Note: Descriptions are shown in the official language in which they were submitted.
CA 02330623 2000-10-30
WO 99/58521 PC'T/iJS99/10185
-1-
lI-ARYL-BENZO[B]NAPHTHO[2,3-DJFURANS AND 11-ARYL
BENZO[BJNAPHTHO[2,3-D]THIOPHENES USEFUL IN THE TREATMENT
OF INSULIN RESISTANCE AND HYPERGLYCEMIA
BACKGROUND OF THE INVENTION
The prevalence of insulin resistance in glucose intolerant subjects has long
been recognized. Reaven et al (American Journal of Medicine 1976, 60, 80) used
a
continuous infusion of glucose and insulin (insulin/glucose clamp technique)
and oral
glucose tolerance tests to demonstrate that insulin resistance existed in a
diverse
group of nonobese, nonketotic subjects. These subjects ranged from borderline
glucose tolerant to overt, fasting hyperglycemia. The diabetic groups in these
studies
included both insulin dependent (IDDM) and noninsulin dependent (NIDDM)
subjects.
Coincident with sustained insulin resistance is the more easily determined
hyperinsulinenua, which can be measured by accurate determination of
circulating
plasma insulin concentration in the plasma of subjects. Hyperinsulinemia can
be
present as a result of insulin resistance, such as is in obese and/or diabetic
(NIDDM)
subjects and/or glucose intolerant subjects, or in IDDM subjects, as a
consequence of
over injection of insulin compared with normal physiological release of the
hormone
by the endocrine pancreas.
The association of hyperinsulinemia with obesity and with ischemic diseases
of the large blood vessels (e.g. atherosclerosis) has been well established by
numerous
experimental, clinical and epidemiological studies (summarized by Stout,
Metabolism
1985, 34, 7, and in more detail by Pyorala et al, DiabeteslMetabolism Reviews
1987,
3, 463). Statistically significant plasma insulin elevations at 1 and 2 hours
after oral
glucose load correlates with an increased risk of coronary heart disease.
Since most of these studies actually excluded diabetic subjects, data relating
the risk of atherosclerotic diseases to the diabetic condition are not as
numerous, but
point in the same direction as for nondiabetic subjects (Pyorala et al).
However, the
incidence of atherosclerotic diseases in morbidity and mortality statistics in
the
diabetic population exceeds that of the nondiabetic population (Pyorala et al;
Jarrett
CA 02330623 2000-10-30
WO 99/58521 PCT/17S99/10185
-2-
DiabeteslMetabolism Reviews 1989,5, 547; Harris et al, Mortality from
diabetes, in
Diabetes in America 1985).
The independent risk factors obesity and hypertension for atherosclerotic
diseases are also associated with insulin resistance. Using a combination of
insulin/glucose clamps, tracer glucose infusion and indirect calorimetry, it
has been
demonstrated that the insulin resistance of essential hypertension is located
in
peripheral tissues (principally muscle) and correlates directly with the
severity of
hypertension (DeFronzo and Ferrannini, Diabetes Care 1991, 14, 173). In
hypertension of the obese, insulin resistance generates hyperinsulinemia,
which is
recruited as a mechanism to limit further weight gain via thermogenesis, but
insulin
also increases renal sodium reabsorption and stimulates the sympathetic
nervous
system in kidneys, heart, and vasculature, creating hypertension.
It is now appreciated that insulin resistance is usually the result of a
defect in
the insulin receptor signaling system, at a site post binding of insulin to
the receptor.
Accumulated scientific evidence demonstrating insulin resistance in the major
tissues
which respond to insulin (muscle, Iiver, adipose), strongly suggests that a
defect in
insulin signal transduction resides at an early step in this cascade,
specifically at the
insulin receptor kinase activity, which appears to be diminished (reviewed by
Haring,
Diabetalogia 1991, 34, 848).
Protein-tyrosine phosphatases (PTPases) play an important role in the
regulation of phosphorylation of proteins. The interaction of insulin with its
receptor
leads to phosphorylation of certain tyrosine molecules within the receptor
protein,
thus activating the receptor kinase. PTPases dephosphorylate the activated
insulin
receptor, attenuating the tyrosine kinase activity. PTPases can also modulate
post-
receptor signaling by catalyzing the dephosphorylation of cellular substrates
of the
insulin receptor kinase. The enzymes that appear most likely to closely
associate with
the insulin receptor and therefore, most likely to regulate the insulin
receptor kinase
activity, include PTP1B, LAR, PTPa and SH-PTPZ (B. J. Goldstein, J. Cellular
Biochemistry 1992, 48, 33; B. J. Goldstein, Receptor 1993, 3, 1-15,; F. Ahmad
and B.
J. Goldstein Biochim. Biophys Acta 1995, 1248, 57-69).
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-3-
McGuire et al. (Diabetes 1991, 40, 939), demonstrated that nondiabetic
glucose intolerant subjects possessed significantly elevated levels of PTPase
activity
in muscle tissue vs. normal subjects, and that insulin infusion failed to
suppress
PTPase activity as it did in insulin sensitive subjects.
Meyerovitch et al (J. Clinical Invest. 1989, 84, 976) observed significantly
increased PTPase activity in the livers of two rodent models of IDDM, the
genetically
diabetic BB rat, and the STZ-induced diabetic rat. Sredy et al (Metabolism,
44, 1074,
1995) observed sinular increased PTPase activity in the livers of obese,
diabetic ob/ob
mice, a genetic rodent model of NIDDM.
The compounds of this invention have been shown to inhibit PTPases derived
from rat liver microsomes and human-derived recombinant PTPase-1B (hPTP-1B) in
vitro. They are useful in the treatment of insulin resistance associated with
obesity,
glucose intolerance, diabetes mellitus, hypertension and ischemic diseases of
the large
and small blood vessels.
K. Shinzo, et al., Heterocylces 1982, 19, 1033-1037 disclosed a synthesis of
benzo(b]naphtho[2,3-d]thiophenes of which two examples also had a 11-phenyl
substituent as shown by structure A below. None of the examples in this
Heterocylces
article contained the appropriate substitution, nor any subtitution on the 11-
phenyl
group necessary for in vitro PTPase inhibition activity or in vivo
antidiabetic activity.
(A), R = H, CH
z
J. Hastings, et al., J. Chem. Soc., Perkin Trans. 1 1975, 19, 1995-1998 and J.
Hastings, et al., J. Chem. Soc., Perkin Trans. 1 1972, 14, 1839-1842 disclosed
three
examples of benzo[b]naphtho[2,3-d]furans that also had a 11-phenyl substituent
as
shown by structure B below. None of the examples in these J. Chem. Soc.,
Perkin
CA 02330623 2000-10-30
WO 99/58521 PCTNS99/10185
-4-
Traps. 1 articles contained the appropriate substitution on the 11-phenyl
group
necessary for in vitro PTPase inhibition activity or in vivo antidiabetic
activity.
1 ) R = CH3, R'= H
2)R,R'=H
3)R=H, R'=CH3
(B)
DESCRIPTION OF THE INVENTION
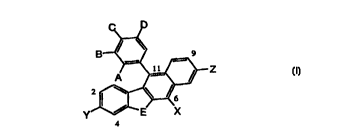
This invention provides a compound of formula I having the structure
Z
4
(I)
wherein
A is hydrogen, halogen, or OH;
B and D are each, independently, hydrogen, halogen, CN, alkyl of 1-6 carbon
atoms,
aryl, aralkyl of 6-12 carbon atoms, nitro, amino or OR;
R is hydrogen, alkyl of 1-6 carbon atoms, -COR1, -CH2C02R1, -CH(Rla)C02R1, or
-SOZR ~ ;
R1 and Rla are each, independently, hydrogen, alkyl of 1-6 carbon atoms,
aralkyl of
6-12 carbon atoms or aryl;
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-5-
E is S, SO, S02, O;
X is hydrogen, halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6
carbon
atoms, alkoxy of 1-6 carbon atoms, aryloxy, arylalkoxy of 6-12 carbon
atoms, nitro, amino, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl, or -OCHZCOZR~b;
Rlb is hydrogen or alkyl of 1-6 carbon atoms;
Y and Z are each, independently, hydrogen or OR2;
R2 is hydrogen, alkyl of 1-6 carbon atoms, aralkyl of 6-12 carbon atoms, or
-CH2C02R3;
R3 is hydrogen or alkyl of 1-6 carbon atoms;
C is hydrogen, halogen or OR4;
R4 is hydrogen, alkyl of 1-6 carbon atoms, -CH(RS)W, -C(CHg)2C02R6,
5-thiazolidine-2,4-dione, -CH(R~)CHZC02R6, -CORE, P03(R6)2, or -S02R6;
RS is hydrogen, alkyl of 1-6 carbon atoms, aralkyl, aryl, CHZ(1H-imidazol-4-
yl),
-CH2(3-1H-indolyl), -CH2CH2(1,3-dioxo-1,3-dihydro-isoindol-2-yl),
-CH2CH2(1-oxo-1,3-dihydro-isoindol-2-yl), -CH2(3-pyridyl), or
-CH2C02H;
W is -COZR6, -CONH2, -CONHOH, CN, -CONH(CHZ)2CN, 5-tetrazole, -P03(R6)2,
-CH20H, or -CH2Br, -CONR6CHR~C02Rg,
R6 is hydrogen, alkyl of 1-6 carbon atoms, aryl or aralkyl;
R~ is hydrogen, alkyl of 1-6 carbon atoms, aryl or aralkyl;
Rg is hydrogen, alkyl of 1-6 carbon atoms, aryl or aralkyl;
or a pharmaceutically acceptable salt thereof, which are useful in treating
metabolic
disorders related to insulin resistance or hyperglycemia.
Pharmaceutically acceptable salts can be formed from organic and inorganic
acids, for example, acetic, propionic, lactic, citric, tartaric, succinic,
fumaric, malefic,
malonic, mandelic, malic, phthalic, hydrochloric, hydrobromic, phosphoric,
nitric,
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
-6-
sulfuric, methanesulfonic, napthalenesulfonic, benzenesulfonic,
toluenesulfonic,
camphorsulfonic, and similarly known acceptable acids when a compound of this
invention contains a basic moiety, such as when RS is -CH2(3-pyridyI) or
contains
similar basic moieties. Salts may also be formed from organic and inorganic
bases,
preferably alkali metal salts, for example, sodium, lithium, or potassium,
when a
compound of this invention contains a carboxylate or phenolic moiety.
Alkyl includes both straight chain as well as branched moieties. Halogen
means bromine, chlorine, fluorine, and iodine. It is preferred that the aryl
portion of
the aryl or aralkyl substituent is a phenyl or naphthy; with phenyl being most
preferred. The aryl moiety may be optionally mono-, di-, or tri- substituted
with a
substituent selected from the group consisting of alkyl of 1-6 carbon atoms,
alkoxy of
1-6 carbon atoms, trifluoromethyl, halogen, alkoxycarbonyl of 2-7 carbon
atoms,
alkylamino of 1-6 carbon atoms, and dialkylamino in which each of the alkyl
groups
is of 1-6 carbon atoms, nitro, cyano, -C02H, alkylcarbonyloxy of 2-7 carbon
atoms,
and alkylcarbonyl of 2-7 carbon atoms.
The compounds of this invention may contain an asymmetric carbon atom and
some of the compounds of this invention may contain one or more asymmetric
centers
and may thus give rise to optical isomers and diastereomers. While shown
without
respect to stereochemistry in Formula I, the present invention includes such
optical
isomers and diastereomers; as well as the racemic and resolved,
enantiomerically pure
R and S stereoisomers; as well as other mixtures of the R and S stereaisomers
and
pharmaceutically acceptable salts thereof.
The compounds of this invention may be atropisomers by virtue of possible
restricted or slow rotation about the aryl-tetracyclic single bond. This
restricted
rotation creates additional chirality and leads to enantiomeric forms. If
there is an
additional chiral center in the molecule, diasteriomers exist and can be seen
in the
NMR and via other analytical technigues. While shown without respect to
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
atropisomer stereochemistry in Formula I, the present invention includes such
atoropisomers (enantiomers and diastereomers; as well as the racemic,
resolved, pure
diastereomers and mixutures of diasteomers) and pharmaceutically acceptable
salts
thereof.
which
Preferred compounds of this invention include compounds of formula (I) in
A and B are each, independently, hydrogen, or bromine;
C and D are OH;
E is S, or O;
X is hydrogen, halogen, alkyl of 1-6 carbon atoms, CN, alkoxy of 1-6 carbon
atoms,
aryloxy of 6-12 carbon atoms; arylalkoxy of 6-12 carbon atoms, arylsulfanyl,
or pyridylsulfanyl;
YandZareH;
or a pharmaceutically acceptable salt thereof.
Other preferred compounds of this invention include compounds of formula
(I) in which
A is hydrogen;
B and D are each, independently, halogen, alkyl of 1-6 carbon atoms, aryl or
aralkyl
of 6-12 carbon atoms, or alkoxy of 1-6 carbon atoms;
C is OR4
E is S, O;
X is hydrogen, halogen, alkyl of 1-6 carbon atoms, perfluoroalkyl of 1-6
carbon
atoms, CN, alkoxy of 1-6 carbon atoms, aryloxy, arylalkoxy of 6-12 carbon
atoms, arylsulfanyl, pyridylsulfanyl;
Y and Z are H;
R4 is H, alkyl of 1-6 carbon atoms, -CH(RS)W, or 5-thiazolidine-2,4-dione;
CA 02330623 2000-10-30
WO 99/58521 PCT/tIS99/10185
-g_
RS is H, alkyl of 1-6 carbon atoms, aralkyl of 6-12 carbon atoms, aryl, -CH2(3-
1H-
indolyl), -CH2CH2(1,3-dioxo-1,3-dihydro-isoindol-2-yl), -CHZCH2(1-oxo-
1,3-dihydro-isoindol-2-yl), or -CHZ(3-pyridyl);
W is -C02R6, -CONH2, -CONHOH, -5-tetrazole, or -POg(R6)2;
R6 is hydrogen or alkyl of 1-6 carbon atoms;
or a pharmaceutically acceptable salt thereof.
More preferred compounds of this invention include:
(R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]furan-11-yl)-phenoxy]-3-
phenyl-propionic acid;
(R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-
phenyl-propionic acid;
(5'-benzo[b]naphtho[2,3-d]thiophen-11-yl)-[ 1,1';3',1 "]terphenyl-2'-yloxy)-
acetic
acid;
4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6-diisopropyl-phenoxy)-
acetic
acid;
(R)-2-[2,6-dibromo-4-(6-iodo-benzo [b] naphtho [2, 3-d] thiophen-11-yl)-
phenoxy]-3-
phenyl-propionic acid;
2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-(4-
fluorophenyl-propionic acid;
(R)-2-[2-bromo-4-(6-bromo-benzo [b]naphtho [2, 3-d] thiophen-11-yl )-6-methoxy-
phenoxy]-3-phenyl-propionic acid;
(R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-
propionic acid;
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-9-
(R)-2-[2,6-dibromo-4-(6-iodo-benzo[b]naphtho[2,3-d]thiophen-11-yl}-phenoxy]-
propionic acid;
2-(2,6-dibromo-4.-(6-bromo-benzo[b)naphtho(2,3-d]thiophen-11-yl)-phenoxy]-
hexanoic acid;
(R)-2-[2,6-dibromo-4-(6-methoxy-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-
3-phenyl-propionic acid;
(R)-2-[2,6-dibromo-4-(6-chloro-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-
3-
phenyl-propionic acid;
(R)-2-[2,6-dibromo-4-(6-phenylsulfanyl-benzo[b]naphtho[2,3-d]thiophen-11-yl)-
phenoxy]-propionic acid;
(R)-2-[2,6-dibromo-4-(6-phenylsulfanyl-benzo[b]naphtho[2,3-d]thiophen-11-yl)-
phenoxy]-3-phenyl-propionic acid;
2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-(1H-
indol-3-yl)-propionic acid;
(R)-2-[2,6-diiodo-4-(benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-phenyl-
propionic acid;
(R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-
phenyl-butyric acid;
(R)-2-[2,6-dibromo-4-(6-trifluoromethyl-benzo[b]naphtho[2,3-d]thiophen-11-yl)-
phenoxy]-3-phenyl-propionic acid;
(S}-2-(2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-
phenyl-butyric acid;
CA 02330623 2000-10-30
WO 99158521 PCT/1JS99/10185
- 10-
(R)-2-(4-benzo[b]naphtho[2,3-d]thiophen-11-yl-2,6-dibromo-phenoxy)-4-( 1, 3-
dioxo-
1, 3-dihydro-isoindol-2-yl)-butyric acid;
(R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-4-
(1, 3-dioxo-l, 3-dihydro-isoindol-2-yl)-butyric acid;
{ 1-[2,6-dibromo-4-(6-bromo-benzo(b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-
phenyl-propyl}-phosphonic acid;
(R)-2-[2,6-dibromo-4-(6-(pyridin-4-ylsulfanyl)-benzo[b]naphtho(2,3-d]thiophen-
11-
yl)-phenoxy]-3-phenyl-propionic acid;
(R)-2-[2,6-dibromo-4-(6-benzyloxy-benzo[b]naphtho[2,3-d]thiophen-11-yl)-
phenoxy]-3-phenyl-propionic acid;
(S}-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-
phenyl-propionic acid;
(R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho(2,3-d]thiophen-11-yl)-phenoxy]-2-
phenyl-acetic acid;
[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-acetic
acid;
[2,6-dibromo-4-(6-brorno-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxymethyl]-
phosphonic acid;
(S}-2-[2,6-dibromo-4-(6-cyano-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-
phenyl-propionic acid;
2-[2,6-dibromo-4-(6-bromo-benzo [b] naphtho [2,3-d] thiophen-11-yl )-phenoxy]-
3-
(naphthalen-2y1)-propionic acid;
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-11-
(R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-4-
(1-oxo-1, 3-dihydro-isoindol-2-yl)-butyric acid;
(R)-2-[2,6-dibromo-4-(6-methyl-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-
3-
phenyl-propionic acid;
(R)-S-{ 1-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-
phenoxy]-2-phenyl-ethyl } -1 H-tetrazole;
(R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-N-
hydroxy-3-phenyl-propionamide;
5-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-
thiazolidine-2,4-dione;
(R)-2-[2,6-diiodo-4-(benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]--propionic
acid;
2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho [2,3-d]thiophen-11-yl)-phenoxy]-3-
pyridin-3-yl-propionic acid;
(R)-2-[4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6-diisopropyl-
phenoxy)-
3-phenyl-propionic acid;
(R)-2-[4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6-diisopropyl-
phenoxy)-
propionic acid;
4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-benzene-1,2-diol;
3-bromo-5-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-benzene-1,2-diol;
4-bromo-5-(6-bromo-benzo[b]naphtho [2,3-d] thiophen-11-yl)-benzene-1,2-diol;
[2, 6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]furan-11-yl)-phenoxy]-acetic
acid;
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
- 12-
2, 6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]furan-l lyl)-phenol;
or pharmaceutically acceptable salts thereof.
The compounds of this invention can be prepared according to the following
schemes from commercially available starting materials or starting materials
which
can be prepared using to literature procedures. These schemes show the
preparation
of representative compounds of this invention.
C \
Scheme lA I
Z B / COCI
I \ ~ .~.- I \ ~ A
/ E Y / E Q (IV)
(II) (III)
Z
(V) (Ia)
Z
In Scheme lA, commercially available thianaphthene (II: Y = H; E is S) or
benzofuran (II: Y = H; E is O) is treated with one to 1.3 molar equivalents of
an alkyl
lithium reagent such as N-butyl lithium most preferably in a nonpratic solvent
such as
THF at temperatures ranging from -78°C to room temperature under
an inert
atmosphere such as nitrogen or .argon to provide the 2-lithiated-thianaphthene
or
benzofuran derivative. This lithiated analog is reacted in situ with one or
more molar
equivalents of benzaldehyde, generally at -78°C to room temperature for
5 min to 3 h
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-13-
to provide the compound of formula (III: Y, Z = H; Q = OH; E is S or O). The
hydroxy group (Q = OH) of (III) can be removed by a number of reduction
procedures such as hydrogenation using palladium catalysts to produce the
compound
of formula (III: Q, Y, Z = H; E is S or O) but is most conveniently removed
using the
method of Nutaitis, et. al. (Org. Prep. and Proceed. Int.1991, 23, 403-411) in
which
(III: Y, Z = H; Q = OH; E is S or O) is stirred with one to ten molar
equivalents of
sodium borohydride in a suitable solvent such as ether, THF or dichloromethane
at
0°C to room temperature and one to fifty molar equivalents of
trifluoroacetic acid is
slowly added over a 15 min to 3 h period to produce the compound of formula
(III: Q,
Y, Z = H; E is S or O). Alternatively, the 2-lithiated analog of thianaphthene
(II: Y =
H; E is S) or benzofuran (II: Y = H; E is O), in a nonprotic solvent such as
THF, can
be reacted with one or more molar equivalents of a benzyl halide such as
benzyl
bromide (PhCHZBr} at -78°C to room temperature to directly provide the
compound
of formula (III: Q, Y, Z = H; E is S or O).
In an analogous fashion, 6-methoxythianapthene (II: Y = OMe; E is S, S. L.
Graham, et al., J. Med. Chem. 1989, 32, 2548-2554) can be used as starting
material
using the above sequences to provide the compound of formula (III: Q, Z = H; Y
=
OMe; E is S). Still, alternatively, using the above sequences and starting
from
thianapthene (II: Y = H; E is S) or benzofuran (II: Y = H; E is O), 3-
methoxybenzaldehyde (o-anisaldehyde) can be used in place of benzaldehyde to
prepare the compound of formula (III: Q, Y = H; Z = OMe; E is S or O). The
latter
compound (III: Q, Y = H, Z = OMe; E is S or O) can also be prepared from a 3
methoxybenzyl halide such as 3-methoxybenzyl bromide and the 2-lithiated
analog of
thianaphthene (II: Y = H; E is S) or benzofuran (II: Y = H; E is O) as
described
above.
The compounds of formula (III: Q = H; Y, Z is H or OMe; E is S or O) can be
acylated with one or more molar equivalents of a commercially available
benzoic acid
chloride of formula (IV: A, B, C, D is H or OMe; with the A, B, C, D,
combination of
substituents having at least one OMe group but not more than three OMe groups)
to
produce the acylated derivative of formula (V: A, B, C, D is H or OMe; with
the A, B,
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 14-
C, D, combination of substituents having at least one OMe group but not more
than
three OMe groups; Y, Z is H or OMe; E is S or O). This acylation is
accomplished
most readily using a one to five molar equivalents of a Lewis acid catalyst
such as tin
tetrachloride or aluminum chloride in an inert solvent such as
dichloromethane, 1, 2-
dichloroethane or carbon disulfide, generally at temperatures such as -
78°C to room
temperature.
Cyclization of the compounds of formula (V: A, B, C, D is H or OMe; with
the A, B, C, D, combination of substituents having at least one OMe group but
not
more than three OMe groups; Y, Z is H or OMe; E is S or O) is generally best
accomplished using one to ten molar equivalents of a strong Lewis acid such as
a
trihaloborane, most conveniently tribromoborane. The reaction is best
performed at -
78°C with warming to room temperature in a halocarbon solvent such as
dichloromethane under an inert atmosphere such as nitrogen or argon. These
procedures not only effect cyclization and aromatization with concomitant loss
of
water, but also result in demethylation of any pendant methoxy moieties and
result in
the production of compounds of formula (Ia: A, B, C, D is H or OH; with the A,
B, C,
D, combination of substituents having at least one OH group but not more than
three
OH groups; Y, Z is H or OH; E is S or O).
In the cases in which the compound of formula (III: Q, Y = H; Z = OMe; E is
S or O) contains a methoxy moiety in position Z, acylation with the compound
of
formula (IV: A, B, D is H; C is OMe; E is S or O) is effected as usual with a
Lewis
acid catalyst such as tin tetrachloride to produce the compound of formula (V:
A, B,
D, Y is H; C, Z is OMe; E is S or O) in situ. This compound, by virtue of its
Z = OMe
moiety, is activated to further facile cyclization under the acylation
conditions to
provide directly the compound of formula (Ia: A, B, D, Y is H; C, Z is OMe; E
is S or
O). This compound can then be demethylated using boron tribromide or boron
trichloride to produce the compound of formula (Ia: A, B, D, Y is H; C, Z is
OH; E is
S or O).
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-15-
In an analogous fashion to the reactions above in Scheme lA, the compounds
of formula (Ia: A is H; B, D is alkyl of 1-6 carbon atoms; C is OH; Y, Z is H;
E is S
or O) can be prepared starting from the compound of formula (III: Q, Y, Z is
H; E is S
or O) and the appropriate benzoic acid chloride (IV: A is H; B, D is alkyl of
1-6
carbon atoms; C is OMe). The benzoic acid chloride (N: A is H; B, D is alkyl
of 1-6
carbon atoms; C is OMe) is prepared from the corresponding benzoic acid by
standard
procedures using reagents such as oxalyl chloride and thionyl chloride. The
starting
benzoic acid of the benzoic acid chloride (IV: A is H; B, D is alkyl of 1-6
carbon
atoms; C is OMe) is commercially available or can be easily prepared by known
procedures. For example, the acid starting material for benzoic acid chloride
(IV: A is
H; B, D is isopropyl; C is OMe) can be prepared using a modification of the
method
of Schuster, et al., J. Org. Chem 1988, 53, 5819. Thus commercially available
2, 6-
diisopropyl phenol is brominated in the 4-position (bromine / acetic acid),
methylated
(iodomethane / potassium carbonate / DMF), reacted with n-butyl lithium to
effect
lithium halogen exchange and the resultant organolithium species is reacted
with
carbon dioxide to provide 3, 5-diisopropyl, 4-methoxy benzoic acid.
The compounds of formula (Ia: A, C is F; D is H; B is OH; Y, Z is H; E is S or
O) can be prepared starting from the compound of formula (III: Q, Y, Z is H; E
is S or
O) and the appropriate benzoic acid chloride (IV: A, C is F; D is H; B is
OMe). The
benzoic acid chloride (IV: A, C is F; D is H; B is OMe) is prepared from the
corresponding benzoic acid by standard procedures using reagents such as
oxalyl
chloride and thionyl chloride. The starting benzoic acid of the benzoic acid
chloride
(IV: A, C is F; D is H; B is OMe) can be easily prepared from the known, 4-
bromo-2,
6-difluoroaniline (L. I. Kruse, et al., Biockemistry 1986, 25, 7271-7278) by
reacting
the latter compound with n-butyl lithium to effect deprotonation ortho to the
bromine
and fluorine atoms, reaction of the resultant organolithium species with
carbon
dioxide to install the carboxy moiety ortho to the bromine and fluorine atoms,
and
further reaction with n-bultyl lithium to effect lithium-bromine exchange and
reaction
of the final, resultant organolithium species with a proton source upon
aqueous
workup to provide 2, 4-difluoro, 3-methoxy benzoic acid. Precedence for the
fluorine
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 16-
directed ortholithiation reaction over lithium-bromine exchange reaction is
found in
the following paper: F. Mongin and M. Schlosser, Tetrahedron Lett. 1996, 37,
6551-
6554.
D
C
Scheme 1B ~ Br
/ / ~ B / CoCi
CHO ~ ~ q
/ OH (VII) ( / O~~ (IX)
Q
(VI) (VIII)
B
(X) (Ia')
In Scheme 1B, according to a procedure in Syn. Comm. 1987, 17, 341-354
commercially available salicylaldehyde (VI) is reacted with one molar
equivalent of,
2-bromacetophenone (VII), one or more molar equivalents of potassium carbonate
and 5 mole % of tetrabutylammonium sulfate in a biphasic mixture of water and
dichloromethane at room temperature to provide the compound of formula (VIII:
Q =
O). The ketone group (Q = O) of (VIII) can be reduced under Wolf-Kishner
conditions (hydrazine, followed by potassium hydroxide in diethylene glycol
reflux)
to produce the compound of formula (VIII: Q = HZ).
The compounds of formula (VIII: Q = HZ) can be acylated with one or more
molar, equivalents of a commercially available benzoic acid chloride of
formula (IX:
A, B, C, D is H or OMe; with the A, B, C, D, combination of substituents
having at
least one OMe group but not more than three OMe groups) to produce the
acylated
derivative of formula (X: A, B, C, D is H or OMe; with the A, B, C, D,
combination
CA 02330623 2000-10-30
WO 99/58521 PCT/11599/10185
-17-
of substituents having at least one OMe group but not more than three OMe
groups).
This acylation is accomplished most readily using a one to five molar
equivalents of a
Lewis acid catalyst such as tin tetrachloride or aluminum chloride in an inert
solvent
such as dichloromethane, 1, 2-dichloroethane or carbon disulfide, generally at
temperatures such as -78°C to room temperature.
Cyclization of the compounds of formula (X: A, B, C, D is H or OMe; with
the A, B, C, D, combination of substituents having at least one OMe group but
not
more than three OMe groups) is generally best accomplished using one to ten
molar
equivalents of a strong Lewis acid such as a trihaloborane, most conveniently
tribromoborane. The reaction is best performed at -78°C with warming to
room
temperature in a halocarbon solvent such as dichloromethane under an inert
atmosphere such as nitrogen or argon. These procedures not only effect
cyclization
and aromatization with concomitant loss of water, but also result in
demethylation of
any pendant methoxy moieties and result in the production of compounds of
formula
(Ia': A, B, C, D is H or OMe; with the A, B, C, D, combination of substituents
having
at least one OH group but not more than three OH groups).
In an analogous fashion to the reactions above in Scheme 1B, the compounds
of formula (Ia': A is H; B, D is alkyl of 1-6 carbon atoms; C is OH) can be
prepared
starting from the compound of formula (VIII: Q is HZ) and the appropriate
benzoic
acid chloride (IX: A is H; B, D is alkyl of 1-6 carbon atoms; C is OMe). The
benzoic
acid chloride (IX: A is H; B, D is alkyl of 1-6 carbon atoms; C is OMe). is
prepared
from the corresponding benzoic acid by standard procedures using reagents such
as
oxalyl chloride and thionyl chloride. The starting benzoic acid of the benzoic
acid
chloride (IX is H; B, D is alkyl of 1-6 carbon atoms; C is OMe) is
commercially
available or can be easily prepared by known procedures. For example, the acid
starting material for benzoic acid chloride (IX: A is H; B, D is isopropyl; C
is OMe)
can be prepared using a modification of the method of Schuster, et al., J.
Org. Chem
1988, 53, 5819. Thus commercially available 2, 6-diisopropyl phenol is
brominated in
the 4-position (bromine / acetic acid), methylated (iodomethane / potassium
carbonate
/ DMF}, reacted with n-butyl lithium to effect lithium halogen exchange and
the
CA 02330623 2000-10-30
WO 99158521 PCT/I1S99/10185
-18-
resultant organolithium species is reacted with carbon dioxide to provide 3, 5-
diisopropyl, 4-methoxy benzoic acid.
Scheme 2
(Ib) (Ic)
Further derivatives of the compounds of formula (I) in Scheme 2 can be
prepared by the following methods. The phenol of formula (Ib: B, D, X is H; C
is OH;
E is S, O) can be brominated in three positions to afford the tribromophenol
of
formula (Ib: B, D, X is Br; C is OH; E is S, O) using at least 3 molar
equivalents of
molecular bromine in an appropriate solvent such as acetic acid. One to fifty
molar
equivalents of a salt of acetic acid such as potassium or sodium acetate can
also be
used as a co-reagent in this reaction although it is not absolutely required.
The
tribromophenol of formula (Ib: B, D, X is Br; C is OH; E is S, O) can be
methylated
to produce the methyl ether of formula (Ib: B, D, X is Br; C is OMe; E is S,
O) by
reacting the phenol moiety with a suitable methylating agent such as one or
more
molar equivalents of methyl iodide or dimethylsulfate employing a base such an
alkali
methyl carbonate or hydroxide such as potassium carbonate or sodium hydroxide
in a
suitable solvent such as THF, DMF or DMSO. The reaction is generally performed
at
temperatures ranging from 0°C to 60°C.
The methyl ether of formula (Ib: B, D, X is Br; C is OMe; E is S, O) can be
reacted with three or more molar equivalents of lower tetra-alkyltin in the
presence of
a palladium catalyst such as 1 to 10 mole % of
bis(triphenylphosphine)palladium II
chloride in a suitable solvent such as DMF, DMA or 1-methyl-2-pyrrolidinone at
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 19-
temperatures ranging from 140°C to 200°C to provide the
trialkylmethoxy derivative
of formula (Ib: B, D, X is alkyl of 1-6 carbon atoms; C is OMe; E is S, O).
This
methoxy analog can be converted to the corresponding phenol analog of formula
(Ic:
B, D, X is alkyl of 1-6 carbon atoms; E is S, O) using standard demethylation
procedures including one or more molar equivalents of boron tribromide or
boron
trichloride in dichloromethane at -78°C to room temperature; excess
neat pyridinium
hydrochloride at 190 to 280°C; hydrobromic acid in acetic acid at
0°C to 50°C; excess
trimethylsilylbromide or trimethylsilyliodide in dichloromethane, carbon
tetrachloride
or acetonitrile at -78°C to 50°C; lithium iodide in pyridine or
quinoline at
temperatures from 100° to 250°C and one or more molar
equivalents of ethyl, methyl
or isopropyl mercaptan in the presence of one or more molar equivalents of a
Lewis
acid such as aluminum trichloride or boron trifluoride in a solvent such as
dichloromethane at temperatures ranging from -78°C to 50°C.
The phenol of formula (Ib: B, D, X is H; C is OH; E is S, O) (Scheme 2) can
be conveniently iodinated to the diiodophenol of formula (Ib: B, D is I; X is
H; C is
OH; E is S, O) using at least two molar equivalents of iodine in the presence
of two or
more molar equivalents of an alkali metal hydroxide such as NaOH in an alcohol
solvent such as methanol at -20°C to room temperature. Similarly the
monoiodophenol (Ib: B is I; X, D is H; C is OH; E is S, O) can be prepared
from the
phenol of formula (Ib: B, D, X is H; C is OH; E is S, O) (Scheme 2) using one
to 1.5
molar equivalents of iodine in the presence of at least one equivalent of an
alkali
metal hydroxide such as NaOH in an alcohol solvent such as methanol at -
20°C to
room temperature. Either the monoiodophenol (Ib: B is I; X, D is H; C is OH; E
is S,
O) or the diiodophenol (Ib: B, D is I; X is H; C is OH; E is S, O) can be
converted to
the respective methyl ether derivatives of formula (Ib: B is I; X, D is H; C
is OMe; E
is S, O) or (Ib: B, D is I; X is H; C is OMe; E is S, O) by reacting the
phenol moiety
with a suitable methylating agent such as one or more molar equivalents of
methyl
iodide or dimethylsulfate employing a base such an alkali methyl carbonate or
hydroxide such as potassium carbonate or sodium hydroxide in a suitable
solvent such
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-20-
as THF, DMF or DMSO. The reaction is generally performed at temperatures
ranging
from 0°C to 60°C.
The monoiodo methylether derivative of formula (Ib: B is I; X, D is H; C is
OMe; E is S, O) or the diiodo methylether of formula (Ib: B, D is I; X is H; C
is OMe;
E is S, O) can be reacted with one or more molar equivalents of copper (I)
cyanide for
the monoiodo analog or two or more molar equivalents of copper (I) cyanide for
the
diiodo derivative to produce the monocyanomethyl ether of formula (Ib: B is
CN; X,
D is H; C is OMe; E is S, O) or the dicyanomethyl ether of formula (Ib: B, D
is CN; X
is H; C is OMe; E is S, O). The cyanation reaction is generally performed at
temperatures ranging from 100°C to 250°C employing polar aprotic
solvents such as
DMF, 1-methyl-2-pyrrolidinone or HMPA. Quinoline or pyridine can also be used.
The mono or dicyano methoxy analogs of formula (Ib: B is CN; D is H or CN; X
is H;
C is OMe; E is S, O) can be converted to the corresponding mono or dicyano
phenol
analogs of formula (Ic: B is CN; D is H or CN; X is H; E is S, O) (Scheme 2)
using
standard demethylation procedures including one or more molar equivalents of
boron
tribromide or boron trichloride in dichloromethane at -78°C to room
temperature;
excess neat pyridinium hydrochloride at 190 to 280°C; hydrobromic acid
in acetic
acid at 0°C to 50°C; excess trimethylsilylbromide or
trimethylsilyliodide in
dichloromethane, carbon tetrachloride or acetonitrile at -78°C to
50°C; lithium iodide
in pyridine or quinoline at temperatures from 100° to 250°C and
one or more molar
equivalents of ethyl, methyl or isopropyl mercaptan in the presence of one or
more
molar equivalents of a Lewis acid such as aluminum trichloride or boron
trifluoride in
a solvent such as dichloromethane at temperatures ranging from -78°C to
50°C.
The monoiodo methylether derivative of formula (Ib: B is I; X, D is H; C is
OMe; E is S, O) or the diiodo methylether of formula (Ib: B, D is I; X is H; C
is OMe;
E is S, O) (Scheme 2) can be reacted with one or more molar equivalents of
copper (I)
bromide for the monoiodo analog or two or more molar equivalents of copper (I)
bromide for the diiodo derivative to produce the monobromo methyl ether of
formula
(Ib: B is Br; X, D is H; C is OMe; E is S, O) or the dibromo-methyl ether of
formula
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-21 -
(Ib: B, D is Br; X is H; C is OMe; E is S, O). The bromine/idodine exchange
reaction
is generally performed at temperatures ranging from 100°C to
250°C employing polar
aprotic solvents such as DMF, 1-methyl-2-pyrrolidinone or HMPA. Quinoline or
pyridine can also be used. The mono or dibromo methoxy analogs of formula (Ib:
B is
Br; D is H or Br; X is H; C is OMe; E is S, O) can be converted to the
corresponding
mono or dibromo phenol analogs of formula (Ic: B is Br; D is H or Br; X is H;
E~is S,
O) (Scheme 2) using standard demethylation procedures including one or more
molar
equivalents of boron tribromide or boron trichloride in dichloromethane at -
78°C to
room temperature; excess neat pyridinium hydrochloride at 190 to 280°C;
hydrobromic acid in acetic acid at 0°C to 50°C; excess
trimethylsilylbromide or
trimethylsilyliodide in dichloromethane, carbon tetrachloride or acetonitrile
at -78°C
to 50°C; lithium iodide in pyridine or quinoline at temperatures from
100° to 250°C
and one or more molar equivalents of ethyl, methyl or isopropyl mercaptan in
the
presence of one or more molar equivalents of a Lewis acid such as aluminum
trichloride or boron trifluoride in a solvent such as dichloromethane at
temperatures
ranging from -78°C to 50°C.
The monoiodo or monobromo methylether or phenol derivatives of formula
(Ib: B is Br, I; X, D is H; C is , OH, OMe; E is S, O) or the diiodo or
dibromo
methylether or phenols of formula (Ib: B, D is Br, I; X is H; C is OH, OMe; E
is S, O)
(Scheme 2) can be reacted with one or more molar equivalents of an aryl
boronic acid
for the monoiodo or monobromo analog or two or more molar equivalents of aryl
boronic acid for the diiodo or dbromo derivative to produce the monophenyl
methyl
ether or phenols of formula (Ib: B is phenyl; X, D is H; C is OH, OMe; E is S,
O) or
the dibromo-methyl ethers or phenols of formula (Ib: B, D is phenyl; X is H; C
is OH,
OMe; E is S, O). This reaction is best known as the Suzuki reaction (N.
Miyaura, T.
Yanagi , A Suzuki, Synthetic Comm. 1981,11, S 13-319) and further involves the
use
of 0.5 to 10 mol% of a palladium catalyst such as tetakis(triphenylphosphine)
palladium or a palladium (II) species such as palladium acetate or [1,1'-
bis(diphenyphosphino)ferroceneJpalladium(II). One or more equivalents of an
alkali-
metal base is also needed and some of the more common bases include sodium,
CA 02330623 2000-10-30
WO 99/58521 PCT/iJS99/10185
-22-
potassium or cesium carbonate; sodium, potassium, barium or thalium hydroxide
and
potassium phosphate. The reaction can be run in a variety of solvents
including
benzene, THF, dioxane, DME or DMF. For some of these solvents, such as THF and
benzene, water or methanol can be used as a colvent. The reaction is generally
run at
temperatures ranging from room temperature to 120°C.
The mono or dibromo methoxy analogs of formula (Ib: B is Br; D is H or Br;
X is H; C is OMe; E is S, O) and the mono and diphenyl methoxy analogs of
formula
(Ib: B is Ph; D is H or Ph; X is H; C is OMe; E is S, O) can be converted to
the
corresponding mono or dibromo phenol analogs of formula (Ic: B is Br; D is H
or Br;
X is H; E is S, O) or the mono and diphenyl phenol analogs of formula (Ib: B
is Ph; D
is H or Ph; X is H; C is OH; E is S, O) (Scheme 2) using standard
demethylation
procedures including one or more molar equivalents of boron tribromide or
boron
trichloride in dichloromethane at -78°C to room temperature; excess
neat pyridinium
hydrochloride at 190 to 280°C; hydrobromic acid in acetic acid at
0°C to 50°C; excess
trimethylsilylbromide or trimethylsilyliodide in dichloromethane, carbon
tetrachloride
or acetonitrile at -78°C to 50°C; lithium iodide in pyridine or
quinoline at
temperatures from 100° to 250°C and one or more molar
equivalents of ethyl, methyl
or isopropyl mercaptan in the presence of one or more molar equivalents of a
Lewis
acid such as aluminum trichloride or boron trifluoride in a solvent such as
dichloromethane at temperatures ranging from -78°C to 50°C.
Scheme 3
(Id) (Ie)
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-23-
Further derivatives of the compounds of formula (I) in Scheme 3 can be
prepared by the following methods. The compounds of formula (Id: B, C, D is H
or
OH; with the B, C, D combination having at least one OH group; E is S, O)
(Scheme
3) can be acylated on the phenolic oxygen using one or more molar equivalents
of
suitable acylating agent to provide the compounds of formula (Id: B, C, D is H
or
OCOR; with the B, C, D combination having at least one OCOR group; R is alkyl
of
1-6 carbon atoms, aryl; E is S, O). The acylating agent is generally a alkyl
of 1-6
carbon atoms or aryl carboxylic acid anhydride or a alkyl of 1-6 carbon atoms
or aryl
carboxylic acid chloride. The reaction is run under standard conditions, for
example,
the use of pyridine as solvent with or without a co-solvent such as
dichloromethane at
0°C to room temperature. The acylated phenols of formula (Id: B, C, D
is H or
OCOR; with the B, C, D combination having at least one OCOR group; R is alkyl
of
1-6 carbon atoms, aryl; E is S, O) can then be brominated in the 6-position of
the
benzo[b]naphtho[2,3-dJthiophene or benzo[b]naphtho[2,3-d]furan ring to form
the
acylated bromophenols of formula (Ie: B, C, D is H or OCOR; with the B, C, D
combination having at least one OCOR group; R is alkyl of 1-6 carbon atoms,
aryl; X
is Br; E is S, O) (Scheme 3). This bromination reaction is generally done
using 1 to
1.3 molar equivalents of molecular bromine in an inert solvent such as
dichloromethane or carbon tetrachloride at temperatures ranging from -78
°C to room
temperature.
Using a similar bromination reaction, the phenols of formula (Id: B, D is
alkyl
of 1-6 carbon atoms, C is OH; E is S, O) can then be brominated in the 6-
position of
the benzo[b]naphtho[2,3-d]thiophene or benzo[b]naphtho[2,3-dJfuran ring to
form the
bromophenols of formula (Ie: B, D is alkyl of 1-6 carbon atoms, C is OH; X is
Br; E
is S, O} (Scheme 3). This bromination reaction is generally done using 1 to
1.3 molar
equivalents of molecular bromine in an inert solvent such as dichloromethane
or
carbon tetrachloride at temperatures ranging from -78 °C to room
temperature.
The acyl group can then be removed from the acylated bromophenols of
formula (Ie: B, C, D is H or OCOR; with the B, C, D combination having at
least one
OCOR group; R is alkyl of 1-6 carbon atoms, aryl; X is Br; E is S, O) to
provide the
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-24-
bromophenols of formula (Ie: B, C, D is H or OH; with the B, C, D combination
having at least one OH group; X is Br; E is S, O) (Scheme 3) using standard
conditions. These conditions include aqueous base in which one or more molar
equivalents of alkali metal hydroxide such as sodium hydroxide is used in
water with
a co-solvent such as THF, dioxane or a lower alcohol such as methanol or
mixtures
of THF and a lower alcohol at temperatures ranging from 0°C to
40°C. acid
conditions may also be employed in which the compound is reacted with one or
more
molar equivalents of a mineral acid such as HCl or sulfuric acid in water with
or
without a co-solvent such as THF at temperatures ranging from room temperature
to
80°C.
The acylated phenols of formula (Id: B, C, D is H or OCOR; with the B, C, D
combination having at least one OCOR group; R is alkyl of 1-6 carbon atoms,
aryl; E
is S, O) can be nitrated to provide the nitro compounds of formula (Ie: B, C,
D is H or
OCOR; with the B, C, D combination having at least one OCOR group; R is alkyl
of
I-6 carbon atoms, aryl; X is NO2; E is S, O) (Scheme 3). Dilute nitric acid at
temperatures ranging from 0°C to room temperature is suitable to effect
this
transformation. The nitro compounds of formula (Ie: B, C, D is H or OCOR; with
the
B, C, D combination having at least one OCOR group; R is alkyl of I-6 carbon
atoms,
aryl; X is NO2; E is S, O) can be further reduced to the primary amine of
formula (le:
B, C, D is H or OCOR; with the B, C, D combination having at least one OCOR
group; R is alkyl of 1-6 carbon atoms, aryl; X is NH2; E is S, O) using a
suitable
reducing agent such as catalytic hydrogenation with a palladium or platinum
catalyst,
tin dichloride in aqueous HCl or in ethyl acetate. The acyl group of the
compounds of
formula (Ie: B, C, D is H or OCOR; with the B, C, D combination having at
least one
OCOR group; R is alkyl of I-6 carbon atoms, aryl; X is N02 or NH2; E is S, O)
can be
removed by using standard conditions to provide the phenols of formula (Ie: B,
C, D
is H or OH; with the B, C, D combination having at least one OH group; R is
alkyl of
1-6 carbon atoms, aryl; X is NOZ or NHZ; E is S, O).
The acylated bromophenols of formula (Ie: B, C, D is H or OCOR; with the B,
C, D combination having at least one OCOR group; R is alkyl of I-b carbon
atoms,
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 25 -
aryl; X is Br; E is S, O) (Scheme 3) can be converted to the acylated
cyanophenols of
formula (Ie: B, C, D is H or OCOR; with the B, C, D combination having at
least one
OCOR group; R is alkyl of 1-6 carbon atoms, aryl; X is CN; E is S, O) by
reaction
with one or more molar equivalents of copper (I) cyanide. The cyanation
reaction is
generally performed at temperatures ranging from 100°C to 250°C
employing polar
aprotic solvents such as DMF, 1-methyl-2-pyrrolidinone or HMPA. Quinoline or
pyridine can also be used. Often the acyl group of (Ie: B, C, D is H or OCOR;
with
the B, C, D combination having at least one OCOR group; R is alkyl of 1-6
carbon
atoms, aryl; X is CN; E is S, O) is liberated under the cyanation reaction
conditions to
afford the cyanophenols of formula (Ie: B, C, D is H or OH; with the B, C, D
combination having at least one OCOR group; X is CN; E is S, O). This
liberation of
the acyl group to afford the cyanophenols of formula (Ie: B, C, D is H or OH;
with the
B, C, D combination having at least one OH group; X is CN; E is S, O) can be
effected most readily by addition of one or more molar equivalents of alkali
metal
hydroxide in water to the reaction mixture containing (Ie: B, C, D is H or
OCOR;
with the B, C, D combination having at least one OCOR group; R is alkyl of 1-6
carbon atoms, aryl; X is CN; E is S, O) prior to workup. The acyl group can
also be
removed from the isolated acylated cyanophenols of formula (Ie: B, C, D is H
or
OCOR; with the B, C, D combination having at least one OCOR group; R is alkyl
of
1-6 carbon atoms, aryl; X is CN; E is S, O) to provide the cyanophenols of
formula
(Ie: B, C, D is H or OH; with the B, C, D combination having at least one OH
group;
X is CN; E is S, O) by using standard conditions. These conditions include
aqueous
base in which one or more molar equivalents of alkali metal hydroxide such as
sodium hydroxide is used in water with a co-solvent such as THF, dioxane or a
lower
alcohol such as methanol or mixtures of THF and a lower alcohol at
temperatures
ranging from 0°C to 40°C. acid conditions may also be employed
in which the
compound is reacted with one or more molar equivalents of a mineral acid such
as
HCl or sulfuric acid in water with or without a co-solvent such as THF at
temperatures ranging from room temperature to 80°C.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-26-
The compounds of formula (Id: B, C, D is H or OH; with the B, C, D
combination having at least one OH group; E is S, O) (Scheme 3) can be
sulfonylated
on the phenolic oxygen using one or more molar equivalents of suitable
sulfonylating
agent to provide the sulfonic acid esters of formula (Id: B, C, D is H or
OSOZR; with
the B, C, D combination having at least one OSOzR group; R is alkyl of 1-6
carbon
atoms, aryl; E is S, O). The sulfonylating agent is generally a alkyl of 1-6
carbon
atoms or aryl sulfonic acid anhydride or a alkyl of 1-6 carbon atoms or aryl
sulfonic
acid chloride. The reaction is run under standard conditions such as using
pyridine as
solvent with or without a co-solvent such as dichloromethane at 0°C to
room
temperature.
The sulfonic acid esters of formula (Id: B, C, D is H or OS02R; with the B, C,
D combination having at least one OSOZR group; R is alkyl of 1-6 carbon atoms,
aryl;
E is S, O) can be treated with a chlorinating agent to effect chlorination at
the 6-
position of the benzo[b]naphtho[2,3-d]thiophene or benzo[b]naphtho[2,3-d]furan
ring
to afford the chloro-sulfonic acid esters of formula (Ie: B, C, D is H or
OSOZR; with
the B, C, D combination having at least one OSOZR group; R is alkyl of 1-6
carbon
atoms, aryl; X is Cl; E is S, O). Suitable chlorinating agents include one or
more
molar equivalents of sulfuryl chloride, chlorine gas or N-chlorosuccinimide in
suitable halocarbon solvents such as dichloromethane or chloroform at
temperatures
ranging from -78°C to 40 °C. The sulfonic ester group can then
be removed from the
chloro-sulfonic acid esters of formula (Ie: B, C, D is H or OSOZR; with the B,
C, D
combination having at least one OSOZR group; R is alkyl of 1-6 carbon atoms,
aryl; X
is Cl; E is S, O) to provide the chlorophenols of formula (Ie: B, C, D is H or
OH; with
the B, C, D combination having at least one OH group; X is CI; E is S, O)
(Scheme 3)
using standard conditions. These conditions include aqueous base in which one
or
more molar equivalents of alkali metal hydroxide such as sodium hydroxide is
used in
water with a co-solvent such as THF, dioxane or a lower alcohol such as
methanol or
mixtures of THF and a lower alcohol at temperatures ranging from room
temperature
to 110°C.
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-27-
The sulfonic acid esters of formula (Id: B, C, D is H or OSOZR; with the B, C,
D combination having at least one OSOzR group; R is alkyl of 1-6 carbon atoms,
aryl;
E is S, O) can also be treated with iodinating reagents to effect iodination
at the 6-
position of the benzo[b]naphtho[2,3-d)thiophene or benzo[b)naphtho[2,3-d]furan
ring
to afford the iodo-sulfonic acid esters of formula (Ie: B, C, D is H or OS02R;
with the
B, C, D combination having at least one OS02R group; R is alkyl of 1-6 carbon
atoms, aryl; X is I; E is S, O). A suitable iodinating reagent includes a
mixture of 0.7
or more molar equivalents of molecular iodine and 0.25 or more molar
equivalents of
iodic acid in a mixture of THF and 80% aqueous acetic acid with a small amount
of
concentrated sulfuric acid at temperatures ranging from room temperature to
80°C.
The sulfonic ester group can then be removed from the iodo-sulfonic acid
esters of
formula (Ie: B, C, D is H or OSOZR; with the B, C, D combination having at
least one
OSOZR group; R is alkyl of 1-6 carbon atoms, aryl; X is I; E is S, O) to
provide the
iodophenols of formula (Ie: B, C, D is H or OH; with the B, C, D combination
having
at least one OH group; X is I; E is S, O) (Scheme 3) using standard
conditions. These
conditions include aqueous base in which one or more molar equivalents of
alkali
metal hydroxide such as sodium hydroxide is used in water with a co-solvent
such as
THF, dioxane or a lower alcohol such as methanol or mixtures of THF and a
lower
alcohol at temperatures ranging from room temperature to 110°C.
Scheme 4
~I~ n
The iodo sulfonic acid esters of formula (If: C, D is H or OS02R; C, D cannot
both be H; R is alkyl of 1-6 carbon atoms, aryl; E is S, O) were a convenient
starting
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-28-
point for further derivatives of the compounds of formula (I) as shown in
Scheme 4
and the methods below. The compounds (If: C, D is H or OSOZR; C, D cannot both
be
H; R is alkyl of 1-6 carbon atoms, aryl; E is S, O) can be reacted with a
reagent that
catalyzes the exchange of the iodine atom in (If) with a perfluoroalkyl of 1-6
carbon
atoms group to afford the compound of formula (Ig: C, D is H or OSOzR; C, D
cannot
both be H; R is alkyl of 1-6 carbon atoms, aryl; X is perfluoroalkyl of 1-6
carbon
atoms; E is S, O) (Scheme 4). The reagent and conditions to effect this
exchange
include reacting (Ifj under anhydrous conditions with one to ten molar molar
equivalents of a sodium perfluorocarboxylate (RCOZNa: R is perfluoroalkyl) and
one
to five molar molar equivalents of copper (I) iodide in a high boiling inert
solvent
such as DMF, DMA or 1-methyl-2-pyrrolidinone at temperatures ranging from
140°C
to 200°C. Alternatively, the compound of formula (Ig: C, D is H or
OSOZR; C, D
cannot both be H; R is alkyl of 1-6 carbon atoms, aryl; X is perfluoroalkyl of
1-6
carbon atoms; E is S, O) can be prepared from the compound of formula (If: C,
D is H
or OS02R; C, D cannot both be H; R is alkyl of 1-6 carbon atoms, aryl; E is S,
O) by
reacting the former with one to ten molar molar equivalents of a
perfluoroalkyl iodide
and one to five molar molar equivalents of activated Cu° in a high
boiling inert
solvent such as DMF, DMA or 1-methyl-2-pyrrolidinone at temperatures ranging
from 140°C to 200°C. Still, alternatively, the compound of
formula (If: C, D is H or
OSOZR; C, D cannot both be H; R is alkyl of 1-6 carbon atoms, aryl; E is S, O)
can be
reacted with 0.5 to two molar equivalents of bis(trifluoromethylmercury) and
two to
four molar equivalents of activated Cu° in a high boiling inert solvent
such as DMF,
DMA or 1-methyl-2-pyrrolidinone at temperatures ranging from 140°C to
200°C to
produce the compound of (Ig: C, D is H or OSOZR; C, D cannot both be H; R is
alkyl
of 1-6 carbon atoms, aryl; X is CF3; E is S, O).
6-Alkyl of 1-6 carbon atoms derivatives of the compound of formula (Ig: C, D
is H or OSOZR; C, D cannot both be H; R is alkyl of 1-6 carbon atoms, aryl; X
is alkyl
of 1-6 carbon atoms; E is S, O) (Scheme 4) can be prepared by reaction of (If:
C, D is
H or OS02R; C, D cannot both be H; R is alkyl of 1-6 carbon atoms, aryl; E is
S, O)
with three or more molar equivalents of lower tetra-alkyltin in the presence
of a
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-29-
palladium catalyst such as 1 to 10 mole % of bis(triphenylphosphine)palladium
II
chloride in a suitable solvent such as DMF, DMA or 1-methyl-2-pyrrolidinone at
temperatures ranging from 140°C to 200°C.
The sulfonic ester group can then be removed from the sulfonic acid esters of
formula (Ig: C, D is H or OSOZR; C, D cannot both be H; R is alkyl of 1-6
carbon
atoms, aryl; X is alkyl of 1-6 carbon atoms or perfluoroalkyl of 1-6 carbon
atoms; E is
S, O) to provide the phenols of formula (Ig: C, D is H or OH; C, D cannot both
be H;
X is alkyl of 1-6 carbon atoms or perfluoroalkyl of 1-6 carbon atoms; E is S,
O) using
standard conditions. These conditions include aqueous base in which one or
more
molar equivalents of alkali metal hydroxide such as sodium hydroxide is used
in
water with a co-solvent such as THF, dioxane or a lower alcohol such as
methanol or
mixtures of THF and a lower alcohol at temperatures ranging from room
temperature
to 110°C.
6-Alkoxy derivatives of the compound of formula (Ig: C, D is H, OH; C, D
cannot both be H; X is alkoxy of 1-6 carbon atoms; E is S, O) can be prepared
by
reaction of (If: C, D is H or OSOZR; C, D cannot both be H; R is alkyl of 1-6
carbon
atoms, aryl; E is S, O) with three or more molar equivalents of lower alkali
metal
alkoxide in the presence of a copper (I) or copper (II) catalyst such as 1 to
10 mole
copper (II) chloride in a suitable solvent such as DMF, DMA or 1-methyl-2-
pyrrolidinone at temperatures ranging from 80°C to 180°C. Under
the reaction
conditions, the sulfonic acid group of (If: C, D is H or OSOZR; C, D cannot
both be
H; R is alkyl of 1-6 carbon atoms, aryl; E is S, O) is removed.
6-Sulfanyl derivatives of the compound of formula (Ig: C, D is H or OH; C, D
cannot both be H; X is alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl; E is S, O) can be prepared
by
reaction of (If: C, D is H or OSOzR; C, D cannot both be H; R is alkyl of 1-6
carbon
atoms, aryl; E is S, O) with one or more molar equivalents of the appropriate
alkyl of
1-6 carbon atomsthiol, arylthiol, thiopyridine or 2-N,N-dimethylaminoethyl-
mercaptan, one or more molar equivalents of an alkali metal hydroxide such as
sodium hydroxide, one or more molar equivalents of a copper (I) or copper (II)
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/t0I85
-30-
catalyst such as copper (I) oxide in a suitable solvent such as DMF, DMA or 1-
methyl-2-pyrrolidinone at temperatures ranging from 100°C to
180°C. Under the
reaction conditions, the sulfonic acid group of (If: C, D is H or OSOZR; C, D
cannot
both be H; R is alkyl of 1-6 carbon atoms, aryl; E is S, O) is removed.
Scheme 5
(Ih) (Ii)
Further derivatives of the compounds of formula (I) in Scheme 5 can be
prepared by the following methods. The phenols of formula (Ih: A is H or OH; X
is
H, halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon
atoms,
arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl; E is S, O)
can be
brominated in two positions to afford the dibromphenols of formula (Ii: A is H
or OH;
B, D is Br; X is H, halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of
1-6
carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-
6
carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl;
E is
S, O) using at least 2 molar equivalents of molecular bromine in an
appropriate
solvent such as acetic acid. One to fifty molar equivalents of a salt of
acetic acid such
as potassium or sodium acetate can also be used as a co-reagent in this
reaction
although it is not absolutely required.
The phenols of formula (Ih: A is H or OH; X is H, halogen, alkyl of 1-6
carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms,
aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-31-
N,N-dimethylaminoethylsulfanyl; E is S, O) can be chlorinated in two positions
to
afford the dichlorophenols of formula (Ii: A is H or OH; B, D is Cl; X is H,
halogen,
alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-
6
carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl; E is S, O) using two or
more
molar equivalents of chlorine in an appropriate solvent such as a lower
alcohol
solvent, most conveniently, methanol. The reaction is run at temperatures
ranging
from -78°C to room temperature.
The phenols of formula (Ih: A is H; X is H, halogen, alkyl of 1-6 carbon
atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms,
aralkoxy,
nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylaminoethylsulfanyl; E is S, O) can be mononitrated to the phenols of
formula
(Ii: A is H; B is NO2; D is H; X is H, halogen, alkyl of 1-6 carbon atoms, CN,
perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy,
nitro,
alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; E is S, O) most conveniently using iron (III)
trinitrate in
a lower alcohol solvent.
The nitro compounds of formula (Ii: A is H; B is NO2; D is H; X is H,
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; E is S, O) can be reduced
to the
amino compounds of formula (Ii: A is H; B is NH2; D is H; X is H, halogen,
alkyl of
1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms, aralkoxy, amino, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; E is S, O) most readily
using tin
dichloride in ethyl acetate at 40 to 100°C or with hydrazine and
Montmorillinite clay
in ethanol at 40 to 100°C.
The nitro compounds of formula (Ii: A is H; B is NO2; D is H; X is H,
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
-32-
pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl; E is S, O) can also be
brominated to the compounds of formula (Ii: A is H; B is NO2; D is Br; X is H,
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl; E is S, O) using at least 2
molar
equivalents of molecular bromine in an appropriate solvent such as acetic
acid. One to
fifty molar equivalents of a salt of acetic acid such as potassium or sodium
acetate can
also be used as a co-reagent in this reaction although it is not absolutely
required. The
bromo nitro compounds of formula (Ii: A is H; B is NO2; D is Br; X is H,
halogen,
alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-
6
carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl; E is S, O) can be reduced
to the
bromo amino compounds of formula (Ii: A is H; B is NH2; D is Br; X is H,
halogen,
alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-
6
carbon atoms, aralkoxy, amino, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl; E is S, O) most readily
using tin
dichloride in ethyl acetate at 40 to 100°C or with hydrazine and
Montmorillinite clay
in ethanol at 40 to 100°C.
The dibromo-bisphenols of formula (Ii: A is OH; B, D is Br; X is H; E is S, O)
can be further brominated in the 6-position of the benzo[bJnaphtho[2,3-
d]thiophene
ring to form the bisphenols of formula (Ii: A is OH; B, D, X is Br; E is S,
O). This
bromination reaction is generally done using 1 to 1.3 molar equivalents of
molecular
bromine in an inert solvent such as dichloromethane or carbon tetrachloride at
temperatures ranging from -78 °C to room temperature.
CA 02330623 2000-10-30
WO 99/58521 PCT/(JS99/10185
-33-
Scheme 6
(Ij) (Ik)
Further derivatives of the compounds of formula (I) in Scheme 6 can be
prepared by the following methods. The phenols of formula (Ij: C is H; D is
OH; X is
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl; E is S, O) can be mono-
brominated to provide the provide the bromophenols of formula (Ik: A, B is H;
C is
Br; D is OH; X is halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-
6
carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-
6
carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-
ethylsulfanyl; E is
S, O) using at least 1 molar equivalent of molecular bromine in an appropriate
solvent
such as acetic acid or dibrominated to provide the bromophenols of formula
(Ik: B is
H; A, C is Br; D is OH; X is halogen, alkyl of 1-6 carbon atoms, CN,
perfluoroalkyl
of I-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy, vitro,
alkylsulfanyl of 1-6
carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl;
E is
S, O) using at least 2 molar equivalents of molecular bromine in an
appropriate
solvent such as acetic acid. Similarly, the bisphenols of formula (Ij: C, D is
OH; X
is halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of I-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl; E is S, O) can be
monobrominated to provide a mixture of the bromobisphenols of formula (Ik: A
is H;
B is Br; C, D is OH; X is halogen, alkyl of 1-6 carbon atoms, CN,
perfluoroalkyl of 1-
6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of
1-6
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-34-
carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-
ethylsulfanyl; E is
S, O) and (Ik: A is Br; B is H; C, D is OH; X is halogen, alkyl of 1-6 carbon
atoms,
CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy,
vitro,
alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethyl-
amino-ethylsulfanyl; E is S, O) using at 1 molar equivalent of molecular
bromine in
an appropriate solvent such as acetic acid. This mixture can be separated into
pure
monobromo products by conventional means.
The bromobisphenols of formula (Ik: A is H; B is Br; C, D is OH; X is
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; E is S, O) can be
alkylated
regioselectively with a alkyl of 1-6 carbon atoms, allyl or benzyl halide on
the
phenolic hydroxyl occupied by position C to provide the monoalkylated products
of
formula (Ik: A is H; B is Br; C is alkoxy of 1-6 carbon atomsl, allyloxy or
benzyloxy,
D is OH; X is halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6
carbon
atoms, alkoxy of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6
carbon atoms,
arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; E is S, O)
using
one or more molar equivalents of an alkali metal carbonate such as potassium
carbonate in a polar aprotic solvent such as DMF.
The monobenzylated products of formula (Ik: A is H; B is Br; C is benzyloxy,
D is OH; X is halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6
carbon
atoms, alkoxy of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6
carbon atoms,
arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; E is S, O)
can be
further alkylated with a alkyl of 1-6 carbon atoms halide to provide the
dialkylated
product of formula (Ik: A is H; B is Br; C is benzyloxy, D is alkoxy of 1-6
carbon
atoms; X is halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6
carbon
atoms, alkoxy of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6
carbon atoms,
arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; E is S, O)
using
one or more molar equivalents of an alkali metal carbonate such as potassium
carbonate in a polar aprotic solvent such as DMF.
CA 02330623 2000-10-30
WO 99/58521 PCT/iJS99/10185
-35-
The benzyl group of the compounds of formula (Ik: A is H; B is Br; C is
benzyloxy, D is alkoxy of 1-6 carbon atoms; X is halogen, alkyl of 1-6 carbon
atoms,
CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy,
nitro,
alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; E is S, O) can be removed using standard
hydrogenolysis conditions, for example, hydrogen gas with a 5 to 10% palladium
on
carbon catalyst in a lower alcohol solvent or in ethyl acetate it THF to
provide the
phenols of formula (Ik: A is H; B is Br; C is OH, D is alkoxy of 1-6 carbon
atoms; X
is halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; E is S, O).
cheme 7
Z Z
(Il) (Im)
Further derivatives of the compounds of formula (I) in Scheme 7 can be
prepared by the following methods. The bisphenol of formula (Il: Y, C is OH; Z
is H;
E is S) can be reacted with one molar equivalent of methyl bromoacetate and
with one
or more molar equivalents of an alkali metal carbonate such as potassium
carbonate in
a polar aprotic solvent such as DMF to afford the monoalkylated product of
formula
(Im: Y is OCH2C02CH3; C is OH; Z is H; E is S). This product may be
contaminated
with small amounts (<10%) of the regioisomer of formula (Im: C is OCHzCOZCH3;
Y
is OH; Z is H; E is S). The regioisomers can be separated by conventional
means.
Alternatively, the bisphenols of formula (Il: Y, C is OH; Z is H; E is S) or
(Z,
C is OH; Y is H; E is S or O) can be diaklylated with two or more molar
equivalents
of an alkyl haloacetate of formula (XZCHzCO~Rb where XZ is Cl, Br or I and R6
is
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-36-
alkyl of 1-6 carbon atoms) and with two or more molar equivalents of an alkali
metal
carbonate such as potassium carbonate in a polar aprotic solvent such as DMF
to
afford the dialkylated product of formula (Im: Y, C is OCH2COzR6; Z is H; E is
S; R6
is alkyl of 1-6 carbon atoms) or (Z, C is OCHZCOZR6; Y is H; E is S or O; R6
is alkyl
of 1-6 carbon atoms).
The monoesters of formula (Im: Y is OCHZCOzCH3; C is OH; Z is H; E is S)
as well as the diesters of formula (Im: Y, C is OCHzCO2R6; Z is H; E is S; E
is S) or
(Z, C is OCHZCOzRb; Y is H; E is S or O) can be transformed into their
carboxylic
acid analogs using standard conditions to afford the moncarboxylic acids of
formula
(Im: Y is OCHZCOZH; C is OH; Z is H; E is S) and the dicarboxylic acids of
formula
(Im: Y, C is OCHZCOZH; Z is H; E is S) or (Z, C is OCHZCOZH; Y is H; E is S or
O).
The conditions to effect these transformations include aqueous base in which
one or
more molar equivalents of alkali metal hydroxide such as sodium hydroxide is
used in
water with a co-solvent such as THF, dioxane or a lower alcohol such as
methanol or
mixtures of THF and a lower alcohol at temperatures ranging from 0°C to
40°C.
~s
cheme
(In) (Io)
Further derivatives of the compounds of formula (I) in Scheme 8 can be
prepared by the following methods. The phenols of formula (In: B is H; A, C is
H, Br
or alkoxy of 1-6 carbon atoms; D is OH; X is halogen, alkyl of 1-6 carbon
atoms, CN,
perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy,
nitro,
alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethyl-
aminoethylsulfanyI; E is S, O) can be alkylated with one or more molar
equivalents of
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-37-
an alkyl haloacetate of formula (XZCHR6'COZR6 where XZ is Cl, Br or I and R6
is alkyl
of 1-6 carbon atoms, R6' is H) and with one or more molar equivalents of an
alkali
metal carbonate such as potassium carbonate in a polar aprotic solvent such as
DMF
to afford the alkylated product of formula (Io: B is H; A, C is H, Br or
alkoxy of 1-6
carbon atoms; X is halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-
6
carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-
6
carbon atoms, arylsulfanyl, pyridylsulfanyI, 2-N,N-dimethylaminoethylsulfanyl;
R6 is
alkyl of 1-6 carbon atoms, R6' is H; E is S, O).
Alternatively the bisphenols of formula (In: A, B is H or Br; C, D is OH; X is
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl; E is S, O) can be
diaklylated
with two or more molar equivalents of an alkyl haloacetate of formula
(XZCHR6'COZR6 where X2 is Cl, Br or I and R6 is alkyl of 1-6 carbon atoms, R6'
is H)
and with two or more molar equivalents of an alkali metal carbonate such as
potassium carbonate in a polar aprotic solvent such as DMF to afford the
dialkylated
esters of formula (Io: A, B is H or Br; C is OCHR6'COzRb; X is halogen, alkyl
of 1-6
carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms,
aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-
N,N-dimethylamino-ethylsulfanyl; R6 is alkyl of I-6 carbon atoms, R6' is H; E
is S,
O).
Still alternatively, the phenols of formula (In: B is H or halogen; A is H or
halogen; C is H, Br or alkoxy of 1-6 carbon atoms; D is OH; X is halogen,
alkyl of 1-
6 carbon atoms, CN, perfluoroalkyl of I-6 carbon atoms, alkoxy of 1-6 carbon
atoms,
aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-
N,N-dimethylaminoethylsulfanyl; E is S, O) can be reacted with a 2-hydroxy
carboxylic acid ester of formula CH(OH)(R6')C02R6 (R6, R6' is alkyl of 1-6
carbon
atoms, aralkyl, aryl) to afford the esters of formula (Io: B is H or halogen;
A is H or
halogen; C is H, Br or alkoxy of 1-6 carbon atoms; X is halogen, alkyl of 1-6
carbon
atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms,
aralkoxy,
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-38-
nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylaminoethylsulfanyl; R6, R6' is alkyl of I-6 carbon atoms, aralkyl,
aryl; E is S,
O) under the conditions of the Mitsunobu Reactions (for a review see Oyo
Mitsunobu
Synthesis. 1981, I-27). The other co-reagents necessary to effect the
Mitsunobu
Reaction include one or more molar equivalents of a alkyl of I-6 carbon atoms
azodicarboxylate diester such as diethyl azodicarboxylate or diisopropyl
azodicarboxylate and one or more molar equivalents of triarylphosphine such as
triphenylphosphine in a suitable solvent such as diethyl ether, THF, benzene
or
toluene at temperatures ranging from -20°C to 120°C.
The monoesters of formula (Io: A, B is H or halogen; C is H, Br or alkoxy of
1-6 carbon atoms; X is halogen, alkyl of I-6 carbon atoms, CN, perfluoroalkyl
of 1-6
carbon atoms, alkoxy of I-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-
6
carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl;
R6,
R6' is alkyl of I-6 carbon atoms, aralkyl, aryl; E is S, O} as well as the
diesters of
IS formula (Io: A, B is H or Br; C is OCHR6'COZR6, X is halogen, alkyl of 1-6
carbon
atoms, CN, perfluoroalkyl of I-6 carbon atoms, alkoxy of 1-6 carbon atoms,
aralkoxy,
nitro, alkylsulfanyl of I-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; R6 is alkyl of I-6 carbon atoms, R6' is H; E is
S, O) can
be transformed into their carboxylic acid analogs using standard conditions to
afford
the moncarboxylic acids of formula (Io: A, B is H or halogen; C is H, Br or
alkoxy of
I-6 carbon atoms; X is halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl
of 1-6
carbon atoms, alkoxy of I-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-
6
carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl;
R6 is
H; R6' is alkyl of 1-6 carbon atoms, aralkyl, aryl; E is S, O) and the
dicarboxylic acids
of formula (Io: A, B is H or Br; C is OCHR6'COZR6, X is halogen, alkyl of 1-6
carbon
atoms, CN, perfluoroalkyl of I-6 carbon atoms, alkoxy of 1-6 carbon atoms,
aralkoxy,
nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; R6, R6' is H; E is S, O). The conditions to
effect these
transformations include aqueous base in which one or more molar equivalents of
alkali metal hydroxide such as sodium hydroxide is used in water with a co-
solvent
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-39-
such as THF, dioxane or a lower alcohol such as methanol or mixtures of THF
and a
lower alcohol at temperatures ranging from 0°C to 40°C.
Z Z
(IP) (I9)
Further derivatives of the compounds of formula (I) in Scheme 9 can be
prepared by the following methods. The phenols of formula (Ip: A is H; B, D is
H,
halogen, CN, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon
atoms,
vitro; Y, Z is H or OCHZCOZR6, but Y and Z are not concurrently OCHZCOZR6; X
is
H, halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon
atoms,
arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; R6 is alkyl
of 1-6
carbon atoms; E is S, O) can be alkylated with one or more molar equivalents
of an
alkyl haloacetate of formula (XZCHZCOZR6 where XZ is Cl, Br or I and R6 is
alkyl of
1-6 carbon atoms) and with one or more molar equivalents of an alkali metal
carbonate such as potassium carbonate in a polar aprotic solvent such as DMF
to
afford the alkylated product of formula (Iq: A is H; B, D is H, halogen, CN,
alkyl of
1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, vitro; Y, Z is H
or
OCHZCOZR6, but Y and Z are not concurrently OCHZC02R6; X is H, halogen, alkyl
of
1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridyl-
sulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is COZR6; RS is H; R6 is alkyl
of 1-6
carbon atoms; E is S, O).
cheme 9
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-40-
The phenols of formula (Ip: A is H or OH; B, D is H, halogen, CN, alkyl
of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, vitro; Y, Z is
H or
OCHZCOZR6, but Y and Z are not concurrently OCHZCOZR6; X is H, halogen, alkyl
of
1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; R6 is alkyl of 1-6 carbon
atoms;
E is S, O) can be reacted with a 2-hydroxy carboxylic acid ester of formula
CH(OH)(Rs)COZR6 (RS is H, alkyl of 1-6 carbon atoms, aralkyl, aryl, CH2(1H-
imidazol-4-yl), CH2(3-1H-indolyl), CHzCHz(1,3-dioxo-1,3-dihydro-isoindol-2-
yl),
CH~CHZ(1-oxo-1,3-dihydro-isoindol-2-yl), CHZ(3-pyridyl), CHzCOzR6, R6 is alkyl
of
1-6 carbon atoms) to afford the esters of formula (Iq: A is H or OH; B, D is
H,
halogen, CN, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon
atoms,
vitro; Y, Z is H or OCHzCO2R6, but Y and Z are not concurrently OCH2COZR6; X
is
H, halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon
atoms,
arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is COZR6;
RS is
H, alkyl of 1-6 carbon atoms, aralkyl, aryl, CHZ(1H-imidazol-4-yl), CHz(3-1H-
indolyl), CHzCH2(1,3-dioxo-1,3-dihydro-isoindol-2-yl), CHZCHZ(1-oxo-1,3-
dihydro-
isoindol-2-yl), CHZ(3-pyridyl), CH2COZR6, R6 is alkyl of 1-6 carbon atoms; E
is S, O)
under the conditions of the Mitsunobu Reactions (for a review see Oyo
Mitsunobu
Synthesis. 1981, 1-27). The other co-reagents necessary to effect the
Mitsunobu
Reaction include one or more molar equivalents of a alkyl of 1-6 carbon atoms
azodicarboxylate diester such as diethyl azodicarboxylate or diisopropyl
azodicarboxylate and one or more molar equivalents of triarylphosphine such as
triphenylphosphine in a suitable solvent such as diethyl ether, THF, benzene
or
toluene at temperatures ranging from -20°C to 120°C.
The 2-hydroxy carboxylic acid ester of formula CH(OH)(Rs)COzRb (RS is
H, alkyl of 1-6 carbon atoms, aralkyl, aryl, CHz(1H-imidazol-4-yl), CHZ(3-1H-
indolyl), CH2CH2(1,3-dioxo-1,3-dihydro-isoindol-2-yl), CHzCH2(1-oxo-1,3-
dihydro-
isoindol-2-yl), CHZ(3-pyridyl), CHZCOZR6, R6 is alkyl of 1-6 carbon atoms) are
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
-41 -
commercially available or can be prepared from commercially available
carboxylic
acid precursors under standard esterification conditions. (S)-(+)-2-Hydroxy-1-
oxo-3-
dihydro-2-isoindolinebutyric acid, methyl ester can be prepared from (S)-(+)-2-
hydroxy-1,3-dioxo-2-isoindolinebutyric acid, methyl ester via sequential
treatment
S with 1) sodium borohydride in THF-water; 2) trifluoroacetic acid /
chloroform; 3)
triethylsilane / trifluoroacetic acid and 4) aqueous sodium bicarbonate.
3-(Pyridin-3-yl)-phenyllactic acid, ethyl ester can be prepared according to
the two
step procedure of B.A. Lefker, W.A. Hada, P.J. McGarry Tetrahedron Lett. 1994,
35,
S20S-5208, from commericially available 3-pyridinecarboxaldehyde and ethyl
chloroacetate.
The esters of formula (Iq: A is H; B, D is H, halogen, alkyl of 1-6 carbon
atoms, aryl, aralkyl, alkoxy of I-6 carbon atoms; Y, Z is H; X is H, halogen,
alkyl of
1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms, aralkoxy, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl; W is COZtBu;
RS is
1 S H; E is S, O) can be treated with one or more molar equivalents of a
strong base such
as lithium diisopropyl amide in a suitable solvent such as THF at temperatures
ranging from -78°C to room temperature. This procedure produces an
anion alpha to
the ester carbonyl. The resultant anion is treated with one or more molar
equivalents
of an alkyl halide of formula X285 (where XZ is halogen; RS is alkyl and
aralkyl) and
warmed to room temperature to produce the alkylated ester of formula (Iq: A is
H; B,
D is H, halogen, alkyl of 1-6 carbon atoms, aryl, aralkyl alkoxy of 1-6 carbon
atoms;
Y, Z is H; X is H, halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-
6
carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy, alkylsulfanyl of 1-6
carbon
atoms, arylsulfanyl; W is COZtBu; RS is alkyl and aralkyl; E is S, O).
2S The esters of formula (Iq: A is H or OH; B, D is H, halogen, CN, alkyl of
1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; Y, Z is H
or
OCH2COZR6, but Y and Z are not concurrently OCHzC02R6; X is H, halogen, alkyl
of
1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is COZR6; RS is H, alkyl
of 1-
CA 02330623 2000-10-30
WO 99/58521 PCT/11599/10185
-42-
6 carbon atoms, aralkyl, aryl, CHZ(1H-imidazol-4-yl), CH2(3-1H-indolyl),
CHZCH2(1,3-dioxo-1,3-dihydro-isoindol-2-yl), CHZCH2(1-oxo-1,3-dihydro-isoindol-
2-yl), CHz(3-pyridyl), CHZCOZR6, R6 is alkyl of 1-6 carbon atoms; E is S, O)
can be
transformed into their carboxylic acid analogs using standard conditions to
afford the
carboxylic acids of formula (Iq: A is H or OH; B, D is H, halogen, alkyl of 1-
6 carbon
atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, vitro; Y, Z is H or
OCHZCO~H, but
Y and Z are not concurrently OCH2COZH; X is H, halogen, alkyl of 1-6 carbon
atoms,
CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy,
vitro,
alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; W is COZH; RS is H, alkyl of 1-6 carbon atoms,
aralkyl,
aryl, CHZ(1H-imidazol-4-yl), CHZ(3-1H-indolyl), CH2CHz(1,3-dioxo-1,3-dihydro-
isoindol-2-yl), CHzCH2(1-oxo-1,3-dihydro-isoindol-2-yl), CHZ(3-pyridyl),
CHZCOZH;
E is S, O). The conditions to effect these transformations include aqueous
base in
which one or more molar equivalents of alkali metal hydroxide such as sodium
1 S hydroxide is used in water with a co-solvent such as THF, dioxane or a
lower alcohol
such as methanol or mixtures of THF and a lower alcohol at temperatures
ranging
from 0°C to 40°C. Alternatively, acid conditions may also be
employed in which the
above mentioned carboxylic acid ester of formula (Iq) is reacted with one or
more
molar equivalents of a mineral acid such as HCl or sulfuric acid in water with
or
without a co-solvent such as THF at temperatures ranging from room temperature
to
80°C. Still alternatively, many other conditions may be employed to
effect the above
mentioned ester to acid transformation leading to (Iq). These include reacting
the
carboxylic acid ester of formula (Iq) with one or more molar equivalents of
boron
tribromide or boron trichloride in dichloromethane at -78°C to room
temperature; one
or more molar equivalents hydrobromic acid in acetic acid at 0°C to
50°C; one or
more molar equivalents trimethylsilylbromide or trimethylsilyliodide in
dichloro-
methane, carbon tetrachloride or acetonitrile at -78°C to 50°C;
one or more molar
equivalents lithium iodide in pyridine or quinoline at temperatures from
100° to
250°C.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 43 -
When the esters of formula (Iq: A is H; B, D is H, halogen, CN, alkyl of
1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; Y, Z is H;
X is Br
or I; W is COZR6; RS is H, alkyl of 1-6 carbon atoms, aralkyl, aryl, CHZ(1H-
imidazol-
4-yi), CHZ(3-1H-indolyl), CHZCH2(1,3-dioxo-1,3-dihydro-isoindol-2-yl),
CHZCHZ(1-
oxo-1,3-dihydro-isoindol-2-yl), CHz(3-pyridyl), CH2COZR6, R6 is alkyl of 1-6
carbon
atoms; E is S, O) are reacted with two or more molar equivalents of
trimethylsilyliodide in dichloromethane at temperatures ranging from
0°C to room
temperature, conversion to the carboxylic acids takes place (i.e., W is COzH)
but also
the 6-halogen (X is Br or I) is reduced to give the carboxylic acids of
formula (Iq: A
is H; B, D is H, halogen, CN, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy
of 1-6
carbon atoms, nitro; Y, Z is H; X is H; W is COZH; RS is H, alkyl of 1-6
carbon atoms,
aralkyl, aryl, CHZ(1H-imidazol-4-yl), CHZ(3-1H-indolyl), CH2CH2(1,3-dioxo-1,3-
dihydro-isoindol-2-yl), CHZCHZ(1-oxo-1,3-dihydro-isoindol-2-yl), CH2(3-
pyridyl),
CHzCOzH; E is S, O).
The phenols of formula (Ip: A is H; B, D is H, halogen, CN, alkyl of 1-6
carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; Y, Z is H or
OCHzCO2R6, but Y and Z are not concurrently OCHZC2R6; X is H, halogen, alkyl
of
1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; R6 is alkyl of 1-6 carbon
atoms;
E is S, O) can be alkylated with one or more molar equivalents of diethyl
trifluoromethylsulfonyloxymethylphosphanate (D.P. Phillion and S.S. Andrew
Tet.
Lett. 1986, 1477-1480) and with one or more molar equivalents of an alkali
metal
hydride such as sodium hydride in a suitable solvent such as THF or DMF to
afford
the diethylphosphonate product of formula (Iq: A is H; B, D is H, halogen, CN,
alkyl
of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; Y, Z is
H or
OCHZCOZR6, but Y and Z are not concurrently OCHzCO2R6; X is H, halogen, alkyl
of
1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridyl-
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
_4.ø_
sulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is P03Etz; Rs is H; R6 is alkyl
of 1-6
carbon atoms; E is S, O).
The phenols of formula (Ig: A is H or OH; B, D is H, halogen, CN, alkyl
of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; Y, Z is
H or
OCHZCOZR6, but Y and Z are not concurrently OCHZCOzRb; X is H, halogen, alkyl
of
1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; R6 is alkyl of 1-6 carbon
atoms;
E is S, O) can be reacted with a 2-hydroxy phosphonic acid diester of formula
CH(OH)(RS)P03(R6)z, (RS is H, alkyl of 1-6 carbon atoms, aralkyl, aryl, R6 is
alkyl of
1-6 carbon atoms) to afford the phosphonic acid diesters of formula (Iq: A is
H or
OH; B, D is H, halogen, CN, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy
of 1-6
carbon atoms, nitro; Y, Z is H or OCH2COZR6, but Y and Z are not concurrently
OCHzCOzRb; X is H, halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-
6
carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-
6
carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-
ethylsulfanyI; W
is PO~(R6)2; R5 is H, alkyl of 1-6 carbon atoms, aralkyl, aryl, R6 is alkyl of
1-6 carbon
atoms; E is S, O) under the conditions of the Mitsunobu Reactions (for a
review see
Oyo Mitsunobu Synthesis 1981, 1-27). The other co-reagents necessary to effect
the
Mitsunobu Reaction include one or more molar equivalents of a alkyl of 1-6
carbon
atoms azodicarboxylate diester such as diethyl azodicarboxylate or diisopropyl
azodicarboxylate and one or more molar equivalents of triarylphosphine such as
triphenylphosphine in a suitable solvent such as diethyl ether, THF, benzene
or
toluene at temperatures ranging from -20°C to 120°C. The 2-
hydroxy phosphonic acid
diester of formula CH(OH)(RS)P03R6 (RS is H, alkyl of 1-6 carbon atoms,
aralkyl,
aryl, R6 is alkyl of 1-6 carbon atoms) can be prepared by reacting a
dialklylphosphonate of formula HP(O)(OR6)2 (R6 is alkyl of 1-6 carbon atoms)
with
an aldehyde of formula RSCHO (RS is alkyl of 1-6 carbon atoms, aryl, aralkyl)
under
standard conditions.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 45 -
The phosphoric acid diesters of formula (Iq: A is H or OH; B, D is H,
halogen, CN, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon
atoms,
nitro; Y, Z is H or OCHZCOZR6, but Y and Z are not concurrently OCHZCOZR6; X
is
H, halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon
atoms,
arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is
P03(R6)Z; Rs
is H, alkyl of 1-6 carbon atoms, aralkyl, aryl, R6 is H, alkyl of 1-6 carbon
atoms; E is
S, O) can be transformed into their phosphoric acid analogs using standard
conditions
to afford the phosphoric acids acids of formula (Iq: A is H or OH; B, D is H,
halogen,
CN, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms,
nitro; Y, Z
is H or OCHZCOzR6, but Y and Z are not concurrently OCHZCOZR6; X is H,
halogen,
alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-
6
carbon atoms, aralkoxy, nitro, alkylsuIfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is P03H2; Rs is H, alkyl
of 1-
6 carbon atoms, aralkyl, aryl, R6 is alkyl of 1-6 carbon atoms; E is S, O).
The
conditions that may also be employed in which the above mentioned phosphoric
acid
diester of formula (Iq) is reacted with two or more molar equivalents of a
mineral acid
such as HCl or sulfuric acid in water with or without a co-solvent such as THF
at
temperatures ranging from 40 to 100°C. Still alternatively, many other
conditions
may be employed to effect the above mentioned diester to acid transformation
leading
to (Iq). These include reacting the phosphoric acid diester of formula (Iq)
with two or
more molar equivalents of boron tribromide or boron trichloride in
dichloromethane
at -78°C to room temperature; two or more molar equivalents hydrobromic
acid in
acetic acid at 0°C to 50°C; two or more molar equivalents
trimethylsilylbromide or
trimethylsilyliodide in dichloromethane, carbon tetrachloride or acetonitrile
at -78°C
to 50°C; two or more molar equivalents lithium iodide in pyridine or
quinoline at
temperatures from 60° to 250°C.
The esters of formula (Iq: A is H or OH; B, D is H, halogen, CN, alkyl of 1-6
carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; Y, Z is H; X
is H,
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
CA 02330623 2000-10-30
WO 99/58521 PCT/fJS99/10185
-46-
of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is COZR6; RS is H, alkyl
of 1-
6 carbon atoms, aralkyl, aryl, CHZ(1H-imidazol-4-yl), CHZ(3-1H-indolyl),
CHZCHZ(1-oxo-1,3-dihydro-isoindol-2-yl), CHZ(3-pyridyl), R6 is alkyl of 1-6
carbon
S atoms; E is S, O) can be transformed into their primary carboxylic acid
amide analogs
of formula (Iq: A is H or OH; B, D is H, halogen, alkyl of 1-6 carbon atoms,
aryl,
aralkyl, alkoxy of 1-6 carbon atoms, nitro; Y, Z is H; X is H, halogen, alkyl
of 1-6
carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms,
aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-
N,N-dimethylamino-ethylsulfanyl; W is CONH2; RS is H, alkyl of 1-6 carbon
atoms,
aralkyl, aryl, CHZ(1H-imidazol-4-yl), CHZ(3-1H-indolyl), , CHZCH2(1-oxo-1,3-
dihydro-isoindol-2-yl), CHZ(3-pyridyl) ; E is S, O} by reacting the ester
starting
material with ammonia gas dissolved in a lower alcohol solvent such as
methanol or
ethanol at temperatures ranging from 0°C to 100°C.
1S Alternatively, the carboxylic acids of formula (Iq: A is H or OH; B, D is
H,
halogen, CN, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon
atoms,
nitro; Y, Z is H; X is H, halogen, alkyl of 1-6 carbon atoms, CN,
perfluoroalkyl of 1-6
carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-
6
carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-
ethylsulfanyl; W
is COzH; RS is H, alkyl of 1-6 carbon atoms, aralkyl, aryl, CHZ(1H-imidazol-4-
yl),
CHZ(3-1H-indolyl), CHZCHZ(1,3-dioxo-1,3-dihydro-isoindol-2-yl), CHZCHZ(1-oxo-
1,3-dihydro-isoindol-2-yl), CHZ(3-pyridyl) ; E is S, O) can be transformed
into their
carboxylic acid amide analogs of formula (Iq: A is H or OH; B, D is H,
halogen, alkyl
of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; Y, Z is
H; X is
2S H, halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon
atoms,
alkoxy of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon
atoms,
arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is CONH2,
CONHOH, CONH(CHZ),CN; RS is H, alkyl of 1-6 carbon atoms, aralkyl, aryl,
CHZ(1H-imidazol-4-yl), CHZ(3-1H-indolyl), CHZCHZ(1,3-dioxo-1,3-dihydro-
isoindol-
2-yl), CHZCHZ(1-oxo-1,3-dihydro-isoindol-2-yl), CHZ(3-pyridyl) ; E is S, O).
This
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
-47-
transformation can be accomplished using standard methods to effect carboxylic
acid
to carboxylic acid amide transformations. These methods include converting the
acid
to an activated acid and reacting with one or more molar equivalents of the
desired
amine. Amines in this category include ammonia in the form of ammonium
hydroxide, hydroxyl amine and 2-aminopropionitrile. Methods to activate the
carboxylic acid include reacting said acid with one or more molar equivalents
of
oxalyl chloride or thionyl chloride to afford the carboxylic acid chloride in
a suitable
solvent such as dichloromethane, chloroform or diethyl ether. This reaction is
often
catalyzed by adding small amounts (0.01 to 0.1 molar equivalents) of
dimethylformamide. Other methods to activate the carboxylic acid include
reacting
said acid with one or more molar equivalents dicyclohexylcarbodiimide with or
without one or more molar equivalents of hydroxybenzotriazole in a suitable
solvent
such as dichloromethane or dimethylformamide at temperatures ranging from
0°C to
60°C.
The phenols of formula (Ip: A is H; B, D is H, halogen, CN, alkyl of 1-6
carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, vitro; Y, Z is H; X
is H,
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylaminoethylsulfanyl; E is S, O) can be alkylated
with
one or more molar equivalents of a haloacetonitrile of formula (XZCHZCN where
X2 is
Cl, Br or I) and with one or more molar equivalents of an alkali metal
carbonate such
as potassium carbonate in a polar aprotic solvent such as DMF to afford the
nitrites of
formula (Iq: A is H; B, D is H, halogen, CN, alkyl of 1-6 carbon atoms, aryl,
aralkyl,
alkoxy of 1-6 carbon atoms, vitro; Y, Z is H; X is H, halogen, alkyl of 1-6
carbon
atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms,
aralkoxy,
vitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; W is CN; RS is H; E is S, O).
Alternatively, the carboxylic acid amide analogs of formula (Iq: A is H or OH;
B, D is H, halogen, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6
carbon
atoms, vitro; Y, Z is H; X is H, halogen, alkyl of 1-6 carbon atoms, CN,
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
-48-
perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy,
nitro,
alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; W is CONHZ; RS is H, alkyl of 1-6 carbon atoms,
aralkyl, aryl, CHZ(1H-imidazol-4-yl), CH2(3-1H-indolyl), CHZCHZ(1,3-dioxo-1,3-
dihydro-isoindol-2-yl), CHzCH2(1-oxo-I,3-dihydro-isoindol-2-yl), CHz(3-
pyridyl) ; E
is S, O) can be converted to their nitrite analogs of formula (Iq: A is H or
OH; B, D is
H, halogen, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon
atoms,
nitro; Y, Z is H; X is H, halogen, alkyl of 1-6 carbon atoms, CN,
perfluoroalkyl of 1-6
carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-
6
carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylarnino-
ethylsulfanyl; W
is CN; RS is H, alkyl of 1-6 carbon atoms, aralkyl, aryl, CHZ(1H-imidazol-4.-
yl),
CHZ(3-1H-indolyl), CHZCHZ(1,3-dioxo-1,3-dihydro-isoindol-2-yl), CHZCHZ(1-oxo-
1,3-dihydro-isoindol-2-yl), CHz(3-pyridyl) ; E is S, O) by using reagents that
dehydrate the primary carboxamide function to the nitrite function. One set of
conditions to effect this transformation include reacting the said primary
carboxylic
acid amide with one or more molar equivalents of trifluoroacetic anhydride and
two
or more molar equivalents of pyridine in a suitable solvent such as dioxane at
temperatures ranging from 60°C to 120°C.
The nitrite analogs of formula (Iq: A is H or OH; B, D is H, halogen, alkyl of
1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; Y, Z is H;
X is H,
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is CN; RS is H, alkyl of
1-6
carbon atoms, aralkyl, aryl, CHZ( 1 H-imidazot-4-yl), CHZ(3-1 H-indolyl),
CHZCHZ( 1,3
dioxo-1,3-dihydro-isoindol-2-yl), CHzCHz(1-oxo-1,3-dihydro-isoindol-2-yl),
CHZ(3-
pyridyl) ; E is S, O) can be converted to the tetrazoles of formula (Iq: A is
H or OH;
B, D is H, halogen, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6
carbon
atoms, nitro; Y, Z is H; X is H, halogen, alkyl of 1-6 carbon atoms, CN,
perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy,
nitro,
alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethyl-
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-49-
amino-ethylsulfanyl; W is S-tetrazole; RS is H, alkyl of 1-6 carbon atoms,
aralkyl,
aryl, CHz(1H-imidazol-4-yl), CHZ(3-1H-indolyl), CHZCHZ(1,3-dioxo-1,3-dihydro-
isoindol-2-yl), CHZCHZ(1-oxo-1,3-dihydro-isoindol-2-yl), CH2(3-pyridyl) ; E is
S, O)
by reacting the nitrile function with one or more molar equivalents of
trimethylaluminum and one or more molar equivalents of trimethylsilyl azide in
a
suitable solvent such as benzene or toluene at temperatures ranging from
60°C to
120°C. Alternatively, the nitrite fuction can be reacted with one or
more molar
equivalents of ammonium azide in a suitable solvent such as dimethylformamide
at
temperatures ranging from 60°C to 160°C.
The esters of formula (Iq: A is H; B, D is H, halogen, alkyl of 1-6 carbon
atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms; Y, Z is H; X is H, halogen,
alkyl of
1-6 carbon atoms, CN, perfluoroalkyl of I-6 carbon atoms, alkoxy of 1-6 carbon
atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is COZR6; RS is H, alkyl
of 1-
6 carbon atoms, aralkyl, aryl; R6 is alkyl of 1-6 carbon atoms; E is S, O) can
be
transformed into their primary alcohol analogs of formula (Iq: A is H; B, D is
H,
halogen, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms;
Y, Z is
H; X is H, halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6
carbon
atoms, alkoxy of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6
carbon atoms,
arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is CH20H;
RS is
H, alkyl of 1-6 carbon atoms, aralkyl, aryl; R6 is alkyl of 1-6 carbon atoms;
E is S, O)
by reacting said ester with one or more molar equivalents of lithium aluminum
hydride and one or more molar equivalents of aluminum chloride in a suitable
solvent
such as THF at temperatures ranging from -78 to room temperature.
The primary alcohol analogs of formula (Iq: A is H; B, D is H, halogen, alkyl
of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms; Y, Z is H; X
is H,
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is CHZOH; RS is H, alkyl
of
1-6 carbon atoms, aralkyl, aryl; R6 is alkyl of I-6 carbon atoms; E is S, O)
can be
CA 02330623 2000-10-30
WO 99/58521 PCT/I)S99/10185
-50-
converted to the primary bromides of formula (Iq: A is H; B, D is H, halogen,
alkyl of
1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms; Y, Z is H; X is
H,
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; W is CHzBr; Rs is H, alkyl
of 1-
6 carbon atoms, aralkyl, aryl; R6 is alkyl of 1-6 carbon atoms; E is S, O) by
reacting
the said primary alcohol with one or more molar equivalents of lithium bromide
with
one or more molar equivalents of a alkyl of 1-6 carbon atoms azocarboxylate
diester
such as diethyl azodicarbxylate or diisopropyl azodicarboxylate and one or
more
molar equivalents of triarylphosphine such as triphenylphosphine in a suitable
solvent
such as diethyl ether, THF, benzene or toluene at temperatures ranging from -
20°C to
120°C.
Scheme 10
(Ir) (Is)
Further derivatives of the compounds of formula (I) in Scheme 10 can be
prepared by the following methods. The phenols of formula (Ir: A is H or
halogen; C
is halogen or methoxy; X is halogen, alkyl of 1-6 carbon atoms, CN,
perfluoroalkyl of
1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl
of 1-6
carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-dimethylamino-
ethylsulfanyl; E is
S, O) can be reacted with a 2-hydroxy carboxylic acid ester of formula
CH(OH)(R'a)COZR' (R', R'a is alkyl of 1-6 carbon atoms, aralkyl, aryl) to
afford the
esters of formula (Is: A is H or halogen; C is halogen or methoxy; X is
halogen, alkyl
of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6
carbon
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-51 -
atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridyl-
sulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; R' is alkyl of 1-6 carbon atoms,
aralkyl, aryl; R'a is H or alkyl of 1-6 carbon atoms, aralkyl, aryl; E is S,
O) under the
conditions of the Mitsunobu Reactions (for a review see Oyo Mitsunobu
Synthesis
1981, 1-27). The other co-reagents necessary to effect the Mitsunobu Reaction
include one or more molar equivalents of a alkyl of 1-6 carbon atoms
azodicarboxylate diester such as diethyl azodicarboxylate or diisopropyl
azodicarboxylate and one or more molar equivalents of triarylphosphine such as
triphenylphosphine in a suitable solvent such as diethyl ether, THF, benzene
or
toluene at temperatures ranging from -20°C to 120°C at
temperatures ranging from
-20°C to 120°C.
The 2-hydroxy carboxylic acid ester of formula CH(OH)(R'a)COZR' (R',
R'e s alkyl of 1-6 carbon atoms, aralkyl, aryl) are commercially available or
can be
prepared from commercially available carboxylic acid precursors under standard
esterification conditions.
The esters of formula (Is: A is H or halogen; C is halogen or methoxy; X is
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl R' is alkyl of 1-6 carbon
atoms,
aralkyl, aryl; R'a is H or alkyl of 1-6 carbon atoms, aralkyl, aryl; E is S,
O) can be
transformed into their carboxylic acid analogs using standard conditions to
afford the
carboxylic acids of formula (Is: A is H or halogen; C is halogen or methoxy; X
is
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; R' H; R'a is H or alkyl of
1-6
carbon atoms, aralkyl, aryl; E is S, O). The conditions to effect these
transformations
include aqueous base in which one or more molar equivalents of alkali metal
hydroxide such as sodium hydroxide is used in water with a co-solvent such as
THF,
dioxane or a lower alcohol such as methanol or mixtures of THF and a lower
alcohol
at temperatures ranging from 0°C to 40°C.
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-52-
Scheme 11
(It) (Iu)
Further derivatives of the compounds of formula (I) in Scheme 11 can be
prepared by the following methods. The phenols of formula (It: B, D is H,
halogen,
CN, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms,
vitro; X is
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; E is S, O) can be reacted
with
one or more molar equivalents of lithium (bis)trimethylsilylamide at
temperautres
ranging from -78°C to room temperature and the lithium salt can be
further reacted
with one or more molar equivalents of 5-bromothiazolidine-2, 4-dione (prepared
according to the method of Zask, et al., J. Med Chem, 1990, 33, 1418-1423)
using a
suitable solvent such as THF under an inert atmosphere at temperautres ranging
from
-78°C to room temperature to provide the compounds of formula (Iu: R4
is (R, S)-5-
thiazolidine-2,4-dione; B, D is H, halogen, CN, alkyl of 1-6 carbon atoms,
aryl,
aralkyl, alkoxy of 1-6 carbon atoms, vitro; X is halogen, alkyl of 1-6 carbon
atoms,
CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy,
vitro,
alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; E is S, O).
Alternatively, the phenols of formula (It: B, D is H, halogen, CN, alkyl of 1-
6
carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, vitro; X is halogen,
alkyl of
1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms, aralkoxy, vitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridyl-
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-53-
sulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; E is S, O) can be reacted with
one or
more molar equivalents of tetrazole and di-tert-butyl N,N-
diethylphosporamidate in
THF at room temperature followed by addition of one or more molar equivalents
of
meta-chlorobenzoic acid at -40°C according to the procedure of J. W.
Perich and R.
B. Johns, Synthesis, 1988, 142-144} to afford the phosphate diesters of
formula (Iu: R4
is P(O)(OtBu)2; B, D is H, halogen, CN, alkyl of 1-6 carbon atoms, aryl,
aralkyl,
alkoxy of 1-6 carbon atoms, nitro; X is halogen, alkyl of 1-6 carbon atoms,
CN,
perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy,
nitro,
alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; E is S, O). These phosphate diesters are then
treated
with one or more molar equivalents hydrochloric acid in a suitable solvent
such as
dioxane to provide the phosphonic acids of formula (Iu: R4 is P(O)(OH}2; B, D
is H,
halogen, CN, alkyl of 1-6 carbon atoms, aryl, aralkyl, nitro; X is halogen,
alkyl of 1-6
carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms,
aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-
N,N-dimethylamino-ethylsulfanyl; E is S, O).
The phenols of formula (It: B, D is H, halogen, CN, alkyl of 1-6 carbon atoms,
aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; X is halogen, alkyl of 1-6
carbon
atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms,
aralkoxy,
nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; E is S, O) can be transformed to the carboxylic
acids of
formula (Iu: R4 is C(CH3)ZCOZH; B, D is H, halogen, CN, alkyl of 1-6 carbon
atoms,
aryl, aralkyl, aIkoxy of 1-6 carbon atoms, nitro; X is halogen, alkyl of 1-6
carbon
atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms,
aralkoxy,
nitro, alkylsulfanyl of I-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; E is S, O) by treatment of the phenols with two
or more
molar equivalents of solid sodium hydroxide followed by one or more molar
equivalents of 1,1,I-trichloro-2-methyl-2-propanol tetrahydrate in the
presence of a
large excess of acetone which also serves as solvent.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-54-
The phenols of formula (It: B, D is H, halogen, CN, alkyl of 1-6 carbon atoms,
aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; X is halogen, alkyl of 1-6
carbon
atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms,
aralkoxy,
nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; E is S, O) can be transformed to the carboxylic
acids of
formula (Iu: R4 is CHZCH2COZH; B, D is H, halogen, CN, alkyl of I-6 carbon
atoms,
aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; X is halogen, alkyl of 1-6
carbon
atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms,
aralkoxy,
nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; E is S, O) by treatment with one or more molar
equivalents of ~i-propiolactone and treatment with one or more molar
equivalents of
potassium tert-butoxide in a suitable solvent such as THF.
The phenols of formula (It: B, D is H, halogen, CN, alkyl of 1-6 carbon
atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; X is halogen, alkyl
of 1-6
carbon atoms, CN, perfluoroalkyl of I-6 carbon atoms, alkoxy of 1-6 carbon
atoms,
aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-
N,N-dimethylamino-ethylsulfanyl; E is S, O) can be reacted with a 3-hydroxy
carboxylic acid ester of formula CH(OH)(R')CHZCOZR6 (R' is H or alkyl of 1-6
carbon atoms; R6 is alkyl of 1-6 carbon atoms) to afford the esters of formula
(Iu: R4
is (R)-CH(R')CHzCO2R6; B, D is H, halogen, CN, alkyl of 1-6 carbon atoms,
aryl,
aralkyl, alkoxy of 1-6 carbon atoms, nitro; X is halogen, alkyl of I-6 carbon
atoms,
CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy,
nitro,
alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl, pyridylsulfanyl, 2-N,N-
dimethylamino-ethylsulfanyl; R' is H or alkyl of 1-6 carbon atoms; R6 is alkyl
of 1-6
carbon atoms; E is S, O) under the conditions of the Mitsunobu Reactions (for
a
review see Oyo Mitsunobu Synthesis 1981, I-27). The other co-reagents
necessary to
effect the Mitsunobu Reaction include one or more molar equivalents of a alkyl
of I-6
carbon atoms azodicarboxylate diester such as diethyl azodicarboxylate or
diisopropyl
azodicarboxylate and one or more molar equivalents of triarylphosphine such as
triphenylphosphine in a suitable solvent such as diethyl ether, THF, benzene
or
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-55-
toluene at temperatures ranging from -20°C to 120°C at
temperatures ranging from
-20°C to 120°C.
The 3-hydroxy carboxylic acid ester of formula CH(OH)(R')CHZCOZR6 (R' is
H or alkyl of 1-6 carbon atoms; R6 is alkyl of 1-6 carbon atoms) are
commercially
available or can be prepared from commercially available carboxylic acid
precursors
under standard esterification conditions.
The esters of formula (Iu: R4 is (R)-CH(R')CHZCOZR6; B, D is H, halogen,
CN, alkyl of 1-6 carbon atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms,
nitro; X is
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms,
arylsulfanyl,
pyridylsulfanyl, 2-N,N-dimethylamino-ethylsulfanyl; R' is H or alkyl of 1-6
carbon
atoms; R6 is alkyl of 1-6 carbon atoms; E is S, O) can be transformed to the
acids of
formula (Iu: R° is (R)-CH(R')CHZCOZH; B, D is H, halogen, CN, alkyl of
1-6 carbon
atoms, aryl, aralkyl, alkoxy of 1-6 carbon atoms, nitro; X is halogen, alkyl
of 1-6
carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms,
aralkoxy, nitro, alkylsulfanyl of 1-6 carbon atoms, arylsulfanyl,
pyridylsulfanyl, 2-
N,N-dimethylamino-ethylsulfanyl; R' is H or alkyl of 1-6 carbon atoms; E is S,
O) by
several standard conditions which include reacting the ester of formula (Iu)
with two
or more molar equivalents of a mineral acid such as HCl or sulfuric acid in
one or
more solvents or a combination of two or more solvents such as water, THF or
dioxane at temperatures ranging from 40 to 120°C. Still alternatively,
many other
conditions may be employed to effect the above mentioned ester to acid
transformation leading to (Iu). These include reacting the esters of formula
(Iu) with
two or more molar equivalents of boron tribromide or boron trichloride in
dichloromethane at -78°C to room temperature; two or more molar
equivalents
hydrobromic acid in acetic acid at 0°C to 50°C; two or more
molar equivalents
trimethylsilylbromide or trimethylsilyliodide in dichloromethane, carbon
tetrachloride
or acetonitrile at -78°C to SO°C; two or more molar equivalents
lithium iodide in
pyridine or quinoline at temperatures from 60° to 250°C.
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-56-
Scheme 12
R,.
~s
'' ~~2 5
R
(Iv) (Iw)
Further derivatives of the compounds of formula (I) in Scheme 12 can be
prepared by the following methods. The esters of formula (Iv: B, D is H,
halogen,
alkyl of 1-6 carbon atoms; RS is H, alkyl of 1-6 carbon atoms, aryl or
aralkyl; R6 is
alkyl of 1-6 carbon atoms; E is S, O) can be reacted with one or more molar
equivalents of boron tribromide in a halocarbon solvent such as
dichloromethane at
temperatures ranging from -78 to room temperature to provide the phenols of
formula
(Iw: B, D is H, halogen, alkyl of I-6 carbon atoms; RS is H, alkyl of 1-6
carbon atoms,
aryl or aralkyl; R6 is alkyl of 1-6 carbon atoms; R8 is H; E is S, O).
The phenols of formula (Iw: B, D is H, halogen, alkyl of 1-6 carbon atoms; RS
is H, alkyl of 1-6 carbon atoms, aryl or aralkyl; R6 is alkyl of 1-6 carbon
atoms; Rg is
H; E is S, O) can be alkylated with one or more molar equivalents of an
alkylating
agent of formula RgX (R8 is alkyl of 1-6 carbon atoms, lower aralkyl and
CH2COZCH3; X is halogen; E is S, O) and with one or more molar equivalents of
an
alkali metal carbonate such as potassium carbonate in a polar aprotic solvent
such as
DMF to afford the alkylated phenol of formula (Iw: B, D is H, halogen, alkyl
of 1-6
carbon atoms; RS is H, alkyl of 1-6 carbon atoms, aryl or aralkyl; R6 is alkyl
of 1-6
carbon atoms; R$ is alkyl of 1-6 carbon atoms, lower aralkyl, CHZCOzCH3; E is
S, O).
The esters of formula (Iw: B, D is H, halogen, alkyl of 1-6 carbon atoms; RS
is
H, alkyl of 1-6 carbon atoms, aryl or aralkyl; R6 is alkyl of I-6 carbon
atoms; Ra is
alkyl of 1-6 carbon atoms, lower aralkyl, CHZCOZCH3; E is S, O) can be
transformed
into their carboxylic acid analogs using standard conditions to afford the
carboxylic
CA 02330623 2000-10-30
WO 99/58521 PCT/tJS99/10185
-57-
acids of formula (Iw: B, D is H, halogen, alkyl of 1-6 carbon atoms; R5 is H,
alkyl of
1-6 carbon atoms, aryl or aralkyl; R6 is H; R8 is alkyl of 1-6 carbon atoms,
lower
aralkyl, CHzC02H; E is S, O). The conditions to effect these transformations
include
aqueous base in which one or more molar equivalents of alkali metal hydroxide
such
as sodium hydroxide is used in water with a co-solvent such as THF, dioxane or
a
lower alcohol such as methanol or mixtures of THF and a lower alcohol at
temperatures ranging from 0°C to 40°C.
Scheme 13
CO R65
~- R
~~~n
(Ix) (Iy)
Further derivatives of the compounds of formula (I) in Scheme 13 can be
prepared by the following methods. The compounds of formula (Ix: B, D is H,
halogen, alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms; X is halogen,
alkyl
of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6
carbon
atoms, aralkoxy, Rs is H, alkyl of 1-6 carbon atoms, aryl or aralkyl; R6 is H,
alkyl of
1-6 carbon atoms) can be transformed into their sulfoxide derivatives of
formula (Iy:
n is l; B, D is H, halogen, alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms; X is
halogen, alkyl of 1-6 carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms,
alkoxy
of 1-6 carbon atoms, aralkoxy, RS is H, alkyl of 1-6 carbon atoms, aryl or
aralkyl; R6
is H, alkyl of 1-6 carbon atoms) using one molar equivalent of an oxidizing
agent
such as m-chloroperbenzoic acid in dichloromethane at temperatures ranging
from
-20°C to 40°C or peracetic acid in acetic acid and water at
temperatures ranging from
room temperature to 100°C.
CA 02330623 2000-10-30
WO 99/58521 PCT/IlS99/10185
-58-
The compounds of formula (Ix: B, D is H, halogen, alkyl of 1-6 carbon atoms,
alkoxy of 1-6 carbon atoms; X is halogen, alkyl of 1-6 carbon atoms, CN,
perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms, aralkoxy, RS
is H,
alkyl of 1-6 carbon atoms, aryl or aralkyl; R6 is H, alkyl of 1-6 carbon
atoms) can be
transformed into their sulfone derivatives of formula (Iy: n is 2; B, D is H,
halogen,
alkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon atoms; X is halogen, alkyl of
1-6
carbon atoms, CN, perfluoroalkyl of 1-6 carbon atoms, alkoxy of 1-6 carbon
atoms,
aralkoxy, RS is H, alkyl of 1-6 carbon atoms, aryl or aralkyl; R6 is H, alkyl
of 1-6
carbon atoms) using two or more molar equivalents of an oxidizing agent such
as m-
chloroperbenzoic acid in dichloromethane at temperatures ranging from -
20°C to
60°C or peracetic acid in acetic acid and water at temperatures ranging
from room
temperature to 100°C.
The compounds of this invention are useful in treating metabolic disorders
related to insulin resistance or hyperglycemia, typically associated with
obesity or
glucose intolerance. The compounds of this invention are therefore,
particularly
useful in the treatment or inhibition of type II diabetes. The compounds of
this
invention are also useful in modulating glucose levels in disorders such as
type I
diabetes.
The ability of compounds of this invention to treat or inhibit disorders
related
to insulin resistance or hyperglycemia was established with representative
compounds
of this invention in the following two standard pharmacological test
procedures which
measure the inhibition of PTPase.
Inhibition of tri-nhosphorylated insulin receptor dodecaphospho~eptide
~enhosnho_rylation by rat hepatic protein- rosine phosphatases~PTPases)
This standard pharmacological test procedure assess the inhibition of rat
hepatic microsomal PTPase activity using, as substrate, the phosphotyrosyl
dodecapeptide corresponding to the 1142-1153 insulin receptor kinase domain,
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-59-
phosphorylated on the 1146, 1150 and 1151 tyrosine residues. The procedure
used
and results obtained are briefly outlined below.
Preparation of Microsomal Fraction Rats (Male Sprague-Dawley rats (Charles
River,
Kingston, NY) weighing 100-150 g, maintained on standard rodent chow (Purina))
are sacrificed by asphyxiation with C02 and bilateral thoracotomy. The liver
is
removed and washed in cold 0.85% (w/v) saline and weighed. The tissue is
homogenized on ice in 10 volumes of Buffer A and the microsomes are isolated
essentially as described by Meyerovitch J, Rothenberg P, Shechter Y, Bonner-
Weir S,
IO Kahn CR. Vanadate normalizes hyperglycemia in two mouse models of non-
insulin-
dependent diabetes mellitus. J Clin Invest 1991; 87:1286-1294 and Alberts B,
Bray
D, Lewis J, Raff M, Roberts K, Watson JD, editors. Molecular biology of the
cell.
New York: Garland Publishing, Inc., 1989 with minor modifications. The liver
homogenate is filtered through silk to remove any remaining tissue debris and
then is
centrifuged at 10,000xg for 20 minutes at 40C. The supernatant is decanted and
centrifuged at 100,000xgfor 60 minutes at 40C. The pellet, microsomes and
small
vesicles, is resuspended and lightly homogenized in : 20 mM TRIS-HCl (pH 7.4),
50
mM 2-mercaptoethanol, 250 mM sucrose, 2 mM EDTA, 10 mM EGTA, 2 mM
AEBSF, 0.1 mM TLCK, 0.1 mM TPCK, 0.5 mM benzamidine, 25 ug/ml leupeptin, 5
ug/ml pepstatin A, 5 ug/mI;HSB antipain, 5 ug/ml chymostatin, IO ug/ml
aprotinin
(Buffer A}, to a final concentration of approximately 850 ug protein/ml.
Protein
concentration is determined by the Pierce Coomassie Plus Protein Assay using
crystalline bovine serum albumin as a standard (Pierce Chemical Co., Rockford,
IL).
Measurement of PTPase activity The malachite green-ammonium molybdate method,
as described by Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA was used. An
improved assay for nanomolar amounts of inorganic phosphate. Anal. Biochem.
1979;100:95-97, and adapted for the platereader, is used for the nanomolar
detection
of liberated phosphate by rat hepatic microsomal PTPases. The test procedure
uses,
as substrate, a dodecaphosphopeptide custom synthesized by AnaSpec, Inc. (San
Jose,
CA). The peptide, TRDIYETDYYRK, corresponding to the 1142-1153 catalytic
CA 02330623 2000-10-30
WO 99/58521 PCT/(JS99/10185
-60-
domain of the insulin receptor, is tyrosine phosphorylated on the 1146, 1150
and 1151
tyrosine residues. The microsomal fraction (83.25 ul) is preincubated for 10
min at
37deg.C with or without test compound (6.25u1) and 305.5 ul of the 81.83 mM
HEPES reaction buffer, pH 7.4. Peptide substrate, 10.5 ul at a final
concentration of
50 uM, is equilibrated to 37deg.C in a LABLINE Multi-Blok heater equipped with
a
titerplate adapter. The preincubated microsomal preparation (39.5 ul) with or
without
drug is added to initiate the dephosphorylation reaction, which proceeds at
37deg.C
for 30 min. The reaction is terminated by the addition of 200 ul of the
malachite
green-ammonium molybdate-Tween 20 stopping reagent (MG/AM/Tw). The
stopping reagent consists of 3 parts 0.45% malachite green hydrochloride, 1
part 4.2%
ammonium molybdate tetrahydrate in 4 N HCl and 0.5% Tween 20. Sample blanks
are prepared by the addition of 200 ul MG/AM/Tw to substrate and followed by
39.5
ul of the preincubated membrane with or without drug. The color is allowed to
develop at room temperature for 30 min and the sample absorbances are
determined at
650 nm using a platereader (Molecular Devices). Samples and blanks are
prepared in
quadruplicates. Screening activity of 50 uM (final) drug is accessed for
inhibition of
microsomal PTPases.
Calculations: PTPase activities, based on a potassium phosphate standard
curve, are
expressed as nmoles of phosphate released/min/mg protein. Test compound PTPase
inhibition is calculated as percent of control. A four parameter non-linear
logistic
regression of PTPase activities using SAS release 6.08, PROC NLIN, is used for
determining IC50 values of test compounds. All compounds were administered at
a
concentration of 50 ~.~M. The following results were obtained using
representative
compounds of this invention.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-61-
% Change from
Exam le Control
7 1.03
14 -34.19
15 -43.68
18 -15.22
20 -26.86
21 -32.41
22 -16.83
23 -8.92
24 3.16
25 -38.31
26 -8.68
27 -35.78
30 1.00
32 -58.12
34 -3.40
37 -3.02
40 -1.40
41 -39.32
42 -21.28
43 -14.62
47 -3.50
58 -43.32
59 -35.46
60 -25.07
61 -54.82
62 -40.14
63 -47.53
-15.90
68 -26.32
70 -29.03
71 -35.74
74 -21.61
76 -18.89
79 -82.58
81 -47.69
82 -39.80
83 -57.89
84 -73.91
85 -71.67
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-62-
lo Change from
Exam le Control
86 -69.35
87 -63.18
88 -67.03
89 -61.04
90 -79.04
91 -97.10
92 -98.16
93 -58.35
g4 -95.80
95 -75.45
108 -72.94
109 -66.67
110 -12.78
111 -76.45
113 -101.01
114 -68.03
115 -55.43
116 -61.89
117 -79.06
118 -82.00
119 -75.29
120 -17.35
121 -74.70
122 -85.46
123 -g7,~
124 -70.01
125 -73.96
126 -78.77
127 -37.08
128 -50.94
129 -59.03
130 -72.14
131 -66.21
132 -49.27
133 -27.89
134 -69.86
135 -59.75
136 -63.42
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-63-
% Change from
Example Control
137 -64.92
138 -69.13
139 -6.q,g9
140 -71.19
141 -76.58
142 -104.68
143 -76.98
1 ~ -85.24
146 -71.95
147 -66.60
148 -82.62
149 -59.82
150 -92.46
152 -95.22
155 -82.25
156 -71.12
161 -8.03
162 -60.67
163 -38.40
1 ~ -70.32
165 -3.12
166 -26.86
167 -16.99
168 -17.85
169 -9.30
170 -18.79
171 -71.04
172 -70.95
174 0.39
175 -69.20
177 -69.35
178 -42.41
179 -66.27
180 -50.64
184 -46.44
185 -98.45
186 -74.87
187 -57.64
189 -89.32
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
_6q._
% Change from
Example Control
194 -79.35
195 -29.34
196 -80.08
200 -50.62
201 -75.75
203 -86.47
210 -51.55
211 -36.82
213 -54.40
215 -80.93
216 -60.67
217 -80.42
Phenylarsine oxide -57.06
(reference standard)
Inhibition of Tri-Phosnhorylated Insulin Receptor Dodecapho_sphopentide
Dephosphor~rlation by hPTPIB
This standard pharmacological test procedure assess the inhibition of
recombinant rat protein tyrosine phosphatase, PTP1B, activity using, as
substrate, the
phosphotyrosyl dodecapeptide corresponding to the 1142-1153 insulin receptor
kinase
domain, phosphorylated on the 1146, 1150 and 1151 tyrosine residues. The
procedure used and results obtained are briefly described below.
Human recombinant PTP1B was prepared as described by Goldstein (see
Goldstein et al. Mol. Cell. Biochem. 109, 107, 1992). The enzyme preparation
used
was in microtubes containing 500-700 pg/ml protein in 33 mM Tris-HCI, 2 mM
EDTA, 10% glycerol and 10 mM 2-mercaptoethanol.
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-65-
Measurement of PTPase activity. The malachite green-ammonium molybdate
method, as described (Lanzetta et al. Anal. Biochem. 100, 95, 1979) and
adapted for
a platereader, is used for the nanomolar detection of liberated phosphate by
recombinant PTP1B. The test procedure uses, as substrate, a
dodecaphosphopeptide
custom synthesized by AnaSpec, Inc. (San Jose, CA). the peptide,
TRDIYETDYYRK, corresponding to the 1142-1153 catalytic domain of the insulin
receptor, is tyrosine phosphorylated on the 1146, 11 S0, and 1151 tyrosine
residues.
The recombinant rPTPIB is diluted with buffer (pH 7.4, containing 33 mM Tris-
HCI,
2 mM EDTA and 50 mM b-mercaptoethanol) to obtain an approximate activity of
1000-2000 nmoles/min/mg protein. The diluted enzyme (83.25 mL) is preincubated
for 10 min at 37°C with or without test compound (6.25 mL) and 305.5 mL
of the
81.83 mM HEPES reaction buffer, pH 7.4 peptide substrate, 10.5 ml at a final
concentration of 50 mM, and is equilibrated to 37°C. in a LABLINE Multi-
Blok
heater equipped with a titerplate adapter. The preincubated recombinant enzyme
preparation (39.5 ml) with or without drug is added to initiate the
dephosphorylation
reaction, which proceeds at 37°C for 30 min. The reaction is terminated
by the
addition of 200 mL of the malachite green-ammonium molybdate-Tween 20 stopping
reagent (MG/AM/Tw). The stopping reagent consists of 3 parts 0.45% malachite
green hydrochloride, 1 part 4.2% ammonium molybdate tetrahydrate in 4 N HCl
and
0.5% Tween 20. Sample blanks are prepared by the addition of 200 mL MG/AM/1'w
to substrate and followed by 39.5 ml of the preincubated recombinant enzyme
with or
without drug. The color is allowed to develop at room temperature for 30 min.
and
the sample absorbances are determined at 650 nm using a platereader (Molecular
Devices). Sample and blanks are prepared in quadruplicates.
Calculations: PTPase activities, based on a potassium phosphate standard
curve, are
expressed as nmoles of phosphate released/min/mg protein. Inhibition of
recombinant
PTPIB by test compounds is calculated as percent of phosphatase control. A
four
parameter non-linear logistic regression of PTPase activities using SAS
release 6.08,
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-66-
PROC NLIN, is used for determining ICsp values of test compounds. The
following
results were obtained.
Exam I~ IC50 ( )
18 0.554
21 0.384
37 1.I8
40 1.10
41 1.08
42 1.50
43 0.189
58 1.01
59 0.612
60 0.129
62 0.654
63 0.904
64 0.347
68 1.02
70 0.074
71 0.079
79 0.386
81 1.99
82 2.00
87 1.68
89 0.126
91 1.30
92 0.644
108 0.061
109 0.071
I I O 1.52
111 0.062
1 I3 0.045
114 0.589
1 I5 0,279
116 0.765
117 1.51
118 0.031
120 0.54 I
121 0.184
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
-67-
Exam le IC50 ( )
122 0.036
123 0.082
124 0.085
126 0.298
127 0.064
128 0.025
129 0.046
130 0.80
132 0.311
133 0.506
I 34 0.093
135 0.209
136 0.050
137 0.341
138 0.636
139 0.061
140 0.204
141 0.126
142 0.103
143 1.17
144 1.13
146 0.064
148 1.23
150 0.207
152 0.994
155 0.056
156 0.026
162 0.145
165 0.665
166 0.565
168 0.994
169 1.22
170 0.607
171 0.302
172 0.076
177 1.08
178 0.480
_ 179 0.203
180 0.384
184 0.045
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-68-
Exam le ~ IC50 (~rM)
189 1.39
190 ~ 2.00
19I 0.118
194 0.217
195 0.889
196 0.174
200 1.17
203 0.402
210 1.06
215 0.49
216 0.083
Phenylarsine oxide 39.7
(reference standard)
Sodium orthovanadate 244.8
(reference standard)
Ammonium molybdate 8.7
tetrahydrate
(reference standard)
The blood glucose lowering activity of representative compounds of this
invention were demonstrated in an in vivo standard procedure using diabetic
(ob/ob)
mice. The procedures used and results obtained are briefly described below.
The non-insulin dependent diabetic (NIDDM) syndrome can be typically
characterizes by obesity, hyperglycemia, abnormal insulin secretion, hyper-
insulinemia and insulin resistance. The genetically obese-hyperglycemic ob/ob
mouse exhibits many of these metabolic abnormalities and is thought to be a
useful
model to search for hypoglycemic agents to treat NIDDM [Coleman, D.:
Diabetologia 14: 141-148, 1978).
In each test procedure, mice [Male or female ob/ob (C57 Bl/6J) and their lean
litermates (ob/+ or +/+, Jackson Laboratories) ages 2 to 5 months ( 10 to 65
g)) of a
similar age were randomized according to body weight into 4 groups of 10 mice.
The
mice were housed 5 per cage and are maintained on normal rodent chow with
water
ad libitum. Mice received test compound daily by gavage (suspended in 0.5 ml
of
0.5% methyl cellulose); dissolved in the drinking water; or admixed in the
diet. The
dose of compounds given ranges from 2.5 to 200 mg/kg body weight/day. The dose
is
calculated based on the fed weekly body weight and is expressed as active
moiety.
CA 02330623 2000-10-30
WO 99/58521 PCT/t1S99/10185
-69-
The positive control, ciglitazone (5-(4-(1-methylcyclohexylmethoxy)benzyl)-2,4-
dione, see Chang, A., Wyse, B., Gilchrist, B., Peterson, T. and Diani, A.
Diabetes 32:
830-838, 1983.) was given at a dose of 100 mg/kg/day, which produces a
significant
lowering in plasma glucose. Control mice received vehicle only.
On the morning of Day 4, 7 or 14 two drops of blood (approximetly 50 ul)
were collected into sodium fluoride containing tubes either from the tail vein
or after
decapitation. For those studies in which the compound was administered daily
by
gavage the blood samples were collected two hours after compound
administration.
The plasma was isolated by centrifugation and the concentration of glucose is
measured enzymatically on an Abbott V.P. Analyzer.
For each mouse, the percentage change in plasma glucose on Day 4, 7 or 14 is
calculated relative to the mean plasma glucose of the vehicle treated mice.
Analysis
of variance followed by Dunett's Comparison Test (one-tailed) are used to
estimate
the significant difference between the plasma glucose values from the control
group
and the individual compound treated groups ( CMS SAS Release 5.18).
The results shown in the table below shows that the compounds of this
invention are antihyperglycemic agents as they lower blood glucose levels in
diabetic
mice.
% Change
Exam le Dose (m Glucose from lo Change Insulin
K ) Vehicle from
Vehicle
70 25 -28.55 45.68 (a)
79 100 -22.6 -60.9
79 100 -22.60 -60.85
87 100 -15.77 (a) -87.97
89 10 -24.9 b
108 100 -45.83 -72.56
108 75 -49.74 -89.61
108 50 -34.79 -84.77
108 25 -25.66 -7I .03
108 10 -26.09 -30.33 (a)
109 25 -26.31 -71.64
111 100 -39.36 -17.85 (a)
113 25 -0.50 (a) -55.84
115 100 -33.9 52.00
117 100 -14.5 (a) -83.8
CA 02330623 2000-10-30
WO 99/58SZ1 PCT/1JS99/10185
-70-
% Change
Exam le Dose (m Glucose from % Change Insulin
K ) Vehicle from
Vehicle
121 100 -54.57 -87.76
121 10 1.37 (a) -41.81
122 100 -28.13 -37.40
123 75 -23.96 -40.94
125 95 -27.58 -89.68
126 25 -28.55 -62.39
127 25 -18.75 (a) -76.54
128 25 3.10 (a) -38.83
129 25 -14.86 (a) -54.58
131 25 -3.63 (a) -67.55
135 25 -15.83 (a) -57.43
136 25 -23.63 -52.05
138 25 -13.84 (a) -91.90
139 25 -18.5 (a) -22.00
146 100 -45.79 -56.19
155 25 -28.89 -63.23
172 100 5.62 (a) -52.05
189 100 -46.8 11.00 (a)
191 25 -36.5 -64.3
191 10 -22.8 b
194 25 -32.0 b
203 100 -16.1 (a) -91.0
215 100 -39.36 -4.66 (a)
216 25 -42.4 -85.6
216 25 -38.7 -84.0
216 10 -28.7 -69.4
216 5 -14.8 (a) -55.9
Ciglitazone
(reference 100 -43 -39
standard
a - no significant activity (p<0.05) at this dose.
b - not measured
Based on the results obtained in the standard pharmacological test procedures,
representative compounds of this invention have been shown to inhibit PTPase
activity and lower blood glucose levels in diabetic mice, and are therefore
useful in
treating metabolic disorders related to insulin resistance or hyperglycemia,
typically
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-71 -
associated with obesity or glucose intolerance. More particularly, the
compounds of
this invention useful in the treatment or inhibition of type II diabetes, and
in
modulating glucose levels in disorders such as type I diabetes. As used
herein, the
term modulating means maintaining glucose levels within clinically normal
ranges.
Effective administration of these compounds may be given at a daily dosage
of from about 1 mg/kg to about 250 mg/kg, and may given in a single dose or in
two
or more divided doses. Such doses may be administered in any manner useful in
directing the active compounds herein to the recipient's bloodstream,
including orally,
via implants, parenterally (including intravenous, intraperitoneal and
subcutaneous
injections), rectally, vaginally, and transdermally. For the purposes of this
disclosure,
transdermal administrations are understood to include all administrations
across the
surface of the body and the inner linings of bodily passages including
epithelial and
mucosal tissues. Such administrations may be carried out using the present
compounds, or pharmaceutically acceptable salts thereof, in lotions, creams,
foams,
patches, suspensions, solutions, and suppositories (rectal and vaginal).
Oral formulations containing the active compounds of this invention may
comprise any conventionally used oral forms, including tablets, capsules,
buccal
forms, troches, lozenges and oral liquids, suspensions or solutions. Capsules
may
contain mixtures of the active compounds) with inert fillers and/or diluents
such as
the pharmaceutically acceptable starches (e.g. corn, potato or tapioca
starch), sugars,
artificial sweetening agents, powdered celluloses, such as crystalline and
microcrystalline celluloses, flours, gelatins, gums, etc. Useful tablet
formulations
may be made by conventional compression, wet granulation or dry granulation
methods and utilize pharmaceutically acceptable diluents, binding agents,
lubricants,
disintegrants, suspending or stabilizing agents, including, but not limited
to,
magnesium stearate, stearic acid, talc, sodium lauryl sulfate,
microcrystalline
cellulose, carboxymethylcellulose calcium, polyvinylpyrrolidone, gelatin,
alginic
acid, acacia gum, , xanthan gum, sodium citrate, complex silicates, calcium
carbonate,
glycine, dextrin, sucrose, sorbitol, dicalcium phosphate, calcium sulfate,
lactose,
kaolin, mannitol, sodium chloride, talc, dry starches and powdered sugar. Oral
formulations herein may utilize standard delay or time release formulations to
alter
the absorption of the active compound(s). Suppository formulations may be made
from traditional materials, including cocoa butter, with or without the
addition of
CA 02330623 2000-10-30
WO 99/58521 PCT/11S99/10185
-72-
waxes to alter the suppository's melting point, and glycerin. Water soluble
suppository bases, such as polyethylene glycols of various molecular weights,
may
also be used.
It is understood that the dosage, regimen and mode of administration of these
compounds will vary according to the malady and the individual being treated
and
will be subject to the judgment of the medical practitioner involved. It is
preferred
that the administration of one or more of the compounds herein begin at a low
dose
and be increased until the desired effects are achieved.
The following procedures describe the preparation of representative examples
of this invention.
Example 1.
Benzofblthiophene-2-y~phen~rll-methanol
n-Butyl lithium (35 ml, 2.5 N in hexanes) was added dropwise to a stirred
solution of thianaphthene (11.5 g, 85.6 mmol) in THF (300 mL) at -78°C
under a dry
N2 atmosphere. After 1 h , benzaldehyde (9.6 mL, 94.4 mmol ) was added and the
cold bath was removed. After an additional 30 minutes, sat. aq. NH4C1 was
added and
the reaction mixture was partitioned between water and ether. The ether phase
was
washed with brine and concentrated. The resultant solid was triturated with
pet. ether
and filtered to provide the title compound as a white solid ( 17.7 g, 86%):
NMR
(CDC13); 8 7.78 (m, 1H, thiopheneH), 7.68 (m, 1H, thiopheneH), 7.22-7.56 (m,
7H},
7.12 (s, 1H, thiopheneH), 6.12 (d, 1H, OH), 2.51 (d, 1H, CH).
Example 2.
(6-Methoxy-benzo,~'blthiophene-2-yl-(phenyl)-methanol
n-Butyl lithium ( 12.5 ml, 2.5 N in hexanes) was added dropwise to a stirred
solution of 6-methoxythianaphthene (5.0 g, 30.4 mmol, S. L. Graham, et al., J.
Med.
Chem. 1989, 32, 2548-2554} in THF (70 mL) at -78°C under a dry N2
atmosphere.
After 1 h , benzaldehyde (3.42 mL, 33.4 mmol ) was added. After an additional
45
minutes, sat. aq. NH4C1 was added and the reaction mixture was partitioned
between
CA 02330623 2000-10-30
WO 99/58521 PCT/11S99110185
-73-
water and ether. The ether phase was washed with brine and silica gel was
added to it.
The solvent was removed and the adsorbate was flash chromatograghed (eluent:
9:1
petroleum ether: ethyl acetate) to provide the title compound as a white solid
(5.5 g,
66%): mp 79-80. NMR (CDC13); b 7.55 (d, J = 9 Hz, 1H), d 7.49 (ddd, J = 7, 1,
1 Hz,
2H), 7.39 (ddd, J = 7, 7, 1 Hz, 2H), 7.26 (m, 1H), 7.01 (s, 1H), 6.94 (d, J =
8 Hz, 1H},
6.09 (d, J = 4 Hz, 1H), 3.85 (s, 3H), 2.46 (d, J = 4 Hz, 1H); MS (EI): 270
(25%, MI).
Example 3.
B enzo f blthiophen-2-yl-~3-methoxy-phenXl)-methanol
Prepared according to the procedure of Example 1 and substituing m-
anisaldehyde for bezaldehyde. White solid: mp 63-65°C; MS (+FAB): [M+]
270;
Anal. Calc. for C 16H 14O2S: C, 71.08, H, 5.22, N, 0.00. Found: C, 71.07, H,
5.16, N,
0.13.
Example 4.
2-Benzyl-benzoLlthio,~hene
Trifluoroacetic acid (105 mL) was added dropwise over a 35 minute period to
a stirred suspension of benzo[b]thiophene-2-yl-(phenyl)-methanol (17.6 g, 73.2
mmol), sodium borohydride ( 13.75 g, 364 mmol) and ether ( 1.3 L). After an
additional 5 hours the reaction mixture was added to 10 % aqueous sodium
hydroxide ( 1.3 L) and stirred for 30 minutes. The layers were separated and
the ether
phase was washed with brine (500 mL) and dried (MgS04). The ether phase was
concentrated to provide the title compound as a white solid (15.2 g, 92%): NMR
(CDCl3); b 7.73 (d, J = 6 Hz, 1 H, thiopheneH), 7.65 (d, J = 7 Hz, 1 H,
thiopheneH),
7.20-7.38 (m, 7H), 7.00 (s, 1H, thiopheneH), 4.22 (s, 2H, CH2).
Example 5.
2-Benzyl-6-methoxy-benzo[blthiophene
Prepared from (6-methoxy-benzo[b]thiophene-2-yl-(phenyl)-methanol
(Example 2) according to the procedure in Example 4. White solid: mp 60-61
°C:
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-74-
NMR (CDC13); b 7.53 (d, J = 9 Hz, 1H), d 7.35-7.22 (m, 6H), 6.93 (dd, J = 8, 2
Hz,
1H), 6.91 (d, J = 1 I~z, 1H), 4.19 (s, 2H), 3.84 (s, 3H); MS (EI): 254 (50%,
MI); Anal.
Calc. for C 16H 140S: C, 75.56, H, 5.55, N, 0.00. Found: C, 75.62, H, 5.44, N,
0.02.
Example 6.
2-(3-Methoxy-benzyl)-benzofblthiophene
Prepared from benzo[b]thiophen-2-yl-(3-methoxy-phenyl)-methanol (Example
3) according to the procedure in Example 4. White solid: mp 74-75.5°C:
MS (+FAB):
[M+] 254; Anal. Calc. for C 16H 140S: C, 75.56, H, 5.55, N, 0.00. Found: C,
75.85,
H, 5.48, N, 0.01.
Example 7.
(2-Benzyl-benzo[b]thiophen-3-~)-(4-methox -phenyl)-methanone
Tin tetrachloride (9.0 mL, 76.91 mmol) was added dropwise over a 25 minute
period to a stirred solution of 2-benzyl-benzo[b]thiophene (14.87 g, 96.79
mmol), p-
anisoyl chloride (11.75 g, 68.87 mmol) and carbon disulfide (75 mL) under a
dry
nitrogen atmosphere. After 6 hours, the reaction mixture was added to water
and
extracted with dichloromethane. The dichloromethane extract was washed with
sat.
aq. sodium bicarbonate and brine. The solvent was removed and the resultant
solid
was triturated with pet. ether to give the title compound as a white solid
(20.2 g,
85%): mp 135-137°C: NMR (CDC13); 8 7.85 (dm, J = 9 Hz, 2H), 7.73 (dm,
1H), 7.42
(dm, 1H), 7.18-7.30 (m, 7H), 6.93 (dm, J = 9 Hz, 2H), 4.21 (s, 2H, CH2), 3.88
(s, 3H,
CH3): IR (KBr, cm-1): 1650; MS (EI): 358 (100%, MI), 343 (15%), 327 (75%j;
Anal.
Calc. for C23H18O2S: C, 77.07, H, 5.06, N, 0.00. Found: C, 76.91, H, 5.02, N, -
0.12.
Example 8.
L2-Benzvl-6-methoxy-benzofblthiophen-3-v1Z(4-methox -y_nhenyl)-methanone
Tin tetrachloride (2.0 mL, 17.09 mmol) was added dropwise over a 10 minute
period to a stirred, -78°C solution of 2-benzyl-6-methoxy-
benzo[b]thiophene (2.71g,
10.65 mmol), anisoyl chloride ( 1.93 g, 11.29 mmol) and dichloromethane (41
mL)
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
-75-
under a dry nitrogen atmosphere. After 1 hours at -78°C, the reaction
mixture was
slowly warmed to room temperature and stirred for 16 h period. The reaction
mixture
was added to water and extracted with ether. The ether phase was washed with
brine
and silica gel was added to it. The solvent was removed and the adsorbate was
flash
S chromatographed (eluent: 9:1 petroleum ether: ethyl acetate) to provide the
title
compound as a white solid (2.42 g, 58%): mp 110-112°C: NMR (CDC13); 8
7.84 (d,
J = 9 Hz, 2H), 7.31-7.18 (m, 7H), 7.92 (d, J = 9 Hz, 2H), 6.97 (dd, J = 9, 2
Hz, 1H),
4.17 (s, 2H), 3.88 (s, 3H), 3.883 (s, 3H); MS (FAB+): 389 (80%, M+H); Anal.
Calc.
for C24H2003S: C, 74.20, H, 5.19, N, 0.00. Found: C, 74.94, H, 5.10, N, 0.03.
Example 9.
4-Bromo-2. 6-diisoproRylanisole
This is a modification of the procedure of Schuster, Ingeborg L; Parvez,
Masood; Freyer, Alan J. J. Org. Chem. 1988, 53, 5819. A solution of bromine
(6.3
mL, 119 mmol) in acetic acid (40 mL) was added dropwise to a stirred, room
temperature solution of 2, 6-diisopropylphenol ( 20 mL, 97.1 mmol of 90% tech.
grade) in acetic acid (280 mL). After 6 h, water was added and the mixture was
extracted with ether. The ether phase was dried and concentrated and the
residue was
flash chromatographed (petroleum ether: eluent) to provide 4-bromo-2, 6-
diisopropylphenol (16.2 g, 65%) as a red oil. This oil was dissolved in DMF
(50 mL),
iodomethane (11.7 mL, 189 mmol) and potassium carbonate (26.3 g, 117 mL) were
added to it. This reaction mixture was stirred for 5 h and diluted with water.
This
mixture was extracted with ether and the ether extracts were dried,
concentrated and
flash chromatographed (petroleum ether: eluent) to provide the title compound
as a
colorless oil ( 15.4 g, 90%}: NMR (CDCl3); b 7.17 (s, 2H), 3.70 (s, 3H), 3.29
(septet,
1H), 1.20 (d, 12 H}.
CA 02330623 2000-10-30
WO 99/58521 PCT/11599/10185
-76-
Example 10.
3 5-Diisopropvl. 4-metho~benzoic acid
This is a modification of the procedure of Schuster, Ingeborg L; Parvez,
Masood; Freyer, Alan J. J. Org. Chem. 1988, 53, 5819. A solution of n-
butyllithium
(2.5 N in hexanes, 13.0 mL, 32.5 mmol) was added dropwise over 20 min to a
stirred,
-78°C solution of 4-bromo-2, 6-diisopropylanisole (8.0 g, 29.5 mmol) in
THF ( 185
mL). After 2 h at -78°C , the reaction mixture was cautiously added to
finely ground
dry ice. The resulting suspension was stirred for 20 min at room temperature
and
cautiously added to water. The water phase was acidified with 10% aqueous HCI
and extracted with ether. The ether extracts were dried and concentrated. The
resulting oil solidified upon standing. This solid was triturated with
petroleum ether to
provide the title compound as a white solid (5.38 g, 77%): NMR (CDCl3); b 7.87
(s,
2H), 3.76 (s, 3H), 3.35 (septet, 1H), I.25 (d, 12 H).
Example 11.
(2-Benzvl-benzofblthiophen-3-yl)-C3 5-diisopropvl-4-methoxy-phenyl)-methanone
A drop of DMF was added to a solution of oxalyl chloride ( 1.4 mL, 16.2
mmmol), 3, 5-diisopropyl-4-methoxybenzoic acid (4.0 g , 14.7 mmol) in dichloro-
methane under a dry nitrogen atmosphere. After 4h the solvent was removed and
the
resulting solid was triturated with petroleum ether and dried in vacuo. To
this solid
was added 2-benzyl-benzo[b]thiophene (3.32 g, I3.4 mmol) and dichloromethane
(75
mL). The resulting solution was stirred under a dry nitrogen atmospher and
cooled to
-78°C. Tin tetrachloride (3.44 mL, 29.48 mmol) was added dropwise over
a 20
minute period. The reaction mixture was warmed to room temperature and stirred
overnight. The reaction mixture was added to water and extracted with ether.
The
ether extract was washed with water and brine. Silica gel was added and the
solvent
was removed. The adsorbate was flash chromatographed (gradient, petroleum
ether to
95:5 petroleum ether : ethyl acetate). The solvent was removed and the solid
was
triturated with ether to give the title compound as a a white solid (3.68 g,
62%): mp
124-125°C; NMR (CDCl3); b 7.75 (ddd, J = 8, 2, 1 Hz, IH), 7.64 (s, 2H),
7.48 (ddd, J
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
_77_
= 8, 2, 1, 1H), 7.30-7.19 (m, 7H), 4.22 (s, 2H), 3.80 (s, 3H) 3.35 (septuplet,
J = 7 Hz,
2H}, 1.19 (d, J = 7 Hz, 12H); MS (+FAB): 443 (100%, M+H); Anal. Calc. for
C29H30O2S: C, 78.69, H, 6.83, N, 0.00. Found: C, 78.57, H, 6.88, N, 0.14.
S Example I2.
L-Benzyl-benzo [blthiophen-3-yl_)~3-methoxy-phenyl_)-methanone
Prepared from 2-benzyl-benzo[b]thiophene and m-anisoyl chloride according
to the procedure in Example 8. White solid: MS (EI): [M+], 358.
Example 13.
(2-Benzvl-benzo(~b],thiophen-3-yl)-(3.4-dimethoxy-nhen~}-methanone
Prepared from 2-benzyl-benzo[b]thiophene and 3,4-dimethoxybenzoyl
chloride according to the procedure in Example 8. White solid: MS (EI): [M+],
388.
Example 14.
4-Benzofb]naphtho[2,3-dlthiophen-11-yl- henol
A 1.0 M solution of boron tribromide in dichlorornethane ( 130 mL, 130
mmol) was added slowly to a stirrred solution of (2-benzyl-benzo[b]thiophen-3-
yl)-
(4-methoxy-phenyl)-methanone ( 14.5 g, 40.45 mmol) in dichloromethane ( 130
mL) at
-78°C under a dry nitrogen atomosphere. The solution was allowed to
warm to
ambient temperature and was stirred overnight. The reaction mixture was
quenched
with water and partitioned between water and dichloromethane. Silica gel was
added
to the dichloromethane phase and the solvent was removed, The adsorbate was
flash
chromatographed (eluent 4:1 pet. ether: ethyl acetate) to provide a white
solid (9.75
g). This solid was recrystalized from acetic acid to provide off white needles
(8.78,
56%): mp: 112-116°C; NMR (CDC13); 8 8.33 (s, 1H), 7.94 (dt, J = 8 Hz,
1H), 7.77
(dm, J = 8 Hz, 1 H), 7.64 (dm, J = 8 Hz, 1 H), 7.52 (ddd, J = 8, 7, 1 Hz, 1
H), 7.37 (m,
2H), 7.29 (d, J = 9 Hz, 2H), 7.11 (d, J = 9 Hz, 2H), 7.08 (m, 1H}, 6.85 (dm, J
= 8 Hz,
1H), 2.11 (s, 3H, acetic acid CH3); MS (EI): 326 (100%, MI); Anal. Calc. for
C22H140S~C2H4O2: C, 74.59, H, 4.69, N, 0.00. Found: C, 74.40, H, 4.59, N,
0.15.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
_78_
Example 15.
11-(4-Hvdroxy-phenyl)-benzojb]naphtho(2 3-dlthi~hen-3-of
Boron tribromide (5 mL, 52.9 mmol) was added slowly to a stirrred solution
of (2-benzyl-6-methoxy-benzo[b]thiophen-3-yl)-(4-methoxy-phenyl)-methanone
(2.30 g, 5.92 mmol) in dichloromethane (30 mL) at -78°C under a dry
nitrogen
atomosphere. The solution was allowed to warm to ambient temperature and was
stirred for 4h. The reaction mixture was quenched with water and partitioned
between
water and ether. Silica gel was added to the ether phase and the solvent was
removed,
The adsorbate was flash chromatographed (eluent 7:3 pet. ether: ethyl acetate)
to
provide the title compound as a white solid (2.08 g, 96%): mp: 197-
199°C; NMR
(CDC13); 8 8.28 (s, 1H), 7.92 (d, J = 8 Hz, 1H), 7.59 (dm, J = 8 Hz, 1H), 7.50
(ddd, J
= 8, 7, 1 Hz, 1H), 7.39-7.25 (m, 5H), 7.21 (d, J = 2 Hz, 1H), 7.11 (d, J = 9
Hz, 2H),
6.69 (d, J = 9 Hz, 1 H), 6.57 (dd, J = 9, 2 Hz, 1 H), 5.03 (s, 1 H), 4.94 (s,
1 H); MS
(FAB+): 343 (15%, M+H); Anal. Calc. for C22H1402S: C, 77.17, H, 4.12, N, 0.00.
Found: C, 76.74, H, 4.04, N, 0.02.
Example 16.
4-(6-Benzolblnaphthof2.3-dlthiophen-1 I-yl)-2 6-diisoprop ~~1- henol
Neat boron tribromide (4.3 mL, 45.2 mmol) was added dropwise to a stirrred
suspension of (2-benzyl-benzo(b]thiophen-3-yl)-(3,5-diisopropyl-4-methoxy-
phenyl)-
methanone (3.57 g, 8.07 mmol) in dichloromethane (30 mL) at -78°C under
a dry
nitrogen atomosphere. The solution was allowed to warm to ambient temperature
and
was stirred for 1.5 h. The reaction mixture was cooled to 0°C and
carefully quenched
with water . The reaction mixture was partitioned between water and ether. The
ether
phase was washed with water and brine. Silica gel was added to the ether phase
and
the solvent was removed. The adsorbate was flash chromatographed (gradient:
99:1 to
97:1 petroleum ether : ethyl acetate) to provide the title compound as a white
solid
(1.46 g, 44%): NMR (CDC13); 8 8.33 (s, 1H), 7.95 (ddd, J = 8, 1, l, 1H), 7.77
(ddd, J
= 8, 1, 1 Hz, 1H), 7.73 (ddd, J = 8, 1, 1 Hz, 1H), 7.53 (ddd, J =8, 8, l, 1H),
7.40 (ddd,
J = 8, 8, 1 Hz, 1H), 7.34 (ddd, J = 8, 8, 1 Hz, 1H), 7.10 (s, 2H), 7.04 (ddd,
J = 8, 8, I
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-79-
Hz, 1H), 6.75 (ddd, J = 8, 1, 1 Hz, 1H), 5.01 (s, 1H), 3.31 (septuplet, J = 7
Hz, 2H),
1.29 (s, J = 7 Hz, 6H), 1.27 (s, J = 7 Hz, 6H); MS (+FAB): 411 (100%, M+H);
Anal.
Calc. for C28H260S: C, 81.91, H, 6:38, N, 0.00. Found: C, 81.10, H, 6.54, N,
0.40.
Example 17.
3-Benzofblnaphtho[2.3-dlthiophen-11-ylZphenol
Prepared from (2-benzyl-benzo[b]thiophen-3-yl)-(3-methoxy-phenyl)-
methanone (Example 12) according to the procedure for Example 15. White solid:
mp
92-94°C: MS (EI): [M+], 326; Anal. Calc. for C22H140S: C, 80.95, H,
4.32, N, 0.00.
Found: C, 80.01, H, 4.18, N, 0.04.
Example 18.
~Benzofb]naphtho[2.3-d]thiophen-11-yll-benzene-1.2-diol
Prepared from (2-benzyl-benzo[b]thiophen-3-yl)-(3,4-dimethoxy-phenyl)-
methanone (Example 13) according to the procedure for Example 15. White solid:
mp
188-189°C: MS (EI}: [M+], 342; Anal. Calc. for C22H14O2S: C, 77.17, H,
4.12, N,
0.00. Found: C, 76.47, H, 3.85, N, 0.00.
Example 19.
8-Methox -y 11-(4-methox~y-nhenyl)-benzo[blnaphtho[2 3-d]thiophene
To a cold (-78°C) solution of 2-(3-methoxybenzyl)-benzo[b]thiophene
(2.10 g,
8.26 mmol) and p-anisoyl chloride ( 1.48 g, 8.67 mmol) in anhydrous methylene
chloride (31 mL) was added tin IV chloride (2.90 mL, 24.8 mmol, 3 eq) dropwise
over a period of 24 minutes. After stirring overnight in the warming dry ice
bath and
at ambient temperature for 7 hours the reaction mixture was poured onto water
( 175
mL,) and the organics were extracted with diethyl ether (2x300 mL). The
extracts
were combined, and washed with brine. Silica gel was added and the solvent was
removed. The adsorbate was flash chromatographed (97/3 petroleum ehter/ethyl
acetate) to give the title compound as a white solid (1.92 g, 63%): mp 158-
159°C; MS
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-80-
(+FAB): [M+] 370; Anal. Calc. for C24H18O2S: C, 77.81, H, 4.90, N, 0.00.
Found:
C, 78.00, H, 4.76, N, 0.03.
Example 20.
11-(4-H droxv-phenyl)-benzo[b]naphtho[2.3-d]thiophen-8-of
To a cold (-75°C) solution of 8-methoxy-11-(4-methoxy-phenyl)-
benzo[b]-
naphtho[2,3-d]thiophene (1.92 g, 5.18 mmol) in anhydrous methylene chloride
was
added a solution of boron tribromide ( 1 M in methylene chloride, 6.74 mL,
6.74
mmol, 1.3 eq) dropwise over a period of 18 minutes. After stirring in the cold
for 3.5
hours and at ambient temperature for approximately 19 hours the reaction
mixture
was quenched with water ( 100 mL), diluted with methylene chloride (50 mL) and
the
organics were extracted with diethyl ether ( 1 x 100 mL, 1 x75 mL). The
extracts were
combined, washed with brine, and combined with silica gel. The solvents were
removed and the adsorbate was flash chromatographed (74/26 petrolem
ether/ethyl
acetate) and dried at 92°C overnight to the title compound as an off
white solid ( 1.07
g, 91%): mp 233-235°C; NMR (DMSO-d6); 8 9.91 (broad s, 1H), 9.77 (broad
s, 1H),
8.30 (s, 1H), 7.88 (d, J = 8 Hz, 1H), 7.40 (d, J = 8 Hz, 1H), 7.35 (ddd, J =
8, 8, 1 Hz,
1H), 7.23 (d, J = 2 Hz, 1H), 7.15 (d, J = 8 Hz, 2H), 7.09 (ddd, J = 8, 8, 1
Hz, 1H), 7.06
- 7.00 (multiplet containing a doublet at 7.05, J = 8 Hz, 3 H), 6.71 (d, J = 8
Hz, 1H);
MS (+FAB): [M+] 343; Anal. Calc. for C22H14O2S: C, 77.17, H, 4.12, N, 0.00.
Found: C, 76.43, H, 3.82, N, 0.01.
Example 21.
2.6-Dibromo-4-(6-bromo-benzofblnaphthof2 3-d]Ithiophen-11- 1)-phenol
A solution of bromine (3.1 mL, 60.17 mmol) in glacial acetic acid (30 mL)
was added over a five minute period to a stirred cloudy solution of 4-benzo[b]-
naphtho[2,3-d]thiophen-1 I-yl-phenol (5.0 g, 15.32 mmol) and potassium acetate
(15.0
g, 153 mmol) in acetic acid (92 mL) at ambient temperature. An exotherm was
noted
and yellow precipitate resulted. After 45 minutes, the reaction mixture was
added to
water ( 1 L), solid sodium thiosulfate (2 g) was added and the suspension was
stirred
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-81 -
for five minutes. The solid was filtered and washed with water (1L) , pet.
ether (2 x
300 mL), 3:1 pet. ether:ether (3 x 50 mL) and pet. ether (2 x 300 mL) and
dried in
vacuo to provide a tan solid (7.00 g}. An additional amount crystallized from
the
mother liquor and was added to the total (tan solid, 8.31 g, 96%). The product
was
95% pure at this stage, however it could be further purified by
recrystallization from
acetic acid to provide the title compound as a white solid: mp 226.5-
227°C: NMR
(CDC13); 8 8.35 (ddd, J = 8, 1, 1 Hz, 1H), 7.84 (ddd, J = 8, l, 1 Hz, 1H),
7.67 (ddd, J
= 8, 7, 1 Hz, 1H), 7.60 (ddd, J = 8, l, 1 Hz, 1H), 7.55 (s, 2H), 7.49 (ddd, J
= 8, 7, 1
Hz, 1H), 7.45 (ddd, J = 8, 7, 1 Hz, 1H), 7.21 (ddd, J = 8, 7, 1 Hz, 1H}, 6.87
(ddd, J =
8, 1, 1 Hz, 1H), 6.19 (s, 1H, OH); MS (-APCI): [M-H]-, 3 bromine isotope
pattern,
559 (25%), 561 ( 75%) 563 (100%) 565 (45%); Anal. Calc. for C22H11Br30S: C,
46.92, H, 1.97, N, 0.00. Found: C, 46.67, H, 1.85, N, 0.03.
Example 22.
11-(3.5-dibromo-4-methoxy-phenyl)-benzo[b]!naphthof2 3-dlthiophene
Iodomethane (0.383 mL, 6.16.mmo1) was added to a stirred suspension of 2,6-
dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenol (2.13 g, 4.4
mmol), potassium carbobnate (0.669 g, 4.34 mmol) and DMF ( 15 mL) at room
temperature under a dry nitrogen atmosphere. After 3h, the reaction mixture
was
diluted with water and the resulting solid was filtered and washed with water.
The
solid was taken up in dichloromethane and silica gel (40 mL) was added. The
solvent
was removed and the adsorbate was flash chromatographed (90:10 petroleum ether
dichloromethane) to provide the title compound as a white solid (2.0 g, 91 %):
mp
238.5-239.5°C: NMR (CDCl3); 8 8.36 (ddd, J = 8, 1, 1 Hz, 1H), 7.84
(ddd, J = 8, I, 1
Hz, 1H), 7.67 (ddd, J = 8, 7, 1 Hz, 1H), 7.60 (s, 2H), 7.58 (ddd, J = 8, 1, 1
Hz, 1H),
7.50 (ddd, J = 8, 7, 1 Hz, 1 H), 7.45 (ddd, J = 8, 7, 1 Hz, 1 H), 7.20 (ddd, J
= 8, 7, 1 Hz,
1H), 6.80 (ddd, J = 8, 1, 1 Hz, 1H), 4.11 (s, 3H, CH3); MS (EI): [M+], 3
bromine
isotope pattern, 574 (35%), 576 ( 95%) 578 (100%) 580 (45%); Anal. Calc. for
C23H13Br30S: C, 47.87, H, 2.27, N, 0.00. Found: C, 47.73, H, 1.88, N, 0.03.
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-82-
Example 23.
1~4-Methox3r_,phen r~l -benzo[bLnaphtho[2.3-d]'thionhene
Prepared from benzo[bJnaphtho[2,3-d]thiophen-11-yl-phenol (Example 14)
according to the procedure in Example 22. White solid (0.516 g, SO%): mp: 220
221°C; NMR (DMSO-d6); 8 8.60 (s, 1H), 8.04 (d, J = 8 Hz, 1H), 7.96 (d,
J = 8 Hz,
1H), 7.57 (ddd, J = 8, 7, 1 Hz, 1H), 7.50 (ddd, J = 8, 1, 1 Hz, 1H), 7.46-7.40
(m, 3H),
7.33 (d, J = 9 Hz, 2H), 7.24 (d, J = 9 Hz, 2H), 7.14 (ddd, J = 8, 7, 1 Hz,
1H), 6.75 (d, J
= 8 Hz, 1H), 3.33 (s, 3H}; MS (EI): 340 (100%, MI); Anal. Calc. for C23H160S:
C,
81.14, H, 4.74, N, 0.00. Found: C, 81.11, H, 4.57, N, 0.14.
Example 24.
11-(4-Methoxy-3 5-dimet~l ~henvl)-6-methyl-benzo[b]naphtho[2 3-d]thionhene
A suspension of 6-bromo-11-(3,5-dibromo-4-methoxy-phenyl)-benzo[b]
naphtho[2,3-d]thiophene (1.0 g, 1.733 mmol), tetramethyl tin (2.0 mL, 14.38
mmol),
bis(triphenylphosphine)palladium II chloride (100 mg, 8 mol%) and DMF (8 mL)
was
heated in a sealed pressure vessel under argon at 100°C for 17 hours
(dissolution
occured after 30 min). The reaction mixture was added water and the water was
extracted with ether. Silica gel was added to the ether phase and the ether
was
removed. The adsorbate was flash chromatographed (96:4 petroleum ether:ethyl
acetate) to provide the title compound as a white solid (0.63 g, 86%): mp 154-
156°C:
NMR (CDC13); 8 8.16 (ddd, J = 8, 1, 1 Hz, 1H), 7.80 (dd, J = 8, 1 Hz, 1H),
7.70 (dd, J
= 8, 1 Hz, 1 H),7.57 (ddd, J = 8, 7, 1 Hz, 1 H), 7.41 (ddd, J = 8, 7, 1 Hz, 1
H), 7.35
(ddd, J = 8, 8, 1 Hz, 1 H), 7.07 (ddd, J = 8, 7, 1 Hz, 1 H), 7.04 (s, 2H),
6.79 (dd, J = 8,
1 Hz, 1H), 3.92 (s, 3H), 2.97 (s, 3H), 2.39 (s, 6H); MS (EI): 382 (100%, MI);
Anal.
Calc. for C26H220S: C, 81.64, H, 5.80, N, 0.00. Found: C, 81.30, H, 5.99, N,
0.38.
Example 25.
2,6-Dimeth~~6-methyl-benzoLlnaphthof2 3-d]thiophen-l h~phenol
A mixture of 11-(4-methoxy-3,S-dimethyl-phenyl)-6-methyl-benzo[b]-
naphtho[2,3-d]thiophene (0.55 g, 1.44 mmol) and pyridinium hydrochloride ( 1.0
g,
CA 02330623 2000-10-30
WO 99/58521 PCT/tJS99/10185
-83-
8.64 mmol) was heated in a 240°C oil bath for 1.25 hour. During this
time an
additional amount of pyridinium hydrochloride was added ( 1.0 g, 8.64 mmol).
The
reaction mixture was cooled to room temperature and partitioned between dilute
HCl
and ether. Silica gel was added to the ether phase and the solvent was
removed. The
adsorbate was flash chromatographed (9:1 petroleum ether:ethyl acetate) to
provide a
light yellow solid (340 mg). This solid was recrystallized from acetic acid to
provide
the title compound as a light yellow solid (0.215 g, 41%): mp 147-
149°C: NMR
(CDCI3); 8 8.15 (ddd, J = 8, 1, 1 Hz, 1H), 7.80 (ddd, J = 8, 1, 1 Hz, 1H),
7.71 (ddd, J
= 8, 1, 1 Hz, 1 H), 7.56 (ddd, J = 8, 7, 1 Hz, 1 H), 7.39 (ddd, J = 8, 7, 1
Hz, 1 H), 7.35
(ddd, J = 8, 8, 1 Hz, 1H), 7.09 (ddd, J = 8, 7, 1 Hz, 1H), 7.01 (s, 2H), 6.79
(ddd, J = 8,
1, 1 Hz, 1H), 2.96 (s, 3H), 2.36 (s, 6H); MS (EI): 368 (100%, MI); Anal. Calc.
for
C25H200S: C, 81.49, H, 5.47, N, 0.00. Found: C, 81.62, H, 5.32, N, -0.03.
Example 26.
~Benzo[b]nahhtho[2.3-d]thiophen-11-~)-2.6-diiodo-phenol
Iodine (4.5 g, 17.6 mmol) was added portionwise to a stirred, 0°C
solution of
4-(benzo[b]naphtho[2,3-d]thiophen-11-yl-phenol (2.3 mL, 7.05 mmol), sodium
hydroxide (97%, 0.581 g, 14.1 mmol) in methanol (46 mL) over a period of one
hour
and the mixture was stirred at 0°C for 1 h. and at ambient temperature
for 6 h. The
reaction mixture was diluted with water (200 mL) and aqueous mixture was
extracted
with ethyl ether (2 X 200 mL). The ethyl ether extracts were washed with 5%
sodium
bisulfite and water, dried with brine and anhydrous MgS04. Silica gel (50 mL)
was
added. Solvent was removed and the adsorbate was flash chrornatographed
(eluent 8:2
petroleum ether: methylene chloride) to provide the title compound as a white
solid
(2.2 g, 54%): mp 213-214 °C: MS (-FAB}: [M-H]-, 576.8; Anal. Calc. for
C22H12I20S: C, 45.70, H, 2.09, N, 0.00. Found: C, 45.82, H, 2.07, N, 0.30.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-84-
Example 27.
4-(Benzo(b]na hn thoj2.3-d]thiophen-11-vl -2-iodo-phenol
Iodine (4.5 g, 17.6 mmol) was added portionwise to a stirred, 0°C
solution of
4-(benzo[b]naphtho[2,3-d]thiophen-11-yl-phenol (2.3 mL, 7.05 mmol), sodium
hydroxide (97%, 0.581 g, 14.1 mmol) in methanol (46 mL) over a period of one
hour
and the mixture was stirred at 0°C for 1 h. and at ambient temperature
for 6 h. The
reaction mixture was diluted with water (200 mL) and aqueous mixture was
extracted
with ethyl ether (2 X 200 mL). The ethyl ether extracts were washed with 5%
sodium
bisulfite and water, dried with brine and anhydrous MgS04. Silica gel (50 mL)
was
added. Solvent was removed and the adsorbate was flash chromatographed (eluent
8:2
petroleum ether: methylene chloride) to provide a off white solid (0.624 g,
20%): mp
125-128 °C: MS (EI): [M+], 452; Anal. Calc. for C22H13IOS: C, 58.42, H,
2.90, N,
0.00. Found: C, 58.46, H, 3.00, N, 0.09.
Example 28.
11-(4-Methoxy-3.S-diiodo-phen,~l-benzo[b]naphtho~,2.3-d]thiophene
Prepared from 4-(benzo[b]naphtho[2,3-d]thiophen-11-yI)-2,6-diiodo-phenol
(Example 26) according to the procedure on Example 22. White solid: mp 228-
230°C: MS (EI): [M+], 592; Anal. Calc. for C23H14I20S: C, 46.65, H,
2.38, N, 0.00.
Found: C, 45.95, H, 2.25, N, 0.19.
Example 29.
11-(3-Iodo-4-methox~phenyl)-benzo[blnaphtho[2.3-d]thiophene
Prepared from 4-(benzo[b]naphtho[2,3-d]thiophen-11-yl)-2-iodo-phenol
(Example 27) according to the procedure on Example 22. White solid: mp 274-
275°C: MS (EI): [M+], 466 (100%, MI); Anal. Calc. for C23H15IOS: C,
59.24, H,
3.24, N, 0.00. Found: C, 58.53, H, 3.11, N, 0.11.
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
-85-
Example 30.
5-BenzoLblnaphthof2.3-dlthio~hen-11-yl-2-methox -~isonhthalonitrile
A suspension of 11-(3,5-diiodo-4-methoxy-phenyl)-benzo[b]naphtho[2,3-
d]thiophene (4.55 g, 7.68 mmol) and copper (I) cyanide 3.13 g, 38.4 mmol) in 1-
methyl-2-pyrrolidinone ( 18 mL) was heated in an 150°C oil bath under a
dry nitrogen
atmosphere. After 1 h the solution was added to water (200 mL) and acidified
with
10% aqueous HCI. The solid was filtered and flash chromatographed (silica gel:
eluent: methylene chloride) to provide the title compound as a yellow solid
(2.47 g,
82%): mp 214-215 °C: MS (EI): [M+], 390 (100%, MI); Anal. Calc. for
C25H14N20S: C, 76.90, H, 3.61, N, 7.17. Found: C, 76.48, H, 3.46, N, 7.17.
Example 31.
5-yB enzo [bl naphtho f 2.3-dl thiophen-11-vl )-2-methoxy-benzonitrile
Prepared from 11-(3-iodo-4-methoxy-phenyl)-benzo[b]naphtho[2,3-d]thio-
phene (Example 29) according to the procedure on Example 30. White solid: mp
230-
232 °C: MS (EI): [M+], 365 (100%, MI); Anal. Calc. for C24H15NOS: C,
78.88, H,
4.41, N, 3.83. Found: C, 77.61, H, 4.23, N, 4.10.
Example 32.
5-Benzo[b]naphthoj2.3-dlthio~hen-11-~ydrox -y isophthalonitrile
Prepared from 5-benzo[b]naphtho[2,3-d]thiophen-11-yl-2-methoxy-iso-
phthalonitrile (Example 30) according to the procedure on Example 20. White
solid:
mp 274-276 °C: MS (EI): [M+], 376 (80%, MI); Anal. Calc. for C24H
12N20S: C,
76.58, H, 3.21, N, 7.44. Found: C, 76.20, H, 3.19, N, 7.35.
Example 33.
5-Benzo[blnahhthof2.3-dlthiophen-11-yl-2-h dery-benzonitrile
Prepared from 5-(benzo[b]naphtho[2,3-d]thiophen-11-yl)-2-methoxy-benzo-
nitrite (Example 31) according to the procedure on Example 20. White solid: mp
231-
CA 02330623 2000-10-30
WO 99/58521 PCT/17S99/10185
-86-
232 °C: MS (EI): [M+], 351 (100%, MI); Anal. Calc. for C23Hi3NOS: C,
78.61, H,
3.73, N, 3.99. Found: C, 78.27, H, 3.59, N, 3.89.
Example 34.
4-(Benzojblnaphthof2.3-d]thiophen-11-yl-phenol) acetate ester
Acetic anhydride (0.62 mL, 6.57 mmol) was added to a 0°C, stirred
solution
of 4-benzo[b]naphtho[2,3-d]thiophen-11-yl-phenol (2.0 g, 6.13 mmol) in
pyridine (8
mL). After 7 h the reaction mixture was added to water and the resulting solid
was
filtered and washed with water and dried in vacuo to provide the title
compound as a
white solid (2.23 g, 99%): mp: 160-161°C; NMR (CDC13); 8 8.36 (s, 1H),
7.95 (d, J =
8 Hz, 1H), 7.78 (d, J = 8 Hz, 1H), 7.61 (d, J = 8 Hz, 1H), 7.53 (ddd, J = 8,
7, 1 Hz,
1H), 7.47-7.33 (m, 6H), 7.08 (ddd, J = 8, 7, 1 Hz, 1H), 6.76 (d, J = 8 Hz,
1H), 2.42 (s,
3H, CH3); MS (EI): 368 (100%, MI); Anal. Calc. for C24H16O2S: C, 78.24, H,
4.38,
N, 0.00. Found: C, 77.99, H, 4.29, N, 0.02.
Example 35.
Acetic acid 3-Benzofb]naphthof2,3-dlthiophen-11-~)-phenyl ester
Prepared from 3-benzo[b]naphtho[2,3-d]thiophen-11-yl}-phenol (Example 17)
according to the procedure of Example 34. White solid: mp 122-124°C: MS
(EI):
[M+], 368; Anal. Calc. for C24H16O2S: C, 78.24, H, 4.38, N, 0.00. Found: C,
77.47,
H, 4.19, N, 0.27.
Example 36.
Acetic acid 2-Acetoxy~benzojb]naphthoj2,3-d]thiophen-11-vl)-nhenfester
Prepared from 4-(benzo[b]naphtho[2,3-d]thiophen-11-yl)-benzene-1,2-diol
(Example 18) according to the procedure of Example 34. White solid: mp 179-
180°C:
MS (EI): [M+], 426; Anal. Calc. for C26H18O4S: C, 73.22, H, 4.25, N, 0.00.
Found:
C, 73.17, H, 4.30, N, 0.12.
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
_87_
Example 37.
4~- 6-Bromo-benzoLlnaphthof2.3-dlthiophen-11-yl-phenol( acetate (ester)
A solution of bromine ( 0.35 mL, 6.63 mmol) in dichloromethane ( 10 mL)
was added dropwise over a 15 min. period to a stirred, -20°C solution
of 4
(benzo[b]naphtho[2,3-d]thiophen-11-yl-phenol) acetate (ester) (2.22 g, 6.03
mmol) in
dichloromethane (45 mL,). This solution was stirred for 1.5 h then quenched
with
dilute aqueous sodium thiosulfate. The organic solvents were removed, water
was
added and the resulting solid was filtered, washed with water, triturated with
pet.
ether and dried in vacuo to provide the title compound as a white solid (2.60,
96%):
mp: 204-205°C; NMR (CDC13); 8 8.35 (ddd, J = 8, l, 1 Hz, 1H), 7.81
(ddd, J = 8, 1, 1
Hz, 1H), 7.68-7.61 (m, 2H), 7.47-7.36 (m, 6H), 7.08 (ddd, J = 8, 8, 1 Hz, 1H),
6.69
(ddd, J = 8,1,1 Hz, 1H), 2.42 (s, 3H, CH3); MS (EI): [M+], 1 bromine isotope
pattern,
446 (60%, MI), 448 ( 65%, MI), 404 (100%), 406 (95%); Anal. Calc. for
C24H15Br02S: C, 64.44, H, 3.38, N, 0.00. Found: C, 64.18, H, 3.34, N, -0.03.
Example 38.
Acetic acid 3-(6-Bromo-benzo[blnaphthof2,3-d]thiophen-11-yll-phenyl ester
Prepared from acetic acid 3-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenyl
ester (Example 35) according to the procedure of Example 37. White solid: mp
74
76°C: MS (EI): [M+], 1 bromine isotope pattern, 446, 448; Anal. Calc.
for
C24H15Br02S: C, 64.44, H, 3.38, N, 0.00. Found: C, 63.77, H, 3.08, N, 0.12.
Example 39.
Acetic acid 2-Acetox~~4-(6-bromo-benzofblnaphtho f2.3-d]'thiophen-11-
ylLphen~rl
ester
Prepared from acetic acid 2-acetoxy-4-(benzo[b]naphtho[2,3-d]thiophen-11-
yl)-phenyl ester (Example 36) according to the procedure of Example 37. White
solid:
mp 178-179°C: MS (EI): [M+], 1 bromine isotope pattern, 504, 506; Anal.
Calc. for
C26H17Br04S: C, 61.79, H, 3.39, N, 0.00. Found: C, 61.37, H, 3.32, N, 0.11.
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
_88_
Example 40.
4-(6-Bromo-benzof bl naphtho f 2.3-d] thiophen-11-~)-2.6-diisopro~yl_-phenol
A solution of bromine (0.185 mL, 3.50 mmol) in dichloromethane (7 mL) was
added dropwise over a 40 minute period to a solution of 4-(6-
benzo[b]naphtho[2,3
d]thiophen-11-yl)-2,6-diisopropyl-phenol (1.31 g, 3.18 mmol) in
dichloromethane (26
mL) at -20°C under a dry nitrogen atmosphere that was stirred in the
absence of light.
After 15 minutes, a dilute aqueous sodium bisulfite solution was added and the
reaction mixture was partitioned between water and ether. The ether phase was
washed with brine and concentrated to provide the title compound as a white
solid
(1.65 g, 100%): mp: 189-190°C; NMR (CDC13); b 8.35 (ddd, J = 8, 1, 1,
1H), 7.80
(ddd, J = 8, 1, 1 Hz, 1H), 7.74 (ddd, J = 8, 1, 1 Hz, 1H), 7.65 (ddd, J = 8,
8, 1, 1H),
7.45 (ddd, J = 8, 8, 1 Hz, 1H), 7.37 (ddd, J = 8, 8, 1 Hz, 1H), 7.08 (s, 2H),
7.06 (ddd, J
= 8, 8, 1 Hz, 1H), 6.68 (ddd, J = 8, l, 1 Hz, 1H), 5.03 (s, 1H), 3.31
(septuplet, J = 7
Hz, 2H), 1.29 (d, J = 7 Hz, 6H), 1.26 (d, J = 7 Hz, 6H); MS (EI): 488 (90%),
490
(100); Anal. Calc. for C28H25BrOS: C, 68.71, H, 5.15, N, 0.00. Found: C,
67.74, H,
5.02, N, 0.07.
Example 41.
4-~6-Bromo-benzofblnaphthof2.3-dlthiophen-11-5r1)-phenol
Aqueous potassium hydroxide (6.0 mL, 6.0 mmol) was added to a stirred,
room temperature suspension of 4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl-
phenol) acetate (ester) (2.60 g, 5.81 mmol) in THF (20 mL) / methanol ( 15
mL).
Dissolution occurred immediately and the reaction mixture turned green. After
1 h, the
organic solvents were removed, water was added, the reaction mixture was
acidified
with 10% HCl and the resulting solid was washed with water and triturated with
pet.
ether and then dried in vacuo to provide the title compound as a white solid (
2.18 g,
93%). A portion of this solid (0.5 g) was recrystalized from acetic acid
/water and
then cyclohexane / acetonitrile: mp: 211-213°C; NMR (CDC13); 8 8.34
(ddd, J = 8, 1,
1 Hz, 1H), 7.80 (ddd, J = 8, 1, 1 Hz, 1H), 7.67-7.62 (m, 2H), 7.43 (ddd, J =
8, 8, 1 Hz,
1H), 7.39 (ddd, J = 8, 8, 1 Hz, 1H), 7.27 (d, J = 9 Hz, 2H), 7.12 (d, J = 9
Hz, 2H), 7.11
CA 02330623 2000-10-30
WO 99/58521 PCT/(JS99/10185
-89-
(m, 1H), 6.78 (ddd, J = 8, 1, 1 Hz, 1H) 4.99 (s, 1H, OH); MS (EI): [M+], 1
bromine
isotope pattern, 404 ( 100%, MI), 406 (96%, MI); Anal. Calc. for C22H l3BrOS:
C,
65.19, H, 3.23, N, 0.00. Found: C, 64.87, H, 3.00, N, 0.03.
Example 42.
3-(6-Bromo-benzoLblnaphtho[2.3-dlthiophen-11-yl)-phenol
Prepared from acetic acid 3-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-
phenyl ester (Example 38) according to the procedure for Example 41. White
solid:
mp 110-111°C: NMR (CDCl3); 8 8.35 (dd, J = 8, 1 Hz, 1H), 7.81 (d, J =
8, Hz, 1H),
7.67-7.63 (m, 2H), 7.53 (dd, J = 8, 7 Hz, 1H), 7.44 (ddd, J = 8, 7, 1 Hz, 1H},
7.39
(ddd, J = 8, 7, 1 Hz, 1H), 7.13-7.09 (m, 2H), 7.00 (dd, J = 8, 1 Hz, 1H), 6.88
(dd, J =
1, 1 Hz, 1H), 6.78 ( dd, J =8, 1 Hz, 1H), 4.99 (s, 1H, OH); MS (EI): [M+], 1
bromine
isotope pattern, 404 (95%), 406 ( 100%); Anal. Calc. for C22H l3BrOS: C,
65.19, H,
3.23, N, 0.00. Found: C, 64.85, H, 3.51, N, 0.43.
Example 43.
4-l6-bromo-benzo lbl naphtho [2.3-dlthiophen-11-yl)-benzene-1.2-diol
Prepared from acetic acid 2-acetoxy-4-(6-bromo-benzo[b]naphtho[2,3-
d]thiophen-11-yl)-phenyl ester (Example 39) according to the procedure for
Example
41. White solid: mp 181-182°C: MS (EI): [M+], 1 bromine isotope
pattern, 420
(95%); Anal. Calc. for C22H13Br02S: C, 62.72, H, 3.11, N, 0.00. Found: C,
62.11,
H, 3.10, N, 0.13.
Example 44.
11-(4-Hydroxy-phen~)-benzo f blnaphtho [2.3-d)thio~hene-6-carbonitrile
A suspension of 4-(6-bromo-benzo[b)naphtho[2,3-d]thiophen-11-yl-phenol)
acetate (ester) (2.0 g, 4.47 mmol), copper (I) cyanide (2.0 g, 22.3 mmol) and
N-
methylpyrrolidinone ( 10 mL) was heated in a sealed vessel in a 150°C
oil bath for 9
h. The reaction mixture was cooled to room temperature, added to water and
extracted
with ethyl acetate. The aqueous phase was filtered and the resulting solid was
taken
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-90-
up in THF. The THF and ethyl acetate phases were combined and concentrated.
THF
( 100 mL), methanol (50 mL) and aqueous potassium hydroxide ( 1.0 N, 4.5 mL,
4.5
mmol) were added to the residue. After 5 minutes, water was added and the
reaction
mixture was acidified with 10% HCl and the resulting solid was filtered,
washed with
water and tritrurated with ether (3X) and then pet. ether. The solid was dried
in vacuo
to provide the title compound as a tan solid (1.27 g, 82%): mp: 307-
309°C; NMR
(CDCl3); 8 8.35 (ddd, J = 8, l, 1 Hz, 1H), 7.84 (ddd, J = 8, 1, 1 Hz, 1H),
7.76-7.69
(m, 2H), 7.51 (ddd, J = 8, 8, 1 Hz, 1H), 7.44 (ddd, J = 8, 8, 1 Hz, 1H), 7.27
(d, J = 9
Hz, 2H), 7.15 (d, J = 9 Hz, 2H), 7.14 (m, 1H), 6.82 (ddd, J = 8, 1, 1 Hz, 1H),
5.08 (s,
1H); IR (KBr, cm-1): 2210 (CN); MS (EI): [M+], 451(40%, MI); Anal. Cak. for
C23H13NOS: C, 78.61, H, 3.73, N, 3.99. Found: C, 77.84, H, 3.46 , N, 3.89.
Example 45.
Methanesulfonic acid 4-Benzo[b]naphthof2.3-dlthiophen-11-~=phenyl Ester
Methylsulfonyl chloride (0.63 mL, 8.14 mmol) was added dropwise to a cold
(ice bath) solution of 4-benzo[b]naphtho[2,3-d]thiophen-11-yl-phenol (2.00 g,
5.43
mmol) in dry methylene chloride (10 mL) and pyridine (2.08 mL). After stiring
at
ambient temperature for ca. 36 h. the reaction mixture was combined with water
( 100
mL). The organics were extracted with ether ( 100 mL), washed with 10% HCl (
100
mL) and concentrated to give the title compound as a white solid (2.51 g,
100%); mp
136-139°C; NMR (CDC13); 8 8.38 (s, 1H), 7.97 - 7.95 (m, 1H), 7.80 -
7.78 (m, 1H),
7.60 - 7.50 (m, 6H), 7.43 - 7.35 (m, 2H), 7.07 (ddd, J = 8, 7, 1 Hz, 1 H),
6.68 (d, J = 8
Hz, 1H), 3.33 (s, 3H); MS (EI): [M+] 404(100%); Anal. calc. for C23H16O3S2 +
0.07 C6H14, C, 68.52, H, 4.17, N, 0.03. Found: C, 67.91, H, 3.85, N, 0.06.
Example 46.
Methanesulfonic acid 4-l6-Chloro-benzofblnaphtho~2 3-dlthiophen-11-yl)-phenyl
ester
To a solution of methanesulfonic acid 4-benzo[b]naphtho[2,3-d]thiophen-11-
yl-phenyl ester ( 1.00 g, 2.47 mmol) in chloroform ( 10 mL) was added sulfuryl
chloride (0.21 mL, 2.60 mmol, 1.05 eq) dropwise at room temperature under a
dry
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-91 -
nitrogen atmosphere. After stirring 19 hours the reaction mixture was added to
water
(100 mL) and the organics were extracted with ether (2x100 mL). The extracts
were
combined, washed with brine, combined with silica gel and the solvent was
removed.
The adsorbate was flash chromatrographed ($5/15 petroleum ether/ethyl acetate)
to
give the title compound as a white solid (0.957 g, 88%): mp 155-158°C;
NMR
(CDCl3 ); 8 8.41 (ddd, J=8,1,1 Hz, 1 H), 7.82 {ddd, J=8, I ,1 Hz, 1 H), 7.67
(ddd,
J=8,7,1 Hz, 1H), 7.61-7.56 (m, 3H), 7.51-7.45 (m, 3H), 7.42-7.38 (ddd, J=8,7,1
Hz,
1H), 7.10 (ddd, J=8,7,1 Hz, 1H), 6.64 (d, J=8 Hz, 1H), 3.34 (s, 3H); MS {+EI)
: [M+],
1 chlorine isotope pattern, 438 (100%) , 440 (40%); Anal. Calc. for
C23H15C1O3S2:
C, 62.93, H, 3.49, N, 0.00. Found: C, 62.72, H, 3.25, N, 0.03.
Example 47.
Methanesulfonic acid 4-l6-Iodo-benzolb]naphthoj2 3-dlthionhen-11-,~~1)-phenXl
ester
To a solution of methanesulfonic acid 4-benzo[b]naphtho[2,3-d]thiophen-11-
yl-phenyl ester (2.24 g, 5.54 mmole) in tetrahydrofuran (22.4 mL), 80% aqueous
acetic acid ( 11.2 mL) and sulfuric acid (0.6 mL) was added iodine (0.984 g,
3.87
mmol) and iodic acid (0.244 g, 1.39 mmol). The reaction mixture was stirred
for 88 h
at room temperature then combined with an aqueous solution of sodium bisulfate
( 100
mL). The organics were extracted with ether (500 mL). The extracts were
concentrated and chased with benzene and pet ether to provide the title
compound as
a yellow solid (2.75 g, 94%): mp 176-186°C; NMR (CDCl3); S 8.24 (ddd, J
= 8, 1, I
Hz, 1H), 7.81 (ddd, J = 8, 1, 1 Hz, IH), 7.65 - 7.58 (m, 3H), 7.52 - 7.38 (m,
5H), 7.11
- 7.07 (ddd, J = 8, 7, I Hz, 1H), 6.57 (ddd, J = 8, 1, 1 Hz, 1H), 3.33 (s,
3H); MS
(+FAB): [M+] m/z 530 (65%), [M + H]+ an/z 531 (25%); Anal. calc. for
C23H 15I03S2: C, 52.08, H, 2.85, N, 0.00. Found: C, 51.75, H, 2.75, N, 0.06.
CA 02330623 2000-10-30
WO 99/58521 PCT/t1S99/l0185
-92-
Example 48.
Methanesulfonic acid 4-(6-trifluoromethyl-benzofblnaphthof2.3-dlthiophen-11
~11-
phenhr ester
A mixture of freshly activated copper (0.359 g, 5.66 mmol) and bis
(trifluoromethyl)mercury (0.993g, 3.78 mmol) in N,N-dimethylacetamide ( 12 mL)
was heated at 144°C under a dry nitrogen atmosphere for 2 hours with
stirring. After
cooling a solution of methanesulfonic acid 4-(6-iodo-benzo[b]naphtho[2,3-d]-
thiophen-11-yl)-phenyl ester (I.OOg, 1.89 mmol) in N,N-dimethylacetamide (12
mL)
was added and the mixture was heated at 160-168°C for 3 hours 20 min.
After cooling
the mixture was poured into water and the organics were extracted with ether
(2 x 200
mL). The extracts were combined, silica gel (ca. 30 mL) was added and the
solvent
was removed. The adsorbate was flash chromatographed (eluant: 80 : 20 pet
ether
ethyl acetate) to provide the title compound as a white solid (0.697 g, 85%):
mp 215 -
216°C; NMR (CDCl3); b 8.39 - 8.36 (m, 1H), 7.81 - 7.79 (m, 1H), 7.71 -
7.67 (m,
1H), 7.61 - 7.59 (m, 3H), 7.51 - 7.46 (m, 3H), 7.41 (ddd, J = 8, 7, 1 Hz, 1H),
7.08
(ddd, J = 8, 7, 1 Hz), 6.54 (d, J = 8 Hz, 1H), 3.35 (s, 3H); MS +FAB [M+H]+
m/z 473
and m+ 472; Anal. Calc. for C24H15F303S2~0.15C6H6: C, 61.76, H, 3.31, N, 0.00.
Found: C, 61.20, H, 3.11, N, 0.16.
Example 49.
Methanesulfonic acid 4-(6-methyl-benzofb]naphtho[2 3-dlthiophen-11-yll-phen
ly~ester
A suspension of methanesulfonic acid 4-(6-iodo-benzo[b]naphtho[2,3-
d]thiophen-11-yl)-phenyl ester (1.65g, 3.11 mmole), tetramethyltin (3.58, 25.8
mmole), and bis(triphenylphosphine)palladium II chloride (0.218g, 0.311 mmole
10
mole %) in dry N,N dimethylformamide ( l6mL} was heated in a sealed vessel
under
argon at 103°C for 4 hours and left at room temperature overnight. The
reaction
mixture was added to water '(200 mL) and extracted with ether. Silica gel (
40mL)
was added and the solvent removed. The adsorbate was flash chromatographed (85
15 Petroleum ether : ethyl acetate) to give the title compound as a pale
yellow solid
(1.12 g, 86%): mp 172-173°C; NMR (CDCl3); S 8.18 (ddd, J = 8, 1, lHz,
1H), 7.81
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 93 -
(ddd, J = 1, 1, 8 Hz, 1H), 7.62-7.55 (m, 3H), 7.50 - 7.48 (m, 2H), 7.44 -
7.34(m, 2H),
7.06 (ddd, J = 8, 7, lHz, 1H), 6.64 (ddd, J = 8,1, 1Hz), 3.32(s, 3H), 2.99 (s,
3H); MS:
EI [m/z] 418( 100%); Anal. Calcd. for C24H 18O3S2: C, 68.87, H, 4.34, N, 0.00.
Found: C, 68.60, H, 4.26, N, 0.02.
Example 50.
4-(6-Chloro-benzo [b] naphtho f 2.3-dl thiophen-11-~l-phenol
A biphasic mixture of methanesulfonic acid 4-(6-chloro-benzo[b]naphtho[2,3
d]thiophen-11-yl)-phenyl ester (0.917 g, 2.09 mmol) in dioxane (13 mL) and a
solution of sodium hydroxide (2.5 N, 6.7 mL, 16.7 mmol, 8 eq) was heated at
reflux
overnight. After cooling to room temperature the reaction mixture was combined
with water (50 mL,), acidified with concentrated hydrochloric acid, and
stirred for 15
minutes. The crude white solid product was collected by filtration,
redissolved in
ether, combined with silica gel, and the solvent was removed. The adsorbate
was flash
chromatographed (gradient 85/15-80/20 petroleum ether/ethyl acetate) to give
the title
compound as a white solid (0.705 g, 94%); mp 193-195°C; NMR (CDCl3); 8
8.37
(ddd, J = 8, 1, Hz, 1H), 7.81 (ddd, J = 8, 1, 1 Hz, 1H), 7.68-7.b3 (m, 2H),
7.44 (ddd, J
= 8, 7, 1 Hz, 1H), 7.39 (ddd, J = 8, 7, 1 Hz, 1H), 7.29-7.26 (m, 2H,), 7.14-
7.09 (m,
3H}, 6.81 (ddd, J = 8, 1, 1 Hz, 1H), 5.01 (d, J = 4 Hz, 1H); MS (+EI): [M+], 1
chlorine isotope pattern, 360 (100%), 362 (45%); Anal. calc. for C22H13C10S:
C,
73.23, H, 3.63, N, 0.00. Found: C, 72.86, H, 3.24, N, 0.04.
Example 51.
4-(6-Iodo-benzo_Iblnaphtho[2.3-dlthiophen-11-yl)-phenol
To a solution of methanesulfonic acid 4-(6-iodo-benzo[b]naphtho[2,3-
d]thiophen-11-yl)-phenyl ester ( 1.00 g, 1.89 mmol) in tetrahydrofuran (5 mL)
was
added a 2.5 N aqueous solution of sodium hydroxide (6.0 rnL) and the biphasic
reaction mixture was heated at reflux for 5 hours and then heated in a sealed
pressure
tube at 110C for about 18 hours. The reaction mixture was diluted with water
(100
mL), acidified with 10% hydrochloric acid and the organics were extracted with
ether
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-94-
(2x 100 mL). The extracts were combined, washed with water ( 100 mL),
concentrated
and chased with petroleum ether to give the title compound (0.869 g, over
theoretical); NMR (DMSO-d6); 8 9.85 (s, 1H, OH), 8.15 (d, J = 8 Hz, 1H), 8.02
(d, J
= 8 Hz, 1H), 7.73 (ddd, J = 8,7,1 Hz, 1H), 7.69-7.44 (m, 3H), 7.21-7.16 (m,
3H),
7.09-7.06 (m, 2H), 6.68 (d, J = 8Hz, 1H).
Example 52.
4-(6-Trifluoromethyl-benzof blnanhtho T2.3-dl thiophen-11-phenol
Prepared from methanesulfonic acid 4-(6-trifluoromethyl-benzo[bJnaphtho
[2,3-dJthiophen-11-yl)-phenyl ester (Example 48) according to the procedure of
Example 51. White solid: mp 210 -211°C; NMR ( CDC13); 8 8.35 (m,
1H), 7.80
7.77 (m, 1H), 7.72 - 7,64 (m, 2H), 7.47 (ddd, J = 8, 7, 1 Hz, 1H), 7.39 (ddd,
J = 8, 7,
lHz, 1H), 7.28 - 7.24 (m, 2H), 7.16 - 7.08 (m, 3H), 6.75 (d, J = 8 Hz, 1H),
5.03 (s,
1H); MS: (+} EI (direct probe) [M+): 394 (100%); Anal. Calc. for: C23H13F30S:
C,
70.04, H, 3.32, N, 0.00. Found: C, 69.68, 3.12, N, 0.10.
Example 53.
~6-Methyl-benzofblnaphthof2.3-dlthiophen-11-yl)-phenol
Prepared from methanesulfonic acid 4-(6-methyl-benzo[bJnaphtho[2,3-dJthio-
phen-11-yl)-phenyl ester (Example 49) according to the procedure of Example
S1.
White solid: mp 184-185°C; NMR (CDCI3); b 8.16 (ddd, J = 8,1,1Hz, 1H),
7.81 (ddd,
J =8, 1, lHz, 1H), 7.67 (ddd, J =8, 1, lHz, 1H), ?.58 (ddd, J = 8, 7, lHz,
1H), 7.42-
7.34 (m, 2H), 7.29 - 7.26 (m, 3H), 7.12-7.06 (m, 3H), 6.82 (ddd, J = 8, 1, 1
Hz, 1H),
4.94(s, IH), 2.98 (s, 3H}; MS(EI): [M+], 340(100%); Anal. Calcd. for C23H160S:
C,
81.14, H, 4.74, N, 0.00, Found C, 81.47, H, 4.59, N, 0.02.
Example 54.
4-(6-Methoxy-benzof blnaphthof 2.3-dlthiophen-11-~~phenol
To cold (ice bath) anhydrous methanol ( 13.2 mL) was added sodium hydride
(80% by weight suspension in mineral oil, 1.70 g, 56.6 mmol) in three
portions. After
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
-9S-
stirring in the cold for 0.5 hours and at ambient temperature for 50 minutes
copper II
chloride (0.251 g, 1.87 mmol) and solution of methanesulfonic acid 4-(6-iodo-
benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenyl ester (3.00 g, 5.66 mmol) in dry
N,N-
dimethylformamide (24 mL) were added. The reaction mixture was heated at
reflux
for approximately 3 hours, cooled to room temperature, diluted with water (400
mL),
acidified with 10% hydrochloric acid and extracted with diethyl ether. The
extracts
were combined, silica gel was added and the solvent removed. The adsorbate was
flash chromatographed (40/60 petroleum ether/methylene chloride) to provide
the title
compound as a white solid (1.82 g, 90%): mp 218-223°C (dec); NMR
(CDCl3); b
8.27 (ddd,J = 8, 1, 1 Hz, 1H), 7.80 (ddd, J = 8, 1, 1 Hz, 1H), 7.65 (ddd, J =
8, 1, 1 Hz,
1 H), 7.57 (ddd, J = 8, 7, 1 Hz, 1 H), 7.40 (ddd, J = 8, 7, 1 Hz, 1H), 7.36
(ddd, J = 8, 7,
1 Hz, 1H), 7.30-7.26 (m, 2H), 7.13-7.07 (m, 3H), 6.85 (ddd, J = 8, 1, 1 Hz,
1H), 4.98
(s, 1H), 4.21 (S, 3H); MS (EI): [M+], 356 (100%); Anal. Calc. for C23H16O2S:
C,
77.50, H, 4.52, N, 0.00; Found C, 76.79, H, 4.61, N, 0.11.
Example 55.
4-(6-Phenylsufanyl-benzo[blnaphthof2.3-dlthiophen-11-yl)-phenol
Sodium hydroxide (0.183 g, 4.58 mmol) was added to a stirred, room
temperature solution of methanesulfonic acid 4-(6-iodo-benzo[b]naphtho[2,3-d]-
thiophen-11-yl)-phenyl ester (1.04 g, 1.95 mmol), thiophenol (0.47 mL, 4.578
mmol),
copper (I) oxide (0.326 g, 2.28 mmol) and dimethylformamide (20 mL). The
vessel
was heated in a 155-160°C oil bath under an argon atmosphere. After 7h,
the reaction
mixture was cooled to room temperature, added to water, acidified with 10% HCl
and
extracted with ether. The ether extract was filtered to remove copper salts
and silica
gel was added. The solvent was removed and the adsorbate was flash
chromatographed (eluent: gradient: 9:1 to 85:15 petroleum ether: ethyl
acetate) to
provide the title compound as a white solid (0.721 g, 86%): mp 162-
164°C; NMR
(DMSO-d6); 8 9.87 (s, 1H), 8.48 (ddd, J = 8, 1, 1 Hz, 1H), 7.95 (ddd, J = 8,
l, 1 Hz,
1H), 7.66 (m, 2H), 7.52 (ddd, J = 8, 7, 1 Hz, 1H), 7.44 (ddd, J = 8, 7, 1 Hz,
1H), 7.27-
CA 02330623 2000-10-30
WO 99/58521 PCT/t1S99/10185
-96-
7.07 (m, lOH), 6.76 (d, J = 8 Hz, 1H); MS (EI): 434 (M+, 100%); Anal. Calc.
for
C28H180S: C, 77.39, H, 4.19, N, 0.00. Found: C, 76.82, H, 3.95, N, 0.16.
Example 56.
4 j6-(2-Dimethylamino-ethylsulfan~)-benzo[b]naphthof2.3-dithiophen-11-vl]-
phenol
To a suspension of methanesulfonic acid 4-(6-iodo-benzo[b]naphtho[2,3-
d]thiophen-11-yl)-phenyl ester (1.00 g, 1.89 mmol), dimethylaminoethanethiol
hydrochloride (0.614 g, 4.34 mmol, 2.3 eq) and copper I oxide (0.316 g, 8.69
mmol,
1.17 eq) in anhydrous N,N-dimethylformamide (24 mL) was added finely ground
sodium hydroxide (0.348 g, 8.69 mmol, 4.6 eq) at room temperature in a
pressure
vessel. The vessel was flushed with argon, sealed and heated with stirring at
155°C
(oil bath) for 6 hours and at ambient temperature for 12 hours. The reaction
mixture
was poured into water ( 100 mL) and extracted with ether. The solid which
remained
as a suspension in the aqueous phase was filtered. The filtrate was extracted
once
more with ether. The extracts were combined and silica gel was added. The
solvents
were removed and the adsorbate was flash chromatographed (gradient 94/6 - 95/
S)
methylene chloride: isopropanol to give the title compound as a solid (0.707
g, 87%):
NMR ( DMSO-d6); S 9.83 (s, 1H, OH), 8.65 (d, J = 8 Hz, 1H), 7.98 (d, J = 8 Hz,
1H),
7.71 (ddd, J = 8, 7, 1 Hz, 1H), 7.61 (d, J = 8 Hz, 1H), 7.50 (ddd, J = 8, 7, 1
Hz, 1H),
7.43 (ddd, J = 8, 7, 1 Hz, 1H), 7.21-7.05 (m, 3H), 7.08-7.04 (m, 2H), 6.73 (d,
J = 8
Hz, 1H), 3.35-3.27 (m,), 3.10 (t, J = 6 Hz, 2H), 2.21-2.08 (broad singlet,
6H); MS(EI):
[ M+] 429.
Example 57.
4-~Pyridin-4-, ls~lfanXl_)-benzoLlnaphthof2.3-d]thiophen-11-vll-phenol
To a suspension of methanesulfonic acid 4-(6-iodo-benzo[b]naphtho[2,3-
d]thiophen-11-yl)-phenyl ester (1.00 g, 1.89 mmol), 4 mercaptopyridine (0.614
g,
4.34 mmol, 2.3 eq) and copper I oxide (0.316 g, 2.21 mmol, 1.17 eq) in dry N,N-
dimethyformamide (24 mL) was added finely ground sodium hydroxide {0.174 g,
4.34 mmol, 2.3 eq) and the mixture was heated in a pressure bottle at
157°C (oil bath)
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-97-
under argon for 5 hours and then stirred at ambient temperature overnight. The
reaction mixture was poured into water ( 100 mL) and the organics were
extracted
with ether (2.300 mL). The extracts were combined, dried with brine, and
silica gel
was added. The solvent was removed and the adsorbate was flash chromatographed
(97/3 methylene chloride/isopropanol) to give a solid which was stirred in
water ( 100
mL) overnight and collected on a sintered glass funnel and air dried to give
the title
compound as a solid (0.587 g, 73%); NMR (DMSO-d6); 8 9.90 (s, 1H), 8.37 (d, J
= 8
Hz, 1H), 8.30 (d, J = 5 Hz, 2H), 7.95 (d, J = 8 Hz, 1H), 7.70 (m, 2H), 7.57
(m, 1H),
7.45 (t, J = 8 Hz, 1H), 7.28 (d, J = Hz, 2H), 7.20 (t, J = 8 Hz, 1H), 7.11 (d,
J = 8 Hz,
2H), 6.96 (d, J = 6 Hz, 2H), 6.77 (d, J = 8 Hz, 1H); MS (EI): [M+] 435.
Example 58.
11-l3 5-Dibromo-4-h~y_phenyl)-benzofblnaphthof2.3-d~thiophene-6-carbonitrile
A solution of bromine (0.21 mL, 4.07 mmol) in acetic acid (3 mL) was added
dropwise to a room temperature, stirred suspension of 11-(4-hydroxy-phenyl)-
benzo[b]naphtho[2,3-d]thiophene-6-carbonitrile (650 mg, 1.85 mmol), potassium
acetate ( 1.82 g, 18.5 mmol) and acetic acid ( 17 mL). After 35 minutes, water
( 100
mL) and a small amount of solid sodium sulfite were added. The suspension was
filtered and the solid was washed with water, triturated with pet. ether and
dried in
vacou to provide the title compound as a tan solid (1.04 g, 96%): mp 321-
323°C:
NMR (CDCl3); S 8.36 (ddd, J = 8, l, 1 Hz, 1H), 7.87 (ddd, J = 8, 1, 1 Hz, 1H),
7.76
(ddd, J = 8, 7, 1 Hz, 1H), 7.67 (ddd, J = 8, 1, 1 Hz, 1H), 7.56 (ddd, J = 8,
7, 1 Hz, 1H),
7.54 (s, 2H), 7.50 (ddd, J = 8, 7, 1 Hz, 1H), 7.27 (ddd, J = 8, 7, 1 Hz, 1H),
6.90 (ddd, J
= 8, 1, 1 Hz, 1H), 6.24 (s, 1H, OH); MS (EI): [M+], 2 bromine isotope pattern,
507
(55%), 509 ( 100%), 511 (55%); Anal. Calc. for C23H11Br2NOS~0.5 H20: C, 53.31,
H, 2.33, N, 2.70. Found: C, 53.51, H, 2.28, N, 2.70.
The compounds in Examples 59-66 were prepared using the procedure in Example
58
and the appropriate starting material.
CA 02330623 2000-10-30
WO 99/58521 PCT/11S99/10185
-98-
Example 59.
2 6-Dibromo-4-(6-iodo-benzofb]naphthof2.3-d]thiophen-11-phenol
From 4-(6-iodo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenol (Example 5I).
White solid: mp 221-222°C; NMR (CDCl3); a 8.22 (d,J = 8 Hz, 1H), 7.83
(d, J = 8
Hz, 1H), 7.63 (ddd, J = 8,7,1 Hz, 1H), 7.55 (d, J = 8 Hz, 1H), 7.54 (s, 2H),
7.50-7.43
(m, 2H), 7.20 (ddd, J = 8,7,1 Hz, 1H), 6.83 (d, J = 8 Hz, 1H); MS (+FAB):
[M+], 2
bromine isotope pattern, 608 (25%), 609.7 (100%), 612 (35%); Anal. Calc. for
C22H11Br2IOS: C, 43.31, H, 1.82, N, 0.00. Found: C, 42.98, H, 1.93, N, 0.26.
Example 60.
2,6-Dibromo-4-(6-chloro-benzo f b]naphtho (2,3-d]thiophen-11-phenol
From 4-(6-chloro-benzo[b]naphtho(2,3-d]thiophen-11-yl)-phenol (Example
50). White solid: mp 222-223°C; NMR (CDC13); 8 8.39 (ddd, J=8,I,1 Hz,
IH), 7.84
(ddd, J = 8, I, 1 Hz, 1H), 7.67 (ddd, J = 8, 7, 1 Hz, 1H), 7.61 (ddd, J = 8,
1, 1 Hz, 1H),
7.55 (s, 2H,), 7.52-7.43 (m, 2H), 7.23 (ddd, J = 8, 7, 1 Hz, 1H), 6.89 (ddd, J
= 8, 1, 1
Hz, 1H,), 6.19 (s, 1H); MS (EI): [M+], 2 bromine, 1 chlorine isotope pattern,
516
(38%), 518 (100%), 520 (72%), 52I (20%); Anal. Calc. for C22H11Br2C10S: C,
50.95, H, 2.14. Found: C, 51.12, H, 2.20.
Example 61.
2,6-Dibromo-4-(6-trifluoromethyl-benzojbinaphthof2.3-dlthiophen-11-yll-phenol
From 4-(6-trifluoromethyl)-benzo[b]naphtho[2,3-d]thiophen-11-yl-phenol
(Example 52). White solid: mp 223-225°C; NMR (CDCl3); 8 8.37 (ddd, J =
8, l, 1
Hz, 1H), 7.82 (m, 2H), 7.54 - 7.50 (m, 2H), 7.53 (s, 2H), 7.45 (ddd, J = 8, 7,
1 Hz,
1H), 7.20 (ddd, J = 8, 7, 1 Hz, 1H), 6.83 (d, J = 8 Hz, IH), 6.22 (s, 1H, OH);
MS:
(+)EI (direct probe) [M+], 2 bromine isotope pattern, 550 (52%), 552 ( 100%)
554
(58%); Anal. Calc. for C23H11Br2F30S: C, 50.03, H, 2.01, N, 0.00. Found: C,
49.66,
H, 2.07, N, 0.08.
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-99-
Example 62.
2 6-Dibromo-4-(6-methyl-benzofblnaphthof2 3-dLhionhen-l lxl)-phenol
From 4-methyl[b]naphtho[2,3-d]thiophen-11-yl-phenol (Example 53). White
solid: mp 224-226°C: NMR (CDC13); 8 8.17 (ddd, J = 8, 1, 1 Hz, 1H),
7.84 (ddd, J =
8, 1, 1 Hz, 1H), 7.62 - 7.58 (m, 2H), 7.55 (s, 2H), 7.47 - 7.39 (m, 3H), 7.18
(ddd, J =
8, 7, 1 Hz, 1H), 6.89 (ddd, J = 8, 1, 1 Hz, 1H), 6.16 (s, 1H,); MS (-ESI): m/z
495.2
(40%), 497.1 ( 100%) 499.2 (27%); Anal. Calc. for C23H14Br20S: C, 55.45, H,
2.83,
N, 0.00. Found: C, 56.17, H, 2.78, N, 0.13.
Example 63.
2.6-Dibromo-4-(6-methoxybenzo[b]naphthof2.3-dlthiophen-11-yl- hp enol
From 4-(6-methoxy-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenol (Example
54). White solid: mp 233-234°C; NMR (DMSO-d6); 810.35 (broad s, 1H),
8.23 (d, J
= 8 Hz, 1H), 8.03 (d, J = 8 Hz, 1H), 7.69 - 7.64 (m, 1H), 7.64 (s, 2H), ?.54
(d, J = 4
Hz, 2H), 7.48 (ddd, J = 8, 7, 1 Hz, 1H), 7.26 (ddd, J = 8, 7, 1 Hz, 1H), 6.84
(d, J = 8
Hz, 1H), 4.13 (s, 3H); MS (EI): [M+], 2 bromine isotope pattern, 512 (48%),
514
(100%), 516 (54%); Anal. Calc. for C23H14Br2O2S: C, 53.72, H, 2.74, N, 0.00.
Found: C, 53.62, H, 2.55, N, 0.18.
Example 64.
2.6-Dibromo-4,~6-Dhenylsulfanyl-benzolb]naphthof 2.3-d]thiophen-11-yl)-phenol
From 4-(6-phenylsulfanyl-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenol
(Example 55). White solid: mp 152-155°C: NMR (DMSO-d6); b 10.42 (s,lH),
8.49
(d, J = 8 Hz, 1H), 7.99 (d, J = 8, Hz, 1H), 7.75 (s, 2H),7.69 (ddd, J = 8, 7,
1 Hz, 1H),
7.63 (d, J = 8 Hz, 1H), 7.57 (ddd, J = 8, 7, 1 Hz, 1H), 7.48 (ddd, J = 8, 7, 1
Hz, 1H),
7.28 (ddd, J = 8, 7, 1 Hz; 1H), 7.25-7.21 (m, 2H), 7.14 (ddd, J = 8, 7, 1 Hz,
1H), 7.09
(d, J = 8 Hz, 2H), 6.8I (d, J = 8 Hz, 1H), 1.90 (s, 3H, AcOH); MS (EI): [M+],
2
bromine isotope pattern, 590 (50%), 592 ( 100%), 594 (60%); Anal. Calc. for
C28H16Br20S2~ 1.0 CH3C02H~ 0.5 H20: C, 54.48, H, 3.20, N, 0.00. Found: C,
54.56, H, 2.91, N, 0.23.
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 100 -
Example 65.
2.6-Dibromo-4-j6-(pyridin-4- ls~ ulfan~rl)-benzofblnaphthof2.3-dlthiophen-11-
yll-
hp enol
From 4-[6-(pyridin-4-ylsulfanyl)-benzo[bJnaphthoj2,3-d)thiophen-11-ylJ-
phenol (Example 57). Yellow solid : mp 225-270°C (dec); NMR (DMSO-d6);
8 10.48
(s, 1H, -OH), 8.40 (dd, J = 8, 1 Hz, 2H), 8.37 (d, J = 8 Hz, 1H), 8.00 (d, J =
8 Hz, 1H),
7.76 (s, 2H), 7.75-7.68 (m, 2H), 7.62 (ddd, J = 8, 7, 1 Hz, 1 H), 7.50 (ddd, J
= 8, 7, 1
Hz, 1H), 7.31 (ddd, J = 8, 7, 1 Hz, IH), 7.20 (dd, J = 6, 1 Hz, 2H), 6.83 (d,
J = 8 Hz,
1H); Anal. _Calc. for C27H15Br2NOS2: C, 54.65, H, 2.55, N, 2.36. Found: C,
48.94,
H, 2.46, N, 2.22.
Example 66.
2.6-Dibromo-4-f6-(2-dimethylaminoethylsulfan3rl)-benzo[b]naphthof2.3-dl
thiophen-
11-,~~1]-phenol
From 4-[6-(2-dimethylaminoethylsulfanyl)-benzo[b]naphtho[2,3-dJ thiophen-
11-ylJ-phenol (Example 56). Yellow solid : NMR (DMSO-d6); S 8.65 (d, J = 8 Hz,
1H), 8.04 (d, J = 8 Hz, 1H), 7.77 (septet, J = 3 Hz, 1H), 7.65 (s, 2H), 7.60-
7.58 (m,
2H), 7.50 (dd, J = 8,1 Hz, 1H), 7.27(ddd, J = 8,7,1 Hz 1H), 6.86 and 6.63 (s,
isomers,
1H), 6.79 (d, J = 8Hz, 1H), 3.24(d, m, 2H), 2.85(m, 2H); MS (EI): [M+J, 2
bronune
isotope pattern, 585 (3%), 587 (7%), 589 (4%).
Example 67.
2.6-Dichloro-4-(6-bromo-benzolblnanhthof2.3-dlthi_ophen-11-v1)-phenol
Methanol (20 mL) was purged with chlorine gas for 2 min. This solution was
cooled to -78°C and added to a -78°C solution of 4-(6-bromo-
benzo[b]naphtho[2,3-
d)thiophen-11-yl)-phenol (0.609 g, 1.50 mmol) in methanol (lSmL). After 25
min,
the solution was added to a rapidly stirred biphasic mixture of dilute aqueous
sodium
thiiosulfate and ether. The layers were separated and silica gel was added to
the ether
phase. The ether was removed and the adsorbate was flashed (gradient: 9:1 to
1:1
CA 02330623 2000-10-30
WO 99/58521 PCT/LJS99/10185
- 101 -
petroleum ether:ethyl acetate). Silica gel was added to the fractions
containing the
desired product and the solvent was removed. The adsorbate was flashed (9:1
petroleum ether:ethyl acetate). Silica gel was added to the fractions
containing the
desired product and the solvent was removed. The adsorbate was flash
chromatographed (7:3 petroleum ether:dichloromethane) to provide the title
compound as a white solid (0.082 g, 12%): NMR (DMSO-d6); 810.65 (s, 1H), 8.28
(ddd, J = 8, 1, 1 Hz, 1H), 8.07 (ddd, J = 8, 1, 1 Hz, 1H), 7.78 (ddd, J = 9,
5, 2 Hz, 1H),
7.63-7.58 (m, 2H), 7.53 (s, 2H), 7.52 (ddd, J = 8, 8, 1 Hz, 1H), 7.31 (ddd, J
= 8, 7, 1
Hz, 1H), 6.69 (d, J = 8 Hz, 1H): MS (EI): [M+], 1 bromine, 2 chlorine isotope
pattern,
472 (60%), 474 (100%), 476 (50%).
Example 68.
2-Bromo-5-(6-bromo-benzo~]naphtho[2.3-dlthiophen-11-yll-phenol
Prepared from 3-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenol
(Example 42) according to the procedure of Example 58. White solid: mp 111-
112°C:
MS (+FAB): [M+], 482; Anal. Calc. for C22H 12Br20S: C, 54.57, H, 2.50, N,
0.00.
Found: C, 53.72, H, 2.35, N, 0.41.
Example 69.
~- 4-Dibromo-5-l6-bromo-benzofblnaphtho12.3-d]thiophen-11-yl)-phenol
Prepared from 3-(6-bromo-benzo[b]naphtho(2,3-d]thiophen-11-yl)-phenol
(Example 42) according to the procedure of Example 58. White solid: 269-
270°C:
MS (+FAB): [M+], 560, 562, 564, 566; Anal. Calc. for C22H11Br30S: C, 46.93, H,
1.97, N, 0.00. Found: C, 46.43, H, 2.20, N, 0.11.
Example 70.
3-Bromo-5~6-bromo-benzofblnaphtho[2.3-dlthiophen-11-Yl_l-benzene-1.2-diol
Prepared from 4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-benzene-
1,2-diol (Example 43) according to the procedure of Example 58. White solid:
mp
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 102 -
212-213°C: MS (EI): [M+], 2 bromine isotope pattern, 498, 500, 502;
Anal. Calc. for
C22H12Br2O2S: C, 52.83, H, 2.42, N, 0.00. Found: C, 52.08, H, 2.55, N, 0.01.
Example 71.
4-Bromo-~6-bromo-benzo[b~naphthof2.3-dlthiophen-11-yl)-benzene-1.2-diol
Prepared from 4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-benzene-
1,2-diol (Example 43} according to the procedure of Example 58. White solid:
mp
138-140°C: MS (EI): [M+], 2 bromine isotope pattern, 498, 500, 502;
Anal. Calc. for
C22H12Br2O2S: C, 52.83, H, 2.42, N, 0.00. Found: C, 52.17, H, 2.71, N, 0.05.
Example 72.
I11-(4-Hydrox~phenyl)-benzofblnaphthof2.3-dlthionhen-3-vloxy]-acetic acid
methyl_
ester
11-(4-Hydroxy-phenyl)-benzo[b]naphtho[2,3-d]thiophen-3-of ( I .36g, 3.96
mmol), methyl bromoacetate (0.38 mL, 4.01 mmol), potassium carbonate (0.548 g,
3.97 mmol) and N,N-dimethylformamide (21 mL) were combined and stirred at
ambient temperatures for 2.Sh. The reaction mixture was added to water and
extracted
with ethyl acetate and THF. Silica gel was added to the organic phase and the
solvent
was removed. The adsorbate was flash chromatographed (eluent: gradient
dichloromethane to 98:2 dichloromethane: acetonitrile) to provide the title
compound
as a white solid (0.953 g, 58%): mp: 229-231°C: NMR (DMSO-d6); 8 9.78
(s, 1H),
8.51 (s, 1H), 8.00 (d, J = 8 Hz, 1H), 7.56-7.39 (m, 4H), 7.18 (d, J = 9 Hz,
2H), 7.05
(d, J = 9 Hz, 2H), 6.79 (dd, J = 9, 2 Hz, 1H), 6.66 (d, J = 9 Hz, 1H), 4.86
(s, 2H), 3.68
(s, 3H); MS (EI): 414 (100%, M+); Anal. Calc. for C25H18O4S: C, 72.45, H,
4.38,
N, 0.00. Found: C, 71.78, H, 4.41, N, 0.13.
Example 73.
I~4-Methoxycarbonylmethoxy-nhen~)-benzof binaphtho[2y3-d]thiophen-3-3rloxy~-
acetic acid meth3rl ester
11-(4-Hydroxy-phenyl)-benzo[b]naphtho[2,3-d]thiophen-3-of (0.60 g, 1.752
mmol), methyl bromoacetate (0.70 mL, 7.39 mmol), potassium carbonate (1.2 g,
8.76
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 103 -
mmol) and N,N-dirnethylformamide (8 mL) were combined and stirred at ambient
temperatures overnight. The reaction mixture was added to water and filtered.
The
solid was washed with water and dried in vacuo to provide the title compound
as a
white solid ( 0.82 g, 96%): mp: 152-154°C: NMR (CDCl3); 8 8.27 (s, 1H),
7.91 (dt, J
= 8, 1 Hz, 1H), 7.61 (dm, J = 8 Hz, 1H), 7.49 (ddd, J = 8, 7, 1 Hz, 1H), 7.39-
7.26 (m,
3H), 7.18 (d, J = 9 Hz, 2H), 6.66 (dd, J = 1 Hz, 2H), 4.82 (s, 2H), 4.67 (s,
2H), 3.90
(s, 3H), 3.81 (s, 3H); MS (FAB+): 487 (10%, M+H); Anal. Calc. for C28H22O6S:
C,
69.12, H, 4.56, N, 0.00. Found: C, 67.52, H, 4.40, N, 0.07.
Example 74.
X11-~4-Carboxxmethox, -~~nhenyll-benzofblnaphtho[2.3-d)thiophen-3-vloxyl-
acetic acid
1.0 N Aqueous potassium hydroxide (8.0 mL, 8.0 mmol) was added to a
stirred suspension of [11-(4-methoxycarbonylmethoxy-phenyl)-
benzo[b]naphtho[2,3
d]thiophen-3-yloxy]-acetic acid methyl ester (0.750 g, 1.54 mmol) in THF (20
mL)
and methanol ( 13 mL). Dissolution occurred. After 3 h at ambient temperature,
the
reaction mixture was diluted with water and extracted with ether ( 100 mL).
The
aqueous phase was acidified with 10% aqueous HCI and filtered. The solid was
washed with water and triturated with pet: ether. The solid was dried in vacuo
at 70°C
to provide a grey solid. This solid was recrystalized from acetic acid to
provide the
title compound as a white solid (0.502, 71%): mp 220-222°C: NMR (DMSO-
d6); 8
12.8 (broad s, 2H), 8.54 (s, 1H), 8.02 {d, J = 8 Hz, 1H), 7.56-7.40 {m, 4H),
7.31 (d, J
= 8 Hz, 2H), 7.23 (d, J = 8 Hz, 2H), 6.73 (dd, J = 9, 1 Hz, 1H), 6.56 (d, J =
9 Hz, 1H),
4.85 (s, 2H), 4.74 (s, 2H), 1.97 (s, 2.49H, 0.83 mol acetic acid); MS (FAB+):
459
(20%, M+H); Anal. Calc. for C26H18O6S~0.83CH3C02H: C, 65.46, H, 4.24, N,
0.00. Found: C, 64.47, H, 4.05, N, 0.03.
Example 75.
f 11-(4-Methoxycarbon3rlmethox~phenyl)-benzofblnaphthof2.3-dlthiophen-8-yloxy]-
acetic acid. methyl ester
To a suspension of 11-(4-hydroxy-phenyl)-benzo[b]naphtho[2,3-d]thiophen-8-
ol (0.600 g, 1.75 mmol) and potassuim carbonate (0.605 g, 4.38 mmol, 2.5 eq)
in
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 104 -
anhydrous N,N-dimethylformamide (3 mL) was added methylbromoacetate (0.50 mL,
5.26 rnmol} dropwise at room temperature. After stirring 26 hours additional
methylbromoacetate (0.166 mL, 1.75 mmol) was added and stirring continued for
64
hours. The solvents were removed and water ( 100 mL) was added. The solid was
dissolved in a mixture of diethyl ether and methylene chloride and combined
with
silica gel. The solvents were removed and the adsorbate was flash
chromatographed
(70/30 petroleum ether/ethyl acetate) to give the title compound as a yellow
solid
(0.300 g, 35%).
Example 76.
f 11-(4-Carboxymethox~nhen,LZbenzofb]~hthof2.3-d,~thiophen-8-3rloxy]-acetic
acid
To a solution of [11-(4-methoxycarbonylmethoxy-phenyl)-benzo[b]naphtho
[2,3-d]thiophen-8-yloxy]-acetic acid, methyl ester (0.268 g, 0.551 mmol} in
tetrahydrofuran (8 mL) and methanol {5 mL) was added an aqueous solution of
potassuim hydroxide (1N, 2.2 mL, 2.2 mmol) dropwise at room temperature. After
stirring 2 hours an addition of potassuim hydroxide (1N, 1 mL, 1 mmol) was
introduced. After stirring another 14 hours the solvents were removed and the
residue
was disolved in water (SO mL) and was acidified with 10% aqueous hydrochloric
acid, and the organics were extracted with diethyl ether. The solvent was
removed and
chased with benzene and petroleum ether and dried at 53°C to give the
title compound
as a white solid (0.18 g, 43%): mp 245-246°C; NMR (DMSO-d6); S 13.08
(broad s,
2H), 8.44 (s, 1H), 7.93 (d, J = 8 Hz, 1H), 7.45 - 7.35 (m, 3H), 7.31 (d, J = 9
Hz, 2H),
7.22 (d, J = 9 Hz, 2H), 7.17 {dd, J = 3,9 Hz, 1H), 7.10 (ddd, J = 9, 9, I Hz,
1H), 6.67
(d, J = 8 Hz, 1H}, 4.84 (d, J = 7 Hz, 4H); MS (EI): [M+] 458; Anal. Calc. for
C26H1806S: C, 68.11, H, 3.96, N, 0.00. Found: C, 66.41, H, 3.95, N, 0.05.
Example 77.
j2.6-Dibromo-4-(6-bromo-benzof blnaphtho[2.3-d]thiophen-11-~phenoxy]-acetic
acid, methyl ester
2,6-Dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenol (1.0
g, 1.78 mmol), methyl bromoacetate (0.35 mL, 3.70 mmol), potassium carbonate
CA 02330623 2000-10-30
WO 99/58521 PCTNS99/10185
- 105 -
(0.50 g, 3.62 mmol) and N,N-dimethylformamide (5 mL) were combined and stirred
at ambient temperatures overnight. The reaction mixture was added to water and
filtered. The solid was washed with water and dried in vacuo to provide the
title
compound as a white solid (1.11 g, 98%): mp 183-184°C: NMR (CDC13); 8
8.36
(ddd, J = 8, 1, 1 Hz, 1H), 7.84 (ddd, J = 8, 1, 1 Hz, 1H), ?.68 (ddd, J = 8,
7, 1 Hz, 1H),
7.62 (s, 2H), 7.57 (ddd, J = 8, 1, 1 Hz, 1H), 7.51 (ddd, J = 8, 7, 1 Hz, 1H),
7.42 (ddd, J
= 8, 7, 1 Hz, 1H), 7.18 (ddd, J = 8, 7, 1 Hz, 1H), 6.72 (ddd, J = 8, 1, 1 Hz,
1H), 4.88
(s, 2H), 3.94 (s, 3H); MS (EI): [M+], 3 bromine isotope pattern, 632 (30%),
634
90%) 636 (100%) 638 (35%); Anal. Calc. for C25H15Br303S: C, 47.27, H, 2.38, N,
0.00. Found: C, 47.17, H, 2.19, N, 0.09.
Example 78.
j2 6 Dibromo-4 (6 bromo-benzofblnaphthof2 3-dlthiophen-11-3rl)-phenoxyl-acetic
acid. tert-butyl ester
To a suspension of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-
11-yl)-phenol ( 1.50 g, 2.66 mmol) in anhydrous DMF ( IOmL) was added
potassium
carbonate (0.496 g, 3.59 mmol) followed by the dropwise addition of tert-butyl
bromo
acetate (0.79 mL, 3.59 mmol) at room temperature under a dry nitrogen
atmosphere.
After stirring for 3 h. the reaction mixture was poured into water (250 mL)
and
filtered. The white solid was washed with water then taken up in methylene
chloride
and silica gel was added. The solvent was removed and the silica adsorbate was
flash
chromatographed (96 : 4 petroleum ether : ethyl acetate) to provide the title
compound as a white solid (1.14 g, 64%): NMR (CDC13); 8 8.36 (d, J = 8 Hz, 1H,
ArH), 7.85 (d, J = 8 Hz, 1H, ArH), 7.67 (ddd, J = 8, 7, 1 Hz, 1H, ArH), 7.60
(s, 2H,
ArH), 7.59-7.36 (m, 3H, ArH), 7.19 (t, J = 7 Hz, 1H, ArH), 6.75 (d, J = 7 Hz,
1H,
ArH), 4.73 (s, 2H, CH2), 1.60 (s, 9H, 3(CH3); MS (EI): [M+], 3 bromine pattern
674
(29%), 676 (85%), 678 (85%), 680 (35%); Anal. Calc. for C28H21Br303S: C,
49.66,
H, 3.12, N, 0.00; found: C, 49.55, H, 2.84, N, 0.06.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 106 -
Example 79.
I2.6-Dibromo-4-(6-bromo-benzo[ ~lnaphtho[2 3-dlthiophen-11-ylZphenox~l-acetic
acid
1.0 N Aqueous potassium hydroxide (2.66 mL, 2.66 mmol) was added to a
stirred suspension of [2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-
11-
yl)-phenoxy]-acetic acid, methyl ester (1.51 g, 2.37 mmol) in THF (13 mL) and
methanol (4 mL). Dissolution occurred. After 2.5 h at ambient temperature, the
reaction mixture was diluted with water and extracted with ether (100 mL). The
aqueous phase was acidified with 10% aqueous HCl and filtered. The solid was
washed with water and triturated with pet. ether. The solid was dried in vacuo
at 70°C
to provide the title compound as a white solid ( 1.34 g, 91 %): mp 259-261
°C: NMR
(DMSO-d6): 8 8.29 (dd, J = 8, 1 Hz, 1H), 8.06 (dd, J = 8, 1 Hz, 1H), 7.82 (s,
2H),
7.79 (ddd, J = 8, 6, 2 Hz, 1H), 7.61 (m, 2H), 7.52 (ddd, J = 8, 7, 1 Hz, 1H),
7.32 (ddd,
J = 8, 7, 1 Hz, 1H), 6.73 (dd, J = 8, 1 Hz, 1H), 4.78 (s, 2H); MS (EI): [M+],
3 bromine
isotope pattern, 618 (30%), 620 ( 90%) 622 ( 100%) 624 (50%}; Anal. Calc. for
C24H13Br303S: C, 46.41, H, 2.11, N, 0.00. Found: C, 46.38, H, 1.99, N, 0.01.
Example 80.
I2 6-Dibromo-4-(6-bromo-benzofblna_phthof2 3-d]thio hp en-11-yl)- henoxX]
acetic
acid, sodium salt
Aqueous sodium hydroxide (1.00 N, 0.315 mL, 0.315 mmol) was added to a
stirred solution of [2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-
yl)-
phenoxy]-acetic acid ( 194 mg, 0.315 mmol) in THF ( 1 mL,) / methanol ( 1 mL).
The
reaction mixture was concentrated, water (2.5 mL) was added and the solid was
filtered ( 101 mg) . The aqeous phase was extracted with ether (25 mL) and the
ether
phase was evaporated to dryness to provide a second solid (77 mg). The solids
were
combined and triturated with toluene and benzene and dried in vacuo to provide
the
tltle compound as a tan solid (152 mg, 76%): mp 315-317°C: NMR (DMSO-
d6);, b
8.28 (d, J = 8.5 Hz, 1 H), 8.07 (d, J = 8 Hz, 1 H), 7.79 (ddd, J = 8, 7, 2 Hz,
1 H), 7.76 (s,
2H), 7.62 (m, 2H), 7.52 (ddd, J = 8, 8, 1 Hz, 1H), 7.33 (ddd, J = 8, 8, 1 Hz,
1H), 6.69
(d, J = 8 Hz, 1H), 4.27 (s, 2H); MS (-FAB}: [M-Na], 3 bromine isotope pattern,
617,
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-107-
619, 621, 622; Anal. Calc. for C24H12Br3O3SNa~0.75 H20: C, 43.90, H, 2.07, N,
0.00. Found: C, 44.09, H, 2.18, N, 0.03.
Example 81.
jj4-Benzofblnaphtho[2 3-dlthiophen-11-~ -2.~yano-phenox~-acetic acid
S-(Benzo[b]naphtho[2,3-d]thiophen-11-yl)-2-hydroxy-isophthalonitrile (0.455
g, 1.21 mmol), methyl bromoacetate (0.351 mL, 3.63 mmol), potassium carbonate
(0.251 g, 1.81 mmol) and N,N-dimethylformamide (5.0 mL) were combined and
stirred at ambient temperature for two days. The reaction mixture was diluted
with
water (60 mL) and acidified with 10% aqueous HCl to pH 1 and aqueous was
extracted with ethyl acetate ( 100 mL). The ethyl acetate extract was washed
with
water, dried with brine and anhydrous MgS04, and concentrated to provide a
white
solid. The solid was dissolved in ethyl acetate (60 mL) and silica gel was
added.
Solvent was removed and the adsorbate was flash chromatographed (eluent 8:2
petroleum ether: ethyl acetate) to provide the methyl ester as a white solid
(0.370 g,
83%): Aqueous potassium carbonate (170 mg in 5 mL of water, 1.23 mmol) was
added to a stirred solution of this methyl ester (0.275 g, 0.613 mmol) in THF
( 10 mL)
at ambient temperature. After 31 h the reaction mixture was diluted with water
( 100
mL} and acidified with 10% aqueous HCl to pH 1 and aqueous was extracted with
ethyl acetate ( 150 mL). The ethyl acetate extract was washed with water,
dried with
brine and anhydrous MgS04, and concentrated to provide the title compound as a
white solid (0.207 g, 78%): mp 134-136°C: NMR (CDC13); S 8.43 (s, 1H),
7.98 (dd J
= 8, 1 Hz, 1H), 7.91 (s, 2H), 7.8 (dd J = 8, 1 Hz, 1H), 7.58 (ddd, J = 8, 7, 1
Hz, 1H),
7.49-7.42 (m, 3H), 7.36 (dd, J = 8, 1 Hz, 1H), 7.18 (ddd, J = 8, 7, 1 Hz, 1H},
6.70 (dd,
3 =8 ,1 Hz, 1H), 4.47 (s, 2H); MS (EI): 434 (100%, MI); High resolution MS
(EI)
Calc. for C26H14N2O3S: High resolution MS (EI) Calc. for C26H14N2O3S:
434.072516, Found: 434.078475 ; Anal. Calc. for C26H14N2O3S: C, 71.88, H,
3.25,
N, 6.45. Found: C, 70.80, H, 3.14, N, 6.18.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 108 -
Example 82.
If4-benzofblnaphtho[2.3-d]thiophen-11-3r11-2-c ano-phenoxY]-acetic acid
5-(Benzo[b]naphtho[2,3-d]thiophen-11-yl)-2-hydroxy-benzonitrile (0.386 g,
1.1 mmol), methyl bromoacetate (0.266 mL, 2.75 mmol), potassium carbonate
(0.228
g, 1.65 mmol) and N,N-dimethylformamide (5.0 mL) were combined and stirred at
ambient temperature for 1.5 h. The reaction mixture was diluted with water (
120 mL)
and acidified with 10% aqueous HCl to pH 1 and aqueous mixture was extracted
with
ethyl acetate ( 150 mL). The ethyl acetate extract was washed with water,
dried with
brine and anhydrous MgS04, and concentrated to provide a white solid. The
solid
was dissolved in ethyl acetate (60 mL) and silica gel was added. Solvent was
removed
and the adsorbate was flash chromatographed (eluent 7:3 petroleum ether: ethyl
acetate) to provide the methyl ester as a white solid (0.195 g, 42%). Aqueous
potassium carbonate ( 124 mg in 4 mL of water, 0.90 mmol) was added to a
stirred
solution of this methyl ester (0.190 g, 0.45 mmol) in THF (5 mL) at ambient
temperature. After 23 h the reaction mixture was diluted with water (80 mL)
and
acidified with 10% aqueous HCl to pH 1 and aqueous was extracted with ethyl
acetate ( 100 mL). The ethyl acetate extract was washed with water, dried with
brine
and anhydrous MgS04, and concentrated to providethe title compound as a white
solid (0.116 g, 63%): mp 235-236°C: NMR (CDC13); S 8.38 (s, 1H), 7.96
(d J = 8 Hz,
1H), 7.81 (dd, J = 8, 1 Hz, 1H), 7.72 (d, J = 2 Hz, 1H), 7.61 (dd J = 8, 1 Hz,
1H), 7.55
(ddd, J = 8, 7, 1 Hz, 1H), 7.45-7.38 (m, 3H), 7.19 (d, J = 8 Hz, 1H), 7.12
(ddd, J = 8,
7, 1 Hz, 1 H), 6.73 (d, J =8 Hz, 1 H), 5.04 (s, 2H); MS (EI): 409 ( 100%, MI);
Anal.
Calc. for C25H15N03S: C, 73.33, H, 3.69, N, 3.42. Found: C, 71.66, H, 3.33, N,
3.31.
Example 83.
j4-Benzofblnaphtho(2 3-dlthiophen-11-yl-2 6-diiodo-phenoxy)-acetic acid
4-(Benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6-diiodo-phenol (0.543 g, 0.94
mmol), methyl bromoacetate (0.181 mL,, 1.88 mmol), potassium carbonate (0.141
g,
1.03 mmol) and N,N-dimethylformamide (5.4 mL) were combined and stirred at
CA 02330623 2000-10-30
WO 99/58521 PCT/tJS99/10185
- 109 -
ambient temperature for 1 h. The reaction mixture was added to water and
filtered.
The solid was washed with water and dried in vacuo to provide the (4-benzo[b]-
naphtho[2,3-d]thiophen-11-yl-2,6-diiodo-phenoxy)-acetic acid methyl ester as a
white
solid (0.537 g, 88%): mp 203-205 °C. Aqueous potassium hydroxide (0.5
N, 2.54 mL,
I.28 mmol) was added to a stirred solution of this methyl ester (0.55 g, 0.85
mmol) in
THF (5.0 mL) at ambient temperature. After 1 h the solution was concentrated,
diluted with water (75 mL) and acidified with 10% aqueous HCI. The solid was
filtered and washed with water to provide the title compounds as a white solid
(0.428
g, 80%): mp 250-252°C: NMR (DMSO-d6); 8 8.66 (s, 1H), 8.07 (d, J = 8
Hz, 1H),
8.01 (d, J = 8 Hz, 1H), 7.93 (s, 2H), 7.61 (ddd, 3 = 8, 7, 1 Hz, 1H}, 7.54-
7.48 (m, 2H),
7.47 (ddd, J = 8, 7, 1 Hz, 1 H), 7.27 (ddd, J = 8, 7, 1 Hz, 1 H), 6.71 (d, J =
8 Hz, 1 H),
4.69 (s, 2H); MS (EI): 636 (100%, MI); Anal. Calc. for C24H14I2O3S: C, 45.31,
H,
2.22, N, 0.00. Found: C, 44.95, H, 1.99, N, 0.23.
The compounds in Examples 84-95 were prepared using the procedure in Example
83
and the appropriate starting material.
Example 84.
j4-Benzofb]naphthof2 3-dlthiophen-11-L-~henoxy]-acetic acid
From 4-benzo[b]naphtho[2,3-d]thiophen-11-yl-phenol (Example 14). White
solid: mp 221-223°C: NMR (DMSO-d6); b 13.05 (broad s, 1H), 8.61 (s,
1H), 8.05 (d,
J = 8 Hz, 1H), 7.96 (d, J = 7 Hz, IH), 7.58 (ddd, J = 8, 7, 1 Hz, 1H), 7.51-
7.40 (m,
3H), 7.33 (d, J = 9 Hz, 2H}, 7.23 (d, J = 9 Hz, 2H), 7.12 (ddd, J = 8, 7, 1
Hz, 1H), 6.71
(ddd, J = 8, 1, 1 Hz, 1H), 4.86 (s, 2H); MS (EI): [M+], 384 (100%); Anal.
Calc. for
C24H16O3S: C, 74.98, H, 4.20, N, 0.00. Found: C, 74.62, H, 4.14, N, 0.08.
Example 85.
(4-Benzo[b]naphthol2 3-dlthioRhen-11-yl-2-iodo-phenoxy)-acetic acid
From 4-(benzo[b]naphtho[2,3-d]thiophen-11-yl)-2-iodo-phenol (Example 27).
White solid: mp 248-249°C: NMR (DMSO-d6); 8 8.63 (s, 1H), 8.05 (d, J
= 8 Hz,
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 110 -
1H), 7.98 (d, J = 7 Hz, 1H), 7.81 (d, J = 2 Hz, 1H), 7.61-7.56 (m, 1H), 7.49-
7.48 (m,
2H), 7.44 (ddd, J = 8, 7, 1 Hz, 1H), 7.41 (dd, J = 8, 2 Hz, 1H), 7.20 (d, J =
8, Hz, 1H),
7.17 (ddd, J = 8, 7, 1 Hz, 1H), 6.75-(d, J = 8, Hz, 1H), 4.96 (s, 2H); MS
(EI): 510
(100%, MI); Anal. Calc. for C24H15I03S: C, 56.48, H, 2.96, N, 0.00. Found: C,
56.35, H, 2.84, N, 0.27.
Example 86.
{ 2.6-Dimethvl-4-f 6-methy~benzo [blnaphtho [2, 3-d]thioDhen-11-yl)1-phenol 1-
acetic acid
From {2,6-dimethyl-4-[6-methyl-(benzo[b]naphtho[2,3-d]thiophen-11-yl)]-
phenoxy }-acetic acid methyl ester (Example 25). White solid: mp 155-181
°C; NMR
(DMSO-d6); S 12.95 (broad s, 1H), 8.23 (d, J = 8 Hz 1H), 7.97 (d, J = 8 Hz,
1H), 7.63
(d, J = 8, 7, 1 Hz, 1 H), 7.58 (d, J = 8 Hz, 1 H), 7.47 (ddd, J = 8, 7, 1 Hz,
1 H), 7.41
(ddd, J = 8, 8, 1 Hz, 1H), 7.15 (ddd, J = 8, 7, 1 Hz, 1H), 7.05 (s, 2H), 6.67
(d, J = 8
Hz, 1H), 4.59 (s, 2H), 2.92 (s, 3H), 2.34 (s, 6H).MS (EI): [M+] 426 (100%);
Anal.
Calc. for C27H22O3S: C, 76.03, H, 5.20, N, 0.00. Found: C, 75.64, H, 5.31, N,
0.02.
Example 87.
I4-l6-Bromo-benzo(b]naphthof2,3-dlthiophen-11-~ henoxyl acetic acid
From [4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-acetic
acid, methyl ester ester (Example 41). White solid: mp 198-200°C: NMR
(CDC13); b
8.34 (ddd, J = 8, 1, 1 Hz, 1H), 7.80 (ddd, J = 8, 1, 1 Hz, 1H), 7.66-7.61 (m,
2H), 7.45
7.38 (m, 2H), 7.36 (d, J = 9 Hz, 2 H), 7.22 (d, J = 9 Hz, 2H), 7.09 (ddd, J =
8, 7, 1 Hz,
TH), 6.71 (ddd, J = 8, 1, 1 Hz, 1H), 4.89 (s, 2H); MS (EI): [M+], 1 bromine
isotope
pattern, 462 (95%), 464 ( 100%);
Anal. Calc. for C24H15Br03S~0.6CH3C02H~0.28H20: C, 60.00, H, 3.37, N, 0.00.
Found: C, 59.82, H, 3.42, N, 0.03.
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185.
- 111 -
Example 88.
[2 6-Dibromo-4-(6-~ano-benzofb]naphthof2.3-dlthiophen-11-~phenox~-acetic acid
From [2,6-dibromo-4-(6-cyano-benzo[b]naphtho[2,3-d]thiophen-I1-yl)-phen-
oxy]-acetic acid, methyl ester (Example 58). White solid: mp 259-261°C:
NMR
(DMSO-d6); 813.2 (broads s, 1H), 8.27 (d, J = 8 Hz, 1H), 8.16 (d, J = 8, 1H),
7.91
(ddd, J = 8, 7, 1 Hz, IH),7.89 (s, 2H), 7.74-7.65 (m, 2H), 7.59 (ddd, J = 8,
7, 1 Hz,
1H), 7.38 (ddd, J = 8, 7, 1 Hz, 1H), 6.75 (d, J = 8 Hz, IH), 4.78 (s, 2H); MS
(EI):
[M+], 2 bromine isotope pattern, 565 (45%), 567 (100%) 569 (50%); Anal. Calc.
for
C25H 13Br32N03S: C, 52.93, H, 2.31, N, 2.47. Found: C, S 1.96, H, 2.07, N,
2.31.
Example 89.
j4-{6-Bromo-benzojblnaphtho[2.3-dlthiophen-11-yl)-2.6-diisopropyl-phenoxy)-
acetic
acid
From 4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6-diisopropyl-
phenol (Example 40). White solid: mp: 220-221 °C; NMR (CDCl3); b 8.37
(ddd, 3 =
8, 1, 1, 1H), 7.81 (ddd, J = 8, 1, 1 Hz, 1H), 7.69 (ddd, J = 8, 1, 1 Hz, 1H),
7.66 (ddd, J
= 8, 8, 1, 1H), 7.48 (ddd, J = 8, 8, 1 Hz, 1H), 7.38 (ddd, J = 8, 8, 1 Hz,
1H), 7.19 (s,
2H), 7.02 (ddd, J = 8, 8, 1 Hz, 1H), 6.54 (d, J = 8 Hz, 1H), 4.69 (s, 2H),
3.42
(septuplet, J = 7 Hz, 2H), 1.28 (d, J = 7 Hz, 6H), I.23 {d, J = 7 Hz, 6H); MS
(+FAB):
546 (90%), 548 (100); Anal. Calc. for C30H27Br03S: C, 65.81, H, 4.97, N, 0.00.
Found: C, 65.56, H, 4.89, N, 0.10.
Example 90.
j~6-Bromo-benzofblnaphtho(2.3-d]thiophen-11-Yll-phenoxyll-acetic acid
From 3-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-I1-yl)-phenol (Example
42). White solid: mp 180-181°C: MS (+FAB): [M+], 462; Anal. Calc. for
C24H15Br03S: C, 62.21, H, 3.26, N, 0.00. Found: C, 61.90, H, 3.19, N, 0.13.
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 112 -
Example 91.
f2 Bromo-5-~6-bromo-benzofblnaphthof2 3-dlthiophen-I I-y_1)-phenoxyll-acetic
acid
From 2-bromo-5-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenol
(Example 68). White solid: mp 174-175°C: MS (+FAB): [M+], 2 bromine
isotope
pattern, 540, 542, 544; Anal. Calc. for C24H14Br203S: C, 53.16, H, 2.60, N,
0.00.
Found: C, 52.91, H, 2.75, N, 0.48.
Example 92.
[2 4-Dibromo-5-f6-bromo-benzofb]naphthof2,3-dlthiophen-I I-yl)-phenox3r11-
acetic
a-cid
From 2,4-dibromo-5-(6-bromo-benzo[bJnaphtho[2,3-d]thiophen-11-yl)-phenol
(Example 69). White solid: mp 256-258°C: MS (+FAB): [M+], 618; Anal.
Calc. for
' C24H13Br303S: C, 46.41, H, 2.11, N, 0.00. Found: C, 46.26, H, 2.17, N, 0.07.
Example 93.
~6-Bromo-benzofblnanhtho[2 3-dlthiophen-11-yl)-2-carboxymethoxy-phenoxyll-
acetic acid
From 4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-benzene-1,2-diol
(Example 42). White solid: mp 222-224°C: MS (+FAB): [M+], 536; Anal.
Calc. for
C26H17Br06S: C, 58.11, H, 3.19, N, 0.00. Found: C, 57.58, H, 3.00, N, 0.15.
Example 94.
3-Bromo-5-l6-bromo-benzofblnaphthof 2,3-dlthiophen-11-yl)-2-carboxymethox~
phenox~rl]-acetic acid
From 3-bromo-5-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-benzene-
1,2-diol (Example 70). White solid: mp 135-137°C: MS (EI): [M+], 2
bromine
isotope pattern, 614, 616, 618; Anal. Calc. for C26H 16Br206S: C, 50.67, H,
2.26, N,
0.00. Found: C, 49.45, H, 2.73, N, 0.11.
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 113 -
Example 95.
4-Bromo-5-l6-bromo-benzo[~]naphthof2.3-d]thiophen-11-yl)-2-carboxymethoxy-
phenoxyli-acetic acid
From 4-Bromo-5-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-benzene-
1,2-diol (Example 71). White solid: mp 256-257°C: MS (EI): [M+], 2
bromine
isotope pattern, 614, 616, 618; Anal. CaIc. for C26H16Br206S: C, 50.67, H,
2.26, N,
0.00. Found: C, 50.88, H, 2.96, N, 0.04.
Example 96.
(Sl-2-Hydroxy-3-phenylpropionic acid. meth I~ter
A solution of commercially available (S)-2-hydroxy-3-phenylpropionic acid
(5.0 g, 30.1 mmol) and p-toluenesulfonic acid hydrate ( 1 g) in methanol ( 125
mL)
was refluxed with removal of water using 3A molecular sieves for 17 h. The
solution
was concentrated and dissolved in ether. The ether solution was washed with
saturated sodium bicarbonate, brine and concentrated to provide the title
compound as
a white solid {5.32 g, 98%): NMR (CDCl3); 8 7.36-7.20 (m, 5H), 4.47 (ddd, J =
5, 6,
7 Hz, 1H), 3.78 (s, 3H), 3.14 (dd, J = 5, 14 Hz, 1H), 2.97 ( dd, J = 7, 14
Hz), 2.69 (d, J
= 6Hz, 1H).
Example 97.
~R)-2-Hydroxy-3-ghenylpropionic acid, methyl ester
Prepared from commercially available (R)-2-hydroxy-3-phenylpropionic acid
according to the procedure in Example 96. White solid: NMR {CDC13); 8 7.34-
7.20
(m, 5H), 4.47 (ddd, J = 5, 6, 7 Hz, 1H), 3.78 (s, 3H), 3.14 (dd, J = 5, 14 Hz,
1H), 2.97
(dd, J = 7, 14 Hz), 2.69 (d, J = 6Hz, 1H).
Example 98.
D.L-Indole-3-lactic acid methyl ester
Prepared from commercially available D, L-indole-3-lactic acid according to
the procedure in Example 96. White solid: mp 42-44 °C: NMR (CDC13); 8
8.07 (m,
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 114 -
1H, NH), 7.61 (d, J = 8 Hz, 1H), 7.34 (d, J = 8 Hz, 1H), 7.19-7.10 (m, 3H),
5.45 (q, J
= 6 Hz, 1H), 3.72 (s, 3H), 3.25 (dd, J = 7, 6 Hz, 2H), 2.75 (d, J = 6 Hz, 1H,
OH); MS
(EI): [M+], 219.
Example 99.
(Sl-(+1-a-Hydrox3r-1 3-dioxo-2-isoindolinebutvric acid. methyl ester
Prepared from commercially available (S)-(+)-a-hydroxy-1,3-dioxo-2-
isoindolinebutyric acid according to the procedure in Example 9. White solid:
mp
123-124.5°C: MS (EI): [M+], 263; Anal. Calc. for C13H13N05: C, 59.31,
H, 4.98, N,
5.32. Found: C, 59.04, H, 5.02, N, 5.06.
Example 100.
L-~i-Imidazolelactic acid methyl ester. hydrochloride
Thionyl chloride (4.8 mL, 68.4 mmol) was added dropwise to a stirred,
ambient temperature suspension of commercially available L-(i-imidazolelactic
acid,
hydrochloride ( 1.11 g, 5.7 mmol) in methanol (7 mL) under a dry nitrogen
atmosphere over a period of 10 min. The solution was heated in an 60°C
oil bath for 2
days. Upon cooling to room temperature, the reaction mixture was concentrated,
chased with ethyl ether to provide the title compound as a sticky white solid
( 1.07 g,
90%); NMR (DMSO-d6); 8 8.63 (s, 1H), 7.23 (s, 1H), 4.35 (t, J = 6 Hz, 1H),
3.62 (s,
3H, OCH3), 2.90 (d, J = 6 Hz, 2H); MS (EI): 170 ( 10%, MI), 111 (40%), 81 (
100%).
Example 101.
N-t-BOC-L-~i -Imidazolelactic acid, meth ly_ester
Triethylamine (0.878 mL, 6.3 mmol) was added dropwise to a stirred, ambient
temperature solution of L-[3-imidazolelactic acid, methyl ester, hydrochloride
(0.87 g,
4.2 mmol) in methanol ( 12 mL) under a dry nitrogen atmosphere. The stirring
was
continued at ambient temperature for 40 min. After 6 h., the reaction mixture
was
concentrated to yield an oil and dichloromethane ( 150 mL) was added. The
dichloromethane was washed with water and brine. Silica gel (12 mL) was added.
CA 02330623 2000-10-30
WO 99/58521 PCT/17S99/10185
- 115 -
Solvents were removed and the silica adsorbate was flash chromatographed
(eluent 4
6 petroleum ether : ethyl acetate) to provide the title compound as an oil
(0.86 g,
75%): MS (EI): [M+], 270.
Example 102.
(S)-2-f4-Nitrobenzoyl]-4-phen l~utyric acid ethyl ester
To a cold (ice bath) solution of commercially available (R)-2-hydroxy-4-
phenyl-butyrate, ethyl ester ( 1.86mL, 9.60mmole), p-nitrobenzoic acid (6.42g,
38.4mmole, 4 eq) and triphenylphosphine (10.07g, 38.4 mmole, 4 eq.) in
anhydrous
tetrahydrofuran (110 mL) was added diethyl azodicarboxylate (6.05 mL, 38.4
mmole,
4 eq) dropwise over a period of 40 minutes keeping the internal temperature
between
4 and 5°C. After stirring for one additional hour, the ice bath was
removed and the
solution was allowed to stir at ambient temperature for 5 days. The solvents
were
removed and the residue was redissolved in a mixture of ether and ethyl
acetate (600
mL). Silica gel (200 mL) was added and the solvents removed. The adsorbate was
flash chromatographed (gradient: 80/20 - 70/30 petroleum ether / ethyl
acetate) to
give the title compound as a yellow oil (4.03g}: NMR (CDC13); 8 8.30 (d, J = 9
Hz,
2H), 8.18 (d, J = 9 Hz, 2H), 7.38 - 7.18 (m, SH), 5.28 (t, J = 2Hz, 1H), 4.23
(q, J =
7Hz, 2H), 2.85 (t, J = 8Hz, 2H), 2.40 - 2.33(m, 2H), 1.29 (t, J = 7H, 3H); MS
[ (+)
FAB]: [M +H] m/z = 358.
Example 103.
LS)-2-H~droxy-4-phenylbutyric acid ethyl ester
To a suspension of potassium cyanide (0.176g, 2.70 mmole) in absolute
ethanol (43mL) was added a solution of (S}-2-[4-nitrobenzoyl]-4-
phenylbutyrate,
ethyl ester (3.868, 10.8 mmole) in absolute ethanol (38mL) dropwise over a
period of
0.5 hours. After stirring 2.25 hours the solvent was removed and the reside
was
diluted with water and acidified with dilute hydrochloric acid. The organics
were
extracted with ether. The extracts were combined, silica gel (60 mL) was added
and
the solvent was removed. The adsorbate was flash chromatographed, eluent
(gradient
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 116-
90/10 - 80/20 petroleum ether / ethyl acetate) and the solvents were chased
with
benzene to give the title compound as a yellow oil (1.67g, 74%): [aJ25D
+178.23
(10.98mg/mL CHC13}; NMR (CDCl3); 8 7.38 - 7.16 (m, SH), 4.30 - 4.10 (m, 3H),
2.9
- 2.6 (m, 3H), 2.2 - 1.9 (m, 2H), 1.15 (t, 4Hz, 3H);.
Example 104.
3-Pyridin-3-yl-propionic acid. ethyl ester
According to the procedure of B.A. Lefker, W.A.Hada, P.J. McGarry
Tetrahedron Lett. 1994, 35, 5205-5208, a solution of sodium
bis(trimethylsilyl)amide
( 1.0 N in THF, 44.3 mL, 44.3 mmol) was added dropwise at a rate to keep the
temperature below -50°C to a stirred solution of 3-pyridine
carboxaldehyde (4.41 mL,
46.7 mmol), ethyl chloroacetate (4.93 mL, 46.7 mmol) and THF (34 mL) under a
dry
nitrogen atmosphere. After 45 min at -78°C, the reaction mixture was
warmed to 0°C
and then quenched with water and concentrated. The residue was partitioned
between
ether and water. The ether phase was dried with brine and concentrated. The
residue
was dissolved in erthyl acetate and palladium hydroxide on carbon (wet,
Degussa
type, 20% Pd content, 1 g) was added. The mixture was hydrogenated at 45 psi
hydrogen pressure for 2 h. The reaction mixture was filtered thru sulka floc
and the
solvent was removed. The residue was flash chromatographed (2:3 petroleum
ether:
ethyl acetate, eluent) to provide the title compound as an oil (3.83 g, 42%):
NMR
(CDC13); 8.24 (m, 2H), 7.60 (d, 1H), 7.22 (dd, 1H), 4.45 (t, 1H), 4.22 (q,
2H), 3.15
(dd, 1H), 2.95 (dd, 1H), 1.27 (t, 3H).
Example 105.
fS)-l+)-a.3-Dihydroxy-1-oxo-2-isoindolinebutyric acid methyl ester
Sodium borohydride (0.474 g, 12.54 mmol) was added portionwise to a -20
°C, stirred solution of (s)-(+)-a-hydroxy-1,3-dioxo-2-
isoindolinebutyric acid methyl
ester (3.0 g, 11.4 mmol) in THF/water (30/1.38 mL) for 6 hours. Upon cooling
to
room temperature, the reaction mixture was carefully quenched and acidified
with
10% aqueous HCI. Aqueous mixture was extracted with ethyl acetate (300 mL).
The
CA 02330623 2000-10-30
WO 99/58521 PCT/tlS99/10185
- 117 -
ethyl acetate extract was washed with water and brine. Silica gel ( 15 mL) was
added.
Solvents were removed and the silica adsorbate was flash chromatographed
(eluent 95
petroleum ether: dichloromethane) to provide the title compound as an oil
(0.96 g,
32%): MS (EI}: [M+J, 265.
5
Example 106.
(s)-(+)-a-Hydroxy-1-oxo-2-isoindolinebutyric acid methyl ester
Trifluoroacetic anhydride ( 1.1 mL, 7.66 mmol) was added dropwise to a
stirred suspension of (s)-(+)-a,3-dihydroxy-1-oxo-2-isoindolinebutyric acid,
methyl
ester (0.84 g, 3.19 mmol) in chloroform (10 mL) under a try N2 atmosphere for
2
hours. After the reaction mixture was concentrated to yield an oil,
trifluoroacetic acid
(3.4 mL) and triethylsilane ( 1.1 mL, 3.83 mmol) were added at ambient
temperature
under a dry nitrogen atmosphere. After 6 hours, the reaction mixture was
carefully
quenched with 10% aqueous sodium bicarbonate. Aqueous mixture was extracted
with dichloromethane (150 mL). The dichloromethane extract was washed with
water
and brine. Silica gel (12 mL) was added. Solvents were removed and the silica
adsorbate was flash chromatographed (eluent 15 : 85 petroleum ether : ethyl
acetate)
to provide an oil (0.50 g, 63%): mp 73.5-74.5°C: MS (EI): [M+], 247.
Example 107.
(R)-2-f2.6-Dibromo-4-(6-bromo-benzofblnaphtho[2 3-dlthiophen-11 yl) nhenoxyl 3
phen r~l-propionic acid, methyl ester
Diethylazodicarboxylate (0.437 mL, 2.74 mmol) was added dropwise to a
stirred, room temperature suspension of 2,6-dibromo-4-(6-bromo-benzo[b)naphtho-
[2,3-dJthiophen-11-yl)-phenol ( 1.00 g, 1.83 mmol), (S)-2-hydroxy-3-
phenylpropionic
acid, methyl ester (0.494 g, 2.74 mmol), triphenylphosphine (0.72 g, 2.74
mmol) and
benzene ( 12 mL) under a dry nitrogen atmosphere. Dissolution occurred and the
solution was heated in an 80°C oil bath for 3.5 h. Upon cooling to room
temperature,
the reaction mixture was diluted with dichloromethane and silica gel ( 30 mL)
was
added. The reaction mixture was concentrated and the silica adsorbate was
flash
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 118 -
chromatographed (96 : 4 petroleum ether : ethyl acetate) to provide the title
compound as a white solid (1.20 g, 90%): mp 138-139.5°C: NMR (CDC13); b
8.36
(ddd, J = 8, I, 1 Hz, 1H), 7.83 (ddd.; J = 8, I, 1 Hz, 1H), 7.68 (ddd, J = 8,
7, 1 Hz,
1H}, 7.60 (dd, J = 5, 2 Hz, 2H), 7.60-7.48 (m, 2H), 7.46-7.28 (m, 6H), 7.18
(ddd, J =
8, 7, 1 Hz, 1H), 6.74 (ddd, J = 8, 1, 1 Hz, 1H), 5.26 ( t, J = 8 Hz, 1H), 3.76
(s, 3H),
3.59 (dd, J = 8, 5 Hz, 2H); MS (FAB+): [M+], 3 bromine isotope pattern, 722
(30%),
724 ( 70%) 726 (100%) 728 (35%); Anal. Calc. for C32H21Br3O3S: C, 52.99, H,
2.99, N, 0.00. Found: C, 52.60, H, 2.68, N, 0.07.
Example 108.
IR)-2-f2.6-Dibromo-4-f6-bromo-benzo[b]naphthof2 3-d]thiophen-1 I-~,)-,phenoxyl
3
nhenvl-propionic acid
Aqueous potassium hydroxide ( 1 N, 6.37 mL, 6.37 mmol) was added to a
stirred solution of (R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-
d]thiophen-
11-yl)-phenoxy]-3-phenyl-propionic acid, methyl ester (2.31 g, 3.19 mmol) in
THF
(22 mL)/methanol ( 15 mL). After 2h the solution was concentrated, diluted
with
water ( 100 mL) and acidified with 10% aqueous HCI. The solid was filtered,
washed
with water and triturated with petroleum ether. It was then recrystalyzed from
methanol to provide the title compound as a white solid (I.52 g, 67%): mp 140-
I42°C: [a]D25=+25.66 (10.52 mg/mL CHCl3); NMR (DMSO-d6);8 13.26
(broad s,
1 H), 8.29 (d, J = 8 Hz, 1 H), 8.07 (d, J = 8 Hz, 1 H), 7.80 (m, 1 H), 7.79
(dd, J = 7, 3
Hz, 2H), 7.63 (ddd, J = 8, 7, 1 Hz, 1 H), 7.55 (d, J = 8 Hz, 1 H), 7.51 (ddd,
J = 8, 7, 1
Hz, 1H), 7.41 (d, J = 7 Hz, 2H), 7.38-7.32 (m, 3H), 7.31-7.22 (m, 3H), 6.64
(d, J = 8
Hz, 1H), 5.32 (t, J = 7 Hz, 1H), 3.41 (d, J = 7 Hz, 2H); MS (EI): [M+], 3
bromine
isotope pattern, 708 (35%), 710 ( 90%) 712 (100%) 714 (40%); Anal. Calc. for
C31H19Br3O3S: C, 52.35, H, 2.69, N, 0.00. Found: C, 52.46, H, 2.59, N, 0.10.
Chiral
analytical HPLC determined that this compound had approx. 100% EE
[column:Chirobiotic V, 5 micron (4.6 x 250 mm); isocratic, 1:1 ethanol:
hexane; flow
rate = 0.80 mL/min; injection volume = 0.3 pL; sample conc. = 0.25 mg/mL;
retention time, R-enantiomer = 21 min; retention time, S-enantiomer = 16
min.].
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 119 -
Example 109.
(R)-2-f2.6-Dibromo-4-l6-bromo-benzo[b]na hn tho[2 3-dLhiophen-11-girl)-
phenoxy]-3
phen rL1-propionic acid, sodium salt
Sodium hydroxide ( 1 N, 0.417 mL, 0.417 mrnol) was added to a stirred room
temperature solution of (R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-dJ-
thiophen-11-yl)-phenoxy]-3-phenyl-propionic acid (0.297 g, 0.418 mmol) in THF
(1.3 mL)/methanol (1.3). After 30 min, the solvent was removed and the solvent
was
repeatedly chased with benzene via rotoevaporation and dried overnight at
70°C to
provide the title compound as a white solid (0.31 g, 100%): mp 261-
265°C: NMR
(DMSO-d6); b 8.27 (d, J = 8 Hz, 1 H), 8.03 (d, J = 8 Hz, 1 H), 7.79 (ddd, J =
8, 8, 1
Hz, 1H), 7.63 (ddd, J = 8, 7, 1 Hz, 1H), 7.58-7.47 (m, 4H), 7.41 (d, J = 7 Hz,
2H),
7.35-7.32 (m, 1H), 7.22 (t, J = 7 Hz, 1H), 6.77 (d, J = 8 Hz, 1H), 5.42 (dd, J
= 8, 4
Hz, 1H), 3.4 (m, 2H); MS (ESI): [M-H]-, 3 bromine isotope pattern, 707, 709,
711,
713; Anal. Calc. for C31H18Br3O3SNa: C, 50.78, H, 2.47, N, 0.00. Found: C,
50.82,
H, 2.79, N, 0.02.
Example 110.
(S)-2-f2.6-Dibromo-4-(6-bromo-benzofb)naphthof2 3-d]thio"phen-11-vl)-phenoxy~-
3-
phenyl_propionic acid methyl ester
Diethylazodicarboxylate (0.839 mL, 5.50 mmol) was added dropwise to a
stirred, room temperature suspension of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho-
[2,3-d]thiophen-11-yl)-phenol (2.00 g, 3.55 mmol), (R)-2-hydroxy-3-
phenylpropionic
acid, methyl ester (0.96 g, 5.50 mmol), triphenylphosphine ( 1.40 g, 5.50
mmol) and
benzene ( 15 mL) under a dry nitrogen atmosphere. Dissolution occurred and the
solution was heated in an 80°C oil bath for 19 h. Upon cooling to room
temperature,
the reaction mixture was diluted with ether and silica gel (60 mL) was added.
The
reaction mixture was concentrated and the silica adsorbate was flash
chromatographed (95:5 petroleum ether : ethyl acetate) to provide the title
compound
as a white solid (2.48 g, 94 %): mp 140-143°C: [a]D25=-56.87°
(10.02 mg/mL
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 120 -
CHC13); NMR (CDC13); 8 8.36 (ddd, J = 8, 1, 1 Hz, 1H), 7.83 (ddd, J = 8, l, 1
Hz,
1H), 7.68 (ddd, J = 8, 7, 1 Hz, 1H), 7.60 (dd, J = 5, 2 Hz, 2H), 7.60-7.49 (m,
2H),
7.46-7.27 (m, 6H), 7.18 (ddd, J = 8; 7, 1 Hz, 1H), 6.74 (ddd, J = 8, 1, 1 Hz,
1H), 5.26
( dd, J = 8, 6 Hz, 1H), 3.76 (s, 3H), 3.59 (dd, J = 8, 5 Hz, 2H); MS (FAB+):
[M+], 3
bromine isotope pattern, 722 (31%), 724 ( 94 %) 726 (100%) 728 (40%); Anal.
Calc.
for C32H21Br303S: C, 52.99, H, 2.99, N, 0.00. Found: C, 52.99, H, 2.85, N,
0.03.
Example 111.
(S)-2-f2.6-Dibromo-4-(6-bromo-benzofblnaphthof2 3-d]thionhen-11-Yl~-phenoxv]-3-
phen3rl-propionic acid
Aqueous potassium hydroxide ( 1 N, 2.40 mL, 2.40 mmol) was added to a
stirred solution of (S)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-
d]thiophen-
11-yl)-phenoxy)-3-phenyl-propionic acid, methyl ester (0.88 g, 1.21 mmol) in
THF
(12 mL)/methanol (8 mL). After 2h the solution was concentrated, diluted with
water
(50 mL) and acidified with 10% aqueous HCI. The reaction mixture was then
partitioned between water and ether. The ether phase was concentrated and
triturated
with ether and pet. ether. It was then recrystalized from methanol to provide
the title
compound as a white solid (0.54 g, 63%): [a)D25 = -24.81° (10.08 mg/mL
CHCI3);
NMR (CDCl3); b 8.36 (d, J = 8 Hz, 1H), 7.81 (d, J = 8 Hz, 1H), 7.68 (ddd, J =
8, 5, 3
Hz, 1 H), 7.58 (dd, J = 10, 2 Hz, 2H), 7.54-7.48 (m, 2H), 7.44-7.27 (m, 6H),
7.16 (ddd,
J = 8, 7, 1), 6.72 (d, J = 8 Hz, 1H), 5.45 (t, J = 7 Hz, 1H), 3.59 (d, J = 7
Hz, 2H); MS
(EI): [M+], 3 bromine isotope pattern, 708 (24%), 710 ( 80%) 712 ( 100%) 714
(40%);
Anal. Calc. for C31H19Br303S: C, 52.35, H, 2.69, N, 0.00. Found: C, 52.05, H,
2.59,
N, 0.10. Chiral analytical HPLC determined that this compound had approx. 100%
EE [column:Chirobiotic V, 5 micron (4.6 x 250 mm); isocratic, 1:1 ethanol:
hexane;
flow rate = 0.80 mL/min; injection volume = 0.3 pL,; sample conc. = 0.25
mg/mL;
retention time, R-enantiomer = 21 min; retention time, S-enantiomer = 16 min.)
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 121 -
Example 112.
(R)-2-f2.6-Dibromo-4-(6-methoxv-benzofblnaphthof2 3-d)thionhen-11-~- henox,~
3-nhen3rl-~ropionic acid methyl ester
Prepared from 2,6-dibromo-4-(6-methoxy-benzo[b]naphtho[2,3-d)thiophen-
I1-yl)-phenol (Example 63) and (S)-2-hydroxy-3-phenylpropionic acid, methyl
ester
(Example 96) according to the procedure of Example 107. White solid (0.608 g,
96%): NMR (THF-d8); 8 8.29-8.26 (m , 1H), 7.87 (d., J = 8 Hz, 1H), 7.72 - 7.68
(dd,
2H, J = 4, 2 Hz), 7.60 - 7.56 (m, 2H), 7.48-7.37 (m, 4H), 7.32 - 7.28 (m, 2H),
7.25-
7.23 (m, 2H), 7.20 - 7.15 (m, 2H), 6.85 - 6.82 (m, I H), 5.22(dd, 1 H, J = 14,
2, Hz),
4.17 (s, 1H), 3.71 (s, IH), 3.58(s, 6H), ; MS (FAB+): [M+), 2 bromine isotope
pattern, 674 (40%), 676 ( 66%), 678 (45%); Anal. Calc. for C33H24Br2O4S: C,
58.60, H, 3.58, N, 0.00. Found: C, 58.11, H, 3.53, N, 0.19.
Example 113.
(R)-2-f2.6-Dibromo-4-(6-bromo-benzo[b]naphthof2 3-dlthiophen-I 1-~rl)_phenoxv]
3
phen~-but ric acid
Diethylazodicarboxylate (DEAD, 0.210 mL, 1.33 mmol) was added to a
stirred, room temperature solution of 2,6-dibromo-4-(6-bromo-
benzo[b]naphtho[2,3-
d]thiophen-11-yl)-phenol ( 0.500 g, 0.888 mmol), (S)-2-hydroxy-4-phenyl-
butyrate,
ethyl ester (0.277 g, 1.33 mmol), triphenylphosphine (0.350 g, 1.33 mmol) and
benzene (3.8 mL) under a dry nitrogen atmosphere. Dissolution occurred and the
solution was heated in an 80°C oil bath for 2 h. Upon cooling to room
temperature,
the reaction nuxture was diluted with ether and silica gel was added. The
reaction
mixture was concentrated and the silica adsorbate was flash chromatographed
(95 : 5
petroleum ether : ethyl acetate) to provide a white solid (0. 570 g, 85%).
Aqueous
potassium hydroxide ( 1 N, 1.4 mL, 1.4 mmol) was added to a stirred solution
of this
solid (0.562 g, 0.746 mmol) in THF (12 mL)/methanol (5 mL). After 3h the
solution
was concentrated, diluted with water and acidified with IO% aqueous HCI. The
solid
was filtered, washed with water and triturated with petroleum ether to provide
the title
compound as a white solid (0.526 g, 97%): mp 115-120°C: NMR (DMSO-d6);
8 13.3
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 122 -
(broad s, 1H), 8.29 (d, J = 8 Hz, 1H), 8.07 (d, J = 8 Hz, 1H), 7.83 (d, J = 2
Hz, 1H),
7.81 (d, J = 2 Hz, 1H), 7.80 (ddd, J = 8, 7, 1 Hz, 1H), 7.65-7.58 (m, 2H),
7.51 (ddd, J
= 8, 8, 1 Hz, 1H), 7.33-7.20 (m, 6H), b.67 (d, J = 8 Hz, 1H), 5.15 (dd, J = 6
Hz, 1H),
3.00 (m, 1H), 2.79 (m, 1H), 2.34 (m, 2H); MS (FAB+): [M+), 3 bromine isotope
pattern, 722, 724, 726, 728; Anal. Calc. for C32H21Br303S: C, 52.99, H, 2.92,
N,
0.00. Found: C, 52.49, H, 2.69, N, 0.17.
The compounds in Examples 114-144 were prepared using the procedure in Example
113 and the appropriate starting materials.
Example 114.
fS)-2-f4-(6-bromo-benzofblnaphthof2 3-d]thiophen-11-yl)-nhenoxyl 3 phenyl
propionic acid
Prepared from 4-(6-bromo-benzo[b)naphtho[2,3-d]thiophen-11-yl)-phenol
(Example 41 ) and (R)-2-hydroxy-3-phenylpropionic acid, methyl ester (Example
97).
White solid: [a]D25=+17.94° (10.30 mg/mL CHC13): NMR (CDC13); b 8.31
(ddd, J =
8, 1, 1 Hz, 1H), 7.76 (ddd., J = 8, 1, 1 Hz, 1H), 7.60 (ddd, J = 8, 8, 1 Hz,
1H), 7.54 (d,
J = 8 Hz, 1H), 7.44-7.29 (m, 6H), 7.24-7.20 (m, 4H), 7.I9-7.04 (m, 3H), 6.62
(d, J =
8 Hz, 1 H), 5.08 ( dd, J = 7, 5 Hz, 1 H), 3.44 (d, J = 5 Hz, 1 H), 3.43 (d, J
= 7 Hz, 1 H);
MS (FAB+): [M+HJ, bromine isotope pattern, 553 (11%), 569 ( 12%); Anal. Calc.
for
C31H21Br03S: C, 67.27, H, 3.82, N, 0.00. Found: C, 65.17, H, 3.64, N, 0.04.
Analytical HPLC determined that this compound was 98.9% pure [column:novapak,
S
micron (4.6 x 250 mm); isocratic, 7:3 accetonitrile: 0.01 M potassium
dihydrogen
phosphate, pH =3.5; flow rate = 1.0 mL/min).
Example 115.
(S)-2-f2.6-Dibromo-4-(6-cvano-benzofblnaphthof2 3-dlthiophen-11-yl)-phenoxy] 3
phen ~~1-propionic acid
Prepared from 11-(3,5-dibromo-4-hydroxy-phenyl)-benzo[b)naphtho[2,3-d]-
thiophene-6-carbonitrile (Example 58) and (R)-2-hydroxy-3-phenylpropionic
acid,
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 123 -
methyl ester (Example 97). White solid: mp 176-178°C: NMR {CDCl3); 8
8.36 (ddd,
J = 8,1,1 Hz, 1H), 7.85 (d, J = $ Hz, 1H), 7.77 (ddd, J = 8, 8, 2 Hz, 1H),
7.58 (d, J = 2
Hz, 1H), 7.61-7.54 (m, 2H), 7.56 (d, J = 2 Hz, 1H), 7.47 (ddd, J = 8, 8, 1 Hz,
1 H),
7.41 (ddd, J = 8, 1, 1, 2H ), 7.36-7.26 (m, 3H), 7.20 (ddd, J = 8, 8, 1, 1 H),
6.75 (ddd,
S J = 8, 1, 1 Hz, 1H), 5.46 (t, J = 7 Hz, 1H), 3.59 (d, J = 7 Hz, 2H); MS (-
FAB): [M-H]
2 bromine isotope pattern, 6S4 (2%), 6S6 ( 4%) 6S8 (2%); Anal. Calc. for
C32H19Br2N03S: C, 58.47, H, 2.91, N, 2.13 Found: C, 58.23, H, 2.69, N, 2.03.
Example 116.
(R)-2-f4-(6-Cvano-benzofblnaphthof2 3-d]thiophen-11-~)-phenoxyl 3 phen~
propionic acid
Prepared from 11-(4-hydroxy-phenyl)-benzo[b]naphtho[2,3-d]thiophene-6-
carbonitrile (Example 44) and (S)-2-hydroxy-3-phenylpropionic acid, methyl
ester
(Example 96). White solid: mp 14S-148°C: [a]D2S= -2.03° (7.883
mglmL CHCl3);
1S NMR (CDCl3); 8 8.31 (ddd, J = 8, 1, 1 Hz, 1H), 7.78 (ddd., J = 8, 1, 1 Hz,
1H), 7.70
(ddd, J = 8, 8, 1 Hz, 1H), 7.56 (ddd, J = 8, 1, 1 Hz, 1H), 7.47-7.37 (m, 6H),
7.32 (ddd,
J = 8, 7, 1 Hz, 1H), 7.20 (dd., J = 8, 2 Hz, 1H),7.14-7.OS (m, 4H), 6.62 (ddd,
J = 8, 1,
1 Hz, 1 H), 5.06 ( dd, J = 7, S Hz, 1 H), 3.44 (d, J = S Hz, 1 H), 3.42 (d, J
= 7 Hz, 1 H);
MS (EI): [M+), 499 ( 100%); Anal. Calc. for C32H21N03S: C, 76.93, H, 4.24, N,
2.80. Found: C, 75.77, H, 4.22, N, 2.70.
Example 117.
(R)-2-f4-(Benzofblnaphthof2 3-dlthiophen-11-yl)-phenoxYl-3-phen~propionic acid
Prepared from 4-benzo[b)naphtho[2,3-d]thiophen-11-yl-phenol (Example 14)
2S and (S)-2-hydroxy-3-phenylpropionic acid, methyl ester (Example 96). White
solid:
[a]2S/D = -19.09° (8.538 mg/mL, CHC13); NMR (CDC13); b 8.30 (s, 1H),
7.91 (d, J =
8 Hz, 1H), 7.73 (d, J = 8 Hz, 1H), 7.SS-7.24 (m, lOH), 7.14-7.09 (m, 2H), 7.OS
(ddd, J
= 8, 7, 1 Hz, 1H), 6.70 (d, J = 8 Hz, 1H), 5.09 (dd, J = 8,S Hz, 1H,), 3.42
(m, 2H);
MS (EI): [M+] 474 (100%); Anal Calc. for C31H22O3S: C, 78.46, H, 4.67, N,
0.00.
Found: C, 77.09, H, 4.60, N, 0.03 Analytical HPLC purity (98.5%).
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 124 -
Example 118.
IS)-2-f2,6-Dibromo-4-(6-bromo-benzofblnaphthoj2 3-djth~i phen-113rlZ~henox3r]-
3-
phenyl-butyric acid
Prepared from of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-
11-yl}-phenol (Example 21) and commercially available (R)-2-hydroxy-4-phenyl-
butyrate, ethyl ester. White solid: mp 180-181°C: [a]D25=+5.83°
(10.3 mg/mL
CHC13); NMR (CDC13); b 8.36 (d, J = 8 Hz, 1H), 7.83 (d, J = 8 Hz, 1H), 7.67
(ddd, J
= 8, 7, 1 Hz, 1 H), 7.64 (dd, J = 5, 2 Hz, 2H), 7.63-7.49 (m, 2H), 7.42 (ddd,
J = 8, 7, 1
Hz, 1 H), 7.34-7.22 (m, SH), 7.12 (ddd, J = 8, 7, 1 Hz, 1 H), 6.76 ( d, J = 8
Hz, 1 H),
5.30 (t, 1H), 3.21-2.87(m, 2H), 2.59-2.49 (m, 2H); MS (FAB-): [M-H]-, 3
bromine
isotope pattern, 721, 723, 725, 727; Anal. Calc. for C32H21Br3O3S: C, 53.00,
H,
2.92, N, 0.00. Found: C, 52.63, H, 2.68, N, 0.09.
Example 119.
(Rl-2-f4-(3-Carboxymethaxy-benzo[blnaphtho[2 3-d]thionhen-11-~)-phenox ~-~3-
phenyl-propionic acid
Prepared from of (11-(4-hydroxy-phenyl)-benzo[b]naphtho[2,3-d]thiophen-3-
yloxy]-acetic acid methyl ester (Example 72) and (S)-2-hydroxy-3-
phenylpropionic
acid, methyl ester (Example 96). White solid: mp: 191-201 °C: NMR (DMSO-
d6); 8
13.1 (broad s, 2H), 8.45 (s, 1H), 8.01 (d, J = 8 Hz, 1H), 7.55-7.25 (m, 11H),
7.16 (d, J
= 9 Hz, 2H), 6.67 (dd, J = 9, 2 Hz, 1H), 6.49 (d, J = 9 Hz, 1H), 5.18 (dd, J =
7, 4 Hz,
1 H), 4.74 (s, 2H), 3.28 (dd, J = 7, 4 Hz, 1 H), 3.21 (dd, J = 14, 7 Hz, 1 H);
MS (EI):
458 (80%, M+); Anal. Calc. for C33H24O6S: C, 72.25, H, 4.41, N, 0.00. Found:
C,
69.57, H, 4.15, N, 0.32; Analytical HPLC :92% purity.
Example 120.
(R)-2-f2.6-Dibromo-4-(6-bromo-benzofblnaphthof2 3-dlthiophen-11-~)-phenoxyj-3-
( 1 H-imidazol-4. ~rly-pro~onic acid h~rdrochloride
Prepared from of 2,6-dibromo-4-(6-bromo-benzo(b]naphtho[2,3-d]thiophen-
11-yl)-phenol (Example 21 ) and N-t-BOC-L-(3 -imidazolelactic acid, methyl
ester
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-125-
(Example 101). White solid: mp >265°C (dec): NMR (CDCl3); 89.00 (s,
1H), 8.29
(d, J = 8 Hz, 1H), 8.08 (d, J = 8 Hz, 1H), 7.84-7.78 (m, 3H), 7.65-7.51 (m,
4H), 7.28
(dd, J = 8, 1 Hz, 1H), 6.68 (d, J = 8 Hz, 1H), 5.40 (t, J = 7 Hz, 1H), 3.49-
3.47 (m, 2H);
MS (+FAB): [M+H]+, 3 bromine isotope pattern, 699, 701, 703, 705; Anal. Calc.
for
C28H17Br3N2O3S: C, 45.59, H, 2.46, N, 3.80. Found: C, 45.69, H, 2.38, N, 3.76.
Example 121.
(R)-2-f2,6-Dibromo-4-(6-bromo-benzofb]naphthof2 3-dlthiophen-11- r~l) nhenoxvl
propionic acid
Prepared from of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-
11-yl)-phenol (Example 21) and commercially available (S)-lactic acid, methyl
ester.
White solid: mp 131-133 °C: NMR (CDC13);8 8.37 (d, J = 9 Hz, 1H), 7.84
(d, J = 8
Hz, 1H), 7.71-7.65 (m, 3H), 7.57-7.50(m, 2H), 7.45 (ddd, J = 8, 7, 1 Hz, 1H),
7.18
(ddd, J = 8, 7, 1 Hz, 1H), 6.73 ( d, J =8 Hz, 1H), 5.36 (q, J = 7 Hz, 1H),
1.82 (d, J = 7
Hz, 3H); MS (EI): [M-H]+, 3 bromine isotope pattern, 631 (14%), 633 (44 %),
635
(42%), 637 (16%); Anal. Calc. for C25H15Br3O3S: C, 47.27, H, 2.38, N, 0.00.
Found: C, 47.57, H, 2.33, N, 0.03.
Example 122.
(R.S)-2-f2.6-Dibromo-4-(6-bromo-benzofblnaphthof2 3-d]thiophen-11-yl)-nhenoxvl
3-(1H-indol-3-yl)-~ropionic acid
Prepared from of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d)thiophen-
11-yl)-phenol (Example 21) and D,L-indole-3-lactic acid methyl ester (Example
98).
White solid: mp 146-147 °C: NMR (CDC13): 8 8.36 (d, J = 9 Hz, 1H), 8.04-
8.03 (m,
1H, NH), 7.81 (d, J = 8 Hz, 1H), 7.72-7.65 (m, 2H), 7.60 (s, 2H), 7.59-7.59
(m, 2H),
7.42-7.37 (m, 2Hz), 7.28 (d, 2 Hz, 1 H), 7.22 (ddd, J = 8, 7, 1 Hz, 1 H), 7.16
(dd, J =8,1
Hz, 1H), 7.12 (dd, J = 8, 1 Hz, 1H), 6.73 (d, J = 8 Hz, 1H), 5.45 (t, J = 6
Hz, 1H), 3.75
(d, J = 7 Hz, 2H); MS (FAB+): [M+], 3 bromine isotope pattern, 747, 749, 751,
753;
Anal. Calc. for C33H20Br3N03S: C, 52.83, H, 2.69, N, 1.87. Found: C, 53.08, H,
2.73, N, 1.19.
CA 02330623 2000-10-30
WO 99/58521 PCT/11S99/10185
- 126 -
Example 123.
2-f2.6-Dibromo-4-(6-bromo-benzofblnaphthof2 3-d]thionhen-11-yll-nhenoxX]
hexanoic acid
Prepared from of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-
11-yl)-phenol (Example 21) and commercially available (R,S)-2-
hydroxypentanoic,
methyl ester. White solid: mp 212-213°C: NMR (CDC13); 8 8.36 (d, J = 8
Hz, 1H),
7.83 (d, J = 8 Hz, 1H), 7.67 (ddd, J = 8, 5, 1 Hz, 1H), 7.63 (dd, J = 2, 1 Hz,
2H), 7.58-
7.42 (m, 3H), 7.16 (t, J = 8, 1H), 6.75 (d, J = 8 Hz, 1H), 5.23 (t, J = 5 Hz,
1H), 2.26 -
2.16 (m, 2H), 1.8 - 1.73 (m, 1H), 1.60 - 1.41 (m, 3H), 0.99 (t, 3H,); MS (EI):
[M+], 3
bromine isotope pattern, 674 (35%), 676 ( 90%) 678 (100%) 680 (35%); Anal.
Calc.
for C28H21Br3O3S: C, 49.66, H, 3.12, N, 0.00. Found: C, 49.28, H, 2.90, N,
0.09.
Example 124.
(2R~-2-f2.6-Dibromo-4-(6-bromo-benzofblnaphthol2 3-djthiophen-11-~)-nhenoxvl
4-(1-oxo-1. 3-dihydro-isoindol-2-yl)-butyric acid
Prepared from of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-
11-yl)-phenol (Example 21) and (S)-(+)-a-hydroxy-1-oxo-2-isoindolinebutyric
acid
methyl ester (Example 106). White solid: mp 239-241 °C: NMR (CDC13); 8
8.34 (d, J
= 8 Hz, 1 H), 7.90 (d, J = 8 Hz, 1 H), 7.80 (d, J = 8 Hz, 1 H), 7.66 (ddd, J =
8, 7, 1 Hz,
1H), 7.62-7.58 (m, 4H), 7.52-7.47 (m, 3H), 7.39 (ddd, J = 8, 7, 1 Hz, 1H),
7.14 (ddd, J
= 8, 7, 1 Hz, 1H), 6.72 (d, J = 8, Hz, 1H), 5.39 (t, J = 7 Hz, 1H), 4.59 (m,
2H), 4.22-
3.93 (m, 2H), 2.65 (t, J = 6 Hz, 2H); MS (+FAB): [M+H]+, 3 bromine isotope
pattern,
778, 780, 782, 784); Anal. Calc. for C34H22Br3N04S: C, 52.33, H, 2.84, N,
1.79.
Found: C, 52.1 l, H, 2.73, N, 1.79.
Example 125.
Rl-2-f2,6-Dibromo-4-(6-trifluoromethyl-benzolb]n~hthoj2 3-d~thiophen-11 ~)
phenoxyl-3-phenyl- propionic acid
Prepared from of 2,6-dibromo-4-(6-trifluoromethyl-benzo[b]naphtho[2,3-d]-
thiophen-11-yl)-phenol (Example 61) and (S)-2-hydroxy-3-phenylpropionic acid,
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 127 -
methyl ester (Example 96). White solid: mp 109-143°C;
[aJD2S=+47.99° (10.002
mg/mL CH30H); NMR (DMSO-d6); S 13.26 ( s, 1H), 8.30 - 8.27 (m, 1H), 8.06 (d, J
= 7 Hz, 1 H), 7.87 - 7.81 (m, 3H), 7.71 - 7.60 (m, 2H), 7.52 (dd, J = 8, 1 Hz,
1 H), 7.43
- 7.24 (m, 6H), 6.60 (d, J = 8 Hz, 1H), 5.33 (t, J = 7 Hz, 1H), 3.41 (d, J = 7
Hz, 2H);
S MS (EI): [M+], 2 bromine isotope pattern, 698 (8%), 700 ( 20%) 702 (1S%);
Anal.
Calc. for C32H19Br2F3O3S: C, 54.88, H, 2.74; N, 0.00. Found: C, SS.29, H,
3.11, N,
0.10.
Example 126.
(R)-2-f2.6-Dibromo-4-(6-methoxy-benzo[blna hp tho[2 3-d]thiophen 11 ~phenoxy]
3-phenyl-propionic acid
Prepared from of 2,6-dibromo-4-(6-methoxybenzo[b]naphtho[2,3-d]thiophen-
11-yl-phenol (Example 63) and (S)-2-hydroxy-3-phenylpropionic acid, methyl
ester
(Example 96). White solid: mp 102-110°C: NMR (DMSO-d6); 8 13.2 (broad
peak,
1 S 1 H), 8.23 (d, J = 8 Hz, 1 H), 8.02 (d, J = 8 Hz, 1 H), 7.74 (dd, J = 6, 2
Hz, 2H), 7.56
(ddd, J'= 8, 7, 1, 1H), 7.56 (ddd, J = 8, 7, 1 1H), 7.51-7.45 (m, 2H), 7.42-
7.40 (m,
2H), 7.36 - 7.32 (m, 4H), 7.30 - 7.20 (m, 2H), 6.68 (d, J = 8Hz, 1H), 5.30(t,
J = 7Hz,
1H), 4.13(s, 3H), 3.41 (d, J = 7 Hz, 2H); MS (EI): [M+J, M/z 660 (46%), 662
(100%), 664 (S4%); Hi Res MS, Calc. Sample Mass for C32H22Br2O4S:
659.960SS3, Measured Mass: 659.953875, Mass deviation 6.7 nam; Anal. Calc. for
C32H22Br2O4S~0.23C6H6: C, 58.08, H, 3.37 N, 0.00. Found: C, 59.34, H, 3.24, N,
0.04.
Example I27.
2S fR)-2-f2,6-Dibromo-4-(6-chloro-benzo[blnaphthof2 3-dlthiophen-11 yll
nhenoxvl
3-phenyl-propionic acid
Prepared from of 2,6-dibromo-4-(6-chloro-benzo(b]naphtho[2,3-d]thiophen-
11-yl)-phenol (Example 60) and (S)-2-hydroxy-3-phenylpropionic acid, methyl
ester
(Example 96). White solid: (a]2S/D = +19.63° (8.805 mg/mL, CHCl3); NMR
(DMSO-d6); 8 13.25-13.22 (broad singlet, 1H), 8.32 (d, J = 8 Hz, 1H,), 8.70
(d, J = 8
CA 02330623 2000-10-30
WO 99/58521 PCT/11599/i0185
- 128 -
Hz, 1H), 7.81-7.79 (ddd, J = 8,7, 1 Hz, 1H), 7.79 (dd, J 10, 2 Hz, 2H), 7.64
(ddd, J =
8,7, 1 Hz, 1 H), 7.58-7.49 (m, 2H), 7.42-7.32 (m, 4H, ), 7.29-7.24 (m, 2H),
6.66 (d, J =
8 Hz, 1H), 5.32 (t, J = 6 Hz, 1H,}, 3.41 (d, J=7 Hz, 2H); MS (-ESI): [(M-H)+],
2
bromine, 1 chlorine isotope pattern, 663 (40%),665 (100%), 667 (60%), 669
(17%);
Anal Calc. for C31H19Br2C1O3S: C, 55.84, H, 2.87, N, 0.00. Found: C, 56.95; H,
3.00, N, 0.24.
Example 128.
(R)-2-12,6-Dibromo-4-(6-phenylsulfanyl-benzo[b]naphthof2 3-d]thiophen~l l vI)
phenoxYl-3-phenyl-propionic acid
Prepared from of 2,6-dibromo-4-(6-phenylsulfanyl-benzo[b]naphtho[2,3-
d]thiophen-11-yl)-phenol (Example 64) and (S)-2-hydroxy-3-phenylpropionic
acid,
methyl ester (Example 96). White solid: NMR (DMSO-d6); 8 8.50 (d, J = 8 Hz,
1H),
7.98 (d, J = 8 Hz, 1H), 7.83 (d, J = 2 Hz, 1H), 7.8I (d, J = 2 Hz, 1H),7.71
(ddd, J = 6,
5, 2 Hz, 2H), 7.58 (m, 2H), 7.47 (ddd, J = 8, 8, 1 Hz, 1H), 7.42 (d, J = 8 Hz,
2H}, 7.34
(t, J = 7 Hz, 2H), 7.29-7.20 (m, 4H), 7.17-7.08 (m, 3H), 6.67 (d, J = 8 Hz,
1H), 5.35
(t, J = 7 Hz, 1H, CH), 3.41 (d, J = 7 Hz, 2H); MS (+FAB): [M+], 2 bromine
isotope
pattern, 738 (35%), 740 ( 90%) 742 (60%); Anal. Calc. for C37H24Br2O3S2: C,
60.01, H, 3.27, N, 0.00. Found: C, 58.57, H, 3.04, N, 0.22.
Example 129.
(R)-2-f2,6-Dibromo-4-(6-phen lsulfanyl-benzo[b]naphtho[2 3-dlthiophen 11 yll
phenoxylpronionic acid
Prepared from of 2,6-dibromo-4-(6-phenylsulfanyl-benzo[b]naphtho[2,3-
d]thiophen-11-yl)-phenol (Example 64) and commercially available (S)-lactic
acid,
methyl ester. White solid: mp 232-234°C: NMR (DMSO-d6); 8 8.51 (d, J =
8 Hz,
1H), 8.00 (d, J = 8 Hz, 1H), 7.89 (d, J = 2 Hz, 1H), 7.89 (d, J = 2 Hz, 1H),
7.71 (ddd, J
= 8, 7, 1 Hz, 1H), 7.65 (d, J = 8 Hz, 1H), 7.60 (ddd, J = $, 7, 1 Hz, 1H),
7.49 (ddd, J =
8, 7, 1 Hz, 1H), 7.28-7. (m, 4H), 7.17-7.09 (m, 4H), 6.68 (d, J = 8 Hz, 1H),
S.I3 (dd, J
= 7, 14 Hz, 1H), 1.64 (d, J = 7 Hz, 2H); MS (+FAB): [M+J, 2 bromine isotope
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 129 -
pattern, 662 (35%), 664 ( 100%) 666 (60%); Anal. Calc. for C31H20Br2O3S2: C,
56.04, H, 3.03, N, 0.00. Found: C, 55.53, H, 2.86, N, 0.24.
Example 130.
(R)-2-f2.6-Dichloro-4-(6-bromo-benzofblnaphthof2 3-d]thiophen-11- rte- hn
enoxy] 3
phen ~~1-propionic acid
Prepared from of 2,6-dichloro-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-
11-yl)-phenol (Example 67) and (S)-2-hydroxy-3-phenylpropionic acid, methyl
ester
(Example 96). White solid: NMR (DMSO-d6); 8 13.25 (broad s, 1H), 8.29 (d, J =
8
Hz, 1 H), 8.07 (d, J = 8 Hz, 1 H), 7.80 (ddd, J = 8, 8, 1, 1 H), 7.65-7.61 (m,
1 H) 7.63 (d,
J = 2 Hz, 1 H), 7.61 (d, J = 2 Hz, 1 H), 7.55 (dd, J = 8, 1, 1 H), 7.51 (dd, J
= 8, 1, 1 H),
7.41 (dd, J = 8, 1, 2H), 7.34 {ddd, J = 8, 8, 1, 2H), 7.27 (ddd, J = 8, 8, l,
2H), 6.66 (d,
J = 8 Hz, 1H}, 5.28 (t, J = 7 Hz, 1H), 3.44-3.30 (m, 2H); MS (+FAB): [M+], 1
bromine, 2 chlorine isotope pattern, 620, 622, 624; Anal. Calc. for
C31H19BrC12O3S: C, 59.83, H, 3.08, N, 0.00. Found: C, 59.31, H, 2.93, N, 0.40.
Example 131.
(R)-Benzofblnaphthoj2 3-d]'thiophen-11-yl-2 6-diiodo-phenoyl-3-phenyl-
nropionic
acid
Prepared from of 4-(benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6-diiodo-phenol
(Example 26) and (S)-2-hydroxy-3-phenylpropionic acid, methyl ester (Example
96).
White solid: mp 115-117°C: NMR (CDC13); 8 8.36 (s, 1H), 7.95 (dd J =
8, 1 Hz,
1H), 7.87 (d, J = 2 Hz, 1H), 7.85 (d, J = 2 Hz, 1H), 7.78 (dd, J = 8, 1 Hz,
1H), 7.58-
7.27 (m, 9 H), 7.12 (ddd, J = 8, 7, 1 Hz, 1H), 6.77 (d, J = 8, 1 Hz, 1H), 5.56
(t, J =7
Hz, 1H), 3.67-3.55 (m, 2H); MS (EI): [M+], 726; Anal. Calc. for C31H20I2O3S:
C,
51.26, H, 2.77, N, 0.00. Found: C, 51.49, H, 2.87, N, 0.13.
Example 132.
(R)-2-(4-Benzofblnaphthof2 3-dlthiophen-11-yl-2 6-diiodo=phenoxy)-propionic
acid
Prepared from of 4-(benzo[b]naphtho[2,3-d]thiophen-11-yl}-2,6-diiodo-phenol
(Example 26) and commercially available (S)-lactic acid, methyl ester. White
solid:
CA 02330623 2000-10-30
WO 99/58521 PCT/11S99/10185
- 130 -
mp 134-136: NMR (CDCl3): 8 8.38 (s, IH), 7.95 (dd J = 8, 6 Hz, 1H), 7.93 (d, J
= 1
Hz, 2H), 7.81 (d, J = 8 Hz, 1H), 7.58 (ddd, J = 8, 7, 1 Hz, 1H), 7.59-7.54 (m,
2H),
7.49-7.40 (m, 2H), 7.15(ddd, J = 8, 7; 1 Hz, 1H), 6.77 (d, J = 8, 1 Hz, 1H),
5.48 (q, J
=7 Hz, 1H), 1.82 (d, J = 7 Hz, 2H); MS (+FAB): [M+H]+, 651; Anal. Calc. for
C26H16I2O3S: C, 46.18, H, 2.48, N, 0.00. Found: C, 46.60, H, 2.50, N; 0.21.
Example 133.
(R)-2-I2.6-Dibromo-4-f6-l2-dimethylamino-eth~rlsulfanvl)-benzo,[b~naphtho[2 3-
d]thiophen-11-yl]_ henoxy]-3-phenyl-propionic acid
Prepared from of 2,6-dibromo-4-[6-(2-dimethylamino-ethylsulfanyl)-benzo-
[b]naphtho[2,3-d]thiophen-11-yl]-phenol (Example 66) and (S)-2-hydroxy-3-
phenyl-
propionic acid, methyl ester (Example 96). White solid: mp 169-174°C;
NMR
(DMSO-d6); 8 13.3 (broad s, 1H), 8.64 (d, J = 8 Hz, 1H), 8.04 (d, J = 8 Hz,
1H), 7.81
(ddd, J = 8,7,1 Hz, 1H), 7.76 (dd, J = 16,1 Hz), 7.72-7.48 (m, 4H), 7.42-7.22
(m, 7H),
6.64 (d, J = 8 Hz, 1H), 5.32 (t, J=7Hz, 1H), 3.36-3.14 {m, 6H); 2.64 (s, 6H);
MS
(+FAB): [(M+H)+], 2 bromine isotope pattern, 734 (55%), 736 (100%), 738 (70%).
Anal. calc. for C35H29Br2N03S2; C, 54.45, H, 3.92, N, 1.81. Found: C, 54.46,
H,
3.76, N, 1.66.
Example I34.
(R)-2-12,6-Dibromo-4-f6-(p~rridin-4-ylsulfanyl)-benzofblnaphtho[2 3-dlthionhen-
11-
~phenoxy~-3-phenvl-propionic acid
Prepared from of 2,6-dihromo-4-[6-(pyridin-4-ylsulfanyl)-benzo[b]naphtho-
[2,3-d]thiophen-11-yl]-phenol (Example 65) and (S)-2-hydroxy-3-phenylpropionic
acid, methyl ester (Example 96). White solid: NMR (DMSO- d6); b I3.3 (broad
singlet, 1H), 8: 45 (m, 2H), 8.37 (d, J = 8 Hz, 1H), 7.99 (d, J = 8 Hz, 1H),
7.85 (dd,
J= 7, 2 Hz, 2H), 7.7?-7.74 (m, 1 H), 7.66-7.65 (m, 2H), 7.50 (t, J 7 Hz, 1 H),
7.43-7.25
(m, 8H), 6.68 (d, J = 8 Hz; 1H), 5.35 (dd, J 8 ,2 Hz, 1H), 3.43 (d, J = 7 Hz,
2H); MS
[+FABJ: [(M+H)+], 739.9 (70%), 741.9 (100%), 743.9 (90%); MS High resolution
(FAB)+ve, calc mass for C36H24Br2N03S2 739.95644; measured mass 739.96224,
CA 02330623 2000-10-30
WO 99/58521 PCT/iJS99/10185
-131-
mass deviation 5.80 mmu. Anal. Calc. for C36H23Br2N03S2: C, 55.58, H, 3.11, N,
1.80. Found: C, 55.38, H, 3.37, N, 1.92.
Example 135.
(R)-2-f2 6-Dibromo-4-(6-iodo-benzo[blnaphthof2 3-dlthiophen-1 l~rll-phenoxvl-
propionic acid
Prepared from of 2,6-dibromo-4-(6-iodo-benzo[b]naphtho[2,3-d]thiophen-11-
yl)-phenol (Example 59) and commercially available (S)-lactic acid, methyl
ester.
White solid: mp 129-196°C dec; Opt. rot. [a]25lD = + 4.312°
(8.812 mg/mL,
CHCl3); NMR (DMSO-d6); 8 13.12 (broad s, IH), 8.12 (d, J = 8, 1 Hz, 1H), 8.06
(d,
J = 7 Hz, IH), 7.82 (q, J = Hz, 2H), 7.75 (ddd, J = 8,7, 1 Hz, 1H), 7.62-7.49
(m, 3H),
7.27 (ddd, J =8, 7, 1 Hz, 1H), 6.61 (d, J = 8 Hz, 1H), 5.12 (q, J = 7 Hz, 1H),
1.63 (d, J
= 7 Hz, 3H); MS (+FAB): [M+], 2 bromine isotope pattern, 680 (45%), 682
(100%),
684 (60%); Anal. Calc. for C25H15Br2I03S: C, 44.02, H, 2.22, N, 0.00; Found:
C,
I S 44.66, H, 2. 54, N, 0.21.
Example 136.
(R)-2-f2.6-Dibromo-4-l6-iodo-benzofblnaphtho[2 3-d]thiophen-11-yl~- henoxY]-3-
phenvl-propionic acid
Prepared from of 2,6-dibromo-4-(6-iodo-benzo[b]naphtho[2,3-d)thiophen-11-
yl)-phenol (Example 59) and (S)-2-hydroxy-3-phenylpropionic acid, methyl ester
(Example 96). White solid: [a]25/D = + 31.791 ° (9.940 mg / mL CHC13);
NMR
(CDC13); 8 8.23 (ddd, J =8, 1, 1Hz), 7.81 (ddd, J = 8, 1, lHz, 1H), 7.64 (ddd,
J = 8,7,
lHz, 1H), 7.58 (dd, J = 9, 2Hz, 2H), 7.52-7.39 (m, 7H), 7.37-7.28 (m, 2H),
6.68 (ddd,
J = 8,1, lHz, 1H), 5.48 (t, J = 7 Hz, 1H, -CH), 3.5 (d, J = 7Hz, 2H); MS
(+FAB):
[M+], 2 bromine isotope pattern, 756 (65%), 758 (100%), 760 (90%); Anal. calc.
for
C31H19Br2I03S: C, 49.10, H, 2.53, N, 0.00. Found: C, 50.75, H, 3.(?0, N, 0.09.
CA 02330623 2000-10-30
WO 99/58521 PCTJIJS99/10185
- 132 -
Example 137.
2-f2,6-Dibromo-4-f6-bromo-benzofblnaphtho[2 3-dlthiophen-11 yl) phenoxy]' 3
pyridin-3-yl-propionic acid
Prepared from 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-
yl)-phenol (Example 21) and 3-pyridin-3-yl-propionic acid, ethyl ester
(Example
104). White solid: NMR (DMSO-d6); S 8.98 (s, 1H), 8.82 (d., J = 5 Hz, 1H),
8.57 (d,
J = 8 Hz, 1H), 8.28 (d, J = 8 Hz, 1H), 8.07 (d, J = 8 Hz, 1H), 7.96 (dd, J =
8, 6 Hz,
1H), 7.82 (d, J = 2 Hz, 1H), 7.79 (m, 1H), 7.78 (d, J = 2 Hz, 1H),7.62 (dd, J
= 8, 1 Hz,
1 H), 7.57-7.50 ( m, 2H), 7.27 (dd, J = 8, 1 Hz, 1 H), 6.67 (d, J = 8 Hz, 1
H), 5.45 (dd, J
= 6, 2, 1H), 3.60 (m, 2H); MS (+FAB): [M+H]+, 3 bromine isotope pattern, 710,
712,
714 , 716; Anal. Calc. for C30H18Br3N03S~HCI: C, 48.13, H, 2.56, N, 1.87.
Found:
C, 48.12, H, 2.86, N, 1.65.
Example 138.
(R)-2-f2.6-Dimethyl-4-(6-methyl-benzo[b]naphthof2 3-dLhiophen-11-yl)
nhenox,~rl
3-phenyl-propionic acid
Prepared from of 2,6-dimethyl-4-(6-methyl-benzo[b]naphtho[2,3-d]thiophen-
11-yl)-phenol (Example 25) and (S)-2-hydroxy-3-phenylpropionic acid, methyl
ester
(Example 96). White solid: NMR (DMSO-d6); 8 13.0 (broad band, 1H), 8.18 (d, J
= 8
Hz, 1 H), 7.91 (d, J = 8 Hz, 1 H), 7.61 - 7.53 (m, 2H), 7.43 (ddd, J = 8, 7, 1
Hz, 1 H),
7.45 - 7.28(m, SH), 7.25 - 7.21 m, 1H), 7.01 (ddd, J = 8, 7, 1 Hz, 1H), 6.95
(d, J = 5
Hz, 2H), 6.53 (d, J = 8Hz, 1 H), 4.75 (t, J = 7 Hz, 1 H), 3.32 - 3.24 (m, 2H),
2.87 (s,
3H), 2.56 (s, 3H), 2.18 (s, 3H): MS(EI): [M+] 516; Anal. Calc. for C34H28O3S:
C,
79.04, H, 5.46, N, 0.00. Found: C, 79.13, H, 5.41, N, 0.11.
Example 139.
2R)-2-f4-(6-Bromo-benzofblnaphtho[2 3-d]thiophen-11-ylZ2 6-diisopropyl
phenoxy)-3-phenyl-pronionic acid
Prepared from of 4-(6-bromo-benzo[b)naphtho[2,3-d]thiophen-11-yl)-2,6-
diisopropyl-phenol (Example 40) and (S)-2-hydroxy-3-phenylpropionic acid,
methyl
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-133-
ester (Example 96). White solid: NMR (DMSO-d6); b 13.08 (broad s, 1H), 8.27
(d, J
= 8 Hz, 1H), 8.00 (d, J = 8 Hz, 1H), 7.76 (ddd, J = 8, 8, l, 1H), 7.66-7.58
(m, 2H),
7.43 (ddd, J = 8, 8, 1 Hz, 1H), 7.44-7.34 (m, 4H), 7.31-7.25 (m, 1H), 7.12 (s,
2H),
7.08 (ddd, J = 8, 8, 1, 1 H), 6.36 (d, J = 8 Hz, 1 H), 4.55 (t, J = 7 Hz, 1
H), 3.45
(septuplet, J = 7 Hz, 2H), 3.32 (d, J = 7 Hz, 2H), 1.14 (d, J = 7 Hz, 3H),
1.09 (d, J = 7
Hz, 3H), 1.05 (d, J = 7 Hz, 3H), 1.03 (d, J = 7 Hz, 3H); MS (+FAB): [M+] 636
(95%),
638 (100%); Anal. Calc. for C37H33Br03S: C, 69.70, H, 5.11, N, 0.00. Found: C,
69.48, H, 5.11, N, 0.11.
Example 140.
(R)-2-f4-l6-Bromo-benzofb]naphtho[2 3-d]thiophen-11 yl)-2 6-diiso~rovvl
phenoxyZpropionic acid
Prepared from of 4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6-
diisopropyl-phenol (Example 40) and commercially available (S)-lactic acid,
methyl
ester. White solid: mp: 214-215°C; NMR (CDC13); 8 8.37 (ddd, J = 8, l,
1, 1H), 7.80
(d, J = 8 Hz, 1 H), 7.70 (ddd, J = 8, 1, 1 Hz, 1 H), 7.66 (ddd, J = 8, 8, 1, 1
H), 7.48 (ddd,
J = 8, 8, 1 Hz, 1H), 7.37 (ddd, J = 8, 8, 1 Hz, 1H), 7.18 (s, 2H), 7.01 (ddd,
J = 8, 8, 1
Hz; 1H), 6.53 (d, J = 8 Hz, 1H), 4.75 (q, J = 7 Hz, IH), 3.47 (septuplet, J =
7 Hz,
1 H), 3.41 (septuplet, J = 7 Hz, 1 H), 1.71 (d, J = 7 Hz, 3H), 1.25 (d, J = 7
Hz, 6H),
1.20 (d, J = 7 Hz, 3H), 1.19 (d, J = 7 Hz, 3H); MS (EI): 560 (90%), 562 (100);
Anal.
Calc. for C31H29Br03S: C, 66.31, H, 5.21, N, 0.00. Found: C, 65.90, H, 5.18,
N,
0.02.
Example 141.
(R)-2-f2,6-Dibromo-4-(6-methyl-benzo[blnaphthof2 3-d]thiophen-11-ylZphenox3r]
3
phenyl-propionic acid
Prepared from 2,6-dibromo-4-(6-methyl-benzo[b]naphtho[2,3-d]thiophen-11-
yl)-phenol (Example 62) and (S)-2-hydroxy-3-phenylpropionic acid, methyl ester
(Example 96). White solid: mp 117-122°C: [a]D25=+34.13° (9.963
mg/mL CHCl3);
NMR (DMSO-d6): 8 8.17 (d, J = 8 Hz, 1H), 7.81 (d, J = 8 Hz, 1H), 7.63-7.58 (m,
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 134 -
1H), 7.57 (dd, J = 6, 2 Hz, 2H), 7.52-7.46 (m, 2H), 7.42-7.38 (m, 3H), 7.37-
7.28 (m,
3H), 7.16-7.10 (dt, J = 1, 6 Hz, 1H), 6.76 (d, J = 8 Hz, 1H), 5.45 (t, J = 7
Hz, 1H,
CH), 3.59 (d, J = 7 Hz, 2H), 2.98 (s, 3H); MS (EI): [M+], 643.6 (20%), 644.4 (
50%),
645.5 (100%) 647.7 (30%); Anal. Calc. for C32H22Br203S: C, 59.46, H, 3.43, N,
0.00. Found: C, 59.34, H, 3.24, N, 0.04.
Example 142.
(R)-f2,6-Dibromo-4-(6-bromo-benzo[blnaphtho[2 3-dlthio~hen-11-ylZ~phenox~rl-
phenyl-acetic acid
Prepared from 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-
yl)-phenol (Example 21 ) and commercially avialable methly (s)-(+)-mandelate.
White
solid: mp 135-137°C: MS (FAB-): [M-H]-, 693; Anal. Calc. for
C30H17Br303S: C,
51.68, H, 2.46, N, 0.00. Found: C S 1.57, H, 2.89, N, 0.14.
Example 143.
LS)-2-f2-Bromo-5-f6-bromo-benzoLblnaphtho~2 3-djthiophen-11-yl)-phenox~rll-
propionic acid
Prepared from of 2-bromo-5-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-
yl)-phenol (Example 68) and commercially available (R)-lactic acid, methyl
ester.
White solid: mp 132-134°C: MS (+EI): [M+], 2 bromine isotope pattern,
554, 556,
558; Anal. Calc. for C25H16Br203S: C, 53.98, H, 2.90, N, 0.00. Found: C,52.97,
H,
2.98, N, 0.04.
Example 144.
LR)-2-f2-bromo-5-l6-Bromo-benzofblnaphtho[2 3-d]thiophen-11-girl)-phenoxvll-
propionic acid
Prepared from of 2-bromo-5-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-
yl)-phenol (Example 68) and commercially available (R)-lactic acid, methyl
ester.
White solid: mp 133-134.5°C: MS (-ESI): (M-H]-, 2 bromine isotope
pattern 553,
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 135 -
555, 557; Anal. Calc. for C25H16Br2O3S: C, 53.98, H, 2.90, N, 0.00. Found: C,
53.18, H, 2.82, N, 0.04.
Example 145.
(R)-2-f2.6-Dibromo-4-(6-bromo-benzofb]na hn tho,[2 3-dlthiophen-11-yl)-
_phenoxy) 4
( 1. 3-dioxo-1. 3-dihydro-isoindol-2=yl)-butyric acid methyl ester
Diethylazodicarboxylate (0.293 mL, 1.86 mrnol) was added dropwise to a
stirred, ambient temperature suspension of 2,6-dibromo-4-(6-bromo-benzo[b]-
naphtho[2,3-d]thiophen-11-yl)-phenol (0.700 g, 1.24 mmol), (S)-(+)-2-hydroxy-
1,3-
dioxo-2-isoindolinebutyric acid, methyl ester (0.490 g, 1.86 mmol), triphenyl-
phosphine (0.488 g, 1.86 mmol) and benzene (5.5 mL) under a dry nitrogen
atmosphere. Dissolution occurred and the solution was heated in an 80°C
oil bath for
1.5 h. Upon cooling to room temperature, the reaction mixture was diluted with
dicloromethane and silica gel ( 20 mL) was added. The reaction mixture was
concentrated and the silica adsorbate was flash chromatographed (83:17
petroleum
ether : ethyl acetate) to provide the title compound as a white solid (0.822
g, 81%):
mp 221-222°C; NMR (DMSO-d6); 8 8.29 (d, J = 8 Hz, 1H), 8.08 (d., J = 8
Hz, 1H),
7.98 (ddd, J = 8, 7, 1 Hz, 2H), 7.87-7.83 (m, 4H), 7.80 (ddd, J = 8, 7, 1 Hz,
1H), 7.66-
7.58 (m, 2H}, 7.53 (ddd, J = 8, 7, 1 Hz, 1H), 7.29 (ddd, J = 8, 7, 1 Hz, 1H),
6.71 ( d, J
= 8 Hz, 1H), 5.16 (t, 1H), 3.94 (m, 2H), 3.71 (s, 3H), 2.50 (m, 2H); MS
(FAB+):
[M+], 3 bromine isotope pattern, 805 (22%), 807 ( 88%), 809 (100%), 811 (42
%);
Anal. Calc. for C35H22Br3N05S: C, 52.01, H, 2.74, N, 1.73. Found: C, 52.02, H,
2.70, N, 1.73.
Example 146.
(R)-2-(4-Benzofblnaphthof2 3-dlthiophen-11-yl-2 6-dibromo-phenoxy) 4 (1 3
dioxo
1 3-dihydro-isoindol-2-xl)-butyric acid
Iodotrimethylsilane (0.203 mL, 1.43 mmol) was added to a rt, stirred solution
of (R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-
phenoxy]-
4-(l, 3-dioxo-1, 3-dihydro-isoindol-2-yl)-butyric acid, methyl ester (0.770 g,
0.953
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 136 -
mmol} in methylene chloride (8 mL) under a dry nitrogen atmosphere. After 2 h,
iodotrimethylsilane (3 x 0.203 mL, 4.29 mmol) was added to the resulted dark
brown
solution at rt three times every 12 h.The solution was quenched with water
(0.4 mL),
concentrated, chased with benzene (3 x 50 mL). The resulted residue was
dissolved
in ethyl acetate (60 mL) and silica gel (acid washed, 8 mL) was added. Solvent
was
removed and the adsorbate was flash chromatographed (eluent 7:3 pet. ether:
ethyl
acetate) to provide the title compound as a yellow solid (0.304 g, 45 %): mp:
222-
223°C; NMR (CDCl3); 8 8.37 (s, 1H), 7.95(d, J = 8 Hz, 1H),7.89-7.85 (m,
2H), 7.79
(dd, J = 8,1 Hz, 1 H), 7.78-7.71 (m, 2H), 7.64 (d, J = 10 Hz, 1 H), 7.62 (d, J
= 10 Hz,
1 H), 7.59 (dd, J = 8, 1 Hz, 1 H}, 7.55 (ddd, J = 8, 7, 1 Hz, 1 H), 7.46 (ddd,
J = 8, 7, 1
Hz, 1H), 7.40 (ddd, J = 8, 7, 1 Hz, 1H), 7.19(ddd, J = 8, 7, 1 Hz, 1H),
6.88(d, J = 8
Hz, 1 H), 5.27 (t, J = 7 Hz, 1 H, 1 H, CH), 4.12 (m, 2H), 2.65 (t, J = 6 Hz,
2H); MS
(FAB-): [M-H]-, 2 bromine isotope pattern, 713, 714, 716; Anal. Calc. for
C34H21Br2N05S: C, 57.08, H, 2.96, N, 1.96. Found: C, 56.43, H, 2.74, N, 1.90.
Example 147.
(R)-2-f2.6-Dibromo-4-(6-bromo-benzofb]naphthof2 3-d]thiophen-11-yl)-phenoxyl 4
(1. 3-dioxo-1. 3-dihvdro-isoindoI-2-~)-butyric acid
Iodotrimethylsilane (2.8 mL, 1 N in methylene chloride) was added dropwise
to a -10°C, stirred solution of (R)-2-[2,6-dibromo-4-(6-bromo-
benzo[b]naphtho[2,3-
d]thiophen-11-yl)-phenoxy]-4-(1, 3-dioxo-1, 3-dihydro-isoindol-2-yl)-butyric
acid,
methyl ester (0.770 g, 0.953 mmol) in methylene chloride ( 14 mL) under a try
N2
atmosphere. The solution was allowed to warm to ambient temperature and was
stirred overnight. The reaction mixture was quenched, further diluted with
water (100
mL), and partitioned between water and methylene chloride (2 x 75 mL).
Methylene
chloride extracts were concentrated and triturated with pet. ether. It was
then
recrystallized from acetic acid to provide the title compound as a grey solid
(0.228 g,
52%): mp 215-217°C: NMR (CDC13); 8 8.35 (d, J = 8 Hz, 1H), 7.89-7.86
(m, 2H),
7.82 (dd, J = 8, 1 Hz, 1 H), 7.76-7.71 (m, 2H), 7.67(ddd, J = 8, 7, 1 Hz, 1
H), 7.63-7.59
(m, 3H), 7.58 (ddd, J = 8, 7, 1 Hz, 1 H), 7.42 (ddd, J = 8, 7, 1 Hz, 1 H),
7.20 (ddd, J =
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 137 -
8, 7, 1 Hz, 1 H), 6.81 (d, J = 8 Hz, 1 H), 5.27 (t, J = 7 Hz, 1 H, 1 H), 4. I2
(m, 2H), 2.65
(t, J = 7 Hz, 2); MS (EI): [M+), 3 bromine isotope pattern, 791 (75%), 793
(98%), 795
(100%), 797 (38%); Anal. Calc. for C34H20Br3N05S: C, 51.41, H, 2.54, N, 1.76.
Found: C, 51.65, H, 2.37, N, 1.70.
Example 148.
(R)-2-l4-benzo~blnaphtho~2 3-dlthiophen-11-yl)-nhenoxy)-4-(1 3 dioxo 1 3
dihydro
isoindol-2- ly )-butyric acid
Iodotrimethylsilane (1.31 mL, 9.23 mmol) was added dropwise to a
0°C,
stirred solution of (R)-2-(4-benzo[b)naphtho[2,3-d]thiophen-11-yl)-phenoxy)-4-
(1, 3-
dioxo-1, 3-dihydro-isoindol-2-yl)-butyric acid, methyl ester (1.17 g, 2.05
mmol) in
methylene chloride ( 15 mL) under a try N2 atmosphere. After 1 h. the solution
was
allowed to warm to ambient temperature. After 1 h. the reaction mixture was
quenched with 10% aqueous sodium bisulfide and further diluted with water (
100
mL). Aqueous mixture was extracted with ethyl acetate ( 150 mL). The ethyl
acetate
extract was washed with water, dried with brine and anhydrous MgS04, and
concentrated to provide a white solid. The solid was recrystallized from
aqueous
acetic acid and purified by HPLC (Dynamax C18 Column, 90:10 acetonitrile:water
(both with 0.1 % TFA) to provide the title compound as a light yellow solid
(0.420 g,
32%): mp I45-14°C 7: NMR (CDC13); b 8.31 (s, 1H), 7.92 (d, J = 8 Hz,
IH), 7.87-
7.84 (2 distored d, J = 8 Hz, 2H), 7.75 (d, J = 8 Hz, 1H), 7.72-7.69 (2
distored d, J = 8
Hz, 2H), 7.59 (d, J = 8 Hz, 1H), 7.49 (ddd, J = 8, 7, 1 Hz, 1H), 7.39-7.33 (m,
2H),
7.29-7.22 (m, 2H), 7.15 (ddd, J = 8, 7, 1 Hz, IH), 7.05 (dd, J = 9, 8 Hz, 1H),
7.01 (dd,
J = 9, 8 Hz, 1 H), 6.75 (d, J = 8 Hz, 1 H), 4.99 (t, J = 6 Hz, 1 H), 4.09 (t,
J = 6 Hz, 2H),
2.52 (q, J = 6 Hz, 2H); MS (+FAB): [M+H]+, 558; Anal. Calc. for C34H23N05S: C,
73.23, H, 4.16, N, 2.51. Found: C, 71.82, H, 4.00, N, 2.61.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 138 -
Example 149.
LR)-2-f4-(3-Carboxymethoxy-benzo~blnaphthof2 3-d]thiophen-11-vl)- henoxy 4 ll
3-dioxo-1. 3-dihydro-isoindol-2-yl)-bu ric acid
Diethylazodicarboxylate (DEAD, 0.133 mL, 0.845 mmol) was added to a
stirred, room temperature solution of I1-(4-hydroxy-phenyl)-
benzo[b]naphtho[2,3-
d]thiophen-3-yloxy]-acetic acid methyl ester ( 0.140 g, 0.338 mmol), L-3-
phenyllactic
acid, methyl ester (0.133 g, 0.845 mmol), triphenylphosphine (0.222 g, 0.845
mmol)
and benzene ( 1.5 mL) under a dry nitrogen atmosphere. Dissolution occurred
and the
solution was heated in an 80°C oil bath for 6 h. More
diethylazodicarboxylate
(DEAD, 0.053 mL, 0.338 mmol), L-3-phenyllactic acid, methyl ester (0.070 g,
0.338
mmol) and triphenylphosphine (0.103 g, 0.338 mmol) were added. The reaction
mixture was cooled to room temperature after 5.5h. The reaction mixture was
diluted
with ether and silica gel was added. The reaction mixture was concentrated and
the
silica adsorbate was flash chromatographed (gradient 99:1 to 98:2
dichloromethane:acetonitrile) to provide a white solid (0.190g). This solid
was
recrystalized from acetonitrile containing 0:1 % of trifluoroacetic acid to
provide a
white solid ( I44 mg). 1.0 M solution of boron tribromide ( I .4 mL, 1.4 mmol)
in
dichlorornethane was added to a stirred, -78°C solution of this solid
in
dichloromethane (3. 0 mL). After 45 min the reaction mixture was warmed to
room
temperature. Afer 3.5 h ,the reaction mixture was recooled to -78°C and
an additional
amount of I.ON boron tribromide (0.8 mL, 0.8 mmol) was added. The reaction
mixture was warmed to room temperature. After an additional 2h, water was
added
and the reaction mixture was extracted with ethyl acetate. The ethyl acetate
phase was
concentrated and purified by prep HPLC (reverse phase: 7:3 acetonitrile: water
with
0.1 % trifluoroacetic acid). The resultant compound ( 13 mg) was recrystalized
from
acetic acid/water to provide the tilte compound as a white solid (0.005 g, 2%)
: mp:
259-260°C: NMR (DMSO-d6); 8 13.2 (broad s, 2H), 8.54 (s, 1H), 8.02 (d,
J = 8 Hz,
1H), 7.88-7.8I (m, 4H), 7.55-7.44 (m, 4H), 7.25 (dd, J = 9, 2 Hz, 2H),7.I1-
7.04 (m,
2H) 6.79 (dd, J = 9, 2 Hz, 1 H), 6.52 (d, J = 9 Hz, 1 H), 5.03 (dd, J = 8, 2
Hz, 1 H), 4.75
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
- 139 -
(s, 2H), 3.92 (m, 2H), 2.32 (m, 2H); MS (FAB+): 632 ( I O%, M+H); Analytical
HPLC:98.8% pure.
Example 150.
LR)-2-f4-(6-Bromo-benzofblnaphthol2 3-dlthiophen-11-yl)-phenoxyl-4~1 3-dioxo-1
3-dihydro-isoindol-2=yll-butyric acid
Boron tribromide (1 M solution in methylene chloride, 10.4 mL, 10.4 mmol)
was added dropwise to a -10°C, stirred solution of (R)-2-[4-(6-bromo-
benzo[b]-
naphtho[2,3-d]thiophen-11-yl)-phenoxy]-4-(1, 3-dioxo-1, 3-dihydro-isoindol-2-
yl)-
butyric acid, methyl ester ( 1.35 g, 2.08 mmol) in methylene chloride (35 mL)
under a
try N2 atmosphere. After 1 h. the solution was allowed to warm to ambient
temperature. After 1 h. the reaction mixture was quenched, further diluted
with water
( 100 mL), and partitioned between water and methylene chloride. Methylene
chloride
extracts were concentrated to give a solid, The solid was separated and
purified by
HPLC (Dynamax C 18 Column, 95:5 acetonitrile:water (both with 0.1 % TFA) to
provide the title compound as a light yellow solid (0.420 g, 32%): mp
>69°C (dec):
NMR (CDCI3): 8 8.32 (d, J = 8 Hz, 1H), 7.86-7.84 (2 d, J = 8 Hz, 2H), 7.77 (d,
J = 8
Hz, I H), 7.72-7.70 (d, J = 8 Hz, 2H), 7.64-7.59 (m, 2H), 7.43-7.36 (m, 2H),
7.25-
7.16 (m, 3H), 7.05 {dd, J = 9, 8 Hz, IH), 7.01 (dd, J = 9, 8 Hz, 1H), 6.68(d,
J = 8 Hz,
1H), 4.99 (t, J = 6 Hz, 1H), 4.09 (t, J = 6 Hz, 2H), 2.52 (q, J = 6 Hz, 2H);
MS (EI):
[M+], 1 bromine isotope pattern, 635 (90%), 637 ( 100%); Anal. Calc. for
C34H22BrNO5S: C, 64.16, H, 3.48, N, 2.20. Found: C, 64.19, H, 4.04, N, 2.08.
Example 151.
(R)-2-f2.6-Dibromo-4-(6-bromo-benzofblnaphtho[2 3-dlthiophen-11-yl)-phenoxy]
succinic acid dimethvl ester
Diethylazodicarboxylate (0.254 mL, 1.61 mmol) was added dropwise to a
stirred, room temperature suspension of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho-
[2,3-d]thiophen-11-yl)-phenol ( 0.600 g, 1.10 mmol), dimethyl (S)-(-)-malate
(0.213
mL, 1.61 mmol) and triphenylphosphine (0.421 g, 1.61 mmol) in benzene (7.5 mL)
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 140 -
under a dry nitrogen atmosphere. The solution was heated in an 79°C oil
bath for 23
h. Additional Dimethyl (S)-(-)-malate (0.043 mL, 0.321 mmol) and
triphenylphosphine (0.084 g, 0.321 mmol) and diethylazodicarboxylate (0.050
mL,
0.321 mmol) were added and the reaction mixture was heated for an additional
10
hours.The reaction mixture was cooled to room temperature, diluted with
methylene
chloride combined with silica gel (20 mL) and concentrated. The silica
adsorbate was
flash chromatographed (92 : 8 petroleum ether : ethyl acetate) to provide the
title
compound as a white solid (0.389 g, 51 %): mp 118-121 °C: NMR (CDC13);
S 8.36
(dd, J = 8, 0.5 Hz, 1H), 7.83 (dd, J = 8, 0.5 Hz, 1H), 7.68 (ddd, J = 8, 7, 1
Hz, 1H),
7.61 - 7.59 (m, 3H), 7.55 - 7.43 (m, 2H), 7.26 - 7.21 (m, 1H), 6.79 (d, J = 8
Hz, 1H),
5.46 ( t, J = 6 Hz, 1H), 3.90 (s, 3H), 3.77 (s, 3H), 3.32 - 3.30 (m, 1H); MS
(EI): [M+],
3 bromine isotope pattern, 704 (5%), 706 ( 15%), 708 (15%), 710 (4%); Anal.
Calc.
for C28H19Br3O5S: C, 47.55, H, 2.71, N, 0.00. Found: C, 47.93, H, 2.65, N,
0.14.
Example 152.
(R)-2-12.6-Dibromo-4-(6-bromo-benzofb]naphtho~2 3-~'thiophen-11-vl)-phenoxyl_
succinic acid
A solution of (R)-2-[2,6-Dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]-
thiophen-11-yl)-phenoxy]- succinic acid, dimethly ester (0.317 g, 0.448 mmol)
in 4M
HCl in dioxane (5 mL) was combined with water (5 mL) and concentrated HCl ( 1
mL) in a sealed pressure bottle and heated for 9 hours. After remaining at
ambient
temperature for an additional 14 hours the reaction mixture was partitioned
between
water and ether. The layers were separated and acid treated silica gel (7 mL)
was
added to the ether layer. The solvent was removed and the adsorbate was
purified by
flash chromatography (acid treated silica gel, eluant 80 : 20 pet. ether :
ethyl acetate)
to provide the title compound as a white solid (0.142 g, 47%), mp 163-
164°C;
[a]D25=+14.16° (8.825 mg/mL, methanol); NMR (DMSO-d6); 8 13.6 - 13.2
(broad
singlet, 1H,), 12.7 - 12.6 (broad singlet, 1H), 8.28 (d, J = 8 Hz, 1H), 8.07
(d, J = 8 Hz,
1H), 7.82 - 7.78(m, 3H}, 7.65 - 7.57(m, 2H,), 7.52(dd, J = 7, 1 Hz, 1H},
7.31(dd, J =
7, 1, 1H), 6.70(d, J = 8 Hz, 1H), 5.32 (t, J = 8 Hz), 3.67(m, 2H); MS (-FAB)
[M-H]-;
CA 02330623 2000-10-30
WO 99/58521 PGT/IJS99/10185
- 141 -
3 bromine pattern detected, 675(25%), 677(10%), 679(25%), 681(10%); Anal.
Calc.
for C26H15Br3O5S: C, 45.78, H, 2.23, N, 0.00. Found: C, 46.42, H, 2.40, N,
0.05.
Example 153.
2-12.6-Dibromo-4-(6-bromo-benzolblnaphtho[2 3-dlthiophen-11-3r1)=phenoxy]~4
fluoro-phen ~~1-pro,~ionic acid tert-but 1 ester
Diisopropylamine (distilled over CaH2, 0.169 mL, 1.2 mmol) was added to
anhydrous tetrahydrofuran (0.65 mL) and cooled to -74°C under a dry
argon
atmosphere. n-Butyllithium {2.SM in hexane, 0.516 mL, 1.29 mmole) was added
dropwise and the mixture was warmed to 0°C for 10 minutes then recooled
to -74°C.
Hexamethylphosphoramide (0.83 mL) was added followed S minutes later by the
slow (0.5 hour) dropwise addition of a solution of (R)-2-[2,6-dibromo-4-(6-
bromo-
benzo[b)naphtho[2,3-d]thiophen-11-yl)-phenoxy]-propionic acid, tert-butyl
ester
(0.715g, 1.06 mmole) in anhydrous tetrahydrofuran (2.8 mL). After stiring one
hour
at -75°C, 4-fluorobenzylbromide (0.158 mL, 1.29 mmol) was added
dropwise. After
stirring 1 hour at -80°C the reaction mixture was allowed to warm to
room
temperature overnight. The reaction mixture was quenched with dilute aqueous
ammonium chloride and was partitioned between water ( 100 mL) and a 1 : 1
methylene chloride : ether solution ( 130 mL). After one more extraction with
methylene chloride (SOmL) the organic layers were combined, silica gel was
added,
and the solvents removed. The adsorbate was purified by flash chromatography
(eluent 7 : 3 pet ether : methylene chloride) and HPLC (Column: Waters Silica
Prep
Pak; gradient, 99 : 1 to 95 : 5 petroleum ether : ethyl acetate ; Flow rate =
225
mL/min ) to provide the title compound as a white solid (0.265 g, 32%): mp: 80-
100°C; NMR(CDC13); 8 8.35 (dd, J = 8, 1 Hz, 1H), 7.83 (dd, J = 6, 1 Hz,
1H), 7.66
(ddd, J = 6, 5, 1 Hz, 1H), 7.58 (dd, J = 4, 2 Hz, 2H), 7.53 - 7.38 (m, J = 2
Hz, SH),
7.18 (ddd, J = 7, 1, 1, 1H), 7.04 (t, J = 9 Hz, 2H), 6.83 (d, J = 8 Hz, 1H),
5.31 (t, J = 6
Hz, 1 H), 3.49 (t, J = 6 Hz, 2H), 1.40 (s, 9H); MS (FAB+): [M+], 3 bromine
pattern,
782 (20%), 784 (70%), 786 (80%), 788 (35%); Anal. Calc. for C35H26Br3F03S: C,
53.53, H, 3.34, N, O.OO.Found: C, 53.25; H, 3.21, N, 0.04.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 142 -
Example 154.
(R)-2-f2.6-Dibromo-4-f6-bromo-benzolblnaphtho[2 3-d]'thiophen-11-~)-phenoxyl-3-
napthalen-2-yl-propionic acid tert-butyl ester
Diisopropylamine (distilled over CaH2, 0.233 mL, 1.78 mmol) was added to
anhydrous tetrahydrofuran (0.91 mL) and cooled to -74°C under a dry
argon
atmosphere. n-Butyllithium (2.SM in hexane, 0.71 mL, 1.78 mmole) was added
dropwise and the mixture was warmed to 0°C for ca. 20 minutes then
recooled to -
75°C. Hexamethylphosphoramide (2 mL) was added followed 10 minutes
later by the
slow (0.5 hour) dropwise addition of a solution of (R)-2-[2,6-dibromo-4-(6-
bromo-
benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-propionic acid, tert-butyl
ester
( 1.004 g, 1.48 mmole) in anhydrous tetrahydrofuran (3 mL). After stirring one
hour
at -75°C, 2 bromomethylnaphthalene (0.393g, 1.78 mmol) was added
dropwise. After
stirring 0.5 hour at -80°C the reaction mixture was allowed warm to
room temperature
overnight. The reaction mixture was quenched with dilute aqueous ammonium
chloride and was partritioned between water ( 100 mL) and a 1 : 1 methylene
chloride
ether solution (200 mL). After one more extraction with methylene chloride the
organic layers were combined, washed with water ( 100 mL) and purification by
flash
chromatographed (eluent 7 : 3 pet ether : ethyl acetate) and HPLC (2 times)
(First
column: Waters Silica Prep Pak; isocratic, 98 : 2 hexane : ethyl acetate; Flow
rate =
225 mL/min; Second column: Waters Silica Prep Pak; gradient, 75 : 25 to 60 :
40
petroleum ether : methylene chloride) to provide the title compound as a white
solid
(0.280 g, 23%); NMR (CDC13); 8 8.35 (d, J = 8 Hz, 1H), 7.88-7.80 (m, SH), 7.66
(ddd, J = 6, 5, 1 Hz, 1H), 7.61 (d, J = 2 Hz, 1H), 7.60 (d, J = 2 Hz 1H) 7.58-
7.56 (m,
2H), 7.51-7.41 (m, 4H), 7.18 (ddd, J = 7, 1, 1 Hz, 1H), 6.83 (dd, J = 7, 1 Hz,
1H) 5.44
(t, J = 8 Hz, 1H), 1.34 (s, 9H); MS (FAB+): [M+], 3 bromine pattern, 814 (30%)
816
(95%) 818 (100%) 820 (42%); Anal. Calc. for C39H29Br303S: C, 57.30; H, 3.58,
N,
0.00. Found: C, 57.55; H, 3.75, N, 0.02.
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 143 -
Example 155.
2-f2.6-Dibromo-4-(6-bromo-benzofblnaphtho[2 3-dlthiophen-11-yl)-phenoxyl 3 y4
fluoro- henyl)=propionic acid
A suspension of 2-[2,6-dibromo-4-(6-bromo-benzo[bJnaphtho[2,3-d]thiophen-
11-yl)-phenoxy]-3-(4-fluoro-phenyl)-propionic acid tert-butyl ester (0.232g
0.295
mmol) in trifluoroacetic acid (5 mL) was heated at 55°C for 11 hours.
After cooling
to room temperature the reaction mixture was poured into water (60mL) and
extracted
with ether. The combined ether extracts were washed with water and brine,
concentrated, and dried in vacuo at 60°C to provide the title compound
as an off white
solid (0.187 g, 98%): NMR (CDC13); b 8.36 (d, J = 7 Hz, 1H), 7.82 (d, J = 7
Hz,
1H), 7.68 (ddd, J = 8, 6, 2 Hz, 1H), 7.58 (dd, J = 8, 2 Hz, 2H), 7.55-7.48 (m,
2H),
7.45-7.35 (m, 3H), 7.15 (dt, J = 7, 1, 1H), 7.03 (tt J = 10, 3, 1, 2H), 6.71
(d, J = 8 Hz,
IH), 5.44 (t, J = 6 Hz, 1H), 3.56 (d, J = 7 Hz, 2H); MS (+ FAB): [M+], 3
bromine
isotope pattern, 726 (15%), 728 ( 30%) 730 (35%) 732 (15%); Anal. Calc. for
C31H18Br3F03S: C, 51.06, H, 2.49, N, 0.00. Found: C, 50.80, H, 2.46, N, 0.10.
Example 156.
2-f2.6-Dibromo-4-(6-bromo-benzorb]naphthof2 3-dlthio~phen-11-3rlZphenox,~
nanhthalen-2v1-propionic acid
A solution of 2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-
l l-yl)-phenoxy]-3-naphthalen-2y1--propionic acid, tert butyl ester (0.221 g,
0.270
mmol) in trifluoroacetic acid (10 mL) was heated to 50°C for 15 hours.
The mixture
was cooled to room temperature and combined with water. Ether was added and
the
resulting organic suspension was separated from the water layer and
concentrated.
The residue was dried in vacuo at 46C to provide the title compound as an off
white
solid (0.186 g, 90%) mp 270-271°C; NMR (DMSO-d6); b 13.25 (broad
singlet, 1H),
8.28 (d, J = 8 Hz, 1 H,), 8.06 (d, J = 8 Hz, I H), 7.91 (m, 4H), 7.8I (d, J =
2 Hz, 1 H),
7.79(d, J = 2 Hz, 1H) 7.82-7.77(m, 1H), 7.63-7.47(m, 6H), 7.25 (dd, J = 7, 1
Hz, ,
1H), 6.64 (d, J = 8 Hz, 1H) 5.42 (t, J = 7 Hz, 1H), 3.59 (d, J = 7 Hz, 2H); MS
(FAB+):
[M+], 3 bromine pattern , 757.8 (21%) 759.8 (100%) 761.8 (100%) 763.8 (50%);
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 144 -
Anal. Calcd. for C31H19Br3O3S: C, 55.07; H,3.04, N, 0.00. Found: C, 55.36; H,
2.89, N, 0.06.
Example 157.
Diethyl Trifluoromethanesulfonoxymethylphosphonate
Prepared according to the procedure of D.P. Phillion and S.S. Andrew Tet.
Lett. 1986, 1477-1480. A solution of trifluoromethanesulfonic anhydride (11.6
mL,
68.9 mmol) in methylene chloride (45 mL) was added dropwise to a stirred, -10
°C
solution of diethyl hydroxymethylphosphonate ( 10.82 g, 64.4 mmol} and
pyridine
(5.76 mL, 68.9 mmol) in methylene chloride (170 mL) under N2 over a period of
20
min. The mixture was stirred at -10 °C for lh. and placed in the
freezer (-15 to -20 °C)
overnight. The reaction mixture was diluted with cold methylene chloride and
washed with cold 1 N HCl and ice water. The organic layer was separated, dried
with
brine, anhydrous MgS04 and concentrated to provide an oil (12.58 g, 65 %): MS
(+FAB): [M+H]+, 423 .
Example 158.
12,6-Dibromo-4-f 6-bromo-benzo f bl naphtho f 2.3-dl thiophen-11-ylZ)-
phenoxymethyl } -
phosphonic acid diethyl ester
A solution of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-
yl)-phenol ( 1.00 g, 1.78 mmol) in THF (5 mL) was added dropwise to a stirred,
0°C
solution of 80 % sodium hydride (0.08 g, 2.67 mmol) in THF ( 10 mL) under a
dry
nitrogen atmosphere over a period of 15 min. After the mixture was stirred in
an 0°C
oil bath for 40 min., a solution of diethyl trifluoromethanesulfonoxymethyl-
phosphonate (0.80 g, 2.67 mmol) was added dropwise over a period of 15 min.
The
reaction mixture was then warmed to, and stirred at, ambient temperature for 1
h. The
reaction mixture was quenched and diluted with water (80 mL). The resulting
solid
was filtered, washed with water and tritrurated with pet. ether. The solid was
dried in
vacuo at 50°C to provide the title compound as a white solid (1.12 g,
88%): mp 178-
180°C: NMR (CDCl3); 8 8.36 (d, J = 9 Hz, 1H), 7.84 (d, J = 8 Hz, 1H),
7.68 (ddd, J
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 145 -
= 7, 6, 1 Hz, 1H), 7.62 (s, 2H), 7.57-7.49 (m, 2H), 7.45 (ddd, J = 8, 7, 1 Hz,
1H), 7.18
(ddd, J = 8, 7, 1 Hz, 1H), 6.73 (d, J = 8, Hz, 1H), 4.61 (s, 2H), 4.41 (q,
4H), 1.49 (t,
6H, CH3); MS (EI): [M+], 3 bromine isotope pattern, 710 (35%), 712 ( 95 %) 714
(100%), 716 (40%); Anal. Calc, for C27H22Br304PS: C, 45.47, H, 3.11, N, 0.00.
Found: C, 45.30, H, 2.95, N, 0.07.
Example 159.
I4-lBenzofblnaphthoj2 3-djthiophen-11 yl)-nhenox~meth 11 nhosphonic acid
diethvl
ester
A solution of 4-(benzo[b)naphtho[2,3-d]thiophen-11-yl-phenol (1.00 g, 3.06
mmol) in THF (5 mL) was added dropwise to a stirred, 0°C solution of 80
% sodium
hydride (0.138 g, 4.95 mmol) in THF ( 10 mL) under a dry nitrogen atmosphere
over a
period of 15 nun. After the mixture was stirred in an 0°C oil bath for
1 h., a solution
of diethyl trifluoromethanesulfonoxymethylphosphonate ( 1.4 g, 4.95 mmol) was
added dropwise over a period of 15 min. The reaction mixture was then warmed
to
and stirred at ambient temperature for 2 h. The reaction mixture was quenched
and
diluted with water ( 150 mL). Aqueous mixture was extracted with ethyl acetate
(2 X
1 SO mL). The ethyl acetate extracts were washed with water, dried with brine
and
anhydrous MgS04, and concentrated to provide a white solid. The solid was
purified
by flash chromatography (eluent 7:3 pet. ether: ethyl acetate) to provide the
title
compound as a white solid (0.922 g, 63 %): mp 129-130°C; MS (EI): [M+],
476
( 100% MI); Anal. Calc. for C27H25O4PS: C, 68.05, H, 5.29, N, 0.00. Found: C,
66.58, H, 5.34, N, 0.00.
Example 160.
3-Phenyl-1-h3rdro-1-prod ly_nho_sphonic acid diethyl ester
Aluminum oxide ( 15 g) was added to a stirred, ambient temperature solution
of hydrocinnamaldehyde ( 3.93 mL, 28.3 mmol) and diethylphosphite (3.72 mL,
28.3
mmol) in methylene chloride (30 mL). The mixture was stirred at ambient
temperature for 2 days. The reaction mixture was filtered and dichloromethane
filtrate
CA 02330623 2000-10-30
WO 99/58521 PCT/t1S99/10185
- 146 -
was concentrated and triturated with petroleum ether to provide the title
compound as
a white solid (4.10 g, 53%): mp 60-61°C: MS (+FAB): [M+H]+, 273 (100%,
MI);
Anal. Calc. for C 13H2104P: C, 57.35, H, 7.77, N, 0.00. Found: C, 57.48, H,
7.87, N,
0.04.
S
Example 161.
~ 1-f2.6-Dibromo-4-(6-bromo-benzofblnaphtho[2 3-dlthiophen-11-yl)-phenoxy]-3-
phen ~Ll-propyl }-phosphonic acid, dieth I ester
Diethylazodicarboxylate (0.420 mL, 2.67 mmol) was added dropwise to a
stirred, ambient temperature suspension of 2,6-dibromo-4-(6-bromo-benzo[b]-
naphtho[2,3-d]thiophen-11-yl)-phenol (1.00 g, 1.78 mmol), 3-phenyl-1-hydro-1-
propylphosphonic acid, diethyl ester (0.730 g, 2.67 mmol), triphenylphosphine
(0.700
g, 2.67 mmol) and benzene (9 mL) under a dry nitrogen atmosphere. The solution
was
heated in an 80°C oil bath for 4 h. Upon cooling to room temperature,
the reaction
mixture was partitioned between water (80 mL) and dichloromethane ( 100 mL).
Dichloromethane phase was washed with 10 % aq HCl ( 80 mL) and silica gel ( 20
mL) was added. Solvent was removed and the silica adsorbate was flash
chromatographed (95:5 Dichloromethane : acetonitrile) to provide the title
compound
as a white solid (1.17 g, 73%): mp 161-162°C: MS (EI+): [M+], 3 bromine
isotope
pattern, 814, 816, 818, 820; Anal. Calc. for C35H30Br304.PS: C, 51.43, H,
3.70, N,
0.00. Found: C, 51.36, H, 3.67, N, 0.03.
Example I62.
I2_.6-Dibromo-4-(6-bromo-benzolblnaphtho(2 3-dlthionhen-11-vl)-nhenox~h
phosphonic acid
Iodotrimethylsilane (0.63 mL, 4.41 mmol) was added dropwise to a stirred,
0°C solution of {2,6-dibromo-4-[6-bromo-benzo[b]naphtho[2,3-d]thiophen-
11-yl)]-
phenoxymethyl }-phosphonic acid diethyl ester ( 1.05 g, 1.47 mmol) in
methylene
chloride (29 mL) under a dry nitrogen atmosphere over a period of 10 min.
After 1 h
at 0°C, the reaction mixture was quenched with water (0.5 mL), stirred
at ambient
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
-I47-
temperature for 30 min., and then diluted with water (SOmL). The solid was
filtered
and partitioned in ethyl acetate ( 100 mL) and 20 % aquous HCl (60 mL) with
stirring
for 3 h. The solid was filtered ,washed with water and triturated with pet.
ether. The
solid was dried in vacuo at 100°C to provide the title compound as an
off white solid
(0.428 g, 44 %): mp 334-335°C: NMR (DMSO-d6); b 8.28 (d, J = 9 Hz, 1H),
8.07 (d,
J = 8, Hz, 1H), 7.84 (s, 2H), 7.79 (ddd, J = 8, 7, 1 Hz, 1H), 7.65-7.57 (m,
2H), 7.52
(dd, J = 7, 1 Hz, 1H), 7.32 (ddd, J = 8, 7, 1 Hz, 1H), 6.67 (d, J = 8 Hz, 1H),
4.32 (d, J
- 1 Hz, 2H); MS (ESI): [M-H]-, 3 bromine isotope pattern; Anal. Calc. for
C23H14Br304PS: C, 42.04, H, 2.15, N, 0.00. Found: C, 41.26, H, 2.12 , N, 0.05;
Karl Fischer: 0.58 mol H20.
Example 163.
L4-(Benzofblnaphthoj2.3-dlthiophen-11 xl)whenoxymethXl_l-uhosphonic acid
Iodotrimethylsilane (0.78 mL, 5.49 mmol) was added to a 0-5 °C,
stirred
solution of [4-(benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxymethyl]-
phosphonic
acid diethyl ester (0.871 g, 1.83 mmol) in methylene chloride (24 mL) under a
try N2
atmosphere over a period of 10 min. After 1 h, the solution was quenched with
water
{0.6 mL), 10 % aqueous sodium carbonate ( 100 mL) was added and mixture was
washed with methylene chloride. The aqueous layer was acidified with 10%
aqueous
HCI. The solid was filtered and recrystallized from methanol (75 mL) to
provide the
title compound as a white solid (0.65 g, 85%): mp 240-242°C: MS (EI):
[M+], 420;
Anal. Calc. for C23H17O4PS: C, 65.17, H, 4.08, N, 0.00. Found: C, 63.95, H,
3.89,
N, 0.41.
Example 164.
( 1-f2.6-Dibromo-4-(6-bromo-benzofblnanhthof2.3-dlthionhen-11-v~-phenoxvl-3-
phenyl-propyl}-,phosphonic acid
Iodotrimethylsilane (0.56 mL, 3.93 mmol) was added dropwise to a stirred,
0°C solution of { 1-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-
d]thiophen-11-yl)
phenoxy]-3-phenyl-propyl }-phosphonic acid, diethyl ester ( 1.07 g, 1.31 mmol)
in
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 148 -
methylene chloride (26 mL) under a dry nitrogen atmosphere over a period of 10
min.
After 1 h at 0°C, the reaction mixture was quenched with 10 % aq sodium
bisulfite ( 1
mL), stirred at ambient temperature for 30 min. The mixture was partitioned in
methylene chloride ( 100 mI,) and water ( 100 mL). The methylene chloride
phase was
washed with 18 % aq HCI ( 100 mL), concentrated, and triturated with pet.
ether to
afford a crude solid (0.894 g). The solid was then recrystalized from 85 % aq.
acetic
acid (50 mL) to provide the title compound as a off white solid (0.466 g,
47%): NMR
(CDC13); 8 8.33 (d, J = 9 Hz, 1H), 7.76 (d, J = 7 Hz, 1H), 7.59 (ddd, J = 8,
7, 1 Hz,
1H), 7.48-7.40 (m, 3H), 7.36-7.28 (m, 2H), 7.06-6.98 (m, SH), 6.91-6.83 (m,
1H),
6.77 (d, J = 8 Hz, 1H), 5.47-5.42(m, 1H), 4.50 (s, 2H), 3.05-2.85 (m, 2H),
2.59-2.41
(m, ZH); MS (FAB-): [M-H]-, 3 bromine isotope pattern, 756; Anal. Calc. for
C31H22Br3O4PS: C, 48.91, H, 2.91, N, 0.00. Found: C, 49.29, H, 2.90, N, -0.02.
Example 165.
2-f2.6-Dibromo-4-(6-bromo-benzofblnaphthof2,3-dlthiophen-11-ylZ,pheno~rl-
acetamide
Methanol ( 10 mL) was purged with ammonia gas for 10 min at 0°C.
[2,6-
dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-acetic acid,
methyl ester (0.50 g, 0.787 mmol) was added and the vessel was sealed, warmed
to
room temperature and stirred for two days. The reaction mixture was
concentrated,
diluted with ether and filtered. The solid was boiled in ethyl acetate (8 mL),
hot
filtered and washed with ethyl acteate and pentane and dried in vacou to
provide the
title compound as an off white solid (0.22 g, 45%): mp 263-265°C: NMR
(DMSO-
d6); 8 8.29 (d, J = 8 Hz, 1H), 8.09 (d, J = 8 Hz, 1H), 7.88 (s, 2H), 7.80
(ddd, J = 8, 7,
1 Hz, 1H), 7.67-?.51 (m, SH), 7.30 (ddd, J = 8, 7, 1 Hz, 1H), 6.71 (d, J = 8
Hz, 1H),
4.56 (s, 2H); MS (EI): [M+], 3 bromine isotope pattern, 617 (30%), 619 ( 90%)
621
(100%) 623 (40%); Anal. Calc. for C24H14Br3N02S: C, 46.48, H, 2.28, N, 2.26.
Found: C, 45.69, H, 2.02 , N, 2.14.
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 149 -
Example 166.
(Rl-2-[2.6-Dibromo-4-f 6-bromo-benzof blnaphthof 2.3-d]thiophen-11-yl)-
phenoxY]-3-
phen ~~1-~ropionamide
Three drops of DMF were added to a stirred, room temperature solution of
(R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-
phenyl-propionic acid (2.09 g, 2.94 mmol), oxalyl chloride (0.31 mL, 3.53
mmol) and
dichloromethane (31 mL) under a dry nitrogen atmosphere. Oxalyl chloride (0.10
mL,
1.14 mmol) was added after 2.5 h and again (0.10 mL, 1.14 mmol) at 6h. After
6.5 h,
the reaction mixture was concentrated and dried in vacuo. The solid was
dissolved in
dichloromethane (25 mL) and added over a 2 minute period to stirred, cold
(0°C)
concentrated ammonium hydroxide (50 mL). The dichloromethane was removed and
the resulting solid was filtered and washed with water and dried in vacuo to
provide
the title Impound as a white solid (1.99 g, 95%): mp 220-222°C: NMR
(DMSO-d6); s
8.28 (d, J = 8 Hz, 1H), 8.07 (d, J = 8 Hz, 1H), 7.80 (ddd, 8, 7, 1 Hz, 1H),
7.75 (d, J =
2 Hz, 1H), 7.74 (d, J = 2 Hz, 1H), 7.67-7.62 (m, 2H), 7.53-7.48 (m, 2H), 7.41-
7.24
(m, 7H), 6.69 (d, J = 8 Hz, 1H), 5.74 (s, 1H, 0.5 eq CH2C12), 5.23 (t, J = 6
Hz, 1H),
3.46-3.27 (m, 2H); MS (+FAB): [M+], 3 bromine isotope pattern, 707 (30%), 709
75%) 711 (100%) 713 (40%); Anal. Calc. for C31H20Br3N02S~0.56CH2C12: C,
50.02, H, 2.82, N, 1.85. Found: C, 49.67, H, 2.67, N, 1.96.
Example 167.
~R)-2-[2.6-Dibromo-4-(6-bromo-benzo f blnaphtho[2.3-dlthiophen-11-3r11-
phenoxyL
N-hydrox«-,-3-phenyl-propionamide
DMF (2 drops) was added to a stirred solution of (R)-2-[2,6-dibromo-4-(6-
bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-phenyl-propionic acid
(0.736 g, 1.03 mmol), oxalyl chloride (0.11 mL, 1.27 mmol) and dichloromethane
( 11
mL). After 1 h, the solvent was removed. The residue was dissolved in
chloroform
(3.5 mL) and added dropwise to a rapidly stirred, 0°C suspension of
hydroxylamine
hydrochloride (0.097 g, 1.34 mmol), sodium carbonate (0.191, 1.65 mmol), water
(3.5
mL) and chloroform (3.5 mL). This stirred suspension was warmed to room
CA 02330623 2000-10-30
WO 99/58521 PCT/tJS99/10185
- 150 -
temperature and stirred overnight. Water was added and the reaction mixture
was
extracted with chloroform. 2% phosphoric acid/methanol treated silica gel was
added
to the chloroform phase and the solvent was removed. The adsorbate was flashed
on
2% phosphoric acid/methanol treated silica gel (gradient: 4:1 to 3:2 petroleum
ether:ethyl acetate) to provide the title compounds as a white solid (0.464 g,
64%):
mp 143-144°C: NMR (DMSO-d6); 8 9.03 (d, J = 2 Hz, 1H), 8.29 (d, J = 8
Hz, 1H),
8.07 (d, J = 8 Hz, 1H), 7.81 (ddd, J = 8, 1,1 Hz, 1H), 7.78 (s, 2H), 7.63
(ddd, J = 8, 7,
1 Hz, 1H), 7.56 (d, J = 8 Hz, 1H), 7.52 (ddd, J = 8, 7, 1 Hz, 1H), 7.36-7.25
(m, 6H),
6.71 (d, J = 8 Hz, 1H), 5.32 (dd, J = 9, 5 Hz, 1H), 3.51-3.39 (m, 2H); MS
(FAB+):
[M+], 3 bromine isotope pattern, 723 (20%), 725 ( 55%) 727 (50%) 729 (30%);
Anal.
Calc. for C31H20NBr3O3S: C, 51.27, H, 2.78, N, 1.93. Found: C, 51.19, H, 2.69,
N,
1.86.
Example 168.
(R~-2-f2.6-Dibromo-4-(6-bromo-benzofblnaphthof2.3-d]thiophen-11-~)-phenox~rl-
N-(3-nitrolo-propyll3-phenyl=propionamide
Dicyclohexylcarbodiirnide (0.605 g, 2.926 mmol) was added to a 0°C
stirred
solution of (R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-
yl)-
phenoxy]-3-phenyl-propionic acid (2.08 g, 2.926 mmol), 2-aminopropionitrile
(0.215
mL, 2.926 mmol), HOBT (0.448 g, 2.926 mmol) in DMF (7.2 m L) undera dry
nitrogen atmosphere. After 16.5 h, the reaction mixture was added to water and
partitioned between water/ethyl acetate and THF. Silica gel was added to the
organic
phase and the solvent was removed. The adsorbate was flashed (7:3,
petroleum:ethyl
acetate) to provide an off-white solid (2.23 g). This solid was dissolved in
dichloromethane and silica gel was added. The solvent was removed and the
adsorbate was flashed (gradient: dichloromethane to 97.5:2.5 dichloro-
methane:acetonitrile) to provide the title compound as a white solid (1.89 g,
85%): mp
194-195°C: NMR (DMSO-d6); 8 8.70 (t, J = 6 Hz, 1 H), 8.28 (ddd, J = 8,
1, 1 Hz,
1H), 8.07 (dd, J = 8, 1 Hz, 1H), 7.80 (ddd, J = 8, 7, 1 Hz, 1H), 7.76 (s, 2H),
7.63 (ddd,
J = 8, 7, 1 Hz, 1H), 7.52 (ddd, J = 8, 7, 1 Hz, 1H), 7.48 (dd, J = 8, 1, 1 Hz,
1H), 7.37-
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
-151-
7.24 (m, 6H), 6.74 (d, J = 8 Hz, 1H), 5.16 (t, J = 6 Hz, 1 H), 3.51-3.26 (m,
2H), 2.62
(t, J = 6 Hz, 1 H); MS (EI): [M+], 3 bromine isotope pattern, 760, 762, 764,
766;
Anal. Calc. for C34H23Br3N2O2S: C, 53.50, H, 3.04, N, 3.67. Found: C, 53.24,
H,
2.86, N, 4.02.
Example 169.
j2,6-Dibromo-4-(6-bromo-benzofblnaphthof2,3-d]t~ hiophen-11-3r1)-phenox~
acetonitrile
Bromoacetonitrile (0.25 mL, 3.56 mmol) was added to a room temperature,
stirred suspension of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-
yl)-phenol ( 1.0 g, 1.78 mmol) and potassium carbonate (0.615 g, 4.45 mmol) in
DMF
(5 mL). After 2.5 h, the reaction mixture was added to water, filtered, washed
with
water and triturated with petroleum ether. The solid was dried in vacuo at
80°C to
provide the title compound as a white solid (1.03 g, 96%a): NMR (DMSO-d6);
88.29
(d, J = 8 Hz, 1H), 8.08 (d, J = 8 Hz, 1H), 7.92 (s, 2H), 7.80 (ddd, J = 8, 7,
1 Hz, 1H),
7.65-7.51 (m, 3H), 7.27 (ddd, J = 8, 7, 1 Hz, 1H), 6.68 (d, J = 8 Hz, 1H),
5.36 (s, 2H);
MS (+FAB): [M+], 3 bromine isotope pattern, 599, 601, 603, 605; Anal. Calc.
for
C24H12Br3NOS: C, 47.87, H, 2.01, N, 2.33. Found: C, 47.83, H, 1.92, N, 2.32.
Example 170.
jR)-2-[2.6-Dibromo-4-(6-bromo-benzofblnaphthof 2.3-dlthiophen-11-ylZphenoxy]-3-
phenyl-propionitrile
Trifluoroacetic anhydride (0.375, 2.65 mmol) was added to a stirred, room
temperature suspension of (R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3
d]thiophen-11-yl)-phenoxy]-3-phenyl-propionamide (1.68 g, 2.37 mmol), pyridine
(0.393 mL, 4.74 mmol) and dioxane (5.4 mL). Dissolution occurred and the
solution
was heated in a 105°C oil bath for 2h. The reaction mixture was cooled
to room
temperature, diluted with ether, washed with 5% HCl and brine..Silica gel was
added
to the ether phase and the solvent was removed. The adsorbate was flash
chromatographed (ether as eluent) to provide the title compound as a white
solid ( 1.38
CA 02330623 2000-10-30
WO 99/58521 PCT/US99I10185
- 152 -
g, 84%): mp 160-165°C: NMR (DMSO-d6): 8 8.29 (d, J = 8 Hz, 1H), 8.07
(d, J = 8
Hz, 1H), 7.93 (d, J = 2 Hz, 1H), 7.91 (d, J = 2 Hz, 1H), 7.80 (ddd, 8, 7, 1
Hz, 1H),
7.65-7.33 (m, 8H), 7.23 (ddd, 8, 7; 1 Hz, 1H), 6.65 (d, J = 8 Hz, 1H), 5.87
(t, J = 7
Hz, 1H), 3.58 (m, 2H); MS (EI): [M+], 3 bromine isotope pattern, 689 (30%),
691
95%) 693 (100%) 695 (40%); Anal. Calc. for C31H18Br3NOS: C, 53.74, H, 2.62, N,
2.02. Found: C, 53.60, H, 2.60, N, 1.83.
Example 171.
S-f 2.6-Dibromo-4-(6-bromo-benzoLblna~phtho j2.3-d] thiophen-11-,
phenox,~xll-1H-tetrazole
Trimethylaluminum (2.55 mL, 3.84mmol, 2.0 M solution in toluene) was
added to [2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-
phenoxy]-
acetonitrile (0.615 g, 1.02 mmol) under a dry nitrogen atmosphere.
Trimethylsilyl
azide (0.510 mL, 3.84 mmol) was then added and the solution was heated in a
80°C
oil bath. After l6.Sh the reaction mixture was cooled to room temperature and
water
was cautiously added.The mixture was partitioned between dilute aqueous HCl
and
ethyl acetate. The layers were separated and the 2% phosphoric acid in
methanol
washed silica gel was added to the organic phase.The solvent was removed and
the
adsorbate was flashed using 2% phosphoric acid in methanol washed silica gel
(eluent: gradient: 4:1 to 7:3 petroleum ether:ethyl acetate) to provide the
title
compound as a white solid, which was recrystallized from cyclohexane (0.365g,
55%): mp 243-245°C: NMR (DMSO-d6); 8 8.29 (d, J = 8 Hz, 1H), 8.09 (d, J
= 8 Hz,
1H), 7.89 (s, 2H), 7.80 (ddd, J = 8, 7, 1 Hz, 1H), 7.66-7.57 {m, 2H), 7.55
(ddd, J = 8,
7, 1 Hz, 1H), 7.40 (ddd, J = 8, 7, 1 Hz, 1H), 6.71 (d, J = 8 Hz, 1H), 5.64 (s,
2H), 1.34
(cyclohexane, 0.3 mole eq); MS (EI): [M+], 3 bromine isotope pattern, 642,
644, 646,
648; Anal. Calc. for C24H13Br3N40S~0.33 C6H12: C, 46.39, H, 2.55, N, 8.32.
Found: C, 46.28, H, 2.43 , N, 7.90.
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 153 -
Example 172.
~Ry-5-~( 1-[2.6-Dibromo-4-(6-bromo-benzofblnaphtho[2.3-dlthiophen-11-yll-
phenoxy]-2=phenyl-ethyl j~-1 H-tetrazole
Trimethylaluminum (7.24 mL, 14.4 mmol, 2.0 M solution in toluene) was
added to (R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl}-
phenoxy]-3-phenyl-propionamide ( 1.33 g, 1.93 mmol) under a dry nitrogen
atmosphere. Trimethylsilyl azide ( 1.92 mL, 14.4 mmol) was then added and the
solution was heated in a 85°C oil bath. After 7h the reaction mixture
was cooled to
room temperature and diluted with ether. Water was cautiously added and after
bubbling subsided, the mixture was partitioned between dilute aqueous HCl and
ether.
The ether phase was concentrated and triturated with petroleum ether, a small
amount
of methanol, a small amount of ether and finally petroleum ether to provide
the title
compound as a white solid (0.802 g, 57%): mp 238-239°C: NMR (DMSO-d6);
8 8.28
(d, J = 8 Hz, 1H}, 8.07 (d, J = 8 Hz, 1H), 7.81-7.74 (m, 3H), 7.62 (ddd, 8, 7,
1 Hz,
1H), 7.57-7.53 (m, 2H), 7.44 (ddd, 8, 7, 1 Hz, 1H), 7.38-7.22 (m, SH), 6.60
(d, J = 8
Hz, 1 H), 5.87 (dd, J = 9, 7 Hz, 1 H), 3.90 (dd, J = 13, 7, 1 H), 3.80 (dd, J
= 13, 8, I H);
MS (-ESI): [M-H], 3 bromine isotope pattern, 731 (60%), 733 ( 90%) 735 ( I00%)
737
(60%); Anal. Calc. for C31H19Br3N40S: C, 50.64, H, 2.61, N, 7.62. Found: C,
49.65, H, 2.434, N, 7.16.
Example 173.
(Rl-6-Bromo-11-f 3.5-dibromo-4-( 1-hydroxymethyl-2-phenyl-ethoxyl-phen3rl]-
benzofb]naphtho[2.3-d,'thiophene
A solution of R)-2-[2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]-
thiophen-11-yl)-phenoxy]-3-phenyl-propionic acid, methyl ester (0.30 g, 0.414
mmol)
in THF (4.2 mL) was added dropwise to a -78 °C, stirred solution of
mixed hydride
(0.435 mL, 0.414 mmol) of lithium aluminum hydride / aluminum chloride ( 1.0 M
solution in THF) in THF (3 mL) under a dry nitrogen atmosphere over a period
of 5
min. After 1 h., the solution was allowed to warm to ambient temperature.
After 2 h.
the reaction mixture was quenched carefully with methanol (3 mL) and further
diluted
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 154 -
with water (80 mL). Aqueous mixture was extracted with ethyl acetate (2 X 80
mL).
The ethyl acetate extracts were washed with water, dried with brine and
anhydrous
MgS04, and concentrated to provide the title compound as a white solid (0.28
g,
97%): mp 83-85: MS (EI): [M+], 3 bromine isotope pattern, 694, 696, 698, 700.
Example 174.
~R)-6-Bromo-11-j3 5-dibromo-4-(1-bromomethyl-2-phenyl-ethoxy)-phenvll-
benzofblnaphtho(_2.3-d]thiophene
Diethylazodicarboxylate (0.151 mL, 0.96 mmol) was added dropwise to a
0°C,
stirred solution of triphenylphosphine (0.257 g, 0.98 mmol) in THF (6 mL)
under a
dry nitrogen atmosphere. After 20 min., lithium bromide was added to a nearly
colorless reation mixture, followed by adding a solution of (R)-6-bromo-11-
[3,5-
dibromo-4-( 1-hydroxymethyl-2-phenyl-ethoxy)-phenyl]-benzo[b]naphtho[2,3-
d]thio-
phene (0.273 g, 0.392 mmol) in THF (3 mL). After 1 h, the solution was allowed
to
warm to ambient temperature. After 2 h, the reaction mixture was quenched with
water (0.2 mL) and further diluted with ethyl ether (50 mL). Silica gel ( 8
mL) was
added. Solvent was removed and the silica adsorbate was flash chromatographed
(eluent 98 : 2 petroleum ether : ethyl acetate) to provide the title compound
as a white
solid (0.23 g, 73%): mp > 90°C (dec.): MS (EI): jM+], 4 bromine isotope
pattern,
756, 758, 760, 762, 764; Anal. Calc. for C28H17Br3N2O3S ~ 0.25 hexane: C,
49.97,
H, 2.97, N, 0.00. Found: C, 49.97, H, 2.83, N, 0.02.
Example 175.
~,j2 6-Dibromo-4-(6-bromo-benzojb]naphthoj2.3-dlthiophen-11-y)-phenox~
thiazolidinedione-2.4-dione
Lithium (bis)trimethylsilylamide ( 1.0 M in THF, 5.32 mL, 5.32 mmol) was
added dropwise over a 20 min period to a -78°C stirred solution of 2,6-
dibromo-4-(6-
bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenol (1.50 g, 2.66 mmol), 5-
bromothiazolidine dione (Zask, et al., J. Med Chem, 1990, 33, 1418-1423, 0.522
g,
2.66 mmol) and THF (20 mL) under a dry nitrogen atmosphere. After 45 min, the
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 155 -
reaction mixture was warmed to room temperature. After 2h, the reaction
mixture
was added to water and acidified with 10% HCl and extracted with ether. Silica
gel
was added to the ether phase and the solvent was removed. The adsorbate was
flashed
(7:3 petroleum ether: ethyl acetate) to provide the title compound a white
solid (0.882
g, 49%): NMR (DMSO-d6); 8 12.75 (s, 1H), 8.30 (dd, J = 8, 1 Hz, 1H), 8.09
(ddd, J =
8, 1, 1 Hz, 1H), 7.92 (s, 2H), 7.81 (ddd, J = 8, 7, 1 Hz, 1H), 7.67-7.59 (m,
2H), 7.54
(ddd, J = 8, 7, 1 Hz, 1H), 7.27 (ddd, J = 8, 7, 1 Hz, 1H), 6.96 (s, 1H), 6.65
(d, J = 8
Hz, 1H); MS (-ESI): [M-H]-, 3 bromine isotope pattern, 674, 676, 678, 680;
Anal.
Calc. for C25H12Br3N03S2: C, 44.27, H, 1.78, N, 2.07. Found: C, 44.20, H, 1.90
,
N, 2.I4.
Example 176.
Phosphoric acid Di-tert-Butvl ester 2.6-Dibromo-4-f6-bromo-benzofblnaphthof2.3-
dlthiophen-1 hyl)-phenyl ester
Tetrazole (0.215 g, 3.0 mmol) was added in one portion to a stirred suspension
of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenol (0.527
g,
1.0 mmol), di-tert-butyl N,N-diethyhlphosporamidate (93%, 0.353 mL, 1.0 mmol)
in
THF at room temperature under a dry nitrogen atmosphere. Dissolution occurred.
After lh, the solution was cooled to -40°C and a suspension occurred. A
solution of
meta-chlorobenzoic aid (80%, 0.26 g, 1.2 mmol) was added slowly so as not to
raise
the temperature. The resultant suspension was slowly warmed to room
temperature
where dissolution occurred. Aqueous 10% sodium bisulfate was added and the
biphasic mixture was stirred for 20 min. The reaction mixture was taken up in
ether
and wasshed with aqeous sodium bisulfate and saturated aqueous sodium
bicarbonate.
The ether phase was concentrated and filtered. The white solid was triturated
with
ether and then flash chromatographed (gradient: dichloromethane to 97.5:2.5
dichloromethane:acetonitrile) to provide the title compound as a white solid
(0.324 g,
43%): NMR (DMSO-d6);: 8 8.25 (d, J = 9 Hz, 1H), 8.06 (d, J = 8 Hz, 1H), 7.86
(s,
2H), 7.75 (ddd, J = 8, 7, 1 Hz, 1H), 7.56 (ddd, J = 8, 7, 1 Hz, 1H), 7.50
(ddd, J = 8, 7,
1 Hz, 1H), 7.44 (d, J = 8 Hz, 1H), 7.14 (ddd, J = 8, 7, 1 Hz, 1H), 6.77 (d, J
= 8 Hz,
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
- 156 -
1H), 1.51 (s, 18H); MS (+FAB): [M+], 3 bromine isotope pattern, 752, 754, 756,
758;
Anal. Calc. for C30H28Br3O4PS: C, 47.71, H, 3.74, N, 0.00. Found: C, 47.87, H,
3.69, N, 0.10.
Example 177.
Phosphoric acid Mono-f2 6-dibromo-4-(6-bromo-benzojb]naphtho[2.3-dlthiophen-
11 girl)-phen,~~ ester
HCl (4N in dioxane, 1.5 mL, 6 mmol) was added to a room temperature,
stirred solution of phosphoric acid di-tert-butyl ester 2,6-dibromo-4-(6-bromo
benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenyl ester (0.290 g, 0.384 mmol) in
dioxane (6 mL). After 4.5 h, the solvent was removed and the solid was
triturated
with ether, petroleum ether and dried in vacuo to provide the title compound
as a
white solid (0.220, 86%): NMR (DMSO-d6); b 8.29 (d, J = 8 Hz, 1H), 8.08 (d, J
= 8
Hz, 1H), 7.83 (s, 2H), 7.80 (ddd, J = 8, 7, 1 Hz, 1H), 7.64 (ddd, J = 8, 7, 1
Hz, 1H),
7.56-7.51 (m, 2H), 7.26 (ddd, J = 8, 7, 1 Hz, 1H), 6.71 (d, J = 8 Hz, 1H); MS
(+FAB):
[M+], 3 bromine isotope pattern, 640, 642, 644, 646; Anal. Calc. for
C22H12Br304PS: C, 41.09, H, 1.88, N, 0.00. Found: C, 41.71, H, 2.22, N, 0.07.
Example 178.
2-[2 6-Dibromo-4-(6-bromo-benzo[b]naphthof2.3-dlthiophen-11-~phenoxyl-2-
methyl-propionic acid
Solid sodium hydroxide (0.682 g, 17.05 mmol) was added in three equal
portions to a 0°C, stirred suspension of 2,6-dibromo-4-(6-bromo-
benzo[b]naphtho-
[2,3-d]thiophen-11-yl)-phenol (0.800 g, 1.421 mmol), 1,1,1-trichloro-2-methyl-
2-
propanol tetrahydrate (1.06 g, 4.263 mmol) in acteone (7.5 mL) over 3 h
period. The
resulting suspension was warmed to room temperature and stirred for 15 h.The
reaction mixture was added to water, acidified with 10% aqueous HCl and
extracted
with ether. To the ether phase was added acid washed (2% phosphoric acid in
methanol) silica gel and the solvent was removed. The adsorbate was flash
chromatographed (2% phosphoric acid in methanol treated silica gel ; eluent:
gradient: 9:1 to 8:2 petroleum ether:ethyl acetate) to provide the title
compound as an
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 157 -
white solid (0.620g, 67%): mp 197-199°C: NMR (DMSO-d6); S 13.00 (broad
s, 1H),
8.29 (d, J = 8 Hz, IH), 8.09 (d, J = 8 Hz, 1H), 7.85 (s, 2H), 7.81 (ddd, J =
7, 6, 1 Hz,
1H), 7.67-7.60 (m, 2H), 7.52 (ddd, J = 8, 7, 1 Hz, 1H), 7.23 (ddd, J = 8, 7, 1
Hz, 1H),
6.60 (d, J = 8 Hz, 1H), 1.70 (s, 6H); MS (-ESI): [M-H], 3 bromine isotope
pattern,
645 (20%), 647 ( 60%) 649 (1000%) 651 (30%); Anal. Calc. for C26H17Br3O3S: C,
48.10, H, 2.64, N, 0.00. Found: C, 47.78, H, 2.76 , N, 0.31.
Example 179.
3-j2_ 6-Dibromo-4-l6-bromo-benzojb)naphthof2.3-dlthiophen-11-vl)-phenoxvl-
p~o_pionic acid
[i-Propiolactone ( 0.186 mL, 2.66 mmol) was added to a stirred, room
temperature solution of 2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-
11-
yl)-phenol ( 1.5 g, 2.66 mmol), potassium tent-butoxide (0.314 g, 2.66 mmol}
in THF
(33 mL) under a dry nitrogen atmosphere. After 2 days the reaction mixture was
added to water, acidified with 10% aqueous HCl and extracted with ethyl
acetate. To
the ethyl acetate phase was added acid washed (2% phosphoric acid in methanol)
silica gel and the solvent was removed. The adsorbate was flash
chromatographed
(2% phosphoric acid in methanol treated silica gel ; eluent: gradient: 9:1 to
8:2
petroleum ether:ethyl acetate) to provide the title compound as an off white
solid
(0.748 g, 44 %): mp 218-220°C: NMR (DMSO-d6);8 12.48 (broad s, 1H),
8.28 (d, J =
8 Hz, 1H), 8.08 (d, J = 8 Hz, 1H), 7.83 (s, 2H), 7.79 (ddd, J = 7, 6, 1 Hz,
1H), 7.64-
7.57 (m, 2H), 7.52 (ddd, J = 8, 7, 1 Hz, 1 H), 7.31 (ddd, J = 8, 7, 1 Hz, 1
H), 6.69 (d, J
= 8 Hz, 1H), 4.44 (t, J = 6 Hz, 2H), 2.89 (t, J = 6 Hz, 2H); MS (+FAB): [M+],
3
bromine isotope pattern, 632 (30%), 634 ( 70%) 636 (80%) 638 (40%}; Anal.
Calc.
for C25H 15Br3O3S: C, 47.27, H, 2.38, N, 0.00. Found: C, 47.68, H, 2.36, N,
0.01.
Example 180.
~~3-[2 6-Dibromo-4-(6-bromo-benzofblnaphthof2.3-d]thiophen-11-yl)-phenoxyl-
butyric acid
Diethylazodicarboxylate (0.420 mL, 2.66 mmol) was added dropwise to a
stirred, ambient temperature suspension of 2,6-dibromo-4-(6-bromo-benzo[b]-
CA 02330623 2000-10-30
wo ~isss2i rc~rn~s99noiss
- 158 -
naphtho[2,3-d]thiophen-11-yl)-phenol ( 0.50 g, 0.89 mmol), methyl-(S)-(+)-3-
hydroxybutyrate (0.30 mL, 2.66 mmol), and triphenylphosphine (0.70 g, 2.66
mmol}
in benzene (6 mL) under a dry nitrogen atmosphere. Dissolution occurred and
the
solution was heated in an 80°C oil bath for 4.0 h. Upon cooling to room
temperature,
the reaction mixture was diluted with dichloromethane and silica gel (15 mL)
was
added. Solvents were removed and the silica adsorbate was flash
chromatographed
(eluent 9 : 1 petroleum ether : ethyl acetate) to provide the methyl ester as
a white
solid (0.345 g, 52 %): mp 143-144°C: 10 % Aqueous hydrochloride (3.0
mL) was
added to a stirred solution of this methyl ester (0.308 g, 0.464 mmol) in 4.0
M
hydrogen chloride / dioxane (6.0 mL) at ambient temperature. The suspension in
a
presure reactor was immersed in an 80°C oil bath for 2.0 h. The
solution was
concentrated and ethyl ether (40 mL) was added to the resulted residue. Silica
gel (3
mL) was added. Solvents were removed and the silica adsorbate was flash
chromatographed (eluent: ethyl acetate) to provide the title compound as a
white solid
(0.143 g, 48 %): mp 175-176°C: NMR (CDC13); 8 8.36 (d, J = 8 Hz, 1H),
7.83 (d, J =
8 Hz, 1H), 7.68 (ddd, 3 = 8, 7, 1 Hz, 1H}, 7.61-7.58 (m, 3H), 7.52 (dd, J = 8
Hz, 1H),
7.43 (ddd, J = 8, 7, 1 Hz, 1H), 7.17 (ddd, J = 8, 7, 1 Hz, 1H), 6.78 (d, J =8
Hz, 1H),
5.29 (q, J = 7 Hz, 1H,), 3.20 (dd, J = 6 Hz, 1H), 2.91 (dd, J = 6 Hz, 1H),
1.62 (d, J = 7
Hz, 3H); MS (EI): M+, 3 bromine isotope pattern, 646, 648, 650, 652; Anal.
Calc, for
C26H17Br303S: C, 48.10, H, 2.64, N, 0.00. Found: C, 48.49, H, 2.63, N, 0.16.
Example 181.
jR)-2-[4-(6-H.Ydrox3r-benzofblnaphthoj2.3-d]thi~hen-11-y~-2.6-dibromo-phenoxy~-
3-phenyl-propionic acid. meth,1
To a cold (-70°C dry ice/isopropanol bath) solution of (R)-2-[4-(6-
methoxy-
benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6-dibromo-phenoxyJ-3-phenyl-propionic
acid, methyl ester ( 1.10 g, 1.63 mmol) in dry methylene chloride ( 11 mL) was
added a
1 M solution of boron tribromide in methylene chloride (5.20 mL, 5.20 mmol,
3.2 eq)
dropwise over a period of 25 minutes under a dry nitrogen atmosphere. After
standing at -55°C overnight the reaction mixture was kept stirring
between -20° and
CA 02330623 2000-10-30
WO 99/58521 PCT/I1S99/10185
- 159 -
-30°C for five hours, then poured into water (50 mL} and the organics
were extracted
with diethyl ether ( 100 mL). The diethyl ether layer was washed with water
and
brine, concentrated, and chased with petroleum ether to the title compound as
a
yellow solid ( 1.10, 100%).
Example 182.
LRl-2-~4-(6-Benzyloxy-benzofblnaphthof2.3-d]thiophen-11-yl]-2.6-dibromo-
phenoxy]-3-phenyl-propionic acid. meth, lr~ester
To a solution of (R)-2-[4-(6-hydroxy-benzo[b]naphtho[2,3-d]thiophen-11-yl]-
2,6-dibromo-phenoxy]-3-phenyl-propionic acid, methyl ester (O.SOg, 0.755 mmol)
in
dry N,N-dimethylformamide (5 mL) was added benzyl bromide (0.27 mL, 2.27
mmol, 3 eq) dropwise at room temperature under a dry nitrogen atmosphere.
After
stirring about 17 hours the reaction was quenched with water (50 mL) and the
organics were extracted with ether. The extracts were washed with water, and
brine
and combined with silica gel. The solvent was removed and the adsorbate was
flash
chromatographed (97/3 petroleum ether/ethyl acetate) and the solvents chased
with
benzene and petroleum ether to provide the title compound as a pale yellow
solid
(0.572 g, 84%): NMR (CDCl3); 8 8.30 (ddd, J =8, 1, 1 Hz, 1H), 7.82 (ddd, J =
8,1,1
Hz, 1H), 7.68 (ddd, J = 8, 1, 1 Hz, 2H), 7.62 (dd, J = 3, 2 Hz, 2H), 7.60-7.55
(m, 2H),
7.51-7.26 (m, lOH), 7.16 (ddd, J = 8, 7,1 Hz, 1H), 6.81 (ddd, J = 8, 1, Hz,
1H), 5.34
(s, 2H), 5.25 (dd, J = 6, 2 Hz, 1H), 3.76 (s, 3H), 3.59 (septet, J = Hz, 2H);
MS (EI):
[M+], 2 bromine isotope pattern, 750 (2%), 752 (3.5%), 754 (2.5%); Anal Calc.
for
C39H28Br204S: C, 62.25, H, 3.75, N, 0.00; Found C, 61.66, H, 3.53, N, 0.25.
Example 183.
11~-2-f2,6-Dibromo-4-(6-methoxvcarbonvlmethoxv-benzofblnanhthof2.3-dlthionhen-
11-yl)-phenox~phenyl~propionic acid methyl ester
To a solution of (R)-2-[2,6-dibromo-4-(6-hydroxy-benzo[b]naphtho[2,3-d]
thiophen-11-yl)-phenoxy]-3-phenyl-propionic acid methyl ester (0.60 g, 0.906
mmol)
in anhydrous N,N-dimethylformamide was added potassuim carbonate (0.376 g,
2.72
mmol, 3 eq) and methylbromoacetate (0.26 mL, 2.72 mmol, 3 eq) at room
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 160 -
temperature under a dry nitrogen atmosphere. After stirring for 24 hours the
reaction
mixture was combined with water (60 mL) the organics were extracted with
diethyl
ether (2x 100 mL). The extracts were combined and washed with water (2x 100
mL)
and brine (100 mL). Silica gel was added and the solvents removed. The
adsorbate
was twice flash chromatographed (eluent 88/12 petroleum ether/ethyl acetate
and
85/15 ethyl ether/ethyl acetate) to provide the title compound as a white
solid (0.493
g, 73%): NMR (CDC13); 8 8.38 (d, J = 8 Hz, 1H), 7.81 (d, J = Hz, 1H), 7.64-
7.59 (m,
3H), 7.55 (d, J = 8 Hz, 1H), 7.48 (ddd, J = 8, 7, 1 Hz, 1H), 7.43-7.26 (m,
6H), 7.16
(ddd, J = 8,7,1 Hz, 1H), 6.79 (d, J = 8 Hz, 1H), 5.25 (dd, J = 8, 6 Hz, 1H),
4.94 (s,
2H), 3.92 (s, 3H), 3.76 (s, 3H), 3.57 (septet, J = 7 Hz, 2H); MS (EI): [M+], 2
bromine
isotope pattern, 732 ( 1.8%), 734 (4%), 736 (0.8%); Anal. CaIc. for
C35H26Br206S:,
57.24, H, 3.57, N, 0.00. Found: C, 57.01, H, 3.42, N, -0.07.
Example 184.
LR7-2-[4-(6-Benz.~y-benzolb]naphthol2.3-dlthiophen-11-yll-2-6-dibromo-
phenoxy_}-3=phenyl-propionic acid
To a solution of (R)-2-[4-(6-benzyloxy-benzo[b]naphtho[2,3-d]thiophen-11-
yl]-2-6-dibromo-phenoxy}-3-phenyl-propionic acid methyl ester (0.484 g, 0.644
mmol) in tetrahydrofuran (9 mL) and methanol (3 mL) was added an aqueous
solution
of potassuim hydroxide ( 1 N, 1.29 mL, 1.29 mmol, 2 eq) dropwise at room
temperature. After stirring 2.5 hours the solvents were removed and the
residue was
partitioned between dilute aqueous hydrochloric acid and ether. This biphasic
system
was shaken vigorously and the layers were separated. The ether layer was
washed
with water and brine and combined with acid treated (2% phosphoric acid in
methanol) silica gel. The ether was removed and the adsorbate was flash
chromatographed (acid treated silica gel, 90/10 petroleum ether/ethyl acetate)
to
provide the title compound as a white solid (0.354 g, 74.5%): [a]25/D = +
24.77°
(10.091 mg/mL, CHC13); NMR (CDCI3); 8 8.29 (d, J = 8 Hz, 1H), 7.80 (d, J = 8
Hz,
1H), 7.68 (d, J =8 Hz, 2H), 7.61 (dd, J = 10, 2 Hz, 2H), 7.59-7.56 (m, 1H),
7.51-7.47
(m, 3H), 7.46-7.27 (m, 8H), 7.15 (ddd, J = 8, 7, 1 Hz, 1H), 6.80 (d, J = Hz,
1H), 5.47
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-161-
(t, J = 7 Hz, 1H), 5.34 (s, 2H), 3.59 (d, J = 7 Hz, 2H); MS (CI): [(M+H)+], 2
bromine
isotope pattern, 737 (6%), 739 (10%), 741 (4%); Anal. Calc. for C38H26Br2O4S:
C,
61.80, H, 3.55, N, 0.00. Found: C, 62.15, H, 3.52, N, 0.07.
Example 185.
(R)-2-[2 6-Dibromo-4-(6-carboxymethox3r-benzofblnaphthof2,3-dlthiophen-11-yll-
phenoxyl-3-phenyl-pronionic acid
To a solution of (R)-2-[2,6-dibromo-4-(6-carboxymethoxy-benzo[b]naphtho
[2,3-d]thiophen-11-yl)-phenoxy]-3-phenyl-propionic acid, methyl ester (0.436
g,
0.594 mmol) in tetrahydrofuran (9 mL) and methanol (3 mL)~ was added an
aqueous
solution of potassuim hydroxide (1N, 2.37 mL, 2.37 mmol, 4 eq) dropwise at
room
temperature. After stirring 3.5 hours the solvents were removed and the
residue was
combined with water. The suspension was acidified with 10% aqueous
hydrochloric
acid and diethyl ether was added. The biphasic mixture was shaken well before
the
layers were separated. The organic phase was washed with water and
concentrated.
The residue was triturated with petroleum ether and dried in vacuo to provide
the title
compound as a white solid (0.366 g, 87%): mp 110-120°C; [a]25/D =
+49.62°
( 10.076 mg/mL, methanol); NMR (DMSO-d6): 813.21 (broad s, 2H), 8.40 (d, J = 8
Hz, 1H), 8.03 (d, J = 8 Hz, 1H), 7.74 (dd, J = 2, 8 Hz, 2H), 7.68 (ddd, J = 8,
7, 1 Hz,
1H), 7.57 (ddd, J = 8, 7, 1 Hz, 1H), 7.52 - 7.45 (m, 2H), 7.43 - 7.38 (m, 2H),
7.37 -
7.11 (m, 2H), 7.10 - 7.20 (m, 2H), 6.69 (d, J = 8 Hz, 1H), 5.30 (t, J = 7 Hz,
1H), 4.90
(s, 2H), 3.41 (d, J = 7 Hz, 2H); MS (+FAB): [M+], 2 bromine isotope pattern,
704
(9%), 706 (22%), 708 (22%); Anal. Calc. for C33212Br2O6S: C, 56.11, H, 3.14,
N,
0.00. Found: C, 55.93, H, 3.28, N, 0.09.
Example 186.
2.6-Dibromo- 4-(6-bromo-5,5-dioxo-SH-6-benzofbLna~of2,3-d]thiophen-11-
phenoxyl-acetic acid
To a stirred suspension of [2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3
d]thiophen-11-yl)-phenoxy]-acetic acid (0.050 g, 0.0805 mmol) in glacial
acetic acid
(2 mL) was added a 30% aqueous solution of hydrogen peroxide (0.082 mL, 0.805
CA 02330623 2000-10-30
WO 99/58521 PCT/1IS99/10185
- 162 -
mmol, 10 eq) dropwise at room temperature. The suspension was heated at 105-
107°C for 2.5 hours. The reaction mixture was cooled to room
temperature and the
white solid was filtered, and washed with petroleum ether to give the title
compound
(0.042 g, 79%): mp 281-282.5°C; NMR (DMSO-d6): b 8.42 (d, J = 8Hz, 1H),
7.6b-
7.61 (m, 2H), 7.50 (d, J = 8Hz, 1H), 6.51-6.48 (m, 1H), 4.75 (s, 2H), 1.90 (s,
3H);
MS (EI): M+], 3 bromine isotope pattern, 650 (32%), 652 (95%), 654 (100%), 656
(38%); Anal. Calc. for C24H13Br3O5S ~ CH3COOH: C, 43.79, H, 2.40, N, 0.00.
Found: C, 43.66, H, 2.26, N, 0.08.
Example 187.
j2.6-Dibromo-4-f 6-bromo-5-oxo-5H-4-benzojblnaphthof 2.3-dlthionhen-11-ylll-
phenoxy]-acetic acid
To a stirred suspension of [2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3
d]thiophen-11-yl)]-phenoxy]-acetic acid (0.450 g, 0.725 mmol) in glacial
acetic acid
(4 mL) was added a 30% aqueous solution of hydrogen peroxide (0.75 mL, 7.25
mmol, 10 eq) dropwise at room temperature. The suspension was heated to
105°C
and when dissolution did not occur an additional 15 mL of acetic acid was
added.
Dissolution occurred rapidly and the solution was heated at 105°C for
5.5 hours. On
cooling to room temperature a yellow solid precipitated. The solid was removed
and
combined with the diethyl ether extracts taken from the diluted filtrate. The
solid did
not completely dissolve in the diethyl ether nor when ethyl acetate was added
and was
removed by filtration. The solid was recrystallized from acetic acid with hot
filtration
to the title compound as a yellow solid (0.055g, 12%): mp 287-289°C;
NMR (DMSO-
d6); 8 13.3 (broad band, 1H), 8.39 (d, J = 8, 1H), 8.14 (ddd, J = 8, 1, 1 Hz,
1H), 7.91
(d, J = 2 Hz, 1H), 7.84 (ddd, J = 8, 7, 1 Hz, 1H), 7.77-7.72 (m, 2H), 7.60-
7.50 (m,
3H), 6.45 (ddd, J = 8, 1, 1 Hz, 1H), 4.75 (s, 2H); MS (EI): [M+], 3 bromine
isotope
pattern, 634 (25%a), 636 (70%), 638 (75%), 640 (30%); Anal. calc. for
C24H13Br3O4S: C, 45.24, H, 2.06, N, 0.00. Found: C, 44.89, H, 1.76, N, 0.06.
CA 02330623 2000-10-30
WO 99/58521 PCT/l7S99/10185
- 163 -
Example 188.
(R)-2-[2.6-Dibromo-4-(6-bromo-5.5-dioxo-5H-50,61-benzofblnaphthoj2,3-dl-
thiophen-11-ylZphenoxyl-3-phenyl_-propionic acid. methyl ester
A stirred suspension of (R)-2-[2,6-Dibromo-4-(6-bromo-benzo[b]naphtho(2,3-
d]thiophen-11-yl)-phenoxy]-3-phenyl-propionic acid, methyl ester (0.94g, 1.30
mmol) in glacial acetic acid (38 mL) and a 30% solution of hydrogen peroxide (
1.5
mL, 13 mmol) was heated at 100 - 110°C (dissolution occurred) for 5.5
hours and
then remained at ambient temperature overnight. The solvents were removed. The
solid residue was dissolved in methylene chloride and silica gel was added.
The
solvent was removed and the adsorbate was flash chromatographed (80 : 20
Petroleum ether : ethyl acetate) to the title compound as a yellow solid
0.937g, 95%):
mp 156-157°C: [a]D25 = +47.92° (10.017 mg/mL CHCl3); NMR
(CDC13); S 8.48 (d,
J = 7 Hz, 1 H), 7.89 (d, J = 6 Hz, 1H), 7.74 (ddd, J = 8, 7, 1 Hz, 1 H,), 7.64
(ddd, J =
8, 7, 1 Hz, 1H), 7.54 (dd,J = 7, 4, Hz, 2H), 7.50 - 7.27 (m, 8 H), 6.44 (dd, J
= 7, 1
Hz), 5.27 (dd, J = 6, 8 Hz, 1 H), 3.75 (s, 3H), 3.56 (m, 2H); MS (+FAB):
[M+H]+, 3
Bromine pattern, 755 (8%), 757 (20%), 759 (30%), 761 ( 10%}; Anal. Calcd. for
C32H21Br305S: C, 50.75, H, 2.80, N, 0.00. Found: C, 50.75, H, 2.61, N, -0.04.
Example 189.
~R)-2-[2.6-Dibromo-4-(6-bromo-5.5-dioxo-5H-~~,6)-benzo[blnaphthoj2.3-d~thio-
phen-11-yl)~henox~-3-nhenYl_-propionic acid
Aqueous potassium hydroxide ( 1 N, 2.22 mL, ~ 2.22 mmol) was added to a
stirred solution of (R)-2-[2,6-dibromo-4-(6-bromo-5,5-dioxo-5H-5(x,6)-benzo[b]-
naphtho[2,3-d]thiophen-11-yl)-phenoxy]-3-phenyl-propionic acid, methyl ester
(0.832
g, 1.11 mmol) in tetrahydrofuran (7.5 mL) / methanol (5.5 mL,). After lh the
solution
was concentrated, diluted with water and acidified with 10% aqueous HCI. The
organics were extracted with ether. The ether was removed and the residue was
dried
in vacuo overnight at 53C to give the title compound as a white solid (0.534
g,
65%): mp 173-193°C: [a]D25=+42.73°; NMR (CDCI3); 8 8.47 (dd, J =
8, 1 Hz, 1H),
7.76 (dd, J = 8, 1 Hz, 1 H), 7.74 (ddd, J = 7, 3, 1 Hz, 1 H,), 7.64 (ddd, J =
7, 3, 1 Hz,
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 164 -
1H), 7.55 - 7.46 (m, 3H,), 7.41 - 7.27 (m, 7H), 6.42 (d, J = 8 Hz, 1H), 5.44
(t, J = 7
Hz, 1H), 3.57 (d, J = 7 Hz, 2H); MS (EI): [M+] 3 bromine pattern 740 (2%), 742
(8%), 744 (6% ), 746 (2%); Anal. Calc. for: C3IH19Br3O5S: C, 50.09, H, 2.58, N
0.00. Found: C, 50.18, H, 2.71, N, 0.00.
Example 190.
5'-Benzo[blnaphthof2,3-d~thiophen-11-,~~1-[1.1':3',1"lterphen,1-r~2'-of
A solution of 4-(benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6-diiodo-phenol
( I .26 g, 2.18 mmol), phenylboronic acid (0.584 g, 4.80 mmol), barium
hydroxide
octahydrate (2.75 g, 8.72 mmol), palladium acetate ( 10 mg, 0.087 mmol) and 6:
I
dimethoxyethane:water (49 ml) was heated to 80°C overnight. An
additional amount
of phenylboronic acid (0.29 g, 2.40 mmol) and a catalytic amount of palladium
acetate were added, and the solution was heated for four additional hours. The
reaction mixture was acidified to pH 1 with conc. HCI, diluted with ethyl
acetate and
washed with water. The solvents was removed and the crude product was flash
chromatographed (99:1 ethyl acetate: pet. ether) to provide the title compound
as a
white solid (0.948 g, 91%): NMR (DMSO-d6); 8 8.76 (s, 1H), 8.61 (s, 1H), 8.05
(d, J
= 8 Hz, 1H), 7.99 (d, J = 8 Hz, 1H), 7.77 (d, J = 9 Hz, 1H), 7.63-7.30 (m,
13H), 7.25
(s, 2H), 7.22 (ddd, J = 8, 8, 1 Hz, 1H), 7.08 (d, J = 9 Hz, IH); MS (EI): 492
(100%,
MI); Anal. Calc. for C34H220S~1.6H20: C, 80.48, H, 5.01, N, 0.00. Found: C,
80.26, H, 4.63, N, 0.05.
Example 191.
S'-Benzofb]naphtho[2.3-dlthiophen-1 I 3r1)-f 1.1':3'.1 "]terphenyl-2'-yloxYl-
acetic acid
5'-Benzo[b]naphtho[2,3-d]thiophen-11-yl-[1,1';3',1"]terphenyl-2'-0l (0.145
g, 0.30 mmol), methyl bromoacetate (0.057 mL, 0.61 mmol), potassium carbonate
(0.046 g, 0.33 mmol) and N,N-dimethylformamide (5 mL) were combined and
stirred
at ambient temperature for 3 day. The reaction mixture was added to water and
extracted with ethyl acetate. The ethyl acetate layer was washed with aqueous
1N Hcl,
sat. sodium bicarbaonte and dried *(magnesium sulfate). The ethyl acetate was
removed and and the crude product was flash chromatographed (95:5 ethyl
acetate:
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 165 -
pet. ether) to provide (S'-benzo[b]naphtho[2,3-d]thiophen-11-yl)-[1,1';3',1"]-
terphenyl-2'-yloxy)-acetic acid, methyl ester as a white solid (0.177 g).
Aqueous
potassium hydroxide ( 1 N, 1.61 mL, 1.61 mmol} was added to a stirred solution
of
this methyl ester in 3:2 THF:methanol (5.0 mL) at ambient temperature. After 2
h the
solution was concentrated, diluted with water (75 mL) and acidified with 10%
aqueous HCI. The solid was filtered and washed with water to provide the title
compound as a white solid (0.119 g, 69%): mp >132°C (dec): NMR (DMSO-
d6); 8
12.60 (s, 1H), 8.63 (s, 1H), 8.07 (d, J = 8 Hz, 1H), 7.99 (d, J = 8 Hz, 1H),
7.76 (d, J =
8 Hz, 1H), 7.67-7.41 (m, 11H), 7.40-7.33 (m, 2H), 7.24 (ddd, J = 8, 8, 1 Hz,
1H), 6.91
(d, J = 9 Hz, 1H); MS (EI): 536 (100%, MI); Anal. Calc. for C36H24O3S~O.SH20:
C,
79.24, H, 4.62, N, 0.00. Found: C, 78.80, H, 4.57, N, 0.09.
Example 192.
3-Bromo-5-(6-bromo-benzo[b]naphthof2.3-dlthiophen-11-yl)-2-benzyloxy-phenol
A suspension of 3-bromo-5-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-
benzene-1,2-diol (0.390 g, 0.78 mmol) and potassium carbonate (0.108 g, 0.78
mmol)
in DMF (4 mL) was stirred at 0 °C under a dry N2 atmosphere for 20 min.
Benzyl
bromide (0.093 mL, 0.78 mmol) was added dropwise to this mixture over a period
of
ten minutes. After the mixture was stirred at 0 °C for 6.5 h., the
reaction mixture was
quenched with aqueous hydrochloric acid to pH 1 and further diluted with water
(60
mL) and aqueous mixture was extracted with methylene chloride (2 X 60 mL). The
combined organic extracts were washed with water and dried with brine. Silica
gel (5
mL) was added. Solvent was removed and the adsorbate was flash chromatographed
(eluents: petroleum ether : methyiene chloride 6:4 to petroleum ether : ethyl
acetate
6:4) to provide a mixture (271 mg, 59%) of the title compound (87%) and 2-
bromo-4-
(b-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-6-benzyloxy-phenol (13%). The
mixture was used for next reaction without separation: NMR (CDCl3): 8 8.35 (d,
J =
8 Hz, 1H), 7.79 (d, J = 8 Hz, 1H), 7.72-7.33 (m, 9 H), 7.18 (d, J = 2 Hz, 1H),
7.17
(ddd, J = 8, 7, 1 Hz, 1 H), 6.93 (d, J = 2 Hz, 1 H), 6.87 (d, J = 8 Hz, 1 H),
5.71 (s, 1 H),
5.32 (t, J = 7 Hz, 2H).
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 166 -
Example 193.
2-Bromo-4-(6-bromo-benzolb]naphtho[2,3-dlthiophen-I1-yll-6-methoxy- h"n e~ol
Iodomethane (0.086 mL, 1.38 mmol) was added dropwise to a room
temperature, stirred light suspension of a mixture [3-bromo-5-(6-bromo-
benzo[b]-
naphtho[2,3-d]thiophen-ll-yl)-2-benzyloxy-phenol (87% pure, contaminated with
2-bromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-6-benzyloxy-phenol
(13%), 0.271 g, 0.46 mmol), potassium carbonate (0.191 g, 1.38 mmol} in DMF (2
mL) over a period of twenty minutes. After the mixture was stirred at ambient
temperature for 3 h., the reaction mixture was quenched with aqueous
hydrochloride
to pH 1 and further diluted with water (40 mL) and aqueous mixture was
extracted
with methylene chloride (80 mL). The organic extract was washed with water,
dried
with brine and anhydrous MgS04, and concentrated to provide a mixture (279 mg,
100%) of 11-(3-methoxy-4-benzyloxy-5-bromo-phenyl)-6-bromo-benzo[b]naphtho-
[2,3-d]thiophene (87%) and I1-(4-methoxy-3-benzyloxy-5-bromo-phenyl)-6-bromo-
benzo[b)naphtho[2,3-d)thiophene(13%). This mixture was used for next reaction
without separation. A solution of a this mixture (279 mg, 0.49 mmol) and 10%
palladium on carbon (28 mg) in ethyl acetate: ethanol ( 1.5:10, 15 mL) was
hydrogenated in a Parr vessel at 51 psi at ambient temperature for 6 h. The
reaction
mixture was filtered through a bed of Solka Floc and washed with hot ethyl
acetate:
ethanol ( 1.5:10). Silica gel (5 mL) was added to the filtrate. Solvent was
removed and
the adsorbate was flash chromatographed (eluents: petroleum ether : methylene
chloride 6:4 to petroleum ether : ethyl acetate 7:3) to provide a white solid
( 145 mg)
that contained about 73% 2-bromo-4-(benzo[b]naphtho[2,3-d]thiophen-I1-yl)-6-
methoxy-phenol and 27% of the title compound. The bromine was re-introduced to
the 6 position of the majority (73 %) of the crude according to the procedures
outlined by methods in Examples 34 (acetylation of the phenol), Example 37
(bromination in the 6 position) and Example 41 (saponification of the acetyl
moiety)
to provide the title compound as a white solid: mp 216-218°C; NMR
(CDCl3); 8 8.36
(d, J = 8 Hz, 1H), 7.83 (d, J = 8 Hz, IH), 7.68-7.64 (m, 2H), 7.50-7.43 (m,
2H), 7.21
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
- 167 -
(d, J = 2 Hz, 1H), 7.18 (d, J = 8 Hz, 1H), 6.93 (d, J = 8 Hz, 1H), 6.87 (d, J
= 8 Hz,
1H), 6.20 (s, 1H), 3.86 (s, 3H); MS (+FAB): [M+], 2 bromine isotope pattern;
512.
Example 194.
(R)-2-f2-Bromo-4-(6-bromo-benzolblnaphthof2.3-dlthiophen-11-yl)-6-methoxy-
phenoxyl-3-phenyl-pro~ionic acid
Prepared from 2-bromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-
6-methoxy-phenol (Example 193) and (S)-2-hydroxy-3-phenylpropionic acid,
methyl
ester (Example 96) according to the procedure in Example 113. White solid: mp
>103°C (dec.): NMR (CDC13); 8 8.36 (ddd, J = 8, 1, 1 Hz, 1H), 7.83
(ddd, J = 8, 7, 1
Hz, IH), 7.67 (ddd, J = 8, 7, 1 Hz, 1H), 7.57-7.26 (m, 9H), 7.18 (ddd, J = 8,
7, 1 Hz,
1H), 6.89 (ddd, J = 8, 1, 1 Hz, 1H), 6.79 (ddd, J = 8, 7, 1 Hz, 1H), 5.29 (t,
1H), 3.76,
3.74 (ds, 3H}, 3.39-3.46 (m, 2H); MS (EI): [M+], 2 bromine isotope pattern,
660;
Anal. Calc. for C32H22Br204S: C, 58.02, H, 3.35, N, 0.00. Found: C, 58.04, H,
3.73,
N, 0.02.
Example 195.
3-B romo-5-(6-bromo-benzo~blnaphtho f 2.3-d] thiophen-11-vl)-2-methoxy-phenol
Iodomethane (0.074 mL, 1.2 mmol) was added dropwise to a rt, stirred light
suspension of 3-bromo-5-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-benzene-
1,2-diol (0.30 g, 0.60 mmol), potassium carbonate (0.083 g, 0.6 mmol) in DMF
(1.5
mL) over a period of five minutes. After the mixture was stirred at ambient
temperature for 1.5 h., the reaction mixture was quenched with aqueous
hydrochloric
acid to pH 1 and further diluted with water (80 mL) and aqueous mixture was
extracted with methylene chloride ( 120 mL). The organic extract was washed
with
water and dried with brine. Silica gel (5 mL) was added. Solvent was removed
and the
adsorbate was flash chromatographed (eluents: petroleum ether : methylene
chloride
7:3 to 1:1 and then petroleum ether : ethyl acetate 7:3) to provide the title
compound
as a white solid (85 mg, 29°l0): mp 233-234°C: NMR (CDC13); 8
8.35 (ddd, J = 8, 1, 1
Hz, 1H), 7.82 (ddd, J = 8, 1, 1 Hz, 1H), 7.66 (ddd, J = 8, 7, 1 Hz, 1H), 7.65
(dd, J = 8,
1, Hz, 1H), 7.48 (ddd, J = 8, 7, 1 Hz, 1H), 7.43 (ddd, J = 8, 7, 1 Hz, 1H),
7.17 (ddd, J
CA 02330623 2000-10-30
WO 99/58521 PCT/US99/10185
-168-
= 8, 7, 1 Hz, 1H), 7.15 (d, J = 2 Hz, 1H), 7.00 (d, J = 2 Hz, 1H), 6.87 (ddd,
J = 8, l, 1
Hz, 1H), 5.93 (s, 1H), 4.14 (s, 3); MS (EI): [M+], 2 bromine isotope pattern,
512;
Anal. Calc. for C23H14Br202S: C, 53.72, H, 2.74, N, 0.00. Found: C, 53.85, H,
2.98,
N, 0.02.
Example 196.
SR)-2-f 3-Bromo-5-(6-bromo-benzo[~lnaphthof 2.3-dlthiophen-11-yl)-2-methoxy-
phenoxyl-3-phenXl-nropionic acid
Prepared from 3-Bromo-5-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-
2-methoxy-phenol (Example 195) and (S}-2-hydroxy-3-phenylpropionic acid,
methyl
ester (Example 96) according to the procedure in Example 113. White solid: mp
>99°C (dec.): NMR (CDCl3); 8 8.34 (ddd, J = 8, 1, 1 Hz, 1H), 7.79 (ddd,
J = 8, 7, 1
Hz, 1H), 7.64 (ddd, J = 8, 7, 1 Hz, 1H), 7.52(dd, J = 8, 1, Hz, 1H), 7.45
(ddd, J = 8, 7,
1 Hz, 1H), 7.39 (ddd, J = 8, 7, 1 Hz, 1H), 7.28-7.07 (m, 7H), 6.67 (ddd, J =
8, 7, 1 Hz,
1H), 6.63 (d, J = 2 Hz, 1H), 4.91-4.84 (m, 1H), 3.33-3.20 (m, 2H), 3.95, 3.89
(ds,
3H); MS (+FAB): [M+HJ+, 2 bromine isotope pattern, 660, 662, 664; Anal. Calc.
for
C32H22Br204S: C, 58.02, H, 3.35, N, 0.00. Found: C, 58.37, H, 3.63, N, 0.03.
Example 197.
2,4-Difluoro-3-methox,~-benzoic acid
A solution of 5-bromo-1,3-difluoro-2-methoxy-benzene (12.358, 55.4 mmole,
L. I. Kruse, et al., Biochemistry 1986, 25, 7271-7278) in anhydrous
tetrahydrofuran
(350 mL) was transferred via canulla into a 1L flask which had been flame
dried. The
solution was cooled to -80°C and a solution of n-butyl lithium (2.5 M
in hexanes,
24.35 mL, 60.9 mmol) was added dropwise via syringe pump over a 1 hour period
with stirring under a dry nitrogen atmosphere. After stirring 2.5 hours, dry
carbon
dioxide gas was bubbled into the cold reaction mixture for 0.5 hour. The
solution was
then poured onto crushed dry ice and strirred for 0.5 hours. The mixture was
causiously added to water (600mL) and acidified with 10% HCI. The organics
were
extracted with ether. The extracts were combined and washed with brine. After
concentrating and standing at room temperature for two days the residue was
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 169 -
redissolved in ether and combined with acid treated (2% H3P04 in methanol)
silica
gel. The solvent was removed and the adsorbate was flash chromatographed to
give
the title compound as an off white solid: mp 191-192°C: NMR (400MHz,
DMSO-
d6); 8 13.37 (broad singlet, 1H}, 7.63-7.58 (m, 1H,), 7.25 - 7.20 (m, 1H),
3.92(s, 3H);
MS (+ FAB): (M + H): 189 (12%), 154 (100%), 136 (75%); Anal. Calc. for
C8H6F203: C, 51.08; H, 3.21. Found: C, 50.98, H, 3.15.
Concentration of less polar fractions gave 6-bromo-2,4-difluoro-3-methoxy-
benzoic
acid (3.44g, 23%) as a white solid: mp 92-94°C: NMR (400MHz, DMSO-d6);
8 14.18
(broad singlet, 1H), 7.65 (dd, J = 3, 1 Hz, 1H), 3.94 (s, 3H}; MS: (+FAB) (M +
H:
267/269 (38%), 9I (100%); Anal. calc. for CBHSBrF2O3: C, 35.98, H, 1.89, N,
0.00.
Found: C, 36.26, H, 1.79, N 0.03.
Example 198
(2-Benzyl-benzofblthio~,hen-3-ylZ~2-4-difluoro-3-methox -~nhenyll-methanone
To a suspension of 2,4-difluoro-3-methoxy-benzoic acid (3.SSg., 18.9 mmol)
and N,N-dimethylformamide (3 drops) in anhydrous methylene chloride (70 mL)
was
added oxalyl chloride (2.80 mL, 32.1 mmol) dropwise under a dry nitrogen
atmosphere. After stirring 3 hours additional oxalyl chloride ( 1.6 mL, 16.1
mmol) was
added. After stirring another hour the solvents and excess oxalyl chloride
were
removed to give a semi-solid residue which was used in the following reaction.
To a thick suspension of 2 benzylbenzo(b]thiophene (3.85g, 17.2 mmole) and
the above acid chloride (3.90g, 18.9 mmole) in methylene chloride (56 mL)
cooled to
-80°C was added tin IV chloride (4.43 mL, 37.8 mmol} dropwise over a
period of 55
minutes under a dry nitrogen atmosphere. After stirring for an additional hour
the ice-
bath was removed. Dissolution occurred as the solution warmed to room
temperature.
After stirring ca. 15 hours the reaction mixture was added to water (200 mL)
and the
organics were extracted with ether. The extracts were combined, washed with
brine
and combined with silica gel. The solvents were removed and the adsorbate was
flash
chromatographed (gradient 100% petroleum ether to 97 / 3 petroleum ether /
ethyl
acetate) to give the title compound as a yellow oil (2.19g, 34% yield): NMR
(400
CA 02330623 2000-10-30
WO 99/58521 PCT/tlS99/10I85
- 170 -
MHz, DMSO-d6): b 7.97-7.94 (m, 1H}, 7.77-7.20 (m, lOH), 4.33 (s, 2H), 4.24 (s,
3H);~MS {EI) (M+): 394 ( 100%).
Example 199.
3-(Benzo[blnaphtho[2.3-d~thiophen-11-yl)-2.6 difluoro-phenol
To a cold (-78°C) solution of (2-benzyl-benzo[b]thiophen-3-yl)-(2-4-
difluoro-
3-methoxy-phenyl)-methanone (2.07g, 5.28 mmole) in anhydrous methylene
chloride
(20 mL) was added boron tribromide ( 1.60 mL, 16.9 mmol) dropwise via syringe
pump over a period of 43 minutes under a dry nitrogen atmosphere. After
stirring an
additional 14 minutes the ice bath was removed and the reaction was allowed to
stir at
room temperature for about 4 hours. The dark red mixture was cooled in an ice
bath
and causiously quenched with water and the organics were extracted with ether.
The
extract was washed with brine and concentrated to give the crude product as a
yellow
foam (2.2 g). The solid was redissolved in a mixture of ether,
tetrahydrofuran, and
methylene chloride and combined with silica gel (60 mL). The solvents were
removed
and the adsorbate was flash chromatographed (90/10 petroleum ether / ethyl
acetate)
to give the title compound as a white solid (1.4g, 74%): NMR (300 MHz, DMSO-
d6);
8 10.57 (s, 1H), 8.69 (s, 1H), 8.09 (d, J = 8 Hz, 1H), 8.02 (d, J = 8 Hz, 1H},
7.64-7.23
(m, 6H), 6.94-6.83 (m, 2H).
Example 200.
~6-Bromo-benzofblnaphtho[2.3-dlthiophen-11-yll-2.6 difluoro-phenol
To a cold (-23°C dry ice / carbon tetrachloride bath) stirred
solution of 3
(benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6 difluoro-phenol (1.38 g, 3.81 mmol)
in
methylene chloride (35 mL) was added a solution of bromine (0.22 mL, 4.19
mmol)
in methylene chloride (7 mL) dropwise, very slowly, over a period of 28
minutes.
After stirring an additional 1.5 h the reaction was quenched with dilute
sodium
bisulfite and the organics were extracted with ether. The extract was
concentrated to
give a yellow solid ( 1.64 g, 98% crude yield). A small portion was taken up
in
methylene chloride and combined with silica gel. The solvent was removed and
the
adsorbate was flash chromatographed (85/15 petroleum ether / ethyl acetate) to
give
CA 02330623 2000-10-30
WO 99/58521 PCTlI1S99/10185
-171-
the title compound as an off white solid: mp 180-182°C; NMR (400 MHz,
DMSO-
d6); 8 10.64 (s, 1H), 8.30 (d, J = 9 Hz, 1H), 8.09 (d, J = 9 Hz, IH), 7.82-
7.78 (m, 1H),
7.64-7.51 (m, 3H), 7.42-7.37 (m, 1H), 7.33-7.29 (m, 1H), 6.96-6.91 (m, 1H),
6.81-
6.78 (m, 1H); MS (FAB): (M-H): one bromine pattern observed; 439 / 441 (8%);
Anal. Calc. for C22H11BrF20S: C, 59.88, H, 2.51, N, 0.00%. Found: C, 59.82, H,
2.59, N, 0.06.
Example 201.
[~6-Bromo-benzofblnaphtho[2.3-dlthiophen-1 I-3r1)-2.6-difluoro-~henoxvl-acetic
acid
To a suspension 3-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6
difluoro-phenol (0.200 g, 0.453 mmol) and potassium carbonate (0.085, 0.612
mmol)
in N,N-dimethylformamide (2 mL) was added methyl bromoacetate (0.086 mL, 0.906
mmol) dropwise at room temperature under a dry nitrogen atmosphere. After
stirring
2.5 hours the reaction mixture was combined with water (SO mL) and the
organics
were extracted with ether. The extract was combined with silica gel, the
solvent was
removed and the adsorbate was flash chromatographed (90/IO petroleum ether /
ethyl
acetate). The solvents were chased with benzene (2x) and petroleum ether to
give [3-
(6-bromo-benzo[b]naphtho[2, 3-d]thiophen-11-yl)-2, 6-difluoro-phenoxy]-acetic
acid,
methyl ester as a white solid (0.198 g, 85%). To a solution of this methyl
ester (0.190
g, 0.370 mmol) in tetrahydrofuran (3 mL) and methanol (I mL) was added a IN
aqueous solution of potassium hydroxide (0.55 mL, 0.55 mmol) dropwise at room
temperature. After stirring 1 hour the solvents were removed and water was
added to
the solid residue. The aqueous mixture was acidified with 10% HCl and the
organics
were extracted with ether. After several minutes of vigorous shaking the
layers were
separated and the organic layer was washed with water and concentrated. The
residue
was chased with benzene and dried in vacuo to give the title compound as a
white
solid (0.177g, 95%}: mp 195-I97°C: NMR (400 MHz, DMSO-d6): 8 13.11
(broad s,
IH), 8.31 (d, J = 8 Hz, 1H), 8.09 (d, J = 8 Hz, 1H), 7.83-7.79 (m, IH), 7.64-
7.46 (m,
4H), 7.31-7.27 (m, IH), 7.23-7.17 (m, 1H), 6.81 (d, J = 8 Hz, 1H), 4.89 (s,
2H); MS(-
FAB): (M-H): one bromine pattern observed: 497/499 (35%/38%); HRMS: Calc. for
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 172 -
C24H13BrF2O3S M+: 497.97368, measured mass: 497.97787, mass deviation 4.19
mmu; Anal. HPLC 97% pure; Anal. Calc. for C24H13BrF2O3S: C, 57.72, H, 2.62%
N, 0.00. Found: C, 56.77, H, 2.79% N, 0.00.
Example 202.
~Rl-2-[3-(6-Bromo-benzo[blnaphth~2.3-dlthiophen-11 ~y-2.6-difluoro-phenoxy]-
propionic acid
To a solution of 3-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6
difluoro-phenol (0.570 g, 1.29 mmol), commercially available (S)-lactic acid,
methyl
ester. (0.246 mL, 2.58 mmol) and triphenylphosphine (0.6778, 2.58 mmol) in dry
benzene (7 mL) was added diethylazodicarboxylate (0.406 mL, 2.58 mmol)
dropwise
at room temperature under a dry nitrogen atmosphere. The reaction mixture was
sealed in a pressure bottle and immersed in a pre-heated oil bath at
105°C. After
heating for 2.5 hours the mixture was stirred at ambient temperature for about
14
hours. The reaction mixture was then diluted with methylene chloride and
combined
with silica gel. The solvents were removed and the adsorbate was flash
chromatographed (90/10 petroleum ether / ethyl acetate) to give (R)-2-[3-(6-
bromo-
benzo[b]naphtho[2,3-d]thiophen-11-yl)-2,6-difluoro-phenoxy]-propionic acid,
methyl
ester as a white solid (0.59 g, 86%). To a solution of this methyl ester (0.46
g, 0.929
mmol) in tetrahydrofuran ( 18 mL) and methanol (6 mi.) was added a 1 N aqueous
solution of potassuim hydroxide ( I .39 mL, 1.39 mmol) dropwise at room
temperature.
After stirring for 2 hours the solvents were removed and the residue was
combined
with water (50 mL) and acidified with 10% HCI. The solid was extracted into
ether.
The layers were shaken together well, separated, and the organic layer was
washed
with water and concentrated to give the title compound as a white solid (0.396
g,
88%): [a]D25=+13.22 (9.383 mg/mL methanol); NMR (400 MHz, DMSO-d6): b
13.11 (s, 1 H), 8.31 (d, J = 8 Hz, 1 H), 8.10-8.08 (m, 1 H), 7.83-7.78 (m, 1
H), 7.65-7.46
(m, 4H), 7.32-7.19 (m, 2H), 6.82-6.75 (m, 1 H), 4.98-4.93 (m, 1 H), 1.53-1.50
(m, 3H);
MS (-FAB): (M-H): one bromine pattern observed: 511 / 513 (2%); Anal. Calc.
for
C25H15BrF2O3S: C, 58.49, H, 2.95, N, 0.00. Found: C, 58.41, H, 3.44, N, 0.02.
CA 02330623 2000-10-30
WO 99/58521 PCT/11S99/10185
-173-
Example 203.
i-2-[~6-Bromo-benzofblnaphtho(2.3-d)thiophen-11-3r1)-2,6-difluoro-phenox3rl-3-
phenxl-nropionic acid
To a solution of 3-(6-bromo-benzo[b]naphtho[2,3-d)thiophen-11-yl)-2,6
difluoro-phenol (0.700, 1.59 mmol), (S)-2-hydroxy-3-phenylpropionic acid,
methyl
ester (0.572g, 3. l7mmol) and triphenylphosphine (0.831 g, 3.17 mmol) in dry
benzene
( 10 mL) was added diethylazodicarboxylate (0.50 mL, 3. l7mmol) dropwise at
room
temperature under a dry nitrogen atmosphere. The reaction mixture was sealed
in a
pressure bottle and immersed in a pre-heated oil bath at 105°C and
heated for 2.5
hours. After stirring at ambient temperature for 14 hours the reaction mixture
was
diluted with methylene chloride and combined with silica gel. The solvents
were
removed and the adsorbate was flash chromatographed (90/10 petroleum ether /
ethyl
acetate) to give (R)-2-[3-(6-bromo-benzo[bJnaphtho[2,3-d)thiophen-11-yl)-2,6-
difluoro-phenoxy]-3-phenyl-propionic acid, methyl ester as an off white solid
(0.73g.,
76%). To a solution of this methyl ester (0.66g, 1.09 mmol) in tetrahydrofuran
(2lmL) and methanol (7 mL) was added a 1N aqueous solution of potassium
hydroxide ( 1.64mL, 1.64mmo1}. After stirring for two hours the solvents were
removed and the residue was combined with water (50 mL) and acidified with 10%
HCI. The solid was extracted into ether and the layers were shaken well
together for
several minutes before they were separated. The organic layer was washed with
water
and concentrated to give the title compound as a white solid (0.613g, 95%):
[a]D25 =
-13.81 (9.413 mg/mL chloroform); NMR (400MHz, DMSO-d6): 8 13.21 (s, 1H), 8.30
(d, J = 8 Hz, 1H), 8.08 (d, J = 8 Hz, 1H), 7.82 - 7.78 (m, 1H), 7.62 - 7.41
(m, 4H),
7.35 - 7.14 (m, 7H), 6.74 (dd, J = 8, 9 Hz, 1H), 5.17 - 5.11 (m, 1H), 3.32 -
3.24 (m,
1H), 3.19 - 3.12(m, 1H), NMR indicates that 0.22 mole eq. of benzene is
present; MS
(+FAB): (M:+): one bromine pattern observed, 588 / 590 (78%, 75%); Anal. Calc.
for
C31H19BrF2O3S~0.22 C6H6: C, 63.99, H, 3.38, N, 0.00. Found: C, 64.52, H, 3.48.
N, 0.06.
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 174 -
Example 204.
2-Benzofuran r~lphenyl-methanone
According to the procedure in Syn. Comm. 1987,17, 341-354, salicylaldehyde
(21.3 mL, 0.20 mol), 2-bromoacetophenone {39.8 g, 0.20 mol), potassium
carbonate
(30%, 300 g in 700 mL water), tetrabutylammonium sulfate ( 3.5 g, 5 mol%) and
dichloromethane ( 1.5 L) were stirred vigorously for 19 h. The layers were
separated,
the dichloromethane phase was washed with water and brine. It was then
concentrated
and the residue was recrytallized from ethanol (200 mL) to provide the title
compound as white crystals (37.9 g, 85%): mp 88-90°C: NMR (DMSO-d6); 8
8.00
(m, 2H), 7.86 (ddd, J = 8, 1.5, 0.5 Hz, 1H), 7.80 (d, J = 1 Hz, 1H), 7.77
(ddd, J = 8, 2,
1 Hz, 1H), 7.77 (ddd, J = 8, 2, 1 Hz, 1H), 7.72 (m, 1H), 7.63-7.55 (m, 3H),
6.73 (dd,
J = 8, 1 Hz, 1H), 7.39 (ddd, J = 8, 7, 1 Hz, 1H); MS (EI): [M+], 222 (100%);
Anal.
Calc. for C 15H 1002: C, 81.07, H, 4.54, N, 0.00. Found: C, 81.05, H, 4.44, N,
-0.09.
Example 205.
2-B enzyl-benzofuran
A suspension of 2-benzofuranylphenyl-methanone (34.8 g, 0.157 mol),
hydrazine monohydrate (31 mL, 0.639 mol) and diethylene glycol (72 mL) was
heated to reflux for 10 min and cooled to room temperature. Potassium
hydroxide
(22.9 g, 0.408 mol) was added. The reaction mixture was heated in 130°C
oil bath for
1 h., cooled to room temperature and added to water. The oil was extracted
with ether.
Silica ge was added to the ether phase and the solvent was removed. The
adsorbate
was flash chromatographed eluent: (petroleum ether) to provide the title
compound as
an oil (23.9 g, 90%): NMR (CDCl3); 8 7.5-7.1 (m, 9H), 6.38 (s, 1H), 4.10 (s,
2H).
Example 206.
{2-Benzyl-benzofuran-3-,~~ll-(4-methox~phenyll-methanone
Tin tetrachloride (6.5 mL, 55.5 mmol) was added dropwise over a 30 minute
periodto a stirred solution of 2-benzyl-benzofuran ( 10.0 g, 48.01 mmol),
anisoyl
chloride (8.51 g, 49.93 mmol) and carbon disulfide (53 mL) at room temperature
under a dry nitrogen atmosphere. After 15 h the reaction mixture was added to
water
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 175 -
and extracted with dichloromethane. Silica gel was added to the
dichloromethane
phase and the solvent was removed. The adsorbate was flash chromatographed
(95:5
petroleum ether:ethyl acetate as eluent) to provide the title compond as a
white solid
(13.84 g, 84%): mp 84-85°C: NMR (CDCl3); 8 7.87 (dm, J = 9 Hz, 2H),
7.46 (dm,
1H), 7.36-7.12 (m, 8H), 6.97 (dm, J = 9 Hz, 2H), 4.29 (s, 2H), 3.90 (s, 3H):
MS (EI):
342 (100%, MI); Anal. Calc. for C23H1803: C, 80.68, H, 5.30, N, 0.00. Found:
C,
80.61, H, 5.25, N, 0.10.
Example 207.
~2-Benzyl-benzofuran-3-~l_L(2.4-dimethox~phenyll-methanone
Prepared from 2-benzyl-benzofuran and 2, 4-dimethoxybenzoyl chloride
according to the procedure in Example 207. White solid (6.88g): mp 74-76C; NMR
(CDC13); 8 7.47(d, J = 8Hz, 1H), 7.40 (dd, J = 2,1 Hz, 1H), 7.31 - 7.24 (m,
5H), 7.24 -
7.19 (m, 2H), 7.16 - 7.12 (m, 1H), 6.55 (d, J = 2, Hz, 1H), 6.48 (s, 1H), 4.29
(s, 2H),
3.89 (s, 3H), 3.58 (s, 3H), MS (EI): [M* + m/z] 372(55%), 165(100%), 234(88%),
Anal. Calc. for C24H2004, C,77.40, H, 5.41, N, 0.00. Found: C, 77.48, H, 5.44,
N,
0.09.
Example 208.
4-Benzofb]naphthof2 3-d]furan-l lxl)-phenol
Boron tribromide ( 1.0 M in dichloromethane, 130 mL, 130 mmol) was added
dropwise over a 30 minute period to a stirred, -78°C solution of (2-
benzyl-
benzofuran-3-yl)-(4-methoxy-phenyl)-methanone ( 12.0 g, 35.05 mmol) in
dichloro-
methane ( 140 mL) under a dry nitrogen atmosphere. The solution was warmed to
room temperature. After 23 h, water was cautiously added. The layers were
separated
and the dichloromethane layer was washed with water (3X), brine and silica gel
was
added to it. The solvent was removed and the adsorbate was flash
chromatographed
(gradient: 9:1 to 1:1 petroleum ether :ethyl acetate) to provide the title
compound as
an off white solid (4.56 g, 42%): mp 137-138°C: NMR (CDCl3); 8 8.01
(dt, J = 8 Hz,
1H), 7.94 (s, 1H) , 7.89 (dm, J = 8 Hz, 1H), 7.53 (m, 2H), 7.44-7.36 (m, 2H),
7.38 (d,
J = 9 Hz, 2H), 7.10 (d, J = 9 Hz, 2H), 7.13-7.06 (m, 1H), 7.01 (dm, J = 8 Hz,
1H); MS
CA 02330623 2000-10-30
WO 99/58521 PCTlllS99/10185
- 176 -
(EI): 310 ( 100%, MI); High Resolution MS (EI) Calc. for C22H 1402:
310.0993803,
Found:310.09878; Anal. Calc. for C22H 1402: C, 85.14, H, 4.55, N, 0.00. Found:
C,
84.33, H, 4.30, N, 0.03.
Example 209.
4-fBenzofblnaphtho[2,3-dlfuran-11-yl~-benzene-1.3-diol
To a cold (-76°C dry ice, isopropanol bath) solution of (2-benzyl-
benzofuran-
3-yl)-(2,4-dimethoxy-phenyl)-methanone (6.13 g, 16.5 mmol) in anhydrous
methylene chloride (60 mL) was added a 1 M solution of boron tribromide in
methylene chloride ( 100 mL, 100 mmol, 6.06 eq) dropwise aver a period of 20
minutes under a dry nitrogen atmosphere. The dry ice bath was removed and the
reaction mixture was stirred at ambient temperature for 45 hours. After
cooling in an
ice bath water was carefully added and after quenching the reaction mixture
was
further diluted with water (300 mL). The organics were extracted with diethyl
ether
and methylene chloride. The extracts were combined, washed with water and
brine,
and combined with silica gel. The solvents were removed and the adsorbate was
flash
chromatographed (80/20 petroleum ether/ethyl acetate) to provide the title
compound
as a white solid (2.07 g, 38%): mp 201-202°C; NMR (CDC13); S 8.03 (ddd,
J = 8, 7,
1 1H), 8.01 (s, 1H), 7.82 - 7.79 (m, 1H), 7.58 - 7.54 (m, 2H), 7.48 - 7.43 (m,
2H), 7.20
(d, J = 8 Hz, 1H), 7.18 - 7.15 (m, 2H), 6.70 (m, 2H), 5.00 (s, 1H), 4.70 (s,
1H); Hi Res
MS, Calc. Sample Mass for C22H1403, 326.0942951, Measured Mass 326.09019,
mas deviation 4 mmu. Anal. Calc. for C22H 1403: C, 80.97, H, 4.32, N, 0.00.
Found:
C, 79.80, H, 4.10, N, 0.07.
Example 210.
2.6-Dibromo- _4-(6-bromo-benzofblnaphthof2,3-dlfuran-1~~ hu enol
A stirred suspension of 4-benzo[b]naphtho[2,3-d]furan-llyl)-phenol (3.0 g,
9.67 mmol) in acetic acid (85 mL) and water (6 mL) was heated slightly to
effect
dissolution. Bromine ( 1.8 mL, 34.01 mmol) in acetic acid (20 mL) was then
added
dropwise over a 10 minute period. The resultant suspension was stirred at room
temperature for 2 h. Water and solid sodium thiosulfite were added and the
reaction
CA 02330623 2000-10-30
WO 99/58521 PCT/U599/10185
- 177 -
mixture was filtered. The solid was wasshed with water and triturated with
petroleum
ether to provide a white solid (4.37 g, 83 %). A portion ( 1.0 g) of this
solid was
recrystallized from acetic acid (45 mL) and then hexane to provide the title
compound
as a white solid: mp 175-176°C; NMR (CDCl3); 8 8.45 (ddd, J = 8, 1, 1
Hz, 1H),7.74
(ddd, J = 8, 1, 1 Hz, 1H), 7.70-7.65 (m, 2H), 7.62 (s, 2H), 7.53-7.48 (m, 2H),
7.20
(ddd, J = 8, 7, 1 Hz, 1H), 7.02 (ddd, J = 8, 1, 1 Hz, 1H), 6.17 (s, 1H); MS
(EI): [M+],
3 bromine isotope pattern, 544 (30%), 546 (100%), 548 (100%) 550 (30%); Anal.
Calc. for C22H11Br302: C, 48.30, H, 2.03, N, 0.00. Found: C, 48.22, H, 1.79,
N,
0.11.
Example 211.
2 6-Dibromo-4-l6-bromo-benzofblnaphthof2,3-dlfuran-11-yl)-3-hydroxy- hp enol
To a solution of the 4-(benzo[b]naphtho[2,3-d]furan-11-yl)-benzene-1,3-diol
( 1.48g, 4.55 mmole) and acetic acid potassium salt (4.468, 45.5 mmole) in
glacial
acetic acid ( 15 mL) was added bromine (0.75 ml,, 14.6 mmole) dropwise over a
period of 20 minutes at room temperature. After stirring 0.5 hours the mixture
was
concentrated and the residue was diluted with water (20mL). The resulting
solid was
filtered, washed with water and petroleum ether and dried in vacuo at 40C to
give the
crude product (2.Sg). The solid was taken up in ethyl acetate, combined with
silica gel
and the solvent was removed. The adsorbate was flash chromatographed (gradient
85/15 petroleum ether / ethyl acetate) to give a yellow solid (0.990g, mixture
of di and
tri brominated products). To a cold (-10°C,) solution of this solid
(0.437 g, 1.11
mmoI) in anhydrous methylene chloride ( 10 mL) was added a solution of bromine
(0.057 mL, 1.11 mmol) in anhydrous methylene chloride (2 mL) dropwise over a
period of 30 minutes under nitrogen. After stirring in the warming bath
overnight the
reaction mixture was poured into water (80 mL) and extracted with diethyl
ether. The
extracts were combined, washed well with a dilute aqueous solution of sodium
bisulfate and brine and silica gel was added. The solvents were removed and
the
adsorbate was flash chromatographed (87/13 petroleum ether/ethyl acetate) to
give
the title compound as an off-white solid (0.429 g): mp 226-228°C; NMR
(CDC13); 8
CA 02330623 2000-10-30
WO 99/58521 PCT/IJS99/10185
- 178 -
8.48 (ddd, J = 8, l, 1 Hz, 1H), 7.73-7.69 (m, 2H), 7.66 (ddd, J = 8, 1, 1 Hz,
1H), 7.51
(ddd, J = 8, 1, 1 Hz, 2H), 7.49 (s, 1H), 7.22 (ddd, J = 8, 7, 1 Hz, 1H), 7.04
(ddd, J = 8,
1, 1 Hz, 1H), 6.18 (s, 1H), 5.47 (s, 1H); MS (EI): [M+], 3 bromine isotope
pattern,
560 (20%), 562 (75%), 564 (44%), 566 (25%); Anal. Calc. for C22H11Br3O3: C,
46.93, H, 1.97, N, 0.00. Found: C, 46.63, H, 1.93, N, 0.09.
Example 212.
~BenzofbLnanhtho~2 3-d]furan-11-yl-phenoxy)-acetic acid methy_ester
Methyl bromoacetate (0.554 mL, 5.8 mmol) was added to a stirred, room
temperature suspension of 4-benzo[b]naphtho[2,3-d]furan-l lyl)-phenol (0.90 g,
2.90
mmol), potassium carbonate (0.81 g, 5.8 mmol) and dimethylformamide (7 mL).
After 20 h, the reaction mixture was added to water and extracted with ether .
Silica
gel was added to the ether phase and the solvent was removed. The adsorbate
was
flash chromatographed (9:1 petroleum ether: ethyl acetate as eluent) to
provide the
title compound as a white solid (0.845 g, 76%): mp 146-147°C: NMR (DMSO-
d6); b
8.19 (s, 1H) 8.13 (d, J = 8 Hz, 1H) , 7.69 (d, J = 8 Hz, 1H), 7.67 (d, J = 8
Hz, 1H),
7.58 (ddd, J = 8,7,1 Hz, 1H), 7.50 (ddd, J = 8,7,1 Hz, 1H), 7.47-7.43 (m, 1H),
7.43 (d,
J = 9 Hz, 2H), 7.24 (d, J = 9 Hz, 2H), 7.17 (ddd, J = 8,7,1 Hz, 1H), 6.88 (dd,
J = 8, .5
Hz, 1H), 4.98 (s, 2H), 3.76 (s, 3H); MS (EI): 382 (100%, MI); Anal. Calc. for
C25H18O4: C, 78.52, H, 4.74, N, 0.00. Found: C, 77.88, H, 4.71, N, 0.07.
Example 213.
4-(BenzoLlnaphthof2 3-dlfuran-11-yl-phenoxy )-acetic acid
Potasium hydroxide ( 1.0 M, 2.85 mL, 2.85 mmol) was added to a stirred
solution of 4-(benzo[b]naphtho[2,3-d]furan-11-yl-phenoxy)-acetic acid methyl
ester
(0.80 g, 2.09 mmol) in THF (9 mL) and methanol (9 mL). After 2 h, the solvents
were
removed, water was added and the reaction mixture was acidified with 10% HCI.
After stirring overnight, the solid was filtered and washed with water,
triturated with
hexane and dried in vacuo at 100°C to provide a white solid (0.735 g,
95%). This
solid was recrystalized from acetic acid and then hexane/ethyl acetate to
provide the
title compound as a white solid (0.338 g, 44%): mp 205-207°C: NMR
(CDCl3); 8
CA 02330623 2000-10-30
WO 99/58521 PCT/tJS99/10185
- 179 -
8.01 (d, J = 8, 1, 0.5 Hz, 1H), 7.95 (s, 1H), 7.76 (ddd, J = 8, 1, 0.5 Hz,
1H), 7.55-7.36
(m, 4H), 7.47 (d, J = 9 Hz, 2H), 7.21 (d, J = 9 Hz, 2H), 7.08 (ddd, J = 9,8,1
Hz, 1H),
6.88 (ddd, J = 8, 1, .5 Hz, 1H), 4:89 (s, 2H); MS (EI): 368 (100%, MI); High
Resolution MS (EI) Calc. for C24H 1604: 368.10486 Found:368.10867. Anal. Calc.
for C24H 1604: C, 78.25, H, 4.38, N, 0.00. Found: C, 77.84, H, 4.30, N; 0.14.
Example 214.
j2 6-Dibromo-4-(6-bromo-benzofblnanhthof2 3-d]furan -11-yl_)-phenoxyl-acetic
acid
methyl ester
Methyl bromoacetate (.554 mL, 5.8 mmol) was added to a stirred, room
temperature suspension of 2, 6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]furan-
llyl)-phenol (1.6 g, 2.92 mmol), potassium carbonate (0.81 g, 5.8 mmol) and
dimethylformamide (7 mL}. After 21 h, the reaction mixture was added to water
and
filtered. The solid was taken up in THF and silica gel was added. The solvent
was
removed. The adsorbate was flash chromatographed (9:1 petroleum ether: ethyl
acetate as eluent) to provide the title compound as a white solid (0.987 g,
55%): mp
188-189°C: NMR (DMSO-d6); 8 8.37 (d, J = 8 Hz, 1H), 7.91 (s, ZH), 7.84
(d, J = 8
Hz, 1H), 7.80 (ddd, J = 8, 7, 1 Hz, 1H), 7.70 (d, J = 8 Hz, 1H), 7.64-7.58 (m,
2H),
7.31 (t, J = 8 Hz, 1H), 6.92 (d, J = 8, 1H), 4.88 (s, 2H), 3.80 (s, 3H); MS
(EI): [M+], 3
bromine isotope pattern, 616 (30%), 618 ( 100%) 620 ( 100%) 622 (30%); Anal.
Calc.
for C25H 1 SBr304: C, 48.50, H, 2.44, N, 0.00. Found: C, 48.53, H, 2.29, N,
0.00.
Example 215.
j_2 6-Dibromo-4~6-bromo-benzo[blnaphthof2 3-d]furan -11-xl'i-phenoxyl acetic
acid
Potasium hydroxide ( 1.0 M, 1.60 mL, 1.60 mmol) was added to a stirred
solution of [2, 6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]furan-11-yl)-
phenoxy]-
acetic acid methyl ester (0.90 g, 1.45 mmol) in THF (9 mL) and methanol (5
mL).
After 3 h, the solvent was removed, water was added and the reaction mixture
was
acidified with 10% HCI. After stirring overnight, the solid was filtered and
washed
with water, triturated with hexane and dried in vacuo at 100°C to
provide the title
compound as a white solid (0.821 g, 94%): mp 250-252°C: NMR (DMSO-d6};
8 8.37
CA 02330623 2000-10-30
WO 99/58521 PCT/1JS99/10185
- 180 -
(d, J = 8 Hz, 1H), 7.90 (s, 2H}, 7.84 (d, J = 8 Hz, 1H), 7.70 (ddd, J = 8, 6,
1 Hz, 1H),
7.61 (ddd, J = 8, 1, 1 Hz, 1H)), 7.62-7.58 (m, 2H), 7.31 (ddd, J = 8, 7, 1 Hz,
1H), 6.92
(ddd, J = 8, 1,1 Hz, 1H), 4.75 (s, 2H); MS (EI): [M+], 3 bromine isotope
pattern, 602
(40%), 604 ( 9S%) 606 (100%) 608 (40%); Anal. Calc. for C24H13Br3O4: C, 47.64,
S H, 2.17, N, 0.00. Found: C, 47.33, H, 1.95, N, 0.04.
Example 216.
(R)-2-j2 6-Dibromo-4.-(6-bromo-benzofblnaphthol2 3-dlfuran-11-~phenox~-3-
phen~rl-propionic acid
Diethylazodicarboxylate (DEAD, 0.108 mL, 0.686 mmol) was added to a
stirred, room temperature solution of 2, 6-dibromo-4-(6-bromo-
benzo[b]naphtho[2,3-
d]furan-llyl)-phenol ( 0.250 g, 0.457 mmol), (S)-2-hydroxy-3-phenylpropionic
acid,
methyl ester (0.124 g, 0.686 mmol), triphenylphosphine (0.180 g, 0.686 mmol)
and
benzene (2 mL) under a dry nitrogen atmosphere. Dissolution occurred and the
1S solution was heated in an 80°C oil bath for 4.5 h. Upon cooling to
room temperature,
the reaction mixture was diluted with ether and silica gel was added. The
reaction
mixture was concentrated and the silica adsorbate was flash chramatographed
(9S : S
petroleum ether : ethyl acetate) to provide a white solid (0. 266 g, 82%).
Aqueous
potassium hydroxide ( 1 N, 1.3 mL, 1.3 mmol) was added to a stirred solution
of this
oil in THF (3 mL)/methanol ( 1.3 mL). After 1.Sh the solution was
concentrated,
diluted with water ( 100 mL) and acidified with 10% aqueous HCI. The solid was
filtered, washed with water and triturated with petroleum ether to provide the
title
compound as a white solid (0.256 g, 98%): NMR (DMSO-d6); 13.25 (broad s, 1H),
8.36 (d, J = 8 Hz, 1H), 7.84-7.77 (m, 3H), 7.67-7.56 (m, 3H), 7.40 (d, J = 8
Hz, 2H),
2S 7.33 (t, J = 8 Hz, 2H), 7.27 (t, J = 8 Hz, 2H), 6.85 (ddd, J = 8, l, 1 Hz,
1H), 5.31 (t, J
= 7 Hz, 1H), 3.41 (d, J = 7 Hz, 2H}; MS (+FAB): [M+], 3 bromine isotope
pattern,
692 (3S%), 694 ( 90%) 696 (100%) 698(SO%); Anal. Calc. for C31H19Br3O4: C,
S3.S6, H, 2.75, N, 0.00. Found: C, 52.44, H, 2.82, N, -0.13.
CA 02330623 2000-10-30
WO 99/58521 PCT/iJS99/10185
- I$1 -
Example 217.
,L 6 Dibromo-4 (6 bromo-benzo(blnaphthof2 3-dlfuran-11-vl)-3-hvdroxv-nhenoxvl-
acetic acid
To a solution of [2,6-dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]furan-11-
yI)-3-hydroxy-phenol ( 1.288, 2.27mmole) in anhydrous tetrahydrofuran (64mL)
was
added triphenylphosphine (1.1938, 4.SSmmole), methyl glycolate (0.351mL,
4.SSmmol) and diethylazodicarboxylate (0.305mL, 4.55 mmole) at room
temperature
under a dry nitrogen atmosphene. After stiring at room temperature for 8 days
the
reaction was quenched with water ( IOmL) and the solvents were removed. The
yellow
residual solid was taken up in a mixture of methylene chloride, ether, and
ethyl
acetate and combined with silica gel. The solvents were removed and the
adsorbate
was flash chromatographed (40 / 60 petroleum ether / methylene chloride) to
provide
a white solid (0.3428, 24%). To a solution of this solid (0.490 g, 0.772 mmol)
in
tetrahydrofuran (6 mL) and methanol (2 mL) was added a 0.5 M aqueous solution
of
potassuim hydroxide (3.24 mL, 1.62 mmol, 2.1 eq) dropwise at room temperature.
After stirring 1.5 hours at room temperature the solvents were removed and the
residue was combined with water. After removing impurities with diethyl ether
(20
mL), the aqueous phase was acidified with 10% aqueous hydrochloric acid. The
organics were extracted with diethyl ether. The extracts were combined,
concentrated,
and chased several times with benzene and dried in vacuo at 60°C to
provide the title
compound as an off white solid (0.380 g, 79%): mp 194-240°C; NMR (DMSO,
d6):
8 13.15 (s, 1 H), 9.58 (s, 1 H, OH), 8.35 (d, J = 8 Hz, 1 H), 7.83 (d, J = 8
Hz, 1 H), 7.78
(ddd, J = 8, 7, 1 Hz, 1H), 7.67 - 7.57 (m, 4H), 7.31 (t, J = 8 Hz, 1H), 6.94 -
6.92 (m,
1H), 4.70 (s, 2H); MS (+FAB): [M+], 3 bromine isotope pattern, 618 (34%), 620
(100%), 622 (100%), 624 (34%); Anal. Calc. for C24HI3Br3O5: C, 46.41, H, 2.11,
N, 0.00. Found: C, 46.78, H, 2.05,