Note: Descriptions are shown in the official language in which they were submitted.
CA 02336436 2001-03-O1
PROCESS FOR PRODUCING GYPSUM FROM A
CALCIUM SULFITE GAS DESULFURIZATION SLURRY
Field of the Invention
The present invention relates to a process for producing
gypsum (dehydrate gypsum: CaS04~2Hz0) from an aqueous flue gas
desulfurization slurry containing calcium sulfite, in an efficient
manner and with production of high purity gypsum.
Background of the Invention
Wet scrubbing process for removing sulfur dioxide from
sulfur dioxide-containing gases, such as gases from power plants or
other coal combustion units, have been perfected which use lime or
limestone as the scrubbing component in aqueous medium, with the
production of aqueous waste sludge. The aqueous waste sludge
contains primarily calcium sulfite, which is difficult to dewater
and is often deposited into settling ponds for extended periods of
time before solidification occurs.
Processes have been developed for the production of a
useful byproduct from such calcium sulfite sludges, such as
oxidizing the calcium sulfite to produce a useful calcium sulfate
product such as gypsum.
In conventional processes where calcium sulfite sludges
from a sulfur dioxide scrubbing process are oxidized to calcium
sulfate, the slurries usually contain less than 25 percent solids.
Direct oxidation of a calcium sulfite slurry from a
thickener of a desulfurization system generally requires dilution
of the slurry solids content to reduce viscosity and improve
oxidation (air bubble/slurry contact), with calcium sulfite
slurries over 20 percent solids generally being so viscous as to
slow oxidation. Limestone processes also generally require long
1
CA 02336436 2003-07-25
74445-69
residence times of 16-24 hours ~.n an oxidizing unit and an
air/sulfite stoichiometric ratio of greater than 3:1 (moles oxygen
used/moles oxygen required). T:n lirne oxidation pracesses,
generally a solids content of between about 15-20 percent is
required, with use of 2.5 to 3 hours residence tune and an
air/sulfite staichiometric ratio of 2:1 to 3:1 far oxidation of the
calcium sulfite to gypsum.
In U.S. 5,312,609,
there is described a metrzod of removing sulfur
dioxide from a gaseous stream with th.e production of alpha
hernihydrate gypsum (aCaS04~?~HaO) as a byproduct. In the method
described therein, a sulfur dioxide-containing gaseous stream is
contacted with an aqueous scrubbing medium containing calcium and
magnesium scrubbing components, in a wet scrubbing unit, with
sulfur dioxide converted to calcium and magnesium sulfites. A
portion of the aqueous medium is remo°ved fx:°om the wet
scrubbing
unit and passed, at a sol~.ds ce~ntent of ~aetween about 5-35 weight
percent to a pressurized oxidatiozz vessel. In the pressurized
oxidation vessel, the calcium arzd magnesi~.xm :Pulfites are contacted
with an oxidizing gas, at an elevated temperature of about 100
145°C (212-293°F) , a superatmosphex:i.c pressure of between
about 20
60 pounds per square inch, and a pH of b~:;tween 2.5-5.5 to convert
the calcium sulfite to alpha~hemihydrate ~yp~~um, and the magnesium
sulfite present t.hexein to magnesium sv~.lfate. ~'he alpha
hemihydrate gypsum precipitates fxom the aqueous medium while the
magnesium sulfate remains in solution i.n the aqueous medium. After
removal of the aqueous medium contair~.ing precipitated alpha
hemihydrate gypsum and dissolved magraesi~~xm sulfate from the
oxidation vessel, the alpharhemihydrate gypsum is separated
3D therefrom.
2
CA 02336436 2001-03-O1
While alpha-hemihydrate gypsum is an especially useful
form of gypsum and has specific uses for which conventional
dehydrate gypsum, calcium sulfate dehydrate (CaS04~2Hz0), cannot be
used, the production of conventional gypsum in efficient processes
is also desirable.
It is an object of the present invention to provide a
process of producing dehydrate gypsum, from an aqueous flue gas
desulfurization slurry containing primarily calcium sulfite, in a
manner that minimizes the air requirement for oxidation, reduces
residence time in an oxidation vessel, and provides for oxidation
of such slurries containing high solids concentration (25-35%).
It is another object of the present invention to provide
a process of producing dehydrate gypsum, from an aqueous flue gas
desulfurization slurry containing primarily calcium sulfite, using
a pressurized oxidation vessel that effects gas bubble sizes and
the mass transfer by providing a much higher surface area and thus
better oxygen utilization, along with increased oxygen solubility.
SUMMARY OF THE INVENTION
The present process produces calcium sulfate dehydrate
(dehydrate gypsum) from an aqueous flue gas desulfurization slurry
containing primarily calcium sulfite. The aqueous slurry,
containing calcium sulfite in a major amount by weight of the
slurry solids, is charged, at a pH of about 5.8 to 6.0 into the top
of a vertically oriented pressurized oxidation vessel and passed
downwardly therethrough while contacting the aqueous slurry with a
countercurrent flow of oxygen-containing gas. During the
contacting, a pressure in the bottom portion of the oxidation
vessel is maintained at about 22-32 pounds per square inch (psig),
and a pressure in the top portion of the oxidation vessel is
3
CA 02336436 2001-03-O1
maintained at about 10-17 prig. Also, in the oxidation vessel, the
aqueous slurry is maintained at a pH of about 4.0-5.5 and at a
temperature of between about 180-217°F. Calcium sulfite in the
aqueous slurry is oxidized to dehydrate gypsum and the slurry is
removed from the bottom of the oxidation vessel at a pH of between
5.0-6Ø
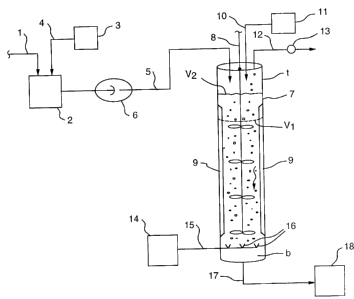
BRIEF DESCRIPTION OF THE DRAWING
The drawing is a schematic illustration of the present
process for producing dehydrate gypsum from an aqueous flue-gas
desulfurization slurry containing primarily calcium sulfite.
DETAILED DESCRIPTION
The present process provides for the production of
dehydrate gypsum from a flue gas desulfurization slurry containing
primarily calcium sulfite by using a pressurized oxidation vessel
under carefully controlled process conditions.
The process is for producing dehydrate gypsum from flue
gas desulfurization slurries, such as calcium sulfite-containing
slurries resulting from desulfurization systems using an aqueous
slurry of lime or limestone as the reactant to remove sulfur
dioxide, which reactants provide calcium sulfite by reaction with
the sulfur dioxide. Such desulfurization systems produce an
aqueous discharge slurry that contains primarily calcium sulfite as
a major portion of the solids content of the slurry, and may
contain a total, by weight, of about 10-35 percent solids.
The aqueous slurry, containing calcium sulfite is passed
to the top portion of a vertically oriented pressurized oxidation
vessel, with the pH of the aqueous slurry adjusted, if necessary to
a pH of between about 5.8 to 6.0, preferably about 6.0, by addition
4
CA 02336436 2001-03-O1
of sulfuric acid prior to introduction into the pressurized
oxidation vessel. The aqueous slurry passes downwardly through the
vertically oriented pressurized oxidation vessel while an oxygen-
containing gas is charged to the bottom portion of the pressurized
oxidation vessel and passed upwardly, countercurrent to the aqueous
slurry, the oxygen oxidizing the calcium sulfite to dehydrate
gypsum.
The production of dehydrate gypsum in the pressurized
oxidation vessel is provided by careful control of the conditions
within the pressurized oxidation vessel. It has been found that
the pressure at the bottom portion of the pressurized oxidation
vessel must be maintained at between about 22 to 32 prig,
preferably about 30 prig, while the pressure at the top portion
thereof is maintained at between about 10-17 psig., most preferably
between 13-17 prig. In addition, the temperature of the aqueous
slurry in the pressurized oxidation vessel must be maintained
between about 180-217°F, while the pH is maintained, by addition of
sulfuric acid, at a pH of between about 4.0-5.5.
The calcium sulfite is oxidized, through contact with the
oxygen of the oxygen-containing gas, to dehydrate gypsum under
these controlled conditions, with the slurry containing resultant
dehydrate gypsum discharged from the bottom portion of the
pressurized oxidation vessel at a pH of between about 5.0-6Ø By
use of the present process conditions, an oxidation efficiency of
about 1.26:1 is achieved. That is, an air/sulfite stoichiometric
ratio of about 1.26:1 is achieved, versus the earlier ratios of 2:1
to 3:1 known in the art, and referred to on page 2 hereof.
Referring now to the drawing, which schematically
illustrates the present process, an aqueous flue gas
desulfurization slurry containing solids that are primarily calcium
5
CA 02336436 2001-03-O1
sulfite is fed from a source, through line 1, to a supply unit 2.
Sulfuric acid is also fed to the supply unit 2 from source 3
through line 4, if needed, in an amount that will adjust the pH of
the slurry to between about 5.8 to 6.0, preferably about 6Ø The
aqueous flue gas desulfurization slurry, containing primarily
calcium sulfite and at a pH of about 6.0, is then charged through
line 5, by means of a higher pressure pump 6 into the top portion
t of a vertically oriented pressurized oxidation vessel 7 which may
contain a stirring mechanism 8 and wall baffles 9 which will aid in
mixing of the slurry. Further sulfuric acid may be added to the
pressurized oxidation vessel 7 through line 10 from a source 11.
The pressurized oxidation vessel 7 is a closed vertically oriented
vessel which has a pressure release line 12 containing a pressure
release valve 13. An oxygen-containing gas, preferably air, from
a source 14 is charged to the bottom portion b of the pressurized
oxidation vessel 7 through line 15 and spargers 16 so as to flow
upwardly through the downwardly flowing aqueous slurry, i.e.
countercurrently. As shown by the dotted line, the aqueous slurry
would have a volume V~ in the absence of air flow and a volume
when air is charged to the pressurized oxidation vessel and flows
countercurrently to the flow of aqueous slurry. The pressure at
the bottom portion b of the pressurized oxidation vessel is
maintained at about 22-32 psig, while the pressure at the top
portion t of the pressurized oxidation vessel is maintained at a
pressure of about 10-17 psig by regulation of the pressure release
valve 13 on pressure release line 12 which release gases from the
pressurized oxidation vessel 7. Also, the pH of the aqueous slurry
is maintained at between about 4.0-5.5 by addition of sulfuric acid
through line 10, and the temperature of the aqueous slurry
maintained at between about 180-217°F. Under these conditions, the
6
CA 02336436 2001-03-O1
calcium sulfite in the aqueous slurry is oxidized to dehydrate
gypsum, with the slurry containing the resultant dehydrate gypsum
discharged from the bottom portion b of the pressurized oxidation
vessel 7 through line 17 for collection at 18.
Example I
As an example of the present process, a sulfur dioxide
flue gas desulfurization slurry containing calcium sulfite (23.9-
31.9% solids) was charged to the top of a 40 foot long oxidation
vessel having a diameter of 6 feet, at a pH of 5.0-5.5, and at a
rate of 45-60 gallons/minute (GPM), and passed downwardly through
the oxidation vessel, the slurry filling the reactor to a height of
26-28 feet with the height increased to 34-36 feet by injection of
air, as an oxidizing gas, to the bottom of the oxidation vessel at
a rate of 1475 standard cubic feet/minute (SCFM). The pressure in
the oxidation vessel was maintained such that a pressure at the top
thereof (vent pressure) was 13-15 psig, and the pressure at the
bottom was 22-26 prig. The temperature was maintained between 190-
205°F, and a pH of 4.0-5.0 maintained, with a mixer stirring the
reactor slurry contents during flow downwardly therethrough.
The residence time, based on 28 feet of slurry was 1.64
hours at 60 GPM.
The oxidized slurry, containing calcium sulfate dehydrate
(dehydrate gypsum), was discharged from the bottom of the vessel at
a pH of 5.0-5.5, and contained uniform crystals of calcium sulfate
dehydrate of a particle size of about 123 microns.
Example II
A further series of test runs were made using starting
material as in Example I, under the conditions listed in Table I,
with the results listed therein.
7
~n n~~~Ha~H ~nni-n~-ni
0 o o o
I I ~ M M M M
-I-~ N ~ rl r-~ f-I .--I
U U1
r~-I r-I I I I I
N -rl .~'-,
rd U -~-I co c0 00 00
E O
f~ -r-I o1 O1 01 01
Ul '-'
~-I
I
I d' l0 L~ I~
I~
o\o I . p~ pl
. .
Lll l0 /~ /\
01 01
~) U7 01 01
01 01
U I N cu I I LI1
N
r~ .1-~ r~ ~--iO a1
~ ~ O O ~'
0
~ \
U7 4-I O O O O
M N ~ O
'b I ~ ~ ~ I
I (v Ul rl v-i rl L~
~ ~ O
r-I ~I -r-II I I .
~-I o\a
N O N r-I
N
C~ 1.J ~ r-W I r-~ t--i
J-1 N
Lf1 Lf1 O O
I ~ x I I I I
0 0 0 0
117 Lf1 Lfl l.n
-ri r-I co c0 \o \o
~
~ -H M M M M
I I ~ I
-rl N " l0 l0 V' N
~l 'b ~ M M M M
I~ L~ N N
I N I N N M M
N ,LI J-) I I I I
~ ~
S-I ~ J-1 N N Lll Lfl
O O P-I
P-i U1 ~ N N N N
~ -L~ '-'
,
E-t -rl N
ca -r-I
1-1 '~
-
-ri I N 1~ C,' ri t-I rl
,'~,'
~-I ~ J-1 M M Lf1 L(7
,'7
~ UWL~ " rl n--1r~ r-i
Ul N
N
I I I I
Ci.i O O 111 O
In N L(1 l0
O O O O
O v-I rl rl
E-i v N N N N
N N N N
O ~
I I I
O Lf1 O Ll1
Lll O O
Ln d' L.f~ d'
Lfl U1 LC1
I
x O O O 00
O
O ~o ~ ~o ~n
~o
~b
O W n u n uo
1~ N O ~ V~ t!7 l~ f~
-r-I ~-1 J-~ L1J I ~ I I
O ~-I ~ ((S U~ O O O O
1~ ~ N (x " di V~ ~' V
3 ~~
~ ~ ~ N l0 d'
1 -~
C
r '~ M lD N N
-
J
0
-rl I N I I
r-1 ~ l0 O v--I
O o\o N N N
rl N M
N '-'
H
CA 02336436 2001-03-O1
As is seen from the above, the present process provides
for the production of dehydrate gypsum in an efficient manner and
high purity. Air or oxygen requirements are minimized, as are the
residence time in the oxidation vessel. The process affects aas
bubble sizes and the mass transfer by providing a higher surface
area, along with increased oxygen solubility so as to effect
efficient oxidation of calcium sulfite to dehydrate gypsum.
9