Note: Descriptions are shown in the official language in which they were submitted.
CA 02335773 2001-02-13
FIELD OF THE INVENTION
This invention pertains to the field of cancer diagnosis, research and
therapeutics.
BACKGROUND OF THE INVENTION
Colorectal carcinoma is the second most frequent cause of cancer mortality in
men
and women, causing nearly one third of all malignancy-related deaths in North
America. It has been estimated that ultimately as many as 6% of Canadians and
l0 Americans will develop malignancy in the lower bowel, and over 50% of them
will
die within 5 years of diagnosis. Because there are no realistic prospects of
significantly improving the cure rate once the cancer has spread beyond the
bowel
wall, many authorities believe that colorectal cancer can be controlled only
by
preventive measures (Lieberman D. A, Amer. J. Gastroenterol. 1992, 87, 1085).
Primary prevention, i.e. averting the development of the tumour by altering
biological
risk factors, is not yet feasible since so little is understood of the
etiology of the
disease. Alternatively, secondary preventive measures, i.e. detection at an
asymptomatic, treatable state, would be possible should an effective screening
test
2 o be available. Indeed, neoplasms of the lower bowel have the
characteristics that
make them a suitable candidate for the development of a screening test: 1 )
because
it is a common cause of cancer-related deaths; and 2) whereas once the stage
of
true cancer is reached, leading to symptoms, the mortality rate is over 50%,
removal
of bowel neoplasms at its earliest, asymptomatic stage can be done by non-
surgical
endoscopic polypectomy, without any significant risk. Moreover, it requires at
least
four to six years before an adenomatous polyp reaches the cancer stage, so
there
is ample opportunity to detect these neoplasms at their treatable stage.
Ulcerative colitis is a chronic, idiopathic inflammatory process of the colon
which
3 o affects about 0.05% of the population of the Northern industrialized
world. The
disease is characterized by recurrent bouts of diarrhea and rectal bleeding
that may
require lifelong medical management. More importantly, it is now well
recognized
that ulcerative colitis is a premalignant condition, and it has been estimated
that
about 13% of patients with pancolitis will develop carcinomas. About 1 % of
all new
3 5 cases of colon cancer in this country arise as a complication of chronic
inflammatory
bowel disease. Compared to most colonic malignancies, these cancers tend to
occur in a younger age group, to be multifocal, and to behave in a more
aggressive
fashion. It is believed that the majority of malignancies in ulcerative
colitis can be
prevented, because the epithelium of the affected colon undergoes premalignant
4 0 dysplastic changes prior to the development or carcinoma and these
premalignant
-2-
CA 02335773 2001-02-13
changes can be detected by regular surveillance biopsies. Since the risk of
malignancy increases with duration of disease, being about 5% at 15 years and
increasing by 20% with each subsequent decade, yearly colonoscopy and
surveillance biopsies are recommended for every patient with ulcerative
colitis
beginning at 7 to 10 years after diagnosis.
The problem that remains to-date, however, is that polyps can be reliably
detected
only by endoscopy. Six or seven random biopsies from different regions of the
colon are usually taken at each colonoscopy. Typically, histological
preparations
1 o are made of the biopsies, constituting thin sections that are examined
under the
microscope. The microscopic image of the colon is usually quite regular and
predictable. In precancer and cancer, focal irregularities, so called lesions,
may be
found within the otherwise normal looking tissue. The pathologists then
classify the
lesions) found and accordingly, the degree of cancer development is
determined.
Dysplasia cannot be recognized with the naked eye. Thus, directed biopsy is
precluded, and sampling error is a major limitation to the efficacy of the
procedure.
The biopsy specimens themselves are, in turn, difficult for pathologists to
interpret
2 o due to the atypical cytologic changes produced by acute inflammation or
epithelial
regeneration (healing) that resemble dysplasia. Interobserver variation in
interpretation of surveillance biopsies varies by 4 to 8% among experienced
pathologists and is undoubtedly much higher among non-expert pathologists.
These
problems will be greatly alleviated or even eliminated if dysplasia can be
recognized
2 5 both grossly and microscopically.
Screening tests are different from histological biopsies. The aforementioned
endoscopic methods, such as sigmoidoscopy or entire-length colonoscopy, are
diagnostic rather than screening techniques.
Recent clinical studies document a decrease in mortality from colorectal
cancer
screening. Present techniques such as HemOccult II involve smearing a sample
of stool onto guaiac impregnated paper which, after treatment with hydrogen
peroxide containing developer, exhibits blue colour if blood, haemoglobin, is
3 5 present. After almost two decades of experience with this methodology, it
has
become clear that even in expert centres, the sensitivity is less than 50% for
curable
neoplasms, and that the positive predictive value approximates, at best, only
40%
in a clinic population. An update from the large-scale (n=97, 205) University
of
Minnesota, Minnesota, United States, prospective trial indicates a positive
predictive
4 0 value for colorectal cancer of only 2.2% when HemOccult is used in
asymptomatic
subjects, aged 50-80, with an overall disease prevalence of 0.2%. (Mandel J.
S.,
-3-
CA 02335773 2001-02-13
Gastroenterol. 1989, 97, 597.) Furthermore, factors such as medications,
multiple
dietary constituents, delays in specimen handling, variabilities in faecal
hydration,
and storage of assay materials commonly confound results. Analysis of one of
the
three randomized controlled studies assessing the value of HemOccult suggests
comparable mortality rates in the screened and control populations (Selby J.
V.,.Ann. Intern. Med. 1993, 118, 1.). Newer methods of detecting occult blood,
e.g.
methods based either on porphyrin analysis [HemoQuant] or antibody specific
for
human haemoglobin, may improve on these results.
l0 However, three limiting problems remain unlikely to be overcome. These are
that
colorectal malignancies shed blood only intermittently, upper gastrointestinal
tract
bleeding may make the results (falsely) positive, and multiple lesions in the
lower
bowel, apart from colorectal neoplasms, commonly bleed. Such lesions include
haemorrhoids, diverticulae, ulcers, and vascular ectasie. Compliance in
unselected
populations has been estimated to be less than 30%, at least partly because
the
technique requires patients themselves to smear their stool onto a slide or a
strip,
a task most people find not only distasteful, but also technically difficult.
Despite
this, HemOccult continues to be widely used because the American Cancer
Society
has recommended occult blood testing yearly for all individuals over 50 years
of
2 o age, arguing that even an imperfect test will save many lives. Implicit in
all
arguments over the value of HemOccult is that any improvement in screening
techniques for bowel malignancy would have a dramatic impact on colorectal
cancer
mortality rates from the disease, since the screening for occult blood even in
the
present form leads to reducing mortality from colorectal cancer (Mandel J.
S.,New
2 5 Engl. J. Med. 1993, 328, 1365) .
Screening for colorectal cancer by stool DNA analysis (Lancet 1992, 339, 1141
) is
based on the presence in stool of neoplastic cells shed in large numbers into
the
colonic lumen. In principle, a mutation which is common to neoplasms could be
3 0 detected with high precision by analysing DNA from these cells. Therefore,
the
existence of a detectable mutation in the colorectal tumour is a prerequisite
for
developing such a method of screening. Unfortunately, this technique can
recognize
a mutation based only on a new or altered oligonucleotide sequence, but not on
a
loss of its portion. Thus, neoplasia-related mutations based on deletion in
genes,
3 5 e.g. allele losses on chromosomes such as are commonly found in colorectal
tumours, are beyond the limits of the methodology. Currently, the most common
mutation is the K-ras oncogene mutation present, in about 40% of colorectal
carcinomas and adenomas. Screening for K-ras gene can therefore detect, at
best,
only 40% of all neoplasias. This methodology is at present technically very
complex
and expensive.
-4-
CA 02335773 2001-02-13
Screening for the presence in colonic mucin of a cancer-related disaccharide,
D-Galp((31-3)-D-GaIpNAc(a I,Ser/Thr), T-(Thomsen-Friedenreich) antigen, since
it
is widely known that T-antigen is not expressed by cells in healthy colons,
whereas
it is expressed by cancer (Boland C. R., Proc. Natl. Acad. Sci. USA 1982, 79,
2051 ).
Monoclonal antibodies and lectins:
It has been shown that monoclonal antibodies raised against synthetic T-
antigen
recognize and bind to cancer cells. Similarly, peanut agglutinin (PNA), a
lectin, binds
strongly to the same disaccharide, but recognizes malignancy with lesser
specificity.
l0 Amaranthin, a lectin from Amaranthus caudatus, has been reported to have
better
specificity for T-antigen than PNA. Neither amaranthin nor PNA bind to
histological
sections of normal mucosa, but both bind to mucin in the goblet cells of
tumours and
certain polyps, and in the transitional mucosa. The visualization of the
binding
utilizes fluorescently labelled antibodies and lectins (Rinderle S. J., J.
Biol. Chem.
1989, 264, 16123.).
Galactose oxidase test.
T-antigen is also reported to be detectable colorimetrically after oxidation
of OH-6
of galactose using galactose oxidase and visualization of the resulting
aldehyde with
2 0 Schiffs reagent (U.S. Pat. No. 4,857,457, issued Aug. 15, 1989 to
Shamsuddin et
al.). In contrast with the tests using lectins, this test is performed on
mucus samples
obtained by digital rectal examination and smeared onto a support. This system
demonstrated a sensitivity of 74% and specificity of 50% for colorectal
neoplasms,
i.e. adenomatous polyps and cancer, in one study with only 1 false negative
result
2 5 among 59 patients with cancer. Since then a number of reports of basically
the
same test have appeared with sensitivity ranging from 35% to 100% and
specificity
ranging from 15% to 76%. Some investigators found that the test was more
sensitive, but less specific, than HemOccult. The lesser specificity has been
ascribed to the positivity of test in individuals with certain inflammatory
condition,
3 o such as diverticulitis and ulcerative colitis (Sakamoto K., Cancer
Biotherapy 1993,
8, 49).
Prior art methods such as that taught in U.S. Patent No. 5416025 teach methods
for detecting the presence of cancer of the colon or rectum by treating a
sample of
3 5 colorectal mucus from the rectum of a patient with Schiff's reagent,
wherein
colouration produced in the sample indicates the presence of cancer. This
method,
however, can only indicate the presence of cancer somewhere in the colon
without
indicating the source because the mucus from various sites tend to accumulate
in
the rectum. The results of this test are likely influenced by bowl habits, the
way
4 o mucus is collected, drugs taken, etc. Most importantly however, the
results are not
proportional to the degree of cancer and will only pass a threshold reading of
-5-
CA 02335773 2001-02-13
positivity once the cancer reaches a sufficiently advanced stage of
development,
that the marker substance is released into the mucus. Moreover, the area of
the
cancerous colon that is responsible for releasing the Schiff positive marker
substance is unknown.
The current diagnostic and screening methods entail detecting the presence or
absence of a marker that collects in the mucus, stool or blood. These methods
usually are limited to provide a qualitative positive or negative reading,
which can
be significantly affected by other factors present in the colon. Moreover,
these
methods fail to provide predictive information and they do not provide
understanding
of the etiology of the disease.
-6-
CA 02335773 2001-02-13
BRIEF DESCRIPTION OF THE DRAWINGS
Table 1 presents the Plasmalogen Indices Recorded for each case. The results
from histometric measurements on individual samples from various groups are
listed, where an index of 10 to 30% plasmalogen index is considered to be
within
a normal range, an index of 30 and 40% is considered to be moderately elevated
and an index above 40% is considered to be high.
l0
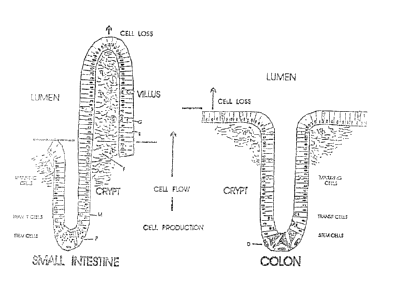
Figure 1 depicts the basic structure of the intestinal epithelium. The level
between
the intestinal lumen and the tissue surface is indicated by the horizontal
heavy lines.
In the small intestine, the epithelium is thrown into finger like projections,
the villi.
Villi are not present in the colon. In both, small intestine and colon, the
epithelium
deeps down into the tissue to form the cylindrical structures, the crypts. The
epithelium is simple columnar, it is continuous so that it covers the luminal
surface
of the tissue including the villi and it lines the crypts. The main cell type
of the
epithelium is the enterocyte that is the absorptive cell (E). The mucus-
producing
goblet cells (G) are scattered singly within the epithelium. Fibrous
connective tissue
2 0 (F) fills up the space in the core of the villi and between the crypts.
The whole
epithelium is in a continuous movement as new cells are being added to it in
the
lower half of the crypts by the cells dividing there (M). The newly added
cells are
mainly immature enterocytes which move upwards to the crypt and cease dividing
after about 3 transit divisions. They then reach the upper crypt, the
compartment
of maturing cells. They continue migrating and become mature by the time they
reach the villus orthe surface epithelium of the colon. They then continue
migrating
and function at the same time. They are lost by exfoliation after reaching the
villus
tip or the colonic surface epithelium at midpoint between adjacent crypt
openings.
The crypt base is organized differently: slender stem cells are located
between large
3 0 "nurse" cells which are the Paneth cells in the small intestine and the
deep crypt
secretory (DCS) cells (Altmann, 1990) in the colon. The stem and nurse cells
do
not participate in the upward migration of cells.
Figure 2 illustrates details of a crypt. The cells are localized in well
distinguished
3 5 compartments.
Figure 3 shows the basic concept of the renewal of the intestinal epithelium.
The
stem cells orchestrate the renewal as their divisions produce the early
transit cells
which then enter the transit compartment. Stem cell divisions also provide for
new
4 o stem cells so that the stem cell compartment is maintained for life. The
transit cell
divisions provide for daughter cells that are more mature than the parental
cells.
CA 02335773 2001-02-13
After such maturation and divisions, the daughter cells reach a
nonproliferative
stage and enter the maturing compartment where the maturation toward
functional
end cells continues.
Figure 4 illustrates the concept of precancerous hyperplasia. The primary
change
is in the stem cell compartment which is enlarged by the addition of initiated
stem
(IS) cells. These then produce their progeny of initiated cells which renew
similarly
to the normal enterocytes (compare with Figure 3). They thus go through the
transit, maturing and mature compartments and then exfoliate. The initiated
progeny coexists with the normal enterocyte population which is not affected
by the
carcinogen. One can visualize that if the IS cells enter the dormant
nonproliferative
Go stage, the initiated progeny will exfoliate and hyperplasia will regress.
After
subsequent activation of the IS cells, they will reconstitute the initiated
progeny.
Figure 5 illustrates the genesis of tumours. I. Normal crypt base. Paneth and
stem
cells alternate in a regular manner. II. Irregular crypt base. An altered
focus
appears from which one or few basophilic small cancer cells arise. I I I.
Early cancer
cell accumulation. IV. The cancer cells spread from the focus and fill most of
the
crypt base. V. Carcinoma in situ. The accumulation takes up the shape of a
2 o tumour. At the same time, streams of cells proceed toward nearby blood or
lymphatic vessels. VI. Metastasis. Some cancer cells reaching the vessels
develop secondary tumours which eventually disseminate via the circulation.
Figure 6 shows a generalized illustration of our conclusions on cancer
development.
2 5 Primarily, the stem cells are affected by various environmental agents and
they go
through various stages until finally cancer (neoplastic) stem cells arise.
Each type
of stem cells produce a different cell type and a different lesion type. There
are 2
major categories of preneoplastic (precancerous) cell types as shown. The
preneoplastic and the neoplastic stem cells are inhibited by NK cells from
producing
3 o their progeny.
Figure 7 shows the life cycle of the colonic enterocytes. From the stem cell
stage,
they proceed to deep crypt secretory cells which accumulate glycoprotein
containing
secretory granules (DCS orvacuolated cell). They soon degranulate and
transform
3 5 into enterocytes, one of the functions of which is phospholipid
production, mainly in
the apical cytoplasm, the rate of which is apparently determined by
environmental
influences. Since plasmalogens are phospholipids, the histological procedure
should not use lipid solvents. The goblet cell line is independent but it is
difficult to
distinguish goblet from DCS cells in the deep crypt region. In midcrypt and
above,
4 o the DCS cells lose granularity whereas the goblet cells retain it.
_g_
CA 02335773 2001-02-13
Figure 8 presents a drawing illustrating well developed enterocytes containing
large
amounts of plasmalogen in their apex. This figure shows that the phospholipid
formation is exaggerated in areas where promoters are accumulating. Here the
probability of cancer arising is high. The morphological signs of these areas
include
(i) enterocytes distended with lipid (lipo-, or I-enterocytes) (ii) apical
bands caused
by accumulated I-enterocytes, and (iii) elevations caused by highly active
accumulated I-enterocytes. We have called these cells also lipo-, or 1-
enterocytes
as the plasmalogen content is lipid-like. The presence of these cells mark out
cancer prone areas. Together they provide for a "band" or a "film" over the
colonic
1 o surface. Some parts of these bands also appear as elevations.
Figure 9 presents PLATE 1, comprising frozen sections from the human colon
biopsies stained with Schiff after mordanting the sections with mercuric
chloride.
There is a general pink tissue staining whereas the plasmalogen positive areas
display strong magenta colour, with typical apical bands an delevations. There
was
a patchy distribution of positive areas but their frequency was high in the
high
cancer risk colon. The three pictures on the left, display largely negative
surface
epithelium which, however, still contains about 10-20% plasmalogen. The
pictures
on the right, display highly positive surface epithelium with 40-60%
plasmalogen.
2 0 Plasmalogen bands and elevations are clearly visible. All X 75.
Figure 10 shows mouse ascending colon, fixed in Carnoy solution, embedded in
Historesin, simithin section, iron-hematoxylin (IH) stain. This region of the
mouse
colon shows clearly the three main regions of the epithelium: deep crypt
secretory
2 5 (DCS) (lower arrow), intermediate, and surface epithelial (upper arrow).
In the DCS
region, the large secretory cells (lower arrow) filled with granules are most
prominent. Occasionally a small group of narrow columnar cells without
granules
can also be made out near the crypt bottom (not shown here); these are the
stem
cells. According to the histological evidence, also shown earlier
(Altmann,1990), the
3 o immediate derivatives of the stem cells fill up with granules probably of
glycoprotein
nature. Such cells with relatively few granules have been referred to
traditionally as
vacuolated cells. In some areas, as here for example, the granules are
abundant,
in which case we refer to the cells as DCS cells. In vacuolated as well as DCS
cells,
most granules eventually exocytose. This activity is very prominent with the
DCS
3 5 cells. The lower third of crypt lumen is usually seen to fill up with the
granules.
After this exocytosis, the cells get into the intermediate zone as columnar or
transit
cells, increase in size gradually and reach the surface as surface enterocytes
(upper
arrow). X 900.
4 o Figure 11 shows mouse ascending colon fixed in osmium-permanganate.
Semithin
section stained with IH. Almost through the entire lower crypt, DCS cells are
seen
-9-
CA 02335773 2001-02-13
to be releasing granules en mass into the crypt lumen. Few cells still full of
granules
are seen to reach the midcrypt in the upper part of the picture. These cells
are
goblet cells which remain filled with granules until their exfoliation near or
at the
luminal surface of the colon. X 1000.
Figure 12 shows the same tissue as in Figure 11 but in the midcrypt position.
The
several cells retaining a few DCS granules are transit cells. The few that
still retain
full granularity are goblet cells. The goblet cells retain their morphology
after
deriving from stem cells; they form thereby a lineage separate from that of
the DCS
l0 enterocyte line. x 1000.
Figure 13 shows mouse colon frozen section stained for plasmalogen. The most
positive reaction is in the surface enterocytes. The reactive area seen as
black was
magenta in the original section. X 450.
Figure 14 shows human colon, frozen section, Schiff plasmalogen staining. Part
of
the surface epithelium is shown from a "cancer prone" area. Most plasmalogen
is
in the apical portion of enterocytes where it may form "bulbous" elevations
(B).
Many of these cells also contain remnants of DCS cell granules (D), which did
not
2 o exocytose with the rest. X 950.
Figure 15 shows human colon, frozen section, Schiff plasmalogen staining but
now
counterstained by hematoxylin to improve visible cellular detail. Clear-cut
I-enterocytes can be observed in this picture. Their apex is filled with the
lipid-like
2 5 plasmalogen. These apices face the colonic lumen (Lu). Because of this
cellular
plasmalogen accumulation, these is a plasmalogen "band" or "film" over the
colonic
surface except where goblet cells (G) are present. x 1020.
Figure 16 shows human colon fixed in Carnoy solution. Semithin section stained
3 0 with IH. Large well developed DCS cells can be seen in lower crypt. Most
reach the
crypt lumen, some are passing several secretory granules into this lumen. In
the
upper crypt, the granularity of the individual DCS cells is much reduced as
those
cells become transit cells. X 1220.
SUMMARY OF THE INVENTION
The method of this invention provides a novel approach to diagnosing and
characterizing the stage of cancer, which also assists in designing and
monitoring
4 o the course of therapy. This approach uses a histopathological biomarker,
the
activated enterocyte (I-enterocyte), to accurately characterize the status of
cancer
-10-
CA 02335773 2001-02-13
development with more accuracy and efficiency than standard techniques, and
can
even enable the determination of the potential of cancer to develop in
response to
carcinogens.
Another object of the present invention is to provide a diagnostic test for
colorectal
carcinoma which detects a biochemical change, such as the accumulation of
plasmalogen in activated enterocytes, that is associated with the stage of
cancer
development. According to Webster Third New International Dictionary,
plasmalogen is a phosphatide that is the precursor of plasmal in tissue.
to
A further object of the present invention is to provide a kit by means of
which such
tests can be conducted to determine the stage of cancer development.
It is a further object of this invention to provide a method to determine the
effects
of different therapeutic regimens that will enable one to design the most
effective
course of treatment for a patient.
In a further embodiment, the method of this invention provides a means for
studying
the underlying cause/changes that lead to cancer.
In yet another embodiment, the method of this invention provides a means to
identify pre-cancer point or individuals with likely propensity to develop
cancer.
In yet a further embodiment, this invention provides an intermediate endpoint
which
2 5 can be used as an indicator of health and the likelihood of developing of
cancer and
the effect of a prophylactic therapy.
Another embodiment of this invention can be used to develop animal models in
which the effects of agents, genes, environmental factors, can be researched
via
3 o their effects on the intermediate endpoint.
Upon study of the specification and appended claims, further objects, features
and
advantages of the present invention will become more fully apparent to those
skilled
in the art to which this invention pertains.
DETAILED DESCRIPTION OF THE INVENTION
The first object of the present invention is a new and efficient method of
determining
4 o whether or not an animal or a human is at risk of developing a pathology
such as
a cancer pathology and more particularly a colorectal cancer pathology. This
-11-
CA 02335773 2001-02-13
method comprises the steps of
(i) obtaining a sample from an animal or from a human source,
which sample is preferably selected in the group constituted by
samples of intestinal tissue, mucus samples and samples of
washings collected from the colon;
(ii) determining if said sample comprises activated I-enterocytes; and
l0 (iii) concluding if the animal or human is at risk of developing
colorectal cancer.
A second object of the present invention is a method of determining whether or
not
an individual is at risk of developing a colorectal cancer comprising the
steps of
(i) obtaining a sample from the individual;
(ii) determining if said sample comprises activated I-enterocytes; and
(iii) concluding if the individual is at risk of developing colorectal
cancer.
2 o According to a preferred embodiment of the invention, in step (i), the
sample is a
sample of epithelial tissue such as samples of intestinal tissues, mucus
samples
and samples of washings collected from the colon. The samples are preferably
recovered by biopsy, by surgical excision or by mucosal scrapping.
2 5 According to a further preferred embodiment, in step (ii), the activated I-
enterocytes
is selected in the group constituted by I-enterocytes with altered morphology.
This
altered
I-enterocytes may be I-enterocytes with aprical abnormalities such as I-
enterocytes
with exaggerated phospholipid formation in areas wherein promoters of cancer
3 0 pathology, preferably in areas wherein cancer promoters are accumulating.
According to a preferential embodiment of the invention, the intestinal tissue
which
is recovered in step (ii) by biopsy is embedded into paraffin or plastic
blocks and
then cut into thick sections, preferably cut into about 5 micrometer thick
sections.
According to another preferred embodiment the thick sections recovered in step
(ii)
are mounted on glass slides, stained by immersing them into specific staining
solutions and then examined under the microscope for the presence of activated
I-enterocytes.
The thick sections are advantageously prepared and treated by mild acid
hydrolysis
-12
CA 02335773 2001-02-13
or by dilute solutions of mercuric chloride before characterisation with a
Schiff's
reagent or with a modified Schiff's reagent in order to obtain an activated
I-enterocytes biomarker.
A preferential modified Schiff's reagent is obtained from a traditional
fuchsin solution
by the following 15 steps process:
1. Filtering the fuchsin solution through pleated filter paper and collect
in a 1000 mL Erlenmyer flask
l0
2. Measuring 68 mL of IN Hydrochloric acid. Record actual quantity
above.
3. Adding 1 N hydrochloric acid to the solution while stirring. Allowing
to dissolve completely. Letting solution cool to room temperature.
Recording temperature.
4. Weighting out 4.68 g of sodium bisulfite. Recording actual quantity
above.
5. Adding the sodium bisulfite while stirring. Allow to dissolve
completely.
6. Covering the Erlenmyer flask with parafilm and store in a dark
cupboard at room temperature for 4 days.
7. Confirming colour of solution, light straw colour.
8. Weighting out 0.60 g of decolorizing charcoal. Recording actual
3 o quantity above.
9. Adding the charcoal to the solution. Stir for 1-2 minutes.
10. Filtering solution into a 500 mL graduated cylinder through two
3 5 Whatman filters. The bottom filter is a disc and top filter is pleated.
Stir as required.
11. Measuring volume. Recording the volume.
4 0 12. Measuring the pH, (it should be approximately 1.1 ). Recording the
pH.
-13-
CA 02335773 2001-02-13
13. Transfering solution into 500 mL brown glass bottle. Close bottle
with screw cap. Label with name (Schiff Reagent), date, and lot
number.
14. Labelling bottle as "Quarantined" and storing the bottle in a
refrigerator at 2-6 °C.
15. Thirty days after production is completed, taking of samples and
performing of QC release tests.
l0
The corresponding modified Schiff's reagent thereby obtained, may still be
generally
qualified as a Schiff's reagent which is characterised by an enhanced storage
stability and colour development ability.
According to the present method, it is possible in step (iii) to conclude that
the
animal or human is classified at risk of developing a pathology if a deep
magenta
colouring may be detected in any part of the sample prepared in step (ii) and
to
conclude that the individual is classified at no risk of developing cancer if
no deep
magenta may be detected in the sample prepared in step (ii).
A third object of the present invention is constituted by a new method of
determining
the stage a pathology in an animal or human with a pathology such as neoplasia
or
such as a cancer pathology(for example a cancer pathology). This method
comprises the steps of
2 5 (i) obtaining a sample from the animal or from the human, which
sample is selected in the group constituted by samples of intestinal
tissue, mucus samples and samples of washings collected from the
colon;
3 0 (ii) determining if said sample comprises elevated levels of activated
I-enterocytes; and
(iii) quantifying the elevated level of activated I-enterocytes and
correlating said elevated level of activated I-enterocytes to the stage
3 5 of the pathology.
According to a preferred embodiment, the sample of intestinal tissue is, after
staining by a Schiff's reagent or after staining by a modified Schiff's
reagent,
evaluated for its level of activated I-enterocytes which is proportional to
the
4 o percentage area of the sample showing a deep magenta colouring.
-14-
CA 02335773 2001-02-13
According to another preferred embodiment, the level of activated I-
enterocytes is
qualified of high if more than 30 % of the area of the sample shows a deep
magenta
colouring.
A fourth object of the present invention is constituted by a new method of
determining the localisation and the stage of a pathology in an animal or
human:
(i) obtaining samples from different and identified parts of the
animal or human, which samples are selected in the group
constituted by samples of epithelial tissues such as intestinal tissues,
mucus samples and samples of washings collected from the colon;
(ii) determining which of the samples collected in step (i) comprise
activated I-enterocytes;
(iii) quantifying the level of activated I-enterocytes present in the
samples identified in step (ii) as comprising activated I-enterocytes;
and
2 0 (iv) correlating the results obtained in step (iii) to the localisation
and to the stage of the pathology.
A fifth object of the present invention is a new method of determining the
risk for a
patient to develop a pathology such as a cancer pathology (for example
neoplasia
2 5 or colorectal cancer). This method comprises the steps of
(i) obtaining a sample of an epithelial tissue from the animal or from
the human;
3 0 (ii) determining if said sample comprises elevated levels of
plasmalogen in the intestinal epithelial tissue;
(iii) correlating said elevated level of plasmalogen to observations
made in normal non-initiated tissue; and
(iv) quantifying the stage of pathology if the individual has developed
pathology.
A sixth object of the present invention is a kit for working one of the five
above
4 o defined method of diagnostic. This kit comprises:
-15-
CA 02335773 2001-02-13
(i) at least one reagent for visualising the presence of plasmalogen
(preferably the presence of I-enterocytes) in the sample(s); and
(ii) a reagent for identifying the presence of activated I-enterocytes
in the sample(s).
According to a preferred embodiment of the kit, the reagent for visualising
the
presence activated I-enterocytes in the samples) is a mild acid or dilute
mercuric
chloride.
A seventh object of the present invention is a kit for working any of the
above
defined diagnostic methods. This kit comprises at least one reagent for
oxydizing
said tissue; and a Schiff's reagent or a modified Schiff's reagent.
The reagent for oxidising said tissue is preferably selected in the group
constituted
by inorganic acids, mercuric chlorides and solutions (preferably aqueous)
solutions
thereof.
This kit may be advantageously completed by a third component which is
2 0 instructions for working the diagnostic method.
A eight object of the present invention is the use of the above defined
methods of
the invention as a tool to investigate pathologic development such as pre-
cancerous
states in animal and cell culture models of disease.
The method of this invention provides a novel approach to diagnosing and
characterizing the stage of cancer, which also assists in designing and
monitoring
the course of therapy. This approach uses a histopathological biomarker, the
activated enterocyte (I-enterocyte) in the intestinal epithelium, to
characterize the
3 0 status of cancer development with more accuracy and efficiency than
standard
techniques, and can even enable the determination of the potential of cancer
to
develop in response to carcinogens. This biomarker derives from the following
model of carcinogenesis.
3 5 According to this model, carcinogens do not cause cancer directly. Rather,
they
establish a permanent set of enterocytes in the intestinal epithelium, that is
slightly
different from the normal ones but being still functional as well as
nonmalignant.
These cells are called initiated cells. Their presence causes no ill effects.
However,
these cells can respond to another set of environmental substances, the
promoters,
4 0 by becoming cells responsive to mutagens. Some of these in the presence of
mutagens may transform into typical cancer cells. While initiation does not
-16-
CA 02335773 2001-02-13
necessarily lead to cancer, cancer development necessarily involves the prior
sequence of initiation, promotion, and then mutagenesis. The method of this
invention uses various techniques to determine the presence of activated I-
enterocytes which enables one skilled in the art to define the status of
cancer
development with more accuracy and efficiency, in addition to providing a
multifaceted understanding of the progression of the disease in accordance
with this
model, which is explained in further detail below.
The method of the invention derives from the following model of cancer
l0 development, which is a unified view of the sequence of events from
initiation and
latency period to metastatic lesions development (Altmann, G., Epith. Cell
Biol.,
1995). The histopathological marker enables the method of this invention is a
method of staining the cellular tissue to evidence the activated I-
enterocytes,
primarily by determining the elevated levels of plasmalogen contained within
the
enterocytes, indicating a cellular status representing stages of cancer
development
that are relative to this model.
In particular, this invention provides a method for indicating a high or low
risk of
developing cancer at specific sites along the colon. The method measures the
2 0 presence and the intensity of a "promoting environment," a region of
intestinal
epithelial cells which are biochemically programed as activated enterocytes to
develop cancer in response to certain signals. The model of this invention
predicts
that cancer can develop only in such an environment when the promoting
influence
is sufficiently intense.
At present, there are no clinical methods of testing for the presence of this
environment. The method of this invention, provides a means for determining
the
presence of this type of environment by visualizing the activated enterocytes
of
intestinal epithelial tissue and correlating the signal measured to the
propensity to
3 0 develop cancer.
There are two important results obtainable from this kind of in situ test; 1 )
the
degree of staining is proportional to the propensity to develop cancer, so
this
method can be used both qualitatively and quantitatively; and 2) the
distribution of
3 5 positivity is not uniform along the epithelium, so this method can be used
to indicate
regions of the colon at risk to develop cancer. Thus, this method can be
conducted
in vitro, analysing histological samples obtained from routine biopsies.
In one embodiment, the method entails treating a frozen or chemically fixed
tissue
4 0 biopsy slice with a solution that enables visualization of plasmalogens in
the tissue,
by either mild acid hydrolysis or by treatment with a dilute solution of
mercuric
-17-
CA 02335773 2001-02-13
chloride to liberate aldehydes which are then reacted with a Schiff reagent.
One
skilled in the art would appreciate the many means possible for first
oxidizing the
cellular material prior to reacting it with a Schiff reagent. This method can
be used
both qualitatively and quantitatively.
A Model of Carcinogenesis
Carcinogenesis, or cancer development, involves the essential stages of
initiation,
promotion, and progression. The basis of initiation seems to be the
establishment
of initiated stem cells which appears to be a high probability event involving
most
if not all crypts along the entire target organ of DMH, the whole intestinal
tract. A
genetic factor seems to be involved apparently regarding the nature of the
stem
cells, whether they are responsive or not to a carcinogen.
Stem cells are proliferative "transit" or "progenitor" cells which further
differentiate
as well as divide, then become nonproliferative maturing cells. These then
mature
into functional "end" cells. Although stem cells represent a small percentage
(0.1 -
0.01 %) of a renewing cell population, they are essential for the maintenance
of this
population as they renew themselves as well as produce the earliest cell types
that
2 o become the renewing cell population itself. In the intestinal epithelium,
the stem
cells are at the bottom of the crypts and the transit cells occupy about the
lower half
of the crypts (Figures1 and 2). The transit cells mature as they divide and
after a
set number of divisions, they reach a stage when divisions cease but
maturation
continues. These nonproliferative maturing cells occupy the upper half of the
2 5 crypts. By the time they reach the surface epithelium, they become mature
"end"
cells which exfoliate after functioning for 3-5 days. There are thus at least
four
compartments, stem, transit, maturing, and mature (functional) in a renewing
cell
population (Figure 3). The same tissue may have more than one renewing system.
3 0 The stem cells are the ultimate source of renewal as their proliferation
results in the
self-renewal of the stem cell population itself as well as in the production
of the
earliest transit cells. These transit cells divide about three times, each
division
resulting in a pair of daughter cells more differentiated than the parent
cell. Transit
cells therefore cannot maintain their own compartment, rather their renewal is
3 5 provided by the output of early transit cells from the stem cell
compartment. If the
divisions of the stem cells cease for any reason, that is the stem cell
compartment
is inactivated, the transit cell population eventually depletes, renewal stops
and
epithelial denudation follows as a result.
4 o Stem cell function may naturally cease by the stem cells entering a
resting or
dormant phase. This is prevalent in slowly renewing tissues such as liver or
muscle
-18-
CA 02335773 2001-02-13
where the functional cells are long lived and need to be replaced mostly in
case of
injury, at which time, the dormant stem cells are reactivated. In the case of
rapidly
renewing cell populations, such as the intestinal epithelium, most stem cells
are
believed to be active. External influences seem to determine whether stem
cells are
in the active or in the dormant state.
Hyperplasias are one of the earliest detectable lesions of precancer. There
are two
aspects of hyperplasia sometimes evaluated separately: 1) expansion of the
cell
content of a particular cell population of the tissue; and 2) an increase in
the
l0 proliferation rate, where the surge of proliferation has been considered to
be a
prerequisite of cancerous transformation. In the current model, hyperplasia is
caused by the appearance of additional cells in the renewing population and
the
pattern of renewal of these new cells is similar to normal. Consequently,
aside from
the overall enlargement of the tissue, there is no change in the relative
content of
the component cell populations and there is no disturbance in regular tissue
architecture. The appearance of such added cells requires that the transit
cells go
through more than their set number of divisions or alternatively, new active
stem
cells make their appearance. In fact, there are new stem cells, "initiated
stem" (IS)
cells associated with the new population. They apparently arise from
proliferation
2 0 of normal stem cells which add the additional mitosis. Hyperplasia is thus
visualized
as an increase in all compartments of renewal, each compartment having an
additional "initiated" component deriving from the newly added IS cells
(Figure 4).
These cells are thus present all along the small and large intestines and
follow the
signals for renewal, that is, they migrate, differentiate and exfoliate. It is
also
2 5 important to note that they coexist with normal enterocytes, and that it
is possible
to measure the number of initiated cells by the degree of hyperplasia.
The natural killer (NK) cells are unique lymphocyte-like cells of the immune
system,
that have the ability to kill cancer and virus-infected cells. They react
against not
3 0 only cancer cells but also against the still noncancerous initiated and
promoted
cells. Under normal physiological conditions, the NK cells render all the
three
aberrant stem cell types nonproliferative. This state of the stem cells is
referred to
as "dormant" or "latent". The NK cells secrete a paracrine factor which can
bring
all three populations, precursor I and II and cancerous, into remission so
that only
3 5 the stem cells survive in a harmless nonproliferative form. This factor is
referred to
as the remission factor. In the active state, the NK cells kill the initiated
and the
other aberrant cell types including their stem cells. NK cells are routinely
activated
by the cytokines interferon gamma or interleukin 2.
4 0 Hyperplasia is the lesion found to represent initiation by being composed
of initiated
enterocytes, which are the progeny of IS cells. A major control mechanism
-19-
CA 02335773 2001-02-13
regulating the presence of this progeny comes from the NK cells which, if not
suppressed, cause the dormancy of IS cells. Such dormant IS cells are the
characteristic but elusive structures present in the otherwise normal tissue
during
the latency period ensuing initiation. They are responsible for the "memory"
of the
initial carcinogenic insult but they are not detectable by existing
pathological tests.
It appears that individuals may accumulate such dormant IS cells during their
life
span, that is being in the initiated state, with no ill effects.
While the IS cells constitute the basis for further progression, only a few
may be
l0 involved, most remaining in dormancy. A pivotal local event in stimulating
dormant
IS cells appeared to be promotion. Promotion may be defined as a local mitotic
stimulus on the IS cells. Under this stimulus, some mutagen-sensitive stem
cells
may arise, the PS cells, which may also give rise to local precancerous
lesions. The
PS cells may be much less frequent than the IS cells but still of high enough
frequency which typical mutation could not produce. Promoters act by
activating the
proliferation of dormant IS cells, and this activation results in a
nonmalignant
transformation of some. It appears that this transformation may not be a
mutation
but rather a switch in stem cell regulation.
2 0 The progression stage involves the neoplastic transformation which can be
elicited
rapidly in initiated animals. The initiated state, previous promotion,
mutagens, and
NK inhibition are necessary for this to occur. Apparently, IS cells sensitized
by
promoters, that is PS cells, transform into NS cells. This time a very few
cells are
involved and true mutational events are probable. The connecting link between
2 5 these lesions is their stem cells which can exist at least at three main
levels of
transformation. Whether or not these three stem cell types remain dormant or
are
activated to form lesions is dependent on environmental factors including a
factor
from the NK cells. A strong influence from the NK cells is capable of keeping
most
if not all of the transformed stem cells in the dormant state. Three basic
stem cell
3 0 types may be involved and all are under a strong control derived from the
NK cells.
The renewal of the colonic epithelium is known to start in the deep crypt from
stem
cells which produce still proliferative transit cells. These gradually develop
into non-
proliferative surface absorptive cells which exfoliate after functioning for 4
- 5 days.
3 5 The first transit cells arise from stem cells accumulate glycoprotein
granules which
then gradually release into the crypt lumen. This process is referred to as
deep
crypt secretion, and the cells accomplishing this task have been named deep
crypt
secretory cells (DCS).
4 o DCS, transit, and surface epithelial cells make up the epithelial
continuum and
represent the three major phenotypes of epithelial cells. The goblet cells
arise
-20-
CA 02335773 2001-02-13
separately from stem cells and continue to migrate as such to the surface.
These
cells produce large quantities of phospholipids. This production is increased
in the
promoted state as shown after feeding promoter to the animals. The mechanism
appears to be as follows: under the influence of the promoter, a large number
of
DCS cells form but they soon degranulate and thus become transit and then
surface
cells. The extent of the phospholipid producing epithelium is much increased.
Positivity is shown among some stem and progenitor cells in the crypt and as
these
cells approach the surface, they accumulate more plasmalogens. Some cells in
high positive cases clearly overproduce this substance. This model predicts
that at
some stage of an enterocyte life cycle, large cytoplasmic areas switch from
glycoprotein production to lipid production and this process is enhanced in
cancer
prone individuals. This is referred to as the "lipid switch".
In this model, cancer does not arise directly from the normal cells. Rather,
there are
at least two groups of precursor cell populations: precursor cell type I or
"initiated",
and precursor cell type II or "promoted". The initiated cells arise after
brief exposure
to carcinogen, in its target organ. They have minor alterations but are still
functional;
they are additional to normal type cells, mingled with them adding 10-20% to
the cell
2 0 number comprised by the normal cells. Thereby, they cause an increase in
tissue
dimensions (hyperplasia) under repeated exposures to high concentrations of
promoters, some initiated cells transform into promoted cells which form
structures
that are still nonmalignant but posses various degrees of typia. Few promoted
cells
may transform into cancer cells upon the influence of mutagens. There is thus
a line
2 5 of transformation from normal cells to initiated, promoted and then
cancerous ones
under environmental influences. Transformations take place primarily at the
level
of stem cells. Only stem cells can populate or repopulate cell populations.
There are
thus three groups of aberrant stem cell types: initiated ( type I), promoted
(type II),
and neoplastic or cancerous (type III). They produce the following populations
of
3 0 enterocytes, respectively: hyperplastic (i.e. additional to normal),
atypic
(premalignant), and neoplastic (malignant or cancerous).
The nature of the latent period precedes cancer. Essentially, the latent
period is the
presence of aberrant stem cells in their latent form while no obvious lesions
can be
3 5 demonstrated. Stem cells in general are few (about one among 1000-10000
functional cells) and inconspicuous so that the latent period appears to be
fully
normal, the presence of a few aberrant stem cells cannot be demonstrated by
routine pathological methods. Stage I of the latent period is when type I stem
cells
are only present. Stage II refers to the situation when type II stem cells are
also
4 o present and stage III refers to the presence of latent cancer (type III)
stem cells.
Stages II and III are actually remission states as the stem cells sources of
-21-
CA 02335773 2001-02-13
premalignant and/or malignant lesions are kept at bay. only when this rather
effective immune control is breached at some location, cancer, which is a
localized
lesion, may develop.
In contrast to known diagnostic and screening techniques wherein the
manifestation
of cancer is observed from the perspective of the neoplastic growth, the
method of
this invention, diagnoses cancer from the point of view of the source of the
neoplastic growth according to the model presented herein. Accordingly, the
method of this invention uses biomarkers associated with stem cell progeny to
l0 analyse mammalian tissue samples for indications of the biological health
status of
stem cells. A key aspect of this invention draws upon the relationship between
stem
cells and their progeny vis-a-vis carcinogenesis; the aberrant stem cells give
rise
to foci of neoplastic growth, which provides biomarkers that can be detected
as an
indicator of aberrant stem cells.
Due to its focus on the source of neoplastic growth, the method of this
invention can
also be used to provide a means for studying the underlying cause/changes that
lead to the development of cancer. It enables one to develop early diagnostic
method to identify pre-cancer point or individuals with likely propensity to
develop
2 0 cancer. This method also provides an intermediate endpoint which can be
used as
an indicator of health and the likelihood of developing of cancer and the
effect of a
prophylactic therapy in addition to enabling one to develop animal models in
which
the effects of agents, genes, environmental factors, can be researched via
their
effects on the intermediate endpoint.
Given the traditional approach of diagnostic methodologies, it is truly
surprising that
specific histopathological markers could be determined and correlated a stage
of
cancer development with emphasis on the precancerous state. The new
biomarkers are based on the observation that these focal changes are preceded
by
3 0 and/or coexist with subtle changes in the tissue housing the lesions.
Thus, the method of this invention comprises using biomarkers to predict the
likelihood of cancer development according to the model presented herein, in
the
intestinal epithelium, which is the location of more than 90% of all cancers
in the
3 5 intestine. In this model, cancer, however scarce it may be in relation to
total tissue
mass, develops in relatively widespread precancerous populations present
within
the normal tissue side by side with normal cells. These populations arise from
altered stem cells and possess some specific morphological and/or chemical
features by which they can be recognized. Each of these specific population
fits into
4 0 and characterizes a stage of carcinogenesis.
-22-
CA 02335773 2001-02-13
These stages are defined and are shown to arise in genetically predisposed
individuals exposed to a particular group of environmental substances as shown
in
Figure 6. Under the influence of carcinogens, the normal stem cells give rise
to
initiated stem (IS) cells. This high probability event takes place all along
the
intestinal tract. In some intestinal areas under the influence of promoters,
some
initiated stem cells transform into preneoplastic stem (PS) cells some of
which in
turn are prone to mutate and thereby to transform into neoplastic stem (NS)
cells
under the influence of mutagens. This last transformation is a low probability
event.
All these aberrant stem cells are recognized by the progenies they form and
also
l0 by the lesions the progenies may form. The NK cells were found to affect
the
aberrant stem cells, not the normal ones. They can elicit the entrance of the
aberrant stem cells into the Go stage so that these stem cells are inhibited
from
forming progenies or lesions even though they are present. This inhibitory
influence
is exerted by a factor produced by the NK cells. A further effect of the NK
cells is
the ability to kill the aberrant stem cells when activated by cytokines. The
aberrant
stem cells and their progeny are thus NK-dependent.
Lipid materials gradually accumulate in the enterocytes as they migrated along
the
crypts toward the colonic luminal surface. This accumulation occurs mainly in
the
2 o apical cytoplasm of the enterocytes but could also take place in other
cytoplasmic
areas (e.g. infranuclear areas). In the course of the natural cell renewal
process,
these lipid material containing enterocytes reached the colon surface and
eventually
exfoliate into the colon lumen. They then break up and release their lipid
material
which then becomes part of the phospholipid content of the colonic mucus.
Distinct
2 5 glycoprotein containing mucus cells (goblet cells) also exfoliate and
contribute to the
glycoprotein portion of the colonic mucus. A third type of mucus secretory
cell
occupies most of the deep portions of the crypts: the "deep crypt secretory"
(DCS)
cells. These cells contain large numbers of prominent secretory granules which
are
secreted into the deep crypt lumen. These granules stain at least partially by
the
3 o PAS (periodic acid Schiff) method, therefore they contain glycoprotein.
The interrelation between stem cells, enterocytes, DCS and goblet cells is
illustrated
in Figure 7. The stem cells in the deep crypts first transform into cells
containing
numerous large secretory granules, that is DCS cells. On reaching about the
3 5 midcrypt level, most granules are expelled ("degranulation"), the cells
proliferate and
then start to produce the phospholipids in the cytoplasm left free by the
expelled
granules. Electron microscopic evidence indicates that the endoplasmic
reticulum
is the site of the lipid material production, the lipid material being
released and
contained in small granules. Around the midcrypt level a metabolic switch
takes
4 0 place in enterocytes switching from glycoprotein to lipid material
synthesis. This
event is prominent morphologically.
-23-
CA 02335773 2001-02-13
Some areas, especially around tumours, contain enterocytes with a high content
of
lipid materials, referred to as lipo-enterocytes or I-enterocytes (Figure 8).
Areas with
a high probability of developing cancer show the lipo-enterocytes and
associated
pathological landmarks. These areas appear to be under the influence of a high
concentration of promoters and therefore, the development of cancer is highly
probable following exposure to promoters. The high lipo-enterocyte content
causes
specific histopathological landmarks (Figure 8) such as a surface band in
epithelium
positive for Schiff and showing special colour, prominent elevations on the
surface
of the epithelium. Further histological evidence showed that in highly
promoted
1 o areas, stem cell number increases and the rate of formation of DCS cells
is also
elevated. These cells degranulate relatively early while still in the lower
crypt.
Consequently, large number of lipid material producing enterocytes are
produced
which spend more than average time in the crypts and therefore accumulate more
than average amounts of lipid material. A high rate of DCS cell degranulation
and
the early metabolic switch from DCS cells to I-enterocytes are additional
pathological markers of highly promoted areas.
The Method of the Invention
2 o The present invention provides a means for assessing or identifying the
stage of
carcinogenesis for intestinal epithelial tissue. The sample used to use such
method,
may be a tissue sample such as biopsy regularly taken during colonoscopic
examinations. There is a potential that the method can be developed for mucus
samples, or for washings collected from the colon.
The first step of this method entails obtaining epithelial biopsies from
several
regions of the colon. From the biopsies, histological preparations are made
using
standard procedures well known to one skilled in the art. Routinely the
biopsies are
embedded into paraffin or plastic blocks and then cut into about 5 micrometer
thick
3 0 sections. These sections are then mounted on glass slides, stained by
immersing
them into specific staining solutions and then examining them under the
microscope.
The Activated I-Enterocyte Biomarker
This biomarker is promoted in high cancer risk areas and is recognized by the
presence of active lipo-enterocytes and the associated pathological signs
(elevations, early metabolic switch, apical band). This biomarker requires
specific
histological preparation of the samples so that lipids are preserved and then
stained
4 o specifically. Frozen sections are prepared and treated by mild acid
hydrolysis or by
dilute solution of mercuric chloride. Aldehydes are liberated from the lipid
materials
-24-
CA 02335773 2001-02-13
in the lipo-enterocytes which are then reacted with Schiff reagent. The
mercuric
pretreatment has a further advantage in that it leaves mercuric ions built
into the
lipid material. The lipid material can then be visualized thereafter by any of
the
conventional mercury stains. The lipo-enterocytes stained by the Schiff
reagent
have provided special pathological markers which in turn allow for the
recognition
of high risk sites as described earlier. The Schiff positive atypical band is
most
conspicuous and the elevations are most frequent in highly positive areas of
the
tissue. In such positive sites, the changeover from DCS cells to lipo-
enterocytes
takes place lower in the crypt than normal, i.e. earlier in the process of
cell renewal.
1 o The site of the changeover is thus lower in the crypts that normal. These
crypts
also have a higher density of lipo-enterocytes towards the surface so that
goblet
cells tend to exfoliate in these areas while still in the upper crypt. These
markers can
therefore be used in the recognition of "promoted" or "high risk" sites.
This biomarker can also be expressed in a quantitative manner as the percent
area
occupied by Schiff positive lipid material within the boundaries of the
epithelium in
representative histological sections. There is a normal level of expression
which is
around 15-20%, and there is an abnormal level of expression in promoted areas,
approaching twice the normal level of expression. These percent values can be
2 o determined by computerized analysis of microscopic images. In highly
positive
areas, there is also a higher density of staining and there is some shift in
the colour.
Higher percent values indicate stronger promoting influence and therefore a
higher
risk of the patient developing cancer. The observations demonstrate that
enterocytes under stimulation by promoters or promoter-like substances produce
2 5 higher than normal lipid material in proportion to the intensity of the
stimulus. The
amount and intensity of the Schiff positive content of the enterocytes of the
surface
epithelium thus reflects the intensity of the stimulus. In the microscopic
images
using representative segments of the surface epithelium are outlined. Also,
the
area of the Schiff positive regions within the epithelium is outlined. The
ratio of the
3 0 two types of areas is then expressed as a percentage, or in any other
arbitrary units.
This can be done by any method of image analysis, including computerized ones.
An index of positivity can thereby be constructed. Measurements of staining
intensity can also be included in such an index. The staining of the
intestinal tissue
by the Schiff reagent imparts a pink colour to the tissue, including the
epithelium.
3 5 The truly Schiff positive areas stain magenta and deep magenta in the high
density
areas. Such colour differences can be accented by computerized methods.
The activated I-enterocyte is a histopathological marker requiring that tissue
samples or biopsies be taken mainly from the epithelial lining of the
intestine.
4 o These samples are to undergo histological processing and the histological
preparations then are subjected to microscopic analysis. This analysis may be
-25-
CA 02335773 2001-02-13
carried out through the microscope or on the photographic or computerized
image
of the sample made under the microscope. Routine analysis of biopsies with the
staining for lipid materials can mark out the high cancer-probability areas in
the
colon as shown in Figure 9. Image analysis can also assign a numerical value
to
these samples expressing the relative proportion and intensity of lipid
material
containing areas in the epithelium.
The Method Embodied in a Kit
l0 Kits to work the method are also embodiments of this invention. The
materials for
use in the method of the invention are ideally suited for the preparation of a
kit.
Such a kit may comprise a carrier means compartmentalized to receive in close
confinement one or more container means, such as vials, tubes, and the like,
each
of the container means comprising one of the separate elements to be used in
the
method. Components of the kits would include specific materials necessary to
work
the methods. Reagents for fixing the tissue samples would be included in the
kits.
For example, the kits could contain, within separate container means,
fixatives such
as a dilute acid or mercuric chloride fixatives, that enable chemical
preparation of
the histological sections. In an alternative procedure, the biopsies can be
frozen,
2 o so kits using this procedure would not contain chemical fixatives.
The frozen or chemically fixed histological sections are placed on specially
coated
slides to preserve their integrity during processing, so specially coated
slides that
would sufficiently adhere the samples to the glass slide would be provided.
Plastic
2 5 or polymer slides may also be used.
Various pre-measured reagents would be provided within separate container
means. One example of such reagents is a specialized Schiff reagent, which
would
be standardized so that optimal and standard colours yield upon development.
3 0 Another reagent would be the silver intensifier solutions and/or physical
and
chemical developer solutions would be provided, which surround the mercury
built
into the tissue (at lipid material sites) with silver atoms. This is an
alternative way
to visualize the lipid material. Alternatively, other solutions may be
provided which
provide mercury-staining. Newly developed stains might also be included in
these
3 5 kits.
Finally, material items such as slides, cover slips and mounting media would
be
provided to convert the stained slides to permanent preparations.
4 o Advantages of the Invention
-26-
CA 02335773 2001-02-13
The method of this invention is particularly important for determining the
promotion
stage of cancer development, as demonstrated in Figure 9. Current techniques
can
only determine the presence of cancer and determine its stage of development
to
some extent. In contrast, the method of this invention provides a means to
determine the propensity of the tissue to develop cancer in addition to
providing
information relative to the particular etiology in that patient.
This method of using biomarkers to assess the biological status of cancer
development: 1 ) enables one to study the effects of different therapeutic
regimes
l0 to design the most effective course of chemo-therapeutic or radio-
therapeutic
treatment for a patient; 2) provides a means for studying the underlying
cause/changes that lead to the development of cancer; 3) enables one to
develop
early diagnostic method to identify pre-cancer point or individuals with
likely
propensity to develop cancer; 4) provides an intermediate endpoint which can
be
used as an indicator of health and the likelihood of developing of cancer and
the
effect of a prophylactic therapy; 5) enables one to develop animal models in
which
the effects of agents, genes, environmental factors, can be researched via
their
effects on the intermediate endpoint.
2 o Moreover, the method ofthis invention allows the practitioner to
distinguish between
cellular changes associated with inflammatory diseases which are not life-
threatening and carcinomatous changes which are life-threatening or may
progress
to be life-threatening.
2 5 To assist in understanding the current invention, the following non-
limiting examples
are provided. The following examples should not be construed as specifically
limiting the present invention, variations presently known or later developed,
which
would be in the understanding of one skilled in the art and considered to fall
within
the scope of the present invention as described herein.
EXAMPLES
EXAMPLE I: PREPARATION OF FRESH COLONIC BIOPSIES FOR LIGHT
MICROSCOPIC EXAMINATION
Biopsies are frozen on dry ice and sectioned by cryotome in proper
longitudinal
orientation. The sections are mounted on glass slides and stored in the
freezer.
-27-
CA 02335773 2001-02-13
To stain, the slides are taken from the freezer and thawed briefly. The
sections are
treated with a proper mordanting solution that oxidise specific lipids so that
some
aldehyde compounds are released to allow for staining with Schiff reagent. The
sections are mordanted with 1 % mercuric chloride or with 6M hydrochloric acid
for
about one minute, followed by rinsing. The sections are stained with Schiff
reagent
for approximately five minutes. The colour is developed in running water for
15
minutes. The samples are then rinsed, dried and a cover slip is applied for
examination.
EXAMPLE II : DEMONSTRATING THE USE OF THE ACTIVATED
L-ENTEROCYTE BIOMARKER
The method using biomarker 3 was employed in a study involving 80 patients,
wherein an average of 4 frozen samples were collected from each case. The
frozen
sections were stained using the method of the invention and analysed. Among
the
80 patients who had former colon cancer, who were having colon cancer and
those
with frequent occurrence of colon cancer in the family, all showed biopsies
which
were predominantly positive for biomarker 3. This study demonstrates that a
positive reading indicates "the proneness" to develop cancer.
Biopsies were taken from the colonic mucosa at various regions of the colon.
The
biopsy is a piece of the mucosa of about 1-2 mm diameter. The biopsy is
dropped
into prepared fixative such as formalin.
2 5 In this example, the biopsy was placed flattened into O.C.T. compound in a
small
aluminum dish and was then quickly frozen on dry ice. More O.C.T. compound was
then added to enlarge the frozen block. Using a freezing microtome, 8-10
micrometer thick sections were then cut and placed on a glass slide and the
whole
preparation was then kept frozen.
In preparation for staining, the slides were quickly thawed and one drop of 1%
mercuric chloride solution was placed over the sections for 1 minute. The
slides
were quickly rinsed and then stained in Schiff solution for 10 minutes. The
slides
-28-
CA 02335773 2001-02-13
were then placed in running tap water for 10 minutes and then dried and cover
slipped.
The resulting slides were examined under the microscope and the relevant
images
were captured in an image analysis software for later analysis. Using the
markers
listed earlier, the samples were assessed either as positive or negative and
compared to the diagnosis made by other means by the gasteroenterologist.
Positive tissue was always associated with individuals whose colon contained
l0 cancer at the time of biopsy or had cancer earlier, or the family had
frequent
occurrence of colon cancer. In the positive colons, there was thus some agent
which made the probability of cancer high. Diverticulitis and ulcerative
colitis were
also positive, but there were other markers which could distinguish these
cases.
The results indicated that in general, highly cancer prone cases demonstrated
a
strong staining, most prominent in the colonic surface epithelium. Here, in
addition
to the general light background staining, a strong staining component was
revealed
which could be enhanced to some degree by computerized imaging methods. In
the most serious cases, most surface epithelium had the magenta component and
2 0 this component was accumulating so much that there were bumps on the
surface.
This accumulation of the magenta component is the critical histopathological
marker.
The degree of staining is proportional to the degree of propensity to develop
cancer
2 5 so the results can be collected and analysed using a computerized method
of
evaluating the relative amount of the magenta area. Thus, a computer measured
reading can be compared against a background reading to indicate an
quantitated
value for the propensity of carcinogenesis.
3 0 Visual analysis of the representative images obtained from the 80 patient
study
indicate the following distinct patterns:
1. No positive reading: slices showing mucosa not involved in any of the
traditional lesions;
-29-
CA 02335773 2001-02-13
2. Low positive reading: slices are results from colons which have a condition
not related to cancer, as for example, stricture, diverticulitis, etc. These
samples reveal a low level of positive material in the surface epithelium.
Such low levels may also be found in cancer or polyp bearing colons in
some but not in the majority of samples. This in turn indicates that the
promoter responsible is not of uniform distribution;
3. Variable positive reading: polyp bearing colons display variably sized but
sometimes heavy positive areas. It is indicated that polyps arise in such
heavily promoted areas. Slices taken from regions of former cancer: a good
number of areas show low positivity. Some heavy positivity however, is still
shown at many places indicating that although the tumour was removed, the
promoting environment was not cleared up;
4. Heavy positive reading: slices wherein a large proportion of the surface
epithelial areas shows heavy positivity. Crypts at many places also show
positivity indicating that response to promoters start early in the life of
the
enterocytes in these cases.
Figure 9 is exemplary of the findings. Positive samples have clearly shown a
clear
and prominent apical band on the surface of the colon, i.e. in the surface
epithelium.
2 o In colour photographs, there is a deep magenta layer. In negative samples,
this
layer is thinner, and pinkish, very much as the rest of the tissue stained
nonspecifically by Schiff. Positive areas stain with the specific magenta
colour.
30
EXAMPLE III: QUANTITATIVE ANALYSIS OF THE ACTIVATED ENTEROCYTES
Biopsies were obtained from 24 cases, including 3 cases of ulcerative colitis
and
one of diverticulitis. Four are still to be studied. Among the remaining 16
cases, 11
positive readings and 5 negative readings were determined using the
histological
method of visualizing activated enterocytes. The microscopic images were
converted into digital images which were then analysed for the percentage of
activated enterocytes in the epithelium and in the crypts. The staining was
performed by first oxidizing the specimens and then staining with Schiff
reagent on
frozen specimens. The images were stored in computer and were evaluated using
-3 0-
CA 02335773 2001-02-13
the Northern Exposure software. The boundaries of the epithelium were traced.
In
this area, some of the cells were carrying a heavy load of the staining
substance
(plasmalogen). The computer was adjusted so that it measured the percentage of
the areas within the surface epithelium.
EXAMPLE IV: DEMONSTRATION OF METHOD
I. Collecting histological samales
Fresh colon tissue was obtained from surgical resections and from biopsies
obtained during endoscopic examinations. The tissue was embedded into O.C.T.
compound and then frozen on dry ice. Frozen sections were cut at 9-10
micrometer
thickness , attached to glass slides and then kept frozen in a humid
atmosphere.
The samples collected represented various disease categories:
I tumor-bearing colon
II polyp-bearing colon
III former cancer in the colon
IV former polyp in the colon
V family history of cancer or polyp of colon
VI various non-cancerous cases (obstruction, nonspecific inflammation,
2 0 etc. )
We took several samples from each case (Kiernan, J.A., Histological and
Histochemical Methods: Theory and Practice, 2"d edn. Oxford: Pergamon Press,
1990; Rapport, M.M., Lipid Res. 1984 ; 25: 1522-1527; Altmann, G.G., Amer. J.
Anat. 1983; 167: 95-117; Altmann, G.G., Electron Microsc. Tech. 1990; 16: 2-
14;
Chang, W.W.L., et al., Am J. Anat. 1971; 131: 73-99; Altmann, G.G., Epith.
Cell.
Biol. 1995; 4:171-183; Terner, J.Y., et al., Stain Technol. 1961; 36: 265-278)
depending on availability. Only occasional samples were taken from the tumours
or
the polyps themselves. We were mostly interested in the non-involved so called
3 0 "normal" mucosa. It was taken at various distances along the available
colon pieces
and when possible, at various given distances from the tumour.
II. Processin~i of the histological samples
-31-
CA 02335773 2001-02-13
The sections were thawed by slight finger pressure against the glass side.
The sections were then flooded with a 1 % solution of mercuric chloride for 1
minute.
The sections were then briefly washed in distilled water then flooded with
Schiff
solution for 5 minutes. The Schiff reagent was then washed out, the sections
were
washed in running tap water for further 5-10 minutes, then dryed and
coverslipped.
III. Qualitive evaluation of the samples
The histochemical reaction we used is specific for plasmalogens (Kiernan,
J.A., Histological and Histochemical Methods: Theory and Practice, 2"d edn.
Oxford:
1 o Pergamon Press, 1990; Terner, J.Y., et al., Stain Technol. 1961; 36: 265-
278). It
is based on the conversion of the ether bond by attachment of mercury and
subsequent formation of aldehyde which is then stained by the Schiff reagent.
The
histochemical procedure had to preserve lipids and was only effective when the
tissue was pretreated with the mercury solution. The Schiff reagent provided
for an
overall pinkish staining of the tissue so that there was no need for
counterstaining.
The actual positive substance was magenta, that is having at least one color
component additional to pink. The Schiff positive substance was confined to
the
cytoplasm in enterocytes and was gradually spreading as the enterocytes
matured
in the course of their natural life cycle. The extent of accumulation of
plasmalogen
2 0 was assessed in the enterocytes, those that were heavily laden with
plasmalogen
were termed "lipo" or "1" enterocytes.
IV. Histometrv
The spread of the plasmalogen within the enterocytes was measured in the
surface
epithelium. in the histological images. Representative epithelial areas were
selected. Total such area versus area occupied by plasmalogen was then
quantified
by area measurements. The percent of enterocyte images occupied by plasmalogen
was called the plasmalogen index (PI).
3 o V. Cytological assessments
The colon epithelium is continually renewed by new cells arising from the
stem cells of the crypt bases. The new cells go through various stages and
give rise
to various derivatives (e.g. 6). In order to evaluate these lineages, more
cytological
-32
CA 02335773 2001-02-13
detail than provided by frozen sections was needed. For these purpose, several
samples were taken in addition to the frozen ones. Also, some colon samples
were
obtained from mice and pigs. The samples were fixed by Carnoy's fluid and/or
by
a mixture of osmium tetroxide and potassium permanganate (Kiernan, J.A.,
Histological and Histochemical Methods: Theory and Practice, 2"d edn. Oxford:
Pergamon Press,1990). After fixation for 2-4 hours, the fixative was washed
out and
the samples were embedded in plastic (Historesin). Sections were cut at 1-2
micrometers and stained with toluidine blue or with iron hematoxylin (Kiernan,
J.A.,
Histological and Histochemical Methods: Theory and Practice, 2"d edn. Oxford:
1 o Pergamon Press, 1990).
Results
Plasmalog~en is the source of Schiff positivity
Upon sixty years of experience of histochemists with the Schiff reagent, it
has been found to be a reliable indicator of the presence of aldehydes
(Kiernan,
J.A., Histological and Histochemical Methods: Theory and Practice, 2"d edn.
Oxford:
Pergamon Press,1990). In general, oxidation of carbohydrates by periodic acid
and
oxidation of plasmalogens by mercuric chloride lead to aldehydes demonstrable
by
2 o conversion of the colourless Schiff reagent into magenta colour. Clear cut
histochemical demonstration techniques have thereby been provided for these
two
types of substances (Kiernan, J.A., Histological and Histochemical Methods:
Theory
and Practice, 2"d edn. Oxford: Pergamon Press, 1990). In view of Yeung's
finding
of Schiff positive mucus in human rectum, we tried to identify Schiff positive
structures in frozen samples of human colonic mucosa. In general, no such
structures were found, the frozen sections remained unreactive in the presence
of
Schiff. With prior periodic acid oxidation, for example, the mucus content of
the
goblet and the DCS cells stained. The DCS ("deep crypt secretory") cells were
first
demonstrated in our laboratory (Altmann, G.G., Amer. J. Anat. 1983; 167: 95-
117;
3 0 Altmann, G.G., Electron Microsc. Tech. 1990; 16: 2-14) as cells somewhat
similar
to goblet cells but accumulating in the deep crypts where they tend eventually
to
release their glycoprotein granules en mass leading to a phenomenon which we
referred to as "deep crypt secretion". To our surprise, the mercuric chloride
-33-
CA 02335773 2001-02-13
pretreatment brought out entirely different structures without demonstrating
goblet
or DCS cells. Several of the epithelial absorptive cells or "enterocytes"
showed
highly Schiff positive areas (Figure 13-16). According to the histochemical
evidence, these areas consisted of plasmalogens, the colonic occurrence and
location of these substances being unknown at the time. These substances were
furthermore soluble in lipid solvents as frozen sections were needed to
preserve
them. Pilot EM evidence indicated that they were produced in the cytoplasmic
endoplasmic reticulum and were therefore of phospholipid nature.
Relation to the renewal of the enterocytes
Following the well elaborated concept of the renewal of enterocytes (Chang,
W.W.L., et al., Am J. Anat. 1971; 131: 73-99), no enterocyte is of more than 6
days
old in the colonic epithelium. The average life span of these cells is about 6
days
after which they exfoliate from the epithelium and are being replaced by
younger
cells forming from the mitosis and the phenotypic transformation of stem cells
and
derivatives. Positional analysis of cells along the crypt axis is a reliable
source of
the age of the enterocytes as with age they migrate and occupy higher
positions.
Plasmalogen patches appear as a rule in young enterocytes at the lower crypt
level.
At this time, the patch is mostly infranuclear but some parts may already be
partially
2 0 supranuclear. With age, the patches appear to expand and fill up more and
more
of the upper cytoplasm of the cells. When this apical content reaches quite an
extent, the cell apex may appear as a lipid containing elevation in the
otherwise
normal looking epithelium (Figure 8). Our general conclusion was that
practically all
enterocytes entered a plasmalogen - producing phase towards the end of their
life
2 5 cycle. However, the amount of plasmalogen produced varied in a patchy
fashion
that is in some colonic areas, the cells were relatively low producing whereas
in
some regions they were very high producing. The possible reasons will be
elaborated below.
3 0 The histometric measurements
These measurements have confirmed the observation that some enterocytes are
low plasmalogen producers whereas in others, this production is high (Table1
). The
histological observations have indicated furthermore that when the enterocytes
-34-
CA 02335773 2001-02-13
exfoliate at the end of their life span, the plasmalogen content is carried
with them
for a short while but soon the cells break up and release the content the
colonic
mucus. The colonic mucus thus appears to be composed of goblet and DCS cell
secretions which are most probably glycoproteins and also of phospholipids,
the
latter coming mainly from enterocytes in the above described manner.
The results on the individual samples are shown in Table 1. from 10 to 30%
plasmalogen index may be considered as the normal range. An index between 30
and 40% may be considered as moderately elevated. Above 40%, it is considered
as high.
l0 Considering the polyp-bearing colons, the average plasmalogen index was
the highest, 39.6%. More than half of the samples (54%) had high index. About
17%
of the samples had moderately elevated index.
Considering the cancer- bearing colons, the average index was much lower,
22.6%. Only 9% of the samples were in the high category, and 17% in the
elevated
one.
Considering the colons with former cancer, the average index was slightly
elevated, 30.5%. About 15% of the samples were in the high category and 26%
were in the moderately elevated category. In the colons in families with
history of
cancer or polyp, the average index was 25.9%. About 11 % of the samples were
in
2 o the high index category, and 26% in the moderately elevated category.
In the non-cancerous group, most values were in the normal range, the
average index being at 22.7%. About 10% of them were moderately elevated with
practically none in the high range.
In conclusion, high plasmalogen indices with high occurrence were
2 5 exclusively in cancer-bearing colons. Poly-bearing colon had a few but
much less
of these high indices. In the case of former cancer or polyp, several samples
were
moderately elevated but most were within normal range.
In conclusion, the results have shown that the tendency of having highly
elevated plasmalogen content was very high in the cancer-bearing colons. This
3 0 tendency was much more moderate in the other groups but some elevation
still
lingered after removal of tumours or polyps or even when the tumours were only
in
the family.
Considering the position of various samples along the colon, the distribution
-35-
CA 02335773 2001-02-13
of the positive sample was patchy, only some were seen in close proximity of
tumours, others were at quite a distance. In other words, positivity did not
mean that
there was an overall stimulatory factor present which stimulated all
enterocytes. The
positive enterocytes did occur in groups so that they were predominant in a
given
sample but positive and negative samples alternated.
Cytological events
The plasmalogen production by enterocytes and the connection of this
1 o phenomenon to cancer development are new findings. The semithin
histological
material along with already published results (Altmann, G.G., Amer. J. Anat.
1983;
167: 95-117; Altmann, G.G., Electron Microsc. Tech. 1990; 16: 2-14) are
sufficient
to decipher the main cytological stages in reaching the plasmalogen producing
stage (Figures 8, 10-12, and 16). As shown by the semithin sectioned material
(from
human, pig and mouse), the immediate stem cell derivatives become filled with
prominent secretory granules. These are the deep crypt secretory (DCS ) cells
which we have reported earlier (Altmann, G.G., Amer. J. Anat. 1983; 167: 95-
117;
Altmann, G.G., Electron Microsc. Tech. 1990; 16: 2-14). These cells migrate
along
the crypt axis and lose their granules into the crypt lumen via a process
which we
2 o call "deep crypt secretion". After most of these granules are lost, the
cells enter a
brief period of mitosis and then the transit stage when absorptive and
plasmalogen-
producing properties develop. On light microscopic examination, these
enterocytes
are large cells much of the apical cytoplasm is being filled with plasmalogen
(Figures 8 and 13-16). This is the type of cell which is finally extrudes and
2 5 contributes its plasmalogen to the colonic mucus.
In short, the DCS cells are the immediate precursors of the plasmalogen
producing enterocytes, the loss of their secretory granules ( "deep crypt
secretion")
is being the critical and well visible event. It is to be acknowledged at this
point that
the presence of a vacuolated cell type was notice earlier in the rodent ileum
(Chang,
3 o W.W.L., et al., Am J. Anat. 1971; 131: 73-99), the loss of the vacuoles
leading to
the transit enterocytes. It seems that in other regions of the colon,
especially in the
ascending region, this phenomenon is much more prevalent involving the much
more prominent DCS cells and the phenomenon of deep crypt secretion which may
-36-
CA 02335773 2001-02-13
be quite impressive (Altmann, G.G., Amer. J. Anat. 1983; 167: 95-117).
DISCUSSION
Plasmalogens, a class of phospholipids with an unsaturated ether bond, are
still enigmatic as to their exact biological role. They are known to occur in
a number
of tissues (e.g. nerve, muscle) (Rapport, M.M., Lipid Res. 1984 ; 25: 1522-
1527).
This is the first report on their occurrence in the colon epithelium and that
they are
produced by the enterocytes. The latter cells are also called absorptive
epithelial
cells indicating that their main role was thought to be in absorbing water
from the
faecal content. As our observations indicate, an additional and rather
significant
role would be the production and accumulation of plasmalogens and after their
exfoliation, adding these accumulated phospholipids to the colonic mucus. The
single main source of colonic mucus has been thought to be the glycoprotein
secretion from the goblet cells. We confirm here our previous report (Altmann,
G.G., Amer. J. Anat. 1983; 167: 95-117; Altmann, G.G., Electron Microsc. Tech.
1990; 16: 2-14) that DCS cell secretions are also added to the mucus and we
report
for the first time that plasmalogen-type phospholipids are also added from the
enterocytes. It was surprising to see how large the enterocytes were when
their
phospholipid content was preserved in the histological preparations. This was
2 o suggestive that the contribution of these cells to phospholipid metabolism
may
indeed be quite substantial. The histochemical basis of demonstrating
plasmalogens has been worked out (Terner, J.Y., et al., Stain Technol. 1961;
36:
265-278). Mercury is built into the double bond of the ether linkage. There is
an
intermediate product which soon dissociates into an alcohol and an aldehyde.
The
aldehyde is then demonstrated by its reaction with the Schiff reagent. By
histochemical definition then, the areas seen to be stained magenta were
plasmalogens. There was unspecific pink tissue staining as well which provided
an
overall differentiation of tissue parts without the need for counterstaining.
Traditionally, the epithelium of the colon is regarded as one of the fastest
3 o renewing cell populations of the body: stem cells produce transit cells
that embark
on 3 possible lines of differentiation, enterocyte-line, goblet cell line, and
enteroendocrine cell line (Chang, W.W.L., et al., Am J. Anat. 1971; 131: 73-
99).
Our histological evidence indicates that the enterocyte-line is more complex
as
-37
CA 02335773 2001-02-13
intermediary cell types are interposed. Such a cell type, called "vaculoated
cell" was
recognized a while ago (Chang, W.W.L., et al., Am J. Anat. 1971; 131: 73-99)
and
was postulated that the immediate stem cell derivative acquired vacuoles which
gradually disappeared before mitosis and differentiation into enterocyte took
place.
This phenomenon is indeed observable in many areas of the colon. As we have
observed however, in many colonic areas and under various circumstances, this
phenomenon is much more intensive, the immediate stem cell derivative fills up
with
prominent secretory granules which eventually exocytose, this often happening
en
mass. We refer to the secretory cells so formed as DCS cells and to the
exocytosis
1 o of their granules as deep crypt secretion. This secretion apparently fills
the lower
crypt lumen with a special type of glycoprotein different in its staining
properties
from that of the goblet cells. The cells mitose and then differentiate soon
after this
event. A pilot study of ours with the electron microscope (to be published
separately) has shown that as the secretory granules disappearfrom the
cytoplasm,
osmiophilic areas of endoplasmic reticulum appear which apparently produce the
plasmalogens well demonstrated in parallel light microscopic preparations. The
various observations indicated furthermore that there are two levels of
plasmalogen
content in enterocytes: normal and elevated. The elevated seems to be in
groups
of enterocytes that are prevalent in "cancer prone" areas of the colon. Cancer
2 0 proneness has a specific definition derived from another extensive line of
investigation in our laboratory (summarized in Altmann, G.G., Epith. Cell.
Biol.
1995; 4:171-183). In this work on experimental carcinogenesis, we have built
up a
working model of intestinal cancers at will and within weeks and progression
of
precancer to cancer could also be halted. The work has shown that practically
all
2 5 individuals predisposed genetically to intestinal cancer harbour a small
percentage
(about 10%) of slightly changed "initiated" epithelial cells. The presence of
these
cells in itself is harmless but these cells if exposed long enough to another
group
of substances, "promoters", they become sensitized to mutagens so that in the
presence of mutagens they are likely to transform into cancer cells. Even one
such
3 0 cell can establish the disease. We found, furthermore, that long enough
prior
exposure to promoters was needed without which such transformation into cancer
cells did not take place. Promotion was thus found to be a key event in
carcinogenesis. Promoters in the intestine are known to derive from bile
and/or the
-3 8
CA 02335773 2001-02-13
bacterial flora. They tend to accumulate in some parts of the intestine making
these
parts prone to cancer formation. Statistical and experimental evidence is
accumulating that these areas indeed are the ones that respond with the
elevated
plasmalogen production. The enterocytes involved are probably the initiated
cells
which tend to proliferate locally in the presence of promoters.
It may be concluded that the plasmalogen reaction described in the present
work, if made on intestinal biopsies, provides a new histopathological tool by
which
the presence and the location of cancer prone areas can be determined. Ways
devised to clear these areas from promoters may also lead to new methods of
reducing cancer risk. Since the enterocytes accumulate plasmalogen mainly in
their
apical portion, a plasmalogen-film is present in those areas of the colonic
surface
that are lined by enterocytes (Figure 8) . Some areas are lined by groups of
goblet
cells but these groups are in minority especially in high risk areas. Instead
of using
just histopatholgical tests, there can be ways of measuring the thickness of
the
plasmalogen-film itself thereby outlining the location of the high risk areas.
In all,
new aspects of enterocyte metabolism have surfaced in the present work and
they
seem to be closely connected with some aspects of carcinogenesis. This may
eventually lead us to clues to the connection between normal and carcinogenic
metabolism.
2 0 A further implication of the connection with promoters is that the protein
kinase C system may be involved. Connection of promoters with the protein
kinase
C regulation of mitosis as well as with phospholipid turnover has been
reported
(DeRubertis, F.R., et al., C. Prev. Med. 1987; 16: 572-579; Lafave, L.M.Z., et
al.,
Lipids 1994; 29: 693-700). It is thus probable that this newly found
"plasmalogen
2 5 phenomenon" together with other findings on carcinogenesis and
phospholipid
metabolism may provide new insights into mitotic regulation and the cancer
problem
itself.
-39-