Note: Descriptions are shown in the official language in which they were submitted.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
1
ENHANCED STORAGE ORGAN PRODUCTION IN PLANTS
FIELD OF THE INVENTION
The invention relates to genetically transformed plants that develop larger
storage organs. More specifically this invention relates to plants comprising
a
heterologous gene encoding an enzyme involved in NAD(P)H consumption. These
plants develop larger roots, exhibit increased growth, and increased stress
tolerance.
BACKGROUND OF THE INVENTION
Perennial crops, including many forage crops, persist in cultivated fields for
several years. Because these plants are capable of multiple cycles of regrowth
and
harvest, the growth and development are distinctly different than annual grain
crops.
In most perennial forage production systems, for example alfalfa (Medicago
sativa),
plants are defoliated before any seed is produced and, unlike annual crops,
regrow
new vegetative shoots from crown or axillary buds. The energy, carbon,
nitrogen and
other reserves necessary to support this regrowth come from the root system
and
crown. The reserves in the root and crown are depleted as the new shoots
develop
new leaves, which capture light energy for photosynthesis. At a certain stage
of
development the shoot becomes self-sufficient and obtains its energy and
carbon
requirements from photosynthesis. At a later stage, the shoot has excess
energy and
carbon from photosynthesis and exports the excess to the root and crown system
to
support nitrogen fixation, nutrient uptake and replenishment of reserves.
Replenishment of reserves continues until the plant is defoliated again by
either
grazing, harvesting or natural stresses, for example, freezing.
Roots or other storage organs of most forage legumes convert the imported
sucrose to starch, whereas forage grasses store fructans. The rate of starch
or fructan
accumulation in these storage organs is usually not controlled by the supply
of sucrose
from the leaf. Instead, sink strength is determined by the ability of the
storage organ
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
2
to synthesize and store starch or fructans. This is determined in turn by the
number of
amyloplasts (sites of starch storage) in the cell, the number of storage cells
in the root,
and the metabolism of the cell.
The size of the root system and the quantity of stored nutrient reserves that
are
available to support new shoot growth determines the rate and amount of shoot
regrowth and the economic yield of perennial plants. The agronomic management
of
forage crops for example is specifically designed to maximize the quantities
of these
reserves that may accumulate between harvests (Hanson et al., 1988; Barnes et
al.,
1995). The quantity of stored nutrient reserves determines the ability of the
plant to
survive winter. Therefore, in northern climates, crop production
recommendations
include clear guidelines to avoid harvesting alfalfa and other forage crops
during late
summer and early autumn because these reserves are being replenished for
winter.
The performance index of alfalfa for example after several winter stresses has
been related to the size of the roots. Significant correlations of root mass
were found
with performance after flooding and icing stress, and a correlation (not
significant at
the 5% level) was found with freezing stress (Bowley and McKersie, 1990). Root
size
alone appears to have an effect on the performance of alfalfa following
winter.
Therefore, there is a need to increase the sink strength of the roots of
plants.
Increased sink strength would increase the amount of carbohydrate and other
stored
nutrients in roots or other storage organs. The increased levels of reserves
would
increase the regrowth rate, yield potential, and the likelihood that a
perennial plant
would survive winter. Similarly, increased reserve levels would ensure yield
potential
OF an annual under varrying environmental conditions.
Genetic transformation has been previously used to modify source-sink
relationships in plants. Although attempts to improve photosynthesis and
thereby
3 0 increase the export of sucrose to sink organs have not been successful,
the
modification of carbohydrate metabolism in the sink organ has increased the
size of
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
3
potato tubers. U.S. 5,436,394 discloses the modification of the distribution
of
photoassimilates, including sucrose, in transgenic potato plants that
expressed a yeast
invertase in either the cytosol or apoplast of tubers. Cytosolic localization
gave rise to
a reduction in tuber size and an increase in tuber number per plant whereas
apoplastic
targeting led to an increase in tuber size and a decrease in tuber number per
plant.
Several plants exhibited phenotypes comprising reduced internode distances and
severly reduced root growth.
U.S. 5,723,757 discloses the use of a patatin promoter to drive the expression
of gene of interest within a sink organ, such as a root, within transgenic
plants. In
U.S. 5,750,869, transgenic plants that ectopically express sucrose phophate
synthase
are shown to exhibit altered sink capacities, with increased levels of
sucrose, starch
and cellulose observed within the sink tissue. Neither of these documents
observed
increased root growth, or root size, in the transformed plants. Furthermore,
the ability
of the transgenic plant to withstand stresses WAS not contemplated.
In order to increase the sink strength and size of roots and other storage
organs, it may be necessary to directly stimulate the growth and development
of the
organ. In WO 98/03631, increased growth of main and lateral roots was noted in
Arabidopsis transformed with a nucleic acid encoding mitotic cyclin proteins,
preferrably the cyclaAt protein. However, there is no indication that these
plants
exhibited increased regrowth potential, nor that they had increased stress
tolerance.
U.S. 5,554,530 discloses an increased tolerance to salt stress and drought
resistance in plants transformed with 8-pyrroline-5-carboxylic synthetase.
Transformed plants exhibited higher levels of proline and improved root growth
under
salt stress conditions. However, regrowth potential in pernnial plants was not
considered. Other stress tolerant plants have been produced by transforming
plants
with superoxide dismutase (EP 359,617 and EP 356,061), however, no increase in
root growth or root size was observed. The regrowth potential in perennial
plants was
also not considered.
CA 02331375 2000-12-11
'TENCHEN 05 ;11- 6- 0 to : zz *613 563 9869-+ +49 89 - " "
14-06-2000 :ZZ FROWOWLIMG +613-553-8868 T-842 P"05/I1 CA 009900553
4
Tanaka et al (1996, Biocttem Soc. Transact.24:200S) and Aono et al (1995, Plam
Cell Physiol. 36:1687-1691) disclose the increase in stress tolerance, as
indicated by
improved resistance to photooxidative stress, in tobacco expressing enhanced
glutathione
reductase and superoxide dismutase activity. There is no teaching of any
effect of the
expression of these enzymes on increase storage organ dry weight or mass.
McKersi.e et al (1993, Platt Phys. 103:115-1163) teach the increase in
freezing
tolereance in transgeaic alfalfa expressing enhanced levels of superoxide
dismutase,
however, there is no disclosure of increased storage organ dry weight or mass.
US 5,821,398 discloses the production of transgenic plants expressing alchohol
dehydrogenase (ADH) under the control of a fruit specific, inducible promoter,
preferrably the tomato ADH2 promoter. Expression of ADH within ft its results
in
controlled fruit softening and increased flavor content.
In US 5,855,881, the expression of mammalian ADH within plants to produce a
ready source of ADH for use as a dietary supplement to ameliorate the effects
of alcohol
consumption in an animal is discussed. There is no teaching of producing
plants
expressing ADH that exhibit the properties of increased stress tolerance or
increased
regrowth potential.
It is an object of the invention to overcome disadvantages of the prior art.
This
object is met by the combinations of features of the main claims, the sub-
claims disclose
further advantageous embodiments of the invention.
The present invention is directed to introducing at least one heterologous
gene
into a plant encoding an enzyme involved in consuming NAD(P)H, for example,
but not
limited to, alcohol dehydrogenase. These plants have increased storage organ
mass, such
as roots, and the sink strength of the plant is also increased. Furthermore,
these plants
exhibit increased stress tolerance, and exhibit increased regrowth potential,
within and in
perennial plants, between growth seasons.
SUMMARY OF THE AVVENTION
This invention relates to genetically transformed plants that develop larger
storage organs. More specifically this invention relates to plaints comprising
a
heterologous gene encoding an enzyme involved in NAD(P)H consumption. These
plants develop larger roots, exhibit increased growth, and increased stress
tolerance.
According to the present invention there is provided a method of increasing
the
mass of a storage organ of a plant, compri sing:
AMENDED SHEET
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
i) transforming the plant with at least one heterologous gene that encodes at
least one enzyme that results in NAD(P)H consumption to produce a transformed
plant;
ii) selecting the transformed plant for occurrence of the heterologous gene;
5 iii) growing the transformed plant.
Preferably, the heterologous gene encodes an enzyme that is directly involved
in
NAD(P)H consumption, selected from the group consisting of alcohol
dehydrogenase, glutathione reductase, dehydroxyascorbate reductase,
monodehydroascorbate reductase, mitochondrial alternative oxidase, NADH
oxidase, and NADPH oxidase. However, the heterologous gene may also encode
an enzyme indirectly involved in NAD(P)H consumption, selected from the group
consisting of superoxide dismutase, ascorbate peroxidase, and
dehydroxyascorbate
reductase.
The present invention also pertains to a method for increasing the mass of a
storage organ of a plant, comprising:
i) transforming the plant with two distinct heterologous genes that encode
enzymes that results in NAD(P)H consumption to produce a transformed plant;
ii) selecting the transformed plant for occurrence of the heterologous genes;
iii) growing the transformed plant.
This invention also relates to a vector comprising a regulatory element in
operative association with at least one heterologous gene, wherein the
heterologous
gene, when expressed in a plant, encodes an enzyme that consumes NAD(P)H and
produces a transformed plant characterized in having increased storage organ
mass.
Preferably, the regulatory element is active in the storage organ, and is root
specific.
Furthermore, this invention also relates to a vector comprising two
regulatory elements in operative association with two distinct heterologous
genes,
wherein expression of the heterologous genes in a plant results in the plant
having
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
6
increased storage organ mass. At least one of the regulatory elements is
active in
the storage organ, and is root specific.
This invention is directed to a transgenic plant, transgenic plant cell, and
transgenic seed comprising the either of the vectors defined above. The
transgenic
plant may be a perennial or an annual plant. If perennial, the plant is
selected from
the group consisting of strawberries, raspberries, grapevines, apple, roses,
orchard
grass, brome grass, timothy, ryegrass, fescue, alfalfa, clover, birdsfoot
trefoil,
turfgrass, bentgrass and bluegrass. If annual, the plant is a winter annual
plant or
a root crop selected from the group consisting of Brassica spp., wheat,
barley, oats,
rye, canola, maize, rice, barely, soybean, potatoes and Phaseolus spp.
The present invention embraces a method of increasing the tolerance to an
environmental stress of a plant, comprising:
i) transforming the plant with at least one heterologous gene that encodes an
enzyme that results in NAD(P)H consumption;
ii) selecting the transformed plant for occurrence of the heterologous gene;
iii) growing the transformed plant.
This method also pertains to increasing flooding, freezing, desication, or
drought
resistance or a combination thereof.
The invention includes an expression system for increasing the size of the
storage organs of a plant or to impart greater sink strength in the storage
organs, for
example, including but not limited to roots, crowns, rhizomes, stolons,
tubers, culmns,
basal stems and tap roots. The invention also includes a transgenic plant, a
plant part,
a seed, a plant cell and a plant tissue that includes the expression system.
The
invention includes the use of the expression system to increase the size of
storage
organs and the sink strength of the plant.
This summary of the invention does not necessarily describe all necessary
features of the invention but that the invention may also reside in a sub-
combination
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
7
of the described features.
DESCRIPTION OF THE DRAWINGS
Preferred embodiments of the invention will be described in relation to the
drawings in which:
Figure 1 shows a restriction map of the T-DNA region in pADH3 vector used
to transform Medicago sativa.
Figure 2 shows alcohol dehydrogenase activity (ADH) in flooded and aerobic
roots of three alfalfa plants. Figure 2(A), ADH activity over a pH range of 6
to 9 in
roots obtained from plants flooded for 24 hr. Figure 2(B), ADH activity over
pH 6 to
9 within aerobic roots. Control (=) is N-4-4-2; (M) transgenic plant 3 (ADH-3-
3), and
(o) transgenic plant 5 (ADH-3-5).
Figure 3 shows native PAGE of alfalfa root extract stained for alcohol
dehydrogenase. Lanes 1-4: no flooding treatment; lanes 5-8: 24 h flooding;
lanes
9-12: 48 h flooding; lanes 1, 5 and 9: control N4-4-2; lanes 2, 6, and 10:
transgenic 3
(ADH-3-3); lanes 3, 7, and 11: transgenic 4 (ADH-3-4); lanes 4, 8 and 12:
transgenic
5 (ADH-3-5).
Figure 4 shows native PAGE of alcohol dehydrogenase in callus cultures of
control and transformed alfalfa. The arrows indicates the presence of an
additional
isozyme in the transgenic plants. Lane 2 is the control, RA3, and lanes 1 and
3 are two
transgenic plants.
Figure 5 shows winter survival and herbage yield in field trials at Elora
Ontario of two alfalfa primary transgenic plants, ADH-3-2 and ADH-3-3,
expressing
alcohol dehydrogenase. Figure 5 (A) shows winter survival, indicated as stand
counts
as % of original. Figure 5 (B) shows herbage yield (g / m2).
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
8
Figure 6 shows Polymerase Chain Reaction (PCR) products formed in the
analysis of the transgenic Medicago sativa plants containing pADH3. Figure
6(A)
shows the PCR products from the adh primers. Lanes I and 2 DNA from transgenic
plants, lanes 3 and 4, negative control, lane 5, adh3 plasmid (positive
control). Figure
6(B) shows the PCR products from the bar primers. Lanes 3 and 4 DNA obtained
from transgenic plants, lane 1, markers, lanes 5 and 6 negative control, lane
7 positive
control (bar plasmid), lane 2, no sample. Similar results were obtained in all
progeny
and the results were used to separate the plants into two populations with and
without
the T-DNA insertion.
Figure 7 shows Southern analysis for adh transgene from pADH3 in plants of
Medicago sativa. Lane 1 (left) is marker lane; lanes 2 and 3 represent
independent
transgenic plants, lanes 4 and 5 are non-transgenic control plants. The arrow
indicates
the unique transgene bands. The adh probe used hybridizes to both the adh
transgene
and the adh native genes. The bands that are unique to each plant represent
the
transgenes; the bands that are common to the plants represent the native adh
gene
family.
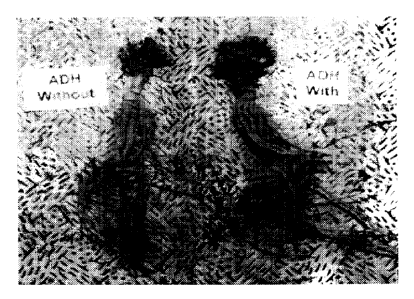
Figure 8 shows a photograph of the crown and roots of two sibling Medicago
sativa plants - one containing the ADH transgene, the other not containing the
ADH
transgene. Note the dramatic difference in size of the root.
Figure 9 is a map of the T-DNA region in pGR36 vector used to transform
Medicago sativa. The T-DNA has the glutathione reductase gene that encodes a
peptide with a transit peptide targeting the protein to the chloroplast.
Figure 10 is a map of the T-DNA region in pGR40 vector used to transform
Medicago sativa. The T-DNA has the glutathione reductase gene that encodes a
peptide with a transit peptide targeting the protein to the. mitochondria.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
9
Figure 11 is a map of the T-DNA region in pGR46 vector used to transform
Medicago sativa. The T-DNA has the glutathione reductase gene that encodes a
peptide with a transit peptide targeting the protein to the chloroplast and to
the
mitochondria.
Figure 12 is a map of the T-DNA region in pAPX vector used to transform
Medicago sativa. The T-DNA has the ascorbate peroxidase cDNA that encodes a
peptide with a transit peptide targeting the protein to the chloroplast.
Figure 13 shows a restriction map of the T-DNA region in pMitSOD vector
used to transform Medicago sativa. The T-DNA has the Mn-SOD cDNA that encodes
a peptide with a transit peptide targeting the protein to the mitochondria
Figure 14 shaows a restriction map of the T-DNA region in pChISOD vector
used to transform Medicago sativa. The T-DNA has the Mn-SOD cDNA that encodes
a peptide with a transit peptide targeting the protein to the chloroplast
Figure 15 shows a restriction map of the T-DNA region in pFeSOD vector
used to transform Medicago sativa. The T-DNA has the Fe-SOD cDNA that encodes
a
peptide with a transit peptide targeting the protein to the chloroplast
Figure 16 shows PCR detection of nos-nptIl transgene from pMitSOD and
pChISOD in individual transgenic plants of Medicago sativa. Lanes 1 to 5 are
different independent transgenic plants; lane 6 is a non-transgenic control;
lane 7
contains markers
Figure 17 shows Southern analysis for nos-nptll transgene from pMitSOD in
four individual transgenic plants of Medicago sativa (labelled as 3, 4, 5 and
6).
Figure 18 shows scans of native PAGE gels showing SOD activity in leaf
extracts of independent transgenic Medicago sativa plants expressing pMitSOD
or
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
pCh1SOD compared to control N4. Note the presence of a relative increase in
the area
of the Mn-SOD in the pMitSOD transgenic and the presence of a new Mn-SOD band
in the pCh1SOD transgenic
5 Figure 19 shows plant samples of alfalfa roots dug in April from a field
trial
(see Example 12).
Figure 20 shows PCR analysis of dual transgenics containing SOD and ADH
transgenes. Figure 20 (A):Chl MnSOD (pSOD4) PCR amplification (700bp band)
10 Figure 20(B):BAR (pADH3) PCR amplification (41 Obp) (Samples are in same
order
on each gel). Lane 1:100bp ladder; lanes 2-19 Fl progeny of C30; Lane 20:
Parent
N4 Chl MnSOD; Lane 21:Parent H19-8; Lane 22; H,O control; Lane 23:100bp
ladder. Double Transgenics: Lanes 4, 5, 11, 16, 19; Progeny 30-4, 30-5, 30-11,
30-16,
30-20.
DESCRIPTION OF THE INVENTION
The present invention relates to the expression of transgenes in storage
organs
of plants that alters the sink strength of storage organs such as the root.
More
specifically this invention relates to plants comprising a heterologous gene
encoding
an enzyme involved in NAD(P)H consumption. The size and mass of the storage
organs is increased, and this increases the herbage yield, and for perennials
and winter
annuals, their persistence and longevity. In addition these plants exhibit
increased
stress tolerance.
The following description is of a preferred embodiment by way of example
only and without limitation to the combination of features necessary for
carrying the
invention into effect.
Without wishing to be bound by theory, it is contemplated that the beneficial
properties observed in these transgenic plants result from lowering the redox
potential,
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
11
defined as the NAD(P)H to NAD(P) ratio, within plant cells, or increasing the
flux
through the NAD(P)H pools. To increase the size of a plant's root system or
any
desired storage organ, it is necessary to introduce a modified gene to cause
the cells of
the desired tissue to have a lower steady-state redox potential. Alternately,
it may be
sufficient to increase the flux of NAD(P)H production by the stimulation of
specific
metabolic pathways. One strategy to accomplish this is to cause the cell to
directly
oxidize NADPH or NADH. An example of a gene that oxidizes NADH, which is not
to be considered limiting in any manner, is alcohol dehydrogenase. Other
examples
are given below. An alternate strategy for lowering the steady-state redox
potential of
an organ is to indirectly decrease the redox potential within a cell, for
example, but
not limited to, increasing the production of ascorbate free radicals,
oxidizing
glutathione or increasing the utilization of NAD(P)H. Alternatively, it may be
suffient to increase the flux of NAD(P)H production by the stimulation of
specific
metabolic pathways. However, other mechanisms may also account for the
beneficial
properties disclosed herein, that arise as a result of plants transformed with
constructs
that encode enzymes that consume NAD(P)H directly or indirectly.
The preferred transgene that is introduced into the plant produces an enzyme
that modifies the metabolism of a desired storage organ, for example, but not
limited
to the root, to increase its consumption of NADH or NADPH, possibly modifying
the
cell's redox potential, and increasing the flux through the NAD(P)H pool.
Examples
of enzymes that may be used for this purpose include, but are not limited to,
the
following:
alcohol dehydrogenase
superoxide dismutase
ascorbate peroxidase
glutathione reductase
dehydroascorbate reductase
monodehydroascorbate reductase
mitochondrial alternative oxidase
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
12
NADH oxidase
NADPH oxidase
Glutathione peroxidase
The above transgenes may be expressed singly or in various combinations in a
tissue specific manner, for example within plant roots or storage organs.
Preferably,
the selected transgene encodes alcohol dehydrogenase (ADH), or a combination
of
ADH and another desired transgene that consumes NAD(P)H either directly or
indirectly. The enzyme alcohol dehydrogenase (E.C. 1.1.1.1) is non-specific
and
reversible. This is the terminal reaction in fermentation or anaerobic
respiration
through glycolysis. thgis enzyme converts an aldehyde, for example
acetaldehyde, to
an alcohol, for example ethanol, using reducing equivalents from NADH,
however,
the enzyme is fully reversible and also converts an alcohol to an aldehyde,
generating
NADH. ADH is considered to be an anaerobic peptide because transcription is
enhanced in anaerobic or hypoxic growth conditions. ADH may be obtained from
any
source and used as described herein, however, preferably the ADH is of plant
origin.
By "regulatory element" it is meant those that include developmentally
regulated, tissue specific, inducible and constitutive regulatory elements. A
regulatory element that is developmentally regulated, or controls the
differential
expression of a gene under its control, is activated within certain organs or
tissues of
an organ at specific times during the development of that organ or tissue.
However,
some regulatory elements that are developmentally regulated may preferentially
be
active within certain organs or tissues at specific developmental stages, they
may also
be active in a developmentally regulated manner, or at a basal level in other
organs or
tissues within the plant as well, such regulatory elements are also considered
"tissue
specific". It is to be understood that tissue specific regulatory elements may
also be
preferentially active within a tissue throughout development, and exhibit a
basal level
of activity in other organs. Regulatory elements may be found either upstream,
within, downstream, or a combination thereof, of the coding region of a gene.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
13
An inducible regulatory element is one that is capable of directly or
indirectly
activating transcription of one or more DNA sequences or genes in response to
an
inducer. In the absence of an inducer the DNA sequences or genes will not be
transcribed. Typically the protein factor, that binds specifically to an
inducible
regulatory element to activate transcription, is present in an inactive form
which is
then directly or indirectly converted to the active form by the inducer. The
inducer
can be a chemical agent such as a protein, metabolite, growth regulator,
herbicide or
phenolic compound or a physiological stress imposed directly by heat, cold,
salt, or
toxic elements or indirectly through the action of a pathogen or disease agent
such as
a virus. A plant cell containing an inducible regulatory element may be
exposed to an
inducer by externally applying the inducer to the cell or plant such as by
spraying,
watering, heating or similar methods.
A constitutive regulatory element directs the expression of a gene throughout
the various parts of a plant and continuously throughout plant development.
Examples of known constitutive regulatory elements include promoters
associated
with the CaMV 35S transcript. (Odell et al., 1985, Nature, 313: 810-812), the
rice
actin 1 (Zhang et al, 1991, Plant Cell, 3: 1155-1165) and triosephosphate
isomerase 1
(Xu et al, 1994, Plant Physiol. 106: 459-467) genes, the maize ubiquitin 1
gene
(Comejo et al, 1993, Plant Mol. Biol. 29: 637-646), the Arabidopsis ubiquitin
1 and 6
genes (Holtorf et al, 1995, Plant Mol. Biol. 29: 637-646), and the tobacco
translational
initiation factor 4A gene (Mandel et al, 1995 Plant Mol. Biol. 29: 995-1004).
The expression of a transgene of the present invention is controlled by a
regulatory element as defined above. Preferably, the regulatory element
enables
transcription in a-tissue specific manner, within root or storage organ cells.
Examples
of root specific regulatory elements include, but are not limited to the
bidirectional
promoter TR1' andTR2' (Velten et al 1984), or other regulatory elements known
to be
expressed in a root specific manner (e.g. Tingey et al., EMBO J. 6:1, 1987; An
et al.,
Plant Physiol. 88:547, 1988; Oppenheimer et al., Gene 63:87, 1988; Conkling et
al.,
Plant Physiol. 93:1203, 1990; Ohl et al., Cell 2:837, 1990; van der Zaal et
al., Plant
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
14
Mol. Biol. 16:983, 1991), or those disclosed in U.S. 5,436,393, U.S.
5,837,848, U.S.
5,837,876, U.S. 5,659,026; US 5856467, U.S. 5,824,798, U.S. 5,837,848, and
U.S.
5,837,876.. However, regulatory elements may be employed that result in
transcription in other tissues and organs, or that are induced by external
stimuli.
The chimeric gene construct of the present invention, comprising at least one
desired transgene and at least one regulatory element, can further comprise a
3'
untranslated region. A 3' untranslated region refers to that portion of a gene
comprising a DNA segment that contains a polyadenylation signal and any other
regulatory signals capable of effecting mRNA processing or gene expression.
The
polyadenylation signal is usually characterized by effecting the addition of
polyadenylic acid tracks to the 3' end of the mRNA precursor. Polyadenylation
signals are commonly recognized by the presence of homology to the canonical
form 5' AATAAA-3' although variations are not uncommon. Examples of suitable
3' regions are the 3' transcribed non-translated regions containing a
polyadenylation
signal of Agrobacterium tumor inducing (Ti) plasmid genes, such as the
nopaline
synthase (Nos gene) and plant genes such as the soybean storage protein genes
and
the small subunit of the ribulose-1, 5-bisphosphate carboxylase (ssRUBISCO)
gene.
The 3' untranslated region from the structural gene of the present construct
can
therefore be used to construct chimeric genes for expression in plants.
The chimeric gene construct of the present invention can also include
enhancers, either translation or transcription enhancers, as may be required.
These
enhancer regions are well known to persons skilled in the art. The initiation
codon
must be in phase with the reading frame of the coding sequence to ensure
translation
of the entire sequence. The translation control signals and initiation codons
can be
from a variety of origins, both natural and synthetic. Translational
initiation
regions may be provided from the source of the transcriptional initiation
region, or
from the structural gene. The sequence can also be derived from the regulatory
element selected to express the gene, and can be specifically modified so as
to
increase translation of the mRNA.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
The transgene of the present invention may further comprise a transit peptide
sequence that targets the peptide to accumulate in a specific subcellular
compartment
or membrane system in the root or storage organ cell such as the cytosol,
mitochondria, apoplasm, plastic, or plasmalemma. Such transit sequences are
well
5 known to one of skill in the art.
To aid in identification of transformed plant cells, the constructs of this
invention may be further manipulated to include plant selectable markers.
Useful
selectable markers include enzymes which provide for resistance to an
antibiotic
10 such as gentamycin, hygromycin, kanamycin, and the like, or
phosphinothricin
resistance, for example the bar gene. Similarly, enzymes providing for
production
of a compound identifiable by colour change such as GUS (P-glucuronidase),
fluorescence, or luminescence, such as luciferase are useful.
15 Also considered part of this invention are transgenic plants containing a
chimeric gene construct of the present invention comprising a desired
transgene in
operative association with a regulatory element. Methods of regenerating whole
plants from plant cells are known in the art. In general, transformed plant
cells are
cultured in an appropriate medium, which may contain selective agents such as
antibiotics, where selectable markers are used to facilitate identification of
transformed plant cells. Once callus forms, shoot formation can be encouraged
by
employing the appropriate plant hormones in accordance with known methods and
the shoots transferred to rooting medium for regeneration of plants. The
plants may
then be used to establish repetitive generations, either from seeds or using
vegetative propagation techniques. The constructs of the present invention can
be
introduced into plant cells using Ti plasmids, Ri plasmids, plant virus
vectors,
direct DNA transformation, micro-injection, electroporation, biolistics etc.
For
reviews of such techniques see for example Weissbach and Weissbach, Methods
for
Plant Molecular Biology, Academy Press, New York VIII, pp. 421-463 (1988);
Geierson and Corey, Plant Molecular Biology, 2d Ed. (1988); and Miki and lyer,
Fundamentals of Gene Transfer in Plants. In Plant Metabolism, 2d Ed. DT.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
16
Dennis, DH Turpin, DD Lefebrve, DB Layzell (eds), Addison Wesly, Langmans
Ltd. London, pp. 561-579 (1997). The present invention further includes a
suitable
vector comprising the transgene or the chimeric gene construct.
Suitable plants that may benefit from the transgenes disclosed herein include
both perennial and annual plants that are grown for a variety of reasons.
Examples of
perennial plants that are grown for forage and repeatedly harvested by
defoliation
include, but are not limited to:
= legumes, for example, alfalfa, clover, birdsfoot trefoilgrasses;
= grasses, for example orchardgrass, bromegrass, timothy, ryegrass, fescue.
Other perennial plants include those that are grown for fruit and for
ornamental or
recreational purposes such as but not limited to:
= fruit crops, for example, strawberry, raspberry, grapevines, apple;
= turfgrass, for example, ryegrass, bentgrass, fescue, bluegrass;
= flowers, for example roses.
Annual plants and winter annual plants that are grown for forage or seed can
also be transformed in accordance with the present invention. These plants
include,
but are not limited to:
= cereals, for example, wheat, barley, oats, rye;
= Brassica spp. for example, canola.
Annual plants that are grown for forage or seed may also benefit from the
methods
disclosed herein. These plants include:
3 0 = cereals, for example, maize, wheat, rice and barley,
= legumes, for example, soybean and Phaseolus spp.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
17
It is to be understood that wheat, barley oats and canola include all types or
varieties of wheat, barley, oats and canola.
Root crops exhibiting larger root growth and increased stress tolerance may
also be produced using the constructs and methods as described herein. Such
plants
include, but are not limited to:
= carrots, turnips, ginseng, potatoes, sugarbeet and cassava.
The above description is not intended to limit the claimed invention in any
manner, furthermore the discussed combination of features might not be
absolutely
necessary for the inventive solution.
While this invention is described in detail with particular reference to
preferred embodiments thereof, the embodiments are offered to illustrate but
not limit
the invention.
EXAMPLE 1: Preparation of Medicago sativa transformed with ADH
The enzyme alcohol dehydrogenase (ADH; E.C. 1.1.1.1) converts
acetaldehyde to ethanol using reducing equivalents from NADH. This is the
terminal
reaction in fermentation or anaerobic respiration through glycolysis. The
enzyme is
fully reversible and is considered to be an anaerobic peptide because
transcription is
enhanced in anaerobic or hypoxic growth conditions. ADH is a non-
photosynthetic
enzyme. Without wishing to be bound by theory, because ADH oxidizes NADH in
this reaction, the redox potential, or redox flux, of the plant cell will be
reduced and
this may restrict the activity of monodehydroascorbate reductase and other
enzymes
that.metabolize hydrogen peroxide or NADH under conditions when NADH is
limiting. ADH may therefore act directly within the root to lower the redox
potential
in the root and increase the mass of the root.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
18
The adh gene from Arabidopsis thaliana was described by Chang and
Meyerowitz (1986). To remove the promoter from adh, a BamH1 restriction site
was
inserted prior to the ATG start codon by M 13 site specific mutagenesis to
give the
sequence ... ATC GGA TCC ATG TCT .... The gene was isolated as a BamH 1
fragment and inserted into a binary transformation vector pDB2 12 that is
related to
pGV94 1 described by DeBlaere et al. (1987). The binary vector pADH3 contains
the
bar gene coding for phosphinothricin resistance (DeBlock et al. 1987) and the
neo
gene under the control of the bidirectional promoter sequence TR1=and TR2'
(Velten
et al. 1984), respectively. pDB212 was digested with Clal, Hindlil to remove
the neo
gene and pADH2 was digested with BamHl to remove the adh gene; both were
treated
with Klenow reagent to remove single stranded regions and ligated to form
pADH3
(Figure 1). This plasmid contains the bar gene under the control of the Trl'
promoter
as a selectable marker, and the Arabidopsis adh gene under the control of the
TR2'
promoter. However, other promoters can be used to express the ADH gene in a
tissue
specific manner plants according to the present invention.
The binary vector pADH3 was transferred to Agrobacterium tumefaciens
C58C 1 Rif pMP90 by triparental mating. Alfalfa (Medicago sativa L.) clone RA3
was transformed as previously described (D'Halluin et al. 1990). Putatively
transgenic plants were regenerated and screened for resistance to
phosphinothricin and
expression of PAT activity from the bar gene (DeBlock et al. 1987). Occurrence
of
the transgene within plants was verified via Southern or PCR analysis (see
below,
Example 8). Only plants exhibiting tolerance and positive marker activity were
transplanted to soil.
EXAMPLE 2: Expression of ADH activity in roots
ADH activity in extracts of alfalfa roots was resolved into three distinct
forms
based pH optima for the reduction of acetaldehyde . One form of ADH had a
broad
optima from pH 6 to 7. A second had maximum activity at pH 8 and a third at pH
9.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
19
The same three forms were present in two transgenic alfalfa plants, which had
similar
activities when the roots were maintained aerobic. When flooded for 24 h (see
Flooding Tolerance), the three forms of ADH increased in activity
approximately 10
fold in all plants. Transgenic plant 5 (ADH -3-5) had higher activity than the
control,
but the flooding tolerant transgenic 3 (ADH-3-3) did not have higher ADH
activity at
any pH (Figure 2).
ADH in the extracts from non-transgenic alfalfa roots could be resolved into
eight isozymes by native PAGE (Figure 3). When the roots were flooded for 24 h
(see Example 4), the isozyme banding pattern did not change dramatically and
the
activity of all isozymes increased. Similarly, in the three transgenic plants,
the ADH
isozyme banding pattern was not altered by the anaerobic treatment. In
transgenic
plant 3 (ADH 3-3), which exhibited enhanced flooding tolerance, there were no
new
ADH isozymes detected in the root extracts. The relative intensity of isozymes
6, 7,
and 8 decreased whereas the activity of isozymes 1 and 2 had increased. There
were
additional ADH isozymes in the root extracts from transgenic plants 4 (ADH 3-4-
)
and 5 (ADH-3-5), but the appearance of these new isozymes was not associated
with
flooding tolerance. The relative activity of the slow moving isozymes 6, 7 and
8 was
greatly reduced in both and the activity of band 2 was greatly increased in
the extracts
from both transgenics.
EXAMPLE 3: Expression of alcohol dehydrogenase in callus cultures
Callus was produced from petiole explants of each of the transgenic alfalfa
plants and the non-transgenic RA3 as described previously by Senaratna et al.
(1989)
on SH medium supplemented with 1 mg/L 2,4-D.
ADH had a broad pH activity range when assayed using ethanol as a substrate
with an optimum at pH 8.5. ADH activity increased in the callus cultures of
alfalfa
with time reaching a maximum at 30 days. On average the transgenic plants had
consistently higher rates of ADH activity than the non-transgenic control at
all
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
sampling times, but the differences were most pronounced at the later times .
At day
30, the callus from all transgenic plants had higher activity and some
transgenic callus
had over 2.4 times the ADH activity of the nontransgenic control (Table 1).
5 Table 1
Expression of alcohol dehydrogenase activity (moles NADH produced per g
protein) in alfalfa callus cultures at 15 and 30 days.
Day 15 Day 30
Plant Activity Std. Dev. Activity Std. Dev.
CONTROL 4.10 0.57 7.80 0.88
2 4.05 0.31 10.67 0.80
3 4.60 0.42 18.03 1.80
4 4.33 0.032 18.00 2.65
5 4.70 0.75 18.93 3.41
6 3.88 0.67 10.40 3.26
7 4.63 0.45 14.00 0.44
8 4.98 0.60 9.27 1.00
10 4.70 0.10 12.13 2.58
The callus contained at least four major ADH isoenzymes which could be
resolved by native PAGE. There was a slow moving relatively faint band, a
large,
more intense band, which was actually composed of a family of isoenzymes, a
third
faint band, and a more intense fast moving band, which could also be resolved
into
several component isozymes if the native gel was run for longer periods of
time
(Figure 4). The transgenic plants contained an additional ADH activity band
which
had a faster migration rate than any of the alfalfa ADH bands (arrow in Figure
4).
This band was present in all transgenic alfalfa samples and absent from all
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
21
non-transgenic alfalfa samples.
EXAMPLE 4: Flooding tolerance
Each of the transgenic plants and the original nontransgenic plant, RA3, were
propagated by cuttings. The alfalfa plants were grown from cuttings with a 16
h
photoperiod, PPFD of 250 Azmol m-' s'', 22/17 C (day/night) temperature. After
approximately 6 weeks, the plants were defoliated, and grown for an additional
14
days to ensure uniform development and size. Plants were randomly divided
between
a non-stressed control and the flooding treatment. Preliminary experiments had
shown a high degree of variability (experimental error) in assessing an
alfalfa plant's
response to flooding. Therefore, to minimize experimental variability, a split-
plot in
time arrangement with a systematic control was employed in this experiment.
Within
a block, vegetative propagules were arranged in 3 x 3 cells. The central cell
was a
propagule of the systematic control (untransformed RA3) and propagules of the
11
entries (10 transgenic and I control) were randomly assigned to all cells
surrounding
the systematic control. Two 3 x 3 cells comprised a block providing 16 entry
cells per
block. Since there were only 11 genotypes (entries), extra copies of the
genotypes
were randomly allocated to the remaining five cells in each block so that a
similar
number of propagules of each genotype were used over the entire experiment.
This
varied between 15 and 21 over the 3 repetitions of the experiment.
In the first and third repetitions, six blocks were used for the flooding
stress
and three blocks were used for the non-stress controls. The second repetition
had four
blocks for the flooding stress and three blocks for the non-stress control.
The
non-stressed control plants were grown in the same conditions as above. The
flooding
treatments were imposed in plastic trays which allowed the plants to be
submersed to
2 cm above soil level, and were otherwise grown under the same conditions as
the
control plants for the 21 days of the flooding treatment. Prior to the
flooding
treatment the water was allowed to stand at room temperature for 7 days to
equilibrate
in temperature and oxygen content.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
22
The plants were removed from the flooding treatment, defoliated and regrown
for 3 weeks under the above growth conditions. The amount of shoot regrowth
over
this vegetative growth period is a measure of the quantity of carbohydrate and
other
root reserves, and the general vigor of the root system. Stressed plants or
plants with
a damaged root system would be expected to regrow slowly or not at all. The
plants
were defoliated, shoot dry matter weighed and the plants allowed to regrow for
2
more cycles of growth with harvesting at 3 week intervals. Dead plants were
included
in the calculations and assigned a regrowth value of 0.
Variance analyses and means were calculated using the general linear model
procedure of the Statistical Analysis System, PC version (1988). Analyses were
conducted within each stress level using a split plot in time arrangement with
covariate (systematic control) model combined over repetitions. Genotypes and
regrowths were assumed to be fixed effects. Means were compared to the
untransformed control mean using a protected LSD test at p=0.05 (Steel and
Torrie
1980). Significant (P<0.05) differences were detected among genotypes for both
the
non-stress and flooded stress treatments. No differences were detected between
repetitions and interactions. In both the no-stress and flooding stress
treatments, no
significant (P>0.05) genotype x harvest effect was detected. This indicated
that
2 0 genotypes responded similarly over the three harvests and it was valid to
compare
genotype means when averaged over all harvests.
Under the non-stress treatment, the transformed genotypes were not
significantly (P>0.05) different from the non-transformed RA3 control and
averaged
292 mg/plant over the 3 harvests (data not shown). Significant differences
were
detected among transgenic plants because plant I was consistently stunted and
had
low shoot growth (108 mg/plant). The average shoot regrowth across all
genotypes
declined from the first to the third harvest because the 3 week interval is
insufficient
time to replenish root reserves for maximal regrowth. This effect was imposed
3 0 intentionally to apply additional stress on the root reserves of the
plants.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
23
Following a 21 day flooding stress, shoot dry matter yield declined, on
average to 78% of the non-stressed yield, averaging 64 mg/plant across all
genotypes
and harvests. The nontransgenic control plant had an average yield reduction
of 80%.
Among the transgenics, a diverse response to the stress was found - some
appeared
more sensitive, others more tolerant. Transgenics 2 (ADH-3-2) and 3 (ADH-3-3)
were significantly (P<0.05) greater in regrowth yield compared to the
untransformed
control (Table 2). The regrowth of transgenic 3 (ADH-3-3) was more than double
that
of the non-transformed control after the flooding treatment.
Table 2
Shoot regrowth (mg/plant) after 21 days flooding over three cycles of regrowth
Transgenic Plant Regrowth (mg/plant)
CONTROL 55
1 20
2 98*
3 156*
4 73
5 60
6 46
7 21
8 48
9 79
10 43
*Significantly (P<0.05) different from the non-transformed control using a
protected
least-significant difference (LSD) means comparison.
The amount of shoot regrowth that occured after flooding stress is a measure
of the
general vigor of the root system and the quantity of carbohydrate and other
root
reserves.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
24
EXAMPLE 5: Icing Tolerance
Transgenic and non-transgenic control plants were propagated by cuttings and
grown as described above (Flooding Tolerance). The plants were arranged with a
systematic control as before with the central cell of the 3x3 tray containing
a
non-transgenic control. The plants were grown for 6 weeks, defoliated, allowed
to
grow for 1 week and transferred to a low temperature acclimation chamber at
constant
2 C, PPFD 250 mol in-' s-', 12h photoperiod for 4 weeks. This acclimation
treatment induces the expression of ice-encasement tolerance as well as
freezing
tolerance. The plants were again defoliated and completely encased in ice at -
5 C in
the dark for 10 days. The plants were then thawed and allowed to regrow under
the
standard growth conditions. Shoot dry matter was determined for three cycles
of
growth of three weeks duration each, as explained for flooding tolerance.
Because of
the low survival rate, the results were not statistically analyzed by analysis
of
variance, but standard deviations were calculated for the regrowth of each
plant.
The ice-encasement stress was relatively severe and on average less than 50%
of the plants of any genotype survived (Table 3). The non-transgenic control
plant
had only 19% survival and produced 5 mg/plant shoot regrowth. Two of the
transgenic plants, 2 (ADH-3-2) and 3 (ADH-3-3), exhibited significantly higher
survival and regrowth, whereas the remainder were injured to a similar extent
as the
non-transgenic control.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
Table 3
Shoot regrowth and plant survival of transgenic alfalfa plants after
ice-encasement for 10 days.
5
Transgenic Plant Regrowth (mg/plant) Survival (%)
Mean Std. Dev.
CONTROL 5 12 19
10 2 26 66 33
3 32 79 50
4 5 17 7
5 9 19 18
6 1 2 10
15 7 0 0 0
8 1 2 18
9 0 0 0
10 6 14 33
20 n = 12 for each transgenic and 16 for control
The amount of shoot regrowth that occured after freezing stress, as indicated
in
Example 1, is a measure of the general vigor of the root system and the
quantity of
25 carbohydrate and other root reserves.
EXAMPLE 6: Field Trials
A field trial of two primary transgenic plants (ADH-3-2 and ADH-3-3) was
conducted at the Elora Research Station. The plots were established in the
spring by
transplanting rooted cuttings of each transgenic and control genotype. The
test was
arranged in a randomized complete block design with 4 replicates of 50 plants.
Plot
size was 1 x 1.5 in. Plants were harvested and dry matter herbage yields
determined
once in the year of transplanting, and twice for each of two subsequent years.
Stand
counts were taken in fall and the following two years in the spring to
determine
survival. Over the three year period, the adh3 primary transgenics had greater
forage
yield and persistence compared to the control plants (Figure 5).
In November, 25 random plants of each genotype were excavated and
subjected to freezing stresses in an indoor freezing chamber. Plants were
washed,
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
26
acclimated at -2 in the dark for 24 hours and cooled at a rate of -2'C/h.
Five random
plants of each genotype were removed at -6, -8, -10, and -12 C. The plants
were
thawed overnight at 2'C and potted and transferred to a greenhouse. Herbage
yields
were determined for each individual plant for two subsequent regrowth (28 day
regrowth cycles). Average regrowth yields and LT50 values (lethal temperature
causing 50% death) were estimated for each genotype.
There were significant differences in plant survival for plants expressing the
ADD transgene compared to the control non-transgenic genotype (Table 4). The
plants with the ADH transgene had significantly higher regrowth yields. Both
increased survival and increased herbage yield in the transgenic plants are a
consequence of improved vigour of the root system and the quantity of
carbohydrate
and other root reserves.
Table 4. LT50 estimates and regrowth yield over two harvests of alfalfa plants
expressing an alcohol dehydrogenase transgene subjected to a laboratory
freezing stress. Plants were field acclimated prior to exposure to freezing
stress.
Regrowth yield (g DM/plant
Genotype LD50 -6 C -8 C -10 C -12 C
RA3 -5.9 0.5 0.2 0 0
RA3-ADH3-3 -9.9 4.1 2.7 0.3 0
RA3-ADH3-3 -11.8 3.4 1.4 1.5 0.4
se 1.20
EXAMPLE 7: Inheritance of Transgenic Traits
The inheritance of phosphinothricin resistance was assessed in the transgenic
plant ADH-3-3. This transgenic plant had higher ADH activity in extracts from
aerobic roots (see Figure 2) and was used as the paternal parent and another
regenerating alfalfa genotype, C2-4, was the maternal parent.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
27
The plants were cross pollinated by hand and seed collected and bulked for
analysis. Sixty seven plants were grown from the seed under conditions as
described
above. A representative leaf from each plant was streaked with 100 mg/L
phosphinothricin. The leaves were visually assessed for injury on a scale of 1
to 5,
with 5 being severe burning and necrosis. The treatments were repeated twice
over.
time. Ratings of l or 2 were considered tolerant, ratings of 3 to 5 were
susceptible,
and plants with variable responses between the two tests were considered
susceptible.
Since the majority of readings were either 1, 4, or 5, the distinction between
tolerance
and susceptibility was usually obvious. In terms of resistance to
phosphinothricin, 35
plants were tolerant, 32 were susceptible (23 + 9 variable), indicating an
inheritance
ratio of 1:1.
EXAMPLE 8: Inheritance of Transgene
T, generation.
The inheritance of phorphinothricin resistance was assessed suing the
transgenic plant RA3-ADH3-3. The transgenic was used as the paternal parent
and a
non-transgenic regenerating alfalfa genotype, C2-4, 4 from the University of
Guelph
alfalfa embryogenesis breeding program was used as the maternal parent. The
alfalfa
plants were grown with a 16 h photoperiod, PPFD of 250 /2mol m'2s-', 22/17 C
(day/night) temperature. The plants were cross pollinated by hand and F, seed
collected and bulked for analysis. Sixty seven F, plants were grown from the
seed
and a representative leaf from each plant was streaked with 100 mg/L
phosphinothricin. The leaves were visually assessed for injury on a scale of 1
to 5,
with 5 being severe burning and necrosis. The treatments were repeated twice
over
time. Ratings of I or 2 were considered tolerant, ratings of 3 to 5 were
susceptible,
and plants with variable responses between the two tests were considered
susceptible.
Since the majority of readings were higher 1, 4, or 5, the distinction between
tolerance
and susceptibility was usually obvious. In terms of resistance to
phosphinothricin, 35
plants were tolerant, 32 were susceptible (23 + 9 variable). This ratio did
not differ
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
28
from a 1:1 ratio (Yate's x2 = 0.06, P=0.8070) expected for a single transgene
insertion.
Backcross Breeding Program
Two F, genotypes, Bast-50 and Bast-61, were selected from the cross C2-4 x
RA3-ADH-3-3 based on expression of bar and adh3. These two F, genotypes were
used to initiate three backcross populations involving University of Guelph
non-
transgenic breeding materials and produce the following backcross (BC1)
populations:
BC-A: crosses to six N3 plants selected for low self-seed set from the
University of
Guelph embryogenic breeding population ('92);
BC-B: crosses to 10 plants selected from the University of Guelph high
persistence
and seed yield;
BC-C: crosses to 12 plants selected from a population which combined the
branched
root character with multileaf-higher forage quality.
For each BC 1 family, 18-72 plants were screened for the herbicide marker,
and the best plants from each family selected for a subsequent set of crosses.
To
produce BC2 populations:
BC2, BC-A: selected BC I progenies of BC-A were crossed to selected plants
from
University of Guelph population `92 with high yield & persistence.
BC2, BC-B: selected BC 1 progenies were crossed to selected plants from
University of Guelph population A29 (Syn-1) with high yield &
persistence.
BC2, BC-C: selected BC I progenies were crossed to another group of plants
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
29
selected for combined branched root and multileaf attributes.
In the spring, seedlings of each BC2 family were grown in the greenhouse,
sprayed with phosphinothricin (Ignite) at 5 wks age, and all survivors planted
at the
Elora research station following protocols authorized by Plant Products
Division,
Agriculture and Agri-Food Canada. Plants were defoliated at the end of June to
determine dry matter herbage yields. Within each population, the best families
for
herbage yield and persistence were identified and the best plants within each
selected
family were identified during the regrowth period.
Vegetative propagules of selected plants were obtained and returned to the
University of Guelph transgenic containment facility. These plants were used
to
initiate a half-sib family recurrent selection program in 1998. The selected
BC2 plants
were intercrossed by hand in the greenhouse and half-sib progeny, 24 plants
per
family, were planted in flats in the winter. At 5 weeks of age the seedlings
were
sprayed with the herbicide Liberty at an application rate of 0.6 kg ai/ha as
described
previously. The foliwoing spring, surviving half-sib progeny were transplanted
to a
nursery in May at the Elora research station following protocols authorized by
Plant
Products Division, Agriculture and Agri-Food Canada (test 98-UoG 1-075-AL01-
288-
2 0 ONO 1-0 l ). Plants were defoliated twice that season.
Test cross of BC, generation
Paired reciprocal crosses were made between BC, transgenic plants (H 19,
H20-3) and two unrelated non-transgenic plants (B4 16-1, B4 40-2) using hand
emasculation by suction. Genotypes B4 16-1 and B4 40-2 were non-transgenic
plants
possessing a multileaf, branched root, twin unifoliate phenotype.
Approximately 60
F, progeny for each cross were evaluated by PCT for the presence or absence of
the
Adh3/Bar transgene. DNA was extracted with 400 mL homogenizing buffer (250
mM NaCl, 25 mM EDTA, 0.5% SDS, 200 mM tris-HCI, pH 7.4). For the PCR
reaction, 25 ng of DNA was combined with 1.5 ml of 15 mM MgC 12, 1 unit taq
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
polymerase, 2.5 ml 10 x buffer, 2.5 ml dNTP and 2 ml of each primer made to a
final
volume of 25 ml with water. One primer pair were used for an internal region
of the
bar gene (41 1 bp product) and a second pair were used for an internal region
of the
adh3 gene (558 bp product). PCR products were visualized on a 0.8% agarose gel
5 with ethidium bromide (Figure 6).
DNA was extracted for Southern hybridization according to Dellaporta et at
(1983). Plasmid DNA preparations were prepared using the Promega Whizard mini-
prep kit and diluted in water to a final concentration of 100-125 ng/111.
Purified DNA
10 was treated with the restriction enzymes at 5-10 fold excess. All samples
were
separated using a 0.8% agarose gel. After electrophoresis the gel was blotted
overnight onto positively charged nylon membrane as outlined in Assubel et al.
(1991). After blotting, the membranes were cross-linked using W light.
Subsequent
Southern analysis was based on the Boehringer Mannheim (BM) Digoxigenin
15 chemiluminescent system (van Miltenburg et al, 1995). Analysis of the
transgenic
plants confirmed that there were 1 or 2 full insertions of the T-DNA in the
chromosomes of each of the transgenic plants. Two fragments (-6264 and -5385
bp)
of the Adh3/Bar transgene insert were observed in plant H 19-8 and only one
fragment
(-5385 bp) in plant H20-3 (Figure 7).
A series of F 1 crosses were performed between three selected BC2 plants, as
female parents, and a series of other plants not carrying the bar-adh
transgene.
Crosses were performed by hand without pollen control. F, progeny were grown
in a
greenhouse and classified using PCR as described earlier. The results are
presented in
Table 5. For most crosses, the observed segregation ratio followed the 1:1
ratio
expected for a single-locus transgene insertion.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
31
Table 5. Observed segregation ratios and chi-square goodness of fit tests for
the
F, test-cross progeny of BC2 alfalfa plants carrying the bar-adh transgene.
The
expected ratio for a single transgene insertion was 1:1 (with:without).
Test-cross Observed number
Female Cross With bar-adh Without bar-adh Yate'sx2 P
92-109-P2 C38 35 28 0.57 0.4497
92-112-P1 C48 40 50 0.90 0.3428
92-120-PI C39 32 31 0.00 1.000
C47 34 62 7.59 0.0059
Experimental varieties
A population of BC2 plants were divided into two subpopulations, with- and
without-the Bar/ADH transgene, using PCR analysis as described earlier. These
BC2
plants were used as parents to create two experimental synthetic varieties,
one with
the gene (24-parent synthetic) and one without the gene (25-parent synthetic).
Hand
pollinations were performed to produce the Syn-I generation seed of the two
synthetic
varieties in the fall and winter. Crosses were performed by hand as well as
using
leafcutter bees (Megachile rotundata) as pollinators.
In the fall, I 1 plants were selected from within the half-sib progeny field
test
established in the following spring. Plants were selected based on their
tolerance to
Liberty, visual herbage yield and vigour, and freedom from potato leafhopper
feeding
injury. Three vegetative propagules of each selected plant were returned to
the
greenhouse and intercrossed by hand and by leafcutter bees (Megachile
rotundata) to
produce the Syn-1 generation in during winter.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
32
Syn-1 generation
Seed of the Syn-1 generation of the experimental variety derived from selected
BC2 plants carrying the transgene was used for this study. Four sets of 96 Syn-
1
generation progeny were planted in a greenhouse and scored using PCT using
primers
for the ADH transgene as described earlier. The expected segregation ratio in
the
Syn-1 generation was 3:1 (with:without the transgene). The observed number in
each
class and the result of the Yate's corrected chi-square test is presented in
Table 6. The
goodness-of-fit test indicated that the observed ratio followed that expected
for a 3:1
segregation ratio. In accordance with previous types of progeny tested, the
transgene
segregated as a single-gene insertion.
Table 6. Observed segregation ratios and chi-square goodness of fit tests for
the
Syn-1 progeny of BC2 alfalfa plants carrying the bar-adh transgene. The
expected ratio for a single transgene insertion was 3:1 (with:without).
Observed Number
Group With bar-adh Without bar-adh Yate'sx2 P
1 65 26 0.44 0.5056
2 67 22 0.01 0.9512
3 69 20 0.18 0.6684
4 55 31 5.02 0.00250
Pooled 256 99 1.58 0.2090
EXAMPLE 9: Field Trial
Two subpopulations, one comprising Bar/ADH transgene, the other without,
were vegetatively propagated and transplanted to the field in the spring for
evaluation
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
33
of performance in competitive plantings. The field trial was conducted at the
Elora
Research Station following protocols authorized by Plant Products Division,
Agriculture and Agri-Food Canada. Replicated plots were established by
transplanting rooted cuttings of each transgenic and control genotype in 1 x 2
metre
rectangular plots at 100 plants per plot. Plants were harvested twice in the
year of
transplanting on 1 July and 2 Sept.
Root growth
Samples were dug from the field on 14 April. Fresh and dry weights of
crowns and taproots were determined, the latter after drying for two weeks at
70 C.
Plants that contained the ADH transgene had larger roots than those that did
not have
the ADH transgene (Figures 5 and Table 7).
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
34
Table 7
Increased size of roots and crowns from two sibling Medicago sativa
populations
- one containing the ADH transgene, the other not containing the ADH
transgene.
Vector Crown Root
Fresh Weight (g)
With ADH 1.53 3.84
Without ADH 1.11 1.86
LSD(0.05) NS 1.50
Vector Crown Root
Dry Weight (g)
With ADH 0.41 0.93
Without ADH 0.32 0.56
LSD(0.05) NS 0.34
LSD(0.05) is the Least Significant Difference between means at the 5% level of
probability; n = 5; NS - not significantly different
Herbage yield of the alfalfa plots transplanted under oats was measured at
Elora. Seasonal herbage yields were significantly higher for plants carrying
the ADH
transgene compared to plants not carrying the transgene (Table 8). There was
an
average 24% difference in seasonal herbage yields between the two
subpopulations.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
Table 8. Herbage yield of alfalfa expressing an alcohol dehydrogenase
transgene
at Elora in 1998. Alfalfa plants were transplanted in 1997 under oats and
three
5 forage harvests taken in 1998.
Entry Harvest 1 Seasonal Total
Without ADH 311 501
With ADH 363 617
std error 22.5 35.1
A second test was established at Elora with alfalfa planted as pure stands.
This experiment was harvested for forage yield on a three-cut management. The
persistence of plants carrying the ADH transgene was greater than those not
carrying
the transgene when grown in pure stand (Table 9).
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
36
Table 9. Herbage yield of alfalfa expressing an alcohol dehydrogenase
transgene
grown in pure stand and in binary mixtures with timothy. Test established 1997
and herbage yields measured 1998, Elora, Ontario.
Herbage yield (g/m2) Percent survival
Entry Harvest 1 Total 60
Without ADH 194 417 60
With ADH 242 545 72
se 28.7 45.4 5.9
In addition, the seasonal herbage yields were higher for plants carrying the
2 0 ADH transgene. Te yield enhancement was on the order of 30%. In mixture
with
timothy, there was no difference detected between the two groups presumably
because
of the growth compensation ability of the timothy plants. Plant survival was
no
different between the two groups when grown in mixture with timothy. This
indicated that plants carrying the transgene did not negatively affect the
growth of
timothy.
EXAMPLE 10: Dig Field Trial
From January to March, cuttings were made of the two populations one with
and the other without ADH. The trial was established with 2 replications at
Elora,
Ontario in May and 2 replications at New Liskeard in June. The plots were
established with rooted propagules of the same transgenic plant per row, 11
different
transgenic plants per plot (other transgenic plants were included in this
trial but the
data are not shown) and 24 plots per replication. One plot was dug on each
sampling
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
37
date from June to October to measure root (including crown) and shoot dry
weight.
The plants were defoliated twice at Elora and twice at New Liskeard prior to
flowering; these periods correspond to the first and second growth cycles
(Tables 10
and 11, respectively). Statistical analysis was conducted as a split-plot
factorial
experiment of location x plant x date. At both locations, there was no
difference in the
growth of the shoots between the two populations (Tables 10 and 11). However,
the
roots of the population with the ADH transgene were larger than those without
the
transgene. This effect was apparent throughout the growing season although the
difference was larger later in the season (Table 11).
Table 10: Shoot and root (including crown) dry weights (g/plant) of two
alfalfa
populations, with and without the ADH transgene, sampled at Elora and New
Liskeard (NL) during the first growth cycle after transplanting.
Shoot Root
Elora NL Elora NL
Without ADH 2.4 1.1 0.4 0.3
With ADH 2.3 1.1 0.6 0.4
std error 0.20 0.31 0.05 0.11
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
38
Table 11: Shoot and root (including crown) dry weights (g/plant) of two
alfalfa
populations, with and without the ADH transgene, sampled at Elora and New
Liskeard (NL) during the second growth cycle after transplanting
Shoot Root
Elora NL Elora NL
Without ADH 3.4 3.0 1.8 2.2
With ADH 3.1 3.2 2.0 2.8
std error 0.18 0.31 0.15 0.39
In the following spring, the larger root system the previous year caused
differences in
the rate of shoot regrowth and consequently, those plants with the ADH
transgene had
greater shoot and root dry weights (Table 12).
Table 12: Shoot and root (including crown) dry weights (g/plant) of two
alfalfa
populations, with and without the ADH transgene, sampled at Elora and New
Liskeard (NL) during the second growth cycle after transplanting.
Shoot Root
Elora NL Elora NL
Without ADH 3.85 2.55 2.15 3.23
With ADH 7.52 3.98 3.61 4.11
std error 0.93 0.93 0.45 0.45
EXAMPLE 11: Increased root development and storage organ (stolon) size in
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
39
grasses and legumes.
Altering the sink strength of stolons and roots in grasses and legumes is
accomplished by introducing an ADH transgene into desired plants. In
greenhouse
trials, creeping bentgrass (Agrostis palustris) and white clover (Trifolium
repens L.)
plants with and without an ADH transgene are propagated. Control plants of the
same
clone are included. Growth and development of plants are measured at weekly
intervals over a number of regrowth cycles by sampling plants and separating
them
into leaves, stolons and roots, for determination of weight.
EXAMPLE 12: Preparation of Medicago saliva transformed with SOD
The enzyme superoxide dismutase (SOD; EC 1.15.1.1) is a metalloprotein
which catalyzes the dismutation of superoxide to hydrogen peroxide and
molecular
oxygen (Scandalios, 1993; Bowler et al., 1992; Bowler et al., 1994). This is
the initial
step in the Asada-Halliwell pathway that has been well characterized in
chloroplasts.
In plants, it can take three forms: Cu/Zn-, Mn- and Fe-SOD, which are
primarily
targeted to the chloroplast, mitochondria and cytosol, respectively, and it
has long
been deemed an essential component of the oxidative detoxification pathway.
Transformation Vectors
pMitSOD (pSOD 1) is a binary vector with the NPT II gene under control of
the NOS promoter and the Mn-SOD gene under control of the 35S promoter.
Details
of vector construction are given by Bowler et al. (1991). The MnSOD cDNA is
from
Nicotiana plumbaginifolia (GenBank Accession X14482). NPT II, coding for
neomycin phosphotransferase, was obtained from E. coli. The NOS promoter is
from
the Nopaline synthase gene from wild-type Agrobacterium and the 35S promoter
is
from the Cauliflower mosaic virus (CaMV). The pMitSOD construct has a transit
peptide leader sequence which targets the gene product to the mitochondria.
The
respective genes are followed by fragments encoding termination and
polyadenylation
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
signals of octopine synthase (3'OCS) and T-DNA gene 7 (3'g7), both from
Agrobacterium. Details are shown in Figure 13.
pCh1SOD (pSOD4) is a binary vector with the NPT II gene under control of
5 the NOS promoter and the Mn-SOD gene under control of the 35-S promoter.
Details
of vector construction are given by Bowler et al. (1991). The MnSOD cDNA is
the
same as pMit SOD. The pChISOD construct has a transit peptide leader sequence
which targets the gene product to the chloroplast (GeneBank Accession A09029).
The
respective genes are followed by fragments encoding termination and
polyadenylation
10 signals of octopine synthase (3'OCS) and T-DNA gene 7 (3'g7), both from
Agrobacterium. Details are shown in Figure 14.
pSOD 10 (pFeSOD) is a binary vector with the NPT II gene under control of
the NOS promoter and the Fe-SOD gene under control of the 35S promoter.
Details of
15 vector construction given by Van Camp, et al. (1996). The Fe-SOD cDNA was
obtained from Arabidopsis (GenBank Accession M55910) and other details are as
described above. The Fe-SOD cDNA from Arabidopsis has a transit peptide leader
sequence, which was retained, which targets the gene product to the
chloroplast.
Details are shown in Figure 15.
Plant Transformation and Screening
Four different clones of alfalfa, designated N4-4-2, V4-11-3, S4-15 and
S4-16, were selected from the University of Guelph plant breeding program.
Petiole
explants of Medicago sativa were co-cultivated with an overnight culture of
Agrobacterium tumefaciens C58C 1 Rif pMP90 containing the binary vectors
pMitSOD, pChISOD or pSOD10. The explants were co-cultivated on SH induction
medium (Shetty and McKersie, 1993) containing 288 mg/L proline, 53 mg/L
thioproline, 4.35 g/L K2SO4 and 100,uM acetosyringinone for 3 days in the
dark.
The explants were washed in half-strength MS medium (Murashige and Skoog,
1962)
and plated on the same SH induction medium without acetosyringinone but
CA 02331375 2001-06-11
WO 99/64612 PCT/CA99/00553
4
containing 500 mg/L claforan and 50 mg/L kanamycin. After several weeks,
somatic
embryos were transferred to BOi2Y development medium (Bingham et al., 1975)
containing no growth regulators. no antibiotics and 50 g/L sucrose. Somatic
embryos
were subsequently germinated on half-strength MS medium. Rooted seedlings were
transplanted into pots containing Turface (Plant Products, Mississauga.
Ontario) in a
greenhouse at approximately 23?C/18?C (day/night) and a minimum 16 h
photoperiod.
PCR Screening
Prior to transfer to the greenhouse, the putatively transgenic plants were
screened for the presence of the nos-nptll transgene using PCR. DNA was
extracted
with 400 ?L homogenizing buffer (250 mM NaCl, 25 mM EDTA, 0.5% SDS, 200
mM tris-HC1, pH 7.4). The supernatant of a 13,000 x g centrifugation was mixed
with
300 ?1 isopropanol. DNA was collected at the interface, washed and resuspended
in
water. The quality and concentration of the DNA was confirmed using a 0.8%
agarose gel with ethidium bromide staining. For the PCR reaction, 25 ng of DNA
was
combined with 1.5 41 of 15 mM MgC12, I unit taq polymerase, 2.5 4110 x buffer,
2.5
L41 dNTP and 2 ?1 of each primer made to a final volume of 25 al with water.
The
2 0 primers used were 5' AGCTGTGCTCGACGTTGTCAG - 3' (SEQ ID NO: 1) and 5'
GGTGGGCGAAGAACTCCAGCA - 3' (SEQ ID NO: 2). The PCR program was 5 min at 94 C,
then
cycles of 94 C for 15 sec., 65 C for 30 sec., and 72 C for 60 sec., followed
by 5
min at 72 C and holding at 4 C. PCR products were visualized on a 0.8% agarose
gel with ethidium bromide (Figure 16).
Approximately 90% of the regenerated plants scored positive in this screen.
Only PCR positive plants were transferred to the greenhouse for further study.
Southern Hybridization
Purified DNA from alfalfa was treated with the restriction enzymes EcoRV
CA 02331375 2001-06-11
WO 99/64612 PCT/CA99/00553
42
and EcoRI at 5-10 fold excess. All samples were separated using a 0.8% agarose
gel.
After electrophoresis the gel was blotted overnight onto positively charged
nylon
membrane as outlined in Ausubel et al. (1991). After blotting, the membranes
were
UV cross-linked. Subsequent Southern analysis was based on the Boehringer
Mannheim digoxigenin chemiluminescent system (van- Miltenburg et al., 1995).
The
DIG-labeled DNA hybridization probes were synthesized by using the "Expand"
enzyme to enzymatically label PCR products with DIG-dUTP as described by
Boehringer Mannheim (PCR DIG Probe Synthesis Kit) . Probes were synthesized
for
both the kanamycin and the mitochondrial SOD genes using 200 pg purified
plasmid
DNA as the template. The PCR primers for synthesis of the kanamycin probe were
1
M each of 5'-AGCTGTGCTCGACGTTGTCAC-3' (SEQ ID NO: 3) and
5'-GGTGGGCGAAGAACTCCAGCA-3' (SEQ ID NO: 4). Annealing temperature was, 65 C
and the
product size was 732 bp. The primers for the SOD gene were
5'GAGCAGACGGACCTTAGC-3' (SEQ ID NO: 5) and
5'-AGAAACCAAAGGGTCCTG-3' (SEQ ID NO: 6), with a 55 C annealing
temperature and a 5 11 bp product.
Southern analysis of eight transgenic plants confirmed that there were I or 2
full insertions of the T-DNA in the chromosomes of each of the transgenic
plants. Not
all transgenic plants were tested (Figure 17).
Superoxide Dismutase Activity
SOD was extracted from 2-3 fully expanded leaf blades (or other tissue as
indicated) from a vegetative stage shoot. The sample was frozen in liquid
nitrogen,
ground and resuspended in 150 ?L of 50 mM KH2PO4, pH 7.8. The homogenate was
centrifuged at 13,000 x g for 15 min and the protein content of the
supernatant was
determined (Bradford, 1976). A constant volume (20 ?L) was applied to a 13%
polyacrylamide gel with a 4% stacking gel (McKersie et al., 1993). One lane of
each
gel contained 0.5 units of bovine Cu/Zn-SOD (Sigma Chemical) as an internal
3 0 standard. The gel was stained with nitroblue tetrazolium and riboflavin
(Sigma
Chemical) at 4?C, then developed on a light box for 20 min. Areas of
superoxide
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
43
dismutase activity were negatively stained against a blue background.
An image of the gel was captured using a CCD video camera and Northern
Exposure Software (Empix Imaging, Mississauga, Ontario). The area under each
SOD isozyme peak was calculated using Microsoft Excel. The data were expressed
as
a percentage of total activity by calculating area of isozyme peak/total area
of all SOD
peaks x 100%. Alternatively, the data were expressed as units of SOD activity
per g
protein, calculated as area of individual peak / area of internal standard x
concentration of internal standard.
The non-transgenic control N4 plant had three major SOD bands - a fast
moving chloroplastic form of Cu/Zn-SOD, a slower moving cytoslic form of
Cu/Zn-SOD and a mitochondrial Mn-SOD (Figure 18). A small Fe-SOD peak was
occasionally detected between the Mn-SOD and cytosolic Cu/Zn-SOD, but its
activity
was quite labile and not included in the calculations of total SOD activity.
The
transgenic plants had an additional Mn-SOD enzyme superimposed on the native
Mn-SOD isozyme in the native gels. In the case of the pMitSOD, the two Mn-SOD
forms were not resolved by PAGE, but in the case of the pCh1SOD, two distinct
Mn-SOD bands were apparent. The difference in the mobility of the
mitochondrial
and chloroplast targeted forms of Mn-SOD possibly reflects differences in the
cleavage site of the transit peptide, or another post-transcriptional
modification.
The amount of each SOD isozyme was quantified in two ways. The area of each
peak
from the linescan was calculated and expressed relative to the total area of
SOD
activity in each lane to determine its proportion of total SOD activity. This
method
compensated for differences in the amount of the extract applied to the gel
and for
differences in the staining intensity among gels. However, the method assumed
that
Cu/Zn-SOD was not affected by expression of the Mn-SOD transgene. So in some
experiments, the amount of each SOD isozyme was quantified by expressing its
area
relative to the area of an internal standard (bovine Cu/Zn-SOD) on the same
gel to
calculate specific activity (units g-1 protein). The amount of bovine Cu/Zn-
SOD
applied was linearly related to the area of the peak over the range used in
these
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
44
experiments (data not shown).
Based on native PAGE analysis, Mn-SOD activity was increased by a variable
amount among the independent transgenic plants containing either pMitSOD or
pCh1SOD. In about 25% of the transgenic plants from either transformation
vector,
Mn-SOD activity was reduced or not changed. In the majority of the transgenic
plants,
Mn-SOD activity was increased less than two-fold. In only a small proportion
of the
transgenic plants, Mn-SOD activity was doubled relative to total SOD activity.
Field trial
A field trial was conducted at the Elora Research Station following protocols
authorised by Plant Products Division, Agriculture and Agri-Food Canada (tests
97-UOG1-075-ALF02-177-ONO1-01; -ALF03-236-ONO1; -ALF04-224-ONO1-01).
Four replicated plots of 1 x 1.5 in rectangular plots were established in May
by
transplanting 100 rooted propagules of each transgenic per plot. Each plot
consisted
of a population of independent transgenic plants for each construct. Plants in
the
direct seeding trial were harvested twice in the year of transplanting on 1
July and 2
September.
Root Growth
In November samples were dug from the field trial. Fresh and dry weights of
crowns and taproots were determined, the latter after it was dried for two
weeks at
70 C. A second set of samples was dug from the same plots in April the
follwoing
year. In November samples, the root and crown systems were larger in all the
transgenic plants than in the controls (Table 13).
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
Table 13: Increased size of roots and crowns of transgenic Medicago saliva
plants expressing different superoxide dismutase transgenes. Plants were dug
in
November and the following April.
5
November April
Vector Crown Root Crown Root
Fresh Weight (g/plant)
Control 1.16 3.67 0.69 2.56
MitSOD 1.52 7.09 3.27 8.96
ChISOD 1.08 4.76 0.75 2.65
FeSOD 1.35 5.40 0.81 2.38
LSD(0.05) 0.31 1.42 1.50 1.50
Dry Weight (g/plant)
Control 0.30 1.31 0.21 0.79
MitSOD 0.44 2.62 1.03 2.64
Ch1SOD 0.29 1.77 0.22 0.84
FeSOD 0.38 1.93 0.24 0.73
LSD(0.05) 0.10 0.53 0.44 0.44
LSD(0.05) is the Least Significant Difference between means at the 5% level of
probability; n = 15 for fall; n = 5 for spring
The roots and crowns of the plants containing the T-DNA from pMitSOD had
greater
fresh and dry weight than the other plants (Figure 19). Those that contained
the
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
46
T-DNA from pChISOD and pFe-SOD were slightly larger than the control but
smaller
than those with pMitSOD. Note that both pChISOD and pFe-SOD produced a SOD
enzyme that was targeted to the chloroplast.
The roots and crowns of the plants containing the T-DNA from pMitSOD did
not have a greater proportion of their dry matter associated with protein or
carbohydrate reserves (data not presented). On a dry weight basis the root and
crown
of all SOD transgenic plants contained the same amount of glucose, fructose,
raffinose, starch and protein as the control. Therefore, the total available
quantity of
reserves available to support the growth of a new shoot from the crown is
considerably greater in the transgenic plants due their larger mass.
Dig Field Trial: Root Growth
From January to March cuttings were made of the primary transgenic plants.
Because of the large numbers of plants required to establish the field trial,
some
transgenic plants were bulked together and randomly propagated. A similar
procedure
was used for control N4-4-2 plants. Other transgenic plants were included in
this trial
but the data are not shown.
Permits to conduct the field trails were obtained from Canadian Food
Inspection Agency (98-UOGI-075-ALF # -ONO 1-01 for trials at Elora and
98-UOGI-075-ALF # -ON3O-01 for trials at New Liskeard where # is 03-177 for
MitSOD, 04-083 for ChISOD and 02-224 for FeSOD). The trial was established
with
2 replications at Elora, Ontario on May, and at 2 replications at New Liskeard
on
June. The plots were established with 5 rooted propagules of the same
transgenic
plant per row, 11 different transgenic plants per plot and 24 plots per
replication. One
plot was dug on each sampling date from June to October 1998 to measure root,
crown and shoot dry weight. The plants were defoliated twice at Elora and once
at
3 0 New Liskeard prior to flowering.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
47
Statistical analysis was conducted as a split-plot factorial experiment of
location x plant * date. The main effects and most interactions were
statistically
significant at the 5% level of probability. The plant* location interaction is
shown in
Table for the three tissues and total dry weight. ChISOD-J48 had larger roots,
shoots
and crowns averaged over all sampling times than N4 control plants. The effect
was
greater at New Liskeard than Elora (Table 14).
Table 14: Shoot and Root (includes crown) dry weights (g/plant) of transgenic
alfalfa expressing SOD transgenes sampled at Elora and New Liskeard from
June to October. Values are averaged over 2 reps of 15 samples at Elora and of
11 samples at New Liskeard.
Location Plant N Root Shoot Total
Elora N4 control 30 1.41 2.59 4.00
MitSOD-J4 29 1.39 2.62 4.02
Ch1SOD448 30 1.87 3.21 5.08
FeSOD-J13 30 1.28 2.42 3.69
Location Mean 119 1.49 2.71 4.20
NL N4 control 21 1.54 2.11 3.65
MitSOD-J4 24 1.64 2.37 4.01
ChISOD-J48 22 2.36 2.81 5.17
FeSOD-J13 18 2.15 2.47 4.62
Location Mean 85 1.91 2.44 4.35
LSD at 5% are 0.58 (shoot) and 0.47 (root).
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
48
EXAMPLE 13: Transgenic plants expressing super oxide dismutase, ascorbate
peroxidase and glutathione reductase have greater root growth.
The expression of other transgenes may also alter the redox potential of root
cells, change the sink strength of roots and increases the mass of the roots.
A field
trial was conducted using rooted cuttings made from alfalfa plants transformed
(as per
Example 1) with glutathione reductase transgene targeted to the chloroplast
(Figure
9), mitochondria (Figure 10), or both (Figure 11), or with an ascorbate
peroxidase
transgene (Figure 12). Control plants of the same clone, but without the
transgenes
are included as a control.
The rooted cuttings are transplanted to the field. Samples of each plant are
taken at two week intervals from June until December and divided into leaves,
stems,
crown and root, and weighed. The field plots are grown under normal alfalfa
management and are defoliated at the late bud stage of development. The field
trial
was established and sampled as described in Example 10, (Elora only).
As indicated in Table 15, the transformed plants had larger root mass.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
49
Table 15 : Root (including crown) dry weights (g/plant) of primary transgenic
alfalfa sampled at Elora during the first and second growth cycles after
transplanting and in the spring of the following year.
First cycle Second cycle spring
Control, non-transgenic 0.65 1.92 2.54
superoxide dismutase 0.92 2.50 3.64
glutathione reductase 0.68 2.00 2.98
ascorbate peroxidase 0.78 2.05 3.58
std error 0.06 0.16 0.25
All vectors were controlled by the CaMC35S promoter and had a trasnit peptide
to
target the protein to the chloroplast.
First and second regrowth cycles are the average of 6 and 9 sampled (n=12 and
18),
respectively. Spring is the mean of n=4.
EXAMPLE 14: Increased association with mycorrhizal fungi
A field trial was established as described in example 10 at Elora. The plant's
roots were sampled in August. Roots were stained and examined for mycorrhizal
fungi according to the method of McGonigle et al (1990). A random section of
root
was selected. The cross hair of the ocular was rotated to pass through the
section
perpendicular to the long axis of the root. If the cross hair contacted an
arbuscle,
vesicle or hypha, it was scored positive. This was repeated 100 times to
determine the
percentage data shown in Table 16.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
Table 16: Association of mycorrhizal fungi with the roots of two alfalfa
populations,
with and without the ADH transgene, sampled at Elora in the year of
transplanting.
5 Hypha (%)
Without ADH 49
With ADH 80
EXAMPLE 15: Preparation of Medicago saliva dual transgenics
The inheritance and performance of alcohol dehydrogenase in combination
with superoxide dismutase was assessed through paired cross-pollination
between
seven independent transgenic individuals. Cross-pollinations were made between
one
of two transgenic plants containing pADH3, H 19-8 or H20-3; and one of four
transgenic plants transformed with superoxide dismutase, N4 Mit MnSOD (pSODI),
N4 Chi MnSOD (pSOD4), S4 Chl MnSOD (pSOD4), N4 Chi FeSOD (pSOD10) or
V4 Chl Fe SOD (pSOD 10) where N4, S4 and V4 designate different alfalfa
genotypes. 189 F 1 progeny were grown from seed (Table 17).
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
51
Table 17: List of cross pollinations made between transgenic alfalfa plants
expressing alcohol dehydrogenase and superoxide dismutase
Cross Family Code Pollen donor Number of
Progeny
H19-8 x N4 Mit MnSOD C11 H19-8 32
H20-3 x N4 Chi MnSOD C17 H20-3 28
H20-3 x S4 Chi MnSOD C26 H20-3 21
H19-8 x S4 Chi MnSOD C30 H19-8 18
H20-3 x N4 Chi FeSOD COI, C02 Both 34
H20-3 x V4 Chi FeSOD C27, C28 Both 31
H19-8 x V4 Chi FeSOD C31, C32 Both 25
Total 189
The presence of pADH 3, pSOD1, 4 or 10 in the parental and progeny plants
was verified by PCR (Figure 19) and Southern hybridization using primers and
probes
specific to pADH3 and each pSOD. Of the 189 individuals assessed, 22.75%
(43/189) were found to have inherited both parental transgenes. The remaining
77.25
inherited either one of the parental transgenes or neither transgene.
A field trial of all F 1 progeny was established at Elora and New Liskeard.
Individual rooted cuttings were transplanted into a randomized split plot
design with
three or four replicates of each family. Plot size was 1 x 1.5m. The plants
are
analyzed for root and crown growth in the year of transplanting, for winter
survival
and yield in subsequent years.
Similar cross pollinations have been made among other primary transgenic
alfalfa plants with glutathione reductase and superoxide dismutase in various
combinations.
CA 02331375 2008-12-02
52
The progeny with the transgenes have larger root mass than those without the
transgenes.
Although the invention has been described with preferred embodiments, it is
to be understood that modifications may be resorted to as will be apparent to
those
skilled in the art. Such modifications and variations are to be considered
within the
purview and scope of the present invention.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
53
REFERENCES
Allen, R. D. (1995). Dissection of oxidative stress tolerance using transgenic
plants.
Plant Physiol 107: 1049- 1054.
Asada, K. (1992). Ascorbate peroxidase - a hydrogen peroxide-scavenging enzyme
in plants. Physiologia Plantarum 85: 235-241.
Ausubel, F.M., Roger, B., Kingston, RE., Moore, D. D., Seidman, J.G., Smith,
J.
A., and Struhl, K. Eds. 1991. Current Protocols In Molecular Biology.
Chapter 2.9. Green Publishing Associates and Wiley-Interscience, Toronto
Canada.
Barnes, RF., D.A. Miller, and C.J. Nelson. (1995) Forages. Vol 1. An
Introduction
to Grassland Agriculture. Iowa State University Press, Ames, Iowa. p. 157-
160.
Bingham, E. T., L. V. Hurley, D. M. Kaatz and J. W. Saunders (1975). Breeding
alfalfa which regenerates from callus tissue in culture. Crop Science 15: 719-
721.
Boerjan, W., M. Cevera, M. Delarue, T. Beeckman, W. Dewitte, C. Bellini, M.
Caboche, H. Van Onckelen, M van Montagu, and D. Inze (1995)
superroot, a recessive mutation in Arabidopsis, confers auxin overproduction.
Plant Cell 7: 1405-1419
Bowler, C., D. Inze and M. van Montagu (1992). Superoxide dismutase.and stress
tolerance. Annual Review of Plant Physiology and Plant Molecular Biology
43:83-116.
Bowler, C., L. Slooten, S. Vandenbranden, R de Rycke, J. Botterman, C.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
54
Sybesma, M. van Montagu and D. Inze (1991). Manganese superoxide
dismutase can reduce cellular damage mediated by oxygen radicals in
transgenic plants. Embo J 10: 1723-1732.
Bowler, C., W. van Camp, M. van Montagu and D. Inze (1994). Superoxide
dismutase in plants. Critical Reviews in Plant-Sciences 13: 199-218.
Bowley, S. R. and B. D. McKersie (1990). Relationships among freezing, low
temperature flooding, and ice encasement tolerance in alfalfa. Can J Plant
Science 70: 227-235.
Bowley, S. R. G. A. Kielly, K. Anandarajah, B. D. McKersie and T. Senaratna
(1993). Field evaluation following two cycles of backcross transfer of somatic
embryogenesis to commercial alfalfa germplasm. Canadian Journal of Plant
Science 73: 131-137.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of
microgram quantities of protein utilizing the principle of protein-dye
binding.
Analytical Biochemistry 72: 248-254.
Chang, C. and E.M. Meyerowitz (1986) Molecular cloning and DNA sequence of
the Arabidopsis thaliana alcohol dehydrogenase. Proc Nat Acad Sci (USA)
83: 1408-1412
Chen, Z., H. Silva and D. F. Klessig (1993). Active oxygen species in the
induction
of plant systemic acquired resistance by salicylic acid. Science 262: 1883-
1886.
D' Halluin, K.J.Botterman, and W.De Greef (1990) Engineering of herbicide-
resistant alfalfa and evaluation under field conditions. Crop Sci. 30:866-871
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
De Block, M., et at. (1987) Engineering herbicide resistance in plants by
expression
of a detoxifying enzyme. EMBO J 6:2513-2518
De Marco, A. and K A. Roubelakis-Angelakis (1996). The complexity of enzymic
5 control of hydrogen peroxide concentration may affect the regeneration
potential of plant protoplasts. Plant Physiol. 110: 137-145.
Debaere R. et at. (1987) Vectors for cloning in plant cells. Methods Enzymol.
153
:277-292
Dellaporta, S.L. et al. (1983) A plant minipreparation: Version II. Plant
Molecular
Biology Reporter.
Fahrendorf, T., W. T. Ni, B. S. Shorrosh and R A. Dixon (1995). Stress
responses
in alfalfa (Medicago sativa L) .19. Transcriptional activation of oxidative
pentose phosphate pathway genes at the onset of the isoflavonoid phytoalexin
response. Plant Mol Biol 28: 885-900.
Foyer, C. (1993). Ascorbic acid. IN: R.G. Alscher and J. L. Hess
(eds)_Antioxidants
in Higher Plants. CRC Press, Boca Raton. pp. 31-58.
Foyer, C. H., P. Descourvieres and K. J. Kunert (1994). Protection against
oxygen
radicals: an important defence mechanism studied in transgenic plants. Plant,
Cell and Environment 17: 507-523.
Gonzalez-Reyes, J.A., F.J. Alcain, J.A. Caler, A. Serrano, F. Cordoba and P.
Navas. (1995). Stimulation of onion root elongation by ascorbate and
ascorbate free radical inAllium cepa L. Protoplasma 184:31-35.
Gupta, A. S., J. L. Heinen, A. S. Holaday, J. J. Burke and R. D. Allen (1993).
Increased resistance to oxidative stress in transgenic plants that overexpress
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
56
chloroplastic Cu/Zn superoxide dismutase. Proceedings of the National
Academy of Sciences of the United States of America 90: 1629-1633.
Gupta, A. S., R P. Webb, A. S. Holaday and R D. Allen (1993). Overexpression
of
superoxide dismutase protects plants from oxidative stress. Induction of
ascorbate peroxidase in superoxide dismutase-overexpressing plants. Plant
Physiology 103: 1067- 1073.
Harourt, D., M.Van Montagu and D. Inze. (1993). Redox-activated expression of
the cytosolic cooper/zinc superoxide dismutase gene in Nicotiana. Proc. Natl.
Acad. Sci. USA. 90:3103-3112.
Hanson, A.A., D.K. Barnes, and R.R. Hill (1988) Alfalfa and Alfalfa
Improvement.
American Society of Agronomy, Madison, WI. pp. 195-228, 423-426.
Herouart, D., C. Bowler, H. Willekens, W. van Camp, L. Slooten, M. van
Montagu, and D. laze (1993). Genetic engineering of oxidative stress
resistance in higher plants. Philosophical Transactions of the Royal Society
of
London. Series B, Biological Sciences 342: 235-240.
Hidalgo, A., G. Garcia-Herdugo, J.A. Gonzalez-Reyes, D.J. MorrS and P. Navas.
(1991). Ascorbate free radical stimulates onion root growth by increasing cell
elongation. Bot. Gaz. 152(3):282-288.
Ho, L.C. (1988) Metabolism and compartmentation of imported sugars in sink
organs in relation to sink strength. Annual Review of Plant Physiology and
Plant Molecular Biology 39: 355-378.
Huner, N. P. A., D. P. Maxwell, G. R Gray, L. V. Savitch, M. Krol, A. G.
Ivanov
3 0 and S. Falk (1996). Sensing environmental temperature change through
imbalances between energy supply and energy consumption: Redox state of
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
57
photosystem II. Physiol Plant 98: 358-364.
Maxwell, D. P., D. E. Laudenbach and N. P. A. Huner (1995). Redox regulation
of
light-harvesting complex II and cab mRNA abundance in Dunaliella salina.
Plant Physiol 109: 787-795.
McKersie, B. D. and Y. Y. Leshem (1994). Stress and stress coping in
cultivated
plants. Dordrecht, Netherlands, Kluwer Academic Publishers.
McKersie, B. D., S. R. Bowley, E. Hacjanto and O. Leprince (1996). Water-
deficit
tolerance and field performance of transgenic alfalfa overexpressing
superoxide dismutase. Plant Physiol 111: 1177-1181.
McKersie, B. D., Y. R. Chen, M. deBeus, S. R. Bowley, C. Bowler, D. Inze, K.
D'Halluin and J. Botterman (1993). Superoxide dismutase enhances
tolerance of freezing stress in transgenic alfalfa (Medicago sativa L.). Plant
Physiology 103: 1155-1163.
McKersie, B.D., J. Murnaghan and S.R Bowley. (1997) Manipulating freezing
tolerance in transgenic plants. Acta Physiol. Plant 19: 485495
Moore, D.J., P. Navas, C. Penel and F.J. Castillo. (1986). Auxin-stimulated
NADH Oxidase (Semidehydroascorbate Reductase) of Soybean Plasma
Membrane: Role in Acidification of Cytoplasm? Protoplasma 133:195-197.
Murashige, T. and F. Skoog (1962). A revised medium for rapid growth and
bioassays with tobacco cultures. Physiologia Plantarum 15: 473-479.
Navas, P. and C. G. mez-Diaz. (1995). Ascorbate free radical and its role in
growth
control. Protoplasma. 184:8-13.
OMAFRA (1997) Field Crop Recommendations. Publication 296. Ontario Ministry
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
58
of Agiculture and Food.
Payton, P., R.D. Allen, N. Trolinder and A. S. Holaday (1997). Over-expression
of
chloroplast-targeted Mn superoxide dismutase in cotton (Gossypium hirsutum
L., cv. Coker 312) does not alter the reduction of photosynthesis after short
exposures to low temperature and high light intensity. Photosynth Res 52:
233-244.
Perl, A., R. Perl Treves, S. Galili, D. Aviv, E. Shalgi, S. Malkin and E.
Galun
(1993). Enhanced oxidative-stress defense in transgenic potato expressing
tomato Cu,Zn superoxide dismutases. Theoretical and Applied Genetics 85:
568-576.
Pitcher, L. H., E. Brennan, A. Hurley, P. Dunsmair, J. M. Tepperman and B. A.
Zilinskas (1991). Overproduction of petunia chloroplastic copper/zinc
superoxide dismutase does not confer ozone tolerance in transgenic tobacco.
Plant Physiology 97: 452-455.
Prasad, T. K., M. D. Anderson, B. A. Martin and C. R Stewart (1994). Evidence
for chilling-induced oxidative stress in maize seedliongs and a regulatory
role
for hydrogen peroxide. The Plant Cell 6: 65-74.
Price, A. H., A. Taylor, S. J. Ripley, A. Griffiths, A. J. Trewavas and M. R.
Knight (1994). Oxidative signals in tobacco increase cytosolic calcium. Plant
Ce116: 1301-1310.
Rayle, D.L. and R.E. Cleland (1992) The acid growth theory of auxin-induced
cell
elongation is alive and well. Plant Physiol. 99:1271-1274.
Regad, F., C. Herve and O. Marinx. (1995). The tefl box, a ubiquitous cis-
acting
element involved in the activation of plant genes that are highly expressed in
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
59
cycling cells. Mol. Gen. Genet. 248:703-711.
Robertson, D., D. R. Davies, C. Gerrish, S. C. Jupe and G. P. Bolwell (1995).
Rapid changes in oxidative metabolism as a consequence of elicitor treatment
of suspension-cultured cells of French bean (Phaseolus vulgaris L). Plant Mol
Biol 27: 59-67.
Scandalios, J. G. (1993). Oxygen stress and superoxide dismutases. Plant
Physiology 101: 7-12.
Seo, M., S. Akaba, T. Oritani, M. Delarue, C. Bellini, M. Caboche, and T.
Koshiba (1998) Higher activity of an adehyde oxidase in the auxin-
overproducing superroot mutant of Arabidopsis thaliana. Plant Physiol
116:687-693.
Shetty, K. and B. D. McKersie (1993). Proline, thioproline and potassium
mediated
stimulation of somatic embryogenesis in alfalfa (Medicago sativa L.). Plant
Science 88: 185-193.
Slekar, K.H., D.J. Kosman and V.C. Culotta (1996). The Yeast Cooper/Zinc
Superoxide Dismutase and the Pentose Phosphate Pathway Play Overlapping Roles
in
Oxidative Stress Protection. The Journal of Biological Chemistry,Vol.
271(46):28831-28836.
Sonnewald, U., M. R Hajirezaci, J. Kossmann, A. Heyer, RN. Trethewey, and L.
Willmitzer (1997) Increased potato tuber size resulting from apoplastic
expression of a yeast invertase. Nat Biotechnol 15:794-797.
Steponkus, P. L. and F. O. Lanphear (1967). Refinement of the triphenyl
tetrazolium chloride method of determining cold injury. Plant Physiol. 42:
1423-1426.
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
Tepperman, J. M. and P. Dunsmuir (1990). Transformed plants with elevated
level
of chloroplastic SOD are not more resistant to superoxide toxicity. Plant
Molecular Biology 14: 501 -511.
5 Thompson, J. E., R. L. Legge and R F. Barber (1987). The role of free
radicals in
senescence and wounding. New Phytologist 105: 317-344.
Toledo, I., A.A. Noronha-Dutra and W. Hansberg. (1991). Loss of NAD(P)-
Reducing Power and Glutathione Disulfide Excretion at the Start of Induction
10 of Aerial Gowth in Neurospora crassa. J. of Bacteriaology. 173(10):3243-
3249.
Van Camp, W., et al. (1996). Enhancement of oxidative stress tolerance in
transgenic tobacco plants overproducing Fe-superoxide dismutase in
15 chioroplasts. Plant Physiol 112: 1703-1714
Van Camp, W., H. Willekens, C. Bowler, M. v. Montagu, D. Inze, P. Reupold
Popp, H. Sandermann, Jr. and C. Langebartels (1994). Elevated levels of
superoxide dismutase protect transgenic plants against ozone damage. Bio
20 Technology 12: 165-168.
Van Camp, W., K. Capiau, M. Van Montagu, D. Inze and L. Slooten (1996).
Enhancement of oxidative stress tolerance in transgenic tobacco plants
overproducing Fe-superoxide dismutase in chioroplasts. Plant Physiol. 112:
25 1703-1714.
van Miltenburg, R. Ruger, B., Grunewald-Janho, S., Leons, M., Schroder, C.,
Eds. 1995. The Dig System User's Guide for Filter Hybridization. Boehringer
Mannheim GmbH, Biochemia, Germany.
Velten, J., L. Velten, R Hain and J Schell (1984) Isolation of a dual promoter
CA 02331375 2000-12-11
WO 99/64612 PCT/CA99/00553
61
fragment from the Ti plasmid of Agrobacterium tumefaciens. EMBO J
3:2723-2730
CA 02331375 2011-10-06
62
SEQUENCE LISTING
<110> University of Guelph
<120> Enhanced Storage Organ Production in Plants
<130> 08-882992CA
<140> PCT/CA99/00553
<141> 1999-06-11
<160> 6
<170> Patentln version 3.0
<210> 1
<211> 21
<212> DNA
<213> primer
<400> 1
agctgtgctc gacgttgtca 9 21
<210> 2
<211> 21
<212> DNA
<213> primer
<400> 2
ggtgggcgaa gaactccagc a 21
<210> 3
<211> 21
<212> DNA
<213> primer
<400> 3
agctgtgctc gacgttgtca c 21
<210> 4
<211> 21
<212> DNA
<213> primer
<400> 4
ggtgggcgaa gaactccagc a 21
<210> 5
<211> 18
<212> DNA
<213> primer
<400> 5
gagcagacgg accttagc 18
CA 02331375 2011-10-06
63
<210> 6
<211> 18
<212> DNA
<213> primer
<400> 6
agaaaccaaa gggtcctg 18