Note: Descriptions are shown in the official language in which they were submitted.
CA 02326311 2000-11-17
D-20361-1
- 1 -
METHOD FOR APPLYING ACTIVATED OZONE IN PULP BLEACHING
FIELD OF THE INVENTION
This invention relates to a method for removing
lignin from wood pulp, and more particularly to a method
for bleaching lignocellulosic materials using activated
ozone.
BACKGROUND OF THE INVENTION
Most pulps obtained from wood pulping processes are
dark colored due to the presence of a small fraction of
lignin. This residual lignin should be completely
removed in the bleaching process to produce a high
brightness paper. Until recently, molecular chlorine,
sodium hypochlorite and chlorine dioxide were the main
reagents used in cellulosic pulp bleaching, in sequences
such as C/DEDED, CEHDED, CEHD, CEHDH and CEHED. The pulp
quality and the bleaching cost were the main factors that
determined the bleaching sequence to be used. However,
with the discovery of o~rganochlorinated substances in the
effluents, the pulp and paper industry is devoting
significant efforts in recent years to search for new
technologies that would reduce the discharge of these
substances together with the effluents.
It has been reported that the main source of
effluents comes from the cellulosic pulp bleaching
process by reaction of molecular chlorine with lignin
degradation compounds. See, C. Rappe et al., "On the
formation of PCDDs and PCDFs in the bleaching of pulp",
Pulp and paper Canada, August, 1989.
These chlorinated substances are difficult to
degrade naturally because they contain carbon-carbon
covalent bonds. From among a broad variety of these
materials, the substances 2,3,7,8-tetrachloro-dibenzo-
CA 02326311 2000-11-17
D-20361-1
- 2 -
furane (TCDF) and 2,3,7,8-tetrachloro-dibenzo-dioxine
(TODD) have shown to be bioaccumulative, potentially
toxic and not environmentally sound.
In response to the pressures resulting from the
discovery of these substances in the effluents, the pulp
and paper industry is searching for bleaching
technologies that allow the production of pulp having
good quality while generating an effluent having
qualities within the limits imposed by current
environmental laws. Thus, bleaching technologies have
developed to a point where they are consistently
searching for a reduction or elimination of molecular
chlorine by the employment of other reagent, such as
chlorine dioxide, oxygen, hydrogen peroxide, ozone,
enzymes and peracids. The use of these reagents led to
the bleaching sequences called ECF (Elemental Chlorine
Free) and TCF (Totally Chlorine Free).
However, such alterations do not seem to be
sufficient to meet governmental legislation and public
concerns over the environment. Closing the water circuit
for the processes used in the pulp and paper industry may
soon be a requirement. The employment of ECF and TCF
sequences has given the pulp and paper industry the
option of closing the water circuit in the bleaching
plant through partial or total re-circulation of the
effluent to the recovery cycle. Several worldwide kraft
pulp concerns are evaluating the closed circuit operation
aiming at achieving at reasonable cost the environmental
quality and acceptability of their products in the
marketplace.
Attempts have been made to control this problem. It
has been known that utilization of oxygen delignification
and/or secondary treatment of the effluent were the first
CA 02326311 2000-11-17
D-20361-1
- 3 -
measures taken after the detrimental effect of the
organo-chlorinated material on the aquatic organisms was
detected. The oxygen delignification stage can remove
about 40 to 50% of the residual lignin without
significantly affecting the carbohydrates. Therefore,
bleaching may be carried out with lower reagent
consumption, and consequently a lower discharge of
chlorinated organic matter into resulting effluent.
An important modification in the conventional
bleaching sequences is the decrease in molecular chlorine
use and the increase in chlorine dioxide consumption. In
most pulp mills, replacing molecular chlorine for
chlorine dioxide in the first bleaching stage carries out
this process modification.
It has been reported that the AOX (absorbable
organic halides) levels in the effluent are significantly
reduced by the implementation of oxygen delignification,
by the substitution of chlorine by chlorine dioxide, and
by the biological treatment of the effluent. Other
important environmental pollution indicators, such as
color, BOD and COD of the effluent, were also decreased
by the utilization of these procedures. See, J.W. Graves
et al., "Effect of chlorine dioxide substitution, oxygen
delignification and biological treatment on bleach plant
effluent", Tappi Journal, July 1993.
Ozone, a three oxygen based compound, is a strong
oxidative agent and highly reactive with lignin. The
first kraft pulp treatments were effected in low and high
consistencies and it was observed that the ozone
dissolution in the fibrous suspension was a determining
factor in the process. See, M. Byrd et al.,
"Delignification and bleaching of chemical pulps with
ozone: a literature review", Tappi Journal, March 1992.
CA 02326311 2000-11-17
D-20361-1
- 4 -
Work relating to the use of ozone in ECF and TCF
bleaching sequences has been performed by varying
reaction consistency employed and the dosage of ozone
applied. The ozone stage is preceded by a stage with
oxygen, in alkaline medium, and followed by a simple
alkaline extraction stage. The final bleaching can be
effected by chlorine dioxide (ECF sequences) or hydrogen
peroxide (TCF sequences) stages. See, Patent No. WO
91/18145 assigned to B.F. Gregg et al.
Also, U.S. Patent No. 4,959,124 to Tsai treats
softwood kraft pulp with the steps of chlorine dioxide
delignification (D), ozone bleaching (Z), alkaline
extraction (E, Eo, Ep, Eo) and dissolution.
Despite advances in the art, there is a need in the
industry for a better bleaching technology that is low in
operating cost, produces effluent having a lower
environmental impact and results in a final product of
similar or superior quality.
Ozone application according to all aforementioned
bleaching methods suffers from two major drawbacks.
These include: (1) low bleaching efficiency and (2) low
bleaching selectivity with regard to pulp lignin. The
ozone bleaching efficiency, defined as the units of kappa
number dropped across the ozone stage per kilogram of
ozone consumed, is usually too low to justify the high
ozone bleaching cost. On the other hand, the ozone
bleaching selectivity, defined as the ratio of units of
kappa number per units of viscosity dropped across the
ozone stage, is usually too low, thus impairing pulp
quality. Hence, there is a need to find alternative ways
to apply ozone in pulp bleaching so that the efficiency
and selectivity of the process are improved.
CA 02326311 2000-11-17
D-20361-1
- 5 -
OBJECTS OF THE INVENTION
It is therefore an object of the invention to
provide a novel method for pulp bleaching with ozone,
such that the overall efficiency and selectivity of the
ozone treatment stage is improved. As a consequence, the
ozone consumption to reach a target kappa number is lower
as compared to conventional ozone bleaching methods. In
addition, the final pulp viscosity of the ozone treated
pulp obtained with the method of this invention is higher
than that achieved with conventional ozone methods.
It is a further object of the invention to provide a
new bleaching process containing an ozone stage which,
when compared to conventional pulp bleaching processes,
is more efficient and selective, produces an effluent of
lower environmental impact, and results in a final
product of similar or superior quality.
SUMMARY OF THE INVENTION
A method for bleaching lignocellulosic materials
with activated ozone, comprising a bleach sequence of at
least four sequential stages. The sequential stages
include an oxidative treatment stage, an alkaline
extraction stage, an activated ozone bleaching stage
performed in the presence of a mixture of ethanol and
dimethyl sulfoxide (DMSO) in appropriate proportions, and
a final bleaching stage.
The oxidative treatment stage may be performed with
chlorine, chorine dioxide, ozone, hydrogen peroxide,
peracids or any other oxidant, under conditions that are
well known to the skilled in the art.
The alkaline extraction stage may be performed with
any source of alkali, preferably sodium hydroxide, under
conditions conventionally used in the pulp industry.
CA 02326311 2000-11-17
D-20361-1
- 6 -
The activated ozone bleaching stage comprises the
acidification of the pulp with a mineral acid to render
its pH to a value in the range of from about 1.5 to about
5, the treatment of the acidified pulp with an
ethanol/DMSO additive mixture, the treatment of the
additive treated pulp with ozone, and the subsequent
neutralization of the ozone treated pulp with alkali to
render the pulp pH to a value in the range of from about
to about 10.
The activated ozone bleaching stage is carried out
at a reaction consistency of from about to to about 150,
at a reaction temperature of from about 20oC to about
90oC, and at a reaction time of from about 1 to about 120
minutes. The mineral acid dose ranges from about 0.5o to
about 4%, the ethanol dose ranges from about 0.001 to
about 200, the DMSO dose ranges from about 0.001 to about
80, the ozone dosage ranges from about 0.1% to about l.Oo
and the alkali dose ranges from about 0.5 to about 30.
All dosages are based on dry pulp fiber weight.
The activated ozone treated pulp is then washed
and/or directly conveyed to the final bleaching
operation, wherein it is further treated with chorine
dioxide and/or hydrogen peroxide, in one or more steps,
under conditions conventionally known to the skilled in
the art, to render a product of desired final quality.
For purpose of this invention, "kappa number" shall
mean the number of milliliters of a O.1N KMn04 solution
consumed by 1 gram of bone dry pulp and correlates with
the pulp bleachability. Viscosity is an indirect
measurement of the average degree of polymerization of
the pulp cellulose chains and correlates with the pulp
strength properties.
CA 02326311 2000-11-17
D-20361-1
For purpose of this invention, "efficiency" shall
mean the number of kappa units (kappa numbers) dropped
across the (ZE)-stage per kilogram of ozone applied
(kg/t) .
For purpose of this invention, "selectivity" shall
mean the percent kappa number divided by the percent
viscosity dropped across the (ZE)-stage. The values of
pulp kappa number, viscosity and brightness were measured
according to Tappi standard procedures.
BRIEF DESCRIPTION OF THE DRAWINGS
Other objects, features and advantages will occur to
those skilled in the art from the following description
of preferred embodiment and the accompanying drawing.
Figure 1 is a schematic representation of a sequence
of bleaching stages that shows the preferred mode of
activated ozone application according to the process of
this invention.
DETAILED DESCRIPTION OF THE INVENTION
This invention may be accomplished by pulp treatment
with activated ozone after it has been treated with an
oxidizing agent and extracted with alkali. After the
activated ozone treatment, the pulp is bleached with
chlorine dioxide and/or hydrogen peroxide until the
desired brightness is achieved.
Unlike the work performed previously in this area,
in the present invention, the ozone stage is activated
with a mixture of organic solvents, namely, ethanol and
dimethyl sulfoxide (DMSO) in appropriate proportions.
The ethanol/DMSO mixture act synergistically in the ozone
stage improving both the efficiency and selectivity of
ozone reaction with the pulp.
The role of the ethanol is to improve the efficiency
of ozone bleaching by increasing the rate of lignin
CA 02326311 2000-11-17
D-20361-1
- g _
removal (kappa units removed/ mass unit of ozone
consumed). Ethanol functions as a free radical scavenger
during ozone bleaching by capturing certain free
radicals, namely hydroxyl and superoxide, which
propagates ozone decomposition reactions. By capturing
these free radicals, ethanol minimizes ozone losses in
undesirable reactions, thus increasing overall process
efficiency.
On the other hand, the role of the DMSO is to
improve the selectivity of the ozone stage by decreasing
the rate of carbohydrate degradation (viscosity units
lost/kappa unit removed). Dimethyl sulfoxide (DMSO) has
the property of both functioning as a free radical
scavenger and increasing the uniformity of ozone contact
with pulp fibers. Consequently, it protects pulp
carbohydrates against degradation. DMSO is known to
disrupt the ordered water structure by breaking the
intra-molecular hydrogen bonds and complexing with water.
A change in water structure enhances the diffusion rate
of ozone into the water and, consequently, reduces the
build up of ozone concentration in localized areas.
The use of the organic solvent mixture
(ethanol/DMSO) in the ozone stage has the unexpected
effect of synergistically improving both efficiency and
selectivity of the ozone stage at the same time. The
organic solvents must be added to the pulp together so
that the synergistic effect can be obtained.
Furthermore, unlike the work performed previously in
this area, in the present invention, the treatment with
activated ozone is preceded by oxidation and alkali
extraction stages, said oxidation and alkali extraction
stages being followed by pulp washing. Thus, ozone does
not react with previously oxidized lignin compounds that
CA 02326311 2000-11-17
D-20361-1
- 9 -
are extracted by the alkali, nor with a pulp having a
high kappa number and a high content of transition metals
that are partially removed in the oxidation stage.
In accordance to this invention, the activated ozone
reacts more efficiently and selectively with a pulp
having a low kappa number that contains a residual lignin
low in carry-over material and low in structures
containing free phenolic units (or "phenolic lignin").
"Carry-over material" is defined as non-oxidized and
oxidized organic matter coming with the pulp from
previous stages of the operation. "Phenolic lignin" is
defined as that fraction of the lignin incoming with the
pulp that contains a free phenolic hydroxyl group. Both
carry-over material and phenolic lignin are responsible
for the low efficiency and selectivity of the ozone
bleaching stage. The low content of carry-over material
and the low phenolic character of the pulp treated in
accordance with the process of this invention arise from
the fact that the pulp is previously treated with an
oxidizing agent and alkali in separate stages.
This invention shows that the activated ozone
bleaching stage is most. efficient and selective when
carried with a pulp that has been previously treated with
an oxidizing agent and extracted with alkali.
This invention relates to a method for bleaching
lignocellulosic material from non-wood fibers, hardwoods,
softwoods, their mixture, or recycled fibers. The
proposed bleaching method is comprised of a number of
stages with possible variants in and between the stages.
The present invention allows employment from different
types of lignocellulosic materials obtained by different
types of pulping processes. The lignocellulosic
materials may be treated with oxygen prior to the
CA 02326311 2000-11-17
D-20361-1
- 10 -
bleaching method of this invention. Oxygen treated
lignocellulosic materials are actually preferred in
relation to regular ones.
The first stage of the process involves pulp
treatment in an oxidative stage with an oxidant that is
aimed at dissolving transition metals, and attacking
lignin containing free phenolics units (or phenolic
lignin) and carry-over material incoming with the pulp
from previous stages.
The second stage of the process involves pulp
treatment in an alkaline extraction stage with any source
of alkali. This treatment aims at extracting and
solubilizing the compounds oxidized in the previous
oxidative stage. It has been suggested that a
nucleophilic substitution caused by the alkaline stage is
necessary to create new electrophilic attack sites in the
remaining lignin structure. See, "The chemistry of
delignification". A general concept: Part II", J.
Gierer, January, 1982, Holzforschung.
The third stage of the process, which is the novelty
of the present invention, consists of an activated ozone
treatment (aZE) of the pulp in acid medium, under
conditions that result in maximum decrease of the pulp
kappa number with a minimal degradation of its
carbohydrates. Among these conditions it is included the
addition of a mixture o,f ethanol/DMSO to the pulp prior
to the ozone reaction. The ethanol/DMSO mixture acts
synergistically so as to maximize ozone reaction with
pulp lignin and minimize ozone reaction with pulp
carbohydrates.
The activated ozone treatment stage is effected at a
consistency of from about 1o to about 15o and a
temperature of from about 20°C to about 90°C, for reaction
CA 02326311 2000-11-17
D-20361-1
- 11 -
periods of from about 1 to about 120 min, with ozone
doses of from about O.lo to about l.Oo based of pulp
fiber dry weight. The ethanol/DMSO mixture is added to
the pulp immediately before ozone injection. Ideally,
the two solvents should be added together as a mixture.
The doses of DMSO may vary in the range of from about
O.Olo to about 8o based on pulp fiber weight and that of
ethanol in the range of from about O.Olo to about 200.
After ozone addition the pulp is neutralized with a
suitable alkali source, preferably sodium hydroxide, to a
pH in the range of from about 5 to about 10. This
neutralization is aimed at destroying the excess acidity
and solubilizing the degradation products of ozone
reaction with lignin.
The activated ozone treated pulp is then
subsequently bleached with chorine dioxide and/or
hydrogen peroxide, to render a product of desired
brightness. The process conditions for chorine dioxide
and hydrogen peroxide bleaching are well known to those
skilled in the art.
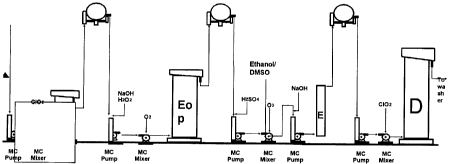
A preferred process of this invention is depicted in
Figure 1, which shows the bleaching sequence 10 of
DEop(aZE)D. The unbleached pulp 20 is fed into medium
consistency pump 30, and is then passed into medium
consistency mixer 32 with chlorine dioxide 62 prior to
proceeding to D-stage 34. All the pumps and mixers
referred to herein are of medium consistency. After
treatment and washing in washer 36, the pulp is mixed
with sodium hydroxide and hydrogen peroxide 64 prior to
pump 38, and is then mixed with oxygen 66 in mixer 40
prior to the Eop extraction stage 42. After washing in
washer 44, the pulp is mixed with sulfuric acid 68 and
proceeds to pump 46, where it receives the mixture
CA 02326311 2000-11-17
D-20361-1
- 12 -
ethanol/DMSO 70, and mixer 48, where the pulp undergoes
treatment with ozone 72. The activated ozone treated
pulp is then mixed with sodium hydroxide 74 and is
transferred to the neutralization tower 52 via pump 50,
completing the (aZE)-stage. The pulp is then washed in
washer 54 and pumped through pump 56 to mixer 58, where
it is treated again with chlorine dioxide 76 in D-stage
60. The resulting bleached pulp 78 is then passed to the
final washer.
The following examples will further clarify the
benefits of this invention:
Examples 1-8:
The pulp employed in this series of examples was
obtained from a kraft pulp mill, in the last washing
stage after the oxygen delignification, having a kappa
number of 9.1, a viscosity of 36.1 mPa.s and a brightness
55o ISO. Example 1 (reference) refers to a pulp
treatment with 0.40 ozone based on pulp weight at 100
consistency, 30°C and pH 2.5, followed by alkali
neutralization with 1.2.o NaOH at loo consistency, 60°C
and 30 min, and pulp washing with excess distilled water.
The combination of these chemical treatments is
designated from here on as the (ZE)-stage. Example 2
refers to the same treatments described in example 1,
except that 100 of ethanol based on fiber weight was
added in the ozone stage. Example 3 was effected under
the same conditions as example 1, except for the addition
of 4o DMSO in the ozone stage. Example 4 was performed
similarly to example l, except for the addition of a
mixture of loo ethanol and 4% DMSO in the ozone stage.
Examples 5-8 were done under the same conditions as
example 4, except for the dosages of the ethanol/DMSO
mixtures, which were varied in a wide range as shown in
CA 02326311 2000-11-17
D-20361-1
- 13 -
Table 1. The results of the examples 1 to 8 were
interpreted on the basis of (ZE)-stage efficiency and
selectivity and final pulp brightness. The values of
pulp kappa number and viscosity measured after the (ZE)-
stage were used to calculate the values of efficiency and
selectivity.
Table 1. Performance of the (ZE)-stage carried out with
and without additives for hardwood kraft pulp previously
delignified with oxygen,
Example Additive EfficiencySelectivityBrightness,
# $ ISO
1 No additive (reference)1.09 1.28 70.8
2 10$ EtOH 1.54 1.33 73.0
3 4$ DMSO 1.21 1.65 72.1
4 10$ EtOH + 4$DMSO 1.71 1.79 74.7
7.5$ EtOH + 3$DMSO 1.50 1.61 72.6
6 5.0$ EtOH + 2$DMSO 1.48 1.53 72.5
7 2.5$ EtOH + 1$DMSO 1.37 1.44 72.3
8 1.25$ EtOH + 0.5$DMSO1.23 1.37 71.0
Comparing examples 1 and 2, it is seen that ethanol
addition to the pulp prior to the ozone treatment
substantially increases the efficiency of the (ZE)-stage
while having only a slight effect on process selectivity.
On the other hand, a comparison of examples 1 and 3
indicates that the addition of DMSO prior to the ozone
treatment increases (ZE)-stage selectivity substantially
while slightly improving efficiency. A comparison of
examples 1, 2, 3 with example 4 shows that the efficiency
and selectivity gains derived from the addition of the
mixture ethanol/DMSO to the pulp prior to the ozone
treatment are more than additive. This suggests that
these two additives act synergistically to enhance ozone
reaction with the pulp. The gain in (ZE)-stage
efficiency obtained with the mixture of ethanol/DMSO
(570) is higher than the sum of the gains achieved with
CA 02326311 2000-11-17
D-20361-1
- 14 -
ethanol alone (410) and DMSO alone (llo) which adds up to
only 520. Also, the increase in selectivity derived from
the use of the mixture of ethanol/DMSO (40o) is higher
than the sum of the selectivity improvements caused by
the ethanol (40) or DMSO (290) alone, which amounts to
33% only.
It is obvious from examples 1 to 4 that ethanol is
effective to improve the efficiency of ozone reaction
with pulp lignin whereas DMSO minimizes ozone attack to
pulp carbohydrates, thus increasing selectivity. The
combination of these additives in a mixture improves both
efficiency and selectivity of ozone bleaching.
Furthermore, when these additives are added as a mixture,
their combined effects ,surmount the sum of each additive
individual effect. Hence, it is characterized a
synergism between the two additives to improve the
efficiency and selectivity of the ozone treatment.
It should be noted that the synergistic effect
appears also when evaluating pulp brightness. The sum of
the brightness gains achieved (in relation to the
reference) with ethanol alone (2.2% ISO) and DMSO alone
(1.3o ISO), which amounts to 3.5o ISO, is lower than the
3.9o brightness gain obtained when these additives are
added as a mixture in the ozone treatment.
Examples 5 to 8 further shows that the (ZE)-stage
efficiency, selectivity and brightness improvements
derived from pulp treatment with ethanol/DMSO mixture
prior to ozone treatment holds true even for additive
doses much lower than those described in the example 4.
However, the benefits of using the additive mixture tend
to decrease as its doses are diminished.
Bleaching of chemical pulp is usually effected in a
sequence of multiple stages. The (ZE)-stage will
CA 02326311 2000-11-17
D-20361-1
- 15 -
normally be one of the stages of such a sequence. It is
important to determine whether or not the enhanced
efficiency, selectivity and brightness gain obtained in
the (ZE)-stage, derived from the addition of the
DMSO/ethanol mixture to the pulp prior to the ozone
reaction, holds true when this stage is applied in a
sequence of multiple stages. In other words, it is
important to determine the impact of using such additive
mixture in the ozone stage on the total bleaching
chemical requirement and quality of the finally b eached
pulp. This is demonstrated through examples 9 to 13
shown below.
Examples 9 to 13:
The pulp sample employed in this series of examples
was obtained from a kraft pulp mill, in the last washing
stage after the oxygen delignification, having a kappa
number of 9.1, viscosity 36.1 mPa.s and brightness 550
ISO. The pulp was bleached using five different
bleaching protocols.
Example 9 refers to the DEopDD ECF bleaching
sequence that has been used commercially by many pulp
mills (reference). The first D-stage was carried out at
loo consistency, 60°C temperature, 30-min reaction and
final pH 3.0 (adjusted with sulfuric acid). The Eop-
stage was carried with l.ls NaOH, 0.5o OZ and 0.5% Hz02
based on fiber weight at loo consistency, 200 kPa
pressure, 90°C temperature, 90 min reaction and pH 11Ø
The second and third D-stages were carried out at l00
consistency, 70°C temperature, 180-min reaction and final
pH 3.8 (adjusted with sodium hydroxide). Pulp washing
between stages was effected with excess distilled water.
Evaluation of pulp final brightness and viscosity was
done according to Tappi~ standard procedures.
CA 02326311 2000-11-17
D-20361-1
- 16 -
Example 10 refers to a bleaching sequence using
ozone in the first stage of the process, (ZE)DEopD
sequence. The (ZE)-stage was performed with 0.40 ozone
based on pulp weight at loo consistency, 30°C and pH 2.5,
followed by pulp treatment with 1.2o NaOH at 10%
consistency, 60°C and 30 min, and pulp washing with
excess distilled water. The first and second D-stages
and the Eop-stage were performed under the same
conditions and using the same procedures described in
example 9.
Example 11 refers to the same sequence of Example
10, except that the ozone treatment was activated with a
mixture of ethanol/DMSO. All bleaching stage conditions
were kept the same, except those of the (ZE)-stage where
a mixture of loo ethanol and 4% DMSO was added to the
pulp slurry prior to the ozone treatment.
Example 12 refers to an ECF bleaching sequence using
ozone in the third stage of the bleaching process,
DEop(ZE)D sequence. This sequence is exactly the same as
the one described in example 10, except for the fact that
the (ZE)-stage was re-located from the first to the third
stage of the sequence. . All process conditions and
procedures were kept the same as described for example
10.
Example 13 refers to the same sequence depicted in
example 12, except that a mixture of ethanol/DMSO was
added to the pulp prior to the ozone treatment. The
stage-by-stage conditions used in the various bleaching
stages were the same as described for example 12.
Table 2. Effect of ozone stage activation with a
mixture of ethanol/DMSO on overall performance of the
sequences (ZE)DEopD and DEop(ZE)D applied to an oxygen
delignified hardwood kraft pulp.
CA 02326311 2000-11-17
D-20361-1
- 17 -
Exam- SequenceZ- Final Final ClOz ClOz/03
Consumption,
$
on
ple stage Bright,Visco- pulp Repla-
# weight
Addi- ISO sity, cement
tive mPa.s Ratio
Do D1 D2 Total
9 DEopDD 90.1 15.4 0.6920.7250.1251.542-
(ZE)DEopDnone 90.0 11.8 0.3810.679- 1.0601.20
11 (ZE)DEopDEtOH/ 90.2 16.5 0.3810.419- 0.8001.86
DMSO
12 DEop(ZE)Dnone 90.0 13.5 0.3810.362- 0.7422.00
13 DEop(ZE)DEtOH/ 90.1 18.1 0.3810.102- 0.4822.65
DMSO
A comparison of examples 9 and 10 indicates that
ozone application in the first stage of the ECF bleaching
process decreases chlorine dioxide requirement in the
order of 1.2 kg ClOzper kg of ozone applied. However, in
this application mode ozone reduces pulp final viscosity
(240) in relation to the reference sequence, without
ozone. On the other hand, if ozone is applied in the
third stage, the replacement ratio of ozone for chlorine
dioxide is substantially increased. A comparison of
examples 9 and 12 shows that each kg of ozone applied in
the third stage displaces about 2 kg of chlorine dioxide.
Furthermore, the viscosity penalty derived from the ozone
treatment is much smaller in this case, being the final
viscosity value only 120 lower than that of the
reference.
Thus, the proper location of the Z-stage in the
bleaching sequence has a significant impact on its
efficiency and selectivity.
CA 02326311 2000-11-17
D-20361-1
- 18 -
Further and significant improvements on ozone stage
efficiency and selectivity is achieved through the
addition an ethanol/DMSO mixture to the pulp slurry prior
to the ozone reaction. This was clearly shown in
examples 1 to 8 (Table 1). It is important to note that
the benefits derived from the additive mixture are
maintained across the bleaching process. A comparison of
examples 10 and 11 shows that the additive mixture
improved the C102/03 replacement ratio from 1.2 to 1.86,
i.e., about 55o whereas the final viscosity of the pulp
was increased about 280. This same trend is observed by
comparing examples 12 and 13 where the use of the
additive mixture resulted in a 33o increase in the ClOz/03
replacement ratio and 34o increase in the final pulp
viscosity.
The final pulp viscosity obtained when the additive
mixture was used in the ozone stage (examples 11 and 13)
were actually higher than that of the pulp bleached with
the reference sequence (example 9). On the other hand,
the total amount of chlorine dioxide used in the
sequences containing the activated-ozone stage were
substantially lower than that of the reference, which
makes a good case for the so-called ECF-light bleaching,
i.e., ECF bleaching using lower quantities of chlorine
dioxide.
It is obvious from the examples 1 to 13 that the use
of ozone according to the process of this invention is
much more efficient and selective than with those of
conventional ozone bleaching methods. Also, the benefits
obtained in the ozone stage (ZE) carries through the
overall bleaching sequence. As a result, the total
requirement of chlorine dioxide to bleach the pulp is
substantially decreased and the pulp final viscosity is
CA 02326311 2000-11-17
D-20361-1
- 19 -
significantly increased. By the process of this
invention it is understood the addition of an
ethanol/DMSO mixture to the pulp prior to the ozone
treatment and the location of the ozone treatment in the
third stage of the bleaching sequence.
To further confirm the benefits of the process of
this invention, the experimental approach used for
examples 1-13 was applied to another type of
lignocellulosic material. In the previous examples a
hardwood kraft pulp previously delignified with oxygen
was used. Examples 14 to 17 were carried out with an
oxygen delignified softwood (spruce/pine) kraft pulp,
which is more typical of North American pulp mills.
Examples 14 to 17:
The pulp employed in this series of examples was
obtained from a softwood kraft pulp mill, in the last
washing stage after the oxygen delignification. The
sample had a kappa number of 18.9, viscosity 28.9 mPa.s
and brightness of 28.Oo ISO. Example 14 (reference)
refers to a pulp treatment with 0.30 ozone based on pulp
weight at 10% consistency, 30°C and pH 2.5, followed by
alkali treatment with 1.2o NaOH at loo consistency, 60°C
and 30 min, and pulp washing with excess distilled water.
The combination of these two chemical treatments is
designated from here on as the (ZE)-stage. Example 15
refers to the very same treatments expressed in example
14, except that l00 of ethanol based on fiber weight was
added to the pulp prior to the ozone treatment. Example
16 was effected under the same conditions as example 14,
except for the addition of 4o DMSO to the pulp prior to
the ozone reaction. Example 17 was performed similarly
to example 14, except for the addition of a mixture of
CA 02326311 2000-11-17
D-20361-1
- 20 -
loo ethanol and 4o DMSO to the pulp prior to the ozone
treatment.
The results of the examples 14-17 were interpreted
on the basis of (ZE)-stage efficiency and selectivity and
final pulp brightness. The values of kappa number and
viscosity measured after the (ZE)-stage were used to
calculate the values of efficiency and selectivity.
Table 3. Performance of. the (ZE)-stage carried out with
and without additives for softwood kraft pulp previously
delignified with oxygen
ExampleAdditive EfficiencySelectivityBrightness,
ISO
14 No additive (reference)1.15 1.27 37.7
15 10$ EtOH 1.55 1.29 40.1
16 4$ DMSO 1.20 1.65 38.2
17 10$ EtOH + 4$DMSO 1.73 1.81 41.5
A comparison between examples 14 and 15 indicates
that addition of ethanol to the ozone treatment
substantially increases the efficiency of the (ZE)-stage
while having only a slight effect on process selectivity.
On the other hand, a comparison between examples 14 and
16 shows that the addition of DMSO to the pulp prior to
the ozone treatment increases (ZE)-stage selectivity
substantially while slightly improving efficiency. A
comparison of examples 14, 15 and 16 with example 17
shows that the benefits of the ethanol and DMSO addition
to the ozone stage are more than additive, suggesting
that these additives act synergistically to improve ozone
bleaching performance. The increase in (ZE)-stage
efficiency obtained with the mixture of ethanol plus DMSO
(50.40) is higher than the sum of the gains achieved with
ethanol alone (34.80) and DMSO alone (4.30), which adds
up to 390 only. Also, the increase in selectivity
CA 02326311 2000-11-17
D-20361-1
- 21 -
derived from the use of the mixture of Ethanol/DMSO
(42.50) is higher than the sum of the selectivity
improvements caused by the ethanol (1.60) or DMSO (300)
alone, which amounts to 31.6$ only.
It is obvious from examples 14-17 that ethanol is
effective to improve the efficiency of the (ZE)-stage
whereas DMSO enhances (ZE)-stage selectivity. The
combination of these additives in a mixture improves both
efficiency and selectivity of the (ZE)-stage.
Furthermore, when the additives are added together, the
gains derived from each individual additive is surmounted
by the sum of their individual gains. Hence, it
characterizes a synergistic effect between the two
additives to improve the efficiency and selectivity of
the ozone treatment.
It should be noted that the synergistic effect
appears also when evaluating pulp brightness. The sum of
the brightness gains achieved with ethanol alone (2.40
ISO) and DMSO alone (0.5o ISO) which amounts to 2.9o ISO
is lower than the 3.8o brightness gain obtained when
these additives are added as a mixture in the ozone
treatment.
The benefits of adding the ethanol/DMSO mixture in
the ozone stage of multiple-stage ECF bleaching sequences
is shown in the examples 18 to 22. The benefits of such
a treatment are quantified by its impact of final pulp
viscosity and total chlorine dioxide requirement to reach
a target brightness of .90% ISO.
Examples 18 to 22:
The softwood pulp (spruce/pine) sample employed in
this series of examples was obtained from a kraft pulp
mill, in the last washing stage after the oxygen
delignification. The pulp had a kappa number of 18.9,
CA 02326311 2000-11-17
D-20361-1
- 22 -
viscosity of 28.9 mPa.s and brightness of 28.0% ISO. The
pulp was bleached using five bleaching protocols.
Example 18 refers to the DEopDD ECF bleaching
sequence that has been used commercially by many pulp
mills. The first D-stage was carried out at 100
consistency, 60°C temperature, 30-min reaction and final
pH 3.0 (adjusted with sulfuric acid). The Eop-stage was
carried with 1.1o NaOH, 0.5% Oz and 0.5o Hz02 based on
fiber weight at loo consistency, 200 kPa pressure, 90°C
temperature, 90 min reaction and pH 11Ø The second and
third D-stages were carried out at loo consistency, 70°C
temperature, 180-min reaction and final pH 3.8 (adjusted
with sodium hydroxide). Pulp washing between stages was
effected with excess distilled water. Evaluation of pulp
final brightness and viscosity was done according to
Tappi standard procedures.
Example 19 refers to a bleaching sequence using
ozone in the first stage of the process, (ZE)DEopD
sequence. The (ZE)-stage was performed with 0.30 ozone
based on pulp dry weight at 10$ consistency, 30°C and pH
2.5, followed by alkali treatment with 1.2o NaOH at 100
consistency, 60°C and 30 min, and pulp washing with
excess distilled water. The first and second D-stages
and the Eop-stage were performed under the same
conditions and procedures as described for example 18.
Example 20 refers to the same sequence of example
19, except for the fact that a mixture of 10% ethanol and
4o DMSO was added to the pulp slurry prior to the ozone
treatment. All other bleaching conditions were kept the
same as described in example 19.
Example 21 refers to an ECF bleaching sequence using
ozone in the third stage of the bleaching process,
DEop(ZE)D sequence. This sequence is exactly the same as
CA 02326311 2000-11-17
D-20361-1
- 23 -
the one described in example 19, except that the ozone
treatment was re-located from the first to the third
stage of the sequence. All process conditions and
procedures were kept the same as described for example
19.
Example 22 refers to the same sequence depicted in
Example 21, except that a mixture of loo ethanol and 40
DMSO was added to the pulp slurry prior to the ozone
treatment. The stage-by-stage conditions used in this
sequence were the same as in example 21.
Table 3. Effect of ozone stage activation with a mixture
of ethanol/DMSO on overall performance of the sequences
(ZE)DEopD and DEop(ZE)D for an oxygen delignified
softwood kraft pulp.
Exam- Sequence Z- Final Final C102 C102/03
Consumption,
$
on
ple stage Bright,Vis- pulp Repla-
# weight
Addi- ISO cosity, cement
tive mPa.s Ratio
Do D1 D2 Total
18 DeopDD 90.0 19.9 1.7250.7640.1912.680-
19 (ZE)DeopDnone 90.1 14.3 1.0061.114- 2.1201.87
20 (ZE)DeopDEtOH/ 90.1 18.5 1.0060.909- 1.9152.55
DMSO
21 DEop(ZE)Dnone 90.1 18.8 1.0060,884- 1.8902.63
22 DEop(ZE)DEtOH/ 90.0 23.9 1.0060.651- 1.6573.41
DMSO '
A comparison of examples 18 and 19 indicates that
ozone application in the first stage of the ECF bleaching
sequence, for the softwood pulp, decreases chlorine
dioxide requirement in the order of 1.87 kg C102 per kg
of ozone applied. However, in this application mode
ozone causes a penalty on pulp viscosity of about 280, in
relation to the reference sequence without ozone. On the
other hand, if ozone is applied in the third stage the
replacement ratio of ozone for chlorine dioxide is
substantially increased. A comparison of examples 18 and
CA 02326311 2000-11-17
D-20361-1
- 24 -
21 shows that each kg of ozone applied in the third stage
displaces about 2.63 kg of chlorine dioxide.
Furthermore, the viscosity penalty derived from ozone
application in the third stage is much smaller, being
only 50 lower than that of the reference.
Thus, the results for the softwood kraft pulp sample
also indicate that proper location of the Z-stage in the
bleaching sequence has a significant impact on overall
efficiency and selectivity of the bleaching process.
Significant improvements on ozone stage efficiency
and selectivity can also be achieved through the addition
of a mixture of ethanoh/DMSO to the softwood pulp slurry,
prior to the ozone reaction. This was clearly shown in
examples 14 to 17. What is important to note is that
these benefits are maintained across the bleaching
process. A comparison between examples 19 and 20 shows
that the use of such additive mixture improved in 36% the
ClOz/03 replacement ratio, from 1.87 to 2.55, whereas the
final viscosity of the pulp was increased about 290.
This same trend is observed by comparing examples 21 and
22 where the use of the additive mixture resulted in a
30o increase in the C102/03 replacement ratio and 27$
increase in the final pulp viscosity. The final pulp
viscosity obtained when the additive mixture was added to
the pulp prior to the ozone treatment (example 22) was
actually higher than that of the pulp bleached with the
reference sequence without ozone (example 18).
Furthermore, the total amount of chlorine dioxide used in
the sequences containing the activated ozone stage was
substantially lower than that of the reference.
Specific features of the invention, particularly as
provided in Figure l, are for convenience only, as each
feature may be combined with other features in accordance
CA 02326311 2000-11-17
D-20361-1
- 25 -
with the invention. Alternative embodiments will be
recognized by those skilled in the art and are intended
to be included within the scope of the claims.