Note: Descriptions are shown in the official language in which they were submitted.
CA 02346379 2001-04-06
WO 00/21971 PCT/EP99/07084
Substituted isophosphindolines and their use
Description
The invention relates to substituted isophosphindolines and their metal
complexes.
Phosphorus compounds, in particular trisubstituted phosphines, have great
importance as ligands in homogeneous catalysis. Variation of the
substituents on the phosphorus in such phosphorus compounds enables
the electronic and stearic properties of the phosphorus ligand to be
influenced in a targeted way, so that selectivity and activity in
homogeneously catalyzed processes can be controlled.
The variety of structures of phosphorus ligands known hitherto is very
great. These ligands can, for example, be classified by class of compound,
and examples of such classes of compounds are trialkylphosphines and
triarylphosphines, phosphites, phosphonites, etc. This classification
according to classes of compounds is particularly useful for describing the
electronic properties of the ligands.
Classification of phosphorus ligands according to their symmetry properties
or according to the number of coordination positions occupied by the
ligands is also possible. This structuring takes account of, in particular,
the
stability, activity and (potential) stereoselectivity of metal complexes
bearing phosphorus ligands as catalyst precursors/catalysts.
Enantiomerically enriched chiral ligands are used in asymmetric synthesis
or asymmetric catalysis; an important aspect here is that the electronic and
the stereochemical properties of the ligand are optimally matched to the
respective catalysis problem. There is a great need for chiral ligands which
differ stereochemically or/and electronically in order to find the optimum
"tailored" ligands for a particular asymmetric catalysis. In the ideal case,
therefore, one has available a flexibly modifiable, chiral ligand skeleton
whose stearic and electronic properties can be varied within a wide range.
Examples of such a basic ligand skeleton are metallocene catalysts for
stereoselective olefin polymerization or for asymmetric catalysis.
REPLACEMENT SHEET (RULE 26)
CA 02346379 2001-04-06
WO 00/21971 2 PCT/EP99/07084
Within the class of cyclic phosphines, the phospholines have achieved
particular importance. Examples of bidentate, chiral phospholines are the
DuPhos and BPE ligands used in asymmetric catalysis (Burk et al.,
Specialty Chemicals, 1998, 58). Although these can be varied stearically
within a relatively broad range, they can be varied electronically to only a
very limited extent, i.e. by replacement of the arenediyl backbone in the
DuPhos ligands by an ethane-1,2-diyl backbone in the BPE ligands.
The class of isophosphindolines of the formula (I) is quite similar to that of
the phospholines, but is known from the literature in the form of only a few
representatives: thus, known compounds are, for example the parent
compound isophosphindoline of the formula (la) (Robinson et al., J.
Heterocycl. Chem., 1973, 395) and alkyl or aryl derivatives of
isophosphindoline of the formula (Ib, Ic) (Mann et al., J. Chem. Soc., 1954,
2832; Schmidbaur et al., J. Organomet. Chem. 250, 1983, 171; Breen et
al., J. Am. Chem. Soc., 1995, 11914), also phosphonium salts of the
isophosphindoline of the formula (II) (Mann et al., J. Chem. Soc., 1954,
2832; ibid. 1958, 2516; Schmidbaur et al., J. Organomet. Chem. 250, 1983,
171).
-- ~ , _ n:
t
~.' /\~'~ \ R
In formula (la), R is hydrogen, while in the formulae (Ib/c) and (II), R is an
alkyl or aryl radical.
Representatives which are substituted on the aliphatic carbon skeleton of
the isophosphindoline are likewise known, but the compounds known
hitherto are either monosubstituted derivatives of the formula (III) (Fluck et
al., Phosphorous Sulfur, 1987, 121; Ouin et al., J. Org. Chem., 1986, 3235)
or derivatives of the formula (IV) which are mentioned in WO 96/16100 and
WO 96/23829 but have obviously not been synthesized hitherto.
CA 02346379 2001-04-06
WO 00/21971 3 PCT/EP99/07084
R' R"
P-R i ~/P-R
/ /
R, R... R,."
(III) (IV)
1,3-Disubstituted isophosphindolines (V) have previously only been known
in the form of their phosphine oxides (Vla, Vlb) (Holland et al., J. Chem.
Soc. Perkin Trans. 1, 1973, 927):
R~~ H R,\ .H
W
P-R ; ! P
i , ~~ ~- ,
R
R' ~/ 'H R' ~ H
R' = Ph. R = Me: (V1a)
R' = Ph, R ~ Ph: (Vlb)
(V) (V1)
The 1,3-disubstituted isophosphindolines of the formula (V), which have not
been described hitherto, are achiral if the two asymmetric centers on C1
and C3 have different (opposite) absolute configurations (meso form). The
remaining representatives are chiral (c2 symmetry). The chiral
representatives are of particular importance since they can be used as
ligands in asymmetric, catalytic syntheses.
Like the substances of the formula (V), their derivatives substituted on the
aromatic carbon skeleton have also not been described hitherto.
The previously known and used phospholine ligands DuPhos and BPE
have, as mentioned above, the disadvantage that their electronic properties
can be varied to only a very small extent. It is therefore an object of the
present invention to provide a basic ligand skeleton which can be varied in
a manner analogous to the previously known phospholine ligands but also
be varied electronically within a wide range.
CA 02346379 2001-04-06
November 16, 2000 4 EP 009907084
This object is achieved by the provision of substituted isophosphindolines
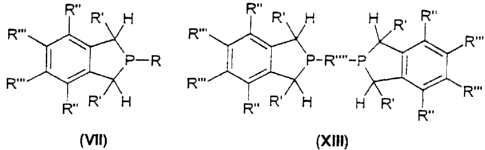
of the formula (VII) or (X111),
R.. R, H R~~ R, H H R, R..
R.., \ R.., \ \ . R.,.
~P_R I ~ ~P_R...._P
R... ~ R.,. ~ ~ R,..
R,. R' H R.. R' H H R' R..
(vn) (xui)
where, in the formula (VII),
R is hydrogen or an alkyl, aryl, haloaryl or haloalkyl group,
R' are alkyl, aryl, haloaryl or haloalkyl groups,
R" and R"' are each, independently of one another, hydrogen, alkyl, aryl,
haloalkyl or haloaryl, alkoxy, amino, dialkylamino or sulfonate
groups or fluorine
and two adjacent radicals R"/R", R"/R"' or R"'/R"' may also be
bridged,
and, in the formula (X111)
R' are alkyl, aryl, haloaryl or haloalkyl groups,
R" and R"' are each, independently of one another, hydrogen, alkyl, aryl,
haloalkyl or haloaryl, alkoxy, amino, dialkylamino or sulfonate
groups or fluorine
and two adjacent radicals R"/R", R"/R"' or R"'/R"' may also be
bridged, and
R"" is an alkanediyl, arenediyl or heteroarenediyl group.
For the present purposes, alkyl and haloalkyl groups include the
corresponding cyclo compounds. Particular preference is given to chiral
substituted isophosphindolines.
The substituted isophosphindoline of the invention preferably bears alkyl,
aryl, haloaryl, haloalkyl, alkoxy or/and dialkylamino groups which are
AMENDED SHEET
CA 02346379 2001-04-06
November 16, 2000 5 EP 009907084
selected independently of one another and each contain from 1 to 20, in
particular from 1 to 6, carbon atoms.
The haloalkyl or/and haloaryl groups preferably have the formulae CHal3,
CH2CHal3, C2Hal5, where Hal can be, in particular, F, CI or Br. Particular
preference is given to haloalkyl or/and haloaryl groups of the formulae CF3,
CH2CFg, C2F5.
It is also preferred that the alkanediyl, arenediyl or heteroarenediyl group
R"" of the substituted isophosphindoline has from 2 to 20 carbon atoms,
more preferably 2, 3, 4, 5 or 6 carbon atoms, in particular 2 or 6 carbon
atoms. Ethane-1,2-diyl, benzene-1,2-diyl or furan-3,4-diyl are particularly
preferred examples of alkanediyl, arenediyl or heteroarenediyl groups R"".
Furthermore, preference is given to a substituted isophosphindoline in
which R is phenyl, R' is methyl or ethyl, R" and R"' is hydrogen, methyl
or/and phenylene, R"" is benzene-1,2-diyl. Particular preference is given to
a substituted isophosphindoline in which R is phenyl, R' is methyl or ethyl,
R" and R"' are hydrogen, methyl or/and phenylene, R"" is benzene-1,2-diyl,
in which R" and R"' are not hydrogen or in which either R" or R"' is
hydrogen.
Furthermore, preference is given to substituted isophosphindolines whose
asymmetric centers in the 1 and 3 positions have the same absolute
configuration. The substituted isophosphindoline of the formula (VII) has
two asymmetric centers having the same absolute configuration in
positions 1 and 3, while in the case of those of the formula (X111), each has
two or four asymmetric centers having the same absolute configuration in
the positions 1, 1', 3 and 3'.
Finally, preference is given to substituted chiral isophosphindolines which
are enriched in one enantiomer.
AMENDED SHEET
CA 02346379 2001-04-06
WO 00/21971 5a PCT/EP99/07084
r and each contain from t~
particular from 1 to 6, carbon atoms.
The haloalkyl or/and haloaryl groups preferably have the for ulae CHal3,
CH2CHal3, C2Hal5, where Hal can be, in particular, F, CI r Br. Particular
preference is given to haloalkyl or/and haloaryl groups o he formulae CFg,
CH2CFg, C2F5.
It is also preferred that the alkanediyl, arened~ or heteroarenediyl group
R"" of the substituted isophosphindoline ha~rom 2 to 20 carbon atoms,
more preferably 2, 3, 4, 5 or 6 carbon atp~Fns, in particular 2 or 6 carbon
atoms. Ethane-1,2-diyl, benzene-1,2-d~~ or furan-3,4-diyl are particularly
preferred examples of alkanediyl, areyfediyl or heteroarenediyl groups R"".
Furthermore, preference is giv n to a substituted isophosphindoline in
which R is phenyl, R' is met I or ethyl, R" and R"' is hydrogen, methyl
or/and phenylene, R"" is benzene-1,2-diyl. Particular preference is given to
a substituted isophosphin~oline in which R is phenyl, R' is methyl or ethyl,
R" and R"' are hydroge~ methyl or/and phenylene, R"" is benzene-1,2-diyl,
in which R" and R"'; pare not hydrogen or in which either R" or R"' is
hydrogen.
Furthermore, pr ference is given to substituted isophosphindolines whose
asymmetric c nters in the 1 and 3 positions have the same absolute
configuratio . The substituted isophosphindoline of the formula (VII) has
two asy etric centers having the same absolute configuration in
position 1 and 3, while in the case of those of the formula (X111), each has
two our asymmetric centers having the same absolute configuration in
the plositions 1, 1', 3 and 3'.
~nally, preference is given to substituted chiral isophosphindolines which
are enriched in_onP Pnantiomer.
The substituents R' in the substances of the formulae (VII) and (X111) are
essential for the steric properties of the ligands, while the other
substituents
R, R"> R"' and R"" essentially determine the electronic properties by means
of their donor or acceptor capabilities, and thus allow, as explained above,
CA 02346379 2001-04-06
WO 00/21971 6 PCT/EP99/07084
the reactivity, selectivity and application range in respect of substrates in
catalytic reactions to be influenced over a wide range.
A further substantial difference between the compounds of the formulae
(VII) and (X111) on the one hand and phospholines on the other hand is the
greater rigidity of the five-membered heterocyclic ring in the compounds of
the formulae (VII) and (X111), which in attributable to the replacement of the
ethanediyl backbone in the phospholines by the benzene-1,2-diyl
substituent in the substances of the formulae (VII) and (X111).
Representatives of the class of compounds of the formula (VII), which
encompasses not only compounds of the formula (V) but also
corresponding compounds substituted in the fused-on aromatic ring, have
been prepared. In cases where these are chiral, the compounds have also
been synthesized in a form enriched in one enantiomer.
R., R. H
R." ~\~~
a.., ~~ ; P R
R"
(VII)
A number of routes are available for the synthesis of the substituted
isophosphindolines of the formula (VII): for example, either the
corresponding phthalic dialdehyde of the formula (VIII) or the
corresponding diketone of the formula (IX) can be used as starting material.
The reaction of the phthalic dialdehyde of the formula (VIII) with two
equivalents of Grignard R'-M gives firstly the corresponding dialkoxide of
the formula (X) which can be reacted further to give the cyclic sulfate of the
formula (XII).
Starting from the diketone of the formula (IX), this is firstly reduced by
means of boron compounds or analogous compounds to give the boronic
CA 02346379 2001-04-06
WO 00/21971 7 PCT/EP99/07084
acid derivative (XI) which can likewise be converted into the cyclic sulfate
(XII).
A single representative of the sulfates of the formula (XII) is a previously
known compound in which R' = methyl and R" - R"' - H and which is
mentioned in WO 97/13763, page 7.
Depending on reaction conditions, synthetic route and reagent, the sulfate
of the formula (XII) is obtained as a mixture of diastereomers (meso/rac
form) or/and enantiomers; separation of the isomers gives the
stereochemically pure sulfate of the formula (XII).
R" O R" OM
R.,. \ H R... \ R,
R... ( / H R... I / R'
R.. R.
R" O R" OM R"' / O
(vul) (x)
soy
R.. O R.. R.1 R..~~O
R... I\ R, R... ~ ~ O ~ R.. R.~
R... ~ ~ R~ ~R..,. \ ~ oB X {XII) .
i ;
R" O R" R' 1. BuLi / RPH
(IX) (Xl) ~ 2. BuLi
R.. R. H
R,., \ \
I'i /~P-R
R.,, i
R" R' / \H
(VII)
In a modification of k wn processes (Burk US 5,386,061 ), reaction of the
sulfate of the for a (XII) with phosphines of the formula RPH2 (R = alkyl,
aryl, hetero I) gives, via the corresponding phosphides, the
correspon ' g substituted isophosphindoline.
CA 02346379 2001-04-06
November 16, 2000 7U EP 009907084
10
R" O R" OM
l
R... ~ \ H ~ R... ~ \ R.
R... ~ H R... ~ R'
R.. R.
R... / O
R" O R" OM
(VIII)
(X) I ~ soy
R.. O R.. R~ R...i ~ O
R... I R... I '
R, / O R~~ R.
R... ~ ~Y R~~ R..., ~ ~ 0 6 X ~ (XII)
i /
R" O R" R' ' 1. BuLi / RPHz
(IX) (XI) I 2. BuLi
R.. R. H
R,., ~
~/~P-R
R... ~~~
R., R' H
(VII)
In a modification of known processes (Burk US 5,386,061 ), reaction of the
sulfate of the formula (XII) with phosphines of the formula RPH2 (R = alkyl,
aryl, heteroarenediyl) gives, via the corresponding phosphides, the
corresponding substituted isophosphindoline.
It is likewise possible to convert the isomer mixture of the sulfate of the
formula (XII) into the corresponding mixture of isomeric isophosphindolines
AMENDED SHEET
CA 02346379 2001-04-06
November 16, 2000 8 EP 009~a07084
and then to carry out a separation in order to obtain stereochemica'~y pure
substituted isophosphindolines. This separation can be achie~r :d, for
example, by fractional crystallization and/or chromatographically.
As phosphines of the formula RPH2, it is possible to use all types of
arylphosphines and alkylphosphines as starting materials (R = aryl, alkyl).
If, instead of these, diphosphines of the formula H2P=R""-PH2 are used,
the products are chelating ligands of the formula (X111) which in the case of
c2-symmetric sulfates of the formula (XII) lead to chiral and then likewise
c2-symmetric chelating ligands.
R, H H R, R,.
R... \ \ R...
I '
R... % \ " ~ R."
R' N H R'
R~~ R.,
(X111)
The compounds of the formulae (VII) and (X111) can be used as ligands on
metals in asymmetric, metal-catalyzed reactions (e.g. asymmetric
hydrogenation, transfer hydrogenations, asymmetric rearrangement,
asymmetric cyclopropanation or Heck reactions) and in polymerizations.
They are particularly useful far asymmetric reactions.
The ligands of the formulae (VII) and (X111) form complexes of the type
(XIV),
[MxPm~nSq~Yr (XIV)
where, in the formula (XIV), M is a metal center, preferably a transition
metal center, L are identical or different coordinating organic or inorganic
ligands and P are organic ligands, according to the invention
isophosphindolines of the type (VII) or (X111), S are coordinating solvent
molecules and Y are equivalents of noncoordinating anions, where x and m
are integers greater than or equal to 1, n, q and r are integers greater than
or equal to 0.
AMENDED SHEET
CA 02346379 2001-04-06
November 16, 2000 9 EP 009907084
The maximum value of the sum m + n + q is determined by the number of
coordination sites available on the metal centers, but not all coordination
AMENDED SHEET
sites have to be occupied. Preference is given to complexes having an
"~ CA 02346379 2001-04-06
WO 00/21971 9a PCT/EP99/07084
0 1~, n, ~q and r are integers~gr~a~r~~ttl'a
r equal to 0.
~'he maximum value of t~,fa,~-scrrr~i~m~+ n + q is determined by the number c
oordination s~available on the metal centers, but not all coordinatio
~te$ .ba'~Te t~O'°~-'O'CCUDfe~l--RfA#A~t'-~RE6....~.~.u~A. fn
nnrr,n~ac.~c haVjrlll a
~c#a~h~a~l,, pseudo octahedral, tetrahedral, pseudo tetrahedral or square
planar coordination sphere, which may also be distorted, around the
respective transition metal center. In such complexes, the sum m + n + q is
smaller than or equal to 6x.
The complexes of the present invention contain at least one metal atom or
ion, preferably a transition metal atom or ion, in particular one selected
from the group consisting of palladium, platinum, rhodium, ruthenium,
osmium, iridium, cobalt, nickel and copper.
Preference is given to complexes having less than four metal centers,
particularly preferably ones having one or more two metal centers. The
metal centers can be occupied by various metal atoms and ions.
Preferred ligands L in such complexes are halide, particularly CI, Br and I,
diene, particularly cyclooctadiene, norbornadiene, olefin, particularly
ethylene and cyclooctene, acetato, trifluoroacetato, acetylacetonato, allyl,
methylallyl, alkyl, particularly methyl and ethyl, nitrite, particularly
acetonitrile and benzonitrile, and also carbonyl and hydrido ligands.
Preferred coordinating solvents S are amines, particularly triethylamine,
alcohols, particularly methanol, and aromatics, particularly benzene and
cumene.
Preferred noncoordinating anions Y are trifluoroacetate, BF4, C104, PFg
and BAr4.
In the individual complexes, different molecules, atoms or ions of the
individual constituents M, P, L, S and Y may be present.
CA 02346379 2001-04-06
WO 00/21971 10 PCT/EP99/070$4
Among the ionic complexes, preference is given to compounds of the type
[RhPm(diene)]+Y , where Pm represents either two isophosphindolines of
the type (VII) or one isophosphindoline of the type (X111).
These metal-ligand complexes can be prepared in situ by reaction of a
metal salt or an appropriate precursor complex with the ligands of the
formulae (VII) and (X111). Alternatively, a metal-ligand complex can be
obtained by reaction of a metal salt or an appropriate precursor complex
with the ligands of the formula (VII) and (X111) and subsequent isolation.
Examples of metal salts are metal chlorides, bromides, iodides, cyanides,
nitrates, acetates, acetylacetonates, hexafluoroacetylacetonates,
perfluoroacetates or triflates, in particular of palladium, platinum, rhodium,
ruthenium, osmium, iridium, cobalt, nickel and/or copper.
Examples of precursor complexes are:
cyclooctadienepalladium chloride, cyclooctadienepalladium iodide,
1,5-hexadienepalladium chloride, 1,5-hexadienepalladium iodide,
bis(dibenzylideneacetone)palladium, bis(benzonitrile)palladium(II) chloride,
bis(benzonitrile)palladium(II) bromide, bis(benzonitrile)palladium(II) iodide,
bis(allyl)palladium, bis(methallyl)palladium, allylpalladium chloride dimer,
methallylpalladium chloride dimer, tetramethylethylenediaminepalladium
dichloride,
tetramethylethylenediaminepalladium dibromide,
tetramethylethylenediaminepalladium diiodide,
(tetramethylethylenediamine)dimethylpalladium,
cyclooctadieneplatinum chloride, cyclooctadieneplatinum iodide,
1,5-hexadieneplatinum chloride,
1,5-hexadieneplatinum iodide, bis(cyclooctadiene)platinum, potassium
ethylenetrichloroplatinate,
cyclooctadienerhodium(I) chloride dimer, norbornadienerhodium(I) chloride
dimer,
1,5-hexadienerhodium(I) chloride dimer, tris(triphenylphosphine)rhodium(I)
chloride,
hydridocarbonyltris(triphenylphosphine)rhodium(I) chloride,
bis(cyclooctadiene)rhodium(I) perchlorate, bis(cyclooctadiene)rhodium(I)
tetrafluoroborate,
-' CA 02346379 2001-04-06
WO 00/21971 11 PCT/EP99/07084
bis(cyclooctadiene)rhodium(I) triflate,
bis(acetonitrile)cyclooctadienerhodium(I) perchlorate,
bis(acetonitrile)cyclooctadienerhodium(I) tetrafluoroborate,
bis(acetonitrile)cyclooctadienerhodium(I) triflate,
cyclopentadienerhodium(III) chloride dimer,
pentamethylcyclopentadienerhodium(III) chloride dimer,
(cyclooctadiene)Ru(rt3-allyl)2, ((cyclooctadiene)Ru)2(acetate)4,
((cyclooctadiene)Ru)2(trifluoroacetate)4, RuCl2(arene) dimer,
tris(triphenylphosphine)ruthenium(II) chloride, cyclooctadieneruthenium(II)
chloride,
OsCl2(arene) dimer, cyclooctadieneiridium(I) chloride dimer,
bis(cyclooctene)iridium(I) chloride dimer,
bis(cyclooctadiene)nickel, (cyclododecatriene)nickel,
tris(norbornene)nickel,
nickel tetracarbonyl, nickel(II) acetylacetonate,
(arene)copper triflate, (arene)copper perchlorate, (arene)copper
trifluoroacetate, cobalt carbonyl.
The complexes based on one or more metals, in particular metals selected
from the group consisting of Ru, Co, Rh, Ir, Ni, Pd, Pt, Cu, can be catalysts
themselves or can be used for preparing catalysts based on one or more
metals, in particular metals selected from the group consisting of Ru, Co,
Rh, Ir, Ni, Pd, Pt, Cu. All these complexes are particularly useful in the
asymmetric hydrogenation of C=C, C=O or C=N bonds, in which they
display high activities and selectivities. In particular, it is found to be
advantageous that the ligands of the formula (X111) can be very well
matched in stearic and electronic terms to the respective substrate due to
the fact that they can easily be modified over a wide range.
Corresponding catalysts comprise at least one of the complexes of the
invention.
Examples:
1. 1,2-bis(a-hydroxyethyl)benzene:
The preparation of 1,2-bis(u.-hydroxyethyl)benzene was carried out by
literature methods: Goldschmidt et al., Chem. Ber. 1961, 94, 169.
CA 02346379 2001-04-06
WO 00/21971 12 PCT/EP99/07084
2. Cyclic sulfate:
The preparation of the cyclic sulfate was carried out by the method of
Burk (J. Am. Chem. Soc. 1993, 115, 10125) starting from 1,2-bis(a-
hydroxyethyl)benzene.
38 mmol of thionyl chloride in 10 ml of CC14 are added dropwise to
31 mmol (5.1 g) of 1,2-bis(a-hydroxyethyl)benzene dissolved in 40 ml of
CC14 over a period of 30 minutes. After refluxing for 2.5 hours and
cooling, the solution is evaporated virtually to dryness. The residue is
then taken up in CC14, acetonitrile, water (25 ml/25 mU35 ml) and
cooled to 0°C. 0.20 mmol of RuCIg~H20 and 61 mml of Na104 are
added one after the other to the cold mixture. After a reaction time of
one hour, the reaction mixture is diluted with 170 ml of water, extracted
four times with 100 ml each time of diethyl ether and the combined
ether extracts are washed twice with 60 ml each time of saturated NaCI
solution. The extract solution is dried overnight over Na2S04 and
subsequently evaporated to a reddish brown serum. Column
chromatography using silica gel 60 as stationary phase and n-
hexane/ethyl acetate 10:1 as mobile phase gives 1.0 g of pure racemic
1,2-bis(a-hydroxyethyl)benzene cyclosulfate (14°~0 of theory).
As a second method, the procedure of Zhang WO 97/13763 was
employed. The yield of pure bis(a-hydroxyethyl)benzene cyclosulfate
was 25%, and the 'H-NMR data reported in the literature could be
reproduced.
~ H-NMR (400, 13 MHz, CDC13): b 1.92 (d, 6H, CH3), 5.56 (q, 2H, CH),
7.35 (m, 2H, CHar), 7.56 (m, 2H, CHar) ppm.
3. Isophosphindoline synthesis
(modification of the method of Burk et al., J. Am. Chem. Soc. 1993, 115,
10125)
1.5 mmol of phenylphosphine are dissolved in 35 ml of kethyl-dried THF
and admixed with 0.94 ml of 1.6 M n-BuLi solution in n-hexane. After
stirring for two hours at room temperature, this solution is added
dropwise at -78°C to a mixture of 1.5 mmol (340 mg) of the cyclic
sulfate and 20 ml of THF. Four hours after the addition was complete,
another 1.09 ml of 1.6 M n-BuLi solution in n-hexane is added dropwise.
The solution is slowly warmed to room temperature overnight, and
°°
CA 02346379 2001-04-06
WO 00/21971 13 PCT/EP99/07084
excess organolithium compound is hydrolyzed using 1 ml of oxygen-
free methanol. Complete evaporation of the solution gives a white
residue which was firstly examined by means of 3'P-NMR as a guide.
This crude product contains many by-products in addition to the desired
isophosphindoline.
31 P NMR (161.99 MHz, CDC13): S+21.7 ppm.
GC/MS (70 eV): m/e = 240 (100%, M+), 225 (25%, M+-15), 212 (9%),
192 (5%), 178 (6%), 165 (3%), 147 (16%), 131 (17%), 120 (10%), 115
(14%), 109 (12%), 91 (27%), 77 (32%), 65 (2%), 51 (2%), 39 (2%).
4. Purification of the isophosphindoline by oxidation/reduction
The crude isophosphindoline product is oxidized for the purposes of
purification and separation of the enantiomers.
The crude product is dissolved in THF and stirred overnight at 20°C
in
the presence of air, the solution is shaken with 20 ml of saturated
NH4C1 solution, the aqueous phase is washed three times with 20 ml
each time of diethyl ether and the combined organic phases are dried
over anhydrous sodium sulfate and evaporated to dryness. The crude
product obtained in this way comprises, inter alia, 1,3-dimethyl-2
phenylisophosphindoline oxide. Washing with n-hexane and
subsequent separate column chromatography of the filtrate and of the
filter residue (silica gel, 1. n-hexane/ethyl acetate 10:4, 2. MeOH) gives
various fractions of the oxide having purities of from 70 to 90%.
Analytical data:
MS (70 eV): m/e = 256 (100%, M+), 241 (5% M+-15), 228 (5%), 200
(4%), 178 (4%), 165 (2%), 147 (1%), 132 (18%), 131 (19%), 129 (5%),
117 (58%), 115 (19%), 91 (14°~0), 77 (6%), 65 (2%), 51 (4%), 39 (2%);
t H NMR (400.13 MHz, CDC13): ~ 1.18 (dd JHH: 7.1 Hz, JpH: 16.7 Hz,
3H, CH3), 1.55 (dd JHH: 7.6 Hz, JpH: 14.8 Hz, 3H, CHg), 3.28, 3.30 (2 x
m, 1 H, CH), 3.54, 3.59 (2 x m, 1 H, CH), 7.18-7.41 (m, 9H, CHar) ppm.
3~ P NMR (161.99 MHz, CgDg): cS +60.4 ppm.
Reduction
0.2 mmol (52 mg) of 1,3-dimethyl-2-phenylisophosphindoline oxide are,
without additional solvent, heated with 0.13 mmol of freshly distilled
phenylsilane for two hours at 90°C under a protective argon
CA 02346379 2001-04-06
WO 00/21971 14 PCT/EP99/07084
atmosphere. The reaction mixture is cooled and then extracted with
3 x 2 ml of absolute, oxygen-free diethyl ether and filtered. The filtrate is
evaporated and the 1,3-dimethyl-2-phenylisophosphindoline is isolated
in a purity of about 80%. Phosphorus-free by-products which have not
been identified up to now were not able to be separated off.
~ H NMR (400.13 MHz, CgDg: 8 1.14 (dd JHH: 7.3 Hz, JpH: 11.2 Hz, 3H,
CHg), 1.49 (dd JHH: 7.7 Hz, JpH: 14.8 Hz, 3H, CH3), 3.34, 3.40 (2 x m,
1 H, CH), 3.50, 3.62 (2 x m, 1 H, CH), 6.72....7.39 {m, 9H, CHa~) ppm;
3~ P NMR (161.99 MHz, CDC13): 8 +21.5 ppm.
5. Resolution of racemic 1,3-dimethyl-2-phenylisophosphindoline 2-oxide
The analytical separation of an ethanolic solution of the racemate of
1,3-dimethyl-2-phenylisophosphindoline 2-oxide is carried out by HPLC
(CHIRALCEL OD-H, n-hexane/EtOH 97:3, flow rate: 0.8 ml/min). The
retention times are 13.5 min and 20.0 min.
For the preparative separation of the enantiomers of the
isophosphindoline oxide and for removing the by-products, a silica gel
precolumn was installed and multiple injection into the above-described
analytical HPLC column and combination of a total of 75 fractions of
each of the two enantiomers gave 5.3 mg of the enantiomer which
eluted first and 6.2 mg of the enantiomerically pure isophosphindoline
oxide which eluted second. The optical rotation of the two enantiomers
could not be determined precisely.
Complexes of 1,3-dimethyl-2-phenylisophosphindoline
6. cis-bis(1,3-dimethyl-2-phenylisophosphindoline)dichloropalladium
0.07 mmol (17 mg) of 1,3-dimethyl-2-phenylisophosphindoline dissolved
in 0.3 ml of absolute THF is combined with 0.035 mmol of
[PdCl2(PhCH)2J dissolved in 0.6 ml of absolute THF under a protective
argon atmosphere, the mixture is stirred briefly, filtered through a
Pasteur pipette filled with silica gel and evaporated completely. The
orange residue is analyzed. This is the cis complex of the formula
PdCl2(isophosphindoline)2.
Analytical data:
.. CA 02346379 2001-04-06
WO 00/21971 15 PCT/EP99/07084
MS (electron impact ionization (70 eV)): m/e = 586 (2%, PdP2+), 584
(2%, PdP2- 2H+), 317 (3%), 240 (100%, M+), 225 (28%, M+-15), 212
(3%), 192 (10%), 178 (14%), 165 (7%), 147 (24%), 131 (34%), 129
(10%), 115 (46%), 109 (12%), 91 (59%), 77 (32%), 65 (2%), 51 (2%),
39 (2%)
3~ P NMR (161.99 MHz, CgD6/THF): 8 +74.04 ppm.
7. cis-Bis(1,3-dimethyl-2-phenylisophosphindoline)cyclooctadienerhodium
tetrafluoroborate
0.05 mmol of [Rh(COD)2]BF4 is slurried in 1.5 ml of THF and, under a
protective argon atmosphere, 0.1 mmol (24 mg) of 1,3-dimethyl-2-
phenylisophosphindoline dissolved in 0.1 ml of CgDg is added thereto.
After being allowed to stand for about one hour at room temperature,
the rhodium COD complex dissolves to form the phospholine complex.
The compound is present as a rapidly exchanging diastereomer mixture
of the formulae Rh(COD)(R-Isophos)(S-Isophos) and Rh(COD)(R-
Isophos)(R-Isophos) or Rh(COD)(S-Isophos)(S-Isophos).
3~ P NMR (161.99 MHz, CgDg): 8 +62.3 ppm (broad signal).
8. (1,3-Dimethyl-2-phenylisophosphindoline)cyclooctadienechlororhodium
0.025 mmol of [Rh(COD)C12] is slurried in 1.0 ml of THF and, under a
protective argon atmosphere, 0.05 mmol (12 mg) of 1,3-dimethyl-2-
phenylisophosphindoline dissolved in 0.1 ml of CgDg is added thereto.
After stirring for about 30 minutes at room temperature, the rhodium-
COD-CI complex dissolves to form the isophosphindoline complex.
3~ P NMR (161.99 MHz, CgDg): 8 +61.8 ppm (d, JpRh = 154 Hz).