Note: Descriptions are shown in the official language in which they were submitted.
CA 02336507 2001-02-14
Flow-Through Electrochemical Reactor for Wastewater
Treatment
Field of the Invention
This invention relates to wastewater treatment where
levels of organic contaminants, such as phenols and related
compounds, are to be decreased.
Background of the Invention
Several industrial processes require the use of large
quantities of water for their operations. The water may
come from natural sources such as rivers or from treated
city water. As a consequence of the industrial activities,
the used water may become contaminated with organic
pollutants beyond permissible, environmentally acceptable
limits.
Organic contaminants can be removed to a limited degree
by adsorption on activated carbon, ozonation, or a
combination of these methods. After use, activated carbon,
if filled with contaminants, requires destruction or
disposal to a special landfill. In addition, activated
carbon is not necessarily selective enough to efficiently
absorb the problem compounds, and when the active sites are
full, the adsorption capacity goes down to zero. Ozone is a
dangerous chemical and it would be preferred if its use
could be avoided in wastewater treatment.
Electrochemical treatment of wastewater can reduce the
level of organic contaminants by oxidation. Noding (United
States Patent 4,652,355) discloses an electrochemical
reactor in which the anode and cathode in a reaction chamber
1
CA 02336507 2001-02-14
are in the same plane as the direction of flow of aryl-
containing wastewater. This reactor predominantly produces
aryl hydroquinones, which are not ideal end products for
environmental release.
Similarly, Cole (United States Patent 5,531,865)
discloses an electrochemical reactor having a cathode and a
plurality of sacrificial anodes elongated in a chamber,
parallel to the direction of flow of contaminated water.
With such a configuration of electrodes, charge density will
vary across the cross-section of the reaction chamber, and
it is possible that a significant amount of aryl compounds
will not contact an anode, and experience sufficient charge
density to be oxidized, while flowing through the chamber.
Several patents have issued relating to reactors that,
in attempting to optimize the possibility of electrochemical
reaction, make available significant electrode surface area
by having multiple solid electrodes in various
configurations and/or requiring meandering flow of
wastewater over the surface the electrodes (for example
United States Patents 5,549,812 (Witty; 5,587,057 (Metzler
et al.); 5,611,907 (Herbst et al.); 5,746,904 (Lee); and
5,928,493 (Morkovsky et al.)). The reactors found in these
patents tend to be of relatively complex construction and
the flowpath of the wastewater over solid electrodes, in
each case, does not guarantee intimate contact with an anode
surf ace .
Sampson et al. (United States Patent 5,705,050)
discloses a packed bed reactor, which includes an ion
exchange material packed between an anode and a cathode.
However, ion exchange materials require special handling and
specific reactor conditions to tolerate higher back
pressures that can occur.
2
CA 02336507 2001-02-14
Summary of the Invention
By using at least one porous anode, the electrochemical
reactor of the present invention addresses limitations in
known reactors. By directing the flow of wastewater through
the pores of at least one porous anode, the reactor
disclosed herein provides a high probability that
contaminant molecules will experience intimate contact with
an anode and thus encounter the necessary current density
for oxidation. This advantage is coupled with the
relatively simple construction of the reactor and ease of
maintenance.
The invention provides an electrochemical reactor
(cell) for reducing the concentration of organic compounds,
such as aryl compounds, found in wastewater from industrial
processes. Breakdown of the organic compounds occurs by
oxidation at the anode of the electrochemical reactor.
More specifically, the present invention provides a
flow-through electrochemical reactor comprising:
a body having an internal chamber, and an inlet port
and an outlet port in communication with said internal
chamber to permit flow of wastewater therethrough;
at least one porous anode arranged in said internal
chamber such that the wastewater flowing between said inlet
port and said outlet port flows through the pores of said at
least one porous anode, said at least one porous anode
having activity for the destruction of a target substance;
and
at least one cathode disposed in the internal chamber
to permit an electric current to be established between said
at least one cathode and said at least one anode, said
3
CA 02336507 2001-02-14
electric current reducing the concentration of said target
substance in the wastewater flowing through the chamber.
The reactor, when in use, reduces TOC content of
industrial wastewater by oxidizing target substances, such
as aryl compounds, efficiently. Efficient oxidation
minimizes the possibility of competing side reactions. The
side reactions are unfavorable since they might produce
compounds that are as harmful as, or more harmful than, the
compounds to be destroyed.
Thus, the electrochemical reactor can treat a
wastewater stream to reduce the concentration of aryl
compounds to an environmentally acceptable level. The
reactor of the present invention also offers the advantage
that it can be installed within an existing piping system.
Brief Description of the Drawings
Further features of the present invention will become
apparent, to those skilled in the art to which the present
invention relates, from reading the following specification
with reference to the accompanying drawings, in which:
Figure 1 is a schematic diagram of an embodiment of the
flow-through reactor of the present invention;
Figure 2 is a top view of an electrode of Figure 1;
Figure 3 is a schematic side view of an electrode and
holder of Figure 1;
Figure 4 is an image of a titanium foam used as an
anode substrate;
Figure 5 shows the morphology of an antimony-doped tin
dioxide (Sn02) dimentionally-stable anode (DSA) coating;
Figure 6 is a graph of the efficiency of phenol
destruction by an embodiment of an electrochemical reactor
4
CA 02336507 2001-02-14
of the invention, at current densities of 1.4 (white), 2.8
(black) and 5.6 (cross-hatched) mA/cm2, using 3D foam anodes
of either antimony-doped tin dioxide, platinum or tantalum-
doped iridium dioxide; and
Figure 7 is a graph of the efficiency of destruction of
a mixture of m- and p-cresol by an embodiment of an
electrochemical reactor of the invention, at current
densities of 1.4 (white), 2.8 (black) and 5.6 (cross-
hatched) mA/cm2, using 3D foam anodes of either antimony-
doped tin dioxide, platinum or tantalum-doped iridium
dioxide.
Detailed Description of the Invention
The following description illustrates the manner in
which the principles of this invention are applied but is
not to be construed as, in any sense, limiting the scope of
the invention.
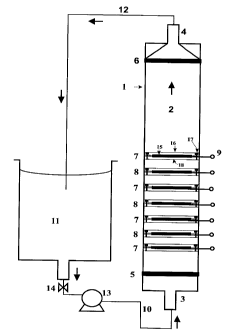
Referring to the embodiment of Figure 1, an
electrochemical reactor 1 in accordance with the present
invention includes a tubular body 2 having an inlet port 3
and an outlet port 4. The inlet port 3 is retained on the
tubular body 2 with a first retaining means (not shown). An
inlet O-ring 5 is disposed between the inlet port 3 and the
tubular body 2 in a sealing engagement. Similarly, the
outlet port 4 is retained on the tubular body 2 with a
second retaining means (not shown). An outlet O-ring 6 is
disposed between the outlet port 4 and the tubular body 2 in
a sealing engagement.
Inside the tubular body are a series of porous cathodes
7 and anodes 8 in alternating arrangement, each having a
contact wire 9 in the form of a screw passing, in a liquid-
5
CA 02336507 2001-02-14
tight manner, through holes in the wall of the tubular body
2. The screws also serve to secure the cathodes and anodes
in place and are further provided with electrical
communication to a DC power supply (not shown). Wastewater
is introduced into the reactor through an inlet pipe 10 from
a reservoir 11. Treated wastewater leaves the reactor
through an outlet pipe 12 and is returned to the reservoir
11. A pump 13 is used to move the wastewater through the
reactor. The wastewater supply from the reservoir 11 is
controlled by a valve 14.
Referring to Figure 2, a cathode 7 is shown which
includes an circular, 3D foam-type electrode 15 retained in
an insulating electrode holder having a top 16 and bottom 18
(see Figure 1) held together with screws 17. Each electrode
holder is sized to provide a snug fit within the tubular
body 2 so that essentially all wastewater introduced into
the reactor passes through the porous anodes 8 and cathodes
7.
Figure 3 shows a foam-type electrode 15 and contact
wire 9 between the top 16 and bottom 18 of the electrode
holder prior to assembly by screwing the top 16 and bottom
18 together with the screw 17.
In use, the reactor 1 can be mounted vertically or
horizontally. The reactor should be placed in an open
recirculation circuit, thus allowing evolved gases, such as
carbon dioxide, to escape.
The body of the reactor can have a variety of shapes
but preferably is tubular and the internal chamber
cylindrical, with a generally circular cross-section. While
Figure 1 shows detachable inlet and outlet ports 3 and 4,
which permit convenient access to the electrodes in the
tubular body 2, a unitary construction is also possible.
6
CA 02336507 2001-02-14
The electrode holder (see Figure 3) serves as a mechanical
device to install electrodes within the electrochemical
reactor 1, as well as an electrical insulator. The
insulating holder preferably is sized, conveniently in a
disc shape, for close-fitting insertion into the internal
chamber. The holders can be held in place within the body 2
by screws passing through the wall of the body, or by some
other suitable means. It is preferred that the electrical
connection is also provided by the screw which can be
connected electrically to a suitable power supply external
to the reactor. Conveniently, the power supply is a DC
supply.
By removing the inlet port 3 or outlet port 4, or both,
the number, and arrangement, of electrodes in the reactor 1
can be conveniently changed. In addition, the electrodes
can be removed from the reactor for periodic cleaning. The
cleaning process can also be performed in situ and may
involve the use of an organic solvent, such as methanol or
ethanol, or an alkaline cleaner, with or without current.
The current may be inverted if needed. It is preferred that
the anode material is platinum and the cathode material is
nickel, because a current polarity inversion to clean them
will not result in damage to the electrode materials.
The electrodes are preferably stacked in an alternating
arrangement, such that an anode is placed next to a cathode
and vice-versa (i.e. C/A/C/A/C/A/C/A/C...). As such, the
number of anodes and cathodes in the reactor can be varied,
from a minimum of one anode and one cathode to many tens of
anodes and cathodes. It is preferred that the alternating
arrangement begins and ends with a cathode, to ensure
optimum activity of the anode at the start and end of the
series. More preferably, there are two to ten anodes and
CA 02336507 2001-02-14
three to eleven cathodes, respectively. Conveniently, there
are seven cathodes and six anodes. The number of electrodes
used depends upon the volume of the solution to be treated
and the desired treatment time. Each anode is isolated from
each cathode, to avoid a short-circuit. The anodes and
cathodes typically are each connected to corresponding bus
bars that in turn are connected to a DC power supply.
The reactor is made from any material that has the
necessary mechanical strength for the chosen dimensions of
the reactor, and resistance to corrosion by the wastewater
stream of interest. Such materials can be glass, polymer-
coated stainless steel, reinforced fiberglass or polymer,
and the like.
Preferably, the wastewater is filtered before treatment
in the reactor in order to minimize the possibility of
blockage of the electrodes with solid materials. The
wastewater to be treated flows through the porous electrodes
in the reactor, and therefore the liquid can be treated then
conducted to a holding tank. While the solution to be
treated flows through the reactor, and hence through the
electrodes, a DC current passes within the reactor, between
the anodes and the cathodes. The pore openings in the foam
electrodes allow a free flow, of the wastewater to be
treated, with a minimum of flow restriction.
Depending upon the anode material, a current density
that can vary between 0.7 and 70 (mA/cm2) is applied,
although for phenolic compounds, a current density of about
1.4 mA/cm2 is preferred. For wastewater having several
target compounds, zones of different current densities can
be established within the reactor in order to optimize the
destruction of each target compound. The distance between
a
CA 02336507 2001-02-14
certain electrodes can be selected based on the desired
current density at a particular location in the reactor.
The electrolysis (or treatment) time depends upon the
initial concentration of the problem compounds and the final
concentration desired, as well as the flow rate. This
latter variable can be between 1 to 60 liters per minute of
reactor capacity, although a flow of about 8 liters per
minute is preferred. The dimensions of the electrodes, and
the reactor generally, can be varied depending on specific
requirements. Electrode diameter conveniently can be up to
about 1.5 m. Electrode thickness conveniently can be up to
3 cm, preferably about 0.5 cm for a titanium substrate.
The wastewater to be treated can circulate for a
variable number of cycles through the reactor, or make a
single pass, depending upon the level of initial
contamination level and final desired (or required) final
level and desired (or required) treatment time.
Conveniently, the reactor is used at ambient temperature and
pressure, although other conditions can be selected as
appropriate.
Wastewater to be treated can come from industrial
sources, such as debarking effluent, and pulp and
papermaking effluent. Preferred target aryl compounds in
such wastewater are phenol and o-, m- and p-cresol. The
reactor described herein has the capability of destroying
the target compounds even in the presence of other organic
compounds, such as butanoic acid, pentanoic acid, hexanoic
acid, butanedioic acid, camphor, borneol, linalyl
propanoate, furan carboxaldehyde, cyclohexanecarboxylic
acid, 2-(2-hydroxy-2-propyl)-5-methyl-cyclohexanol, benzoic
acid, 4-hydroxy-benzenepropanoic acid, or inorganic species
9
CA 02336507 2001-02-14
such as calcium, iron, magnesium, manganese, aluminum, zinc,
sodium and potassium.
The total organic carbon (TOC) level of the wastewater
to be treated is preferably less than 7500ppb, more
preferably less than 1500ppb.
The Anode
The anode conveniently should be made from a material
that is stable in the wastewater to be treated, and that
provides reasonable activity for the destruction of the
target compounds. The anode is preferably non-sacrificial.
The anode typically is constituted by a coated
substrate, the substrate preferably being a valve metal,
such as tantalum or titanium. Although various anode
substrates could be used, such as nickel, stainless steel
alloys or other corrosion resistant materials, titanium is
preferred. The anode substrate should be in the form of a
porous or 3D medium (sponge, foam, felt or mesh). A foam-
type is preferred, such as the Astro Met ~ materials (Astro
Met, Cincinnati, Ohio), in a configuration similar to that
shown in Figures 2 and 3. Each anode should have a pore
opening value of up to 40 pores per linear inch (ppi),
preferably 20ppi, to allow liquid flow with minimal
resistance.
When titanium is used as anode substrate, it is
preferably first activated through a process that removes
the surface oxide layer. Treating the titanium with boiling
concentrated hydrochloric acid is one such process. The
treated titanium is then quickly coated with the selected
anode material.
io
CA 02336507 2001-02-14
The anode is where the electrooxidative processes take
place. Destruction of an organic compound by oxidation is
a two-fold process:
Step 1. H20 + M -~ M-OH- + H+ + e-
In Step 1, the water molecule is split into hydrogen
and hydroxyl radicals. The anode (M) serves as a base to
the formation of these two species (it acts as an
electrocatalyst). The second step involves the oxidation of
an organic compound (R):
Step 2. R + M-OH' -~ M + RO + H+ + e-
where RO corresponds to the oxidized organic compound.
This overall reaction competes with the reaction that
forms oxygen. Electrochemical efficiency is defined as the
ratio between the two main anodic reactions.
Anode materials were tested for stability and
efficiency to destroy organic contaminants in high TOC
wastewater. The results of these tests are summarized in
Table 1:
Anode Material Efficiency for Electrochemical
Organic Destruction Stability
Platinum Very good Very good
Tantalum doped Very good Poor
Iridium Dioxide
Antimony doped Good Very good
Tin Dioxide
Table 1
m
CA 02336507 2001-02-14
Platinum, electrodeposited on a titanium substrate (see
Figure 4), exhibited high efficiency, together with high
stability. Platinum was efficient in electrolyzing
wastewater contaminated with phenol compounds, and is thus
the preferred anode material. A summary of the evaluated
efficiencies of the anode materials described in Table 1 is
given in Figure 6 and Figure 7.
As well as platinum exemplified above, other metals
such as palladium, rhodium, iridium or ruthenium, alone or
in alloys with themselves or other suitable metals, can be
used as the anode material.
Antimony-doped tin dioxide (see Figure 5) coated anodes
have been shown to be good at destroying organic compounds.
However, it was the least efficient anode material that was
tested in the reactor of the invention, most probably due to
the presence of numerous other organic species in the
wastewater to be treated.
Although tantalum-doped iridium dioxide-coated anodes
showed a very good efficiency for destroying organic
compounds, it was found that, over a period of time, the
coating tends to spall off the anode substrate.
Although it is preferred that the tin dioxide and
iridium dioxide coatings are doped as described above, they
can each generally be doped with a dopant selected from Sb,
Ta, F, C1, Mo, W and Nb, and mixtures thereof, if required.
Known coating methods can be used to coat the anodes.
The invention is augmented when the coating is uniform and
homogeneous on the substrate.
12
CA 02336507 2001-02-14
The Cathode
A cathode is necessary to complete the electrical
circuit and allow the electrochemical oxidation process to
be possible. The cathode can be formed from a porous or 3D
medium (foam, sponge, felt or mesh) and is preferably of a
structure similar to that shown in Figures 2 and 3. Each
porous cathode should have a pore opening value of up to 40
pores per linear inch (ppi), preferably 20ppi, to allow
liquid flow with minimal resistance. The cathode can also
have other structures, such as a ring-like structure.
The cathode material can be nickel, nickel alloys,
stainless steel or even titanium, or any other corrosion
resistant material. Nickel is preferred because of its
acceptable cost, stability in water and because it is
commercially available in a porous-type structure such as
found in Astro Met ~ materials (Astro Met, Cincinnati,
Ohio) .
Examples
Example 1
A solution from origin A, containing a total
concentration of 7051 ppb of phenolic contaminant compounds,
was treated in a reactor built with antimony-doped tin
dioxide anodes for 72 hours. The anodic current density was
5.6 mA/cm2, the flow rate was 8.2 1/minute, corresponding to
a volume to treat of 6.8 liters of solution per volume liter
of reactor, and the total applied current was 300 mA,
corresponding to 76104 coulombs. After the treatment
period, the final total concentration of the phenolic
13
CA 02336507 2001-02-14
compounds went down to 26 ppb. The concentration decrease
of each species is shown in Table 2.
Compound\ 0 4925 24077 31738 50890 76104
charge (C)
Phenol 2600 1500 140 63 0 0
0-cresol 51 24 0 0 0 0
m-cresol 600 270 20 10 0 0
p-cresol 3800 1100 71 44 30 26
Table 2
Example 2
A solution from origin A, containing a total
concentration of 7519 ppb of phenolic contaminant compounds,
was treated in a reactor built with antimony-doped tin
dioxide anodes for 48 hours. The anodic current density was
2.8 mA/cm2, the flow rate was 8.2 1/min., corresponding to a
volume to treat of 32.8 liters of solution per volume liter
of reactor, and the total applied current was 600 mA,
corresponding to 77760 coulombs. After the treatment
period, the final total concentration of the phenolic
compounds went down to 23 ppb. The concentration decrease
of each species is shown in Table 3.
14
CA 02336507 2001-02-14
Compound\ 0 5683 10543 38362 48752 77760
charge (C)
Phenol 2800 910 630 23 0 0
0-cresol 39 12 0 0 0 0
m-cresol 480 210 140 0 0 0
p-cresol 4200 730 220 38 29 23
Table 3
Example 3
A solution from origin B, containing a total
concentration of 2783 ppb of phenolic contaminant compounds,
was treated in a reactor built with antimony-doped tin
dioxide anodes for 12 hours. The anodic current density was
1.4 mA/cm2, the flow rate was 8.2 1/min., corresponding to a
volume to treat of 32.8 liters of solution per volume liter
of reactor, and the total applied current was 150 mA,
corresponding to 6480 coulombs. After the treatment period,
the final total concentration of the phenolic compounds went
down to 710 ppb. The concentration decrease of each species
is shown in Table 4.
Compound\ 0 540 1060 3240 4860 6480
charge (C)
Phenol 1174 1298 1015 643 586 540
0-cresol 25 13 13 9 8 7
m- + p-cresol 1219 762 593 385 259 163
Total 2418 2073 1621 1038 853 710
Table 4
Example 4
A solution from origin B, containing a total
concentration of 2374 ppb of phenolic contaminant compounds,
is
CA 02336507 2001-02-14
was treated in a reactor built with tantalum-doped iridium
dioxide anodes for 6 hours. The anodic current density was
5.6 mA/cm2, the flow rate was 8.2 1/min., corresponding to a
volume to treat of 32.8 liters of solution per volume liter
of reactor, and the total applied current was 300 mA,
corresponding to 6480 coulombs. After the treatment period,
the final total concentration of the phenolic compounds went
down to 922 ppb. The concentration decrease of each
species is shown in Table 5.
Compound\ 0 540 1080 2160 4320 6480
charge (C)
Phenol 822 772 754 665 486 358
0-cresol 27 25 25 20 14 10
m- + p- 1525 1454 1474 1176 801 554
cresol
Total 2374 2251 2253 1861 1301 922
Table 5
Example 5
A solution from origin B, containing a total
concentration of 2343 ppb of phenolic contaminant compounds,
was treated in a reactor built with tantalum-doped iridium
dioxide anodes for 12 hours. The anodic current density was
2.8 mA/cm2, the flow rate was 8.2 1/min., corresponding to a
volume to treat of 32.8 liters of solution per volume liter
of reactor, and the total applied current was 150 mA,
corresponding to 6480 coulombs. After the treatment period,
the final total concentration of the phenolic compounds went
down to 272 ppb. The concentration decrease of each species
is shown in Table 6.
16
CA 02336507 2001-02-14
Compound\ 0 540 1620 3330 4860 6480
charge (C)
Phenol 771 600 432 272 202 116
0-cresol 28 17 14 8 8 6
m- + p- 1545 951 733 380 298 151
cresol
Total 2343 1568 1179 660 509 272
Table 6
Example 6
A solution from origin B, containing a total
concentration of 2783 ppb of phenolic contaminant compounds,
was treated in a reactor built with tantalum-doped iridium
dioxide anodes for 12 hours. The anodic current density was
1.4 mA/cm2, the flow rate was 8.2 1/min., corresponding to a
volume to treat of 32.8 liters of solution per volume liter
of reactor, and the total applied current was 75 mA,
corresponding to 6480 coulombs. After the treatment period,
the final total concentration of the phenolic compounds went
down to 115 ppb. The concentration decrease of each species
is shown in Table 7.
i7
CA 02336507 2001-02-14
Compound\ 0 270 540 1080 1620 6480
charge (C)
Phenol 788 704 524 380 285 37
0-cresol 36 31 21 17 13 7
m- + p- 1960 1551 1066 723 515 70
cresol
Total 2783 2286 1612 1121 813 115
Table 7
Example 7
A solution from origin B, containing a total
concentration of 1369 ppb of phenolic contaminant compounds,
was treated in a reactor built with anodes made of platinum
electroplated on sponge titanium substrate for 9 hours. The
anodic current density was 5.6 mA/cm2, the flow rate was 8.2
1/min., corresponding to a volume to treat of 32.8 liters of
solution per volume liter of reactor, and the total applied
current was 200 mA, corresponding to 6480 coulombs. After
the treatment period, the final total concentration of the
phenolic compounds went down to 48 ppb. The concentration
decrease of each species is shown in Table 8.
Compound\ 0 720 1440 3120 4560 6480
charge (C)
Phenol 359 286 253 173 119 16
0-cresol 22 15 15 11 8 4
m- + p- 988 690 595 373 250 29
cresol
Total 1369 990 863 557 377 48
Table 8
ie
CA 02336507 2001-02-14
Example 8
A solution from origin B, containing a total
concentration of 1994 ppb of phenolic contaminant compounds,
was treated in a reactor built with anodes made of platinum
electroplated on foam titanium substrate for 18 hours. The
anodic current density was 2.8 mA/cm2, the flow rate was 8.2
1/min., corresponding to a volume to treat of 32.8 liters of
solution per volume liter of reactor, and the total applied
current was 100 mA, corresponding to 6480 coulombs. After
the treatment period, the final total concentration of the
phenolic compounds went down to 49 ppb. The concentration
decrease of each species is shown in Table 9.
Compound\ 0 360 1440 2880 4680 6480
charge (C)
Phenol 519 416 246 97 54 23
0-cresol 29 20 13 6 3 2
m- + p- 1445 1078 585 156 46 24
cresol
Total 1994 1514 844 259 103 49
Table 9
Example 9
A solution from origin B, containing a total
concentration of 1829 ppb of phenolic contaminant compounds,
was treated in a reactor built with anodes made of platinum
electroplated on foam titanium substrate for 18 hours. The
anodic current density was 1.4 mA/cm2, the flow rate was 8.2
1/min., corresponding to a volume to treat of 32.8 liters of
solution per volume liter of reactor, and the total applied
current was 50 mA, corresponding to 6480 coulombs. After
the treatment period, the final total concentration of the
19
CA 02336507 2001-02-14
phenolic compounds went down to 52 ppb. The concentration
decrease of each species is shown in Table 10.
Compound\ 0 360 720 1260 4320 6480
charge (C)
Phenol 479 375 255 137 23 29
0-cresol 24 19 13 9 3 2
m- + p- 1356 957 651 381 38 21
cresol
Total 1829 1351 919 528 64 52
Table 10