Note: Descriptions are shown in the official language in which they were submitted.
CA 02327806 2000-10-06
''' Le A 32 986-Foreign Countries Kri/wa/NT
-1-
Substituted uhenylpyridazinones
The invention relates to novel substituted phenylpyridazinones, to processes
for their
preparation and to their use as crop protection agents, in particular as
herbicides and
S as insecticides.
It is already known that certain substituted phenylpyridazinones have
herbicidal
properties (cf. DE-A-1105232, DE-A-1670309, DE-A-1670315, DE-A-1695840,
DE-A-2526643, DE-A-2706700, DE-A-2808193, DE-A-2821809, DE-A-19754348,
US-A-5298502, WO-A-96/39392, WO-A-97/07104). The herbicidal activity of these
compounds is not entirely satisfactory.
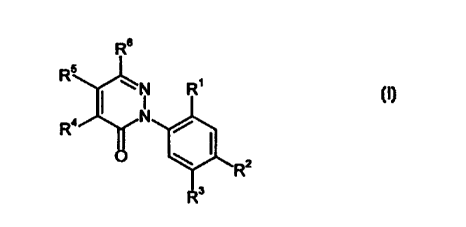
This invention, accordingly, provides the novel substituted
phenylpyridazinones of
the general formula (I)
Rs
R5
N R'
R4
O ~ R2 (I)
Rs
in which
R1 represents hydrogen, fluorine, chlorine or bromine,
R2 represents cyano, carbamoyl, thiocarbamoyl, fluorine, chlorine, bromine, or
represents in each case optionally fluorine- and/or chlorine-substituted
alkyl,
alkoxy or alkylthio having in each case 1 or 2 carbon atoms,
R3 represents the grouping -A1-A2-A3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-2-
in which
A1 represents a single bond, represents oxygen, sulphur, -SO-, -S02-,
-CO- or the grouping -N-A4-, in which A4 represents hydrogen,
hydroxyl, C1-C4-alkyl, C3-C4-alkenyl, C3-C4-alkinyl, C1-Cq.-alkoxy,
phenyl, C1-C4-alkylsulphonyl or phenylsulphonyl,
A1 furthermore represents in each case optionally fluorine-, chlorine- or
bromine-substituted C1-C6-alkanediyl, C2-C6-alkenediyl, C2-C6-aza-
alkenediyl, C2-C6-alkinediyl, C3-C6-cycloalkanediyl, C3-C6-cyclo-
alkenediyl or phenylene,
A2 represents a single bond, represents oxygen, sulphur, -SO-, -S02-,
-CO- or the grouping -N-A4-, in which A4 represents hydrogen,
hydroxyl, C 1-C4-alkyl, C 1-C4-alkoxy, phenyl, C 1-C4-alkylsulphonyl
or phenylsulphonyl,
A2 furthermore represents in each case optionally fluorine-, chlorine-,
bromine- or C~-C4-alkoxy-substituted C1-C6-alkanediyl, C2-C6-
alkenediyl, C2-C6-azaalkenediyl, C2-C6-alkinediyl, C3-C6-cyclo
alkanediyl, C3-C6-cycloalkenediyl or phenylene,
A3 represents hydrogen, hydroxyl, amino, cyano, isocyano, thiocyanato,
nitro, formyl, carboxyl, carbamoyl, thiocarbamoyl, sulpho, chloro-
sulphonyl, fluorine, chlorine, bromine, represents in each case
optionally hydroxyl-, fluorine-, chlorine-, C1-C4-alkoxy-, C~-C4-alkyl-
carbonyloxy- or C,-C4-alkoxy-carbonyloxy-substituted alkyl, alkoxy,
alkylthio, alkylsulphinyl, alkylsulphonyl, alkylamino, dialkylamino,
alkoxycarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl or di-
alkoxy(thio)phosphoryl having in each case 1 to 6 carbon atoms in the
alkyl groups, represents in each case optionally fluorine- or chlorine-
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-3-
substituted alkenyl, alkenyloxy, alkenylamino, alkylideneamino,
alkenyloxycarbonyl, alkinyl, alkinyloxy, alkinylamino or alkinyloxy-
carbonyl having in each case 2 to 6 carbon atoms in the alkenyl,
alkylidene or alkinyl groups, represents in each case optionally
fluorine-, chlorine-, cyano-, carboxyl-, C1-C4-alkyl- and/or C1-C4-
alkoxy-carbonyl-substituted cycloalkyl, cycloalkyloxy, cycloalkyl-
amino, cycloalkylalkyl, cycloalkylalkoxy, cycloalkylalkylamino,
cycloalkylideneamino, cycloalkyloxycarbonyl or cycloalkylalkoxy-
carbonyl having in each case 3 to 6 carbon atoms in the cycloalkyl
groups and optionally 1 to 4 carbon atoms in the alkyl groups, or
represents in each case optionally nitro-, cyano-, carboxyl-, fluorine-,
chlorine-, bromine-, C1-C4-alkyl-, C1-C4-halogenoalkyl, C1-C4-
alkyloxy, C1-C4-halogenoalkyloxy- and/or C1-C4-alkoxy-carbonyl-
substituted phenyl, phenyloxy, phenyl-C1-C4-alkyl, phenyl-C1-C4-
alkoxy, phenyloxycarbonyl or phenyl-C 1-C4-alkoxycarbonyl,
A3 furthermore represents in each case optionally fully or partially
hydrogenated pyrrolyl, pyrazolyl, imidazolyl, triazolyl, furyl, oxiranyl,
oxetanyl, dioxolanyl, dioxanyl, thienyl, oxazolyl, isoxazolyl, thiazolyl,
isothiazolyl, oxadiazolyl, thiadiazolyl, pyridinyl, pyrimidinyl, triazinyl,
pyrazolyl-C1-C4-alkyl, furyl-C1-C4-alkyl, thienyl-C1-C4-alkyl, oxa-
zolyl-C1-C4-alkyl, isoxazole-C1-C4-alkyl, thiazole-C1-C4-alkyl,
pyridinyl-C1-C4-alkyl, pyrimidinyl-C1-C4-alkyl, pyrazolylmethoxy,
furylmethoxy, represents perhydropyranylmethoxy or pyridylmethoxy,
R4 represents hydrogen, carboxyl, cyano, carbamoyl, thiocarbamoyl, nitro,
hydroxyl, mercapto, amino, fluorine, chlorine, bromine, or represents in each
case optionally fluorine- and/or chlorine-substituted alkyl, alkoxy,
alkylthio,
alkylamino, dialkylamino or alkoxycarbonyl having in each case 1 to 4 carbon
atoms in the alkyl groups,
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-4-
RS represents hydrogen, carboxyl, cyano, carbamoyl, thiocarbamoyl, vitro,
hydroxyl, mercapto, amino, fluorine, chlorine, bromine, or represents in each
case optionally fluorine- and/or chlorine-substituted alkyl, alkoxy,
alkylthio,
alkylamino, dialkylamino or alkoxycarbonyl having in each case 1 to 4 carbon
atoms in the alkyl groups, and
R6 represents hydrogen, carboxyl, cyano, carbamoyl, thiocarbamoyl, vitro,
hydroxyl, mercapto, amino, fluorine, chlorine, bromine, or represents in each
case optionally fluorine- and/or chlorine-substituted alkyl, alkoxy,
alkylthio,
alkylamino, dialkylamino or alkoxycarbonyl having in each case 1 to 4 carbon
atoms in the alkyl groups.
The novel substituted phenylpyridazinones of the general formula (I) have
interesting
biological properties, in particular strong herbicidal and insecticidal
activity.
In the definitions, the saturated or unsaturated hydrocarbon chains, such as
alkyl,
alkenyl or alkinyl, are in each case straight-chain or branched.
Halogen generally represents fluorine, chlorine, bromine or iodine, preferably
fluorine, chlorine or bromine, in particular fluorine or chlorine.
R1 preferably represents hydrogen, fluorine or chlorine;
R2 preferably represents cyano, carbamoyl, thiocarbamoyl, fluorine, chlorine,
bromine, methyl or trifluoromethyl;
R3 preferably represents the grouping -A1-A2-A3
in which
CA 02327806 2000-10-06
Le A 32 986-Foreim Countries
-5-
A1 represents a single bond, represents oxygen, sulphur, -SO-, -S02-,
-CO- or the grouping -N-A4-, in which A4 represents hydrogen,
hydroxyl, methyl, ethyl, n- or i-propyl, methoxy, ethoxy, n- or
i-propoxy, methylsulphonyl or ethylsulphonyl,
S
A1 furthermore represents methylene, ethane-1,1-diyl, ethane-1,2-diyl,
propane-1,1-diyl, propane-1,2-diyl, propane-1,3-diyl, ethene-1,2-diyl,
propene-1,2-diyl, propene-1,3-diyl, ethine-1,2-diyl, propine-1,2-diyl or
propine-1,3-diyl,
A2 represents a single bond, represents oxygen, sulphur, -SO-, -S02-,
-CO- or the grouping -N-A4-, in which A4 represents hydrogen,
hydroxyl, methyl, ethyl, n- or i-propyl, methoxy, ethoxy, n- or
i-propoxy, methylsulphonyl, ethylsulphonyl, n- or i-propylsulphonyl or
phenylsulphonyl,
A2 furthermore represents methylene, ethane-1,1-diyl, ethane-1,2-diyl,
propane-1,1-diyl, propane-1,2-diyl, propane-1,3-diyl, ethene-1,2-diyl,
propene-1,2-diyl, propene-1,3-diyl, ethine-1,2-diyl, propine-1,2-diyl or
propine-1,3-diyl,
A3 represents hydrogen, hydroxyl, amino, cyano, nitro, formyl, carboxyl,
carbamoyl, sulpho, fluorine, chlorine, bromine, represents in each case
optionally fluorine-, chlorine-, methoxy- or ethoxy-substituted methyl,
ethyl, n- or i-propyl, n-, i-, s- or t-butyl, n-, i-, s- or t-pentyl, methoxy,
ethoxy, n- or i-propoxy, n-, i-, s- or t-butoxy, n-, i-, s- or t-pentyloxy,
methylthio, ethylthio, n- or i-propylthio, n-, i-, s- or t-butylthio,
methylsulphinyl, ethylsulphinyl, n- or i-propylsulphinyl, methyl-
sulphonyl, ethylsulphonyl, n- or i-propylsulphonyl, methylamino,
ethylamino, n- or i-propylamino, n-, i-, s- or t-butylamino, dimethyl-
amino, diethylamino, methoxycarbonyl, ethoxycarbonyl, n- or i-
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-6-
propoxycarbonyl, dimethoxyphosphoryl, diethoxyphosphoryl or di-
propoxyphosphoryl, diisopropoxyphosphoryl, represents in each case
optionally fluorine- or chlorine- substituted ethenyl, propenyl, butenyl,
propenyloxy, butenyloxy, propenylamino, butenylamino, propylidene-
amino, butylideneamino, propenyloxycarbonyl, butenyloxycarbonyl,
ethinyl, propinyl, butinyl, propinyloxy, butinyloxy, propinylamino,
butinylamino, propinyloxycarbonyl or butinyloxycarbonyl, represents
in each case optionally fluorine-, chlorine-, cyano-, carboxyl-, methyl-,
ethyl-, n- or i-propyl-, methoxycarbonyl- or ethoxycarbonyl-
substituted cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclo-
propyloxy, cyclobutyloxy, cyclopentyloxy, cyclohexyloxy, cyclo-
propylmethyl, cyclobutylmethyl, cyclopentylmethyl, cyclohexyl-
methyl, cyclopropylmethoxy, cyclobutylmethoxy, cyclopentyl-
methoxy, cyclohexylmethoxy, cyclopentylideneamino, cyclo-
hexylideneamino, cyclopentyloxycarbonyl, cyclohexyloxycarbonyl,
cyclopentylmethoxycarbonyl or cyclohexylmethoxycarbonyl, or
represents in each case optionally nitro-, cyano-, carboxyl-, fluorine-,
chlorine-, bromine-, methyl-, ethyl-, n- or i-propyl, trifluoromethyl-,
methoxy-, ethoxy-, n- or i-propoxy-, difluoromethoxy-, trifluoro-
methoxy-, methoxycarbonyl- and/or ethoxycarbonyl-substituted
phenyl, phenyloxy, benzyl, phenylethyl, benzyloxy, phenyloxy-
carbonyl, benzyloxycarbonyl,
A3 furthermore represents (in each case optionally fully or partially
hydrogenated) dioxolanyl, dioxanyl, pyrrolyl, pyrazolyl, imidazolyl,
triazolyl, furyl, thienyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl,
oxadiazolyl, thiadiazolyl, pyridinyl, pyrimidinyl, triazinyl, pyrazolyl-
methyl, furylmethyl, thienylmethyl, oxazolylmethyl, isoxazolemethyl,
thiazolemethyl, pyridinylmethyl, pyrirnidinylmethyl, pyrazolyl-
methoxy, furylmethoxy or pyridylmethoxy;
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-
R4 preferably represents hydrogen; carboxyl, cyano, carbamoyl, thiocarbamoyl,
nitro, hydroxyl, mercapto, amino, fluorine, chlorine, bromine, or represents
in
each case optionally fluorine- and/or chlorine-substituted methyl, ethyl, n-
or
i-propyl, n-, i-, s- or t-butyl, methoxy, ethoxy, methylthio, ethylthio,
methyl-
amino, ethylamino, dimethylamino, methoxycarbonyl or ethoxycarbonyl;
RS preferably represents hydrogen, carboxyl, cyano, carbamoyl, thiocarbamoyl,
nitro, hydroxyl, mercapto, amino, fluorine, chlorine, bromine, or represents
in
each case optionally fluorine- and/or chlorine-substituted methyl, ethyl, n-
or
i-propyl, n-, i-, s- or t-butyl, methoxy, ethoxy, methylthio, ethylthio,
methyl-
amino, ethylamino, dimethylamino, methoxycarbonyl or ethoxycarbonyl; and
R6 preferably represents hydrogen, carboxyl, cyano, carbamoyl, thiocarbamoyl,
nitro, hydroxyl, mercapto, amino, fluorine, chlorine, bromine, or represents
in
each case optionally fluorine- and/or chlorine-substituted methyl, ethyl, n-
or
i-propyl, n-, i-, s- or t-butyl, methoxy, ethoxy, methylthio, ethylthio,
methyl-
amino, ethylamino, dimethylamino, methoxycarbonyl or ethoxycarbonyl.
R1 particularly preferably represents hydrogen, fluorine or chlorine;
R2 particularly preferably represents cyano or thiocarbamoyl;
R3 particularly preferably represents the grouping -A1-A2-A3,
in which
A1 represents a single bond, represents oxygen, sulphur, -SO-, -S02-
-CO- or the grouping -N-A4-, in which A4 represents hydrogen,
methyl, ethyl, n- or i-propyl, methylsulphonyl or ethylsulphonyl,
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
_8_
A1 furthermore represents -methylene, ethane-1,1-diyl, ethane-1,2-diyl,
propane-1,1-diyl, propane-1,2-diyl, propane-1,3-diyl, ethene-1,2-diyl,
propene-1,2-diyl or propene-1,3-diyl,
A2 represents a single bond, represents oxygen, sulphur, -SO-, -S02-,
-CO- or the grouping -N-A4-, in which A4 represents hydrogen,
methyl, ethyl, n- or i-propyl, methoxy, ethoxy, n- or i-propoxy,
methylsulphonyl, ethylsulphonyl, n- or i-propylsulphonyl or phenyl-
sulphonyl, -
A2 furthermore represents methylene, ethane-1,1-diyl, ethane-1,2-diyl,
propane-1,1-diyl, propane-1,2-diyl, propane-1,3-diyl, ethene-1,2-diyl,
propene-1,2-diyl or propene-1,3-diyl,
A3 represents hydrogen, hydroxyl, amino, cyano, nitro, carboxyl,
carbamoyl, sulpho, fluorine, chlorine, bromine, represents in each case
optionally fluorine-, chlorine-, methoxy- or ethoxy-substituted methyl,
ethyl, n- or i-propyl, n-, i-, s- or t-butyl, n-, i-, s- or t-pentyl, methoxy,
ethoxy, n- or i-propoxy, n-, i-, s- or t-butoxy, n-, i-, s- or t-pentyloxy,
methylthio, ethylthio, n- or i-propylthio, n-, i-, s- or t-butylthio,
methylsulphinyl, ethylsulphinyl, n- or i-propylsulphinyl, methyl-
sulphonyl, ethylsulphonyl, n- or i-propylsulphonyl, methylamino,
ethylamino, n- or i-propylamino, n-, i-, s- or t-butylamino, dimethyl-
amino, diethylamino, methoxycarbonyl, ethoxycarbonyl, n- or i-
propoxycarbonyl, represents in each case optionally fluorine- or
chlorine-substituted propenyl, butenyl, propenyloxy, butenyloxy,
propenylamino, butenylamino, propylideneamino, butylideneamino,
propenyloxycarbonyl, butenyloxycarbonyl, propinyl, butinyl, propinyl-
oxy, butinyloxy, propinylamino, butinylamino, propinyloxycarbonyl or
butinyloxycarbonyl, represents in each case optionally fluorine-,
chlorine-, cyano-, carboxyl-, methyl-, ethyl-, n- or i-propyl-, methoxy-
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-9-
carbonyl- or ethoxycarbonyl-substituted cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, cyclopropyloxy, cyclobutyloxy, cyclopentyl-
oxy, cyclohexyloxy, cyclopropylmethyl, cyclobutylmethyl, cyclo-
pentylmethyl, cyclohexylmethyl, cyclo-propylmethoxy, cyclobutyl-
methoxy, cyclopentylmethoxy, cyclohexylmethoxy, cyclopentylidene-
amino, cyclohexylidenamino, cyclopentyloxycarbonyl, cyclohexyloxy-
carbonyl, cyclopentylmethoxycarbonyl or cyclohexylmethoxy-
carbonyl, or represents in each case optionally nitro-, cyano-,
carboxyl-, fluorine-, chlorine-, bromine-, methyl-, ethyl-, n- or i-
propyl-, trifluoromethyl-, methoxy-, ethoxy-, n- or i-propoxy-, di-
fluoromethoxy-, trifluoromethoxy-, methoxycarbonyl- and/or ethoxy-
carbonyl-substituted phenyl, phenyloxy, benzyl, phenylethyl, benzyl-
oxy, phenyloxycarbonyl or benzyloxycarbonyl,
A3 furthermore represents pyrazolyl, imidazolyl, triazolyl, furyl, thienyl,
oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl, thiadiazolyl,
pyridinyl, pyrimidinyl, triazinyl, pyrazolylmethyl, furylmethyl, thienyl
methyl, oxazolylmethyl, isoxazolemethyl, thiazolemethyl, pyridinyl
methyl, pyrimidinylmethyl, pyrazolylmethoxy, furylmethoxy or
pyridylmethoxy;
R4 particularly preferably represents hydrogen, cyano, thiocarbamoyl, nitro,
fluorine, chlorine, bromine, or represents in each case optionally fluorine-
and/or chlorine- substituted methyl, ethyl, n- or i-propyl, methoxy, ethoxy,
methylthio, ethylthio, methylamino, ethylamino or dimethylamino;
R5 particularly preferably represents in each case fluorine- and/or chlorine-
substituted methyl, ethyl, n- or i-propyl; and
R6 particularly preferably represents hydrogen, cyano, thiocarbamoyl, nitro,
fluorine, chlorine, bromine, or represents in each case optionally fluorine-
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
- 10-
and/or chlorine-substituted methyl, ethyl, n- or i-propyl, methoxy, ethoxy,
methylthio, ethylthio, methylamino, ethylamino, dimethylamino, methoxy-
carbonyl or ethoxycarbonyl.
The abovementioned general or preferred radical definitions apply both to the
end
products of the formula (I) and, correspondingly, to the starting materials or
inter-
mediates required in each case for the preparation. These radical definitions
can be
combined with one another as desired, i.e. including combinations between the
given
preferred ranges.
Preference according to the invention is given to the compounds of the formula
(I)
which contain a combination of the meanings listed above as being preferred.
Particular preference according to the invention is given to the compounds of
the
formula (I) which contain a combination of the meanings listed above as being
particularly preferred.
Very particularly preferred compounds of the formula (I) are listed in the
groups
below.
Group 1
F
N-
Ar -N / CF3
(~-1 )
O
Ar has here, for example, the meanings listed below:
4-cyano-phenyl, 2-fluoro-4-cyano-phenyl, 2-chloro-4-cyano-phenyl, 3-fluoro-4-
cyano-phenyl, 3-chloro-4-cyano-phenyl, 2-fluoro-5-chloro-4-cyano-phenyl, 2,5-
dichloro-4-cyano-phenyl, 2-chloro-5-fluoro-4-cyano-phenyl, 2-chloro-4,5-
dicyano-
phenyl, 2-chloro-4-fluoro-5-cyano-phenyl, 2,5-difluoro-4-cyano-phenyl, 2-
chloro-4-
CA 02327806 2000-10-06
Le A 32 986-Forei~~n Countries
-11-
cyano-5-methyl-phenyl, 2-fluoro-4-cyano-S-methyl-phenyl, 2-chloro-4-cyano-5-
tri-
fluoromethyl-phenyl, 2-fluoro-4-cyano-5-trifluoromethyl-phenyl, 2,5-difluoro-4-
thio-
carbamoyl-phenyl, 2-fluoro-4-cyano-5-methoxy-phenyl, 2-fluoro-4-cyano-S-i-
pro-poxy-phenyl, 2-chloro-4-cyano-S-(2-propinyloxy)-phenyl, 2-fluoro-4-cyano-5-
(1-
methyl-2-propinyloxy)-phenyl, 2-chloro-4-thiocarbamoyl-5-i-propoxy-phenyl,
2-fluoro-4-cyano-5-(2-propenyloxy)-phenyl, 2-chloro-4-cyano-5-methylsulphonyl-
amino-phenyl, 2-fluoro-4-cyano-5-ethylsulphonylamino-phenyl, 2-fluoro-4-thio-
carbamoyl-5-methylsulphonylamino-phenyl, 2-chloro-4-cyano-5-ethylsulphonyl-
amino-phenyl, 2-fluoro-4-cyano-5-cyclopropylsulphonylamino-phenyl, 2-fluoro-4-
cyano-5-i-propylsulphonylamino-phenyl, 2-chloro-4-thiocarbamoyl-5-ethyl-
sulphonylamino-phenyl, 2-chloro-4-cyano-S-cyanamino-phenyl, 2-fluoro-4-cyano-5-
trifluoromethylsulphonylamino-phenyl, 2-fluoro-4-cyano-5-(2,2-difluoroethyl-
sulphonylamino)-phenyl, 2-fluoro-4-cyano-S-phenylsulphonylamino-phenyl, 2-
fluoro-4-cyano-5-t-butylsulphonylamino-phenyl, 2-chloro-4-cyano-S-methoxy-
carbonyl-phenyl, 2-fluoro-4-cyano-5-ethoxycarbonyl-phenyl, 2-fluoro-4-thio-
carbamoyl-S-methoxycarbonyl-phenyl, 2-fluoro-4-cyano-S-(1-methyl-2-propinyl-
thio)-phenyl, 2-fluoro-4-cyano-5-methylamino-phenyl, 2-chloro-4-thiocarbamoyl-
5-
methoxycarbonylmethyl-phenyl, 2-chloro-4-cyano-S-(N-methyl-ethylsulphonyl-
amino)-phenyl, 2-chloro-4-cyano-5-i-propoxycarbonyl-phenyl, 2-fluoro-4-cyano-5-
(bis-ethylsulphonyl-amino)-phenyl, 2-fluoro-4-cyano-5-(N-methylsulphonyl-N-
ethyl-
sulphonylamino)-phenyl, 2-fluoro-4-cyano-5-( 1-methoxycarbonyl-ethoxy)-phenyl,
2-
fluoro-4-cyano-5-cyclopropyloxy-phenyl, 2-chloro-4-cyano-5-dimethylamino-
phenyl,
2-fluoro-4-cyano-5-tetrahydrofurylmethoxy-phenyl, 2-fluoro-4-cyano-5-amino-
phenyl, 2-fluoro-4-cyano-S-methylaminocarbonyl-phenyl, 2-fluoro-4-cyano-5-
methylsulphonyloxy-phenyl, 2-chloro-4-cyano-5-difluoromethoxy-phenyl, 2-fluoro-
4-cyano-5-ethoxycarbonylmethoxy-phenyl, 2-fluoro-4-cyano-S-dimethylamino-
carbonyl-phenyl, 2-fluoro-4-cyano-5-cyanomethoxy-phenyl, 2-fluoro-4-cyano-5-(2-
chloro-2-propenyloxy)-phenyl, 2-fluoro-4-cyano-5-hydroxy-phenyl, 2-fluoro-4-
cyano-5-nitro-phenyl, 2-fluoro-4-cyano-5-diethoxyphosphorylamino-phenyl, 2-
fluoro-4-cyano-5-chlorosulphonyl-phenyl, 2-fluoro-4-cyano-5-formylamino-
phenyl,
2-chloro-4-cyano-5-ethoxycarbonyloxy-phenyl, 2-fluoro-4-cyano-5-diethoxy-
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-12-
phosphorylinethoxy-phenyl, 2-chloro-4-cyano-S-hydroxy-phenyl, 2-fluoro-4-cyano-
S-(N,N-diacetyl-amino)-phenyl, 2-fluoro-4-cyano-S-acetylamino-phenyl, 2-chloro-
4-
cyano-S-thiocyanato-phenyl, 2-fluoro-4-cyano-S-diethylaminooxy-phenyl, 2-
fluoro-
4-cyano-S-tetrahydrofuryloxy-phenyl, 2-fluoro-4-cyano-S-ureido-phenyl, 2-
fluoro-4-
S cyano-S-dimethoxymethyleneamino-phenyl, 2-chloro-4-cyano-S-ethoxymethylene-
amino-phenyl, 2-fluoro-4-cyano-S-(2-chloro-ethoxycarbonyloxy)-phenyl, 2-chloro-
4-
cyano-S-dimethylaminomethylenamino-phenyl, 2-chloro-4-cyano-S-(perhydropyran-
4-yloxy)-phenyl, 2-fluoro-4-cyano-S-(2-methoxycarbonyl-ethyl)-phenyl, 2-chloro-
4-
cyano-S-(2-carboxy-2-chloro-ethyl)-phenyl, 2-fluoro-4-cyano-S-(2-chloro-2-
methoxycarbonyl-ethyl)-phenyl, 2-fluoro-4-cyano-S-(2-chloro-2-s-
butoxycarbonyl)-
phenyl, 2-fluoro-4-cyano-S-(2-chloro-2-carbamoyl-ethyl)-phenyl, 2-fluoro-4-
cyano-
S-(2-chloro-2-methoxycarbonyl-1-methyl-ethyl)-phenyl, 2-fluoro-4-cyano-S-(1,2-
dibromo-2-methoxycarbonyl-ethyl)-phenyl, 2-chloro-4-cyano-S-(2-chloro-2-i-
propoxy-carbonyl-ethyl)-phenyl, 2-fluoro-4-cyano-S-(2-carboxy-2-chloro-ethyl)-
1 S phenyl, 2-fluoro-4-cyano-S-(2-chloro-2-ethylaminocarbonyl-ethyl)-phenyl, 2-
fluoro-
4-cyano-S-(2-allylaminocarbonyl-2-chloro-ethyl)-phenyl, 2-fluoro-4-cyano-S-(2-
ethoxycarbonyl-ethenyl)-phenyl, 2-fluoro-4-cyano-S-(2-chloro-2-
cyclopropylamino-
carbonyl-ethyl)-phenyl, 2-fluoro-4-cyano-S-(2-chloro-2-dimethylaminocarbonyl-
ethyl)-phenyl, 2-chloro-4-cyano-S-(2-chloro-2-ethylsulphonylaminocarbonyl-
ethyl)-
phenyl, 2-fluoro-4-thiocarbamoyl-S-(2-ethylaminocarbonyl-ethenyl)-phenyl, 2-
fluoro-4-cyano-S-(1-ethoxycarbonyl-ethyl)-phenyl, 2-chloro-4-cyano-S-(1-ethoxy-
carbonylethyl)-phenyl, 2-chloro-4-cyano-S-carboxy-phenyl, 2-fluoro-4-cyano-S-i-
butoxy-phenyl, 2-chloro-4-cyano-S-i-butoxy-phenyl, 2-chloro-4-cyano-S-(2-
methoxy-
ethoxy)-phenyl, 2-fluoro-4-cyano-S-(N-acetyl-N-methylsulphonyl-amino)-phenyl,
2-
2S fluoro-4-cyano-S-(N-propionyl-N-methylsulphonyl-amino)-phenyl, 2-fluoro-4-
cyano-
S-(N-i-butyroyl-N-methylsulphonyl-amino)-phenyl, 2-fluoro-4-cyano-S-(N-
pivaloyl-
N-methylsulphonyl-amino)-phenyl, 2-fluoro-4-cyano-S-(N-benzoyl-N-methylsulph-
onyl-amino)-phenyl, 2-fluoro-4-cyano-S-(N-(4-methoxy-benzoyl)-N-methyl-
sulphonyl-amino)-phenyl, 2-fluoro-4-cyano-S-(N-acetyl-N-ethylsulphonyl-amino)-
phenyl, 2-fluoro-4-cyano-S-(N-propionyl-N-ethylsulphonyl-amino)-phenyl, 2-
fluoro-
4-cyano-S-(N-i-butyroyl-N-ethylsulphonyl-amino)-phenyl, 2-fluoro-4-cyano-S-(N-
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-13-
pivaloyl-N-ethylsulphonyl-amino)-phenyl, 2-fluoro-4-cyano-S-(N-benzoyl-N-ethyl-
sulphonyl-amino)-phenyl, 2-fluoro-4-cyano-5-(N-(4-methoxy-benzoyl)-N-ethyl-
sulphonyl-amino)-phenyl, 2-fluoro-4-thiocarbamoyl-5-(N-acetyl-N-
methylsulphonyl-
amino)-phenyl, 2-fluoro-4-thiocarbamoyl-S-(N-propionyl-N-methylsulphonyl-
S amino)-phenyl, 2-fluoro-4-thiocarbamoyl-5-(N-i-butyroyl-N-methylsulphonyl-
amino)-phenyl, 2-fluoro-4-thiocarbamoyl-5-(N-pivaloyl-N-methylsulphonyl-amino)-
phenyl, 2-fluoro-4-thiocarbamoyl-S-(N-benzoyl-N-methylsulphonyl-amino)-phenyl,
2-fluoro-4 thiocarbamoyl-5-(N-(4-methoxy-benzoyl)-N-methylsulphonyl-amino)-
phenyl, 2-fluoro-4-thiocarbamoyl-5-(N-acetyl-N-ethylsulphonyl-amino)-phenyl, 2-
fluoro-4-thiocarbamoyl-5-(N-propionyl-N-ethylsulphonyl-amino)-phenyl, 2-fluoro-
4-
thiocarbamoyl-S-(N-i-butyroyl-N-ethylsulphonyl-amino)-phenyl, 2-fluoro-4-thio-
carbamoyl-5-(N-pivaloyl-N-ethylsulphonyl-amino)-phenyl, 2-fluoro-4-thio-
carbamoyl-5-(N-benzoyl-N-ethylsulphonyl-amino)-phenyl, 2-fluoro-4-thio-
carbamoyl-5-(N-(4-methoxy-benzoyl)-N-ethylsulphonyl-amino)-phenyl.
Grou 2
N-
i
Ar-N ~ CF3
W-2)
O
Ar has here, for example, the meanings listed above in Group 1.
Group 3
N-
i
Ar-N ~ CF3
O H (~-3)
3
Ar has here, for example, the meanings listed above in Group 1.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
- 14-
Grou 4
CH3
N-
Ar-N / CF3 (lA-4)
O CH3
S Ar has here, for example, the meanings listed above in Group 1.
Group 5
N-
/
Ar-N
CF3 (IA-5)
O CH3
Ar has here, for example, the meanings listed above in Group 1.
Group 6
CH3
N-
/
Ar-N
CF3 (~-6)
O
Ar has here, for example, the meanings listed above in Group 1.
Group 7
CI
N-
/
Ar-N / CF3
O
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-15-
Ar has here, for example, the meanings listed above in Group 1.
Group 8
N-
Ar-N ~ CHF2
(~-8)
O
Ar has here, for example, the meanings listed above in Group 1.
Gr_ oup 9
CI
N-
Ar-N ~ CF3
O CH3
Ar has here, for example, the meanings listed above in Group 1.
Group 10
CH3
N-
i
Ar-N / CF3
(IA-10)
O
Ar has here, for example, the meanings listed above in Group 1.
Groin 11
N-
Ar-N / CHFZ
(IA-11 )
O CH3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-16-
Ar has here, for example, the meanings-listed above in Group 1.
Group 12
CH3
N-
Ar-N / CHFZ
(IA-12)
O
Ar has here, for example, the meanings listed above in Group 1.
Group 13
CH3
N-
Ar-N / CFZCI (IA-13)
O
Ar has here, for example, the meanings listed above in Group 1.
Group 14
N-
Ar-N ~ CFZCI
(IA-14)
O CH3
Ar has here, for example, the meanings listed above in Group 1.
Group 1 S
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-17-
N- -
Ar-N / CFZCI
(IA-15)
O
Ar has here, for example, the meanings listed above in Group 1.
Group 16
N-
Ar-N ~ CF3
(IA-16)
O Br
Ar has here, for example, the meanings listed above in Group 1.
Group 17
N-
/
Ar-N ~ CF3
(IA-17)
O CI
Ar has here, for example, the meanings listed above in Group 1.
Group 18
CF3
N-
/
Ar-N
(IA-18)
O
Ar has here, for example, the meanings listed above in Group 1.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
- 18-
Group 19
CF3
N-
Ar-I~ ~ CH3
(IA-19)
O
Ar has here, for example, the meanings listed above in Group 1.
Grou~20
CH3
N-
Ar-N ~ CF3
(IA-20)
O C2H5
Ar has here, for example, the meanings listed above in Group 1.
Group 21
CH3
N-
Ar-N / CHF2
(IA-21 )
O CH3
Ar has here, for example, the meanings listed above in Group 1.
Group 22
CH3
N-
Ar-N / CF2CI
(IA-22)
O CH3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-19-
Ar has here, for example, the meanings-listed above in Group 1.
Group 23
'zFs
(IA-23)
Ar has here, for example, the meanings listed above in Group 1.
Group 24
CH3
N-
Ar-N ~ CF3
(IA-24)
O Br
Ar has here, for example, the meanings listed above in Group 1.
Group 25
N-
/
Ar-N / CF3
(IA-25)
O C2H5
Ar has here, for example, the meanings listed above in Group 1.
Group 26
COOCzH$
N-
Ar-N ~ CF3
O CH3 (~-26)
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-20-
Ar has here, for example, the meanings listed above in Group 1.
S Group 27
COOCZHS
N-
Ar-N ~ CF3
(IA.27)
O
Ar has here, for example, the meanings listed above in Group 1.
Group.2 8
COOH
N-
i
Ar-N ~ CF3
(IA-28)
O CH3
Ar has here, for example, the meanings listed above in Group 1.
Group 29
CN
N-
Ar-N ~ CF3
(IA-29)
O CH3
Ar has here, for example, the meanings listed above in Group 1.
CA 02327806 2000-10-06
' Le A 32 986-Foreign Countries
-21 -
GrouQ 30
CN
N-
Ar-N ~ CF3
(IA-30)
O
Ar has here, for example, the meanings listed above in Group 1.
Groin 31
CSNH2
N-
i
Ar-N / CF3
(IA-31 )
O CH3
Ar has here, for example, the meanings listed above in Group 1.
Group 32
CSNH2
N-
Ar-N ~ CF3
(IA-32)
O
Ar has here, for example, the meanings listed above in Group 1.
The novel substituted phenylpyridazinones of the general formula (I) are
obtained
when
(a) halogenoarenes of the general formula (II)
CA 02327806 2000-10-06
' Le A 32 986-Foreign Countries
-22-
R'
X~
R2 (B)
R3
in which
R1, R2 and R3 are as defined above and
X 1 represents halogen
are reacted with pyridazinones of the general formula (III)
Rs
R5
~N
I
Ra NCH (.III)
O
in which
R4, RS and R6 are as defined above,
or with acid adducts or alkali metal salts of compounds of the formula (III)
if appropriate in the presence of a reaction auxiliary and if appropriate in
the presence
of a diluent,
or when
(b) arylhydrazines of the general formula (IV)
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
- 23 -
NHZ R'
HN
R2
R3
in which
R1, R2 and R3 are as defined above
are reacted with 13-trihalomethyl-enones of the general formula (V)
Rs
R5
O (V)
R4 0x2)3
in which
R4, RS and R6 are as defined above and
X2 represents halogen,
if appropriate in the presence of a reaction auxiliary and if appropriate in
the presence
1 S of a diluent,
or when
(c) hydrazonecarboxylic acids of the general formula (VI)
Rs
R5
N R'
N /
R
COOH ~ ~ 2 (VI)
'R
Rs
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-24-
in which
R1, R2, R3, R4, RS and R6 are as defined above
are condensed with ring-closure, i.e. reacted with a dehydrating agent,
S
or when
(d) 2,4-disubstituted phenylpyridazinones of the general formula (Ia)
Rs
R5
N R'
N /
R ~ (Ia)
O \ z
R
in which
R1, R2, R4, RS and R6 are as defined above
are nitrated, i.e. reacted with a nitrating agent,
or when
(e) hydrazonecarbonyl compounds of the general formula (VII)
Rs
Rs
~ N R'
I
O HN /
R2 (VII)
R3
in which
R1, R2, R3, RS and R6 are as defined above
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-25-
are reacted with alkoxycarbonylmethylenephosphoranes of the general formula
(VIII)
COOR
4 ~
R "P(C6H5)s
(VIII)
in which
R4 is as defined above and
R represents alkyl having 1 to 6 carbon atoms,
or with trialkyl phosphonocarboxylates of the general formula (IX)
COOR
R4' _PO(OR)2
in which
R4 is as defined above and
R represents alkyl having 1 to 6 carbon atoms,
in each case optionally in the presence of a reaction auxiliary and optionally
in the
presence of a diluent.
The compounds of the general formula (I) can also be converted by other
customary
methods into other compounds of the general formula (I) according to the above
definition, for example by nucleophilic substitution (e.g. R3: F -~ OH, SH,
NH2,
OCH3, NHS02CH3) or by further transformations of functional groups (e.g. R2:
F/CIBr -~ CN, CONH2 ~ CN, CN ~ CSNH2, N02 ~ NH2; R3: N02 -~ NH2,
NH2 ~ F, C1, Br, CN, NHS02CH3, S02C1, OCH3 -~ OH, OH ~ OCHzCH=CHZ) -
cf. also the Preparation Examples.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
' -26-
Using, for example, 4-fluoro-6-methyl-5-trifluoromethyl-pyridazin-3-one and
4,5-di-
fluoro-2-methoxy-benzonitrile as starting materials, the course of the
reaction in the
process (a) according to the invention can be illustrated by the following
formula
scheme:
CH3
CH3 F F3C ~ N F
FsC ~ N F / -.. I N
I N~ + ~ I - HF F / I
F ~ H ~CN ~ O
O OCH3 CN
OCH3
Using, for example, 4,4,4-trichloro-3-methyl-2-difluoromethyl-crotonaldehyde
and
4-cyano-2-fluoro-5-propargyloxy-phenylhydrazine as starting materials, the
course of
the reaction in the process (b) according to the invention can be illustrated
by the
following formula scheme:
F H
F H
F ~ F I ~N F
F ~O
+ / I - 3 HCI H C N /
3
H3C CCI3 ~ CN O
~CN
O
Using, for example, 2-chloro-3-chlorodifluoromethyl-4-oxo-2-butenoic acid N-(4-
cyano-2-fluoro-5-methylsulphonylamino-phenyl)-hydrazone as starting material,
the
course of the reaction in the process (c) according to the invention can be
illustrated
by the following formula scheme:
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
' -27-
H . H
CIF2C w N CIF2C ~ N F
F
i I I
N --~ N
CI COOH / I - H20 CI
\ CN CN
H~N~SOZ H~N~SO2
CH3 CH3
Using, for example, 2-(2-chloro-4-cyano-phenyl)-4,6-dichloro-5-
pentafluoroethyl-
pyridazin-3-one and nitric acid as starting materials, the course of the
reaction in the
process (d) according to the invention can be illustrated by the following
formula
scheme:
CI
F5C2 ~ N CI + HN03 F
CI / ~ - HZO
~CN CN
Using, for example, 3,3,3-trifluoro-2-oxo-propanal I-(4-cyano-2,5-
difluorophenyl-
hydrazone) and ethyl triphenylphosphoranylidene-acetate as starting materials,
the
course of the reaction in the process (e) according to the invention can be
illustrated
by the following formula scheme:
F3C~N F + COOC2H5 F3C ~ ~ N F
'OI N ~ ~ ~ N
I H P~C6H5~3
CN O ~ CN
F F
The formula (II) provides a general definition of the halogenoarenes to be
used as
starting materials in the process (a) according to the invention for preparing
the
CA 02327806 2000-10-06
' . Le A 32 986-Foreign Countries
-28-
compounds of the formula (I). In the formula (II), R1, R2 and R3 preferably or
in
particular have that meaning which has already been mentioned above, in the
description of the compounds of the formula (I) to be prepared according to
the
invention, as being preferred or as being particularly preferred for R1, R2
and R3; X1
S preferably represents fluorine, chlorine or bromine, in particular fluorine.
The starting materials of the formula (II) are known and/or can be prepared by
processes known per se (cf. EP 191181, EP 370332, EP 431373, EP 441004).
The formula (III) provides a general definition of the pyridazinones further
to be used
as starting materials in the process (a) according to the invention. In the
formula (III),
R4, RS and R6 preferably have that meaning which has already been mentioned
above, in the description of the compounds of the formula (I) to be prepared
according to the invention, as being preferred, particularly preferred or very
particularly preferred for R4, RS and R6.
The starting materials of the formula (III) are known and/or can be prepared
by
processes known per se (cf. J. Chem. Soc. 1947, 239; Angew. Chem. 77 ( 1965),
282;
Monatsh. Chem. 120 (1989), 329).
The formula (IV) provides a general definition of the arylhydrazines to be
used as
starting materials in the process (b) according to the invention for preparing
the
compounds of the formula (I). In the formula (IV), R1, R2 and R3 preferably
have
that meaning which has already been mentioned above, in the description of the
compounds of the formula (I) to be prepared according to the invention, as
being
preferred, particularly preferred or very particularly preferred for R1, R2
and R3.
The starting materials of the formula (IV) are known and/or can be prepared by
processes known per se (cf. EP 370332).
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-29-
The formula (V) provides a general definition of the ~i-trihalogenomethyl-
enones
further to be used as starting materials in the process (b) according to the
invention.
1n the formula (V), R4, RS and R6 preferably have that meaning which has
already
been mentioned above, in the description of the compounds of the formula (I)
to be
S prepared according to the invention, as being preferred, particularly
preferred or very
particularly preferred for R4, RS and R6; X2 preferably represents fluorine,
chlorine
or bromine, in particular chlorine.
The starting materials of the formula (V) are known and/or can be prepared by
I O processes known per se (cf. DE 2706700).
The formula (VI) provides a general definition of the hydrazonecarboxylic
acids to be
used as starting materials in the process (c) according to the invention for
preparing
the compounds of the formula (I). In the formula (VI), Rl, R2, R3, R4, RS and
R6
I S preferably have that meaning which has already been mentioned above, in
the
description of the compounds of the formula (I) to be prepared according to
the
invention, as being preferred, particularly preferred or very particularly
preferred for
Rl, R2, R3, R4, RS and R6.
20 The starting materials of the formula (VI) are known and/or can be prepared
by
processes known per se (cf. WO 9639392).
The hydrazonecarboxylic acids of the general formula (VI) are obtained when
arylhydrazines of the general formula (IV)
RZ (.N)
in which
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-30-
R1, R2 and R3 are as defined above
are reacted with ~i-carboxy-enones of the general formula (X)
Rs
R5
(X)
R4 COOH
in which
R4, RS and R6 are as defined above,
if appropriate in the presence of a diluent, such as, for example, ethanol,
and if
appropriate in the presence of a reaction auxiliary, such as, for example,
p-toluenesulphonic acid, at temperatures between 0°C and 100°C.
The formula (Ia) provides a general definition of the 2,4-disubstituted phenyl-
pyridazinones to be used as starting materials in the process (d) according to
the
invention for preparing compounds of the formula (I). In the formula (Ia), R1,
R2,
R4, RS and R6 preferably have that meaning which has already been mentioned
above, in the description of the compounds of the formula (I) to be prepared
according to the invention, as being preferred, particularly preferred or very
particularly preferred for R1, R2, R4, RS and R6.
The starting materials of the formula (Ia) are known and/or can be prepared by
processes known per se (cf. the processes (a) to (c) according to the
invention).
The formula (VII) provides a general definition of the hydrazonecarbonyl
compounds
to be used as starting materials in the process (e) according to the invention
for
preparing compounds of the formula (I). In the formula (VII), R1, R2, R3, RS
and R6
preferably or in particular have those meanings which have already been
mentioned
above, in connection with the description of the compounds of the formula (I)
CA 02327806 2000-10-06
' Le A 32 986-Foreign Countries
-31-
according to the invention, as being preferred, particularly preferred or very
particularly preferred for R1, R2, R3, RS and R6.
The starting materials of the formula (VII) are known and/or can be prepared
by
processes known per se (cf. WO 9707104).
Not yet known from the literature and, as novel compounds, part of the subject-
matter of the present application, are the hydrazonecarbonyl compounds of the
general formula (VIIa)
Rs
R5
~ N R'
I
O HN
\ ~ R2_i
(VIIa)
Ra
in which
R1 represents fluorine, chlorine or bromine,
1 S R2-1 represents cyano, carbamoyl, thiocarbamoyl, or represents in each
case
optionally fluorine- andlor chlorine-substituted alkyl, alkoxy or alkylthio
having in each case 1 or 2 carbon atoms,
R3 represents the grouping -A1-A2-A3
in which
Al represents a single bond, represents oxygen, sulphur, -SO-, -S02-,
-CO- or the grouping -N-A4-, in which A4 represents hydrogen,
hydroxyl, C1-C4-alkyl, C3-C4-alkenyl, C3-C4-alkinyl, C1-C4-alkoxy,
phenyl, Cl-C4-alkylsulphonyl orphenylsulphonyl,
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
' -32-
A1 furthermore represents in each case optionally fluorine-, chlorine- or
bromine-substituted C1-C6-alkanediyl, C2-C6-alkenediyl, C2-C6-aza
alkenediyl, C2-C6-alkinediyl, C3-C6-cycloalkanediyl, C3-C6-cyclo
alkenediyl or phenylene,
A2 represents a single bond, represents oxygen, sulphur, -SO-, -S02-,
-CO- or the grouping -N-A4-, in which A4 represents hydrogen,
hydroxyl, C1-C4-alkyl, C1-C4-alkoxy, phenyl, C1-C4-alkylsulphonyl
or phenylsulphonyl,
A2 furthermore represents in each case optionally fluorine-, chlorine- or
bromine-substituted C1-C6-alkanediyl, C2-C6-alkenediyl, C2-C6-aza
alkenediyl, C2-C6-alkinediyl, C3-C6-cycloalkanediyl, C3-C6-cyclo
I S alkenediyl or phenylene,
A3 represents hydrogen, hydroxyl, amino, cyano, isocyano, thiocyanato,
nitro, carboxyl, carbamoyl, thiocarbamoyl, sulpho, chlorosulphonyl,
fluorine, chlorine, bromine, represents in each case optionally
fluorine-, chlorine- or C1-C4-alkoxy-substituted alkyl, alkoxy, alkyl-
thio, alkylsulphinyl, alkylsulphonyl, alkylamino, dialkylamino,
alkoxycarbonyl or dialkoxy(thio)phosphoryl having in each case 1 to 6
carbon atoms in the alkyl groups, represents in each case optionally
fluorine- or chlorine-substituted alkenyl, alkenyloxy, alkenylamino,
alkylideneamino, alkenyloxycarbonyl, alkinyl, alkinyloxy, alkinyl-
amino or alkinyloxycarbonyl having in each case 2 to 6 carbon atoms
in the alkenyl, alkylidene or alkinyl groups, represents in each case
optionally fluorine-, chlorine-, cyano-, carboxyl-, CI-C4-alkyl- and/or
CI-C4-alkoxy-carbonyl-substituted cycloalkyl, cycloalkyloxy, cyclo-
alkylalkyl, cycloalkylalkoxy, cycloalkylideneamino, cycloalkyloxy-
carbonyl or cycloalkylalkoxycarbonyl having in each case 3 to 6
CA 02327806 2000-10-06
. Le A 32 986-Foreign Countries
-33-
carbon atoms in the cycloalkyl groups and optionally 1 to 4 carbon
atoms in the alkyl groups, or represents in each case optionally nitro-,
cyano-, carboxyl-, fluorine-, chlorine-, bromine-, Cl-C4-alkyl-, Cl-
C4-halogenoalkyl-, Cl-C4-alkyloxy-, Cl-C4-halogenoalkyloxy-
and/or C1-C4-alkoxy-carbonyl-substituted phenyl, phenyloxy, phenyl-
C 1-C4-alkyl, phenyl-C 1-C4-alkoxy, phenyloxycarbonyl or phenyl-C 1-
C4-alkoxycarbonyl,
A3 furthermore represents in each case optionally fully or partially
hydrogenated pyrrolyl, pyrazolyl, imidazolyl, triazolyl, furyl, oxiranyl,
oxetanyl, dioxolanyl, thienyl, oxazolyl, isoxazolyl, thiazolyl, iso-
thiazolyl, oxadiazolyl, thiadiazolyl, pyridinyl, pyrimidinyl, triazinyl,
pyrazolyl-Cl-C4-alkyl, furyl-Cl-C4-alkyl, thienyl-Cl-C4-alkyl, oxa-
zolyl-C1-C4-alkyl, isoxazole-Cl-C4-alkyl, thiazole-C1-C4-alkyl, pyri-
dinyl-C1-C4-alkyl, pyrimidinyl-Cl-C4-alkyl, pyrazolylmethoxy, furyl-
methoxy, represents perhydropyranylmethoxy or pyridylmethoxy,
R5 represents hydrogen, cyano, thiocarbamoyl, nitro, hydroxyl, mercapto,
amino,
fluorine, chlorine, bromine, or represents in each case optionally fluorine-
andlor chlorine-substituted alkyl, alkoxy, alkylthio, alkylamino or dialkyl-
amino having in each case 1 to 4 carbon atoms in the alkyl groups, and
R6 represents hydrogen, cyano, thiocarbamoyl, nitro, hydroxyl, mercapto,
amino,
fluorine, chlorine, bromine, or represents in each case optionally fluorine-
and/or chlorine-substituted alkyl, alkoxy, alkylthio, alkylamino or dialkyl-
amino having in each case 1 to 4 carbon atoms in the alkyl groups.
The novel hydrazonecarbonyl compounds of the general formula (VIIa) are
obtained
when
(a) arylhydrazines of the general formula (Na)
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
' -34-
N H2 R'
HN
\ ~ Rz_~
(IVa)
R3
in which
R1, R2-1 and R3 are as defined above
are reacted with a-dihalogeno-carbonyl compounds of the general formula (XI)
Rs
R5 X3
X3 (XI)
O
in which
RS and R6 are as defined above and
X3 represents halogen (in particular chlorine or bromine),
if appropriate in the presence of a diluent, such as, for example, water, and
if
appropriate in the presence of a reaction auxiliary, such as, for example,
sodium
acetate, at temperatures between 0°C and 100°C (cf. the
Preparation Examples),
or when - in the case that R6 represents hydrogen -
(~3) arylamines of the general formula (XII)
R'
H2N
\ ~ R2_~
(XII)
R3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
' -35-
in which
Rl, R2-1 and R3 are as defined above
are diazotized in a customary manner (for example by reaction with sodium
nitrite
and hydrochloric acid and/or acetic acid), the resulting diazonium compounds
are
reacted in a customary manner with 1,3-dicarbonyl compounds of the general
formula (XIII)
O O
R5'J v _OR
in which
RS is as defined above and
R represents alkyl (preferably methyl or ethyl)
and the resulting compounds of the general formula (XIV)
O OR
R5
~ N R'
I
O HN
\ ~ R2_~ (XIV)
R3
in which
R, R1, R2-1 , R3 and RS are as defined above
are hydrolyzed and decarboxylated in a customary manner.
The formulae (VIII) and (IX) provide general definitions of the
alkoxycarbonylmethylenephosphoranes and trialkyl phosphonocarboxylates,
CA 02327806 2000-10-06
-, ~ Le A 32 986-Foreign Countries
-36-
respectively, furthermore required as starting materials in the process (e)
according to
the invention for preparing compounds of the fornmla (I). In the formulae
(VIII) and
(IX), R4 in each case preferably has that meaning which has already been
mentioned
above, in connection with the description of the compounds of the formula (I)
according to the invention, as being preferred, particularly preferred or very
particularly preferred for R4; R in each case preferably represents alkyl
having 1 to 4
carbon atoms, in particular methyl or ethyl.
The starting materials of the formulae (VIII) and (IX) are known chemicals for
synthesis.
The processes (a), (b), (c), (d) and (e) according to the invention for
preparing the
compounds of the formula (I) are preferably carried out in the presence of a
diluent.
Suitable diluents are, in general, the customary organic solvents. These
preferably
include aliphatic, alicyclic and aromatic, optionally halogenated
hydrocarbons, such
as, for example, pentane, hexane, heptane, petroleum ether, ligroin, benzine,
benzene,
toluene, xylene, chlorobenzene, dichlorobenzene, cyclohexane,
methylcyclohexane,
dichloromethane (methylene chloride), trichloromethane (chloroform) or carbon
tetrachloride, dialkyl ethers, such as, for example, diethyl ether,
diisopropyl ether,
methyl t-butyl ether, ethyl t-butyl ether, methyl t-pentyl ether (MTBE), ethyl
t-pentyl
ether, tetrahydrofuran (THF), 1,4-dioxane, ethylene glycol dimethyl ether or
ethylene
glycol diethyl ether, diethylene glycol dimethyl ether or diethylene glycol
diethyl
ether; dialkyl ketones, such as, for example, acetone, butanone (methyl ethyl
ketone),
methyl i-propyl ketone or methyl i-butyl ketone, nitriles, such as, for
example,
acetonitrile, propionitrile, butyronitrile or benzonitrile; amides, such as,
for example,
N,N-dimethyl-formamide (DMF), N,N-dimethyl-acetamide, N-methyl-formanilide,
N-methyl-pyrrolidone or hexamethyl-phosphoric triamide; esters, such as, for
example, methyl acetate, ethyl acetate, -n- or -i-propyl acetate, -n-, -i- or -
s-butyl
acetate; sulphoxides, such as, for example, dimethyl sulphoxide; alkanols,
such as,
for example, methanol, ethanol, n- or i-propanol, n-, i-, s- or t-butanol,
ethylene
glycol monomethyl ether or ethylene glycol monoethyl ether, diethylene glycol
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-37-
monomethyl ether or diethylene glycol ~monoethyl ether; mixtures thereof with
water
or pure water. In the process (b) according to the invention it is
advantageously also
possible to use acetic acid as diluent.
S The processes (a), (b) and (e) according to the invention for preparing the
compounds
of the formula (I) are preferably carried out in the presence of a suitable
reaction
auxiliary. Suitable reaction auxiliaries are, in general, the customary
inorganic or
organic bases or acid acceptors. These preferably include alkali metal or
alkaline
earth metal acetates, amides, carbonates, bicarbonates, hydrides, hydroxides
or
alkoxides, such as, for example, sodium acetate, potassium acetate or calcium
acetate, lithium amide, sodium amide, potassium amide or calcium amide, sodium
carbonate, potassium carbonate or calcium carbonate, sodium bicarbonate,
potassium
bicarbonate or calcium bicarbonate, lithium hydride, sodium hydride, potassium
hydride or calcium hydride, lithium hydroxide, sodium hydroxide, potassium
hydroxide or calcium hydroxide, sodium methoxide, ethoxide, n- or i-propoxide,
n-,
i-, s- or t-butoxide or potassium methoxide, ethoxide, n- or i-propoxide, n-,
i-, s- or t-
butoxide; furthermore also basic organic nitrogen compounds, such as, for
example,
trimethylamine, triethylamine, tripropylamine, tributylamine, ethyl-
diisopropylamine,
N,N-dimethyl-cyclohexylamine, dicyclohexylamine, ethyl-dicyclohexylamine, N,N-
dimethyl-aniline, N,N-dimethyl-benzylamine, pyridine, 2-methyl-, 3-methyl-, 4-
methyl-, 2,4-dimethyl-, 2,6-dimethyl-, 3,4-dimethyl- and 3,5-dimethyl-
pyridine, 5-
ethyl-2-methyl-pyridine, 4-dimethylamino-pyridine, N-methyl-piperidine, 1,4-
diaza-
bicyclo[2,2,2]-octane (DABCO), 1,5-diazabicyclo[4,3,0]-non-S-ene (DBN), and
1,8-diazabicyclo[5,4,0]-undec-7-ene (DBU).
The process (c) according to the invention for preparing the compounds of the
formula (I) is carried out in the presence of a dehydrating agent. Suitable
dehydrating
agents are the customary dehydrating agents, such as, for example, sulphuric
acid,
methanesulphonic acid, benzenesulphonic acid, p-toluenesulphonic acid, acetic
anhydride and phosphorus (V) oxide.
CA 02327806 2000-10-06
Le A 32 986-Foreisn Countries
-38-
The process (d) according to the invention for preparing the compounds of the
formula (n is carried out using a nitrating agent. Suitable nitrating agents
are the
customary nitrating agents, such as, for example, nitric acid and its mixtures
with
nitrating auxiliaries, such as, for example, sulphuric acid.
When carrying out the processes (a), (b), (c), (d) and (e) according to the
invention,
the reaction temperatures can be varied within a relatively wide range. In
general, the
processes are carried out at temperatures between -20°C and
+200°C, preferably
between 0°C and 150°C, in particular between 10°C and
120°C.
The processes according to the invention are generally carned out under
atmospheric
pressure. However, it is also possible to carry out the processes according to
the
invention under elevated or reduced pressure - in general between 0.1 bar and
10 bar.
For carrying out the processes (a), (b), (c), (d) and (e) according to the
invention, the
starting materials are generally employed in approximately equimolar amounts.
However, it is also possible to use a relatively large excess of one of the
components.
The reaction is generally carried out in a suitable diluent, if appropriate in
the
presence of a reaction auxiliary, and the reaction mixture is generally
stirred at the
required temperature for several hours. Work-up is carried out by customary
methods
(cf. the Preparation Examples).
The active compounds according to the invention can be used as defoliants,
desiccants, haulm killers and, especially, as weed killers. By weeds in the
broadest
sense there are to be understood all plants which grow in locations where they
are
undesired. Whether the substances according to the invention act as total or
selective
herbicides depends essentially on the amount used. The active compounds
according
to the invention can be used, for example, in connection with the following
plants:
Dicotyledonous weeds of the e~ nera: Sinapis, Lepidium, Galium, Stellaria,
Matricaria, Anthemis, Galinsoga, Chenopodium, Urtica, Senecio, Amaranthus,
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-39-
Portulaca, Xanthium, Convolvulus, Ipomoea, Polygonum, Sesbania, Ambrosia,
Cirsium, Carduus, Sonchus, Solanum, Rorippa, Rotala, Lindernia, Lamium,
Veronica, Abutilon, Emex, Datura, Viola, Galeopsis, Papaver, Centaurea,
Trifolium,
Ranunculus, Taraxacum.
Dico~rledonous crops of the genera: Gossypium, Glycine, Beta, Daucus,
Phaseolus,
Pisum, Solanum, Linum, Ipomoea, Vicia, Nicotiana, Lycopersicon, Arachis,
Brassica, Lactuca, Cucumis, Cucurbita.
Monocotyledonous weeds of the eg nera: Echinochloa, Setaria, Panicum,
Digitaria,
Phleum, Poa, Festuca, Eleusine, Brachiaria, Lolium, Bromus, Avena, Cyperus,
Sorghum, Agropyron, Cynodon, Monochoria, Fimbristylis, Sagittaria, Eleocharis,
Scirpus, Paspalum, Ischaemum, Sphenoclea, Dactyloctenium, Agrostis,
Alopecurus,
Apera.
Monocotyledonous crops of the eg nera: Oryza, Zea, Triticum, Hordeum, Avena,
Secale, Sorghum, Panicum, Saccharum, Ananas, Asparagus, Allium.
However, the use of the active compounds according to the invention is in no
way
restricted to these genera, but also extends in the same manner to other
plants.
Depending on the concentration, the compounds are suitable for total weed
control,
for example on industrial terrain and rail tracks and on paths and areas with
or
without tree growth. Equally, the compounds can be employed for controlling
weeds
in perennial crops, for example forests, ornamental tree plantings, orchards,
vineyards, citrus groves, nut orchards, banana plantations, coffee
plantations, tea
plantations, rubber plantations, oil palm plantations, cocoa plantations, soft
fruit
plantings and hop fields, on lawns and turf and pastures and for selective
weed
control in annual crops.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-40-
The compounds of the formula (n according to the invention have strong
herbicidal
activity and a broad activity spectrum when applied to the soil and to above-
ground
parts of plants.
The active compounds can be converted into the customary formulations, such as
solutions, emulsions, wettable powders, suspensions, powders, dusts, pastes,
soluble
powders, granules, suspo-emulsion concentrates, natural and synthetic
substances
impregnated with active compound, and microencapsulations in polymeric
substances.
These formulations are produced in a known manner, for example by mixing the
active compounds with extenders, that is to say liquid solvents and/or solid
Garners,
optionally with the use of surfactants, that is to say emulsifiers and/or
dispersants
and/or foam formers.
If the extender used is water, it is also possible to use, for example,
organic solvents
as auxiliary solvents. Liquid solvents which are mainly suitable are:
aromatics, such
as xylene, toluene or alkylnaphthalenes, chlorinated aromatics and chlorinated
aliphatic hydrocarbons, such as chlorobenzenes, chloroethylenes or methylene
chloride, aliphatic hydrocarbons, such as cyclohexane or paraffins, for
example
petroleum fractions, mineral and vegetable oils, alcohols, such as butanol or
glycol,
and also their ethers and esters, ketones, such as acetone, methyl ethyl
ketone, methyl
isobutyl ketone or cyclohexanone, strongly polar solvents, such as
dimethylformamide and dimethyl sulphoxide, and water.
Suitable solid Garners are: for example ammonium salts and ground natural
minerals,
such as kaolins, clays, talc, chalk, quartz, attapulgite, montmorillonite or
diatomaceous earth, and ground synthetic minerals, such as finely divided
silica,
alumina and silicates; suitable solid Garners for granules are: for example
crushed
and fractionated natural rocks, such as calcite, marble, pumice, sepiolite,
dolomite
and synthetic granules of inorganic and organic meals, and granules of organic
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-41 -
material, such as sawdust, coconut shells, maize cobs and tobacco stalks;
suitable
emulsifiers and/or foam formers are: for example nonionic and anionic
emulsifiers,
such as polyoxyethylene fatty acid esters, polyoxyethylene fatty alcohol
ethers, for
example alkylaryl polyglycol ethers, alkylsulphonates, alkyl sulphates,
S arylsulphonates and protein hydrolysates; suitable dispersants are: for
example
lignosulphite waste liquors and methylcellulose.
Tackifiers, such as carboxymethylcellulose, natural and synthetic polymers in
the
form of powders, granules or latices, such as gum arabic, polyvinyl alcohol
and
polyvinyl acetate, and also natural phospholipids, such as cephalins and
lecithins, and
synthetic phospholipids can be used in the formulations. Other possible
additives are
mineral and vegetable oils.
It is possible to use colorants, such as inorganic pigments, for example iron
oxide,
1 S titanium oxide, Prussian blue, and organic dyestuffs, such as alizarin
dyestuffs, azo
dyestuffs and metal phthalocyanine dyestuffs, and trace nutrients, such as
salts of
iron, manganese, boron, copper, cobalt, molybdenum and zinc.
The formulations generally comprise between 0.1 and 95 per cent by weight of
active
compound, preferably between 0.5 and 90%.
For controlling weeds, the active compounds according to the invention, as
such or in
the form of their formulations, can also be used as mixtures with known
herbicides,
finished formulations or tank mixes being possible.
Possible components for the mixtures are known herbicides, for example
acetochlor, acifluorfen(-sodium), aclonifen, alachlor, alloxydim(-sodium),
ametryne,
amidochlor, amidosulphuron, anilofos, asulam, atrazine, azafenidin,
azimsulphuron,
benazolin(-ethyl), benfuresate, bensulphuron(-methyl), bentazone, benzofenap,
benzoylprop(-ethyl), bialaphos, bifenox, bispyribac(-sodium), bromobutide,
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-42-
bromofenoxim, bromoxynil, butachlor, -butroxydim, butylate, cafenstrole,
caloxydim,
carbetamide, carfentrazone(-ethyl), chlomethoxyfen, chloramben, chloridazon,
chlorimuron(-ethyl), chlornitrofen, chlorsulphuron, chlortoluron, cinidon(-
ethyl),
cinmethylin, cinosulphuron, clethodim, clodinafop(-propargyl), clomazone,
clomeprop, clopyralid, clopyrasulphuron(-methyl), cloransulam(-methyl),
cumyluron,
cyanazine, cybutryne, cycloate, cyclosulphamuron, cycloxydim, cyhalofop(-
butyl),
2,4-D, 2,4-DB, 2,4-DP, desmedipham, diallate, dicamba, diclofop(-methyl),
diclosulam, diethatyl(-ethyl), difenzoquat, diflufenican, diflufenzopyr,
dimefuron,
dimepiperate, dimethachlor, dimethametryn, dimethenamid, dimexyflam,
dinitramine, diphenamid, diquat, dithiopyr, diuron, dymron, epoprodan, EPTC,
esprocarb, ethalfluralin, ethametsulphuron(-methyl), ethofumesate, ethoxyfen,
ethoxysulphuron, etobenzanid, fenoxaprop(-P-ethyl), flamprop(-isopropyl),
flamprop(-isopropyl-L), flamprop(-methyl), flazasulphuron, fluazifop(-P-
butyl),
fluazolate, flucarbazone, flufenacet, flumetsulam, flumiclorac(-pentyl),
flumioxazin,
flumipropyn, flumetsulam, fluometuron, fluorochloridone, fluoroglycofen(-
ethyl),
flupoxam, flupropacil, flurpyrsulphuron(-methyl, -sodium), flurenol(-butyl),
fluridone, fluroxypyr(-meptyl), flurprimidol, flurtamone, fluthiacet(-methyl),
fluthiamide, fomesafen, glufosinate(-ammonium), glyphosate(-
isopropylammonium),
halosafen, haloxyfop(-ethoxyethyl), haloxyfop(-P-methyl), hexazinone,
imazamethabenz(-methyl), imazamethapyr, imazamox, imazapic, imazapyr,
imazaquin, imazethapyr, imazosulphuron, iodosulphron, ioxynil, isopropalin,
isoproturon, isouron, isoxaben, isoxachlortole, isoxaflutole, isoxapyrifop,
lactofen,
lenacil, linuron, MCPA, MCPP, mefenacet, mesotrione, metamitron, metazachlor,
methabenzthiazuron, metobenzuron, metobromuron, (alpha-)metolachlor,
metosulam, metoxuron, metribuzin, metsulphuron(-methyl), molinate,
monolinuron,
naproanilide, napropamide, neburon, nicosulphuron, norflurazon, orbencarb,
oryzalin, oxadiargyl, oxadiazon, oxasulphuron, oxaziclomefone, oxyfluorfen,
paraquat, pelargonic acid, pendimethalin, pentoxazone, phenmedipham,
piperophos,
pretilachlor, primisulphuron(-methyl), prometryn, propachlor, propanil,
propaquizafop, propisochlor, propyzamide, prosulphocarb, prosulphuron,
pyraflufen(-ethyl), pyrazolate, pyrazosulphuron(-ethyl), pyrazoxyfen,
pyribenzoxim,
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
- 43 -
pyributicarb, pyridate, pyriminobac(-methyl), pyrithiobac(-sodium),
quinchlorac,
quinmerac, quinoclamine, quizalofop(-P-ethyl), quizalofop(-P-tefuryl),
rimsulphuron,
sethoxydim, simazine, simetryn, sulcotrione, sulphentrazone,
sulphometuron(-methyl), sulphosate, sulphosulphuron, tebutam, tebuthiuron,
tepraloxydim, terbuthylazine, terbutryn, thenylchlor, thiafluamide, thiazopyr,
thidiazimin, thifensulphuron(-methyl), thiobencarb, tiocarbazil, tralkoxydim,
triallate,
triasulphuron, tribenuron(-methyl), triclopyr, tridiphane, trifluralin and
triflusulphuron.
A mixture with other known active compounds, such as fungicides, insecticides,
acaricides, nematicides, bird repellents, plant nutrients and agents which
improve soil
structure, is also possible.
The active compounds can be used as such, in the form of their formulations or
in the
use forms prepared therefrom by further dilution, such as ready-to-use
solutions,
suspensions, emulsions, powders, pastes and granules. They are used in the
customary manner, for example by watering, spraying, atomizing, scattering.
The active compounds according to the invention can be applied both before and
after emergence of the plants. They can also be incorporated into the soil
before
sowing.
The amount of active compound used can vary within a relatively wide range. It
depends essentially on the nature of the desired effect. In general, the
amounts used
are between 1 g and 10 kg of active compound per hectare of soil surface,
preferably
between 5 g and S kg per ha.
The preparation and the use of the active compounds according to the invention
can
be seen from the examples below.
CA 02327806 2000-10-06
. Le A 32 986-Foreign Countries
Preparation Examples:
Example 1
F3C
~N F
N \
O
'CN
F
5.0 g (18 mmol) of 3,3,3-trifluoro-2-oxo-propanal-1-(2,5-difluoro-4-cyano-
phenyl-
hydrazone) in 150 ml of toluene are admixed with 8.7 g (26 mmol) of methyl
(triphenylphosphoranylidene)acetate, and the mixture is stirred at reflux
temperature
for 3 hours. The mixture is concentrated using a rotary evaporator, and the
residue is
recrystallized from isopropanol.
This gives 1.3 g (24% of theory) of 2-(2,5-difluoro-4-cyano-phenyl)-S-
trifluoro-
methyl-pyridazin-3-one of melting point 143°C.
Example 2
F3C
~N F
HaC N ~ \
O /
CN
F
5.0 g (18 mmol) of 3,3,3-trifluoro-2-oxopropanal-1-(2,5-difluoro-4-cyano-
phenyl-
hydrazone) in 150 ml of toluene are admixed with 9.4 g (26 mmol) of (1-ethoxy-
carbonylethylidene)triphenylphosphorane, and the mixture is stirred at reflux
temperature for 3 hours. The mixture is concentrated using a rotary evaporator
and
the residue is recrystallized from isopropanol.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
- 45 -
This gives 2.41 g (42.5% of theory) of 2-(2,5-difluoro-4-cyano-phenyl)-4-
methyl-5-
trifluoromethyl-pyridazin-3-one of melting point 135°C.
Analogously to Example 1 and 2, and in accordance with the general description
of
the preparation processes according to the invention, it is also possible to
prepare the
compounds of the formula (I) listed in Table 1 below.
Rs
Rs
I ~ N R'
O I / R2 (I)
R3
Table 1: Examples of the compounds of the formula (I)
Ex.
No. R1 R2 R3 R4 RS R6
3 F CN N02 H CF3 H
4 Cl CN N02 H CF3 H
5 F CN N02 H CF3 CH3
6 Cl CN N02 H CF3 CH3
7 F CN N02 CH3 CF3 H
8 Cl CN N02 CH3 CF3 H
9 Cl CN OH H CF3 H
10 F CN OH CH3 CF3 H
11 F CN OCH3 CH3 CF3 H
12 F CN OCZHS CH3 CF3 H
13 F CN OCH(CH3)2 CH3 CF3 H
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-46-
Ex.
No. Rl R2 R3 R4 RS R6
14 F CN OCH2CH=CH2 CH3 CF3 H
1 F CN OCH2C---CH CH3 CF3 H
S
16 F CN OCH(CH3)C---CH CH3 CF3 H
17 F CN OCH2COOCH3 CH3 CF3 H
18 F CN OCH2COOC2H5 CH3 CF3 H
19 F CN OCH2COOCH(CH3)2 CH3 CF3 H
20 F CN OCH2COOCSH" CH3 CF3 H
21 F CN OCH(CH3)COOC2H5 CH3 CF3 H
22 F CN OH H CF3 H
23 F CN OCH3 H CF3 H
24 F CN OC2H5 H CF3 H
25 F CN OCH(CH3)z H CF3 H
26 F CN OCH2CH=CH2 H CF3 H
27 F CN OCHZC=CH H CF3 H
28 F CN OCH(CH3)C=CH H CF3 H
29 F CN OCH2COOCH3 H CF3 H
30 F CN OCH2COOC2H5 H CF3 H
31 F CN OCH2COOCH(CH3)2 H CF3 H
32 F CN OCH2COOCSH" H CF3 H
33 F CN OCH(CH3)COOC2H5 H CF3 H
34 F CN COOC2H5 CH3 CF3 H
35 F CN COOCzHs H CF3 H
36 F CN CONHCH3 CH3 CF3 H
37 F CN CON(CH3)z CH3 CF3 H
38 F CN NHZ CH3 CF3 H
39 F CN NH2 H CF3 H
40 F CN NHSOZCH3 CH3 CF3 H
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-47-
Ex.
No. R' R2 R3 R RS R6
41 F CN NHS02C2H5 CH3 CF3 H
42 F CN NHS02CH2C1 CH3 CF3 H
43 F CN NHS02CF3 CH3 CF3 H
44 F CN NHS02cyclopropyl CH3 CF3 H
45 F CN NHS02CH3 H CF3 H
46 F CN NHS02C2H5 H CF3 H
47 F CN NHS02CH2C1 H CF3 H
48 F CN NHS02CF3 H CF3 H
49 F CN NHS02cyclopropyl H CF3 H
50 Cl CN NH2 H CF3 H
S Cl CN OCH2C---CH H CF3 H
1
52 Cl CN OCH(CH3)C---CH H CF3 H
53 C1 CN OCH2COOCH3 H CF3 H
54 C1 CN OCHZCOOC2H5 H CF3 H
55 C1 CN OCH2COOCH(CH3)2 H CF3 H
56 Cl CN OCHZCOOCSH~ 1 H CF3 H
57 Cl CN NHSOZCH3 CH3 CF3 H
58 Cl CN NHSOZC2H5 CH3 CF3 H
59 C1 CN OCH2C=CH CH3 CF3 H
60 Cl CN OCH(CH3)C---CH CH3 CF3 H
61 Cl CN OCH2COOCH3 CH3 CF3 H
62 C1 CN OCH2COOC2H5 CH3 CF3 H
63 Cl CN OCHZCOOCH(CH3)2 CH3 CF3 H
64 Cl CN OCHZCOOCSH" CH3 CF3 H
65 F CSNHz NHSOzCH3 CH3 CF3 H
66 F CSNHz NHS02C2H5 CH3 CF3 H
67 F CSNHZ NHS02CH3 H CF3 H
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-48-
Ex. -
No. R' R2 R3 R4 RS R6
68 F CSNH2 NHS02C2H5 H CF3 H
69 Cl CSNH2 OCH(CH3)C---CH CH3 CF3 H
70 Cl CSNH2 OCH2COOCSH~, CH3 CF3 H
71 F CSNH2 OCH2CH=CH2 H CF3 H
72 F CSNH2 OCH2C---CH H CF3 H
73 F CSNH2 OCH(CH3)C---CH H CF3 H
74 Cl CSNH2 NHS02CH3 H CF3 H
75 Cl CSNH2 NHS02C2H5 H CF3 H
76 F CSNH2 OCH2CH=CH2 CH3 CF3 H
77 F CSNH2 OCH2C---CH CH3 CF3 H
78 F CSNH2 OCH(CH3)C---CH CH3 CF3 H
79 Cl CSNH2 NHSOZCH3 CH3 CF3 H
80 Cl CSNH2 NHS02CzH5 CH3 CF3 H
81 F CN N(S02CH3)z CH3 CF3 H
82 F CN N(S02C2H5)z CH3 CF3 H
83 F CN N(CH3)S02CH3 CH3 CF3 H
84 F CN N(CH3)S02C2H5 CH3 CF3 H
85 F CSNHZ N(SOzCH3)2 CH3 CF3 H
86 F CSNH2 N(S02CzH5)2 CH3 CF3 H
87 F CN SCH2COOCH3 CH3 CF3 H
88 F CN SCH2COOC2H5 CH3 CF3 H
89 F CF3 NHSOZCH3 CH3 CF3 H
90 F CF3 NHS02C2H5 CH3 CF3 H
91 F CF3 OCH2CH=CHZ CH3 CF3 H
92 F CF3 OCH2C=CH CH3 CF3 H
93 F CF3 OCH2COOCSH,1 CH3 CF3 H
94 F CSNH2 N(CH3)SOZCH3 CH3 CF3 H
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-49-
Ex.
No. Rl R2 R3 R4 RS R6
95 F CSNH2 N(CH3)S02C2H5 CH3 CF3 H
96 F CSNH2 N(S02CH3)z CH3 CF3 H
97 F CSNH2 N(S02C2H5)2 CH3 CF3 H
98 F CN OCH2CH=CH2 H CF3 CH3
99 F CN OCH2C---CH H CF3 CH3
100 F CN OCH(CH3)C--__CH H CF3 CH3
101 F CN OCH2COOCH3 H CF3 CH3
102 F CN OCH2COOC2H5 H CF3 CH3
103 F CN OCH2COOCH(CH3)2 H CF3 CH3
104 F CN OCH2COOCSH1, H CF3 CH3
105 F CN OCH(CH3)COOC2H5 H CF3 CH3
106 F CN NHSOZCH3 H CF3 CH3
107 F CN NHSOZC2H5 H CF3 CH3
108 F CN NHS02CH2C1 H CF3 CH3
109 F CN NHS02CF3 H CF3 CH3
110 F CSNH2 NHS02CH3 H CF3 CH3
111 F CSNH2 NHS02C2H5 H CF3 CH3
112 F CN H H CF3 H
113 F CSNHZ H H CF3 H
114 F CN H CH3 CF3 H
115 F CSNH2 H CH3 CF3 H
116 H CN F H CF3 H
117 H CSNH2 F H CF3 H
118 H CN F CH3 CF3 H
119 H CSNH2 F CH3 CF3 H
120 F CSNH2 F CH3 CF3 H
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-50-
Ex.
No. R' R2 R3 R4 RS R6
121 F CN O CH3 CF3 H
\
~
N
I
~SOz
H C
3
122 F CN O CH3 CF3 H
\
~
N
I
SO
z
HsCi
123 F CN CHs CH3 CF3 H
\NiSOz
I
SO
2
HsCz
124 F CN O CH3 CF3 H
\N I \
SOz /
H5C2
125 F CN O CH3 CF3 H
\N
I
SO
H
Cz z
S
126 F CN O CH3 CF3 H
\N
I
SOz
HSCZ
CI
127 F CN O CH3 CF3 H
\N
I
SO CI
/ z
HsCz
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
- S1 -
Ex.
No. R' R2 R3 R4 RS R6
128 F CN O CH3 CH3 CF3 H
~N~
I
/SOz
HsCz
129 F CN NHS02C2H5 CH3 CF3 CH3
130 F CONH2 OCH3 CH3 CF3 H
131 F CN OCH(CH3)COOCH(CH3)2 CH3 CF3 H
132 F CN OCH(C2H5)COOCH(CH3)2CH3 CF3 H
133 F CN NHS02CH3 CH3 CF3 COOC2H5
134 F CN NHS02C2H5 CH3 CF3 COOC2H5
135 F CN OCH(C2H5)COOCH3 CH3 CF3 H
136 F CN OCH(C2H5)COOC2H5 CH3 CF3 H
137 F CN OCH(CH3)COOCH3 CH3 CF3 H
138 F CN OCH2CN CH3 CF3 H
139 F CN O~ H CF3 H
140 F CN O~ CH3 CF3 H
141 F CN O~ H CF3 CH3
142 F CN O~ CH3 CF3 CH3
143 F CN O~ H CF3 H
144 F CN O~ CH3 CF3 H
145 F CN O~ H CF3 CH3
CA 02327806 2000-10-06
Le A 32 986-Forei~~n Countries
-52-
Ex.
No. R' R2 R3 R4 RS R6
146 F CN O~ CH3 CF3 CH3
147 F CN O ~ H CF3 H
O
148 F CN ~ CH3 CF3 H
O
149 F CN ~ H CF3 CH3
O
150 F CN ~ CH3 CF3 CH3
0
151 F CN I , H CF3 H
CI
O
152 F CN I , CH3 CF3 H
CI
O
153 F CN I , H CF3 CH3
CI
O
154 F CN I , CH3 CF3 CH3
CI
155 F CN O ~ I CH3 CF3 H
O
O~O \
156 F
CN I H CF3 H
O
/
157 O
F CN I H CF3 CH3
O
,
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-53-
Ex.
No. R' R2 R3 R4 RS R6
158 F CN o~o \
O ~ , CH3 CF3 H
O~O \
159 F CN O ~ / CH3 CF3 CH3
160 F CN O O \ I H CF3 H
O
i
161 F CN O O \ I CH3 CF3 H
O
162 F CN O O \ I H CF3 CH3
O
163 F CN O O \ ( CH3 CF3 CH3
O
O~O~CH
164 F CN O\ 3 H CF3 H
CH3
O~O~CH3
165 F CN O\ CH3 CF3 H
CH3
O~O~CH3
166 F CN O\ H CF3 CH3
CH3
O~O~CH3
167 F CN O\ CH3 CF3 CH3
CH3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-54-
Ex.
No. R' R2 R3 R4 RS R6
O'
O~
CzHS
168 F CN o\ H CF3 H
CzHs
O~O~CzHs
169 F CN o\ CH3 CF3 H
CzHs
O~O\CzHS
170 F CN o\ H CF3 CH3
CzHs
O~O'CzHs -
171 F CN o' CH3 CF3 CH3
CzHs
O
172 F CN o/J , H CF3 H
173 F CN o~~ CH3 CF3 H
0
O
174 F CN o~~ H CF3 CH3
O
175 F CN o~~ CH3 CF3 CH3
~/O
O
176 F CN O~ H CF3 H
J
O
o
177 F CN ~ CH3 CF3 H
J
0
O
178 F CN ~ H CF3 CH3
J
0
O
179 F CN
~ CH3 CF3 CH3
O
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-55-
Ex. -
No. R' R2 R3 R4 Rs R6
O O
180 F CN ~ H CF3 H
J
O o
181 F CN ~ CH3 CF3 H
J
O o -
182 F CN ~ H CF3 CH3
J
O O
183 F CN ~ CH3 CF3 CH3
J
184 F CN O~CHO H CF3 H
185 F CN O~CHO CH3 CF3 H
186 F CN O~CHO H CF3 CH3
187 F CN O~CHO CH3 CF3 CH3
O
188 F CN OOH H CF3 H
O
189 F CN OOH CH3 CF3 H
O
190 F CN OOH H CF3 CH3
O
191 F CN OOH CH3 CF3 CH3
O -
192 F CN O~O~ H CF3 H
CH3
O
193 F CN O~O~ CH3 CF3 H
CH3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-56-
Ex.
No. R' R2 R3 R4 RS R6
O
194 F CN O O~ H CF3 CH3
CH3
O
195 F CN O O~ CH3 CF3 CH3
CH3
O
196 F CN p~ ~ H CF3 H
O
O
197 F CN O~ ~ CH3 CF3 H
O
0
198 F CN O~ ~ H CF3 CH3
O
O
199 F CN O~ ~ CH3 CF3 CH3
O
O
200 F CN O~ ~ H CF3 H
O
O
201 F CN O~ ~ CH3 CF3 H
O
O
202 F CN O~ ~ H CF3 CH3
O
O
203 F CN O~ CH3 CF3 CH3
~
O
O
204 F CN O O~ H CF3 CH3
CH3
O
205 F CN O~O~ CH3 CF3 H
I
CH3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-57-
Ex.
No. Rl R2 R3 R4 RS R6
O
206 F CN O O~ H CF3 CH3
CH3
O
207 F CN O O~ CH3 CF3 CH3
CH3
O
208 F CN O~N~CH3 H CF3 H
H
O
209 F CN O~N~CH3 CH3 CF3 H
H
O
210 F CN O~N~CH3 H CF3 CH3
H
O
211 F CN O~N~CH3 CH3 CF3 CH3
H
O
212 F CN O H~CH3 H CF3 H
CH3
O
213 F CN O H'CH3 CH3 CF3 H
CH3
O
214 F CN O~N~CH3 H CF3 CH3
H
CH3
O
21 S F CN O~N~CH3 CH3 CF3 CH3
H
CH3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-58-
Ex.
No. R' R2 R3 R4 RS R6
O
216 F CN O N~CH(CH3)Z H CF3 H
H
CH3
O
217 F CN O N~CH(CH3)2 CH3 CF3 H
H
CH3
O
218 F CN O N~CH(CH3)2 H CF3 CH3
H
CH3
O
219 F CN O N~CH(CH3)z CH3 CF3 CH3
H
CH3
O
220 F CN O~N~Q H CF3 H
H
O
221 F CN O~N~ CH3 CF3 H
H
O
222 F CN O~N~ H CF3 CH3
H
O
223 F CN O~N~Q CH3 CF3 CH3
H
O
224 F CN O N~ H CF3 H
H
CH3
O
225 F CN O~H~ CH3 CF3 H
CH3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-59-
Ex.
No. RI R2 R3 R4 RS R6
O
226 F CN ~ H~ H CF3 CH3
CH3
O
227 F CN ~ H~ CH3 CF3 CH3
CH3
O
O '
228 F CN H / ~ H CF3 H
CH3
O
O
229 F CN CH H / ~ CH3 CF3 H
3
O
O
230 F CN ~N H CF CH
H ~ ~ 3 3
CH3
O
O
231 F CN H / ~ CH3 CF3 CH3
CH3
O
232 F CN ~ N(CH3)2 H CF3 H
CH3
O
233 F CN ~ N(CH3)Z CH3 CF3 H
CH3
O
234 F CN ~~N(CH3)2 H CF3 CH3
CH3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-60-
Ex.
No. R' R2 R3 R4 RS R6
O
235 F CN ~~N(CH3)2 CH3 CF3 CH3
CH3
O
236 F CN O N(C2H5)Z H CF3 H
CH3
O
237 F CN ~~N(CZHS)2 CH3 CF3 H
CH3
O
238 F CN O Y 'N(CZHS)2 H CF3 CH3
'
CH3
O
239 F CN O Y 'N(CZHS)z CH3 CF3 CH3
I
CH3
O
240 F CN C~N~ H CF3 H
I H
CH3
O
241 F CN ~~N~ CH3 CF3 H
H
CH3
O
242 F CN ~~N~ H CF3 CH3
' H
CH3
O
243 F CN C~N~ CH3 CF3 CH3
H
CH3
CA 02327806 2000-10-06
Le A 32 986-Foreman Countries
-61 -
Ex.
No. Rl R2 R3 R4 RS R6
O
244 F CN O H~OH H CF3 H
CH3
O
245 F CN O H~OH CH3 CF3 H
CH3
O
246 F CN O H~OH H CF3 CH3
CH3
O
247 F CN O H~OH CH3 CF3 CH3
CH3
O
248 F CN O~N~O~CH3 H CF3 H
(~
H
CH3 O
O
249 F CN O~N~O~CH3 CH3 CF3 H
II
H
CH3 O
O
250 F CN O H~O~CH3 H CF3 CH3
I I
CH3
O
O
251 F CN O~N~O CH3 CH3 CF3 CH3
H
CH3 O
O
252 F CN O~N~ H CF3 H
I H
CH3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-62-
Ex. -
No. R' R2 R3 R4 RS R6
O
253 F CN O H~ CH3 CF3 H
CH3
O -
254 F CN O H~ H CF3 CH3
CH3
O
255 F CN O H~ CH3 CF3 CH3
CH3
256 F CN OC3H~-n CH3 CF3 H
O
O
257 F CN ~ ~ CH3 CF3 H
CI
O
258 F CN O O~CH3 CH3 CF3 H
CH3
~O N~
259 F CN ~ S02 CH3 CF3 H
O
260 F CN ~ N \ ~ CH3 CF3 H
SOZ CH3
O
261 F CN O~ C H H CF3 H
2 s
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-63-
The compound listed above in Table 1 as Example 10 can, for example, be
prepared
as follows:
F3C ~ ~ N F
N
H3C
C I ~ CN
~~H
S 2.0 g (6.1 mmol) of 2-(4-cyano-2-fluoro-5-methoxy-phenyl)-4-methyl-5-
trifluoro-
methyl-pyridazin-3-one in 100 ml of dichloromethane are admixed with 18.4 g
(18.3
mmol) of boron tribromide (1 molar solution in dichloromethane), the mixture
is
stirred at 25°C for 16 hours, 150 ml of water are then added slowly and
the organic
phase is dried over sodium sulphate and concentrated using a rotary
evaporator.
This gives 1.8 g (94% of theory) of 2-(4-cyano-2-fluoro-5-hydroxy-phenyl)-4-
methyl-5-trifluoromethyl-pyridazin-3-one of melting point 189°C.
The compound listed above in Table 1 as Example 11 can, for example, be
prepared
as follows:
F3C ~ ~ N F
N
H3C
C ~ ~ CN
~~CH3
3.9 g (13.5 mmol) of 3,3,3-trifluoro-2-oxopropanal-1-(4-cyano-2-fluoro-S-
methoxy-
phenylhydrazone) in 150 ml of toluene are admixed with 7.0 g (19.3 mmol) of
(1-ethoxycarbonyl-ethylidene)-triphenylphosphorane, and the mixture is stirred
under
argon at reflux temperature for 2 hours. After concentration using a rotary
evaporator,
the residue is recrystallized from isopropanol.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-64-
This gives 2.2 g (50% of theory) of 2-(4-cyano-2-fluoro-5-methoxy-phenyl)-4-
methyl-S-trifluoromethyl-pyridazin-3-one of melting point 178°C.
The compound listed above in Table 1 as Example 13 can, for example, be
prepared
as follows:
F3C ~ ~ N F
N
H3C
~ CN
~~CH(CH3)2
3.8 g (8 mmol) of 2-(4-bromo-2-fluoro-5-isopropoxy-phenyl)-4-methyl-S-
trifluoro-
methyl-pyridazin-3-one are dissolved in 10 ml of N-methyl-pyrrolidone, admixed
with 0.9 g (9.6 mmol) of copper(I) cyanide and heated under reduced pressure
(0.1
mbar) at 90°C, with distillative removal of 2 ml of solvent. The
mixture is then
heated under atmospheric pressure (under argon) to 170°C and stirred at
this
temperature for 6 hours. After cooling to 20°C, the mixture is stirred
with 100 ml of
ethyl acetate, admixed with 10% strength ammonia solution and filtered. The
filtrate
is washed with water, dried with sodium sulphate and carefully concentrated
under
waterpump vacuum.
This gives 3.0 g (89% of theory) of 2-(4-cyano-2-fluoro-5-isopropoxy-phenyl)-4-
methyl-S-trifluoromethyl-pyridazin-3-one as crude product (content: 85%
according
to HPLC).
For further purification, 2 g of this crude product are chromatographed over
silica gel
using dichloromethane. This gives the pure product (0.7 g, content: 97%
according to
HPLC) of melting point 121 °C.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-65-
The compound listed above in Table 1 as Example 1 S can, for example, be
prepared
as follows:
N
S
0.6 g (1.9 mmol) of 2-(4-cyano-2-fluoro-5-hydroxy-phenyl)-4-methyl-S-trifluoro-
methyl-pyridazin-3-one in 30 ml of N,N-dimethyl-formamide are stirred at
25°C with
0.4 g (3 mmol) of potassium carbonate and 0.31 g (2.1 mmol) of propargyl
bromide
(80% pure) for 60 minutes, and the mixture is then admixed with water and
adjusted
to pH 6 using conc. hydrochloric acid, and precipitated product is filtered
off with
suction, washed with water and dried. For purification, the product is
recrystallized
from isopropanol.
This gives 0.3 g (44% of theory) of 2-(4-cyano-2-fluoro-S-propargyloxy-phenyl)-
4-
methyl-5-trifluoromethyl-pyridazin-3-one of melting point 145°C.
The compound listed above in Table 1 as Example 18 can, for example, be
prepared
as follows:
F3C
~~N F
N
H3C
O ~ /
~CN
O
O ~O-CZHS
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-66-
0.7 g (2.24 mmol) of 2-(4-cyano-2-fluoro-5-hydroxy-phenyl)-4-methyl-5-
trifluoro-
methyl-pyridazin-3-one in 30 ml of N,N-dimethyl-formamide are stirred at
25°C with
0.48 g (3.5 mmol) of potassium carbonate and 0.42 g (2.5 mmol) of ethyl
bromoacetate for 60 minutes, the mixture is then admixed with water and
adjusted to
pH 6 using conc. hydrochloric acid, and precipitated product is filtered off
with
suction, washed with water and dried.
This gives 0.64 g (71 % of theory) of 2-[4-cyano-5-(ethoxycarbonylmethoxy)-2-
fluoro-phenyl]-4-methyl-5-trifluoromethyl-pyridazin-3-one of melting point
95°C.
The compound listed above in Table 1 as Example 41 can, for example, be
prepared
as follows:
F3C ~ ~ N F
N
H3C
O ~ ~ CN
,NH
H5C2 S02
5.5 g (15 mmol) of 3,3,3-trifluoro-2-oxo-propanal-1-(4-cyano-5-ethylsulphonyl
amino-2-fluoro-phenylhydrazone) in 100 ml of toluene are admixed with 8.2 g
(22.5
mmol) of (1-ethoxycarbonyl-ethylidene)-triphenylphosphorane, and the mixture
is
stirred under argon at reflux temperature for 2 hours. After concentration
using a
rotary evaporator, the residue is chromatographed over silica gel using
toluene/ethyl
acetate 3:1.
This gives 3.6 g (59.5% of theory) of 2-(4-cyano-5-ethanesulphonamino-2-fluoro-
phenyl)-4-methyl-5-trifluoromethyl-pyridazin-3-one of melting point
198°C.
The compound listed above in Table 1 as Example 66 can, for example, be
prepared
as follows:
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-67-
F3r
H3
1.6 g (4 mmol) of 2-(4-cyano-5-ethylsulphonylamino-2-fluoro-phenyl)-4-methyl-S-
trifluoromethyl-pyridazin-3-one are initially charged in 30 ml of pyridine and
12 ml
of triethylamine and, at 60-70°C, hydrogen sulphide is introduced
underneath the
surface of the liquid for 90 minutes. After the reaction has ended, the
mixture is
flushed with nitrogen for 60 minutes, cooled to 25°C and stirred with
ice-water,
precipitated product is filtered off with suction and the residue is washed
with water
and dried.
This gives 0.9 g (51% of theory) of 2-(2-fluoro-5-ethylsulphonylamino-4-thio-
carbamoyl-phenyl)-4-methyl-5-trifluoromethyl-pyridazin-3-one of melting point
88°C
The compound listed above in Table 1 as Example 122 can, for example, be
prepared
as follows:
F3C I ~ N F
N
H3C
O I ~ CN
N
HSCZ S0 ~ -2
O
1.2 g (3 mmol) of 2-(4-cyano-S-ethylsulphonylamino-2-fluoro-phenyl)-4-methyl-5-
trifluoromethyl-pyridazin-3-one in 50 ml of acetonitrile are admixed with 0.6
g
(6 mmol) of triethylamine and 0.77 g (6 mmol) of 3-chloro-propionyl chloride,
and
the mixture is stirred at 25°C for 12 hours. After concentration using
a rotary
H5C2 S02 '
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-68-
evaporator, the residue is stirred with-water and acidified with conc.
hydrochloric
acid, and precipitated product is filtered off with suction, washed with water
and
dried.
This gives 1.1 g of (2-[5-(N-ethenylcarbonyl-N-ethylsulphonylamino)-4-cyano-2-
fluoro-phenyl]-4-methyl-5-trifluoromethyl-pyridazin-3-one of melting point
96°C.
The compound listed above in Table 1 as Example 129 can, for example, be
prepared
as follows:
CH3
F3C ~ ~ N F
N
H3C
O ~ ~ CN
,NH
H5C2 S02
1.22 g (3.2 mmol) of 1-[(4-cyano-S-methylsulphonylamino-2-fluoro-phenyl)-
hydrazono]-3-ethoxycarbonyl-1-chloro-1,1-difluoro-propan-2-one in 50 ml of
toluene
are admixed with 1.74 g (4.8 mmol) of (1-ethoxycarbonyl-ethylidene)-triphenyl-
1 S phosphorane, and the mixture is stirred under argon at reflux temperature
for 2 hours.
After concentration using a rotary evaporator, the residue is chromatographed
over
silica gel using toluene/ethyl acetate 3:1.
This gives 0.2 g (15% of theory) of 2-(4-cyano-S-ethanesulphonamino-2-fluoro-
phenyl)-4,6-dimethyl-5-trifluoromethyl-pyridazin-3-one of melting point 191
°C.
The compound listed above in Table 1 as Example 107 can, for example, be
prepared
as follows:
CA 02327806 2000-10-06
' Le A 32 986-Forei~ Countries
-69-
-Hs
F3C ~ ~ N F
N
C ~ ~ CN
,NH
H5C2 S02
1.22 g (3.2 mmol) of 1-[(4-cyano-5-methylsulphonylamino-2-fluoro-phenyl)-
hydrazono]-3-ethoxycarbonyl-1-chloro-1,1-difluoro-propan-2-one in 50 ml of
toluene
are admixed with 1.7 g (4.8 mmol) of (1-ethoxycarbonyl-methylidene)-triphenyl-
phosphorane, and the mixture is stirred under argon at reflux temperature for
2 hours.
After concentration using a rotary evaporator, the residue is chromatographed
over
silica gel using toluene/ethyl acetate 3:1.
This gives 0.2 g (15.5% of theory) of 2-(4-cyano-5-ethylsulphonylamino-2-
fluoro-
phenyl)-6-methyl-5-trifluoromethyl-pyridazin-3-one of melting point
143°C.
Physical data for compounds listed in Table 1 are listed in Table 1 a below.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-70-
Table 1 a:
Ex. No. Physical data
10 m.p.: 189C
log.P = 2.27 a~
11
m.p.: 178C
log.P = 2.76 a~
12 log.P = 3.12 a~
13
m.p.: 121C
log.P = 3.40 a~
log.P = 3.24 a'
14
NMR(CDC13): 2.44-2.45; 4.65-4.68; 7.46-7.49;
8.03 ppm
15
m.p.: 145C
Iog.P = 2.92 a~
18
m.p.: 95C
Iog.P = 3.04 a~
21 m.p.: 118C
23
m.p.: 174C
log.P = 2.41 a~
24
m.p.: 140C
log.P = 2.76 a~
40
m.p.: 202C
log.P = 2.91 a~
41
m.p.: 198C
log.P = 2.42 a~
46 m.p.: 174C
66
m.p.: 88C
Iog.P = 2.44 a~
84
m.p.: 98C
Iog.P = 2.68 a~
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-71-
Ex. No. Physical data
m.p.: 143C
107
log.P = 2.31 a)
112 m.p.: 160C
m.p.: 183C
120
log.P = 2.43 a)
m.p.: 149C
121
log.P = 2.70~a)
m.p.: 95C
122
log.P = 2.89 a)
123 m.p.: 220C
m.p.: 135C
124
log.P = 3.39 a)
125 log.P = 3.03 a)
m.p.: 165C
126
log.P = 3.29 a)
m.p.: 116C
127
log.P = 2.97 a)
128 m.p.: 131 C
m.p.: 189C
129
log.P = 2.65 a)
m.p.: 142C
130
log.P = 1.94 a)
log.P = 3.62 a~
131 NMR(CDC13): 1.17-1.19; 1.25-1.27; 1.70-1.72;
2.43;
4.72-4.78; 6.94-6.96; 7.99 m
log.P = 3.93 a)
132 NMR(CDC13): 1.13-1.26; 2.04-2.13; 2.43;
4.56-4.60;
7.46-7.49 m
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-72-
Ex. No. Physical data
133 m.p.: 134C
134 m.p.: 60C
135 NMR(CDC13): 2.43-2.44; 3.77; 4.61-4.63;
8.01 ppm
~(CDC13): 2.07-2.12; 2.43-2.44; 4.19-4.24;
7.46-
136
7.49; 8.00 ppm
NMR(CDCl3): 1.72-1.74; 2.36; 4.77-4.82;
7.47-7.50;
137
8.01 ppm
138 NMR(CDC13): 2.44-2.46; 4.93; 7.55-7.58;
8.05 ppm
log.P = 3.51
158
NMR(CDC13): 2.44-2.54; 5.03; 7.26; 8.03
ppm
m.p.: 83C
161
log.P = 3.65
256 m.p.: 97C
m.p.: 22 I C
257
log.P = 4.17
log.P = 4.64
258 NMR(CDC13):1.54-I.55; 1.99-2.09;
2.42-2.44;4.25-4.26; 6.91-6.93 ppm
m.p.: 161C
259
log.P = 3.10
log.P = 2.93
260 NMR(CDC13): 1.49-1.54; 2.01; 2.44-2.46;
4.51-4.52; 7.60-7.63; 8.05 ppm
m.p.:
261
log.P = 2.91
CA 02327806 2000-10-06
Le A 32 986-Forei~~n Countries
-73-
The loge values given above in Table 1 were determined in accordance with EEC
Directive 79/831 Annex V.A8 by HPLC (High Performance Liquid Chromatography)
using a reverse-phase column (C 18). Temperature: 43°C.
S (a) Mobile phases for the determination in the acidic range: 0.1 % aqueous
phosphoric
acid, acetonitrile; linear gradient from 10% acetonitrile to 90% acetonitrile -
the
corresponding test results in Table 1 are labelled a).
(b) Mobile phases for the determination in the neutral range: 0.01 molar
aqueous
phosphate buffer solution, acetonitrile; linear gradient from 10% acetonitrile
to 90%
acetonitrile - the corresponding test results in Table 1 are labelled b).
Calibration was carned out using unbranched alkan-2-ones (having 3 to 16
carbon
atoms) with known loge values (determination of the loge values by the
retention
times using linear interpolation between two successive alkanones).
The lambda max values were determined in the maxima of the chromatographic
signals using the UV spectra from 200 nm to 400 nm.
CA 02327806 2000-10-06
Le A 32 986-Forei,~n Countries
-74-
Starting materials of the formula (IV):
Example (IV-1)
H2N
H5C2 S02
15 g (50 mmol) of 81% pure 4-cyano-2-fluoro-5-ethylsulphonylamino-aniline
(known from DE-A-4414568) in a mixture with 100 ml of acetic acid and 20 ml of
conc. sulphuric acid are cooled to 5°C and admixed a little at a time
with 12.7 g (100
mmol) of nitrosylsulphuric acid, and the mixture is stirred at from 5°C
to 10°C for 6
hours; a solution of 33.6 g (140 mmol) of tin(II) chloride dihydrate -
dissolved in 15
ml of cone hydrochloric acid - is then added dropwise at 10°C. The
mixture is stirred
at 25°C for 12 hours, then stirred with ice-water and made alkaline
using conc.
ammonia. The precipitated salt is filtered off with suction, the filtrate is
made slightly
acidic using conc. hydrochloric acid and extracted with ethyl acetate, and the
extract
is dried over sodium sulphate and concentrated using a rotary evaporator. The
residue
is recrystallized from isopropanol.
This gives 12.9 g (33% of theory) of 4-cyano-S-ethylsulphonylamino-2-fluoro-
phenylhydrazine.
Example (IV-1)
F
H2N~
'CN
~~CH3
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-75-
Step 1
H
3
9.2 g (0.4 mol) of sodium metal are dissolved in 200 ml of methanol, and the
solution
is admixed with 30.8 g (0.2 mol) of 2,5-difluoro-4-cyano-aniline. The stirring
apparatus is fitted with a distillation head and heated to 90-95°C, and
about 4/S of the
amount of metanol is distilled off. After an extra stirring time of 30 minutes
at 95°C,
heating is removed, the content of the flask is admixed with water, cooled to
25°C
and adjusted to pH 5 using conc. hydrochloric acid, and precipitated product
is
filtered off with suction, washed with water and dried.
This gives 31.2 g (94% of theory) of 4-cyano-2-fluoro-S-methoxy-aniline of
melting
point 99°C, purity (method): 99% (HPLC, IogP 1.34).
1 S Step 2 - Variant a
F
HzN~~ \
~CN
~~CH3
4.7 g (2.8 mmol) of 4-cyano-2-fluoro-S-methoxy-aniline are admixed with 100 ml
of
conc. hydrochloric acid and 30 ml of water, and the mixture is briefly heated
to 40°C
and then cooled to 0°C. A solution of 2.6 g of sodium nitrite -
dissolved in 30 ml of
water - is then added dropwise, and the mixture is stirred at from 5°C
to 10°C for 60
minutes. The mixture is then filtered and the filtrate is, at 0°C,
added dropwise to a
solution of 18 g (8 mmol) of tin(II) chloride dihydrate in 100 ml of conc.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-76-
hydrochloric acid. The suspension is then stirred at room temperature
(20°C) for 60
minutes, made alkaline using 45% strength aqueous sodium hydroxide solution
and
extracted repeatedly with ethyl acetate. The organic phase is dried over
sodium
sulphate and concentrated under waterpump vacuum using a rotary evaporator.
This gives 3 g (60% of theory) of 4-cyano-2-fluoro-S-methoxy-phenylhydrazine
of
melting point 160°C.
Step 2 - Variant b
F
H2N~
~CN
O\CH3
g (0.5 mol) of sodium metal are dissolved in 400 ml of methanol, and the
solution
is admixed with 68 g (0.4 mol) of 2,5-difluoro-4-cyano-phenylhydrazine. The
stirnng
apparatus is fitted with a distillation head and heated at from 80°C to
85°C, and
about 80% of the methanol are distilled off. After an extra stirnng time of 3
hours at
15 85°C, heating is removed, the content of the flask is admixed with
water, cooled to
25°C and neutralized using conc. hydrochloric acid, and precipitated
product is
filtered off with suction, washed with water and dried. For purification, the
product is
recrystallized from ethanol.
20 This gives 39 g (54% of theory) of 4-cyano-2-fluoro-5-methoxy-
phenylhydrazine of
melting point 163°C.
CA 02327806 2000-10-06
Le A 32 986-Forei;~n Countries
_77_
Starting materials of the formula (VIII
Exampl~VII-1 )
F3C
O
30 g (370 mmol) of sodium acetate are initially charged in 200 ml of water.
With ice-
cooling, 25 g (93 mmol) of 1,1-dibromo-3,3,3-trifluoroacetone are added
dropwise,
and the mixture is stirred at room temperature (about 20°C) for 30
minutes. The
reaction mixture is then admixed with 12 g (72 mmol) of 2,5-difluoro-4-cyano-
phenylhydrazine and stirred at from 40°C to 50°C for 2 hours.
The cold precipitated
product is filtered off with suction, washed with water and dried.
This gives 19 g (95.4% of theory) of 3,3,3-trifluoro-2-oxopropanal-1-(2,5-
difluoro-4-
cyano-phenylhydrazone) of melting point 181 °C.
1 S Example (VII-2)
F
FsC w N~~ \
O
CN
O~CH3
7 g (87 mmol) of sodium acetate in 100 ml of water are admixed with 5.94 g
(22 mmol) of 1,1-dibromo-3,3,3-trifluoro-acetone, and the mixture is stirred
at room
temperature (about 20°C) for 30 minutes, admixed with 3.0 g (17 mmol)
of 4-cyano-
2-fluoro-5-methoxy-phenylhydrazine and stirred at from 40°C to
50°C for 60 minutes
and at 95°C for 10 minutes. After cooling to 20°C, the
precipitated product is filtered
off with suction, washed with water and dried.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
_78_
This gives 4.1 g (83% of theory) of 3,3,3-trifluoro-2-oxo-propanal-1-(4-cyano-
2-
fluoro-5-methoxy-phenylhydrazone) of melting point 168°C.
Example (VII-3)
F
FsC w N~~ \
O / CN
,NH
HSCz S02
4.1 g (50 mmol) of sodium acetate in 50 ml of water are stirred at 80°C
with 3.6 g of
1,1-dibromo-3,3,3-trifluoro-acetone, the mixture is admixed at 25°C
with 2.58 g (10
mmol) of 4-cyano-5-ethylsulphonylamino-2-fluoro-phenylhydrazine, stirred at
from
40°C to 50°C for 90 minutes and cooled to 10°C, and
precipitated product is filtered
off with suction, washed with water and dried.
This gives 3.03 g (83% of theory) of 3,3,3-trifluoro-2-oxopropanal-1-(4-cyano-
5-
ethylsulphonylamino-2-fluoro-phenylhydrazone) of melting point 127°C.
Example (VII-41
CH3 F
H
FsC ~NiN \
O ~ /
~CN
~NH
H5C2 SOz
4.1 g (50 mmol) of sodium acetate in SO ml of water are stirred at 80°C
with 3.6 g of
3,3-dibromo-1,1,1-trifluoro-2-butanone for 30 minutes, and the mixture is
admixed at
25°C with 2.58 g (10 mmol) of 4-cyano-5-ethylsulphonylamino-2-fluoro-
phenyl-
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-79-
hydrazine, stirred at from 40°C to 50°C for 2 hours and cooled
to 10°C. The
precipitated product is filtered off with suction, washed with water and
dried.
This gives 3.2 g (84.2% of theory) of 1,1,1-trifluoro-2-oxo-butanal-3-(4-cyano-
5-
ethylsulphonylamino-2-fluoro-phenylhydrazone) of melting point 211 °C.
Precursors of the formula I;XIV
Example (XIV-1~
CzHs
O O
F
F3C-CF2 w ~~
~N
O
~CN
,NH
H3C-SOZ
6.87 g (30 mmol) of 4-cyano-5-methylsulphonylamino-2-fluoro-aniline are
initially
charged in 100 ml of acetic acid, 4.7 g (34 mmol) of nitrosylsulphuric acid
(92%
strength) are introduced a little at a time at 20°C with ice-cooling,
and the mixture is
stirred at 25°C for 2 hours. At 5-10°C, the mixture is
subsequently added dropwise to
a suspension of 10.6 g (45 mmol) of ethyl pentafluoropropinylacetate and 16.4
g (200
mmol) of sodium acetate in 200 ml of ethanol, the mixture is stirred at
25°C for 12
hours and stirred into water, and precipitated product is filtered off with
suction,
washed with water and dried. For purification, the product is recrystallized
from
isopropanol.
This gives 8.6 g (60.5% of theory) of 1-[(4-cyano-5-methylsulphonylamino-2-
fluoro-
phenyl)hydrazono]-4-ethoxycarbonyl-1,1,1,2,2-pentafluorobutan-3-one of melting
point 165°C.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-80-
Example (XIV-2)
C2Hs
O O
F
FZCIC ~N.~ \
O ~ ~ CN
,NH
H3C-S02
S 10.7 g (47 mmol) of 4-cyano-5-methylsulphonylamino-2-fluoro-aniline are
initially
charged in 100 ml of acetic acid, 7.3 g (53 mmol) of nitrosylsulphuric acid
(92%
strength) are introduced at 20°C a little at a time with ice-cooling,
and the mixture is
stirred at 25°C for 2 hours. At from 5°C to 10°C, the
mixture is then added dropwise
to a suspension of 14.2 g (71 mmol) of ethyl 1,1,1-difluorochloroacetoacetate
and 26
g (313 mmol) of sodium acetate in 200 ml of ethanol, the mixture is stirred at
25°C
for 12 hours and stirred into water, and precipitated product is filtered off
with
suction, washed with water and dried.
This gives 13.7 g (66% of theory) of 1-[(4-cyano-S-methylsulphonylamino-2-
fluoro-
phenyl)hydrazono]-3-ethoxycarbonyl-1,1-difluoro-1-chloro-propan-2-one of
melting
point 175°C.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-81-
Use Examples:
In the use examples, the following known compounds are used for comparison:
H3C
CI
(A)
2-(5-amino-2,4-dichloro-phenyl)-5-methyl-3(2H)-pyridazinone
- known from WO 9639392.
H3C I ~ N CI
B N I \
O /
CI
N02 (B)
4-bromo-2-(2,4-dichloro-5-nitro-phenyl)-5-methyl-3(2H)-pyridazinone
- known from WO 9639392.
H3C ( ~ N CI
Br N I \
O /
CI
NH2
(C)
2-(5-amino-2,4-dichloro-phenyl)-4-bromo-S-methyl-3(2H)-pyridazinone
- known from WO 9639392.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-82-
H3C ~ ~ N CI
N
O ~ /
~CI
N02
(D)
2-(2,4-dichloro-5-nitro-phenyl)-5-methyl-3(2H)-pyridazinone
- known from WO 9639392.
H3
CN
(E)
2,5-difluoro-4-(4-methyl-6-oxo-1 (6H)-pyridazinyl)-benzonitrile
- known from WO 9639392.
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-83-
Example A
Pre-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent, the stated amount of
emulsifier is added and the concentrate is diluted with water to the desired
concentration.
Seeds of the test plants are sown in normal soil. After about 24 hours, the
soil is
sprayed with the preparation of active compound such that the particular
amount of
1 S active compound desired is applied per unit area. The concentration of the
spray
liquor is chosen so that the particular amount of active compound desired is
applied
in 1000 litres of water per hectare.
After three weeks, the degree of damage to the plants is rated in % damage in
comparison to the development of the untreated control.
The figures denote:
0% - no effect (like untreated control)
100% - total destruction
In this test, for example, the compounds of Preparation Example l, 2, 12, 13,
24, 40,
41, 46, 66, 84, 107, 112, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129 and
133
show considerably stronger activity against weeds than the known compounds
(A),
(B), (C), (D) and (E).
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-84-
Example B
Post-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent, the stated amount of
emulsifier is added and the concentrate is diluted with water to the desired
concentration.
Test plants of a height of 5-15 cm are sprayed with the preparation of active
compound such that the particular amounts of active compound desired are
applied
per unit area. The concentration of the spray liquor is chosen so that the
particular
amounts of active compound desired are applied in 10001 of water/ha.
After three weeks, the degree of damage to the plants is rated in % damage in
comparison to the development of the untreated control.
The figures denote:
0% - no effect (like untreated control)
100% - total destruction
In this test, for example, the compounds of Preparation Example 1, 2, 12, 13,
24, 40,
41, 46, 66, 84, 107, 112, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129 and
133
show considerably stronger activity against weeds than the known compounds
(A),
(B), (C), (D) and (E).
CA 02327806 2000-10-06
Le A 32 986-Foreign Countries
-85-
Example C
Phaedon larvae test
Solvent: 7 parts by weight of dimethylformamide
Emulsifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent and the stated amount of
emulsifier, and the concentrate is diluted with water to the desired
concentration.
Cabbage leaves (Brassica oleracea) are treated by being dipped into the
preparation
of active compound of the desired concentration and are populated with mustard
beetle larvae (Phaedon cochleariae) while the leaves are still moist.
After the desired period of time, the kill in % is determined. 100% means that
all
beetle larvae have been killed; 0% means that none of the beetle larvae have
been
killed.
In this test, for example, the compounds of Preparation Examples 1 and 2 show
good
activity.