Note: Descriptions are shown in the official language in which they were submitted.
CA 02328229 2006-04-24
TNTER)!30DY DEVTCE AND Iv~ET>EiOD FOTt TREATIvIFNT OF
OSTEOPOROTIC VETtT)CBRAL COLLAPSE
AAC)KGROUNp
1. Technical Field
The present disclosure relates to an apparatus and method for treating a
structural collapse of the hzunan vertebrae, and more particularly, to an
intervertebral
device for treating collapsed vertebrae due to osteoporotic weakening.
2. Bs~ck~round of Related Art
Osteopenia is a bone condition resulting in a reduction in the normal content
of
the mineral calcium within a bone. The lack of calcium and associated collagen
matrix, which binds the calcium into the bone structure, results in a
weakening of the
overall bone strength. Osteoporosis, the pathological weakening of bone by
severe
demineralization, is brought about by advanced osteopenia and gives rise to
CA 02328229 2000-10-13
WO 99/02214 PCTlUS98/14146
significantly higher incidences of bone fractures. Osteopenia or osteoporosis
in the
spine can result in fracture collapse of one or more vertebrae or bone
segments thereby
shortening and deforming the spine. In some cases, these deformities inhibit a
person's ability to function normally and may also affect a person's ability
to breathe
normally due to the collapse of the vertebral segments. These fractures or
deformities
are found most commonly among post-menopausal women, since it is known that
the
body's circulating level of the female hormone estrogen has a direct effect on
osteopenia. Thus, when a woman's ovaries are either removed or stop
manufacturing
the estrogen hormone, osteoporosis is more likely to occur which could result
in
multiple bone fractures throughout the body.
Osteoporotic fractures are a common health problem and generally occur
principally at the wrists, hip joints, ribs and collar bones. However,
collapses
involving the vertebrae, while the most common, are the least understood of
the
various fractures. Upon collapse of a spinal vertebra, the collapsing vertebra
is
transformed into the shape of a wedge having the narrow portion directed
towards an
anterior direction (front), thus causing the spine to exhibit the classic
forward bending
and the formation of a posterior hump. The collapse is usually opposite and
away
from the posterior compartment or spinal canal housing the spinal cord. A
lesser
occurring collapse of the posterior compartment of a spinal vertebra may
result in a
patient suffering from myelopathy due to cord compression. This total collapse
of the
vertebra with nearly complete loss of the vertebral body mass in all
dimensions is
quite rare except in some cases of metastatic cancer where the collapse may
compress
-2-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
the spinal cord resulting in paraplegia or death.
Once the osteoporotic bone of the spine has become soft enough to permit a
collapse under a relatively normal load, other bones or additional levels of
the spine
often fracture as well. This cascade of fractures creates a deformed,
shortened spinal
column. Secondary problems may then arise, such as interference with normal
breathing, gait disturbance and a social stigma against the person's
appearance.
Collapse of a vertebral body occurs when there is a sudden increase in loading
beyond
that which the bone can tolerate, sometimes as the result of a normal event
like
sneezing or picking up a light object. The membrane surrounding the vertebral
bone,
the periosteum, is richly innervated with pain fibers which when disturbed by
vertebral
collapse administer pain signals, as well as, incite the formation of new bone
growth.
The vertebral collapse causes a loss of the contained vertebral bone marrow
and an
associated loss of vertebral body height. Due to a difference in construction
and
metabolism, the outer hard cortical enclosure of the vertebral bone does not
suffer as
much loss of mineral or strength as the softer interior cancellous bone.
The most important risk factors for bone fractures are an individual's: (1)
age,
(2) genetic factors, (3) environmental factors, (4) hormone levels, (5)
presence of
chronic diseases, and (6) the physical or radiologic characteristics of the
bone.
Although the true incidence of vertebral fracture is unlalown, the evidence is
clear that
it increases exponentially with age in much the same way as for hip fractures.
Between the ages of 60 and 90 years the incidence of vertebral fracture rises
approximately 20-fold in women compared to a 50-fold increase in the risk of
hip
-3-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
fracture. The problem of vertebral collapse is not limited to women alone,
studies
have shown that vertebral osteoporosis is seen in over 20% of men and women
and is
correlated with low dietary calcium intake and low serum vitamin D levels.
Additional significant risk factors included cigarette smoking, low physical
activity and
long-term immobilization. The lowest levels of bone density were seen in women
who
suffered vertebral collapse fractures, most commonly in those having early
menopause.
It has also been shown that when the deformity or collapse of the vertebral
bone
segment is 4 cm or greater in vertebral height the likelihood of back pain is
2.5 times
greater than when the collapse is of a lesser height. This likelihood is
independent of
how many vertebral levels are involved in fractures or whether or not the
deformity
involves anterior wedging, end plate failure or vertebral body crush. It is
clear that
vertebral collapse fractures are a significant clinical and economic problem.
The best treatment for osteoporosis is prevention particularly since the loss
of
bone strength that accompanies bone loss is not known to be reversible.
Identification
1 S of those at risk by measurement of risk factors may help target prevention
efforts.
Many of the factors that are known to increase fracture risk in susceptible
patients can
be treated. Appropriate care or correction of risk factors include cigarette
smoking,
low circulating estrogen (usually associated with menopause), low physical
activity and
long-term immobilization, low dietary calcium intake and low serum vitamin D
levels.
Other treatable risk factors include: peptic ulcer, tuberculosis and illnesses
or
conditions that may cause dizziness, weakness and falling. These factors are
particularly important in the elderly. It is clear that appropriate diet,
exercise and
-4-
CA 02328229 2000-10-13
WO 99/02214 PCTNS98/14146
supportive treatments are helpful in nearly all cases. However, a very large
number of
cases are not preventable since they are strongly influenced by genetic,
medical or
environmental circumstances. In such cases, certain new drugs including oral
alendronate, an aminobisphosphonate or bisphosphonated etidronate taken daily,
can
S progressively increase the bone mass (strength) in the body, including the
spine and
hip areas. Such treatments can reduce the incidence of vertebral fractures,
the
progression of vertebral fracture deformities and height loss in
postmenopausal
osteoporotic women. Unfortunately, these drugs have no beneficial effect to
reverse
the collapse after it has occurred. In fact, regardless of the predisposing
factors, once
the collapse has occurred, pain control and immobilization are essentially the
only
current treatments available. There exists no present method that can acutely
reverse
the collapse, lead to reconstitution of the vertebra and relieve the severe
associated
pain. The current mode of treatments include bed rest, the wearing of a rigid
brace,
sedatives, muscle relaxers, physical therapy modalities and other palliative
measures.
These treatments exhibit some value in pain reduction but generally the
fractured or
collapsed vertebra must be stabilized or fused for the severe pain to
effectively
subside.
More recently, spinal supporting injections of fast setting substances into
the
collapsed vertebrae have been used to fixate the vertebral collapse in order
to stop the
pain and suffering. Such injection substances include tricalcium phosphate,
calcium
carbonate, calcium hydroxyapatite, all of which act essentially like plaster
of Paris.
These injection materials will stop the progression of the vertebral collapse
and
-5-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
subsequently be slowly converted into bone and thereby restore the strength of
the
collapsed. segment. Also used as injection materials are polymerics, such as,
fast
setting polymethylmethacrylate mixed with powdered barium making the injected
materials visible on X-ray images. However, none of these fast-setting
materials and
associated methods of use restore vertebrae height. In order to re-inflate or
re-form
the collapsed vertebra and restore the vertical height, the materials would
have to be
injected within the vertebrae under considerable pressure (up to 8 or 10
atmospheres,
116 to 145 psi, 510 to 638 Newtons) so as to overcome the collapsing force,
muscle
pull and tissue recoil subjected upon the vertebra. At such high injection
pressures,
the injected material may leak through the fractured or collapsed portions of
the
vertebra and enter the adjacent major vessels, possibly causing an immediate
and
potentially lethal blockage. Further, these materials and other self curing
thermoplastics are highly viscous and cannot be injected through a reasonably
sized
hypodermic tube, cannula, catheter or introducer. The use of these materials
also
generate significant heat which may damage the sensitive bone cells leading to
bone
atrophy and delayed integration. In addition, the above-mentioned polymerics
do not
form or integrate into new bone and as such may create a new problem where the
bone and the plastic material have a zone of non-union or pseudoarthrosis.
The embodiments of the present disclosure are described here to overcome the
above limitations and achieve the goals of re-inflating or re-forming
partially collapsed
vertebrae, to restore the vertebral height, stabilize the fracture, integrate
the injected
material into bone and alleviate the severe pain associated with osteoporotic
collapse.
-6-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
In addition, the techniques described herein may also be used in certain cases
of
complete or partial vertebral body collapse from erosion of the bone by a
metastatic
cancer or the like.
SUMMARY
S The present disclosure is directed to an intervertebral apparatus and method
for
treating collapsed vertebrae due to osteoporotic weakening and collapse of
vertebrae.
The interbody device and method for treatment of osteoporotic vertebral
collapse is
specifically designed to re-establish or reform the lost vertebrae height
attributable to
debilitating orthopedic diseases such as osteoporosis and osteopenia.
Accordingly, an apparatus for repairing a collapsed space within vertebral
bodies is disclosed. The apparatus includes an introduces including an
elongate
member having proximal and distal ends and defining a longitudinal bore. The
introduces further includes a projection along an external length thereof, the
projection
facilitating the rotation of the threaded portion into the vertebral bodies.
The elongate
member includes a threaded portion adjacent the distal end and being
configured for
insertion into vertebral bodies to facilitate mounting of the elongate member
to the
vertebral bodies and a catheter at least partially positionable within the
longitudinal
bore of the elongate member of the introduces. The threaded portion of the
elongate
member further includes a collar, the collar having an elastic seal adapted to
form a
seal along an external portion of'the vertebral bodies.
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
The catheter includes a catheter body member having proximal and distal ends,
an inflation lumen extending along at least a portion of the catheter body and
an
expandable membrane adjacent the distal end of the catheter body member in
fluid
communication with the inflation lumen. The expandable membrane is extendible
beyond the distal end of the introduces and positionable between the vertebral
bodies.
The expandable membrane is expandable in response to inflation fluids conveyed
by
the inflation lumen to exert a force on the vertebral bodies to achieve a
desired spacing
therewithin. The apparatus includes a source of inflation fluid in
communication with
the inflation lumen to expand the expandable membrane. The source of inflation
fluid
includes an injected bone growth inducing material.
Preferably, the apparatus also includes a treating agent delivery lumen
extending along at least a portion of the catheter body and in fluid
communication
with an interior of the vertebral bodies. An injection device is coupled to at
least one
of the inflation lumen and treating agent delivery lumen for providing the
inflation
fluids to the expandable membrane and to the treating agent delivery lumen for
providing bone growth inducing materials within the interior of the vertebral
bodies.
The injection device is preferably a high injection pressure syringe.
Preferably, the expandable membrane is releasably attached to the catheter
body
member. An uncoupling sleeve is mounted about the elongate member of the
introduces, wherein the uncoupling sleeve is movable to separate the
expandable
membrane from the catheter body member.
_g_
CA 02328229 2006-04-24
A method Cor refom~ing a collapsed vertebra is also disclosed. The method
includes the steps of mounting an introduces to vertebral body portions to
access a
collapsed area therewithin, the introduces defining a longitudinal bore.
Inserting a
catheter within the longitudinal bore of the introduces, wherein the catheter
includes a
S catheter body having an expandable membrane mounted adjacent a distal end
thereof.
Positioning the expandable membrane within the collapsed area of the vertebral
body
portions and e.~cpanding the expandable membrane whereby the expandable
membrane
exerts a force on the vertebral body portions to increase a dimension of the
collapsed
area to achieve a desired spacing therewithin. The expanding step includes
inflating
the expandable member with inflation fluids. The catheter body includes a
delivery
lumen terminating in an orening in the catheter body member and wherein the
step of
injecting includes introducing the treating agent into the delivery lumen to
be conveyed
thereby and dispensed through the opening.
Preferably, the method further includes the step of injecting a treating agent
into the collapsed area of tl~e vertebral body portions to facilitate bone
growth within
the collapsed area of the vertebral bodies.
Bl2IEr bESCR~rTION OE THE DRA'fVINGS
The present disclosure, both as to its organization and mariner of operation
together with further objectives and advantages may best be understood by
reference
to the following description.
_9_
CA 02328229 2000-10-13
WO 99/02214 PCT/US98I14146
taken in connection with the accompanying drawings, in which:
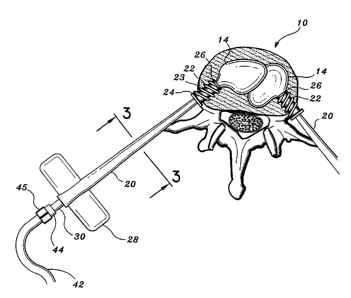
Fig. 1 is a schematic view of the interbody device according to the
present disclosure illustrating a guide needle and associated introducer;
FIG. 2 is a view illustrating a cross-sectional diagrammatic view of a
typical vertebra of the spine;
FIG. 3 is a cross-sectional view of the introducer along lines 3-3 of
FIG. 2;
FIG. 4 is an oblique isometric view illustrating a hand-held, three-ring
pressure syringe according to the present disclosure;
FIG. S is a lateral isometric view illustrating a partially collapsed
vertebra prior to reforming; and
FIG. 5A is lateral isomeric view illustrating a reformed vertebra
utilizing the interbody device according to the present disclosure.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The preferred embodiments of the apparatus and methods disclosed herein are
discussed in terms of orthopedic vertebral procedures and instrumentation
thereof. It is
envisioned, however, that the disclosure is applicable to a wide variety of
procedures
including, but, not limited to joint repair, non-union fractures, spinal
stabilization and
the like. In addition, it is believed that the present method and
instrumentation finds
application in both open and minimally invasive procedures including
endoscopic and
arthroscopic procedures wherein access to the surgical site is achieved
through a
-10-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
cannula or small incision.
In the discussion which follows, the term "proximal", as is traditional, will
refer to the portion of the structure which is closer to the operator, while
the term
"distal" will refer to the portion which is further from the operator.
S The following discussion includes a description of the interbody vertebral
device followed by a description of the preferred method for treatment of
osteoporotic
vertebral collapse in accordance with the present disclosure.
Reference will now be made in detail to the preferred embodiments of the
disclosure, which are illustrated in the accompanying figures. Turning now to
the
figures, wherein like components are designated by like reference numerals
throughout
the various figures, attention is first directed to FIGS. 1-3. In preferred
embodiments,
one or more interbody devices may be simultaneously used to re-inflate and/or
reform
a collapsed spinal vertebrae. FIG. 1 is a cross-sectional representation of a
collapsed
vertebral spinal segment 10 with guide needle 12 engaged within its collapsed
interior
11. The percutaneous insertion of each guide needle 12 is preferably performed
under
the visual aide of a continuous fluoroscopic contrast agent to ensure the
proper
alignment of guide needles 12 within vertebral interior 11. Endoscopic visual
techniques are contemplated as well. Preferably, a first and second guide
needle 12
(not shown) is placed from each side of the back or chest into the collapsed
vertebra
IO along an insertion path through a narrow access between the costovertebral
junctions. Once both guide needles 12 are in a correct position, an introduces
20 with
auger tip 22 and sealing member 24 is inserted over each needle 12 and
subsequently
-11-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98l14146
screwed into the vertebral body 10.
With reference to FIG. 2, each introduces 20 includes butterfly shaped
thumbscrew projections 28 for manually twisting the auger-like threaded tip 22
into
the outer shell of the vertebral body 10. Each auger threaded tip 22 includes
double
start threads 23 having a high pitch so as to facilitate the initial biting
into and
penetration through the tough outer cortex of the vertebra 10. The auger
threaded tip
22 facilitates in positioning, fixating or mounting introduces 20 to the
vertebral body
10. At the base of the threaded portion 23 of the auger tip 22, there is a
sealing
member 24 which includes a small collar 24a and an elastic seal 24b adjacent
the
collar. During insertion of the auger tip 22 within the vertebra 10, the
collar 24a acts
as a stop against the vertebral body 10 while the elastic seal 24b assists in
preventing
the escape of marrow and injected materials from leaking out through the bore
created
by the introduces 20. It is contemplated that the interbody device according
to the
present disclosure can be manufactured in various sizes appropriate for the
safe
1 S insertion of the needles 12 and introducers 20 through the lateral
structures of vertebral
bodies of various dimensions.
Upon proper seating of introducers 20 within vertebra 10, guide needles 12 are
removed from the introducers 20 through a proximal end thereof. As is best
shown in
FIGS. 2 and 3, each catheter 30 includes a first lumen 32 and a second lumen
34
located in each introduces 20. At a distal end of catheter 30 is attached at
least one
balloon or cuff 14 preferably manufactured of a thin, flexible, high-pressure
polymeric
material as is known in the art. Once inflated, the balloons 14 are
dimensioned to
-12-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
conform to the pre-collapse interior dimensions of the particular vertebra
being
reformed. The two balloons 14 are positioned bilaterally into the central
marrow area
of vertebral space 11 of the collapsed vertebra 10. To aide in the
visualization of the
internal structure of vertebral space 1 l and in the proper placement of auger
tips 22
and balloons 14, the fluoroscopic contrast agent may be injected through the
lumens
32 or 34 into vertebral space 11 and be viewed through X-ray images as is
known in
the art.
The balloons 14 are preferably manufactured to withstand high pressures (up to
atmospheres) and retain a volume of up to 10 ml., although balloons meeting
other
10 pressures and volumes are contemplated. In other preferred embodiments, the
balloon
attachment to catheter 30 and associated lumens 32 and 34 is separable by an
uncoupling member or device to ,permit the balloons 14 to be permanently left
within
the vertebral space 11. One example of an uncoupling member includes a sleeve
26
(FIG. 3) which is slidably mounted over catheter 30 and movable in a distal
direction
I S to slide the balloon 14 off the distal end of the catheter 30. With this
arrangement,
balloon 14 would be self sealing, whereby upon removal, the proximal end of
the
balloon 14 attached to the catheter 30 would close or seal. The uncoupling
sleeves 26
are especially beneficial when the balloons 14 are filled with a hardening
material, as
will be discussed below. In addition, the balloon membranes may be
manufactured
from a biodegradable material so as to permit time controlled dissolving of
the
balloons 14 to thereby expose the hardening materials contained therein to the
interior
of vertebral space 11. Such biodegradable balloon membranes may be
manufactured
-13-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
from known materials such as a polylactic acid polymer, a polygalactone
biodegradable film, a hydrogel membrane such as polyvinyl acetate or an
acrylonitrile.
Further, the balloons 14 can be fabricated where only selected segments of the
balloon's membrane would slowly dissolve when exposed to body fluids. This
feature
initially maintains the internal balloon pressure but allows the contained
injected
material to slowly integrate into the recipient bone of vertebra 10.
With particular reference to FIG. 4, a single hand operated syringe 40 is
shown
although, as will be discussed below, as many as four syringes 40 may be used
to
inject materials through lumens 32 and 34 of catheter 30. As such, the
syringes 40 of
the present disclosure are preferably used to hydraulically inflate balloons
14 and to
inject medicants within vertebral space 11, although other similar
injection/inflation
devices such as pumps, squeezable membranes or the like may be used. Each
lumen
32 and 34 engages a separate syringe 40 which acts to inflate balloon 14 and
inject
medicants within vertebral space 11, respectively. Syringes 40 are preferably
three-
ring pressure syringes having finger rings 46 on a collar 48 and a thumb ring
47 on
plunger portion 49. As noted above, the syringes 40 may be filled with a
combination
of injectable materials and/or solutions including sterile saline solution,
fluoroscopic
contrast agent, bone growth inducing materials, hardening materials and the
like. The
injectable materials may be a slurry of calcium complex known to integrate
into bone
with a supporting polymeric filler to improve strength until the fracture has
healed or
fused. Additionally, a bone growth factor, such as bone morphogenic protein
may be
added to the injectate to facilitate the rapid growth of firm bone within
vertebra i0.
-14-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
As will be discussed below, each balloon 14 is inflated separately with a
particular
solution or combination thereof dependent upon the anatomical conditions of
the
collapsed vertebrae 10. The syringes 40 are connected to catheter 30 and
lumens 32
and 34 via high-pressure flexible polymeric tubing 42. The tubing 42 is
attached to
each lumen 32 and 34 and respective syringes 40 with Luer connections 44.
Valves
structures 45 are placed in-line along Luer connections 44, tubing 42 or
syringes 40 to
maintain the inflation pressure in each balloon 14 once inflated. Syringes 40
are
capable of manually providing high amounts of injection pressure (8 or 10
atmospheres) to balloons 14. Through these high injection pressures, the
syringe
solution inflates balloons 14, as well as, cause each balloon 14 to internally
dissect or
collapse the cancellous matrix of the vertebral marrow within vertebral space
11
thereby creating a cavity within the vertebral body 10.
As best seen in FIGS. S and SA, the inflation of balloons 14 create a cavity
within vertebral body 10 and cause the hardened end plates 52 of vertebra 10
to
separate and expand to a point 50 restoring the normal pre-collapse vertebral
height of
vertebra 10. In cases where the side cortical wall 54 of vertebra 10 is
imperfect or
broken, the dimensions of the balloons 14 are such that, upon inflation, the
balloons 14
are maintained within the confines of the vertebral space 11.
The method of treating osteoporotic vertebral collapse according to the
present
disclosure will now be described. The method utilizes a controlled and
monitored
technique which is simple to perform and provides relative safe effective
treatment for
the patient. Preferably, the method is performed percutaneously as opposed to
open
-15-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
surgery. The procedure is performed under aseptic conditions in the operating
room or
in a standard cardiac catheterization room in the X-ray department. The
patient is
partly anesthetized and sedated using appropriate intravenous medications. The
patient
is suspended in a chest and underarm supporting harness to overcome forces
such as
gravity and muscle spasms in the thoracic and lumbar spinal segments. These
forces
participate in the collapsing force imparted on the vertebrae and must be
overcome to
facilitate the re-expansion of the collapsed vertebral bodies. Utilizing an
image-amplifying fluoroscope, X-ray, CT scanner or the like, the points of
entry and
traj ectory to the target vertebrae are noted and marked on the overlying
skin.
Attention to the patient's anatomical detail is necessary to avoid potential
serious
damage to structures normally found adjacent to the vertebrae, such as,
segmental
blood vessels and spinal nerves, as well as, avoiding penetration of the lungs
and other
tissues.
Upon proper alignment of the patient and through guided images (X-ray or the
like) guiding needles 12 are placed well into the center of the affected
vertebral body
10 from a posterolateral approach. A small amount of X-ray opaque contrast dye
such
as OMNIPAQUE (TM) or HYPAQUE (TM) is injected through each needle 12 to
ensure that the needles 12 are properly situated within the vertebrae. A small
amount
of local anesthetic may also be injected within vertebra 10 to reduce the pain
and to
determine that the particular collapsed vertebra is causing the pain
experienced by the
patient. Subsequent to proper insertion of needles 12 within the collapsed
vertebra 10,
introducers 20 are passed over the needles at the insertion points of the
vertebra 10.
-16-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
With the aid of the auger-like threaded tips 22 of introducers 20, each
introducer 20 is
screwed into the cortical or lateral wall 54 of the collapsed vertebral body
10 using
thumbscrew wings 28 positioned proximally on the introducers 20. The guide
needles
12 are then removed. Catheter 30 includes at least one balloon 14 distally
situated and
coupled to a first 32 or second 34 lumen which is introduced within each
catheter 30.
One lumen 32 may be used to inflate balloon 14 while a second lumen 34 may be
provided for the injection of materials such as contrast agent or bone
fixation materials
into the surrounding vertebral space 11.
After insertion within the.collapsed vertebra 10, bath balloons 14 are
hydraulically inflated using a solution of sterile saline and fluoroscopic
contrast agent.
It is contemplated that the other solutions or mixtures previously described
herein may
also be used to inflate the balloons 14 or be injected within the vertebral
space 11.
While under close observation via an X-ray monitor, the syringe 40 is
compressed
creating a pressure, this pressure inflates balloons 14 and correspondingly
expands
vertebral space 11. As this pressure increases, the expanding balloons 14
create a
cavity within the central soft bone area of vertebral space 11. As the
balloons 14 are
further inflated, the pressure resistant end plates 52 of vertebra 10 are
pushed apart
from their collapsed form to a point that substantially restores the original
vertebral
disc height of the collapsed vertebra 10.
Once the balloons 14 are inflated, the tissue is allowed several minutes to
accommodate to the pressures and alterations in the restored vertebral bone
shape. A
first balloon 14 is then deflated leaving a cavity. Into this cavity, rapidly
hardening
-17-
CA 02328229 2000-10-13
WO 99/02214 PCT/US98/14146
materials such as bone growth inducing materials are injected through lumen 34
of
catheter 30 with the use of, e.g., a syringe 40 discussed above. These
hardening
materials may be a calcium based self curing material combined with bone
morphogenic protein or similar fusion-inducing bone growth factor, as
previously
S described. Alternatively, either or all of the balloons 14 fabricated from
an absorbable
material may be filled with the rapid hardening, bone growth inducing material
and
left permanently within the vertebral space 11, as discussed hereinabove. Due
to the
use of two or more inflated balloons 14 within the vertebral space 11, the
deflation of
a first balloon 14 does not render a re-collapse of the vertebra 10 because
the
remaining one or more inflated balloons 14 provide sufficient vertical support
to
vertebra 10. Therefore, a first balloon 14 is deflated and removed. The second
balloon 14 is then deflated and its cavity is likewise injected with bone
growth
inducing substance or the like. The introducers 20 with auger-like tips 22 are
then
unscrewed and removed from the body.
1 S The patient will preferably remain in the traction rigging, or wear a
rigid
supporting brace for a matter of Several minutes or hours as the setting
process
proceeds to completion. A brace might be required for a matter of weeks in
some
cases. Over time the injected bone-inducing hardened material will be replaced
by
bone material providing a rigid vertebral segment. The pain and deformity are
thus
treated rapidly with a desired long-term result.
It will be understood that various modifications may be made to the
embodiments disclosed herein. For example, the number and size of balloons 14
-18-
a
CA 02328229 2006-04-24
inflated within the vertebral spaoe 11 may vary dependent upon the specific
ailment,
dimensions, and anatomical variants of the diseased vertebrae. Also, the
number of
lumens 32, 34 within introduces 20 and corresponding materials transported
therein
may vary to accommodate delivery of solutions, bone growth inducing
substances,
S anesthetic, oontrast agent (fluoroscopic solution) and any combination
thereof to the
vertebral space 11 of vertebra 10. Therefore, the above description should not
be
construed as limiting, bait merely as exemplifications of preferred
embodiments. Those
skilled in the art will envision other modifications within the scope and
spirit of
the present invention.
-19-