Note: Descriptions are shown in the official language in which they were submitted.
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
CELL ADHESION-INHIBITING ANTIINFLAMMATORY COMPOUNDS
Technical Field
The present invention relates to compounds that are useful for treating
inflammatory diseases, to pharmaceutical compositions comprising these
compounds, and
to methods of inhibiting inflammation in a mammal.
Background of The Invention
Inflammation results from a cascade of events that includes vasodilation
accompanied by increased vascular permeability and exudation of fluid and
plasma
proteins. This disruption of vascular integrity precedes or coincides with an
infiltration of
inflammatory cells. Inflammatory mediators generated at the site of the
initial lesion serve
to recruit inflammatory cells to the site of injury. These mediators
(chemokines such as
IL,-8, MCP-1, MIP-1, and RANTES, complement fragments and lipid mediators)
have
chemotactic activity for leukocytes and attract the inflammatory cells to the
inflamed
lesion. These chemotactic mediators which cause circulating leukocytes to
localize at the
site of inflammation require the cells to cross the vascular endothelium at a
precise
location. This leukocyte recruitment is accomplished by a process called cell
adhesion.
Cell adhesion occurs through a coordinately regulated series of steps that
allow the
leukocytes to first adhere to a specific region of the vascular endothelium
and then cross
the endothelial barrier to migrate to the inflamed tissue (Springer, T.A.,
1994, Traffic
Signals for Lymphocyte Recirculation and Leukocyte Emigration: The Multistep
Paradigm, Cell 76: 301-314; Lawrence, M.B., and Springer, 'T.A., 1991,
Leukocytes' Roll
on a Selectin at Physiologic Flow Rates: Distinction from and Prerequisite for
Adhesion
Through Integrins, Ce11.65: 859-873; von Adrian, U., Chambers, J.D., McEnvoy,
L.M.,
Bargatze, R.F., Arfos, K.E, and Butcher, E.C., 1991, Two-Step Model of
Leukocyte-
Endothelial Cell Interactions in Inflammation, Proc. Natl. Acad. Sci. USA 88:
7538-
7542; and Ley, K., Gaehtgens, P., Fennie, C., Singer, M.S., Lasky, L.H. and
Rosen,
S.D.,1991, Lectin-Like Cell Adhesion Molecule 1 Mediates Rolling in Mesenteric
Venules
in vivo, Blood 77: 2553-2555). These steps are mediated by families of
adhesion
molecules such as integrin, Ig supergene family members, and selectins which
are
expressed on the surface of the circulating leukocytes and on the vascular
endothelial cells.
The first step consists of leukocyte rolling along the vascular endothelial
cell lining in the
CA 02333770 2000-11-30
WO 99/62908 PCT/CTS99/12419
region of inflammation. The rolling step is mediated by an interaction between
leukocyte
surface oligosaccharides (such as Sialylated Lewis-X antigen (Slex)) and a
selectin
molecule expressed on the surface of the endothelial cell in the region of
inflammation.
The selectin molecule is not normally expressed on the surface of endothelial
cells but
rather is induced by the action of inflammatory mediators such as TNF-a and
interleukin-
1. Rolling decreases the velocity of the circulating leukocyte in the region
of
inflammation and allows the cells to more firmly adhere to the endothelial
cell. The firm
adhesion is accomplished by the interaction of integrin molecules that are
present on the
surface of the rolling leukocytes and their counter-receptors-the Ig
superfamily molecule-
on the surface of the endothelial cell. The Ig superfamily molecules or CAMS
(Cell
Adhesion Molecules) are either not expressed or are expressed at low levels on
normal
vascular endothelial cells. The CAM's, like the selectins, are induced by the
action of
inflammatory mediators like TNF-alpha and IL-1. The final event in the
adhesion process
is the extravasation of the leukocyte through the endothelial cell barrier and
the migration
of the leukocyte along the chemotactic gradient to the site of inflammation.
This
transmigration is mediated by the conversion of the leukocyte integrin from a
low avidity
state to a high avidity state. The adhesion process relies on the induced
expression of
selectins and CAM's on the surface of vascular endothelial cells to mediate
the rolling and
firm adhesion of leukocytes to the vascular endothelium.
The induced expression of e-selectin and CAM's is mediated by the
transcription
factor NFkB. NFkB is a family of dimeric transcription factors made from
monomers
containing the 300 amino acid Rel domain. These factors can bind to DNA,
interact with
each other and bind to an inhibitor molecule termed IkB (Vermaa, LM.,
Stevenson, J.K.,
Schwarz, E.M., Antwerp, D.V., and Miyamoto, S, 1995, Rel/NF1B/IkB Family:
Intimate
Tales of Association and Dissociation, Genes Dev. 9: 2723-2735; and BaIdwin,
A.S.
1996, The NFkB and IkB proteins: New Discoveries and Insights, Annu. Rev.
Immunol.
14: 649-681). NFkB is found in the cytoplasm complexed with IkB. Activation of
NFkB
occurs in response to inflammatory mediators such as TNF-a, IL-l, and
lipopolysaccharide. Activation of NFkB requires phosphorylation of IkB
followed by
ubiquitinylation of the IkB molecule and subsequent degradation by
proteosomes. Release
of NFkB from association with IkB results in translocation of the dimer to the
nucleus
where it can associate with specific DNA sequences. The e-selectin gene and
CAM's
contain NFkB-recognition sequences upstream from their coding regions. The DNA-
bound NFkB acting with other proteins in the transcription complex directs the
expression
of the e-selectin and CAM genes among others controlled by this transcription
factor.
2
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
The present invention discloses compounds that inhibit the expression of e-
selectin
and ICAM-1 relative to VCAM-1. These compounds are useful for the treatment or
prophylaxis of diseases caused by expression of adhesion molecules. These
diseases
include those in which leukocyte trafficking plays a role, notably acute and
chronic
inflammatory diseases, autoimmune diseases, tumor metastasis, allograft
rejection, and
reperfusion injury.
Summary of The Invention
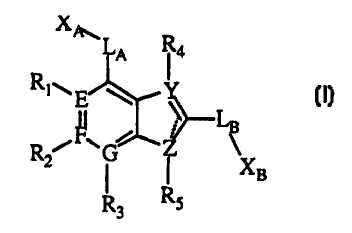
In one embodiment of the present invention are disclosed compounds represented
by structural Formula I:
I,
or a pharmaceutically acceptable salt or prodrug thereof, where the symbol -'
represents
a single bond or a double bond, provided that when one bond is a double bond,
the
adjacent bond is a single bond;
E, F, and G are independently selected from
(1) carbon,
(2) nitrogen, and
(3) N+-O-,
provided that at Least one of E, F or G is nitrogen or N+-O-, and
further provided that at least one of E, F or G is carbon;
Y and Z are independently selected from
( carbon,
1 )
(2) nitrogen,
(3) oxygen, and
(4) S(O)t where t is an integer 0-2,
provi ded that at least one of Y or Z
is other than carbon;
LA is selected from
( 1 ) a covalent bond,
(2) -O-,
3
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
(3) -S(O)S-,
(4) -NR6- where R6 is selected from
(a) hydrogen,
(b) alkyl of one to ten carbons optionally substituted with 1 or 2
substituents
independently selected from,
(i) aryl and
(ii) cycloalkyl of three to ten carbons,
(c) alkanoyl where the alkyl part is of one to ten carbons, and
(d) cycloalkyl of three to ten carbons,
(5) -C(W)- where W is selected from
(a) O and
(b) S, and
(6) alkenylene;
XA is selected from
( 1 ) halo,
(2) alkyl of one to ten carbons optionally substituted with 1, 2, or 3
substituents
independently selected from
(a) oxo,
(b) cycloalkyl of three to ten carbons,
(c) -COZR~ where R~ is selected from
(i) hydrogen and
(ii) alkyl of one to ten carbons optionally substituted with 1, or 2
substituents independently selected from
aryl and
cycloalkyl of three to ten carbons,
(d) -NRgR9 where Rg and R9 are independently selected from
(i) hydrogen,
(ii) alkyl of one to six carbons optionally substituted with 1 or 2
substituents independently selected from
-OH,
aryl,
heterocycle,
cycloalkyl of three to ten carbons, and
-NRARB where Ra and RB are independently selected from
hydrogen and
4
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
alkyl of one to six carbons optionally substituted with
1 or 2 substituents selected from -OH,
(iii) alkanoyl where the alkyl part is of one to to
ten carbons,
(iv) cycloalkyl of three to ten carbons,
(v) alkoxy,
(vi) heterocycle, and
(vii) aryl,
where (vi) and
(vii) are substituted
with 1 or 2 substituents
independently
selected from
alkyl of one to six carbons and
halo,
(e) -C(W)R,o where
W is previously
defined and Rlo
is selected from
(i) hydrogen,
(ii) alkyl of one to ten carbons optionally substituted
with l, or 2
substituents independently selected from
aryl ana
cycloalkyl of three to ten carbons,
(iii) -NR8R9, and
(iv) -ORS,
(f) -OH,
(g) aryl, and
(h) heterocycle,
where (g) and (h)
can be optionally
substituted with
l, 2, 3, 4, or
5 substituents
independently selected
from
(i) alkyl of one to twenty carbons,
(ii) -NR8R9,
(iii) alkoxy of one to ten carbons,
{iv) thioalkoxy of one to ten carbons,
(v) halo,
(vi) perfluoroalkyl of one to three carbons,
(vii) alkenyl of two to ten carbons,
(viii) alkyl of one to ten carbons optionally substituted
with 1 or 2
substituents independently selected from
alkoxy of one to ten carbons and
-OH,
(ix) -COZR~,
S
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
(x) aryl, and
(xi) -CHO,
(3) cycloalkyl of three to ten carbons,
(4) aryl,
(5) heterocycle
where (4) and (5) can be optionally substituted with l, 2, 3, 4, or 5
substituents
independently selected from
(a) alkyl of one to twenty carbons,
(b) alkyl of one to ten carbons substituted with 1, 2, or 3 substituents
independently selected from
(i) -OR> > where R, ~ is selected from
hydrogen,
-C(W)R12 where R~2 is selected from
alkyl of one to ten carbons,
cycloalkyl of three to ten carbons,
aryl, and
heterocycle, and
heterocycle optionally substituted with l, 2, 3, or 4
substituents independently selected from
-OH and
alkyl of one to six carbons optionally substituted with
1 or 2 substituents selected from -OH,
(ii) alkoxy of one to ten carbons optionally substituted with 1 or 2
substituents independently selected from
alkoxy and
alkoxyalkoxy,
(iii) spiroalkyl of three to ten carbons, and
(iv) halo,
(c) alkoxy of one to ten carbons optionally substituted with 1 or 2
substituents
independently selected from
(i) alkoxy, and
(ii) alkoxyalkoxy,
(d) thioalkoxy of one to ten carbons,
(e) halo,
(f) perfluoroalkyl of one to three carbons,
(g) alkenyl of two to ten carbons optionally substituted with 1 or 2
substituents
6
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
independently selected from
(i) -C(W)R,o and
Gi) -C(W)R~2
(h) -COZR~,
(i) -NR8R9,
G ) ~'Yl,
(k) -C(W)R12,
(1) -CHO,
(rn) -C(O)NR8R9,
(n) -CN,
(o) heterocycle optionally substituted with 1 or
2 substituents independently
selected from
(i) alkyl of one to ten carbons and
(ii) perfluoroalkyl of one to three carbons,
(p) -C(W)Rio
(q) ethylenedioxy, and
(r) -OCF3,
(6) -ORS,
(7) hydrogen,
and
(8) -NR8R9;
LB is selected from
( 1 ) a covalent bond,
(2) -O-,
(3) -S(O),-,
(4) -NR6-,
(5) -C(W)-, and
(6) -C(=NR,3)- where R,3 is selected from
(a) hydrogen,
(b) -NOz,
(c) -CN, and
(d) -OR,4 where R,4 is selected from
(i) hydrogen,
(ii) aryl, and
(iii) alkyl of one to ten carbons optionally substituted with 1 or 2
substituents independently selected from
7
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
aryl and
-C(O)R~5 where R,S is selected from
hydrogen,
-OH,
alkoxy, and
NRARB;
XB is selected from
(1) hydrogen,
(2) alkyl of one to ten carbons optionally substituted with 1, 2, or 3
substituents
independently selected from
(a) -COZR~,
(b) -NR8R9,
(c) -C(W)NR8R9,
(d) heterocycle,
(e) aryl optionally substituted with 1 or 2 substituents independently
selected
from
(i) alkyl of one to ten carbons,
(ii) -NOZ, and
(iii) -NRARB,
(f) -OR,6 where R,6 is selected from
(i) hydrogen and
(ii) -C(W)NRARB, and
(g) -NRAC(W)NR8R9,
(3) alkenyl of two to six carbons optionally substituted with 1 or 2
substituents
independently selected from
(a) -C(W)NRARB,
(b) -C02R~, and
(c) heterocycle,
(4) -NRI~R~g where R» and R18 are independently selected from
(a) hydrogen,
(b) alkyl of one to ten carbons optionally substituted with l, 2, or 3
substituents
independently selected from
(i) -OH,
(ii) -C(W)R~o
(iii) -NRAC(=NRI~)NRBR~9 where RA, RB, and Ri3 are previously defined
8
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
and R~9 is selected from
hydrogen,
alkyl of one to ten carbons, and
-NOa
(iv) heterocycle,
(v) aryl,
(vi) halo, and
(vii) -NRARB,
(c) alkoxy,
(d) aryl optionally substituted with 1, 2, or 3 substituents
independently selected
from
(i} halo,
(ii) alkyl of one to ten carbons,
(iii) alkoxy of one to ten carbons, and
(iv) perfluoroalkyl of one to three carbons,
(e) heterocycle,
(f) -NR,,RB,
(g) -C(O)R2o where R2o is selected from
(i) hydrogen,
(ii) alkyl of one to ten carbons,
(iii) -ORIZ, and
(iv) -NRARB,
(h) cycloalkyl of three to ten carbons, and
(i) -OH,
(5} alkoxy,
(6) -OH,
(7) -NRAC(=NRj3)NRBR,9,
(8} -C(W)NR8R9,
(9) aryl,
(10) heterocycle,
where
(9)
and
(10)
can
be
optionally
substituted
with
1,
2,
3,
4,
or
5
substituents
independently
selected
from
(a) halo,
(b) alkyl of one to ten carbons optionally substituted
with 1, 2, or 3 substituents
independently selected from
(i) halo,
9
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
(ii) aIkoxy of one to ten carbons,
(iii) -NRARB,
(iv) -OH,
(v) -COZR~,
(vi) -C(W)NRARB, and
(vii) aryl,
{c) -NRARB,
(d) alkoxy of one to ten carbons,
(e) thioalkoxy of one to ten carbons,
IO (f) perfluoroalkyl of one to three carbons,
(g) -OH,
(h) -C(W)NR8R9,
(i) -COZR~,
(j) -NRAC(W)ORZ, where RA is previously defined and
RZI is selected from
(i) alkyl of one to ten carbons optionally substituted
with 1 or 2
substituents selected from
aryl and
cycloalkyl of three to ten carbons,
(ii) aryl, and
(iii) cycloalkyl of three to ten carbons,
(k) alkenyl of two to ten carbons,
(1) heterocycle,
(m) aryl, and
(n) -NO2,
( 11 ) -CN,
(12) -CHO,
(I3} halo, and
(14) -B(ORA}(ORB);
provided that when RI, R2, R3, R4, and RS are hydrogen or absent, -L,,- is a
covalent bond,
and -LB- is a covalent bond, then one of XA or XB is other than hydrogen; and
R~, RZ, R3, R4, and RS are absent or independently selected from
{ 1 ) hydrogen,
(2) alkyl of one to six carbons optionally substituted with 1 or 2
substituents
independently selected from
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
(a) -OC(O)R22, where R2z is selected from
(i) alkyl,
(ii) alkoxy, and
(iii) NRARB,
(b) alkoxy,
(c) -OH,
(d) -NRARB,
(e) heterocycle, and
aryh
(3) -C02R~,
(4) -C(O )NRARB,
(5) -SR23
where R23
is selected
from
(a) hydrogen,
(b) alkyl of one to six carbons,
(c) aryl optionally substituted with I or 2 substituents
selected from
(i) alkyl of one to six carbons and
(ii) halo,
(6) -NRARB,
(7) halo,
(8) alkoxy,
(9) perfluoroalkyl of one to three carbons,
( IO) -OH, and
( 11 ) heterocycle,
provided that when E, F, and Y are carbon, G is nitrogen, Z is sulfur, -LA- is
a covalent
bond, and XA is halo, R1 is other than -C02R~.
In another embodiment of the invention are disclosed methods of treating
diseases
comprising administering an effective amount of a compound having Formula I.
In yet another embodiment of the invention are disclosed pharmaceutical
compositions containing compounds of Formula I.
11
CA 02333770 2000-11-30
WO 99!62908 PCT/US99/12419
Detailed Description of The Invention
Definition of Terms
The term "alkanoyl" as used herein refers to an alkyl group attached to the
parent
molecular group through a carbonyl group.
The term "alkenyl" as used herein refers to a monovalent straight or branched
chain
group of 2-12 carbon atoms containing at least one carbon-carbon double bond
derived
from an alkene by the removal of one hydrogen atom.
The term "alkenylene" as used herein refers to a divalent straight or branched
chain
group of 2-10 carbon atoms containing a carbon-carbon double bond derived from
an
alkene by the removal of two hydrogen atoms.
The term "alkoxy" as used herein refers to an alkyl group attached to the
parent
molecular group through an oxygen atom.
The term "alkoxyalkoxy" as used herein refers to an alkoxy group attached to
the
parent molecular group through another alkoxy group.
IS The term "alkoxycarbonyloxy," as used herein, refers to an alkoxy group, as
defined herein, appended to the parent molecular moiety through a carbonyloxy
group, as
defined herein.
The term "alkoxycarbonyloxymethylene," as used herein, refers to an
alkoxycarbonyloxy group, as defined herein, appended to the parent molecular
moiety
through a methylene group, as defined herein.
The term "alkyl" as used herein refers to a saturated straight or branched
chain
group of 1-20 carbon atoms derived from an alkane by the removal of one
hydrogen atom.
The term "alkylcarbonyl," as used herein, refers to an alkyl group, as defined
herein, appended to the parent molecular moiety through a carbonyl group.
The term "alkylcarbonyloxy," as used herein, refers to an alkyl group, as
defined
herein, appended to the parent molecular moiety through a carbonyloxy group,
as defined
herein.
The term "alkylcarbonyloxymethylene," as used herein, refers to an
aIkylcarbonyloxy group, as defined herein, appended to the parent molecular
moiety
through a methylene group, as defined herein.
The term "alkylene," denotes a divalent group derived from a straight or
branched
chain hydrocarbon of from 1 to 10 carbon atoms. Representative examples of
alkyiene
include, but are not limited to, -CHZ-, -CHZCHZ-, -CHZCHZCH2-, -CH2CH2CH2CH2-,
-CHzCH(CH3)CHZ-, and the like.
The term "amino," as used herein, refers to a-NR8oR8, group, where R8o and Rg,
are independently selected from hydrogen and alkyl.
12
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
The term "aminocarbonyl," as used herein, refers to an amino group, as defined
herein, appended to the parent molecular moiety through a carbonyl group, as
defined
herein.
The term "aminocarbonyloxy," as used herein, refers to an aminocarbonyl group,
as
defined herein, appended to the parent molecular moiety through an oxy group,
as defined
herein.
The term "aminocarbonyloxymethylene," as used herein, refers to an
aminocarbonyloxy group, as defined herein, appended to the parent molecular
moiety
through a methylene group, as defined herein.
The term "aryl" refers to a mono- or bicyclic carbocyclic ring system having
one or
two aromatic rings. The aryl group can also be fused to a cyclohexane,
cyclohexene,
cyclopentane or cyclopentene ring. The aryl groups of this invention can be
optionally
substituted.
The term "carbonyl," as used herein, refers to a -C(O)- group.
The term "carbonyloxy," as used herein, refers to a carbonyl group as defined
herein, appended to the parent molecular moiety through an oxy group, as
defined herein.
The term "cycloalkyl" as used herein refers to a monovalent saturated cyclic
hydrocarbon group of 3-12 carbons derived from a cycloalkane by the removal of
a single
hydrogen atom.
The term "ethylenedioxy," as used herein, refers to a -O(CH2)20- group wherein
the oxygen atoms of the ethylenedioxy group are attached to the parent
molecular moiety
through one carbon atom forming a 5 membered ring or the oxygen atoms of the
ethylenedioxy group are attached to the parent molecular moiety through two
adjacent
carbon atoms forming a six membered ring.
The terms "halo" or "halogen" as used herein refers to F, CI, Br, or I.
The term "heterocycle" represents a represents a 4-, 5-, 6- or 7-membered ring
containing one, two or three heteroatoms independently selected from the group
consisting
of nitrogen, oxygen and sulfur. The 4- and 5-membered rings have zero to two
double
bonds and the 6- and 7-membered rings have zero to three double bonds. The
term
"heterocycle" also includes bicyclic, tricyclic and tetracyclic groups in
which any of the
above heterocyclic rings is fused to one or two rings independently selected
from an aryl
ring, a cyclohexane ring, a cyclohexene ring, a cyclopentane ring, a
cyclopentene ring or
another monocyclic heterocyclic ring. Heterocycles include acridinyl,
benzimidazolyl,
benzofuryl, benzothiazolyl, benzothienyl, benzoxazolyl, biotinyl, cinnolinyl,
dihydrofuryl,
dihydroindolyl, dihydropyranyl, dihydrothienyl, dithiazolyl, furyl,
homopiperidinyl,
imidazolidinyl, imidazolinyl, imidazolyl, indolyl, isoquinolyl,
isothiazolidinyl,
13
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
isothiazolyl, isoxazolidinyl, isoxazolyl, morphoIinyl, oxadiazolyl,
oxazolidinyl, oxazolyl,
oxadiazolyl, piperazinyl, piperidinyl, pyranyl, pyrazolidinyl, pyrazinyl,
pyrazolyl,
pyrazolinyl, pyridazinyl, pyridyl, pyrimidinyl, pyrimidyl, pyrrolidinyl,
pyrrolinyl, pyrrolyl,
quinolinyl, quinoxaloyl, tetrahydrofuryl, tetrahydroisoquinolyl,
tetrahydroquinolyl,
tetrazolyl, thiadiazolyl, thiazolidinyl, thiazolyl, thienyl, thiomorpholinyl,
triazolyl, and the
like.
Heterocyclics also include bridged bicyclic groups where a monocyclic
heterocyclic group is bridged by an alkylene group such as
, , , and the like.
Heterocyclics also include compounds of the formula
where X* is selected from -CHZ-, -CH20- and -O-, and Y* is selected from
--C(O)- and -(C(R")2)~-, where R" is hydrogen or alkyl of one to four carbons,
and v is 1
3. These heterocycles include 1,3-benzodioxolyl, 1,4-benzodioxanyl, and the
like. The
1 S heterocycle groups of this invention can be optionally substituted.
The term "oxo," as used herein, refers to =O.
The term "oxy," as used herein, refers to -O-.
The term "methylene," as used herein, refers to a -CHI- group.
The term "perfluoroalkyl" as used herein refers to an alkyl group in which all
of the
hydrogen atoms have been replaced by fluoride atoms.
The term "phenyl" as used herein refers to a monocyclic carbocyclic ring
system
having one aromatic ring. The aryl group can also be fused to a cyclohexane or
cyclopentane ring. The phenyl groups of this invention can be optionally
substituted.
The term "pharmaceutically acceptable prodrugs" as used herein represents
those
prodrugs of the compounds of the present invention which are, within the scope
of sound
medical judgment, suitable for use in contact with the tissues of humans and
lower animals
with undue toxicity, irritation, allergic response, and the like, commensurate
with a
reasonable benefit/risk ratio, and effective for their intended use, as well
as the
zwitterionic forms, where possible, of the compounds of the invention.
14
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
The term "prodrug," as used herein, represents compounds which are rapidly
transformed in vivo to the parent compound of the above formula, for example,
by
hydrolysis in blood. A thorough discussion is provided in T. Higuchi and V.
Stella, Pro-
drugs as Novel Delivery Systems, Vol. 14 of the A.C.S. Symposium Series, and
in
S Edward B. Roche, ed., BioreversibIe Carriers in Drug Design, American
Pharmaceutical
Association and Pergamon Press, 1987, both of which are incorporated herein by
reference.
The term "spiroalkyl" as used herein refers to an alkylene group wherein two
carbon atoms of the alkylene group are attached to one carbon atom of the
parent
molecular group thereby forming a carbocyclic ring of three to eleven carbon
atoms.
The term "tautomer" as used herein refers to a proton shift from one atom of a
molecule to another atom of the same molecule wherein two or more structurally
distinct
compounds are in equilibrium with each other.
The term "thioalkoxy" as used herein refers to an alkyl group attached to the
parent
1 S molecular group through a sulfur atom.
Compounds of the present invention can exist as stereoisomers wherein
asymmetric or chiral centers are present. These compounds are designated by
the symbols
"R" or "S," depending on the configuration of substituents around the chiral
carbon atom.
The present invention contemplates various stereoisomers and mixtures thereof.
Stereoisomers include enantiomers and diastereomers, and mixtures of
enantiomers nr
diastereomers are designated Individual stereoisomers of compounds of
the present invention can be prepared synthetically from commercially
available starting
materials which contain asymmetric or chiral centers or by preparation of
racemic
mixtures followed by resolution well-known to those of ordinary skill in the
art. These
methods of resolution are exemplified by ( 1 ) attachment of a mixture of
enantiomers to a
chiral auxiliary, separation of the resulting mixture of diastereomers by
recrystallization or
chromatography and liberation of the optically pure product from the auxiliary
or (2) direct
separation of the mixture of optical enantiomers on chiral chromatographic
columns.
Geometric isomers can exist in the compounds of the present invention. The
present invention contemplates the various geometric isomers and mixtures
thereof
resulting from the arrangement of substituents around a carbon-carbon double
bond or
arrangement of substituents around a carbocyclic ring. Substituents around a
carbon-
carbon double bond are designated as being in the Z or E configuration wherein
the term
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
"Z" represents substituents on the same side of the carbon-carbon double bond
and the
term "E" represents substituents on opposite sides of the carbon-carbon double
bond. The
arrangement of substituents around a carbocyclic ring are designated as cis or
trans
wherein the term "cis" represents substituents on the same side of the plane
of the ring and
the term "trans" represents substituents on opposite sides of the plane of the
ring.
Mixtures of compounds wherein the substituents are disposed on both the same
and
opposite sides of plane of the ring are designated cis/trans.
Tautomers can also exist in the compounds of the present invention. The
present
invention contemplates tautomers due to proton shifts from one atom to another
atom of
the same molecule generating two distinct compounds that are in equilibrium
with each
other.
Compounds of the present invention include, but are not limited to
methyl 2-[(6-ethylthieno[2,3-d]pyrimidin-4-yl)thio]acetate,
6-ethyl-4-[(4-methylphenyl)thio]thieno[2,3-d]pyrimidine,
6-ethyl-4-(2-pyridinylthio)thieno[2,3-d]pyrimidine,
6-ethyl-4-[(2-methylethyl)thio]thieno[2,3-d]pyrimidine,
6-ethyl-4-[(phenylmethyl)thio]thieno[2,3-d]pyrimidine,
6-ethyl-4-[(S-methyl-1,3,4-thiadiazol-2-yl)thio]thieno[2,3-d]pyrimidine,
ethyl 6-ethyl-4-[(4-methylphenyl)thio]thieno[2,3-d]pyrimidine-6-carboxylate,
6-ethyl-N-(phenylmethyl)thieno[2,3-d]pyrimidin-4-amine,
6-ethyl-N-(S-methyl-1,3,4-thiadiazol-2-yl)thieno[2,3-d]pyrimidin-4-amine,
4-[(5-amino-1,3,4-thiadiazol-2-yl)thio]-6-ethyl-2-(phenylmethyl)thieno(2,3-
d)pyrimidine,
4-chloro-6-ethyl-2-(phenylmethyl)thieno[2,3-d]pyrimidine,
4-[(5-amino-1,3,4-thiadiazol-2-yl)thio]-6-ethyl-2-(phenylmethyl)thieno[2,3-
d]pyrimidine,
7-methyl-4-[(4-methylphenyl)thio]thieno[3,2-d]pyrimidine,
7-methyl-4-[(5-methyl-1,3,4-thiadiazol-2-yl)thio]thieno(3,2-d]pyrimidine,
7-methyl-4-[[5-(methylthio)-1,3,4-thiadiazol-2-yl)thio]thieno [3,2-
d]pyrimidine,
4-[(5-amino-1,3,4-thiadiazol-2-yl)thio]-7-methylthieno[3,2-d]pyrimidine,
7-methyl-N-[(4-(methylthio)phenyl]thieno[3,2-d]pyrimidin-7-amine,
7-methyl-4-[(4-methylphenyl)thin]thieno[3,2-d]pyrimidine-6-carboxamide,
methyl 4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-carboxylate,
4-[(4-methylphenyl)thio]thieno[2,3-c)pyridine-2-carboxylic acid,
4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-carboxamide,
16
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
4-(2-pyridinylthio}thieno[2,3-c]pyridine-2-carboxamide,
4-[(4-chlorophenyl)thio]thieno[2,3-c]pyridine-2-carboxamide,
N-methoxy-N-methyl-4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-
carboxamide,
N-methoxy-4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-carboxamide,
N-(4-chlorophenyl)-4-[(4-methylphenyl)thin]thieno[2,3-c]pyridine-2-
carboxamide,
4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-carboxaldehyde,
4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-carboxaldehyde, O-
methyloxime,
4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-carboxaldehyde, O-
(phenylmethyl)oxime,
2-[[[4-[(4-methylphenyl)thio]thieno[2,3-c]pyridin-2-yImethylene]
amino]oxy]acetic acid,
4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-carboxaldehyde, O-
phenyloxime,
4-[(4-methylphenyl)thin]thieno[2,3-c]pyridine-2-carboxaldehyde, oxime,
2-[[[4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-ylmethylene]-
amino]oxy]acetamide,
(E)-3-[(4-methylphenyl)thio]thieno[2,3-c]pyridin-2-yI]-2-propenamide,
1-[4-[(4-methylphenyl)thio]thieno[2,3-c]pyridin-2-yl]ethanone,
2-benzoyl-4-[(4-methylphenyl)thin]thieno[2,3-c]pyridine,
2-ethyl-4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine,
1-[4-[(4-methylphenyl)thio]thieno[2,3-c]pyridin-2-yl]ethanone, oxime,
N-(2,3-dihydroxypropyl}-4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-
carboxamide,
4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-carboxylic acid, hydrazide,
N2-4-[(4-methylphenyl)thio]thieno[2,3-c]pyridin-2-yl]carbonyl]-N6-
[(nitroamino)iminomethyl]-L-lysine, methyl ester,
N-(aminoiminomethyl)-4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-
carboxamide,
4-[(4-methylphenyl)thio)thieno[2,3-c]pyridine-2-carbothioamide,
4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine,
methyl 4-[(2-methoxy-2-oxoethyl)thio]thieno[2,3-c]pyridine-2-carboxylate,
4-[(2-amino-2-oxoethyl)thio]thieno[2,3-c]pyridine-2-carboxamide,
4-[(4-bromophenyl)thio]thieno[2,3-c]pyridine-2-carboxamide,
17
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-(phenylthio)thieno[2,3-c]pyridine-2-carboxamide,
4-[[4-(trifluoromethyl)phenyl]thio]thieno[2,3-c]pyridine-2-carboxamide,
4-[(2-methylphenyl)thio]thieno[2,3-c]pyridine-2-carboxamide,
4-[(3-methylphenyl)thio]thieno(2,3-c]pyridine-2-carboxanude,
4-[(3,4-dimethylphenyl)thio]thieno[2,3-c]pyridine-2-carboxamide,
4-[(3,5-dimethylphenyl)thio]thieno[2,3-c]pyridine-2-carboxamide,
4-[(2,4-dimethylphenyl)thio]thieno[2,3-c]pyridine-2-carboxamide,
4-[(2-methyl-3-furanyl)thio]thieno[2,3-c]pyridine-2-carboxamide,
4-[[(4-chlorophenyl)methyl]thio]thieno[2,3-c]pyridine-2-carboxamide,
4-((3,4-dichlorophenyl)thio]thieno[2,3-c]pyridine-2-carboxamide,
4-[(4-methoxyphenyl)thio]thieno[2,3-cJpyridine-2-carboxamide,
4-(cyclohexylthio)thieno[2,3-c]pyridine-2-carboxamide,
4-[{4-methylphenyl)thio]-N-[3-(4-morpholinyl)propyl]thieno[2,3-
c]pyridine-2-carboxamide,trifluoromethylacetate salt,
4-[(4-methylphenyl)sulfinyl]thieno[2,3-cJpyridine-2-carboxamide,
methyl 4-[(4-methylphenyl)sulfinyl]thieno[2,3-c]pyridine-2-carboxylate,
4-(4-methylphenoxy)thieno[2,3-c]pyridine-2-carboxamide,
methyl 4-(4-methylphenoxy)thieno[2,3-c]pyridine-2-carboxylate,
4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
methyl4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxylate,
4-[4-(trifluoromethyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
4-(4-octylphenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-[4-( I-methylethyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
4-(2-bromo-4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-ethylphenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-ethenylphenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-[4-( 1,2-dihydroxyethyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
4-[2-(2-propenyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
4-[2-(2,3-dihydroxypropyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
4-[4-(trifluoromethyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide, 1-
oxide,
4-[3-(pentadecyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
4-(4-bromophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(3-chlorophenoxy)thieno[2,3-cJpyridine-2-carboxamide,
4-(4-t-butylphenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-chloro-3-methylphenoxy)thieno[2,3-c]pyridine-2-carboxamide,
18
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-(4-chloro-2-methylphenoxy)thieno(2,3-c]pyridine-2-carboxamide,
4-(4-methoxyphenoxy)thieno [2, 3-c] pyridine-2-carboxamide,
ethyl 3-([2-(aminocarbonyl)thieno[2,3-c]pyridin-4-yl]oxy]benzoate,
4-phenoxythieno (2, 3-c] pyridine-2-carboxamide,
4-(3-bromophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-fluorophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(3,5-dimethylphenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(3-chloro-4-methylphenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-iodophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-(methoxymethyl)phenoxy)thieno[2,3-c]pyridine-2-carboxamide,
2-(aminocarbonyl)-4-(4-chlorophenoxy)thieno[2,3-c]pyridinium, iodide,
4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxylic acid,
N-(4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl)-O-(3-
tetrahydrofuranyl)carbamate,
4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-methanol,
(E)-3-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl:]-2-propenoic acid,
4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxaldehyde,
(E)-3-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-2-propenamide,
4-bromothieno[2,3-c]pyridine-2-carboxamide,
methyl4-bromothieno[2,3-c]pyridine-2-carboxylate,
4-chlorothieno[2,3-c]pyridine-2-carboxamide,
4-[4-(trifluoromethyl)phenyl]thieno[2,3-c]pyridine-2-carboxamide,
methyl 4-[4-(trifluoromethyl)phenyl]thieno[2,3-c]pyridine-2-carboxylate,
N-methyl-4-[4-(trifluoromethyl)phenyl]thieno[2,3-c]pyridine-2-carboxamide,
4-phenylthieno[2,3-c]pyridine-2-carboxamide,
methyl 4-phenylthieno[2,3-c]pyridine-2-carboxylate,
4-([ 1,1'-biphenyl]-4-ylthio)thieno[2,3-c]pyridine-2-carboxamide,
4-(5-formyl-2-furanyl)thieno[2,3-c]pyridine-2-carboxamide,
ethyl 4-[[2-(aminocarbonyl)thieno[2,3-c]pyridin-4-yl]oxy]benzoate,
4-[(2-(aminocarbonyl)thieno[2,3-c]pyridin-4-yl]oxy]benzoic acid,
4-( 1-phenylethenyl)thieno[2,3-c]pyridine-2-carboxamide,
methyl 4-( 1-phenylethenyl)thieno[2,3-c]pyridine-2-carboxalate,
4-[(4-methylphenyl)thio]thieno[2,3-c]pyridine-2-methanol,
4-(4-Chlorophenoxy)-N-methylthieno[2,3-c]pyridine-2-carboxamide,
4-(4-chlorophenoxy)-N,N-dimethylthieno[2,3-c]pyridine-2-carboxamide,
4-(4-chlorophenoxy)-N,N-diethylthieno[2,3-c]pyridine-2-carboxamide,
19
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/I2419
4-(4-chlorophenoxy)-N-cyclopropylthieno[2,3-c]pyridine-2-carboxamide,
1-[[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]carbonyl]pyrrolidine,
1-[[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-ylJcarbonylJpiperidine,
4-[[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yI]carbonyl]morpholine,
1-[[4-(4-chlorophenoxy)thieno[2,3-c)pyridin-2-yl]carbonyl]-4-
methylpiperazine,
1-[[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]carbonyl]-4-
phenyllpiperazine,
1-[[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]carbonyl)-4-(phenylmethyl)-
piperazine,
1-[[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]carbonyl]-4-(2-pyridinyl)-
piperazine,
4-(4-chlorophenoxy)-N-(2-hydroxyethyl)lthieno[2,3-c]pyridine-2-carboxamide,
4-[[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yI]carbonyl]-N-( 1-
methylethyl}-1-piperazineacetamide, trifluoroacetate salt,
4-(4-chlorophenoxy)-N-( 1-(hydroxymethyl)ethyl]thieno[2,3-c]pyridine-2-
carboxamide,
4-(4-chlorophenoxy)-N-[ 1,1-bis(hydroxymethyl)ethyl]thieno[2,3-
c]pyridine-2-carboxamide,
(D,L)-4-(4-chlorophenoxy)-N-(2-hydroxypropyl)thieno[2,3-c]pyridine-2-
carboxamide,
4-(4-chlorophenoxy)-N-[2-(4-morpholinyl)ethyl)thieno[2,3-c]pyridine-2-
carboxamide,
4-(4-chlorophenoxy)thieno[2,3-c)pyridine-2-sulfonamide,
4-((4-methylphenyl)methyl]thieno[2,3-c]pyridine-2-carboxamide,
methyl4-[(4-methylphenyl)methyl)thieno[2,3-c]pyridine-2-carboxylate,
4-(4-morpholinyl)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxamide, N-oxide,
methyl (4-chlorophenoxy)thieno[2,3-c)pyridine-2-carboxylic acid, N-oxide,
4-(4-chlorophenoxy)-2-(2-methoxyphenyl)thieno[2,3-c]pyridine,
4-(4-Chlorophenoxy)thieno[2,3-cJpyridine,
4-(4-Chlorophenoxy)-3-methylthieno(2,3-cJpyridine-2-carboxamide,
Methyl 4-(4-chlorophenoxy)-3-methylthieno[2,3-c]pyridine-2-carboxylate,
4-(4-Chlorophenoxy)-3-hydroxythieno[2,3-c]pyridine-2-carboxamide,
methyl 4-(4-chlorophenoxy)-3-hydroxythieno[2,3-c]pyridine-2-carboxylate,
4-(4-Chlorophenoxy)-3-(1-methylethoxy)thieno[2,3-c]pyridine-2-carboxamide,
3-bromo-4-(4-chlorophenoxy)thieno[2,3-c]pyridine,
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-(4-Chlorophenoxy)thieno[2,3-c]pyridine-3-carboxylic acid,
4-(4-Chlorophenoxy)thieno[2,3-c]pyridine-3-carboxamide,
3-amino-4-(4-chlorophenoxy)thieno[2,3-c)pyridine-2-carboxamide,
methyl 3-amino-4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxylate,
3-amino-4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxylic acid,
4-((4-methylphenyl)thio)thieno[2,3-b]pyridine,
4-[(4-methylphenyl)thin]thieno[2,3-b]pyridine-2-carboxamide,
4-chloro-N-(4-chlorophenyl)thieno[2,3-b]pyridine-5-carboxamide,
ethyl 4-[(5-methyl-1,3,4-thiadiazol-2-yl}thio]thieno[2,3-b]pyridine-5-
carboxylate,
7-[(4-methylphenyl)thio)thieno[3,2-b]pyridine-2-carboxamide,
methyl 6-[{4-methylphenyl)thioJthieno[2,3-b]pyridine-2-carboxylate,
methyl 3-amino-6-chlorothieno[2,3-bJpyridine-2-carboxylate,
6-[(4-methylphenyl)thin]thieno[2,3-bJpyridine-2-carboxamide,
2-bromo-4-[(4-methylphenyl)thio]thieno[3,2-c]pyridine,
4-[(4-methylphenyI)thin)thieno[3,2-c]pyridine-2-carboxamide,
4-[(4-methylphenyl)thio)thieno[3,2-c]pyridine-2-carbonitrile,
4-(4-Methylphenoxy)thieno[3,2-c]pyridine-2-carboxamide,
4-(4-Methylphenoxy)thieno[3,2-cJpyridine-2-carbonitrile,
7-(4-methylphenoxy)oxazolo[5,4-c)pyridine-2-carboxamide,
methyl7-(4-methylphenoxy)oxazolo[5,4-c]pyridine-2-carboxylate,
7-(4-methylphenoxy)[ 1,3]thiazolo [5,4-c]pyridine-2-carboxamide,
methyl 7-(4-methylphenoxy)[1,3]thiazolo[5,4-cJpyridine-2-carboxylate,
7-(4-methylphenoxy)-3H-inudazo[4,5-c]pyridine-2-carboxamide,
methyl 7-(4-methylphenoxy)-3H-imidazo[4,5-c]pyridine-2-carboxylate,
4-(4-chlorophenoxy)thieno[2,3-d]pyridazine-2-carboxamide,
4-(4-chlorophenoxy)thieno[2,3-d]pyridazine-2-carboxylic acid,
7-(4-chlorophenoxy)thieno[3,2-c]pyridine-2-carbamide,
7-(4-chlorophenoxy)thieno[3,2-c]pyridine-2-carboxylic acid,
4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carbothioamide,
4-(4-chlorophenoxy)-N-ethylthieno[2,3-c)pyridine-2-carboxamide,
4-(4-chlorophenoxy)-N-(2,3-dihydroxypropyl)thieno[2,3-c]pyridine-2-
carboxamide,
4-(4-bromophenoxy)-N-(2,3-dihydroxypropyl)thieno[2,3-c]pyridine-2-
carboxamide,
N-(2-chloroethyl)-4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-bromophenoxy)-N-(2-hydroxyethyl)thieno[2,3-c]pyridine-2-carboxamide,
21
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-(2-bromo-4.-chlorophenoxy)-N-(2-hydroxyethyl)thieno[2,3-c]pyridine-2-
carboxamide,
N-(2-hydroxyethyl)-4-[4-(trifluoromethyl)phenoxy]thieno[2,3-c]pyridine-2-
carboxamide,
N-(2-aminoethyl)-4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-chlorophenoxy)-N-hydroxythieno[2,3-cJpyridine-2-carboxamide,
4-(4-chlorophenoxy)thieno[2,3-c)pyridine-2-carbohydrazide,
4-(4-bromophenoxy)thieno[2,3-c]pyridine-2-carbohydrazide,
4-[4-(trifluoromethyl)phenoxy]thieno[2,3-c)pyridine-2-carbahydrazide,
4-(4-chlorophenoxy)-N-hydroxythieno[2,3-c]pyridine-2-carboxamide,
2-({[4-(4-chlorophenoxy)thieno[2,3-cJpyr'idin-2-yl]carbonyl}amino)acetic acid,
N-(2-amino-2-oxoethyl)-4-(4-chlorophenoxy)thieno[2,3-cJpyridine-2-
carboxamide,
N-(2-amino-2-oxoethyl}-4-(4-bromophenoxy)thieno[2,3-c]pyridine-2-
carboxamide,
(2S)-2-( { (4-(4-chlorophenoxy)thieno[2,3-cJpyridin-2-ylJcarbonyl } amino)-3-
hydroxypropanoic acid,
N-[( 1 S)-2-amino- I -(hydroxymethyl)-2-oxoethylJ-4-(4-
chlorophenoxy)thieno[2,3-
c]pyridine-2-carboxamide,
(2R)-2-( { [4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]carbonyl } amino)-3-
hydroxypropanoic acid,
(2R)-2-({ [4-(4-chlorophenoxy)thieno[2,3-cJpyridin-2-
yl]carbonyl}amino)propanoic acid,
4-(4-chlorophenoxy)-N-[( I R)- I-methyl-2-(methylamino)-2-oxoethyl]thieno[2,3-
c]pyridine-2-carboxamide,
(2S)-2-( { [4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-
yl)carbonyl}amino)propanoic acid,
4-(4-chlorophenoxy)-N-[( 1 S)-I-methyl-2-(methylamino)-2-oxoethyl]thieno[2,3-
c]pyridine-2-carboxamide,
4-(4-chlorophenoxy)-N-[(1R)-1-(hydroxymethyl)-2-(methylamino)-2
oxoethylJthieno[2,3-c)pyridine-2-carboxamide,
4-(4-chlorophenoxy)-N-[( 1 S)- I -(hydroxymethyl)-2-(methylamino)-2-
oxoethyl)thieno[2,3-c]pyridine-2-carboxamide,
4-(3-pyridinyloxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-bromophenoxy)-N-methylthieno[2,3-c]pyridine-2-carboxamide,
4-(4-bromophenoxy)-N,N-dimethylthieno[2,3-c)pyridine-2-carboxamide,
22
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
N,N-dimethyl-4-[4-(trifluoromethyl)phenoxy]thieno[2,3-c]pyridine-2-
carboxamide,
4-(4-chloro-3-fluorophenoxy)-N-methylthieno[2,3-c]pyridine-2-carboxamide,
4-(4-chloro-3-fluorophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-chloro-3-ethylphenoxy)thieno[2,3-a]pyridine-2-carboxamide,
4-(3-fluorophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(2,3-difluorophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(2,3-difluorophenoxy)-N-methylthieno[2,3-c]pyridine-2-carboxamide,
4-(3-fluorophenoxy)-N-methylthieno[2,3-c)pyridine-2-carboxamide,
N-methyl-4-(2,3,4-trifluorophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(2,3,4-trifluorophenoxy)thieno[2,3-c)pyridine-2-carboxamide,
N-methyl-4-[4-(trifluoromethyl)phenoxy)thieno[2,3-c)pyridine-2-carboxamide,
4-[3-(trifluoromethyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
N,N-dimethyl-4-(4-vinylphenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-cyanophenoxy)-N-methylthieno[2,3-c]pyridine-2-carboxamide,
4-(4-cyanophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-aminophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
4-[4-(acetylamino)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
N-methyl-4-[4-(4-morpholinyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
4-[4-(hydroxymethyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
4-[4-(hydroxymethyl)phenoxy]-N-methylthieno[2,3-c]pyridine-2-carboxamide,
4-[4-(methoxymethyl)phenoxy]-N-methylthieno[2,3-c]pyridine-2-carboxamide,
4-{4-[(2-methoxyethoxy)methyl]phenoxy }thieno[2,3-c]pyridine-2-carboxamide,
4-{4-[(2-methoxyethoxy)methyI]phenoxy }-N-methylthieno[2,3-c]pyridine-2-
carboxamide,
4-(4-{ [2-(2-methoxyethoxy)ethoxy]methyl } phenoxy)thieno[2,3-c]pyridine-2-
carboxamide,
4-(4-{ [2-(2-methoxyethoxy)ethoxy]methyl }phenoxy)-N-methylthieno[2,3-
c]pyridine-2-carboxamide,
4-{4-[(tetrahydro-2H-pyran-2-yloxy)methyl]phenoxy}thieno[2,3-c)pyridine-2-
carboxamide,
N-methyl-4-{ 4-((tetrahydro-2H-pyran-2-yloxy)methyl]phenoxy } thieno[2,3-
c]pyridine-2-carboxamide,
4-{ [2-(aminocarbonyI)thieno[2,3-c)pyridin-4-yl]oxy }benzyl 2-furoate,
4-[4-( { [(2R,4R,SS,6R)-4,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-
yl]oxy } methyl)phenoxy]-N-methylthieno[2,3-c]pyridine-2-carboxamide,
23
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/124I9
4-(4-acetylphenoxy)-N-methylthieno[2,3-c]pyridine-2-carboxamide,
4-[4-(4-morpholinylcarbonyl)phenoxyJthieno[2,3-c]pyridine-2-carboxamide,
N-methyl-4-[4-(4-morpholinylcarbonyl)phenoxy)thieno[2,3-c]pyridine-2-
carboxamide,
4-[4-( { [2-(4-morpholinyl)ethyl]amino } carbonyl)phenoxy)thieno[2,3-
c]pyridine-2-
carboxamide,
N-methyl-4-[4-({ [2-(4-morpholinyl)ethyl)amino }carbonyl)phenoxy]thieno[2,3-
c]pyridine-2-carboxamide,
4- { 4-[(E)-3-(4-morpholinyl)-3-oxo-1-propenyl)phenoxy } thieno [2,3-
c)pyridine-2-
carboxamide,
4-[4-((E)-3-{ [2-(4-morpholinyl)ethylJamino}-3-oxo-1-
propenyl)phenoxy]thieno[2,3-cJpyridine-2-carboxamide,
N-methyl-4-[4-((E)-3-{ [2-(4-morpholinyl)ethyl)amino }-3-oxo-I-
propenyl)phenoxy]thieno[2,3-c)pyridine-2-carboxamide,
4-(4-{(E)-3-[(2,3-dihydroxypropyl)amino]-3-oxo-1-propenyl}phenoxy)thieno[2,3-
c]pyridine-2-carboxamide,
4-(4- { (E)-3-[(2,3-dihydroxypropyl)amino]-3-oxo- I -propenyl } phenoxy)-N-
methylthieno[2,3-c]pyridine-2-carboxamide,
4-[4-((E)-3-{ [2-( 1H-imidazol-4-yl)ethyl]amino }-3-oxo-I-propenyl)phenoxy]-N-
methylthieno[2,3-c]pyridine-2-carboxamide,
4- { 4-[(E)-3-( { 2-[bis(2-hydroxyethyl)amino]ethyl } amino)-3-oxo- I -
propenyl]phenoxy }-N-methylthieno[2,3-c]pyridine-2-carboxamide,
4- { 4-[(E)-3-( { 2-[bis(2-hydroxyethyl)amino]ethyl } amino)-3-oxo-1-
propenyl]phenoxy } thieno[2,3-c)pyridine-2-carboxamide,
4-[4-(1H-imidazol-1-yl)phenoxy]thieno[2,3-c)pyridine-2-carboxamide,
N-methyl-4-[4-( 1 H-pyrazol-1-yl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
N-methyl-4-[4-( 1 H-1,2,4-triazol-1-yl)phenoxyJthieno [2,3-c]pyridine-2-
carboxarnide,
N-methyl-4-{ 4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]phenoxy } thieno[2,3-
c)pyridine-2-carboxamide,
4-[4-(4,5-dihydro-1 H-imidazol-2-yl)phenoxy]-N-methylthieno[2,3-c]pyridine-2-
carboxamide,
N-methyl-4-[4-(2-thienyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
4-([ 1,1'-biphenyl]-4-yloxy)-N-methylthieno[2,3-c]pyridine-2-carboxamide,
N-methyl-4-[4-(I-methyl-1H-imidazol-5-yl)phenoxy]thieno[2,3-c)pyridine-2-
carboxamide,
24
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-{ 4-[ 1-(hydroxymethyl)cyclopropyl]phenoxy }-N-methylthieno [2,3-c]pyridine-
2-
carboxamide,
4-[4-( 1- { [2-(2-ethoxyethoxy)ethoxy]methyl } cyclopropyl)phenoxy]-N-
methylthieno[2,3-c]pyridine-2-carboxamide,
N-methyl-4-[4-(trifluoromethoxy)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
5-{ 4-[4-( 1-{ [2-(2-ethoxyethoxy)ethoxy]methyl
}cyclopropyl)phenoxy]thieno[2,3-
c]pyridin-2-yl}-1,3,4-oxadiazol-2-amine,
4-[4-( 1,1-difluoro-2-hydroxyethyl)phenoxy]-N-methylthieno[2,3-c]pyridine-2-
carboxamide,
4-(4-{2-[2-(2-ethoxyethoxy)ethoxy]-1,1-difluoroethyl}phenoxy)-N-
methylthieno[2,3-c]pyridine-2-carboxamide,
4-(4-chlorophenoxy)-6-{ [(2,2-dimethylpropanoyl)oxy]methyl }-2-
[(methylamino)carbonyl]thieno[2,3-c]pyridin-6-ium,
4-{4-bromophenoxy)-6-{ [(2,2-dimethylpropanoyl)oxy]methyl }-2-
[(methylamino)carbonyl]thieno[2,3-c]pyridin-6-ium,
2-(aminocarbonyl)-4-(4-chlorophenoxy)-6-
{ [(isopropoxycarbonyl)oxy]methyl } thieno[2,3-c]pyridin-6-ium,
4-(benzyloxy)thieno[2,3-c]pyridine-2-carboxamide,
4-[(4-chlorophenyl)(hydroxy)methyl]thieno[2,3-c]pyridine-2-carboxamide,
4-(4-chlorobenzoyl)-N-methylthieno[2,3-c]pyridine-2-carboxamide,
N4-(4-chlorophenyl)thieno[2,3-cjpyridine-2,4-dicarboxamide,
[4-(4-bromophenoxy)thieno[2,3-c]pyridin-2-yl]methanol,
4-(4-bromophenoxy)thieno[2,3-c)pyridine-2-carbaldehyde,
4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carbaldehyde oxime,
4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carbaldehyde O-methyloxime,
1-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-1-ethanone O-methyloxime,
1-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-1-ethanone O-methyloxirne,
1-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-1-ethanone oxime,
1-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-1-ethanone oxime,
1-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-1-propanone,
1-[4-(4-chlorophenoxy)thieno[2,3-c)pyridin-2-yl]-1-propanone oxime,
2-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-N-methoxy-N-methyl-2-
oxoacetamide,
4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carbonitrile,
4-(4-chlorophenoxy)-N'-hydroxythieno[2,3-c]pyridine-2-carboximidamide,
4-(4-chlorophenoxy)-N'-cyanothieno[2,3-c]pyridine-2-carboximidamide,
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl](2-nitrophenyl)methanol,
[4-(4-chlorophenoxy)thieno[2,3-cJpyridin-2-yl](2-nitrophenyl)methanone,
(2-aminophenyl)[4-(4-chlorophenoxy}thieno[2,3-cJpyridin-2-yl]methanone,
(2-aminophenyl)[4-(4-chlorophenoxy)thieno[2,3-cJpyridin-2-yl]methanol,
[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl](3-nitrophenyl)methanol,
(3-aminophenyl)[4-(4-chlorophenoxy)thieno[2,3-cJpyridin-2-ylJmethanone,
(3-aminophenyl)[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]methanol,
4-(4-bromophenoxy)-2-vinylthieno[2,3-c]pyridine,
1-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-1,2-ethanediol,
1-[4-(4-bromophenoxy)thieno[2,3-c]pyridin-2-yl]-1,2-ethanediol,
[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]methanamine,
[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]methyl carbamate,
N-{ [4-(4-chlorophenoxy)thieno[2,3-cJpyridin-2-yl]methyl }urea,
(E)-3-[4-(4-bromophenoxy)thieno[2,3-c]pyridin-2-yl]-2-propenamide,
(E)-3-[4-(4-bromophenoxy)thieno[2,3-c]pyridin-2-yl]-N-methyl-2-propenamide,
3-[4-(4-bromophenoxy)thieno[2,3-c]pyridin-2-yl]-2,3-dihydroxy-N-
methylpropanamide,
3-[4-(4-bromophenoxy)thieno[2,3-c]pyridin-2-yl]-2,3-dihydroxypropanamide,
4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-ylamine,
4-(4-chlorophenoxy)thieno[2,3-cJpyridin-2-ylformamide,
N-(4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]urea,
N-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-y1]-N'-methylthiourea,
4-(4-chlorophenoxy)-N-methylthieno[2,3-c]pyridine-2-sulfonamide,
4-(4-chlorophenoxy)-N-(2,3-dihydroxypropyl)thieno[2,3-c]pyridine-2-
sulfonamide,
4-(4-chlorophenoxy)-N-(2-hydroxyethyl)thieno[2,3-cJpyridine-2-sulfonamide,
4-(4-(4-chlorophenoxy)thieno(2,3-cJpyridin-2-yl]phenol,
3-(4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]aniline,
4-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-ylJaniline,
4-(4-chlorophenoxy)-2-(5-nitro-2-pyridinyl)thieno[2,3-c]pyridine,
6-[4-(4-chlorophenoxy)thieno[2,3-cJpyridin-2-yl]-3-pyridinamine,
5-[4-(4-chlorophenoxy)thieno[2,3-cJpyridin-2-yI]-2-pyridinamine,
5-[4-(4-chlorophenoxy)thiena[2,3-c]pyridin-2-yl]-1,3,4-oxadiazol-2-amine,
5-[4-(4-bromophenoxy)thieno[2,3-c]pyridin-2-ylJ-1,3,4-oxadiazol-2-ylamine,
5-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-4H-1,2,4-triazol-3-amine,
5-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-1,3,4-thiadiazol-2-amine,
26
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
4-(4-chlorophenoxy)-2-(5-methyl-1,2,4-oxadiazol-3-yl)thieno[2,3-c]pyridine,
5- { 4-[4-(trifluoromethyl)phenoxyJthieno[2,3-c]pyridin-2-yl } -1,3,4-
oxadiazol-2-
amine,
4-(4-chlorophenoxy)-2-[5-(methylsulfanyl)-1,3,4-oxadiazol-2-yl]thieno[2,3-
c]pyridine,
4-(4-chlorophenoxy)-2-(2-methyl-2H- I ,2,3,4-tetraazol-5-yl)thieno[2,3-
c]pyridine,
5-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-4-methyl-4H-1,2,4-triazol-3-
amine,
4-(4-chlorophenoxy)-2-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]thieno(2,3-
c]pyridine,
5-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-I,2,4-oxadiazol-3-amine,
5-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-N-methyl-I,3,4-thiadiazol-2-
arnme,
4-(4-chlorophenoxy)-2-( 1,2,4-oxadiazol-3-yl)thieno[2,3-c]pyridine,
2-(1,3,4-oxadiazol-2-yl)-4-[4-(trifluoromethyl)phenoxy]thieno[2,3-c]pyridine,
3-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-1,2,4-oxadiazol-5-amine,
2-(5-methyl-I,3,4-oxadiazol-2-yl)-4-[4-(trifluoromethyl)phenoxy]thieno[2,3-
c]pyridine,
methyl 2-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-1,3-thiazole-4-
carboxylate,
2-[4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]-1,3-thiazole-4-carboxamide,
tert-butyl 2-(4-(4-chlorophenoxy)thieno[2,3-c]pyridin-2-yl]- I ,3-thiazol-4-
ylcarbamate,
2-[4-(4-chlorophenoxy)thieno [2,3-c]pyridin-2-yl]-1,3-thiazol-4-amine,
4-(4-chlorophenoxy)-2-(1,3-oxazol-2-yl)thieno[2,3-c]pyridine,
4-(4-chlorophenoxy)-2-( 1 H-imidazol-2-yl)thieno[2,3-c]pyridine,
4-chloro-3-methylthieno[2,3-c]pyridine-2-carboxamide,
3-amino-4-chlorothieno[2,3-c]pyridine-2-carboxamide,
4-(4-chIorophenoxy)-N,3-dimethylthieno[2,3-c]pyridine-2-carboxamide,
4-(4-bromophenoxy)-3-methylthieno[2,3-c]pyridine-2-carboxamide,
7-chloro-4-(4-chlorophenoxy)-3-methylthieno[2,3-c]pyridine-2-carboxamide,
tert-butyl 2-(aminocarbonyl)-4-(4-chlorophenoxy)thieno[2,3-c]pyridine-3-
carboxylate,
N-methyl-4-(4-toluidino)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-chloroanilino)-N-methylthieno[2,3-c]pyridine-2-carboxamide,
N-methyl-4-(4-morpholinyl)thieno[2,3-c]pyridine-2-carboxamide,
27
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
7-chloro-4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
7-chloro-4-(4-chlorophenoxy)-N-methylthieno[2,3-c]pyridine-2-carboxamide,
7-chloro-4-(4-chlorophenoxy)-N-(2-hydroxyethyl)thieno[2,3-c]pyridine-2-
carboxanude,
7-bromo-4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxamide,
7-bromo-4-(4-chlorophenoxy)-N-methylthieno[2,3-c]pyridine-2-carboxamide,
4-(4-bromophenoxy)-7-chlorothieno(2,3-c]pyridine-2-carboxamide,
4-(4-bromophenoxy)-7-chloro-N-methylthieno[2,3-c]pyridine-2-carboxamide,
7-chloro-4-[4-(trifluoromethyl)phenoxy]thieno[2,3-c]pyridine-2-carboxamide,
7-chloro-N-methyl-4-[4-(trifluoromethyl)phenoxy]thieno[2,3-c]pyridine-2-
carboxamide,
7-chloro-N-(2-hydroxyethyl)-4-[4-(trifluoromethyl)phenoxy]thieno[2,3-
c]pyridine-
2-carboxamide,
4-(4-chlorophenoxy)-N,7-dimethylthieno[2,3-c]pyridine-2-carboxamide,
4-(4-chlorophenoxy)-7-methoxythieno[2,3-c]pyridine-2-carboxamide,
4-(4-chlorophenoxy)-7-oxo-6,7-dihydrothieno[2,3-c]pyridine-2-carboxamide,
4-(4-chlorophenoxy)-N-methyl-7-(methylamino)thieno[2,3-c]pyridine-2-
carboxamide,
7-(4-methylphenoxy) [ 1,3)thiazolo[5,4-c]pyridine-2-carboxamide,
N-methyl-7-(4-methylphenoxy)[1,3]thiazolo[5,4-c]pyridine-2-carboxamide,
4-(4-chlorophenoxy)furo[2,3-c]pyridine-2-carboxamide,
4-(4-chlorophenoxy)furo[2,3-c]pyridine-2-carbothioamide,
4-[(E}-2-phenylethenyl]thieno[2,3-c]pyridine-2-carboxamide,
4-(4-chlorophenyl)thieno [2,3-c]pyridine-2-carboxamide,
4-[3-(trifluoromethyl)phenyl]thieno[2,3-c]pyridine-2-carboxamide,
4-(3-chlorophenyl)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-bromophenyl)thieno[2,3-c]pyridine-2-carboxamide,
4-(3-aminophenyl)thieno[2,3-c]pyridine-2-carboxamide,
4-(3,5-dichlorophenyl)thieno[2,3-c]pyridine-2-carboxamide,
4-(2,4-dichlorophenyl)thieno[2,3-c]pyridine-2-carboxamide,
4-(3,4-dichlorophenyl)thieno[2,3-c]pyridine-2-carboxamide,
4-(2,4-difluorophenyl)thieno[2,3-c]pyridine-2-carboxamide,
4-(4-fluorophenyl)thieno[2,3-c]pyridine-2-carboxamide, and
4-(4-bromophenoxy)-5-chlorothieno[2,3-c]pyridine-2-carboxamide.
Determination of Biological Activity
28
CA 02333770 2000-11-30
WO 99/62908 PCT/(JS99/12419
Pooled primary human umbilical vein endothelial cells (HUVEC's) (Clonetics)
between passages 3 and 7 were plated in 96-well plates (Costar), 100 IrL/well,
5x 104
cells/mL in Clonetics EBM/2% FBS/EGFBovine brain extract/gentamicin in the
absence
of hydrocortisone. The following day, compounds of the invention were added in
10
ftL/well medium, and the plates were incubated at 37 °C. 24 hours after
compound
addition, TNF (GibcoBRL) in 10 pL/well medium was added to a final
concentration of 5
ng/mL, and the cells were incubated an additional 6 hours at 37 °C.
Then media was
removed, and the plates were washed once with D-PBS (GibcoBRL) and treated
with
primary antibodies (Becton Dickinson, City), 100 ~L/well in D-PBS/2% BSA
(Sigma)/0.01% azide. Primary antibodies at an initial concentration of 1 mg/mL
were
used at the following dilutions: anti-ELAM-1, 1:2000, anti-ICAM-1, 1:2000 and
anti-
VCAM-I, 1:3000. Plates were stored overnight at 4 °C, washed 3 times
with D-PBS, and
treated with secondary antibody (Jackson Labs), 100 pL/well 1:8000 dilution
HRP-
conjugated donkey anti-mouse IgG(H+L) in D-PBS/2%BSA. Plates were incubated a
minimum of 1 hour at room temperature, washed 3 times with D-PBS and treated
with 100
pL, of ortho-phenylene diamine~2 HCl reagent per well. Plates were developed
approximately 15 minutes and neutralized with 100 pL,/well 1N sulfuric acid.
Absorbance
was read at 490 nm. Inhibitory potencies for representative compounds of the
invention
are shown in Table 1.
Table 1
CAM ELISA
% Inhibition
at 1
Exam le E-Selectin ICAM-1 VCAM-1
2 28 35 4
3 37 32 19
16 35 32 19
17 29 30 14
19 79 67 41
20 55 46 26
2I 63 55 23
22 64 64 33
32 75 63 41
33 69 60 28
53 70 67 48
29
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
54 74 59 33
60 74 71 42
61 82 74 50
79 66 63 38
89 62 64 50
90 47 51 27
95 85 71 61
102 78 68 53
104 78 72 53
110 44 48 39
119 59 61 36
120 72 72 49
135 29 22 34
123 39 31 12
124 18 29 26
125 72 69 44
142
151 69 73 44
158 61 65 26
159 30 45 13
161 51 58 51
170 66 67 53
171 67 72 50
183 63 69 48
184 52 56 30
187 78 72 54
190C 70 65 37
202 80 68 49
210 64 58 42
217 64 63 42
218 66 64 51
219 62 68 50
220 60 51 32
222 40 42 44
223 34 36 36
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/1Z419
224 61 55 41
225 75 78 60
226 77 74 56
227 61 62 44
228 70 68 54
228 67 64 52
229 54 55 46
230 56 53 45
233 74 79 62
236 59 59 33
237I 71 77 54
238 46 47 17
249 75 69 61
254 24 28 24
255 67 65 19
259 63 59 57
261 20 16 0
274 65 71 44
275 73 72 64
276 66 61 52
279 30 40 24
280 73 77 69
281 18 25 20
282A 8 15 5
283 39 57 52
284 56 68 45
285 56 65 50
286 54 64 46
287 49 49 27
289 64 62 52
290 42 53 48
294 46 45 24
295 59 56 44
301 44 48 34
303 65 66 33
31
CA 02333770 2000-11-30
WO 99162908 PCT/US99/12419
311 72 77 36
312 64 76 42
313 57 68 39
316 76 68 42
320 64 67 55
325 76 68 42
329 72 62 64
340 18 33 20
Thus the compounds of the present invention act as antiinflammatory agents
with
potencies below 1 p,M and are therefore useful for treating inflammatory
diseases.
Pharmaceutical Compositions and Methods of Treatment
The present invention also provides pharmaceutical compositions which comprise
compounds of the present invention formulated together with one or more non-
toxic
pharmaceutically acceptable carriers. The pharmaceutical compositions may be
specially
formulated for oral administration m solid or liquid form, for parenteral
injection, or for
rectal administration.
The pharmaceutical compositions of this invention can be administered to
humans
and other animals orally, rectally, parenterally , intracisternally,
intravaginally,
intraperitoneally, topically (as by powders, ointments, or drops), bucally, or
as an oral or
nasal spray. The term "parenteral" administration as used herein refers to
modes of
administration which include intravenous, intramuscular, intraperitoneal,
intrasternal,
subcutaneous and intraarticular injection and infusion.
Pharmaceutical compositions of this invention for parenteral injection
comprise
pharmaceutically acceptable sterile aqueous or nonaqueous solutions,
dispersions,
suspensions or emulsions as well as sterile powders for reconstitution into
sterile
injectable solutions or dispersions just prior to use. Examples of suitable
aqueous and
nonaqueous carriers, diluents, solvents or vehicles include water, ethanol,
polyols (such as
glycerol, propylene glycol, polyethylene glycol, and the like), and suitable
mixtures
thereof, vegetable oils (such as olive oil), and injectable organic esters
such as ethyl oleate.
Proper fluidity can be maintained, for example, by the use of coating
materials such as
lecithin, by the maintenance of the required particle size in the case of
dispersions, and by
the use of surfactants.
These compositions may also contain adjuvants such as preservative, wetting
agents, emulsifying agents, and dispersing agents. Prevention of the action of
microorganisms may be ensured by the inclusion of various antibacterial and
antifungal
32
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
agents, for example, paraben, chlorobutanol, phenol sorbic acid, and the like.
It may also
be desirable to include isotonic agents such as sugars, sodium chloride, and
the like,
Prolonged absorption of the injectable pharmaceutical form may be brought
about by the
inclusion of agents which delay absorption such as aluminum monostearate and
gelatin.
In some cases, in order to prolong the effect of the drug, it is desirable to
slow the
absorption of the drug from subcutaneous or intramuscular injection. This may
be
accomplished by the use of a liquid suspension of crystalline or amorphous
material with
poor water solubility. The rate of absorption of the drug then depends upon
its rate of
dissolution which, in turn, may depend upon crystal size and crystalline form.
Alternatively, delayed absorption of a parenterally administered drug form is
accomplished by dissolving or suspending the drug in an oil vehicle.
Injectable depot forms are made by forming microencapsule matrices of the drug
in
biodegradable polymers such as polylactide-polyglycolide. Depending upon the
ratio of
drug to polymer and the nature of the particular polymer employed, the rate of
drug release
can be controlled. Examples of other biodegradable polymers include
poly{orthoesters)
and poly(anhydrides) Depot injectable formulations are also prepared by
entrapping the
drug in liposomes or microemulsions which are compatible with body tissues.
The injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter, or by incorporating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile
injectable medium just prior to use.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders,
and granules. In such solid dosage forms, the active compound is mixed with at
least one
inert, pharmaceutically acceptable excipient or carrier such as sodium citrate
or dicalcium
phosphate and/or a) fillers or extenders such as starches, lactose, sucrose,
glucose,
mannitol, and silicic acid, b) binders such as, for example,
carboxymethylcellulose,
alginates, gelatin, polyvinylpyrrolidone, sucrose, and acacia, c) humectants
such as
glycerol, d) disintegrating agents such as agar-agar, calcium carbonate,
potato or tapioca
starch, alginic acid, certain silicates, and sodium carbonate, e) solution
retarding agents
such as paraffin, f) absorption accelerators such as quaternary ammonium
compounds, g)
wetting agents such as, for example, cetyl alcohol and glycerol monostearate,
h)
absorbents such as kaolin and bentonite clay, and i} lubricants such as talc,
calcium
stearate, magnesium stearate, solid polyethylene glycols, sodium lauryl
sulfate, and
mixtures thereof. In the case of capsules, tablets and pills, the dosage form
may also
comprise buffering agents.
33
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
Solid compositions of a similar type may also be employed as fillers in soft
and
hard-filled gelatin capsules using such excipients as lactose or milk sugar as
well as high
molecular weight polyethylene glycols and the like.
The solid dosage forms of tablets, dragees, capsules, pills, and granules can
be
S prepared with coatings and shells such as enteric coatings and other
coatings well known
in the pharmaceutical formulating art. They may optionally contain opacifying
agents and
can also be of a composition that they release the active ingredients) only,
or
preferentially, in a certain part of the intestinal tract, optionally, in a
delayed manner.
Examples of embedding compositions which can be used include polymeric
substances
and waxes.
The active compounds can also be in micro-encapsulated form, if appropriate,
with
one or more of the above-mentioned excipients.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, solutions, suspensions, syrups and elixirs. In addition to the
active compounds,
the liquid dosage forms may contain inert diluents commonly used in the art
such as, for
example, water or other solvents, solubilizing agents and emulsifiers such as
ethyl alcohol,
isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl
benzoate,
propylene glycol, 1,3-butylene glycol, dimethyl formamide, oils (in
particular, cottonseed,
groundnut, corn, germ, olive, castor, and sesame oils), glycerol,
tetrahydrofurfuryl alcohol,
polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof.
Besides inert diluents, the oral compositions can also include adjuvants such
as
wetting agents, emulsifying and suspending agents, sweetening, flavoring, and
perfuming
agents.
Suspensions, in addition to the active compounds, may contain suspending
agents
as, for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and
sorbitan
esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-
agar, and
tragacanth, and mixtures thereof.
Compositions for rectal or vaginal administration are preferably suppositories
which can be prepared by mixing the compounds of this invention with suitable
non-
irritating excipients or carriers such as cocoa butter, polyethylene glycol or
a suppository
wax which are solid at room temperature but liquid at body temperature and
therefore melt
in the rectum or vaginal cavity and release the active compound.
Compounds of the present invention can also be administered in the form of
liposomes. As is known in the art, liposomes are generally derived from
phospholipids or
other lipid substances. Liposomes are formed by mono- or multi-lamellar
hydrated liquid
crystals that are dispersed in an aqueous medium. Any non-toxic,
physiologically
34
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
acceptable and metabolizable lipid capable of forming liposomes can be used.
The present
compositions in liposome form can contain, in addition to a compound of the
present
invention, stabilizers, preservatives, excipients, and the like. The preferred
lipids are the
phospholipids and the phosphatidyl cholines (lecithins), both natural and
synthetic.
Methods to form liposomes are known in the art. See, for example, Prescott,
Ed.,
Methods in Cell Biology, Volume XIV, Academic Press, New York, N.Y. (1976), p.
33 et
seq.
The compounds of the present invention may be used in the form of
pharmaceutically acceptable salts derived from inorganic or organic acids. By
"pharmaceutically acceptable salt" is meant those salts which are, within the
scope of
sound medical judgment, suitable for use ir< contact with the tissues of
humans and lower
animals without undue toxicity, irritation, allergic response and the like and
are
commensurate with a reasonable benefit/risk ratio. Pharmaceutically acceptable
salts are
well-known in the art. For example, S. M. Berge, et al. describe
pharmaceutically
acceptable salts in detail in J. Pharmaceutical Sciences, 1977, 66: 1 et seq.
The salts may
be prepared in situ during the final isolation and purification of the
compounds of the
invention or separately by reacting a free base function with a suitable acid.
Representative acid addition salts include, but are not limited to acetate,
adipate, alginate,
citrate, aspartate, benzoate, benzenesulfonate, bisulfate, butyrate,
camphorate,
camphorsufonate, digluconate, glycerophosphate, hemisulfate, heptanoate,
hexanoate,
fumarate, hydrochloride, hydrobromide, hydroiodide, 2-hydroxyethansulfonate
(isethionate), lactate, maleate, methanesulfonate, nicotinate, 2-
naphthalenesulfonate,
oxalate, pamoate, pectinate, persulfate, 3-phenylpropionate, picrate,
pivalate, propionate,
succinate, tartrate, thiocyanate, phosphate, glutamate, bicarbonate, p-
toluenesulfonate and
undecanoate. Also, the basic nitrogen-containing groups can be quaternized
with such
agents as lower alkyl halides such as methyl, ethyl, propyl, and butyl
chlorides, bromides
and iodides; dialkyl sulfates like dimethyl, diethyl, dibutyl and diamyl
sulfates; long chain
halides such as decyl, lauryl, myristyl and stearyl chlorides, bromides and
iodides;
arylalkyl halides like benzyl and phenethyl bromides and others. Water or oil-
soluble or
dispersible products are thereby obtained. Examples of acids which may be
employed to
form pharmaceutically acceptable acid addition salts include such inorganic
acids as
hydrochloric acid, hydrobromic acid, sulphuric acid and phosphoric acid and
such organic
acids as oxalic acid, malefic acid, succinic acid and citric acid.
Basic addition salts can be prepared in situ during the final isolation and
purification of compounds of this invention by reacting a carboxylic acid-
containing
moiety with a suitable base such as the hydroxide, carbonate or bicarbonate of
a
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
pharmaceutically acceptable metal cation or with ammonia or an organic
primary,
secondary or tertiary amine. Pharmaceutically acceptable salts include, but
are not limited
to, cations based on alkali metals or alkaline earth metals such as lithium,
sodium,
potassium, calcium, magnesium and aluminum salts and the like and nontoxic
quaternary
ammonia and amine cations including ammonium, tetramethylammonium,
tetraethylammonium, methylamine, dimethylamine, trimethylamine, triethylamine,
diethylamine, ethylamine and the like. Other representative organic amines
useful for the
formation of base addition salts include ethylenediamine, ethanolamine,
diethanolamine,
piperidine, piperazine and the like. Preferred salts of the compounds of the
invention
include phosphate, tris and acetate.
Dosage forms for topical administration of a compound of this invention
include
powders, sprays, ointments and inhalants. The active compound is mixed under
sterile
conditions with a pharmaceutically acceptable carrier and any needed
preservatives,
buffers, or propellants which may be required. Opthalmic formulations, eye
ointments,
powders and solutions are also contemplated as being within the scope of this
invention.
Actual dosage levels of active ingredients in the pharmaceutical compositions
of
this invention may be varied so as to obtain an amount of the active
compounds) that is
effective to achieve the desired therapeutic response for a particular
patient, compositions,
and mode of administration. The selected dosage level will depend upon the
activity of
the particular compound, the route of administration, the severity of the
condition being
treated, and the condition and prior medical history of the patient being
treated. However,
it is within the skill of the art to start doses of the compound at levels
lower than required
for to achieve the desired therapeutic effect and to gradually increase the
dosage until the
desired effect is achieved.
Generally dosage levels of about 1 to about 50, more preferably of about 5 to
about
20 mg of active compound per kilogram of body weight per day are administered
orally to
a mammalian patient. If desired, the effective daily dose may be divided into
multiple
doses for purposes of administration, e.g. two to four separate doses per day.
Preparation of Compounds of this Invention
Abbreviations
Abbreviations which have been used in the descriptions of the schemes and the
examples that follow are: BH3 for borane, BH3~DMS for borane dimethylsulfide
complex,
BINAP for 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl, BF~OEt2 for boron
trifluoride
diethyl ether complex, n-BuLi for n-butyllithium, CC14 for carbon
tetrachloride, Cs2C03
36
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
for cesium carbonate, DBU for 1,8-diazabicyclo[5.4.0]undec-7-ene, DMA for N,N-
dimethylacetamide, DIBAL for diisobutylaluminum hydride, DME for
dimethoxyethane,
DMF for N,N-dimethylformamide, DMSO for dimethylsulfoxide, DIPEA for
diisopropylethylamine, DPPA for diphenylphosphoryl azide, EDCI or EDC for 1-
ethyl-3-
[3-(dimethylamino)propyl]-carbodiimide hydrochloride, Et3N for triethylamine,
Et20 for
diethyl ether, EtOAc for ethyl acetate, EtOH for ethanol, K2C03 for potassium
carbonate,
LiAlH4 for lithium aluminum hydride, LDA for lithium diisopropylamide, MeOH
for
methanol, NaOMe for sodium methoxide, NaOH for sodium hydroxide, HCl for
hydrochloric acid, NMP for 1-methyl-2-pyrrolidinone, H2/Pd for hydrogen and a
palladium catalyst, iPrOH for isopropyl alcohol, PPh3 for triphenylphosphine,
THF for
tetrahydrofuran, THP for tetrahydropyran, TFA for trifluoroacetic acid, and
pyBOP for
benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate.
Synthetic Methods
The compounds and processes of the present invention will be better understood
in
connection with the following synthetic schemes which illustrate the methods
by which
the compounds of the invention can be prepared. Further, all citations herein
are
incorporated by reference.
Scheme 1
Scheme 1 shows the preparation of thieno[2,3-d]pyrimidines from esters of
general
formula 1 by published procedures. 4-Chlorothieno[2,3-d]pyrimidines of general
formula
3 were substituted with thiols to provide 4-thioethers of general formula 4 or
substituted
with amines to provide 4-aminothieno[2,3-d]pyrimidines of general formula 5.
37
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 2
Scheme 2 shows the preparation of 2-carboxy-substituted thieno[2,3-
d]pyrimidines. Pyrimidinones of general formula 1 weres reacted with
phosphoryl
chloride to produce 4-chloro pyrimidines of general formula 6, which were then
substituted with thiols to provide thioethers of general formula 7.
Scheme 3
Scheme 3 shows the preparation of thieno[3,2-d]pyrimidines derived from
chloropyrimidines of general formula 8. Substitution of the chlorides with
thiols provided
thioethers of general formula 9, and substitution of the chlorides with amines
provided
aminopyrimidines of general formula 10.
Scheme 4
Analogs having 2-carboxamide groups on the thieno[3,2-d]pyrimidine were
prepared as shown in Scheme 4. The thiophene-2-position was deprotonated with
a strong
base such as lithium diisopropylamide, and the corresponding carbanion was
treated with
carbon dioxide to provide acids of general formula 11. The acids were
converted to the
corresponding amides of general formula 12 through the intermediate acid
chlorides.
38
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 5
Scheme 5 shows the preparation of 6-alkyl substituted thieno[2,3-d]pyrimidines
with alkylthio groups at the 4-position. Using known procedures, 2-
aminothiophene 13
was acylated to provide amide 14 which was cyclized to provide
thienopyrimidinone 15.
The pyrimidinone was converted to chloride 16 and further to thioether 17 by
standard
procedures.
Scheme 6
The general process for preparing 2,4-disubstituted thieno[2,3-c]pyridines is
shown
in Scheme 6. Commercially available 3,5-dichlorpyridine was treated with
strong base
such as lithium diisopropylanude in an anhydrous solvent at low temperature
followed by
reaction with methyl formate (or alternatively, dimethylformamide) to provide
the known
pyridine-4-carboxaldehyde 18. Aldehyde 18 was then substituted with one
equivalent of
thiol (R' = substituted or unsubstituted aryl, alkyl, or heterocyclic) to
produce
chloroaldehyde of general formula 19. Treatment of 19 with methyl
thioglycolate and a
base such as cesium carbonate or potassium carbonate provided the 4-thioether
[2,3-c]-
thienopyridine esters of general formula 20. The esters were converted to the
corresponding acids of general formula 21 by basic hydrolysis, for example
using lithium,
sodium, or potassium hydroxide in a mixture of water and alcohol or
tetrahydrofuran.
Acids of general formula 21 may also be converted to amides of general formula
22 or 23
through the intermediate acid chlorides by treatment first with oxalyl
chloride or thionyl
chloride, then with the amine of choice (RZ, R3 can be substituted or
unsubstituted alkyl,
39
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
aryl, heterocyclic). Alternatively, aicds 21 may be converted to amides 22 or
23 by other
coupling methods, such as carbodiimide (for example, N-ethyl-N'-(3-
dimethylamino)propyl carbodiimide hydrochloride (EDC)), mixed anhydrides
(derived
from pivaloyl chloride or isobutyl chloroformate treatment), and active esters
(for
example, derived from N-hydroxysuccinimide, p-nitrophenol).
Scheme 7
Scheme 7 illustrates the analogous preparation of 4-ether-substituted
thienopyridines of general formula 30. Aldehyde 18 was substituted with
alcohols (R1 =
substituted or unsubstituted aryl, heterocyclic) under basic conditions (for
example,
potassium tert-butoxide or cesium carbonate in anhydrous tetrahydrofuran or
dimethylformamide) to give pyridine-ethers of general formula 24, and then
further
reacted with methyl thioglycolate to provide the thieno[2,3-c]pyridine esters
of general
formula 25. These esters may be converted to the corresponding primary amides
of
general formula 26 by heating in methanolic ammonia solution. Alternatively,
esters of
general formula 25 may be reacted with mono or disubstituted amines in polar
solvents
such as dimethylformamide or methanol. Esters of general formula 25 were
hydrolyzed to
carboxylic acids of general formula 28 by basic hydrolysis with sodium or
lithium
hydroxide in aqueous methanol or tetrahydrofuran. The acids were then
converted to
amides of general formula 30 by reaction of the corresponding acid chlorides
of general
formula 29 with amines. Alternatively, the acids 30 were coupled to amines by
standard
peptide-coupling conditions as described in Scheme 6 for amides 22 or 23.
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/1Z419
Scheme 8
Similar methods may be utilized for the preparation of 4-bromothieno[2,3-
c]pyridine 32, as shown in Scheme 8. 3,5-Dibromopyridine was converted to
aldehyde 31
by the procedure described for preparation of compound 18 in Scheme 6.
Reaction of 31
with methyl thioglycolate, for example in the presence of cesium carbonate in
DMF,
produced 4-bromothieno[2,3-c]pyridine ester 32. Bromide 32 served as starting
material
for the preparation of 4-aryl, heterocyclic, alkyl, or alkenyl derivatives of
general formula
33, using Suzuki coupling methodology as shown. Bromide 32 may also be coupled
with
terminal alkynes to provide alkynyl derivatives (R' = alkynyl) following
Sonogashira
methodology (Sonogashira, K.; Tohda, Y.; Hagihara, N. Tetrahedron Lett. 1975,
4467-70).
Esters of general formula 33 were converted to amides of general formula 34 by
the
procedures described for 26, 27 or 30 in Scheme 7.
Scheme 9
Scheme 9 shows the conversion of acids of general formula 21 to aldehyde or
ketone-derived compounds. For example, aidehydes of general formula 36 were
produced
by reduction of the N-methyl-N-methoxylamides of general formula 35. The
amides of
general formula 35 were also reacted with Grignard reagents to produce
unsymmetrical
ketones of general formula 39. Aldehydes of general formula 36 and Ketones of
general
formula 39 were utilized for the production of oximes of general formula 37 or
40 by
reaction with hydroxylamine derivatives. Aldehyde of general formula 36 were
reacted
with phosphoranes (or phosphonoacetate salts) to produce 2-alkenyl substituted
derivatives of general formula 38. Ketones of general formula 39 were reduced
to the
corresponding alkanes of general formula 41 by treatment with hydrazine and
strong base,
such as potassium hydroxide. Analogous 2-position derivatives of
thienopyridine ethers of
general formula 28 in Scheme 7 would follow similar synthetic routes as
described in
41
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
Scheme 9. Thus, acids 28 may be converted to aldehydes, ketones, oximes,
alkenes, or
alkanes substitutions at the 2-position.
Scheme 10
Amides of general formula 34 (or 26, 27 or 30), may be converted to the
corresponding thionoamides of general formula 42 by treatment with Lawesson's
reagent
as shown in Scheme 10.
Scheme 11
As shown in Scheme 11, 4-sulfoxides of general formula 43 were produced by
reaction of thioethers of general formula 20 with an oxidant such as
m-chloroperoxybenzoic acid under controlled conditions.
Scheme 12
Alternative functionality was introduced at the 2-position of thienopyridines
of
general formula 44 by metallation of the 2-position, followed by reaction with
appropriate
electrophiles, as shown in Scheme 12. Acids of general formula 21 (LAXA =
thioalkoxy,
alkoxy, alkyl, alkenyl, aryl, heterocyclic) were decarboxylated at elevated
temperatures
(optionally in the presence of copper powder) to afford 2-unsubstituted
derivatives of
general formula 44. Compounds of general formula 44 were deprotonated with
strong
42
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
organic bases such as n-butyllithium, and then reacted with electrophiles such
as borates,
cyanoformates, aldehydes, or trialkyltin chlorides according to standard
procedures
(Masakatsu, N.; Kazuhiro, N.; Ichiro, M.; Iwao; W. Chem. Lett. 1983, 6, 905-
908).
Scheme 13
Scheme 13 illustrates an alternative method for the preparation of
2-carboxaldehydes of general formula 36 or 47. Esters of general formula 20 or
25 (R' _
thioalkoxy, alkoxy, alkyl, alkenyl, aryl, heterocyclic) were reduced to the
corresponding
alcohols of general formula 46 using calcium borohydride. The alcohols were
then
oxidized to the aldehydes using Swern conditions. The aldehydes were then
reacted with
Wittig reagents (for example phosphoranes to produce the acrylate derivatives
of general
formula 48 (Jung, M. E. and Kiankarami, M. J. Org. Chem. 1998, 63, 2968-2974).
Scheme 14
As shown in Scheme 14, thienopyridines of general formula 27 or 30 were
alkylated on the pyridine nitrogen using alkyl iodides (or alkyl bromides or
triflates) to
produce the pyridinium salts of general formula 49. For example, R may be
alkylcarbonyloxymethylene, aminocarbonyloxymethylene,
alkoxycarbonyloxymethylene,
or alkyl. Such derivatives may serve as prodrug forms of the thienopyridine
amides 27 or
30.
43
CA 02333770 2000-11-30
WO 99/62908 PCT/ITS99/12419
Scheme 15
A variety of 2-aminothieno[2,3-c]pyridine derivatives were available starting
from
the 2-carboxylic acids of general formula 21 or 28, as shown in Scheme 15,
wherein R'
may be thioalkoxy, alkoxy, alkyl, alkenyl, aryl, heterocyclic. Curtius
rearrangement led to
isocyanates of general formula 50, which were reacted with alcohols (alkyl,
aryl,
heterocyclic, or dialkylaminoalkyl) to provide carbamates of general formula
51.
Isocyanates 50 may be reacted with amines (ammonia, primary alkyl or secondary
alkyl) to
provide ureas of general formula 52. Also, 50 may be reacted with alkyl or
arylmagnesium halides, or alkyl lithium salts, to provide amides of general
formula 53.
Isocyanate 50 was hydrolyzed under aqueous conditions to produce 2-amino
derivatives of
general formula 54.
Scheme 16
Amines of general formula 54 were reacted with appropriate electrophiles to
further derivatize this position. Thus reaction of 54 with aryl or
alkylsulfonyl chlorides
(R2 = alkyl, aryl, heterocyclic) or sulfamoyl chlorides, (R2 = NH2, mono- or
di-alkylamino)
to give sulfonamides of general formula 55. Amino derivative 54 was also
reacted with
acyl chlorides (RZ = alkyl, heterocyclic, or aryl) to produce amides of
general formula 53
as shown in Scheme 16.
44
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 17
Functionality present on the aryl rings attached at the 4-position of the
thieno[2,3-
c]pyridines may be further reacted to advantage, as illustrated in Scheme 17.
For example,
styryl derivative 56 was converted to the 1,2-diol 57 by treatment with osmium
tetroxide
under standard conditions. The 4-(4-bromophenoxy) derivative 58 underwent
fascile
substitution with aryl boronic acids under palladium catalysis under Suzuki
conditions to
provide biaryl derivatives of general formula 59. Alternatively,
alkoxycarbonylation under
palladium catalysis efficiently provides esters of general formula 61.
Scheme 19
IS
Scheme 19 shows the use of boronic acid derivatives to functionalize the 4-
position of the thieno[2,3-cJpyridines. The chemistry depicted may be applied
to a broad
range of aryl olefins analogous to bromostyrene 62. In the case shown,
bromostyrene 62
was converted to the boronic acid 63 under standard conditions, and the
boronic acid was
coupled to 4-bromothienopyridine 32 under Suzuki conditions, affording the
styryl analog
64. The ester 64 was converted to the amide 65 by the previously described
method
(Miyara, N and Suzuki, A. Chem. Rev. 1995, 95, 2457-2463). The olefinic group
may
then be converted to the epoxide 66, which can undergo reactions with
nucleophilic
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
reagents at the less-hindered position of the epoxide to producing analogs of
general
formula 67. Alternatively, styryl derivative 65 may be converted to the diol
68 by standard
methods.
Scheme 20
Another method for introduction of substituents at the 4-position of the
thieno[2,3-
cJpyridines is shown in Scheme 20. Bromide 32 may be converted to the
corresponding
cuprate through the intermediate zinc bromide reagent, which then may be
reacted with
appropriate electrophiles (acid chlorides, alkyl halides, aldehydes, ketones)
to afford the
substituted compounds of general formula 69 (Zhu, L.; Wehmeyer, R. M.; Rieke,
R. D. J.
Org. Chem. 1991, 56, 1445-1453).
Scheme 21
Scheme 21 depicts the preparation of 5-halo thienopyridine derivatives, as
exemplified by the preparation of the 5-chloro analog 75. Formylation of
lithiated 2,3,5-
trichloropyridine with methyl formate gave aldehyde 72. Displacement of the 3
and 5
chlorines with excess 4-bromophenol and reaction with methylthioglycolate gave
the
5-chlorothienopyridine 74 in low yield, together with the major product 73.
The 5-chloro
isomer was treated with ammonia in methanol in a pressure tube to generate the
amide 75.
It should be noted that this chemistry may be applied using a range of phenols
or hydroxy
heterocyclic compounds in place of 4-bromophenol.
46
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 22
The 2-position of thienopyridines was substituted with aryl, vinyl, acetylenic
or
alkyl groups using the procedure shown in Scheme 22. Boronic acids of general
formula
79 prepared according to Scheme 12, was coupled to aryl halides under
palladium catalysis
to produce 2-aryl derivatives of general formula 80.
Scheme 23
Scheme 23 illustrates the preparation of 4-acyl derivatives of thieno[2,3-
c]pyridines. Carboxylic acid 85 was converted to amide 86 via the acid
chloride, then the
hydroxyamide 86 underwent thionyl chloride-mediated cyclization to the
oxazoline 87
(Meyers, A. L; Stoianova, D. J. Org. Chem. 1997, 62, 5219-5221). Palladium
mediated
alkoxycarbonylation of 87 yielded ester 88 (Heck, R. F.; et al. J. Org. Chem.
1974, 39,
3318). Ester 88 may be converted to the Weinreb amide 89 by standard methods.
Amide
89 was reacted with the appropriate Grignard reagents to provide the desired 4-
acyl
products of general formula 90. Hydrolysis of the oxazoline and conversion of
the
resultant carboxylic acid to the amide yields the desired products of general
formula 91.
47
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
Scheme 24
Scheme 24 depicts a proposed method for the formation of 4-hydroxy substituted
thieno[2,3-c]pyridine. Reaction of phenol 92 with dihydropyran under acidic
conditions
yields tetrahydropyranyl ether 93 (Grant, H. N., et al. Helv. Chim. Acta.
1963, 46, 41 ).
Lithiation of 93 and subsequent quench with methyl formate gives aldehyde 94.
Displacement of the halide with methyl thioglycolate and subsequent
cyclization with
cesium carbonate yields the ester 95. Removal of the tetrahydropyranyl ether
with
aqueous HCl gives hydroxypyridine 96, which may be converted to the amide as
described
previously.
Scheme 25
Scheme 25 proposes the use of the 4-hydroxy group for introduction of
functionality to the 4-position of the thieno[2,3-cJpyridines. The 2-
carboxylic acid group
is first protected as the oxazoline 99 (through the intermediate amide 98),
then the hydroxy
group is converted to the aryl triflate 100 by standard conditions. Triflate
100 may then be
converted to the N-methyl-N-methoxy amide 89 under conditions similar to that
for
bromide 87. It should be noted that 4-triflate 100 may serve as a coupling
partner in a
variety of transition-metal mediated couplings with appropriate nucleophilic
partners (for
example, boronic acids, boranes, alkyl or aryl-zinc reagents).
48
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 26
Compounds with amino substitutions at the 3-position of the thieno[2,3-
c]pyridines
were prepared according to the procedures outlined in Scheme 26. Aldehyde 18
was
converted to the cyanopyridine 107 by treatment with hydroxylamine under
dehydrating
conditions. In a manner similar to the reactions involving aldehyde 18,
cyanopyridine 107
was sequentially substituted with phenols and methyl thioglycolate to provide
the 3-amino
thieno[2,3-c]pyridines of general formula 108. Esters of general formula 108
were then
converted to amides of general formula 109 by standard procedures.
Scheme 27
Scheme 27 illustrates additional derivatives which can be derived from amino
esters of general formula 108 or amino amides of general formula 109. For
example,
amino amides of general formula 109 may be treated with 1,1'-
carbonyldiimidazole to
produce cyclic imides of general formula 110. The 3-amino group was acylated
(for
example, with acid chlorides and weak base, or by coupling with acids using
carbodiimides} to give the diamides of general formula 111. The 3-amino group
may be
alkylated under reductive conditions using aldehydes and a reducing agent
(such as
triacetoxyborohydride), to provide alkylated amines of general formula 112.
49
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 28
Scheme 28 shows the preparation of compounds bearing alkyl substituents at the
3-
position of the thieno[2,3-c]pyridines. Using similar chemistry to that
described for
compound 30, 3,5-dichloropyridine was deprotonated with strong base (for
example,
lithium diisopropylamide), and then reacted with an acylating reagent (ester,
N-methyl-N-
methoxyamide, acyl pyrazole, or others) to provide ketone 113. Alternatively,
the anion
can be reacted with an aldehyde (for example, acetaldehyde) and then
subsequently the
product may be oxidized (for example, with tetrapropylammonium perruthenate)
to
provide ketone 113. Following the protocol outlined for example 30, the
dichloroketone
was reacted sequentially with phenols and then with methyl thioglycolate to
provide cyclic
products of general formula 114. The esters of general formula 114 may be
converted to
various derivatives as outlined previously.
Scheme 29
Scheme 29 describes a similar strategy used to obtain derivatives with alkoxy
groups at the 3-position of thieno[2,3-c]pyridines. Ester 116 was substituted
and cyclized
to provide 3-hydroxy analogs of general formula 117. The hydroxy group may be
left
unsubstituted, leading to amides of general formula 118 (or other
derivatives).
Alternatively, hydroxy esters of general formula 117 may be alkylated by
standard
procedures to produce 3-alkoxy derivates of general formula 119, followed by
amide
formation to provide compounds of general formula 120.
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 30
Scheme 30 shows the procedure used for the transformation of a commercially
available furo[2,3-b]pyridine 121 into an amide 122.
Scheme 31
Scheme 31 proposes the preparation of thienopyridine derivatives containing an
amide group at the 3-position. Thienopyridines of general formula 44 are
halogenated
using electophilic halide sources (for example, N-bromosuccinimide, I2), to
produce
3-halothienopyridines of general formula I23. Metal-halogen exchange, followed
by
trapping with carbon dioxide, provides acids of general formula 124. The acids
are
converted into amides of general formula I25 by standard procedures, or may be
homologated to esters of general formula 126 (for example, by the Arndt-Eisert
procedure}. Esters of general formula 126 may then be converted to amides or
other
functionality by the methods described above.
Scheme 32
Scheme 32 describes the methods used for the preparation of a variety of
thieno[2,3-b]pyridines starting from the known 4-chloro-5-ester 127.
Displacement of the
chlorine of I27 proceeded with thiols in the presence of potassium carbonate
to provide
4-thioethers of general formula 128. Ester 127 was also hydrolyzed to the acid
129, which
was converted to amides of general formula 130 by standard coupling
conditions.
51
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 33
Scheme 33 shows the conversion of thioethers of general formula 128 to
2,4-disubstituted analogs. The corresponding acids of general formula 131 may
be
thermally decarboxylated to provide the 5-unsubstituted analogs of general
formula 132.
Compounds of general formula 132 were treated with strong base (for example, n-
butyllithium), and reacted with carbon dioxide to provide the 2-carboxylic
acids of general
formula 133. The acids were then transformed into amides of general formula
134 by
previously described procedures.
Scheme 34
Scheme 34 illustrates the preparation of thieno[3,2-b]pyridines. Chloride 135
was
transformed by a similar set of conditions to that described in Scheme 33 to
produce acids
of general formula 139 and amides of general formula 140.
Scheme 35
Scheme 35 depicts the preparation of thieno[3,2-c]pyridines. Starting with
thienopyridone 141, 4-oxo-4,5-dihydrothieno[3,2-c]pyridine-2-nitrite 144 was
prepared
according to literature methods (Eloy, F.; Deryckere, A. Bul. Soc. Chim. Belg.
1970, 79,
301; Troxler, F.; Wiskott. E. US Patent 3,998,835). Treatment of
thienopyridone 144 with
52
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
phosphoryl chloride at 130 °C provided chloride 145, which upon
exposure to thiols under
basic conditions afforded thioethers of general formula 146. Hydrolysis of the
nitrile
group with polyphosphoric acid gave the corresponding amides of general
formula 147.
Scheme 36
Scheme 36 show that ethers of general formula 149 were prepared in an
analogous
fashion as previously described in Scheme 35.
Scheme 37
Scheme 37 shows the preparation of intermediates of general formula 151 useful
for the preparation of alternative 2-derivatives. Treatment of the known 2-
bromo-4-
chlorothieno[3,2-c]pyridine 150 with 1 equivalent of a thiol afforded 2-
bromothieno[3,2-
c]pyridines of general formula 151.
Scheme 38
Scheme 38 depicts an example of the preparation of a related class of
inhibitors
based on an oxazolopyridine structure. Commercially available 3-chloropyridine
is
oxidized to the N-oxide 152 with peracetic acid, which is then nitrated in a
mixture of
concentrated nitric, concentrated sulfuric, and fuming sulfuric acids to give
the 4-nitro
derivative 153. The chlorine in 153 is then displaced with the sodium salt of
p-cresol, and
the resulting biaryl ether 154 is hydrogenated (Raney nickel catalysis) to
reduce both the
nitro functional group and the N-oxide to give 155. The amino group of 155 is
protected
53
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
with an N-trimethylacetyl group, and the 5-hydroxyl group is introduced
according the
procedure of Chu-Moyer and Berger (J. Org. Chem. 1995, 60, 5721 ) by formation
of the
dianion of 156, quenching with trimethyl borate, and oxidation of the
intermediate
boronate ester followed by hydrolysis with basic hydrogen peroxide to give
hydroxypyridine 157. The amide is hydrolyzed with hydrochloric acid to give
158, which
is condensed with methyl oxalyl chloride to give the oxazolapyridine 158. The
methyl
ester in 159 was then converted to the primary amide by treatment with ammonia
in
methanol to give the target compound 160.
Scheme 39
Scheme 39 illustrates an example of the preparation of an analogous
thiazolopyridine-based inhibitor. The scheme illustrates the use of para-
cresol substituted
pyridine as starting material, but the synthesis may be generalized to other
aryl,
heterocyclic, or alkyl ethers. The dianion of 4-(N-trimethylacetyl)-amino-3-(4-
methylphenoxy)-pyridine is quenched with tetramethylthiuram disulfide to
introduce the 5-
mercapto group into the substituted pyridine ring as dithiocarbamate 161.
Subsequent
deprotection of the amine under acidic conditions is followed by acylation of
the free
aniline 162 with methyl oxalyl chloride to afford the oxalamide 163. The
thiazolopyridine
bicyclic core 164 is then prepared by treatment with mild acid (for example
formic acid at
reflux). The ester functionality was converted to the corresponding amide 165
with a
solution of an amine in methanol with warming.
Scheme 40
A related imidazopyridine class of compounds may be prepared from
intermediates
shown in Scheme 40. 5-Hydraxypyridine 157 may be converted to the
corresponding
aniline 166 by heating in ammonium hydroxide saturated with sulfur dioxide in
a pressure
54
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
vessel (Newman and Galt, J. Org. Chem. 1960, 25, 214). After removal of the
pivaloyl
group of 166 with hydrochloric acid, the resulting dianunopyridine 167 may be
condensed
with methyl oxalyl chloride to give the imidazopyridine 168. The ester
functionality may
then be converted to amide 169 by treatment with ammonia in methanol, as
described
previously.
Scheme 41
Scheme 41 is an illustration of the preparation of thienopyridazine-containing
inhibitors. The protected thiophene acid 170 was deprotonated with strong base
(e.g. n-
butyllithium), and reacted with a formylating reagent. The resultant oxazoline
aldehyde
171 was hydrolyzed and cyclized with hydrazine to provide the hydroxy
thienopyridazine
172. The hydroxy group was converted to the chloride 173 by the action of
phosphorous
oxychloride and subsequent substitution by alkoxides produced the ethers 174.
The amide
group is introduced in a manner analogous to that described above for the
thienopyridines,
providing amide 176.
Scheme 42
The syntheses of water-soluble glycosyl amide derivatives of general formula
178
are shown in Scheme 42. Tributylphosphine-mediated coupling of thienopyridine
carboxylic acids of general formula 21 or 28 with 2,3,4,6-tetra-O-acetyl-D-
glucopyranosyl
azide with assistance of PyBOP smoothly furnished the protected (3-glycosyl
amides of
general formula 177. No other isomers were detected in the reaction. Cleavage
of the
acetyl groups with methyl amine provided compounds of general formula 178.
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 43
Scheme 43 outlines the preparation of 4-(4-aminophenoxy)thieno[2,3-c]pyridine-
2-
carboxamide using a modification of the route described in Scheme 7. A two
step
sequence was adopted for assembling the thienopyridine core in the synthesis
of 182.
Treatment of the dichloropyridine aldehyde with one equivalent of N-BOC-
protected 4-
hydroxyaniline afforded compound 179, which was cyclized to give ester 180.
Transformation to the amide 181 followed the previously described procedure,
and the
Boc-group was removed by treatment with trifluoroacetic acid. It should be
noted that
aniline 182 also serves as starting material for Sandmeyer reactions via the
diazonium salt,
in which the amino group may be converted to a variety of functional groups,
including
halo, hydroxy, cyano, among other standard Sandmeyer products.
Scheme 44
Scheme 44 exemplifies a general method for the preparation of 4-substituted
aminophenoxythieno(2,3-c)pyridines using methodology described by Buchwald, et
al.
(Wolfe, John; Buchwald, Stephen L. J. Org. Chem. 1997, 62, 6066). Iodide 183,
prepared
by the previously described methods, was coupled with disubstituted amines
(such as
morpholine in the above example) in the presence of
bis(dibenzylideneacetone)dipalladium and BINAP to provide the substituted
aniline 184.
56
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/1Z419
Scheme 45
Scheme 45 describes the preparation of 4-(4-hydroxymethylphenyl)thieno[2,3-
c]pyridine 188, utilizing a modification of the route shown in Scheme 7.
Starting with
mono-tritylated 4-hydroxybenzyl alcohol, condensation with dichloroaldehyde
18,
followed by cyclization with methyl thioglycolate, provided protected benzyl
alcohol 188.
Standard transformations provided alcohol 188.
Scheme 46
Scheme 46 describes the preparation of a protected benzyl alcohol 190,
starting
from mono-tetrahydropyran-protected hydroxybenzyl alcohol I89. Standard acid-
catalyzed hydrolysis of the THP group can also yield the benzyl alcohol analog
188.
Scheme 47
25
As shown in Scheme 47, benzyl alcohol 188 may be further derivatized to
esters,
using standard coupling procedures, for example by carbodiimide conditions
shown above,
or by use of acid chloride. In addition, the alcohol may be converted to
carbamates (R =
NH2, mono or disubstituted amino) by treatment with isocyanates or carbamoyl
chlorides.
57
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 48
Glycosides of benzyl alcohol 187 may be produced using the procedure outlined
in
Scheme 48. Treatment of the alcohol 187 and tri-O-acetyl-D-glucal with
stoichiometric
scandium triflate afforded stereo-specifically protected glycoside 192, which
was
deprotected with methyl amine to give the free glycoside 193.
Scheme 49
Stille coupling of iodophenyl derivative 194 (or the corresponding bromophenyl
analogs) with tributylethoxyvinyltin allows for the introduction of an acetyl
group onto the
4-position of the phenyl ether as shown in Scheme 49. The intermediate vinyl
ether is
hydrolyzed during the work up conditions, thus providing acetophenone
derivative 195.
Addition of methylmagnesium bromide to ketone 195 at -50 °C did not
give the expected
addition product, but rather aldol adduct 196 was isolated in 40% yield. The
palladium
catalyzed carbonylation of bromophenoxy methyl ester 197 in aqueous media
afforded the
acid 198 in modest yield. PyBOP- or EDC-mediated coupling with amines
(illustrated
above with morpholine), followed by amination of the 2-methyl ester, provided
the
diamides 199.
58
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
Scheme 50
In Scheme 50 the preparation of cinnamide ethers is
described. Heck reaction of the bromophenoxy methyl ester 197 with t-butyl
acrylate
using tritolylphosphine as ligand furnished cinnamate 200 in good yield.
Hydrolysis of the
t-butyl ester with trifluoroacetic acid, followed by PyBOP or EDC-mediated
coupling with
an amine, and then amination of the methyl ester, provided diamide 201.
Scheme 51
Scheme 51 exemplifies the preparation of 4-heterocyclephenoxythienopyridines.
Treatment of para-cyanophenyl derivative 202 with hydroxyamine in a mixture of
DMF
and ethanol smoothly gave hydroxyimidamide 203, which was heated with
trifluoroacetic
anhydride in pyridine to provide the oxadiazole 204. Cyano derivative 202 was
transformed into imidazoline 205 under the conditions described above.
Incorporation of
other heterocycles was accomplished using known Stifle, Suzuki, or Heck
conditions as
exemplified by the Stifle coupling of iodo compound 194 and
tributylstannylthiophene to
provide compound 205A. The aryl coupling reactions described above may be
applied to
compounds with a variety of substituents at C-2 of the thienopyridine, in
addition to the
methyl amides exemplified in Scheme 51.
Scheme 52
Cyclopropylcarbinyl alcohol derivatives of the 4-phenyl ethers may be prepared
according to the procedure described in Schemes 52 and 53. Commercially
available
59
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
phenylcyclopropane carboxylic acid is converted to the corresponding alcohol
206 by LAH
reduction, then demethylation and selective protection of the hydroxymethyl
group affords
phenol 207. Using the procedure of Scheme 7, phenol 207 was condensed with
dichloroaldehyde 18 and then methyl thioglycolate, to produce thienopyridine
208.
Standard transformations led to the desired compounds 209 and 210.
Scheme 53
As shown in Scheme 53, alcohol 206 was alkylated to produce the polyether
phenol 211 which was converted into cyclopropylcarbinyl polyether 212 using
similar
procedures described in Scheme 52. Such alkylation chemistry may be broadly
applied by
replacement of the diether tosylate shown with other alkyl halides or
sulfonate esters.
Scheme 54
In Scheme 54, difluoroacetic acid derivative 213 was synthesized by a
copper mediated coupling between iodide 194 and ethyl iododifluoroacetate in
the
presence of phenol, which was found to remarkably inhibit side reactions.
Reduction of
the ester 213 gave difluoroethyl alcohol 214. Alkylation of alcohol 214 with
ethoxyethyl
tosylate in the presence of sodium hydride and 15-crown-5 afforded polyether
215.
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 55
An alternative synthesis of 4-alkoxythieno[2,3-c]pyridines through a phenolic
alkylation strategy is outlined in Scheme 55. Mitsunobu alkylation of 5-chloro-
3-
hydroxypyridine provided benzyl ether 216, which was deprotonated with
alkyllithium
base and the resulting anion was treated with methyl formate to produce the
pyridine
carboxaldehyde 217. Construction of the thienopyridine core is accomplished by
condensation with methyl thioglycolate under previously described conditions,
leading to
ester 218. This method may be applied to other alkyl ethers analogous to 216
to provide a
variety of 4-alkoxy derivatives related to 218. Esters 218 were then converted
to other
active derivatives such as amides using previously described procedures.
Benzyl ether 218
was hydrogenolyzed to phenol 219, which was converted to the corresponding
amide 220
by standard procedures. Phenol 219 also serves as a partner in Mitsunobu
reactions with a
variety of primary or secondary alcohols, to provide alkyl ethers of general
formula 221
(Huang, F., et al. J. Med. Chem. 1998, 41, 4216-4223). Esters of general
formula 221
were converted to amides of general formula 222 by treatment under the
standard
conditions of reflux with methanolic amine solutions.
Scheme 56
Thienopyridine analogs bearing a 4-carbonyl group may be prepared by the
procedures described in Scheme 56. Dichloropyridine aldehyde 18 was treated
with
methyl thioglycolate under previously described conditions to produce the
4-chlorothienopyridine ester 223. Ester exchange was accomplished by base
catalyzed
hydrolysis to acid 224, and tert-butyl esterification to 225 was accomplished
with
O-t-butyl trichloroacetimidate under Lewis acid catalysis. Palladium-catalyzed
carbethoxylation proceeded under previously described conditions to give
diester 226.
Reduction/oxidation reactions led to the aldehyde 227, which was then
condensed with
61
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
arylmagnesium halide reagents (exemplified above with 4-chlorophenyl magnesium
chloride) to produce alcohol 228. The ester 228 may be transformed to various
2-
substituted thienopyridine analogs directly, or oxidized to the corresponding
4-keto
derivative 230. Standard transformation of ester 230 to amide 231 completes
the
synthesis. It should be noted that ester 226 may be selectively converted to
amide
derivatives by initial alkaline hydroysis of the ethyl ester, coupling to an
amine, then acidic
hydrolysis of the tert-butyl ester, and finally coupling to another amine to
produce 308.
Scheme 57
A variety of 2-substituted thieno[2,3-c~pyridines are accessible from 2-esters
of
general formula 20 and 25. Scheme 57 exemplifies products which may be
obtained from
hydroxymethyl derivatives of general formula 232. Calcium borohydride
reduction of
esters of general formula 20 or 25 provided alcohols of general formula 232.
Swern
oxidation cleanly provided aldehydes of general formula 233. This versatile
intermediate
may be converted to olefins by standard Wittig conditions, as exemplified by
the
preparation of 2-vinylthienopyridines of general formula 234 (Hibino, S. J.
Org. Chem.
1984, 49, 5006-5008). Additional modification to dihydroxyethyl compound 235
was
accomplished through the use of catalytic osmium tetroxide with 4-
methylmorpholine N-
oxide as stoichiometric oxidant.
Scheme 58
62
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/124I9
Scheme 58 illustrates the conversion of aldehyde 233 to the acrylate 236,
which
was accomplished by Horner-Emmons condensation with trimethyl phosphonoacetate
(Jung, M. E. and Kiankarami, M. J. Org. Chem. 1998, 63, 2968-2974). The
described
methods may be extended to analogs bearing a wide variety of C-4 substituents,
including
aryloxy, alkoxy, arylamino, aryl, alkyl. The derived ester 236 was then
subjected to
hydrolysis to provide acid 237. Carboxylic acid 237 was subjected to standard
coupling
conditions to produce amide 238. The derived acrylates of general formula 239
may be
oxidized to the corresponding diols of general formula 240 with catalytic
osmium
tetroxide in the presence of 4-methylmorpholine-N-oxide.
Scheme 59
Scheme 59 illustrates the use of aldehyde 233, with LAXA=aryloxy, as starting
material for the preparation of oxime derivatives of general formula 241. The
strategy
outlined is generally applicable to analogs with a variety of LAXA
substituents. Aldehydes
of general formula 233 were also reacted with organomagnesium (or
organolithium)
reagents to produce secondary alcohols which were oxidized to the
corresponding ketones
of general formula 242. Oxidation is preferably accomplished by standard Swern
conditions (DMSO and oxalyl chloride in CHZC12 solution at low temperature,
followed by
treatment with tertiary amines such as ethyldiisopropyl amine), but other
conditions (tetra-
n-propyl perruthenate, manganese dioxide) may be employed. The ketones were
then
converted to oximes of general formula 243 by previously described methods.
Scheme 60
The preparation of 2-heterocyclic thienopyridines of general formula 249 is
illustrated in Scheme 60. Primary amides of general formula 26 were treated
with excess
63
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
trifluoroacetic anhydride in pyridine to smoothly produce nitrites of general
formula 244,
which serves as a convenient intermediate for the preparation of amidine and
azole
derivatives. Thus, nitrites of general formula 244 were converted to the
amidoximes of
general formula 245 by treatment with hydroxylamine hydrochloride and
triethylamine.
Reaction of amidoximes of general formula 245 with acetyl chloride,
trifluoroacetic
anhydride, triethylorthoformate or trichloroacetyl chloride in pyridine
produced the
oxadiazoles of general formula 246 with variations in X dictated by the choice
of
acylating/dehydrating reagent. The trichloromethyl oxadiazoles of general
formula 246
(X=CCl3) were converted to the 5-amino-1,2,4-oxadiazoles of general formula
247 by
heating with ammonia in a sealed tube. Nitrites of general formula 244 were
also
converted to cyanoamidines of general formula 248 when subjected to excess
cyanamide
in THF with DBU as the base. The 3-amino-1,2,4-oxadiazoles of general formula
249
were then generated by treatment of cyanoamidines of general formula 248 with
hydroxylamine hydrochloride and triethyamine in methanol.
Scheme 61
Scheme 61 depicts the preparation of 2-arylcarbonylthienopyridines.
Thienopyridines of general formula 44 were deprotonated with alkyllithium base
and
condensed with nitrobenzaldehdyes to produce the benzyl alcohols of general
formula 250.
Tin(II)-induced reduction of the nitrophenyls to the anilines of general
formula 251 were
followed by selective alcohol oxidation with pyridinium chlorochromate. The
nitro benzyl
alcohols of general formula 250 were also converted to the corresponding
ketones of
general formula 253 under Swern conditions (for example, oxalyl
chloride/DMSO/CH2C12
at low temperature, followed by treatment with amine bases).
64
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/124I9
Scheme 62
Scheme 62 depicts the preparation of 2-carbamatethienopyridines and 2-
ureathienopyridines. Alcohols of general formula 232 were converted to the
amines of
general formula 254 by Mitsunobu reaction with phthalimide, followed by
deprotection
with hydrazine. The amines of general formula 254 were converted to the
corresponding
ureas of general formula 255 by reaction with potassium isocyanate under
acidic
conditions. Similarly, the alcohols of general formula 232 were converted to
the
corresponding carbamates of general formula 256. This chemistry is generally
applicable
to the use of substituted isocyanates or carbamoyl chlorides, leading to mono
or
disubstituted carbamates or ureas.
Scheme 63
Scheme 63 illustrates the preparation of 2-thioureathienopyridines, starting
from
2-aminothienpyridines of general formula 54. Reaction of 54 with substituted
isothiocyanates in pyridine at reflux provided the thioureas of general
formula 257.
Scheme 64
Scheme 64 exemplifies the synthesis of sulfonamides at the 2-position of the
thienopyridines. An improved procedure for decarboxylation of thienopyridine-2-
carboxylic acids is shown, wherein acids of general formula 21 were heated in
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
diphenylether at 210 °C to provide thienopyridines of general formula
44 in high yield.
Compounds 44 were deprotonated with strong base, then treated with sulfur
dioxide to
produce intermediate sulfinic acids. Addition of N-chlorosuccinimide produced
sulfonyl
chlorides of general formula 258, from which a variety of sulfonamides of
general formula
259 were prepared by reaction with ammonia, primary or secondary amines in the
presence
of diisopropylethylamine in protic solvents such as methanol (Prugh, J. D., et
al. J. Med.
Chem. 1991, 34, 1805-1818; Davidsen, S. K., et al. J. Med. Chem. 1998, 41, 74-
95).
Scheme 65
Scheme 65 provides the outline of the synthesis of additional 2-
arylthienopyridines
with amino or hydroxy groups on the aryl ring. Using the method of Scheme 24,
Suzuki
coupling of boronic acids of general formula 79 with nitro-substituted aryl
iodides
produced biaryls of general formula 260. Biaryls of general formula 260 were
reduced to
the ammophenyl derivatives of general formula 261 with tin(II) chloride.
Methyl ethers of
general formula 262 were prepared from boronic acids 79 by coupling with
methoxy
iodobenzenes, and were converted to the hydroxy derivatives of general formula
263
through the use of boron tribromide to demethylate the methyl ethers.
Scheme 66
Schemes 66-71 illustrate syntheses for additional 5-membered heterocycles at
the
2-position of the thienopyridines. Scheme 66 outlines a method for producing
1,3,4-
oxadiazoles. Hydrazides of general formula 264 were prepared by treating
esters of
66
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
general formula 20 or 25 with hydrazine in methylene chloride. The hydrazides
were
converted to 5-amino-1,3,4-oxadiazoles of general formula 265 by reaction with
cyanogen
bromide. Hydrazides of general formula 264 may be converted to 5-unsubstituted
or 5-
alkyl-substituted-1,3,4-oxadiazoles of general formula 266 by condensation
with
orthoesters under reflux conditions.
Scheme 67
Scheme 67 indicates a method of preparing 1,3,4-triazoles from methyl esters
of
general formula 20 or 25. Condensation with aminoguanidine under basic
conditions (for
example sodium methoxide in methanol) produced the 2-amino-1,3,4-triazoles of
general
formula 267. Nonspecific methylation was performed on 1,3,4 triazoles of
general
formula 267 using sodium hydride and methyl iodide, which provided mono-methyl
triazoles of general formula 268, dimethyl triazoles of general formula 269,
and trimethyl
triazoles of general formula 270, which were chromatographically separable.
Scheme 68
Scheme 68 indicates a method for the preparation of 1,3,4-thiadiazoles of
general
formula 272. The acid chlorides derived from acids of general formula 21 or 28
were
reacted with thiosemicarbazide or substituted thiosemicarbazides to give
intermediate
acylated thiosemicarbazides of general formula 271, which were cyclized under
acid
catalysis (for example methanesulfonic acid in refluxing toluene) to provide
thiadiazoles
of general formula 272.
67
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 69
Scheme 69 provides a method for the preparation of 1,3,4-oxadiazol-2-thiones
and
the derived alkylthio-substituted oxadiazoles of general formula 274.
I~ydrazides of
general formula 264 were treated with carbon disulfide in potassium hydroxide
in aqueous
ethanol solution to give the cyclic thiocarbamates of general formula 273. The
thiocarbonyl group was alkylated in low yield with alkyl halides to give the
alkylthio
1,3,4-oxadiazoles of general formula 274.
Scheme 70
Scheme 70 shows the preparation of tetrazoles at the 2-position of
thienopyridines.
2-Cyano derivatives of general formula 244 were converted to tetrazoles of
general
formula 275 using trimethylsilyl azide in the presence of catalytic dibutyltin
oxide. The
tetrazoles were converted to the N-methyl derivatives of general formula 276
by use of a
solution of diazomethane in methanol.
Scheme 71
Scheme 71 illustrates the syntheses of 2-oxazole and 2-imidazole
thienopyridines.
Chloroethyl amides of general formula 277 were prepared by chlorination of the
corresponding hydroxyethyl amides and then cyclized to oxazolines of general
formula
278 under basic catalysis (for example, diazabicycloundecane in
dichloromethane). The
oxazolines of general formula 278 may be converted to the oxazoles of general
formula
68
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
278 by dehydrogenation according to the procedure of Meyers (Meyers, A. L, et
al. J.
Amer. Chem. Soc. 1975, 97, 7383). Aminoethyl amides of general formula 280
were
cyclized to the imidazolines of general formula 281 by treatment with calcium
oxide at
high temperature in diphenyl ether. Imidazolines of general formula 28I may be
converted to imidazoles of general formula 282 by literature methods (Hughey,
J. L., et al.
Synthesis [SYNTBF] 1980, (6), 489).
Scheme 72
In Scheme 72, an improved preparation of 3-alkyl-substituted thienopyridines
of
general formula 115 is disclosed. Aldehyde 18 was condensed with preformed
potassium
phenoxides to produce a mixture of mono- and disubstituted aryloxyaldehydes.
This
mixture was then reacted with the desired Grignard reagent, followed by a
Swern
oxidation of the resultant mixture of secondary alcohols to give the desired
aryloxy ketone
compounds. The mixture of aryloxy ketones were further reacted with methyl
thioglycolate in the presence of cesium carbonate to provide the 2,3,4-
trisubstituted
thieno[2,3-c]pyridine esters of general formula 1 I4. These esters were
converted to the
corresponding acids by hydrolysis with lithium hydroxide, and then the acids
were coupled
with various amines, for example by carbodiimides, to give the desired amides
of general
formula 115.
Scheme 73
Scheme 73 provides a synthesis for 3-carboxythienopyridines.
Using methodology analogous to that described for the preparation of 20 or 25,
3,5-
dichloropyridine was treated with strong base such as lithium diisopropylamide
in
anhydrous ethereal solvent at low temperature, followed by the addition of t-
butyl
chlorooxoacetate to provide the
69
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
4-tert-butyl-2-ketoester of 3,5-dichloropyridine 283. The ester 283 was then
reacted with
1.25 equivalents of preformed potassium phenoxides at ambient temperature, to
give the
monoaryloxy derivatives as the main product. Without purification, the
monoaryloxy
esters were treated with methyl thioglycolate and base such as potassium t-
butoxide or
cesium carbonate to provide the desired thienopyridine diesters of general
formula 284.
The diesters of general formula 284 were then treated with methanoIic amines
to give the
corresponding 3-tert-butyl ester amides of general formula 285. The tert-butyl
ester
amides of general formula 285 were converted to the corresponding acid amides
of general
formula 287 by solvolysis with trifluoroacetic acid. Diesters of general
formula 284 may
also be converted to the acids of general formula 286 by a similar solvolysis
reaction.
Scheme 74
Scheme 74 describs the use of 4-bromothienopyridine 32 to prepare 4-amino
substituted thienopyridine derivatives. Ester 32 was converted to amides of
general
formula 288 by standard procedures, and then coupled to a variety of amines
using
palladium(0) catalysis, as described by Buchwald (J. Org. Chem. 1997, 62, 6066-
6068),
producing 4-amino derivatives of general formula 289.
Scheme 75
Scheme 75 outlines the preparation and reactions of 7-chloro and 7-
bromothienopyridine derivatives. The analogs are useful for preparing active
derivatives
as well as serving as synthetic intermediates for a variety of 7-substituted
thienopyridines.
Esters of general formula 25 were oxidized to the pyridine-N-oxides of general
formula
290 with meta-chloroperbenzoic acid. The N-oxides were rearranged to the 7-
halo
derivatives of general formula 291 by warming in phosphorous oxychloride or
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
phosphorous oxybromide. The resultant 7-halides could be converted to the
amide
derivatives of general formula 292 by standard methods without reaction of the
7-chloro or
7-bromo moieties. However, under more forcing conditions, the chloro or bromo
groups
could be substituted with amines or alcohols to provide 7-amino derivatives of
general
formula 293 and 7-alkoxy derivatives of general formula 294 respectively.
Esters of
general formula 294 were converted to amides of general formula 295 using
standard
methods. 7-Hydroxy analogs of general formula 296 were prepared from 291
derivatives
using acetic anhydride followed by hydrolysis with water. In addition, the 7-
halo
derivatives 291, in particular the 7-bromo derivatives, were effective educts
in Suzuki
reactions with aryl boronic acids, similar to those described in Scheme 19 and
65.
Scheme 76
Scheme 76 describes the preparation of furopyridine analogs such as 299
(Example
327). By a procedure analogous to that of the preparation of 20 or 25,
diehloropyridinecarboxaldehyde 18 was reacted sequentially with a potassium
phenoxide,
then with ethyl glycolate, followed by cyclization under basic conditions,
affording
furopyridine ester 298 in low yield. Standard hydrolysis and coupling
conditions afforded
the amide 299. The amide was converted to the thioamide 300 by treatment with
Lawesson's reagent in hot toluene.
Scheme 77
Scheme 77 illustrates the preparation N-alkyl 5-amino-1,3,4,-oxadiazoles. The
treatment of 265 in refluxing trimethylorthoformate followed by reduction of
the eneamine
intermediate with sodium borohydride in refluxing ethanol provides the N-
alkylated 5-
amino-1,3,4,-oxadiazoles of general formula 301.
71
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Scheme 78
Scheme 78 exemplifies the synthesis of substituted vinyl moieties at the 4-
position
of thienopyridines. Treatment of aldehyde 227 with the appropriate
diethylphosphonate in
the presence of potassium bis(trimethylsilyl)amide provided 302. Compound 302
was
then treated with sulfuric acid in methanol to yield the methyl ester 303,
followed by
standard amide formation with ammonia and methanol to produce 4-
vinylsubstituted
thienopyridines of general formula 304. Installation of a substituted vinyl
can also be
accomplished by using Wittig phosphorane chemistry.
Scheme 79
20
Scheme 79 demonstrates the preparation of 4-substituted alkyl thienopyridines.
Alcohol 228 was subjected to palladium on carbon in acetic acid to generate
the methylene
derivative 305. Treatment of 305 with sulfuric acid in methanol yields 306.
Formation of
the amide is accomplished by treatment of 306 with ammonia in methanol to
yield 307
Scheme 80
Scheme 80 illustrates the preparation of thiazole derivatives at the 2-
position of
thienopyridines. Thioamides of general formula 309 were alkylated and cyclized
with
ethyl bromopyruvate, providing thiazole esters of general formula 310.
Standard amide
formation led to amides of general formula 311.. Other amines may be used to
produce a
72
CA 02333770 2000-11-30
WO 99/62908 PCT/US99112419
variety of substituted amides. In addition, esters of general formula 310 may
be converted
to carbamates of general formula 312 through Curtius rearrangement of the
intermediate
acid. The tert-butyl carbamates of general formula 312 were converted to the
primary
amines of general formula 313 by the action of trifluoroacetic acid.
Scheme 81
Scheme 81 outlines an alternative method for preparing 3-substituted
thienopyridines, wherein Ar = unsubstituted or substituted aryl, or
heterocycle, and R =
alkyl, alkoxy, substituted alkyl, aryl, arylalkyl. Aldehyde 18 was reacted
with the
appropriate organomagnesium halide, to give an intermediate secondary alcohol,
which
was oxidized to the corresponding ketone 314. The method of oxidation was the
Swern
procedure, although other standard oxidations of this type may be employed
(e.g PCC,
TPAP). The procedure then follows that previously described for the 3-
unsubstituted
analogs, leading to ester 315. Ester 315 then served as starting material for
the preparation
of amides, or other heterocyclic derivatives at the 2-position of the
thienopyridines.
Scheme 82
Scheme 82 describes a method for producing cyclic derivatives between the 2-
and
3-positions of thienopyridines. 3-Methyl derivative 115 was treated with N-
bromosuccinimide (or alternatively N-chlorosuccinimide) in carbon
tetrachloride to give
bromomethyl (or chloromethyl) compound 316 (X= Br, Cl). Compound 316 can then
be
reacted with a primary amine, through alkylation and acylation, leading to the
tricyclic
lactam 317. Compound 316 may also be treated with sodium alkoxides or
aryloxides R'' _
alkyl, aryl, or heterocycle) leading to the 3-position extended alkoxymethyl
derivatives
73
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
318. These esters in turn can be reacted with substituted amines to yield the
corresponding
amides 319.
The compounds and processes of the present inventian will be better understood
in
connection with the following examples which are intended as an illustration
of and not a
limitation upon the scope of the invention.
Example 1
methyl 2-f (6-ethylthienof 2,3-dlpyrimidin-4-yl)thiolacetate
Exam~pIe 1 A
methyl 6-ethyl-3,4-dihydro-4-oxothienof2,3-dlpyrimidine-2-carbox
The desired compound was prepared as described in J. Heterocylic Chem. 1987,
24, 581-587.
Example 1B
6-ethylthienof 2,3-dlpyrimidin-4(3H)-one
Example lA (35 g, 140 mmol) and LiCI (6.5 g, 153 mmol) in DMSO (80 mL) and
water (8 mL) was heated to 150 °C for 18 hours, cooled to room
temperature, diluted with
water, and extracted with ethyl acetate. The extract was dried (MgS04),
filtered, and
concentrated to provide the designated compound.
Example 1 C
4-chloro-6-ethylthienof2,3-dlp~rimidine
Example 1B (3.97 g, 22.0 mmol) in POCI3 (22 mL) was heated to reflux for 2
hours, cooled, poured onto ice, diluted with water, made basic with
concentrated
ammonium hydroxide, and extracted with ethyl acetate. The extract was dried
(MgS04),
filtered, and concentrated. The residue was purified by flash chromatography
on silica gel
with 10% ethyl acetate-hexane to provide the designated compound.
Example ID methyl2-f(6-ethylthienof2,3-dlpyrimidin-4-yl)thio)acetate
Example 1 C (0.25 g, 1.26 mmol) in DMF ( 1.2 mL) was treated sequentially with
methyl thioglycolate (0.134 g, 1.26 mmol) and potassium carbonate (0.174 g,
1.26 mmol),
stirred at room temperature for 18 hours, cooled, poured into water, diluted
with brine, and
extracted with dichloromethane. The extract was washed with water and brine,
dried
(MgS04), filtered, and concentrated. The residue was triturated then washed
with 10%
ethyl acetate/hexanes to provide the title compound.
74
CA 02333770 2000-11-30
WO 99/62908 PCTIUS99/12419
mp 36-58 °C;
MS (DCI/NH3) m/z 269 (M+H)+;
~H NMR (300 MHz, DMSO-d6) 8 1.33 (t, 3H}, 2.99 (q, 2H), 3.75 (s, 2H), 4.26 (s,
3H),
7.23 (s, 1 H), 8.76 (s, 1H).
Example 2
6-ethyl-4-1(4-methylphenyl)thiolthienof2 3-dlpyrimidine
Example 1C was processed as in Example 1D but substituting thiocresol for
methyl thioglycolate to provide the title compound.
mp 56-58 °C;
MS (DCI/NH3) m/z 286 (M+H)+;
tH NMR (300 MHz, DMSO-d6) 8 1.32 (t, 3H), 2.38 (s, 3H), 2.99 (q, 2H}, 7.20 (s,
1H),
7.33 (m, 2H), 7.52 (m, 2H), 8.63 (s, 1H);
Anal. calcd for C15H14N2s2~ C~ 62.90; H, 4.93; N, 9.78. Found: C, 63.11; H,
4.82 N,
9.63.
Example 3
6-ethyl-4-(2-pyridinylthio)thieno f 2,3-dlpyrimidine
Example 1C was processed as in Example 1D but substituting 2-mercaptopyridine
for methyl thioglycolate to provide the title compound.
mp 76.5-79 °C;
MS (DCI/NH3) m/z 274 (M+H)+;
~H NMR (300 MHz, DMSO-d6) S 1.31 (t, 3H), 2.99 (q, 2H), 7.18 (s, 1H), 7.46
(dt, 1H),
7.81 (d, 1H), 7.90 (dt, 1H), 8.60 (m, 1H), 8.74 (s, 1H).
Example 4
6-ethyl-4-f(2-methylethyl)thiolthienof2 3-dlpyrimidine
Example 1C was processed as in Example 1D but substituting isobutyl mercaptan
for methyl thioglycolate to provide the title compound.
MS (DCI/NH3) m/z 253 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 1.08 (d, 6H), 1.39 (t, 3H), 2.03 (hep, 1H), 2.95
(q, 2H),
3.28 (d, 2H), 7.01 (s, 1H), 8.71 (s, 1H};
Anal. calcd for CI2H16N2S2~ C, 57.12; H, 6.38; N, 11.09. Found: C, 57.22; H,
6.29 N,
11.08.
Example 5
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
6-ethyl-4-((nhenylmethyl)thiolthieno f 2 3-dlpyrimidine
Example 1C was processed as in Example 1D but substituting benzyl mercaptan
for methyl thioglycolate to provide the title compound.
mp 54-60 °C;
MS (DCI/NH3) m/z 287 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 1.30 (t, 3H), 2.96 (q, 2H), 4.65 (s, 2H), 7.16 (s,
1H),
7.21-7.36 (m, 3H), 7.46 (m, 2H), 8.83 (s, 1H);
Anal. calcd for C15H14N2S2~ C, 62.90; H, 4.93; N, 9.78. Found: C, 62.11; H,
4.94 N,
9.71.
Example 6
6-ethyl-4-((5-methyl-1,3,4-thiadiazol-2-yl)thiolthieno(2 3-dlpyrimidine
Example 1 C was processed as in Example 1 D but substituting 5-methyl- I,3,4-
thiadiazol-2-thiol for methyl thioglycolate to provide the title compound.
mp 132-135 °C;
MS (DCI/NH3) m/z 295 (M+H}+;
1H NMR (300 MHz, DMSO-d6) 8 1.35 (t, 3H), 2.82, (s, 3H), 3.05 (q, 2H), 7.42
(s,lH),
8.88 (s, 1 H);
Anal. calcd for C 1 i H ~ pN4S3: C, 44.88; H, 3.42; N, I 9.03. Found: C,
44.61; H, 3.47 N,
18.92.
Example 7
ethyl 6-ethyl-4-((4-methylphenyl)thiolthieno(2 3-dlpyrimidine-6-carboxylate
Example 1 A was processed as in examples 1 C and 2 and to provide the title
compound.
mp 87.5-90 °C;
MS (DCI/NH3) m/z 359 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 1.26 (t, 3H), 1.31 (t, 3H), 2.39 (s, 3H), 3.02 (q,
2H),
4.27 (q, 2H), 7.17 (s, 1 H), 7.33 (m, 2H), 7.57 (m, 2H);
Anal. calcd for ClgH~gN202S2: C, 60.31; H, 5.06; N, 7.81. Found: C, 60.44; H,
4.88 N,
7.65.
Example 8
6-ethyl-N-(nhenylmethyl)thieno(2 3-d]pyrimidin-4-amine
Example 1C (0.27 g, 1.37 mmol) in isopropanol (1.5 mL,) was treated with
benzylamine (0.19 mL, 1.71 mmol) and sodium carbonate (0.24 g, 2.3 mmol),
stirred at
76
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
room temperature overnight, filtered, and concentrated. The residue was
purified by flash
chromatography on silica gel with 25% ethyl acetate/hexanes to provide the
title
compound.
mp 128-131 °C;
MS (DCI/NH3) m/z 270 (M+H)+;
iH NMR (300 MHz, DMSO-d6) 8 1.30 (t, 3H), 2.88 (q, 2H), 4.72 (d, 2H), 7.20-
7.40 (m, 6
H), 8.26 (s, 1H), 8.34 (t, 2H);
Anal. calcd for C15Ht5N3S2: C, 66.89; H, 5.61; N, 15.60. Found: C, 66.66; H,
5.43 N,
15.43.
Example 9
6-ethyl-N-(5-methyl-1,3,4-thiadiazol-2-yl)thienof2 3-dlpyrimidin-4-amine
A solution of Example 1 C (0.20 g, 1.01 mmol) in isopropanol (2 mL) was
treated
with 2-amino-5-methyl-1,3,4-thiadiazole (0.15 g, 1.26 mmol} and sodium
carbonate (0.18
g, 1.7 mmol), stirred at room temperature for 48 hours, treated with cesium
carbonate
(0.55 g, 1.7 mmol), stirred at reflux for 24 hours, concentrated, treated with
water, and
extracted with dichloromethane. The extract was dried (MgS04), filtered, and
concentrated. The residue was recrystallized with ethanol/water to provide the
title
compound.
mp 277-280 °C;
MS (DCI/NH3) m/z 278 (M+H)+;
IH NMR (300 MHz, DMSO-d6) S 1.33 (t, 3H), 2.63 (s, 3H), 2.96 (q, 1H), 7.81 (br
s, 1H),
8.65 (s, 1H);
Anal. calcd for Ct 1Hi 1N5S2: C, 47.63; H, 4.00; N, 25.25. Found: C, 47.48; H,
3.68 N,
24.89.
Example 10
4-f(5-amino-1,3 4-thiadiazol-2-yl)thiol-6-ethyl-2-(phe~lmethyl)thienof2 3-
dlpyrimidine
Example l0A
2-amino-5-ethylthiophene-3-carboxamide
The designated compound was prepared as described in J. Heterocylic Chem.
1987, 24, pp 581-587.
Example lOB
5-ethyl-2-f (phenylacetyl)aminol-3-thio~henecarboxamide
77
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example l0A was processed as in Bull. Soc. Chim. France 1975, p 815 to provide
the designated compound.
Example lOC
6-ethyl-2-phenylmethylthienof2,3-dlpyrimidin-4(3H)-one
Example lOB was stirred in dioxane/water in the presence of 10% Na2C03 to
provide the designated compound.
Example lOD
4-chloro-6-ethyl-2-(phenylmethyl)thienof2 3-d'[pyrimidine
Example l OC was processed as in Example 1 C to provide the designated
compound.
Example 10E
4-((5-amino-1,3,4-thiadiazol-2-yl)thiol-6-ethyl-2-(phenylmethyl)thienof2 3-
dlpyrimidine
Example lOD and 5-amino-1,3,4-thiadiazole-2-thiol were processed as in Example
1D to provide the title compound.
MS (DCI/NH3) m/z 386 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 1.31 (t, 3H), 2.97 (q, 2H), 4.17 (s, 2H), 7.17-
7.30 (m,
6H), 7.70 (br s, 2H);
Anal. calcd for Cl~Hi5N5S3: C, 52.96; H, 3.92; N, 18.17. Found: C, 53.10; H,
3.74 N,
18.03.
Example 11
7-methyl-4-f(4-meth3rlphenyl)thiolthieno_ f3 2-dlpyrimidine
3-Methyl-7-chlorothieno[3,2-d]pyrimidine was processed as in Example 1D but
substituting p-thiocresol for methyl glycolate to provide the title compound.
mp 103-107 °C;
MS (DCI/NH3) m/z 273 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.40 (s, 6H), 7.36 (m, 2H), 7.57 (m, 2H), 8.04 (s,
1H),
8.88 (s, 1H);
Anal. calcd for C ~ SH ~ 2N20S2 : C, 61.73; H, 4.44; N, 10.28. Found: C,
61.73; H, 4.50 N,
10.21.
Example 12
7-methyl-4-((5-methyl-1,3,4-thiadiazol-2-yl)thiolthienof3 2-dlpyrimidine
78
CA 02333770 2000-11-30
WO 99/62908 PCT/U599/12419
3-Methyl-7-chlorothieno[3,2-d]pyrimidine and 5-methyl-1,3,4-thiadiazol-2-thiol
were processed as in Example 1D to provide the title compound.
mp 144-147 °C;
MS (DCI/NH3) m/z 281 (M+H)+;
~H NMR (300 MHz, DMSO-d6) b 2.44 (s, 3H), 2.83 (s, 3H), 8.20 (s, 1H), 9.08 (s,
1H);
Anal. calcd for CIpHgN4S3: C, 42.84; H, 2.88; N, 19.98. Found: C, 42.72; H,
2.83 N,
19.64.
Example 13
7-methyl-4-ff5-(methylthio)-1,3,4-thiadiazol-2-yllthiolthienof3 2-dlpYrimidine
3-Methyl-7-chlorothieno[3,2-d]pyrimidine and 5-(methylthio)-1,3,4-thiadiazol-2-
thiol were processed as in Example 1D to provide the title compound.
mp 163-166 °C;
MS (DC1JNH3) m/z 313 (M+H)+;
IH NMR (300 MHz, DMSO-d6) 8 2.45 (s, 3H), 2.83 (s, 3H), 8.22 (s, 1H), 9.11 (s,
1H);
Anal. calcd for C~pHgN4S4: C, 38.44; H, 2.58; N, 17.93. Found: C, 38.46; H,
2.63 N,
17.82.
Example 14
4-f(5-amino-1,3,4-thiadiazol-2-yl)thiol-7-methylthienof3 2-dlpyrimidine
3-Methyl-7-chlorothieno[3,2-d]pyrimidine and 5-amino-1,3,4-thiadiazole-2-thiol
were processed as in Example 1 D to provide the title compound.
mp 221-223 °C;
MS (DCI1NH3) m/z 282 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.43 (s, 3H), 7.80 (br s, 2H), 8.15 (s, 1H), 9.02
(s, 1H);
Anal. calcd for C9H~NSS3: C, 38.42; H, 2.51; N, 24.89. Found: C, 38.41; H,
2.42 N,
24.97.
Example 15
7-methyl-N-f(4-(meth lt~phenyllthienof3 2-dlpyrimidin-4-amine
A solution of 3-methyl-7-chlorothieno[3,2-d]pyrimidine in ethanol was treated
with 4-(methylmercapto)aniline, stirred at reflux for 45 minutes, cooled to
room
temperature, and filtered. The precipitate was recrystallized from
ethanol/water to provide
the title compound.
mp 212-215 °C;
MS (DCI/NH3) m/z 288 (M+H)+;
79
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, DMSO-d6) 8 2.37 (s, 3H), 2.48 (s, 3H), 7.29 (m, 2H), 7.76 (m,
2H),
7.87 (br s, 1H), 8.60 (s, 1H), 9.63 (br s, 1H);
Anal. calcd for C14H13N3S2~ C. 58.51; H, 4.56; N, 14.62. Found: C, 58.31; H,
4.49 N,
14.47.
Example 16
7-methyl-4-f (4-methylnhenyl)thiolthienof 3,2-dlpyrimidine-6-carboxamide
Example 16A
7-methyl-4-f(4-methylphenyl)thiolthienof3,2-dlpyrimidine-2-carboxylic acid
A solution of LDA (O.1M in THF, 9.6 mL) at -78 °C was treated with
Example 11
(0.26 g, 0.96 mmol), warmed to 0 °C over 1 hour, poured onto dry ice
with constant
swirling, quenched with saturated NH4C1, and extracted with 3:1
chloroform/isopropanol.
The extract was concentrated, and the residue was purified by flash
chromotograpy on
silica gel with 7% methanol/dichloromethane to provide the designated
compound.
Example 16B
7-methyl-4-f (4-methylnhenyl)thiolthienof 3,2-dlpyrimidine-2-carboxamide
A suspension of Example 16A in dichloromethane (3.3 mL) was treated
sequentially with oxalyl chloride (0.03 mL, 0.33 mmol) and DMF ( 1 drop), and
concentrated after formation of the acid chloride. The residue was suspended
in THF ( 10
mL), transferred to a vigorously stirred solution of 1:1 ammonium
hydroxide/water ( 10
mL), and extracted with dichloromethane. The extract was dried (MgS04),
filtered, and
concentrated. The residue was recrystallized from ethyl acetate/hexanes to
provide the
title compound.
mp 243-246 °C;
MS (DCI/NH3) m/z 316 (M+H)+;
~H NMR (300 MHz, DMSO-d6) b 2.40 (s, 3H), 2.58 (s, 3H), 7.35 (m, 2H), 7.57 (m,
2H),
8.01 (br s, 2H), 8.93 (s, 1H);
Anal. calcd for C~SH13N30S2: C, 57.12; H, 4.15; N, 13.32. Found: C, 56.81; H,
4.06 N,
13.25.
Example 17
methyl4-f(4-methylnhenyl)thiolthienof2 3-cl~yridine-2-carbox~ate
Example 17A
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
3,5-dichloropyridine-4-carboxaldeh~rde
Diisopropyl amine ( 15.6 mL, 0.111 mol) in dry THF (25 mL) at 0 °C was
treated
with n-BuLi (44.6 mL, 2.5 M in hexane, 0.111 mol) over 35 minutes, stirred for
30
minutes, cooled to -78 °C, diluted with THF (100 mL), and a solution of
3,5-
dichloropyridine ( 15.0 g, 0.101 mol) in THF ( 175 mL) was added slowly over
3.5 hours in
order to maintain an internal temperature < -74 °C. The solution was
stirred at -78 °C for
30 minutes, treated dropwise over 35 minutes with methyl formate (12.5 mL,
0.203 mmol)
in THF (50 mL) maintaining an internal temperature of < -74 °C, stirred
at -78 °C for I .4
hours, rapidly cannulated into a ice cold solution of saturated NaHC03 with
vigorous
stirring, partitioned with ethyl acetate (500 mL), extracted sequentially with
saturated
NaHC03 (2x100 mL), brine (3x150 mL), dried (MgS04), and concentrated. The
residue
was purified by flash chromatography on silica gel with 10% acetone/hexane.
MS (DC1/NH3) m/z 176, 178, 180 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.80 (s, 2H), 10.31 (s, 1H).
Example 17B
3-(4-methYlphenylthio)-5-chloro-4-pyridinecarbox~raldeh~de
Example 17A (5.05 g, 28.7 mmol) in DMF (70 mL) was treated with p-thiocresol
(3.56 g, 28.7 mmol) and potassium carbonate (4.36 g, 31.6 mmol), stirred for
0.5 hours at
0 °C then for I hour at room temperature, poured into water, diluted
with brine, and
extracted with dichloromethane. The extract was washed sequentially with water
and
brine, dried (MgSOq.}, filtered, and concentrated to provide the designated
compound.
Example 17C
methyl 4- f (4-methylphenyl)thiolthieno f 2,3-clpyridine-2-carboxylate
A solution of Example 17B was processed as in Example ID to provide the title
compound.
mp 116-119 °C;
MS (DCI/NH3) m/z 316 (M+H)+;
tH NMR (300 MHz, DMSO-d6) 8 2.28 (s, 3H), 3.91 (s, 3H), 7.20 (m, 2H), 7.29 (m,
2H),
8.00 (s, 1H), 8.44 (s, IH), 9.36 (s, 1H);
Anal. calcd for C16H13N02S2~0.25 H20 C, 60.07; H, 4.25; N, 4.37. Found: C,
60.04; H,
4.08 N, 4.27.
Example 18
4-[(4-methylphenyl)thiolthienof2,3-clpyridine-2-carboxylic acid
81
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
A suspension of Example 17C (2.0 g, 6.35 mmol) and LiOH~H20 ( 1.4 g, 32 mmol)
in isopropanol (25 mL) and water ( 15 mL) was heated to 75 °C for 1
hour, cooled, treated
with water, and washed with diethyl ether. The aqueous layer was cooled in an
ice bath
and adjusted to pH 2 with 10% HCI. The resulting solid was collected, washed
with
water, dried, and recrystallized from ethanol/water to provide the title
compound.
mp 272-274 °C;
MS (DCI/NH3) m/z 302 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.29 (s, 3H), 7.20 (m, 2H), 7.28 (m, 2H), 7.92 (s,
1H),
8.44 (s, 1H), 9.34 (s, 1H);
Anal. calcd for C1gH12N2OS2: C, 59.78; H, 3.67; N, 4.64. Found: C, 59.48; H,
3.58 N,
4.54.
Example 19
4-1(4-methylphenyl)thiolthieno f 2,3-clpyridine-2-carboxamide
A suspension of Example 18 (0.535 g, 1.78 mmol) in dichloromethane (25 mL) at
0 °C was treated sequentially with oxalyl chloride (0.34 g, 2.67 mmol)
and DMF ( 1 drop),
stirred at room temperature for 0.5 hours, and concentrated. The residue was
suspended in
THF, treated with THF (60 mL), water (30 mL), and concentrated NH40H (30 mL),
and
stirred for 0.5 hours. The THF layer was separated, washed with brine,
partially dried
(MgS04), filtered, and concentrated. The residue was purified by flash
chromatograpy on
silica gel with 5% methanol/dichloromethane and recrystallized from 95%
ethanol to
provide the title compound.
mp 198-199 °C;
MS (DCI/NH3) m/z 301 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.29 (s, 3H), 7.20 (m, 2H), 7.30 (m, 2H), 7.89 (br
s,
1H), 8.26 (s, 1H), 8.35 (s, 1H), 8.54 (br s, 1H) 9.16 (s, 1H);
Anal. calcd for C15H12N20S2: C, 59.97; H, 4.02; N, 9.32. Found: C, 59.84; H,
4.12 N,
9.31.
Example 20
4-~-pyridinylthio)thieno12,3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B, 17C, 18, and 19 but substituting
2-mercaptopyridine for p-thiocresol in Example 17B to provide the title
compound.
mp 239-242 °C;
MS (DCIINH3) m/z 305 (M+NH4)+;
82
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, DMSO-d6) b 6.99 (d, 1H), 7.17 (dd, 1H), 7.65 (dt, 1H), 7.85
(br s,
1H), 8.18 (s, 1H), 8.36 (m, 1H), 8.49 (br s, 1H), 8.69 (s, 1H}, 9.23 (s, 1H);
Anal. calcd for C13H9N30S2: C, 54.34; H, 3.16; N, 14.47. Found: C, 54.10; H,
3.14; N,
14.62.
Example 21
4-f (4-chlorophenyl)thiolthienof 2,3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B, 17C, 18, and 19 but substituting
4-chlorothiophenol for p-thiocresol in Example 17B to provide the title
compound.
mp 239-241 °C;
MS (DCI/NH3) m/z 321 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.31 (m, 2H), 7.43 (m, 2H), 7.89 (br s, 1H), 8.24
(s,
1H), 8.54 (br s, 1H), 8.56 (s, 1H), 9.38 (s, 1H);
Anal. calcd for C~4H9C1N20S2: C, 52.42; H, 2.83; N, 8.73. Found: C, 52.33; H,
2.80; N,
8.63.
Example 22
N-methox~N-methyl-4-f (4-methvlphenyl)thiolthienof 2,3-clpyridine-2-
carboxamide
A solution of Example 18 (0.66 g, 2.2 mmol) in dichloromethane was treated
sequentially with oxalyl chloride (0.29 mL, 3.3 mmol) and DMF ( 1 drop),
stirred for 30
minutes, and concentrated. The residue was suspended in THF, transferred to a
solution of
N,O-dimethylhydroxylamine hydrochloride (0.32 g, 3.3 mmol) and triethylamine
(0.92
mL, 6.6 mmol) in 1:1 THF water, and stirred for 5 minutes. The THF layer was
separated,
dried (MgS04), filtered, and concentrated. The residue was purified by flash
chromatography on silica gel with 20°lo ethyl acetate/hexanes to
provide the title
compound.
mp 103-107 °C;
MS (DCI/NH3) m/z 345 (M+H)+;
~H NMR (300 MHz, DMSO-d6) b 2.27 (s, 3H), 3.34 (s, 3H), 3.74 (s, 3H), 7.19 (m,
2H),
7.27 (m, 2H), 8.02 (s, 1H), 8.46 (s, 1H);
Anal. calcd for C1~H~6N202S2: C, 59.28; H, 4.68; N, 8.13. Found; C, 58.76; H,
4.58; N,
8.06.
Example 23
N-methoxy-4-f (4-methylphenyl)thiolthienof 2,3-clpyridine-2-carboxamide
83
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 18 was processed as in Example 22 but substituting O-methylhydroxyl-
aminehydrochloride for N,O-dimethylhydroxylamine hydrochloride to provide the
title
compound.
mp 200-203 °C;
MS (DCI/NH3) m/z 331 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.29 (s, 3H), 3.76 (s, 3H), 7.20 (m, 2H), 7.30 (m,
2H),
7.89 (br s, 1H), 8.15 (s, 1H), 8.4 (s, 1H), 9.3 (s, 1H);
Anal. calcd for C~6H~4N202S2~0.25.H20C, 58.16; H, 4.27; N, 8.48. Found: C,
57.46; H,
4.1 N, 8.01.
Example 24
N-(4-chlorophenyl)-4-f (4-methylphenyl)thiolthienof2,3-clpyridine-2-
carboxamide
A solution of Example 18 (0.1 g, 0.33 mmol) in dichloromethane was treated
with
oxalyl chloride (0.03 mL, 0.33 mmol) and DMF (1 drop) stirred at reflux for 20
minutes,
and concentrated. The residue was suspended in (3:1 ) benzene/dichloromethane
(4 mL),
treated with triethylamine (0.5 mL) and 4-chloroaniline (46 mg, 0.36 mmol),
stirred at
reflux overnight, and concentrated. The residue was treated with water and
extracted with
dichloromethane. The extract was dried (MgS04), filtered, and concentrated.
The residue
was purified by flash chromatography on silica gel with ethyl acetate/hexanes
to provide
the title compound.
mp 208-211 °C;
MS (DCI/NH3) m/z 411 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.29 (s, 3H), 7.23 (m, 2H), 7.33 (m, 2H), 7.47 (m,
2H),
7.81 (m, 2H), 8.34 (s, 1H), 8.57 (s, 1H), 9.31 (s, 1H), 10.90 (br s, 1H);
Anal. calcd for C~2H15CIN20S2: C, 61.38; H, 3.68; N, 6.82. Found: C, 61.22; H,
3.67;
N, 6.79.
Example 25
4-f (4-methylphenyl)thiolthienof2,3-clpyridine-2-carboxaldehyde
A solution of Example 22 (3.33 g, 9.6 mmol) in THF at -S °C was treated
dropwise
with 1M DIBAI-H in THF (14.5 mL, 14.5 mmol), stirred for 45 minutes, poured
into
ice/HCl with constant stirring, and extracted with dichloromethane. The
extract was dried
(MgS04), filtered, and concentrated to provide the title compound.
MS (DCI/NH3) m/z 303 (M+H)+;
~H NMR (300 MHz, DMSO-d6) 8 2.29 (s, 3H), 7.22 (m, 2H), 7.34 (m, 2H), 8.40 (s,
1H),
8.48 (s, 1H), 9.38 (s, 1H), 10.23 (s, 1H);
84
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Anal. calcd for C15Ht1NOS2: C, 63.13; H, 3.33; N, 4.91. Found: C, 62.81; H,
3.97; N,
5.01.
Example 26
4-f(4-methylphenyl)thiolthienof2,3-clpyridine-2-carboxaldehyde, O-methyloxime
A solution of Example 25 (0.22 g, 0.76 mmol) in 1:1 pyridine:ethanol (8 mL)
was
treated with methoxylamine~hydrochloride (0.51 mL, 1.52 mmol), stirred at room
temperature for 3 hours, concentrated, treated with water and extracted with
dichloromethane. The extract was washed with 1N HCI, dried (MgS04), filtered,
and
concentrated. The residue was purified by flash chromatography on silica gel
with 20%
ethyl acetate/hexanes to provide the title compound.
mp 95-98 °C;
MS (DCI/NH3) m/z 315 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 2.28 (s, 3H), 3.95 (s, 1.8H), 4.08 (s, 1.2H), 7.18
(m, 2H),
7.25 (m, 2H), 7.79 (s, 0.6H), 7.95 (s, 0.4H), 8.27 (s, 0.4H), 8.36 (s, 0.6H),
8.38 (s, 0.4H),
8.68 (s, 0.6H), 9.20 (s, 0.6H), 9.30 (s, 0.4H);
Anal. calcd for C16H14N20S2: C, 61.12; H, 4.49; N, 8.91. Found: C, 60.93; H,
4.55; N,
8.98.
Example 27
4-f(4-meth~lphenyl)thiolthienof2,3-clpyridine-2-carboxaldehyde, O-
(phenylmethyl)oxime
Example 25 and O-benzylhydroxylamine hydrochloride were processed as in
Example 26 but for 18 hours instead of 3 hours to provide the title compound.
mp 127-133 °C;
MS (DCI/NH3) m/z 391 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 2.27 (s, 3H), 5.22 (s, 1.2H), 5.38 (s, 0.8H), 7.15-
7.26 (m,
4H), 7.31-7.47 (m, 5H), 7.78 (s, 0.6H), 7.96 (s, 0.4H), 8.31 (s, 0.4H), 8.36
(s, 0.6H), 8.39
(s, 0.4H), 8.74 (s, 0.6H), 9.20 (s, 0.6H), 9.30 (s, 0.4H);
Anal. calcd for C22H1gN20S2: C, 67.66; H, 4.65; N, 7.17. Found: C, 67.45; H,
4.80; N,
7.13.
Example 28
2-f f f4-f (4-methylphenyl)thio~~thieno f 2,3-clpyridin-2-
ylmethylenelaminoloxylacetic acid
Example 25 was processed as in Example 26 but substituting carboxymethoxyl-
amine hemihydrochloride for methoxylamine~hydrochloride to provide the title
compound.
mp 227-230 °C;
CA 02333770 2000-11-30
WO 99/62908 PC'T/tJS99/12419
MS (DCI/NH3) m/z 359 (M+H)+;
iH NMR (300 MHz, DMSO-d6) 8 2.28 (s, 3H), 4.71 (s, 2H), 7.19 (m, 2H), 7.25 (m,
2H),
7.84 (s, 1H), 8.36 (s, 1H), 8.79 (s, 1H), 9.20 (s, 1H);
Anal. calcd for C1~H~4N2O3S2: C, 56.97; H, 3.94; N 7.82. Found C, 56.90; H,
4.10; N,
7.97.
Example 29
4-f(4-meth~nhenYl)thiolthienof2,3-clpyridine-2-carboxaldehyde, O phenyloxime
Example 25 was processed as in Example 26 but substituting O-phenylhydroxyl-
amine hydrochloride for methoxylamine~hydrochloride to provide the title
compound.
mp 94-97 °C;
MS (DCI/NH3) m/z 377 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 2.38 (s, 3H), 7.09-7.50 (m, 9H), 7.98 (s, 0.5H),
8.16 (s,
0.5H), 8.39 (s, O.SH), 8.42 (s, 05H), 8.71 (s, 0.5H), 9.16 (s, O.SH), 9.27 (s,
O.SH), 9.37 (s,
0.5);
Anal. calcd for C21 H 16N20S2: C, 67.00; H 4.28; N, 7.44. Found: C, 67.14; H,
4.50; N,
7.57.
Example 30
4-f(4-methyl~henyl)thiolthienoT2,3-clpyridine-2-carboxaldehyde, oxime
Example 25 was processed as in Example 26 but substituting hydroxylamine
hydrochloride for methoxylamine~hydrochloride to provide the title compound.
mp 209-210 °C;
MS (DCI/NH3) m/z 301 (M+H)+;
ZH NMR (300 MHz, DMSO-d6) S 2.28 (s, 3H), 7.18 (m, 2H), 7.70 (s, 0.3H), 7.87
(s,
0.7H), 8.19 (s, 0.7H), 8.35 (s, 0.3H), 8.38, (s, 0.7H), 8.56 (s, 0.3H), 9.17
(s, 0.3H), 9.27 (s,
0.7H);
Anal. calcd for C~SH~2N20S2: C, 59.98; H, 4.03; N, 9.33. Found: C, 59.80; H,
4.08, N,
9.30.
Example 31
2-if f4-f (4-methylphenyl)thiolthienof 2,3-clpyridine-2-
ylmethylenelaminoloxylacetamide
Example 28 was processed as in Example 19 to provide the title compound.
mp 152-156 °C;
MS (DCI/NH3) m/z 358 (M+H)+;
86
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, DMSO-d6) S 2.27 (s, 3H), 4.52 (s, 0.6H), 4.66 (s, 0.4H), 7.19
(m,
2H), 7.25 (m, 2H), 7.32 (br s, 1H), 7.40 (br s, 1H), 7.84 (s, 0.6H), 7.97 (s,
0.4H), 8.32 (s,
0.4H), 8.37 (s, 0.6H), 8.40 (s, 0.4H), 8.75 (s, 0.6H), 9.21 (s, 0.6H), 9.32
(s, 0.4H);
Anal. calcd for CI~H15N302S2~(1.25 H20): C, 57.12; H, 4.23; N, 11.76. Found C,
56.19;
H, 4.48; N, 10.94
Example 32
(E)-3-f (4-methylphenyl)thiolthienof2,3-clpyridin-2-yll-2-propenamide
Example 25 (0.23 g, 1.27 mmol) in chloroform ( 10 mL) was treated with
carbamoylmethylenetriphenylphosphorane (0.41 g, 1.27 mmol), heated to reflux
for 30
minutes, cooled, and concentrated. The residue was purified with flash
chromatography
on silica gel with 2% methanol/dichloromethane to provide the title compound.
mp 171-174 °C;
MS (DCI/NH3) m/z 327 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.28 (s, 3H), 6.64 (d IH), 7.19 (m, 2H), 7.25-7.37
(m,
3H), 7.68-7.82 (m, 3H), 8.35 (s, 1H), 9.19 (s, 1H);
Anal. calcd for C1~H~4N20S2 ~H20: C, 62.55; H, 4.32; N, 8.58. Found: C, 59.78;
H,
4.50; N, 8.20.
Example 33
1-f4-f (4-methylphenyl)thiolthienof 2,3-clpyridin-2-yllethanone
A solution of Example 22 in THF (25 mL) at 0 °C was treated with
methyl
magnesium bromide ( 1.4M in toluene/THF, 1.85 mL, 2.6 mmol), warmed to room
temperature, stirred overnight, treated with methylmagnesiumbromide ( 1.4M in
toluene/THF, 0.7 mL, 1.3 mmol) stirred for 1 hour, poured with constant
swirling onto
ice/NH4Cl, and extracted with ethyl acetate. The extract was dried (MgS04),
filtered, and
concentrated. The residue was purified by flash chromatography on silica gel
with 20%
ethyl acetate/hexanes to provide the title compound.
mp 134-138 °C;
MS (DCI/NH3) m/z 317 (M+NH4)+;
iH NMR (300 MHz, DMSO-d6) 8 2.33 (s, 3H), 2.71 (s, 3H), 7.24 (m, 2H), 7.38 (m,
2H),
8.28 (s, 1H), 8.31 (s, 1H), 9.29 (s, IH);
Anal. calcd for C16Ht3NOS2: C, 64.19; H, 4.38; N, 4.68. Found C, 64.11; H,
4.41; N,
4.61.
Example 34
87
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
2-benzoyl-4-f (4-methylphenyl)thiolthienof 2,3-clpyridine
Example 22 and phenyl lithium were processed as in Example 33 to provide the
title compound.
mp 103-107 C;
MS (DCI/NH3) m/z 362(M+H)+;
1H NMR (300 MHz, DMSO d6) b 2.33 (2, 3H), 7.26 (m, 4H), 7.57 (m, 2H), 7.71 (m,
4H),
8.49 (s, 1H), 9.40 (s, 1H);
Anal. Calcd for C21H15NOS2~1.25 H20: C, 65.68;, H, 4.59; N, 3.64. Found: C,
65.67; H,
4.09; N, 3.46.
Example 35
2-eth r~l-4-f(4-meth~phenyl)thiolthienof2,3-clpyridine
A solution of Example 33 in ethylene glycol ( 10 mL) was treated with
hydrazine
hydrate (0.18 mL, 5.8 mmol), stirred at 160 °C for 30 minutes, cooled
to room
temperature, treated with potassium hydroxide, stirred at 150 °C for 45
minutes, cooled to
room temperature, treated with water, and extracted with ethyl acetate. The
extract was
washed with water, dried (MgS04), filtered, and concentrated. The residue was
purified
by flash chromatography on silica geI with 10% ethyl acetate/hexanes to
provide the title
compound.
MS (DCI/NH3) m/z 286 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 1.28 (t , 3H), 2.26 (s, 3H), 2.99 (q, 2H), 7.14-
7.27 (m,
5H), 8.34 (s, 1H), 9.11 (s, 1H);
Anal. calcd. for C ~ 6Hj gNS2~0.25 H20: C, 67.33; Hi, 5.30; N, 4.91. Found: C,
66.63; H,
5.38; N, 4.72.
Example 36
1-f4-f(4-meth~rlphenyl)thiolthienof2,3-clpyridin-2-~lethanone, oxime
Example 33 and hydroxylamine hydrochloride were processed as in Example 26 to
provide the title compound.
mp 209-213 °C;
MS (DCI/NH3) m/z 315 (M+H)+;
tH NMR (300 MHz, DMSO-d6) b 2.22 (s, 1.5H}, 2.28 (s, 3H), 2.32 (s, 1.SH), 7.20
(m,
2H), 7.30 (m, 2H), 7.62 (s, 0.5H), 7.70 (s, 0.5H), 8.30 (s, O.SH), 8.34 (s,
0.5H), 9.12 (s,
0.5H), 9.24 (s, O.SH);
Anal. calcd for C16H~4N20S2: C, 61.16; H, 4.49, N, 8.91. Found C, 60.83, H,
4.61; N,
9.03.
88
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
Example 37
N-(2 3-dihydroxypropyl)-4-f(4-methylphenyl)thiolthienof2,3-clpyridine-2-
carboxamide
A solution of Example 18 (2.5 g, 8.3 mmol) and N-hydroxysuccinimide (0.95 g,
8.3 mmol) in dichloromethane (35 mL) was treated with DCC ( 1.882 g, 9.13
mmol) in
methylene chloride ( 15 mL), stirred at room temperature for 18 hours, and
concentrated.
The residue was dissolved in ethyl acetate, washed with water, dried (MgS04),
filtered,
and concentrated. The residue was added to a solution of 3-amino-1,2-propane
diol (0.144
g, 1.6 mmol) in 3:1 dioxane/methanol (20 mL), was stirred at room temperature
for 18
hours, concentrated, dissolved in ethyl acetate, washed with water, dried
(MgS04),
filtered, and concentrated. The residue was purified by flash chromotograpy on
silica gel
with 6% methanol/dichloromethane to provide the title compound.
mp 120-122 °C;
MS (DCI/NH3) m/z 375 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.29 (s, 3 H), 3.19 (m, 1H), 3.4 (m, 1 H), 3.65
(m, 1 H),
4.62, (t, 1 H), 4.88 (d, 1 H) 7.20 (m, 2 H), 7.30 (m, 2 H), 8.38 (s, 1 H), 9.1
(s, 1 H), 9.28
(s, 1H) ;
Anal. calcd for C~gH~gN203S2~0.75H20: C, 57.73; H, 4.84; N, 7.48. Found: C,
55.54, H,
5.23 N, 6.7.
Example 38
4-f (4-methy~henyl)thiolthieno(2,3-clpyridine-2-carboxylic acid, hydrazide
Example 18 was processed as in Example 37 but substituting hydrazine for 3
amino-1,2-propanediol to provide the title compound.
mp 176-178 °C;
MS (DCI/NH3) m/z 316 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.29 (s, 3 H), 4.68 (br s, 2H), 7.20 (m, 2 H),
7.30 (m, 2
H), 8.2 (s, 1 H), 8.4 (s, 1 H), 9.28 (s, 1 H) 10.4 (br s, 1 H);
Anal. calcd for C15H~3N30S2-0.25.H20: C, 57.12; H, 4.15; N, 13.32. Found: C,
56.49;
H, 4.19 N, 12.29.
Example 39
N2-4-f (4-methylnhenyl)thiolthienof2,3-clpyridin-2-yllcarbonyll-N6-
[(nitroamino)iminomethlysine, methyl ester
89
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
N-c~nitroarginine methyl ester hydrochloride and NaHC03 were processed as in
Example 37. The residue was purified by flash chromotograpy on silica gel with
5%
methanol/dichloromethane to provide the title compound.
mp 84-87 °C;
MS (DCI/NH3) m/z 517 (M+H)+;
tH NMR (300 MHz, DMSO-d6) 8 1.60 (m, 2H), 1.85 (m, 2H), 2.29 (s, 3 H), 3.20,
(m,
2H), 3.68, (s, 3H), 4.35, (t, IH), 4.48, (m, IH, 7.20 (m, 2 H), 7.30 (m, 2 H),
8.32 (s, 1 H),
8.48 (s, 1 H), 8.52, (br s, IH), 9.30 (s, I H), 9.42 (d, 1H) ;
Anal. calcd for C22H24N605S2~0~25 H20: C, 51.15; H, 4.68; N, 16.27. Found: C,
50.95;
I O H,4.89; N,15.73.
Example 40
N-(aminoiminomethyl)-4-j(4-methyluhenyl)thiolthienoj2 3-clpyridine-2-
carboxamide
A solution of guanidine hydrochloride (0.095 g, 1 mmol) in methanol was
treated
IS with potassium t-butoxide (0.112 g, 1 mmol), stirred at room temperature
for 30 minutes,
treated with Example 17 (0.1 g, 0.3 mmol), warmed to room temperature for 16
hours and
concentrated. The concentrate was dissolved in ethyl acetate ( 100 mL), washed
with
water, dried (MgS04), filtered, and concentrated. The residue was purified by
flash
chromotograpy on silica gel with 6% methanol/dichloromethane to provide the
title
20 compound.
mp 202-205 °C;
MS (DCI/NH3) m/z 343 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 2.29 (s, 3H), 6.90 (br s, 2H), 7.20 (m, 4H), 7.80
(S,
1H), 8.00 (br s, 2H), 8.20 (s, IH), 8.40 (s, 1H), 9.24 (s, IH).
Example 41
4-j(4-methylnhenyl)thiolthienof 2,3-clpyridine-2-carbothioamide
A solution of Example 19 (190 mg, 0.63 mmol) and Lawsesson's reagent (383 mg,
9.48 mmol) in toluene ( 15 mL) was heated to room temperature for 5 hours and
concentrated. The residue purified by flash chromotograpy on silica gel with
4%
methanol/dichloromethane to provide the title compound.
mp 18I-183 °C;
MS (DCI/NH3) m/z 317 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.29 (s, 3H), 7.20 (m, 2H), 7.30 (m, 2H), 8.18 (br
s,
1H), 8.32 (s, IH), 9.2 (s, IH) 10.I (br s, IH) 10.2 (br s, 1H);
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Anal. calcd for Ct5H12N2S3' 0.25 H20: C, 56.93; H, 3.82; N, 8.85. Found: C,
55.89;
H,3.83 N, 8.48.
Example 42
4-f(4-methylphenyl)thiolthienof2 3-clpyridine
Boiling Dowtherm A (2 mL) was treated sequentially with Example 18 (0.6 g,
1.99
mmol) and copper powder (0.3 g), stirred for 5 minutes, cooled, diluted with
hexanes, and
purified by flash chromatography on silica gel with 15% ethyl acetate/hexanes.
The
product was then recrystallized from hexanes to provide the title compound.
mp 94-95 °C;
MS (DCI/NH3) m/z 258 (M+H)+;
1H NMR (300 MHz, DMSO-db) 8 2.27 (s, 3H), 7.16 (m, 2H), 7.23 (m, 2H), 7.44 (d,
1H),
8.20 (d, 1H), 8.40 (s, 1H), 9.27 (s, 1H);
Anal. calcd for C~4H11NS2: C, 65.33; H, 4.30; N, 5.44. Found: C, 65.44; H,
4.20, N,
5.26.
Example 43
methyl 4-f(2-methoxy-2-oxoethyl)thiolthienof2 3-c~pyridine-2-carboxylate
Example 93A was processed as in examples 17B and 17C, but substituting methyl
thioglycolate for p-thiocresol in Example 17B to provide the title compound.
MS (DCI/NH3) m/z 298 (M+H)+;
1H NMR (500 MHz, DMSO-d6) S 3.59 (s, 3H), 3.94 (s, 3H), 4.04 (s, 2H), 8.14 (s,
1H),
8.55 (s, 1H), 9.27 (s, 1H).
Example 44
4-((2-amino-2-oxoethvl)thiolthienof2 3-cipyridine-2-carboxamide
Example 43 was dissolved in 2M methanolic ammonia and warmed to 45
°C in a
sealed tube for 18 hours. The precipitate was filtered, washed with methanol-
diethyl ether
(1:1) and dried under vacuum to provide the title compound.
MS (APCI) m/z 268 (M+H)+;
~H NMR (400 MHz, DMSO-d6) $ 3.81 (s, 2H), 7.17 (br s, 1H), 7.59 (br s, 1H),
7.82 (br s,
1H), 8.29 (br s, 1H), 8.46 (s, 1H), 8.52 (br s, 1H), 9.14 (s, 1H).
Example 45
4-f(4-bromophenyl)thiolthienof2 3-clpyridine-2-carboxamide
91
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 17A was processed as in examples 17B and 17C, and 44, but substituting
4-bromothiophenol for p-thiocresol in Example 17B to provide the title
compound.
MS (DCI/NH3) m/z 365 (M+H}+;
1H NMR (300 MHz, DMSO-d6) b 7.20 (dt, 2H), 7.53 (dt, 2H), 7.87 (br s, 1H),
8.21 (s,
1H), 8.51 (br s, 1H), 8.54 (s, 1H), 9.36 (s, 1H);
Anal. calcd for C~4H9BrN20S2: C, 46.04; H, 2.48; N, 7.67. Found: C,45.86;
H,2.30; N,
7.51.
Example 46
4-(phenylthio)thienof 2,3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B, 17C, and 44 but substituting
thiophenol for p-thiocresol in Example 17B to provide the title compound.
MS (DC1/NH3) m/z 287 (M+H)+;
iH NMR (300 MHz, DMSO-d6) 8 7.29-7.40 (m, SH), 7.86 (br s, 1H), 8.25 (s, 1H),
8.46 (s,
IS 1H), 8.52 (br s, 1H), 9.31 (s, 1H);
Anal. calcd for Cl4HtoN20S2: C, 58.72; H, 3.52; N, 9.28. Found: C, 58.62; H,
3.42; N,
9.48.
Example 47
4-j f 4-(trifluoromethyl)phenyllthiolthieno f 2,3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B, 17C, and 44 but substituting
a,a,a-trifluorothiocresol for p-thiocresol in Example 17B to provide the title
compound.
MS (DCI/NH3) m/z 355 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 7.31 (d, 2H), 7.65 (d, 2H), 7.85 (br s, 1H), 8.19
(s, 1H),
8.50 (br s, 1H), 8.68 (s, 1H), 9.44 (s 1H);
Anal. calcd for CISH9F3N20S2: C,50.84; H, 2.56; N, 7.91. Found: C, 50.63; H,
2.44; N,
7.82.
Example 48
4-f (2-methylphenyl)thiolthienof 2,3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B and 17C, and 44, but substituting
2-methylthiophenol for p-thiocresol in Example 17B. The residue was purified
by column
chromatography, eluting with 5°lo methanol in dichloromethane to
provide the title
compound.
mp 170-172 °C;
MS (DCI/NH3) m/z 301 (M+H)+;
92
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
1H NMR (300 MHz, DMSO-d6) 8 2.41 (s, 3H}, 7.04 {dd, 1H), 7.15 (dt, 1H), 7.27
(dt, 1H),
7.38 (br d, 1H), 7.86 (br s, 1H}, 8.20 (s, 1H}, 8.23 (s, 1H), 8.53 (br s, 1H),
9.28 (s, 1H);
Anal. calcd for C~SH12N20S2: C, 59.97; H, 4.03; N, 9.33. Found: C, 59.86; H,
4.16; N,
9.11.
Example 49
4- f (3-methylnhenyl)thiolthienof 2,3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B and 17C, and 44, but substituting
3-methylthiophenol for p-thiocresol in Example 17B. The residue was purified
by flash
chromatography with 5% methanol/dichloromethane to provide the title compound.
mp 171-173 °C;
MS (DCI/NH3) m/z 301 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 2.27 (s, 3H), 7.06-713 {m, 2H), 7.21-7.27 (m, 2H),
7.89
{br s, 1H), 8.26 (s, 1H), 8.42 (s, 1H), 8.55 (br s, 1H), 9.30 (s, 1H);
Anal. calcd for C~SH12N20S2~0.25H20: C, 59.08; H, 4.13; N, 9.19. Found: C,
59.10; H,
4.16; N, 9.11.
Example 50
4-f (3,4-dimethylnhenyl)thiolthienof2,3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B and 17C, and 44, but substituting
3,4-dimethylthiophenol for p-thiocresol in Example 17B. The residue was
purified by
flash chromatography with 5% methanol/dichloromethane to provide the title
compound.
mp 192-194 °C;
MS (APCI) m/z 315 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 2.09 (s, 3H), 2.11 (s, 3H), 7.05 (m, 2H), 7.19 {s,
1H)
7.81 (br s, 1 H), 8.12 (d, 2H), 8.49 (br s, 1 H), 9.15 (s, 1 H);
Anal. calcd for C~6H14N20S2~0.25H20: C, 60.25; H, 4.58; N, 8.78. Found: C,
60.34; H,
4.52; N, 8.75.
Example 51
4-f(3,5-dimethylnhenyl)thiolthienof2 3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B and 17C, and 44, but substituting
3,5-dimethylthiophenol for p-thiocresol in Example 17B. The residue was
purified by
flash chromatography with 5% methanol /dichloromethane to provide the title
compound.
mp 177-179 °C;
MS (DCI/NH3) m/z 315 (M+H)+;
93
CA 02333770 2000-11-30
WO 99/62908 PCT/IJS99/12419
1H NMR (300 MHz, DMSO-d6) 8 2.13 (s, 6H), 6.83 (s, IH), 6.92 (s, IH), 7.81 (br
s, 1H),
8.21 (s, 1H), 8.30 (s, 1H), 8.50 (br s, 1H), 9.19 (s, 1H);
Anal. calcd for C~6H14N20S2: C, 61.12; H, 4.49; N, 8.91. Found: C, 60.82; H,
4.48; N,
8.75.
Example 52
4-j(2,4-dimethylphenyl)thiolthienof 2,3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B and I7C, and 44, but substituting
2,4-dimethylthiophenol for p-thiocresol in Example 17B. The residue was
purified by
flash chromatography with 5% methanol/dichloromethane to provide the title
compound.
mp I93-195 °C;
MS (APCI) m/z 315 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.28 (s, 3H), 2.38 (s, 3H), 7.02 (d, 1H), 7.13 (d,
1H),
7.20 (s, 1H), 7.91 (br s, 1H), 8.05 (s, 1H), 8.58 (br s, 1H), 9.22 (s, 1H);
Anal. calcd for C~6H14N20S2~0.25H20: C, 60.25; H, 4.58; N, 8.78. Found: C,
60.40; H,
4.52; N, 8.72.
Example 53
4-j(2-methyl-3-furanyl)thiolthieno j2,3-clpyridine-2-carboxamide
Example 17A was processed as in examples I7B and 17C, and 44, but substituting
2-methyl-3-furanthiol for p-thiocresol in Example 17B. The residue was
purified by flash
chromatography with 5% methanol/dichloromethane to provide the title compound.
mp 236-239 °C (decomposes);
MS (ESI) m/z 291 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.41 (s, 3H), 6.68 (d, 1H), 7.74 (d, 1H), 7.93 (br
s, 1H),
8.19 (s, IH), 8.38 (s, 1H), 8.60 (br s, 1H), 9.15 (s, 1H);
Anal. calcd for C13H1oN202S2~0.25H20: C, 52.95; H, 3.59; N, 9.50. Found: C,
52.57; H,
3.41; N, 9.30.
Example 54
4-ff(4-chlorophenyl)methyllthiolthienoj2 3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B and I7C, and 44, but substituting
4-chlorobenzylmercaptan for p-thiocresol in Example I7B. The residue was
purified by
flash chromatography with 5% methanol/dichloromethane to provide the title
compound.
mp 198-199 °C;
MS (APCI) m/z 335 (M+H)+;
94
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, DMSO-d6) b 4.40 (s, 2H), 7.31 (s, 4H), 7.86 (br s, 1H), 8.26
(s, 1H),
8.41 (s, 1H), 8.52 (br s, 1H), 9.15 (s, 1H);
Anal. calcd for CigH11C1N20S2: C, 53.80; H, 3.31; N, 8.37. Found: C, 53.52; H,
3.18;
N, 8.31.
Example 55
4- f (3,4-dichlorophenyl)thiolthieno f 2,3-clpyridine-2-carboxamide
Example 17A was processed as in examples I7B and 17C, and 44, but substituting
3,4-dichlorothiophenol for p-thiocresol in Example 17B. The residue was
purified by
flash chromatography with 5% methanol/dichloromethane to provide the title
compound.
MS (ESI) m/z 355 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.10 (dd, 1H), 7.55 (d, 1H), 7.59 (d, 1H), 7.91
(br s,
1H), 8.21 (s, IH), 8.53 (br s, IH), 8.62 (s, 1H), 9.41 (s, IH);
Anal. calcd for Cl4HgC12N20S2: C, 47.33; H, 2.27; N, 7.89. Found: C, 47.34; H,
2.52;
N, 8.05.
Example 56
4-f(4-methoxynhenyl)thiolthieno~2,3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B and 17C, and 44, but substituting
4-methoxythiophenol for p-thiocresol in Example 17B. The residue was purified
by flash
chromatography with 5% methanol/dichloromethane to provide the title compound.
mp 219-22I °C;
MS (ESI) m/z 317 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 3.76 (s, 3H), 6.99 (d, 2H), 7.46 (d, 2H), 7.89 (br
s, IH),
8.17 (s, 1H), 8.30 (s, 1H), 8.54 (br s, 1H), 9.18 (s, 1H);
Anal. calcd for C15H12N202S2~ C, 56.94; H, 3.82; N, 8.85. Found: C, 56.80; H,
3.78; N,
8.79.
Example 57
4-(cyclohexylthio)thieno f 2,3-clpyridine-2-carboxamide
Example 17A was processed as in examples 17B and 17C, and 44, but substituting
cyclohexylmercaptan for p-thiocresol in Example 17B. The residue was purified
by flash
chromatography with 5% methanol/dichloromethane to provide the title compound.
mp 205-207 °C;
MS (ESI) m/z 293 (M+H)+;
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, DMSO-d6) 8 1.14-1.43 (br m, 6H), 1.51-1.61 (br m, 1H), 1.66-
1.78
(br m, 2H), 1.83-1.98 (br m, 2H), 7.90 (br s, 1H), 8.33 (s, 1H), 8.52 (s, 1H),
8.57 (br s,
1H), 9.22 (s, 1H);
Anal. calcd for C~4H16N20S2: C, 57.50; H, 5.51; N, 9.58. Found: C, 57.53; H,
5.39; N,
9.51.
Example 58
4-f(4-methylnhenyl)thiol-N-f3-(4-morpholinyl)propyllthienof2 3-clpyridine-2
carboxamide.trifluoromethylacetate (salt)
IO Example 17C (200 mg, 0.635 mmol) in 9:1 4-(3-aminopropyl)morpholine/acetic
acid (2 mL) was warmed at 70 °C for 4 hours, diluted with acetonitrile
(6 mL), and
purified by C-18 reverse phase HPLC with a gradient of 20% acetonitrile/water
to 100%
CH3CN containing 0.1 % trifluoroacetic acid to provide the title compound.
MS (APCI) m/z 428 (M+H)+;
1H NMR (400 MHz, DMSO-d6) 8 1.95 (m, 2H), 3.08 (m, 2H), 3.18 (m, 2H), 3.36 (m,
2H), 3.43 (m, 2H), 3.68 (m, 4H), 7.20 (d, 2H), 7.28 (d, 2H), 8.0 (br s, 1H),
8.27 (s, 1H),
8.34 (m, 1H), 9.27 (m, 1H).
Example 59
4-f (4-methylphenyl)sulfinyllthienof2,3-clpyridine-2-carboxamide
Example 59A
Methyl 4-f (4-methylnhenyl)sulfinyllthienof 2,3-clpyridine-2-carboxylate
A solution of Example 17C ( 144 mg, 0.46 mmol) in dichloromethane ( 10 mL) at
0
°C was treated with 3-chloroperoxybenzoic acid (57-86%, 82 mg), warmed
to room
temperature over 4 hours, treated with dichloromethane (50mL), washed
sequentially with
1N NaOH, water, and brine, dried (MgS04), filtered, and concentrated. The
residue was
purified by flash chromatography on silica gel with 50% ethyl acetate/hexane
to provide
the title compound.
MS (DCI/NH3) m/z 332 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.25 (s, 3H), 3.84 (s, 3H), 7.38 (d, 2H), 7.65 (d,
2H),
8.41 (s, 1H), 9.0 (s, 1H), 9.58 (s, 1H).
Example 59B
4-f(4-methylphen 1)y sulfin,yllthienof2 3-c_]pyridine-2-carboxamide
Example 59A was processed as in Example 44 to provide the title compound.
96
CA 02333770 2000-11-30
WO 99!62908 PCT/US99/12419
MS (DC1/NH3) m/z 317 (M+H)+;
tH NMR (300 MHz, DMSO-d6) b 2.31 (s, 3H), 7.38 (d, 2H), 7.79 (d, 2H), 7.94 (br
s, 1H),
8.43 (s, 1H), 8.62 (br s, 1H), 8.85 (s, 1H), 9.43 (s, 1H).
Example 60
4-(4-methylnhenoxy)thienof2,3-clpyridine-2-carboxamide
Example 60A
methyl 4-(4-methylnhenoxy)thienof2,3-clpyridine-2-carboxylate
Example 17A was processed as in examples 17B and 17C, but substituting p-
cresol
for p-thiocresol in Example 17B to provide the title compound.
mp 96-98 °C;
MS (DCI/NH3) m/z 317 (M+NH4)+, 300 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.32 (s, 3 H), 3.91 (s, 3 H), 7.05 (m, 2 H), 7.24
(m, 2
H), 7.95 (s, 1 H), 8.12 (s, 1 H), 9.17 (s, 1 H);
Anal. calcd for C16H13N03S: C, 64.19; H, 4.37; N, 4.67. Found: C, 64.05; H,
4.34 N,
4.52.
Example 60B
4-(4-methylphenoxy)thienof2,3-clpyridine-2-carboxamide
Example 60A was processed as in examples 18 and 19 to provide the title
compound.
mp 196-197 °C;
MS (DCI/NH3) m/z 285 (M+H)+, 302 (M+NH4)+;
1H NMR (300 MHz, DMSO-d6) S 2.3I (s, 3 H), 7.04 (m, 2 H), 7.25 (m, 2 H), 7.82
(br s, 1
H), 8.00 (s, 1 H), 8.21 (s, 1 H), 8.42 (br s, 1 H) 9.07 (s, 1 H);
Anal. calcd for Ci5H12N2O2S. C, 63.36; H, 4.25; N, 9.85. Found: C, 63.29; H,
4.28 N,
9.68.
Example 61
4-(4-chlorophenoxy)thienof 2,3-clpyridine-2-carboxamide
Example 61 A
methyl 4-(4-chlorophenoxy)thienof2 3-clpyridine-2-carboxylate
A solution of 4-chlorophenol (2.63 g, 20.5 mmol) in THF (20 mL) at 0
°C was
treated dropwise with a solution of potassium tert-butoxide (1.0 M solution in
THF, 20.4
97
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
mL, 20.5 mmol), stirred at 25 °C for 1 hour, cooled to 0 °C,
treated with a solution of
Example 17A (3.54 g, 20.23 mmol) in THF (40 mL), warmed at 60 °C for
0.5 hours,
cooled to 0 °C, treated with methylthioglycolate ( 1.989 mL, 22.25
mmol) and Cs2C03
(6.59 g, 20.23 mmol), warmed at 60 °C for 0.25 hours, cooled to room
temperature and
filtered. The filtrate was diluted with ethyl acetate, washed sequentially
with water and
brine, dried (MgS04), filtered, and concentrated. Purification of the residue
by flash
chromatography on silica gel with 4% acetone/hexane provided the title
compound.
mp 99-100 °C;
MS (APCI) m/z 320 (M+H)+;
iH NMR (300 MHz, DMSO-d6) b 3.91 (s, 3H, OCH3), 7.14 (d, 2H), 7.48 (d, 2H),
7.95 (s,
1H), 8.23 (s,lH), 9.23 (s, 1H);
13C NMR (100 MHz, DMSO-d6) 8 56.45 (OCH3), 120.19 (CH), 123.06 (Ar-CH), 128.04
(Ar-CH), 131.34 (C), 132.37 (Ar-CH), 133.38 (Ar-CH), 136.40 (Ar-CH), 139.38
(C),
141.75 (C), 142.09 (C), 144.89 (Ar-CH), 150.91 (C), 158.64 (C), 164.95 (CO);
Anal. calcd for C15H1oC1N03S: C, 56.34; H, 3.15; N, 4.38. Found: C, 56.23; H,
3.16; N,
4.38.
Example 61B
4-(4-chlorophenoxy)thienof 2,3-clpyridine-2-carboxamide
Example 61 A was processed as in Example 44 to provide the title compound.
mp 176-177 °C;
MS (DCI/NH3) m/z 305 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.15 (m, 2H), 7.50 (m, 2H), 7.95 (b, 1H), 8.25 (d,
2H),
8.45 (b, 1H), 9.15 (s, 1H);
Anal. calcd for C~4H9C1N202S~0.25H20: C, 54.37; H, 3.10; N, 9.06. Found: C,
54.44;
H, 2.74; N, 9.06.
Example 62
4-14-(trifluoromethyl)phenoxylthieno12,3-clpyridine-2-carboxamide
Example 17A was processed as in Example 61 but substituting
4-(trifluoromethyl)phenol for 4-chlorophenol to provide the title compound.
MS (DCI/NH3) m/z 339 (M+H)+;
IH NMR (300 MHz, DMSO-d6) b 7.24 (d, 2H), 7.77 (d, 2H), 7.88 (br s, 1H), 8.10
(s, 1H),
8.33 (s, 1H), 8.45 (br s, 1H), 9.24 (s, 1H);
Anal. calcd for ClgH9F3N202S: C,53.26; H, 2.68; N, 8.28. Found: C, 53.06; H,
2.55; N,
8.19.
98
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 63
4-(4-octylphenoxy)thienof2 3-clpyridine-2-carboxamide
Example 17A and 4-octylphenol were processed as in Example 61 to provide the
title compound.
MS (DCI/NH3) m/z 383 (M+H)+;
1H NMR (300 MHz, CDC13) 8 0.88 (t, 3H), 1.22-1.38 (m, lOH), 1.62 (m, 2H), 2.61
(t,
2H), 6.05 (br s, 2H), 6.99 (d, 2H), 7.20 (d, 2H), 7.87 (s, 1 H), 8.07 (br s, 1
H), 8.92 (br s,
1 H);
Anal. calcd for C22H26N202S: C, 69.08; H, 6.85; N, 7.32. Found: C, 69.04; H,
6.82; N,
7.22.
Example 64
4-f4-(1-methylethyl)nhenoxylthieno~2 3-clpyridine-2-carboxamide
Example 17A and 4-(1-methylethyl)phenol were processed as in Example 61 to
provide the title compound.
MS (DCI/NH3} m/z 313 (M+H)+;
~H NMR (300 MHz, DMSO-d6) b 1.21 (d, 6H), 2.92 (septet, 1H), 7.05 (d, 2H),
7.30 (d,
2H), 7.82 (br s, 1H), 8.03 (s, 1H), 8.21 (s, 1H), 8.44 (br s, 1Fl), 9.09 (s,
1H).
Example 65
4-(2-bromo-4-chlorophenoxy)thienol2 3-clpyridine-2-carboxamide
Example 17A and 2-bromo-4-chlorophenol were processed as in Example 61 to
provide the title compound.
MS (DCI/NH3) m/z 383 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 7.18 (d, 1H), 7.49 (dd, 1H), 7.90 (br s, 1H), 7.98
(s,
2H}, 8.23 (s, 1 H), 8.49 (br s, 1 H), 9.14 (s, 1 H);
Anal. calcd for Cl4HgBrC1N202S: C, 43.83; H, 2.10; N, 7.30. Found: C, 43.53;
H, 1.97;
N, 6.99.
Example 66
4-(4-ethylnhenoxy)thienof2 3-clpyridine-2-carboxamide
Example 17A and 4-ethylphenol were processed as in Example 61 to provide the
title compound.
MS (DCI/NH3) m/z 299 (M+H)+;
99
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, DMSO-d6) b 1.19 (t, 3H), 2.62 (q, 2H), 7.05 (dt, 2H), 7.26
(dt, 2H),
7.81 (br s, 1H), 8.07 (s, 1H), 8.21 (s, 1H), 8.43 (br s, 1H), 9.08 (s, 1H);
Anal. calcd for C16H14N202S~CH30H: C, 63.71; H, 4.69; N, 9.14. Found: C,
63.34; H,
4.51; N, 9.51.
Example 67
4-(4-ethenylnhenoxy)thieno f 2,3-clpyridine-2-carboxamide
Example 67A
4-Vinylphenol
A solution of 4-vinylphenol in propylene glycol was treated with water and
extracted with diethyl ether in order to remove the propylene glycol and
provide the
designated compound in diethyl ether.
Example 67B
4-(4-ethenylphenoxy)thienof2,3-clpyridine-2-carboxamide
Example 17A and Example 67A were processed as in Example 61 to provide the
title compound.
MS (DCI/NH3) m/z 297 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 5.24 (d, IH), 5.79 (d, 1H), 6.75 (dd, 1H), 7.10
(d, 2H),
7.54 (d, 2H), 7.87 (br s, 1H), 8.12 (s, IH), 8.18 (s, 1H), 8.45 (br s, IH),
9.13 (s, IH);
Anal. calcd for C16H12N202S~0.25CH30H: C, 64.13; H, 4.06; N, 9.20. Found: C,
64.40;
H, 4.12; N, 9.27.
Example 68
4-f4-(1,2-dihydroxyethyl)nhenoxylthienof2 3-clpyridine-2-carboxamide
A solution of Example 67B (35 mg, 0.118 mmol) in pyridine (5 mL) was treated
with Os04 (90 mg, 0.354 mmol), stirred for 5 hours, treated with 10% aqueous
NaHS03,
stirred for 5 hours, treated with brine, and extracted with ethyl acetate. The
extract was
dried (MgS04), filtered, and concentrated. The residue was purified by flash
chromatography on silica gel with 1:10 methanol/dichloromethane to provide the
title
compound.
MS (DCI/NH3) m/z 331 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 3.44 (t, 2H), 4.55 (q, 1H}, 4.73 (t, 1H), 5.27 (d,
IH),
7.08 (d, 2H), 7.39 (d, 2H), 7.85 (br s, 1H), 8.03 (s, IH), 8.21 (s, 1H), 8.47
(br s, 1H), 9.10
(s, 1H);
100
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/124I9
Anal. calcd for C~6H~4N204S~0.25CH30H: C, 57.68; H, 4.24; N, 8.28. Found: C,
57.92;
H, 4.35; N, 8.24.
Example 69
4-(2-(2-propenyl)phenoxylthienof2 3-clpyridine-2-carboxamide
Example 17A and 2-allylphenol were processed as in Example 61 to provide the
title compound.
MS (DCI/NH3) m/z 311 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 3.43 (d, 2H), 5.01 (m, 1H), 5.05 (m, 1H), 5.98 (m,
IH),
IO 7.00 (dd, 1H), 7.27 (m, 2H), 7.39 (dd, 1H), 7.82 (s, 1H), 7.88 (br s, 1H),
8.27 (s, 1H), 8.49
(br s, 1H), 9.05 (s, IH);
Anal. calcd for C~~H14N202S: C, 65.79; H, 4.55; N, 9.03. Found: C, 65.53; H,
4.37; N,
8.95.
Example 70
4-[2-(2,3-dihydroxypropyl)phenoxylthienof2 3-clpyridine-2-carboxamide
Example 69 was processed as in Example 68 to provide the title compound.
MS (DCI/NH3) m/z 345 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.60 (dd, 1H), 2.88 (dd, 1H), 3.29 (t, 2H), 3.76
(m, 1H),
4.55 (t, 1 H), 4.63 (d, 1 H), 6.94 (dd, 1 H), 7.22 (m, 2H), 7.45 (dd, 1 H),
7.84 (s, 1 H), 7.88
(br s, IH), 8.26 (s, I H), 8.46 (br s, 1 H}, 9.04 (s, 1 H);
Anal. calcd for C~~H~6N204S: C, 59.29; H, 4.68; N, 8.13. Found: C, 59.16; H,
4.51; N,
8.06.
Example 71
4-f4-(trifluoromethyl)phenoxylthieno(2 3-clpyridine-2-carboxamide 1-oxide
A solution of Example 62 (26 mg, 0.077 mmol) in ( 1 mL) and dichloromethane (5
mL), at 0 °C was treated with m-CPBA (80-85%, 30 mg, 0.14 mmol),
stirred at 0 °C for 1
hour, and at room temperature for 10 hours. The precipitate that formed was
collected by
filtration and washed with dichloromethane. HPLC analysis of the material (C-
18, reverse
phase) showed a mixture of desired sulfoxide and starting thiephene in a 8:1
ratio. The
mixture was recrystallized from DMF/methanol/dichloromethane to provide the
title
compound (97.5% pure by HPLC analysis).
MS (HPCI/NH3) m/z 355 (M+H)+;
iH NMR (300 MHz, DMSO-d6) 8 7.39 (d, 2H), 7.79 (br s, 1H), 7.81 (d, 2H), 8.02
(s, 1H},
8.05 (d, 1H), 8.36 (br s, 1H), 9.02 (s, 1H);
101
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Anal. calcd for CIgH9F3N03S~0.25CH30H: C,50.55; H, 2.57; N, 7.73. Found: C,
50.55;
H, 2.59; N, 7.69.
Example 72
4-f3-(pentadecyl)phenoxylthienof2 3-clpyridine-2-carboxamide
Example 17A and 3-pentadecylphenol were processed as in Example 61 to provide
the title compound.
MS (DCI/NH3) m/z 481 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 0.84 (t, 3H), 1.20-1.28 (m, 24H), 1.54 (m, 2H),
2.57 (t,
2H), 6.92 (m, 1H), 6.97 (t, 1H), 7.03 (d, 1H), 7.33 (t, 1H), 7.85 (br s, 1H),
8.03 (s, 1H),
8.23 (s, 1 H), 8.44 (br s, 1 H), 9.09 (s, 1 H);
Anal. calcd for C29H4oN202S: C, 72.46; H, 8.39; N, 5.83. Found: C, 72.69; H,
8.18; N,
5.47.
Example 73 Methyl 4-(4-Bromophenoxy)thienof2 3-clpyridine-2 carboxylate
To a solution of 4-bromophenol (4.94 g., 28.55 mmol) in anhydrous
tetrahydrofuran (10 mL) under nitrogen atmosphere was added dropwise a
solution of
potassium t-butoxide (1 M solution in THF, 28.6 mL, 28.6 mmol). The reaction
mixture
was stirred at ambient temperature for 30 minutes, then a solution of Example
17A (2 g,
11.4 mmol) in anhydrous tetrahydrofuran (20 mL) was added and refluxed for 8
hours.
The reaction mixture was allowed to cool to 25 °C, methyl thioglycolate
( 1.23 mL, 13.7
mmol) was added and refluxed for 15 minutes. The cooled reaction mixture was
diluted
with ethyl acetate (300 mL) and partitioned with an ice cold solution of 1 N
NaOH (3x75
mL). The organic layer was washed with brine (3x100 mL), dried (MgS04) and
solvents
were removed under reduced pressure to obtain the crude product (4.2 g). This
was
purified by flash chromatography on silica gel eluting with 10% acetone-hexane
to obtain
the title compound ( 1.81 g) in 44% yield.
'H NMR (300 MHz, DMSO-d6) 8 3.91 (s, 3H), 7.10 (d, J=9Hz, 2H), 7.59 (d, J=9Hz,
2H),
7.94 (s, 1 H}, 8.25 (s, 1 H), 9.24 (s, 1 H);
MS (APCI) m/e 364;366 (M+H)'.
Example 74
4-(3-chloronhenoxy)thienof2 3-clpyridine-2-carboxamide
Example 17A and 3-chlorophenol were processed as in Example 61 to provide the
title compound.
MS (DCI/NH3) m/z 305 (M+H)+;
102
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, DMSO-d6) 8 7.10 (m, 1H), 7.30 (m, 2H), 7.45 (b, 1H), 7.95 (b,
1H),
8.20 (d, 1H), 8.30 (s, 1H), 8.6 (b, 1H), 9.30 (s, 1H).
Example 75
4-(4-t-butylphenoxy)thienol2,3-clpyridine-2-carboxamide
Example 17A and 4-tent-butylphenol were processed as in Example 61 to provide
the title compound.
MS (DCI-NH3) m/z 327 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 1.3 (s, 9H), 7.10 (d, 2H), 7.45 (d, 2H), 7.85 (br
s, 1H),
8.05 (s, 1H), 8.20 (s, 1H), 8.45 (br s, 1H), 9.1 (s, 1H).
Example 76
4-(4-chloro-3-methylphenoxy)thienof2 3-clpyridine-2-carboxamide
Example 17A and 4-chloro-3-methylphenol were processed as in Example 61 to
provide the title compound.
MS (DCI/NH3) m/z 319 (M+H}+;
1H NMR (300 MHz, DMSO-d6) 8 2.30 (s, 3H), 6.95 (dd, 1H), 7.20 (d, 1H), 7.45
(d, 1H),
7.85 (br s, 1H), 8.15 (s, 1H), 8.19 (s, 1H), 8.45 (br s, 1H), 9.15 (s, 1H).
Example 77
4-(4-chloro-2-methylphenoxy)thieno(2 3-cl!pyridine-2-carboxamide
Example 17A and 4-chloro-2-methylphenol were processed as in Example 61 to
provide the title compound.
MS (DCI/NH3) m/z 319 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 2.30 (s, 3H), 6.95 (dd, 1H), 7.30 (d, 1H), 7.50
(d, 1H),
7.85 (br s, 1H), 7.95 (s, 1H), 8.25 (s, 1H), 8.45 (br s, 1H), 9.15 (s, 1H).
Example 78
4-(4-methoxyphenoxy)thienof2 3-clpyridine-2-carboxamide
Example 17A and 4-methoxyphenol were processed as in Example 61 to provide
the title compound.
MS (DCI/NH3) m/z 30I (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 3.78 (s, 3H), 7.00 (dd, 2H), 7.15 (d, 2H), 7.85
(b, 1H),
7.90 (s, 1H), 8.30 (s, 1H), 8.45 (b, 1H), 9.05 (s, 1H).
Example 79
103
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
ethyl3-ff2-(aminocarbonyl)thienof2,3-clp riy din-4- lloxyibenzoate
Example 17A and ethyl 3-hydroxybenzoate were processed as in Example 61 to
provide the title compound.
MS (DCI/NH3) m/z 343 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 1.30 (t, 3 H), 4.30 (s, 3 H), 7.40 (dd, 1 H), 7.60
(m, 2
H), 7.80 (dd, 1H), 7.85 (b,lH), 8.15 (s, 1 H), 8.20 (s, 1 H), 8.42 (b,lH),
9.17 (s,lH).
Example 80
4-phenoxythienof 2,3-clpyridine-2-carboxamide
Example 17A and phenol were processed as in Example 61 to provide the title
compound.
MS (DCI/NH3) m/z 271 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.15 (dd, 2H), 7.20 (t, 1H), 7.45 (t, 2H), 7.85
(b, 1H),
8.10 (s, 1 H), 8.20 (s, 1 H), 8.45 (b, 1 H), 9.15 (s, 1 H).
Example 81
4-(3-bromophenoxy)thienof2 3-clpyridine-2-carboxamide
Example 17A and 3-bromophenol were processed as in Example 61 to provide the
title compound.
MS (DCI/NH3) m/z 349, 351 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.07 (dt, 2 Hz, 1H), 7.36-?.39 (m, 3H), 7.87 (br
s, 1H),
8.15 (s, 1H), 8.20 (s, 1H), 8.45 (br s, 1H), 9.17 (s, 1H);
Anal calcd for C14H9N202S'CH30H: C, 47.26; H, 2.64; N, 7.35. Found: C, 47.26;
H,
3.21; N, 7.29.
Example 82
4-(4-fluorophenoxy)thienof2 3-clpyridine-2-carboxamide
Example 17A and 4-fluorophenol were processed as in Example 61 to provide the
title compound.
MS (DCI/NH3) m/z 289 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.25 (m, 4 H), 7.85 (b, 1H), 8.05 (s, 1H), 8.20
(s, 1 H),
8.42 (b, 1H), 9.10 (s, 1H).
Example 83
4-(3,5-dimethylphenoxy)thienof2 3-clpyridine-2-carboxamide
104
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 17A and 3,5-dimethylphenol were processed as in Example 61 to provide
the title compound.
MS (DCI/NH3) m/z 299 (M+H)+;
IH NMR (300 MHz, DMSO-d6) 8 3.30 (s, 6H), 6.75 (s, 2H), 6.85 (s, 1H), 7.80 (b,
1H),
8.05 (s, 1 H), 8.18 (s, 1 H), 8.45 (b, 1 H), 9.10 (s, l H).
Example 84
4-(3-chloro-4-methylphenoxy)thienof2 3-clpyridine-2-carboxamide
Example 17A and 3-chloro-4-methylphenol were processed as in Example 61 to
provide the title compound.
MS (DCI-NH3) m/z 3I9 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 2.35 (s, 3H), 7.00 (dd, 1H), 7.25 (d, 1H), 7.45
(d, 1H),
7.85 (b, 1H), 8.15 (s, 1H), 8.20 (s, 1H), 8.45 (b, 1H), 9.15 (s, 1H).
Example 85
4-(4-iodophenoxy)thienof2 3-c~[pyridine-2-carboxamide
Example 17A and 4-iodophenol were processed as in Example 61 to provide the
title compound.
MS (DCI/NH3) m/z 397 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 6.94 (d, 2H), 7.74 (d, 2H), 7.86 (br s, 1H), 8.i3
(s, 1H),
8.17 (s, 1H), 8.44 (br s, 1H), 9.16 (s, 1H);
Anal calcd for C~4H9IN202S: C, 42.44; H, 2.29; N, 7.07. Found: C, 42.58; H,
2.27; N,
7.08.
Example 86
4-(4-(methoxymethyl)phenoxy)thieno f 2 3-clpyridine-2-carboxamide
Example 17A and 4-(methoxymethyl)phenol were processed as in Example 61 to
provide the title compound.
mp 168-168.5 °C
MS (DCI/NH3) m/z 315 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 3.30 (s, 3H), 4.41 (s, 2H), 7.10 (d, 2H), 7.37 (d,
2H),
7.86 (s, 1H), 8.08 (s, 1H), 8.19 (s, 1H), 8.45 (br s, 1H), 9.12 (s, 1H).
Example 87
2-(aminocarbonyl)-4-(4-chlorophenoxy)thienof2 3-clpyridinium iodide
105
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 61 (0.1 lg, 0.0033 mole) was treated with methyl iodide (0.2 mL,
0.0033
mmol) at reflux for 2 hours and filtered. The precipitate was washed with
ether, dried, and
recrystallized from acetonitrile to provide the title compound.
MS (DCI/NH3) m/z 305 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 4.40 (s, 3H), 7.40 (dd, 2H), 7.65 (dd, 2H), 8.25
(br s,
1H), 8.55 (s, 1H), 8.65 (s, 1H), 8.70 (br s, 1H), 9.70 (s, 1H).
Example 88
4-(4-chlorophenoxy)thieno f 2 3-clpyridine-2-carboxylic acid
Example 61A (354 mg, 1.11 mmol), lithium hydroxide monohydrate (98 mg, 2.33
mmol) in 3:1 methanol/water (4 mL) was stirred at room temperature for 20
hours,
acidified with 90% formic acid (0.13 mL), and filtered to provide the title
compound.
MS (DCI/NH3) m/z 306, 308 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.26 (m, 2H), 7.47 (m, 2H)> 7.83 (s, 1H), 8.23 (s,
1H),
9.21 (s, 1H);
Anal. calcd for Cl4HgC1N03S: C, 55.00; H, 2.64; N, 4.58. Found: C, 54.77; H,
2.60; N,
4.44.
Example 89
N-(4-(4-chlorophenoxy)thienof2 3-clnyridin-2-yl)-O-(3-
tetrahydrofuranyl)carbamate
A suspension of Example 88 ( 100 mg, 0.327 mmol) in toluene (2 mL) was treated
with ethyldiisopropylamine (63 mg, 0.49 mmol) and diphenylphosphorylazide (109
mg,
0.394 mmol) at 63 °C, stirred for 1, treated with (~)-3-
hydroxytetrahydrofuran (130 mg,
1.47 mmol) at 110 °C, stirred for 18 hours, and concentrated. The
residue was purified by
flash chromatography on silica gel with 30% ethyl acetate/hexane and
recrystallized from
ethyl acetate to provide the title compound.
mp 194-201;
MS (APCI) m/z 391 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 1.93-2.04 (m, 1H), 2.13-2.28 (m, 1H), 3.29-3.34
(m,
1H plus HOD), 3.70-3.86 (m, 4H), 5.33 (m, 1H), 6.56 (s, 1H), 7.02 (dt, 2H),
?.43 (dt, 2H),
8.14 (s, 1H), 8.91 (s, 1H);
Anal. calcd for CIgH~SC1N204S: C, 55.32; H, 3.87; N, 7.17. Found: C, 55.08; H,
3.69;
N, 7.05.
Example 90
4-(4-chlorophenoxy)thienof2 3-clpyridine-2-methanol
106
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
A solution of Example 61A (254 mg, 0.793 mmol) in absolute ethanol (4 mL} was
treated with anhydrous CaCl2 ( 177 mg, 1.59 mmol), stirred for 1 hour, cooled
to 0 °C,
treated with NaBH4 ( 123 mg, 3.25 mmol), stirred at 0 °C for 4 hours
and at room
temperature for 18 hours, treated with water and extracted with
dichloromethane. The
extract was washed with brine, dried (Na2S04), filtered and concentrated. The
residue
was purified on silica gel with 30% ethyl acetate/hexane to provided the title
compound.
mp 90-91 °C;
MS (APCI) m/z 292, 294 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 2.2-2.65 (vbr s, 1H), 4.97 (d, 2H), 6.95 (dt, 2H),
7.43 (m,
1H), 7.31 (dt, 2H), 8.13 (s, 1H), 8.89 (s, 1H);
Anal. calcd for C14H1oCIN02S: C, 57.64'; H, 3.45; N, 4.80. Found: C, 57.50; H,
3.58; N,
4.66.
Example 91
(E)-3-f4-(4-chloronhenoxy)thienof2,3-clpyridin-2- l~propenoic acid
Example 91 A
4-(4-chlorophenoxy)thienof2,3-clpyridine-2-carboxaldeh~de
A solution of DMSO (77 mg, 0.99 mmol) in 1.7 mL dichloromethane at -78
°C
was treated dropwise with oxalyl chloride ( 109 mg, 0.86 mmol), stirred for 5
minutes,
treated dropwise with Example 90 (123 mg, 0.420 mmol) in 2 mL dichloromethane,
stirred at -78 °C for 1 hour, treated with ethyldiisopropylamine (326
mg, 2.53 mmol)
warmed to -20 °C, stirred 1.5 hours and partitioned between 10 mL
dichloromethane and 5
mL water, and extracted. The extract was washed with water (5 mL) and brine (5
mL),
dried (NaZS04), and filtered. The residue was rotoevaporated and dried under
high
vacuum to provide the title compound.
MS (APCI) m/z 290, 292 (M+H)+.
Example 91 B
(E)-methyl 3-f4-(4-chlorophenoxy)thieno f 2,3-clpyridin-2-yll-2-propenoate
Example 91A (138 mg, 0.42 mmol) and methyl triphenylphosphoranylidene
acetate (210 mg, 0.628 mmol) in dichloroethane (2 mL) was stirred at 65
°C for 3 hours
and concentrated. The residue was purified by flash chromatography on silica
gel with
25% ethyl acetate/hexane to provide the title compound.
MS (APCI) m/z 346, 348 (M+H)+;
107
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, CDCl3) b 3.83 (s, 3H), 6.43 (d, 1H), 7.00 (dt, 2H), 7.35 (dt,
2H), 7.48
(s, 1 H), 7.84 (d, 1 H), 8.11 (s, 1 H), 8.88 (s, 1 H);
Anal. calcd for C1~H~~C1N03S: C, 59.05; H, 3.50; N, 4.05. Found: C, 58.82; H,
3.46; N,
3.86.
Example 91C
(E)-3-f4-(4-chlorophenoxy)thienof2 3-clp ridin-2-yll-2-propenoic acid
Example 91B was processed as in Example 88 to provide the title compound.
MS (ESI-) m/z 330, 332 (M-H)-;
1H NMR (300 MHz, DMSO-d6) 8 6.46 (d, 1H), 7.14 (dt, 2H), 7.46 (dt, 2H), 7.83
(s, 1H),
7.92 (d, 1H), 8.15 (s, 1H), 9.10 (s, 1H);
Anal. calcd for Ci~HI~C1N03S: C, 59.05; H, 3.50; N, 4.05. Found: C, 58.82; H,
3.46; N,
3.86.
Example 92
(E)-3-f4-(4-chlorophenoxy)thienof2 3-clpyridin 2 yll 2 propenamide
A solution of Example 91 C (51.5 mg, 0.1 SS mmol), N-hydroxybenzotriazole
monohydrate (34.5 mg, 0.225 mmol), 4-methylmorpholine (47 mg, 0.464 mmol) and
NH4CI (31.6 mg, 0.591 mmol) in DMF ( 1 mL) at 0 °C was treated with EDC
(45.0 mg,
0.235 mmoI), stirred at 0 °C for 4 hours and at room temperature for 10
hours, treated with
chloroform (5 mL), washed sequentially with 1M NaHC03 and brine, dried
(Na2S04),
filtered, and concentrated. The residue was purified by flash chromatography
on silica gel
with 5% methanol/dichloromethane to provide the title compound.
mp 176-178 °C;
MS (ESI) m/z 331, 333 (M+H)+;
IH NMR (300 MHz, CDCl3) b 5.60 (br s, 2H), 6.46 (d, 1H), 7.01 (m, 2H), 7.35
(m, 2H),
7.46 (s, 1H), 7.84 (d, 1H), 8.11 (s, 1H), 8.88 (s, 1H);
Anal. calcd for C~6H11C1N202S: C, 58.10; H, 3.35; N, 8.47. Found: C, 57.98; H,
3.24;
N, 8.45.
Example 93
4-bromothienof2 3-clpyridine-2-carboxamide
Example 93A
3 5-dibromopyridine-4-carboxaldeh~e
108
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
A solution of diisopropylamine (6.6 mL, 46.43 mmol) in THF (40 mL) at 0
°C was
treated with n-butyllithium in hexanes (2.50 M solution, 18.6 mL, 46.43 mmol)
over 15
minutes, stirred at 0 °C for 30 minutes, diluted with THF (200 mL),
cooled to -78 °C,
treated with 3,5-dibromopyridine ( 10 g, 42.21 mmol) in THF ( 110 mL) over 95
minutes,
stirred at -78 °C for 30 minutes, treated dropwise with methylformate
(5.2 mL, 84.42
mmol), stirred at -78 °C for 2 hours, transferred to ice-cold saturated
NaHC03 solution,
stirred for I5 minutes, and extracted with diethyl ether. The extract was
washed with
brine, dried (MgS04), filtered, and concentrated. The residue was purified by
flash
chromatography on silica gel with 10% acetone/hexane to provide the title
compound.
MS (DCI/NHg) m/z 266 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.91 (s; 2H), 10.09 (s, IH).
Example 93B
methyl 4-bromothienof 2.3-clpyridine-2-carboxylate
Example 93A was processed as in Example 17C except at 0- 25 °C to
provide the
title compound.
MS (DCI/NH3) m/z 274 (M+H)+;
1H NMR (500 MHz, DMSO-d6) b 3.95 (s, 3H), 7.99 (s, 1H), 8.67 (s, IH), 9.31 (s,
IH).
Example 93C
4-bromothienof2,3-clpyridine-2-carboxamide
Example 93B was processed as in Example 44 to provide the title compound.
MS (DCl/NH3) m/z 257 (M+H)+;
tH NMR (400 MHz, DMSO-d6) 8 7.97 (br s, IH), 8.11 (s, IH), 8.33 (br s, 1H),
8.43 (s,
1H), 9.24 (s, IH).
Example 94
4-chlorothienof2,3-clpyridine-2-carboxamide
3,5-Dichloropyridine was processed as in Example 93 to provide the title
compound.
MS (DCI/NH3) m/z 213 (M+H)+;
~H NMR (300 MHz, DMSO-d6) b 7.93 (br s, 1H, NH), 8.28 (s, 1H), 8.55 (br s, 1H,
NH),
8.58 (s, 1H), 9.28 (s, 1H).
Example 95
4-f4-(trifluoromethyl)phenyllthienof2 3-clpyridine-2-carboxamide
I09
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 95A
methyl 4-f4-(trifluoromethyl)phenyllthienof2 3-clpyridine 2 carboxylate
A solution of Example 93B (272 mg, 1 mmol), 4-(trifluoromethyl)phenyl boronic
acid (209 mg, 1.1 mmol) and cesium fluoride (347 mg, 2.1 mmol) in DME (5 mL)
was
degassed for 15 minutes, treated with tetrakis(triphenylphosphine)palladium(0)
(35 mg,
0.03 mmol), warmed at 80 °C for 6 hours, stirred at room temperature
for 12 hours,
filtered through Celite~, and concentrated. The residue was purified by flash
chromatography on silica gel with 5% acetone/hexane to provide the title
compound.
MS (DC1/NH3) m/z 338 (M+H)+;
IH NMR (300 MHz, DMSO-d6) 8 3.92 (s; 3H), 7.94 (m, 4H), 8.06 (s, 1H), 8.66 (s,
1H),
9.47 (s; 1H).
Example 95B
4-j4-(trifluoromethyl)phenyllthienof2 3-clpyridine-2-carboxamide
Example 95A was processed as in Example 44 to provide the title compound.
MS (APCI) m/z 323 (M+H) +, 321 (M-H)-, and 357 (M+Cl)-;
1H NMR (400 MHz, DMSO-d6) 8 7.81 (br s, 1H), 7.93 (m, 4H), 8.24 (s, 3H), 8.45
(br s,
1H), 8.59 (br s, 1H), 9.37 (br s, 1H).
Example 96
N-methyl-4-j4-(trifluoromethyl)phenyllthienof2 3-clpyridine 2 carboxamide
Example 95A was processed as in Example 44 but substituting methylamine (2.0
M in methanol) for methanolic ammonia to provide the title compound.
MS (APCI) m/z 337 (M+H) +, 335 (M-H)-, and 371 (M+Cl)-;
1H NMR (400 MHz, DMSO-d6) 8 2.82 (d, 3H), 7.90 (d, 2H), 7.94 (d, 2H), 8.17 (s,
1H),
8.58 (s, 1H), 8.93 (br d, 1H), 9.36 (s, 1H);
13C NMR (I00 MHz, DMSO-d~) 8: 26.1 (CH3), 121.6 (Ar-CH), 123.1, 125.3 (C),
125.7
(CH), 125.8 (CH), 128.3, 128.6, 128.8, 129.1 (CF3), 129.9 (2xAr-CH), 136.6
(C), 140.6
(C), 142.4 (C), 142.5 (CH), 145.0 (2xCH), 146.6 (C), 161.1 (C).
Example 97
4-phenylthieno f 2,3-clpyridine-2-carboxamide
Example 97A
methyl 4-phenylthienof2 3-clpyridine-2-carboxylate
110
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 93B and phenylboronic acid were processed as in Example 95 to provide
the designated compound.
MS (DCUNH3) m/z 338 (M+H) +;
~H NMR (300 MHz, DMSO-d6} b 3.92 (s, 3H), 7.94 (m, 4H), 8.06 (s, IH), 8.66 (s,
1H),
9.47 (s, 1H).
Example 97B
4-nhenylthienof 2,3-clpyridine-2-carboxamide
Example 97A was processed as in Example 44 to provide the title compound.
MS (DCI/NH3) m/z 255 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.52-7.69 (m, SH), 7.78 (br s, IH), 8.23 (s, 1H),
8.44
(br s, 1H), 8.52 (s, 1H), 9.30 (s, IH);
Anal. calcd for C ~ 4H ~ pN20S: C, 66.12; H, 3.96; N, 11.02. Found: C, 66.02;
H, 3.94; N,
11.00.
Example 98
4-(fl,l'-binhenyll-4-ylthio)thienof2 3-clpyridine-2-carboxamide
Example 73 and phenylboronic acid were processed and purified as in Example 95
then repurified by HPLC (C 18 reverse phase, 0-90% acetonitrile gradient in
water
containing 0.1 % TFA) to provide the title compound.
MS (DCI/NH3) mlz 363 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.36-7.48 (m, SH), 7.63-7.68 (m, 4H), 7.91 (br s,
1H),
8.30 (s, 1H), 8.54 (s, 1H), 8.57 (br s, 1H), 9.36 (s, 1H).
Example 99
Methyl 4-f3-(2,3,4,5-Tetrahydrofuranyl)oxylthienof2 3-clpyridine-2-carboxamide
Example 99A
Methyl 4-f3-(2,3,4,5-Tetrahydrofuranyl)oxylthienof2 3-clpyridine-2-carboxylate
To a solution of Example 236E ( 1 l Omg, 0.53 mmol} in anhydrous
tetrahydrofuran
( 10 mL) at room temperature under nitrogen atmosphere was added 3-hydroxy
tetrahydrofuran (0.043 mL, 0.53 mmol), triphenylphosphene ( 138 mg, 0.53 mmol)
and
diethyl azodicarboxylate (0.083 mL, 0.53 mmol). After 22 hr the reaction
mixture was
diluted with ethyl acetate ( 100 mL), filtered and the filtrate was
concentrated under
reduced pressure. The residue obtained was purified by flash chromatography on
silica gel
111
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
(Biotage Flash 40 S) eluting with 10 % acetone-hexane to obtain the title
compound in
22% yield.
'H NMR (400 MHz, DMSO-d6) b 2.05-2.18 (m, 1H), 2.26-2.49 (m, 1H), 3.61-3.77
(m,
2H), 3.93 (s, 2H), 4.25-4.31 (m, 2H), 5.32-5.39 (m, 1H), 8.10 9s, 1H), 8.26
(s, 1H), 8.99
(s, 1 H);
MS (APCI) m/e 280 (M+H)+.
Example 99B
Methyl 4-f3-(2,3,4,5-Tetrahydrofuranyl)oxylthienof2 3-clpyridine-2-carboxamide
The title compound (5.7 mg, 19%) was prepared from Example 99A (30 mg, 0.108
mmol) as described in Example 171. The product was isolated by C-18 reverse
phase
HPLC eluting with a gradient of 20% CH3CN-H20 containing 0.1 % trifluoroacetic
acid.
'H NMR (400 MHz, DMSO-d6) b 2.05-22.13 (m, 1H), 2.30-2.40 (m, 1H), 2.81 (d,
J=SHz,
3H), 3.78-3.84 (m, 1 H), 3.90-4.01 (m, 3H), 5.32-5.37 (m, 1 H), 8.13 (s, 1 H),
8.21 (s, 1 H),
8.90 (s, 1 H), 8.85 (d, J=SHz, 1 H);
'3C NMR (75 MHz, DMSO-d6) 8 26.2 (CH3), 32.5 (CH2), 66.4 (CH2), 72.3 (CHz),
78.5
(CH),119.6 (CH), 126.7 (CH), 135.6 (C),137.3 (C), 137.8 (CH),144.3 (C), 148.6
(C),
161.1 (CO);
MS (APCI) m/e 279 (M+H)+, 313 (M+Cl)-.
Example 100
ethyl4-ff2-(aminocarbonyl)thienof2,3-clpyridin-4- lloxylbenzoate
A solution of Example 73 ( 120 mg, 0.33 mmol), palladium(II)acetate ( 11 mg,
0.05
mmol), 1,3-bis(diphenylphosphino)propane (20.6 mg, 0.05 mmol), and
triethylamine (100
mg, 0.99 mmol) in DMF (6 mL) and ethanol (3 mL) were purged with carbon
monoxide,
heated at 105 °C under a carbon monoxide atmosphere (balloon) for 12
hours, treated with
ether, washed sequentially with brine and water, dried (Na2SU4), filtered, and
concentrated. The residue was purified by flash chromatography (20% ethyl
acetate/hexane) to provide the title compound.
MS (DCI/NH3) m/z 358 (M+H)+.
Example 101
4-[f2-(aminocarbonyl)thienof2,3-clpyridin-4-ylloxylbenzoic acid
A solution of Example 100 (50 mg) in DMF (5 mL) and methanol ( 10 mL) was
treated with a solution of NaOH (200 mg) in water (0.5 mL), stirred for 13
hours, treated
112
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
sequentially with acetic acid (S00 mg) and water, and filtered. The residue
was
recrystallized from DMF/water to provide the title compound.
MS (DCI/NH3) m/z 315 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 7.13 (dt, l.BHz, 2H), 7.86 (br s, 1H), 7.98 (dt,
2H), 8.09
(s, 1H), 8.31 (s, 1H), 8.44 (br s, 1H), 9.22 (s, 1H);
Anal. calcd for ClSHION204S: C, 57.32; H, 3.21; N, 8.91. Found: C,57.32; H,
3.30; N,
8.92.
Example 102 4-(1-phenylethenyl)thieno~2,3-clpyridine-2-carboxamide
Example 102A
styrene a-boronic acid
A solution of oc-bromo styrene (5.5 g, 30 mmol) in diethyl ether (30 mL) at -
78 °C
was treated with a solution of tert-BuLi (1.7 M solution, 21.2 mL, 36 mmol),
stirred at
-78 °C for 0.5 hours, treated with triisopropyl borate (8.31 mL, 36
mmol) over 48 minutes,
stirred for 1 hour, warmed to room temperature over 18 hours, diluted with
diethyl ether
( 100 mL), treated with 1 M HCl ( 100 mL), stirred at room temperature for 5
hours,
concentrated to remove the THF, adjusted pH 14 with 1N NaOH, washed with
hexane,
adjusted to pH 1 with 1M HCI, and extracted with ethyl acetate. The extract
was dried
(Na2S04), filtered and concentrated to provide the title compound.
iH NMR (300 MHz, DMSO-d6) 8 5.75 (d, 1H), 5.83 (d, 1H), 7.2-7.39 (m, SH, Ar-
CH).
Example 102B
methyl 4-( 1-phenylethenyl)thienof2,3-clpyridine-2-carboxalate
Example 93B and styrene-a-boronic acid were processed as in Example 95 to
provide the title compound.
MS (APCI) m/z 296(M+H) +;
1H NMR (400 MHz, DMSO-d6) 8 3.84 (s, 3H), 5.56 (s, 1H), 5.95 (s, 1H), 7.31 (m,
2H),
7.36 (m, 3H), 7.47 (s, 1H), 8.5 (s, 1H), 9,40 (s, 1H);
13C NMR ( 100 MHz, DMSO-d6) 8 53.03 (OCH3), 118.37 (vinylic CH2), 126.79 (Ar-
CH),
127.60 (Ar-CH), 128.38 (Ar-CH), 128.75 (Ar-CH), 132.55 (Ar-CH), 137.20 (C),
138.10
(C), 139.59 (C), 141.88 (C), 142.97 (3-CH), 144.03 (C), 145.39 (CH), 161.69
(CO).
Example 102C
4-( 1-phenylethenyl)thienof2,3-c~pyridine-2-carboxamide
Example 102B was processed as in Example 44 to provide the title compound.
113
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
MS (DCI) m/z 281 (M+H) ~;
1H NMR (400 MHz, DMSO-d6) 8 5.53 (S, 1H), 6.04 (S, 1H), 7.31 (m, 2H), 7.35 (m,
3H),
7.72 (br s, 1H), 7.82 (s, 1H), 8.33 (s, 1H), 8.37 (br s, 1H), 9.29 (s, 1H);
13C NMR (100 MHz, DMSO-d6) 8 118.0 (CH2), 123.10 (CH), 126.73 (Ar-CH), 128.22
(CH), 128.60 (Ar-CH), 132.41 (C), 136.59 (C), 139.42 (C), 142.73 (3-CH),
143.41 (C),
144.01 (C), 144.66 (5-CH), 146.0 (C), 162.5 (CO).
Example 103
4-f(4-methylphenyl)thiolthieno(2 3-clpyridine-2-methanol
A suspension of NaBH4 (28 mg, 0.743 mmol) in 2:3 THF/ethanol (2mL) was
stirred at 0 °C for 10 minutes, treated with CaCl2 (41.2 mg, 0.37
mmol), stirred for 15
minutes, treated with a solution of Example I7C ( 117 mg, 0.37 mmol) in 2:3
THF/ethanol
(3 mL), stirred at 0 °C for 4 hours, treated with 20% aqueous acetic
acid (5 mL), and
concentrated to remove the lower boiling solvents. The resulting mixture was
adjusted to
pH 7 with saturated NaHCOg and extracted with ethyl acetate . The extract was
dried
(MgS04), filtered, and concentrated. The residue was purified by column
chromatography
on silica gel eluting with 15% acetone/hexane to provide the title compound.
MS (DCI/NHg) m/z 288 (M+H)+;
1H NMR (S00 MHz, DMSO-d6) b 2.25 (s, 3H), 4.80 (s, 2H), 5.90 (br s, 1H), 7.14
(d, 2H),
7.18 (d, 2H), 7.32 (s, 1H), 8.36 (s, 1H), 9.15 (s, 1H).
Example 103A
4-(4-chlorophenoxy)-N-methylthienof2 3-clpyridine-2-carboxamide
A solution of Example 61 A ( 100 mg, 0.3135 mmol) and methylamine (2M
solution in THF, 0.467 mL, 0.941 mmol) in THF (2 mL) at 0 °C was
treated with NaH (12
mg, 0.47 mmol), stirred at room temperature for 1 hour, treated with water
(0.1 mL), and
concentrated. The residue was purified by flash chromatography on silica gel
with 20%
acetone/hexane to provide the title compound.
MS (APCI) m/z 319 (M+H)~;
1H NMR (300 MHz, DMSO-d6) b 2.80 (d, 3H), 7.13 (d, 2H), 7.45 (d, 2H), 8.06 (s,
1H),
8.19 (s, 1H), 8.94 (d, 1H), 9.16 (s, 1H).
Example 104
4-(4-chloronhenoxy)-N N-dimethylthienof2 3-clpyridine-2 carboxamide
Example 61 A and dimethylamine were processed as in Example 103A to provide
the title compound.
114
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
MS (APCI) m/z 333 (M+H)+;
t H NMR (300 MHz, DMSO-d~) $ 3.03 (br s, 3H), 3.12 (br s, 3H), 7. I? (d, 2H),
7.46 (d,
2H), 7.62 (s, 1H), 8.18 (s, 1H), 9.15 (s, IH).
Example 105
4-(4-chlorophenoxy)-N N-diethylthienof2 3-clpyridine-2 carboxamide
Example 61A and diethylamine were processed as in Example I03A to provide the
title compound.
MS (APCI) m/z 361 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 1.09 (m, 6H), 3.42 (m, 4H), 7.15 (d, 2H), 7.45 (d,
2H),
7.49 (s, 1H), 8.74 (s, 1H), 9.I7 (s, 1H).
Example 106
4-(4-chlorophenoxy)-N-cyclonropylthienof2 3-clpyridine 2 carboxamide
Example 6IA and cyclopropylamine were processed as in Example 103A to
provide the title compound.
MS (APCI) m/z 345 (M+H)+;
1H NMR (500 MHz, DMSO-d6) 8 2.85 (m, 1H), 7.12 (d, 2H), 7.46 (d, 2H), 8.I 1
(s, IH),
8.13 (s, 1H), 8.93 (d, IH), 9.12 (s, IH).
Example 107
I-ff4-(4-chloronhenoxy)thienof2 3-clpyridin-2-yllcarbonyllpyrrolidine
Example 61A and pyrrolidine were processed as in Example 103A to provide the
title compound.
MS (APCI) m/z 359 (M+H)+;
tH NMR (400 MHz, DMSO-d6) 8 1.83-1.93 (m, 4H), 3.53 (t, 2H), 3.71 (t, 2H),
7.17 (d,
2H), 7.47 (d, 2H), 7.70 (s, 1 H), 8.16 (s, 1 H), 9.12 (s, I H).
Example 108
I-ff4-(4-chloronhenoxy)thienof2 3-clpyridin-2-yllcarbonvllpiperidine
Example 61A and piperidine were processed as in Example 103A to provide the
title compound.
MS (APCI) m/z 373 (M+H)+;
tH NMR (400 MHz, DMSO-d6) b 1.52 (m, 3H), 1.62 (m, 2H), 3.53 (m, 5H), 7.14 (d,
2H),
7.46 (d, 2H), 7.47 (s, 1H), 8.20 (s, IH), 9.14 (s, IH).
115
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
Example 109
4-f f4-(4-chlorophenoxy)thienof 2,3-clpyridin-2-yllcarbonyllmorpholine
Example 61A and morpholine were processed as in Example 103A to provide the
title compound.
MS (APCI) m/z 375 (M+H)+;
1H NMR (500 MHz, DMSO-d6) 8 3.6 (m, 8H), 7.14 (d, 2H), 7.45 (d, 2H}, 7.55 (s,
1H),
8.17 (s, 1H), 9.14 (s, 1H).
ExamQle 110
1-f f4-(4-chloro_phenoxy)thienof 2.3-clpyridin-2-~lcarbonyll-4-
methylpiperazine
Example 61A and methylpiperazine were processed as in Example 103A to
provide the title compound.
MS (APCI) m/z 388 (M+H)+;
1H NMR (400 MHz, DMSO-d6) 8 2.2 (s, 3H), 2.32 (br s, 4H;), 8.58 (br s, 4H),
7.15 (dd,
1H), 7.47 (dd, 1H), 7.49 (s, 1H), 8.2 (d, 1H), 9.15 (s, 1H).
Example 111
1-f f4-(4-chlorophenoxy)thienof2,3-clpyridin-2-yllcarbonyll-4-
phenyllpiperazine
Example 61A and phenylpiperazine were processed as in Example 103A to
provide the title compound.
MS (APCI) m/z 450 (M+H)+;
1H NMR (500 MHz, DMSO-d6) b 3.18 (br s, 4H), 3.73 (br s, 4H), 6.81 (t, 1H),
6.95 (d,
2H), 7.15 (d, 2H), 7.24 (d, 2H), 7.46 (d, 2H), 7.57 (s, 1H), 8.20 (s, 1H),
9.17 (s, 1H).
Example 112
1-f f4-(4-chlorophenoxy)thienol2,3-clpyridin-2-yllcarbonyll-4-(phen ly
methyl)piperazine
Example 61A and benzylpiperazine were processed as in Example 103A to provide
the title compound.
MS (APCI) m/z 464 (M+H)+;
1H NMR (500 MHz, DMSO-d6) 8 2.38 (br s, 4H), 3.51 (s, 2H), 3.58 (br s, 4H),
7.13 (d,
2H), 7.32 (m, 5H), 7.45 (d, 2H), 7.47 (s, 1H), 8.91 (s, 1H), 9.13 (s, 1H).
Example 113
1-f f4-(4-chlorophenoxy)thienof 2,3-clpyridin-2-yllcarbonyll-4-(2-
pyridin~~perazine
Example 61A and 2-pyridylpiperazine were processed as in Example 103A to
provide the title compound.
116
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
MS (APCI) m/z 45I (M+H)+;
~H NMR (500 MHz, CDC13-d) 8 3.65 (br s, 4H), 3.85 (br s, 4H), 6.70 (m, 2H),
7.07 (d,
2H), 7.34 (d, 2H), 7.50 (s, 1 H}, 7.54 (m, 1 H), 8.15 (s, 1 H), 8.29 (m, 1 H),
8.96 (s, 1 H}.
Example l l4
4-(4-chlorophenoxy)-N-(2-hydroxyethyl)lthienof2 3-clpyridine-2-carboxamide
Example 61A and ethanolamine were processed as in Example 103A to provide
the title compound.
MS (DCI/NH3) m/z 349 (M+H) +;
1H NMR (400 MHz, DMSO-d6) 8 3.33 (m, 2H), 3.51 (m, 2H), 5.76 (t, 1H), 7.I2 (d,
2H),
7.26 (d, 2H), 8.17 (s, 2H), 8.98 (br.t, 1H), 9.14 (s, 1H);
13C NMR ( 100 MHz, DMSO-d6) S 42.3 (N-CH2), 59.4 (O-CH2), 119.2 (CH), 119.3
(Ar-
CH), 127.6 (C), 130.0 (Ar-CH), 133.2 (CH), 137.5 (C), 137.9 (C), 141.4 (CH),
146.4 (C),
147.1 (C), 155.6 (C), 160.6 (CO}.
IS
Example 115
4-ff4-(4-chlorophenoxy)thienof2 3-clpyridin-2-yllcarbonyll-N-(1 methyleth 1~
piperazineacetamide, trifluoroacetate (salt)
Example 6IA and N-isopropylpiperazine acetamide were processed as in Example
103A to provide the title compound. The residue was purified by C-18 reverse
phase
HPLC with a gradient of 20% CH3CN/water and I00% CH3CN containing 0.1 %
trifluoroacetic acid to provide the title compound.
MS (APCI) m/z 473 (M+H}+;
tH NMR (400 MHz, DMSO-d6) 8 1.09 (m, 6H), 3.05 (br s, 4H), 3.43 (s, 2H), 3.87
(br s,
4H), 7.16 (d, 2H), 7.67 (d, 2H), 7.68 (s, 1H), 8.20 (s, 1H), 9.18 (s, 1H).
Example 116 4-(4-chloronhenoxv)-N-f 1-(hydroxymeth I~eth~rllthienof2 3
clpyridine 2
carboxamide
Example 61A and DL-2-amino-I-propanol were processed as in Example 103A to
provide the title compound.
MS (APCI) m/z 363 (M+H) ~, 361 (M-H)-, 397 (M+Cl)-;
1H NMR (400 MHz, DMSO-d6) 8 1.14 (d, 3H), 3.36-3.40 (m, 1H), 3.43-3.5 (m, 1H),
3.97-4.04 (m, i H), 4.77 (t, 1 H), 7.15 (d, 2H), 7.48 (d, 2H), 8.14 (s, 1 H),
8.26 (s, 1 H), 8.67
(d, IH), 9.14 (s, 1H);
117
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
13C NMR ( 100 MHz, DMSO-d6) b 16.87 (CH3), 47.74 (CH), 64.06 CH20H), 119.16 (3-
CH), 119.46 (2xAr-CH), 127.72 (C), 130.08 (2xAr-CH), 132.84 {5-CH), 137.51
(C),
137.91 (C), 141.20 (7-CH), 146.62 (C), 147.28 (C), 155.53 (C), 160.01 (CO).
Example 117
4-(4-chlorophenoxy)-N-(1,1-bis(hydroxymethyl)ethyllthieno(2 3-clpyridine-2-
carboxamide
Example 61A and 2-amino-2-methyl-1,3-propanediol were processed as in
Example 103A to provide the title compound.
MS (APCI) m/z 393 (M+H) +, 39I (M-H)-, 393 {M+Cl)-;
1H NMR (400 MHz, DMSO-d6) b 1.28 (s, 3H), 3.56-3.66 (m, 4H), 4.71 (t, 2H),
7.16 (d,
2H), 7.48 (d, 2H), 7.92 (s, IH), 8.11 (s, 1H), 8.31 (s, 1H), 9.12 (s, 1H);
13C NMR (100 MHz, DMSO-d6) S 18.45 (CH3), 59.80 (C), 63.08 (CH2), 119.46 (CH),
119.74 (2xAr-CH), 127.83 (C), 130.09 (2xAr-CH), 132.53 (CH), 137.43 (C),
137.84 (C),
140.99 (CH}, 147.38 (C), 147.50 (C), 155.40 (C), 160.59 (CO);
Example 118
(D,L)-4-(4-chlorophenoxy)-N-(2-hydroxypropyl)thienof2 3-clpyridine-2-
carboxamide
Example 61A and DL-1-amino-2-propanol were processed as in Example 103A to
provide the title compound.
MS (APCI) m/z 363 (M+H) + and 397 (M+Cl)-;
1H NMR (400 MHz, DMSO-d6) 8 1.08 (d, 3H), 3.21 (m, 2H), 3.75-3.84 (m, 1H), 4.8
(br s,
1H), 7.14 (d, 2H), 7.48 (d, 2H), 8.17 (s, 1H), 8.22 (s, 1H), 8.98 (br s, 1H),
9.15 (s, 1H);
13C NMR ( 100 MHz, DMSO-d6) S 21.13 (CH3), 47.24 (CH2), 64.84 (CH), 119.30 (3-
CH), 119.42 (2xAr-CH), 127.66 (C), 130.04 (2xAr-CH), 133.05 (CH), 137.48 (C),
137.92
(C), 141.30 (CH), 146.37 (C), 147.16 (C), 155.58 (C), 160.59 (CO).
Example 119
4-(4-chlorophenoxy)-N-f2-(4-morpholinyl)ethyllthienof2 3-clnyridine-2-
carboxamide
Example 61 A and 4-(2-aminoethyl)morpholine were processed as in Example
103A to provide the title compound.
MS (APCI) m/z 418 (M+H) +, 452 (M+Cl)-;
1H NMR (400 MHz, DMSO-db) 8 2.41 (t, 4H), 2.48 (m, 2H), 3.40 (m, 2H), 3.56 (t,
4H),
7.15 (d, 2H), 7.47 (d, 2H), 8.13 (s, 1H), 8.17 (s, 1H), 8.94 (t, 1H), 9.04 (s,
1H);
13C NMR ( 100 MHz, DMSO-d6) 8 36.73 (N-CH2), 53.21 (morpholine ring 2xN-CH2),
57.07 (N-CH2), 66.12 (morphline ring 2x-OCH2}, 119.14 (3-CH), 119.50 (2xAr-
CH),
118
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
127.73 (C), 130.06 (2xAr-CH), 132.96 (pyridyl ring CH), 137.35 (C), 137.91
(C), 141.29
(pyridyl ring CH), 146.20 (C), 147.20 (C), 155.40 (C), 160.45 (CO).
Example 120
4-(4-Chlorophenoxy)thienof 2,3-clpyridine-2-sulfonamide
To a solution of Example 124A (261 mg, 1 mmol) in anhydrous THF (5 mL) at -78
°C was added tent-BuLi ( 1.7 M solution in hexanes, 0.647 mL, 1.1 mmol)
under nitrogen
atmosphere. The reaction mixture was stirred at -78 °C for 15 minutes
and S02 gas was
bubbled into the solution for 15 minutes. Then this was stirred at -72
°C for 2.5 hours and
at 0 °C for 4 hours. The reaction mixture was diluted with hexane ( 10
mL) and
evaporated, and the residue obtained was suspended in CHZC12 (5 mL) and
treated with N-
chlorosuccinimide (214 mg, 1.6 mmol) at 0 °C. After 2 hours at ambient
temperature the
reaction mixture was diluted with CH2C12, washed with 10% aqueous NaHS03
solution
(3x25 mL) followed by brine (3x25 mL). The dried (MgS04} organic layer was
evaporated to dryness under reduced pressure to obtain the crude sulfonyl
chloride as an
oil. This was dissolved in acetone (5 mL) and treated with ice cold solution
of NH40H (5
mL) at 0 °C. After 2 hr at 0 °C reaction mixture was evaporated
from toluene to obtain the
crude product as an oil. The title compound (57 g, 16%) was obtained by flash
chromatography on silica gel eluting with 20% acetone-hexane followed by 40%
acetone-
hexane.
'H NMR (400 MHz, DMSO-db) 8 7.21 (d, J=9Hz, 2H), 7.51 (d, J=9Hz, 2H), 7.79 (s,
1H),
8.14 (br.s, 2H), 8.30 (s, 1H), 9.27 (s, 1H);
'3C NMR (100 MHz, DMSO-d6) 8 I 19.65 (CH}, 120.88 (CH), 127.94 (C), 130.12
(CH),
133.67 (CH), 135.61 (C), 141.65 (CH) 152.11 (C), 155.41 (C );
MS (APCI) m/e 341 (M+H)+, 339 (M-H)~, 375 (M+CI)-.
Example 121
4-f (4-methylphenyl)methyllthienof2,3-clp~rridine-2-carboxamide
Example 121A
methyl 4-f (4-methylphenyl)methyllthieno f 2,3-clpyridine-2-carboxylate
Example 121A is processed as in J. Org. Chem, 1988, 53, pp. 2392-2394.
For example, a suspension of Zn dust (92 mg, 1.4 mM) in THF (2 ml) containing
1,2-
dibromoethane (0.05 ml, 0.054 mmol) is heated at 65 °C for 2 minutes,
cooled to 25 °C ,
treated with trimethylsilyl chloride (0.0(?9 ml, 0.043 mM), stirred at room
temperature for
25 minutes, cooled to 0 °C , slowly treated with a solution of 4-
methylbenzyl bromide
119
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
(0.248 mL, 1.0 mmol) in THF (5 mL), warmed to 40 °C for 3 hours, cooled
to -10 °C,
treated with CuCN ( 106 mg, I .18 mM) and LiCI ( 100 mg, 2.35 mM) in THF ( 10
mL),
stirred at 0- °C for 30 minutes, treated slowly with a solution of
Example 93B (272 mg, 1
mmol) in THF (5 mL), stirred at 0 °C for 3 hours, warmed to 25
°C over 18 hours, treated
with ethyl acetate, washed sequentially with saturated NH4C1 and brine, dried
(MgS04),
filtered and concentrated. The residue is purified by flash chromatography on
silica gel to
provide the title compound.
Example 121B
4-I(4-methylnhenyl)methyllthienof2,3-clpyridine-2-carboxamide
Example I21A is processed as in Example 44 to provide the title compound.
Example 122
Methyl 4-(Momholino)thienof2,3-clpyridine-2-carboxamide
Example 122 (241 mg, 72°10) was prepared as described in Example
308,
substituting 1,4-dioxo-8-azaspiro[4,5Jdecane (0.256 mL, 2 mmol) for 4-
methylaniline.
1H NMR (400 MHz, DMSO-d6) b 1.91 (m, 4H), 2.85 (d, J=4Hz, 3H), 3.25 (m, 4H),
3.96
(s, 4H), 8.10 (s, 1 H), 8.12 (s, I H), 8.87 (s, 1 H), 8.96 (d, J=4Hz, 1 H);
i3C NMR ( 100 MHz, DMSO-d6) S 26.1 (CH3), 35.0 (CHZ), 49.7 (CHZ), 63.8 (CH2),
69.8
(CH2), 106.1 (C), 121.3 (CH), 132.0 (CH), 136.9 (C), 138.3 (C), 138.7 (CH),
143.6 (C),
143.8 (C), 161.3 (C);
MS (APCI) m/e 334 (M+H)+, 368 (M+Cl)-.
Example 123
4-(4-chlorophenoxy)thieno(2,3-clpyridine-2-carboxamide N-oxide
Example 123A
methyl (4-chloronhenoxy)thieno~2,3-clpyridine-2-carboxylic acid N-oxide
A solution of methyl 4-(4-chlorophenoxy)thieno[2,3-c]pyridine-2-carboxylate
(319
mg, 1 mmol) in dichloromethane ( 15 mL) at 0 °C was treated with 3-
chloroperoxybenzoic
acid (302 mg, 1.75 mmol), stirred 0.5 hours at 0 °C and 4 hours at room
temperature
washed sequentially with water, saturated sodium bicarbonate, water, and
brine, dried
(Na2S04), filtered and concentrated to provide the title compound.
MS (DCI/NH3) m/z 336 (M+H)+ ;
tH NMR (300 MHz, DMSO-d6) 8 3.89 (s, 3H), 7.30 (m, 2H), 7.52 (m, 2H), 7.84 (s,
IH),
7.88 (s, 1 H), 9.02 (s, 1 H).
I20
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 123B
4-(4-chlorophenoxy)thienof2,3-clpyridine-2-carboxamide N-oxide
Example 123A was processed as in Example 44 to provide the title compound.
1H NMR (300 MHz, DMSO-d6) 8 7.30 (m, 2H), 7.52 (m, 2H), 7.74 (d, 1H), 7.81 (br
s,
1H), 8.10 (s, 1H), 8.34 (br s, 1H), 8.93 (s, 1H).
Example 124
4-(4-chloronhenoxy)-2-(2-methoxyphenyl)thieno f 2 3-clpyridine
Example 124A
4-(4-Chlorophenoxy)thieno~2,3-c~[pyridine
Example 88 (4.5 g, 14.75 mmol) was added to a solution of diphenyl ether
(SSmI)
at 210 °C and kept at this temperature for 10 hours. The cooled
reaction mixture was
directly purified by flash chromatography on silica gel eluting with hexane
followed by
10% acetone-hexane to obtain the title compound (3.83 g, 99.5%).
mp 87-89 °C;
MS (DCI/NH3) mle 262 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 7.09 (d, J=9Hz, 2H), 7.35 (d, J=6Hz, 1H), 7.45 (d,
J=9Hz, 2H), 8.13 (d, J=6Hz, 1 H), 8.18 (s, 1 H), 9.15 (s, 1 H);
Anal. calcd for C~3HgCI,NIO~S1: C, 59.66; H, 3.08; N, 5.35. Found : C, 59.52;
H, 3.08; N,
5.15.
Example 124B
4-(4-chlorophenoxy)thienof2,3-clpyridine-2-boronic acid
A solution of sec-butyllithium (0.92 mmol) in THF (2 mL) at -78 °C was
treated
dropwise with Example 124A in THF ( 1 mL), stirred at -78 °C for 30
minutes, treated
dropwise with tributylborate, stirred for 5 minutes at -78 °C, stirred
at room temperature
for 45 minutes, treated with 2M NaOH (3 mL), stirred for 5 minutes, washed
with
hexanes, and acidified to pH 2 with 6M HCI. The precipitate that formed was
collected
and dried under vacuum to provide the title compound.
MS (APCI) m/z 262, 264 (M+H-B(OH)2)+, 340, and 342 (M+Cl-)-;
1H NMR (300 MHz, CD30D) 8 7.29 (d, 2H), 7.53 (d, 2H), 8.08 (s, 1H), 8.11 (s,
1H), 9.40
(s, 1H).
Example 124C
121
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-(4-chiorophenoxy)-2-(2-methoxyphenyl)thienof2 3-clp ridine
Example 124B is processed as in Example 95 but substituting of 2-iodoanisole
for
Example 93B and Example 124B for 4-(trifluoromethyl)phenylboronic acid to
provide the
title compound.
S
Example 1254-(4-Chlorouhenoxy)-3-methylthienolf2,3-c]pyridine-2-carboxamide
Example 125A
Methyl 4-(4-Chloronhenoxy)-3-methylthienol f 2,3-clpyridine-2-carboxylate
4-Chlorophenol was dissolved in THF (20 mL) and treated with 1 M potassium t-
butoxide ( 13 mL, 13 mmol) and stirred at room temperature for 1 hour. To this
solution
was added Example 17A (1.76g, 10 mmol) in THF (5 mL). The reaction was heated
at 70
°C for 4 hours, and cooled to room temperature. Poured into water
diluted with brine and
extracted with ethyl acetate. The ethyl acetate was then washed (3x20 mL),
dried and
evaporated. The residue was dissolved in THF (20 mL) and cooled in an ice
bath, to
which 3 M methyl magnesium bromide in ethyl ether (4 mL, 12 mmol) was added.
The
reaction was stirred at room temperature overnight. The excess Grignard
reagent was
decomposed with a saturated ammonia chloride solution (25 mL) and then
extracted with
ethyl acetate (3x25 mL). The ethyl acetate was washed with brine (3x25 mL) ,
dried and
evaporated to give the desired phenoxy alcohol. This alcohol was then
subjected to an
Swern oxidation, using the following conditions. To a solution of oxalyl
chloride ( 1.1
mL, 12 mmol) in anhydrous methylene chloride (20m1) cooled to -78 °C
was added
dimethylsulfoxide ( 1.85 mL, 24 mmol) over 30 minutes. Then a solution of the
above
phenoxy alcohol in methylene chloride (20 mL) was added over 15 minutes.
Triethylamine (7.5 mL) was added and the reaction allowed to come to room
temperature
over 2 hours. Ice water was then added and the mixture was extracted with
ethyl acetate.
The ethyl acetate was washed with brine (3x20 mL), dried and evaporated. To a
0 °C
solution of this residue in THF (20 mL) was added methyl thioglycolate (0.88
mL, 10
mmol) and cesium carbonate (3.2 g, 10 mmol). The reaction was then heated at
70 °C for
1 hour, cooled, poured into water, diluted with brine and extracted with ethyl
acetate. The
ethyl acetate was then washed with 1 N NaOH (2x20 mL), brine (3x20 mL), dried
and
evaporated to give an oil. This oil was triturated with methanol to give the
desired
compound.
mp 140-141 °C;
MS (DCI/NH3) m/e 334 (M+H)+;
122
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
'H NMR (300 MHz, DMSO-db} 8 2.80 (s, 3H), 3.90 (s, 3H), 7.22 (d, 2H), 7.45 (d,
2H),
8.12 (s, 1H), 9.15 (s, 1H);
Anal. Calcd for C~6H,2C1N03S~0.5 HzO: C, 56.06; H, 3.82; N, 4.09. Found: C,
56.03; H,
3.43; N, 3.71.
Example 125B
4-(4-Chlorophenoxy)-3-methylthienolf2,3-clpyridine-2-carbolic acid
A solution of Example 125A {1.1 g, 3.3mmol) and LiOH~H20 (0.30 g, 6.9 mmol)
in THF (20 mL) and H20 ( 10 mL) was heated at 50 °C for 1 hour, then
cooled, acidified
with formic acid, and extracted with ethyl acetate. The ethyl acetate extract
was washed
with brine (3x15 mL), then dried and evaporated to give the desired product as
white solid.
mp 315-317 °C;
MS (DCI/NH3) m/e 320 (M+H)+;
IH NMR (300 MHz, DMSO-d6) 8 2.76 (s, 3H), 3.30 (m, 1H), 7.10 (d, 2H), 7.45 (d,
2H),
8.12 (s, 1H), 9.15 (s, 1H);
Example 125C4-(4-Chlorophenoxy)-3-methylthienol~2,3-clpyridine-2-carboxamide
Example 125B was processed as in Example 92 to provide the title compound.
mp 174-175 °C;
MS (DCI/NH3) m/e 319 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.55 (s, 3H), 7.05 (d, 2H), 7.45 (d, 2H), 7.90 (m,
1H),
7.95 (m, 1H), 8.15 (s, 1H), 9.12 (s, 1H);
Anal. calcd for C,SH,1C1N20ZS: C, 56.52; H, 3.48; N, 8.79. Found: C, 56.36; H,
3.50; N,
8.69.
Example 126
4-(4-chlorophenoxy)-3-hydroxythienof2,3-clpyridine-2-carboxamide
Example 126A
ethyl 3,5-dichlorpyridine-4-carboxylate
To a stirred solution of lithium diisopropylamide (45 mL, 1.5 M in THF, 67.6
mmol) in 150 mL THF at -78 °C is treated with 3,5-dichloropyridine ( 10
g, 67.6 mmol) in
mL THF over 1.5 hours, stirred for 1 hour at -78 °C, treated with ethyl
chloroformate
(9.5 mL, 100 mmol}, stirred for 2 hours, transferred into saturated sodium
bicarbonate
35 (200 ml) at 0 °C, treated with diethyl ether (200 ml), and extracted
with ethyl ether (2x100
mL). The extract is washed sequentially with saturated sodium bicarbonate
solution
123
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
(2x100 mL), brine (2x100 mL), dried (MgS04), and concentrated. The residue is
purified
by flash chromatography on silica gel with hexane/ethyl acetate to provide the
title
compound.
Example 126B
methyl 4-(4-chlorophenoxy)-3-hydroxythienof2,3-clpyridine-2-carboxylate
Example 126A is treated as in Example 61 A to provide the title compound.
Example 126C
4-(4-chlorophenoxy)-3-hydroxythienof 2,3-clpyridine-2-carboxamide
Example 126B is treated as in Example 61 B to provide the title compound.
Example 127
4-(4-chlorophenoxy)-3-( 1-methylethoxy)thieno f 2,3-clpyridine-2-carboxamide
Example 127 is processed as in J. Medicinal. Chem. 1992, 35, p. 958. Example
126C (0.10 g, 0.3 mmol) in 50 ml of THF and cesium carbonate ( 1.0 g, 0.1
nunol) are
treated with 2-bromopropane (0.37 g, 0.3 mmol), heated for 2 hours, poured on
ice,
extracted with ethyl ether, washed sequentially with 1 N aqueous sodium
hydroxide and
brine, and concentrated. The residue is purified by flash chromatorgraphy on
silica gel
with hexane-acetone (7:3) to provide the title compound.
Example 128
3-bromo-4-(4-chlorophenoxy)thienof 2,3-clpyridine
The method described in (Arkiv For Kemi ( 1970-74), 32, p. 249) may be
adapted.
Example 124A in thionyl chloride is treated with bromine at 90 °C for 4
hours to provide
the title compound.
Example 129
4-(4-chlorophenoxy)thienof2,3-clpyridine-3-carboxylic acid
The method described in (Arkiv For Kemi ( 1970-74), 32, p. 249) may be
adapted.
Example 128 cooled to -78°C is treated with ethyllithium followed by
reaction with
carbon dioxide to provide the title compound.
Example 130
4-(4-chlorophenoxy)thienof 2,3-clpyridine-3-carboxamide
Example 129 may be treated as in Example 19 to provide the title compound.
124
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 131
3-amino-4-(4-chlorophenoxy)thienof2 3-clpyridine-2-carboxamide
Example 131 A
3,5-Dichloropyridine-4-carbonitrile
Example 17A (2.0 g, 1 I .4 mmol) in formic acid ( 10 mL) is treated with
hydroxylamine hydrochloride ( 1.04 g, 11.4 mmol) and concentrated sulfuric
acid (0.05
mL}, stirred at reflux for 18 hours and concentrated. The residue was
partitioned between
1:1 ethyl acetate-water, and washed sequentially with saturated sodium
bicarbonate, water,
brine, dried (Na2S04), and concentrated. The residue is recrystallized from
hexanes to
provide the title compound.
mp 117-118 °C;
IH NMR (300 MHz, CDC13) 8 8.70 (s);
IR (KBr, v cm-1) 1?10, 1525, 1400, 1250, I 190, 1100, 920, 820, 800, 750.
Example 131 B
methyl 3-amino-4-(4-chloronhenoxy)thienof2 3-clpyridine-2-carboxylate
A solution of 4-chlorophenol ( 1.12 g, 8.72 mmol) in THF (20 mL) at 0
°C is
treated with a solution of potassium t-butoxide (8.72 mL, 8.72 mmol, 1 M in
THF), stirred
at 0 °C for I hour, treated with Example 131 A ( 1.5 g, 8.72 mmol) in
THF ( 10 mL) at 0 °C,
warmed to room temperature, stirred overnight, concentrated, partitioned
between 1:1
ethyl acetate-water, and extracted. The extract is washed with brine, dried
(Na2S04), and
concentrated. A solution of concentrate in DMF (50 mL) at 0 °C was
treated with
potassium carbonate (2.42 g, 17.51 mmol) and methyl thioglycolate (778 pL,,
8.72 mmol),
warmed to room temperature, stirred overnight, and poured into ether (400 mL).
The
organic layer was washed with brine, dried (Na2S04), and concentrated. The
residue was
purified by flash chromatography on silica gel with 0-5°lo acetone-
hexane to provide the
title compound.
mp 194-196 °C;
MS (APCI) m/z 335 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 3.81 (s, 3H), 6.86 (br s, 2H), 7.22-7.32 (m, 2H),
7.45-
7.56 (m, 2H), 7.88 (s, IH), 8.89 (s,lH);
Anal. Calcd for C15H1 ~C1N203S: C, 53.81; H, 3.31; N, 8.36. Found: C, 53.80;
H, 3.27;
N, 8.27.
125
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 131C3-Amino-4-(4-chloronhenoxy)thienof2,3-clpyridine-2-carboxylic acid
The title compound was prepared from Example 131B using the procedure of
Example 18.
mp 173-176 °C (dec);
'H NMR (300 MHz, DMSO-d6) S 7.29 (m, 2H), 7.52 (m, 2H), 7.88 (s, 1H), 8.89 (s,
1H);
MS (ESI) m/e 321 (M+H)+;
Anal. Calcd for C~4H9C1N20~S'0.25H20: C, 51.70; H, 2.94; N, 8.61. Found??
Example 131D
3-amino-4-(4-chloronhenoxy)thienof 2,3-clpyridine-2-carboxanvde
A solution of Example 131C (96 mg, 0.3 mmol) in DMF (2 mL) is treated with 1-
hydroxybenzotriazole hydrate (67 mg, 0.44 mmol), NH4Cl (61 mg, 1.14 mmol) and
4-
methylmorpholine (100 pl,, 0.9 mmol), cooled to 0 °C, treated with 1-[3-
(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (86 mg, 0.45 mmol),
warmed
to room temperature, stirred overnight, poured into saturated NaHC03,
collected, washed
with water and dried. The residue is recrystallized from
methanol/toluene/hexanes to
provide the title compound.
mp 202-204 °C;
MS (APCI) m/z 320 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 6.84 (br s, 2H), 7.21-7.30 (m, 2H), 7.39 (br s,
2H),
7.47-7.56 (m, 2H), 7.88 (s, 1H), 8.90 (s,lH);
Anal. Calcd for C 14H ~ pC1N302S: C, 52.58; H, 3.15; N, 13.14. Found: C,
52.63; H, 3.18;
N, 13.12.
Example 132A
ethyl 4-chlorothienof2,3-blpyridine-5-carboxylate
Example 132A was processed as in J. Heterocyclic C'.hem. 1977, 14, pp. 807-812
to provide the title compound.
mp 71-72 °C;
MS (DCI/NH3) m/z 259 (M+H)+; 242 (M+NH4)+;
iH NMR (300 MHz, DMSO-d6) S 1.37 (t, 3 H), 4.41 (q, 2 H), 7.64 (d, 1 H), 8.17
(d, 1 H),
8.95 (s, 1 H);
Anal. calcd for CIpHgC1N02S: C, 49.69; H, 3.33; N, 5.79; S, 13.26. Found: C,
49.46; H,
3.13; N, 5.62; S, 13.42.
Example 132B
126
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
ethyl 4-f (4-methylnhenyl)thiolthienof 2,3-b]pyridine-5-carboxylate
Example 132A and thiocresol were processed as in Example 2 to provide the
title
compound.
mp 60-63 °C;
MS (DCI/NH3) m/z 347 {M+NH4)+ and 330 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 1.30 (t, 3H), 2.28 (s, 3H), 4.26 (q, 2H), 7.00 (d,
1H), 7.17
(m, 2H), 7.24 (m, 2H), 7.91 (d, 1H), 8.81 (s, 1H);
Anal. calcd for Cl~H1gN02S2: C, 61.98, H, 4.59; N, 4.25. Found C, 61.92, H,
4.53, N,
4.21.
Example 132C
4-f (4-methylphenyl)thiolthienof 2,3-b)pyridine
Example 132B was processed as in Example 18 and 42 to provide the title
compound.
mp 90-92 °C;
MS (DCI/NH3) m/z 275 (M+NH4)+ and 258 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.39 (s, 3 H), 6.66 (d, 1 H), 7.38 (m, 2 H), 7.46
(d, 1
H), 7.53 (m, 2 H), 7.46 (d, 1 H}, 7.53 (m, 2 H), 7.95 (d, 1 H), 8.12 (d, 1 H);
Anal. calcd for C~4H~ INS2: C, 65.33; H, 4.30; N, 5.44. Found: C, 65.40; H,
4.26, N,
5.26.
Examale 132D
4- f (4-methylphenyl)thiolthieno f 2,3-blpyridine-2-carboxamide
Diisopropylamine (0.056 g, 0.56 mmol) in THF ( 10 mL) at -78 °C was
treated with
n-butyllithium (0.22 mL, 0.56 mmol, 2.5 M in hexanes), stirred for 15 minutes,
treated
with Example 132C (0.13 g, 0.51 mmol) in THF (5 mL), stirred for 0.5 hours,
warmed to
0 °C for 1 minutes, recooled to -78 °C, poured onto solid C02,
stirred for 0.5 hours,
diluted with saturated NH4Cl, and extracted with ethyl acetate. The extract
was washed
with brine, dried (MgS04) and concentrated to provide the title compound.
mp 280-282 °C;
MS (DCI/NH3) m/z 318 (M+NH4)+ and 301 (M+H)+;
tH NMR (300 MHz, DMSO-d6) 8 2.41 (s, 3 H), 6.62 (d, 1 H), 7.40 (m, 2 H), 7.57
(m, 2
H), 7.77 (br s, 1 H), 8.26 (s, 1 H), 8.36 (d, 1 H), 8.43 (br s, 1 H);
Anal. calcd for C ~ SH ~ 2N2OS2: C, 59.97; H, 4.02; N, 9.32. Found: C, 59.83;
H, 4.03 N,
9.11.
127
CA 02333770 2000-11-30
WO 99/62908 PCT/CTS99/12419
Example 133
4-chloro-N-(4-chlorophenyl)thienof 2,3-blpyridine-5-carboxamide
Example 132A was processed as in examples 18 and 19 but substituting 4-
chloroaniline for concentrated NH40H in Example 19 to provide the title
compound.
mp 199-202 °C;
MS (DCI/NH3) m/z 340 (M+NH4)+, 342 (M+NH4)+, 323 (M+H)+, 325 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.6 (m, 2H), 7.62 (d, 1H), 7.77 (m, 2H}, 8.19 (d,
1H),
8.79 (s, 1H);
Anal. calcd for CI4HgC12N20S2: C, 52.03; H, 2.49; N, 8.67. Found: C, 52.02; H,
2.15;
N, 8.50.
Example 134
ethyl 4-f(5-methyl-1,3,4-thiadiazol-2-Yl)thiolthienof2 3-blpyridine-5-
carboxvlate
Example 132A and 5-methyl-1,3,4-thiadiazol-2-thiol were processed as in
Example 17B to provide the title compound.
mp 93-94 °C;
MS (DCI/NH3) m/z 355 (M+NH4)+ and 238 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 1.32 (t, 3 H), 2.66 (s, 3H}, 4.36 (g, 2 H), 7.34
(d, 1H),
8.13 (d, 1H), 9.00 (s, 1H);
Anal. calcd for C13H1 ~N302S3: C, 46.27; H, 3.28; N, 12.45; S, 28.50. Found:
C, 46.04;
H, 3.20; N, 12.32; S, 28.39.
Example 135
7-f(4-methylphenyl)thiolthienof3 2-blpyridine-2-carboxamide
Ethyl 7-chlorothieno[3,2-b]pyridine-6-carboxylate was processed as in Example
17B, 18, and 19 to provide the title compound.
MS (DCl/NH3) m/z 301 (M+H)+;
IH NMR (300 MHz, DMSO-d6) 8 2.38 (s, 3H), 6.83 (d, 1H), 7.37 (m, 2H), 7.56 (m,
2H),
7.83 (br s, 1H}, 8.25 {s, 1H), 8.41 (br s, 1H), 8.53 (d, 1H);
Anal. calcd for CISH12N20S2: C, 59.98; H, 4.03; N, 9.33. Found: C, 59.79; H,
4.01; N,
9.16.
Example 136
methyl 6-f(4-methylphenyl)thiolthienof2 3-blnyridine-2-carboxylate
Example 136A
128
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
2,6,dichloro-3-pyridinecarbonitrile
Example 136B
methyl 3-amino-6-chlorothienof 2,3-blpyridine-2-carboxylate
Example 136A and methyl thiogycolate were processed as in Example 1D to
provide the designated compound.
Example 136C
methyl 6-f (4-methylphenyl)thiolthienof 2.3-b~[pyridine-2-carboxylate
Example 136B (32 g, 1.34 mmoI) in 75% H2S04 (7.4 mL) at 0 °C was
treated
dropwise with aqueous NaN02 (0.24 g/1.5 mL, 3.5 mmol), stirred for 30 minutes,
poured
into cold 50% H2P03 ( 11.8 mL,), stirred for 30 minutes, stored at 0 °C
for 60 hours,
warmed to room temperature, treated with NaHC03, and extracted with ether. The
extract
was dried (MgS04), filtered, and concentrated. The residue was dissolved in
methanol (7
mL), heated to 50 °C, treated sequentially with NaOCH3 (0.08 g, 1.45
mmol) and p-
thiocresol (0.18 g, 1.45 mmol), stirred at room temperature for 18 hours, and
concentrated.
The residue was treated with 10% citric acid and extracted with
dichloromethane. The
extract was dried (MgS04), filtered, and concentrated. The residue was
purified by flash
chromatography on silica gel with 25% ethyl acetate/hexanes to provide the
title
compound.
mp 127-130 °C;
MS (DCI/NH3) m/z 316 (M+H)+;
1HNMR (300 MHz, DMSO-d6) 2.39 (s, 3H), 3.89 (s, 3H) 7.02 (d, 7.36 (m, 2H),
7.57 (m,
2H), 8.13 (s, 1 H), 8.23 (d, 1 H);
Anal. calcd for Ct6H13N02S2: C, 60.93; H, 4.15; N, 4.44. Found: C, 60.79; H,
4.18; N,
4.35.
Example 137
6-f(4-methylphenyl)thiolthienof2 3-blpyridine-2-carboxamide
The title compound can be prepared from Example 136C using the procedure of
Example 44.
Example 138
2-bromo-4-f(4-methylphenyl)thiolthienof3 2-clpyridine
Para-thiocresol (500 mg, 4 mmol} in DMF ( 10 mL) was treated with potassium t-
butoxide (451 mg, 4 mmol) at room temperature, after 15 minutes cooled to 0
°C, treated
129
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
with 2-bromo-4-chlorothieno(3,2-c]pyridine (prepared in 6 steps according to
the method
of F. Eloy and A. Deryckere (Bull. Soc. Chim. Belg. 1970, 79, 301 ) ( 1.0 g,
4.0 mmol),
stirred at 0 °C for 2 hours and at room temperature for 12 hours,
poured into water and
extracted with diethyl ether. The extract was washed with water, dried
(Na2S04), and
concentrated under vacuum. The residue was purified by flash chromatography on
silica
gel with 1:20 ethyl acetate-hexane to provide the title compound.
MS (DCI/NH3) m1z 336, 338 (M+H)+;
1H NMR (300Hz, CDC13) 8 2.36 (s, 3H), 7.18(d, 2H), 7.40 (d, 2H), 7.48 (br s,
1H), 7.52
(br d, I H), 8.16 (d, 1 H).
Example 139
4-f (4-meth~lphenyl)thiolthienof 3,2-clpyridine-2-carboxamide
Example 139A
4-chlorothienof 3,2-clpyridine-2-carbonitrile
A solution of 4-oxo-4,5-dihydrothieno[3,2-c]pyridine-2-nitrite (500 mg, 2.84
mmol) prepared as in F. Eloy and A. Deryckere, Bull. Soc, Chim. Belg. 1970,
79, 301)
and phosphoryl chloride (5 mL) was heated at reflux for 1 hour. The formed red
solution
was poured onto ice and extracted with methylene chloride (2x150 mL). The
dichloromethane solution was dried over anhydrous Na2S04, filtered, and
concentrated.
The residue was purified by flash chromatography with 1:10 EtOAc/hexanes to
provide
the title compound.
MS (DC1/NH3) m/z I95 (M+H)+;
IH NMR (300MHz, CDC13) 8 7.74 (d, 1H), 8.10 (s, 1H), 8.41 (d, 1H).
Example I39B
4-f (4-meth~nhenyl)thiolthienof 3,2-clpyridine-2-carbonitrile
Para-thiocresol ( 192 mg, 1.54 mmol) in DMF (5 mL) at room temperature was
treated with potassium tent-butoxide (173 mg, 1.54 mmol) , stirred for 15
minutes, cooled
to 0 °C, treated with Example 139A (200 mg, 1.03 mmol), stirred first
at 0 °C and then at
room temperature for 48 hours, treated with water, and extracted with
dicholomethane.
The extract was dried (MgS04), filtered, and concentrated. The residue was
purified by
flash chromatography on silca gel with 1:7 ethyl acetate-hexane to provide the
title
compound.
IR (KBr, cm-1) 2200 (w, CN), 1550 (s), 1520 (s) cm-1;
MS (DCI/NH3) m/z 283 (M+H)+;
130
CA 02333770 2000-11-30
WO 99162908 PCT/US99/12419
1H NMR (300 MHz, CDC13) b 2.40 (s, 3H), 7.25 (d, 2H), 7.47 {d, 2H), 7.49 (d,
1H), 8.07
(s, 1H), 8.33 (d, 1H);
Anal. calcd for ClSHtoN2S2: C, 63.80; H, 3.57; N, 9.92. Faund: C, 63.80; H,
3.52; N,
9.98.
Example 139C
4-f (4-methylphenyl)thiolthienof 3.2-clpyridine-2-carboxamide
Example 139B ( 198 mg, 0.7 mmol) in polyphosphoric acid (5 mL) was heated at
110 °C for 3 hours, cooled, treated with water, and extracted with
dichloromethane. The
extract was dried (MgS04), filtered, and concentrated. The residue was
purified by flash
chromatography on silca gel with 4:5 ethyl acetate-hexane to provide the title
compound.
IR(KBr) 3300 (m), 3130 (s), 1660 (s), 1600 (s);
MS (DCI/NH3) m/z 301 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 2.36 (s, 3H), 7.28 (d, 2H), 7.47 (d, 2H), 7.78 (br
s, 1H),
7.84 (d, 1H), 8.19 (d, 1H), 8.34 (s, 1H), 8.46 (br s, 1H);
Anal. calcd for C15H12N20S2: C, 59.98; H, 4.03; N, 9.33. Found: C, 59.77; H,
3.88; N,
9.15.
Example 140
4-(4-meth~phenoxy)thienof 3,2-clpyridine-2-carboxamide
Example 140A
4-(4-methylnhenoxy)thieno(3,2-clpyridine-2-carbonitrile
Example 139A and 4-methylphenol were processed as in Example 139B to provide
the title compound.
IR (KBr) 2200 (w), 1580 (s), 1540 (s), 1500 (s), 1440 (s) cm-t;
MS (DCI/NH3) m/z 267 (M+H)+;
1H NMR (300 MHz, CDCl3) b 2.39 (s, 3H), 7.09 (dt, 2H), 7.25 (br d, 2H), 7.43
(dd, 1H),
8.10 (d, 1H), 8.21 (s, 1H);
Anal. calcd for ClgHtoN2OS: C, 67.65; H, 3.78; N, 10.52. Found: C, 67.60; H,
3.66; N,
10.48.
Example 140B
4-(4-methylphenoxy)thienof 3.2-clpYridine-2-carboxamide
Example 140A was processed as in Example 139C to provide the title compound.
IR (KBr) 3400 (m), 1680 (m), 1650 (s), 1600 (s), 1500 (s) cm-t;
131
CA 02333770 2000-11-30
WO 99162908 PCT/CTS99/12419
MS (DCI/NH3) m/z 285 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.34 (s, 3H), 7.13 (d, 2H), 7.25 (d, 2H), 7.75 (d,
1H),
7.76 (br s, 1H), 7.95 (d, 1H), 8.38 (br s, 1H), 8.41 (s, 1H);
Anal. calcd for C15H12N202S: C, 63.36; H, 4.25; N, 9.85. Found: C, 63.16; H,
4.18; N,
9.77.
Example 141
7-(4-meth~nhenoxy)oxazolof 5,4-clpyridine-2-carboxamide
Example 141 A
3-chloropyridine-N-oxide
Example 141A was processed as in Caldwell and Martin (J. Heterocyclic Chem.,
1980, 17, 989). A solution of 3-chloropyridine ( 15.0 g, 132 mmol) in acetic
anhydride (75
mL) with cooling to maintain an internal temperature below 30 °C was
treated with
hydrogen peroxide (75 mL of 30% aqueous solution), stirred at room temperature
for 3
hours, heated at 60 °C for 18 hours, diluted with water (200 mL),
concentrated, and solid
sodium bisulfate added in portions until peroxides could no longer be detected
(by
enzymatic peroxide test), and the remaining solvent was removed under reduced
pressure.
The residue was triturated with ethyl acetate. The washes were filtered and
concentrated
to provide the designated compound.
Example 141B
4-nitro-3-chloronyridine-N-oxide
Example 141B was processed as in Caldwell and Martin (J. Heterocyclic Chem.,
1980, 17, 989). Example 141A (16.8 g, 130 mmol) in sulfuric acid (25 mL, 98%),
fuming
sulfuric acid (30% 503, 10 mL), and nitric acid (60 mL, 90%~) was heated at
120 °C for 2
hours, cooled to room temperature, poured into ice water (200 mL), solid
ammonium
carbonate was added to bring the solution to pH = 9, and extracted with
methylene
chloride (4 x 100 mL). The extracts were dried (Na2S04) and concentrated. The
residue
was recrystallized from ethyl acetate-hexanes to provide a clean first crop of
the title
compound. Second crop recrystallizations provided mixtures of title compound
and side
products.
MS (DCI/NH3) m/z 194 (3~C1)/192 (35C1), (M+NH4)+; 177 (3~C1)/175 (35C1),
(M+H)+;
1H NMR (CDC13, 300 MHz) 8 8.01 (d, 1H), 8.14 (dd, 1H), 8.32 (d, 1H).
Example 141C
132
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-nitro-3-(4-methylphenox~pyridine-N-oxide
NaH (834 mg, 34.8 mmol) in DMF (20 mL) at room temperature was treated
sequentially with p-Cresol (3.57 g, 33.0 nunol) Example 141B (5.75 g, 32.9
mmol), stirred
for 10 minutes at room temperature and partitioned between ethyl acetate and 1
N aqueous
HCI. The aqueous phase was separated and washed with ethyl acetate. The
organic
phases were washed with I N aqueous HCI, dried (Na2S04), and concentrated.
Recrystallization from diethyl ether provided the title compound.
MS (DC1/NH3) m/z 264 (M+NH4)+, 247 (M+H)+;
1H NMR (CDC13, 300 MHz) 8 2.39 (s, 3H), 7.02 (d, 2H), ?.26 (d, 2H), 7.78 (d,
1H), 7.89
(dd, 1H), 7.98 (d, 1H).
Example 141 D
3-(4-methylphenoxy)-4-pvridinamine
Example 141 C (3.65 g, 14.8 mmol) was dissolved in methanol ( 100 mL), treated
with raney nickel ( 1.00 g), flushed with hydrogen, pressurized to 4 atm., at
37 °C for 2.5
hours, and filtered. The filtrate was concentrated to provide the title
compound.
MS (DCI/NH3) m/z 201 (M+H)+;
1H NMR (CDC13, 300 MHz) 8 2.32 (s, 3H), 4.40 (br s, 2H), 6.68 (br s, 1H), 6.88
(d, 2H),
7.I2 (d, 2H), 8.01 (m, 2H).
Example 141 E
2,2-dimethyl-N-f3-(4-meth~phenoxy)-4-pyridinyllpropanamide
Example 141D (2.80 g, 14.0 mmol) was dissolved in methylene chloride (50 mL},
cooled to 0 °C, treated sequentially with triethylamine ( 1.78 g, 17.6
mmol) and
trimethylacetyl chloride (1.86 g, 15.4 mmol), stirred at room temperature for
15 hours, and
poured into water (100 mL) containing a trace of sodium chloride. The organic
layer was
separated, treated with activated charcoal, filtered through Celite~, washed
with saturated
sodium bicarbonate, dried (Na2S04), and concentrated to provide the title
compound.
MS (DCI/NH3) m/z 285 (M+H)+;
1H NMR (CDCl3, 300 MHz) b 1.24 (s, 9H), 2.35 (s, 3H), 6.93 (d, 2H), 7.18 (d,
2H), 8.14
(br s, 1H), 8.15 (s, 1H), 8.32 (d, 1H), 8.42 (d, IH).
Example 141F
5-Hydroxy-4-(N-trimethylacetyl)amino-3-(4-meth~rlphenoxy)pyridine
The procedure of Chu-Moyer and Berger (J. Org. Chem. 1995, 60, 5721 ) was
adapted. Example 141E (5.50 g, 19.3 mmol) was dissolved in diethyl ether and
cooled to
133
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
-78 °C. tert-Butyllithium (24.0 mL of 1.7 M soluton in pentane, 40.8
mmol) was added
dropwise and let stir 2 h at -78 °C. Trimethylborate (5.01 g, 48.3
mmol) was added, and
the reaction was slowly warmed to room temperature and stirred for 18 h.
Glacial acetic
acid (3.9 mL) was added, followed by the addition of 30% aqueous hydrogen
peroxide
(5.8 mL). The reaction was stirred 2 h at room temperature and then poured
into water.
The resulting mixture was washed twice with CH2C12, and the organic layers
were treated
with activated charcoal and filtered through celite. The filtrate was washed
once with
water, once with brine, dried (Na2S04), and concentrated under reduced
pressure to give a
mixture of two compounds, the higher Rf being desired. The mixture was
purified by flash
silica chromatography using a 40M Biotage cartridge, I.5% methanol in CH2C12
eluent, to
give 0.15 mmol (15% yield) of the desired product and 0.73 mmol (73%) of the
title
compound.
MS (DCI/NH3) m/e 301 (M+H)+;
1H NMR (CDC13, 300 MHz) 8 1.20 (s, 9H), 2.27 (s, 3H), 6.84 (d, 2H), 7.09 (d,
2H), 7.65
{br s, 1H), 8.08 (s, 1H), 8.14 (br s, 1H), 10.26 (br s, 1H).
Example I41 G
5-Hydroxy-4-amino-3-(4-methylphenoxy)pyridine
Example 141F (850 mg, 2.83 mmol) was suspended in 3 N aqueous HCl and
stirred at 90 °C for 18 h. The reaction was then cooled to 0 °C,
neutralized with 6 N
aqueous NaOH, and extracted with CH2Cl2. The organic layers were combined,
dried
(Na2S04), and concentrated under reduced pressure to give the desired product
(612 mg,
100% yield).
MS (DCI/NH3) mle 217 (M+H)+;
1H NMR {300 MHz, DMSO-db) b 2.25 (s, 3H), 5.16 (s, 2H), 6.81 (d, 2H), 7.12 (d,
2H)
7.50 (s, IH), 7.71 (s, 1H), 8.14 (s, IH), 9.55 (br s, 1H).
Example 141H
Methyl Oxazolof5,4-c1-4-(4-methylphenox~pyridine-2-carboxylate
To Example 141 G ( 1.00 mmol) in DMF is added pyridine ( 1. I O mmol) and
methyl
oxalyl chloride ( 1.10 mmol), and the resulting solution is stirred at room
temperature
overnight. The reaction is then partitioned between methylene chloride and 1 N
aqueous
HCI, and the organic phase is separated, dried (Na2S04), and concentrated
under reduced
pressure to give the title compound.
Example 14II
134
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Oxazolof5,4-cl -4-(4-methylphenox~)pyridine-2-carboxamide
Example 141H may be treated according to the procedure of Example 44 to give
the title compound.
Example 142
7-(4-Meth r~lphenoxy)f 1,31thiazolof 5,4-clpyridine-2-carboxamide
Example 142A
5-(N.N-Dimethylthiuram)sulfide-4-(N-trimethylacetyl)amino-3-(4-
meth~phenox~pyridine
Example 141E (284 mg, 1.00 mmol) in diethylether (12 mL) at -78 °C was
treated
dropwise with tert-butyllithium ( 1.3 mL of 1.7 M solution in pentanes, 2.21
mmol,
Aldrich) followed by stirring for 3 h at -78 °C. Tetramethyl thiuram
disulfide (529 mg,
2.20 mmol) was added to the resultant dianion, and stirring and temperature
elevation was
continued for 18 h. The reaction was quenched with water and extracted with
CHzCl2.
The organic phase is dried (Na2S04) and concentrated under reduced pressure.
The title
compound (50 mg, 12% yield) was isolated by flash silica gel column
chromatography.
MS (DCI/NH3) m/e 404 (M+H)+;
1H NMR (CDCl3, 300 MHz) 8 2.32 (s, 3H), 3.55 (s, 3H), 3.58 (s, 3H), 6.92 (d,
2H), 7.12
(d, 2H), 7.93 (s, 1H), 8.43 (s, 1H), 8.45 (s, 1H}.
Example 142B
5-(N,N-Dimethylthiuram)sulfide-4-amino-3-(4-methylphenoxy)pyridine
Example 142A (270 mg, 0.67 mmol) was combined with formic acid (20 mL of
96%, Aldrich) and stirred at 90 °C for 72 hr. The reaction is then
cooled to room
temperature, and the formic acid is removed under reduced pressure. The
resulting residue
is purified by flash silica gel chromatography (70% EtOAc in hexane) to
provide the title
compound (96 mg, 45% yield}.
MS (DCI/NH3) m/e 320 (M+H)+;
'H NMR (CDCI~, 300 MHz) 8 2.33 (s, 3H), 3.56 (s, 3H), 3.58 (s, 3H), 4.93 (br
s, 2H),
6.94 (d, 2H), 7.15 (d, 2H), 8.04 (s, 1H), 8.12 (s, 1H).
Example 142C
5-(N N-Dimethylthiuram)sulfide-4-methyloxamate-3-(4-methylphenoxy)pyridine
135
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 142B (90 mg, 0.28 mmol) was combined with CHzCl2 (7.0 mL). Triethyl
amine {0.39 mL, 2.2 mmol) was added, followed by the addition of methyl oxalyl
chloride
( 120 mL, 1.30 mmol, Aldrich). After 6 h, the mixture was combined with
saturated
aqueous sodium bicarbonate and extracted thrice with CH2C12. The organic
layers were
combined, dried (Na2S04), filtered, and concentrated under reduced pressure.
MS (DCI/NH3) m/e 406 (M+H)+;
'H NMR (CDC13, 300 MHz) 8 2.33 (s, 3H), 3.54 (s, 3H), 3.58 (s, 3H), 3.90 (s,
3H), 7.00
{d, 2H), 7.15 (d, 2H), 8.37 (s, 1H), 8.41 (s, 1H), 9.20 (br s, 1H).
Example 142D
Methyl 4-(4-Methylphenoxy)thiazolof5,4-cl-pyridine-2-carboxylate
Example 142C (50 mg, 0.12 mmol) was dissolved in formic acid ( 14 mL, 96%,
Aldrich), and heated to reflux. After 4 h, the reaction was cooled, and
volatiles were
removed. Flash silica gel column chromatography (60% EtOAc in hexane) provided
the
title compound ( 15 mg, 39 % yield) as a white solid.
MS (DCI/NH3) m/e 301 (M+H)+, 318 (M+NH3)+;
'H NMR (CDC13, 300 MHz) 8 2.39 (s, 3H), 4.10 (s, 3H), 7.08 (d, J=8.5 Hz, 2H),
7.22 (d,
J=8.5 Hz, 2H), 8.14 (s, 1H), 9.00 (s, 1H).
Example 142E
4-(4-Methylphenoxy)thiazolof5,4-cl-pyridine-2-carboxamide
Example 142D (2.0 mg, 6.7 mmol) was treated according to the procedure of
Example 44 to give the title compound (1.5 mg, 75%) as a white solid.
MS (DCUNH3) m/e 286 (M+H)+, 303 {M+NH3)+;
1H NMR (CDCI~, 300 MHz) b 2.39 (s, 3H), 5.66 (br s, 1H), 7.06 (d, J=8.2 Hz,
2H), 7.22
(d, J=7.8 Hz, 2H}, 7.32 (br s, 1H), 8.20 (s, 1H), 9.04 (s, 1H).
Example 143
7-(4-methylnhenoxy)-3H-imidazof4.5-clpyridine-2-carboxamide
Example 143A
N-f3-amino-5-(4-meth~phenoxy)-4-pyridinyll-2 2-dimethylpropanamide
Example 141F (1.00 mmol) is suspended in ammonium hydroxide (28%),
saturated with sulfur dioxide, heated to 150 °C in a pressure vessel
for 27 hours, and
cooled, and extracted with ethyl acetate. The extract is concentrated to
provide the title
compound.
136
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 143B
5-(4-meth~phenoxy)-3,4-pyridinediamine
Example 143A ( 1.00 mmol) is suspended in HCl (3N aqueous) and stirred at 90
°C
for 18 hours, cooled to 0 °C, neutralized with 6 N aqueous NaOH, and
the water removed.
The resulting residue is triturated with methanol. The washes are concentrated
to provide
the title compound.
Example 143C
methyl 7-(4-methylphenox~r)-3H-imidazo(4,5-clpyridine-2-carbo~late
Example 143B ( 1.00 mmol) in DMF is treated with pyridine ( 1.10 mmol) and
methyl oxalyl chloride ( I .10 mmol), stirred at room temperature overnight,
and partitioned
between methylene chloride and 1 N aqueous HCI. The organic phase is
separated, dried
(Na2S04), and concentrated to provide the title compound.
Example 143D
7-(4-methylnhenoxy)-3H-imidazo(4,5-clpyridine-2-carboxamide
Example 143C can be treated as in Example 44 to give the title compound.
Example 144
4-(4-chlorophenox~)thieno(2,3-dlpyridazine-2-carboxamide
Example 144A
3-Bromothiophene-2-carboxaldehyde
Example 144A can be processed as in Prugh, et. al. (J. Med. Chem. 1991, 34,
1805). A solution of dibromothiophene ( 14 g, 58 mmol) in THF ( 100 mL) at -78
°C was
treated with n-butyliithium (24 mL, 59 mmol), stirred for 15 minutes, treated
with dry
DMF (6.8 mL, 87 mmol), stirred at -78 °C for 10 minutes, slowly warmed
to 0 °C over a
period of 15 minutes, poured into cold 1 N aqueous HCI, and extracted with
diethyl ether.
The extract was washed with I N aqueous HCI, water, and saturated sodium
bicarbonate.
The washes were extracted with diethyl ether. The organic layers were
combined, dried
(MgS04), filtered, and concentrated under reduced pressure. The residue was
purified by
flash chromatography on silica gel with 5% ethyl acetate/hexanes to provide
the title
compound.
1H NMR (CDC13, 300 MHz) S 7.16 (d, 1H), 7.74 (d, 1H), 10.0 (s, 1H).
137
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
Example 144B
3-bromo-2-(2-dioxolan I)thiophene
Example 144B can be processed as in Prugh, et. al. (J. Med. Chem. 1991, 34,
1805). A three-necked flask equipped with a Dean-Stark trap is charged with
Example
144A (5.24 g, 27.4 mmol), ethylene glycol (6.2 mL, 110 mmol), pyridinium
tosylate (276
mg, 1.10 mmol), and toluene (30 mL), heated at reflex for 14 hours, cooled to
room
temperature, poured into water, and extracted with diethyl ether. The organic
layer is
washed with water and saturated sodium bicarbonate, dried (MgS04), filtered,
and
concentrated. The residue is purified by flash chromatography on silica gel
with 5% ethyl
acetate/hexanes to provide the title compound.
MS (DCI/NH3) m/z 252 (~9Br)/254 (glBr), (M+NH4)+; 235 (~9Br)/237 (glBr),
(M+H)+;
iH NMR (CDCl3, 300 MHz) 8 4.11-4.02 (m, 4H), 6.14 (s, 1H), 6.97 (d, 1H), 7.30
(d, 2H).
Example 144C
ethyl-2-(2-dioxylan 1)~phene-3-carboxylate
Example 144C can be processed as in Prugh et al. (J. Med. Chem., 1991, 34,
1805). Example 144B ( 1.00 g, 4.25 mmol) in THF ( 12 mL) is treated with n-
butyllithium
(1.7 mL, 4.25 mmol) while maintaining temperature between -105 and -95
°C, treated with
diethyl carbonate (0.57 mL, 4.68 mmol), and warmed to room temperature. The
solution
is poured into water and extracted with diethyl ether. The extract is washed
with brine,
dried (MgS04), filtered, and concentrated under reduced pressure. The residue
is purified
by flash chromatography on silica gel 5% ethyl acetate/hexanes to provide the
title
compound.
Example 144D
2-formylthiophene-3-carboxylic acid
Example 144C ( 1.0 mmol) and ethanol ( 1.0 mL) are treated with 1 N aqueous
sodium hydroxide solution ( 100 mmol), stirred for 1 hour, brought to pH=5 by
the addition
of glacial acetic acid, stirred for 1 hour, diluted with water, and extracted
with ethyl
acetate. The extracts are combined, washed with brine, washed with saturated
sodium
bicarbonate, dried (MgS04), filtered, and concentrated. The designated
compound is used
without further purification.
Example 144E
4-oxothieno f 2,3-dlpyridazine
Example 144D is processed as in Bull. Soc. Chim. Fr. 1967, 2495.
138
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 144F
4-chlorothienof2,3-dlpyridazine
Example 144E is processed as in Bull. Soc. Chim. Fr. 1967, 2495.
Example 1446
4-(4-Chlorophenoxy)thienof2,3-dlpyridazine
Adapting the method of Robba and others (Bull. Soc. Chim. Fr., 1967, 4220),
Example 144F ( I OOmg, 0.59 mmol) was combined with 4-chlorophenol ( 1.0 mL,
10.0
mmol) and sodium metal (21 mg, 0.90 mmol). The mixture was heated at 100
°C for 14
h. After cooling to room temperature, the residue was diluted with chloroform
and
washed once with 2 N aqueous sodium hydroxide and once with brine. The organic
layer
was dried (MgS04), filtered, and concentrated under reduced pressure.
Recrystallization
from diethyl ether gave the title compound (124 mg, 85%) as a white solid.
MS (DCI/NH3) mle: 363 (M+H)+;
'H NMR (CDC13, 300 MHz) d 7.27 (d, J=8.9 Hz, 2H), 7.42 (d, J=8.9 Hz, 2H), 7.72
(d,
J=5.5 Hz, 1H), 7.88 (d, J=5.2 Hz, 1H), 9.41 (s, IH).
Example 144H
4-(4-chlorophenoxy)thienof2,3-dlpyridazine-2-carbox lic acid
Example 1446 (1.0 mmol) in THF (1.0 mL) at -78 °C is treated with
n-
Butyllithium ( 1.0 mmol), stirred for 15 minutes, saturated with C02, slowly
warmed to
room temperature, partitioned between 1 N aqueous sodium hydroxide and diethyl
ether,
separated, and glacial acetic acid added to the aqueous layer until pH=5. The
acidic
solution is extracted thrice with methylene chloride. The extracts are
combined, washed
with dilute sodium bicarbonate, dried (MgS04), filtered, and concentrated to
provide the
title compound.
Example 144I
4-(4-chlorophenoxy)thieno f 2,3-dlpyridazine-2-carbamide
Example 144I can be processed as in Desai and Stramiello (Tetrahedron Lett,
1993, 34, 7685). Example I44H ( 1.0 mmol), 1-hydroxybenzotriazol ( 1.4 mmol),
N-
methylmorpholine ( 12 mmol), and DMF ( 1.0 mL) are combined and cooled to 0
°C,
treated with 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (1.4
mmol),
stirred at 0 °C for 1 hour, and partitioned between methylene chloride,
saturated sodium
139
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
bicarbonate, and separated. The extract is dried (MgS04), filtered, and
concentrated.
Recrystallization from hot methanol will provide the title compound.
Example 145
7-(4-chlorophenoxy)thienof 3,2-clpyridine-2-carbamide
Example 145A
2,5-dibromo-3-thiophenecarboxaldehyde
A solution of 3-thiophenecarboxaldehyde is processed as in Bull. Soc. Chim.
Fr.,
1974, 3040 to provide the title compound.
Example 145B
dimethylacetyl-(2,5-dibromo-3-thienyl)carboximino ethane
Example 145A is processed as in Bull. Soc. Chim. Fr., 1974, 3040 to provide
the
title compound.
Example 145C
6.7-dihydro-7-oxothieno(3,2-clpyridine
Example 145B is processed as in Bull. Soc. Chim. Fr., 1974, 3040 to provide
the
title compound.
Example 145D
7-(4-chloro~henoxy)thienof 3,2-clp~idine
Example 145D can be processed as in Lindley (Tetrahedron, 1983, 1433).
A solution of Example 145C ( 1.0 mmol) and DMF (2.0 mL) is treated at 0
°C with sodium
hydride ( 1.0 mmol), slowly warmed to room temperature, treated with 1-chloro-
4-
iodobenzene (1.0 mmol) and copper iodide (0.1 mmol), heated at 80 °C
overnight, and
cooled. The solution is poured into water and extracted with diethyl ether.
The extracts
are combined, dried (MgS04), filtered, and concentrated. Recrystallization
from ethyl
acetate/hexanes provides the title compound.
Example 145E
7-(4-chlorophenoxy)thienof3,2-clpyridine-2-carbox lic acid
The designated compound is processed in the manner described for Example 144H.
Example 145F
140
CA 02333770 2000-11-30
WO 99/62908 PGTNS99/12419
7-(4-chlorophenoxy)thienof3,2-clpyridine-2-carboxamide
The designated compound is processed in the manner described for Example 144I.
Example 146
4-(4-Chlorophenoxy)thienof2,3-clpyridine-2-carbothioamide A solution of
Example
61 (50 mg, 0.16 mmol) and Lawesson's reagent (73 mg, (0.18 mmol) in toluene {2
mL)
was heated at reflux for 4 hours. The solvent was removed under reduced
pressure to
obtain the crude product (150 mg) as a yellow residue. The purified title
compound (24
mg, 47%) was obtained by flash chromatography on silica gel eluting with 10%
methanol
in dichloromethane.
MS (DCI/NH3) m/e 321 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 7.14 (m, 2H), 7.47 (m, 2H), 8.18 (s, 1H), 8.21 (s,
1H),
9.14 (s, 1H); 9.86 (s, 1H);10.15 (s, 1H).
Example 147
4-(4-Chlorophenoxy)-N-ethylthieno(2,3-clpyridine-2-carboxamide
Example 61A (200 mg, 0.627 mmol) was prepared as in Example 171 but
substituting ethylamine ( 1 ml, 17.65 mmol} for methylamine to provide the
title compound
(209 mg, 100%).
MS (DCI/NH3) m/e 333 (M+H)+, 303;
'H NMR (400 MHz, DMSO-d6) 8 1.14 (t, J=8Hz, 3H), 3.30 {m, 2H), 7.14 (d, J=9Hz,
2H),
7.47 (d, J=9Hz, 2H, 8.13 (s, 1H), 8.17 (s, 1H), 8.91 (t, J=6Hz, 1H), 9.15 (s,
1H).
Example 148
4-(4-Chloronhenoxy)-N-(2,3-dihvdroxypropyl)thienof2 3-clpyridine-2-carboxamide
Example 148 was prepared in a similar manner as Example 103A, by combining 3-
amino-1,2-propanediol (60 mL, 0.782 mmol) with Example 61A (250 mg, 0.782
mmol) to
provide the title compound (133 mg, 45% yield) as a white solid.
mp 106-115 °C;
MS (DCIlNH3) m/e: 379 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 3.16 (m, 2H), 3.38 (m, 2H), 3.63 (m, 1H), 4.56 (t,
J=5.7
Hz, 1H), 4.81 (d, J=5.1 Hz, IH), 7.13 (d, J=9.2, 2H), 7.47 (d, J=9.2 Hz, 2H),
8.18 (s, 1H),
8.21 (s, 1H), 8.95 (t, J=5.7 Hz, 1H), 9.16 (s, 1H);
Anal. calcd for C»H,5C1N204S~0.25 HZO: C, 53.27; H, 4.08; N, 7.31. Found: C,
53.19; H,
4.22; N, 6.22.
141
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 149 4-(4-Bromophenoxy)-N-(2,3-dihydroxypropyl)thienof2,3-clpyridine-2
carboxamide
Example 149 was prepared according to the procedure of Example 114, with the
substitution of methyl 4-(4-bromophenoxy)-thieno[2,3-c]pyridine-2-carboxylate
for
Example 61 A.
mp 76-77 °C;
MS (DCI/NH3) m/e: 423,425 (M+H)+;
IH NMR (300 MHz, DMSO-d6) b 3.12 (m, 2H), 3.41 (m, 3H), 3.63 (m, 2H), 7.06 (d,
2H,
J=8.8 Hz), 7.59 (d, 2H, J=8.8 Hz), 8.20 (s, 1H), 8.22 (s, 1H), 8.99 (t, 1H,
J=5.5 Hz), 9.18
(s, 1H).
Example 150
N-(2-Chloroethyl)-4-(4-chlorophenoxy)thienof 2,3-clpyridine-2-carboxamide
To a solution of Example 114 (0.32 g, 0.92 mmol) in anhydrous THF (5 mL) was
slowly added thionyl chloride (0.34 mL, 4.60 mmol). The reaction was heated to
50 °C
for 18 hr, cooled to room temperature, and neutralized with sat. NaHC03. The
aqueous
suspension was extracted with dichloromethane (100 mL) arid the organic layer
washed
with dilute NaHC03 (2 x 100 mL), brine (50 mL), partially dried (Na2S04), and
concentrated to a solid. The crude product was purified by flash
chromatography on silica
gel using EtOAc/hexane as eluent to yield the title compound as a solid (0.27
g, 80%).
mp 60-62 °C (dec);
MS (DCI-NH3) m/e 367 (M+H)+;
IH NMR (300 MHz, DMSO-d6) 8 3.60 (m, 2H), 3.75 (t, J=6.lHz, 2H), 7.15 (m, 2H),
7.48
(m, 2H), 8.18 (s, 1H), 8.18 (s, 1H), 9.17 (s, 1H), 9.26 (m, 1H);
Anal. calcd for C~6H~ZC12N2OZS: C, 52.33; H, 3.29; N, 7.63. Found: C, 52.22;
H, 3.47;
N, 7.35.
Example 151
4-(4-Bromophenoxy)-N-(2-h~yethyl)thienof2,3-clpyridine-2-carboxamide
Example 151 was prepared according to the procedure of Example I 14, with the
substitution of methyl 4-(4-bromophenoxy)-thieno[2,3-c]pyridine-2-carboxylate
for
Example 61 A.
mp 158-159 °C;
MS (DCI/NH3) m/e: 393,395 (M+H)+;
142
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
1H NMR (300 MHz, DMSO-db) S 3.32 (m, 2H), 3.51 (m, 3H), 4.79 (t, 1H, J=5.9
Hz), 7.06
(d, 2H, J=8.8 Hz), 7.59 (d, 2H, J=8.8 Hz), 8.17 (s, 1H), 8.2U (s, 1H), 9.02
(t, 1H, J=5.5
Hz), 9.17 (s, 1H).
Example 152
4-(2-Bromo-4-chloronhenoxy)-N-(2-hydroxyethyl)thienof 2,3-clpyridine-2-
carboxamide
Example 152 was prepared according to the procedure of Example 114, with the
substitution of 2-bromo-4-chlorophenol for 4-chlorophenol.
MS (DCI/NH3) m/e: 428 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.86 (q, 1H, J=5.4 Hz), 3.34 (m, 2H), 3.55 (m,
2H);
7.15 (d, 1H, J=8.8 Hz), 7.48 (dd, 1H, J=2:4, 8.8 Hz), 7.97 (d, 1H, J=2.7 Hz},
8.02 (s, 1H),
8.25 (s, 1H), 9.05 (t, 1H, J=5.4 Hz), 9.16 (s, 1H).
Example 153
N-(2-Hydroxyethyl)-4-f4-(trifluoromethyl)nhenoxylthienof2 3-clpyridine-2-
carboxamide
A solution of Example 62A (322 mg, 0.912 mmol) in 10 mL of dichloromethane
was treated with 226 mg (3.65 mmol) of ethanolamine. The solution was heated
to reflux
for 4 hours. Upon cooling crystals formed. Recrystallization from ethyl
acetate afforded
170 mg (48.8%) of white crystals.
mp 181-182 °C;
MS (DCI/NH3) m/e: 383 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 3.3-3.4 (m, 2H), 3.5-3.6 (m, 2H), 4.80 (t, 1H),
7.21 (d,
2H), 7.79 (d, 2H), 8.15 (s, 1H}, 8.38 (s, 1H), 9.01 (t, 1H), 9.25 (s, 1H);
Anal. calcd for C17H13F3N2O3S: C, 53.40; H, 3.43; N, 7.33. Found: C, 53.41; H,
3.62; N,
7.30.
Example 154
N-(2-Aminoethyl)-4-(4-chloronhenoxy)thieno f 2,3-clpyridine-2-carboxamide
To a suspension of Example 88 (0.50 g, 1.64 mmol} in anhydrous methylene
chloride ( 15 mL) was added N,N-diisopropylethylamine (0.57 mL, 3.28 mmol).
The
reaction was cooled in an ice bath and pivaloyl chloride (0.24 mL, 1.97 mmol)
was slowly
added. After 10 minutes, the ice bath was removed and reaction stirred at
ambient
temperature for 1.5 hr. The reaction contents were slowly transferred via
cannula into an
anhydrous solution of ethylenediamine (0.33 mL, 4.92 mmol) in methylene
chloride (5
mL) at 0 °C over a period of 5 minutes. The reaction was stirred 15
minutes and the cold
bath was removed. The reaction was stirred 1 hr and then partitioned between
143
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
CHCI~/Sat.NaHC03. The organic layer was washed with dilute aqueous NaHC03,
brine,
dried (Na2S04), and concentrated to yield a light yellow foam. The crude
product was
purified by preparative HPLC using a gradient of 25%-65% acetonitrile/water +
0.1 % TFA
over 40 minutes. The product was neutralized with saturated aqueous NaHC03,
precipitate collected by filtration, and dried in a desiccator to yield the
title compound as a
white solid (0.45 g, 79%).
mp 111-114 °C;
MS (APCI) m/e 348 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.69 (t, J=6.4Hz, 2H), 3.25 (t, J=6.4Hz, 2H), 7.14
(m,
2H), 7.47 (m, 2H), 8.16 (s, 1H), 8.18 (s, 1H), 9.15 (s, 1H).
Example 155
4-(4-Chlorophenoxy)-N-hydroxythienof 2,3-clpyridine-2-carboxamide
Example 155 was prepared as in Example 92 by combining Example 88 ( 161 mg,
0.527 mmol) with hydroxylamine hydrochloride (37.0 mg, 0.527 mmol) to provide
the
title product (40 mg, 24% yield) as a white solid.
mp 145 °C (decomposes);
MS (DCI/NH3) m/e: 321 (M+H)+;
'H NMR (300 MHz, CD30D) S 7.11 (d, J=9.2 Hz, 2H), 7.42 (d, J=9.2 Hz, 2H), 7.90
(s,
1H), 8.05 (s, 1H), 9.01 (s, 1H);
IR (KBr) 3200-2800 (m), 1660 (s), 1640 (s), 1560 (m), 1485 (s), 1420 (s) cm-1;
Anal. calcd for C14H9CIN203S+0.25 H20: C, 51.70; H, 2.94; N, 8.61. Found: C,
51.64; H,
2.71; N, 8.80.
Example 156
4-(4-Chlorophenoxy)thieno f 2,3-clpyridine-2-carbohydrazide
Example 61 A (0.50 g, 1.56 mmol) was dissolved in dichloromethane ( 10 mL) and
anhydrous hydrazine (1 mL) was added. After 24 hours, the precipitate was
collected by
filtration and washed with dichloromethane (2 x 25 mL) and water (2 x 50 mL).
The
product was dried in a desiccator to yield the title compound as a white solid
(0.35 g,
70%).
mp 197-199 °C;
MS (APCI) m/e: 320 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 4.62 (broad s, 2H), 7.12 (m, 2H), 7.46 (m, 2H),
8.03 (s,
1H), 8.19 (s, 1H), 9.15 (s, 1H), 10.24 (br s, 1H);
144
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Anal. calcd for Cl4HioC1N3O2S: C, 52.59; H, 3.15; N, 13.14. Found: C, 52.59;
H, 3.12; N,
13.18.
Example 157
4-(4-BromophenoxY)thienof2,3-clpyridine-2-carboh drazide
Example 73A (0.21 g, 0.58 mmol) was dissolved in dichloromethane (2 mL) and
anhydrous hydrazine ( 1 mL) added. After 18 hours, the precipitate was
collected by
filtration and washed with dichloromethane (2x5 mL), water (2x5 mL),
acetonitrile (2x5
mL} to yield white solid. The washes were combined, diluted with sat. NaHC03
(100 mL)
and extracted with dichloromethane (4x25 mL). Organic extracts were combined,
partially dried (Na2S04), and concentrated to yield a white solid which was
combined with
the collected precipitate and dried in a desiccator (0.21 g, 99%).
mp 176-178 °C;
MS (APCI) m/e: 364 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 4.62 (br s, 2H), 7.06 (m, 2H), 7.58 (m, 2H), 8.02
(s,
1H), 8.21 (s, 1H), 9.16 (s, 1H), 10.24 (br s, 1H);
Anal calcd for C~4H~oBrN302S: C, 46.17; H, 2.77; N, 11.54. Found: C, 46.08; H,
2.90; N,
11.41.
Example 158
4-f4-(Trifluoromethyl)phenoxylthieno f 2,3-clpyridine-2-carbohydrazide
Example 62A was treated according to the procedure of Example 157 to provide
the title compound.
mp 146-147 °C;
MS (ESI) m/e: 353.9 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 3.18 (d, 2H), 4.11 (t, 1H), 7.19 (d, 2H}, 7.77 (d,
2H),
7.78 (s, 1H), 8.36 (s, 1H), 9.21 (s, 1H).
Example 159
2-(~ f4-(4-Chlorophenoxy)thienof2,3-clpyridin-2~rllcarbonyl?amino)acetic acid
Example 159A4-(4-Chlorophenoxy)thienof2,3-clpyridin-2-carboxylic acid
A suspension of Example 61A (354 mg, 1.11 mmol) in 3 mL methanol and 1 mL
water was treated with lithium hydroxide monohydrate (98 mg, 2.33 mmol) and
the
resulting mixture was stirred at room temperature (under NZ) for 20 hours. The
reaction
was acidified with 0.13 mL of 90% formic acid, and the white suspension was
stirred for 5
145
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
minutes, then the solid was isolated by suction filtration. The solid was
washed
sequentially with 15 mL water and 5 mL diethyl ether, then dried under vacuum
to provide
302 mg (89%) of the title compound.
MS (DCI-NH3) m/e: 306, 308 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 7.15 (m, 2H), 7.47 (rn, 2H), 7.82 (s, 1H}, 8.23
(s, 1H),
9.21 (s, 1H), 13-15 (vbr s, 1H);
Anal. calcd for CI~HgC1N03S: C, 55.00; H, 2.64; N, 4.58. Found: C, 54.77; H,
2.60; N,
4.44.
Example 159B
Methyl 2-( ( f4-(4-Chloronhenoxy)thienof 2,3-clpyridin-2-yllcarbonyl l
amino)acetate
The title compound was prepared from Example 159A in analogy to Example 92,
with the substitution of glycine methyl ester hydrochloride for ammonium
chloride.
HPLC: Supelco C-18 column, eluent gradient of water:acetonitrile 0:90-90:0
over 30
minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 19.04 minutes;
MS (APCI) m/e: 377 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 3.68 (s, 3H), 4.05 (d, J=6 Hz, 2H), 7.13 (m, 2H),
7.49
(m, 2H), 8.19 (s, 1H), 8.21 (s, 1H), 9.19 (s, 1H), 9.50 (br t, J=6 Hz, 1H);
Anal. calcd for C1~H13C1N204S: C, 54.19; H, 3.48; N, 7.43. Found: C, 53.92; H,
3.61; N,
7.52.
Example 159C
2-(( f4-(4-Chlorophenoxy)thienof2,3-clnyridin-2-yllcarbonyllamino)acetic acid
The title compound was prepared from Example 159B using the procedure of
Example 18 to provide the title compound.
MS (APCI) m/e: 363 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 3.95 (d, J=6 Hz, 2H), 7.13 (m, 2H), 7.48 (m, 2H),
8.19
(s, 1H), 8.21 (s, 1H), 9.19 (s, 1H), 9.41 (br t, J=6 Hz, 1H), 12.77 (br s,
1H);
Anal. calcd for C~6Hi1C1N204S~1 H20: C, 50.46; H, 3.44; N, 7.36. Found: C,
50.33; H,
3.38; N, 7.29.
Example 160
N-(2-Amino-2-oxoethyl )-4-(4-chlorophenoxy)thienof 2,3-clpyridine-2-
carboxamide
The title compound was prepared from Example 159C in analogy to Example 92.
mp 222-225 °C;
MS (APCI) m/e: 362 (M+H)+;
146
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
'H NMR (300 MHz, DMSO-d6) 8 3.82 (br s, 2H), 7.12 (m, 2H), 7.49 (m, 2H), 8.19
(s,
1H), 8.21 (s,lH), 9.20 (s,lH), 9.29 (br s, 1H);
Anal. caled for C~6H,ZCIN303S~1.35 HzO: C, 49.77; H, 3.84; N, 10.88. Found: C,
49.86;
H, 3.79; N, 10.59.
Example 161
N-(2-Amino-2-oxoethyl )-4-(4-bromouhenoxy)thienof 2,3-clpyridine-2-carboxamide
Example 161 A
Methyl N-(2-amino-2-oxoethyl)-4-(4-bromonhenoxy)thieno(2 3-clpyridine-2-
carboxylate
Example 73A was hydrolyzed according to the procedure of Example 18. The
derived carboxylic acid was coupled to glycine methyl ester hydrochloride in
analogy to
Example 92.
MS (DCI/NH3) m/e: 421 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 370 (s, 3H), 4.11 (br d, 2H), 7.11 (br d, 2H),
7.61 (br d,
2H), 8.22 (br d, 2H}, 9.19 (s, 1H), 9.51 (br t, 1H);
i3C NMR ( 100 MHz, DMSO-d6) 8 41.15, 51.84, 104.95, 115.57, 119.74, 119.97,
132.93,
133.32, 137.36, 138.04, 141.45, 145.06, 147.03, 156.10, 161.09, 169.80;
Anal. calcd for C1~H~3BrN204S: C, 48.47; H, 3.1 l; N, 6.65. Found: C, 48.16;
H, 3.27; N,
6.65.
Example 161 B
2-(if4-(4-Bromophenoxy)thienof2,3-clpyridin-2-yllcarbonyl~amino)acetic acid
The title compound was prepared from Example 161 A using the procedure of
Example 18 to provide the title compound.
MS (DCI/NH3) m/e: 407 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 4.11 (br d, 2H), 7.10 (br d, 2H), 7.60 (br d, 2H),
8.21
(br d, 2H), 9.19 (s, 1H), 9.40 (br t, 1H);
"C NMR ( 100 MHz, DMSO-d6) 8 41.,50, 115.85, 120.00, 120.09, 133.25, 133.67,
137.76,
138.34, 141.79, 145.74, 147.29, 156.46, 161.28, 171.05;
Anal. calcd for C16H> >BrN204S~HZO: C, 45.19; H, 3.08; N, 6.59. Found: C,
45.20; H,
3.15; N, 6.45.
Example 161 C
N-(2-Amino-2-oxoethyl)-4-(4-bromophenoxy)thienof2 3-clpyridine-2-carboxamide
The title compound was prepared from Example 161B in analogy to Example 92.
147
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
MS (APCI) m/e: 406 (M+H)+;
~H NMR (300 MHz, DMSO-d6) 8 3.81 (br d, J= 6 Hz, 2H), 7.08 (m, 2H), 7.10 (br
s, 1H),
7.60 (m, 2H), 8.19 (s, 1H), 8.22 (s, 1H), 9.20 (s, 1H), 9.28 (br t, J= 6 Hz,
1H);
Anal. calcd for Cl6HizBrN303S~0.3 H20: C, 46.68; H, 3.09; N, 10.21. Found: C,
46.68;
H, 3.30; N, 10.16.
Example 162
N-f(1S)-2-Amino-1-(hydroxymethyl)-2-oxoethyll-4-(4-chlorophenoxy)thienof2 3
clpyridine-2-carboxamide
Example 162A
(2S)-2-(f f4-(4-Chlorophenoxy)thienof2,3-clpyridin-2-yllcarbonyllamino)-3
hydroxypropanoic acid
Example 162A was prepared in analogy to Example 92, with the substitution of L-
serine ethyl ester hydrochloride for ammonium chloride. The intermediate ester
was then
hydrolyzed according to the procedure of Example 18 to provide the title
compound.
HPLC: Supelco C-18 column, eluent gradient of water:acetonitrile 0:90- 90:0
over 30
minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 15.93 minutes;
MS (APCI) m/e: 392 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 3.70 (m, 2H), 4.43 (br t, 2H), 5.04 (br s, 1H),
7.13 (m,
3H), 7.49 (m, 3H), 8.18 (s, 1H), 8.37 (s, 1H), 8.98 (br s, 1H), 9.18 (s, 1H).
Example 162B
N-f ( 1 S)-2-Amino-1-(hydroxymethyl)-2-oxoethyll-4-(4-chlorophenoxy)thieno f 2
3-
cip~yridine-2-carboxamide
Example 162B was prepared from Example 162A in analogy to Example 92.
HPLC: Supelco C-18 column, eluent gradient of water:acetonitrile 0:90- 90:0
over 30
minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 15.93 minutes;
MS (APCI) m/e: 392 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 3.70 (m, 2H), 4.43 (br t, 2H), 5.04 (br s, 1H),
7.13 (m,
3H), 7.49 (m, 3H), 8.18 (s, 1H), 8.37 (s, 1H), 8.98 (br s, 1H), 9.18 (s, 1H).
Example 163
148
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
(2R)-2-( ( f4-(4-Chlorophenoxy)thienof 2,3-clpyridin-2-yllcarbonyl ) amino)-3
hydroxynropanoic acid
Example 163 was prepared in analogy to Example 92, using D-serine methyl ester
hydrochloride in place of ammonium chloride. The derived ester was hydrolyzed
according to the procedure of Example 18 to give the title compound.
HPLC: Supelco C-18 column, eluent gradient of water:acetonitrile 0:90- 90:0
over 30
minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 16.47 minutes;
MS (ESI} m/e: 393 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 3.78 (br d, 2H), 4.49 (m, IH), 5.02 (br s, 1H),
7.13 (m,
2H), 7.48 (m, 2H), 8.18 (s, 1H), 8.39 (s, 1H), 9.14 (d, 1H), 9.18 (s, 1H),
12.81 (br s, 1H).
Exa J~le 164
4-(4-Chlorophenoxy)-N-f ( 1 R)- I -methyl-2-(methylamino)-2-oxoethyllthienof
2.3
c~[pyridine-2-carboxamide
Example 164A
2R)-2-(~~-(4-Chloronhenoxy)thienof2,3-clnyridin-2-yllcarbonvllamino)nronanoic
acid
Example 164A was prepared in analogy to Example 92, using D-alanine methyl
ester hydrochloride in place of ammonium chloride. The derived ester was
hydrolyzed
according to the procedure of Example 18 to give the title compound.
HPLC: Supelco C-18 column, eluent gradient of water:acetonitrile 0:90- 90:0
over 30
minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 18.36 minutes;
MS (DCI/NH3) m/e: 377 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 1.40 (d, J=7 Hz, 3H), 4.41 (g, J=7 Hz, 1H), 7.15
(m,
2H), 7.49 (m, 2H), 8.19 (s, 1H), 8.32 (s, 1H), 9.17 (s, 1H), 9.23 (d, J=7 Hz,
1H), 12.71 (br
s, 1H).
Example 164B
4-(4-Chloronhenoxy)-N-f(1R)-1-methyl-2-(methylamino)-2-oxoethyllthienof2 3-
clpyridine-2-carboxamide
Example 164 was prepared in analogy to Example 92, using D-alanine methyl
ester
hydrochloride in place of ammonium chloride. The derived ester was treated
according to
the procedure of Example 171 to give the title compound.
HPLC: Supelco C-18 column, eluent gradient of water:acetonitrile 0:90- 90:0
over 30
minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 17.46 minutes;
MS (DCI/NH3) m/e: 390 (M+H)+;
I49
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
'H NMR (300 MHz, DMSO-d6) b 1.32 (d, 3H), 2.60 (d, 3H), 4.41 (m, 1H), 7.13 (m,
2H),
7.49 (m, 2H), 7.96 (br d, 1H), 8.19 (s, 1H), 8.38 (s, 1H), 9.13 (br d, 1H),
9.19 (s, 1H).
Example 165
4-(4-Chloro~henoxy)-N-f(1S)-1-methyl-2-(methylamino)-2-oxoethyllthienof2,3-
clnyridine-2-carboxamide
Example 165A
(2S)-2-( f f4-(4-Chlorophenoxy)thienof 2,3-clpyridin-2-yllcarbonyl ~
amino)propanoic acid
Example 165A was prepared in analogy to Example 92, using L-alanine methyl
ester hydrochloride in place of ammonium chloride. The derived ester was
hydrolyzed
according to the procedure of Example 18 to give the title compound.
HPLC: Supelco C-18 column, eluent gradient of water:acetonitrile 0:90- 90:0
over 30
minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 18.40 minutes;
MS (DC1/NH3} m/e: 377 (M+H)+;
'H NMR (300 MHz, DMSO-db) b 1.40 (d, J=7 Hz, 3H), 4.41 (q, J=7 Hz, 1H), 7.15
(m,
2H), 7.48 (m, 2H), 8.18 (s, 1 H), 8.31 (s, 1 H), 9.16 (s, 1 H), 9.21 (d, J=7
Hz, 1 H), 12.71 (br
s, 1H);
'3C NMR (100 MHz, DMSO-d6) 8 16.78, 48.46, 119.52, 119.80, 119.97, 127.84,
130.13,
132.97, 137.54, 137.60, 138.09, 141.30, 145.59, 147.38, 155.59, 160.54,
173.68.
Example 165B
4-(4-Chlorophenoxy)-N-f ( 1 S )- I -methyl-2-(methylamino)-2-oxoethyllthieno f
2,3
clpyridine-2-carboxamide
Example 165B was prepared from Example 165A in analogy to Example 92, with
the substitution of L-alanine methyl ester for ammonium chloride. The
intermediate ester
was converted to the title compound according to the procedure or Example 171,
using
methanolic methylamine.
HPLC: Supelco C-18 column, eluent gradient of water:acetonitrile 0:90- 90:0
over 30
minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 17.48 minutes;
MS (DCI/NH3) m/e: 390 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 1.32 (d, 3H), 2.59 (d, 3H), 4.41 (m, 1H), 7.13 (m,
2H),
7.49 (m, 2H), 7.94 (br d, 1H), 8.18 (s, 1H), 8.36 (s, 1H), 9.12 (br d, IH),
9.19 (s, 1H).
Example 166
150
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-(4-Chlorophenoxy}-N-f ( I R)- I -(hydroxymethyl)-2-(meth~lamino)-2
oxoethyllthienof2,3-clpyridine-2-carboxamide
The title compound was made in analogy to the procedure of Example 164B, using
D-serine methyl ester.
HPLC: Supelco C-18 column, eluent gradient of water:acetonitrile 0:90- 90:0
over 30
minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 16.10 minutes;
MS (APCI} m/e: 404 (M-H)-;
'H NMR (300 MHz, DMSO-db) 8 2.59 (d, 2H), 3.69 (m, 2H), 4.45 (m, IH), 4.96,
(t, 1H),
7.14 (m, 2H), 7.49 (m, 2H), 7.97 (m, 1H), 8.I8 (s, 1H), 8.49 (s, 1H), 9.01 (br
d, 1H), 9.19
(s, 1 H).
Example 167
4-(4-Chlorophenoxy)-N-f(1S)-1-(hydroxymethyl)-2-(methylamino)-2-
oxoethyllthienof2 3
clpyridine-2-carboxamide
The title compound was made in analogy to the procedure of Example 164B, using
L-serine methyl ester.
HPLC: Supelco C-18 column, eluent gradient of water:acetonitrile 0:90- 90:0
over 30
minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 16.18;
MS (APCI) m/e: 404 (M-H)-;
1H NMR (300 MHz, DMSO-d6) 8 2.59 (d, 2H), 3.69 {m, 2H), 4.45 (m, 1H), 4.96,
(t, 1H),
7.14 (m, 2H), 7.49 (m, 2H), 7.97 (br d, 1H), 8.18 (s, 1H), 8.49 (s, 1H), 9.OI
(br d, 1H),
9.19 (s, 1H).
Example 170 4-(3-Pyridinyloxy)thienof2,3-clpyridine-2-carboxamide
Example 17A and 3-Hydroxypyridine were processed as in Example 61 to provide
the title compound.
MS (DC1/NH3) m/e: 272 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.50 (m, 2H), 7.85 (m, 1H), 8.20 (s, 2H), 8.45
(dd, 2H),
8.55 (d, 1H), 9.20 (s, IH);
Anal. Calcd for C13H9N302S~0.25 H20: C, 56.61; H, 3.47; N, 15.24. Found: C,
57.01; H,
3.50; N, 15.16.
Example 171
Methyl 4-(4-Bromophenoxy)thienof 2,3-clpyridine-2-carboxamide
151
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 73A (2 g, 5.5 mmol) was suspended in a solution of methylamine in
methanol (2 M solution, 15 mL) and refluxed for 1 hour under nitrogen
atmosphere. Then
the solvent was removed and the residue was purified by flash chromatography
on silica
gel eluting with 30% acetone in hexane to obtain the title compound (1.96 g,
98%).
mp 78-80 °C;
MS (DC1/NH3) m/e: 363,365 (M+H)+;
1H NMR (300 MHz, DMSO-db) b 2.79 (d, 3H, J=4.8 Hz), 7.06 (d, 2H, J=8.8 Hz),
7.59 (d,
2H, J=8.8 Hz), 8.06 (s, 1H), 8.20 (s, 1H), 8.96 (q, 1H, J=4.8 Hz), 9.17 (s,
1H).
Example 172
4-(4-Bromo~henoxy)-N,N-dimeEhylthienof2,3-clpyridine-2-carboxamide
Example 172 was prepared according to the procedure of Example 104, with the
substitution of methyl 4-(4-bromophenoxy)-thieno[2,3-c]pyridine-2-carboxylate
for
Example 61 A.
mp 93-95 °C;
MS (DC1/NH3) m/e: 377, 379 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 3.02 (br s, 3H), 3.13 (br s, 3H), 7.09 (d, 2H,
J=8.8 Hz),
7.5 d, 2H, J=8.8 Hz), 7.60 (s, 1H), 8.19 (s, 1H), 9.15 (s, 1H)ppm.
Example 173
N~N-Dimethyl-4-f4-(trifluoromethyl)phenoxylthienof2,3-clpyridine-2-carboxamide
Example 173 was prepared according to the procedure of Example 104, with the
substitution of methyl 4-(4-trifluoromethylphenoxy)-thieno[2,3-c]pyridine-2-
carboxylate
for Example 61 A.
mp 74-76 °C;
MS (DCI/NH3) m/e: 367 (M+H)+;
1H NMR (300 MHz, CDC13) 8 3.17 (br s, 6H), 7.11 (d, 2H, J=8.0 Hz), 7.45 (s,
1H), 7.63
(d, 2H, J=8.0 Hz), 8.24 (br s, 1H), 9.01 (br s, 1H).
Example 174
4-(4-Chloro-3-fluoronhenoxy)-N-methylthienof 2,3-clpyridine-2-carboxamide
Example 174 was prepared as in Example 103 but substituting 4-chloro-3
fluorophenol for 4-chlorophenol to provide the title compound.
mp 62-64 °C;
MS (DCI/NH3) m/e: 337 (M+H)+;
152
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
'H NMR (300 MHz, DMSO-d6) 8 3.05 (d, 3H, J=4.7 Hz), 6.24 (br s, 1H), 6.77
(d,lH,
J=9.8 Hz), 6.84 (dd, 1H, J=2.4, 9.5 Hz), 7.26 (s,2H), 7.37 (t, 1H, J=8.5 Hz),
7.69 (s,lH),
8.21 (s, 1H), 9.00 (s, 1H);
Anal. calcd for C,SHION2C1F02S: C, 53.50; H, 2.99; N, 8.32. Found: C, 53.78;
H, 3.26; N,
8.02.
Example 175
4-(4-Chloro-3-fluorophenoxy)thienof 2,3-clpyridine-2-carboxamide
Example 175 was prepared as in Example 61 but substituting 4-chloro-3-
fluorophenol for 4-chlorophenol to provide the title compound.
mp 227-228 °C;
MS (DCI/NH3) m/e: 323 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 6.94 (m,lH), 7.34 (dd, 1H, J=3.0, 10.5 Hz), 7.60
(t, 1H,
J=8.7 Hz), 7.87 (s,lH), 8.11 (s, 1H), 8.26 (s, 1H), 8.44 (s, 1H), 9.19 (s,
1H);
Anal. calcd for C~4HgN2C1FO2S: C, 52.10; H, 2.50; N, 8.68. Found: C, 52.06; H,
2.49; N,
8.52.
Example 176
4-(4-Chloro-3-ethylphenoxy)thienof 2,3-clpyridine-2-carboxamide
Example 17A and 4-Chloro-3-ethylphenol were processed as in Example 61 to
provide the title compound.
mp 185-187 °C;
MS (DCI/NH3) m/e: 333 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 1.15 (t, 3H), 2.70 (q, 2H), 6.95 (dd, 1H), 7.20
(d, 1H),
7.45 (d, 1H), 7.85 (br s, 1H), 8.15 (s, 1H), 8.45 (m, 1H), 9.15 (s, 1H).
Example 177
4-(3-Fluorophenoxy)thienof2, 3-clpyridine-2-carboxamide
Example 177 was prepared according to the procedure of Example 61, with the
substitution 3-fluorophenol for 4-chlorophenol.
mp 215-216 °C;
MS (DCI/NH3) m/e: 289 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 6.90 (m, 1H), 7.05 (m, 2H), 7.43 (q, 1H, J=8.6
Hz),
7.86 (br s, 1H), 8.14 (s, 1H), 8.20 (s, 1H), 8.45 (br s, 1H), 9.17 (s, 1H).
Example 178
153
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-(2. 3-Difluorophenoxy)thienof2, 3-clpyridine-2-carboxamide
Example I78 was prepared according to the procedure of Example 61, with the
substitution 2, 3-difluorophenol for 4-chlorophenol.
mp 207-209 °C;
MS (DC1/NH3) m/e: 307 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 6.97 (t, 1H, J=8.5 Hz), 7.22 (q, 1H, J=8.5 Hz),
7.32 (q,
1H, J=8.5 Hz), 7.87 (br s, 1H), 8.18 (s, 1H}, 8.21 (s, 1H), 8.43 (br s, 1H),
9.17 (s, 1H).
Example 179
4-(2, 3-Difluorophenoxy)-N-methylthieno f 2, 3-clpyridine-2-carboxamide
Example 179 was prepared according to the procedure of Example 103, with the
substitution 2, 3-difluorophenol for 4-chlorophenol.
mp 169-171 °C;
MS (DC1/NH3) m/e: 321 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 2.88 (d, 3H, J=4.4 Hz), 6.94 (t, 1H, J=8.5 Hz),
7.21 (q,
1 H, J=8.5 Hz), 7.31 (q, 1 H, J=8.5 Hz), 8.14 (s, 1 H), 8.21 (s, 1 H), 8.95
(q, 1 H, J=4.5 Hz),
9.17 (s, 1H).
Example 180
4-(3-Fluorophenoxy)-N-methylthienol2, 3-clpyridine-2-carboxamide
Example 180 was prepared according to the procedure of Example 103, with the
substitution 3-fluorophenol for 4-chlorophenol.
mp 194-195 °C;
MS (DCI/NH3) m/e: 303 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.79 (d, 3H, J=4.4 Hz), 6.87 (d, 1H, J=8.5 Hz),
7.05 (m,
2H), 7.42 (q, 1 H, J=8.5 Hz), 8.05 (s, 1 H), 8.23 (s, 1 H), 8.95 (q, 1 H,
J=4.4 Hz), 9.17 (s,
1 H).
Example 181
N-Methyl-4-(2, 3, 4-trifluorophenoxy)thieno(2, 3-clpyridine-2-carboxamide
Example 181 was prepared according to the procedure of Example 103, with the
substitution 2, 3, 4-trifluorophenol for 4-chlorophenol.
mp 170-171 °C;
MS (DCI/NH3) m/e: 339 (M+H)+;
'H NMR (300 MHz, DMSO-d6) S 2.82 (d, 3H, J=4.4 Hz), 7.13 (m, 1H), 7.35 (q, 1H,
J=8.5
Hz), 8.16 (s, 1H), 8.17 (s, 1H), 8.97 (q, 1H, J=4.5 Hz), 9.16 (s, 1H).
154
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 182 4-(2, 3, 4-Trifluorophenoxy)thienof2, 3-clpyridine-2-carboxamide
Example 182 was prepared according to the procedure of Example 61, with the
substitution 2, 3, 4-trifluorophenol for 4-chlorophenol.
mp 218-219 °C;
MS (DCI/NH3) m/e: 325 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 7.15 (m, 1H), 7.38 (q, IH, J=8.5 Hz), 7.89 (br s,
1H),
8.15 (s, 1H), 8.23 (s, IH), 8.45 (br s, 1H), 9.15 (s, 1H).
Example 183
N-Methyl-4-f4-(trifluoromethyl)phenoxylthienoF2, 3-clpyridine-2-carboxamide
Example 183 was prepared according to the procedure of Example 103, with the
substitution 4-trifluoromethylphenol for 4-chlorophenol.
mp 157-158 °C;
MS (DCI/NH3) m/e: 353 (M+H)+;
'H NMR (300 MHz, DMSO-db) S 2.78 (d, 3H, J=4.4 Hz), 7.22 (d, 2H, J=8.5 Hz),
7.76 (d,
2H, J=8.5 Hz), 8.01 (s, 1H), 8.34 (s, 1H), 8.92 (q, 1H, J=4.4 Hz), 9.24 (s,
1H).
Example 184
4-f3-(Trifluorometh~phenoxylthienof2, 3-clpyridine-2-carboxamide
Example 17A and 3-trifluoromethylphenol were processed as in Example 183 to
provide the title compound.
mp 175-176 °C;
MS (DCI/NH3) mle: 353 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.80 (d, 3H), 7.35 (d, 1H), 7.20 (d, 1H), 7.60 (m,
3H),
8.10 (s, 1H), 8.30 (s, 1H), 9.00 (b, 2H), 9.25 (s, 1H);
Anal. Calcd for C,6H~,F3N202S'0.25 HZO: C, 53.86; H, 3.25; N, 7.85. Found: C,
53.60;
H, 3.06; N, 7.78.
Example 185
N, N-Dimethyl-4-(4-vinylnhenoxy)thieno(2. 3-clpyridine-2-carboxamide
Example 185 was prepared according to the procedure of Example 104, with the
substitution 4-vinylphenol for 4-chlorophenol.
mp 80-81 °C;
MS (DCI/NH3) m/e: 325 (M+H)+;
155
CA 02333770 2000-11-30
WO 99/62908 PCT/US99112419
'H NMR (300 MHz, DMSO-d6) 8 3.02 (br s, 3H), 3.13 (br s, 3H), 5.24 (d, 1H,
J=11.4 Hz)
5.78 (d, 1H, J=17.3 Hz), 6.74 (dd, 1H, J=11.4, 17.3 Hz), 7.09 (d, 2H, J=8.5
Hz), 7.53 (d,
2H, J=8.5 Hz), 7.61 (s, 1H), 8.16 (s, 1H), 9.13 (s, 1H).
Example 186
4-(4-Cyanophenoxy)-N-methylthienof2, 3-clpyridine-2-carboxamide
Example 186 was prepared as in Example 103 but substituting 4-cyanophenol for
4-chlorophenol to provide the title compound.
MS (ESI/NH3) m/e: 310 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.78 (d, 3H, J=4.4 Hz), 7.20 (d, 2H, J=8.8 Hz),
7.89 (d,
2H, J=8.8 Hz), 7.97 (s, 1H), 8.37 (s, 1H), 8.94 (q, 1H, J=4.4 Hz), 9.26 (s,
1H);
Anal. calcd for C~6H1~N302SØ05 CHzCl2: C, 61.46; H, 3.56; N, 13.30. Found:
C, 61.29;
H, 3.53; N, 13.23.
Example 187
4-(4-Cyanophenoxy)thienof2, 3-clpyridine-2-carboxamide
Example 187 was prepared as in Example 61 but substituting 4-cyanophenol for 4-
chlorophenol to provide the title compound.
mp 255-257 °C;
MS (ESI/NH3) m/e: 296 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.20 (d, 2H, J=8.8 Hz), 7.84 (s, 1H), 7.89 (d, 2H,
J=8.8
Hz), 8.05 (s, 1H), 8.36 (s, 1H}, 8.41 (q, 1H, J=4.4 Hz}, 9.26 (s, 1H);
Anal. calcd for C,SH9N30zS.1.5CH30H: C, 57.71; H, 3.08; N, 12.24. Found: C,
57.45; H,
3.28; N, 12.43.
Example 188
4-(4-Aminophenoxy)thienof2, 3-clpyridine-2-carboxamide
Example 188A
3-Chloro-5-(4-(tert-butvloxvcarbonyl)amino)phenoxy-4-pyridinecarboxaldehvde
A solution of Example 17A (2.Og, I l.4mmo1) and tort-butyl N-(4-
hydroxyphenyl)carbamate (2.38 g, 11.4 mmol) which was prepared according to
literature
method (A. Vigroux, M. Bergon, C. Zedde; J. Med. Chem. 1995, 38, 3983) in DMF
(30
mL) was treated with CsCO~ (3.70 g, 11.4 mmol) at room temperature for 1 hour,
and at
70 °C for 30 minutes. Brine ( 150mL) was added, and the mixture was
extracted with
ether/ethyl acetate (2x200mL). The combined organic phases were dried (MgS04),
and
156
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
concentrated. Flash chromatography of the residue on silica gel with 1:6 ethyl
acetate/hexane provided the designated compound (2.65 g, 67%).
MS (ESI/NH3) m/e: 349 (M+H)+.
Example 188B
Methyl 4-((4-tert-butyloxycarbonylamino)phenoxy)thienof2, 3-clpyridine-2-
carboxylate
A solution of Example 188A (2.64 g, 7.58 mmol) in THF (30 mL) was treated with
methyl thioglycolate (804 mg, 7.58 mmol) at 0 °C for 0.5 hour, and at
room temperature
for 1 hour, after which Cs2C03 (2.47 g, 7.58 mmol) was added. The reaction
mixture was
stirred at room temperature for 1 hour, and at 70 °C for 0.5 hour.
Brine ( 150 mL) was
added and the mixture was extracted with ethyl acetate (2x 150 mL). The
combined
organic phases were dried (MgS04) and concentrated. The residue was flash
chromatographed on silica gel with 20% ethyl acetate in hexane to provide
designated
compound (1.29 g, 43%).
MS (ESI/NH3) m/e: 401 (M+H)+.
Example 188C
4-f(4-tent-Butyloxycarbonylamino)phenoxylthienof2, 3-clpyridine-2-carboxamide
Example 188C was prepared as in Example 44 but substituting Example 188B for
Example 43 to provide the title compound.
MS (ESI/NH3) m/e: 386 (M+H)+.
Example 188D
4-(4-Aminophenoxy)thienof2, 3-clpyridine-2-carboxamide
Example 188C was dissolved in trifluoroacetic acid (20 mL), and the solution
was
kept at room temperature for 1 hour before TFA was removed. The residual oil
was
treated with a mixture of ethyl acetate and aqueous NaHCO~ solution. The
formed solid
was collected by filtration, washed successively with ethyl acetate, aqueous
NaHC03
solution, water, methanol and ethyl acetate, and dried to provide the title
compound (492
mg, 86% from Example 188B) as a yellow solid.
mp >250 °C;
MS (DCI/NH3) m/e: 286 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 5.62 (br s, 2H), 6.65 (d, 2H, J=8.8 Hz), 6.93 (d,
2H,
J=8.8 Hz}, 7.86 (s, 1H), 8.30 (s, 1H), 8.44 (s, 1H), 9.00 (br s, 1H);
Anal. calcd for C,4H"N~02SØ5 CH30H: C, 57.79; H, 3.85; N, 13.94. Found: C,
57.69;
H, 3.95; N, 13.57.
157
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 189
4-f4-(Acetylamino)phenoxylthienof2, 3-clpyridine-2-carboxamide
Example 189 was prepared as in Example 188C but substituting 4-
(acetylamino)phenol for t-butyl N-(4-hydroxyphenyl)carbamate to provide the
title
compound.
MS (DCI/NH3) m/e: 328 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 2.04 (s, 3H), 7.10 (d, 2H, J=8.8 Hz), 7.65 (d, 2H,
J=8.8
Hz), 7.82 (br s, 1H), 7.99 (s, 1H), 8.20 (s, 1H), 8.43 (br s, 1H), 9.06 (s,
1H), 9.99 (s, 1H);
Anal. calcd for C~6H~3N303S~1.O CH3OH: C, 56.81; H, 3.93; N, 11.69. Found: C,
56.51;
H, 3.93; N, 11.57.
Example 190
N-Methyl-4-f4-(4-morpholinyl)phenoxylthienof2, 3-clpyridine-2-carboxamide
Example 190A
Methyl-4-f4-(4-iodophenoxylthienof2, 3-clpyridine-2-carboxylate
Example 190A was prepared as in Example 61A but substituting 4-iodophenol for
4-chlorophenol to provide the designated compound.
MS (DCI/NH3) m/e: 412 (M+H)+.
Example 190B
N-Methyl-4-f4-(4-iodophenoxy)lthienof2, 3-clpyridine-2-carboxamide
A solution of Example 190A ( 1.4 g, 3.4 mmol) in methylamine/methanol (2.0 M
solution,
70 mL) was stirred at 45 °C for 15 hours, and concentrated under
vacuum. The residue
was flash chromatographed on silica gel with EtOAc/hexane ( 1.5/ 1 ) to
provide the
designated compound ( 1.3 g, 93%).
MS (DCI/NH3) m/e: 411 (M+H)+.
Example 190C
N-Methyl-4-f4-(4-mornholinyl)phenoxylthienof2, 3-clpyridine-2-carboxamide
A two necked flask was charged with Example 190B ( 150 mg, 0.37 mmol),
NaOBu-t (71 mg, 0.74 mmol), Pd2(dba)3 ( 14 mg, 0.014 mmol), BINAP (27 mg,
0.044
mmol) and 18-crown-6 (196 mg, 0.74 mmol), and purged with nitrogen. Anhydrous
degassed THF ( 10 mL) and morpholine (64 mg, 0.74 mmol) were added
successively.
The clear dark red solution was heated at 60 °C for 70 hours, and
quenched with brine.
158
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
The mixture was extracted with methylene chloride. The organic layer was dried
(MgS04)
and concentrated. The crude was flash chromatographed on silica gel
(EtOAc/hexane) and
was further purified on HPLC (C-18, CH3CN/H20) to provide the title compound
(26 mg).
MS (DCI/NH3) m/e: 370 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 2.81 (d, 3H, J=4.5 Hz), 3.1 (m, 2H), 3.74 (m, 2H),
6.99
(d, 2H, J=8.8 Hz), 7.05 (d, 2H, J=8.8 Hz), 7.92 (s, 1H), 8.20 (s, 1H), 8.98
(q, 1H, J=4.8
Hz), 9.04 (s, 1H).
Example 191
4-f4-(Hydroxymethyl)phenoxylthienof2, 3-clpyridine-2-carboxamide
Example 191 A
Methyl 4-~4-f(trityloxy)methylluhenoxy)thienof2, 3-clpyridine-2-carboxylate
Example 191A was prepared as in Example 61A but substituting 4-
trityloxymethylphenol which was prepared according to literature method
(Frank, R.;
Doring, R. Tetrahedron 1988, 44, 6031) for 4-chlorophenol to provide the title
compound.
MS (DCI/NH3) m/e: 558 (M+H)+.
Example 191B
4- f 4- f (Trityloxy)methyllphenoxy ) thienof2, 3-clpyridine-2-carboxamide
Example 191A was prepared as in Example 61 but substituting
4-trityloxymethylphenol for 4-chlorophenol to provide the title compound.
MS (DCI/NH3) m/e: 543 (M+H)+;
'H NMR (300 MHz, DMSO-db) S 4.10 (s, 2H), 7.11 (d, 2H, J=8.5 Hz), 7.26-7.46
(m,
17H), 7.87 (brs, 1H), 8.09 (s, 1H), 8.21 (s, 1H), 8.46 (brs, 1H), 9.12 (s,
1H)ppm.
Anal. calcd for C34H26NZO3S: C, 75.25; H, 4.83; N, 5.16. Found: C, 75.17; H,
4.76; N,
5.15.
Example 19I C
Methyl4-f4-(hydroxymethyl)nhenoxylthienof2 3-clpyridine-2-carboxylate
A solution of Example 191A (5.05g, 9 mmol) in a mixture of chloroform (20 mL)
and methanol (8 mL) was treated with trifluoroacetic acid ( 10 mL) at 0
°C for 6 hours, and
was then poured into a mixture of ice and saturated NaHC0;3. The mixture was
extracted
with methylene chloride (2x200 mL). The combined organic layers were dried
(MgS04),
and concentrated. The residue was flash chromatographed on silica gel with 66%
EtOAc/hexane to give the designated compound (2.11 g, 74%).
159
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
MS (DCI/NH3) m/e: 316 (M+H}+.
Example 191 D
4-f4-(Hydroxymethyl)phenoxylthienof2, 3-clpyridine-2-carboxamide
Example 191 D was prepared as in Example 61 but substituting Example 191 C for
Example 61A to provide the title compound.
MS (DC1/NH3) m/e: 301 (M+H)+;
1H NMR (300 MHz, DMSO-db) b 4.50 (d, 2H, J=5.8 Hz), 5.19 (t, 1H, J=5.8 Hz),
7.10 (d,
2H, J=8.5 Hz), 7.37 (d, 2H, J=8.5 Hz), 7.82 (br s, 1 H), 8.03 (s, 1 H), 8.20
(s, 1 H), 8.43 (br
s, 1H), 9.09 (s, 1H);
Anal. calcd for C,SH,2Nz03S: C, 59.99; H, 4.03; N, 9.33. Found: C, 59.82; H,
3.93; N,
8.82.
Example 192
4-f4-(H~drox~methyl)phenoxyl-N-methylthienof2, 3-clpyridine-2-carboxamide
Example 192 was prepared as in Example 103 but substituting Example 191 C for
Example 61A to provide the title compound.
mp 195-196 °C;
MS (DCI/NH3) m/e: 315 (M+H)+;
'I~ NMR (300 MHz, DMSO-d6) 8 2.80 (d, 3H, J=4.5 Hz), 4.49 (d, 2H, J=4.5 Hz),
5.19 (t,
1H, J=4.5 Hz), 7.08 (d, 2H, J=8.5 Hz), 7.37 (d, 2H, J=8.5 Hz), 8.07 (s, 1H),
8.11 (s, 1H),
8.94 (q, 1H, J=4.5 Hz), 9.10 (s, 1H);
Anal. calcd for Ci6H~4N203SØ75 CH30H: C, 59.45; H, 4.39; N, 8.28. Found: C,
59.31;
H, 4.35; N, 8.49.
Example 193
4-f4-(Methoxymeth~~phenoxyl-N-methylthienof2, 3-clpyridine-2-carboxamide
Example 193 was prepared as in Example 188C but substituting
4-methoxymethylphenol for 4-tent-butyloxycarbonylaminophenol and substituting
methylamine for ammonia to provide the title compound.
mp 163-164 °C;
MS (DCI/NH3) m/e: 329 (M+H)+;
1H NMR (300 MHz, DMSO-db) b 2.79 (d, 3H, J=4.4 Hz), 3.29 (s, 3H), 4.40 (s,
2H), 7.08
(d, 2H, J=8.5 Hz), 7.37 (d, 2H, J=8.5 Hz), 8.09 (s, 1H), 8.12 (s, 1H), 8.94
(q, 1H, J=4.4
Hz), 9.12 (s, 1 H);
160
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Anal. calcd for C,~H16N2O3S: C, 62.18; H, 4.91; N, 8.53. Found: C, 61.86; H,
4.79; N,
8.40.
Example 194
4-~4-f(2-Methoxyethoxy)methyllphenoxy~thienof2.3-clpyridine-2-carboxamide
Example 194 was prepared as in Example 188C but: substituting 4-(2-
methoxyethoxymethyl)phenol for 4-tent-butyloxycarbonylaminophenol to provide
the title
compound.
MS (DCI/NH3) m/e: 359 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 3.40 (s, 3H), 3.60 (m, 2H), 3.65 (m, 2H), 4.56 (s,
2H), 7.02
(d, 2H, J=8.5 Hz), 7.36 (d, 2H, J=8.5 Hz}, 7.80 (s, 1H), 8.13 {s, 1H), 8.94
(s, 1H};
Anal. calcd for C,sH,gN204S: C, 60.32; H, 5.06; N, 7.82. Found: C, 60.33; H,
5.03; N,
7.63.
Example 195
4-14-f(2-Methoxyethoxy)meth~llphenoxyl-N-methylthienof2, 3-clpyridme-2
carboxamide
Example 195 was prepared as in Example 188C but substituting 4-(2-
methoxyethoxymethyl)phenol for 4-tert-butyloxycarbonylaminophenol and
substituting
methylamine for ammonia to provide the title compound.
mp 133-134 °C;
MS (DCI/NH3) mle: 373 (M+H)+;
'H NMR (300 MHz, CDCl3) b 3.01 (d, 3H, J=5.1 Hz), 3.40 (s, 3H), 3.60 (m, 2H),
3.65 (m,
2H), 4.54 (s, 2H), 6.51 (q, 1 H, J=5.1 Hz), 7.00 (d, 2H, J=8.5 Hz), 7.34 (d,
2H, J=8.5 Hz),
7.73 (s, 1H), 8.14 (s, 1H), 8.94 (s, 1H);
Anal. calcd for Ci9H2oN2O4S: C, 61.27; H, 5.41; N, 7.52. Found: C, 61.28; H,
5.35; N,
7.46.
Example 196
4-(4-( 12-(2-Methox~ethoxy)ethox l~methyllphenoxy)thienol2, 3-clpyridine-2-
carboxamide
Example 196 was prepared as in Example 188C but substituting 4-{2-(2-
methoxyethoxy)ethoxymethyl}phenol for 4-tert-butyloxycarbonylaminophenol to
provide
the title compound.
MS (DCI/NH3) m/e: 403 (M+H)+;
161
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, CDC13) 8 3.38 (s, 3H), 3.57 (m, 2H), 3.63-3.70 (m, 6H), 4.55
(s, 2H),
7.02 (d, 2H, J=8.5 Hz}, 7.36 (d, 2H, J=8.5 Hz), 7.71 (s, 1H), 8.15 (s, 1H),
8.95 (s, 1H).
Example 197
4-(4-( f2-(2-Methoxyethoxy)ethoxylmethyllphenoxy)-N-methylthienof2, 3-
clpyridine-2-
carboxamide
Example 197 was prepared as in Example 188C but substituting 4-{2-(2-
methoxyethoxy)ethoxymethyl}phenol for 4-tert-butyloxycarbonylaminophenol and
substituting methylamine for ammonia to provide the title compound.
MS (DCI/NH3) m/e: 417 (M+H)+;
'H NMR (300 MHz, CDCl3) b 3.02 (d, 3I~, J=4.8 Hz), 3.38 (s, 3H), 3.57 (m, 2H),
3.63-
3.70 (m, 6H), 4.54 (s, 2H), 6.45 (m, 1H), 7.00 (d, 2H, J=8.5 Hz), 7.34 (d, 2H,
J=8.5 Hz),
7.72 (s, 1H), 8.15 (s, 1H), 8.94 (s, 1H).
Example 198
4-(4-f(2. 3, 4, 5-Tetrahydro-2H-pyran-2-~y)methyllphenox~~thienof2, 3-
clpyridine-2
carboxamide
Example 198A
Methyl 4-f 4-( f(2, 3, 4, 5-tetrahydro-2H-pyran-2-
yl)oxylmethyllphenoxylthienof2, 3-
clpyridine-2-carboxylate
Example 198A was prepared as in Example 188B but substituting 4-[(2, 3, 4, 5-
tetrahydro-2H-pyran-2-yl)oxy]methylphenol (P.A.Grieco, et al, J. Org. Chem.
1977, 42,
3772) for 4-tert-butyloxycarbonylaminophenol to provide the designated
compound.
MS (DCI/NH3) m/e: 400 (M+H)+.
Example 198B
4-14-f(2, 3. 4, 5-Tetrahvdro-2Hwran-2-yloxv)methvllphenoxv)thienof2. 3-
clnvridine-2-
carboxamide
Example 198B was prepared as in Example 61 but substituting Example 198A for
Example 61 A to provide the designated compound.
mp 95-9b °C;
MS (DCI/NH3) m/e: 385 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 1.49 (m, 4H), 1.69 (m, 2H), 3.49 (m, 1H), 3.80 (m,
1H),
4.44 (d, 1H, J=12.1 Hz), 4.67 {d, 1H, J=12.1 Hz), 4.70 (m, 1H), 7.10 (d, 2H,
J=8.8 Hz),
7.41 (d, 2H, J=8.8 Hz), 7.87 (s, 1H), 8.08 (s, 1H), 8.20 (s, 1H), 8.46 (s,
1H), 9.12 (s, 1H);
162
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Anal. calcd for C2oH2oN204S'CH~OH: C, 60.56; H, 5.08; N, 6.73. Found: C,
60.51; H,
5.07; N, 6.59.
Example 199
N-Methyl-4-(4-f(tetrahydro-2H-pyran-2-yloxy)methylluhenoxylthienof2 3
clnyridine 2
carboxamide
Example 199 was prepared as in Example I03 but substituting Example 198A for
Example 61A to provide the designated compound.
mp 195-196 °C;
MS (DCI/NH3) m/e: 399 (M+H)+;
jH NMR (300 MHz, DMSO-d6) S 1.49 (m, 4H), 1.69 (m, 2H), 2.79 (d, 3H, J=4.8
Hz),
3.50 (m, 1H), 3.80 (m, 1H), 4.44 (d, 1H, J=12.1 Hz), 4.67 (d, 1H, J=I2.I Hz),
4.70 (m,
1H), 7.09 (d, 2H, J=8.8 Hz), 7.40 (d, 2H, J=8.8 Hz), 8.1 I (s, 1H), 8.12 (s,
IH), 8.97 (q,
1H, J=4.8 Hz}, 9.I3 (s, IH);
Anal. calcd for CZ,H22NZO4S: C, 63.30; H, 5.56; N, 7.03. Found: C, 63.22; H,
5.58; N,
6.93.
Example 200
4-( f2-(Aminocarbonyl)thienof2 3-clpyridin-4-ylloxy?benzyl 2 furoate
A solution of Example I91D (40 mg, 0.133 mmol) in DMF (5 mL) was treated
with 2-furoic acid (45 mg, 0.4 mmol), HOBt (54 mg, 0.4 mmol), EDC (77 mg, 0.4
mmol)
and 2 drops of triethylamine at room temperature for 48 hours. Brine was added
and the
mixture was extracted with EtOAc. The combined organic phases were washed with
water, dried (MgS04) and concentrated. The residue was flash chromatographed
on silica
gel with 65% EtOAc/hexane to provide the designated compound.
mp 180-182 °C;
MS (DCI/NH3) m/e: 395 (M+H)+;
1H NMR (300 MHz, DMSO-d6} 8 5.31 (s, 2H), 6.70 (dd, 1H, J=1.7, 3.4 Hz), 7.14
(d, 2H,
J=8.5 Hz), 7.36 (d, IH, J=3.4 Hz), 7.50 (d, 2H, J=8.5 Hz), 7.84 (s, 1H}, 8.00
(dd, 1H,
J=1.1, 3.7 Hz), 8.13 (s, IH), 8.17 (s, 1H), 8.44 (s, IH), 9.14 (s, 1H).
Example 201
4-(4-((((2R,4R SS 6R)-4 5-Dihydroxy-6-(hydroxymethyl)tetrahydro 2H pyran 2
]oxy)methyl)uhenoxyl-N-methylthienof2 3-c]pyridine 2 carboxamide
Example 201A
163
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Methvl 4-f4-( 1 ((2R, 4R, SS, 6R)-4, 5-Diacetoxy-6-(acetoxymethyl)tetrahydro-
2H-pyran-2-
~loxylmet~l)phenoxylthienol2, 3-clpyridine-2-carboxylate
A solution of Example 191C (200 mg, 0.63 mmol) and tri-O-acetyl-D-glycal (520
mg, 1.92 mmol) in dry CHzCl2 ( 10 mL) was treated with Sc(OTf)3 (380 mg, 0.75
mmol) at
room temperature for 12 hours, and was directly flash chromatographed on
silica gel with
40% EtOAc/hexane to provide the designated compound.
M5 (DCI/NH3) m/e: 528 (M-OAc)+.
Example 201 B
4-f4-(( f(2R, 4R, 5S, 6R)-4, 5-Dihydroxy-6-(h d~rox~yl)tetrahydro-2H-pyran-2-
~lloxylmethyl)phenoxyl-N-mefhylthienof2, 3-clpyridine-2-carboxamide
A solution of Example 201 A ( I 67 mg} in a 2M solution of methylamine in
methanol ( 10 mL) was heated at 45 °C for 12 hours, and was
concentrated. The residual
oil was chromatographed on silica gel with 8% MeOH/CH2Cl~ to give the
designated
compound ( 120 mg, 91 %).
MS (ESI/NH3) m/e: 443 (M-OH)+;
1H NMR (300 MHz, DMSO-d6) b 2.79 (d, 3H, J=4.8 Hz), 3.53 (m, 3H), 3.67 (m,
1H),
3.87 (m, 1H), 4.50 (d, 1H, J=11.5 Hz), 4.64 (t, 1H, J=5.4 Hz}, 4.76 (d, 1H,
J=11.5 Hz),
5.06 (m, 2H), 5.70 (dt, 1H, J=10.2, 2.4 Hz), 5.86 (d, 1H, J=10.2 Hz), 7.08 (d,
2H, J=8.5
Hz), 7.40 (d, 2H, J=8.5 Hz), 8.10 (s, 1H), 8.11 (s, 1H), 8.95 (q, 1H, J=4.8
Hz), 9.12 (s,
1 H).
Example 202
4-(4-Acet~phenoxyl-N-methylthienof2, 3-clpyridine-2-carboxamide
A flask, purged with nitrogen, was charged with Example 190B (500 mg, 1.2
mmol), Pd(OAc)2 (27 mg, 0.12 mmol), (Tol)3P ( 110 mg, 0.36 mmol), dry degassed
DMF
(20 mL), tributylethoxyvinyltin (810 mL, 2.4 mmol), and triethylamine (835 mL,
6 mmol).
This suspension was stirred at 80 °C for 14 hours. After diluting with
ethyl acetate, the
reaction mixture was washed with 1 % HCl aqeous solution, water, dried
(MgS04), and
concentrated. The residue was separated by HPLC (C-18, CH3CN/H20 containing
0.1%
TFA) to provide the title compound (476 mg, 89%).
MS (DCI/NH3) m/e: 327 (M+H)+;
iH NMR (300 MHz, DMSO-d6) b 2.56 (s, 3H), 2.78 (d, 3H, J=4.8 Hz), 7.15 (d, 2H,
J=8.8
Hz), 8.00 (d, 2H, J=8.8 Hz), 8.03 (s, 1H), 8.36 (s, 1H), 8.98 (q, 1H, J=4.8
Hz), 9.28 (s,
1H);
164
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/1Z419
Anal, calcd for C~7H,4Nz03S. 1.35 CF3COZH: C, 49.25; H, 3.45; N, 5.83. Found:
C,
49.31; H, 3.60; N, 5.93.
Example 203
4-f4-(4-Morpholinylcarbonyl)phenoxylthienof2 3-clpyridine-2-carboxamide
Example 203A
Methvl4-(4-(4-carboxy)phenoxylthienof2 3-clpyridine-2-carboxylate
A suspension of methyl 4-bromophenoxythieno[2, 3-c]pyridine-2-carboxylate (
1.0
g, 2.74 mmol}, PdChDPPFCH2Cl2 (0.284 g), and triethylamine (0.55 g) in a
mixture of
THF( 15 mL) and HBO ( 1 S mL) was heated at 130 °C under CO atmosphere
(400 psi) for
19 hours. EtOAc (200 mL) was added, and the mixture was washed with brine,
dried
(MgS04), and concentrated. The residue was flash chromatographed on silica gel
with 5%
CH30H/CHZC12 to provide the designated compound (311 mg, 34%).
MS (DCI/NH3) m/e: 330 (M+H)+.
Example 203B
Methyl4-f4-(4-momholinylcarbonyl)phenoxylthieno[2 3-clpyridine-2-carboxylate
A solution of Example 203A (200 mg, 0.61 mmol) in a mixture DMF (SmL) and
CH2C12 (15 mL) was treated with morpholine (80 mg, 0.91 mmol), PyBOP (474 mg,
0.91
mmol) and DIPEA (296 mg, 2.28 mmol) at room temperature for 2 hours. After
diluting
with CHZCI2, the solution was washed with brine, dried (MgS04), and
concentrated. The
residue was flash chromatographed on silica gel with 90% EtOAc/hexane to give
the
designated compound (277 mg, 100%).
MS (DCI/NH3) m/e: 399 (M+H)+.
Example 203C
4-(4-(4-morr~holinylcarbonyl)nhenoxy~thienof2 3-clpyridine-2-carboxamide
Example 203C was prepared as in Example 61 but substituting Example 203B for
Example 61 A to provide the title compound.
mp >260 °C;
MS (DC1/NH3) m/e: 401 (M+NH4)+;
'H NMR (300 MHz, DMSO-d6) 8 3.50 (m, 4H), 3.60 (m, 4H), 7.14 (d, 2H, J=8.5
Hz),
7.48 (d, 2H, J=8.5 Hz), 7.86 (s, 1H), 8.15 (s,.1H), 8.22 (s, 1H), 8.45 (s,
1H), 9.17 (s, 1H);
Anal. calcd for C,9H,~N304S: C, 59.52; H, 4.47; N, 10.96. Found: C, 59.64; H,
4.52; N,
10.90.
165
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 204
N-Methvl-4-f4-(4-morpholinylcarbonyl)phenoxylthieno f 2 3-c]pyridine-2-
carboxamide
Example 204 was prepared as in Example 103 but substituting Example 203B for
Example 61 A to provide the title compound.
mp 173-175 °C;
MS (DCI/NH3) m/e: 415 (M+NH4)+;
1H NMR (300 MHz, DMSO-db) 8 2.79 (d, 3H, J=4.4 Hz), 3.50 (m, 4H), 3.60 (m,
4H),
7.12 (d, 2H, J=8.5 Hz), 7.48 (d, 2H, J=8.5 Hz), 8.07 (s, 1H), 8.24 (s, 1H),
8.96 (q, 1H,
J=4.4 Hz), 9.18 (s, 1 H);
Anal. calcd for CZOH,9N3O4S. 1.5 CH30H: C, 57.96; H, 4.64; N, 9.43. Found: C,
57.99;
H, 4.86; N, 9.63.
Example 206
4-f4-(( f2-(4-Mornholinyl)ethyllaminolcarbonyl)phenoxylthienof2 3-clpyridine-2
carboxamide
Example 206A
Methyl 4-f4-(( f2-(4-morpholinyl)ethyllaminolcarbonyl)phenoxylthienof2 3-
clpyridine-2-
carboxylate
A solution of Example 203A (200 mg, 0.61 mmol) in DMF (llmL) was treated
with 4-(2-aminoethyl)morpholine (158 mg, 1.21 mmol), EDC (232 mg, 1.21 mmol),
HOBt
(164 mg, 1.21 mmol) and triethylamine (122 mg, 1.21 mmol) at room temperature
for 18
hours. After diluting with EtOAc, the reaction mixture was washed with brine,
dried
(MgS04) and concentrated. The residue was flash chromatographed on silica gel
with
10% MeOH/EtOAc to provide the designated compound (239 mg, 89%).
MS (DCI/NH3) m/e: 442 (M+H)+.
Example 206B
4-f4-(( f2-(4-Moroholinyl)ethyllamino~carbonyl)phenoxylthienof2 3 clpyridine 2
carboxamide
Example 206B was prepared as in Example 61 but substituting Example 206A for
Example 61 A to provide the title compound.
mp 214-216 °C;
MS (DC1/NH3) m/e: 427 (M+H)+;
166
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, DMSO-db) 8 2.41 (t, 4H, J=4.8 Hz}, 3.37 (q, 2H, J=6.1 Hz),
3.56 (t,
4H, J=4.8 Hz), 7.14 (d, 2H, J=8.8 Hz), 7.84 (s, 1 H), 7.87 (d, 2H, J=8.8 Hz),
8.11 (s, 1 H),
8.22 (s, IH), 8.39 (t, IH, J=6.0 Hz), 8.43 (s, 1H), 9.17 (s, IH).
Example 207
N-Methvl-4-f4-( ( f 2-(4-moruholinyl)ethyllamino ~ carbonyl)phenoxylthieno f 2
3
clpyridine-2-carboxamide
Example 207 was prepared as in Example 103 but substituting Example 206A for
Example 61 A to provide the title compound.
mp 226-228 °C;
MS (DCI/NH3) m/e: 441 (M+H)+;
'H NMR (300 MHz, DMSO-d6} b 2.42 (m, 4H), 2.78 (d, 3H, J=4.4 Hz), 3.36 (q, 2H,
J=6.1
Hz), 3.56 (t, 4H, J=4.8 Hz), 7.12 (d, 2H, J=8.5 Hz), 7.89 (d, 2H, J=8.5 Hz),
8.03 (s, 1H),
8.26 (s, IH), 8.41 (t, 1H, J=6.0 Hz), 8.95 (q, 1H, J=4.4 Hz), 9.20 (s, IH).
Example 208
4-14-f(E)-3-(4-Mornholinyl)-3-oxo-I-propenyllphenoxylthienof2 3-clpyridine-2
carboxamide
Example 208A
Methyl4-(4-f(E)-3-(tert-butyloxy)-3-oxo-1-pro~enyllphenoxylthienof2 3-
clpyridine 2
carboxvlate
A flask, purged with nitrogen, was charged with Example 73 (50 mg, I.37 mmol),
Pd2{dba)3 (63 mg, 0.069 mmol), tri-o-tolylphosphine (64 mg, 0.21 mmol), dry
degassed
DMF (20 mL)> t-butyl acrylate (602 mL, 4.11 mmol), and triethylamine (575 mL,
4.1 I
mmol). This suspension was stirred at 100 °C under nitrogen for I2
hours. After diluting
with ethyl acetate, the reaction mixture was washed with brine, water, dried
(MgS04), and
concentrated. The residue was flash chromatographed on silica gel with 20%
EtOAc/hexane to provide the title compound (323 mg, 57%).
MS (DCI/NH3) m/e: 4I2 (M+H)+.
Examule 208B
Methyl 4-d4-f(E)-nropenoic acid-I-yllphenoxy)thienof2 3-clpyridine-2-
carboxylate
A solution of Example 208A ( 1.76 g, 4.2 mmol) in chloroform (50 mL) was
treated
with trifluoroacetic acid ( I O mL) at room temperature for 4 hours, and was
then poured
into ice-cold aqeous NaHCO~. The formed white solid was collected by
filtration, washed
167
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
with water, MeOH, CH2C12, and dried to provide the designated compound ( 1.38
g,
100%).
MS (DCI/NH~) m/e: 356 (M+H)+.
Example 208C
Methyl4-~[4-((E)-3-(4-morpholinyl)-3-oxo-1-propenyllphenoxylthienof2, 3-
clnvridine-2-
carboxylate
A solution of Example 208B (260 mg, 0.73 mmol) in a mixture of DMF (5 mL)
and CH2C12 ( 10 mL) was treated with morpholine ( 127 mg, 1.46 mmol), PyBOP
(760 mg,
1.46 mmol} and DIPEA (380 mg, 2.92 mmol) at room temperature for 12 hours.
After
diluting with CH2C12, the solution was washed with brine, dried (MgS04), and
concentrated. The residue was flash chromatographed on silica gel with 90%
EtOAc/hexane to give the designated compound.
MS (DCI/NH3) m/e: 425 (M+H)+.
Example 208D
4-~4-f(E)-3-(4-morc~holinyl)-3-oxo-1-propenyl]phenoxy)thienof2 3-clpyridine-2
carboxamide
Example 208D was prepared as in Example 61 but substituting Example 208C for
Example 61A to provide the title compound.
MS (DCI/NH3) m/e: 410 (M+H)+;
~H NMR (300 MHz, DMSO-d6} b 3.59 (m, 6H), 3.70 (m, 2H), 7.13 (d, 2H, J=8.5
Hz),
7.20 (d, 1H, J=15.5 Hz}, 7.52 (d, 1H, J=15.5 Hz), 7.79 (d, 2H, J=8.5 Hz), 7.86
(s, 1H},
8.16 (s, IH), 8.I8 (s, 1H), 8.45 (s, 1H), 9.17 (s, IH).
Example 209
4-f4-((E)-3-1f2-(4-mornholinyl)ethyllaminol-3-oxo-1-propenyl)phenoxylthienof2
3
clpyridine-2-carboxamide
Example 209A
Methyl 4-f4-((E)-3-( f2-(4-momholinyl)ethyllaminol-3-oxo-
lpropenyl)phenoxylthienof2
3-clpyridine-2-carboxylate
Example 209A was prepared as in Example 208C but substituting 4-(2-
aminoethyl)morpholine for morpholine to provide the designated compound.
MS (DCI/NH3) m/e: 468 (M+H)+.
168
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 209B
4-f4-((E)-3-df2-(4-morpholinyl)ethyllamino~-3-oxo-1-propenyl~phenoxylthienof2
3-
clpyridine-2-carboxamide
Example 209B was prepared as in Example 61 but substituting Example 209A for
Example 61A to provide the title compound.
MS (DCI/NH3) m/e: 453 (M+H)+;
'H NMR (300 MHz, DMSO-db) 8 2.44 (m, 4H), 3.30 (m, 4H), 3.59 (t, 4H, J=4.8
Hz), 6.60
(d, 1H, J=15.8 Hz), 7.13 (d, 2H, J=8.8 Hz), 7.42 (d, 1H, J=15.8 Hz), 7.61 (d,
2H, J=8.8
Hz), 7.87 (s, 1H), 8.06 (t, 1H, J=4.8 Hz), 8.16 (s, 1H), 8.21 (s, 1H), 8.45
(s, 1H), 9.17 (s,
1H).
Example 210
N-methyl-4-f4-((E)-3-( f 2-(4-mo holinyl)ethyllamino ~-3-oxo-1-
propenyl)nhenoxylthienof2, 3-clpyridine-2-carboxamide
Example 210 was prepared as in Example 103 but substituting Example 209A for
Example 61 A to provide the title compound.
MS (DCI/NH3) m/e: 467 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.38 (m, 4H), 2.79 (d, 3H, J=4.4 Hz), 3.59 (m,
8H),
6.58 (d, IH, J=15.8 Hz), 7.11 (d, 2H, J=8.8 Hz), 7.42 (d, 1H, J=15.8 Hz), 7.61
(d, 2H,
J=8.8 Hz), 8.05 (s, IH), 8.23 (s, 1H), 8.95 (q, 1H, J=4.4 Hz), 9.17 (s, 1H).
Example 211
4-(4-1(E)-3-f(2 3-Dihydroxypropyl)aminol-3-oxo-1 propen~phenoxy)thienof2 3
clpyridine-2-carboxamide
Example 211 A
Methyl4-(4-~(E)-3-f(2 3-Dihydroxypronyl)aminol-3-oxo-1-propen
r~phenoxy)thienof2
3-clpyridine-2-carboxylate
A solution of Example 208B (250 mg, 0.71 mmol)) in DMF ( 10 mL) was treated
with 3-amino-1, 2-propanediol (128 mg, 1.41 mmol), EDC (270 mg, 1.41 mmol),
HOBt
( 191 mg, 1.41 mmol) and triethylamine ( 142 mg, 1.41 mmol) at room
temperature for I 8
hours. After diluting with EtOAc, the reaction mixture was washed with brine,
dried
(MgS04) and concentrated. The residue was flash chromatographed on silica gel
to
provide the designated compound ( 189 mg, 63%).
MS (DCI/NH3) m/e: 429 (M+H)+.
169
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 211B
4-(4-((E)-3-f(2, 3-Dihydroxynronyl)aminol-3-oxo-1 propenyllphenoxy)thienof2 3
c~pyridine-2-carboxamide
Example 211B was prepared as in Example 61 but substituting Example 211A for
Example 61A to provide the title compound.
mp 185-187 °C;
MS (DC1/NH3) m/e: 4I4 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 3.10 (m, IH), 3.30 (m, 2H), 3.54 (m, 1H), 4.60 (t,
IH,
J=5.9 Hz), 4.84 (d, 1H, J=4.8 Hz), 6.66 (d, IH, J=I5.8 Hz), 7.13 (d, 2H, J=8.8
Hz), 7.42
(d, 1H, J=15.8 Hz), 7.61 (d, 2H, J=8.8 Hz), 7.86 (s, 1H), 8.08 (t, IH, J=5.5
Hz), 8.16 (s,
1 H), 8.21 (s, 1 H), 8.45 (s, 1 H), 9.17 (s, 1 H);
Anal. calcd for C2pH~9N3O5S: C, 58.10; H, 4.63; N, 10.16. Found: C, 57.99; H,
4.54; N,
10.08.
Example 212
4-(4-((E)-3-f(2, 3-Dihydroxypropyl)aminol-3-oxo-1 propenyllphenoxy) N
methylthienof2, 3-clpyridine-2-carboxamide
Example 212 was prepared as in Example 103 but substituting Example 211A for
Example 61 A to provide the title compound.
mp 225-226 °C;
MS (DCI/NH3) m/e: 428 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.79 (d, 3H, J=4.8 Hz), 3.10 (m, 1H), 3.30 (m,
2H),
3.54 (m, 1H), 4.60 (t, 1H, J=5.5 Hz), 4.84 (d, 1H, J=4.8 Hz), 6.66 (d, IH,
J=15.8 Hz), 7.11
(d, 2H, J=8.8 Hz), 7.42 (d, IH, J=15.8 Hz), 7.61 (d, 2H, J=8.8 Hz), 8.06 (s,
1H), 8.08 (t,
IH, J=5.5 Hz), 8.23 (s, IH), 8.97 (q, IH, J=4.8 Hz), 9.18 (s, 1H);
Anal. calcd for CZ~H21N305S: C, 59.00; H, 4.95; N, 9.83. Found: C, 58.85; H,
4.90; N,
9.58.
Example 2I3
4-(4-((E)-3-((2-(IH-Imidazol-4-yl)ethyllamino)-3-oxo-I-propen rZl)phenoxyl N
methylthienof2, 3-clQyridine-2-carboxamide
Example 2I3 was prepared as in Example 212 but substituting 2-(1H-imidazol-5-
yl)ethylamine for 3-amino-I, 2-propanediol to provide the title compound.
MS (DCI/NH3) m/e: 448 (M+H)+;
'H NMR (300 MHz, DMSO-d6) S 2.79 (d, 3H, J=4.5 Hz), 2.85 (t, 2H, J=6.6 Hz),
3.49 (q,
2H, J=6.0 Hz), 6.53 (d, 1 H, J=I 5.8 Hz), 7.11 (d, 2H, J=8.5 Hz), 7.42 (d, 1
H, J=15.8 Hz),
170
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
7.47 (s, 1H), 7.61 (d, 2H, J=8.5 Hz), 8.07 (s, 1H), 8.24 (s, 1H), 8.27 (t, 1H,
J=5.5 Hz), 8.97
(q, 1H, J=4.8 Hz), 9.01 (s, !H), 9.21 (s, 1H).
Example 214
4-(4-f(E)-3-((2-fbis(2-Hydroxyethyl)aminolethyl)amino)-3-oxo-1-
propen~l]phenoxy~-N-
methylthienof2, 3-clpyridine-2-carboxamide
Example 214 was prepared as in Example 212 but substituting 2-[bis(2-
hydroxyethyl)amino]ethylamine for 3-amino-l, 2-propanediol to provide the
title
compound.
MS (DCI/NH3) m/e: 485 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.79 (d, 3H, J=4.8 Hz), 3.34 (m, 6H), 3.58 (q, 2H,
J=6.1
Hz), 3.77 (t, 4H, J=5.1 Hz), 6.55 (d, 1H, J=15.6 Hz), 7.13 (d, 2H, J=8.5 Hz),
7.48 (d, 1H,
J=15.6 Hz), 7.64 (d, 2H, J=8.5 Hz), 8.07 (s, 1H), 8.24 (s, 1H), 8.43 (t, 1H,
J=4.8 Hz), 8.97
(q, 1H, J=4.8 Hz), 9.20 (s, 1H).
Example 215
4-~ 4- f (E)-3-( ( 2-fBis(2-hydroxyethyl)aminolethyl 1 amino)-3-oxo-1
pronenyllphenoxylthienof2, 3-clpyridine-2-carboxamide
Example 215 was prepared as in Example 211 but substituting bis(2-
hydroxyethyl)aminoethylamine for 3-amino-l, 2-propanediol to provide the title
compound.
MS (DCI/NH3) m/e: 471 (M+H)+;
'H NMR (300 MHz, DMSO-db) S 2.56 (m, 4H), 3.21 (m, 2H), 3.41 (m, 4H), 4.37 (t,
2H,
J=5.6 Hz), 6.56 (d, 1H, J=15.4 Hz), 7.13 (d, 2H, J=8.8 Hz), 7.42 (d, 1H,
J=15.4 Hz), 7.61
(d, 2H, J=8.8 Hz), 7.86 (s, 1H), 8.00 (t, 1H, J=5.5 Hz), 8.15 (s, 1H), 8.21
(s, 1H), 8.45 (s,
1 H), 9.17 (s, 1 H);
Anal. calcd for C23H26N4OSS.CH3OH: C, 56.36; H, 5.41; N, 11.15. Found: C,
56.40; H,
5.76; N, 11.40.
Example 216
4-(4-{3-Hydroxy-3-f4-((2-f(methylamino)carbo~llthienof2 3-clpyridin-4
yl ~ oxy)phenyllbutanoyl 1 nhenoxy)-N-methylthienof 2 3-clpyridine-2-
carboxamide
A solution of Example 202 (200 mg, 0.45 mmol) in THF (5 mL) was treated with
methylmagnesium bromide (3 M solution in ether, 0.18 mL, 0.55 mmol) at -50
°C for 30
minutes, and slowly warmed up to room temperature for 10 minutes. An aqueous
NH4C1
solution was added, and the mixture was extracted with ether. The combined
organic
171
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
phases were washed with brine, water, dried (MgS04), and concentrated. The
residue was
flash chromatographed on silica gel with 5% MeOH/EtOAc to provide the title
compound
(60 mg, 40%).
MS (ESI/NH3) m/e: 653 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 1.58 (s, 3H), 2.77 (d, 3H, J=4.8 Hz), 2.80 (d, 3H,
J=4.8
Hz), 3.35 (d, 1H, J=13.0 Hz), 3.49 (d, 1H, J=13.0 Hz), 5.27 (s, 1H), 7.02 (d,
2H, J=8.8
Hz), 7.08 (d, 2H, J=8.8 Hz}, 7.53 (d, 2H, J=8.8 Hz), 7.96 (d, 2H, J=8.8 Hz),
7.99 (s, 2H),
8.I3 (s, 1H), 8.30 (s, 1H), 8.94 {m, 2H), 9.08 (s, 1H), 9.21 (s, 1H);
Anal. calcd for C34HZgN4O6S2.CH3OH: C, 61.39; H, 4.27; N, 8.18. Found: C,
61.26; H,
4.29; N, 7.95.
Example 217
4-f4-(1H-Imidazol-1-yl)phenoxylthieno(2, 3-clpyridine-2-carboxamide
Example 217A
Methyl4-f4-(1H-imidazol-1-yl)phenoxylthienof2, 3-clpyridine-2-carboxylate
Example 17A (0.88 g, 5 mmol) in THF ( 15 mL) and DMF (5 mL) was treated with
4-( 1-imidazolyl)phenol and potassium t-butoxide ( 1 N in THF, 5.0 mL, 5 mmol)
at 70 °C
for 4 hours, then cooled to 0 °C, added methyl thioglycolate (0.4 mL, 5
mmol) and cesium
carbonate ( 1.62 g, 5 mmol) then refluxed for 1 hour. The reaction was poured
into water,
diluted with brine and extracted with ethyl acetate. The ethyl acetate was
then washed
with 1 N NaOH (2x20 mL), then brine (3x20 mL), dried (MgS04) to give the
titled
compound.
MS (DCI/NH3) m/e: 352 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 3.90 (s, 3H), 7.10 (s, 1H), 7.30 (d, 2H), 7.70 (s,
1H),
7.25 (d, 2H), 8.00 (s, 1H), 8.25 (d, 2H), 9.25 (s, 1H).
Example 217B
4-E4-(1H-Imidazol-1-yl)phenoxylthieno(2, 3-clpyridine-2-carboxamide
Example 217A was dissolved in 2 M methanolic ammonia and warmed at 50
°C in
a sealed tube for 24 hours. The reaction was then evaporated and crystallized
from
methanol to give the titled compound.
mp 310-312 °C;
MS (DCI/NH~) m/e: 337 (M+H)+;
'H NMR (300 MHz, DMSO-db) b 7.10 (s, 1H), 7.28 (m, 2H), 7.68 (t, 1H), 7.25
(dd, 2H),
7.85 (br s, 1H), 8.15 (s, 1H), 8.20 (d, 2H), 8.45 (br s, 1H), 9.15 (s, 1H);
172
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Anal. Calcd for C,7H,ZN4OZS O.SO HZO: C, 59.12; H, 3.79; N, 16.22. Found: C,
59.40; H,
3.63; N, 16.30.
Example 218
N-Methyl-4-f4-(1H-pyrazol-1-~phenoxylthienof2, 3-clpyridine-2-carboxamide
Example 218A
Meth-4-(4-(1H-pyrazol-1-~phenoxy)thienolf2, 3-clpyridine-2-carboxylate
Example 17A (0.8 g, 5 mmol) in THF (15 mL) was treated with 4-(1H-pyrazol-1-
yl)phenol and cesium carbonate ( 1.6 g, 5 mmol) under reflux for 4 hours, then
cooled to 0
°C, then methyl thioglycolate (0.4 mL, 5 mmol) and cesium carbonate (
1.62 g, 5 mmol)
were added, then the mixture was refluxed for 1 hour. The mixture was poured
into water,
diluted with brine and extracted with ethyl acetate. The ethyl acetate was
then washed
with 1 N NaOH (2x20 mL), then brine (3x20 mL), dried (MgS04) to give the
titled
compound.
MS (DCI/NH3) m/e: 352 (M+H)+;
'H NMR (300 MHz, DM50-d6) 8 3.80 (s, 3H}, 6.55 (m, 1H), 7.30 (d, 2H), 7.42 (d,
1H),
7.75 (d, 1H), 7.90 (d, 2H), 8.25 (s, 1H), 8.50 (d, 1H}, 9.22 (s, 1H).
Example 218B
N-Methyl-4-f4-(1H-pyrazol-1-~phenoxylthienof2, 3-clpyridine-2-carboxamide
Example 218A was dissolved in 2 M methanolic methylamine and warmed at 50
°C in a round bottom flask for 4 hours. The reaction was then
evaporated and crystallized
from methanol to give the titled compound.
mp 192-194 °C;
MS (DCI/NH3) m/e: 351 (M + H)+;
'H NMR (300 MHz, DMSO-d6) S 2.70 (d, 3H), 6.55 (m, 1H), 7.25 (d, 2H), 7.75 (br
s, 1H),
7.90 (d, 2H), 8.12 (s, 1H), 8.20 (s, 1H), 8.50 (d, 1H), 9.00 (m, 1H), 9.18 (s,
1H);
Anal. Calcd for C,gHz4N402S 0.25 HBO: C, 60.15; H, 4.21; N, 15.59. Found: C,
60.30; H,
3.93; N, 15.73.
Example 219
N-Methyl-4-f4-(1H-1, 2, 4-triazol-1-yl)phenoxylthienof2, 3-clpyridine-2-
carboxamide
Example 17A and 4-(1H-l, 2, 4-triazol-1-yl)phenol Were processed as in Example
218 to provide the title compound.
mp 214-215 °C;
173
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
MS (DCI/NH3) m/e: 352 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.70 (d, 3H), 7.30 (d, 2H), 7.55 (b, 1H), 7.90 (d,
2H),
8.12 (s, 1 H), 8.25 (d, 1 H), 9.00 (q, 1 H), 9.30 (s, 1 H).
Example 220
N-Methyl-4-~4-f5-(trifluoromethyl)-1 2 4-oxadiazol-3-~]phenoxy~thienof2 3
clpyridine-2-carboxamide
Example 220A
N-Methyl-4-f4-(N-hydroxyamindino)phenoxylthienof2 3-clpyridine 2 carboxamide
A solution of Example 186 (500 mg, 1.62 mmol) in a nuxture of DMF (10 mL)
and EtOH (10 mL) was treated with triethylamine (279 mg, 2.75 mmol) and
hydroxylamine hydrochloride ( 169 mg, 2.43 mmol) at room temperature for 18
hours. The
formed white solid was collected by filtration, washed with EtOH, dried to
provide the
designated compound (376 mg, 68%).
MS (ESI/NH3) m/e: 343 (M+H)+.
Example 220B
N-Methyl-4-~4-f5-(trifluoromethyl)-1 2 4-oxadiazol-3-yllphenoxy)thienof2 3
clpyridine-2-carboxamide
A suspension of Example 220A (200 mg, 0.58 mmol) in pyridine (8 mL) was
treated with trifluoroacetic anhydride ( 178 mg, 0.85 mmol) at room
temperature for 1
hour. The resultant yellow solution was heated at 120 °C for 18 hours,
and was then
concentrated. The residue was separated by HPLC (C-18, CH3CN/H20 containing
0.1%
TFA) to provide the title compound (169 mg, 69%).
mp 174-176 °C;
MS (ESI/NH3) m/e: 421 (M+H)+;
'H NMR (300 MHz, DMSO-db) b 2.78 (d, 3H, J=4.4 Hz), 7.26 (d, 2H, J=8.8 Hz),
8.03 (s,
1H), 8.10 (d, 2H, J=8.8 Hz), 8.38 (s, 2H), 8.96 (q, 1H, J=4.4 Hz), 9.25 (s,
1H);
Anal. calcd for C~gH1~N403SF3: C, 51.43; H, 2.64; N, 13.33. Found: C, 51, 56;
H, 2.76;
N, 13.32.
Example 22I
4-j4-(4, 5-Dihydro-1H-imidazol-2-yl)phenoxy -N-methylthienof2 3 clpyridine 2
carboxamide
174
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
A solution of Example I86 (800 mg, 2.6 mmol) in a mixture of MeOH (30 mL),
Et20 (20 mL), and CH2Cl2 (30 mL) was introduced hydrogen chloride gas at 0
°C for I.5
hours, stirred at room temperature for 24 hours, and concentrated. The residue
was
dissolved in MeOH (30 mL) and ethylenediamine (3 mL), and heated at 70
°C for 2 hours.
After the reaction was cooled, the resultant white solid was collected by
filtration, washed
with methanol, and dried to provide the title compound (804 mg, 88%).
mp >280 °C;
MS (ESI/NH3) m/e: 353 (M+H)+;
IH NMR (300 MHz, DMSO-d6) S 2.78 (d, 3H, J=4.4 Hz), 3.32 (br s, 4H), 6.88 (br
s, 1H),
7.11 (d, 2H, J=8.8 Hz), 7.85 (d, 2H, J=8.8 Hz), 8.04 (s, IH), 8.22 (s, 2H),
8.93 (q, 1H,
J=4.4 Hz), 9.17 (s, 1H);
Anal. calcd for C,gH~6N402S: C, 59.36; H, 4.46; N, 14.57. Found: C, 59.60; H,
4.55; N,
14.40.
Example 222
N-Methyl-4-f4-(2-thien~phenoxylthienof2, 3-clpyridine-2-carboxamide
A flask, purged with nitrogen, was charged with Example 190B (200 mg, 0.48
mmol), Pd(OAc)2 ( 1 I mg, 0.048 mmol), tri-o-tolylphosphine (44 mg, 0.14
mmol), dry
degassed DMF ( 10 mL), 2-tributylstannylthiophene (305 mL, 0.96 mmol), and
triethylamine (334 mL, 2.4 mmol). This suspension was stirred at 80 °C
for 15 hours.
After diluting with ethyl acetate, the reaction mixture was washed with brine,
H20, dried
(MgS04), and concentrated. The residue was separated by HPLC (C-18, CH3CN/H20
containing 0.1% TFA) to provide the title compound (212 mg, 90%).
MS (ESI/NH3) m/e: 367 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.80 (d, 3H, J=4.4 Hz), 7.13 (m, 1H), 7.17 (d, 2H,
J=8.8
Hz), 7.48 (d, 1 H, J=3.7 Hz), 7.54 (d, 1 H, J=5.1 Hz), 7.71 (d, 2H, J=8.8 Hz),
8.1 S (s, 1 H),
8.24 (s, 1H), 9.02 (q, 1H, J=4.4 Hz), 9.22 (s, IH).
Example 223
4-(f 1. 1'-Binhenvll-4-vloxv)-N-methvlthienof2, 3-cltwridine-2-carboxamide
Example 223 was prepared as in Example 222 but substituting tributylphenyltin
for
(tributylstannyl)thiophene to provide the title compound.
MS (ESI/NH3) m/e: 361 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.80 (d, 3H, J=4.5 Hz), 7.19 (d, 2H, J=8.8 Hz),
7.36 (t,
1H, J=7.4 Hz), 7.47 (t, 2H, J=7.3 Hz), 7.66 (d, 2H, J=7.3 Hz), 7.72 (d, 2H,
J=8.8 Hz), 8.15
(s, IH), 8.23 (s, IH), 9.00 (q, 1H, J=4.4 Hz), 9.19 (s, LH}.
175
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 224
N-Methyl-4-(4-(1-methyl-1H-imidazol-5-yl)nhenoxylthienof2, 3-clpyridine-2
carboxamide
Example 224 was prepared as in Example 222 but substituting 1-methyl-(5-
tributylstannyl)imidazole, which was prepared according to a literature method
(K. Gaare,
et al., Acta Chem. Scand. 1993, 47, 57), for 2-tributylstannylthiophene to
provide the title
compound.
mp 256-258°C;
MS (ESI/NH3) m/e 365(M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.80 (d, 3H, J=2.1 Hz), 3.67 (s, 3H), 7.03 (s,
1H), 7.17
(d, 2H, J=8.8 Hz), 7.53 (d, 2H, J=8.8 Hz), 7.69 (s, 1H), 8.12 (s, 1H), 8.22
(s, 1H), 9.00 (q,
1H, J=2.I Hz), 9.16 (s, 1H);
Anal. calcd for C19H~6N402S: C, 62.62; H, 4.43; N, 15.37. Found: C, 62.38; H,
4.23; N,
15.13.
Example 225
4-(4-f 1-(Hydro~methyl)cyclopropyllphenoxy 1-N-methylthienof2, 3-clpyridine-2
carboxamide
Example 225A
4-( 1-Hydroxymethylcyclopropyl)anisole
A solution of 1-(4-rnethoxyphenyl)-1-cyclopropane-carboxylic acid (5.0 g, 26
mmol) in THF ( 100 mL) was slowly treated with LiAlH4 (0.95 g, 25 mmol) at -20
°C for
0.5 hour, and was then warmed to room temperature for 2 hours. The excess
LiAlH4 was
consumed by slowly adding EtOH. After diluting with ether, the reaction
mixture was
washed with 2% HCl in brine, water, dried (MgS04) and concentrated to provide
the
designated compound (5.0 g, 100%).
MS (DCI/NH3) m/e: 196 (M+NH4)+.
Example 225B
4-( 1-Hydrox~methylcyclopropyl)phenol
To a suspension of NaH (60% in mineral oil, 392 mg, 9.8 mmol) in DMF (10 mL)
was slowly added neat ethanethiol (610 mg, 9.8 mmol) at room temperature. The
reaction
mixture was stirred for 10 minutes to form a clear solution. Example 225A
(500mg, 2.8
mmol) was then added, and the mixture was heated at 145 °C for 4 hours.
After diluting
176
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
with ether, the reaction mixture was washed with 2% HCl in brine, dried
(MgS04) and
concentrated. The residue was flash chromatographed on silica gel with 50%
EtOAc/hexane to provide the designated compound (373 mg, 81 %).
MS (DCI/NH3) m/e: 182 (M+NH4)+.
Example 225C
4- 1-Triphenylmethoxymeth~!lcyclopropyl)nhenol
A solution of Example 225B ( 1.0 g, 6 mmol) in pyridine (7 mL) was treated
with
triphenylmethyl chloride ( 1.87g, 6.7 mmol) at room temperature for 18 hours.
After
diluting with ether, the reaction mixture was washed 1 % aqeous HCI, water and
dried
(MgS04). The residue was flash chromatographed on silica gel with 12%
EtOAc/hexane
to provide the designated compound.
MS (DCI/NH3) m/e: 407 (M+H)+.
Example 225D
Methyl4-f4-(1-triphenylmethoxymethyl)cyclopropyllphenoxy-f2, 3-clpyridine-2
carboxylate
Example 225D was prepared as in Example 61 A but substituting Example 225C
for 4-chlorophenol to provide the designated compound.
MS (DCI/NH3) m/e: 598 (M+H)+.
Example 225E
Methyl4-f4-(1-hydroxvmethyl)cyclopropyllnhenoxy-f2, 3-clpyridine-2-carboxylate
A solution of Example 225D (230 mg, 0.38 mmol) in a mixture of CH2C12. ( 10
mL)
and MeOH (5 mL) was treated with trifluoroacetic acid (1 mL) at 0 °C
for 1 hour, allowed
to warm to room temperature and stir for for 1 hour, and was poured into
aqeous NaHC03
solution. The mixture was extracted with methylene chloride. The combined
organic
phases were washed with water, and dried (MgS04). The residue was flash
chromatographed on silica gel with 65% EtOAc/hexane to provide the designated
compound (78 mg, 58%).
MS (DCI/NH~) m/e: 356 (M+H)+.
Example 225F
4-f4-(1-H~droxymethyl)cycloprop ~~llphenoxy-N-methylthieno(2, 3-clpyridine-2
carboxamide
177
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 225F was prepared as in Example 103 but substituting Example 225E for
Example 61 A to provide the designated compound.
MS (ESI/NH3) m/e: 355 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 0.72 (m, 2H), 0.82 (m, 2H), 2.80 (d, 3H, J=4.7
Hz),
3.51 (d, 2H, J=5.8 Hz), 4.66 (t, 1 H, J=5.8 Hz), 7.01 (d, 2H, J=8.8 Hz), 7.34
(d, 2H, J=8.8
Hz), 8.06 (s, 1H), 8.13 (s, 1H), 8.96 (g, 1H, J=4.7 Hz), 9.10 (s, 1H).
Example 226
4-f4-(1-(f2-(2-Ethoxyethox )e~thoxylmethyllcyclopropyl)phenoxyl-N-
methylthienof2, 3-
c]pyridine-2-carboxamide
Example 226A
4-(2-(2-Ethoxyethoxy)ethoxy)methylcyclopropyl)anisole
A solution of Example 225A ( 1.0 g, 5.6 mmol) in THF ( 15 mL) was treated with
NaH (60% in mineral oil, 312 mg, 7.8 mmol) and 15-crown-5 (1.33 mL, 6.7 mmol)
at
room temperature for 15 minutes follwed by addition of 2-(2-ethoxyethoxy)ethyl
tosylate
( 1.93 g, 6.7 mmol) which was prepared according to literature method (C.
Almansa, et al.,
Tetrahedron 1991, 47, 5867). The brown slurry was stirred at room temperature
for 5
hours and was poured into brine. The mixture was extracted with CHZCl2, and
the
combined organic phases were dried (MgS04) and concentrated. The residue was
flash
chromatographed on silica gel with 25% EtOAc/hexane to provide the designated
compound ( 1.58 g, 95%).
MS (ESI/NH3) m/e: 312(M+NH4)+.
Example 226B
4-(2-(2-Ethoxyethoxy)ethox )~ylc~propyl)phenol
A solution of Example 226A ( 1.5 g, 5.1 mmol) in DMF ( 15 mL) was treated with
sodium thiomethoxide ( 1.25 g, 17.8 mmol) at 145°C for 5 hours. After
cooling to room
temperature methylene chloride ( 100mL) was added, and the mixture was washed
with 2%
HCl in brine. The organic layer was dried (MgS04), concentrated and the
residue was
flash chromatographed on silica gel with 35% EtOAc/hexane to provide the
designated
compound (1.33g, 93%).
MS (ES1/NH3) m/e: 298(M+NH4)+.
Example 226C
178
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Methyl 4-f4-( 1-( f 2-(2-Ethoxyethoxy)ethoxylmethyl )cyclopronyl)nhenoxyl-N
methylthienof2, 3-clpyridine-2-carboxylate
Example 226C was prepared as in Example 61A but substituting Example 226B
for 4-chlorophenol to provide the designated compound.
MS (ESI/NH3) m/e: 472(M+H)+.
Example 226D
4-f4-(1-lf2-(2-Ethoxyethoxy~ethoxylmethyllcyclopropyl)nhenoxyl-N-
methylthienof2, 3
~pyridine-2-carboxamide
Example 226D was prepared as in Example 103 but substituting Example 226C for
Example 61A to provide the title compound.
MS (ESI/NH3) m/e: 471(M+H)+.
tH NMR (300 MHz, DMSO-d6) 8 0.84 (m, 2H), 0.87 (m, 2H), 1.06 (t, 3H, J=6.7Hz),
2.82
(d, 3H, J=4.4Hz), 3.37 (t, 2H, J=6.7Hz), 3.39-3.55 (m, lOH), 7.07 (d, 2H,
J=8.8Hz), 7.36
{d, 2H, J=8.8Hz), 8.13 (s, 1H), 8.26 (s, 1H), 9.10 (q, 1H, J=4.4Hz), 9.28 (s,
1H);
Anal. calcd for C2gH3pNq.OSS: C, 59.22; H, 6.16; N, 5.52. Found: C, 59.50; H,
6.16; N,
5.26.
Example 227
N-Methyl-4-f4-(trifluoromethoxy)phenoxylthienof2, 3-clyyridine-2-carboxamide
Example 227 was prepared as in Example 103 but substituting 4-
trifluoromethoxyphenol for 4-chlorophenol to provide the title compound.
mp 132-133 °C;
MS (ESI/NH3) m/e: 368 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.80 (d, 3H, J=4.4 Hz), 7.20 (d, 2H, J=9.2 Hz),
7.41 (d,
2H, J=9.2 Hz), 8.08 (s, 1H), 8.21 (s, 1H), 8.95 (q, 1H, J=4.4 Hz), 9.18 (s,
1H);
Anal. calcd for C,6H~,NZO~SF3: C, 52.17; H, 3.01; N, 7.61. Found: C, 52.21; H,
3.26; N,
7.29.
Examele 228
S-f 4-f4-(1-( f2-(2-Ethox~ethoxy)ethoxylmethyl}cycloprop~phenoxylthienof2, 3
~pyridin-2-yll-1, 3, 4-oxadiazol-2-amine
Example 228 was prepared as in Example 275 and Example 156 but substituting
Example 226C for Example 61 A to provide the title compound.
mp 113-114 °C;
MS (ESI/NH3) m/e: 497 (M+H)+;
179
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
'H NMR (300 MHz, DMSO-d6) b 0.82 (m, 2H), 0.85 (m, 2H), 1.05 (t, 3H, J=7.1
Hz), 3.40
(t, 2H, J=7.1 Hz), 3.42-3.54 (m, lOH), 7.00 (d, 2H, J=8.8 Hz), 7.33 (d, 2H,
J=8.8 Hz), 7.55
(s, IH), 7.58 (s, 2H), 8.18 (br s, 1H), 9.15 (br s, 1H).
Example 229
4-f4-(1 I-Difluoro-2-hydroxyethyI)phenoxyl-N-methylthienof2, 3-clpyridine-2
carboxamide
Example 229A
4-f4-(1 1-Ddifluoro-2-ethoxy-2-oxoethyl)phenoxyl-N-methylthienol2, 3-
clpyridine-2-
carboxamide
A suspension of activated copper (512 mg, 8 mmol) in dry DMSO (5 mL) was
treated with ethyl iododifluoroacetate ( I .0 g, 4 mmol) at roam temperature
for 10 minutes.
Phenol (188 mg, 2 mmol) and Example 190B were then added. The reaction mixture
was
stirred at room temperature for 20 hours. After diluted with 1:1 ether/EtOAc,
the mixture
was washed with 1%HCI in brine, water, dried (MgS04) and concentrated. The
residue
was flash chromatographed on silica gel with 65% EtOAc/hexane, and was further
purified
on HPLC (C-18, CH3CN/H20 containing 0.1% TFA) to provide the designated
compound
(85 mg, 15%).
MS (ESI/NH3) m/e: 407 (M+H)+.
Example 229B
4-f4-(1 1-Difluoro-2-h d~yeth~)nhenoxyl-N-methylthienof2, 3-clpyridine-2
carboxamide
A solution of Example 229A (40 mg, 0.1 mmol) in MeOH (5 mL) was treated
NaBH4 (50 mg) at room temperature for 2 hours. Brine was added, and the
mixture was
extracted with EtOAc. The combined organic phases were dried (Na2S04) and
concentrated. The residue was purified by HPLC (C-18, CH3CN/H~O containing
0.1%
TFA) to provide the title compound (44.4 mg, 94%).
MS (ESI/NH3) m/e: 365 (M+H)+;
1H NMR (300 MHz, CD~OD) S 2.94 (s, 3H), 3.93 (t, 2H, J=13.5 Hz), 7.27 (d, 2H,
J=9.2
Hz), 7.65 (d, 2H, J=9.2 Hz), 8.15 (s, 2H), 9.24 (s, 1H);
Anal. calcd for C,~Hi4N203SFz.TFA: C, 47.70; H, 3.16; N, 5.86. Found: C,
47.67; H,
3.10; N, 5.76.
Example 230
180
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-(4-12-f2-(2-Ethoxyethoxy)ethoxyl-1, 1-difluoroethyllphenoxy)-N-
methvlthienof2, 3
clpyridine-2-carboxamide
A solution of Example 229B (40 mg, 0.11 mmol) in THF (3 mL) was treated with
NaH (60% in mineral oil, 7 mg, 0.16 mmol) and 15-crown-5 (35 mg, 0.16 mmol) at
room
temperature for 15 minutes. 2-(2-ethoxyethoxy)ethyl tosylate (46 mg, 0.16
mmol) which
was prepared according to literature method (C. Almansa, et al, Tetrahedron
1991, 47,
5867), was then added. The reaction mixture was stirred at room temperature
for 15
hours, and was then directly separated on HPLC (C-18, CH~CN /H20 containing
0.1%
TFA) to provide the title compound (46 mg, 81%).
MS (ESI/NH3) m/e: 481 (M+H)+;
1H NMR (300 MHz, CD30D) 8 1.16 (t, 3H, J=7.1 Hz), 2.97 (s, 3H), 3.49 (q, 2H,
J=7.1
Hz), 3.57 (m, 6H), 3.70 (m, 2H), 4.01 (t, 2H, J=12.7 Hz), 7.37 (d, 2H, J=8.8
Hz), 7.72 (d,
2H, J=8.8 Hz), 8.22 (s, 1H), 8.33 (s, 1H), 9.46 (s, 1H).
Example 231
4-(4-Chlorophenoxy)-6-( f (2, 2-dimethylpropanoyl)oxylmethyl ) -2-
1(methylamino)carbonyllthienof2, 3-clpyridin-6-ium Trifluoroacetate
Example 103 (47.4 mg, 0.149 mmol) was dissolved (under Nz atmosphere) in 1.5
mL dry acetonitrile (with warming), and chloromethyl pivalate (25 mg, 0.167
mmol) was
added at room temperature. The reaction was stirred for 16 hours, then
tetrabutylammonium iodide ( 1 mg) was added, and then the solution was warmed
to reflux
for 48 hours. The reaction mixture was concentrated under reduced pressure,
and the
residue was purified by preparative HPLC (C-18 column, gradient eluent 20-70%
acetonitrile-0.1 % aqueous TFA, 60 minutes elution) to provide 24 mg (34%) of
the title
compound as a foam.
HPLC: Supelco C-18 column, gradient eluent of 0.1% aqueous TFA:acetonitrile
0:90-
90:0 over 30 minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 20.0
minutes.
MS (APCI+) m/e: 433 (M)+;
1H NMR (300 MHz, DMSO-d6) 8 1.18 (s, 9H), 3.01 (s, 3H), 6.4-6.6 (br s, 2H),
7.1-7.3 (br
s, approx. 2H), 7.49 (br s, 2H), 7.74 (br s, 1H), 8.41 (br s, 1 H), 8.64 (br
s, 1 H), 9.3-9.9 (vbr
s, 1H);
Anal. calcd for C23H22F3C1zO6S: C, 45.43; H, 3.51; N, 4.24. Found: C, 45.93;
H, 3.70; N,
4.34.
Example 232
181
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
4-(4-Bromophenoxy)-6-f f(2, 2-dimethylpropanoyl)oxylmethylJ~-2
I(methylamino)carbonyllthienof2, 3-clpyridin-6-ium
Example 17I (69.4 mg, 0.191 mmol) was dissolved in 2 mL, acetonitrile, then
tetrabutylammonium iodide ( 1 mg) was added, followed by chloromethyl pivalate
(22 mg,
0.146 mmol). The reaction solution was warmed to reflex for 24 hours. An
additional
portion of chloromethyl pivalate ( 11 mg, 0.073 mmol) was added, and the
reaction was
refluxed for an additional 72 hours. The reaction was concentrated under
reduced
pressure, and the solid was partitioned between 15 mL water and 15 mL EtOAc.
The
aqueous phase was extracted with 2x15 mL, EtOAc, then the aqueous phase was
concentrated under reduced pressure to a yellow solid (93.5 mg). HPLC
purification (C-
18 column, 20-75% acetonitrile-O.I% aqueous TFA) provided pure title compound
(55.9
mg, 49%}.
HPLC: Supelco C-18 column, gradient eluent of 0.1 % aqueous TFA:acetonitrile
0:90
90:0 over 30 minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 20.3
minutes;
MS (APCI-) m/e: 475, 477 (M-H)-;
1H NMR (300 MHz, DMSO-d6) b I.17 (s, 9H), 3.02 (br m, 3H), 6.0-6.7 (vbr s,
2H), 7.14
(br d, 2H), 7.64 (br d, 2H), 7.78 (s, 1H}, 8.07-8.17 (br s, 1H), 8.34 (s, IH),
9.44-9.65 (vbr
s, IH);
Anal. calcd for Cz3H22F3BrNzO6S~I.5 H20: C, 40.99; H, 3.58; N, 3.82. Found: C,
40.94;
H, 3.25; N, 3.76.
Example 233
2-(Aminocarbonyl)-4-(4-chlorophenoxy)-6-1 ((isopropoxycarbonyl)oxy~methvl
lthienof2
3-clpyridin-6-ium
To a solution of Example 61 (300mg, 0.94mmo1) in acetonitrile ( ISmL) under a
nitrogen atmosphere was added tetraphenylboron sodium (387mg, 1. l3mmol),
sodium
iodide (169mg, 1.13mmol), and [(isopropyloxycarbonyl}oxyjmethyl chloride
(172mg,
1. l3mmol). The reaction mixture was heated at reflex for 4 hours, cooled to
room
temperature and diluted with acetonitrile ( 100 mL) before filtering through
Celite°. The
filtrate was concentrated to a foam that was triturated in methanol to afford
the pyridinium
tetraphenylborate salt (550 mg) as a yellow solid. The tetraphenylborate salt
dissolved in
l: I CH3CN:i-PrOH and passed over an ion exchange column using Dowex 1 X 2
chloride,
50-100 mesh. The eluent was concentrated and the resulting residue triturated
with Et20
to afford example 233 as a white solid (210mg, 49%).
MS (FAB} m/e: 421 (M+H)+;
182
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
'H NMR (300 MHz, DMSO-d6) 8 1.26 (d, J= 7 Hz, 6H), 4.81 (q, J=7 Hz, 1H), 6.44
(s,
2H), 7.41 (m, 2H), 7.62 (m, 2H), 8.31 (br s, 1H), 8.59 (s, 1H), 8.68 (s, 1H),
8.80 (br s, 1H),
9.98 (s, 1H);
Anal. calcd for Cl9H,gC1zN205S-: C, 49.90; H, 3.96; N, 6.13. Found: C, 49.74;
H, 3.95; N,
6.14.
Example 234
4- Cyclopentyloxy)-N-methylthienof2, 3-clpyridine-2-carboxamide
Example 234A
5-chloro-3-cyclopentyloxypyridine
The title compound (5.91 g, 77%) was prepared as described in Example 236A
except substituting cyclopentanol (4.2 mL, 46.31 mmol) for benzyl alcohol.
MS (APCI) m/e: 198 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 1.53-1.76 (m, 6H), 1.86-2.02 (m, 2H), 4.92-4.99
(m,
1H), 7.55 (t, J=2.25 Hz, 1H), 8.18 (d, J=2.25 Hz, 1H), 8.22 (d, J=3 Hz, 1H}.
Example 234B
5-Chloro 3-cyclopentylloxy pyridine-4-carboxaldehyde
The title compound (5.22 g, 77%) was prepared as described in Example 236B
except substituting Example 234A ( 5.9 g, 30 mmol) for Example 236A.
MS (APCI) m/e: 226 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 1.55-1.85 (m, 6H), 1.93-2.04 (m, 2H), 5.14-5.22
(m,
1H), 8.36 (s, 1H), 8.63 (s, 1H), 10.31 (s, IH).
Example 234C
Methyl 4-fcyclopentyloxylthienof2, 3-clpyridine-2-carboxylate
The title compound (4.31 g, 67%) was prepared as described in Example 236C
except substituting 234B (5.2 g, 23.11 mmol) for Example 236B.
MS (APCI) m/e: 278 (M+H)+;
'H NMR (400 MHz, DMSO-db) 8 1.59-1.69 (m, 2H), 1.74-1.81 (m, 2H), 1.83-1.90
(m,
2H), 1.97-2.07 (m, 2H), 3.93 (s, 3H), 5.12-5.17 (m, 1H), 8.05 (s, 1H), 8.24
(s, 1H), 8.94 (s,
1H);
'3C NMR ( 100 MHz, DMSO-db) 8 23.63 (CHZ), 32.30 (CH2), 52.98 (OCH3}, 80.33
(CH),
125.52 (CH), 127.09 (CH), 134.72 (C), 136.45 (C), 137.44 (CH), 138.05 (C),
149.27 (C),
161.90 (C=O).
183
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 234D
4- Cyclopentyloxylthienof2, 3-clpyridine-2-N-methylamide
Example 234C ( 1.6 g, 61 %) was prepared as described in Example 171 except
substituting Example 234C (2.6 g, 9.4 mmol) for Example 73A.
mp 216-217 °C;
MS (APCI) m/e: 277 (M+H)+, 244 (M+Cl)';
1H NMR {400 MHz, DMSO-d6) 8 1.60-1.69 (m, 2H), 1.73-1.90 (m, 4H), 1.97-2.08
(m,
2H), 2.83 (d, J=4 Hz, 3H), 5.10-5.17 (m, 1H), 8.11 (s, 1H), 8.19 (s, 1H), 8.85
(s, 1H), 8.93
(d, J=4 Hz, 1H);
'3C NMR ( 100 MHz, DMSO-db) 8 23.60 (CH2), 26.14 (NCH3), 32.34 (CH2}, 80.14
(CH),
119.68 (CH), 126.91 (CH), 135.73 (C), 137.17 (CH), 144.2 (C), 149.02 (C),
161.18 (C).
Example 235
4-(2-Cvclohexen-1-yloxy)-N-meth~thienof2, 3-clpyridine-2-carboxamide
Example 235A
Methyl4-f2-cyclohexene-3-oxyllthienof2, 3-clnvridine-2-carboxylate
The title compound (158 mg, 57%) was prepared as described in Example 99A
except substituting 2-cyclohexenol (0.113 mL, 0.115 mmol) for 3-hydroxy
tetrahydrofuran. Pure product was obtained by flash chromatography on silica
gel eluting
with 10% acetone-hexane.
MS (APCI) m/e: 290 (M+H)+, 288 (M-H)', 324 (M+Cl)-;
'H NMR (300 MHz, DMSO-db) S 1.95-1.62 (m, 1H), 1.66-2.14 (m, 5H), 3.92 (s, 3H,
OCH3}, 5.22-5.26 (m, 1H), 5.91-5.99 (m, 2H), 8.03 (s, 1H, CH), 8.36 (s, 1H,
CH), 8.95 (s,
1H, CH).
Example 235B
4-(2-Cyclohexen-1-yloxy)-N-methylthienof2, 3-clpyridine-2-carboxamide
The title compound Example 235 (59 mg, 40%) was prepared from Example 235A
( 150 mg, 0.519 mmol) as described in Example 171.
MS (APCI) m/e: 289 (M+H)+, 287 (M-H)', 323 (M+Cl)';
1H NMR (500 MHz, DMSO-d6) S 1.62-1.70 (m, 1H), 1.78-1.91 (m, 2H), 1.96-2.17
(m,
3H), 2.82 (d, J=5 Hz, 3H), 5.21 (br s, 1H), 5.93-5.98 (m, 1H), 6.01-6.05 (m,
1H), 8.14 (s,
1H), 8.30 (s, 1H), 8.87 (s, 1H), 8.75 (d, J=5 Hz, 1H);
184
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
'3C NMR (75 MHz, DMSO-db) b 18.2 (CH2), 24.6 (CH2), 26.2 (CH3), 27.7 (CH2),
71.3
(CH), 119.7 (CH), 125.6 (CH), 127.4 (CH), 132.6 (CH), 136.0 (C), 137.3 (C),
137.4 (CH),
144.1 (C), 149.0 (C), 161.2 (C).
Example 236
4-lBenzyloxy)thienof2, 3-clpyridine-2-carboxamide
Example 236A
5-Chloro-3-benzyloxypyridine
To a stirred solution of 5-chloro-3-pyridinol ( 10 g, 77.19 mmol) in anhydrous
tetrahydrofuran ( 155 mL ) at 0 °C and under nitrogen atmosphere was
added benzyl
alcohol (9.6 mL, 92.63 mmol), triphenylphosphine (26.32 g, 100.35 mmol)) and
diethyl
azodicarboxylate (I5.8 mL, 100.35 mmol). The reaction mixture was stirred at
room
temperature overnight and the solvent was removed under reduced pressure. The
residue
(70 g) obtained was treated with diethyl ether (2x300 mL) and the solids were
removed by
filtration. The filtrate obtained was concentrated under reduced pressure and
the residue
was purified by silica gel flash chromatography eluting with 5% acetone and
hexane to
obtain the title compound in 36% (5.8 g) yield.
MS (APCI) m/e: 220 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 5.22 (s, 2H), 7.33-7.49 (m, 5H), 7.77 (t, J=5 Hz,
1H),
8.23 (d, J=5 Hz, 1H), 8.34 (d, J=5 Hz, 1H).
Example 236B
5-Chloro 3-benzyloxy pyridine-4-carboxaldehyde
To a stirred solution of diisopropylamine (4.5 mL, 31.78 mmol) in anhydrous
tetrahydrofuran (20 mL) under nitrogen atmosphere at -5 °C was added
dropwise n-BuLi
in hexanes (2.5 M solution, 12.8 mL, 31.78 mmol) maintaining internal
temperature of the
reaction mixture below 0 °C. The reaction mixture was stirred at -10
°C for 10 minutes,
then at 0 °C for 30 minutes. This was cooled to -78 °C and a
solution of Example 236A
(5.8 g, 26.5 mmol) in anhydrous tetrahydrofuran (30 mL) was added slowly.
Stirring at -
78 °C was continued for 1 hour. Then the reaction was quenched with
dropwise addition
of methyl formate (5 mL, 79.5 mmol) in anhydrous THF (15 mL) and stirred at -
78 °C for
3.5 hours. Internal temperature of the reaction mixture was maintained at or
below -74 °C
throughout the reaction. After 3.5 hours, the reaction mixture was poured into
an ice cold
saturated aqueous solution of NaHC03 (200 mL) and stirred for 15 minutes. The
mixture
was partitioned with ethyl acetate (250 mL), organic layer was separated and
washed with
185
CA 02333770 2000-11-30
WO 99!62908 PCT/US99/12419
brine (2x60 mL}. The dried (MgS04) organic layer was concentrated under
reduced
pressure to obtain the crude product (8.5 g). The title compound was obtained
in 75%
yield (4.2 g) by flash chromatography on silica gel eluting with 6% acetone-
hexane.
MS (APCI) m/e: 248 (M+H)+;
'H NMR (300 MHz, DMSO-db) d: 5.42 (s, 2H), 7.33-7.45 (m, 3H), 7.48-7.52 (in,
2H),
8.41 (s, 1H}, 8.72 (s, 1H), 10.39 (s, 1H).
Example 236C
Methyl 4-benzylox,~thienol2, 3-clpyridine-2-carboxylate
To an ice cold solution of Example 236B (4.2 g, 17 mmol) in anhydrous
tetrahydrofuran (42 mL) under nitrogen atmosphere was added methyl
thioglycolate ( 1.83
mL, 20.4 mmol) followed by powdered cesium carbonate (6.65 g, 20.4 mmol). Then
the
reaction mixture was allowed to warm to room temperature with stirring under
nitrogen.
After 30 minutes the reaction was refluxed for 15 minutes and cooled to room
temperature. The reaction mixture was quenched with ice (50 mL) and
partitioned with
ethyl acetate (250 mL). The organic layer separated was washed with an ice
cold solution
of saturated NaCI (3x60 mL), dried (Na2S04), and concentrated under reduced
pressure to
obtain the crude product which was recrystallized from methanol. The mother
liquor was
purified by flash chromatography on silica gel eluting with 7% acetone-hexane.
The
combined fractions gave the title compound in 55% yield (3.07 g).
MS (APCI) m/e: 300 (M+H)+;
IH NMR (300 MHz, DMSO-d6) d: 3.92 (s, 3H), 5.42 (s, 2H), 7.35-7.47 (m, 3H),
7.52-7.57
(m, 2H, Ar-CH), 8.12 (s, 1H, ), 8.36 (s, 1H), 8.98 (s, 1H}.
Example 236D
Methyl 4-hydroxylthienof2, 3-clpyridine-2-carboxylate
To a suspension of 10 wt % Pd on activated carbon (38 mg, 10% w/w) in absolute
ethanol (3 mL) was added a cold solution of Example 236C (380 mg, 1.3 mmol) in
ethanol
(82 mL) under nitrogen atmosphere. Then the reaction mixture was degassed and
stirred
at room temperature under hydrogen atmosphere. After one over night reaction
mixture
was treated with additional 10 wt % Pd on activated carbon ( 190 mg, 50% w/w)
and
stirred under hydrogen atmosphere. Additional catalyst ( 100 mg, 26% w/w) was
added to
the reaction mixture after 48 hours. The reaction mixture was stirred under
hydrogen
atmosphere for further 24 hours and filtered through Celite~. The filtrate was
evaporated
to dryness under reduced pressure to obtain the crude product ( 320 mg). The
title
186
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
compound Example 236E was obtained in 75% yield (200 mg) by flash
chromatography
on silica gel eluting with 5% acetone-hexane followed by 40 % acetone-hexane.
MS (APCI) m/e: 210 (M+H)+, 208 (M-H)-, 244 (M+Cl)-;
1H NMR (300 MHz, DMSO-d6) 8 3.93 (s, 3H), 8.08 (s, 1H), 8.21 (s, 1H), 8.81 (s,
1H),
10.66-10.90 (br.s, 1H).
Example 236E
4-Benzyloxythienof2, 3-clpyridine-2-carboxamide
The title compound ( 50 mg, 67%) was prepared as described in Example 44 using
Example 236C (75 mg, 0.25 mmol).
MS (APCI) m/e: 285 (M+H)+, 319 (M+Clj-;
1H NMR (400 MHz, DMSO-d6) 8 5.43 (s, 2H), 7.49-7.50 (m, 3H), 7.58-7.63 (m,
2H), 7.79
(br s, 1H), 8.30 (s, 1H), 8.36 (s, 1H, ), 8.48 (br s, 1H), 8.93 (s, 1H);
i3C NMR ( 100 MHz, DMSO-d6) d: 70.13 (CH2), 120.56 (CH), 126.21 (CH), 127.5
(CH),
128.14 (CH), 128.51 (CH), 135.30 (C), 136.35 (C), 137.48 (C), 137.86 (CH),
144.75 (C),
149.82 (C), 162.61 (C).
Example 237
4-(4-Chlorobenzoyl)-N-methylthienof2, 3-clpyridine-2-carboxamide
Example 237A
Methyl 4-chlorothienof2, 3-clpyridine-2-carboxylate
Example 17A (15.00 g, 85.22 mmol) was dissolved in THF (80 mL) and cesium
carbonate (27.77 g, 85.22 mmol} added. Methyl thioglycolate (7.62 mL, 85.22)
diluted in
THF (20 mL) was added dropwise over a period of 20 minutes. The reaction was
stirred
for 1.5 hours then heated to 40 °C for 1 hour. The reaction mixture was
poured into 850
mL of stirring water. After 10 minutes, the precipitate was collected by
filtration and
washed twice with water. The product was dried in a desiccator to yield the
title
compound as a solid ( 15.2 g, 78%).
MS (DCI/NH3) m/e: 228 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 3.96 (s, 3H), 8.15 (s, 1H), 8.65 (s, 1H), 9.38 (s,
1H).
Example 237B
4-Chlorothienof2, 3-clpyridine-2-carboxylic acid
Example 237A ( 15.17 g, 66.63 mmol) was suspended in a solution of 1:4
MeOH/water (500 mL) and LiOH hydrate (4.34 g, 103.50 mmol) was added. The
reaction
187
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
was stirred 1.5 hours then concentrated ( 100 mL). The aqueous phase was
washed with
Et20 and then acidified to pH 5 with 1 N HCl (aq). The precipitate was
isolated by
filtration, washed once with water, then twice with acetonitrile. The product
was dried in
a desiccator to yield 4-chlorothieno[2, 3-c]pyridine-2-carboxylic acid as a
solid (12.10 g,
85%).
MS (DCI/NH3) m/e: 214 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 8.05 (s, 1H), 8.62 (s, 1H), 9.34 (s, 1H).
Example 237C
Dimethylethyl 4-chlorothienof2, 3-clpyridine-2-carboxylate
To a suspension of Example 237B ( 12.07 g, 56.50 mmoI) in THF (200 mL) at 0
°C, was added tert-butyl 2, 2, 2-trichloroacetamidate (25.00 g, 114.41
mmol) followed by
dropwise addition of boron trifluoride-diethyl etherate (2.14 mL, 16.95 mmol).
The
reaction was allowed to warm to room temperature and stirred 18 hours.
Additional tert-
butyl 2, 2, 2-trichloroacetamidate ( 12.50 g, 57.21 mmol) was added and the
reaction was
stirred 3 hours. The stirred reaction was treated with NaHC03 ( 14 g) and then
diluted with
water (300 mL). The reaction was partitioned between water (300 mL) and 50%
EtOAc/Et20. Organic layer was washed with sat. NaHC03, washed with brine,
partially
dried (Na2S04), filtered, and concentrated. The residue was purified by flash
chromatography on silica gel neutralized with Et3N using EtOAc/hexane as
eluent. The
title compound was isolated as a solid ( 10.04 g, 66%).
MS (DCI/NH3) m/e: 270 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 1.59 (s, 9H), 8.04 (s, IH), 8.64 (s, 1H), 9.35 (s,
1H).
Example 237D
Dimethylethyl4-(ethoxycarbonyl)thienof2, 3-clpyridine-2-carbox, late
To a solution of Example 237C (1.00 g, 3.71 mmol), 1, 3-
bis(diphenylphosphino)propane (0.46 g, 1.1 I mmol), triethylamine ( 1.55 mL,
11.13
mmol), in 2:3 EtOH/DMF (25 mL), was added palladium(II) acetate (0.25 g, 1.11
mmol).
The reaction was aspirated with a stream of CO(g) for 15 minutes. A balloon of
CO(g)
was applied and reaction heated to 105 °C for 16 hours then cooled to
room temperature.
The reaction was poured into water (400 mL). The aqueous was diluted with
brine (25
mL) and sat. NaHC03 (25 mL), then extracted with EtOAc (4 x 50 mL). The
organic
extracts were combined, washed with 20% sat.NaHC03 (2 x 200 mL), brine (2 x
100 mL),
partially dried (Na2S04), filtered, and concentrated. The residue was purified
by flash
188
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
chromatography on silica gel using EtOAc/hexane as eluent to provide the title
compound
as a solid (0.60 g, 53%),
MS (APCI) m/e: 308 (M+H)+;
'H NMR (300 MHz, CD2Cl2) 8 1.44 (t, J = 7.1 Hz, 3H), 1.59 (s, 9H), 4.44 (q, J
= 7.1 Hz,
2H), 8.65 (s, 1H), 9.12 (s, 1H), 9.25 (s, 1H).
Example 237E
Dimethylethyl 4-formylthienof2, 3-clpyridine-2-carboxylate
To a stirred solution of NaBH4 (0.18 g, 4.89 mmol) in anhydrous 50% MeOH/THF
at 0 °C was added powdered CaCl2 (0.54 g, 4.89 mmol). The suspension
was stirred 20
minutes and a solution of Example 237D (0.50 g, 1.63 mmol) in anhydrous 50%
MeOH/THF was slowly added over a period of 10 minutes. The reaction stirred 1
hour at
0 °C followed by 16 hours at room temperature. Reaction was quenched
into a slurry of
dilute AcOH (aq)/ice. After all gas evolution ceased with occasional stirring,
aqueous was
made basic with sat. NaHC03. Aqueous was extracted with dichloromethane (3x40
mL)
and extracts combined. The organic phase was dried (NaZS04), filtered, and
concentrated.
The residue was purified by flash chromatography on silica gel using
EtOAc/hexane as
eluent to provide dimethylethyl 4-(hydroxymethyl)-thieno[2, 3-c]pyridine-2-
carboxylate
compound as a solid (0.14 g, 32%).
MS (APCI) m/e: 266 (M+H)+;
1H NMR (300 MHz, CD2C12) 8 1.54 (s, 9H), 4.94 (s, 2H), 8.08 (s, 1H), 8.40 (s,
1H), 9.02
(s, 1H).
To a stirred solution of oxalyl chloride (0.10 mL, 1.17 mmol) in anhydrous
dichloromethane ( 1 mL) at -78 °C was added DMSO (0.19 mL, 2.65 mmol).
After 20
minutes, a solution of dimethylethyl 4-(hydroxymethyl)-thieno[2, 3-c]pyridine-
2-
carboxylate (0.28 g, 1.06 mmol) in anhydrous dichloromethane (4 mL) was added
dropwise. The reaction was stirred 1 hours at -78 °C then treated with
triethylamine (0.74
mL, 5.30 mmol). After 5 minutes, reaction was allowed to warm to room
temperature
over 30 minutes. The reaction was quenched with water (5 mL) and partitioned
between
dichloromethane (50 mL) and 50% sat. aq NaHC03 (50 mL). The organic phase was
washed with 50% sat. aq NaHC03 (1x50 mL), dried (Na2S04), filtered, and
concentrated
and dried in a desiccator to yield the title as a solid (0.25 g, 90%).
MS (APCI) m/e: 264 (M+H)+;
'H NMR (300 MHz, CD2Cl2) 8 1.59 (s, 9H), 8.74 (s, 1H), 8.91 (s, 1H), 9.31 (s,
1H), 10.24
(s, 1H).
189
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
ExamQle 237F
_Dimethylethyl4-f(4-chlorophenyl)(hydroxylmethyllthienof2, 3-clpyridine-2-
carboxylate
To a solution of Example 237E (0.25 g, 0.95 mmol) in anhydrous THF (5 mL) at
-5 °C was slowly added a 1 M solution of p-chlorophenylmagnesium
bromide in diethyl
ether (2.85 mL, 2.85 mmol). The reaction was quench after 10 minutes with
dropwise
addition of water ( 1 mL) and partitioned between dichloromethane (25 mL) and
50% sat.
aq NaHC03 (50 mL). The aqueous phase was extracted with dichloromethane (25
mL)
and the organic extracts combined, dried (Na2S04), filtered, and concentrated.
The residue
was purified by flash chromatography on silica gel using EtOAc/hexane as
eluent to
provide the title compound as a foam, which was crushed and dried in a
desiccator to yield
a powder (0.36 g, 100%).
MS (APCI) m/e: 376 (M+H)+;
1H NMR (300 MHz, CD2C12) 8 1.52 (s, 9H), 6.18 (d, 1H), 7.25-7.34 (series of m,
4H),
7.96 (s, 1H), 8.47 (s, 1H), 9.03 (s, 1H).
Example 2376
Methyl 4-f(4-chlorophenyl)(hydroxy)methyllthienof2, 3-clpyridine-2-carboxylate
Example 237F (0.12 g, 0.32 mmol) was dissolved in a solution of 10%
H2S04/MeOH (10 mL) and heated to 50 °C for 18 hours. The reaction was
quenched into
sat. NaHC03 ( 100 mL). The aqueous phase was extracted with dichloromethane (2
x 50
mL) and the organic extracts combined. The organic layer was washed with sat.
NaHC03
( 1 x 100 mL), brine ( 1 x 100 mL), partially dried (NazS04), filtered, and
concentrated.
The product was dried in a desiccator to yield the title compound as a solid
(0.10 g, 94%).
MS (APCI) m/e: 334 (M+H)+;
1H NMR (300 MHz, CD2Cl2) 8 3.85 (s, 3H), 6.17 (d, 1H), 7.23-7.33 (series of m,
4H),
8.05 (s, 1H), 8.48 (s, 1H), 9.05 (s, 1H).
Example 237H
Methyl 4-(4-chlorobenzoyl)-N-methylthienof2, 3-clpyridine-2-carboxylate
To a stirred solution of oxalyl chloride (0.023 mL, 0.26 mmol) in anhydrous
dichloromethane (1 mL) at -78 °C was added DMSO (0.045 mL, 0.63 mmol).
After 15
minutes, a solution of Example 2376 (0.07 g, 0.21 nunol) in anhydrous 1:4
DMSO/dichloromethane (5 mL) was added dropwise. The reaction was stirred 1
hour at
78 °C then treated with triethylamine (0.15 mL, 1.05 mmol). After 5
minutes, reaction
was allowed to warm to room temperature over 1 hour. The reaction was quenched
with
water (2 mL) and partitioned between EtOAc (50 mL) and sat. aq. NaHC03 (50
mL). The
190
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
organic layer was washed with 50% sat. aq. NaHC03 (2 x 50 mL), brine ( 1 x 50
mL),
dried (Na2S04), filtered, and concentrated. The product was dried in a
desiccator to yield
the title compound as a white solid (0.07 g, 100%).
MS (APCI) m/e: 332 (M+H)+;
IH NMR (300 MHz, DMSO-d6) 8 3.95 (s, 3H), 7.68 (m, 2H), 7.87 (m, 2H), 8.31 (s,
1H},
8.74 (s, 1H), 9.66 (s, 1H); )+;
IR (KBr) 3208, 2959, 1719, 1657, 1585, 1567, 1434, 1308, 1268 crri ~.
Example 237I
4-(4-Chlorobenzoyl)-N-methylthienol2, 3-clpyridine-2-carboxamide
Example 237H (70 mg, 0.21 mmol) was suspended in MeOH (5 mL) and
chloroform was added until the solid dissolved. A balloon of ammonia was
applied and
reaction heated to 50 °C for 20 hours. The reaction was concentrated
and the residue was
purified by flash chromatography on silica gel using EtOAc/hexane as eluent.
The
obtained title compound was dried in a desiccator to yield a white solid (35
mg, 53%).
mp 216-218 °C;
MS (APCI) m/e: 317 (M+H)+;
1H NMR (DMSO-d6) 8 7.68 (m, 2H), 7.86 (br s, IH), 7.88 (m, 2H), 8.38 (s, IH),
8.53 (br
s, 1H), 8.67 (s, 1H), 9.55 (s, 1H);
'3C NMR (DMSO-d6) b 123.4, 128.0, 129.3, 132.0, 135.9, 137.9, 138.9, 143.2,
145.1,
148.8, 148.9, 162.7, 193.6;
IR (KBr) 3289, 3145, 1681, 1655, 1399, 1270 cni';
Anal calcd for C,SH9C1NZO2S~O.1 C6H,4: C, 57.59; H, 3.22; N, 8:61. Found: C,
57.58; H,
3.22; N, 8.41.
Example 238
N-4-(4-Chlorophenyl)thienof2, 3-clpyridine-2,4-dicarboxamide
Example 238A
4-(Ethoxycarbonyl)thienol2. 3-clpyridine-2-carboxamide
Example 94 was treated according to the procedure of Example 237D to provide
the title compound.
Example 238B
4-(Carboxy)thieno(2, 3-clpyridine-2-carboxamide
191
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 238A was treated according to the procedure of Example 159A to provide
the title compound.
Example 238C
N-4-(4-Chlorophenyl)thienof2, 3-clpyridine-2,4-dicarboxamide
Example 238B was treated according to the procedure of Example 24 to provide
the title compound.
mp > 270 °C;
MS (ESI) m/e: 332 (M+H)+;
'H NMR (300 MHz, DMSO-db) 8 7.46 (m, 2H), 7.84 (br s, 1H), 7.85 (m, 2H), 8.52
(m,
1H), 8.56 (br s, 1H), 8.90 (m, 1H), 9.47 (m, 1H), 10.79 (br s, 1H).
Example 239
f4-(4-Bromophenoxy)thienof2, 3-clpyridin-2~Ilmethanol
Example 239 (900 mg, 97%) was prepared as described in Example 90 except
substituting Example 73 (1 g, 2.74 mmol) for Example 61A.
MS (APCI) m/e: 336; 338 (M+H)+, 370; 372 (M+Cl)-;
~H NMR (300 MHz, DMSO-d6) b 4.78 (d, J=6 Hz, 2H), 5.88 (t, J=6 Hz, 1H), 6.98
(d, J=9
Hz, 2H), 7.14 (s, 1H), 7.55 (d, J=9 Hz, 2H), 8.19 (s, 1H), 9.06 (s, 1H).
Example 240
4-(4-Bromophenoxy)thienof2, 3-clpyridine-2-carbaldehyde
Example 240 (400 mg, 80%) was prepared as described in Example 91A except
substituting Example 239 (500 mg, 1.49 mmol) for Example 90.
MS (APCI) m/e: 334; 336 (M+H)+, 333; 335 (M-H)-;
1H NMR (300 MHz, DMSO-d6) b 7.62 (d, J=9 Hz, 2H), 7.62 (d, J=9 Hz, 2H), 8.26
(s,
1H), 8.40 (s, 1H), 9.27 (s, 1H), 10.21 (s, 1H).
Example 241
4-(4-Chlorophenoxy)thienof2. 3-clpyridine-2-carbaldehyde oxime
The title compound was prepared from Example 91A in a manner similar to
Example 30.
HPLC: Supelco C-18 column, water:acetonitrile 0:90- 90:0, 30 minute elution,
flow rate
0.8 mL/min, rt 19.61min. and 20.28 min;
MS (DCI/NH3) m/e: 305 (M+H)+;
192
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
'H NMR (300 MHz, DMSO-d6) b 2.40 (s, 3H, toluene), 7.15 (m, 4H), 7.25 (m, 5H,
toluene), 7.48 (m, 4H), 7.58 (s, 1H), 7.75 (s, 1H), 8.16 (m, 3H), 8.51 (s,
IH), 9.05 (s, 1H),
9.14 (s, 1H), 11.91 (s, 1H), 12.66 (s, 1H);
i3C NMR (100 MHz, DMSO-d6) b 119.28, 119.33,120.58, 122.21, 125.25, 127.51,
128.13, 128.82, 129.99, 133.03, 133.84, 135.37, 136.10, 136.60, 137.11,
139.40, 139.95,
140.83, 141.28, 143.24, 143.66, 146.31, 146.58, 155.69;
Anal. calcd for C14H9C1N202S ~ 0.4 toluene: C, 59.07; H, 3.60; N, 8.20. Found:
C,
59.15; H, 3.65; N, 8.25.
Example 242
4-(4-Chlorophenoxy)thienof2, 3-clpyridine-2-carbaldehyde O-methyloxime
Example 242 was prepared from Example 91A similarly to Example 26.
Spectral data for E isomer:
HPLC: Supelco C-18 column, water:acetonitrile 0:90- 90:0, 30 minute elution,
flow rate
0.8 mL/min, rt 22.72 min. and 23.60 min.;
MS (ESI) m/e 319 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 3.94 (s, 3H), 7.12 (m, 2H}, 7.47 (m, 2H), 7.b5 (s,
1H),
8.18 (s, 1H), 8.61 (s, 1H), 9.08 (s, 1H);
1~C NMR (100 MHz, DMSO-d6) 8 62.29, 119.35, 122.26, 124.01, 127.60, 130.01,
133.77,
136.85, 140.91, 141.38, 144.34, 146.46, 155.58.
Example 243A Mike Staeger
1-f4-(4-Chloronhenoxy)thienof2 3-clpyridin-2-yll-1-ethanone O-methvloxime
Example 159A was treated similarly to the Example 22 procedure. The derived
amide was treated according to Example 33 procedure, to produce the
corresponding
methyl ketone. This ketone was treated according to the procedure of Example
26 to
provide the title compound as a mixture of E- and Z-isomers. The isomers were
separated
by column chromatography using type H (Sigma) silica gel and eluting with
25°10
EtOAc:hexanes.
Spectral data for Z-isomer:
mp 126-128 °C;
MS (APCI) m/e: 333 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 2.29 (s, 3H), 3.97 (s, 3H), 7.15 (m, 2H), 7.48 (m,
2H),
7.72 (s, 1H), 8.09 (s, 1H), 9.01 (s, IH);
193
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
'3C NMR ( 100 MHz, DMSO-d6) S 12.18, 62.32, 118.85, 119.88, 127.77, 130.01,
132.98,
136.94, 136.98, 140.32, 145.60, 146.94, 150.84, 155.40;
Anal. calcd for C16H~3C1N202S: C, 57.74; H, 3.94; N, 8.42. Found: C, 58.03; H,
3.92; N,
8.14.
Example 243B
1-f4-(4-Chlorophenoxy)thienof2 3-clpyridin-2-yll-1-ethanone O-methyloxime
E-isomer isolated from Example 243A preparation:
MS (APCI) m/e: 333 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 2.38 (s, 3H), 4.04 (s, 3H), 7.18 (m, 2H), 7.48 (m,
2H),
7.82 (s, 1H), 8.11 (s, 1H), 9.12 (s, 1H);
i3C NMR (100 MHz, DMSO-db) 8 19.25, 62.16, 119.88, 120.17, I2I.60, 127;95,
130.04,
131.98, 134.49, 136.52, 138.85, 140.68, 146.21, 147.39, 155.22;
Example 244A
I-f4-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-yll-1-ethanone oxime
Example 244A was prepared similarly to Example 243A, with the substitution of
hydroxylamine hydrochloride for methoxylamine hydrochloride.
MS (APCI) m/e: 319 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.23 (s, 3H), 7.15 (m, ZH), 7.48 (m, 2H), 7.62 (s,
1H),
8.10 (s, 1H), 9.02 (s, 1H), 11.89 (s, IH);
'3C NMR (100 MHz, DMSO-d6) b 11.41, 117.45, 119.73, 124.45, 127.65, 129.97,
133.14,
136.78, 137.17, 140.28, 146.72, 147.39, 149.85, 155.51;
Anal. calcd for C15H,1C1N02S: C, 54.96; H, 3.69; N, 8.55. Found: C, 55.37; H,
3.47; N,
8.37.
Example 244B
1-f4-(4-Chloronhenoxy)thienof2, 3-clpyridin-2-yll-1-ethanone oxime
Z-isomer isolated from Example 244A.
MS (APCI) m/e: 319 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 2.38 (s, 3H), 7.15 (d, J=9 Hz, 2H), 7.48 (d, J=9
Hz,
2H), 7.73 (s, IH), 8.10 (s, 1H), 9.12 (s, 1H), 12.35 {s, 1H);
isC NMR (100 MHz, DMSO-d6) b 19.32, 118.36, 119.96, 120.01, 127.81, 130.02,
132.08,
134.60, 136.99, 138.95, 140.73, 145.08, 147.18, 155.40.
Example 245
194
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1-f4-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-yll-1-nropanone
Title compound was prepared in analogy to Example 33, substituting
ethylmagnesium bromide for methylmagnesium bromide.
mp 101-102 °C;
MS (APCI) m/e: 318 (M+H)+;
'H NMR (300 MHz, DMSO-d6) S 1.11 (t, J=8 Hz, 3H), 3.18 (d, J=8 Hz, 2H); 7.21
(m,
2H), 7.51 (m, 2H), 8.13 (s, 1H), 8.32 (s, 1H), 9.19 (s, 1H).
Example 246
1-f4-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-yll-1-propanone oxime
The title compound was prepared from Example 245 in analogy to Example 26,
substituting hydroxylamine hydrochloride to provide a mixture of E- and Z-
oxime
isomers.
mp 195-198 °C (dec);
MS (APCI) m/e: 333 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 1.10 (m, 6H), 2.77 (m, 4H), 7.17 (m, 4H), 7.48 (m,
4H),
7.61 (s, 1H), 7.71 (s, 1H), 8.09 (s, 1H), 8.11 (s, 1H), 9.00 (s, 1H), 9.12 (s,
1H), 11.88 (s,
1H), 12.42 (s, 1H);
HPLC Supelco C-18 column, water:acetonitrile 0:90- 90:0 in 30 minutes,
detection at 254
nm, flow rate of 0.8 mL/min, RT = 20.20 min and 21.10 min (E- and Z-isomers);
Anal. calcd for C,6H,~C1Nz02S: C, 57.74; H, 3.94; N, 8.42. Found: C, 57.51; H,
4.12; N,
8.22.
Example 247
2-f4-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-yll-N-methoxy-N-methyl-2-
oxoacetamide
Example 88 (0.38 mmol) was prepared as reported in Example 42 and then
combined with THF ( 1.0 mL) and LDA (0.92 mL of a freshly prepared 0.5 M
solution in
THF, 0.46 mmol) at -78 °C. The clear, pale yellow solution was stirred
at -78 °C for 1.25
hours before the solution was transferred by canula to a solution of bis(N, O-
dimethylhydroxyl)oxamide (88 mg, 0.50 mmol) in THF ( 1.0 mL) at -78 °C.
The solution
was slowly warmed to room temperature, diluted with 2N aqueous HCl (20 mL),
and
extracted with CH~C12 (3x20 mL). The organic extracts were combined, washed
with
brine (1x,0 mL), dried (MgSO.~), and concentrated to a yellow solid. Flash
silica gel
column chromatography (15% acetone in hexane) gave the title compound (25 mg,
17%
yield) as a yellow solid.
mp 135.0-137.8 °C;
195
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
MS (DCI/NH3) m/e: 377 (35C1)/379 (3'Cl);
1H NMR (300 MHz, DMSO-d6) 8 3.31 (s, 3H), 3.64 (s, 3H), 7.27 (d, J=8.8 Hz,
2H), 7.52
(d, J=8.8 Hz, 2H), 8.15 (s, 1H), 8.22 {s, 1H), 9.26 (s, 1H).
Example 248
4-(4-Chlorophenoxy)thienof2, 3-clpyridine-2-carbonitrile
A solution of the resultant compound from Example 61B (500 mg, 1.64 mmol) in
pyridine (7 mL) under nitrogen at -78 °C was treated with
trifluoroacetic anhydride ( 1 mL,
6.6 mmol), stirred at -78 °C for 40 minutes, allowed to slowly warm to
room temperature,
and stirred an additional two hours. This mixture was diluted with ethyl
acetate, washed
with saturated NaHC03, brine, dried (MgS04) and concentrated. The resultant
light
purple solid was dissolved in a minimal amount of ethyl acetate, filtered
through a plug of
silica, washed through with 50/50 hexane/ethyl acetate and concentrated to
give 395 mg of
the title compound as a white solid (84%).
mp 140-142 °C;
MS (APCI-NH3) m/e: 287 (M+H)+;
'H NMR (300 MHz, CDCI3) 8 7.04 (d, 2H), 7.40 (d, 2H), 8.00 (s, 1H), 8.14 (s,
1H), 8.96
(s, 1H);
Anal. calcd for C~4H~CIN20S: C, 58.64; H, 2.46; N, 9.77. Found: C, 58.45; H,
2.62; N,
9.52.
Example 249
4-(4-Chlorophenoxy)-N'-hydroxythienol2, 3-clpyridine-2-carboximidamide
A solution of the resultant compound from Example 248 (100 rng, 0.35 mmol) in
ethanol (2 mL) under nitrogen at room temperature was treated with
triethylamine (90 mL,
0.6 mmol), hydroxylamine hydrochloride (40 mg, 0.53 mmol) and stirred for 18
hours.
The resultant white, heterogeneous mixture was diluted with ethyl acetate,
washed with
saturated NaHC03, brine, dried (MgS04), and concentrated to give 120 mg of an
off-white
foam. This foam was dissolved in ethyl acetate, filtered through a plug of
silica and
concentrated to give the title compound as a white solid ( 110 mg, 98%).
mp 194-196 °C;
MS (APCI-NH3) m/e: 320 (M+H)+;
1H NMR (300 MHz, DMSO-db) 8 10.23 {s, 1H), 9.02 (s, 1H), 8.13 (s, 1H), 7.87
(s, 1H),
7.46 (d, 2H), 7.11 (d, 2H), 6.26 (br s, 2H);
Anal. calcd for C,4HioC1N30~S: C, 52.59; H, 3.15; N, 13.14. Found: C, 52.72;
H, 3.05; N,
12.82.
196
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 250
4-(4-Chlorophenoxy)-N'-cyanothieno(2, 3-clnyridine-2-carboximidamide
A solution of the resultant compound from Example 248 ( 100 mg, 0.35 mmol) in
THF (2 mL) under nitrogen at ambient temperature was treated with cyanamide
(74 mg,
1.75 mmol), 1, 8-diazabicylco [5.4.0]undec-7-ene (52 mL, 0.35 mmol) and
stirred for 24
hours. The resultant yellow, homogeneous solution was diluted with ethyl
acetate, washed
with saturated NaHC03, brine, dried (MgS04), concentrated to give a light
yellow solid,
triturated with CHZC12 to give 123 mg of a white powder, dissolved in ethyl
acetate and
THF, washed with distilled water, brine, dried (MgS04), concentrated to give
99 mg of a
white powder (MgS04) which was triturated with CH2Clz and then placed in a
vacuum
oven overnight at 60 °C to give the title compound as a white powder
(78 mg, 69%).
mp 265-268 °C;
MS (APCI-NH3) m/e: 329 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.58 (br s, 1H), 9.17 (s, 1H), 9.05 (br s, 1H),
8.41 (s,
1H), 8.19 (s, 1H), 7.48 (d, 2H), 7.15 (d, 2H);
Anal. calcd for Ci5H9C1N40S: C, 54.80; H, 2.76; N, 17.04. Found: C, 54.50; H,
3.01; N,
17.16.
Example 251
(2-Aminophenyl)f4-(4-chlorophenoxy)thienof2, 3-clpyridin-2-yllmethanone
Example 251 A
f4-(4-ChloroQhenoxy)thieno(2 3-clpyridin-2-yll(2-nitronhenyl)methanol
To a stirred solution of Example 124A ( 1.00 g, 3.82 mmol) in THF (40 mL) at -
78
°C, a 1.3 M suspension of sec-butyllithium in cyclohexane (3.52 mL,
4.58 mmol) was
added dropwise over a 10-minute period. After 40 minutes, the reaction was
transferred
via cannula into a stirred solution of 2-nitrobenzaldehyde (1.43 g, 9.55 mmol)
in THF (10
mL) at -48 °C. After 20 minutes, the reaction was quenched by slow
addition of MeOH (6
mL). The reaction was diluted with EtOAc ( 125 mL) and the organic washed with
1:1 sat.
NaHC03/water ( 1 x75 mL), brine ( I x75 mL), partially dried (Na2S04), and
concentrated.
The residue was purified by flash chromatography on silica gel using
EtOAc/hexane as
eluant to yield the title compound as a solid (1.49 g, 95%).
mp 85-90 °C;
MS (APCI) m/e: 413 (M+H)+;
197
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
~H NMR (300 MHz, DMSO-d6) 8 6.54 (s, 1H), 6.95 (s, 1H), 7.03 (m, 1H), 7.06 (m,
2H),
7.42 (m, 2H), 7.60 (m, 1H), 7.78 (m, 1H), 7.85 (m, 1H}, 7.98 (m, 1H), 8.12 (s,
1H), 9.02
(s, 1H);
Anal. calcd for C2oH»C1N204S ~ 0.3 H20: C, 57.43; H, 3.28; N, 6.70. Found : C,
57.42; H,
3.45; N, 6.42.
Example 251B
~2-Aminonhenyl)14-(4-chlorophenoxy)thienof2, 3-clnyridin-2-yllmethanol
Example 25 i A (0.10 g, 0.24 mmol) was dissolved in EtOH ( 1.7 mL) and a
solution
of tin(II) chloride dihydrate (0.43 g, 1.92 mmol} in concentrated HCI (0.70
mL) was
slowly added. The reaction was stirred 18 hours, then partitioned between
CHC13 (50 mL)
and sat. NaHC03 (75 mL). The aqueous layer was extracted with EtOAc ( 1 x 50
mL) and
all organic extracts combined, partially dried (Na2SOa), and concentrated. The
residue was
purified by flash chromatography on silica gel using EtOAc/hexane as eluent to
yield the
title compound as a lightly colored solid (0.08 g, 87%).
mp 92-96 °C;
MS (APCI) m/e: 383 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 5.08 (br s, 2H), 6.10 (d, J=4.3 Hz, IH), 6.54 (d,
J=4.3
Hz, 1H), 6.55 (m, 1H), 6.61 (m, 1H), 6.98 (m, 1H), 7.05 (m, 2H}, 7.13 (m, IH),
7.19 (m,
1H), 7.42 (m, 2H), 8.09 (s, 1H), 8.98 (s, 1H);
Anal. calcd for CZOH,5C1N20zS: C, 62.74; H, 3.95; N, 7.32. Found : C, 63.09;
H, 4.05; N,
7.06.
Example 251C
(2-Amin~henyl)f4-(4-chlorophenoxy)thienof2, 3-clpyridin-2-yllmethanone
To an anhydrous stirred suspension of silica gel (0.13 g) and celite (0. I3 g)
in
dichloromethane (6 mL) was added pyridinium chloroehromate (0.13 g, 0.59
mmol). A
solution of Example 251B (0.15 g, 0.39 mmol) in anhydrous dichloromethane (9
mL) was
slowly added dropwise. After 1 hour, sat. NaHC03 (5 mL) was added, and the
reaction
stirred for 1 hour. The reaction was filtered and the black solid was crushed
and washed
with 5% MeOH/ dichloromethane (3x20 mL). The organic filtrate and washes were
combined and washed with sat. NaHC03 (2x 100 mL), brine ( 1 x75 mL), partially
dried
(Na2S04), and concentrated. The residue was purified by flash chromatography
on silica
gel using EtOAc/hexane as eluant to yield the title compound as a brightly
colored solid
(45 mg, 39%).
mp 152-154 °C;
198
CA 02333770 2000-11-30
WO 99/62908
PCTlUS99/12419
MS (APCI) m/e: 381 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 6.54 (m, 1H), 6.87 (m, 1H), 7.05 (broad s, 2H),
7.18 (m,
2H), 7.33 (m, 1H), 7.47 (m, 2H), 7.57 (s, 1H), 7.58 (m, 1H), 8.24 (s, 1H),
9.21 (s, 1H);
'3C NMR (300 MHz, DMSO-d6) b 114.5, 115.9, 117.1, 120.1, 123.8, 128.0, 130.0,
132.5,
133.0, 134.9, 136.0, 138.2, 141.2, 147.8, 148.5, 151.8, 155.3, 188.5;
IR (KBr) 3440, 3411, 3293, 3190, 1616, 1587, 1552, 1483, 1409, 1267, 1245,
1219, 1155
cm ';
Anal. calcd for C2oH,3C1N2O2S: C, 63.08; H, 3.44; N, 7.36. Found: C, 62.94; H,
3.51; N,
7.25.
Example 252
(3 Aminophenyl)[4-(4-chlorophenox~thieno[2 3-clpyridin-2-yllmethanone
Example 252A
[4 (4 Chloronhenoxy)thienof2 3-clpyridin-2-yll(3-nitronhenyl)rnethanol
The procedure of Example 251 A was used, substituting 3-nitrobenzaldehyde for
2-
nitrobenzaldehyde.
mp 79-83 °C;
MS (APCI) m/e: 413 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 6.33 (d, J=4.4 Hz, 1H), 6.99 (d, J=4.4 Hz, 1H),
7.07 (m,
2H), 7.27 (m, 1H), 7.43 (m, 2H), 7.66 (m, 1H), 7.92 (m, 1H), 8.13 (s, 1H),
8.15 (m, 1H),
8.34 (m, 1H), 9.01 (s, 1H);
Anal. calcd for C2oH~3C1N20aS: C, 58.19; H, 3.17; N, 6.79. Found: C, 57.97; H,
3.23; N,
6.70.
Example 252B
(3 Aminophenvl)[4-(4-chlorophenox~)thienof2 3-clpyridin-2-yllmethanol
Example 252A was treated according to the procedure of Example 251B to provide
the title compound.
mp 73-78 °C;
MS (APCI) m/e: 383 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 5.03 (s, 2H), 5.88 (s, 1H), 6.45 (series of m,
2H), 6.57
(m, 1H}, 6.64 (m, 1H), 6.96 (m, 1H), 7.06 (m, 2H), 7.11 (m, 1H), 7.42 (m, 2H),
8.08 (s,
1H), 8.97 (s, 1H);
Anal. calcd for CZOH,5C1N~02S: C, 62.74; H, 3.95; N, 7.32. Found: C, 63.06; H,
4.22; N,
6.92.
199
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
Example 252C
(3-Aminophenyl)!4-(4-chlorophenoxy)thienol2. 3-clnyridin-2-yllmethanone
Example 252B was treated according to the procedure of Example 251 C to
provide
the title compound.
mp 174-178 °C;
MS (APCI) m/e: 481 (M+H)+;
'H NMR (300 MHz, DMSO-d6) S 5.47 (br s, 2H), 6.86-6.96 (series of m, 2H), 7.06
(m,
1H), 7.16-7.23 (series of m, 3H), 7.48 (m, 2H), 7.77 (s, 1H), 8.20 (s, 1H),
9.22 (s, 1H).
Example 253
4-(4-Bromophenoxy)-2-vinylthienol2, 3-clpyridine
To a stirred suspension of methyl triphenylphosphonium bromide ( 113 mg, 0.314
mmol) in anhydrous tetrahydrofuran (2 mL) at -78 °C was added dropwise
a solution of n-
BuLi (2.5 M solution in hexanes, 0.125 mL, 0.314 mmol) under nitrogen
atmosphere.
Then the reaction mixture was stirred at 0 °C for 40 minutes and cooled
down to -78 °C.
To this a solution of Example 240 { 100 mg, 0.3 mmol) in anhydrous
tetrahydrofuran (2
mL) was added while maintaining the internal temperature below -72 °C.
Once the
addition was completed the reaction mixture was stirred at 0 °C for 15
minutes and at
ambient temperature for 1 hour. Then the reaction mixture was partitioned
between ethyl
acetate (60 mL) and brine (20 mL). The organic layer was washed with brine
(2x20 mL),
dried (MgS04) and evaporated to dryness under reduced pressure to obtain the
crude
product (145 mg). The title compound was obtained in 26 % yield (26 mg) by
flash
chromatography on silica gel eluting with 25% acetone-hexane.
MS (APCI) m/e: 332; 334 (M+H)+;
~H NMR (400 MHz, DMSO-db) 8 5.53 (d, J=10 Hz, 1H), 5.86 (d, J=16 Hz, IH), 7.02
(d,
J=9 Hz, 2H), 7.06-7.14 (m, 1H), 7.37 (s, 1H), 7.57 (d, J=9 Hz, 1H), 8.17 (s,
1H), 9.04 (s,
1H).
Example 254
1-!4-(4-Chlorophenoxy)thienol2, 3-clpyridin-2-yll-l, 2-ethanediol
Example 254A
4-(4-ChloroQhenoxy)-2-ethenylthienol2, 3-clnyridine
Example 254A (70 mg, 10%) was prepared as in Example 253 except substituting
Example 91A (700 mg, 2.42 mmol) for Example 240.
200
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
'H NMR (300 MHz, DMSO-d6) 8 5.53 (d, J=10.5 Hz, 1H), 5.86 (d, J=18 Hz, 1H),
7.04-
7.14 (m, 1H), 7.10 (d, J=9 Hz, 2H), 7.38 (s, 1H), 7.45 (d, J=9 Hz, 2H), 8.16
(s, 1H), 9.04
(s, 1H).
Example 254B
1-j4-(4-Chlorophenoxy)thienof 2. 3-clpyridin-2-yll-1 2-ethanediol
Example 254A (22 mg, 28%) was prepared as in Example 255 except substituting
Example 254A (70 mg, 0.26 mmol) for Example 253.
MS (APCI) m/e: 322 (M+H)+, 356 (M+Cl)-;
'H NMR (400 MHz, DMSO-d6) 8 3.50-3.64 (m, 2H), 4.86-4.91 (m, 1H), 5.0 (t, J=6
Hz,
1H), 6.04 (d, J=4 Hz, 1H), 7.07 (d, J=9 Hz, 2H}, 7.21 (s, 1H), 7.43 (d, J=9
Hz, 2H), 8.14
(s, 1H), 9.04 (s, 1H);
'3C NMR (100 MHz, DMSO-d6) d: 66.52 (CH2), 70.24 (CH), 114.60 (CH), 119.06
(CH),
127.22 (C), 129.91 (CH), 133.36 (CH), 137.16 (C), 137.42 (C), 140.79 (CH),
145.79 (C),
155.91 (C), 156.59 (C).
Example 255
1-f4-(4-Bromonhenoxy)thienof2, 3-clp ridin-2-yll-1 2-ethanediol
To a solution of Example 253 (90 mg, 0.271 mmol) in tetrahydrofuran (2 mL) was
added 4-methylmorpholine N-oxide (63.5 mg, 0.542 mmol) and osmium tetroxide (
14 mg,
0.054 mmol) in water (0.5 mL) at room temperature. The reaction mixture was
stirred for
48 hours and solvents were removed. The residue obtained was directly purified
by flash
chromatography on silica gel eluting with 20 % acetone-hexane to obtain the
title
compound (52 mg, 53%).
MS (APCI) m/e: 366;368 (M+H)+, 402 (M+Cl)-;
'H NMR (400 MHz, DMSO-d6) 8 3.50-3.64 (m, 2H), 4.89 (m, 1H), 5.01 (t, J=6 Hz,
1H),
6.05 (d, J=4 Hz, 1H), 7.01 (d, J=9 Hz, 2H), 7.21 (s, 1H), 7.55 (d, J=9 Hz,
2H), 8.15 (s,
1H), 9.04 (s, 1H);
'3C NMR (100 MHz, DMSO-db) 8 66.52 (CH2), 70.25 (CH), 114.60 (CH), 115.08 (C),
119.24 (CH), 132.84 (CH), 133.49 (CH), 137.18 (C), 137.48 (C), 140.$7 (CH),
145.67
(C), 156.46 (C), 156.65 (C).
Example 256
j4-(4-Chlorot~henoxy)thieno f 2 3-clpyridin-2-yllmethanamine
Diethyl azodicarboxylate ( 180 mL, 1.13 mmol) was added to Example 90 (220 mg,
0.750 mmol), THF (7.5 mL), triphenyl phosphine (297 mg, 1.13 mmol), and
phthalimide
201
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
(166 mg, 1.13 mmol). After 16 hours, the orange solution was concentrated
under vacuum
to an orange solid. Flash silica gel column chromatography (20% acetone in
hexane)
provided one compound (100% yield) as the major product, which was combined
with
hydrazine hydrate (230 mL, 7.50 mmol) and ethanol (75 mL) and heated at
reflux. After
four hours, the solution was cooled to room temperature, concentrated, diluted
with 5 N
HCl (30 mL), and filtered through a fritted glass funnel. The filtrate was
combined with 3
N NaOH until pH>12 and extracted with EtOAc (3x30 mL). The organic extracts
were
combined, washed once with brine (30 mL), dried (MgS04), filtered, and
concentrated
under vacuum to provide the title compound (190 mg, 87% yield) as a white
solid.
mp 78.6-79.8 °C;
MS (DCI/NH3) m/e: 321 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.24 (br s, 2H), 4.02 (s, 2H), 7.03 (d, J=9.1 Hz,
2H),
7.15 (s, 1H), 7.42 (d, J=9.1 Hz, 2H), 8.14 (s, 1H), 9.01 (s, 1H);
Anal. calcd for C,4H,1C1NZOS~0.25 HBO: C, 56.95; H, 3.93; N, 9.49. Found: C,
56.86; H,
3.81; N, 9.62.
Example 257
j4-(4-Chlorophenoxy)thienof2 3-clpyridin-2-yllmethyl carbamate
Example 90 (50 mg, 0.17 mmol) was combined with CHZCl2 (0.5 mL), sodium
cyanate (22 mg, 0.34 mmol), and trifluoroacetic acid (40 mL, 0.34 mmol); gas
evolution
was observed. After 24 hours, the mixture was partitioned between distilled
water (15
mL) and CH2C12 (50 mL). The layers were separated, and the organic layer was
dried
(MgS04), filtered, and concentrated. Silica gel column chromatography (30%
acetone in
hexane) provided the title compound (21 mg, 37% yield) as a white solid.
mp 113-115 °C;
MS (DCI/NH3) m/e: 335 (35C1)/337 (3~C1);
1H NMR (300 MHz, DMSO-d6) b 5.30 (s, 2H), 6.80 (br s, 2H), 7.06 (d, J=9.2 Hz,
2H),
7.37 (s, 1H), 7.45 (d, J=8.8 Hz, 2H), 8.18 (s, 1H), 9.10 (s, 1H).
Example 258
N-([4-(4-Chlorophenoxy)thieno[2, 3-clpyridin-2-yllmethyl~urea
Potassium cyanate (41 mg, 0.50 mmol) was added to a mixture of Example 256
( 130 mg, 0.45 mmol), distilled water (2.0 mL), and concentrated HCl (40 mL,
0.45 mmol),
and the solution was heated at 50 °C. After 3 hours, the solution was
slowly cooled to 0
°C, and the resulting precipitate was isolated by filtration. Flash
silica gel column
202
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
chromatography (20% acetone in hexane, switching to 10% MeOH in EtOAc)
provided
the title compound (63 mg, 42% yield) as a white solid.
mp 202-204 °C;
MS (DC1/NH3) m/e: 334 (35CI)+/336 (37C1)+;
1H NMR (300 MHz, DMSO-d6) 8 4.47 (d, J=6.1 Hz, 2H), 5.68 (s, 2H), 6.68 (t,
J=6.1 Hz,
1H), 7.05 (d, J=8.8 Hz, 2H}, 7.14 (s, 1H), 7.43 (d, J=8.8 Hz, 2H), 8.14 (s,
1H), 9.02 (s,
1H);
Anal. calcd for C~SH12C1N302S: C, 53.97; H, 3.62; N, 12.59. Found: C, 53.80;
H, 3.67; N,
12.37.
Example 259
(E)-3-f4-(4-Bromophenoxy)thienof2, 3-clpyridin-2-yllpropenamide
Example 259A
Methyl 3-(4-(4-Bromophenoxy)thienof2, 3-clpyridine-2- l~propenoate
Example 259A (590 mg, 57%) was prepared as in Example 91B except
substituting Example 240 (890 mg, 2.67 mmol) for Example 91A.
MS (APCI) m/e: 390;392 (M+H)+, 389;391 (M-H)-;
'H NMR (300 MHz, DMSO-d6) b 3.75 (s, 3H), 6.58 (d, J=16 Hz, 1H), 7.07 (d, J=9
Hz,
2H), 7.59 (d, J=9 Hz, 2H), 7.89 (s, 1H), 8.02 (d, J=16 Hz, IH), 8.17 (s, IH),
9.12 (s, IH).
Example 259B
3-(4-(4-Bromophenoxy)thienof2, 3-clpyridine-2-yl)propenoic acid
Example 259A and the procedure of Example 88 was used to provide the title
compound (270 mg, 93%).
MS (APCI) m/e: 376;378 (M+H)+;
1H NMR (300 MHz, DMSO-d6) d: 6.45 (d, J=16 Hz, 1H), 7.07 (d, J=9 Hz, 2H), 7.58
(d,
J=9 Hz, 2H), 7.81 (s, IH), 7.90 (d, J=16 Hz, IH), 8.16 (s, 1H), 9.10 (s, 1H).
Example 259C
3-(4-(4-Bromophenoxy)thienof2. 3-clpyridine-2-~propenamide
Example 259B (222 mg, 81 %) and the procedure in Example 92, except
substituting Example 259B for Example 91C, was used to provide the title
compound.
mp 195-196 °C;
MS (APCI) m/e: 375;377 (M+H)+, 409;411 (M+Cl)-;
203
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
'H NMR (400 MHz, DMSO-d6) S 6.62 (d, 16 Hz, 1H), 7.04 (d, J=9 Hz, 2H), 7.26
(s, 1H),
7.56 (d, J=9 Hz, 2H), 7.64 (s, 1H), 7.68 (s, 1H), 7.72 (d, J=16 Hz, 1H), 8.13
(s, 1H), 9.05
(s, 1H);
'3C NMR (100 MHz, DMSO-d6) 8 115.55 (C), 119.98 (CH), 122.11 (CH), 126.68
(CH),
131.80 (CH), 132.91 (CH), 133.71 (CH}, 136.69 (C), 137.48 (C), 140.84 (CH),
145.79
(C), 146.43 (C), 156.07 (C), 165.37 (C).
Example 260
Methyl (E)-3-f4-(4-Bromophenoxy)thienof2, 3-clpyridin-2-yllnronenamide
Example 260 (25 mg, 50%) was prepared from Example 259A (50 mg, 0.13 mmol)
as described in Example 171.
MS (APCI) m/e: 389 (M+H)+;
'H NMR (400 MHz, DMSO-db) 8 2.71 (d, J=4.5 Hz, 3H), 6.62 (d, J=16 Hz, IH),
7.06 (d,
J=9 Hz, 2H}, 7.58 (d, J=9 Hz, 2H), 7.69 (s, 1H), 7.74 (d, J=16 Hz, 1H), 8.15
(s, 1H), 8.27
(d, J=4.5 Hz, 1H), 9.08 (s, 1H);
'3C NMR (100 MHz, DMSO-d6) b 25.7 (CH3), 115.6 (C), 120.0 (CH), 122.1 (CH),
126.4
(CH), 131.1 (CH), 132.9 (CH), 133.7 (CH), 136.7 (C), 137.5 (C), 140.9 (CH),
145.9 (C),
146.4 (C), 156.1 (C), 164.3 (C).
Example 261
Methyl 3-f4-(4-Bromophenoxy)thienof2, 3-clpyridin-2-yll-2, 3-
dihydroxypropanamide
Example 260 (52 mg, 53%) was prepared as described in Example 255, except
substituting Example 260 (90 mg, 0.232 mmol), to provide the title compound.
MS (APCI) m/e: 423;425 (M+H)+, 456 (M+Cl)-;
'H NMR (300 MHz, DMSO-db) 8 2.61 (d, J=4.5 Hz, 3H), 4.09 (br d, J=3 Hz, 1H),
5.27 (br
d, J=3 Hz, 1H), 5.68 (d, J=6 Hz, 1H), 6.09 (d, J=6 Hz, 1H), 7.00 (d, J=9 Hz,
2H), 7.23 (s,
1H), 7.56 (d, J=9 Hz, 2H), 7.77 (d, J=4.5 Hz, 1H), 8.14 (s, 1H), 9.04 (s, 1H);
'3C NMR (75 MHz, DMSO-d6) 8 25.5 (CH3), 70.3 (CH), 75.1 (CH), 115.0 (CH),
115.1
(C), 119.5 (CH), 132.9 (CH), 133.5 (CH), 137.5 (C), 137.5 (C), 140.9 (CH),
145.8 (C),
156.3 (C), 156.5 (C), 171.7 (C).
Example 262
3-f4-(4-Bromophenoxy)thienof2, 3-clpyridin-2-yll-2, 3-dihydroxypropanamide
The procedure of Example 261 and the product from Example 259C may be used
to prepare the title compound.
204
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 263
4~4-Chlorophenoxy)thienof2, 3-clpyridin-2- famine
A mixture of Example 159A {0.500 g, 1.64 mmol) and 1, 8-bis(dimethyl-
amino)naphthalene, N, N, N', N'-tetramethyl-I, 8-naphthalenediamine (0.350 g,
1.64
mmol) in 90 mL of anhydrous THF was warmed until all the solids went into
solution.
The solution was stirred for 15 m, whereupon diphenyiphosphoryl azide (0.450
g, 1.64
mmol) was added. The solution was heated to reflux for 18 hours. The resulting
deep red
solution was evaporated to dryness under reduced pressure. The product was
passed
through 5 g of silica gel, eluting with 20% ethyl acetate/hexanes to provide
422 mg of the
intermediate isocyanate as a bright orange solid. The resulting product was
dissolved in
100 mL of toluene and the solution was heated to reflux for 6 hours. The
product was
evaporated to dryness. The resulting dark orange solid was dissolved in 20 mL
of 2.0 M
hydrogen chloride in dioxane. The solution was evaporated, leaving 313 mg
(84.9%) of
the title compound.
MS (APCI) m/e: 277 (M+H)+;
'H NMR (300 MHz, DMSO-db) b 6.91 (s, 1H), 7.18 (d, 2H), 7.47 (d, 2H), 8.34 (s,
1H),
9.21 (s, 1H).
Example 264
4-(4-Chlorophenoxy)thienof2, 3-c~pyridin-2-ylformamide
A mixture of 5 mL of acetic anhydride and 1.8 mL of 96% formic acid was heated
to 70° C for 3 hours. The solution was allowed to cool whereupon the
obtained amine
from Example 263 (32 mg, 0.12 mmol) was added. The mixture was stirred for 4
days
and then poured into 50 mL of dilute HCI. The mixture was extracted with ethyl
acetate,
the combined extracts were washed with saturated sodium carbonate and then
water, dried
(MgS04), and evaporated. Purification of the resulting product by preparative
HPLC
using a gradient of 30%-70% acetonitrile/water + 0.1 % TFA over 40 minutes
afforded 18
mg (49 %) of the title compound.
MS (APCI) m/e: 305 (M+H)+;
'H NMR (300 MHz, CD30D) b 7.16 (s, 1H), 7.24 (d, 2H), 7.46 (d, 2H), 8.08 (s,
IH), 8.61
(s, 1H), 9.13 (s, IH).
Example 265
N-[4-(4-Chloronhenoxy)thienof2 3-clp ridin-2-, llurea
A mixture of the obtained isocyanate from Example 263 ( 1 l Omg, 0.364 mmol)
in
10 mL of ammonium hydroxide was vigorously stirred for 18 hours. The resulting
red
205
CA 02333770 2000-11-30
WO 99!62908 PCT/US99/12419
solid was collected and dried under vacuum, affording 60.7 g (52.2°!0)
of the title
compound.
MS (APCI) m/e: 320 (M+H)+;
1H NMR (300 MHz, DMSO-db) 8 7.20 (d, 2H), 7.34 (s, 1H), 7.50 (d, 2H), 7.65-
7.79 (m,
4H), 8.06-8.16 (m, 2H), 9.18 (s, 1H).
Example 266
N-f4-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-yll-N'-methylthiourea
A solution of obtained amine from Example 263 ( 150 mg, 0.542 mmol) in 5 mL of
pyridine was treated with methyl isothiocyanate ( 198 mg, 2.7 I mmol). The
solution
heated to 100 °C under a nitrogen atmosphere for 5 days. All volatiles
were removed
under reduced pressure. The resulting product was purified by flash column
chromatography on silica gel eluting with chloroform/NH40H, affording 110 mg
(58. I °lo)
of the title compound.
MS (APCI) m/e: 350 (M+H)+;
1H NMR (300 MHz, CD30D) 8 3.31 (s, 3H), 6.61 (bs, 1H), 6.96 (d, 2H), 7.34 (d,
2H),
7.88 (s, 1H), 7.96 (s, 1H), 8.67 (s, 1H).
Example 267
Methyl 4-(4-Chlorophenoxy)thienof2, 3-clpyridine-2-sulfonamide
To a solution of Example 124A (261 mg, 1 mmol) in anhydrous THF (2 mL) at -78
°C was added n-BuLi (2.5 M solution in hexanes, 0.60 mL, 1.5 mmol)
under nitrogen
atmosphere. This was stirred at -78 °C for 2 hours and a rapid stream
of S02 was
introduced on the surface of the reaction mixture. After 15 minutes the
reaction mixture
was allowed to warm to 0 °C with continuous introduction of 502. The
stream of S02 gas
was discontinued after 10 minutes at 0 °C and the reaction mixture was
allowed to warm
to 10 °C. Then the solvent and excess S02 gas were removed under
reduced pressure to
obtain the sulfinic acid lithium salt as a cream colored solid. This material
was dissolved
in a saturated aqueous solution of NaHC03 ( 1 mL) and treated with N-
chlorosuccinimide
(200 mg, 1.5 mmol) at 0 °C. The reaction mixture was stirred at 0
°C for 1 hour and at
room temperature for 2 hours. The product formed was extracted into CH2C12
(2x50 mL)
and washed with water (2x20 mL). The dried (Na2S04) organic layer was
evaporated to
dryness under reduced pressure to obtain the sulfonyl chloride derivative. A
portion of
this material ( 143 mg, 0.398 mmol) was dissolved in CH2C12 ( I mL) at -5
°C and treated
with diisopropylethylamine (0.483 mL, 0.478 mmol) and 2 M solution of
methylamine in
methanol (0.239 mL, 1.2 mmol) under nitrogen atmosphere. The reaction mixture
was
206
CA 02333770 2000-11-30
WO 99/62908 PCT/US99112419
stirred at room temperature for 1 hour. This was directly purified by silica
gel flash
chromatography eluting with 10% acetone-hexane followed by 25% acetone-hexane
to
obtain the title compound (19 mg, 13.5%).
MS (APCI) m/e: 355 (M+H)+, 353 (M-H)-;
'H NMR (300 MHz, DMSO-d6) 8 2.57 (s, 3H), 7.19 (d, J=9 Hz, 2H), 7.49 (d, J=9
Hz,
2H), 7.76 (s, 1H), 8.16 (br d, J=3 Hz, 1H), 8.24 (s, 1H), 9.24 (s, 1H).
Example 268
2, 3-Dihydroxypropyl 4-(4-chlorophenoxy)thienof2, 3-clpyridine-2-sulfonamide
Example 268 (8.6 mg, 7.5%) was prepared as described in Example 267 except
substituting 3-amino-1, 2-propanediol (0.086 mL, 1.12 mmol) for methylamine.
MS (APCI) m/e: 415 (M+H)+, 413 (M-H)-;
'H NMR (300 MHz, DMSO-d6) 8 2.76 (d, J=7.5 Hz, 1H), 2.81 (d, J=7.5 Hz, 1H),
3.02 (d,
J=4.5 Hz, 1H), 3.08 (d, J=4.5 Hz, 1H), 3.44-3.55 (m, 1H), 4.47-4.64 (m, 1H),
4.80 (d, J=6
Hz, 1H), 7.17 (d, J=9 Hz, 2H), 7.48 (d, J=9 Hz, 2H), 7.77 (s, 1H), 8.23 (s,
1H), 9.22 (s,
1 H).
Example 269
2-Hydroxyethyl 4-(4-chlorophenoxy)thieno f 2, 3-clp~ridine-2-sulfonamide
Example 269 (25 mg, 16%) was prepared s described in Example 267 except
substituting 2-hydroxyethylamine (0.072 mL, 1.2 mmol) for methylamine.
MS (APCI) m/e: 385 (M+H)+, 383 (M-H)-;
'H NMR (300 MHz, DMSO-d6) 8 2.97 (t, J=6 Hz, 2H), 3.38-3.45 (m, 2H), 4.69-4.78
(m,
1H), 7.18 (d, J=9 Hz, 2H), 7.48 (d, J=9 Hz, 2H), 7.78 (s, 1H), 8.23 (s, 1H},
9.21 (s, 1H);
'3C NMR (75 MHz, DMSO-d6) 8 45.4 9CH2), 59.7 (CHZ), 119.9 (CH), 122.2 (CH),
128.0
(C), 130.1 (CH), 133.3 (CH), 135.5 (C), 138.2 (C), 141.4 (CH), 147.5 (C},
148.4 (C),
155.2 (C).
Example270
4-f4-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-yllphenol
Example 270A
4-f4-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-boronic acid
A 1.3 M suspension of sec-butyllithium in cyclohexane (3.52 mL, 4.58 mmol) was
added to THF ( 10 mL) at -78 °C . A solution of Example 124A ( 1.00 g,
3.82 mmol) in
THF (5 mL) was added dropwise. The reaction was stirred 30 minutes and
tributyl borate
207
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
( 1.55 mL, 5.73 mmol) was slowly added. The cold bath was removed and the
reaction
stirred 45 minutes while warming to room temperature. A solution of 2 N NaOH (
15 mL)
was added. After 10 minutes, the reaction was diluted with hexane ( 15 mL) and
the
aqueous layer collected. The organic layer was extracted with 2 N NaOH (2x5
mL) and all
aqueous extracts combined, acidified to pH 2 with 6 N HCI, and extracted with
10%
MeOH/CH2C12 (4x25 mL). The organic extracts were combined and concentrated.
The
resulting solid was washed with acetonitrile (1x25 mL) and dried in a
desiccator to yield
the title compound as a tan solid (0.83 g, 71%).
MS (APCI) m/e: 262 (M+H-B(OH)2)+, m/e: 340 (M+CI-)-;
'H NMR (300 MHz, DMSO-db) 8 7.15 (m, 2H), 7.48 (m, 2H), 8.03 (s, 1H), 8.24 (s,
1H),
9.29 (s, 1H).
Example 270B
4-f4-(4-Chloro~henoxy)thienof2, 3-clpyridin-2-yllanisol
A mixture of Example 270A (0.25 g, 0.82 mmol), 4-iodoanisole (0.19 g, 0.82
mmol), [1, 1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II)
dichloromethane
complex ( 1:1 ) (0.10 g, 0.12 mmol), cesium fluoride (0.37 g, 2.46 mmol),
triethylamine
(0.11 mL, 0.82 mmol), in DME (7 mL) was aspirated 10 minutes with anhydrous
nitrogen.
The reaction was heated to 75 °C for 18 hours and then partitioned
between EtOAc ( 100
mL) and sat. NaHC03 ( 100 mL). The organic layer was washed with sat. NaHC03 (
100
mL), brine (75 mL), partially dried (Na2S04), and concentrated to a colored
wet solid. The
residue was purified by flash chromatography on silica gel using EtOAc/hexane
as eluant
to yield a colored solid. The title product was crystallized from hot
acetonitrile (0.11 g,
37%).
mp 121-123 °C;
MS (APCI) m/e: 368 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 3.82 (s, 3H), 7.05 (m, 2H), 7.13 (m, 2H), 7.45 (m,
2H),
7.67 (s, 1H), 7.81 (m, 2H), 8.13 (s, 1H), 9.05 (s, 1H);
Anal. calcd for C2oH,4CINOzS: C, 65.30; H, 3.84; N, 3.81. Found : C, 65.06; H,
3.69; N,
4.05.
Examp1e270C
4-f4-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-yllphenol
To a solution of Example 270B (0.09 g, 0.24 mmol) in anhydrous CH2Ch (4 mL)
was added a 1 M solution of boron tribromide in CHZC12 (0.96 mL, 0.96 mmol).
After 2
hours, the reaction was quenched by slow addition of MeOH (2 mL) and then
208
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
concentrated. The residue was diluted with CH2C12 (50 mL) and organic washed
with 1:1
sat. NaHC03/brine (50 mL), partially dried (Na2S04), then concentrated. The
residue was
purified by flash chromatography on silica gel using EtOAclhexane as eluent to
yield the
title compound as a solid (0.08, 96%).
mp 213-215 °C;
MS (ESI) m/e: 354 (M+H)+;
1H NMR (300 MHz, DMSO-db) 8 6.86 (m, 2H), 7.11 (m, 2H), 7.45 (m, 2H), 7.56 (s,
1H},
7.68 {m, 2H), 8.13 (s, 1H), 9.03 (s, 1H), 9.99 (s, 1H);
Anal. calcd for C~9H~ZC1N02S ~ 0.5 H20: C, 62.90; H, 3.61; N, 3.86. Found: C,
62.96; H,
3.61; N, 3.52.
Examele 271
3-14-(4-Chloro~henoxy}thieno(2 3-clnyridin-2-yllaniline
Example 272
4-(4-(4-Chlorophenoxy)thienol2, 3-clpyridin-2-yllaniline
Example 272A
4-14-(4-Clorophenoxy)thienof2 3-clpyridin-2-yllnitrobenzene
A mixture of Example 170A (0.25 g, 0.82 mmol), 1-iodo-4-nitrobenzene (0.20 g,
0.82 mmol), [1, 1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II)
dichloromethane complex (1:1) (0.10 g, 0.12 mmol), cesium fluoride (0.37 g,
2.46 mmol),
triethylamine (0.11 mL, 0.82 mmol), in DME (8 mL) was aspirated 10 minutes
with
anhydrous nitrogen. The reaction was heated to 70 °C for 18 hours and
then partitioned
between EtOAc ( 100 mL)and sat. NaHC03 ( 100 mL). The organic layer was washed
with
sat. NaHC03 ( 100 mL), brine (75 mL), partially dried (Na2SOa}, and
concentrated to a
colored oil. The residue was purified by flash chromatography on silica gel
using
EtOAc/hexane as eluent to yield the title compound as a colored solid (0.15 g,
48%).
mp 193-195 °C;
MS (ESI) m/e: 383 (M+H)+;
'H NMR (300 MHz, DMSO-d6) S 7.17 (m, 2H), 7.48 (m, 2H), 8.15 (m, 1H}, 8.17 (s,
1H),
8.19 (m, 2H), 8.32 (m, 2H), 9.17 (s, 1H);
Anal. calcd for C~9H"C1NZO~S: C, 59.61; H, 2.90; N, ?.32. Found : C, 59.35; H,
2.94; N,
7.22.
Example 272B
209
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-(4-(4-Chlorophenoxy)thienof2, 3-clnyridin-2-yllaniline
To a suspension of Example 272A (0.13 g, 0.34 mmol) in EtOH (3.5 mL), a
solution of tin(II) chloride dihydrate (0.31 g, 1.36 mmol) in conc. HCl (0.68
mL) was
slowly added. The reaction was stirred for 22 hours and partitioned between
dichloromethane (75 mL) and 1 N NaOH (75 mL). The organic layer was washed
with 1 N
NaOH (1 x 50 mL), brine (1 x 50 mL), partially dried (Na2S04), and
concentrated to a
colored solid (0.13 g). The desired product was crystallized from acetonitrile
to yield
colored crystals (0.08 g, 68%).
mp 178-182 °C;
MS (APCI) m/e: 353 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 5.68 (br s, 2H), 6.62 (m, 2H), 7.09 (m, 2H), 7.39
(s,
1H), 7.44 (m, 2H), 7.51 (m, 2H), 8.11 (s, 1H), 8.97 (s, 1H);
Anal. calcd for C,9H~3C1N20S: C, 64.68; H, 3.71; N, 7.94. Found : C, 64.65; H,
3.73; N,
8.13.
Example 273
6-f4-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-yll-3-pyridinamine
Example 273A
6-j4- 4-Chlorophenoxy)thienof2,3-clpyridin-2-yll-3-nitrouyridine
Example 273A ( 120 mg, 32%) was prepared as in Example 272A substituting 2-
bromo-5-nitropyridine for 1-iodo-4-nitrobenzene.
mp 221-223 °C;
MS (APCI) m/e: 384 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.20 (m, 2 H), 7.49 (m, 2 H), 8.16 (s, 1 H), 8.50
(s, 1
H), 8.61 (d, J=8.8Hz, 1 H), 8.70 (dd, J=8.8, 2.4Hz, 1 H), 9.17 (s, 1 H), 9.44
(d, J=2.4Hz, 1
H).
Example 273B
6- f 4-(4-Chlorophenoxy)thieno f 2,3-clpyridin-2-yll-3-pyridinamine
Example 273B (0.07g, 59%) was prepared as in Example 272B
mp 225-227 °C;
MS (APCI) m/e: 354 (M+H)+;
'H NMR (300 MHz, DMSO-d6) S 5.82 (broad s, 2 H), 6.92 (dd, J=8.5, 2.6Hz, 1 H),
7.05
(m, 2 H), 7.39 (m, 2 H), 7.60 (m, 1 H), 7.79 (d, J=8.5Hz, 1 H), 7.91 (d,
J=2.6Hz, 1 H),
8.04 (s, l H), 8.93 (s, 1 H);
210
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Anal. calcd for C~gH,2CIN~OS: C, 61.10; H, 3.42; N, 11.88. Found: C, 60.97; H,
3.39;
N, 12.08.
Example 274
S 14-(4-Chloronhenoxy)thienof2, 3-clpyridin-2-yll-2-pyridinamine
A mixture of Example 270A (0.20 g, 0.65 mmol), 2-amino-5-bromopyridine (0.11
g, 0.65 mmol), [1, 1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II)
dichloromethane complex ( 1:1 ) (0.11 g, 0.13 mmol), cesium fluoride (0.30 g,
1.95 mmol),
triethylamine (0.09 mL, 0.65 mmol), in DME (6 mL) was aspirated 20 minutes
with
anhydrous nitrogen. The reaction was heated to reflux for 4 hours then
concentrated. The
residue was dissolved in 10% 'PrOH/CHCl3 ( 100 mL), filtered, and the organic
washed
with sat. NaHC03 (2 x 100 mL), partially dried (Na2S04), then concentrated to
yield a
crude colored solid (0.25 g). The crude material was purified by preparative
HPLC using a
gradient of 25%-65% acetonitrile/water + 0.1 % TFA over 40 minutes. The
product was
neutralized with sat NaHC03, precipitate collected by filtration, and dried in
a desiccator
to yield the title compound as a lightly colored solid (54 mg, 23%).
mp 208-210 °C;
MS (APCI) m/e: 254 (M+H)+;
1H NMR (300 MHz, DMSO-db) 8 6.50 (br s, 2H), 6.53 (dd, J=8.9, 0.9 Hz, 1H),
7.11 (m,
2H), 7.44 (m, 2H), 7.55 (s, 1 H), 7.85 (dd, J=8.9, 2.6 Hz, 1 H), 8.11 (s, 1
H), $.41 (dd, J=2.6,
0.9 Hz, 1H), 9.00 (s, 1H);
Anal. calcd for C18H~2C1N30S: C, 61.10; H, 3.42; N, 11.88. Found : C, 60.92;
H, 3.45; N,
11.90.
Example 275
5-(4-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-yll-1 3 4-oxadiazol-2-amine
Example 156 (0.15 g, 0.47 mmol) was suspended in l, 4-dioxane (3 mL) and a 5 M
solution of cyanogen bromide in acetonitrile (0.10 mL, 0.50 mmol) was added.
The
reaction was allowed to stir 10 minutes and a solution of NaHC03 (0.04 g, 0.50
mmol) in
water ( 1.4 mL) was added dropwise. The colored reaction was stirred 2 hours,
then
poured into sat. NaHC03 (75 mL). The aqueous phase was extracted with 10%
isopropanol/CHC13 (4 x 25 mL). The organic extracts were combined, dried
(Na2S04), and
concentrated to yield a solid (0.13 g). A portion of the crude was purified by
HPLC using
a gradient of 30%-70% acetonitrile/water + 0.1 % TFA over 40 minutes to yield
the title
compound as a tan solid.
211
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
mp 262-263 °C;
MS (APCI} m/e: 345 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 7.15 (m, 2H), 7.47 (m, 2H), 7.52 (s, 1H), 7.60
(broad s,
2H), 8.24 (s, 1H), 9.17 (s, 1H);
'3C NMR (DMSO-d6) S 164.3, 155.6, 153.1, 146.6, 141.2, 137.0, 136.9, 134.0,
131.4,
130.1 (2C), 127.7, 119.5 (2C), 117.5;
IR (KBr) 3325, 3234, 3080, 1665, 1578, 1547, 1486, 1411, 1287, 1257, 1229,
1203crri';
Anal calcd for CISH9C1N402S: C, 52.26; H, 2.63; N, 16.25. Found: C, 52.40; H,
2.68; N,
16.23.
Example 276
5-f4-(4-BromophenoxK)thienof2 3-clpyridin-2-yll-1, 3, 4-oxadiazol-2-ylamine
Example 157 (0.15 g, 0.41 mmol) was suspended in 1, 4-dioxane (4 mL) and a 5 M
solution of cyanogen bromide in acetonitrile (0.10 mL, 0.50 mmol) was added.
The
reaction was allowed to stir 10 minutes and a solution of NaHC03 (0.04 g, 0.50
mmol) in
water ( 1.4 mL) was added dropwise. The colored reaction was stirred 3 hours,
then
poured into sat. NaHC03 (75 mL). The aqueous was extracted with 10% IPA/CHCl3
(4 x
mL). The organic extracts were combined, partially dried (Na2S04), and
concentrated
to yield a solid. A portion of the crude was purified by HPLC using a gradient
of 30%-
20 70% acetonitrile/water + 0.1 % TFA over 40 minutes to yield the title
compound as a tan
solid.
mp 270-273 °C;
MS (APCI) m/e: 389 (M+H)+;
'H NMR (300 MHz, DMSO-db) 8 7.08 (m, 2H), 7.52 (s, IH}, 7.56 (br s, 2H), 7.59
(m,
25 2H), 8.27 (s, 1H), 9.19 (s, 1H);
Anal calcd for C~5H9BrN4O2S: C, 46.29; H, 2.33; N, 14.39. Found: C, 46.08; H,
2.59; N,
14.12.
Example 277
5-f4-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-yll-4H-1, 2, 4-triazol-3-amine
To a dry flask containing 61A (0.33 g, 1.03 mmol) and aminoguanidine
hydrochloride (3.45 g, 31.20 mmol) was added slowly a solution of 25% wt
NaOMe/MeOH (7.13 mL, 34.32 mmol). The reaction was stirred at ambient
temperature
for 1 hour then heated to 50 °C for 20 hours, then 70 °C for 24
hours. The reaction was
poured into water (200 mL) and aqueous neutralized with 3 N HCl ( 10 mL). The
precipitate was collected by filtration, washed with water (2 x 20 mL), and
dried in a
212
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
desiccator. The crude material was purified by preparative HPLC using a
gradient of 25%-
65% acetonitrile/water + 0.1% TFA over 40 minutes. The product was neutralized
with
sat. NaHC03, and the precipitate was collected by filtration then dried in a
desiccator to
yield the title compound as a white solid (0.16 g, 45%).
mp >270 °C;
MS (APCI) m/e: 344 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 6.46 (br s, 2H), 7.27 (m, 2H), 7.58 (m, 2H), 7.67
(s,
1H), 8.31 (s, 1H), 9.21 (s, 1H);
Anal. calcd for CISH~oC1N50S: C, 52.41; H, 2.93; N, 20.37. Found: C, 52.21; H,
3.02; N,
20.45.
Example 278
5-f4- 4-Chlorophenoxy)thienof2, 3-clpyridin-2-yll-l, 3, 4-thiadiazol-2-amine
Example 88 (0.36 g, 1.18 mmol) was suspended in thionyl chloride (4 mL) and
the
suspension was heated to 45 °C for 2 hours. The reaction was
concentrated and the
residue diluted with CHzCIz (2 x 5 mL) and concentrated to yield a colored
solid. The
crude solid was dissolved in DMF (5 mL) and thiosemicarbazide (2.69 g, 29.50
mmol)
was added and the reaction stirred 24 hours. The reaction was poured into
water (250 mL,)
and the aqueous suspension treated with sat. aq. NaHC03 ( 10 mL) until pH > 7.
The
precipitate was collected, washed with water (2 x 20 mL), and dried in a
desiccator to yield
the corresponding acyl semicarbazate as a solid (0.30 g) [MS (APCI) m/e: 377
(M+H)+].
The crude material (0.20 g) was suspended in toluene (2 mL) and
methanesulfonic acid
(0.10 mL, 1.60 mmol) added. The reaction was heated to reflux for 4 hours,
then allowed
to cool to room temperature. The heterogeneous mixture was diluted with hexane
(5 mL)
and the solvent decanted away from the colored residue. The residue was
triturated with
hexane (2 x 10 mL) and dried in-vacuo. The solid was suspended in water ( 15
mL) and
treated with NH40H until pH 9 was attained. The precipitate was collected and
washed
with water (2 x 5 mL). The crude material was partially purified on silica gel
by flash
chromatography using acetone as eluent. This product was further purified by
preparative
HPLC using a gradient of 25%-65% acetonitrile/water + 0.1 % TFA over 40
minutes. The
product was neutralized with sat. aq. NaHC03, and the precipitate collected by
filtration,
then dried in a desiccator to yield the title compound as a tan solid (0.04
mg, 14% overall).
mp > 270 °C;
MS (APCI) m/e: 361 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 7.15 (m, 2H), 7.46 (m, 2H), 7.68 (s, 1H), 7.75 (br
s,
2H), 8.11 (s, 1H), 9.06 (s, IH);
213
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Anal. calcd for C~SH9CIN40S2: C, 49.93; H, 2.51; N, 15.53. Found: C, 49.82; H,
2.64; N,
15.58.
Example 279
4-(4-Chlorophenoxy)-2-(5-meth-1 2 4-oxadiazol-3-y1)thienof2, 3-clpyridine
A solution of the resultant compound from Example 249 ( 160 mg, 0.5 mmol) in
pyridine (2.0 mL) under nitrogen at ambient temperature was treated with
acetyl chloride
(50 mL, 0.55 mmol) and heated to reflux for 15 hours. The resultant dark
yellow
homogeneous solution was diluted with ethyl acetate, washed with saturated
NaHC03,
brine, dried (MgS04), filtered through a plug of silica and concentrated to
give 169 mg of
an off-white powder. This solid was flash chromatographed on silica gel with
30-SO%
ethyl acetate/hexane to provide the title compound (150 mg, 87%).
mp I20-121 °C;
MS (APCI-NH3} m/e: 344 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 9.24 (s, 1H), 8.27 (s, 1H}, 7.89 (s, 1H), 7.48 (d,
2H),
7.16 (d, 2H), 2.69 (s, 3H);
Anal. Calcd for Cl6H,oCIN302S: C, 55.90; H, 2.93; N, 12.22. Found: C, 56.10;
H, 3.16;
N, 12.01.
Example 280
5-14-f4-(Trifluoromethyl)nhenoxylthienof2 3-clpyridin-2-y11-l, 3, 4-oxadiazol-
2-amine
Example 158 was treated according to the procedure of Example 275 to provide
the title compound.
MS (APCI) m/e: 358.9 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 7.22 (d, 2H), 7.51 (s, 1H), 7.60 {s, 2H) 7.79 (d,
2H),
8.40 (s, 1H), 9.25 (s, 1H).
Example 281
4-(4-Chlorophenoxy)-2-f5-(methylsulfanyl)-1, 3, 4-oxadiazol-2-yllthienof2, 3-
clpyridine
Example 281 A
5-f4-l4-chloronhenoxy)thienof2,3-clpyridin-211-1,3,4-oxadiazole-2-thiol
The compound from Example 156 ( 100 mg, 0.31 mmol) was suspended in ethanol
(2 ml) and cooled to 0 °C. Carbon disulfide (0.04 ml, 0.71 mmol) was
added follow by
potassium hydroxide (20 mg, 0.31 mmol). The reaction was stirred 1 hr and the
cold bath
removed. After 1 hr at ambient temperature, the reaction was refluxed for 3
hours then
214
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
concentrated to a solid. The crude solid was triturated with chloroform (1 x 5
ml) and
concentrated. The residue was dissolved in water (15 ml) and acidified with
formic acid.
The resulting precipitate was isolated by filtration, washed with water (2 x
15 ml), and
dried in a desiccator to yield the title compound (106 mg, 94%).
mp 236-240 °C;
MS (ESI) m/e: 362 (M+H)+;
1H NMR (300 MHz, CDZCl2) S 7.15 (m, 2H), 7.46 (m, 2H), 7.65 (m, IH), 8.24 (s,
1H),
9.18 (s, 1 H);
Anal. calcd for C15H8C1N302Sz: C, 47.89; H, 2.57; N, 11.17. Found: C, 47.89;
H, 2.49;
N, 10.97.
Example 281B
4-(4-ChloroQhenoxy)-2-[5-(methylsulfanyl)-1,3,4-oxadiazol-2-yllthieno(2,3-
clnyridine
To a stirred suspension of 281 A ( 100 mg, 0.28 mmol) in THF ( I ml) at 0
°C was
added an aqueous 1 M sodium hydroxide solution (0.28 ml, 0.28 mmol). After 30
minutes
all solid had dissolved and iodomethane (0.02 ml, 0.31 mmol) was slowly added
dropwise.
The reaction was stirred 30 minutes and water (8 ml) was added. Solid was
collected by
filtration, washed with water (2 x 15 ml), and dried in a desiccator to yield
80 mg of a pale
yellow solid. The crude product was purified by flash chromatography on silica
gel using
acetone/hexane as eluent to yield the title compound as a solid (41 mg, 39%).
mp 158-160 °C;
MS (ESI) m/e: 376 (M+H)+;
1H NMR (300 MHz, CD2C12} 8 2.75 (s, 3 H), 7.01 (m, 2 H), 7.33 (m, 2 H), 7.91
(s, 1 H),
8.12 (s, 1 H), 8.93 (s, 1 H);
Anal. calcd for C~6H~pC1N3O2S2: C, 51.13; H, 2.68; N, 11.18. Found: C, 51.25;
H, 3.02;
N, 10.89.
Example 282
4-(4-Chlorophenoxy)-2-(2-methyl-1, 2, 3, 4-tetrazol-5-yl)thieno[2, 3-
clpyridine
Example 282A
4-(4-Chlorophenoxy)-2-(1, 2, 3, 4-tetrazol-5-yl)thieno[2, 3-clpyridine
A solution of the resultant compound from Example 248 (90 mg, 0.314 mmol) in
toluene ( 1.5 mL) under nitrogen at room temperature was treated with
dibutyltin oxide (8
mg, 0.031 mmol), trimethylsilylazide ( 125 mL, 0.942 mmol) and heated to
reflux for 24
hours, then stirred at room temperature an additional 2.5 days. The resultant
yellow
215
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
heterogeneous mixture was concentrated and then flash chromatographed with 20%
methanol/dichloromethane to give 105 mg of a light yellow powder. This powder
was
dissolved in ethyl acetate, extracted with 10% NaHC03 (2x), the aqueous
extracts
combined, acidified to pH 2 with 6 N HCI, extracted with ethyl acetate, dried
(Na2S04)
and concentrated to give the title compound as a white powder (63 mg, 61%).
mp 250-254 °C;
MS (APCI-NH3) m/e: 330 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 9.26 (s, 1H), 8.27 (s, 1H), 8.01 (s, 1H), 7.48 (d,
2H),
7.19 (d, 2H);
Anal. Calcd for C,4H8C1NSOS 0.25 H20: C, 50.30; H, 2.56; N, 20.95. Found: C,
50.27;
H, 2.69; N, 20.78.
Example 282B
4-(4-Chloronhenoxy)-2-(2-methyl-1. 2, 3, 4-tetraazol-5-yl)thienof2 3-
clpyridine
A solution of the resultant compound from Example 282A ( 100 mg, 0.3 mmol) in
methanol (4 mL) under nitrogen at room temperature was treated with
diazomethane in
diethyl ether (generated from N-methyl-N'-nitro-N-nitrosoguanidine in diethyl
ether and
40% KOH) until a yellow color persisted for more than 5 minutes, stirred an
additional 15
minutes and then quenched by slow, dropwise addition of glacial acetic acid
till yellow
coloi disappeared (vigorous gas evolution) and then concentrated. The
resultant light
yellow solid was filtered through a plug of silica gel with 5%
methanol/dichloromethane
and then flash chromatographed on reverse phase silica gel (Dynamax 21.4 mm C-
18
column) with 25-65 % CH3CN with 0.1 % TFA/H20 with 0.1 % TFA to provide the
title
compound as a white powder (40 mg, 39%).
mp 131-133 °C;
MS (APCI-NH3) m/e: 344 (M+H)+;
'H NMR (300 MHz, DMSO-db) b 9.25 (s, 1H), 8.28 (s, 1H), 7.89 (s, 1H), 7.48 (d,
2H),
7.18 (d, 2H), 4.47 (s, 3H);
Anal. Calcd for C,SHIOCIN50S 0.25 H20: C, 51.73; H, 3.04; N, 20.11. Found: C,
51.74;
H, 2.93; N, 19.93.
Example 283
5-[4-(4-Chlorophenoxy)thienof2, 3-cluyridin-2-yll-4-methyl-4H-1 2 4-triazol-3-
amine
Sodium hydride (60% in oil, 0.02 g, 0.42 mmol) was suspended in DMF (1 mL) at
0 °C. A solution of Example 277 (0.11 g, 0.32 mmol) in DMF ( 1 mL) was
added dropwise
and the reaction stirred 20 minutes. Iodomethane (0.06 mL, 0.96 mmol) was
added and
216
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
after 30 minutes the reaction was poured into water (75 mL). The resulting
precipitate was
collected by filtration, washed with water ( 1 x 20 mL), and with 50%
EtOAc/hexane (2 x
25 mL). The crude solid was dried to yield a colored solid (0.11 g). The title
compound
was isolated by preparative HPLC using a gradient of 25%-65%
acetonitrile/water + 0.1 %
TFA over 40 minutes. The product was neutralized with sat. aq. NaHC03, and the
precipitates collected by filtration, then dried in a desiccator to provide
(31 mg, 27%).
mp 233-235 °C;
MS (APCI) m/e: 358 (M+H)+;
'H NMR (300 MHz, DMF-d~) 8 3.75 (s, 3H), 6.58 (br s, 2H), 7.23 (m, 2H), 7.54
(m, 2H),
7.61 (s, 1H), 8.27 (s, 1H), 9.18 (s, IH);
Anal. calcd for C,6H,2C1N5OS: C, 53.71; H, 3.38; N, 19.57. Found: C, 54.00; H,
3.56; N,
19.68.
Example 284
4-(4-Chlorophenoxy)-2-f5-(trifluoromethyl)-l, 2, 4-oxadiazol-3-yllthienof2, 3-
clpyridine
A solution of the resultant compound from Example 249 ( 100 mg, 0.31 mmol) in
pyridine ( 1.5 mL) under nitrogen at room temperature was treated with
trifluoroacetic
anhydride (50 mL, 0.31 mmol), stirred for 20.5 hours, then heated to reflux
for three hours.
The brown solution was diluted with ethyl acetate, washed with saturated
NaHC03, brine,
dried (MgS04), concentrated, then filtered through a plug of silica gel with
50/50
hexane/ethyl acetate and concentrated to give 120 mg of a yellow residue. This
residue
was flash chromatographed on silica gel twice using 20-33% ethyl
acetate/hexane and
then 0-1% methanol/CHZC12 to give the title compound as a white solid (67 mg,
54%).
mp 52-54 °C;
MS (APCI-NH3) m/e: 398 (M+H)+, 416 (M+NH4)+;
'H NMR (300 MHz, DMSO-d6) 8 9.30 (s, 1H), 8.29 (s, IH), 8.10 (s, 1H), 7.48 (d,
2H),
7.20 (d, 2H);
Anal. Calcd for C~6H~C1F3N302S'0.25 H20: C, 47.77; H, 1.88; N, 10.45. Found:
C,
48.15; H, 2.09; N, 10.14.
Example 285
5-f4-(4-Chloro~henoxy)thienof2, 3-clpyridin-2-yll-1, 2, 4-oxadiazol-3-amine
A solution of the resultant compound from Example 250 ( 100 mg, 0.3 mmol) in
methanol ( 1.5 mL) under nitrogen at ambient temperature was treated with
hydroxylamine
hydrochloride (40 mg, 0.45 mmol), triethylamine (70 mL, 0.5 mmol) and stirred
for 18
hours, 4 mL THF was added and stirred for two days, solvent switched to 50/50
217
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
dichloromethane/methanol, additional hydroxylamine hydrochloride (100 mg, 1.4
mmol)
and triethylamine (200 mL, 2.7 mmol) added, stirred at ambient temperature for
24 hours
and then reflux for 8 hours. The reaction mixture was diluted with ethyl
acetate, washed
with dilute NaHCOa, brine, dried (MgS04) and concentrated to give 105 mg of an
off-
white solid. The resultant solid was flash chromatographed twice on reverse
phase silica
gel (Dynamax 21.4 mm C-18 column) with 25-65% CH3CN with 0.1% TFA/H20 with
0.1 % TFA, followed by 20-80% CH3CN with 0.1 % TFA/H20 with 0.1 % TFA to
provide
the title compound as a white powder (26 mg, 25%).
mp 217-219 °C;
MS (APCI-NH3) m/e: 345 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.26 (s, 1H), 8.25 (s, 1H), 8.02 (s, 1H), 7.49 (d,
2H),
7.14 (d, 2H), 6.62 (br s, 2H);
Anal. Calcd for C~SH9C1N402S: C, 52.25; H, 2.63; N, 16.25. Found: C, 51.94; H,
2.88;
N, 15.98.
Example 286
5-f4-(4-Chloro~henoxy~thienof2, 3-clpyridin-2-yll-N-methyl-1, 3, 4-thiadiazol-
2-amine
The title compound (4% overall yield) was prepared as in Example 278,
substituting 4-methylthiosemicarbazide for thiosernicarbazide.
mp 226-229 °C;
MS (APCI) m/e 375 (M+H)+;
;H NMR (300 MHz, DMSO-d6) 8 2.95(d, J=l.7Hz, 3 H), 7.14 (m, 2 H), 7.45 (m, 2
H),
7.69 (s, 1 H), 8.17 (s, 1 H), 8.19 (br m, 1 H}, 9.07 (s, 1 H);
Anal. calcd for C~6H»C1N40S2: C, 51.27; H, 2.96; N, 14.95. Found: C, 51.24; H,
3.03;
N, 14.85.
Example 287
4-(4-Chlorophenoxy)-2-(l, 2, 4-oxadiazol-3-yl)thienof2, 3-clpyridine
Example 287A
4-(4-Chlorophenoxv~-N' ~ethoxymethoxy)thienof2, 3,-clpvridine-2-
carboxaimidamide
A solution of the resultant compound from Example 249 ( 100 mg, 0.31 mmol) in
triethylorthoformate ( 1.3 mL) under nitrogen was heated to 140 °C for
5 hours, 160 °C for
2 hours and stirred at room temperature for 14 hours. The resultant light
yellow oil ( 110
mg) was flash chromatographed on silica gel 20-70% ethyl acetate/hexane to
provide the
title compound as a white solid (50 mg, 38%).
218
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
MS (APCI-NH3) m/e: 422 (M+H)+;
~H NMR (300 MHz, DMSO-d6) 8 9.05 (s, 1H), 8.15 (s, 1H), 7.97 (s, 1H), 7.46 (d,
2H),
7.10 (d, 2H), 6.65 (br s, 2H), 5.66 (s, 1H), 3.62-3.71 (m, 4H), 1.15 (t, 6H).
Examvle 287B
4-(4-Chlorophenoxy)-2-(1 2 4-oxadiazol-3-yl)thienof2, 3-cltwridine
A solution of the resultant compound from Example 287A (SOmg, 0.119 mmol) in
toluene (6 mL) under nitrogen was heated at reflux for 20 hours then allowed
to cool and
concentrated. The yellow residue (46 mg) was flash chromatographed on silica
gel 25-
50% ethyl acetate/hexane to provide the title compound as a white solid (39
mg, 100%).
Analytical HPLC: 4.6x250 mm C-18 column, 0.8 mL/min, 254 nm, CH3CN:H20 with
0.1% TFA, 0:100 (0-3 min), ramp to 90:10 (3-30 min), 90:10 (30-35 min), ramp
to 0:100
(35-40 min), Rt = 22.47 min ( 100% peak area);
mp 151-152 °C;
MS (APCI-NH3) m/e: 330 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 9.86 (s, 1H), 9.26 (s, 1H), 8.27 (s, 1H}, 7.98 (s,
1H),
7.48 (d, 2H}, 7.19 (d, 2H}.
Example 288
2-(1 3 4-Oxadiazol-2-~)-4-f4-(trifluoromethyl)phenoxylthienof2, 3-clpyridine
A solution of Example 183 ( 100 mg, 0.283 mmol) in triethyl orthoformate ( 15
mL)
was heated to reflux under an atmosphere of nitrogen for 28 hours. All
volatiles were
removed under reduced pressure. The resulting oil was purified by flash column
chromatography eluting with hexane/ethyl acetate (2:1), affording 65 mg (63%)
of the title
compound as a colorless oil that solidified on standing.
MS (ESI) m/e: 364 (M+H)+;
'H NMR (300 MHz, CDC13) 8 7.14 (d, 2H), 7.66 (d, 2H), 8.05 (s, 1H), 8.24 (s,
1H), 8.50
(s, 1H), 9.07 (s, 1H);
Anal. calcd for C,6HgN3F302S: C, 52.89; H, 2.22; N, I 1.57. Found: C, 53.03;
H, 2.25; N,
11.48.
ExamRle 289
3-f4-(4-Chlorophenoxy)thienof2 3-clpyridin-2-yll-1, 2, 4-oxadiazol-5-amine
Example 289A
3-f4-(4-Chlorophenoxy)thienof2 3-clpyridin-2-yll-5-(trichloromethyl-l, 2, 4-
oxadiazole
2I9
CA 02333770 2000-11-30
WO 99162908 PCTJUS99/12419
A solution of the resultant compound from Example 249 (50 mg, 0.156 mmol)
pyridine (2 mL) under nitrogen at ambient temperature was treated with
trichloroacetyl
chloride (20 mL, 0. I7 mmol) and heated at reflux for 1.5 hours, allowed to
cool to room
temperature, stirred overnight, treated with additional trichloroacetyl
chloride ( 100 mL,
0.86 mmol) and stirred 4 hours. The reaction mixture was then diluted with
ethyl acetate,
washed with saturated NaHC03, brine, dried (MgS04) and concentrated. The
resultant
brown residue was flash chromatographed on silica gel with 25-50% ethyl
acetate/hexane
to give the title compound as a clear residue (37 mg, 53%).
MS (APCI-NH3) m/e: 448 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.25 (s, IH), 8.24 (s, 1H), 8.09 (s, 1H), 7.46 (d,
2H),
7.18 (d, 2H).
Example 289B
3-14-(4-Chlorophenoxy)thienof2, 3-clpyridin-2-yll-l, 2, 4-oxadiazol-5-amine
A solution of the resultant compound from Example 289A (30 mg, 0.067 mmol) in
2.0 M ammonia in methanol (6 mL) in a pressure tube was heated to 60 °C
for 15 hours
then allowed to cool ambient temperature, rinsed out of the flask with
methanol and
distilled water. The bulk of the methanol was removed under vacuum and the
cloudy
white mixture was filtered and washed with distilled water. The resultant
yellow-brown
solid was flash chromatographed twice on reverse phase silica gel (Dyanamax C-
18, 21.4
mm column) with 25-65 %, and then 20-80% CH3CN with 0.1 % TFA/H20 with 0.1 %
TFA
to give the title compound as a white solid (4 mg, 17%).
Analytical HPLC: 4.6x250 mm C-18 column, 0.8 mL/min, 254 nm, CH3CN:HzO with
0.1 % TFA, 0:100 (0-3 min), ramp to 90:10 (3-30 min), 90:10 (30-35 min), ramp
to 0:100
(35-40 min), Rt = 19.44 min ( 100% peak area);
mp 268-270 °C;
MS (APCI-NH3) m/e: 345 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.21 (s, 1H), 8.27 (s, 1H), 8.22 (br s, 2H), 7.67
(s, 1H),
7.47 (d, 2H), 7.15 (d, 2H).
Example 290
2-(5-Methyl-1, 3, 4-oxadiazol-2-yl)-4-~4-(trifluoromethyl)pheno~)thienof2 3-
clpyridine
The title compound was prepared as described for Example 288 using triethyl
orthoacetate as the solvent. Reflux was maintained for 5 days with the yield
being 29%.
MS (APCI) m/e: 377 (M+H)+;
220
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, CDCl3) 8 2.65 (s, 3H), 7.13 (d, 2H}, 7.65 (d, 2H), 7.94 (s,
1H), 8.27
(s, 1H), 9.06 (s, 1H}.
Example 291
4-(4-Chlorophenoxy)-2-(2-furyl)thienof2 3-clpyridine
A mixture of Example 270A (0.30 g, 0.98 mmol), 2,5-dibromofuran (0.66 g, 2.94
mmol), cesium fluoride (0.45 g, 2.94 mmol), triethylamine (0.14 mL, 0.98
mmol), in DME
(9 mL) was aspirated 25 minutes with anhydrous nitrogen and [1,1'-
bis(diphenylphosphino)ferrocene]dichloropalladium(II) dichloromethane complex
( 1:1 )
(0.16 g, 0.20 mmol) was added. The reaction was heated to reflux for 4 hr and
then stirred
at ambient temperture overnight. The reaction was diluted with EtOAc (100 mL)
and
filtered. The organic layer was washed with sat. NaHC03 (3 x 50 mL), brine (75
mL),
partially dried (Na2S04), and concentrated to a colored oil. The residue was
purified by
flash chromatography on silica gel using EtOAc/hexane as eluent to yield a
colored oil
(0.12g, 0.30 mmol) [MS (APCI) m/e 406 (M+H)+]. This material was dissolved in
EtOH
(10 mL) and 5% Pd/C (3 mg, 0.02 mmol) was added. A balloon of hydrogen gas was
applied and reaction stirred 3 days at ambient temperature. Reaction was
filtered through
celite and celite wash with MeOH ( 10 mL) and dichloromethane ( 10 mL). The
filtrate and
washes were combined and concentrated to a colored wet foam. The crude
material was
purified by preparative HPLC using a gradient of 30%-70% acetonitrile/water +
0.1 % TFA
over 40 minutes. The product was neutralized with sat NaHC03, precipitate
collected by
filtration, and dried in a desiccator to yield the title compound as a lightly
colored solid (15
mg, 5% overall yield).
mp 75-77 °C;
MS (APCI) m/e: 328 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 6.70 (dd, J=3.4, l.7Hz, 1 H), 7.12 (m, 2 H), 7.24
(d,
J=3.4Hz, 1 H), 7.46 (m, 2 H), 7.59 (s, 1 H), 7.88 (d, J=l.7Hz, 1 H), 8.16 (s,
1 H), 9.08 (s, 1
H).
Example 292
4~4-Chlorophenoxy)-2-(2-thienyl)thienof2 3-clpyridine
Example 292 ( 50 mg, 22%) was prepared as in Example 272A, substituting 2-
iodothiophene for 1-iodo-4-nitrobenzene.
mp 101-103 °C;
MS (APCI) m/e: 344 (M+H)+;
221
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, DMSO-d6) S 7.14 (m, 2 H), 7.19 (dd, J=5.0, 3.4Hz, 1 H), 7.46
(m, 2
H), 7.54 (d, J=0.9Hz, 1 H), 7.67 (dd, J=3.4, l.3Hz, 1 H), 7.73 (dd, J=5.0,
l.3Hz, 1 H), 8.14
(s, 1 H), 9.05 (s, 1 H);
Anal. calcd for CI~H~oCINOS2'0.2H20: C, 58.77; H, 3.02; N, 4.03. Found: C,
58.74; H,
2.84; N, 3.72.
Example 293
2-f4-(4-Chloronhenoxy)thienof2 3-clpyridin-2-yll-1 3-thiazole-4 carboxamide
Example 293A
Ethyl 2-f4-(4-Chlorophenoxy)thienof2 3-clpyridin-2-yll-1 3-thiazole-4
carboxylate
Ethyl bromopyruvate (390 mL, 2.30 mmol) was combined with Example 146 (672
mg, 2.09 mmol) and absolute ethanol (100 mL), and the orange, homogenous
solution was
heated at 60 °C. After 48 hours, the mixture was cooled to ambient
temperature and
concentrated under vacuum. Purification was achieved by means of flash silica
gel
chromatography (15% acetone in hexane), and the title compound (297 mg, 34%
yield)
was obtained as a white solid.
MS (DCI/NH3) m/e: 417 (35C1)/419 (3~C1);
'H NMR (300 MHz, CDCl3) S 8.94 (s, 1H), 8.24 (s, IH), 8.12 (s, 1H), 7.88 (s,
1H), 7.36
(d, J=8.8 Hz, 2H), 7.04 (d, J=8.8 Hz, 2H), 4.45 (q, J=7.0 Hz, 2H), 1.45 (t,
J=7 Hz, 3H).
Example 293B
2-f4-(4-Chlorophenoxy)thienof2 3-clpyridin-2-yll-1 3-thiazole-4 carboxamide
Example 293A (32 mg, 77 mmol) was combined with ammonia in methanol (4 mL
of a 2.0 M solution), and the solution was heated in a sealed tube at 40
°C. After 16 hours,
the homogeneous solution was cooled to room temperature and concentrated to a
brown
solid that was dry-loaded on flash silica gel and eluted with 20% acetone in
hexane,
followed by 40% acetone in hexane to recover the title compound (8 mg, 27%
yield).
mp 215-218 °C;
MS (DCI/NH3) m/e: 388 (M+H)/405 (M+NH3), 407 (3~C1+NH3);
1H NMR (300 MHz, CDC13) 8 5.67 (br s, 2H), 7.06 (d, J=8.5 Hz, 2H), 7.38 (d,
J=8.8 Hz,
2H), 8.09 (s, 1H), 8.25 (s, 1H), 8.94 (s, 1H).
Example 294
tent-Butyl 2-f4-(4-Chlorophenoxy)thienof2 3-clpyridin-2-yll-1 3 thiazol 4
ylcarbamate
222
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 293 was converted to the corresponding carboxylic acid in a similar
manner as described in Example 18. biphenyl phosphorylazide (25 p.L,, 0.10
mmol) was
added to a mixture of the carboxylic acid (40 mg, 0.10 mmol), tert-butanol (10
mL), and
triethyl amine (20 ~t.L,, 0.10 mmol), and the solution was heated at 80
°C. After 18 hours,
the solution was cooled and concentrated. The yellow residue was dissolved in
CH2C12
(30 mL), washed sequentially with 0.5 N aqueous HCl (40 mL) and saturated
aqueous
NaHC03 (25 mL), and brine (25 mL). The combined aqueous washes were back-
extracted
with CH2C12 (2x25 mL). The organic layers were combined, dried (MgS04),
filtered, and
concentrated to a yellow residue. Flash silica gel flash column chromatography
(15%
acetone in hexane) provided the title compound ( 13 mg, 28 % yield) as a
bright yellow
solid.
MS (APCI) m/e: 460 (35C1)/462 (3'C);
1H NMR (300 MHz, CDCl3) S 8.91 {s, IH), 8.11 (s, 1H), 7.71 (s, 1H), 7.35 (d,
J=8.9 Hz,
2H), 7.03 (d, J=8.9 Hz, 2H), 3.97 (d, J=11.4 Hz, 2H), 1.54 (s, 9H).
Example 295
2-f4-(4-Chloronhenoxy)thienof2 3-clpyridin-2-yll-1 3-thiazol-4 amine
Trifluoroacetic acid (0.5 mL) was added to Example 294 (9.0 mg, 20 p,mol) in
CH2Cl2 ( 1.5 mL) at 0 °C. After 1 h, the volatiles were removed, and
the orange residue
was dissolved in 0.5 N aqueous HCl (35 mL). The aqueous phase was washed once
with
Et20 ( 10 mL); the ether wash was extracted with 1 N HCl (2x20 mL). The acidic
layers
were combined, and saturated aqueous potassium carbonate was added until the
solution
was basic (pH>12). The alkaline phase was extracted with EtOAc (3x40 mL); the
organic
extracts were combined, dried (MgS04), filtered, and concentrated to a dark
brown solid
(9 mg). Purification by flash silica gel column chromatography (20% acetone in
hexane)
provided the title compound as a bright yellow solid (6.8 mg, 97% yield).
mp 168-170 °C (decomposes);
MS (APCI) m/e: 360 (35C1)/362 (3~C1);
'H NMR {300 MHz, CDCl3) S 8.90 (s, 1H), 8.I I (s, 1H), 7.68 (s, 1H), 7.35 (d,
J=9.2 Hz,
2H), 7.02 (d, J=8.9 Hz, 2H), 6.07 (s, IH), 3.89 {br s, 2H).
Example 296
4-(4-Chlorophenoxy)-2-(4 5-dihydro-1 3-oxazol-2-yl)thieno~2 3 clpyridine
To a solution of Example 150 (0.14 g, 0.38 mmol) in anhydrous dichloromethane
(4 mL) was added 1,8-diazabicyclo[5.4.0)undec-7-ene (DBU) (0.09 mL, 0.57
mmol). The
reaction was stirred 24 hours and an excess of morpholine (0.2 mL) was added
to react
223
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
with any remaining starting material. The reaction was stirred 4 hours then
partitioned
between EtOAc ( 100 mL) and H20 ( 100 mL). The organic layer was washed with
dilute
NaH2P04 ( 100 mL), sat. NaHC03 ( 100 mL), brine ( 100 mL), partially dried
(Na2S04), and
concentrated to a solid. The crude product was purified by flash
chromatography on silica
gel using EtOAc/hexane as eluant to yield the title compound as a solid (0.07
g, 55%).
mp 158-160 °C (dec);
MS (APCI) m/e: 331 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 4.01 (t, J=9.6Hz, 2 H), 4.48 (t, J=9.6Hz, 2 H),
7.14 (m,
2 H), 7.47 (m, 2 H), 7.62 (s, 1 H), 8.24 (s, 1 H}, 9.19 (s, 1 H);
IO Anal. calcd for C16H~1CIN202S: C, 58.09; H, 3.35; N, 8.47. Found: C, 58.16;
H, 3.31; N,
8.27.
Example 297
4-(4-Chlorophenoxy)-2-(1 3-oxazol-2-yl)thienof2 3-clp ridine
The title compound may be produced from Example 296 according to the
procedure of Ishibashi, Y., et al. (Tetrahedron Lett. 1996, 37(17), 2997-
3000).
Example 298
4-(4-Chlorophenoxy)-2-(4 5-dihydro-1H-imidazol-2-yl)thieno(2 3 clpyridine
A suspension of Example 154 (0.15 g, 0.43 mmol) and calcium oxide (0.12 g,
2.15
mmol) in phenyl ether ( 10 mL) was heated to 220-250 °C. The reaction
was stirred 45
minutes over which reaction volume decreased due to evaporation of solvent.
The
reaction was cooled to room temperature and diluted with 10%
MeOH/dichloromethane
(25 mL) then filtered. The filtrate was concentrated and residue was purified
by
preparative HPLC using a gradient of 30%-90% acetonitrile/water + 0.1 % TFA
over 40
minutes. The product containing fractions were combined and neutralized with
sat
NaHC03. The product crystalized upon standing for 3 days and was collected by
filtration, and dried in a desiccator to yield the title compound as a tan
needles (23 mg,
16%).
mp 154-155 °C;
MS (APCI) m/e: 330 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 3.46 (td, J=9.9, 1.7 Hz, 2H), 3.83 (t, J=9.9 Hz,
H), 7.12
(m, 2H), 7.34 (br s, 1H), 7.46 (m, 2H), 7.81 (s, 1H), 8.17 (s, lH), 9.11 (s,
1H);
Anal. calcd for C~6H,ZCIN30S: C, 58.27; H, 3.67; N, 12.74. Found: C, 58.15; H,
3.50;
N, 12.73.
224
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 299
4-(4-chlorophenoxy)-2-(1H-imidazol-2-yl)thienof2 3-clpyridine
The title compound may be produced from Example 298 according to the
procedure of Zimmerman, S. C., et al. (J. Org. Chem. 1989, 54(6), 1256-1264).
Example 300
4-Chloro-3-methylthienof2 3-clpyridine-2-carboxamide
Example 300 was prepared as in Example 125 from the corresponding 4-chloro
methyl ester which was isolated as a by-product from example 125A.
MS (DCI/NH3) m/e: 227 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 2.55 (s, 3H), 6.95 (d, 2H), 7.57 (d, 2H), 7.90 (b,
1H),
8.00 (b, 1H), 8.27 (s, 1H), 9.15 (s, 1H).
Example 301
3-Amino-4-chlorothienof2 3-clpyridine-2-carboxamide
Methyl 3-amino-4-chlorothieno[2, 3-c]pyridine-2-carboxylate was isolated from
the crude product mixture of Example 131B. The mixture was hydrolyzed as
described in
Example 18, and the resultant acid was coupled with ammonium chloride using
the
procedure for Example 92. The product was isolated by filtration and washed
with water
after precipitating the reaction mixture by pouring it into 5% sodium
bicarbonate solution.
MS (DC1/NH3) m/e: 228 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 7.01 (br s, 2H), 7.49 (br s, 2H), 8.42 (s, 1H),
9.11 (s,
1 H).
Example 302
9-(4-Chlorophenoxy)pyridof4' 3':4 Slthienof3 2-dlpyrimidine-2 4(1H 3H) dione
To a suspension of Example 131 D (70mg, 0.22 mmol) in anhydrous
tetrahydrofuran (5 mL) under nitrogen atmosphere was added 1,1-
carbonyldiimidazole
(7lmg, 0.44 mmol) and triethylamine (60 p.L, 0.44 mmol). The reaction mixture
was
stirred at reflux for 48 hours and then ambient temperature for an additional
24 hours. The
reaction mixture was poured into a 1:1 solution of water: saturated NH4CI and
the
resulting solid collected by filtration. This material was purified by flash
chromatography
on silica gel eluting with 20% acetone-hexane. The desired fractions were
combined,
evaporated and slurried in hot EtOAC to obtain title compound (39mg) in 51 %
yield.
MS (APCI) m/e: (M-H)- 344;
225
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
1H NMR (300 MHz, DMSO-d6) 8 7.32 (m, 2H), 7.55 (m, 2H), 7.92 (s, 1H), 9.09
(s,lH),
11.22 (br s, 1H), 11.72 (br s, 1H);
HPLC: Supelco C-18 column, water:acetonitrile 0:90- 90:0, 30 minute elution,
flow rate
0.8 mL/min, rt 20.33 min.
Example 303
4-(4-Chlorophenoxy)-N, 3-dimethylthienof2 3-clpyridine-2-carboxamide
Example 125A was prepared as in Example 218 to provide the title compound.
MS (DCI/NH3) rn/e: 333 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 2.55 (s, 3H), 2.80 (d, 3H), 7.05 (d, 2H), 7.45 (d,
2H),
8.20 (s, 1 H), 8.55 (b, 1 H), 9.18 (s, 1 H);
Example 304
4-(4-Bromophenoxy)-3-methylthienof2 3-clpyridine-2-carboxamide
Example 17A and 4-bromophenol were processed as in Example 125 to provide
the title compound.
mp 177-178 °C;
MS (DCI/NH3) m/e: 364 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.55 (s, 3H), 6.95 (d, 2H), 7.57 (d, 2H), 7.90 (m,
1H),
8.00 (m, 1H), 8.27 (s, 1H), 9.15 (s, 1H);
Anal. Calcd for C,SH, ~BrNz02S: C, 49.60; H, 3.05; N, 7.71. Found: C, 49.36;
H, 3.24; N,
7.61.
Example 305
7-Chloro-4-(4-chlorophenoxy)-3-methylthienof2 3-c]pvridine-2-carboxamide
Example 305A Methyl 4-(4-Chlorophenoxy)-3-methylthienof2 3-clpvridine-2-
carboxylate, N-oxide
Example 125A was prepared according to the procedure of Example 123A to
provide the title compound.
MS (DCI/NH3) m/e: 350 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.78 (s, 3H), 3.88 (s, 3H), 7.28 (m, 2H), 7.51 (m,
2H),
7.68 (br s, 1H), 8.92 (br s, 1H).
Example 305B Methyl 7-Chloro-4-(4-chlorophenoxy)-3-methylthienof2 3 clpyridine
2
carboxamide
226
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 305A was prepared according to the procedure of Example 1 C to provide
the title compound.
HPLC: Supelco C-18 column, gradient elution 0.1 % aqueous TFA:acetonitrile
0:90- 90:0
over 30 minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 31.64
minutes;
MS (DCI/NH3) m/e: 368 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.78 (s, 3H), 3.92 (s, 3H), 7.18 (m, 2H), 7.48 (m,
2H),
8.01 (s, 1H).
Example 305C 7-Chloro-4-(4-chloronhenoxy)-3-methylthienof2 3-clpyridine-2-
carboxylic acid
Example 3058 was prepared according to the procedure of Example 18
substituting tetrahydrofuran for isopropanol to provide the title compound.
HPLC: Supelco C-18 column, gradient elution 0.1% aqueous TFA:acetonitrile 0:90-
90:0
over 30 minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 27.25
minutes;
MS (APCI) m/e: 354 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 2.72 (s, 3H), 7.16 (m, 2H), 7.49 (m, 2H), 8.01 (s,
1H).
Example 305D
7-Chloro-4-(4-chlorophenoxy)-3-methylthienof2 3-clpyridine-2-carboxamide
Example 305C was prepared according to Example 92 to provide the title
compound.
HPLC: Supelco C-18 column, gradient elution 0.1 % aqueous TFA:acetonitrile
0:90- 90:0
over 30 minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 24.75
minutes;
MS (DCI/NH3) m/e: 353 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.58 (s, 3H), 7.10 (m, 2H), 7.47 (m, 2H), 8.0 (br
s, 1H),
8.02 (s, 1H), 8.03 (br s, 1H).
Example 306
tert-Butyl2-(aminocarbonyl)-4-(4-chlorophenoxy)thienof2 3-clpyridine-3-
carboxylate
Example 306A
tert-Butyl 3, 5-Dichloropyridine-4-oxalate
Example 17A was followed except for replacing the methyl formate with t-Butyl
chlorooxalate to provide the titled compound.
MS (DCI/NH3) m/e: 241 (M+H)+;
'H NMR (300 MHz, DMSO-d6) S 1.5 (s, 9H), 8.85 (s, 2H).
227
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
Example 306B
tert-Butyl2-(Methoxycarbonyl)-4-(4-chloronhenoxy)thienof2 3-clpyridine-3-
carboxylate
Example 306A and 4-chlorophenol were processed as in Example 61 to provide
the title compound.
MS (DCI/NH3) m/e: 420 (M+H)+;
IH NMR (300 MHz, DMSO-d6) 8 1.42 (s, 9H), 3.95 (s, 3H), 7.18 (d, 2H), 7.50 (d,
2H),
8.05 (s, 1H), 9.20 (s, 1H).
Example 306C
tert-Butyl 2-(Aminocarbonyl)-4-(4-chlorophenoxy)thienol2 3-clpyridine 3
carboxylate
Example 306B was prepared as in Example 217 to provide the title compound.
MS (DCI/NH3) m/e: 405 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 1.35 (s, 9H), 7.10 (d, 2H), 7.45 (d, 2H), 7.95 (m,
1H),
8.08 (s, 1H), 8.14 (m, 1H), 9.18 (s, 1H).
Example 306D
2-(Aminocarbonyl)-4-(4-chlorophenoxy)thienof2 3-clpyridine-3-carboxylic acid
Example 306C (0.08 g, 0.2mmol) was placed in a cold solution of
trifluoroacetic
acid (0.5 mL) and methylene chloride (0.5 mL) and stirred for 1 hour. The
solution was
evaporated and treated slowly with a cold solution of sodium bicarbonate (20
mL), and
then the mixture was extracted with ethyl acetate (3x20 mL). The ethyl acetate
extract
was dried and evaporated to provide the title compound.
MS (DC1/NH3) m/e: 349 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 7.05 (d, 2H), 7.45 (d, 2H), 7.95 (b, 1H), 8.05 (m,
1H),
8.15 (s, 1H), 9.20 (s, 1H).
Example 307
Methyl4-(4-toluidino)thienof2 3-clpyridine-2-carboxamide
A mixture of Example 93C (271 mg, 1 mmol), 4-methyl aniline ( 150 mg, 1.4
mmol), sodium t-butoxide ( 134.5 mg, 1.4 mmol), 18-crown-6 (370 mg, 1.4 mmol),
Pd2(dba)3 (46 mg, 5 mol%) and BINAP (31 mg, 5 mol%) were combined in a three
necked
round bottom flask equipped with a condenser, internal temperature probe and a
N2 inlet.
This was evacuated under nitrogen and anhydrous tetrahydrofuran (5 mL) was
added. The
reaction mixture was warmed at 45 °C for three days, solid materials
were filtered through
celite and washed with a mixture of ethyl acetate and acetone. The filtrate
was diluted
228
CA 02333770 2000-11-30
WO 99/62908 PC'T/US99/12419
with ethyl acetate (100 mL), washed with brine (2x50 mL), dried (MgS04) and
evaporated
to dryness under reduced pressure. The title compound was obtained in 29%
yield (86
mg), by flash chromatography on silica gel eluting with 30% acetone-hexane.
MS (DC1/NH3) m/e: 298 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 2.25 (s, 3H), 2.80 (d, J=6 Hz, 3H), 7.02 (d, J=9
Hz,
2H), 7.10 (d, J=9 Hz, 2H), 8.13 (s, 1H), 8.26 (s, 1H), 8.36 (m, 1H), 8.76 (br
s, 1H);
'3C NMR (75 MHz, DMSO-d.6) b 20.4 (CH3), 26.4 (CH2), 118.2 (CH), 118.2 (CH),
122.1
(CH), 129.8 (CH), 130.0 (C), 130.6 (CH), 130.7 (C), 135.9 (C), 136.5 (CH),
137.1 (C),
140.3 (C), 142.3 (C), 161.6 (CO).
Example 308
4-(4-Chloroanilino)-N-methylthienof2 3-clpyridine-2-carboxamide
The title compound (500 mg, 84%) was prepared as in Example 307 except
substituting 4-chloroaniline (510 mg, 4 mmol) for 4-methylaniline and reaction
was
warmed at 60 °C for 20 hours.
MS (APCI) m/e: 318 (M+H)+, 352 (M+Cl)-;
'H NMR (400 MHz, DMSO-d6) d: 2.83 (d, J=4 Hz, 3H), 7.07 (d, J=9 Hz, 2H), 7.32
(d,
J=9 Hz, 2H), 8.11 (s, 1H), 8.38 (s, 1H), 8.67 (s, 1H), 8.85 (d, J=4 Hz, 1H),
8.91 (s, 1H);
'3C NMR (100 MHz, DMSO-d6) 8 26.3 (CH3), 118.1 (2xCH), 121.7 (CH), 123.6 (C),
129.1 (2xCH), 133.0 (CH), 134.4 (C), 137.1 (C), 137.2 (C), 138.2 (CH), 142.6
(C), 143.2
(C), 161.4 (C).
Example 309
Methyl4-(4-momholinyl)thienof2 3-clpyridine-2-carboxamide
The title compound (105 mg, 38%) was prepared as in Example 308 except
substituting morpholine (0. I75 mL, 2 mmol) for 4-chloroaniline.
MS (APCI) m/e: 278 (M+H)+, 312 (M+Cl)-;
'H NMR (400 MHz, DMSO-d6) 8 2.91 (d, J=4 Hz; 3H), 3.23 (m, 4H), 3.91 (m, 4H),
8.14
(s, 1H), 8.18 (s, 1H), 8.96 (s, 1H), 8.99 (d, J=4 Hz, 1H);
'3C NMR (100 MHz, DMSO-d6) 8 26.1 (CH3), 51.6 (2xCH2), 66.3 (CH2), 121.2 (CH),
131.6 (CH), 137.1 (C), 137.9 (C), 139.0 (C), 143.3 (C), 143.9 (C), 161.3 (CO).
Example 311 7-Chloro-4-(4-chloronhenoxy)thienof2 3-clpyridine-2-carboxamide
Example 311 A
Methyl7-chloro-4-(4-chloronhenoxy)thienof2 3-clnyridine-2-carbox late
229
CA 02333770 2000-11-30
WO 99162908 PCT/US99/12419
Example 61 A was prepared as in Example 1 C to provide the title compound.
HPLC: Supelco C-18 column, gradient elution of 0.1% aqueous TFA:acetonitrile
0:90-
90:0 over 30 minutes, detection at 254 nm, flow rate of 0.8 mL/minutes RT
30.35 minutes;
MS (DCI/NH3) m/e: 354 (M+H)+;
'H NMR (300 MHz, DMSO-d6) S 3.91 (s, 3H), 7.14 (m, 2H), 7.45 (m, 2H), 7.91 (s,
1H);
8.24 (s, 1H); 9.21 (s, 1H).
Example 311B 7-Chloro-4-(4-chlorophenoxy)thienof2 3-clpyridine-2-carboxamide
Example 311 A was prepared according to the procedure of Example 44 to provide
the title compound.
MS (DCI/NH3) m/e: 339 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 7.20 (m, 2H), 748 (m, 2H), 7.94 (br s, 1H), 8.04
(s, 1H)
8.22 (s, 1H), 8.49 (br s, 1H);
Anal. calcd for C14H8C1zN202S: C, 56.42; H, 3.28; N, 10.12. Found: C, 56.31;
H, 3.22; N,
10.01;
Example 312
7-Chloro-4-(4-chlorot~henoxy)-N-methylthienof2 3-cltwridine-2-carboxamide
Example 311 A was prepared as described in Example 171 to provide the title
compound.
MS (DCI/NH3) m/e: 353 {M+H)+;
'H NMR (300 MHz, DMSO-db) b 2.80 (d, 3H); 7.16 (m, 2H), 7.49 (m, 2H), 8.05 (s,
1H),
8.17 (s, 1H), 9.04 (br s, 2H).
Example 313
7-Chloro-4-(4-chlorophenoxy)-N-(2-hydroxyethyl)thienof2 3-clpyridine 2
carboxamide
Example 311 A was prepared according to the procedure of Example 114 to
provide the title compound.
HPLC: Supelco C-18 column, gradient elution of 0.1% aqueous TFA:acetonitrile
0:90-
90:0 over 30 minutes, detection at 254 nm, flow rate of 0.8 mL/minutes RT
23.49 minutes;
mp 129-132 °C;
MS (DCI/NH3) m/e: 382 (M+H)+;
1H NMR (300 MHz, DMSO-db) 8 3.33 (m, 2H), 3.51 (m, 2H), 4.82 (t, 1H), 7.19 (m,
2H),
7.48 (m, 2H), 8.08 (s, 1H), 8.27 (s, 1H), 9.12 (br t, 1H), 9.18 (s, 1H), 12.81
(br s, 1H).
Example 314
230
CA 02333770 2000-11-30
WO 99/62908 PCTNS99/12419
7-Bromo-4-(4-chloronhenoxy)thienof2 3-clpyridine-2-carboxamide
Example 314A
Methyl7-Bromo-4-(4-chlorophenoxy)thienof2 3-clpyridine-2-carboxylate
Example 123A was prepared as in Example 1C substituting phosphorous
oxybromide for phosphorous oxychloride to provide the title compound.
MS (ESI) m/e: 400 (M+H)+;
'H NMR (300 MHz, DMSO-d6) & 3.91 (s, 3H), 7.22 (m, 2H), 7.48 (m, 2H), 8.19 (s,
1H),
8.20 (s, 1H).
Example 314B
7-Bromo-4-(4-chlorophenoxy)thienof2 3-clpyridine-2-carboxamide
Example 314A was prepared according to Example 44 to provide the title
compound.
HPLC: Supelco C-18 column, gradient elution of 0.1% aqueous TFA:acetonitrile
0:90-
90:0 over 30 minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 24.95
minutes;
MS (DCI/NH3) m/e: 385 (M+H)+;
'H NMR (300 MHz, DMSO-d6) S 7.22 (m, 2H), 7.44 (m, 2H), 7.95 (s, 1H), 8.02 (s,
1H),
8.29 (s, 1H); 8.51 (br s, 1H).
Example 315
7-Bromo-4-(4-chlorophenoxy)-N-methylthienof2 3-c]pyridine-2 carboxamide
Example 314A was prepared according to the procedure of Example 171 to
provide the title compound.
HPLC: Supelco C-18 column, gradient elution of 0.1% aqueous TFA:acetonitrile
0:90-
90:0 over 30 minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 25.40
minutes;
MS (DCI/NH3) m/e: 397 (M+H)+;
'H NMR (300 MHz, DMSO-db) 8 2.81 (d, 3H), 3.97 (s, 3H), 7.19 (m, 2H), 7.48 (m,
2H),
8.04 (s, 1H), 8.21 (s, 1H), 9.05 (br s, 1H).
Example 316
4-(4-Bromophenoxy)-7-chlorothienof2 3-clpyridine-2-carboxamide
Example 316 was prepared as in Example 311 but substituting 4-bromophenol for
4-chlorophenol to provide the title compound.
MS (DCI/NH3) m/e: 383, 385 (M+H)+;
231
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
'H NMR (300 MHz, DMSO-d6) b 7.14 (d, 2H, J=8.9 Hz), 7.61 (d, 2H, J=8.8 Hz),
7.98 (br
s, 1H), 8.05 (s, 1H), 8.22 (s, 1H), 8.52 (br s, 1H);
Anal. calcd for C,4H8N202SBrC1Ø5 HZO: C, 42.82; H, 2.31; N, 7.10. Found: C,
42.62;
H, 2.26; N, 6.82.
Example 317
4-(4-Bromonhenoxy)-7-chloro-N-methylthienof2 3-clpyridine-2-carboxamide
Example 317 was prepared as in Example 312 but substituting 4-bromophenol for
4-chlorophenol to provide the title compound.
MS (DCI/NH3) m/e: 397, 399 (M+H)+;
~H NMR (300 MHz, DMSO-db) 8 2.80 (d, 3H, J=4.7 Hz), 7.13 (d, 2H, J=9.2 Hz),
7.60 (d,
2H, J=9.2 Hz), 8.07 (s, 1H), 8.13 (s, 1H), 9.03 (q, 1H, J=4.7 Hz);
Anal. calcd for CISH~oN202SBrCl: C, 45.30; H, 2.53; N, 7.04. Found: C, 45.25;
H, 2.31;
N, 6.86.
Example 318
7-Chloro-4-f4-(trifluoromethyl)phenoxylthienof2 3-cipyridine-2-carboxamide
Example 17A and 4-trifluoromethylphenol were processed as in Example 311 to
provide the title compound.
mp 175-176 °C;
MS (DCI/NH3) m/e: 373 (M+H)+;
1H NMR (300 MHz, DMSO-db) b 7.30 (d, 2H), 7.80 (d, 2H), 8.00 (s, 1H), 8.20 (s,
1H),
8.25 (s, 1 H), 8.55 (s, 1 H);
Anal. Calcd for Cl6H~pCIF3N2O2S: C, 48.33; H, 2.16; N, 7.52. Found: C, 48.26;
H, 2.25;
N, 7.40.
Example 319
7-Chloro-N-methyl-4-f4-(trifluoromethyl)phenoxylthienof2 3-clpyridine-2
carboxamide
Example 17A and 4-trifluoromethylphenol were processed as in Example 312 to
provide the title compound.
mp 178-179 °C;
MS (DCI/NH3) m/e: 387 (M+H)+;
IH NMR (300 MHz, DMSO-d6) b 2.80 (s, 3H), 7.30 (d, 2H), 7.80 (d, 2H), 8.00 (s,
1H),
8.25 (s, 1H), 8.55 (m, 1H);
Anal. Calcd for C~6H~oC1F3NZOZS: C, 49.68; H, 2.61; N, 7:24. Found: C, 49.58;
H, 2.54;
N, 6.94.
232
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 320
7-Chloro-N-(2-hydroxyethyl)-4-f4-(trifluoromethyl)phenoxylthienof2 3-
clpyridine 2
carboxamide
Example 320 was prepared according to the procedure of Example 319, with the
substitution of aminoethanol for methylamine.
mp 96-97 °C;
MS (ESI/NH3) m/e: 415 (M+H)+;
~H NMR (300 MHz, CDCI3) b 3.66 (t, 2H, J=4.8 Hz), 3.87 (t, 2H, J=4.8 Hz), 6.63
(m,
1H), 7.11 (d, 2H, J=8.5 Hz), 7.64 (d, 2H, J=8.5 Hz), 7.72 (s, IH), 8.02 (s,
1H).
Example 321
4-(4-Chloronhenoxy)-N 7-dimethylthienof2 3-clpyridine-2-carboxamide
Example 321A
Methyl 4-(4-Chloropheno ~)-N 7-dimethylthienof 2 3-clpyridine 2 carbox. date
Example 3I lA was prepared as Example 95A but substituting methyl boronic acid
for 4-(triflouoromethyl)phenyl boronic acd and substituting
dichlorobis(tricyclo-
hexylphosphine) palladium for tetrakis(triphenylphosphine)palladium and
substituting
NMP for DME to provide the title compound.
MS (DCUNH3) m/e: 334 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 2.74 (d, 3H), 3.91 (s, 3H), 7.14, (m, 2H), 7.44
(m, 2H),
7.9I (s, 1 H), 8.22 (s, 1 H).
Example 321B
4-(4-Chlorophenoxy)-N 7-dimethylthienof2 3-clpyridine-2-carboxamide
Example 321A was prepared according to the procedure of Example 171 to
provide the title compound.
HPLC Supelco C-18 column, gradient elution of 0.1 % aqueous TFA:acetonitrile
0:90-
90:0 over 30 minutes, detection at 254 nm, flow rate of 0.8 mL,/minutes RT
20.70 minutes;
MS (DC1/NH~) m/e: 334 (M+H)+;
~H NMR (300 MHz, DMSO-d6) 8 2.82 (d, 3H), 7.04 (m, 2H), 7.41 (m, 2H), 8.04,
(s, 1H),
8.11 (s, 1H), 8.92 (br s, 1H).
Example 322
4-(4-Chlorophenoxy)-7-methoxythieno(2 3-clpyridine-2-carboxamide
233
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 322A
4-(4-Chlorophenoxy)-7-methoxythienof2,3-clpyridine-2-carboxylic acid
Example 311 A ( 100 mg, 0.28mmol) was dissolved in 2S% sodium methoxide in
S methanol ( 10 mL) and was warmed to 60 °C in a pressure tube for 3
days. The solvent
was removed under reduced pressure and the residue was redissolved in
methylene
chloride and acidified with formic acid. The organic layer was washed with H20
and
brine, dried (Na2S04) and the solvent removed under reduced pressure to afford
the title
compound (SO mg, S4%) as an off-white solid.
MS (DCI) m/e 336 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 4.18 (s, 3H), 6.92 (m, 2H), 7.31 (m, 2H), 7.54 (s,
1H),
7.69 (s, 1H).
Example 322B
1S 4-(4-Chlorophenoxy)-7-methoxythienof2, 3-clpyridine-2-carboxamide
Example 322A (40 mg, 0.12mmol) was treated according to the procedure of
Example 92 to provide the title compound (23 mg, O.S8 mmol) as a white solid.
mp >2S0 °C;
MS (DCI/NH3) m/e: 33S (M+H)+;
IH NMR (300 MHz, DMSO-d6) b 4.08 (s, 3H), 7.01 (m, 2H), 7.38 (m, 2H), 7.82 (br
s,
1H), 7.90 (s, 1H), 8.04 (s, 1H), 8.43 (s, 1H).
Example 323
4-(4-Chlorophenoxy)-7-oxo-6, 7-dihydrothienof2, 3-c]p~rridine-2-carboxamide
2S
Example 323A
Methyl4-(4-Chloronhenoxy)-7-oxo-6 7-dihydrothienof2 3-clpyridine-2-carboxylate
A solution of Example 311A (200 mg, O.S97 mmol) in acetic anhydride (20 mL)
was heated at reflux for 18 hours. The reaction was cooled and poured over
ice. The
mixture was allowed to stir 1 hour before CH2CI2 ( 100 mL) was added. The
organic
extracts were washed with 1 N NaOH ( 100 mL), H20 (SO mL) brine (S0 mL), dried
(NazS04), filtered and rotoevaporated to afford a crude brown residue. This
residue was
directly dissolved in DMF (20 mL,) and H20 (3 mL), treated with K2C03 and
warmed to
60 °C for 2 hours. The reaction was allowed to cool to room temperature
and then was
3S rotoevaporated. The crude residue was purified by column chromatography on
silica gel
234
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
eluting with 10% ethyl acetate/hexane with a gradient to 50% ethyl
acetate/hexane to
provide the title compound.
MS (DCI/NH3) m/e: 336 {M+H)+;
'H NMR {300 MHz, DMSO-db) S 3.82 (s, 3H), 7.09 (m, 2H), 7.38 (m, 2H), 7.42 (s,
1H),
7.52 (s, 1H), 11.84 (br s, 1H).
Example 323B
4-(4-Chlorophenoxy)-7-oxo-6, 7-dihydrothienof2 3-clpyridine-2-carboxamide
Example 323A was prepared according to Example 44 to provide the title
compound.
HPLC: Supelco C-18 column, eluent gradient of water:acetonitrile 0:90- 90:0
over 30
minutes, detection at 254 nm, flow rate of 0.8 mL/min, RT 18.61 minutes;
mp >250 °C;
MS (APCI) m/e: 321 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 7.04 (m, 2H), 7.38 (m, 2H), 7.40 (s, 1H), 7.75 (s,
1H),
8.32 (br s, 1H).
Example 324
4-(4-Chlorophenoxy)-N-methyl-7-(methylamino)thienof2 3-clpyridine-2-
carboxamide
Example 311A (27 mg, 76 mmol) was treated according to the procedure of
Barraclough, et. al (J. Med. Chem. 1990, 33, 2231 ) to afford the title
compound ( 12 mg,
45% yield).
MS (DC1/NH3) m/e: 348 (35C1)/ 350 (3~C1);
'H NMR (CDC13, 300 MHz) 8 2.98 (d, 3H), 3.16 (d, 3H), 4.65 (d,lH), 6.56 (d,
1H), 6.83
(d, 2H), 7.27 (d, 2H), 7.47 (s, 1H), 7.86 (s, 1H).
Example 325
N-Methyl-7-(4-methylphenoxy)f 1 3lthiazolof5 4-clpyridine-2-carboxamide
Example 142D ( 10 mg, 33 mmol) was treated according to the procedure of
Example 96 to give the title compound (1.5 mg, 75%) as a white solid.
MS (DCI/NH~) m/e: 300 (M+H)+, 317 (M+NH3)+;
IH NMR (CDCI3, 300 MHz) b 2.39 (s, 3H), 3.07 (d, J=5.1 Hz, 3H), 7.06 (d, J=8.8
Hz,
2H), 7.23 (d, J=8.5 Hz, 2H), 7.52 (d, J=5.5 Hz, 1H), 8.18 (s, IH), 9.02 (s,
1H).
Example 327
4-(4-Chloronhenoxy)furof2 3-clpvridine-2-carboxamide
235
CA 02333770 2000-11-30
WO 99162908 PCT/US99/12419
Example 327A
Ethyl4-(4-Chlorophenoxy)furof2,3-clpyridine-2-carbox late
To a solution of 4-chlorophenol ( 1.08 g, 8.7 mmol) in anhydrous
tetrahydrofuran
(25 mL) under nitrogen atmosphere at 0 °C was added dropwise a solution
of potassium
tent-butoxide (1.0 M solution in THF, 8.7 mL, 8.7 mmol). The reaction mixture
was then
stirred and heated at 65 °C for 2 hours, cooled to 0 °C, then
treated with Example 17A ( 1.0
g, 5.7 mmol) in anhydrous tetrahydrofuran ( 10 mL) and warmed at 65 °C
for 2 hours. The
reaction was cooled to 0 °C, ethyl glycolate ( 1.07 mL, 11.4 mmol) and
cesium carbonate
(3.Og, 9.2 mmol) were added, and the mixture was heated at 65 °C for 3
hours. The
reaction was cooled and concentrated, and the residue was then diluted with
ethyl acetate
(50 mL) and washed with brine (3x50 mL), then dried (MgSO4). The ethyl acetate
was
then evaporated to give an oil. Purification by flash chromatography on silica
gel eluting
with 10% ethyl acetate-hexane yielded 0.1 lOg (6.1 %) of the title compound as
a glassy
residue.
MS (DCI/NH3) mle: 318 (M+H)+;
'H NMR (300 MHz, DMSO-d6) S 1.35 (t, 2H, CH2), 4.4 (q, 3H, CH3), 7.2 (d, J=9
Hz, 2H),
7.5 (d, J=9 Hz, 2H), 8.09 (s, 1H), 8.23 (s, 1H), 9.25 (s, 1H).
Example 327B
4-(4-Chlorophenoxy)furo f 2,3-clpyridine-2-carboxylic acid
To a solution of lithium hydroxide monohydrate (0.0113 g, 0.5 mmol ), in
tetrahydrofuran (5 mL) and water ( 1 mL) was added Example 327A (0.1 g, 0.3
mmol), and
the mixture was heated at 50 °C for 2 hours. The mixture was cooled,
and then formic
acid was added until it was acidic (pH?). The mixture was then extracted with
ethyl
acetate (50 mL), and the extract was washed with brine (2x20 mL), dried
(MgS04), and
evaporated. Purification by flask chromatography on silica gel eluting with
20% acetone-
hexane yielded 0.710 g (81.6%) of the title compound as a glassy residue.
MS (DCI/NH3) m/e: 290 (M+H)+;
'H NMR (300 MHz, DMSO-db) 8 3.4 (br s, 1H), 7.2 (d, J=9 Hz, 2H), 7.5 (d, J=9
Hz, 2H),
8.09 (s, 1H), 8.23 (s, 1H), 9.25 (s, 1H).
Example 327C
4-(4-Chlorophenoxy)furof2 3-clpyridine-2-carboxamide
A solution of Example 327B (0.15 g, 0.5 mmol) in DMF ( 10 mL) was treated with
1-hydroxybenzotriazole hydrate ( 0.104g, 0.66 mmol), NH4C1 (0.0948 g, 0.017
mmol), and
236
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-methylmorpholine (0.141 g, 0. l4mmol). The solution was cooled to 0
°C and treated
with 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (0.115 g,
0.6 mmol),
warmed to room temperature, stirred overnight, poured into saturated NaHC03,
filtered,
washed with brine (3x20 mL}, dried (MgS04), and evaporated. Purification by
flask
chromatography on silica gel eluting with 20% acetone-hexane yielded 0.030g
(21 %) of
the title compound as a glassy residue.
MS (DCI/NH3) m/e: 289 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 7.18 (d, 2H), 7.29 (s, 1H), 7.5 (d, 2H), 7.82 (br
s, 1H),
8.25 (s, 1H), 8.35 (br s, 1H), 8.95 (s, 1H);
Example 328
4-(4-Chlorophenoxy)furo(2,3-clpYridine-2-carbothioamide
To a solution of Example 327 (0.06 g, 0.2 mmol) in toluene (5 mL) was added
Lawesson's reagent (0.1 g, 0.2 mmol}. The reaction was then refluxed for 1
hour, then
cooled, evaporated and dissolved in ethyl acetate. The ethyl acetate solution
was washed
with brine (3x15 mL), dried (MgS04), and evaporated. Purification by flask
chromatography on silica gel eluting with 20% acetone-hexane yielded 0.022 g
(37%) of
the title compound as a light yellow solid.
MS (DCI/NH3) m/e: 305 (M+H)+;
'H NMR (300 MHz, DMSO-d6) b 7.20 (d, 2H), 7.29 (s, 1H), 7.5 (d, 2H), 8.25 (s,
1H),
8.82 (s, 1H), 9.95 (b, 2H).
Example 329
4-(2-Phenylethenyl)thieno f 2,3-clpyridine-2-carboxamide
Example 329A
tert-Butyl E- and Z-4-f 2-phenylethenyl)thieno(2,3-clpYridine-2-carboxylate
To a stirred solution of diethyl benzylphosphonate (0.08 mL, 0.38 mmol) in
dichloromethane (2 mL) at -78 °C, a 0.5 M solution of potassium
bis(trimethyisilyl)amide
in toluene (0.84 mL, 0.42 mmol) was added dropwise. After 45 min, a solution
of
Example 237E (0.10 g, 0.38 mmol) in dichloromethane (3 mL} was slowly added
and
reaction stirred 1 hour. Bath was removed and reaction stirred 20 minutes.
Reaction was
quenched into a dilute aqueous solution of NaHC03. Aqueous was extracted with
dichloromethane (2 x 25 mL), ethyl acetate (2 x 25 mL). All organic phases
were
combined, dried (Na2S04), and concentrated to yield a colored oil. The residue
was
purified by flash chromatography on silica gel using EtOAc/hexane as eluent.
The mixture
237
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
of stereoisomers was dried in a desiccator to yield a solid (0.07 g, 55%): MS
(APCI) m/e:
338 (M+H)+.
Example 3298
E-4-(2-Phenylethenyl)thienof 2,3-clpyridine-2-carboxamide
Example 329A (0.07 g, 0.21 mmol) was dissolved in a solution of 10%
HZSOa/MeOH (10 mL). Solution was heated to reflux for 6 hours then stirred at
room
temperature for 16 hours. The reaction was concentrated under reduced
pressure, then
basified with sat. NaHC03 (50 mL). The aqueous phase was extracted with
dichloromethane (2x50 mL) and the organic extracts were combined. The organic
layer
was washed with a dilute brine solution ( 100 mL), dried (NaZS04), filtered,
and
concentrated under reduced pressure to a colored residue. The residue was
dissolved in
methanol (8 mL) and chloroform ( 1 mL). A balloon of ammonia gas was applied
and
reaction heated to 35 °C for 24 hours. The reaction was concentrated
and the residue was
purified by HPLC using a gradient of 25%-65% acetonitrilelwater + 0.1 % TFA
over 40
minutes. Products were neutralized with Sat. NaHC03 to yield the title
compound (27 mg,
46%), and additionally the corresponding Z-isomer ( 14 mg, 24%).
mp 257-258 °C'
MS (DCI/NH3) m/e: 281 (M+H)+;
~ H NMR (300 MHz, DMSO-d6) 8 7.34 (dd, J=7.6, 7.2 Hz, 1 H), 7.46 (dd, J=7.6,
7.2 Hz,
2H), 7.55 (d, J=16.5 Hz, 1H), 7.64 (d, J=16.5 Hz, 1H), 7.73 (d, J=7.2 Hz, 2H),
7.88 (br s,
1H), 8.37 (br s, 1H), 8.62 (s, 1H), 8.85 (br s, 1H), 9.18 (s, 1H);
Anal calcd for (C16H~2N20S 0.2 H20): C, 67.68; H, 4.40; N, 9.87. Found: C,
67.47; H,
4.18; N, 9.84.
Example 330
4-(4-Chlorophenyl)thienof 2,3-clpyridine-2-carboxamide
Example 330A
4-(4-Chlorophenyl)thienof2,3-clpyridine-2-carboxalate
The title compound (160 mg, 53%) was prepared as in Example 95A but
substituting 4-cholorophenyl boronic acid for 4-(trifluorome.thyl)phenyl
boronic acid.
MS (APCI) m/e: 304 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 3.92 (s, 3H), 7.69 (m, 4H), 8.30 (s, 1H), 8.60 (s,
IH),
9.42 (s, 1H).
238
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
Example 330B
4-(4-Chlorophenyl)thieno(2,3-clpyridine-2-carboxamide
Example 330A was prepared as in Example 44 to provide the title compound (60
mg, 60%).
MS (APCI) m/e: 289 (M+H)+;
'H NMR (400 MHz, DMSO-d6) 8 7.63 (d, J=8Hz, 2H), 7.69 (d, J=8Hz, 2H), 7.77 (s,
1H),
8.19 (s, 1H), 8.41 (s, 1H), 8.51 (s, 1H), 9.30 (s, 1H).
Example 331
4-[3-(Trifluoromethyl)nhenyllthienof2 3-clpyridine-2-carboxalamide
Example 331 A
4-f3-(trifluoromethyl)phenyllthienol2 3-clpyridine-2-carboxalate
The title compound (100 mg, 30%) was prepared as in Example 95A but
substituting 4-(trifluoromethy)lphenyl boronic acid for 4-
(trifluoromethyl)phenyl boronic
acid.
MS (APCI) m/e: 338 (M+H}+;
'H NMR (300 MHz, DMSO-d6) 8 3.92 (s, 3H), 7.81-7.93 (m, 4H), 8.01 (s, 1H),
8.67 (s,
1H), 9.46 (s, 1H).
Example331B
4-f3-(trifluoromethyl)phenyllthienof2 3-clpyridine-2-carboxalamide
Example 331A was prepared as in Example 44 to provide the title compound
(90mg, 94%}.
MS (APCI) m/e: 323(M+H)+;
'H NMR (400 MHz, DMSO-db) 8 7.85 (s, 1H), 7.90-7.97 (m, 4H), 8.25 (s, 1H),
8.46 (s,
1H), 8.69 (s, 1H), 9.38 (s, 1H).
Example332
4-(3-Chlorophenyl)thienof2 3-clpyridine-2-carboxamide
Example 332A
4-(3-Chlorophenyl)thienof2 3-clpyridine-2-carboxalate
The title compound (130 mg, 43%) was prepared as in Example 95A but
substituting 3-chlorophenyl boronic acid for 4-chlorophenyl boronic acid.
239
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
MS (APCI) m/e: 304 (M+H)+;
jH NMR (300 MHz, DMSO-d6) S 3.92 (s, 3H), 7.59-7.68 (m, 3H), 7.75 (s, 1H),
8.02 (s,
1H), 8.62 (s, 1H), 9.43 (s, 1H).
Example 332B
4-(3-Chloroghenyl)thienof2,3-clpyridine-2-carboxamide
Example 332A was prepared as in Example 44 to provide the title compound
(82mg, 86%).
MS (APCI) m/e: 288 (M+H)+;
IH NMR (400 MHz, DMSO-d6) b 7.58-7.62 (m, 3H), 7.62 (s, 1H), 7.69 (s, 1H),
8.19 (s,
1H), 8.49 (s, 1H), 8.51 (s, 1H), 9.31 (s, 1H).
Example 333
4-(4-Bromo~henyl)thienof2,3-clpyridine-2-carboxamide
Example 333A
4-(4-Bromophenyl)thienof 2,3-clpyridine-2-carboxalate
The title compound (148mg, 42%) was prepared as in Example 95A but
substituting 4-bromophenyl boronic acid for 4-(trifluoromethyl)phenyl boronic
acid.
MS (APCI) m/e 305 (M+H)+;
IH NMR (300 MHz, DMSO-d6) 8 3.91 (s, 3H), 7.61 (d, J=7.SHz, 2H), 7.77 (d,
J=7.SHz,
2H), 8.02 (s, 1H), 8.57 (s, 1H), 9.40 (s, 1H).
Example 333B
4-(4-bromophenyl)thienof 2,3-clpyridine-2-carboxamide
Example 333A was prepared as in Example 44 to provide the title compound
( 1 l8mg, 88%).
MS (APCI) m/e: 333, 335 ( 1:1 ) (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.63 (d, J=7.5 Hz, 2H), 7.79 (d, J=7.SHz, 2H),
7.84 (s,
1H), 8.22 (s, 1H), 8.46 (s, 1H), 9.33 (s, 1H).
Example 334
4-(3-Aminophenyl)thienof 2,3-clpyridine-2-carboxamide
Example 334A
4-(3-Aminoo_phenyl)thienof 2,3-c]pyridine-2-carboxalate
240
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
The title compound (90 mg, 32%) was prepared as in Example 95A but
substituting 3-aminophenyl boronic acid for 4-(trifluoromethyl)phenyl boronic
acid.
MS (APCI) m/e: 285 (M+H)+;
~H NMR (300 MHz, DMSO-d6) b 3.92 (s, 3H), 5.34 (s, 2H), 6.67-6.76 (m, 2H),
6.81 (m,
1H), 7.22 (t, J=7.SHz, 1H), 8.07 (s, 1H), 8.53 (s, 1H), 9.36 (s, 1H).
Example 334B
4-(3-Aminophenyl)thienof2.3-clpyridine-2-carboxamide
Example 334A was prepared as in Example 44 to provide the title compound
(83mg, 98%).
MS (APCI} m/e 270 (M+H)+;
~H NMR (300 MHz, DMSO-d6) b 5.30 (s, 2H), 6.67-6.82 (m, 3H), 7.22 (t, J=7.SHz,
IH),
7.79 (s, 1H), 8.23 (s, 1H), 8.43 (s, 1H), 8.51 (s, 1H), 9.25 (s, 1H).
Example 335
4-(3,5-Dichlorohenyl)thienof2,3-clpyridine-2-carboxamide
Example 335A
4-(3,5-Dichlorophenyl)thienof2.3-clpyridine-2-carboxalate
The title compound (90 mg, 27%) was prepared as in Example 95A but
substituting 3,5-dichlorophenyl boronic acid for 4-(trifluoromethyl)phenyl
boronic acid.
MS (APCI) m/e: 338 (M+H)+.
Example 335B
4-(3,5-Dichlorohenyl)thienof2 3-clpyridine-2-carboxamide
Example 335A was prepared as in Example 44 to provide the title compound (21
mg, 24%).
MS (APCI) m/e: 323 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.73 (d, J=2.25Hz, 2H), 7.80 (m, IH), 7.88 (s,
1H), 8.20
(s, 1H), 8.53 (s, 1H), 8.56 (s, 1H), 9.36 (s, IH).
Example 336
4-(2,4-Dichlorohenyl)thienof2 3-clpyridine-2-carboxamide
Example 336A
4-(2,4-Dichlorophenyl)thieno_ f 2 3-clpyridine-2-carboxalate
241
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
The title compound (100 mg, 30%) was prepared as in Example 95A but
substituting 2,4-dichlorophenyl boronic acid far 4-(trifluoromethyl)phenyl
boronic acid.
MS (APCI) m/e: 338 (M+H)+;
~H NMR (300 MHz, DMSO-d6) 8 3.38 (s, 3H), 7.59 (s, 1H), 7.61 (d, J=2.25Hz,
1H), 7.70
(s, 1H), 7.86 (d, J=2.25Hz, 1H), 8.49 (s, 1H), 9.45 (s, 1H).
Example336B
4-(2,4-Dichlorohenyl)thienof2,3-clpyridine-2-carboxamide
Example 336A was prepared as in Example 44 to provide the title compound.
'H NMR (300 MHz, DMSO-d6) b 7.60 (s, 1H), 7.64 (m, 1H), 7.81 (br.s, 1H), 7.87
(s, 1H),
7.91 (m, 1H), 8.37 (br.s, 1H), 8.45 (s, 1H), 9.37(s, 1H).
Example 337
4-l3,4-Dichlorohenyl)thienof 2,3-clpyridine-2-carboxamide
Example 337A
4-(3,4-Dichlorophenyl)thienof2,3-clpyridine-2-carboxalate
The title compound (130 mg, 39%) was prepared as in Example 95A but
substituting 3,4-dichlorophenyl boronic acid for 4-(trifluoromethyl)phenyl
boronic acid.
MS (APCI) m/e: 338 (M+H)+;
'H NMR (300 MHz, DMSO-db) b 3.94 (s, 3H), 7.67-7.76 (m, 1H), 7.85 (m, 1H),
7.79 (d,
J=2.25Hz, 1 H), 8.06 (s, 1 H), 8.63 (s, 1 H), 9.44 (s, 1 H).
Example 337B
4-(3,4-Dichlorohenyl)thienof2 3-clpyridine-2-carboxamide
Example 337A was prepared as in Example 44 to provide the title compound (44
mg, 46%).
MS (APCI) m/e: 323(M+H)+;
~H NMR (300 MHz, DMSO-d6) 8 7.65-7.68 (m, 1H), 7.84-7.87 (m, 2H), 8.96 (d,
J=2.25Hz, 1 H), 8.21 (s, l H), 8.47 (s, 1 H), 8.56 {s, 1 H), 9.35 (s, 1 H).
Example 338
4-X2,4-Difluorophenyllthienof2 3-c]pyridine-2-carboxamide
Example 338A
242
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
4-f2,4-Difluorophenyllthienof2 3-clpyridine-2-carboxalate
The title compound (130 mg, 42%) was prepared as in Example 95A but
substituting 2,4-difluorophenyl boronic acid for 4-(trifluoromethyl)phenyl
boronic acid.
MS (APCI) m/e: 306 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 3.90 (s, 3H), 7.26 (m, 1H), 7.45 (m, 1H), 7.63 (m,
1H),
7.81 (d, J=3Hz, 1H), 8.55 (s, 1H), 9.44(s, 1H).
Example 338B
4- f 2,4-Difluorophenyllthieno f 2,3-clpyridine-2-carboxamide
Example 338A was processeded as in Example 44 to provide the title compound.
MS (APCI) m/e: 291 (M+H)+;
'H NMR (300 MHz, DMSO-db) 8 7.30 (m, 1H), 7.49 (m,lH), 7.66 (m, 1H), 7.77 (s,
1H),
7.99 (s, 1H), 8.39 (s, 1H), 8.47 (s, 1H), 9.34 (s, 1H).
Example 339
4- f 4-Fluorphenyllthienof 2,3-clpyridine-2-carboxamide
Example 339A
4-(4-Fluoronhenyl)thienof2,3-clpyridine-2-carboxalate
The title compound (100 mg, 35%) was prepared as in Example 95A but
substituting 4-fluorophenyl boronic acid for 4-(trifluoromethyl)phenyl boronic
acid.
MS (APCI) m/e: 288 (M+H)+;
'H NMR (300 MHz, DMSO-db) 8 3.89 (s, 3H), 7.38-7.48 (m, 2H), 7.55-7.64 (m,
1H), 7.78
(d, J=3Hz, 1H), 8.57 (s, 1H), 9.44 (s, 1H).
Exam~e 339B
4- f 4-Fluorophenyllthienof 2,3-clpyridine-2-carboxamide
Example 339A was processed as in Example 44 to provide the title compound.
MS (APCI) m/e: 273 (M+H)+;
'H NMR (300 MHz, DMSO-d6) 8 7.40-7.50 (m, 2H), 7.57-7.65 (m, 2H), 7.81 (s,
1H), 8.01
(s, 1H), 8.47 (s, 1H), 8.51 (s, 1H), 9.36 (s, 1H).
Example 340 5-Chloro-4-(4-chlorophenoxy)thienof2 3-clpyridine-2-carboxamide
Example 340A
2,3,5-Trichloro-4-formylpyridine
243
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
A solution of lithium diisopropylamide (7.3 mL, 1.5 M in cyclohexane, 11 mmol)
in 10 mL of dry THF under nitrogen at -78 °C was treated with 2,3,5-
trichloropyridine (2
g, 11 mmol) in 20 mL THF over a 30 minute period, stirred an additional 30
minutes, then
methyl formate ( 1.4 mL, 1.3 g, 22 mmol) in 14 mL THF was added slowly to the
brown
solution over 1 S minutes, allowed to slowly warm to room temperature and
stirred
overnight. The resultant dark brown solution was poured onto ice and saturated
NaHC03,
extracted with ethyl acetate, washed with brine, dried (NazS04) and
concentrated. The
brown oil was flash chromatographed on silica gel with 20-33% ethyl
acetate/hexane to
provide the title compound ( 1.7g, 74%).
MS (APCI-NH3) m/e: 211 (M+H)+, 229 (M+NH4)+;
'H NMR (300 MHz, DMSO-db) 8 10.26 (s, 1H), 8.70 (s, IH).
Example 340B
2-Chloro-3,5-bis(4-bromophenoxy)-4-p~rridinecarboxaldeh,~e
A solution of 4-bromophenol (1.04 g, 6 mmol) in 4 rr>L. THF at 0 °C was
treated
with potassium t-butoxide (4 mL, 1 M in THF, 4 mmol) via syringe, allowed to
warm to
room temperature and stirred one hour, cooled to 0 °C, Example 340A
(390 mg, 2 mmol)
in 2 mL THF was added, the reaction heated to 60 °C for two hours, and
then allowed to
cool to room temperature. The reaction mixture was diluted with ethyl acetate,
washed
with 1 N NaOH, brine, dried (Na2S04) and concentrated. The brown residue was
flash
chromatographed on silica gel twice with 1-2% methanoUdichloromethane and then
5-
20% ethyl acetate/hexane to provide the title compound (235 mg, 24%).
MS (APCI-NH3) m/e: 483 (M-H)-, 517 (M+Cl)-;
1H NMR (300 MHz, DMSO-d6) 8 10.20 (s, 1H), 8.24 (s, 1H), 7.63 (m, 2H), 7.53
(m, 2H),
7.24 (m, 2H), 6.99 (m, 2H).
Example 340C
Methyl 5-Chloro-4-(4-chlorophenoxy)thienof2 3-clpyridine-2-carboxylate
A solution of 340B (227 mg, 0.47 mmol} in 2 mL THF was treated with methyl
thioglycolate (50 ltL,, 0.52 mmol) followed by powdered CsZC03 ( 179 mg, 0.55
mmol),
stirred at room temperature for 21 hours, heated to 60 °C for 15
minutes, then allowed to
cool to room temperature. The reaction was diluted with ethyl acetate and
distilled water,
washed with 1 M KZC03, brine, dried (MgS04) and concentrated. The residue was
flash
chromatographed on silica gel with 5-20% ethyl acetate/hexane followed by HPLC
purification (C-18), gradient eluent of 30-90% CH3CN/H~O with 0.1% TFA to
provide the
title compound (6 mg, 3%).
244
CA 02333770 2000-11-30
WO 99/62908 PCT/US99/12419
MS (APCI-NH3) m/e: 400 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.20 (s, 1H), 7.78 (s, 1H), 7.51 (d, 2H), 6.93 (d,
2H),
3.90 (s, 3H).
Example 340D
5-Chloro-4-(4-chloronhenoxy)thienof2 3-clpyridine-2-carboxamide
A solution of 340C (S mg, 0.013 mmol) in 1 mL methanol and 1 mL
dichloromethane was treated with 2M ammonia in methanol (3 mL, 6 mmol) in a
pressure
tube and heated to 60 °C for 4 hours, allowed to cool to room
temperature, and
concentrated. The residue was filtered through a plug of silica with 95/5
dichloromethane/methanol, concentrated, then purified by reverse phase HPLC (C-
18) 20-
75% CH3CN/H20 with 0.1 % TFA to provide the title compound (4.2 mg, 84%).
HPLC (C-18, 4.6 x 250 mm), 0.8 mL/min, ~, = 254 nm, CH3CN:H20 with 0.1 % TFA 0-
90%, RT 23.3 min (98.52% area);
MS (APCI-NH3) m/e: 385 (M+H)+;
1H NMR (300 MHz, MeOH-d4) b 8.98 (s, 1H), 7.9 (s, 1H}, 7.49 (d, 2H), 6.83 (d,
2H).
The foregoing is merely illustrative and is not intended to limit the
invention to the
disclosed compounds. Variations and changes which are obvious to one skilled
in the art
are to be within the scope and nature of the invention which are defined in
the appended
claims.
245