Note: Descriptions are shown in the official language in which they were submitted.
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
TITLE OF THE INVENTION
ENHANCEMENT OF RETURN TO INDEPENDENT LIVING STATUS
WITH A GROWTH HORMONE SECRETAGOGUE
BACKGROUND OF THE INVENTION
Many patients who receive surgery or experience a major
illness or injury require additional care and supervision following such
surgery or illness. Patients who receive surgery or experience a major
illness or injury such as hip fracture may have prolonged recovery times
due to deconditioning, a bed-rest associated syndrome comprising, e.g.:
decreased cardiac output, hypotension, muscular atrophy and acute
muscle loss, and joint contractures (Hoenig and Rubenstein, ~Tournal of
the nerican Geriatrics Society, 39, 220-221. (1991)). In the elderly the
loss of muscle mass and function as a result of illness or immobilization
may be up to 5% per day at bedrest. Among the elderly in particular,
deconditioning associated with acute illness is believed to lead to
recovery times far in excess of that expected for the acute illness itself.
In addition to prolonged recovery times, functional losses may result
from deconditioning, the acute illness itself, and untoward effects of
treatment. Although many functional losses are often reversible with
activity and exercise interventions, recovery times may vary widely
(Vorhies and Riley, Clinical Geriatric Medi ~~~e, _9, 745-763 (1993)). There
are direct and indirect costs associated with both the acute illness and
recovery periods. These costs may be considerable.
As a result of their deconditioned physical state, patients
who had once been able to live independently may require additional
assistance and care. In particular, patients who had previously lived
independently in a private home or apartment may find that following
surgery or a major illness or injury they require the formal care
provided by an assisted living center, a nursing home, a rehabilitation
hospital/center, an acute care hospital or a chronic medical care center.
This increase in the degree of care which is required by an
individual following such deconditioning imposes increased financial
costs. Moreover, such restrictions in their lifestyle may impose
-1-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
detrimental psychological impact with respect to the patient's self
esteem and independence.
Many patients suffering from acute deconditioning, such as
following a hip fracture, never regain their premorbid level of function.
The recovery of a patient following a hip fracture as represented by their
independent living status is generally a very difficult process. Data on
the percentage of patients living independently following a hip fracture
has been presented (Jette, et al., Arch. Phv~. Med Rehab , x;735 (I987)).
Approximately 48% of the patients studied were living independently
prior to their hip fracture. Immediately following reconstructive hip
surgery only about 5% were living independently; at 3 months and at 6
months after their hip fracture only about 25% were living
independently; and at 12 months after their hip fracture only about 22%
were living independently.
Very few compounds are known in the art to be useful for
the enhancing the return of patients to independent living status
following deconditioning. Moreover, these therapeutic regimens suffer
from numerous problems and a more effective, physiological way to
enhance the return of patients to independent living status following
deconditioning would be highly desirable.
Growth hormone, which is secreted from the pituitary,
stimulates growth of all tissues of the body that are capable of growing.
In addition, growth hormone is known to have the following basic effects
on the metabolic processes of the body: (1) Increased rate of protein
synthesis in all cells of the body; (2) Decreased rate of carbohydrate
utilization in cells of the body; (3) Increased mobilization of free fatty
acids and use of fatty acids for energy.
Various ways are known to release growth hormone. For
example, chemicals such as arginine, L-3,4-dihydroxyphenylalanine
(L-DOPA), glucagon, vasopressin, and insulin induced hypoglycemia, as
well as activities such as sleep and exercise, indirectly cause growth
hormone to be released from the pituitary by acting in some fashion on the
hypothalamus perhaps either to decrease somatostatin secretion or to
increase the secretion of the known growth hormone secretagogue growth
-2-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
hormone releasing factor (GRF) or an unknown endogenous growth
hormone-releasing hormone or all of these.
In cases where increased levels of growth hormone were
desired, the problem was generally solved by providing exogenous growth
hormone or by administering GRF, IGF-I or a peptidal compound which
stimulated growth hormone production and/or release. In either case the
peptidyl nature of the compound necessitated that it be administered by
injection. Initially the source of growth hormone was the extraction of the
pituitary glands of cadavers. This resulted in a very expensive product and
carried with it the risk that a disease associated with the source of the
pituitary gland could be transmitted to the recipient of the growth hormone.
Recombinant growth hormone has become available which, while no longer
carrying any risk of disease transmission, is still a very expensive product
which must be given by injection or by a nasal spray. In addition,
administration of exogenous growth hormone may result in side-effects,
including edema, and does not correlate with the pulsitile release seen in
the endogenous release of growth hormone.
Certain compounds have been developed which stimulate
the release of endogenous growth hormone. Peptides which are known
to stimulate the release of endogenous growth hormone include growth
hormone releasing hormone, the growth hormone releasing peptides
GHRP-6 and GHRP-1 (described in U.S. Patent No. 4,411,890, PCT Patent
Pub. No. WO 89/07110, and PCT Patent Pub. No. WO 89/07111) and
GHRP-2 (described in PCT Patent Pub. No. WO 93/04081), as well as
hexarelin (~T. Endoc 'nol Inve t., 1~5(Suppl 4), 45 (1992)). Other
compounds possessing growth hormone secretagogue activity are
disclosed in the following: U.S. Patent No. 3,239,345; U.S. Patent No.
4,036,979; U.S. Patent No. 4,411,890; U.S. Patent No. 5,206,235; U.S.
Patent No. 5,283,241; U.S. Patent No. 5,284,841; U.S. Patent No. 5,310,737;
U.S. Patent No. 5,317,017; U.S. Patent No. 5,374,721; U.S. Patent No.
5,430,144; U.S. Patent No. 5,434,261; U.S. Patent No. 5,438,136; U.S.
Patent No. 5,494,919; U.S. Patent No. 5,494,920; U.S. Patent No. 5,492,916;
U.S. Patent No. 5,536,716; EPO Patent Pub. No. 0,144,230; EPO Patent
Pub. No. 0,513,974; PCT Patent Pub. No. WO 94/07486; PCT Patent Pub.
3 5 No. WO 94/08583; PCT Patent Pub. No. WO 94/11012; PCT Patent Pub. No.
-3-
CA 02341649 2001-02-26
WO 00/I2047 PCT/US99/19996
WO 94/13696; PCT Patent Pub. No. WO 94/I9367; PCT Patent Pub. No. WO
95/03289; PCT Patent Pub. No. WO 95/03290; PCT Patent Pub. No. WO
95/09633; PCT Patent Pub. No. WO 95/11029; PCT Patent Pub. No. WO
95/12598; PCT Patent Pub. No. WO 95/13069; PCT Patent Pub. No. WO
95/14666; PCT Patent Pub. No. WO 95/16675; PCT Patent Pub. No. WO
95/16692; PCT Patent Pub. No. WO 95/17422; PCT Patent Pub. No. WO
95/17423; PCT Patent Pub. No. WO 95/34311; PCT Patent Pub. No. WO
96/02530; PCT Patent Pub. No. WO 96/05195; PCT Patent Pub. No. WO
96/15148; PCT Patent Pub. No. WO 96/22?82; PCT Patent Pub. No. WO
96/22997; PCT Patent Pub. No. WO 96/24580; PCT Patent Pub. No. WO
96/24587; PCT Patent Pub. No. WO 96/35713; PCT Patent Pub. No. WO
96/38471; PCT Patent Pub. No. WO 97/00894; PCT Patent Pub. No. WO
97/06803; PCT Patent Pub. No. WO 97/07117; Science, ~,0 1640-1643
(June 11, 1993); Ann. Rep ~Vled Che~n., x,177-186 (1993); Bioorg Med
Chem Ltrs., 4(22), 2709-2714 (1994); and Proc. Nati. Acad Sci USA ~,
7001-7005 {July 1995). Additional compounds with growth hormone
secretagogue activity are described herein.
SUMMARY OF THE INVENTION
The present invention is directed to the use of a compound
which has the ability to stimulate or amplify the release of natural or
endogenous growth hormone for enhancing the return of patients to
independent living status following deconditioning, in a warm-blooded
animal. The advantage of this method is that in contrast to injections of
growth hormone it provides a physiological-like pulsatile profile of
growth hormone release from the pituitary gland. Accordingly, the
present invention provides a method for enhancing the return of patients
to independent living status following deconditioning in a warm-blooded
animal comprising the administration of a growth hormone
secretagogue. The present invention further provides a pharmaceutical
composition for enhancing the return of patients to independent living
status following deconditioning.
-4-
CA 02341649 2001-02-26
WO 00/12047 PC'TNS99/19996
DETAILED DESCRIPTION OF THE DRAWING
A more complete understanding of the present invention
may be obtained by reading the following description in conjunction with
the appended figures which like elements are labeled similarly.
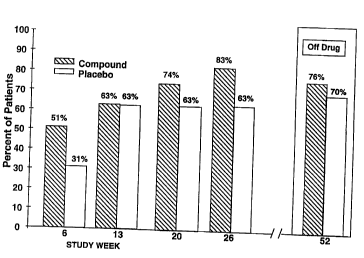
FIG. 1 depicts a summary of the data from a double-blind,
placebo-controlled, parallel-group study to determine the effect of a
growth hormone secretagogue on the return to independent living status
in patients suffering a hip fracture. Following their hip fracture, a
greater percentage of the patients receiving growth hormone
secretagogue were living independently.
FIG. 2 depicts a summary of the data from a double-blind,
placebo-controlled, parallel-group study to determine the effect of a
growth hormone secretagogue on the return to independent living status
in patients suffering a hip fracture. Of the patients who were living
independently prior to their hip fracture, a greater percentage of the
patients receiving growth hormone secretagogue returned to
independence following their hip fracture.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to the use of a compound
which has the ability to stimulate or amplify the release of natural or
endogenous growth hormone for enhancing the return of patients to
independent living status following deconditioning. In particular, the
present invention provides a method for enhancing the return of a
patient to independent living status following deconditioning of the
patient comprising the administration of a growth hormone
secretagogue. In a preferred aspect, the present invention provides a
method for enhancing the return of a patient to independent living
status following acute deconditioning of the patient comprising the
administration of a growth hormone secretagogue.
The present invention is further directed to a method for
ameliorating an acute deconditioned physiological state of in a mammal
which comprises administering an effective amount of a growth
hormone secretagogue. The acute deconditioned physical state in the
mammal may result from immobilization, surgery, major injury such
-5-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
as hip fracture or other bone fracture, gunshot wound or automobile
accident, and the like. The present invention currently has found
greatest application in enhancing the return of patients to independent
Living status following hip fracture.
In the present invention, it is preferred that the subject
mammal is a human. Although the present invention is applicable both
old and young people, it may find greater application in elderly people
especially people aged 70 years and older.
In a preferred embodiment of the present invention to
enhance the return of patients to independent living status following
acute deconditioning, therapy with the growth hormone secretagogue is
supplemented or employed in conjunction with physical rehabilitation.
In the present invention, it is preferred that the patient be
living independently prior to their acute deconditioning. Nevertheless,
the present invention may also be applicable in helping patients who
were not living independently prior to their acute deconditioning to
achieve independent living status.
By the term "growth hormone secretagogue" is meant any
exogenously administered compound or agent that directly or indirectly
stimulates or increases the endogenous release of growth hormone,
growth hormone-releasing hormone or somatostatin in an animal, in
particular, a human.
. The growth hormone secretagogue may be peptidal or non-
peptidal in nature, however, the use of a orally active growth hormone
secretagogue is preferred. In addition, it is preferred that the growth
hormone secretagogue induce or amplify a pulsatile release of
endogenous growth hormone.
The growth hormone secretagogue may be used alone or in
combination with other growth hormone secretagogues or with other
agents which are known to be beneficial for enhancing the return of
patients to independent living status following deconditioning. The
growth hormone secretagogue and the other agent may be
coadministered, either in concomitant therapy or in a fixed combination.
For example, the growth hormone secretagogue may be administered in
combination with other compounds which are known in the art to be
-6-
CA 02341649 2001-02-26
WO 00/12047 PCTNS99/19996
useful for enhancing the return of patients to independent living status
following deconditioning.
Representative growth hormone secretagogues are
disclosed in: U.S. Patent No. 3,239,345; U.S. Patent No. 4,036,979; U.S.
Patent No. 4,411,890; U.S. Patent No. 5,206,235; U.S. Patent No. 5,283,241;
U.S. Patent No. 5,284,841; U.S. Patent No. 5,310,737; U.S. Patent No.
5,317,017; U.S. Patent No. 5,374,721; U.S. Patent No. 5,430,144; U.S.
Patent No. 5,434,261; U.S. Patent No. 5,438,136; U.S. Patent No. 5,494,919;
U.S. Patent No. 5,494,920; U.S. Patent No. 5,492,916; U.S. Patent No.
5,536,716; EPO Patent Pub. No. 0,144,230; EPO Patent Pub. No. 0,513,974;
PCT Patent Pub. No. WO 89/07110; PCT Patent Pub. No. WO 89/07111;
PCT Patent Pub. No. WO 93/04081; PCT Patent Pub. No. WO 94/07486;
PCT Patent Pub. No. WO 94/08583; PCT Patent Pub. No. WO 94/11012;
PCT Patent Pub. No. WO 94/13696; PCT Patent Pub. No. WO 94/19367;
PCT Patent Pub. No. WO 95/03289; PCT Patent Pub. No. WO 95/03290;
PCT Patent Pub. No. WO 95/09633; PCT Patent Pub. No. WO 95/11029;
PCT Patent Pub. No. WO 95/I2598; PCT Patent Pub. No. WO 95/13069;
PCT Patent Pub. No. WO 95/14666; PCT Patent Pub. No. WO 95/16675;
PCT Patent Pub. No. WO 95/16692; PCT Patent Pub. No. WO 95/17422;
PCT Patent Pub. No. WO 95/17423; PCT Patent Pub. No. WO 95/34311;
PCT Patent Pub. No. WO 96/02530; PCT Patent Pub. No. WO 96/05195;
PCT Patent Pub. No. WO 96/15148; PCT Patent Pub. No. WO 96/22782;
PCT Patent Pub. No. WO 96/22997; PCT Patent Pub. No. WO 96/24580;
PCT Patent Pub. No. WO 96/24587; PCT Patent Pub. No. WO 96/35713;
PCT Patent Pub. No. WO 96/38471; PCT Patent Pub. No. WO 97/00894;
PCT Patent Pub. No. WO 97/06803; PCT Patent Pub. No. WO 97/07117; ~
Endocrinol Invest., ~5(Suppl 4), 45 (1992)); Science. ~, 1640-1643 (June
11,1993); Ann Rep Med Chem., 28,177-186 (1993); B~'oorg. Med Ch m
Ltrs., 4_(22), 2709-2714 (1994); and Proc. Natl. Acad Sci USA 9~, 7001_7005
(July 1995).
A representative first class of growth hormone
secretagogues is set forth in U.S. Patent No. 5,206,235 as follows:
_7_
CA 02341649 2001-02-26
WO 00/12047 PGT/US99/19996
R~. ~X~n-~CH2~p 4
R
I * N-C-A-N~
N ~ ~~ ~ Rs
R I O Rs O
~C~2)q
~w
R1a ~/
R2a R3a
wherein the various substituents are as defined in U.S. Patent 5,206,235.
The most preferred compounds within this first class are
identified as having the following structures:
H I I CH ~ ~H3
I / N-C-CH2 C-NH2
N O N-N
Nw , NH
or
H II CH\ ~H3 OH
I / N-C-CH~--C -NHCH2CHCH3
N N- N
O
NwNH
_g_
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
A representative second class of growth hormone
secretagogues is set forth in U.S. Patent No. 5,283,241 and PCT Patent
Publication No. 94/05634 as having the following structural formula:
R~ (X)n-(CH2)p
/R
N-C-A-N
I II ~s
R2 N O R6 O
(CI-~2)q
(~)w
l/v
R1a
R2a ~ 3a
R
5 wherein the various substituents are as defined in U.S. Patent 5,283,241
and PCT Patent Publication No. 94/05fi34.
A representative third class of growth hormone
secretagogues is disclosed in U.S. Patent No. 5,536,716 and PCT Patent
10 Pub. No. WO 94/13696 as compounds of the following structural
Formulas I and II:
-9-
CA 02341649 2001-02-26
WO 00/12047 PC'T/US99/19996
* R2 Rs Ra * R2 Rs Ra
R1-C-N-C-A-N,R R1-C-N-C-A-N
R5
C=O C=O
N
N
~n ~n
B F B F
C. E ~sa p / ~Rsa
H
I J, ; I
R3b
R3b
Formula I Formula II
wherein:
R1 is selected from the group consisting of
-C1-C10 alkyl, -aryl, -aryl-(C1-Cg alkyl),
-Cg-C7 cycloalkyl-(C1-Cgalkyl), -C1-C~alkyl-K-C1-C5 alkyl,
-aryl(CO-C5alkyl)-K-(C1-C5 alkyl),
-C3-C7 cycloalkyl(CO-C5 alkyl)-K-(C1-C5 alkyl),
wherein K is O, S(O)m, N(R2)C(O), C(O)N(R2), OC(O), C(O)O, or
-CR2=CR2-, or -C--_C-,
and wherein the aryl groups are as defined below and the R2 and alkyl
groups may be futher substituted by 1 to 9 halogen, S(O)mR2a, 1 to 3
OR2a, or C(O)OR2a, and the aryl groups may be further substituted by
phenyl, phenoxy, halophenyl, 1-3 C1-Cg alkyl, 1 to 3 halogen, 1 to 2 -OR2,
methylenedioxy, -S(O)mR2, 1 to 2 -CFg, -OCFg, nitro, -N(R2)(R2),
-N(R2)C(O)R2, -C(O)OR2, -C(O)N(R2)(R2), -S02N(R2)(R2), -N(R2)S(O)2
aryl, and -N(R2)S02R2;
R2 is selected from the group consisting of
hydrogen, C1-C6 alkyl, Cg-C7 cycloalkyl, and where two C1-Cg alkyl
groups are present on one atom, they may be optionally joined to form a
C3-Cg cyclic ring optionally including oxygen, sulfur or NR2a;
R2a is hydrogen, or C1-Cg alkyl;
- 10-
CA 02341649 2001-02-26
WO 00/12047 PCTNS99/19996
Rga and Rgb are independently selected from the group consisting of
hydrogen, halogen, -C1-C6 alkyl, -OR2, cyano, -OCFg, methylenedioxy,
vitro, -S(O)mR, -CFg or -C(O)OR2 and when Rga and Rgb are in an ortho
arrangement, they may be joined to form a C5 to Cg aliphatic or
aromatic ring optionally including 1 or 2 heteroatoms selected from
oxygen, sulfur or nitrogen;
R4 and R5 are independently selected from the group consisting of
hydrogen, -C1-Cg alkyl, substituted C1-C6 alkyl wherein the substituents
are selected from 1 to 5 halo, 1 to 3 hydroxy, 1 to 3
C1-C10 alkanoyloxy, 1 to 3 C1-C6 alkoxy, phenyl, phenoxy, 2-furyl, C1-Cg
alkoxycarbonyl, -S(O)m(C1-Cg alkyl); or R4 and R5 can be taken together
to form -(CH2)rLa (CH2)s_ where La is -C(R2)2-, -O-, -S(O)m-, or -N(R2)-,
where r and s are independently 1 to 3 and RZ is as defined above;
Rg is hydrogen or C1-C6 alkyl;
A is:
R7
- (CH2)X i -(CH2)Y
Rya
or
R~
- Z-(CH2)x" i -(CH2)Y-
R7a
wherein x and y are independently 0-3;
Z is N-R2 or O;
R7 and R7a are independently selected from the group consisting of
hydrogen, -C1-Cg alkyl, -OR2, trifluoromethyl, phenyl, substituted
C 1-C6 alkyl where the substituents are selected from imidazolyl, phenyl,
indolyl, p-hydroxyphenyl, -OR2, 1 to 3 fluoro, -S(O)mR2, -C(O)OR2, -C3-
C7 cycloalkyl, -N(RZ)(R2), -C(O)N(RZ)(R2); or R7 and R7a can
independently be joined to one or both of R4 and R~ groups to form
- 11-
CA 02341649 2001-02-26
WO 00/12047 PCTNS99/19996
alkylene bridges between the terminal nitrogen and the alkyl portion of
the R7 or Rya groups, wherein the bridge contains 1 to 5 carbons atoms;
B, D, E, and F are independently selected from the group consisting of
-C(Rg)(Rlp)-, -O-, C=O~ -S(O)m-, or -NRg_, such that one or two of B, D, E,
or F may be optionally absent to provide a 5, fi, or ? membered ring; and
provided that B, D, E and F can be -C(Rg)(Rlp)- or C=O only when one of
the remaining B, D, E and F groups is simultaneously -O-, -S(O)m-, or
-NRg_, or B and D, or D and E taken together may be -N=CRlO- or
-CRlp=N-, or B and D, or D and E taken together may be -CRg=CR10-,
provided one of the other of B and E or F is simultaneously -O-, -S(O)m-,
or -NRg-;
Rg and R1p are independently selected from the group consisting of
hydrogen, -R2, -OR2~ (-CH2)q-aryl, -(CH2)q-C(O)OR2, -(CH2)q-
C(O)O(CH2)q-aryl, or -(CH2)q-(1H-tetrazol-5-yl), where the aryl may be
optionally substituted by 1 to 3 halo, 1 to 2 C1-Cg alkyl, 1 to 3 -OR2 or 1 to
2
-C(O)OR2;
Rg is selected from the group consisting of
-R2, -(CH2)q-aryl, -C(O)R2, -C(O)(CH2)q-aryl, -S02R2,
-S02(CH2)q-aryl, -C(O)N(R2)(R2), -C(O)N(R2)(CH2)q-aryl, -C(O)OR2,
1-H-tetrazol-5-yl, -SOgH, -S02NHC--_N, -S02N(R2)aryl, -S02N(R2)(R2),
and wherein the (CH2)q may be optionally substituted by 1 to 2 C1-C4
alkyl, and the R2 and aryl may be optionally further substituted by 1 to 3
-OR2a, -O(CH2)q aryl, l to 2 -C(O)OR2a, 1 to 2 -C(O)O(CH2)q aryl, 1 to 2
-C(O)N(R2a)(R2a), 1 to 2 -C(O)N(R2a)(CH2)q aryl, 1 to 5 halogen, 1 to 3
C1-C4 alkyl, 1,2,4-triazolyl, 1-H-tetrazol-5-yl, -C(O)NHS02R2a,
-S(O)mR2a, -C(O)NHS02(CH2)q-aryl, -S02NHC-_-_-N, -S02NHC(O)R2a,
-S02NHC(O)(CH2)qaryl, -N(R2)C(O)N(R2a)(R2a), '
-N(R2a)C(O)N(R2a)(CH2)q-aryl, -N(R2a)(R2a), -N(R2a)C(O)R2a,
-N(R2a)C(O)(CH2)q aryl, -OC(O)N(R2a)(R2a), -OC(O)N(R2a)(CH2)q aryl,
-S02(CH2)qCONH-(CH2)wNHC(O)R11,
wherein w is 2-6 and R11 may be biotin, aryl, or aryl substituted by 1 or 2
OR2, 1-2 halogen, azido or nitro;
-12-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
m is 0, 1 or 2;
n is 1, or 2;
q may optionally be 0, 1, 2, 3, or 4; and
G, H, I and J are carbon, nitrogen, sulfur or oxygen atoms, such that at
least one is a heteroatom and one of G, H, I or J may be optionally
missing to afford a 5 or 6 membered heterocyclic aromatic ring;
and pharmaceutically acceptable salts and individual diastereomers
thereof.
Within this third class, the most preferred growth hormone
secretagogues employed in the instant invention are realized in
structural Formula V:
CH3 CH3
- N
NH2
O
N
~/ R3a
V
wherein R1 is selected from the group consisting of
- 13-
CA 02341649 2001-02-26
WO 00/I2047 PCT/US99/19996
CH2CH2-,
CH2CH2CH2-, ~ ~ CH20CH2-,
/ CH2- F / CH2- F
\ ~ ~ ~ ~ CH20CH2-,
N '
H H F
CH2CH2-, \
F ~ ~ CH2CH2-,
N CH2CH2CH2-,
F ~ ~ CH2CH2CH2- ;
R3a is H, or fluoro;
D is is selected from the group consisting of
-O-~ -S-~ -S(O)m-, N(R2), NS02(R2), NSOZ(CH2)tai'Yh NC(O)(R2),
5 NS02(CH2)qOH, NS02(CH2)qCOOR2, NS02(CHZ)qC(O)-N(R2)(R2),
N-SOZ(CH2)qC(O)-N(R2)(CH2)~,~,OH,
O
H S
N-SO CH C
2( 2)q (~)-N(R2)(CH2)w'N
HN NH
O
O OH
N-S02(CH2)qC(O)-N(R2)(CH2)w "-N ~ ~ N3
I
N- NH
N-S02{CH2)q ----C~
N=N '
- 14-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
and the aryl is phenyl or pyridyl and the phenyl may be
substituted by 1-2 halogen;
R2 is H, or C1-C4 alkyl;
m is 1, 2;
t is 0, 1, or 2;
q is 1, 2, or 3;
w is 2, 3, 4, 5, or 6;
and the pharmaceutically acceptable salts and individual diastereomers
thereof.
Representative most preferred growth hormone
secretagoues within this third class which may be employed in the
present invention include the following:
1) N-[1(R)-((1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-2-(IH-indol-3-yl)ethyl]-2-amino-2-methyl-
propanamide;
2) N-[1(R)-((1,2-Dihydro-1-methanecarbonylspiro[3H-indole-3,4'-
piperidin)-1'-yl)carbonyl]-2-(1H-indol-3-yl)ethyl]-2-amino-2-methyl-
propanamide;
3) N-[1(R)-[(1,2-Dihydro-1-benzenesulfonylspiro(3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-2-(1H-indol-3-yl)ethyl]-2-amino-2-methyl-
propanamide;
4) N-[1(R)-[(3,4-Dihydro-spiro[2H-1-benzopyran-2,4'-piperidin]-1'-yl)
carbonyl]-2-(1H-indol-3-yl)ethyl]-2-amino-2-methylpropanamide;
5) N-[I(R)-[(2-Acetyl-1,2,3,4-tetrahydrospiro[isoquinolin-4,4'-piperidin]-
1'-yl)carbonyl]-2-(indol-3-yl)ethyl]-2-amino-2-methyl-propanamide;
- 15-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
6) N-[1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-2-(phenylmethyloxy)ethyl]-2-amino-2-
methylpropanamide;
7) N-[1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-2-(phenylmethyloxy)ethyl]-2-amino-2-
methylpropanamide mesylate salt;
$) N-[1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonylJ-2-(2',6'-difluorophenylmethyloxy)ethyl]-2-
amino-2-methylpropanamide;
9) N-[1(R)-((1,2-Dihydro-1-methanesulfonyl-5-fluorospiro[3H-indole-3,4'-
piperidinJ-1'-yl)carbonyl]-2-(phenylmethyloxy)ethyl]-2-amino-2-
methylpropanamide;
10) N-[1(S)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl) carbonyl]-2-(phenylmethylthio)ethyl]-2-amino-2-
methylpropanamide;
11) N-[1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidinJ-1'-yl)carbonyl]-3-phenylpropylJ-2-amino-2-methyl-
propanamide;
12) N-(1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-3-cyclohexylpropyl]-2-amino-2-methyl-
propanamide;
13) N-[1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-4-phenylbutyl]-2-amino-2-methyl-
propanamide;
14) N-[1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonylJ-2-(5-fluoro-1H-indol-3-yl)ethylJ-2-amino-2-
methylpropanamide;
- 16-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
15) N-[1(R)-((1,2-Dihydro-1-methanesulfonyl-5-fluorospiro[3H-indole-
3,4'-piperidin]-1'-yl)carbonyl]-2-(5-fluoro-1H-indol-3-yl)ethyl]-2-amino-2-
methylpropanamide;
16) N-[1(R)-[(1,2-Dihydro-1-(2-ethoxycarbonyl)methylsulfonylspiro-[3H-
indole-3,4'-piperidin]-1'-yl)carbonyl]-2-(1H-indol-3-yl)ethyl]-2-amino-2-
methylpropanamide;
17) N-[1(R)-[(1,2-Dihydro-1,1-dioxospiro[3H-benzothiophene-3,4'-
piperidin]-1'-yl)carbonyl]-2-(phenylmethyloxy)ethyl]-2-amino-2-
methylpropanamide;
and pharmaceutically acceptable salts thereof.
Expecially preferred growth hormone secretagogues within
this third class which may be employed in the present invention include:
N-[1(R)-[(1,2-dihydro-1-methanesulfonylspiro[3H-indole-3,4'-piperidin]-
1'-yl)carbonyl]-2-(phenylmethyloxy)ethyl]-2-amino-2-methylpropan-
amide;
N-[1(R)-[( 1,2-dihydro-1-methanesulfonylspiro[3H-indole-3,4'-piperidin]-
1'-yl)carbonyl]-2-(phenylmethyloxy)ethyl]-2-amino-2-methylpropanamide
methanesulfonate; (ibutamoren mesylate)
N-(1(R)-((1,2-dihydro-1-methanesulfonylspiro[3H-indole-3,4'-piperidin]-
1'-yl)carbonyl]-3-phenylpropyl]-2-amino-2-methyl-propanamide;
and pharmaceutically acceptable salts thereof.
The most preferred compounds within this third class
which may be employed in the present invention are identified as having
the following structure:
- 17-
CA 02341649 2001-02-26
WO 00/I2047 PCT/US99/19996
H H
O~CIN\ ~~%~
t C N H~
C=O O
I
N,
N-S02-CH3
and pharmaceutically acceptable salts thereof, in particular, the
methanesulfonate salt.
A representative fourth class of growth hormone
secretagogues is disclosed in U.S. Patent No. 5,492,916 as being
compounds of the structural formula I:
H H O R4
R~~--N-C-A-N\
C=O R5
N
(CH2~n
X
Y
Formula I
wherein the various substituents are as defined in U.S. Patent 5,492,916.
Full descriptions of the preparation of the growth hormone
secretagogues which may be employed in the present invention may be
found in art, particularly the references cited herein.
In the above structural formulas and throughout the
instant specification, the following terms have the indicated meanings:
The alkyl groups specified above are intended to include
those alkyl groups of the designated length in either a straight or
branched configuration which may optionally contain double or triple
-18-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
bonds. Exemplary of such alkyl groups are methyl, ethyl, propyl,
ethinyl, isopropyl, butyl, sec-butyl, tertiary butyl, pentyl, isopentyl,
hexyl, isohexyl, allyl, propenyl, butenyl, butadienyl and the like. The
alkoxy groups specified above are intended to include those alkoxy
groups of the designated length in either a straight or branched
configuration which may optionally contain double or triple bonds.
Exemplary of such alkoxy groups are methoxy, ethoxy, propoxy,
isopropoxy, butoxy, isobutoxy, tertiary butoxy, pentoxy, isopentoxy,
hexoxy, isohexoxy allyloxy, propinyloxy, isobutenyloxy, 2-hexenyloxy,
and the like. The term "halogen" is intended to include the halogen
atom fluorine, chlorine, bromine and iodine. The term "aryl" is
intended to include phenyl and naphthyl and aromatic residues of 5- and
6- membered rings with 1 to 3 heteroatoms or fused 5 or 6 membered
bicyclic rings with 1 to 3 heteroatoms of nitrogen, sulfur or oxygen.
Examples of such heterocyclic aromatic rings are pyridine, thiophene,
benzothiophene, tetrazole, indole, N-methylindole, dihydroindole,
indazole, N-formylindole, benzimidazole, thiazole, furan, pyrimidine,
and thiadiazole.
Certain of the above defined terms may occur more than
once in the above formula and upon such occurrence each term shall be
defined independently of the other. Similarly, the use of a particular
variable within a noted structural formula is intended to be independent
of the use of such variable within a different structural formula.
For use in medicine, the salts of the compounds of this
invention refer to non-toxic "pharmaceutically acceptable salts." Other
salts may, however, be useful in the preparation of the compounds
according to the invention or of their pharmaceutically acceptable salts.
Salts encompassed within the term "pharmaceutically acceptable salts"
refer to non-toxic salts of the compounds of this invention which are
generally prepared by reacting the free base with a suitable organic or
inorganic acid. Representative salts include the following: Acetate,
Benzenesulfonate, Benzoate, Bicarbonate, Bisulfate, Bitartrate, Borate,
Bromide, Calcium, Camsylate, Carbonate, Chloride, Clavulanate,
Citrate, Dihydrochloride, Edetate, Edisylate, Estolate, Esylate,
3~ Fumarate, Gluceptate, Gluconate, Glutamate, Glycollylarsanilate,
- 19-
CA 02341649 2001-02-26
WO 00/12047 PC'T/US99/19996
Hexylresorcinate, Hydrabamine, Hydrobromide, Hydrochloride,
Hydroxynaphthoate, Iodide, Isothionate, Lactate, Lactobionate, Laurate,
Malate, Maleate, Mandelate, Mesylate, Methylbromide, Methylnitrate,
Methylsulfate, Mucate, Napsylate, Nitrate, N-methylglucamine
ammonium salt, Oleate, Oxalate, Pamoate (Embonate), Palmitate,
Pantothenate, Phosphate/diphosphate, Polygalacturonate, Salicylate,
Stearate, Subacetate, Succinate, Sulfate, Sulfonate, Tannate, Tartrate,
Teoclate, Tosylate, Triethiodide and Valerate. Furthermore, where the
compounds of the invention carry an acidic moiety, suitable
pharmaceutically acceptable salts thereof may include alkali metal
salts, e.g., sodium or potassium salts; alkaline earth metal salts, e.g.,
calcium or magnesium salts; and salts formed with suitable organic
ligands, e.g., quaternary ammonium salts.
The compounds employed in the present invention, may
have chiral centers and occur as racemates, racemic mixtures and as
individual diastereomers, or enantiomers with all isomeric forms being
included in the present invention. Therefore, where a compound is
chiral, the separate enantiomers, substantially free of the other, are
included within the scope of the invention; further included are all
mixtures of the two enantiomers.
Full descriptions of the preparation of the growth hormone
secretagoue employed in the present invention may be found e.g., in:
U.S. Patent No. 3,239,345; U.S. Patent No. 4,036,979; U.S. Patent No.
4,411,890; U.S. Patent No. 5,206,235; U.S. Patent No. 5,283,241; U.S.
Patent No. 5,284,841; U.S. Patent No. 5,310,737; U.S. Patent No. 5,317,01?;
U.S. Patent No. 5,374,721; U.S. Patent No. 5,430,144; U.S. Patent No.
5,434,261; U.S. Patent No. 5,438,136; U.S. Patent No. 5,494,919; U.S.
Patent No. 5,494,920; U.S. Patent No. 5,492,916; U.S. Patent No. 5,536,716;
EPO Patent Pub. No. 0,144,230; EPO Patent Pub. No. 0,513,974; PCT
Patent Pub. No. WO 89/07110; PCT Patent Pub. No. WO 89/07111; PCT
Patent Pub. No. WO 93/04081; PCT Patent Pub. No. WO 94/07486; PCT
Patent Pub. No. WO 94/08583; PCT Patent Pub. No. WO 94/11012; PCT
Patent Pub. No. WO 94/13696; PCT Patent Pub. No. WO 94/19367; PCT
Patent Pub. No. WO 95/03289; PCT Patent Pub. No. WO 95/03290; PCT
Patent Pub. No. WO 95/09633; PCT Patent Pub. No. WO 95/11029; PCT
-20-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
Patent Pub. No. WO 95/12598; PCT Patent Pub. No. WO 95/13069; PCT
Patent Pub. No. WO 95/14666; PCT Patent Pub. No. WO 95/16675; PCT
Patent Pub. No. WO 95/16692; PCT Patent Pub. No. WO 95/17422; PCT
Patent Pub. No. WO 95/17423; PCT Patent Pub. No. WO 95/34311; PCT
S Patent Pub. No. WO 96/02530; PCT Patent Pub. No. WO 96/05195; PCT
Patent Pub. No. WO 96/15148; PCT Patent Pub. No. WO 96/22782; PCT
Patent Pub. No. WO 96/22997; PCT Patent Pub. No. WO 96/24580; PCT
Patent Pub. No. WO 96/24587; PCT Patent Pub. No. WO 96/35713; PCT
Patent Pub. No. WO 96/38471; PCT Patent Pub. No. WO 97/00894; PCT
Patent Pub. No. WO 97/06803; PCT Patent Pub. No. WO 97/07117; ~
Endocryo~ Inve ., ~5(Suppl 4), 45 (1992)); c' c ~, 1640-1643 (June
11,1993); ~n ~Re~ Med Chgm., x,177-186 (1993); Rioore. Med. Ch m.
t s., 4(22), 2709-2714 (1994); and Proc. Natl Acad Sci SA g2~ 7001-7005
(July 1995), as well as herein.
1S Methods to obtain the growth hormone releasing peptides
GHRP-6 and GHRP-1 are described in U.S. Patent Nos. 4,411,890 and
PCT Patent Publications WO 89/07110, WO 89/07111, methods to obtain
the growth hormone releasing peptide GHRP-2 are described in PCT
Patent Publication WO 93/04081, and methods to obtain hexarelin are
described in J. Endocrinol Inv ~t., ~(guppl 4), 45 (1992).
The identification of a compound as a "growth hormone
secretagogue" and thus able to directly or indirectly stimulate or
increase the endogenous release of growth hormone in an animal may
be readily determined without undue experimentation by methodology
2S well known in the art, such as the assay described by Smith, et al.,
Science, 2fi0, 1640-1643 (1993) (see text of Figure 2 therein). In a typical
experiment pituitary glands are aseptically removed from 150-200 g
Wistar male rats and cultures of pituitary cells are prepared according
to Cheng et al. Endocrinol. ,124, 2791-2798 (1989). The cells are treated
30, with the subject compound and assayed for growth hormone secreting
activity and intracellular cAMP levels as described by Chang et al. In
particular, the intrinsic growth hormone secretagogue activity of a
compounds which may be used in the present invention may be
determined by this assay.
-21-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
The term "therapeutically effective amount" shall mean
that amount of a drug or pharmaceutical agent that will elicit the
biological or medical response of a tissue, system, animal or human that
is being sought by a researcher or clinician.
The term "acute deconditioning" is meant to indicate the
presence of a diminished physical state in a patient characterized by
muscle atrophy and muscle loss which results from specific insult such
as immobilization or inactivity brought on by acute illness or injury. In
contrast, chronic deconditioning refers to long-term muscle loss or
wasting, i.e. sarcopenia.
The term "independent living" is meant to indicate that the
patient is able to handle the physical demands of daily living with a
minimal level of physical assistance from other persons. Patients living
independently include, for example, patients who live in a private home
or apartment and who do not receive formal or nonformal home health
care services.
Accordingly, the present invention includes within its scope
the use of a growth hormone secretagogue for enhancing the return of
patients to independent living status following deconditioning, alone or
in combination with other agents such as growth promoting and
anabolic agents including TRH, diethylstilbesterol, amino acids,
estrogens, ~i-agonists, theophylline, anabolic steroids, enkephalins, E
series prostaglandins, retinoic acid, compounds disclosed in U.S. Patent
No. 3,239,345, e.g., zeranol, and compounds disclosed in U.S. Patent No.
4,036,979, e.g., sulbenox. or peptides disclosed in U.S. Patent No.
4,411,890, growth hormone releasing peptides GHRP-6, GHRP-1 and B-
HT920 as well as hexarelin and GHRP-2 or growth hormone releasing
hormone (GHRH, also designated GRF) and its analogs or growth
hormone and its analogs or somatomedins including IGF-1 and IGF-2,
a-adrenergic agonists such as clonidine, serotonin SHTID agonists such
as sumitriptan or agents which inhibit somatostatin or its release such
as physostigmine and pyridostigmine. In particular, the growth
hormone secretagogue may be used in combination with growth
hormone releasing factor, an analog of growth hormone releasing
3 ~ factor, IGF-1, or IGF-2.
-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
This particular application of growth hormone
secretagogues provides unexpected benefit relative to the administration
of exogenous growth hormone. In particular, the growth hormone
secretagogue enhances the normal pulsatile releases of endogenous
growth hormone and thus is more likely to reproduce the natural
pattern of endogenous growth hormone release, especially with regard
to increasing the level of endogenous growth hormone prior to or in
during the initial onset of sleep. Growth hormone secregagogues which
are orally active also have the benefit being able to be administered
orally, rather than just intravenously, intraperitoneally or
subcutaneously.
In addition, the present invention includes within its scope
a pharmaceutical composition for enhancing the return of patients to
independent living status following acute deconditioning comprising, as
IS an active ingredient, at least one growth hormone secretagogues in
association with a pharmaceutical carrier or diluent. Optionally, the
active ingredient of the pharmaceutical compositions can comprise an
anabolic agent in addition to at least one growth hormone secretagogue
or another composition which exhibits a different activity, e.g., an
antibiotic growth promoting agent or in combination with a
corticosteroid to minimize the catabolic side effects or with other
pharmaceutically active materials wherein the combination enhances
efficacy and minimizes side effects. Growth promoting and anabolic
agents include, but are not limited to, TRH, diethylstilbesterol,
estrogens, (3-agonists, theophylline, anabolic steroids,
dehydroepiandrosterone, enkephalins, E series prostaglandins, retinoic
acid, compounds disclosed in U.S. Patent No. 3,239,345, e.g., zeranol,
and compounds disclosed in U.S. Patent No. 4,036,979, e.g., sulbenox. or
peptides disclosed in U.S. Patent No. 4,411,890.
The present invention further includes the use of a growth
hormone secretagogue, alone or in combination with another agent, in
the manufacture of a medicament for enhancing the return of patients
to independent living status following acute deconditioning.
In addition, the present invention contemplates the use of a
growth hormone secretagogue for enhancing the return of patients to
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
independent living status following deconditioning in combination with
another growth hormone secretagogues such as those referenced
herein, including the growth hormone releasing peptides GHRP-6 and
GHRP-1 (described in U.S. Patent No. 4,411,890 and PCT publications
WO 89/07110, WO 89/07111) and GHRP-2 (described in WO 93/04081) and
B-HT920, as well as hexarelin or growth hormone releasing hormone
(GHRH, also designated GRF) and its analogs, or growth hormone and
its analogs, or somatomedins including IGF-1 and IGF-2, or with a-
adrenergic agonists such as clonidine or serotonin SHTD agonists such
as sumatriptan, or agents which inhibit somatostatin or its release such
as physostigmine and pyridostigmine. For example, a growth hormone
secretagogue may be used in combination with IGF-1 for enhancing the
return of patients to independent living status following deconditioning.
It will be known to those skilled in the art that other
compounds may be used in an effort to enhance the return of patients to
independent living status following deconditioning. Combinations of
these therapeutic agents some of which have also been mentioned herein
with a growth hormone secretagogue will bring additional,
complementary, and often synergistic properties to enhance the
desirable properties of these various therapeutic agents. In these
combinations, the growth hormone secretagogue and the therapeutic
agents may be independently present in dose ranges from one one-
hundredth to one times the dose levels which are effective when these
compounds and secretagogues are used singly.
Typically, the individual daily dosages for these
combinations may range from about one-fifth of the minimally
recommended clinical dosages to the maximum recommended levels for
the entities when they are given singly.
To illustrate these combinations, a growth hormone
secretagogue effective clinically effective clinically at a given daily dose
range may be effectively combined, at levels which are equal or less than
the daily dose range, with the following compounds at the indicated per
day dose range: and salts thereof, and combinations thereof, and the
like.
CA 02341649 2001-02-26
WO 00/12047 PCTNS99/19996
Naturally, these dose ranges may be adjusted on a unit
basis as necessary to permit divided daily dosage and, as noted above, the
dose will vary depending on the nature and severity of the disease,
weight of patient, special diets and other factors.
Anabolic effects especially in the treatment of geriatric male
patients are obtained with compounds of this invention in combination
with anabolic steroids such as dehydroepiandrosterone, oxymetholone,
methyltesterone, fluoxymesterone, restosterone and stanozolol.
These combinations may be formulated into
pharmaceutical compositions as known in the art and as discussed
below.
A growth hormone secretagogue may be administered
alone or in combination by oral, parenteral (e.g., intramuscular,
intraperitoneal, intravenous or subcutaneous injection, or implant),
nasal, vaginal, rectal, sublingual, or topical routes of administration
and can be formulated in dosage forms appropriate for each route of
administration.
Solid dosage forms for oral administration include
capsules, tablets, pills, powders and granules. In such solid dosage
forms, the active compound is admixed with at least one inert
pharmaceutically acceptable carrier such as sucrose, lactose, or starch.
Such dosage forms can also comprise, as is normal practice, additional
substances other than inert diluents, e.g., lubricating agents such as
magnesium stearate. Illustrative of the adjuvants which may be
incorporated in tablets, capsules and the Iike are the following: a binder
such as gum tragacanth, acacia, corn starch or gelatin; an excipient
such as microcrystalline cellulose; a disintegrating agent such as corn
starch, pregelatinized starch, alginic acid and the like; a lubricant such
as magnesium stearate; a sweetening agent such as sucrose, lactose or
saccharin; a flavoring agent such as peppermint, oil of wintergreen or
cherry. In the case of capsules, tablets and pills, the dosage forms may
also comprise buffering agents. When the dosage unitform is a capsule,
it may contain, in addition to materials of the above type, a liquid carrier
such as fatty oil. Various other materials may be present as coatings or
to otherwise modify the physical form of the dosage unit. Tablets and
-25-
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
pills can additionally be prepared with enteric coatings and tablets may
be coated with shellac, sugar or both.
Liquid dosage forms for oral administration include
pharmaceutically acceptable emulsions, solutions, suspensions, syrups,
S the elixirs containing inert diluents commonly used in the art, such as
water. Besides such inert diluents, compositions can also include
adjuvants, such as wetting agents, emulsifying and suspending agents,
and sweetening, flavoring, and perfuming agents. A syrup or elixir
may contain the active compound, sucrose as a sweetening agent,
methyl and propyl parabens as preservatives, a dye and a flavoring such
as cherry or orange flavor.
Preparations according to this invention for parenteral
administration include sterile aqueous or non-aqueous solutions,
suspensions, or emulsions. Sterile compositions for injection may be
formulated according to conventional pharmaceutical practice by
dissolving or suspending the active substance in a vehicle such as water
for injection, a naturally occurring vegetable oil like sesame oil, coconut
oil, peanut oil, cottonseed oil, etc., or a synthetic fatty vehicle like ethyl
oleate or the like. Bufl'ers, preservatives, antioxidants and the like may
be incorporated as required. Examples of non-aqueous solvents or
vehicles are propylene glycol, polyethylene glycol, vegetable oils, such as
olive oil and corn oil, gelatin, and injectable organic esters such as ethyl
oleate. Such dosage forms may also contain adjuvants such as
preserving, wetting, emulsifying, and dispersing agents. They may be
sterilized by, for example, filtration through a bacteria-retaining filter,
by incorporating sterilizing agents into the compositions, by irradiating
the compositions, or by heating the compositions. They can also be
manufactured in the form of sterile solid compositions which can be
dissolved in sterile water, or some other sterile injectable medium
immediately before use. Compositions for rectal or vaginal
administration are preferably suppositories which may contain, in
addition to the active substance, excipients such as cocoa butter or a
suppository wax. Compositions for nasal or sublingual administration
are also prepared with standard excipients well known in the art.
- 26 -
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
The dosage of active ingredient in the compositions of this
invention may be varied, however, it is necessary that the amount of the
active ingredient be such that a suitable dosage form is obtained. The
active ingredient may be administered to patients (animals and human)
in need of such treatment in dosages that will provide optimal
pharmaceutical efficacy. The selected dosage depends upon the desired
therapeutic effect, on the route of administration, and on the duration of
the treatment. The dose will vary from patient to patient depending
upon the nature and severity of disease, the patient's weight, special
diets then being followed by a patient, concurrent medication, and other
factors which those skilled in the art will recognize. Generally, dosage
levels of between 0.0001 to 10 mg/kg. of body weight daily are
administered to patients and animals, e.g., mammals, to obtain effective
release of growth hormone. The dosage range will generally be about 0.5
mg to 1.0 g. per patient per day which may be administered in single or
multiple doses. Perferably, the dosage range will be about 0.5 mg to 500
mg per patient per day; more preferably about 0.5 mg to 200 mg per
patient per day; and even more preferably about 5 mg to 50 mg per
patient per day. Pharmaceutical compositions of the present invention
may be provided in a solid dosage formulation preferably comprising
about 0.5 mg to 500 mg active ingredient, more preferably comprising
about 1 mg to 250 mg active ingredient. The pharmaceutical
composition is preferably provided in a solid dosage formulation
comprising about 1 mg, 5 mg, 10 mg, 25 mg, 50 mg, 100 mg, 200 mg or
250 mg active ingredient.
The following examples are provided for the purpose of
further illustration only and are not intended to be limitations on the
disclosed invention.
3 0 EXAMPLE 1
Double-Blind, Placebo-Controlled, Parallel-Group Study to Determine the
Effect of a Growth Hormone Secretagogue on the Return to Independent
Liv' to ~ P i ff in i Fr c r
-27-
CA 02341649 2001-02-26
WO 00/12047 PCTNS99/19996
In this study, hip fracture is a model state of acute
deconditioning in the elderly for determining the effect of a growth
hormone secretagogue in return of patients to independent living status.
Approximately 168 postoperative hip fracture patients were
enrolled in this study. Patients were betweeen 3 and 14 days
postoperative hip repair. Patients included both males and females over
65 years of age who have ambulatory prefracture (with or without
assistance), with need for assistance and level of premorbid ambulation
documented. Their fracture was of nonpathological origin, e.g., it
resulted from a fall or accident rather than from neoplasm.
The subjects received either a placebo or 25 mg of the growth
hormone secretagogue N-[1(R)-[(1,2-Dihydro-1-methanesulfonyl-
spiro[3H-indole-3,4'-piperidin]-1'-yl)carbonyl]-2-(phenylmethy-
loxy)ethyl]-2-amino-2-methyl-propanamide mesylate P.O., daily in the
morning, for a total duration of 6 months. The study was initiated
between 3 and 14 days following surgery to repair their hip fracture. All
subjects received standard-of care post-operative physical rehabilitation.
Follow-up of subjects was conducted 6 months after cessation of
treatment (i.e. in Months 7-12).
Patients completed a personal profile questionnaire at 2, 6,
13, 20, and 26 weeks following initiation of treatment and at 52 weeks
following initiation of treatment (i.e. 6 months after cessation of
treatment). The questionnaires compared the subject's pre-fracture
status and post-fracture status with respect to housing and degree of
care which they received. The questionnaire for pre-fracture status
determined where the particular subject was living before their hip
fracture (i.e. in a private home/apartment, an assisted living center, a
nursing home, or other living situation) and whether they received
home health care services. The questionnaire for post-fracture status
determined where the particular subject was living and had lived after
their hip fracture (i.e. in a private home/apartment, an assisted living
center, a nursing home, a rehabilitation hospitallcenter, an acute care
hospital, a chronic medical care center, or other living situation) and
whether they received home health care services.
-28_
CA 02341649 2001-02-26
WO 00/12047 PCT/US99/19996
Specific demographics for the group receiving the growth
hormone secretagogue: Initial randomization ~ 84 patients; Completed
Month 6 = 62 patients; Completed Month 12 = 61 patients; Age = 79.1~ 7.3
yrs.; Gender = 79 Females, 21 Males; Independent Pre-Fracture = 64%.
Specific demographics for the group receiving placebo:
Initial randomization = 77 patients; Completed Month 6 = 69 patients;
Completed Month 12 = 65 patients; Age = 79.1~ 7.2 yrs.; Gender = 78
Females, 22 Males; Independent Pre-Fracture = 72%.
The data from this study is summarized in Figures 1 and 2.
FIG. 1 depicts a summary of the data regarding living
independence from a double-blind, placebo-controlled, parallel-group
study to determine the effect of a growth hormone secretagogue on the
return to independent living status in patients suffering a hip fracture.
The percentage of patients living independently is presented with respect
to the number of weeks since the patient initiated the study.
Following their hip fracture, a greater percentage of the
patients receiving growth hormone secretagogue ("Compound") were
living independently (i.e. living at home with no assistance) following
their hip fracture.
FIG. 2 depicts a summary of the data regarding the return
to independent living status from a double-blind, placebo-controlled,
parallel-group study to determine the effect of a growth hormone
secretagogue on the return to independent living status in patients
suffering a hip fracture. The percentage of patients living independently
is presented with respect to the number of weeks since the patient
initiated the study.
Of the patients who were living independently prior to their
hip fracture, a greater percentage of these patients receiving growth
hormone secretagogue ("Compound") returned to independence (i.e.
living at home with no assistance) following their hip fracture.
Overall, of the patients who were living independently at
baseline, approximately 64% of the placebo group versus 83% of the
group receiving the growth hormone secretagogue (p=0.036) returned to
independent function at the end of the study.
- 29 -
CA 02341649 2001-02-26
WO 00/t2047 PCT/US99/19996
The results of the foregoing study indicate that the
administration of the growth hormone secretagogue had a positive ef~'ect
with respect to enhancing the return of patients to independent living
status following acute deconditioning, specifically hip fracture. The
results observed in this study would clearly extend to other growth
hormone secretagogues and for other conditions associated with acute
deconditioning.
While the invention has .been described and illustrated with
reference to certain particular embodiments thereof, those skilled in the
art will appreciate that various adaptations, changes, modifications,
substitutions, deletions, or additions of procedures and protocols may be
made without departing from the spirit and scope of the invention. For
example, effective dosages other than the particular dosages as set forth
herein above may be applicable as a consequence of variations in the
responsiveness of the mammal being treated for any of the indications
with the compounds of the invention indicated above. Likewise, the
specific pharmacological responses observed may vary according to and
depending upon the particular active compounds selected or whether
there are present pharmaceutical carriers, as well as the type of
formulation and mode of administration employed, and such expected
variations or differences in the results are contemplated in accordance
with the objects and practices of the present invention. It is intended,
therefore, that the invention be defined by the scope of the claims which
follow and that such claims be interpreted as broadly as is reasonable.
-30-