Note: Descriptions are shown in the official language in which they were submitted.
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
SOMATOSTATIN RECEPTOR RADIOLIGAND WITH INCREASED UPTAKE
The invention relates to a radioligand, its use as a
pharmaceutical (therapeutic or diagnostic) and a method for
the detection of peptide receptors in cell material.
Radiolabelled compounds are being used in modern
medicine for both diagnostic and therapeutic purposes. In the
case of radionuclidetherapy, the radioactive compound is used
to treat, for instance, tumours or other groups of
malignancies or unwanted cells. In diagnostic methods
radioactive compounds are being used to visualise certain
parts of the body or specific tissues, for instance bones, or
again, a tumour.
One of the disadvantages of radiolabelled compounds
is the radiotoxicity of these compounds for normal tissue.
The selective interaction of radioactive compounds is
expressed in the target/non-target ratio of these compounds.
In case of a high target/non-target ratio, the radiotoxicity
of the radioactive compounds can increase to an undesirable
high level, too high for normal and healthy tissue
Minimisation of radiotoxicity and/or exposure of tissue to
radiation is therefore an ongoing incentive for research in
this area. Minimisation can, in general, be accomplished by
using smaller doses of radioactive compounds and/or by using
more effective radioactive compounds. Effectiveness of a
radioactive compound used for these purposes regards two
aspects. One aspect is the characteristics of the radioactive
compound in relation to its purpose (diagnosis or therapy).
Half-life and emitted energy are the main features. The other
aspect is concerned with the characteristics of the
radioactive compound in relation to the ability to express
its activities at a specific location, for instance a tumour
or a specific cell-type.
A disadvantage related to the second aspect of the
use of radiolabelled compounds is that the radiolabelled
compound will spread to a certain extent throughout the
entire body or material causing a lower target/non-target
ratio. A low target/non-target ratio (e. g. tumour/non-tumour
ratio) may result in a smaller therapeutic window.
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
2
It is a significant advantage if the uptake of
radiolabelled compounds by the targeted tissue, whether a
tumour or otherwise, for diagnostic or therapeutic purposes
is increased and the target/non-target ratio and/or
therapeutic window is enhanced.
The radiolabelled compounds which are used for the
diagnostic and therapeutic purposes generally comprises a
ligand which contains a radiolabel.
The radiolabel is usually a radionuclide specifically
selected for the purpose. In case of diagnosis, in general
radionuclides with relative low radiotoxicity can be used,
the most important criterion is the detection of the nuclide.
This can be accomplished with radiolabels with a lower energy
over a larger distance such as y-emitter or low energy
emitters. For radiotherapy nuclides are needed for which an
important criterion is the ability to express anti-
proliferative activities in a tumour, e.g. attack the DNA of
a tumourcell. In general this requires radiolabels which emit
particles with higher energy over a relative small distance.
Particles which have such high Linear Energy Transfer are a-
emitters or high energy p-emitters.
The radioligand in general consists of a part which
associates with parts of tissue or cell material by, for
instance, binding to a substance associated with a cell
surface such as a receptor. By carefully selecting the
characteristics of the binding part, selective labelling of
proteins, receptors, tissue etc. is achieved. The radiolabel
coupled to the binding part subsequently provides the
detectability or the therapeutic effect of the radioligand.
The radiolabel can be detected using conventional techniques.
One of the techniques which uses this concept is
peptide receptor scintigraphy. Peptide receptor scintigraphy
is a technique in which a receptor for a peptide is detected
by contacting the receptor with a radiolabelled peptide or
analogue of the peptide, which radiolabelled peptide or its
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
3
analogue is capable of binding to the receptor. The labelled
peptide is subsequently detected by scintigraphy.
Peptide receptor scintigraphy with radiolabelled
peptides or analogues is a sensitive and specific technique
to show in vivo and in vitro the presence and abundance of
peptide receptors in cell material.
Mammalian tissues containing (peptide) receptors can
be visualised or treated using this technique. For instance,
many endocrine tumours express somatostatin (SS) receptors
(SSR)(Reubi et al. 1992). The presence of a high density of
SSR on these tumours has been the basis for the successful
development of the technique of SSR scintigraphy.
In this method an SS-analogue carrying a Y-emitting
radiolabel is injected. The SS-analogues will subsequently
bind to the SSRs. In this way a radiolabel can be targeted to
tissue having SSRs. Because of the presence of the radiolabel
the tissue can be visualised using a y-camera (Krenning et
al. 1993). By this technique the SSR-positive tumours and
their metastases can be localised.
One of the known SS-analogues is octreotide~ (OCT,
Formula 1).
D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr(ol)
Formula 1: Octreotide~ (OCT).
Radioiodinated OCT derivatives are in general
attractive radioligands for use in nuclear medicine. The
short retention time of radioactivity following the
internalisation of radioiodinated OCT derivatives by such
tumours is a serious drawback in this respect. It has been
found (Breeman et al. 1998) that the retention time of
radioactivity is increased in tumours by administration of
radioiodinated OCT analogues derivatised with a DTPA
(Diethylene TriaminePentaacetic Acid) group. When comparing
[1251-D-Tyrl]OCT [DTPA~, 1251-D-Tyrl]OCT [1251-Tyr3]OCT and
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
4
[DTPA~ 1251-Tyr3]OCT, the last one was found to have the
greatest retention time in OCT receptor positive tissue.
Unfortunately, the kidney to tumour ratio was also very high,
which seriously hinders the potential application of [DTPA~,
1251-Tyr3]OCT in peptide receptor radionuclide therapy of OCT
expressing tumours.
An important disadvantage of the currently known
radiolabelled chelated peptides is, as addressed earlier,
that the increased uptake in the target-organ or -tissue, is
combined with a corresponding uptake in the kidneys. The
maximum level of radiolabelled compounds in the kidneys
determine the therapeutic window wherein the compounds can be
applied. A high kidney-uptake therefor limits the window
wherein these peptides can be used.
Surprisingly, we have now found a group of compounds
which, when coupled to an SS or -analogue, result in a
radioligand with an increased internalisation rate. The
internalisation rate is the relative amount of the
radioligand which is incorporated in the cell instead of
remaining bound to the receptor on the cell membrane. For
instance in nuclear therapy, a radioligand with an increased
internalisation rate will transport the radioactive compound
closer to the nucleus of the cell (increasing the target/non-
target ratio) and hence be more effective in its anti-
proliferative effects. The coupling of such a compound to an
SS-analogue does not prevent the internalisation of OCT after
binding to SSR. The OCT analogues retain an intact high
affinity binding characteristics to the somatostatin
receptor.
The invention accordingly comprises a peptide capable
of binding to a receptor wherein the peptide is coupled to at
least one chelating agent, which chelating agent is not
coordinated to a metal ion under physiological conditions.
An important advantage of the present invention is
that the radioligand according to the invention not only has
an increased uptake in the receptor positive tissue but also
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
expresses a lower uptake in the kidney. This results in an
increased tumour/kidney ratio and hence in a larger window
for the application of these peptides.
The in vivo uptake of radioactivity in somatostatin
5 receptor positive tissues at several time points after the
injection of the radioligand also depends on its rate of
metabolism, locally as well as in the kidneys and liver which
are major clearance sites for the clearance of OCT
derivatives. The radioligand according to the invention
therefore has an increased uptake and an improved
tumour/kidney ratio. Without being bound by theory, the
chelating agent bond to the ligand according to the invention
is thought to interfere with the degradation of the OCT
analogues by peptidases and to induce the accumulation of the
radioligand or its metabolic products in tissue or
cellmaterial.
A preffered chelating agent according to the
invention.is DOTA (Tetraazacyclododecane tetraacetic acid ).
Chelating agents in general are organic compounds
with the capability of capturing (ir)reversibly metal ions.
Usually these compounds have coordinating atoms or groups in
their structure such as N, O, S, P, acid groups (GOO-), ester
groups (COOR) and the like. In order to bind the positive
metal ions, these coordinating atoms or groups carry negative
charges (COO-)or are free-electron donors (N~). Chelating
agents are known in many varieties such as crown ether, crown
thioethers, EDTA (Ethylene diaminetetraacetic acid) and many
others. For the purpose of the present invention a chelating
agent which is coordinated or bound to a metal ion is
considered 'full', whereas a chelating agent which is not
bound to a metal ion is called 'empty'. For the purpose of
the invention a 'empty' ligand means that a charge remains on
the chelating agent.
The receptor mediated uptake of the radioligands is
favoured by the presence of the 'empty' chelating agent. The
empty chelating agent may be able to influence the charge
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
6
distribution of the a-amino acid to which the chelating agent
is bound. The charge present at the a-amino acid can be
neutralised, delocalised or dissipated by the chelating
agent.
Accordingly, another aspect of the invention relates
to a radioligand comprising a peptide capable of binding to a
receptor wherein the peptide is coupled to a compound which
can neutralise, delocalise or dissipate positive or negative
charges in the peptide.
In a different embodiment of the invention the
peptide can also be coupled to a succinimide-containing group
or the like. The succinimide group is an example of a charge
dissipating group.
In another embodiment of the invention a spacer is
positioned between the peptide and the ligand. The spacer
itself can also attribute to the charge dissipating,
delocalising or neutralising effect. As a spacer any compound
known in the art will suffice.
In another embodiment of the invention, a toxin is
coupled to the peptide. The toxin can then be transported to
the receptor. In this way the toxin can be selectively
delivered. Possible toxins are small toxins, such as
doxorubicine, vincrystine, vinblastine, adriamycine and the
like. A preferred embodiment of the invention is one wherein
the receptor is a peptide binding receptor, preferably a
tumour associated receptor.
In principle any receptor will suffice. The
requirement for the receptor is mostly based on the presence
of the receptor in the targeted organ or tissue and that the
receptor can be sufficiently labelled to allow for the
detection or treatment of the tumour.
Without being bound by theory, the difference in
behaviour can be thought to arise from a difference in
affinity for metals. DTPA has a very high affinity for a
widespread range of metal ions, which it will bind with a
high rate and a high yield. DOTA is a preferred chelating
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
7
agent because of the different affinity for metal ions. DOTA
expresses more selective binding characteristics and only
under enforced conditions, such as increased temperatures, is
the ligand coordinating towards metal ions. It is therefore
likely that after administration originally empty DTPA
becomes full by coordination or binding to metal ions present
in the tissue or body such as for example Ca2+, Mg2+ and the
like.
The peptide according to the invention can be any
peptide capable of binding to its receptor. With a
radioligand according to he invention the receptor can be
selectively labelled. The peptide having affinity for a
receptor is preferably a peptide receptor in a tumour, more
preferably a somatostatin receptor.
The peptide according to the invention can also
comprise peptides such as adrenocorticotrope hormone (ACTH),
Molecular recognition units (MRU), Gonadotropine releasing
(GnRH), Bombesine (gastro-releasing hormone),
cholesystokinine (Substance-P) and the like and analogues
thereof.
According to the invention, the radioligand has not
only an enhanced internalisation and/or binding rate with
somatostatin receptors but also in other receptors, for
instance those for the peptides mentioned above.
A preferred embodiment of the invention is therefor one
wherein the peptide contains between about 6 and about 40
aminoacids. The lower limit is determined by the minimal
desired selectivity of the peptide for the receptor. A more
preferred embodiment of the invention is one wherein the
peptide is somatostatin or a somatostatin analogue,
preferably wherein the somatostatin analogue is octreotide or
analogues thereof, preferably tyrosine-octreotide, more
preferably 3-tyrosine-octreotide.
To impart detectability or a therapeutic effect of the
radioligand, a radiolabel is coupled to the peptide. The
replacement of Phe3 by Tyr in OCT allows the radio-iodination
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
8
of the phenolic group of the molecule and results in the
1251-labelled radioligand [DOTA~-125I-Tyr3]OCT.
Other radiolabels are also suitable for imparting
radiodetectability or therapeutic effects. A preferred
embodiment of the invention therefore is a radioligand
wherein the radiolabel is selected from 123I~ 125I~ 2ilAt.
Chelating agents can also be coupled to other
positions at the peptide. These chelating agents are coupled
independently from the substituent at the a-position and thus
provide alternate positions for the incorporation of other
radiolabels than iodine or the like, such as ~i-emitters
(Yttrium-90) or Auger-electron emitting isotopes, which make
it possible, depending on the specific characteristics of the
radiolabel, to create radiolabelled radioligands with an
increased uptake, which are applicable in radiotherapy.
Thus another embodiment of the invention is a
radioligand wherein two or more chelating agents are coupled
to the peptide. One of the chelating agents is empty and
coupled to the a-position, the other is coupled at any other
position of the peptide and can contain a radiolabel.
A preferred embodiment of the invention is therefor a
radioligand wherein two or more chelating agents are coupled
to the peptide wherein one of the chelating agents contains a
radionuclide.
In a preferred embodiment of the invention the
radioligand encompasses a radiolabel suitable for diagnostic
or therapeutic purposes. The invention accordingly
encompasses compositions which are suitable as a
pharmaceutical and/or a diagnostic.
By using compounds with an increased internalisation
rate according to the invention the detection of somatostatin
receptors and the like can be achieved with a higher signal
to noise ratio.
An aspect of the invention therefor comprises an
method for the detection of somatostatin receptors in cell
material by contacting said cell material or tissue with a
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
9
composition comprising the radioligand according to the
invention. An embodiment of the invention comprises also a
kit for the detection of somatostatin receptor positive
material in vivo or in vitro. The invention is further
illustrated by the examples without limiting the scope of the
invention.
Materials and methods
Abbreviations: DMEM, Dulbecco's Minimal Essential Medium;
FCS, foetal calf serum; MEM, Minimal Essential Medium.
Compounds: [Tyr3]OCT and [DTPA~,Tyr3]OCT were synthesised
according to the solid phase method using Fmoc-Threoninol
attached to Rink acid resin (Schmidt et al. 1997). The
corresponding DTPA-derivatives were synthesised using
tributyl-DTPA as the acylating agent by solid phase method
(Srinavasan et al. 1997). [DOTA~,Tyr3]OCT was synthesised by
an analogous route (Desphande et al., J. Nucl. Med. 31, 1990,
473-479; Otte et al. Eur. Journ. Nucl. Med. 1997, 24, 792-
795) .
125I-labelling of the SS-analogues was performed as
described (Bakker et al. 1990). OCT (octreotide } was
obtained from Sandoz (Basel, Switzerland, Pertussis toxin
(PT) and phenyl arsine oxide (PAO) were purchased form Sigma
(St. Louis, USA).
Cell culture: AtT20 mouse pituitary tumour cell were cultured
in DMEM with 10% foetal calf serum as described (Hofland et
al. 1995). Human insulinoma cell were dissociated with
collagenase and cultured in MEM with 10% FCS as described
(Lamberts et al. 1990).
Internalisation studies: The cells were washed twice with
internalisation medium (DMEM supplemented with HEPES (30 mMj,
L-glutamine (2 mM), sodium pyruvate (1 mM), penicillin (105
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
U/L), fungizone (0.5 mg/L) and 0.2~ bovine serum albumin(
Fraction V, Sigma Chemical Co., St. Louis and allowed to
adjust to the medium for 1 h at 37 °C. Approximately 200.00
cpm radioligand (0.1 nM final concentration) were added to
5 the medium and the cells were incubated at 37 °C for a period
up to 4 h in quadruplicate without or with excess unlabelled
OCT (100nM) to determine non-specific internalisation.
Treatment of the cells with low pH was used to distinguish
between surface bound (acid-releasable )and internalised
10 (acid resistant) radioligand. Controls for the
internalisation experiments have been described in detail in
(Hofland et al. 1995). SSR binding studies on membrane
homogenates of AtT20 cells were carried out as described in
detail previously in (Hofland et al. 1995).
Animal studies: The in vivo studies of tissue
distribution of radioactivity after the injection of 2 MBq
(0.5 ~tg) of the radioiodinated compounds were carried out in
male Wistar rats as described in detail previously (de Jong
et al. 1997). Rats (n=3 per treatment group) were sacrificed
at several time points after the injection of the
radioisotopes and radioactivity in the pituitary, pancreas
and adrenals was measured in a y-counter. Animals were kept,
treated, and cared for in accordance with the guidelines
approved by the European Community on November 24, 1986.
Analysis of Data: Statistical analysis of data was
performed using one way analysis of variance (ANOVA),
multiple comparisons were made by the Newman-Keuls test. Data
are expressed as mean ~ SE.
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
11
Results
Saturation experiments revealed that [1251-Tyr3]OCT,
[DTPA~~125I-Tyr3]OCT and [DOTA~~125I-Tyr3]OCT bound with
comparable high affinity (Kd-values of 0.2, 0.2,and 0.3 nM,
respectively) to membrane preparations of AtT2o cells.
The three radioligands were internalised by AtT20
cells in a time-dependent manner (Figure 1). In comparison
with [1251-Tyr3]OCT and [DTPA~,125I-Tyr3]OCT, [DOTA~,125I-
Tyr3]OCT was internalised in a significantly higher amount
(approximately 5-fold higher after 4h of incubation, p<0.01
at all time points studied). Internalised radioactivity
dissociated rapidly from the cells to 62'; .24 and 40% of
radioactivity t=0 min, after 4 h.
In agreement with the high internalisation rate of
[DOTA~,125I-Tyr3]OCT, human insulinoma cells also
internalised [DOTA~,125I-Tyr3]OCT in a significant (p<0.01)
higher amount (6.7-fold) in comparison with [DTPA~~125I-
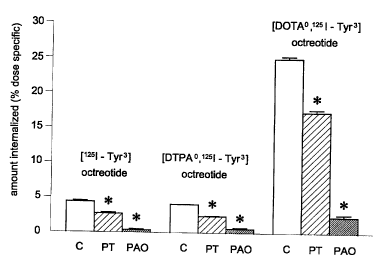
Tyr3]OCT (Table 1). PT or PAO (an inhibitor of the process of
receptor mediated endocytosis) (Gibson et al. 1989, Nouel et
al. 1997) significantly reduced the amount of internalisation
of the three radioligands to the same extent, demonstrating
that all three compounds are internalised by a receptor
mediated process (Figure 2).
Consonant with the high amount of internalisation of
[DOTA~,125I-Tyr3]OCT by AtT20 and human insulinoma cells a
considerable higher uptake of this radioligand was found in
SSR-positive organs in vivo in rats. Figure 3 shows that
uptake, 4 h post-injection, of [DOTA~,125I-Tyr3]OCT was
significantly higher (p<0.01) in comparison, with that of
[1251-Tyr3]OCT and [DTPA~~125I-Tyr3]OCT in the pituitary (10-
fold), pancreas (5-fold} and adrenals (8-fold). Also inline
with the in vitro studies is the rapid dissociation of
radioactivity from these organs.
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
12
Table 1. Internalisation of [DTPA~,125I-Tyr3]OCT and by
[DOTA~,125I-Tyr3]OCT a primary culture of human insulinoma
cells.
radioligand amount internalised
(% dose specific f SE)
[DTPA~,125I-Tyr3]OCT 0.09 ~ 0.00
[DOTA~,1251-Tyr3]OCT 0.60 ~ 0.04a
a' p<0.01 vs [DTPA~,125I_Tyr3]OCT. Incubation time:
4h; n=4 wells per treatment group; 106 cells per well. Value
in bracket represents the fold-difference with [DTPA~,125I-
Tyr3][125I-Tyr3]OCT.
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
13
Description of the Figures:
Figure 1: Time dependent internalisation of [1251-Tyr3]OCT
(0) , [DTPA~,125I_Tyr3] OCT (O) , and [DOTA~,125I_Tyr3]OCT (0)
by mouse AtT20 pituitary tumour cells.
Figure 2: Effect of pertussis toxin (PT) and phenyl arsine
oxide (PAO) on the internalisation of [1251-Tyr3]OCT,
[DTPA~,125I-Tyr3]OCT, and [DOTA~,125I_Tyr3]OCT by mouse AtT20
pituitary tumour cells. PAO (10~M) was added simultaneously
with the radioligand, while the cells were pre-treated for 24
h with pertussis toxin (100 ~tg/L) prior to the
internalisation experiment. p<0.01 vs. control (C).
Figure 3: Uptake of radioactivity in pituitary, pancreas and
adrenals in rats after the injection of 2 MBq (0.5 ~,g) [125I-
Tyr3 ] OCT ( D ) , [ DTPA~, 1251-Tyr3 ] OCT ( O ) or [ DOTA~ ~ 125I_
Tyr3JOCT (D). Values are expressed in % injected dose (%ID)
of radioactivity per gram tissue. Uptake of radioactivity in
these organs could be displaced by more than 90 % by co-
injection with 0.5 mg unlabelled octreotide~, demonstrating
that the uptake represented specific binding to SSR.
CA 02341489 2001-02-23
WO 00/18440 PCTlUS99/22337
14
REFERENCES
Reubi JC, Laissue J, Krenning E, Lamberts SWJ. 1992.
Somastatin receptors in human cancer: incidence
characteristics functional correlates and clinical
implications. J. Steroid Biochem. Mol. Biol. 43: 27-35.
Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WAP, Kooij
PPM, Oei HY, van Hagen M, Postema PTE, de Jong M, Reubi JC,
Visser TJ, Reijs AEM, Hofland LJ, Koper JW, Lamberts SWJ.
1993. Somastatin receptor scintigraphy with 111In_DTPA-D-
Phel]- and 1231-Tyr3-octreotide: The Rotterdam experience
with more than 1000 patients. Eur. J. Nucl. Med. 20: 716-731.
Breeman WAP, de Jong M, Bernard B, Hofland LJ, Srinavasan A,
van der Pluijm M, Bakker WH, Visser TJ, Krenning EP.
1998.Tissue distribution and metabolism of radioiodinated
DTPA~, D-Tyrl and Tyr3 derivatives of octreotide in rats.
Anticancer research 18: 83-90.
Schmidt MA, Wilhelm RR, Srinavasan A. 1997. Synthesis of
peptide alcohols using Rink acid resin. American Peptide
symposium, Nashville Tennessee, P159.
Srinavasan A, Schmidt MA. 1997.Tri-t-butyl-DTPA: a versatile
synthon for the synthesis of DTPA containing peptides.
American Peptide Symposium Nashville Tennessee, P110.
Bakker WH, Krenning EP, Breeman WAP, Koper JW, Kooij PPM,
Reubi JC, Klijn JG, Visser TJ, Docter R, Lamberts SWJ. 1990.
Receptor scintigraphy with a radioiodinated somastatin
analogue: radiolabelling, purification, biologic activity,
and in vivo application in animals. J. Nucl. Med. 31: 1501-
1509.
CA 02341489 2001-02-23
WO 00/18440 PCT/US99/22337
Hofland LJ, van Koetsveld PM, Waaijers M, Zuyderwijk J,
Breeman WAP, Lamberts SWJ. 1995. Internalisation of the
radioiodinated somatostatin analogue [l2sl_Tyr3]octreotide by
mouse and human pituitary tumour cells: increase by
5 unlabelled octreotide. Endocrinology 136: 3698-3706.
Lamberts SWJ, Hofland LJ, Reubi JC, Bruining HA, Bakker WA,
Krenning EP. 1990. Parallel in vivo and in vitro detection of
functional somatostatin receptors in human endocrine
10 pancreatic tumours: consequences with regard to diagnosis,
localisation and therapy. J. Endocrin. Metab. 71: 566-574.
de Jong M., Bakker WH, Krenning EP, Breeman WAP, van der
Pluijm ME, Bernard BF, Visser TJ, Jermann E, Behe M, Powell
15 P, Macke HR. 1997. Yttrium-90 and indium-111 labelling,
receptor binding and biodistribution of [DOTA~, D-Phel,
Tyr3]octreotide, a promising somatostatin analogue for
radionuclide therapy. Eur. J. Nucl. Med. 24: 368-371.
Gibson AE, Noel RJ, Herlihy JT, Ward, WF. 1989. Phenylarsine
oxide inhibition of endocytosis: effects on asialofetuin
internalisation. Am. J. Physiol. 257:C182-C184.
Nouel D, Gaudriault G, Houle M, Reisine T, Vincent J-P,
Mazella J, Beaudet A. 1997. Differential internalisation of
somatostatin in COS-7 cell transfected with sstl and sst2
receptor subtypes: a confocal microscopic study using novel
fluorescent somatostatin derivatives. Endocrinology 138:296-
306.