Note: Descriptions are shown in the official language in which they were submitted.
CA 02337615 2001-O1-03
WO 00/66009 PCT/IJS00/12073
METHODS AND DEVICES FOR FORMING A CONDUIT BETWEEN
A TARGET VESSEL AND A BLOOD SOURCE
CROSS-REFERENCES TO RELATED APPLICATIONS
This application is a continuation-in-part of the following U.S. patent
applications: application serial no. 09/023,492, filed on February 13, 1998
and entitled
"Methods and Devices Providing Transmyocardial Blood Flow to the Arterial
Vascular
System of the Heart," the entire subject matter of which is incorporated
herein by
reference; application serial no. 09/232,103, filed on January 15, 1999 and
entitled
"Methods and Devices for Forming Vascular Anastomoses," the entire subject
matter of
which is incorporated herein by reference; and application serial no.
09/232,062, filed on
January 15, 1999 and entitled "Methods and Devices For Bypassing an Obstructed
Target
Vessel by Placing the Vessel in Communication with a Heart Chamber Containing
Blood," the entire subject matter of which is incorporated herein by
reference.
BACKGROUND OF THE INVENTION
Field of the Invention
The invention relates to methods and devices for forming a conduit
between a target vessel and a source of blood, and more particularly methods
and devices
for forming a conduit constructed at least in part of synthetic vascular graft
material.
2. Description of the Background Art
Despite the considerable advances that have been realized in cardiology
and cardiovascular surgery, heart disease remains the leading cause of death
throughout
much of the world. Coronary artery disease, or arteriosclerosis, is the single
leading
cause of death in the United States today. As a result, those in the
cardiovascular field
continue to search for new and improved treatments.
Coronary artery disease is can ently treated by interventional procedures
such as percutaneous transluminal coronary angioplasty (PTCA), atherectomy and
placement of coronary stents, as well as surgical procedures including
coronary artery
bypass grafting (CABG). The goal of these procedures is to reestablish or
improve blood
flow through occluded (or partially occluded) coronary arteries, and is
accomplished, for
example, by enlarging the blood flow lumen of the artery or forming a bypass
that allows
CA 02337615 2001-O1-03
WO 00/66009 PCTNS00/12073
blood to circumvent the occlusion. What procedures) is used typically depends
on the
severity and location of the blockages. When successful, these procedures
restore blood
flow to myocardial tissue that had not been sufficiently perfused due to the
occlusion.
CABG, the most common surgical procedure to treat coronary artery
disease, uses a graft vessel to deliver oxygenated blood to a coronary artery
downstream
of an obstruction in the artery. For example, in a typical CABG procedure one
end of a
graft vessel is attached to the aorta (proximal anastomosis) and another end
is attached to
the coronary artery (distal anastomosis). The anastomoses are formed by
suturing the
graft vessel to the coronary artery and aorta, typically in an end-to-side
manner.
Although suturing produces a strong anastomosis when done correctly, the
procedure is highly technical and time consuming due to the small size of the
vessels
being joined. The procedure is particularly difficult when carned out
minimally
invasively because of limited access to the heart and coronary arteries.
Further, forming a
hand-sewn anastomosis on a beating heart is very challenging for a majority of
surgeons.
Most CABG procedures are performed on a stopped heart despite recognized
drawbacks
associated with cardiopulmonary bypass.
Another one of the difficulties associated with CABG procedures, and in
particular multiple bypass procedures, is obtaining a sufficient number of
conduits to
bypass obstructions in several arteries. The conduits may comprise hollow
tissue
structures, e.g., the left and right internal mammary arteries, a saphenous
vein or
epigastroplegic artery. The conduits may also comprise synthetic vascular
graft material,
such as Dacron (polyester) or ePTFE (expanded polytetrafluoroethylene).
Synthetic vascular grafts having a diameter greater than about S mm have
been shown to generally remain free of thrombus over an extended period of
time. Grafts
of this size are used to treat lesions in larger peripheral vessels including
the distal
abdominal aorta, the aortoiliac segment, and the renal arteries. The
performance of
smaller synthetic vascular grafts, such as those with a diameter of S mm or
less, has been
unsatisfactory as the grafts typically become occluded over a relatively short
period of
time. See, for example, Eric T. Choi and Allan D. Callow, The Effect of
Biomarerials on
the Host, in Implantation Biology, pp. 40-S0, Ralph S. Greco Ed., (1994).
In addition to the cross-sectional size, length is another factor that may
adversely affect the patency of synthetic vascular grafts as, for a given
small diameter
graft vessel, increasing the length will usually increase the likelihood of
thrombosis.
Other factors affecting patency include the particular material used for the
conduit and its
2
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
characteristics, for example, the pore size or internodal distance of an ePTFE
conduit.
Patency may also be affected by joining the conduit to additional components,
for
example, an additional layers) of material, an internal andlor external
support such as a
stmt, etc.
These limitations have effectively prevented the use of synthetic vascular
grafts as conduits in CABG procedures. The coronary arteries that are treated
during a
CABG procedure typically have a diameter in the range of about 2-4 mm. In
addition, the
conduits must be long enough to extend from the blood source to a site on the
artery distal
to the lesion, for example, from the aorta to the distal left anterior
descending artery
(LAD). As such, the conduit size requirements associated with CABG procedures
have
limited the application of synthetic vascular graft materials in coronary
procedures.
Given the great number of CABG procedures performed every year, those in the
art have
attempted to develop conduits formed of synthetic vascular graft material that
are suitable
for use in bypassing obstructed coronary arteries.
As a result, graft vessels formed of tissue remain the most preferred and
widely used conduits in CABG procedures. Using tissue graft vessels, though,
has
several drawbacks. For example, harvesting graft vessels from the patient's
body is
painful and traumatic and increases the length and recovery time associated
with the
procedure. Another drawback arises when treating patients who do not have a
sufficient
number of peripheral vessels that can be harvested and used as bypass
conduits. The lack
of conduits, for example, may be due to the patient already having undergone a
CABG
procedures) or having peripheral vascular disease. In either case the
surgeon's options
are limited due to the unavailability of tissue grafts for use as conduits.
Accordingly, there remains a need in the art for methods and devices for
forming conduits between a target vessel, such as a coronary artery, and a
source of
blood, such as the aorta or a heart chamber, that may be used precisely and
relatively
quickly compared with current technology, and wherein the conduit is formed at
least in
part of synthetic vascular graft material.
SUMMARY OF THE INVENTION
According to one embodiment of the invention, a method is provided for
forming a conduit to place a coronary artery in fluid communication with a
source of
blood. The method includes steps of providing a first conduit comprising
synthetic
vascular graft material, and securing the first conduit to a coronary artery
to place the first
CA 02337615 2001-O1-03
WO 00/66009 PGT/US00/12073
conduit in fluid communication with the coronary artery. A second conduit
comprising a
hollow tissue structure is adapted to be placed in communication with a source
of blood
and is secured to the first conduit. The second conduit is placed in fluid
communication
with a source of blood.
According to another embodiment, the invention provides a method for
forming a conduit that is in fluid communication with a target vessel. This
method
includes steps of providing a tubular connector having a lumen, the tubular
connector
comprising a length of synthetic vascular graft material, and securing the
tubular
connector to a target vessel having a lumen. A graft vessel has a lumen and
comprises a
length of a hollow tissue structure. The graft vessel is secured to the
tubular connector to
form a conduit that places the lumens of the graft vessel and the target
vessel in fluid
communication.
According to yet another embodiment, the invention provides a method for
increasing the flow of blood to a selected site in a patient's arterial
vascular system. This
method includes steps of inserting a first end of a conduit into a heart
chamber containing
blood, and inserting a second end of the conduit into the arterial vascular
system at a
selected site in the vascular system. The first and second ends of the conduit
are
connected together, and the conduit is maintained in an open position for
blood flow
through at least one of the diastolic and systolic phases of the heart cycle.
According to another embodiment, the invention provides a method for
forming a conduit that is in fluid communication with a target vessel. The
method
includes steps of providing a tubular connector having a lumen and being
movable
between collapsed and expanded orientations, the tubular connector comprising
a length
of synthetic-vascular graft material, and disposing a sheath over at least a
portion of the
tubular connector to hold the portion of the tubular connector in the
collapsed orientation.
The collapsed portion of the tubular member is placed at least partially into
a lumen of a
target vessel, and relative movement is imparted to the sheath and a sheath
removal
mechanism to remove the sheath and allow the collapsed portion of the tubular
connector
to expand against a wall of a target vessel.
Pursuant to another embodiment of the invention, a device is provided for
being secured to a target vessel in fluid communication with the vessel lumen.
The
device includes a conduit adapted to be secured to a target vessel so as to be
in fluid
communication therewith, the conduit comprising synthetic vascular graft
material. A
vessel coupling is attached to the conduit in fluid communication with the
conduit, and
4
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
the vessel coupling is movable to an expanded orientation to secure the vessel
coupling
and the conduit to a target vessel. The conduit may be secured to a graft
vessel to
establish a conduit that is in fluid communication with the target vessel.
According to another embodiment, the invention provides a system for
forming a conduit that is in fluid communication with a target vessel having a
lumen.
The system includes a graft vessel comprising a hollow tissue structure, and a
tubular
connector secured to the graft vessel so as to be in fluid communication with
the graft
vessel. The tubular connector comprises synthetic vascular graft material, and
a vessel
coupling is attached to the tubular connector so as to be in fluid
communication
therewith. The vessel coupling is configured to be coupled to a target vessel
so as to
place the graft vessel in fluid communication with the target vessel.
According to another embodiment, the invention provides a device for
being secured to a target vessel in fluid communication with a lumen of the
target vessel.
The device includes a tubular connector having a lumen and being movable
between
I 5 collapsed and expanded orientations, the tubular connector comprising a
length of
synthetic vascular graft material. A sheath is disposed over at least a
portion of the
tubular connector to hold the portion of the tubular connector in the
collapsed orientation,
and a sheath removal mechanism is disposed adjacent the sheath. The sheath and
the
sheath removal mechanism are relatively movable with respect to one another,
and an
actuator is coupled to at least one of the sheath and the sheath removal
mechanism for
imparting relative movement to the sheath and the sheath removal mechanism,
which
allows the portion of the tubular connector held collapsed by the sheath to
expand against
the wall of the target vessel wall.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will be better understood from the following detailed
description of preferred embodiments thereof, taken in conjunction with the
accompanying drawing figures, wherein:
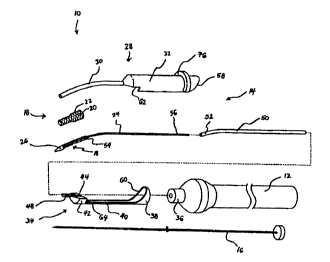
Fig. 1 is a perspective view of a device constructed according to a first
embodiment of the invention for forming a conduit to place a target vessel in
fluid
communication with a source of blood;
Fig. 2 is an exploded perspective view of the device shown in Fig. 1;
Fig. 3 is an enlarged perspective view of a distal portion of the device
shown in Fig. 1;
CA 02337615 2001-O1-03
WO 00/66009 PCTNS00/12073
Fig. 4 is an exploded perspective view of the distal portion of the device
shown in Fig. 3;
Fig. 5 is a fragmentary, longitudinal sectional view of the distal portion of
the device shown in Figs. 3 and 4;
Figs. 6A-6B are schematic perspective views sequentially illustrating the
removal of a sheath that forms part of the device shown in Fig. 1;
Fig. 7 is a schematic view of a patient prepared to undergo a
cardiovascular surgical procedure, the patient's heart being exposed via a
retractor
positioned in a thoracotomy foamed in the patient's chest;
Fig. 8 is a perspective view of the heart shown in Fig. 7 including an
obstructed coronary artery, wherein the distal end of the device shown in Fig.
1 is
positioned in the coronary artery;
Figs. 9A-9E are enlarged sectional views of a portion of the heart shown in
Fig. 8 sequentially illustrating using the device shown in Fig. 1 to secure a
conduit within
the lumen of the coronary artery, wherein Fig. 9E shows the conduit after it
has been
placed in fluid communication with the artery;
Fig. 10 is a perspective view of the heart shown in Fig. 8 illustrating the
conduit placed according to the steps shown in Figs. 9A-9D secured to the
target vessel in
fluid communication with the vessel lumen;
Fig. 1 OA is a perspective view showing the attachment of a conduit
constructed according to another embodiment of the invention secured to a
target vessel,
wherein the conduit is configured to preserve native blood flow through the
target vessel;
Fig. 11 is a perspective view of the exterior of the heart shown in Fig. 8
illustrating a first conduit secured to the artery and a second conduit
adapted to be
attached to the first conduit, wherein the second conduit is also adapted to
be coupled to
the aorta;
Fig. 12 is a perspective view of the heart shown in Fig. 11 illustrating a
completed proximal anastomosis formed between the second conduit and the
aorta;
Fig. 12A is a schematic perspective view of an instrument for coupling the
second conduit to the first conduit, wherein an end of the second conduit is
held in the
instrument;
Fig. 13 is a perspective view of the heart shown in Fig. 12 after the first
and second conduits have been coupled together to form a conduit that places
the
coronary artery in fluid communication with the aorta;
6
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
Fig. 14 is a perspective view of the exterior of the heart shown in Fig. 8
illustrating a first conduit secured to the artery;
Fig. 14A is a side elevation view of a second conduit adapted to be
attached to the first conduit shown in Fig. 14, wherein the second conduit is
also adapted
to be placed in fluid communication with a heart chamber containing blood;
Fig. 15 is a perspective view of the heart shown in Fig. 14 illustrating the
second conduit positioned in the myocardium in fluid communication with a
heart
chamber containing blood;
Fig. 1 SA is a schematic perspective view of an instrument for coupling the
second conduit to the first conduit, wherein an end of the second conduit is
held in the
instrument;
Fig. 16 is a perspective view of the heart shown in Fig. 15 after the first
and second conduits have been coupled together to form a conduit that places
the
coronary artery in fluid communication with a heart chamber containing blood;
and
Fig. 16A is a sectional view taken through the myocardium illustrating the
second conduit positioned in the myocardium so as to be in fluid communication
with the
heart chamber.
DESCRIPTION OF THE SPECIFIC EMBODIMENTS
Refernng to Figs. 1-5, a device constructed according to one preferred
embodiment of the invention is indicated generally by the reference numeral 10
and is
used to place a target vessel in fluid communication with a source of blood.
The device
10 comprises a handle 12, a shaft assembly 14 and an optional incising
component 16
(Fig. 2). The incising component 16 is used to penetrate tissue in order to
introduce the
device into a target vessel. While a separate incising component is shown in
the Figures,
the incising component could instead be formed as an integral part of the
device. Further,
it will be appreciated that the device 10 may be used without an incising
assembly for
piercing tissue, for example, by placing the shaft assembly 14 through a
surgical incision
in the vessel wall.
If used with an incising element, such as component 16, the shaft assembly
of the device may be provided with a bore that receives the incising element
and allows it
to be extended and retracted with respect to the device. One benefit of
providing a bore
through the shaft assembly of the device is that the conduit is protected from
contact with
any element located in the bore. Thus, an incising element, guide wire, guide
catheter,
7
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
etc., may be used without risk of damage to the conduit, the bore allowing the
device to
be passed over a guide wire or catheter that has been introduced into the
lumen of a
vessel. Moreover, the bore may be configured to act as a flashback lumen to
indicate to
the user that the device has entered a lumen containing blood, for example, a
coronary
artery or heart chamber.
The shaft assembly 14 may be relatively flexible to permit the shaft
assembly to bend during the procedure, or it may be substantially rigid. The
degree of
flexibility imparted to the shaft assembly 14 of the device 10, as well as the
dimensions of
the device 10, will vary depending on the application and user preference. The
device 10
could be formed with a shaft assembly 14 that is curved, malleable so as to be
bendable to
a selected configuration, or articulated with a movable portion that may be
controlled or
steered, for example, by known mechanisms used to steer catheters or guide
wires.
As an example of a range of possible constructions, the device 10 may be
relatively short with the shaft assembly 14 substantially rigid for use in an
open-chest
procedure. Alternatively, the device 10 may be relatively long with the shaft
assembly 14
rigid or flexible for use in a minimally invasive procedure. As yet another
alternative, the
device may be even longer (with the shaft assembly 14 flexible or rigid) for
use in an
endoscopic procedure, wherein the actuators for controlling the device
components are
located adjacent the proximal end of the device to allow remote deployment of
the vessel
coupling. An example of a device configured for use in a minimally invasive
procedure
is disclosed in co-pending, commonly owned application no. 09/304,140, filed
on
May 3, 1999, and entitled "Methods and Devices for Placing a Conduit in Fluid
Communication With a Target Vessel," the entire subject matter of which is
incorporated
herein by reference.
In the application illustrated in the Figures, the invention is used to
establish a conduit between a target vessel and a source of blood. The conduit
may
comprise one member, or it may comprise multiple conduits coupled to form a
conduit
between the target vessel and the blood source. The conduit is preferably
formed, either
partially or completely, of synthetic vascular graft material. It should thus
be appreciated
that reference to a first or a second conduit in connection with describing
one preferred
embodiment is not intended to limit the scope or application of the invention.
The conduit preferably comprises a tubular connector that is at least
partially constructed of synthetic vascular graft material and is configured
to be placed in
fluid communication with the target vessel. The tubular connector preferably
includes a
8
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
vessel coupling adapted to be secured to the target vessel. One preferred
vessel coupling
is constructed so that it may be secured to the target vessel by a
substantially suture-free
attachment, which means that the attachment is not a conventional hand-sewn
anastomosis created by suturing the members together. As such, although some
suture
may be used, in this preferred embodiment the vessel coupling (or conduit
without a
vessel coupling) is attached to the target vessel by means other than a
typical, hand-sewn
sutured connection. The invention, however, may be practiced using a conduit
without a
vessel coupling, for example, by using suture to secure the conduit to the
target vessel.
Further, the vessel coupling used to attach the conduit to the target vessel
is preferably expandable so that the coupling may be collapsed for
introduction into the
target vessel and then expanded into contact with the vessel wall.
Nonetheless, it will be
understood that the invention may be carried out by using a vessel coupling
that
comprises a non-expandable structure; for example, the vessel wall could be
dilated to
receive a tubular element and then allowed to move against the element to hold
it in place
in the vessel.
The illustrated device 10 is adapted to place a conduit that is coupled to
another conduit to establish a conduit. As noted above, the conduit may
comprise any
number of sections or portions. The conduit 18 includes a vessel coupling in
the form of
a stmt 20 and a liner 22 formed of synthetic vascular graft material coupled
to the stem
20. As an example, the stmt 20 may be a self expanding nitinol stent 20 and
the liner or
layer 22 may be a tubular length of teflon (PTFE) or expanded teflon (ePTFE).
The stmt
may be laser cut from a tube of material so as to include a plurality of
struts that permit
the stmt to move between collapsed and expanded orientations. Other stmt
constructions
may be used instead; for example, the stent could be wire-formed or could
comprise a flat
sheet of material that is unrolled to an expanded orientation. The liner 22 is
secured to
the stmt 20 by any suitable means, for example, suture (not shown) passing
through the
liner wall and the wall of the stent. Other suitable means for securing the
two
components include biologically compatible adhesives, ultrasonic welding,
clips or
fasteners, weaving the liner through the stmt elements, tying the liner to the
stmt
elements, etc.
As an example, the stmt may be cut or formed by subjecting a tube of
suitable material to any of various procedures such as laser cutting, EDM
(electrical
discharge machining), photochemical etching, etc. The stent/tube material is
preferably
nitinol, but may be titanium, tantalum, etc. It may be desirable to further
process or finish
9
CA 02337615 2001-O1-03
WO 00/66009 PCTNS00/12073
the cut stent to remove burrs or surface irregularities, for example, by acid
etching,
electropolishing, or abrasive blasting. Next, the frame sections that engage
the wall of the
target vessel are shape-set to their expanded orientation. This may be done by
placing the
frame sections in that orientation and applying sufficient heat to produce a
structure that
will assume the desired configuration above a certain temperature, e.g., 5
° below body
temperature. The stmt may then be placed in its collapsed orientation by
cooling (e.g.,
with liquid nitrogen), coupled to a liner and loaded onto a delivery device,
and then
deployed in a target vessel.
As shown in Fig. 2, the proximal and distal ends of the stmt 20 and the
liner 22 are preferably aligned so that the two components are generally
coextensive.
Rather than being coextensive, though, the stent 20 and liner 22 could
partially overlap
each other a desired extent so that either has a portion that is uncovered.
For example, the
distal portion of the stent 20 could extend beyond the distal end of the liner
22 so as to be
exposed for engagement with the target vessel. The extent that the stmt 20 and
liner 22
overlap may be different from that shown, and the stmt may be disposed within
the liner,
as shown, or it may be disposed outside the Liner. Finally, while the
illustrated conduit 18
includes only the stmt 20 and liner 22, an additional Iayer(s) of material,
such as a layer
of ePTFE, silicone, or another stmt, may be included along with the stmt 20
and the
liner 22.
The invention may be carried out by establishing a conduit that comprises
any suitable synthetic vascular graft material, either alone or in combination
with another
component or material, synthetic or tissue. The conduit may comprise a
relatively short
length of a suitable synthetic vascular graft material, for example, a woven
or knitted
material such as Dacron {polyester), PTFE or ePTFE. However, as used herein
the phrase
synthetic vascular graft material encompasses any material suitable for use as
a substitute
for natural blood vessels. As an example, the conduit may comprise ePTFE
having an
inner diameter within the range of from about 1 mm to about 5 mm, and more
preferably
about 2 mm to about 4 mm, a wall thickness of about 0.2 mm, and an internodal
distance
or pore size in the range of from about 20 us to about 100 ps.
The conduit of the invention preferably comprises a relatively short length
of synthetic vascular graft material in order to avoid thrombosis problems
that arise with
longer vessels formed of the same materials (as discussed above). If the
procedure being
carned out requires a longer length conduit to extend between the target
vessel and blood
source, the invention may provide a second conduit that is coupled to the
first conduit.
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
The second conduit is preferably a tissue vessel in order to avoid thrombosis
problems
associated with longer vessels formed of synthetic graft materials, although
the conduit
could comprise only synthetic vascular graft material. It should also be
appreciated that
the diameter, length and material of construction of the conduit may vary.
As shown in Figs. 1 and 2, the conduit 18 is preferably somewhat bell-
shaped when in its expanded orientation: This shape may be provided to
facilitate
coupling the conduit 18 to a second conduit, for example, by docking an end of
the
second conduit into the larger end of the bell-shaped first conduit. In use,
the liner 22 is
preferably secured to the stent 20 prior to collapsing the stmt from the
expanded
orientation to a smaller profile (shown in phantom in Fig. 2), in which the
conduit 18 is
not bell-shaped. It will be recognized that alternative configurations, with
or without a
larger diameter opening for coupling the conduit, may be used if desired.
The conduit 18 is supported by a support member 24 which may be in the
form of a shaft sized and configured to mount the stmt 20 and liner 22. The
support shaft
24 is preferably provided with a nose cone dilator 26 having one or more
tapered surfaces
for introducing the device into the lumen of a target vessel, the dilator
preferably being
formed of a soft, floppy atraumatic material. Alternative or additional means
for dilating
the target vessel may be used as well.
The illustrated device 10 utilizes a self expanding stmt 20; thus, the device
is not provided with an expansion mechanism for deploying the stmt. If
desired, though,
the device may include such a mechanism, for example, an inflatable balloon,
in order to
use the device with a stmt that is forced to an expanded orientation. This
embodiment of
the invention may be practiced using the teachings disclosed in application
serial no.
09/232,102, filed on January 15, 1999, and entitled "Methods and Devices for
Forming
Vascular Anastomoses," the entire subject matter of which has been
incorporated herein
by reference. Additionally, the device could be provided with a separate
inflation lumen
for inflating the balloon to expand the stmt, or the design in the
aforementioned
application may be used with suitable seals such as O-rings or the like.
The shaft assembly 14 of the illustrated embodiment also includes a
retention mechanism, indicated generally at reference numeral 28, for
retaining the self
expanding stmt 20 in its collapsed orientation during introduction into the
lumen of the
target vessel. The retention mechanism 28 comprises a sheath 30 carned by a
sheath hub
32 which is adapted to be removably supported by the device 10. The device 10
is
provided with an actuator for imparting relative motion to the sheath 30 and
the conduit
11
CA 02337615 2001-O1-03
WO 00/66009 PCTNS00/12073
18 to deploy the conduit in the target vessel. In the illustrated embodiment,
the sheath
hub 32 acts as the actuator; however, it will be appreciated that alternative
actuators may
be used to impart the relative motion, e.g., actuators using cables, lever
assemblies, etc.
The sheath 30 is preferably formed of a thin-walled, flexible material, e.g.,
polyolefin,
nylon, polyimide, PEEK, Hytrel, etc.
The device 10 also includes a positioning mechanism, indicated generally
by the reference numeral 34, for engaging the retention mechanism 28 and the
conduit 18.
The positioning mechanism 34 serves to maintain proper orientation of the
conduit 18 and
the retention mechanism 28, with respect to each other and with respect to the
remaining
components of the device.
As shown in Figs. 1 and 2, the distal end of the handle 12 has a boss 36
which is used to mount the positioning mechanism 34. In particular, the
positioning
mechanism 34 has a proximal end portion 38 with an opening sized and
configured to
receive the boss 36. The mechanism 34 is preferably fixed to the handle 12 by
any
suitable means, for example, adhesives, brazing, welding, fasteners, etc. The
positioning
mechanism 34 includes a base 40 which extends away from the handle 12 and
leads to a
distal end portion 42 which supports the conduit 18 and conduit support member
24. In
the illustrated embodiment, the positioning mechanism 34 includes a mechanism
for
removing the sheath 30 from the device 10. The distal end portion 42 has a
groove 44
sized to receive the shaft of the support member 24, a slot 46 that
accommodates the
sheath removal mechanism, and a positioning member 48 that engages the conduit
18 and
the sheath 30 to maintain the components in position during use. See Figs. 2-
4.
The conduit 18 is mounted on the support member 24 and retained in its
collapsed orientation (Fig. 2) by the sheath 30. The sheath 30 preferably
covers the entire
length of the conduit 18, although it could overlie only a portion of the
conduit. For
example, the sheath could be constructed, positioned and actuated in a manner
the same
as or similar to that disclosed in the aforementioned co-pending, commonly
owned
application no. 09/304,140, filed on May 3, 1999, the entire subject matter of
which
application has been incorporated herein by reference.
The illustrated embodiment also includes a sleeve 50 for maintaining the
conduit in position on the support member 24 during use. The sleeve 50 is
sized and
shaped so that it can be slid over the support member 24 until the distal end
52 of the
sleeve abuts the proximal end 54 of the conduit 18. The opposite end of the
sleeve SO
abuts (or is fixed to) the boss 36 of the handle 12. The distal end of the
conduit 18 abuts
12
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
the nose cone dilator 26 so that the conduit 18 is held in position on the
support member
24 between the nose cone dilator 26 and the sleeve 50.
The support member 24, with the conduit 18, sheath 30, hub 32 and sleeve
50 mounted thereon, is passed through the opening in the proximal end 38 of
the
positioning mechanism 34. The proximal portion 56 of the support member 24 is
inserted
into the bore in the handle 12 and then fixed in position. The shaft assembly
14 thus
extends distally away from the handle 12, and the proximal end of the support
member 24
may be either movably or immovably secured to the handle.
The sheath hub 32 is provided with a surface 58 configured to mate with a
complementarily formed surface 60 on the positioning mechanism 34, which
allows the
components to nest together and present a smooth outer profile, as shown in
Fig. 1.
Similarly, the sheath hub 32 has a cutout 62 that mates with a step 64 formed
on the
positioning mechanism 34. When assembled as shown in Fig. 1, the positioning
member
48 preferably extends between the outer surface of the conduit 18 (and in
particular the
outer surface of liner 22) and the inner surface of the sheath 30.
As viewed best in Figs. 6A-6B, the lower portion of the sheath 30 rests
directly beneath the positioning member 48, which preferably has an arcuate
shape that
substantially conforms to the contour of the conduit 18 and the sheath 30. The
positioning member 48 serves to shield the conduit 18 (and in particular the
liner 22)
from the mechanism for removing the sheath, which is disposed in the slot 46
formed in
the distal end portion 42 of the positioning mechanism 34.
Referring to Figs. 3-5, the sheath removal mechanism is indicated
generally by reference numeral 66 in Fig. 4 and comprises a cutting blade 68
with a
cutting edge 70 for splitting the sheath 30. The cutting blade 68 is fixed to
the positioning
member 48 and extends into the slot 46 in the distal end portion 42 of the
positioning
mechanism 34. In the illustrated embodiment, the cutting blade 68 and
positioning
member 48 are separate from the distal end portion 42. The cutting blade 68
has an
aperture 72 which is aligned with an aperture 74 in the distal end portion 42
to receive a
set screw (not shown) that fixes the components in position. Fig. 5 shows the
cutting
blade 68 located in the slot 46 with cutting edge 70 facing the distal end of
the device.
The sheath hub 32 is preferably provided with a portion, such as the flange 76
shown in
Figs. l and 2, that may be grasped to move the sheath 30 with respect to the
sheath
removal mechanism 66.
13
CA 02337615 2001-O1-03
WO 00/66009 PCT/~JS00/12073
Figs. 6A-6B show schematically the sheath 30 being removed from the
conduit 18. It should be noted that the sheath 30 may be integral with the hub
32 or it
may be a separate element attached thereto. The sheath hub 32, support member
24 and
sleeve 50 are omitted from Figs. 6A-6B for sake of clarity; however, in use
the flange 76
would be grasped to move the hub 32 in a proximal direction to split the
sheath 30. Fig.
6A shows the positioning member 48 located between the sheath 30 and conduit
18 such
that the proximal end of the sheath is located within the slot 46 so as to
rest against the
cutting edge 70 of the blade 68. The lower wall of the proximal end of the
sheath 30 may
have a slit that is placed over the cutting blade 68.
The sheath 30 is moved in the direction of the arrow shown in Fig. 6B
which moves the cutting edge 70 through the sheath to form a split 78 that
runs along the
length of the sheath. As the sheath 30 is moved proximally against the cutting
blade 68,
the sleeve 50 retains the conduit 18 in position and the positioning member 48
ensures
that the conduit 18 does not contact the cutting blade 68. As shown in Fig.
6B, the sheath
30 is split so that it can be removed from the device. This allows the conduit
18, and in
particular the stmt 20 which forms the vessel coupling in the illustrated
embodiment, to
expand into contact with the target vessel wall in order to secure the conduit
to the target
vessel.
The sheath removal mechanism 66 may be modified or omitted depending
on the specific construction of the device. As an example, it should be
understood that
the cutting blade 68 (or alternative sheath removal mechanism) and positioning
member
48, rather than being attached to the device 10, may instead be integrally
formed with the
device 10. Further, the sheath 30 may have an alternative construction, for
example, the
sheath may be a peel-away type that is pulled apart and removed, or a non-
splitting
member that undergoes relative motion with respect to the conduit 18 in order
to expose
the conduit. It will be appreciated that if the conduit utilizes a vessel
coupling that does
not have to be expanded to secure the conduit to the target vessel, then the
sheath may be
omitted altogether. A sheath may nonetheless be desirable in order to protect
the tissue of
the target vessel during introduction of the device.
It should be appreciated that a device constructed according to the
invention could include removable or detachable components, or could be
constructed as
a one-piece instrument with no separable components. The device may be formed
as a
disposable instrument, a reusable instrument capable of being sterilized, or a
combination
of disposable and reusable components.
14
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
Referring now to Figs. 7-9E, an exemplary method for placing a conduit in
fluid communication with a target vessel according to one embodiment of the
invention
will be described. Fig. 7 schematically depicts a patient who has been
prepared to
undergo a cardiovascular surgical procedure. A thoracotomy T is formed in the
patient's
chest by making an incision between two ribs to provide access to the thoracic
cavity. A
retractor R may be used to spread the ribs and increase access to the heart H
and great
vessels. The retractor preferably raises one side of the incision with respect
to the other
side to increase the working space around the heart. Any suitable retractor
may be used,
for example, one of the commercially available rib retractors currently used
in minimally
invasive cardiac surgery. As shown, the retractor R provides considerable
access to the
heart H and great vessels including the aorta A. The left side of the heart as
well as the
left coronary artery LCA is easily accessible via the thoracotomy T.
Fig. 8 shows the heart H in isolation along with a device 10 constructed as
described above. Fig. 8 is an anterior view of the heart H showing the left
ventricle LV,
right atrium RA, aorta A, pulmonary trunk PT and pulmonary veins PV. The left
coronary artery, including the circumflex branch and the left anterior
descending branch
LAD, is visible in this view, as is the right coronary artery RCA. The
coronary arteries
run along the heart wall and deliver oxygenated blood to the myocardial
tissue. An
occlusion or blockage O partially (or completely) obstructs the lumen of the
LAD, which
results in inadequate or no blood flow to the heart wall tissue fed by the
portion of the
LAD that is downstream of the occlusion O. It will be appreciated that the
particular
target vessel and source of blood shown in the Figures are exemplary only as
there will be
numerous applications for the methods and devices disclosed herein.
As shown in Fig. 8, the distal end of the device 10, and in particular the tip
of the incising component 16 and the nose cone dilator 26, is passed through
the wall of
the LAD. The device 10 may be manipulated with respect to the heart H in order
to
obtain the most advantageous angle of entry into the coronary artery. The
illustrated
device 10 has a curved shaft assembly 14 configured to allow easier
cannulation of the
target vessel. The particular manner in which the device 10 is oriented will
of course
depend on the specific application, including the particular vessel being
treated and
whether the procedure is being carried out, for example, in an open-chest
manner via a
median sternotomy or a minimally invasive manner via one or more smaller
surgical
openings (such as the thoracotomy T in Fig. 7). In any event, the device 10 is
held and
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
manipulated to achieve an optimal position for passing the distal end smoothly
into the
lumen of the LAD.
Figs. 9A-9D are sectional views corresponding to Fig. 8 but showing only
the portion of the LAD and the heart wall M adjacent the point of entry of the
device 10.
As can be seen in Fig. 9A, the sharpened tip of the incising component 16 is
exposed
inside the lumen of the LAD. The incising component 16 is retracted once the
distal end
of the device 10 has been passed through the wall of the LAD. Once this has
been done,
the device 10 is introduced further into the LAD, preferably by angling the
device as
shown in Fig. 9B. The device 10 is moved into the lumen of the LAD a
sufficient amount
to place the conduit 18 at a predetermined location within the LAD.
The device 10 may be provided with means for indexing the position of
the device in order to control the position of the conduit 18 with respect to
the target
vessel. Suitable means for indexing the position of the device 10 include
markings (not
shown) placed along the shafi assembly 14, for example, the sheath 30, that
may be read
with respect to the wall of the target vessel to determine the position of the
conduit 18
with respect to the target vessel. Other means include one or more stops
carried by the
shaft assembly 14 for engaging or contacting tissue to control the position of
the conduit
18 in the target vessel. Additionally, the sheath 30 may be transparent to
allow the user to
view the conduit 18 and visually confirm its position in the target vessel.
Fig. 9C shows the sheath 30 partially split as a result of moving the sheath
against the cutting blade 68. As described above, the flange 76 (not shown in
Fig. 9C) is
grasped and moved in a proximal direction. This pulls the sheath 30 against
the edge 70
of cutting blade 68, which forms a split 78 in the sheath. The split 78 allows
the sheath
to be removed from the device, and in particular the conduit 18 which is
either
25 partially or completely covered by the sheath. As the remaining length of
the sheath 30 is
split and removed the formerly covered portion of the conduit 18 is exposed.
The conduit
18 moves to its expanded orientation once the stmt 20 is no longer restrained
by the
sheath 30.
Once the conduit 18 has been uncovered and assumes its expanded
30 orientation, the size of the conduit is sufficient to allow the nose cone
dilator 26 to be
passed through the conduit (as can be seen from Fig. 9C). Alternatively, the
nose cone
dilator could be collapsible in order to remove it through the conduit. After
removing the
sheath 30, the remainder of the device 10, i.e., the support member 24, nose
cone dilator
26, positioning member 48, and sleeve 50 are removed from the conduit 18. The
16
CA 02337615 2001-O1-03
WO 00/66009 PCTNS00/12073
resulting configuration is shown in Fig. 9D, wherein the expanded conduit 18
engages the
wall of the LAD and provides a secure attachment.
In the illustrated embodiment, the reduced diameter distal portion 80 of the
conduit 18 is disposed substantially entirely within the lumen of the LAD. The
larger
diameter proximal portion 82 of the conduit 18 is disposed entirely outside
the lumen of
the LAD. The larger diameter portion 82 of the conduit 18. which is bell-
shaped in the
illustrated embodiment but could be shaped differently if desired, is
presented for
attachment to a second conduit (not shown in Fig. 9D). It is preferred to have
a portion of
the conduit 18 extend through the opening in the wall of the target vessel to
enhance the
seal formed at the junction.
Fig. 10 shows the completed conduit as viewed from the exterior of the
heart H. The conduit 18 extends through the wall of the LAD and is adapted to
be
coupled to another conduit placed in communication with a source of blood. It
should be
recognized that position and orientation of the conduit with respect to the
target vessel
may be varied from the exemplary configuration illustrated in Figs. 9D and 10.
Depending on the construction of the device, securing the conduit to the
target vessel may partially or completely obstruct the lumen of the target
vessel. For
example, as shown best in Fig. 9D, the lumen of the LAD may be occluded by the
conduit
18, and in particular by the liner 22, once the conduit has expanded to its
final position.
As a result, native blood flow through the target vessel may be hindered or
prevented
from moving distally past the attachment site between the conduit and the
vessel. In the
case of a coronary artery, the conduit 18 could limit or block native blood
flow through
the artery, e.g., blood from the aorta flowing through the artery. Many
patients
undergoing a CABG procedure will have some native proximal blood flow in one
or more
obstructed arteries. It therefore would be desirable to place a conduit in
fluid
communication with the target vessel in a manner that preserves such native
blood flow in
the target vessel.
Accordingly, another embodiment of the invention provides methods and
devices for attaching a conduit comprising synthetic vascular graft material
to a target
vessel while preserving native blood flow through the target vessel. That is,
blood
flowing through the target vessel prior to placing the conduit is free to flow
past the site
of the attachment. One way of achieving this is by forming the conduit 18 with
a portion
comprising uncovered stmt that is placed in the target vessel to allow flow
past the
junction. Another way to preserve flow is by forming an opening in the portion
of the
17
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
conduit that is placed in the target vessel, for example, an opening through
the stent 20
and the liner 22.
Fig. 1 OA shows a conduit constructed according to this embodiment of the
invention for preserving native flow in the target vessel. The conduit is
designated by the
reference numeral 90 and has a similar construction as the conduit 18
described above
with respect to the previous embodiments. The conduit 90 comprises a stem 92
and a
liner 94, each of which may be constructed as described above regarding the
stmt 20 and
liner 22. The stmt 92 of the conduit 90, however, has a different construction
in that the
distal portion is configured to engage the target vessel without blocking
blood flow
through the target vessel.
In particular, the stmt 92 has a frame structure 96 including one or more
sets of frame elements 98 that secure that conduit 90 to the target vessel yet
allow blood
flow through the vessel, as indicated by the arrows in Fig. 10A. The conduit
90 may be
collapsed (in whole or in part) for introduction into the target vessel and
then expanded to
engage the vessel wall. The embodiments of the invention that preserve native
blood
flow through the vessel may be practiced using the methods and devices
disclosed in the
aforementioned application serial no. 09/232,103, filed on January 15, 1999,
as well as
the aforementioned co-pending, commonly owned application no. 09/304,140,
filed on
May 3, 1999, the entire subject matter of which applications has been
incorporated herein
by reference.
The illustrated embodiment of the invention that preserves native blood
flow through the target vessel has a construction that does not cover a major
portion of
the inner or posterior wall of the vessel. As can be seen from Fig. 10A, the
frame
structure 96 contacts the inner wall of the vessel but leaves the majority of
the vessel wall
uncovered. This allows blood flowing through the target vessel to feed septal
perforators
(not shown but extending downward as viewed in Fig. l0A), which feed blood to
the
myocardial tissue. This feature thus prevents the myocardial tissue perfused
by the septal
perforators from becoming ischemic due to the conduit located in the target
vessel. It
should be appreciated that while it is preferred to leave the majority of the
vessel wall
unexposed to perfuse as many septal perforators as possible, the invention may
be
practiced with a conduit that covers more or less of the vessel wall than that
shown.
An exemplary method for establishing a conduit according to the invention
to place a target vessel in fluid communication with a source of blood will be
described in
connection with Figs. 11-13. Fig. 11 illustrates a heart wherein a first
conduit 18 has
18
CA 02337615 2001-O1-03
WO 00/66009 PCTNS00/12073
been secured to the LAD, for example, by the method described above with
respect to
Figs. 8-9D. The first conduit 18 is adapted to be coupled to a second conduit
to establish
the conduit that communicates the artery and blood source. Figs. 11-13 depict
the
invention being used to carry out a CABG procedure. The first conduit 18,
shown
S attached to the LAD downstream of the obstruction O in Figs. 11-13, is
adapted to be
secured to a second conduit 100 that is placed in fluid communication with the
source of
blood. However, if desired the first conduit 18 may have a length sufficient
to allow it to
be placed in fluid communication with the LAD and the aorta. The second
conduit 100
preferably comprises a hollow tissue structure, and most preferably an
autologous tissue
structure, for example, a saphenous vein harvested from the patient. The
second conduit
I00 could alternatively comprise synthetic vascular graft material, such as
PTFE or
ePTFE.
The proximal end 102 of the second conduit 100 may be prepared as is
known in the art for anastomosis to a source of oxygenated blood, which in
this
embodiment is the aorta A. An aortotomy AO is formed in the wall of the aorta,
for
example, by making an incision and using an aortic punch (not shown). The
distal end
104 of the second conduit 100 is preferably joined to a vessel coupling
adapted to be
secured to the first conduit 18. The vessel coupling may be in the form of a
stmt 106 that
is collapsed for introduction into the first conduit 18 and then expanded to
secure the two
conduits together.
Fig. 12 shows the proximal end 102 of the second conduit 100 sutured to
the aorta in conventional fashion to form a proximal anastomosis. It should be
appreciated that the proximal end 102 of the conduit 100 could be secured to
the aorta (or
other blood source) by alternative means, such as a vessel coupling carried by
the end
102, which vessel coupling may be in the form of a stmt.
Next, the conduit 100 is clamped off to block blood flow from the aorta A
(not shown). The stmt 106 carried by the distal end 104 of the second conduit
100 may
be collapsed by any suitable mechanism for introduction into the first conduit
18. As an
example, an instrument 108 may be in the form of forceps with a pair of jaws
that are
used to collapse a portion of or the entire stmt 106 so that it may be
positioned in the first
conduit 18 (Fig. 12A). If necessary, a second instrument may be used to
gradually
expand the stmt 106 in the first conduit 18 in order to allow the instrument
108 to be
removed. The illustrated second conduit 100 is secured to the stent 106 so
that a portion
of the stmt 106 is exposed. The portion of the stent 106 that is exposed may
be more or
19
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
less than that shown; for example, the stent 106 may be entirely covered by
the distal end
104 of the second conduit 100. In any case, the distal end 104 of the second
conduit 100
is coupled to the first conduit 18 via a secure, fluid-tight connection.
Fig. 13 shows the completed conduit that is formed by the conduits 18,
100 in order to communicate the LAD with the aorta. In the illustrated
procedure the
proximal end 102 of the conduit 100 is first coupled to the blood source and
then coupled
to the conduit 18; this may be desirable in order to expel air from the
interior of the
conduit. The specific manner in which the procedure is carned out may be
varied, for
example, by first coupling the distal end 104 of the conduit 100 to the
conduit 18 and then
to the blood source. Also, as an example, the length of the conduit extending
between the
aorta and the coronary vessel may be in the range of from about S cm to about
8 cm
(including the portion of the conduit disposed in the vessel).
The procedure according to this embodiment of the invention provides
several benefits over conventional CABG procedures. For example, utilizing a
conduit
that comprises synthetic vascular graft material allows the device to be
supplied with the
conduit loaded on the device. Also, the relatively short length of synthetic
vascular graft
material that is exposed to blood minimizes the risk of or prevents thrombosis
in the
conduit. Further, the invention enables the user to secure a conduit to the
target vessel
without (or substantially without) using suture.
One aspect of the invention thus obviates the need to create a hand-sewn
sutured distal anastomosis between the conduit and the target vessel, which is
a highly
technical component of CABG procedures, particularly if carried out minimally
invasively. As cardiovascular treatments have continued to become more and
more
minimally invasive with reduced access to the heart, suturing extremely small
blood
vessels together has become more difficult and time-consuming. Thus, a
significant
advantage of the invention is that the distal anastomosis may be formed
relatively quickly
and easily during a minimally invasive, beating heart procedure. Finally, if
an autologous
tissue structure is used as a conduit, then the user need only harvest the
tissue graft (as is
conventionally done) and secure same to a vessel coupling, such as the stmt
106, that is
adapted to be secured to the first conduit.
Turning now to Figs. 14-16A, another exemplary method for placing a
conduit constructed according to the invention in fluid communication with a
target
vessel and a source of blood will be described. In the procedure exemplified
in Figs. 14-
16A, though, the target vessel (LAD) is placed in fluid communication with a
heart
CA 02337615 2001-O1-03
WO 00/66009 PCTNS00/12073
chamber containing blood, rather than the aorta. Thus, the aorta A in Figs. 11-
I3 does not
have an aortotomy; otherwise, the heart and the first conduit 18 are depicted
the same in
the respective groups of Figures.
Fig. 14A illustrates in detail a second conduit 110 which is adapted to be
coupled to the first conduit 18 and placed in fluid communication with a heart
chamber
containing blood. In the Figures, the heart chamber is the left ventricle;
however, it will
be understood that another heart chamber could be used if desired. The second
conduit
110 comprises a graft vessel 112 preferably formed of synthetic vascular graft
material,
for example, the same material used to form the first conduit 18. The graft
vessel 112,
though, could be formed of tissue or a different synthetic vascular graft
material. The
distal (or blood outlet) end 116 of the second conduit 110 is preferably
provided with a
vessel coupling that may be in the form of a stmt 118 constructed in the same
manner as
the stmt 106 described above with respect to the embodiment of Figs. 11-13.
The
proximal (or blood inlet) end 120 of the second conduit 110 is preferably
provided with a
fitting 114 configured to be positioned in the myocardium such that the vessel
112 is in
fluid communication with the heart chamber.
The fitting 114 is preferably a hollow cylinder formed of any suitable
biocompatible material, such as stainless steel, titanium, tantalum, polymers,
etc. The
graft vessel 112, which may be a length of ePTFE, is positioned in the fitting
114 with the
proximal end 120 everted over the end of the fitting. The proximal end 120 is
secured to
the fitting 114 by suitable means, such as the sutures shown in Fig. 14A. The
fitting 114
may be provided with recesses 122 (or alternative structure) adapted to
receive or support
the suture. The attachment of the vessel 112 to the fitting 114 may also be
achieved using
silicone, biologically compatible adhesives, clamps rings, etc. An internal
component,
such as a stmt, may also be provided to force the graft vessel 112 against the
fitting 114
in order to ensure the vessel wall does not kink or collapse within the
fitting.
The fitting 114 also is preferably provided with a mechanism to assist in
maintaining the proximal end 120 of the conduit 110 patent once positioned in
the
myocardium, for example, by preventing tissue or other matter moving over and
blocking
flow into the proximal end. For instance, the end of the conduit that is
placed in the
ventricle will be located near tissue such as the chordae tendineae, papillary
muscle or
other myocardial tissue, thereby creating the risk of such tissue blocking the
flow of
blood into the conduit.
21
CA 02337615 2001-O1-03
WO 00/66009 PCTNS00/12073
One preferred component for preventing blockage of the conduit is shown
in Fig. 14A and comprises a structure comprising a plurality of struts
defining open areas
through which blood may flow. As shown, a plurality of struts 124 are secured
to the
fitting 114, each strut 124 having one end 126 fixed to the fitting 114 and
another end 128
positioned adjacent the proximal end 120 of the conduit 110. The struts 124
extend away
from the fitting 114 and then converge to a location at which the ends 128 are
joined.
The resulting configuration forms a frame or cage around the end of the
conduit 110 that
will be placed in fluid communication with the heart chamber. In addition to
preventing
blockage by the aforementioned tissue structures, the component will prevent
or minimize
tissue being forced into the fitting 92 during placement of the fitting in the
myocardium.
While the illustrated mechanism includes four curved struts 124, fewer or
more struts may be used, and the struts may be straight, curved, or otherwise
shaped, and
may be rigid or flexible. Further, it will be readily appreciated that
alternative
mechanisms for preventing blockage of the end of the conduit that communicates
with the
1 S heart chamber (or other blood source) may be used in lieu of that
illustrated in Fig. 14A.
For example, rather than a plurality of individual struts, the mechanism could
comprise a
grid or mesh that allows blood to flow into the conduit 110.
The dimensions of the fitting 114 may vary depending on the application.
As an example, the fitting 114 may be formed from 8 gauge thin wall 304
stainless steel
hypo tube stock and be approximately 23 mm long. The struts 124 may be formed
from
304 full hard stainless steel wire and be approximately 18 mm long with an
outer
diameter of approximately 0.375 mm. If constructed as in the illustrated
embodiment, the
length of the portion of each strut 124 extending beyond the proximal end 120
of the graft
vessel 112 may be approximately 8 mm.
Fig. 15 shows the conduit 110 after it has been positioned in the
myocardium M such that the proximal end 120 is in fluid communication with the
left
ventricle LV. This may be achieved by making a stab incision partially or
entirely
through the myocardium M and then placing the fitting 114 in the tissue at a
location that
permits blood to flow from the ventricle into the conduit 110. The fitting 114
may be
designed to rest entirely within the myocardium, which would allow the user to
visually
confirm proper placement of the conduit 110. Alternatively, the fitting 114
(or another
part of the conduit 110) may be provided with markings or other means for
indicating the
position of the end of the conduit that is placed in the left ventricle LV.
22
CA 02337615 2001-O1-03
WO 00/66009 PCT/US00/12073
The fitting 114 is preferably sized and configured to securely engage the
tissue of the myocardium without requiring suture or other fastening means. To
that end,
the fitting 114 may be provided with a roughened surface or a layers) of
material that
enhances fixation in the tissue. Of course, the fitting 114 could be
positively secured to
the tissue by suture or other fasteners if desired. Further, the fitting 114,
or another
portion of the conduit 110 that engages the tissue of the myocardium, may be
used to
deliver various drugs to the tissue. For example, the fitting 114 itself or a
sleeve of
material provided on the fitting may be impregnated with a pharmaceutical
composition,
such as angiogenic growth factor, heparin, etc. The sleeve (not shown) could
comprise a
metal or a polymer, for example, ePTFE, PTFE, Dacron, etc.
From the position shown in Fig. 15, the second conduit 110 is clamped off
(not shown) and its distal end 116 is coupled to the open end of the first
conduit 18 that
has been secured to the LAD. In the illustrated embodiment, the distal end 116
is
provided with a stmt 118, and for that reason may be coupled to the conduit 18
in the
same manner described above with respect to Figs. I 1-13. As shown in Fig.
15A, the
instrument 108 with movable jaws may be used to collapse a portion of or the
entire stmt
118 so that it may be positioned in the conduit 18. As explained above, if
necessary, a
second instrument may be used to gradually expand the stem 118 in the first
conduit 18 in
order to allow the instrument 108 to be removed. The illustrated second
conduit 110 has
a portion of the stmt I 18 exposed. Also as explained above, the exposed
portion of the
stmt I 18 may be greater or less than that shown. If desired, the stent 118
may be entirely
covered by the distal end of the conduit 110. In any case, the distal end of
the conduit
110 is preferably coupled to the conduit 18 to provide a secure, fluid-tight
connection.
Fig. 16 shows the completed conduit that is formed by the conduits I 8,
110 in order to communicate the target vessel (the LAD) with a heart chamber
(the left
ventricle LV). In the illustrated procedure the proximal end 120 of the
conduit 110 is first
coupled to the blood source, and the distal end I 16 is then coupled to the
conduit 18, as
discussed above with respect to the previous embodiment. It should be noted,
though,
that the distal end 116 of the conduit 100 could be coupled to the conduit 18
first, with the
proximal end 120 then secured to the myocardium. In either case, the result is
a fluid
tight, preferably suture-free attachment between the conduit 110 and the
conduit 18 and
between the conduit 110 and the blood source. As an example, the length of the
conduit
extending between the heart chamber and the coronary vessel may be in the
range of from
23
CA 02337615 2001-O1-03
WO 00/66009 PCTNS00/12073
about 3.5 cm to about 5 cm (including the portion of the conduit disposed in
the
myocardium).
Fig. 16A is a sectional view of the proximal end 120 of the conduit 110
positioned in the myocardium M. The fitting 114 is generally coextensive with
the
myocardium M; however, the length of the fitting 114 could instead be less or
greater
than the thickness of the myocardium. Similarly, the struts 124 could be
positioned
partially within the myocardium M, as shown, or completely outside the
myocardium
within the heart chamber LV. Also, as rioted above, the conduit 1 i 0 could be
provided
with an internal support such as a stmt (not shown).
Those in the art will recognize many possible variations of the invention as
described and illustrated herein. The Figures show a coronary artery being
placed in fluid
communication with a source of blood selected from the aorta and the left
ventricle. It
should be understood that the target vessel may be any hollow body structure
having a
lumen, such as any coronary or peripheral vessel, and any blood-containing
hollow body
structure, such as heart chamber, any of the great vessels, or another vessel.
Also, while an expandable vessel coupling is illustrated, a rigid or non-
expandable vessel coupling may be used to establish the conduit. The coupling
may
comprise a rigid tube that is sized and configured to be secured to the target
vessel. For
example, the conduit could be oversized with respect to the target vessel and
the vessel
dilated up to receive the conduit. The target vessel would then close back
down around
the conduit to securely hold the components together without using suture.
Further, the
conduit (including one or more of the conduits) could have means for
preventing kinking
of the conduit, for example, by providing the conduit with a pre-formed shape
and/or by
disposing a strain relief type element, such as a spring wire, on one or more
areas of the
conduit, as disclosed in the aforementioned co-pending, commonly owned
application no.
09/304,140, filed on May 3, 1999, the entire subject matter of which has been
incorporated herein by reference.
The conduit also could be provided with a valve or other means for
controlling or regulating blood flow. A valve could be provided in one or more
of the
conduits and could take the form, for example, of any of the valves disclosed
in co-
pending, commonly owned application serial no. 09/023,492, filed on February
13, 1998,
and entitled "Methods and Devices Providing Transmyocardial Bload Flow to the
Arterial Vascular System of the Heart," the entire subject matter of which has
been
incorporated herein by reference.
24
CA 02337615 2001-O1-03
WO 00/66009 PGT/US00/12073
Similarly, it will be appreciated that a conduit or vessel coupling
configured to preserve native blood flow in a target vessel may be constructed
differently
than that shown. For example, the portion of the vessel coupling that is
disposed in the
target vessel could take the form of a conventional coronary stmt joined to
the portion of
the coupling disposed in the graft vessel. Further, the portion of the vessel
coupling that
permits native flow through the target vessel could control or meter the flow.
Other
variations may be used as well.
It will be appreciated that the features of the various preferred
embodiments described herein may be used together or separately, while the
illustrated
methods and devices may be modified or combined in whole or in part. As an
example,
the attachment formed between the conduit and the target vessel may be suture-
free while
allowing or blocking native flow through the target vessel; alternatively, the
attachment
may be formed to allow native flow through the target vessel but be created
using to some
extent conventional suturing techniques.
Further, it will be understood that the embodiments may be used in various
types of procedures, for example, the surgical approach depicted in the
Figures, an open
surgical procedure including a median sternotomy, or a minimally invasive
procedure
utilizing one or more relatively small access openings or ports. Endoscopes or
thoracoscopes may be used for visualization if the procedure is truly
minimally invasive.
Similarly, the different embodiments may be used in beating heart procedures,
stopped-
heart procedures utilizing cardiopulmonary bypass (CPB), or procedures during
which the
heart is intermittently stopped and started. Finally, any suitable delivery
device,
instrument or catheter may be used in conjunction with the invention.
It also will be recognized that the invention is not limited to the
illustrated
applications. For example, the invention may be used to establish
arteriovenous shunts,
perform a femoral-femoral bypass, treat peripheral arterial disease in the
distal abdominal
aorta including the infrarenal aorta and aortoiliac segment, aortofemoral, or
carotid, and
to treat disease in the iliac and renal arteries.
The preferred embodiments of the invention are described above in detail
for the purpose of setting forth a complete disclosure and for sake of
explanation and
clarity. It will be readily understood that the scope of the invention defined
by the
appended claims will encompass numerous changes and modifications.