347 2000-09-19
WO 99148495 PCTIUS99/06220
16
penetrants appropriate to the barrier to be permeated are used in the
formulation. Such
penetrants are generally known in the art.
For oral administration, the compounds of Formula I can be formulated
readily by combining with pharmaceutically acceptable carriers that are well
known in the
art. Such carriers enable the compounds to be formulated as tablets, pills,
dragees,
capsules, emulsions, lipophilic and hydrophilic suspensions, liquids, gels,
syrups, slurries,
suspensions and the like, for oral ingestion by a patient to be treated.
Pharmaceutical
preparations for oral use can be obtained by mixing the compounds with a solid
excipient,
optionally grinding a resulting mixture, and processing the mixture of
granules, after
adding suitable auxiliaries, if desired, to obtain tablets or dragee cores.
Suitable
excipients are, in particular, fillers such as sugars, including lactose,
sucrose, rnannitol, or
sorbitol; cellulose preparations such as, for example, maize starch, wheat
starch, rice
starch, potato starch, gelatin, gum tragacanth, methyl cellulose,
hydroxypropylmethyl-
cellulose, sodium carboxymethylcellulose, and/or polyvinylpyrrolidone (PVP).
If
desired, disintegrating agents may be added, such as the cross-linked
polyvinyl
pyrrolidone, agar, or alginic acid or a salt thereof such as sodium alginate.
Dragee cores are provided with suitable coatings. For this purpose,
concentrated sugar solutions may be used, which may optionally contain gum
arabic, talc,
polyvinyl pyrrolidone, carbopol gel, polyethylene glycol, and/or titanium
dioxide, lacquer
solutions, and suitable organic solvents or solvent mixtures. Dyestuffs or
pigments may
be added to the tablets or dragee coatings for identification or to
characterize different
combinations of active compound doses.
Pharmaceutical preparations which can be used orally include push-fit
capsules made of gelatin, as well as soft, sealed capsules made of gelatin and
a plasticizes,
such as glycerol or sorbitol. The push-fit capsules can contain the active
ingredients in
admixture with filler such as lactose, binders such as starches, and/or
lubricants such as
talc or magnesium stearate and, optionally, stabilizers. In soft capsules, the
active
compounds may be dissolved or suspended in suitable liquids, such as fatty
oils, liquid
paraffin, or liquid polyethylene glycols. In addition, stabilizers may be
added. All
formulations for oral administration should be in dosages suitable for such
administration.
For buccal administration, the compositions may take the form of tablets
or lozenges formulated in conventional manner.
For administration by inhalation, the compounds for use according to the
present invention are conveniently delivered in the form of an aerosol spray
presentation
from pressurized packs or a nebulizer, with the use of a suitable propellant,
e.g.,
dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane,
carbon
dioxide or other suitable gas, or from propellant-free, dry-powder inhalers.
In the case of
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
17
a pressurized aerosol the dosage unit may be determined by providing a valve
to deliver a
metered amount. Capsules and cartridges of, e.g., gelatin for use in an
inhaler or
insufflator may be formulated containing a powder mix of the compound and a
suitable
powder base such as lactose or starch.
The compounds may be formulated for parenteral administration by
injection, e.g., by bolus injection or continuous infusion. Formulations for
injection may
be presented in unit dosage form, e.g., in ampules or in multidose containers,
with an
added preservative. The compositions may take such forms as suspensions,
solutions or
emulsions in oily or aqueous vehicles, and may contain formulator agents such
as
suspending, stabilizing and/or dispersing agents.
Pharmaceutical formulations far parenteral administration include aqueous
solutions of the active compounds in water-soluble form. Additionally,
suspensions of
the active compounds may be prepared as appropriate oily injection
suspensions.
Suitable lipophilic solvents or vehicles include fatty oils such as sesame
oil, or synthetic
fatty acid esters, such as ethyl oleate or triglycerides, or liposomes.
Aqueous injection
suspensions may contain substances which increase the viscosity of the
suspension, such
as sodium carboxymethyl cellulose, sorbitol, or dextran. Optionally, the
suspension may
also contain suitable stabilizers or agents which increase the solubility of
the compounds
to allow for the preparation of highly concentrated solutions. Alternatively,
the active
ingredient may be in powder form for constitution with a suitable vehicle,
e.g., sterile
pyrogen-free water, before use.
The compounds may also be formulated in rectal compositions such as
suppositories or retention enemas, e.g., containing conventional suppository
bases such as
cocoa butter, carbowaxes, polyethylene glycols or other glycerides, all of
which melt at
body temperature, yet are solidified at room temperature.
In addition to the formulations described previously, the compounds may
also be formulated as a depot preparation. Such long acting formulations may
be
administered by implantation (for example subcutaneously or intramuscularly)
or by
intramuscular injection. Thus, for example, the compounds may be formulated
with
suitable polymeric or hydrophobic materials (for example as an emulsion in an
acceptable
oil) or ion exchange resins, or as sparingly soluble derivatives, for example,
as a sparingly
soluble salt.
Alternatively, other delivery systems for hydrophobic pharmaceutical
compounds may be employed. Liposomes and emulsions are well known examples of
delivery vehicles or Garners for hydrophobic drugs. In a presently preferred
embodiment,
long-circulating, i.e., stealth, liposomes are employed. Such liposomes are
generally
CA 02324347 2000-09-19
WO 99/48495 PCTIUS99106220
18
described in Woodle, et al., U.S. Patent No. 5,013,556, the teaching of which
is hereby
incorporated by reference.
Monoclonal antibodies optionally conjugated to liposomes and directed
against a tumor marker, TNF-a, or a TNF-a receptor, is another strategy that
can be
employed. In addition, targeting of a marker on abnormal tumor vasculature can
be
employed. The targeting moiety when coupled to a toxic drug or radioisotope
will act to
concentrate the drug where it is needed. Ligands for tumor-associated vessel
markers can
also be used. For example, a cell adhesion molecule that binds to a tumor
vascular
element surface marker can be employed. Liposomes and other drug delivery
systems
can also be used, especially if their surface contains a ligand to direct the
carrier
preferentially to the tumor vasculature. Liposomes offer the added advantage
of shielding
the drug from most normal tissues, thereby reducing the inherent toxicity of
many
compounds. When coated with polyethylene glycol {PEG) (i.e., stealth
liposomes) to
minimize uptake by phagocytes and with a tumor vasculature-specific targeting
moiety,
liposomes offer longer plasma half lives, lower non-target tissue toxicity,
and increased
efficacy over non-targeted drug. Other targeting strategies include, but are
not limited to,
ADEPT (antibody-directed enzyme prodrug therapy), GDEPT (gene-directed EPT)
and
VDEPT (virus-directed EPT). In ADEPT, the targeting of an inactive prodrug to
a tumor
mass is effected by an antibody against a tumor-associated marker. The enzyme
milieu in
or about the tumor transforms the prodrug into an active toxic agent that then
acts on the
tumor tissue. Similarly, differential gene expression or viral targeting at
the tumor site is
used to activate a prodrug into its active, toxic form in GDEPT and VDEPT,
respectively.
Other strategies include targeting differentially expressed genes, enzymes or
surface
markers that appear on tumor-associated vasculature, to effect control of
tumor growth.
Using the foregoing methods, the compounds of Formula I can be targeted to the
tumor
vasculature to effect control of tumor progression or to other sites of
interest (e.g.,
endothelial cells).
Certain organic solvents such as dimethylsulfoxide also may be employed,
although usually at the cost of greater toxicity. Additionally, the compounds
may be
delivered using a sustained-release system, such as semipermeable matrices of
solid
hydrophobic polymers containing the therapeutic agent. Various types of
sustained-
release materials have been established and are well known by those skilled in
the art.
Sustained-release capsules may, depending on their chemical nature, release
the
compounds for a few weeks up to over 100 days.
The pharmaceutical compositions also may comprise suitable solid or gel
phase Garners or excipients. Examples of such Garners or excipients include
but are not
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
19
limited to calcium carbonate, calcium phosphate, various sugars, starches,
cellulose
derivatives, gelatin, and polymers such as polyethylene glycols.
Pharmaceutical compositions suitable for use in the present invention
include compositions wherein the active ingredients are contained in a
therapeutically
effective amount. The amount of composition administered will, of course, be
dependent
on the subject being treated, on the subject's weight, the severity of the
affliction, the
manner of administration and the judgment of the prescribing physician.
Determination
of an effective amount is well within the capability of those skilled in the
art, especially in
light of the detailed disclosure provided herein.
For any compound used in the method of the invention, a therapeutically
effective dose can be estimated initially from cell culture assays. For
example, a dose can
be formulated in animal models to achieve a circulating concentration range
that includes
the ICSO as determined in cell culture (i.e., the concentration of test
compound that is
lethal to 50% of a cell culture), or the IC1~ as determined in cell culture
(i.e., the
concentration of compound that is lethal to 100% of a cell culture). Such
information can
be used to more accurately determine useful doses in humans. Initial dosages
can also be
estimated from in vivo data.
Moreover, toxicity and therapeutic efficacy of the compounds described
herein can be determined by standard pharmaceutical procedures in cell
cultures or
experimental animals, e.g., by determining the LDso, (the dose lethal to 50%
of the
population) and the EDSO (the dose therapeutically effective in 50% of the
population).
The dose ratio between toxic and therapeutic effect is the therapeutic index
and can be
expressed as the ratio between LDso and EDso. Compounds which exhibit high
therapeutic indices are preferred. The data obtained from these cell culture
assays and
animal studies can be used in formulating a dosage range that is not toxic for
use in
human. The dosage of such compounds lies preferably within a range of
circulating
concentrations that include the EDSO with little or no toxicity. The dosage
may vary
within this range depending upon the dosage form employed and the route of
administration utilized. The exact formulation, route of administration and
dosage can be
chosen by the individual physician in view of the patient's condition. (See,
e.g., Fingl et
al., 1975, In: The Pharmacological Basis of Therapeutics, Ch. l, p. 1).
Dosage amount and interval may be adjusted individually to provide
plasma levels of the active compound which are sufficient to maintain
therapeutic effect.
Usual patient dosages for oral administration range from about 50-2000
mg/kg/day,
, commonly from about 100-1000 rnglkg/day, preferably from about 150-700
mg/kg/day
and most preferably from about 250-500 mg/kg/day. Preferably, therapeutically
effective
serum levels will be achieved by administering multiple doses each day. In
cases of local
CA 02324347 2000-09-19
WO 99148495 PCT/US99/06220
administration or selective uptake, the effective local concentration of the
drug may not
be related to plasma concentration. One having skill in the art will be able
to optimize
therapeutically effective local dosages without undue experimentation.
5 F. EXAMPLES
The invention will be described in greater detail by way of specific
examples. The following examples are offered for illustrative purposes, and
are not
intended to limit the invention in any manner. Those of skill in the art will
readily
recognize a variety of noncritical parameters which can be changed or modified
to yield
10 essentially the same results.
EXAMPLE I
This example illustrates the synthesis of methyl 3,5-diiodo-4-(4-
methoxyphenoxy)benzoate (BTO-956).
15 The synthesis of BTO-956 is accomplished in a series of steps, first
yielding methyl 3,5-dinitro-4-(4-methoxyphenoxy)benzoate, the vitro groups of
which are
then reduced to amines and subsequently replaced by iodine. The method for
preparing
BTO-956 is essentially as described in: Masuda, K., Imashiro, Y., and Okada,
Y.
Synthesis of triiodothyroformic acid and its derivatives. J. Takeda Res. Lab.
1970, 29,
20 545-552.
1. Methy13.5-dinitro-4-l4-methoxypheno~lbenzoate
N20 KOH N02
OH C~ \ \
\ \ ~ O
+ ~ H20
CH3O 02N ~ CO2CH3 15d°C CH3O ~ 02N ~ CO2CH3
M.W.124.14 M.W.260.59 M.W.348.27
Methyl 3,5-dinitro-4-chlorobenzoate 20.2 g; 77.5 mmol
4-Methoxyphenol 10.0 g; 80.6 mmol
Potassium hydroxide 4.7 g; 82.0 mmol
Water 20 mL; solvent
To a 100 mL round-bottomed flask containing potassium hydroxide (4.7 g;
82.0 mmol) dissolved in water (20 mL) was successively added 4-methoxyphenol
(10.0 g;
80.6 mmol) and methyl 4-chloro-3,5-dinitrobenzoate (20.2 g; 77.5 mmol). The
flask was
fitted with a reflux condenser and the reaction heated at 150°C (oil
bath) for 3 hours.
After cooling to room temp, the reaction mixture was transferred to a large
mortar and
CA 02324347 2000-09-19
WO 99/48495 PCTIUS99106220
21
triturated with cold 2N NaOH (100 mL) to remove unreacted phenol. The solid
was
collected by filtration and air-dried to give 21.5 g of crude product.
Crystallization from
absolute ethanol gave 17.7 g (65.6 %) of pure methyl 3,5-dinitro-4-(4-
methoxyphenoxy)benzoate as light yellow needles. 300 MHz 1H NMR (CDC13) d 3.77
(s, 3H, OCH3), 4.02 (s, 3H, OCH3), 6.82 (m, 4H, ArH), 8.70 (s, 2H, ArH).
2. Methv13.5-diamino-4-(4-methox'~phenoxx)benzoate
N02 NH2
\ O I \ H2/Pd_C O
\ \
AcOH I
CH3O '~OZN ~ CO2CH3 CH30 ~ H2N ~ C02CH3
M.W. 348.27 M. W. 288.27
Methyl 3,5-dinitro-4-(4-methoxyphenoxy)benzoate 20.2 g; 77.5 nunol
10% palladium on charcoal 0.7 g; catalyst
Glacial acetic acid 80 mL; solvent
To a Parr shaker bottle containing a suspension of methyl 3,5-di-vitro-4-
(4-methoxyphenoxy)-benzoate (20.2 g; 77.5 mmol) in glacial acetic acid {80 mL)
was
added 10% palladium on charcoal (0.7 g). The bottle was shaken under an
atmosphere of
hydrogen (3 atm) until no more hydrogen was consumed. The catalyst was
filtered off
and the resulting solution concentrated to approximately 10 ml,. The residue
was
dissolved in acetone (50 mL) and heated on a steam bath while water (100 mL)
was
added in portions. Upon cooling, medium brown needles formed which were
collected
by suction filtration and dried to give 7.1 g (86%) of methyl 3,5-diamino-4-(4-
methoxyphenoxy)benzoate. 300 MHz 1H NMR (CDCl3) d 3.73 (s, 3H, OCH3), 3.80
(bs,
4H, ArNH2) 3.86 (s, 3H, OCH3), 6.84 (m, 4H, ArH), 6.91 (s, 2H, ArH).
3. Methv13.5-diiodo-4-l4-methoxyphenoxyybenzoate
NH2 (1) H2S04, NaN02 I
\ O I \ AcOH ( \ O ~ \
CH30 ~ H2N ~ C02CH3 (2) Kl, H20 CH30 ~ I ~ C02CH3
M.W. 288.27 M.W. 510.06
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
22
Methyl 3,5-diamino-4-(4-methoxyphenoxy)benzoate4.3 g; 14.2 mmol
Sodium nitrite 2.6 g; 37.4 mmol
Glacial acetic acid 80 mL; solvent
Sulfuric acid 26 mL; solvent
Potassium iodide 20 g; 120.0 mmol
Water 30 mL; solvent
Sulfuric acid' (26 mL) was placed in a three-necked flask equipped with a
mechanical stirrer and cooled in an ice bath. Sodium nitrite (2.58 g, 37.4
mmol) was
added in small portions, and the mixture was stirred for 20 min to form a
thick solution.
To this was added a slurry of methyl 3,S-diamino-4-{4-methoxyphenoxy)benzoate
(4.30
g, 14.22 mmol) in glacial acetic acid (80 mL), dropwise over a period of 30
min, keeping
the temperature below 10°C with the ice bath. The reddish brown
solution was stirred at
below 10°C for 45 min, after which it was poured slowly inta an aqueous
(30 mL)
solution of potassium iodide (20 g) at room temperature, with vigorous
stirring. A thick
suspension formed, and was allowed to stir at RT for lh. The reaction mixture
was then
heated in an oil-bath to 80°C (internal temperature) for 15 min, and
then allowed to cool
to RT. The solution was filtered and the black gummy residue was dissolved in
300 mL
of acetone. The dark filtrate, when refrigerated overnight, deposited a dark
residue,
which was collected by decanting the supernatant, and the residue was
dissolved in 100
mL of acetone. The combined acetone solution was filtered over a pad of basic
alumina
(5 cm) in a 150 mL sintered glass funnel to remove some colored impurities.
The
alumina pad was washed with 100 mL acetone and the red filtrate was evaporated
to
dryness, to give a dark solid as crude product. This was purified by flash
chromatography
on silica gel, eluting with hexanes: CH2C12 (60:40). Initial fractions
containing the pure
product were pooled and evaporated to yield 1.67 g of the desired product as
an off white
solid. This compound gave a single, clean spot on TLC (Hexanes: CH2C12; 1:1;
Rf 0.35).
Impure fractions were pooled and triturated with absolute EtOH for 16 hours at
room
temperature. The solid was filtered and dried to yield another 0.3 g of the
product as a
cream solid, which contained ~5% of the slow-moving impurity as evidenced by
TLC (Rf
0.29). Total yield of the product was 27%. 300 MHz'H NMR (CDC13) d 3.78 (s,
3H,
OCH3), 3.94 (s, 3H, COOCH3), 6.70 and 6.83 (two d, AA'XX', 4H, p-subs. Ar-H),
8.51
(s, 2H, Ar-H).
EXAMPLE II
This example illustrates the anti-angiogenic properties of methyl 3,5-
diiodo-4-(4-methoxyphenoxy)benzoate in the chick chorioallantoic membrane
assay
(CAM). The endpoint of the CAM assay was a quantitative determination of
basement
membrane biosynthesis by measuring the incorporation of '4C-proline into Type
IV
collagenous protein.
CA 02324347 2000-09-19
WO 99148495 PCT/US99/06220
23
A. Approach
The CAM assay involves the development of live chick embryos in Petri
dishes under special sterile conditions. Therefore, only limited numbers of
embryos can
be used for evaluation of compounds in a single experiment. For this reason,
two
separate assays were conducted to test the three Biosource compounds at three
concentrations per compound. In this assay, a known angiogenesis inhibitor, 2-
methoxyestradiol (2-ME), was used as the positive control, and human
fibroblast growth
factor (hFGF) was used to induce angiogenesis in the CAM.
B. Materials
Fertilized eggs were supplied by Melody Ranch, Aptos, CA. L-[LJ-14C]
proline (specific activity, 290 mCi/mmol) was purchased from New England
Nuclear,
Boston, MA. Collagenase and 2-ME were obtained from Sigma Chemical Co., St.
Louis,
MO. Silicone ring cups were obtained by cutting silicone tubing {3 mm
diameter) into
small "O" rings 1 mm in thickness. These silicone ring cups can be reused many
times if
they are sterilized prior to each assay. Plastic Petri dishes (20 x 100 mm)
were purchased
from Baxter Diagnostics, Inc., Hayward, CA. hFGF-B was obtained from Clonetics
Corporation, San Diego, CA.
For testing, a minimum amount of acetone-methanol (1:1) was added to
the test compounds for sterilization. The acetone-methanol mixture was then
evaporated
to dryness in a sterile hood. The compounds were dissolved dimethyl sulfoxide
(DMSO)
first and then diluted with saline containing methylcellulose. The final
concentrations
were 2% DMSO and 0.5% methylcellulose. All test solutions were added to each
CAM
in 20-ml aliquots.
C. Development of the CAM for Measuring ~gioeenesis Inhibition
The method of Folkman, et al., Dev. Biol. 41: 391-394 (1974), with some
modifications, was used to cultivate chicken embryos as follows:
Fresh fertile eggs were incubated for three days in a standard egg
incubator. On Day 3, eggs were cracked under sterile conditions and embryos
were
placed in 20 x 100 mrn plastic Petri dishes and cultivated at 37°C in
an embryo incubator
with a water reservoir on the bottom shelf. Air was continuously bubbled into
the water
reservoir by using a small pump so that the humidity in the incubator was kept
constant.
Observations were made daily to ensure that all embryos were healthy. Dead or
unhealthy embryos were removed from the incubator immediately to avoid
contamination. On Day 9, a sterile silicone ring cup was placed on each CAM
and 0.5
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
24
mCi of 14C-proline with or without the test compound plus 2.5 ng of hFGF
dissolved in
saline containing 0.5% methylcellulose was delivered into each ring cup in a
sterile hood.
2-ME was tested in parallel to serve as a reference compound. After addition
of test
materials, the embryos were returned to the incubator and cultivation
continued. On Day
12, all embryos were transferred to a cold room at 4-10°C. The
antiangiogenic effect of
each test compound was determined by using the collagenase assay
(Maragoudakis, et al.,
J. Pharm. Exp. Ther. 251:679-682 (1989)) to measure '4C-proline incorporation
into
collagenous protein.
D. Collagenase Assay for Measurement of '4C-Proline Incorporation
into Collaaenous Protein
With the embryos placed on ice, a piece of CAM 10 mm in diameter was
cut off under each ring cup and placed in a separate tube. To each tube was
added 1.0 ml
of phosphate-buffered saline (PBS, pH 7.3) containing 0.11 mg cycloheximide
and 0.17
mg dipyridyl. The tubes were placed in a boiling water bath for 10 min and
then cooled
to room temperature. The PBS in each tube was discarded after centrifugation
at 3000 x
g for 10 min. The CAM residue was washed once with 3 ml of 1 S% TCA and then
three
times with 3 ml of 5% TCA. Centrifugation was carried out as described above
between
each washing. At this point all non-protein bound radioactivity was removed,
and the
CAM containing the newly synthesized 14C-collagenous protein was suspended in
0.9 ml
of 0.1 N NaOH and 1.1 ml of HEPES buffer at pH 7.4. The pH of the sample was
neutralized with 0.8 N HCI, using phenol red as indicator.
To digest the 14C-collagenous protein, 7.5 units of collagenase and 500
nrnol of calcium chloride in 40 ml of HEPES buffer was added to the above
samples, and
mixtures were incubated at 37°C for 4 h. The reaction was stopped by
adding 1.0 ml of
20% TCA containing S mg of tannic acid into each tube. After vortex mixing,
the
samples were centrifuged at 3000 x g for 10 min. An aliquot of the clear
supernatant was
taken for scintillation counting to quantitate the radiolabeled tripeptides
corresponding to
basement membrane collagen and other collagenous materials synthesized by the
CAM
from 14C-proline. The CAM pellets in each tube were solubilized in 0.5 ml of
1.0 N
NaOH by boiling in a water bath for 5 min. An aliquot of the dissolved CAM was
used
for protein determination using the method provided by Pierce Chenucal Co.
(Instruction
manual for protein assay using bicinchoninic acid (BCA) Pierce Chemical Co.,
Rockford,
IL.). The radioactivity per milligram of protein from the CAM treated with a
test
compound relative to that from the control CAM gave the percent of
angiogenesis
inhibition.
CA 02324347 2000-09-19
WO 99148495 PCT/US99/06220
E. Resul s
Tables 1 and 2 summarize the results of the two separate experiments.
BTO-956 showed statistically significant inhibitory effects on hFGF-B-induced
angiogenesis. At 75 mg/CAM, the inhibition caused by BTO-956 was 30%, compared
to
5 38% caused by the same concentration of the known antiangiogenic agent, 2-
methoxyestradiol. The results from these two experiments suggest that the
antiangiogenic
effect of BTO-956 in the CAM assay is on the same order as that of 2-
methoxyestradiol, a
drug that is under development as an antiangiogenic agent.
Table 1
INHIBITORY EFFECTS OF
COMPOUNDS ON ANGIOGENESIS INDUCED BY hFGF-B
iaC-Proline Incorporated
into Collagenous Protein
(cprn/mg-protein)
Compound Dose (p,g/CA1V1) (mean + S.E.) % Inhibition
BTO-956 75 4374 + 651 31
25 5098 785 19
8.3 b884 1395 0
2-Methoxyestradiol 75 3875 + 891 38
25 5068 1609 20
8.3 5711 1469 9
Control - 6300 + 696 -
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
26
Table 2
INHIBITORY EFFECTS OF
COMPOUNDS ON ANGIOGENESIS INDUCED BY hFGF-B
i4C-Proline Incorporated
into Collagenous Protein
(cpm/mg-protein)
Compound Dose (pg/CA1VI) {mean + S.E.) % Inhibition
BTO-956 75 7534 ~ 1099a 32
25 9100 ~ 1664 18
8.3 10138 + 1625 10
2-Methoxyestradiol 75 6984 + 1022b 38
25 7303 ~ 1424 35
8.3 10499 ~ 1372 6
Control - 11200 + $29 -
Significantly lower than control, P < 0.02.
b Significantly lower than control, P < 0.01.
EXAMPLE III
This example illustrates the determination IDsa of BTO-956 on the
proliferation of human microvascular endothelial cells (HMVEC).
Human microvascular endothelial cells (HMVEC) (Clonetics Corporation,
#CC-2505) were seeded into 96-well plates at a concentration of 5 x 103
cells/well in a
volume of 100 p,l/well of Endothelial Growth Medium (EGM-2-MV, Clonetics
Corporation #CC-3162). Plates were incubated at 37°C in 5% C02 for 24 h
and then
covered with 100 p,l/well of EGM-2-WV. BTO-956 was diluted to 20 mM in
dimethyl
sulfoxide (DMSO) and further diluted with EGM-2-MV to 2x the concentrations
reported
below. Then 100 p,l aliquots of BTO-956/BGM-2-MV dilutions were added to the
HMVEC preparations and plates were incubated at 37°C in 5% C02 for 3
days. The
relative number of cells was determined by adding 20 p,l/ml of Alamar Blue
(BioSource
International #DAL-1025) for 3-6 h at 37°C and measuring color changes
indicating
metabolic activity by using a Millipore Cytofluor 2350 Fluorescence
Measurement
System at an excitation wavelength of 530 nm and an emission wavelength of 590
nm. In
this assay, the intensity of the fluorophore signal is directly proportional
to cell number.
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
27
A broad range curve was first established for BTO-956 to determine the
concentrations to use for a narrow range curve. A narrow range curve was then
generated
to find a region around the IDso point at which the curve is linear. The >Dso
was
calculated by using the linear portion of the curve in the immediate area
around the dose
giving 50% inhibition and using the equation y = ax+ b, where x is the
calculated m5o, Y
is 50% of the maximum optical density (OD), and a and b are constants.
To establish an exact Ipso for BTO-956, a narrow concentration range was
tested on HMVECs (from 450 nM to 50 nM). Maximum inhibition was determined by
using an additional upper concentration limit of 10 p,M. The baseline value
was
determined by using medium without compound. The IDso was determined to be 201
nM
on Day 3.
,aoo
aso ~,. .
S aoo S aso t
~so aao + .. .
aao ,
~o +
y=-,.,aa~.,o4ss
aoo
EX
100 200 .30D 100 9DQ 100 1fi0 20D 2
AM ~ ' ~n s~u.ess
LE IV
A. BTO-956 is orally bioavailable and has minimal normal tissue
tox' i
Gavage administration of BTO-956 to nude mice at doses as high as 1,000
mg/kg-day for 60 to 180 days produced only slight and transient increases in
liver and
kidney weights. No other gross pathology was observed. No mortality was seen
at a
daily administration of 4,000 mg/kg-day of BTO-956 for 3 weeks. Because a
common
toxic effect of anti-proliferative chemotherapeutic agents is myelosuppression
caused by
depletion of bone marrow stem cells, we tested the possibility that BTO-956
may have
bone marrow toxicity. Swiss Webster mice were exposed to the drug at 500 mg/kg-
day
by gavage for a period of 4 weeks, and bone marrow was examined
histologically. The
bone marrow profiles of treated animals did not differ from those of control
animals
exposed only to the carrier vehicle, and no hematopoietic abnormalities were
found. Both
myeloid and erythroid lineages were observed at various stages of maturation.
These
results demonstrate that oral administration of BTO-956 is well tolerated by
animals
exposed to the drug for periods of up to six months, and has no obvious
myelosuppressive
activity after one month of daily administration at a therapeutic dose.
Preliminary studies
CA 02324347 2000-09-19
WO 99/48495 PCT/US99/06220
28
(not shown) indicate that therapeutic oral doses of BTO-956 are within the
range of 500
to 1,000 mg/kg-day.
B. BTO-956 has strong growth delay effects in human breast and
S ovarian carcinoma xenografts
Swiss NCr nude (nu/nu) female mice implanted with MDA MB-231
human breast carcinoma xenografts showed a strong growth delay response to
oral
treatment with BTO-956 (FIG. 1). Intraperitoneally administered
cyclophosphamide
{CTX; Cytoxan; I50 mg/kg), which is used in some regimens for adjuvant breast
cancer
therapy was used as a positive control. Growth of tumors in the BTO-956-
treated group
was suppressed during 6 weeks of therapy while those in the control vehicle-
treated
animals increased approximately 5-fold on the average (relative mean tumor
volume;
FIG. lA). BTO-956 was also effective in inhibiting the growth of OVCAR-3 human
ovarian carcinoma xenografts (FIG. 1B). Using the same schedules and doses as
those
described for the MDA MB-231 breast tumor study, but with shorter study
duration, the
relative mean tumor volume at 5 weeks of animals exposed to BTO-956 was again
approximately 5-fold less than that of the vehicle-treated controls. Two
important
conclusions of this study are the following: (1) BTO-956 is effective as a
cytostatic/cytotoxic antitumor drug when administered orally; and (2) BTO-956
is
essentially nontoxic even at very high orally administered doses.
C. BTO-956 induces a mitotic arrest in MCF-7 human breast
carcinoma cells
Having demonstrated significant antitumor effects of BTO-956 in vivo, we
investigated its cytotoxicity toward human breast carcinoma cells in vitro.
The ICSo
{concentration for SO% reduction of cell number relative to that of a vehicle-
treated
control) of BTO-956 in MDA MB-231 and MCF-7 cell cultures treated for 48 h was
0.3
pM and 0.6 lr.M, respectively. Investigation of the effect of BTO-956 (1 ~ for
48 h) on
the nuclear morphology of these cells demonstrated that a significant number
had
condensed or fragmented nuclei characteristic of apoptotic cell death. To test
whether
BTO-956 exerts its cytotoxicity by perturbing the cell cycle, MCF-7 cultures
were
exposed to the drug at 1 p,M far 24 h (FIG. 2). A large fraction of these
cells showed
chromosome condensation characteristic of a prometaphase arrest (FIG. 2B). In
agreement with this conclusion, treatment of MCF-7 cells with the antimitotic
drug
colchicine (1 pM) for 24 h produced identical chromosome condensation patterns
(FIG.
2C). After 48 h of exposure to 1 pM BTO-956, MCF-7 cells showed substantial
cytotoxicity based on the criterion of loss of membrane integrity measured by
a trypan
blue exclusion assay. This result is consistent with that of the tumor growth
delay study
CA 02324347 2000-09-19
WO 99148495 PCT/US99/06220
29
described above, and demonstrates that BTO-956-induced cell death occurs
subsequent to
a prometaphase arrest in human breast carcinoma cell cultures.
To investigate the mitotic arrest caused by BTO-956 in more detail, flow
cytometry was used to determine the effect of the drug on the cell-cycle
progression of
MCF-7 cells. These experiments showed that at least 60% of these cells
arrested at G2/M
within 24 h of treatment with 1 ~M BTO-956. The same finding was obtained for
MDA
MB-231 cells exposed to the drug under the same conditions. These results
demonstrate
that BTO-956 causes human breast carcinoma cells to accumulate at the G2/M
cell-cycle
interphase, presumably by activating a mitotic spindle-assembly checkpoint.
Such
arrested cells eventually undergo cell death which can occur by an apoptotic
mechanism.
D. BTO-956 inhibits microtubule dynamics both in vitro and in
cultured cells
Microtubules are major cytoskeletal components composed of a- and ~i-
tubulin heterodimers bound with GTP. Antimitotic drugs such colchicine, Yinca
alkaloids, and paclitaxel (Taxol} perturb the intrinsic dynamic instability of
microtubules
arising from GTP hydrolysis by binding directly to tubulin and causing
inhibition of
spindle formation with consequent mitotic arrest. To determine whether the
antimitotic
effect of BTO-956 exposure could be explained by tubulin-binding activity, two
studies
were performed. The effect of the drug on microtubule assembly was
investigated
directly by using a cell-free fluorescence assay involving the visualization
of microtubule
formation from rhodamine-labeled tubulin and microtubule seeds in the presence
of GTP.
In this assay, 15 wM tubulin rapidly polymerized to form long, bright
microtubule threads
in the absence of a tubulin-binding agent (FIG. 3A), whereas in the presence
of 1 ~M
colchicine microtubule formation was completely inhibited (FIG. 3B). The same
effect
was observed in the presence of 10 p,M BTO-956 (FIG. 3D). At a concentration
of 1 p.M
BTO-956 was less potent, but nonetheless produced much shorter structures than
those
visible in the control experiment (FIG. 3C). In a second study, the ability of
BTO-956 to
perturb cellular microtubule assembly was investigated by using HeLa cells
(FIG. 4),
which also arrest at mitosis when exposed to the drug (FIG. 4) shows that HeLa
cells
exposed to 10 ~M BTO-956 for 1 h had aberrant microtubule networks resembling
those
created by exposure to 1 pM colchicine. Taken together, these findings
demonstrate that
BTO-956 can interact directly with tubulin in vitro and in cells to inhibit
microtubule
formation, much like the antimitotic drug colchicine.
E. BTO-956 competes with colchicine for bindin~~ to tubulin in vitro
Tubulin-binding antimitotic drugs interact with the protein at diverse sites.
Colchicine binds to soluble tubulin heterodimers at a single high-affinity
site (the
CA 02324347 2000-09-19
WO 99148495 PCT/C1S99/06220
colchicine binding site) to form a kinetically inert complex. Vinblastine
binds to one or
two identical high-affinity sites on tubulin (Vinca alkaloid binding sites)
that are different
from the colchicine site. To determine whether the effect of BTO-956 on
microtubule
assembly is mediated by a specific site on tubulin, the ability of '4C-labeled
drug to
5 compete with colchicine or vinhlastine for binding to purified tubulin was
measured as a
function of colchicine or vinblastine concentration. FIG. 5 demonstrates that
colchicine
inhibited the binding of BTO-956 to tubulin, whereas vinblastine had no
effect. This
finding indicates that BTO-956 interacts directly or indirectly with the
colchicine site but
not the Vinca alkaloid high-affinity binding sites of tubulin in vitro.
EXAMPLE V
This example demonstrates that BTO-956 can downreguiate the expression
of cytokines in lipopolysaccharide-stimulated marine macrophages. The
following is an
assay for measuring the ability of Compounds of Formula I to reduce the levels
of Tumor
Necrosis Factor (TNF-a).
A. II Line
The marine macrophage PUS-1.8 cell line was purchased from the
American Type Culture Collection (ATCC, Rockville, MD). Cells were grown in
DMEM medium supplemented with 100 mM sodium pyruvate, O.ImM nonessential
amino acids, 2mM glutamine and S% fetal bovine serum (Life Technologies,
Staten
Island, NIA. Cells were maintained in a humidifed atmosphere of 5% C02-95% air
at
37°C. Cells were passages twice weekly by firmly tapping the side of
the flask to
dislodge the adherent cells. Both nonadherent and adherent cells were
passages.
Exponentially growing cells were seeded at 5 x 105/mL, 4mL per 60-mm dish 24 h
prior
to the experiment. Test compounds were delivered in 1mL volumes of the medium
added
to each dish at the start of the experiment. All dishes were incubated at
37°C in 5% C02-
95% air for 3 h.
B. Reagents
The Tumor Necrosis Factor (TNF-a) cDNA was obtained from the ATCC
(Rockville, MD). [a 32P]-dCTP (250 pCi) and nylon membranes (Hybond N) were
obtained from Amersham (Arlington Heights, Il.). Colchicine (used as a
control) was
purchased from the Sigma Chemical Company (St. Louis, MO). Lipopolysaccharide
(LPS) from Escherichia coli was purchased from DIFCO Laboratories (Detroit,
MI). All
plastic supplies were from VWR Scientific products (San Francisco, CA).
CA 02324347 2000-09-19
WO 99/48495 PCT/C1S99/06220
31
C. Northern blotting
Total RNA was isolated by the guanidinium-cesium chloride method as
described in N.S. Waleh, J. Gallo, T.D. Grant, B.J. Murphy, R.H. Kramer and
R.M.
Sutherland. {1994) "Selective downregulation of integrin receptors in
spheroids of
S squamous cell carcinoma" Cancer Rer., 54: 838-843. Five to 10 p.g of total
RNA was
electrophoresed in 1% agarose gels containing 6% formaldehyde. Following
electrophoresis, gels were stained with ethidium bromide to visualize the
positions of 28S
and 18S RNA. The RNAs were then transferred to nylon membranes (Amersham
Hybond N) by capillary blotting and fixed to the filter by exposure to UV
light. The blots
were probed with 32P-labeled cDNA sequences of human TNF-a obtained from the
American Type Culture Collection (ATCC). The TNF-a cDNA was a I .1 kb PstI
fragment of plasmid pE4 in E. coli MM294 (ATCC 39894). Hybridizations were
carried
out at 42°C in 50% formamide, 5 X SSC, SX Denhardt's solution, 0.1 %
SDS, and
0.3 mg/mL salmon sperm DNA. Filters were washed by 1 X SSC, 0.1% SDS, twice at
room temperature for 15 min and once at 55°C in O.I X SSC, 0.1% SDS for
1 hr. Filters
were exposed to X-ray film at -70°C using an intensifying screen
(Coronex Hi-Plus).
Hybridized bands were quantified by analyzing the images obtained by
using a video densitometer (Applied Imaging Corporation, Santa Clara, CA).
Film
densities were calibrated using an optical-density wedge.
D. RE. SUL."T,,~
Treatment of PUS-1.8 marine macrophages with LPS (100 ng/mL) for 3 h
resulted in a significant increase (> 7 fold) in the level of TNF-a mRNA as
determined by
Northern blot analysis (see, Fig. 1). Treatment of cells with only BTO-956 or
colchicine
at 10 p.M concentration had no effect on TNF-a mRNA expression. However,
addition of
colchicine or BTO-956 at 10 ~,M to the LPS treated cultures resulted in
substantial
reduction of TNF-a mRNA accumulation. The inhibition levels were 68% for
colchicine
and 61 % for BTO-956, respectively.
To establish a concentration-effect relationship, macrophages were
exposed to various concentrations of BTO-956 in the presence of the stimulus
LPS for 3
h. Fig. 2 illustrates that the amounts of TNF-a mRNA declined with the
increasing
concentrations of BTO-956. The maximum effect was observed at 10 pM
concentration
of BTO-956. Total RNA was intact in cells treated with different
concentrations of BTO-
956 and colchicine, suggesting toxicity was not problematic.
The results indicate that BTO-956 at 10 ~eM partially suppresses, but does
not completely inhibit, the expression of TNF-a mRNA in the marine macrophage
cell
CA 02324347 2000-09-19
WO 99/48495 PG"T/US99/06220
32
line PUS-1.8. This indicates that BTO-956 downregulates LPS-stimulated
responses by
affecting the microtubule-dependent signaling pathways.
This fording is of clinical interest because BTO-956 can be used as a new
class of compounds to prevent LPS-mediated excessive TNF-a production and its
undesired side effects. This is especially attractive because BTO-956 has been
shown to
be a safe and well tolerated drug when administered orally to animals.
It is to be understood that the above description is intended to be
illustrative and not restrictive. Many embodiments will be apparent to those
of skill in the
art upon reading the above description. The scope of the invention should,
therefore, be
determined not with reference to the above description, but should instead be
determined
with reference to the appended claims, along with the full scope of
equivalents to which
such claims are entitled. The disclosures of all articles and references,
including patent
applications and publications, are incorporated herein by reference for all
purposes.