Note: Descriptions are shown in the official language in which they were submitted.
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361 -
NEW COMPOUNDS FOR DNA-TRANSFECTION
The present invention is concerned with novel compounds especially useful for
non-viral
introduction of biologically active molecules such as DNA, RNA, peptides or
proteins into
eukaryotic cells.
Non-viral systems have been developed to carry DNA into cells, e.g., the
transfection tech-
nique based on a cationic lipid, the dioleoyloxypropyl trimethylammonium
(Felgner et al.,
Proc. Natl., Acad. Sci. USA, 1987, 84, 7413-7417) commercialized as
LipofectinTM. Since the
discovery of this transfection technique, many more cationic lipids have been
synthesized and
some are commercially available as transfecting reagent for laboratory use:
DOGS (Trans-
fectamTM), DOSPA (LipofectamineTM), DOTAP (DOTAPT"'~.
Transfection of cells with oligonucleotides such as DNA can be used, for
example to express
in a host cell or organism, a protein which is not normally expressed by that
cell or organism.
For example, a DNA molecule called a plasmid may be introduced into a cell
that does not
normally contain the gene (s) encoded by that plasmid in order to express a
marker gene
product in that cell, or to express a protein of interest such as a
recombinant protein which is
later harvested from such cells (See Sambrook, et al., Molecular Cloning: A
Laboratory
Manual, 2nd ed. (Cold Spring Harbor, 1989), ch. 1.). The transfection of
oligonucleotides
into cells can also be used therapeutically. For example, antisense
oligonucleotides, once in
the cell or cell nucleus, bind to target single-stranded nucleic acid
molecules by Watson-Crick
base pairing or to double stranded nucleic acids by Hoogsteen base pairing,
and in doing so
disrupt the function of the target by one of several mechanisms: by preventing
the binding of
factors required for normal transcription, splicing, or translation; by
triggering the enzymatic
degradation of mRNA by RNAse H, or by destroying the target via reactive
groups attached
directly to the antisense oligonucleotide. (See Zamecnic et al., Proc. Natl.
Acad. Sci.-USA,
1978, 75, 280-284). Gene therapy or DNA based vaccination are other
therapeutic appli-
cations.
Proteins and other macromolecules are transfected into cells for therapeutic
and screening
purposes. For example, immunization is enhanced by introducing an immunogenic
protein
into a cell, so that it is more efficiently processed and presented on the
surface of the cells,
thereby enhancing the immunogenic response. Negatively charged macromolecules
which act
CONFIRMATION COPY
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361 _
-2-
inside a cell are transported past the cell membrane into the cytoplasm where
they exert their
effect. Factors which enhance or hinder transcription of DNA can be used in a
screening test
to verify the transcription of a gene of interest. These transcription assays
are very well
known for use in screening compounds to determine their effect against a
particular macro-
molecule, for example a cell receptor.
Cell-lytic antibacterial peptides that act by perturbing the barrier function
of membranes are
reviewed in Saberwal, et al., Biochim. Biophys. Acts, 1994, 1197, 109-131.
Certain fatty
acid-bearing basic peptides having antibacterial activity are disclosed in
Vogler, et al., Helv.
Chim. Acta, 1964, 47, 526-543. Poly(lysine-serine) random polymers for use as
carriers to
transport oligonucleotides into cells is disclosed in European Patent
Publication No. EP-A-
727 223.
European Patent Publication No. EP-A-784 984 and Legendre et al. (Bioconjugate
Chem.,
1997, 8, 57-63) describe conjugates of a lipid and a basic, membrane
disturbing peptide that
bind polynucleotides and anionic macromolecules can be used for transfection
of cells. The
peptide portion of the conjugate consists of natural amino acids linked by a
natural amide
binding.
Nevertheless, despite important progress in the formulation of non-viral gene
delivery sys-
tems, there remains a need for more efficient techniques, since the
transfection efficiency of
synthetic systems is usually below that of viral vectors. Furthermore, still
many problems
arise in vivo and in vitro due in part to the poor stability of the non-viral
systems in biological
fluids and culture media does not allow high and reproducible levels of
transfection.
Many known compounds used for transfection experiments are toxic for
eukaryotic cells. In
addition problems may occur in scaling up where the conditioned medium reduces
the effect
of known transfecting agents.
The present invention is directed to novel compounds which avoid disadvantages
associated
with known transfection agents.
In one aspect, the invention is concerned with novel compounds which are
conjugates of
lipids and a modified basic membrane disturbing peptide, characterized in that
the peptides
comprise a reversed amide backbone.
The term "conjugates" means compounds consisting of a lipid chemically bound
to the pep-
tide, e.g., via a disulfide bond formed between a sulfur atom present in or
attached to the lipid
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361
-3-
and a sulfur atom present in or attached to the peptide; or an amide bond
formed between the
carboxyl group present in or attached to the lipid and an amino group of the
peptide.
The term "lipid" as used herein comprises straight-chain, branched-chain,
saturated or un-
saturated aliphatic carboxylic acids and phospholipids. Examples of aliphatic
carboxylic acids
are lauric acid, palmitic acid, stearic acid, oleic acid and (CH3(CH2)")2CH
COOH, where n is
an integer from 3 to 19. Examples of phospholipids are
phosphatidylethanolamines such as
dioleoylphosphatidylethanolamine.
The term "basic peptides" denotes peptides containing at least one basic amino
acid.
Examples of such basic amino acids are natural and unnatural diamino-
monocarboxylic acids,
such as alpha-, beta-diaminopropionic acid, alpha-, gamma-diaminobutyric acid,
lysine,
arginine, ornithine and p-aminophenylalanine, etc.
The term "membrane disturbing peptides" denotes cell-lytic or antibacterial
peptides that
perturb the barrier function of membranes (G. Saberwal and R. Nagaraj, BBA,
1994, 1197,
109-131). Examples of basic, cell-lytic peptides are melittin, hemolysin,
mastoparan,
bombolitin, crabrolin and derivatives thereof. Examples of basic antibacterial
peptides are.
cecropins, magainins, gramicidin S and tyrocidine and derivatives thereof.
The term "derivatives" refers to peptides wherein one or more amino acid
residues are mis-
sing, have been added or have been replaced by another amino acid residue
without sub-
stantially changing the biological activity of the original peptide concerned,
i.e. allow trans-
fection of a macromolecule, preferably a polynucleotide, into a cell. The term
"derivatives"
also refers to peptides wherein the terminal carboxyl group is esterified,
particularly to form
lower alkyl esters such as the methyl and ethyl ester; or converted into an
amide, lower alkyl
amide or di-lower alkyl amide or hydrazide. The term "derivatives" also
relates to peptides
wherein the NH2-group of the N-terminus may be acylated to form an amide or a
lower alkyl
amide. In particular, the NH2-group is acetylated. The term "lower" denotes
groups contai-
ning from 1-6 carbon atoms.
The term "reversed amide backbone" refers to retro-peptides which
characteristically have the
same composition as its parent peptide, but the sequence is reversed, i.e., n,
... 3, 2, 1 instead
of 1, 2, 3, ... n when reading both in the N to C direction. Both have normal
peptide bonds.
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361
-4~-
Throughout this application the following standard abbreviations are used to
refer to amino
acids:
Alanine Ala A
Glutamate Glu
Glutamine Gln Q
Aspartate Asp D
Asparagine Asn N
Leucine Leu L
Glycine Gly G
Lysine Lys K
Serine Ser S
Valine Val V
Arginine Arg R
Threonine Thr T
Proline Pro P
Isoleucine Ile 1
Methionine Met M
Phenylalanine Phe
Tyrosine Tyr
Cysteine . Cys C
Tryptophan Trp
Histidine His H
Ornithine Orn O
D-Alanine D-Ala a
D-Glutamate D-Glu a
D-Glutamine D-Gln q
D-Aspartate D-Asp d
D-Asparagine D-Asn n
D-Leucine D-Leu I
D-Lysine D-Lys k
D-Serine D-Ser s
D-Valine D-Val v
D-Arginine D-Arg r
D-Threonine D-Thr t
D-Proline D-Pro p
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361 _
-5-
D-Isoleucine D-Ile i
D-Methionine D-Met m
D-Phenylalanine D-Phe f
D-Tyrosine D-Tyr y
D-Cysteine D-Cys c
D-Tryptophan D-Trp w
D-Histidine D-His
D-Ornithine D-Orn a
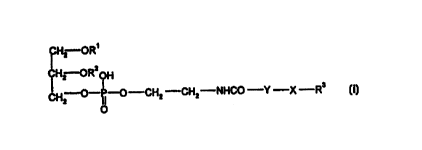
In a preferred embodiment, the novel compounds of the present invention are
compounds of
formula
~Hz OR'
H2 ORZOH
CH-O-~-O-CHZ -CHZ-NHCO -Y -X -R'
2
O (I)
wherein Rl and R2 are a hydrocarbyl moiety of a straight-chain or branched-
chain, saturated
or unsaturated aliphatic carboxylic acid or a phospholipid moiety, R3 is a
basic, membran
edisturbing peptide with a reversed amide backbone, Y is C2-10 alkylene, X is -
C(O}-NH- or
-S-S-, and salts thereof.
Particularly preferred compounds are those wherein Rl and R2 independently are
an acyl
moiety of a C 12_zo carboxylic acid. The term "C 12-20~~ denotes a number of
carbon atoms of
from 12 to 20. The acyl moieties R1 and R2 can be a straight-chain ar branched-
chain,
saturated or unsaturated moiety. Examples of such moieties are lauroyl,
palmitoyl, stearoyl
and oleoyl. In a preferred aspect, R~ and R2 are oleoyl. Y is preferably
ethylene, propylene or
decamethylene. X is preferably -S-S-.
R3 is a basic, membrane disturbing peptide with a reversed amide backbone. For
example, R3
is Gln-Gln-Arg-Lys-Arg-Lys-Ile-Trp-Ser-Ile-Leu-Ala-Pro-Leu-Gly-Thr-Thr-Leu-Val-
Lys-
Leu-Val-Ala-Gly-Ile-NH-CH[CONH2]-(CH2)-. The peptide has a reversed amide
backbone.
Preferably, R3 comprises at least 50%, more preferably 65%, and even more
preferably 80%
of D-amino acids or derivatives thereof. In the most preferred embodiment all
amino acids are
D-amino acids. The term "D-amino acid" refers to naturally as well as non-
naturally D-a-
amino carbonic acids or derivatives thereof. In addition R3 also comprises
membrane-distur-
bing peptide sequences such as magainin, cecropin, defensins, etc., or chimers
of such e.g.,
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361 _
-6-
cecropin-melittin (Hancock & Lehrer TIBTECH, 1998, vol. 16, 82-88) synthesized
as the
retro-inverso peptides and derivadzed to allow conjugation to lipids,
analogous to the
methods as described below, especially analogous in fashion to Example i.
The term "retro-inverso peptide" is the all -D analogue of an all -L retro
peptide.
In a further preferred embodiment, R3 is D-Gln-D-Gln-D-Arg-D-Lys-D-Arg-D-Lys-D-
Ile-D-
Trp-D-Ser-D-Ile-D-Leu-D-Ala-D-Pro-D-Leu-Gly-D-Thr-D-Thr-D-Leu-D-Val-D-Lys-D-
Leu-
D-Val-D-Ala-Gly-D-Ile-NH-[CONH2]-CH-(CH2)-.
The most preferred compound is
O qqrkrkiwsilaplGttivkIvaGi\ O
OH O H NH
O O..~O_P_O~N~ _ z
0 S S
O
A further embodiment of the present invention refers to the peptide portion of
R3, especially
to the intermediate peptide Gln-Gln-Arg-Lys-Arg-Lys-Ile-Trp-Ser-Ile-Leu-Ala-
Pro-Leu-Gly-
Thr-Thr-Leu-Val-Lys-Leu-Val-Ala-Gly-Ile-Cys-NH2 or derivatives and/or salts
and/or sol-
vates thereof having a reversed amide backbone and consisting of at least 50%,
more pre-
ferably 65%, and even more preferably 80% of D-amino acids or derivatives
thereof. In the
most preferred embodiment all amino acids are D-amino acids. The term "D-amino
acid"
refers to naturally as well as non-naturally D-a-amino carbonic acids or
derivatives thereof.
In a preferred embodiment the peptide is D-Gln-D-Gln-D-Arg-D-Lys-D-Arg-D-Lys-D-
Ile-D-
Trp-D-Ser-D-Ile-D-Leu-D-Ala-D-Pro-D-Leu-Gly-D-Thr-D-Thr-D-Leu-D-Val-D-Lys-D-
Leu-
D-V al-D-Ala-Gly-D-Ile-D-Cys-NH2.
In another aspect, this invention relates to a process for preparing the novel
compounds de-
fined above, i.e., conjugates of lipids and basic, membrane disturbing
peptides wherein the
peptides comprise a reversed amide backbone, and compositions comprising at
least one such
compound, a polynucleotide or any other anionic macromolecule, and,
optionally, a helper
lipid and/or a short chain phospholipid, and/or a cationic lipid and
optionally an additional
known transfection reagent other than a conjugate of this invention (i.e. a
compound of for-
mula I or II). In still another aspect, this invention relates to compositions
comprising con-
jugates of lipids and basic, membrane disturbing peptides and a helper lipid
andlor a short
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361
chain phospholipid, and/or a cationic lipid or and optionally an additional
known transfection
reagent other than a conjugate of this invention, e.g. a compound of formula
I. The invention
further relates to the use of the novel compounds as a carrier for
transfecting a cell with a
polynucleotide or any other anionic macromolecule.
The compounds provided by this invention can be prepared by reacting a peptide
of the
formula R3NH2 with a lipid of the formula
~HZ OR'
~HZ ORZOH II
CHZ O-P-O-CHZ -CH2-NHCO -Y -COOH
O
or a peptide of the formula R3SH with a lipid of the formula
~HZ OR'
HZ ORZOH , III
CH2 O-P-O-CH2 -CHZ-NHCO -Y -SZ
O
wherein R1, R2, R3 and Y are as defined above and Z is a leaving group such as
2-
pyridinethio. These reactions can be carned out in a manner known per se.
Compounds of the formula R3NH2 wherein the peptide part is comprises a
reversed amide
backbone may be prepared by methods known in the art. The corresponding
methods have
been described for example for the preparation of synthetic melittin, its
enantio, retro, and
retroenantio isomers and derivatives thereof (Juwadi et al. (1996) J. Am.
Chem. Soc. 118,
8989-8997).
The peptides may be prepared by the solid-phase synthesis technique. In this
technique, syn-
thesis is occurring while the peptide is attached to a polymeric support,
therefore allowing for
the separation of product from byproduct by washing steps. The completion of
the acylation
reaction is ensured by using large excesses of soluble reagents. Synthesis
involves the cova-
lent anchorage of the first amino acid in the sequence to the solid support
followed by the de-
protection of the protected amino function for the subsequent coupling to the
incoming amino
acid derivative. After n cycles of deprotection and coupling, the peptide is
released from the
solid phase by a chemical cleavage reaction. In a preferred embodiment, D-
amino acids are
used.
CA 02327367 2000-10-OS
WO 99/51629 PC'T/EP99/02361
_g-
Thus, the coupling of peptide of the formula R3NH2 with a lipid of the formula
II can be
accomplished by reacting the compounds wherein amino group other than the
amino group to
be reacted are protected in a suitable solvent in the presence of a
condensation agent such as
dicyclohexylcarbodiimide in analogy to methods known for producing peptide
bonds.
The reaction of a peptide of the formula R3SH with a compound of formula R-SZ
can be
carried out in an appropriate solvent or solvent mixture which solubilizes
both reactants. The
compound of formula R-SZ can be dissolved in an organic solvent, e.g., in
chloroform. The
peptide R3SH is suitably dissolved in aqueous buffer solution, such as
phosphate buffer, that
contains an appropriate amount of an water-miscible organic solvent such as
acetonitrile to
accomplish the formation of a single phase reaction system.
Compounds of the formula II and III are known or can be prepared by known
methods, e.g. as
described in Biochim. Biophys. Acta, 1986 862, 435-439. For example, compounds
of the
formula
~HZ OR'
CH2 OR2pH
CHZ O-P-O-CHz -CHZ-NHCO -Y -COOH
O
wherein R1 and R2 are oleolyl, and Y is ethylene, propylene or decamethylene
and the
compound of the formula
~HZ OR'
H2 ORZOH III
CH2 O-P-O-CH2 -CHZ-NHCO -Y -SZ
O
wherein R1 and R2 are oleolyl, Y is ethylene and Z is 2-pyridinethio are
commercially
available as N-Succinyl-PE, N-Glutaryl-PE , N-Dodecanyl-PE and N-PDP-PE from
Avanti
Polar Lipids, Alabaster, Alabama, USA.
Any anionic macromolecule can be transfected into a cell using a compound of
formula I. An
anionic macromolecule is a macromolecule which contains at least one negative
charge per
molecule. Examples of anionic macromolecules which can be transfected in
accordance with
this invention include polynucleotides , such as deoxyribonucleic acids (DNA)
and ribo-
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361
_g_
nucleic acids (RNA); and proteins, such as ribonucleoproteins and proteins
used for immuni-
zation, e.g. viral proteins. Examples of DNA for use in the present invention
are plasmids
and genes, especially those for which gene therapy protocols have been
launched such as
cystic fibrosis transmembrane regulator (CFTR), adenosine deaminase (ADA),
thymidine
kinase (tk) and HLA B7; as well as reporter genes such as beta-galactosidase,
luciferase,
green fluorescence protein (gfp), chloramphenicol transferase and alpha-1
antitrypsin. Other
examples of DNA are oligodeoxynucleotides and their analogues used as
antisense, aptamer
or 'triple-helix' agents. Examples of RNA are ribozymes or oligoribonucleotide
antisense
molecules.
The nature of the cell which is to be transfected is not narrowly crucial. The
cell can be a
prokaryotic or eukaryotic cell, a mammalian or a plant cell.
In transfecting a cell using a conjugate of this invention, e.g. a compound of
formula I, the
cell is contacted with the anionic macromolecule in the presence of an
appropriate amount of
such compound. The appropriate amount of the conjugate, e.g. a compound of
formula I for a
given amount of anionic macromolecule depends on their respective charges. The
+1- charge
ratio between the conjugate and the molecule to be transfected generally
varies between 0.1-
10, preferably between 0.5-5. The value of "+/- charge ratio" is calculated by
dividing the
number of positively charged groups on the amino acids in the group R3 by the
number of
negative charges of the molecule to be transfected. When the molecule to be
transfected is a
polynucleotide for example, number of negative charges means the number of
negatively
charged phosphates in the backbone. The optimal ratio within these ranges
depends on the
cell to be transfected and is readily ascertained by one of skill in the art
to which this inven-
tion pertains.
The amount of anionic macromolecules to the number of cells is such that the
amount of
anionic macromolecule for transfecting 104 cells is from 0.1 ng to 10 mg,
preferably from 0.2
mg to 2 mg. When the anionic macromolecule is DNA the preferred amount of DNA
for
transfecting 104 cells in vitro is from 0.1 mg to 10 mg. When cells are being
transfected in
vivo, the preferred amount of DNA is from 0.1 pg to 1 g.
In a preferred aspect of the invention the transfection is further carned out
in the presence of a
helper lipid and/or short chain phospholipid, and/or a cationic lipid or any
other known trans-
fection competent molecule other than a conjugate of this invention. Any
conventional helper
lipid can be used. "Helper lipids" are phospholipids which are known to
increase delivery of
macromolecules to cells when used together with known transfection competent
molecules.
Examples of helper lipids are phospholipids, such as phosphatidylcholines or
phosphatidyl-
CA 02327367 2000-10-OS
WO 99/51629 PC'T/EP99/02361 _
-10-
ethanolamines or mixtures thereof. Preferred helper lipids are
phosphatidylethanolamines,
such as dioleoylphosphatidylethanolamine. Any conventional short chain
phospholipid can be
used. "Short chain phospholipids" are phospholipids containing fatty acid
residues, which
fatty acid residues contain from 6 to 12 carbon atoms in their backbone.
Examples of short
chain phospholipids are phosphatidylcholines that carry two C6_12 fatty acid
residues. Pre-
ferred short chain phospholipids are dicapryl- and dicapryloyl
phosphatidylcholine.
Examples of transfection competent molecules include cationic lipids as
described by J.B.
Behr in Bioconjugate Chem., 1994, 5,382-389 and X. Gao and L. Huang in Gene
Ther., 1995,
2, 710-722; polycations as described by A.V. Kabanov and V.A.: Kabanov in
Bioconjugate
Chem., 1995, 6, 7-20; peptides and polymers and other non-viral gene delivery
systems as
described by F.D. Ledley in Human Gene Therapy, 1995, 6, 1129-1144.
The helper lipid and/or short chain phospholipid and/or a cationic lipid and
optionally another
additional known transfection competent molecule other than a conjugate of
this invention is
suitably in the form of a liposome, micelles, organic or aqueous dispersions,
or organic or
aqueous solutions. The optimal molar ratio between the compound of formula I
and the helper
lipid is 0.1:50, preferably 1:10. The optimal molar ratio between helper lipid
and short-chain
phospholipid is 2:20. The optimal molar ratio between the compound of formula
I or II and
additional transfection competent molecules is 0.1:10.
The present invention also comprises the use of a composition as defined above
for transfec-
ting an eukaryotic or prokaryotic cell in vivo or in vitro with an anionic
macromolecule, pre-
ferably with a polynucleotide.
The invention also comprises the use of compounds of formula (I) as defined
above for trans-
fecting a eukaryotic or prokaryotic cell in vivo or in vitro with an anionic
macromolecule,
preferably with a polynucleotide.
A further embodiment of the present invention is a process for transfecting a
cell in vivo or in
vitro with an anionic macromolecule, preferably a polynucleotide, comprising
contacting a
cell in vivo or in vitro with the anionic macromolecule in the presence of a
compound of for-
mula (I) or with a composition as defined above.
In addition, the invention is directed to a process for introducing a
biologically active anionic
molecule into a cell in vivo or in vitro with an anionic macromolecule,
comprising contacting
a cell in vivo or in vitro with the anionac macromolecule in the presence of a
composition or a
compound as defined above. This includes also a process for introducing in
vivo or in vitro a
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99102361 T
-11-
biologically active anionic molecule into a cell, comprising contacting in
vivo or in vitro a
cell with the anionic macromolecule in the presence of a composition or
compound as defined
above.
Further, the present invention is directed to the use of a compound or
composition as defined
above for introducing in vivo or in vitro a biologically active molecule into
cells.
For transfection, an appropriate amount of a conjugate of this invention,
e.g., a compound of
formula I is added to the molecule to be transfected (e.g., plasmid DNA),
suitably in an
aqueous solution. Optionally, a helper lipid and, if desired, a short chain
phospholipid and/or
a cationic lipid and optionally another additional known transfection
competent molecule
other than a conjugate of this invention is then added, either in form of
liposomes, micelles,
or as an organic solution or aqueous dispersion. Alternatively, the molecule
to be transfected
may be added to a composition comprising a compound in accordance with this
invention, a
helper lipid, and, if desired, a short chain phospholipid and/or a cationic
lipid and optionally
another additional known transfection competent molecule other than a
conjugate of this
invention. The composition may be in solid, liquid, semisolid or aerosol form,
suitably in
form of liposomes, micelles, or as an organic solution or aqueous dispersion.
For transfecting cells in an animal or human patient the composition can be
administered by
oral, parenteral (i.v., i.m., s.c., i.d., i.p.) transdermal, pulmonary, nasal,
rectal, ocular, ven-
tricular, vascular (catheter) and intratumoral route. Furthermore, the
composition can be
administered by high velocity impaction administration to the skin surface.
The progress of
transfection can be measured by appropriate testing protocols which are known
to those
skilled in the art.
The present invention also refers to a composition comprising at least one
compound as de-
fined above and a helper lipid and/or a short chain phospholipid and/or a
cationic lipid or
optionally another additional transfection competent molecule. In addition,
the composition
may comprise an anionic macromolecule, preferably a polynucleotide. These
compositions
may also comprise a polycationic polymer, preferably polyethyleneimine (PEI).
The compo-
nents of these compositions may be in the form of an aqueous or organic
solution, an aqueous
or organic dispersion, or a liposome or a micelle.
The term "polyethylenimine (PEI)" refers to a synthetic organic, generally
branched macro-
molecule with a high cationic charge/density potential, preferably with a
molecular weight of
about 25 kDA.
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361
-12-
The invention also relates to the use of a compound as above for transfecting
a cell with an
anionic macromolecule, preferably a polynucleotide.
In accordance with the present invention it has been found that conjugating a
lipid to a basic,
membrane disturbing peptide having a reversed amide bond results in novel
compounds that
bind polynucleotides and other anionic macromolecules and can be used for
transfection of
cells. Thus, the invention is concerned with a process for transfecting a cell
with an anionic
macromolecule comprising, contacting the cell with the anionic macromolecule
in the pre-
sence of a compound as described above, so as to transfect the cell with the
anionic macro-
molecule.
The invention also relates to a process for production of large quantities of
recombinant pro-
teins in PEI-mediated transfected cells. For example, high-level expression of
both G protein
coupled receptors an ligand-gated ion channels by the use of Semliki Forest
virus (SFV) has
been realized. Generally, B""x values of more than 50 pmol receptor per mg
protein and re-
ceptor densities of more than 3 x 1 O6 receptors per cell have been achieved.
To further faci-
litate large scale protein production optimal conditions for mammalian serum-
free suspension
cultures have been obtained. Adaptation of BHK, CHO and HEK293 cells to these
conditions
has allowed efficient infection with SFV vectors to produce large volumes of
recombinant
protein expressing cell cultures.
Surprisingly, the growth temperature of the cell cultures can have a dramatic
effect on the
duration and levels of recombinant protein-expression. Expression of
recombinant luciferase
is increased 5 to 10-fold by lowering the growth temperature of BHK and CHO
cells from
37°C to 33°C. The expression time is much longer at 33°C
with still high expression 65 hours
post-infection.
Moreover, the effect of the temperature on the expression of two 7TM
receptors, human neu-
rokinin-1 receptor and rat metabotropic glutamate receptor-2 and the 5-HT3
ligend-gated ion
channel could be shown. A similar effect as observed for luciferase has also
been obtained for
the receptors. The receptor density is much higher in cells grown at
33°C compared to 37°C.
The expression time for receptors is usually restricted to 24 hours in SFV-
infected cells, but
can be prolonged to 65 hours when cells are cultured at 33°C. This
improvement can greatly
facilitate production of large quantities of recombinant proteins. Moreover,
the utility of the
inventive transfection for the rapid expression of heterologous proteins in
large scale for ad-
herent cells (CFU) and suspension cells (121, 23 1, 601 fermentors) has been
realized. In par-
ticular helper lipids in combination with PEI exhibit a high transient gene
expression in
HEK293 cells and other mammalian cell lines at low DNA concentration. In
addition the fre-
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361
-13-
quently occurring inhibitory effect of conditioned medium which is a serious
problem for
scale up expression methods is reduced or even avoided.
The following examples which are not limitative illustrate the invention
further.
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361 __
-14-
EXAMPLES
Example 1
a) Preparation of the retro-inverso peptide R3SH
Continuous-flow solid-phase synthesis was performed on a Pioneers Peptide
Synthesis
System, starting from Tenta Gel S RAM resin (0.25 mmole/g [Rapp Polymere GmbH,
Tiibingen, Germany] according to the method described by Atherton and
Sheppard, Solid
Phase Peptide Synthesis : A Practical Approach (IRL Press Oxford 1989). The
base-labile
Fmoc group was used for a-amino protection. Side chains were protected with
the following
protection groups: D-Arg(Pbfj,D-Cys(Trt),D-Gln(Trt),D- Lys(Boc),D-Ser(But), D-
Thr(But)
and D-Trp(Boc). Fmoc-amino acids (2.5 equiv.) were activated with an
equivalent amount of
TATU (L. A. Carpino J. Am. Chem. Soc. 1993, I 15, 4397-4398) and DIPEA. Fmoc
depro-
tection was achieved with 20% piperidine in DMF. D-Gln(Trt)- D-Gln(Trt)- D-
Arg(Pbf)- D-
Lys(Boc)- D-Arg(Pbf)- D- Lys(Boc)-D-Ile- D-Trp(Boc)- D-Ser(But)-D-Ile-D-Leu-D-
Ala-D-
Pro-D-Leu-Gly-D-Thr(But)- D-Thr(But)-D-Leu-D-Val- D- Lys(Boc)-D-Leu-DVaI-D-Ala-
Gly-D-Ile-D-Cys(Trt) -amide Tenta Gel S-resin {2.0 g) was treated with a
mixture (100 ml) of
90% TFA, 2% EDT, 5% H20, 3% triisopropylsilane for 6 hours. The reaction
mixture was
concentrated and poured into diethyl ether and the precipitate was collected
by filtration and
lyophilized from water. The crude peptide was purified by preparative RP-HPLC.
There was
obtained homogenous D-Gln-D-Gln-D-Arg-D-Lys-D-Arg-D-Lys-D-Ile-D-Trp-D-Ser-D-
Ile-
D-Leu-D-Ala-D-Pro-D-Leu-Gly-D-Thr-D-Thr-D-Leu-D-Val-D-Lys-D-Leu-D-Val-D-Ala-
Gly-D-Ile-D-Cys-NH2. 6 TFA.
b) Preparation of a compound of formula IV
The homogenous peptide obtained in paragraph a) above (24.2 mg, 7 mmole) was
dissolved
in a mixture of 2 ml of 100 mM ammonium acetate buffer, pH 6.5, and 2 ml of
acetonitrile.
To this solution there was added 7.4 mg of 1,2-dioleoyl-sn-glycero-3-
phosphoethanolamine-
N-[3-(2-pyridyidithio)propionate] in 0.5 ml of chloroform. The mixture was
stirred at room
temperature for 1 hour and the organic solvent was removed by evaporation. The
remaining
solution was washed 3 times with ethyl acetate and the aqueous phase was
lyophilized. There
was obtained 28.5 mg of a compound of formula IV wherein R~ and R2 are oleoyl
and D-
Gln-D-Gln-D-Arg-D-Lys-D-Arg-D-Lys-D-Ile-D-Trp-D-Ser-D-Ile-D-Leu-D-Ala-D-Pro-D-
Leu-Gly-D-Thr-D-Thr-D-Leu-D-Val-D-Lys-D-Leu-DVaI-D-Ala-Gly-D-Ile-NH-
CH[CONH2]-(CH2)- is R3. ISP-MS: M= 3722.
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361 _
-15-
Example 2
1 mg of the compound obtained in Example 1 was dissolved in 500 p,l
acetonitrile and diluted
in 500 ~l sterile pure water (lmg/ml) and stored at 4°C. 15 ~1 of a
plasmid solution (lmg/ml)
and stored at 4°C. 15 ~1 of a plasmid solution encoding the soluble
human tumor necrosis
factor receptor p55 gene was transferred into 1.5 ml medium and mixed. Various
amounts of
the compound obtained in a) was added , mixed and after 10 min at room
temperature the
mixture was transferred to 15 ml HEK293(EBNA) cells in suspension.
Table 1 shows the transfection efficiency of the compound as described in
Example 1 and the
cell viability in serum-free suspension culture.
Table 1
Transient
tranfection
of HEK293(EBNA)
cells
Compound 1 2 4 6 10
(mg/ml)
viability 90 - 90 - 90 - 90 - 90 -
(%) 95 95 95 95 95
TNFRp55 0.1 0.2 2.5 6.3 83
(ng/ml)
The data clearly indicate that compound-mediated transfection can be achieved
in the absence
of detectable cell toxicity effects in serum-free suspension culture of 293
(EBNA) cells.
Example 3
1 mg of the compound obtained in Example 1 was dissolved in 500 p,l
acetonitrile and diluted
in S00 ~1 sterile pure water (lmg/ml) and stored at 4°C. 90 mg
polyethyleneimine (PEI), with
a molecular weight of 25 kDa, were dissolved in sterile pure water (0.9
mg/ml), neutralized
with HCl and sterile filtered and stored at room temperature.
3 ~1 of a plasmid solution ( 1 mg/ml) encoding the soluble human tumor
necrosis factor
receptor p55 gene was diluted in 1.5 ml culture medium and mixed. Various
amounts of the
compound (lmglml) as described in Example 1 were added, mixed, followed by 8.3
~,l PEI
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361
-16-
(0.9 mg/ml). After mixing and incubation for 10 min at room temperature the
mixture was
transferred into 15 ml of HEK293 (EBNA) cells in suspension. Cells which were
grown in
serum-free suspension culture were transferred into fresh medium before adding
the com-
plexes. The released receptor protein was measured 72 hrs post-transfection in
the culture
medium and expressed as ng receptor per milliliter culture.
Table 2 shows the transfection efficiency of the compound described in Example
1 in
combination with PEI by measuring the released receptor protein in serum free
suspension
culture.
Table 2
Transient
transfection
of HEK293(EBNA)
cells together
with
PEI
Compound + 0.02 0.05 0.1 0.15 0 +
+ + + +
polyethyleneimine0.5 0.5 0.5 0.5 0.5
(m~~)
TNFRp55 (ng/ml)490 800 1120 1100 180
The results clearly indicate the synergisitc enhancement of the coumpound
together with PEI.
The sequence of adding first the compound followed by PEI to the DNA or vice
versa has no
detectable infludence on the transfection effeciency. No significant changes
of the expression
levels were observed when the cells were cultured in the presence of 10%
serum.
Example 4
1 mg of the compound obtained in Example 1 was dissolved in S00 ~1
acetonitrile and diluted
in 500 p,l sterile pure water (lmg/ml) and stored at 4°C. 90 mg
polyethelenimine, with a mole-
cular weight of 25 kDa, was dissolved in sterile pure water (0.9 mg/ml),
neutralized with HCl
and sterile filtered and stored at room temperature.
3 pl of a plasmid solution (1 mg/ml) encoding the luciferase gene was diluted
in 1.5 ml cul-
ture medium and mixed. 2.25 ~l compound (lmg/ml) as described in Example 1 was
added,
mixed, and followed by 8.3 p,l PEI (0.9 mg/ml). After mixing and incubation
for 10 min at
room temperature the mixture was transferred into 15 ml of various cells.
Cells which were
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361
-17-
grown in serum-free suspension culture were transferred into fresh medium
before adding the
complexes. The luciferase activity was measured the soluble cell extract after
24 hrs incu-
bation and expressed as relative light unit (RLU) per milligram protein.
Table 3 shows the transfection efficiency of the compound described in Example
1 in combi-
nation with PEI by measuring the luciferase activity in serum free suspension
culture.
Table 3
Luciferase
activity
(RLU/m1)
Compound
PEI
Compound
+ PEI
BHK 21 C 1 5.2 1 O5 2.8 10~ 1.8 109
x x x
CHO dhfr- 1.4 106 3.5 10~ 5.3 109
x x x
HEK293 (EBNA) 2.2 106 3.2 109 6.7 lOlo
x x x
HEK293 1.8 106 2.9 109 6.3 10 ~
x x x o
Glioma C6 5.8 106 6.5 10~ 3.4 109
x x x
The results clearly indicate the synergistic enhancement of the compound
together with PEI
in various cell lines.
Example 5
1 mg of the compound obtained in Example 1 was dissolved in SO ~1 acetonitrile
and diluted
in 500 ~1 sterile pure water (lmg/ml) and stored at 4°C. 90 mg
polyethelenimine, with a
molecular weight of 25 kDa, was dissolved in sterile pure water (0.9 mg/ml),
neutralized
with HCl and sterile filtered and stored at room temperature.
3 ml of a plasmid solution (lmg/ml) encoding the green fluorescence protein
was diluted in
1.5 ml culture medium and mixed. 2.25 ~,1 compound ( 1 mg/ml) was added,
mixed, and
followed by 8.3 ~,1 PEI (0.9 mg/ml). After mixing and incubation for 10 min at
room tem-
perature the mixture was transferred into 15 ml of various cells. Cells which
were grown in
serum-free suspension culture were transferred into fresh medium before adding
the com-
CA 02327367 2000-10-OS
WO 99/S1b29 PCT/EP99/02361
-18-
plexes. The transfection efficiency was measured by counting the number of
fluorescent
cells after 24 hours incubation and expressed as percent transfected cells.
Table 4 shows the transfection efficiency of the compound described in Example
1 in combi-
nation with PEI by measuring the green fluorescence within various cell lines
in serum-free
suspension culture.
Table 4
Amount of fluorescence
cells (%) - compound
+ PEI
BHK 21 C1 25 ( 5)
CHO dhfr- 15 ( 5)
HEK293(EBNA) 85 (t 5)
Example 6
1 mg of the compound obtained in Example 1 was dissolved in 500 ~l
acetonitrile and diluted
in 500 p.l sterile pure water (1 mg/ml) and stored at 4°C. 90 mg
polyethylenimine, with a
molecular weight of 25 kDa, was dissolved in sterile pure water (0.9 mg/ml),
neutralized with
HCI and sterile filtered and stored at room temperature.
3 mI of a plasmid solution (1 mg/ml) encoding the luciferase gene was diluted
in 1.5 ml
culture medium and mixed. 2.25 ~tl compound ( 1 mg/ml) as described in Example
1 was
added, mixed, and followed by 8.3 pl PEI (0.9 mg/ml). After mixing and
incubation for 10
min at room temperature 0,1 ml of the mixture was transferred into a well
containing 1 m1
medium of a 12 well plate with various adherent cell lines. Cells which were
grown in the
presence of 10% serum were incubated during 4 hours post-transfection in serum-
free me-
dium. The luciferase activity was measured in the soluble cell extract after
24 hours in-
cubation and expressed as relative light unit (RLU) per milligram protein.
Table 5 shows the transfection effciency of the compound described in Example
1 in
combination with PEI by measuring the luciferase activity in various adherent
cell lines.
CA 02327367 2000-10-OS
WO 99/51629 PCT/EP99/02361 _
-19-
Table 5
Luciferase activity
(RLU/mg Protein)
PEI Compound PEI
HEK293 5.4 x 109 5.3 x 10'
glioma C6 3.1 x 10' 1.9 x 10'
IMR32 2.2 x 109 7.7 x 109
COS7 2.9x108 1.1x10'
HepG2 <1 x 108 8 x 108
The results clearly indicate also the synergistic enhancement of the compound
together with
PEI in various adherent cell lines.