Note: Descriptions are shown in the official language in which they were submitted.
CA 02335980 2000-12-22
Autologous epithelialisation or endothelialisation of hollow organs or
vessels.
The invention concerns natural or artificial hollow organs and all their
parts, in particular
vessels and their valves, of which the inner or luminal surface is lined with
patient autologous
epithelium, in particular endothelial cells, a process for producing these
hollow organs and the
use of these hollow organs in surgery, in particular cardiac and vascular
surgery.
The cryopreservation and banking of organs and human tissue in order to
conserve these for
utilisation at a later stage is known and in the framework of transplantations
to a large degree
has become standard procedure, only the techniques used have irrelevant
differences.
(Brockbank KGM. Basic Principles of Viable Preservation. In: Transplantation
Techniques
and Use of Cryopreserved Allograft Cardiac Vessels and Vascular Tissue. DR
Clarke (ed.),
Adams Publishing Group Ltd., Boston.P9-23, American Association of Tissue
Banks
Standards for Tissue Banking (1995), A.A.T.B., McLean, VA, U.S.A. European
Association
of Tissue Banks General Standards for Tissue Banking (1995), E.A.T.B.,
(Vienna, Austria).
The utilisation of cryopreserved venous allografts is an established procedure
in bypass
surgery (Brockbank KGM et al., Cryopreserved vein transplantation. J. Cardiac
Surg.7:170-
176, 1992; Gelbfish J et al., Cryopreserved homologous saphenous vein: Early
and late
patency in coronary artery bypass surgical procedures. Ann. Thorac. Surg. 42:
70, 1986
Fujitani RM et al., Cryopreserved saphenous vein allogenic homografts: An
alternative
conduit in lower extremity arterial reconstruction in infected fields. J.
Vasc. Surg. 15: S 19-
526, 1992) and is utilised for patients who do not have sufficient vessel
material of their on
available or the vessels are qualitatively not suitable.
The utilisation of these types of veins show a poor longevity (Bilfinger TV et
al.,
Cryopreserved Veins in Myocardial Revasculization: Possible Mechanisms for
Their
Increased Failure. Ann. Thorac. Surg 63: 1063-69, 1997 and commentary Ann.
Thorac. Surg:
64: 1524-5, 1997. Marshin RS et al., Cryopreserved Saphenous Vein Allografts
for Below
Knee Lower Extremity Revascularization. Ann. Surg. 219: 664-72, 1994). The
cause for this
may be mainly due to an immunologically conditioned degeneration (Carpenter
JP,
Tomaszewski. JE, Immunosuppresion for Human Saphenous Vein Allograft Bypass
Surgery:
A Prospective Randomised Trial. J Vasc. Surg. 26: 32-42, 1997. Carpenter JP,
Tomaszewski
JE, Human Saphenous Vein Allograft Bypass Grafts: Immune Responses. J Vasc.
Surg. 27:
492-9, 1998). In addition premature thrombotic occlusions are often observed.
Till now in the
CA 02335980 2000-12-22
2
framework of cryopreservation these two processes have been traced back to
damage of the
donor endothelium, which can lead to the total absence of the same or a
limited functioning of
the preserved endothelium (Brockbank KGM et al, Cryopreserved vein
transplantation. J
Cardiac Surg. 7:170-176, 1992 Brockbank KGM et al. Functional analysis of
cryopreserved
veins. J. Vasc. Surg. 11: 94-102, 1990. Laub GW et al, Cryopreserved allograft
veins as
alternative coronary conduits: early phase results. Ann. Thorac. Surg. 54: 826-
31, 1992.
Louagie YA et al., Viability of long term cryopreserved human saphenous veins.
J.
Cardiovasc. Surg. 31: 92-100, 1990). As a result of this known and already
patented
cryopreserved technologies for allografts and xenografts aim at guaranteeing a
possibly higher
degree of preservation regarding the vascularity and microvascularity of the
donor
endothelium after the cleansing process. The longevity of the donor
endothelium of
cryopreserved tissue is given in the literature as being SO% -80%. (Bambang LS
et al., Effects
of cryopreservation on the proliferation and anticoagulant activity of human
saphenous vein
endothelial cells. J Thorac. Surg. 110: 998-1004).
However a key position has recently especially been assigned to the vascular
and
micovascular endothelium in the framework of the acute and chronic organ
rejection.
Endothelium specific non HLA antigen, which leads to the activation of CD4 T-
Cells, enables
the donor endothelium to supply - in conjunction with other accessory
molecules - the
recipient's immune system with foreign antigens. The release of non-HLA
antigen by
damaged endothelial cells leads to a chronic immune reaction and possibly to
graph
vasculopathy and chronic rejection. (Rose ML, Role of endothelial cells in
allograft rejection.
Vasc. Med. 2(2): 105-14, 1997; Reul, RM, Fang JC, Denton MD, et al., CD 40 and
CD 40
ligand (CD 154) are coexpressed in microvessels in vivo in human cardiac
allograft rejection.
Transplantation 64(12): 1765-74, 1997 Salom RN, Maguire JA, Hancock WW,
Endothelial
activation and cytokine expression in human acute cardiac allograft rejection.
Pathology 30
(1): 24-29, 1998). A process for the manufacturing of a non-immunogenic tissue
matrix was
proposed in the US patents No. 5,843,182 and No. 5,613,982. In this process at
first
decellularisation to removal native cells with the aid of hydrolytic enzymes
(e.g. proteases,
lipases, nucleosidases, glycosidases etc.) is carried out, and the matrix
obtained is treated with
adhesion and growth factors to enable the repopulation of fibroblasts.
The main disadvantage of these measures however is that it can not be ruled
out that this
treatment does not also negatively influence the strength of the other equally
decisive
CA 02335980 2000-12-22
components regarding the structural integrity of the hollow organ's walls, for
example type I
collagen, proteoglycans or glycoproteins.
This is even more important, as it is very well W own that the most serious
complication
concerning weakness of the cryopreserved veins' walls, for example tears of
the walls of the
veins or wall ballooning (aneurysms) lead to repeat operations resulting from
complications a
long-time after implantation (Lehalle B et al., Early rupture and degeneration
of
cryopreserved arterial grafts J Vasc. Surg. 25: 751-2, 1997. Couvelard A et
al., Human
allograft failure. Hum Pathol. 26: 1313-20, 1995). When decellularised tissue
serves as a
matrix for recellularisation measures, it is necessary to incubate the tissue
to be transplanted
with high concentrations of special adhesion or growth factors to enable cell
repopulation in
the wall. (US Pat. No. 5,632,778,and 5,613,928 or U.S. Pat. No. 5,192,312,
U.S. Pat. No.
5,843,182, WO 95/2483). These types of preparations are expensive, are mostly
not allowed
in clinical use and to a large degree, the amount of influence that the high
non-physiological
concentrations of these substances have on the functional differentiation of
tissue is unknown.
Pre-treatment of the matrix which leads to the decellularisation poses - just
as is the case when
treating the decellularised matrix with adhesion or growth factors -
immunological risks as
well as risks with regard to cell biology, that are not insignificant.
Apart from these disadvantages until now he literature concerned has hardly
taken into
consideration any knowledge from literature regarding the distribution of
antithrombogenic or
prothrombogenic activities or the active structures in the walls of hollow
organs. While
vascular endothelium (as the tissue covering the inner or luminal side of all
blood vessels and
valves in the vessels) is characterised for example by numerous anti-
aggregatory,
anticoagulatory and profibrinolytical activities (Z Kardiol. 82:Suppl. 5, 13-
21, 1993 FASEB J.
2:116-123, 1998) cellular components of the deep vessel walls are mainly
characterised by the
expression of tissue factor, which initiate an immediate coagulation reaction
when coming
into contact with plasma factors (Thrombosis Res. 81: 1-41, 1996, J Clin.
Invest. 100: 2276-
2285, 1997, FASEB J 8: 385-390; 1994, Arterioscler. Thromb. Vasc. Biol. 17: 1-
9, 1997).
Shielding of the walls' prothrombogenic activities is not only of
physiological importance
regarding blood vessels and their valves but also with all other cryopreserved
and non-
cryopreserved natural or artificial hollow organs or vessels.
CA 02335980 2000-12-22
4
The invention's underlying task is therefore to supply improved vessel
material for cardiac
and vascular surgery.
Another task of this invention in the future is to provide a suitable process
for producing the
type of material for vessels - in particular blood vessels and their valves -
necessary for
surgical transplantation purposes.
This problem is solved in accordance with the invention, by natural or
artificial hollow organs
and their components where the inner surface or the luminal surface is lined
recipient (patient)
autologous epithelium. Preferred in accordance with the invention are hollow
organs where
the inner surface or the luminal surface is lined with patient autologous
endothelial cells. A
particularly preferred model of the invention includes vessels and their
valves where the inner
or luminal surface is lined with recipient autologous endothelial cells
Hollow organs in accordance with the invention, in particular vessels, in
accordance with the
invention where the inner surface is lined with patient autologous endothelial
cells, show a
better long term patency than hollow organs or vessels that are not lined
Examples of hollow organs, in accordance with the invention, where the inner
or luminal
surface is lined with patient autologous epithelium are natural blood vessels
and their valves;
lymph vessels and their valves; ureter and urinary bladder; seminal ducts,
bronchi, the heart as
a special blood vessel with its valves and any prosthetic replacement of such
hollow organs.
As particularly preferred vessels in accordance with the invention,
cryopreserved or non-
cryopreserved allogeneic or xenogeneic vessels (arteries, veins, lymph
vessels) where the
inner surface is lined with autologous endothelial cells come into
consideration.
Another advantage of the invention is that the anchoring of the epithelial
covering (lining ) of
the hollow organs, in accordance with the invention is confluent and long-
lasting.
This is of particular importance as the endothelium of all the larger blood
vessels, vascular
valves and heart chambers, when in a healthy intact condition, functions
simultaneously as an
anatomical, physical and metabolic barrier between the blood and the deeper
layers of the
CA 02335980 2000-12-22
walls of the vascular structures mentioned and thus allows independent,
separated regulation
of numerous physiological processes in both these body compartments.
In particular the formation of thromboemboli and the triggering off of an
immuno reaction on
the luminal surface as well as an acute infiltration of the deep layers of the
wall by unspecific
effective defence cells (granulocytes, monocytes)is prevented through the to
be transplanted
blood vessels, valves and heart chambers, in accordance with the invention,
where the luminal
surface is lined with patient autologous endothelium. As the self collected
clinical experience
has already shown this is the reason why clinically .relevant rejection
reactions of transplanted
autologous endothelised blood vessels do not occur.
In addition the invention is concerned with a process for the production of
hollow organs in
accordance with the invention. The lining process includes the lining,
particularly both the
long lasting lining of cryopreserved or non-cryopreserved donor hollow organs
with patient
autologous epithelium and the lining of cryopreserved or non-cryopreserved
donor vessels,
with patient autologous endothelial cells.
A preferred design, in accordance with the invention includes the lining
process, in
accordance with the invention, as well as the pre-coating of cryopreserved or
non-
cryopreserved vessels with patient autologous serum.
In a particularly preferred design the lining of the vessel (or hollow organ )
with patient
autologous endothelial cells is carried out with the help of a special
cultivation device, which
is characterised by being able to create a pressure gradient between the lumen
of the vessel (or
hollow organ) and the outer area of the vessel, this prevents the walls of the
vessels or the
hollow organ from collapsing.
The hollow organs that are produced in this way in accordance with the
invention have
considerable advantages - an endothelial layer can be established on the
luminal surface e.g.
of a blood vessel This layer is absolutely confluent and is anchored to
withstand the shearing
force of the blood flowing past in the long term. This does not only act as a
complicated
antithrombogenic catalyst to prevent the thromboembolisation of he hollow
organs, but also
as effective protection against a mass scale recruiting of the defence cells
from the blood,
CA 02335980 2000-12-22
6
which is inalienable to the deleterious rejection of the deep wall structures
of the hollow organ
in the sense of an acute infective reaction
The process, in accordance with the invention leads to a long term re-
endothelisation with
patient autologous endothelium of the recipient.
In a preferred design a possible selective de-epithelisation of the
cryopreserved or non-
cryopreserved donor hollow organ (= removal of the donor epithelium) before
the lining with
patient autologous epithelial cells (= application of the recipient's
epithelium) is carried out.
Particularly preferred is the production of blood vessels lined with patient
autologous
endothelium, where the donor epithelium is gently removed beforehand,
therefore in this way
the other structures in the wall are totally retained. This selective removal
of the donor
epithelium is carried out mechanically or immunologically (by complement
mediated lysis).
Enzymatic methods for removing the donor epithelium in particular are to be
avoided.
The clinical results show that the clinically relevant rejection reaction is
not found when the
standard cytoimmunological monitoring is earned out, if the pre-treatment in
particular with
enzymatic means of the matrix which leads to decellularisation is not earned
out.
In another design, the process, in accordance with the invention" is included
as well as the
precoating of the cryopreserved or non- cryopreserved vessels with patient
autologous serum.
A pre-coating with patient autologous serum, where physiological
concentrations are used,
promotes not only the adhesion but also the functional differentiation of the
seeded
endothelium layer.
In vitro studies that were earned out with regard to the endothelisation of
cryopreserved and
non-cryopreserved veins showed that a pre-coating of the veins with serum
presents an ideal
matrix for the cell repopulation on the veins.
This pre-coating was, with what was at the time the standardised coating i.e.
fibronectin with
and without proteoglycan (e.g. heparin sulphate, see U.S. Pat. No. 5,192,312;
U.S. Pat. No.
5,632,778; U.S. Pat. 5,613,982; U.S. Pat. No. 5,483,182; WO 95/24873, Zilla P.
et al.,
CA 02335980 2000-12-22
Endothelial seeding of polytetrafluoroethylene grafts in humans. J Vasc. Surg.
6: 535-541,
1987) clearly superior. This presents an advantage worth mentioning, as the
clinical use of
fibronectin is not permitted in Europe.
In a particularly preferred design, the process in accordance with the
invention, for coating the
vessel (or hollow organ) with patient autologous endothelial cells is carried
out with the help
of a special cultivation device, which is characterised by being able to
create a pressure
gradient between the lumen of the vessel (or hollow organ ) and the outer area
of the vessel,
which prevents the walls of the vessels or the hollow organ from collapsing.
In addition it
specifically enables unwanted metabolic products of the vessel's wall to be
washed out by
transmural filtration.
The lining process in accordance with the invention, can be used for numerous
natural or
artificial hollow organs and their components, for example natural blood
vessels. The expert
can also use the process, in accordance with the invention, for
endothelisation of blood vessels
and heart valves; lymphatic vessels and their valves; ureters and urinary
bladders; seminal
ducts, bronchi, the heart as a special blood vessel with its valves and any
prosthetic
replacement of such hollow organs.
It is preferable to use the coating process in accordance with the invention,
for cryopreserved
donor vessels (veins and arteries) and non-cryopreserved donor vessels (veins
and arteries) as
well as with allogeneic heart valve replacement. In addition the lining
process, in accordance
with the invention, can be used for relevant xenografts.
In a design that includes the process, in accordance with the invention, for
producing hollow
organs correspondingly the cryopreservation of hollow organs till the time
that they are used;
their defrosting; the selective removal of the epithelial or endothelial
lining, where an
enzymatic treatment is to be avoided; the isolation of patient autologous
epithelium,
preferably endothelial cells and particularly preferred vascular endothelial
cells; the pre-
coating of the cryopreserved or non-cryopreserved hollow organs, preferably of
a donor
vessel, particularly a cryopreserved donor vein, with patient autologous serum
and the long
lasting lining of the hollow organ (or the donor vessel) with patient
autologous epithelial or
endothelial cells, with the help of the cultivation device in accordance with
the invention -
this is particularly preferred.
CA 02335980 2000-12-22
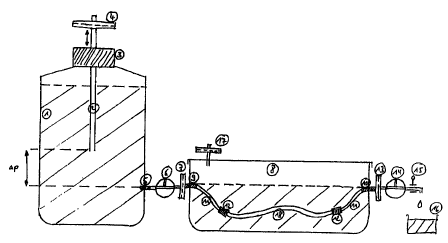
A particularly preferred cultivation device is shown in figure . This
cultivation device includes
a culture vessel that is filled with a medium, in which the vessel (e.g. vein)
is placed. The
lumen of both vein ends are connected to both the vents of the cultivation
vessel by means of
two hoses. There are two sterifilters have been placed (7, 13) in between
these and outside the
vessel there are two three-way taps (6, 14). The one hose is connected to a
Boyle-Marnott
vessel filled with a medium, the other ends in a drainage vessel. The pressure
gradient p
(dependant on cannula (2) and the hose clamp (15) are set so as to prevent the
vein collapsing
and provide the endothelial cells with a constant flow of culture medium as a
source of
nutrition. By opening the hose clamp (1 S) a complete exchange of the medium
within the vein
can be carned out as often as required. The medium exchange can also be
automated by
means of an electronically controlled pump.
The vessels, in accordance with the invention, can be used in cardiac or
vascular surgery,
especially in aortacoronary bypass in the case of cardiac disease and as the
graft in the case of
every type of vessel reconstruction. This, for example includes peripheral
arterial occlusion,
aneurysmal changes to vessels which result in the replacement of vessels as
well as numerous
repeat cardiac or vascular surgery. The vessels are an ideal conduit for usage
in infected areas.
Another indication for using this type of vessel is the numerous inborn
malformations (for
example any form of shunt operations can be mentioned). In addition these
types of vessels
are suitable for pure scientific research, for example arteriosclerosis
research or permeation
testing of pharmaceutical drugs.
Another design of the invention concerns vessels, whose inner surface have
been lined with
patient endothelial cells which were obtained from a different source (e.g.
peripheral blood,
bone marrow, fatty tissue, genetically modified or manufactured endothelium,
xenogenous
and when necessary genetically modified xenogenous endothelium.
In another design the patient autologous epithelium is manufactured using gene
technology ,
so that the epithelium imitates the surface's characteristics of the patient
autologous
epithelium and its immunological properties.
An additional design involves the utilisation of matrixes of xenogenous origin
for the
construction of new vessels and their endothelisation (e.g. destroying the
tissue of bovine
CA 02335980 2000-12-22
chest wall arteries by means of e.g. forced cryopreservation of these vessels
to the basic
structure of the vessel's connective tissue and the seeding of endothelium on
these vessels).
In another design the outer surface of the hollow organ, in accordance with
the invention, is
enclosed by a supplementary coat made of synthetic (man-made) material.
The definition of synthetic material as used here, means any organic and/or
inorganic product
that is suitable for such purposes.
In an a design that is especially preferred the hollow organ, in accordance
with the invention,
is enclosed in a supplementary coat made of reabsorbable synthetic material.
In a design that is especially preferred the coat is made of synthetic
polyglycon acids.
The hollow organ, in accordance with the invention is enclosed by a
supplementary coat for
example of polyglycon acids, has the advantage of being stabilised for many
months.
Another design involves the seeding of epithelium on reabsorbable material
(e.g.
polydioxanon) for the purpose of tissue engineering of a vessel.
The figure serves as an illustration of the invention.
Figure 1 shows the culture device used for the process, in accordance with the
invention, for
cryopreserved and non-cryopreserved vessels.
The following examples explain the invention and are must not limit the
conception.
Example 1: Patient autolo~ous endothelisation of cr~preserved veins
During the pre-operative phase approx. SOOmI of whole blood without
coagulation inhibitors
is taken from the patient, stored at 4°C for 24 hours and then the
solid components are
removed centrifugally. The serum is frozen until required. Simultaneously a
donor vein, if not
already available, is cryopreserved according to a specific scheme (Brockbank
KGM et al.,
Cryopreserved Vein Transplantation. J. Cardiac Surg. 7: 170-176, 1992; Gelbish
J, et al.,
CA 02335980 2000-12-22
1~
Cryopreserved homologous saphenous vein: Early and late patency in coronary
artery bypass
surgical procedures. Ann Thorac. Surg. 42:70, 1986; Brockbank KGM et al.
Functional
analysis of cryopreserved veins. J Vasc. Surg. 11: 94-102, 1990)
During a pre-operation a piece of vein, about S cm in length is removed under
local
anaesthetic from the patient who is to receive the treated/coated prosthesis.
The cell isolation
and the multiplication of isolated endothelial cells results from the current
cell culture
techniques (Jaffe EA, Nachman RL, Becker CG, et al. Culture of human
endothelial cells
derived from umbilical veins. Identification by morphologic and immunological
criteria. J
Clin. Invest. 52: 2745-56, 1973). Medium 199 (Seromed) supplemented with 20%
autologous
serum and 2ng/ml of recombined bFGF (basic fibroblast growth factor) for
example can be
used as a culture medium. After the required number of cells for the lining
have been
obtained, the vein that had been cryopreserved is defrosted in a bath of water
at 37°C.
Preferably the donor vein is freed of all residual donor epithelium by pulling
a blown up
balloon catheter (e.g. Fogarty's catheter, Fa. Baxter) through the vein in the
direction of the
blood flow (note venous valves!). In other approaches the endothelium can also
be
specifically removed by antibody induced complementary lysis. The vein is
filled with patient
autologous serum and in this state is incubated for 12 - 24 hours. Here the
two ends of the
vein are sealed by a universal adapter stopper (which is tied in), which in
turn is closed off
with a removable stopper. Finally the serum is drained by removing the
stopper, the pre-
coated vein is then filled with autologous patient endothelium having a
defined number of
cells (80.000-120.000 cells/cm' graft surface) and is closed again by
reintroducing the
stopper. Now the vein is placed in a rotating device, based on the one which
has been
described numerous times in the literature [Kadletz M, Moser R, Preiss, P et
al., In vitro lining
of fibronectin coated PTFE grafts with cryopreserved saphenous vein
endothelial cells.
Thorac. Surg. 35 Spec No. 2 143-147, 11/1987] and rotated in an incubator
(Functionline,
Heraeus Instruments) at 37°C. This results in regular adhesion of the
cells to the graft's
surface. After this the vein is taken out of the rotation devise and placed
into the special
cultivation device (see Fig. 1).
Figure 1 shows the special cultivation device that was especially developed
for the cultivation
of endothelised vessels, that mainly is made of biologically inert
autoclavible parts. With this
device a constant pressure gradient (0 - 20cm H20) can be built up between the
vein's walls
CA 02335980 2000-12-22
II
to prevent the vein to be cultivated from collapsing. In addition a continual
exchange of the
medium under sterile conditions can be carried out.
The Boyle Marriott vessel (1) comprises of a SOOmI glass bottle which is
equipped with a
universal stopper (3) that admits a cannula (2). This cannula which serves to
adjust the
pressure gradient is equipped with a sterifilter (Millipore) (4) to the
atmosphere. In the lower
third of the bottle there is an opening (5) which by means of a three way tap
(6) and another
sterifilter (7) is linked to the actual culture vessel (8). A sealable glass
culture dish serves as
the culture vessel, which mid-way up the sides is equipped with two supports
one leading to
(9) and the other away (10) from the cultivation vessel, thus the wall of the
vein has
penetrating supports. In order to cultivate veins of varying lengths a viton
hose (11) is
attached to both sides of the supports which can later be connected to a vein
adapter (12) so
that a continual flow of the medium from the Boyle Marriott vessel to the
drainage vessel.
A vitron hose (Labokron)with the appropriate adapters (identical to those that
will be used for
the vein) is placed exactly where the vein will go. The outlet is connected to
a drainage vessel
(16) via a sterifilter (13) and an adjustable three-way tap (14) and a
regulatable hose clamp
(15).In a sealed state the necessary gaseous exchange (5% C02, saturated water
vapour) for
the cultivation of cells occurs via a sterifilter (17) mounted on the cover of
the bowl. When
preparing the system the Boyle-Marriott bottle and the culture vessel are 2/3
filled with a
culture medium and the hose system is bled by opening the three-way tap. The
preparations
for the cultivation of the vein are complete.
The culture vessel it is opened in order to place the vein (18) that is to be
coated in it. The
adapter of the vein is connected to the culture vessel after the removal of
the sealed stopper
and the viton hose which had served the purpose of keeping the vein's place.
During this
procedure attention must be paid to prevent the entry of air bubbles
(disconnection and
reconnection are done under the level of the level of the medium). The daily
exchange of the
medium is carried out by opening the hose clamp and the draining off approx.
20m1 of
medium. In addition it is necessary to monitor the level of the medium in the
Boyle-Marnott
bottle and the culture vessel, in order to detect any possible leakage from
the vein. After 4 - 9,
preferably 6 - 9 days of cultivation in the incubator the implantation of the
graph is carried
out according to the standard surgical procedure. (Kirklin JW, Barratt-Boyes
BG: Cardiac
surgery: p. 299-311. New York, Edinburgh, London, Madrid, Melbourne, Tokyo.
1993).
CA 02335980 2000-12-22
IZ
The utilisation of the described cultivation device (Fig. 1) in the lining
process, in accordance
with the invention, is particularly preferred, as it provides the following
advantages:
A constant pressure gradient across the vein's walls is maintained. Through
this the collapsing
of the vein is prevented. In addition the medium is transported across the
vein's wall, which
serves the purpose of nourishing the seeded endothelial cells and the Vein's
walls. The
pressure gradient is kept constant even when the level of the medium sinks as
a result of the
Boyle-Marriott bottle's principle. The complete exchange of the medium which
is necessary
for the nutrition of the endothelial cells which are establishing themselves
(newly acquired
intima of the vessel) can be done easily and under sterile conditions by means
of the
regulatable hose clamp. This procedure can also be automated by means of a
computer
controlled pump. This device is a simple, easy to operate, cheap and safe aid
for the
epithelisation of every type of vessel.
Perfusion trials carried out with endothelised cryopreserved or non-
cryopreserved donor
vessels show no difference in the morphology of the endothelium and the
stability against the
shearing power when compared to totally intact newly gained veins or arteries.
With regard to judging the long-term patency of the treated vessels on
statistic significant
levels, a sufficiently large clinical study has not yet been carried out. The
first clinical usage
of this type of bypass, earned out in January 1993 was unusually successful.
Example 2: Patient autologous endothelisation of non- crvopreserved veins
The endothelisation of a non-cryopreserved vein was carried out according to
the process in
example 1.
Example 3: Patient autolo~ous endothelisation of another vessel a ~ an artery
The endothelisation of an artery was carried out in exactly the same way as
the described
endothelisation of the vein in example 1.
CA 02335980 2000-12-22
l
Example 4 Epithelisation of another hollow organ i a a ureter
The epithelisation of a ureter carried out according to the epithelisation of
a cryopreserved
vein described in example 1, the only difference being that urothelium was
used.
Example 5: Lining process where the endothelial cells are gained from a
different source (see
above .
Lining process as in example 1. The isolation of the corresponding endothelial
cells were
obtained from peripheral blood, bone marrow and abdominal fat. This isolation
of endothelial
cells is of importance to the patient, as this provides a process for those
patients who do not
have sufficient vascular substrate for the extraction of autologous
endothelium. In addition
this process is less invasive for the patient.