Note: Descriptions are shown in the official language in which they were submitted.
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
TREATMENT OF ARTHRITIS WITH MEK INHIBITORS
FIELD OF THE INVENTION
This invention relates to methods for preventing and treating rheumatoid
arthritis or osteoarthritis by administering a compound characterized as an
inhibitor of a kinase enzyme known as MEK (MAP kinase or ERK Kinase). MEK
phosphorylates and activates MAP kinase (also known as Erk). The method is
ideally practiced by administering a phenyl amine MEK inhibitor.
BACKGROUND OF THE INVENTION
Arthritis is a debilitating disease that afflicts millions of people, and for
which there currently are no cures. Several forms of arthritis are known.
Rheumatoid arthritis is characterized as a chronic systemic inflammatory
disease,
primarily of the joints, and generally marked by inflammatory changes in the
synovial membranes and articular structures and by atrophy and rarefaction of
the
bones. Osteoarthritis is a noninflammatory degenerative joint disease
occurring
most often in older persons. Characterized by degeneration of the articular
cartilage, hypertrophy of bone at the margins, and changes in the synovial
membrane, osteoarthritis is accompanied by pain and stiffness, particularly
after
prolonged physical activity. Osteoarthritis is also referred to as
degenerative
arthritis, hypertrophic arthritis, and degenerative joint disease. The current
treatments are designed to relieve the pain, and to diminish the symptoms.
Most of
the known treatments are anti-inflammatory agents such as NSAIDs and
cyclooxygenase inhibitors.
We have now discovered that a series of compounds that are said to be
selective MEK inhibitors are useful to prevent and treat arthritis. Many of
the
compounds are described in WO 98/37881 as being useful to treat septic shock.
CA 02346448 2001-04-06
WO 00/35436 PCTNS99/29783
SUMMARY OF THE INVENTION
This invention provides a method for preventing and treating arthritis,
wherein the method comprises the step of administering to a mammal suspected
of
developing arthritis, or in need of treatment, an effective anti-arthritic
amount of a
MEK inhibitor, preferably a selective MEK inhibitor. Selective MEK inhibitors
are those compounds which inhibit the MEK 1 and MEK 2 enzymes without
substantial inhibition of other related enzymes. One aspect of the invention
provides a method for treating rheumatoid arthritis, said method comprising
the
step of administering a MEK inhibitor to a patient. In another aspect, the
invention
provides a method for treating osteoarthritis, said method comprising
administering a MEK inhibitor to a patient. In further embodiments of these
aspects, the invention provides a method for preventing and/or treating
arthritis
comprising the step of administering a therapeutically effective amount of a
selective MEK inhibitor described in US 5,525,625, incorporated herein by
reference in its entirety. An example of a selective MEK inhibitor is 2-(2-
amino-
3-methoxyphenyl)-4-oxo-4H-[ 1 ]benzopyran.
MEK inhibitors are compounds which inhibit one or more of the family of
mammalian enzymes known as MAP kinase kinases, which phosphorylate the
MAP kinase subfamily of enzymes (mitogen-associated protein kinase enzymes)
referred to as MAP kinases or ERKs (extracellular signal-regulating enzymes
such
as ERKl and ERK 2). These enzymes regulate phosphorylation of other enzymes
and proteins within the mammalian body. MEK 1 and MEK 2 are dual specificity
kinases that are present in all cell types and play a critical role in the
regulation of
cell proliferation and differentiation in response to mitogens and a wide
variety of
growth factors and cytokines
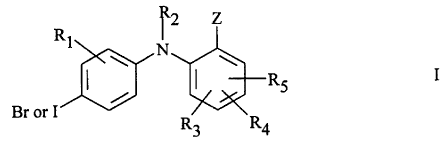
In a preferred embodiment, the MEK inhibitor to be administered is a
phenyl amine derivative of Formula I
2
CA 02346448 2001-04-06
WO 00/35436 PCTNS99/29783
~2 Z
RI
N
RS
Br or I
R3 R4
In formula (I), R1 is hydrogen, hydroxy, C1-Cg alkyl, C1-Cg alkoxy, halo,
trifluoromethyl, or CN. R2 is hydrogen. R3, R4, and RS are independently
selected from hydrogen, hydroxy, halo, trifluoromethyl, C1-Cg alkyl,
C1-Cg alkoxy, vitro, CN, and -(O or NH)m-(CH2)n Rg. Rg is hydrogen, hydroxy,
COOH, or NR1pR11; n is 0-4; m is 0 or 1. Each of R10 and R11 is independently
selected from hydrogen and C1-Cg alkyl, or taken together with the nitrogen to
which they are attached can complete a 3-10 member cyclic ring optionally
containing 1, 2, or 3 additional heteroatoms selected from O, S, NH, or N-(C1-
Cg
alkyl). Z is COORS, tetrazolyl, CONR6R~, CONHNR1pR11, or CH20R~.
R6 and R~ independently are hydrogen, C1-Cg alkyl, C2-Cg alkenyl,
C2-Cg alkynyl, (CO)-C1-Cg alkyl, aryl, heteroaryl, C3-C10 cycloalkyl, or
C3-C10 (cycloalkyl optionally containing one, two, or three heteroatoms
selected
from O, S, NH, or N alkyl); or R6 and R~ together with the nitrogen to which
they
are attached complete a 3-10 member cyclic ring optionally containing 1, 2, or
3
additional heteroatoms selected from O, S, NH, or N alkyl. In formula (I), any
of
the foregoing alkyl, alkenyl, aryl, heteroaryl, heterocyclic, and alkynyl
groups can
be unsubstituted or substituted by halo, hydroxy, C~-C~alkoxy, amino, vitro,
C1-
C4 alkylamino, di(C1-C4)alkylamino, C3-C6 cycloalkyl, phenyl,phenoxy, C3-CS
heteroaryl, or C3-CS heteroaryloxy; or a pharmaceutically acceptable salt,
ester,
amide, or prodrug thereof.
Preferred embodiments of Formula (I) have a structure wherein: (a) Rl is
hydrogen, methyl, methoxy, fluoro, chloro, or bromo; (b) RZ is hydrogen; (c)
R3,
R4, and RS independently are hydrogen, fluoro, chloro, bromo, iodo, methyl,
methoxy, or vitro; (d) Rio and R> > independently are hydrogen or methyl; (e)
Z is
COORS, tetrazolyl, CONR6R~, CONHNRIOR~ 1, or CH~OR~; R6 and R~
3
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/Z9783
' independently are hydrogen, C 1~ alkyl, heteroaryl, or C 3_5 cycloalkyl
optionally
containing one or two heteroatoms selected from O, S, or NH; or R4 and R~
together with the nitrogen to which they are attached complete a S-6 member
cyclic ring optionally containing 1 or 2 additional heteroatoms selected from
O,
NH or N-alkyl; and wherein any of the foregoing alkyl or aryl groups can be
unsubstituted or substituted by halo, hydroxy, methoxy, ethoxy, or
heteroaryloxy
(such as the synthetic intermediate 2,3,4,5,6-pentafluorophenyl); (f) Z is
COORS;
(g) R7 is H, pentafluorophenyl, or tetrazolyl; (h) R3, R4, and RS are
independently
H, fluoro, or chloro; (i) R4 is fluoro; (j) two of R3, R4, and RS are fluoro;
(k) or
combinations of the above. In another preferred embodiment of Formula (I), R~
is
methyl, fluoro, chloro, or bromo.
Examples of preferred embodiments include methods comprising a MEK
inhibitor selected from Formula (I) Compound Table below.
4
CA 02346448 2001-04-06
WO 00/35436 PC'T/US99/29783
' FORMULA (I) COMPOUND TABLE
(page 1 of 10)
[4-Chloro-2-( 1 H-tetrazol-5-yl)-phenyl-(4-iodo-2-methyl-phenyl)-amine
(4-iodo-2-methyl-phenyl)-[2-( 1 H-tetrazol-5-yl)-phenyl]amine
[4-nitro-2-( 1 H-tetrazol-5-yl)-phenyl-(4-iodo-2-methyl-phenyl)-amine
4-Fluoro-2-(4-iodo-2-methylphenylamino)benzoic acid
3,4,5-Trifluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid
S-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino}-benzoic acid
5-Chloro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid
Sodium 5-Chloro-2-(4-iodo-2-methyl-phenylamino)-benzoate
5-Bromo-2-(4-iodo-2-methyl-phenylamino)-benzoic acid
2-(4-Iodo-2-methyl-phenylamino)-5-nitro-benzoic acid
4-Chloro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid
2-(4-Iodo-2-methyl-phenylamino)-benzoic acid
5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid
5-Iodo-2-{4-iodo-2-methyl-phenylamino)-benzoic acid
2,3,5-Trifluoro-4-{4-iodo-2-methyl-phenylamino}-benzoic acid
2-(4-Iodo-phenylamino)-5-methoxy-benzoic acid
S-Methyl-2-(4-iodo-2-methyl-phenylamino)-benzoic acid
2-(4-Iodo-2-methyl-phenylamino)-4-nitro-benzoic acid
2-(4-Bromo-2-methyl-phenylamino)-4-fluoro-benzoic acid
2-(2-Bromo-4-iodo-phenylamino)-5-nitro-benzoic acid
2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-benzoic acid
5-Chloro-N-(2-hydroxyethyl)-2-(4-iodo-2-methyl-phenylamino}-
benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-methyl-benzamide
N-Ethyl-4-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N,N-dimethyl-benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-( 1 H-tetrazol-5-yl)-
benzamide
5
CA 02346448 2001-04-06
WO 00135436 PCT/US99/29783
FORMULA (I) COMPOUND TABLE
(continued, page 2 of 10)
5-Bromo-2-(4-iodo-2-methyl-phenylamino)-benzamide
S-Chloro-2-(4-iodo-2-methyl-phenylamino)-N,N-dimethyl-benzamide
[5-Chloro-2-(4-iodo-2-methyl-phenylamino)-benzoylamino]-acetic acid
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-propyl-benzamide
5-Bromo-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
N,N-Diethyl-4-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide
4-Fluoro-N- { 3-[4-(2-hydroxy-ethyl)-piperazin-1-yl]-propyl } -2-(4-iodo-
2-methyl-phenylamino)-benzamide
N,N-Diethyl-2-(4-iodo-2-methyl-phenylamino)-S-nitro-benzamide
N-Butyl-4-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Chloro-N,N-diethyl-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N,N-dimethyl-benzamide
5-Bromo-3,4-difluoro-N-{2-hydroxy-ethyl)-2-(4-iodo-2-methyl-
phenylamino)-benzamide
N-(2,3-Dihydroxy-propyl)-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-
benzamide
S-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-piperidin-1-
yl-ethyl)-benzamide
3,4-Difluoro-N-(2-hydroxy-ethyl)-2-{4-iodo-2-methyl-phenylamino)-
benzamide
N-(2,3-Dihydroxy-propyl)-4-fluoro-2-(4-iodo-2-methyl-phenylamino)-
benzamide
3,4-Difluoro-N-(3-hydroxy-propyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyrrolidin-
1-yl-ethyl)-benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyridin-4-
yl-ethyl)-benzamide
6
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
FORMULA (I) COMPOUND TABLE
(continued, page 3 of 10)
4-Fluoro-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
S-Bromo-N-(3-dimethylamino-propyl)-3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-morpholin-
4-yl-ethyl)-benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-morpholin-4-yl-
ethyl}-benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyrrolidin-1-yl-
ethyl)-benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyridin-4-yl-ethyl)-
benzamide
N-(3-Dimethylamino-propyl)-3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-benzamide
N-Benzyl-4-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide
2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(2-hydroxy-ethyl)-
benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-morpholin-4-yl-ethyl)-
benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-piperidin-1-yl-propyl)-
benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-piperidin-1-yl-
propyl)-benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-thiophen-2-yl-ethyl)-
benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyrrolidin-1-yl-ethyl)-
benzamide
2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(2-morpholin-4-yl-
ethyl)-benzamide
7
CA 02346448 2001-04-06
WO 00/35436 PCT/US99129783
FORMULA (I) COMPOUND TABLE
(continued, page 4 of 10)
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-pyridin-4-
ylmethyl-benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-pyridin-4-ylmethyl-
benzamide
2-(4-Bromo-2-methyl-phenylamino)-N-(3-dimethylamino-
propyl}-3,4-difluoro-benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-pyridin-4-ylmethyl-
benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyridin-4-yl-ethyl)-
benzamide
2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(2-pyridin-4-yl-ethyl)-
benzamide
2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(3-hydroxy-propyl)-
benzamide
2-(4-Bromo-2-methyl-phenylamino}-3,4-difluoro-N-(2-pyrrolidin-1-yl-
ethyl)-benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-phenethyl-benzamide
2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(2-thiophen-2-yl-
ethyl)-benzamide
2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-pyridin-4-ylmethyl-
benzamide
2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-phenethyl-benzamide
2-(4-Bromo-2-methyl-phenylamino}-3,4-difluoro-N-(2-piperidin-1-yl-
ethyl)-benzamide
5-Chloro-N-{3-[4-(2-hydroxy-ethyl)-piperazin-1-yl]-propyl}-2-(4-iodo-2-
methyl- phenylamino)- benzamide
5-Fluoro-N-{3-[4-(2-hydroxy-ethyl)-piperazin-1-ylJ-propyl}-2-(4-iodo-2-
methyl- phenylamino)- benzamide
2-(4-Iodo-2-methyl-phenylamino)-5-nitro-N-pyridin-4-yl methyl-
benzamide
8
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
FORMULA (I) COMPOUND TABLE
(continued, page 5 of 10)
5-Bromo-N- { 3-[4-(2-hydroxy-ethyl)-piperazin-1-yl]-propyl } -2-(4-iodo-2-
methyl- phenylamino)- benzamide
S-Chloro-N-(2-diethylamino-ethyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-(2-piperidin-1-yl-ethyl)-
benzamide
(3-Hydroxy-pyrrolidin-1-yl)-[5-nitro-2-(4-iodo-2-methyl-phenylamino)-
phenyl)-methanone
5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyrrolidin-1-yl-ethyl)-
benzamide
S-Bromo-N-(2-diethylamino-ethyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
N- { 2-[Bis-(2-hydroxy-ethyl)-amino)-ethyl } -5-chloro-2-(4-iodo-2-methyl-
phenylamino)- benzamide
N- { 2-[Bis-(2-hydroxy-ethyl)-amino]-ethyl } -5-bromo-2-(4-iodo-2-methyl-
phenylamino)- benzamide
N-{ 3-[4-(2-Hydroxy-ethyl)-piperazin-1-yl]-propyl }-2-(4-iodo-2-methyl-
phenylamino)- benzamide
5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-pyridin-4-ylmethyl-
benzamide
5-Bromo-2-(4-iodo-2-ethyl-phenylamino)-N-(2-pyrrolidin-1-yl-ethyl)-
henzamide
5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(2-piperidin-1-yl-ethyl)-
benzamide
5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyrrolidin-1-yl-ethyl)-
benzamide
5-Chloro-N-(3-dimethylamino-propyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
N- { 2-[Bis-(2-hydroxy-ethyl)-amino}-ethyl } -5-fluoro-2-(4-iodo-2-methyl-
phenylamino)- benzamide
9
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
FORMULA (I) COMPOUND TABLE
(continued, page 6 of 10)
S-Chloro-N-(3-hydroxy-propyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
5-Chloro-N-(3-diethylamino-2-hydroxy-propyl)-2-(4-iodo-2-methyl-
phenylamino)- benzamide
5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-piperidin-1-yl-ethyl)-
benzamide
5-Bromo-N-(3-hydroxy-propyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
S-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(3-piperidin-1-yl-propyl)-
benzamide
N- { 2-[Bis-(2-hydroxy-ethyl)-amino]-ethyl } -2-(4-iodo-2-methyl-
phenylamino)-5-nitro- benzamide
5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-(2-morpholin-4-yl-ethyl)-
benzamide
5-Chloro-N-(3-diethylamino-propyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
S-Ghloro-N-(2-diisopropylamino-ethyl)-2-(4-iodo-2-methyl-phenylamino)-
henzamide
5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-(3-piperidin-1-yl-propyl)-
benzamide
2-(4-Iodo-2-methyl-phenylamino)-5-nitro-N-(2-piperidin-1-yl-ethyl)-
benzamide
5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(2-piperazin-1-yl-ethyl)-
benzamide
N-(2-Diethylamino-ethyl)-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-
benzamide
3 0 5-Bromo-N-(3-dimethylamino-propyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
N-(3-Hydroxy-propyl)-2-(4-iodo-2-methyl-phenylamino)-S-nitro-
benzamide
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
FORMULA (I) COMPOUND TABLE
(continued, page 7 of 10)
5-Fluoro-N-(3-hydroxy-propyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
N-(3-Diethylamino-propyl)-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-
benzamide
N-(3-Diethylamino-propyl)-2-(4-iodo-2-methyl-phenylamino)-5-nitro-
benzamide
5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(2-morpholin-4-yl-ethyl)-
benzamide
2-(4-Iodo-2-methyl-phenylamino)-5-nitro-N-(3-piperidin-1-yl-propyl)-
benzamide
[5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-(2 or 3-hydroxy-
pyrrolidin-1-yl)-methanone
5-Bromo-N-(2-diisopropylamino-ethyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
5-Fluoro-2-(4-iodo-2-methyl-phenylamino}-N-(2-morpholin-4-yl-ethyl)-
benzamide
5-Fiuoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-piperidin-1-yl-propyl)-
benzamide
[5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-[4-(2-hydroxy-ethyl)-
piperazin-1-yl)-methanone
N-(3-Diethylamino-2-hydroxy-propyl)-5-fluoro-2-(4-iodo-2-methyl-
phenylamino}- benzamide
N-Cyclopropyl-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide;
5-Chloro-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
5-Fluoro-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
N-Benzyloxy-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide
N-Benzyloxy-5-bromo-2-(4-iodo-2-methyl-phenylamino)-benzamide
11
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
FORMULA (I) COMPOUND TABLE
(continued, page 8 of 10)
2-(4-Iodo-2-methyl-phenylamino)-5-vitro-N-(4-sulfamoyl-benzyl)-
benzamide
5-Bromo-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
N-(2-Hydroxy-ethyl)-S-iodo-2-(4-iodo-2-methyl-phenylamino)-benzamide
N-(2-Hydroxy-ethyl)-2-(4-iodo-2-ethyl-phenylamino)-5-vitro-benzamide
2-(4-Iodo-2-methyl-phenylamino)-N-methyl-5-vitro-N-phenyl-benzamide
5-Chloro-N-cyclopropyl-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-
benzamide
N-Allyl-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide
N-Benzyloxy-S-iodo-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(4-sulfamoyl-benzyl)-
benzamide
N-Allyl-S-chloro-2-(4-iodo-2-methyl-phenylamino)-benzamide
N-Cyclopropyl-2-(4-iodo-2-methyl-phenylamino)-5-vitro-benzamide
5-Bromo-N-cyclopropyl-2-(4-iodo-2-methyl-phenylamino}-benzamide
5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-
benzamide
5-Iodo-2-(4-iodo-2-methyl-phenylamino)-N-{4-sulfamoyl-benzyl)-
benzamide
5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(4-sulfamoyl-benzyl)-
benzamide
N-Allyl-2-(4-iodo-2-methyl-phenylamino)-5-vitro-benzamide
2-(4-Iodo-2-methyl-phenylamino)-5-vitro-N-(4-sulfamoyl-benzyl)-
benzamide
N-Allyl-5-bromo-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-methyl-benzyl)-
benzamide
N-Cyclopropyl-S-iodo-2-(4-iodo-2-methyl-phenylamino)-benzamide
I2
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
FORMULA (I) COMPOUND TABLE
(continued, page 9 of 10)
5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-
benzamide
N-Benzyloxy-2-(4-iodo-2-methyl-phenylamino)-5-vitro-benzamide
N-Cyclohexyl-5-iodo-2-(4-iodo-2-methyl-phenylamino)-benzamide
N-Allyl-S-iodo-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Iodo-2-(4-iodo-2-methyl-phenylamino)-N-(3-methyl-benzyl)-benzamide
2-(4-Iodo-2-methyl-phenylamino)-N-(3-methyl-benzyl)-5-vitro-benzamide
5-Iodo-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-benzamide
N-Cyclohexyl-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Chloro-N-cyciohexyl-2-(4-iodo-2-methyl-phenylamino}-benzamide
5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(3-methyl-benzyl)-
benzamide
5-Bromo-N-cyclohexyl-2-(4-iodo-2-methyl-phenylamino)-benzamide
S-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-(3-methyl-benzyl)-
benzamide
N-Cyclohexyl-2-(4-iodo-2-methyl-phenylamino)-S-vitro-benzamide
N-Benzyloxy-5-bromo-2-(4-iodo-2-methyl-phenylamino)-benzamide
N-Benzyloxy-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Chloro-N-(2-hydroxy-ethyl}-2-(4-iodo-2-methyl-phenylamino)-
benzamide
S-Bromo-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
2-(4-Iodo-2-methyl-phenylamino)-N-methyl-5-vitro-N-phenyl-benzamide
S-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-
benzamide
N-(2-Hydroxy-ethyl)-S-iodo-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Chloro-N-cyclopropyl-2-(4-iodo-2-methyl-phenylamino)-benzamide
N-Allyl-5-chloro-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-
benzamide
13
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
FORMULA (I) COMPOUND TABLE
(continued, page 10 of 10)
N-(2-Hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-5-vitro-benzamide
5-Fluoro-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-
benzamide
5-Bromo-N-cyclopropyl-2-(4-iodo-2-methyl-phenylamino)-benzamide
N-Cyclopropyl-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(4-sulfamoyl-benzyl)-
benzamide
N-Cyclopropyl-2-(4-iodo-2-methyl-phenylamino)-5-vitro-benzamide
N-Allyl-S-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzatnide
N-Benzyloxy-5-iodo-2-(4-iodo-2-methyl-phenylamino)-benzamide
N-Allyl-5-bromo-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(4-sulfamoyl-benzyl)-
benzamide
5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-
benzamide
N-Allyl-2-(4-iodo-2-methyl-phenylamino)-5-vitro-benzamide
4-Fiuoro-2-(4-iodo-2-methyl-phenylamino)-benzyl alcohol
[S-Chloro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-methanol
[2-(4-Iodo-2-methyl-phenylamino)-S-vitro-phenyl]-methanol
[5-Bromo-2-(4-iodo-2-methyl-phenylamino)-phenyl]-methanol
N-Allyl-2-(4-iodo-2-methyl-phenylamino)-5-vitro-benzamide.
14
CA 02346448 2001-04-06
WO 00/35436 PCTNS99/29783
In another preferred embodiment, the MEK inhibitor is a compound of
Formula II
~6a
~2a C-N-O- Rya
Rla N
II
RSa
Br or I
R3a R4a
In Formula (II), Rla is hydrogen, hydroxy, C1-Cg alkyl, C1-Cg alkoxy,
halo, trifluoromethyl, or CN. R2a is hydrogen. Each of R3a, R4a, and Rsa is
independently selected from hydrogen, hydroxy, halo, trifluoromethyl,
C 1-Cg alkyl, C 1-Cg alkoxy, nitro, CN, and (O or NH)m-(CH2)n-Rga. Rga is
hydrogen, hydroxy, C02H or NR10aR1 la~ n is 0-4; and m is 0 or 1. Each of
RlOa ~d R1 la is independently hydrogen or C1-Cg alkyl, or taken together with
the nitrogen to which they are attached can complete a 3- to 10-member cyclic
ring optionally containing one, two, or three additional heteroatoms selected
from
O, S, NH, or N-(C1-Cg alkyl). R6a is hydrogen, C1-Cg alkyl, (CO}-(C1-Cg
alkyl), aryl, aralkyl, or C3-C10 cycloalkyl. Rya is hydrogen, C1-Cg alkyl,
C2-Cg alkenyl, C2-Cg alkynyl, C3-C10 (cycloalkyl or cycloalkyl optionally
containing a heteroatom selected from O, S, or NRg~. In Formula (II), any of
the
alkyl, alkenyl, aryl, heterocyclic, and alkynyl groups can be unsubstituted or
substituted by halo, hydroxy, C~-C6 alkoxy, amino, nitro, C,-C4 alkylamino,
di(C~-
C4)alkylamino, C3-C6 cycloalkyl, phenyl,phenoxy, C3-CS heteroaryl, or C3-CS
heteroaryloxy; or R.6a and Rya taken together with the N to which they are
attached can complete a S- to 10-membered cyclic ring, optionally containing
one,
two, or three additional heteroatoms selected from O, S, or NR10aR1 la~ The
invention also encompasses pharmaceutically acceptable salts, esters, amides
or
prodrugs of each of the disclosed compounds.
Preferred embodiments of Formula (II) are those structures wherein:
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
(a) R,e is H, methyl, fluoro, or chloro; (b) R2a is H; R3a, R4a, and Rsaare
each H,
Cl, nitro, or F; (c) Rba is H; (d) R~e is methyl, ethyl, 2-propenyl, propyl,
butyl,
pentyl, hexyl, cyclopropylmethyl, cyclobutylmethyl, cyclopropylmethyl, or
cyclopropylethyl;
(e) the 4' position is I, rather than Br; (f) R4a is F at the 4 position, para
to the CO-
N-R68-OR~$ group and meta to the bridging nitrogen; (f) R3a or Rsa is F; (g)
at least
one of R3a, R4a, and Rsa is F; (h) bt,e is methyl or chloro; or (i) or a
combination
of the above.
In a more preferred embodiment the MEK inhibitor is a compound
selected from Formula (II) Compound Table below.
16
CA 02346448 2001-04-06
WO 00/3543b PCT/US99/29783
FORMULA (II) COMPOUND TABLE
(page 1 of 7)
4-Fluoro-N-hydroxy-2-(4-iodo-2-methyl-phenylamino)-benzamide
S 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(methoxy)-benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(prop-2-ynyloxy)-
benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-phenoxyethoxy}-
benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-thienylmethoxy)-
benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(prop-2-enyloxy)-
benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino}-N-(cyclopropylmethoxy)-
benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(cyclopentoxy)-benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino}-N-(3-furylmethoxy)-
benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-ethoxy-benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(but-2-enyloxy)-
benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(cyclopropylmethoxy)-
benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-( 1-methylprop-
2-ynyloxy)-benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-phenylprop-
2-ynyloxy)-benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-methyl-
5-phenylpent-2-en-4-ynyloxy)-benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(prop-2-ynyloxy)-
benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(propoxy)-benzamide
17
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
FORMULA (II) COMPOUND TABLE
(continued, page 2 of 7)
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(cyclobutyloxy)-
benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-thienylmethoxy)-
benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-methyl-prop-
2-enyloxy)-benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-phenoxyethoxy)-
benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(but-2-enyloxy)-
benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(but-3-ynyloxy)-
benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(cyclopentyloxy)-
benzamide
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-(2-fluorophenyl)-
prop-2-ynyloxy)-benzamide
5-Bromo-3,4-difluoro-N-hydroxy-2-(4-iodo-2-methyl-phenylamino)-
benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(n-propoxy)-
benzamide
5-Bromo-3,4-difluoro-N-(furan-3-ylmethoxy)-2-(4-iodo-2-methyl-
phenylamino)-benzamide
5-Bromo-N-(but-2-enyloxy)-3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-benzamide
5-Bromo-N-butoxy-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-
benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-methyl-but-
2-enyloxy)-benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-methyl-
pent-2-en-4-ynyloxy)-benzamide
18
CA 02346448 2001-04-06
WO 00/35436 PC'T/US99/29783
FORMULA (II) COMPOUND TABLE
(continued, page 3 of 7}
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-benzyl}-N-[5-(3-methoxy-
S phenyl)-3-methyl-pent-2-en-4-ynyloxy]-benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(prop-
2-ynyloxy)-benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-
[3-(3-methoxy-phenyl)-prop-2-ynyloxy]-benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(thiopen-
2-ylmethoxy)-benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(pyridin-
3-ylmethoxy)-benzamide
5-Bromo-3-4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-
(3-(2-fluorophenyl)-prop-2-ynyloxy)-benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(ethoxy)-
benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-
(cyclopropylmethoxy)-benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(isopropoxy)-
benzamide
5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino}-N-but-
3-ynyloxy)-benzamide
S-Chloro-N-hydroxy-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-(tetrahydro-pyran-2-yloxy}-
benzamide
S-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-methoxy-benzamide
4-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-phenylmethoxy-benzamide
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-phenylmethoxy-benzamide
5-Fluoro-N-hydroxy-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Iodo-2-(4-iodo-2-methyl-phenylamino)-N-phenylmethoxy-benzamide
5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(tetrahydropyran-2-yloxy)-
benzamide
19
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/Z9783
' FORMULA (II) COMPOUND TABLE
(continued, page 4 of 7)
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(3-phenylprop-
2-ynyloxy)-benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(3-furylmethoxy)-
benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(2-thienylmethoxy)-
benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(but-3-ynyloxy)-
benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(2-methyl-prop-
2-enyloxy)-benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(but-2-enyloxy)-
benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(methoxy)-benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(ethoxy)-benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(cyclobutoxy)-
benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(isoprogoxy)-
benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(2-phenoxyethoxy)-
benzamide
3,4-Difluoro-2-{4-bromo-2-methyl-phenylamino)-N-(cyclopropyl-
methoxy)-benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(n-propoxy)-
benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-( 1-methyl-prop-
2-ynyloxy)-benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(3-(3-fluorophenyl)-
prop-2-ynyloxy)-benzamide
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(4,4-dimethylpent-
2-ynyloxy)-benzamide
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
FORMULA (II) COMPOUND TABLE
(continued, page S of 7)
3,4-Difluoro-2-(4-bromo-2-methyl-phenylamino)-N-(cyclopentoxy)-
benzamide
3,4,5-Trifluoro-N-hydroxy-2-(4-iodo-2-methyl-phenylamino)-benzamide
5-Chloro-3,4-difluoro-N-hydroxy-2-{4-iodo-2-methyl-phenylamino)-
benzamide
5-Bromo-3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-N-hydroxy-
benzamide
N-Hydroxy-2-(4-iodo-2-methyl-phenylamino)-4-vitro-benzamide
3,4,5-Trifluoro-2-{2-fluoro-4-iodo-phenylamino)-N-hydroxy-benzamide
5-Chloro-3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-N-hydroxy-
benzamide
1 S 5-Bromo-2-(2-chloro-4-iodo-phenylamino)-3,4-difluoro-N-hydroxy-
benzamide
2-{2-Fluoro-4-iodo-phenylamino)-N-hydroxy-4-vitro-benzamide
2-(2-Chloro-4-iodo-phenylamino)-3,4,5-trifluoro-N-hydroxy-benzamide
5-Chloro-2-(2-chloro-4-iodo-phenylamino)-3,4-difluoro N-hydroxy-
benzamide
5-Bromo-2-(2-bromo-4-iodo-phenylamino)-3,4-difluoro-N-hydroxy-
benzamide
2-(2-Chloro-4-iodo-phenylamino)-N-hydroxy-4-methyl-benzamide
2-{2-Bromo-4-iodo-phenylamino)-3,4,5-trifluoro-N-hydroxy-benzamide
2-{2-Bromo-4-iodo-phenylamino)-5-chloro-3,4-difluoro-N-hydroxy-
benzamide
2-(2-Bromo-4-iodo-phenylamino)-N-hydroxy-4-vitro-benzamide
4-Fluoro-2-(2-fluoro-4-iodo-phenylamino)-N-hydroxy-benzamide
3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-N-hydroxy-benzamide
2-(2-Chloro-4-iodo-phenylamino)-4-fluoro-N-hydroxy-benzamide
2-(2-Chloro-4-iodo-phenylamino)-3,4-difluoro-N-hydroxy-benzamide
2-(2-Bromo-4-iodo-phenylamino)-4-fluoro-N-hydroxy-benzamide
2-(2-Bromo-4-iodo-phenylamino)-3,4-difluoro-N-hydroxy-benzamide
21
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
FORMULA (II) COMPOUND TABLE
(continued, page 6 of 7)
N-Cyclopropylmethoxy-3,4,5-trifluoro-2-(4-iodo-2-methyl-phenylamino)-
benzamide
S-Chloro-N-cyclopropylmethoxy-3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-benzamide
S-Bromo-N-cyclopropylmethoxy-3,4-difluoro-2-(2-fluoro-4-iodo-
phenylamino)-benzamide
N-Cyclopropylmethoxy-2-(4-iodo-2-methyl-phenylamino)-4-nitro-
benzamide
N-Cyclopropylmethoxy-3,4,5-trifluoro-2-(2-fluoro-4-iodo-phenylamino)-
benzamide
5-Chloro-N-cyclopropylmethoxy-3,4-difluoro-2-(2-fluoro-4-iodo-
phenylamino)-benzamide
S-Bromo-2-(2-chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-
3,4-difluoro-benzamide
N-Cyclopropylmethoxy-2-(2-fluoro-4-iodo-phenylamino)-4-nitro-
benzamide
2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4,5-trifluoro-
benzamide
5-Chloro-2-(2-chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-
3,4-difluoro-benzamide
S-Bromo-2-(2-bromo-4-iodo-phenylamino)-N-ethoxy-3,4-difluoro-
benzamide
2-(2-Chloro-4-iodo-phenylamino)-N-ethoxy-4-vitro-benzamide
2-(2-Bromo-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4,5-trifluoro-
benzamide
2-(2-Bromo-4-iodo-phenylamino)-S-chloro-N-cyclopropylmethoxy-
3,4-difluoro-benzamide
2-(2-Bromo-4-iodo-phenylamino)-N-cyclopropylmethoxy-4-nitro-
benzamide
22
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29~83
FORMULA (II) COMPOUND TABLE
(continued, page 7 of 7)
N-Cyclopropylmethoxy-4-fluoro-2-(2-fluoro-4-iodo-phenylamino)-
benzamide
N-Cyclopropylmethoxy-3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-
benzamide
2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-4-fluoro-
benzamide
2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4-difluoro-
benzamide
2-(2-Bromo-4-iodo-phenylamino)-N-cyclopropylmethoxy-4-fluoro-
benzamide
2-(2-Bromo-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4-difluoro-
benzamide.
In the most preferred embodiment of this invention, a compound selected
from the following is administered to a patient (ie, a mammal) in an amount
that is effective to prevent or treat rheumatoid arthritis or osteoarthritis:
2-(2-Chloro-4-iodophenylamino)-N-cyclopropylmethoxy-
3,4-difluorobenzamide (PD184352); 2-(2-Methyl-4-iodophenylamino}-N-
hydroxy-4-fluorobenzamide (PD 170611 ); 2-(2-Methyl-4-iodophenylamino)-N-
hydroxy-3,4-difluoro-5-bromobenzamide (PD171984); 2-(2-Methyl-
4-iodophenylamino)-N-cyclopropylmethoxy-3,4-difluoro-5-bromobenzamide
(PD177168); 2-(2-Methyl-4-iodophenylamino)-N-cyclobutylmethoxy-
3,4-difluoro-5-bromobenzamide (PD 180841 ); 2-(2-Chloro-
4-iodophenylamino)-N-cyclopropylmethoxy-3,4-difluoro-5-bromobenzamide
(PD 184161 ); 2-(2-Chloro-4-iodophenylamino}-N-hydroxy-3,4-difluoro-
5-bromobenzamide (PD184386); 2-(2-Chloro-4-iodophenylamino)-N-
cyclobutylmethoxy-3,4-difluorobenzamide (PD 185625); 2-(2-Chloro-
4-iodophenylamino)-N-hydroxy-4-fluorobenzamide (PD 185848);
2-(2-Methyl-4-iodophenylamino)-N-hydroxy-3,4-difluorobenzamide
(PD 188563); 2-(2-Methyl-4-iodophenylamino)-N-cyclopropylmethoxy-
23
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
- 3,4,5-trifluorobenzamide (PD 198306); and 2-(2-Chloro-4-iodophenylamino)-
N-cyclopropylmethoxy-4-fluorobenzamide (PD 203311 ); and the benzoic acid
derivatives thereof. For example, the benzoic acid derivative of PD 198306 is
2-(2-Methyl-4-iodophenylamino)-3,4,5-trifluorobenzoic acid.
S Additional preferred compounds include 2-(2-chloro-4-
iodophenylamino)-5-chloro-N-cyclopropylmethoxy -3,4-difluorobenzamide
(PD 297189), 2-(4-iodophenylamino)-N-cyclopropylmethoxy-5-chloro-3,4-
difluorobenzamide (PD 297190), 2-(4-iodophenylamino)-5-chloro-3,4-
difluorobenzoic acid (PD 296771 ), 2-(2-chloro-4-iodophenylamino)-5-chloro-
3,4-difluorobenzoic acid (PD 296770), 5-chloro-3,4-difluoro-2-(4-iodo-2-
methylphenylamino)-benzoic acid (PD 296767); and 5-chloro-N-
cyclopropylmethoxy -3,4-difluoro-2-(4-iodo-2-methylphenylamino)-benzamide
(PD ~.
The invention further provides methods of synthesis and synthetic
intermediates.
Other features and advantages of the invention are apparent from the
detailed description, examples, and claims set forth.
DETAILED DESCRIPTION OF THE INVENTION
This invention provides a method of preventing or treating arthritis in a
patient which comprises the step of administering to a patient suffering from
arthritis and in need of treatment, or to a patient at risk for developing
arthritis, an
effective anti-arthritic amount of a MEK inhibitor. The invention provides a
method of preventing and treating both rheumatoid arthritis and
osteoarthritis. The
invention is preferably practiced by administering a phenyl amine MEK
inhibitor
of Formula (I) or Formula (II). Many of these MEK-inhibiting phenyl amine
compounds are specific or selective MEK 1 and MEK 2 inhibitors.
Selective MEK 1 or MEK 2 inhibitors are those compounds which inhibit
the MEK 1 or MEK 2 enzymes without substantially inhibiting other enzymes
such as MKK3, ERK, PKC, Cdk2A, phosphorylase kinase, EGF and PDGF
receptor kinases, and C-src. In general, a selective MEK 1 or MEK 2 inhibitor
has
24
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
' an ICso for MEK 1 or MEK 2 that is at least one-fiftieth (1/50) that of its
ICso for
one of the above-named other enzymes. Preferably, a selective inhibitor has an
ICSO that is at least 1/100, more preferably 1/500, and even more preferably
1/1000, 1/5000 or less than that of its ICSO for one or more of the above-
named
enzymes.
T'he mammals to be treated according to this invention are patients, not
only humans but also animals such as horses and dogs, who have developed
arthritis and are suffering from the pain and disfiguration associated with
arthritis,
or who are at risk for developing the disease, for example, those who have a
family history of arthritis. Those skilled in the medical art are readily able
to
identify individual patients who are afflicted with arthritis, as well as
those who
are susceptible to developing the disease.
The term "patient" means all animals including humans. Examples of
patients include humans, cows, dogs, cats, goats, sheep, horses, and pigs.
The compounds of the present invention, which can be used to treat septic
shock, are MEK inhibitors. A MEK inhibitor is a compound that shows MEK
inhibition when tested in the assays titled "Enzyme Assays" in United States
Patent Number 5,525,625, column 6, beginning at line 35. The complete
disclosure-of United States Patent Number 5,525,625 is hereby incorporated by
reference. An example of a MEK inhibitor is 2-(2-amino-3-methoxyphenyl)-
4-oxo-4H-( 1 )benzopyran. Specifically, a compound is a MEK inhibitor if a
compound shows activity in the assay titled "Cascade Assay for Inhibitors of
the
MAP Kinase Pathway," column 6, line 36 to column 7, line 4 of the United
States
Patent Number 5,525,625 and/or shows activity in the assay titled "In Vitro
MEK
Assay" at column 7, lines 4 to 27 of the above-referenced patent.
A. Terms
Some of the terms used herein are defined below in combination with their
usage throughout this disclosure.
As used herein, the term "aryl" means a cyclic, bicyclic, or tricyclic
aromatic ring moiety having from five to twelve carbon atoms. Examples of
typical aryl groups include phenyl, naphthyl, and fluorenyl. The aryl may be
substituted by one, two, or three groups selected from fluoro, chloro, bromo,
iodo,
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
alkyl, hydroxy, alkoxy, vitro, amino, alkylamino, or dialkylamino. Typical
substituted aryl groups include 3-fluorophenyl, 3,5-dimethoxyphenyl,
4-nitronaphthyl, 2-methyl-4-chloro-7-aminofluorenyl, and the like.
The term "aryloxy" means an aryl group bonded through an oxygen atom,
for example phenoxy, 3-bromophenoxy, naphthyloxy, and 4-methyl-
1-fluorenyloxy.
"Heteroaryl" means a cyclic, bicyclic, or tricyclic aromatic ring moiety
having from four to eleven carbon atoms and one, two, or three heteroatoms
selected from O, S, or N. Examples include furyl, thienyl, pyrrolyl,
pyrazolyl,
imidazolyl, triazolyl, thiazolyl, oxazolyl, xanthenyl, pyronyl, indolyl,
pyrimidyl,
naphthyridyl, pyridyl, benzinnidazolyl, and triazinyl. The heteroaryl groups
can be
unsubstituted or substituted by one, two, or three groups selected from
fluoro,
chloro, bromo, iodo, alkyl, hydroxy, alkoxy, vitro, amino, alkylamino, or
dialkylamino. Examples of substituted heteroaryl groups include chloropyranyl,
methylthienyl, fluoropyridyl, amino-1,4-benzisoxazinyl, nitroisoquinolinyl,
and
hydroxyindolyl.
The heteroaryl groups can be bonded through oxygen to make
heteroaryloxy groups, for example thienyloxy, isothiazolyloxy,
benzofuranyloxy,
pyridyloxy, and 4-methylisoquinolinyloxy.
The term "alkyl" means straight and branched chain aliphatic groups.
Typical alkyl groups include methyl, ethyl, isopropyl, tert.-butyl,
2,3-dimethylhexyl, and l,l-dimethylpentyl. The alkyl groups can be
unsubstituted
or substituted by halo, hydroxy, alkoxy, amino, alkylamino, dialkylamino,
cycloalkyl, aryl, aryloxy, heteroaryl, or heteroaryloxy, as those terms are
defined
herein. Typical substituted alkyl groups include chloromethyl, 3-
hydroxypropyl,
2-dimethylaminobutyl, and 2-(hydroxymethylamino)ethyl. Examples of aryl and
aryloxy substituted alkyl groups include phenylmethyl, 2-phenylethyl,
3-chlorophenylmethyl, 1,1-dimethyl-3-(2-nitrophenoxy)butyl, and
3,4,5-trifluoronaphthylmethyl. Examples of alkyl groups substituted by a
heteroaryl or heteroaryloxy group include thienylmethyl, 2-furylethyl,
6-furyloxyoctyl, 4-methylquinolyloxymethyl, and 6-isothiazolylhexyl.
Cycloalkyl
substituted alkyl groups include cyclopropylmethyl, 2-cyclohexyethyl,
piperidyl-
2-methyl, 2-(piperidin-1-yl)-ethyl, 3-(morpholin-4-yl)propyl.
26
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
"Alkenyl" means a straight or branched carbon chain having one or more
double bonds. Examples include but-2-enyl, 2-methyl-prop-2-enyl, 1,1-dimethyl-
hex-4-enyl, 3-ethyl-4-methyl-pent-2-enyl, and 3-isopropyl-pent-4-enyl. The
alkenyl groups can be substituted with halo, hydroxy, alkoxy, amino,
alkylamino,
S dialkylamino, aryl, aryloxy, heteroaryl, or heteroyloxy, for example
2-bromoethenyl, 3-hydroxy-2-butenyl, 1-aminoethenyl, 3-phenylprop-2-enyl,
6-thienyl-hex-2-enyl, 2-furyloxy-but-2-enyl, and 4-naphthyloxy-hex-2-enyl.
"AlkynyI" means a straight or branched carbon chain having at least one
triple bond. Typical alkynyl groups include prop-2-ynyl, 2-methyl-hex-5-ynyl,
3,4-dimethyl-hex-5-ynyl, and 2-ethyl-but-3-ynyl. The alkynyl groups can be
substituted as the alkyl and alkenyl groups, for example, by aryl, aryloxy,
heteroaryl, or heteroaryloxy, for example 4-(2-fluorophenyl)-but-3-ynyl,
3-methyl-5-thienylpent-4-ynyl, 3-phenoxy-hex-4-ynyl, and 2-furyloxy-3-methyl-
hex-4-ynyl.
The alkenyl and alkynyl groups can have one or more double bonds or
triple bonds, respectively, or a combination of double and triple bonds. For
example, typical groups having both double and triple bonds include hex-2-en-
4-ynyl, 3-methyl-5-phenylpent-2-en-4-ynyl, and 3-thienyloxy-hex-3-en-5-ynyl.
The term "cycloalkyl" means a nonaromatic ring or fused rings. Examples
include cyclopropyl, cyclobutyl, cyclopenyl, cyclooctyl, bicycloheptyl,
adamantyl,
and cyclohexyl. The ring can optionally contain one, two, or three heteroatoms
selected from O, S, or N. Such groups include tetrahydrofuryl,
tetrahydropyrrolyl,
octahydrobenzofuranyl, morpholinyl, piperazinyl, pyrrolidinyl, piperidinyl,
octahydroindolyl, and octahydrobenzothiofuranyl. The cycloalkyl groups can be
substituted with the same substituents as an alkyl and alkenyl groups, for
example,
halo, hydroxy, aryl, and heteroaryloxy. Examples include 3-hydroxycyclohexyl,
2-aminocyclopropyl, 2-phenylpyrrolidinyl, and 3-thienylmorpholine-1-yl.
B. Administration and Formulation
The MEK inhibitors of the present method can be administered to a patient
as part of a pharmaceutically acceptable composition. The compositions can be
administered to humans and animals either orally, rectally, parenterally
(intravenously, intramuscularly, or subcutaneously), intracisternally,
27
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29?83
intravaginally, intraperitoneally, intravesically, locally (powders,
ointments, or
drops), or as a buccal or nasal spray.
Compositions suitable for parenteral injection may comprise
physiologically acceptable sterile aqueous or nonaqueous solutions,
dispersions,
S suspensions or emulsions, and sterile powders for reconstitution into
sterile
injectable solutions or dispersions. Examples of suitable aqueous and
nonaqueous
carriers, diluents, solvents, or vehicles include water, ethanol, polyols
(propyleneglycol, polyethyleneglycol, glycerol, and the like), suitable
mixtures
thereof, vegetable oils (such as olive oil), and injectable organic esters
such as
ethyl oleate. Proper fluidity can be maintained, for example, by the use of a
coating such as lecithin, by the maintenance of the required particle size in
the
case of dispersions and by the use of surfactants.
These compositions may also contain adjuvants such as preserving,
wetting, emulsifying, and dispensing agents. Prevention of the action of
microorganisms can be ensured by various antibacterial and antifungal agents,
for
example, parabens, chlorobutanol, phenol, sorbic acid, and the like. It may
also be
desirable to include isotonic agents, for example sugars, sodium chloride, and
the
like. Prolonged absorption of the injectable pharmaceutical form can be
brought
about by the use of agents delaying absorption, for example, aluminum
monostearate and gelatin.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders, and granules. In such solid dosage forms, the active compound is
admixed with at least one inert customary excipient (or Garner) such as sodium
citrate or dicalcium phosphate or (a) fillers or extenders, as for example,
starches,
lactose, sucrose, glucose, mannitol, and silicic acid, (b) binders, as for
example,
carboxymethylcelluiose, alignates, gelatin, polyvinylpyrrolidone, sucrose, and
acacia, (c) humectants, as for example, glycerol, (d) disintegrating agents,
as for
example, agar-agar, calcium carbonate, potato or tapioca starch, alginic acid,
certain complex silicates, and sodium carbonate, (e) solution retarders, as
for
example paraffin, (f) absorption accelerators, as far example, quaternary
ammonium compounds, (g) wetting agents, as for example, cetyl alcohol and
glycerol monostearate, (h) adsorbents, as for example, kaolin and bentonite,
and
(i) lubricants, as for example, talc, calcium stearate, magnesium stearate,
solid
28
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
polyethylene glycols, sodium lauryl sulfate, or mixtures thereof. In the case
of
capsules, tablets, and pills, the dosage forms may also comprise buffering
agents.
Solid compositions of a similar type may also be employed as fillers in
soft and hard-filled gelatin capsules using such excipients as lactose or milk
sugar
as well as high molecular weight polyethyleneglycols, and the like.
Solid dosage forms such as tablets, dragees, capsules, pills, and granules
can be prepared with coatings and shells, such as enteric coatings and others
well-
known in the art. They may contain opacifying agents, and can also be of such
composition that they release the active compound or compounds in a certain
part
of the intestinal tract in a delayed manner. Examples of embedding
compositions
which can be used are polymeric substances and waxes. The active compounds
can also be in micro-encapsulated form, if appropriate, with one or more of
the
above-mentioned excipients.
Liquid dosage forms for oral administration include pharmaceutically
acceptable emulsions, solutions, suspensions, syrups, and elixirs. In addition
to the
active compounds, the liquid dosage forms may contain inert diluents commonly
used in the art, such as water or other solvents, solubilizing agents and
emulsifiers,
as for example, ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl
acetate,
benzyl alcohol, benzyl benzoate, propyleneglycol, I,3-butyleneglycol,
dimethylformamide, oils, in particular, cottonseed oil, groundnut oil, corn
germ
oil, olive oil, castor oil and sesame oil, glycerol, tetrahydrofurfuryl
alcohol,
polyethyleneglycols, and fatty acid esters of sorbitan or mixtures of these
substances, and the like.
Besides such inert diluents, the composition can also include adjuvants,
such as wetting agents, emulsifying and suspending agents, sweetening,
flavoring,
and perfuming agents.
Suspensions, in addition to the active compounds, may contain suspending
agents, as for example, ethoxylated isostearyl alcohols, polyoxyethylene
sorbitol
and sorbitan esters, microcrystalline cellulose, aluminum metahydroxide,
bentonite, agar-agar and tragacanth, or mixtures of these substances, and the
like.
Compositions for rectal administrations are preferably suppositories which
can be prepared by mixing the compounds of the present invention with suitable
non-irntating excipients or carriers such as cocoa butter, polyethyleneglycol,
or a
29
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
' suppository wax, which are solid at ordinary temperatures but liquid at body
temperature and therefore, melt in the rectum or vaginal cavity and release
the
active component.
Dosage forms for topical administration of a compound of this invention
include ointments, powders, sprays, and inhalants. T'he active component is
admixed under sterile conditions with a physiologically acceptable carrier and
any
preservatives, buffers, or propellants as may be required. Ophthalamic
formulations, eye ointments, powders, and solutions are also contemplated as
being within the scope of this invention.
The compounds of the present method can be administered to a patient at
dosage levels in the range of about 0.1 to about 1000 mg per day. For a normal
human adult having a body weight of about 70 kg, a dosage in the range of
about
0.01 to about 100 mg per kg of body weight per day is preferable. The specific
dosage used, however, can vary. For example, the dosage can depend on a
I S numbers of factors including the requirements of the patient, the severity
of the
condition being treated, and the pharmacological activity of the compound
being
used. The determination of optimum dosages for a particular patient is well-
known to those skilled in the art.
The compounds of the present method can be administered as
pharmaceutically acceptable salts, esters, amides, or prodrugs. The term
"pharmaceutically acceptable salts, esters, amides, and prodrugs" as used
herein
refers to those carboxylate salts, amino acid addition salts, esters, amides,
and
prodrugs of the compounds of the present invention which are, within the scope
of
sound medical judgment, suitable for contact with the tissues of patients
without
undue toxicity, irritation, allergic response, and the like, commensurate with
a
reasonable benefit/risk ratio, and effective for their intended use, as well
as the
zwitterionic forms, where possible, of the compounds of the invention. The
term
"salts" refers to the relatively non-toxic, inorganic and organic acid
addition salts
of compounds of the present invention. These salts can be prepared in situ
during
the final isolation and purification of the compounds or by separately
reacting the
purified compound in its free base form with a suitable organic or inorganic
acid
and isolating the salt thus formed. Representative salts include the
hydrobromide,
hydrochloride, sulfate, bisulfate, nitrate, acetate, oxalate, valerate,
oleate,
CA 02346448 2001-04-06
WO 00135436 PCT/US99/29783
' palmitate, stearate, laurate, borate, benzoate, lactate, phosphate,
tosylate, citrate,
maleate, fumarate, succinate, tartrate, naphthylate, mesylate, glucoheptonate,
lactiobionate and laurylsulphonate salts, and the like. These may include
cations
based on the alkali and alkaline earth metals, such as sodium, lithium,
potassium,
calcium, magnesium and the like, as well as nontoxic ammonium, quaternary
ammonium, and amine cations including, but not limited to ammonium,
tetramethylammonium, tetraethylammonium, methylamine, dimethylamine,
trimethylamine, triethylamine, ethylamine, and the like. (See, for example,
S.M.
Berge, et al., "Pharmaceutical Salts," J. Pharm. Sci., 1977;66:1-19 which is
incorporated herein by reference.)
Examples of pharmaceutically acceptable, non-toxic esters of the
compounds of this invention include C 1-C6 alkyl esters wherein the alkyl
group is
a straight or branched chain. Acceptable esters also include CS-C7 cycloalkyl
esters as well as arylalkyl esters such as, but not limited to benzyl. C 1-C4
alkyl
esters are preferred. Esters of the compounds of the present invention may be
prepared according to conventional methods.
Examples of pharmaceutically acceptable, non-toxic amides of the
compounds of this invention include amides derived from ammonia, primary
C1-C6 alkyl amines and secondary C1-C6 dialkyl amines wherein the alkyl
groups are straight or branched chain. In the case of secondary amines the
amine
may also be in the form of a 5 or 6 membered heterocycle containing one
nitrogen
atom. Amides derived from ammonia, C1-C3 alkyl primary amines and C1-C2
dialkyl secondary amines are preferred. Amides of the compounds of the
invention may be prepared according to conventional methods.
The term "prodrug" refers to compounds that are rapidly transformed
in vivo to yield the parent compound of the above formula, for example, by
hydrolysis in blood. A thorough discussion is provided in T. Higuchi and
V. Stella, "Pro-drugs as Novel Delivery Systems," Vol. 14 of the A.C.S.
Symposium Series, and in Bioreversible Carriers in Drug Design, ed. Edward B.
Roche, American Pharmaceutical Association and Pergamon Press, 1987, both of
which are incorporated herein by reference.
In addition, the compounds of the present method can exist in unsolvated
31
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
' as well as solvated forms with pharmaceutically acceptable solvents such as
water,
ethanol, and the like. In general, the solvated forms are considered
equivalent to
the unsolvated forms for the purposes of the present invention.
Some of the compounds of the present method can exist in different
stereoisometric forms by virtue of the presence of chiral centers. It is
contemplated that all stereoisometric forms of the compounds as well as
mixtures
thereof, including racemic mixtures, form part of this invention.
32
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
C. Synthesis
The examples presented below are intended to illustrate particular
embodiments of the invention and are not intended to limit the scope of the
specification, including the claims, in any way. After the priority date of
the
present disclosure, related syntheses and MEK inhibition data were also
published
in WO 99/01421 and WO 99/01426, hereby incorporated by reference.
The 2-(4-bromo and 4-iodo phenylamino)-benzoic acid derivatives of
Formula (I ) can be prepared from commercially available starting materials
utilizing synthetic methodologies well-known to those skilled in organic
chemistry. A typical synthesis is carried out by reacting a 4-bromo or 4-iodo
aniline with a benzoic acid having a leaving group at the 2-position to give a
2-(phenylamino)-benzoic acid. This process is depicted in Scheme 1.
Scheme 1
O
2 C-OH
Rl
L
/ + I RS
BrorI v\
R3 R4
base
O
I I
~2 C - OH
R1 N
I / I Rs
BrorI v\
R3 R4
where L is a leaving group, for example halo such as fluoro.
The reaction of aniline and the benzoic acid derivative generally is
accomplished by mixing the benzoic acid with an equimolar quantity or excess
of
33
CA 02346448 2001-04-06
WO 00/35436 PCTNS99129783
- the aniline in an unreactive organic solvent such as tetrahydrofuran or
toluene, in
the presence of a base such as lithium diisopropylamide, n-butyl lithium,
sodium
hydride, triethylamine, and Hunig's base. The reaction generally is carried
out at a
temperature of about -78°C to about 100°C, and normally is
complete within
about 2 hours to about 4 days. The product can be isolated by removing the
solvent, for example by evaporation under reduced pressure, and further
purified,
if desired, by standard methods such as chromatography, crystallization, or
distillation.
The 2-(phenylamino)-benzoic acid (e.g., Formula I, where R~ is hydrogen)
can be reacted with an organic or inorganic base such as pyridine,
triethylamine,
calcium carbonate, or sodium hydroxide to produce a pharmaceutically
acceptable
salt. The free acids can also be reacted with an alcohol of the formula HORS
(where R~ is other than hydrogen, for example methyl) to produce the
corresponding ester. Reaction of the benzoic acid with an alcohol can be
carned
out in the presence of a coupling agent. Typical coupling reagents include
2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline (EEDQ),
1,3-dicyclohexylcarbodiimide (DCC), bromo-tris(pyrrolidino)- phosphonium
hexafluorophosphate (PyBrOP), and (benzotriazolyloxy) tripyrrolidino
phosphonium hexafluorophosphate (PyBOP). The phenylamino benzoic acid and
alcohol derivative normally are mixed in approximately equimolar quantities in
an
unreactive organic solvent such as dichloromethane, tetrahydrofuran,
chloroform,
or xylene, and an equimolar quantity of the coupling reagent is added. A base
such
as triethylamine or diisopropylethylamine can be added to act as an acid
scavenger
if desired. The coupling reaction generally is complete after about 10 minutes
to
2 hours, and the product is readily isolated by removing the reaction solvent,
for
instance by evaporation under reduced pressure, and purifying the product by
standard methods such as chromatography or crystallizations from solvents such
as acetone, diethyl ether, or ethanol.
The benzamides of the invention, Formula (I )where Z is CONR6R~, are
readily prepared by reacting the foregoing benzoic acids with an amine of the
formula HNR6R~. The reaction is carried out by reacting approximately
equimolar quantities of the benzoic acid and amine in an unreactive organic
34
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
' solvent in the presence of a coupling reagent. Typical solvents are
chloroform,
dichloromethane, tetrahydrofuran, benzene, toluene, and xylene. Typical
coupling
reagents include DCC, EEDQ, PyBrOP, and PyBOP. The reaction is generally
complete after about 10 minutes to about 2 hours when carried out at a
S temperature of about 0°C to about 60°C. The product amide is
readily isolated by
removing the reaction solvent, for instance by evaporation, and further
purification can be accomplished by normal methods such as chromatography,
crystallization, or distillation. The hydrazides (z = CONHNRIpRI 1) are
similarly
prepared by coupling a benzoic acid with a hydrazine of the formula
H2HNR1 OR11
The benzyl alcohols of the invention, compounds of Formula (I) where
Z is CH20R6 and R6 is hydrogen, are readily prepared by reduction of the
corresponding benzoic acid according to the following Scheme 2.
Scheme 2
O
I I
RI - ~2 C-OH RI ~2 H20H
reducing
RS ~ ~ / ~ RS
Br or I v ~ Br or I
R3 R4 R3 R4
Typical reducing agents commonly employed include borane in tetrahydrofuran.
The reduction normally is carried out in an unreactive organic solvent such as
tetrahydrofuran, and generally is complete within about 2 hours to about 24
hours
when conducted at a temperature of about 0°C to about 40°C.
The following detailed examples illustrate specific compounds provided
by this invention.
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
EXAMPLE 1
4-Fluoro-2-(4-iodo-2-methylnhenylamino)benzoic acid
To a stirring solution comprised of 3.16 g (0.0133 mol) of 2-amino-5-
iodotoluene in S mL of tetrahydrofuran at -78°C was added 10 mL (0.020
mol) of
a 2.0 M lithium diisopropylamide in tetrahydrofuran/heptane/ethenylbenzene
(Aldrich) solution. The resulting green suspension was stirred vigorously for
minutes, after which time a solution of 1.00 g (0.00632 mol) of
2,4-difluorobenzoic acid in 10 mL of tetrahydrofuran was added. The reaction
temperature was allowed to increase slowly to room temperature, at which
10 temperature it was stirred for 2 days. The reaction mixture was
concentrated.
Aqueous HCl (10%) was added to the concentrate, and the solution was extracted
with dichloromethane. The organic phase was dried (MgS04) and then boiled
over a steambath to low volume and cooled to room temperature. The off white
fibers were collected by vacuum filtration, rinsed with hexanes, and vacuum-
oven
15 dried. (76°C; ca. 10 mm of Hg) to afford 1.10 g (47%) of the desired
material;
mp 224-229.5°C;
1 H NMR (400 MHz; DMSO): 8 9.72 (s, 1 H), 7.97 (dd, 1 H, J = 7.0, 8.7 Hz},
7.70
(d, 1 H, J = 1.5 Hz), 7.57 (dd, 1 H, J = 8.4, 1.9 Hz}, 7.17 (d, 1 H, J = 8.2
Hz),
6.61-6.53 (m, 2H), 2.18 (s, 3H);
13C NMR (100 MHz; DMSO): 8 169.87, 167.60, 165.12, 150.17, 150.05, 139.83,
138.49, 136.07, 135.31, 135.20, 135.07, 125.60, 109.32, 105.09, 104.87, 99.72,
99.46, 89.43, 17.52;
19F NMR (376 MHz; DMSO): 8 -104.00 to -104.07 (m);
IR (KBr) 1670 (C = O stretch) cm-1;
MS (CI) M+1 = 372.
Analysis calculated for C 14H 11 F~02
C, 45.31; H, 2.99; N, 3.77.
Found: C, 45.21; H, 2.77; N, 3 .64.
EXAMPLES 2-30
By following the general procedure of Example 1, the following benzoic
acids and salts of Formula (I) were prepared.
36
CA 02346448 2001-04-06
WO 00/35436 PCT/US99I29783
' Example Compound Mp ~C
No.
2 3,4,5-Trifluoro-2-(4-iodo-2-methyl-phenylamino)-206-210
benzoic acid
3 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic240.5-244.5
acid
4 5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-259.5-262
phenylamino)-benzoic acid
S-Chloro-2-(2-chloro-4-iodo-phenylamino')_benzoic255-260
acid
6 S-Chloro-2-(4-iodo-2-methyl-phenylamino}-benzoic234-238
acid
7 Sodium 5-Chloro-2-(4-iodo-2-methyl-phenylamino)-310-320
DEC
benzoate
8 5-Bromo-2-(4-iodo-2-methyl-phenylamino)-benzoic239.5-240
acid
9 2-(2-Chloro-4-iodo-phenylamino~5-nitro-benzoic289-293
acid
4-Fluoro-2-(3-fluoro-4-iodo-2-methyl-phenylamino}-233-235
benzoic acid
11 2-(4-Iodo-2-methyl-phenylamino)-5-nitro-benzoic264-267
acid
12 2-(2-Fluoro-4-iodo-phenylamino)-5-nitro-benzoic256-258
acid
13 2-(4-Bromo-2-methyl-phenylamino)-4-fluoro-benzoic218.5-220
acid
14 ~ 2-(2-Bromo-4-iodo-phenylamino}-5-nitro-benzoic285-288
acid DEC
2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-230-234
benzoic acid
16 3-Fluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic218-221
acid
17 3,4-Difluoro-2-(4-iodo-2-methoxy-phenylamino)-230-233
benzoic acid
18 4-Chloro-2-(4-iodo-2-methyl-phenylamino)-benzoic245-255
acid DEC
37
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound Mp C
No.
19 2-(4-Iodo-2-methyl-phenylamino)-benzoic218-223
acid
20 5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic243-46
acid
21 5-lodo-2-(4-iodo-2-methyl-phenylamino)-benzoic241-245
acid
22 2,3,5-Trifluoro-4-(4-iodo-2-methyl-phenylamino)-218-222
benzoic acid
23 4-Fluoro-2-(3-chloro-4-iodo-2-methyl-phenylamino)-248-252.5
benzoic acid
24 2-(4-Iodo-phenylamino)-5-methoxy-benzoic208-211
acid
25 3-Chloro-2-(2-chloro-4-iodo-phenylamino)-benzoic232-233
acid
26 2-Fluoro-6-(4-iodo-2-methyl-phenylamino)-benzoic179-182
acid
27 4-Fluoro2-(2,3-dimethyl-4-iodo-2-methyl-258-261
phenylamino)benzoic acid
28 5-Methyl-2-(4-iodo-2-methyl-phenylamino)-benzoic209.5-211
acid
29 2-Chloro-6-(4-iodo-2-methyl-phenylamino)-benzoic acid 171-175
30 2-(4-lodo-2-methyl-phenylamino)-4-nitro-benzoic acid 251-263
EXAMPLE 31
5-Chloro-N-(2-h~yeth~l-~4-iodo-2-methyl-phenylaminol-benzamide
To a stirring solution comprised of 0.1020 g (0.2632 mmol) of 5-chloro-
2-(4-iodo-2-methyl-phenylamino)-benzoic acid, 0.1 mL (1.7 mmol) of
ethanolamine, and 0.05 mL (0.29 mmol) of diisopropylethylamine in 5 mL of a
1:1 (v/v) tetrahydrofuran-dichloromethane solution was added 0.15 g (0.29
mmol)
of solid PyBOP powder directly. The reaction mixture was stirred at room
temperature overnight. The solvent was removed in vacuo. The crude residue was
partitioned between ether (50 mL) and 10% aqueous hydrochloric acid (50 mL).
The organic phase was washed with 10% aqueous sodium hydroxide (50 mL),
dried (MgS04) and concentrated in vacuo to afford a yellow-brown oil which was
crystallized from hexanes-ether to afford 0.0831 g (73%) of a green-yellow
powder; mp 120-121°C;
38
CA 02346448 2001-04-06
WO 00/35436 PC'T/US99/29783
1 H NMR (400 MHz; CDCl3): 8 9.11 (s, 1 H), 7.56 (d, 1 H, J = 1.4 Hz), 7.46-
7.41
(m, 2H), 7.20 (dd, 1H, J = 8.9, 2.4 Hz), 7.00 (t, 2H, J = 9.6 Hz), 6.55 (broad
t,
1H), 3.86 (t, 2H, J = 5.0 Hz), 3.61 (dd, 2H, J = 10.1, 5.5 Hz), 2.23 (s, 3H),
1.56
(broad s, 1 H);
IR (KBr) 3297 (O-H stretch), 1627 (C = O stretch) cm-1;
MS (CI) M+1 = 431.
Analysis calculated for C16H16C1~202~
C, 44.62; H, 3.74; N, 6.50.
Found: 44.63; H, 3.67; N, 6.30.
EXAMPLES 32-48
By following the general procedure of Example 31, the following
benzamides were prepared by reacting the corresponding benzoic acid with the
corresponding amine.
Example Compound MP C
No.
32 4-Methoxy-N-(4-methoxy-phenyl)-3-nitro-153.5-156
benzamide
33 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-158
benzamide
34 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-102.5-104.5
methyl-benzamide
35 N-Ethyl-4-fluoro-2-(4-iodo-2-methyl-phenylamino)-90-91
benzamide
36 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N,N-oil
dimethyl-benzamide
37 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(1H-285-288
DEC
tetrazol-S-yl)-benzamide
38 5-Bromo-2-(4-iodo-2-methyl-phenylamino)-180-182
benzamide
39 5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N,N-137-138
dimethyl-benzamide
39
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
' Example Compound MP °C
No.
40 [5-Chloro-2-(4-iodo-2-methyl-phenylamino~ 170-173
benzoylamino]-acetic acid
41 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N- 69-71
propyl-benzamide
42 5-Bromo-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-132-133.4
phenylamino)-benzamide
43 N,N-Diethyl-4-fluoro-2-(4-iodo-2-methyl-oil
phenylamino)-benzamide
44 4-Fluoro-N-{3-[4-(2-hydroxy-ethyl)-piperazin-1-yl]-122-124
propyl}-2-(4-iodo-2-methyl-phenylamino)-
benzamide
45 N,N-Diethyl-2-(4-iodo-2-methyl-phenylamino)-5-91-93
nitro-benzamide
46 N-Butyl-4-fluoro-2-(4-iodo-2-methyl-phenylamino)-97-99
benzamide
47 5-Chloro-N,N-diethyl-2-(4-iodo-2-methyl-118-120
phenylamino)-benzamide
4$ 5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N,N-142.5-144
dimethyl-benzamide
EXAMPLE 49
4-Fluoro-2-(4-iodo-2-meth ~~l-phenylamino)-benzyl alcohol
4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid (0.50 g,
1.35 mmol) was dissolved in 6 mL (6 mmol) of cold 1.0 M borane-
tetrahydrofuran complex in tetrahydrofuran solution. The reaction mixture was
stirred under nitrogen atmosphere at room temperature overnight. The reaction
was quenched with 80 mL of methanol. Concentration in vacuo produced a clear
tan oil which was purified by MPLC. Elution with dichloromethane afforded
0.4285 g (89%) of a white solid; mp 99-100.5°C;
1 H NMR (400 MHz; DMSO): 8 7.57 (d, 1 H, J=1.7 Hz), 7.45 (dd, 1 H, J=8.4,
1.9 Hz), 7.39 (s, 1 H), 7.29 (t, 1 H, J=7.5 Hz), 6.89 (d, 1 H, J=8.4 Hz), 6.67-
6.60 (m,
1 H), 5.47 (t, 1 H, J=5.5 Hz), 4.49 (d, 2H, 5.1 Hz), 2.14 (s, 3 H);
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
IR (KBr) 3372 (O-H stretch) cm-1;
MS (CI) M+1 = 358.
Analysis calculated for C14H13FIN0:
C, 47.08; H, 3.67; N, 3.92.
Found: C, 47.17; H, 3.75; N, 3.72.
EXAMPLE 50-52
The following benzyl alcohols were prepared by the general procedure of
Example 49.
Example No. Compound MP °C
50 [5-Chloro-2-(4-iodo-2-methyl-phenylamino)- 82-85
phenyl]-methanol
51 [2-(4-Iodo-2-methyl-phenylamino)-5-nitro-phenyl]- 126.5-128.5
methanol
52 [5-Bromo-2-(4-iodo-2-methyl-phenylamino)- 60.5-63.5
phenyl]-methanol
Several invention compounds of Formula (I) were prepared utilizing
combinatorial synthetic techniques. The general procedure is as follows:
To a 0.8-mL autosampler vial in a metal block was added 40 u.L, of a
0.5 M solution of the acid in DMF and 40 ~L of the reagent amine {2 M solution
in Hunig's base and 1 M in amine in DMF). A 0.5 M solution of PyBrop was
freshly prepared and 50 ~L were added to the autosampler vial. The reaction
was
allowed to stand for 24 hours.
The reaction mixture was transferred to a 2-dram vial and diluted with
2 mL of ethyl acetate. The organic layer was washed with 3 mL of distilled
water
and the water layer washed again with 2 mL of ethyl acetate. The combined
organic layers were allowed to evaporate to dryness in an open fume hood.
The residue was taken up in 2 mL of SO% acetonitrile in water and injected
on a semi-prep reversed phase column (10 mm x 25 cm, S N.M spherical silica,
pore size 115 A derivatized with C-18, the sample was eluted at 4.7 mL/min
with
41
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
a linear ramp to 100% acetonitrile over 8.5 minutes. Elution with 100%
acetonitrile continued for 8 minutes}. Fractions were collected by monitoring
at
214 nM. The residue was dissolved in chloroform and transferred to a
preweighed
vial, evaporated, and weighed again to determine the yield.
EXAMPLES 53-206
The following compounds of Formula I were prepared by combinatorial
methodology:
Example Compound MS
No. M-H
53 5-Bromo-3,4-difluoro-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-510
phenylamino)-benzamide
54 N-(2,3-Dihydroxy-propyl)-3,4-difluoro-2-(4-iodo-2-methyl-462
phenylamino)-benzamide
55 5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-577
piperidin-I-yl-ethyl)-benzamide
56 3,4-Difluoro-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-432
phenylamino)-benzamide
57 N-(2,3-Dihydroxy-propyl~4-fluoro-2-(4-iodo-2-methyl-444
phenylamino)-benzamide
58 3,4-Difluoro-N-(3-hydroxy-propyl)-2-(4-iodo-2-methyl-446
phenylamino)-benzamide
59 S-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-564
(2-pyrrolidin-1-yl-ethyl)-benzamide
60 S-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-571
(2-pyridin-4-yl-ethyl)-benzamide
61 4-Fluoro-N-(2-hydroxy-ethyl~2-(4-iodo-2-methyl-phenylamino)-414
benzamide
62 5-Bromo-N-(3-dimethylamino-propyl)-3,4-difluoro-2-(4-iodo-551
2-methyl-phenylamino)-benzamide
42
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound MS
No. M-H
63 5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-580
(2-morpholin-4-yl-ethyl)-benzamide
64 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-morpholin-501
4-yl-ethyl)-benzamide
65 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino~N-(2-pyrrolidin-485
I -yl-ethyl)-benzamide
66 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyridin-4-yl-493
ethyl)-benzamide
67 N-(3-Dimethylamino-propyl)-3,4-difluoro-2-(4-iodo-2-methyl-473
phenylamino)-benzamide
68 N-Benzyl-4-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide460
69 2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(2-hydroxy-384
ethyl~benzamide
70 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-morpholin-4-yl-483
ethyl~benzamide
71 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-piperidin-1-yl-495
propyl)-benzamide
72 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-piperidin-513
1-yl-propyl)-benzamide
73 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-thiophen-2-yl-480
ethyl)-benzamide
74 4-Fluoro-2-(4-iodo-2-methyl-phenylamino~N-(2-pyrrolidin-1-yl-467
ethyl)-benzamide
75 2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(2-morpholin-453
4-yl-ethyl)-benzamide
76 5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-N-557
pyridin-4-ylmethyl-benzamide
77 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-N-pyridin-479
4-ylmethyl-benzamide
78 2-(4-Bromo-2-methyl-phenylamino)-N-(3-dimethylamino-propyl)-425
3,4-difluoro-benzamide
43
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound MS
No. M-H
79 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-pyridin-4-ylmethyl- 461
benzamide
80 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyridin-4-yl-475
ethyl)-benzamide
81 2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(2-pyridin-445
4-yl-ethyl)-benzamide
82 2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(3-hydroxy-400
propyl)-benzamide
83 2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(2-pyrrolidin-437
1-yl-ethyl)-benzamide
84 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-phenethyl-474
benzamide
85 2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(2-thiophen-450
2-yl-ethyl)-benzamide
86 2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-pyridin-431
4-ylmethyl-benzamide
87 2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-phenethyl-444
.
benzamide
88 2-(4-Bromo-2-methyl-phenylamino)-3,4-difluoro-N-(2-piperidin-451
1-yl-ethyl)-benzamide
89 5-Chloro-N-{3-[4-(2-hydroxy-ethyl~piperazin-1-yl]-propyl}-557*
2-(4-iodo-2-methyl- phenylamino)- benzamide
90 5-Fluoro-N-{3-[4-(2-hydroxy-ethyl)-piperazin-1-yl]-propyl}-541
2-(4-iodo-2-methyl- phenylamino)- benzamide
91 2-(4-Iodo-2-methyl-phenylamino)-S-vitro-N-pyridin-4-yl487
methyl-
benzamide
92 5-Bromo-N-{3-[4-(2-hydroxy-ethyl)-piperazin-1-yl]-propyl}-601
2-(4-iodo-2-methyl- phenylamino)- benzamide
93 5-Chloro-N-(2-diethylamino-ethyl)-2-(4-iodo-2-methyl-486*
phenylamino)- benzamide
44
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29~83
Example Compound MS
No. M-H
94 5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-(2-piperidin-1-yl- 497*
ethyl~benzamide
95 (3-Hydroxy-pyrrolidin-1-yl)-[2-(4-iodo-2-methyl-phenylamino)-466
5-nitro-phenyl]-methanone
96 5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyrrolidin-1-yl-484*
ethyl)-benzamide
97 5-Bromo-N-(2-diethylamino-ethyl)-2-(4-iodo-2-methyl-530*
phenylamino)- benzamide
98 N-{2-[Bis-(2-hydroxy-ethyl)-amino]-ethyl}-5-chloro-2-(4-iodo-518*
2-methyl- phenylamino}- benzamide
99 N-{2-[Bis-(2-hydroxy-ethyl)-amino]-ethyl}-5-bromo-2-(4-iodo-562*
2-methyl- phenylamino)- benzamide
100 [5-Bromo-2-(4-iodo-2-methyl-phenylamino)-phenyl]-(3-hydroxy-499
pyrrolidin-1-yl)-methanone
101 2-(4-Iodo-2-methyl-phenylamino)-5-nitro-benzoic501
acid phenethyl
ester
102. N-{3-[4-(2-Hydroxy-ethyl)-piperazin-l-yl]-propyl}-2-(4-iodo-568*
2-methyl-phenylamino)- benzamide
103 [5-Chloro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-(3-hydroxy-455
pyrrolidin-1-yl)-methanone
104 5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-pyridin-4-ylmethyl-460
benzamide
105 S-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyrrolidin-I-yl-528*
ethyl)-benzamide
106 5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(2-piperidin-1-yl-542*
ethyl~benzamide
107 5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-pyrrolidin-1-yl-468*
ethyl)-benzamide
108 5-Chloro-N-(3-dimethylamino-propyl)-2-(4-iodo-2-methyl-472*
phenylamino)-benzamide
109 N-{2-[Bis-(2-hydroxy-ethyl)-amino]-ethyl}-5-fluoro-2-(4-iodo-502*
2-methyl- phenylamino)- benzamide
45
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound MS
No. M-H
110 5-Chloro-N-(3-hydroxy-propyl)-2-(4-iodo-2-methyl-445*
phenylamino)-benzamide
111 S-Chloro-N-(3-diethylamino-2-hydroxy-propyl)-2-(4-iodo-516*
2-methyl-phenylamino)- benzamide
112 5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-piperidin-1-yl-482*
ethyl~benzamide
113 5-Bromo-N-(3-hydroxy-propyl)-2-(4-iodo-2-methyl-489*
phenylamino)-benzamide
114 5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(3-piperidin-1-yl-556*
propyl)-benzamide
115 N-{2-[Bis-(2-hydroxy-ethyl)-aminoJ-ethyl}-2-(4-iodo-2-methyl-529*
phenylamino)-5-vitro- benzamide
116 5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-(2-morpholin-4-yl-500*
ethyl)-benzamide
117 5-Chloro-N-(3-diethylamino-propyl)-2-(4-iodo-2-methyl-500*
phenylamino)-benzamide
118 5-Chloro-N-(2-diisopropylamino-ethyl)-2-(4-iodo-2-methyl-514*
_
phenylamino)-benzamide
119 5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-(3-piperidin-1-yl-512*
propyl)-benzamide
120 2-(4-Iodo-2-methyl-phenylamino)-5-vitro-N-(2-piperidin-1-yl-509*
ethyl)-benzamide
121 5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(2-piperazin-1-yl-544*
ethyl)-benzamide
122 N-(2-Diethylamino-ethyl)-5-fluoro-2-(4-iodo-2-methyl-470*
phenylamino)-benzamide
123 5-Bromo-N-(3-dimethylamino-propyl)-2-(4-iodo-2-methyl-516*
phenylamino)-benzamide
124 N-(3-Hydroxy-propyl)-2-(4-iodo-2-methyl-phenylamino)-5-vitro-456*
benzamide
125 5-Fluoro-N-(3-hydroxy-propylr2-(4-iodo-2-methyl-429*
phenylamino)-benzamide
46
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound MS
No. M-H
12b N-(3-Diethylamino-propyl)-5-fluoro-2-(4-iodo-2-methyl-484*
phenylamino)-benzamide
127 N-(3-Diethylamino-propyl)-2-(4-iodo-2-methyl-phenylamino)-511
5-nitro-benzamide
128 5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(2-morpholin-4-yl-544*
ethyl)-benzamide
129 2-(4-Iodo-2-methyl-phenylamino)-5-nitro-N-(3-piperidin-1-yl-523*
propyl)-benzamide
130 [5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-(3-hydroxy-439
pyrrolidin-1-yl)-methanone
131 S-Bromo-N-(2-diisopropylamino-ethyl)-2-(4-iodo-2-methyl-558*
phenylamino)-benzamide
132 5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(2-morpholin-4-yl-484*
ethyl)-benzamide
133 5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-piperidin-1-yl-496*
propyl)-benzamide
134. [5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-482
[4-(2-hydroxy-ethyl)-piperazin-1-
135 N-(3-Diethylamino-2-hydroxy-propyl)-5-fluoro-2-(4-iodo-500*
2-methyl-phenylamino)- benzamide
136 [5-Chloro-2-(4-iodo-2-methyl-phenylamino)-benzoylamino]-443
acetic acid
137 2-(4-Iodo-2-methyl-phenylamino)-5-nitro-N-(2-pyrrolidin-1-yl-495*
ethyl)-benzamide
138 N-(3-Dimethylamino-propyl)-2-(4-iodo-2-methyl-phenylamino)-483*
5-vitro-benzamide
139 N-(2-Diisopropylamino-ethyl)-S-fluoro-2-(4-iodo-2-methyl-498*
phenylamino)- benzamide
140 5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-thiobenzoic490
acid S-
phenethyl ester
141 5-Chloro-2-(4-iodo-2-methyl-phenylamino)-thiobenzoic506
acid S-
phenethyl ester
47
CA 02346448 2001-04-06
WO 00/35436 PCTNS99/29783
Example Compound MS
No. M-H
142 S-Bromo-2-(4-iodo-2-methyl-phenylamino)-thiobenzoic536
acid S-
benzyl ester
143 2-(4-Iodo-2-methyl-phenylamino)-5-nitro-thiobenzoic503
acid S-
benzyl ester
144 S-Fluoro-2-(4-iodo-2-methyl-phenylamino)-thiobenzoic476
acid S-
benzyl ester
145 S-Chloro-2-(4-iodo-2-methyl-phenylamino)-thiobenzoic492
acid S-
benzyl ester
146 N-Cyclopropyl-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-409
benzamide
147 S-Chloro-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-429
benzamide
148 5-Fluoro-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenyiamino)-413
benzamide
149 N-Benzyloxy-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-475
benzamide
150 N-Benzyloxy-5-bromo-2-(4-iodo-2-methyl-phenylamino)-593*
benzamide
151 2-(4-Iodo-2-methyl-phenylamino)-5-nitro-N-(4-sulfamoyl-567
benzyl)-benzamide
152 S-Bromo-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-473
benzamide
153 N-(2-Hydroxy-ethyl)-5-iodo-2-(4-iodo-2-methyl-phenylamino)-521
benzamide
48
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound MS
No. M-H
154 N-(2-Hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-5-nitro-440
benzamide
155 2-(4-Iodo-2-methyl-phenylamino)-N-methyl-5-nitro-N-phenyl-486
benzamide
156 5-Chloro-N-cyclopropyl-2-(4-iodo-2-methyl-phenylamino)-425
benzamide
157 5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-459
benzamide
158 N-Allyl-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide409
159 N-Benzyloxy-5-iodo-2-(4-iodo-2-methyl-phenylamino)-583
benzamide
160 5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-{4-sulfamoyl-538
benzyl)-benzamide
161 N-Allyl-S-chloro-2-(4-iodo-2-methyl-phenylamino)-benzamide425
162 N-Cyclopropyl-2-(4-iodo-2-methyl-phenylamino)-5-nitro-436
benzamide
163 5-Bromo-N-cyclopropyl-2-(4-iodo-2-methyl-phenylamino)-469
benzamide
164 S-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-475
benzamide
165 5-Iodo-2-(4-iodo-2-methyl-phenylamino)-N-(4-sulfamoyl-benzyl)-646
benzamide
166 5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(4-sulfamoyl-598
benzyl)-benzamide
167 N-Allyl-2-(4-iodo-2-methyl-phenylamino)-5-nitro-benzamide436
49
CA 02346448 2001-04-06
WO 00/35436 PCTNS99/29?83
Example Compound MS
No. M-H
168 2-(4-Iodo-2-methyl-phenylamino)-5-nitro-N-(4-sulfamoyl-565
benzyl)-benzamide
169 N-Allyl-S-bromo-2-(4-iodo-2-methyl-phenylamino)-benzamide469
170 S-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(3-methyl-benzyl)-473
benzamide
I71 N-Cyclopropyl-5-iodo-2-(4-iodo-2-methyl-phenylamino)-517
benzamide
172 5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-519
benzamide
173 N-Benzyloxy-2-(4-iodo-2-methyl-phenylamino)-5-nitro-502
benzamide
174 N-Cyclohexyl-5-iodo-2-(4-iodo-2-methyl-phenylamino)-559
benzamide
175 N-Allyl-5-iodo-2-(4-iodo-2-methyl-phenylamino)-benzamideS
17
176 5-Iodo-2-(4-iodo-2-methyl-phenylamino)-N-(3-methyl-benzyl)-581
benzamide
177 2-(4-Iodo-2-methyl-phenylamino)-N-(3-methyl-benzyl)-5-nitro-500
benzamide
178 5-Iodo-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-567
benzamide
179 N-Cyclohexyl-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-451
benzamide
180 5-Chloro-N-cyclohexyl-2-(4-iodo-2-methyl-phenylamino)-467
benzamide
181 5-Bromo-2-(4-iodo-2-methyl-phenylarnino)-N-(3-methyl-benzyl)-533
benzamide
182 5-Bromo-N-cyclohexyl-2-(4-iodo-2-methyl-phenylamino)-511
benzamide
183 5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-(3-methyl-benzyl)-489
benzamide
184 N-Cyclohexyl-2-(4-iodo-2-methyl-phenylamino)-5-nitro-478
benzamide
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound MS
No. M-H
185 N-Benzyloxy-5-bromo-2-(4-iodo-2-methyl-phenylamino)-538
benzamide
186 N-Benzyloxy-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-477
benzamide
187 5-Chloro-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-431
benzamide
188 5-Bromo-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-475
benzamide
189 2-(4-Iodo-2-methyl-phenylamino~N-methyl-5-vitro-N-phenyl=488
benzamide
190 5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-477
benzamide
191 N-(2-Hydroxy-ethyl)-5-iodo-2-(4-iodo-2-methyl-phenylamino)-523
benzamide
192 5-Chloro-N-cyclopropyl-2-(4-iodo-2-methyl-phenylamino)-425
benzamide
193 N-AIly1-5-chloro-2-(4-iodo-2-methyl-phenylamino)-benzamide427
194 5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl-461
benzamide
195 N-(2-Hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-S-vitro-442
benzamide
196 5-Fluoro-N-(2-hydroxy-ethyl)-2-(4-iodo-2-methyl-phenylamino)-41
S
benzamide
197 5-Bromo-N-cyclopropyl-2-(4-iodo-2-methyl-phenylamino)-472
benzamide
198 N-Cyclopropyl-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-411
benzamide
199 5-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N-(4-sulfamoyl-540
benzyl)-benzamide
200 N-Cyclopropyl-2-(4-iodo-2-methyl-phenylamino)-5-vitro-438
benzamide
201 N-Allyl-5-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzamide411
Sl
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound MS
No. M-H
202 N-Benzyloxy-5-iodo-2-(4-iodo-2-methyl-phenylamino)- 585
benzamide
203 N-Allyl-5-bromo-2-(4-iodo-2-methyl-phenylamino)-benzamide 472
204 5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-(4-sulfamoyl- 601
benzyl)-benzamide
205 5-Bromo-2-(4-iodo-2-methyl-phenylamino)-N-methyl-N-phenyl- 522
benzamide
206 N-Allyl-2-(4-iodo-2-methyl-phenylamino)-5-nitro-benzamide 438
* M+H
EXAMPLE 207
Preparation of [4-Chloro-2-( 1 H-tetrazol-5-yl)-(4-iodo-2-methyl-phenyl)-amine
Step a: Preparation of 5-chloro-2-fluoro-benzaldehyde
To a solution of 1-chloro-4-fluorobenzne (13.06 g, 0.1 mol) in THF
(180 mL), at -78°C, LDA (2M solution in THF, 50 mL, 0.1 mol) was added
drop
wise. After stirring at -78°C for 1.5 hours, DMF (8 mL) was added to
the reaction
mixture and allowed to warm up to room temperature overnight. The reaction
mixture was partitioned between water and Et20. The Et20 layer was dried
(MgS04) and the solvent removed in vacuum to give 14.95 g (94%) yield of
crude aldehyde: 1H NMR (CDC13): 8, 10.3 (s, -C(=O)H).
Step b: Preparation of 5-chloro-2-fluoro-benzaldehyde oxime
A solution of 5-chloro-2-fluoro-benzaldehyde (10 g, 0.0631 mol),
hydroxylamine hydrochloride (6.57 g, 0.0946 mol) and pyridine (8.3 mL,
0.1010 moI) in EtOH (100 mL) was heated at 75°C (oil bath temperature)
for
1 hour and the solvent removed under vacuum to give an oil. The oil was
partitioned between water and CH2C12. The CH2C12 layer was dried (MgS04)
and the solvent removed under vacuum to give crude aldoxime as a solid. The
solid was purified by medium pressure liquid chromatography on silica. Elution
52
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
with CH2Cl2 gave 4.87 g (28%) of the aldoxime as white solid: mp 95-
97°C;
Analysis calculated for C7HSNOFCI:
C, 48.44; H, 2.90; N, 8.07.
Found: C, 48.55; H, 2.69, N, 7.90.
Step c: Preparation of 5-chloro-2-fluoro-benzonirile
A solution of the 5-chlaro-2-fluoro-benzaldehyde oxime (3.15 g,
0.0182 mol) in acetic anhydride (150 mL) was refluxed for 16 hours. The
reaction
mixture was cooled to room temperature and poured into saturated aqueous
NaHC03 (200 mL) solution. The mixture was extracted with Et20. The Et20
layer was dried (K2C03) and the solvent removed to give the product as an oily
solid. The product was used without further purification in the next step.
Step d: Preparation of 5-(5-chloro-2-fluoro-phenyl)-1H-tetrazole
A mixture of 5-chloro-2-fluoro-benzonitrile (2.84 g, 0.01823 mol), butanol
(15 mL), sodium azide (1.543 g, 0.0237 mol), acetic acid (1.36 mL, 0.0237 mol)
was refluxed for 24 hours. The reaction mixture was cooled to room
temperature,
additional 1.543 g sodium azide added, and the reaction mixture refluxed for
additional 24 hours. After cooling to room temperature, Et20 (100 mL) and 10%
aqueous NaOH (200 mL) were added sequentially. The mixture was vigorously
stirred. The aqueous layer was separated, cooled with ice-methanol bath (-
15°C)
and acidified to pH 1 with conc. HCI. A gray solid precipitated. The solid was
dried in vacuum at 50°C to give 1.76 g (49%) of 5-(5-chloro-2-fluoro-
phenyl)-1H-
tetrazole: mp partial melt at 110°C, complete melting at 124°C);
1 H (400 Mz, CDCl3): 8 8.19-8.08 (m, 1 H), 7.77-7.71 (m, 1 H), 7.61-7.52 (m, 1
H);
13C (100 Mz, CDCl3): b 159.00, 156.49, 140.88, 133.02, 132.93, 130.73, 129.23,
129.21, 129.08, 126.05, 118.96, 118.73, 114.50;
MS (CI) M+1 = 199 (100), M = 198 (6).
53
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Step e: Preparation of [4-Chloro-2-( 1 H-tetrazol-5-~)-(4-iodo-2-methyl-
phenyl)-
amine
To a solution of 2-methyl-4-iodoaniline (3.52 g, 0.01 S 1 mol) in THF
(25 mL) at -78°C, LDA (2 molar solution in THF, 11.33 mL, 0.02267 mol)
was
added dropwise. After stirring for 0.5 hours, a solution of 1-(tetrazol-5-yl)-
2-
fluoro-5-chlorobenzene (1.5 g, 0.00756 mol) in THF (15 mL) was added
dropwise. The reaction was stirred for 16 hours as it warmed up to room
temperature. The reaction mixture was quenched with aqueous conc. NH4C1
solution and extracted with CH2C12. The organic layer was dried (MgS04) and
the solvent removed giving a crude product as an oil. The oil with CH2C12-
>CH2C12:MeOH (9.7:0.3) gave 1.5 g (48%) of the desired product:
mp 205-208°C; 1 H (400 Mz, DMSO): 8 9.13 (s, 1 H), 8.00-7.99 (s, 1 H),
?.69 (s,
1 H), 7.55-7.52 (m, 1 H), 7.43-7.40 (m, 1 H), 7.12-7.05 (m, 1 H), 2.24 (s,
3H);
13C (100 Mz, CDC13): 8 141.87, 139.28, 138.88, 135.47, 133.71, 131.65, 128.15,
1 S 123.69, 121.94, 116.68, 87.79, 17.22; MS (CI) M+2 = 413 (44), M+1 = 412
(85),
M = 411 ( 100).
Analysis calculated for C14H11NSC1hO.SH20:
C, 39.97; H, 2.87; N, 16.65.
Found: C, 38.87, H, 2.77; N, 16.47.
The following tetrazole substituted phenylamines were prepared by
following the general procedure of Example 207.
EXAMPLE 208
!4-iodo-2-methyl-phen~j2~lH-tetrazol-5-yl)-phenyl]amine, mp 231°C (dec)
EXAMPLE 209
L4-vitro-2-(1H-tetrazol-5-~)-(4-iodo-2-methyl-phenyl -amine, mp 205-
208°C.
The 4-bromo and 4-iodo phenylamino benzhydroxamic acid derivatives of
Formula II can be prepared from commercially available starting materials
utilizing synthetic methodologies well-known to those skilled in organic
54
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29?83
chemistry. A typical synthesis is carried out by reacting a 4-bromo or 4-iodo
aniline with a benzoic acid having a leaving group at the 2-position to give a
phenylamino benzoic acid, and then reacting the benzoic acid phenylamino
derivative with a hydroxylamine derivative (Scheme 3).
Scheme 3
O
~2a C-OH
Rla ~ NH
/ + ~ RSa
Br or I
R3a R4a
base
O
I I
~2a C-OH
Rla
N
/ ~ RSa
Br or I
R3a R4a
~6a
HN O R7a
~6a
~2a C-N-O- Rya
Rla
N
RSa
BrorI
R3a R4a
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
where L is a leaving group, for example halo such as fluoro, chloro, bromo or
iodo, or an activated hydroxy group such as a diethylphosphate,
trimethylsilyloxy,
p-nitrophenoxy, or phenylsulfonoxy.
The reaction of aniline and the benzoic acid derivative generally is
accomplished by mixing the benzoic acid with an equimolar quantity or excess
of
the aniline in an unreactive organic solvent such as tetrahydrofuran, or
toluene, in
the presence of a base such as lithium diisopropylamide, n-butyl lithium,
sodium
hydride, and sodium amide. The reaction generally is carried out at a
temperature
of about -78°C to about 25°C, and normally is complete within
about 2 hours to
about 4 days. The product can be isolated by removing the solvent, for example
by
evaporation under reduced pressure, and further purified, if desired, by
standard
methods such as chromatography, crystallization, or distillation.
The phenylamino benzoic acid next is reacted with a hydroxylamine
derivative HNR6aOR~a in the presence of a peptide coupling reagent.
Hydroxylamine derivatives that can be employed include methoxylamine,
N-ethyl-isopropoxy amine, and tetrahydro-oxazine. Typical coupling reagents
include 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline (EEDQ),
1,3-dicyclohexylcarbodiimide (DCC), bromo-tris(pyrrolidino)-phosphonium
hexafluorophosphate (PyBrOP) and (benzotriazolyloxy)tripyrrolidino
phosphonium hexafluorophosphate (PyBOP). The phenylamino benzoic acid and
hydroxylamino derivative normally are mixed in approximately equimolar
quantities in an unreactive organic solvent such as dichloromethane,
tetrahydrofuran, chloroform, or xylene, and an equimolar quantity of the
coupling
reagent is added. A base such as triethylamine or diisopropylethylamine can be
added to act as an acid scavenger if desired. The coupling reaction generally
is
complete after about 10 minutes to 2 hours, and the product is readily
isolated by
removing the reaction solvent, for instance by evaporation under reduced
pressure,
and purifying the product by standard methods such as chromatography or
crystallizations from solvents such as acetone, diethyl ether, or ethanol.
An alternative method for making the invention compounds involves first
converting a benzoic acid to a hydroxamic acid derivative, and then reacting
the
56
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
hydroxamic acid derivative with an aniline. This synthetic sequence is
depicted in
Scheme 4
Scheme 4
O ~ ~6a
C-OH ~6a C-N-O-R7a
L \ HN-O- R7a L I \
Rsa RSa
R3a 'R4a R3a R4a
NHR2
\
Rla
Br or I
~6a
~2a C-N-O- R7a
Rla
\ N \
/ ~ RSa
Br or I
R3a R4a
where L is a leaving group. The general reaction conditions for both of the
steps in
Scheme 4 are the same as those described above for Scheme 3.
Yet another method for making invention compounds comprises reacting a
phenylamino benzhydroxamic acid with an ester forming group as depicted in
Scheme 5.
57
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Scheme S
~6a
~2a C-N-OH
Rla
N
RSa + L - R7a
Br or I
R3a R4a
base
~6a
~2a C-N-O- Rya
Rla
N
RSa
Br or I
R3a R4a
where L is a leaving group such as halo, and a base is triethylamine or
diisopropylarnine.
The synthesis of compounds of Formula (II) is further illustrated by the
following detailed examples.
EXAMPLE 1 a
4-Fluoro-N-hydroxy-2-(4-iodo-2-methyl-phenylaminol-benzamide
(a) Preparation of 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid
To a stirred solution containing 3.16 g {0.0133 mol) of 2-amino-
5-iodotoluene in 5 mL of tetrahydrofi~ran at -78°C was added 10 mL
(0.020 mol)
of a 2.0 M lithium diisopropylamide in tetrahydrofuran/heptane/ethylbenzene
(Aldrich) solution. The resulting green suspension was stirred vigorously for
1 S minutes, after which time a solution of 1.00 g (0.00632 mol) of
2,4-difluorobenzoic acid in 10 mL of tetrahydrofuran was added. The reaction
temperature was allowed to increase slowly to room temperature, at which
58
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
temperature the mixture was stirred for 2 days. The reaction mixture was
concentrated by evaporation of the solvent under reduced pressure. Aqueous HCl
(10%) was added to the concentrate, and the solution was extracted with
dichloromethane. The organic phase was dried (MgS04) and then concentrated
over a steambath to low volume (10 mL) and cooled to room temperature. The
off white fibers which formed were collected by vacuum filtration, rinsed with
hexane, and dried in a vacuum-oven (76°C; ca. 10 mm of Hg) to afford
1.10 g
(47%) of the desired material; mp 224-229.5°C;
1H NMR (400 MHz, DMSO): S 9.72 (s, 1H), 7.97 (dd, 1H, J=7.0, 8.7 Hz),
7.70 (d, 1 H, J=1.5 Hz), 7.57 (dd, 1 H, J=8.4, 1.9 Hz), 7.17 (d, 1 H, J=8.2
Hz),
6.61-6.53 (m, 2H), 2.18 (s, 3H);
13C NMR (100 MHz, DMSO): 8 169.87, 166.36 (d, JC_F=249.4 Hz), 150.11 (d,
JC-F=11.4 Hz), 139.83, 138.49, 136.07, 135.26 (d, JC-F=11.5 Hz), 135.07,
125.60, 109.32, 104.98 (d, JC_p=21.1 Hz), 99.54 (d, JC_F=26.0 Hz), 89.43,
17.52;
19F NMR (376 MHz, DMSO): 8 -104.00 to -104.07 (m);
IR (KBr) 1670 (C=O stretch)cm-1;
MS (CI) M+1 = 372.
Analysis calculated for C 14H 11 F~02
C, 45.31; H, 2.99; N, 3.77.
Found: C, 45.21; H, 2.77; N, 3.64.
(b) Preparation of 4-Fluoro-N-hvdroxy-2~4-iodo-2-methyl-phenylamino)-
benzamide
To a stirred solution of 4-fluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic
acid (0.6495 g, 0.001750 mol), O-(tetrahydro-2H-pyran-2-yl)-hydroxylamine
(0.2590 g, 0.002211 mol), and diisopropylethylamine (0.40 mL, 0.0023 mol) in
31 mL of an equivolume tetrahydrofuran-dichloromethane solution was added
1.18 g (0.00227 mol) of solid PyBOP ([benzotriazolyloxy]tripyrrolidino
phosphonium hexafluorophosphate, Advanced ChemTech) directly. The reaction
mixture was stirred for 30 minutes after which time it was concentrated in
vacuo.
The brown oil was treated with 10% aqueous hydrochloric acid. The suspension
59
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
was extracted with ether. The organic extraction was washed with 10% sodium
hydroxide followed by another 10% hydrochloric acid wash, was dried (MgS04)
and concentrated in vacuo to afford 1.0 g of a light-brown foam. This
intermediate
was dissolved in 25 mL of ethanolic hydrogen chloride, and the solution was
allowed to stand at room temperature for 15 minutes. The reaction mixture was
concentrated in vacuo to a brown oil that was purif ed by flash silica
chromatography. Elution with a gradient ( 100 % dichloromethane to 0.6
methanol in dichloromethane) afforded 0.2284 g of a light-brown viscous oil.
Scratching with pentane-hexanes and drying under high vacuum afforded
0.1541 g (23%) of an off white foam; mp 61-75°C;
1 H NMR (400 MHz, DMSO): 8 11.34 (s, 1 H), 9.68 (s, 1 H), 9.18 (s, 1 H), 7.65
(d,
1 H, J=1.5 Hz), 7.58 (dd, 1 H, J=8.7, 6.8 Hz), 7.52 (dd, 1 H, J=8.4, 1.9 Hz),
7.15 (d,
1 H, J=8.4 Hz), 6.74 (dd, 1 H, J=11.8, 2.4 Hz), 6.62 (ddd, 1 H, J=8.4, 8.4,
2.7 Hz),
2.18 (s, 3H);
I3C NMR (100 MHz, DMSO): 8 165.91, 164.36 (d, JC-F=247.1 Hz), 146.78,
139.18, 138.77, 135.43, 132.64, 130.60 (d, JC_F=11.5 Hz), 122.23, 112.52,
104.72 (d, J=22.1 Hz), 100.45 (d, JC_F=25.2 Hz), 86.77, 17.03;
19F NMR (376 MHz, DMSO): S -107.20 to -107.27 (m);
IR (KBr) 3307 (broad, O-H stretch), 1636 (C=O stretch) cm-I;
MS (CI) M+1 = 387.
Analysis calculated for C14H12F~202~
C, 43.54; H, 3.13; N, 7.25.
Found: C, 43.62; H, 3.24; N, 6.98.
EXAMPLE 2a
5 Bromo-3 4-difluoro-N-hydroxy-2-(4-iodo-2-methyl-phenylamino)-benzamide
(a) Preparation of 5-Bromo-2,3,4-trifluorobenzoic acid
To a stirred solution comprised of 1-bromo-2,3,4-trifluorobenzene
(Aldrich, 99%; 5.30 g, 0.0249 mol) in 95 mL of anhydrous tetrahydrofuran
cooled
to -78°C was slowly added 12.5 mL of 2.0 M lithium diisopropylamide in
heptane/tetrahydrofuran/ethylbenzene solution (Aldrich). The mixture was
stirred
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
for 1 hour and transferred by canula into 700 mL of a stirred saturated
ethereal
carbon dioxide solution cooled to -78°C. The cold bath was removed, and
the
reaction mixture was stirred for 18 hours at ambient temperature. Dilute (10%)
aqueous hydrochloric acid (ca. 500 mL) was poured into the reaction mixture,
and
the mixture was subsequently concentrated on a rotary evaporator to a crude
solid.
The solid product was partitioned between diethyl ether (150 mL) and aq. HCl
(330 mL, pH 0). The aqueous phase was extracted with a second portion (100 mL)
of diethyl ether, and the combined ethereal extracts were washed with 5%
aqueous
sodium hydroxide (200 mL) and water (100 mL, pH 12). These combined alkaline
aqueous extractions were acidified to pH 0 with concentrated aqueous
hydrochloric acid. The resulting suspension was extracted with ether (2 x
200 mL). The combined organic extracts were dried (MgS04), concentrated
in vacuo, and subjected to high vacuum until constant mass was achieved to
afford
5.60 g (88% yield) of an off white powder; mp 139-142.5°C;
1 H NMR (400 MHz, DMSO): 8 13.97 (broad s, 1 H, 8.00-7.96 (m, 1 H);
13C NMR (100 MHz, DMSO): b 162.96, 129.34, 118.47, 104.54 (d,
JC_F=22.9 Hz);
19F NMR (376 MHz, DMSO): 8 -120.20 to -120.31 (m), -131.75 to -131.86 (m),
-154.95 to -155.07 (m);
IR (KBr) 1696 (C=O stretch)cm-1;
MS (CI) M+1 = 255.
Analysis calculated for C74H21BrF302:
C, 32.97; H, 0.79; N, 0.00; Br, 31.34; F, 22.35.
Found: C, 33.18; H, 0.64; N, 0.01; Br, 30.14; F, 22.75.
(b) Preparation of 5-Bromo-3 4-difluoro-2-f4-iodo-2-methyl-ahenylamino)-
benzoic acid
To a stirred solution comprised of 1.88 g (0.00791 mol) of 2-amino-
5-iodotoluene in 10 mL of tetrahydrofuran at -78°C was added 6 mL
(0.012 mol)
of a 2.0 M lithium diisopropylamide in tetrahydrofuran/heptane/ethylbenzene
(Aldrich) solution. The resulting green suspension was stirred vigorously for
61
CA 02346448 2001-04-06
WO 00/35436 PCTNS99/29783
minutes, after which time a solution of 1.00 g (0.00392 mol) of 5-bromo-
2,3,4-trifluorobenzoic acid in 15 mL of tetrahydrofuran was added. The cold
bath
was subsequently removed, and the reaction mixture stirred for 18 hours. The
mixture was concentrated, and the concentrate was treated with 100 mL of
dilute
5 (10%) aqueous hydrochloric acid. The resulting suspension was extracted with
ether (2 x 150 mL), and the combined organic extractions were dried (MgS04}
and concentrated in vacuo to give an orange solid. The solid was triturated
with
boiling dichloromethane, cooled to ambient temperature, and collected by
filtration. The solid was rinsed with dichloromethane, and dried in the vacuum-
10 oven (80°C) to afford 1.39 g (76%) of a yellow-green powder; mp
259.5-262°C;
1 H NMR (400 MHz, DMSO): 8 9.03 (s, 1 H), 7.99 (dd, 1 H, J=7.5, 1.9 Hz),
7.57 (dd, 1 H, J=1.5 Hz), 7.42 (dd, 1 H, J=8.4, 1.9 Hz), 6.70 (dd, 1 H, J=8.4,
6.0 Hz), 2.24 (s, 3H);
19F NMR (376 MHz, DMSO): 8 -123.40 to -123.47 (m); -139.00 to -139.14 (m);
IR (KBr) 1667 (C=O stretch)cm-1;
MS (CI) M+1 = 469.
Analysis calculated for C 14H9BrF2IN02:
C, 35.93; H, 1.94; N, 2.99; Br, 17.07; F, 8.12; I, 27.11.
Found: C, 36.15; H, 1.91; N, 2.70; Br, 16.40; F, 8.46; I, 26.05.
(c) Preparation of 5-Bromo-3 4-difluoro-N-hydroxy-~4-iodo-2-methvl-
phenylaminol-benzamide
To a stirred solution comprised of 5-bromo-3,4-difluoro-2-(4-iodo-
2-methyl-phenylamino)-benzoic acid (0.51 g, 0.0011 mol), O-(tetrahydro-2H-
pyran-2-yl)-hydroxylamine (0.15 g, 0.0013 mol), and diisopropylethylamine
(0.25 mL, 0.0014 mol) in 20 mL of an equivolume tetrahydrofuran-
dichloromethane solution was added 0.6794 g (0.001306 mol) of solid PyBOP
(Advanced ChemTech) directly. The reaction mixture was stirred at 24°C
for
10 minutes, and then was concentrated to dryness in vacuo. The concentrate was
suspended in 100 mL of 10% aqueous hydrochloric acid. The suspension was
extracted with 125 mL of diethyl ether. The ether layer was separated, washed
with 75 mL of 10% aqueous sodium hydroxide, and then with 100 mL of dilute
62
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
acid. The ether solution was dried (MgS04) and concentrated in vacuo to afford
0.62 g (100%) of an off white foam. The foam was dissolved in ca. 15 mL of
methanolic hydrogen chloride. After 5 minutes, the solution was concentrated
in vacuo to an oil, and the oil was purified by flash silica chromatography.
Elution
S with dichloromethane: dichloromethane-methanol (99:1) afforded 0.2233 g
(42%)
of a yellow powder. The powder was dissolved in diethyl ether and washed with
dilute hydrochloric acid. The organic phase was dried (MgS04) and concentrated
in vacuo to afford 0.200 g of a foam. This product was triturated with pentane
to
afford 0.1525 g of a powder that was repurified by flash silica
chromatography.
Elution with dichloromethane afforded 0.0783 g (15%) of an analytically pure
title
compound, mp 80-90°C;
1H NMR (400 MHz, DMSO): 8 11.53 (s, 1H), 9.38 (s, 1H), 8.82 (s, 1H), 7.70 (dd,
1H, J=7.0, 1.9 Hz), 7.53 (s, 1H), 7.37 (dd, 1H, J=8.4, 1.9 Hz), 6.55 (dd, 1H,
J=8.2,
6.5 Hz), 2.22 (s, 3H);
19F NMR (376 MHz, DMSO): 8 -126.24 to -126.29 (m), -137.71 to -137.77 (m);
IR (KBr) 3346 (broad, O-H stretch), 1651 (C=O stretch)cm-1;
MS (CI) M+1 = 484.
Analysis calculated for C 14H 1 OBrF2~202
C, 34.81; H, 2.09; N, 5.80.
Found: C, 34.53; H, 1.73; N, 5.52.
Examples 3a to 12a in the table below were prepared by the general
procedure of Examples 1 a and 2a.
EXAMPLES 13a-77a
Examples 13a to 77a were prepared utilizing combinatorial synthetic
methodology by reacting appropriately substituted phenylamino benzoic acids
(e.g., as shown in Scheme 1) and hydroxylamines (e.g., (NHR6a )-O-R7~. A
general method is given below:
To a 0.8-mL autosampler vial in a metal block was added 40 pL of a
0.5 M solution of the acid in DMF and 40 pL of the hydroxylamine (2 M solution
63
CA 02346448 2001-04-06
WO 00/35436 PCTNS99/29783
in Hunig's base and 1 M in amine in DMF). A 0.5 M solution of PyBrOP was
freshly prepared, and 50 ~L were added to the autosampler vial. The reaction
was
allowed to stand for 24 hours.
The reaction mixture was transferred to a 2-dram vial and diluted with
2 mL of ethyl acetate. The organic layer was washed with 3 mL of distilled
water
and the water layer washed again with 2 mL of ethyl acetate. The combined
organic layers were allowed to evaporate to dryness in an open fume hood.
The residue was taken up in 2 mL of 50% acetonitrile in water and injected
on a semi-prep reversed phase column ( 10 mm x 25 cm, 5 pM spherical silica,
pore Size 115 A derivatized with C-18, the sample was eluted at 4.7 mL/min
with
a linear ramp to 100% acetonitrile over 8.5 minutes. Elution with 100%
acetonitrile continued for 8 minutes.) Fractions were collected by monitoring
at
214 nM. The desired fractions were evaporated using a Zymark Turbovap. The
product was dissolved in chloroform and transferred to a preweighed vial,
evaporated, and weighed again to determine the yield. The structure was
confirmed by mass spectroscopy.
64
CA 02346448 2001-04-06
WO 00/35436 PCT/US99l29783
EXAMPLES 3a-77a
Example Compound Melting MS
No. Point (°C) (M-H+)
3a 2-(4-bromo-2-methyl-phenylamino)-4-fluoro-N- 56-75 dec 523
hydroxy-benzamide
4a 5-Chloro-N-hydroxy-2-(4-iodo-2-methyl- 65 dec
phenylamino)-benzamide
Sa 5-Chloro-N-hydroxy-2-(4-iodo-2-methyl- 62-67
phenylamino)-N-methyl-benzamide
6a 5-Chloro-2-(4-iodo-2-methyl-phenylamino)-N- 105-108
(terahydropyran-2-yloxy)benzamide
7a S-Chloro-2-(4-iodo-2-methyl-phenylamino)-N- 64-68
methoxybenzamide
8a 4-Fluoro-N-hydroxy-2-(4-fluoro-2-methyl- 119-135
phenylamino)-benzamide
9a 4-Fluoro-N-hydroxy-2-(2-methyl phenylamino)- 101-103
benzamide
l0a 4-Fluoro-2-(4-fluor-2-methyl-phenylamino)-N- 142-146
(terahydropyran-2-yloxy)benzamide
lla 4-Fluoro-N-hydroxy-2-(4-cluoro-2-methyl- 133.5-135
phenylamino)-benzamide
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound Melting MS
No. Point (°C) (M-H+)
12a 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N- 107-109.5
phenylmethoxy-benzamide
13a 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N- 399
methoxy-benzamide
14a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)- 417
N-methoxy-benzamide
1 Sa 2-(4-Bromo-2-methyl-phenylamino)- 369
3,4-difluoro-N-methoxy-benzamide
16a 2-(4-Bromo-2-methyl-phenylamino)-N-ethoxy- 342*
3,4-difluoro-benzamide (M-Et0)
17a 5-Bromo-N-ethoxy-3,4-difluoro-2-(4-iodo- 509
2-methyl-phenylamino)-benzamide
18a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)- 445
N-isopropoxy-benzamide
19a 2-(4-Bromo-2-methyl-phenylamino)- 397
3,4-difluoro-N-isopropoxy-benzamide
20a 4-Fluoro-N-(furan-3-ylmethoxy)-2-(4-iodo- 465
2-methyl-phenylamino)-benzamide
66
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound Melting MS
No. Point (°C) (M-H+)
21 a 3,4-Difluoro-N-(furan-3-ylmethoxy)-2-(4-iodo- 483
2-methyl-phenylamino)-benzamide
22a 2-(4-Bromo-2-methyl-phenylamino)- 435
3,4-difluoro-N-(furan-3-ylmethoxy)-benzamide
23a 5-Bromo-3,4-difluoro-N-(furan-3-ylmethoxy)- 561
2-(4-iodo-2-methyl-phenylamino)-benzamide
24a 5-Bromo-N-(but-2-enyloxy)-3,4-difluoro- 536
2-(4-iodo-2-methyl-phenylamino)-benzamide
25a 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N- 423
(prop-2-ynyloxy)-benzamide
26a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)- 441
N-(prop-2-ynyloxy)-benzamide
27a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)- 455
N-( 1-methyl-prop-2-ynyloxy)-benzamide
28a 2-(4-Bromo-2-methyl-phenylamino)- 4U7
3,4-difluoro-N-( 1-methyl-prop-2-ynyloxy)-
benzamide
29a N-(But-3-ynyloxy)-3,4-difluoro-2-(4-iodo- 455
2-methyl-phenylamino)-benzamide
67
CA 02346448 2001-04-06
WO 00/35436 PCT/US99l29783
Example Compound Melting MS
No. Point (°C) {M_H+)
30a 2-(4-Bromo-2-methyl-phenylamino)-N-(but- 407
3-ynyloxy)-3,4-difluoro-benzamide
31a S-Bromo-N-(but-3-ynyloxy)-3,4-difluoro- 533
2-{4-iodo-2-methyl-phenylamino)-benzamide
32a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)- 517
N-(3-phenyl-prop-2-ynyloxy)-benzamide
33a 3,4-Difluoro-2-(4-bromo-2-methyl- 469
phenylamino)-N-(3-phenyl-prop-2-ynyloxy)-
benzamide
34a 3,4-Difluoro-N-[3-(3-fluoro-phenyl)-prop- 535
2-ynyloxy]-2-(4-iodo-2-methyl-phenylamino)-
benzamide
35a 2-(4-Bromo-2-methyl-phenylamino)- 487
3,4-difluoro-N-[3-(3-fluoro-phenyl)-prop-
2-ynyloxy]-benzamide
36a 3,4-Difluoro-N-[3-(2-fluoro-phenyl)-prop- 535
2-ynyloxy]-2-(4-iodo-2-methyl-phenylamino)-
benzamide
37a S-Bromo-3,4-difluoro-N-[3-(2-fluoro-phenyl)- 613
prop-2-ynyloxy]-2-(4-iodo-2-methyl-
phenylamino)-benzamide
68
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound Melting MS
No. Point (°C) (M-H+)
38a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)- 557*
N-(3-methyl-5-phenyl-pent-2-en-4-ynyloxy)- * (M+H)
benzamide
39a 2-(4-Bromo-2-methyl-phenylamino)- 510
3,4-difluoro-N-(3-methyl-5-phenyl-pent-2-en-
4-ynyloxy)-benzamide
40a N-Ethoxy-3,4-difluoro-2-(4-iodo-2-methyl- 431
phenylamino)-benzamide
41a 2-(4-Bromo-2-methyl-phenylamino)-N-ethoxy- 383
3,4-difluoro-benzamide
42a 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N- 427
propoxy-benzamide
43a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)- 445
N-propoxy-benzamide
44a 2-(4-Bromo-2-methyl-phenylamino)- 397
3,4-difluoro-N-propoxy-benzamide
45a 5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl- 523
phenylamino)-N-propoxy-benzamide
46a 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N- 427
isopropoxy-benzamide
69
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound Melting MS
No. Point (°C) (M-H+)
47a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)- 445
N-isopropoxy-benzamide
48a 2-(4-Bromo-2-methyl-phenylamino)- 397
3,4-difluoro-N-isopropoxy-benzamide
49a 5-Bromo-3,4-difluoro-2-(4-iodo-2-methyl- 523
phenylamino)-N-isopropoxy-benzamide
SOa N-Cyclobutyloxy-3,4-difluoro-2-(4-iodo- 457
2-methyl-phenylamino)-benzamide
Sla 2-(4-Bromo-2-methyl-phenylamino)-N- 409
cyclobutyloxy-3,4-difluoro-benzamide
52a N-Cyclopentyloxy-4-fluoro-2-(4-iodo-2-methyl- 453
phenylamino)-benzamide
53a N-Cyclopentyloxy-3,4-difluoro-2-(4-iodo- 47I
2-methyl-phenylamino)-benzamide
54a 2-(4-Bromo-2-methyl-phenylamino)-N- 423
cyclopentyloxy-3,4-difluoro-benzamide
SSa N-Cyclopropylmethoxy-4-fluoro-2-(4-iodo- 439
2-methyl-phenylamino)-benzamide
56a N-Cyclopropylmethoxy-3,4-difluoro-2-(4-iodo- 457
2-methyl-phenylamino)-benzamide
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound Melting MS
No. Point (°C;) (M-H+)
57a 2-(4-Bromo-2-methyl-phenylamino)-N- 409
cyclopropylmethoxy-3,4-difluoro-benzamide
58a 5-Bromo-N-cyclopropylmethoxy-3,4-difluoro- 435
2-(4-iodo-2-methyl-phenylamino)
59a 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N- SOS
(2-phenoxy-ethoxy)-benzamide
60a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)- 523
N-(2-phenoxy-ethoxy)-benzamide
61 a 2-(4-Bromo-2-methyl-phenylamino)- 475
3,4-difluoro-N-(2-phenoxy-ethoxy)-benzamide
62a 4-Fluoro-2-(4-iodo-2-methyl-phenylamino}-N- 481
(thiophen-2-ylmethoxy)-benzamide
63a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)- 499
N-(thiophen-2-ylmethoxy)-benzamide
64a 2-(4-Bromo-2-methyl-phenylamino}- 451
3,4-difluoro-N-(thiophen-2-ylmethoxy)-
benzamide
65a 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N- 439
(2-methyl-allyloxy)-benzamide
71
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
' Example Compound Melting MS
No. Point (C) (M-H+)
66a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-457
N-(2-methyl-allyloxy)-benzamide
67a 2-(4-Bromo-2-methyl-phenylamino)-410
3,4-difluoro-N-(2-methyl-allyloxy)-benzamide
68a N-(But-2-enyloxy)-4-fluoro-2-(4-iodo-2-methyl-439
phenylamino)-benzamide
69a N-(But-2-enyloxy)-3,4-difluoro-2-(4-iodo-457
2-methyl-phenylamino)-benzamide
70a 2-(4-Bromo-2-methyl-phenylamino)-N-(but- 410
2-enyloxy)-3,4-difluoro-benzamide
71 a 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)- 441
N-(prop-2-ynyloxy)-benzamide
72a N-(But-3-ynyloxy)-3,4-difluoro-2-(4-iodo- 455
2-methyl-phenylamino}-benzamide
73a 2-(4-Bromo-2-methyl-phenylamino)-N- 449
(4,4-dimethyl-pent-2-ynyloxy)-3,4-difluoro-
benzamide
74a N-(But-2-enyloxy)-3,4-difluoro-2-(4-iodo- 457
2-methyl-phenylamino)-benzamide
72
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Example Compound Melting MS
No. Point (°C) (M-H+)
75a 2-(4-Bromo-2-methyl-phenylamino)-N-(but- 410
2-enyloxy}-3,4-difluoro-benzamide
76a N-(3-tert-butyl-propyn-2-yl)oxy-4-fluoro- 479
2-(4-iodo-2-methyl-phenylamino)-benzamide
77a 4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-N- 577*
phenylmethoxy-benzamide *CI
D. Pharmacological Activity
Several of the compounds described above have been evaluated in both
in vitro and in vivo assays which are designed to measure anti-arthritic
activity,
and are recognized by those skilled in the art to be valid predictors of
clinical
efficacy.
Type II-collagen-induced arthritis (CIA) in mice is recognized as an
experimental model of arthritis that has a number of pathologic, immunologic,
and
genetic features in common with rheumatoid arthritis in humans. The disease is
induced by immunization of DBA/1 inbred strain of mice with 100 micrograms of
type II collagen (C II), which is the major component of joint cartilage. The
collagen was delivered to the mice by intradermal injection of a solution made
up
in Freund's complete adjuvant. A progressive and inflammatory arthritis
develops
in the majority of the mice immunized, characterized by paw width increases of
up to 100%. A clinical scoring index is used to assess disease progression
from
erythema and edema (stage 1), joint distortion (stage 2), to joint ankylosis
(stage 3). The disease is variable in that it can affect one or all of the
paws of the
animal, resulting in total possible score of 12 for each mouse. Histopathology
of
arthritic joints revealed synovitis, pannus formation, and cartilage and bone
erosions. All mouse strains that are susceptible to CIA are high antibody
responders to type II collagen, and there is a marked cellular response to C
II.
73
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29~83
The foregoing assay was carried out to evaluate the anti-arthritic activity
of several doses of the compound 2-(2-chloro-4-iodophenylamino)-N-
cyclopropylmethoxy-3,4-difluorobenzamide. The compound (also referred to
as "PD 184352") was suspended in an aqueous mixture of 0.5%
hydroxypropylmethyl cellulose (HPMC) and 0.2% Tween 80. The suspension was
administered orally twice daily (once in the morning and once in the evening)
in
equally divided doses. All animals were fed laboratory chow, and given water
ad libitum. The assay was continued for 63 days, with disease scores being
taken
periodically throughout the study, and on Day 63, and averaged at the end of
the
study. The results of the assay are presented in Pharmacological Table 1
below:
Pharmacological Table 1
Collagen-Induced Arthritis in Mice
Treatment Number of Percent Average Average No.
Mice Per Arthritis Severity of Arthritic
Group Incidence Score Paws
Vehicle 10 100 6.3 3.5
PD 184352 9 22 0.333 0.333
200 mg/kg/day
PD 184352 10 10 0.6 0.4
60 mg/kg/day
PD 184352 10 60 2.9 2
mg/kg/day
The foregoing data establish that the compound is a potent anti-arthritic
agent.
In another standard assay, monoarticular arthritis was induced in rats. Rats
were given 6 microgram doses of sonicated Streptoccocal cell wall (SCW) } in
1 S 10 microliters of Dulbecco's phosphate buffered saline (DPBS) by infra-
articular
injection into the right tibiotalar joint on Day 0. SCW induces paw swelling
in the
animals. On Day 21, the delayed-type hypersensitivity (DTH) was initiated with
100 micrograms of SCW administered intravenously. Test compounds were
suspended in an aqueous mixture of 0.5% HPMC and 0.2% Tween 80, sonicated,
74
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
' and administered twice daily in equally divided doses ( 10 mL/kg volume)
beginning 1 hour prior to reactivation with SCW. The amount of edema was
determined by measuring the baseline volumes of the sensitized hindpaw before
reactivation on Day 21, and comparing them with the volumes at subsequent time
points. Paw volumes were measured by mercury plethysmography. The
antiarthritic activities of several of the phenyl amine compounds described
above,
when evaluated in the foregoing assay, are presented below in Pharmacological
Table 2. In the Table, compound "PD 184352" is 2-(2-chloro-
4-iodophenylamino)-N-cyclopropylmethoxy-3,4-difluorobenzamide, and
compound "PD 170611" is 2-(2-methyl-4-iodophenylamino)-N-hydroxy-
4-fluorobenzamide.
Pharmacological Table 2
SCW-Induced Monoarticular Arthritis
Percent
Inhibition
of Paw
Swelling
at Indicated Time Points
Treatment (n) Day 22 Day 23 Day 24 Day 25
PD 184352 10 59 57 68 53
200 mg/kg/day
PD 184352 10 30 34 40 31
60 mg/kg/day
PD 184352 10 17 17 28 17
mg/kg/day
PD 170611 10 40 4 0 0
300 mg/kg/day
PD 170611 10 80 9 20 32
100 mg/kg/day
The foregoing data establish that the phenyl amine compounds of
15 Formulas I and II are potent anti-arthritic agents, and can be used to
prevent and
CA 02346448 2001-04-06
WO 00/35436 PCTNS99/29783
treat various forms of arthritis, including rheumatoid arthritis and
osteoarthritis.
Several of the phenyl amine MEK inhibitors have been evaluated in an
in vitro cell culture assay designed to measure the effect of MEK inhibitors
on
interleukin-1 (IL-1 ) induced stromelysin production and phospo-ERK levels in
rabbit synovial fibroblasts. The stromelysin is a matrix metalloproteinase
enzyme
that is a causative factor in arthritis. The phospho-ERK is an enzyme that is
phosphorylated by a MEK enzyme, and is thus an indicator of MEK activity in
cells.
New England White rabbits were euthanized with B-euthanasia
administered IV with a 25 gauge needle in the marginal ear vein. The synovium
was immediately removed by the incision of the quadracep tendon and retracting
the patella. The synovium, with the infrapellar fat body, was then cut away
from
the patellar ligament and placed in sterile phosphate buffered saline (PBS)
(Gibco BRL, Gaithersberg, MD). The synovium was finely minced with a sterile
scalpel and placed in a 50 mL tube containing 6 mL of a solution of 4 mg
collagenase type I (Gibco BRL, Gaithersberg, MD)/mL PBS. The mixture was
incubated for 3 hours at 37°C. During the incubation, the 50 mL tube
was gently
swirled 4 to 6 times. The synoviocytes were then washed twice in media (the
media composition is described below). Washed cells were seeded into one
T-75 plastic cell culture flask and incubated at 37°C in 5% C02. After
reaching
90-100% confluency, the cells were seeded into appropriate containers for the
assay. Synovial fibroblasts were allowed to grow in 96 well plates for three
days
after confluency before testing. Vehicle (0.1 % dimethylsulfoxide in media),
or a
phenyl amine MEK inhibitor test compound dissolved in vehicle, was added to
the
synovial fibroblasts 30 minutes before addition of IL-la. Interleukin-la
( 100 U/mL) (Genzyme, Cambridge, MA) was suspended in media and added in a
volume of 10 pL/well. The cells were then incubated for 24 hours before the
media was removed and stored at -20°C. Prostromelysin-1 levels were
measured
using an ELISA from Amersham (Cat. No. RPN2615). Percent inhibition was
determined by comparing the stromelysin-1 concentration of drug-treated cells
to
that of vehicle-treated controls. The drug concentration at which 50%
inhibition of
76
CA 02346448 2001-04-06
WO 00/35436 PCTNS99/29783
' stromelysin-1 production was measured (ICSO) was determined using linear
regression analysis.
The media used in the foregoing assay was prepared as follows, utilizing
commercial reagents acquired from Gibco BRL (Gaithersberg, MD) unless
otherwise stated. To each 500 mL bottle of alpha-modified Eagles medium
(a-MEM, Cat. No. 12561-023) was added 10 mL of 1 Molar N-2-
hydroxyethylpiperazine-N-2-ethane sulfonic acid (1 M HEPES, Cat.
No. 15630-023), 10 mL of Penicillin/Streptomycin Stock (Cat. No. 15070-030,
5,000 U/mL Pen./5,000 p,g/mL Strep), 500 p,L Gentamicin Stock (50 mg/mL)
(Cat. No. 15750-Ol 1), 40 mL Fetal Calf Serum from Hyclone Inc. (Cat.
No. A1111-L). The results of the foregoing assays are presented in
Pharmacological Tables 3 and 4. Pharmacological Table 3 presents the nanomolar
dose of test compound required to cause a SO% inhibition of stromelysin
expression (ICSO).
Pharmacological Table 3
Effect of MEK Inhibitors on IL-I-induced Stromeylsin Expression
in Rabbit Synovial Fibroblast Cell Cultures
Compound Tested ICSO (nM)
PD 171984 59
PD 177168 20
PD 180841 61
PD 184161 192
PD 184352 28
PD 184386 18
PD 185625 24
PD 185848 9
PD 188563 11
PD 198306 18
PD 199601 24
PD 20331 I 20
77
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
In addition, a Western blot analysis of phospho-ERK levels in cell cultures
was performed. Pharmacological Table 4 presents the % inhibition of ERK
1/2 phosphorylation caused by a phenyl amine MEK inhibitor. Cells were lysed
with 1mL lysis buffer (containing NaCI (70 mM), B-glycerol phosphate (50 mM),
1 M HEPES ( 10 mM}, Triton X-100 ( 1 %)) per T25. The mixture was transferred
to microcentrifuge tubes, and spun at 2500 x g for 15 minutes. After removing
the
supernatant, the protein assay was performed. The samples were run on a 10%
Tris-Glycine gel, and transferred to nitrocellulose. The blots were then
probed
with a phospho-p44/42 MAP kinase antibody followed by the secondary Ab (goat
anti rabbit HRP conjugated), coated with the ECL detection reagent, and
exposed
to film. The amount of phospo-ERK present was determined by relative
densitometry.
Pharmacological Table 4
Inhibition of ERK Phosphorylation by PD I 84352 in IL-2
Stimulated Rabbit Synovial Fibroblast Cell Cultures
PD 184352 (nM) % Inhibition of Phospho-ERK
10 22
10 81
1,000 100
10,000 97
The data presented in Pharmacological Tables 3 and 4 establish the phenyl
amino MEK inhibitors of Formula I and Formula II are potent inhibitors of
cellular enzymes which are causative factors in arthritis.
The method of this invention has also been established in in vivo assays
utilizing New Zealand White rabbits in which cartilage degradation was induced
by interleukin 1-alpha injections into the knee joints.. Adult mate rabbits
weighing
about 3 kg were anesthetized with 5 mg/kg of rompun and 10-1 S mg/kg of
78
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
ketamine. Test compounds were suspended in a vehicle of 0.5% aqueous
hydroxypropyl methyl cellulose and 0.2% Tween 80. The suspensions were
administered by oral gavage to the animals. Thirty minutes following dosing
with
the MEK inhibitors, human recombinant IL-la (Genzyme, Cambridge, MA) was
injected (25 ng) into one knee joint space through the suprapatella ligament.
The
contralateral joint received an equal volume of vehicle (phosphate buffered
saline/0.2% fetal bovine serum). The animals were euthanized after 24 hours
after
the IL-1 injection, and the extent of cartilage degradation was determined by
measuring the proteoglycan content of the articular cartilage from the femoral
condyles with a dimethylene blue dye assay kit. Analysis was done
spectrophotometrically, and the percent of inhibition of proteoglycan loss in
the
treated joint compared to the non-treated joint was determined. The results of
this
in vivo assay for several of the selective MEK inhibitors of Formulas I and II
are
presented below in Pharmacological Table 5.
Pharmacological Table 5
Inhibition of Proteoglycan Loss in Rabbits
PD No. Dose Dosed (n) % Inhibition of
(mg/kg Proteoglycan Loss
184352 30 2x 6 75
10 2x 6 48
3 2x 6 13
185625 30 lx 6 63
185848 30 lx 6 43
Additional support for the claimed methods was obtained using the SCW
model again and also three other in vivo models of in>Zammation and/or
arthritis.
The data for each of the additional experiments is shown in Pharmacological
Table 6 below. In a carrageenan-induced footpad edema (CFE) model, male
outbred Wistar rats (135-150g, Charles River Labs) were dosed orally with
l Oml/kg vehicle or test compound one hour prior to administration of a
sonicated
suspension of carrageenan (lmg/0.1 ml saline). Carrageenan was injected into
the
79
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
subplantar region of the right hind paw. Paw volume was determined by mercury
plethysmography immediately after injection and again five hours after
carrageenan injection. Percent inhibition of edema was determined, and the
ID4o
calculated by linear regression. Differences in swelling compared to control
5 animals were assessed by a 1-way ANOVA, followed by Dunnett's test.
In another model, rat adjuvant-induced polyarthritis (rat AIP) was induced
following published procedures. Outbred male Wistar rats (100-115 gms) were
obtained from Charles River Labs 2 - S days prior to initiation of the study.
Rats
were injected subcutaneously in the distal third of the tail with 1 mg
10 Mycobacterium butyricum suspended in paraffin oil using glass tuberculin
syringes and 25 gauge needles on day 0. The Mycobacterium butyricum
suspension was achieved by sonicating in paraffin oil for 10 minutes with the
vessel immersed in an ice bath. After all the rats in the study were
immunized,
they were randomized into groups. In the therapeutic study, randomization was
15 done on day 14. Dosing started on day 14 and ended on day 27. Vehicle or
drug
suspended in vehicle was administered orally in 10 ml/kg volume. Hindpaw
swelling was assessed by mercury plethysmography, beginning on the 11 '" day
of
the study and occurring every third or fourth day subsequently. The change in
edema was determined by the difference between hindpaw volume on the day in
20 which it was assessed and the day 14 volume. Percent inhibition was based
on a
comparison of the treatment group to the vehicle group. The number of animals
in a treatment group was 10 while that in the vehicle was 20.
Finally, in a rabbit IL-1 arthritis (IL-1 ) model, adult male New Zealand
White rabbits were anesthetized with rompun (10 mg/kg) and ketamine (50
25 mg/kg) (im). Twenty-five nanograms IL-1 was injected into one knee joint
space
through the suprapatella ligament (using sterile techniques). The
contralateral joint
received an equal volume of vehicle. The knees were first shaved and then
swabbed with a surgical disinfectant prior to intraarticular injection. The
animals
were euthanized after 24 hours, the articular cartilage scraped from the
femoral
30 condyles and tibial plateaus and weighed, and the extent of cartilage
degradation
determined by a standard dimethylene blue assay. Test compound was
administered by oral gavage one hour prior to IL-1 administration.
80
CA 02346448 2001-04-06
WO 00/35436 PCT/US99/29783
Pharmacological Table 6
Activity of MEK Inhibitors in animal models
of arthritis and inflammation
S
Model 184352198306203311
Rat carrageenan footpad edema (CFE) 75.8 14.7 18.9 m
(ID4o)
Rat SCW arthritis (SCW) (IDS) 10.0 11.2 >100 "
Rat adjuvant arthritis (AIP) (IDS) 6.9 6.6 > 30 "
Rabbit IL-1 arthritis (IL-1 )
(% Inh proteoglycan loss @ 30mg/kg) 57.9 42.9 29.2
81