Note: Descriptions are shown in the official language in which they were submitted.
CA 02344197 2004-09-22
METHODS AND SYSTEMS FOR TREATING BREAST TISSUE
BACKGROUND OF THE INVENTION
I. Field of the Invention
The present invention relates generally to medical methods and
S apparatus for treating breast tissues. More particularly, the present
invention relates to
methods and apparatus for ablating or inhibiting the proliferation of
epithelial and other
cells lining a breast duct.
Breast cancer is the most common cancer in women, with well over
100,000 new cases being diagnosed each year in the United States alone. Breast
cancer
usually begins in the cells lining a breast duct, with the first stage thought
to be excessive
proliferation of individual cells) leading to "ductal hyperplasia." Some of
the
hyperplastic cells may then become atypical, with a significant risk of the
atypical
hyperplastic cells becoming neoplastic or cancerous. Initially, the cancerous
cells remain
in the breast ducts, and the condition is referred to as ductal carcinoma in
situ (DCIS).
After a time, however, the cancerous cells are able to invade outside of the
ductal
environment, presenting the risk of metastases, which can be fatal to the
patient.
While breast cancer through the DCIS phase is in theory quite
treatable, effective treatment requires both early diagnosis and an effective
treatment
modality. At present, mammography is the state-of the-art diagnostic tool for
detecting
breast cancer. Often, however, mammography is only able to detect tumors,
which have
reached a size in the range from 0.1 cm to 1 cm. Such a tumor mass may be
reached as
long as from 8 to 10 years following initiation of the disease process.
Detection of breast
cancer at such a late stage is often too late to permit effective treatment.
Alternative diagnostic modalities, which promise much earlier
detection of breast cancer and DCIS, are described in U.S. Patent Nos.
6,168,779;
6,221,622 and 6,494,859. Together, these applications describe techniques for
identifying
one or more (usually all) individual ductal orifices on a nipple in a breast
and for
collecting cellular and other materials from individual ductal networks to
determine if
hyperplasia, DCIS, or other abnormal conditions are present in that network.
While these
techniques will be very useful in providing early and accurate diagnosis of
breast cancer
and other diseased
CA 02344197 2004-09-22
conditions, they do not directly provide for treatment of the condition once
it is
diagnosed. Conventional treatments for breast cancer have been focused on the
treatment of a latter stage disease and include removal of the breast,
localized removal of
the tumor ("lumpectomy"), radiation, and chemotherapy. While these techniques
are often
S very effective, they suffer from certain deficiencies. Removal of the breast
provides the
best assurance against recurrence of the cancer, but is' disfiguring and
requires the patient
to make a very difficult choice. Lumpectomy is less disfiguring, but is
associated with
greater risk of recurrence of the cancer. Radiation and chemotherapy are
arduous and are
not completely effective against recurrence. Such conventional treatments will
not always
be able to take full advantage of emerging diagnostic techniques, which
promise to allow
detection of pre-cancerous and cancerous conditions in the breast at a very
early stage.
For these reasons, it would be desirable to provide improved and
alternative techniques for treating breast cancer and pre-cancerous conditions
such as
ductal carcinoma in situ (DCIS) and atypical ductal hyperplasia (ADH). In
particular, it
would be desirable to provide treatment modalities, which can be used in
conjunction
with the newly developed techniques for diagnosing DCIS and other abnormal
conditions
within individual breast ducts. Such techniques should be less invasive and
traumatic to
the patient than the present techniques, should result in minimum or no
disfigurement of
the breast, and should be effective locally within target sites within the
breast duct and/or
throughout an entire ductal network. Preferably, the techniques should be
capable of
being performed in a single or very few treatment session(s). At least some of
these
objectives will be met by the invention described hereinafter.
2. Description of the Background Art
Related U.S. Patent Nos 6,168,779; 6,221,622 and 6,494,859 have been
described above. Publications by the inventor herein relating to breast duct
access include
Love and Barsky (1996) Lancet 348: 997-999; Love (1992) "Breast duct
endoscopy: a
pilot study of a potential technique for evaluating intraductal disease,"
presented at 15th
Annual San Antonio Breast Cancer Symposium, San Antonio, TX, Abstract 197;
Barsky
and Love (1996) "Pathological analysis of breast duct endoscoped
mastectomies,"
Laboratory Investigation, Modern Pathology, Abstract 67. A description
2
CA 02344197 2001-03-16
WO 00/16708 PCT/US99/21378
of the inventor's earlier breast duct access work was presented in Lewis
(1997)
Biophotonics International, pages 27-28, May/June 1997.
Nipple aspiration and/or the introduction of contrast medium into breast
ducts prior to imaging are described in Sartorius ( 1995) Breast Cancer Res.
Treat. 35:
255-266; Satorius et al. (1977) "Contrast ductography for the recognition and
localization
of benign and malignant breast lesions: An improved technique," in: Logan
(ed.), Breast
Carcinoma, New York, Wiley, pp. 281-300; Petrakis (1993) Cancer Epidem.
Biomarker
Prev. 2: 3-10; Petrakis (1993) Epidem. Rev. 15: 188-195; Petrakis (1986)
Breast Cancer
Res. Treat. 8: 7-19; Wrensch et al. (1992) Am. J. Epidem. 135: 130-141;
Wrensch et al.
( 1990) Breast Cancer Res. Treat. 15: 39-S 1; and Wrensch et al. ( I 989)
Cancer Res. 49:
2168-2174. The presence of abnormal biomarkers in fine needle breast aspirates
is
described in Fabian et al. (1993) Proc. Ann. Meet. Am. Assoc. Cancer Res. 34:
A1556.
The use of a rigid 1.2 mm ductoscope to identify intraductal papillomas in
women with
nipple discharge is described in Makita et al. ( 1991 ) Breast Cancer Res.
Treat. 18: 179-
1 S 188. The use of a 0.4 mm flexible scope to investigate nipple discharge is
described in
Okazaki et al. ( 1991 ) Jpn. J Clin. Oncol. 21: 188-193. The detection of CEA
in fluids
obtained by a nipple blot is described in Imayama et al. (1996) Cancer 78:
1229-1234.
Delivery of epithelium-destroying agents to breasts by ductal cannulation is
described in
WO 97/05898 and U.S. Patent No. 5,763,41 S.
A company called Diagnostics Inc., formed in 1968, produced devices to
obtain breast ductal fluid for cytological evaluation. The devices included a
breast nipple
aspiration device to collect NAF (nipple aspirate fluid) from subjects, and
catheters to
retrieve ductal fluid from breast ducts. The devices were sold prior to May
28, 1976 for
the purpose of collecting breast ductal fluid for cytological evaluation.
Energy-mediated ablation of the uterus, gall bladder, blood vessels, and
other hollow body organs are described in the following U.S. Patent Nos.:
4,776,349;
4,869,248; 4,872,458; 4,979,948; 5,045,056; 5,100,388; 5,159,925; 5,222,938;
5,277,201;
5,242,390; 5,403,311; 5,433,708; 5,507,744; and 5,709,224.
SUMMARY OF THE INVENTION
The present invention provides improved methods for treating individual
milk ducts in human and animal breasts. Such treatments will usually be
performed in
patients diagnosed with cancer or precancerous conditions but may also find
use
prophylactically in patients at risk of cancer or other ductal diseases.
Treatment is
3
CA 02344197 2004-09-22
directed at individual ducts in ductal networks within the breast and
typically comprises
transfernng energy to or from a lumen of the duct in an amount sufficient to
destroy
(ablate) or inhibit proliferation of cells lining the duct, such as epithelial
cells which are
atypical, excessively proliferating (neoplastic), and/or at risk of excessive
proliferation. In
an exemplary embodiment, high frequency electrical current is directed to the
lumen in
order to ablate or necrose at least a portion of the cellular lining of the
duct. The present
invention also encompasses directing other forms of energy to the lumen of the
duct,
including light energy, vibrational energy (e.g., ultrasonic or sonographic),
radiation
(electromagnetic, ultraviolet, infrared, nuclear (typically (3 but sometimes a
and/or y), x-
ray, etc.), heat, direct electrical current, microwave, ferromagnetic, and the
like.
Cryogenic treatment may also find use with suitable cryogenic delivery systems
as
described, for example, in U.S. Patent Nos. 5,899,898 and 5,147,355.
Photodynamic
therapy employing light with wavelengths from ultraviolet to infrared may find
use with
known photoactive agents, such as porfimer sodium (PHOTOFRIN'~), lutetium
texaphrin
(Antrin ), temoporfin (Foscan~), aminolevulinic acid HC 1 (Levulan~), and the
like.
Exemplary phorphyrins and methods of making and other potential aids in the
process of
using them against breast cancer are described in U.S. Patent Nos. 4,935,498,
5,159,065,
5,292,414, 5,369,101, 5,439,570, 5,451,576, 5,457,183, 5,530,122, 5,567,687,
5,587,371,
5,587,463, 5,607,924, 5,756,726, 5,776,925, 5,801,229, 5,817,017, and
5,837,866.
Usually, the photoactive agent will be directed into an individual ductal
network and light
radiation directed to the ductal network and/or the entire breast. For
example, the light
may be introduced into the ductal network using a light fiber or waveguide in
the form of
a ductal access tool. Alternatively or additionally, light can be directed
onto and through
the exterior of the breast.
Other forms of energy and radiation may also be utilized to activate or
enhance the activity of drugs and active agents which have been introduced
into the
ductal lumen(s). For example, ultrasonic energy may be used, e.g., to excite a
fluid or
material that is inside the duct. The fluid or material can then act upon the
duct, including
the ductal lining. For example, polymers which are sensitive to ultrasonic
energy can be
administered to a breast duct. The present invention provides intraductal
delivery of the
polymer spheres, and localized or intraductal exposure of the duct or breast
to ultrasound
to achieve the diagnostic or therapeutic purpose. If the polymers are beads
that house a
cavity, the cavity can be filled with a diagnostic or therapeutic material
which is delivered
4
CA 02344197 2004-09-22
to the breast duct. When exposed to ultrasonic energy the polymer wall breaks
down and
the bead disperses the material that was carried inside the polymer. Such
beads are
available from Point Biomedical located in San Carlos.
Photodynamic therapy (PDT) may be practiced by intravenous
S (systemic) delivery or intraductal delivery of a photoactivatable material
followed by
exposure to light that activates the material. In this invention, a PDT drug,
such as, for
example, Lutex is delivered via catheter into one or more mammary ducts. At
the desired
time, light of the appropriate frequency is applied to the outer surface of
the breast or
intraductally using a fiber optic. The most desirable frequencies of light are
in the range
of 700 nm to 800 nm which is the range that provides the highest penetration
depth in
tissue and blood. The light activates the PDT drug and the therapeutic effects
begin a8er
the drug is activated.
The invention also provides for the delivery of radiosensitizers that
enhance radiation treatment. A radiation sensitizer can be administered to the
duct or
systemically. The sensitizer can be, for example, gadolinium which goes though
an
electron reduction forming a free radical when exposed to x-ray or gamma
radiation.
Texaphyrin can be combined with gadolinium to form a metallotexaphyrin (see
U.S.
Patent No. 5,801,229) which is available from Pharmacyclics, Inc. The toxicity
of
gadolinium is further increased when exposed to the gamma or x-rays. The
radiation
sensitizer need not be metallic-based, but may also be a drug or chemical
which enhances
a cell's susceptibility to radiation, such as CMNa (a nitroimidazole compound,
see U.S.
Patent Nos. 5,650,442, and 4,820,844), carboplatin (see U.S. Patent No.
5,780,653), or
gemcitabine.
Radioisotopes can be delivered to the duct. A radioisotope can be
delivered alone or conjugated to an antibody that is specific for a tumor or
lesion antigen.
The antigen can be, for example, a ductal epithelial cell surface molecule, or
a cell surface
protein that is expressed on the surface of transformed. cells. The
radioisotope can be
conjugated to a monoclonal antibody which is capable of targeting breast ducal
epithelial
cell epitopes, e.g., the antibodies HER2 (see Ross and Fletcher, Oncologist
3(4):237-252
(1998); Pegram et al., Oncogene 18(3):2241-51 (1999)) or MUCI (C,'hu and
Chang,
Cancer Lett 142:121-7(1999); Goldenberg and Nabi, Semin Nuci Med 29:41-
(1999)).
The radioisotope can be a particle emitting isotope, e.g., an alpha particle
emitting
radioisotope, e.g., Bismuth-213 (Bi-2 13). Alpha emitting radioisotopes are
preferred
because alpha particles have a short penetration depth (typica115r 3-fi layers
of cells), are
CA 02344197 2001-03-16
WO 00/16708 PCT!US99/21378
easily shielded, the present minimal radiation risks during handling. The
alpha particles
generated during the degradation of Bi-213 are particles with sufficient
energy to ablate a
limited radius of cells in the ductal lumen and surrounding the duct (e.g.,
myoepithelial
cells, basal cells or stromal cells), and has a short half life, about 45
minutes.
Additionally, Bi-213 decays into stable Bi-209 which is approved for use in
humans (and
is biocompatible) and is routinely used in pharmaceuticals. To target specific
lesions
identified in a duct, any atypical ductal cells retrieved from a duct can be
tested for
antigens, and an appropriate antibody conjugated to the Bi-213 molecules for
administration into the duct and specific targeting to the lesion.
Heating the breast duct (andlor fluid in the duct) may be provided in a
number of ways. Heating the duct may be provided by intraductal access of a
laser
equipped ductal access tool for generating enough laser energy intraductally
to ablate the
ductal lumen tissue, including any lesions present in the duct. Preliminary
work by
radiologist Steven Harms at University of Arkansas has shown that laser
ablation of
tumors is possible and effective. Additionally, laser heating to ablate breast
lesions is
described in Robinson et al., JAm Coll Surg 186(3):284-292 (1998).
Radiowaves at particular frequencies or microwaves may be used to
preferentially heat a medium in the breast ducts and thus ablate the ductal
tissue but not
other breast tissue distal from the duct. For example a fluid or material with
a resonance
frequency of that of an electromagnetic source (e.g., radiofrequency and
microwave, etc.)
can be administered to a breast milk duct. Upon application of the
electromagnetic
energy (either intraductally, or to the entire breast) the fluid or material
in the duct with
the resonance frequency that corresponds to that of the electromagnetic source
would
resonate the heat. The heat would destroy the ductal system that contains the
resonating
fluid or material. The amount of heating is controlled by the amount of
electromagnetic
energy applied. Metallic fluids such as gold or silver colloid can be used,
for example, as
the fluid or material placed into the duct to resonate at a particular
electromagnetic
frequency.
Microwave heating of the breast duct can also be accomplished with a
breast duct access tool capable of generating a local heat that affects only
the lesion and
surrounding tissue of the ductal lumen. A company called Celsion, located at
Columbia,
MD, is developing this particular version of hyperthermia therapy, phone 410-
290-5390.
The methods are described for application in broken tissue, whereas the
present invention
provides methods to apply the ablative therapy intraductally.
6
CA 02344197 2004-09-22
The present invention still comprises removing energy from the
cellular lining of the duct, e.g., freezing the cellular lining, using
cryogenic apparatus and
methods. Agents of extreme cold, or agents which draw heat from surrounding
tissue that
contact the agent can be applied locally to the breast duct using a cryo-probe
adapted for
ductal access to ablate a lesion in the duct or other luminal tissue. A
company called
Endocare, Inc. is developing a CRYOcare 1- Probe Surgical system for
administration to
surgical incision sites. The Endocare product may be adapted for
administration to an
accessed breast duct instead of a surgical site. Extreme cold is applied
though
administration of a cryo agent to the ductal lumen resulting in an ablation of
the ductal
tissue that contacts the agent. See article by Brown, J. Nat'l Cancer Inst.
90(5):351-353
(1998).
Additionally, Titan Corp. of San Diego and TomoTherapeutics has
developed an x-ray needle its subsidiary. The tool may be applied or modified
for use
intraductally, to therapeutically ablate ductal tissue comprising a lesion.
Brachytherapy has been described to treat local breast disease. The
brachytherapy described can be modified for administration intraductally,
without
incision or tissue removal. Dr. Robert Kuske is completing work along these
lines at
Ochsner Clinic in New Orleans, LA.
Finally, radiofrequency (RF) therapy can be applied intraductally. A
ductal access tool capable of delivering radiofrequency waves can be
introduced into a
breast duct and radiofrequency waves can be delivered to the duct to ablate
tissue in the
ductal lumen, including any lesion.
In a first specific aspect of the present invention, a method comprises
selecting an individual duct and transferring energy to or from cells lining
the duct in an
amount sufficient to destroy or inhibit proliferation of said cells. The
individual duct is
usually selected based on a prior diagnosis or evaluation of that duct
indicating a presence
or risk of DCIS or other cancer within the duct. Particular methods for
accessing
individual ducts through an associated orifice in the nipple and for
diagnosing abnormal
conditions within the duct are described in U.S. Patent Nos. 6,168,779;
6,221,622 and
6,494,859.
Briefly, at least one and usually all ductal orifices in
7
CA 02344197 2004-09-22
the nipple of a breast are first located and marked using localization
techniques. The
lumens of each of the ductal networks can then be accessed using small
diameter
catheters which permit collection of cellular and other materials. By
evaluating the
cellular and other materials which are collected, the presence or risk of
disease within an
individual ductal network can be evaluated. Those individual ducts which are
diseased or
at risk of disease can then be selected for treatment according to the methods
of the
present invention.
While it will frequently be desirable to locate and screen all of the
ductal orifices as just described, in certain cases it will be necessary to
screen only pre-
selected ductal orifices, e.g. those which display indications suggesting that
they may be
pre-cancerous or otherwise of particular interest. For example, it may be
possible to
identify regions of the breast or even particular ductal orifices which have
conditions such
as calcification indicating that only certain ductal orifices need to be
screened. In some
instances, it may even be possible to identify particular ductal networks and
associated
network orifices for treatment by the methods of the present invention without
performing
a specific ductal screening technique, e.g. using mammography, ultrasound,
magnetic
resonance imaging (MRI), or other ductal non-specific screening technique.
Depending on the nature of the diagnosis, the entire ductal network or
only a portion thereof can be treated. To treat a portion of the ductal
network, the transfer
of energy will preferably be limited to the cells lining only that portion.
Usually,
however, it will be desirable to treat substantially the entire ductal network
and energy
will be transferred to or from the cells lining the entire ductal lumen.
When applying high frequency or other forms of electrical energy to
the ductal lumen, it will usually be desirable to first introduce an
electrically conductive
medium, such as saline, electrically conductive contrast medium, or the like,
to
substantially fill (and usually distend) the entire duct. Methods utilizing
dual lumen
catheters for flushing and filling ductal networks are described in U.S.
Patent Nos.
6,221,622 and 6,494,859. Once the ductal network has been filled with the
electrically
conductive medium, an electrode or other electrically conductive member can be
introduced to the lumen, typically through the ductal orifice, and current
flow established
through the conductive medium to reach substantially all portions of the
cellular lining. In
the case of radiofrequency and other electrical energy treatments, a
monopolar" energy or
current flow will usually be established. That is, the electrically conductive
medium
within the ductal network will be connected to one pole of a suitable
8
CA 02344197 2001-03-16
WO 00/16708 PCT/US99/21378
radiofrequency or other power supply, while the other pole is connected to a
dispersive
electrode which is placed on the patient's skin, preferably over at least a
portion of the
breast, and more preferably over a region which circumscribes the breast.
Alternatively,
the dispersive electrode can be placed on the patient's thigh, or over the
lower back,
percutaneously with the breast tissue, or in any other manner typically used
in monopoiar
electrosurgical procedures.
Electrical energy could also be applied in a bipolar fashion with a first pole
spaced-apart from a second pole within the lumen. In that way, electrical
energy could be
focused with a well defined length of the lumen.
While electrical energy will usually be delivered into the electrically
conductive medium within the ductal network directly using electrode(s), it
will also be
possible to selectively heat the medium within the ductal network using
radiofrequency
induction. A radiofrequency antenna or other source, optionally shaped to
conform to the
exterior of the breast, may be used to selectively excite and heat the
conductive medium
1 S which has been delivered into the breast. As breast tissue is primarily
water, such
conductive heating will generally be useful to raise the ductal temperature to
very high
temperatures, but rather be useful to selectively increase the temperature
relative to the
rest of the duct to inhibit cellular proliferation. Energy may also be
transferred to the
breast by introducing energy active species, such as photodynamic species,
followed by a
subsequent treatment of the breast using ultraviolet or other light or
radiation having a
wavelength selected to excite the introduced photodynamic or other species. It
will be
appreciated that the present invention is directed at the ductal-specific
delivery of energy
to the ductal network and may comprise a wide variety of specific techniques
for such
delivery.
The agent can comprise an agent sensitive to vibrational energy, for
example, collagen spheres that break-up upon a diagnostic level of ultrasound
applied to
the breast or ductal lumen.
The energy will be applied in an amount and over a time sufficient to at
least inhibit cellular proliferation of cells lining the ductal lumen, usually
epithelial cells
lining the lumen. More usually, the energy will be applied in order to necrose
all cells
lining the ductal lumen. Usually, at least the inner Layer of epithelial cells
which line and
are directly exposed in the ductal lumen will be effected, usually being
destroyed. Often,
it will be further desirable to treat and usually destroy an epithelial layer
below the first
layer, and in some instances it may even be desirable to destroy the
myoepithelial Layer or
9
CA 02344197 2001-03-16
WO 00/16708 PCT/US99/21378
beyond. The electrical energy will typically be applied at a power level from
about 50 W
to 300 W, usually from about 120 W to 200 W. The power may be applied
continuously,
or may be applied at a duty cycle typically in the range from 10 percent to 90
percent,
often from 25 percent to 75 percent. Optionaliy, the amount of power delivered
can be
controlled based on preselected algorithms and/or feedback control algorithms.
For
example, the amount of power delivered to the duct could be controlled based
on the
temperature of the ductal lining, the temperature of the electrically
conductive medium
within the duct, electrical impedance bet<veen electrodes, or other measured
values taken
during the treatment protocol.
In addition to various forms of electrical energy, the present invention may
provide for delivery of other forms of radiation, including electromagnetic,
ultraviolet,
infrared, nuclear, x-ray, and the like. In particular, nuclear radiation can
be delivered
using a solution, dispersion, sol, seeds, or other form of radio isotopic
medium which can
be introduced into and throughout the ducta) lumen, typically using a syringe,
a infusion
1 S catheter, or other device. Nuclear radiation may also be delivered to the
ductal lumen
using a radioactive catheter, wire, stem, implantable seed, or the like.
Catheters may be
designed to immobilize a nuclear radiation source, where the source may then
be
advanced through the ductal lumen to selectively expose portions of the
luminai wall.
Alternatively, or additionally, catheters may provide delivery paths for
introducing
nuclear materials, where the materials may be transported within the catheter
to provide
the desired treatment times and regions. Infrared and ultraviolet radiation
may be
delivered using suitable light fibers and other optical systems. X-ray energy
can be
delivered using a miniaturized x-ray source which can be introduced and
translated
through at least selected portions of the ductal lumen system.
Systems according to the present invention for applying high frequency or
other electrical current to a breast duct comprise a lumen electrode, a
dispersive electrode,
and optionally conductive fluids to be introduced into the ductal network. The
lumen
electrode will be adapted to enter a lumen of the duct, typically through the
associated
ductal orifice, but alternatively percutaneously through tissue into the duct.
The lumen
electrode may be a simple single wire or filament electrode, with the primary
requirement
being the ability to carry sufficient power to the ductal lumen, i.e., within
the power
ranges set forth above. The dispersive electrode will be adapted for placement
on and
conformance to a region on the patient's skin, usually on the breast, more
usually
circumscribing the breast, but alternatively on other locations, such as the
lower back,
CA 02344197 2004-09-22
thigh, abdomen, or the like. The system may further comprise a sheath having a
lumen for introducing the lumen electrode through the ductal orifice. The
sheath will
typically be composed of an electrically insulating material, and the
electrode will be
advanced out a distal tip of the sheath in order to contact the electrically
conductive
medium that has been introduced to the ductal lumen, as described above.
Suitable
electrically conductive fluids include saline, Ringer's medium, lactate
solution, and
other physiologically acceptable electrolyte. Usually, from 0.1 ml to 10 ml,
typically
0.5 ml to 5 ml will be introduced into a single ductal network, and systems
and kits
may include vials, syringes and other containers holding pre-measured amounts
of the
conductive fluids. Optionally, the system may further comprise a high
frequency
electrical power supply having a first pole for connection to the lumen
electrode and a
second pole for connection to the dispersive electrode. The power supply may
incorporate control features to implement the control protocols described
above.
The present invention further comprises kits for treating a breast duct.
The kits will usually comprise a probe,' such as a lumen electrode, and
optionally a
dispersive electrode and conductive fluids as just described. In addition, the
kits will
include at least instructions for use, usually in a printed format, more
usually being
printed on a separate piece of paper packaged together with the kit, or
printed on a
portion of the kit packaging. The instructions will set forth a method for
treating a
breast duct as generally described above, where the probes used to transform
energy
to or from the duct. The kit may further comprise a suitable package, usually
in the
form of a pouch, tray, box, tube, or other conventional package structure.
Usually,
although not necessarily, at least the electrode component of the kit will be
sterilized
and maintained in a sterile condition within the kit, e.g., usually being
maintained in a
sterile package or sterile portion of the package. The instructions for use
may set
forth any of the protocols set forth above in connection with the description
of the
methods of the present invention.
While the present invention is particularly useful for delivering energy
to the ductal network in an amount and for a time sufficient to destroy at
least a
portion of the epithelial or other cellular lining of the ductal lumens, as
described
above, the invention will also find use in treating cancers and other
conditions which
extend beyond the cellular lining of the ductal network.
11
CA 02344197 2004-09-22
According to an aspect of the invention, there is provided the use of
energy in amounts sufficient to ablate or inhibit the proliferation of cells
lining a
breast duct.
According to another aspect of the invention, there is provided the use
of energy for delivery through a lumen of a duct of a breast for the treatment
of breast
tissue.
According to another aspect of the invention, there is provided a
system for applying high frequency electrical current to a breast duct, the
system
comprises:
a lumen electrode adapted to enter a lumen of the duct; and
a dispersive electrode adapted to conform to a patient's skin.
According to another aspect of the invention, there is provided a kit
for treating a breast duct, the kit comprises:
a probe adapted for introduction to a lumen of a breast duct; and
instructions for treating the breast duct with the probe according to
claim 8, wherein the probe is used for transferring energy to or from the
duct.
According to another aspect of the invention, there is provided the use
of a radioactive material for delivery into a least a portion of a ductal
lumen for the
treatment of tissue surrounding the ductal lumen.
According to another aspect of the invention, there is provided the use
of an energy sensitive agent for delivery into and treatment of a breast duct
of a
patient, the agent being sensitive to at least one energy selected from the
group
consisting of light energy, electrical energy, electromagnetic energy,
radiation energy
and vibrational energy, the energy being capable of disrupting the agent which
acts on
target cells lining the breast duct.
According to another aspect of the invention, there is provided the use
of a radioisotope for delivery into a breast duct for the treatment of the
breast duct
wherein the radioisotope emits a particle capable of contacting a cell lining
the duct
and ablating it.
lla
CA 02344197 2006-10-11
According to a further aspect, there is provided use of energy to treat a
breast
duct having a device positioned therein, the energy capable of being
transferred to or
from cells lining the duct in an amount sufficient to ablate or inhibit
proliferation of
said cells.
According to another aspect, there is provided use of energy to treat breast
tissue, wherein a breast duct has a conductive medium therein, the energy
capable of
being applied to the breast tissue through the conductive medium located
within the
breast duct.
According to a further aspect, there is provided use of an agent sensitive to
at
least one energy selected from the group consisting of light energy,
electrical energy,
electromagnetic energy, radiation energy and vibrational energy in a breast
duct
targeted for treatment in a patient, the agent capable of receiving specific
light,
electrical, electromagnetic, radiation or vibrational energy in an amount Buff
dent to
disrupt the agent such that the agent acts on target cells lining the breast
duct.
According to another aspect, there is provided use of a device for treating a
breast duct, the device capable of being positioned within an individual duct
and said
device being capable of transferring energy to or from cells lining the duct
in an
amount sufficient to ablate or inhibit proliferation of said cells.
According to a further aspect, there is provided use of energy to treat a
breast
duct having a medium therein, the energy capable of being transferred to or
from cells
lining the duct in an amount sufficient to ablate or inhibit proliferation of
said cells.
According to another aspect, there is provided use of energy to treat a breast
duct, wherein the breast duct contains a medium and a device, contacting the
medium,
the energy capable of being transferred to or from cells lining the duct in an
amount
sufficient to ablate or inhibit proliferation of the cells.
According to a further aspect of the present invention, there is provided a
use
of a tool and an agent for treating a breast duct of a patient, said agent
sensitive to at
least one form of energy and said agent capable of being systemically
introduced into
said patient targeted for treatment, said tool capable of being introduced
into said
breast duct through a ductal opening and said tool capable of transferring
said at least
one form of energy to the agent in the duct in an amount sufficient to disrupt
the agent
whereupon the agent acts on target cells lining the breast duct.
llb
CA 02344197 2006-10-11
According to another aspect of the present invention, there is provided a use
of
an energy delivering tool and an agent for treating a breast duct of a
patient, said
agent sensitive to at least one form of energy and capable of being
systemically
introduced into said patient targeted for treatment, said tool capable of
being
introduced into said breast duct and said tool capable of transferring said at
least one
form of energy to the agent in the duct in an amount sufficient to disrupt the
agent
whereupon the agent acts on target cells lining the breast duct, said target
cells
including benign cells and at least one of the following types of cells:
cancerous and
precancerous cells.
llc
CA 02344197 2001-03-16
WO 00/16708 PCT/US99/21378
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is an anterior view of a human female breast, shown in section, and
illustrating three of the six to nine ductal networks extending inwardly from
the nipple.
Fig. 2 is an enlarged view of the nipple of Fig. 1 illustrating the orifices
leading to each of the three ductal networks.
Figs. 3A-3C illustrate a catheter suitable for accessing and diagnosing an
individual ductal network as well as for introducing an electrode for high
frequency
energy therapeutic treatment according to the method of the present invention.
Fig. 4A illustrates use of the catheter of Fig. 3 for accessing and
diagnosing a ductal lumen as a preliminary step to performing the therapeutic
treatment
of the present invention.
Fig. 4B illustrates use of the catheter of Fig. 3 for introducing an electrode
for performing high frequency electrical treatment according to the methods of
the
present mvent~on.
I S Fig. 5 illustrates a system according to the present invention in use
during
a therapeutic treatment.
Fig. 6 illustrates a kit comprising a lumen electrode, a dispersive electrode,
and instructions for use according to the methods of the present invention.
Figs. 7A-7D illustrate a photodynamic therapy for ablation of the ductal
epithelium.
Figs. 8A-8C illustrate ultrasound activation of biopolymers.
Figs. 9A and 9B illustrate a ductal access device comprising fiber optic
capacity.
Figs. 1 OA and l OB illustrate devices and materials for administration of a
radioactive panicle emitter.
DESCRIPT10N OF THE SPECIFIC EMBODIMENTS
The present invention comprises methods, systems, and kits for treating
the cellular linings of ductal networks in a human or animal breast. A typical
breast B, as
illustrated in Fig. 1, includes a nipple N and from six to nine ducts D. Three
ductal
networks D,_3 extending inwardly from the nipple N into the breast tissue are
illustrated.
As best seen in Fig. 2, each ductal network D,_3 begins with an orifice O~_3
which lies at
the surface of the nipple N and extends inwardly through a ductal sinus S,_3
and then into
a branching network. Each network D comprises a series of successively smaller
lumens
12
CA 02344197 2004-09-22
which are arranged in complex, three-dimensional patterns. The networks of
each duct
will overlap within the breast tissue but will not be interconnected. 'the
total volume of
each network is usually in the range from 0.1 ml to 0.5 ml, but the walls are
somewhat
compliant so the internal volume may increase as fluid is introduced. The
treatment
methods of the present invention generally rely on accessing the ductal
networks)
through the orifice 0 of the duct D within the nipple N. Usually, there will
be from six to
nine orifices which open into a like number of ductal networks. If desired,
confirmation
of the number and location of the ductal orifices for any individual patient
can be made
by labeling the nipple as described below.
The therapeutic methods of the present invention will usually be
performed following a diagnosis or evaluation of cancer or a pre-cancerous
condition or
other disease in one or more of the ducts) of the patient. In some cases,
however, the
methods could be used prophylactically in asymptomatic patients at significant
risk of
cancer or other breast diseases. The manner of making a diagnosis does not
form part of
the present invention and may be performed as described in U. S. Patent Nos.
6,221,622
and 6,494,859. Briefly, such diagnosis of individual breast ducts relies on
collecting
endogenous ductal fluids and cellular and non-cellular marker materials from
the
individual ductal networks on a duct-by-duct basis. That is, fluids and marker
materials
are obtained from a single duct without obtaining material from any other
ducts. This is in
contrast to other techniques which, in some instances, are able to obtain
cellular and other
materials from all milk ducts at once by applying a mild vacuum to the nipple.
It should
be noted, however, that in some instances such screening of all ducts in a
single step may
be appropriate in order to identify patients showing abnormalities for whom
further, duct-
specific testing is appropriate. Other identification techniques may also be
employed. For
example, calcification may be identified using mammography followed by duct-
specific
diagnosis to confirm the identity of the associated duct and to confirm that
the
calcification are associated with DCIS or other abnormal conditions.
In order to carry out the diagnosis, a location of at least one duct will
be determined, typically by labeling at least one and usually all ductal
orifices as
described in U.S. Patent No. 6,168,779. Briefly, a portion of the epithelial
lining present
exposed at the ductal orifice may be labeled with a visible marker which
allows the
13
CA 02344197 2004-09-22
treating professional to identify the entry orifice for each of the ductal
networks in the
breast. Following identification of the ductal orifice, a washing fluid will
be introduced
into the duct in order to loosen and mobilize cellular material from the
ductal lining,
primarily epithelial cells from the lining. The washing fluid is introduced in
an amount
and a manner such that substantially the entire volume of the duct will be
washed with the
fluid in order to obtain a sample which is representative of the entire ductal
network.
Cellular components from the sample will usually be of the most interest, but
ductal
fluids and secreted molecular species (both small molecules and more usually
biological
macromolecules such as proteins and carbohydrates) may also be analyzed. The
washing
fluid carrying the cells and other materials is then collected, and the
materials
morphologically, (i.e., cytologically), histologically, immunohistologically,
chemically,
immunologically, enzymatically, or otherwise examined in order to determine
any
abnormal or disease conditions within the ductal network, particularly cancer
or a pre-
cancerous condition.
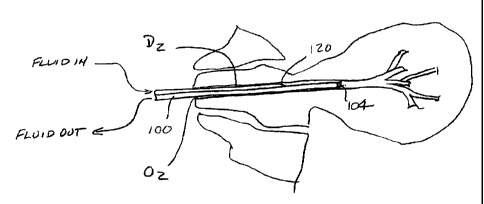
1 S An exemplary catheter 100 suitable for accessing ductal lumens for
both diagnosis and therapy according to the present invention is illustrated
in Figs. 3A-
3C. The catheter 100 is a three French double lumen catheter with a length
from hub 102
to distal tip or port 104 of about 30 cm, and outer diameter Do (Fig. 3B) of
about 1 mm, a
guidewire DGW of about 0.5 mm, and a crescent-shaped lumen 110. The outer tip
diameter
DoT is about 0.8 mm and the luminal tip diameter D,,T is about 0.4 mm, with
the distal end
116 being tapered. A sideport 120 having an oval geometry opens from the
crescent-
shaped lumen 110 and is spaced proximally of the tip 104 by a distance DS of
about 4
mm. Fluid for washing the duct is introduced through port 130 into the lumen
110 and out
through the side port 120 into the ductal lumen. Fluid may be collected
through port 140
on the hub 102 via the guidewire lumen 142 which extends to the distal tip
104. The
catheter may be formed from a wide variety of polymeric materials which
possess
sufficient flexibility and hoop strength, such as polyethylenes, polyimides,
and the like.
The particular dimensions and geometry set forth above have been found to be
suitable
for accessing and diagnosing the breast and would further be suitable for
introducing an
electrode into the ductal lumen, as described in more detail hereinafter.
14
CA 02344197 2004-09-22
As illustrated in Fig. 4A, the catheter 100 is used for collecting the
cellular and other marker materials from a ductal network DZ by first
accessing the duct
with a guidewire, such as a conventional 0.014 inch guidewire (not shown).
After the
guidewire is introduced, typically by a distance in the range from 0.25 cm to
2.5 cm past
the orifice O2, the catheter 100 will be introduced over the guidewire by
passing the distal
port 104 thereover. The distal port 104 is introduced, also typically to a
depth from about
0.25 cm to 2.5 cm, usually from about 0.5 cm to 1.5 cm. Fluid is first
introduced through
port 120 to substantially fill and slightly distend the ductal lumen,
typically at a gauge
pressure from 1 psi to 20 psi. The fluid may then be collected through distal
port 104.
Typically, fluid will be recirculated continuously from the port 120, through
the ductal
network, and then collected into the distal port 104. Particular techniques
and alternative
techniques for performing the washing and analysis of the ductal lumens is
described in
U.S. Patent 6,221,622 and 6,494,859.
After the diagnosis is complete, the methods, systems, and kits of the
present invention may be used to treat individual ductal lumens which are
diagnosed as
having cancerous, pre-cancerous, or other abnormal cells or disease
conditions. A
preferred method for treating the duct DZ is illustrated in Fig. 4B. A sheath
or other
cannula which may be the catheter 100 described above, is reintroduced to the
duct DZ,
usually to a depth substantially equivalent to that used for diagnosis. A
luminal electrode
150 is then introduced through the guidewire lumen so that it extends distally
of proximal
port 104 into the ductal lumen. Before or after introducing the electrode, an
electrically
conductive medium will be introduced to substantially completely fill the
entire ductal
network. Unlike the diagnosis step, however, the fluid will typically be
maintained in a
static condition, i.e., without recirculation. Usually, a slight static
pressure will be
maintained on the fluid in order to completely fill and slightly distend the
ductal network,
typically at least about I psi, often at least about 3 psi, and sometimes 5
psi or higher.
Refernng now to Fig. S, the electrode 1 SO may be connected to a first
pole 152 of a high frequency power supply, such as a conventional
electrosurgical power
supply. A dispersive electrode 154 may be placed about the exterior of the
breast B,
typically circumscribing the breast, as illustrated in Fig. 5. The dispersive
electrode will
be connected to the other pole 156 of the power supply 160. Suitable
electrosurgical
power supplies are available from a number of commercial vendors, such as
Valleylab,
Aspen, Bovie, and Butcher. The power supply will usually provide energy at
high
frequencies in the range from about 200 kiHz to 4 MHz, and may employ
conventional
CA 02344197 2001-03-16
WO 00/16708 PCT/U S99/2 t 378
sinusoidal or non-sinusoidal waveforms. The total power delivered to each
ductal lumen
may be in the range from SO W to 300 W, usually from about 120 W to 200 W. The
electrical energy will be applied for a time sufficient to inhibit
proliferation of the cells
lining the breast duct, usually for a time sufficient to ablate or necrose
substantially the
S entire cellular layer lining the breast duct.
As illustrated in Fig. S, operation of the electrosurgical system is
"monopolar." That is, the current flows between the lumen electrode, which is
considered
an active electrode to the dispersive electrode disposed on the exterior of
the patient's
skin. In other cases, it might be possible to perform the procedure in a
bipolar manner.
In such cases, two or more electrodes may be penetrated into the ductal lumen,
where the
electrodes are energized with opposite polarities. In some cases, at least one
of the two
electrodes might be introduced percutaneously, e.g., using a needle stick,
into the breast
tissue so that it is disposed at or near a distal terminus of the ductal
network. In general,
however, monopolar operation as illustrated in Fig. S will be preferred.
1 S As described thus far, the electrically conductive fluid is directly
energized
by contact with at least a single electrode disposed in the duct lumen. It
will also be
possible to indirectly heat the fluid by inductive heating where an external
antenna or
electrode is brought into proximity with the breast and energized to excite
and heat the
fluid which fills the ductal lumen. Ultrasonic energy may be used, e.g., to
excite a fluid
or material that is inside the duct. The fluid or material can then act upon
the duct,
including the ductal lining.
Kits 200 according to the present invention are illustrated in Fig. 6. The
exemplary kit 200 comprises a lumen electrode, such as electrode 1 S0, a
dispersive
electrode, such as electrode 1 S4, and instructions for use 1 S6 setting forth
a treatment
2S protocol for an individual breast duct according to the principals
described above. The
components of the kit will typically be packaged in a conventional medical
device
package, such as pouch 210, where some or all of the components may be
maintained in a
sterile environment.
Fig. 7 depicts photodynamic therapy using light sensitive chemical
(e.g., texaphyrins (porphyrins)) delivered locally to the breast duct, and
applying the light
source stimulation to the breast, either in the duct or at the breast
generally. Fig. 7A
depicts the chemical lutetium texaphyrin that after exposure to light emits
cytotoxic
signlet oxygen which attacks tumor cells. Fig. 7B depicts breast duct 300,
nipple and
breast 301, ductal orifice 302, and intraductal lesion 304. The duct 300 is
accessed at the
16
CA 02344197 2004-09-22
orifice 302 by catheter 307 containing a chemical 306, for example, lutetium
texaphyrins
in syringe delivery receptacle 308, delivered to the duct and contacting the
lesion 304.
Fig. 7C depicts the application of general light from a light source 309 to
the external
portions of the breast to excite the chemicals 306 in the duct 300. Fig. 7D
depicts the
S application of an intraductally delivered light from a source 310 configured
to access a
breast duct 300 in order to excite chemicals 306 which in turn act on the
lesion 304.
The device depicted in Fig. 7 may also be applied to administer a radiation
sensitizer to a breast duct and activate it there. For example duct 300 is
accessed at the
orifice 302 by catheter 307 containing chemical 306, for example, the
radiation sensitizer
XCYTRIN, a metallotexaphyrin, can be administered to the duct in a syringe
delivery
receptacle 308, delivered to the duct and contacting lesion 304. An x-ray or
gamma ray
source is applied to the breast to activate the radiation sensitizer and
provide toxic effects
for intraductal tumor cells. The XCYTRIN molecules can capture electrons
produced by
x-ray or gamma radiation, resulting in one electron reduction of the complex.
A pi (7c) -
1 S radical cation is created that is reactive and capable of destroying
neighboring
biomolecules such as DNA. The radiation therapy is preformed by irradiation of
the
tumor site with x-rays or gamma rays (while shielding adjacent normal tissue
to minimize
toxicity). The x-rays and gamma rays interact with molecules in the duct such
as water to
generate high energy electrons and free radicals which are highly reactive and
short-lived
molecules. Radiation sensitizers are chemicals that increase the lethal
effects of radiation
when administered in conjunction with it. Tumor cells, which are hypoxic, are
2.3 to 3.0
times more resistant than normal cells to the damaging effects of ionizing
radiation.
Administration of 'C~'YCT 1N l:rov:des ue agen: of strong c'.~c.r<,a z:fnity
ezp,able of
reacting with hydrated electrons to prevent them from neutralizing cytotoxic
hydroxyl
2S radicals, and thus promote radiation sensitization of hypoxic cells.
Chemical 306 can
comprise a chelating agent, such as e.g., ETPA or DTPA, to bind the metallic
radioactive
ions making them chemically inert, but still radioactive.
Fig. 8 depicts ultrasound activation of high molecular weight collagen
derived biopolymers to better visualize the breast duct or to deliver
diagnostic or
therapeutic agents to the breast duct. Fig. 8A shows a polymer sphere housing
either air
or a diagnostic or therapeutic chemical 403. The sphere has an outer wall 402
and an
inner wall 401 that can be designed to be sensitive to ultrasonic energy and
to break open
to release particles 403 as depicted. Fig. 8B shows a breast 404 containing a
breast duct
17
trade-mark
CA 02344197 2001-03-16
WO 00/16708 PCT/US99/21378
405 and spheres 400 with particles 403 inside. The spheres are delivered in a
ductal
access tool 406 from a syringe delivery receptacle 407 in order to treat or
diagnose lesion
408. Fig. 8C depicts the same duct 405 accessed by ductal access tool 410
having an
ultrasonic signal transmitter connected to energy source 409. Lesion 408 is
diagnosed or
treated when particles 403 are released upon application of the ultrasonic
wave energy.
Fig. 8C also depicts delivery of a fluid or substance that resonates at a
particular frequency. Different mediums will resonate at different
frequencies, and a
medium selected to resonate at the frequency of applied radiofrequency or
microwave
energy will be preferentially heated over the surrounding breast tissue. In
this
embodiment, lesion 408 is ablated when medium 403 is delivered (for example, a
gold
colloid) to breast duct 405. Energy delivery tool 410 connected to energy
source 409
supplies the radiofrequency waves or the microwaves that heat the medium
preferentially
and thus destroy some of the lining of the duct including the lesion 408. The
resonant
energy can also be applied externally to the outside of the whole breast and
the effects of
the energy would be felt where the medium was administered, i.e., in the
target breast
ducts.
Fig. 9 depicts a breast duct S00 in a breast 501 accessed by a needle
containing ductal access tool 505 through orifice 502, so that fiberoptic
light and energy
source 504 can be threaded through the needle or lumen SOS to contact the
lesion 503 and
apply laser generated heat to the lesion. Fig. 9B shows the follow-up
procedure to check
that all the lesion is removed using a ductal access tool 506 with fiberoptic
scope
apparatus 507 placed into duct 500 in breast 501 to visualize that the lesion
has been
removed by laser-generated heat. See Robinson et al. JAm Coll Smg. 186(3):284-
292,
for details of the procedure with regard to systemic delivery of the
ultrasonic sensitive
agent and application of laser hyperthermia. A similar procedure can be
performed using
a cryo probe in place of the ductal access tool emitting laser-generated heat.
Referring
now to Fig. 9A, the cryo probe 504 is placed in the duct S00 and extremely
cold
temperatures are delivered to the duct and preferably contacting the lesion
and killing the
neoplastic cells of the lesion. The heat therapy can also be performed using a
probe that
delivers by microwave energy a concentrated heat that burns the ductal lumen
including
the lesion, but does not substantially damage other breast tissue.
Figs. l0A and 10B depict administration of a radioactive alpha emitter to
the breast duct. The radioactive alpha particle emitter can be, for example,
Bismuth 213
(Bi-213) (half life 45 minutes), Bi-212 (half life 60 minutes), Tb-I49 (half
life 4.13
18
CA 02344197 2001-03-16
WO 00/16708 PCT1US99/2I378
hours), At-211 (half life 7.21 hours), Fm-256 (half life 20.1 hours), Ac-225
(half life 10
days), and Ra-223 (half life 1 1.4 days). Pure Bismuth 213 (Bi-213) may be
coupled to an
alpha particle emitter. Fig. l0A indicates Bi-213 conjugated to an antibody
specific for a
tumor antigen. Either the compound is administered to breast duct 600 in
breast 601 by
accessing the ductal orifice 602 with breast duct access tool and therapeutic
drug
administrator 603. The radioactive alpha emitter Bi-213 either alone or
conjugated to a
tumor or lesion specific antibody is contained in delivery receptacle 604 and
administered
through tool 603 from the receptacle. Inside the duct, the radioactive alpha
emitter
decays emitting an alpha particle capable of penetrating a cell wall and
violating the
integrity of a cell, including the hyperplastic or neoplastic cells of a tumor
or lesion.
While the above is a complete description of the preferred embodiments of
the invention, various alternatives, modifications, and equivalents may be
used.
Therefore, the above description should not be taken as limiting the scope of
the
invention which is defined by the appended claims.
19