Note: Descriptions are shown in the official language in which they were submitted.
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-1-
CHEMOKINE RECEPTOR ANTAGONISTS
AND METHODS OF USE THEREFOR
RELATED APPLICATION
This application is a continuation-in-part of U.S.
Serial No. 09/146,827, filed September 4, 1998, the entire
teaching of which are incorporated herein by reference.
BACKGROUND OF THE INVENTION
Chemoattractant cytokines or chemokines are a family
of proinflammatory mediators that promote recruitment and
10 activation of multiple lineages of leukocytes and
lymphocytes. They can be released by many kinds of tissue
cells after activation. Continuous release of chemokines at
sites of inflammation mediates the ongoing migration of
effector cells in chronic inflammation. The chemokines
15 characterized to date are related in primary structure.
They share four conserved cysteines, which form disulfide
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-2-
chemokines (a-chemokines), and the C-C chemokines
(~3-chemokines), in which the first two conserved cysteines
are separated by an intervening residue, or adjacent
respectively (Baggiolini, M. and Dahinden, C. A.,
Immunology Today, 15:127-133 (1994)).
The C-X-C chemokines include a number of potent
chemoattractants and activators of neutrophils, such as
interleukin 8 (IL-8), PF4 and neutrophil-activating
peptide-2 (NAP-2). The C-C chemokines include RANTES
10 (gegulated on 8ctivation, formal T Expressed and
secreted), the macrophage inflammatory proteins la and la
(MIP-1a and MIP-1(3), eotaxin, and human monocyte
chemotactic proteins 1-3 (MCP-1, MCP-2, MCP-3), which have
been characterized as chemoattractants and activators of
15 monocytes or lymphocytes but do not appear to be
chemoattractants for neutrophils. Chemokines, such as
RANTES and MIP-la, have been implicated in a wide range of
human acute and chronic inflammatory diseases including
respiratory diseases, such as asthma and allergic
20 disorders.
The chemokine receptors are members of a superfamily
of G protein-coupled receptors (GPCR) which share
structural features that reflect a common mechanism of
action of signal transduction (Gerard, C. and Gerard, N.P.,
25 Annu Rev. Immunol., 12:775-808 (1994); Gerard, C. and
Gerard, N. P., Curr. Opin. Immunol., 6:140-I45 (1994)).
Conserved features include seven hydrophobic domains
spanning the plasma membrane, which are connected by
hydrophilic extracellular and intracellular loops. The
30 majority of the primary sequence homology occurs in the
CA 02343536 2001-03-05
WO 00/14089 PC'f/US99/01235
-3-
hydrophobic transmembrane regions with the hydrophilic
regions being more diverse. The first receptor for the C-C
chemokines that was cloned and expressed binds the
chemokines MIP-la and RANTES. Accordingly, this
MIP-la/RANTES receptor was designated C-C chemokine
receptor 1 (also referred to as CCR-1; Neote, K., et al.,
Cell, 72:415-425 (1993); Horuk, R. et al., WO 94/11504, May
26, 1994; Gao, J.-I. et al., J. Exp. Med., 177:1421-1427
(1993)). Three receptors have been characterized which
bind and/or signal in response to RANTES: CCR3 mediates
binding and signaling of chemokines including eotaxin,
RANTES, and MCP-3 (Ponath et al., J. Exp. Med., 183:2437
(1996)), CCR4 binds chemokines including RANTES, MIP-la,
and MCP-1 (Power, et al., J. Biol. Chem., 270:19495
(1995)), and CCR5 binds chemokines including MIP-la,
RANTES, and MIP-1(3 (Samson, et al., Biochem. 35: 3362-3367
(1996)). RANTES is a chemotactic chemokine for a variety
of cell types, including monocytes, eosinophils, and a
subset of T-cells. The responses of these different cells
20 may not all be mediated by the same receptor, and it is
possible that the receptors CCR1, CCR4 and CCR5 will show
some selectivity in receptor distribution and function
between leukocyte types, as has already been shown for CCR3
(Ponath et aI.). In particular, the ability of RANTES to
25 induce the directed migration of monocytes and a memory
population of circulating T-cells (Schall, T, et al.,
Nature, 347:669-71 (1990)) suggests this chemokine and its
receptors) may play a critical role in chronic
inflammatory diseases, since these diseases are
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-4-
characterized by destructive infiltrates of T cells and
monocytes.
Many existing drugs have been developed as antagonists
of the receptors for biogenic amines, for example, as
5 antagonists of the dopamine and histamine receptors. No
successful antagonists have yet been developed to the
receptors for the larger proteins such as chemokines and
CSa. Small molecule antagonists of the interaction between
C-C chemokine receptors and their ligands, including RANTES
10 and MIP-la, would provide compounds useful for inhibiting
harmful inflammatory processes "triggered" by receptor
ligand interaction, as well as valuable tools for the
investigation of receptor-ligand interactions.
SUMMARY OF THE INVENTION
15 It has now been found that a class of small organic
molecules are antagonists of chemokine receptor function
and can inhibit leukocyte activation and/or recruitment.
An antagonist of chemokine receptor function is a molecule
which can inhibit the binding and/or activation of one or
20 more chemokines, including C-C chemokines such as RANTES
and/or MIP-la, to one or more chemokine receptors on
leukocytes and/or other cell types. As a consequence,
processes and cellular responses mediated by chemokine
receptors can be inhibited with these small organic
25 molecules. Based on this discovery, a method of treating a
subject with a disease associated with aberrant leukocyte
recruitment and/or activation is disclosed as well as a
method of treating a disease mediated by chemokine receptor
function. The method comprises administering to the
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-5-
subject a therapeutically effective amount of a compound or
small organic molecule which is an antagonist of chemokine
receptor function. Compounds or small organic molecules
which have been identified as antagonists of chemokine
5 receptor function are discussed in detail herein below, and
can be used for the manufacture of a medicament for
treating or for preventing a disease associated with
aberrant leukocyte recruitment and/or activation. The
invention also relates to the disclosed compounds and small
10 organic molecules for use in treating or preventing a
disease associated with aberrant leukocyte recruitment
and/or activation. The invention also includes
pharmaceutical compositions comprising one or more of the
compounds or small organic molecules which have been
15 identified herein as antagonists of chemokine function and
a suitable pharmaceutical carrier. The invention further
relates to novel compounds which can be used to treat an
individual with a disease associated with aberrant
leukocyte recruitment and/or activation and methods for
20 their preparation.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic showing the preparation of the
compounds represented by Structural Formula (I), (III) and
( IV) .
25 Figure 2 is a schematic showing the preparation of
representative compounds of Structural Formula (I),(III)
and (IV) wherein Z is represented by Structural Formulas
(VIII) and wherein Ring A and/or Ring B in Z can be
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-6-
substituted with - (O) "- (CHz) t-COORz°, - (O) "- (CHz) t-OC (O)
Rz°-
(O) u- (CHz) t-C (O) -NRzlRzz or _ (O) U_ (CHz) t-NHC (O) O-Rz° .
Figure 3 is a schematic showing the preparation of the
compounds represented by Structural Formula (I), (III) and
5 (IV), wherein Z is represented by Structural Formula
(VIII) .
Figure 4 is a schematic showing the preparation of
compounds represented by Structural Formulas (I) , (III)
and (IV), wherein Z is represented by Structural Formula
(VIII), wherein W is H.
Figure 5 is a schematic showing the preparation of
compounds represented by Structural Formulas (I) , (III)
and (IV), wherein Z is represented by Structural Formula
(VIII), wherein W is H.
15 Figure 6 shows the preparation of compounds
represented by Structural Formula (I), where in Z is
represented by Structural Formulas (VIII) and wherein Ring
A and/or Ring B in Z is substituted with - (O) "- (CHz) t-COORz°,
a is one.
20 Figure 7 shows the preparation of compounds
represented by Structural Formula (I), wherein Z is
represented by Structural Formulas (VIII) and wherein Ring
A or Ring B in Z is substituted with - (O) "- (CHz) t-COORz°, a
is zero.
25 DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to small molecule
compounds which are modulators of chemokine receptor
function. In a preferred embodiment, the small molecule
CA 02343536 2001-03-05
WO 00/14089 PCTNS99/01235_
compounds are antagonists of chemokine receptor function.
Accordingly, processes or cellular responses mediated by
the binding of a chemokine to a receptor can be inhibited
(reduced or prevented, in whole or in part), including
5 leukocyte migration, integrin activation, transient
increases in the concentration of intracellular free
calcium [Ca"];, and/or granule release of proinflammatory
mediators.
The invention further relates to a method of
treatment, including prophylactic and therapeutic
treatments, of a disease associated with aberrant leukocyte
recruitment and/or activation or mediated by chemokines or
chemokine receptor function, including chronic inflammatory
disorders characterized by~the presence of RANTES, MIP-la,
15 MCP-2, MCP-3 and/or MCP-4 responsive T cells, monocytes
and/or eosinophils, including but not limited to diseases
such as arthritis (e. g., rheumatoid arthritis),
atherosclerosis, arteriosclerosis, ischemia/reperfusion
injury, diabetes mellitus (e. g., type 1 diabetes mellitus),
20 psoriasis, multiple sclerosis, inflammatory bowel diseases
such as ulcerative colitis and Crohn's disease, rejection
of transplanted organs and tissues (i.e., acute allograft
rejection, chronic allograft rejection), graft versus host
disease, as well as allergies and asthma. Other diseases
25 associated with aberrant leukocyte recruitment and/or
activation which can be treated (including prophylactic
treatments) with the methods disclosed herein are
inflammatory diseases associated with Human
Immunodeficiency Virus (HIV) infection, e.g., AIDS
CA 02343536 2001-03-05
wo oonaos9 rcTms99ioiz3s
_8_
associated encephalitis, AIDS related maculopapular skin
eruption, AIDS related interstitial pneumonia, AIDS related
enteropathy, AIDS related periportal hepatic inflammation
and AIDS related glomerulo nephritis. The method comprises
5 administering to the subject in need of treatment an
effective amount of a compound (i.e., one or more
compounds) which inhibits chemokine receptor function,
inhibits the binding of a chemokine to leukocytes and/or
other cell types, and/or which inhibits leukocyte migration
10 to, and/or activation at, sites of inflammation.
The invention further relates to methods of
antagonizing a chemokine receptor, such as CCR1, in a
mammal comprising administering to the mammal a compound as
described herein.
15 According to the method, chemokine-mediated chemotaxis
and/or activation of pro-inflammatory cells bearing
receptors for chemokines can be inhibited. As used herein,
"pro-inflammatory cells" includes but is not limited to
leukocytes, since chemokine receptors can be expressed on
20 other cell types, such as neurons and epithelial cells.
While not wishing to be bound by any particular theory
or mechanism, it is believed that compounds of the
invention are antagonists of the chemokine receptor CCR1,
and that therapeutic benefits derived from the method of
25 the invention are the result of antagonism of CCR1
function. Thus, the method and compounds of the invention
can be used to treat a medical condition involving cells
which express CCR1 on their surface and which respond to
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-9-
signals transduced through CCR1, as well as the specific
conditions recited above.
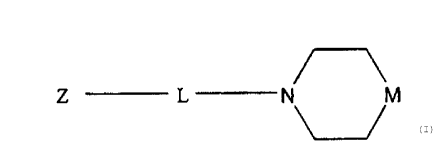
In one embodiment, the antagonist of chemokine
receptor function is represented by the structural formula
(I)
Z L N M
(I)
Z is a cycloalkyl or non-aromatic heterocyclic ring
group fused to a pyridine ring and to a carbocyclic
aromatic or heteroaromatic ring, wherein each ring in Z is
independently substituted or unsubstituted.
L is a C1-C18 hydrocarbyl group wherein, optionally one
or more of the carbon atoms is replaced by a heteroatom
such as nitrogen, oxygen or sulfur.
M is >NRZ or >CR1R2.
R1 is -H, -OH, -N3, halogen, an aliphatic group, -O-
(aliphatic group), -O-(substituted aliphatic group), -SH,
-S-(aliphatic group), -S-(substituted aliphatic group),
-OC(O)-(aliphatic group), -O-C(O)-(substituted aliphatic
group), -C(O)O-(aliphatic group), -C(O)O-(substituted
aliphatic group) , -COOH, -CN, -CO-NR3R', -NR3R'; or R1 can be
a covalent bond between the ring atom at M and an adjacent
carbon atom in the ring which contains M. R1 is preferably
-H or -OH.
RZ is -H, -OH, an acyl group, a substituted acyl group,
-NRSR6, an aliphatic group, a substituted aliphatic group,
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-10-
an aromatic group, a substituted aromatic group, a benzyl
group, a substituted benzyl group, a non-aromatic
heterocyclic group or a substituted non-aromatic
heterocyclic group. R2 is preferably an aromatic group or
5 a substituted aromatic group.
R3, R4, RS and R6 are independently -H, an acyl group, a
substituted acyl group, an aliphatic group, a substituted
aliphatic group, an aromatic group, a substituted aromatic
group, a benzyl group, a substituted benzyl group, a non-
10 aromatic heterocyclic group or a substituted non-aromatic
heterocyclic group.
R1 and Rz, R' and R4, or RS and R6 taken together with
the atom to which they are bonded, can alternatively form a
substituted or unsubstituted non-aromatic carbocyclic or
15 heterocyclic ring.
In a preferred embodiment, L in Structural Formula (I)
is a chemical group represented by Structural Formula (II):
Y ~CH2)n
20 (II)
Y is a covalent bond, -0-, -CO- or =CH-.
n is an integer from one to eighteen, more preferably
n is an integer from one to about five, most preferably n
is three.
25 X is a single covalent bond or -CO-, and the
antagonist of chemokine receptor function is represented by.
Structural Formula (III):
CA 02343536 2001-03-05
WO 00/14089 PCTJl1S99/01235
-11-
Y (CH2)n
(III)
Z and M are as described above for Structural Formula
(I) .
Y, n and X are as described above for Structural
5 Formula (II).
In another preferred embodiment, X and Y in Structural
Formula (III) are each a covalent bond and the antagonist
of chemokine receptor function is a compound represented by
Structural Formula (IV):
10
Z (CHz)n
(IV)
n is an integer from one to about five. n is
preferably three.
Z and M are as described above for Structural Formula
15 (I) .
In another preferred embodiment, X is a covalent bond,
Y is -CO- and the antagonist of chemokine receptor function
is a compound represented by Structural Formula (V):
CA 02343536 2001-03-05
WO 00114089 PCT/US99/01235
-12-
O
Z (CHZ)n N M
(v>
Z, M and n are as described above for Structural
Formula (IV).
In another preferred embodiment, X is a covalent bond,
Y is a double bond and the antagonist of chemokine receptor
function is a compound represented by Structural formula
(VI)
Z
( CH2 ) n
15 (VI)
Z, M and n are as described above for Structural
Formula (IV). Preferably n is two.
In embodiments where M is >CR1R2 and R1 is a covalent
bond between the carbon atom at M and an adjacent carbon
20 atom in the ring which contains M, the antagonist of
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-13-
chemokine function can be represented by Structural
Formulas (IVa) and (VIa) .
5
_ z C ~ - Rz
Z-(C~~N /C R
Z
(IVa) (VIa)
Z, n, and Rz are as described in Structural Formula
(I) .
Preferably, Z is a tricyclic ring system comprising a
10 six, seven or eight membered cycloalkyl or a non-aromatic
heterocyclic ring group fused to a pyridine ring and to a
carbocyclic aromatic group. In one example, Z is
represented by Structural Formula (VII):
15
(VII)
The pyridine ring labeled with an "A", and the phenyl
ring labeled with a "B" are herein referred to as "Ring A"
20 and "Ring B" respectively. The central ring labeled with a
CA 02343536 2001-03-05
WO 00/14089 PC1'/US99/01235
-14-
"C", is herein referred to as "Ring C" and can be, for
example, a six, seven or eight membered non-aromatic
carbocyclic ring (e. g., a cycloheptane or cyclooctane ring)
or a non-aromatic heterocyclic ring. When Ring C is a non-
5 aromatic heterocyclic ring, it can contain one or two
heteroatoms such as nitrogen, sulfur or oxygen. When Z is
represented by Structural Formula (VII), the tricyclic ring
system can be connected to Y in Structural Formula (III) by
a single or double covalent bond between Y and a ring atom
10 in Ring C.
Each ring can be unsubstituted or can have one or more
substituents. Suitable substituents are as described
herein below for substituted aromatic groups. In one
example, Ring B is substituted with - (O) u- (CHz) t-COORzo,
15 - (O) "- (CHz) t-OC (0) RZ°' - (O) "- (CHz) t-C (0) -NRalRza or
- (O) "- (CH2) t-NHC (O) O-Rz°.
a is zero or one.
t is an integer, such as an integer from zero to about
three, and the methylene group, -(CH2)t-, can be substituted
20 or unsubstituted.
R2°, R~1 or R22 are independently -H, an aliphatic group,
a substituted aliphatic group, an aromatic group, a
substituted aromatic group or a substituted or
unsubstituted non-aromatic heterocyclic group.
25 Alternatively, R21 and R2z, taken together with the nitrogen
atom to which they are bonded, form a non-aromatic
heterocyclic ring. In another example, Ring B is
substituted with R$ and R9, wherein Ra and R9 are
CA 02343536 2001-03-05
WO 00114089 PCT/US99/01235
-15-
independently -H, a halogen, alkoxy or alkyl; or, taken
together with Ring B, form a naphthyl group
Ring C optionally contains one or more additional
substituents as described herein below. Preferably, Ring C
is substituted with an electron withdrawing group or is
unsubstituted. Suitable electron withdrawing groups
include -CN, -CH=NH, alkylimines, alkylsulfonyl,
carboxamido, carboxylic alkyl esters, -N02 and halogens
(e. g., -Br and -C1). Alternatively, Ring C is substituted
with a group selected from -CH2-NR11R=', -CH2-OR1',
-CH2-NH-CO-NR11R1z, -CH2-O-CO-NRIIRIZ or -CH2-NHC (O) -O-R11 .
Rll and R12 are independently -H, an aliphatic group, a
substituted aliphatic group, an aromatic group, a
substituted aromatic group or a non-aromatic heterocyclic
group. Alternatively, R1- and R12, taken together with the
nitrogen atom to which they are bonded, form a non-aromatic
heterocyclic ring.
Examples of suitable tricyclic ring systems
represented by Structural Formula (VII) are provided by
Structural Formulas (VIII)-(X), shown below:
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-16-
W
W
A\) C ~ B A C l B\
1 ' and
\X / ~ N X1
N z
(VIII) (IX}
W
A C ~ B
X1
(X)
X1 is a covalent bond, -S-, -CHz-, -CHz-CHz-, -CHz-S-,
-S-CH2-, -O-CHZ-, -CHZ-O-, -NR~-CHZ-, -CHZ-NR~-, -SO-CHz-,
-CHz-SO-, -S (O) z-CHz-, -CH2-S (0) 2-, -CH=CH-, -NR~-CO- or
5 =CO-NR~-
R~ is hydrogen, an aliphatic group, a substituted
aliphatic group, an aromatic group, a substituted aromatic
group, a benzylic group or a substituted benzylic group.
In one example, R~ is - (CHz) s-COOR'°, - (CHz) e-C (O) -NR31R'z
or
10 - (CHz) g-NHC (O) -O-R3°.
s is an integer from zero to about 3; and
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-17-
R'o, R'1 or R32 are independently -H, an aliphatic group,
a substituted aliphatic group, an aromatic group, a
substituted aromatic group or a non-aromatic heterocyclic
group. Alternatively, R31 and R32, taken together with the
5 nitrogen atom to which they are bonded, form a non-aromatic
heterocyclic ring.
W is -H, an electron withdrawing group or is selected
from -CH2-NRl'Rl~, -CH2-OR11, -CH2-NH-CO-NRllRla, -CH2-O-CO-NR"R1'
or -CHz-NHC (O) -O-Rll .
10 Rll and R12 are as defined above in Structural Formula
(VII) .
Ring B in Structural Formulas (VIII}-(X) can be
unsubstituted or substituted as described in Structural
Formula (VII).
15 In a preferred embodiment Ring B in Structural
Formulas (VIII)-(X} is substituted para to the carbon atom
in Ring B which is bonded to X1 in Ring C, and the
tricyclic ring system is represented by Structural Formulas
(XI) - (XIII) shown below:
20
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-18-
W f
R4o W
A~~ C ~ B A C B\ R4o
N~ ~ X ~ ' ~ ~ I and
1 N X1 /
(XI ) j,,l ~ (XII )
R4o
A C I B
N Xi ,~-'
(XIII)
X1 and w are as defined above in Structural Formulas
(VI II ) - (X) .
R°° is a substituent as described herein. Preferably
5 R4° is an aliphatic group, substituted aliphatic group,
-O-(aliphatic group) or -O-(substituted aliphatic group).
More preferably R4° is an -O-alkyl, such as -O-CH3, -O-C2H5,
-O-C3H, or -O-C4H9.
In this preferred embodiment the antagonist of
10 chemokine receptor function is a compound represented by
Structural Formulas (XIV) - (XVI) shown below:
CA 02343536 2001-03-05
_ WO 00/14089 PCT/US99/01235
-19-
M M
c~ c~
N N
n(HyC)
n(HZC) qr
Rao
Rno
N~~x~ ~ ' ~ A / X ~ ~ and
N
M
(XIV) ~ ~ (XV)
Rao
(XVI)
n is as defined above in Structural Formula (II). M is
as described above in Structural Formula (I).
X1, W and R'° are as described above in Structural
Formulas (XI) - (XIII). Preferably in Structural Formulas
(XIV) - (XVI ) X1 is -CHZ-O-, W is -CN, M is >C (OH) R2, Rq° is
-0-CH3and n is three.
In another embodiment, the antagonist of chemokine
activity can be represented by Structural Formula (XVII):
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-20-
Z Y ( CHz )-n - X N M
q
(XVII)
and physiologically acceptable salts thereof.
n, Y, X and M are as described in Structural Formula
5 (I) .
Z is as described herein, preferably Z is as described
in Structural Formulas (XI) - (XIII).
q is an integer, such as an integer from zero to about
three, and the ring containing M can be substituted or
10 unsubstituted.
Thus, the antagonist of chemokine function can be
represent by, for example, Structural Formulas (XVIIa)-
(XVIId)
Z ( CF~ n ~ Z ( C~ n M
M
15 (XVIIa) (XVIIb)
R2
~C~
M Z-. ( c~ n
Z-( cx~ n
(XVIIc) (XVIId)
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-21-
and physiologically acceptable salts thereof, wherein Z, n
and M are as described in Structural Formula (VII), and the
ring which contains M is substituted or unsubstituted.
Another embodiment of the invention provides novel
5 compounds employed in these methods.
Also included in the present invention are
physiologically acceptable salts of the compounds
represented by Structural Formulas (I) through (XVIId).
Salts of compounds containing an amine or other basic group
10 can be obtained, for example, by reacting with a suitable
organic or inorganic acid, such as hydrogen chloride,
hydrogen bromide, acetic acid, citric acid, perchloric acid
and the like. Compounds with a quaternary ammonium group
also contain a counteranion such as chloride, bromide,
15 iodide, acetate, perchlorate and the like. Salts of
compounds containing a carboxylic acid or other acidic
functional group can be prepared by reacting with a
suitable base, for example, a hydroxide base. Salts of
acidic functional groups contain a countercation such as
20 sodium, potassium, ammonium, calcium and the like.
As used herein, aliphatic groups include straight
chained, branched or cyclic C1-Czo hydrocarbons which are
completely saturated or which contain one or more units of
unsaturation. For example, suitable aliphatic groups
25 include substituted or unsubstituted linear, branched or
cyclic C1-C2o alkyl, alkenyl or alkynyl groups.
A hydrocarbyl group includes straight chain C1-ClB
hydrocarbons which are completely saturated or which
contain one or more units of unsaturation. Optionally, one
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-22-
or more of the carbon atoms in a hydrocarbyl group may be
replaced with a heteroatom such as oxygen, nitrogen or
sulfur. An "alkyl group" is a saturated aliphatic group,
as defined above. The term "alkoxy" refers to an alkyl
5 ether chain with an alkyl group. "Alkanoyl" refers to
alkyl substituted carbonyl; "aralkanoyl" refers to
phenyl-alkyl-CO- and "aroyl" refers to arylcarbonyl
including benzoyl, naphthoyl and the like. The term
"halogen" means fluoro, chloro, bramo and iodo. The term
10 "substituted phenyl" means phenyl substituted by alkyl,
halogen, alkoxy, nitro, amino, acetamido, cyano and
trifluoromethyl and naphthyl. "Aralkyl" means -(CHz)X-aryl,
wherein x is an integer from one to four including benzyl.
Aromatic or aryl groups include carbocyclic aromatic
15 groups such as phenyl, 1-naphthyl, 2-naphthyl, 1-anthracyl
and 2-anthracyl, and heterocyclic aromatic or heteroaryl
groups such as N-imidazolyl, 2-imidazolyl, 4-imidazolyl,
5-imidazolyl, 2-thienyl, 3-thienyl, 2-furanyl, 3-furanyl,
2-pyrrolyl, 3-pyrrolyl, 2-pyridyl, 3-pyridyl, 4-pyridyl,
20 2-pyrimidyl, 4-pyrimidyl, 5-pyrimidyl, 3-pyridazinyl,
4-pyridazinyl, 3-pyrazolyl, 4-pyrazolyl, 5-pyrazolyl,
2-pyrazinyl, 2-thiazolyl, 4-thiazolyl, 5-thiazolyl,
5-tetrazolyl, 2-oxazolyl, 4-oxazolyl and 5-oxazolyl.
Where these rings are fused, for example, to Ring C, the
25 stated point of attachment can be either of the two fused
bonds.
Aromatic groups also include fused polycyclic aromatic
ring systems in which a carbocyclic aromatic ring or
heteroaryl ring is fused to one or more other heteroaryl
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-23-
rings. Examples include tetrahydronapthyl, 2-benzothienyl,
3-benzothienyl, 2-benzofuranyl, 3-benzofuranyl, 2-indolyl,
3-indolyl, 2-quinolinyl, 3-quinolinyl, 2-benzothiazolyl,
2-benzooxazolyl, 2-benzimidazolyl, 2-quinolinyl,
S 3-quinolinyl, 1-isoquinolinyl, 3-isoquinolinyl,
1-isoindolyl, 3-isoindolyl, and acridinyl. Also included
within the scope of the term "aromatic group", as it is
used herein, is a group in which one or more carbocyclic
aromatic rings and/or heteroaromatic rings are fused to a
10 cycloalkyl or non-aromatic heterocyclic ring. Examples
include decalin, phthalimido, benzodiazepines,
benzooxazepines, benzooxazines, phenothiazines, and groups
represented by the following structural formulas:
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-24-
i~ S i~ i~ y
w ° ~ w
i i> >
i~ i~ i~°
or
The term "non-aromatic ring" includes non-aromatic
carbocyclic rings and non-aromatic heterocyclic rings.
Non-aromatic heterocyclic rings are non-aromatic
carbocyclic rings which include one or more heteroatoms
5 such as nitrogen, oxygen or sulfur in the ring. The ring
can be five, six, seven or eight-membered and/or fused to
another ring, such as a cycloalkyl or aromatic ring.
Examples of non-aromatic rings include, for example,
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-25-
3-1H-benzimidazol-2-one, 3-1-alkyl-benzimidazol-2-one,
3-1-methyl-benzimidazol-2-one, 2-tetrahydrofuranyl,
3-tetrahydrofuranyl, 2-tetrahyrothiophenyl,
3-tetrahyrothiophenyl, 2-morpholino, 3-morpholino,
5 4-morpholino, 2-thiomorpholino,
3-thiomorpholino, 4-thiomorpholino, 1-pyrrolidinyl,
2-pyrrolidinyl, 3-pyrrolidinyl, 1-piperazinyl,
2-piperazinyl, 1-piperidinyl, 2-piperidinyl, 3-piperidinyl,
4-piperidinyl, 4-thiazolidinyl, diazolonyl, N-substituted
10 diazolonyl, 1-phthalimidyl, 1-3-alkyl-phthalimidyl,
tetrahydronapthyl, benzocyclopentane, benzocyclohexane,
benzoxane, benzopyrolidine, benzopiperidine, benzoxolane,
benzothiolane, benzothiane,
a n O 0 S S HN
o O O O O
O O O ~O NH ~NH ~:7H H/O~
HH NN O
\ / \ \ \ \ ~ \ ~ \ ~ \
C1 ~ C1 ~ ~ ~ '' ~ ~ ~ '" ~ ~ and ~
15 "Heterocyclic ring" includes "heteroaryl group" and
"non-aromatic heterocylic ring". Examples of heterocyclic
rings include imidazole, benzimidazole, pyridine,
pyrimidine, thiazole, benzothiazole, thienyl, benzothienyl.
Suitable substituents on an alkyl, aliphatic,
20 aromatic, non-aromatic heterocyclic ring or benzyl group
include, for example, an electron withdrawing group, an
CA 02343536 2001-03-05
WO 00/14089 PCT/US99101235
-26-
aliphatic group, substituted aliphatic group, azido, -OH, a
halogen (-Br, -C1, -I and -F), -0-(aliphatic, substituted
aliphatic, benzyl, substituted benzyl, aromatic or
substituted aromatic group), -CN, -NOz, -COOH, -NH2,
5 -NH(aliphatic group, substituted aliphatic, benzyl,
substituted benzyl, aromatic or substituted aromatic
group), -N-(aliphatic group, substituted aliphatic, benzyl,
substituted benzyl, aromatic or substituted aromatic
group)Z, -COO(aliphatic group, substituted aliphatic,
10 benzyl, substituted benzyl, aromatic or substituted
aromatic group), -CONH~, -CONH(aliphatic, substituted
aliphatic group, benzyl, substituted benzyl, aromatic or
substituted aromatic group). -CON(aliphatic, substituted
aliphatic group, benzyl, substituted benzyl, aromatic or
15 substituted aromatic group)z, -SH, -SOk(aliphatic,
substituted aliphatic, benzyl, substituted benzyl, aromatic
or substituted aromatic group) (k is 0, 1 or 2),
-NH-C (=NH) -NH2, - (O) ~- (CHz) t-COOR2° , - (O) u- (CH2) t-OC (O)
Rz°,
- (0) "- (CHZ) t-C (0) -NRZIRzz or _ (0) u- (CH2) t-NHC (O) O-RZ°
20 Rz°, R21 or R22 are independently -H, an aliphatic group,
a substituted aliphatic group, an aromatic group, a
substituted aromatic group or a non-aromatic heterocyclic
group, and wherein RZ1 and Rzz, taken together with the
nitrogen atom to which they are bonded, can form a non-
25 aromatic heterocyclic ring.
a is an integer such as zero or one.
t is an integer such as an integer from zero to about
three, and the methylene group, -(CH2)t-, can be substituted
or unsubstituted.
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-27-
A substituted non-aromatic heterocyclic ring, benzyl
group or aromatic group can also have an aliphatic or
substituted aliphatic group, as a substituent. A
substituted alkyl or aliphatic group can also have a non-
5 aromatic heterocyclic ring, benzyl, substituted benzyl,
aromatic or substituted aromatic group as a substituent. A
substituted non-aromatic heterocyclic ring can also have
=O, =S, =NH or =N(aliphatic, aromatic or substituted
aromatic group) as a substituent. A substituted aliphatic,
10 substituted aromatic, substituted non-aromatic heterocyclic
ring or substituted benzyl group can have more than one
substituent.
Acyl groups include substituted and unsubstituted
aliphatic carbonyl, aromatic carbonyl, aliphatic sulfonyl
15 and aromatic sulfonyl.
Suitable electron withdrawing groups include, for
example, alkylimines, alkylsulfonyl, carboxamido,
carboxylic alkyl esters, -CH=NH, -CN, -NOZ and halogens.
The compounds disclosed herein may be obtained as
20 different sterioisomers (e.g., diastereomers and
enantiomers). For example, when the antagonist of
.chemokine receptor function is represented by Structural
Formula (III) and Z is represented by Structural Formula
(VII), the carbon atom in Ring C which is bonded to Y may
25 be in the R or S sterioconfiguration. It is pointed out
that the invention includes all isomeric forms and racemic
mixtures of the disclosed compounds and a method of
treating a subject with both pure isomers and mixtures
thereof, including racemic mixtures.
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-28-
It is understood that one sterioisomer can have
greater activity than another, The desired isomer can be
determined by screening for activity, employing the methods
described herein.
5 In the structural formulas depicted herein, the single
or double bond by which a chemical group or moiety is
connected to the remainder of the molecule or compound is
indicated by the following symbol:
10 For example, the corresponding symbol in Structural Formula
(VIII) or (IX) indicates that the tricyclic ring system,
which represent Z in Structural Formula (IV), is connected
to the alkylene group in Structural Formula (IV) by a
single covalent bond between the alkylene group and the
15 ring carbon in Ring C which is bonded to W.
A "subject" is preferably a bird or a mammal, such as
a human, but can also be an animal in need of veterinary
treatment, e.g., domestic animals (e.g., dogs, cats, and
the like), farm animals (e. g., cows, fowl, sheep, pigs,
20 horses, and the like) and laboratory animals (e. g., rats,
mice, guinea pigs, and the like).
An "effective amount" of a compound is an amount which
results in the inhibition of one or more processes mediated
by the binding of a chemokine to a receptor in a subject
25 with a disease associated with aberrant leukocyte
recruitment and/or activation. Examples of such processes
include leukocyte migration, integrin activation, transient
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-29-
increases in the concentration of intracellular free
calcium [Caz']; and granule release of proinflammatory
mediators. Alternatively, an "effective amount" of a
compound is a quantity sufficient to achieve a desired
5 therapeutic and/or prophylactic effect, such as an amount
which results in the prevention of or a decrease in the
symptoms associated with a disease associated with aberrant
leukocyte recruitment and/or activation.
The amount of compound administered to the individual
10 will depend on the type and severity of the disease and on
the characteristics of the individual, such as general
health, age, sex, body weight and tolerance to drugs. It
will also depend on the degree, severity and type of
disease. The skilled artisan will be able to determine
15 appropriate dosages depending on these and other factors.
Typically, an effective amount of the compound can range
from about 0.1 mg per day to about 100 mg per day for an
adult. Preferably, the dosage ranges from about 1 mg per
day to about 100 mg per day. An antagonist of chemokine
20 receptor function can also be administered in combination
with one or more additional therapeutic agents, e.g.
theophylline, (3-adrenergic bronchodilators,
corticosteroids, antihistamines, antiallergic agents,
immunosuppressive agents (e. g., cyclosporin A, FK-506,
25 prednisone, methylprednisolone) and the like.
The compound can be administered by any suitable
route, including, for example, orally in capsules,
suspensions or tablets or by parenteral administration.
Parenteral administration can include, for example,
CA 02343536 2001-03-05
WO 00/14089 PCTIUS99/01235
-30-
systemic administration, such as by intramuscular,
intravenous, subcutaneous, or intraperitoneal injection.
The compound can also be administered orally (e. g.,
dietary), transdermally, topically, by inhalation (e. g.,
5 intrabronchial, intranasal, oral inhalation or intranasal
drops), or rectally, depending on the disease or condition
to be treated. Oral or parenteral administration are
preferred modes of administration.
The compound can be administered to the individual in
10 conjunction with an acceptable pharmaceutical or
physiological carrier as part of a pharmaceutical
composition for treatment of HIV infection, inflammatory
disease, or the other diseases discussed above.
Formulation of a compound to be administered will vary
15 according to the route of administration selected (e. g.,
solution, emulsion, capsule). Suitable carriers may
contain inert ingredients which do not interact with the
compound. Standard formulation techniques can be employed,
such as those described in Remington's Pharmaceutical
20 Sciences, Mack Publishing Company, Easton, PA. Suitable
pharmaceutical carriers for parenteral administration
include, for example, sterile water, physiological saline,
bacteriostatic saline (saline containing about 0.9% mg/ml
benzyl alcohol), phosphate-buffered saline, Hank's
25 solution, Ringer's-lactate and the like. Methods for
encapsulating compositions (such as in a coating of hard
gelatin or cyclodextran) are known in the art (Baker, et
al., "Controlled Release of Biological Active Agents", John
Wiley and Sons, 1986).
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-31-
The activity of compounds of the present invention can
be assessed using suitable assays, such as receptor binding
assays and chemotaxis assays. For example, as described in
the Exemplification Section, small molecule antagonists of
5 RANTES and MIP-1a binding have been identified utilizing
THP-1 cells which bind RANTES and chemotax in response to
RANTES and MIP-la as a model for leukocyte chemotaxis.
Specifically, a high through-put receptor binding assay,
which monitors ~25I-RANTES and 125I-MIP-la binding to THP-1
10 cell membranes, was used to identify small molecule
antagonists which block binding of RANTES and MIP-la.
Compounds of the present invention can also be identified
by virtue of their ability to inhibit the activation steps
triggered by binding of a chemokine.to its receptor, such
15 as chemotaxis, integrin activation and granule mediator
release. They can also be identified by virtue of their
ability to block R.ANTES and MIP-la mediated HL-60, T-cell,
peripheral blood mononuclear cell, and eosinophil
chemotactic response.
20 The compounds disclosed herein can be prepared
accordingly to the schemes shown in Figures 1-5. The
schemes are described in greater detail. below.
Figure 1 is a schematic showing the preparation of
compounds represented by Structural Formulas (I) and (II),
25 wherein Z is represented by Structural Formula (IV),
wherein W is CN.
L1, L2 and L' in Figure 1 are suitable leaving groups
such as halogen; p-toluene sulfonate, mesylate, alkoxy and
phenoxy. The other symbols are as defined above.
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-32-
The reduction reaction in Step 1 of Figure 1 is
performed with a reducing agent such as sodium borohydride
or lithium aluminum hydride (LAH) in an inert solvent such
as methanol or tetrahydrofuran (THF). The reaction is
5 carried out at temperatures ranging from 0°C up to the
reflux temperature and for 5 minutes to 72 h.
Compounds represented by formula II in Figure 1 can be
prepared by procedures disclosed in JP 61/152673, U.S.
Patent 5089496, WO 89/10369, WO 92/20681 and WO 93/02081,
10 the entire teachings of which are incorporated herein by
reference.
A chlorination reaction in step 2 of Figure 1 can be
performed with reagents such as thionyl chloride. The
reaction can be carried out in an inert solvent such as
15 methylene chloride at 0°C up to the reflux temperature for
5 minutes to 72 h. The hydroxy group can be also be
converted to other leaving groups by methods familiar to
those skilled in the art.
The cyanation reaction in step 3 of Figure 1 can be
20 carried out using reagents such as copper cyanide, silver
cyanide or sodium cyanide in an inert solvent such as
benzene or toluene. Reaction temperatures range from 0°C
up to the reflux temperature for 5 minutes to 72 h.
Compounds represented by Formula V in Figure 1 can also be
25 prepared by the procedures described in J. Med. Chem. 1994,
37, 804-810 and U.S. Patent 5672611, the entire teachings
of which are incorporated herein by reference.
The alkylation reactions in steps 4 and 5 of Figure 1 can
be carried out in a solvent such as acetone, methyl ethyl
CA 02343536 2001-03-05
WO 00114089 PCT/US99/OI235
-33-
ketone, ethyl acetate, toluene, tetrahydrofuran (THF) or
dimethylformamide (DMF) in the presence of a base such as
potassium carbonate or sodium hydride and a catalyst such
as an alkali metal iodide (when necessary). The reaction
5 temperature can range from room temperature up to the
reflux temperature and for 5 minutes to 72 h.
The product of the synthetic scheme shown in Figure 1
can be decyanated using a reducing agent such as lithium
aluminum hydride (L,AH) in an inert solvent such as ether or
10 tetrahydrofuran (THF) at 0°C up to the reflux temperature
for the solvent used for 5 minutes to 72 h.
Figure 2 is a schematic showing the preparation of
representative compounds of Structural Formula (I), (III)
and (IV), wherein Z is represented by Structural Formula
15 (VIII) and wherein Ring A and/or Ring B in Z can be
substituted with - (O),;- (CHz) ~-COORz°, - (O),,- (CHz) t-OC (O) Rzo,
- (O) u- (CHz) t-C (O) -NRzlRzz or' _ (O) "_ (CHz) t-NHC (O) -O-Rzo .
In Figure 2, the hydrolysis reaction may be carried
out in a mixture of aqueous alkali metal hydroxide solution
20 and a solvent such as methanol, ethanol, tetrahydrofuran
(THF) or dioxane at room temperature up to the reflux
temperature for the solvent used for 5 minutes to 72 h.
The acylation reaction can be carried out using
dicyclohexylcarbodiimide (DCC) or
25 (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (DEC) in a
solvent such as tetrahydrofuran (THF), dimethylformamide
(DMF) or methylene chloride in the presence of a base such
as pyridine or triethylamine (when necessary) at
temperatures of 0 to 100°C for 5 minutes to 72 h.
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-34-
Compounds represented by Structural Formulas
(I),(III) and (IV) wherein Z is represented by Structural
Formulas (VIII ) - (XI ) , wherein X1 is -CO-N (R~) - and R~ is
- (CHZ} $-COORS°, - (CHZ} s-C (O) -NR'1R'2 or - (CHZ) s-NHC (0) -O-
R3° Cari
S be prepared by suitable modification of the scheme shown in
Figures 1 and 2. One modification utilizes the starting
material shown in Figures 1 and 2, wherein X1 is -CO-NH-.
The amide is then alkylated with L3- (CH2) g-COOR'° using the
alkylation procedures described above. L3 is a suitable
leaving group. The remainder of the synthesis is as
described in Figures 1 and 2.
Figure 3 is a schematic showing the preparation of
the compounds represented by Structural Formula (I),(III)
and (IV) wherein Z is represented by Structural Formula
(VIII).
The reduction of the cyano group to an amine in Figure
3 can be carried out using metal hydrides or by catalytic
reduction processes. Suitable reducing agents include
lithium aluminum hydride (LAH), diisobutyl aluminum hydride
(DIBAL-H), borane-methyl sulfide complex or sodium
borohydride. The reduction can be carried out in an inert
solvent such as ether, tetrahydrofuran (THF), methylene
chloride or methanol at -78°C up to the reflux temperature
for 5 minutes to 72 h. It is also possible to isolate the
corresponding imine intermediate, which can be converted to
the amine using similar reduction processes.
Figure 4 is a schematic showing the preparation of
compounds represented by Structural Formulas (I), (III) and
(IV), wherein Z is represented by Structural Formula
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235_
-35-
(VIII), wherein W is H. The reduction of the double bond
in step 1 of Figure 4 can be carried out using the
catalytic reduction process. Suitable catalyst include
palladium-carbon, platinum oxide or Ranney-nickel. The
5 reduction can be carried out in an inert solvent such as
methanol, ethanol or acetic acid at temperatures of 0 to
70°C under a hydrogen pressure of 1 to 100 atm for 5
minuets to 72 h. The alkylation reactions in step 2 of
Figure 4 can be carried out using the same as those in step
10 5 of Figure 1.
Figure 5 is a schematic showing the preparation of
compounds represented by Structural Formulas (I), (III) and
(IV), wherein Z is represented by Structural Formula
(VIII), wherein W is H. The alkylation reaction in step 1
15 of Figure 5 can be carried out using the same as those in
step 5 of Figure 1. The reduction of the double bond in
step 2 of Figure 5 can be carried out using the same as
those in step 1 of Figure 4.
Figure 6 shows the preparation of compounds represented
20 by Structural Formula (I), where in Z is represented by
Structural Formulas (VIII) and wherein Ring A and/or Ring B
in Z is substituted with - (0) "- (CH2) t-COOR2°, a is one . In
Figure 6, the alkylation reaction can be carried out in a
solvent such as acetone, methyl ethyl ketone, ethyl
25 acetate, toluene, tetrahydrofuran (THF) or
dimethylformamide (DMF) in the presence of a base such as
potassium carbonate or sodium hydride and a catalyst such
as an alkali metal iodide at room temperature up to the
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-36-
reflux temperature for the solvent used for 5 minutes to 72
h.
Figure 7 shows the preparation of compounds represented
by Structural Formula (I), wherein Z is represented by
5 Structural Formulas (VIII) and wherein Ring A or Ring B in
Z is substituted with - (O) "- (CHZ) t-COORZ°, a is zero. L4 is
a suitable leaving group such as halogen or
trifluoromethylsulfonate. In Figure 7, a palladium
coupling reaction such as Stille coupling, Suzuki coupling,
l0 Heck reaction, or carboxylation using carbon monoxide can
be carried out using a palladium catalyst such as
tetrakis(triphenylphosphine)palladium,
bis(triphenylphosphine)palladium chloride, and palladium
acetate in a solvent such as tetrahydrofuran (THF), 1,4-
15 dioxane, toluene, dimethylformamide (DMF), or
dimethylsufoxide (DMSO) in the presence of additive (when
necessary) such as triphenylphosphine, l,l'-
bis(diphenylphosphino)ferrocene, triethylamine, sodium
bicarbonate, tetraethylammonium chloride, or lithium
20 chloride at room temperature up to the reflux temperature
for the solvent used for 5 minutes to 72 h.
Although Figures 1-7 show the preparation of compounds
in which B is a phenyl ring, analogous compounds with
heteroaryl groups for Ring B can be prepared by using the
25 starting materials with heteroaryl groups in the
corresponding positions, which can be prepared according to
methods disclosed in JP 61/152673, U.S. Patent 5089496, WO
89/10369, WO 92/20681 and WO 93/02081.
CA 02343536 2001-03-05
WO 00!14089 PCT/US99/01235
-37-
The invention is illustrated by the following
examples which are not intended to be limiting in any way.
EXEMPLIFICATION
Example 1
5 4-(4-Chlorophenyl)-1-[3-(5,11-dihydro-7-methoxypyrido[2,3-
c][1]benzoxepin-5-propyl]piperidin-4-of
Step 1
To a solution of 5,11-dihydro-7-methoxypyrido[2,3-
c][1]benzoxepin-5-one (S.Og) in THF (50m1) was added 1.1M
l0 cyclopropylmagnesium bromide THF solution (25m1) at 0°C.
The reaction mixture was warmed to room temperature, and
stirred for 30 minutes. Aqueous ammonium chloride and ethyl
acetate were added to the reaction mixture, the organic
layer was separated and washed with saturated aqueous
15 sodium chloride, and dried with magnesium sulfate. The
solvent was distilled off under reduced pressure. The
residue was filtered and washed with ethyl acetate-hexane
(1:2) to give 5-cyclopropyl-5,11-dihydro-7-
methoxypyrido[2,3-c][1]benzoxepin-5-of (5.Og).
20 Step 2
To a solution of the product of step 1 (4.3g) in
acetic acid (30m1) was added 48~ aqueous HBr (25m1) at
10°C. The reaction mixture was warmed to room temperature,
and stirred for 12 hours. Water and ethyl acetate were
25 added to the reaction mixture and neutralized with dilute
NaOH solution. The organic layer was separated and washed
with saturated aqueous sodium chloride, and dried over
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-38-
magnesium sulfate. The solvent was distilled off under
reduced pressure. The residue was purified by silica gel
chromatography eluting with ethyl acetate-hexane (1:4) to
give 5-(3-bromopropylidene)-5,11-dihydro-7-
methoxypyrido [2 , 3-c] [1] benzoxepine (5 . 6g) .
1H-NMR (CDC13) 8: 2 . 74 (2H, q) , 3 .46 (2H, t) , 3 . 78 (3H, s) ,
5.25 (2H,brs) , 6.07 (lH,t) , 6.72-6.82 (3H,m) , 7.21-7.42 (5H,m) ,
7.56(lH,dd), 8.45(lH,dd).
Step 3
10 To a solution of the product of step 2 (160mg) in
ethanol (3m1) and acetic acid (lml) were added 10~ Pd-C
(79mg) was stirred under hydrogen (under a balloon) at room
temperature for 24 hour. The mixture was filtered through
the celite and distilled off under reduced pressure. The
15 residue was purified by preparative thin layer
chromatography eluting with ethyl acetate-hexane (1:2) to
give 5-(3-bromopropyl)-5,11-dihydro-7-methoxypyrido[2,3-
c] [1] benzoxepine (48mg) .
1H-NMR (CDC13) 8: 1.80-2 .45 (4H,m) , 3 .33-3.39 (2H,m) ,
20 3.59 (lh,dd) , 3.77 (3H, s) , 4.98 (lH,d) , 5.44 (lH,d) , 6.70-
6.79 (2H,m) , 7.08-7.14 (SH,m) , 7.52 (lH,dd) , 8.41 (lH,dd) .
Step 4
To a solution the product of step 3 (45mg) in DMF
(lml) were added 4-(4-chlorophenyl)-4-hydroxypiperidine
25 (54mg) and potassium carbonate (l9mg) and the mixture was
stirred at 50°C for 1 hour. Water and ethyl acetate were
added to the reaction mixture, the organic layer was
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-39-
separated and washed with saturated aqueous sodium
chloride, and dried with magnesium sulfate. The solvent was
distilled off under reduced pressure. The residue was
purified by silica gel chromatography eluting with ethyl
5 acetate-methanol (10:1) to give the titled compound (l9mg).
1H-NMR (CDC11) 8: 1.50 (lH,brs) , 1.67-1.72 (2H,m) , 2.00-
2.47(lOH,m), 2.76-2.81(2H,m), 3.59(lH,dd), 3.77(3H,s),
4.97(lH,d), 5.43(lH,d), 6.72-6.78(2H,m), 7.06-7.13(2H,m),
7.26-7 .44 (4H,m) , 7. 52 (1H, dd) , 8.37 (1H, dd) .
10 MS m/z: 479(M+1)
Examples 2 - 157 which can be represented by Structural
Formulas (XIV) and (XVI) and are presented in Table 1 and
Table la, can be prepared by methods set forth in the
schemes in Figure 1-5 and the procedures described above.
CA 02343536 2001-03-05
WO 00/14089 PCT/1JS99/01235
-40-
~rahle
SUBSTITUTE SHEET (RULE 26)
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-41
Table 1 (cont.)
26 H2 -H R R ~I H~
27 - z- ~cl -_ _C~H -_
....~ ..- -..,a ~..(~~.
28 -0H~- -H CEt'R~ -0H I -CI-I~Oi -0H~
29 -CHz-O- -H - CR'R' -0H I -CH_CO~H
\ /
30 -CHz-O- -H CR'R' -0H I -CHiCHiCO~H
\ /
31 -CHz-O- -H CR'R' -0H ; / cl -CH~CH~CH=COiH
32 -CHz-O- -H CR'R- -0H -OCHs
\ /
33 -CHz-O- -H CR'R' -OH -OCH~
\ /
34 -C~-0_ _ _ _ _H __ CR~R' ~H _~~'_
\ / r
_ 35 -0~~ _ =H R~Rz ~~ - H' -_
-CHz-O__ _ -_~ CRTR' - -0H H:
37 -CHz-O- -H CR'R' -0H -OCH,
\ / I
38 -CHz-O- -H CR'R' -0H I -OCH~
\ /
I
39 -CH2-O- _H -- CR'R' -0H I _~H~ -
\ /
-C:H2-U- -H C:LZ'K' -UH -UC:H3
\ /
I
41 -CHz-O- -H CR'R' -OH -OCH~
\ /
c
4 -CHz-O- -H CR R' H -OCH~
\ /
43 -CH?-0- -H CR'R' -0H H ~ -OCH3
_ ~~_CR'R' =CN- - H'
45 -CHz-O- -H CR'R' -OCH3 I -OCH~
\ /
\
_CHz-0- -. _ - -H CR~R= ~COCN3 _ - H'
47 -CHz-O- -H CR'R' -H -OCH,
\ /
~/
48 _CHz-0_ ~_CR'R- _ _~CI
49 -CHz-O- -H CR'R' -H -N~ -OCH~
50 -CHz-O- -H CR'R- -H a -OCHj
H
i
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-42-
Table 1 (cont . )
2_ -H .R' F'1's
~i
5
_ -H R R'
w
i
-
61 -H R ' ( -w.n~
HN
i
6
_ ~_O_ -H NR'
.. /
6~ - H=-O- -w.n,
_H \ / i
66 -CH:- - N R ci '
\ /
67~ _ -CN CR H \ / ~ H~CH,
R-
(g -CH,- -CN CR'R- \ / C~ - ~HzCN
6 - ' N \ / Ci
H,
-w.ny.vy..~.:~.
H
R. -vL.raZw
_ \ / CI
71 H=_ -CN R R' -OH ~ c
\ /
.. _ R \ / c t O.H
z_ R- CH,
\ / '
SUBSTITUTE SHEET (RULE 26}
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-43-
Table 1 (cont.)
. . _, .
.i w
5 R _E ~.
\ /
76 - H~- R'R- H \ / c~ H'
77 -CHz- -CN CR R' -H H -0CH~
7 - N CR' R' ~ -"..",
\ /
79 -CHZ-O_ -CN CR R- ~ -. _OCHl .
0 -CH=-O- -CN CR'R' ' ~ ( w
t
-CN CR'R -O~
_ ~ _ N ~ I w _ _OC~
- R~~ o
84 -CHz-O- R,R ~ -0~1
\ /
~-0- ~NH R R ZH
c ' -1H -v
87 -CH=-O- ~ CR'R- -0H \ / ~ -O HsCH3
~'~ OOH
- H=- ~NH ~R- -OH \ / ~~ -w,n~
~I~ c~ H3
w _sH
,...~.~.~" \ /
9 -C --S- -H R R- - H \ / ~ Cxs
-Vl-l1jl.
H5
1 . \ / i
N ~ / ~t - ~H,
- ~.~MoH R \ /
i R, - =OH \ / ~~ -m.r~
S Hi Hi -H CR R- -OH \ / ~ H2CH3
96 - HOC : - N R R- - H \ / -0CH~
SUBSTITUTE SHEET (RULE 26)
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-44-
Table 1 (cont . )
- CR,R' -OH . \ / d -v...~
9 _N _ _H . CR \ / ~C~
-N ~- _H .. R R _ H \ / ' _OCH~
1
OCH,
~ / a
1 1 \ / c~ -~~H'
1 2 - Hs-NH- -H CR R' - H ~i
103 H=- ,- R H ~\ / 'c'i
1 \ ~ ct
-
1 5 -N ; _ R R \ / c'
_OCH,
SUBSTITUTE SHEET (RULE 26)
CA 02343536 2001-03-05
WO 00/14089 -45- PCT/US99/01235
Table la
Exam (~. W girl R~ Rz Rao
le
106 -CH2-0--H CR1 R2 _OH -OCH2CH20H
107 -CH2-O--H CR1 R2 _OH -OCH2CH20CH3 O
108 -CH2-0--H CR1R2 _OH B A ~O~N~
109 -CH2-0--H CR1 R2 _OH C I
110 -CH2-O--H CR1 R2 _OH D
111 -CH2-O--H CR1 R2 _OH E
112 -CH2-0--H CR1 R2 -0H F
113 -CH2-0--H CR1 R2 _H -OH O
114 -CH2-0--H CR1 R2 _H -OCH2CH20H
~
115 -CH2-0--H CR1 R2 _H -OCH2CH20CH3 g ~O
N~
116 -CH2-O--H CR1 R2 _H _ A H
117 -CH2-O--H CR1 R2 _H Cl C
118 -CH2-0--H CR1 R2 _H \ ~ D
119 -CH2-0--H CR1 R2 _H F
120 -CH2-O-CN CR1 R2 _OH -OCH2CH20H O
121 -CH2-O--CN CR1 R2 _OH -OCH2CH20CH3
122 -CH2-0--CN CR1R2 _OH g C NH2
123 -CH2-0--CN CR1 R2 _OH C
124 -CH2-O--CN CR1 R2 _OH D
125 -CH2-O--CN CR1 R2 _OH E
126 -CH2-0--CN CR1 R2 _OH F p
127 -CH2-O--CN CR1 R2 _H -OH O
128 -CH2-O--CN CR1 R2 _H -OCH2CH20H D ~ ~N
129 -CH2-0--CN CR1 R2 _H -OCH2CH20CH3
130 -CH2-0--CN CR1 R2 _H ~ A
131 -CH2-0--CN CR1 R2 _H C
132 -CH2-O--CN CR1 R2 _H D
133 -CH2-0--CN CR1 R2 _H F O
134 -CH2-0--H CR1R2 -OH E ~0~~~
135 -CH2-0--H CR1 R2 -OCH2CH20H
136 -CH2-O--H CR1 R2 -OCH2CH20CH3 H
137 -CH2-O--H CR1 R2 A
138 -CH2-O--H CR1 R2 C
139 -CH2-0--H CR1 R2 D 0
140 -CH2-0--H CR1 R2 F ll
141 -CH2-O--CN CR1 R2 M -OH F ~O~NH~
~
142 -CH2-O--CN CR1 R2 O ~ i H / \
-OCH2CH20
143 -CH2~0--CN CR1 R2 CI -OCH2CH20CH3
(example-80)
144 -CH2-0--CN CR1 R2 A
145 -CH2-0--CN CR1 R2 C
~
146 -CH2-O--CN CR1 R2 D
147 -CH2-O--CN CR1 R2 F
H
~O
148 -CH2-CH2- CR1 R2 _OH -OCH2CH20H ~O
-H G
149 -CH2-CH2--CNCR1 R2 _OH F
150 -CH2-CH2- CR1 R2 _OH -OCH2CH20H
-H
151 -CH2-CH2--CNCR1 R2 _OH F
152 -CH2-S--H CR1 R2 _OH CI -OCH2CH20H
~
~
153 -CH2-S--CN CR1 R2 _OH \ F
154 -CH2-S--H CR1 R2 _OH -OCH2CH20H
155 -CH2-S--CN CR1 R2 _OH F
156 -CH2-0--H CR1 R2 _OH G
157 -CH2-O--CN CR1 R2 _OH G
_
SUBSTITUTE S~itcT (RULE 26)
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235
-46-
Example 158
Membrane Preparations for Chemokine Binding and Binding
Assays
Membranes are prepared from THP-1 cells (ATCC #TIB202).
5 Cells are harvested by centrifugation, washed twice with
PBS (phosphate-buffered saline), and the cell pellets are
frozen at -70 to -85°C. The frozen pellet is thawed in
ice-cold lysis buffer consisting of 5 mM HEPES (N-2-
hydroxyethylpiperazine-N'-2-ethane-sulfonic acid) pH 7.5, 2
10 mM EDTA (ethylenediaminetetraacetic acid), 5 ~,g/ml each
aprotinin, leupeptin, and chymostatin (protease
inhibitors), and 100 ~.g/ml PMSF (phenyl methane sulfonyl
fluoride - also a protease inhibitor), at a concentration
of 1 to 5 x 10' cells/ml. This procedure results in cell
15 lysis. The suspension is mixed well to resuspend all of
the frozen cell pellet. Nuclei and cell debris are removed
by centrifugation of 400 x g for 10 minutes at 4°C. The
supernatant is transferred to a fresh tube and the membrane
fragments are collected by centrifugation at 25,000 x g for
20 30 minutes at 4°C. The supernatant is aspirated and the
pellet is resuspended in freezing buffer consisting of 10
mM HEPES pH 7.5, 300 mM sucrose, l~Cg/ml each aprotinin,
leupeptin, and chymostatin, and 10 ~g/ml PMSF
(approximately 0.1 ml per each 108 cells). All clumps are
25 resolved using a minihomogenizer, and the total protein
concentration is determined using a protein assay kit (Bio-
Rad, Hercules, CA, cat #500-0002). The membrane solution
is then aliquoted and frozen at -70 to -85°C until needed.
CA 02343536 2001-03-05
WO 00/14089 PCT/US99/01235_
-47-
Binding Assays utilize the membranes described above.
Membrane protein (2 to 20 ~g total membrane protein) is
incubated with 0.1 to 0.2 nM 125I_labeled RANTES or MIP-la
with or without unlabeled competitor (RANTES or MIP-la) or
5 various concentrations of compounds. The binding reactions
are performed in 60 to 100 ~.1 of a binding buffer
consisting of 10 mM HEPES pH 7.2, 1 mM CaCl2, 5 mM MgCl2,
and 0.5% BSA (bovine serum albumin), for 60 min at room
temperature. The binding reactions are terminated by
10 harvesting the membranes by rapid filtration through glass
fiber filters (GF/B or GF/C, Packard) which are presoaked
in 0.3% polyethyleneimine. The filters are rinsed with
approximately 600 ~cl of binding buffer containing 0.5 M
NaCl, dried, and the amount of bound radioactivity is
15 determined by scintillation counting in a Topcount beta-
plate counter.
The activities of test compounds can be reported as
ICSO values or the inhibitor concentration required for 50%
inhibition of specific binding in receptor binding assays
20 using l2sl-RANTES or l2sMIP-la as ligand and THP-1 cell
membranes. Specific binding can be defined as the total
binding minus the non-specific binding; non-specific
binding can be the amount of cpm still detected in the
presence of excess unlabeled RANTES or l2sMIP-la.
CA 02343536 2001-03-05
PCT/US99/01235
WO 00/14089 -
-48-
Table 2
BIOLOGICAL DATA
Example ICso
<1
Those skilled in the art will be able to recognize, or
be able to ascertain, using no more than routine
experimentation, many equivalents to the specific
embodiments of the invention described herein. Such
equivalents are intended to be encompassed by the following
l0 claims.