Note: Descriptions are shown in the official language in which they were submitted.
CA 02329064 2000-10-19
FUEL CELL
Description
Technical Field
The invention relates to a fuel cell, including at least
a housing, at least one layer of a proton conducting,
polymeric material, which is covered on both sides by
catalyst layers, gas permeable electrodes on the catalyst
layers, and bi-polar plates which closely electrically
contact the electrodes and together with the electrodes
define gas guiding channels.
Prior Art
Such a fuel cell is known from Spektrum der
Wissenschaft, July 1995, Page 98. The channels thereby
extend parallel to each other and are formed into the
bi-polar plates. Their manufacture is correspondingly
expendable and expensive. Furthermore, the high weight to
power ratio of the known fuel cell is unsatisfactory.
Description of the Invention
It is an object of the invention to provide a fuel cell
of the above-mentioned type which is technically simpler and
can be manufactured at lower cost.
This object is achieved in accordance with the invention
with a fuel cell of the above-mentioned type including the
characterizing features from claim 1. Preferred embodiments
are dealt with in the dependent claims.
In the fuel cell in accordance with the invention, it is
provided that the first layer or the second layers are
provided with an undulation, pleating and/or embossing and
that the respectively other layer or layers are of planar
construction. The respectively opposite surfaces of the
first and second layers extend parallel to one another
resulting in an enlargement of the active surface or a
mutual overlapping of the profiles of laterally adjacent
channels, which then results in a significant increase of
the power density and thereby at the same time an increase
of the power to weight ratio. This is of great advantage
-~. I
CA 02329064 2000-10-19
especially for mobile applications.
Which of the two layers is provided with the undulation,
pleating and/or embossing and which is of planar
construction is of little importance within the meaning of
the present invention. However, for technical reasons, an
embodiment is preferred wherein the first layer including
the catalyst layers and electrodes thereon is of planar
construction and the second layer which mainly consists of
metal is provided with the undulation, pleating and/or
embossing. The functional reliability of such embodiments
is generally higher than in the other variant.
The cross-section of the fuel cell can be of similar
construction as undulated cardboard wherein an undulated,
pleated or embossed layer is alternated with a planar layer
until the desired total thickness or total performance is
achieved. The successive channels between the individual
layers are ultimately filed with hydrogen and oxygen
containing gas during the desired use which at the same time
can be used to remove from the fuel cell excess heat and the
water created during the chemical reaction. It is thereby
practical when at least those channels in which water
generation takes place are oriented vertically during the
desired use and if the gas flows therethrough from top to
bottom. At sufficiently high temperatures of the fuel cell,
the water is in the gaseous state, which means in the form
of water vapour. The actual water separation can in such a
case be carried out outside the channels and the remaining,
unused gas temperature adjusted, if necessary, and returned
in a circuit into the channels. The remaining channels
preferably extend parallel to the "water conducting"
channels. However, they can also extend transverse to those
channels.
In embodiments wherein either the first or second layer
is elastically compressible and the outer' layer respectively
in contact therewith is non-compressible, a good sealing of
adjacent channels is automatically achieved, which allows
for compensation of manufacturing variances and achievement
'3- of
CA 02329064 2000-10-19
of good efficiency. When the undulation, pleating and/or
embossing forms a component of the compressible layer, the
manufacture is especially simple. It can be carried out by
using methods applied in textile processing, especially by
using deep drawing and/or pleating processes. Similar
methods are known from sheet metal processing. They can be
used accordingly.
The first layer can be made of a porous foil, a woven
fabric or knitted fabric or a fleece of short or endless
fibres which is filled to saturation with. a perfluorated
ionomer, whereby the perfluorated ionomer can be a
polytetrafluoro ethylene with sulfonated perfluorovinyl
ether side chains. As an alternative, the microfibre fleece
can be filled with a 1 to 5 molar, aqueous sulfuric acid
solution or with concentrated phosphoric acid. It is
further possible to use hydrated zirconium phosphate and
ammonium dihydrogen phosphate.
An improvement of the efficiency of the fuel cell is
achieved with decreasing thickness of the first layer.
Under this-aspect it has been shown advantageous when a
fleece included in the first layer is made of microfibres
from fibrils or microfilaments. However, the use of porous
foils is also possible. Materials which have been proven
are especially PTFE (polytetrafluoroethylene) and
polysulfone.
When a microfibre fleece is used as the proton
conductor, it is impregnated to saturation with an
electrolyte; whereby the microfibre fleece is chemically and
physically inert relative to the electrolyte at temperatures
up to 200°C as well as under oxidizing and reducing
conditions, whereby the weight of the microfibre fleece is
20 to 200 g/m2; whereby the fleece material thickness is a
maximum of 1 mm and the pore volume is 65 to 920.
The mean pore radius of the microfibre fleece shall be
20 nm to 10 Vim.
The first layer can be directly laminated with the
electrodes, for example by direct, mutual adhesion in spaced
~3
CA 02329064 2000-10-19
apart areas. The electrodes can thereby be made of
carbonized fibres of polymeric material, for example of
carbonized polyacrylonitrile or pitch fibres and have a
surface weight of 20 to 100 g/m2 and a thickness of less than
0.5 mm.
The second layer in the simplest case consists of a
sheet metal of planar shape. The heat generated in the fuel
cell during the intended use can be conducted away
therethrough exclusively or in addition and parallel to the
current generated.
Independent of the specific construction of the first
and second layer, the channels at least at one end can open
into openings of comb-shaped interengaging protrusions of
the housing which sealingly engage on both sides the layer
provided with the undulation, pleating and/or embossing. It
is thereby ensured that the reaction gases conducted through
the fuel cell during the intended use can only react by
proton conduction through the first layer.
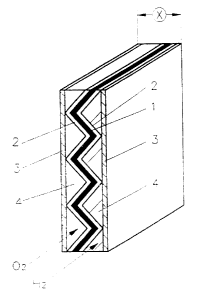
An exemplary embodiment of the fuel cell is shown in
Figure 1. It includes a not illustrated housing, a first
layer 1 of a proton conducting, polymeric material, which is
covered on both sides by catalyst layers, gas permeable
electrodes 2 on the catalyst layers and second layers 3
positioned both sides of layer 1 and in the form of
electrically conductive plates which electrically
conductively engage the electrodes 2 at closely spaced
locations and together with the electrodes 2 define gas
conducting channels 4, whereby the first layer 1 is provided
with a pleating for the forming of channels 4 and whereby
the second layers 3 are formed by planar metallic sheets
which directly engage the folded edges of the electrodes 2
on the first layer 1. During the intended use, oxygen and
hydrogen containing gases are conducted through the channels
separated by the layer 1 and after reaction at the catalyst
surface are reacted with one another through the first layer
1. The current thereby generated is conducted by the
electrodes 2 to the second layer 3 and conducted off
-5~-
CA 02329064 2000-10-19
therethrough. The heat liberated during the chemical
process fallows the same path.
The fuel cell schematically illustrated in the drawing
shows the smallest functional unit. Its current generation
is principally dependent on the size of the first layer as
well as the cross-section of the channels 4. It can be
increased by increase of the corresponding values or by
parallel connection of identically constructed units in a
closed off package.
In the simplest case in a fuel cell in accordance with
the invention, the first layer is provided with an
undulation, pleating and/or embossing for the formation of
the channels and the second layers 3 are of planar
construction and formed by metallic sheets. It is thereby
advantageous when the first layer 1 includes a fleece of
short or endless fibres in order to provide the first layer
1 with the required mechanical stability. The electrolyte
included in the interstices of such a fleece is thereby not
loaded in a mechanical respect. It can be optimized in type
and amount for the achievement of an especially good
electrochemical efficiency. The use of a porous foil which
pores include a corresponding electrolyte is also possible.
The temperature resistance of the first layer 1 is
essentially determined by the type of the fleece or porous
foil contained therein. An especially high temperature
resistance is achieved when a fleece or porous foil of PTFE
or polysulfone is used in the first layer. The operating
temperatures can exceed 90°C in such an embodiment without
the generation of catalyst poisons in the course of chemical
side reactions during an operation with preformed methanol
and without a reduction of the surface life of the fuel
cell. Fleece materials of microfibres have the advantage
that the pore structure takes up an extremely large,
relative volume, is continuous and has large pores which are
covered by fibres in direction of the surfaces. The
undesired washout of the electrolyte by the water generated
during the intended use is hereby suppressed.
CA 02329064 2000-10-19
The layer of polymeric material can be formed by a
microfibre fleece impregnated to saturation with an
electrolyte or by a plastic foil which is sintered or drawn
for the generation of pores. The fibres or the foil are
thereby made of a polymeric material which is chemically
inert relative to the electrolyte under the conditions of
the intended use, whereby temperatures up to 200°C can be
present under oxidizing as well as reducing conditions.
Polytetrafluoroethylene is especially suited for the
manufacture of this layer.
The fibres can be endless or mutually adhered without
the use of secondary adhesives, for example by welding
and/or a mutual amalgamation of the fibres.
A fleece is preferably used which has a longitudinal/
transverse tension strength of more than 50 MPa, an
elongation capacity of 50 to 100% and an E-module of 2 to 4
GPa, and which is physically stable at ambient temperatures
of up to 200°C. The fleece weight should be 20 to 200 g/m2
at a thickness of less than 1 mm when impregnated with the
electrolyte, a mean pore radius of 0.1 to 10 ~m and a pore
volume of 65 to 920. The dielectric constant can be 0.3200
to 3500 Hz.
The fleece framework ensures the mechanical stability of
the membrane so that the electrolyte no longer has to
perform this task and thereby can be used just for the
control of the electrochemical processes in the cell and at
a significantly lower concentration. The material cost for
the membrane is thereby reduced by up to 90% compared to the
cost of manufacturing, for example, a correspondingly
dimensioned foil of perfluorated ionomer.
The temperature resistance of the membrane in accordance
with the invention is essentially determined by the fleece
material, unless affected by other factors. This condition
allows the use of the membrane even in fuel cells operated
with reformed methanol; the amount of catalyst poisons
generated during the course of chemical side reactions is
further reduced at any rate at operating temperatures above
4
CA 02329064 2000-10-19
90°C, which results in a longer service life of the cell.
The following examples are meant to illustrate that the
invention in different variants is always superior to a pure
polymer membrane of perfluorated ionomer. The basic
materials are common to all examples and are described in
the following:
Fleece material: polysulfone fibres with rectangular cross-
section (width 6 to 13 ~,m, height 1.7 to 2.4 Vim).
Mechanical properties of the polysulfone material: Melting
range: 343 to 399°C.
Tension strength: 70 MPa
Elongation capacity: 50 to 100%
E-module: 2.4 GPa
Bending temperature under 1.8 MPa load: 174°C
Dielectric constant: 3100 Hz
Manufacture of the fibres: spinning of a solution of
polysulfone in methylene chloride in an electrostatic field.
An apparatus according to DE-OS 26 20 399 can be used for
this purpose, for example. The fibres are collected on a
linearly continuously moving textile carrier.
Fleece properties:
Weight: 150 g/m2
Thickness (compressed): 0.05 mm
Thickness (impregnated with electrolyte): 0.25 mm
Mean pore radius in the uncompressed condition: 8 ~,m
Mean pore radius in the compressed condition: 4 ~,m
Pore volume: 83%
The temperature resistance of the membrane in accordance
with the invention is essentially determined by the fleece
material unless affected by other factors and therefore only
ends at about 174°C for the pure fibre material polysulfone.
Because of the mutual mechanical connection of the fibres in
the fleece, the mechanical stability is further increased up
to temperatures of 250°C. A high temperature operation of
the fuel cell is thereby possible, which, for example, is
important for reducing the generation of catalyst poisons.
-1
CA 02329064 2000-10-19
Example 1
The microfibre fleece is layered in a glass frit of 16
mm diameter with Nafion, a commercially available
perfluorated ionomer of the company DuPont. The liquid
phase is sucked into the pore structure of the fleece by the
application of a slight vacuum. The resulting impregnated
membrane is treated in the drying oven at 60°C for the
removal of solvents. The subsequent storage before further
processing is possible under distilled water.
Examples 2 to 4:
The microfibre fleece is impregnated with three aqueous
sulfuric acid solutions of different molarity analogous to
Example 1, whereby however the sulfuric acid is heated to
about 70°C to lower its viscosity. The fleece can also be
boiled for several minutes in the acid heated to 70°C
without achieving a different result.
Storage of the membrane so obtained is advantageously
carried out in the corresponding impregnating medium.
The following conductivities were determined for the
membrane prepared in this manner using a method according to
DIN 53779 of March 1979:
Example Measuring Specific
Temperature C Conductivity S/cm
1 23 0.016
2
1 M HZSO9 18 0.031
3
3 M H2S09 18 0.041
4
M H2S04 18 0.080
5
(Comparative Example) 25 0.070
Example 5 in this Table represents a Comparative Example
for corresponding measurements on a self-supporting polymer
CA 02329064 2000-10-19
membrane of 125 ~,m thickness according to the prior art made
of perfluorated ionomer (Nafion-117, DuPont).
The values for specific conductivity S/cm clearly show
that the operation of a fuel cell of a power output
according to the prior art is possible with a membrane in
accordance with the invention which is significantly
cheaper, constructively simpler and mechanically more stable
than pure Nafion.
Compared to a swollen Nafion membrane of, for example,
125 ~,m thickness, the electrolyte impregnated fleeces used
in Examples 1 to 4 are twice as thick.
The power output of the fuel cell, which is the product
of voltage and current can be achieved not only by higher
acid concentrations, which means higher specific
conductivities S/cm, but also by diffusion inhibition, using
thinner fleece materials.
As an example, the corresponding current/voltage curves
at room temperature are shown in Figure 2 for the Examples
1, 3 and 5. It is apparent that, compared with the prior
art (Example 5), comparable curve shapes can be achieved
with the membrane in accordance with the invention. The
above-mentioned effect of a higher cell output at higher
acid concentrations or with thinner fleece materials would
in this illustration result in a shift of the curves in the
positive direction of the y-axis.
~- °I