Note: Descriptions are shown in the official language in which they were submitted.
CA 02324885 2000-11-O1
990006 FA/al
1
Polyesters
The invention relates to polyesters, a process for their
preparation and their use.
Polyesters are known compounds which can be used inter alia
for the production of textiles.
They are described in Ullmann's Encyclopedia of Industrial
Chemistry vol A 21 (1992) pages 227 to 251.
The preparation of polyester fibres is known from Ullmann's
Encyclopedia of Industrial Chemistry vol A 10 (1992) pages
579 to 613.
A glycol, inter alia, is employed as the alcohol in the
preparation of polyesters or polyester fibres.
A filler is added to this glycol in order to establish
certain physico-chemical properties of the polymer.
The invention provides polyesters, which are characterized
in that they contain a silicon dioxide doped by means of
aerosol.
A silicon dioxide doped with aluminium oxide by means of
aerosol, such as is described in the patent application DE
19847161.0-41, can be employed as pyrogenically prepared
silicon dioxide doped by means of aerosol.
The pyrogenically prepared silicon dioxide doped with
aluminium oxide by means of aerosol is characterized in
that the base component is a silica which is prepared
pyrogenically in the manner of flame oxidation or,
preferably, flame hydrolysis and is doped with a doping
component of 1~10-4 and up to 20 wt.%, the amount of doping
preferably being in the range from 1 to 10,000 ppm and the
doping component being a salt or a salt mixture of
aluminium or a suspension of an aluminium compound or
CA 02324885 2000-11-O1
990006 FA/al
2
metallic aluminium or mixtures thereof, the BET surface
area of the doped oxide being between 5 and 600 m2/g,
preferably in the range between 40 and 100 m2/g.
The doped silica can have a DBP number of less than
100g/100g.
The pyrogenically prepared silicon dioxide doped with
aluminium oxide by means of aerosol can be prepared by
feeding an aerosol into a flame such as is used for the
pyrogenic preparation of silica in the manner of flame
oxidation or, preferably, flame hydrolysis, mixing the -
aerosol homogeneously with the gas mixture of the flame
oxidation or flame hydrolysis before the reaction,
subsequently allowing the aerosol-gas mixture to react in
the flame and separating the resulting pyrogenically
prepared silicas doped with aluminium oxide by means of
aerosol off from the gas stream in a known manner, an
aqueous solution which comprises salts or salt mixtures of
aluminium or the metal itself in dissolved or suspended
form or mixtures thereof being used for the preparation of
the aerosol, and the aerosol being prepared by atomization
by means of a two-component nozzle or by another method of
aerosol preparation, preferably by an aerosol generator
after ultrasonic atomization.
Salts which can be employed are: A1C13, Alz (SO9) 3, A1 (N03) 3-
The processes of flame hydrolysis for the preparation of
pyrogenic oxides and thus also for the preparation of
silicon dioxide (silica) are known from Ullmanns
Enzyklopadie der technischen Chemie [Ullmann's
Encyclopaedia of Industrial Chemistry), 4th edition, volume
21, page 464.
According to the invention, mixtures of 0.1 to 100 per cent
of the silicas which can be employed according to the
invention with other pyrogenically prepared or precipitated
CA 02324885 2000-11-O1
990006 FA/al
3
silicas or bentonites or other conventional fillers in the
preparation of polyesters or mixtures of these fillers can
be employed.
The silica according to the invention which can be
employed, which is obtained as the product, for example,
using aluminium chloride salts dissolved in water for the
preparation of the aerosol to be fed in, can be dispersed
extremely well in polar media, such as e. g. water.
The polyester according to the invention is suitable for
the production of fibres (polyester fibres). .
The invention also provides a process for the preparation
of polyesters, in which glycol, preferably ethylene glycol,
is esterified with an organic acid by a known route, which
is characterized in that a pyrogenically prepared silicon
dioxide doped by means of aerosol is added to the glycol.
The invention also provides a glycol, preferably ethylene
glycol, which is characterized in that it contains a
pyrogenically prepared silicon dioxide doped by means of
aerosol, optionally up to max. 60 parts per part of glycol.
In a preferred embodiment of the invention, the glycol can
contain the pyrogenically prepared silicon dioxide doped by
means of aerosol in a concentration of 50 to 60 parts per
part of glycol.
The preparation of the polyester can be carried out by a
known route, such as is described in Ullmann's Encyclopedia
of Industrial Chemistry, vol. A21 (1992) pages 227 to 251.
The production of the polyester fibres can be carried out
by a known route, such as is described in Ullmann's
Encyclopedia of Industrial Chemistry vol. A10 (1992) pages
579 to 613.
CA 02324885 2000-11-O1
990006 FA/al
4
According to the invention, the silicon dioxide doped with
aluminium oxide by means of aerosol in the polyester
fibres, which can have a diameter of 10 ~m diameter, can
have an average particle size of 0.5 Vim.
The silicon dioxide according to the invention which can be
employed and the process for its preparation and its use
are explained and described in more detail with the aid of
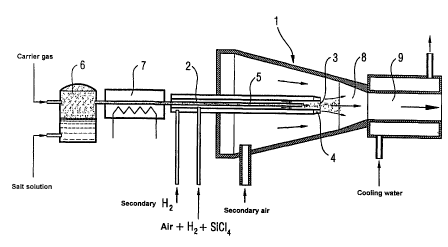
figure 1 and the following examples:
Figure 1 shows a diagram of the doping apparatus. The core
piece of the apparatus is a burner of known construction
for the preparation of pyrogenic oxides.
The burner 1 comprises the central pipe 2 which ends in the
nozzle 3, from which the main gas stream flows out into the
burner chamber and burns off there. The nozzle 3 is
surrounded by the annular nozzle 4, out of which (ring or
secondary) hydrogen flows.
In the central pipe 2 is the axial pipe 5, which ends a few
centimetres before the nozzle of the central pipe 2. The
aerosol is fed into the axial pipe 5.
The aerosol, which comprises an aqueous aluminium chloride
solution, is produced in the aerosol generator 6
(ultrasonic atomizer).
The aluminium chloride-water aerosol produced in the
aerosol generator 6 is passed by means of a light carrier
gas stream through the heating zone 7, in which the water
carried along evaporates, small salt crystals in finely
divided form remaining in the gas phase.
Example 1
Preparation of a pyrogenically prepared silica of low BET
surface area doped with aluminium oxide by means of aerosol
CA 02324885 2000-11-O1
990006 FA/al
5.25 kg/h SiCl9 are vaporized at approx. 130 °C and
transferred into the central pipe 2 of the burner 1.
3.47 Nm3/h (primary) hydrogen and 3.76 Nm3/h air are
additionally fed into the central pipe 2. 0.95 Nm3/h oxygen
5 are additionally added to this mixture.
The gas mixture flows out of the nozzle 3 of the burner 1
and burns in the burner chamber and the water-cooled flame
pipe connected thereto.
0.5 Nm3/h (jacket or secondary) hydrogen and 0.3 Nm3/h
nitrogen are fed into the annular nozzle 4.
Nm3/h (secondary) air are also additionally fed into the
burner chamber.
The second gas stream flows out of the axial pipe 5 into
the central pipe 2.
15 The second gas stream comprises the aerosol, which is
produced in the aerosol generator 6 by ultrasonic
atomization of A1C13 solution. The aerosol generator 6
atomizes here 460 g/h 2.29 per cent aqueous aluminium
trichloride solution. The aluminium chloride aerosol is led
20 with the aid of the carrier gas of 0.5 Nm3/h air through
the heated line, the aqueous aerosol being converted into a
gas and a salt crystal aerosol at temperatures of approx.
180 °C.
At the burner mouth the temperature of the gas mixture
(SiClq-air-hydrogen, water-aerosol) is 156 °C.
The reaction gases and the pyrogenic silica doped with
aluminium oxide by means of aerosol are sucked through the
cooling system by applying a reduced pressure. As a result
of this, the particles-gas stream is cooled to approx. 100
to 160 °C. The solid is separated off from the waste gas
stream in a cyclone.
CA 02324885 2000-11-O1
990006 FA/al
6
The pyrogenically prepared sil,'_ca doped with aluminium
oxide by means of aerosol is obtained as a white finely
divided powder.
In a further step, still adhering hydrochloric acid
residues are removed from the silica by treatment with air
containing steam at elevated temperature.
The BET surface area of the pyrogenic silica doped with
aluminium oxide is 55 m2/g.
The preparation conditions are summarized in table 1.
Further analytical data of the silica according to the
invention are given in table 2.
Example 2
Preparation of a pyrogenically prepared silica of high BET
surface area doped with aluminium oxide by means of aerosol
4.44 kg/h SiCl4 are vaporized at approx. 130 °C and
transferred into the central pipe 2 of the burner 1 of
known construction. 3.15 Nm3/h (primary) hydrogen and
8.2 Nm3/h air are additionally fed into the central pipe 2.
The gas mixture flows out of the nozzle 3 of the burner 1
and burns in the burner chamber and the water-cooled flame
pipe connected thereto.
0.5 Nm3/h (jacket or secondary) hydrogen and 0.3 Nm3/h
nitrogen are fed into the annular nozzle 4.
12 Nm3/h (secondary) air are also additionally fed into the
burner chamber.
The second gas stream flows out of the axial pipe 5 into
the central pipe 2.
The second gas stream comprises_the aerosol, which is
produced in the separate atomizing unit 6 by ultrasonic
CA 02324885 2000-11-O1
990006 FA/al
7
atomization of A1C13 solution. The aerosol generator 6
atomizes here 450 g/h 2.29 per cent aqueous aluminium
trichloride solution. The aluminium chloride aerosol is led
with the aid of the carrier gas~of 0.5 Nm3/h air through
the heated line, the aqueous aerosol being converted into a
gas and a salt crystal aerosol at temperatures of approx.
180 °C.
At the burner mouth the temperature of the gas mixture
(SiCl9-air-hydrogen, water-aerosol) is 180 °C.
The reaction gases and the pyrogenically prepared silica
doped with aluminium oxide by means of aerosol are sucked
through a cooling system by applying a reduced pressure. As
a result of this, the particles-gas stream is cooled to
approx. 100 to 160 °C. The solid is separated off from the
waste gas stream in a cyclone.
The pyrogenically prepared silica doped with aluminium
oxide by means of aerosol is obtained as a white finely
divided powder. In a further step, still adhering
hydrochloric acid residues are removed from the silica by
treatment with air containing steam at elevated
temperature.
The BET surface area of the pyrogenic silica doped with
aluminium oxide by means of aerosol is 203 m2/g.
The preparation conditions are shown in table 1. Further
analytical data of the silica which can be employed
according to the invention are given in table 2.
CA 02324885 2000-11-O1
ro
a w
O ,,
v
. U
-1
.
x
o '
w \ ~n o
a 'n '_'w
l ~ N ~ O
v .-Oi
~
-rl . tr,w
O
a
a ~ ~ ~ n
\ N
~ c
0 ro
0
,I a
~
+~
a w
C
O
_
o ~ m n ~ o
.~ .,
~
a
~ \ ~r ~ x ro
m
O ~ .
0 0
p., ~ rt1 .. o
~
O ~ o
ro
Z3 ro a
a,
tn U7 ~, ~
(IJ ~ oho~ ov~ a
~''~ ~"~
a
U ~ '~ ~ O ~ O ro
~ ~
N N N N b a
U U p
_
,
p N b" N b' ~ p.
FC FC U
t/7 rn t~ rt3 ~ ~
w
ro
U a O
a
OD -~ U
O
b
O U ~
w
II
W
~1, N x M M .. ri
\ O
O
r,
0 o a
'
-
I C1nN
1 N
N
ro
O a ~
.i
r-I ~ x 1.~N
X \ l!7 l.n
N
0 0
rr3 -,~ .~ w
N a
~ G ~
-u o
C1 N u v' ~ ro
, \ o
a a
a
- a.
a ro
00
w ~
. y o o
p '~ -~
''
~ N .--t c ro
o v
1
x ~
O ~ '~ ~ o ~
c
z ~
o
O _~ N
ro
O ~ _~ ~ N ~ v
U \ . ~
"'
. ~ w
z
M CL
~
rd
w
w ~ ~s
r-1
~ U N ~' o
t0 - c a a
-I ~
O N r . u7 ~r
t~
O '-I O b
O
O ~ ~ z r1 N Q.
1
E-~ w w
CA 02324885 2000-11-O1
a~ +~
0
-,-1 ~ W
U N
~
~I -.~ O N
+-~
L1.
o ~n
~ a.
-,~o
U U
(' LO
N ~ ~
~
o~
O O o~ ~ n
U
w oo ~
O
N O
N
'~' -I
+~
W
o O o
V
x
0
-~ tr
w .u ~0 0
o 0
CO Oy~''~ N ~1 O
~ lD
A O M
~
~n CT
-r-I 'L3 ~ a
0
U b ~
U ~ -.~ O o
.-i
~ N
~ CT rl
H ~ ro
N
s~
a
rif
U
Ul cH Ir
O
x ~n M ~ o
cn C1 aw . . ~ sr
~r
'
ao w
(2,
M v
t~
w
O
E._, ~,.~ x
~, ~ O
w
W ~ O ~ II
CO ~
N ~
O
ra m
+.~
N r-iN O O cr
'O U7
N . . -,~ x
'
0 0 ~I x
w ~ ~
~ n, O
N -r-I N N ~ ,-i
l0 ~ .~ r-iO -rl
O N >, C1,fl~U cn
O .-i .-i ~ ~ O
O .~ ~ rtirtS~ !-I
H r.>r W W t~ ~ w
CA 02324885 2000-11-O1
990006
- EM photograph:
Figure 2 shows an EM photograph of the pyrogenic silica
doped with aluminium oxide by means of aerosol according to
example 1.
5 It is noticeable that there are many individual spherical
primary particles which have not coalesced with one
another.
The difference between the pyrogenic silicas which are
doped with aluminium oxide by means of aerosol and can be
10 employed according to the invention and pyrogenic silicas
of known preparation and the same specific surface area
manifests itself in particular in the DBP absorption, which
is a measure of the structuring of the pyrogenic silica
(i.e. of the degree of coalescence thereof).
The commercially obtainable silica OX 50 prepared by the
pyrogenic high-temperature flame hydrolysis process thu s
has (at a BET surface area of 50 m2/g) a DBP absorption of
approx. 160 (g/100g), while the pyrogenic silica which is
doped with 0.187 wt.o A1203 and can be employed according
to the invention has a DBP absorption of only 81 (g/100g).
The consequence of the very low DBP absorption is that
dispersions of low viscosity can be prepared from the
pyrogenic silica doped with aluminium oxide. Dispersions
with a high degree of filling with solid are easy to
prepare because of these properties.
Mixtures of the silicas which can be employed according to
the invention with other pyrogenically prepared or
precipitated silicas or bentonites or other conventional
fillers for the preparation of polyesters are in principle
also possible.
Example 3
Dispersion of--the silica in ethylene glycol
CA 02324885 2000-11-O1
990006
11
Ethylene glycol, which is a starting material for the
production of polyester fibres, is mixed with silicon
dioxide doped with aluminium oxide by means of aerosol.
The dispersibility is considerably better compared with
Aerosil OX50, Aerosil 50, MOX 80, A1z03 C.
A concentration of up to 60 parts per 100 parts of ethylene
glycol can thus be achieved according to the invention with
only a slight increase in viscosity. (Figure 4)
The viscosity values determined in ethylene glycol are
shown on a graph in figure 4. Figure 3 shows the particle
size distribution of the various pyrogenic oxides dispersed
in ethylene glycol at a concentration of 20 parts per 100
parts of ethylene glycol, and figure 4 shows the various
viscosities as a function of the concentration.
According to figure 3, the silica doped with aluminium
oxide by means of aerosol employed according to the
invention (example 1) has the lowest average particle size
of 0.14 Vim.
According to figure 4, the silica doped with aluminium
oxide by means of aerosol employed according to the
invention (example 1) has the lowest increase in viscosity.