Note: Descriptions are shown in the official language in which they were submitted.
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
PROTEASE INHIBITORS
FIELD OF THE INVENTION
This invention relates to novel protease inhibitors, particularly inhibitors
of
cysteine and serine proteases, more particularly compounds which inhibit
cysteine
proteases. The compounds of this invention even more particularly relate to
those
compounds which inhibit cysteine proteases of the papain superfamily, and
particularly
cysteine proteases of the cathepsin family. In the most preferred embodiment,
this
invention relates to compounds which inhibit cathepsin K. Such compounds are
particularly useful for treating diseases in which cysteine proteases are
implicated,
especially diseases of excessive bone or cartilage loss, e.g., osteoporosis,
periodontitis, and
arthritis.
BACKGROUND OF THE INVENTION
Cathepsin K is a member of the family of enzymes which are part of the papain
superfamily of cysteine proteases. Cathepsins B, H, L, N and S have been
described in the
literature. Recently, cathepsin K polypeptide and the cDNA encoding such
polypeptide
were disclosed in U.S. Patent No. 5,501,969 (called cathepsin O therein).
Cathepsin K has
been recently expressed, purified, and characterized. Bossard, M. J., et al.,
(1996) J. Biol.
Chem: 271, 12517-12524; Drake, F.H., et al., (1996) J. Biol. Chem. 271, 12511-
12516;
Bromme, D., et al., (1996) J. Biol. Chem. 271, 2126-2132.
Cathepsin K has been variously denoted as cathepsin O, cathepsin X or
cathepsin
02 in the literature. The designation cathepsin K is considered to be the more
appropriate
one (name assigned by Nomenclature Committee of the International Union of
Biochemistry and Molecular Biology).
Cathepsins of the papain superfamily of cysteine proteases function in the
normal
physiological process of protein degradation in animals, including humans,
e.g., in the
degradation of connective tissue. However, elevated levels of these enzymes in
the body
can result in pathological conditions leading to disease. Thus, cathepsins
have been
implicated in various disease states, including but not limited to, infections
by
pneumocystis carinii, trypsanoma cruzi, trypsanoma brucei brucei, and
Crithidia fusiculata;
as well as in schistosomiasis malaria, tumor metastasis, metachromatic
leukodystrophy,
muscular dystrophy, amytrophy, and the like. See International Publication
Number WO
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
94/04172, published on March 3, 1994, and references cited therein. See also
European
Patent Application EP 0 603 873 A1, and references cited therein. Two
bacterial cysteine
proteases from P. gingivallis, called gingipains, have been implicated in the
pathogenesis of
gingivitis. Potempa, J., et al. ( 1994) Perspectives in Drug Discovery and
Design, 2, 445-
458.
Cathepsin K is believed to play a causative role in diseases of excessive bone
or
cartilage loss. Bone is composed of a protein matrix in which spindle- or
plate-shaped
crystals of hydroxyapatite are incorporated. Type I Collagen represents the
major structural
protein of bone comprising approximately 90% of the structural protein. The
remaining
10% of matrix is composed of a number of non-collagenous proteins, including
osteocalcin,
proteoglycans, osteopontin, osteonectin, thrombospondin, fibronectin, and bone
sialoprotein. Skeletal bone undergoes remodeling at discrete foci throughout
life. These
foci, or remodeling units, undergo a cycle consisting of a bone resorption
phase followed
by a phase of bone replacement.
Bone resorption is carried out by osteoclasts, which are multinuclear cells of
hematopoietic lineage. The osteoclasts adhere to the bone surface and form a
tight sealing
zone, followed by extensive membrane ruffling on their apical (i.e.,
resorbing) surface.
This creates an enclosed extracellular compartment on~the bone surface that is
acidified by
proton pumps in the ruffled membrane, and into which the osteoclast secretes
proteolytic
enzymes. The low pH of the compartment dissolves hydroxyapatite crystals at
the bone
surface, while the proteolytic enzymes digest the protein matrix. In this way,
a resorption
lacuna, or pit, is formed. At the end of this phase of the cycle, osteoblasts
lay down a new
protein matrix that is subsequently mineralized. In several disease states,
such as
osteoporosis and Paget's disease, the normal balance between bone resorption
and
formation is disrupted, and there is a net loss of bone at each cycle.
Ultimately, this leads
to weakening of the bone and may result in increased fracture risk with
minimal trauma.
The abundant selective expression of cathepsin K in osteoclasts strongly
suggests
that this enzyme is essential for bone resorption. Thus, selective inhibition
of cathepsin K
may provide an effective treatment for diseases of excessive bone loss,
including, but not
limited to, osteoporosis, gingival diseases such as gingivitis and
periodontitis, Paget's
disease, hypercalcemia of malignancy, and metabolic bone disease. Cathepsin K
levels
have also been demonstrated to be elevated in chondroclasts of osteoarthritic
synovium.
Thus, selective inhibition of cathepsin K may also be useful for treating
diseases of
excessive cartilage or matrix degradation, including, but not limited to,
osteoarthritis and
2
CA 02334652 2000-12-07
WO 99/64399 PCTIUS99/13334
rheumatoid arthritis. Metastatic neoplastic cells also typically express high
levels of
proteolytic enzymes that degrade the sunrounding matrix. Thus, selective
inhibition of
cathepsin K may also be useful for treating certain neoplastic diseases.
It now has been discovered that a novel class of compounds are protease
inhibitors,
most particularly inhibitors of cathepsin K, and these compounds are useful
for treating
diseases in which inhibition of bone resorption is indicated, such as
osteoporosis and
periodontal disease.
SUMMARY OF THE INVENTION
An object of the present invention is to provide protease inhibitors, such as
inhibitors of cysteine and serine proteases. In particular, the present
invention relates to
compounds which inhibit cysteine proteases, and particularly cysteine
proteases of the
papain superfamily. Preferably, this invention relates to compounds which
inhibit cysteine
proteases of the cathepsin family and particularly, compounds which inhibit
cathepsin K.
The compounds of the present invention are useful for treating diseases, which
may be
therapeutically modified by altering the activity of such proteases.
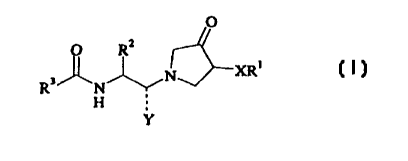
Accordingly, in the first aspect, this invention provides a compound according
to
formula (I).
O R~ O
R' N IV~XR~
H
Y
(I)
In another aspect, this invention provides a pharmaceutical composition
comprising
a compound according to formula (I) and a pharmaceutically acceptable carrier.
In yet another aspect, this invention provides a method of treating diseases
in which
the disease pathology may be therapeutically modified by inhibiting proteases,
such as
cysteine and serine proteases. In particular, the method includes treating
diseases by
inhibiting cysteine proteases, and particularly cysteine proteases of the
papain superfamily.
More particularly, the inhibition of cysteine proteases of the cathepsin
family, such as
cathepsin K is described.
In another aspect, the compounds of this invention are especially useful for
treating
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
diseases characterized by bone loss, such as osteoporosis and gingival
diseases, such as
gingivitis and periodontitis, or by excessive cartilage or matrix degradation,
such as
osteoarthritis and rheumatoid arthritis.
In yet another aspect of this invention, this invention provides a method of
producing the compounds having the formula (I) above.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides alkoxypyrrolidinone compounds of formula (I):
O
O RZ
R3~N~N~y
~H ;
Y
(I)
wherein:
X is selected from the group consisting of oxygen, sulfur, SO, and SOZ;
Y is selected from the group consisting of HZ and oxygen; where if Y is Hz,
then the
----- bond represents two single bonds and where if Y is O, then the -----
bond represents a
double bond;
R' is selected from the group consisting of hydrogen, C1_6 alkyl, CZ_6
alkenyl, C2_
6 alkynyl, C3_6 cycloalkyl-Cp_6 alkyl, Ar-Cp_6 alkyl, Het-Cp_6 alkyl,
(CHZ)~,~COzR", and
(CHz)o-eAr;
Rz is selected from the group consisting of H, C1_6 alkyl, C2_6 alkenyl, C2_6
alkynyl, C3_6
cycloalkyl-Cp_6 alkyl, Ar-Cp_6 alkyl, and Het-Cp_6 alkyl;
R' is selected from the group consisting of H, Cl_6 alkyl, C2_6 alkenyl, C2_6
alkynyl, C3_6 cycloalkyl-Cp_6 alkyl, Ar-Cp_6 alkyl, and Het-Cp_6 alkyl;
or pharmaceutically acceptable salts, hydrates, and isomers thereof.
Preferably, X is O.
Preferably, Y is O.
Preferably, R~ is selected from the group consisting of
4
CA 02334652 2000-12-07
WO 99/64399 PCTNS99/13334
OCH3 COZCH~
H'-C ~ ~ ~ sHZC ~ ~ ,
O O
-CHZCOH , -CHZCO-'-'~ ~ -HZ ~~~ OCH3
and i O
-H2C- v CH3
Suitably, RZ is isobutyl or a substituted isobutyl.
Suitably, R' is selected from the group consisting of
~D/ /
and \ /
Suitably, R" is selected from the group consisting of hydrogen, C1_6 alkyl,
C2_6 alkenyl,
C2_6 alkynyl, C3_6 cycloalkyl-Cp_6 alkyl, Ar-Cp_6 alkyl, and Het-Cp_6 alkyl;
The present invention includes all hydrates, solvates, complexes and prodrugs
of
the compounds of this invention. Prodrugs are any covalently bonded compounds
which
release the active parent drug according to formula (I) in vivo. If a chiral
center or another
form of an isomeric center is present in a compound of the present invention,
all forms of
such isomer or isomers, including enantiomers and diastereomers, are intended
to be
covered herein. Inventive compounds containing a chiral center may be used as
a racemic
mixture, an enantiomerically enriched mixture, or the racemic mixture may be
separated
using well-known techniques and an individual enantiomer may be used alone. In
cases in
which compounds have unsaturated carbon-carbon double bonds, both the cis (Z)
and traps
(E) isomers are within the scope of this invention. In cases wherein compounds
may exist
in tautomeric forms, such as keto-enol tautomers, each tautomeric form is
contemplated as
being included within this invention whether existing in equilibrium or
predominantly in
one form.
The meaning of any substituent at any one occurrence in formula (I) or any
subformula thereof is independent of its meaning, or any other substituent's
meaning, at any
other occurrence, unless specified otherwise.
CA 02334652 2000-12-07
WO 99/64399 PC'f/US99/13334
Specific representative compounds of this invention include:
3-Benzyloxy-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one;
3-Benzylthio-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one;
3-Benzylsulfiny I-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one;
3-Benzylsulfonyl-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one;
1-[2-(Benzyloxycarbonylamino)-4-methylpentyl]-3-benzylthiopyrrolidin-4-one;
3-Benzylthio-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidin-4-one;
3-tert-Butoxycarbonylmethoxy-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one;
3-(3-Methoxybenzyloxy)-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one;
3-(3-Methoxycarbonylbenzyloxy)-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one;
3-tert-Butoxycarbonylmethoxy-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidin-4-
one;
1-[N-(2-Quinolinecarbonyl)-L-leucyl]-3-oxo-4-pyrrolidineooxyacetic acid;
3-(3-Methoxybenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidin-4-one;
3-(4-Methoxybenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidin-4-one;
3-(3-Methoxycarbonylbenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidin-
4-one;
3-(4-Nitrobenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidin-4-one;
3-(5-Methyl-3-isoxazolylmethoxy)-1-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrolidin-4-one;
3-(3-Methoxybenzyloxy)-1-[N-(2-naphthalenecarbonyl)-L-leucyl]pyrrolidin-4-one;
and
3-(4-Methoxyphenoxy)-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one
and pharmaceutically acceptable salts thereof.
~ In yet another aspect, this invention provides novel intermediates useful in
the
preparation of formula (I) compounds represented by:
3-Benzyloxy-4-hydroxy-1-(N-carbobenzyloxy-L-leucyl)pyrrolidine;
3-Benzylthio-4-hydroxy-1-(N-carbobenzyloxy-L-leucyl)pyrrolidine;
1-[2-(Benzyloxycarbonylamino)-4-methyl-pentyl]-3-benzylthio-4-
hydroxypyrrolidine;
3-Benzylthio-4-hydroxy-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidine;
3-tert-Butoxycarbonylmethoxy-4-hydroxy-1-(N-carbobenzyloxy-L-
leucyl)pyrrolidine;
6
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
3-Hydroxy-4-(3-methoxybenzyloxy)-I-(N-carbo-benzyloxy-L-leucyl)pyrrolidine;
3-Hydroxy-4-(3-methoxy-carbonylbenzyloxy)-I-(N-carbobenzyloxy-L-
leucyl)pyrrolidine;
3-tert-Butoxycarbonylmethoxy-I-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidine;
3-Hydroxy-4-(3-methoxycarbonylbenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrolidine;
3-Hydroxy-4-(4-nitrobenzyloxy)- I -[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrolidine;
3-Hydroxy-4-(5-Methyl-3-isoxazolylmethoxy)-1-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrolidine;
3-Hydroxy-4-(3-methoxybenzyloxy)-1-[N-(2-naphthalenecarbonyl)-L-
leucyl]pyrrolidine;
3-Hydroxy-4-(4-methoxyphenoxy)-1-(N-carbobenzyloxy-L-leucyl)pyrrolidine;
1-(N-Phthaloyl-L-leucyl}-3-pyrroline; and
3,4-Dihydroxy-1-(N-phthaloyl-L-leucyl)pyrrolidine)
or salts thereof.
These intermediates are prepared using methods analogous to that described in
Scheme 1, Scheme 2, Scheme 3 and the Examples described hereinafter.
Abbreviations and symbols commonly used in the peptide and chemical arts are
used herein to describe the compounds of the present invention. In general,
the amino acid
abbreviations follow the IUPAC-IUB Joint Commission on Biochemical
Nomenclature as
described in Eur. J. Biochem., 158, 9 (1984). The term "amino acid" as used
herein refers
to the D- or L- isomers of alanine, arginine, asparagine, aspartic acid,
cysteine, glutamine,
glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine,
phenylalanine,
proline, serine, threonine, tryptophan, tyrosine and valine.
Certain radical groups are abbreviated herein. t-Bu refers to the tertiary
butyl
radical; Boc or BOC refers to the t-butyloxycarbonyl radical; Fmoc refers to
the
fluorenylmethoxycarbonyl radical; Ph refers to the phenyl radical; and Cbz or
CBZ or Z
refers to the benzyloxycarbonyl radical.
Certain reagents are abbreviated herein. DCC refers to
dicyclohexylcarbodiimide;
EDC or EDCI refers to N-ethyl-N'(dimethylaminopropyl)-carbodiimide; HOBT or
HOBt
refers to I-hydroxybenzotriazole; DMF refers to dimethyl formamide; DIEA
refers to di-
isopropylethylamine; HoAt refers to 1-hydroxy-7-aza-benzotriazote; Dess-
Martin's reagent
is I,1,I-triacetoxy-1,1-dihydro-1,2-benziodoxol-3(1H)-one; TFA refers to
trifluoroacetic
acid; and THF refers to tetrahydrofuran.
7
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
"Cl_6 alkyl" as applied herein is meant to include substituted and
unsubstituted
methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl and t-butyl, pentyl, n-
pentyl, isopentyl,
neopentyl and hexyl and the simple aliphatic isomers thereof. Any Cl_6alkyl
group may be
optionally substituted independently by one or two halogens, SR', OR', N(R~2,
C(O)N(R~2,
carbamyl or Cl_4alkyI, where R' is H or CI_6alkyl. Cpalkyl means that no alkyl
group is
present in the moiety. Thus, Ar-COalkyl is equivalent to Ar.
"C3_6 cycloalkyl" as applied herein is meant to include substituted [i.e.,
alkyl, OR,
SR or halogen) and unsubstituted cyclopropane, cyclobutane, cyclopentane, and
cyclohexane.
"C2_6 alkenyl" as applied herein means an alkyl group of 2 to 6 carbons,
wherein a
carbon-carbon single bond is replaced by a carbon-carbon double bond.
C2_6alkenyl
includes ethylene, 1-propene, 2-propene, 1-butene, 2-butene, isobutene and the
several
isomeric pentenes and hexenes. Both cis and trans isomers are included.
"C2_6 alkynyl" means an alkyl group of 2 to 6 carbons, wherein one carbon-
carbon
single bond is replaced by a carbon-carbon triple bond. C2_6 alkynyl includes
acetylene, 1-
propyne, 2-propyne, 1-butyne, 2-butyne, 3-butyne, and the simple isomers of
pentyne and
hexyne.
"Ar" or "aryl" means unsubstituted phenyl or naphthyl; or phenyl or naphthyl
substituted by one or more of Ph-CO_6 alkyl, Het-CO_6 alkyl, CI_6 alkoxy, Ph-
Cp_6 alkoxy,
Het-CO_6 alkoxy, OH, (CH2)o-bC02R", where R" is as defined above, (CH2) 1-6NR
R',
O(CH2)~_6NR R'; wherein each R' independently is H, CI-6 alkyl, Ar-CO_6 alkyl,
or Het-
CO_6 alkyl; or phenyl or naphthyl substituted by one to three moieties
selected from
Cl_4alkyl, OR', N(R')z, SR', CF3, NOZ, CN, COZR', CON(R'), F, Cl, Br and I, or
substituted
by a methylenedioxy group.
As used herein "Het" or "heterocyclic" represents a stable 5- to 7-membered
monocyclic or a stable 7- to 10-membered bicyclic heterocyclic ring, which is
either
saturated or unsaturated, and which consists of carbon atoms and from one to
four
heteroatoms selected from the group consisting of N, O and S, and wherein the
nitrogen and
sulfur heteroatoms may optionally be oxidized, and the nitrogen heteroatom may
optionally
be quaternized, and including any bicyclic group in which any of the above-
defined
heterocyclic rings is fused to a benzene ring. The heterocyclic ring may be
attached at any
heteroatom or carbon atom which results in the creation of a stable structure,
and may
optionally be substituted with one or two moieties selected from C 1 _4alkyl,
OR', N(R')2,
SR', CF3, N02, CN, C02R', CON(R'), F, CI, Br and I, where R' is as defined
hereinbefore.
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
Examples of such heterocycles include piperidinyI, piperazinyl, 2-
oxopiperazinyl, 2-
oxopiperidinyl, 2-oxopyrrolodinyl, 2-oxoazepinyl, azepinyl, thienyl, pyrrolyl,
4-
piperidonyl, pyrrolidinyl, pyrazolyl, pyrazolidinyl, imidazolyl, pyridyl,
pyrazinyl,
oxazolidinyl, oxazolinyl, oxazolyl, isoxazolyl, morpholinyl, thiazolidinyl,
thiazolinyl,
isothiazolyl, thiazolyl, quinuclidinyl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzothienyl, benzopyranyl, benzoxazolyl, benzofuranyl, furyl, pyranyl,
tetrahydrofuryl,
tetrahydropyranyl, thienyl, benzoxazolyl, thiamorpholinyl sulfoxide,
thiamorpholinyl
sulfone, oxadiazolyl, benzothiazolyl, benzoisothiazolyl, benzisoxazolyl,
pyrimidinyl,
cinnolinyl, quinazolinyl, quinoxalinyl, 1,5-napthyridinyl, 1,6-napthyridinyl,
1,7-
napthyridinyl, 1,8-napthyridinyl, tetrazolyl, 1,2,3-triazolyl, and 1,2,4-
triazolyl.
Compounds of the formula (I) are prepared by methods analogous to those
described in the
solution synthesis method of Scheme 1, 2 or 3.
Scheme 1
N~ OH
~ O
HN' > ~ ~-a- N N~OH
O
(1) ~ O
OH
O
N O N~OR~ OH O OH
O ~ ~N N OR RyH O N OR
O
(4) (S) (6)
o
--,. o /
R ~N N~~OR~
H O
~~)
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
a) N-Phthaloyl-IeuOH, N-methylmorpholine, EDC, HOBt, CHZC12; b) NMMO, Os04; c)
NaH, RBr, DMF; d) HZNNH2, EtOH; e) Where R = Z: PhCHzOCOCI, Et3N, CHZC12,
otherwise RCOOH, N-methylmorpholine, EDC, HOBt, CHZCIz; f) Dess-Martin
reagent,
CHZC12
Compounds of the general formula (n, wherein X is O, are prepared by methods
shown in Scheme 1. 3-Pyrroline 1-Scheme-1 is reacted with N-Phthaloyl-
leuOH,.in the
presence of N-methylmorpholine, N-ethyl-N'(dimethylaminopropyl}-carbodiimide,
and 1-
hydroxybenzotriazole in dichloromethane (CHZCIz) to give 2-Scheme-1. The
compound 2
is then reacted with osmium tetroxide and N-methylmorpholine N-oxide to give 3-
Scheme-
1. This is subsequently treated with sodium hydride and R'-bronude in dimethyl
formamide to give 4-Scheme-1. Compound 4 is then reacted with hydrazine
hydrate in
ethanol to afford the amine 5-Scheme-1. Where R3 is Z, compound 5 is reacted
with a
benzyl-chloroformate and triethylamine in dichloromethane. Where R3 is not Z,
the
compound 5 is reacted with an appropriate carboxylic acid in the presence of N-
methyimorpholine, N-ethyl-N'(dimethylaminopropyl)-carbodiimide, and 1-
hydroxybenzo-
triazole in dichloromethane to give compound 6-Scheme-1. Compound 6 is then
treated
with Dess-Martin reagent in dichloromethane to give the final product 7-Scheme-
1.
Scheme 2
O OH OH
b g
N -~ N~ ---~ TFA.HN~ -~. o ot:
Bac~ Boc~ XR~ XR~ y N
R HN XR
(1) (2) (3) O
(6)
c
a f
OH
d OH O
O
N~XR~ ~ N
BocNH N~ ~ 3 i
O (4) TFA.H2N ~ XR R ~ O XR
O (5) (7)
a) R~XH/Na; b) TFA/CHZC12; c) N-Boc-LeuOH, N-methylmorpholine, EDC, HOAt,
CHZC12; d) TFA/CHZC12; e) R3COOH, N-methylmorpholine, EDC, HOAt, CHZCIz; fj
Dess-
Martin reageant, CHzCl2; g) for R3 = Z: Z-leuOH, EDC, HOAt, CHZC12
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
Compounds of the general formula (I), wherein XR' is OCH2Ph, SCHzPh, are
prepared by the method shown in Scheme 2. The epoxide 1-scheme-2 is treated
with the in
situ generated sodium salt of the alcohol or thiol, R'XH, in excess of the
alcohol as solvent,
or in an appropriate solvent such as methanol, for the thiol reagent, to
afford 2-Scheme-2.
This compound is deprotected using trifluoroacetic acid in dichloromethane to
give 3-
Scheme-2. Compound 3 is reacted with N-t-butyloxycarbonyl leucine in the
presence of N-
methylmorpholine, N-ethyl-N'-butyloxycarbonyl leucine in the presence of N-
methylmorpholine, N-ethyl-N'-(dimethylaminopropyl)-carbodiimide and 1-hydroxy-
7-
azabenzotriazole in dichloromethane to give 4-Scheme-2. Compound 4 is then
deprotected
with trifluoroacetic acid in dichloromethane to give 5-Scheme-2. This compound
is then
treated with a carboxylic acid having the formula R'COOH, N-methylmorpholine,
N-ethyl-
N'(dimethylaminopropyl)-carbodiimide, and HOAt in dichloromethane to give 6-
Scheme-2.
Alternatively, 6-Scheme-2 can be produced directly from 2-Scheme-2, where R'
is Z, by
treating 2-Scheme-2 with Z-IeuOH, N-ethyl-N'{dimethylaminopropyl)-
carbodiimide, and
HOAt in dichloromethane. Compound 6 is then treated with Dess-Martin reagent
in
dichloromethane to give the final product 7-Scheme-2.
Scheme 3
0 0
O MCPBA.CHZCI2 O
R3~H N~SR~ R3~H
H V 'S(O)"R
O O
(1) (2)
Compounds of the general formula (I), wherein X is S, SO, or SOz are prepared
by
the method shown in Scheme 3. According to this method, 1-Scheme-3 in
dichloromethane
is treated with m-chloroperoxybenzoic acid to give 2-Scheme-3.
11
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
S Scheme 4
O O
II O
\ a Y R3~N N~- b-~. R~~N N
HN~ H H
(1) (2)O (30
O OH O O
. R3' \N NOR' ~ R3~ N i
H N ~ OR
O H
O
(4) (5)
a) eg for R3 =Z: Z-IeuOH, N-methylmorpholine, EDC, HOBt, CHZC12; b) MCPBA,
CH2C12;
c) R'OH, KO'Bu, THF; d) Dess-Martin reagent, CHZCl2.
Compounds of the general formula (n, wherein R' is a substituted phenyl group
are
prepared by the method shown in Scheme 4. Where R' is Z, 3-pyrroline 1-Scheme-
4 is
treated with Z-leuOH, N-methylmorpholine, N-ethyl-N'(dimethylaminopropyl)-
carbodiimide, and HOBt (1-hydroxy benzotriazole) in dichIoromethane to give 2-
Scheme-
4. This compound in dichloromethane is then treated with m-chloroperoxybenzoic
acid to
give the epoxide 3-Scheme-4.. Compound 3 is then reacted with the alcohol R20H
and
potassium tent-butoxide in tetrahydrofuran to afford 4-Scheme-4, which is
subsequently
treated with Dess-Martin reagent in dichloromethane to provide the final
product
cheme-4
Scheme
H OH
i O O
HN XR --.~ R~~N N~XR~ --~~ O
H Rs~ ~N~'XR~
N
H
(3)
a) eg for R = Z: Z-leu-H, NaH(OAc),,Et3N, CH30H; b) (COCI)z, DMSO, Et3N,
CHZCIz
I2
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
Compounds having the general formula (I), wherein Y is Hz are prepared
according
to the method of Scheme 5. In particular, where R' is Z, 1-Scheme-S is treated
with Z-
leucinal, sodium triacetoxyborohydride, and triethylamine in methanol to
afford 2-Scheme-
5. This compound is then is then oxidized using Swern conditions (oxalyl
chloride, DMSO,
triethyl-amine) to afford 2-Scheme-5.
The starting materials used herein are commercially available or are prepared
by
routine methods well known to those of ordinary skill in the art and can be
found-in
standard reference books, such as the COMPENDIUM OF ORGANIC SYNTHETIC
METHODS, Vol. I-VI (published by Wiley-Interscience).
Coupling methods to form amide bonds herein are generally well-known to the
art.
IS The methods of peptide synthesis generally set forth by Bodansky et al.,
THE PRACTICE
OF PEPTIDE SYNTHESIS, Springer-Verlag, Berlin, 1984; E. Gross and J.
Meienhofer,
THE PEPTIDES, Vol. 1, 1-284 (1979); and J.M. Stewart and J.D. Young, SOLID
PHASE
PEPTIDE SYN'i'I~SIS, 2d Ed., Pierce Chemical Co., Rockford, Ill., 1984, are
generally
illustrative of the technique and are incorporated herein by reference.
Synthetic methods to prepare the compounds of this invention frequently employ
protective groups to mask a reactive functionality or minimize unwanted side
reactions.
Such protective groups are described generally in Green, T.W, PROTECTIVE
GROUPS IN
ORGANIC SYNTHESIS, John Wiley & Sons, New York (1981). The term "amino
protecting groups" generally refers to the Boc, acetyl, benzoyl, Fmoc and Cbz
groups and
derivatives thereof as known to the art. Methods for protection and
deprotection, and
replacement of an amino protecting group with another moiety are well known.
Acid addition salts of the compounds of formula (I) are prepared in a standard
manner in a suitable solvent from the parent compound and an excess of an
acid, such as
hydrochloric, hydrobromic, hydrofluoric, sulfuric, phosphoric, acetic,
trifluoroacetic,
malefic, succinic or methanesulfonic acid. Certain of the compounds foam inner
salts or
zwitterions which may be acceptable. Cationic salts are prepared by treating
the parent
compound with an excess of an alkaline reagent, such as a hydroxide,
carbonate, or
alkoxide, containing the appropriate cation; or with an appropriate organic
amine. Cations
such as Li+, Na+, K+, Ca++, Mg++ and NH4+ are specific examples of cations
present in
pharmaceutically acceptable salts. Halides, sulfate, phosphate, alkanoates
(such as acetate
and trifluoroacetate), benzoates, and sulfonates (such as mesylate) are
examples of anions
present in pharmaceutically acceptable salts.
13
CA 02334652 2000-12-07
WO 99/64399 PGT/US99/13334
This invention also provides a pharmaceutical composition which comprises a
compound according to formula (I) and a pharmaceutically acceptable carrier,
diluent or
excipient. Accordingly, the compounds of formula (I) may be used in the
manufacture of a
medicament. Pharmaceutical compositions of the compounds of formula (I)
prepared as
hereinbefore described may be formulated as solutions or lyophilized powders
for
parenteral administration. Powders may be reconstituted by addition of a
suitable diluent
or other pharmaceutically acceptable carrier prior to use. The liquid
formulation may be a
buffered, isotonic, aqueous solution. Examples of suitable diluents are normal
isotonic
saline solution, standard 5% dextrose in water, or buffered sodium or ammonium
acetate
solution. Such formulation is especially suitable for parenteral
administration, but may also
be used for oral administration or contained in a metered dose inhaler or
nebulizer for
insufflation. It may be desirable to add excipients such as
polyvinylpyrrolidone, gelatin,
hydroxy cellulose, acacia, polyethylene glycol, mannitol, sodium chloride, or
sodium
citrate.
Alternately, these compounds may be encapsulated, tableted, or prepared in an
emulsion or syrup for oral administration. Pharmaceutically acceptable solid
or liquid
carriers may be added to enhance or stabilize the composition, or to
facilitate preparation of
the composition. Solid carriers include starch, lactose, calcium sulfate
dihydrate, terra alba,
magnesium stearate or stearic acid, talc, pectin, acacia, agar or gelatin.
Liquid carriers
include syrup, peanut oil, olive oil, saline and water. The carrier may also
include a
sustained release material such as glyceryl monostearate or glyceryl
distearate, alone or
with a wax. The amount of solid Garner varies hut, preferably, will be between
about 20
mg to about 1 g per dosage unit. The pharmaceutical preparations are made
following the
conventional techniques of pharmacy involving milling, mixing, granulating,
and
compressing, when necessary, for tablet forms; or milling, mixing and filling
for hard
gelatin capsule forms. When a liquid Garner is used, the preparation will be
in the form of
a syrup, elixir, emulsion or an aqueous or non-aqueous suspension. Such a
liquid
formulation may be administered directly or filled into a soft gelatin
capsule.
For rectal administration, the compounds of this invention may also be
combined
with excipients such as cocoa butter, glycerin, gelatin or polyethylene
giycols and molded
into a suppository.
The compounds of formula (I) are useful as protease inhibitors, particularly
as
inhibitors of cysteine and serine proteases, more particularly as inhibitors
of cysteine
proteases, even more particularly as inhibitors of cysteine proteases of the
papain
14
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
superfamily, yet more particularly as inhibitors of cysteine proteases of the
cathepsin
family, most particularly as inhibitors of cathepsin K. The present invention
also provides
useful compositions and formulations of said compounds, including
pharmaceutical
compositions and formulations of said compounds.
The present compounds are useful for treating diseases in which cysteine
proteases
are implicated, including infections by pneumocystis carinii, trypsanoma
cruzi, trypsanoma
brucei, and Crithidia fusiculata; as well as in schistosomiasis, malaria,
tumor metastasis,
metachromatic leukodystrophy, muscular dystrophy, amytrophy; and especially
diseases in
which cathepsin K is implicated, most particularly diseases of excessive bone
or cartilage
loss, including osteoporosis, gingival disease including gingivitis and
periodontitis,
arthritis, more specifically, osteoarthritis and rheumatoid arthritis, Paget's
disease;
hypercalcemia of malignancy, and metabolic bone disease.
Metastatic neoplastic cells also typically express high levels of proteolytic
enzymes
that degrade the surrounding matrix, and certain tumors and metastatic
neoplasias may be
effectively treated with the compounds of this invention.
The present invention also provides methods of treatment of diseases caused by
pathological levels of proteases, particularly cysteine and serine proteases,
more
particularly cysteine proteases, even more particularly as inhibitors of
cysteine proteases of
the papain superfamily, yet more particularly cysteine proteases of the
cathepsin family,
which methods comprise administering to an animal, particularly a mammal, most
particularly a human in need thereof a compound of the present invention. The
present
invention especially provides methods of treatment of diseases caused by
pathological
levels of cathepsin K, which methods comprise administering to an animal,
particularly a
mammal, most particularly a human in need thereof, an inhibitor of cathepsin
K, including
a compound of the present invention. The present invention particularly
provides methods
for treating diseases in which cysteine proteases are implicated, including
infections by
pneumocystis carinii, trypsanoma cruzi, trypsanoma brucei, and Crithidia
fusiculata; as
well as in schistosomiasis, malaria, tumor metastasis, metachromatic
leukodystrophy,
muscular dystrophy, amytrophy, and especially diseases in which cathepsin K is
implicated,
most particularly diseases of excessive bone or cartilage loss, including
osteoporosis,
gingival disease including gingivitis and periodontitis, arthritis, more
specifically,
osteoarthritis and rheumatoid arthritis, Paget's disease, hypercalcemia of
malignancy, and
metabolic bone disease.
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
This invention further provides a method for treating osteoporosis or
inhibiting
bone loss which comprises internal administration to a patient of an effective
amount of a
compound of formula (I), alone or in combination with other inhibitors of bone
resorption,
such as bisphosphonates (i.e., allendronate), hormone replacement therapy,
anti-estrogens,
or calcitonin. In addition, treatment with a compound of this invention and an
anabolic
agent, such as bone morphogenic protein, iproflavone, may be used to prevent
bone loss or
to increase bone mass.
In accordance with this invention, an effective amount of the compounds of
formula (I) is administered to inhibit the protease implicated with a
particular condition or
disease. Of course, this dosage amount will further be modified according to
the type of
administration of the compound. For example, "effective amount" for acute
therapy,
parenteral administration of a compound of formula (I) is preferred. An
intravenous
infusion of the compound in 5% dextrose in water or normal saline, or a
similar
formulation with suitable excipients, is most effective, although an
intramuscular bolus
injection is also useful. Typically, the parenteral dose will be about 0.01 to
about 100
mg/kg; preferably between O.l and 20 mg/kg, in a manner to maintain the
concentration of
drug in the plasma at a concentration effective to inhibit cathepsin K. The
compounds are
administered one to four times daily at a level to achieve a total daily dose
of about 0.4 to
about 400 mg/kg/day. The precise amount of an inventive compound which is
therapeutically effective, and the route by which such compound is best
administered, is
readily determined by one of ordinary skill in the art by comparing the blood
level of the
agent to the concentration required to have a therapeutic effect.
Prodrugs of compounds of the present invention may be prepared by any suitable
method. For those compounds in which the prodrug moiety is a ketone
functionality,
specifically ketals and/or hemiacetals, the conversion may be effected in
accordance with
conventional methods.
The compounds of this invention may also be administered orally to the
patient, in
a manner such that the concentration of drug is sufficient to inhibit bone
resorption or to
achieve any other therapeutic indication as disclosed herein. Typically, a
pharmaceutical
composition containing the compound is administered at an oral dose of between
about 0.1
to about 50 mg/kg in a manner consistent with the condition of the patient.
Preferably the
oral dose would be about 0.5 to about 20 mg/kg.
16
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
No unacceptable toxicological effects are expected when compounds of the
present
invention are administered in accordance with the present invention.
The compounds of this invention may be tested in one of several biological
assays
to determine the concentration of a compound which is required to have a given
pharmacological effect.
Determination of cathepsin K proteolytic catalytic activity
All assays for cathepsin K were carried out with human recombinant enzyme.
Standard assay conditions for the determination of kinetic constants used a
fluorogenic
peptide substrate, typically Cbz-Phe-Arg-AMC, and were determined in 100 mM Na
acetate at pH 5.5 containing 20 mM cysteine and 5 mM EDTA. Stock substrate
solutions
were prepared at concentrations of 10 or 20 mM in DMSO with 20 p.M final
substrate
concentration in the assays. All assays contained 10% DMSO. Independent
experiments
found that this level of DMSO had no effect on enzyme activity or kinetic
constants. All
assays were conducted at ambient temperature. Product fluorescence (excitation
at 360
nM; emission at 460 nM) was monitored with a Perceptive Biosystems Cytofluor
Ii
fluorescent plate reader. Product progress curves were generated over 20 to 30
minutes
following formation of AMC product.
Inhibition studies
Potential inhibitors were evaluated using the progress curve method. Assays
were
carried out in the presence of variable concentrations of test compound.
Reactions were
initiated by addition of enzyme to buffered solutions of inhibitor and
substrate. Data
analysis was conducted according to one of two procedures depending on the
appearance of
the progress curves in the presence of inhibitors. For those compounds whose
progress
curves were linear, apparent inhibition constants (Ki,aPP) were calculated
according to
equation 1 (Brandt et al., Biochemitsry, 1989, 28, 140):
v - V,nA l (Ka( 1 + IIK~~ aPP~ +A] ( 1 )
where v is the velocity of the reaction with maximal velocity Vm , A is the
concentration of
substrate with Michaelis constant of Ka, and I is the concentration of
inhibitor.
17
CA 02334652 2000-12-07
WO 99!64399 PCT/US99/13334
For those compounds whose progress curves showed downward curvature
characteristic of time-dependent inhibition, the data from individual sets was
analyzed to
give kobs according to equation 2:
[~C] = vss t + (v0 - vss) l1- exP l-kobst)I ~kobs (2)
where [AMC] is the concentration of product formed over time t, vp is the
initial reaction
velocity, and vss is the final steady state rate. Values for kobs were then
analyzed as a
linear function of inhibitor concentration to generate an apparent second
order rate constant
(kobs / inhibitor concentration or kobs / [I]) describing the time-dependent
inhibition. A
complete discussion of this kinetic treatment has been fully described
(Morrison et al., Adv.
Enrymol. Relat. Areas Mol. Biol., 1988, 61, 201 ).
One skilled in the art would consider any compound with a Ki of less than 50
micromolar to be a potential lead compound. Preferably, the compounds used in
the
method of the present invention have a K; value of less than 1 micromolar.
Most
preferably, said compounds have a Ki value of less than 200 nanomolar.
Human Osteoclast Resorption Assay
Aliquots of osteoclastoma-derived cell suspensions were removed from liquid
nitrogen storage, warmed rapidly at 37°C and washed xl in RPMI-1640
medium by
centrifugation ( 1000 rpm, 5 min at 4°C). The medium was aspirated and
replaced with
murine anti-HLA-DR antibody, diluted 1:3 in RPMI-1640 medium, and incubated
for 30
minutes on ice. The cell suspension was mixed frequently.
The cells were washed x2 with cold RPMI-1640 by centrifugation ( 1000 rpm, 5
min at 4°C) and then transferred to a sterile 15 mL centrifuge tube.
The number of
mononuclear cells were enumerated in an improved Neubauer counting chamber.
Sufficient magnetic beads (5 / mononuclear cell), coated with goat anti-mouse
IgG,
were removed from their stock bottle and placed into 5 mL of fresh medium
(this washes
away the toxic azide preservative). The medium was removed by immobilizing the
beads
on a magnet and is replaced with fresh medium.
The beads were mixed with the cells and the suspension was incubated for 30
minutes on ice. The suspension was mixed frequently. The bead-coated cells
were
immobilized on a magnet and the remaining cells (osteoclast-rich fraction)
were decanted
18
CA 02334652 2000-12-07
W O 99/64399 PCT/US99/13334
into a sterile 50 mL centrifuge tube. Fresh medium was added to the bead-
coated cells to
dislodge, any trapped osteoclasts. This wash process was repeated x 10. The
bead-coated
cells were discarded.
The osteoclasts were enumerated in a counting chamber, using a large-bore
disposable plastic pasteur pipette to charge the chamber with the sample. The
cells were
pelleted by centrifugation and the density of osteoclasts adjusted to
l.Sx104/mL in EMEM
medium, supplemented with 10% fetal calf serum and 1.7g/litre of sodium
bicarbonate. 3
mL aliquots of the cell suspension ( per treatment) were decanted into 15 mL
centrifuge
tubes. These cells were pelleted by centrifugation. To each tube 3 mL of the
appropriate
treatment was added (diluted to 50 ~tM in the EMEM medium). Also included were
appropriate vehicle controls, a positive control (87MEM 1 diluted to 100
ug/mL) and an
isotype control (IgG2a diluted to 100 uglmL). The tubes were incubated at
37°C for 30
minutes.
0.5 mL aliquots of the cells were seeded onto sterile dentine slices in a 48-
well
plate and incubated at 37°C for 2 hours. Each treatment was screened in
quadruplicate. The
slices were washed in six changes of warm PBS (10 mL / well in a 6-well plate)
and then
placed into fresh treatment or control and incubated at 37°C for 48
hours. The slices were
then washed in phosphate buffered saline and fixed in 2% glutaraldehyde (in
0.2M sodium
cacodylate) for 5 minutes, following which they were washed in water and
incubated in
buffer for 5 minutes at 37°C. The slices were then washed in cold water
and incubated in
cold acetate buffer / fast red garnet for 5 minutes at 4°C. Excess
buffer was aspirated, and
the slices were air dried following a wash in water.
The TRAP positive osteoclasts were enumerated by bright-field microscopy and
were then removed from the surface of the dentine by sonication. Pit volumes
were
determined using the Nikon/Lasertec ILM21 W confocal microscope.
Examples
In the following synthetic examples, unless otherwise indicated, all of the
starting
materials were obtained from commercial sources. Without further elaboration,
it is
believed that one skilled in the art can, using the preceding description,
utilize the present
invention to its fullest extent. These Examples are given to illustrate the
invention, not to
limit its scope. Reference is made to the claims for what is reserved to the
inventors
hereunder.
19
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
Example 1
Preparation of 3-Benzylox~l-fN-carbobenzyloxY L-leucyl)pyrrolidin-4-one
(a) 3-Benzyloxy-1-tert-butoxycarbonyl-4-hydroxypytrolidine
To a solution of sodium (0.12 g) in benzyl alcohol (3.69 g) was added 1-tert-
butoxy-
carbonyl-3,4-epoxypyrrolidine (1.07 g) and the mixture stirred at 60°
for 15 hours. Water
was added and the mixture extracted with dichloromethane. The organic extract
was dried
(magnesium sulphate) and evaporated down under reduced pressure. The residue
was
washed with cold hexane and dried under vacuum to give the title compound
(0.88 g) as a
white solid.'H NMR (CDCI3) 8: 1.46 (s, 9H), 3.25-3.7 (m, 4H), 3.91 (s, IH),
4.30 (s, 1H),
4.54 (m, 2H), 4.70 (d, 1H), 7.3-7.45 (m, SH).
(b) 3-Benzyloxy-4-hydroxypyrolidine trifluoroacetate
A solution of 3-benzyloxy-1-tert-butoxycarbonyl-4-hydroxypyrrolidine (0.81 g)
and
trifluoroacetic acid (4 ml) in dichloromethane (16 ml) was stirred at room
temperature for
IS hours. The solution was evaporated down under reduced pressure to give the
title
compound (1.31g) as a tan-coloured liquid.'H NMR (D20) S: 3.15-3.5 (m, 4H),
4.09 (d,
1H), 4.41 (d, 1H), 4.50 (s, 2H), 7.28 (s, SH).
(c) 3-Benzyloxy-4-hydroxy-I-(N-carbobenzyloxy-L-leucyl)pyrrolidine
A solution of 3-benzyloxy-4-hydroxypyrrolidine trifluoroacetate (0.29 g), N-
methyl-
morpholine (0.42 g), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (0.16
g), 1-hydroxy-7-azabenzotriazole (0.10 g) and N-carbobenzyloxy-L-leucine (0.18
g) in
dichloromethane (25 ml) was stirred at room temperature for 15 hours. Solvent
was
evaporated off under reduced pressure and the residue dissolved in ethyl
acetate (25 ml).
The solution was washed successively with 1N hydrochloric acid, saturated
potassium
carbonate solution, water and brine, dried (magnesium sulphate) and evaporated
down
under reduced pressure to give the title compound (0.28g) as a pale tan oil.'H
NMR
(CDC13) 8: 0.9-1.05 (m, 6H), 1.4-1.6 (m, 3H), 3.35-4.05 (m, 6H). 4.25-4.7 (m,
4H), 5.08 (m,
2H), 5.51 (m, 1H), 7.35 (m, lOH).
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
(d) 3-Benzyloxy-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one
A mixture of 3-benzyloxy-4-hydroxy-1-(N-carbobenzyloxy-L-leucyl)pyrrolidine
(0.15 g)
and Dess-Martin's reagent (0.31 g) in dichloromethane ( 10 ml) was stirred at
room
temperature for 2 hours. The solution was diluted with ether and treated with
saturated
sodium bicarbonate solution containing an excess of sodium thiosulphate. The
ether layer
was washed successively with saturated sodium bicarbonate solution and water,
dried
(magnesium sulphate) and evaporated down under reduced pressure to give the
itle
compound (85mg) as a colourless oil.'H NMR {CDCl3) 8: 0.85-1.0 (m, 6H), 1.35-
1.7 (m,
3H), 3.45-3.75 (m, 1H), 3.75-4.0 (m, 2H), 4.0-4.35 (m, 2H), 4.35-4.5 (m, 1H),
4.5-4.75 (m,
1H), 4.75-4.95 (m, 1H), 5.08 (s, 2H), 5.4 (m, 1H}, 7.35 (m, lOH).
Example 2
Preparation of 3-Benzvlthio-1-(N-carbobenzyloxy-L-leucvl)pvrrolidin-4-one
(a} 3-Benzylthio-1-tert-butoxycarbonyl-4-hydroxypyrrolidine
A sodium methoxide solution (50 mg sodium in 5 ml methanol) was added to a
solution of
benzyl mercaptan (0.68 g) and 1-tert-butoxycarbonyl-3,4-epoxypyrrolidine
(l.Og) in dry
methanol (5 ml) and the solution stirred at 50° for 24 hours. The
solution was evaporated
down under reduced pressure and water (20 ml) added. The mixture was extracted
with di-
chloromethane (3 x 20m1) and the combined extracts washed with water and
brine, dried
(magnesium sulphate) and evaporated down under reduced pressure to give the
title
compound (1.54g) as a white solid, m.p. 97-8°.'H NMR (CDC13) b: 1.45
(s, 9H), 3.02 {br.s,
1H), 3.2-3.4 (m, 2H), 3.7 (m, 2H}, 3.80 (d, 2H), 4.15 (m, 1H), 7.30 (m, SH).
(b) 3-Benzylthio-4-hydroxypyrrolidine hydrochloride
To a solution of 3-benzylthio-1-tert-butoxycarbonyl-4-hydroxypyrrolidine (0.93
g) in dry
ethyl acetate ( 10 ml) at 0° was introduced dry hydrogen chloride for 4
minutes and the
solution stirred at 0° for 1 hour. Solvent was removed under reduced
pressure to give the
title compound (0.77g) as a pale buff gum.'H NMR (DMSO-db) 8: 2.95-3.25 (m,
4H), 3.88
(d, 2H), 3.95 (d, 1H), 4.23 (m, 1H), 7.3 (m, SH), 9.45 (br.s, 2H).
21
CA 02334652 2000-12-07
WO 99/64399 PC'T/US99/13334
(c) 3-Benzylthio-4-hydroxy-1-(N-carbobenzyloxy-L-leucyl)pyrrolidine
In a manner similar to Example I (c) reaction of 3-benzylthio-4-
hydroxypyrrolidine
hydrochloride (246 mg), N-methylmorpholine (506 mg), I-(3-dimethylaminopropyl)-
3-
ethyl-carbodiimide hydrochloride (230 mg), I-hydroxybenzotriazole {153 mg) and
N-
carbobenzyloxy-L-leucine (265 mg) in dichloromethane (20 ml) followed by
chromatography over silica using 1:1 ethyl acetate: hexane gave the title
compound
(284mg) as a colourless gum.'H NMR (CDCl3) b: 0.95 (m, 6H), 1.3-1.8 (m, 3H),
2.95-3.2
(m, IH), 3.25-3.55 (m, 2H), 3.55-3.9 (m, 4H), 3.9-4.3 (m, 2H), 4.45 (m, 1H),
5.05 (m, 2H),
5,45 (d, 1H), 7.33 (m, lOH).
IS (d) 3-Benzylthio-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one
In a manner similar to Example 1 (d) reaction of 3-benzylthio-4-hydroxy-1-(N-
carbobenzyloxy-L-leucyl)pyrrolidine (0.58 g) and Dess-Martin's reagent (1.07
g) in
dichloromethane (10 ml) followed by chromatography over silica using 4:1
hexane: ethyl
acetate gave the title compound (0.40g) as a pale yellow oil.'H NMR (DMSO-db)
8: 0.87
(m, 6H), 1.50 (m, 2H), 1.64 (m, 1H), 3.61 (m, 2H), 3.87 (m, 2H), 4.1 (m, 2H),
4.27 (m,
1 H), 5.03 (s, 2H), 6.91 (d, 1 H), 7.3 (m, l OH).
Example 3
Preparation of 3-Benzylsuifinyl-1-(N-carbobenz~y-L-leu~l)pyrrolidin-4-one
To a solution of 3-benzylthio-I-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one
(0.16 g) in
dichloromethane (5 ml) at -60° was added m-chloroperoxybenzoic acid (77
mg) and the
solution stirred at -60° for 1 hour. The solution was washed
successively with 5% NaH2SO3
solution, saturated sodium bicarbonate solution and water, dried (magnesium
sulphate) and
evaporated down under reduced pressure to give the title compound (93mg) as a
glassy
yellow solid.'H NMR (CDCi3) b: 0.95 (m, 6H), 1.4-1.8 (m, 3H), 3.8-4.7 (m, 8H),
5.05 (m,
2H), 5.3 (m, IH), 7.3-7.45 (m, lOH).
22
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
Example 4
Preparation of 3-Benzylsulfonvl-1-(N-carbobenz~y-L-leucyl)pyrrolidin-4-one
To a solution of 3-benzylthio-I-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one
(0.16 g) in
dichloromethane (5 ml) at room temperature was added m-chloroperoxybenzoic
acid ( 147
mg) and the solution stirred for 1 hour. The solution was washed successively
with 5%
NaHzS03 solution, saturated sodium bicarbonate solution and water, dried
(magnesium
sulphate) and evaporated down under reduced pressure to give the title
compound (0.13g)
as a glassy yellow solid.'H NMR (CDC13) 8: 0.95 (m, 6H), 1.4-1.85 (m, 3H), 3.9-
4.25 (m,
3H), 4.25-4.6 (m, 4H), 4.6-4.8 (m, 1H), 5.05 (m, 2H), 5.3 (m, 1H), 7.3-7.55
(m, lOH).
Example 5
Preparation of 1-f2-(Benzyloxvcarbonylamino)-4-methylpentyll-3-
benz~rlthiopyrrolidin-4-
one
(a) 1-[2-(Benzyloxycarbonylamino)-4-methylpentylJ-3-benzylthio-4-
hydroxypyrrolidine
To a stirring solution of 3-benzylthio-4-hydroxypyrrolidine hydrochloride
(0.25 g) in
dichloromethane (10 ml) was added triethylamine (0.12 g) and a solution of N-
carbobenzyl-
oxy-L-leucinaldehyde (0.30 g) in methanol ( 10 ml). The solution was stirred
for 30 minutes,
sodium triacetoxyborohydride (0.53 g) added and the mixture stirred for 18
hours. Water
( 10 ml) was added and the mixture stirred for 15 minutes. The organic layer
was separated,
washed successively with saturated sodium bicarbonate solution and brine,
dried
(magnesium sulphate) and evaporated down under reduced pressure.
Chromatography over
silica using 1:1 ethyl acetate: hexane gave the title compound (0.31 g) as a
colourless oil.'H
NMR (CDCl3) b: 0.95 (m, 6H), 1.30 (m, 2H), 1.65 (m, 1H), 2.05 (m, 1H), 2.35-
2.6 (m, 3H),
2.70 (m, 1H),2.95 (m, 1H), 3.15-3.3 (m, 1H), 3.78 (m, 3H), 4 03 (m, IH), 4.59
(m,lH), 5.11
(m, 2H), 7.33 (m, lOH).
(b) 1-[2-(Benzyloxycarbonylamino)-4-methylpentylJ-3-benzylthiopyrrolidin-4-one
To a stirring solution of oxalyl chloride (90 mg) in dichloromethane (3 ml) at
-65° was
slowly added a solution of dimethyl sulphoxide (0.12 g) in dichloromethane (3
ml), the
mixture stirred for 5 minutes, then a solution of 1-[2-
(benzyloxycarbonylamino)-4-methyl-
23
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
pentylJ-3-benzylthio-4-hydroxypyrrolidine (285 mg) in dichloromethane (4 ml)
slowly
added. The mixture was stirred at -65° for 20 minutes, triethylamine
(325 mg) slowly added
and stirring continued for 18 hours at room temperature. Water (5 ml) was
added, the
mixture stirred for 10 minutes and the aqueous layer extracted with
dichloromethane (2 x 5
ml). The combined dichloromethane layers were dried (magnesium sulphate) and
evaporated down under reduced pressure. Chromatography over silica using 4: I
hexane:
ethyl acetate gave the title compound (90mg) as a tan gum.'H NMR (CDC13) 8:
0.90 (d,
6H), 1.33 (m, 2H), 1.67 (m, IH), 2.50 (m, 2H), 2.6-2.8 (m, 2H), 2.95-3.3 (m,
3H), 3.78 (d,
2H), 3.95 (d, 1H), 4.6 (m, 1H), 5.10, (s, 2H), 7.31 (m, IOH).
Example 6
Preparation of 3-Benzylthio-1-fN-(2-quinolinecarbon~l)-L-leucyljpyrrolidin-4-
one
(a) 3-Benzylthio-4-hydroxy-1-[N-(tert-butoxycarbonyl)-L-leucyl]pyrrolidine
In a manner similar to Example 1 (c) reaction of 3-benzylthio-4-
hydroxypyrrolidine
trifluoroacetate {1.42 g), N-methylmorpholine (2.3 g), 1-{3-
dimethylaminopropyl)-3-ethyl-
carbodiimide hydrochloride (1.06 g), I-hydroxybenzotriazole (0.71 g) and N-
(tert-
butoxycarbonyl)-L-leucine ( 1.11 g) in dichloromethane (50 ml) followed by
chromatography over silica using 1:1 ethyl acetate: hexane gave the title
compound (l.lOg)
as a colourless oil.'H NMR (CDC13) b: 0.95 (m, 6H), 1.42 (d, 9H), 1.64 (m,
3H), 2.95-3.2
(m, 1H), 3.3-3.55 (m, 2H), 3.8 (m, 4H), 3.95-4.25 (m, 2H), 4.35 (m, 1H), 5.15
(m, IH), 7.3
(m, SH).
(b) 3-Benzylthio-4-hydroxy-1-L-leucylpyrrolidine trifluoroacetate
In a manner similar to Example 1(b) reaction of 3-benzylthio-4-hydroxy-I-[N-
(tert-
butoxycarbonyl)-L-leucyl]pyrrolidine (0.92 g) and trifluoroacetic acid (2 ml)
in
dichloromethane (8 ml) gave the title compound (1.35g) as a tan-coloured oil.
(c) 3-Benzylthio-4-hydroxy-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidine
In a manner similar to Example I (c) reaction of 3-benzylthio-4-hydroxy-1-L-
leucyl-
pyrrolidine trifluoroacetate (0.70 g), N-methylmorpholine (0.83 g), 1-(3-
dimethylamino-
propyl)-3-ethylcarbodiimide hydrochloride (0.37 g), 1-hydroxybenzotriazole
(0.25 g) and 2-
quinolinecarboxylic acid (0.29 g) in dichloromethane (15 ml) followed by
chromatography
24
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
over silica using I :1 ethyl acetate: hexane gave the title compound (0.27g)
as a colourless
oil.'H NMR (CDCI;) 8: 0.95 (m, 6H), 1.65-1.85 (m, 3H), 3.0-3.25 (m, 1H), 3.4-
3.6 (m, 2H),
3.7-4.05 (m, 4H), 4.15-4.3 (m, 2H), 4.95 (m, 1H), 7.32 (m, SH), 7.61 (t, 1H),
7.75 (t, 1H),
7.85 (d, 1 H), 8.15 (t, 1 H), 8.35 (m, 2H), 8.80 (t, 1 H).
(d) 3-Benzylthio-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidin-4-one
In a manner similar to Example 1 (d) reaction of 3-benzyithio-4-hydroxy-1-[N-
(2-
quinolinecarbonyl)-L-leucyl]pyrrolidine (0.25 g) and Dess-Martin's reagent
(0.46 g) in
dichloromethane (15 ml) followed by chromatography over silica using l:l
hexane: ethyl
acetate gave the title compound (0.12g) as a colourless oil.'H NMR (CDCl3) 8:
1.0 (m,
6H), 1.75 (m, 3H), 3.2-3.4 (m, 1H), 3.7-4.05 (m, 4H), 4.25-4.45 (m, 2H), 4.85-
5.15 (m, IH),
7.35 (m, SH), 7.62 (t, 1H), 7.77 (t, 1H), 7.85 (d, 1H), 8.15-8.35 (m, 3H),
8.75 (m, IH).
Example 7
Preparation of 3-tert-Butox caY rbonylmethox -~~1-(N-carbobenzyloxy-L-
leuc~pvrrolidin-4-
one
(a) 1-(N-Phthaloyl-L-leucyl)-3-pyrroline
In a manner similar to Example I(c) reaction of 3-pyrroline (2.23 g), N-
methylmorpholine
( 16.6 g), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (7.45
g), I-
hydroxybenzotriazole (5.99 g) and N-phthaloyl-L-leucine {8.62 g) in
dichloromethane (100
ml) gave the title compound (9.75g) as a white solid.'H NMR (CDCI~) 8: 0.95
(m, 6H),
I.55 (m, 1H), 1.7-1,85 (m, IH), 2.62 (m, IH), 4.28 (m, 4H), 5.00 (d of d, 1H),
5.82 (d of d,
2H), 7.75 (m, 2H), 7.85 (m, 2H).
(b) 3,4-Dihydroxy-1-(N-phthaloyl-L-leucyl)pyrrolidine
To a solution of N-methylmorpholine-N-oxide (4.41 g) and osmium tetroxide (80
mg) in
water (50 ml), acetone (20 ml) and tert-butanol (10 ml) was added 1-(N-
phthaloyl-L-
leucyl)-3-pyrroline (9.24 g) and the solution stirred at room temperature for
3 days. Sodium
metabisulphite {2.3 g), magnesium silicate (fluorisil, 2.3 g) and sodium
sulphate (4.6 g)
were added and the mixture stirred for 30 nunutes. Solid was filtered through
Celite and
washed with acetone. The filtrate was evaporated down under reduced pressure
and
dichloromethane (100 ml) was added. The mixture was washed successively with
sodium
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
thiosulphate solution, 1N hydrochloric acid, saturated sodium bicarbonate
solution and
brine, dried (magnesium sulphate) and evaporated down under reduced pressure
to give the
title compound (8.87g) as a white solid.'H NMR (CDCl3) 8: 0.95 (m, 6H),1.55
(m, 1H),
1.75 (m, 1H), 2.35-2.65 (m, 1H), 3.1 (m, 2H), 3.3-3.6 (m, 2H), 3.7 (m, 2H),
4.25 (d, 2H),
4.95 (m, 1H), 7.75 (m, 2H), 7.9 (m, 2H).
(c) 3-tert-Butoxycarbonylmethoxy-4-hydroxy-1-(N-phthaloyl-L-leucyl)pyrrolidine
To a solution of 3,4-dihydroxy-I-(N-phthaloyl-L-leucyl)pyrrolidine (0.80 g) in
dry
dimethylformamide ( 10 ml) at -5° was added 60% sodium hydride in oil
(0.10 g) and the
mixture stirred for 30 minutes at -5°. Tert-butyl bromoacetate (568 mg)
was added and the
solution stirred for 16 hours at room temperature. The bulk of the solvent was
removed
under reduced pressure and dichloromethane (50 ml) and ice-water (50 ml)
added. The
aqueous layer was washed with dichloromethane (20 ml) and the combined organic
layers
washed with water, dried (magnesium sulphate) and evaporated down under
reduced
pressure. Chromatography over silica using 3:1 ethyl acetate: hexane gave the
title
compound (458mg) as a colourless oil.'H NMR (CDC13) b: 0.95 (m, 6H), I.5 (m,
9H), 1.75
(m, 2H), 2.3-2.75 (m, 1H), 3.35 (m, 1H), 3.5-3.8 (m, 3H), 3.8-4.0 (m, 2H), 4.1-
4.3 (m, 2H),
5.0 (m, 2H), 7.75 (m, 2H), 7.85 (m, 2H).
(d) 3-tert-Butoxycarbonylmethoxy-4-hydroxy-I-L-leucylpyrrolidine
A solution of 3-tert-butoxycarbonylmethoxy-4-hydroxy-1-(N-phthaloyl-L-
leucyl)pyrrol-
idine (440 mg) and hydrazine hydrate (0.26 g) in ethanol (10 ml) was stirred
under reflux
for I hour. The white solid that formed was filtered off, washed with ethanol
and
dichloromethane and the filtrate evaporated down under reduced pressure. Water
(5 ml) and
dichloromethane (5 ml) were added, the aqueous layer washed with
dichloromethane (5 ml)
and the combined organic layers washed with water, dried (magnesium sulphate)
and
evaporated down under reduced pressure to give the title compound (202mg) as a
colourless
oil.'H NMR (CDC13) 8: 0.95 (m, 6H), 1.4 (m, IH), 1.50 (s, 9H), 1.85 (m, 2H),
3.4-3.85 (m,
7H), 3.95 (m, 2H), 4.3 (m, 2H).
(e) 3-tert-Butoxycarbonylmethoxy-4-hydroxy-1-(N-carbobenzyloxy-L-
leucyl)pyrrolidine
To a solution of 3-tert-butoxycarbonylmethoxy-4-hydroxy-1-L-leucylpyrrolidine
(96 mg)
and triethylamine (73 mg) in dichloromethane (2.5 ml) at 0° was slowly
added a solution of
26
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
benzyl chloroformate (60 mg) in dichloromethane (2.5 ml) and the solution
stirred at 4° for
3 hours. The solution was was washed with water, dried (magnesium sulphate)
and
evaporated down under reduced pressure to give the title compound (129mg) as a
colourless
gum.'H NMR (CDC13) S: 0.95 (m, 6H), 1.4 (m, 1H), 1.50 (s, 9H), 1.75 (m, 2H),
3.45-3.85
(m, 3H), 3.85-4.15 (m, 3H), 4.15-4.4 (m, 2H), 4.5 (m, 1H), 4.71 (d, 1H), 5.09
(d, 2H), 5.45
(m, 1H), 7.35 (m, SH).
(f) 3-tert-Butoxycarbonylmethoxy-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one
In a manner similar to Example 1 (d) reaction of 3-tert-butoxycarbonylmethoxy-
4-hydroxy-
1-(N-carbobenzyloxy-L-leucyl)pyrrolidine (119 mg) and Dess-Martin's reagent
(500 mg) in
dichloromethane (5 ml) followed by chromatography over silica using 2:1
hexane: ethyl
acetate gave the title compound (7lmg)as a pale yellow oil.'H NMR (CDC13) b:
0.95 (m,
6H), 1.4 (m, 1H), 1.48 (d, 9H), 1.7 (m, 2H), 3.55-4.05 (m, 3H), 4.05-4.3 (m,
3H), 4.3-4..7
(m, 2H), 5.09 (s, 2H), 5.35 (m, 1H}, 7.34 (s, 5H).
Example 8
Preuaration of 3-(3-Methoxybenz~y)-1-(N~arbobenzylox -L-leucyl)pyrrolidin-4-
one
(a) 3-Hydroxy-4-(3-methoxybenzyloxy)-1-(N-phthaloyl-L-leucyl)pyrrolidine
In a manner similar to Example 7(c) reaction of 3,4-dihydroxy-1-(N-phthaloyl-L-
leucyl)-
pyrrolidine (0.83 g), 60°lo sodium hydride in oil (0.12 g) and 1-
bromomethyl-3-methoxy-
benzene (0.72 g} in dry dimethylformamide (50 ml) followed by chromatography
over silica
using 4:1 ethyl acetate: hexane gave the title compound (0.29g) as a
colourless oil. .'H
NMR (CDCl3) S: 0.95 (m, 6H), 1,55 (m, 1H), 1.65 (m, 1H), 2.35-2.75 (m, 2H),
3.45-3.75
(m, 4H), 3.81 (m, 3H), 4.0 (m, 1H), 4.25 (m, IH), 4.4-4.7 (m, 2H), 4.95 (m,
1H), 6.85 (m,
3H), 7.3 (m, 1H), 7.35 (m, 2H), 7 45 (m, 2H).
(b) 3-Hydroxy-4-(3-methoxybenzyloxy)-1-L-leucylpyrrolidine
In a manner similar to Example 7(d) reaction of 3 hydroxy-4-(3-
methoxybenzyloxy)-1-(N-
phthaloyl-L-leucyl)pyrrolidine (0.26 g) and hydrazine hydrate (0.15 g) in
ethanol ( 10 ml)
gave the title compound (0.1 lg) as a colourless oil.'H NMR (CDC13) 8: 0.93
(m, 6H), 1.4
27
CA 02334652 2000-12-07
WO 99/64399 PCT/ITS99/13334
(m, 2H)> 1.85 (m, 1H), 3.35-3.8 (m, 6H), 3.82 (s, 3H), 4.05 (m, 1H), 4.3 (m,
1H), 4.5-4.7
(m, 2H), 6.9 (m, 4H), 7.3 (m, 2H).
(c) 3-Hydroxy-4-(3-methoxybenzyloxy)-1-(N-carbobenzyloxy-L-leucyl)pyrrolidine
In a manner similar to Example 7(e) reaction of 3-hydroxy-4-(3-
methoxybenzyloxy)-I-L-
leucylpyrrolidine (90 mg), triethylamine (73 mg) and benzyl chloroformate (60
mg) in
dichloromethane (5 ml) gave the title compound (123mg) as a colourless gum.'H
NMR
(CDC13) 8: 0.95 (m, 6H), 1.3-1.55 (m, 2H), 1.7 (m, 1H), 2.5-2.8 (m, IH), 3.4-
3.95 (m, 3H),
3.82 (s, 3H), 4.05 (m, 1H), 4.3 (m, 1H), 4.4-4.55 (m, 1H), 4.60 (s, 2H), 4.7
(m, IH), 5.07 (s,
2H), 5.4 (m, 1H), 6.9 (m, 3H), 7.35 (m, 6H).
(d) 3-(3-Methoxybenzyloxy)-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one
In a manner similar to Example 1(d) reaction of 3-hydroxy-4-(3-
methoxybenzyloxy)-1-(N-
carbobenzyloxy-L-leucyl)pyrrolidine (113 mg) and Dess-Martin's reagent (500
mg) in
dichioromethane (5 ml) followed by chromatography over silica using 2:1
hexane: ethyl
acetate gave the title compound (SOmg) as a colourless oil.'H NMR (CDC13) 8:
0.95 (m,
6H), 1.3-1.8 (m, 3H), 3.45-3.65 (m, 1H), 3.81 (s, 3H), 3.85-4.0 (m, IH), 4.0-
4.25 (m, 2H),
4.25-4.45 (m, 1 H), 4.5-4.75 (m, 2H), 4.85 (m, I H), 5.08 (m, 2H), 5.4 (m, 1
H), 6.9 (m, 3H),
7.33 (m, 6H).
Example 9
Preparation of 3-(3-Methoxvcarbonvlbenz~y)-1-lN~arbobenzylox -y L-
leuc~pvrrolidin-
4-one
(a) 3-Hydroxy-4-(3-methoxycarbonylbenzyloxy)-1-(N-phthaloyl-L-
leucyl)pyrrolidine
In a manner similar to Example 7(c) reaction of 3,4-dihydroxy-I-(N-phthaloyl-L-
leucyl)-
pyrrolidine ( I .40 g), 60% sodium hydride in oil (0.18 g) and methyl-3-
bromomethylbenzoate ( 1.08 g) in dry dimethylformamide ( 100 ml) followed by
chromatography over silica using 3:1 ethyl acetate: hexane gave the title
compound (0.92g)
as a colourless oil.'H NMR (CDC13) b: 0.95 (m, 6H), 1.45-1.8 (m, 3H), 3.3-3.8
(m, 3H),
3.95 (m, 3H), 4.05 (m, 1H), 4.3 (m, 1H), 4.5-4.75 (m, 2H), 4.95 (m, IH), 7.5
(m, 2H), 7.75
{m, 2H), 7.85 (m, 2H), 8.0 (m, 2H).
28
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
(b) 3-Hydroxy-4-(3-methoxycarbonylbenzyloxy)-I-L-leucyl-pyrrolidine
In a manner similar to Example 7(d) reaction of 3-hydroxy-4-(3-methoxycarbonyl-
benzyloxy)-1-(N-phthaloyl-L-leucyl)pyrrolidine (0.91 g) and hydrazine hydrate
(0.45 g) in
ethanol (15 ml) gave the title compound (0.64g) as a pale buff gum.'H NMR
(CDC13) $:
0.95 (m, 6H), 1.35 (m, 2H), 1.8 (m, IH), 3.4-3.8 (m, 5H), 3.94 (s, 3H), 4.05
(m, 1H), 4.35
(m, 1H), 4.55-4.75 (m, 2H), 7.5 (m, 2H), 8.0 (d, 2H).
(c) 3-Hydroxy-4-(3-methoxycarbonylbenzyloxy)-1-(N-carbobenzyloxy-L-
leucyl)pyrrolidine
In a manner similar to Example 7(e) reaction of 3-hydroxy-4-{3-methoxycarbonyl-
benzyloxy)-1-L-leucyl-pyrrolidine (315 mg), triethylamine (218 mg) and benzyl
IS chloroformate (179 mg) in dichloromethane (15 ml) gave the title compound
(429mg) as a
colourless gum.'H NMR (CDC13) 8: 0.95 (m, 6H), 1.35-1.6 {m, 3H), 2.6 (m, 1H),
3.4-3.85
(m, 3H), 3.94 (s, 3H), 4.05 (m, 1H), 4.3 (m, IH), 4.45 (m, 1H), 4.6 (m, 1H),
4.7 (d, 2H),
5.07 (s, 2H), 5.45 (m, 1H), 7.3 (m, 5H)> 7.5 (m, 2H), 8.01 (m, 2H).
(d) 3-(3-Methoxycarbonylbenzyloxy}-1-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-
one
In a manner similar to Example 1 (d) reaction of 3-hydroxy-4-(3-
methoxycarbonyl-
benzyloxy)-1-(N-carbobenzyloxy-L-leucyl)pyrrolidine (419 mg) and Dess-Martin's
reagent
(1.5 g) in dichloromethane (15 ml) followed by chromatography over silica
using 2:1
hexane: ethyl acetate gave the title compound (176mg) as a colourless oil.'H
NMR
(CDCl3) b: 0.95 (m, 6H), 1.35-1.6 (m, 3H), 3.35-3.6 (m,lH), 3.9b (s, 3H), 4.0-
4.3 {m, 2H),
4.3-4.5 (m, 2H), 4.6 (m, 1 H), 4.7 (d, I H), 4.85-5.0 (m, 1 H), 5.08 (s, 2H),
5.4 (m, 1 H), 7.34
(s, 5H), 7.45 (m, 1H), 7.55 (m, 1H), 8.05 (m, 2H).
Example 10
Preparation of 3-tert-Butox~rcarbon)rlmethoxy-1-fN-(2-quinolinecarbonyl)-L-
leucyllpyrrolidin-4-one
(a) 3-tert-Butoxycarbonylmethoxy-4-hydroxy-I-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrroiidine
In a manner similar to Example 1 (c) reaction of 3-tert-butoxycarbonylmethoxy-
4-hydroxy-
I-L-leucylpyrrolidine (96 mg), N-methylmorpholine (120 mg), I-(3-dimethylamino-
propyl)-
3-ethylcarbodiimide hydrochloride (67 mg), I-hydroxybenzotriazoie (45 mg) and
2-
29
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
quinolinecarboxylic acid (51 mg) in dichloromethane (5 ml) followed by
chromatography
over silica using 1:1 ethyl acetate: hexane gave the title compound ( 1 l5mg)
as a colourless
gum.'H NMR (CDC13) S: 1.0 (m, 6H), 1.5 (m, 9H), 1.75 (m, 3H), 3.5-3.8 (m, 2H)>
3.85-
4.35 (m, 7H), 5.0 (m, 1H), 7.6 (t, 1H), 7.75 (t, 1H), 7.85 (d, 1H), 8.15 (d,
1H), 8.25 (m, 2H),
8.75 (m, 1H).
(b) 3-tert-Butoxycarbonylmethoxy-I-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrolidin-4-one
In a manner similar to Example 1 (d) reaction of 3-tent-butoxycarbonylmethoxy-
4-hydroxy-
I-[N-(2-quinolinecarbonyl)-L-leucyl)pyrrolidine (105 mg) and Dess-Martin's
reagent (650
mg) in dichloromethane (5 ml) followed by chromatography over silica using 2:1
hexane:
ethyl acetate gave the title compound (87mg) as a colourless gum.'H NMR
(CDC13) 8:
1.05 (m, 6H), 1.5 (m, 9H), 1.8 (m, 3H), 3.55-3.8 (m, 1H), 3.8-4.0 (m, 1H), 4.0-
4.5 (m, 4H),
4.5-4.75 (m, 1 H), 4.85-5.25 (m, 1 H), 7.62 (t, 1 H), 7.79 (t, I H), 7.87 (d,
1 H), 8. I S (d, 1 H),
8.25 (m, 2H), 8.75 (m, 1H).
Example I 1
Preparation of 1-fN-(2-Ouinolinecarbonyl)-L-leucvll-3-oxo-4-
p~rrrolidineoxyacetic acid
A solution of 3-tent-butoxycarbonylmethoxy-1-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrol-
idin-4-one (14 mg) and trifluoroacetic acid (0.1 ml) in dichloromethane (0.5
ml) was stirred
at room temperature for 3 hours. Solvent was removed under reduced pressure
and traces
azeotroped with toluene to give the title compound (l2mg) as a buff foam.'H
NMR
(CDCI3) 8: 0.95 (m, 6H), 1.5-1.9 (m, 3H), 3.5-4.5 (m, 6H), 4.5-4.7 (m, 1H),
4.75-5.15 (m,
Example 12
Preparation of 3-(3-Methoxybenzyloxy)-1-1-jN-(2~uinolinecarbon,~l)-L-
leucvllpyrrolidin-4-
one
(a) 3-Hydroxy-4-(3-methoxybenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-
leucylJpyrrolidine
In a manner similar to Example 1(c) reaction of 3-hydroxy-4-(3-
methoxybenzyloxy)-1-L-
leucylpyrrolidine (404 mg), N-methylmorpholine (488 mg), I-(3-
dimethylaminopropyl)-3-
ethylcarbodiimide hydrochloride (275 mg), 1-hydroxybenzotriazole (184 mg) and
2-
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
quinolinecarboxylic acid (208 mg) in dichloromethane (20 ml) followed by
chromatography
over silica using 3:1 ethyl acetate: hexane gave the title compound (519mg) as
a colourless
gum.'H NMR (CDC13) b: 1.0 (m, 6H), 1.75 (m, 3H), 3.6 (m, 2H), 3.85 (m, 4H),
4.0-4.2 (m,
2H), 4.3 (m, 1H), 4.45-4.7 (m,. 2H), 5.0 (m, 1H}, 6.9 (m, 3H), 7.3 (m, IH),
7.61 (t, 1H),
7.76 (t, 1 H), 7.86 (d, 1 H), 8. I 5 (d, 1 H), 8.25 (m, 2H), 8.75 (m, 1 H).
{b) 3-(3-Meihoxybenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidin-4-
one
In a manner similar to Example 1(d) reaction of 3-hydroxy-4-(3-
methoxybenzyloxy)-1-[N-
(2-quinolinecarbonyl)-L-leucylJpyrrolidine (504 mg) and Dess-Martin's reagent
( I .65 g) in
dichloromethane ( 18 ml) followed by chromatography over silica using 2:1
hexane: ethyl
acetate gave the title compound (448mg) as a colourless oil.'H NMR (CDCIj) 8:
1.0 (m,
6H), 1.75 (m, 3H), 3.45-3.75 (m, 1H), 3.85 (m, 3H), 3.9-4.45 (m, 3H), 4.5-4.75
(m, 2H),
4.75-5.2 (m, 2H), 6.95 (m, 3H), 7.3 (m, 1H), 7.62 (t, 1H), 7.78 (t, 1H), 7.86
(d, 1H), 8.14 (d,
1H), 8.3 {m, 2H), 8.7 (m, IH).
Example 13
Preparation of 3-(4-Methoxybenzyloxy)-I-fN-(2-quinolinecarbonyl)-L-
leucyllpyrrolidin-4-
one
{a) 3-Hydroxy-4-(4-methoxybenzyloxy)-1-(N-phthaloyl-L-leucyl)pyrrolidine
In a manner similar to Example 7(c) reaction of 3,4-dihydroxy-1-(N-phthaloyl-L-
leucyl)-
pyrrolidine (0.80 g), 60% sodium hydride in oil (0.10 g) and 1-bromomethyl-4-
methoxy-
benzene (0.58 g) in dry dimethylformamide (10 ml) followed by chromatography
over silica
using 3:1 ethyl acetate: hexane gave the title compound (322mg) as a
colourless oil.'H
NMR (CDC13) 8: 0.95 {m, 6H), 1.55 (m, 2H), 1.75 (m, 1H), 2.35-2.7 (m, 2H),
3.45-3.75 (m,
3H), 3.8 (m, 3H), 4.0 (m, 1 H), 4.25 {m, 1 H), 4.35-4.6 (m, 2H), 4.95 (m, 1
H),6.9 (m, 2H),
7.25 (m, 2H), 7.7 (m, 2H), 7.85 (m, 2H).
(b) 3-Hydroxy-4-(4-methoxybenzyloxy)-1-L-leucylpyrrolidine
In a manner similar to Example 7(d) reaction of 3 hydroxy-4-(4-
methoxybenzyloxy)-1-{N-
phthaloyl-L-leucyl)pyrrolidine (0.31 g) and hydrazine hydrate (0.16 g) in
ethanol (6 ml)
gave the title compound (213mg) as a colourless oil.'H NMR (CDCl3) 8: 0.95 (m,
6H), 1.4
31
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
(m, 2H), 1.8 (m, 1H), 3.3-3.8 (m, SH), 3.81 (s, 3H), 4.0 (m, 1H), 4.25 (m,
1H), 4.4-4.65 (m,
2H), 6.9 (m, 2H), 7.3 (m, 2H}.
(c) 3-Hydroxy-4-(4-methoxybenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrolidine
in a manner similar to Example 1(c} reaction of 3-hydroxy-4-(4-
methoxybenzyloxy)-1-L-
leucylpyrroiidine (203 mg), N-methylmorpholine (248 mg), 1-(3-
dimethylaminopropyl)-3-
ethylcarbodiimide hydrochloride (140 mg), 1-hydroxybenzotriazole (93 mg) and 2-
quinolinecarboxylic acid (105 mg) in dichloromethane (10 ml) followed by
chromatography
over silica using 3:1 ethyl acetate: hexane gave the title compound (273mg) as
a colourless
gum. ~H NMR (CDCI3) 8: 1.0 (m, 6H), 1.5-1.9 (m, 3H), 3.5-.37 (m, 2H), 3.8 (m,
3H), 3.95-
4.2 (m, 2H), 4.3 (m, 1H), 4.5 (m, 1H), 4.6 (m, 2H), 5.0 (m, 1H), 6.9 (m, 2H),
7.3 (m, 2H),
7.61 (t, 1 H), 7.76 (t, 1 H), 7.86 (d, 1 H), 8.15 (d, 1 H), 8.25 (m> 2H), 8.75
(m, 1 H).
(d) 3-(4-Methoxybenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-leucyl]pynrolidin-4-
one
In a manner similar to Example 1(d) reaction of 3-hydroxy-4-(4-
methoxybenzyloxy)-1-[N-
(2-quinolinecarbonyl)-L-leucyl]pyrrolidine (262 mg) and Dess-Martin's reagent
( 1.20 g) in
dichloromethane (10 ml) followed by chromatography over silica using 2:1
hexane: ethyl
acetate gave 138 mg of the title compound as a colourless oil. ~H NMR (CDC13)
8: 1.0 (m,
6H), 1.6-1.9 {m, 3H), 3.45-3.75 (m, 1H), 3.81 (m, 3H), 4.0-4.25 (m, 3H), 4.5-
4.7 (m, 2H),
4.7-5.0 (m, 1H), 5.1 (m, 1H), 6.9 (m, 2H), 7.3 (m, 2H), 7.62 (t, 1H), 7.78 (t,
1H), 7.88 (d,
1H), 8.15 (d, 1H), 8.3 (m, 2H), 8.7 (m, 1H).
Example 14
Preparation of 3-~3-Methoxycarbonylbenz loxy~-1-fN-(2-quinolinecarbon l
leucyl]nyrrolidin-4-one
(a) 3-Hydroxy-4-(3-methoxycarbonylbenzyloxy}-1-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrolidine
In a manner similar to Example 1(c) reaction of 3-hydroxy-4-(3-methoxycarbonyl-
benzyloxy)-1-L-leucylpyrrolidine (315 mg), N-methylmorpholine (350 mg), I-(3-
dimethyl-
aminopropyl)-3-ethylcarbodiimide hydrochloride (199 mg), I-
hydroxybenzotriazole (132
mg) and 2-quinolinecarboxylic acid (150 mg) in dichloromethane (15 ml)
followed by
chromatography over silica using 3: I ethyl acetate: hexane gave the title
compound
32
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
(337mg) as a colourless gum.'H NMR (CDC13) 8: 1.05 (m, 6H), l.8 (m, 3H), 3.65
(m, 2H),
3.8 (m, l H), 3.95 (m, 3H), 4.0-4.25 (m, 2H), 4.35 (m, 1 H), 4.55-4.8 (m..
2H), 5.0 (m, 1 H),
7.4-7.75 (m, 3H), 7.80 (t, 1H), 7.90 (d, 1H), 8.05 (m, 2H), 8.20 (d, 1H), 8.3
(m, 2H), 8.75
(m, I H).
(b) 3-(3-Methoxycarbonylbenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrolidin-4-one
In a manner similar to Example 1 (d) reaction of 3-hydroxy-4-(3-
methoxycarbonyl-
benzyloxy)-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidine (327 mg) and Dess-
Martin's
reagent ( 1.1 g) in dichloromethane ( 12 ml) followed by chromatography over
silica using
2:1 hexane: ethyl acetate gave the title compound {259mg) as a colourless
oil.'H NMR
(CDCi~) 8: 1.05 (m, 6H), 1.8 (m, 3H), 3.5-3.8 (m, 1H), 3.93 {m, 3H), 4.0-4.5
(m, 3H), 4.5-
4.8 (m, 2H), 4.85-5.2 (m, 2H), 7.45 (m, 1 H), 7.5-7.7 (m, 2H), 7.80 (t, 1 H),
7.90 (d, 1 H), 8.0
(m, 2H), 8.15 (d, IH), 8.25 (m, 2H), 8.7 (m, 1H).
Example 15
Preparation of 3~4-Nitrobenzyloxy)-1-fN-(2-quinolinecarbonyl)-L-
leucyllpyrrolidin-4-one
(a) 3-Hydroxy-4-(4-nitrobenzyloxy)-1-(N-phthaloyl-L-leucyl)pyrrolidine
In a manner similar to Example 7(c) reaction of 3,4-dihydroxy-1-(N-phthaloyl-L-
leucyl)-
pyrrolidine (0.80 g), 60% sodium hydride in oil (0.10 g) and 4-nitrobenzyl
bromide (0.64 g)
in dry dimethylfonmamide (8 ml) followed by chromatography over silica using
3:1 ethyl
acetate: hexane gave the title compound (190mg) as a colourless oil.'H NMR
(CDCI~) 8:
1.0 (m, 6H), 1.55 (m, 2H), 1.75 (m, 1H), 2.3-2.7 (m, 2H), 3.4-3.8 (m, 3H),
4.05 (m, 1H),
4.35 (m, 1H), 4.6-4.8 (m, 2H), 4.95 (m, 1H), 7.48 (m, 2H), 7.75 (m, 2H), 7.85
(m, 2H), 8.2
(m, 2H).
(b) 3-Hydroxy-4-(4-nitrobenzyloxy)-1-L-leucylpyrrolidine
In a manner similar to Example 7{d) reaction of 3 hydroxy-4-(4-nitrobenzyloxy)-
I-(N-
phthaloyl-L-leucyl)pyrrolidine (225 mg) and hydrazine hydrate (0.12 g) in
ethanol (4 ml)
gave the title compound (109mg) as a colourless oil.'H NMR (CDC13) b: 0.95 (m,
6H), 1.3-
1.5 (m, 2H), 1.8 (m, IH), 3.4-3.9 (m, SH), 4.1 (m> 1H), 4.4 (m, IH), 4.65-4.9
(m, 2H), 7.5
(m, 2H), 8.25 (m, 2H).
33
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
(c) 3-Hydroxy-4-(4-nitrobenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrolidine
In a manner similar to Example 1 (c) reaction of 3-hydroxy-4-(4-
nitrobenzyloxy)-1-L-
leucylpyrrolidine ( 100 mg), N-methylmorpholine ( 115 mg), 1-(3-dimethylamino-
propyl)-3-
ethylcarbodiimide hydrochloride (65 mg), l-hydroxybenzotriazole (44 mg) and 2-
quinoline-
carboxylic acid (49 mg) in dichloromethane {5 ml) followed by chromatography
over silica
using 3:1 ethyl acetate: hexane gave the title compound (124mg) as a pale buff
gum.'H
NMR (CDCl3) 8: 1.05 (m, 6H),1.6-1.9 (m, 3H), 3.55-3.9 (m, 3H), 4.05-4.3 (m,
2H), 4.45
(m, 1H), 4.65-4.85 (m, 2H), 5.05 (m, 1H), 7.4-7.7 (m, 3H), 7.78 (t, IH), 7.87
(d, 1H), 8.2
(m, 2H), 8.3 (m, 3H), 8.75 (m, 1H).
(d) 3-(4-Nitrobenzyloxy)-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidin-4-one
In a manner similar to Example 1 (d) reaction of 3-hydroxy-4-(4-
nitrobenzyloxy)-1-[N-(2-
quinolinecarbonyl)-L-leucyl]pyrrolidine (114 mg) and Dess-Martin's reagent
(0.80 g) in
dichloromethane (6 ml) followed by chromatography over silica using 2:1
hexane: ethyl
acetate gave the title compound (40mg) as a colourless oil.'H NMR (CDCI3) b:
1.0 (m,
6H),1.65-1.9 (m, 3H), 3.55-3.9 (m, 1H), 4.05-4.3 (m, 3H), 4.4-4.65 (m, 1H),
4.7-5.25 (m,
3H), 7.4-7.7 (m, 3H), 7.79 (t, 1H), 7.87 (d, 1H), 8.05-8.35 (m, 5H), 8.7 (m,
1H).
Example 16
Pre,.paration of 3-!5-Methyl-3-isoxazol~methoxy)-1-fN-(2-guinolinecarbon l
leucyllpyrrolidin-4-one
(a) 3-Hydroxy-4-(5-methyl-3-isoxazolylmethoxy)-1-(N-phthaloyl-L-
leucyl)pyrrolidine
In a manner similar to Example 7(c) reaction of 3,4-dihydroxy-1-(N-phthaloyl-L-
leucyl)-
pyrrolidine (0.40 g), 60% sodium hydride in oil (50 mg) and 3-bromomethyl-5-
methyl-
isoxazole (0.25 g) in dry dimethylformamide (5 ml) followed by chromatography
over silica
using 3:1 ethyl acetate: hexane gave the title compound (116mg) as a glassy
solid.'H NMR
(CDC13) b: 1.0 (m, 6H), 1.55 (m, 2H), 1.75 (m, 1H), 2.45 (m, 3H), 2.5-2.8 (m,
2H), 3.45-3.8
(m, 3H), 4.0 (m, 1 H), 4.25 (m, 1 H), 4.55-4.8 (m, 2H), 4.95 (m, l H), 6.00
(t, 1 H)> 7.75 (m,
2H), 7.85 (m, 2H).
34
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
(b) 3-Hydroxy-4-(5-methyl-3-isoxazolylmethoxy)-1-L-leucyl-pyrrolidine
In a manner similar to Example 7(d) reaction of 3-hydroxy-4-(5-methyl-3-
isoxazolyl-
methoxy)-1-(N-phthaloyl-L-leucyl)pyrrolidine (106 mg) and hydrazine hydrate
(60 mg) in
ethanol (2.5 mI) gave the title compound (66mg) as a colourless oil.'H NMR
(CDCI~) 8:
0.95 (m, 6H), 1.25-1.55 (m, 2H), 1.85 (m, 1H), 2.44 (s, 3H), 3.35-3.7 (m, 4H),
3.75 (m, 1H),
4.05 (m, IH), 4.3 (m, 1H), 4.6-4.8 (m, 2H), 6.02 (m, 1H).
(c) 3-Hydroxy-4-(5-methyl-3-isoxazolyimethoxy)-1-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrolidine
In a manner similar to Example 1 (c) reaction of 3-hydroxy-4-(5-methyl-3-
isoxazolyl-
methoxy)-1-L-leucylpyrrolidine (56 mg), N-methylmorpholine (74 mg), I-(3-
dimethyl-
aminopropyl)-3-ethylcarbodiimide hydrochloride (41 mg), I-hydroxybenzotriazole
(28 mg)
and 2-quinolinecarboxylic acid (32 mg) in dichloromethane (3 ml) followed by
chromato-
graphy over silica using 3:1 ethyl acetate: hexane gave the title compound
(6lmg) as a pale
buff gum.'H NMR {CDC13) b: 0.95-1.1 (m, 6H), 1.6-1.9 (m, 3H), 2.45 (m, 3H),
2.75-2.95
(m, IH), 3.5-3.7 (m, 2H), 3.8 (m, 1H), 4.0-4.2 (m, 2H), 4.3 (m, 1H), 4.55-4.8
(m ,2H), 5.0
(m, 1 H), 6.02 (d, 1 H), 7.61 {t, 1 H), 7.77 (t, 1 H), 7.86 (d, 1 H), 8.15 (d,
1 H), 8.25 (m, 2H),
8.75 (m, 1H).
(d) 3-(5-Methyl-3-isoxazolylmethoxy)-1-[N-(2-quinolinecarbonyl)-L-
leucyl]pyrrolidin-4-
one
In a manner similar to Example I (d) reaction of 3-hydroxy-4-(5-methyl-3-
isoxazolyl-
methoxy)-1-[N-(2-quinolinecarbonyl)-L-leucyl]pyrrolidine (51 mg) and Dess-
Martin's
reagent {0.29 g) in dichloromethane (2 ml) followed by chromatography over
silica using
I :1 hexane: ethyl acetate gave the title compound (37mg) as a colourless
gum.'H NMR
(CDCl3) 8: 0.95 (m, 6H), 1.6-1.9 (m, 3H), 2.34 (d, 3H), 3.35-3.55 (m, IH), 3.4-
4.4 (m, 3H),
4.5-4.95 (m, 3H), 5.05 (m, 1H), 5.99 (d, IH), 7.55 (t, 1H), 7.70 (t, 1H), 7.80
(d, 1H), 8.07
(d, IH), 8.2 (m, 2H), 8.65 (m, IH).
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
Example 17
Preparation of 3-(3-Methoxybenzyloxv)-1-IN-(2-naphthalenecarbonyl)-L-
leucvl>pyrrolidin-
4-one
(a) 3-Hydroxy-4-(3-methoxybenzyloxy)-1-[N-(2-naphthalenecarbonyl)-L-
leucyl]pyrrolidine
In a manner similar to Example 1 (c) reaction of 3-hydroxy-4-(3-
methoxybenzyloxy)-l-L-
leucylpyrrolidine (228 mg), N-methylmorpholine (276 mg), I-(3-
dimethylaminopropyl)-3-
ethylcarbodiimide hydrochloride (156 mg), I-hydroxybenzotriazole (104 mg) and
2-
naphthoic acid ( 117 mg) in dichloromethane { 12 ml) followed by
chromatography over
silica using 4:1 ethyl acetate: hexane gave the title compound (295mg) as a
colourless gum.
~H NMR (CDCl3) b: 0.95 (m, 6H), 1.45-1.9 (m, 3H), 3.5-3.7 (m, 3H), 3.84 (t,
3H), 3.95-4.2
(m, 2H), 4.35 (m, 1H), 4.5-4.7 (m, 2H), 5.05 (m, IH), 6.9 (m, 3H), 7.1 (m,
1H), 7.3 (m, 1H),
7.55 (m, 2H), 7.9 (m, 4H), 8.35 (s, 1H).
(b) 3-(3-Methoxybenzyloxy)-1-[N-(2-naphthalenecarbonyl)-L-leucyl]pyrrolidin-4-
one
In a manner similar to Example 1(d) reaction of 3-hydroxy-4-(3-
methoxybenzyloxy)-1-[N-
(2-naphthalenecarbonyl)-L-leucyl]pyrrolidine (280 mg) and Dess-Martin's
reagent (0.48 g)
in dichloromethane (7.5 ml) followed by chromatography over silica using 2:1
hexane:
ethyl acetate gave the title compound (246mg) as a colourless gum. IH NMR
(CDC13) 8:
1.05 (m, 6H), 1.5-1.9 (m, 3H), 3.45-3.75 (m, 1H), 3.83 (m, 3H), 3.9-4.05 (m,
1H), 4.05-4.2
(m, 1H), 4.2-4.4 (m, 1H), 4.4-4.7 (m, 2H), 4.75-5.05 (m, 1H), 5.15 (m, 1H),
6.95 (m, 4H),
7.25 (m, IH), 7.55 (m, 2H), 7.85 (m, 4H), 8.3 (s, IH).
Example 18
Preparation of 3-(4-Methoxyphenoxy)-1-(N-carbobenz~~L-leucyl)nyrrolidin-4-one
(a) N-(carbobenzyloxy-L-leucyl)-3-pyrroline
In a manner similar to Example I(c) reaction of 3-pytroline (0.21g), N-
methylmorpholine
(1.66 g), I-(3-dimethyiaminopropyl)-3-ethylcarbodiimide hydrochloride (0.70
g), I-
hydroxybenzotriazole (0.47 g) and N-carbobenzyloxy-L-leucine (0.80 g) in
dichloromethane (10 ml) followed by chromatography over silica using 2:3 ethyl
acetate:
hexane gave the title compound (0.70g) as a white solid. ~H NMR (CDC13) 8 0.97
(d of d,
36
CA 02334652 2000-12-07
WO 99/64399 PCT/US99/13334
6H). 1.35-1.65 (m, 2H), 1.75 (m, IH), 4.1-4.4 (m, 3H), 4.5 (m, 2H), 5.08 (s,
2H), 5.45 (d,
1H)> 5.85 {m, 2H), 7.34 (s, SH).
(b) N-(carbobenzyloxy-L-leucyl)-3,4-epoxypyrrolidine
To a solution of N-(carbobenzyloxy-L-leucyl)-3-pyrroline (0.64 g) in
dichloromethane (20
ml) was added m-chloroperoxybenzoic acid (1.45 g) and the solution stirred for
48 hours:
The mixture.was filtered and the filtrate washed successively with saturated
NaH2S0~ in 2N
sodium hydroxide solution, saturated sodium bicarbonate solution and water,
dried
(magnesium sulphate) and evaporated down under reduced pressure to give the
title
compound (0.31g) as a colourless oil.'H NMR (CDCl3) 8 0.95 (d of d, 6H), 1.3-
1.65 (m,
2H), 1.7 (m, 1H), 3.3-3.6 (m, 2H), 3.7-3.95 (m, 3H), 4.0-4.15 (m, 1H), 4.3-4.5
(m, 1H), 5.07
{s, 2H), 5.4 (d of d, 1H), 7.34 (s, SH).
(c) 3-hydroxy-4-(4-methoxyphenoxy)-1-(N-carbobenzyloxy-L-leucyl)pyrrolidine
To a solution of 4-methoxyphenol (150 mg) in dry tetrahydrofuran (0.5 ml) was
added IM
potassium ten-butoxide in tetrahydrofuran ( 1.8 ml) followed by a solution of
N-
(carbobenzyloxy-L-leucyl)-3,4-epoxypyrrolidine ( 195 mg) in dry
tetrahydrofuran { 1.5 ml)
and the mixture stirred at 50° for 16 hours then under reflux for 3
hours. Solvent was
evaporated off under reduced pressure and the residue dissolved in
dichloromethane (8 ml).
The solution was washed successively with water and brine, dried (magnesium
sulphate)
and evaporated down under reduced pressure. Chromatography over silica using
1:1 ethyl
acetate: hexane gave the title compound (88mg) as a pale buff gum.'H NMR
{CDCl3) 8
0.95 (m, 6H), 1.3-1.6 (m, 3H), 3.3-3.75 (m, 2H), 3.77 (s, 3H), 3.85-4.05 (m,
1H), 4.3-4.7
(m, 3H), 5.05 (m, 2H), 5.3-5.55 (m, 1H), 6.82 (m, 4H), 7.33 (s, SH).
(d) 3-(4-Methoxyphenoxy)-I-(N-carbobenzyloxy-L-leucyl)pyrrolidin-4-one
In a manner similar to Example 1(d) reaction of 3-hydroxy-4-(4-methoxyphenoxy)-
1-(N-
carbobenzyloxy-L-leucyl)pyrrolidine (78 mg) and Dess-Martin's reagent (300 mg)
in
dichloromethane (3 ml) followed by chromatography over silica using 2:1
hexane: ethyl
acetate gave the title compound (45mg) as a colourless oil.'H NMR (CDCl3) 8
0.95 (m,
6H), 1.4-1.8 (m, 3H), 3.5-3.7 (m, 1H), 3.78 (d, 3H), 3.9-4.1 (m, 1H), 4.2-4.55
(m, 2H),
4.55-4.7 (m, 1H), 4.7-4.9 (m, 1H), 5.09 (s, 2H), 5.4 (m, 1H), 6.75-6.95 (m,
4H), 7.34 (s,
SH).
37