Note: Descriptions are shown in the official language in which they were submitted.
CA 02325829 2000-11-16
75304-15D
1
This is a Division of our co-pending Canadian Patent
Application No. 2,210,65 filed 7 February 1996
The present invention pertains generally to an
apparatus for providing access to a living body. More
particularly, the invention relates to an improved implantable
patient access device which allows for repeated access to a
region within the body of a patient.
During a course of treatment, it may be necessary to
gain repeat access to specific sites, devices, tissues, or
fluids within the body of a patient. This may be effected for
the temporary or sustained infusion of various therapeutic
agents, the removal and treatment of fluids, the injection of
contrast agents, as well as the insertion of various treatment
devices such as fiber-optic cameras and light sources,
ultrasound probes, and thrombectomy catheters. A number of
strategies are currently used to gain such access, including
direct vessel cannulation, short and long term catheterization,
as well as subcutaneous port and pump implantation.
Direct cannulation of a native or artificial vessel
with a needle provides perhaps the least expensive and simplest
form of access. However, repeat cannulation of superficial
vessels has been shown to result in vessel thrombosis, and in
case of hemodialysis graft cannulation, access stenosis and the
formation of pseudoaneurisms. A patient's accessible vessels
can quickly be eliminated by repeat direct cannulation during
the course of some aggressive treatment regimens, limiting
treatment options and worsening prognosis. The use of large
needles also leaves behind substantial lacerations in the
vessel, requiring the application of pressure for a number of
minutes to regain hemostasis, particularly in the case of high
flow or high pressure vessels such as arteries, central veins,
CA 02325829 2000-11-16
75304-15D
2
and primary or prosthetic fistulas. This pressure is
uncomfortable for the patient and may result in early vessel
thrombosis independent of other causes.
Short and long term catheters have been used to
address the many problems of direct cannulation. These
transcutaneous devices are generally flexible cannulae that are
inserted percutaneously into the region of interest such as a
blood vessel or the peritoneal cavity. Catheters have one or
more lumens through which various fluids or devices can pass.
While catheters allow repeat access with a reduced risk of
vessel thrombosis, they suffer from a number of significant
drawbacks. Aside from being unsightly and prone to inadvertent
withdrawal, catheters often have complications with infection.
The location of the infection is commonly the exit site or
point at which the catheter passes through the skin. This
essentially open wound provides a path for various hazardous
organisms to migrate into the body and cause infections, either
local or systemic. Infection has also been shown by a number
of authors to increase the occurrence of both catheter and
vessel thrombosis, other common complications of indwelling
catheters.
Subcutaneously implanted ports have increasingly been
used as an alternative to transcutaneous catheterization.
These devices provide a site beneath the skin that can be
accessed by special non-coring needles through a percutaneous
puncture at the time of treatment. The devices generally
comprise a housing that forms a reservoir which communicates
with a catheter that leads to the area requiring treatment. A
self-sealing septum formed from a high density silicone
elastomer spans the top of this reservoir, creating a
continuous barrier against the passage of fluids such as blood
that are in communication with the port. This septum is
punctured by the needle to permit access to the reservoir.
CA 02325829 2000-11-16
75304-15D
3
Once the needle is withdrawn, the septum closes, restoring the
continuous barrier. By being completely implanted (that is,
requiring no open passage through the skin) ports avoid many of
the infection complications of catheters. Ports are also
generally better accepted by the patient because they are less
obtrusive, cannot be accidentally withdrawn, and are easy to
maintain.
Subcutaneously implanted ports are also used as a
means of communicating with other implanted medical devices.
For example, implantable infusion pumps that provide a
sustained infusion of therapeutic agents into the body of a
patient often use one or more integral ports as refilling and
flushing sites. Various other devices, such as implanted
inflatable prostheses, have exploited or may have benefited
from the use of such ports as well.
Subcutaneously implanted ports do have a number of
significant drawbacks that limit their application. First,
their useful life is limited by the number of punctures that
the septum can withstand before it leaks. Repeat access slowly
degrades the silicone septum until ultimately it is unable to
resist the passage of fluids or other elements that are in
communication with the port. Secondly, they cannot be accessed
by normal needles, requiring special, relatively expensive non-
coring needles to reduce the damage done to the septum. This
expense may seem minimal, but can be significant when
aggressive therapies are required or when the therapies are
primarily Medicare funded. Thirdly, only small needle gauges
can be used even with non-coring needles because larger bore
needles quickly destroy the septum. However, small needles are
not appropriate for many treatments such as transfusion or
hemodialysis which require high blood flows.
CA 02325829 2000-11-16
75304-15D
4
creating a potential site for infection. Another limitation of
prior art concepts is the durability of the valve assembly when
sharp needles or trocars are used for access. While there
exist various concepts that allow access by either flexible
filaments such as catheters or rigid filaments like needles,
all of the valve assemblies allowing access specifically by
rigid filaments are either subject to direct contact with the
sharp tip of the accessing needle promoting wear or do not
specifically seal around the accessing filament before the
valve assembly is open or before it closes. In certain known
devices, elastomeric members which form the valve assembly are
in the direct path of the accessing needle. The hole in the
first elastomeric member is smaller in diameter than the
accessing filament, and hence will suffer damage every time the
accessing needle is inserted. This damage could ultimately
lead to valve failure, which can have castastrophic
consequences for the patient.
In certain prior art designs, movement of the valve
components is directly linked with movement of the sealing
components so that creation of a seal around the accessing
filament requires the valve to be opened. The leaflets of the
valve are either in direct sealing engagement with the filament
sealing element or the motions of the two elements are directly
linked through an intervening rigid member. These designs
imply that some throw or partial opening of the valve is
required before the seal is created around the accessing
filament or, more importantly, that flow is potentially allowed
through this partially open valve and around the accessing
filament until the valve has been opened far enough to generate
an effective seal. This could potentially lead to the repeat
formation of hematomas or passage of other fluids into the
tissue surrounding the device as a result of access.
CA 02325829 2000-11-16
75304-15D
Some prior art concepts disclose an implantable
patient access port which allows the introduction of various
filaments including catheters and needles into the body of a
patient without the use of a standard septum. By employing a
5 variety of different valuing mechanisms, the port presumably
has broader applications to more rigorous therapies requiring
frequent access or high flow, i.e. therapies previously
restricted to transcutaneous catheters and direct cannulation.
All of these ports incorporate a housing having a generally
funnel-shaped entrance orifice, a valuing mechanism that is
opened by the accessing filament, allowing its passage, and an
exit passageway.
One significant limitation of the foregoing prior
concepts is in the strike area, or the region that the medical
professional attempting access must hit with the accessing
filament to enter the device. A large strike area is critical
for simple cannulation and for allowing each insertion wound to
heal before that region must be re-cannulated. By nature, to
increase the strike area of a funnel such as that described in
the art, one must also increase its overall size in three
dimensions. A dimension of particular importance with ports is
height, or depth from the skin inward. The taller a port, the
more tension it places on the insertion wound, the more obvious
its presence to observers, and potentially the greater chance
for erosion and infection. So increasing the strike area of
the funnel, increases the size of the port in three dimensions,
potentially leading to complications.
The funnel-shaped entrance orifice further limits the
strike area by providing only a single focal point or entry
point for the accessing filament. Because the filament is
always focused to the same site, the same tissue proximal to
that entry site must be traumatized during each access. Repeat
trauma to tissue can lead to devascularization and necrosis,
CA 02325829 2000-11-16
75304-15D
5a
Summary of the Invention
The primary objective of the present invention is to
provide an implantable patient access device which overcomes
many of the deficiencies of prior art ports. Specifically the
invention provides an implantable access device comprising a
housing having at least one entry port and at least one exit
port with a passageway extending therebetween, said entry port
being adapted to receive a filament for passage into said
passageway, said housing further including and disposed in said
passageway a valve assembly comprising a valve and a sealing
element, said valve assembly adapted to be activated by said
filament after passage of said filament through said entry port
whereupon a seal, independent of activation of said valve, is
created by said sealing element about said filament before said
valve opens to allow access through said exit port
characterized in that said sealing element comprises an
elastomeric member with a first and second end and an open
conduit therebetween, said first end being substantially fixed
in position within said housing and said second end having a
resilient cap affixed thereto, said cap being adapted to
withstand repeat contact with said filament, resisting passage
of said filament such that when said filament is advanced
through said conduit the filament makes contact with said cap
causing said elastomeric member to stretch and collapse around
said filament.
In one embodiment, the implantable access device
employs an open guidance channel that allows for increases in
accessing filament strike area without increasing the overall
height of the device. Further, the device employs a valve
assembly that provides access to the patient while at all times
maintaining a fluid tight seal around the accessing filament,
normally a needle. The valve assembly does not allow contact
of the accessing filament's sharp leading edges,
CA 02325829 2000-11-16
75304-15D
5b
particularly in the case of a needle, with any soft elastomeric
member of the valve assembly. In this way, the valve assembly
allows repeat access by standard needles of either small or
large gauge, eliminating many of the access problems that have
limited the use of standard ports with septums and some other
prior art devices. Further, the valve assembly ensures that a
seal around the accessing filament will be formed prior to the
valve assembly opening to allow access to the patient. This is
accomplished in one embodiment of the invention by ensuring
that less movement of the accessing filament is required to
create a seal about the filament than is required to begin
opening the valve, and in another embodiment of the invention
by completely decoupling creation of the seal from motion of
the valve. The assembly thus ensures that there is no leakage
of fluids around the assessing filament at any time during
access. Other advantages of the present invention are
described below.
The valve might comprise a miter valve or a slit
valve, with each valve adapted to be opened by movement of the
filament into the valve assembly. The valve might comprise an
elastomeric plug seated in sealing engagement within the
passageway, the plug being forced from sealing engagement with
the passageway by movement of the filament through the
passageway. The sealing element comprises an elastomeric
member with a first and second end and an open conduit
therebetween, the first end being substantially fixed in
position within the housing and the second end having a
resilient cap affixed thereto, the cap being adapted to
withstand repeat contact with the filament, resisting passage
of the filament such that when the filament is advanced through
the conduit the filament makes contact with the cap causing the
elastomeric member to stretch and collapse around the filament.
The elastomeric member has an outer dimension, the outer
CA 02325829 2000-11-16
75304-15D
5c
dimension at a first location having a first magnitude which
decreases to an outer dimension of a second magnitude at a
second location, the decrease corresponding to a decrease in
dimension of the passageway such that when the elastomeric
member is stretched by advancement of the filament, the larger
outer dimension of the elastomeric member is compressed against
the accessing filament within the smaller dimension of the
passageway creating a seal about the filament. The housing
might further comprise means for retaining an accessing
filament in a fixed position within the housing. The filament
might be a needle having a point and the housing might further
include means for guiding the needle through the conduit and
into the resilient cap such that the point of the needle
contacts only the resilient cap. The exit ports in these
devices are adapted to be connected to a catheter, a graft or
an implanted medical device.
The valve assembly preferably comprises a sealing
element and a valve disposed in the passageway, with the
sealing element first creating a seal about the filament before
the valve assembly opens to allow access to the patient by the
filament. The sealing element maintains the seal about the
filament until after the valve assembly closes. The channel
might have a generally V-shaped cross section or it might have
a generally U-shaped cross section such as a parabola. The
valve might comprise a miter valve or a slit valve, with each
valve adapted to be opened by movement of the filament into the
CA 02325829 2000-11-16
5304-15
6
valve assembly. Alternatively, the valve might comprise in
combination a plug seated in sealing engagement within the
passageway and a slit valve, the plug and the slit valve being
forced from sealing engagement with the passageway by movement
of the filament through the passageway. Additionally, the
valve might comprise a plug seated in sealing engagement within
the passageway and an opening proximate to the plug such that
when the plug is forced from sealing engagement with the
passageway by movement of the filament through the passageway
the opening allows access to the patient or site, space,
device, or other object, tissue, or fluid within the patient by
the filament. The sealing element comprises an elastomeric
member with a first and second end and an open conduit
therebetween, with the first end being substantially fixed in
position within the housing and with the second end having a
resilient cap affixed thereto, the cap being adapted to
withstand repeat contact with the filament, resisting passage
of the filament such that when the filament is advanced through
the conduit the filament makes contact with the cap causing the
elastomeric member to stretch and collapse around the filament.
The elastomeric member has an outer dimension, the outer
dimension at a first location having a first magnitude which
decreases to an outer dimension of a second magnitude at a
second location, the decrease corresponding to a decrease in
dimension of the passageway such that when the elastomeric
member is stretched by advancement of the filament, the larger
outer dimension of the elastomeric member is compressed against
the accessing filament within the smaller dimension of the
passageway. The housing further comprises means for retaining
an accessing filament in a fixed position within the housing.
The exit port is adapted to be connected to a catheter, a graft
or an implanted medical device.
CA 02325829 2000-11-16
W oI25196 PCT11896/00095
-7-
member to stretch and collapse around the filament. The elastomeric member has
an
outer dimension, the outer dimension at a first location having a first
magnitude which
decreases to an outer dimension of a second magnitude at a second location,
the
decrease corresponding to a decrease in dimension of the passageway such that
when
the elastomeric member is stretched by advancement of the filament, the larger
outer
dimension of the elastomeric member is compressed against the accessing
filament
within the smaller dimension of the passageway creating a seal about the
filament.
The housing might further comprise means for retaining an accessing filament
in a fixed
position within the housing. The filament might be a needle having a point and
the
housing might further include means for guiding the needle through the conduit
and into
the resilient cap such that the point of the needle contacts only the
resilient cap. The
exit ports in these devices are adapted to be connected to a catheter, a graft
or an
implanted medical device.
The invention additionally embodies an implantable patient access device
comprising a housing having a plurality of entry ports and a plurality of exit
ports with
a passageway extending between each entry port and each exit port, with the
housing
further comprising a plurality of elongated open guidancE channels disposed
therein,
each of the guidance channels communicating with an entry port, each of the
guidance
channels having a substantially constant cross sectional area, with each of
the
guidance channels further being adaptable to receive a filament for guiding
the filament
toward and into an associated entry port, the housing further including a
valve
assembly disposed in each passageway, the valve assembly adapted to be
activated
by the filament after passage of the filament through the entry port, the
valve assembly
being normally closed but adapted to be opened by the filament to allow access
to the
patient or site, space, device, or other object, tissue, or fluid within the
patient by the
filament.
Brief Description of the Drawin4s
Fig. 1 is a schematic perspective view of a first embodiment of an implantable
patient access device in accordance with the principles of the present
invention and
illustrating an elongated open generally V-shaped entrance guidance channel.
Fig. 2 is an enlarged longitudinal sectional view of the device depicted in
Fig.
1.
CA 02325829 2000-11-16
W(. x/25196 PC'T/1896/00095
_g_
Fig. 2A is a further enlarged view of a portion of the device illustrated in
Fig. 2
showing a partial view of the valve assembly of the device.
Fig. 3 is a view much like that of Fig. 2 but further showing the valve
assembly
of the device being activated by an accessing filament.
Fig. 3A is an enlarged view much like that depicted in Fig. 2A but further
showing the valve assembly after activation by the accessing filament.
Fig. 3B is an enlarged view of another portion of the device illustrated in
Fig. 3
showing a seal created about the accessing filament.
Fig. 4 is a view substantially like that of Fig. 2 but depicting an alternate
embodiment of the valve of the invention.
Fig. 5 is a view substantially like that of Fig. 3 but depicting the valve
arrangement of Fig. 4
Fig. 6 is a view much like that of Fig. 1 but showing an elongated open
generally U-shaped entrance guidance channel.
Fig. 7 is a view similar to Fig. 1 but illustrating a device having multiple
entrance
guidance channels and exit ports.
Fig. 8 is a view much like that of Figs. 2 and 4 but depicting an alternate
embodiment of a valve assembly with the valve assembly closed.
Fig. 9 is a view much like that of Fig. 8 but depicting a seal created about
the
accessing filament but with the valve closed.
Fig. 10 is a view much like that of Fig. 9 with the seal maintained but the
valve
open.
Fig. 11 schematically depicts an embodiment of the present inventive device as
an integral part of an implanted medical apparatus.
Detailed Description of the Invention
The description herein presented refers to the accompanying drawings in which
like reference numerals refer to like parts throughout the several views.
Referring to
Fig. 1, in accordance with the principles of the present invention, there is
illustrated a
schematic perspective view of a first embodiment of an implantable patient
access
device 10. The access device 10 includes a housing 12 having defined therein
an
elongated open guidance channel 14 communicating with entry port 16 of the
housing.
In this figure the guidance channel is shown to be of a generally V-shaped
configuration but other configurations would be possible. Port 16 in turn is
in fluid
CA 02325829 2000-11-16
vVC: :6125196 PCT/IB96l00095
_g_
communication with housing exit port 18. The internal structure of device 10
will be
shown in greater detail in subsequent views.
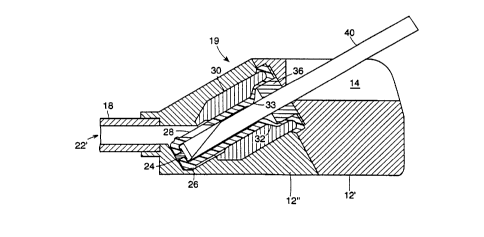
Turning now to Fig.2, there is depicted an enlarged longitudinal sectional
view
of implantable patient access device 10 depicted in Fig. 1. Here there is
shown a valve
assembly 19 comprising an elastomeric member 20 disposed in passageway 22 of
device 10. Elastomeric member or sealing element 20, in this embodiment,
includes
a plug 26, a slit valve 28 and terminates in a cap 24. Cap 24 may be titanium,
stainless steel or any other suitable resilient metal. Elastomeric member 20
is
positioned within a housing insert 30. Housing insert 30 is employed for ease
of
manufacture, but it should be understood that it could also be integral in the
geometry
of housing 12. Here housing 12, for ease of manufacture, is shown to be
composed
of part 12' and part 12". Elastomeric member 20 further has a transition
region 32
along which the outer diameter of the elastomeric member 20 decreases from a
first
larger diameter to a second smaller diameter. The interaction between the
elastomeric
member 20, specifically its transition region 32, and the housing insert 30
will create
a seal around an accessing filament as will be further described below.
Elastomeric
member 20 has a substantially thinner walled section 34 above transition
region 32.
Also within passageway 22 is a filament retention piece 36. Exit port 18
extends from
housing part 12" and forms lumen 22' which is in fluid communication with
passageway
22. Exit port 18 is adaptable to be coupled to a catheter, graft, another
device or
conduit that is within andlor in communication with the body of a patient.
Also shown
here as part of housing part 12", is a limner 38 which stops the downward
movement
of the activated valve assembly. Fig. 2A is an enlarged view of the left
portion of Fig.
2. Fig. 2A shows the plug 26 at the distal end of the elastomeric member 20 in
a
sealing engagement with passageway 22, and slit valve 28 in a closed position.
Fig.
ZA also depicts cap 24 and filament landing 24'.
Turning now to Fig. 3, there is shown the patient access device of Fig. 2 with
an accessing filament 40 opening the plug 26 and the slit valve 28. Preferably
the
filament is substantially rigid. Typically the filament would be a needle but
a catheter
or other substantially rigid member could be used. Before movement of plug 26
out of
passageway 22 and the opening of slit valve 28 which would allow communication
between filament 40 and lumen 22', a seal 33 is first created about filament
40. Seal
33 is maintained at all times when plug 26 and slit valve 28 allow
communication
CA 02325829 2000-11-16
V4'O x/25196 PCT/IB96I00095
-10-
between the filament 40 and lumen 22' and the seal is released only after plug
26
returns to a sealing engagement within passageway 22. Fig. 3A shows an
enlarged
view of the valve comprising plug 26 and slit valve 28 in an open position.
Fig. 3B is
an enlarged view which shows in greater detail the seal 33 about accessing
filament
40. Seal 33 is generated when the transition region 32 of elastomeric member
20 is
pulled into the smaller diameter 32' of housing insert element 30, compressing
the
elastomeric member 20 against the accessing filament 40. Further in Fig. 38 is
shown
the filament retention piece 36 engaging accessing filament 40. The filament
retention
piece 36 is configured with an inner dimension smaller than the outer
dimension of the
accessing filament 40, such that as the accessing filament 40 is introduced
into entry
port 16, the filament retention piece 36 expands and applies a force against
the
accessing filament 40 to resist its withdrawal from entry port 16. Filament
retention
piece 36 may employ a strain release slot or slots 37 to tune the force
applied to
accessing filament 40 and increase its useful life span. Figs. 4 and 5 are
substantially
the same as Figs. 2 and 3 and illustrate valve assembly 19' with the primary
difference
being that slit valve 28 has been replaced by an opening 42 located in
elastomeric
member 20.
Fig. 6 is substantially the same view as that shown in Fig. 1 except that here
the device has been designated 10' and the guidance channel 14' has a
generally
parabolic or generally U-shaped cross section. A guidance channel having a
flat rather
than a curved bottom is also considered to be of a generally U-shaped
configuration.
The generally U-shaped configuration is but one of the many possible
configurations
suitable for the elongated open guidance channel of the invention.
Fig. 7 depicts a dual patient access device 10" configuration with two
complete
devices (each having any of the valve assemblies described herein) fixedly
coupled in
a housing 13 to simplify the implantation of two devices. Fig. 7 also shows
two suture
holes 44 for anchoring the device to the patient. Suture holes 44 are only one
of the
many possible anchoring means for these devices. While not shown, any of the
devices that form this invention can employ an anchoring means such as suture
holes
44.
Figs. 8 through 10 depict another embodiment of the present invention and
illustrate valve assembly 19" which employs a duck bill or miter valve 46 in
place of
plug 26 and slit valve 28 or opening 42. Cap 48, having a filament landing or
strike
CA 02325829 2000-11-16
WC ;25196 PC'TliB96/00095
_11_
area 48', has replaced cap 24. A fastener 50 assists in maintaining the
coupling
between elastomeric member 20' and cap 48. Elastomeric member 20' has all of
the
attributes of elastomeric member 20. Fig. 8 depicts the valve assembly prior
to
activation. Also shown in Fig. 8, is housing insert 30' which is substantially
like housing
insert 30. The remaining structural elements are like those herein described
in respect
to the other embodiments of the invention. Fig. 9 additionally depicts an
accessing
filament 40 which moves cap 48 and elastomeric element 20' to create a seal 33
about
filament 40 before valve 46 is opened. Fig. 10 shows further advancement of
filament
40 and cap 48 which opens valve 46 to provide access to a patient or a site,
space,
device, or other object, tissue, or fluid within the patient. As shown here
and as is
shown in all other embodiments of the invention, seal 33 is created about the
accessing
member before the respective valve is opened, the seal is maintained during
the time
that the valve is open and the seal is not released until after the valve is
closed.
Turning lastly to Fig. 11, there is shown a schematic view of device 10 of the
present invention as an integral functioning part of an impiantable medical
apparatus
52, such as a sustained infusion pump 54. Here two devices are shown. However,
it
should be understood that one or a number of devices could be employed, such
as 10,
10', 10". In this view, pump 54 has been implanted below skin line 56 of a
patient.
Additionally shown is catheter 58 fluidly coupled to lumen 22'(not depicted in
this view).
The catheter is in fluid communication with a vessel 60, however,
communication could
be with a site, space, tissue, fluid, organ or another implanted device.
Although not
shown in this view, it also should also be understood that, like in Fig. 11,
each of the
devices of Figs. 1-10 are adaptable for inclusion as an integral part of an
implanted
medical apparatus or adaptable for independent implantation underthe skin of a
patient
for communication with a site, space, tissue, fluid, vessel, organ, or the
like.
An important characteristic of the various valve assemblies is the timing of
the
valve opening and closing relative to the seal formed around the accessing
filament.
Each valve assembly forms a seal around the accessing filament before the
valve
opens allowing access to the patient, and then releases that seal only after
the valve
has again been closed. This prevents any possibility of hemorrhage or reflux
of fluids
or gases out the device.
The open guidance channels that are part of this invention have a number of
advantages over the funnels described in the prior art. First, they allow for
increases
CA 02325829 2000-11-16
Wl. ~I25196 ' PCT/IB96/00095
_12_
in strike area without an increase in overall device height. With a device of
the
configuration shown in Figure 1, the strike area is increased simply by
increasing the
length of the device. Another advantage of the channel is that it allows the
device to
better simulate a natural vessel both in shape and the way in which it is
accessed.
This may make the device and its use more readily apparent to the accessing
nurse
or physician. Finally, an elongated open channel could allow for multiple
entry sites
along the channel's length, unlike a funnel which is limited to a single focal
point. By
accessing different entrance orifices during a treatment that requires repeat
access
procedures, trauma to the same tissue can be minimized relative to the funnel
with its
single focal orifice.
The device in Fig. 3 consists of a three-part housing, a needle retention
piece,
and a wedge seal and plug valve assembly. A first piece 12' of the housing
could be
made of a resilient material such as titanium that could endure frequent
contact with
the sharp tip of an accessing filament such as a needle. The guide channel
that is an
integral part of piece 12' is one of the many possible open channel forms
described by
this invention. The channel depicted in Fig. 3 could be employed as a filament
guide.
The base of this guide channel could be sloped from a first end towards the
entrance
orifice at an angle suitable for allowing the accessing filament to slide
easily upon
contact as well as for decreasing the overall volume of the device. The walls
of this
channel may be, to name but a few configurations, vertical, sloped or rounded.
Extending laterally from either side of piece 12' at its base could be two
suture loop
attachment sites for facilitating fixation of the device within the body. Any
suitable
number of attachment points can be used. Fig. 7 illustrates but one potential
fixation
configuration. Alternatively, the exterior surface of the housing can be
roughened or
porous, promoting tissue ingrowth to help fix the device within the patient.
A second piece 12" of the housing can be made either of a resilient material
or
of a more easily molded material such as plastic. This piece forms much of the
flow
path for the fluids that could be infused or removed through the complete
device. To
decrease the necessary flush volume and the risk of fluid pooling, the
diameter of the
flow path is closely matched to the diameter of the accessing filament. A
third piece
18 is a simple tube insert that provides a surface along which a catheter or
graft may
be joined with the patient access device. Again, this piece could be
constructed from
either a resilient or moldable plastic material. The exit port may provide
communication
CA 02325829 2000-11-16
Wt , x/25196 PCT/1896l00095
-13-
with an implantable medical device and may be of another configuration more
suitable
to optimizing its function in a certain application.
Filament retention piece 36 is a simple tube with a flanged end. It should be
constructed of a resilient material capable of withstanding frequent contact
with a sharp
accessing filament. The tube is slotted along all or part of its axial length
and is of a
diameter to some degree less than the diameter of the accessing filament.
Hence
when the accessing filament such as a needle is inserted, the tube expands
elastically,
applying a force normal to the filament about its circumference. This force
creates a
friction that is sufficient to retain the filament in an engaged position
during the access
procedure.
The wedge seal and plug valve assembly consists of three functional parts. The
first is a tube-like structure (20) formed from an elastomer such as silicone
rubber. The
second is a small cap (24) formed of a resilient material which is fixed to
the distal end
of the tube, but can be fixed to the tube at any appropriate site. The third
piece is a
simple insert (30) that is either a separate piece as depicted or is part of
the geometry
of the second piece of the housing. The tube is clamped into place at its
proximal end
just beyond the entrance orifice and filament retention piece. The tube fits
within the
internal structure of the insert. The outer diameter of the tube minors the
interior
shape of the insert along most of its length, being greatest at the most
proximal end,
narrowing along a short transitional length, and then remaining constant up to
a point
near the distal end. It should be understood that the term proximal, when
referring to
Fig. 2 for example, is that location towards the right of the figure while the
tens distal
refers to that location towards the left of the figure. At the distal location
of the tube,
an annular plug (26) bulges radially from the tube to a diameter greater than
the
corresponding interior diameter of the insert. This plug acts as the valve,
sealing
against fluids or gases when the tube is recessed within the insert and the
plug is
compressed against the insert's interior. Just above this plug is either a
hole or slit
through the wall of the tube which becomes a passageway for fluids or
filaments when
the valve is open. The tube has an internal diameter that is larger than that
of the
specified accessing filament. The proximal portion has the largest internal
diameter to
allow the filament retention piece to fit recessed within the tube. This
portion of the
tube also has the thinnest wall, making it the most flexible section. When an
accessing
CA 02325829 2000-11-16
WG ./25196 PC'T/>$96100095
-14-
filament is inserted into the device it makes contact only with the retention
piece and
the cap at the tube's distal end. Further advancement of the filament causes
the
elastomeric tube to stretch, particularly in the thinner proximal section.
This stretch
pulls the thicker transitional length of tube into the narrower portion of the
insert,
compressing the tube between the wall of the insert and the circumference of
the
filament. This compression creates a seal. When the annular plug at the distal
portion
of the tube is pushed beyond the distal portion of the insert, the opening
above this
plug is exposed to the exit port allowing fluids to be infused and withdrawn
or
instruments to be inserted into the body of the patient.
The valve only opens once the seal has been created about the accessing
filament and closes before that seal is broken. This is ensured by the travel
necessary
to push the annular plug out of sealing engagement with the interior wall of
the insert.
This travel is specified to be longer than the travel necessary to generate a
seal around
the accessing filament.
The device depicted in Figs. 8-10 uses a miter or duck bill valve (46) as the
valuing element. Typically the miter valve comprises elastomeric elements or
components. The valve is opened as the cap at the distal end of the
elastomeric tube
is pushed into the valve by the advancing filament or needle. This cap would
again be
formed from a resilient material such as stainless steel, titanium or other
suitable metal.
The cap has a simple step decrease in internal diameter ftom the proximal
portion to
the distal portion. The larger diameter allows passage of certain specked
filaments or
needle gauges, while the smaller diameter acts to limit passage of those
filaments or
needles, but allows for fluid flow.
The duck bill valve may have some advantages over the side hole valve of Fig.
4 or the slit valve of Fig. 2. It provides a more direct and potentially
smoother fluid flow
and instrument insertion pathway. This may ease insertion of various devices
and
allow for higher infusion flow rates at lower pressures. Another distinct
advantage of
this valve assembly is that creation of the seat about the accessing filament
requires
no motion of the valve. By decoupling the sealing element from the valve and
by
separating the two elements, the design ensures that the seal will be created
about the
filament before the valve opening is initiated.
The use of a channel in these devices allows the overall device to better
simulate a natural artery or vein. By running down the central axis of the
device, a
CA 02325829 2000-11-16
W O .:25196 PC'T/1896/00095
-15-
channel, as herein described, would allow the accessing medical professional
to access
the port in much the same way they access peripheral vessels, i.e. by placing
fingers
on either side of the vessel and sticking for its center. The length of this
channel can
be chosen to fit the requirements of the specific therapy, allowing for an
increase in
overall strike area by increasing the size of the implantable access device in
only a
single dimension.