Note: Descriptions are shown in the official language in which they were submitted.
CA 02333404 2000-11-24
WO 99/61522 PCT/US99/11610
NUCLEATION OF POLYAMIDES IN THE PRESENCE OF
HYPOPHOSPHITE
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to the production of synthetic polyamide
compositions
having a high degree of whiteness and color stability and improved molding
cycle time.
2. Description of Related Art
Polymerization of suitable diamines with dicarboxylic acids to form polyamides
is well
io known in the art and is of considerable commercial significance. Polyamides
have a variety of
uses. One important commercial use is resin for molding articles, especially
articles that require
toughness and the ability to withstand heat.
Nucleants have often been used to improved the molding cycle time or
crystallization
rate of polyamides. For example, U.S. Patent No. 3,080,345 discloses using as
a nucleating agent
~s sodium phenylphosphinate, sodium isobutylphosphinate, magnesium oxide,
mercuric bromide,
mercuric chloride, cadmium acetate, lead acetate, or phenolphthalein. U.S.
Patent Nos.
3,585,264 and 4,866,115 also disclose using nucleating agents for improving
the rate of
crystallization of polyamides.
Hypophosphite compounds have been used as catalysts for polymerization of the
Zo polyamides, for example in U.S. Patent Nos. 3,860,558; 3,173,898; and
3,691,131. In U.S.
Patent Nos. 3,860,558 and 3,691,131, metal hypophosphites are used along with
a hindered
phenolic compound as an antioxidant. In U.S. Patent 3,173,898, hypophosphites
of certain
metals are used in small concentrations. The use of hypophosphites in the
polymerization
process also gives the polyamide a greater degree of whiteness and color
stability.
zs One problem in polyamide manufacturing is that most nucleants are rendered
much less
effective in the presence of hypophosphite. Therefore, a need exists for
polyamide compositions
and manufacturing processes that will have the advantages imparted by the use
of a nucleant,
such as reduced molding cycle time, while still permitting the use of
hypophosphites.
CA 02333404 2000-11-24
WO 99/61522 PCT/US99/11610
-2-
SUMMARY OF THE INVENTION
One aspect of the present invention is a polyamide composition that comprises
(a) a
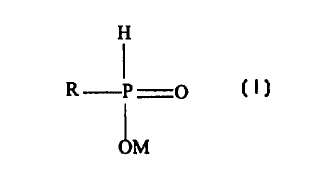
polyamide other than nylon 2,2, (b) a phosphorous-containing whitening agent
having Formula
I:
Formula I:
H
R P O
OM
wherein R is hydrogen, an alkyl group with 1 to 6 carbons, a cycloalkyl group
with 5 to 6
carbons, or a phenyl or methylphenyl aromatic group, and M is hydrogen or a
metal, and (c)
nylon 2,2 in an amount effective to cause nucleation of the polyamide other
than nylon 2,2. The
io polyamide of part (a) preferably is nylon 6,6, and the phosphorous-
containing whitening agent of
part (b) is preferably selected from the group consisting of hypophosphorous
acid and metal salts
thereof. More preferably, the phosphorous-containing whitening agent is a
metal hypophosphite
wherein the metal is selected from groups Ia, IIa, or IIb of the periodic
table. Sodium
hypophosphite is especially preferred.
i s In a particular embodiment, the composition contains the phosphorous-
containing
whitening agent in an amount between about 5 ppm and 500 ppm phosphorous by
weight, more
preferably between about 20 ppm and $0 ppm phosphorous by weight. The nylon
2,2 preferably
is present in an amount between about 2 ppm and 2000 ppm by weight, more
preferably between
about 5 ppm and SO ppm by weight.
Zo Another aspect of the invention is a process for producing nucleated
polyamide,
comprising cooling a molten polyamide other than nylon 2,2 in the presence of
a phosphorous-
containing whitening agent and nylon 2,2 in an amount effective to cause
nucleation of the
polyamide.
CA 02333404 2000-11-24
WO 99/61522 PCT/US99/11G10
-3-
The compositions of the present invention exhibit commercially desirable
toughness,
whiteness and color-stability, yet also have improved molding cycle times. The
polyamide
compositions of the invention can recrystallize at relatively high
temperatures without
substantial deterioration of the toughness of the end product. Higher
recrystallization
s temperatures mean that the material needs less time to cool from the melt,
because it hardens
earlier as temperature drops from the melt. This improves molding cycle time
and increases
throughput and productivity.
DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
The polyamides of the present invention are condensation polymers obtained by
the
io polycondensation of amino carboxylic acids or of mixtures of diamines and
dicarboxylic acids
including interpolyamides obtained by the polycondensation of different
polyamide forming
components. Preferred polyamides are the class generally known as nylons. A
particularly
preferred polyamide is polyhexamethylene adipamide, nylon 6,6. This invention
is useful for
polyamides in all forms, but is especially useful for polyamide resins which
are to be used for
~s molding articles. Useful forms of polyamides include blends, alloys, and
copolymers thereof.
Preferred copolymers are copolymers of nylon 6,6 with nylon 6, nylon 6IA,
nylon 6TA, and the
like.
The phosphorous-containing whitening agents of the present invention are
preferably
hypophosphites, and more preferably hypophosphorous acid or metal
hypophosphites, wherein
Zo the metal preferably is from group Ia, IIa or IIb of the periodic table.
For example, the metal can
be lithium, sodium, potassium, barium, magnesium, calcium, strontium or zinc.
The transition
metal manganese can also be used. The preferred metal hypophosphite is sodium
hypophosphite.
The nylon 2,2 and the phosphorous-containing whitening agent can be
incorporated into
the polyamide before, during or after the polycondensation step. Preferably,
the phosphorous-
Zs containing whitening agent is added during the polycondensation step to
result in a white
polymer. Yellowing of polymer due to oxidation during polycondensation cannot
be reversed by
subsequent addition of phosphorous-containing whitening agent; however,
phosphorous-
containing whitening agents do show efficacy as color stabilizers in
subsequent melting and melt
processing of the polymer. Thus the nylon 2,2 and phosphorous-containing
whitening agent can
CA 02333404 2000-11-24
WO 99/61522 PCT/US99/11610
-4-
be added to the polymer forming ingredients before the polycondensation step
or during the
polycondensation process and the reaction completed by heating. The nylon 2,2
can also be
added to the already formed polyamide by adding it to the molten polyamide.
Preferably, nylon
2,2 can be mixed with the solid polyamide. Alternatively, the solid polyamide
in the form of
s lumps, pellets or chips may be coated or dusted with the ingredients and the
polyamide then
melted.
Preferably, nylon 2,2 can be incorporated into one set of polyamide pellets,
while the
phosphorous-containing whitening agent is incorporated into a second set of
polyamide pellets.
The two sets of polyamide pellets are then mixed and melted. In yet another
embodiment, one
io set of polyamide pellets containing nylon 2,2 and the phosphorous-
containing whitening agent
are mixed and melted with a second set of polyamide pellets containing only
the phosphorous-
containing whitening agent.
The polyamide compositions of the present invention can further comprise
conventional
polymer additives known to those of skill in the art, including fillers,
reinforcing agents,
~s stabilizers, dyes, flame retarding agents, mold-release agents,
plasticizers, pigments, ultraviolet
light absorption agents, antistatic agents, lubricants, and the like which may
be added in effective
amounts and which do not deleteriously affect the compositions of the present
invention.
The following examples are included to demonstrate preferred embodiments of
the
invention. It should be appreciated by those of skill in the art that the
techniques disclosed in the
Zo examples which follow represent techniques discovered by the inventor to
function well in the
practice of the invention, and thus can be considered to constitute preferred
modes for its
practice. However, those of skill in the art should, in light of the present
disclosure, appreciate
that many changes can be made in the specific embodiments which are disclosed
and still obtain
a like or similar result without departing from the spirit and scope of the
invention.
Zs Comparative Example 1
Nylon 6,6 molding resin compositions comprising a phosphorous-containing
whitening
agent, specifically sodium hypophosphite, were prepared to compare the results
of addition of
talc, a known nucleating agent, to the results when no talc was added. A
control composition
was formed from pellets of conventional nylon 6,6 commercially available from
Solutia Inc.
CA 02333404 2000-11-24
WO 99/61522 PCT/US99/11610
-S-
under the name VYDYNE~. To this control composition was added 70 ppm
phosphorous by
weight of sodium hypophosphite. The control composition will herein be termed
Composition
1. Two masterbatch concentrates were formed by dusting 5% of either Cimpact
699 or Mistron
Superfrost, both types of talc commercially available from Luzenac, onto
VYDYNE pellets as
s described above. Both formulations of dusted pellets were then processed on
a ZSK-40 twin
screw extruder at a barrel temperature of 275°C. Parts were molded by a
process-blend of
Composition 1 pellets and masterbatch pellets fed into an injection molding
machine.
Percentages of masterbatch pellets added to formulations of the process blend
were either 0% for
a control formulation or 2% of either type of the masterbatch to produce two
talc-containing
~ o formulations, each with a final talc concentration of 0.1 %.
Tensile strength, elongation at failure, and recrystallization temperature for
molded parts
formed from the control and the two talc-containing formulations were measured
by techniques
known in the art. The results are given in Table 1.
t5 TABLE 1
%MasterbatchTalc conc. Tensile strengthElongation Recrystallization
/ at fail
talc type (%) ISO 527 ISO 527 temperature
(MPa) (%)
0% 0 79.9 51.4 219.3
2%/Cimpact 0.1 92.7 23.4 230.3
699
2%/Mistron 0.1 93.3 22.3 230.6
Superfrost
As Table 1 shows, the addition of talc as a nucleating agent raised the
recrystallization
temperature relative to the control without added nucleating agent, which
result is desirable as it
would translate into reduced molding cycle times. However, the addition of
talc to nylon 6,6 in
2o the presence of sodium hypophosphite resulted in a reduction in elongation
at fail of over SO%
relative to the control. Such a reduced elongation at fail is unacceptable for
a commercial
product that requires a significant retention of toughness.
CA 02333404 2000-11-24
WO 99/61522 PCT/US99/11610
-6-
Example 2
Nylon 6,6 molding resin compositions with added sodium hypophosphite were
prepared
in the presence of from 0 ppm to 22 ppm nylon 2,2. A masterbatch was formed by
dusting 500
ppm of an approximately 88% by weight nylon 2,2-containing powder,
commercially available
s from L. Bruggemann as P22, onto VYDYNE pellets as described above. The
masterbatch was
approximately 440 ppm nylon 2,2. The dusted pellets were then compounded on a
Killion 3.81
cm {1 '/2 in) barrel diameter, 24/1 length/diameter ratio, single screw
extruder equipped with
meter pump, and Maillifer-type screw at barrel temperatures of 280°C to
285°C. Also provided
were pellets of Composition I as described under Comparative Example 1.
Molding was
io achieved by a process-blend of Composition 1 pellets and masterbatch
pellets fed into an
injection molding machine. Percentages of masterbatch pellets added to
formulations of the
process blend ranged from 0% to 5%, resulting in nylon 2,2 concentrations of 0
to 22 ppm.
Tensile strength, elongation at failure, and recrystallization temperature for
five
formulations of between 0 ppm and 22 ppm nylon 2,2 were measured by techniques
known in
is the art. For example, recrystallization was measured on a DSC-7 (Perkin-
Elmer), at peak of
exotherm after 1 min at 50°C, heating from 50°C to 285°C
at 20°C/min, 5 min at 285°C, and
cooling from 285°C to 50°C at 20°C/min. The results are
given in Table 2.
TABLE 2
%MasterbatchNylon 2,2 Tensile StrengthElongation Recrystallization
concentrationISO at fail (C)
(Ppm) 527 ISO 527
(MPa) (%)
0 0 81.2 48.9 2 l 7.3
I 4.4 83.7 51.7 225.3
2 8.8 84.3 46.1 225.6
3 13.2 84.9 41.4 225.9
22 85.5 40.6 226.3
zo
In all cases in which nylon 2,2 was added, the recrystallization temperature
increased by
at least 8.0°C. Unlike the comparative example, however, the use of
nylon 2,2 at between 4.4
CA 02333404 2000-11-24
WO 99/61522 PCT/US99/11610
ppm and 22 ppm in the presence of a phosphorous-containing whitening agent,
specifically
sodium hypophosphite, did not lead to unacceptable decreases in the elongation
percentage at
fail. For all four of the test formulations, the elongation percentage at fail
was between 83% and
106% of the corresponding value for the control formulation. Tensile strength
was similarly not
s impaired by use of nylon 2,2 as a nucleating agent in the presence of sodium
hypophosphite.
All of the compositions and methods disclosed and claimed herein can be made
and
executed without undue experimentation in light of the present disclosure.
While the
compositions and methods of this invention have been described in terms of
preferred
embodiments, it will be apparent to those of skill in the art that variations
may be applied to the
io compositions and methods and in the steps or in the sequence of steps of
the method described
herein without departing from the concept, spirit and scope of the invention.
More specifically,
it will be apparent that certain agents which are chemically related may be
substituted for the
agents described herein while the same or similar results would be achieved.
All such similar
substitutes and modifications apparent to those skilled in the art are deemed
to be within the
is spirit, scope and concept of the invention as defined by the appended
claims.