Note: Descriptions are shown in the official language in which they were submitted.
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Inhibitors of Neuraminidases
Technical Field
The present invention relates to novel compounds, compositions and
methods for inhibiting neuraminidase, especially influenza neuraminidase. The
invention also contemplates a composition and methods for preventing and
treating an influenza infection and processes for making such compounds and
synthetic intermediates employed in these processes.
Background of the Invention
Many disease-causing microorganisms possess a neuraminidase
neuraminidase (also known as sialidase) which is involved in the replication
process of the microorganism. In particular, viruses of the orthomyxovirus and
paramyxovirus groups possess a neuraminidase. Diseases associated with
paramyxoviruses include RSV (respiratory syncytial virus-related diseases),
pneumonia and bronchiolitis (associated with paramyxovirus type 3) and
laryngotracheobronchitis (associated with paramyxovirus type 1 ). Some of the
more important disease-causing microorganisms in man and/or animals which
possess a neuraminidase include Vibrio cholerae, Clostridium perfringens,
Streptococcus pneumoniae, Arthrobacter sialophilus, influenza virus,
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
parainfluenza virus, mumps virus, Newcastle disease virus, fowl plague virus,
equine influenza virus and Sendai virus.
Mortality due to influenza is a serious problem throughout the world. The
disease is devastating to man, lower mammals and some birds. Although
vaccines containing attenuated influenza virus are available, those vaccines
only
provide immunological protection toward a few influenza strains and are less
effective in otherwise immunologically compromised populations such as the
elderly, young children, and in those who suffer from chronic respiratory
illness.
The productivity loss from absence due to sickness from influenza virus
infection
has been estimated to be more than $1 billion per year.
There are two major strains of influenza virus (designated A and B).
Currently, there are only a few pharmaceutical products approved for treating
influenza. These include amantadine and rimantadine, which are active only
against the A strain of influenza viruses, and ribavirin, which suffers from
dose-limiting toxicity. Mutant virus which is resistant to amantadine and
rirnantadine emerges quickly during treatment with these agents.
Neuraminidase is one of two major viral proteins which protrude from the
envelope of influenza virus. During the release of progeny virus from infected
cells, neuraminidase cleaves terminal sialic acid residues from glycoproteins,
glycalipids and oligosaccharides on the cell surface. Inhibition of
neuraminidase
enzymatic activity leads to aggregation of progeny virus at the surface. Such
virus is incapable of infecting new cells, and viral replication is therefore
retarded
or blocked. X-ray crystallographic studies and sequence alignments have shown
that the residues which directly contact the sialic acid portion of the
substrate are
strictly conserved in the neuraminidase from all A and B influenza strains.
Thus,
a compound which binds to the sialic acid binding region of the neuraminidase
active site will block the replication of both the A and B strains of
influenza virus.
Compounds which are influenza neuraminidase inhibitors will be useful for the
_2_
CA 02329660 2000-10-20
WO 99/54290 PCT1US99/07949
prevention of influenza infection and will be useful for the treatment of
influenza
infection.
Y. Babu, et al., International Patent Application No. W097/47194,
published December 18, 1997 discloses substituted cyclopentanes which are
useful as neuraminidase inhibitors and treatments for influenza.
The following references disclose neuraminic acid derivatives with the
disclosed utility listed after each reference:
L. Von Itzstein, et al., European Patent Application No. EP539204, published
April 28, 1993 (antiviral agent);
T. Honda, et al., European Patent Application No. EP823428, published February
11, 1998 (sialidase inhibitor; influenza treatment);
T. Honda, et al., International Patent Application No. W098/06712, published
February 19, 1998 {sialidase inhibitor; influenza remedy);
L. Von Itzstein, et al., International Patent Application No. W095/20583,
published August 3, 1995 (viral neuraminidase inhibitor; influenza treatment);
P. Smith, International Patent Application No. W095I18800, published July 13,
1995 (viral neuraminidase inhibitor);
P. Colman, et al., International Patent Application No. W092/06691, published
April 30, 1992 (viral neuraminidase inhibitor);
L. Von Itzstein, et al., U.S. Patent No. 5,648,379, issued July 15, 1997
(influenza
treatment);
P. Reece, et al., International Patent Application No. W097/32214, published
September 4, 1997 (bind to influenza virus neuraminidase active site); and
P. Reece, et al., International Patent Application No. W098/21243, published
May 23, 1998 (anti-influenza agent).
-3-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
The following references disclose sialic acid derivatives with the disclosed
utility fisted after each reference:
Y. Ohira, et al., International Patent Application No. W098/11083, published
March 19, 1998 (antiviral agent);
Y. Ohira, European Patent Application No. EP882721, published December 9,
1998 (antiviral agent); and
B. Glanzer, et al., Helvetica Chimica Acta 74 343-369 (1991 ) (Vibrio cholerae
neuraminidase inhibitor).
The following references disclose benzene derivatives, cyclohexane
derivatives or cyclohexene derivatives with the disclosed utility listed after
each
reference:
Y. Babu, et al., U.S. Patent No. 5,602,277, issued February 11, 1997
(neuraminidase inhibitors);
M. Luo, et al., U.S. Patent No. 5,453,533, issued September 26, 1995
(influenza
neuraminidase inhibitor; influenza treatment);
Y. Babu, et al., International Patent Application No. W096/30329, published
October 3, 1996 (neuraminidase inhibitor; viral infection treatment);
N. Bischofberger, et al., U.S. Patent No. 5,763,483, issued June 9, 1998
(neuraminidase inhibitor); and
K. Kent, et al., International Patent Application No. 98/07685, published
February
26, 1998 (intermediates for the preparation of neuraminidase inhibitors).
C. Kim, et al., International Patent Application No. W098/17647, published
April 30, 1998 discloses piperidine derivatives which are useful as
neuraminidase
inhibitors.
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
N. Bischofberger, et al., international Patent Application No. W096/26933,
published September 6, 1996 discloses various substituted 6-membered ring
compounds which are useful as neuraminidase inhibitors.
The following references disclose dihydropyran derivatives which are
useful as viral neuraminidase inhibitors:
D. Andrews, et al., International Patent Application No. W097/06157, published
February 20, 1997; and
P. Cherry, et al., International Patent Application No. W096/36628, published
November 21, 1996.
C. Kim, et al., U.S. Patent No. 5,512,596, issued April 30, 1996 discloses
6-membered aromatic ring derivatives which are useful as neuraminidase
inhibitors.
G. Diana, et al., International Patent Application No. W098/03487,
published January 29, 1998 discloses substituted pyridazines which are useful
for
treatment of influenza.
B. Horenstein, et al., International Patent Application No. W099/06369,
published February 11, 1999 discloses piperazine derivatives which are useful
as
neuraminidase inhibitors.
L. CZOllner, et al., Helvetica Chimica Acta 73 1338-1358 (1990) discloses
pyrrolidine analogs of neuraminic acid which are useful as Vibrio cholerae
sialidase inhibitors.
The following references disclose siastatin B analogs which are useful as
neuraminidase inhibitors:
Y. Nishimura, et al., Natural Product Letters 1 39-44 (1992); and
Y. Nishimura, et al., Natural Product Letters 1 33-38 (1992).
-5-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
C. Penn, UK Patent Application No. GB2292081, published February 14, 1996
discloses the use of a neuraminidase inhibitor in combination with an
infiuenza
vaccine.
An object of the invention is to provide compounds which inhibit
neuraminidase of disease-causing microorganisms; especially, viral
neuraminidase; and, most especially, influenza neuraminidase.
An object of the invention is also to provide compounds which inhibit
neuraminidase from both A and B strains of influenza.
Another object of the invention is to provide prohylaxis of influenza
infection in humans and other mammals.
Another object of the invention is to provide treatment of influenza infection
in humans and other mammals.
Another object of the invention is to provide compounds which exhibit
activity against influenza A virus and and influenza B virus by virtue of
inhibiting
influenza neuraminidase when such compounds are administered orally.
Another object of the invention is to provide a compound which can be
effectively transported from the plasma into the lung bronchoaveolar fluid of
humans and other mammals in order to block the replication of influenza virus
in
that tissue.
Disclosure of the Invention
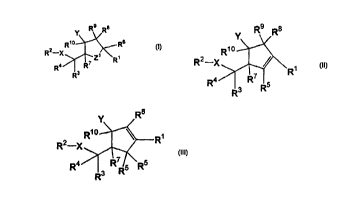
The present invention discloses compounds having Formula I, II or III
Y
R9
Rio Rs
R2-X
Z R~
Ra R'
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
R9
Y.
R1o
R2-X ~ 1
( ~ R
R4 ( R7 s
R3 R
Y
R1o ~ 1
R
R2-X,
R4 / ~ R7 ~5
R3 R
or a pharmaceutically acceptable salt, ester or prodrug thereof,
wherein R' is selected from the group consisting of
(a) -C02H, (b) -CH2C02H, (c) -S03H, (d) -CH2S03H, (e) -S02H,
(f) -CH2S02H, (g) -P03H2, (h) -CH2P03H2, (i) -P02H, (j) -CH2P02H,
(k) tetrazolyl, (I) -CH2-tetrazolyl, (m) -C(=O)-NH-S(O)2-R",
(n) -CH2C(=O)-NH-S(O)2-R", (o) -S02N(T-R")R'Z and
(p) -CH2S02N(T-R")R'2
wherein T is selected from the group consisting of
-7-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
(i) a bond, (ii) -C(=O)-, (iii) -C(=O)O-, (iv) -C(=O)S-, (v) -C(=O)NR3s-,
(vi) -C(=S)O-, (vii) -C(=S)S-, and (viii) -C(=S)NR3s-,
R"~ is selected from the group consisting of
(i) C~-C~2 alkyl, (ii) C2-C~2 alkenyl, (iii) cycloalkyl, (iv) (cyclo-
alkyl)alkyl,
(v) (cycloalkyl)alkenyl, (vi) cycloalkenyl, (vii) (cycloalkenyl)alkyl,
(viii) (cycloalkenyl)alkenyl, (ix) aryl, {x) (aryl)alkyl, (xi)
(aryl)alkenyl,
(xii) heterocyclic, (xiii) (heterocyclic)alkyl and
(xiii) (xiv) (heterocyclic)alkenyl; and
R'2 and R3s are independently selected from the group consisting of
(i) hydrogen, (ii) C,-C~2 alkyl, (iii) C2-C~2 alkenyl, (iv) cycloalkyl,
(v) (cycloalkyl)alkyl, (vi) (cycloalkyl)alkenyl, (vii) cycloalkenyl,
(viii) (cycloalkenyl)alkyl, (ix) (cycloalkenyl)alkenyl, (x) aryl,
(xi) (aryl)alkyl, (xii) (aryl)alkenyl, (xiii) heterocyclic,
(xiv) (heterocyclic)alkyl and (xv) (heterocyclic)alkenyl;
X is selected from the group consisting of
(a) -C(=O)-N(R*)-, (b) -N(R*)-C(=O)-, (c) -C(=S)-N(R*)-, (d) -N(R*)-C(=S)-,
(e) -N(R*)-S02-, and (f) -S02-N(R*)- wherein R* is hydrogen, C~-C3 loweralkyl
or
cyclopropyl;
R2 is selected from the group consisting of
(a) hydrogen, (b) C~-Cs alkyl, (c) C2-Cs alkenyl, (d) C3-Cs cycloalkyl,
(e) CS-Cs cycloalkenyl, (f) halo C~-Cs alkyl and (g) halo CZ-Cs alkenyl;
or Rz-X- is
_g_
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
,o
Y~'
wherein Y' is -CHZ-, -O-, -S- or -NH- and Y2 is -C(=O)- or -C(Raa)(Rbb)-
wherein
Raa and Rbb are indepedently selected from the group consisting of hydrogen,
C~-C3 loweralkyl, hydroxymethyl, 1-hydroxyethyl, 2-hydroxyethyl, aminomethyl,
1-aminoethyl, 2-aminoethyl, thiolmethyl, 1-thiolethyl, 2-thiolethyl,
methoxymethyl,
N-methylaminomethyl and methylthiomethyl;
Z' Is -O-, -S-, Of C(R5)2;
R3 and R4 are independently selected from the group consisting of
(a) hydrogen, (b) cycloalkyl, (c) cycloaikenyl, (d) heterocyclic, (e) aryl and
(~ -Z-R'a
wherein Z is
(i) -C(R37a)(R37b)- (ii) -C(R47)=C(R48)- (jll) -C=C-, (IV) -C(=O)-,
(v) -C(=S)-, (vi) -C(=NR'S)-, (vii) -C(Rs'a)(OR3'c)_, (viii) _
C(Rs~a)(SRs~c)-
(iX) _C(R37a)(N(R37b)(R37c))_~ (X) -C(R37a)(R37b)-(~-
(Xi) _C(R3~a)(Rs~b)-N(R37c)-, (Xii) -C(R3~a)(Rs~b)_N(O)(Rs~c)-,
(xiii) -C(Rs'a)(Rs'b)-N(OH)-, (xiv) -C(R37a)(R37b)-S-
(~) -C(R37a)(R37b)-S(O)_, (XVI) -C(R37a)(R37b)-S(0)2-
_g-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
(XVII) -C(R37a)(R37b)-C(=O)-, (XVItI) -C(R37a)(R37b)-C(=S)-~ .
(xix) -C(Rs~a)(Rsm)-C(=NR~5)-, (xx) -C(R3~a)(ORs~c)-C(=O)-,
(Xxl) -C(R3~a)(SR37c)-C(=O)- (Xxii) -C(Rs~a)(~R3~c)-C(=S)-
(xxiii) -C(R3~a)(SRs~c)-C(=S)-, (xxiv) -C(=O)-C(Rs~a)(ORs~~)-,
(xxv) -C(=O)-C(Rs~a)(SRs~c)-, (xxvi) -C(=S)-C(R3~a)(OR3~c)-,
(xxvii) -C(=S)-C(Rs~a)(SRs~c)-, (Xxviii) -C(Rs~a)(OFts~c)-C(Rs~a)(ORs~c)-,
(xxix) -C(Rs~a)(SRs~c)-C(R3~a)(ORs~c)-,
(xxx) -C(Rs~a)(ORs7c)-C(R3~a)(SRs~c)-
(xxxi) -C(Rs~a)(SRs~c)-C(R3~a)(SR3~c)-, (Xxxii) -C(=O)-C(=O)-,
(xxxiii) -C(=S)-C(=S)-, (xxxiv) -C(=O)-O-, (xxxv) -C(=O)-S-,
(xxxvi) -C(=S)-O-, (xxxvii) -C(=S)-S-, (xxxviii) -C(=O)-N(R3'a)-,
(xxxix) -C(=S)-N(Rs~a)-, (x~) -C(R3~a)(Rs7b)-C(=O)-N(Rs~a)-,
(X~I) -C(Rs~a)(Rs~b)-C(=S)-N(Rs~a)-, (x~ll) -C(R37a)(R37b)-C(=O)-O-
(X~III) -C(R37a)(R37b)-C(=O)-S-, (x~IV) -C(R37a)(R37b)-C(=S)-O-
(X~V) -C(R37a)(R37b)-C(=S)-S-, (X~VI) -C(R37a)(R37b)-N(R37b)-C(=O)-,
(X~VII) -C(Rs~a)(Rs~b)-N(R37b)-C(=S)-, (Xwlll) -C(R37a)(R37b)-O-C(=O)-
(xilx) -C(R37a)(R37b)-S-C(=O)-~ (i) -C(R37a)(R37b)-O-C(=S)-
(Ii) -C(R37a)(R37b)-S-C(=S)-~ (iii) -C(Rs~a)(R3~b)-N(Rs~b)-C(=~)-N(Rs~a)-,
(liii) -C(R37a)(Rs~b)-N(R37b)-C(=S)-N(Rs~a)-,
(IIV) -C(R37a)(R37b)-N(R37b)-C(=O)-O-
-10-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
(Iv) _C(R37a)(R37b)-N(R37b)-C(=O)-S-
(IVI) -C(R37a)(R37b)-N(R37b)-C(=S)-O-
(IVII) -C(R37a)(R37b)-N(R37b)-C(=S)-S-
(Iviii) -C(R3'a)(R3~b)-O-C(=O)-N(R3'a)-,
(lix) -C(Rs~a)(R3~n)-S-C(=O)-N(R3~a)-,
(Ix) _C(Rs~a)(Rs~b)-O-C(=S)-N(Rs~a)-,
(IXI) -C(Rs~a)(Rs~b)-S-C(=S)-N(Rs~a)-, (1X11) -C(R37a)(R37b)-O-C(=O)-O-
(1X111) -C(Rs~a)(R37b)-S-C(=O)-O-, (IXIV) -C(Rs~a)(R37b)-~-C(=O)-S-,
(IXV) -C(R37a)(R37b)-S-C(=O)-S-~ (IXVI) -C(R37a)(R37b)-~-C(=S)-~-
(IXVII) -C(R37a)(R37b)-S-C(=S)-O-, (IxVlll) -C(R37a)(R37b)-~-C(= ,~~~, )-S-
(IXIX) -C(R37a)(R37b)-S-C(=S)-S- Or (IXX) -C(R37a)(R37b)-C(R37a)(OR37c)-_
R' 4 is
(i) hydrogen, (ii) C1-C~2 alkyl, (iii) haloalkyl, (iv) hydroxyalkyl,
(v) thiol-substituted alkyl, (vi) R3'°O-substituted alkyl,
(vii) R3'°S-substituted alkyl, (viii) aminoalkyl,
(ix) (R3'°)NH-substituted alkyl, (x) (R3'a)(R3~')N_susbstituted alkyl,
(xi) R3'a0-(O=)C-substituted alkyl, (xii) R3'aS-(O=)C-substituted
alkyl, (xiii) R3'a0-(S=)C-substituted alkyl,
(xiv) R3'aS-(S=)C-substituted alkyl,
(xv) (R3'a0)2-P(=O)-substituted alkyl, (xvi) cyanoalkyl,
(xvi) CZ-C~z alkenyl, (xviii) haloalkenyl, (xix) C2-C~2 alkynyl,
-11-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
(xx) cycloalkyl, (xxi) (cycloalkyl)alkyl, (xxii) (cycloalkyl)alkenyl,
(xxiii) (cycloalkyl)alkynyl, (xxiv) cycloalkenyl,
(xxv) (cycloalkenyl)alkyl,
(xxvi) (cycloalkenyl)alkenyl, (xxvii) (cycloalkenyl)alkynyl, (xxviii)
aryl,
(xxix) (aryl)alkyl, (xxx) (aryl)alkenyl, (xxxi) (aryl)alkynyl,
(xxxii) heterocyclic, (xxxiii) (heterocyclic)alkyl,
(xxxiv) (heterocyclic)alkenyl or (xxxv) (heterocyclic)alkynyl,
with the proviso that R'4 is other than hydrogen when Z is
_C(R37a)(R37b)-N(R37b)-C(=O)-O- -C(R37a)(R37b)-N(R37b)-C(=S)-O-
TC(R37a)(R37b)-N(R37b)-C(=O)-S- _C(R3~a)~R3~b)-N(Rs~b)-C(=S)-S-
_C(R37a)(R37b)-O-C(=O)-O- _C(R37a)(R37b)-O-C(=S)-O-
'C(R37a)(R37b)-S-C(=O)-O- rC(R37a)(R37b)-S-C(-S)-O-
!C(R37a)(R37b)-O-C(=O)-S- ~C(Rs~a)(Rs~b)-~-C(=S)-S-
_C(R37a)(R37b)-S-C(=O)-S- ~r _C(R37a)(R37b)-S-C(=S)-S-
Rs~a, R37b Ray, and R4$ at each occurrence are independently selected
from the group consisting of
(i) hydrogen, (ii) Ci-C~2 alkyl, (iii) haloalkyl, (iv) hydroxyalkyl, (v)
alkoxyalkyl, (vi) C2-C~2 alkenyl, (vii) haloalkenyl, (viii) C2-C~2 alkynyl,
(ix) cycloalkyl,
(x) (cycloalkyl)alkyl, (xi) (cycloalkyl)alkenyl, (xii) (cycloalkyl)alkynyl,
-12-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
(xiii) cycloalkenyl, {xiv) (cycloalkenyl)alkyl, (xv) (cycloalkenyl)-
alkenyl,
(xvi) (cycloalkenyl)alkynyl, (xvii) aryl, (xviii) (aryl)alkyl,
(xix) (aryl)alkenyl, (xx) (aryl)alkynyl, (xxi) heterocyclic,
(xxii) (heterocyclic)alkyl, (xxiii) (heterocyclic)alkenyl and
(xxiv) (heterocyclic)alkynyl;
R3'' at each occurrence is independently selected from the group
consisting of
(i) hydrogen, (ii) C~-C~z alkyl, (iii) haloalkyl, {iv) C2-C~2 alkenyl,
(v) haloalkenyl, (vi) C2-C~2 alkynyl, (vii) cycloalkyl,
(viii) (cycloalkyl)alkyl, (ix) (cycloalkyl)alkenyl, (x) (cycloalkyl)alkynyl,
(xi) cycloalkenyl, (xii) (cycloalkenyl)alkyl, (xiii) (cycloalkenyl)alkenyl,
(xiv) (cycloalkenyl)alkynyl, (xv) aryl, (xvi) (aryl)alkyl,
(xvii) (aryi)alkenyl, (xviii) (aryl)alkynyl, (xix) heterocyclic,
(xx) (heterocyclic)alkyl, (xxi) (heterocyclic)alkenyl,
(xxii) (heterocyclic)alkynyl, (xxiii) -C(=O)-R'4, (xxiv) -C(=S)-R'4,
(xxv) -S(O)2-R'4 and (xxvi) hydroxyalkyl;
or when Z is -C(R3'a)(R3'b)-N(R3'c)-, then N(R3'') and R'4 when
taken together are an azido group;
-13-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
or when Z is -C(R3'a)(R3~b)-N(O)(R3'°)-, then N(O)(R3~') and R'4
when taken together are an N-oxidized 3-7 membered heterocyclic
ring having at least one N-oxidized ring nitrogen atom;
or when Z is -C(R3~a)(OR3~c)-, --C(R3~a)(SRs'c)- or
-C(R3~a)(N(R3'b)(R3'c))_, then R3~a, R'4 and the carbon atom to
which they are bonded when taken together form a cyclopentyl,
cyclopentenyf, cyclohexyl or cyclohexenyl ring;
R'~ is selected from the group consisting of
(i) hydrogen, (ii) hydroxy, (iii) amino, (iv) C~-C~2 alkyl, (v) haloalkyl,
(vi) C2-C~2 alkenyl, (vii) haloalkenyl, (viii) cycloalkyl,
(ix) (cycloalkyl)alkyl, (x) (cycloalkyl)alkenyl, (xi) cycloalkenyl,
(xii) (cycloalkenyl)alkyl, (xiii) (cycloalkenyl)alkenyl, (xiv) aryl,
(xv) (aryl)alkyl, (xvi) (aryl)alkenyl, (xvii) heterocyclic,
(xviii) (heterocyclic)alkyl and (xix) (heterocyclic)alkenyl;
or R3 and R4 taken together, with the atom to which they are attached, form a
carbocyclic or heterocyclic ring having from 3 to 8 ring atoms;
R~ at each occurrence is independently selected from the group consisting
of
(a) hydrogen, (b) -CH(R3$)2, (c) -(CH2),-O-R4°, (d) C2-C4 alkynyl, (e)
cyclopropyl,
(f) cyclobutyl, (g) -C(=Q')-R", and (h) -(CHZ)~-N(R'9)2
-14-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
wherein r is 0, 1 or 2; with the proviso that when one R5 is -O-R4° or -
N(R'9)2,
then the
other R5 is other than -O-R4° or -N(R'9)2;
wherein Q' is O, S, or N(R'B);
R" and R'8 are independently selected, at each occurrence, from the
group consisting of hydrogen, methyl, and ethyl;
R'9, R38, and R4° are independently selected, at each occurrence,
from the
group consisting of
(i) hydrogen, (ii) C,-C~2 alkyl, {iii) haloalkyl, (iv) Cz-C,2 alkenyl,
(v) haloalkenyi, (vi) cycloalkyl, (vii) (cycioalkyl)alkyl,
(viii) (cycloalkyl)alkenyl, (ix) cycloalkenyl, (x) (cycloalkenyl)alkyl,
(xi) (cycloalkenyl)alkenyl, {xii) aryl, (xiii) (aryl)alkyl, (xiv)
(aryl)alkenyl,
(xv) heterocyclic, (xvi) (heterocyclic)alkyl and
(xvii) (heterocyclic)alkenyl;
or one R~9 is an N-protecting group;
or the two R~ groups taken together with the carbon atom to which they are
bonded, form a carbocyclic or heterocyclic ring having from 3 to 6 ring atoms;
Y is selected from the group consisting of
(a) C~-C5 alkyl, (b) C,-C5 haloalkyl, (c) C2-C5 alkenyl, (d) C2-C5
haloalkenyl,
(e) CZ-C5 alkynyl, (f) C3-C5 cycloalkyl, (g) C3-C5 cycloalkyl-C~-to-C3-alkyl,
(h) C5
cycloalkenyl, (i) C5 cycloalkenyl-C~-to-C3-alkyl, (j) C5 cycloalkenyl-C2-to-C3-
alkenyl, (k) -(CHR39)~OR2°, (I) -CH(OR2°)-CHz(OR2°), (m) -
(CHR39)~SR2', (n)
phenyl, (o) halo-substituted phenyl, {p) -(CHR39)~C(=Q2)R22, {q)
-15-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
-(CHR39)~N(=Q3), (r) -N(O)=CHCH3, (s) -(CHR39)~N(CH3)R24 and (t) a
heterocyclic ring having from 3 to 6 ring atoms;
wherein n is 0, 1, or 2; Q2 is O, S, NRZ5, or CHR26; and Q3 is NR4', or CHR42;
R2° at each occurrence is independently
(i) methyl, (ii) ethyl, (iii) n-propyl, (iv) isopropyl,
(v) C~-C3 haloalkyl, (vi) vinyl, (vii) propenyl, (viii) isopropenyl,
(ix) ally!, (x) C2-C3 haloalkenyl, (xi) amino, (xii) -NHCH3, (xiii) -N(CH3)2,
(xiv) -NHCH2CH3, (xv) -N(CH3)(CH2CH3), (xvi) -N(CH2CH3)2 or
(xvii) -N(=CH2);
R2' is
(i) hydrogen, (ii) methyl, (iii) ethyl, (iv) n-propyl, (v) isopropyl,
(vi) C,-C3 haloalkyl, (vii) vinyl, (viii) propenyl, (ix) isopropenyl,
(x) ally! or (xi) C2-C3 haloalkenyl;
RZ2 is
(i) hydrogen, (ii) methyl, (iii) ethyl, (iv) n-propyl, (v) isopropyl,
(vi) hydroxy, (vii) thiol, (viii) methoxy, (ix) ethoxy, (x) n-propoxy,
(xi) isopropoxy, (xii) cyclopropyloxy, (xiii) methylthio, (xiv) ethylthio,
(xv) n-propylthio, (xvi) isopropylthio, {xvii) cyclopropylthio, (xviii)
vinyl, (xix) propenyl, (xx) isopropenyl, (xxi) ally!, (xxii) -N(R28a)(RZSb),
(xxiii) -CH2Rz9, (xxiv) aminomethyl, (xxv) hydroxymethyl,
(xxvi) thiolmethyl, (xxvii) -NHNH2, (xxviii) -N{CH3)NH2 or
(xxix) -NHNH(CH3);
R23 and R39 are independently hydrogen or methyl;
-16-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
R4' and R42 are independently hydrogen, methyl, or ethyl;
R24 is selected from the group consisting of
(i) hydrogen, (ii) C~-C4 alkyl, (iii) C2-C4 alkenyl, (iv) C2-C4 alkynyl,
(v) cyclopropyl, (vi) -C{=Q4)-R3°, (v) -OR3', and (vi) -N(R3Z)2,
wherein Q4 is O, S, or N(R3s);
Rz5 is hydroxy, methyl, ethyl, amino, -CN, or -N02;
R26 group is hydrogen, methyl or ethyl ;
RZ8a hydrogen, hydroxy, methyl, ethyl, amino, -NHCH3, -N(CH3)2, methoxy,
ethoxy, or -CN;
RZ8b is hydrogen, methyl or ethyl;
or RZaa, RZab and the nitrogen to which they are bonded taken together
represent azetidinyl;
R29 group is hydrogen, hydroxy, thiol, methyl, ethyl, amino, methoxy,
ethoxy, methylthio, ethylthio, methylamino or ethylamino;
R3° group is hydrogen, methyl, ethyl, -OR3'°, -SR34, -
N(R35)z, -NHOH,
-NHNH2, -N(CH3)NHZ, or -N(CHZCH3)NH2;
R3' and R32 substituents, at each occurrence, are independently hydrogen,
methyl or ethyl;
R33 group is hydrogen, hydroxy, methyl, ethyl, amino, -CN, or -N02;
R34 group is methyl or ethyl;
R35 group is independently hydrogen, methyl or ethyl;
-17-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
with the proviso that when Qz is CHRzs then Rzz is selected from the group
consisting of hydrogen, -CH3, -CZH$, -C3H~, -OCH3, -SCH3, -O-CZH5, and
-S-CzHs;
Rs and R' are independently selected from the group consisting of
(a) hydrogen, (b) C~-C~z alkyl, (c) Cz-C~2 alkenyl, (d) cycloalkyl,
(e) (cycloalkyl)alkyl, (f) (cycloalkyl)alkenyl, (g) cycloalkenyl, (h)
(cycloalkenyl)alkyl,
(i) (cycloalkenyl)alkenyl, (j) aryl, (k) (aryl)alkyl, (I) (aryl)alkenyl, (m)
heterocyclic,
(n) (heterocyclic)alkyl, (o) (heterocyclic)alkenyl, (p) -OR~'a and (q) -
N(R3'a)z; and
R8, R9, and R'° are independently selected from the group
consisting of
(a) hydrogen, (b) C~-C6 alkyl, (c) Cz-C6 alkenyl, (d) C3-C6 cycloalkyl,
(e) C3-C6 cycloalkenyl, and (f) fluorine, with the proviso that the total
number of
atoms, other than hydrogen, in each of R8, R9, and R'°, is 6 atoms or
less.
Preferred compounds of the invention include compounds wherein R3 and
R4 are not the same and which have the relative stereochemistry depicted by
Formula IV, V, Vl, Vll, VIII or IX:
R9 .R8 ., R9 Rs
R1o \ _Rs R1o Rs
R2-.X Z1~...,,,,.~R1
R2'_X~~~'~~. Z1 °~'~~R1
R4 ~ R7 Ra ~ R7
R3 Rs
IV V
-18-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
8
R8 Yes.. /R
R~ o ..,.
Rio ~ R~
R~ R2-X
R2-X~~.,,,. a R~
5
Ra ~ R~ ~s ERs R ~ 3 ~5 R
R R R
R3
VI VII
8
R
R9 Q$ .. R .
Rio ~ Rio R~
R R2-X
R2 -X~~.,,,.
Ra ~ R~ (s Ra Is R R5
R3 R R
VIII IX
or a pharmaceutically acceptable salt, ester or prodrug thereof, wherein R',
R2,
R3, R4, R5, R6, R', R8, R9, R'°, X , Y and Z' are as defined
above.
Other preferred compounds of the invention are compounds having
Formula I, I1, III, IV, V, VI, VII, VIII or IX or a salt, ester or prodrug
thereof wherein
R' is defined as above;
-X-R2 is RZ-C(=O)-NH-, R2-NH-C(=O)-, RZ-NH-SO~- or R2-S02-NH-
wherein R2 is C,-C3 loweralkyl, halo C~-C3 loweralkyl, C2-C3 alkenyl or halo
C2-C3
alkenyl or -X-R2 is
-19-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
~o
Y~
\ ~N_~_
Yz
wherein Y' is -CHZ-, -O-, -S- or -NH- and Y2 is -C(=O)- or -C(Raa)( Rbb)-
wherein
Raa and Rbb are independently selected from the group consisting of hydrogen,
C~-C3 loweralkyl, hydroxymethyl, 1-hydroxyethyl, 2-hydroxyethyl, aminomethyl,
1-aminoethyl, 2-aminoethyl, thiolmethyl, 1-thiolethyl, 2-thiolethyl,
methoxymethyl,
N-methylaminomethyl and methylthiomethyl;
R3 and R4 are independently selected from hydrogen, heterocyclic and
-Z-R'4 wherein Z and R'4 are defined as above and wherein one of R3 and R4 is
other than hydrogen;
Z' is -O-, -S- or -CH(R5)- wherein R5 is hydrogen, loweralkyl or -
(CH2)~N(R'9)2 wherein r and R,9 are defined as above; or R5 is hydrogen,
loweralkyl or -(CH2)~N(R'9)2 wherein r and R~9 are defined as above;
R6 and R' are independently hydrogen or loweralkyl;
R8 and Rg are independently hydrogen, fluoro or loweralkyl;
R'° is hydrogen, fluoro or loweralkyl; and
Y is C2-C5 alkenyl, C2_C5 haloalkenyl, -C(=Qz)R22,
-N(=Q3), -N(O)=CHCH3, -NR23R2a or a heterocyclic ring having from 3 to 6 ring
atoms, wherein R22, R23, RZa, Q2 and Q3 are defined as above.
More preferred compounds of the invention are compounds having
Formula I, II, III. 1V, V, VI, VII, VIII or IX or a salt, ester or prodrug
thereof wherein
R' is defined as above;
-20-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
-X-R2 is R2-C(=O)-NH-, R2-NH-C(=O)-, R2-NH-S02- or R2-SOZ-NH-
wherein R2 is C~-C3 loweralkyl, halo C~-C3 loweralkyl, C2-C3 alkenyl or halo
CZ-C3
alkenyl or -X-R2 is
O
Y~
,N-
Y2
wherein Y' is -CH2- and Y2 is -C(=O)- or -C(Raa)( Rbb)- wherein Raa and Rbb
are
independently selected from the group consisting of hydrogen,
C~-C3 loweralkyl, hydroxymethyl, 1-hydroxyethyl and 2-hydroxyethyl;
R3 and R4 are independently selected from hydrogen, heterocyclic and
-Z-R'4 wherein Z and R'4 are defined as above and wherein one of R3 and R4 is
other than hydrogen;
Z' is -O-, -S- or -CH(R5)- wherein R5 is hydrogen, loweralkyl or -
(CH2)~N(R'9)Z wherein r and R'9 are defined as above; or R5 is hydrogen,
loweralkyl or-(CH2)~N(R'9)2 wherein r and R'9 are defined as above;
Rs and R' are independently hydrogen or loweralkyl;
R$ and R9 are independently hydrogen or loweralkyl;
R'° is hydrogen or loweralkyl; and
Y is C2-C~ alkenyl, Cz_C5 haloalkenyl, -C(=QZ)R22,
-N(=Q3), -N(O)=CHCH3 or a heterocyclic ring having 5 ring atoms and also
containing one or two double bonds, wherein R22, Q2 and Q3 are defined as
above.
-21-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
Even more preferred compounds of the invention are compounds having
Formula Formula I, II, 111, IV, V, VI, VII, VIII or IX or a salt, ester or
prodrug thereof
wherein R' is defined as above;
-X-R2 is R2-C(=O)-NH-, R2-NH-C(=O)-, R2-NH-SOZ- or R2-SOZ-NH-
wherein R2 is C~-C3 loweralkyl, halo C~-C3 loweralkyl, CZ-C3 alkenyl or halo
C~-C3
alkenyl or -X-R2 is
O
Y~
,N-
Y2
wherein Y' is -CH2- and Y2 is -C(=O)- or -C(Raa)( Rbb)- wherein Raa and Rbb
are
independently selected from the group consisting of hydrogen,
C~-C3 loweralkyl, hydroxymethyl, 1-hydroxyethyl and 2-hydroxyethyl;
R3 and R4 are independently selected from hydrogen, heterocyclic and
-Z-R'4 wherein Z and R'4 are defined as above and wherein one of R3 and R4 is
other than hydrogen;
Z' is -O-, -S- or -CH(R5)- wherein R5 is hydrogen, loweralkyl or -
(CHZ)~N(R'9)2 wherein r and R~9 are defined as above; or R5 is hydrogen,
loweralkyl or -(CH2)~N(R'9)2 wherein r and R~9 are defined as above;
R6 and R' are independently hydrogen or loweralkyl;
R$ and R9 are independently hydrogen or loweralkyl;
R'° is hydrogen or loweralkyl; and
Y is C2-C5 alkenyl, C2_C5 haloalkenyl or a heterocyclic ring having 5 ring
atoms and also containing one or two double bonds.
-22-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
More highly preferred compounds of the invention are compounds having
Formula I, II, III, IV, V, VI, VII, VIII or IX or a salt, ester or prod rug
thereof wherein
R' is -C02H;
-X-Rz is R2-C(=O)-NH-, R2-NH-C(=O)-, R2-NH-S02- or RZ-S02-NH- wherein Rz is
C,-C3 loweralkyl or halo- C~-C3 loweralkyl;
R3 and R4 are independently selected from hydrogen, heterocyclic and
-Z-R'4 wherein Z and R'4 are defined as above and wherein one of R3 and R4 is
other than hydrogen;
Z' is -O-, -S- or -CH2-; or R5 is hydrogen;
R6 and R' are independently hydrogen or loweralkyl;
Ra and R9 are hydrogen independently hydrogen or loweralkyl;
R'° is hydrogen or loweralkyl; and
Y is C2-C5 alkenyl, C2_C5 haloalkenyl or a heterocyclic ring having 5 ring
atoms and also containing one or two double bonds.
Even more highly preferred compounds of the invention are compounds
having Formula l, II, III, IV, V, VI, VII, VIII or IX or a salt, ester or
prodrug thereof
wherein R' is -C02H;
-X-R2 is R2-C(=O)-NH-, R2-NH-C(=O)-, R2-NH-S02- or R2-S02-NH- wherein R2 is
C,-C3 loweralkyl or halo- C,-C3 ioweralkyl;
R4 is hydrogen or loweralkyl and R3 is heterocyclic or -Z-R'4 wherein Z
and R'4 are defined as above;
Z' is -O-, -S- or -CH2-; or R5 is hydrogen;
Rs and R' are hydrogen;
-23-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
R$ and R9 are hydrogen;
R'° is hydrogen; and
Y is C2-C5 alkenyl, C2_C5 haloalkenyl or a heterocyclic ring having 5 ring
atoms and also containing one or two double bonds.
Other even more highly preferred compounds of the invention are
compounds having Formula I, II, Ill, IV, V, VI, VII, VIII or IX or a salt,
ester or
prodrug thereof wherein R' is -C02H;
-X-R2 is R2-C(=O)-NH-, RZ-NH-C(=O)-, RZ-NH-S02- or RZ-S02-NH- wherein RZ is
C~-C3 loweralkyl or halo C~-C3 loweralkyl;
R4 is hydrogen or loweralkyl and R3 is (a) heterocyclic, (b) alkyl,
(c) cycloalkyl, (d) cycloalkylalkyl, (e) alkenyl, (f) alkynyl, (g) -C(=O)-R'4,
(h) -C(Rs~a)(OR3~c)-R,a or (i) -C(Rs~a)(Rs~b)-N(O)(R3~c)R~4 wherein R'4 is
(i) alkyl, (ii) cycloalkyl, (iii) cycloalkylalkyl, (iv) alkenyl, (v)
haloalkyl,
(vi) haloalkenyl, (vii) aryl, (viii) arylaikyl, (ix) heterocyclic,
(x) (heterocyclic)alkyl, (xi) hydroxyalkyl, (xii) alkoxyalkyl, (xiii)
cyanoalkyl,
(xiv) (R3'a0)-(O=)C-substituted alkyl or (xv) (R3~a0)2-P(=O)-substituted
alkyl;
R3'a and R3'b are independently selected from the group consisting of
(i) hydrogen, (ii) loweralkyl and (iii) loweralkenyl; and
Rs~o is
hydrogen, (ii) ioweralkyl or (iii) loweralkenyl;
Z' is -O-, -S- or -CH2-; or R5 is hydrogen;
-24-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
R6 and R7 are hydrogen;
R8 and R9 are hydrogen;
R'° is hydrogen; and
Y is C2-C5 alkenyl, C2_C5 haloalkenyi or a heterocyclic ring having 5 ring
atoms and also containing one or two double bonds.
Most highly preferred compounds of the invention are compounds having
Formula I, II, III, IV, V, V1, VII, VIII or IX or a salt, ester or prodrug
thereof wherein
R' is -C02H;
-X-Rz is R2-C(=O)-NH-, R2-NH-C(=O)-, R2-NH-S02- or R2-SOZ-NH- wherein R2 is
C,-C3 loweralkyl or halo C,-C3 loweralkyl;
R4 is hydrogen and R3 is (a) heterocyclic, (b) alkyl or (c) -
C(R3'a)(OR3'°)-
R'4 wherein R'4 is
(i) alkyl, (ii) cycloalkyl, (iii) cycloalkylalkyl, (iv) alkenyl, (v)
haloalkyl,
(vi) haloalkenyl, (vii) aryl, (viii) arylalkyl, (ix) heterocyclic,
(x) (heterocyclic)alkyl, (xi) hydroxyalkyl, (xii) alkoxyalkyl, (xiii)
cyanoalkyl,
(xiv) (R3~a0)-(O=)C-substituted alkyl or (xv) (R3'a0)2-P(=O)-substituted
alkyl;
R3'a and R3'b are independently selected from the group consisting of
(i) hydrogen, (ii) loweralkyl and (iii) loweralkenyl; and
R3'' is
hydrogen, (ii) C~-C3 loweralkyl or (iii) allyl;
Z' is -O-, -S- or -CH2-; or R5 is hydrogen;
R6 and R' are hydrogen;
-25-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
R8 and R9 are hydrogen ;
R'° is hydrogen; and
Y is C2-C5 alkenyl, C2_C5 haloalkenyl or a heterocyclic ring having 5 ring
atoms and also containing one or two double bonds.
Other most highly preferred compounds of the invention are compounds
having Formula I, II, III, IV, V, VI, VII, VIII or 1X or a salt, ester or
prodrug thereof
wherein R' is -C02H;
-X-RZ is Rz-C(=O)-NH- or R2-SOZ-NH- wherein R2 is C,-C3 loweralkyl or halo C~-
C3 loweralkyl;
R4 is hydrogen and R3 is (a) heterocyclic, (b) alkyl or (c) -C(R3'a)(OR3'c)-
R'4 wherein R'4 is
(i) loweralkyl, (ii) loweralkenyl, (iii) hydroxy-substituted loweralkyl or
(iv) alkoxy-substituted loweralkyl;
R37a jS
(i) hydrogen, (ii) loweralkyl or (iii) loweralkenyl; and
R3'' is
(i) hydrogen, (ii) C~-C3 loweralkyl or (iii) allyl;
Z' is -O-, -S- or -CH2-; or R5 is hydrogen;
R6 and R' are hydrogen;
R8 and R9 are hydrogen;
R'° is hydrogen; and
-26-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Y is C2-C5 alkenyl, CZ_C5 haloalkenyl or a heterocyclic ring having 5 ring
atoms and also containing one or two double bonds.
Other most highly preferred compounds of the invention are compounds
having Formula I, II, III, IV, V, VI, VII, VIII or IX or a salt, ester or
prodrug thereof
wherein R' is -C02H;
-X-Rz is R2-C(=O)-NH- or R2-S02-NH- wherein R2 is C~-C3 loweralkyl or halo C~-
C3 loweralkyl;
R4 is hydrogen and R3 is -C(R3~a)(OR3'')-R'4 wherein R'4 is
loweralkyl or loweralkenyl;
R37a jS
loweralkyl or loweralkenyl; and
R3~~ is
hydrogen, C,-C3 loweralkyl or allyl;
Z' is -O-, -S- or -CHZ-; or R5 is hydrogen;
R6 and R' are hydrogen;
R8 and R9 are hydrogen;
R'° is hydrogen; and
Y is C2-C5 alkenyl, C2_C5 haloalkenyl or a heterocyclic ring having 5 ring
atoms and also containing one or two double bonds.
Preferred substituents R' include -C02H or esters or prodrugs thereof.
Preferred esters include C2-C6 loweralkyl esters or substituted or
unsubstituted
-27-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
benzyl esters. Preferred substituents R' also include-S(O)2NHC(=O)R"
wherein R" is defined as above.
Most highly preferred substituents R' include -C02H or esters or prodrugs
thereof. Most highly preferred esters include C2-C6 loweralkyl esters or
substituted or unsubstituted benzyl esters.
Preferred substituents -X-R2 include RZ-C(=O)-NH-, R2-NH-C{=O)-,
R2-NH-S02- or R2-S02-NH- wherein R2 is C,-C3 loweralkyl, halo C~-C3
loweralkyl,
Cz-C3 alkenyl or halo C2-C3 alkenyl or -X-R2 is
~o
Y~
\ ~N_~_
~,2
wherein Y' is -CHz-, -O-, -S- or -NH- and Y2 is -C(=O)- or -C(Raa)( Rbb)-
wherein
Raa and Rbb are independently selected from the group consisting of hydrogen,
C~-C3 loweralkyl, hydroxymethyl, 1-hydroxyethyl, 2-hydroxyethyl, aminomethyl,
1-aminoethyl, 2-aminoethyl, thiolmethyl, 1-thiolethyl, 2-thiolethyl,
methoxymethyl,
N-methylaminomethyl and methylthiomethyl.
More preferred substituents -X-R2 include R2-C(=O)-NH-, R2-NH-C(=O)-,
RZ-NH-SOz- or RZ-SOz-NH- wherein R2 is C,-C3 loweralkyl, halo C,-C3
loweralkyl,
C2-C3 alkenyl or halo C2-C3 alkenyl or -X-Rz is
/o
Yi'-'.~~
\ ~N_~_
Yz
wherein Y' is -CHZ- and Y2 is -C(=O)- or -C(Raa)( Rbb)- wherein Raa and Rbb
are
independently selected from the group consisting of hydrogen,
_28_
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
C,-C3 loweralkyl, hydroxymethyl, 1-hydroxyethyl and 2-hydroxyethyl.
Even more preferred substituents -X-R2 include R2-C(=O)-NH-,
R2-NH-C(=O)-, R2-NH-SOZ- or R2-SOZ-NH- wherein R2 is C1-C3 loweralkyl, halo
C~-C3 loweralkyl, C2-C3 alkenyl or halo C2-C3 alkenyl.
More highly preferred substituents -X-R2 include R2-C(=O)-NH-,
RZ-NH-C(=O)-, R2-NH-SO~- or R2-SOZ-NH- wherein R2 is C~-C3 loweralkyl or
halo- C,-C3 loweralkyl.
Even more highly preferred substituents -X-R2 include R2-C(=O)-NH-,
R2-NH-C(=O)-, R2-NH-SOZ- or R2-S02-NH- wherein R2 is C,-C2 loweralkyl or halo
C~-C2 loweralkyl, and especially, CH3-C(=O)-NH-, CF3-C(=O)-NH-, CH3-S02-NH-
or CF3-S02-NH-.
Preferred substituents Z' include -O-, -S- or -CH(R5)- wherein R5 is
hydrogen, loweralkyl or-(CHz)fN(R'9)2 wherein r and R~9 are defined as above;
or R5 is hydrogen, loweralkyl or-(CH2)~N(R'9)2 wherein r and R~9 are defined
as
above;
More preferred substituents Z' are -O-, -S- or -CH(R5)- wherein R5 is
hydrogen or loweratkyl.
More highly preferred substituents Z' are -O-, -S- or -CH2-.
Even more highly preferred substituents Z' are -O- or -CH2-.
Preferred substituents R3 and R4 are independently selected from the
group consisting of hydrogen, heterocyclic and -Z-R'4 wherein Z and R'4 are
defined as most broadly defined previously herein and wherein one of R3 and R4
is other than hydrogen.
_29_
CA 02329660 2000-10-20
WO 99/54290 PCT/L1S99/07949
More highly preferred, substituent R4 is hydrogen or loweralkyl and R3
includes heterocyclic or -Z-R'4 wherein Z and R'4 are defined as most broadly
defined previously herein.
Even more highly preferred, substituent R4 is hydrogen or loweralkyl and
R3 includes
(a) heterocyclic, (b) alkyl, (c) cycloalkyl, (d) cycloalkylalkyl, (e) alkenyl,
(f) alkynyl,
(g) -C{=O)-R~4~ (h) -C(R3~a)(OR3~c)-R~4 or {i) -C(Rs~a)(Rs~b)-N(O)(Rs~c)R~4
wherein R'4 is
(i) alkyl, (ii) cycloalkyl, (iii) cycloalkylalkyl, (iv) alkenyl, (v)
haloalkyl,
(vi) haloalkenyl, (vii) aryl, (viii) arylalkyl, (ix) heterocyclic,
(x) (heterocyclic)alkyl, (xi) hydroxyalkyl, (xii) alkoxyalkyl, (xiii)
cyanoalkyl,
(xiv) (R3'a0)-{O=)C-substituted alkyl or (xv) (R3'a0)2-P(=O)-substituted
alkyl;
R3'a and R3'b are independently selected from the group consisting of
(i) hydrogen, (ii) loweralkyl and (iii) loweralkenyl; and
R3'° is (i) hydrogen, (ii) loweralkyl or (iii) loweralkenyl.
Most highly preferred, substituent R4 is hydrogen and R3 includes
{a) heterocyclic, (b) alkyl or (c) -C(R3'a){OR3'°)-R'4 wherein R'4 is
(i) alkyl, (ii) cycloalkyl, (iii) cycloalkylalkyl, (iv) aikenyl, (v)
haloalkyl,
(vi) haloalkenyl, (vii) aryl, (viii) arylalkyl, (ix) heterocyclic,
(x) (heterocyclic)alkyl, (xi) hydroxyalkyl, (xii) alkoxyalkyl, (xiii)
cyanoalkyl,
(xiv) (R3'a0)-(O=)C-substituted alkyl or (xv) {R3'a0)2-P(=0)-substituted
alkyl;
-30-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
R3'a and R3'b are independently selected from the group consisting of
(i) hydrogen, (ii) loweralkyl and (iii) loweralkenyl; and
R3'' is (i) hydrogen, (ii) C~-C3 loweralkyl or (iii) alfyl.
Also most highly preferred, substituent R4 is hydrogen and R3 includes
(a) heterocyclic, (b) alkyl or (c) -C(R3'a)(OR3'°)-R'4 wherein R'4 is
(i) loweralkyl, (ii) loweralkenyl, (iii) hy~lroxy-substituted loweralkyl or
(iv) alkoxy-substituted ioweralkyl;
R3'a is (i) hydrogen, (ii) loweralkyl or (iii) loweralkenyl; and
R3'' is (i) hydrogen, (ii) C~-C3 loweralkyl or (iii) allyl.
Also most highly preferred, substituent R4 is hydrogen and R3 includes
-C(R3'a)(OR3'~)-R'4 wherein R'4 is loweralkyl or loweralkenyl;
R3'a is loweralkyl or loweralkenyl; and
R3'' is hydrogen, C,-C3 loweralkyl or allyl, and especially, wherein R3'' is
hydrogen or methyl.
Preferred substituents R5 include those independently selected from
hydrogen, loweralkyl and -(CH2)~N(R'9)2 wherein r and R'9 are defined as
above.
More preferred substituents R5 are independently selected from hydrogen,
lowerafkyl and -(CH2)~N(R'9)2 wherein r is 0 or 1 and one R'9 is hydrogen or
loweralkyl and the other R'9 is hydrogen, loweralkyl or an N-protecting group.
Even more preferred substituents R5 are independently selected from
hydrogen and loweralkyl.
Most highly preferred, R5 is hydrogen.
-31-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Preferred substituents R6 and R' include independently hydrogen and
loweralkyl. Most highly preferred, R6 and R' are hydrogen.
Preferred substituents R8, R9 and R'° include independently
hydrogen,
fluoro and loweralkyl. Most highly preferred, R8, R9 and R'° are
hydrogen.
Preferred substituent Y includes CZ-C5 alkenyl, C2_C5 haloalkenyl,
-C(=Q2)R22, -N(=Q3), -N(O)=CHCH3, -NR23R2a or a heterocyclic ring having from
3 to 6 ring atoms, wherein R22, R2s, Rza, Q2 and Q3 are defined as above.
More preferred substituent Y includes C2-C5 alkenyl,
C2_C5 haloalkenyl, -C(=Q2)R22, -N(=Q3), -N(O)=CHCH3 or a heterocyclic ring
having 5 ring atoms and also containing one or two double bonds, wherein R22,
Q2 and Q3 are defined as above.
Even more preferred substituent Y includes Cz-C5 alkenyl, CZ_C5
haloalkenyl or a heterocyclic ring having 5 ring atoms and also containing one
or
two double bonds. Representative alkenyl and haloalkenyl substituents Y
include:
-CH=CH2, -CH=CHF, -CH=CH-CH3, -CH=CH-CFa, -CH=CHCI, -CH=CHBr,
-CH=CF2, -CH=CF(CH3), -CH=CF(CF3), -CH=CFCI, -CH=CFBr, -CH=C(CH3)2,
-CH=C(CH3)(CF3), -CH=CCI(CH3), -CH=CBr(CH3), -CH=G(CF3)Z, -CH=CCl(CF3),
-CH=CBr(CF3), -CH=CCIZ, -CH=CCIBr, -CF=CH2, -CF=CHF, -CF=CH-CH3,
-CF=CH-CF3, -CF=CHCI, -CF=CHBr, -CF=CF2, -CF=CF(CH3), -CF=CF(CF3),
-CF=CFCI, -CF=CFBr, -CF=C(CH3)2, -CF=C(CH3)(CF3), -CF=CCI(CH3),
-CF=CBr(CH3), -CF=C{CF3)Z, -CF=CCI(CF3), -CF=CBr(CF3), -CF=CCIZ,
-CF=CCIBr, -C(CH3)=CH2. -C(CH3)=CHF, -C{CH3)=CH-CH3, -C(CH3)=CH-CF3,
-32-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
-C(CH3)=CHCi, -C(CH3)=CHBr,-C(CH3)=CFz, -C(CH3)=CF(CH3),
-C(CH3)=CF(CF3), -C(CH3)=CFC1,
-C(CH3)=CFBr, -C(CH3)=C(CH3)z, -C(CH3)=C(CH3)(CF3), -C(CH3)=CCl{CH3),
-C(CH3)=CBr(CH3), -C(CH3)=C(CF3)z, -C{CH3)=CCI(CF3), -C(CH3)=CBr(CF3),
-C(CH3)=CCIz, -C(CH3}=CCIBr,
-C(CF3)=CHz, -C(CF3)=CHF, -C(CF3)=CH-CH3, -C(CF3)=CH-CF3, -C(CF3)=CHCI,
-C(CF3)=CHBr, -C(CF3)=CFz, -C(CF3)=CF(CH3), -C(CF3)=CF(CF3), -
C(CF3)=CFCI, -C(CF3)=CFBr, -C(CF3)=C(CH3)z, -C(CF3)=C(CH3)(CF3), -
C(CF3)=CCI(CH3),
-C(CF3)=CBr(CH3), -C(CF3)=C(CF3)z, -C(CF3)=CCI{CF3), -C(CF3)=CBr(CF3),
-C(CF3)=CCiz, -C(CF3)=CCIBr,
-CCI=CHz, -CCI=CHF, -CCI=CH-CH3, -CCI=CH-CF3, -CCI=CHCI, -CCI=CHBr,
-CCI=CFZ, -CCI=CF(CH3), -CCI=CF(CF3), -CCI=CFCI, -CCI=CFBr, -CCI=C(CHa)z,
-CCI=C(CH3)(CF3), -CCI=CCI(CH3), -CCI=CBr{CH3), -CCI=C(CF3)z,
-CCI=CCI(CF3), -CCI=CBr(CF3), -CCI=CCIz, -CCI=CCIBr,
-CH=CH-CH2CH3, -CH=CF-CHZCH3, -CF=CH-CH2CH3, -CF=CF-CH2CH3,
-CH=C(CH3)(CHzCH3), -CF=C(CH3)(CH2CH3), -CH=CCI(CH2CH3),
-CF=CCI(CH2CH3), -C(CH3)=CH-CH2CH3, -C(CH3)=CF-CHZCH3,
-CCI=CH-CH2CH3, -CCI=CF-CHZCH3, -C(CH2CH3)=CHz, -C(CHZCH3)=CHF,
-C(CHZCH3)=CFz, -C(CH2CH3)=CH-CH3, -C(CH2CH3)=CF-CH3,
-C(CH2CH3)=CH-CI, -C(CH2CH3)=CFCI.
-33-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
Representative Y substituents which are heterocyclic rings having 5 ring
atoms and also containing one or two double bonds include:
furanyl, dihydrofuranyl, didehydrodioxolanyl, dithiolyl, imidazolyl,
imidazolinyl,
isothiazolyl, isothiazolinyl, isoxazolyl, isoxazolinyl, oxadiazolyl,
oxadiazolinyl,
oxathiolyl, oxazolyl, oxazolinyl, pyrazolyl, pyrazolinyl, pyrrolyl,
dihydropyrrolyl,
tetrazolyl, tetrazoiinyl, thiadiazolyl, thiadiazolinyi, thiazolyl,
thiazolinyl, thienyl,
dihydrothienyl, triazolyl, triazolinyl.
More highly preferred substituents Y include cis-propenyl, trans-propenyl,
isobutenyl, cis-2-chlorovinyl, vinyl, 2,2-difluorovinyl, imidazolyl,
pyrazolyl,
oxazolyl, isoxazoiyl, thiazolyl, isoxazolyl.
Most highly preferred substituents Y include cis-propenyl, cis-2-chlorovinyl,
vinyl, imidazolyl, pyrazolyl, oxazolyl, isoxazolyl, thiazolyl, isoxazolyl.
The term "acid protecting group" as used herein refers to groups used to
protect acid groups (for example, -C02H, -S03H, -SOZH, -P03H2, -PO2H groups
and the like) against undesirable reactions during synthetic procedures.
Commonly used acid protecting groups are disclosed in T.H. Greene and P.G.M.
Wuts, Protective Groins in Organic Synthesis. 2nd edition, John Wiley & Sons,
New York (1991 ). Most frequently, such acid protecting groups are esters.
Such esters include:
alkyl esters, especially loweralkyl esters, including, but not limited to,
ethyl,
n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, t-butyl, n-pentyl esters
and the
like;
arylalkyl esters including, but not limited to, benzyl, phenethyl, 3-
phenylpropyl, naphthylmethyl esters and the like, wherein the aryl part of the
arylalkyl group is unsubstituted or substituted as previously defined herein;
-34-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
silylesters, especially, (tri-loweralkyl)silyl esters, (di-
loweralkyl)(aryl)silyl
esters and (loweralkyl)(di-aryl)silyl esters, including, but not limited to,
trimethylsilyl, triethylsilyl, isopropyldimethylsilyl, t-butyldimethylsilyl,
methyldiisopropylsilyl, methyldi-t-butylsilyl, triisopropylsilyl,
methyldiphenylsilyl,
isopropyldiphenylsilyl, butyldiphenylsilyl, phenyldiisopropylsilyl esters and
the like;
and the like.
Preferred acid protecting groups are loweralkyl esters.
The term "activated carboxylic acid group" as used herein refers to acid
halides such as acid chlorides and also refers to activated ester derivatives
including, but not limited to, formic and acetic acid derived anhydrides,
anhydrides derived from alkoxycarbonyl halides such as
isobutyloxycarbonylchloride and the like, anhydrides derived from reaction of
the
carboxylic acid with N,N'-carbonyldiimidazole and the like, N-
hydroxysuccinimide
derived esters, N-hydroxyphthalimide derived esters, N-hydroxybenzotriazole
derived esters, N-hydroxy-5-norbornene-2,3-dicarboximide derived esters, 2,4,5-
trichlorophenol derived esters, p-nitrophenol derived esters, phenol derived
esters, pentachlorophenol derived esters, 8-hydroxyquinoline derived esters
and
the like.
The term "acyl" as used herein, refers to groups having the formula
-C(=O)-R95 wherein R95 is hydrogen or an alkyl group. Preferred alkyl groups
as
R95 are loweralkyl groups. Representative examples of acyl groups include
groups such as, for example, formyl, acetyl, propionyl, and the like.
The term "acyfamino" as used herein, refers to groups having the formula
-NHR89 wherein R89 is an acyl group. Representative examples of acylamino
include acetylamino, propionylamino, and the like.
-35-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
The term "alkenyl" as used herein, refers to a straight or branched chain
hydrocarbon radical containing from 2 to 15 carbon atoms and also containing
at
least one carbon-carbon double bond. The term "lower alkenyl" refers to
straight
or branched chain alkenyl radicals containing from 2 to 6 carbon atoms.
Representative examples of alkenyl groups include groups such as, for example,
vinyl, 2-propenyl, 2-methyl-1-propenyl, 3-butenyl, 4-pentenyl, 5-hexenyl and
the
like.
The term "alkenylene" as used herein, refers to a divalent group derived
from a straight or branched chain hydrocarbon containing from 2 to 15 carbon
atoms and also containing at least one carbon-carbon double bond. The term
"lower alkenylene" refers to a divalent group derived from a straight or
branched
chain alkene group having from 2 to 6 carbon atoms. Representative examples
of alkenylene groups include groups such as, for example, -CH=CH-,
-CH2CH=CH-, -C(CH3)=CH-, -CH2CH=CHCH2-, and the like.
The term "alkenyloxy" as used herein, refers to groups having the formula
-OR8' where RB' is an alkenyl group.
The term "alkoxy" as used herein, refers to groups having the formula
-OR99 wherein R99 is an alkyl group. Preferred R99 groups are loweralkyl
groups.
Representative examples of alkoxy groups include groups such as, for example,
methoxy, ethoxy, tent-butoxy, and the like.
The term "alkoxyalkoxy" as used herein, refers to groups having the
formula -O-R9s-O-R9' wherein R9' is loweralkyl, as defined herein, and R96 is
a
lower alkylene group. Representative examples of alkoxyalkoxy groups include
groups such as, for example, rnethoxymethoxy, ethoxymethoxy, t butoxymethoxy
and the like.
-36-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
The term "alkoxyaikyl" as used herein refers to an alkyl radical to which is
appended an alkoxy group, for example, methoxymethyl, methoxylpropyl and the
like.
The term "alkoxycarbonyl" as used herein, refers to groups having the
formula, -C(=O)- R8°, where R$° is an alkoxy group.
The term "alkoxycarbonylalkyl" as used herein, refers to groups having the
formula, -C(=O)- R'9, appended to the parent molecular moiety through an
alkylene linkage, where R'9 is an alkoxy group.
As used herein, the term "alkyl" refers to straight or branched chain
hydrocarbon radicals containing from 1 to 12 carbon atoms. The term
"loweralkyl" refers to straight or branched chain alkyl radicals containing
from 1 to
6 carbon atoms. Representative examples of alkyl groups include groups such
as, for example, methyl, ethyl, n-propyl, iso-propyl, n-butyl, iso-butyl, sec-
butyl,
t-butyl n-pentyl, 1-methylbutyl, 2,2-dimethylbutyl, 2-methylpentyl, 2,2-
dimethyl-
propyl, n-hell, and the like. The hydrocarbon chains in alkyl groups or the
alkyl
portion of an alkyl-containing substituent can be optionally interrupted by
one or
two heteroatoms or heterogroups independently selected from the group
consisting of oxygen, -N(R2')- and sulfur wherein R2' at each occurrence is
independently hydrogen, loweralkyl, cylcoalkyl, cycloalkylalkyl or arylalkyl
and
wherein two such heteroatoms or heterogroups are separated by at least one
carbon atom.
The term "alkylamino" as used herein, refers to groups having the formula
-NHR9' wherein R9' is an alkyl group. Preferred R9' groups are loweralkyl
groups. Representative examples of alkylamino include methylamino,
ethylamino, and the like.
The term "alkylene" as used herein, refers to a divalent group derived from
a straight or branched chain saturated hydrocarbon group having from 1 to 15
-37-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
carbon. The term "lower alkylene" refers to a divalent group derived from a
straight or branched chain saturated hydrocarbon group having from 1 to 6
carbon atoms. Representative examples of alkylene groups include groups such
as, for example, methylene (-CH2-), 1,2-ethylene (-CH2CH2-), 1,1-ethylene
(-CH(CH3)-), 1,3-propylene (-CH2CH2CHz-), 2,2-dimethylpropylene
(-CH2C(CH3)2CH2-), and the like. The hydrocarbon chains in alkylene groups or
the alkylene portion of an alkylene-containing substituent can be optionally
interrupted by one or two heteroatoms or heterogroups independently selected
from the group consisting of oxygen, -N(R2')- and sulfur wherein R2' at each
occurrence is independently hydrogen, loweralkyl, cylcoalkyl, cycloalkylalkyl
or
arylalkyl and wherein two such heteroatoms or heterogroups are separated by at
least one carbon atom.
The term "alkylsulfonyl" as used herein refers to the group having the
formula, -S02-R'$, where R'8 is an alkyl group. Preferred groups R'8 are
loweralkyl groups.
The term "alkylsulfonylamino" as used herein refers to the group having
the formula, -S02-R", appended to the parent molecular moiety through an
amino linkage (-NH-), where R" is an alkyl group. Preferred groups R" are
loweralkyl groups.
The term "alkynyl" as used herein, refers to a straight or branched chain
hydrocarbon radical containing from 2 to 15 carbon atoms and also containing
at
least one carbon-carbon triple bond. The term "lower alkynyl" refers to
straight or
branched chain alkynyl radicals containing from 2 to 6 carbon atoms.
Representative examples of alkynyl groups include groups such as, for example,
acetylenyl, 1-propynyl, 2- propynyl, 3-butynyl, 2-pentynyl, 1-butynyl and the
like.
The term "alkynylene" as used herein, refers to a divalent group derived
from a straight or branched chain hydrocarbon containing from 2 to 15 carbon
-38-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
atoms and also containing at least one carbon-carbon triple bond. The term
"lower alkynylene" refers to a divalent group derived from a straight or
branched
chain alkynylene group from 2 to 6 carbon atoms. Representative examples of
alkynyfene groups include groups such as, for example, -C=C-, -CHZ-C---C-,
-C---C-CH2-, -CH{CH3)-C=C-, and the like.
The term "aminoalkyl" as used herein refers to an alkyl radical to which is
appended an amino (-NHZ) group.
The term "aryl" as used herein refers to a carbocyclic ring system having
6-10 ring atoms and one or two aromatic rings. Representative examples of aryl
groups include groups such as, for example, phenyl, naphthyl,
tetrahydronaphthyl, indanyl, indenyl and the like.
The aryl groups can be unsubstituted or substituted with one, two or three
substituents, each independently selected from loweralkyl, halo, haloalkyl,
haloalkoxy, hydroxy, oxo (=O), hydroxyalkyl, afkenyloxy, alkoxy, alkoxyalkoxy,
alkoxycarbonyl, alkoxycarbonylalkyl, thioalkoxy, amino, alkylamino,
alkylsulfonyl,
dialkylamino, acylamino, unsubstituted aryl, unsubstituted arylalkyl,
unsubstituted
arylalkoxy, unsubstituted aryloxy, mercapto, cyano, vitro, carboxy,
carboxaldehyde, NH2C(=O)-, cycloalkyl, carboxyalkyl, alkylsulfonylamino,
unsubstituted heterocyclic, unsubstituted (heterocyclic)alkyl, unsubstituted
(heterocyclic)alkoxy, unsubstituted (heterocyclic)oxy and -SOaH. Preferred
aryl
substituents are each independently selected from the group consisting of
loweralkyl, halo, haloalkyl, hydroxy, hydroxyalkyl, alkenyloxy, alkoxy,
alkoxyalkoxy, thioalkoxy, amino, alkylamino, dialkylamino, alkylsulfonyl,
acylamino, cyano and vitro. Examples of substituted aryl include 3-
chlorophenyl,
3-fluorophenyl, 4-chlorophenyl, 4-fluorophenyl, 3,4-dichlorophenyl,
3-chloro-4-fluoro-phenyl, 4-methylsulfonylphenyl, and the like.
-39-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
The term "(aryl)alkenyl" refers to a lower alkenyl group having appended
thereto an aryl group. Representative examples of (aryl)alkenyl groups include
groups such as, for example phenylethylenyl, phenylpropenyl, and the like.
The term "(aryl)alkyl" refers to a loweralkyl group having appended thereto
an aryl group. Representative examples of (aryl)alkyl groups include groups
such
as, for example benzyl and phenylethyl.
The term "arylalkoxy" as used herein refers to the group having the
formula, -O-R'6 where R'6 is an arylalkyl group.
The term "(aryl)alkynyl" refers to an alkynylene group having appended
thereto an aryl group. Representative examples of (aryl)alkynyl groups include
groups such as, for example phenylacetylenyl, phenylpropynyl, and the like.
The term "aryloxy" as used herein refers to the group having the formula,
-O-R'z, where R'2 is an aryl group.
The term "carbamoyl" as used herein refers to the group having the
formula, -C(=O)-NH2.
The term "carboxyalkyl" as used herein, refers to the group having the
formula, -R~-COOH, where Rfia is a lower alkylene group.
The term "cyanoalkyl" as used herein refers to an alkyl radical to which is
appended a cyano group (-CN).
The term "cycloalkenyl" as used herein refers to an aliphatic ring system
having 5 to 10 carbon atoms and 1 or 2 rings containing at least one double
bond
in the ring structure. Representative examples of cycloalkenyl groups include
groups such as, for example, cyclohexene, cyclopentene, norbornene and the
like.
-40-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Cycloalkenyl groups can be unsubstituted or substituted with one, two or
three substituents independently selected hydroxy, halo, amino, alkylamino,
dialkylamino, alkoxy, alkoxyalkoxy, thioalkoxy, haloalkyl, mercapto,
loweralkenyl
and loweralkyl. Preferred substitutents are independently selected from
loweralkyl, loweralkenyl, haloalkyl, halo, hydroxy and alkoxy.
The term "(cycloalkenyl)alkenyl" as used herein refers to a cycloalkenyl
group appended to a lower alkenyl radical. Representative examples of
(cycloalkenyl)alkenyl groups include groups such as, for example,
cyclohexenylethylene, cyclopentenylethylene, and the like.
The term "(cycloalkenyl)alkyl" as used herein refers to a cycloalkenyl group
appended to a lower alkyl radical. Representative examples of
(cycloalkenyl)alkyl
groups include groups such as, for example, cyclohexenylmethyl,
cyclopentenylmethyl, cyclohexenylethyl, cyclopentenylethyl, and the like.
The term "(cycloalkenyl)alkynyl" as used herein refers to a cycloalkenyl
group appended to a lower alkynyl radical. Representative examples of
(cycloalkenyl)alkynyl groups include groups such as, for example,
cyclohexenylacetylenyl, cyclopentenylpropynyl, and the like.
The term "cycloalkyl" as used herein refers to an aliphatic ring system
having 3 to 10 carbon atoms and 1 or 2 rings. Representative cylcoalkyl groups
include, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl,
norbornane, bicyclo[2.2.2]octane and the like.
Cycloalkyl groups can be unsubstituted or substituted with one, two or
three substituents independently selected hydroxy, halo, amino, alkylamino,
dialkylamino, alkoxy, alkoxyalkoxy, thioalkoxy, haloalkyl, mercapto,
ioweralkenyl
and Ioweralkyl. Preferred substitutents are independently selected from
loweralkyl, loweralkenyl, haloalkyl, halo, hydroxy and alkoxy.
-41-
CA 02329660 2000-10-20
WO 99/5429U PCT/US99/07949
The term "(cycloalkyl)alkyl" as used herein refers to a cycloalkyl group
appended to a loweralkyl radical. Representative examples of (cycloalkyl)alkyl
groups include groups such as, for example, cyclohexylmethyl,
cyclopentylmethyl, cyclohexylethyl, cyclopentylethyl, and the like.
The term "(cycloalkyl)alkenyl" as used herein refers to a cycloalkyl group
appended to a lower alkenyl radical. Representative examples of (cycloalkyl)-
alkenyl groups include groups such as, for example, cyclohexylethylene,
cyclopentylethylene, and the tike.
The term "(cycloalkyl)alkynyl" as used herein refers to a cycloalkyl group
appended to a lower alkynyl radical. Representative examples of (cycloalkyl)-
alkynyl groups include groups such as, for example, cyclohexylacetylenyl,
cyclopentylpropynyl, and the like.
The term "dialkylamino" as used herein, refers to groups having the
formula -N(R9°)2 wherein each R9° is independently a lower alkyl
group.
Representative examples of dialkylamino include dimethylamino, diethylamino, N-
methyl-N-isopropylamino and the like.
The term "halo" as used herein refers to F, C1, Br or I.
The term "haloalkenyl" as used herein refers to a lowerafkenyl group in
which one or more hydrogen atoms is replaced with a halogen. Examples of
haloalkenyl groups include 2-fluoroethylene, 1-chloroethylene, 1,2-
difluoroethylene, trifluoroethylene, 1,1,1-trifluoro-2-propylene and the like.
The term "haloalkoxy" as used herein refers to the group having the
formula, -OR69, where R69 is a haloalkyl group as defined herein. Examples of
haloalkoxy include chloromethoxy, fluoromethoxy, dichloromethoxy,
trifluoromethoxy and the like.
-42-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
The term "haloalkyl" as used herein, refers to a loweralkyl group in which
one or more hydrogen atoms has been replaced with a halogen including, but not
limited to, trifluoromethyl, trichloromethyl, difluoromethyl, dichloromethyl,
fluoromethyl, chloromethyl, chloroethyl, 2,2-dichloroethyl, pentafluoroethyl
and
the like.
The term "heterocyclic ring" or "heterocyclic" or "heterocycle" as used
herein, refers to any 3- or 4-membered ring containing a heteroatom selected
from oxygen, nitrogen and sulfur; or a 5-, 6- or 7-mernbered ring containing
one,
two, three, or four nitrogen atoms; one oxygen atom; one sulfur atom; one
nitrogen atom and one sulfur atom; two nitrogen atoms and one sulfur atom; one
nitrogen atom and one oxygen atom; two nitrogen atoms and one oxygen atom;
two oxygen atoms in non-adjacent positions; one oxygen atom and one sulfur
atom in non-adjacent positions; or two sulfur atoms in non-adjacent positions.
The 5-membered ring has 0-2 double bonds and the 6- and 7-membered rings
have 0-3 double bonds. The nitrogen heteroatoms can be optionally quaternized.
The term "heterocyclic" also includes bicyclic groups in which any of the
above
heterocyclic rings is fused to a benzene ring or a cyclohexane ring or another
heterocycfic ring, such as, for example, indolyl, dihydroindolyl, quinolyl,
isoquinolyl, tetrahydroquinolyl, tetrahydroisoquinolyl, decahydroquinolyl,
decahydroisoquinolyl, benzofuryl, dihydrobenzofuryl or benzothienyl and the
like.
Heterocyclic groups include, but are not limited to groups such as, for
example, aziridinyl, azetidinyl, epoxide, oxetanyl, thietanyl, pyrrolyl,
pyrrolinyl,
pyrrolidinyl, pyrazolyl, pyrazolinyl, pyrazolidinyl, imidazolyl, imidazolinyl,
imidazolidinyl, pyridyl, tetrahydropyridyl, piperidinyl, homopiperidinyl,
pyrazinyl,
piperazinyl, pyrimidinyl, pyridazinyl, oxazolyl, oxazolinyl, oxazofidinyl,
isoxazolyl,
isoxazolidinyl, morpholinyl, thiomorpholinyl, thiazolyl, thiazolinyl,
thiazolidinyl,
isothiazolyl, isothiazolidinyl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzothiazolyl, benzoxazolyl, oxetanyl, dihydrofuranyl, tetrahydrofuranyl,
-43-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
dihydropyranyl, tetrahydropyranyl, thienyl, dihydrothienyl, tetrahydrothienyl,
triazolyl, triazolinyl, tetrazolyl, tetrazolinyl, isoxazolyl, 1,2,3-
oxadiazoiyl, 1,2,4-
oxadiazolyl, 1,3,4-oxadiazolyl, 1,2,5-oxadiazolyl, oxadiazolinyl, , 1,2,3-
thiadiazolyl, 1,2,4-thiadiazolyl, 1,3,4-thiadiazolyl, 1,2,5-thiadiazolyl,
thiadiazolinyl,l,3-dithiolinyl, 1,2-dithiolyl, 1,3-dithiolyl, 1,3-dioxolinyl,
didehydrodioxolanyl, 1,3-oxathiolinyl, oxathiolyl, pyrimidyl, benzothienyl and
the
like. Heterocyclic groups also include compounds of the formula
x*
Y*
/ i
O
where X* is -CH2 or -O- and Y* is -C(O)- or [-C(R92)2-]~ where R92 is
hydrogen or C~-C4 alkyl where v is 1, 2, or 3 such as 1,3-benzodioxolyl,
1,4-benzodioxanyl and the like. Heterocyclic groups also include bicyclic
rings
such as quinuclidinyl and the like.
Heterocyclic groups can be unsubstituted or substituted with from one to
three substituents, each independently selected from loweralkyl, hydroxy,
alkoxy,
thioalkoxy, amino, alkylamino, dialkylamino and halogen. In addition, nitrogen
containing heterocyclic rings can be N-protected.
The term "(heterocyclic)alkenyl" as used herein refers to a heterocyclic
group appended to a lower alkenyl radical including, but not limited to,
pyrrolidinylethenyl, morpholinylethenyl and the like.
The term "(heterocyclic)alkoxy" as used herein refers to the group having
the formula, -OR68, where R6$ is a (heterocyclic)alkyl group.
The term "(heterocyclic)alkyl" as used herein refers to a heterocyclic group
appended to a loweralkyl radical including, but not limited to,
pyrrolidinylmethyl,
morpholinylmethyl and the like.
-44-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
The term "(heterocyclic)alkynyl" as used herein refers to a heterocyclic
group appended to a lower alkynyl radical including, but not limited to,
pyrrolidinylacetylenyl, morpholinylpropynyl and the like.
The term "(heterocyclic)oxy" as used herein refers to a heterocyclic group
appended to the parent molecular moiety through an oxygen atom (-O-)
The term "hydroxy protecting group", "hydroxyl protecting group" or "-OH
protecting group" as used herein refers to refers to groups used to hydroxy
groups against undesirable reactions during synthetic procedures. Commonly
used hydroxy protecting groups are disclosed in T.H. Greene and P.G.M. Wuts,
Protective Groups in Organic Synthesis. 2nd edition, John Wiley & Sons, New
York (1991 ). Such hydroxy protecting groups include:
methyl ether;
substituted methyl ethers, including, but not limited to, methoxymethyl,
methylthiomethyl, t-butylthiomethyl, (phenyldimethylsilyl)methoxymethyl,
benzyloxymethyl, p-methoxybenzyloxymethyl, (4-methoxyphenoxy)methyl, t-
butoxymethyl, 2-methoxyethoxymethyl, 2,2,2-trichloroethoxymethyl, 2-
(trimethylsilyl)ethoxymethyl, tetrahydropyranyl, tetrahydrothiopyranyi,
tetrahydrofuranyl, tetrahydrothiofuranyl ether and the like;
substituted ethyl ethers, including, but not limited to, 1-ethoxyethyl, 1-
methyl-1-methoxyethyl, 1-methyl-1-benzyloxyethyl, 2,2,2-trichloroethyl,
trimethylsilylethyl, t-butyl ether and the like;
benzyl ether;
substituted benzyl ethers, including, but not limited to, p-methoxybenzyl,
3,4-dimethoxybenzyl, o-nitorbenzyl, p-halobenzyl, p-cyanobenzyl,
diphenylmethyl, triphenylmethyl ether and the like;
-45-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
silyl ethers, including, but not limited to, trimethylsilyl, triethylsilyl,
triisopropylsilyl, dimethylisopropylsilyl, diethylisopropylsilyl,
dimethylthexylsilyl, t-
butyldimethylsilyl, t-butyldiphenylsilyl, tribenzylsilyl, triphenylsilyl,
diphenylmethylsilyl ether and the like;
esters, including, but not limited to, formate, acetate, chloroacetate,
dichloroacetate, trichloroacetate, trifluoroacetate, methoxyacetate,
phenoxyacetate, pivaloate, benzoate ester and the like; and the like.
Preferred hydroxy protecting groups include substituted methyl ethers,
benzyl ether, substituted benzyl ethers, silyl ethers and esters.
The term "hydroxyalkyl" as used herein refers to the group having the
formula, -R65-OH, where R65 is an alkylene group
The term "leaving group" as used herein refers to a group which is easily
displaced from the compound by a nucleophile. Examples of leaving groups
include a halide (for example, CI, Br or I) or a sulfonate (for example,
mesylate,
tosylate, triflate and the like) and the like.
The term "N-protecting group" or "N-protected" as used herein refers to
those groups intended to protect the N-terminus of an amino acid or peptide or
to
protect an amino group against undesirable reactions during synthetic
procedures. Commonly used N-protecting groups are disclosed in T.H. Greene
and P.G.M. Wuts, Protective Groups in Organic Synthesis, 2nd edition, John
Wiley & Sons, New York (1991). N-protecting groups comprise acyl groups such
as formyl, acetyl, propionyl, pivaloyl, t-butylacetyl, 2-chloroacetyl, 2-
bromoacetyl,
trifluoroacetyl, trichloroacetyl, phthalyl, o-nitrophenoxyacetyl, a-
chlorobutyryl,
benzoyl, 4-chlorobenzoyl, 4-bromobenzoyl, 4-nitrobenzoyl, and the like;
sulfonyl
groups such as benzenesulfonyl, p-toluenesulfonyl and the like; carbamate
forming groups such as benzyloxycarbonyl, p-chlorobenzyloxycarbonyl, p-meth-
oxybenzyloxycarbonyl, p-nitrobenzyloxycarbonyl, 2-nitrobenzyloxycarbonyi,
-46-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
p-bromobenzyloxycarbonyl, 3,4-dimethoxybenzyloxycarbonyl, 3,5-dimethoxy-
benzyioxycarbonyl, 2,4-dimethoxybenzyloxycarbonyl, 4-methoxybenzyloxycar-
bonyl, 2-vitro-4,5-dimethoxybenzyloxycarbonyl, 3,4,5-trimethoxybenzyloxycar-
bonyl, 1-(p-biphenylyl)-1-methylethoxycarbonyl, a,a-dimethyl-3,5-dimethoxy-
benzyloxycarbonyl, benzhydryloxycarbonyl, t-butyloxycarbonyl, diisopropyl-
methoxycarbonyl, isopropyloxycarbonyl, ethoxycarbonyl, methoxycarbonyl, allyl-
oxycarbonyl, 2,2,2-trichloroethoxycarbonyl, phenoxycarbonyl, 4-vitro-phen-
oxycarbonyl, fluorenyl-9-methoxycarbonyl, cyclopentyloxycarbonyl, adamantyl-
oxycarbonyl, cyclohexyloxycarbonyl, phenylthiocarbonyl and the like; alkyl
groups
such as benzyl, triphenylmethyl, benzyloxymethyl and the like; and silyl
groups
such as trimethylsilyl and the like. Preferred N-protecting groups are formyl,
acetyl, benzoyl, pivaloyl, t-butylacetyl, phenylsulfonyl, benzyl, t-
butyloxycarbonyl
(Boc) and benzyloxycarbonyl (Cbz).
The term "thioalkoxy" as used herein refers to groups having the formula
-SR98 wherein R98 is an alkyl group. Preferred groups R9$ are loweralkyl
groups.
The term "thio-substituted alkyl" as used herein refers to an alkyl radical to
which is appended a thiol group (-SH).
As used herein, the terms "S" and "R" configuration are as defined by the
IUPAC 1974 Recommendations for Section E, Fundamental Stereochemistry,
Pure Appl. Chem. (1976) 45, 13 - 30.
The compounds of the invention can comprise asymmetrically substituted
carbon atoms. As a result, all stereoisomers of the compounds of the invention
are meant to be included in the invention, including racemic mixtures,
mixtures of
diastereomers, as well as individual optical isomers, including, enantiomers
and
single diastereomers of the compounds of the invention substantially free from
their enantiomers or other diastereomers. By "substantially free" is meant
greater
than about 80% free of other enantiomers or diastereomers of the compound,
-47-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
more preferably greater than about 90% free of other enantiomers or
diastereomers of the compound, even more preferably greater than about 95%
free of other enantiomers or diastereomers of the compound, even more highly
preferably greater than about 98% free of other enantiomers or diastereomers
of
the compound and most preferably greater than about 99% free of other
enantiomers or diastereomers of the compound.
In addition, compounds comprising the possible geometric isomers of
carbon-carbon double bonds and carbon-nitrogen double are also meant to be
included in this invention.
Individual stereoisomers of the compounds of this invention can be
prepared by any one of a number of methods which are within the knowledge of
one of ordinary skill in the art. These methods include stereospecific
synthesis,
chromatographic separation of diastereomers, chromatographic resolution of
enantiomers, conversion of enantiomers in an enantiomeric mixture to
diastereomers and then chromatographically separating the diastereomers and
regeneration of the individual enantiomers, enzymatic resolution and the like.
Stereospecific synthesis involves the use of appropriate chiral starting
materials and synthetic reactions which do not cause racemization or inversion
of
stereochemistry at the chiral centers.
Diastereomeric mixtures of compounds resulting from a synthetic reaction
can often be separated by chromatographic techniques which are well-known to
those of ordinary skill in the art.
Chromatographic resolution of enantiomers can be accomplished on chiral
chromatography resins. Chromatography columns containing chiral resins are
commercially available. In practice, the racemate is placed in solution and
loaded
-48-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
onto the column containing the chiral stationary phase. The enantiomers are
then separated by HPLC.
Resolution of enantiomers can also be accomplished by converting the
enantiomers in the mixture to diastereomers by reaction with chiral
auxiliaries.
The resulting diastereomers can then be separated by column chromatography.
This technique is especially useful when the compounds to be separated contain
a carboxyl, amino or hydroxyl group that will form a salt or covalent bond
with the
chiral auxiliary. Chirally pure amino acids, organic carboxylic acids or
organosulfonic acids are especially useful as chiral auxiliaries. Once the
diastereorners have been separated by chromatography, the individual
enantiomers can be regenerated. Frequently, the chiral auxiliary can be
recovered and used again.
Enzymes, such as esterases, phosphatases and lipases, can be useful for
resolution of derivatives of the enantiomers in an enantiomeric mixture. For
example, an ester derivative of a carboxyl group in the compounds to be
separated can be prepared. Certain enzymes will selectively hydrolyze only one
of the enantiomers in the mixture. Then the resulting enantiomerically pure
acid
can be separated from the unhydrolyzed ester.
In addition, solvates and hydrates of the compounds of Formula I, II, III, IV,
V, VI, VII, VIII or IX are meant to be included in this invention.
When any variable (for example R', R2, R3, m, n, etc.) occurs more than
one time in any substituent or in the compound of Formula I, II, II1, IV, V,
VI, VII,
VIII or IX or any other formula herein, its definition on each occurrence is
independent of its definition at every other occurrence. In addition,
combinations
of substituents are permissible only if such combinations result in stable
-49-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
compounds. Stable compounds are compounds which can be isolated in a useful
degree of purity from a reaction mixture.
This invention is intended to encompass compounds having Formula I, I1,
III, IV, V, VI, VII, VIII or IX when prepared by synthetic processes or by
metabolic
processes. Preparation of the compounds of the invention by metabolic
processes include those occurring in the human or animal body (in vivo) or
processes occurring in vitro.
Compounds of the invention can be prepared according to the methods
described in Schemes 1-8 as shown below.
Throughout the schemes, methods will be illustrated wherein R' is a
carboxylic acid or carboxylic acid ester substituent. It will be understood by
those
skilled in the art that other R' substituents can (a) be obtained either from
the
carboxylic acid or carboxylic acid .ester group, (b) can be introduced by
similar
methods to those used to introduce the carboxylic acid or carboxylic acid
ester
group or (c) can be introduced by other methods generally known in the art.
In adddition, throughout the schemes, methods will be illustrated wherein
R4, R6, R', R8, R9 and R'° are hydrogen. It will be understood by those
skilled in
the art that compounds wherein one or more of these substituents is other than
hydrogen can be prepared by methods analogous to those disclosed in the
schemes or by other methods generally known in the art.
In addition, throughout the schemes, methods will be illustrated for
obtaining compounds of the invention having the preferred relative
stereochemistry. It will be understood by those skilled in the art that
compounds
of the invention having other relative stereochemistry can be prepared by
methods analogous to those disclosed in the schemes or by other methods
generally known in the art.
-50-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
In addition, throughout the schemes, methods will be illustrated wherein X
is -C(=O)-NH-. It will be understood by those skilled in the art that other X
groups can be prepared by methods analogous to those disclosed in the
schemes or by other methods generally known in the art.
Compounds of the invention can be prepared according to the procedure
described in Scheme 1. N-protected amino acid 1 (P' is an N-protecting group,
for example, t-butoxycarbonyl or the like) can be prepared by N-protection of
amino acid ((t)-(2R,3S)-2-Amino-bicyclo[2.2.1]hept-5-ene-3-carboxylic acid;
Stajer, G. et al. Tetrahedron, 40, 2385 {1984)). Formation of an anhydride
derivative of the acid (for example, by reaction with ethyl chloroformate or
the
like), followed by reduction (for example, with sodium borohydride or the
like)
provides alcohol 2. The alcohol group is oxidized (for example, by Swern
oxidation or the like) to provide aldehyde 3. Reductive amination of the
aldehyde
(for example with benzyl amine and Na(Ac0)3BH or the like) provides N-
protected amine 4 (P2 is an N-protecting group, such as benzyl and the like).
A
second N-protecting group can be introduced to give 5 (P3 is an N-protecting
group, for example, benzyioxycarbonyl or the like). Optionally, the mono-
protected amino group can be alkylated (for example, by reaction with a non-
nucleophilic base and an alkyl halide). The bicyclic ring is oxidatively
cleaved (for
example, with RuOZ and Na104 or the like) to give a diacid which is esterified
to
give diester 6 (P4 is a carboxylic acid protecting group, for example,
loweralkyl,
such as methyl, ethyl or the like). The N-protecting groups P2 and P3 are
selectively removed (for example, by hydrogenation or the like) to provide
amine
7. Amine 7 can be further functionalized to complete the introduction of the
R2-
X- substituent (for example, by reaction of the amine with an acylating agent
such
as acetic anhydride or the like) to give 8. Removal of the acid protecting
groups
P4 (for example, by base hydrolysis) provides diacid 9. Diacid 9 can be
monoprotected (for example, by treatment with acetic anhydride, followed by
methanol and triethylamine) and chromatographic separation to give 10 (P5 is a
-51-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
carboxylic acid protecting group, for example, loweralkyl or the like). The
acid
group of 10 can also be transformed to a variety of substituents Y of the
compounds of the invention using methods known to those skilled in the art to
give 11, followed by N-deprotection, to give compounds of the invention 12.
As shown in Scheme 2, substituents R3 can be introduced via reaction of
aldehyde 3 with a Grignard reagent (for example, R3MgBr or the like) to give
alcohol 13. Oxidation of alcohol 13 (for example, Swern oxidation or the like)
provides ketone 14. Reductive amination of ketone 14 (for example, by reaction
with ammonium acetate and sodium cyanoborohydride in methanol or the like)
gives amine 15. Amine 15 can be further functionalized to complete
introduction
of the R2-X- substituent (for example, by reaction of the amine with an
acylating
agent such as acetic anhydride or the like or by other acylation methods),
followed by chromatographic separation of the diastereomers to give 16a. The
other diastereomer (16b) can also be isolated and further transformed
according
to the scheme.
Oxidation of 16a and esterification gives 17 (in a manner analogous to that
disclosed in Scheme 1). Also similar to Scheme 1, the diacid resulting from
hydrolysis of diester 17 can be selectively protected to give give 1$, which
can
then be transformed to compounds of the invention 19.
As shown in Scheme 3, diol 20 is selectively diprotected (Culbertson, et
al., Journal of the American Chemical Society 82, 2541-2547 (1960)) by
reaction
with one equivalent of a hydroxy protecting agent, followed by reaction with a
second hydroxy protecting agent, to give 21 (P6 is a hydroxy protecting group,
for
example, acetyl or the like and P' is a hydroxy protecting group, for example,
benzyl or the like). Oxidation and esterification provide 22. Removal of
protecting group P', followed by transformation of the hydroxy group to an
amine,
which is then N-protected, provides 24. Transformation of 24 in a manner
-52-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
analogous to the transformation of 2 to 11 or 12 in Scheme 1 provides
compounds of the invention 27 or 28.
As shown in Scheme 4, alcohol 31 can be transformed to compounds of
the invention 38 in a manner analogous to the transformation of 13 to 19 in
Scheme 2.
As shown in Scheme 5, aldehyde 39 can be reacted with a Grignard
reagent to introduce substituent R3 to give 40. Oxidation (for exampie, by
Swern
oxidation or the like) provides 41a, which can be epimerized (for example,
with
sodium methoxide or the like) and 41 b can be obtained by chromatography.
Ketone 41b can be transformed to compounds of the invention 47 in a manner
analogous to the transformation of 14 to 19 in Scheme 2.
As shown in Scheme 6, olefin 48 (P8 is a hydroxy protecting group) is
converted to alcohol 48a (for example, by reaction with borane dimethylsulfide
complex and hydrogen peroxide or the like). Oxidation of the alcohol to a
carboxylic acid, followed by esterification with a carboxylic acid protecting
group
P9 and deprotection of the diol, provides 49. Selective protection of the
primary
alcohol with a hydroxy protecting group P'° gives 50. Oxidation of 50
(for
example, Swern oxidation or the like) provides ketone 51. Reductive amination
of
ketone 51 (for example, by reaction with ammonium acetate and sodium
cyanoborohydride in methanol or the like) gives amine 52. Amine 52 can be
further functionalized to complete the introduction of the R2-X- substituent (
for
example, by reaction with an acylating agent such as acetic anhydride or the
like
or by other acylation methods), followed by chromatographic separation of the
diastereomers to give 53a. The other diastereomeric amide (53b) can also be
isolated and further functionalized according to this scheme.
Selective removal of the P8 hydroxy protecting group in 53a provides
alcohol 54. Oxidation of the alcohol to an aldehyde (for example, Swern
-53-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
oxidation or the like) provides 55. The aldehyde can serve as a precursor for
various substituents Y in the compounds of the invention. For example,
olefination of 55 (for example, with Ph3PCH2, or triphenylphosine/methylene
chlorideln-BuLi, or I-Ph3P+CH2CH~/KOtBu, or the like) provides 56 wherein Y
is an olefinic substituent. Removal of the P'° hydroxy protecting group
gives
alcohol 57.
The alcohol can serve as a precursor for a variety of R3 substituents in the
compounds of the invention. For example, the alcohol of 57 can be oxidized to
an aldehyde -(for example, by Dess-Martin oxidation or the like) to give 58.
Aldehyde 58 can be reacted with Grignard reagents (R'4MgBr or the like) or
other
organometallic reagents (for example, organolithium reagents such as R'aLi or
the like) to provide 59 as a mixture of alcohol diastereomers which can be
separated chromatographically to provide the major isomer 59a and the other
isomer 59b. Isomer 59a or the mixture of isomers 59 can be oxidized (for
example, by Dess-Martin oxidation or the like) to give ketone 62. Reduction of
ketone 62 (for example, with sodium borohydride in ethanol or the like)
provides
alcohol 59b as the major isomer, which can be isolated by chromatography.
Ester hydrolysis provides compounds of the invention 63a or fi3b,
respectively,
wherein Y is an olefinic substituent.
Alkylation of alcohol 59a or 59b provides ethers 60a or 60b, respectively.
Ester hydrolysis provides compounds of the invention 61a or 61b, respectively,
wherein Y is an olefinic substituent.
As shown in Scheme 7, reaction of ketone 62 with with Grignard reagents
(R3'aMgBr or the like) or other organometallic reagents (for example,
organolithium reagents such as R3'aLi or the like) provides alcohols 64a and
64b
as a mixture of alcohol diastereomers which can be separated
chromatographically. Ester hydrolysis provides compounds of the invention fi5a
or 65b, respectively, wherein Y is an olefinic substituent.
-54-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Alkylation of alcohol 64a or 64b provides ethers 66a or 66b, respectively.
Ester hydrolysis provides compounds of the invention 67a or 67b, respectively,
wherein Y is an olefinic substituent.
Scheme 8 shows the preparation of precursor 74 for compounds of the
invention which are substituted tetrahydrofurans. Alcohol 68 is oxidized to a
ketone (for example, by Swern oxidation or the like), followed by oxidation of
the
olefin to a diol (for example, by treatment with Os04 and N-methylmorpholine N-
oxide or the like) to give 69. Diol 69 is protected as the acetonide 70.
Oxidation
of 70 (for example, with MCPBA -or the like) provides lactone 71. Iodination
via
the enolate .-of 71 provides 72. Reaction of 72 with potassium carbonate and
methanol provides ester 73. Reduction of the ester provides aldehyde 74. The
aldehyde provides a functional group via which substituents R3 and R2-X- can
be
introduced. Deprotection of the diol and oxidation of the diol provides
functional
groups via which substituents Y and R' can be introduced.
Esters or prodrugs of the compounds of the invention can be prepared by
methods known in the art.
-55-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 1
HO HO H
O NHP~ NHP~ ~ O NHP~
3
H ~ p3 w
P2N P2N
3
NHP~ NHP~
4
P40~0 P40~0
~ P2~N OP4 ~ H2N ..,~~, OP4
NPR O Np~ O
H
H
7
6
P40~0 HO~O
OP4 R2C(O)HN ., ~~I~OH
..., N
R2C(O)H
7 NPR O Np~ O
H
H 9
8 _
-56-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 1 ycont'd.~
HO~O Y,
s
R2C(O)HN OH R2C(O)HN ...",'~ p
NP' O ~~ Np~ O
H
H 11
10
Y,
R2C(O)HN .",~I~ H
NH2 O
12
-57-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 2
w ~ w
H ~HO ~. O
0 NHPi R3 NHP~ R3 NHP~
13 14
H2N
_14 _
R3 NHP~
w w
R2C{O)HN~,, ~ R2C(O)HN
R3 NHP~ + R3 NHP~
16a 16b
1 0
HO-.l/
5
RZC(O)HN~,. .., ~ OP4 R2C(O)HN~,. ..,aI~OP
ll
R3 NP' 0 R3 Np~ O
H H
17 1
_58_
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 2 (cont'd.)
0
HO.-.!/ Y.
R2C(O)HN~,, ~~~~~I~OP R2C(O)HN~,, ...~~y~OH
R3 NPR O R3 NHZ 'O
18 H 19
-59-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 3
w w Pa0?,, ,,
HO Ps0 _ PsOp OPa
OH OP7 ~ O
20 21
22
a
P O?.,,.. P O?.,,..
4
Ps0 _,,~pPa Ps0 ..,~OP
22 O O
OH NHP1
23 24
4
P O?.,,.. H 0~.,,..
~OH
R2C(O)HN .,~OPa R2C(O)HN
p ----- O
24 NHP~
NHP~
25 26
-60-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 3 ycont'd.)
H~~.,,,,
26 R2C(O)HN OP5
~''~O
NHP~
2T
Y.,,
R2C(O)HN .,,~ H
O
NH2
28
-61-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 4
HO
NHP~ R3 HzNHP'
21 30 31
O Hz
31 ' R3 ~H2NHP~ ~ R3 H2NHP~
33
32
w
R2C(O)HN~,, RzC(O)HN
R3 CH2NHP~ + R3 CHZNHP~
34a 34b
1 0
paO~O HO-..l/
R2C(O)HN.,, ." ~ OP4. RzC(O)HN~,. ..,~~'I~OH
R3 ~ R3 ~ O
CH NHP~ CH2NHP~
2
36
O
HO-l/ Y,
5
R2C(O)HN~,, ."" OP R2C(O)FiN~,, .."" OH
n
R3 I O R3 I O
CH2NHP~ CH2NHP~
37 3$
-62-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 5
w ,.. w
-- 0
O R R3
39 4~ 41a
O H2N
R3 ~ Rs
41 b 42
2
RZC(O)HN~,, R C(O)HN
R3 + Rs
43a 43b
1 p
P40~0 HO-.~/
RzC(O)HN~,. .."" OP4 R2C(O)HN~,,
I
3
Rs O R O
44 45
-63-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 5 ,(cont'd.~
HO~O Y,
RZC(O)HN~,.~-~"~~I~OP R2C(O)HN~,.~w.~~'I~OH
Rr3 v O~ R~[ s '''' O
46 47
-64-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 6
Pap ;, P80-.
",~~OH
O O
48 48a
Pap-., P80-.
HO Op9 HO OP9
48a ~~~ -- II
HO O plop O
49 50
P80- P80-,,
O OP9 H2N OP9
p~o0 O plop O
51 52
Pap-,, P80-
R2C(O)HN~,, ..,~I~OP9 RZC(O)HN~,, .,,~I~OP9
52 PioO Or + p,o0 1O
53a 53b
-65-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 6 (cont'd.)
HO-,, H-!O
R2C(O)HN,,, Op9 R2C(O)HN, OP9
53a - I~ -
p~o0 O p~o0 O
54 55
Y, Y,
R2C(O)HN,,, OP9 R2C(O)HN,, Op9
- p~o0 O HO O
_56 Y is an alkene
or haloalkene
Y,
R2C(O)HN,,, OP9
57
H O
O
58
-66-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 6 ~cont'd.)
Y. Y.
R2C(O)HN,,, OP9 R2C(O)HN,,,, OP9
58 ---
Rya .~~OH O + Rya OH O
59a 59b
Y. Y.
R2C(O)HN,,, OP9 RZC(O)HN , OP9
Rya O Ria O
%pR37a pR37a
60a 60b
Y. Y,
R2C(O)HN,,, OH R2C(O)HN'~. OH
~I~
Rya O R14 O
'~R37a pR37a
61 a 61 b
_57_
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 6 ~cont'd.1
Y. Y,
R2C(O)HN,,, OP9 R2C(O)HN,,, OP9
59a ~''~~ il
Rya O O ~. Rya OH O
62 59b
Y,
R2C(O)HN,,, OH
Y.
Rya O RZC(O)HN,,, OH
.~OH
63a R,a OH O
63b
-68-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 7
Y, Y,
R C(O)HN,,,
OP9 R2C(O)HN,,, OP9
s2 ~f ~i~
- R1a OH O + Rya O
R3~a R3~a ~OH
64a 64b
Y, Y,
R2C(O)HN,, OH R2C(O)HN,,, OH
~I~ ~~I~
Rya OH O Rya O
R3~a R37a ~OH
65a 65b
-69-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 7 i(cont'd.)
Y, Y,
R C(O)HN,,,
OP9 R2C(O)HN, OP9
~I~ ~'I~
Rya OH O + Rya O
R37a R37a ~OH
64a 64b
Y, Y,
R2C(O)HN,,, OP9 R2C(O)HN,,, OP9
~ ~I~~I~ ~I~
Rya O Rya O
37c 37c
R37a OR R37a ~OR
66a 66b
Y, Y,
R2C(O)HN,,, OH R2C(O)HN,,, OH
~I~ ~'I~
Rya O Ria O
37c 37c
R37a OR ~OR
R37a
67a 67b
-70-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
SCHEME 8
i .-~ HO O
OH HO O
68 o O
69 70
o ~, ~ i
0
0 0
0 0 0 0
71 72
~o ~ o
0 0 ~o
C02Me O
CHO
73 74
-71-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
The other compounds of the invention can be readily prepared from the
compounds available through commercial sources, in the chemical (literature or
as described herein using techniques well known in the chemical literature.
The
procedures required are well known and can be readily practiced by those
having
ordinary skill in the art.
Afl patents, patent applications, and literature references cited in the
specification are hereby incorporated by reference in their entirety. In the
case of
inconsistencies, the present disclosure, including definitions, will prevail.
The reagents required for the synthesis of the compounds of the invention
are readily available from a number of commercial sources such as Aldrich
Chemical Co. (Milwaukee, WI, USA); Sigma Chemical Co. (St. Louis, MO, USA);
and Fluka Chemical Corp. (Ronkonkoma, NY, USA); Alfa Aesar (Ward Hill, MA
01835-9953); Eastman Chemical Company (Rochester, New York 14fi52-3512);
Lancaster Synthesis Inc. (Windham, NH 03087-9977); Spectrum Chemical
Manufacturing Corp. (Janssen Chemical) (New Brunswick, NJ 08901 ); Pfaltz and
Bauer (Waterbury, CT. 06708). Compounds which are not commercially
available can be prepared by employing known methods from the chemical
literature.
The following examples will serve to further illustrate the preparation
of the compounds of the invention, without limitation.
_72_
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 1
fit) (1 S 2S 3R 4R) 2 (N Met~l-N-f-butylo~carbonylamino)-3-acetamidomethyl-4-
methoxycarbonyl-c~clopentane-1-carboxylic Acid
HO
O NHZ
1A. ~,t) (2R 3S)-2-Aminobicyclo[-2 2 1]hept-5-ene-3-carboxylic acid.
The title compound was synthesized from norbornadiene by a
cycloaddition reaction with chlorosulfonyl isocyanate followed by a reduction
and
acidic hydrolysis as reported by Stajer, G. ef al. Tetrahedron; 40, 2385
(1984).
HO
O NHBoc
1 B. (t) (2R 3S)-2-(t-Butylox~carbonylamino~bicyclo[2.2.11hept-5-ene-3-
carboxylic acid.
A solution of (t)-(2R,3S)-2-aminobicyclo[2.2.1]hept-5-ene-3-carboxylic
acid (6.3 g, 0.04 mole) , NaOH (3.3 g, 0.082 mole), and di-fert-butyl
Bicarbonate
(0.082 mmole) in water (200 mL) was stirred at room temperature for 48 hours.
The reaction mixture was acidified using 1 M aqueous HCI, while cooling the
solution in an ice/water bath. The reaction mixture was extracted with
dichloromethane {3 X 250 mL). The organic layer was dried over MgS04, filtered
and concentrated to provide the title compound as a white solid (yield: 5.6 g,
55%).
' H NMR (CDCi3) 81.44 (s, 9H), 1.64 (d, 1 H), 2.08 (d, 1 H), 2.58 (m, 1 H),
2.72 (s, 1 H), 2.97 (s,1 H), 6.18 (m, 2H), 6.96 (d, 1 H).
-73-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
HO
NHBoc
1 C. ~t~~2R 3S)-2- t Butyloxycarbonylamino)-3-hydroxymethvlbicyclof2.2.11hept-
5-ene.
Ethyl chloroformate (2.3 mL, 23.7 mmole) was added slowly to a solution
of (t)-(2R,3S)-2-(t-butyloxycarbonylamino)bicyclo[2.2.1]hept-5-ene-3-
carboxylic
acid (6 g, 23.7 mmole) and N-methylmorpholine (2.6 mL, 23.~ mmole) in THF
(110 mL) at -20 °C . The reaction mixture was warmed to 0 °C,
and the slurry-like
reaction became nearly homogeneous. The reaction mixture was cooled to -20
°C, and treated with sodium borohydride ( 3.7g, 66 mmole). Methanol (10
mL)
was added dropwise, over 20 minutes. The reaction mixture was neutralized with
1 N HCI and the reaction mixture was concentrated by removal of volatile
materials, in vacuo. The residue was partitioned between ethyl acetate and
water. The organic layer was washed with 1 N HCI, water, and brine, dried over
MgS04, filtered and concentrated in vacuo. Purification by silica gel column
chromatography using 15% ethyl acetate/hexanes provided the title compound
(yield: 4.27 g, 75 %).
' H NMR (CDC13) b 1.45 (s, 9H), 1.52 (m, 2H), 1.85 {q, 1 H), 2.09 (bs, 1 H},
2.68 {m, 2H), 3.66 (m, 3H), 4.83 (bs, 1 H), 6.11 (m, 1 H), 6.27 (m, 1 H).
-74-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
W
O
H NHBoc
1 D. fit)-(2R 3S)-2-(t Butyloxycarbonylamino -3-formylbicyclof2.2.11hept-5-
ene.
DMSO (3.4 mL) in dichloromethane (5 mL) was added slowly to oxalyl
chloride (2 mL, 22 mmole) in dichloromethane (50 mL) at -78 ~C. After 5
minutes,
(t)-(2R,3S)-2-(t-butyloxycarbonylamino)-3-hydroxymethyl-bicycloj2.2.1 ]hept-5-
ene (4.27g, 17.8 mmole) in 15 mL of dichioromethane and 10 mL of DMSO was
added. Stirring was continued for 20 minutes at -78 ~C. The solution was
treated
with triethylamine (14 mL). After 5 minutes, the cooling bath was removed and
the reaction stirred for an additional 30 minutes. The reaction mixture was
partitioned with water (100 mL). The aqueous layer was extracted with
dichloromethane (3 x 50 mL). The organic layers were combined, washed with
brine, dried over MgS04, filtered and concentrated. The crude product was
chromatographed on silica gel with 0-10% ethyl acetate/dichloromethane, to
provide the title compound. (yield: 3.8 g, 90%)
H NMR (CDC13) b1.43 (s, 9H), 1.63 (m, 2H), 2.74 (m, 2H), 3.11 (s, 1 H),
3.99 (t, 1 H). 4.85 (d, 1 H), 6.22 (m, 2H), 9.81 (s, 1 H).
H
BnN
NHBoc
1 E. (t)-(2R 3R)-2-~Bu loxycarbonylamino)-3-N-ben~laminomethylbicyclo-
j2.2.1~he~t-5-ene.
A solution of (t)-(2R,3S)-2-(f-butyloxycarbonylamino)-3-formyl-
bicyclo[2.2.1]kept-5-ene (3.8g, 16.0 mmole), benzylamine (1.9 mL, 17.6 mmole)
and acetic acid (1 mL) in dichloromethane (80 mL) was stirred at 0 °C
for 10
-75-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
minutes followed by addition of Na(Ac0)sBH (5.09g, 24 mmole). The reaction
mixture was stirred at 0 °C for 2 hours then allowed to warm slowly to
room
temperature for 2 hours. After completion, the reaction mixture was washed
with
aqueous sodium bicarbonate, dried over MgS04, filtered and concentrated. The
crude product was purified by silica gel chromatography using 0-2.5%
methanollchloroform to provide the title compound (yield: 4.0 g, 77%).
' H NMR (CDC13) 81.47 (s, 9H), 1.51 (m, 2H), 1.78 (t, 1 H), 2.72 (m, 4H),
3.57 (t, 1 H), 3.78 (s, 1 H), 6.10 (m, 1 H), 6.21 (m, 1 H), 7.30 (m, 5H).
MS: (M+H)+ = 329.
Cbz
BnN
NHSoc
1 F. {t~~2R 3RL2 (f But~rloxycarbonylamino~ 3-(N-benzyl-N-(benzyloxycarbonyl-
amino)methyl bicyclo(2.2.11hept-5-ene.
A solution of (t)-(2R,3R)-2-(t-butyloxycarbonylamino)-3-N-
benzylaminomethylbicyclo[2.2.1]kept-5-ene (2.8 g, 8.5 mmole) and N-
(benzyloxycarbonyloxy)succinimide (2.3 g) in 50 mL of dichloromethane was
reacted at room temperature for 6 hours. The reaction mixture was diluted with
ethyl acetate, washed with saturated aqueous NaHC03 and brine, dried over
MgS04 and concentrated in vacuo. The product was purified by silica gel
chromatography using hexanes/ethyl acetate (3:1) to provide the title compound
(yield: 3.37 g, 85%).
' H NMR (CDC13) 51.32 (m,2H), 1.36 (s, 9H), 1.56 (m, 1 H), 1.88 (m, 1 H),
2.63 (m, 2H), 3.06 (m, 1 H), 4.34 {m, 1 H), 4.63 (m, 1 H), 5.18 (m, 2H), 6.05
(bs,
2H), 7.28 (m, 11 H).
-76-
CA 02329660 2000-10-20
WO 99/54290 PCT/U599/07949
CbzN NBoc
Bn CH3
1 G. (t)-(2R 3R)-2-(N-Methyl-N-t butyloxycarbonylamino)-3-(N-benzyl-N-benzyl-
oxycarbonylamino)methLrl-bicyclo[2.2.1 ]hept-5-ene.
Sodium hydride (300 mg , 60% in oil) was added to a solution of (t)-
(2R,3R}-2-(t butyloxycarbonylamino)-3-(N-benzyl-N-benzyloxycarbonylamino)-
methylbicyclo[2.2.1]hept-5-ene (3.37 g, 7.27 mmole) and iodomethane (0.9 mL)
in anhydrous DMF (60 mL) at 0 °C. After 4 hours, the reaction mixture
was
concentrated in vacuo and purified by silica gel chromatography using
hexanes/ethyl acetate (4:1 ) to provide the title compound (yield: 3.3 g,
95%).
H NMR (CDC13) b1.40 (s, 9H}, 1.82 (m, 2H), 2.26 (m, 1 H), 2.78 (m, 1 H),
2.85 (m, 1 H), 2,90 (s, 3H), 2.94 (m, 2H), 3.63 (m, 1 H), 4.22 (m, 2H), 5.34
(s, 2H),
5.60 (m, 2H), 7.15 (m, 10H).
MS: (M+H)+ = 477.
-77-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
O
CH30-l~
OCH3
CbzN NBoc O
Bn 'CH3
1 H. !t)-( 1 S 2S 3R 4R~2-(N-Methyl-N-t-butyloxycarbonylamino)-3-(N-benzyl-N-
benzyloxycarbonylamino)meth~cyclopentane-1 4-dicarboxylic Acid Dimethyl
Este r.
A mixture of Na104 (10 g, 47 mmole) and Ru02 (45 mg, 0.3 mmol) in 25
mL of carbon tetrachloride 50 mL of acetonitrile, and 75 mL of water was
rapidly
stirred for 30 minutes. A solution of (t)-(2R,3R)-2-(N-methyl-N-t
butyloxycarbonylamino)-3-(N-benzyl-N-benzyloxycarbonylamino)methyl-
bicyclo[2.2.1]hept-5-ene (3.3 g, 6.9 mmole) in 25 mL of carbon tetrachloride
was
added to the bright yellow mixture. The resultant black mixture was stirred at
room temperature for 1.5 hours and diluted with 250 mL of water. The aqueous
layer was extracted with ethyl acetate (5 x 200 mL). The combined organic
layers were washed with brine, dried over MgS04, filtered and concentrated in
vacuo. The residue was dissolved in ethyl acetate and treated with a solution
of
diazomethane in ethyl ether. The reaction mixture was concentrated in vacuo,
and the crude product purified by silica gel chromatography using ethyl
acetate/hexanes (1:1} to provide the title compound as a white solid (yield:
1.91
g, 50%).
'H NMR (CDC13) 81.41 (s, 9H), 2.21 (m, 2H), 2.33 (m, 1H), 2.90 (s, 3H),
2.92 (m, 2H), 2.96 (m, 1 H), 3.38 (t, 1 H), 3.67 (s, 6H), 4.23 (m, 3H), 5.38
(s, 2H),
7.12 (m, 10H).
MS: (M+H)+ = 569.
-78-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O
CH30-'l~
OCH3
'"If
NH2 NBoc O
CH3
11. fit) (1S 2S 3R 4R) 2 (N Methyl N t Bu~loxycarbonylamino)-3-aminomethvl-
cyclopentane-1 4-dicarboxylic Acid Dimethvl Ester.
A mixture of (t)-(1S,2S,3S,4R)-2-(N-methyl-N-t-butyloxycarbonylamino)-3-
(N-benzyl-N-benzyloxycarbonylamino)methyl-cyclopentane-1,5-dicarboxylic acid
dimethyl ester (1.91 g, 3.36 mmole) and Pd(OH)z (380 mg, 20% on carbon) in 50
mL of isopropanol was stirred at room temperature under an hydrogen
atmosphere, for 16 hours. The catalyst was removed by vacuum filtration
through a bed of Celite~, and the filtrate concentrated in vacuo to provide
the title
compound (yield: 1.2 g 100 %).
'H NMR (CDC13) 61.48 (s, 9H), 2.00 (m, 2H), 2.42 (m, 1H), 2.64 (m, 2H),
2.90 ( bs, 3H), 3.00 (m, 2H), 3.71 (s, 3H), 3.73 (s, 3H), 4.4 (bs, 1 H), 5.4
(bs, 2H).
MS: (M+H)+ = 345.
_79_
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O
CH30-~~
OCH3
AcHN Ngoc O
CH3
1 J. (~1 S 2S 3R 4R)-~N-Methyl-N-t but~ycarbonylamino)-3-acetamido-
methyl-cyclopentane-1 4-dicarboxylic Acid Dimethv I E~ stet.
A solution of (t)-(1S,2S,3R,4R)-2-(N-methyl-t
butyloxycarbonylamino)-3-aminomethylcyclopentane-1,5-dicarboxylic acid
dimethyl ester (1.2 g, 3.5 mmole), acetic anhydride (0.5 mL) and triethylamine
(1
mL) in 25 mL of dichloromethane was stirred at room temperature for 2 hours.
The reaction mixture was diluted with ethyl acetate and washed successively
with
1 N HCI, water and brine, dried over MgS04, and concentrated in vacuo. The
residue was purified by silica gel chromatography using ethyl acetate/hexanes
(1:1) to provide the title compound (yield: 790 mg, 58 %).
' H NMR (CDCI~) 61.45 (s, 9H), 1.92 (s, 3H), 2.05 (m, 1 H), 2.40 (m, 1 H),
2.62 (m, 1 H), 2.85 (s, 3H), 3.1 (m, 1 H), 3.3 {m, 1 H), 3.42 (m, 1 H), 3.71
(s, 6H),
4.62 (bs, 1 H), 6.38 (bs, 1 H).
MS: (M+H)+= 387.
-80-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99107949
O
HO--~~
OH
'''I
AcHN Ngoc O
CH3
1 K. fit) (1 S 2S 3R 4R)-2-(N-Methyl-N-f but~oxLrcarbonylamino)-3-acetamido-
methyl-cyclopentane-1 4-dicarboxylic Acid.
A solution of (t)-(1 S,2S,3S,4R)-2-(N-methyl-N-t-
butyloxycarbonylamino)-3-acetamidomethylcyclopentane-1,5-dicarboxylic acid
dimethyl ester (720 mg, 1.86 mmole) and 5 equivalents of lithium hydroxide in
methanol (40 mL) and H20 (10 mL) was stirred at room temperature for two
hours. The solution was concentrated in vacuo. The residue was partitioned
between 1 N HCI and ethyl acetate. The aqueous layer was back extracted with
ethyl acetate (3 x 50 mL). The organic layers were combined and washed with
brine, dried over Na2S04, filtered, and concentrated in vacuo to provide the
title
compound {yield: 500 mg, 75 %).
H NMR (CDC13) 81.45 (s, 9H), 1.92 (s, 3H), 2.05 (m, 1 H), 2.40 (m, 1 H),
2.62 (m, 1 H), 2.85 (s, 3H), 3.1 (m, 1 H), 3.3 (m, 1 H), 3.42 (m, 1 H), 3.71
(s, 6H),
4.62 (bs, 1 H), 6.38 (bs, 1 H).
-81-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O
CH30-l/
..~~~~oH
AcHN Ngoc ~
CH3
1 L. ,fit)-(1 S 2S 3R 4R)-2-lN-Methyl-N-t-butylox~carbonylamino)-3-acetamido-
methyl-4-methox cay rbonylcyclopentane-1-carboxylic Acid
A solution of (t)-(1 S,2S,3R,4R)-2-(N-methyl-N-t
butyloxycarbonylamino)-3-acetamidomethylcyclopentane-1,5-dicarboxylic acid
(500 mg, 11.4 mmole), in 5 mL of dichloromethane and 5 mL of acetic anhydride,
was stirred at room temperature for two hours. The solution was concentrated
in
vacuo, at 20 °C. The residue was dissolved in 10 mL of methanol and 0.2
mL of
triethylamine and reacted for 16 hours at room temperature, under a nitrogen
atmosphere. The reaction mixture was diluted with 100 mL of chloroform, and
washed successively with 0.1 N HCI and brine. The solution was dried over
MgS04, filtered and concentrated in vacuo. The residue was purified by silica
gel
chromatography using chloroformlmethanol/acetic acid (97:2:1 ) to provide the
title compound (yield: 155 mg, 30 %).
'H NMR (CDC13) 81.47 (s, 9H), 1.95 (s, 3H), 2.10 (m, 2H), 2.43 (m, 1H),
2.64 (m, 1 H), 2.88 ( m, 4H), 3.20 (m, 1 H), 3.30 (m, 1 H), 3.44 (m, 1 H),
3.72 (s,
3H), 4.66 (t, 1 H), 6.48 (bs, 1 H).
MS (M+H)+= 373.
-82-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 2
0
CH30-'l~
OH
~~~''If
AcHN NH O
CH3 HCI
!t)-(1 S 2S 3R 4Rl-2-N-Methyl-3-acetamidomethyl-4-methoxycarbonyl-
cyclo~entanecarboxylic Acid Hydrochloride.
A solution of (t)-(1 S,2S,3R,4R)-2-(N-methyl-N-t-butyloxycarbonylamino)-3-
acetamidomethyl-4-methoxycarbonylcyclopentanecarboxylic acid (130 mg, 0.35
mmole) in 4.5 mL of dichloromethane was reacted with trifluoroacetic acid (1.5
mL) for 1 hr, at room temperature. The solution was concentrated in vacuo, at
25
°C. The residue was treated with 1 N HCI and concentrated in vacuo to
provide
the title compound as a white solid (yield: 100 mg, 92 %).
'H NMR (D20) 82.02 (s, 3H), 2.08 (m, 1H), 2.54 (m, 2H), 2.76 (s, 3H), 2.85
(m, 2H), 3.36 (m, 2H), 3.76 (s, 3H), 3.81 (t, 1 H).
Example 3
0
CH30~~
OH
AcHN NCbz O
CH3
fit)-(1 S 2S 3R 4R)-2~N-Methyl-N-benzyloxycarbonylamino)-3-acetamidomethyl-
4-methoxycarbonyl-cyclopentanecarboxLrlic Acid.
A solution of (t)-{1S,2S,3R,4R)-2-N-methyl-3-acetamidomethyl-4-
methoxycarbonyl-cyclopentanecarboxylic acid hydrochloride (100 mg), N-
-83-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
(benyloxycarbonyloxy)succinimide (183 mg) and triethylamine (0.160 mL) in
dichloromethane (10 mL) was stirred at room temperature for 16 hours. The
mixture was diluted with ethyl acetate and washed successively with 1 N HCI,
and brine, dried over MgS04, and concentrated in vacuo. The residue was
purified by silica gel chromatography using chloroform/methanol/acetic acid
(97:2:1 ) to provide the title compound as a white solid.
~ H NMR (CDC13) b1.88 (s, 3H), 2.08 (m, 2H), 2.46 (m, 1 H), 2.66 (m, 1 H),
2.92 (m, 1 H), 2.96 (s, 3H), 3.18 (m, 1 H), 3.35 (m, 1 H), 3.70 (s, 3H), 4.64
(bs, 1 H),
5.14 (s, 2H), 6.29 (bs, 1 H), 7.34 (m, 5H).
MS: (M+H)+ = 407.
Example 4
fit) (1 S 2S 3R 4R)-2-lt-Butyloxycarbonylamino)-3-(acetamidomethyl)-4-(methoxy-
carbonyl -cyclopentane-1-carboxylic Acid.
Ac0
NHBoc
4A. ~t~~2R 3S~ 2 t But~loxycarbonylamino-3-acetoxymethylbicyclof2.2.11hept-5-
ene.
A solution of (t)-(2R,3S)-2-(t butyloxycarbonylamino)-3-
hydroxymethylbicyclo[2.2.1]hept-5-ene (1.0 g, 4.18 mmole), acetic anhydride
(0.55 mL), triethylamine (2 mL) and N,N-dimethylaminopyridine (catalytic) in
50
mL of dichloromethane was reacted for 2 hours, at room temperature. The
reaction mixture was diluted with 200 mL of ethyl acetate, washed with 0.5 N
HCI,
water, saturated sodium bicarbonate, and brine. The organic layer was dried
over MgS04, filtered and concentrated. The crude product was purified by
silica
-84-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
gel chromatography using 10% ethyl acetate/hexanes to provide the title
compound. (yield: .92 g, 78 %)
MS: (M+H)+ = 282.
0
H O-J
Ac0 OH
BocHN O
4B. (t) 1 S 2S 3S 4R) 2 (t Butt'loxycarbonylamino)-3-(acetoxymethyl)-cycio-
pentane-1 4-dicarboxylic Acid
Ruthenium dioxide hydrate (43mg, 0.32 mmole) was added to a vigorously
stirred mixture of Na104 (7.12 g, 33 mmole) in 33 mL of acetonitrile, 33 mL of
carbon tetrachloride and 58 mL of water. The mixture was stirred at room
temperature for 5 minutes or until a homogeneous yellow color was attained. A
solution of (t)-(2R,3S)-2-(t-butyloxycarbonylamino)-3-acetoxymethyl-
bicyclo[2.2.1 ]hept-5-ene (2.28 g, 8.11 mmole) in 10 mL of (1:1 )
acetonitrile:carbon tetrachloride was added rapidly to the reaction mixture.
The
mixture was stirred vigorously for 1 hour at room temperature. The reaction
mixture was partitioned between ethyl acetate and 0.5 N HCI. The organic layer
was washed with brine, dried over Na2S04, filtered and concentrated to provide
the diacid compound which was used in the following reaction without
additional
purification.
_gb_
CA 02329660 2000-10-20
WO 99/54290 PCT/1JS99/07949
O
HO--~~
HO OH
BocHN O
4C. (t) (1S 2S 3S 4R)-2-(t-ButYloxycarbon~rlamino -3~-(h_ydroxymethyl)-cyclo-
pentane-1 4-dicarboxylic Acid.
The crude diacid, (t)-(1 S,2S,3S,4R)-2-(t butyloxycarbonylamino)-3-
(acetoxymethyl)-cyclopentane-1,4-dicarboxylic acid, prepared in example 4B,
and
sodium hydroxide (1.2 g) in 45 mL of water was reacted for 6 hours, at room
temperature. The reaction mixture was acidified to pH 1 with and extracted
with
ethyl acetate (3 x 40 mL). The organic layer was washed with brine and dried
over Na2S04, filtered and concentrated. The crude diacid-alcohol was used in
the following reaction without additional purification.
0
CH30--!~
HO ,,~~~OCH3
BocHN 1O
4D. (t,~ (1 S 2S 3S 4R~ 2-(t Butyloxycarbonylamino)-3-(hydroxymethyl)-cyclo-
pentane-1 4-dicarboxylic Acid Dimethyl Ester.
The diacid-alcohol, (t)-(1S,2S,3S,4R)-2-(t-butyloxycarbonylamino)-3-
(hydroxymethyl)-cyclopentane-1,5-dicarboxyiic acid, prepared in Example 4C,
was dissolved in 40 mL of tetrahydrofuran {THF) and reacted with diazomethane
in ethyl ether until complete conversion to the dimethyl ester. The reaction
mixture was monitored by TLC, using 10% methanol in chloroform with 1 % acetic
acid. The reaction was concentrated in vacuo to provide the title compound as
a
colorless oil (yield: 1.3 g, 85 %).
-86-
CA 02329660 2000-10-20
WO 99154290 PCT/US99/07949
MS: (M+H)+ = 331.
O
CH30--l~
N3 .,~~~~ CH3
BocHN
4E. ,~~1S 2S 3R 4R)-2-(f Butyloxycarbonylamino)-3-(azidomethyl)-cyclo-
pentane-1 4-dicarboxylic Acid Dimethyl Ester.
Methanesulfonyl chloride (1.3 mL, 17.0 mmole) was added slowly to a
solution of (t)-(1S,2S,3S,4R)-2-(t butyloxycarbonylamino)-3-(hydroxymethyl)-
cyclopentane-1,4-dicarboxylic acid dimethyl ester (2.76g, 8.34 mmole) and
triethylamine (2.4 mL, 17.0 mmole) in 80 mL of 1:1
dichloromethaneaetrahydrofuran, maintained at -30 °C. The reaction
mixture was
stirred for 2.5 hours, at -30 °C then diluted with ethyl acetate washed
with 0.1 N
HCI and brine, dried over MgS04, filtered and concentrated in vacuo to provide
the crude mesyiate. The mesylate and lithium azide (4 g) were reacted in 35 mL
of N,N-dimethylformamide for 1 hour, at 90 °C. The reaction mixture was
partitioned between ethyl acetate and water. The organic layer was washed with
brine, dried over MgS04, filtered and concentrated. The crude product was
purified by silica gel chromatography using 20% ethyl acetate in hexanes to
provide the title compound. (yield: 1.8 g, 48%)
MS: (M+H)+ = 373.
-87-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O
CH30-~~
AcHN OCH3
BocHN O
4F. (t)_(1 S 2S 3R 4R~-2-(t-But~loxycarbonylamino)-3-(acetamidomethyl)-
cycfopentane-1 5-dicarboxylic Acid Dimethyl Ester.
(t)-(1 S,2S,3R,4R)-2-(t-Butyloxycarbonylamino)-3-(azidomethyl)-
cyclopentane-1,4-dicarboxylic acid dimethyl ester (506 mg, 1.42 mmole) and
thiolacetic acid (0.4 mL) were reacted at room temperature for 6 hours. The
reaction mixture was concentrated in vacuo and the crude product was purified
by silica gel chromatography using 3% methanol in chloroform to provide the
title
compound (yield: 255 mg, 48%).
MS: (M+H)+ = 373.
o
HO--l~
AcHN OH
O
NHBoc
4G. (t)~1S 2S 3R 4R)-2-(t Butyloxycarbonylamino)-3-(acetamidomethyl)-
~clopentane-1.4-dicarboxylic Acid
(t)-(1 S,2S,3R,4R)-2-(t-Butyloxycarbonylamino)-3-(acetamidomethyl)-
cyclopentane-1,4-dicarboxylic acid dimethyl ester (255 mg, 0.68 mmole) and
lithium hydroxide (2.2 equivalents) in 15 mL of 4:1 methanol:water were
reacted
at room temperature for 2 hours. The reaction was acidified with dilute HCI
and
extracted with ethyl acetate (3 X 60 mL). The organic layers were combined,
dried over Na2S04, filtered and concentrated to provide the title compound.
-88_
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O
CH30--U
AcHN OH
BocHN O
4H. fit) (1 S 2S 3R 4R)-2-(t Buyloxycarbonylamino)-3-(acetamidomethvl)-4-
(methoxycarbonyl)-cyclopentane-1-carboxylic Acid
The crude diacid, (t)-(1S,2S,3R,4R)-2-(t-butyloxycarbonylamino)-3-
(acetamidomethyl)-cyclopentane-1,4-dicarboxylic acid, prepared in Example 4G
was reacted with acetic anhydride (20 mL) for approximately 1 hour at 60
°C to
provide the bicyclic anhydride. The reaction mixture was concentrated in vacuo
and the crude anhydride was treated with methanol (50 mL) and triethylamine (2-
3 equivalents) at room temperature for 3 hours. The reaction mixture was
diluted
with ethyl acetate and washed with 0.5N HCl and brine. The organic solution
was
dried over Na2S04, filtered and concentrated. Chromatographic separation of
the
diastereomers was accomplished by silica gel chromatography using 25% ethyl
acetate in hexanes and 0.5% acetic acid to provide the title compound (yield:
146
mg, 60 %).
'H NMR (methanol-d4) 5 1.44 (s, 9H), 1.90 (s, 3H), 2.04 (m, 1H), 2.32
(m,1 H), 2.54 (m,1 H), 2.72 (m,2H), 3.11 (m, 1 H), 3.36 (m,1 H), 4.38 (m,1 H),
6.92
(broad d, 1 H), 7.8 (broad s, 1 H).
MS: (M+H)'' = 359.
_89_
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 5
0
CH30--!~
AcH N OH
~'I~
NH2 O
HCI
(t)-(1 S.2S.3R.4R~-2-Amino-~acetamidomethyl)-4-(methoxycarbonyl)-
cyclopentane-1-carboxylic Acid Hydrochloride
A solution of (t)-(1S,2S,3R,4R)-2-(t butyloxycarbonylamino)-3-(acetamido-
methyl)-4-(methoxycarbonyl)-cyclopentane-1-carboxylic acid (66 mg, 0.18
mmole) in 3 mL of dichloromethane was reacted with trifluoroacetic acid (1.0
mL)
for 1 hr, at room temperature. The solution was concentrated in vacuo at 25
°C.
The crude product was treated with 1 N HCI and concentrated in vacuo to
provide
the title compound as a white solid.
' H NMR {D20) 82.01 (s, 3H), 2.19 (m, 1 H), 2.58 (m, 1 H), 2.81 (t, 1 H), 2.95
{m, 1 H), 3.15 (m, 1 H), 3.38 (m, 3H), 3.75 (s, 3H), 4.08 (t, 1 H).
MS = (M+H)+ = 259.
-90-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
Example 6
(~S 2S 3R 4R) 3 Acetamidomethyl-2-(N-t-butoxycarbonylamino)methyl-4-
metho~carbonyl-cyclopentane-1-carboxylic Acid
Ac0
OBn
6A. (t) exo exo 3 Acetoxymethyl-2-benzyioxymethyl-bicyclof2.2.11hept-5-ene.
(t)-exo-exo-2,3-Dihydroxymethylbicyclo[2.2.1]hept-5-ene (620 mg, 4.0
mmole), prepared according to the procedure described by Culberson, C. et al.,
Journal of the American Chemical Society 82, 2541-2547, (1960), was reacted
with sodium hydride (300mg, 60% oil dispersion) in 10 mL of N,N-dimethyl-
formamide (DMF) for 15 min, at 0 °C. This was followed by treatment of
the
dianion with benzyl bromide (0.5 mL) for an additional 2 hours. The reaction
mixture was diluted with ethyl acetate washed with wafer, and brine, dried
over
MgS04, filtered and concentrated in vacuo.
The crude benzylated product was reacted with acetic anhydride (0.7 mL),
triethylamine (3 mL) and N,N-dimethylaminopyridine (catalytic) in
dichloromethane (20 mL) at room temperature for 1 hour. The reaction mixture
was concentrated in vacuo and purified by silica gel chromatography using 10%
ethyl acetate in hexanes to provide the title compound (yield: .845 g, 74%).
'H NMR (CDC13) 81.41 (m, 2H), 1.85 (m, 2H), 2.05 (s, 3H), 2.73 (brs, 1H),
2.78(brs, 1 H), 3.38 (m, 1 H), 3.57 (m, 1 H), 3.95 (m, 1 H), 4.31 (m, 1 H),
4.51 (m,
2H), 6.18 (m, 2H), 7.33 (m, 5H).
MS: (M+H)+ = 287.
-91-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
O
CH30~/
Ac0 OCH3
0
OBn
6B. fit)-( 1 S 2S 3R 4RZ 3-AcetoxvmethYl-2-benzvlo~methyl-cyclopentane-1.4-
dicarboxylic Acid Dimethyl Ester.
A rapidly stirred mixture of Na104 (10 g) and Ru02 (45 mg) in 25 mL of
carbon tetrachloride, 50 mL of acetonitrile, and 75 mL of water was reacted
for 30
minutes. A solution of (t)-(2R,3S)-2-acetoxymethyl-3-benzyloxymethyl-
bicyclo[2.2.1 ]hept-5-ene (3.3 g, 11.5 mmole) in 25 mL of carbon tetrachloride
was
added to the bright yellow mixture. The resultant black mixture was stirred
for 1.5
hours, at room temperature. Water (250 mL) was added to the reaction mixture
and the aqueous layer was extracted with ethyl acetate (5 x 200 mL) . The
combined organic layers were washed with brine, dried over MgS04, and
concentrated in vacuo.
The residue was dissolved in ethyl acetate and treated with a solution of
diazomethane in ethyl ether. The reaction mixture was concentrated in vacuo,
and the crude product purified by silica gel chromatography using ethyl
acetate:
hexanes (1:1 ) to provide the title compound as a white solid (yield: 1.91 g,
44%).
' H NMR (CDC13) 82.00 (s, 3H), 2.14 (m, 1 H), 2.34 (m, 1 H), 2.77 (m, 3H),
2.89 (m, 1 H), 3.51 (m, 2H), 3.67 (s, 3H), 3.70 (s, 3H), 4.18 (m, 2H), 4.47
(s, 2H),
7.32 (m, 5H).
MS: (M+H)+ = 379.
-92-
CA 02329660 2000-10-20
WO 99/54290 PCT/1JS99/07949
O
CH30-l
Ac0 ,,~~~OCH3
tO
OH
6C. SOS 2S 3R 4R) -3-Acetox~methyl-2-~droxymethYl_cyclopentane-1.4-
dicarboxylic Acid Dimethyl Ester.
A mixture of (t)-(1S,2S,3R,4R)-3-acetoxymethyl-2-
benzyloxymethylcyclopentane-1,4-dicarboxylic acid dimethyl ester {3.3 g, 8.72
mmole) and palladium (600 mg, 10% on carbon) in ethanol (100 mL) was stirred
vigorously at room temperature under an hydrogen atmosphere. Upon
completion, as determined by TLC, the reaction mixture was filtered and
concentrated in vacuo to provide the title compound (yield: 2.56 g, 100%).
' H NMR (CDC13) 81.84 (t, 1 H), 2.05 (s, 3H), 2.16 (m, 1 H), 2.36 (m, 1 H),
2.75 (m, 4H), 3.70 (s, 3H), 3.71 (s, 3H), 3.73 (m, 1 H), 4.19 {m, 2H).
MS = (M+H)+ = 289.
O
CH30~
Ac0 .,,~~~OCH3
O
N3
6D. {t)-{1 S 2S 3R 4R~ 3-Acetoxymethyl-2-azidomethylcyclopentane-1,4-di-
carboxylic Acid Dimethyl Ester.
Methanesulfonyl chloride (2.1 mL, 26.6 mmole) was added slowly to
a solution of (t)-(1S,2S,3R,4R)-3-acetoxymethyl-2-hydroxymethylcyclopentane-
1,4-dicarboxylic acid dimethyl ester (2.56g, 8.72 mmole) and triethylamine
{3.7
-93-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
mL, 26.6 mmole) in 100 mL of dichloromethane at -30 °C. The reaction
mixture
was stirred at -30 °C for 0.5 hour then allowed to warm to 0 °C
over 1 hour. The
reaction mixture was diluted with ethyl acetate washed with 0.1 N HCI and
brine,
dried over MgS04, filtered and concentrated in vacuo to provide 4.32 g of
crude
mesylate.
The crude mesylate, prepared above, and lithium azide (4.2 g, 87.2
mmole) were reacted at 90 °C in 50 mL of N,N-dimethylformamide for 1
hour.
The reaction mixture was cooled and partitioned between ethyl acetate and
water. The organic layer was washed with brine, dried over MgS04, filtered and
concentrated. The crude product was purified by silica gel chromatography
using
25 % ethyl acetate in hexanes to provide the title compound (yield: 2.0 g,
72%).
' H NMR (CDC13) 82.07 (s, 3H), 2.17 (m, 1 H), 2.37 (m, 1 H), 2.76 (m, 4H),
3.47 (m, 2H), 3.71 (s, 3H), 3.73 (s, 3H), 4.16 (m, 2H).
0
CH30-l/
Ac0 OCH3
~'If
0
NHBoc
6E. (t)~1S 2S 3R 4R~-3-Acetoxymethyl-2-(N-t butoxycarbonylamino)methyl-
cycloeentane-1 4-dicarboxvlic Acid Dimethyl Ester.
A mixture of (t)-(1 S,2S,3R,4R)-3-acetoxymethyl-2-azidomethylcyclo-
pentane-1,4-dicarboxylic acid dimethyl ester (2.0 g, 6.6 mmole), di-t-butyl-
dicarbonate (3.79 g), and palladium (900mg, 10% on carbon) in 100 mL of ethyl
acetate was stirred vigorously at room temperature under an hydrogen
atmosphere. Upon completion, as determined by TLC, the reaction mixture was
-94-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
filtered and concentrated in vacuo. The residue was purified by chromatography
on silica gel using 50% ethyl acetate in hexanes to provide the title compound
(Yield: 2.3 g, 94%).
' H NMR (CDC13) 81.43 (s, 9H), 2.06 (s, 3H), 2.17 (m, 1 H), 2.35 (m, 1 H),
2.72 (m, 4H), 3.21 (m, 2H), 3.70 (s, 3H), 3.72 {s, 3H), 4.12 (m, 2H), 4.74 (t,
1 H).
MS = (M+H)+ = 388.
0
CH30-'~
HO .,~~~~ CH3
O
NHBoc
6F. (t)-(1S 2S 3R 4R)-2-(N-t Butoxycarbonylamino),methyl-3-hydroxymethyl-
cyclopentane-1 4-dicarboxylic Acid Dimethyl Ester.
(t)-(1 S,2S,3R,4R)-3-Acetoxymethyf-2-(N-t-butoxycarbonylamino)-
methylcyclopentane-1,4-dicarboxylic acid dimethyl ester (600 mg,11.55 mmole)
was treated with potassium carbonate (catalytic) in 10 mL of methanol, at room
temperature for Bh. The reaction mixture was concentrated in vacuo. The
residue was partitioned between ethyl acetate and brine. The organic layer was
dried over MgS04, filtered and concentrated to provide the title compound.
(yield:
510 mg, 95%)
'H NMR (CDC13) 81.44 (s, 9H), 2.24 (m, 2H), 2.59 (m, 2H), 2.77 (m, 2H),
3.28 (m, 2H), 3.73 (m, 8H), 5.04 (t, 1 H).
-95-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O
CH30--!~
N3 OCH3
O
NHBoc
6G. (t,~1 S 2S 3R 4R)-3-Azidomethyl-2-(N-t butox~carbonylamino)methylcyc-
lopentane-1 4-dicarboxylic Acid Dimethyl Ester.
Methanesulfonyl chloride (0.35 mL, 4.5 mmole) was added slowly to a
solution of (t)-(1 S,2S,3R,4R)-2-(N-t-butoxycarbonylamino)methyl-3-
hydroxymethyl-cyclopentane-1,4-dicarboxylic acid dimethyl ester {560 mg, 1.48
mmole) and triethylamine (0.6 mL) in dichloromethane (10 mL) at -30 °C.
The
reaction mixture was stirred at -30 °C for 2 hours then allowed to warm
to 0 °C
over 1 hour. The reaction mixture was then diluted with ethyl acetate washed
with 0.1 N HCI and brine, dried over MgS04, filtered and concentrated in vacuo
to
provide the crude mesylate.
The crude mesylate, prepared above, and lithium azide (0.7 g, 14.3
mmole) were reacted at 85 °C in 10 mL of N,N-dimethylformamide for 1
hour.
The reaction mixture was cooled to room temperature and partitioned between
ethyl acetate and water. The organic layer was washed with brine, dried over
MgS04, filtered and concentrated. The crude product was purified by silica gel
chromatography using 25% ethyl acetate in hexanes to provide the title
compound (yield: 386 mg, 70%).
'H NMR (CDC13) 81.43 (s, 1H), 2.26 {m, 2H), 2.69 (m, 4H), 3.23 (m, 2H),
3.46 (m, 2H), 3.70 (s, 3H), 3.71 (s, 3H), 4.74 (bs, 1 H).
-96-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O
CH30~
AcHN OCH3
O
NHBoc
6H. ,{t)~1 S 2S 3R 4R)-3-Acetamidomethyl-2-LN-t butoxycarbonylamino)methyl-
~clopentane-1,4-dicarboxylic Acid Dimethyl Ester.
A mixture of (t)-(1S,2S,3R,4R)-3-azidomethyl-2-(N-t butoxycarbonyl-
amino)methylcyclopentane-1,4-dicarboxylic acid dimethyl ester (386 mg, 1.04
mmole) , acetic anhydride (0.25 mL) and palladium (25 mg, 10% on carbon) in 10
mL of ethyl acetate was stirred vigorously at room temperature under an
hydrogen atmosphere. Upon completion, as determined by TLC, the reaction
mixture was filtered and concentrated in vacuo. The residue was purified by
chromatography on silica gel using 75% ethyl acetate in hexanes to provide the
title compound. (yield: 232 mg, 58%).
'H NMR (CDC13) x1.44 (s, 9H), 1.97 (s, 3H), 2.27 (m, 2H), 2.64 (m, 2H),
3.18 (m, 2H), 3.32 (t, 1 H), 3.71 (s, 3H), 3.73 (s, 3H), 7.78 {bs, 1 H), 6.19
(bs, 1 H).
MS = (M+H)+ = 387.
_97_
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O
CH30-~~
AcHN OH
O
NHBoc
61. (,t~~1 S 2S 3R 4R~-3-Acetamidomethvl-2-~N-t butoxycarbonylamino)methyl-4-
methoxycarbonylcyclopentane-1-carboxylic Acid.
(t)-( 1 S,2S, 3R,4 R)-3-Acetamidomethyl-2-(N-t
butoxycarbonylamino)methylcyclopentane-1,4-dicarboxylic acid dimethyl ester
(232 mg, 0.60 mmole) was reacted with 5 equivalents of lithium hydroxide in 5
mL
of (4:1 ) methanol:water for 2 hours, at room temperature. The reaction
mixture
was neutralized with 0.1 N HCI and partitioned between ethyl acetate and
brine.
The organic layer was concentrated to provide 194 mg of crude diacid.
The crude diacid (190 mg), prepared above, was reacted with acetic
anhydride (10 mL) for 3 hours, at room temperature. The reaction mixture was
concentrated in vacuo. The crude product was treated with methanol (10 mL)
and triethylamine (.250 mL) for 16 hours. The reaction mixture was
concentrated
and partitioned between ethyl acetate and 0.1 N HCI. The organic layer was
dried
over NaZS04, filtered and concentrated. The diastereomeric mixture of methyl
esters (174 mg) was chromatographed on silica gel using (5-10%) methanol in
chloroform and acetic acid (0.5%) to provide the title compound. (yield: 72
mg,
32%)
'H NMR (CD30D) 81.44 (s, 9H), 1.93 (s, 3H), 2.24 (m, 2H), 2.64 (m, 4H),
3.12 (m, 3H), 3.67 (s, 3H).
MS = (M+H)+ = 373.
_98_
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 7
0
CH30-!~
AcHN OH
O
HCI NHZ
fit) (1 S 2S 3R 4R~-2-Aminomethyl-3-acetamidomethyl-4-methoxycarbonyl-cyclo-
pentane-1-carboxylic Acid Hydrochloride.
A solution of (t)-(1 S,2S,3R,4R)-3-Acetamidomethyl-2-(N-t-butoxycarbonyl-
amino)methyl-4-methoxycarbonylcyclopentane-1-carboxylic acid (62 mg, 0.16
mrnol) in of dichloromethane (4 mL) was reacted with trifluoroacetic acid (1.0
mL)
for 1 hour, at room temperature. The solution was concentrated in vacuo, at 25
°C. The residue was treated with 1 N HCI and concentrated in vacuo to
provide
the title compound as a white solid (yield: 39 mg, 75 %).
' H NMR (D20) 81.95 (s, 3H), 2.22 (m, 1 H), 2.46 (m, 1 H), 2.71 (m, 2H),
2.83 (m, 1 H), 2.96 (q, 1 H), 3.10 (m, 2H), 2.31 (m, 2H), 3.72 (s, 3H).
MS : (M+H)+ = 273.
-99_
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 8
St)-(1S 2S 3R 4R)-2-N-t-Butoxycarbonylamino-3-(acetamidomethyl}-4-carbamoyl-
c rclopentane-1-carboxylic Acid.
0
H2N-J
AcHN OCH3
O
NHBoc
8A. (t~~1 S 2S 3R 4R)-2-N-t-Butoxycarbonyiamino-3 ~acetarnidomethyl)-4-
carbamoyl-cyclo~entane-1-carboxylic Acid Methyl Ester.
The title compound was prepared ih two steps starting by reacting (t)-
(1 S,2S,3R,4R)-2-(N-t-butyloxycarbonylamino)-3-(acetamidomethyl)-
cyclopentane-1,4-dicarboxylic acid in place of (t)-(1 S,2S,3R,4R)-2-(t
butyloxycarbonylamino)-3-(acetamidomethyl)-cyclopentane-1,4-dicarboxylic acid,
according to the method described in Example 4H, and substituting anhydrous
liquid ammonia for methanol and triethylamine.
In the second step the crude product (54 mg, 0.16 mmole), prepared
above, in 4 ml of THF was cooled to 0 °C and treated with an ethereal
solution of
CHZN2 until the yellow reaction color persisted. The reaction mixture was
warmed slowly to room temperature and concentrated in vacuo to provide a
colorless oil. Purification using flash chromatography eluting with 5%
methanol/chloroform provided the title compound as a white solid (yield: 16
mg,
28%).
'H NMR (CDC13) 51.43 (s, 9H), 1.90 (s, 3H), 2.03 (m, 1 H), 2.24 (m, 1 H),
2.65 (m, 4H), 3.68 (s, 3H), 4.30 (m, 2H).
MS: (M+H)+ = 358.
-100-
CA 02329660 2000-10-20
WO 99/54290 PCT/1JS99/07949
O
HZN~/
AcHN OH
~'If
0
NHBoc
8B. ~t~~1S 2S,3R 4R)-2-N-t-Butox)rcarbonYlamino-3-(acetamidomethyl)-4-
carbamoyl-cyclopentane-1-carboxylic Acid.
A solution of (t)-(1 S,2S,3R,4R)-2-N-t butoxycarbonylamino-3-(acetamido-
methyl)-4- carbamoyl-cyclopentane-1-carboxylic acid methyl ester (16 mg, 0.045
mrnole) in 0.2 ml methanoI/H20 (3:1 ) was treated with (1 mg, 0.045 mmole) of
LiOH. After stirring at room temperature overnight, the reaction mixture was
quenched with 5% HCI and extracted 3 times with ethyl acetate. The combined
extracts were dried over Na2SOa, filtered and concentrated in vacuo to provide
the title compound as a white solid (yield: 11 mg, 73%).
'H NMR (methanol-d4) 81.44 (s, 9H), 1.91 (s, 3H), 2.02 (m, 1H), 2.24 (m,
1 H), 2.60 (m, 5H), 4.32 (m, 1 H), 6.90 (br d, 1 H).
MS (M-H)- = 342.
-101-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 9
~t)-(1S,2S,3R.4R)-2.3-Acetamidomethyl-4-methoxycarbon r~yclopentane-1-
carboxylic Acid.
Ac0
OAc
9A. (t)-exo-exo-2.3-diacetoxymethyl-bi~clo[2.2.1Lept-5-ene.
(t)-exo-exo-2,3-dihydroxymethyl-bicyclo[2.2.1]hept-5-ene was treated with
acetic anhydride and triethylamine in dichloromethane. Standard workup
provided the title compound.
MS: (M+H)+ = 329.
O
CH30-!~
Ac0 OCH3
.,,,
OAc
9B. Lt)-(1 S 2S.3R 4R~-2.3-Diacetoxymethylcyclopentane-1,4-dicarboxylic Acid
Dimethvi Ester.
The title compound was prepared according to the method described in
Example 6B, substituting (t)-exo-exo-2,3-diacetoxymethylbicyclo[2.2.1]hept-5-
ene (1 g, 4.2 mmol) in place of (t)-(2R,3S)-2-acetoxymethyl-3-benzyloxymethyl-
bicyclo[2.2.1]hept-5-ene. Purification, using flash chromatography eluting
with
50% ethyl acetate/hexanes, provided the title compound as a colorless oil.
(yield:
0.8 g, 58%).
' H NMR (CDC13) 62.05 (s, 6H), 2.29 (m, 2H), 2.79 (m, 4H), 3.72 (s, 6H),
4.14 (m, 4H).
-102-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
MS: (M+H)+ = 331.
O
CH30-l~
HO OCH3
0
OH
9C. fit)-~1S 2S 3R 4R~2 3-DihYdroxymet~fcyclopentane-1.4-dicarboxylicAcid
Dimethyl Ester.
A solution of (t)-(1S,2S,3R,4R)-2,3-diacetoxymethylcyclopentane-1,4-
dicarboxylic acid dimethyl ester (5.0 g, 0.015 mole) in 60 ml of methanol was
treated with a catalytic amount of K2C03. The reaction mixture was stirred,
under
a nirogen amosphere, at room temperature, for 16 hours. The reaction mixture
was diluted with ethyl acetate and quenched with 5% HCI. The organic layer was
separated, dried over Na2S04, filtered, and concentrated in vacuo, at 30
°C to
provide the title compound as a colorless oil. (yield: 3.3 g, 89%)
'H NMR (CDC13) 82.06 (m, 1H), 2.29 (m, 2H), 2.54 (m, 3H), 2.80 (m, 2H),
3.67 (m, 8H).
MS: (M+H)+ = 247.
-103-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
0
CH30-J~
Ms0 OCH3
..,,
OMs
9D. ~f)-(1 S.2S.3R.4R)-2.3-Dimethansulfonyloxymeth rLlcyclopentane-1.4-di-
carbox~rlic Acid Dimethyl Ester.
A solution of (t)-(1 S,2S,3R,4R)-2,3-dihydroxymethylcyciopentane-1,4-
dicarboxylic acid dimethyl ester (200 mg, 0.81 mmole) in 4 ml of THF and 4 ml
of
dichloromethane was cooled to -30 °C and treated with methanesulfonyl
chloride
(0.19 mL, 2.4 mmole) followed by dropwise addition of triethylamine (0.34 mL,
2.4
mmole). After 30 minutes of stirring under a nirogen amosphere, at -30
°C, the
mixture was diluted with ethyl acetate and washed with water and brine. The
organic layer was dried over Na2S04 , filtered and concentrated in vacuo to
provide the title compound as a colorless oil (yield: 302 mg, 93%).
'H NMR (CDC13) b 62.16 (m, 1 H), 2.45 (m, 1 H), 2.87 (m, 4H), 3.06 (s, 6H),
3.72 (s, 6H), 4.38 (m, 4H).
MS: (M+H)+ = 403.
-104-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O
CH30-l~
N3 OCH3
~'I~
0
N3
9E. (t)-(1 S 2S 3R 4R~-2 3-Diazidometh~-cyclopentane-1 4-dicarboxylic Acid
Dimeth~ Ester.
A solution of (t)-(1 S,2S,3R,4R)-2,3-dimethansulfonyloxymethyl-
cyclopentane-1,4-dicarboxylic acid dimethyl ester (300 mg, 0.75 mmole) in 3 mL
of DMF was treated with LiN3 (400 mg, 8.2 mmole) and heated to 100 °C
under a
nitrogen amosphere. After heating for 1 hour, the reaction mixture was diluted
with ethyl acetate and washed twice with water, brine, dried over Na2S04,
filtered
and concentrated in vacuo to provide the title compound as a copper colored
oil
(yield: 204 mg, 92%).
'H NMR (CDC13) 8 62.12 (m, 1 H), 2.37 (m, 1 H), 2.71 (m, 4H), 3.50 (m, 4H),
3.72 (m, 6H).
MS: (M+H)+ = 297.
O
CH30~~
AcHN OCH3
0
NHAc
9F. (t)-(1 S 2S 3R 4R)-2 3-Acetamidomethyl-cyclopentane-1 4-dicarboxylic Acid
Dimethyl Ester.
A solution of (t)-(1S,2S,3R,4R)-2,3-diazidomethyl-cyclopentane-
1,4-dicarboxylic acid dimethyl ester (145 mg, 0.49 mmole) in 2 ml of isopropyl
-105-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
alcohol was treated with Pd/C. The reaction mixture stirred vigorously
overnight
under a hydrogen atmosphere, at room temperature. The reaction mixture was
filtered over Celite'~ and concentrated in vacuo to provide a pale yellow oil.
The
crude product was then taken up in dichloromethane and treated with excess
acetic anhydride and N,N-dimethylaminopyridine. The reaction mixture was
stirred at room temperature for 1 hour before concentrating in vacuo to
provide a
pale yellow oil. Purification, by flash chromatography eluting with 10%
methanol/chloroform provided the title compound as a colorless oil (yield: 40
mg,
25%).
'H NMR (CDC13) X1.98 (s, 6H), 2.30 (m, 1H), 2.36 (m, 1H), 2.56 (m, 2H),
2.68 (m, 2H), 3.30~(m, 4H), 3.71 (s, 6H), 6.16 (br s, 2H).
MS: (M+H)* = 329.
O
CH30--l~
AcHN ,,,~~OH
1O
NHAc
9F. ~(1S 2S 3R4R)-2 3-Acetamidomethyl-4-methoxYcarbonylcvclopentane-1-
carboxylic Acid.
A solution of (t)-(1S,2S,3R,4R)-2,3-acetamidomethyl-cyclopentane-
1,4-dicarboxylic acid dimethyl ester (100 mg, 0.33 mmole) in 1 m! of a mixture
of
methanoI/H20 (3:1 ) was treated with LiOH (16 mg, 0.66 mmole). After stirring
for
1 hour, at room temperature, the reaction mixture was acidified with 5% HCI
and
extracted three times with ethyl acetate. The organic extracts were dried over
Na2S04, filtered and concentrated in vacuo to provide the diacid intermediate
as
a white solid (69 mg, 70%). The diacid was taken up in acetic anhydride (2 ml)
-106-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
and heated for 1 hour at 60°C. The reaction mixture was then
concentrated in
vacuo, taken up in 1 mL of methanol and treated with triethyl amine. The
mixture
was stirred at room temperature for 3 hours, under a nitrogen atmosphere. The
reaction mixture was diluted with ethyl acetate and washed 3 times with 5%
HCI.
The organic layer was dried over Na2S04 , filtered and concentrated in vacuo.
Purification, using flash chromatography, eluting with 20% ethyl
acetate/hexanes
with 1 % acetic acid provided the title compound as a white solid. (yield: 6
mg,
6%)
'H NMR (methanol-d4) 81.92 (d, J=3 Hz, 6H), 2.15 (m, 2H), 2.35 (m, 2H),
2.67 (m, 4H), 3.15 (m, 2H), 3.67 (s, 3H).
MS: (M+H)+ = 315.
-107-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 10
_{t)-(1S 2S 3R 4R~2-N-t Butoxycarbonylamino-3-Lacetamidomethyl)-4-N-
methylcarboxamido-cyclopentane-1-carboxylic Acid and~t~1 R,2R.3S,4S)-3-N-t
butoxycarbonylamino-2-{acetamidomethyl)-4-N-methylcarboxamido-cyclopent-
ane-1-carboxylic Acid.
0 0
H3CHN-~~ HO-~~
AcHN OH AcHN NHCH3
O O
NHBoc NHBoc
10A. {t~1S 2S 3R 4R~2-N-t butox~carbonylamino-3- acetamidomethyl)-4-N-
methylcarboxamido-cyclopentane-1-carboxylic Acid Methyl Ester and (t)-(1 R.-
~R 3S 4Sy-3-N-t butoxycarbonylamino-2-(acetamidomethyl)-4-N-methylcarbox-
amido-cvclopentane-1-carboxylic Acid Methyl Ester.
A solution of (t)-(1S,2S,3R,4R)-2-N-t butoxycarbonylamino-3-(acetamido-
methyl)-4-N-methylcarboxamido-cyclopentane-1-carboxylic acid methyl ester and
(t)-(1 R,2R,3S,4S)-3-N-t-butoxycarbonylamino-2-{acetamidomethyl)-4-N-methyl-
carboxamido-cyclopentane-1-carboxylic acid methyl ester (1:1) (260 mg, 0.72
mmole) in 3 ml of dichloromethane was treated with hydroxybenzotriazole (1
equiv.), 1-(3-dimethyiaminopropyl)-3-ethylcarbodiimide (1.5 equivalents), and
methylamine (5 equivalents). After stirring at room temperature for 2 hours,
the
reaction mixture was diluted with ethyl acetate and washed 3 times with 5%
HCI.
The organic layer was dried over Na2S04 , filtered and concentrated in vacuo
to
provide the title ester-amide compounds as a white solid. (yield: 111 mg, 42%)
MS: (M+H)+ = 372.
-108-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O O
CH3N-~~ HO~
Ac0 OH Ac0 NHCH3
~''I'~ ~'I~
NHBo NHBo
1 OB. (t)-(1 S 2S 3R 4R)-2-N-t Butoxycarbonylamino-3-(acetamidomethvl)-4-N-
methylcarboxamido-cyclopentane-1-carboxylic Acid and ~t)~1 R,2SR,3S,4S)-3-N-
t-butoxycarbonylamino-2 ~acetamidomethyl)-4-N-methYlcarboxamido-cyclopent-
ane-1-carboxylic Acid.
A solution of the ester-amide mixture (125 mg, 0.34 mmole) prepared in
Example 10A, in 1 ml of methanoI/H20 (3:1 ) was treated with LiOH (8 mg, 0.34
mmole). After stirring for 1 hour at room temperature, the reaction mixture
was
quenched with 5% HCI and extracted 3 times with ethyl acetate. The combined
organic extracts were dried over Na2S04 , filtered and concentrated in vacuo
to
provide the title compound as a white solid. (yield: 85 mg, 70%)
1 H NMR (methanol-d4) 81.44 (br d, 18H), 1.91 (d, J=5 Hz, 6H), 2.00 {m,
2H), 2.20 (m, 2H), 2.60 (m, 6H), 2.72 (br s, 6H), 3.08 (m, 4H), 4.35 (m, 2H).
MS: (M+H)+ = 358.
-109-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 11
(t)-(1 S 2S 3R 4RZ 3-AcetamidomethYl-2 4-diamino-cyclopentane-1-carboxylic
Acid Hydrochloride.
O O
CH30-l~ HO~~
Ns~,. ,~~~I~OH N3~,. .,, ~~OCH3
BocHN O BocHN rO
11A. (t)-y1S 2S 3R 4R)-2-(t-Butyloxycarbonylamino)-3-(azidomethyl)-4-meth-
oxycarbonyl-cyclopentane-1-carboxylic Acid and (t)-(1 S 2S.3R.4R)-2-(t-butyl-
oxycarbonylamino)-3-~azidomethyl)-1-methox~carbonyl-cyclopentane-4-carbox-
ylic Acid.
The title compound was prepared, in two steps, first following the
procedure described in Example 4G and 4H substituting (t)-(1S,2S,3R,4R)-2-(i-
butyloxycarbonylamino)-3-(azidomethyl)-cyclopentane-1,4-dicarboxylic acid
dimethyl ester ( 98 mg, 0.28 mmole) in place of (t)-(1 S,2S,3R,4R)-2-(t-
butyloxycarbonylamino)-3-(acetamidomethyl)-cyclopentane-1,4-dicarboxylic acid
dimethyl ester (yield: 76 mg, 79 %).
MS: '(M+H)+ = 343
O
CbzHN, H3C0-~~
N3 ,"~~I~OCHg N3 "'~NHCbZ
BocHN O BocHN
11 B. StL(1 S 2S 3S 4R)-2-(f-Butyloxycarbonylamino)-3-(azidomethyl)-4-benzyl-
oxycarbonylamino-cyclo~entane-1-carboxylic Acid Methyl Ester and (t)-(1 R.2R.-
-110-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
3S 4S) 3 t ButYloxycarbon la~mino)-2-(azidomethyl)-4-benzvloxycarbonylamino-
cyclopentane-1-carboxylic Acid Methyl Ester.
A solution of (250 mg,0.73 mmole) of the above mixture of regioisomers of
methyl ester azide cycfopentanes, prepared in Example 11A, was treated with
diphenylphosphorylazide, in 8 ml of toluene, Et3N, ( 0.2 mL) and 0.75 mL of
benzyl alcohol, under a nitrogen atmosphere, and heated to 80 °C for 3
hours.
The reaction mixture was diluted with ethyl acetate and washed 3 times with 5%
HC1. The organic layer was dried over Na2SOa, filtered and concentrating in
vacuo to provide a pale yellow oil. Purification using flash chromatography on
silica gel eluting with 20% ethyl acetate/hexanes provided the title compounds
(mixture of regioisomers) as a white solid (yield: 278 mg, 85%)
MS: (M+H)+ = 448.
CbzHN,
AcHN~,, ",~~~~OCH3
BocHN O
11 C. ft)~1 S 2S 3S 4R)-2-ff But~oxycarbonylaminoZ 3-(acetamido)methyl-4-
benzyloxycarbonylamino-cyclo~pentane-1-carboxylic Acid Methyl Ester.
A solution of the methyl ester diastereomers (278 mg, 0.62 mmoie),
prepared in Example 11 B, in 0.2 ml of thiolacetic acid was stirred under a
nitrogen atmosphere at room temperature for 6 hours. The reaction mixture was
concentrated in vacuo to provide a yellow oil. The crude oil was purified
using
flash chromatography, eluting with 50% ethyl acetatelhexanes to provide the
title
compound as a white solid. (yield: 70 mg, 24%)
MS: (M+H)+ = 464.
-111-
CA 02329660 2000-10-20
WO 99/54290 PCTlUS99/07949
CbzHN,
AcHN~,, O
BocHN O
11 D. ~t}-(1 S 2S 3S 4R)-2-~t Butylox~rcarbonylamino)-3-(acetamido)methyl-4-
benzyloxycarbonylamino-cyclopentane-1-carboxylic Acid.
A solution of (t)-(1S,2S,3S,4R)-2-(t-butyloxycarbonylamino)-3-
(acetamido)methyl-4-benzyloxycarbonylamino-cyclopentane-1-carboxylic acid
methyl ester (70 mg, 0.15 mmole) in 1 mL of methanoI/H20 (3:1 ) was treated
with
(4 mg, 0.15 mmole) of LiOH. After stirring at room temperature for 2 hours the
reaction mixture was quenched with 5% HCI and extracted 3 times with ethyl
acetate. Standard workup, as described above, provided the title compound as a
white solid (yield: 51 mg, 76%).
MS: (M+H)+ = 450.
H2N,
AcHN OH
NH ,/'G 2 HC~
2
11 E. (rt)-(1 S 2S 3S 4R~-3-Acetamidomethyl-2 4-diamino-cyclopentane-1-car-
boxylic Acid Dihrdrochloride
A solution of (t)-(1 S,2S,3S,4R)-2-(t-butyloxycarbonylamino)-3-
(acetamido)methyl-4-benzyloxycarbonylaminocyclopentane-1-carboxylic acid (51
mg, 0.11 mmole) in 0.2 ml of isopropyl alcohol and Pd/C was reacted under 1
atmosphere of hydrogen for 18 hours. The reaction mixture was filtered through
Celite° and concentrated in vacuo to provide the title compound as a
tan foam.
(yield: 30 mg, 86%)
-112-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
MS: (M+H)+ = 216.
Example 12
(t)-(1 R.2R,4R,1'S~-4-(1'-Acetamido-3'-ethy~pentyl-3-methoxycarbonyl-
cKclopentane-1-carboxylic Acid.
12A. {t~exo,1'RS~and (endo.1'RS)-2-(~3-eth~rl-1-
hydroxy~pentylbicyclo[2.2.1 jhept-5-ene.
1-bromo-2-ethylbutane (12.5 mL) and a catalytic amount of 1,2-
dibromoethane were added to a suspension of 2.1 g Mg turnings in 200 mL of
tetrahydrofuran. This mixture was heated to 50 °C for 2 hours, and
cooled to -78
°C. A solution of 5.4 mL (t)-endo-2-formylbicyclo[2.2.1 jhept-5-ene, in
75 mL
tetrahydrofuran, was added dropwise to the Grignard solution. The mixture was
warmed to 0 °C and stirred for 1 hour. The reaction was quenched by
addition of
20 mL saturated ammonium chloride and ethyl acetate. The organic layer was
separated, washed with brine, dried over magnesium sulfate, filtered and the
solvent is evaporated. The crude material was purified by flash chromatography
using 10% ethyl acetate:hexanes (1:9) to provide the title compounds. (yield:
4.9
g, 53%)
'H NMR (CDC13) 8 0.78-0.91 (m, 6H), 1.15-1.50 (m, 11H ), 1.70-2.10 (m,
1 H), 2.60-2.95 {m, 2H), 2.95-3.55 (m, 1 H),6.0-6.2(m,2H),
MS: (M+H)~' = 208.
-113-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
12B. (t)-exo and endo-2-(1'-oxo-3'-ethyl)pentyl-bic~rcloL2.2.1]hept-5-ene.
A solution of of oxalyl chloride, (2.3 mL) in 100 mL dichloromethane,
maintained at -78 °C, was treated dropwise with dimethyl sulfoxide,
(4.0 mL).
The mixture was stirred under nitrogen for 20 minutes and a solution of (t)-
(2S,1'R) and (2S,1'S)-2-(1'-hydroxy-3'ethyl)pentyl-bicyclo[2. 2.1]hept-5-ene
(4.9 g)
in 50 mL of dichloromethane was added. The mixture was stirred at -78
°C for
0.5 hours warmed to 0 °C for 15 minutes, treated with 16.4 mL
triethylamine at 0
°C and stirred for 10 minutes. Water (100 mL), at 25 °C, was
added over 10
minutes. The organic layer was separated, washed with brine, dried over
MgS04, filtered and the solvent evaporated. The crude ketone mixture was
purified by flash chromatography using chloroform:hexanes (1:3) to provide the
products (yield: 3.72 g, 77%}.
The ketone mixture prepared above (3.1 g, 14.9 mmole) was dissolved in
50 mL of methanol and combined with 22 mL of 1 M sodium methoxide. This
mixture is heated to 70 °C for 18 hours. The solvent was evaporated and
the
residue dissolved in 300 mL ethyl acetate. The organic layer was washed with
100 mL of 0.5M HC1, brine, dried over magnesium sulfate, filtered, and the
solvent is evaporated. The ketones were separated by flash chromatography
using chloroform:hexanes (1:3) to provide title compound (t) (2R)-2-(3-ethyl-1-
oxo)pentyl-bicyclo[2.2.1 Jhept-5-ene (yield: 0.99 g, exo ketone) (higher Rf)
and (t)
(2S)-2-(3-ethyl-1-oxo)pentyl-bicyclo[2.2.1]hept-5-ene (yield: 1.7 g, endo
ketone,
87%).
-114-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
~ H NMR (CDC13) 8 0.85 (2t, 6H), 1.19-1.42 (m, 7H ), 1.70-1.92 (m, 2H),
2.32-2.39 (m, 1 H), 2.39-2.47 (2d, 2H), 2.88-2.98 (d,2H), 6.10-6.17 (m, 2H).
12C. (t)-f2R)-2-(1-Hydroxyimino-3-ether pentyl-bicyclo(2.2.11heat-5-ene.
A solution of (t) (2R)-2-(1'-oxo-3'ethyl)pentyl-bicyclo[2.2.1]hept-5-ene (1.1
g, 5.4 mmole) in 45 mL methanol was reacted with hydroxylamine hydrochloride
(1.5 g, 21.6 mmole) and 1 N NaOH (16.3 mL). This mixture was heated at 40
°C
for 2 days. The solvent was evaporated and the residue was partitioned between
ethyl acetate and brine. The organic layer was dried over MgS04, filtered, and
the solvent evaporated to provide the title compound (yield: 1.16 g, 97%).
~ H NMR (CDC13) 8 0.87 (2t, 6H), 1.24-1.40 (m, 8H ), 1.50 (d, 1 H), 1.65 (m,
1 H), 1.78 (m, 1 H), 2.15 (m,1 H), 2.35 (m, 2H), 2.90 (m,2H), 6.13 (m, 2H),
2.87(m,
1 H), 3.68 (s,3H), 3.85 (m, 1 H )
MS: (M+H)+= 222
-115-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
HZN~,
H
12D. ~)-(2R 1'S)-2-(1-Amino-3-ethyl)pentyl-bicyclof2.2.11hept-5-ene.
The crude (t)-(2R)-2-(1-hydroxyimino-3-ethyl)pentylbicyclo[2.2.1]hept-5-
ene (0.49 g, 2.13 mmole), prepared in Example 12C, in 10 mL toluene, was
treated with 1 M lithium aluminum hydride bis(tetrahydrofuran) (4.3 mL). The
mixture was heated to 100 °C for 2 hours, under a nitrogen atmosphere.
The
mixture was cooled to 0 °C, and consecutively combined with water (0.16
mL),
15% NaOH (0.16 mL), and water (0.49 mL). The solids were filtered, and the
solution diluted with 150 mL ethyl acetate. The organic layer was washed with
water, and brine, dried over MgS04, filtered and the solvent evaporated. The
crude amines were separated by flash chromatography using
ether:methanol:ammonium hydroxide (98:2:0.2) to provide the title compound,
(t)-(2R,1'S)-2-(1-amino-3-ethyl)pentylbicyclo[2.2.1]hept-5-ene (yield: 79 mg),
and
the (t)-(2R,1'R)- isomer (77 mg) and 77 mg of a mixture of the amines overall
yield 52%.
AcHN,,,
H
12E. (t)-(2R 1'S)-2-(1-Acetamido-3-ethyl-)pentyl-bicYclo[2.2.1]hept-5-ene.
(t)-(2R,1'S)-2-(1'-amino-3'-ethyl-)pentyl-bicyclo[2.2.1]hept-5-ene (78 mg,
0.38 mmole) was dissolved in 3 mL dichloromethane . Triethylamine (0.16 mL)
-116-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99107949
and acetic anhydride (0.07 mL) were added to the mixture. After 1 hour at 25
°C
the solvent was evaporated and the residue purified by flash chromatography
using ethyl acetate:hexanes (1:4) to provide the title compound (yield: 78 mg,
83%).
~ H NMR (CDC13, 300 MHz) b 0.77-0.88 (m, 6H), 1.10-1.57 (m, 11 H ), 2.02
(s, 3H), 2.65 (s, 1 H), 2.84 (s, 1 H), 3.80-3.95 (m,1 H), 5.0-5.15(m, 1 H),
6.05 (s,2H).
MS: (M+H)+= 250.
12F (t)-(1 R 3R 4R 1'S)-4-(1-Acetamido-3-eth r~l pent~cyclopentane-1,3-
Dicarboxylic Acid.
A solution of ruthenium tetroxide was prepared from 5.5 mg of ruthenium
dioxide, suspended in carbon tetrachloride:acetonitriie (2:1 ), and 219 mg
sodium
periodate in 3 mL of water. The mixture was stirred 15 minutes at 0 °C.
A
solution of (t)-(2R,1'S)-2-(1'-acetamido-3'-ethyl)pentylbicyclo[2.2.1]hept-5-
ene
(64 mg, 0.256 mmol) in 1 mL of carbon tetrachloride was added to the ruthenium
mature. The reaction mixture was allowed to warm to 25 °C and stirred
for 3
hours. 1 M Sodium bicarbonate (2 mL) was added and the aqueous layer was
separated and acidified with 5 mL 1 M HCI and extracted with ethyl acetate.
The
organic layer was filtered through Celite~ and the solvents were evaporated in
vacuo. The crude mixture was purified by flash chromatography using
-117-
CA 02329660 2000-10-20
WO 99!54290 PC'T/US99/07949
methanol:dichloromethane :acetic acid (3:20:0.5) to provide the title compound
(yield: 50 mg, 62%).
~ H NMR (CD30D) s 0.78-0.92 (2t, 6H), 1.14-1.47 (m, 7H ), 1.62-1.75 (m,
1 H), 1.92 (s, 3H), 1.95-2.03 {m, 1 H), 2.08-2.20 (m,1 H), 2.22-2.33 (m, 1 H),
2.35-
2.49 (m,1 H), 2.58-2.68 (m, 1 H), 2.82-2.94 (m, 1 H), 3.83-3.95 (m,1 H),.
MS: (M+H)+= 314
0
CH30-f~
AcHN~,, .,,~~~OH
H O
12G. (t) (1R 3R 4R 1'S)-4-(1'-Acetamido-3'-ethyl)pentyl-3-methoovcarbonyl-
~clopentane-1-carboxylic Acid.
(t)-(1 R,3R,4R,1'S)-2-(1-Acetamido-3-ethyl}pentylcyclopentane-1,3-
dicarboxylic acid was reacted with acetic anhydride (0.1 mL), suspended in 3
mL
of chloroform, in a sealed tube, and heated to 70 °C for 3 hours. The
solvents
were evaporated and the residue was added to 1 mL of methanol and 0.1 mL of
triethylamine, and heated for 1 hour, at 70 °C. The solvents were
evaporated and
the crude acidlester purified by flash chromatography using ethyl
acetate:methanol:acetic acid (97:2:1) to provide of the mixture of acidlesters
(yield: 30 mg, 57%). The two isomers were separated by preparative thin layer
chromatography using ethyl acetate:methanol:acetic acid (97:2:1) to provide
the
title compound (yield: 6.9mg, 13%).
-118-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
~H NMR (CD30D, 300 MHz) 8 0.83 (2t, 6H), 1.14-1.48 (m, 8H ), 1.67 (m,
1 H), 1.91 (s, 3H), 1.98 (m, 1 H), 2.11 (m,1 H), 2.25 (m, 1 H}, 2.42 {m,1 H),
2.62 (m,
1 H), 2.92 (m, 1 H), 3.68 (s,3H), 3.89 (m, 1 H )
MS: (M+H}+= 328
Example 13
~1 R 3Rj4R 1'S)-3-Hydroxymetf~rl-4~1'-acetamido-3'-ethyl)pentyl-c~clopentane-1-
carboxylic Acid.
0 0
CH30-l~ CH30
O - O
hi ~ H
O O
13A. (t)-1 R 2R 4'S) and (t)-(1 S 2S 4'S)-2~2 2-dimethKl-1,3-dioxolan-4-yl)-4-
methylenecyclopentane-1-carboxylic Acid Methyl Ester.
A solution of 2-[(trimethylsilyl)methyl]-2-propen-1-yl-acetate (5 g, 26.9
mmole), methyl (S}-(+}-3-(2,2-dimethyl-1,3-dioxolan-4-yl)-trans-2-propenoate
(5
g, 26.9 mmole), Pd(OAc)2 {0.42 g, 1.8 mmole) and (i-Pr0)3P (2 mL, 8.1 mmole)
in
27 mL of toluene was heated under an argon atmosphere for 24 hours. The
reaction was cooled and concentrated in vacuo. The crude product was
chromatographed on silica gel with 5-15% ethyl acetate in hexanes to provide
the
title compound (yield: 4.68 g, 73%}.
'H NMR {CDC13) 84.88 (m, 2H), 4.18-3.95 (m, 2H), 3.70 (s), 3.69 (s) [3H
overall], 3.68-3.55 (m, 1 H), 2.78-2.29 (m, 6H), 1.40 (s), 1.38 (s) [3H,
overall], 1.34
(s), 1.32 {s) [3H, overall].
-119-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
MS: (M+H)*=241.
OH
O H
13B. (1R 2R 4'S~-2-Hydroxymethyl-1-(2 2-dimethyl-1 3-dioxolan-4-yl)-4-
methvlene-c~ciopentane
Lithium aluminum hydride (0.63g, 16.6 mmole) was added to a solution of
(1 R,2R,4'S) and (1 R,2S,4'S)-methyl 2-(2,2-dimethyl-1,3-dioxolan-4-yl)-4-
methylene-cyclopentane-1-carboxylate (2.Og, 8.33 mmole) in 40 mL of THF
maintained at -78 °C. The reaction was allowed to warm to 0 °C
and stirred for 1
hour. The reaction was quenched (at 0 °C) by adding sequentially H20
(1.9 mL),
10% NaOH (2.8 mL), and H20 (2.8 mL). The reaction was allowed to warm to
room temperature then dried over MgS04. The reaction was filtered and the
solids were washed with ethyl acetate (200 mL). The filtrate was concentrated
in
vacuo to provide 1.83 g of the crude product mixture. Preparative HPLC on
silica
gel with (0-75%) ethyl acetate in hexanes provided the title compound (yield;
1.15 g, 65%).
' H NMR (CDC13) 84.84 (m, 2H), 4.21 (m, 1 H), 4.03 (dd, J=6, 8 Hz, 1 H),
3.64 (t, J=8 Hz, 1 H), 3.56 (m, 2H), 2.60-2.05 (m, 6H), 1.44 (s, 3H), 1.36 (s,
3H).
MS: (M+H)*=213.
-120-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
OTBDPS
0 H
13C. (1R 2R 4'S)-2-(t-ButXldiphenylsilyloxymethy!)-1-(2 2-dimethyl-1,3-
dioxolan-
4-yl)-4-methylenecyclopentane
A solution of (1 R,2R,4'S)-2-hydroxymethyl-1-(2,2-dimethyl-1,3-dioxolan-4-
yl)-4-methylenecyclopentane (1.158, 5.4 mmole), t-butyldiphenylsilyl chloride
(1.6
mL, 6.8 mmole) and imidazole (1.11g, 16.3 mmole) in 30 mL of dichlorornethane
was stirred at room temperature for 1.5 hours. The reaction mixture was
quenched with methanol (.2 mL) and stirred for 1 hour. The mixture was
partitioned between ethyl acetate and 10% citric acid. The organic layer was
washed with water and saturated NaHC03, dried over MgS04, filtered and
concentrated to provide the title compound.
' H NMR (CDC13) 67.65 (m, 4H), 7.39 (m, 6H), 4.82 (m, 2H), 4.00 (m, 1 H),
3.89 (dd, J=6, 8 Hz, 1 H), 3.62-3.48 (m, 3H), 2.51-1.98 (m, 6H), 1.37 (s, 3H),
1.31
(s, 3H), 1.05 (s, 9H).
MS: (M+H)+= 451.
-121-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
OTBDPS
HO
H
HO
13D. (1 R 2R 1'S~ 2-(t-Butyldiphen~silylox~meth~Z 1-(1 2-dihydroxy)ethyl-4-
methylene-cyclopentane
(1 R,2R,4'S)-2-(t-Butyldiphenylsilyioxymethyl)-1-(2,2-dimethyl-1,3-dioxolan-
4-yl)-4-methylene-cyclopentane and pyridinium p-toluenesulfonate (0.68g, 2.7
mmole) in 110 mL of methanol were heated to 45 °C for 16 hours. The
reaction
was cooled, concentrated in vacuo and partitioned between dichloromethane and
water. The organic layer was washed with brine, dried over MgS04, filtered and
concentrated. The crude product was chromatographed on silica gel with 15%
ethyl acetate in hexanes to provide the title compound and recovered starting
material (yield: 1.2 g, 54%).
'H NMR (CDC13) 87.65 (m, 4H), 7.39 (m, 6H), 4.81 (m, 2H), 3.75-3.46 (m,
5H), 2.54-1.89 (m, 6H), 1.06 (s, 9H).
MS: (M+H)+= 411.
OTBDPS
O_~.
H H
-122-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
13E. ~1 R 2R) 2 (t-Butyldi~henylsi~lox rLmethyl)-1-formyl-4-methylene-
cyclopentane
A solution of (1R,2R,1'S)-2-(t-butyldiphenylsilyloxymethyl)-1-(1,2-
dihydroxy)ethyl-4-methylene-cyclopentane (1.2 g, 2.92 mmole), Na104 (2.5 g,
11.6 mmole) in ethanol {4 mL) and water (4 mL) was stirred at 0 °C for
4 hours.
The reaction was diluted with ethyl ether (90 mL) and filtered through a
celite pad.
The filtrate was concentrated in vacuo. The crude product was dissolved in
toluene and concentrated in vacuo to azeotropically remove water. The reaction
was redissolved in ethyl ether and filtered again followed by concentration to
provide the title compound as a crude oil.
'H NMR (CDC13) 89.68 (d, J=3 Hz, 1H), 7.65 (m, 4H), 7.39 {m, 6H), 4.87
(m, 2H), 3.68 (dd, J=5, 10 Hz, 1 H), 3.56 {dd, J=7, 10 Hz, 1 H), 2.83-2.09 (m,
6H),
1.04 (s, 9H).
MS: (M+H)+= 379.
13F. {1 R 2R 1'R) 2 (t Butyldit~henylsi~loxYmethyl)-1-(3-ethyl-1-
hydroxylpentyl-4-
methylene-cyclopentane
A 0.9 M solution of 2-ethylbutyl magnesium bromide (15.3 mL), prepared
from 1-bromo-2-ethylbutane (3.8 mL, 27.1 mmole), magnesium (1 g, 41.6 mmole)
-123-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
and iodine (170 mg) in ethyl ether (30 mL) and stirred overnight at room
temperature, was added to a solution of (1 R,2R)-2-(t-butyldiphenylsilyloxy-
methyl)-1-formyl-4-methylene-cyclopentane (2.92 mmole) in ethyl ether (15 mL)
at 0 °C for 20 minutes. The reaction was quenched with saturated
ammonium
chloride and stirred for 30 minutes. The reaction was partitioned between
ethyl
acetate and water. The organic layer was washed with brine, dried over MgSOa,
filtrered and concentrated. The crude product was purified by preparative HPLC
on silica gel with (5-50%) ethyl acetate in hexanes to provide the title
compound
(yield: 0.7 g, 51 %).
' H NMR (CDC13) 87.65 (m, 4H), 7.39 (m, 6H), 4.80 (m, 2H), 3.79 (m, 1 H),
3.64 (dd, J=5, 10 Hz, 1 H), 3.56 (dd, J=7, 10 Hz, 1 H), 2.46-1.9 (m, 6H), 1.5-
1.21
(m, 7H), 1.05 (s, 9H), 0.84 (t, J=8 Hz, 3H), 0.83 (t, J=8 Hz, 3H).
MS: {M+H)+= 465.
OTBDPS
13G. {1 R 2R 1'S~-1-jt-Butyldi~,henylsilyloxymethyl)-2-(3-ethyl-1-azido)pentyl-
4-
methylene-cyclopentane
A solution of (1R,2R,1'R)-2-(t-butyldiphenylsiiyloxymethyl)-1-(3-ethyl-1-
hydroxy)pentyl-4-methylene-cyclopentane (0.7g, 1.51 mmole), methanesulfonyl
chloride (.25 mL, 3.23 mmole) and triethylamine {1 mL) in dichloromethane (15
mL) was stirred for 0.5 hours, at 0 °C. The reaction was quenched with
saturated
NaHC03 solution (3 mL) and then partitioned between ethyl acetate and 10%
-124-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
citric acid. The organic layer was washed with saturated NaHC03 and brine,
dried over MgS04, filtered and concentrated in vacuo to provide the
intermediate
mesylate. A solution of the crude mesylate and sodium azide (1g, 15.3 mmole)
in dimethylformamide (15 mL) was reacted at 65 °C for 16 hours. The
reaction
was cooled and diluted with ethyl acetate. The organic layer was washed with
water and brine, dried over MgS04, filtered and concentrated to provide the
title
compound.
'H NMR (CDC13) 87.66 (m, 4H), 7.39 (m, 6H), 4.81 (m, 2H), 3.64 (dd, J=6,
Hz, 1 H), 3.52 (dd, J=7, 10 Hz, 1 H), 3.22 (m, 1 H), 2.58-2.0 (m, 6H), 1.48-
1.11
(m, 7H), 1.05 (s, 9H), 0.86 (t, J=7 Hz, 3H), 0.80 (t, J=7 Hz, 3H) .
OTBDPS
AcH N.
w
U W
H
13H. (1R 2R 1'S)-1-(t-Butyldiphenylsilyloxymethyl)-2-(1'-acetamido-3'-
ethy~pentyl-4-methylene-c~clopentane
A solution of the crude (1R,2R,1'S)-1-(t-butyldiphenylsilyloxymethyl)-2-(3-
ethyl-1-azido)pentyl-4-methylene-cyclopentane (~1.5 mmole), prepared in
Example 13G, and triphenylphosphine (1.39g, 5.3 mmole) in 20% H20 in THF (25
mL) was stirred at 75 °C for 16 hours. The reaction was cooled and
concentrated
in vacuo to provide the crude amine. The amine, acetic anhydride (.25 mL) and
pyridine (.55 mL) in dichloromethane (13 mL) were stirred at room temperature
for 2 hours. The reaction was quenched with methanol (2 mL) and stirred for an
additional 1 hour. The reaction was diluted with ethyl acetate and washed
sequentially with 10% citric acid, saturated NaHC03, and brine, dried over
-125-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
MgS04, filtered and concentrated. Chromatography on silica gel with 2.5 -10%-
ethyl acetate in dichloromethane provided the title compound (yield: 0.404 g,
60%).
'H NMR (CDC13) 57.65 (m, 4H), 7.39 (m, 6H), 4.93 (d, J=10 Hz, 1H), 4.77
(m, 2H), 3.92 (m, 1 H), 3.60 (dd, J=6, 10 Hz, 1 H), 3.52 (dd, J=6, 10 Hz, 1
H}, 2.54-
1.83 (m, 6H), 1.71 (s, 3H), 1.40-1.10 (m, 7H), 1.07 (s, 9H), 0.83 (t, J=7 Hz,
3H),
0.77 (t, J=7 Hz, 3H).
MS: (M+H)+= 506.
DPS
AcH N,
.~~~I
OH
131. ~1 R 2R 4R 1'S)-1-(t-But~diphenylsilyloxymethyl)-2-(1'-acetamido-3'-
ethyl)
pentXl-4.-hydroxymethyl-cyclopentane
A solution of (1R,2R,1'S)-1-(t-butyldiphenylsilyloxymethyl)-2-(1'-acetamido-
3'-ethyl)pentyl-4-methylene-cyclopentane (50 mg, 0.1 mmole) and 2M borane
dimethylsulfide complex, in THF ( 75 uL, 0.15 mmole) in THF (1 mL) was stirred
at 0 °C for 7 hours. Water (0.1 mL) and 1 N NaOH (0.2 mL) were added
and the
reaction allowed to warm to room temperature. After 15 minutes, 30% hydrogen
peroxide (2 mL) was added and the reaction was stirred for an additional 0.5
hour. The reaction was diluted with ethyl acetate washed with water (2X) and
with brine. The organic layer was dried over MgS04, filtered and concentrated
in
vacuo. The crude product was purified by chromatography using preparative
-126-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99107949
HPLC in silica gel using 0-15% ethyl acetate in dichloromethane to provide the
title compound (yield: 22 mg, 42%).
'H NMR (CDC13) 87.65 (m, 4H), 7.43 (m, 6H), (m, H), 5.00 (d, J=10 Hz,
1 H), 3.84 (m, 1 H), 3.66-3.54 (m, 2H), 3.52 (d, J=7 Hz, 2H), 2.20-1.04 (m,
14H),
1.66 (s, 3H), 1.08 (s, 9H), 0.83 (t, J=7 Hz, 3H), 0.78 (t, J=7 Hz, 3H).
MS: (M+H)+= 524.
AcHN,
_ .,,~('O
H OH
13J. (1 Ri3R 4R 1'S), 3-(t-ButyldiphenylsilyloxYmethyl)-4-(1'-acetamido-3'-
eth r~l pentyl-cyclopentane-1-carboxylic Acid
A solution of (1R,2R,4R,1'S) 1-(t-butyldiphenylsilyloxymethyl)-2-(1'-
acetamido-3'-ethyl) pentyl-4-hydroxymethyl-cyclopentane (22 mg, 0.042 mmole)
and pyridinium dichromate (100mg, 0.26 mmole) in N,N-dimethylformamide (0.75
mL) was stirred at room temperature for 48 hours. The reaction was partitioned
between ethyl acetate and 10% citric acid. The organic layer was separated and
washed with brine, dried over MgSOa, filtered and concentrated to provide the
title compound, which was used without further purification.
-127-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
OH
AcH N,
_ ..,,.1 0
H v OH
13K. (1 R 3R 4R 1'S) 3-Hydroxymethyl~-(1'-acetamido-3'-ethyl) oentyl-
~clopentane-1-carboxylic Acid
A solution of (1R,3R,4R,1'S) 3-(t-butyl-diphenylsilyloxymethyl)-4-(1'-
acetamido-3'-ethyl) pentyl-cyclopentane-1-carboxylic acid from 1 L and 1 M
tetrabutylammonium fluoride in THF (0.8 mL) in THF (2 mL) was stirred for 16
hours at room temperature. The reaction was partitioned between ethyl acetate
and 10% citric acid. The organic layer was separated and washed with brine,
dried over MgS04, filtered and concentrated in vacuo. The crude product was
chromatographed using a C-18 reverse phase column with 10-25% acetonitrile in
water to provide the title compound (yield: 6.6 mg, 52 %).
' H NMR (CD30D) 87.81 (d, J=10 Hz, 1 H), 3.88 (m, 1 H), 3.61 (dd, J=5, 11
Hz, 1 H), 3.38 (dd, J=8, 11 Hz, 1 H), 2.75 (m, 1 H), 2.19-1.00 (m, 13H), 1.95
(s,
3H), 0.87 (t, J=7 Hz, 3H), 0.83 (t, J=7 Hz, 3H).
MS. (M+H)+= 300.
-128-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 14
H
AcH N,
OH
(1R 3R 4R 1'S) 3-Formyl-4~1'-acetamido-3'-ethvl)pentyl-cyclopentane-1-
carbolic Acid.
A solution of (1R,3R,4R,1'S)-3-hydroxymethyl-2-(1'-acetamido-3'-ethyl)-
pentyl-cyclopentane-1-carboxylic acid (34 mg, 0.11 mmole) and pyridinium
dichromate (40 mg, 0.1 mmoie) in dichloromethane (1.5 mL) was stirred at room
temperature for 0.5 hours. The reaction was partitioned between ethyl acetate
and 10% citric acid. The organic layer was separated and washed with brine,
dried over MgS(~4, filtered and concentrated. The crude product was
chromatographed on a C18 column with 10-50% acetonitrile in water to provide
the title compound.
-129-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 15
~1 R 3R 4R 1'S) 3 (Imidazol 2 ~)-4 (1-acetamido-3-ethyl)pentyl-cvclopentane-1-
carboxylic Acid
OTBDPS
..
AcHN,
OTrityl
15A. (1 R 2R 4R 1'S) 1 (t Butyldiphenylsilyloxymethyl)-2-(1'-acetamido-3'-
ethy~pentYl-4-triphenylmethyloxymethyl-cyclopentane
A solution of (1 R,2R,4R,1'S) 1-(t-butyldiphenylsilyloxymethyl)-2-(1-
acetamido-3-ethyl)pentyl-4-hydroxymethylcyclopentane (0.357 g, 0.682 mmol) in
pyridine (0.165 mL, 2.05 mmol) and dichloromethane (2 mL) was reacted with
trityl chloride (0.248 g, 0.89 mmol) and DMAP (16.3 mg, 0.13 mmol) at room
temperature for 16 hours. The reaction was partitioned between ethyl acetate
and
10% citric acid. The organic layer was separated and washed with brine, dried
over MgS04, filtered and concentrated in vacuo. The residue was purified by
chromatography on silica gel using 2% methanoildichloromethane to provide the
title compound (yield: 0.5 g, 95%).
' H NMR (CDC13) s 7.62 (m, 4H), 7.4 (m, 12H), 7.23 (m, 9H), 3.57 (s, 2H),
2.96 (s, 2H), 2.26 (m, 1 H), 1.97 (m, 2H), 1.62 (m, 3H), 1.52 (s, 3H), 1.25-
1.5 (m,
7H), 1.06 (s, 9H), 0.83 (br s, 6H).
MS: (M+Na)+ = 788
-130-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
OH
AcH N,
OTrityl
15B. ;~R 2RL4R 1'S)-1-Hydroxymethyl-2-y1'-acetamido-3'-ethvllpentyl-4-
triphenylmethyloxy~meth~lc~clopentane
The title compound was prepared according to the method described in
Example 13K substituting (1R,2R,4R,1'S)-1-(t-butyldiphenylsilyloxymethyl)-2-
(1'-
acetamido-3'-ethyl)pentyl-4-(triphenylmethyloxy)methyl-cyclopentane for
(1R,3R,4R,1'S) 3-(t-butyldiphenylsilyloxymethyl)-4-(1'-acetamido-3'-ethyl)
pentyl-
cyclopentane-1-carboxylic acid (yield: 0.33 g, 98%).
'H NMR (CDC13) b 7.42 (m, 6H), 7.26 (m, 9H), 5.59 (d, 1 H), 3.86 (m, 1 H),
3.6 (2d, 1 H), 3.42 (2d, 1 H), 3.95 (2d, 2H), 2.25 (m, 1 H), 1.95 (s, 3H), 1.7-
2.02 (m,
2H), 1.5-1.7 (m, 4H), 1.1-1.5 (m, 9H), 0.83 (2t, 6H)
MS: (M-H)- = 526, (M+35)- = 562; (M+Na)+ = 550
-131-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O
H ~/
AcH N,
H .~~~~I
OTrityl
15C. (1 R 2R 4R 1'S)-1-Form-5-~1'-acetamido-3'-ethyl)pentyl-3-
(triphenylmethyloxy)methyl-c rLclopentane
The title compound was prepared according to the method described in
Example 13L substituting (1 R,2R,4R,1'S)-1-hydroxymethyl-2-(1'-acetamido-3'-
ethyl)pentyl-4-(triphenylmethyloxy)methyl-cyclopentane for (1 R,3R,4R,1'S)-3-
hydroxymethyl-4-(1'-acetamido-3'-ethyl) pentyl-cyclopentane-1-carboxylic acid
(yield: 0.3 g, 91 %).
H NMR (CDCI~) b 9.56 (d, 1 H), 7.42 (m, 6H), 7.27 (m, 9H), 5.13 (d, 1 H),
3.86 (m, 1 H), 2.94 (m, 2H), 2.72 (m, 1 H), 2.42 (m, 1 H), 2.16 (m, 2H), 1.90
(s, 3H),
1.1-1.7 (m, 10H), 0.83 (2t, 6H)
MS: (M-H)- = 524
~N
HN
AcHN,
~~~~~I
OTrityl
15D. (1 R 3R 4R 1'S)-3-(Imidazol-2-yl)-4-(1-acetarnido-3-ethyl)pentyl-1-
(triphen I~e#h)rloxy)methyl-cyclopentane
( 1 R,3R,4R,1'S)-1-Formyl-2-(1'-acetamido-3'-ethyl)pentyl-4-
(triphenylmethyloxy)methyl-cyclopentane (80 mg, 0.15 mmol) was reacted with
ammonia and 40% glyoxal (0.264mL, 7.5 mmol) in methanol (7 mL) according the
-132-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
procedure of Rothenberg, A. and coworkers in Angew. Chem. Int. Ed. 1983, 22,
p. 560 to provide the title compound (yield: 60 mg, 70%).
' H NMR (CDC13) 8 7.7 (d, 1 H), 7.42 (m, 7H), 7.27 (m, 9H), 5.72 (d, 1 H),
3.86 {m, 1 H), 3.2 (m, 2H), 3.08 (m, 2H), 2.54 (m, 1 H), 2.42 (m, 2H), 1.77
(s, 3H),
1.1-1.7 (m, 10H), 0.78 (2t, 6H).
MS: (M-H)- = 562, (M+35)- = 598; {M+H)+ = 564, (M+Na)+ = 586
~N
HN~,
AcHN,
.~~~I
OH
15E. ~1 R 3R 4R 1'S}-3-~Imidazol-2-yl~-4-(1'-acetamido-3'-ethyt)pentyl-1-
hydroxymeth~-c~clo~entane
(1 R,3R,4R,1'S)-3-(Imidazol-2-yl)-4-(1'-acetamido-3-ethyl)pentyl-1-
(triphenylmethyloxy)methyl-cyclopentane (60 mg, 0.106 mmol) was reacted with
p-toiuenesulfonic acid monohydrate (61 mg, 0.32 mmol) in MeOH (1 mL) for 1
hour. The reaction was quenched with water (10 mL) and diluted with ethyl
acetate (25 mL). The organic layer was washed with water, and brine, dried
over
MgS04, filtered and concentrated in vacuo. The residue was purified by
chromatography on silica gel using 10% methanol/dichloromethane to provide the
title compound (yield: 30 mg, 88%).
' H NMR (CDC13) 8 7.83 (d, 1 H), 7.2 (d, 1 H), 6.47 (d, 1 H), 3.86 (m, 1 H),
3.6
(m, 2H), 3.2 (m, 1 H), 2.61 (m, 1 H), 2.32 (m, 2H), 1.82 {m, 1 H), 1.72 (s,
3H), 1.62
(m, 2H), 1.1-1.4 (m, 7H), 0.78 (2t, 6H)
MS: (M-H)- = 320, (M+35)- = 356; (M+H)+ = 322, (M+Na)+ = 344
-133-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
N
~J..
AcHNH
_ ..,,~~0
H v OH
15F. {1 R 3R 4R 1'S)-3-(Imidazol-2-yl)-4-(1-acetamido-3-ethyl)pentyl-
cyclopentane-1-carboxylic Acid
The title compound was prepared according to the procedure described
ing Example 13J substituting (1R,3R,4R,1'S)-3-(imidazol-2-yl)-4-(1-acetamido-3-
ethyl)pentyl-1-hydroxymethylcyclopentane for (1 R,2R,4R,1'S) 1-(t-
butyldiphenylsilyloxymethyl)-2-(1'-acetamido-3'-ethyl) pentyl-4-hydroxymethyl-
cyclopentane (yield: 5.2 mg, 17%).
H NMR (dg-MeOH) 8 7.12 (br s, 2H), 3.92 (m, 1 H), 2.95 (m, 1 H), 2.42 {m,
1 H), 2.22 (m, 1 H), 1.96 (m, 1 H), 1.82 (m, 1 H), 1.72 (s, 3H), 1.15-1.45 (m,
7H),
0.82 (2t, 6H)
MS: (M-H)- = 334, (M+35)- = 370; (M+H)+ = 33fi, (M+Na)+ = 358
Example 16
~1R 3R 4R 1'SL~oxazol-5-yl)-4-(1'-acetamido-3'-ethyl)pentyl-cvclopentane-1-
carboxylic acid
-134-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
N~O
U
AcH N,
_ ..,,.1 0
H v OCHPh2
16A (1 R 3R 4R 1'S)-Diphenylmethyl 3-(oxazol-5-YI)-4-(1'-acetamido-3'-ethyl)
pentylcyclopentane-1-carboxylate
(1R,3R,4R,1'S)- Diphenylmethyl 3-formyl-4-(1'-acetamido-3'-ethyl) pentyl-
cyclopentane-1-carboxylate is reacted with p-toluensulfonylmethylisocyanide
following the procedure of van Leusen and coworkers in Tetrahedron Letters,
2369 (1977) to provide the title compound.
NCO
.,
AcHN,
_ ..,,.1 0
H v OH
16B. (1 R.3R.4R 1'S)-3-(oxazol-5-yl)-4-(1'-acetamido-3'-ethyl) pentyl-
cyclopentane-1-carbox~c acid
(1 R,3R,4R,1'S)-diphenylmethyl 3-(oxazol-5-yt)-4-(1'-acetamido-3'-
ethyl)pentyl-cyclopentane-1-carboxylate is reacted with anhydrous
trifluoroacetic
acid in dichloromethane concentration in vacuo provides the title compound.
-135-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 17
~1R 3R 4R 1'S)-3-(N N-dimeth~carbamoyl~(1'-acetamido-3'-ethyl)pentyl-
~clopentane-1-carboxylic Acid
0
HO~~
AcH N,
H v OCHPh2
17A (1 R 3R 4R 1'S)-Di~henylmethyl 3-carboxy-4~1'-acetamido-3'-ethyl)pentyl-
c~rclo~~,entane-1-carboxylate
The title compound is prepared according to the procedure described in
Example 14J substituting (1R,3R,4R,1'S)-diphenylmethyl-3-hydroxymethyl-4-(1'-
acetamido-3'-ethyl) pentyl-cyclopentane-1-carboxylate for (1 R,2R,4R,1'S) 1-(t-
butyldiphenylsilyloxymethyl)-2-(1'-acetamido-3'-ethyl) pentyl-4-hydroxymethyl-
cyclopentane
H3C'~N~l/
AcHN,
. ,~iO
H ,OCHPh2
-136-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
17B (1 R 3Ri4R 1'S)-Diphenylmethyl 3-IN N-dimethylcarbamoyl)-4-(1'-
acetamido-3'-ethyl)pentyl-cyclopentane-1-carboxylate
(1R,3R,4R,1'S)- Diphenylmethyl 3-carboxy-4-(1'-acetamido-3'-ethyl)
pentyl-cyclopentane-1-carboxylate is reacted with isobutyl chloroformate in
the
presence of N-methylmorpholine at 0°C. The intermediate activated ester
is
reacted with N,N-dimethylamine to provide the title compound.
H3C,
H3C-N
AcHN,
_ .,,.~0
H OH
17B (1 R~3R 4R 1'S)-3-(N N-dimethxlcarbamoyl)-4-(1'-acetamido-3'-
ethyLpentyl-c rLclopentane-1-carboxylic Acid
The title compound is prepared according to the procedure described in
Example 16B substituting (1R,3R,4R,1'S)- diphenylmethyl-3-(N,N-
dimethylcarbamoyl)-4-(1'-acetamido-3'-ethyl) pentyl-cyciopentane-1-carboxylate
for (1R,3R,4R,1'S)-diphenylmethyl-3-(imidazol-2-yl)-4-(1'-acetamido-3'-ethyl)
pentyl-cyclopentane-1-carboxylate.
Example 18
(1 R 3R 4R 1'SZ-3-propionvl-4-(1'-acetamido-3'-ethyl)pentyl-cyclopentane-1
carboxylic Acid
-137-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
~OH
.,
AcH N,
_ ..,,.ISO
H v OCHPh2
18A (1 R 3R 4R 1'S 1 "S~, and (1 R 3R 4R 1'S 1 "R)-Diphenylmethyl 3-(1-
hydroxypropyl) 4-~1'-acetamido-3'-ethyl) pentyl-cyclopentane-1-carboxylate
(1R,3R,4R,1'S)- Diphenylmethyl 3-formyl-4-(1'-acetamido-3'-ethyl) pentyl-
cyclopentane-1-carboxylate is reacted with ethyl magnesium bromide in THF at
0°C. The reaction is quenched with aqueous ammonium chloride and
partitioned
between ethyl acetate and water. The organic layer is dried over MgS04,
filtered,
and concentrated in vacuo to provide the title compound.
~.!%
AcH N, O
_
H , OCHPhz
18B (1 R 3R 4R 1'S~ Diphen~methyl 3~ropionyl~1'-acetamido-3'-ethyl)
pentyl-cyclopentane-1-carboxylate
The title compound is prepared according to the procedure of Example
12B substituting (1R,3R,4R,1'S,1"S)- and (1R,3R,4R,1'S,1"R)-diphenylmethyl3-
(1-hydroxypropyl)-4-(1'-acetamido-3'-ethyl) pentyl-cyclopentane-1-carboxylate
for
(t)-{2S,1'R) and (2S,1'S)-2-(3'ethyl-1'-hydroxy)pentyl-bicyclo[2.2.1]hept-5-
ene.
-138-
CA 02329660 2000-10-20
WO 99/54290 PC'f/US99/07949
AcH N,
OH
18C ,~1 R 3R 4R 1'S)-3-propionyl-4-,(1'-acetamido-3'-ethYl)pentyl-cyclopentane-
1-carboxylic Acid
The title compound is prepared according to the procedure described in
Example 16B substituting (1R,3R,4R,1'S)-diphenylmethyl 3-propionyl-4-{1'-
acetamido-3'-ethyl) pentyl-cyclopentane-1-carboxylate for (1 R,3R,4R,1'S)-
diphenylmethyl 3-(imidazol-2-yl)-4-(1'-acetamido-3'-ethyl) pentyl-cyclopentane-
1-
carboxylate.
Example 19
(t)-(2R 3R 5R 1'S)-2-{1'-acetamido-3'-methyl)butyl-3-methoxycarbonyl-
tetrahydrofuran-5-carboxylic acid
~~'To
19~t1-Bicyclo(2.2.1 ]hept-5-en-2-one
In a three-necked flask equipped with a mechanical stirrer, a N2 inlet and
an additional funnel, a solution of oxalyl chloride (2.0 M in dichloromethane,
136
mL, 0.272 mole) in dichloromethane (250 mL) was cooled to -78 °C and a
solution of DMSO (40 mL) in 40 mL of dichloromethane was added dropwise over
30 min. After stirring for 5 additional minutes, a solution of 5-norbornen-2-
of (24
g, 0.218 mole) in 40 mL of dichloromethane was added dropwise. The solution
was stirred for another 10 minutes and triethylamine (150 mL) was added over
40
minutes. The mixture was then stirred for 10 minutes at -78 °C and
allowed to
-139-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
warm up to 0 °C over 1 hour. Water (250 mL) was added. Following the
separation of two layers, the organic layer was washed with 0.2 N HCI (4 x 200
mL) and brine (2 x 200 mL). After drying (MgS04), the solution was
concentrated
to about 80 mL. The residue was distilled with a 12" Vigreux column at reduced
pressure to give the title compound b.p. 100-105 °C /15 mmHg, (yield:
20.1 g,
86%).
' H NMR (CDC13): 1.85 (dd, 1 H), 1.90-2.00 (m, 2H), 3.00 (brs, 1 H), 3.20
(b.s, 1 H), 6.01 (t, 1 H), 6.58 (t, 1 H).
HC~~O
H ~O
19~t)-exo exo-5 6-DihydroxYbicyclo~2 .2.1]heptan-2-one
A solution of (t)-bicyclo[2.2.1]hept-5-en-2-one (10.8 g, 0.1 mole) and N-
methyl morpholine oxide (12.7 g, 0.12 mole) in 300 mL of 90% THF-water was
reacted with a solution of osrniurn tetroxide (2.5% wt in t-BuOH, 8.0 mL) for
5
hours at ambient temperature. The solvents were evaporated and the resulting
residue was dried in vacuo. The residue was then taken up in 100 mL of ethyl
acetate, dried (MgS04) and filtered. The filtrate was passed through a short
silica
gel plug, eluting with ethyl acetate. Concentration gave the title compound as
a
thick oil (14.5 g) which was used directly for the next step.
p
19C (t)-exo exo-5 6-Dihydroxyacetonide-bicyclo[2.2.11heptan-2-one
A solution of (t)-exo,exo-5,6-dihydroxybicyclo[2.2.1]heptan-2-one (14.5 g,
crude) in 250 mL of 2,2-dimethoxypropane was cooled to 0 °C and p-
toluensulfonic acid (125 mg) was added. The solution was stirred for 30
minutes
when TLC indicated complete reaction. The solution was loaded on an aluminum
-140-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
oxide (neutral) column and eluted with 15-30% ethyl acetate in hexane, to give
the title compound as a white solid (yield: 11.9 g, 65% for two steps).
MS (DCI-NH3): m/z 200 for (M+NH4), base peak.
1H NMR (CDC13): b 1.34 (s, 3H), 1.50 (s, 3H), 1.63-1.74 (m, 2H), 2.72-2.20
(m, 2H), 2.70-2.76 (m, 2H), 4.28 (d, 1 H), 4.34 (d, 1 H). '3C NMR (CDC13): 8
21.1,
25.3, 31.2, 39.4, 39.5, 55.3, 76.8, 81.2, 111.2, 214Ø Analysis: C~oH,403,
calc.
65.92% C, 7.75% H;: found, 66.01 % C, 7.79% H.
0
0
0
19D ~t~-exo exo-6 7-dihydroxyacetonide-2-oxabicyclof3.2.11octan-3-one
To a solution of (t)-exo,exo-5,6-dihydroxyacetonide-bicyclo[2.2.1]heptan-
2-one (14.76 g, 0.081 mole) in 500 mL of dichloromethane was added NaHC03
(13.6 g, 0.16 mole). The mixture was cooled with a water bath and MCPBA (30.3
g, ~60%) was added portionwise over 30 minutes. The solution was then stirred
for 2 hours at ambient temperature and washed with 10% aqueous NazS205 (500
mL), saturated NaHC03 solution (3 x 200 mL) and brine (3 x 200 mL). The
organic solution was then dried (MgS04}, filtered and concentrated. The
residue
was loaded on an aluminum oxide (neutral) column for 30 minutes, then eluted
with 10-25% ethyl acetate in hexane to give the title compound as a white
solid
(yield: 7.7 g, 50%)
MS (DCI-NH3): mlz 216 for (M+NH4)+, base peak.
'H NMR (CDC13): 8 1.30 (s, 3H), 1.45 (s, 3H), 1.84 (d, 1H), 2.10-2.20 (m;
1 H), 2.48-2.56 (m, 2H), 2.80 (dd, 1 H), 4.56 (d, 1 H), 4.60 (s, 1 H), 4.70
(d, 1 H). '3C
NMR (CDC13): 23.79, 25.58, 29.17, 35.97, 36.51, 80.70, 82.49, 82.55, 83.24,
-141-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
110.83, 167.95. Analysis: C~pH~4O4, cal. 60.59% C, 7.12% H; found, 60.52% C;
6.97% H.
O
O
O
19E t) exo exo-6 7-dihydroxyacetonide-4-exo-iodo-2-oxabicyclof3.2.11octan-3-
one
To a mixture of lithium bis(trimethylsilyl)amide (1.0 M in THF, 32.6 mL) and
distilled THF (60 mL), cooled to -78 °C, was added a solution of (t)-
exo,exo-6,7-
dihydroxyacetonide-2-oxabicyclo[3.2.1]octan-3-one (5.87 g, 29.6 mrnol) in 60
mL
of THF over 30 minutes. After stirring for another 30 minutes, the solution
was
then cannulated into a flask containing a solution of iodine (8.3 g, 32.6
mmol) in
60 mL of THF cooled to -78 °C over 30 minutes. The resulting solution
was
stirred for another 10 minutes and quenched with 300 mL of 5% aqueous citric
acid. The mixture was then extracted with ethyl acetate (3 x 100 mL). The
combined ethyl acetate solution was washed with 10% aqueous Na2S203 solution
(2 x 100 mL) and brine (2 x 200 mL), and dried (MgSOa). After filtration, the
solution was evaporated and the residue chromatograhed on a silica gel column
eluting with 15-25% ethyl acetate in hexane, to give a white crystalline solid
(yield:
7.55 g, 79%).
MS (DCI-NH3): m/z= 342 for (M+NH4)'', base peak.
'H NMR (CDC13): a 1.30 (s, 3H), 1.45 (s, 3H), 1.55 (s, 1H), 2.12-2.20 (m,
1 H), 2.42 (d, 1 H), 2.80 (m, 1 H), 4.56 (d, 1 H), 4.54 (d, 1 H), 4.70 (m,
2H).
Analysis: C~pH~3IO4, cal. 37.06% C, 4.04% H, 39.15% I; found, 37.08% C,
3.98% H, 39.55% I.
-142-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O
0'~>~CO Me
2
19F ~-Methyl-exo exo-5 6-dihydroxyacetonide-2-oxabicyclof2.2.11heptane-3-
exo-carboxylate.
To a solution of (t)-exo,exo-6,7-dihydroxyacetonide-4-exo-iodo-2-
oxabicyclo[3.2.1]octan-3-one (7.55 g, 23.3 mmol) in 300 mL of methanol (pre-
dried with 4 A sieves) was added K2C03 (3.54 g, 25.6 mmol). The mixture was
stirred vigorously for 30 min. The undissolved potassium carbonate was
removed by filtration and the filtrate was concentrated to dryness. The
residue
was triturated with ethyl acetate several times until complete extraction of
the
product (TLC). The ethyl acetate solution was then concentrated and directly
chromatographed on a silica gel column eluting with 10-25% ethyl acetate in
hexane, giving 4.86 g of the title compound as a white solid. Yield: 92.0%.
MS (DCI-NH3): m/z 246 for (M+NH4), base peak.
~ H NMR (CDC13): 8 1.30 (s, 3H), 1.45 (s, 3H), 1.62 (d, 1 H), 1.85 (d, 1 H),
2.82 (s, 1 H), 3.78 (s, 3H), 3.80 (s, 1 H), 4.20 (d, 1 H), 4.30 (d, 1 H), 4.40
(s, 1 H).
Analysis:C»H,605, calc.: 57.88% C, 7.07% H; found: 57.98% C, 7.10% H.
/' O~~'' O
O~~~CHO
19G (t)-exo-3-Form r~l-exo exo-5.6-dihydroxyacetonide-2-
oxabicycloj2.2.1 Leptane
To a well-stirred solution of (t)-methyl-exo,exo-5,6-dihydroxyacetonide-2-
oxabicyclo[2.2.1]heptane-exo-3-carboxylate (0.82 g, 3.62 mmol) in 30 mL of
anhydrous dichloromethane at -78 °C was added a solution of
diisobutylaluminum
hydride in toluene (1.0 M, 5.77 mL) dropwise. The mixture was then stirred at -
78
-143-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
°C for 1 hour and quenched with 2 mL of methanol and 15 mL of saturated
aqueous sodium potassium tartrate solution. The mixture was then stirred
vigorously and allowed to warm to ambient temperature over 1 hour. The phases
were separated and the aqueous phase was extracted with Et20 (5 x 50 mL).
After drying, solvent removal gave the title compound as an oil (0.8 g) which
was
used directly for the next step.
o
0
OH
19H ~,t)-(1'RS)-exo-3-(1'-hydrox~r-3'-methyl~butyl-exo.exo-5.6-
dihYdroxyacetonide-2-oxabicyclo[2.2.1 ]heptane.
To a solution of (t)-exo-3-formyl-exo,exo-5,6-dihydroxyacetonide-2-
oxabicyclo[2.2.1]heptane (0.99 g, 5.0 mmol) in 50 mL of anhydrous THF at -78
°C
was added a solution of isobutylmagnesium chloride (2.0 M in ether, 30 mL)
dropwise over 30 minutes. The mixture was then allowed to warm up to ambient
temperature over 2 hours and quenched with 100 mL of saturated aqueous
NH4C1 solution. The solution was then extracted with EtzO (3x100 mL). The
combined organic layers were washed with brine (3x100 mL) and dried. After
filtration, evaporation of solvent gave title compound as an oil (1.28 g)
which was
used directly for the next step.
-144-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
O
O
O
191 ~t)-exo-3-L'-oxo-3'-methYl)but~-exo exo-5 6-dihydroxyacetonide-2-
oxabic~clof2.2.11he~tane
A solution of oxalyl chloride (2.0 M in dichloromethane, 3.76 mL, 7.3 mmol)
was mixed with 10 mL of anhydrous dichloromethane and the solution was
cooled to -78 °C. A solution of DMSO (1.06 mL) in 10 mL of anhydrous
dichloromethane was then added dropwise. After stirring for 10 minutes, a
solution of (t)-(1'RS)-exo-3-(1'-hydroxy-3'-methyl)butyl-exo,exo-5,6-
dihydroxyacetonide-2-oxabicyclo[2.2.1]heptane 01.28 g, 5.0 mmol) in anhydrous
dichloromethane (10 mL) was added dropwise and the solution was stirred for
another 10 minutes. Triethylamine (4.3 mL) in 10 mL of dichloromethane was
then added. The mixture was then stirred at -78 °C for 2 hours and
allowed to
warm up to ambient temperature. The solution was washed with water (100 mL),
5% aqueous citric acid (100 mL), saturated NaHC03 (100 mL) and brine. After
drying and concentration, the crude material was purified by flash
chromatography on a silica gel column using 5%-20% ethyl acetate-hexane to
give the title compound as a white solid, 0.65 g, 51% for three steps.
MS (DCI-NH3): mlz 272 for (M+NH4)+ base peak.
'H NMR (CDC13): b 0.84 (dd, 6H), 1.30 (s, 3H), 1.34, 1.38 (bd, 1H), 1.43 (s,
3H), 1.77, 1.82 (dd, 1 H), 2.10-2.10 (m, 1 H), 2.30-2.50 (dq, 2H), 2.86 (s, 1
H), 3.62
(s, 1 H), 4.16-4.20 (m, 1 H), 4.28-4.32 (bd, 1 H), 4.37 (s, 1 H).
Analysis: C~4H22O4, cal. 66.12% C, 8.72% H; found, 65.99% C, 8.58% H.
-145-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
HO ~.", O
HO
O
19J (t)-exo-3-(1'-oxo-3'-methyl)butYl-exo exo-5 6-dihydroxy-2-
oxabicyclof2.2.1 ]heptane.
(t)-exo-3-(1'-Oxo-3'-methyl)butyl-exo, exo-5,6-d ihydroxyacetonide-2-
oxabicyclo[2.2.1]heptane (0.64 g, 0.25 mmol) was dissolved in 12.5 mL of
methanol and 12.5 mL of 2M HCI. The solution was stirred at 60 °C for
2.5 hours.
After cooling to ambient temperature, the solution was partially concentrated
and
then extracted with ethyl acetate (4x50 mL). The combined ethyl acetate
solution
was washed with saturated aqueous NaHC03 solution (2x50 mL) and brine (2x50
mL), and dried. After filtration, removal of solvent gave the title compound
as an
off-white crystalline solid, (yield: 0.53 g, 98.0%).
MS (DCl-NHs): mlz 232 for (M+NH4)+, base peak.
' H NMR (CDC13): 8 0.92 (dd, 6H), 1.34, 1.44 (bd, 1 H), 1.77, 1.82 (dd, 1 H),
2.10-2.20 (m, 1 H), 2.30-2.45 (dq, 2H), 2.65 (d, 1 H), 2.74-2.80 (m, 2H), 3.72
(s,
1 H), 3.94-4.00 (m, 1 H), 4.00-4.05 (bd, 1 H), 4.24 (s, 1 H).
Me02C,
O ,
p~~~''C02Me
19~t)-l2R 3R 5R)-Dimethvl 2-(1'-oxo-3'-methyl)butyltetrahydrofuran-3,5-
dicarboxylate
(t)-exo-3-( 1 '-Oxo-3'-methyl) b uty I-exo, exo-5 , 6-d i hyd roxy-2
oxabicyclo[2.2.1]heptane.(0.50 g, 2.3 mmol) was dissolved in a mixture of
-146-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
acetonitrile (6.5 mL), carbon tetrachloride (6.5 mL) and water (10 mL). To
this
solution was added sodium periodate (2.1 g, 10 mmol) and RuC13.H20 (10 mg).
The solution was stirred at ambient temperature for 3 hours and filtered to
remove the solid. The filtrate was loaded on a short silica gel column, eluted
with
5% methanol in ethyl acetate containing 5% acetic acid. The resulting crude
diacid (yellow oil, 0.53 g) was dissolved in ethyl acetate (25 mL). N,N-
diisopropylethylamine (1.73 mL, 12 mmol) and methyl iodide (2.98 mL, 46 mmol)
were added and the mixture was stirred at 50 °C for 2 hours. The
mixture was
filtered to remove the solid and the filtrate was concentrated. The residue
was
purified by flash chromatography on a silica gel column eluting with 10-40%
ethyl
acetate in hexane, giving the title compound as a colorless liquid (0.40g,
68.0%
for two steps).
MS (DCI-NH3): mlz 290 for (M+NH4)+, base peak.
' H NMR (CDC13): 8 0.92 (d, 3H, CH3), 0.93 (d, 3H, CH3), 2.19 (m, 1 H, H~),
2.40-2.60 (m, 4H, H6a, H6b, H3a, H3b), 3.30 (m, 1 H, H3), 3.75 (s, 3H, OCH3),
3.78
(s, 3H, OCH3), 4.65 (dd, 1 H, H5), 4.85 (d, 1 H, H2).
Me02C,
HO'N O~w~COZMe
19L (t~-(2R 3R 5R -Dimethyl-2-(1'-N-hvdroxyimino-3'-methyl)butyl-
tetrahydrofuran-3.5-dicarboxylate
A solution of (t)-(2R,3R,5R)-dimethyl-2-(1'-oxo-3'-methyl)butyl-
tetrahydrofuran-3,5-dicarboxylate(0.3 g, 1.09 mole), hydroxylamine
hydrochloride
(0.23 g, 3.3 mmol), diisopropylethylamine (0.57 mL, 3.3 mmol) and
tetrabutylammonium iodide in 10 mL of methanol was stirred at 40 °C for
2 hours.
-147-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99I07949
The solution was concentrated and purified directed on a 10-g silica gel
cartridge,
eluting with 30% ethyl acetate in hexane, giving the title compound as a
mixture
of cis and trans oximes.
MS (DCI-NH3): m/z 287 (M+H), 305 (M+NH4)+.
' H NMR (CDC13): 8 0.95-1.0 (m, 6H), 2.19 (m, 1 H), 2.40-2.60 (m, 4H,),
3.45 (m, 1 H), 3.65-3.80 (4 s, 6H), 4.50-4.60 (bs, 1 H), 4.70 (m, 1 H), 5.0
(bs, 1 H)
MeO2C,, MeOZC,
H N
Boc'N~,. ~ ~'''COZMe Boc' p)~'''C02Me
19M (t)-(2R 3R 5R 1'S) and (t)-{2R 3R 5R 1'R) Dimethyl 2-(1'-t
butoxycarbonylamino-3'-methyl)but~-tetrah~drofuran-3 5-dicarboxylate.
A solution of (t)-(2R,3R,5R)-dimethyl-2-(1'-N-hydroxyimino-3'-
methyl)butyl-tetrahydrofuran-3,5-dicarboxylate (0.27 g, 0.94 mmol) and Boc20
(2.11 mL, 10x) in 70 mL of isopropanol was hydrogenated with Raney Nickel at 4
atm of hydrogen for 15 hours. After concentration of the solution, separation
on a
silica gel column with 10% ethyl acetate in hexane gave (t)-(2R,3R, 5R,1'S)
isomer (137 mg) and (t)-(2R,3R, 5R,1'R) isomer {176 mg).
(t)-(2R,3R, 5R,1'S) MS (DCI-NH3): mlz 374, 391, 317, 335.
'H NMR (CDC13): 0.9-0.95 (2d, 6H), 1.22-1.31 (m, 1H), 1.49 (s, 9H), 1.50-
1.55 (m, 1 H), 1.62-1.75 (m, 1 H), 2.40-2.60 (m, 2H), 2.95-3.10 (m, 1 H), 3.70
(s,
3H), 3.75 (s, 3H), 4.20 (t, 1 H), 4.37 (bd, 1 H), 4.60 (dd, 1 H).
-148-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
MHO2C,,
~N, '
~.~~'C02Me
O
19N (t)-(2R 3R 5R 1S)-Dimethyl 2-(1'-acetamido-3'-methyl)butvl-
tetrahydrofuran-3.5-dicarboxylate.
A solution of (t)-(2R,3R, 5R,1'S) dimethyl 2-(1'-t-butoxycarbonylamino-3'-
methyl)butyl-tetrahydrofuran-3,5-dicarboxylate (40 mg, 0.11 mmol).in 2 mL of
50% trifluoroacetic acidldichloromethane was stirred at ambient temperature
for 1
hours. The solution was then evaporated to dryness. The residue was dissolved
in 0.5 mL of dichloromethane. Acetic anhydride (104 mL, 10.0 eq.) and
diisopropylethylamine {192 mL, 10.0 eq.) were added. After stirring for 2
hours,
the mixture was directly loaded on a 5-g silica gel cartridge and eluted with
60%
ethyl acetate in hexane, giving the title compound as a solid (34 mg ,100%).
MS (DCI-NH3): mlz 316 (M+H) +, 333 (M+NH4)+
'H NMR (CDC13): b 0.9-0.95 (2d, 6H), 1.25-1.35 (m, 1H), 1.50-1.70 (m,
2H), 1.99 (s, 3H), 2.40-2.60 (m, 2H), 2.95-3.10 (m, 1 H), 3.70 (s, 3H), 3.75
(s, 3H),
4.20-4.40 (m, 2H), 4.60 (dd, 1 H), 5.30 (bd, 1 H).
MH02C,,
~N~ p~~~''C02H
O
190 (t)-(2R 3R~ 5R 1'S}-2-(1'-acetamido-3'-methy~butyl-3-methoxycarbonyl-
tetrahydrofuran-5-carboxylic acid
To a solution of (t)-(2R,3R,5R,1'S}-dimethyl 2-(1'-acetamido-3'
methyl)butyl-tetrahydrofuran-3,5-dicarboxylate (32 mg, 0.1 mmol) in 1.0 mL of
-149-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
THF cooled to 0 °C was added a solution of LiOH (0.1 M, 1.0 mL)
slowly
dropwise. The solution was then stirred for 15 minutes and quenched with 1.0
mL of acetic acid. After extracting with ethyl acetate (3x1 mL), the organic
solution was dried (MgS04) and purified on a 5-g silica gel cartridge, eluting
with
0-20% methanol/ethyl acetate containing 5% acetic acid. The product obtained
was further purified by recrystallization from ethyl acetate and isopropanol.
(yield:
22 mg, 71 %).
HRMS: C~4H24O6N, calculated: 302.1604, found: 302.1592.
'H NMR (CDC13): 8 0.9-0.95 (2d, 6H), 1.25-1.35 (m, 1H), 1.50-1.70 (m,
2H), 1.99 (s, 3H), 2.40-2.50 (m, 1 H), 2.60-2.70 (m, 1 H), 2.95-3.10 (m, 1 H),
3.70
(s, 3H), 4.10-4.40 (m, 2H), 4.60 (dd, 1 H), 5.30 (bd, 1 H). The relative
stereochemistry was confirmed by the X-ray structure of a single crystal.
Example 20
MFi02C,
/N, ,
~~~~'C02t8u
O
20A t~,-(2R 3R 5R 1'S~2 ~1'-Acetamido-3'-methyt)but~l-3-methoxycarbonyl-
tetrahydrofuran-5-carboxylic Acid t-Bu I Ester.
To a solution of (t)-(2R,3R,5R,1'S)-2-(1'-acetamido-3'-methyl)butyl-3-
methoxycarbonyltetrahydrofuran-5-carboxylic acid {25 mg, 0.08 mmol) in 1.0 mL
of dichloromethane was added a solution of tent-butyl 2,2,2-
trichloroacetimidate
(54 mg, 0.24 mmol) in 0.3 mL of cyclohexane. After cooling to 0 °C, 3
drops of
boron trifluoride etherate was added. The mixture was stirred for another 40
minutes and quenched with 2 mL of 5% NaHC03 solution. After diluting with 10
mL of dichloromethane, the mixture was filtered to remove insoluble by-product
-150-
CA 02329660 2000-10-20
WO 99!54290 PCT/US99/07949
and the organic phase was further washed with 5% NaHC03 solution, dried, arid
concentrated. The residue was purified on a 5-g silica gel cartridge to give
the
title compound as an oil (26 mg) which was used directly for the next step.
Ho2c,,
H
\ /N, ,
0~ ~~'C02~Bu
O
20B !t)-(2R 3R 5R 1'Sl-2-(1'-acetamido-3'-methyl)butyl-3-carboxyl-
tetrahydrofuran 5-carboxylic acid t-Butyl Ester
A solution of (t)-(2R,3R,5R,1'S)-2-(1'-acetamido-3'-methyl)butyl-3-
methoxycarbonyl-tetrahydrofuran-5-carboxylic acid t-butyl ester (26 mg, 0.073
mmol) in 1.5 mL of THF was cooled to 0 °C and a solution of 0.1 N LiOH
(0.73
mL) was added. The mixture was allowed to warm up to ambient temperature
over 90 minutes, then acidified to pH 2-3 with 1 N HCI and extracted with
ethyl
acetate. The combined ethyl acetate solution was dried and concentrated. The
residue was purified on a 5-g silica gel cartridge to give the title compound
(10
mg) as a solid.
MS (DCI-NH3): mlz 344 (M+H)+, 361 (M+NH4)+.
' H NMR (methanol-d4): 8 0.87, 0.89 (d, 3H), 0.92, 0.94 (d, 3H), 1.35-1.45
(m, 1 H), 1.47 (s, 9H), 1.50-1.70 (m, 2H), 1.92 (s, 3H), 2.30-2.37 (m, 1 H),
2.45-
2.55 (m, 1 H), 2.90-2.97 (m, 1 H), 4.00-4.07 (m, 1 H), 4.20 (t, 1 H), 4.45
(dd, 1 H).
-151-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
-Br
O->.,
H
"N,
O O
20C (t)-(2R 3R 5R 1'S~-2-~1'-acetamido-3'-methyl butyl-3-(1"-oxo-2"-
bromo)ethyl-tetrahydrofuran-5-carbox~ic Acid t-Butyl Ester
To a solution of (t)-methyl (2R,3R,5R,1'S)-2-(1'-acetamido-3'-methyl)butyl-
3-carboxyl-tetrahydrofuran-5-carboxylic acid t-butyl ester (10 mg, 0.029 mmol)
in
1.0 mL of THF, cooled to 0 °C, was added N-methyl morpholine (0.13 mL)
and
iso-butyl chloroformate (0.15 mL, 0.12 mmol). The mixture was stirred at 0
°C for
1 h. A solution of diazomethane generated from Diazald (0.21 g, 1.0 mmol) and
KOH (0.8 g) in 2 mL of Et20 was then added via a syringe until the yellow
color
persisted. The solution was then stirred for 3 hours, washed with brine (2 x
2.0
mL) and dried (MgS04). Evaporation of solvent gave a yellow solid which was
redissolved in 1.5 mL of dioxane. To this solution was added 48% HBr (aq.)
solution (0.125 mL) and the mixture was stirred for 10 minutes. Saturated
NaHC03 solution (0.5 mL) was then added slowly and the mixture was extracted
with ethyl acetate (5 x 1.0 mL). The combined ethyl acetate solution was dried
(MgS04), filtered and concentrated. Chromatography on a 5-g silica gel
cartridge
eluting with 60% ethyl acetate-hexane give the title compound (7.3 mg) as a
solid
which was used directly for the next step.
-152-
CA 02329660 2000-10-20
WO 99/54290 PC'T/US99/07949
H
/N
<N~..
H
~N~,. f
O
20~t)-(2R 3S 5R 1'S)-2-(1'-acetamido-3'-methyl)bufirl-3-(imidazol-4-yl)-
tetrahydrofuran-5-carboxylic Acid t-Butyl Ester
To a vial containing (t)-(2R,3R,5R,1'S)-2-(1'-acetamido-3'-methyl)butyl-3-
(1"-oxo-2"-bromo)ethyl-tetrahydrofuran-5-carboxylic acid t-butyl ester (7 mg,
0.014 mmol) and formamidine acetate (30 mg, excess) was added liquid
ammonia (~2.0 mL). The vial was sealed and stirred at 45 °C overnight.
The
ammonia was allowed to evaporate slowly. The residue was taken up in 5%
Na2C03 (aq. 1.0 mL) and extracted with ethyl acetate (4x1.0 mL). The combined
ethyl acetate solution was dried and concentrated. The residue was
chromatographed on a 2-g silica gel cartridge eluting with ethyl acetate to
give
the title compound as a solid (1.0 mg).
MS (APCI+): mlz 36fi (M+H)+, 310 (M-C4H9)+, base peak.
'H NMR (methanol-d4): 8 0.87, 0.89 (d, 3H), 0.95, 0.97 (d, 3H), 1.27-1.37
(m, 1 H), 1.47 (s, 9H), 1.42-1.67 (m, 2H), 1.84 (s, 3H), 2.10-2.17 (dt, 1 H),
2.77-
2.87 (dt, 1 H), 3.50 (q, 1 H), 4.02-4.06 (m, 1 H), 4.16 (t, 1 H), 4.55 (t, 1
H), 7.34 (s,
1 H), 8.64 (s, 1 H).
-153-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
H
/N~'
\N
H
~N~,. Ol~.,,I~OH
IOI ,O
HCI
20~t)-(2R 3S 5R 1'S~l-~1'-Acetamido-3'-methyl}butyl-3-(imidazol-4-vl)-
tetrahydrofuran 5-carboxylic Acid
A solution of (t)-(2R,3S,5R,1'S)-2-(1'-acetamido-3'-methyl)butyl-3-
(imidazol-4-yl}-tetrahydrofuran-5-carboxylic acid t-butyl ester (1.0 mg) in
1.0 mL
of 6N aqueous HCi was stirred for 1 h. The solution was then concentrated to
give the title compound as a white solid (0.8 mg).
'H NMR (D20): a 0.81, 0.83 (d, 3H), 0.87, 0.89 (d, 3H), 1.30-1.58 (m, 4H),
1.84 (s, 3H), 2.10-2.22 (m, 1 H), 2.85-2.95 (m, 1 H), 3.58 (q, 1 H), 3.90-3.94
(m,
1 H}, 4.00-4.04 (m, 1 H), 4.20 (t, 1 H), 7.32 (s, 1 H), 8.64 (s, 1 H).
-154-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 21
,~S 3R 4S 1'S)-3-(1'-Acetamido-3'-ethyllpentyl-4-vinyl-cyclopentane-1-
carboxylic
Acid
AcH N,
U
OTrity l
21A-(1 S,3R.4S,1'S)-3-(1'-acetamido-3'-ethyl-)pentyl-1-
(triphen rLlmethylo~)methyl-4-vinyl-cyclopentane
A solution of (1 R.2R,4R,1'S)-1-formyl-2-(1'-acetamido-3'-ethyl)pentyl-4-
(triphenylmethyloxy)methyl-cyclopentane (60 mg, 0.11 mmol) prepared according
to the method of Example 15C in THF (1 mL) was added to a mixture prepared
from the reaction of methyltriphenylphosphonium iodide (69 mg, 0.17 mmol) and
2.5M n-BuLi/hexane (64 mg, 0.16 mmol) in THF (1 mL) for 2 hours at room
temperature. After 1.5 hours, the reaction was partitioned between ethyl
acetate
and 10% citric acid. The organic layer was separated and washed with brine,
dried over MgS04, filtered and concentrated in vacuo. The residue was purified
by chromatography on silica gel using 3% methanolldichloromethane to provide
the title compound (yield: 29 mg, 49%).
'H NMR {CDC13) a 7.52 (m, 6H), 7.24 (m, 9H), 5.64 (m, 1H), 4.84-5.04 {m,
3H), 3.98 (m, 1 H), 2.95 (m, 2H), 2.7 {m, 2H), 2.0 (m, 1 H}, 1.88 (m, 2H),
1.66 (m,
1 H), 1.56 (s, 3H), 1.1-1.5 (m, 9H), 0.83 (m, 6H).
MS: {M-H)- = 522, (M+35)- = 558; (M+Na)+ = 546.
-155-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
= =.
AcHN,
~I~~~I
OH
21B. (1S 3R 4S 1'S~,3-vine-4-(1-acetamido-3-ethyl)pentyl-1-hydroxymethvl-
~clo~entane
The title compound was prepared according to the method described in
Example 15E substituting (1S,3R,4S,1'S)-3-{1-acetamido-3-ethyl)pentyl-1-
(triphenylmethyloxy)methyl-4-vinyl-cyclopentane for (1 R,3R,4R,1'S)-3-
(irnidazol-
2-yl)-4-(1'-acetamido-3'-ethyl)pentyl-1-(triphenylmethyloxy)methyl-
cyclopentane
(yield: 7 mg, 87%).
H NMR (CDC13) b 5.68 (m, 1 H), 4.84-5.05 (m, 3H), 3.98 (m, 1 H), 3.54 (2d,
2H), 2.4 (m, 1 H), 2.18 (m, 1 H), 1.92 (s, 3H), 1.1-1.5 (m, 1 OH), 0.83 {2t,
6H).
MS: (M+H)+ = 282, (M+18)+ = 299
-.
AcHN.
_ ..,..1 0
H v OH
21C. {1S 3R 4S 1'S)-3-(1-acetamido-3-ethyl-)pentyl-4-vinyl-cyclopentane-1-
carboxylic Acid
The title compound was prepared according to the procedure described in
Example 13J substituting (1S,3R,4S,1'S) 3-(1-acetamido-3-ethyi)pentyl-1-
hydroxymethyl-4-vinyl-cyclopentane for (1 R,2R,4R,1'S)-1-(t
butyldiphenylsilyloxymethyl)-2-(1'-acetamido-3'-ethyl)pentyl-4-hydroxymethyl-
cyclopentane (yield: 4.2 mg, 33%).
-156-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
' H NMR (CDC13) a 5.65-5.78 (m, 1 H), 5.05 (d, 1 H), 5.02 (2d, 1 H), 4.92
(2d, 1 H), 3.99 (q, 1 H), 2.88 (m, 1 H), 2.43 (m, 1 H), 2.15 (m, 2H), 1.91 (s,
3H), 1.87
(m, 1 H), 1.71 (m, 1 H), 1.1-1.5 (m, 8H), 0.83 (2t, 6H)
MS: (M-H)- = 294; (M+H)+ = 296, (M+Na)+ = 318
Example 22
(,tL(1 R 3R 4R 1'S)-3-N-methylcarbamoyl-4-(1'-acetamido-3'-ethyl)pentyl-
cyclopentane-1-carboxylic Acid
O
MeHN-~
AcHN.
H 'OH
The title compound was prepared according to the procedure described
ing Example 12G substituting methylamine for methanol (yield: .029 g, 30%).
' H NMR (CD30D) 8 7.52 (d, 1 H), 3.78-3.90 (m, 1 H), 2.80-2.95 (m, 1 H),
2.71 (s, 3H), 2.46-2.50 (m, 2H), 2.09-2.24 (m, 2H), 1.87 (s, 3H), 1.59-1.72
(m,
1 H}, 1.10-1.49 (m, 7H), 0.82, 0.87 (2t, 6H).
MS: (M+H)+= 327, (M+NH4)+=344
-157-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 23
~1 R 3R 4R 1'S) 3 (imidazol-4-yl)-4-~1'-acetamido-3'-methyl)butyl-cyclopentane-
1-
carboxylic Acid
OTBDPS
AcH N,
_ ..,,~('O
H v OH
23A. (1 R 3R 4R 1'S) 3-(t-Butyldiphenylsilyloxymethyl)-4-(1'-acetamido-3'-
methyl)butyl-cycio~entane-1-carboxylic Acid.
The title compound was prepared by the methods described in Examples
13F-13J substituting isobutylmagnesium bromide for 3-pentylmagnesium bromide
in Example 13F and substituting the resulting products in the subsequent
steps.
' H NMR (d4-methanol) 8 7.68 (m, 4H), 7.42 (m, 6H), 3.88 (m, 1 H), 3.74
(2d, 1 H), 3.48 (2d, 1 H), 2.77 (m, 1 H), 2.15-2.27 (m, 2H), 1.68-2.05 (m,
3H), 1.80
(s, 3H), 1.5 (m, 1 H), 1.1-1.32 (m, 2H), 1.03 (s, 9H), 0.84 (2d, J=6.44 Hz,
6H).
MS: (M-H}- = 508; (M+H)+ = 510, (M+Na)+ = 532
OTBDPS
AcH N, ' O
_
H ,O a
23B. L1 R 3R 4R 1'S}-3-(t-Butyldi~henvlsilyloxyrnethYl)-4-(1'-acetamido-3'-
methyl)butyl-cyclopentane-1-carboxylic Acid t-Butyl Ester.
(1 R,3R,4R,1'S)-3-(t-Butyldiphenylsilyloxymethyl)-4-(1'-acetamido-3'-
methyl)butyl-cyclopentane-1-carboxylic acid (0.65 g, 1.27 mmol) was reacted
-158-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
with t-butyl trichloroacetimidate (1.36 mL, 7.56 mmol) in toluene at
100°C for 16
hours. The reaction was quenched with water (20 mL) and diluted with ethyl
acetate {50 mL). The organic layer was washed with water, and brine, dried
over
MgS04, filtered and concentrated in vacuo. The residue was purified by
chromatography on silica gel using 7% methanol/dichloromethane to provide the
title compound (yield: 0.65 g, 90%).
' H NMR (d4-methanol) 8 7.65 (m, 4H), 7.4 (m, 6H), 3.88 (m, 1 H), 3.72
{2d, 1 H), 3.46 (2d, 1 H). 2.68 (m, 1 H), 2.12 (m, 1 H), 1.76-2.0 (m, 2H),
1.79 (s, 3H),
1.68 (m, 1 H), 1.48 (m, 1 H), 1.42 (s, 9H), 1.1-1.32 (m, 2H), 0.84 (2d, J=6.44
Hz,
6H).
MS: (M-H)- = 564, {M+35)- = 600; (M+H)+ = 566, (M+Na)+ = 588
OH
..
AcH N,
H v O'Bu
23C. ~1 R 3R 4R 1'S)-3-H~droxymethyl-4-f~1'-acetamido-3'-methyl)butyl-
cyclopentane-1-carbox rLlic Acid t-Butyl Ester
The title compound was prepared according to the method described in
Example 13K substituting (1R,3R,4R,1'S) 3-(t-butyldiphenylsilyloxymethyl)-4-
(1'-
acetamido-3'-methyl)butyl-cyclopentane-1-carboxylic acid t-butyl ester for
(1 R,3R,4R,1'S)-3- (t-butyldiphenylsilyloxymethyl)-4-(1'-acetamido-3'-
ethyl)pentyl-
cyclopentane-1-carboxylic acid (yield: 0.35 g, 93%).
' H NMR (d4-methanol) 8 3.88 (m, 1 H), 3.58 (2d, 1 H), 3.35 {2d, 1 H), 2.7
(m, 1 H), 2.08 {m, 1 H), 1.95 (s, 3H), 1.94 (m, 1 H), 1.78 (m, 1 H), 1.5-1.7
(m, 3H),
1.43 (s, 9H), 1.2-1.4 (m. 2H), 0.9 (2d, J=6.44 Hz, 6H)
-159-
CA 02329660 2000-10-20
WO 99/54290 PC'T/US99/07949
MS: (M-H)- = 326, (M+35)- = 362; (M+H)+ = 328, (M+Na)+= 350
O
H ~~
AcHN, O
'O a
23D. (1 R 3R 4R 1'S)-3-Form-4-(1'-acetamido-3'-meth~)butyl-cyclopentane-1-
carboxylic Acid t-Butyl Ester
The title compound was prepared according to the method described in
Example 1 D substituting (1 R,3R,4R,1'S)-3-hydroxymethyl-4-(1'-acetamido-3'-
methyl)butyl-cyclopentane-1-carboxylic acid t-butyl ester for (t)-{2R,3S)-2-(t-
butyloxycarbonylamino)-3-hydroxymethylbicyclo(2.2.1]hept-5-ene (yield: 0.32 g,
92%).
' H NMR (d4-methanol) 8 9.5 (d, 1 H), 7.85 (d, 1 H), 3.88 (m, 1 H), 2.85 (m,
1 H), 2.62 (m, 1 H), 2.47 (m, 1 H), 2.05 (m, 2H), 1.91 (m, 1 H), 1.87 (s, 3H),
1.5-1.72
(m, 2H), 1.44 (s, 9H), 1.23-1.4 (m, 2H), 0.9 (2d, J=6.44 Hz, 6H).
MS: (M-H)- = 324; (M+H)+ = 326, (M+Na)+ = 348.
OH
O
AcHN,
_ ..,,.1 0
H v OtBu
23E. (1 R 3R 4R 1'Sl-3-Carbox~-4-~'-acetamido-3'-methyl)butyl-cyclopentane-
1-carboxylic Acid t-Butyl Ester
A solution of (1R,3R,4R,1'S)-3-formyl-4-(1'-acetamido-3'-methyl)butyl-
cyclopentane-1-carboxylic acid t-butyl ester (0.3 g, 0.93 mmol) and 2-methyl-2-
-160-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
butene (1.5 mL, 14.15 mmol) dissolved in t-BuOH (7.5 mL) and acetonitrile (7.5
mL) was reacted with a solution of NaCl02 (0.3 g, 3.31 mmol) and NaH2P04.H20
(0.3 g, 2.17 mmol) in water (6 mL) at 0 °C for 1 hour. The reaction was
quenched
with 10% aqueous NaZS203 and extracted with dichloromethane. The organic
layer was washed with water and brine, dried (MgS04) and concentrated in
vacuo. The residue was purified by chromatography on silica gel using 9%
methanol/dichloromethane to provide the title compound (yield: 0.286 g,
90.8%).
' H NMR (d4-methanol) b 3.86 (m, 1 H), 2.8 (m, 1 H), 2.7 (m, 1 H), 2.4 (m,
1 H), 2.22 (m, 1 H), 2.08 (m, 1 H), 1.97 (m, 1 H), 1.90 {s, 3H), 1.5-1.68 (m,
2H), 1.44
(s, 9H), 1.23-1.4 (m, 2H), 0.9 (2d, J=6.44 Hz, 6H).
MS: (M-H)- = 340, (M+35)- = 376; (M+H)+ = 342, (M+Na)+ = 364.
o--~:
AcH
N
,
_ ..,,~~0
v
H OtBu
23F. (1 R 3R 4R 1'S) 3-(2-Bromo-1-oxo ethyl~1-acetamido-3-methyl)butyl-
cycloaentane-1-carboxylic Acid t-Bu I Ester
(1 R,3R,4R,1'S)-3-Carboxyl-4-(1'-acetamido-3'-methyl)butyl-cyclopentane-
1-carboxylic acid t-butyl ester (286 mg, 0.84 mmol) and N-methylmorpholine
(111
pL, 1.01 mmol) in ether {15 mL) was reacted with isobutyl chloroformate {120
pL,
0.93 mmol) at 0°C for 45 minutes. To the reaction flask was cannulated
a distilled
diazomethane solution in ether prepared from the reaction of Diazald (2.4 g)
in
ether (60 mL) with a solution of potassium hydroxide (2.4 g) in ethanol (15
mL)
and water (15 mL). The reaction was stirred for 8 hours at room temperature
-161-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
then diluted with ether. The organic layer was washed with brine, dried
(Na2S04)
and concentrated to give the intermediate diazo ketone. The diazoketone was
reacted with 48% HBr {90 pL) in dioxane (10 mL) at 23°C for 45 minutes.
The
reaction was quenched with water (50 mL) and diluted with ethyl acetate (200
mL). The organic layer was washed with water, and brine, dried over MgS04,
filtered and concentrated in vacuo. The residue was purified by chromatography
on silica gel using 20% ethyl acetatelhexane to provide the title compound
(yield:
105 mg, 33%).
' H NMR (d4-methanol) 8 4.18 (2d, J=14.07 Hz, 2H), 3.8 (m, 1 H), 3.08 (m,
1 H}, 2.83 (m, 1 H), 2.48 (m, 1 H), 2.3 (m, 1 H), 2.07 (m, 1 H), 1.89 (s, 3H),
1.45 (m,
1 H), 1.5-1.7 (m, 2H), 1.15-1.4fi (m, 2H), 1.43 (s, 9H), 1.2-1.4 (m, 3H), 0.88
(2d,
J=6.44 Hz, 6H).
MS: (M-H)- = 372; (M+H)+ = 374
HN~N
AcH N,
_ .,..1 0
H v OtBu
23G. ~1R 3R 4R 1'S)-3~midazol-4-yl)-4-(1'-acetamido-3'-methyl)butyl-
cyclopentane-1-carboxylic Acid t-Butyl Ester
(1 R,3R,4R,1'S)-3-(2-Brorno-1-oxo)ethyl-4-( 1'-acetamido-3'-methyl)butyl-
cyclopentane-1-carboxylic acid t-butyl ester (105 mg, 0.28 mmol) was reacted
with formamidine acetate (0.52 g, 4.99 mmol) in liquid ammonia (5 mL) and
heated at 45 °C in a sealed tube for 18 hours. The reaction was
concentrated in
vacuo. The residue was treated with aqueous NaHC03 and extracted with
dichloromethane (5x20 mL). The combined organic layers were washed with
brine, dried over sodium sulfate, filtered and concentrated in vacuo. The
residue
-162-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
was purified by chromatography on silica gel using 10% methanol in
dichloromethane to provide the title compound as an oil {40 mg, 39%).
' H NMR(d4-methanol) s 7.57 (br s, 1 H), 6.81 (br s, 1 H), 3.93 (m, 1 H), 2.96
(m, 1 H), 2.7 (m, 1 H), 2.1-2.4 (m, 3H), 1.85 (m, 1 H), 1.72 (s, 3H), 1.48 (m,
1 H),
1.43 (s, 9H), 1.15-1.46 (m, 2H), 0.85 {2d, J=6.44 Hz, 6H).
MS: (M-H)- = 362, (M+35)- = 398; (M+H)+ = 364, (M+Na)+ = 386
HN~N
AcHN,
_ ..,,~~0
H v IOH
HCI
23G. {1 R 3R 4R 1'S)-3-{imidazol-4-vl)-4-(1'-acetamido-3'-meth I)~ butyl-
cyclo~entane-1-carboxylic Acid Hydrochloride
(1R,3R,4R,1'S) 3-(imidazol-4-yl)-4-(1'-acetamido-3'-methyl)butyl-
cyclopentane-1-carboxylic acid t-butyl ester (40 mg, 0.11 mmol) was reacted
with
6N HCI (4 mL) for 1 hour. The reaction concentrated in vacuo to provide the
title
compound (yield: 37mg, 98%).
' H NMR (d4-methanol) b 8.83 (d, J=1.01 Hz, 1 H), 7.36 (d, J=1.01 Hz, 1 H},
3.92 (m, 1 H), 3.18 (m, 1 H), 2.98 (m, 1 H), 2.49 (m, 2H), 2.22 (m, 1 H), 1.90
(m,
2H), 1.77 (s, 3H), 1.53 (m, 1 H), 1.41 (m, 1 H), 1.25 (m, 1 H), 0.86 (2d,
J=6.45 Hz,
6H).
MS: (M-H}- = 306, (M+35)- = 342; (M+H)+ = 308, (M+Na)+ = 330
-163-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Example 24
(3R 4R 1'S)-4-(imidazol-2-yl)-3-(1'-acetamido-3'-ethyl)pentyl-cyclopent-1-ene-
1-
carboxylic Acid
OTBDPS
O
= v
H
24A f3R 4R 1'S)-3-(t-ButYldiphen rLlsilyioxymethyl)-4-(1'-azido-3'-
ethyl)pentyl-
~clopentan-1-one
(3R,4R,1'S)-3-(t-Butyldiphenylsilyloxymethyl)-4-(1'-azido-3'-ethyl)pentyl-1-
methylene-cyclopentane (0.4558, 0.93 mmol) was reacted with 4% osmium
tetroxide in water (0.59 mL, 0.093 mmol) and sodium periodate (0.8 g, 3.73
mmol) in THF (15 mL) and water (3 mL) at room temperature for 3 hours. The
mixture was filtered and the filtrate was diluted with ethyl acetate, washed
with
10% Na2Sz03 solution, water, dried over MgS04, filtered, and concentrated in
vacuo. Chromatography on silica gel using 10% ethyl acetate/hexane afforded
the title compound (yield: 240 mg, 52%).
'H NMR (CDC13) 8 7.64 (m, 4H), 7.4 (m, 6H), 3.68 (m, 2H), 3.36 (m, 1H),
2.3-2.6 (m, 4H), 2.23 (2d, 1 H), 2.08 (m, 1 H), 1.16-1.52 (m, 6H), 1.05 (s,
9H),
0.78-0.88 (2t, J=7.12 Hz, 6H)
MS: (M+18)+ = 509, (M+Na)+= 514
-164-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
OTBDPS OTBDPS
Na,,, / Ns,,,
H ~OTf H ~OTf
24B. ~3R 4R 1'S~-4-(t-Butyldiphe~IsilYloxymethy~-~1'-azido-3'-ethyl)pentyl-1-
trifluoromethansulfonyloxy-cyclopent-1-ene and (3R,4R.1'S)-3-(t-
Butyldiphenylsilyloxymethyl)-4-(,1'-azido-3'-ethyl)~entyl-1-
trifluoromethansulfonyloxy-cyclopent-2-ene
(3R,4R,1'S)-3-(t-Butyldiphenylsilyloxymethyl)-4-(1-azido-3-ethyl)pentyl-
cyclopentan-1-one (230 mg, 0.468 mmol) was reacted with 1M lithium
hexamethyldisilazide (1.17 mL) in THF (1 mL) at -78°C for 45 minutes.
The
reaction mixture was treated with N-phenyltrifluoromethanesuifonimide (0.2 g,
0.56 mmol) and reacted at 0°C for 0.5 hour. The reaction was quenched
with
water (10 mL) and diluted with ethyl acetate (50 mL). The organic layer was
washed with water, brine, dried over MgS04, filtered and concentrated in
vacuo.
The residue was purified by chromatography on silica gel using 5% ethyl
acetate/hexane to provide a mixture of the title compounds in a ratio of 3: 2
(yield:
280 mg, 96%).
'H NMR (CDC13) b 7.64 (m, 4H), 7.4 (m, 6H), 5.58-5.5 (m, 1H), 3.55-3.72
(m, 2H), 3.36 (m, 1 H), 2.7-2.83 (m, 2H), 2.3-2.47 (m, 2H), 1.16-1.5 (m, 6H),
1.05
(s, 9H), 0.78-0.9 (m, 6H)
-165-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
OTBDPS
OCH3
hi O
24C. j3R 4R 1'S)-4-~-Butyldiphenylsilylox~methyl -~1'-azido-3'-ethyl)pentyl-
cyclopent-1-ene-1-carboxylic Acid Methyl Ester and (3R.4R.1'S)-3-(t-
Butyldiphenylsilyloxymethyl)-4- 1'-azido-3'-ethyl)pentyl-1-cyclopent-1-ene1-
carboxylic Acid Methyl Ester
Carbon monoxide was bubbled into a mixture consisting of (3R,4R,1'S)-4-
(t-Butyldiphenylsilyloxymethyl)-3-(1'-azido-3'-ethyl)pentyl-1-
trifluoromethansulfonyloxy-cyclopent-1-ene and (3R,4R,1'S)-3-(t-
Butyldiphenylsiiyioxymethyl)-4-(1'-azido-3'-ethyl)pentyl-1-
trifluoromethansulfonyloxy-cyclopent-1-ene (280 mg, 0.45 mmol), triethylamine
(0.125 mL, 0.9 mmol), bis(acetato)bis(triphenylphosphine)palladium(II) (34 mg,
0.045 mmol) in DMF (4 mL) and methanol (2 mL) at 40°C for 2 hours. The
reaction was quenched with water (10 mL) and diluted with ethyl acetate (50
mL).
The organic layer was washed with water, brine, dried over MgS04, filtered and
concentrated in vacuo. The residue was purified by chromatography on silica
gel
using 5% ethyl acetate/hexane to provide the title compounds in a ratio of 3:
2 as
a mixture (yield: 181 mg, 75%).
'H NMR (CDC13) 8 7.64 (m, 4H), 7.4 (m, 6H), 6.58-6.7 (2 br s, 1 H), 3.74
(br s, 3H), 3.25-3.62 {m, 3H), 2.7-2.95 (m, 2H), 2.22-2.49 (m, 2H), 1.16-1.5
(m,
6H), 1.05 (s, 9H), 0.78-0.9 (m, 6H)
MS: (M+18)+ = 551, (M+Na)+ = 556
-166-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
OTBDPS OTBDPS
.. ..
AcHN, ' / OCH3 AcHN, 1. ~ OCH
3
H O H O
24D. (3R 4R 1'S)-4-(t-Butyldiphen~yloxymeth~~1'-acetamido-3'-
eth~l)pentyl-cyclopent-1-ene-1-carboxylic Acid Methy! Ester and (3R.4R,1'S)-3-
(t-
Butyldiphenylsilyloxymethyl)-4-(1'-acetamido-3'-ethyl~pentyl-cyclopent-1-ene-1-
carboxylic Acid Methyl Ester
A solution of the mixture of (3R,4R,1'S)-4-(t-butyldiphenylsilyloxymethyl)-3-
(1'-azido-3'-ethyl)pentyl-1-trifluoromethansulfonyloxy-cyclopent-1-ene-1-
carboxylic acid methyl ester and (3R,4R,1'S)-3-(t-butyldiphenylsilyloxymethyl)-
4-
(1'-azido-3'-ethyl)pentyl-1-trifluoromethansulfonyloxy-cyclopent-1-ene-1-
carboxylic acid methyl ester (0.18 g, 0.33 mmol) in THF (12 mL) and water (3
mL)
was reacted with triphenylphosphine {0.265 mg, 1.01 mmol) at 65°C for 4
hours.
The reaction mixture was concentrated in vacuo, redissolved in chloroform,
dried
over MgS04, filtered and concentrated in vacuo. The crude amine was reacted
with acetic anhydride (64 ~L, 0.67 mmol) in pyridine (0.135 mL, 1.67 mmol) and
dichloromethane (1 mL) at room temperature for 2 hours. The reaction was
quenched with 10% citric acid solution (10 mL) and diluted with ethyl acetate
(50
mL). The organic layer was washed with water, and brine, dried over MgS04,
filtered and concentrated in vacuo. The residue was purified by chromatography
on silica gel using 40% ethyl acetate/hexane to provide the title compounds
(3R,4R,1'S)-4-(t-butyldiphenylsilyloxymethyl)-3-(1'-acetamido-3'-ethyl)pentyl-
cyclopent-1-ene-1-carboxylic acid methyl ester (yield: 109 mg, 60%) and
(3R,4R,1'S)-3-(t-butyldiphenylsilyloxymethyl)-4-(1'-acetamido-3'-ethyl)pentyl-
cyclopent-1-ene-1-carboxylic acid methyl ester (yield: 37mg, 20%).
-167-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
(3R,4R,1'S)-4-(t-butyldiphenylsilyloxymethyl) 'H NMR (CDC13) 8 7.63
(m, 4H), 7.4 (m, 6H), 6.58 (s, 1 H), 4.95 (br s, 1 H), 4.0 (br s, 1 H), 3.73
(s, 3H),
3.57 (d, 2H), 2.72 (m, 2H), 1.78 (s, 3H}, 1.47 (m, 3H), 1.18 (m, 3H), 1.06 (s,
9H),
0.74-0.88 (2t, J=7.12 Hz, 6H)
MS: (M-H)- = 548, (M+35)- = 584; (M+H)+ = 550, (M+Na)+ = 572
(3R,4R,1'S)-3-(t-butyldiphenylsilyloxymethyl) 'H NMR (CDC13) 8 7.63
(m, 4H), 7.4 (m, 6H), 6.64 (d, 1 H), 4.72 (d, 1 H), 3.95 (m, 1 H), 3.74 (s,
3H), 3.73
(2d, 1 H), 3.57 (2d, 1 H), 2.9 (m, 1 H), 2.75 (m, 1 H), 2.3 (m, 1 H), 2.14 (m,
1 H), 1.71
(s, 3H), 1.45 (m, 3H), 1.18 (m, 3H), 1.05 {s, 9H), 0.72-0.86 (2t, J=7.12 Hz,
6H)
MS: (M-H)- = 548, (M+35)- = 584; (M+H)+ = 550, (M+Na)+ = 574
OH
..
AcHN,
/ OCH3
H O
24E. ~3R 4R 1'S~-4-hydroxymethyl-3-f1'-acetamido-3'-ethyl)pentyl-cycloaent-1-
enel-carbox~ic acid methyl ester
The title compound was prepared according to the method described in
Example 13K substituting (3R,4R,1'S)-4-(t-butyldiphenylsilyloxymethyl)-3-(1-
acetamido-3-ethyl)pentyl-1-trifluoromethansulfonyloxy-cyclopent-1-ene-1-
carboxylic acid methyl ester for (1R,3R,4R,1'S)-3-(t-
butyldiphenylsilyloxymethyl)-
4-(3-ethyl-1-acetamido)pentyl-cyclopentane-1-carboxylic acid (yield: 53 mg,
85%).
' H NMR (CDC13) 8 6.63 (m, 1 H), 5.49 (d, 1 H), 3.98 {m, 1 H), 3.74 (s, 3H),
3.5-3.7 (m, 2H), 2.87 (m, 1 H), 2.75 (m, 1 H), 2.2-2.4 (m, 2H), 1.99 (s, 3H),
1.17-
1.48 (m, 8H), 0.78-0.87 {2t, J=7.12 Hz, 6H)
-168-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
MS: (M-H)- = 310, (M+35)- = 346; (M+H)+ = 312, (M+Na)+ = 334
24F. {3R 4R 1'SZ-4-formvl-3-~-acetamido-3-ethyl)pentyl-cycioaent-1-ene-1-
carboxylic acid methyl ester
The title compound was prepared according to the method described in
Example 1D substituting (3R,4R,1'S)-4-hydroxymethyl-3-(1'-acetamido-3'-
ethyl)pentyl-cyclopent-1-ene-1-carboxylic acid methyl ester for (t)-(2R,3S)-2-
(t-
butyloxycarbonylamino)-3-hydroxymethyl-bicyclo[2.2.1 ]hept-5-ene (yield: 38
mg,
72%).
' H NMR (CDC13) a 9.71 (s, 1 H), 6.62 {m, 1 H), 5.2 (d, 1 H), 4.06 (m, 1 H),
3.75 (s, 3H), 3.25 (m, 1 H), 3.1 (m, 1 H), 2.96 (m, 1 H), 2.83 (m, 1 H), 1.96
(s, 3H),
1.2-1.5 (m, 8H), 0.77-0.9 (2t, J=7.12 Hz, 6H)
MS: (M-H)- = 308; (M+H)+ = 310
-169-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
/ N
~J.;
AcHNH / O
U
H OCH3
24G. ~3R 4R 1'S)-4~imidazol-2-yl)-3-(1'-acetamido-3'-eth rLl)pentyl-cyclopent-
1-
ene1-carboxylic acid methyl ester
The title compound was prepared according to the method described in
Example 15D substituting (3R,4R,1'S)-4-formyl-3-(1'-acetamido-3'-ethyl)pentyl-
cyclopent-1-ene1-carboxylic acid methyl ester for (1 R,3R,4R,1'S)-1-formyl-2-
(1'-
acetamido-3'-ethyl)pentyl-4-(triphenylmethyloxy)methyl-cyclopentane (yield: fi
mg, 14%).
' H NMR (CDC13) 8 7.06 (s, 2H), 6.69 (s, 1 H), 3.95 (m, 1 H), 3.74 (s, 3H),
3.5-3.7 (m, 2H), 3.23 (m, 1 H), 2.8 (m, 1 H), 1.90 (s, 3H), 1.1-1.5 (m, 8H),
0.73-
0.87 (2t, J=7.12 Hz, 6H)
MS: (M-H)- = 346, (M+35)- = 382; (M+H)+ = 348, (M+Na)+ = 370
~J.;
AcHNH / O
a
H OH
24H. ~3R 4R 1'S~-4-(Irnidazol-2-yl)-3-~1'-acetamido-3'-ethyl)pentyl-cyclopent-
1-
ene-1-carboxylic Acid
(3R,4R,1'S)-4-(Imidazol-2-yl)-3-(1'-acetamido-3'-ethyl)pentyl-cyclopent-1-
ene-1-carboxylic acid methyl ester (6 mg, 0.017 mmol) was reacted with lithium
hydroxide (1 mg, 0.026 mmol) in THF (0.5 mL) and water (0.2 mL) at 0°C
for 4
-170-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
hours. The reaction was quenched with 10% citric acid (1 mL) and diluted with
25% isopropanol/dichloromethane (10 mL). The organic layer was washed with
water, and brine, dried over MgS04, filtered and concentrated in vacuo. The
residue was purified by chromatography on silica gel using 1:3:1:1 waterlethyl
acetate/n-butanol/acetic acid followed by extraction into 25%
isopropanol/dichloromethane to provide the title compound (yield: 4.3 mg,
74%).
' H NMR (d4-methanol) 8 7.06 (br s, 2H), 6.48 (br s, 1 H), 4.08 (m, 1 H),
3.45-3.7 (m, 2H), 3.1 (m, 1 H), 2.62 (m, 1 H), 1.98 (br s, 2H), 1.82 (br s,
3H), 1.1-
1.42 (m, 8H), 0.73-0.87 (m, 6H)
MS: (M-H)- = 332, (M+35)- = 368; (M+H)+ = 334, (M+Na)+ = 356
Using the methods described above and the general knowledge of one
skilled in the art, compounds of the invention can be prepared which are
represented by taking one core from Table 1 (wherein Ac is acetyl), one Y
substituent from Table 2, one R substituent from Table 3, one R3 substituent
from
Table 4a, 4b, 4c, 4d, 4e, 4f or 4g and one R5 substituent from Table 5.
Table 1
Y, Y,
AcHN, ~ OR AcHN ~ OR
R3 H O O R3 H O O
1 2
-171-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Y, Y,
CH3S02+iN, 3 H 0,.. ~OR CH3S02i-IN 3 H 0~.. ~OR
R IOI R I'O
4
O Y, O Y,
II 1i
CF3C-HN, 3 H O~. ~OR CF3C-HN 3 H O~ ~OR
R IOI R I IO
6
Y, Y,
AcHN, OR AcHN OR
R3 H O R3 H O
7
Y, Y,
CH3SO2+iN, ). ~OR CH3S02+iN ~OR
R3H IOI R3H IIO
g 10
O Y, O Y,
II II
CF C-HN, OR CF C-HN OR
R3 H ~ 3 R3 H
11 12
Y~ Y
AcHN OR AcHN OR
R3 H / O R3 H / O
13 14
-172-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Y, Y,
CH3S02i~-IN / ~OR CH3S02+iN , ~OR
R3 H IOI R3 H I IO
15 16
O Y, O Y,
II II
CF3C-HN, f~ OR CF3C-HN / OR
R3H O R3H O
16
Y
AcHN ,OR AcHN OR
R3 H 5 O R3 H R5 O
R
18 19
Y, Y,
CH3S02tiN, ~ ~OR CH3S02i-IN ~OR
fI I1
R3 H R5 O R3 H R5 O
2p 21
p Y, p Y,
CF3C-HN, OR CF3C-HN OR
R3 H 5 O R3 H R5 O
R
22 23
-173-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Table 2
~ HN/=(
",.,.,.. CI~ N '
1 2 3 4
/ O
O~ H3
H3C ,~"~ ""."
6 7 8
/v F
H~ S~=--C F
g 10 11~ 12
F3C F
HN~ N~~' N=(O CI~
13 14 15 16
H3 ~ H3 ~ ~ F3
F ~ CI ,~~,. F3C H '~"",'
17 18 19 20
HN~ N NCO N~O
H3C
N=~, ~, N=-C"".",.,
21 22 23 24
F3 ~ F~ F3 ~ NHS
F ,~,.,., F3C .,.""- CI .,.~,., N~,
25 26 27 28
-174-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
-.l H F
Et ~~~~ Et Et
29 30 31 32
F3 ~ I F~CI Et, H3C
,N
C
H
F .",.~",F3C .",."", H ~"",.. .""..
33 34 35 3
36
H\ CH3 F\ HN~ CI,
H~,"".,..C I /~~~~ H~~..--~~~~~~
37 38 39 40
HO O
~ \ N-N
=
~
N N
-
41 42 43 44
HN~~ ~ N~N> ~N~
'
N -N N-N
45 46 47 48
~' N HN~~ HN~ ~ HN =N
N N
49 50 51 52
~ ' N ; ~ o '
H c .
N ~~~~~
N-N
53 54 55 56
S~ S --( N --t
S ~ N ~ S
57 58 59 60
-175-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
O N\ S Nw N-'(
S , ,
61 62 63 64
F~ Hi \
"""N, F ,~",.",. ,~""",. F ,,.,.,."..
H
, 66 67 68
65
F~F F~F ~ H3 H3C, CH3
H~~.-(~~ F~~-.(~~~~ H ~--(~
C
69 70 3 72
71
H3 ~ H ~ I CI"CI CI, CI
H ~~~~ H~~.--~(~~~~~CI~~-~(~~~~~
C ~
3 74 75 76
73
CI, ~ I ~ F3 Fa ~ Fs
C I ~~-.-~,~~~~~C I .""~~~ ,"~",.' .,.""~''
77 78 79 80
F3 ~ CI~ CI, CH3
C .~,...". F3C .~.~. H3C/ ,M,r . CI~~-."(~",."..
F
3 82 83 84
81
I ~ H3 F3 ~ F3 H3 ~ i
C .,.""". CI ,M,r,.. C ..,~,.",. H3C
H F
3 8s 3 88
s5 s7
CI, CH3 H3 ~ I H3 ~ H3 C~ H3
w. C~--,M,w.
CI H
",. 90 ,M, 3
89 91 92
-176-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
F F CH3 H3C CI
F~ H3C~ F ' Ci
93 94 95 96
CI, CI ~ H3 F~CH3
C~~-".",.,. F H3C .""~,.., H3C~~--...(,~...~.
H
3 98 99 100
97
H3 ~ F~CH3 H3 ~ F~F
C~~--
(~
H
.""",., F ...."",. "....
101 102 103 ...
3
104
H3 ~ F3 ~ Hs ~ Fs H3 ~ Hs
."""". C .~,~", C ."~,.",. C .,~,.",.
F H H F
C
3 3 3 3
105 106 107 108
H3 ~ F3 F3 ~ Hs ~ F3 F3 ~ H3
F
C
""". H3C ..~....,..",.,....
109 110 111 3
112
H3 H3 ~ F3 F3 ~ F3 CI~
.,... ~"",.. H F
,r F C
F C
C
. . 3 116
" 3 115
3 114
113
F~CI F~CI CI, F ~ I
F~~-."(.~."~.~-."(~"",.,. F ,"""....
117 118 119 120
Cl, F CI, CI F\ .F
CI ~ CI~~-(~ F~~ CI/u\~~~
121 122 123 124
7-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
CI, .F CI, CI Ci~ CI, ,CF3
F~~-,~(~~~~~ F3C~~--~(~~~~, F3C/ \w~~~~~r
125 126 127 128
F3 ~ I F3 ~ I Fs ~ I F3 ~ Fs
F3C ~ CI ~~~~~- .~..,~. CI ."
129 130 131 132
F3 ~ I CI"CF3 CI, CF3
CI F3C CI ~~--~~ F3C~)--..,.(~",.~.
133 134 735 136
F~CF3 F~~ F3 F~CF3 F~F
F ~-.~~ F3C~~-(~~~ F3 ~~-(~C
137 138 139 140
F3 ~ F3 ~ ~ F3 F3
F ~ ~ F F3C ..."....
141 142 143 144
F ~ F3 ~ Fs H3 ~ H3 ~ I
3C ,~~~~ F ~~~~~ CI ~~~~ F
145 146 147 148
CI, .F F\ ,CI F~CH3 CI, CH3
149 150 151 152
H~ ~ F3 H3C CI CI CF3 F3C CI
CI .~ F3 ~"",~",. H3C ~
153 154 155 156
-178-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
F3 ~ H3 CI/ \ H3 H3 ~ F3 H3
CI ~,~~~~ FsC '~'~~' F """"". FsC .",."",.
157 158 159 160
F~CF3 F~CF3 F3 ~ F3 ~ Hs
H3C~--"~~,.~~ H3C~~-.-,M,w. H3C .~,.,.~~ F "".",.''
161 162 163 164
F\ ,CHs CI, CF3 CI, F F' ,CFs
F3C/~.~(\~~,.~ F~~..(~~ ",~ F3C~~~_~(~~~~~ C
165 166 167 168
Table 3
-H 1 CHs ~ ~CH3 3
~~CH3 /~CH3 ~~CH3
1~/ 4 6
CHs 5
3
--~-CHs ~~ Hs
CHs ~ CHs 8
H C / CH CHs
,f,.,~CH3 ~-~-CHs
J 10 CHs Il CHs 12
,CHs ~~ CHs ~~ CHs
~ N N
,
'-CHs 13 CHs 14 ~CH3 15
-179-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Table 4a
.""". '"""' CH3 ."~".
H3C
~OH OH OH
CH3
1 2 3 4
w ~ H3C w
CHs ~~
HsC OH OH I OH OH
HsC CH
CHs H C~ CHs 3
3
6
CHs ~ ",.."
~OH OH HOH OH
H3C CHs C 3
9 10 11 12
" HsC CH3~ ~ \
H3C
OH ~OH H3C CH~H H C OH
3 CHs
13 14 15 16
CHs C
\~ H3C~ ~ H3C \ OCH3
I OCHs OCHs OCHs
CHs
17 18 19 20
CHs ~~~.
H C \ ~ HsC \ CH OCH
s OCH OCH 1 0 3 3
s HsC CH
CHs HsCJ CHs 3
21 22 23 24
-180-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
CH3
~OCH3 OCH3 CHOCH3 OCH3
H3C CH3 3
25 26 27 28
H C CH3~' ~ \ .,.,.",.
3
H3C~OCH3
~OCH OCH3 OCH3
3 H3C CH3 H3C CH3
29 30 31 32
OH ~ OH ~ ".",.'
HsC HsC
~OCH3
~OCH3 ~OH
~OH H3C CH HsC CH3
H3C CH3 H3C CH3 s
33 34 35 36
H3C.,
H3C ~~ ~~
H3C OH OH H3C _ -OH
OH
37 38 39 40
'OH - _OH
OH 'OH
H3C CH3
41 42 43
.,."". ~~~, H3C
H3~,~ ~
H3C OCH3 OCH3 H3C - -OCH3
OCH3
45 46 47 48
\ _ \
v 'OCH ~ - -OCH3
OCH3 H3C CH ~CH3
49 50 51 52
-181-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Table 4b
~OH ~OCH3 ~O~CH3 ~O~
OH OH OH OH CH3
1 2 3 4
~'OH ~'OCH3 ~'O~CH3 ~'O~
OH OH OH OH CH3
6 7 8
HO~OH HO~OCH3 HO~o~CH3 HO~O
OH OH OH CH3
9 10 11 12
HO' Y 'OH HO' 1"OCH3 HO~o'~CH3 HO~O
OH OH OH OH CH3
13 14 15 16
HO"Y 'OH HO~'OCH3 HO~'O~CH3 HO~'O
OH OH OH OH CH3
17 18 19 20
HO~'OH HO~'OCH3 HO~ O CH3 HO~'O
OH OH OH CH3
21 22 23 24
~O ~'O HO~O HO~O
OH ~ OH ~ OH ~ OH
25 26 27 28
-182-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
HO' Y ~O HO~ ~O ~OCH3 ~ ~OCH3
OH ~ OH ~ OCH3 OCH3
2g 30 31 32
CH30 OCH3 CH30"Y 'OCH3 CH30~ ~OCH3 CH30~ ~OCH3
OCH3 OCH3 OCH3 OCH3
33 34 35 36
H3C~OH H3C~OCH3 H3C~O~CH3 H3C O
H3C OH 3 OH OH H3C
37 38 39 OH CH3
40
CH3""".,.
H3C OH HsC- x _OCH3 H3C~0~CH3 H3C
~O
H3C OH H3C OH HsC OH CH3
41 42 43 44
H3C
H3C~OH H3C~OCH3 H3C~O~CH3
OH OH OH OH CH3
45 46 47 48
HsC OH H3C~OCH3 H3C~O~CH3 H3C~0
OH
OH OH OH CH3
49 50 51 52
~ ~ ~~ HsC Y
H3CY 'OH H3CY _OCH3 H C O~CH3
s ~
OH OH OH OH CH3
53 54 55 56
~ ~ ~~ i ~ HaC
~C~OH HsC Y _OCH3 H3C~0 CH3 O
OH OH ~H OH CH3
57 58 59 60
-183-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
H3C~'OH H3C~ ~OCH3 H3C~ ~O~CHs H3C~ 'O~
HsC OH HsC OH OH OH CHs
61 62 63 64
~~~~ CHI""""
HsC ~OH H3C~ ~OCH3 H3C~ ~O~CHs H3C .O
OH
H C OH HsC OH H3C OH CHs
3
65 66 67 68
H3C~OH H3C~OH H3C~OCH3 H3C~OCH3
[OH ~CH3 [OH ,~CH3 jOH ~CH3 'OH ''CH3
69 70 71 72
H3C~OH H3C~OH H3C---~-OCH3 H3C~OCH3
OH CHs OH ~CH3 OH CHs OH 'CHs
73 74 75 76
H3C~OH H3C~OH H3C~OCH3 H3C~OCH3
HsC~'(OH CHs HsC~OH ,'-CHs H3C OH CHs HsC~'(OH ~'-CHs
77 78 79 80
~~~~' CH~~~~ CHs H3C
H C~OH ~ OH ~OCH3 H3C~OCHs
IOH CHs ~'-CHs H3C~..~OH CHs ''OOH ~~CH3
HsC CHs
81 82 83 84
Table 4c
H3C
H3C/' CH3 ~ CH
OH NH2 OH s
CH3
4
-184-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
~-CH3 ~ CH3 ~CHa ~'CH3
CH3 CH3 CH3 CH3
6 7 8
CH3 ~ CH3
~.CH H3C ~ 'CH3 \ 'CH3 H3C~'CH3
3
CH3 CH3
9 10 11 12
CH3 CH3 ~~~,
~CH3 H3C I\ ~'CH H3C \ 'CH3
3 ~ CH3
HsC CHs HsC CHa
13 14 15 16
H3C 'CH3 H3C~'CH3 ~C~"'~3 'CH3
CH3 H3C CH3
17 18 19 20
CH3 """' CH3 "'."' CH3 "'""
'CH 'CH ''T' 'CH ~CH3
3 3 ,
H3C CH3 CH3 CH3 H3C CH3
21 22 23 24
CH3 '.."" H3C ..."."
i
H3C~'CH3 ~'CH3 .CH3 'CH3
OH OH OH OH
25 26 27 28
CH CH i HsC """,,
Y 'CH 1' 'CH ~~' ~' H
3 ~ 3 'r 'CHg ~ C 3
OH OH ~H OH
29 30 31 32
-185-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
.""". OH OH """' OH """'
~CH3 ~CH3 ~CH3
CH
3
OH OH HsC CH3
33 34 35 36
CH3 H3C _
H3C ~ ~
HsC CH3 CH3 ~CH3
CH3
37 38 39 40
CH3 ~ CH3 ~CH3
CH3 CH3
41 42 43 44
CH. 3 ; ~ CH3 ,~,.
CH3 HsC \ CH3 ~CH3 HsC~CH3
CH3 CH3
45 46 47 48
CH3 ~~~~ CH3 ~~~. \ ,w",,
CHs HaC " 'CH HsC \ CH
3 ~ s CH3
HaC CHs HsC CHs
49 50 51 52
H3C CH3 H3C~CH3 CH3 CH3
CH3 H3C CH3
53 54 55 56
CH3 '~"" CH3 ""r' CH3 '"'~'
CH CH ''r 'CH ~CH3
3 3 ~ 3
H3C CH3 CH3 CH3 H3C CH3
57 58 59 60
-186-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
CH3 '~"' H3C \
H3C~CH3 ~CH3 CH CH3
OH OH
OH OH
61 62 63 64
CH i CH HaC
Y 'CH3 ~CH3 ~ CH
, ~ Y 'CH3 ~ s
OH OH ~H OH
6~ 66 67 68
OH OH OH
Y 'CH ~CH3 ~CH3
CH ,
H3C CH3
OH OH
69 70 71 72
CH, 3 ; HaC
H3C~ . ~CHg HsCwi\~CH3 ~ . ~CH3 . ~CH3
73 74 75 76
~'~CH3 ~. CH3 ~'~CH3 ~.~CH3
CH3
77 78 79 80
CH3 ~~~, i CH3 .~"..
~~ JCH3 ~3~~.~CH3 \ ,~CH3 H3C~.~CH3
CH3
CH3
81 82 83 84
CIH3 I CH3.~..~. \
~. ~CH3 H3C~ _ ~CH3 H C ~ . ~CH3 ~ CH
3 ~ . ,/ 3
HsC CHs HaC CHs
8~ 86 87 88
-187-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
H3C , ~CH3 H3C , ~CH3 , ~CH3 . ~CH3
CH3 H3C CH3
89 90 91 92
CH3 ',."" CH3 i CH3 .,..."..
~ ~CH3 , ~CH3 ~ , ~CH3 ~ , ~CH3
,
H3C CH3 CH3 CH3 H3C CH3
93 94 95 96
CIH3 '~"" H3~ _ \
H3C~.~CH3 ~.~CH3 ~,~GH3 ,~CH3
OOH OH OH OH
97 98 99 100
CH CH H3G \~ ~
~.~CH3 ~.,~CH3 ~..~GH3 ~.~CH3
, , , ,
OH OH OH OH
101 102 103 104
,~~~- OH i OH OH
CH ~/' ~ .~CH3 ~. ~CH3 ~. ~~CH3
~.i
OH OH H3C CH3
105 106 107 108
CH3 HsG
H3C~CH3 HsC~CH3 ~CH3 ~ GH3
109 110 111 112
C H3
C
C~
113 114 115 116
-188-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
C' 3 CH3 ~CH3
~1 .,\ H3C
CH3 CHs
117 118 119 120
w,n. ~~H3 i '''H3 .rwr \ w.w
CHs HsC ~ ~ CHs H C~CH3 CHs
3
H3C CHs HsC CHs
121 122 123 124
HsC CHs H3C I CHs CHs CHs
v
CHs HsC CHs
12~ 12b 127 128
CHs """" CHs j CHs """"
CHs ~CH3 ~CH3 ~ /CHs
H3C CHs CHs CHs H3C CHs
129 130 131 132
CHs j HsC _ \ '"""
H3C~CH3 ~CH3 ~CN3 CHs
TOH - OH ~OH ~ OH
133 134 135 136
CHs i HsCl I \1 I
H3C.~CH3 ~~CH3 ~CH3 1~CH3
OH OH OH OH
137 138 139 140
i OH ~ OH """" OH '""
CHs ~CH3 CHs CHs
OH HsC CHs
141 142 143 144
-189-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
'~""' CHs CHs CH3 ; CHs HsC1 .M... CHs
H3C'l . J H3~J~ . J . J
14~ 146 147 148
CHs \ _ CH '.~"' CHs
J. J ~. ~H3 J..J 3 . J
J J
CHs
149 150 150A 150B
CHs CH CHs CHs ~ CH '~ Hs
J.J s H3C~.J \ .J s HsCJ
CHs
CHs
1~1 1S2 153 154
"'~'.~ Hs HsC C~. CHs CHs ~~ Hs \ CH
H3C~ ~'~ 3
H3C '~(C~~H3 H3C CHs
1>j 156 157 158
HsC "..""~ Hs HsC ...".. J Hs .,."",~ Hs .~.,..~ Hs
CHs HsC CHs
1~9 160 161 162
CHs '".."~ Hs CHs '~"'~ Hs CH, 3 ' j ~ Hs ~ .",."~ Hs
~'Y~.~
H3C CHs CHs CHs H3C CHs
163 164 165 166
' ~ Hs lHs .~.",.~ Hs HsC .~"".~ Hs \ .",~,.~ Hs
H3C
OH OH OH OH
167 168 169 170
-190-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
CH3 j 'J Hs CH3 j 'J Hs H3C~'~ H3 ~'w""~ H3
OH OH OH OH
171 172 173 174
CH Oi H I ' ~ H3 OH ~~ H3 OH ~~ H3
~~ ~J
HC
OH OH 3 CHg
17S 176 177 178
H C~CH3 i ,CH3 CH3 ; CH3 H3C~~CH
H3C'v~vJ [\
3
179 180 181 182
~CH3 ~ ~ CH ~CH3 ~ CH3
\~
CH3
183 184 18S 186
CH3 CH3 ~ ' CH3 CH3 ~ CH3 CH3
HsC ~ \ HsC l
CH3
CH3
187 188 189 190
CH3 CHs CH3 CH3 CH3 \ .",..,.
~~ 1 J CH3
H3C~ H3C
CH3
H3C HsC CH3
191 192 193 194
CH3 CH3 CH3 CH3
H3C H3C
CH3 H3C CH3
19S 196 197 198
-191-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
CHs ,."" CHs CHs """" CHs CH3 "j ' ,CHs ~ CHs
H3C CHs CHs CHs H3C CHs
199 200 201 202
CHs CHs i CHs HsC CHs \ CHs
H3C
~OH ~ OH OH OH
203 204 205 206
CH~~CHs lHs i cHs H3C1 ; CH3 \ CHs
~~J~~~
OH OH OH OH
207 208 209 210
i CHs lH j CHs OH CHs OH "."" CHs
OH OH HsC CHs
211 2x2 213 214
CHs ~ HsC
H3~~ J H3~~ . J ~.J~ . J ~ ,
J
215 216 217 218
UJ ~:, ~ ~. J ~.J
J
CHs
219 220 221 222
cue:. J H3~-,.~. J ~~ . J H3~ \ ~J
CHs CHs
223 224 225 226
-192-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
CH3 ~ CH3 '....." \ ,......,
. J H3~~.~=. J H3~ ~ . ~ . J
HsC CHs HsC CHs
227 228 229 230
H3C ~.J H~C ~.J ~.J . J
CH3 "~C CH3
231 232 233 234
CH3 ~ J l 3 ~~ CHI, J ~ ~:J
H3C CH3 CH3 CH3 H3C CH3
235 236 237 238
H C ~:~ ~3 ~:.J "3c ~:~ \ ~:J
3
OH OH O" OH
239 240 241 242
CHI, J ~~,~ "3~ ~:J ~.J
OH OH ~H OH
243 244 245 246
O~,J OH~~ °"~:J
OH OH "3C CH3
247 248 249 250
HsC
H3C~ ~ C~ "3C
251 252 253 254
-193-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
\ ,w.".
\~
CH3
255 256 257 258
CH3 ; " """'' ~ CH3 ,~~~.
HaC'~ w H3C 1
CH3 CH3
259 260 261 262
CH3 ~ ~ CH3 ,~~~, ~ \
H3C~~ H3C \
HsC CHa HsC CHs
263 264 265 266
H3C ~ ~ H3C
i
CH3 H3C CH3
267 268 269 270
CH3 '""" ( ~H3 '~"~' ~ CH
H3C CH3 CH3 CH3 H3C CH3
271 272 273 274
~H3 ~ ~ H3C
H3C
OH OH OH OH
275 276 277 278
CH3 '~"' , i H~ H3C ~ \
OH OH pH OH
279 280 281 282
-194-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
-".". OH ; ii OH ( OH ".
~I~IJ
OH OH H3C CH3
283 284 285 286
H CH3 H CJ H3CJ
3
287 288 289 290
HsC HsC
CH3 H3C
291 292
Table 4d
1 2 3 4
H3C CH3 H3C CH3 H3C CH3
6 7 8
H3C i
O O H3C' O H3C O
H3C
9 10 11 12
-195-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
H3C ~ H3C ~ H3C
H3C O ~ O O
13 14 15 16
H3C
'O H3C .O H3C' .O H3C .O
17 18 19 20
H3C ~ H3C H3C
H3C .O ~ .O
21 22 23 24
~O ~O ~O ~O
w
25 26 27 28
H3C H3~
'O I ~O H3C ~O H3C ~O
29 30 31 32
H3C H3C H3C
H3C H3C HsC
33 34 35
-196-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Table 4e
I CH~ ~ H3C
H3C~ . OOH HsC~OH ~ , OOH OH
1 2 3 4
"""' \ ,.,.".,
~.~OH ~, ~.~OH ~.~OH
OOH
CH3
6 7 8
CH3 ""~' CH3 "",...
OOH H3C~ . OOH \ , OOH H3C \ ~. OOH
CH3 CH3
9 10 11 12
CH3 CH3 ~ \
~..,iOH H C~ . OOH H C ~ , OOH ~~OH
3 3
HsC CHs HsC CHs
13 14 15 16
H3C . OOH H3C . OOH , OOH , OOH
CH3 H3C CH3
17 18 19 20
CH3 """' CH3 '~"' C'H3 I
.,OOH ,OOH ~,~OH ~~OH
H3C CH3 CH3 CH3 H3C CH3
21 22 23 24
-197-
CA 02329660 2000-10-20
WO 99/54290 PC'fNS99/07949
....." CHs .~",, H3C '.. "."",
H3C~ . OOH ~ , OOH ~~OH , OOH
1OH OH OH OH
25 26 27 28
CH CH HsC
~.,~OH ~.~OH ~~pH .OOH
OH OH pH OH
29 30 31 32
OH OH OH
OH ~~' . .iOH ~. OOH ~. OOH
OH OH HsC CHs
33 34 35 36
CHs H3C
H3C~OH HaC~OH ~OH OH
37 38 39 40
",M,
~OH OH ~OH OH
CHs
41 42 43 44
CHs "~"~- CHs .~,..
~OH H3C~OH W OH HsC ~ ~ OH
CHs CHs
45 46 47 48
'~""' CHs CHs ~~~~
OH H3C~OH HsC ~ OH OH
HsC CHs HsC CHs
49 50 51 52
-198-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
HsC OH H3C OH OH OH
CHs HsC CHs
53 54 55 56
CHs ,M". OH CHs ".",.. OH CH., i OH ~ OH
H3C CHs CHs CHs H3C CHs
57 58 59 60
"""' CHs """' HsC .~..". \
H3C~OH ~OH OH OH
OOH - 'O~H '' OH OH
61 62 63 64
CHs H3C
H3C~.,~OH ~OH OH ~OH
OH OH OH OH
65 66 67 68
i ~ OH i OIH "'"I" OH
\ I OH ~OH ~OH ~OH
'OH _ HsC CCH ~'3
69 70 71 72
OH ",.."" OH ".""
HO~OH ~ HgCO~OCH3
69 70 71 72
-199-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Table 4f
'~"" H3C
H3C' '-CHs 1
OH NH2 OH ~.~-CHs
CHs
1 2 3 4
'""" ~ CHs
~-CH3 ~CH3 ~CH3
CHs CHHs CHs HsC CHs
3
6 7 8
CHs CHs ~ '"""'
~'CH HsC \ '' Hs ~~ -CHs HsC W -CHs
s I -CH ~CH
CHs C~3 CH s CHs 3
3
9 10 11 12
CHs ~ CHs
~CH3 H3C~ CHs HsC ~ -CHs -CHs
CHs CH ~CH3
HsC s CHs H3C CHCHs
13 14 IS 16
H3C -CHs HsC -CHs CHs CHs
CHs H C CHs CHs CHs
CHs s CHs
17 18 19 20
CHs ,M". CHs '"""' CHs """'
_CHs _CH3 ~ CHs ~ -CHs
~CH3 ~CH3 ~ CHs ~CH3
H3C CHs CHs CHs H3C CHs
21 22 23 24
-200-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
CHs '~"' HsC .,...". \ ~~~,
H3C~CHH3 l I CHHs -CHs -CHs
OH s OH s ~ CHs
OH CHs OH
25 26 27 28
CH '"'"' CH HsC \
CHs ~'CH3 ~CH ~ CHs
CHs CHs ~ s CH
OH OH OH CHs pH s
29 30 31 32
~CH ~_CHs 'CHs ,,."",CHs
OH OH """'~' OH
s ~ CH ~CH CHs
CHs OH s OH s HsC CHs
33 34 3~ 36
CHs CHs CHs '.."" CH H3C
HsC 3 CH3
"3~ 1
CHs H3C CHs CH3
37 38 39 40
CHs \ CH \ ~ CHs ~~ CHs
H
CH3 CHs CHs C s
CHs
41 42 43 44
~ ~ CHs
CW ~~CH3 H3C~.~CH3 CW ~J H C W-./CHs
NCH
CHs s CHs CHs CHs CHs
45 46 47 48
CHs ~ CHs CH3 ~ CHs \ .,
~CH3 H3C~J HsC ~ -~ - ~CH3
s CH s
HsC ~CH3 CH 3 CHs H3C CHs CH
49 50 51 52
-201-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
H C CHs H C CHs CHs CHs
3 3
H3C
CHsCHs CH3CHs CHs CHs
53 54 55 56
CHs """' JCHs CHs ~ ~ Hs CH~CH3 I ~ ~ Hs
CHs ~-CHs , CHs ~~-CHs
H3C CHs CHs CHs H3C CHs
57 58 59 60
_ CH3 CHs ~ ' ~ Hs H3C~ CH ~ CH
H3C~CH 1 '-CH ! '-CH CHs
OH 3 OH 3 OH 3 OH
61 6? 63 64
CHs "'.".'~ Hs IH~~CH3 HsC CHs \~~ s
CH
J
CH CHs CHs , CHs
OH s OH OH OH
65 66 67 68
/~ OH j OH "'"~" OH '~"~' CHs
i ~ ~ ! ~CH3 ~.,/CH3 ~-iCH3
~CH3 OH CHs OH CHs H3C CHs CHs
69 70 71 72
H3C "."". H3C H3C
H3C
73 74 75 76
HsC
\ ~ / HsC
77 78 79 80
-202-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
H3C .~",.. H3C H3C
H3C
81 82 83 84
HsC HsC HsC HsC
8J $6 87 88
HsC HsC HsC HsC
89 90 91 92
CH3 ~~,- H3C
H C CH3 H3C CH3 CH3 CH3
3
~'CH3 CH3 CH3 CH
3
93 94 95 96
I CH3 ~ \ CH3 CH3 CH3
i \
CH3
CH3 CH3 CH3
CH3
97 98 99 100
CH3 ~ H3C
\ H3C \ \
H3C
101 102 103 104
I ~ ~ i I ~ I
i ~ I ~H3-Il
los l06 l07 los
-203-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
CH3
HO~_CH3 HO CH3 HO HO
CH
3 CH3
CH3
109 110 111 112
CH3
HO -CHs HO~CH3 HO HO
CH3 'CH3
CH3
113 114 IIS 116
OH ~ OH CH3 ,~."' OH H3C
H3C~ OH
OH
HO OH OH
117 118 119 120
~~~~ .,.",.. H
OH H3C OH CH3 OH 3C OH
H3C
'-OH ~OH OH '-OH
121 122 I23 124
Table 4g
.,....~' CH 3 .,~,.
H3C~ CH3 H 3C ~-CH 3 ~-CH 3 ~..~-CH3
OH OH OH OH
1 2 3 4
-204-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
H3C' ' CHs H C~_ J Hs CH~_ ./ Hs ~ ~ Hs
3 V'
OH OH OH OH
5 6 7 8
~_ I CHs H3C~_~CH3 CH~CH3 ~ ~CH3
H3C~ ~''~'~~/ ~
H OH O
OH H
9 10 11 12
H3C' ' _ ~ H3C~_ ~ CH~_
OH OH pH OH
13 14 15 16
'~~"' CHs _
HsC'' CHs H3C~-CH3 ~-CHs ~-CHs
OCHs OCHs OCHs OCHs
17 18 19 20
CHs I CHs CHs CHs I CHs
H3C/'-_ / HsC~_ J ~_ J ~_ J
'''' ~'
OCH3 OCHs OCHs OCHs
21 22 23 24
H3C'' ~CH3 H C~_.~CH3 CH~CH3 ~_~CHs
3 ''/ ~
OCHs OCHs OCH OCHs
3
25 26 27 28
~ ' ~ CHs
HsC' ' H3C~_ ~ _
OCHs OCHs OCHs OCHs
29 30 31 32
'~"' CHs
/ HsC~-OH ~-OH ~-OH
OH
' CHs CHs CHs
H3C
CHs
33 34 35 36
-205-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
"'~" CHs "~". '"""'
H3C~H HsC~H -OH ~H
~CH3 CHs ~CH CHs
3
37 38 39 40
CHs
HsC ~-OH HsC ~-OH -OH I ~ OH
CHs CHs CHs CHs
41 42 43 44
'~"" CHs .M".
HsC -OH HsC -OH -OH -OH
45 46 47 48
CHs
H3C' ' OCHs HsC.,,.~-OCH3 ~-OCHs ~-OCHs
CHs CHs CHs CHs
49 50 51 52
~ ~ CHs
H3C~CH3 H3C~CH3 -OCHs ~CH3
~--CH3 CHs ~CH CHs
3
53 54 55 56
'~~" CHs ~~~,
H C -OCHs HsC -OCHs -OCHs -OCHs
3
CHs ~ CHs CHs CHs
57 58 59 60
.~.. CHs ."~",
H C ~OCH3 HsC -OCHs -OCHs ~ ~'OCH3
3
61 62 63 64
-206-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
'M" CHs '~"" ~ ,M".
-CH3 ~-CH3 '~ -CH3 ~-CH
OH %' \ OH
~~OH
OH
65 66 67 68
- CH3 '~~" ~ H3 CH3 ~~~, ~ H3 '\ "".". ~ H3
OH OH OH OH
69 70 71 72
_ ! CH3 ~~CH3 CH3 .,.""-~CH3
OH ~ OH ~ pH OH
73 74 75 7s
_ ~ ~ CH3 ~ ~ \ .,.
OH ~ OH OH OH
77 78 79 80
CH3 ~ \
_CHs y_CHs _CHs _CHa
OCH3 ~7~\ 'OCH3 LOCH
OCH3
81 82 83 84
- CH3 .,."" ~ H3 CH3 ,~~~ ~ H3 \ ."",.. ~ H3
OCH3 ~ OCH3 OCH3 OCH3
85 86 87 88
_ I CH3 ~ ~CH3 CH3 ~ r-CH3 \ ,-CH3
J i
OCH3 OCH3 OCH3 OCH3
89 90 91 92
.,."" _ CH3 .~",., _ ~..,
_ ~ _~ _
OCH3 ' ~ OCH3 1 OCH3 OCH3
-207-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
93 94 95 96
CH3 ""~' \
-OH ( -OH -OH ~OH
CH3 ~CH3 CH3 CH3
97 98 99 100
CH3 "~.", \
~-OH ~ I -OH -OH ~-OH
CH3 '~-CH3 CH3 CH3
101 102 103 104
CH3 ,~",. \
-OH ~ I -OH -OH ~-OH
CH3 CH3 CH3 CH3
105 106 107 108
CH3 ,",.", ~\ "".",
-OH -OH -OH -OH
109 110 111 112
CH3 '""" \
~-OCfv3 I -OCH3 -OCH3 ~-OCH3
CH3 ~CH3 CH3 CH3
113 114 115 116
CH3 ,...",. \ ""H.
-OCH3 I -OCH3 -OCH3 -OCH3
CH3 ~-CH3 CH3 CH3
117 118 119 120
CH3 ..",... \
-OCH3 ~ -OCH3 -OCH3 -OCH3
i
CH3 CH3 CH3 CH3
-208-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
121 122 123 124
CHs ,,..",. \ .".,.....
-OCHs I -OCHs -OCHs -OCHs
\ I '\~ \
125 126 127 128
CHs ~ , \ .""".
HsC -OH -OH -OH -OH
OH OH OH OH
129 130 131 132
CHs ~ I \
HsC -OCH3 -OCHs -OCHs -OCHs
OH OH OH OH
133 134 135 136
CHs ~ I \
HsC -OCHs ~ -OCH3 -OCHs ~-OCH
3
CH ~ CH3 OCHs OCH
137 138 139 140
,~"" CHs ~ \ ."
H3C~ H ~~ H O~H -OH
OH OH OH
141 142 143
CHs I ~ \
3
H3C~OH3 l~~-CHs OOHS ~ OCH
OH
145 146 147 148
,,."". CHs I I ,"...", \ .,d.,..
HsC -OCHs l -OCHs CHs -OCHs
~OCH ~- OCHs
3 OCHs OCHs
-209-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
149 150 151 152
-OH '~~ OH -OH -OH
OH ~~OH OH
OH
153 154 155 156
-OCH3 '~~ CH3 -OCH3 -OCH3
~-OH OH OH OOH
157 158 159 160
~ I
-OCH3 \ ~-OCH3
H3C~CHg ~' ~OCH3
N ~~
~
OCH3 OCH3 OCH3 OCH
CH 3
3
161 162 163 164
I CH3 ~I ~ \
HsC~OH ~OH ~OH ~
OH
OH OH OH O
H
165 166 167 168
CH3
HsC~OCH ~-OCH ~OCH3
s 3 OCH3
OH OH OH O
H
169 170 171 172
CH3 I II I \
HaC~OCH ~ ~OCH """,.
~OCH ~
~
~
3 . 3 OCH3
3
OCH OCH3 OCH3
OCH3
173 174 175 176
CH3 ~~~, ( \
H3C~OH ~ ~OH ~
~OH
, ...OH
-OH ~'OH '-OH ~~--OH
-210-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
177 178 179 180
CH3 I I
H3C~-OCH3 ~,~-OCH3 ~OCH3 , OCH3
OH ~-OH OH ,--OH
181 182 183 184
CH3 I I ~ ","",
H3C~OCH3 ~~-OCH3 ~OCH3 ~ ~
~OCH3
OCH 'OCH3 OCH3 ~-OCH
3 3
185 186 187 188
OH OH OH ~pH
~~-OH ~'-OH ~'--OH
'-OH
189 190 191 192
OCH3 OCH3 OCH3 ~ ~
~OCH3
'~OH ~'-OH ~-OH
'-OH
193 194 195 196
HOC pCH3 OCH3 ~ OCH3
~OCH3
~'-OCH ~'-OCH ~-OCH
CH 3 3 s ~-OCH3
3
197 198 199 200
".,.". ,~"", .,..",.
-OH -OCH3 -OH -OCH3
201 202 203 204
-OH OH -OCH3 OCH3
205 206 207 208
-211-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
-OH OH -OCH3 OCH3
209 210 211 212
~OH I OCH3 O - ~-O~
CH3 CH3
213 214 215 216
OCH3 O~CH3 OCH3 O CHs
217 218 219 220
-CH3 r~ 1 , CH3 ,
O ~O CH3 O O CH3
221 ~/ 222 223 224
Table 5
NH2
NH2 OH
1 2 3
-212-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
The ability of the compounds of the invention to inhibit neuraminidase in
vitro can be determined according to the method described below.
Neuraminidase Inhibition Assav:
Influenza virus A/N1/PRIB/34 was grown in the allantoic cavity of fertilized
eggs and purified by sucrose density gradient centrifugation (Layer, W. G.
(1969)
in "Fundamental Techniques in Virology" (K. Habel and N. P. Salzman, eds.) pp.
92-86, Academic Press, New York). Influenza virus A/N2/Tokyo/3/67 was
obtained from the tissue culture supernatents of virus grown on MDCK cells.
Neuraminidase from B/Memphis/3/89 virus was prepared by digestion of the virus
with TPCK-trypsin followed by centrifugation and then purification of the
neuraminidase catalytic fragment using sucrose density gradient centrifugation
and dialysis as described previously (Air, G. M., Layer, W. G., Luo, M.,
Stray,
S. J., Legrone, G., and Webster, R. G. (1990) Virolo 177, 578-587).
The neuraminidase inhibition assays used the neuraminidase enzymatic
activity associated with the A/N11PR/8/34 or A/N2ITokyo/3167 whole virus, or
the
B/Memphisl3/89 catalytic head fragment. The whole virus or catalytic fragment
was diluted appropriately with 20 mM N-ethylmorpholine, 10 mM calcium choride,
pH 7.5 buffer on the day of the experiment. Neuraminidase inhibition assays
were conducted in 20 mM N-ethylmorpholine, 10 mM calcium choride, pH 7.5
buffer with 5% DMSO. Reaction mixtures included neuraminidase, inhibitor (test
compound) and 20-30,uM 4-methylumbeiliferyl sialic acid substrate in a total
volume of 200,uL and were contained in white 96-well U-shaped plates.
Typically,
five to eight concentrations of inhibitor were used for each Ki value
measurement.
The reactions were initiated by the addition of enzyme and allowed to proceed
for
30-60 minutes at room temperature. The fluorescence for each well of the plate
was measured once each minute during the reaction period by a Fluoroskan Il
plate reader (ICN Biomedical) equipped with excitation and emission filters of
355
+/- 35 nm and 460 +/- 25 nm, respectively. The plate reader was under the
control of DeItaSoft II software {Biometallics) and a Macintosh computer. If
the
-213-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
compound exhibited linear reaction velocities during the reaction period, then
ttie
reaction velocities for the dose-response study were fit to equation 1 using a
nonlinear regression program (Kaleidagraph) to determine the overall Ki value
(Segel, I. H. (1975) in Enzyme Kinetics, pp. 105-106, Wiley-Interscience, New
York).
(1 - Vi/Vo) _ [I]/ {[I] + Ki(1 + [S]/Km)} eqn 1
In equation 1, Vi and Vo represent inhibited and uninhibited reaction
velocities,
respectively, and Km = 16 - 40,uM depending on the neuraminidase strain
tested. For those compounds exhibiting slow-binding inhibition (Morrison, J.
F.
{1982) Trends Biochem. Sci. 7, 102-105), a second experiment was performed
in a manner identical to the first except that neuraminidase and inhibitor
were
preincubated in the absence of substrate for 2 hours at room temperature prior
to
initiating the reactions with substrate. Data analysis for the resulting
linear
velocities was conducted as described above.
Equation 2 was used to measure Ki values in the sub-nanomofar range
(Morrison; J. F. And Stone, S. R. (1985) Comments Mol. Cell Biophys. 2, 347-
368).
V = A{sqrt{(Ki' + It -Et)~2 + 4Ki'Et} - (Ki' + It - Et)] eqn. 2
In equation 2, V = velocity; A = akcat[S]I2(Km + [S]); a is a factor to
convert
fluorescence units to molar concentrations; Ki' = Ki(1 + [S]IKm); It = total
inhibitor concentration and Et = total active concentration. of neuraminidase.
The compounds of the invention inhibit influenza A neuraminidase and
influenza B neuraminidase with Ki values between about 24 micromolar and
about 0.9 micromolar.
-214-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
The ability of the compounds of the invention to inhibit plaque formation in
cell culture can be determined by the method described below.
Cell Culture Pla4ue Formation Inhibition Assay
Cell Cultures: MDCK cells obtained from the American Type Culture Collection
were grown in Dulbecco's Modified Eagle Medium (DMEM) high glucose
(GibcoBRL) supplemented with 10% fetal calf serum (JRH Biosciences), 40 mM
HEPES buffer (GibcoBRL) and antibiotics {GibcoBRL). Cells were routinely
cultured in flasks or roller bottles at 37°C and 5% C02. At confluence
cells were
reduced to a density of 500,000 cells in a ml using trypsin/EDTA (GibcoBRL)
treatment of the monolayer followed by cell centrifugation, resuspension, and
dilution into growth media. Cells were planted at a volume to surface area
ratio of
1 ml over 1 cm2 of growth surface.
Plaque Assay Protocol: On MDCK cell confluent 6 well plates growth media
was removed and the cells were overlaid with 1.5 ml of assay media (DMEM with
1 % fetal calf serum, 40 mM HEPES buffer and antibiotics) containing pre-mixed
virus (influenza A/Tokyo/3167 [H2N2]) (40 -100 plaque forming units) and 2x
concentration test compound. The plates were placed on a rocker and incubated
for 2 hours at room temperature. During the virus adsorption period agar
overlay
media was prepared. In a microwave oven 2X agarose (final concentration of
0.6% agarose) in overlay media (DMEM with 40 mM HEPES buffer) was melted
and then placed in a 48°C water bath for temperature equilibration.
After the
virus adsorption period was completed 1.5 ml agar over media was added and
mixed with the 1.5 ml virus and test compound containing media per well.
Cultures were incubated at 35°C for the period required for plaque
development, usually several days. Plaques were fixed with 3.7% formaiin in
PBS for 20 minutes followed by removal of the agar overlay and staining with
0.1 % crystal violet in distilled water for 15 minutes. Plaques were counted
and
-215-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
EC 50 concentration determined from multiple concentrations of the tested
compound using regression analysis.
Viral Stocks: Stocks were prepared in MDCK confluent roller bottles incubated
at 37°C in DMEM supplemented with 1 % FCS, 40mM HEPES buffer, and
antibiotics. Bottles were inoculated with a multiplicity of infection of
approximately
0.1 plaque forming unit for each cell. Roller bottles were harvested after the
cytopathic effect of the virus was observed to be complete. Stocks were
prepared from the supernatant resulting from the low speed centrifugation of
the
media and cell iysate. Stocks were titered and stored at -80°C.
The compounds of the invention can be tested for in vivo antiviral activity
using the method described below.
In Vivo Antiviral Efficac rLMethod
Female BALB/c mice were placed under anesthesia {sevoflurane) and
inoculated intranasally (IN) with 0.1 ml of influenza A VR-95 (Puerto Rico PR8-
34) at 10-2 (diluted from frozen stock). This viral concentration consistently
produced disease in mice within 5 days of inoculation. Animals were treated
4h.
pre-infection and 4h. post-infection, and periodically thereafter, with one of
the
following therapies: no treatment; test compound (100, 25, 6.25, 1.39
mg/kg/day
BID, PO); or vehicle (sterile water BID, PO). A group of ten animals
(designated
as control) was inoculated with 0.9% saline. Percent survival was determined.
On day five, lungs were harvested, weighed and assigned scores of 0,1, 2, 3 or
4
based on percentage consolidation (0; 10-20; 25-50; 50-75; 75-100%,
respectively). In addition, each lung pair was image analyzed to determine
objective lung consolidation percentages.
The compounds of the present invention can be used in the form of salts
derived from inorganic or organic acids. These salts include but are not
limited to
the following: acetate, adipate, alginate, citrate, aspartate, benzoate,
-216-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
benzenesulfonate, bisulfate, butyrate, camphorate, camphorsulfonate,
digluconate, cyclopentanepropionate, dodecylsulfate, ethanesulfonate,
glucoheptanoate, glycerophosphate, hemisulfate, heptanoate, hexanoate,
fumarate, hydrochloride, hydrobromide, hydroiodide, 2-hydroxy-ethanesulfonate
(isethionate), lactate, maleate, methanesulfonate, nicotinate, 2-
naphthalenesulfonate, oxalate, pamoate, pectinate, persulfate, 3-
phenylpropionate, picrate, pivalate, propionate, succinate, tartrate,
thiocyanate, p-
toluenesulfonate and undecanoate. Also, basic nitrogen-containing groups can
be quaternized with such agents as lower alkyl halides, such as methyl, ethyl,
propyl, and butyl chloride, bromides, and iodides; dialkyl sulfates like
dimethyl,
diethyl, dibutyl, and diamyl sulfates, long chain halides such as decyl,
lauryl,
myristyl and stearyl chlorides, bromides and iodides, aralkyl halides like
benzyi
and phenethyl bromides, and others. Water or oil-soluble or dispersible
products
are thereby obtained.
Examples of acids which may be employed to form pharmaceutically
acceptable acid addition salts include such inorganic acids as hydrochloric
acid,
hydrobromic acid, sulfuric acid and phosphoric acid and such organic acids as
oxalic acid, malefic acid, succinic acid and citric acid. Other salts include
salts
with alkali metals or alkaline earth metals, such as sodium, potassium,
Lithium,
calcium or magnesium or with ammonium or N(R**)4+ salts (where R** is
loweralkyl).
In addition, salts of the compounds of this invention with one of the
naturally occurring amino acids are also contemplated.
Preferred salts of the compounds of the invention include hydrochloride,
methanesulfonate, suifonate, phosphonate and isethionate.
The compounds of the Formula I, II, III, IV, V, VI, VII, VIII or IX of this
invention can have a substituent which is an acid group (for example, -COZH,
-217-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
-S03H, -S02H, -P03H2, -P02H). Compounds of the Formula I, II, III, IV, V, VI,
VII,
VIII or IX of this invention having a substituent which is an ester of such an
acidic
group are also encompassed by this invention. Such esters may serve as
prodrugs. The prodrugs of this invention are metabolized in vivo to provide
the
above-mentioned acidic substituent of the parental compound of Formula I, II,
III,
IV, V, VI, VII, VIII or IX. Prodrugs may also serve to increase the solubility
of
these substances and/or absorption from the gastrointestinal tract. These
prodrugs may also serve to increase solubility for intravenous administration
of
the compounds. Prodrugs may also serve to increase the hydrophobicity of the
compounds. Prodrugs may also serve to increase the oral bioavailability of the
compounds by increasing absorption and/or decreasing first-pass metabolism.
Prodrugs may also serve to increase tissue penetration of the compounds,
thereby leading to increased activity in infected tissues andlor reduced rate
of
clearance.
Such esters contemplated by this invention include:
alkyl esters, especially loweralkyl esters, including, but not limited to,
ethyl,
n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, t-butyl, n-pentyl esters
and the
like;
alkoxyalkyl esters, especially, loweralkoxyloweralkyl esters, including, but
not limited to, methoxymethyl, 1-ethoxyethyl, 2-methoxyethyl,
isopropoxymethyl,
t-butoxymethyl esters and the like;
alkoxyalkoxyalkyl esters, especially, alkoxyalkoxy-substituted loweralkyl
esters, including, but not limited to, 2-methoxyethoxymethyl esters and the
like;
aryloxyalkyl esters, especially, aryloxy-substituted loweralkyl esters,
including, but not limited to, phenoxymethyl esters and the like, wherein the
aryl
group is unsubstituted or substituted as previously defined herein;
-218-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
haloalkoxyalkyl esters, especially, haloalkoxy-substituted loweralkyl esters,
including, but not limited to, 2,2,2-trichloroethoxymethyl esters and the
like;
alkoxycarbonylalkyl esters, especially, loweralkoxycarbonyl-substituted
loweralkyl esters, including, but not limited to, methoxycarbonylmethyl esters
and
the like;
cyanoalkyl esters, especially, cyano-substituted loweralkyl esters,
including, but not limited to, cyanomethyl, 2-cyanoethyl esters and the like;
thioalkoxymethyl esters, especially, lowerthioalkoxy-substituted methyl
esters, including, but not limited to, methylthiomethyl, ethylthiomethyl
esters and
the like;
alkylsulfonylalkyl esters, especially, loweralkylsulfonyl-substituted
ioweralkyl esters, including, but not limited to, 2-methanesulfonylethyl
esters and
the like;
arylsulfonylalkyl esters, especially, arylsulfonyl-substituted loweralkyl
esters, including, but not limited to, 2-benzenesulfonylethyl and 2-
toluenesulfonylethyl esters and the like;
acyloxyalkyl esters, especially, loweralkylacyloxy-substituted loweralkyl
esters, including, but not limited to, formyloxymethyl, acetoxymethyl,
pivaloyloxymethyl, acetoxyethyl, pivaioyloxyethyl esters and the like;
cycloalkylcarbonyloxyalkyl esters including, but not limited to,
cyclopentanecarbonyloxymethyl, cyclohexanecarbonyloxymethyl,
cyclopentanecarbonyloxyethyl, cyclohexanecarbonyloxyethyl esters and the like;
arylcarbonyloxyalkyl esters including, but not limited to, benzoyloxymethyl
esters and the like;
-219-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
(alkoxycarbonyloxy)alkyl esters, especially, (loweralkoxycarbonyloxy)-
substituted loweralkyl esters, including, but not limited to,
methoxycarbonyloxymethyl, ethoxycarbonyloxymethyl, 1-
(methoxycarbonyloxy)ethyl, 2-(ethoxycarbonyloxy)ethyi esters and the like;
(cycloalkyloxycarbonyloxy)alkyl esters, especially,
(cycloalkyloxycarbonyloxy)-substituted loweralkyl esters, including, but not
limited
to, cyclohexyloxycarbonyloxymethyl, cyclopentyloxycarbonyloxyethyl,
cyclohexyloxycarbonyloxypropyl esters and the like;
oxodioxolenylmethyl esters including, but not limited to, (5-phenyl-2-oxo-
1,3-dioxolen-4-yl)methyl, [5-(4-methylphenyl)-2-oxo-1,3-dioxolen-4-yl]methyl,
[5-
(4-methoxyphenyl)-2-oxo-1,3-dioxolen-4-yl]methyl, [5-(4-fluorophenyl)-2-oxo-
1,3-
dioxolen-4-yl]methyl, [5-(4-chlorophenyl)-2-oxo-1,3-dioxolen-4-yl]methyl, (2-
oxo-
1,3-dioxolen-4-yl)methyl, (5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl, (5-ethyl-2-
oxo-1,3-dioxolen-4-yl)methyl, (5-propyl-2-oxo-1,3-dioxolen-4-yl)methyl, {5-
isopropyl-2-oxo-1,3-dioxolen-4-yl)methyl, (5-butyl-2-oxo-1,3-dioxolen-4.-
yl)methyl
esters and the like;
phthalidyl esters wherein the phenyl ring of the phthalidyl group is
unsubstituted or substituted as defined previously herein, including, but not
limited to, phthalidyi, dimethylphthalidyl, dimethoxyphthalidyl esters and the
like;
aryl esters including, but not limited to, phenyl, naphthyl, indanyl esters
and the like;
arylalkyl esters, especially, aryl-substitued loweralkyl esters, including,
but
not limited to, benzyl, phenethyl, 3-phenylpropyl, naphthylmethyl esters and
the
like, wherein the aryl part of the arylalkyl group is unsubstituted or
substituted as
previously defined herein;
-220-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
dialkylaminoalkyl esters, especially dialkylamino-substituted loweralkyl
esters, including, but not limited to, 2-{N,N-dimethylamino)ethyl, 2-(N,N-
diethylarnino)ethyl ester and the like
(heterocyclic)alkyl esters, especially, heterocyclic-substituted loweralkyl
esters wherein the heterocycle is a nitrogen-containing heterocycle,
including, but
not limited to, (heterocyclic)methyl esters and the like, wherein the
heterocyclic
part of the (heterocyclic)alkyl group is unsubstituted or substituted as
previously
defined herein; and
carboxyalkyl esters, especially, carboxy-substituted loweralkyl esters,
including, but not limited to carboxymethyl esters and the like;
and the like.
Preferred prodrug esters of acid-containing compounds of the Formula I, II,
III, IV, V, VI, VI1, VIII or IX are loweralkyl esters, including, but not
limited to, ethyl,
n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, t-butyl, n-pentyl esters
and benzyl
esters wherein the phenyl ring is unsubstituted or substituted as previously
defined herein.
Methods for the preparation of prodrug esters of compounds of the
Formula I, II, I11, IV, V, Vl, VI1, VIII or IX are well-known in the art and
include:
reacting the acid with the corresponding halide (for example, chloride or
acyl chloride) and a base (for example, triethylamine, DBU, N,N-
dimethylaminopyridine and the like) in an inert solvent (for example, DMF,
acetonitrile, N-methylpyrrolidone and the like);
reacting an activated derivative of the acid (for example, an acid chloride,
sulfonyl chloride, monochlorophosphonate and the like) with the corresponding
alcohol or alkoxide salt; and the like.
-221-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Other examples of prodrugs of the present invention include esters of
hydroxyl-substituted compounds of Formula I, II, III, IV, V, VI, VI1, VIII or
IX which
have been acylated with a blocked or unblocked amino acid residue, a phosphate
function, a hemisuccinate residue, an acyl residue of the formula
R'°°C(O)- or
R'°°C(S}- wherein R'°° is hydrogen, lower alkyl,
haloalkyl, alkoxy, thioalkoxy,
alkoxyalkyl, thioaikoxyalkyl or haloalkoxy, or an acyl residue of the formula
Ra-C(Rb)(Rd)-C(O)- or Ra-C(Rb)(Rd)-C(S)- wherein Rb and Rd are independently
selected from hydrogen or lower alkyl and Ra is -N(Re)(Rf), -ORe or -SRe
wherein
Re and Rf are independently selected from hydrogen, lower alkyl and haloaikyl,
or
an amino-acyl residue having the formula R'°'NH(CH2)2NHCH2C(O)- or
R'°'NH(CHZ)20CHZC(0}- wherein R'°' is hydrogen, lower
alkyl, (aryl)alkyl,
(cycloalkyl)alkyl, acyl, benzoyl or an a-amino acyl group. The amino acid
esters
of particular interest are of glycine and lysine; however, other amino acid
residues
can also be used, including any of the naturally occuring amino acids and also
including those wherein the amino acyl group is -
C(O)CH2NR'°zR'°3 wherein R'°2
and R'°3 are independently selected from hydrogen and lower alkyl, or
the group
-NR'°2 R'°3, where R'°2 and R'°3, taken together,
forms a nitrogen containing
heterocyclic ring.
Other prodrugs include a hydroxyl-substituted compound of Formula I, II,
III, IV, V, VI, VII, Vlll or IX wherein the hydroxyl group is functionalized
with a
substituent of the formula -CH(R'°4)OC(O)R'°5 or -
CH(R'°4)OC(S)R'o5 wherein
R'°5 is lower alkyl, haloalkyl, alkoxy, thioalkoxy or haloalkoxy and
R'°4 is
hydrogen, lower alkyl, haloalkyl, alkoxycarbonyl, aminocarbonyl,
alkylaminocarbonyl or dialkylaminocarbonyl. Such prodrugs can be prepared
according to the procedure of Schreiber (Tetrahedron Lett. 1983, 24, 2363) by
ozonolysis of the corresponding rnethallyl ether in methanol followed by
treatment
with acetic anhydride.
-222-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
The preparation of esters of hydroxyl-substituted compounds of formula
Formula I, II, III, IV, V, VI, VII, Vlli or IX is carried out by reacting a
hydroxyl-substituted compound of formula Formula I, II, III, IV, V, VI, VII,
VIII or
IX, with an activated amino acyl, phosphoryl, hemisuccinyl or acyl derivative.
Prodrugs of hydroxyl-substituted-compounds of the invention can also be
prepared by alkylation of the hydroxyl substituted compound of formula Formula
I,
II, III, IV, V, VI, VI1, VIII or IX, with (halo)alkyl esters,
transacetalization with
bis-(alkanoyl)acetals or condensation of the hydroxyl group with an activated
aldehyde followed by acylation of the intermediate hemiacetal.
In preparing prodrugs it often is necessary to protect other reactive
functional groups, in order to prevent unwanted side reactions. After
protection of
the reactive groups the desired group can be functionalized. The resulting
functionalized product is then deprotected, to remove the protecting groups
that
were added to prevent unwanted side reactions. This will provide the desired
prodrug. Suitable reaction conditions for preparing protecting groups are well
known in the art. One source for reaction conditions is found in T.H. Greene
and
P.G.M. Wuts, Protective Groups in Or acLnic Synthesis, 2nd edition, John Wiley
&
Sons, New York (1991 ).
This invention also encompasses compounds of the Formula I, II, III, IV, V,
VI, VII, VIII or IX which are esters or prodrugs and which are also salts. For
example, a compound of the invention can be an ester of a carboxylic acid and
also an acid addition salt of an amine or nitrogen-containing substituent in
the
same compound.
The compounds of the present invention are useful for inhibiting
neuraminidase from disease-causing microorganisms which comprise a
neuraminidase. The compounds of the invention are useful (in humans, other
-223-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
mammals and fowl) for treating or preventing diseases caused by
microorganisms which comprise a neuraminidase
The compounds of the present invention are useful for inhibiting influenza
A virus neuraminidase and influenza B virus neuraminidase, in vitro or in vivo
(especially in mammals and, in particular, in humans). The compounds of the
present invention are also useful for the inhibition of influenza viruses,
orthomyxoviruses, and paramyxoviruses in vivo, especially the inhibition of
influenza A viruses and influenza B viruses in humans and other mammals. The
compounds of the present invention are also useful for the treatment of
infections
caused by influenza viruses, orthomyxoviruses, and paramyxoviruses in vivo,
especially the human diseases caused by influenza A and influenza B viruses.
The compounds of the present invention are also useful for the prophylaxis of
infections caused by influenza viruses, orthomyxoviruses, and paramyxoviruses
in vivo in humans and other mammals, especially the prophylaxis of influenza A
and influenza B viral infections; and, in particular, the prophylaxis of
influenza A
and influenza B viral infections in human subjects who are at high risk of
developing other respiratory diseases concurrent with or as a consequence of
influenza virus infections, or who suffer from chronic respiratory illness,
such as
asthma, emphysema, or cystic fibrosis.
Total daily dose administered to a human or other mammal host in single
or divided doses may be in amounts, for example, from 0.001 to 300 mg/kg body
weight daily and more usually 0.1 to 10 mg/kg body weight daily. Dosage unit
compositions may contain such amounts of submultiples thereof to make up the
daily dose.
The amount of active ingredient that may be combined with the carrier
materials to produce a single dosage form will vary depending upon the host
treated and the particular mode of administration.
-224-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
It will be understood, however, that the specific dose level for any
particular patient will depend upon a variety of factors including the
activity of the
specific compound employed, the age, body weight, general health, sex, diet,
time of administration, route of administration, rate of excretion, drug
combination, and the severity of the particular disease undergoing therapy.
Administration of a compound of this invention will begin before or at the
time of infection or after the appearance of established symptoms andlor the
confirmation of infection.
The compounds of the present invention may be administered orally,
parenterally, sublingually, intranasally, by intrapulmonary administration, by
inhalation or insufflation as a solution, suspension or dry powder (for
example, in
a spray), or rectally, in dosage unit formulations containing conventional
nontoxic
pharmaceutically acceptable carriers, adjuvants, and vehicles as desired. The
term parenteral as used herein includes subcutaneous injections, intravenous,
intramuscular, intrasternal injection, or infusion techniques.
Injectable preparations, for example, sterile injectable aqueous or
oleagenous suspensions may be formulated according to the known art using
suitable dispersing or wetting agents and suspending agents. The sterile
injectable preparation may also be a sterile injectable solution or suspension
in a
nontoxic parenterally acceptable diluent or solvent, for example, as a
solution in
1,3-propanediol. Among the acceptable vehicles and solvents that may be
employed are water, Ringer's solution, and isotonic sodium chloride solution.
in
addition, sterile, fixed oils are conventionally employed as a solvent or
suspending medium. For this purpose any bland fixed oil may be employed
including synthetic mono- or diglycerides. In addition, fatty acids such as
oleic
acid find use in the preparation of injectables.
-225-
CA 02329660 2000-10-20
WO 99/54290 PCT/US99/07949
Suppositories for rectal administration of the drug can be prepared by
mixing the drug with a suitable nonirritating excipient such as cocoa butter
and
polyethylene glycols which are solid at ordinary temperatures but liquid at
the
rectal temperature and will therefore melt in the rectum and release the drug.
Solid dosage forms for oral administration may include capsules, tablets,
pills, powders, and granules. In such solid dosage forms, the active compound
may be admixed with at least one inert diluent such as sucrose lactose or
starch.
Such dosage forms may also comprise, as is normal practice, additional
substances other than inert diluents, e.g., lubricating agents such as
magnesium
stearate. In the case of capsules, tablets, and pills, the dosage forms may
also
comprise buffering agents. Tablets and pills can additionally be prepared with
enteric coatings.
Liquid dosage forms for oral administration may include pharmaceutically
acceptable emulsions, solutions, suspensions, syrups, and elixirs containing
inert
diluents commonly used in the art, such as water. Such compositions may also
comprise adjuvants, such as wetting agents, emulsifying and suspending agents,
and sweetening, flavoring, and perfuming agents.
The compounds of the present invention can also be administered in the
form of liposomes. As is known in the art, liposomes are generally derived
from
phospholipids or other lipid substances. Liposomes are formed by mono- or
mufti-lamellar hydrated liquid crystals that are dispersed in an aqueous
medium.
Any non-toxic, physiologically acceptable and metabolizable lipid capable of
forming liposomes can be used. The present compositions in liposome form can
contain, in addition to a compound of the present invention, stabilizers,
preservatives, excipients, and the like. The preferred lipids are the
phospholipids
and phosphatidyl cholines (lecithins), both natural and synthetic.
-226-
CA 02329660 2000-10-20
WO 99/54290 PCTNS99/07949
Methods to form liposomes are known in the art. See, for example,
Prescott, Ed., Methods in Cell Bioloay, Volume XIV, Academic Press, New York,
N.Y. {1976}, p. 33 et seq.
While the compounds of the invention can be administered as the sole
active pharmaceutical agent, they can also be used in combination with one or
more anti-infective agents and/or other agents used to treat other acute or
chronic respiratory ailments. Other agents to be administered in combination
with
a compound of the present invention include: an influenza vaccine; other
influenza inhibitors such as, for example, amantadine, rimantadine, ribavirin,
and
the like; another influenza neuraminidase inhibitor, such as, for example,
zanamivir or GS 4104 and the like; agents used to treat respiratory bacterial
infections and bronchitis, such as, for example, erythromycin, clarithromycin,
azithromycin and the like; and agents used to treat asthma, such as, for
example,
zileuton, albuterol (safbutamol), salmeterol, formoterol, ipratropium bromide,
inhaled steroids and the like, or anti-inflammatory agents for treating asthma
such
as, for example, beclomethasone dipropionate, fluticasone propionate,
budesonide, triamcinolone acetonide, flunisolide, cromolyn, zafirlukast,
montelukast used in combination with a compound of the present invention.
When administered as a combination, the therapeutic agents can be
formulated as separate compositions which are given at the same time or
different times, or the therapeutic agents can be given as a single
composition.
The foregoing is merely illustrative of the invention and is not intended to
limit the invention to the disclosed compounds. Variations and changes which
are obvious to one skilled in the art are intended to be within the scope and
nature of the invention which are defined in the appended claims.
-227-