Note: Descriptions are shown in the official language in which they were submitted.
CA 02343644 2001-04-11
1
1 Title: PACKAGED TABLET SYSTEM
2
3 The invention is described herein particularly as it relates to diet
(i.e nutrition) supplements. However, the invention may be applied
to some types of ingested medicines and drugs. The term
s "supplement" should be un.d.erstood as including both diet ingredients
7 and (some kinds of) medication ingredients.
s
s Some types of diet supplement plan may require the ingredients to be
io taken sequentially for best results. The plan may require the
11 dosage of an ingredients to be ramped up gradually, for instance,
i2 rather than the ingredient to be introduced at the full dosage; or,
13 a supplement plan may require that a certain second ingredient be
14 ingested only after the :person has been taking a first ingredient
for some time.
16
17 The invention recognises 'that supplement plans often relate to a
is system for presenting t:o a person the various pills and tablets,
i9 comprising the mixes of ingredients of the supplement that the
2o person wishes to ingest.
21
22
23 BACKGROUND TO THE INVENT:ICJN
24
People generally are coming to realise that the modern diet is not
2s properly balanced. One :Factor leading to this realisation is that
27 the nature and configuration of the food people eat is primarily
2$ determined more by the nee=_d for the food to be commercially
29 processed for efficient sale in grocery supermarkets, rather than by
3o the requirements for good nutrition. As a result, more people are
31 recognising the need far dietary supplements.
32
33 Many professionals have devised (and are devising) programs, based
34 on recognising the nutrii:ional shortcomings of commercially
available foods, and are seeking to balance those deficiencies with
36 supplements of minerals, vitamins, etc, and to do so especially by
37 means of e.g natural herbs.
38
39 It is a general characteristic of nutritional matters that the
ao effects of correcting an imbalance or deficiency can take a long
41 time. Unlike medical drugs generally, nutritional or diet
42 supplements generally are. not powerful in small doses. Rather, it
43 is only the accumulated effect, over a long period of time, that
CA 02343644 2001-04-11
2
1 makes a diet supplement effective. In fact, where a person
2 continues to ingest the wrong types of food, it may be necessary to
3 continue taking the nutrition-supplement more or less for life.
4
In this sense, nutritional supplements in general are quite unlike
s drugs and medicines. Generally, a medicine is aimed at having a
7 large effect on the body, to cure some ailment. The person is ill,
and wants to be cured as quickly as possible. Generally, a main aim
s of a drug is to get they maximum curative effect, while minimising
io the harmful side effects.
11
12 A nutrition-supplement plan is not intended to be curative, as is a
13 medicine. Rather, the effect of a diet supplement is to restore the
14 imbalance caused by eating the wrong types of foods, whereby the
body is not receiving, on an on-going basis, all the complete range
is of vitamins and minerals :needed for complete nutrition.
17
18 The effect of taking large doses of a nutrition-supplement for a
is short period is quite different from taking small doses over a long
2o period. Generally, the body is not able to store the excess, and
21 taking too much of a diet supplement results in the excess being
22 expelled from the body, and wasted. Therefore, it is not so much
23 the case that an excess o.f a diet supplement might be dangerous
24 (though of course, there are exceptions to that), any more than
taking an excess, over a :short period of time, of a particular kind
2s of food might be regarded as dangerous; but rather, it is the case
27 that the body cannot ass:Lmilate more than the small quantities of
2$ the supplement ingredienl~ over a short period of time.
2s
3o However, even though an excess might cause no harm, an on-going
31 deficiency of a vitamin.rnight be not at all harmless. The small
32 quantities, ingested on a daily basis, can be very important, in
33 that an on-going deficien<:y in some types of vitamins and minerals
34 can cause serious medical problems.
36 Thus, the need for nutrit==~on-supplements may be regarded either as
37 temporary - until the person can move to a different, and more
38 nutritious, way of eating - or as more or less permanent. The
3s changes to be effected by the nutrition-supplements may be intended
4o as correcting the effect:> of some previous deficiencies, or may be
41 intended as ensuring generally that the body is getting a full range
42 of nutritional ingredient::; .
43
CA 02343644 2001-04-11
3
1 In any case, nutrition-supplements tend to be the subject of plans,
2 i.e of plans in which there is an element of change, and the change
3 occurs over a period o.f_ time.
4
In addition to the reasons for plans in which the mix of supplements
6 change over time, as mentioned above, there is also the fact that
7 changes occur in vitamin-needs with the different seasons of the
s year. Also, as people succumb to, and recover from, different
s illnesses, again, the need is for a diet-supplement plan that
1o encompasses changes-over-time as to the mix of the ingredients.
11
12 A supplement plan of the type with which the invention is concerned
13 is one that involves a person taking many ingredient-doses, on many
14 occasions, over a period of time. For the invention to be most
advantageous, preferably the supplement plan is one that calls for
is the ingredient-doses tc::be changed, and especially to be changed
17 gradually. Preferably also, the supplement plan is one that calls
18 for one ingredient to be :replaced by, or added to, another
1s ingredient, again gradually over a period of time.
2i The invention is not app:l.icable to supplement plans in which there
22 is only one ingredient, ~~nd in which the dosage of that ingredient
23 is the same throughout t',ne plan. The invention is also not so
24 advantageous, even where 'the ingredient-doses vary, if the plan is
completed after a small. number of ingredient-doses. One of the
2s benefits of the invention lies in its ability to ensure that a long
27 and complex plan is adhered to by the user, and the invention is at
28 its most advantageous with long and complex plans, especially plans
29 in which the ingredients and the ingredient-doses change gradually
over a period that is measured in weeks or months.
31
32 Another aspect of nutrit_Lon-supplementation plans is that the user
33 cannot tell whether the plan has been effective until the user has
34 been following the plan. for a long period of time. During that
period, if the plan is ai. all complicated, requiring the taking of
3s different types of pills and tablets, in sequence, it may be
37 difficult for the person t;o stick firmly to the plan over the whole
38 period; therefore, if the: results are not as expected, the users
3s (and the professionals) cannot be certain whether the failure was a
4o failure of the plan itse7_f=, i.e of the mix of ingredients and
41 dosages, or a failure due: to the plan not being properly followed.
42
43 Also, in the field of nutrition-supplementation plans, there may be
CA 02343644 2001-04-11
4
1 an element of trial and error. In other words, the dietician can
2 experiment with different types of food supplement, in the Nape of
3 achieving efficacious :rea ults - in a way that cannot be done with
4 drugs, for example. Ex~~eriments can be done with diet-supplements,
on the understanding that an excess of the wrong food will dc. little
s harm, in that it is simply expelled. Such experiments cannot: be
7 done with drugs, because the introduction of the wrong drug, or of
an excess of the right drug, is much more likely to be harmful.
s However, experiments with the food supplements, generally, cannot be
1o completed in a short time, and so the person has to pay attention to
11 getting a perhaps-complex mix, and a perhaps-complex sequence, of
12 the ingredients right, in order for the plan to be evaluated.
13
14 There are many professional dieticians who advertise nutrition-
supplementation plans to users, based on different theories and
is paradigms, and users may wish to evaluate the effects on themselves.
i7 Hitherto, the success of a plan has depended on whether the plan
18 itself is simple for the user to put into effect. If the plan, on
is paper, requires the user to take e.g one of this tablet on mondays,
2o two on tuesdays, two more plus one of that on wednesdays, etc, the
2i plan is doomed simply x>ecause of its complexity, to the user. What
22 is needed is for the necessary complexity to be the concern of, and
23 to be under the control. of, only the professional, not the user.
24
The system of the invention is aimed at permitting the described
2s various manners of usage of nutritional-supplementation plans, in a
27 way that is straightforward to the user and minimises the
2s possibility of mistakes by the user, and yet is simple and
2s inexpensive to manufacture.
31
32 GENERAL FEATURES OF THE :LNVENTION
33
34 When a person wishes to i~a ke a number of nutrition supplementation
ingredients, over a period of time, the person does not wish to have
3s to follow a plan, i.e a plan that is written out on paper, in which
37 the user is called upon i~o take e.g one pill A per day for one week,
3s then half a pill A and one tablet B per day, for another week, and
39 so on. And yet, that is what adhering to a nutrition
4o supplementation plan has entailed, hitherto.
41
42 It is an aim of the invention, to provide a system that presents the
43 various, and changing, ingredients of a diet-supplement plan i~o the
CA 02343644 2001-04-11
1 user, in accordance with a pre-determined program or regime of
2 supplements, in a manner that enables the user to adhere easily to
3 the plan, to see clearly that the plan is indeed being adhered to,
to minimise mistakes, a~~.d to minimise especially the type of mistake
5 whereby the person remains unaware that the mistake has been made.
s
7 In one aspect, the invention lies in a diet-supplement dispensing
8 system, comprising a whole series of pre-prepared tablets. Each one
9 of the tablets that make up the whole series is formulated to
io contain at least one diet supplement ingredient, comprising
11 vitamins, minerals, herbs, or the like.
12
13 A first one of the tablets is formulated to contain a mix
14 composition F1 of dietary minerals and vitamins, and a second one of
the tablets is formulated to contain a corresponding mix composition
is F2. An nth one of the tablets that make up the whole series is
i7 formulated to contain a corresponding mix composition Fn of dietary
is minerals and vitamins. The tablets that comprise a quantity Ql of
is the tablets have a mix composition F-Q1, and those that comprise a
2o quantity Q2 of the tablets have a mix composition F-Q2, the mix
21 composition F-Q1 being substantially different, nutritionally, from
22 the mix composition F-Q2.
23
24 Preferably, the composition of each tablet is based on the sequence
in which the tablets will be ingested, relative to the first tablet
2s taken. Each tablet should contain ingredients that are optimised as
27 to the type of ingredient, and as to the dosage thereof, in
2s pursuance of a pre-determined supplement plan.
29
3o The system includes a di~apensing apparatus, in which the whole
31 series of tablets is contained. The dispensing apparatus includes a
32 means for presenting the tablets to the user in pre-determined
33 sequence, the first one o:E the tablets first, then the second, and
34 so on, the individual tablets in 1-2-3 ordered sequence. The
dispensing apparatus pre:E<:rably is so structured as to make it clear
36 to the user just what i.s the intended sequence of the tablets.
37
3s The nutrition-supplementation plan might be intended as a temporary
3s measure, until the users are sure they are getting the ingredients
from normal food, so the~~ do not need the supplements any more. Or
41 the plan might be intended to go on for ever, i.e the user continues
42 taking the supplements indefinitely.
43
CA 02343644 2001-04-11
6
1 It is an aim of this aspect of the invention to provide all the
2 supplementation ingredients needed for one day in one tablet. All
3 the ingredients needed for the next day are provided in another
4 tablet. The one-tablet-per-day aspect is not essential, in that
other periods of time might be more appropriate. Another favoured
6 tablet-taking regime is three times a day, in which case the pack is
7 labelled e.g monday-morning, monday-afternoon, rnonday-evening,
s tuesday-morning, and so on.
s
io In some cases, it may be important that the ingredients be mixed or
11 blended together just prior to ingestion, but that the ingredients
12 be kept separated until then. In that case, the designer of the
13 plan may call for two (or more) tablets to be taken on one occasion.
14 In that case, the dispensing apparatus would be arranged to dispense
the two tablets together.
16
i7 It is not the case that nutrition supplements can be ingested on the
is basis of supplying an excess of everything, and the body will take
i9 what it needs. People cannot process excess amounts of, for
2o example, some minerals, 'which might accumulate in various organs and
21 become toxic. Also, the ideal mix is different for different
22 people. For example, the mix is different for people who are e.g
23 active/inactive; male/female; old/young; and so on.
24
26 DETAILED DESCRIPTION OF':PREFERRED EMBODIMENTS
27
28 By way of further explanation of the invention, exemplary
29 embodiments of the invention will now be described with reference to
3o the accompanying drawings, in which:
31
32 Fig 1 is a pictorial view of a blister-pack, containing a program of
33 nutritional supplements, which embodies the invention.
34 Fig 2 is a graph showing how the desired amount to be ingested of a
typical ingredient of a supplementation plan changes over a period
36 of time.
37 Fig 3 is a graph showing how the amounts of two ingredients changes
3s over time, for best interaction between the ingredients.
39
4o The systems shown in the accompanying drawings and described below
41 are examples which embody the invention. It should be noted t=hat
42 the scope of the invention is defined by the accompanying claims,
43 and not necessarily by spE:cific features of exemplary embodiments.
CA 02343644 2001-04-11
7
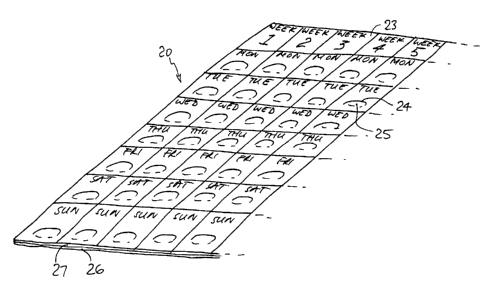
1 Fig 1 shows a dispensing apparatus 20 of a type that may be used in
2 the invention. The apparatus includes a bubble-pack or blister-pack
3 23. Each pocket 24 in the pack contains a single tablet 25. The
4 pockets are marked each with a day of the week. Thus, the tablet
placed {by the manufacturer) in the pocket marked "1st Week Monday"
s contains the ingredients, i.e the mix of different ingredients,
7 appropriate to the commencement of the diet supplement program. The
s tablets for Tuesday, Wednesday, and so on in sequence, may be
s designed, by the dietician, to provide a gradually-increasing dosage
of a particular ingredient. Later, other combinations of
11 ingredients may be introduced, either suddenly, or gradually over a
12 period of days; and later still, ingredients may be discontinued
13 suddenly, or gradually over a period of days, in accordance with the
14 dietician's purpose.
is Knowing the sequence-dispenser will inevitably be used, the
17 dietician can pre-plan the changes, and can make the changes much
18 more complicated than in previous plans, knowing that the user has
is the simple task of just taking one tablet per day, in the correct
2o sequence, from the dispenser.
21
22 The dietician is the one who decides, and controls, the mix of
23 ingredients to be taken every day, and the change of the mix as the
24 days go by. The user cannot do anything about it. The dietician
not only recommends, but actually physically controls, the mix of
2s ingredients, as to what. the ingredients are, and as to quantities
27 and ratios, that the user takes each day of the program. Thus, the
28 program itself becomes a matter that is under the complete control
29 of the dietician. This gives the dietician freedom to put whatever
3o he feels is desirable into the program, knowing that if the user
3i follows the program at all, the user will follow it to the letter.
32 Thus, it is not possible for the user to participate slightly in the
33 program. The user either participates fully in the program, in all
34 its complexity, or not at all.
3s This passiveness by the 'user is very useful in assessing whether a
37 program has been efficacious. The user need only certify that he
38 has taken the correct ta:b.lets every day; if so, the dietician knows
3s exactly what the user has been receiving.
41 Fig 1 shows a blister-pa~~k 23. The pack is made generally with a
42 metal-foil backing sheet 26, having a transparent plastic film 27
43 covering the tablets. The tablets are taken out of the pack by
CA 02343644 2001-04-11
8
1 pressing them through the foil material.
2
3 The user buys the whole ~~et or series of tablets, each tablet: being
already located in its correct sequence in the blister-pack. The
tablets have been manufa.c~tured each with the appropriate mix of
s ingredients for the pa:rt.i.cular day, in sequence.
7
s A number of different supplementation plans may be made available.
s For example, the designer might provide a stress-relief plan, or a
1o muscle-strength plan, or a general-well-being plan, ete. Each plan
11 may be sub-divided inter plans suited for children, adults, seniors,
12 obese, active, sedentary, etc.
13
i4 The dietician might even offer a "secret ingredients" plan,
especially one that has been endorsed by a celebrity. In each case,
16 whether the plan has been put together based on strictly rational
17 scientific principles, err whether the plan's only claim to efficacy
18 is that it is used by a celebrity, the user simply buys the bubble
is pack. Users do not have to buy a series of bottles, and then do
2o their own dispensing, e=very day, one of these, two of those, etc.
21
22 The tablets are set into the packs by the manufacturer, under the
23 direction of the dietician. The user does not do anything at all,
24 from the standpoint of preparing the pack, and the tablets, for use.
Preferably, the structure of the pack is such that it is just as
2s economical for the user' to buy a new pack, as far the user to try to
27 replenish an empty pack.
2s
29 The blister or bubble pack is not essential to the invention.
3o Alternatives to the blister pack include plastic moulded dispensing
31 devices, the conventionally known types of which have been mainly
32 intended for use with pha=rmaceutical drugs. In these dispensers,
33 the tablets are pre-rose=rted, and drop down, in pre-determined
34 sequence, onto a dispens=ing slot or tray. While these types of
devices are not ruled out, they are not preferred, in the invention,
36 on the grounds that they are more expensive than a simple blister-
37 or bubble-pack, and thri:E~y users might be tempted to re-use the
38 pack, i.e to re-fill it l~lzemselves. The disadvantage of this that
3s now the program, and the capacity for mistakes in the program, has
4o passed outside the control of the designer of the plan.
41
42 Fig 2 is a graph of the desired concentration over a period of time
43 of a particular ingredi.eni~ A. The dietician wishes to avoid sudden
CA 02343644 2001-04-11
9
1 introduction at the full. dosage rate, and equally, wishes to avoid
2 sudden cessation of the full dosage. The invention permits the
3 gradual start and stop, under the full and complete control of the
dietician; the user doe,. not even need to know that the change is
gradual. (This is not t:a say the user is better off not knowing the
s program, but rather, that: the plan can be administered without the
7 user needing to know.)
s
s Fig 3 is a graph showing- two ingredients being introduced. The
1o dietician knows that ingredient A serves as a precursor to
tt ingredient B, but is concerned that either A or B (or both) are of
12 such nature that introduction and cessation should be done
13 gradually. Some ingrec:~ients can be toxic at certain dosage levels,
t4 unless accompanied by another ingredient. Again, all of this
~5 complexity is done behind the scenes, in the dietician's laboratory
1s and the tablet factory. Neither the user, nor the pharmacist or
17 other retail outlet at which the user purchased the system, are
18 called upon to use skill and care to put the plan into effect.
Indeed, they need know nothing about it.
21 Nutrition-supplementats.on programs generally go on for a long time.
22 Generally, it does not matter so much whether the user misses a day,
23 or takes a double dose one day. This situation may be contrasted
24 with (strong) medicines, in which time sequencing, if it is
important, is important because missing a dose, or double dosing, is
2s critical, right then arad there. The user must get the dose right,
27 because the dosage is critical. In 'the case of diet
2s supplementation, the car:rectness of a single dosage is not too
2s important, in the sense that it would be rare for a person to be
3o harmed by a single overdose of the diet supplement ingredient, but
31 correctness of dose matt~;rs more as a way of preventing an
32 accumulation of errors of dosing, over a long period of time.
33
34 Equally, in a nutrition supplementation plan, it does not matter so
much on which day the pro<~ram starts. Therefare, the user, having
3s bought the bubble-pack containing the whole series of tablets, then
37 simply waits for the day of the week to come round on which the pack
3$ actually starts. This may be contrasted with a medication or drug
3s plan, especially under prescription, where the need is for
4o treatment, and for the treatment to start right away. The notion of
41 waiting for the days to go by, until the day marked on the package
42 as the start of the plan,. would be unacceptable for a drug treatment
43 plan, but is acceptable enough for a diet or nutrition supplement
CA 02343644 2001-04-11
1 plan.
2
3 It is also contemplated that the indication as to which tablet
4 should be taken on which day can come from markings on the tablets
5 themselves. Thus, a series of seven tablets might be marked each
6 with a day of the week, the marking being applied physically onto
7 the tablet itself. This method of marking is only suitable when
s there is a small number cf tablets i.n the series, however; nutrition
9 supplement programs generally are intended to go on for longer than
1o a few days or a week, and the invention lends itself more to the
11 longer programs.
12
t3 The invention has an area of applicability to other kinds of
14 tablets, besides nutritional supplementation programs, including
(restrictedly) some aspects of medicinal drugs, as will now be
is considered.
17
i8 The trend in medicinal drugs is not to combine several drugs into
1s one pill. The trend is to keep each drug as a physically-separate
2o pill. Pharmacists tend not to combine different drugs into a single
21 pill, on the grounds that such tampering might have serious side
22 effects, and might negate the effect the manufacturer is seeking to
23 provide. Neither the manufacturers, nor the prescribing doctors,
24 would want to lose control of the form of the drug, to the
pharmacist. In the case of prescription drugs, it is not common for
2s the need to arise that the drugs are to be combined in inter-active
27 ways, requiring rampinc~ u.p and down of dosage. But even where
2s prescription drugs are being used like that, it is unusual for the
2s doctor to not require an.y control over the dosage etc. Rather, the
3o doctor wants to be able to refine the dosage, or at least the doctor
31 wants to have the dosage under his/her own direct control, whereby
32 the doctor is the one who determines, and effects, whatever changes
33 might be called for.
34
On the other hand, in th~~ case of some non-prescription drugs, the
3s drug manufacturer may wi;~)z to offer, not just a drug, but a program
37 of changing drugs. By the use of the invention, all the user has to
3s do is to take one tablet per day, taking care to take the tablets in
3s sequence, until the program is finished. There is almost no limit
4o to what the manufacturer can introdu<:e by way of complex refinements
41 into the treatment program, by this means.
42
43 Some types of prescription drugs may be dispensed in the manner of
CA 02343644 2001-04-11
11
1 the invention. General7_y, the prescription of drugs is done by a
2 doctor, who will always wish to have the flexibility to prescribe
3 what he thinks the patiE:nt needs. In cases where doctors wish to
retain flexibility as to the mix of ingredients, dosage, etc, the
invention is not applicax>le. However, such flexibility might not
s always be beneficial, in that a drug manufacturer might be inhibited
7 from releasing a complex drug plan, particularly if the plan is one
s which would have serious side effects if the plan is not adhered to
s exactly. Thus, the manufacturer might only feel able to release the
io drug plan (for prescription by doctors) if the possibility is
11 removed that the doctor, and/or the pharmacist, might make changes.
12
13 When it comes to pharmaceutical drugs, although the invention might
14 be used in respect of prescription drugs, the invention is more
accommodating in the field of over-the-counter, non-prescription
is drugs, where the drugs are not so strong, and tend to be taken over
i7 a longer period of time, and where there is little need for
1$ supervision as to the taking, and where the manufacturer is the sole
is prescriber, and no other professionals involved. This is the case
2o where the manufacturer will wish to, and now can, refine the dosages
21 and mixes of several different ingredients, to suit the particular
22 ailments that can be treatred that way.
23
24 Prescription drugs are beset by the controls that have grown up for
the very good reason that prescription drugs are dangerous if mis-
ts prescribed. However, these considerations do not really apply to
27 vitamins and minerals, where the dangers of over-prescription,
28 though not zero, are quiv~e minimal. It is acceptable for dieticians
2s to devise plans, even on an experimental basis. But the
experimenter wishes to b<, sure, when evaluating the effects of the
31 plan, that, if a person. is taking the plan, at least it is certain
32 what the persan is actually taking.
33
34 In the invention, the dispenser should be such that it constrains
the user to take the correct tablet on the correct day. It would be
3s outside the invention if t:he dispenser were to just dispense a
37 random one from a supply of pills. The order or sequence of the
3s pills has to be built i:nt:o the packaging/dispenser. The invention
3s requires more than just a set of instructions, on paper, as to which
tablets should be taken c>n, which day.
41
~42 The invention is aimed at removing the need for judgement and
~33 decision-making from th<~ user, and indeed from everyone in the chain
CA 02343644 2001-04-11
12
1 through which the dispensing apparatus passes, once it leaves the
2 manufacturer's production line. This is why the invention is highly
3 suitable for vitamins and minerals and other food supplements, is
marginally suitable for over-the-counter, non-prescription drugs and
remedies, but is hardly suitable at all for prescription drugs.
s
7 Examples of the type of supplementation plans that are made possible
s with the invention will now be described.
9
io The first example is f:r_om the field of diet-supplementation. In
11 order for the body to convert beta-carotene into vitamin A, the body
12 needs the pre-cursor vitamin B12. Prior to this, the body needs
i3 vitamin B6, to absorb the vitamin B12. If vitamin A is introduced
14 without the pre-cursor vitamin B6, most of the ingested vitamin A
will be lost. By the 'use of the system of the invention, first the
1s vitamin B6 is introduced into the body; then, the vitamin B12 is
17 introduced, and absorbed; then, once the body has an optimum level
1s of vitamin B12, the beta-carotene can be introduced. Now, the beta-
1s carotene will be readily and properly converted into the functional
2o form of vitamin A.
21
22 A second example is from the field of non-prescription drugs. The
23 common cold has been the subject of many treatment programs, in
24 which a person takes first a nose-drying drug, then a de-congestant
drug, then an anti-couch drug, then an anti-catarrh drug, or as
2s determined. With the system of the invention, the manufacturer
27 would pre-determine the :mix of (non-prescription) drugs needed for
28 the various stages of a typical cold. The mix may be tailored to
2s certain segments of the ;population, such as child, young adult,
3o senior, etc. All the user has to do is simply take one tablet per
31 day, the ingredients of the tablets being made up according to the
32 manufacturer's pre-determined program. Of course, not all ailments
33 can be treated in this common manner, but many can, and the non-
34 prescription drug manufa~~turers may be expected to know which
ailments can be treated ~~nd which cannot.
36
37 A third example is from.the field of prescription drugs. Some of
3s the drugs that reduce blood pressure, such as Norvasc or Vasotec,
3s need to be introduced c;radually. The invention may be used to
4o accomplish this, and thereby to reduce what might otherwise be a
41 shock to the body. Also,, the system of the invention may enable the
42 system designer to gradual ramp an addictive drug. Also, the system
43 of the invention may be used to enable a system designer to provide
CA 02343644 2001-04-11
13
1 a reduction in a drug at: certain points in the day when the drug is
2 not needed, e. g at night:.
3
Examples of suitable applications from the field of prescription
drugs are rare, however, but it is recognised, in the invention,
s that there many more a:re:as of application in the fields of non-
prescription drugs, and especially of nutrition or dietary
8 supplements (vitamins anal minerals, etc).
9
io The invention enables a manufacturer to introduce supplementation
11 plans that involve complex mixes of ingredients, and to control the
12 complexity without the need for the intervention of a doctor,
13 pharmacist, or other professional, between the manufacturer and the
14 user.