Note: Descriptions are shown in the official language in which they were submitted.
CA 02340030 2001-02-28
- 1 -
Scaffold Fixation Device for Use
in Articular Cartilage Repair
Field of the Invention
The present invention relates to scaffold fixation
devices useful in articular cartilage repair and more
specifically to a device for fastening an articular
cartilage scaffold to underlying bone.
Background of the Invention
Tis'sue engineering is defined as the application of
engineering disciplines to either maintain existing
tissue structures or to enable new tissue growth. This
engineering approach generally includes the delivery of
a tissue scaffold that serves as an architectural
support onto which cells may attach, proliferate, and
synthesize new tissue to repair a wound or defect.
Cartilage tissue scaffolds have high open-celled
porosity to allow cell migration throughout the scaffold
and also to allow important nutrient-bearing fluids to
flow through the scaffold to maintain the health of the
cells.
Articular cartilage is a tissue that covers the
articulating surfaces between bones in the joints.
Articular cartilage consists of two principal phases: a
solid matrix and an interstitial fluid phase. The
matrix, which gives cartilage its stiffness and
CA 02340030 2001-02-28
- 2 -
strength, is produced and maintained by chondrocytes.
Many studies have indicated that load has an important
influence on matrix synthesis and on the composition of
articular cartilage. Published studies have described
the effect of mechanical loading on cell activity and
matrix synthesis in cartilage: Hall, Urban, and Gehl,
"The Effects of Hydrostatic Pressure on Matrix Synthesis
in Articular Cartilage", Journal of Orthopaedic
Research, Vol. 9, pp. 1-10,1991; Freeman, Natarajan,
Kimura, and Andriacchi, "Chondrocyte Cells Respond
Mechanically to Compressive Loads", Journal of
Orthopaedic Research, Vol. 12, pp.. 311-320, 1994; Tagil
and Aspenberg, "Cartilage Induction by Controlled
Mechanical Stimulation In Vivo, Journal of Orthopaedic
ls Research, Vol. 17, pp. 200-204, 1999 and; Carver and
Heath, "Semi-continuous Perfusion System for Delivering
Intermittent Physiological Pressure to Regenerating
Cartilage", Tissue Engineering, Vol. 5, pp. 1-11, 1999.
Synthetic absorbable biocompatible polymers are
well known in the art. Such polymers typically are used
to manufacture medical devices which are implanted in
body tissue and absorb over time. Synthetic absorbable
biocompatible aliphatic polyesters include homopolymers,
copolymers (random, block, segmented and graft) of
monomers such as glycolic acid, glycolide, lactic acid,
lactide(d, 1, meso and mixtures thereof), -
caprolactone, trimethylene carbonate and p-dioxanone.
Numerous U.S. Patents describe these polymers, including
5,431,679; 5,403,347; 5,314,989; 5,431,679; 5,403,347;
CA 02340030 2001-02-28
- 3 -
and 5,502,159. Devices made of an absorbable material
have the advantage that they are absorbed by the body
after healing has occurred.
U.S. Patent 5,067,964 describes an articular
cartilage repair piece which includes a backing layer of
non-woven, felted fibrous material which is either
uncoated or covered by a coating of tough, pliable
material. A number of means are disclosed for fastening
the repair piece to the underlying bone. U.S. Patents
5,306,311 and 5,624,463 describe a prosthetic,
resorbable articular cartilage and methods of its
fabrication and insertion. U.S. Patent 5,713,374
describes an attachment method to hold a biomaterial in
place until healing occurs. U.S. Patents 5,632,745 and
5,749,874 and 5,769,899 describe a bioabsorbable
cartilage repair system.
High porosity is a critical design criterion in
engineering of tissue scaffolds. Since a very porous
tissue scaffold will have low stiffness and strength, a
device is needed that will protect the scaffold from
high joint loads. The same device needs to provide
controlled mechanical stimulation of the cells within
the scaffold to increase cell activity and matrix
synthesis to produce new cartilage.
Accordingly, it would be advantageous to provide a
scaffold fixation device which allows limited loading of
the scaffold effective to stimulate tissue regeneration
within the scaffold, while also providing protection of
CA 02340030 2006-02-22
4 -
the scaffold from excessive loading that may damage the
repairing tissue.
Summary of the Invention
The present invention is directed to scaffold
fixation devices comprising means for anchoring the
device to bone, a load support comprising an upper
surface, and means for providing deformation of the
device.
More particularly, the present invention provides a
scaffold fixation device suitable for use in articular
cartilage repair, comprising: means for anchoring said
fixation device to bone, a load support comprising an
upper surface and a lower surface; and means for
providing deformation of said fixation device, wherein
said deformation provides a controlled load on a scaffold
provided with said fixation device, which controlled load
is effective to stimulate growth of cells and synthesis
of a cell matrix in and/or on said scaffold without
substantially damaging said cells, cell matrix or
scaffold.
In one embodiment the means for providing
deformation of the device comprises a flexible structural
member. The flexible structural member may fold,
collapse or otherwise deform in response to load applied
to the load support.
In another form of the invention the fixation device
comprises an upper component comprising said load support
and posts protruding downward from said load support near
an outer perimeter thereof; and a base component
comprising a base platform and post guides protruding
upward from said base platform near an outer perimeter
thereof, said post guides being in axial alignment with
CA 02340030 2006-02-22
- 4a -
said posts, wherein said upper component is free to
deform with respect to said base component through a
controlled deformation distance between an upper surface
of said guide post and said lower surface of said load
support, and wherein said means for providing deformation
comprises said post and post guide.
The device described above may further comprise
means for substantially preventing rotation of the device
and the scaffold.
Brief Description of the Figures
Figure 1 is a side elevation view of a one-piece of the
present invention;
Figure 2 is a perspective view of the device of Figure 1;
Figure 3 is a side elevation view of the device of Figure
1;
Figure 4 is a side elevation view of a device of the
present invention;
Figure 5 is a perspective view of the device of Figure 4;
Figure 6 is a side elevation view of the device of Figure
4 as deployed in bone;
CA 02340030 2001-02-28
- 5 -
Figure 7 is a side elevation view of a two-piece device
of the present invention;
Figure 8 is a side elevation view of the device of
Figure 7 after connection of the pieces;
Figure 9 is a perspective view of the device of Figure
8;
io Figure 10 is a perspective view of the device of Figure
7;
Figure 11 is a cross-sectional side view of a two-piece
device of the present invention when there is no axial
load on the device and the gap is completely open;
Figure 12 is a cross-sectional side view of a portion of
a two-piece device of the present invention when there
is axial load on the device such that the gap is closed;
Figure 13 is.a side elevation view of the device of
Figure 8 as deployed in bone;
Figure 14 is a side elevation view of a two-piece device
of the present invention;
Figure 15 is a side elevation view of the device of
Figure 14 after connection of the pieces;
CA 02340030 2001-02-28
- 6 -
Figure 16 is a perspective view of the device of Figure
15;
Figure 17 is a cross-sectional side view of the device
of Figure 14;
Figure 18 is a cross-sectional side view of the device
of Figure 15 after connection of the pieces.
Figure 19 is a perspective view of a portion of another
two-piece alternative embodiment of the present
invention.
Figure 20 is a side elevation view of the device of
is Figure 19 as deployed in bone.
Figure 21 is a perspective view of an anchoring section
that comprises ribs for preventing rotation of the
device.
Detailed Descrigtion of the Invention
The present invention provides a device for
fastening an articular cartilage scaffold to underlying
bone. The device comprises means for providing
deformation of the device. The deformation is selected
so as to provide a particular controlled load on the
cartilage scaffold. The controlled load is sufficient
t6 stimulate cell growth and matrix synthesis, while at
the same time not excessive so as to cause substantial
CA 02340030 2001-02-28
- 7 -
cell and tissue damage. As used herein, "deformation"
means linear displacement of the upper surface of the
load support relative to the original position of the
upper surface of the load support. The amount of
s deformation required in a particular situation is
determined in part by such factors as the properties of
materials selected for the device and the cartilage
scaffold, respectively, e.g. strength, stiffness, etc.
and the amount of load effective to stimulate cell
io growth and matrix synthesis without substantially
damaging the cells or tissue.
One device of the invention comprises a single
integral part, comprising a load support having an upper
surface, means for providing controlled deformation of
is the device and means for anchoring the device to bone.
The load support resides above the cartilage scaffold,
is flush with the neighboring healthy cartilage and is
in direct contact with the opposing joint surface. The
anchoring means may comprise a fixation post that
20 protrudes from the load support, through the cartilage
scaffold, and into the underlying bone, thereby
anchoring the device, and thus the cartilage scaffold,
within the cartilage defect space. Load applied by the
opposing joint surface is transmitted through the load
25 support to the underlying scaffold and/or bone,
depending on the amount of load transmitted.
The anchor means may comprise means for providing
controlled deformation of the device. Such means permits
selected linear displacement of the upper surface of the
CA 02340030 2001-02-28
- 8 -
load support, relative to its original position, in
response to the load applied by the opposing joint
surface. When the load is sufficiently low, so as not to
cause substantial damage to the cartilage scaffold,
s1 cells or tissue, the device may deform, such that the
load may be borne mostly by the cartilage scaffold. In
this way, a minimum load may be applied to the cartilage
scaffold to stimulate cell growth and matrix synthesis.
When the load becomes excessive, such that damage to the
io cartilage scaffold, or to cells or tissue may occur, the
device is prevented from deforming further and the
excessive load is transferred directly to the underlying
bone via the fixation device, thereby shielding the
scaffold from excessive load.
15 One means of providing controlled deformation
comprises flexible structural members that fold, or
collapse, or otherwise deform in response to load
applied to the load support, thus providing limited
mechanical response, i.e. stiffness, to the load,
20 thereby transferring a substantial portion of the load
to the cartilage scaffold. Once maximum deformation is
achieved, e.g. when the collapsing member can collapse
no further or meets a constraint, the mechanical
response of the device become.s greater and the device
25 bears a greater to a substantial portion of the load,
thereby preventing damage to the cells or scaffold.
Other devices of the invention comprise an assembly
of two parts, between which the cartilage scaffold
resides. The two parts are pressed together to engage
CA 02340030 2001-02-28
- 9 -
mechanical fasteners that, once fully engaged, prevent
the parts from being separated. The connected parts are
free to move with respect to each other, i.e. the device
is free to deform, through a controlled deformation
s distance (CDD) between the assembled parts. The CDD.is
effective to provide a load on the cartilage scaffold
effective to stimulate cell activity and matrix
synthesis without causing substantial damage to the
scaffold or cells and healing tissue. When the load is
io sufficiently low so as not to cause substantial damage
to the scaffold and/or cells, deformation, i.e. relative
travel between the two parts, is less than the CDD and
the applied load is borne substantially by the scaffold.
When the load becomes excessive, the distance between
15 the two parts is closed and the two parts are in
contact, thus preventing additional deformation. A
substantial portion of the load then is.transferred
directly to the underlying bone via the device, thus
protecting the scaffold from excessive load.
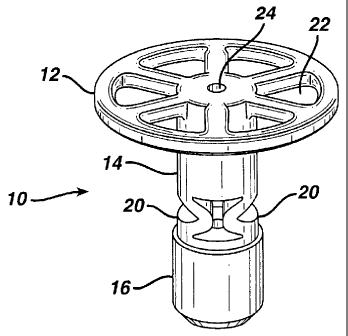
20 One embodiment of the present invention is shown in
Figures 1 through 3. Figure 1 shows a side elevation
view of scaffold fixation device 10, comprising load
support 12 having upper surface 12a and lower surface
12b, fixation post 14, anchoring section 16 and flexible
25 members 20. Flexible members 20 are designed such that
the mechanical response is very nonlinear, having low
stiffness under low load or displacement and much higher
stiffness under high load or displacement. Preferably,
the initial compressive stiffness of the flexible
CA 02340030 2001-02-28
- 10 -
members is less than the compressive stiffness of the
scaffold. A dramatic increase in stiffness occurs when
the displacement of load support 12 is such that
flexible members 20 fold upon themselves or are
constrained from further bending. Load applied by the
opposing joint surface is transmitted through load
support 12 and is shared by fixation post 14 and the
scaffold. In the low load regime in which flexible
members 20 may bend, the scaffold bears the majority of
the total applied load. Once the applied load becomes
excessive, flexible members 20 fold together or are
constrained from further bending and the device will
become stiffer, thus transferring excess load directly
to the underlying bone via the device. The scaffold
thereafter will bear a much lower percentage of the
total applied load.
Figure 2 shows a perspective view of scaffold
fixation device 10 showing perforations 22 in load
support 12 and guide wire channel 24 traveling
longitudinally through the device along the axis of
fixation post 14. Perforations 22 allow fluid to flow
to and from the scaffold and are not limited to the
shape or arrangement show in the figures.
Figure 3 shows a side elevation view of the
surgical placement of scaffold fixation device 10. Bone
hole 30 is drilled in bone tissue 32 to a diameter such
that an interference fit is made between bone hole 30
and anchoring section 16. Cartilage hole 34 is drilled
in cartilage tissue 36 to a diameter at least as large
CA 02340030 2001-02-28
- 11 -
as the outermost diameter of load support 12. The depth
of bone hole 30 is drilled such that when fixation post
14 is inserted completely into the hole, upper surface
12a of load support 12 preferably lies in alignment with
or slightly below upper cartilage surface 38 of adjacent
cartilage tissue 36 when no vertical load is applied to
the device. The scaffold would reside within the space
available between lower surface 12b of load support 12
and top surface 39 of bone tissue 32 and would fill the
diameter of cartilage-hole 34. Anchoring section 16
also may include ribs, serrations, or other surface
roughness or engagement features that improve the
attachme'nt of anchoring section 16 to the surrounding
bone hole 30 and substantially prevent rotation of
device 10 and the scaffold. Anchoring section 16 also
may include chamfer 26, which aids in guiding the
fixation post into bone hole 30. A surgical guide wire
may be passed through guide wire channel 24 during
surgery to align scaffold fixation device 10 with the
cartilage repair site.
Figures 4, 5 and 6 show a scaffold fixation device
of the present invention comprising load support 42
having upper surface 42a and lower surface 42b, fixation
post 44, anchoring section 46 and flexible members 50.
Flexible members 50 are oriented such that they will
bend outwards when a compressive load is applied to load
support 42. The stiffness of the device will be
relatively low until flexible members 50 contact lateral
surface 61 of bone hole 60, at which point the stiffness
CA 02340030 2001-02-28
- 12 -
of the device will increase dramatically, since flexible
members 50 are thereafter constrained from further
bending outwards. Also shown in Figure 6 are cartilage
hole 64, cartilage tissue 68 and bone tissue 62.
A scaffold fixation device of the present invention
is shown in Figures 7 through 13. Figure 7 shows a side
view of the unassembled scaffold fixation device 80
comprising upper component 82 and base component 84.
Upper component 82 comprises load support 86 having
upper surface 86a and lower surface 86b and posts 88
which protrude downward from load support 86 near its
outer perimeter. Each post 88 contains ledge 90 with
outer di'ameter larger than that of post 88. Base
component 84 comprises base platform 92, fixation post
94, and post guides 96 which protrude upward from base
platform 92 near its outer perimeter and in axial
alignment with posts 88 of upper component 82. Figure 8
shows a side view of scaffold fixation device 80 after
connection of upper component 82 and base component 84.
Figures 9 and 10 show perspective views of the
device of Figures 7 and 8. Guide channel 98, with
diameter at least as large as the outermost diameter of
ledge 90 of post 88, is located in axial alignment with
post guide 96 and passes through base platform 92 and
partially through post guide 96. The upper portion of
post guide 96 is designed to be radially flexible by way
of perforations 100 which allow the upper portion of
post guide 96 to bend outwards to.receive ledge 90 of
post 88 of upper component 82.
CA 02340030 2001-02-28
- 13 -
The method of connection between upper component 82
and base component 84 is shown in Figure 11. Figure 11
is a cross-sectional side view of the device of Figures
7 through 10. Upper component 82 and base component 84
s are connected together by mechanical fastening between
posts 88 and post guides 96 by way of ledge 90 on each
post 88 locking.with latch 102 on each post guide 96.
As upper component 82 and base component 84 are pressed
together with the axis of each post 88 aligned with the
axis of each post guide 96, each post guide 96 is forced
to expand outward around ledge 90 of each post 88 until
ledge 90 passes latch 102, at which point the post guide
96 returns to its unloaded configuration so that latch
102 captures ledge 90. Once upper component 82 and base
is component 84 are connected together, they can not be
easily separated. Preferably the geometry of ledge 90
and post guide 96 is such that the elastic limit of post
guide 96 would not be exceeded during connection of
upper component 82 and base component 84.
Once upper component 82 and base component 84 are
connected together, they are free to move with respect
to each other through CDD 104 between upper surface 103
of guide posts 96 of base component 84 and lower surface
86b of load support 86 of upper component 82. CDD 104
between the assembled parts provides a minimum amount of
scaffold deformation effective to stimulate cell
activity and matrix synthesis, i.e. tissue growth, and a
maximum amount of scaffold deformation effective to
prevent substantial damage to the scaffold or to the
CA 02340030 2001-02-28
- 14 -
cells and the healing tissue. While the relative travel
between upper component 82 and base component 84 is less
than CDD 104, the applied load is borne entirely by the
scaffold. When CDD 104 is closed and load platform 86
comes in contact with post guides 96, as shown in Figure
12, the stiffness of the device is much higher than the
stiffness of the scaffold, since post guides 96 would
then act as load-bearing columns to protect the scaffold
from hi-gh displacement and load. Preferably the lengths
of posts 88 are such that they will not protrude beyond
bottom surface 106 of base component 84 in the closed
configuration shown in Figure 12. Once posts 88 are
aligned within guide channels 98 in guide posts 96,
resistance to rotation of upper component 82 relative to
base component 84 is provided. Clearance between ledges
90 of posts 88 and guide channels 98 of post guides 96
allows upper component 82 to displace freely towards
base component 84.
Figure 13 shows a side elevation view of the
surgical placement of scaffold fixation device 80.
Bone hole 110 is drilled in bone tissue 112 to a
diameter such that an interference fit is made between
bone hole 110 and anchoring section 94. Cartilage hole
114 is drilled in cartilage tissue 116 to a diameter at
least as large as the outermost diameter of load support
86. The depth of bone hole 110 is drilled such that
when fixation post 94 is inserted completely into hole
110 upper surface 86a of load support 86 preferably lies
in alignment with or slightly below upper cartilage
CA 02340030 2001-02-28
- 15 -
surface 118 of adjacent cartilage tissue 116 when no
vertical load is applied to the device. The scaffold
resides in the available space between upper component
82 and base component 84 and fills the diameter of
cartilage hole 114. Fixation post 94 also may include
ribs, serrations, or other surface roughness or bone
engagement features that improve the attachment of the
post to the surrounding bone and/or to prevent rotation
of the device and scaffold once implanted. Fixation
device 80 also may comprise guide wire channel 101
passing completely through upper component 82 and base
component 84. A surgical guide wire may be passed
through guide wire channel 101 during surgery to align
base component 84 and upper component 82 with bone hole
110 and cartilage hole 114. Figures 7 through 12 show
an embodiment of the invention having six sets of posts
88 and post guides 96. At least 3 sets of posts 88 and
post guides 96 are preferred for mechanical stability.
Another embodiment of the present invention is
shown in Figures 14 through 20. Figure 14 shows a side
view of unassembled scaffold fixation device 130 that
comprises top component-132 and fixation component 134.
Top component 132 comprises load support 136 and
connecting post 138. Top component 132 also may include
support columns 140 protruding downward from load
platform 136. Connecting post 138 includes radially
flexible members 142 with latches 144 for connection to
fixation component 134. Fixation component 134
comprises shoulder 146, anchor section 148, and also may
CA 02340030 2001-02-28
- 16 -
comprise chamfer 150 on the lower tip of anchor section
148 to help align fixation component 134 during
insertion into a hole in bone. Figure 15 shows a side
view of scaffold fixation device 130 after connection of
top component 132 and fixation component 134. Figure 16
shows a perspective view of scaffold fixation device
130.
As shown in Figures 17 and 18, cross-sectional side
views of the fixation device described in Figures 14, 15
and 16, fixation component 134 also comprises post
channel 152 passing longitudinally therethrough and
latch channels 154, which pass partially through
fixation,component 134 from the bottom end. Post
channel 154 has a diameter that is at least as large as
the diameter of.connecting post 138 and latch channels
154 have the same general shape as latches 144, with at
least the same dimensions so that latches 144 fit in
latch channels 154 without interference. The shape of
latches 144 and latch channels 154 is such that there
are flat surfaces 150 in latch channels 154 that
interact with mating surfaces on latches 144 of flexible
members 142 to prevent rotation of top component 132
with respect to fixation component 134. Figure 19 shows
a perspective view of a portion of scaffold fixation
assembly 130 showing the fit between latches 144 and
latch channels 154.
Assembly of scaffold fixation device 130 is
achieved by inserting connecting post 138 of top
component 132 axially into post channel 152 in fixation
CA 02340030 2001-02-28
- 17 -
component 134 with latches 144 in alignment with latch
channels 154. Flexible members 142 will deflect to
enter post channel 152 and will return to their unloaded
configuration when top component 132 is displaced
s downwards until latches 144 travel beyond the upper
surfaces of the latch channels. Preferably, the
geometry of flexible members 142 and post channel 152
are such that the elastic limit of flexible members 142
will not be exceeded during connection of top component
132 to fixation component 134.
Figure 18 is a cross-sectional side viewof the
device described in Figure 17 after connection of the
pieces. Once top component 132 and fixation component
134 are connected together, they'are free to move with
is respect to each other through CDD 156 between upper
surfac.e 157 of shoulder 146 of fixation component 134
and lower surface 136b of load support 136 of top
component 132. CDD 156 between the assembled parts
provides a minimum amount of scaffold deformation
2o effective to stimulate cell activity and matrix
synthesis, and a maximum amount of scaffold deformation
effective to prevent substantial damage to the scaffold
and/or to the cells and the healing tissue. When the
relative travel between top component 132 and base
25 component 134 is less than CDD 156, the applied load is
borne entirely by the scaffold. When CDD 156 is closed
and load support 136 comes in contact with shoulder 146,
the stiffness of the device is much higher than the
stiffness of the scaffold, since shoulder 146 then acts
CA 02340030 2001-02-28
- 18 -
as a load-bearing column to protect the scaffold from
high displacement and load. Another or an additional
structural support to protect the scaffold would be
provided if support columns 140 of the load support 136
5, were also included in the invention. Preferably, the
device will contain at least one of shoulder 146 or
support columns 140.
Figure 20 shows a side elevation view of the
surgical placement of scaffold fixation device 130.
Bone hole 160 is drilled in bone tissue 162 to a
diameter such that an interference fit is made between
bone hole 160 and anchor section 148. Cartilage hole 164
is drilled in cartilage tissue 166 to a diameter at
least as large as the outermost diameter of upper
component 132. The depth of bone hole 160 is drilled
such that when fixation component 134 is inserted
completely into the hole, upper surface 167a of load
support 167 preferably lies in alignment with or
slightly below upper cartilage surface 168 of adjacent
cartilage tissue 166 when no vertical load is applied to
the device. The scaffold resides in the available space
between top component 132 and bone tissue 162 and fills
the diameter of cartilage hole 164. The fixation
section 148 may also include ribs, serrations, or other
surface roughness or bone engagement features that
improve the attachment of the post to the surrounding
bone and/or prevent rotation of the device and scaffold.
Figure 21 shows anchoring section 170 of the type
disclosed in Figures 14-20, comprising chamfer 172 and
CA 02340030 2001-02-28
19 -
ribs 174 for preventing rotation of the device once
implanted.
Suitable materials from which the scaffold fixation
device may be formed include biocompatible polymers
selected from the group consisting of aliphatic
polyesters, polyorthoesters, polyanhydrides,
polycarbonates, polyurethanes, polyamides and
polyalkylene oxides. The present invention also can be
formed from absorbable glasses or ceramics comprising
calcium phosphates and other biocompatible metal oxides
(i.e., CaO). The present invention can also be formed
from metals. The fastener of the present invention
further can comprise combinations of metals, absorbable
ceramics, glasses and polymers.
In the preferred embodiment, the scaffold fixation
device comprises aliphatic polymer and copolymer
polyesters and blends thereof. The aliphatic polyesters
are typically synthesized in a ring opening
polymerization. Suitable monomers include but are not
limited to lactic acid, lactide (including L-, D-, meso
and D,L mixtures), glycolic acid, glycolide, -
caprolactone, p-dioxanone (1,4-dioxan-2-one),
trimethylene carbonate (1,3-dioxan-2-one), delta-
valerolactone, beta-butyrolactone, epsilon-decalactone,
2,5-diketomorpholine, pivalolactone, alpha, alpha-
diethylpropiolactone, ethylene carbonate, ethylene
oxalate, 3-methyl-l,4-dioxane-2,5-dione, 3,3-diethyl-
1,4-dioxan-2,5-dione, gamma-butyrolactone, 1,4-dioxepan-
2-one, 1,5-dioxepan-2-one, 6,6-dimethyl-dioxepan-2-one,
CA 02340030 2001-02-28
- 20 -
6,8-dioxabicycloctane-7-one and combinations thereof.
These monomers generally are polymerized in the presence
of an organometallic catalyst and an initiator at
elevated temperatures. The organometallic catalyst is
s preferably tin based, e.g., stannous octoate, and is
present in the monomer mixture at a molar ratio of
monomer to catalyst ranging from about 10,000/1 to about
100,000/1. The initiator is typically an alkanol
(including diols and polyols), a glycol, a hydroxyacid,
io or an amine, and is present in the monomer mixture at a
molar ratio of monomer to initiator ranging from about
100/i to about 5000/1. The polymerization typically is
carried'out at a temperature range from about 80 C to
about 240 C, preferably from about 100 C to about 220 C,
is until the desired molecular weight and viscosity are
achieved.