Note: Descriptions are shown in the official language in which they were submitted.
CA 02327140 2000-11-30
Aventis Research & Technologies HOE 1999/F054 Dr.AC
GmbH & Co KG
Novel protein conjugates and process for the preparation thereof
Description
The invention relates to novel protein conjugates and a process for the
preparation
thereof. The novel protein conjugates comprise on the one hand a naturally
occurring, synthetically modified or recombinant protein, enzyme,
immunoglobulin,
antibody, receptor protein or lectin and on the other hand simultaneously
three
further low molecular weight compounds selected from the group consisting of
amines, carboxylic acids, isonitriles, aldehydes or ketones. The invention
further
relates to a process for preparing the novel protein conjugates by reacting a
protein
with the three low molecular weight components in a single reaction step, up
to four
reporter groups or ligands being introduced simultaneously in a defined
stoichiometry and molecular distance and to applications of the protein
conjugates
obtainable in this way.
Background:
Linkage products of proteins and low molecular weight compounds, so-called
protein
conjugates, are applied in many different ways within the life sciences. In
medicine
they include, for example, protein conjugates which can be employed
therapeutically
and which consist of, for example, an antibody and a cytostatic. In
diagnostics and
biochemistry protein conjugates are employed in a great variety of fields for
recognizing, labeling and quantifying the proteins themselves or their
molecular
receptors, messenger molecules and antagonists. In medical immunology protein
conjugates, in particular of carbohydrate and nucleic acid antigens, are used
for
immunization (vaccination). The protein may serve on the one hand as a vehicle
for
presenting antigens and for the targeted transport of one or more low
molecular
weight compounds; on the other hand it is possible to employ the low molecular
weight part of protein conjugates to specifically modify properties of the
proteins
themselves. The latter variant comprises, for example, specific modification
of the
physical properties of the protein, for example thermal stabilization or
solubility
characteristics of a catalytically active enzyme, catalytically active
antibody
(abzyme), therapeutically active protein or antibody or specific modification
of the
actual catalytic activity, specificity or general properties for recognizing a
substrate,
receptor or ligand. Thus the function of the protein part of the protein
conjugates may
CA 02327140 2000-11-30
2
extend from a mere vehicle (prodrug) via targeted transport (drug targeting)
or ligand
presentation (vaccines) to the catalytic activity or actual molecular
recognition.
Conversely, the function of the "low molecular weight" part of the protein
conjugates
extends from physical modification or stabilization via labeling using one or
more
characteristic and analytically quantifiable reporter groups (diagnostics) to
the actual
principle of action (e.g. in prodrugs) or multivalent ligands (e.g. in
vaccines).
Most physiologically active proteins are already in the form of protein
conjugates, in
particular as conjugates with carbohydrates (glycoproteins), which underlines
the
biological importance of carbohydrates as binding partners of proteins. They
are
primarily bound on the protein surface by serine, threonine and asparagine and
take
part, for example, in cell recognition. The artificial attachment of low
molecular
weight structures, in particular also further carbohydrates, to so-called
neoglycoproteins may be employed. for example, to specifically modulate cell
recognition and to study the biological phenomena it is based on.
The available repertoire of methods for linking carbohydrates, active
ingredients,
reporter molecules, dyes, antigens, ligands etc. to proteins is based
essentially on
the presence of reactive amino, carboxylate, thiol or aldehyde groups (after
oxidation) in proteins. Thus it is known already that proteins carrying free
amino,
carboxylate. thiol or aldehyde groups can be linked to low molecular weight
compounds which themselves carry free carboxylate or amino groups to give
protein
conjugates (Advances in Carbohydrate Chemistry and Biochemistry 37, 225-281,
1980). Free protein amino groups are in particular the N termini of the
proteins and
the s-amino groups of lysines whiich can be linked to low molecular weight
carboxylic
acids at least partially by amidation. Free protein carboxylate groups are in
particular
the C termini of the proteins and the carboxylate groups of the amino acids
aspartic
acid and glutamic acid, which are linked to low molecular weight amines at
least
partially by amidation. Cysteines present free thiol groups; free aldehyde
groups of
proteins are in particular oxidized carbohydrate components of proteins which
can be
linked to amines by reductive amination.
Numerous reagents are available as condensing agents for these reactions, in
particular those agents which imay be employed quite generally for amidation
reactions in organic synthesis (Novabiochem catalog, 1999, page 264 ff.) such
as,
for example, carbodiimides, in particular EDC (1-ethyl-3-[3-
dimethylaminopropyl]-
carbodiimide). Additionally, bifunctional coupling reagents having two
equivalent or
two orthogonal active groups are used either as crosslinkers or for
conjugation with
CA 02327140 2000-11-30
3
any additional component (low rnolecular weight compound, additional protein,
solid
phase). Suitable active groups thereof are maleimides, imido esters, pyridyl
disulfides, a-halo carbonyl compounds and aryl azides (Pierce catalog, 1997,
pages
133-154).
Enzyme labeling is needed, for example, in biochemistry for detection in
immunoassays (ELISAs), in immunohistochemistry, for Western blotting and for
DNA
or RNA hybridization assays. ,A variety of biochemical, analytical and
technical
applications use in particular peroxidase (immunoassays, immunoblots,
immunohistochemistry), alkaline phosphatase (immunoassays, immunoblots), [i-
galactosidase (immunoassays, immunoblots, immunocytochemistry of blood
samples) and glucose oxidase (immunohistochemistry, biosensors).
A typical example of the known procedure is the preparation of conjugates of
alkaline
phosphatase (AP) by reacting in an aqueous buffer solution the reagent
succinimidyl
4-[N-(maleimidomethyl)-cyclohexane]-1-carboxylate (SMCC) with free AP amino
groups to give activated AP. It is then possible in a second step for a low
molecular
weight compound or a protein, for example an antibody, to be linked to the
maleimide of the activated AP by free thiol groups. In another example,
horseradish
peroxidase (HRP) can bind to ligands or antibodies quite analogously.
Further known methods for preparing protein conjugates are reductive
amination, for
example by complex borohydrides or the reaction with reactive cumulative
multiple
bond systems. The latter include, for example, isocyanates. Thus so-called
neoglycoproteins are prepared, for example, from BSA (bovine serum albumin) by
reacting the protein with up to 200 equivalents of an isocyanate-modified
sialyl-
Lewis-X tetrasaccharide, yielding an epitope density of up to 16 (J. K.
Welply,
Glycobiology 4, 259-265, 1994). US 5,059,654 describes further known methods
for
linking proteins, in particular to solid phases such as polysaccharides.
Typical
processes for covalently linking polysaccharides to carrier proteins with the
aim of
vaccine production are based on analogous processes, in particular by
employing
bifunctional linkers (WO 99/18121 ). The following reference extensively
describes
common modification processes for glycoproteins and proteoglycans: B.Kuberan
et
al., Glycoconjugate J., 16, 271-281 (1999).
The mentioned known methods for preparing protein conjugates most often use
very
reactive electrophilic reagents (for example isocyanates, carbodiimides)
reacting with
the native nucleophilic groups of the protein (amine, carboxylate, thiol). In
order to
CA 02327140 2000-11-30
4
attach a nucleophilic antigen to a protein, for example for producing
synthetic
vaccines, a suitable bifunctional reagent more or less reverses its reactivity
by
amidating the nucleophile obtained after reductive amination of the
oligosaccharide
antigen, for example, using a succinimide active ester which for its part is
linked via a
linker to an electrophilic maleimide, which reacts subsequently with the
nucleophilic
thiol groups of the protein. Using the known coupling reagents it is in
principle
possible also to attach a plurality of reporter groups or ligands to the
protein,
provided that the latter react as a mixture or successively in multiple
process steps. It
would not be possible, however, to control the stoichiometry of the protein
conjugates to be prepared in this way because not all of the protein's
functional
groups have exactly the same reactivity. It would also be impossible to
control in this
way the molecular proximity of ligands or reporter groups to be introduced in
the
protein.
On the other hand novel protein conjugates containing up to four further
functional
groups (e.g. ligands, antigens) or analytically detectable groups (reporter
groups,
e.g. dyes) would be desirable. All of these groups ought to be linked to the
protein
simultaneously in a single simple process step providing an exactly defined
stoichiometry of the formed protein conjugate and a structurally defined
molecular
distance of the functional or analytical groups.
The novel proteins la-c may also be in the form of mixtures with the aim of
generating from an initial protein mixture, which was obtained from, for
example, a
cell or body fluid and fractionated by means of, for example, high-performance
liquid
chromatography (HPLC) or two-dimensional gel or capillary electrophoresis, a
specific detectable protein pattern significantly different from the pattern
of the
existing protein mixture owing to the performed modification.
The abovementioned methods for preparing protein conjugates using bifunctional
reagents cannot fulfill these technical demands. The same is true for specific
types
of reagents of higher functionality: thus, for example, sulfo-SBED (Pierce,
1997,
page 151 ) is a trifunctional molecule for preparing protein conjugates
combining in
one reagent an amino specific s~uccinimide ester, a biotin ligand and an
unspecific
photoreactive group (phenylazido).
Furthermore, Endeavour 78, 11 ~~ y 122 (1994) has already disclosed a reaction
of
low molecular weight amines with carboxylic acids, isonitriles and a carbonyl
compound to give low molecular weight peptides (Ugi reaction). For this,
soluble
proteins have not been used as amino, carboxylic acid or aldehyde components
up
CA 02327140 2000-11-30
to now. In particular, isonitriles have not been used as condensing agents in
combination with carbonyl compounds for this reaction up to now.
Description:
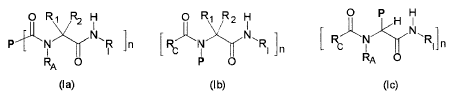
5 The invention relates to novel protein conjugates of the structures given in
the
formulae (la-c)
O R~ R2 H O R~ R2 H
N~
P~N N~R ]~ R~'~N- N~RI n [ R N R~,n
R O ~ n O ~ RA O
A
(la) (Ib) (Ic)
wherein P is a protein carrying free amino, carboxyl or aldehyde groups such
as an
albumin, immunoglobulin, antibody, avidin, streptavidin, hemocyanin, lectin,
enzyme
or serum glycoprotein,
and RA is a radical derived from amines Rq-NH2, where RA is an amino group-
carrying linear or branched alkyl or cycloalkyl radical of up to 18 carbon
atoms, an
amino group-carrying alkaloid, peptide or protein, carbohydrate, nucleotide,
nucleoside, steroid, terpene, porphyrin, chlorin, corrin, eicosanoid,
pheromone,
vitamin, in particular an n-(biotinamido)-n-alkyl radical where n = 2-8, an
antibiotic,
cytostatic, a dye molecule or a cryptand, in particular tris(bipyridinium)-
europium(III).
and R~ is a radical derived from carboxylic acids R~-C02H, where R~ is a
carboxyl
group-carrying linear or branched alkyl or cycloalkyl radical of up to 18
carbon
atoms, carboxyl group-carrying alkaloid, peptide or protein, carbohydrate,
nucleotide,
nucleoside, steroid, terpene, porphyrin, chlorin, corrin, eicosanoid,
pheromone,
vitamin, in particular biotin, an antibiatic, cytostatic, dye molecule or a
cryptand, in
particular tris(bipyridinium)-europium(III).
and R~ is a radical derived from isonitriles R~-NC, where R~ is an isonitrile
group-
carrying linear or branched alkyl or cycloalkyl radical of up to 18 carbon
atoms,
methoxycarbonylethyl, t-butoxycarbonylmethyl, phenyl, o-alkylphenyl, m-
alkylphenyl,
p-alkylphenyl, o-halophenyl, m~-halophenyl, p-halophenyl, 2,3-dihalophenyl,
2,4-
dihalophenyl, 3,4-dihalophenyl, o-alkoxyphenyl, m-alkoxyphenyl, p-
alkoxyphenyl, o-
arylphenyl, m-arylphenyl, p-arylphenyl, o-aryloxyphenyl, m-aryloxyphenyl,
p-aryloxyphenyl, o-nitrophenyl, m-nitrophenyl, p-nitrophenyl, 1-naphthyl, 2-
naphthyl,
CA 02327140 2000-11-30
6
benzenesulfonylmethyl, p-toluenesulfonylmethyl, pyranosyl radical, furanosyl
radical,
nucleosyl radical, n-(biotinamido)-n-alkyl radical Where n = 2-8 or a dye
molecule,
and R~ and R2 are a radical R~-C(=0)-R2 derived from carbonyl compounds and
can be simultaneously or independently of one another hydrogen, methyl, ethyl,
propyl, i-propyl, cyclopropyl, butyl, i-butyl, t-butyl, cyclobutyl, pentyl,
cyclopentyl,
hexyl, cyclohexyl, 2-methylbutyl, heptyl, octyl, nonyl, decyl, undecyl,
dodecyl,
methoxycarbonylethyl, t-butoxycarbonylmethyl, phenyl, o-alkylphenyl, m-
alkylphenyl,
p-alkylphenyl, o-halophenyl, m~-halophenyl, p-halophenyl, 2,3-dihalophenyl,
2,4-
dihalophenyl, 3,4-dihalophenyl, o-alkoxyphenyl, m-alkoxyphenyl, p-
alkoxyphenyl, o-
arylphenyl, m-arylphenyl, p-arylphenyl, o-aryloxyphenyl, m-aryloxyphenyl, p-
aryloxyphenyl, o-nitrophenyl, m-~nitrophenyl, p-nitrophenyl, 1-naphthyl, 2-
naphthyl,
oxiranyl, vinyl, propenyl, propen-2-yl, 2-penten-2-yl, 3-hepten-3-yl, penta-
1,3-dienyl,
phenylmethyl, 1-phenylethyl, 2-phenylethyl, cyclohexen-3-yl, cyclohexen-4-yl,
2-furyl,
3-furyl, 2-thienyl, 3-thienyl, 2-pyrrolyl, 3-pyrrolyl, halomethyl,
dihalomethyl,
trihalomethyl, 2-pyridyl, 3-pyridyl, 4-pyridyl,
and n = 1-15, preferably 1-10.
The invention further relates to a process for preparing the novel protein
conjugates
of the formulae (la-c) by reacting a protein (P) which is soluble or
immobilized on a
solid phase and which carries free amino, carboxylic acid or aldehyde groups
with an
amine RA-NH2, or carboxylic acid R~-C02H, isonitrile R~-NC and carbonyl
compound R~-C(=0)-R2, where RA, R~, R~, R~ and R2 are as stated above, in
aqueous solution to give the corresponding protein conjugates (la), (Ib) or
(Ic).
Here the respective structure variations (la-c) - as can be seen from the
structure
formulae - are obtained from the corresponding components as follows:
(la): from the protein (P), amine RA-NH2, isonitrile R~-NC and carbonyl
compound
R~-C(=O)-R2,
(Ib): from the protein (P), carboxylic acid R~-C02H, isonitrile R~-NC and
carbonyl
compound R~-C(=O)-R2,
(Ic): from the oxidized protein (P), carboxylic acid R~-C02H, amine RA-NH2 and
isonitrile R~-NC.
CA 02327140 2000-11-30
7
The process according to the invention is particularly suitable for reacting
proteins
(P) carrying free amino groups or free carboxylate groups or free aldehyde
groups.
Particularly suitable are the following proteins which are either free or
bound to a
solid phase:
- carrier proteins to be linked to haptens (peptides or carbohydrates) as
immunogenic epitopes, for example KLH (keyhole limpet hemocyanin), CRM
(nontoxic diphtheria toxin variants) or albumins such as bovine serum albumin
(BSA).
- native or recombinant proteins of microbial origin used in immunology, for
example proteins A, G or A/G, which recognize the Fc part of immunoglobulins,
for example in IgG. Furthermore, avidin and streptavidin and lectins such as,
for
example, concanavalin A or wheat-germ agglutinin (WGA) and peptidoglycans.
- immunoglobulins IgG, IgM, IgA, also in oxidized form, for example oxidized
IgG.
- enzymes such as, for example, pepsin, papain, trypsin, ficin, chymotrypsin,
lipases, esterases, oxidoreductases, transaminases, glycosidases, e.g. ~i-
galactosidase, glycosyltransferases, amidases, hydantoinases, horseradish
peroxidase (HRP), alkaline phosphatase (AP).
In the process according to the invention the protein (P) is reacted with the
amine
RA-NH2 or the carboxylic acid R~-C02H, and the isonitrile R~-NC and the
carbonyl
compound R~-C(=O)-R2 in such a way that the amine RA-NH2 or the carboxylic
acid
R~-C02H and the isonitrile R~-NC are added successively to a solution or
suspension of (P) in aqueous buffer containing the other components in at
least
molar amounts to the protein in each case. Here each of the required
components
amine RA-NH2, carboxylic acid R~-C02H, isonitrile R~-NC and carbonyl compound
R~-C(=0)-R2 are employed in a 10-10,000 fold molar excess over protein (P),
preferably in a molar ratio of 100-1,000, particularly preferably in a ratio
of 200-1000.
Suitable reaction solutions are aqueous buffers such as, for example, 0.001 -
1.0
molar solutions of sodium or potassium dihydrogen phosphate and disodium or
dipotassium hydrogen phosphatE~ or solutions of
tris(hydroxymethyl)aminomethane
and hydrochloric acid, and particularly suitable are buffer solutions for the
pH range
between 5 and 9, particularly preferably between pH 6 and pH 8.
In the process according to the invention methanol, ethanol, propanol, i-
propanol,
butanol, ethyl acetate, methyl acetate, dimethylformamide, acetonitrile,
dimethylsulfoxide or sulfolane may be added to the buffer as cosolvents in
quantities
CA 02327140 2000-11-30
8
of 0.1-20% by volume, depending on the solubility requirements of the
reactants.
The reaction temperature is between 0°C and 90°C, preferably
10°C to 40°C, and is
particularly preferably room temperature.
The crude protein conjugates (la-c) obtainable from the process according to
the
invention may be further purified by dialysis using aqueous buffer solutions
or pure
water and biochemical chromatography processes familiar to the skilled worker
and
then put to further use.
To detect the exact structure of the products (la-c), that is to analytically
determine
the mean number (n) of the components bound to the protein (P), the molecular
weight is directly measured by means of MALDI-TOF mass spectrometry or one or
more components bound to the protein are selectively determined. In this way
it is
possible, for example to measure the amount of a dye molecule introduced by
the
components RA-NH2, R~-C02t-I or R~-NC in a simple manner through the UV
extinction. An analogously introduced nucleotide may be quantified according
to the
familiar biochemical methods, where appropriate after amplification by means
of
PCR methods.
The resulting calculated number (n) describes the stoichiometry, i.e. the so-
called
mean epitope density on the protein (P) of formulae (la-c).
Thus the process according to the invention can be carried out in practice,
for
example, such that initially a protein (P) such as, for example, bovine serum
albumin
is dissolved at room temperature (appr. 20°C) in 0.1 molar phosphate
buffer (pH 7.5)
containing the carbonyl compound R~-C(=O)-R2 in a molar ratio of 100 - 4000.
Subsequently, RA-NH2 or R~-C;02H and R~-NC are added successively to this
solution in the appropriate molar ratio of 100 - 4000 (relative to P). After a
reaction
time of two days at room temperature, excess low molecular weight reaction
components are removed by dialysis using buffer or pure water or by
chromatography. The solution or suspension of the novel protein conjugate
obtainable in this way is then analytically characterized as described above.
Variation of the total of 4 reaction components 1. amine RA-NH2, 2. carbonyl
compound R~-C(=O)-R2, 3. carboxylic acid R~-C02H and 4. isonitrile R~-NC, the
protein (P) being employed either as an amine RA-NH2, carbonyl compound R~-
C(=O)-R2 or as carboxylic acid R~-C02H depending on the selection of the
remaining 3 reaction components employed in excess, leads to manifold
possibilities
CA 02327140 2000-11-30
9
of attaching almost any components to (P), up to four different types of
ligands (e.g.
biotin) and/or reporter groups (e.g. dyes) being introduced at an exactly
defined
stoichiometric ratio of 1:1:1:1 and a defined molecular distance relative to
each other
and relative to the protein by varvying the respective radicals R. It is
possible here to
adjust the molecular ratios further by selecting the spacer lengths in the
radicals RA,
R1, R2, RC and R~.
The following formulae illustrate a small selection of the various
possibilities resulting
therefrom, for example for conjugating 2 different dyes (F1 and F2) and any
further
ligand, for example, biotin (B):
O F~ R2 H O R~ B H ~ P H H
N
p~N N~ Z ~n F~~N~/ N~F2 ~ [ F~ N \F~n
B O ~ p ~ B O
O R~ F H O R~ F2 H O P H
P N 2 N~g ]n B~ .N~ NSF n B/ \N H NSF ln
I ~ ~_ ~ I 2J
F~ O p O F~ O
(la) (Ib) (Ic)
One example is, for example, to use a dye label as carboxyl component RC-C02H
and a biotin ligand as amine component RA-NH2:
0
N ~ Horseradish peroxidase
H
O
HN NH
H~H N H
%'~ ~ r
0
n
CA 02327140 2000-11-30
This example illustrates the appliication potential of the protein conjugates
according
to the invention and the simplicity of the process of their preparation: the
four
reaction components
1. Oxidized protein horseradish peroxidase as carbonyl component HC(=O)-R2,
5 2. Rhodamine dye label as carboxyl component R~-C02H
3. Biotin ligand as amine component RA-NH2
4. Cyclohexyl isonitrile as R~-NC
are combined simply in the desired stoichiometric ratio and reacted in aqueous
buffer under mild conditions. The outlined course of reaction and the
structure of the
10 novel protein conjugate can now be characterized exactly using independent
functional determination methods for the two reporter groups (biotin, dye):
thus,
molar mass determination by means of MALDI-TOF gives a mean epitope density of
n = 3.4 which is confirmed by a biotin ELISA assay (n = 3.3) and UV extinction
measurement (n = 3.5). The enzyme activity (residual activity 93%) is to a
large
extent retained in the enzyme assay.
Conversely, it would also be possible to employ rhodamine B as amine component
RA-NH2 via a suitable linker and biotin as carboxyl component R~-C02H, to
mention
only one of the various possible variations.
Thus it is possible for the stoichiometrically present reporter group to be
employed
for direct internal determination of another reporter group and vice versa,
thereby
improving the accuracy of the determinations. Thus, the dye in the
abovementioned
example may serve to determine the degree of biotinylation directly and
internally
and vice versa. This may be much simpler than the familiar methods for
determining
biotin in proteins which employ indirect methods: thus, for example, the HABA-
avidin
method (HABA = 2-hydroxyazobenzene-4'-carboxylic acid) uses binding to avidin
to
form a colored complex (absorption at 500 nm) which is displaced by free
biotin. A
plurality of measurement points form a standard curve from which the degree of
biotinylation is determined.
Thus it is also possible for example to use the protein conjugates (la-c)
according to
the invention in various ways in the biotin-avidin complex (ABC) system for
detecting
and quantifying antigens, for example on solid phases. This naturally also
includes
all biochemical assay methods based on this system such as, for example, the
classical ELISA assay, fluorescence-activated cell sorting (FACS), affinity
chromatography and Western blotting.
Proteins and enzymes frequently used in these systems are avidin,
streptavidin,
horseradish peroxidase, glucose oxidase and alkaline phosphatase. Frequently
used
CA 02327140 2000-11-30
11
dyes are, for example fluorescein, (FITC), rhodamine B and Texas red, but
variants
of these molecules having improved fluorescence properties may also be used in
accordance with the process according to the invention, for example
sulforhodamine
conjugates (US 5,846,737).
It is therefore possible for the protein conjugates (la-c) according to the
invention to
be integrated in various ways into the immunological methods used in clinical
diagnostics, for example, for monitoring plasma levels of analytes in the
blood,
checking hormonal control systems (thyroid function, fertility), detecting
viral
infections (hepatitis, HIV) or tumor-associated proteins (tumor aftercare).
Macromolecules such as peptides, proteins and nucleic acids are determined in
general by heterogeneous immunoassays (e.g. solid phase carrying an antibody),
while for low molecular weight substances homogeneous methods such as
fluorescence polarization assay (FPIA) or enzyme multiplied immunoassay (EMIT)
are used as well. Newer methods have an increased sensitivity by simultaneous
binding of the analyte (e.g. a tumor marker protein) to a monoclonal antibody
conjugated with a europium(III) complex and to a further antibody coupled to a
dye
molecule (allophycocyanin). According to the Forster theory, the absorbed
energy of
monochromatic light, due to the molecular spatial proximity of lanthanide
complex
and dye molecule, leads to a radiation-free energy transfer from the former to
the
latter during binding and thus causes a long-lived and amplified emission
(time-
resolved amplified cryptate emission assay, TRACE) (Bioforum, 1-2, 1998, 14-
16.).
Similarly, the spatial proximity of one or more dyes (multiple chromophoric
donor
groups) to a fluorescence acceptor dye may be used for signal amplification
(US 5,849,489). A particularly sensitive trace analysis of biomolecules is
based on
the so-called fluorescence cross correlation (FCS) spectroscopy comprising the
light
amplification by two fluorogenic dyes in defined spatial proximity. Excitation
by two
corresponding laser sources leads to energy transfers and amplified
fluorescence
emission of the labeled molecule, which is measured by photon detectors in a
confocal arrangement. Examples; of dyes used are rhodamine green and Cy5 (both
available from Amersham Life Sciences). R. Rigler et al., J. Biotechnol., 63,
97-109
(1998) describe the fundamentals of this method which was used to quantify a
few
copies of an amplified DNA.
Since the process according to the invention allows linking of up to 4
reporter groups
in defined stoichiometry and adjustable molecular distance, it is possible to
expect
from corresponding protein conjugates (la-c) having these types of dyes
(examples
of corresponding derivatives in US 5,849,489) a greatly increased sensitivity
in the
CA 02327140 2000-11-30
12
detection of the protein itself or of a bound ligand or of an analyte to be
determined
in an immunoassay.
The process according to the invention is suitable in particular also for
specific
conjugation of saccharides having a defined epitope density on the protein
(see
Examples). The latter may be determined, for example, in one of the reaction
components by the stoichiometric simultaneous attaching of a dye reporter
molecule.
This opens up the possibility of attacking in a controlled manner to, for
example,
suitable proteins such as CRM (nontoxic diphtheria toxin variant) or the KLH
protein
a plurality of saccharide antigens of, for example, various bacterial or viral
serotypes
(e.g. influenza, hepatitis) to givs~ vaccines of even higher valency or a
plurality of
tumor associated antigens for tumor vaccines.
The process according to the invention is further suited to covalently linking
one or
up to three different cytostatics to a protein, for example to a tumor-
specific antibody
or to an enzyme, for example to a glycosidase, or to a glycoprotein recognized
by a
cell-specific lectin such as, for example, asialoglycoproteins recognized by
liver cell
lectins.
The process according to the invention is further suited to reacting protein
mixtures
with the aim of creating a specifically detectable protein pattern after
appropriate
fractionation of this protein mixture in order to analytically characterize
the proteome
of an organism (proteomics). In this case, the process step according to the
invention is simply added to the sample preparation before developing the
usual two-
dimensional electrophoresis chromatogram (so-called 2-DE map). The usual
methods for visualizing the protein spots on gels as stationary phases employ
Coomassie blue or silver nitrate (staining) and fluorescent dyes. The proteins
are
fractionated in two dimensions according to their molar masses and isoelectric
points
(pl values), basic proteins being detected, for example, up to pl 9 and acidic
proteins
up to pl 4-5.
Proteins having a pl beyond these values are separated or detected less well
or not
at all. Here the process according to the invention offers a technical
solution for a
better fractionation and detection by reacting strongly basic proteins as
amine
component with a low molecular weight carboxylic acid R~-C02H, isonitrile R~-
NC
and carbonyl compound R~-C(=O)-R2, at least one of the latter three components
each containing, for example, dyes or biotin label. Analogously, strongly
acidic
proteins are reacted as carboxyl component with the amine RA-NH2, the
isonitrile R~-
CA 02327140 2000-11-30
13
NC and the carbonyl compound I~~-C(=O)-R2. Glycoproteins are reacted in
oxidized
form as carbonyl component with the amine Rq-NH2, the carboxylic acid R~-C02H
and the isonitrile R~-NC. Thus there are three novel possibilities of
generating
patterns of protein mixtures, it being possible to identify the newly formed
protein
groupings by the attached label. Consecutive application of all modifications
would
make it possible to simultaneously classify corresponding groups of proteins
on the
basis of their different labels.
The invention is explained in more detail by the following exemplary
embodiments.
Example 1
Preparation of (la) for P = bovine serum albumin (BSA), R~, R2 = CH3, R~ _
cyclohexyl, RA = 5-((i-D-glucopyranosyloxy)pentyl, n = 7.7.
A solution of bovine serum albumin (1 mg, 0.015 ~,mol) in water (1 ml), 5%
strength
acetone solution in water (67.2 ~,I, 45 ~,mol), 5-aminopentyl ~i-D-
glucopyranoside
(13.59 mg, 45 ~.mol) and 2 drops of cyclohexyl isonitrile are added to 3 ml
0.1 molar
phosphate buffer, pH 7.5. The solution is left at room temperature for 2 days
and
concentrated in an ultrafiltration cell. The crude protein conjugate is
subsequently
dialyzed once against 0.1 molar phosphate buffer, pH 7.5, and twice against
pure
water. The molar mass of the product is determined by means of MALDI-TOF mass
spectrometry: molecular weight: Ei9978 Da, i.e. a mean epitope density of
n=7.7.
0
O~~~N R Bovine serum albumin
NH
HO
HO O
HC~~O
OH n
Example 2
Preparation of (la) for P = bovine serum albumin (BSA), R~, R2 = CH3, RI =
cyclohexyl, RA = 5-(~i-D-glucopyranosyloxy)pentyl, n = 1Ø
CA 02327140 2000-11-30
14
Analogously to Example 1, bovine serum albumin (1 mg, 0.015 ~,mol), 5%
strength
acetone solution in water (2.24 yl, 1.5 ~,mol), 5-aminopentyl ~3-D-
glucopyranoside
(0.45 mg, 1.5 ~,mol) and 1 drop of cyclohexyl isonitrile are reacted in 0.1
molar Tris
buffer, pH 7.5. Result: molecular weight: 66898 Da, mean epitope density:
n=1.0; for
formula see Example 1.
Example 3
Preparation of (la) for P = bovine serum albumin (BSA), R~, R2 = CH3, RI =
methoxycarbonylmethyl, RA = 5-((i-D-glucopyranosyloxy)pentyl, n = 8.8.
Analogously to Example 1, bovine serum albumin (1 mg, 0.015 ~.mol), 5%
strength
acetone solution in water (22.4 g.l, 15 ~.mol), 5-aminopentyl (i-D-
glucopyranoside
(4.5 mg, 15 ~,mol) and 1 drop of methyl isocyanoacetate are reacted in 0.1
molar
phosphate buffer, pH 7.5. Result: molecular weight: 67948 Da, i.e. mean
epitope
density: n=3.4.
O
O
O ~~~N bovine serum albumin
~NH
Me0
HO
HO-~
HO O
OH n
Example 4
Preparation of (la) for P = bovine serum albumin (BSA), R~, R2 = CH3, RI =
cyclohexyl, RA = 5-(2-acetamido-~2-deoxy ~-D-glucopyranosyloxy)pentyl, n =
8.8.
Analogously to Example 1, bovine serum albumin (1 mg, 0.015 ~,mol), 5%
strength
acetone solution in water (44.8 ELI, 30 ~.mol), 5-aminopentyl 2-acetamido-2-
deoxy-~i-
D-glucopyranoside (10.28 mg, 30 ~mol) and 2 drops of cyclohexyl isonitrile are
reacted in 0.1 molar phosphate Ibuffer, pH 7.5. Result: molecular weight:
70480 Da,
i.e. mean epitope density: n=8.8.
CA 02327140 2000-11-30
0
~~~N bovine serum albumin
NH
HO
HO-~
HO O
NHAc n
Example 5
Preparation of (Ib) for P = bovine serum albumin (BSA), R~, R2 = CHg, RI =
5 cyclohexyl, R~ _ (2-O-acetyl-(3-D-glucopyranosyloxy)methyl, n = 9.9.
Analogously to Example 1, bovine serum albumin (1 mg, 0.015 ~,mol), 5%
strength
acetone solution in water (89.6 ~.1, 60 ~,mol), carboxymethyl 2-O-acetyl-a-D
glucopyranoside (19.1 mg, 60 ~.rnol) and 2 drops of cyclohexyl isonitrile are
reacted
in 0.1 molar phosphate buffer, pH 7.5. Molecular weight: 70975 Da, mean
epitope
10 density: n=9.9.
H
N Bovine serum albumin
N
O O
HO
HO O O
HCi
OAC
n
Example 6
15 Preparation of (Ib) for P = bovine serum albumin (BSA), R~, R2 = CH3, RI =
cyclohexyl, R~ = rhodamine B, n = 4.1-4.8.
Analogously to Example 1, bovine serum albumin (1 mg, 0.015 ~,mol), 5%
strength
acetone solution in water (67.2 txl, 45 ~.mol), rhodamine B (21.6 mg, 45
~.mol, in 400
~,I water) and 2 drops of cyclohexyl isonitrile are reacted in 0.1 molar
phosphate
buffer, pH 7.0, (3932.8 ul). Conjugate yield and mean epitope density are
determined
photometrically by measuring the extinctions at 280 nm (bovine serum albumin)
and
575 nm (rhodamine B).
Result after 1 day reaction time:
Bovine serum albumin: 7.4 nrnol Rhodamine B: 30.4 nmol
Conjugate yield: 49% Mean epitope density: n=4.1
Result after 2 days reaction time:
Bovine serum albumin: 1.2 nmol Rhodamine B: 6.0 nmol
CA 02327140 2000-11-30
16
Conjugate yield: 8.3% Mean epitope density: n=4.8
H
N
~~~N E3ovine serum albumin
O
~N I ~ ONO
n
Example 7
Preparation of (Ib) for P = bovine serum albumin (BSA), R~, R2 = CH3, RI = 1(i-
D-
glucopyranosyl, R~ = rhodamine B, n = 6.8-12.5.
Analogously to Example 1, bovine serum albumin (1 mg, 0.015 ~.mol), 5%
strength
acetone solution in water (67.2 ~~I, 45 ~,mol), rhodamine B (400 fig, 0.835
~.mol, in
400 ~.I water) and (i-D-glucopyranosyl isonitrile (1.9 mg, 10 ~mol, in 1 ml
water) are
reacted in 0.1 molar phosphate buffer, pH 7.0 (2932.8 ~.I). (i-D-
glucopyranosyl
isonitrile is freshly prepared by dissolving 2,3,4,6-tetra-0-acetyl-(i-D-
glucopyranosylisonitrile (3.6 mg, 10 ~,mol) in 5 ml methanol, 5 h stirring at
RT with
catalytic amounts of NaOMe, neutralizing by Dowex H+ ion exchanger, filtration
and
concentration of the filtrate. Conjugate yield and mean epitope density are
determined photometrically by measuring the extinctions at 280 nm (bovine
serum
albumin) and 575 nm (rhodamine B).
Result after 2 days reaction time:
Bovine serum albumin: 15 nmol Rhodamine B: 6.8 nmol
Conjugate yield: 100% Mean epitope density: n=0.45
Result after 4 days reaction time:
Bovine serum albumin: 12 nmol Rhodamine B: 12.5 nmol
Conjugate yield: 80% Mean epitope density: n=1.04
CA 02327140 2000-11-30
17
HO H
HO-_S~ N ~.
HO
OH ~ Bovine serum albumin
Example 8
Preparation of (Ib) for P = horseradish peroxidase (HRP), R~ = H, R2 = CH2CH3,
RI
= cyclohexyl, R~ = 5-(biotinoylamino)pentyl, n = 3.0
Analogously to Example 1, horseradish peroxidase (1.9 mg, 0.049 ~.mol HRP),
(+)-
biotinaminocaproic acid (14.5 mg, 45 ~,mol), propionaldehyde (2.4 ~.g, 0.05
~.mol)
and cyclohexyl isonitrile (2 drops) are reacted in 0.1 molar phosphate buffer,
pH 7.5
(4000 ~,I). Conjugate yield is determined photometrically by measuring the
extinction
at 280 nm. The residual activity of the peroxidase conjugate compared to
native
peroxidase is determined photornetrically by oxidation of an ARTS dye solution
and
extinction measurement at 404 nm. The degree of biotinylation is determined
quantitatively in a photometer by displacing HRP-BA from its avidin complex
and
measuring the extinction at 500 nm and qualitatively by an ELISA assay in
streptavidin-coated microtiter plates.
Result after 1 day reaction time:
Peroxidase conjugate: 36 nmol (75%) Residual activity: 62%
Biotin content: 108 nmol Epitope density: n=3.0
CA 02327140 2000-11-30
18
H
N
N--- Horseradish peroxidase
O
O 'D
HN~NH
H H
,,
O
n
Example 9
Preparation of (Ic) for P = ox-horseradish peroxidase (ox-HRP), Ri =
cyclohexyl,
R~ = rhodamine B, Rp, _ = 5-(biotinoylamino)pentyl, n = 3.3-3.4
Analogously to Example 1, NalU4-oxidized horseradish peroxidase (1.8 mg, 0.045
~,mol), (+)-biotinamidopentylamine (14.8 mg, 45 ~.mol), rhodamine B (21.6 mg,
45
~.mol) and cyclohexyl isonitrile (2' drops) are reacted in 0.1 molar phosphate
buffer,
pH 7.5 (4000 ~,I). Conjugate yield is determined photometrically by measuring
the
extinction at 280 nm (peroxidase conjugate) and 575 nm (rhodamine B). The
residual activity of the peroxidase conjugate compared to native peroxidase is
determined photometrically by oxidation of an ABTS solution and extinction
measurement at 404 nm. The degree of biotinylation is determined
quantitatively in a
photometer by displacing ox-HR;P-BA from its avidin complex and measuring the
extinction at 500 nm and qualii:atively by an ELISA assay in streptavidin-
coated
microtiter plates.
Result after 1 day reaction time:
Molecular weight: 46118 Da Epitope density: n = 3.4
Peroxidase conjugate: 4.8 nmol (11 %) Residual activity: 93%
Rhodamine B content: 16.8 nmol Epitope density: n=3.5
Biotin content: 16 nmol Epitope density: n=3.3
CA 02327140 2000-11-30
19
Horseradish peroxidase
O
HN~NH
H H
I
S
O
n
Example 10
Preparation of (Ic) for P = ox-horseradish peroxidase (ox-HRP), RI =
cyclohexyl,
R~ = 5-(biotinoylamino)pentyl, R~ _ = 5-(biotinoylamino)pentyl, n = 1.0
Analogously to Example 1, Na104-oxidized horseradish peroxidase (1 mg, 0.026
~mol), (+)-biotinamidopentylamine (14.8 mg, 45 ~.mol), (+)-biotinaminocaproic
acid
(14.5 mg, 45 ~.mol) and cyclohexyl isonitrile (2 drops) are reacted in 0.1
molar
phosphate buffer, pH 7.5 (4000 yl). Conjugate yield is determined
photometrically by
measuring the extinction at 280 nm. The residual activity of the peroxidase
conjugate
compared to native peroxidase is measured photometrically by oxidation of an
ABTS
solution and extinction measurE:ment at 404 nm. The degree of biotinylation is
determined quantitatively in a photometer by displacing ox-HRP-BA from its
avidin
complex and measuring the extinction at 500 nm and qualitatively by an ELISA
assay in streptavidin-coated microtiter plates.
Result after 1 day reaction time:
Peroxidase conjugate: 16 nmol (Ei7%) Residual activity: 157%
Biotin content: 16 nmol Epitope density: n=1.0
CA 02327140 2000-11-30
o
N r Horseradish peroxidase
H
O N' O
HN NH
H~H NH
~S~
~ NH
HN NH
H H
~vr
s n
Example 11
Proteome analysis: The soluble proteins of a microorganism, for example from
yeast
5 or E.coli cells, or a mammalian organism, for example from human blood
serum, are
obtained according to the familiar methods (cell lysis, extraction, washing in
buffers,
etc.). Instead of being directly applied to an electrophoresis gel the protein
mixture is
derivatized beforehand in aqueous buffer, quite analogously to the description
in one
of the Examples above. After two-dimensional fractionation the gel is
developed as
10 usual, for example by Coomassie blue, silver nitrate or a fluorescent dye.
If at least
one of the three employed low molecular weight reaction components is already
a
derivative of a suitable fluorescent dye, then the corresponding modified
proteins
may be detected directly in a reader. Image processing (e.g. by means of the
Biorad
program Melanie II, PC-NT) and the subsequent computer-based processing (i.e.
15 pattern recognition and analysis) are carried out as is usual for the
analysis of two-
dimensional electrophoresis chromatograms. Additionally, it is possible to
pick up the
gel spots of interest as usual by a gel picker and to analyze them in a mass
spectrometer after trypsin digestion (MALDI-TOF, ESI, MS-MS).