Note: Descriptions are shown in the official language in which they were submitted.
CA 02341375 2001-02-21
WO 00/10536 PCT/US99/19260
EXTENDED RELEASE BUCAL BIOADHESIVE TABLET
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a bioadhesive, bioerodible tablet for the
extended and
controlled release of active ingredients. More particularly, the present
invention relates to a
progressive hydration tablet for adhesion to the wall of a body cavity for the
sustained release
of active ingredients without premature degradation of the active ingredients
caused by
metabolism, or by moisture, enzymes or pH effects.
2. Description of the Related Art
Medications and other pharmaceutical products have traditionally been
administered in
doses via oral ingestion, nasal sprays or injections. These delivery methods
have proven
ineffective for patients needing a prolonged and constant supply of an active
ingredient delivered
to the bloodstream. Particularly difficult are patients needing dosing during
sleep time hours.
For these patients, intravenous venous lines, slow-dissolving pills, and
suppositories or
transdermal patches have been prescribed. However, the inconvenience and
discomfort of IVs,
the short life span of many ingested active ingredients from gastrointestinal
degradation or first-
pass liver metabolism, and the inability of many products to be comfortably
delivered
2 0 transdermally in suitable doses or in controlled concentrations have
proven these methods
unsatisfactory.
Previous artisans have attempted to meet the needs of the art by developing
products for
the transmucosal administration of active ingredients. For example, certain
active ingredients
can be administered quickly into the bloodstream via the walls of a body
cavity, such as the
CA 02341375 2001-02-21
WO 00/10536 PCT/(JS99/19260
buccaI or vaginal cavities, without the risk of first pass hepatic
degradation. Generally, delivery
of active ingredients through mucosal surfaces may be enhanced by the use of
bioadhesive
formulations. However, one particular area where those in the art have
attempted, but heretofore
failed, to meet the needs of the art is in developing a bioadhesive tablet
useful for sustained
release applications without risking degradation of the active ingredient
before it is actually
released.
"Sustained release" generally refers to continuous or sporadic release of an
active
ingredient over an extended time after a single administration, whereby the
level of active
ingredient available to the host patient often is maintained at some constant
level over a period
of time. As used herein, it is also intended to cover the situation where the
release of an active
ingredient is controlled over a period at time wherein the level of active
ingredient available to
the host (bioavailable) may be at a variable but predetermined level at a
particular instant in time
of treatment.
The sustained release bioadhesive tablets known in the art can be generally
broken down
into two categories: (1) tablets consisting ofwater soluble carbomers, and (2)
tablets consisting
of insoluble polymers. Both types of tablets have proven unsatisfactory for
many applications.
For example, numerous artisans have attempted to formulate a suitable
sustained release
bioadhesive tablet from water soluble carbomers, such as carbomer 934P or
CARBOPOLT"" 974
resin (commercially available from B.F. Goodrich, Cleveland, Ohio). However,
such tablets
2 0 often are only able to adhere to the wall of a body cavity for short
periods of time, e.g., six hours
or less. Also, these tablets are easily dislodged from the wall of a body
cavity and thus place
patients using such tablets buccally at risk of asphyxiation. Furthermore,
these prior art tablets
inherently become hydrated relatively quickly and thus may prematurely expose
the reservoir of
-2-
CA 02341375 2001-02-21
WO 00/10536 PCT/US99/19260
active ingredient to degradation by moisture or by enzymes from the host
environment such as
from bacteria in the septic oral or vaginal cavities.
Similarly, tablets comprised of insoluble polymers, such as polycarbophil,
have proven
unsuitable for many applications. For example, although polycarbophil tablets
are capable of
prolonged attachment to the wall of a body cavity, such tablets do not adhere
immediately,
making them impractical for certain treatments such a buccal delivery of
active ingredients to
patients during sleep time hours. Further, such tablets often do not soften
sufficiently to provide
comfort and imperceptibility, or provide safety from potential aspiration of
the tablet.
Furthermore, for example, neither type of prior art tablet is particularly
suitable for
treating many conditions. As alluded to previously, there are numerous medical
conditions in
which a sustained and/or controlled release of active ingredients) is desired
for any of numerous
reasons including, for example, to alleviate the impact of first-pass hepatic
metabolism of the
active ingredient or the risk of premature degradation of the active
ingredient by moisture, pH
effects, or enzymes, or to attain the comfort and convenience offered by a
suitable bioadhesive
tablet. Such conditions include, but are not limited to, for example, those
needing treatment with
an active ingredient that may be, but is not limited to, a glycoprotein,
protein, sex hormone, anti-
hormone, nitrate, beta-agonist, beta-antagonist, opioid, opioid-antagonist,
antidepressant, HMG
CoA (3-hydroxy-3-methyiglutaryl Coenzyme A) reductase inhibitor,
antihistamine, ACE
(angiotensin converting enzyme) inhibitor, and/or prostaglandin. Heretofore
the art has required
2 0 such patents to undergo the more invasive and less suitable techniques and
methods of delivery
described above.
To illustrate the need in the art, consider hypogonadal men, for example.
Hypogonadism
in man is characterized by a deficiency or absence of endogenous testosterone
production.
-3-
CA 02341375 2001-02-21
WO 00/10536 PCT/US99/19260
Abnormally low levels of testosterone may place men at risk of "Andropause",
wherein men are
at greater risk of cardiovascular disease, Alzheimer's disease, and
osteoporosis.
Testosterone has traditionally been used to treat hypogonadal men. However, to
be most
effective, the treatment must be capable of complete physiologic testosterone
replacement.
Moreover, the treatment must be capable of providing sustained levels of
testosterone through
the night, preferably sustaining a peak in the middle of the night.
Transdermal testosterone
patches typically produce only sub-physiologic levels and thus incomplete
relief. Similarly, the
prior art buccal tablets hereintofore described would be ineffective or
impractical for such
sustained testosterone delivery.
The hormone testosterone, like many other drugs, including many other proteins
and
glycoproteins, undergoes high first pass metabolism. Accordingly, as will be
appreciated by one
of ordinary skill in the art, buccal or vaginal tablets consisting of
materials that are incapable of
keeping the interior reservoir of the tablet in the dry state for prolonged
periods are inherently
incapable of preventing dissolution and swallowing or dissolution and rapid
absorption through
the muscosa of the active ingredient. Furthermore, as will be appreciated by
one of ordinary skill
in the art, tablets which are unable to quickly adhere to the target area or
are able to become
dislodged are impractical for treatments which use night-time delivery, such
as testosterone
treatment.
Furthermore, as will be appreciated by one of ordinary skill in the art, the
advantages of
2 0 a sustained release, bioadhesive tablet according to the present invention
are not limited to the
treatment of hypogonadism in men. For example, patients often require
sustained release
hormone treatment for various conditions. In addition, other medications, such
as steroids for
treating such conditions as asthma, involve treatments where desired peak
levels are at night
-4-
CA 02341375 2005-06-07
50202-2
during sleep-time hours. Accordingly, one of ordinary skill
in the art will appreciate that there exists a long-felt,
yet unresolved, need to develop a bioadhesive, sustained
release tablet to overcome the aforementioned needs of the
art, including, but not limited to, the delivery of
therapeutically effective amounts of an active ingredient
which may be metabolized or otherwise degraded by moisture,
enzymes, or pH effects, such as glycoproteins, proteins, sex
hormones, anti-hormones, nitrates, beta-agonists, beta-
antagonists, opioids, opioid-antagonists, antidepressants,
HMG CoA reductase inhibitors, antihistamines, ACE
inhibitors, and/or prostaglandins.
SUMMARY OF THE INVENTION
In one tablet aspect, the invention provides a
sustained release, progressive hydration, bioadhesive
tablet, comprising: an active ingredient; about 5o to about
50o by weight cellulose; about 0.5% to about 25o by weight
starch; about 1% to about 50o by weight lactose; about 0.50
to about loo by weight water insoluble cross-linked
polycarboxylic polymer; and about 1% to about 75% by weight
water soluble polymer.
In a further tablet aspect, the invention provides
a sustained release, progressive hydration, bioadhesive
tablet, comprising: an active ingredient; about 2o to about
30o by weight binder; about 5% to about 40o by weight
lactose; about to to about 3o by weight water insoluble
cross-linked polycarboxylic polymer; and about 5% to about
50o by weight water soluble polymer.
In a still further tablet aspect, the invention
provides as sustained release, progressive hydration,
bioadhesive tablet, for delivering an active ingredient to
-5-
CA 02341375 2004-06-02
50202-2
the bloodstream of a patient to treat a health condition,
comprising a therapeutically effective amount of an active
ingredient, a water insoluble cross-linked polycarboxylic
polymer, and a water soluble polymer.
The invention also provides for the use of the
tablets of the invention for the treatment of a clinical
condition requiring the sustained release administration of
the said active ingredient.
The invention also provides a commercial package
comprising tablets of the invention and associated therewith
instruction for the use thereof for the treatment of a
clinical condition requiring the sustained release
administration of the said active ingredient.
The invention also provides use of an active
ingredient, a water insoluble cross-linked polycarboxylic
polymer and a water soluble polymer for the manufacture of a
progressive hydration, bioadhesive tablet for the treatment
of a clinical condition requiring the sustained release
administration of the said active ingredient.
The invention also provides use of an active
ingredient, from about 5% to about 50% by weight of
cellulose, about 0.5% to about 25% by weight of starch,
about 1% to about 50% by weight of lactose, about 0.5% to
about 10% by weight of a water insoluble cross-linked
polycarboxylic polymer and about 1% to about 75% by weight
of a water soluble polymer, for the manufacture of a
progressive hydration, bioadhesive tablet for the treatment
of a clinical condition requiring the sustained release
administration of the said active ingredient.
The invention provides a bioadhesive tablet that
adheres immediately or almost immediately to the target
-5a-
CA 02341375 2004-06-02
50202-2
tissue area of a body cavity and generally stays attached
substantially throughout treatment. In accordance, with
this aspect of the invention, there is provided a
bioadhesive tablet that can stay attached and deliver active
ingredients in the buccal cavity for as much as eighteen
hours or more. In accordance with a related aspect of the
invention, there is provided a bioadhesive tablet that can
stay attached and deliver active ingredients vaginally for
as much as 72 hours or more.
The invention provides a bioadhesive tablet that
progressively hydrates, whereby the inner core of the tablet
remains protected from moisture and the surrounding
environment. In accordance with this aspect of the
invention there is provided a bioadhesive tablet suitable
for sustained release use in mucosal and other body cavities
even with
-5b-
CA 02341375 2002-10-16
50202-2
active ingredients comprising proteins or glycoproteins or
other treating agents that are particularly susceptible to
metabolism, or to enzymatic, pH, or moisture-induced
degradation.
The invention provides a bioadhesive tablet having
both controlled and sustained release properties due to a
tablet formulation wherein the active ingredient is only
progressively made bioavailable over an extended time period
by the progressive hydration of the tablet's dry reservoir
of active ingredient.
The invention provides a bioadhesive tablet
according to the invention that also gelifies and/or swells
to help protect a patient using the tablet buccally from
asphyxiation, particularly a sleeping patient undergoing
treatment.
The invention provides methods of making
bioadhesive tablets in accordance with the aforementioned
aspects of the invention. In accordance with one aspect of
the invention, there is provided a method of making
bioadhesive tablets wherein an active ingredient resistant
to premature metabolism and/or degradation is added in the
first and/or second step (manufacture of granulate). In
accordance with a related aspect of the invention there is
provided a method of making bioadhesive tablets wherein an
active ingredient prone to premature metabolism and/or
degradation is added in the second step (manufacture of the
tableting mixture) after the granulate is dried and sieved.
Of course, other concerns or factors may affect the choice
of which step or steps are appropriate for adding a
particular active ingredient.
6
CA 02341375 2002-10-16
50202-2
The invention provides methods of using
bioadhesive tablets as described herein. In accordance with
one aspect of the invention, there is provided a method of
using a bioadhesive tablet to administer to a male patient a
sustained release of testosterone. In accordance with a
related aspect of the invention, there is provided a method
of using a
6a
CA 02341375 2002-10-16
50202-2
bioadhesive tablet to administer to a female patient a sustained release of a
hormone, such as
testosterone.
The inventors ofthepresentinventionhave discovered, quiteundly, that these and
other aspects for theinve~tionmaybe achievedbymaking andusingtablets
comprising an active
ingredient, water soluble polymers (e.g., CARBOPOLr" 974P), and insoluble
polycarbophil
(e.g., NOYEON~, available from B ~. Goodrich Specialty Polymers of Cleveland,
Ol~, and
preferably hydroxypropyhnethyl cellulose (I~MG~, lactose, corn starch and
other standard
tablets ingredients, such as magnesium steuatc and talc.
Bioadhesive, progressive hydration tablets according to the invention may be
used with
any suitable active ingredient and may be used to deliver a therapeutic amount
of the active
ingredient to a patient at controlled rates for sustained periods of time.
Tablets according to the
invention may also be constructed in any suitable shape and any suitable size
consistent with the
intended therapeutic use of the tablet.
Tablets according to the invention may comprise any suitable amount of active
~~'~~L S~~ble amounts of active ingredient according to the invention are may
be from
minuscule amounts of active ingredient to about 50%, or more, by weight active
ingredient. As
will be appreciated by one of ordinary skill in the art, "minuscule amounts"
is intended to cover
those amounts of active ingredient that are disproportionately smal! relative
to the tablet, for
example, when only a few micrograms of active ingredient are to-be delivered
via a tablet
2 0 weighing over a hundred milligrams. Accordingly, one of ordinary skill in
the art will appreciate
that any amount of active ingredient; in any ratio, is within the scope of
tire present invention.
7
CA 02341375 2002-10-16
50202-2
The balance of the tablet according to the
invention may comprise water soluble polymers) and water
insoluble cross-linked polycarboxlyic polymer(s). Also,
according to the invention, exemplary tablets preferably
have between about 1% and about 75% by weight water soluble
polymer and between about .5% and about 10% by weight water
insoluble cross-linked polycarboxylic polymer. In
accordance with the invention, such exemplary tablets also
preferably include between about 5% and about 50% cellulose.
Also in accordance with the invention, presently preferred
tablets may have between about .5% and about 25% by weight
starch. These preferred tablets may also have between about
1% and about 50% by weight lactose.
Furthermore, according to the invention, preferred
tablets may comprise from about .O1% up to about 2% silica;
and/or up to about .5% by weight talc; and/or up to about
2.5% by weight magnesium stearate.
Accordingly, one of ordinary skill in the art will
appreciate that the components of the tablets can be varied
to suit a particular purpose. For example, the inventors of
the present invention have discovered that one way of
increasing (decreasing) the time it takes a progressive
hydration tablet to hydrate is by increasing (decreasing)
the amount of lactose and/or starch and decreasing
(increasing) the amount of water soluble polymer.
Alternatively, the density of the tablet may be altered to
affect the hydration period.
Active ingredients suitable for use in the present
invention include any active ingredient or ingredients
requiring sustained or controlled release, any active
8
CA 02341375 2002-10-16
50202-2
ingredient or ingredients requiring extended protection from
premature degradation of the active by moisture, pH effects,
or enzymes, or any active ingredient requiring
administration to a patient with protection from first-pass
hepatic metabolism. Exemplary active ingredients suitable
for use with the present
8a
CA 02341375 2001-02-21
WO 00/10536 PCTNS99/19260
invention include, but are by no means limited to: ( 1 ) glycoproteins, such
as follicle-stimulating
hormone (FSH), luteinizing hormone (LH), human chorionic gonadotropin (HCG),
thryoid-
stimulating hormone (TSH), and the like; (2) proteins, such as GnRH (agonist
and antagonist),
oxytocin analogs, somatostatin analogs, tissue plaminogen activator (TPA),
growth hormone
releasing hormone (GHRH), corticotropin-releasing hormone analogs (CRH
analogs), and the
like; (3) sex hormones, such as estradiol, testosterone, progesterone, and the
like; (4) anti-
hormones, such as tamoxifen, mifepristone, and the like; (5) nitrates, such as
nitroglycerin,
isosorbide, erythrityl tetranitrate, pentaerythritol tetranitrate, and the
like; (6) beta-agonists, such
as terbutaline, albuterol, pirbuterol, bitolterol, ritodrine, and the like;
(7) beta-antagonists, such
as propranolol, metoprolol, nadolol, atenolol, timolol, esmolol, pindolol,
acebutolol, labetalol,
and the like; (8) opioids, such as morphine, hydromorphone, oxymorphone,
codeine,
hydrocodone, oxycodone, leverophanol, levallorphan, buprenorphine, fentanyl,
nalbuphine,
butorphanol, pentazocine, and the like; (9) opioids-antagonists, such as
naloxone, nalmefene, and
the like; (10) antidepressants, such as amitriptyline, amoxapine, desipramine,
doxepin,
imipramine, maprotilen, nortriptyline, protripyline, trimipramine, fluoxetine,
trazodone, and the
like; ( 11 ) HMG CoA reductase inhibitors, such as lovastatin, mevastatin,
simvastatin,
pravastatin, atorvastatin, and the like; (12) antihistamines, such as
loratadine, chlorpheniramine
maleate, brompheniramine maleate, diphenhydramine, dimenhydrinate,
carbinoxamine,
promethazine, tripelannamine, and the like; (13) ACE inhibitors, such as
captopril, enalapril,
2 0 lisinopril, and the like; and, (14) prostaglandins, such as misoprostol
and the like. Accordingly,
one of ordinary skill in the art will appreciate that tablets according to the
invention may be used
with a wide variety of active ingredients to treat a wide variety of
conditions.
_g_
CA 02341375 2004-06-02
50202-2
The aforementioned and other aspects of the invention will become more clear
by
reference to the Figures and descriptions of preferred embodiments.
BRIEF DESCRIPTION OF THE DRAWIrIGS
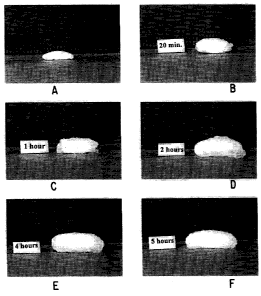
Figure 1 is a series of photographs depicting the progressive hydration of a
bioadhesive tablet according to the invention.
Figure 2 is a flowchart depicting a presently preferred method of making
bioadhesive tablets according to the invention.
Figures 3 to 6 show the data of Tables 2 to 5 for Formulae 1, 3, 5 and 6.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
A presently preferred embodiment of the invention is depicted in Figure 1. As
shown
in the first-frame of Figure 1, before the tablet is administered all of the
active is in the dry
state and thus, not subject to the deleterious action of moisture, pH effects,
enzymes or other
chemicals. It is also not available for absorption (bioavailable). As shown in
frames 2-6 of
Figure 1, over time the residual portion of the active remains in the dry
state which both
protects it from water and the immediate environment as well as allowing it to
serve as a
reservoir for the sustained and controlled release of the active. Such a
delivery system is well
suited for the delivery of proteins, glycoproteins, and other drugs which must
be protected
from metabolism or during prolonged administration from enzymatic, pH, or
moisture-
induced degradation.
2 0 In a preferred embodiment, when used buccally, progressive hydration of
the
bioadhesive tablet protects the patient, should the tablet become dislodged,
by gelifying and
becoming heavier and thus less likely to float in the airway, risking
aspiration. This makes
-10-
CA 02341375 2001-02-21
WO 00/10536 PCT/US99/19260
this embodiment particularly well suited for agents that should reach their
peak levels in the
middle of the night, e.g., hormones like testosterone or steroids to treat
asthma. According to
the invention, the hydration of the tablet can preferably take hours (e.g. I2
to 24 hours) when
formulated for buccal tablets or even days when formulated for vaginal use. As
will be
appreciated by one of ordinary skill in the art, prior art bioadhesive tablets
do not protect the
active ingredient from moisture, pH, or from enzymes produced by bacteria in
the septic oral
and vaginal orifices.
Furthermore, as will be appreciated by one of ordinary skill in the art
following the
teaching of the present application, the tablet can be sized shaped and dosed
to meet the needs
of the particular treatment being undertaken. For example, the buccal
bioadhesive tablet
depicted in Figure 1 was constructed to be only 9mm in diameter for the
comfort of the
patient, but made capable of delivering 7mg of testosterone per day, full
physiologic level.
By contrast, prior art transdermal patches were only capable of delivering Smg
per day, in
other words a sub-physiologic level.
A presently preferred method of manufacturing bioadhesive tablets is diagramed
in
Figure 2. The presently preferred method involves three steps as described
below:
1. First step: manufacture of the granulate.
Hydroxypropylmethyl cellulose 15 000(=HPMC 15 000) is mixed with corn starch
and lactose and in case of an active ingredient non sensitive to moisture the
active is added.
2 0 The mixture is wet with an aqueous solution of hydroxypropylmethyl
cellulose 5 (=HPMC S}
and knead/granulated.
-11-
CA 02341375 2001-02-21
WO 00/10536 PCT/US99/19260
The granulate is dried in an oven under warm air (50°C) until moisture
content is less
than 2.5%
The dried granulate is broken with a stainless steel sieve oscillating
granulator mesh
size 1000 ~cm.
2. Second step: manufacture of the tableting mixture. Talc, silicon dioxide
magnesium stearate, and in a case of an active sensitive to moisture, the
active ingredient is
added. All is sieved through a sieving machine having aperture size 500 ~cm
and then
transferred into a free-fall mixer.
Addition of the granulate of step 1, followed by polycarbophil, carbomer and
lactose.
The whole is mixed until homogenous.
3. Third step: tableting
The tableting mixture is compressed into tablets by means of a rotative
tableting
machine equipped with punches 9mm flat on the upper side and curved (r=9mm) on
the lower
side both with beveled edge. The tablets are dedusted and packed.
As depicted in Figure 2, an active ingredient that is non-sensitive to
moisture is
preferably added during the manufacture of the granulate. However,
alternatively, the active
ingredient can be added during the second step after the granulate is dried
and sieved. Also,
as will be appreciated by one of ordinary skill in the art, this second method
is particularly
preferred when the active ingredient is sensitive to moisture.
2 o In a presently preferred manufacturing process, the active ingredient is
preferably
protected from moisture. A wet granulation is made of lactose, corn starch and
HPMC.
-12-
CA 02341375 2004-06-02
50202-2
Testosterone, polycarbophil, carbomcr 934P, talc and magnesium stcarate are
addod dry for
the final coon.
Furthermore, as w71 be appreaatcd by oae of ordinary sla'Il in the art
following the
teaching of the prGSent application, the mate~als of construction caa be
varied to optr~e the
desired charactaristics of the tabld. For example, tho prat inve~ors have
disoovaed that
by progressively increasing the amount of lactose and oonz starch and
progressively decreasingthe amount of carbomer 934P, the amount of time it
takes a tablet to
hydrate is progressively increased. Accordingly, as will be appreciated by one
of ordinary
skill in the art, tablets suited for specific treatments ('~.e., specific
alive, speci5c aos~
speci5c delivery time) can be manufact~d.
These and other aspects of the invention may be more clearly shown by way of
example_
EXAMPLE: TESTOSTERONE TABLET
The following is an example of a formulation (Formulation 8, batch #0002990 .
designed for complete physiologic replacement of testosterone in men:
Testosterone 30.000 mg 24.0~/0
HPMC 26250 mg 21.f'/.
Corn Starch 22.500 mg ' 18.0~/0
Monohydraied Lactose 30.125 mg 24.1%
S~ica 1.250 mg 1.0'/0
Polycarbopbil (Novcon) 3.125 mg ZS%
Carbomer 974P 9375 mg 7.5%
-13-
50202-2
CA 02341375 2004-06-02
Talc 1.500 mg 1.2%
Magnesium stearate 0.875 mg 0.7%
Formulations like the one above produced sustained release in in vitro
dissolution
tests. When used in female subjects formulas Iike this one also produce a
sustained and
controlled release of testosterone for 12 hours or more.
Table 1 depicts nine different fonmulations of bioadhesive tablets according
to the
invention. The active ingredient, testosterone, was held constant at 30.0 mg
(24% by weight)
so the effect of varying the proportions of the inactive ingredients could be
studied.
The testosterone dissolution rates of selected formulations were.then studied.
Table 2
depicts the testosterone dissolution rate of six tablets selected from Formula
1, batch
#0069904. Table 3 depicts the testosterone dissolution rate of six tablets
selected from
Formula 3, batch #0049904. Table 4 depicts the testosterone dissolution rate
of six tablets
selected from Formula S, batch #0029904. Table 5 depicts the testosterone
dissolution rate of .
Fonmula 6, batch #0019904.
The dissolution rate data was then graphed to illustrate the percent of
testosterone
released per hour. Figure 3 depicts the testosterone release rate for Formula
1 (see
Table 2). Figure 4 depicts the testosterone release rate for Formula 3 (see
Table 3).
Figure 4 depicts the testosterone release rate for Formula 5 (see Table 4).
Figure 6 depicts
the testosterone release rate for Formula 6 (see Table 5).
-14-
CA 02341375 2004-06-02
50202-2
As shown in the Figures and tables, by decreasing
the amount of lactose and corn starch and increasing the
amount of carbomer 974P, the time it takes for the tablet to
hydrate is progressively decreased. Formulation 1 (0069904)
and others like it with high levels of carbomer 974P and low
levels of lactose and corn starch are probably best suited
to buccal administration where 12 hours of delivery is
usually sufficient. In the first example given above
Formulation 8 (0029906), where the levels of lactose and
corn starch are high and carbomer 974P is low, the formula
is probably better suited for vaginal administration where
release is often required over a period of days.
As will be appreciated by one of ordinary skill in
the art, the examples and preferred embodiments are not
intended to be limiting, and the invention applies to
tablets comprised of any active ingredient and any
combination of tablet materials. Furthermore, as will be
appreciated by one of ordinary skill in the art, the
invention is intended to cover the methods of manufacturing
and therapeutic uses of the aforementioned tablets.
-15-
CA 02341375 2001-02-21
WO 00/10536 PCT/US99/19260
S ~ S N n
4 x N N ~ ~ r N
IA r
O
1 O
~~~~ _
Pf r 19 f0 1f1
r O
r~~~J~N
N
~ N
41 x NN~NrNArO S
O O ~ ~ r ~ N A _
~ A pp
~L E ~ O ~.~ ' r O
M N O
PI 01 r p
N
O ~ ~ ~ ~ ~
A
A Y' N N ~ ~ ~-
!V O O O
r
O ~ O 10 IA O
t 1n
Y1 N ~ A W
L E N N ~- h aD
aD ~
N ~ N r ~ r
C O
N
O ~ ~ ~ ~ Ip p
~ ~
0
~ V ~ 01 r n
N
tD f O
IL
~ tN0 m m
N ~ N r ~ ~ N
O O
N
O n
h
N N ~ ~ r N
~ O O
n
p
O O ~. O
!L ~ ' 10 t p
fp N f~ ID
aD
POf~~Nre~a'Op
N_
A ~
r
C x ~ ~ w n ~"'
O Y N O O O
N N
h ~ ~ g ~ ~ ~ H M
O h h LLJ
~ h. .1~
N
N ~ w r O
n n
N N ~ ~ r N
N C O
O Q ~ A t~
1~ '
4, t~ N p
N a0 aD
~~~"' -o
c m
ve N
i
n n p
r N ~ O O O
N O
1f7 O 10 II1 Op
1A
1~ ~f1 ~ ~
f~ A
y, a0 N 1l7 CD p
a0
O C
'O
0
A r ~ lyf
~ N
l
,~ c
9
~ O ~ ~ 10~ O
n
ie . O
N. N N m r
AI O O
O
1A O IA o In O
IA
A
A
N
~
Oa~D
n P
a
'f.N"
"
Op
N
~~~
~ O
t~i~l N
.
O
m
7
m
r0
~~
_
m
m ~ N
L p
O!
d m
m ~ y
N 3
~ o d
V '~
o
s
a o o '
~
m o
-~
~ ~~ ~
i--xcWn
ac~r~
- 16-
SUBSTITUTE SHEET (RULE 26)
CA 02341375 2001-02-21
WO 00/10536 PCT/US99/19260
0
o N
W
J
H
m
t.
4
t4
i
r
G_~
P
0
C
d
C
1
1
0~
O
SUBSTITUTE SHEET (RULE 26)
- 17-
CA 02341375 2001-02-21
WO 00/10536 PCT/US99/19260
~d
p
a
H_
Ca
M
O
W
m
H
a
H
0.
O
V
t~
A
i1
L
C
a
s.
~E
a.
3~
o.
- 1$ _
SUBSTITUTE SHEET (RULE 26)
CA 02341375 2001-02-21
WO 00/10536 PCT/US99119260
F
a~
x
O
W
J
a
0
0
- 19-
SUBSTITUTE SHEET (RULE 26)
CA 02341375 2001-02-21
WO 00/10536 PCTNS99/19260
G
O
A
m
O.
H
r
r
a
8
-20-
SUBSTITUTE SHEET (RULE 26)