Note: Descriptions are shown in the official language in which they were submitted.
CA 02345720 2004-05-17
APPARATUS AND METHOD FOR NON-INVAS1VE,
PASSIVE FETAL HEART MONITORING
BACKGROUND OF THE INVENTION
1. Field of Invention
The invention relates generally to biomedical devices and, in particular,
comprises a non-
invasive and passive apparatus and method that uses sensors and signal
processing techniques to
monitor fetal electrocardiographic waveform (EKGf), heart rate, heart rate
variability and heart .
vector orientation and maternal heart rate and uterine contraction noise
artifacts.
2. Description of the Related Art
Though the perinatal mortality rate in the United States has decreased
significantly in the
past three decades, the vast majority of the current perinatal deaths are
thought to be attributable
to potentially preventable etiologies. Prematurity, intrauterine hypoxia,
perinatal infections, and
maternal complications account for 60 to 80% of perinatal losses.
Maximizing the health and well-being of the mother and fetus by appropriate
medical
intervention is the general goal of obstetrical care. Effective monitoring of
a fetus may require
continuous assessment, and is commonly performed using electronic technology.
However,
recent escalation of the frequency of normal births by cesarean section has
called into question
2o the validity of present monitoring techniques with respect to specificity
of identifying the fetus at
risk. Reducing the number of unnecessary cesarean sections and, in general,
reducing the
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295
-2-
number of babies that are seriously ill at birth has been raised as a national
health care priority in
an effort to reduce the cost of both short- and long-term health care.
Fetal assessment in this context is intended to detect conditions that, if
continued, would
likely result in fetal and newborn damage or death. The condition of the fetus
is reflected by the
cardiovascular responses in utero and may be recognized by monitoring the
fetal heart rate.
The difficulties in monitoring fetal well-being have long been recognized by
the medical
profession. The variable position of the fetus within the womb, surrounded by
the amnion and
amniotic fluids makes direct examination of the fetus impossible or very
difficult using most
examination techniques.
Present electronic fetal heart rate monitoring shows great sensitivity, but
inadequate
specificity, and poor positive predictive value in correlating fetal heart
rate changes with
subsequent adverse neonatal outcome. Such electronic fetal heart rate
monitoring, despite these
limitations, remains an integral part and standard of care in the assessment
of fetal status.
Presently, the primary non-invasive fetal monitoring technique is the
Doppler/tocometer.
i s The technique is cumbersome and subject to data loss as a result of fetal
and maternal movement.
Typically, a Doppler transducer is placed on the mother's abdomen in a
position that focuses the
ultrasound signal at the fetal heart. Should the fetus move relative to the
transducer, it is highly
likely that the transducer will no longer be in proper position and, thus, not
record an accurate
heart signal. In fact, the use of a Doppler monitor is not precise enough for
reliable analysis of
2 o subtle heart rate changes.
L1.S. Patent No. 5,257,627 to Rapoport relates to a portable apparatus for the
non-
invasive, simultaneous, self testing of fetal and maternal signals. The device
has a signal
processing means for simultaneously processing fetal heart rate and maternal
input signals, and
CA 02345720 2001-03-28
WO 00/54650 PCTNS00/06295
-3-
also has a communication linking means for the simultaneous transmission of
the fetal heart rate
and maternal input data to a remote output device. Rapoport's device uses
ultrasonic means to
detect the fetal heart rate.
Other non-invasive techniques are also in use. These include the processing of
s electrocardiograph and electromyogram signals for determination of the
fetus' well-being.
U.S. Patent No. 4,299,234 to Epstein et al. relates to a fetal heart rate
monitor which
combines electrocardiograph and electromyogram type signals to increase
reliability and
accuracy of the resulting heart rate information.
U.S. Patent No. 4,781,200 to Baker relates to a self contained, lightweight
ambulatory
fetal monitoring system for substantially continuous analysis of fetal well-
being. The monitor
includes a sensor garment which is worn by the mother and has a plurality of
sensors. The
sensors detect fetal heartbeats and movements of the fetus within the mother.
Signals developed
by the sensors are processed by signal processing equipment and analyzed by a
programmable
data processing unit which can be provided with a variety of analytical
programs which are
1 s proposed to automatically and continuously analyze fetal well-being. The
sensor belt goes
around the waist of the mother, and thus obstructs the surgical field.
U.S. Patent No. 5,042,499 to Frank et al. relates to a fetal heart rate
monitor that monitors
weak fetal electrocardiogram signals in the presence of strong interfering
noise. Frank et al's
invention non-invasively obtains from the abdomen of a pregnant subject the
fetal EKGr signal,
2o fetal heart rate, and accurate beat-to-beat heart rate variability. An
operator views the EKGf
signal and optimally places the set of thoracic electrodes in an attempt to
adaptively cancel the
maternal EKGf signal from the signal separately derived from a variably
located abdominal
electrocardiograph lead. There is no uniform placement of the abdominal
electrodes for all
CA 02345720 2004-05-17
-4-
patients. Placement of such leads is dependent on prior examination by a
trained medical
professional to identify optimal lead orientation.
Evaluation of the fetal electrocardiographic waveform itself might provide
increased
insight into the status of the fetus. Unfortunately, direct accessibility of
the fetus has limited the
electrocardiogram as an indicator of well-being. During labor, after the
rupture of the amniotic
sac, a fetal scalp electrode may be attached to the fetus's skin. This
requires twisting a wire
corkscrew electrode into the presenting part of the fetus, e.g., scalp or
buttocks, via the vaginal
opening.
In the absence of direct electrode contact with the fetus, a large maternal
signal and the
1 o presence of electrical noise (e.g. muscle artifact) has substantially
precluded recognition of the
fetal electrocardiogram. The placement of a fetal scalp electrode is clearly
invasive, generally
less comfortable for the mother, and has associated increased risks, such as
infection, to the fetus,
mother, and caretakers. The issue of infection has received more attention
recently with
increased risks of serious bloodborne infections such as AIDS.
1 s Regardless of the monitoring technique, critical difficulties frequently
arise when there is
an emergent need to transfer the monitored patient from the labor area to the
operating room.
The monitors are usually detached during this critical interval with the
mother and her fetus
unmonitored during the transfer. Reattachment to monitors in the operating
room (if at all)
requires additional, possibly precious time and attention. Doppler
transducers, if used, are
2 o inevitably in the operative field for an emergency cesarean section.
Likewise, scalp electrodes
must be removed or cut and withdrawn with the baby through the abdominal
incision, again
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295
-5-
increasing the risk of infection.
This established need, therefore, creates a requirement for a reliable,
accurate, and
noninvasive technique to monitor the electrocardiogram of the fetus.
Furthermore, the technique
must maintain a clear operative field, accommodate movement of the mother and
fetus, and be
usable for a relevant portion of gestation. Moreover, it will be very
desirable for the monitor
output to include the fetal electrocardiogram waveform in addition to the
fetal heart rate and
description of heart rate variability. Monitoring of maternal heart rate and
the state of uterine
contractions and noise artifacts attributable to the uterus would also be
desirable.
i o SUMMARY OF THE INVENTION
The present invention provides a method of monitoring a fetal biopotential
waveform.
More particularly, the present invention provides a method for generating a
fetal biopotential
waveform and using the waveform components to monitor many variables
including, but not
limited to, the fetal heart rate, the fetal heart rate variability, and/or the
fetal heart vector
15 orientation of a fetus in a pregnant mother. The method includes the steps
of measwing at least
one biopotential waveform indicative of the mother's heart beat to form a
maternal waveform,
measuring at least one biopotential waveform indicative of the combined
maternal and fetal heart
beats to form a combined biopotential waveform, and using signal processing to
cancel the
maternal waveform from the combined waveform to derive a fetal waveform
indicative of the
2o fetus' biopotential electrocardiographic waveform (EKGr).
The present invention also provides an apparatus for monitoring a fetal
biopotential
waveform. The present invention also provides an apparatus for generating a
fetal biopotential
waveform and using the waveform to monitor the fetal heart rate, the fetal
heart rate variability,
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295 r
-6-
and/or the fetal heart vector orientation of a fetus in a pregnant mother. The
apparatus includes
at least one sensor, e.g., an electrode, for measuring at least one
biopotential waveform indicative
of a maternal heart beat, at least one sensor for measuring at least one
biopotential waveform
indicative of the combined maternal and fetal heart beats taken from a
pregnant mother, and
s signal processing hardware, software, or hybrid mixes that can cancel the
maternal waveform
from the combined waveform to form a waveform indicative of the EKGr.
The present invention non-invasively and passively measures fetal and maternal
electrocardiographic and maternal electromyographic waveforms by using
traditional surface
electrode electrocardiographic and electromyographic techniques combined with
adaptive signal
i o processing methods to solve the problems associated with the
devices/techniques described
above. The invention provides patient information (e.g., fetal heart
rate/variability, taking into
account noise artifacts attributable to uterine contractions) that at least
duplicates current clinical
standards.
In particular, the invention uses, for example, suitable skin contact
electrodes connected
i s to amplifiers to acquire biopotential waveforms and form signals,
preferably differential signals,
indicative of the mother's heart beat from sensors, e.g., electrodes, placed
on her chest, and
indicative of the combined maternal and fetal heart beats from sensors placed
on the mother's
abdomen, lower back, or both, as well as electromyographic signatures
indicative of noise
artifacts attributable to changes in uterine tone. Maternal heart rate, heart
rate variability, and
2 o respiration rate are derived from the chest signals; standard maternal EKG
is derived from planar
leads. Instead of differential signals, more vectors may be formed by
collecting single-ended
signals and creating "differential pairs" therefrom.
The sensors placed on the mother's abdomen, lower back, or both, are
preferably placed
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295
to form pairs of sensors wherein each sensor of the pair is spaced from the
other and each pair is
positioned in a substantially criss-crossed pattern with respect to other
sensor pairs. Substantial
spacing between the sensors of each sensor pair and between pairs of sensors-
it -preferred so as to
achieve a three-dimensional processing of the fetal biopotential waveform. As
mentioned above,
s the sensors are preferably positioned to avoid blocking any surgical fields,
for example, the
abdominal area. By sensing the combined fetal and maternal waveforms with a
multiplicity of
sensors, the uniqueness of the vectors can be used to establish the vector
orientation of the fetus.
Preferably, the number of vectors used is sufficient to achieve a clear signal
indicative of the
combined fetal and maternal waveforms. If a clear enough combined signal is
obtained from a
1 o single sensor, the present invention can operate using a single sensor to
obtain the combined
waveform.
The signals from the abdominal electrodes are divided into a plurality of
channels. After
data validation, an adaptive signal processing filter (ASPF) algorithm or
other suitable algorithm
is used to cancel the estimated maternal waveform from each channel in the
abdominal
15 electrodes, using chest signals as references. The system then selects from
at least one of the
resulting waveforms to serve as the reference fetal waveform, for example, the
waveform with
the highest peak-to-peak amplitude. Using another ASPF or other suitable
algorithm, the
reference waveform is then processed against the other abdominal waveforms
with the maternal
waveforms canceled to form an enhanced fetal signal that is a representation
of the EKGr. The
2 o EKGr can subsequently be used to measure fetal heart rate and other
biophysical profile
parameters. Surface electromyogram (EMG) signals allow for concurrent
monitoring of uterine
contractions and afford improved cancellation of motion artifacts including
noise attributable to
skeletal muscles and uterine contractions.
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295
_g_
The present invention provides a device that is totally non-invasive, passive
and will
supplant the fetal scalp electrode and, therefore, eliminate those risks of
infection. In one
embodiment, all signals are derived from standard EKG electrodes applied to
the patient's skin.
The present invention also provides a device with sensor placement, e.g.,
probe electrode
s placement, that is universal across the patient population. Furthermore, in
embodiments of the
present invention wherein sensor strips or other free floating sensors, e.g.,
non-adhesive, are used
to contact the mother's chest, abdomen, and/or back, the patient's position
can be rotated or
reorientated relative to the sensor field. In such an embodiment, the sensors
must be capable of
sensing a respective wavefonn without the need to be adhered to the patient's
body.
1 o The present invention also provides a device where the placement of the
electrodes
maintains a clear surgical field, thereby facilitating operative procedures
such as cesarean section
deliveries, and will not interfere with resuscitation of the mother, should
either become
necessary.
The present invention also provides a device that overcomes the signal loss
anomaly of
i s ultrasound devices resulting from fetal movement. There is no need to tend
to the device and
reposition electrodes as the fetus moves, thereby allowing health professional
time and attention
to be directed toward more productive patient care activities.
The present invention also provides a device that will achieve a full
representation of the
fetal EKG f waveform which may provide useful information about the fetal
condition.
2o The present invention also provides a device which upon interpretation of
the fetal EKGf
waveform makes the subject device capable of determining the instantaneous
orientation of the
fetal heart vector, thereby indicating the orientation of the fetus and
permitting prediction of
delivery complications associated with atypical presentation.
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295 r
-9-
The present invention also provides a device that routinely collects maternal
EKG signals.
Thus, collateral information about the well-being of the mother and possible
maternal-fetal
interactions are-immediately available:
The present invention also provides a device that will function for an
ambulatory patient,
either pre-term or during prolonged labors where the patient wishes to
ambulate.
The present invention also provides a device that can be used in the case of
non-imminent
deliveries, for example, pre-term patients who may have high risk pregnancies.
The present invention also provides a device that computes and displays a
unique
monitoring reading that provides a measure of the instantaneous processing
performance.
to The present invention also provides a device that computes and displays
heart rate
variability information in at least two forms: i) long term variability trend,
as is available with
current commercial systems; and ii) a unique measure of instantaneous
variability.
The present invention also provides a means to monitor multiple gestations
with no
additional sensors and/or processing techniques being required.
The present invention also provides a device that routinely collects
electromyographic
(EMG) signals as a means for monitoring maternal uterine contractions and for
providing an
additional signal input for noise cancellation. In addition, the device also
permits the
identification and characterization of active (maternal movement) and passive
(surgical
manipulation, uterine contraction) maternal signals from EMG inputs useful for
canceling noise
2o artifacts to even further enhance the EKGf.
The accompanying drawings, which are incorporated in and constitute a part of
this
application, illustrate several embodiments of the present invention and
together with the
description serve to explain the principles of the present invention.
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295
-10-
BRIEF DESCRIPTION OF THE DRAWINGS
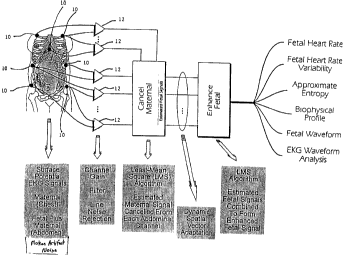
Fig. 1 is a block diagram of the preferred concept of the present invention:
Fig. 2 illustrates exemplary electrode positions and signal channels for the
present
s invention.
Fig. 3 is an illustration of a sensor placement scheme showing ship sensors
positioned on
opposing sides of a pregnant mother's abdomen in accordance with an embodiment
of the present
invention.
Fig. 4 is an illustration of a sensor placement scheme showing a strip sensor
in place and
a strip sensor connected to an electrode interface.
Fig. 5 is a functional block diagram of the signal processing of the
invention.
Fig. 6 illustrates an exemplary adaptive signal processing filter (ASPF) used
in the
invention's signal processing, the ASPF using a Least-Mean-Squares (LMS)
algorithm.
Fig. 7 illustrates results using the present invention with simulated data.
Characteristic
i5 simulated fetal (Baseline Fetal) and maternal EKG signals were summed
together (Abdominal)
with noise in order to validate the signal processing algorithms. Fetal R-
peaks are evident
(Maternal Removed) following the first state of processing; significant noise
reduction is evident
(Fetal Enhanced) following a second stage of processing.
Fig. 8 illustrates results with representative clinical data. Data sample
collected in the
2o clinical environment using the present invention. The three signal traces
correspond to the
simulation data shown in the inset of Fig. 7. The jagged appearance of the
signals is an artifact
of graphics manipulation and not a system limitation.
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295
Fig. 9 is a graph showing the time histories of the fetal heart rate and the
maternal heart
rate derived from waveforms acquired according to the present invention.
DETAILED DESCRIPTION OF THE PRESENT INVENTION
s The present invention provides a method of monitoring a fetal biopotential
waveform.
More particularly, the present invention provides a method for generating a
fetal biopotential
waveform and using the waveform to monitor the fetal heart rate, the fetal
heart rate variability,
and/or the fetal heart vector orientation of a fetus in a pregnant mother. The
method includes the
steps of measuring at least one biopotential waveform indicative of the
mother's heart rate to
1 o form a maternal wavefonm, measuring at least one biopotential waveform
indicative of the
combined maternal and fetal heart rates to form a combined biopotential
waveform, and using
signal processing to cancel the maternal waveform from the combined waveform
to derive a fetal
waveform indicative of the fetal electrocardiographic waveform (EKGf).
The present invention also provides an apparatus for monitoring a fetal
biopotential
1 s waveform and an apparatus for monitoring the fetal heart rate, the fetal
heart rate variability,
and/or the fetal heart vector orientation of a fetus in a pregnant mother. The
apparatus includes
at least one electrode for measuring at least one biopotential waveform
indicative of a maternal
heart rate, at least one electrode for measuring at least one biopotential
waveform indicative of
the combined maternal and fetal biopotential waveform taken from a pregnant
mother, and a
2 o signal processing circuit that can cancel the maternal waveform from the
combined waveform to
form a waveform indicative of the fetal electrocardiographic waveform (EKGf).
According to a preferred method of the present invention, the maternal
biopotential
waveform is preferably acquired from at least one sensor, e.g., a skin contact
electrode; and
CA 02345720 2001-03-28
WO UO/54650 PCTNS00/06295
-12-
preferably from two or more sensors, placed on or in contact with the mother's
chest, upper back,
or both. The combined waveform is acquired from at least one sensor, e.g., a
skin contact
electrode, and-preferably from two or more sensors,-placed on or in contact
with the mother's
abdomen and/or lower back. Preferably, the combined waveforin is acquired by
combining the
signal from at least one sensor placed on or in contact with the mother's
abdomen and at least one
other sensor placed on or in contact with the mother's abdomen, lower back, or
both. Other
devices to acquire the signals or waveforms mentioned throughout can be used
and include
sensors that may be mounted on a table or chair that the mother rests on, or
non-contact sensors
such as magnetometers and the like that can be spaced from the surface of the
mother's skin.
1 o The combined waveform can be preferably derived, according to the
invention, from a
plurality of signals acquired from a plurality of sensors placed on the
mother's abdomen, and by
dividing the signals into a plurality of channels. The method then includes
canceling an acquired
maternal waveform from each channel to form a plurality of resulting
waveforms, and selecting
at least one of the resulting waveforms as a reference fetal waveform. The
selected waveform
can be, for example, the waveform with the highest peak-to-peak amplitude. The
selected
reference fetal waveform is enhanced using an adaptive signal processing
filter algorithm or
another suitable algorithm to remove correlated noise by processing against
the remaining
resulting waveforms that were not selected as the reference feta) waveform.
The signal strength
of the various resulting waveforms can then be compared and the detected
signal characteristics
20 of the resulting waveforms cah be used to infer a heart vector orientation
and the position of the
fetus. The signal processing algorithm can use a least mean squares {LMS)
algorithm to
dynamically weigh all of the waveforms derived from the sensor on the mother's
chest against
each of the waveforms derived from each of the sensors on the mother's abdomen
and/or lower
CA 02345720 2004-05-17
-13-
back. An exemplary teaching of an LMS filter algorithm that can be used in the
methods and
apparatus of the present invention is described in U.S. Patent No. 5,891,045,
and the references
cited therein; including Changxiu et al.; "A New Algorithm for Adaptive Noise
Cancellation
Using Singular Value Decomposition," Ac~a Aucomatica Sinica, Vol. 12, No. 2,
pp. 146-153
s (April 1986); Damen et al., "The Use of the Singular Valve Decomposition in
Electrocardiology," Medical & Biological Engineering & Computing, pp. 473-482
(1982); and
Widrow, "Adaptive Interference Canceling," Adaptive Signal Processing,
Applications Part IY,
Chap. 12, Prentice-Hall, Englewood Cliffs, NJ, pp. 302-367 (1985). ,
The fetal electrocardiographic waveform derived according to the method and
apparatus
of the present invention can be visually analyzed by observing a visual
display of the waveform
or by inspecting other forms of data acquired that correlate with or have a
relationship to the
wavefonm. A trained technician can visually analyze the waveform to determine
any
abnormalities in the visual representation of the waveform and thus can
determine any
abnormalities in the fetus' well-being. Over time, a table or library of
normal EKGs can be
1 s obtained so that technicians can become familiar with normal fetal
electrocardiographic
waveforms and be able to determine abnormalities in subsequently tested
fetuses. Trained
technicians can, at a glance, by visually inspecting the displayed
electrocardiographic waveform,
make a determination of a fetus' well-being (normality of EKG) in a reliable
and non-invasive
manner.
2o According to advantageous embodiments of the present invention, the sensors
placed on
the mother's abdomen and/or lower back are preferably spaced away from the
lower anterior
abdomen or, for example, away from the lower right-side anterior abdomen so as
not to interfere
CA 02345720 2001-03-28
WO 00/54650 PCTNS00/06295
-14-
with a cesarean section delivery of the fetus, an appendectomy operation or
other procedure
should such procedures be necessary. The skin sensors placed on the mother's
chest are
preferably placed, for example, away from the midline of the chest so as not
to interfere with
resuscitation attempts on the mother, should such attempts be necessary.
s The sensors used to transduce the biopotential signals of interest may
preferably be skin
contact electrodes. Exemplary electrodes include silver-silver chloride {Ag-Ag
Cl) dot electrodes
that make contact with the skin on one or more sides and are in contact with
an electrical contact
(e.g., a snap) on the other side. The skin is generally prepared in order to
provide a good
electrical interface with the dot electrode. For instance, the skin may be
wiped with alcohol,
to subject to slight abrasion, and coated with an electrode gel. After the
preparation, the dot
electrode is applied and wired into an amplifier of the signal processing
system. Good technique
in skin preparation is helpful when the sensors employed are skin contact
electrodes. Poor
electrode interfaces can lead to excessive noise on the signal lead, potential
for external pick-up,
and similar problems. These "extra" noises or noise sources are likely to be
of such a character
is that they may interfere with the extraction and enhancement of the fetal
electrocardiographic
waveform.
To eliminate bad signals, the present invention provides a method to
automatically
identify, assess, and validate the signal integrity of the electrode or
sensor. Although an
exemplary device that can be used to make such an assessment is an impedance
meter such as the
2 o Prep-Check, available from General Devices, making an impedance
measurement requires that
an active measurement be made, for example, by imposing a small current on the
circuit and
measuring the voltage drop. Accordingly, the present inventors have developed
a preferred
method of using the passive amplifier data to make an assessment of signal
quality.
CA 02345720 2004-05-17
-1 S-
The signal quality can be tested by a variety of means, including testing the
frequency
character. The frequency character (spectrum) of the EKG signal preferably has
a large low
frequency component followed by a roll-off with increasing frequency.
According to the present
invention, amplifiers are used that preferably have a 60 Hz notch filter for
rejecting line noise
artifacts. After the notch filter, the signal rises slightly to a flat noise
floor. According to the
present invention, "good" EKG signals preferably repeatedly and reliably
exhibit this spectrum,
whereas "bad" EKG signals do not exhibit a 60 Hz notch characteristic and
reach a noise floor at
a lower frequency and at a higher relative amplitude. These distinctions are
used to validate
whether a channel is good or bad. A segment of the EKG signal is preferably
processed by an
t o algorithm known as a fast fourier transfer (FFT) to generate the frequency
spectrum. Then, a
ratio of the signal energy at a low frequency (approximately 2 Hz) to the
signal energy at 60 Hz
(2 Hz energy/60 Hz energy) is measured. Good channels are those determined to
have large ratio
magnitudes whereas bad channels have smaller ratio magnitudes, depending upon
the sensors
used and the characteristics of the various signals that are obtained. Those
channels that do not
exceed a minimum ratio magnitude are deemed to be bad and are not used for
subsequent
processing. The ability to selectively include only "good" signals provides
the apparatus with a
high level of adaptability and robustness.
Signal processing and noise filtering/rejecting devices and components for
such devices
that are suitable for the methods and apparatus of the present invention
include those components
2o described in LLS. Patent Nos. 5,853,364; 5,983,127; and 5,999,845.
In yet other embodiments of the present invention, a device in accordance with
the
invention can be used off site to monitor a pregnant women while going about
her normal daily
CA 02345720 2004-05-17
- I 6-
activities. The device can also include, for example, a thermometer or a
motion sensor. Suitable
motion sensors that can be used include, for example, accelerometers or
inclinometers. These
added devices can be included to provide an indication of the patient's
condition at the time that
certain changes in electrocardiographic waveform occur. The information
acquired by the
s monitor might be stored and forwarded or might be used to identify problem
situations.
According to such an embodiment, collateral measurements of the activities
occurring at the time
a "suspect" event occurs may shed light on the nature of the event. For
instance, if an episode of
low fetal heart rate is identified, it would be helpful to a proper analysis
of the low heart rate to
know whether the mother was lying down or jogging. A possible motion sensor is
described in
1 o U.S. Patent No. 5,999,661.
The apparatus of the present invention is described in more detail below and
includes
electrodes and signal processing circuitry to carry out the methods and
complete the apparatus
described above.
The preferred invention is shown in functional form in Fig. 1. The periodic
beating of the
15 human heart is induced by a biopotential waveform. In one embodiment, the
waveform can be
measured non-invasively by suitable skin contact electrodes 10 connected to
differential
amplifiers 12. The biopotential waveform of an electrically beating fetal
heart, though small in
proportion to its mother, will exist in combination with the maternal
wavefonm.
The invention preferably starts by acquiring maternal and maternal-plus-fetal
biopotential
2o wavefonns. The maternal waveforms can be collected by surface electrodes
preferably placed on
the mother's chest and/or upper back, preferably on both sides, but not in the
middle, of the
mother's chest. By "upper back" what is meant is the portion of the back not
beloov the level
corresponding to the sternum. The maternal-plus-fetal waveforms or "combined
waveforms" are
CA 02345720 2001-03-28
WO 00/54650 PCTNS00/06295
-17-
collected by surface electrodes placed on the mother's abdomen and/or lower
back, preferably on
the sides of the mother's abdomen. By "lower back" what is meant is that
portion of the back
below the sternum. An exemplary electrode placement scheme is shown in Fig. 2.
A clinically significant aspect of the present invention is that the sensors
(electrodes) are
placed in an adaptable pattern, in other words, in a pattern irrespective of
the fetal position, the
maternal condition, or the size and shape of the mother. According to an
advantageous
embodiment of the present invention, the electrodes are preferably positioned
in a manner so as
to remain clear of usual potential operative sites. Equally significant is the
fact that the
successful implementation of the monitor is insensitive to variations in the
placement of the
1 o electrodes; thus, a patient who is monitored at different points in time
need not have the
electrodes placed in the same exact location for each monitoring episode. The
electrodes can be
a plurality of separate electrodes or a small number, for example, two, of
electrode strips. Each
strip can contain a plurality of electrodes and preferably only a single cable
assembly. According
to a preferred embodiment of the present invention, two electrode strips are
used and each strip
contains a plurality of electrodes. Placement of the electrode strips can be
routine, simple, and
rapid.
Fig. 3 is an illustration of a sensor placement scheme useful in accordance
with the
present invention. Chest sensors for monitoring the maternal biophysical
waveform are shown in
the form of skin contact electrodes 30, 32. The abdominal sensors for
acquiring the combined
2 o fetal and maternal biophysical waveform are shown in the form of strip
sensors 34 and 36. As
can be seen in Fig. 3, strip sensor 34 includes a plurality (seven in the
strip sensor shown) of
individual sensors 38 along the length of the strip sensor 34. Likewise, strip
sensor 36 includes a
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295
-18-
plurality of sensors 40 (seven in the strip sensor shown) spaced along the
length of strip sensor
36.
Fig. -4 -shows the operative positioning-of a left-side strip sensor -42
containing seven
sensors 44 spaced along the length of strip sensor 42. The strip sensor 42 is
positioned in the
s same place as the left-side sensor shown in Fig. 3. As shown in Fig. 4, the
strip sensor is
positioned on the pregnant mother along the lower anterior abdomen away from
the operative
field necessary for a cesarean section delivery, and wrapping around the curve
of the mothers
lower abdomen. Also shown in Fig. 4 is a strip sensor 46 containing seven
individual sensors 48
spaced along the length of strip sensor 46. Strip sensor 46 is not in an
operative position but is
1 o shown to demonstrate that a singular lead 50 carrying signals from each of
the seven individual
sensors 48 can be employed and can be interfaced with an electrode interface
52 from which a
lead 54 extends to carry the signals for further signal processing.
According to the present invention, numbers of differently oriented vectors
are collected
for both signal types (maternal only and maternal-plus-fetal). Pairs of
electrodes (channels) are
i s assigned to individual differential amplifiers. An exemplary channel would
include the pair of
electrodes 38', 40' shown in Fig. 3. All channels are preferably amplified and
filtered in order to
reject noise and provide anti-aliasing for subsequent digitization. In one
embodiment, all
channels are sampled at a rate less than or equal to 250 samples/second. When
sampling
involves digitization, a resolution of less than or equal to 16 bits is
preferred. Other sampling
2o methods can be used, including analog methods, hybrid analog/digital
methods, or combinations
of sampling methods. Channel validation as discussed above is preferably used
to assure that
non-informative or corrupt ("bad") chest channels are excluded from the
processing.
CA 02345720 2004-05-17
- I 9-
The first phase of signal processing applies an Adaptive Signal Processing
Filter (ASPF)
algorithm to cancel the estimated maternal (chest) waveform from each
abdominal channel. The
result is a set .of .estimated fetal. signals -plus residual-noise. Maternal
heart rate; heart rate
variability, and respiration race are derived from chest signals. A standard
maternal EKG can be
s derived from planar leads.
The implementation of the ASPF algorithm is shown functionally in Fig. S (for
the
overall configuration) and in Fig. 6 (for each individual filter). All chest
channels .are
dynamically weighted against individual abdominal channels in order to effect
the cancellation of
the estimated maternal (chest) waveform from each abdominal channel. Several
bipolar leads
1 o measure the electrical signal (reference) across the maternal chest (Chest
Channel I ...N). Several
additional bipolar leads measure the signal from the maternal abdomen
(Abdominal Channel
1...Q). The chest leads are used as a basis to cancel the maternal signal from
the abdominal leads
(Estimated Fetal I...K). The abdominal leads are enhanced to derive the
resulting fetal EKGf.
Additional reference signals (e.g., EMG) can be included to optimize noise
cancellation. All
15 components used to implement the algorithms are commercially available
individually. A filter
chip co-processor as described in U.S. Patent No. 5,931,892 can be used, for
example,
.to implement the ASPF algorithm.
Preferably, the resultant estimated fetal waveforms will exhibit a range of
peak-to-peak
amplitudes as a consequence of the orientation of the fetal heart relative to
the abdominal vector
20 orientations. At least one of the resultant waveforms is selected as the
reference for the next
phase of processing, for example, the waveform with the largest peak-to-peak
amplitude.
Channel validation can preferably be used to assure that non-functional or
corrupt abdominal
channels are excluded from the processing.
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/Ob295
-20-
The selected reference fetal waveform happens to be Estimated Fetal 1 in Fig.
5 although
any of the estimated fetal signals can be selected. One method of selecting
the estimated fetal
signal is to choose the signal with the largest peak-to-peak amplitude. Using
the same ASPF
algorithm of Fig. 5, the selected estimated fetal signal is enhanced to remove
correlated noise, by
s processing against the remaining abdominal estimated fetal waveforms. The
enhanced fetal
signal that is the output of this step is a representation of the fetus'
biopotential
electrocardiogram (EKGr) and can be used to assess or monitor fetal conditions
including the
well-being of the fetus. The EKGr can be used to assess fetal well-being by
measuring fetal heart
rate, fetal heart rate variability, and approximate entropy, as well as
defining orientation of the
1 o fetus within the mother, and/or other components of biophysical profile
parameters. Fetal heart
rate and heart rate variability are derived, preferably through R-to-R
interval timing, or by
appropriate auto-correlation processing of either the enhanced fetal signal or
one or more of the
processed abdominal signals. Fetal position, inferred from the heart vector
orientation, is
determined by the signal strength and polarity of the EKGf waveform relative
to the abdominal
1 s electrode pairs. The maternal heart vector can be used as a reference
point for determining the
fetal heart vector. The three-dimensional nature of the abdominal array
readily accommodates
movement of the fetal heart vector without loss of signal. Because maternal
EKG signatures are
collected as an integral part of the process, similar biophysical profiles can
be determined for the
mother. Though not shown in Fig. 1, surface EMG signals allow for monitoring
of uterine
2 o contractions and afford improved cancellation of motion artifacts.
The invention has been validated both with simulated data (Fig. 7) and human
clinical
data (Fig. 8). Analysis from 20 human subjects, collected as part of an
approved research
protocol, have been used to demonstrate reliable determination of fetal heart
rate and fetal heart
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295
-21-
rate variability. The inclusion of an EMG as an additional reference signal
can be used to more
fully refine the EKG f waveform derivation and address noise artifacts that
otherwise mask
smaller waveforriufeatures while eiiliancing the systerii robustness in the
face of skeletal and
uterine muscle noise caused by maternal motor activity.
The monitor concept and algorithms can be validated using simulated data
comprised of a
representative fetal EKGr signal (Fig. 7). To this representative signal a
maternal signal of an
amplitude proportional to a standard signal reported in literature can be
added. The fetal and
maternal EKG's were randomly dithered in both amplitude and repetition rate.
In addition, a
baseline noise level, characterized by what is seen in the clinical situation,
was also added to this
1 o composite. This signal was then processed by the algorithm to first
adaptively cancel the
maternal signal, and then adaptively enhance the resulting fetal signal to
identify the underlying
signal. The results from clinical data (Fig. 8) demonstrate comparable
performance.
The present invention will be further clarified by the following examples,
which are
intended to be exemplary of the present invention.
EXAMPLES
Twenty subjects were monitored using a device according to the present
invention. The
device comprised a MP 100 system (BIOPAC Systems, Inc.), with l 6 ECG 1 OOB
electrocardiogram amplifiers (BIOPAC Systems, Inc.), an AcqKnowledge (BIOPAC
Systems,
2 o Inc.) data acquisition application and MATLAB device and signal processing
toolbox
(MathWorks, Inc.), several Silvon Diaphoretic Electrodes (New Dimensions in
Medicine
(NDM)), and a MATLAB-based application code to implement the various
algorithms. A small
number of the subjects were simultaneously monitored with a Doppler. There was
no
interference between the system of the present invention and the Doppler
ultrasound and the
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295
-22-
results qualitatively showed good correlation between fetal heart rate
measurements. Nineteen of
the 20 subjects were in the range of 28 to 36 weeks of gestation. The
remaining subject was not
pregnant, and she was monitored in order to establish a system noise baseline:
Two of the
subjects were twin gestations and discrimination between the heart signatures
was effected.
All data collected used the universal electrode positions (avoiding potential
surgical sites)
as shown in Fig. 2, as opposed to fetal-specific positions as has been
reported in U.S. Patent No.
5,042,499 to Frank et al. User-attended batch processing was used to
demonstrate feasibility.
Inclusion of a uterine contraction measurement, automation of the dynamic
processes, and
implementation of a real-time output could have been implemented.
1 o Fig. 9 demonstrates a use of the present invention in monitoring the
deceleration of a
fetus' heart rate as, for example, accompanying uterine contractions. Both the
fetal heart rate and
the maternal heart rate shown in the graph of Fig. 9 were derived according to
the method and
apparatus of the present invention. Fig. 9 shows that during the time period
of from about 48 to
about 50 seconds there was a corresponding, and normal, deceleration of the
fetal heart rate.
The present invention is a reliable, accurate, non-invasive, and passive
technique to
measure the electrocardiographic waveform of the fetus. Furthermore, the
present invention
maintains a clear operative field, accommodates movement of the mother and
fetus, and is usable
for a relevant portion of gestation. The monitor's output can include the
fetal
electrocardiographic waveform in addition to the fetal heart rate, and
includes a description of
2 o heart rate variability as well as maternal heart rate and noise artifacts
attributable to uterine
contraction.
CA 02345720 2001-03-28
WO 00/54650 PCT/US00/06295
-23-
Other embodiments of the present invention will be apparent to those skilled
in the art
from consideration of the specification and practice of the present invention
disclosed herein. It
is intended that the specification ~ and examples be considered as exemplary
only, with a true
scope and spirit of the invention being indicated by the following claims and
equivalents thereof.