Note: Descriptions are shown in the official language in which they were submitted.
CA 02343762 2001-03-12
WO 00/15102 PCTNS99I20796
ATTRIBUTE COMPENSATION FOR ANALYTE DETECTION AND/OR CONTINUOUS MONTrORING
This application claims priority to U.S. Provisional Application No.
60/099,733
filed September 10, 1998; U.S. Provisional Application No. 60/140,283 filed
June 18,
1999 and U.S. Provisional Application No. 60/140,285 filed June 18, 1999.
Io BACKGROUND OF THE INVENTION
The present invention relates to a system and method for the compensation of
assay measurements of analytes from small quantities of biological fluids
harvested
from tissue of a subject utilizing conditions at the harvesting and assay or
measurement
site.
I5 Current analyte assay devices suffer from inaccuracies resulting from a
variety
of confounding conditions at the harvesting site. Far example, blood glucose
meters
adjust an assay measurement for ambient temperature conditions associated with
the
glucose test strip when it is inserted in the meter.
As attempts are made to reduce the volume of biological fluid collected or the
2o time required for the assay, these conditions become more and more
detrimental to an
accurate assay measurement. The conditions include, but are not limited to,
humidity,
temperature, ambient light, pressure, etc. For example, this is particularly
the case in a
system that measures a glucose concentration from blood or interstitial fluid
collected
in a harvesting device that is placed in or about the surface of a tissue.
Attribute
25 compensation is even more important in a system that monitors an analyte on
a
continuous basis from a harvesting device that is kept in contact with the
tissue for
several hours, days or even weeks. Through the use of appropriate sensors,
these
conditions may be monitored and compensated for in the desired assay
measurement.
3o SUMMARY OF THE INVENTION
In accordance with the present invention, at least one sensor is provided to
measure an attribute associated with the biological fluid harvesting operation
of a
CA 02343762 2001-03-12
WO 00/15102 PCT/US99/20796
2
device or the assay of the biological fluid for one or more analytes by the
device. A
variety of attributes, or conditions, at the harvesting site of the fluid or
within the fluid
handling portions within the device may affect the accuracy of the assay or
other
operational parameters of the device. The types of sensors used are based upon
the
conditions that are measured. An operational parameter of the harvesting
device is
compensated for (i.e., adjusted) based on the sensed attribute. Examples of
attributes
are temperature, pH, conditions of the tissue affecting fluid productivity,
etc.
The present invention is useful in a system that performs a single (one time)
measurement of an analyte in a biological fluid of a subject from a harvesting
device
1o placed in contact with the tissue, as well as in a system that continually
monitors an
analyte from a subject from such a harvesting device. Thus, it is contemplated
that an
analyte in a biological fluid of a subject may be repeatedly assayed at
regular and
frequent intervals by the system and method of this invention.
The above and other objects and advantages of the present invention will
become more readily apparent when reference is made to the following
description,
taken in conjunction with the accompanying drawings.
BRIEF DESCRIPTION OF DRAWINGS
FIG. 1 is a block diagram illustrating a system according to one embodiment of
2o the present invention.
FIG. 2 is a diagram of a sensor head showing the position of attribute sensors
according to the invention.
FIG. 3 is a diagram showing the use of a vacuum/pressure sensor according to
the invention.
FIG. 4 is a block diagram of the components of an assay meter forming part of
the system of FIG. 1.
FIG. 5 shows the use of compensation data in graphical form to compensate an
assay measurement for temperature.
FIG. 6 is a diagram delineating the steps that may be performed by a process
3o according to the present invention.
CA 02343762 2001-03-12
WO 00/15102 PCT/US99/20796
3
FIG. 7 is a block diagram of a system according to another embodiment of the
present invention.
DETAILED DESCRIPTION OF THE INVENTION
s DEFIrIITIONS
As used in this specification, "a" and "an" may mean one or more than one. For
example, "an" analyte may mean one analyte or more than one analyte.
As used herein, the term "biological membrane" means the structure separating
one area of an organism from another area of the organism, such as a capillary
wall, or
l0 the outer layer of an organism which separates the organism from its
external
environment, such as skin, buccal mucosa or other mucous membrane. The term
"epithelial tissue, " when used herein is mean to mean skin, mucosa and
linings of the
body cavities of an organism.
As used herein, the term "tissue" means an aggregate of cells of a particular
15 kind, together with their intercellular substance, that forms a structural
material. At
least one surface of the tissue is preferably, but not necessarily, accessible
to
electromagnetic radiation so that one embodiment of the invention can be
earned out.
The preferred tissue is the skin. Other tissues suitable for use with this
invention
include mucosal tissue and soft organs.
2o As used herein, the term "suction" or "pressure" relates to the relative
pressure
as compared to the internal pressure of the organism to which the system is
interfaced.
"Vacuum" is used synonymously with the term "suction."
As used herein, the term "biological fluid" means blood serum, whole blood,
interstitial fluid, lymph fluid, spinal fluid, plasma cerebrospinal fluid,
urine, prostatic
2s fluid, bile, pancreatic secretions, or any combination of these fluids.
Other fluids that
may be harvested from the surface of various tissues include fluids selected
from the
group consisting of mucus, saliva, breast milk, tears, gastric secretions and
perspiration.
"Interstitial fluid" means the clear fluid that occupies the space between the
cells in the
body. It is also contemplated that biological fluids can be harvested from
beneath the
30 surface of tissue of other organs, particularly during operative
procedures.
CA 02343762 2001-03-12
WO 00/15102 PCTNS99/20796
4
As used herein, "poration," "microporation," or any such similar term means
the
artificial formation of a small hole, opening or pore to a desired depth in or
through a
biological membrane, such as skin or mucous membrane, or the outer layer of an
organism to lessen the barrier properties of this biological membrane to the
passage of
biological fluids, such as analytes from within the biological membrane or the
passage
of permeants or drugs from without the biological membrane into the body for
selected
purposes, or for certain medical or surgical procedures. The size of the hole
or
"micropore" so formed is approximately 1-1000p.m in diameter. It is to be
understood
that the term "micropore" is used in the singular form for simplicity, but
that multiple
to openings or pores may be formed by the integrated device according to the
present
invention.
As used herein, "artificial opening" means any physical breach of the
biological
membrane of a suitable size for delivering or extraction fluid therethrough,
including
micropores.
As used herein, the term "harvesting device" means a device suitable for being
placed in contact with tissue for collecting a biological fluid sample from
the tissue
(preferably through the micropores so created) and analyzing the biological
fluid to
determine a characteristic thereof. The harvesting device may be designed for
one
time, i.e., discrete use, or may be designed to be placed in contact with the
tissue for
longer periods of time, e.g., hours, days or weeks, for periodic, continual or
continuous
analyte monitoring. The harvesting device may optional include a porating
element (as
defined below) located thereon.
The term "porating element" is meant to include any means of forming a
micropore, hole or opening described above, including by thermal ablation,
mechanically breaching the tissue by lancet or needle, and other known
techniques. An
example of a mechanical porating element is disclosed in published PCT
application
WO 9800193, entitled, "Multiple Mechanical Microporation Of Skin Or Mucosa."
Another porating technique suitable for use in connection with this system is
disclosed
in PCT Application No. PCT/LJS99/15967 entitled "Controlled Removal Of
Biological
3o Membrane By Pyrotechnic Charge For Transmembrane Transport," filed July 14,
1999.
CA 02343762 2001-03-12
WO 00/15102 PCT/US99/20796
The term "continuously" or "continually" when used in connection with a
analyte monitoring system, means acting on an ongoing basis at a frequency or
event
rate that may vary depending on a particular application of the system. For
example,
the output of the sensor may be read on a periodic basis, such as every
minute, several
minutes, hour, several hours, etc. Moreover, at each reading event, the sensor
output is
optionally sampled multiple times, so as to obtain a plurality of readings
relatively
close in time, whereby an average or other adjustment of those multiple
readings is
made for determining a final reading that is displayed or logged.
As used herein, "analyte" means any chemical or biological material or
compound suitable for passage through a biological membrane by the technology
taught in this present invention, or by technology previously known in the
art, of which
an individual might want to know the concentration or activity inside the
body.
Glucose is a specific example of an analyte because it is a sugar suitable for
passage
through the skin, and individuals, for example those having diabetes, might
want to
know their blood glucose levels. Other examples of analytes include, but are
not
limited to, such compounds as sodium, potassium, bilirubin, urea, ammonia,
calcium,
lead, iron, lithium, salicylates, and the like.
An "attribute" is a physical condition present at the harvesting site, assay
site, or
otherwise associated with the operation of the harvesting device. An example
of an
2o attribute is temperature. Other attributes or conditions that are useful to
be measured
are humidity, ambient light, pressure, vacuum, tissue tone, tissue thickness,
tissue
moisture content, oxygen, pH, etc.
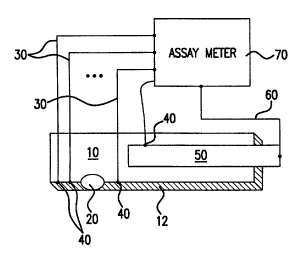
FIG. 1 illustrates one embodiment of a system comprising a harvesting device
10 and an assay meter 70. The harvesting device lU comprises collects a sample
of
biological fluid from tissue such as skin, which fluid is collected through an
opening 20
on the skin contact side 12. The harvesting device 10 may include incorporated
thereon
or therein tissue penetrating or porating means, such as a lancet, thermal
ablation
(optically or electrically heated) such as disclosed in U.S. Patent No.
5,885,211. See
also PCT applications PCT/LTS99/16378, filed July 20, 1999; PCT/US99/04990,
filed
3o March 5, 1999 and PCT/LTS99/04983, filed March S, 1999 for variation
configurations
CA 02343762 2001-03-12
WO 00/15102 PCT/US99/20796
6
of a harvesting device that includes optional on-board tissue penetrating or
porating
elements.
The harvested fluid is moved by vacuum applied over the opening 20 and/or by
capillary action, for example, such that the fluid flows through, across, or
on the analyte
detection strip or sensor 50. The analyte sensor 50 is coupled by an optical
or electrical
link 60 to the assay meter 70. One or more sensors 40 are positioned in the
harvesting
device 10 to measure conditions at the harvesting site at the time the
biological fluid is
harvested. The sensors 40 are coupled by electrical or optical links 30 to the
assay
meter 70.
1o The type of sensor depends on the type of attribute or conditions)
measured.
As explained above, the attribute may be temperature, humidity, ambient light,
pressure, vacuum, tissue conditions indicative of fluid productivity (tissue
tone, tissue
thickness, and/or tissue moisture content) etc., or my combination thereof.
The point
of measurement also depends on the type of attribute or conditions) measured.
Proximity to the assay is important for measuring all environmental
dependencies of
the assay except for those, which are common to the measurement environment
such as
humidity, pressure or vacuum. For example, in one embodiment, a hose is
provided to
supply suction or vacuum to the harvesting device to suck fluid from the
tissue into the
harvesting device and onto the analyte sensor. This hose provides a mechanism
to
2o measure environmental parameters along the hose that would be consistent
with the
environment at the assay such as humidity, pressure and vacuum level. Those
enviroriznental dependencies which should be measured near the assay include
ambient
light, pH and temperature. To correct for assay temperature dependence the
temperature measurement point should be as close to the assay as possible
within the
25 same housing material but usually not in contact with the sample. The pH of
the fluid
being measured can be used to compensate for pH effects on the assay and may
be
changed by the assay process, therefore pH should be measured in the sample
just
before the assay in the flow channel.
Tissue characteristics such as tone, thickness and moisture content should be
3o measured close to the sample site on similar tissue. For example, if the
sample site is
CA 02343762 2001-03-12
WO 00/15102 PCT/US99/20796
7
on the mid-volar forearm tissue characteristics should be measured on the mid-
volar
forearm close to the site. Variations in characteristics have been measured
between
lower mid, and upper volar forearm sites.
Temperature is particularly important when the harvesting device 10 is part of
a
5 discrete or continual glucose monitoring system. For example, an attribute
sensor 40
that is responsive to temperature is preferably placed as close as possible to
the analyte
sensor SO (if not on it) so that the effects of temperature variation on the
analyte sensor
can be minimized. Many types of temperature sensors are known in the art that
are
suitable for use in connection with the present invention. Commonly used
sensors
Io include forward biased semiconductor diodes, thermistors, thermocouples,
Resistance
Temperature Detectors (RTDs), radiation thermometers, fiber optic sensors,
bead
thermocouples and solid state sensors. For this example, a thermistor is used
because
of its known temperature characteristics, availability and low cost.
Preferably, the
response time for the temperature sensor is less than 10 seconds per degree
Celsius to
I5 minimize noise and allow the temperature measured to track the changes at
the assay.
Turning to FIGs. 2 and 3, an embodiment of sensor head S00 of a harvesting
device 10 is shown, wherein the sensor head 500 has one or more attribute
sensors
positioned thereon. The analyte sensor 50 is, for example, a "primary" sensor
for
glucose in this application, and can also measure pH or oxygen content in this
2o configuration through working, reference, etc., electrodes 51. Attribute
sensor 40( 1 ) is a
thermistor placed close to the analyte sensor 50 to measure temperature.
Attribute
sensor 40(2) is an optical sensor-source pair to profile boundaries in the
tissue to which
the device 10 is attached, such as skin. Attribute sensor 40(3) measures
ambient light,
primarily in the ultraviolet (UV) range, where damage to the assay sensor 50
is more
25 common. Attribute sensor 40(4) is a micro-durometer to measure skin
conditions or
properties, including tone/hardness, which is related to tissue moisture
content.
Conditions of the tissue, such as skin, are useful because they indicate the
degree of fluid productivity of the tissue. Dry and hard skin produces less
fluid than
softer skin. If the output of the micro-durometer indicates that the
thickness, hardness
30 and/or dryness of the tissue is more than normal, then the amount of
suction applied to
CA 02343762 2001-03-12
WO 00/15102 PCT/US99/20796
8
the harvesting device 10 is increased to ensure sufficient amounts of fluid is
extracted.
Conversely, if the output of the micro-durometer indicates that the skin is
relatively
soft, then the vacuum level may be maintained or decreased. This is
particularly useful
in a continuous monitoring system in which fluid is harvested on a continual
basis from
a harvesting device located on or about the same harvesting site on the
tissue.
As shown in FIG. 3, the sensor head 500 attaches to an assay meter 70 (FIG. 1
)
by an umbilical cord 510 which carnes vacuum and electrical signals. An
attribute
sensor 40(5) is provided at the meter body which measures pressure and/or
vacuum and
humidity in the hose inside the umbilical cord 510 that carries vacuum to the
sensor
1o head 500.
Referring to FIG. 4, within the assay meter 70 or as a separate component, the
attribute signals) of the attribute sensors) 40 is/are connected to a
compensation
element 110 which determines the appropriate compensation based upon the
attribute
signals) from the attribute sensors) 40. The compensation element 110
generates an
appropriate compensation that is output to a processor 80 such as a
microprocessor or
other computing element. The analyte sensor 50 generates a measurement signal
based
on the type of analyte being measured. The measurement signal is connected to
an
assay element 90 within the assay meter 70. The assay element 90 performs a
traditional assay of the analyte, generates a signal corresponding to this
value and
outputs this signal to the processor 80. The processor 80 generates a
corrected assay
value based upon the compensation signal from the compensation element 110 and
the
assay signal from the assay element 90 and outputs a signal corresponding to
this value
to an output means 100 such as a display, a monitoring device or a signal
processing
device.
The type of compensation to the measurement made by the assay element 90
depends on the conditions sensed at the harvesting site. The compensation
applied may
be linear or non-linear with respect to the confounding conditions, or utilize
a neural
network or fuzzy logic. Alternatively, correction may be implemented using a
lookup
table or an equation-based algorithm. For example, pH effects the efficiency
of a
3o glucose oxidase based assay sensor for glucose measurement. If the pH
varies, a
CA 02343762 2001-03-12
WO 00/15102 PCT/US99/20796
9
correction from a lookup table is applied to the assay result to compensate
for the
variation. Humidity measurements are used in discrete sampling interval
systems
where the sample is assayed and then is disposed of by the system prior to the
next
sample being collected. Humidity measurements are also useful in this case to
5 determine if the sample is being collected and humidity differentials are
used to
quantify change in concentration of the analyte being measured. Humidity
measurements are also useful to quantify transepidermal water loss (TEWL).
Temperature effects on the efficiency of glucose oxidase based assay are
measured and
used to generate a lookup table or formula to compensate the assay results for
10 temperature variation. As described above, tissue tone and thickness
measurements are
useful to estimate the vacuum levels required to maintain sufficient sample
flow for
proper assay function. As tissue at the site becomes hydrated it will thicken
and soften
requiring less vacuum for equivalent sample flux.
As an example, FIG. S illustrates graphical diagrams that represent the
15 measurement compensation process using temperature measured from a
thermistor to
compensate a glucose measurement. The upper graph in FIG. 5 shows the
conversion
from the output of temperature sensor to a temperature value. The lower graph
in FIG.
shows the compensation factor for a given temperature value derived from the
data in
the upper graph of FIG. 5. The compensation factor is applied (added or
subtracted) to
2o the glucose measurement to improve the accuracy of the glucose measurement.
In
actual implementation, the conversion process may be implemented in a variety
of
ways, including a stored lookup table of data representing the graphs shown in
FIG. 5.
It should be understood that each attribute may involve a compensation process
that is
similar to that represented by the diagrams of FIG. 5, but with different
data. Similarly
25 a mufti-dimensioned lookup table may be used to efficiently map the outputs
of
multiple attribute sensors into a single assay compensation factor.
FIG. 6 shows steps in a process according to the present invention. The first
step 200 involves the harvesting of biological fluid for the assay. Step 240
requires the
acquisition of condition measurements (i.e., the attributes) relevant to the
assay such as
3o temperature, humidity, etc. This step may occur before, during or after
step 200. Step
CA 02343762 2001-03-12
WO 00/15102 PCTNS99/20796
280 determines the assay compensation value from the measured conditions. Step
220
involves the performance of a traditional assay of analyte concentration from
the fluid
collected in step 200. Step 260 involves the calculation of a corrected assay
value by
modifying the assay value determined in step 220 with a compensation or
adjustment
5 factor determined in step 280. Finally, step 300 outputs the corrected assay
measure for
subsequent usage such as by a display or processing device.
A particular example of the process of FIG. 6 involves the assay of glucose.
Blood or interstitial fluid is harvested through microporation of the
harvesting site in
step 200. In step 240, the temperature of the analyte sensor 50 is measured.
Step 220
to assays the harvested interstitial fluid for glucose levels using
traditional assay
techniques. A compensation factor for the assay based upon the attribute, such
as
temperature, is made in step 280. The compensated assay value is calculated
from the
traditional assay measure from step 220 and the compensation measure from step
260.
The compensated glucose concentration value is output in step 300.
In a continuous analyte monitoring system, such as that disclosed in PCT
application No. PCT/LJS99/16378 filed July 20, 1999, it is also desirable to
compensate
for fluctuations in attributes at the harvesting site, in the harvesting
device or the
analyte sensor, in particular. The process shown in FIG. 6 is repeated on a
continual
basis. For example, an attribute may be measured continuously, and at each
assay or
2o measurement event from the analyte sensor, the attribute signal from the
one or more
attribute sensors are used to compensate the measurement signal obtained from
the
analyte sensor.
Turning to FIG. 7, another embodiment of the present invention is shown. In
this embodiment, a processor 400 performs all of the calculations necessary
for
deriving a value from the analyte sensor S0, compensated for one or more
attributes
from one or more attribute sensors 40. For example, the processor 400 is a
microprocessor or other programmable processing device that executes an assay
program 410 to derive an assay value, compensated for the one or more
attributes
through the use of a compensation program or data 420. The processor 400 reads
a
measurement signal from the analyte sensor 50 and one or more attribute
signals from
CA 02343762 2001-03-12
WO 00/15102 PCTNS99/20796
11
the attribute sensors 40, executes the assay program 410 together with the
compensation program 420 to obtain a measurement value. The compensation
program
420 may be a mathematical algorithm or one or more lookup tables (for each
attribute)
as described above in conjunction with FIG. 5. This may occur on a discrete or
continual basis, depending on the type of environment the system is used. The
value
generated by the processor 400 may be coupled to a display 430. User
interaction with
the processor may occur through a keypad 440. The system shown in FIG. 7 may
further include memory to store values of attribute signals, particularly in a
continual
monitoring system, where it is desirable to retain an archive of information.
1o In summary, the present invention is directed to a system for detecting and
measuring an analyte in a biological fluid of a animal, comprising: a
harvesting device
suitable for positioning on the surface of tissue of an animal to harvest
biological fluid
therefrom, and comprising an analyte sensor positioned to be contacted by the
harvested biological fluid and which generates a measurement signal
representative of
the analyte; at least one attribute sensor to measure an attribute associated
with the
operation of the harvesting device and which generates an attribute signal
representative of the attribute; and a processor coupled to the attribute
sensor and the
analyte sensor to receive the attribute signal and the measurement signal,
wherein the
processor adjusts for an operational parameter of the harvesting device based
on
2o attribute signal.
In addition, the present invention is directed to a method for detecting and
measuring an analyte in a biological fluid of a subject, comprising steps of
harvesting
biological fluid from the surface of tissue of an animal with a harvesting
device;
contacting an analyte sensor with the biological fluid on the tissue surface;
detecting an
analyte in the biological fluid with the analyte sensor; sensing an attribute
associated
with the operation of the harvesting device; and adjusting an operational
parameter of
the harvesting device based on attribute.
In addition, the present invention is directed to a device suitable for
positioning
on the surface of tissue of an animal to harvest biological fluid therefrom,
and
3o comprising: an analyte sensor positioned to be contacted by the harvested
biological
CA 02343762 2001-03-12
WO 00/15102 PCT/US99/20796
12
fluid and which generates a measurement signal representative of the analyte;
and at
least one attribute sensor to measure.an attribute associated with the
operation of the
harvesting device and which generates an attribute signal representative of
the attribute.
The above description is intended by way of example only and is not intended
to limit the present invention in any way except as set forth in the following
claims.