Note: Descriptions are shown in the official language in which they were submitted.
CA 02339576 2001-02-05
WO 00/07950 PCT/1JS99/16176
CREATING SILICA SOOT WITH A PLUG-FREE SYSTEM
FIELD OF THE INVENTION
The present invention relates to the delivery of liquid reactants to
a combustion zone formed adjacent a burner assembly to create soot used in
the manufacture of glass. More particularly, the present invention relates to
a
system and method of delivering liquid reactants to a combustion zone that
avoids the premature solidification of the liquid reactants within the burner
assembly.
While the invention is subject to a wide range of glass soot
deposition applications, it is especially suited for use in producing soot for
glass
preforms used in the manufacture of optical waveguides, and will be
particularly described in that connection.
BACKGROUND OF THE INVENTION
Various processes are known in the art that involve the production of
metal oxides from vaporous reactants. Such processes require a feedstock
solution or precursor, a means of generating and transporting vapors of the
feedstock solution (hereafter called vaporous reactants) and an oxidant to a
conversion reaction site (also known as a soot reaction zone or combustion
CA 02339576 2001-02-05
WO 00/07950 PCTNS99/16176
2
zone to those skilled in the art), and a means of catalyzing oxidation and
combustion coincidentally to produce finely divided, spherical aggregates,
called soot. This soot can be collected in any number of ways, ranging from
capture in a collection chamber to deposition on a rotating mandrel. The
collected soot may be simultaneously or subsequently heat treated to form a
non-porous, transparent, high purity glass article. This process is usually
carried out with specialized equipment having a unique arrangement of
nozzles, injectors, burners and/or burner assemblies.
Much of the initial research that led to the development of such
processes focused on the production of bulk silica. Selection of the
appropriate feedstock was an important aspect of that work. Consequently, it
was at that time determined that a material capable of generating a vapor
pressure of between 200-300 millimeters of mercury (mm Hg} at temperatures
below approximately 100°C would be useful for making such bulk silica.
The
high vapor pressure of silicon tetrachloride (SiCl4) suggested its usefulness
as
a convenient vapor source for soot generation and launched the discovery and
use of a series of similar chloride-based feedstocks. This factor, more than
any other is responsible for the presently accepted use of SiCl4, GeCl4,
POC13,
and BC13 as feedstock vapor sources.
Use of these and other halide-based feedstocks as vapor sources,
however, does have its drawbacks. The predominate drawback being the
formation of hydrochloric acid (HCI) as a by-product of oxidation. HCI is not
only detrimental to the deposition substrates and the reaction equipment, but
to
the environment as well. Overcoming this drawback, amongst others, led to
the use of halide-free compounds as precursors or feedstocks for the
production of soot for optical waveguides.
Although use of halide-free silicon compounds as feedstocks for fused
silica glass production, as described in U.S. Patent Nos. 5,043,002 and
5,152,819, for example, avoids the formation of HCI, other problems remain,
particularly when the soot is intended for the formation of optical
waveguides. It
has been found that, in the course of delivering a vaporized polyalkylsiloxane
to the burner, high molecular weight species can be deposited as gels in the
2
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
3
lines carrying the vaporous reactants to the burner, or within the burner
itself.
This leads to a reduction in the deposition rate of the soot that is
subsequently
consolidated to a blank from which an optical waveguide fiber is drawn. It
also
leads to imperfections in the blank that often produce defective and/or
unusable optical waveguide fiber from the effected portions of the blank. An
additional problem encountered while forming silica soot using siloxane
feedstocks is the deposition of particulates having high molecular weights and
high boiling points on the optical waveguide fiber blank. The build-up of
these
particulates results in "defect" or "clustered defect" imperfections that
adversely
affect the optical and structural quality of optical waveguides formed using
the
silica soot.
Other feedstocks, some of which are, and others of which may be useful
in forming soot for the manufacture of optical waveguides are not currently
acceptable alternatives to the halide-based and halide-free feedstocks for
delivery via vapor deposition. Materials such as salts and those known as rare-
earth elements, for example, are extremely unstable as vapors and often
decompose before they can be delivered in their vapor phase, or do not have
sufficiently high vapor pressures to be vaporized at accessible temperatures.
Although it is often possible to deliver at least a percentage of these
elements to the combustion zone as a vapor, it is technically very difficult.
Elaborate systems incorporating expensive equipment are necessary to
convert these elements to the vapor phase, and further, to deliver them to the
combustion zone without leaving deposits in the lines leading to the burners
and in the burners themselves. Moreover, if multiple elements are being
delivered as vapors and a specific percentage of each is needed for the
desired composition, it is difficult to control the delivery since different
elements
have different vapor pressures.
U.S. Patent Application Serial Number 08/767,653, discloses that these
and other limitations can be overcome by delivering a feedstock to an injector
or burner in liquid form, atomizing the feedstock to form an aerosol
containing
fine droplets of the liquid feedstock, and converting the atomized liquid
feedstock into soot at the combustion zone. Because the feedstock is
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
4
delivered directly into the burner flame as a liquid rather than a vapor, the
vapor pressure of the feedstock is no longer a limiting factor in the
formation of
soot for use in the manufacture of optical waveguides. The injectors, burners,
and burner assemblies disclosed in U.S. Patent Application Serial Number
08/767,653 and other currently pending applications rely on very small
orifices
to deliver the liquid in a fine stream for proper atomization. Because the
orifices are so small, they are extremely susceptible to plugging. Even a
small
solid particle in the liquid being delivered can partially clog the orifice,
which in
turn adversely effects the soot deposition rate, and the homogeneity of the
soot
collected.
Although materials never before delivered to a combustion zone to form
soot for the manufacture of glass can now be delivered in a liquid solution,
many of these materials have inherent short-comings while in a liquid form.
Most problematic is that many of these liquid materials quickly form solids
when exposed to oxygen andlor water. Thus, any exposure to the air during
liquid delivery of these reactants likely will result in the formation of
solids,
which clog the lines leading to the burners and the small orifices of the
burners
and the burner assemblies themselves. When the orifices become partially
clogged, the flame, and thus the soot stream becomes non-uniform and the
soot deposition rate suffers. As a result, the liquid delivery system must be
shut down so that it can be cleaned. Such cleaning operations typically
require
partial disassembly of the burner assembly, which results in significant
production down time.
In liquid delivery systems, plugging or clogging of the burner assembly
orifices is particularly problematic during the start up and shut down stages
of
the liquid delivery cycle. During these periods, the liquid reactant tends to
trickle or sputter out of the injector orifice. This occurs during the start
up stage
of the liquid delivery cycle before steady state pressure is available, and at
the
shut down stage of the liquid delivery cycle after steady state pressure is no
longer available. These limited pressure stages result in significantly
reduced
liquid flow rates, which in turn can provide the exposure time necessary for
the
slow moving liquid to react with the air to form solids. Alternatively, these
liquid
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
feedstocks can leak and solidify on the burner face or within the burner head
cavity, resulting in increased down time for cleaning or unclogging the
burner.
Because the liquid feedstocks can react with water in the air almost
instantaneously, any amount of slow moving liquid feedstock within the
injector
5 or at the injector orifice can result in deposit of solids. The resultant
partial
plugging degrades burner performance for rate and quality of the soot
produced, and complete plugging will stop soot deposition altogether.
There is a need therefore, for a system and method of delivering liquid
feedstocks or precursors (hereinafter, "liquid reactants") through a burner
assembly to form soot for the manufacture of glass that eliminates the
premature solidification of the liquid reactants and therefore the plugging of
burner assemblies in liquid delivery systems.
SUMMARY OF THE INVENTION
The present invention is directed to a system and method for
delivering liquid reactants to a combustion zone adjacent a burner assembly of
a liquid delivery system to produce soot for use in the manufacture of glass.
In
a liquid delivery system, the liquid reactant, capable of being converted by
thermal oxidative decomposition to glass, is introduced directly into the
combustion zone of a combustion burner; thereby forming fineiy divided
amorphous soot. The amorphous soot is typically deposited on a receptor
surface where, either substantially simultaneously with or subsequent to its
deposition, the soot is consolidated into a body of fused glass. The body of
glass may then be either used to make products directly from the fused body,
or the fused body may be further treated, e.g., by forming an optical
waveguide
such as by drawing to make optical waveguide fiber as further described in,
for
example, U.S. Patent Application No. 08/574,961 entitled, "Method for
Purifying Polyalkylsiloxanes and the Resulting Products", the specification of
which is hereby incorporated by reference.
One advantage of the present invention is that the system and method
facilitates against "plugging" of the burner assembly orifices and the
respective
CA 02339576 2001-02-05
WO 00/07950 PCTNS99/16176
6
liquid delivery lines feeding those burner assembly orifices. The terms,
"plugging" and "plug", as used herein, refer to the effect of solids (formed
by
the chemical reactions that result from exposing liquid reactants to water,
and
specifically, water contained in air) that collect on the inner surfaces of
the lines
leading to the burner assembly and walls of the burner assembly orifices. The
collected solids impede or partially impede liquid flow. The system and
method of the present invention are particularly well suited for eliminating
plugging, and particularly, plugging that typically occurs during periods of
transient liquid flow. As used herein, the phrases, "periods of transient
liquid
flow" and "transient liquid flow conditions" are defined as those times or
periods
when liquid flow is being increased to achieve steady state flow within the
system. Transient liquid flow also includes these times or periods when liquid
flow is being decreased from steady state liquid flow and those times or
periods when liquid flow is maintained at a rate less than the selected steady
state flow rate for soot deposition.
The liquid reactant preferably is prevented from being exposed to air,
and thus water contained within air, during periods when the liquid reactant
is
not being delivered to the combustion zone to form soot. Accordingly, another
advantage of the present invention is that elements previously not capable of
being delivered as precursors for the formation of soot used to manufacturing
glass are now capable of being delivered to form soot having qualities and
properties heretofore unknown in the art. Since the liquid reactant does not
come in contact with water until desired, and since the liquid precursor is
not
delivered as a vapor prior to its exposure to the combustion zone, elements
selected from groups IA, IIA, IIIA, IIIB, 1VA, IVB, VA, VB and the rare earth
series of the periodic table of elements are now available to be converted by
oxidation or flame-hydrolysis to soot for use in the manufacture of glass
preforms.
When soot is being deposited and during other periods of steady state
liquid flow, plugging is not generally a concern as the liquid enters the
combustion zone before it has time to solidify enroute. However, during
transient liquid flow conditions, particularly those times when liquid
delivery is
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
7
started and stopped, the liquid reactant flow rate is greatly reduced and the
liquid reactant tends to trickle or sputter out of the liquid exit orifice of
the
burner assembly. During these conditions, the liquid reactant is available
within the lines and within the liquid exit orifice of the burner assembly for
reaction to a solid. These solids plug the orifice as well as the lines, and
therefore adversely effect the soot deposition rate.
The present invention, however, delivers an evaporative liquid through
the lines and burner assembly liquid exit orifice during the transient liquid
flow
conditions thus removing the liquid reactant from the lines and orifice during
periods of reduced liquid flow rate. Because there is no liquid reactant
available in the lines and orifice during this time, only the evaporative
liquid is
available to trickle and sputter. The evaporative liquid simply vaporizes and
evaporates from the lines and orifice without leaving behind significant
solids to
plug the burner assembly.
To achieve these and other advantages, the system of the present
invention delivers an evaporative liquid to a combustion zone through a
conduit
and an injector. Flow through the conduit is selectively controlled and is
transitioned from the evaporative liquid to a liquid reactant. The liquid
reactant
is then delivered to the combustion zone through the conduit and the injector
to
create soot for use in the manufacture of glass. During this transitioning, a
steady state flow of liquid is maintained within the conduit leading to the
combustion zone. This can be achieved for example, by first delivering
evaporative liquid through the conduit until a flow rate of the evaporative
liquid
is established which is effective to propel a uniform flow of the evaporative
liquid through the conduit and burner assembly without sputtering, or dripping
on or in the burner assembly, etc. Consequently, when the flow of liquid
reactant is first initiated, a continuous flow rate has already been
established
within the conduit (i.e., by the evaporative liquid). Moreover, even though a
relatively small flow rate of liquid reactant is initially present, the method
of the
present invention enables the liquid reactant to be propelled through the
conduit at a higher speed than would otherwise occur without the aid of the
evaporative liquid. When necessary, flow can be transitioned from the liquid
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
8
reactant back to the evaporative liquid to purge the conduit and injector
prior to
terminating liquid flow to the combustion zone.
In another aspect, the invention includes a burner assembly for
delivering a liquid reactant directly into a combustion zone as an aerosol to
create soot for the manufacture of glass, and a dry environment positioned
upstream from the burner assembly to house the liquid reactant. A conduit
extending from the dry environment to the burner assembly carries the liquid
reactant to the combustion zone via a flow control apparatus.
In yet another aspect, an inert gas is introduced into an enclosure to
create a dry environment where a liquid reactant is staged. The enclosure
includes a conduit for selectively transporting the liquid reactant to a
combustion zone through an injector. An evaporative liquid is delivered
through the conduit to the combustion zone and thereafter, the evaporative
liquid is transitioned to the liquid reactant. The reactant is then delivered
to the
combustion zone through the conduit and the injector to form soot used in the
manufacture of glass. The liquid reactant may then be transitioned to the
evaporative liquid, and the evaporative liquid delivered to the combustion
zone
through the conduit and the injector to purge the system.
Additional features and advantages of the invention will be set forth in
the detailed description, which follows, and in part will be apparent from the
description, or may be learned by practice of the invention. It will be
understood by those skilled in the art that both the foregoing general
description and the following detailed description are exemplary and
explanatory in nature and are intended to provide further explanation of the
invention as claimed.
The accompanying drawings are included to provide a further
understanding of the invention and are incorporated in and constitute a part
of
this specification, illustrate several embodiments of the invention and
together
with the description serve to explain the principles of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
9
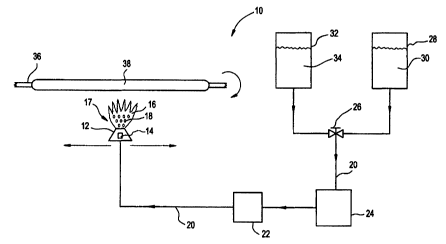
FIG.1 schematically depicts a first preferred embodiment of the system
of the present invention.
FIG. 2 schematically depicts a second preferred embodiment of the
system of the present invention.
DETAILED DESCRIPTION OF THE PERFERRED EMBODIMENTS
A number of soot collection and soot deposition techniques are
employed in the manufacture of glass products. While the present invention is
capable of being employed in a number of these techniques, it is particularly
well suited for those techniques used to deposit soot on a target to form
glass
preforms used in the manufacture of optical waveguides, and specifically
optical waveguide fibers. During the manufacture of optical waveguide fibers,
soot typically is uniformly deposited on or within a target. The collected
soot is
consolidated into a high purity glass preform and thereafter subjected to
further
processing steps such as drawing to form a thin fiber capable of carrying and
directing light. Accordingly, the present invention will be described in this
regard. It will be understood by those skilled in the optical waveguide fiber
art,
however, that there are other systems and variations of the depicted systems
in which the present invention can be incorporated to perform the functions
described and claimed herein. Reference will now be made in detail to the
present preferred embodiments of the invention, examples of which are
illustrated in the accompanying drawings.
A first preferred embodiment of the system for delivering a liquid
reactant into a combustion zone to form soot for use in the manufacture of
glass is depicted in FIG. 1. The liquid reactant delivery system 10 includes a
burner assembly 12 incorporating injector 14 which is capable of delivering
liquids to a combustion zone 16 in the form of atomized liquid droplets 18.
Burner assembly 12 is preferably an atomizing burner assembly such as a
burner assembly disclosed in U.S. Patent Application Serial No. 0$/767,653,
filed December 17, 1996, and entitled "Method and Apparatus for Forming
Fused Silica by Combustion of Liquid Reactants"; U.S. Patent Application
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
Serial No. 08/903,501, filed July 30, 1997, entitled "Method for Forming
Silica
by Combustion of Liquid Reactants Using Oxygen"; U.S. Patent Application
Serial No. 09/089,869, filed June 3, 1998, entitled "Method and Apparatus for
Forming Silica by Combustion of Liquid Reactants Using a Heater"; U.S.
5 Provisional Application Serial No. 60/068,255, filed December 19, 1997,
entitled "Burner and Method for Producing Metal Oxide Soot"; and U.S.
Provisional Application entitled "Method and Apparatus for Forming Soot for
the Manufacture of Glass", filed July 31, 1998, the specifications of which
are
hereby incorporated by reference. However, it will be understood by those
10 skilled in the art that other burner assemblies capable of delivering
liquid
reactants to a combustion zone in liquid form can be incorporated into the
system of the present invention.
Liquid reactant delivery system 10 further includes a liquid reactant 30
and an evaporative liquid 34 capable of being selectively fed to injector 14
of
burner assembly 12 through conduit 20. The term "liquid reactant," as used
herein means any reactant capable of reacting in a combustion zone to form
soot. In a preferred embodiment, the liquid reactant is a glass precursor
capable of making glass soot used to manufacture preforms for optical
waveguide fibers. Likewise, the term "evaporative liquid," as used herein is
defined as a liquid, other than water, capable of evaporating without leaving
significant solids behind. Typically, liquid reactant 30 and evaporative
liquid 34
are stored in liquid reactant reservoir 28 and evaporative liquid reservoir
32,
respectively. Flow control mechanism 26, preferably a 3-way valve, provides
selective control of the liquid delivered to conduit 20. The desired
quantities of
the selected liquid are delivered to injector 14 with a conventional pump 24
and
flow meter 22.
In operation, combustion reactants (not shown) are delivered through
burner assembly 12 and ignited to form a flame 16 at combustion zone 17.
Although not shown, the combustion reactants preferably include, a flame gas,
an additional combustion gas, and a shield gas. Typically, the flame gas is a
mixture of methane and oxygen, the combustion gas is additional oxygen, and
the shield gas is an inert gas such as nitrogen. It will be understood
however,
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
11
that other gases and combination of gases can be used for the combustion
reactants delivered through burner assembly 12. Flow control mechanism 26
is positioned to provide flow of evaporative liquid 34 into conduit 20.
Evaporative liquid 34 is pumped via pump 24 though flow meter 22 to control
the flow rate of evaporative liquid 34 to injector 14. Evaporative liquid 34
is
discharged by injector 14 from burner assembly 12 as atomized liquid droplets
18 into combustion zone 17. During the start-up phase of the present
invention, evaporative liquid 34 initially trickles or sputters out of burner
assembly 12 due to initial low pressure and the corresponding low flow rate in
conduit 20. However, once the flow rate through conduit 20 is increased by
pump 24, evaporative liquid 34 is uniformly delivered into combustion zone 17
as atomized liquid droplets 18, which are quickly combusted in combustion
zone 16 without leaving significant solids behind to plug burner assembly 12.
Once a steady state liquid flow rate is attained (i.e., a flow rate at which
transitioning can occur without significant build-up occurring caused by
solidification of liquid reactant), flow control mechanism 26 transitions
liquid
flow from evaporative liquid 34 to liquid reactant 30. In a preferred
embodiment, a steady state liquid flow rate is a flow rate substantially equal
to
that employed during soot deposition and achieved within the conduit prior to
the transitioning step. As flow of liquid reactant 30 is increased, flow of
evaporative liquid 34 is decreased, thus a "bumpless" transition occurs. The
term "bumpless" as defined herein, means that there are no gaps between the
liquids delivered through conduit 20. Instead, the flow rate through conduit
20,
burner assembly 12, and into combustion zone 17, remains constant during
transition. Shortly after flow into conduit 20 is exclusively liquid reactant
30,
evaporative liquid 34 remaining within conduit 20 is discharged into
combustion
zone 17 followed immediately by liquid reactant 30 which is combusted to form
soot. Burner assembly 12 is directed toward rotating mandrel 36 and soot 38
is deposited on rotating mandrel 36. Although soot 38 is shown in FIG. 1 as
being deposited on rotating mandrel 36 by traversing burner assembly 12 back
and forth along the exterior length of rotating mandrel 36, soot deposition
can
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
12
be achieved by alternative methods, e.g. by moving the mandrel back and forth
with respect to the burner assembly.
Once the desired quantity of soot is deposited on rotating mandrel 36,
liquid flow is transitioned from liquid reactant 30 to evaporative liquid 34
via
flow control mechanism 26. Burner assembly 12 is preferably directed away
from rotating mandrel 26 so that when the remainder of liquid reactant 30 in
conduit 20 is delivered to combustion zone 17 evaporative liquid 34 discharged
immediately thereafter is combusted away from rotating mandrel 36. Flow
control mechanism 26 is can then be engaged to prevent all liquid flow into
conduit 20. Once the remainder of evaporative liquid 34 in conduit 20 is
delivered through burner assembly 12 to combustion zone 17, the trickle flow
from injector 14 evaporates cleanly and the burner assembly flame is turned
off. Since evaporative liquid 34 is the last liquid discharged from burner
assembly 12, there is no liquid reactant 30 available to react with water in
the
air to form solids in the orifices (not shown) of burner assembly 12 or in
conduit
adjacent burner assembly 12. Accordingly, cleaning of burner assembly 12
is not necessary prior to additional runs of the liquid delivery system 10 of
the
present invention.
A second preferred embodiment of the system and method for
20 delivering liquid reactants 40 is schematically depicted in FIG. 2. The
second
preferred embodiment is particularly well suited for the delivery of one or
more
dopants, either independently of, or together with other liquid reactants. It
will
be understood by those skilled in the art, however, that dopants can also be
delivered using the first preferred embodiment of the system and method of the
present invention 10. When delivered using liquid reactant delivery system 10,
dopants can be mixed with liquid reactants 30 in a single reservoir to form a
liquid reactant having the desired proportions of liquid reactants 30 and
dopants. The liquid reactant can then be delivered in a single step to the
combustion zone where it is reacted to form uniformly doped soot. In this way,
the soot can be formed without a separate doping step. As will be described in
detail below, there are practical and economical advantages to delivering
dopants using the second preferred embodiment of the present invention,
CA 02339576 2001-02-05
WO 00/07950 PCT/iJS99/1617b
13
particularly when the optical properties of the fiber to be formed are to be
altered during laydown.
Liquid reactant delivery system 40 includes a burner assembly 42
having an injector 44. It also includes a liquid reactant reservoir 64 and an
evaporative liquid reservoir 68 containing, respectively, liquid reactant 62
and
evaporative liquid 66. Like the first embodiment of the present invention,
evaporative liquid 66 and liquid reactant 62 are selectively delivered through
conduit 60 to injector 44 via flow control mechanism 70. Pump 72 and flow
meter 74 control the flow rate of the selected liquid through conduit 60.
In addition, liquid reactant delivery system 40 includes an enclosure 50,
an inert gas source 56, a syringe pump 78, and an additional flow control
mechanism 76. Like flow control mechanism 70, additional flow control
mechanism 76 is preferably a 3-way valve capable of transitioning liquid flow
into conduit 60 among a number of liquid sources. One preferred syringe
pump 78 is the "Harvard Syringe Pump", model #44, manufactured by Harvard
Inc. of Holliston, Massachusetts. Although other delivery devices would be
acceptable alternatives, syringe pump 78 includes a syringe 80 and metering
device 82 that are particularly well suited for delivering materials in
precise
quantities. This makes syringe pump 78 ideal for delivering dopants for use in
the manufacture of optical waveguides. Inert gas source 56 is preferably a
dispenser capable of delivering an inert gas 54 into enclosure 50 through
passageway 58.
Liquid reactant reservoir 64, evaporative liquid reservoir 68, and
preferably syringe pump 78, are all housed or staged within enclosure 50.
Inert
gas 54 is delivered through passageway 58 into enclosure 50 to displace all
water residing therein and thereby create a dry environment 52. As used
herein, the term "dry environment" means an environment essentially free of
water, i.e., less than an amount of water which would have an adverse effect
on the liquid reactants) contained within the reservoir(s). Although not
required, it is preferred that inert gas 54 be continuously fed into and
exhausted from enclosure 50. Among other benefits, continuously delivering
inert gas 54 into enclosure 50 prevents water containing air from entering
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
14
enclosure 50 if a leak develops therein. Dry environment 52 provides ideal
conditions for working with liquid reactant 62 and dopant 84. Because there is
essentially no water within the enclosure 50 when it is provided with inert
gas
54, solidification of liquid reactant 62 and dopant 84 is avoided. Although
enclosure 50 can be any size, it is preferably a glove box provided with a
pair
of arm length rubber gloves which enable an operator of liquid reactant
delivery
system 40 to access the various liquids staged therein. In this way, the
operator can change the solutions to deliver a different liquid reactant 62
and/or dopant 84 as desired without exposing liquid reactant 62 and dopant 84
to water. Accordingly, solutions can be changed between runs without having
to clean and/or purge the system. Syringe pump 78 offers the additional
advantage of having interchangeable syringe 80. Thus, several syringes 80
containing different dopants 84 can be pre-positioned within enclosure 50 so
that numerous runs can be made before additional dopants 84 must be
introduced and staged within enclosure 50.
In one embodiment, inert gas 54, such as argon, helium or dry air
(comprising approximately 75% nitrogen and 25% oxygen, but no water), but
preferably nitrogen, is introduced into enclosure 50 to create dry environment
52 therein. Liquid reactant 62, and if desired dopant 84 are staged within
enclosure 50 for subsequent delivery through injector 44 of burner assembly
42. Evaporative liquid 66 is also typically housed within enclosure 50 so that
it
does not absorb water from the air outside of the enclosure, but can be
maintained outside of enclosure 50, provided it is not exposed to air or other
water sources. After combustion reactants (not shown) are delivered to burner
assembly 42 to create flame 46 at combustion zone 47, flow control
mechanism 70 is positioned to deliver evaporative liquid 66 into conduit 60.
Evaporative liquid 66 is metered through pump 72 and flow meter 74 to
additional flow control mechanisms 76, which passes evaporative liquid 66 to
injector 44 and into combustion zone 47 as atomized liquid droplets 48. Once
steady state flow of evaporative liquid 66 is attained, flow control mechanism
70 is engaged to transition flow to liquid reactant 62. Again, the transition
is
"bumpless", and once the evaporative liquid 66 has cleared from conduit 60,
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
burner assembly 42 is directed toward rotating mandrel 86. Liquid reactant 62
is discharged from burner assembly 42 as atomized liquid droplets 48 which
are reacted in combustion zone 47 to form soot 88 which is deposited on
rotating mandrel 86. When desired, dopant 84 can be selectively metered
5 through conduit 60 via additional flow control mechanism 76 and into
combustion zone 47 to produce doped soot.
Once the desired quantity of soot 88 is deposited on rotating mandrel
86, burner assembly 42 is directed away from rotating mandrel 86 and liquid
flow into conduit 60 is transitioned to evaporative liquid 68 via flow control
10 mechanism 70. Once liquid reactant 62 has cleared conduit 60, flow of
evaporative liquid 66 is terminated and the combustion flame 46 is turned off.
Because evaporative liquid 66 is the last liquid to exit burner assembly 42,
the
trickled liquid merely evaporates and no reactant is present in burner
assembly
42 for reaction to solids. Thus, plugging the burner assembly 42 and conduit
15 60 adjacent burner assembly 42 is avoided.
In both the first and second preferred embodiments of the present
invention, a preferred liquid reactant 30, 62 for delivery through liquid
reactant
delivery system 10 and 40, respectively, is a silicon alkoxide such as
tetraethoxysilane or tetramethoxysilane. More preferably, liquid reactant 30,
62
is a metal alkoxide such as titanium (IV) propoxide, germanium (1V) ethoxide,
potassium butoxide (made soluble with a suitable organic solvent, such as
ethylene glycol monomethylether) and other metal alkoxides known in the art.
Most preferably liquid reactant 30, 62 is a siloxane, and specifically an
organosiloxane such as octamethylcyclotetrasiloxane. It will be understood by
those skilled in the art, however, that liquid reactant 30, 62 can be
combinations of the above-listed compounds, made soluble with a suitable
organic solvent, such as ethylene glycol monomethylether.
The preferred dopants 84 delivered by syringe pump 78 of liquid
reactant delivery system 40 are ketonates, alkoxides, acetates, ~i-
diketonates,
or fluoro-~3-diketonates of praeseodynium, holmium, and thulium dissolved in a
suitable organic solvent such as ethylene glycol monomethylether. The most
preferred dopant 84, however, is erbium, which is preferably delivered in
liquid
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
16
solution as a ~i-diketonate or fluoro-~3-diketonate. It will be understood by
those
skilled in the art that other rare earth elements, as well as other elements
having properties beneficial for optical waveguides can be delivered by
syringe
pump 78. Although not shown in liquid reactant delivery system 10 of FIG. 1,
it
will also be understood by those skilled in the art that the above-mentioned
elements can also be delivered through liquid reactant delivery system 10
using a syringe pump or other suitable delivery device, provided the selected
dopant solution is not exposed to water.
Evaporative liquid 34, 66 delivered through liquid reactant delivery
system 10 and liquid reactant delivery system 40, respectively, can be organic
nitrogen-containing solutions such as amides, amines, and nitrites, but are
more preferably organic oxygen-containing solutions. Ketones such as
acetone, acetates such as ethylacetate, ethers such as diethylether, and
glycols such as ethylene glycol and ethylene glycol monomethylether are
representative examples of such organic oxygen-containing solutions. More
preferably, alcohol such as ethanol, methanol and propanol are the organic
oxygen-containing solutions delivered as evaporative liquids 34 and 66, with
ethanol and 1-propanol being the most preferred solutions.
It will be apparent to those skilled in the art that several of the organic
oxygen-containing liquids and organic nitrogen-containing liquids described
above can be mixed with organosiloxanes, metal alkoxides, siloxanes, silicon
alkoxides, metal acetates, metal (3-diketonates, metal ketonates, rare earth
acetates, ketonates, alkoxides, ~i-diketonates and/or fluoro-~i-diketonates,
in a
single reservoir, for delivery as liquid reactants 30 and 62. When combusted,
these solutions form various silicon-oxide and metal-oxide snots that can be
captured and used to form optical waveguides having distinct properties.
Examples of such organic oxygen-containing liquids are ketones, alcohols,
glycols, esters, ~i-diketones, and carboxylic acids. Examples of such organic
nitrogen-containing liquids are amides, amines, nitrites and imines.
It will be apparent to those skilled in the art that various modifications
and variations can be made in the system and method for plug-free delivery of
liquid reactants through a burner assembly of the present invention without
CA 02339576 2001-02-05
WO 00/07950 PCT/US99/16176
17
departing from the spirit or scope of the invention. Thus, it is intended that
the
present invention cover the modifications and variations of this invention
provided they come within the scope of the appended claims and their
equivalents. In addition, the corresponding structures, materials, acts and
equivalents of all means or step plus function elements in the claims below
are
intended to include any structure, material, or acts for performing the
function
in combination with other claimed elements as specifically claimed herein.