Note: Descriptions are shown in the official language in which they were submitted.
CA 02338801 2001-01-26
WO 00/06583 PCT/NL98/00429
TIN POLYOXAALKANECARBOXYLATES AND COMPOSITIONS CONTAINING THEM
The invention relates to novel tin
polyoxaalkanecarboxylates and to anti-tumour compositions
containing such compounds.
The anti-tumour activity of tin compounds is known; it
is also known that the anti-tumour activity of tin compounds
could be enhanced by increasing their solubility in water.
The invention now provides water-soluble tin compounds
which show strong in vitro anti-tumour activities against a
broad spectrum of tumours, as appears from the experimental
part, disclosed hereinafter.
More specifically the invention relates to tin
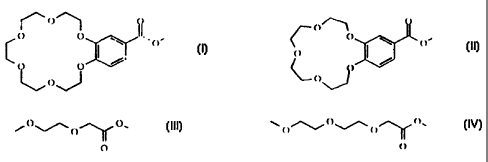
polyoxaalkanecarboxylates having the formula
[ (R'pR2QSn) rOBJ L
wherein
R1 represents C1-C6 alkyl, branched or straight, substituted
or not by one or more hydroxyl groups or halogen atoms, or a
phenyl group, substituted or not by one or more hydroxyl
groups or halogen atoms,
R2 is a carboxylic residue selected from:
("-"- O~ ~ O '
O O ~ / / O Nt* 0/
~
~O
OC)l'_'~Yor O~~iO~/~O~{'O=.
O O
and p, q, r, s and t have the following meanings:
p= 3, q = 1, r= 1, s = 0 and t = 1;
p= 2, q= 2, r = 1, s = 0 and t = 1;
p= 2, q= 1, r= 2, s = 1 and t = 2.
SUBSTITUTE SHEET (RULE-26)
CA 02338801 2001-01-26
WO 00/06583 PCT/NL98/00429
- 2 -
According to a preferred embodiment of the invention,
represents R' a phenyl group or a n-butyl group in a compound
having formula (1), (2) or (3).
The compounds according to the invention can be
synthesized by effecting a condensation reaction between a
carboxylic acid having formula
r o ~ ~H o o , tt
1 o 1
LU ~Oj
U~ ~'~(O~ H
O/~~0~/~0 11
or U
O
with triaryltin hydroxide, trialkyltin acetate or dialkyltin
oxide, preferably triphenyltin hydroxide, tri-n-butyltin
acetate or di-n-butyltin oxide, according to the following
reaction schemes:
a) RCOOH + ( C6H5 ) 3 SnOH > ( C6H5 ) 3SnOCOR + H20
b) RCOOH + Bu3SnOCOCH3 > BuZSnOCOR + CH3COOH
c) 2RCOOH + BuzSnO - > BuZSn (OCOR) 2 + H20
d) 2RCOOH + 2BuZSnO - > 1/2 {[Bu2 (RCOO) Sn] 20}2 + H20
Different media and methods can be used to synthesize such derivatives
1) the condensation can be performed in toluene/cthanol. The water formed
dtiring the
condensation is eliminated by azeotropic distillation (Dean-Stark funnel)
2) benzene can be used instead of toluene/ethanol
3) these compounds can also be prepared by a two-step procedure, dibutyltin
oxide
being first condensed with n-propanol to yield
tetrabutyldipropoxydistannoxnne:
2 Bu2SnO + 2 PrC)H ----- (PrOSnBuz)2O + H20
In a second step, the carboxylic acid is cidded at room tetnperature to this
tetrabutyldipropoxydistannoxane in the desired molar ratio.
SUBSTITUTE SHEET (RULE 26)
CA 02338801 2007-08-01
WO 00/06583 PCT/NL98100429
- 3 -
The compounds synthesized by one of ttlese m~thods were characterized by
elemental
anatysis, 'H, "C and 1'7Sn NMR, electrosfray mass spectron-etry and 191"Sn
Wsshauer spectroscopy. Chromatography on SephadexTM LH-20 proved to be a very
efficient method to separate 3 (or 7) froni 4 (or 8), or 11, 12, 15 and 16
from the
starting carboxylic acid.
The structures of the compounds synthesized are depicted below
10 9
11~0~8 9
10 ~8
0 O 234 7 a SnR3 11 O 0 2~
~ 4 O~ SnR3
O 1 5 0 1
Xo
1 S
6 5 /
14~Oj 17 ~ p 1 0 -1,/
15 16 13 14 15
R C6H51 R!= C6H5 5
R'= CH2CHZCH~CH3 2 R'= CH2CH2CH2CH3 6
0 Bu 0 0 o sno 0
c O
O
~Oj ~..- o")
3
O 0 Bu Bu 0
r' O 1
Ia O a+ Sn, o, S I n, a O O
+ Bu Bu
\ \
O 0 '~
O./ 4 ~O
2
o~ 0 Bu 0 r'o
O Sn 0
I
0
~\ 0' Bu O O
0 ~ O
~ ~0--/
7
CA 02338801 2001-01-26
WO 00/06583 PCT/NL98/00429
- 4 -
4 2
7 lj ON. ~ 10 ~04 2 1 O~
p
O I) SnR3 O ~ SnR3
7 5
5 R=C6H58 Rf=C6H512 p
R'= CH2CHZCHZCH3 9 R' = CHZCH2CHZCH3 13
Bu
O\/'\ O=
O""Y Sn- p)r O--~ O\
O Bu O
10
Bu Bu
O~ O- Sn'O,Sn'O~p/~/
i I
0 Bu Bu 0 2
11
Bu
p/~p\.~O~Y p'Sn -p'~ p~~ p
~(
o B-, o
14
pp~SBun O~Bu O /
~ Sn ~ 0 O\~\ ip
i i
O Bu Bu O 2
Generalities and abbreviations
NMR spectra: CDC13 solutions; IH and 13C chemical shifts S in ppm vs. TMS,
homonuclear coupling constants in Hz, in parentheses; M6ssbauer parameters
(quadrupole splitting QS, isomers shift IS, and band widths T', and 172) in
mrn/s; s:
singlet; d: doublet; dd: doublet of doublets; m: complex pattern; t: triplet;
tq: triplet of
quartets; Ws, pseudo-singlet.
Electrospray mass spectra: positive monoisotopic ions (12C, tH, 160, 23Na,
39K,
120Sn). Na and/or K are already present in the electrospray mass spectra of
the starting
carboxylic acids.
CA 02338801 2001-01-26
WO 00/06583 PCT/NL98/00429
- 5 -
Charaterization of compounds 1 to 15
Compound 1: triphenyltin 4,7,10,13,16,19-
hexaoxadicyclo[16.4.0]dicosa-1,3(20),21-triene-l-carboxylate
prepared according to method 1,
10 h of reflux, recrystallized from diethyl ether/hexane, m.p. 110-112 C,
yield 98%, elemental arialysis: exp. (calc. for C35H38SnO8 ): C: 60.2 (59.60);
H: 5.5
(5.43); IH NMR: 7.7-7.8, m, H(o) & H(5); 7.62, d(4J(iH-tH) = 2), H(3); 7.4-
7.5,
m, H(m) & H(p); 6.91, d(3J(1H-1H) = 9), H(6); 4.1-4.3, m, H(8) & H(17); 3.8-
4.0,
m, H(9) & H(16); 3.6-3.8, m, H(10), H(11), H(14) & H(15); 3.67, yls, H(12) &
H(13); 13C NMR: 172.7, C(7); 152.9, C(1); 148.0, C(2); 138.5, C(i); 137.0,
C(o)
2J(117n19Sn-13C) = 47/49; 130.2, c(p) 4J(1 17/119Sn-13C) = 13; 129.0, C(m)
3J(117/119Sn-
13C) = 61/63; 125.1, C(5); 122.8, C(4); 114.9, C(3); 111.7, C(6); 70.80 &
70.77,
C(10) & C(15); 70.71 & 70.66, C(12) & C(13); 70.53, 70.51, 69.4, 69.2, 68.8 &
68.6, C(8), C(9), C(11), C(14), C(16) & C(17); 117Sn NMR: -115.7; electrospray
MS:
M+K+ (m/z = 745), 14%; M + Na+, 100%; Mossbauer: QS: 2.26; IS: 0.55; I'i:
1.34;
r2: 1.32.
Compound 2: tri-n-butyltin 4,7,10,13,16,19-
hexaoxadicyclo[16.,4.0]dicosa-1,3(20),21-triene-l-carboxylate
prepared according to method 1,
4 h of reflux, recryst. hexane/chioroform, m.p. 45-47 C, yield 80%,
elemental analysis: exp. (calc. for C29H50SnOg - H20): C: 52.5 (52.50); H: 8.3
(7.90);
iH NMR: 7.63, dd (3J(IH-tH) = 8; 4J(IH-iH) = 2), H(5); 7.54, d(4J(IH-tH) = 2),
H(3); 6.82, d(3J(1H- IH) = 8), H(6); 4.1-4.3, m, H(8) & H(17); 3.8-4.0, m,
H(9) &
H(16); 3.6-3.8, m, H(10), H(11), H(14) & H(15); 3.65, yls, H(12) & H(13); = 2,
HOH; 1.6-1.7, m H((3); 1.2-1.3, m, H(a) & H(y); 0.89, t(3J(1H-IH) = 7), H(S);
13C
NMR: 171.4, C(7); 152.2, C(1); 148.1, C(2); 125.0, C(4); 124.2, C(5); 115.0,
C(3);
112.1, C(6); 70.96 & 70.84, C(10) & C(15); 70.77 & 70.73, C(12) & C(13);
70.66,
3 5 70.61, 69.5, 69.4, 68.9 & 68.8, C(8), C(9), C(11), C(14), C(16) & C(17);
27.9,
C(P) 2J(117/19Sn-13C) == 20; 27.0, C(y) 3J(117/119Sn-13C) = 62/65; 16.6, C(oc)
lJ(117/119Sn-13C) = 341/358; 13.7, C(S); 117Sn NMR: 108.2; electrospray MS: M
+ Na*
(m/z = 669), 6%; M + H20 + H+, 9%; M + H+, 11%; Mossbauer: QS: 3.40; IS: 1.39;
r,: 0.72; r2: 0.85.
CA 02338801 2001-01-26
WO 00/06583 PCT/NL98/00429
- 6 -
Compound 3: di-n-butyltin bis[4,7,10,13,16,19-
hexaoxadicyclo[16.4.0]dicosa-1,3(20),21-triene-l-
carboxylate, prepared according to method 1,
6 h of reflux, recryst. hexane/chloroform, m.p. 125-127 C, yield 96%,
elemental analysis: exp. (calc. for C42H64SnO16): C: 53.5 (53.46); H: 7.1
(6.84); IH
NMR: 7.73, d(3J('H-'H) = 8) , H(5); 7.58, s, H(3); 6.86, d(3J(1H-IH) = 8),
H(6);
4.1-4.3, m, H(8) & H(17); 3.8-4.0, m, H(9) & H(16); 3.6-3.8, m, H(10), H(11),
H(14) & H(15); 3.65, Ws, H(12) & H(13); 1.7-1.8, m, H((3) & H(a); 1.37, tq,
(3J(tH-tH) = 7, 3J(1 H-~H) = 7), H(Y); 0.86, t(3J(tH-tH) = 7), H(S); 13C NMR:
175.7, C(7); 153.3, C(1); 148.3, C(2); 124.9, C(5); 122.7, C(4); 115.3, C(3);
112.3,
C(6); 70.9, C(10) & C(15); 70.81 & 70.76, C(12) & C(13); 70.73 & 70.67, 69.5,
69.4, 69.2 & 69.0, C(8), C(9), C(11), C(14), C(16) & C(17); 26.7, C(P) 2J(l
17/19Sn-
13C) = 33; 26.3, C(y) 3J(117/119Sn-13C) = 95; 25.4, C(a) lJ(117/119Sn-13C) =
569/596;
13.5, C(S); 117Sn NMR: -156.2; electrospray MS: M + Na* (m/z = 967), 100%;
Mossbauer: QS: 3.41; IS:: 1.44; I'i: 10.94; 1,2: 0.94.
Compound 4: bis{di-n-butyl-[4,7,10,13,16,19-
hexaoxadicyclo[16.4.0]dicosa-1,3(20),21-triene-l-
carboxylato]tin}oxide, prepared according to method 3
12 h of reflux, Sephadex LH-20, elution with chloroform/methylene
chloride; recryst. hexane/chloroform, m.p. 96-98 C, yield 90%, elemental
analysis:
exp. (calc. for CIooH164Sn4O34): C: 50.0 (50.35); H: 7.1 (6.94); IH NMR: 7.54;
d
(3J(1H-IH) = 8), H(5); 7.49, s, H(3); 6.84, d(3J(tH-iH) = 8), H(6); 4.1-4.3,
m,
H(8) & H(17); 3.8-4.0, m, H(9) & H(16); 3.6-3.8, m, H(10), H(11), H(14) &
H(15);
3.62, yVs, H(12) & H(13); 1.6-1.8, m, H((3 ); 1.4-1.6, m, H(a); 1.1-1.4, m,
H(y); 0.7-
0.9, m, H(&); 13C NMR: 172.6, C(7); 152.5, C(1); 148.3, C(2); 126.3, C(4);
124.0,
C(5); 115.3, C(3); 112.4, C(6); 70.8, C(10) & C(15); 70.7 & 70.6, C(12) &
C(13);
70.6, 70.5, 69.42, 69.35, 69.1 & 68.9, C(8), C(9), C(11), C(14), C(16) &
C(17);
26.7 & 26.6, C((3); 27.6 & 27.4, C(y); 29.5 & 28.3, C(a); 13.5 & 13.4, C(S);
117Sn
NMR: -213.0 & -217.3; electrospray MS: M/2 + K+ (m/z = 1233), 11%; Mossbauer:
QS: 3.36; IS: 1.27; T'i: 0.96; 172: 0.99.
CA 02338801 2001-01-26
WO 00/06583 PCT/NL98/00429
- 7 -
Compound 5: triphenyltin 4,7,10,13,16-
pentaoxadicyclo[13.4.0]cosa-1,3(17),18-triene-l-carboxylate,
prepared according to method 2,
48 h of reflux, recryst. hexane, m.p.: 130-131 C, yield 95%, elemental
analysis: exp. (calc. for C33H34SnO71 H2O): C: 58.4 (58.34); H: 5.7 (5.35); 1H
NMR: 7.7-7.8, m, H(o) & H(5); 7.60, d(4J(IH-iH) = 2), H(3); 7.4-7.5, m, H(m) &
H(p); 6.82, d(3J(IH-IH) = 8), H(6); 4.1-4.2, m, H(8) & H(15); 3.8-3.9, m, H(9)
&
H(14); 3.73, yrs, H(10), H(11), H(12) & H(13); = 2, HOH; 13C NMR: 172.8, C(7);
153.1, C(1); 148.3, C(2); 138.7, C(i); 136.9, C(o) 2J(117/119Sn-13C) = 47/49;
130.1,
C(p) 4J(1 17/119Sn-13C) = 13; 128.9, C(m) 3J(1171119Sn-13C) = 62/65; 125.2,
C(5); 123.3,
C(4); 115.7, C(3); 112.1, C(6); 70.95 & 70.92, C(8) & C(15); 70.31 &'70.23,
C(9)
& C(14); 69.3, 69.2, 69.9 & 68.5, C(10), C(11), C(12) & C(13); 117Sn NMR: -
116.3;
electrospray MS: M+K+ (ni/z=701, 67%); M + Na+, 73%; M6ssbauer: QS: 2.77; IS:
1.23; I'1: 0.94; I'Z: 0.88.
Compound 6: tri-n-butyltin 4,7,10,13,16-
pentaoxadicyclo[13.4.0]cosa-1,3(17),18-triene-l-carboxylate,
prepared according to method 2,
h of reflux, Sephadex LH-20, elution with chloroform/methylene
chloride, liquid; yield 90%; elemental analysis: exp. (calc. for C27H46SnO7 -
1/2 H20):
C: 53.1 (53.14); H: 7.8 (7.77); IH NMR: 7.63, dd (3J(IH-iH) = 8; 4J(1H-1H) =
2),
25 H(5); 7.54, d(4J(1H-IH) = 2), H(3); 6.82, d(3J(1H-1H) = 8), H(6); 4.1-4.2,
m, H(8)
& H(15); 3.8-3.9, m, H(9) & H(14); 3.73, yls, H(10), H(11), H(12) &:H(13); 1.6-
1.7, m, H(P); 1.2-1.4, m, H(a) & H(y); 0.89, t(3J('H-)H) = 7), H(S); 13C NMR:
171.4, C(7); 152.5, C(1. ); 148.4, C(2); 125.1, C(4); 124.4, C(5); 115.5,
C(3); 112.3,
C(6); 71.2, C(8) & C(a5); 70.57 & 70.52, C(9) &'C(14); 69.6, 69.5, 69.1 &
68.9,
C(10), C(11), C(12) & C(13); 27.9, C(P) 2J(l 17/119Sn-13C) = 20; 27.0, C(y)
3J(117/119Sn-13C) = 62/65; 16.6, C(a) lJ(117/119Sn-13C) = 350/362; 13.6, C(S);
117Sn
NMR: 107.4; electrospray MS: M + Na+ (m/z = 625), 5%; M + NH4+, 9%; Mossbauer:
QS: 3.29; IS: 1.45; I,,: 0.94; I'2: 0.88.
Compound 7: di-ri-butyltin bis[4,7,10,13,16-
pentaoxadicyclo [13 .4 . 0] cosa-.1, 3(17) , 18-triene-l-
carboxylate], prepared according to methode 2,
CA 02338801 2001-01-26
WO 00/06583 PCT/NL98/00429
- 8 -
48 h of reflux, recrystallized from petroleum ether/methylene chloride,
m.p.: 130-132 C; yield 98%; elemental analysis: exp. (calc. for C3bH56SnO[4):
C: 53.9
(53.35); H: 6.7 (6.60); 111 NMR: 7.74, dd (3J(1 H-' H) = 8, 4J(1 H-1 H) = 2),
H(5);
7.59, d(4J(1H-1H) = 2) , H(3); 6.86, d(3J(1H-1H) = 8), H(6); 4.1-4.2, m, H(8)
&
H(15); 3.8-4.0, m, H(9) &. H(14); 3.75, yrs, H(10), H(11), H(12) & H(13); 1.7-
1.8,
m, H((3) & H((x); 1.38, tq (3J(1H-1H) = 7, 3J(1H-1H) = 7), H(y); 0.86, t(3J(1H-
1H) =
7), H(8); 13C NMR: 175.8, C(7); 153.5, C(1); 148.5, C(2); 125.0, C(5); 122.9,
C(4);
lo 115.4, C(3); 112.2, C(6); 71.11 & 71.09, C(8) & C(15); 70.39 & 70.31, C(9)
&
C(14); 69.4, 69.2, 69.0 & 68.6, C(10), C(11), C(12) & C(13); 26.7, C(P),
2J(117i119Sn-13C) = 34; 26.4, C(y), 3J(117n19Sn-13C) = 103; 25.5, C(ot),
1 J(117i119Sn-13C) = 561/588; 13.6, C(S); "'Sn NMR: -156.8; electrospray MS: M
+
Na+ (m/z = 879), 27%, M + K+, 27%; M6ssbauer: QS: 3.28; IS: 1.41; I'i: 0.92; 1-
2:
0.93.
Compound 8: tripheriyltin 3,6-diheptanoate, prepared
according to method 2,
8 h of reflux, recrystallized from hexane/chloroform, m.p.: 100-102 C,
yield 95%, elemental analysis: exp. (calc. for C23H1.4SnO4 ): C: 57.4 (57.18);
H: 4.7
(5.01); IH NMR: 7.7-7.8, m, H(o); 7.4-7.5, m, H(m) & H(p); 4.25, s, H(2); 3.7-
3.8,
m, H(4); 3.5-3.6, m, H(5); 3.35, s, H(7); 13C NMR: 176.5, C( i); 137.7, C(i);
136.8,
C(o) 2J(117/119Sn-13C) = 49; 130.2, c(p) 4J(117/19Sn-13C) = 13; 128.9, C(m)
3J(117/119Sn-'3C) = 62/65; 72.0, C(5); 70.6, C(4); 69.0, C(2); 59.0, C(7);
117Sn NMR:
-100.0; electrospray MS: M + Na+ (m/z = 507), 5%, M + H+, 2%; M6ssbauer: QS:
3.60; IS: 1.24; T'r 0.85; I'i: 0.79.
Compound 9: tri-n-butyltin 3,6-diheptanoate, prepared
according to methocde 2,
8 h of reflux, Sephadex LH-20, elution with methylene chloride, liquid,
yield 95% elemental analysis: exp. (calc. for Ci7H36SnO4 - 1/2 H2O): C: 47.4
(47.27);
H: 8.6 (8.63);; 1H NMR: 4.09, s, H(2); 3.6-3.7, m, H(4); 3.5-3.6, m, H(5);
3.36, s,
H(7); = 2, HOH; i.5-1.6, m, H((3); 1.2-1.4, m, H(a) & H(y); 0.88, t(3J(1H-1H)
=
7), H(S); 13C NMR: 175.2, C(1); 71.9, C(5); 70.4, C(4); 69.0, C(2); 59.0,
C(7);
CA 02338801 2001-01-26
WO 00/06583 PCT/NL98/00429
- 9 -
27.8, C((3) 2J(117/119Sn-13C) = 20; 27.1, C(y) 3J(11/119Sn-13C) = 64/67; 16.6,
C(a). -
lJ(i 17/119Sn-13C) = 338/355; 13.7, C(S); 11 7Sn NMR: 120.7; electrospray MS:
M + Na+
(m/z = 447), 7%; Mossbauer: QS: 3.81; IS: 1.47; I,j: 1.15; I72: 1.14.
Compound 10: di-n-butyltin bis(3,6-diheptanoate), prepared
according to method 3,
12 h of reflux, liquid, yield 98%; elemental analysis: exp. (caic. for
CjgH36SnO8): C: 43.4 (43.31); H: 7.5 (7.27); IH NMR: 4.16, s, H(2); 3.6-3.8,
m,
H(4); 3.5-3.6, m, H(5); 3.36, s, H(7); 1.6-1.7, m, H((3) & H(a); 1.34, tq
(3J(tH-IH)
= 7, 3J(1 H-tH) = 7), H(j); 0.87, t(3J(l H-I H) = 7), H(S); 13C NMR: 178.3,
C(1);
71.8, C(5); 70.7, C(4); 68.6, C(2); 59.0, C(7); 26.5, C(O) 2J(l 171119Sn13C) =
34;
26.3, C(y) 3J(117/119Sn-13(") = 98/102; 25.7, C(a) lJ(117/119Sn-13C) =
538/567; 13.4,
C(8);11 7Sn NMR: -124.7; electrospray MS: M + Na+ (m/z = 523), 77%; M + K+,
13%; Mossbauer: QS: 3.90; IS: 1.44; I'l: 1.28; I72: 1.02.
Compound 11: bis[di-n-butyl(3,6-dioxaheptanoato)tin)oxide,
prepared according to method 3,
12 h of reflux, Sephadex LH-20, elution with methylene chloride,
liquid, yield 80%; elemental analysis: exp. (caic. for C52H108Sn4O18 - 2 H20):
C: 40.7
(40.76); H: 7.2 (7.37); lH: NMR: 3.95, s, H(2); 3.6-3.7, m, H(4); 3.5-3.6, m,
H(5);
3.34, s, H(7); = 2, HOH; 1.5-1.7, m, H(P); 1.3-1.5, m, H(a); 1.30, tq, (3J([H-
tH) =
7, 3J(1H-tH) = 7), H(y); 0.86, m, H(S); 13C NMR: 174.9, C(1); 71.8, C(5);
70.2,
C(4); 69.8, C(2); 58.9, C(7); 27.5 & 27.2, C((3); 26.8 & 26.7, C(y); 29.0 &
26.3,
C(a); 13.57 & 13.55, C(8); 117Sn NMR: -204.8 & -215.8; electrospray MS: M12 +
Bu2SnOH+ (m/z = 1001), 40%; Mossbauer: QS: 3.42; IS: 1.34; i'i: 1.22; I',:
1.18.
Compound 12: triphenyltin 3,6,9-trioxadecanoate, prepared
according to method 2,
8 h of reflux, recryst. diethyl ether/methylene chloride, m.p.: 109-
111 C, yield 92%, elemental analysis: exp. (calc. for C25H28SnO5 ): C: 57.1
(56.96);
H: 5.4 (5.36); ; 1H NMR: 7.7-7.8, m, H(o); 7.4-7.5, m, H(m) & H(p); 4.22, s,
H(2);
CA 02338801 2001-01-26
WO 00/06583 PCT/NL98/00429
- 10 -
3.7-3.8, m, H(4); 3.6-3.7, m, H(5); 3.5-3.6, m, H(7); 3.4-3.5, m, H(8); 3.34,
s,
H(10); 13C NMR: 176.4, C(1); 137.8 C(i); 136.9, C(o) 2J(117i119Sn-13C) = 47-
50;
130.2, C(p) 4J(1171119Sn-13C) = 13; 128.9, C(m) 3J(1171119Sn-13C) = 63/66;
72.0, C(8);
70.7, 70.7 & 70.5, C(4), C(5) & C(7); 69.0, C(2); 59.0 C(10);1 17Sn NMR: -
103.2;
electrospray MS: M + K-* (m/z.= 567), 2%; M + Na+, 1 I%; M + H+, 6%;
M6ssbauer:
QS: 3.44; IS: 1.29; I"i: 0.91; 1,2: 0.87.
Compound 13: tri-ri-butyltin 3,6,9-trioxadecanoate, prepared
according to method 2,
8 h of reflux, Sephadex LH-20, elution with methylene chloride, liquid,
yield 92%;elemental analysis: exp. (calc. for C19H40SnO5 ' 1/2 H20): C: 47.7
(47.94);
H: 8.8 (8.68); iH NMR: 4.09, s, H(2); 3.6-3.8, m, H(4), H(5) & H(7); 3.5-3.6,
m,
H(8); 3.36, s, H(10); = 2, HOH; 1.5-1.7, m, H((3); 1.2-1.4, m, H(a) & H(7);
0.89, t
(3J(tH-tH) = 7), H(S); 13C NMR: 175.1, C(1); 72.0, C(8); 70.6, 70.5 & 70.5,
C(4),
C(5) & C(7); 69.0, C(2); 58.9, C(10); 27.8, C(P) 2J(117/119Sn-13C) = 20; 27.0,
C(y)
3J(1171119Sn-13C) = 63/66; 16.6, C(a) lJ(117/119Sn-13C) = 349/355; 13.6, C(5);
117Sn
NMR: 120.7; electrospray MS: M + K+ (m/z = 507), 18%; M + Na+, 51%;
M6ssbauer: QS: 3.84; IS: 1.47; I',: 1.07; I'.: 1.02.
Compound 14: di-n-butyltin bis (3,6,9-trioxadecanoate),
prepared according to method 3,
12 h of reflux, liquid, yield 95%; elemental analysis: exp. (caic. for
C22H44SnOto ): C: 44.8 (44.99); H: 7.8 (7.56); IH NMR: 4.15, s, H(2); 3.6-3.8,
m,
H(4), H(5) & H(7); 3.5-3.6, m, H(8); 3.35, s, H(10); 0.6-0.8, m, H(O) & H(a);
1.35, tq (3J(tH-tH) = 7, 3J(IH-1H) = 7), H(y); 0.88, t(3J(IH-1H) = 7), H(S);
13C
NMR: 175.8, C(1); 71.8, C(8); 71.1, 70.6 & 70.4, C(4), C(5) & C(7); 68.7,
C(2);
59.0, C(10); 26.6, C(P)õ'-J(<<7/119Sn-13C) = 38; 26.3, C(y), 3J(117/119Sn-13C)
= 99;
25.6, C((C), 1J(117/119Sn-1-1C) = 540/567; 13.5, C(S); 117Sn NMR: -124.1;
electrospray
MS: M + K* (m/z = 62'7), 12%; M + Na*, 22%; M6ssbauer: QS: 3.77; IS: 1.42; I'i
:
136; 1-2: 1.18.
CA 02338801 2001-01-26
WO 00/06583 PC"T/NL98/00429
-11-
Compound 15: bis[d:i-n-butyl(3,6,9-trioxadecanoato)tin]oxide,
prepared according to method 3,
12 h of reflux, Sephadex LH-20, elution with methylene chloride,
liquid, elemental analysis: exp. (calc. for C60H124Sn4O22. 2 H20): C: 42.2
(42.18); H:
7.4 (7.56); yield 80%; IH NMR: 3.96, s, H(2); 3.6-3.7, m, H(4), H(5) & H(7);
3.5-
3.6, m, H(8); 3.34, s, H(10); = 2, HOH; 1.57, tt (3J('H-1H) = 7, 3J(1H-IH) =
7),
H(D); 1.4-1.5, m, H(a); 1.27, tq (3J(iH-IH) = 7, 3J(IH-IH) = 7), H(Y); 0.8-
0.9, m,
H(S); 13C NMR: 175.1, C(1); 72.0, C(8); 70.60, 70.55 & 70.4, C(4), C(5) &
C(7);
69.9, C(2); 59.0, C(10); 27.6 & 27.3, C(~); 26.9 & 26.7, C(y); 29.1 & 25.8,
C(a);
13.6, C(S); 117Sn NMR: -204.9 & -217.6; electrospray MS: M/2 + Na+ (m/z =
861),
10%; M12 + Bu2SnOH+, 33%; M6ssbauer: QS: 3.49; IS: 1.32; T'i: 0.90; I'2: 0.90.
Stability in water of organotin polyoxaalkanenecarboxylates
The stability in the presence of water of four compounds, 6, 8, 9 and 12, was
determined. Solutions in CD3CD2OD exhibit a single resonance in 117Sn NMR (at
36.5,
-210.7, 27.9 and -212.0 ppm, respectively) that is regularly slightly shifted
after the
addition of increasing amounts of D20..
CA 02338801 2001-01-26
WO 00/06583 PCT/NL98/00429
- 12 -
Antittmnur activity of coapaunds 1 to 15
The IDSO inhibition doses in ng/ml of the tested ccnpounds are given in
the table as well as thase for scare known reference ccnpounds.
MQ?-7 EVSA- WiI7r IG- M19 A498 H226
T FZOI
1 15 12 13 30 16 43 37
2 35 6 11 30 70 97 100
3 155 128 781 260 219 282 281
4 36 46 239 82 68 126 73
5 <3 <3 <3 <3 <3 <3 <3
6 <3 <3 <3 <3 <3 <3 <3
7 273 237 332 321 286 49 854
8 13 12. 34 37 31 32 33
9 32 40 82 84 90 153 61
10 60 62 379 128 115 134 161
11 <3 <3 6 <3 <3 <3 5
12 9 9 19 33 24 21 25
13 36 25 40 89 78 93 56
14 86 66 495 178 167 145 280
15 <3 <3 3 <3 <3 <3 <3
cisplatin 699 422 967 169 558 2253 3269
do3wrubicin 10 8 11 60 16 90 199
etoposide 2594 = 317 150 580 505 1314 3934
5-fluoro-uracil 750 475 225 297 442 143 340
methotrexate 18 5 <3 7 23 37-L2-28-711
Inhibition doses IDso in vitro (in ng/ml) against some tumoural cell lines
of human origin, two ma.mnaxy cancers, (MCF-7, EVSA-T), a colon carcincxna
(WiDr), an ovarian cancer ( ICROV) , a melancaia (M19 MEL), a renal cancer
(A 498) and a non small. cell lung cancer (H226) of organotin derivatives
of carboxybenzocrown and di- or trioxaalkanecarboxylic acids, and of some
known reference corrpoun(J.s.
SUBSTITUTE SHEET (RULE 26)