Note: Descriptions are shown in the official language in which they were submitted.
CA 02329818 2000-10-25
WO 99/58951 PCT/US99/08460
METHOD FOR ANALYZING TOTAL REACTIVE SULFUR
FIELD OF THE INVENTION
This invention relates to a method for analyzing for total reactive
sulfur in crude oils or fractions thereof using mass spectrometric techniques.
DESCRIPTION OF THE RELATED ART
There has been increasing interest in the determination of total
reactive sulfur (TRS) in crude oils due to the declining supplies of sweet
crudes
and the increasing need to process sour crudes. The prediction of crude
corrosivity is important to the economics of crude purchasing and processing.
It
is known that certain sulfur -containing species or total reactive sulfur are
mainly
responsible for the corrosivity attributed to sulfur compounds present in
crude
oils. The total reactive sulfur includes contributions from the following
sulfur-
containing types: (i) hydrogen sulfide, (ii) mercaptans, (iii) aliphatic
sulfides,
(iv) aliphatic disulfides, (v) elemental sulfur and (vi) polysulfides.
A combination of wet chemical methods are conventionally
required for the determination of total reactive sulfur. The aliphatic
sulfides are
determined by UV measurement of a sulfur-iodine complex. Mercaptans and
hydrogen sulfide are measured by non-aqueous potentiometric titration.
Disulfides are measured by conversion to mercaptans using acidic reduction.
Polysulfides and elemental sulfur are determined by polaragraphic methods.
The main concern of these chemical methods is the determination of aliphatic
sulfides which are believed to account for the majority (about 80% or more) of
total reactive sulfur species in crudes and fractions thereof. Since aliphatic
sulfides contribute at least about 80% or more of the total corrosivity, any
CA 02329818 2000-10-25
WO 99/58951 2 PCT/US99/08460
problems which affect their measurement affects the final TRS and hence final
corrosion values.
The method for determining aliphatic sulfides was originally
developed for the 450 F- fraction, and is based on the selective complexation
of
the "basic" sulfide sulfur atom with iodine. The complex shows a strong band
at
308 nm in the UV region. However, this technique suffers from the
disadvantage that iodine complexation can also occur with basic nitrogen-
containing species which can affect the accuracy of the UV method of analysis.
A more rigorous spectroscopic approach is disclosed by George
and Gorbaty, J. Am. Chem. Soc., 111, 3182 (1989) which is based on the sulfur
K-Edge X-ray absorption near edge structure spectroscopy and X-ray
photoelectron spectroscopy. These techniques have been successfully employed
for elucidating organically bound sulfidic sulfur and thiophenic sulfur.
Unfortunately, the cost of the requisite equipment is very high thus rendering
the
technique unavailable for routine analytical purposes.
It is known to use 70 eV electron ionization and chemical
ionization mass spectrometric techniques for the identification and
quantitation
of aromatic sulfur compounds. However, these methods have not been extended
to the direct analysis of alkyl sulfides and disulfides which are the species
most
directly linked to sulfur corrosivity.
It would be desirable to be able to rapidly and inexpensively
determine TRS in a crude oil or fraction thereof.
SUMMARY OF THE INVENTION
This invention relates to a method for determining total reactive
sulfur in a crude oil or fraction thereof which comprises the steps of
CA 02329818 2000-10-25
WO 99/58951 3 PCT/US99/08460
(1) introducing the crude oil or fraction thereof into a mass
spectrometer;
(2) obtaining a series of mass spectra;
(3) selecting fragment ions which are characteristic of reactive sulfur
species including hydrogen sulfide, mercaptans, hydrocarbyl sulfides,
hydrocarbyl
disulfides, elemental sulfur and polysulfides, said fragment ions being
selected
from at least one of the group consisting of SH+, CSH+;CH3S+, C2H5S+, H2S2+
and
SZ+:
(4) identifying peaks in the mass chromatograms which are
characteristic of at least one of the fragment ions; and
(5) quantifying the reactive sulfur species identified by their
corresponding fragment ions, wherein the total reactive sulfur is the sum of
individual reactive sulfur species.
In another embodiment, this invention relates to a method for
determining total reactive sulfur as a function of boiling point for a crude
oil or
fraction thereof which comprises the steps of:
(1) introducing the crude oil or fraction thereof into a
chromatographic separation means which is interfaced to a mass spectrometer
thereby causing at least a partial separation of the crude oil or fraction
thereof
into constituent chemical components as a function of retention time;
(2) introducing the constituent chemical components into a mass
spectrometer;
(3) obtaining a series of time resolved mass spectra;
(4) selecting fragment ions which are characteristic of reactive
sulfur species including hydrogen sulfides, mercaptans, hydrocarbyl sulfides,
CA 02329818 2000-10-25
WO 99/58951 4 PCT/US99/08460
hydrocarbyl disulfides, elemental sulfur and polysulfides, said fragment ions
being selected from at least one of the group consisting of SH+, CHS+, CH3S+,
CZHSS+, H,SZ+, and S,+;
(5) identifying peaks in the mass chromatogram which are
characteristic of at least one of the fragment ions; and
(6) quantifying the reactive sulfur species identified by their
corresponding fragment ions as a function of retention time, wherein the total
reactive sulfur is the sum of the individual reactive sulfur species.
Yet another embodiment of the invention is directed to a method
for determining total reactive sulfur in a crude oil or fraction thereof which
comprises the steps of:
(1) introducing the crude oil of fraction thereof into a low
resolution mass spectrometer;
(2) obtaining a series of mass spectra;
(3) selecting fragment ions which are characteristic of reactive
sulfur species including hydrogen sulfide, mercaptans, hydrocarbyl sulfides,
hydrocarbyl disulfides, elemental sulfur and polysulfides, said fragment ions
being
selected from at least one of the group consisting of SH+, CSH+, CH3S+,
CzHSS+,
H2S2+ and SZ+:
(4) identifying peaks in the mass chromatograms which are
characteristic of the nominal masses of at least one of the fragment ions;
(5) selecting reference samples having known values of total
reactive sulfur and subjecting said references samples to steps (1) to (4)
above;
(6) calibrating the peaks in the mass chromatograms of the
reference samples which are characteristic of the nominal masses of the at
least
CA 02329818 2000-10-25
WO 99/58951 5 PCT/US99/08460
one fragment ions against the known values of total reactive sulfur species in
the
reference samples; and
(7) quantifying the reactive sulfur species identified by the
nominal masses of their corresponding fragment ions by comparison with the
calibration obtained from the reference samples, wherein the total reactive
sulfur
is the sum of the individual reactive sulfur species.
BRIEF DESCRiPTION OF THE DRAWINGS
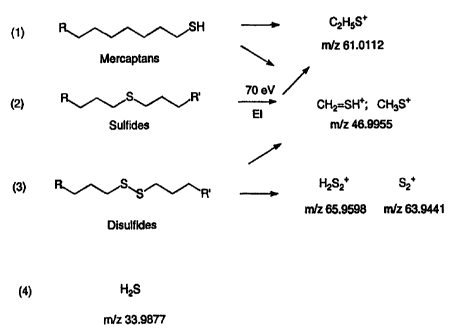
Figure 1 is a schematic showing reactions of parent molecular
species which result in characteristic fragment ions.
Figure 2 is a graph showing the relative abundances of selected
mass ions in the mass spectra of RSR and RSH compounds.
Figure 3 is a graph showing the relative abundances of selected
mass ions in the mass spectra of RSSR compounds.
Figure 4 is a graph showing the relative abundances of selected
mass ions in the mass spectra of thiophenes.
Figure 5 is a graph showing a total reactive sulfur external
calibration curve using a Nigerian medium crude spiked with known amounts of
reactive sulfur compounds.
Figure 6 is a graph showing a comparison of mass spectral areas
and total reactive sulfur measured by standard wet chemical methods.
Figure 7 is a graph showing total reactive sulfur as a function of
time/boiling point for three petroleum samples.
Figure 8 is a graph showing total sulfur as a function of
time/boiling point for three petroleum samples.
CA 02329818 2000-10-25
WO 99/58951 6 PCT/US99/08460
Figure 9 is a graph showing a calibration chart of total reactive
sulfur using model sulfur compounds.
Figure 10 is a graph showing a validation chart for the mass
spectrometry total reactive sulfur method.
DETAILED DESCRIPTION OF THE INVENTION
Crude oils and fractions thereof typically contain many different
kinds of sulfur-containing species. These species are broadly characterized as
being sulfur incorporated into an aromatic ring structure vs. non-aromatic
sulfur.
Aromatic sulfur compounds such as thiophene and the like are generally not as
corrosive when compared to non-aromatic sulfur-containing compounds such as
mercaptans (RSH), hydrocarbyl sulfides (RSR) and hydrocarbyl disulfides
(RSSR) wherein R is a hydrocarbyl radical. By hydrocarbyl is meant aliphatic,
alicyclic, aromatic, aliphatic- and alicyclic-substituted aromatic, and
aromatic-
substituted aliphatic and alicyclic wherein the hydrocarbyl radicals may be
substituted by halogen, nitro, cyano, carboxyl, amino and the like. The nature
of
hydrocarbyl radical is not critical. Total reactive sulfur is then the sum of
the
non-aromatic sulfur-containing species including the mercaptans, sulfides and
disulfides identified above plus hydrogen sulfide, elemental sulfur and
polysulfides (S,,).
In the mass spectrometer, sample molecules are bombarded with
high energy electrons thereby creating molecular ions which fragment in a
pattern characteristic of the molecular species involved. A continuous series
of
mass spectra are obtained over a scan range of about 10 to 800 Daltons. The
mass spectral data may also be acquired in selected ion monitoring mode. In
this
mode, care must be taken to select ions representative of the components of
interest and to operate under repeatable conditions. A variety of mass
spectrometers may be used including low resolution, high resolution, MS/MS,
CA 02329818 2000-10-25
WO 99/58951 7 PCTIUS99/08460
ion cyclotron resonance and time of flight. Any ionization technique may be
used such as electron ionization, chemical ionization, multiphotonionization,
field desorption, field ionization and the like, provided that the technique
provides either molecular or fragment ions which are suitable for use in the
present method.
An important aspect of the present invention is the discovery that
certain fragment ions can be used as quantitative indicators of their parent
sulfur-
containing species. These fragment ions and corresponding sulfur species or
compounds are set forth in the following table:
Fragment Ion Mass/Charge Parent Sulfur-Containing Species
C2H5S+ 61.0112 RSR/RSH
H2S2+ 65.9528 RSSR
S2+ 63.9441 S,, RSSR
CH3S_ 46.9955 RSR/RSH/RSSR
CSH+ 44.9799 RSR/RSH/RSSR, thiophenes
SH+ 32.9799 H2S, RSR
The reactions schemes which produce the fragment ions in the mass
spectrometer are also illustrated in Fig. 1. By monitoring the masses
corresponding to these fragment ions, it is possible to quantitatively
ascertain the
concentration of TRS species in a given sample. Other fragment ions may
substituted be if they are quantitative indicators of parent sulfur-containing
species.
For any given parent species, the relative abundance of each of the
fragment ions over the total ionization of the particular compound making up
the
parent species was discovered to be different depending on the type of parent
species. The results are shown in Figures 2-4 which show the average relative
abundances of selected fragment ions for homologous members of RSR, RSH
CA 02329818 2000-10-25
WO 99/58951 8 PCT/US99/08460
(Figure 2), RSSR (Figure 3) and thiophenes (Figure 4). The hydrocarbyl
sulfides and mercaptans have been grouped together based on their chemical
similarity. The fragment ion with mass/charge 33 is weak in all types of
spectra.
The fragment ion at m/z 61 is intense in the spectra of RSR/RSH. The fragment
ion at m/z 45 is intense in the spectra of thiophenes. Excluding the first
spectra
in RSR/RSH series (CzH6S), the ion m/z 47 is present in both RSR/RSH and
RSSR spectra in approximately the same ratio, and is very weak in the spectra
of
the thiophenes. The ion m/z 66 is more intense in the spectra of RSSR.
As a first approximation, the ion CHS+ (m/z 44.9799) can be used
to measure the total sulfur content in a sample. This approximation has been
made previously by Gallegos, Anal. Chem., 1975, 45, 1150 to monitor all sulfur
compounds in fluid catalytic cracker heavy naphtha. However, this
approximation includes both aromatic and non-aromatic sulfur-containing
species. Ions C2H5S+ (m/s 61.0112) and HZSz+ (m/z 65.9598) can be used to
monitor the amount of RSR/RSH and RSSR, respectively. The ion CH3S+ is
very weak in thiophenes, and since it is approximately in the same ratio in
both
RSR/RSH and RSSR, it can be used to quantitatively monitor the TRS content in
a sample. Thus CH3S+ (m/z 47) is the preferred fragment ion for quantitatively
determining TRS.
As can be seen from Figures 2-4, the relative abundances of ions
with m/z 45, 61 and 66 change with the different homologs in a series. In
addition, both RSR/RSH and RSSR produce m/z 61 and 66 and cannot be used
exclusively for each type. Therefore, there is a certain error in the
evaluation of
the sulfur amount when assuming a direct relationship between the measured
mass spectral area and the actual sulfur quantity. Nevertheless, the
methodology
can be used on a relative basis, when comparing samples of similar origin with
different sulfur types.
CA 02329818 2000-10-25
WO 99/58951 9 PCT/US99/08460
The actual limitation in using the above noted characteristic
fragment ions to determine low concentrations of various reactive sulfur types
is
the working resolution attainable by the mass spectrometer. The resolution
requirements for separation of ions CHS', CH3S+, C,H5S+ and H,S2' vary widely
based on the permutations of C, H, N and O. If all four of C, H, N and 0 are
present, very high resolution (90,000) would be required. In practice, most of
the possible elemental compositions are either very weak or are not present in
typical petroleum samples. Thus an average resolution of 10,000 is adequate to
separate most ions. Attaining higher resolution requires a sacrifice in
sensitivity.
Low voltage electron ionization primarily forms molecular ions,
and the resolution required for separation of higher molecular weight
structures
necessitates higher resolution settings according the equation: Resolution =
m/Am where m is the ion mass and Om is the difference between the mass of the
ion and the mass of the nearest interfering ion.
The precise method of measuring TRS in a given sample may vary
according to the type of mass spectrometer (MS) available. A tandem MS/MS
instrument can be used to directly measure TRS with maximum sensitivity.
High resolution (HR) 70 eV electron ionization can be used for the relative
monitoring of the various sulfur types in crude oils and refinery streams. HR
can
attain more accurate results by using the response factors for each compound
type. The concentrations of various sulfur types are obtained by HR
measurement of ion abundances and a calibration matrix determined from known
sulfur mixtures according to conventional methods. HR can be coupled with a
gas chromatograph to correlate TRS with boiling points for a given sample. Low
resolution (LR) 70 eV electron ionization can predict the concentration of
sulfur
species based on multiple linear regression analysis of samples with known
concentrations of sulfur types. This method is the most practical for field
CA 02329818 2000-10-25
WO 99/58951 10 PCTIUS99/08460
measurements where more complex MS instruments are not available. Mass
spectral data may be acquired in the selected ion monitoring (SIM) mode.
GC/MS utilizes a gas chromatograph interfaced with a mass
spectrometer. While any chromatographic method may be used to separate the
mixture into components, capillary gas chromatography is the preferred means
for interfacing with a mass spectrometer. The sample to be analyzed is first
injected into a GC where the sample components are separated as a function of
retention time and boiling point. Only partial chromatographic resolution of
sample components is necessary. Components may also be identified by a
detector such as a flame ionization detector, thermal conductivity detector,
atomic emission detector or electron capture detector. The separated or
partially
separated components are then transferred to the mass spectrometer by a heated
capillary line interface. In this manner, a series of mass spectra can be
obtained
which are correlated with retention times and hence boiling point distribution
of
the sample under investigation. This provides a correlation of TRS species as
a
function of boiling point.
When using the low resolution (LR) MS method for determining
TRS, one cannot normally directly monitor the exact mass of the characteristic
fragment ion in the presence of interferring hydrocarbon species. As this
method
only measures the nominal mass of an ion, it is coupled with a multiple linear
regression calibration to obtain TRS values. In order to distinguish between
the
signal of the mass spectra of the ion of interest and signal due to
interferences, a
representative set of known samples is subjected to LR MS analysis, and the
contribution of desired and interfering components is mathematically
determined. Due to the complexity of typical samples, it is not feasible to
prepare standard calibration mixtures that would adequately represent the many
different sulfur compounds in petroleum.
CA 02329818 2000-10-25
WO 99/58951 11 PCT/US99/08460
While there are many mathematical tools for handling the data
generated by the known samples, a preferred method is based on a multiple
linear regression approach. In multiple linear regression, a series of
equations is
created by measuring the ion abundances A, of a number of representative
samples I with known TRS concentrations C; . The equations are as follows:
C; = b+ a~Ari + aaA11 + a3A13 +...... + aj.4ri
C, = b + arA2 f+ a2A11 + a jA13 +...... + aA,;
C.; b + aIA31 + ar432 + a3A33 +...... + a.;A3r
... ......... .......... ......... ...... .......
... ......... .......... ......... ...... .......
... ......... .......... ......... ...... .......
C; = b+ a;A;, + a',Ai1 + a jA; j+...... + aiAj
Solutions of the above equations produces the coefficients b and aj . The TRS
concentration for an unknown sample is determined from the measured ion
abundances and the known coefficients. Although for the solution of the system
one needs i= j, generally the condition i>>j is allowed in order to account
for the
presence of the same TRS concentration is samples with different hydrocarbon
chemical compositions. A better analysis includes the ion abundances of the
contributing interfering hydrocarbons. Although a typical LR MS spectrum
using a quadrupole MS can acquire ions with up to 800 Dalton masses, for
reasons of simplicity, the preferred approach is restricted to the minimum
number of possible characteristic TRS ions. Monitoring a smaller set of
selected
ions also increases considerably the detection limit of the instrument because
more time is spent in signal accumulation and less in scanning between peaks.
Based on analysis of a comparison of many known samples with standard wet
chemical analysis vs. LR MS, the LR MS method measures TRS within the
repeatability of wet chemical method with at least a 95% confidence.
The methods of the invention are further illustrated by the
following examples.
CA 02329818 2000-10-25
WO 99/58951 12 PCT/US99/08460
EXAMPLE 1
The instrumental procedures are described in this example. Mass
spectra were obtained using a JEOL JMS-AX505WA double focusing MS. This
instrument contains an electrostatic sector (E) and a magnetic analyzer (B) in
a
forward geometry arrangement (Electric Field/Magnetic Field). The JEOL MS
instrument is based on a virtual image double focusing ion optics design with
a
1:4 image magnification. For a given resolution setting, the main slit width
is
four times wider than the collector slid width. The overall effect is to allow
the
entrance of a wider ion beam into the analyzer and thus increase the maximum
sensitivity.
Samples were introduced into the MS through a heated inlet
system, a Hewlett-Packard 5890 gas chromatograph or a dynamic batch type
inlet system. The dynamic batch system allows the direct introduction of the
sample in a manner analogous to the sample introduction in a GC injection port
using a carrier gas. The advantages of this system are the short time required
for
analysis and the capabilities of automation using an autosampler for the
routine
analysis of samples.
High resolution experiments were performed at 7,500 resolution.
Collision induced dissociation (CID) was done using helium as a collision gas.
The pressure in the collision cell, located in the first field-free region,
was set to
achieve a 50% attenuation of the main beam. MS/MS spectra were obtained
using linked scans at constant B/E (daughter ion scan) and at constant B2 (1-
E)/E2 (neutral loss).
CA 02329818 2000-10-25
WO 99/58951 13 PCT/US99/08460
EXAMPLE 2
This example is directed to the direct.measurement of TRS using
MS/MS. CID spectra of various sulfur fragments were obtained at constant B/E
scan using the instrument described in Example 1. This scan mode allows the
acquisition of the daughter ions produced upon collisional dissociation of the
selected parent ion structure. The B/E CID spectra of the ion CH3S+ at m/z 47
are shown in Table 1 for the designated sulfur compounds: 1=2-
methylthiophene; 2=2,3-dimethylthiophene; 3=2,5-dimethylthiophene;
4=ethyldisulfide; 5=isopropyldisulfide; 6=n-propyl disul fide;
7=benzothiophene;
8=n-butyldisulfide and 9=dibenzothiophene.
Tabte 1
m/z 1 2 3 4 5 6 7 8 9
15 0.2 1.6 0.3 0.1 1.2 0.5 0 0.3
25 1.3 1.0 0.1 0.9 2.6 0.1
26 1.3 0.4 0.6 1.0 1.9 0.8 0.9 0.3 5.2
27 0.3 0.9 0.4 1.7 1.0 1.1 0.3
28 0.6 0.1 0.5 0.2 0.4 1.0 0.1 1.0
29 0.1 0.1 1.5 0.1 0.3 0.5 2.7
30 0.6 0.2 1.1
31 0.3 0.1 1.0
32 4.2 6.1 4.5 4.7 2.9 2. 10.8 1.5 10.4
33 1.2 4.0 1.8 2.9 2.9 2.1 2.1 2.1 3.9
34 1.7 1.0 2.7 1.7 0.6 0.8 4.9 0.6
35 1.4 0.8 2.8 0.1 2.9
36 0.6 0.2 0.3 5.2 0.1 11.9
37 2.9 0.1 2.2 0.3 0.6 0.3 6.4 0.4 18.0
38 2.3 2.4 0.1 0.8 0.2 5.1 0.3 18.7
39 2.1 0.3 3.3 0.2 1.9 0.8 9.8 0.7
40 1.4 0.4 0.8 0.5 0.3 1.3 0.3 1.5
41 0.4 1.2 2.2 0.7 2.1 0.8 0.6 1.0 2.8
42 0.2 . 0. ! 0.1 0.19 0.2
43 0.1 0.1 5.1 2.14 1.0
44 24 7.9 4.4 4.0 5.1 4.14 3.4 3.2 12.4
45 11.5 39.1 14.2 13.3 16.7 15.35 11.2 14.3 13.2
46 7.8 11.5 9.8 7.1 3.4 4.32 13.5 4.5 16.8
47 100 100 100 100 100 100 100 100 100
CA 02329818 2000-10-25
WO 99/58951 PCTIUS99/08460
14
As can be seen from Table 1, an intense transition is observed at
m/z 45 (CHS+). Although this fragment is formed by all sulfur types when
analyzed pure, the relative abundance of the parent ion m/z 47 is very weak in
the spectra of thiophenes. Thus m/z 47 can be used as a TRS diagnostic. Other
characteristic MS/MS transitions are as follows:
(1) CH3S+ -~ CHS+ TRS (loss of H2)
47 m/z 45 mlz
(2) CZH5S+ -~ CHS+ RSR/RSH (loss of CH4)
61 m/z 45 m/z
(3) H2S" ~ S2+ RSSR (loss of HZ)
66 m/z 64 m/z
The RSR/RSH and RSSR transitions are bound by the relative abundance of each
parent ion in the mixture and cannot therefore be used to directly monitor the
amount of the corresponding sulfur type.
Fig. 5 shows an external calibration curve for TRS (standard
addition). A Nigerian medium crude was spiked with varying amounts of a
standard mixture of reactive sulfur compounds. The peak area measured with
the MS is plotted against the known TRS concentration in the sample. The slope
of the line represents the average TRS response factor. The intercept is a
measure of the initial TRS amount in the crude. This calibration curve is for
the
determination of the TRS concentrations in an unknown sample.
Fig. 6 shows a comparison of MS vs. wet chemical methods. The
results of the comparison for a variety of crudes (New Grade, Nigerian, Murban
and Cold Lake) and heavy petroleum cuts (heavy and light gas oils and reduced
crudes) indicate that there is a very good (at least 95%) correlation between
the
two methods.
CA 02329818 2000-10-25
WO 99/58951 15 PCT/US99/08460
EXAMPLE 3
The use of high resolution MS to monitor TRS is shown in this
Example. A kerosene was subjected to high resolution MS in the selected ion
monitoring (SIM) mode. While the SIM can be used to study any of the
characteristic fragment ions noted above, of particular interest is the CH3S+
ion
in view of its correlation with TRS. The results for three different samples
boiling in the kerosene range are shown in Fig. 7 and 8. Fig. 7 is a graph of
TRS
as a function of time for the three samples. Fig. 8 is a graph of total sulfur
as a
function of time.
EXAMPLE 4
This example is directed to the use of low resolution (LR) MS for
the determination of TRS. A Brunfeldt batch type system was used for the
introduction of sample into a Hewlett-Packard 5970 mass spectrometer. Samples
were introduced either through an ampoule which was heated under vacuum or
in the case of light samples, through a gallium frit inlet. The spectrometer
was
calibrated using perfluorobutylamine. Data were obtained by scanning the
desired range (33-80 Daltons) or in the SIM mode. A series of MS for crudes
spiked with different concentrations of standard sulfur mixtures were obtained
and the appropriate coefficients from a multiple linear regression analysis of
the
data were obtained. The calibration equation is
[TRS-Wt.%] = 0.1591 + 0.1100A45 + 1.2596A47 - 0.0116A51
- 0.0568A61 - 0.0006A64 - 0.0644A66
with an average absolute error between the known and measured TRS values of
0.0163S-wt.%. The coefficient of correlation is 0.9908. The calibration chart
is
shown in Fig. 9 which is a graph of TRS using model sulfur compounds. Whole
crudes, distillate and resid cuts are spiked with RSR and RSSR compounds.
CA 02329818 2000-10-25
WO 99/58951 16 PCTIUS99/08460
A series of over 100 samples varying from whole crudes of
differing character to distillate cuts were analyzed using standard wet
chemical
techniques and TRS determined from LR MS. The average absolute difference
of the results obtained with the LR MS method as compared to the wet chemical
method is 0.056%. Fig. 10 is a graph of the TRS difference measured by MS
from the wet method for these samples. The average difference from Fig. 10 is
0.0083 wt.%. This demonstrates that there is virtually no bias between LR MS
and wet methods over a large set of data.
The LR MS method has the advantages that it is simple, rapid,
precise and accurate and is amenable to refinery and field operations where
expensive MS equipment is not available. Crudes with specific gravities
between 0.75 and 1.10, and total sulfur values up to 7.0 S-wt.% have been
successfully analyzed using this technique. The method is applicable to even
wider ranges of crudes and fractions thereof.