Note: Descriptions are shown in the official language in which they were submitted.
WO 00/06522 PCTlUS98/27725
CERAMER CONTAINING A BROMINATED POLYMER AND
INORGANIC OXIDE PARTICLES
". _
Fietd of the Invention
This invention relates to abrasion or scratch resistant protective coatings,
composite structures containing such coatings, and compositions for preparing
and
methods of preparing such coatings and composite structures.
Background
l0 Many important commercial products, including optically functional
products such as lenses, light fibers, optical screens and filters, reflective
sheeting,
and the like, have structures or are prepared from irnaterials that are
susceptible to
physical damage. Alternatively, there may exist some other reason why such
products benefit from protection against physical or mechanical damage. To
15 protect these products, a tough, abrasion resistant "hardcoat" layer may be
coated
and cured onto their structures.
Abrasion resistant hardcoats can include a p~oiymeric binder matrix formed
from a curable material, and inorganic oxide materials suspended or dispersed
within the polymeric binder. See, e.g., WO 96/36669 Al, which describes a
20 hardcoat formed from a "ceramer" used, in one application, to protect the
surfaces
of retroreflective sheeting from abrasion. As is known in the art of ceramer
compositions, ceramers can be derived from aqueous sols of inorganic colloids
according to a process in which a curable binder precursor and other optional
ingredients are blended into the aqueous sol. The resulting curable
composition is
2s dried to remove substantially all of the water. Solvent may then be added,
if
desired, in amounts effective to provide the composition with viscosity
characteristics suitable for coating the composition onto a desired substrate.
After
coating, the composition can be dried to remove the: solvent, and then exposed
to a
suitable source of energy to cure the binder precursor.
3o Optically functional products can include coatings having the primary
function of enhancing or reducing light reflectance from the surface of a
substrate.
When such a coating reduces the amount of light reflected by the substrate, it
is
-1-
CA 02338920 2001-O1-29
:~m~~~rsTR. a
r~.lfUJ~oIGI lL~ 81675 MUNCH,EN.'
Minnesota Mining & Manufacturing Co. ' ,
Our Ref.: D 2210 PCT '
a ~ Ju. 2(;00:
called "antireflective." When the coating enhai<ces the amount of light
reflected by
the substrate it is called "reflective."
Antireflective (AR) coatings in particular are becoming increasingly
important in commercial applications. The transparency of plastic or glass, in
the
form of doors, windows, lenses, filters, display devices (e.g., display
panels) of
electronic equipment, and the like, can be impaired by glare or reflection of
light.
To reduce the amount of glare on plastic or glass, the surface can include a
layer of
a metal oxide (such as silicon dioxide or indium tin oxide (ITO)), or suitably
alternating layers of metal oxides, such as ITO/Si02. li'or example, glass
surfaces
to can typically have about 4% surface reflection, but with the aid of
specialized
coatings, such as muitilayers of sputter deposited ITO/SiOz, surface
reflection can
be reduced to less than about 0.5% in the visible region of the spectrum (400-
700
nm).
Importantly, the reflectivity or antireflectivity of a mufti-layer optically
functional composite article depends not only on the reflectivity of each
layer, but
also on the relative refractive indices of layers that are .adjacent within
the
composite structure. Adjacent layers having similar or identical refractive
indices
will cause little or no additional reflection. But, if the indices of
refraction of
adjacent layers of a multilayer optically functional composite are different,
this
2o will cause reflectance of light at the interface of such adjacent layers,
and diminish
antireflective propertie~'~~~ ~
There is a need for chemical compositions that can function as abrasion-
resistant "hardcoat" compositions. There exists an even more specific need for
such hardcoats having optical properties (e.g., specific indices of
refraction)
wherein the hardcoat composition can be useful in optical product
applications, for
example in optically functional composites having reflective or antireflective
properties.
Summary of the Invention
3o In brief summary, the invention provides ceramer compositions, ceramer
solutions, hardcoat compositions, and optically functional composite
structures
including the hardcoat compositions. The ceramer compositions contain
-2-
AfVLFN~E~ SHEET'
CA 02338920 2001-O1-29
< US-A-5,002,795 describes a method of making a fire resistant and antistatic
film comprising the steps of providing a substrate film material, preparing a
flame retardant antistatic coating by mixing a radiation curable halogenated
prepolymer with an effective amount of a qu<arternary ammonium antistatic
compound and an effective amount of an antimony pentoxide fire retardant
compound, applying said coating to said film material and contacting said
coating with electron radiation in an amount sufficient to cure said coating.
EP-A-0 337 695 discloses a coating composition curable to an abrasion and
weather resistant coating comprising a non-aqueous dispersion of colloidal
silicon dioxide particles of diameters less than 100mpm in a protic group-
substituted ester or amide of acrylic or rnethacryliic acid.
US-A-4,438,190 relates to a photosensitive resin composition comprising (a) at
least one compound selected from the group consisting of benzotriazole, benz-
imidazole, benzthiazole and derivatives thereof and salts thereof, (b) a
phosphate compound having photopolymeric unsaturated bonds, (c) if
necessary, an organic thermoplastic polymeir, (d) a photopolymerizable
unsaturated compound having at least one terminal ethylene group and (e) a
sensitizes andlor a sensitizes system, and a photosensitive element comprising
a layer of said photosensitive resin composition and a support film thereof.
US-A-3,793,293 describes photoconductive coating compositions for
application to solid substrates which are to be utilized in electro-
photographic
operations. The resultant photoconductive coatings comprise a layer of
photoconductive pigment particles bonded to themselves and to the solid
substrate with a binder comprising an interpolymer, at least one of whose
constituent monomers contains bromine.>
-2a-
~~11.!~i~°°~~0 S~~ET
CA 02338920 2001-O1-29
WO 00106622 PCT/US98/27725
ingredients including inorganic oxide particles and a curable binder
precursor,
wherein the binder precursor includes a polymeriz;~ble brominated compound.
The
polymerizable brominated compound can contain a brominated monomer having a
relatively high index of refraction, e.g., at least about 1.5, andlor can
contain at
least one aromatic, brominated (meth)acrylate cam~pound. The ceramer
composition can be cured or polymerized to form a hardcoat composition
including
a brominated polymeric matrix having dispersed Herein, or surrounding the
inorganic oxide particles. The ceramer and hardcoat compositions can have
desirable physical (e.g., mechanical) and optical properties such as hardness,
1o scratch and abrasion resistance, and index of refraction. A desired index
of
refraction may be one that is sufficiently high {e.g., maximized), or one that
is
appropriately chosen to match an adjacent layer of a multilayer composite,
e.g., to
match a substrate to which the ceramer composition is coated.
An aspect of the invention relates to a chemical composition .including
inorganic oxide particles and an aromatic, brominated (meth)acrylate compound.
The binder precursor may further contain polymerizable non-brominated
compounds, and may further comprise a coupling agent, an organic or aqueous
solvent, or both.
A further aspect of the invention relates to a chemical composition
2o containing inorganic oxide particles and a polymeriizable brominated
compound,
wherein the polymerizable brominated compound <;xhibits an index of refraction
of
at least about 1.5.
Yet a further aspect of the invention relates to a cured hardcoat composition
including a brominated polymer and inorganic oxicle particles, wherein the
polymer includes monomeric units derived from a ~polymerizabIe composition
including a poIymerizable brominated compound having an index of refraction of
at least about 1.5.
Yet a further aspect of the invention relates to a cured hardcoat composition
including a brominated polymer and inorganic oxidle particles, wherein the
3o polymer includes monomeric units derived from a lpolymerizable composition
including a polymerizable, aromatic, brominated (nneth)acrylate compound.
-3-
CA 02338920 2001-O1-29
WO 00/06b22 PCT/US98I27725
Yet a further aspect of the invention relates to a composite structure
including a substrate and a hardcoat composition, wherein the hardcoat
includes ~,
inorganic oxide particles and a brominated polymer'. The brominated polymer
comprises monomeric units derived from a polyrnerizable composition comprising
brominated monomers such as a brominated compound having an index of
refraction of at least about 1.5, andlor a polymerizable, aromatic
(meth)acrylate
compound. The composite structure can include other layers or components
including a primer layer, an antireflective layer, or one or more other
optically
functional layers. These composite structures cari be used as optical products
such
1o as antireflective composite structures.
As used within the present description, the i:ollowing terms shall have the
given meanings.
"Ceramer composition" or "ceramer" refers to any mixture comprising
substantially non-aggregated, colloidal or suspended inorganic oxide particles
15 dispersed in a curable binder precursor. The ceram.er composition can
optionally
contain a coupling agent, and can optionally contain organic or aqueous
solvent. A
ceramer composition diluted with solvent, e.g., to facilitate processing and
coating
onto a substrate, can be referred to as a "ceramer solution."
"Curable" refers to a material that can be thickened or solidified e.g., by
20 heating to remove solvent, heating to cause polymerization, chemical
crosslinking,
radiation polymerization or crosslinking, or the like.
"Cured" means a curable material that has been so thickened or solidified.
"PolymerizabIe" refers to chemical compounds such as monomers, dimers,
trimers, oligomers, pre-polymers, or polymers~etc., and chemical compositions,
25 capable of undergoing chemical reaction (e.g., via unsaturated moieties) to
produce
a higher molecular weight material such as a polynner, copolymer, or polymeric
or
copolymeric material.
"(Meth)acrylate" refers to both acrylate anti methacrylate compounds.
"Hardcoat," as in "hardcoat composition," :refers to a composition
3o comprising a polymeric matrix surrounding or containing inorganic oxide
particles.
A hardcoat composition can be prepared, for example, by curing or polymerizing
a
CA 02338920 2001-O1-29
w0 00106622 1'CTIUS98/27725
ceramer composition such that the binder precursor forms a polymeric matrix
having inorganic oxide particles contained or dispersed therein.
Brief Summary of the Di~awin~s
Figure 1 illustrates a side plan view of an antireflective film composite
structure including the hardcoat composition of the present description.
Detailed Description
Binder Precursor
to The ceramer composition includes a curable; binder precursor comprising
any of a variety of polymerizable chemical compounds or materials, e.g.,
monomers, oligomers, prepolymers, or polymers, etc. According to the
invention,
the binder precursor includes a polymerizable brominated compound such as a
high index of refraction polymerizable brominated compound {e.g., having an
~5 index of refraction of at least 1.5), and/or a polymenzable aromatic,
brominated
(meth)acrylate compound (compound means e.g., monomer, dimer, trimer,
oligomer, pre-polymer, or polymer etc.). The term "binder precursor," as used
within the present description, means reactive ingredients of a ceramer
composition that can be reacted to form a polymeric matrix. This includes
2o specifically the polymerizable brominated compound, monofunctional and
multifunctional non-brominated polymerizable comipounds if used, as each is
described in, fro, and does not include inorganic oxide particles, solvent,
any
coupling agent {whether reactive or not with the po:lymerizable compounds), or
other ingredients that cannot react to form the polyrneric matrix.
25 The components of a binder precursor can be chosen to provide a ceramer
or hardcoat composition having desired physical, optical, and mechanical
properties, and can preferably be chosen to provide a binder precursor having
a
desired refractive index, e.g., in the range from about 1.3 to about 1.7.
3o Polvmerizable Brominated Compound
The binder precursor contains a polymerizable brominated compound such
as a polymerizable brominated compound having an index of refraction of at
least
-5-
" _
CA 02338920 2001-O1-29
WO 00/06622 PCTIUS98I27725
1.5, or an aromatic, brominated (meth)acrylate comlaound., Preferred
polymerizable .brominated compounds can exhibit indices of refraction above "
_
about 1.52, 1.53, or 1.55.
Particularly preferred polymerizable bromin;ated compounds comprise
polymerizable aromatic, brominated (meth)acrylate compounds having an aromatic
portion, a brominated portion (which may or may not be the aromatic portion),
and
a (meth)acrylate moiety. An aromatic, brominated I;meth)acrylate compound may
be mono-functional or mufti-functional with respect: to the (meth)acrylate
moiety,
and can exhibit chemical and physical properties that facilitate preparation
of
1o ceramer compositions and.cured hardcoat compositiions having desired
properties.
The index of refraction of an aromatic, brominated (meth)acrylate
compound, and of a ceramer or hardcoat compositic>n prepared from an aromatic,
brominated (meth)acrylate compound; can be ai~ected by the chemical identity
of
the aromatic portion of the compound, and by the amount and position of
bromine.
i5 Bromine generally increases the index of refraction of an aromatic
(meth)acrylate
compound. Bromine can be present on an aromatic, brominated (meth)acrylate
compound at any useful position, and in any amount sufficient to provide a
desired
index of refraction.
In some applications the polymerizable brorninated compound, e.g., a
20 polymerizable, aromatic brominated (meth)acrylate compound, can preferably
have a relatively low melting point to reduce the mc;lting point of the binder
precursor and the ceramer composition, and thereby facilitate reduced
temperature
processing of a ceramer composition or ceramer solution. Some preferred
aromatic, brominated (meth)acrylate compounds, e.g., some monomers of formulas
25 2 and 3 infra; can exhibit relatively low melting points, e.g., below about
60
degrees Celsius (60C), more preferably below about 35C or 30C, even more
preferably below about 25C, and most preferably corn exist in~a liquid state
at about
room temperature (e.g., 23C). Preferably, an aromatic, brominated
(meth)acrylate
compound can also be soluble or miscible in one or more other ingredients of
the
3o ceramer composition; most preferably at room temperature.
An example of a preferred class of polymeriizable aromatic, brominated
(meth)acrylate compound is the class of aromatic, t>rominated (meth)acrylate
.6_
CA 02338920 2001-O1-29
WO 00106622 PCT/US98127725
monomers comprising a six-membered phenyl group preferably substituted by one
or more bromine substituents, and most preferably substituted by an alkyl
substituent. The aromatic portion of the monomer :may be connected directly to
the (meth)acrylate moiety, (e.g., see formula 2, infra), or the aromatic
portion may
s be connected to the (meth)acrylate moiety through a divalent organic linking
group
(L) (e.g., see formulas 1 and 3, infra). The linking group (L) can be any
substituted or unsubstituted divalent organic group;, and is preferably a
straight or
branched, substituted or unsubstituted alkylene (e.~;., methylene),
alkoxylene, or
polyalkoxylene.
to An example of a class of particularly preferred aromatic, brominated
(meth)acrylate monomers are mono-functional bro:minated (meth)acrylate
monomers:
0
R
BrX ~-L-p~
(1).
is
In formula 1, x represents the number of bromine s~ubstituents attached to the
aromatic group (Ar); when Ar is a six membered riing x ca.n be in the range
from 1
to about s. Ar can be substituted by other chemical substituents, such as an
alkyl.
L can be absent or a divalent linking group, and R can be hydrogen or methyl.
2o A preferred sub-class of brominated (meth;lacrylate monomer includes
those of formula 1 wherein L is absent and the aromatic portion of the monomer
comprises a brominated, alkyl-substituted phenyl moiety. These monomers can be
referred to as (alkyl,bramo)phenyl (meth)acrylate :monomers, and can be
described
according to formula 2:
Br
2s
CA 02338920 2001-O1-29
WO 00/06622 PCT/US98127725
wherein R can be hydrogen (-H) or methyl (-CH3), :~ can be in the range from 1
to
- about 4 and is preferably about 2, and Rl can be a straight or' branched
alkyl, w
positioned ortho, meta, or para to the ester. Specific; examples of brominated
monomers according to formula 2 include those having an alkyl group located
ortho to the ester substituent:
R
0 .O
Rl
rx
(2.1),
wherein~R, x, and Rl are defined supra. In a particularly
preferred.emliodiment,
bromines are located at the 4 and 6 positions on the aromatic ring, ortho and
para
to the ester substituent:
Ri
Io (2.2).
Particular monomers according to formula 2.2 include 4,6-dibromo-2-alkyl
phenyl
(meth)acrylates wherein the alkyl has from about 3 to 4 carbons, including the
following:
4,6-dibromo-2-sec-butyl phenyl (meth)acrylate,
'R
O p.
Br
Br
_g_
(2.2.1);
CA 02338920 2001-O1-29
4,6-dibromo-2 tert-butyl phenyl (meth)acrylate,
(2.2.2); and
4,6-dibromo-2-isopropyl phenyl (meth)acrylate,
'R
0 0
Br
13r
(2.2.3).
A second sub-class of preferred aromatic, brominated (meth)acrylate
to monomers includes monomers of formula 1 wherein L comprises a divalent
alkoxy
linking group, with an alkoxy oxygen atom attaching to the aromatic ring, the
aromatic ring preferably being phenyl, and the aromatic; ring being optionally
and
preferably substituted with bromine and an alkyl. Such monomers can be
referred
to as (alkyl,bromo)phenoxy alkylene (meth)acrylate monomers, as shown, e.g.,
by
the structure of formula 3:
Rl
o~~..-o ~R
L'
0
Brx v
(3),
wherein R can be hydrogen or methyl, x can preferably ibe from 1 to 4 and is
more preferably 2, ~-saa-~aa:e~~i.~~,~~
L'~ is s7~niytt a braes checl ~.t ky IeH 9- o ~ prt,~c,.,~ ~ y ~! i~ ~2
Carbons ~
All~El~l~?1'~ ~~l~~T
CA 02338920 2001-O1-29
~~l~o:~ and Rl , , ,
can be a.straight or branched alkyl preferably having u~p to 12 carbon atoms,
positioned ortho, meta, or pare to the phenyl oxygen. :Examples of brominated
monomers according to formula 3 can include monomers wherein Rl is located
ortho to the phenoxy oxygen, as illustrated by formula 3.1:
R1
o~~....0 ~R
La
0
BrX
(3.1)
wherein, R, x,,p, and R' are defined supra. In a particularly preferred
embodiment,
bromine atoms can be located at the 4 and 6 positions on the aromatic ring,
ortho
and para to the attached phenyl oxygen atom, as illustrated by formula 3.2:
Ri
o~'~~.0 ~R
o
0
Br Br
(3 .2).
Particularly preferred monomers of formula 3.2 include 4,6-dibromo-2-
alkyl phenyl alkylene {meth)acrylates wherein the R' alkyl has from 3 to 4
carbons, includ~Ag monomers of the types shown in formulas 3.2.1 and 3.2.2,
wherein R ands are as defined:
0~~~.0
~'R
L'~
0
Br
{3.2.1)
-lo-
Al~~l~a~~~ ~l-ly~'C
CA 02338920 2001-O1-29
O O
\ / \
R
O
Br ~Br
(3.2.2).
These and other aromatic, brominated {meth)a~~rylate monomers and
methods for their preparation are described in Assignee's copending patent
lno0 q8 So3~10
a llcatlon tT...a-.t c~a.,a..., c~,. ,
Pp ~~~~-~.~~a
I and International Patent
A lication W~ o~~''~ ~0
pp ~a~i~~r'o'~~SZiTC ~T:n ~d'r~ fro r=
n t 4lhT -t c~~~nnn
each of which is incorporated herein by
reference.
to Other examples of polymerizable brominated compounds that can be useful
in the binder precursor include but are not limited to tribromo phenyl
(meth)acrylate, pentabromo phenyl (meth)acrylate, tribromo phenyl ethyl
(meth)acrylate, bromo methyl styrene, and brominated bisphenol A
(meth)acrylate
compounds, including for example
Br
c
Ho
0
0
3-14-(1-(4-13-(acryIoyloxy)-2-hydroxypropoxy]-3,5-dibromoF~henyl}-1-
methylethyt}-2,b
dibromophenoxyJ-2-hydroxypropyl acrylate
Polymerizable brominated compounds, including those having an index of
2o refraction of at least 1,5, as well as aromatic, brominated (meth)acrylate
compounds, are commercially available, and can be prepared by methods
generally
known in the art of organic chemistry.
-1 I-
A~~~~~ED SI~IL~ET
CA 02338920 2001-O1-29
WO 00/06622 PCT/US98127725
Brominated (meth)acrylate compounds can be prepared by functionalizing
a (meth)acrylate moiety onto an acceptable brominated precursor. Brominated -
monomers of formula 1, e.g., bromo-phenyl (meth)acryiate monomers and alkyl
bromo-phenyl (meth)acrylate monomers, can be prepared by reacting a brominated
phenol (optionally alkyl-substituted) with a (meth)acrylate compound to attach
the
(meth)acrylate functionality to the phenol.
Brominated phenols are commercially avail.:ble, and can be prepared by
brominating a suitable phenol compound, such as an alkylphenol. Alkylphenols
are commercially available from Schenectady international Inc., Chemical
1o Division, Schenectady, NY. Phenols and alkylphen.ols can be brominated by
methods that are generally known in the chemical art, and as described, for
example, in the Kirk-Othmer Encyclopedia of Chemical Technology, Volume 4,
543 (4~' ed. 1992). An example of this process, as exemplified with ortho-
substituted alkyl phenols, can b~e represented as follows:
OH OH
Br
Br~
Br
As will be appreciated by those skilled in the organic chemistry art, this
bromination reaction can be useful to brominate other phenols or substituted
phenols, and thereby produce other phenols, as desired, for further reaction
toward
an aromatic brominated {meth)acrylate compound.
2o A brominated aromatic alcohol such as a br~aminated alkylphenal can be
esterified to produce a brominated alkylphenyl (meth)acrylate monomer by
reaction with an appropriate acid chloride (e.g., a (rneth)acryloyl chloride).
The
reaction between an alcohol and an acid chloride is well known in the chemical
art,
and is described, for example, in the Kirk-Othmer, :Encyclopedia of Chemical
Technology, Volume 9, 769 {4~' ed.1992); see also United States Patent No.
3,845,102. Inhibitors, such as phenothiazine or 4-naethoxyphenol (MEHQ), can
be
used during the reaction in an amount to provide protection from pre-
-12-
CA 02338920 2001-O1-29
WO 04!46622 PCT/US98/27725
polymerization of the monomer during its synthesis and storage, while not
excessively influencing the subsequent polymerization. , -
w
With respect to preferred brominated monomers described herein, a
brominated alkylphenol can be reacted with (meth)acryloyl chloride. Dlustrated
below is the production of an {alkyl, bromo)phenyl (rneth)acrylate monomer:
Rl Br Ri
yth)aCryloyl
c~~~
As will be appreciated by those skilled in the organiic chemistry art, other
brominated alkylphenol compounds ca.n be reacted with (meth)acryloyl chloride
to
produce dii~erent alkyl,-bromo-substituted (meth)ac;rylate compounds.
i0 Brominated monomers of formulas 1 and 3, which include a divalent
organic linking group L, can be prepared by starting with a brominated
aromatic
alcohol, as described above, and reacting a linking group precursor (including
a
secondary reactive group such as an alcohol, ally!, or epoxy) onto the
alcohol. A
(meth)acrylate moiety can thereafter be attached to the organic linking group
by
15 reaction onto the secondary reactive group.
Specifically, compounds according to formula 1, including a divalent
linking group L, can be prepared, e.g., by alkylatin~; a brominated aromatic
alcohol, by known methods, to produce an alkylated, brominated, aromatic
alkanol
compound (as illustrated below with respect to a bromine-substituted, alkyl-
20 substituted phenol).
OH
L/
OH
Rl Br ~y~~g R1
Agent
Br Br
-I3-
CA 02338920 2001-O1-29
WO 00106622 PCT/US98/27725
Alkylation methods are known in the art of organic chemistry, and are
generally
accomplished by introducing an alkylating agent, for example anyone of an , _
alkylene carbonate (e.g., ethylene carbonate), a chloroalkanol {e.g.,
chloroethanol),
or an alkylene oxide (e.g., ethylene oxide), to a brorninated aromatic
alcohol, under
proper conditions to allow the alkylating agent to react with the aromatic
alcohol
and cause alkylation. See, e.g., United States Patent No. 2,448,767.
The resulting (alkyl,bromo)aromatic alkanol can be esterified to give a
brominated (meth)acrylate monomer, in the exemplified case, an
(alkyl,bromo)phenoxy alkylene (meth)acrylate monomer.
OH
L~ R2
~ O R2 O
HO~
/L
O
R1, Br
0
to Br
A binder precursor can include a polymerizable brominated compound
comprising a reactive moiety different from a {meth)acrylate. For instance, a
binder precursor can contain a brominated epoxy, a brominated olefinic
compound,
a brominated styrene, a brominated (meth)acrylamide, a brominated vinyl ether,
a
15 brominated vinyl ester, a brominated allyl ether, brominated alkyl ether, a
brominated (meth)acrylonitrile, a brominated azalac;tone, a brominated N-vinyl
carbazole, a brominated N-vinyl pyrrolidone, a brominated aziridine monomer,
combinations of these, and the like.
2o Non-Brominated Compounds
The binder precursor can optionally include one or more polymerizable
non-brominated compound (e.g., a monomer, dimes, oligomer, pre-polymer, or
polymer) which can react with other components ofthe binder precursor to
provide
-14-
CA 02338920 2001-O1-29
WO 00!06622 PCTIUS98/27725
a braminated polymeric matrix. Polymerizable non-brominated. compounds are
known in the art of polymerizable compositions, polymers, and ceramer , _
compositions. Polymerizable non-brominated comlpounds can be of any chemical
nature that can be useful within a ceramer composition to react to form a
polymerized matrix suitable to contain or surround inorganic oxide particles.
Such
non-brominated compounds can include low molecular weight reactive diluents
which can modify flow properties of a ceramer composition, and mufti-
functional
crosslinking agents to crosslink polymers upon reaction and provide a highly
crosslinked matrix.
Polymerizable non-brominated compounds can be mono-functional or
mufti-functional with respect to the polymerizable moiety, and when multi-
functional, the two or more polymerizable moieties can be the same or
different.
The polymerizable moiety or moieties can preferably be reactive with another
polymerizable component of the binder precursor. Examples of suitable non-
brominated compounds include monomeric compounds comprising a
polymerizable moiety such as an olefin, a styrene, a vinyl ether, a vinyl
ester, an
ally! ether, an ally! ester, a (meth)acrylate, an acrylonitrile, a
methacrylonitrile, a
(meth)acrylamides, an azalactone, an N-vinyl carba~zole, an N-vinyl
pyrrolidone,
an arizidine, an epoxy, or a combination thereof.
Preferred non-brominated compounds include (meth)acrylate monomers,
e.g., alkyl and/or aryl (meth)acrylate compounds. 'The alkyl group of an alkyl
(meth)acrylate compound can preferably contain on average 1 to about 14 oarbon
atoms. The alkyl group can optionally contain oxygen atoms in the chain,
thereby
forming ethers. Preferably, an aryl group can contain on average from about 6
to
about 20 carbon atoms.
Examples of suitable monofunctional non-brominated polymerizable
compounds include 2-hydroxyethyl (meth)acrylate., 2-methylbutyl
(meth)acrylate,
(meth)acrylic acid, itaconic acid, crotonic acid, ma:leic acid; fumaric acid,
2,2'-
(ethoxyethoxy)ethyl (meth)acrylate, 2-hydroxypropyl (meth)acrylate, 3-
hydroxypropyl (meth)acrylate, t-butyl (meth)acrylate, n-butyl (meth)acrylate,
isobomyl (meth)acrylate, 2-(phenoxy)ethyl (meth)o~crylate, biphenyl
(meth)acrylate, t-butyIphenyl (meth)acrylate, cyclo~hexyl (meth)acrylate,
-is-
CA 02338920 2001-O1-29
WO OOI06622 PCT/US98/27725
dimethyladamantyl (meth)acrylate, 2-naphthyl (meth)acrylate, and phenyl
(meth)acrylate. w _
Useful non-brominated polymerizable compounds can also include, for
example, N-vinyl pyrrolidone, N-vinyl caprolactam, and styrene monomers such
as
methyl styrene monomers including 3-methyl styrene, 4-methyl styrene, alpha
methyl styrene, and mixtures thereof.
Non-brominated monofunctional acryIamide and methacrylamide
((meth)acrylamide) monomers can be useful to increase adhesion between a
ceramer or hardcoat composition and certain substrates, such as polycarbonates
to and PET, and can improve water miscibility of a cer~amer composition.
Exemplary
(meth)acrylamide monomers can have the formula:
R3 O RF
I II
H2C=C-C-N
R2
wherein R1 and R2 are each independently hydrogen" a (C1-Cs)alkyl group
optionally having hydroxy, halide, carbonyl, and amido functionalities, a (Ci-
15 Cg)alkylene group optionally having carbonyl and amido functionalities, a
(Cl-
C4)alkoxymethyl group, a (C4-Cis)aryl group, a (Ci-C3)alk(C4-Cl$)aryl group,
and
a {C4-Cl8)heteroaryl group; with the proviso that anly one of RI and RZ is
hydrogen; and R3 can be hydrogen, a halogen, or a methyl group. Preferably, Rl
is
a (C~-C4)alkyl group; R2 is a (Ci-C4)alkyl group; and R3 is hydrogen or
methyl. Rl
2o and RZ can be the same or different. More preferably, each of Rl and R2 is -
CH3,
and R3 is hydrogen.
Examples of useful {meth)acrylamides include (meth)acrylamide, N-
methylol (meth)acrylamide, N-hydroxyethyl (meth)acrylamide, diacetone
(meth)acrylamide, N-ethyl-N-aminoethyl (meth)acrylamide, N-ethyl-N-
25 hydroxyethyl {meth)acrylamide, N,N-dimethylol (m~eth)acrylamide, N,N-
dihydroxyethyl (meth)acrylamide; dimethylaminoethyl (meth)acrylamide, N-octyl
(meth)acrylamide (normal and branched), 1, I,3,3-tetxamethylbutyl
(meth)acrylamide, N-(3-brornopropionamidomethyl)(meth)acrylamide, N-tert-
butyl(meth)acrylamide, N,N-dimethyl(meth)acrylamide, N,N-
~o diethyl(meth)acrylamide, N-(5,5-dimethylhexyl)(meth)acrylamide, N-(I,1-
-16-
CA 02338920 2001-O1-29
WO 00/06622 PCT/US98/27725
dimethyl-3-oxobutyl)(meth)acrylamide, N-(hydrox~~methyl)(meth)acrylamide, N-
(isobutoxymethyl)(meth)acrylamide, N-isopropyl(meth)acrylamide, N- ,~ _
methyl(meth)acrylamide, N-ethyl(meth)acrylamide., N-methyl-N-
ethyl(meth)acrylamide, N-(fluoren-2-ylXmeth)acrylamide, N-(2-fluorenyl)..2-
methyl(meth)acrylamide, 2,3-bis(2-furyl)(meth)acrylamide, and N,N'-methylene-
bis (meth}acrylamide. A particularly preferred (meth)acrylamide is N,N-
dimethyl
acrylamide.
Multifunctional Poiymerizable Non-Brominated Co~ounds
to The binder precursor can further include a multifunctional polymerizable
non-brominated compound; e.g., a monomer, dimes, oligomer, pre-polymer,
polymer, etc., which can be useful, for example, to improve the amount of
crosslinking within a hardcoat composition. Multifunctional non-brominated
compounds are known in the art of organic chemistry (e.g., as crosslinkers),
and in
15 the art of polymers and ceramer compositions. A multifunctional non-
brominated
compound can be any multifunctional non-brominated compound that can react
with the other components of the binder precursor to produce a polymer.
The multifunctional non-brominated compound can include any two or
more polymerizable moieties such as those previously identified, which can be
the
2o same or different. Preferred multifunctional non-brominated compounds
comprise
ester (meth)acrylate compounds such as difunctional (meth)acrylate esters of a
polyhydric alcohol, and combinations thereof. Of these, trifunctional and
tetrafunctional esters of (meth)acrylate esters of polyhydric alcohol can be
especially preferred.
25 Examples of suitable multifunctional ester (meth)acrylates include
poly(meth)acrylic acid esters of polyhydric alcohols including, for example,
the
di(meth)acryIic acid ester of aliphatic diols such as ethyleneglycol,
triethyleneglycol, 2,2-dimethyl-1,3-propanediol, 1,3-cyclopentanediol, 1-
ethoxy-
2,3-propanediol, 2-methyl-2,4,pentanediol, 1,4-cycl'~,ohexanediol, 1,6-
3o hexamethylenediol, 1,2-cyclohexanediol, 1,6-cyclohexandimethanol; the
tri(meth)acryIic acid esters of aliphatic trials such as glycerin, 1,2,3-
propanetrimethanol, 1,2,4-butanetriol, 1,2,5-pentan~etriol, 1,3,6-hexanetroil,
and
-17-
CA 02338920 2001-O1-29
WO 00/06622 PCT/US98l27725
1,5, IO-decanetriol; the tri{meth)acrylic acid esters o:f
tris(hydroxyethyl)isocyanurate; the tetra(meth)acryl is acid esters of
aliphatic , _
tetrols, such as 1,2,3,4-butanetetrol, 1,1,2,2,-tetramethylolethane, 1,1,3,3-
tetramethylolpropane, and pentaerythritol, the tri(mE~th)acrylic acid esters
of
pentaerythritol, and the penta(meth)acrylic acid esters of dipentaerythritol;
the
penta(meth}acrylic acid esters of aliphatic pentols such as adonitol; the and
hexa(meth)acrylic acid esters of hexanols such as so~rbitot and
dipentaerythritol;
the di(meth)acrylic acid esters of aromatic diols suclh as resorcinol,
pyrocatechol,
bisphenol A, and bis{2-hydroxyethyl) phthalate; the tri(meth}acrylic acid
ester of
to aromatic triols such as pyrogalloi, phloroglucinol, and 2-phenyl-2,2-
methylolethanol; and the hexa(meth)acrylic acid esters of dihydroxy ethyl
hydantion; and mixtures thereof. Particularly preferred multifunctional ester
(meth)acrylic acids can comprise a mixture of di-, tai-, and
tetra(meth)acrylate
esters of pentaerythritol.
i5 The binder precursor can include amounts oiEpolymerizable brominated
compounds and mono- and mufti-functional non-brominated compounds sufficient
to provide a ceramer composition that can be processed, alone or in the
presence of
added solvent, to provide a useful hardcoat composition. While amounts outside
of the following ranges may be useful, preferred binder precursors can include
2o from about 20 to about 80 parts by weight (pbw) polymerizable brominated
compound, e.g., aromatic, brominated (meth)acrylai;e compound, preferably
about
30 to 50 pbw polymerizable brominated compound, based on 100 pbw of the
binder precursor; preferably the binder precursor includes both mono- and
multi-
functional palymerizable brominated (meth)acrylatE; monomer, e.g., from about
10
25 to 20 parts by weight monofunctional polymerizable brominated compound and
from about 20 to 30 pbw difunctional polymerizablE; brominated compound based
on 100 pbw binder precursor. The binder precursor can also contain
polymerizable
non-brominated compound in useful amounts, e.g., from about 20 to 80 pbw,
preferably about 50 to 70 pbw polymerizable non-b:rominated compound based on
30 100 pbw binder precursor; the non-brominated compound can include mono- and
mufti-functional materials, including preferably from about 10 to 20 pbw
monofunctional polymerizable non-brominated compound and from about 40 to 50
-18-
CA 02338920 2001-O1-29
WO 00106622 PCT/US9$/27725
pbw multifunctional polymerizable non-brominatedl compound based on 100 pbw
of the binder precursor.
Coupling Agent
Optionally the ceramer composition can contain a coupling agent. The
coupling agent is thought to provide a link, either b;y chemical bond or a
lesser
chemical interaction, between the polymeric matrix and inorganic oxide
particles.
A wide variety of coupling agents are known in the ceramer art, and a
particular
coupling agent can be selected for use in a given ceramer composition based on
l0 factors such as the chemical compositions of the binder precursor and the
inorganic
oxide particles. Exemplary coupling agents include: organofunctional silane
monomers and carboxylic acid-functional compounds.
Examples of coupling agents include carboxylic acids such as stearic acid,
acrylic acid, oleic acid, silanes such as methyl trimethoxysilane, methyl
triethoxysilane, phenyl trimethoxysilane, phenyl triethoxysilane,
(meth)acryloxyalkyl trimethoxysilanes such as (metth)acryloyloxypropyl
trimethoxysilane and {meth)acryloyloxypropyl trichlorosilane, phenyl
trichlorosilane, vinyl trimethoxysilane, vinyl trietho~xysilane, propyl
trimethoxysilane, propyl triethoxysilane, glycidoxy~propyl trimethoxysilane,
2o glycidoxypropyl triethoxysilane, glycidoxypropyl t~~ichlorosilane, and
fluorinated
or perfluorinated silane compounds such as perfluoroalkyl trimethoxysilane,
perfluoroalkyl triethoxysilane, perfluoromethyl alkyl trimethoxysilanes such
as
tridecafluoro-1,1,2,2-tetrahydrooctyl trimethoxysila.ne, perfluoroalkyl
trichlorosilanes, trifluoromethylpropyl trimethoxysilane,
trifluoromethylpropyl
trichlorosilane, and perfluorinated sulfonimido ethyl trimethoxysilane
(available
from the 3M company, St. Paul, MN under the trade designation FC 405),
combinations of these, and the like.
Inorganic Oxide Particles
The ceramer composition can include colloidal inorganic oxide particles
useful to alter (e.g., increase or control) the refractive index of the
ceramer
composition and the refractive index and physical and mechanical properties of
a
-19-
CA 02338920 2001-O1-29
WO 00/06622 PCT/US98/27725
hardcoat prepared from the ceramer composition. Inorganic oxide particles can
be
crystalline in structure, arid preferably have a relatively high index of
refraction. , _
The addition of a specific volume fraction of inorganic oxide particles to
ceramer
composition allows for a controllable increase of the refractive index of a
ceramer
composition and a hardcoat composition thereof. Specific examples of inorganic
oxide particles include but are not limited to silica, ~alumina, titanic,
zirconia, ceria,
and antimony oxide particles. The inorganic oxide particles are typically
small in
size, functionally being preferably large enough to provide a hardcoat having
good
and useful hardness properties, while at the same tune having useful optical
to properties, e.g., having desired transmissivity, clarity or opacity, index
of
refraction, etc. While particles will generally include a distribution of a
range of
sizes, preferred particles can have an average particle diameter of about 5
nanometers (nm) to about 1000 nm, preferably front about 10 nm to about 50 nm,
and most preferably from about 10 to 30 nm. Aver;~ge particle size of
inorganic
oxide particles can be measured by known methods, e.g., by transmission
electron
microscopy. The inorganic oxide particles ca.n most preferably be dispersible
in a
polymeric matrix in a substantially non-agglomerated form, and can preferably
be
readily dispersed and sterically stabilized in non-polar or polar solvents and
organic monomers, e.g., the ceramer composition.
A variety of inorganic oxide particles are known and are described in the
patent literature. (Meth}acrylate functionalized colloidal silica particles
are
described, for example, in U.S. Pat. Nos. 4,491,508 (Olsen et al.), 4,455,205
(Olsen et al.), 4,478,876 (Chung),.4,486,504 (Chun,g), and 5,258,225
(Katsamberis).
Inorganic oxide particles can be provided in. any form that will allow their
useful incorporation into a ceramer composition, such as in the form of a
powder,
or in the form of a liquid "soL" These forms of inorganic oxide particles are
known in the art of inorganic oxide particles. The germ "sol" as used within
the
present description refers to a colloidal dispersion of substantially non-
3o agglomerated, inorganic oxide particles in a liquid omedium. The liquid
medium
can be water or an organic solvent such as ethanol, methyl ethyl ketone, or an
other
-24-
CA 02338920 2001-O1-29
WO OOI06622 PCT/US98/27725
organic solvent which may or may not be water-miscible. Solvent may be present
in known amounts, e.g., 60 to 80 percent by weight of the sol. ~,
Colloidal inorganic oxide particles dispersed as sols in aqueous solutions
are available commercially, e.g., silica sots are available commercially under
such
trade names as LUDOX (E.I. DuPont de Nemours and Co., Wilmington, DE),
NYACOL (Nyacol Co., Ashland, MA), and NALCC) (Nalco Chemical Co., Oak
Brook, IL). Nonaqueous silica sots (also called silica organosols) are
commercially available under the trade names NALCO 1057 (a silica sol in 2-
propoxyethanol, Nalco Chemical Co.), NALCO 2327 and MA ST, IP-ST, and
to EG-ST (Nissan Chemical Ind., Tokyo, Japan). Addiitional examples of
suitable
colloidal silicas are described in U.S. Pat. No. 5,126,394 (Bilkadi).
Ceramer Composition
The term "ceramer composition" refers to a ~:,omposition minimally
containing binder precursor and inorganic oxide particles, and optionally
containing a coupling agent, solvent, and other optional ingredients as
described.
The ceramer composition can include any amounts of binder precursor, inorganic
oxide particles, coupling agent, solvent, etc., that caan be combined to form
a useful
ceramer composition.
2o The ceramer composition can contain any annount of binder precursor that,
in combination with the other ingredients of the ceramer composition, will
allow
processing and coating of the ceramer composition into a useful hardcoat
composition. Functionally stated, the amount of binder precursor should be
sufficient to provide a ceramer composition that can be processed into a
hardcoat
composition that includes a polymeric matrix effectiive to bind inorganic
oxide
particles. A useful amount of binder precursor in a ceramer composition can
generally be at least 20 parts by weight binder precursor based on 100 parts
by
weight ceramer solids ("ceramer solids" refers to thc~ non-solvent portion of
the
ceramer composition). Preferred amounts of binder precursor can be in the
range
3o from about 45 to about 75 parts by weight, more preferably about 60 to 70
parts by
weight binder precursor, based on 100 parts by weight ceramer solids. Within
these ranges, preferred ceramer compositions can comprise from about 10 to 50
-21-
CA 02338920 2001-O1-29
WO 00!06622 PCT/US98/2??25
pbw polymerizable brominated compound, e.g., (alk:yl, bromo) phenyl ester
(meth)acrylate monomer, and from about 30 to 70 pbw non-brominated monomer , _
based on 100 parts solids.
ffused, a coupling agent can be included in a ceramer composition in an
amount no greater than about 80 wt%, more preferalbly no greater than about 70
wt%, and most preferably no greater than about 60 ~wt% based on the weight of
inorganic oxide particles. Alternatively stated, a coupling agent can be
included in
an amount of at least about 5 parts by weight, more preferably at least about
10
parts by weight, and most preferably, at least about :20 parts by weight based
on
l0 100 parts by weight ceramer solids.
The amount of inorganic oxide particles included in a ceramer composition
can be chosen based on a number of factors such as desired processing
properties
of a ceramer composition and desired optical, physical, and mechanical
properties
of the ceramer and hardcoat compositions. The amount of inorganic oxide
particles relative to binder precursor can be.any amount useful to provide a
processable ceramer composition and a functional, useful, hardcoat. Relatively
higher amounts of inorganic oxide particles can improve hardness and
refractive
properties of a hardcoat. On the other hand, it may not be desired to achieve
a
maximum index of refraction, but instead a controlled level of refractivity,
and
2o excessive particles can prevent efficient or practical processing of a
ceramer
composition and can tend to make a hardcoat brittle. Generally practical
amounts
of inorganic oxide particles in a ceramer compositicm can be greater than zero
and
generally less than about 80 parts by weight inorganic oxide particles, e.g.,
less
than about 60 pbw, based on 100 parts by weight ce,ramer solids. Depending on
the application, preferred ceramer compositions ma;y contain about 25 to 45
parts
by weight, more preferably about 30 to 40 parts by 'weight inorganic oxide
particles based on 100 parts by weight ceramer solids.
A ceramer composition prepared from only binder precursor, inorganic
oxide particles, and optionally a coupling agent, without the addition of
separate
3o solvent, will generally include some but not a large amount of organic or
inorganic
solvent principally present due to the solvent's presence in one of the
ingredients
of the ceramer composition (e.g., if inorganic oxide particles are added in
the form
-22-
CA 02338920 2001-O1-29
WO 00/06622 PCT/US98/27725
of a sol). Such a ceramer composition may or may not be easily or effectively
processed to form a coated ceramer. Moreover, solvent initially present in
these , _
ingredients may be removed from a ceramer composition at some point during
preparation or processing. Accordingly, aqueous or organic solvent may be
added
to the ceramer composition to facilitate processing. A ceramer composition
containing added solvent, e.g., for purposes of improving processing or
coating
properties, can be referred to as a "ceramer solution.."
The solvent can be aqueous or organic, and can be selected to be
compatible with other components of the ceramer composition. As used in this
1o context, compatibility between the solvent and the ceramer composition
means that
there is minimal phase separation between the solvent and the ceramer
composition. Additionally, the solvent can be selected to not adversely affect
the
properties of the cured hardcoat, or undesirably affect (e.g., chemically
attack) the
substrate upon which a ceramer solution is coated.
~5 Examples of suitable solvents include alcoh~ols, preferably the lower
alcohols such as isopropyl alcohol, n-butanol, methanol, ethanol, ketones such
as
methyl ethyl ketone, glycols, and combinations thereof. A particularly
preferred
solvent can include a majority of an alcohol, e.g., isopropyl alcohol, and a
minor
amount of water to facilitate processing and increase stability of the ceramer
2o solution (a preferred weight ratio of alcohol to water can be from about
14:1 to
about 16:1).
Solvent can be included in the ceramer composition in any amount useful
to provide a ceramer solution that can be processed and coated to a useful
degree.
In general, the solids content of the ceramer solution can be anywhere in the
range
25 from about 5-99 weight percent solids, preferably from about 10-70 wt%,
more
preferably from about 30 to about 65 wt% solids, based on the total ceramer
solution.
As will be appreciated by those skilled in the art of ceram'er compositions,
the ceramer composition can contain other useful ingredients such as a
crossIinking
3o agent, reaction initiator, one or more surfactant, pigment, filler, or
other ingredients
that can be useful within a polymerizable composition, ceramer composition,
-23-
CA 02338920 2001-O1-29
WO 00106622 PCTlUS98/27725
hardcoat composition, or optical product. Such ingredients can be included in
the
composition in amounts known to be effective for their respective purposes. -
A crosslinking agent can be useful to increase the glass transition
temperature of the polymer resulting from crosslinkiing the binder precursor
or
ceramer composition. Glass transition temperature of a composition can be
measured by methods known in the art, such as Differential Scanning
Calorimetry
{DSC), modulated DSC (MDSC), or Dynamic Mechanical Analysis (DMA).
Polymeric beads, inorganic fillers, and/or pi~s;ments can be added to the
ceramer composition in order to improve processing;, to impart slip and
scratch
to resistance to the polymerized material, or to affect optical properties of
the
polymerized material. Examples of useful polymeric beads include those made of
polystyrene, polyacrylates, copolymers of styrene and acrylates, polyethylene,
polypropylene, polytetrafluoroethylene, or combinations thereof. Examples of
inorganic fillers and pigments include solid or hollow glass beads, and
aluminum
is trihydroxide.
A reaction initiator can be included in the ceramer composition to facilitate
polymerization or cure. To prepare a hardcoat from the ceramer composition,
the
ceramer composition can be exposed to an energy source to cause polymerization
or cure, and the formation of a polymeric matrix surrounding and containing
20 inorganic particles. Examples of suitable energy include radiant or thermal
energy
such as electromagnetic energy (e.g., infrared energy, microwave energy,
visible
light, ultraviolet light, and the like), accelerated particles (e.g., electron
beam
energy), and energy from electrical discharges (e.g., coronas, plasmas, glow
discharge, or silent discharge). The curing or polymerization process of a
25 (meth)acrylate ceramer composition typically occurs via a free radical
mechanism,
which can require the use of a free radical initiator (simply referred to
herein as an
initiator, e.g., a photoinitiator or a thermal initiator). if the energy
source is an
electron beam, the electron beam generates free radiicals and no initiator is
required. If the energy source is heat, ultraviolet light, visible light, or
infrared
30 light, an initiator can be required to effect efficient polymerization.
When the
initiator is exposed to one of these energy sources, the initiator generates
free
radicals, which then initiates polymerization and crosslinking.
-24-
CA 02338920 2001-O1-29
WO 00106522 PCT/US98l27725
Examples of suitable free radical thermal initiators include, but are not
limited to, peroxides such as benzoyl peroxide, and azo compounds. Examples of
photoinitiators that generate a free radical source wlhen exposed to visible
light
radiation include, but are not limited to mixtures of camphorquinaiies and
organic
amines, and bisacyl phosphoric oxides. Examples of photoinitiators that
generate a
free radical source when exposed to ultraviolet light; include, but are not
limited to,
organic peroxides, azo compounds, quinones, nitroso compounds, acyl halides,
hydrozones, mercapto compounds, pyrylium compounds, triacrylimidazoles,
bisimidazoles, chloroalkytriazines, benzoin, benzoin methyl ether, benzoin
ethyl
to ether, benzoin isopropyl ether, benzoin isobutyl ethers anti methylbenzoin,
diketones such as benzil and diacetyl, phenones such as acetophenone, 2,2,2-
tri-
bromo-1-phenylethanone, 2,2-diethoxyacetophenone, 2,2-dimethoxy-2-
phenylacetophenone, 2,2,2, tribromo-1(2-nitrophenyl) ethanone, benzophenone,
and 4,4-bis(dimethyamino)benzophenone. Examples of commercially available
15 ultraviolet photoinitiators include those available under the trade
designations
IRGACURE 184 (1-hydroxycyclohexyl phenyl ketone), IRGACURE 36i and
DAROCUR 1173 (2-hydroxy-2-methyl-1-phenyl-propan-1-one) from Ciba-Geigy,
1'iawtharn, NY. Typically, if used, an amount of initiator is included in the
precursor composition that is effective to achieve a desired level and rate of
cure.
20 Preferably, the initiator is used in an amount of about 0.1 wt % to about
10 wt %,
and more preferably about 2 wt % to about 4 wt %, based on the total weight of
the
ceramer composition solids. Combinations of different initiators can be used
if
desired.
The ceramer composition can include a photosensitizer to facilitate the
25 formation of free radicals that initiate curing of the binder precursor,
especially in
an air atmosphere. Suitable photosensitizers include, but are not limited to,
aromatic ketones and tertiary amines. Suitable aromatic ketones include, but
are
not limited to, benzophenone, acetaphenone, benzil, camphorquinone,
benzaldehyde, and o-chlorobenzaldehyde, xanthonE:, tioxanthone, 9,10-
3o anthraquinone, and many other aromatic ketones. Suitable tertiary amines
include,
but are not limited to, methyldiethanolamine, ethyldiethanolamine,
triethanolamine, phenylmethyl-ethanolamine, dimethylaminoethylbenzoate, and
-25-
CA 02338920 2001-O1-29
WO 00/06622 PCTIU598/27725
the like. Typically, if used, an amount of initiator is included in the
precursor
compositions to effect the desired level and rate of cure. Preferably, the
amount of -
w
photosensitizer used in the compositions of the present invention is about
O.Oi wt
to about 10 wt %, more preferably about 0.05 wt % to about S wt %, and most-
preferably, about 0.25 wt % to about 3 wt %, based on the weight of the
ceramer
solids. Combinations of different photosensitizers can be used if desired.
The ceramer composition can also preferably include a leveling agent to
improve the flow or wetting of the ceramer composition onto a substrate. If
the
ceramer composition does not properly wet a desired substrate, this can lead
to
visual imperfections (e.g., pin holes and/or ridges). Examples of leveling
agents
include, but are not limited to, alkoxy terminated polysilicones such as that
available under the trade designation DOW 57 (a mixture of dimethyl-, methyl-,
and (polyethylene oxide acetate)-capped siloxane) from Dow Corning, Midland,
MI; and fluorochemical surfactants such as those available under the trade
designations FC430, FC431, and FX313 from 3M Co., St. Paul, MN. Preferably,
the leveling agent is present in an amount up to about 3 wt %, and more
preferably,
about 0.5 wt % to about 1 wt %, based on the weight of the ceramer solids.
Combinations of different leveling agents can be used if desired.
Polymeric materials are known to degrade by a variety of mechanisms.
2o Common additives that can offset such degradation are known as stabilizers,
UV-
absorbers, antioxidants, and the like. The ceramer .compositions can include
one or
more of the following: ultraviolet stabilizer, ultraviolet absorber, ozone
stabilizer,
thermal stabilizer, or antioxidant.
A photostabilizer and/or ultraviolet absorber can be used to improve
weatherability and reduce "yellowing" of a ceramer or hardcoat. An example of
a
photostabilizer includes that available under the trade designation TINUVIN
292
(bis(1,2,2,6,6-penta.methyl-4-piperidinyl)sebacate) and an example of an
ultraviolet
absorber includes that available under the trade designation TINiJVIN 1130
(hydroxyphenyl benzotriazole), both of which are available from Ciba-
Chemicals.
3o Preferably, the ultraviolet stabilizer or absorber is present in an amount
up to about
10 wt %, and more preferably; about 1 wt % to about 5 wt %, based on the
weight
-26-
CA 02338920 2001-O1-29
WO 00106622 PCTlUS98I27725
of the ceramer solids. Combinations of different ultraviolet stabilizers and
absorbers can he used if desired.
Method of Pre»arin~ Ceramer Composition and Ceramer Coating
To prepare a ceramer composition, a binder precursor, inorganic oxide
particles, and the optional coupling agent or other optional ingredients, can
be
combined or mixed together in any manner. The mixture can if desired be heated
to remove volatiles and solvents used for the produ~~tion or processing of
these
ingredients, e.g., water in the sol. The heating can optionally be in
combination
1o with mild vacuum (about 90 mm Hg), leaving behind mainly solid components
of
the ceramer composition. Once substantially all solvents are removed, the
ceramer
composition will generally be in the farm of a liquid; this liquid maybe
suitable
for processing, coating, or other handling, as is, or may benefit from solvent
added
in an amount sui~tcient to produce a ceramer solution having a solids content
that
15 will over useful processing and coating properties. The ceramer solution
can be
filtered prior to application to a substrate, to remove; any gel particles or
other
agglomerated materials. Filtering can be accomplished with a ten-, five-, or
one-
micron filter made of a material that is unreactive v~~ith the solvent or any
ofthe
components of the ceramer solution.
2o To facilitate processing the ceramer composition into a cured hardcoat
composition, it can be preferred, but is not required., that the ceramer
composition
exhibit a relatively low melting point. Most preferably, the ceramer
composition
can be liquid at or near room temperature, and the c:eramer composition or a
ceramer solution thereof can have a viscosity that allows processing, e.g.,
25 pumping, circulation, extrusion, coating, forming, curing, or other
handling, at or
near room temperature. Although viscasities outside of the following ranges
can
be useful, and the viscosity of a ceramer composition or ceramer solution can
be
chosen depending on various conditions such as the: coating thickness,
application
technique, and the type of substrate, the ceramer composition or a solution
thereof
3o can preferably exhibit a room temperature viscosity in the range from about
I-200
centipoise (cps), preferably from about 3-75 centipoise; more preferably about
4-
50 centipoise, and most preferably about ~-20 centipoise. Preferably, the
ceramer
-27-
CA 02338920 2001-O1-29
WO OOI06622 PCT/US98I27725
composition exhibits a high transparency, and preferably a refractive index in
the
range from about 1.3 to about 1.7, as measured with a conventional
refractometer -
using a conventional measurement procedure, such as ASTM D1747-94
("Standard Test Method for Refractive Index of Viscous Materials").
A ceramer composition, opdona.Ily in the form of a ceramer solution
including added solvent, can be applied to a substrate by techniques such as
spray
coating, knife coating, dip coating, flow coating, roll coating, and the like.
The
coating thickness of the ceramer composition will depend on the formulation,
the
amount of solvent, and the desired thickness of the cured hardcoat. Any
solvent
can be dried or flashed off at a temperature suitable for the solvent used.
Once solvent is removed, the ceramer composition can be exposed to an
energy source to initiate curing the ceramer composition to form a hardcoat
composition comprising inorganic oxide particles suspended, dispersed, or
otherwise contained within or bound by a brominate;d polymeric matrix. This
energy source can be thermal energy, electron beam, ultraviolet light, or
visible
light, or the like. The amount of energy required can be primarily dependent
on
the chemistry of the precursor composition, as well as its thickness and
density.
For thermal energy, the oven temperature will typic;aliy range from about
50°C to
about 250°C (preferably about 90°C to about 110°C) for
about I5 minutes to about
2o 16 hours. Electron beam radiation can be used at an energy level of about
0.1
megarad to about 10 megarad (Mrad), preferably at an energy level of about 1
Mrad to about 10 Mrad. Ultraviolet radiation refers to nonparticulate
radiation
typically having a wavelength within the range of about 200 to about 400
nanometers; preferably within the range of about 250 to 400 nanometers. It is
preferred that UV light have an energy level of at least 300 Wattsrnch (120
Watts/cm), preferably at least 600 Wattslinch (240 'Wattslcm). Visible
radiation
refers to nonparticulate radiation having a wavelength within the range from
about
400 nanometers (nm) to about 800 nm, preferably in the range from about 400 nm
to about 550 nm. Optionally, the ceramer composition can be cured in an inert
atmosphere (i.e., minimal oxygen present) such as a~ nitrogen atmosphere.
Typically, a cured ceramer composition (also referred to as "hardcoat" or
"hardcoat composition") can have a thickness of at least about 1 micron,
preferably
-28-
CA 02338920 2001-O1-29
WO oo/Ob622 PCT/US98/27725
at least about 2 microns, more preferably less than about 50 microns, more
preferably less than about 25 microns, even more pn~eferably less than about
10 -
microns, and most preferably less than about 4 microns. The amount of the
ceramer composition, or a solution thereof, applied to a substrate can be
adjusted to
provide this coating thickness.
The hardcoat composition of the invention cyan be useful in applications
where an abrasion or scratch resistant coating is des fired, for example to
protect
other layers of a composite structure. The hardcoat can exhibit advantages of
especially desirable optical properties, e.g., a high index of refraction or
an index
of refraction that can be controlled by changing the relative amount or
chemical
composition of one or more of the ingredients of the ceramer composition, such
as
the polymerizable brominated compound or the inorganic oxide particles.
The hardcoat can be used to alter optical properties, such as reflective
properties, of a substrate which it contacts. That is, the hardcoat may
provide a
i5 reflective or mirror-like property to a substrate, or it may provide an
antireflective
property. The hardcoat can be especially useful in optically functional
composite
structures wherein the index of refraction of the har~dcoat composition can be
made
similar to the index of refraction of an adjacent layc;r, thereby reducing
reflectance
at the interface and enhancing transmission of light through the composite
2o structure.
The hardcoat can have desirable physical, mechanical, and optical
properties, including one or more of a desired index of refraction; good
scratch
resistance; a relatively high hardness; and desired reflective or
antireflective
properties. For instance, the hardcoat can have an index of refraction that
matches
25 an adjacent layer of a composite article (e.g., about 1.58 for
polycarbonate or about
1.65 for PET), or if a maximum refractive index is desired, an index of
refraction
that is at least about 1.5, 1.7, 1.8, or 2.0 or greater. The hardcoat may have
a
hardness that passes the pencil hardness test using a 1H pencil, preferably a
3H
pencil. And the hardcoat can preferably have a reflectivity Less than about
4%,
3o e.g., less than about 1%.
An optically functional structure can comprise the cured ceramer hardcoat
composition contacting a substrate. The substrate can be a component of a
Iight
-29-
CA 02338920 2001-O1-29
WO OO/U6622 PCT/US98I27725
management device, e.g., in the form of a film, sheet; prism; a filter, an
optical
element such as a lens, a light conducting pipe, a computer screen; a CRT face
,. _
plates, any of these including a microstructured surface, or any other form of
light
conducting device. It is possible that more than one. layer of a ceramer
coating
may be applied to a surface of a substrate, and that each layer has the same
or
different physical or optical properties.
Suitable substrates can be flat or microstructured, stiff or flexible, or can
have other physical or structural properties known in the art of optical
materials,
and can be made from any material such as glass, a polymeric material such as
a
1o plastic or thermoplastic, or metal. The substrate care be a laminate of two
or more
different materials adhered together, either with or without an adhesive layer
between. The substrate thickness can vary and typically ranges from about 0.1
mm
to about 1000 mm, more typically from about 10 mm to about 200 mm, and most
preferably from about 10 mm to about 0.25 rnm. Flexible organic film
substrates
are typically no greater than about 1 mm thick.
Optically transparent substrates can be of an optically transmissive
thermoplastic material (e.g., plastic sheets, films, or bodies having
transmissivities
over visible wavelengths of at least 25% to about 99% without marked
absorption
or.reflection peaks in this range). Representative tr,~nsparent substrates
include
2o polyesters such as poly{ethyleneterephthalate) "PE7."", poiycarbonates,
poly(meth)acrylates, polyphenyleneoxide, cellulose esters, such as cellulose
acetate, cellulose diacetate, cellulose triacetate, and cellulose acetate-
butyrate
copolymer "CAB", polystyrene and styrene copolymers such as acrylonitrile-
butadiene-styrene copolymer and acrylonitrile-styrene copolymer, polyolefins,
such as polypropylene and polyethylene, polyvinyl chloride, polyimides, and
the
like. "Poly(meth)acrylate" includes acrylates and rnethacrylates commonly
referred to as cast acrylic sheeting, stretched acrylic.,
poly{methylmethacrylate)
"PMMA," poly(methylacrylate), poly{ethylacrylate), and
poly(methylmethacrylate-co-ethylacrylate), and the like.
3o The substrate may include a primed surface, which can be provided by a
chemical primer layer or by other methods such as chemical etching, eleetron-
beam irradiation, corona treatment, plasma etching, or coextrusion of adhesion
-~o-
CA 02338920 2001-O1-29
WO 00/06622 PCT/US98/27725
promoting layers. Flexible substrates that contain primed surfaces are
commercially available. An example of such a material is a polyethylene
~ _
terephtha~tate film primed with an aqueous acrylic latex, which is available
from
DuPont Films, Hopewell, VA under the trade designation Melinex 453, 454, 505,
or 617. Another particularly preferred substrate is cellulose diacetate from
Courtlands Plastic of Derby England, and marketed under the trade name
Clarifoil.
Optically Functional Coating
An optically functional coating can be a component of an optically
1o functional composite, e.g., as a layer adjacent to the. hardcoat
composition.
As described in Optical Thin Film User's Handbook by James D. Rancourt;
MacMillan Publishing Co., 1987, optically functional coatings may be formed
from suitably deposited thin films of metals (includ:ing metalloids) or alloys
thereof, such as silver, gold, aluminum, palladium, .and palladium-gold. One
of the
15 most versatile classes of materials used in the deposition of optically
functional
coatings are metal oxides such as oxides of single metals (including
metalloids) as
well as oxides of alloys thereof. Examples of particular metal oxides that
have
been used in optical coatings include oxides of aluminum, silican, tin,
titanium,
niobium, zinc, zirconium, tantalum; yttrium, cerium, tungsten, bismuth,
indium,
2o and mixtures thereof, such as A1203, Si02, Sn02, TiOZ, Nb205, ZnO, ZrO2,
Ta2Og, Y2O3, Ce02, W03, Bi205, In203, and ITO (indium tin oxide). Metal
oxides that are depleted in oxygen (i.e., where the amount of oxygen in the
oxide is
less than the stoichiometric amount), such as SiOx, where x is no greater than
2,
have also been used. One method of synthesizing such oxygen deficient oxides
is
25 by reactive sputtering.
One of the reasons for the versatility of metal oxides in optically functional
coatings is that unlike other materials, they may be used to deposit both
reflective
or antireflective coatings depending on the configuration of the oxide coating
and
its chemical composition. Thus, as discussed in International Publication
3o Document WO 96/31343 (Bright), when a single thin layer of metal oxide,
such as
ITO, having a thickness of about 50 Angstroms to about 3000 Angstroms is
deposited over a transparent plastic film, such as polyester or polycarbonate,
the
-31-
CA 02338920 2001-O1-29
WO 00/06622 PCTNS98/27725
amount of light reflected by the polyester or polycarbonate increases
substantially.
In this case the ITO film acts as a "reflective" coating. On the other hand,
when
w
alternating layers of ITO and Si02 or ITO and SiO;;~ with a combined thickness
of
about 50 Angstroms to about and 3000 Angstroms .are deposited over the
polyester
or polycarbonate substrate, the amount of light reflected by the polyester or
polycarbonate decreases substantially. In this case the alternating ITO/SiOx
stack
acts as an "antireflective" coating.
Another reason for the versatility of metal oxide coatings, particularly ITO,
is that they-can be made electrically conductive by doping with a conductive
1o element, such as tin, aluminum, barium, boron, or antimony. When made
conductive, the metal oxides also help reduce static. charge and
electromagnetic
emissions.
Whether an optically functional coating is "reflective" or "antireflective"
depends on its overall refractive index relative to the refractive index of
the
15 underlying substrate. The simplest reflective coating is a single thin
layer of a
transparent material, such as a metal or metal oxide;, having a refractive
index
higher than the refractive index of the underlying substrate. When the
substrate is
a transparent organic polymeric material, such as polyester or polycarbonate,
the
simplest "reflective" coating is generally chosen to be a single thin layer of
a
2o material, such as a metal or metal oxide, having a refractive index of
about 1.6 to
about 2.7. This is because most organic polymeric materials have indices of
refraction of about 1.3 (for fluorinated polymers) to about 1.7 (for aromatic
polymers). Fluorinated thermoplastic polymers, such as TEFLON (1.35), have the
lowest indices of refraction among organic polymers, whereas aromatic
25 thermoplastic polymers, such as polystyrene (i.60) have some ofthe highest.
The simplest antireflective coating is a single layer of a transparent
material
having a refractive index lower than that of the substrate on which it is
disposed.
Multilayer antireflective coatings include two or more layers of dielectric
material
on a substrate, wherein at least one layer has a refractive index higher than
the
3o refractive index of the substrate. The multilayer coatings are generally
deposited
by thermal evaporation, sputtering, or other vacuum deposition techniques.
Such
multilayer coatings are disclosed, far example, in International Publication
No.
-32-
CA 02338920 2001-O1-29
WO 00/06622 PGT/US98I27725
WO 96/31343 (Southwall Technologies Inc..), U.S. Pat. Nos. 5,091,244
(Bjornard),
5,105,310 (Dickey), S,i47,125 (Austin), 5,270,858 (Dickey), 5,372,874 (Dickey
et ~ -
al.), 5,407,733 (Dickey), and 5,450;238 (Bjornard et al.)
Antireflective (AR) film stacks prepared by vacuum sputtering of metal
s oxide thin films on substrates made of substrates, p~uticularly flexible
plastic
substrates, such as polycarbonate, acrylic; polystyrene, and polyesters are
describe, for example, in United States Patent Number 5,579,162 (Bjornard et
al.)
and International Publication No. WO 96/31343 (Sauthwall Technologies Inc.).
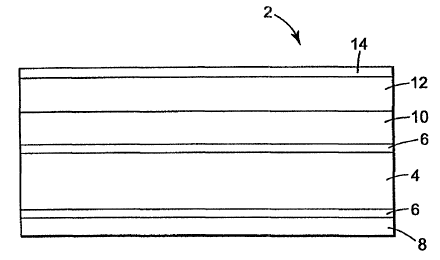
An embodiment of the invention can be an anti-reflective composite as
io shown in Figure 1, which illustrates optically-functional (anti-reflective)
composite
structure 2 comprising substrate 4, optionally primed on one or two sides with
primer 6, and contacting on one side adhesive 8 and on the other side hardcoat
composition 10. An antireflective (AR) layer I2 or stack can be provided on
hardcoat 8, and an anti-smudge layer 14 can be provided on AR stack 12. In
this
is embodiment of the invention, the hardcoat acts as aan adhesion promoter
between
the AR layer and the substrate, which otherwise ma;y not adhere well to a
thermoplastic substrate. The hardcoat also modifies the mechanical properties
of
the AR layer, making it less susceptible to fracture. Preferably in this
embodiment
the index of refraction of the hardcoat can be chosen to match the index of
2o refraction of the substrate, minimizing reflection from occurring at this
interface.
Test Methods
Test Procedure 1: Pencil Hardness
This test was run according to ASTM D336:3-92a (Standard Test Method
25 far Film Hardness by Pencil Test). This test method covers a procedure for
rapid
determination of the film hardness of an organic coating on a substrate in
terms of
drawing leads or pencil leads of known hardness. According to this test
method,
test samples were cut to appraximately 1 inch by 8 finches in size and placed
on a
clean glass sheet. The carefully planarized tip of a 1'.ead pencil of
specified
3o hardness was held firmly against the sample at a 45'° angle and
pushed at about
Scmlsec. The weight on the pencil was 1000g. The: process started with the
hardest pencil and continued down the scale of hardness to the pencil that
will not
scratch the film. Each sample was tested five times at each hardness level. To
-33-
CA 02338920 2001-O1-29
WO 40106622 PCT/US98l27725
pass, at least four of the five tests pass the hardness i.evei. Uni Hardness
Pencils
(manufactured by Mitsubishi, Japan) were used throughout this,test.
Test Procedure 2' Index of Refraction
The refractive index of resin compositions and cured films were measured
using an Abbe Refractometer, made by Erma Inc. of Tokyo Japan and distributed
by Fisher Scientific.
Materials
1o Preparation of 4 6-dibromo-2-sec-but~rl phenyl acrvllate ~DBsBPAI
In an appropriately sized round bottom flask equipped with a mechanical
stirrer, condenser, nitrogen cap, addition funnel and temperature probe, 8508
{grams) of 2-sec-butylphenol was mixed with 50971; of deionized water: The
mixture was stirred with a mechanical mixer and purged with nitrogen for about
10
minutes. 18818 bromine was added to 'the mixture drop-wise through the
addition
funnel. The reaction temperature was maintained at: about 30°C or less
using an
ice bath. Following the addition of the bromine, the; reaction mixture was
stirred
for 30 minutes at room temperature. Reaction completion was determined by gas
chromatography, by monitoring the disappearance of the starting material and
of
2o monobrominated species.
Upon completion of the reaction, 44878 of ethyl acetate was added. The
mixture was stirred for 15 minutes and then allowed to phase split. The bottom
{aqueous) layer was removed and 750.58 of a 13 wt~% aqueous sodium
hydrosulfite
solution was added. The mixture was stirred well and then allowed to phase
split.
The bottom (aqueous) layer was removed and 856 ~Ig of a 13 wt% aqueous sodium
chloride solution was added. The mixture was stirred well and then allowed to
phase split. The bottom (aqueous) layer was removed and solvent was stripped
from the top layer using a rotary evaporator.
The crude product was then distilled using a distillation head and vigeraux
3o column. The product distilled at 0.1 mm Hg, a pot temperature of 1 S 1
°C, and a
head temperature of 97°C. This procedure provided approximately 15008
of 4,6-
dibromo-2-sec-butyl phenol (DBsBP).
-34-
CA 02338920 2001-O1-29
WO 00/06622 PCT/US98127725
In an appropriately sized round bottom flask equipped with a mechanical
stirrer, condenser, addition funnel and temperature probe, 140g of 4,6-
dibrorno-2-
. ° -
sec-butyl phenol, 3608 of t-butyl methyl ether, 55.2,g triethyl amine, and
0.02g
phenothiazine were mixed (in these examples, the base used was triethyl amine;
however, a stoichiometric amount of any other appropriate bases could also be
used, such as sodium hydroxide or pyridine, among others). 47.38 of acryloyl
chloride was added drop wise and, using an ice batty the reaction temperature
was
maintain below 20°C. The reaction was run to completion, taking
approximately
30 minutes.
to The product was then worked up by sequential washings with deionized
water {257g); 0.7% (w/w) aqueous hydrochloric acid (5lg); 16.1% {w/w) aqueous
sodium carbonate (59.6g); and 8.3%(w/w) aqueous sodium chloride (54.Sg).
Solvent was removed using a rotary evaporator and the crude product was vacuum
distilled to yield 155grams {94%) of 4,6-dibromo-2-sec-butyl phenyl acrylate
(DBsBPA).
Preparation of 2-,{4 6-dibromo-2-sec-but~rl phenoxrrli ethyl ac~late (DBsBPEA)
In a 12 liter round bottom flask equipped with a mechanical stirrer,
condenser, nitrogen cap, addition funnel and temperature probe, 1500 g (grams)
of
2-sec-butylphenol was mixed with 4500 g of deionized water. The mixture was
stirred with a mechanical mixer and purged with nitrogen far about 10 minutes.
3319 g bromine was added to the mixture drop-wise through the addition funnel.
The temperature was maintained at about 30C or less using an ice bath.
Following
addition of the bromine, the reaction mixture was starred for one hour at room
temperature: Reaction completion was determined by gas chromatography, by
monitoring the disappearance of the starting material, 2-sec-butylphenol, and
of
monobrominated species.
Upon completion of the reaction; 3960 g of ethyl acetate was added. The
mixture was stirred for 15 minutes and then allowed to phase split. The bottom
(aqueous) layer was removed and 2686 g of a 13 wt% aqueous sodium hydrosulfite
solution was added. The mixture was stirred well a.nd then allowed to phase
split.
The bottom (aqueous) layer was removed and 2760 g of a 15 wt% aqueous sodium
carbonate solution was added. The mixture was stirred well and then allowed to
-35-
CA 02338920 2001-O1-29
WO 00/06622 PCT/U598/27725
phase split. The bottom (aqueous) layer was removed and solvent was stripped
from the top layer using a rotary evaporator. This procedure provided
" _
approximately 2647 g of DBsBP.
A 500 ml round bottom flask was equipped with a magnetic stirrer,
condenser and temperature probe. 40 g of the 4,6-dibromo-2-sec-butylphenol,
12.5
g ethylene carbonate and 13.1 g triethylamine were added to the flask. The
mixture was heated to reflux (~120C) and held at that temperature for about 24
hours. At this point, gas chromatograph analysis showed only 0.9% residual
starting material, so the reaction was cooled to room temperature. 170 g t-
butyl
io methyl ether was added, then 20.1g of 37% HCI in 150 g of DI water was
added.
The mixture was shaken welt and allowed to phase split and the Iower aqueous
phase removed. The mixture was then washed with; a solution of 150 g water and
g of sodium carbonate and the lower aqueous phase was removed. The solvent
was remove using a rotary evaporator to yield about 40 grams of dark
intermediate
15 product. This product batch distilled using a 163C pot, 115C overhead
temperature and 0.2mm Hg vacuum to yield the yellow desired product; 2-(4,6-
dibromo-2-sec-butyl phenoxy) ethanol.
A 500 ml round bottom flask was equipped with a mechanical stirrer,
Dean-Stark trap, condenser, and temperature probe. 25 g of 2-(4,6-dibrorno-2-
sec-
2o butylphenoxy) ethanol, 125 g of toluene, 0.58 g of p-toluene sulfonic acid,
5.5 g of
acrylic acid and 200 ppm each of methyl hydroquiinone and hydroquinone were
mixed together in the flask. The mixture was heated to reflux to azeotrope out
the
water generated during esterification. After 5 hours, gas chromatography
analysis
showed the reaction to be substantially complete (>98%). The reaction mixture
was cooled, then washed three times: first with a solution of HCI in water,
then
with a solution of NaC03 in water and finally with a solution of NaC 1 in
water and
finally the toluene was then stripped in vaccuo. The product was purif ed
using
continuous distillation on a rolled film evaporator (available from UIC Inc.
of
Joliet, IL,) at the following conditions: 1 micron Hg, vacuum and 130C to
obtain the
product with >98% purity by NMR.
-36-
CA 02338920 2001-O1-29
WO OOJ06622 PCT/US98I27725
Component A
50.01 parts by weight of PETA (pentaerythritol triacrylate) were heated to
about 49°C (120°F). 31.48 parts by weight Nalco 2327 colloidal
silica {sold as a
40% sal by Nalco Corp., Naperville, IL) were added to form a first admixture.
In a
separate one liter flask, 7.91 parts by weight of 3-methacryloxypropyl-
trimethoxysilane were mixed with 8.06 parts by weight N,N-dimethyl acrylamide
(DMA) to form a second admixture. The first admi~aure was mixed with the
second admixture to form a third admixture. In a wE;ighing tray, 0.039 parts
by
weight BHT (butylated hydroxytoluene) and 0.004 parts by weight phenothiazine
to were mixed together and then added to the third admixture to form a fourth
admixture.
The fourth admixture was stripped by application of gentle vacuum
distillation (100 t 20 mm Hg) at 52° ~ 2°C until most of the
water from the sal
was removed. At the end ofthe stripping process, the admixture was diluted to
15 50% solids with a 16:1 mixture of isopropyl alcohol:deionized water (wlw).
1.63
parts by weight (solids) photoinitiator {IrgacureT"" 1 F~4) was then added.
Component B
56.2 parts by weight ofthe curable binder precursorPETA (pentaerythritol
2o triacrylate) was heated to about 49°C (120°F) in a o:ne liter
flask. 34.5 parts by
weight Nalco 2327 colloidal silica {sold as a 40% sol by Nalco Corp.,
Naperville;
IL,) and 0.7 parts of sodium aluminate (available from Matheson, Coleman and
Bell, Norwood, OH) were added to the PETA to form a first admixture. In a
separate one liter flask, 7.7, parts by weight of the crosslinkable silane
component
25 3-methacryloxypropyl-trimethoxysilane, (commercially available from Union
Carbide under the trade designation "A-174") were mixed with 0.8 parts by
weight
of FC-405 (a fluorolsiiane component commercially available from Minnesota
Mining and Manufacturing Company, St: Paul, MN) to form a second admixture.
The first and second admixtures were then mixed together to form a third
3o admixture.
In a weighing tray, 0.15 parts by weight BH'T (butylated hydroxytoluene}
and 0.02 parts by weight phenothiazine (both based on the 56.2 parts by weight
-37-
CA 02338920 2001-O1-29
w0 0010b622 PCT/US98/27725
PETA) were mixed together and then added to the third admixture to form a
fourth
admixture. The fourth admixture was then stripped, i:e., subjected to a gentle
w _
vacuum distillation ( 100 t 20 mm Hg) at 52° t 2°C until most of
the water from
the soi was removed. At the end of the stripping process, the admixture was
diluted
to 25% solids with a 14:1 weight-ratio of isopropyl alcohol:distilled water.
About
0.? parts by weight (solids) photoinitiator were also added (commercially
available
from Ciba Geigy Corp.; Hawthorne, NY, under the trade designation IrgacureTM
184).
to Component C
56.2 parts by weight of the curable binder precursor PETA (pentaerythritol
triacrylate) was heated to about 49°C {120°F) in a one liter
flask. 31.7 parts by
weight Nalco 2327 colloidal silica {sold as a 40% sol by Nalco Corp.,
Naperville,
IL) and 3.5 parts of colloidal zirconia (sold as a 20°.i°
colloidal zirconia acetate sol
~s by The PQ Corporation, Ashland, MA) were added to the PETA to form a first
admixture. In a separate one titer flask, 7.7 parts by weight of the
crosslinkable
silane component 3-methacryloxypropyl-trimethoxysilane, (commercially
available from Union Carbide under the trade designation "A-174") were mixed
with 0.8 parts by weight of FC-405 (a fluoro/silane component commercially
2o available from Minnesota Mining and Manufacturing Company, St. Paul, Ml~ to
form a second admixture. The first and second admixtures were then mixed
together to form a third admixture.
In a weighing tray, 0.15 parts by weight BRIT (butylated hydroxytoluene)
and 0.02 parts by weight phenothiazine (both basedl on the 56.2 parts by
weight
25 PETA) were mixed together and then added to the 'third admixture to form a
fourth
admixture. The fourth admixture was then strippedl, i.e., subjected to a
gentle
vacuum distillation {100 ~ 20 mm Hg) at 52° ~ 2°C; until most of
the water from
the sol was removed. At the end of the stripping process, the admixture was
diluted
to 25% solids with a 14:1 weight-ratio of isopropyl alcohoi:distilled water.
About
30 0.7 parts by weight (solids) photoinitiator were also added (commercially
available
from Ciba Geigy Corp., Hawthorne, NY, under the trade designation IrgacureT""
184).
-38-
CA 02338920 2001-O1-29
WO 00!06622 PCT/US98127725
Components D~,E, F. ~G
High index of refraction (HiR) components :for ceramers were prepared by ~
mixing the materials outlined in Table 1 below in a ,glass container. All
values
indicate the weight percent of the material based on the total HIR
composition.
Table 1
I3~ Components
Com onent D E F G
RDX-51027 55 - 55 -
Meth 1 St ene 7 9 7 9
DBsBPA 15 1.5 - -
DBsBPEA - - 15 15
CN-10.4 20 73 20 73
_ 3 ~ 3 3 3
EbercrylTM 3603
~
DBsBPA 4,6-dibromo-2-sec-butyl phenyl acrylate
DBsBPEA 2-(4,6-dibromo-2-sec-butyl p~henoxy) ethyl acrylate
to RDX 51027 brominated epoxy acrylate available from UCB Corporation
CN-104 epoxy acrylate by Sartomer t:ompany
EbercrylTM 3603 novolac epoxy acrylate by UCB Company
Additionally, Lucirin~ TPO (dipheny!(2,4,6-trimethylbenzoyl) phosphine oxide
1s from BASF, Charlotte, NC) and Irgacure'~ 184 (1-hydroxycyclohexyl phenyl
ketone from Ciba Specialty Chemicals Corp., Tarry~town, NY} were each added to
the mixture in amounts of 1.5 parts TPO per hundred parts H1R component, and
3.0 parts Irgacure-184 per hundred parts HIR component.
20 Examples 1-4
Example 1 was prepared by mixing four pats of component A with one
part of component D.
Example 2 was prepared by mixing four parts of component A with one
part of component E.
25 Example 3 was prepared by mixing four pans of component A with one
pair of component F.
Example 4 was prepared by mixing four parts of component A with one
part of component G.
Each mixture, which contained approximately 60% solids, was coated on a
30 10 inch square of 5 mil Melinex 6I7 polyester film (manufactured by DuPont
Films) using a #4 Meyer rod (manufactured by RD Specialties, Rochester, N''S~.
-39-
CA 02338920 2001-O1-29
WO 00/06622 PCT/US98127725
The samples were placed in a 50°C oven for approximately one
minute to
_ evaporate the solvent. After the solvent was evaporated, the coated films
were ~ _
passed twice under two 300 wattslcm W lamps {manufactured by Fusion UV
Curing Systems, Rockville, MD) at a rate of 15 ftlminute. The coating was
cured
in air and had a thickness of 4 to 6 micrometers. The refractive index was
measured on the cured ceramers and the pencil hardness of the cured films was
determine. The results are shown in Table 2 below.
Table 2
Example ~~ HIR Index of Pencil
Com onent Refraction*Hardness
1 D 1.544 3H
2 E 1.525 2H
3 F 1.531 2H
4 G 1.530 1H
l0 * refractive index of cured c;eramer
Examples 5-6
Example 5 was prepared by mixing four pants of component B with one
part of 4,6-dibromo-2-sec-butyl phenyl acrylate (D:BsBPA).
Example 6 was prepared by mixing four parts of component C with one
i5 part of 4,6-dibromo-2-sec-butyl phenyl acrylate (DI3sBPA):
The ceramer compositions were coated on a polyester film substrate at a
thickness of 4 to 5 micrometers using conventional flow coating techniques.
Each
coated substrate was flash dried at 60°C for 2.5 minutes in an air
circulating oven
to ensure that the majority of the isopropanol was driven off. The ceramer
coating
2o was cured on a conveyor belt of a UV light processor using a high pressure
mercury Lamp {Model QC 1202 manufactured by Fusion W Curing Systems,
Rockville, MD); process conditions were 55 feet/m:inute, 410 volts, energy 90
mJ/cmZ and atmospheric air. The index of refraction and pencil hardness of the
cured films were determined. The results are shovv~n in Table 3 below.
25 Table 3
Example Component Index: Pencil
of
Refractionardness
* H
5 B __ _
1.5442 _
1H
6 C 1.51.5 -
*refractive index of cured ceramer ~Im
-40-
CA 02338920 2001-O1-29
WO OU/06622 PCTlUS98l27725
Example 7
Particle Preparation . , _
In a nitrogen filled glovebox, I.7 grams of h<;xanoic acid was rapidly added
to 10 gams tetrabutyl titanate in a 20 milliliters screw cap glass vial and
shaken
vigorously. Outside of the nitrogen filled glovebox, 0.93 gram deionized water
was added to the solution and the solution was vigorously shaken for one
minute,
then transferred to a 23 milliliter Teflon lined non-stirred, pressure vessel
(Pressure
Vessel Model # 4749, commercially available from Parr Instruments Co., Moline,
IL} that was purged with nitrogen for 1 minute. The; reactor was heated to 235
C
to for approximately 5 hours. Upon cooling, the solution was nearly colorless
and
contained a white precipitate. The precipitate was separated from the liquid
by
placing the slurry into centrifuge bottles which were: centrifuged at 2500 rpm
for
minutes using an International Equipment Company Model EXD centrifuge,
commercially available from Fisher Scientific Company, Pittsburgh, PA. The
liquid was decanted. Further purification of the particles was accomplished by
resuspending the particles in a fresh portion of hexane and then centrifuging
the
slurry at 2500 rpm for 10 minutes followed by decaunting the hexane.
Preparation of Counlin_g Agent/Dispersina Agent
Under dry nitrogen, 7.24 grams 4,6-dibromo-2-seo-butylphenyIacrylate,
from Minnesota Mining and Manufacturing Company, St. Paul, MN, was added
dropwise to 1.96 grams of stirred 3-mercaptopropyltrimethoxysilane and 0.18
gram
triethylamine. The solution warmed slightly upon auidition of the acrylate.
The
mixture was allowed to stir 12 hours at room temperature. The triethylamine
was
removed under vacuum (ca. 10-1 Torr) to provide 4,6-Br2-2-C4H9-C6H2-
OC(O)(CH2)2S(CH2)3 Si(OCH3)3.
Attaching Coupling Agent/Dispersing Agent to the Metal Oxide Particle
Metal oxide particles (calculated as 2.28 grauns of titanium dioxide) were
3o added to 40 milliliters 2-butanone containing 0.35 I;ram 4,6-Br2-2-C4H9-
C6H2-
OC(O)(CH2)2S(CH2)3Si(OCH3)3 and 0.35 gram 3~-methacryloyloxypropyl-
-41-
CA 02338920 2001-O1-29
WO OOIObb22 PCT/US98127725
trimethoxysiiane. The mixture was heated to 68°C with continuous
stirring for 1.5
hours. The mixture was combined with 0.35 gram of a dilute ammonium -
hydroxide solution (8 drops of aqueous 30 percent ammonium hydroxide mixed
with 3.64 grams deionized water mixed with 3 millilliters of 2-butanone) at 68
C.
The temperature was reduced to 45 C and the colloid stirred 12 hours. The
transparent colloid was concentrated until the 2-buW none ceased to distill
using a
rotary evaporator (30 C). Approximately 40 milliliters of hexane was-added to
the
mixture and stirred for 1 hour and resulted in the immediate precipitation of
weakly flocced particles. The slurry was transferrec! to centrifuge bottles
and
1o centrifuging the slurry at 2500 rpm for 10 minutes followed by decanting
the
supernate. Further purification of the particles was accomplished by
resuspending
the particles in a fresh portion of hexane and then cE;ntrifuging the slurry
at 2500
rpm for 10 minutes followed by decanting the hexane. The titanic particles
were
dispersed in 30 milliliters of 2-butanone forming a transparent pale yellow,
colloidal solution.
-42-
CA 02338920 2001-O1-29
WO 00/06622 PCT/US98127725
Formation of Ceramer
The colloidal suspension was combined with 0:2 gram 4,6-dibromo-2-sec- -
w
butylphenylacrylate and 0.05 gram IrgacureT"" 4265. A portion of the 2-
butanone
was removed by vacuum distillation until the volume of the sample was
approximately 5 milliliters. For purposes of measuriing the refractive index,
the
ceramer composition of was placed on a silicon substrate (commercially
available
from Monsanto, St. Louis, MO) between two 50 miacrons thick tape strips spaced
2
centimeters apart. The ceramer composition was spread between the silicon
wafer
and a polyester liner by pressing the assembly together on a flat surface with
a
l0 2500 grams steel coating bar. The polyester liner was removed by. peeling
and the
fiHri was cured on the silicon wafer, in the presence of nitrogen, at a
translation
speed of 20 feet/minute using a UV curing station (iModel # MC-6RQN,
commercially available from Fusion System Corp., Reckville, MD, with a "D"
bulb). The cured film was then placed in an oven at 80 C for 2 hours to drive
off
i5 any residual 2-butanone. The refractive index of the cured film was
determined to
be 2.01 by scanning laser confocal microscope available from Leica
Lasertechnik
Gmbh of Fieidelberg, Germany.
-43-
CA 02338920 2001-O1-29