Note: Descriptions are shown in the official language in which they were submitted.
CA 02326039 2000-11-16
CHAIN OF CUSTODY FORM WITH RFID
Field of the Invention
[0001] This invention relates to maintaining a of custody for object handling,
and more particularly, to a chain of custody form or associated label
including a
radio frequency identification chip allowing electronic chain of custody form
processing.
Background And Summary
[0002] Important decisions are often made based on laboratory analyses. For
example, a physician will often decide on a diagnosis or a treatment based on
lab
results. In other contexts, an employer might make a hiring or a firing
decision
based on the results of a drug test, or a court or jury might decide a legal
case
based on lab results of a DNA test, blood alcohol test or the like.
[0003] Important questions that may arise in these (and other) contexts is
whether the laboratory tested the correct sample, and whether the sample was
tampered with before it was tested. While the possibility of intentional
tampering
might seem unlikely, criminal defendants often allege that law enforcement
officials and/or laboratory personnel have tampered with incriminating
evidence in
order to "frame" the defendant. More commonly, there is always the possibility
of
mixing up samples or test results between different people. Imagine a
situation in
which samples from two different patients get mixed up -- resulting in both
patients being given incorrect diagnoses and treatments. It is very important
that
the laboratory and/or hospital or office personnel responsible should take
care to
prevent mix-ups from occurring.
CA 02326039 2000-11-16
2
[0004] The most effective way to avoid such problems is to establish a strict
chain of custody for each laboratory sample. Generally, a chain of custody
process
tracks or audits the sample as it passes through each step. To establish and
evidence chain of custody, laboratories commonly use specialized chain of
custody
forms requiring each and every custodian of a particular sample to certify
certain
information. For example, the person who collects the sample typically
certifies
his or her identity and the time and date on which the sample was collected.
The
courier, common carrier or other entity transporting the sample from the
collector
to the laboratory may provide, on the same or different form, a certification
establishing courier identity, how long the courier had the sample, etc. Seals
applied to the sample or its container can establish that no one has tampered
with
the sample in transit. The laboratory receiving the sample may complete an
additional portion of the chain of custody form to evidence when the sample
was
received and by whom, when the test was performed and by whom -- all of which
information can be used to establish a very strict evidentiary basis and
assurance
that the sample has not been mixed up with another sample, has not become
stale
or spoiled, and has not be tampered with. Commonly-assigned U.S. Patent No.
5,976,014 to Petrick et al. entitled "Integrity Seal Form/Label Combination"
discloses a particularly advantageous business form that can be used to
provide
evidence of chain of custody and absence of sample tampering.
[0005] While existing chain of custody forms and associated procedures are
often successful in establishing a rigorous chain of custody that virtually
eliminates
the possibility of sample mix-ups and tampering, a problem still remains that
such
existing forms and associated procedures are typically time-consuming to
follow
and complete. Commonly, each and every custodian of the sample must take the
time to complete all of the information required on the chain of custody form.
CA 02326039 2000-11-16
3
There is often no backup source for missing information -- requiring a new
sample
to be collected and re-tested. In life-threatening or other time-critical
situations,
the time required for re-testing may mean the difference between a favorable
or an
unfavorable outcome. In legal contexts, it may be impossible to go back and
re-test -- so that even a single missing piece of information on the chain of
custody
form might be outcome-determinative. In addition, chain of custody forms and
procedures (like all human endeavors) are subject to human error.
[0006] Some ability to perform automatic backup checking and/or to
automate some of the chain of custody processes would be highly desirable.
While
computers and other automation equipment have been used in the past in
connection with chain of custody processes, many such arrangements require
extensive data entry and/or optical scanning technology to enter data into any
of
several computer systems used to track the sample and the form. Accordingly,
further improvements are possible and desirable.
[0007] The present invention overcomes these problems by providing a new
chain of custody form and/or associated label that includes a radio frequency
identification chip. The radio frequency identification (RFID) chip allows
pertinent data to be monitored and filed electronically -- minimizing the need
for a
mufti-part form and reducing consumable costs to the customer.
[0008] In accordance with one aspect of the invention, data is tracked
automatically using the RFID chip. The chip/or processes associated therewith
can
prompt a data collector to enter the data correctly. An unbroken chain of
custody
can be automatically maintained accurately through automated means. The RFID
chip allows data to be shared electronically with various parties (e.g.,
employer,
collection site, medical review officer, and third party administrator)
without need
to rekey the information. Automatic entry of the data may occur upon the
CA 02326039 2000-11-16
4
specimen entering the laboratory. Since the RFID chip allows for complete
tracking of the specimen, loss of samples in transit or at a lab is minimized
and
positive identification becomes more reliable.
Brief Descriution Of The Drawings
[0009] These, as well as other features and advantages of this invention, will
be more completely understood and appreciated by careful study of the
following
more detailed description of presently preferred example embodiments of the
invention taken in conjunction with the accompanying drawings, of which:
[0010] Figure 1 shows an example preferred embodiment chain of custody
data collection system using radio frequency identification chip capabilities;
[0011] Figure 2 shows an example chain of custody data logging operation;
and
[0012] Figures 3A and 3B show example chain of custody forms.
Detailed Descriution Of Presently Preferred Examule Embodiments
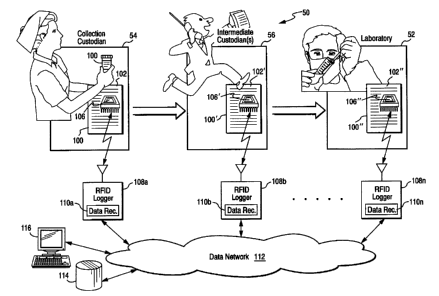
[0013] Figure 1 shows a chain of custody data collection system 50 provided
by an example preferred embodiment of the present invention. This example
system 50 is described, for purposes of illustration, in the context of
establishing a
chain of custody for medical samples being delivered to a laboratory 52 for
analysis, but the invention is not to be limited to this particular example.
[0014] In the example system 50 shown, a collection custodian 54 such as a
nurse or an employer may collect a sample 100 to be tested. The sample may be,
for example, a specimen of bodily fluid (e.g., blood, urine, etc.) that the
collection
custodian 54 seals into a conventional plastic container and matches to a
chain of
custody form 102 such as described in the above-referenced U.S. Patent No.
CA 02326039 2000-11-16
5,976,014. The collection custodian 54 may complete certain information on the
chain of custody form 102 using manual and/or automated means to establish the
time and date of collection; the name, medical record number and/or other
identity
of the owner; and other information associated with the sample 100 or its
collection.
[0015] Unlike in the conventional case, the chain of custody form 102,
sample 100 container, and/or associated label is provided with a radio
frequency
identification (RFID) chip 106. RFID chip 106 is of a conventional design and
may include, for example, a conventional active or passive radio frequency
transponder that, when queried by an RFID logger 108, responds by wirelessly
providing a unique identification value such as a serial number or other
arbitrary
unique or other distinctive identifier that distinguishes that particular RFID
chip
(and thus the form 102 and/or sample 100 associated therewith) from other
forms
and/or samples. Most such conventional RFID chips 106 comprise passive devices
that respond with a characteristic signature indicating an identifying number
or
string upon being exposed to radio waves of the appropriate frequency. Other
such
known RFID chip arrangements are able to store information sent to them, and
to
later reveal the information in response to a query. Still other such devices
operate
based on mechanisms other than radio frequency (e.g., light waves, sound
waves,
etc.). The present invention is intended to encompass all such variations.
[0016] As shown in Figures 3A and 3B, one particularly advantageous
arrangement is to embed the RFID chip 106 within the form 102 and/or within an
associated adhesive label so that it is permanently associated with the form
and/or
label. In this particular example, to de-associate the RFID chip 106 from the
associated form 102 one would need to tear (i.e., destroy) the form -- which
destruction will be evident immediately upon inspection of the form. Through
this
CA 02326039 2000-11-16
6
mechanism, it is difficult if not impossible in the preferred example
embodiment to
lose, de-associate or otherwise tamper with the association between RFID chips
106 and associated forms 102. Furthermore, the form or label described in the
above-referenced U.S. Patent No. 5,976,014 with RFID chip 106 affixed thereto
can be used to positively associate form or label 102 including RFID chip 106
with
a particular sample container 100 so as to establish a positive, permanent
association between the RFID chip and the collected sample.
[0017] Figure 2 is a flowchart of an example data collection process 200 that
may be performed by RFID logger 108 shown in Figure 1. In the Figure 2
example, the RFID logger 108 may query the RFID chip 106 (block 202) to
receive the RFID chip's identification (and other) information and/or to send
information to the RFID chip. The RFID logger 108 (and/or the RFID chip 106 in
some arrangements) may log this identification information along with date,
time
and other pertinent information (block 204).
[0018] In one example embodiment, RFID logger 108 may prompt the
collection (or other) custodian 54 to input additional required information
either
manually (e.g., my writing the information onto form 102 using a pen or
pencil)
and/or automatically (e.g., by inputting information into a computer
workstation or
other electronic device via a keyboard, barcode scanner, optical character
reader,
speech recognition device and/or other data input means) (block 206). This
additional information may become part of form 102 and/or a data record 110
that
RFID logger 108 (and/or chip 106) records. RFID logger 108 may record the
collected information onto form 102 and/or in an associated data record 110
(block
208) -- which data record is associated with the particular RFID chip 106.
[0019] Typically, the collection custodian 54 may, after the data collection
process is complete, provide the sample 100 and form 102 (or one or more parts
CA 02326039 2000-11-16
7
thereof in the case of a mufti-part form) with associated RFID chip 106 to an
intermediate custodian 56. In one example, intermediate custodian 56 might be
a
courier entrusted to deliver sample 100 to a laboratory 52. In other contexts,
intermediate custodian 56 might be a storage facility such as a records room,
some
other intermediate handler of the sample, or any other person or entity
entrusted
with temporary custody of sample 100. There may be any number of intermediate
custodians 56.
[0020] In the example embodiment shown in Figure 1, intermediate custodian
56 also has an associated data logger 108 that may query the RFID chip 106 and
provide an additional data collection process as shown in Figure 2 --
resulting in
additional information being provided on form 102 and/or an additional data
record
110 that is associated with (and may or may not be stored by) RFID chip 106
and
thus also with sample 100.
[0021] In the example embodiment, the sample 100 and associated form 102
eventually reaches a laboratory 52 for analysis. In this example system 50,
laboratory 52 (as the final link of the chain of custody) also -- like all of
the other
custodians before it -- has an RFID logger 108 that queries the RFID chip 106
associated with sample 100 and performs a data collection and recording
process
as shown in Figure 2. Laboratory 52 may thus perform automatic logging of
sample 100 based on the same identification supplied by RFID chip 106 that the
collection custodian 54 (and all intermediate custodians 56) used in their own
respective logging processes.
[0022] As shown in Figure l, one interesting capability provided by system
50 is the ability to exchange data records 110 between custodian sites. For
example, each RFID logger 108 may be coupled to the Internet, an enterprise
intranet, a local or wide area network, the telephone network, or other data
network
CA 02326039 2000-11-16
8
112. Data network 112 allows the various data loggers 108 to share
automatically
collected information and/or record the collected information to a centralized
or
distributed database facility 114 for archival and management purposes. Data
network 112 allows data records 110 associated with an RFID chip 106 to
"follow"
the RFID chip in the sense that any node connected to the network may (if
authorized) access a record tagged to the RFID chip. This capability allows
the
data record 110 to travel with the RFID chip 106 even though the RFID chip
may,
in some embodiments, contain no data storage capability of its own beyond an
identification string. The centralized or distributed database 114 may be used
to,
for example, independently establish a chain of custody of sample 100 based on
the logging information obtained from RFID chips 106. In one example
embodiment, a data terminal 116 (e.g., a workstation, browser-based appliance
or
other display and data input means) may be used to review and/or print
electronic
chain of custody audit trail developed by a network of RFID loggers 108. Such
a
chain of custody audit trail can be used to substantiate the information
provided on
form 102 and/or to independently establish a chain of custody for sample 100.
In
one example, a seal affixed to sample 100 into which RFID chip 106 has been
embedded and the information automatically logged based upon the RFID chip 106
may be all that is required a sufficient chain of custody for certain
purposes.
[0023] Several types of forms 102 can be used with the RFID chip 106. For
example, the Figure 3A form comprises a conventional lab work request form
that,
by itself, does not include enough information to establish a chain of custody
for
the associated sample. In this particular example, RFID chip 106 is embedded
within a label affixed to the Figure 3A form. The chain of custody associated
with
sample 100 is in this case provided mostly or exclusively by automatically
monitoring and interacting with the RFID chip 106.
CA 02326039 2000-11-16
9
[0024] The Figure 3B form includes enough information to establish a strict
chain of custody for an associated sample. For example, the form requires the
donor, the collector, the temporary storage custodian and the laboratory
technician
to all supply and write down or print information establishing a chain of
custody
for the associated sample 100. In addition, the Figure 3B form includes a
separable label portion used for sealing an associated sample 100 container
with
the donor's initials and the date to prevent tampering. In this particular
embodiment, RFID chips 106 are providing within this label for each of three
sample 100 containers so as to automatically, independently and individually
track
the chain of custody of each sample container. Additional, RFID chips 106 may
be
provided in other portions of the label to, for example, provide laboratory
confirmation information, courier information and the like. The Figure 3B form
is
especially advantageous for use in drug testing in connection with potential
employment or the like.
[0025] The chain of custody form 102 with RFID chip 106 results in all data
being monitored and filed electronically -- minimizing the need for a mufti-
part
form and resulting in reduced consumable costs to the customer. Samples 100
are
no longer lost in transit or within laboratory 52, as the RFID chip 106 allows
for
complete tracking of the specimen. Positive location identification is always
available. All data may be tracked using the RFID chip 106 which may prompt
the
collector 54 or other custodian to correctly enter data. The chain of custody
is
always accurate and is never broken. All data may be shared with all parties
electronically including, for example, an employer, a collection site, a
medical
review officer, a third party administrator, etc. No re-keying of the
information is
necessary, eliminating multiple steps, reducing the potential for error, and
providing increased efficiencies and decreased processing time at reduced
costs.
CA 02326039 2000-11-16
1
[0026] While the invention has been described in connection with what is
presently considered to be the most practical and preferred embodiment, it is
to be
understood that the invention is not to be limited to the disclosed
embodiment, but
on the contrary, is intended to cover various modifications and equivalent
arrangements included within the scope of the appended claims.