Note: Descriptions are shown in the official language in which they were submitted.
CA 02346707 2001-04-06
WO 00/20105 PCT/GB99/03318
- 1 -
Membrane Structure
The present invention relates to a membrane structure with improved
performance
characteristics which is particularly useful for zeolite membranes.
A commonly used membrane structure for separating two components consists of a
tubular membrane with the mixture being passed down the tube and a separated
component passing through the membrane and the other component or mixture of
components passing down the tube. The tube can be bent so that it is in the
form of a
continuous zig-zag or other convoluted or similar configuration to increase
the
surface area of the tube contained in a module.
Alternatively or in addition there can be a plurality of tubes arranged
substantially in
parallel to increase the surface area of membrane without having too large a
diameter
of each tube or tube length.
In a module for use in sep~~ration or filtration processes using tubular
membranes, the
size and configuration oFthe membranes is chosen so that the optimum
performance
can be achieved. For a tubular membrane, the larger the diameter of the tube
the
greater the surface area pe;r unit length of the tube and the lower the
pressure drop
down the tube, this is nornially a desired criterion. However the larger the
diameter of
the tube, the greater the possibility, at any given flow rate of streamline
flow down
the tube and the greater the distance from the centre of the tube to the
membrane and
these will lead to a corresponding loss of performance. Whereas a narrower
tube
gives a lower surface area per unit length, and requires a lower flow rate to
give the
same degree of turbulence, but gives a higher pressure drop. In order to
balance these
characteristics, a series of eparallel tubes in a module can be used, with the
diameter of
each tube chosen for optimum performance and the number of tubes chosen to
have
the desired surface area in the module.
SUBSTITUTE SHEET (RULE 26)
CA 02346707 2001-04-06
WO 00/20105 PCT/GB99/0331$
_ 2 _
With ceramic membranes tit is cost efficient and convenient to form a
plurality of
tubes together in the form of a monolith. Hence monolithic assemblies of tubes
have
been developed wherein a single, tubular body comprises a multiplicity of
smaller
channels.
The number and shape of the inner channels can vary. For example, monoliths
with 7,
19 or a greater number of channels have been developed as well as monoliths
with
star or other shaped channels. Typically, such designs have been developed so
as to
maximise the surface area per unit length of monolith, combined with minimum
pressure drop whilst maintaining high overall permeability.
We have found that a particular arrangement of tubular membranes gives
unexpectedly superior results for zeolite membranes in pervaporation over what
would have been expected.
According to the invention there is provided a comprising a tubular porous
ceramic
monolith having at least four tubular conduits formed within the monolith with
a
zeolite membrane formed on the internal surface of the conduits the zeolite
membranes having an internal diameter of 5 to 9 millimetres preferably 6.Q
millimetres and the ceramic monolith having an outer diameter of 20 to 25
millimetres, preferably ?Omm.
In practice the internal diameter will vary along the length of the tubular
membrane
and will vary according to membrane thickness, so the internal diameter of the
tubular membranes is an <~pproximate average along the length of the tube and
the
invention will encompass structures which deviate from the exact measurements
in
accordance with normal practice.
SUBSTITUTE SHEET (RULE 26)
CA 02346707 2001-04-06
WO 00/20105 PCT/GB99/03318
- 3 -
The length of the porous ceramic monolith will depend on the use to which the
zeolite membrane is to be used and the vessel into which it is to be fitted.
In general
lengths of from 1 to 10 metres are useful in may applications.
The tubular zeolite membrane is preferably formed by the methods disclosed in
our
co-pending patent applications PCT/GB96/00243, PCT/GB97/00928 and PCT/GB
97/00635.
Typical zeolites which can b~e used in the present invention include but are
not limited
to, 3A, 4A, SA, 13X, X, Y, Z,SMS, MPOs, SAPOs, SiIicalite, etc.
The porous supports on which zeolite membranes are formed are preferably
formed
of sintered ceramic powders such as alpha alumina, titanic, zirconia or other
suitable
media which are capable of being extruded and sintered upon which the zeolite
will
nucleate and grow.
The present invention can be used with porous supports of any suitable size
although,
for large flux rates through a membrane, large pore sizes are preferred.
Preferably
pore sizes of 0.01 to 2,000 microns, more preferably of 0.1 to 200 and ideally
of 0.1
to 20 microns are used. Pore sizes up to 300 microns can be determined by
bubble
point pressure as specified in ISO 4003. Larger pore sizes can be measured by
microscopic methods.
The membranes which can. be used in the present invention can be formed by any
method, for example by crystallisation from a gel or solution, by plasma
deposition or
by any other method such as electro-deposition of crystals on conducting
substrates
e.g. as described in DE 4109037.
.SUBSTITUTE SHEET (RULE 26)
CA 02346707 2001-04-06
WO 00/20105 PCT/GB99/03318
- 4 -
When the membrane comprising a film of a zeolite material is prepared by
crystallisation from a synthesis gel, any of the methods described in the
prior art can
be used.
The synthesis gel used in the process can be any gel which is capable of
producing
the desired crystalline zeolite membrane. Gels for the synthesis of zeo-type
materials
are well known and are described in the prior art given above or, for example,
in EP-
A-57049, EP-A-104800, E;P-A-2899 and EP-A-2900. Standard text books by D W
Breck ("Zeolites Molecular Sieves, Structure Chemistry and Use") published by
John
Wiley (1974) and P.A Jacobs and J.A Martens (Studies in Surface Science and
Catalysis No. 33, Synthesis of High Silica Alumino silicate Zeolites"
published by
Elsevier (1987). describe many such synthesis gels. The process which can be
used
includes conventional syntheses of zeolite membranes, except that the
synthesis is
carried out in the presence of the porous support. Most commonly, gels are
crystallised by the application of heat.
The membrane can be prepared by a process which comprises deposition or
crystallisation from a growth medium. One method for forming the membrane
preferably has a molar composition in the range of
{ 1.5 - 3.0)'Na20 : ( 1 )A12O3 : (2.0)Si02 : {50-200)H20
and the method used can be used in any of the methods disclosed in the
references
listed above
The conditions which can be used for forming the membrane are with a
temperature
of the growth solution preferably in the range of 50 to 100°C and the
pH can be
adjusted e.g. to pH of 12.5 to 14 by addition of sodium hydroxide or ammonia.
If
desired the sodium ion concentration can be increased without increasing the
pH by
the addition of a sodium salt such as sodium chloride. The growth solution can
be
SUBSTITUTE SHEET (RULE 26)
CA 02346707 2001-04-06
WO 00/20105 PCT/G899/03318
- 5 -
seeded with zeolite crystals of the desired zeolite to be synthesised. The
membrane
can be washed to pH neutral after membrane formation prior to any post-
treatment.
The porous support can be contacted with the growth medium by immersion or by
pouring the growth medium over the support with the support held substantially
horizontal, either face up at the bottom of a container, or face down at the
surface of
the growth medium, or it can be passed over one or both sides of the support,
with the
support held substantially horizontal, or it can be passed over one or both
sides of the
support with the support held substantially vertical or the support can be in
any
intermediate position.
The growth medium can be kept static. stirred, tumbled or passed over or
around the
support, alternatively the growth medium can be passed over both sides of the
support
with the support held substantially horizontal or at any intermediate
position.
Pressure may also be applied but it is usually convenient to conduct the
crystallisation under autogenous pressure. Preferably the porous support is
completely
immersed in the growth medium; alternatively, if desired, only one surface of
the
support may be in contact with the growth medium. This may be useful, for
example,
if it is desired to produce a membrane in the form of a tube, where only the
inside or
outside of the tube need be in contact with the growth medium.
It may be useful if it is desired to produce a membrane containing two
different
zeolites, one on each side of the support. Use of such a bi-functional
membrane
would be equivalent to using two separate membranes, each carrying a different
zeolite.
If desired, the treatment with the gel can be repeated one or more times to
obtain
thicker membrane coatings.
.SUBSTITUTE SHEET (RULE 26)
CA 02346707 2001-04-06
WO 00/20105 PCT/GB99/03318
- 6 -
Preferably the porous support is pre-treated with a zeolite initiating agent.
The zeolite
initiating agent is preferably a cobalt, molybdenum or nickel oxide or it can
be
particles of a zeolite, e.g. the zeolite which it is intended to deposit on
the porous
support, or any combination of these. Another example of an initiating agent
is a
compound which can deposit a zeo-type pre-cursor material e.g. a silicic acid
or
polysilicic acid.
The zeolite initiation agent can be contacted with the porous support by a wet
or dry
process. If a dry process is used, the particles of the zeolite initiation
agent can be
rubbed into the surface of the porous material, or the porous material surface
can be
rubbed in the particles.
Alternatively the particles of the zeolite initiation agent can be caused to
flow over
and/or through the porous support, or pulled into the support by means of a
vacuum.
If a wet process is used, a liquid suspension of powder of the zeolite
initiation agent
is formed and the liquid suspension contacted with the porous support to
deposit the
zeolite initiation agent on the support.
Before contacting the surface of the porous support with the zeolite
initiation agent
the surface is preferably wetted with wetting agent such as an alcohol, water
or a
mixture of these.
After formation the membrane is preferably treated with a surface modifying
agent
which can cross link with the zeolite membrane and thus form a membrane with
substantially no defects, 'l'he preferred surface modifying agents are silicic
acid and
silcates such as alkyl silicates e.g. tetra ethyl orthosilicate (TEOS).
In the present specification by silicic acid is meant monosilicic, low, medium
and
high molecular weight polysilicic acids and mixtures thereof.
SUBSTITUTE SHEET (RULE 26)
CA 02346707 2001-04-06
WO 00/20105 PCT/GB99/03318
Methods of making silicic acids are described in GB Patent Application
2269377.
The silicic acids used can have a "narrow" molecular weight distribution as
formed or
in a combination of different molecular weight ranges.
Greater flexibility can be introduced into the final membranes by treating
them with a
flexibilising agent by adding e.g. a hydroxy terminated polysiloxane into the
silicic
acid solution before treatment of the membrane.
The membrane structures of the present invention can be used in a range of
separation
and catalytic processes, e.g. dehydration of LPG, air, alcohols and natural
gas,
removing linear alkanes, olefins and substituted hydrocarbons from mixtures
with
branched chain compounds, e.g. in reforming, dewaxing, etc., hydrogenation and
dehydrogenation of linear hydrocarbon in admixture with branched chain
compounds.
The invention is described in the Example.
Example
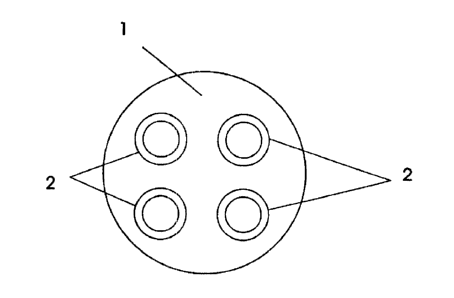
A ceramic substrate of the structure of fig.l of the drawing was pre-treated
so as to
deposit zeolite 4A powder on the inside of the channels using the following
method.
The outer ceramic tube (; l ) had a diameter of 20mm and the inner tubes (2)
had a
diameter 6.4mm
An appropriate sized pipe cleaner, which had been loaded with zeolite 4A
particles
(nominally sized 2-Sum) was inserted into one channel of a porous ceramic tube
60
cm long by 20 mm overall diameter with four channels each 6.4mm diameter and
fed
SUBSTITUTE SHEET (RULE 26)
CA 02346707 2001-04-06
WO 00/20105 PCT/GB99/03318
_ g _
through the bore of one channel until it emerged out of the other end (the
pipe cleaner
was twisted to form a stiffi~r rod so as to aid insertion through the tube).
The pipe
cleaner was pulled backwards and forwards through the channel effecting a
deposit of
4A particles on the internal walls of the channel. This was repeated for each
of the
remaining three channels.
By this method of powder deposition, between 0.435 x10 and 2.39 x10 g/cm2 of
powder were deposited on the total surface of the ceramic support. The total
weight
of powder deposited was found to vary with the pore size of the ceramic
support.
Membrane growth procedure
The zeolite membrane was formed on the inside of the four pre-treated channels
by
allowing a hydrogel suspension to be in contact with the surfaces under the
conditions
described below.
The hydrogel is formed by combining two separate solutions, (solution A) and
(solution B ) to from a homogeneous suspension.
Solution A
24.498 Sodium Aluminate, 3.758 Sodium Hydroxide and 179.748 de-ionised water
were mechanically shaken until dissolved. The Sodium Aluminate had an actual
composition 62.48% A1203., 35.24% Na20, and 2.28% H20.
Solution B
50.578 Sodium Silicate of composition 14.21% Na20, 35.59% Si02 and 50.20%
H20 was dissolved in 148.88 de-ionised water.
SUBSTITUTE SHEET (RULE 26)
CA 02346707 2001-04-06
WO 00/20105 PCT/GB99/03318
_ g _
Solution A was heated to 50°C and added slowly to solution B which had
been pre-
heated to 90°C with stirnng to ensure complete and even mixing (it is
important that
no lumps of hydrogel are formed). The mixture was then heated to 95°C.
This
resulted in a hydrogel having; a molar composition
2.01 Na20 : A1203: 2.0 Si02 : 143.10 H20
The pre-treated tube was w~etaed by immersing it in deionised water for I S
seconds.
The tube was then suspended vertically above the bottom of the growth vessel.
Hot
hydrogel was then added to the growth vessel, care being taken to ensure that
all the
air was expelled from the channels .
The growth vessel was sealed and heated to 100°C for 5 hours.
After 5 hours the tube was removed from the growth vessel, allowed to cool
slightly
and then removed and washed clean using deionised water over a period of 16
hours.
The ceramic tube was then dried at 100°C for 6 hours.
X-ray Analysis showed this to be a Zeolite 4A.
A mixture of polysilicic acids of mean molecular weight of about 800 was
diluted
with ethanol to S% wt. solids. SOOrnI. of this solution was circulated over
the feed
side of the membrane and drawn through the membrane to treat the surface
whilst
being heated to 70° C., with vacuum for 5 hours to cross-link the
silicic acid in the
pores of the membrane.
A comparison of the performance of the four channelled monolith with that of a
single narrow tube in water separation form a water/isopropanol mixture at
70°C.
Care was taken to ensure that the tubes were tested under identical conditions
of
turbulence of the feed solution and the results shown below.
.SUBSTITUTE SHEET (RULE 26)
CA 02346707 2001-04-06
WO 00/20105 PCT/GB99/03318
- 10 -
Tube Type Water Flux Number of Tube price per ~/Kg water
tubes per mZ m2 at ~100 each removed
Kgl m2l day
At Re8582 and
2% wt Water/
IPA at 70°C
4 Channel 21 22 2200 200
Narrow bore 41 100 10,000 243.9
The tube dimensions were
Tube Diameter Tube Inner Tube area per
mm Circumference 58cm length
mm
4 channel 4 x 6.4 7.92 459
Narrow Bore 1 x S.S 1.728 100.2
As can be seen the four tube configuration is surprisingly superior in
performance and
cost per unit area of membrane.
SUBSTITUTE SHEET (RULE 26)