Note: Descriptions are shown in the official language in which they were submitted.
CA 02336654 2001-01-05
The present invention relates to a medicinal composition for
transdermal application of an estrogen in combination with a
gestagen.
in cutaneous application of active substances one aims at the
systemic action of the pharmaceutical substances. By reason
of the limited permeability of the skin, the substances suit-
able and preferred for this purpose are those which are ap-
plied in low dosage (daily doses of up to 10 mg).
Cutaneous application is useful in those cases where after
oral administration a large portion of the active substance
is metabolized during the first passage through the mucous
membranes of the gastrointestinal tract, or is retained by
the liver (first pass effect), and/or where the active sub-
stance has a low plasma half time. By contrast, those sub-
stances having a high allergising potential as well as those
having a locally irritating action are unsuitable. Where the
basic requirements are fulfilled, cutaneous application is an
alternative to oral administration.
The invention relates to an active substance-containing de-
vice, releasing one or more pharmaceutical substances at a
pre-determined rate, continuously, over a fixed period of
time, to a fixed site. Such a device is characterized by an
exact treatment program and is called a therapeutic system.
Since the system according to the present invention is ad-
hered as a patch to the skin to achieve a systemic effect,
the system in this context is called a transdermal therapeu-
tic system (TTS).
CA 02336654 2001-01-05
2
The invention relates to a preparation having a high degree
of action, that is the preparation leads to a high bioavail-
ability of the pharmaceutical substances. This is achieved in
that the presystemic elimination is strongly reduced by
avoiding the digestive tract, the efficacy of the pharmaceu-
tical substances being independent of the gastric emptying
rate and the intestinal motility.
The action of the pharmaceutical substances can be stopped at
any time - with a certain delay - by simply removing the
preparation. The plasma concentration can be set within the
therapeutic range without any peaks and lows. As a conse-
quence, the preparation is characterized by a controllability
of the absorption phenomena.
The preparation according to the invention exhibits high re-
liability as regards the observance of the therapy plan by
the patient since the frequency of application as compared to
conventional oral pharmaceutical forms is strongly reduced.
Furthermore, the active substance amount applied can gener-
ally be reduced. Dose-dependent side effects are thereby re-
duced, too, or they are even eliminated. This results in im-
proved therapy safety.
Estradiol, estrone (estrogens) and progesterone (gestagen)
are the natural female sex hormones. Sex hormones serve to
form the primary and secondary sex characteristics. They in-
fluence growth and body biiilding as well as the water and
mineral balance. Furthermore, sex hormones.determine the se-
quence of the menstrual cycle in women.
Natural sex hormones, their derivatives as well as structure
analogues are used for hormonal contraception, for substitu-
tion therapy or for treatment of various diseases.
CA 02336654 2004-08-06
3
A main area of application of sex hormones is the field of
postmenopausal hormone substitution. The substitution serve:>
to prevent climacteric complaints (hot flushes, giddiness,
tachycardias, sweating, feeling of anxiety, irritability, bad
concentration, sleep disturbances, etc.). Furthermore,
changes occurring in the urinary and genital organs, cardio-
vascular changes caused by hyperlipoproteinemia, skin atro-
phies, or osteoporosis, as well as further pathologic phenom-
ena are meant to be prevented. For this purpose, an estrogen
is administered in combination with a gestagen.
For example, the following estrogens may be used: 17-beta-
estradiol, 17-alpha-estradiol, 17-beta-estradiol cypionate;
17-beta-ethinyl estradiol, 3,17-beta-estradiol dienanthate,
17-beta-estradiol valerate, 17-beta-estradiol benzoate, 17-
beta-estradiol undecylate, 17-deacetyl norgestimate, norges-
timate, mestranol and quinestrol. The estrogens mentioned are
characterized by an aromatic hydroxyl group or the ethers
thereof.
Since the natural gestagen progesterone exhibits insufficient
pharmacokinetic properties for substitution therapy, numerous
modification products have been synthesized. Examples to be
mentioned are: 19-norprogesterone, norethisterone acetate,
norethisterone, ethisterone, melengestrol, norgestrel, levo-
norgestrel, gestodene, hydroxyprogesterone capronate, me-
droxyprogesterone acetate, ethynodiol diacetate, 17-alpha-
hydroxyprogesterone, megestrol acetate, lynestrenol, deso-
gestrel, allyl estrenol, chlormadinone and.chlormadinone ace=-
tate. A typical structural feature of most of the compounds
is a 3-keto-4-ene structure.
If estradiol is administered orally, only a small portion
thereof is absorbed due to its poor water solubility. The
portion absorbed is subject to a strong first pass effect. In
this process, numerous metabolites are disintegrated which no
CA 02336654 2001-01-05
4
longer exhibit an estrogen effect and which lead to side ef-
fects. Furthermore, oral administration leads to unphysi-
ological fluctuation of the hormone blood level. Owing to the
first pass effect it is, in addition, necessary to administer
large amounts of estradiol. This causes further side effects.
The ideal application for estradiol would be a slow intrave-
nous infusion. This is, however, impractical. By transdermal
administration it is possible to achieve almost ideal condi-
tions. in this case the first pass effect is avoided, and
plasma concentrations are obtained which correspond to the
physiological hormone blood level of a premenopausal woman
(40 - 60 pg/ml). A further advantage of transdermal admini-
stration over oral application is that the daily dose can be
reduced to 50 }ig/day.
The main risk connected with postmenopausal estradiol admini-
stration is hyperstimulation of the endometrium, in conjunc-
tion with an increased risk of hyperplasia or degeneration.
Furthermore, in the case of monotherapy with estradiol this
is frequently accompanied by disorders of menstruation. To
reduce these risks it is useful to administer estradiol in
combination with a gestagen. In the case of transdermal ad-
ministration, to prevent hyperplasia, about 200 - 300 Kg of
norethisterone acetate or of a corresponding equivalent are
necessary per day. In comparison thereto, with peroral ad-
ministration, 0.7 - 1 mg/day are required [Wiseman, L.R. and
McTavish, D., Transdermal Estradiol/Norethisterone: A Review
of its Pharmacological Properties and Clinical Use in Post-
menopausal Women, Drug & Aging, Vol. 4, No. 3, 1994, 238-
256] .
Combined TTSs having a composition for transdermal applica-
tion of an estrogen, especially of estradiol, in combination
with a gestagen, especially of norethisterone acetate, are
already known and available on the market (e.g. Estracomb ).
CA 02336654 2001-01-05
Their complex structure is a disadvantage. Thus, Estracomb
contains two compartments, serving as an active substance
reservoir. Furthermore, a control membrane is contained
therein for controlled active substance release. Due to the
space-consuming compartments, the systems is very thick and
is therefore uncomfortable to wear.
Examples for simple-structure matrix systems for transdermal
application of estradiol in combination with gestagen are de-
scribed in EP 0 695 177 B1. WO 96/40087 describes a matrix
system on the basis of a cross-linked acrylate polymer for
transdermal application of estradiol
When storing steroid-containing TTS of the matrix type, two
stability problems occur inter alia: the active substances
may be recrystallized or disintegrated.
Recrystallization occurs when the saturation concentration of
the corresponding active substance is exceeded. If the satu-
ration concentration is exceeded, this can be a result of a
modification change. Thus, estradiol is apt to forming a
semihydrate by absorbing crystal water, said semihydrate re-
crystallizing by reason of its lower solubility. Furthermore,
there is a possibility that mixed crystals are formed. Here,
the solubility of the mixed crystals is lower than the satu-
ration solubility of the individual components. As a result
of the recrystallization, there is a decrease in the thermo-
dynamic activity and the ptrmeation rate of the active sub-
stances through the skin. The therapeutic efficacy of the
preparation is thereby endangered.
To prevent the crystallization of the estradiol, various pos-
sibilities have been described. The system described in the
US patent 5,676,968 contains in the active substance-
containing compartment auxiliary substances which are de-
scribed as "crystallization inhibitors" and which are to
CA 02336654 2001-01-05
6
counteract the crystallization process. In particular, sili-
con dioxide and macromolecular substances, such as polyvinyl-
pyrrolidone or its copolymers with vinyl acetate are men-
tioned there. In US patent 5,711,962 and in WO 97/23227 the
addition of octyl dodecanol is described, for preventing
crystallization. In WO 96/05814 a system is described con-
taining anhydrous glycerin as a component of the matrix. An-
hydrous glycerin is miscible with water in any ratio, it is
hygroscopic and can be employed as a dehydrating agent. If
anhydrous glycerin is stored together with estradiol semihy-
drate, it is capable of withdrawing water therefrom. Anhy-
drous glycerin is thus a suitable agent for preventing re-
crystallization during storage. In WO 96/05815 the addition
of water-binding mineral components to the active substance-
containing matrix is described, so that the recrystallization
of estradiol semihydrate is prevented. As mineral components
are mentioned, for example, the anhydrate of calcium sulfate,
zink oxide, magnesium oxide, silicon dioxide, silica gel,
talcum and further substances. DE 42 37 453 proposes the use
of desiccants in the primary package. EP 0 186 019 Al de-
scribes the use of water-swellable polymers for crystal
growth inhibition of the active substance, which is present
in a concentration above its saturation concentration. EP 0
695 177 B1 describes a system containing estradiol in a con-
centration near the saturation concentration. The saturation
concentration is exceeded, through absorption of water, only
after the system is stuck on the skin, thereby increasing
thermodynamic activity. Iii this process, aipha-tocopherol de-
termines the degree of oversaturation of the hydrogenated ma-
trix and thus the diffusion of active substance through the
skin. Alpha-tocopherol in this context serves to improve ac-
tive substance solubility and to prevent recrystallization
during storage.
Steroids (hormones and corticoids) are disintegrated to a
lesser or greater degree during storage, dependent on the
CA 02336654 2001-01-05
S
7
auxiliary substances used. Various degradation mechanisms
must be taken into consideration. On the one hand, saponifi-
cation reactions occur, which lead to more strongly hydro-
phile compounds, and on the other hand, oxidation reactions
occur, which lead to ineffective products. In particular,
steroids, which have a 3-keto-4-ene partial structure, are
very sensitive. For this instability, acidic-reacting groups
are held responsible inter alia. Thus, WO 97/03629 describes
a system having a carrier that has no acid functions and does
not develop such functions during storage either. DE 195 48
332 Al describes a hormone patch with norethisterone acetate
which exhibits good storage stability. The stability here is
achieved by using a certain polymer mixture. WO 97/23227 de-
scribes that the degradation of norethisterone acetate is de-
pendent on the moisture content of the matrix. Consequently,
it is advantageous to work with dried air during manufacture
and to integrate a desiccant (e.g. sodium sulfate or calcium
sulfate) in the primary package. It is further described that
the stability of norethisterone acetate is improved if during
manufacture the active substance is dissolved in a solvent
mixture, consisting of methyl ethyl ketone and ethanol.
Apart from the stability problems mentioned, it is necessary
to ensure the therapeutic efficacy of the TTSs. Therapeutic
efficacy is determined by the extent of skin permeation. To
guarantee that permeation is sufficient, enhancers are fre-
quently used. For example, in US patent 5,676,968 the use of
1,2-propanediol and isopr6pyl myristate for increasing estra-
diol permeation is described by way of example. EP 0 811 381
Al describes the use of fatty alcohols in combination with a
diethylene glycol monoalkyl ether as enhancer for estradiol
and norethisterone acetate. US patent 5,686,097 mentions the
use of monoglycerides and lactate esters for enhancing pene-
tration of an estrogen/gestagen combination.
CA 02336654 2001-01-05
8
The use of enhancers, however, is not always without prob-
lems. Many of the substances employed lead to skin irrita-
tions. To alleviate such irritations, further substances are
frequently used which are to counteract such irritations.
Glycerol and polyglycerol ether are to be mentioned here, for
example. Besides its lenitive effect, glycerol also has a
positive effect on the permeation rate. it is of disadvantage
that glycerin strongly decreases the cohesion of a poly-
acrylate matrix. A deterioration in cohesion manifests itself
in increased "tack", increased "cold flow", a transfer of ad-
hesive to the protective film, or in the fact that residues
of adhesive remain on the skin after removal of the system.
To generally improve the cohesion of systems having a high
content of liquid or softening components, US patent
5,306,503 mentions the addition of so-called film-formers.
It has been the object of the present invention to provide a
TTS having a simple structure, a so-called matrix system,
which has a low-cost composition, for transdermal application
of an estrogen, especially of estradiol, in combination with
a gestagen, especially of norethisterone acetate, which TTS
guarantees an effective pharmaceutic therapy of climacteric
complaints.
Matrix systems are characterized in that the active sub-
stances are present finely distributed (dissolved or dis-
persed) in a polymer matrix. The matrix here has a reservoir
function and an adhesive function. Whereas in a membrane sys-
tem the transdermal active substance uptake is largely regu-
lated by the integrated control membrane, in matrix systems
this function is performed by the skin. The production of the
active substance-containing matrix is very simple.
This task is solved by a self-adhesive composition based on
polyacrylate for transdermal application according to the
CA 02336654 2001-01-05
9
features of the main claim. Preferred and advantageous em-
bodiments have the features as described in the subclaims.
Polyacrylate-based compositions have a very low allergenic
potential and are therefore also suitable for prolonged ap-
plication. The adhesiveness of the polymers is brought about
here only by the molecular weight distribution and the mono-
mers used.
To produce polyacrylate-based compositions according to the
invention, acrylic acid and/or alkyl acrylic acid, especially
methacrylic acid or their derivatives, especially the alkyl
esters, are employed. Among the alkyl esters of acrylic acid
and/or methacrylic acid are those having 1 to 18 carbon atoms
in the alkyl residue, especially methyl, ethyl, n-butyl, iso-
butyl, pentyl, 2-ethylbutyl, n-hexyl, heptyl, n-octyl, iso-
octyl, 2-ethylhexyl, n-decyl, isodecyl, n-dodecyl and stearyl
acrylate or methacrylate. Apart from these, further comono-
mers can participate in the structure of the poly-
mer/copolymer. Examples are acrylic and/or methacrylic amide,
hydroxyalkyl esters and polyalkylene glycol esters of acrylic
and/or methacrylic acid, nitrogen-containing monomers of
acrylic and/or methacrylic acid or the salts thereof, ethyl-
ene, vinyl acetate, vinyl propionate, vinyl butyrate, vinyl-
pyrrolidone, vinyl chloride, vinyl toluene, acrylonitril,
styrene and the like.
For producing a system according to the invention an acrylate
copolymer is advantageously used which contains 2-ethylhexyl
acrylate, vinyl acetate, hydroxyethyl acrylate and glycidyl
methacrylate.
To ensure the therapeutic efficacy, glycerol is used as en-
hancer. To this end, glycerol must be incorporated into the
matrix in larger amounts. It is problematic that, if present
in larger amounts, glycerol diminishes the cohesion of the
CA 02336654 2006-07-05
matrix. To counteract this effect, a further polymer is added
to the acrylate base, which polymer is non-adhesive itself,
but has very good film-forming properties. This film-forming
polymer has a positive influence on the cohesion of the ma-
trix. Polymers based on polyacrylic or polymethacrylic acid
and their esters have proved to be well suited.
To prepare a system according to the invention, a film-
forming acrylate copolymer having 10 - 90%-wt. of methacrylic
acid and 10 - 90%-wt. of inethyl methacrylate are used. It is
surprising, that the addition of the acid-reacting components
does not lead to increased degradation of the incorporated
hormones having 3-keto-4-ene partial structure. This is in
stark contradiction to the test results described in WO
97/03629. By contrast, the use of the polymer mentioned
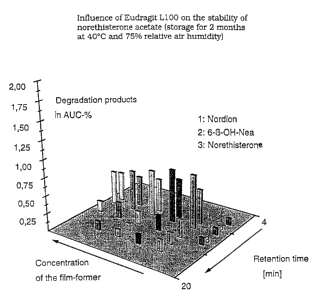
rather contributes to improving stability. FIG. 1 illustrates
the influence of Eudragit*L100 on the formation of degrada-
tion products of norethisterone acetate. The bars designated
with figures 1 - 3 represent known degradation products (Nor-
dion, 6-beta-hydroxynorethisterone acetate and norethister-
one). The z-axis indicates the associated retention times,
corresponding to the RP-HPLC that has been carried through.
The y-axis gives the percentages for the degradation products
formed, in relation to the overall surface in the RP-HPLC
chromatogram. The second row from the right on the x-axis
shows that a formulation containing no Eudragit* L100 has the
largest number of degradation products. With increasing con-
tent of Eudragit'~L100 (from right to left, of 1- 10% Eu-
dragit '%100, relative to the dry matter), the sum of the deQ-
radation products decreases. The TTSs are stored for two
months at 40 C and 27% relative air humidity.
To improve cohesion, advantageously, additives, preferably
metal ions, such as aluminium or titanium are incorporated.
TM
CA 02336654 2001-01-05
11
If the adhesive power of the matrix has been reduced by the
addition of film-forming polymers, this can be effectively
compensated by admixing of strongly tackifying resins. As
tackifying agents, colophony resins, polyterpene resins, pe-
troleum resins, coumarone-indene resins, terpene phenol res-
ins, hydrocarbon resins, liquid polybutene resins and the
like may be used in the production of the composition accord-
ing to the invention.
In the following, the invention will be illustrated by means
of an example:
EXAMPLE:
1,4 g of estradiol, 7.5 g of norethisterone acetate, 105 g of
acrylate adhesive (Durotak 387-2287), 46 g of adhesive resin
(e.g. Hercolyn DE), 10 g of Eudragit L100, 30 g of glycerin,
15 g of acetyl acetone and 0.1 g of aluminium acetyl aceto-
nate (4%-wt. in ethyl acetate) were mixed. The layer was ap-
plied to a siliconized polyester film (e.g. Hostaphan ) with
the aid of a doctor knife, at a wet-coating thickness of 200
pm. The moist film was dried for 30 minutes at 50 C and
subsequently laminated with a polyester film (e.g. Hosta-
phan ). The weight per unit area of an adhesive film prepared
in this manner was about 80.4 g/m2. From the laminate, TTSs
of the desired size were punched out by means of a suitable
punch. The TTSs were stored at various temperatures for ex-
amination of their stabil'rty.