Some of the information on this Web page has been provided by external sources. The Government of Canada is not responsible for the accuracy, reliability or currency of the information supplied by external sources. Users wishing to rely upon this information should consult directly with the source of the information. Content provided by external sources is not subject to official languages, privacy and accessibility requirements.
Any discrepancies in the text and image of the Claims and Abstract are due to differing posting times. Text of the Claims and Abstract are posted:
| (12) Patent: | (11) CA 2336711 |
|---|---|
| (54) English Title: | BLISTER CONTAINING A TRANSDERMAL THERAPEUTIC SYSTEM AND A SINGLE DOSE FORM OF ADMINISTRATION |
| (54) French Title: | PLAQUETTE ALVEOLAIRE CONTENANT UN SYSTEME THERAPEUTIQUE TRANSDERMIQUE ET UNE FORME GALENIQUE INDIVIDUELLE |
| Status: | Deemed expired |
| (51) International Patent Classification (IPC): |
|
|---|---|
| (72) Inventors : |
|
| (73) Owners : |
|
| (71) Applicants : |
|
| (74) Agent: | BLAKE, CASSELS & GRAYDON LLP |
| (74) Associate agent: | |
| (45) Issued: | 2007-05-29 |
| (86) PCT Filing Date: | 1999-07-02 |
| (87) Open to Public Inspection: | 2000-01-20 |
| Examination requested: | 2003-09-11 |
| Availability of licence: | N/A |
| (25) Language of filing: | English |
| Patent Cooperation Treaty (PCT): | Yes |
|---|---|
| (86) PCT Filing Number: | PCT/EP1999/004614 |
| (87) International Publication Number: | WO2000/002538 |
| (85) National Entry: | 2001-01-05 |
| (30) Application Priority Data: | ||||||
|---|---|---|---|---|---|---|
|
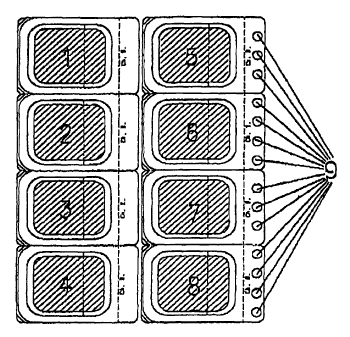
A dosage form for medicinal active ingredients in blister
form is characterized in that it contains a transdermal
therapeutic system alongside at least one single-dose
active ingredient form.
Forme galénique se présentant sous la forme d'une plaquette alvéolaire de substances médicinales et caractérisée par le fait qu'elle contient l'un à côté de l'autre un système thérapeutique transdermique et au moins une dose individuelle de substance médicinale.
Note: Claims are shown in the official language in which they were submitted.
Note: Descriptions are shown in the official language in which they were submitted.
For a clearer understanding of the status of the application/patent presented on this page, the site Disclaimer , as well as the definitions for Patent , Administrative Status , Maintenance Fee and Payment History should be consulted.
| Title | Date |
|---|---|
| Forecasted Issue Date | 2007-05-29 |
| (86) PCT Filing Date | 1999-07-02 |
| (87) PCT Publication Date | 2000-01-20 |
| (85) National Entry | 2001-01-05 |
| Examination Requested | 2003-09-11 |
| (45) Issued | 2007-05-29 |
| Deemed Expired | 2011-07-04 |
There is no abandonment history.
| Fee Type | Anniversary Year | Due Date | Amount Paid | Paid Date |
|---|---|---|---|---|
| Registration of a document - section 124 | $100.00 | 2001-01-02 | ||
| Application Fee | $300.00 | 2001-01-02 | ||
| Maintenance Fee - Application - New Act | 2 | 2001-07-03 | $100.00 | 2001-01-02 |
| Maintenance Fee - Application - New Act | 3 | 2002-07-02 | $100.00 | 2002-06-19 |
| Maintenance Fee - Application - New Act | 4 | 2003-07-02 | $150.00 | 2003-06-20 |
| Request for Examination | $400.00 | 2003-09-11 | ||
| Maintenance Fee - Application - New Act | 5 | 2004-07-02 | $200.00 | 2004-06-30 |
| Maintenance Fee - Application - New Act | 6 | 2005-07-04 | $200.00 | 2005-06-29 |
| Maintenance Fee - Application - New Act | 7 | 2006-07-04 | $200.00 | 2006-06-27 |
| Final Fee | $300.00 | 2007-03-16 | ||
| Maintenance Fee - Patent - New Act | 8 | 2007-07-03 | $200.00 | 2007-06-26 |
| Maintenance Fee - Patent - New Act | 9 | 2008-07-02 | $200.00 | 2008-06-20 |
| Maintenance Fee - Patent - New Act | 10 | 2009-07-02 | $250.00 | 2009-06-22 |
Note: Records showing the ownership history in alphabetical order.
| Current Owners on Record |
|---|
| LTS LOHMANN THERAPIE-SYSTEME AG |
| Past Owners on Record |
|---|
| ASMUSSEN, BODO |
| FRANKE, HANSHERMANN |
| SCHAFER, WOLFGANG |