Note: Descriptions are shown in the official language in which they were submitted.
CA 02346933 2001-04-18
DEMANDES OU BREVETS VOLUMlNEUX
L.~4 PRESENTS PARTIE DE CETTE DEMANDS OU CE BREVET
COMPAEND PLUS D'UN TOME.
CECt EST LE TOME ~ DE
NOTE. Pour les tomes additionels, veuiilez contacter le Bureau canadien des
brevets
JUMBO APPL1CAT10NS/PATENTS
Tl~ ilS SECTION OF THE APPLICATIONIPATENT CONTAINS MORE
T1HAN ONE VOLUME , ,
. THIS !S VOLI~ME ~ ~_ OI= 2_
PdOTE: For additional volumes-plE,ase.contact the Canadian Patent Office
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
TITLE
N-UREIDOALKYL-PIPERIDINES AS MODULATORS OF CHEMOKINE
RECEPTOR ACTIVITY
S FIELD OF THE INVENTION
This invention rel,~tes generally to modulators of
chemokine receptor activity, pharmaceutical compositions
containing the same, and methods of using the same as
agents for treatment and prevention of inflammatcry
diseases such as asthma and allergic diseases, as well as
autoimmune pathologies ::>uch as rheumatoid arthritis and
atherosclerosi:~.
BACKGROT.JIsTD OF THE INVENTION
Chemokines are chemotactic cytokines, of molecular
weight 6-25 kDa, that are released by a wide variety of
cells to attract and activate, among other cell types,
macrophages, T and B lymphocytes, eosinophils, basophils
and neutrophils (reviewed in Luster, New ~ng. ~ Med., 338,
436-445 (1998) and Rollins, Blood, 90, 909-928 (1997)).
There are two major cla~~s~e~; of chemokines, CXC and CC,
depending on whether the first two cysteines in the amino
acid sequence are separated by a single amino acid (CXC) or
are adjacent (CC). The C;~C chemokines, such as
interleukin-8 (IL-8), neuv~rophil-activating protein-2 (NAP-
2) and melanoma growth stimulatory activity protein (MGSA)
are chemotactic primarily for neutrophils and T
lymphocytes, whereas the CC chemokines, such as RANTES,
MIP-loC, MIP-1~, the monocvte chemotactic proteins (MCP-1,
MCP-2, MCP-3, MCP-4, and. MCP-5) and the eotaxins (-1,-2,
and -3) are chemotactic for, among other cell types,
:macrophages, T lymphocytes, eosinophils, dendritic cells,
and basophils. There also exist the chemokines
lymphotactin-1, lymphotact:in-2 (both C chemokines), and
fractalkine (a CXX.XC chemokine) that do not fall into
.either of the major chemol~:ine subfamilies.
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
The chemokines bind to specific cell-surface receptors
belonging to the family of G-protein-coupled seven-
transmembrane-domain proteins (reviewed in Horuk, Trends
Pharm. Sci., 15, 159-165 (1994)) which are termed
"chemokine receptors." On binding their cognate ligands,
chemokine receptors transduce an intracellular signal
through the associated trimeric G proteins, resulting in,
among other responses, a rapid increase in intracellular
calcium concentration, changes in cell shape, increased
expression of cellular adhesion molecules, degranulation,
and promotion of cell migration. There are at least ten
human chemokine receptors that bind or respond to CC
chemokines with the following characteristic patterns: CCR-
1 (or "CKR-1" or "CC-CKR-1") [MIP-la, MCP-3, MCP-4, RANTES]
(Ben-Barruch, et al., Cell, 72, 415-425 (1993), Luster, New
Eng. J. Med., 338, 436-445 (1998)); CCR-2A and CCR-2B (or
"CKR-2A"/"CKR-2B" or "CC-CKR-2A"/"CC-CKR-2B") [MCP-1, MCP-
2, MCP-3, MCP-4, MCP-5] (Charo et al., Proc. Natl. Acad.
Sci. USA, 91, 2752-2756 (1994), Luster, New Eng. J. Med.,
338, 436-445 (1998)); CCR-3 (or "CKR-3" or "CC-CKR-3")
[eotaxin-1, eotaxin-2, RANTES, MCP-3, MCP-4J (Combadiere,
et al., J. Biol. Chem., 270, 16491-16494 (1995), Luster,
New Eng. J. Med., 338, 436-445 (1998)); CCR-4 (or "CKR-4"
or "CC-CKR-4") [TARC, MIP-la, RANTES, MCP-1] (Power et al.,
J. Biol. Chem., 270, 19495-19500 (1995), Luster, New Eng.
J. Med., 338, 436-445 (1998)); CCR-5 (or "CKR-5" OR "CC-
CKR-5") [MIP-1a, RANTES, MIP-1(3] (Sanson, et al.,
Biochemistry, 35, 3362-3367 (1996)); CCR-6 (or "CKR-6" or
"CC-CKR-6") [LARC] (Baba et al., J. Biol. Chem., 272,
14893-14898 (1997)); CCR-7 (or "CKR-7" or "CC-CKR-7") [ELC]
(Yoshie et al., J. Leukoc. Biol. 62, 634-644 (1997)); CCR-8
(or "CKR-8" or "CC-CKR-8") [I-309, TARO, MIP-1~3J
(Napolitano et al., J. Immunol., 157, 2759-2763 (1996), .
Bernardini et al., Eur. J. Immunol., 28, 582-588 (1998));
and CCR-10 (or "CKR-10" or "CC-CKR-10") [MCP-1, MCP-3]
(Bonini et al, DNA and Cell Biol., 16, 1249-1256 (1997)).
2
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
In addition to the mammalian chemokine receptors,
mammalian cytomegaloviru;~es, herpesviruses and poxviruses
have been shown to expre=ss, in infected cells, proteins
with the binding properties of chemokine receptors
(reviewed by Wells and Schwartz, Curr. Opin. Biotech., 8,
741-748 (1997)). Human C'C chemokines, such as RANTES and
MCP-3, can cause rapid mobilization of calcium via these
virally encoded receptors. Receptor expression may be
permissive for infection by allowing for the subversion of
normal immune system surveillance and response to
infection. Additionally, human chemokine receptors, such
as CXCR4, CCR2, CCR3, CCRS and CCR8, can act as co-
receptors for the infection of mammalian cells by microbes
as with, for example, the human immunodeficiency viruses
(HIV) .
Chemokine receptors :have been implicated as being
important mediators of inflammatory, infectious, and
immunoregulatory disorder, and diseases, including asthma
and allergic diseases, as well as autoimmune pathologies
such as rheumatoid arthritis and atherosclerosis. For
example, the chemokine receptor CCR-3 plays a pivotal role
in attracting eosinophils to sites of allergic inflammation
and in subsequently activating these cells. The chemokine
ligands for CCR-3 induce a rapid increase in intracellular
calcium concentration, increased expression of cellular
adhesion molecules, ce:Llu7_ar degranulation, and the
promotion of eosinophil migration. Accordingly, agents
which modulate chemokine receptors would be useful in such
disorders and diseases. I:n addition, agents which modulate
~hemokine receptors would also be useful in infectious
diseases such as by blocking infection of CCR3 expressing
~~ells by HIV or in preventing the manipulation of immune
~~ellular responses by viruses such as cytomegaloviruses.
A substantial body of art has accumulated over the
. 35 past several decades with respect to substituted
piperidines and pyrrolidines. These compounds have
:implicated in the treatment of a variety of disorders.
3
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
WO 98/25604 describes spiro-substituted azacycles
which are useful as modulators of chemokine receptors:
RS ('C H?Jm
R N
(C H2)i
R (C N~k
3
R2
wherein R1 is C1-6 alkyl, optionally substituted with
functional groups such as -NR6CONHR~, wherein R6 and R~ may
be phenyl further substituted with hydroxy, alkyl, cyano,
halo and haloalkyl. Such spiro compounds are not
considered part of the present invention.
WO 95/13069 is directed to certain piperidine,
pyrrolidine, and hexahydro-1H-azepine compounds of general
formula:
H
4
R1--~-NHC O-A-f~
=O R5
N W
X
(CH2~
R3 Y
wherein A may be substituted alkyl or Z-substituted alkyl,
with Z=NR6a or O. Compounds of this type are claimed to
promote the release of growth hormone in humans and
animals.
WO 93/06108 discloses pyrrolobenzoxazine derivatives
as 5-hydroxytryptamine (5-HT) agonists and antagonists:
R5 ~-
N
R2
y Rs
CONH-(A)~ R4
wherein A is lower alkylene and R4 may be phenyl optionally
substituted with halogen.
4
CA 02346933 2001-04-18
WO 00135449 PCT/US99/30292
U.S. Pat. No. 5,66$,251 discloses Neuropeptide Y (NPY)
antagonists comprising 1,4-dihydropyridines with a
piperidinyl or tetrahydropyridinyl-containing moiety
attached to the 3-position of the 4-phenyl ring:
3
. HN \ Ra R~
R2 \ / NHCO-B-(CH~~ N\ X 6
.~J ~w
R~02C \ I~ R
Rs
wherein B may be NH, NR1, 0, or a bond, and R? may be
substituted phenyl, benzyl, phenethyl and the like.
These reference compounds are readily distinguished
structurally by either the nature of the urea
functionality, the attacrunent chain, or the possible
substitution of the present invention. The prior art does
not disclose nor suggest the unique combination of
structural fragments which embody these novel piperidines
and pyrrolidines as having activity toward the chemokine
receptors.
SUMMARY.' OF THE INVENTION
Accordingly, one object of the present invention is to
provide novel agonists or antagonists of CCR-3, or
pharmaceutically acceptable salts or prodrugs thereof.
It is another objects of the present invention to
provide pharmaceutical compositions comprising a
pharmaceutically acceptable carrier and a therapeutically
effective amount of at lf~ast one of the compounds of the
present invention or a pharmaceutically acceptable salt or
prodrug form thereof.
It is another object of the present invention to
provide a method for treating inflammatory diseases and
allergic disorders comprising administering to a host in
. need of such treatment a therapeutically effective amount
of at least one of the compounds of the present invention
5
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
or a pharmaceutically acceptable salt or prodrug form
thereof.
It is another object of the present invention to
provide novel N-ureidoal:kyl-piperidines for use in therapy
It is another object of the present invention to
provide the use of novel N-ureidoalkyl-piperidines for the
manufacture of a medicament for the treatment of allergic
disorders.
In another embodiment, the present invention provides
novel N-ureidoalkyl-piperidines for use in therapy.
In another embodiment, the present invention provides
the use of novel N-ureidoalkyl-piperidines for the
manufacture of a medicame=nt for the treatment of allergic
disorders.
These and other objects, which will become apparent
during the following detailed description, have been
achieved by the inventors' discovery that compounds of
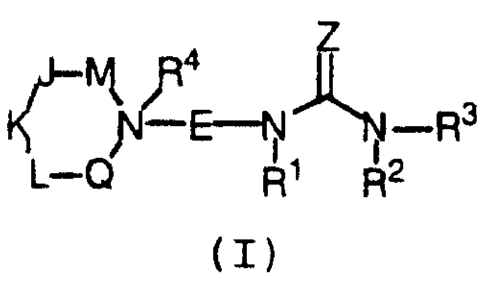
formula (I):
-iV~-R3
i
2 0 L-(~ R~ R2
(I)
or stereoisomers or pharir~aceutically acceptable salts
thereof, wherein E, Z, M, J, K, L, Q, Rl, R', R3, and R4 are
defined below, are effective modulators of chemokine
activity.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
[1] Thus, in a first embodimenr_, the present invention
provides novel compounds of formula (I):
n 4
hi
3
1~ ~E-N~-R
L-C~ R ~ R2
(I)
6
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
or stereoisomers or pharmaceutically acceptable salts
thereof, wherein:
M is absent or selected from CH2, CHRS, CHR13, CR13R13, and
CRSR13 ;
Q is selected from CHR~3, CR13R13 and CR5R13;
J, K, and L are independently selected from CH2, CHRS, CHR6,
CR6R6 and CR5R6;
with the provisos:
1) at least one of M, J, K, L, or Q contains an R5;
and
2) when M is absent, J is selected from CH2, CHRS,
CHR13 , and CR5RI3 ;
Z is selected from O, >, NRla, CHCN, CHN02, and C(CN)2;
Rla is selected from H, C1_6 alkyl, C3-6 cycloalkyl,
CONRIbRib ORib NOz, CN, and(CH2)wphenyl;
Rlb is independently selE_cted from H, C1_3 alkyl, C3_6
cycloalkyl, and phenyl;
E is -(CR~Rg)-(CR9Rlo)~--(CR~1R12)_;
R1 and R2 are independent=ly selected from H, C1-8 alkyl, C2_a
alkenyl, C2_g alkynyl, (CH2)rC3-6 cycloalkyl, and a
(CH2)r-C3-1o carbocyclic residue substituted with 0-5
Ra;
Ra, at each occurrence, is selected from C1_4 alkyl, C2_g
alkenyl, C2_g alkynyl, (CH2)rC3-6 cycloalkyl, C1, Br, I,
F, (CF2)rCF3, N02, C'N, (CH2)rNRbRb, (CH2)rOH, (CH2)rOR~,
( CH2 ) rSH , ( CH2 ) rSR~= , ( CH2 ) rC ( O ) Rb ( CH2 ) rC ( 0 ) NRbRb,
7
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
( CHZ ) rNRbC: ( O ) Rb, ( CH:2 ) rC ( O ) ORS', ( CH2 ) rOC ( O ) Rc ,
( CH2 ) rCH ( ==NRb ) NRbRb, ( CH2 ) rNHC ( =NRb ) NRbRb, ( CH2 ) rS ( O ) pRc
,
(CH2)rS(0;2NRbRb, (CH2)rNRbS(0)2Rc, and (CH2)rphenyl;
Rb, at each occurrence, is selected from H, C1_6 alkyl, C3-6
cycloalkyl, and phenyl;
Rc, at each occurrence, is selected from Cy-6 alkyl, C3-6
cycloalky:l, and phenyl;
alternatively, R2 and R3 join to form a 5, 6, or 7-membered
ring subs~ituted with 0-3 Ra;
R3 is selected from a (CR3'R3")r-C3-to carbocyclic residue
substituted with 0--5~ R1' and a (CR3'R3") r-5-10 membered
heterocyc:lic system containing 1-4 heteroatoms
selected :from N, O, and S, substituted with 0-3 R15;
R3' and R3", at each occui:rence, are selected from H, C1_s
alkyl, (CH2)rC3-6 cYcloalkyl, and phenyl;
R4 is absent, taken with the nitrogen to which it is
attached too form an N-oxide, or selected from C1_8
alkyl, C2-8 alkenyl, C2_~ alkynyl, (CH2)rC3-6
cycloalky:L, (CH2 ) qC (O) R4b, (CHZ ) qC (0) NR4aR4a~ ,
(CHZ) qC (O) OR4b, and a (CHZ ) r-C3_lo carbocyclic residue
substituted with 0-3 R4c;
R4a and R4a' , at each occurrence, are selected from H, C1_6
alkyl, (CFi2)rC3-5 cYcloalkyl, and phenyl;
R4b, at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, ~;CHZ)rC3-5 c'Ycloalkyl, C2_g alkynyl, and
phenyl;
R9c, at each occurrence, :is selected from C1-6 alkyl, C2_g
alkenyl, C:2_8 alkyny:L, C3_6 cycloalkyl, C1, F, Br, I,
8
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
CN, N02, (CF2)rCF3, (CHZ)rOC1_5 alkyl, (CH2)rOH,
(CHz)rSCl_5 alkyl, (CHZ)rNR4aR4a' and (CH2)rphenyl;
alternatively, R4 joins with R~, R5, or R1I to form a 5, 6
or 7
membered piperidinium spirocycle or pyrrolidinium
spirocycle substituted with 0-3 Ra;
R5 is selected from a (C1~5'RS" ) t-C3-1o carbocyclic residue
substituted with 0-5 R16 and a (CRS'RS")t-5-10 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, C), and S, substituted with 0-3 R16;
RS' and R5", at each occurrence, are selected from H, C1-6
alkyl, (CH2)rC3-6 cycloalkyl, and phenyl;
R6, at each occurrence, .is selected from C1_6 alkyl, C2_g
alkenyl, C2_8 alkyny7., (CH2)rC3-6 cYcloalkyl, (CF2)rCF3,
CN, (CH2)rNR6aR6a'. (CH2)rOH, (CH2)rOR6b, (CH2)rSH,
(CH2)rSR6D, (CH2)rC(0)OH, (CH2)rC(O)R6b,
(CH2)rC(O)NR6aR6a', (CH2)rNR6dC(O)R6a (CH2)rC(O)OR6b,
(CH2)rOC(0)R6b, (CH2)rS(O)pR6b (CH2)rS(0)2NR6aR6a'.
(CH2)rNR6dS(O)2R6b, and (CH2)tphenyl substituted with 0-
3 R6c;
R6a and R6a' , at each occurrence, are selected from H, C, _6
alkyl, C3_6 cycloalkyl, and phenyl substituted with 0-3
R6c;
R6b, at each occurrence, is selected from Cz_~ alkyl, C3-6
cycloalkyl, and phenyl substituted with 0-3 R6c;
R6c, at each occurrence, is selected from C1_5 alkyl, C3-6
cycloalkyl, C1, F, ~3r, I, CN, N02, (CFZ)rCF3, (CHZ)rOCl_
5 alkyl, (CH2)rOH, (~~H2)rSCl_5 alkyl, and (CH2)rNR6dR6d;
9
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R6d, at each occurrence, is selected from H, C1_6 alkyl, and
C3-6 cycloalkyl;
with the proviso that when any of J, K, or L is CR6R6 and R6
is halogen, cyano, nitro, or bonded to the carbon to
which it is attached through a heteroatom, the other
R6 is not halogen, cyano, or bonded to the carbon to
which it is attached through a heteroatom;
R~, is selected from H, C1_6 alkyl, CZ_g alkenyl, C2_
alkynyl, (CH2}qOH, (CH2)qSH, (CH2)qOR~d, (CHZ)qSR~d,
(CH2)qNR~aR7a', (CH2)rC(O)OH, (CH2)rC(O)R7b
(CH2)rC(O)NR~aR7a', (CH2)qNR~aC(O)R7a, (CH2)~NR~aC(0)H,
( CH2 ) rC ( O ) OR~b , ( CHZ ) qOC ( O ) Rib , ( CH2 ) qS ( O ) pR~ b ,
(CH2)qS(O)zNR~aR7a', (CH2}qNR~aS(O)2R7b, C1_6 haloalkyl,
a (CH2)r-C3-to carbocyclic residue substituted with 0-3
R~~, and a (CH2)r-5-10 membered heterocyclic system
containing 1-4 heteroatoms selected from N, 0, and S,
substituted with 0-2 R~~;
Rya and Rya', at each occurrence, are selected from H, C1
alkyl, C2_8 alkenyl, C2_8 alkynyl, a (CH2)r-C3-to
carbocyclic residue substituted with 0-5 Rye, and a
(CH2)r-5-10 membered heterocyclic system containing 1-4
heteroatoms selected from N, O, and S, substituted
with 0-3 Rye;
Rib, at each occurrence, is selected from Cl_6 alkyl, C2_8
alkenyl, C2_8 alkynyl, a (CH2)r-C3-s carbocyclic residue
substituted with 0-2 Rye, and a (CH2)r-5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, 0, and S, substituted with 0-3 Rye;
R~~, at each occurrence, is selected from C1_6 alkyl, C2_a
alkenyl, C2_g alkynyl, (CH2)rC3-6 cYcloalkyl, C1, Br, I,
F, (CF2)rCF3, N02, CN, (CHZ)rNR~fR7f, (CH2)rOH,
(CH2)rOCl_4 alkyl, (CH2)rSCl_4 alkyl, (CH2)rC(O)OH,
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
(CH2)rC(O)R7b (CH~)rC(O)NR~fR7f, (CH2)rNR~fC(0)R7a
(CHZ ) rC (O) OC1_4 alkyl, (CH2 ) rOC (O) Rib,
( CHZ ) rC ( =NR~ f ) NR~ f R7 f ~ ( CH2 ) rS ( O ) pR7b
(CH2)rNHC(=NR~f)NR';~fr~7f~ (CH2)rS(0)2NR~fR7f~
(CH2)rNR~fS(O)2R~b, and (CH2)rphenyl substituted with 0-
3 Rye;
Rid, at each occurrence, is selected from C~_~ alkyl
substituted with CD-3 Rye, alkenyl, alkynyl, and a C3_1o
carbocyclic residue substituted with 0-3 R~~;
Rye, at each occurrence, is selected from C1_b alkyl, C2_g
alkenyl, C2_g alkynyl, C3-6 cycloalkyl, Cl, F, Br, I,
CN, N02, (CF2}rCF3, (CH4)rOC1_5 alkyl, OH, SH, (CH2)rSCl_
~ alkyl, (CH2)rNR~fR~-', and (CH2)rphenyl;
Ref, at each occurrence, is selected from H, C1-6 alkyl, and
C3_6 cycloalkyl;
R8 is selected from H, C_~_6 alkyl, C3__6 cycloalkyl, and
(CH2)rphenyl substituted with 0-3 RBa;
R8a, at each occurrence, is selected from C1_~ alkyl, C2_8
alkenyl, C2_a alkynyl, C3_6 cycloalkyl, Cl, F, Br, I,
CN, N02, (CFZ)rCF3, (CH2)rOCl_5 alkyl, OH, SH, (CH2)rSCl_
alkyl , ( CH2 ) rNR~ f R'7 f and ( CH2 ) rphenyl ;
alternatively, R~ and R8 join to form C3_~ cycloalkyl, or
-NR$b;
R8b is selected from H, C_'~-5 alkyl, C3_6 cycloalkyl, OH, CN,
and (CH2)r-phenyl;
R9, is selected from H, C_Z_s alkyl, C2_8 alkenyl, C2_a
alkynyl, F, C1, Br, I, N02, CN, (CH2)rOH, (CH2)rSH,
(CH2 ) rOR93, (CH2 ) rSR'.3d~ (CH2 ) rNR~aR9a' , (CH2 ) rC (0) OH,
(CH2)rC(O)R9b, (CH;r)rC(0)NR9aR9a', (CH2)rNR9aC(O)R9a
11
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
(CH2)rNR9aC(O)H, (CH2)rNR9aC(O)NHR9a, (CH2)rC(O)OR9b,
(CH2)rOC(0)R9b (CH2)rOC(O)NHR9a, (CH2)rS(O)pR9b,
(CH2)rS(0)zNR9aR9a', (CH2)rNR9aS(0)ZR9b, C1_6 haloalkyl,
a (CH2)r-C3-1o carbocyclic residue substituted with 0-5
R9~, and a (CH2)r-5-10 membered heterocyclic system
containing 1-4 heteroatoms selected from N, O, and S,
substituted with 0-3 R9~;
R9a and R9a', at each occurrence, are selected from H, C1-6
alkyl, C2-8 alkenyl, C2_8 alkynyl, a (CH2)r-C3-1o
carbocyclic residue substituted with 0-5 R9e, and a
(CH2)r-5-10 membered heterocyclic system containing 1-4
heteroatoms selected from N, 0, and S, substituted
with 0-3 R9e;
R9b, at each occurrence, is selected from C1-6 alkyl, C2_g
alkenyl, C2_8 alkynyl, a (CH2)r-C3-5 carbocyclic residue
substituted with 0-2 R9e, and a (CH2)r-5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-3 R9e;
R9~, at each occurrence, is selected from C1_6 alkyl, C2_a
alkenyl, C2_g alkynyl, (CH2)rC3-6 cycloalkyl, C1, Br, I,
F, (CF2)rCF3, N02, CN, (CH2)rNR9fR9f (CH2)rOH,
(CH2)rOCl_4 alkyl, (CH2)rSCl-4 alkyl, (CH2)rC(O)OH,
(CH2)rC(O)R9b (CH2)rC(0)NR9fR9f (CH2)rNR9fC(O)R9a
(CH2)rC(O)OC1_4 alkyl, (CHZ)rOC(O)R9b,
(CH2 ) rC (=NR9f ) NR9fR9f (CH2 ) rS (0) pR9b
(CH2)rNHC(=NR9f)NR9fR9f (CH2)rS(O)2NR9fR9f~
(CH2)rNR9fS(0)ZR9b, and (CH2)rphenyl substituted with 0-
3 R9e;
R9d, at each occurrence, is selected from C1_6 alkyl, C2-5
alkenyl, C2-6 alkynyl, a C3-to carbocyclic residue
substituted with 0-3 R9~, and a S-6 membered
heterocyclic system containing 1-4 heteroatoms
12
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
selected from the group consisting of N, O, and S
substituted with 0-3 R9c;
_ R9e, at each occurrence, is selected from C1_6 alkyl, C2-8
alkenyl, C2_8 a7_kynyl, (CH2)rC3-5 cYcloalkyl, C1, F, Br,
I, CN, N02, (CF~)rCF3, (CHZ)rOC1_5 alkyl, OH, SH,
(CH2)rSC1_S alkyl, (CH2)rNR9fR9f, and (CH2)rphenyl;
R9f, at each occurrence, is selected from H, C1-6 alkyl, and
C3-6 cycloalkyl;
Rlo, is selected fronn H, C1_6 alkyl, C2_8 alkenyl, C2_8
alkynyl, F, C1, Br, I, NO?, CN, (CH2)rOH, (CH2)rORlOd~
( CH2 ) rSRlOd ( C_'H2 ) r.NR10aR10a' , ( CHZ ) rC ( O ) OH,
( CH2 ) rC ( 0 ) RlOb r ( CH2 ) rC ( O ) NR1 OaRlOa' ~ ( CHZ ) rNRlOaC ( O )
RlOa
(CH2)rNRlOaC(O)H, (CH2)rC(O)ORlOb (CH2)rOC(O)RlOb
( CH2 ) rS ( O ) pRlOb ( CH2 ) rS ( O ) 2NR10aR10a'
(CH2) rNRloaS (O) Z,Rlob, C1-6 haloalkyl, a (CH2) r-C3-10
carbocyclic residue substituted with 0-5 Rloc, and a
(CHZ)r-5-10 mernbered heterocyclic system containing 1-4
heteroatoms selected from N, O, and S, substituted
with 0-3 Rloc;
Rloa and Rloa' , at each occurrence, are selected from H, C1_6
alkyl, C2_8 alkenyl, C2_g alkynyl, a (CH2)r-C3-10
carbacyclic residue substituted with 0-5 Rloe, and a
(CHZ)r-5-10 membered heterocyclic system containing 1-4
heteroatoms sel~scted from N, O, and S, substituted
with 0-3 RlOe;
Rlob at each occurrence, is selected from C1_6 alkyl, C2_g
alkenyl, C2_g alkynyl, a (CH2)r-C3-6 carbocyclic residue
substituted with 0-2 Rloe, and a (CH2)r-5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-3 Rloe;
13
CA 02346933 2001-04-18
WO 00/35449 PCf/US99/30292
F;loc~ at each occurrence, .is selected from C1_6 alkyl, C2_8
alkenyl, C~>_g alkynyl, (CHZ) rC3-6 cycloalkyl, C1, Br, I,
F, (CF2)rCF'3, N02, CN, (CH2)rNRl~'fRlOf~ (CH2)rOH,
(CH2)rOCl-4 alkyl, (CH2)rSCl_4 alkyl, (CH2)rC(O)OH,
(CH2)rC(O)RlOb~ (CH2)rC(0)NR10fR10f, (CH2)rNRlOfC(0)RlOa~
(CH2)rC(O)OC1_q alkyl, (CH2)i.OC(O)Rlob~
(CH2)rC(=NRlOf)~lOfRlOf~ (CH~,)rS(0)pRlOb~
(CH2 ) rNHC (=NRlOf ) NRlOf~~lOf (CH2 } rS (O) 2NR10fR10f
(CHZ ) rNRlOf;~ (0) 2R10b~ ~~nd (CHa ) rphenyl substituted with
0-3 RlOe;
Rlod, at each oc~~urrence, is selected from C1_6 alkyl, C2-6
alkenyl, C2_6 alkynyl, a C3-1.o carbocyclic residue
substituteoi with 0-3 Rloc, and a 5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from the group consisting of N, 0, and S
substituted. with 0-3 Bloc;
R:loe~ at each occurrence, i.s selected from C1_6 alkyl, C2_8
alkenyl, C2_8 alkynyl, (CH2)rC3-s cYcloalkyl, C1, F, Br,
I, CN, N02, (CF2)rCF3, (CH2)rOCl_~ alkyl, OH, SH,
(CH2)rSCl_5 alkyl, (CHO)rNRlOfRlOf and (CH2)rphenyl;
R:LOf ~ at each occurrence, is seler_ted from H, C1-6 alkyl,
and C3_6 cycloalkyl;
a:Lternatively, R9 and R10 join to form C3_~ cycloalkyl, 5-6-
membered cyclic ketal" or =O;
wuth the proviso that when Rlo is halogen, cyano, nitro, or
bonded to t:he carbon too which it is attached through d
heteroatom, R9 is not halogen, cyano, or bonded to the
carbon to which it is attached through a heteroatom;
R11, is selected from H, C1_~ alkyl, C2_g alkenyl, C2_8
alkynyl , (Cl~z ) qOH, (CH2 ) qSH, (CH2 ) qORlld (CH2 ) qSRlld,
(CHZ}qNR11aR11a'~ (CH2)rC(0)OH, (CH2)rC(O)Rllb
14
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
( CHz ) rC ( O ) NRilaRl la" ( CH2 ) qNRllaC ( O ) Rlla
(CH2)qNRllaC(0)NHRlla~ (CH2)rC(0)ORllb (CHZ)qOC(O)Rllb
(CH2)qS(O)pRllb, (CH;~)qS(0)2NR11aR11a'
(CH2)qNRllaS(O)2Rllb C1-6 haloalkyl, a (CH2)r-C3-10
carbocyclic residue substituted with 0-5 Rllc, and a
(CH2)r-5-10 memberec~ heterocyclic system containing 1-4
heteroatoms selected from N, 0, and S, substituted
with 0-3 Rllc;
Rlla and Rlla', at each occurrence, are selected from H, C1-s
alkyl, Cz_g alkenyl, C2_g alkynyl, a (CH2)r-C3-to
carbocyclic residue substituted with 0-5 Rlle, and a
(CH2)r-5-10 memberec) heterocyclic system containing 1-4
heteroatoms selected from N, 0, and S, substituted
with 0-3 Rlle;
Rllb~ at each occurrence, is selected from C1_6 alkyl, C2_g
alkenyl, C2_g alkynyl, a (CH2)r-C3-s carbocyclic residue
substituted with G-:2 Rlle and a (CH2)r-5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-3 Riles
Rllc~ at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, Cz_8 alkynyl, (CH2)rCz_6 cycloalkyl, Cl, Br, I,
F, (CF2)rCF3% NO?, CN, (CH2)rNRllfRllf (CH2)rOH,
(CH2)rOCl._4 alkyl, (CH~)rSCI_4 alkyl, (CH2)rC(O)OH,
(CH2)rC(O)Rllb (CHZ)rC(O)NRllfRllf~ (CH2)rNRllfC(O)Rlla
(CH2)rC(O)OC1_q alkyl, (CH2)rOC(O)Rllb
(CH2)rC(=NRllf)NRIIfFZIlf (CH2)rNHC(=NRllf)NRllfRllf
(CH2 ) rS ,(O) pRllb (C,HZ ) rS (0) 2NR11fRllf
(CH2)rNRllfS(0)2Rllb and (CH2)rphenyl substituted with
0-3 Rlle
Rlld, at each occurrence, is selected from C1_6 alkyl
substituted with 0-3 Rlle, C2_e alkenyl, CZ_6 alkynyl,
and a C3-to carbocyclic residue substituted with 0-3
Rllc.
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Rlle, at each occurrence, is selected from C1_6 alkyl, Cz_g
alkenyl, Cz_8 alkynyl, C3_6 cycloalkyl, Cl, F, Br, I,
CN, NOz, (CFZ)rCF3, (CHZ)rOCI_5 alkyl, OH, SH, (CHZ)rSCl_
5 alkyl, (CHz)rNRIIfRW f, and (CHZ)rphenyl;
Rlif, at each occurrence, is selected from H, C1_6 alkyl,
and C3_6 cycloalkyl;
R1z is selected from H, C~_6 alkyl, (CHZ)qOH, (CH2)rC3-6
cycloalkyl, and (CHz)tphenyl substituted with 0-3 Rl2a;
Rlza~ at each occurrence, is selected from C1_6 alkyl, C2_g
alkenyl, Cz_8 alkynyl, C3_6 cycloalkyl, C1, F, Br, I,
CN, NO2, (CF2)rCF3, (CH2)rOCl_5 alkyl, OH, SH, (CH2)rSCl_
5 alkyl, (CHz)rNR9fR9f, and (CHZ)rphenyl;
alternatively, R11 and R1z join to form C3_~ cycloalkyl;
R13, at each occurrence, is selec'~-~ from (CHR"°) OH,
( CHRl3a ) ORl3b ! C:ri;;m~ ) SH ,
(CHRlsa) SRl3b, (CHRl3a)NR1%eC (p) ~t='F, and
(CHRl3a)NRl3eS (O) 2R13f;
Rl3a is selected from C1_~ alkyl;
Rl3b, at each occurrence, is selected from C(O)RI3d,
C(O)NHRl3d Cl_E alkyl, C3_6 cycloalkyl, and phenyl
13c.
substituted with 0-3 R
Rl3c, at each occurrence, is selected from C1_6 alkyl, Cz-g
alkenyl, C2_8 alkynyl, C3-6 cycloalkyl, C1, F, Br, I,
CN, NOZ, (CFZ)rCF3, (CHz)rOCl_5 alkyl, OH, SH, (CHZ)rSCl_
5 alkyl, (CHZ)rNR9fR9f, and (CHZ)rphenyl;
Rl3a, at each occurrence, is selected from C1_6 alkyl, C3_6
cycloalkyl, and phenyl substituted with 0-3 R6c;
16
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Ri3e, at each occurrence, is selected from H, C1_6
alkyl, (CH2)rC3-5 cyc:loalkyl, and phenyl where phenyl
is substituted from G-3 R13~
Rl3f, at each occurrence, is selected from C1_s
alkyl, (CH2)rC3-5 cycloalkyl, CF3, and phenyl where
phenyl is substituted from 0-3 Rl3c
R15, at each occurrence, :is independently selected from C1_g
alkyl, (CH2)rC3-s cycl.oalkyl, C1, Br, I, F, N02, CN,
( CHR' ) rNRl 5aR15a' , ( C~iR' ) rOH, ( CHR' ) r.0 ( CHR' ) rRlSd,
(CHR')rSH, (CHR')rC(0)H, (CHR')xS(CHR~)rRlSd,
(CHR')rC(O)OH, (CHR')rC(O)(CHR')rRlSb,
(CHR' ) rC (0)NR15aR15a' , (CHR' ) rNRlSfC (O) (CHR' ) rRlSb,
(CHR' ) rNRlSfC (0) NRlSfFt~iSf, (CHR' ) rC (O) O (CHR' ) rRlSd,
( CHR' ) rOC (O) (CHR' ) ~RlSb, (CHR' ) rC (=NRlSf ) NR15aR15a' ,
(CHR' ) rNHC (=NRlSf ) NRlSfRlsf, (CHR' ) rS (O) p (CHR' ) rRlSb,
(CHR' ) rS (O) 2NR15aR15a' ~ (CHR' ) rNRl5fS (O) 2 (CHR' ) rRlSb, C1-6
haloalkyl, C2_g alkenyl substituted with 0-3 R', C2_s
alkynyl substituted with 0-3 R', (CHR')rphenyl
substituted with 0-3 R,lse, and a (CH2)r-5-10 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-2 RlSe.
R', at each occurrence, i;~ selected from H, C1_6 alkyl, C2-g
alkenyl, C2_g alkynyl, (CH2)rC3-6 cycloalkyl, and
(CH2)rphenyl substituted with RlSe;
RlSa and RlSa', at each occurrence, are selected from H, C1_6
alkyl, C2_g alkenyl, C2_g alkynyl, a (CH2)r-C3-to
carbocyclic residue ssubstituted with 0-5 Rl5e, and a
(CH2)r-5-10 rnembered heterocyclic system containing 1-4
heteroatoms selected from N, 0, and S, substituted
with 0-2 RlSe
I7
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Rlsb at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, C2_g alkynyl, a (CH2)Y-C3_6 carbocyclic residue
substituted with 0-3 Rlse, and (CH2)r-5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-2 RlSe;
Risd~ at each occurrence, is selected from CZ_8 alkenyl, C2_8
alkynyl, C1_6 alkyl substituted with 0-3 RlSe, a
(CH2)r-C3-to carbocyclic residue substituted with 0-3
RlSe~ and a (CH2)r5-6 membered heterocyclic system
containing 1-4 heteroatoms selected from N, O, and S,
substituted with 0-3 Rlse_
Rise at each occurrence, is selected from C1_6 alkyl, C2_g
alkenyl, C2_8 alkynyl, (CH2)rC3-6 cycloalkyl, C1, F, Br,
I, CN, N02, (CF2)rCF3, (CH2)YOC1_5 alkyl, OH, SH,
(CH2)rSCl_s alkyl, (CH2)rNRlSfRlsf and (CHZ)rphenyl;
Rlsf, at each occurrence, is selected from H, C1-6 alkyl,
C3-6 cycloalkyl, and phenyl;
R16, at each occurrence, is selected from C1_g alkyl, CZ_g
alkenyl, C2_g alkynyl, (CHz)rC3-6 cycloalkyl, Cl, Br, I,
F, N02, CN, (CHR')rNR16aR16a' (CHR')rOH,
(CHR')r0(CHR')rRl6d (CHR')rSH, (CHR')rC(O)H,
(CHR')rS(CHR')rRl6d (CHR')rC(O}OH,
( CHR' ) rC ( 0 ) ( CHR' ) rRl6b , ( CHR' ) y C ( O ) NR16aR16a'
(CHR')rNRl6fC(O)(CHR')rRl6b (CHR')rC(0)0(CHR')rRl6d
( CHR' ) rOC ( O ) ( CHR' ) rRl 6b , ( CHR' ) rC ( =NR16 f ) NR16aR16a'
(CHR')rNHC(=NRl6f}NR16fR16f (CHR')rS(0)D(CHR')rRl6b
(CHR')rS(O)2NR16aR16a' (CHR')rNRl6fS(0)2(CHR')rRl6b C1_6
haloalkyl, C2-8 alkenyl substituted with 0-3 R', C2_8
alkynyl substituted with 0-3 R', and (CHR')rphenyl
substituted with 0-3 Rl6e;
Rl6a and Rl6a', at each occurrence, are selected from H, C1_6
alkyl, C2_g alkenyl, C2_g alkynyl, a (CH2)r-C3-1o
18
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
carbocyclic residue ~~ubstituted with 0-5 Rl6e, and a
(CH2)r-5-10 membered heterocyclic system containing 1-4
heteroatoms selected from N, 0, and S, substituted
with 0-2 Rl6e;
,~16b~ at each occurrence, is selected from C1_6 alkyl, C2_g
alkenyl, C2_e alkynyl, a (CH2)rC3-6 carbocyclic residue
substituted with 0-.3 Rl6e, and a (CH2)r-5-6 membered
heterocyclic system containing 2-4 heteroatoms
selected from N, 0, a.nd S, substituted with 0-2 Rl6e;
FZl6d~ at each occurrence, .is selected from C2_8 alkenyl, C2_g
alkynyl, C;1_6 alkyl substituted with 0-3 Rl6e~ a
(CH2)r-C3-1o carbocyclic residue substituted with 0-3
Rl6e and a (CH2)r-5-6 membered heterocyclic system
containing 1-4 heteroatoms selected from N, O, and S,
substituted with 0-3 Rl6e_
F;l6e, at each occurrence, is selected from C1_6 alkyl, C2_g
alkenyl, C2_g alkynyl, (CH2)rC3-6 cYcloalkyl, C1, F, Br,
I, CN, N02, (CF2)rCF3, (CH2)rOCI_5 alkyl, OH, SH,
(CH2)~SC1-5 alkyl, (CHZ)rNR16fR16f, and (CHZ)rphenyl;
R,l6f~ at each occurrence, i.s selected from H, C1_5 alkyl,
and C3-6 cycloalkyl, a.nd phenyl;
v is selected from 0, 1, and 2;
t is selected from 1 and 2;
w is selected from 0 and 1;
r is selected from 0, 1, 2, 3, 4, and 5;
q is selected from 1, 2, 3, 4, and 5; and
p is selected from 0, 1, 2, and 3.
19
CA 02346933 2001-04-18
WO 00/35449 PCT/US99I30292
[2] In a preferred embodiment, the present invention
provides novel compounds of formula (I), wherein:
R4 is absent, taken with the nitrogen to which it is
attached to form an N-oxide, or selected from C1_g
alkyl, (CH2)rC3-6 cycloalkyl, and (CHz)r-phenyl
substituted with 0-3 R4c.
R4c, at each occurrence, is selected from CI_6 alkyl, Cz_8
alkenyl, C2_s alkynyl, C3_6 cycloalkyl, C1, F, Br, I,
CN, N02, (CF2)rCF3, (CH2)rOCl_5 alkyl, (CH2)rOH,
(CHZ)rSCl_5 alkyl, (CH2)rNR4aR4a', and (CH2)rphenyl;
alternatively, R4 joins with R~, R9, or R11 to form a 5, 6
or 7
membered piperidinium spirocycle or pyrrolidinium
spirocycle substituted with 0-3 Ra;
R1 and R2 are independently selected from H and C1_4 alkyl;
R6, at each occurrence, is selected from C1_4 alkyl, C2_8
alkenyl, Cz_8 alkynyl, (CH2)rC3-6 cycloalkyl, (CF2)rCF3,
CN, (CH2 ) rOH, (CH2 ) rOR6b, (CH2 ) rC (O) R6b,
(CHZ)rC(O)NR6aR6a', (CH2)rNR6dC(0)R6a, and (CH2)tphenyl
substituted with 0-3 R6c;
R6a and R6a', at each occurrence, are selected from H, C1_6
alkyl, C3_6 cycloalkyl, and phenyl substituted with 0-3
R6c .
R6b, at each occurrence, is selected from C1_6 alkyl, C3_6
cycloalkyl, and phenyl substituted with 0-3 R6c;
R6c, at each occurrence, is selected from C1_6 alkyl, C3-5
cycloalkyl, Cl, F, Br, I, CN, N02, (CF2)rCF3, (CH2)rOC1-
5 alkyl, (CHZ)rOH, (CH2)rSCl_5 alkyl, and (CHZ)rNR6dR6d;
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30Z92
R6~, at each occurrence, :is selected from H, C1_6 alkyl, and
C3-6 cycloalkyl;
R~, is selected from H, C.L_3 alkyl, (CH2)rC3-6 cYcloalkyl,
(CH2)qOH, (CH2)qOR~d, (CH2)qNR~aR7a', (CH2)rC(0)R~b,
(CH2 ) rC (O) NR~aR7a' , (CH2 ) qNR~aC (O) Rya, Cl_6 haloalkyl,
(CH2)rphenyl with 0-2 R~~;
IQ Rya and Rya', at each occurrence, are selected from H, C1-6
alkyl, (CHZ)rC3-5 cycloalkyl, a (CH2)rphenyl
substituted with 0-3 Rye;
Rib, at each occurrence, i.s selected from C1_6 alkyl, C2_8
alkenyl, C.2_g alkynyl, (CH2)rC3-6 cycloalkYl,
(CH2)rphenyl substitLaed with 0-3 Rye;
R~~, at each occurrence, is selected from C1_4 alkyl, C2_8
alkenyl, C2_8 alkynyl, (CH2)rC3-6 cYcloalkyl, C1, Br, I,
F, (CFZ)rCF3, N02, CN, (CH2)rNR~fR7f, (CHZ)rOH,
(CH2)rOCl_q alkyl, (CH2)rC(O)R~b, (CH2)rC(O)NR~fR7f
(CHZ)rNR~fC(O)R~a, (CH2)rS(O)pR7b (CHZ)rS(O)2NR~fR7f~
(CH2)rNR~fS(O)2R~b, and (CH2)rphenyl substituted with 0-
2 Rye;
Rid, at each occurrence, is selected from C1_6 alkyl,
(CH2)rC3-6 CYcloalkyl, (CH2)rphenyl substituted with 0-
3 Rye;
3E~ l~~e, at each occurrence, is selected from C1_6 alkyl, Cz_8
alkenyl, C2_8 alkynyl, C3-6 cycloalkyl, C1, F, Br, I,
CN, N02 , (CF2 ) rCF3 , (CH2 ) rOCl_5 alkyl , OH, SH, (CH2 ) rSCz_
5 alkyl , ( CH2 ) rNR~ f R~~ f , and ( CH2 ) phenyl ;
~z~f, at each occurrence, is selected from H, C1_5 alkyl, and
C3-6 cycloalkyl;
21
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R8 is H or joins with R? to form C3_~ cycloalkyl or =NR8b;
R11, is selected from H, C1_6 alkyl, (CH2)rC3-5 cycloalkyl
(CH2)qOH, (CH2)qORlld tCH2)qNRllaRlla', (CH2)rC(0)Rllb
(CH2)rC(O)NRllaRlla' (CH2)qNRllaC(O)Rlla C1-6 haloalkyl,
(CH2)rphenyl with 0-2 Rllc, (CH2)r-5-10 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-3 R15;
Rlla and Rlla' , at each occurrence, are selected from H, C1_6
alkyl, (CH2)rC3-s cycloalkyl, a (CH2)rphenyl
substituted with 0-3 Rlle.
Rllb, at each occurrence, is selected from C1_6 alkyl,
alkenyl, C2_8 alkynyl, (CH2)rC3-5 cycloalkyl,
(CHZ)rphenyl substituted with 0-3 Rlle;
Rllc at each occurrence, is selected from C1_4 alkyl, C2_g
alkenyl, C2_8 alkynyl, (CH2)rC3-6 cycloalkyl, C1, Br, I,
F, (CF2)rCF3, N02, CN, (CHZ)rNRllfRllf (CH2)rOH,
(CH2)rOCl_4 alkyl, (CH2)rC(O)Rllb (CH~)rC(O)NRllfRllf
(CHZ)rNRllfC(O)Rlla~ (CH2)rS(O)pRllb
(CHZ ) rS (O) 2NR11fRllf (CH2 ) r~llfS (O) 2Rllb arid
(CH2)rphenyl substituted with 0-2 Rlle;
Rlld, at each occurrence, is selected from C1_6 alkyl,
(CH2)rC3-6 cycloalkyl, (CHZ)rphenyl substituted with 0-
3 Rlle.
Rlle, at each occurrence, is selected from C1_6 alkyl, CZ_8
alkenyl, Cz_8 alkynyl, C3-6 cycloalkyl, C1, F, Br, I,
CN, N02, (CF2)rCF3, (CHz)rOCl_5 alkyl, OH, SH, (CHZ)rSCl_
5 alkyl, (CH2)rNR11fR11f, and (CH2)rphenyl;
Rllf at each occurrence, is selected from H, C1_S alkyl and
C3-6 cycloalkyl;
22
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R12 is H or joins with R11 to form C3_7 cycloalkyl;
v is selected from 1 and 2;
q is selected from 1, 2, and 3; and
r is selected from 0, 1, 2, and 3.
[3] In a more preferred Esmbodiment, the present invention
;..provides novel compounds of formula (I), wherein:
:R3 is selected from a (CR'''H)r-carbocyclic residue
substituted with 0-5 R15, wherein the carbocyclic
residue is selected ,From phenyl, C3_6 cycloalkyl,
naphthyl, and adamant=yl; and a (CR3'H)r-heterocyclic
system substituted with 0-3 R15, wherein the
heterocyclic system .is selected from pyridinyl,
thiophenyl, furanyl, :indazolyl, benzothiazolyl,
benzimidazolyl, benzothiophenyl, benzofuranyl,
benzoxazolyl, benzisoxazolyl, quinolinyl,
isoquinolinyl, imidazolyl, indolyl, indolinyl,
isoindolyl, isothiad_Lazolyl, isoxazolyl, piperidinyl,
pyrrazolyl, 1,2,4-tr_~azolyl, 1,2,3-triazolyl,
tetrazolyl, thiadiazo:lyl, thiazolyl, oxazolyl,
pyrazinyl, and pyrim_Ldinyl; and
l~5 is selected from (CR''H)t-phenyl substituted with 0-5
R16; and a (CRS'H)t-h~aterocyclic system substituted
with 0-3 R16, wherein. the heterocyclic system is
selected from pyridinyl, thiophenyl, furanyl,
indazolyl, benzothia::olyl, benzimidazolyl,
benzothiophenyl, ben::ofuranyl, benzoxazolyl,
benzisoxazolyl, quinolinyl, isoquinolinyl, imidazolyl,
indolyl, isoindolyl, piperidinyl, pyrrazolyl, 1,2,4-
triazolyl, 1,2,3-triazolyl, tetrazolyl, thiazolyl,
oxazolyl, pyrazinyl, and pyrimidinyl.
23
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
[4) In an even more preferred embodiment, the present
invention provides novel compounds of formula (I-i),
wherein the compound of formula (I-i) is:
Z
J II
K ~N-E-N~N-R3
- L-~ H H
(I-i)
R16, at each occurrence, is selected from C1_8 alkyl,
(CH2)rC3-6 cycloalkyl, CF3, C1, Br, I, F,
(CH2)r~16aR16a' N02 CN, OH,, (CHZ)rORl6d
(CH2)rC(O)Rl6b, (CH2)rC(O)NR16aR16a', (CH2)rNRl6fC(O)Rl6b
(CH2)rS(0)pRl6b (CH2)rS(0)2NR16aR16a',
(CH2)rNRl6fS(O)2R16b and (CH2)rphenyl substituted with
0-3 Rl6e-
Rl6a and Rl6a' at each occurrence, are selected from H, C1_6
alkyl, C3_6 cycloalkyl, and (CH2)rphenyl substituted
with 0-3 Rl6e;
Rl6b at each occurrence, is selected from H, C1_6 alkyl,
C3_6 cycloalkyl, and (CH2)rphenyl substituted with 0-3
Rl6e;
Rl6d at each occurrence, is selected from C1_6 alkyl and
phenyl;
Rl6e, at each occurrence, is selected from C1_6 alkyl, C1,
F, Br, I, CN, N02, (CFZ)rCF3, OH, and (CH2)rOCl_5 alkyl;
and
Rl6f, at each occurrence, is selected from H, and C1-5
alkyl.
[5) In another even more preferred embodiment, the present
invention provides novel compounds of formula (I-ii),
wherein the compound formula (I-ii) is:
24
CA 02346933 2001-04-18
WO 00/35449 PCT/US99I30292
z
~K ~N-E-N~N-R3
H H
,
(I-ii)
R16, at each occurrence, is selected from C1_g alkyl,
(CH2)rC:3-6 cYcloalkyl, CF3, C1, Br, I, F,
(CH2)rNR16aR16a', NO~, CN, OH, (CH2)rORl6d~
(CH2)rC:(0)Rl6b, (CH2)=C(O)NR16aR16a', (C:HZ)rNRl6fC(0)Rl6b~
(CH2)rS(O)pRl6b, (CH2)rS(O)2NR16aR16a',
(CH2)rNRl6fs(O)2R16b, and (CH2)rphenyl substituted with
0-3 RlE~e;
Rl6a and Rl6a', at each occurrence, are selected from H, C1_6
alkyl, C3_6 cycloalkyl, and (CH2)rphenyl substituted
with 0--3 Rl6e;
Rl6b, at each occurrence, is selected from H, C1_6 alkyl,
C3_6 cycloalkyl, and (CH2)rphenyl substituted with 0-3
Rl6e;
Rl6d, at each occurrence, is selected from C1-6 alkyl and
phenyl;
Rl6e, at each occurrence, is selected from C1-6 alkyl, C1,
2'.~ F , Br , I , CN , N02 , ( CF2 ) rCF3 , OH , and ( CH2 ) rOC1-5 alkyl ;
and
Rl6f, at each occurrence, is selected from H, and C1-5
alkyl.
[6] In a preferred embodiment, the present invention
provides no~~el compounds of formula (I-i) wherein:
R5 is CH2phenyl substituted with 0-3 R16
3 ~i
E is -CH2- (CR9R1~ ) - ( CR11R12 ) ;
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R9, is selected from H, C1_6 alkyl, (CH2)rC3-6 cycloalkyl, F,
Cl, CN, (CH2)rOH, (CH2)rOR9d, (CH2)rNR9aR9a',
(CH2)rOC(O)NHR9a, (CH2)rphenyl substituted with 0-5 R9e,
and a heterocyclic system substituted with 0-2 R9e,
wherein the heterocyclic system is selected from
pyridyl, thiophenyl, furanyl, oxazolyl, and thiazolyl;
R9a and R9a~ , at each occurrence, are selected from H, Cl_6
alkyl, C3_6 cycloalkyl, and (CH2)rphenyl substituted
with 0-3 R9e;
R9a, at each occurrence, is selected from C1_6 alkyl and
phenyl;
R9e, at each occurrence, is selected from C1_6 alkyl, C1, F,
Br, I, CN, NO2, (CF2)rCF3, OH, and (CH2)rOCl-5 alkyl;
R1~ is selected from H, C1_5 alkyl, OH, and CH20H;
alternatively, R9 and R1~ join to form C3_~ cycloalkyl, 5-6-
membered cyclic ketal or =O;
with the proviso that when R1~ is halogen, cyano, nitro, or
bonded to the carbon to which it is attached through a
heteroatom, R9 is not halogen, cyano, or bonded to the
carbon to which it is attached through a heteroatom;
R11 is selected from H, C1_g alkyl, (CHZ)rphenyl substituted
with 0-5 Rlle, and a (CH2)r-heterocyclic system
substituted with 0-2 Rlie, wherein the heterocyclic
system is selected from pyridinyl, thiophenyl,
furanyl, indazolyl, benzothiazolyl, benzimidazolyl,
benzothiophenyl, benzofuranyl, benzoxazolyl,
benzisoxazolyl, quinolinyl, isoquinolinyl, imidazolyl,
indolyl, isoindolyl, piperidinyl, pyrrazolyl, 1,2,4-
26
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
triazolyl, 1,2,3-triazolyl, tetrazolyl, thiazolyl,
oxazolyl, pyrazinyl, and pyrimidinyl; and
_ Rlle~ at each occurrence, is selected from C1_6 alkyl, C1,
F, Br, I, CN, N02, (CF2)rCF3, OH, and (CH2)rOCl_5 alkyl;
R12 is H;
alternatively, R11 ancL R12 join to form C3_~ cycloalkyl; and
:L 0
r is selected from 0, :L, and 2.
[7]. In another preferred embodiment, the present
invention provides novel compounds of formula (I-ii),
:L5 wherein:
R5 is CH2phenyl substituted with 0-3 Rls;
E is -CH2- (CR9Rlo) - (CR:L1R12 ) ;
R9, is selected from H, C1_6 alkyl, (CH2)rC3-6 cYcloalkyl, F,
C1, CN, (CH2)rOH, (CH2)rOR9d, (CH2)rNR9aR9a'.
(CH2)rOC(O)NHR9a, (CH2)rphenyl substituted with 0-5 R9e,
and a heterocyc:li.c system substituted with 0-2 R9e,
wherein the heterocyclic system is selected from
pyridyl, thiophenyl, furanyl, oxazolyl, and thiazolyl;
R9a and R9a', at each occurrence, are selected from H, C,_6
alkyl, C3_6 cycloalkyl, and (CH2)rphenyl substituted
3 0 with 0-3 R9e;
R9d, at each occurrence, is selected from C1_6 alkyl and
phenyl;
R9e, at each occurrence, is selected from C1_6 alkyl, Cl, F,
Br, I, CN, N02, (CF2)rCF3, OH, and (CH2)rOCl_S alkyl;
27
CA 02346933 2001-04-18
WO 00135449 PCT/US99/30292
R1~ is selected from H, C1_g alkyl, OH, and CH20H;
alternatively, R9 and R1~ join to form C3_~ cycloalkyl, 5-6-
membered cyclic ketal or =0;
with the proviso that when R1~ is halogen, cyano, nitro, or
bonded to the carbon to which it is attached through a
heteroatom, R9 is not halogen, cyano, or bonded to the
carbon to which it is attached through a heteroatom;
R11 is selected from H, CI_8 alkyl, (CH2)rphenyl substituted
with 0-5 Rlle, and a (CH2)r-heterocyclic system
substituted with 0-2 Rlle, wherein the heterocyclic
system is selected from pyridinyl, thiophenyl,
furanyl, indazolyl, benzothiazolyl, benzimidazolyl,
benzothiophenyl, benzofuranyl, benzoxazolyl,
benzisoxazolyl, quinolinyl, isoquinolinyl, imidazolyl,
indolyl, isoindolyl, piperidinyl, pyrrazolyl, 1,2,4-
triazolyl, 1,2,3-triazolyl, tetrazolyl, thiazolyl,
oxazolyl, pyrazinyl, and pyrimidinyl; and
Rlle, at each occurrence, is selected from C1_6 alkyl, Cl,
F, Br, I, CN, NO2, (CF2)rCF3, OH, and (CH2)rOCl_5 alkyl;
R12 is H;
alternatively, R11 and R12 join to form C3_~ cycloalkyi; and
r is selected from 0, 1, and 2.
[8] In a more preferred embodiment, the present invention
provides novel compounds of formula (I-i), wherein:
J is selected from CH2 and CHRS;
K is selected from CH2 and CHRS;
28
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
L is selected from CHZ and CHRS;
R3 is a C3_lU carbocycl:ic residue substituted with 0-3 R15,
wherein the carbocyclic residue is selected from
cyclopropyl, cyclopentyl, cyclohexyl, phenyl, naphthyl
and adamantyl, and a (CR3'H)r-heterocyclic system
substituted with 0-3 R15, wherein the heterocyclic
system is selected from pyridinyl, thiophenyl,
furanyl, indazolyl_, benzothiazolyl, benzimidazolyl,
benzothiophenyl, benzofuranyl, benzoxazolyl,
benzisoxazolyl, c~.~inolinyl, isoquinolinyl, imidazolyl,
indolyl, indolinyl., isoindolyl, isothiadiazolyl,
isoxazolyl, pipe.ri.dinyl, pyrrazolyl, 1,2,4-triazolyl,
1,2,3-t:riazolyl, t.etrazolyl, thiadiazolyl, thiazolyl,
oxazoly:l, pyrazinyl, and pyrimidinyl; and
R15, at each occurrence, is selected from C1_g alkyl,
(CH2)rC3-6 cycloalk:yl, CF3, C1, Br, I, F,
( CH2 ) rNR15aR15a ~ ~ ~~~2 CN , OH , ( CHZ ) rORlSd
(CH2)rC(O)RlSb~ {~~~H,;~)rC(O)NR15aR15a'. {CH2)rNRlSfC(O)RlSb~
{ CH2 ) rS ( O ) pRlSb C CH2 ) rS ( O ) 2NR=5aR15a'
(CH2)rNR.lSfS(O)2RlSb, (CH2)rphenyl substituted with 0-3
RlSe~ and a (CH2).~-S-5 membered heterocyclic system
containing 1-4 heteroatoms selected from N, O, and S,
substituted with 0-2 RlSe;
RlSa and Rlsa~ , at each occurrence, are selected from H, C1_s
alkyl, C3_6 cycloal.kyl, and (CH2)rphenyl substituted
with 0-3 RlSe;
RlSb~ at each occurrence, is selected from H, C1_6 alkyl,
C3_6 cyc:loalkyl, and (CH2)rphenyl substituted with 0-3
RlSe;
RlSd, at each occurrence, is selected from C1_6 alkyl and
phenyl;
29
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Rl5e, at each occurrence, is selected from C1_6 alkyl, C1,
F, Br, I, CN, N02, (CF2)rCF3, OH, and (CH2)rOC1_5 alkyl;
and
RlSf, at each occurrence, is selected from H, and C1-5
alkyl.
[9] In another more preferred embodiment, the present
invention provides novel compounds of formula (I-ii),
wherein:
K is selected from CHZ and CHRS;
L is selected from CH2 and CHRS;
R3 is a C3-to carbocyclic residue substituted with 0-3 R15,
wherein the carbocyclic residue is selected from
cyclopropyl, cyclopentyl, cyclohexyl, phenyl, naphthyl
and adamantyl, and a (CR3'H)r-heterocyclic system
substituted with 0-3 R15, wherein the heterocyclic
system is selected from pyridinyl, thiophenyl,
furanyl, indazolyl, benzothiazolyl, benzimidazolyl,
benzothiophenyl, benzofuranyl, benzoxazolyl,
benzisoxazolyl, quinolinyl, isoquinolinyl, imidazolyl,
indolyl, indolinyl, isoindolyl, isothiadiazolyl,
isoxazolyl, piperidinyl, pyrrazolyl, 1,2,4-triazolyl,
1,2,3-triazolyl, tetrazolyl, thiadiazolyl, thiazolyl,
oxazolyl, pyrazinyl, and pyrimidinyl; and
R15~ at each occurrence, is selected from C1_8 alkyl,
(CH2)rC3-6 cycloalkyl, CF3, Cl, Br, I, F,
(CH2)rNR15aR15a' N02 CN, OH,, (CH2)rpRlSd
(CH2)rC(0)RlSbWCH2)rC(0)NR15aR15a', (CH2)rNRlSfC(O)RlSb
3 5 (CH2)rS(O)pRlSb (CH2)rS(0)ZNR15aR15a',
(CH2)rNRlSfS(O)zRlSb, (CH2)rphenyl substituted with 0-3
RlSe, and a (CH2)r-5-6 membered heterocyclic system
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
containing 1-4 heteroatoms selected from N, O, and S,
substituted with 0-2 RlSe;
RlSa and RlSa~, at each. occurrence, are selected from H, Cz_6
alkyl, C3_6 cyclo~alkyl, and (CH2)rphenyl substituted
with 0-3 RlSe;
RlSb~ at each occurrence, is selected from H, C1_6 alkyl,
C3_6 cycloalkyl, and (CH2)rphenyl substituted with 0-3
1.0 RlSe;
RlSd at each occurrence, is selected from C1_6 alkyl and
phenyl;
RlSe, at each occurrence, is selected from C1_6 alkyl, C1,
F, Br, I, CN, NO2, (CF2)rCF3, OH, and (CHZ)rOCl_5 alkyl;
and
RlSf, at each occurrence, is selected from H, and C1-5
alkyl.
[10] In another more preferred embodiment, the present
invention provides novel compounds of formula (I-ii),
wherein:
Rl3a is selected from H, methyl, ethyl, propyl, butyl,
pentyl, hexyl, isobutyl, isopentyl and isohexyl.
[11] In a further even more preferred embodiment, the
present invention provides novel compounds of formula
(I) and pharmaceutically acceptable salt forms
thereof, wherein the compound of formula (I) is
selected from:
erythro-cis~-1-[3-(3-acetylphenylaminocarbonylamino)-n-prop
1-yl)-~~-benzyl-cx-methyl-2-piperidinemethanol;
31
CA 02346933 2001-04-18
WO 00135449 PCT/US99/30292
erythro-cis-1-[3-(3-acetylphenylaminocarbonylamino)-n-prop-
1-yl]-4-benzyl-a-ethyl-2-piperidinemethanol;
erythro-cis-1-[3-(3-acetylphenylaminocarbonylamino)-n-prop-
1-yl]-4-benzyl-a-(n-prop-1-yl)-2-piperidinemethanol;
erythro-cis-1-[3-(3-acetylphenylaminocarbonylamino)-n-prop-
1-yl]-4-benzyl-a-(n-but-1-yl)-2-piperidinemethanol;
erythro-cis-1-[3-(3-acetylphenylaminocarbonylamino)-n-prop-
1-yl]-4-benzyl-a-(n-prop-2-yl)-2-piperidinemethanol;
erythro-cis-1-[3-(3-acetylphenylaminocarbonylamino)-n-prop-
1-yl]-4-benzyl-a-(3-methyl-n-prop-1-yl)-2-
piperidinemethanol;
(+)-erythro-cis-1-[3-(3-acetylphenylaminocarbonylamino)-n-
prop-1-yl]-4-benzyl-a-(n-but-1-yl)-2-
piperidinemethanol;
erythro-cis-1-[3-(indazol-5-yl)aminocarbonylamino)-n-prop-
1-yl]-4-benzyl-a-(n-but-1-yl)-2-piperidinemethanol;
erythro-cis-1-[3-(3-acetylphenylaminocarbonylamino)-n-prop-
1-yl]-2-[1-(3-acetylphenylaminocarbonyloxy)-n-prop-1-
yl]-4-benzylpiperidine;
erythro-cis-1-[3-(3-acetylphenylaminocarbonylamino)-n-prop-
1-yl]-2-[1-(3-acetylphenylaminocarbonyloxy)-n-but-1-
yl]-4-benzylpiperidine;
erythro-cis-1-[3-(3-acetylphenylaminocarbonylamino)-n-prop-
1-yl]-2-[1-(3-acetylphenylaminocarbonyloxy)-n-pent-1-
yl]-4-benzylpiperidine;
32
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
erythro-cis-1-[3-(3-acE_tylphenylaminocarbonylamino)-n-prop-
1-yl]-2-[1-(3-aces:ylphenylaminocarbonyloxy)-2-methyl-
n-prop-1-yl]-4-benzylpiperidine; and
erythro-cis-1-[3-(3-acetylphenylaminocarbonylamino)-n-prop-
1-yl]-2-[1-(3-acet:ylphenylaminocarbonyloxy)-3-methyl-
n-but-1-ylJ-4-benzylpiperidine.
[12] In a second embodiment, the present invention
provides novel compounds of formula (I):
Z
,E-M R4
I~ ~ E-iV~-R3
l.-Q R 1 R2
(I)
or stereoisomers or pharmaceutically acceptable salts
1_'i thereof, wherein:.
M is absent or selected from CH2, CHRS, CHR13, CR13R13, and
CR'R13 ;
Q is selected from CHR13 , CR13R13 , and CR5R13 ;
J, K, and L are independently selected from CH2, CHRS, CHR6,
CR6R6 and CR5R6;
with the provisos:
1) at least one of M, J, K, L, or Q contains an R5;
and
2) when M is absewt, J is selected from CH2, CHRS,
CHR13
and CRSR13 ;
Z is selected from 4, S; NRla, CHCN, CHNOz, and C(CN)2;
33
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Rla is selected from H, C1-6 alkyl, C3_6 cycloalkyl,
CONRIbRib OR>.b , N02 , CN , and ( CH2 ) Wphenyl ;
R1b is independently selected from H, C1_3 alkyl, C3_s
S cycloalkyl, and phenyl;
E is selected from:
R8 11 12
R' 8 ~ R8
A A A
~R 14)9 ' (R 14)9 ' R9 ~Rlo ~R 14)9 ' R9 ~Rlo (R 14)9
IO
11 12 Rg 10 7 R8 9 10
A ~ A R1i R12 A - R~R12
(R 14)9 (R 14)9 (R 14)
9 ,
7 8 11 R12 7 8 Rs 1o R a Rs R1o
A A R~R12 1o A
R R
(R 14)9 , R R 1 ° (R 14)9 ' (R 14)9 , and
R~ R8 R9 R10 11 R12
R ~Rto
15 (R 14)9
ring A is a C3_6 carbocyclic residue;
with the proviso that when A is phenyl, R14
20 is not ortho to CR~Ra;
R1 and R2 are independently selected from H, C1_6 alkyl, C2_8
alkenyl, C2_8 alkynyl, (CH2)rC3-6 cycloalkyl, and a
(CH2)r-C3-1o carbocyclic residue substituted with 0-5
25 Ra;
34
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Ra, at each occurrence', is selected from C1_6 alkyl, C2_8
alkenyl, C2_8 alkynyl, (CHZ)rC3_6 cycloalkyl, C1, Br, I,
F, (CF2)rCF3, N02. CN, (CH2)rNRbRb. (CH2)rOH, (CH2)rORc.
( CH2 ) rSH, ( CH2 ) L.S:Rc , ( CH2 ) rC ( 0 ) Rb, ( CH2 ) rC ( 0 ) NRbRb ,
( CH2 ) rNRbC ( O ) Rb, ( CH2 ) rC ( 0 ) ORb, ( CHZ ) rOC ( 0 ) Rc ,
(CH2)rCH(=NRb)NRbl~b, (CH2)rNHC(=NRb)NRbRb, (CH2)rS(0)pRc,
(CH2)rS(O)2NRbRb, (CHZ)rNRbS(0)ZRc, and (CH2)rphenyl;
Rb, at each occurrence, is selected from H, C1_6 alkyl, C3-5
cycloalkyl, and phenyl;
Rc, at each occurrence, is selected from C1_6 alkyl, C3-6
cycloalkyl, and phenyl;
alternatively, R2 and 1~3 join to form a 5, 6, or 7-membered
ring substituted with 0-3 Ra;
R3 is selected from a (CR3'R3") r-C3-1o carbocyclic residue
substituted with 0-5 R15 and a (CR3'R3")r-5-10 membered
2~~ heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-3 R15;
R3' and R3", at each occurrence, are selected from H, C1-6
alkyl, (CH2)rC3_ti cycloalkyl, and phenyl;
2 _'i
R4 is absent, taken with the nitrogen to which it is
attached to form an N-oxide, or selected from C1_s
alkyl, C2_8 alkenyl, C2_8 alkynyl, (CH2)rC3-6
cycloalkyl, (CH2)~~C(0)R4b, {CH2)qC(0)NR4aR4a~,
3 Ci (CH2 ) qC (O) OR4b, and a (CHZ ) =-C3_1o carbocyclic residue
substituted with 0-3 R4c;
R4a and R4a', at each occurrence, are selected from H, C1-s
alkyl, (CH2)rC3-5 c=ycloalkyl, and phenyl;
35
CA 02346933 2001-04-18
WO 00/35449 PC'T/US99/30292
R4b, at each occurrence, is selected from C~_6 alkyl, C2_8
alkenyl, (CHZ)rC3-6 cycloalkyl, C2_g alkynyl, and
phenyl;
R4c, at each occurrence, is selected from C1_6 alkyl, C2_g
alkenyl, C2_g alkynyl, C3-6 cycloalkyl, C1, F, Br, I,
CN, N02, (CF2)rCF3, (CH2)rOCl_5 alkyl, (CHZ)rOH,
(CHZ)rSCl_5 alkyl, (CH2)rNR4aR4a', and (CH2)rphenyl;
alternatively, R4 joins with R~, R9, or R11 to form a 5, 6
or 7 membered piperidinium spirocycle or pyrrolidinium
spirocycle substituted with 0-3 Ra;
R5 is selected from a (CR5'R5°)t-C3-1o carbocyclic residue
substituted with 0-5 R16 and a (CRS'RS'~)t-5-10 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-3 R16;
R5' and R5", at each occurrence, are selected from H, C1_6
alkyl, (CHZ)rC3-6 cycloalkyl, and phenyl;
R6, at each occurrence, is selected from C1_6 alkyl, CZ_8
alkenyl, C2_8 alkynyl, (CH2)rC3-6 cycloalkyl, (CFZ)~CF3,
CN, (CH2)rNR6aR6a', (CH2)rOH, (CH2)rOR6b, (CHZ)rSH,
(CH2 ) rSR6b, (CHZ ) rC (O) OH, (CH2 ) rC (O) R6b,
(CH2)rC(O)NR6aR6a', (CH2)rNR6dC(O)R6a, (CH2)rC(O)OR6b,
(CH2)rOC(O)R6b, (CH2)rS(0)pR6b, (CH2)rS(O)2NR6aR6a'.
(CH2)rNR6aS(O)2R6b, and (CH2)tphenyl substituted with 0-
3 R6c;
R6a and R6a', at each occurrence, are selected from H, C1_6
alkyl, C3_6 cycloalkyl, and phenyl substituted with 0-3
R6c.
R6b, at each occurrence, is selected from Ci_o alkyl, C3-6
cycloalkyl, and phenyl substituted with 0-3 R6c;
36
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R6~, at each occurrence, is selected from C1_6 alkyl, C3-6
cycloalkyl, CI, F, Br, I, CN, N02, (CFZ)rCF3, (CH2)rOCl-
alkyl, (CH2)rOH, (CH2)rSCl_5 alkyl, and (CHZ)rNR6dR6d;
5 R6d, at each occurrence, is selected from H, C1_6 alkyl, and
C3-6 cycloalkyl;
with the proviso that.when any of J, K, or L is CR6R6 and R6
is halogen, cyano,, nitro, or bonded to the carbon to
which it is attached through a heteroatom, the other
R6 is not halogen, cyano, or bonded to the carbon to
which it is attached through a heteroatom;
R~, is selected from H, C1-6 alkyl, C2_g alkenyl, C2_8
alkynyl, (CH2)qOH, (CH2)qSH, (CH2)qOR~d, (CH2)qSR~d,
(CH2 ) qNR~aR7a' , (CH:2 ) rC (O) OH, (CHZ ) rC (O) R7b
(CH2)rC(O)NR~aR7a", (CH2)qNR~aC(O)R~a, (CH2)qNR~aC(O)H,
(CH2)rC(O)OR~b, (CHZ)qOC(O)R~b, (CH2)qS(O)pR7b
(CH2)qS(O)2NR~aR7a', (CH2)qNR~aS(O)2R7b, C1_6 haloalkyl,
2« a (CH2),_-C3_1o carbocyclic residue substituted with 0-3
R~~, and a (CH2)r-5-10 membered heterocyclic system
containing 1-4 heteroatoms selected from N, 0, and S,
substituted with 0-2 R~~;
Rya and Rya' , at each occurrence, are selected from H, C1-6
alkyl, (:2_g alkeny7., C2_8 alkynyl, (CH2) rC3-5
cycloalkyl, a (CH2)r-C3-1o carbocyclic residue
substituted with 0-5 Rye, and a (CH2)r-5-10 membered
heterocyclic system containing i-4 heteroatoms
selected from N, O, and S, substituted with 0-3 Rye;
Rib, at each occurrence,. is selected from C1_6 alkyl, C2_$
alkenyl, C2_$ alkynyl, a (CH2)r-C3_6 carbocyclic residue
substituted with 0-2 Rye, and a (CH2)r-5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-3 Rye;
37
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R~~, at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, C2_8 alkynyl, (CHZ)rC3-6 cYcloalkyl, C1, Br, I,
F, (CF2)rCF3, N02, CN, (CH2)rNR~fR7f, (CH2)rOH,
(CH2)rOCl_q alkyl, (CH2)rSCl_4 alkyl, (CH2)rC(O)OH,
(CH2)rC(O)R~b, (CH2)rC(O)NR~fR7f, (CHZ)rNR~fC(0)R~a,
(CH2)rC(O)OC1_q alkyl, (CH2)rOC(O)R7b
(CH2)rC(=NR~f)NR~fR7f, (CH2)rS(O)pR~b,
(CH2 ) rNHC (=NR~f ) NR~fR7f, (CHZ ) rS (O) 2NR7fR7f
(CH2)rNR~fS(0)zR~b, and (CH2)rphenyl substituted with 0-
3 Rye;
Rid, at each occurrence, is selected from C1_6 alkyl
substituted with 0-3 Rye, alkenyl, alkynyl, and a C3-1o
carbocyclic residue substituted with 0-3 R~~;
Rye, at each occurrence, is selected from C1_6 alkyl, CZ_8
alkenyl, C2_8 alkynyl, C3_6 cycloalkyl, C1, F, Br, I,
CN, N02, (CF2)rCF3, (CH2)rOCl_5 alkyl, OH, SH, (CH2)rSCl_
5 alkyl, (CH2}rNR~fR~f, and (CHZ)rphenyl;
Ref, at each occurrence, is selected from H, C1_6 alkyl, and
C3-6 cycloalkyl;
R8 is selected from H, C1_6 alkyl, C3_5 cycloalkyl, and
(CH2)tphenyl substituted with 0-3 RBa;
RBa, at each occurrence, is selected from C1_6 alkyl, CZ_8
alkenyl, C2_8 alkynyl, C3-6 cycloalkyl, C1, F, Br, I,
CN, N02, (CF2);CF3, (CH2)rOCl_5 alkyl, OH, SH, (CH2)rSCl_
5 alkyl, (CH2)rNR~fR~f, and (CH2)rphenyl;
alternatively, R~ and R8 join to form C3_~ cycloalkyl, or
=NRBb;
R8b is selected from H, C1_6 alkyl, C3_6 cycloalkyl, OH, CN,
and
(CH2)r-phenyl;
38
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R9, is selected from H, C1_6 alkyl, C2-8 alkenyl, C2_g
alkynyl, F, C1, Br, I, N02, CN, (CH2)rOH, (CH2)rSH,
(CH2)rC~R9d, (CH2)rSR9d, (CHZ)rNR9aR9a', (CH2)rC(0)OH,
(CH2)rG(0)R9b, (C~C~;)rC(0)NR9aR9a'~ (CH2)rNR9aC(O)R9a,
( CH2 ) rNR9aC ( 0 ) H , ( CHZ ) rNR9aC ( O ) NHR9a , ( CHZ ) rC ( 0 ) OR9b,
(CH2)rOC(0)R9b, (C.'H2)rOC(O)NHR9a, (CH2)rS(O)pR9b~
(CH2)rS(O)2NR9aR9a'. (CH2)rNR9aS(O)ZR9b, C.'1_6 haloalkyl,
a (CHZ)r-C3-1o carbocyclic residue substituted with 0-5
1() R9c, and a (CH2)r-5-10 membered heterocyclic system
containing 1-4 heteroatoms selected from N, O, and S,
substituted with 0-3 R9c
R9a and R9a' , at each occurrence, are selected from H, C1-s
1'. alkyl, C2_8 alkenyl., C2_8 alkynyl, a (CH2) r-C3-1o
carbocyclic residue substituted with 0-5 R9e, and a
(CH2)r-5-10 membered heterocyclic system containing 1-4
heteroatoms selected from N, 0, and S, substituted
with 0-3 R9e;
R9b, at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, C2_8 alkynyl, a (CHZ)~-C3-E carbocyclic residue
substituted with 0-G R9e, and a (CH2)r-5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-3 R9e;
R9c, at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, C2-8 alkynyl, (CHZ)rC3-6 cycloalkyl, C1, Br, I,
F, (CF2)rCF3, N0~7, CN, (CH2)rNR9fR9f (CH2)rOH,
(CH2)rOCl-4 alkyl, (CH2)rSCl_.~ alkyl, (CHZ)rC(O)OH,
(CH2)rC(0)R9b, (CH2)rC(0)NR9fR9f (CH2)rNR9fC(0)R9a
(CHZ)rC(O)OC1_4 alkyl, (CH2)rOC(O)R9b,
(CH2)rC(=NR9f)NR9fR~f, (CH2)rS(O)pR9b
(CH2 ) rNHC (=NR9f ) NR9fR9f , (CH2 ) rS (O) ZNR9fR9f,
(CH2)rNR9fS(0)2R9b, and (CH2)rphenyl substituted with 0-
3 R9e.
39
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R9d, at each occurrence, is selected from C1_6 alkyl, C2-5
alkenyl, C2_6 alkynyl, a C3_1o carbocyclic residue
substituted with 0-3 R9c, and a 5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from the group consisting of N, O, and S
substituted with 0-3 R9c;
R9e, at each occurrence, is selected from C1_6 alkyl, C2_g
alkenyl, C2_8 alkynyl, (CH2)rC3-6 cYcloalkyl, C1, F, Br,
I, CN, N02, (CF2)rCF3, (CH2)rOCl_5 alkyl, OH, SH,
(CHZ)rSCl_5 alkyl, (CH2)rNR9fR9f, and (CH2)rphenyl;
R9f, at each occurrence, is selected from H, C1_6 alkyl, and
C3-o cycloalkyl;
Rlo, is selected from H, C1_6 alkyl, Cz_e alkenyl, C2_8
alkynyl, F, Cl, Br, I, N02, CN, (CH2)rOH, (CH2)rORlod,
(CH2)rSRlOd (CH2)rNRl0aR10a', (CH2)rC(O)OH,
(CH2 ) rC (O) R20b. (CH2 ) rC (O) NR10aR10a' , (CH2 ) rNRlOaC (O) RlOa
2 0 ( CH2 ) rNRl OaC ( O ) H , ( CH2 ) rC ( O ) OR1 Ob , ( CHZ ) rOC ( O ) R1
Ob
(CH2)rS(O)pRlOb (CH2)rS(O)2NR10aR10a',
(CH2) rNRloaS (0) 2RIOb C1-6 haloalkyl, a (CH2 ) r-C3-10
carbocyclic residue substituted with 0-5 Rloc, and a
(CH2)r-5-10 membered heterocyclic system containing 1-4
heteroatoms selected from N, 0, and S, substituted
with 0-3 Rloc;
Rloa and Rloa' , at each occurrence, are selected from H, C1-5
alkyl, C2_g alkenyl, C2_g alkynyl, a (CH2)r-C3-to
carbocyclic residue substituted with 0-5 Rloe, and a
(CH2)r-5-10 membered heterocyclic system containing 1-4
heteroatoms selected from N, O, and S, substituted
with 0-3 RlOe.
RlOb, at each occurrence, is selected from C~_6 alkyl, C2_g
alkenyl, C2_g alkynyl, a (CH2)r-C3-s carbocyclic residue
substituted with 0-2 R2oe, and a (CHZ)~-5-6 membered
CA 02346933 2001-04-18
WO 00/35449 PCTlUS99/30292
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-3 RlOe;
Rloc~ at each occurrence, is selected from C1_6 alkyl, C2_g
alkenyl, C2_8 alkynyl, (CH2)rC3_6 cycloalkyl, C1, Br, I,
F, (CF2)rCF3, NO~, CN, (CH2)rNR10fR10f (CH2)rOH,
(CH2)r0(:1-4 alkyl, (CH2)rSCI_4 alkyl, (CH2)rC(O)OH,
(CH2)rC(0)RlOb~ (CH~)rC(0)NR10fR10r~ (CH2)rNRlOfC(O)RlOa~
(CHZ)rC(0)OC1_q al)cYl, (CH2)rOC(0)RlOb~
(CH2)rC(=NRlOf)NR.lffRlOf~ (CH2)rS(0)pRlOb~
(CH2 ) rNHC (=NRlOf ) NRxOfRlOf (CH2 ) rS (0) zNR10fR10f
(CH2 ) rNRlofS (0) ZR:LOb and (CH2 ) rphenyl substituted with
0-3 Rloe .
Rlod, at each occurrences, is selected from C1_6 alkyl, C2-6
alkenyl, C2_6 alky:nYl, a C3_1o carbocyclic residue
substituted with f--3 Rloc, and a 5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from the group consisting of N, O, and S
substituted with G-3 Rloc;
RlOe, at each. occurrence., is selected from Cl_6 alkyl, C2_8
alkenyl, C2_g alkynyl, (CH2)rC3-6 cycloalkyl, Cl, F, Br,
I, CN, PJO2, (CF2)rCF3, (CH2)rOC1_5 alkyl, OH, SH,
(CH2 ) rSC'1_5 alkyl , (CH2 ) rNR10fR10f and (CHZ) rphenyl;
Rlof at each occurrence, is selected from H, Cl_5 alkyl,
and C3_E cycloalkyl;
alternatively, R9 and Rlo join to form C3_~ cycloalkyl, 5-6-
membered cyclic ketal or =0;
with the proviso that when Rlo is -OH, R9 is not halogen,
cyano, or bonded to the carbon to which it is attached
through a heteroatom;
41
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R11, is selected from H, C1_6 alkyl, C2_g alkenyl, C2_~
alkynyl, (CH2)qOH, (CH2)qSH, (CH2)qORlld, (CH2)qSRlld
(CH2)qNR11aR11a~ (CH2)rC(0)OH, (CHZ)rC(O)Rllb
( CH2 ) rC ( O ) NR11aR11a' , ( CH2 ) qNRllaC ( O ) Rlla
( CHZ ) qNRllaC ( O ) NHRlla ( CH2 ) rC ( O ) ORllb ( CH2 ) qOC ( 0 ) Rllb
(CH2)qS(O)pRllb (CH2)qS(O)2NR11aRlla'
(CH2)qNRllaS(O)2R11b C1-6 haloalkyl, a (CH2)r-C3-1o
carbocyclic residue substituted with 0-5 Rllc, and a
(CH2)r-5-10 membered heterocyclic system containing 1-4
heteroatoms selected from N, O, and S, substituted
with 0-3 Rllc;
Rlla and Rlla', at each occurrence, are selected from H, C1_6
alkyl, Cz_~ alkenyl, C2_8 alkynyl, a (CHZ)r-C3-lc
carbocyclic residue substituted with 0-5 Rlle, and a
(CH2)r-5-10 membered heterocyclic system containing 1-4
heteroatoms selected from N, 0, and S, substituted
with 0-3 Rlle;
Rllb, at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, C2_8 alkynyl, a (CH2)r-C3-6 carbocyclic residue
substituted with 0-2 Rlle, and a (CH2)r-5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-3 Rlle.
Rllc at each occurrence, is selected from C1_6 alkyl, C2-8
alkenyl, C2_g alkynyl, (CH2)rC3-5 cycloalkyl, C1, Br, I,
F, (CF2)rCF3, N02, CN, (CH2)rNRllfRllf (CH2)rpH,
(CH2)rOCl_4 alkyl, (CH2)rSCl_4 alkyl, (CH2)rC(O)OH,
(CH2)rC(O)Rllb (CH2)rC(0)NRllfRllf (CH2)rNRIIfC(0)Rlla
(CH2)rC(O)OC1_q alkyl, (CHZ)rOC(O)Rllb
(CH2)rC(=NRllf)NRllfRllf (CHZ)rNHC(=NRllf)NRllfRllf
(CH2)rS(O)pRllb WCH2)rS(O)2NR11fR11f
(CHZ)rNRllfS(0)2R11b and (CH2)rphenyl substituted with
0-3 Rlle.
42
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Rlld~ at each occurrence, is selected from Cl_6 alkyl
substituted with 0-3 Rlle, C2-5 alkenyl, C2_6 alkynyl,
and a C3_1o carboc_yclic residue substituted with 0-3
Rllc;
Rlle~ at each occurrence, is selected from C1_6 alkyl, C2
alkenyl, C2_8 alky:nyl, C3_6 cycloalkyl, C1, F, Br, I,
CN, N02, (CF2)rCF3,, (CH2)rOCl_5 alkyl, OH, SH, (CH2)rSCl_
alkyl,. (CH2)rNRll.f:Rllf~ and (CH2)rphenyl;
Rllf at each occurrencE=_, is selected from H, C1-6 alkyl,
and C3_E; cycloalkyl;
R12 is selected from H, C1_6 alkyl, (CH2)qOH, (CH2)rC3-5
1'. cycloalkyl, and (CH2)tphenyl substituted with 0-3 Rl2a;
Rl2a, at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl,, C2_8 alkynyl, C3-6 cycloalkyl, C1, F, Br, I,
CN, N02, (CF2)rCF3, (CH2)rOCl_5 alkyl, OH, SH, (CH2)rSCl_
2 0 5 alkyl , ( CH2 ) rNR'3 f R9 f , and ( CH2 ) rphenyl ;
alternatively, R1i and R12 join to form C3_~ cycloalkyl;
R13, at each occurrence, is selected from (CHRl'a)OH,
25 (CHRl3a)O:Rl3b (CHRla") SH,
(CHRl'a) SRl3b, (CHRl3a) ~l3eC (O) Rl3f and
(CHRl3a)PJRI3eS (O) 2R13f;
Rl3a is selected from C1_~ alkyl;
Rl3b~ at each occurrence, is selected from C(O)Rl3d~
C(0)NHRl3d, C1-6 alkyl, C3_6cycloalkyl, and phenyl
substituted with 0--3 Rl3c;
Rl3c, at each occurrence, is selected from C~_6 alkyl, C2_g
alkenyl, C2_~ alkynyl, C3-s cYcloalkyl, Cl, F, Br, I,
43
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
CN, N02, (CF2)rCF3, (CH2)rOCl_5 alkyl, OH, SH, (CH2)rSCl_
alkyl, (CHZ)rNR9fR9f, and (CH2)rphenyl;
Rl3d at each occurrence, is selected from C1_6 alkyl, C3_6
5 cycloalkyl, and phenyl substituted with 0-3 R6c;
Rl3e~ at each occurrence, is selected from H, C1_s
alkyl, (CH2)rC3-5 cycloalkyl, and phenyl where phenyl
is substituted from 0-3 Rz3c;
Rl3f, at each occurrence, is selected from C1-5
alkyl, (CH2)rC3-5 cycloalkyl, CFA, and phenyl where
phenyl is substituted from 0-3 R13~;
alternatively, R14 joins with R4 to form a 5, 6 or 7
membered
piperidinium spirocycle or pyrrolidinium spirocycle
fused to ring A, the spirocycle substituted with 0-3
Ra:
R14, at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, C2_8 alkynyl, (CH2)rC3-s cycloalkyl, C1, Br, I,
F, N02, CN, (CHR')rIVR14aR14a' (CHR')rOH,
(CHR')r0(CHR')rRl4d (CHR')rSH, (CHR')rC(0)H,
(CHR')rS(CHR~)rRl4d, (CHR')rC(O)OH,
( CHR' ) rC { O ) ( CHR' ) rRl4b , ( CHR' ) rC ( O ) NR14aR14a'
(CHR' ) rNRl4fC (O) (CHR~ ) rRl4b {CHR' ) rC (0) O (CHR' ) rRl4d~
( CHR' ) rOC ( O ) ( CHR' ) rRl4b , ( CHR' ) rC ( =NR14 f ) NR14aR14a'
(CHR' ) rNHC (=NRl4f ) NR14fR14f (CHR' ) rS (0) p (CHR' ) rRl4b
(CHR')rS(O)2NR14aR14a', {CHR')rNRl4fS(O)2(CHR')rRl4b Ci_6
haloalkyl, C2_g alkenyl substituted with 0-3 R', C2_g
alkynyl substituted with 0-3 R', (CHR')rphenyl
substituted with 0-3 Rl4e, and a (CH2)r-5-10 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-2 RlSe.
44
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R', at each occurrence, is selected from H, C1_6 alkyl, C2_8
alkenyl, C2_8 alk:ynyl, (CHZ)rC3_o cycloalkyl, and
(CH2)rphenyl substituted with Rl4e;
Rl4a and Rl4a', at each occurrence, are selected from H, C1_s
alkyl, C2_g alkenyl, C2_g alkynyl, a (CHZ)r-C3-1o
carbocyclic residue substituted with 0-5 Rl4e, and a
(CH2)r-5-10 membered heterocyclic system containing 1-4
heteroatoms selected from N, 0, and S, substituted
~-0 with 0-2 Rl4e;
Rl4b, at each occurrence, is selected from C1-6 alkyl, C2_8
alkenyl, C2_8 alkynyl, a (CH2)~-C3-6 carbocyclic residue
substituted with I7-3 Rl4e, and (CH2)r-5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, 0, and S, substituted with 0-2 Rl4e;
Rl4d at each occurrence, is selected from Cz-8 alkenyl, C2_g
alkynyl, C,_6 alkyl substituted with 0-3 Rl4e~ a
(CH2)r--C3-so carbocyclic residue substituted with 0-3
Rl4e~ and a (CH2),~'~-6 membered heterocyclic system
containing 1-4 heteroatoms selected from N, O, and S,
substituted with 0-3 Rl4e;
Rl4e, at each occurrence, is selected from C1_6 alkyl, C2_8
alkeny:l, C2_g alkynyl, (CH2)rC3-6 cYcloalkyl, C1, F, Br,
I, CN, N02, (CF2)rCF3, (CH2)rOC1_5 alkyl, OH, SH,
(CH2 ) r=>C1-5 alkyl, (CH2 ) rNR14fR14f ~ and (CH2 ) rphenyl;
Rl4f, at each occurrence, is selected from H, C1_6 alkyl,
C3-6cyc:loalkyl, and phenyl;
R15, at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, CZ_8 alk5myl, (CH2)rC3-6 cycloalkyl, C1, Br, I,
3!i F, N02, CN, (CHR')rNR15aR15a'~ (CHR')rOH,
(CHR' ) r0 (CHR' ) rRlSd, (CHR' ) rSH, (CHR' ) rC (O) H,
(CHR')rS(CHR~)rRl5d, (CHR')rC(O)OH,
CA 02346933 2001-04-18
WO 00!35449 PCT/US99/30292
(CHR')rC(O)(CHR')rRlSb, (CHR')rC(O)NR15aR15a',
(CHR' ) rNRlSfC (0) (CHR' ) rRlSb, (CHR' ) rC (O) O (CHR' ) ,.RlSd,
(CHR')rOC(O)(CHR')rRlSb, (CHR')rC(O)NR15aR15a'
(CHR')rC(=NRlSf)NR15aR15a', (CHR')rNHC(=NRlSf)NR15fR15f,
(CHR')rS(0)p(CHR')rRl5b, (CHR')rS(0)2NR15aR15a~~
(CHR')rNRlSfS(0)2(CHR')rRlSb, C1-6 haloalkyl, C2-8
alkenyl substituted with 0-3 R', C2_8 alkynyl
substituted with 0-3 R', (CHR')rphenyl substituted with
0-3 RlSe, and a (CH2)r-5-10 membered heterocyclic
system containing 1-4 heteroatoms selected from N, 0,
and S, substituted with 0-2 R~Se;
RlSa and RlSa' , at each occurrence, are selected from H, Cl-6
alkyl, C2-a alkenyl, C2_g alkynyl, a (CH2)r-C3-10
carbocyclic residue substituted with 0-5 RlSe, and a
(CHZ)r-5-10 membered heterocyclic system containing 1-4
heteroatoms selected from N, 0, and S, substituted
with 0-2 RlSe;
RlSb, at each occurrence, is selected from C1-6 alkyl, C2_8
alkenyl, C2_8 alkynyl, a (CHZ)r-C3-s carbocyclic residue
substituted with 0-3 RlSe, and (CHZ)r-5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, O, and S, substituted with 0-2 RlSe
RlSd, at each occurrence, is selected from C2_8 alkenyl, C2-g
alkynyl, C1-6 alkyl substituted with 0-3 R~Se, a
(CH2)r-C3-1o carbocyclic residue substituted with 0-3
R25e, and a (CH2)r5-6 membered heterocyclic system
containing 1-4 heteroatoms selected from N, O, and S,
substituted with 0-3 RlSe;
RlSe~ at each occurrence, is selected from C1_6 alkyl,
alkenyl, C2_8 alkynyl, (CH2)rC3_6 cycloalkyl, C1, F, Br,
I, CN, N02, (CF2)rCF3, (CHZ)rOCl_5 alkyl, OH, SH,
(CH2)rSCl_5 alkyl, (CH2)rNR15fR15f, and (CH2)rphenyl;
46
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
RlSf, at each occurrence, is selected from H, C1_6 alkyl,
C3_6 cycloalkyl, and phenyl;
R16, at each occurrence, is selected from C1_6 alkyl, CZ_8
alkenyl, C2_g alk:ynyl, (CH2)rC3-6 cycloalkyl, C1, Br, I,
F , N0;> , CN, ( CHR' ) rNR16aR16a' ~ ( CHR' ) rOH,
( CHR' ) r0 ( CHR' ) rRl'S d , ( CHR' ) rSH , ( CHR' ) rC ( O ) H ,
(CHR')rS(CHR')rRlnd, (CHR')=C(O)OH,
(CHR')rC(0)(CHR°)rRl6b, (CHR')rC(0)NR16aR16a'~
( CHR' ) rNRl6 fC { O ) ( CHR' ) rRl6b, ( CHR' ) rC ( 0 ) O ( CHR' ) rRl6d
( CHR' ) rOC ( O ) ( CHR' ) rRl 6b , ( CHR' ) rC ( =NR16 f ) ~16aR16a'
( CHR' ) rNHC ( =NR16 f ) NR16 f R16 f ( CHR' ) r S ( 0 ) p ( CHR' ) rRl6b
(CHR' ) rS (0) 2NR16aF~16a' ~ (CHR' ) rNRl6fS {O) 2 (CHR' ) rRl6b~ C1-6
haloalkyl, CZ_8 a:lkenyl substituted with 0-3 R', C2_8
:L5 alkynyl substituted with 0-3 R', and (CHR')rphenyl
substituted with 0-3 Rl6e;
Rl6a and Rl6a' at each occurrence, are selected from H, C1_6
alkyl, C2_g alken5rl, C2_8 alkynyl, a (CH2 ) r-C3_lo
?0 carbocyclic residue substituted with 0-5 Rl6e, and a
(CHZ)r~-5-10 membe:red heterocyclic system containing 1-4
heteroatoms selected from N, O, and S, substituted
with 0-2 Rl6e;
c.5 Rl6b at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, C2_8 alkynyl, a (CH2)rC3-6 carbocyclic residue
substituted with 0-3 Rl6e, and a (CH2)r-5-6 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, 0, and S, substituted with 0-2 Rl6e.
Rl6d, at each occurrence, is selected from C2_g alkenyl, C2_8
alkyny:L, C1_6 alkyl substituted with 0-3 Rl6e, a
(CH2)r-~C3-1o carbo~~yclic residue substituted with 0-3
Rl6e~ and a (CH2)r-5-6 membered heterocyclic system
containing 1-4 he~teroatoms selected from N, O, and S,
substituted with 0-3 Rise;
47
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Rl6e at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, C2_g alkynyl, (CH2)rC3-6 cycloalkyl, Ci, F, Br,
I, CN, NO~, (CF2)rCF3, (CH2)rOCl_7 alkyl, OH, SH,
(CH2)rSCl_S alkyl, (CH2)rNR16fR16f, and (CH2)rphenyl;
Rl6f at each occurrence, is selected from H, C1_6 alkyl,
and C3_6 cycloalkyl, and phenyl;
g is selected from 0, 1, 2, 3, and 4;
v is selected from 0, 1, and 2;
t is selected from 1 and 2;
w is selected from 0 and 1;
r is selected from 0, 1, 2, 3, 4, and 5;
q is selected from 1, 2, 3, 4, and 5; and
p is selected from 1, 2, and 3.
[13] In a preferred embodiment, the present invention
provides novel compounds of formula (I), wherein:
E is selected from:
R8 ~i ~2
R~ a ~ a _
A A A
(R~4~9 Rs Rya (R~a)9 Rs Rio (R'4)9
i~ i2 ~ ~ '
A
3 0 (R ~a)s
48
CA 02346933 2001-04-18
V10 00/35449 PCT/US99/30292
Rs ~o F ~ Rg 9 10 ~ 8 ~ ~ Ri2
R~R12 ~ ~ R~.R12
(R ia)9 ' (R ~a)9 and
R4 is absent, taken with the nitrogen to which it is
attached to form an N-oxide, or selected from C1_8
alkyl, (CH2)rC3-6 cycloalkyl, and (CHZ)r-phenyl
substituted with 0-3 R4c;
R4c, at each occurrence, is selected from C1_6 alkyl, C2_8
.LO alkenyl, C2_g alk:ynyl, C3-6 cycloalkyl, C1, F, Br, I,
CN, NG2, (CF2)rCF'3, (CH2)rOCl_5 alkyl, (CH2)rOH,
(CHz)rSC~_5 alkyl, (CH2)rNR4aR4a', arid (CH2)rphenyl;
alternatively, R4 joins with R~ or R9 to form a 5, 6 or 7
J.5 membered piperid:inium spirocycle substituted with 0-3
Ra_
R1 and R2 are independently selected from H and C1_4 alkyl;
c.0 R6, at each occurrence',. is selected from C1_4 alkyl, C2_8
alkenyl, C2_8 alkynyl, (CH2)rC3_6 cycloalkyl, (CFZ)rCF3,
CN, (CH2 ) rOH, (CH:2 ) ~OR6b, (CH2 ) rC (O) R6b,
(CH2)rC(O)NR6aR6a'', (CH2)rNR6dC(O)R6a, arid (CH2)tphenyl
substituted with 0-3 R6c;
R6a and R6a' , at each occurrence, are selected from H, C1_6
alkyl, C3_6 cycloalkyl, and phenyl substituted with 0-3
R6c
R6b, at each occurrence, is selected from C1_6 alkyl, C3_6
cycloalkyl, and phenyl substituted with 0-3 R6c;
49
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R6~, at each occurrence, is selected from Ci_6 alkyl, C3_E
cycloalkyl, C1, F, Br, I, CN, N02, (CF2)rCF3, (CH2)rOC1-
alkyl, (CH2)rOH, (CH2)rSCl_5 alkyl, and (CH2)rNR6aR6d;
5 R6d, at each occurrence, is selected from H, C1_6 alkyl, and
C3_6 cycloalkyl;
R~, is selected from H, C1_3 alkyl, (CH2)rC3-6 cycloalkyl,
(CHZ)qOH, (CH2)qOR~d, (CH2)qNR~aR7a', (CH?)rC(O)R~b,
(CH2)rC(O)NR~aR~a', (CH2)qNR~aC(O)R~a, C1_6 haloalkyl,
(CH2)rphenyl with 0-2 R~~;
Rya and Rya', at each occurrence, are selected from H, C1_6
alkyl, (CH2)rC3-5 cycloalkyl, a (CH2)rphenyl
substituted with 0-3 Rye;
Rib, at each occurrence, is selected from C1_6 alkyl, C
alkenyl, C2_8 alkynyl, (CH2)rC3-6 cycloalkyl,
(CH2)rphenyl substituted with 0-3 Rye;
R~~, at each occurrence, is selected from C1_4 alkyl, C
alkenyl, C2_8 alkynyl, (CH2)rC3-5 cYcloalkyl, C1, Br, I,
F, (CF2)rCF3, N02, CN, (CH2)rNR~fR7f, (CH2)rOH,
(CH2)rOCl_4 alkyl, (CH2)rC(0)R~b, (CH2)rC(O)NR~fR7f
(CH2)rNR~fC(O)R7a, (CH2)rS(0)pR7b, (CHZ)rS(O)2NR7fR7f
(CH2)rNR~fS(O)2R~b, and (CH2)rphenyl substituted with 0-
2 Rye;
Rid, at each occurrence, is selected from C1_6 alkyl,
(CH2)rC3_6 cycloalkyl, (CH2)rphenyl substituted with 0-
3 Rye;
Rye, at each occurrence, is selected from C1_6 alkyl, C2_g
alkenyl, C2_8 alkynyl, C3_6 cycloalkyl, C1, F, Br, I,
CN, NO2, (CF2)rCF3, (CH2)rOCl_5 alkyl, OH, SH, (CH2)rSCl_
5 alkyl, (CH2)rNR~fR~f, and (CH2)rphenyl;
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Ref, at each occurrence, is selected from H, C1-5 alkyl, and
C3_6 cycloalkyl;
R8 is H or joins with R~ to form C3_~ cycloalkyl or =NReb;
R11, is selected from H, C1_6 alkyl, (CH2)rC3-6 cycloalkyl,
(CH2)qOH, (CH2)q01211d, (CH2)qNRllaRlla', (CHZ)rC(0)Rllb~
(CH2 ) rC (O) NR11aR11.a' ~ (CH2 ) q~llaC (0) Rlla C1-6 haloalkyl,
(CH2)rphenyl with 0-2 Rllc, (CH2)r-5-10 membered
heterocyclic system containing 1-4 heteroatoms
selected from N, 0, and S, substituted with 0-3 Rls;
Rlla and Rlla' , at each occurrence, are selected from H, C1_6
alkyl, (CH2)rC3_6 cycloalkyl, a (CHZ)rphenyl
LS substituted with 0-3 Rlle;
Rllb at each occurrence, is selected from C1_6 alkyl, C2_8
alkenYl, C2_8 alkynyl, (CH2)rC3-s cYcloalkyl,
(CH2)rphenyl substituted with 0-3 Rlle;
Rllc, at each occurrence, is selected from C1_4 alkyl, C2_8
alkenyl, C2_8 alkynyl, (CH2)rC3-6 cYcloalkyl, C1, Br, I,
F, (CF'2)rCF3, N02, CN, (CH2)rNRllfRllf~ (CH2)rOH.
(CH2)rOCl-4 alkyl, (CH2)rC(O)Rllb~ (CH2)rC(0)NRllfRllf~
:25 (CH2 ) rNRllfC (O) Rll.a (CH2 ) rS (O) pRllb~
(CHZ ) rS (0) 2NR11fRl.l.f ~ (CH2 ) rNRllfS (O) ZRllb, and
(CH2)rphenyl substituted with 0-2 Rlle;
Rlld~ at each occurrence, is selected from C1_6 alkyl,
:30 (CH2)rC3-s cYcloa:Lkyl, (CH2)rphenyl substituted with 0-
3 Rlle
Rlle, at each occurrence, is selected from C1_6 alkyl, C2_8
alkenyl, C2_8 alkynyl, C3_6 cycloalkyl, C1, F, Br, I,
:35 CN, NC>2, (CF2)rCF3, (CH2)rOCl_5 alkyl, OH, SH, (CH2)rSCl_
alkyl, (CH2)rNRl:1fR11f~ and (CH2)rphenyl;
51
CA 02346933 2001-04-18
WO 00/35449 PCT/LS99/30292
Rllf, at each occurrence, is selected from H, C1-5 alkyl and
C3_5 cycloalkyl;
R12 is H or joins with R11 to form C3_~ cycloalkyl;
v is selected from 1 and 2;
q is selected from 1, 2, and 3; and
r is selected from 0, 1, 2, and 3.
[14] In a more preferred embodiment, the present invention
provides novel compounds of formula (I), wherein:
ring A is selected from:
(R 14)9 (R 14)9 (R 14)9 (R 14)9
(R 14)9
.r' ~'i
14 14
(R )9 , and (R )9 ;
R3 is selected from a (CR3'H)r-carbocyclic residue
substituted with 0-5 R15, wherein the carbocyclic
residue is selected from phenyl, C3_6 cycloalkyl,
naphthyl, and adamantyl; and a (CR3'H)r-heterocyclic
system substituted with 0-3 R15, wherein the
heterocyclic system is selected from pyridinyl,
thiophenyl, furanyl, indazolyl, benzothiazolyl,
benzimidazolyl, benzothiophenyl, benzofuranyl,
benzoxazolyl, benzisoxazolyl, quinolinyl,
52
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
isoqui.nolinyl, i:midazolyl, indolyl, indolinyl,
isoindolyl, isot.hiadiazolyl, isoxa;:olyl, piperidinyl,
pyrrazolyl, 1,2,4-triazolyl, 1,2,3-triazolyl,
tetrazolyl, thiadiazolyl, thiazolyl, oxazolyl,
pyrazinyl, and p:yrimidinyl; and
R5 is selected from (C:R~'H)t-phenyl substituted with 0-5
R16; and a (CR5'H)t-heterocyclic system substituted
with 0-3 R16, wherein the heterocyclic system is
J_0 selected from pYridinyl, thiophenyl, furanyl,
indazolyl, benzot:hiazolyl, benzimidazolyl,
benzothiophenyl, benzofuranyl, benzoxazolyl,
benzisoxazolyl, quinolinyl, isoquinolinyl, imidazolYl,
indolyl, isoindo7_yl, piperidinyl, pyrrazolyl, 1,2,4-
25 triazolyl, 1,2,3--triazolyl, tetrazolyl, thiazolyl,
oxazol:yl, pyrazinyl, and pyrimidinyl.
[15). In an even more' preferred embodiment, the present
invention provides novel compounds of formula (I-i),
20 wherein the compound of= formula (I-i) is:
Z
K~ N-E-N~N-R3
H H
(I-i)
25 R16, at each occurrence, is selected from C1_8 alkyl,
(CH2)rC3-6 cycloalkyl, CF3, C1, Br, I, F,
(CH2)rNR16aR16a'~ N02, CN, OH, (CH2)rORl6d~
(CH2 ) rC (O) Rl6b~ (CH2 ) rC (O) NR16aR16a' , (CH2 ) rNRl6fC (O) Rl6b
(CH2)rS(O)pRl6b~ (CH2)rS(O)2NR16aR16a'~
31) (CH2 ) rNRl6fS (O) 2R16b, and (CH2 ) rphenyl substituted with
0-3 Rl6e
Rl6a and Rl6a~~ at each occurrence, are selected from H, C1-6
alkyl, C3_6 cycloa~lkyl, and (CH2)rphenyl substituted
3-''~ with 0-3 Rl6e
53
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Rl6b at each occurrence, is selected from H, C1_6 alkyl,
C3_6 cycloalkyl, and (CH2)rphenyl substituted with 0-3
Rl6e;
Rl6d, at each occurrence, is selected from Cl_6 alkyl and
phenyl;
Rl6e, at each occurrence, is selected from C1_6 alkyl, C1,
F, Br, I, CN, N02, (CF2)rCF3, OH, and (CH2)rOCl_~ alkyl;
and
Rl6f at each occurrence, is selected from H, and C1-5
alkyl.
[16] In an another even more preferred embodiment, the
present invention provides novel compounds of formula (I-
ii), wherein (I-ii) is:
Z
K ~N-E-N ~ N-R3
H H
2 0 R's
(I-ii)
R16, at each occurrence, is selected from C1_a alkyl,
(CH2)rC3-6 cycloalkyl, CF3, C1, Br, I, F,
(CH2)rNR16aR16a', NO2, CN, OH, (CH2)rORl6d
(CH2 ) rC (O) Rl6b (CH2 ) rC (O) NR16aR16a' , (CH2 ) rNRl6fC (O) Rl6b
(CH2)rS(p)pRl6b tCH2)rS(O)2NR16aR16a',
(CH2)rNRl6fS(O)2R16b and (CH2)rphenyl substituted with
0-3 Rl6e;
Rl6a and Rl6a' , at each occurrence, are selected from H, C1_6
alkyl, C3_6 cycloalkyl, and (CH2)rphenyl substituted
with 0-3 Rl6e;
54
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Rl6b, at each occurrence, is selected from H, C1_6 alkyl,
C3_6 cycloalkyl, and (CH2)rphenyl substituted with 0-3
Rl6e;
Rl6d, at each occurrence, is selected from C1_6 alkyl and
phenyl;
Rl6e at each occurrence, is selected from C1_6 alkyl, C1,
F, Br, I, CN, NO~, (CF2)rCF3, OH, and (CH2)rOCl_5 alkyl;
and
Rl6f~ at each occurrence, is selected from H, and C1-5
alkyl.
[17] In a preferred embodiment, the present invention
provides navel compounds of formula (I-i), wherein:
R5 is CH2phenyl substituted with 0-3 R16;
:~0 R9, is selected from H, C~_6 alkyl, (CH2)rC3-6 cYcloalkyl, F,
C1, Cnf, (CH2)rOH, (CH2)rOR9d, (CH2)rNR9aR9a'.
(CH2)rOC(O)NHR9a, (CH2)rphenyl substituted with 0-5 R9e,
and a heterocycl_LG System substituted with 0-2 R9e,
wherein the heterocyclic system is selected from
pyridyl, thiophenyl, furanyl, oxazolyl, and thiazolyl;
R9a and R9a'~, at each occurrence, are selected from H, C1-6
alkyl, C3_6 cycloalkyl, and (CHZ)rphenyl substituted
with 0-3 R9e;
:30
R9d, at each occurrence, is selected from C1_6 alkyl and
phenyl;
R9e, at each occurrence, is selected from C1_6 alkyl, C1, F,
a5 Br, I, CN, N02, (CF2)rCF3, OH, and (CH2)rOCZ_5 alkyl;
R1~ is selected from H, C1_5 alkyl, OH, and CH20H;
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
alternatively, R9 and R1~ join to form C3_~ cycloalkyl, 5-6-
membered cyclic ketal or =0;
with the proviso that when R1~ is halogen, cyano, nitro, or
bonded to the carbon to which it is attached through a
heteroatom, R9 is not halogen, cyano, or bonded to the
carbon to which it is attached through a heteroatom;
R11 is selected from H, C1_g alkyl, (CH2)rphenyl substituted
with 0-5 Rlle, and a (CHZ)r-heterocyclic system
substituted with 0-2 Rlle, wherein the heterocyclic
system is selected from pyridinyl, thiophenyl,
furanyl, indazolyl, benzothiazolyl, benzimidazolyl,
benzothiophenyl, benzofuranyl, benzoxazolyl,
benzisoxazolyl, quinolinyl, isoquinolinyl, imidazolyl,
indolyl, isoindolyl, piperidinyl, pyrrazolyl, 1,2,4-
triazolyl, 1,2,3-triazolyl, tetrazolyl, thiazolyl,
oxazolyl, pyrazinyl, and pyrimidinyl; and
Rlle at each occurrence, is selected from C1_6 alkyl, Cl,
F, Br, I, CN, N02, (CF2)rCF3, OH, and (CH2)_OC1_5 alkyl;
R12 is H;
alternatively, R11 and R12 join to form C3_~ cycloalkyl;
R14, at each occurrence, is selected from C1_8 alkyl,
(CH2 ) rC3-6 cYcloalkyl , CF3 , C1, Br, I, F,
(CHZ)rNR14aR14a~ NOZ CN, OH, (CH2)rORl4d
(CH2)rC(O)Rl4b~ (CH2)rC(O)NR14aR14a' (CH2)rNRl4fC(0)Rl4b
(CH2)rS(0)pRl4b~ (CH2)rS(O)2NR14aR14a'~
(CHz)rNRl4fS(O)2R14b (CH2)rphenyl substituted with 0-3
Rl4e~ and a (CH2)r-5-6 membered heterocyclic system
containing 1-4 heteroatoms selected from N, O, and S,
substituted with 0-2 RlSe;
56
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Rl4a and R14~~' , at each occurrence, are selected from H, Cz_6
alkyl, C3-6 cycloalkyl, and (CH2)rphenyl substituted
with 0~-3 Rl4e, anti a (CHZ)r-5-6 membered heterocyclic
system containing 1-4 heteroatoms selected from N, O,
and S, substituted with 0-2 RlSe;
Rl4b~ at each occurrence, is selected from H, C1-6 alkyl,
C3_6 cycloalkyl, and (CHZ)rphenyl substituted with 0-3
Rl4e
Rl4d, at each occurrence, is selected from C1_6 alkyl and
phenyl ;~
Rl4e, at each occurrence, is selected from C1_6 alkyl, C1,
F, Br, I, CN, N02; (CF2)rCF3, OH, and (CH2)rOCl-5 alkyl;
and
Rl4f, at each occurrence, is selected from H, and C1-5
alkyl;
and
r is selected from 0, :1, and 2.
[18) In a preferred embodiment, the present invention
provides novel compounds of formula (I-ii), wherein:
R5 is CH2phenyl substituted with 0-3 R16
R9, is selected from H,. C1_6 alkyl, (CH2)rC3-5 cycloalkyl, F,
C1, CN, (CH2)rOH, (CH2)rOR9d, (CH2)rNR9aR9a',
(CH2)rOC(0)NHR9a, (CH2)rphenyl substituted with 0-5 R9e,
and a heterocyclic system substituted with 0-2 R9e,
wherein the heterocyclic system is selected from
pyridy7., thiophenyl, furanyl, oxazolyl, and thiazolyl;
3'.~
57
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R9a and R9a' , at each occurrence, are selected from H, Cl-5
alkyl, C3_6 cycloalkyl, and (CH2)rphenyl substituted
with 0-3 R9e;
R9d, at each occurrence, is selected from C1_6 alkyl and
phenyl;
R9e, at each occurrence, is selected from C1_6 alkyl, C1, F,
Br, I, CN, N02, (CFZ)rCF3, OH, and (CHz)rOCl_S alkyl;
RIB is selected from H, C1_8 alkyl, OH, and CH20H;
alternatively, R9 and R1~ join to form C3_~ cycloalkyl, 5-6-
membered cyclic ketal or =O;
with the proviso that when R1~ is halogen, cyano, nitro, or
bonded to the carbon to which it is attached through a
heteroatom, R9 is not halogen, cyano, or bonded to the
carbon to which it is attached through a heteroatom;
R11 is selected from H, C1_g alkyl, (CH2)rphenyl substituted
with 0-5 Rile, and a (CH2)r-heterocyclic system
substituted with 0-2 Rlle, wherein the heterocyclic
system is selected from pyridinyl, thiophenyl,
furanyl, indazolyl, benzothiazolyl, benzimidazolyl,
benzothiophenyl, benzofuranyl, benzoxazolyl,
benzisoxazolyl, quinolinyl, isoquinolinyl, imidazolyl,
indolyl, isoindolyl, piperidinyl, pyrrazolyl, 1,2,4-
triazolyl, 1,2,3-triazolyl, tetrazolyl, thiazolyl,
oxazolyl, pyrazinyl, and pyrimidinyl; and
Rlle~ at each occurrence, is selected from Cl_6 alkyl, C1,
F, Br, I, CN, N02, (CF2)rCF3, OH, and (CH2)rOCl_5 alkyl;
R12 is H;
alternatively, R11 and R12 join to form C3_~ cycloalkyl;
58
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R14, at each occurrence, is selected from C1_8 alkyl,
(CH2)rC3-6 cyclaalkyl, CF3, Cl, Br, I, F,
(CH2)rNR14aR14a' NO2~ CN, OH, (CH2)rORl4d~
(CH2)r~(O)Rl4b~ (CH')rC(O)NR14aR14a', (CH2)rNRl4fC(O}Rl4b~
(CH2)rS(O)pRl4b, (CH2)rS(0}2NR14aR14a'~
(CH2 ) r;.'~3R14fS (O) 2R:L4b (CH2 ) rphenyl substituted with 0-3
Rl4e ~~nd a (CH~)r-S-6 membered heterocyclic system
containing 1-4 heteroatoms selected from N, 0, and S,
substituted with 0-2 RlSe;
Rl4a and Rl4a~, at each occurrence, are selected from H, C1_6
alkyl, C3_6 cycloa.lkyl, and (CH2)rphenyl substituted
with 0-3 Rl4e
Rl4b at each occurrence, is selected from H, C1_6 alkyl,
C3_6 cycloalkyl, .and (CH2)rphenyl substituted with 0-3
Rl4e.
Rl4d at each occurrence, is selected from C1_6 alkyl and
phenyl ,;
Rl4e, at each occurrence, is selected from C1_6 alkyl, C1,
F, Br, I, CN, N02, (CF2)rCF3, OH, and (CH2}rOCl_5 alkyl;
Rl4f~ at each occurrence, is selected from H, and C1-5
alkyl;
and
31) r is selected from 0, 1, and 2.
[19] In a more preferred embodiment, the present invention
provides novel compounds of formula (I-i), wherein:
J is selected from CH2 and CHRS;
K is selected from CH2 and CHRS;
59
CA 02346933 2001-04-18
WO 00/35449 PCT/L'S99/30292
L is selected from CH2 and CHRS;
R3 is a C3_to carbocyclic residue substituted with 0-3 R1J,
wherein the carbocyclic residue is selected from
cyclopropyl, cyclopentyl, cyclohexyl, phenyl, naphthyl
and adamantyl, and a (CR3'H}r-heterocyclic system
substituted with 0-3 R15, wherein the heterocyclic
system is selected from pyridinyl, thiophenyl,
furanyl, indazolyl, benzothiazolyl, benzimidazolyl,
benzothiophenyl, benzofuranyl, benzoxazolyl,
benzisoxazolyl, quinolinyl, isoquinolinyl, imidazolyl,
indolyl, indolinyl, isoindolyl, isothiadiazolyl,
isoxazolyl, piperidinyl, pyrrazolyl, 1,2,4-triazolyl,
1,2,3-triazolyl, tetrazolyl, thiadiazolyl, thiazolyl,
oxazolyl, pyrazinyl, and pyrimidinyl; and
R15, at each occurrence, is selected from C1-g alkyl,
(CH2)rC3-6 cYcloalkyl, CF3, C1, Br, I, F,
2 0 (CHZ)rNR15aR15a'. N02, CN, OH, (CH2)rORlSd
(CH2)rC(0)RlSb. (CH2)rC(O)NR15aR15a', (CH2)rNRlSfC(O)RlSb
(CHZ)rS(O)pRlSb. (CHZ)rS(0)2NR15aR15a'.
(CH2)rNRlSfS(O)2R15b (CH2)rphenyl substituted with 0-3
RlSe. and a (CH2)r-5-6 membered heterocyclic system
containing 1-4 heteroatoms selected from N, O, and S,
substituted with 0-2 RlSe;
RlSa and RlSa', at each occurrence, are selected from H, C1-6
alkyl, C3_6 cycloalkyl, and (CH2)rphenyl substituted
with 0-3 RlSe;
Rl5b. at each occurrence, is selected from H, C1_6 alkyl,
C3_6 cycloalkyl, and (CH2)rphenyl substituted with 0-3
RlSe;
RlSd. at each occurrence, is selected from C1_6 alkyl and
phenyl;
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
RlSe, at each occurrence, is selected from C1-6 alkyl, Cl,
F, Br, I, CN, NC)~;, (CF2)rCF3, OH, and (CHZ)rOCl_S alkyl;
and
RlSf, at each occurrence, is selected from H, and C1-5
alkyl.
[20] In a more preferred embodiment, the present invention
provides novel compounds of formula (I-ii), wherein:
K is selected from CH4 and CHRS;
L is selected from CHI; and CHRS;
R3 is a C3_.lo carbocyc;lic residue substituted with 0-3 R15,
wherein the cark>ocyclic residue is selected from
cyclopropyl, cyc:lopentyl, cyclohexyl, phenyl, naphthyl
and adamantyl, and a (CR3'H)r-heterocyclic system
substituted with 0-3 R15, wherein the heterocyclic
system is selected from pyridinyl, thiophenyl,
furanyl, indazo7.yl, benzothiazolyl, benzimidazolyl,
benzothiophenyl, benzofuranyl, benzoxazolyl,
benzisoxazolyl, quinolinyl, isoquinolinyl, imidazolyl,
indolyl, indolinyl, isoindolyl, isothiadiazolyl,
isoxazolyl, pipE:ridinyl, pyrrazolyl, 1,2,4-triazolyl,
1,2,3-triazolyl, tetrazolyl, thiadiazolyl, thiazolyl,
oxazolyl, pyrazinyl, and pyrimidinyl; and
R15, at each occurrence, is selected from C1_g alkyl,
(CH2):rC3-~ cycloalkyl, CF3, Cl, Br, I, F,
(CH2):rNR15aR15a' ~ N02, CN, OH, (CH2)rORlSd
(CH2):rC(0)RlSb~ (CH2)rC(O)NR15aR15a'. (CH2)rNRlSfC(O)RlSb~
(CH2).rS(0)pRlSb~ (CHZ)rS(0)2NR15aR15a'.
(CH2)rNRl5tS(O)2FZ15b, and (CH2)rphenyl substituted with
0-3 RlSe, and a (CH2)r-5-6 membered heterocyclic system
61
CA 02346933 2001-04-18
WO 00/35449 PCT/US99130292
containing 1-4 heteroatoms selected from N, 0, and S,
substituted with 0-2 RlSe.
RlSa and RlSa', at each occurrence, are selected from H, C1-5
alkyl, C3_6 cycloalkyl, and (CH2)rphenyl substituted
with 0-3 RlSe;
RlSb, at each occurrence, is selected from H, C1_6 alkyl,
C3_6 cycloalkyl, and (CH2)rphenyl substituted with 0-3
RlSe;
RlSd at each occurrence, is selected from C1-6 alkyl and
phenyl;
RlSe, at each occurrence, is selected from C1_6 alkyl, C1,
F, Br, I , CN, N02 , ( CF2 ) rCF3 , OH , and ( CH2 ) rOCl-5 alkyl ;
and
RlSf~ at each occurrence, is selected from H and Cz-5 alkyl.
[21] In another embodiment, the present invention provides
a pharmaceutical composition, comprising a pharmaceutically
acceptable carrier and a therapeutically effective amount
of a compound of the present invention.
[22] In another embodiment, the present invention provides
a method for modulation of chemokine receptor activity
comprising administering to a patient in need thereof a
therapeutically effective amount of the compounds of the
present invention.
[23] In another embodiment, the present invention provides
a method for treating or preventing inflammatory diseases,
comprising administering to a patient in need thereof a
therapeutically effective amount of a compound of the
present invention.
62
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
[24) In a fifth embodiment, the present invention provides
a method for treating or preventing asthma, comprising
administering to a patient in need thereof a
therapeutically effective amount of a compound of the
present invention.
In another embodiment, the present invention provides
a pharmaceutical composition, comprising a pharmaceutically
acceptable carrier and a therapeutically effective amount
:10 of a compound of the present invention.
In another embodiment, the present invention provides
a method for modulation of chemokine receptor activity
comprising administering to a patient in need thereof a
J_5 therapeutically effective amount of a compound of the
present invention.
In another embodiment, the present invention provides
a method fo:r treating inflammatory disorders comprising
20 administering to a patient in need thereof a
therapeutically effective amount of a compound of the
present invention
In anot=her embodiment, the present invention provides
25 a method for treating or preventing disorders selected from
asthma, allergic rhinitis, atopic dermatitis, inflammatory
bowel diseases, idiopathic pulmonary fibrosis, bullous
pemphigoid, helminthic parasitic infections, allergic
colitis, eczema, conjunctivitis, transplantation, familial
31) eosinophilia, eosinophilic cellulitis, eosinophilic
pneumonias, eosinoph~l.ic fasciitis, eosinophilic
gastroenteritis, drug :induced eosinophilia, HIV infection,
cystic fibrosis, Churg~-Strauss syndrome, lymphoma,
Hodgkin's disease, and colonic carcinoma.
3 ~~
63
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
DEFINITIONS
The compounds herein described may have asymmetric
centers. Compounds of the present invention containing an
asymmetrically substituted atom may be isolated in
optically active or racemic forms. It is well known in the
art how to prepare optically active forms, such as by
resolution of racemic forms or by synthesis from optically
active starting materials. Many geometric isomers of
olefins, C=N double bonds, and the like can also be present
in the compounds described herein, and all such stable
isomers are contemplated in the present invention. Cis and
trans geometric isomers of the compounds of the present
invention are described and may be isolated as a mixture of
isomers or as separated isomeric forms. All chiral,
diastereomeric, racemic forms and all geometric isomeric
forms of a structure are intended, unless the specific
stereochemistry or isomeric form is specifically indicated.
The term "substituted," as used herein, means that any
one or more hydrogens on the designated atom is replaced
with a selection from the indicated group, provided that
the designated atom's normal valency is not exceeded, and
that the substitution results in a stable compound. When a
substitent is keto (i.e., =O), then 2 hydrogens on the atom
are replaced.
When any variable (e. g., Ra) occurs more than one time
in any constituent or formula for a compound, its
definition at each occurrence is independent of its
definition at every other occurrence. Thus, for example,
if a group is shown to be substituted with 0-2 Ra, then
said group may optionally be substituted with up to two Ra
groups and Ra at each occurrence is selected independently
from the definition of Ra. Also, combinations of
substituents and/or variables are permissible only if such
combinations result in stable compounds.
When a bond to a substituent is shown to cross a bond
connecting two atoms in a ring, then such substituent may
be bonded to any atom on the ring. When a substituent is
64
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
listed without indicating the atom via which such
substituent is bonded to the rest of the compound of a
given formula, then such substituent may be bonded via any
atom in such substituent. Combinations of substituents
and/or variables are permissible only if such combinations
result in :table compounds.
As used herein, "C1-g alkyl" is intended to include
both branched and straight-chain saturated aliphatic
hydrocarbon groups having the specified number of carbon
atoms, examples of which include, but are not limited to,
methyl, ethyl, n-prop:yl, i-propyl, n-butyl, i-butyl, sec-
butyl, t-butyl, pentyl, and hexyl. C1_8 alkyl, is intended
to include C1, Cz, C3, C4, C~, C6, C~, and Cg alkyl groups.
"Alkenyl" i.s intended to include hydrocarbon chains of
.15 either a straight or branched configuration and one or more
unsaturateo. carbon-cao~bon bonds which may occur in any
stable point along the chain, such as ethenyl, propenyl,
and the like. "Alkyny:l" is intended to include hydrocarbon
chains of either a straight or branched configuration and
a:0 one or more unsaturated triple carbon-carbon bonds which
may occur in any stable point along the chain, such as
ethynyl, propynyl, and the like. "C3_6 cycloalkyl" is
intended to include saturated ring groups having the
specified number of carbon atoms in the ring, including
25 mono-, bi-, or poly-cyclic ring systems, such as
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and
cycloheptyl in the case of C~ cycloalkyl. C3_6 cycloalkyl,
is intended to include C3, C4, C5, and C6 cycloalkyl groups
"Halo" or "halogen" as used herein refers to fluoro,
30 chloro, bromo, and iod.o; and "haloalkyl" is intended to
include both branched and straight-chain saturated
aliphatic hydrocarbon groups, for example CF3, having the
specified niunber of carbon atoms, substituted with 1 or
more halogen (for example -C~FW where v = 1 to 3 and w = 1
3:5 to ( 2v+1 ) ) .
The compounds of Formula I can also be quaternized by
standard techniques such as alkylation of the piperidine or
CA 02346933 2001-04-18
WO 00/35449 PCT/US99l30292
pyrrolidine with an alkyl halide to yield quaternary
piperidinium salt products of Formula I. Such quaternary
piperidinium salts would include a counterion. As used
herein, "counterion" is used to represent a small,
negatively charged species such as chloride, bromide,
hydroxide, acetate, sulfate, and the like.
As used herein, the term "piperidinium spirocycle or
pyrrolidinium spirocycle" is intented to mean a stable
spirocycle ring system, in which the two rings form a
quarternary nitrogene at the ring junction.
As used herein, the term "5-6-membered cyclic ketal"
is intended to mean 2,2-disubstituted 1,3-dioxolane or 2,2-
disubstituted 1,3-dioxane and their derivatives.
As used herein, "carbocycle" or "carbocyclic residue"
is intended to mean any stable 3, 4, 5, 6, or 7-membered
monocyclic or bicyclic or 7, 8, 9, 10, 11, 12, or
13-membered bicyclic or tricyclic, any of which may be
saturated, partially unsaturated, or aromatic. Examples of
such carbocycles include, but are not limited to,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl, adamantyl, cyclooctyl,; [3.3.0]bicyclooctane,
[4.3.0]bicyclononane, [4.4.0]bicyclodecane (decalin),
[2.2.2]bicyclooctane, fluorenyl, phenyl, naphthyl, indanyl,
adamantyl, or tetrahydronaphthyl (tetralin).
As used herein, the term "heterocycle" or
"heterocyclic system" is intended to mean a stable 5, 6, or
7-membered monocyclic or bicyclic or 7, 8, 9, or 10-
membered bicyclic heterocyclic ring which is saturated,
partially unsaturated or unsaturated (aromatic), and which
consists of carbon atoms and 1, 2, 3, or 4 heteroatoms
independently selected from the group consisting of N, NH,
O and S and including any bicyclic group in which any of
the above-defined heterocyclic rings is fused to a benzene
ring. The nitrogen and sulfur heteroatoms may optionally
be oxidized. The heterocyclic ring may be attached to its
pendant group at any heteroatom or carbon atom which
results in a stable structure. The heterocyclic rings
66
CA 02346933 2001-04-18
. WO 00/35449 YCT/US99/30292
described herein may be substituted on carbon or on a
nitrogen atom if the resulting compound is stable. If
specifically noted, a nitrogen in the heterocycle may
optionally be quaternized. It is preferred that when the
total number of S and O atoms in the heterocycle exceeds 1,
then these heteroatoms are not adjacent to one another. As
used herein, the term "aromatic heterocyclic system" is
intended to mean a stable 5- to ?- membered monocyclic or
bicyclic ox- 7- to 10-membered bicyclic heterocyclic
aromatic ring which consists of carbon atoms and from 1 to
4 heterotams independently selected from the group
consisting of N, O and S.
Examples of heterocycles include, but are not limited
to, 1H-indazole, 2-pyrrolidonyl, 2H,6H-1,5,2-dithiazinyl,
2H-pyrrolyl, 3H-indol.yl, 4-piperidonyl, 4aH-carbazole, 4H-
quinolizinyl, 6H-1,2,5-thiadiazinyl, acridinyl, azocinyl,
benzimidazolyl, benzofuranyl, benzothiofuranyl,
benzothiophenyl, benzoxazolyl, benzthiazolyl,
benztriazol;yl, benztetrazolyl, benzisoxazolyl,
benzisothiazolyl, benzi.midazalonyl, carbazolyl,
4aH-carbazo:lyl, p-carbolinyl, chromanyl, chromenyl,
cinnolinyl, decahydroquinolinyl, 2H,6H-1,5,2-dithiazinyl,
dihydrofuro[2,3-8)tetrahydrofuran, furanyl, furazanyl,
imidazolidinyl, imidazolinyl, imidazolyl, 1H-indazolyl,
indolenyl, indolinyl, indolizinyl, indolyl,
isobenzofuranyl, isochromanyl, isoindazolyl, isoindolinyl,
isoindolyl, isoquinoli;nyl (benzimidazolyl), isothiazolyl,
isoxazolyl, morpholinyl, naphthyridinyl,
octahydroisoquinolinyl, oxadiazolyl, 1,2,3-oxadiazolyl,
1,2,4-oxadiazolyl, 1,2,5-oxadiazolyl, 1,3,4-oxadiazolyl,
oxazolidinyl., oxazoly:l, oxazo-~idinylperimidinyl,
phenanthridinyl, phenanthrolinyl, phenarsazinyl,
phenazinyl, phenothiazinyl, phenoxathiinyl, phenoxazinyl,
phthalazinyl, piperazinyl, piperidinyl, pteridinyl,
piperidonyl, 4-piperidonyl, pteridinyl, purinyl, pyranyl,
pyrazinyl, pyrazolidinyl, pyrazolinyl, pyrazolyl,
pyridazinyl, pyridooxaz:ale, pyridoimidazole,
67
CA 02346933 2001-04-18
WO 00135449 PCT/1JS99/3v292
pyridothiazole, pyridinyl, pyridyl, pyrimidinyl,
pyrrolidinyl, pyrrolinyl, pyrrolyl, quinazolinyl,
quinolinyl, 4H-quinolizinyl, quinoxalinyl, quinuclidinyl,
carbolinyl, tetrahydrofuranyl, tetrahydroisoquinolinyl,
tetrahydroquinolinyl, 6H-1,2,5-thiadiazinyl, 1,2,3-
thiadiazolyl, 1,2,4-thiadiazolyl, 1,2,5-thiadiazolyl,
1,3,4-thiadiazolyl, thianthrenyl, thiazolyl, thienyl,
thienothiazolyl, thienooxazolyl, thienoimidazolyl,
thiophenyl, triazinyl, 1,2,3-triazolyl, 1,2,4-triazolyl,
1,2,5-triazolyl, 1,3,4-triazolyl, tetrazolyl, and
xanthenyl. Preferred heterocycles include, but are not
limited to, pyridinyl, thiophenyl, furanyl, indazolyl,
benzothiazolyl, benzimidazolyl, benzothiaphenyl,
benzofuranyl, benzoxazolyl, benzisoxazolyl, quinolinyl,
25 isoquinolinyl, imidazolyl, indolyl, isoidolyl, piperidinyl,
piperidonyl, 4-piperidonyl, piperonyl, pyrrazolyl, 1,2,4-
triazolyl, 1,2,3-triazolyl, tetrazolyl, thiazolyl,
oxazolyl, pyrazinyl, and pyrimidinyl. Also included are
fused ring and spiro compounds containing, for example, the
above heterocycles.
The phrase "pharmaceutically acceptable" is employed
herein to refer to those compounds, materials,
compositions, and/or dosage forms which are, within the
scope of sound medical judgment, suitable for use in
contact with the tissues of human beings and animals
without excessive toxicity, irritation, allergic response,
or other problem or complication, commensurate with a
reasonable benefit/risk ratio.
As used herein, "pharmaceutically acceptable salts"
refer to derivatives of the disclosed compounds wherein the
parent compound is modified by making acid or base salts
thereof. Examples of pharmaceutically acceptable salts
include, but are not limited to, mineral or organic acid
salts of basic residues such as amines; alkali or organic
salts of acidic residues such as carboxylic acids; and the
like. The pharmaceutically acceptable salts include the
conventional non-toxic salts or the quaternary ammonium
68
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
salts of the parent compound formed, for example, from non-
toxic inorganic or arganic acids. For example, such
conventional non-toxic salts include those derived from
inorganic acids such as hydrochloric, hydrobromic,
sulfuric, sulfamic, phosphoric, nitric and the like; and
the salts prepared from organic acids such as acetic,
propionic, succinic, glycolic, stearic, lactic, malic,
tartaric, citric, ascorbic, pamoic, malefic, hydroxymaleic,
phenylacetic, glutamic, benzoic, salicylic, sulfanilic, 2-
:10 acetoxyben~:oic, fumaric, toluenesulfonic, methanesulfonic,
ethane disulfonic, oxalic, isethionic, and the like.
The pharmaceutically acceptable salts of the present
invention c:an be synthesized from the parent compound which
contains a basic or acidic moiety by conventional chemical
.l5 methods. Gienerally, such salts can be prepared by reacting
the free acid or base forms of these compounds with a
stoichiomet:ric amount of the appropriate base or acid in
water or in an organi~~ solvent, or in a mixture of the two;
generally, nonaqueous media like ether, ethyl acetate,
~.'0 ethanol, isopropanol, or acetonitrile are preferred. Lists
of suitable salts are found in Remington's Pharmaceutical
Sciences, l.7th ed., Mack Publishing Company, Easton, PA,
1985, p. 1918, the disclosure of which is hereby
incorporated by refer=_nce.
25 Since prodrugs a:re known to enhance numerous desirable
qualities of pharmaceuticals (e. g., solubility,
bioavailability, manufacturing, etc...) the compounds of
the present invention may be delivered in prodrug form.
Thus, the present invention is intended to cover prodrugs
?.0 of the presently claimed compounds, methods of delivering
the same and compositions containing the same. "Prodrugs"
are intended to include any covalently bonded carriers
which release an active parent drug of the present
invention in vivo when such prodrug is administered to a
35 mammalian subject. P:rodrugs the present invention are
prepared by modifying functional groups present in the
compound in such a way that the modifications are cleaved,
69
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
either in routine manipulation or in vivo, to the parent
compound. Prodrugs include compounds of the present
invention wherein a hydroxy, amino, or sulfhydryl group is
bonded to any group that, when the prodrug of the present
S invention is administered to a mammalian subject, it
cleaves to form a free hydroxyl, free amino, or free
sulfhydryl group, respectively. Examples of prodrugs
include, but are not limited to, acetate, formate and
benzoate derivatives of alcohol and amine functional groups
in the compounds of the present invention.
"Stable compound" and "stable structure" are meant to
indicate a compound that is sufficiently robust to survive
isolation to a useful degree of purity from a reaction
mixture, and formulation into an efficacious therapeutic
agent.
SYNTHESIS
The compounds of Formula I can be prepared using the
reactions and techniques described below. The reactions
are performed in a solvent appropriate to the reagents and
materials employed and suitable for the transformations
being effected. It will be understood by those skilled in
the art of organic synthesis that the functionality present
on the molecule should be consistent with the
transformations proposed. This will sometimes require a
judgment to modify the order of the synthetic steps or to
select one particular process scheme over another in order
to obtain a desired compound of the invention. It will
also be recognized that another major consideration in the
planning of any synthetic route in this field is the
judicious choice of the protecting group used for
protection of the reactive functional groups present in the
compounds described in this invention. An authoritative
account describing the many alternatives to the trained
practitioner is Greene and Wuts (Protective Groups In
Organic Synthesis, Wiley and Sons, 1991).
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Generally, compounds described in the scope of this
patent application can be synthesized by the route
described in Scheme 1. The appropriately substituted
pyrrolidine (n=0) or piperidine (n=1) 1 is alkylated by a
N-protected alkylhalide (halide = C1, Br, I), mesylate,
tosylate or triflate, 2, (where E represents a linkage
described within the scope of this application in its fully
elaborated form with the appropriate protecting groups as
understood by one skilled in the art or in a precursor form
which can be later elaborated into its final form by
methods familiar to one skilled in the art) with or without
base or an acid scavenger to yield the piperidinyl- or
pyrrolidinylalkyl protected amine 3. If the halide is not
I, then KI car. also loe added to facilitate the
displacement, provided the solvent is suitable, such as an
alcohol, 2-butanone, DMF or DMSO, amongst others. The
displacement can be performed at room temperature to the
reflux temperature o:E the solvent. The protecting group is
subsequently removed to yield amine _4. Protecting groups
include phthalimide ~Nhich can be removed by hydrazine, a
reaction familiar to one skilled in the art; bis-BOC which
can be removed by eit=her TFA or HC1 dissolved in a suitable
solvent, both procedures being familiar to one skilled in
the art; a nitro group instead of an amine which can be
reduced to yield an amine by conditions familiar to one
skilled in the art; :?,4-dimethyl pyrrole (S. P. Breukelman,
et al. J. Chem. Soc. Perkin Trans. I, 1984, 2801); N-
1,1,4,4-Tetramethyl-<iisilylazacyclopentane (STABASE) (S.
Djuric, J. Venit, and P. Magnus Tet. Lett 1981, 22, 1787)
and other protecting groups. Reaction with an isocyanate or
isothiocyanate 5 (Z =- O,S) yields urea ~r thiourea 6.
Reaction with a chloroformate or chlorothioformate 7
(Z=O,S) such as o-, p-nitrophenyl-chloroformate or
phenylchloroformate (ar their thiocarbonyl equivalents),
followed by diplacement with an amine 9, also yields the
corresponding urea or thiourea 6. Likewise, reaction of
carbamate _8 (X = H, c>r 2- or 4-N02) with disubstituted
71
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
amine 10 yields trisubstituted urea or thiourea 12.
Reaction of the amine 4 with an N,N-disubstituted carbamoyl
chloride 11 (or its thiocarbonyl equivalent) yields the
corresponding N,N-disubstituted urea or thiourea 12.
Amine 4 can also be reductively aminated to yield 13 by
conditions familiar to one skilled in the art and by the
following conditions: Abdel-Magid, A. F., et al. Tet. Lett.
1990, 31, (39) 5595-5598. This secondary amine can
subsequently be reacted with isocyanates or isothiocyanates
to yield trisubstituted ureas 14 or with carbamoyl
chlorides to yield tetrasubstituted ureas 15.
72
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 1
H
1V~ H--.~- P
+ X/,L~N_ P ~ wN~
2
R5 n
1 P=protecting grow 5 ri
P
X=leaving group: CI,Br,I, R 3
n=0, 1 OTs, OMs, OTf, etc
E=linker
y (C'=Z ) -NR2R3 E"~~2
~ Cl- (C=Z) -NR2R3
11 n
R5 :n 12 R5 4
2 3 C1- ( C=Z ) -OPh R3N=C=Z
R R 2J
7 5
~-NH- ( C=Z ) -OPh- Y ,~NH- ( C=Z ) -NH-R3
R3NH2
R5 l:n 8 --.-~ R~ Jn 6
R2CH0
Y = H, o- or p-N02
Na (Ac0) 38H /
'~R2- (C=Z ~ -~3
R3N=C=Z
~,z--NHR 2
5
s
R5 n 14
R5 n 13
~r---NR2- (C=Z ) -NRzR3 C1- (C=Z) -NR2R3
Z=0 or S 11
R5 n 15
One can also convert amine 4 into an isocyanate,
isothiocyanate, carbamoyl chloride or its thiocarbonyl
5 equivalent (isocyanate~: Nowakowski, J. J Prakt. Chem/Chem-
Ztg 1996, 3.38 (7), 667-671; Knoelker, H.-J. et al., Angew.
Chem. 1995, 107 (22), 2746-2749; Nowick, J. S.et al., J.
Org. Chem. :1996, 61 (11), 3929-3934; Staab, H. A.; Benz,
73
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
W.; Angew Chem 1961, 73; isothiocyanate: Strekowski L.et
al., J. Heterocycl. Chem. 1996, 33 (6), 1685-1688;
Kutschy, Pet al., Synlett. 1997, (3), 289-290) carbamoyl
chloride: Hintze, F.; Hoppe, D.; Synthesis (1992) 12, 1216-
1218; thiocarbamoyl chloride: Ried, W.; Hillenbrand, H.;
Oertel, G.; Justus Liebigs Ann Chem 1954, 590) (these
reactions are not shown in Scheme 1). These isocyanates,
isothiocyantes, carbamoyl chlorides or thiocarbamoyl
chlorides can then be reacted with R2R3NH to yield di- or
trisubstituted ureas or thioureas 12. An additional urea
forming reaction involves the reaction of
carbonyldiimidazole (CDI) (Romine, J. L.; Martin, S. W.;
Meanwell, N. A.; Epperson, J. R.; Synthesis 1994 (8), 846-
850) with 4 followed by reaction of the intermediate
imidazolide with 9 or in the reversed sequence (9 + CDI,
followed by 4). Activation of imidazolide intermediates
also facilitates urea formation (Bailey, R. A., et al.,
Tet. Lett. 1998, 39, 6267-6270). One can also use 13 and
10 with CDI. The urea forming reactions are done in a non-
hydroxylic inert solvent such as THF, toluene, DMF, etc.,
at room temperature to the reflux temperature of the
solvent and can employ the use of an acid scavenger or base
when necessary such as carbonate and bicarbonate salts,
triethylamine, DBU, Hunigs base, DMAP, etc.
Substituted pyrrolidines and piperidines 1 can either
be obtained commercially or be prepared as shown in Scheme
2. Commercially available N-benzylpiperid-3-one 16 can be
debenzylated and protected with a BOC group employing
reactions familiar to one skilled in the art. Subsequent
Wittig reaction followed by reduction and deprotection
yields piperidine 20 employing reactions familiar to one
skilled in the art. Substituted pyrrolidines may be made
by a similar reaction sequence. Other isomers and analogs
around the piperidine ring can also be made by a similar
reaction sequence. Chiral pyrrolidines/piperidines can be
synthesized via asymmetric hydrogenation of 18 using chiral
catalysts (see Parshall, G.W. Homogeneous Catalysis, John
74
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Wiley and Sons, New 'York: 1980, pp. 43-45; Collman, J.P.,
Hegedus, h.S. Principles and Applications of
Organotransition Met,ai Chemistry, University Science gooks,
Mill Valley, CA, 1980, pp. 341-348).
SCHEME 2
Hz/Pd Wittig H2/Pd
_--s s -:.
BOC:2 0 R~
BOC BOC
17 18
16
R5
H+
19
BOC 20
H
The ~~yanoguanidines (Z = N-CN) can be synthesized by
the method of K. S. Atwal, et al. and references contained
therein (.J. Med. Chem. (1998) 41, 217-275). The
nitroethylene analog (Z - C-N02) can be synthesized by the
method of F. Moimas, et al. (Synthesis 1985, 509-510) and
references contained therein. The malononitrile analog (Z
- C(CN)2) may be synthesized by the method of S. Sasho, et
al. (J. Med. Chem. 1993, 36, 572-579).
Guanidines (Z=NRla) can be synthesized by the methods
outlined in Scheme 3. Compound ~1 where Z=S can be
methylated -to yield the methylisothiourea 22. Displacement
of the SMe group with amines yields substituted guanidines
?L3 (see H. King and T. M. Tonkin J. Chem. Soc. 1946, 1063
and references therein). Alternatively, reaction of
thiourea ,21 with amines in the presence of triethanolamine
and "lac sulfur" which facilitates the removal of H2S
yields substituted g~uanidines 23 (K. Ramadas, Tet. Lett.
1996, 37, 5161 and references therein). Finally, the use
of carbonimidoyldichloride 24, or 25 followed by sequential
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
displacements by amines yields the corresponding
substituted guanidine 23 (S. Nagarajan, et al., Syn. Comm.
1992, 22, 1191-8 and references therein). In a similar
manner, carbonimidoyldichlorides, R2-N=C(CI)2 (not shown in
Scheme 3) and R3-N=C(C1)2 (not shown) can also be reacted
sequentially with amines to yield di- and trisubstituted
guanidine 23.
SCHEME 3
E-NR' - ( C=S ) -NHR la ~~1 _ ( C=NHRla ) -SMe
CH3 I
R5 n 21 ~ /n
R 22
n=0,1
HNR2R3
N ( CH20H ) 3 ,
"lac sulfur" , F-NR1- (C=NHRla) -NR2R3
R2R3NH
~i
I.H2NR", Et3N R5 n 23
2 . HNR2R3 or
1 . HNR2R3 , Et3N 1 . HNR2R3 , Et3N
2 . HZNR" 2 . 13 or
1 . 13 , Et3N
2 . HNR2R3
N=C (C1) 2
Rla-N=C ( C1 ) 2
~n 24 25
R
A method for introducing substituents in linkage E is
that of A. Chesney et al. (Syn. Comm. 1990, 20 (20), 3167-
3180) as shown in Scheme 4. Michael reaction of
pyrrolidine or piperidine 1 with Michael acceptor 26 yields
intermediate 27 which can undergo subsequent reactions in
the same pot. For example, reduction yields alcohol 28
which can be elaborated to the amine 29 by standard
procedures familiar to one skilled in the art. Some of
76
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
these include mesylation or tosylation followed by
d:.splacement with NaN3 followed by reduction to yield amine
29. Another route as depicted in Scheme 4 involves
reaction with diphenylphosphoryl azide followed by
reduction of the azide to yield amine 29.
SCHEME 9.
g 9
~H 9
+ ~ ~ O Michael
R5 n Rxn iz
Re R11 R5 n R
27
26
n=0, 1 Rl2Li or Rl2MgBr
NaBH4 31
a
Old
R~ n R1 i
2 8 1~
(Ph0)2(P=O):N3 32 (ph0)2(P=O)N3
.H2, Pd/C
H2, Pd/C
2
h
n 33
29 as described
as described
previously previously
1- ( C=~~ ) -NR2 R3 1 _ ( C=Z ) -NR2 R3
34
77
WO 00/35449
CA 02346933 2001-04-18
PCT/US99/30292
The mesylate or tosylate can also be displaced by
other nucleophiles such as NH3, BOC2N-, potassium
phthalimide, etc., with subsequent deprotection where
necessary to yield amines 29. Finally, 29 can be converted
to urea or thiourea 30 by procedures discussed for Scheme 1
or to the compounds of this invention by procedures
previously discussed. Similarly, aldehyde 27 may be
reacted with a lithium or a Grignard reagent 31 to yield
alcohol adduct 32. This in turn can be converted to urea
or thiourea 34 in the same way as discussed for the
conversion of 28 to 30.
Scheme 5 shows that intermediate 36 can be extended
via a Wittig reaction (A. Chesney, et al. Syn. Comm. 1990,
(20), 3167-3180) to yield 37. This adduct can be
15 reduced catalytically to yield 38 or by other procedures
familiar to one skilled in the art. Alkylation yields 39,
followed by saponification and Curtius rearrangement (T. L.
Capson and C. D. Poulter, Tet. Lett., (1984) 25, 3515-3518)
followed by reduction of the benzyl protecting group yields
20 amine 40 which can be elaborated further as was described
earlier in Scheme 1 and elsewhere in this application to
make the compounds of this invention. Dialkyllithium
cuprate, organocopper, or copper-catalyzed Grignard
addition (for a review, see G. H. Posner, "An Introduction
to Synthesis Using Organocopper Reagents", J. Wiley, New
York, 1980; Organic Reactions, 19, 1 (1972)) to alpha,beta-
unsaturated ester 37 yields 41 which can undergo subsequent
transformations just discussed to yield amine 43 which can
be elaborated further to the compounds of this invention as
was described earlier. The intermediate enolate ion
obtained upon cuprate addition to 37 can also be trapped by
an electrophile to yield 42 (for a review, see R. J. K.
Taylor, Synthesis 1985, 364). Likewise, another 2-carbon
homologation is reported by A. Chesney et al. (ibid.) on
intermediate 36 which involves reacting 36 with an enolate
anion to yield aldol condensation product 42 where R~2-OH.
The OH group can undergo synthetic transformations which
78
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
are familiar to one skilled in the art and which will be
discussed in much detail later on in the application.
Chiral au:xilliaries can also be used to introduce stereo-
and enantioselectivi.ty in these aldol condensations,
procedures which are familiar to one skilled in the art.
79
i
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME S
7 8 Rg
H g
O Michael Rxn ~ ~0
R
R5 ~r_ / > R5 Jn Rg
1 R8 Rg 3 6
n=0 , 1 35 R11
Wittig ~ pph~(
\'OMe
Hz Pd/C
1.LDA '°
2 . RlzX 37
7 8 R9
11 12
1 . -OH
2 . ( Ph0 ) z ( P=O ) N3 5 n Rg
R ,~ ~ 2
3.BnOH
39 4.Hz Pd/C 40
to compounds
by methods
8 g 7 8 g previously
discussed
11 as above 11
~n R9~NH2 g OMe
R R R~ wn R Rlo
44 41 ~ (Rlo)zCuLi
1.LDA
to compounds
by methods ~ 8 9 2 ' R12X 8 g 2 . RlzX
previously
discussed ~ R11 12 ag R 11
above ~ Rlz
R~ 'n Rg Rlo NHz R~ ~n Rg Rlo OMe
43 42 0
Examples of such methods are taught in D. A. Evans, et al.,
J. Am. Chem. Soc. 1981, 103, 2127; D. A. Evans, J. Am.
5 Chem.Soc. 1982, 104, 1737; D. A. Evans, J. Am. Chem. Soc.
CA 02346933 2001-04-18
WO 00/35449 PCT/iJS99/30292
1986, 108, 2476; D. A. Evans. et al., J. Am. Chem. Soc.
1986, 108, 657; D. A. Evans, J. Am. Chem. Soc. 1986, 108,
6395; D. A. Evans, J. Am. Chem. Soc. 1985, 107, 4346; A.
G. Myers, et al., J. Am. Chem. Soc. 1997, 119, 6496. One
can also perform an enantioselective alkylation on esters
38 or 41 with Ri2X where X is a leaving group as described
in Scheme 1, provided the ester is first attached to a
chiral auxiliary (see above references of Evans, Myers and
Mauricio de L. Vander..lei, J. et al., Synth. Commum. 1998,
1.0 28, 3047} .
One ca:n also react alpha,beta-unsaturated ester 37
(Scheme 6) with Corey's dimethyloxosulfonium methylide
(E.J. Corey and M. Chaykovsky, J. Am. Chem. Soc. 1965, 87,
1345) to foam a cyclopropane which can undergo eventual
Curtius rearrangement and subsequent elaboration to the
compounds of this invention wherein the carbon containing
R9R1~ is tied up in a cyclopropane ring with the carbon
containing ;R11R12. In addition, compound 48 can also
undergo the analogous reactions just described to form
cyclopropylamine 50 which can be further elaborated into
the compounds of this invention as described previously.
Compound 48 may be synthesized by an alkylation,reaction of
pyrrolidine/piperidine 1 with bromide 47 in an inert
solvent employing the conditions as described for the
alkylation of 2 onto 1 in Scheme 1.
Another way to synthesize the compounds in the scope
of this application is shown in Scheme 7. Michael reaction
of amine 1 with an acrylonitrile 51 (as described by I.
Roufos in J. Med. Chem. 1996, 39, 1514-1520) followed by
Raney-Nicke:i hydrogenation yields amine 53 which can be
elaborated to the compounds of this invention as previously
described.
81
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30252
SCHEME 6
I-
OMe OMe
NaH,
THF
37 '*o
1 . - OH
2 . ( Ph0 ) 2 ( P=O ) N3
3.BnOH
4.H2 Pd/C
~ ~s 9
R11
to compounds
by methods ~ +
previously
discussed R5 ~n R9 NH2
46
7 8 R9 7 8 R9
C02 Me --~ +
C02 Me
~n Rli NaH,
R 48 THF R~ ~n R11
49
s R9 1.-OH
1 + 2 . ( Ph0 ) 2 ( P=O ) N3
Br ~ 3.BnOH
47 C02Me 4. H2 Pd/C
R11
g 9
to compounds
by methods ~ ~ +
previously
discussed R5 n R11
In Schemes 4,5, and 6, we see that there is no gem-
substitution on the alpha-carbon to the electron-
s withdrawing group of what used to be the Michael acceptor.
In other words, in Scheme 4, there is no R~0 gem to R9; in
Scheme 5, there is no R10 gem to one of the R9s and in
82
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Scheme 7 there is no R10 gem to R9. Gem-substitution can
be introduced by :~eact:ing pyrrolidine or piperidine 1 with
the epoxide of Michael acceptors 26, 35, and 51 to yield
the corresponding alcohols (for amines reacting with
epoxides of Michael acceptors, see Charvillon, F. B.;
Amouroux, R.; Tet. Lett. 1996, 37, 5103-5106; Chong, J.
M.; SharplE~ss, K. B.; J Org Chem 1985, 50, 1560). These
alcohols a«entually can be further elaborated into R10 by
one skilled in the art, as, for example, by tosylation of
.LO the alcohol. and cuprate displacement (Hanessian, S.;
Thavonekham, B.; DeHoff, B.; J Org. Chem. 1989, 54, 5831),
etc., and by other displacement reactions which will be
discussed in great detail later on in this application.
SCHEME '1
R Re
H ~3
V
+ R CN Ra_Ni/H2
~CN --~ ' R9
Rs n
1 R8 R5 ~n
5~
n=0,1 51
R~
C:H2 -NH2
'' ~ to compounds
g by methods previously
discussed
Jn
R5
1'S 5 :3
Further_ use of epoxides to synthesize compounds of
this invention are shown in Scheme 8. Reaction of pyrrole
or piperidine 1 with epoxide 54 yields protected- - amino-
2() alcohol 55. This reaction works- exceptionaly well when R~
and R8 are H but is not limited thereto. The reaction is
performed in an inert solvent at room temperature to the
reflux temperature of the solvent. Protecting groups on
the nitrogen atom of 54 include BOC and CBZ but are not
25~ limited thereto. The :hydroxyl group can be optionally
83
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
protected by a variety of protecting groups familiar to one
skilled in the art.
SCHEME 8
H ~ 9or10
R
+ Rg NH- P
R5 n R1
Rlz - P
1 54
h
n=0,1 55
fO] -P
( R9or10=H )
"-7 ~8 p9or10
_P
7 ' ~mnz
R9or10-M R5 n R-11 \R12
56
where M=Li,MgBr,
MgCl, ZnCl, etc.
_P
-7 a R9or10
to compounds
OH by methods
previously
discussed
_P
R5" ~ ~n R11 'Rlz
58
S
Deprotection of the nitrogen by methods familiar to one
skilled in the art yields 56 which can be elaborated to the
compounds of this invention by the procedures previously
discussed. If R9=H, then oxidation, for example, by using
PCC (Corey E.J. and Suggs, J.W., Tet. Lett. 1975, 31, 2647-
2650) or with the Dess-Martin periodinane (Dess, D.B. and
Martin, J.C., J. Org. Chem. 1983, 48, 4155-4156) yields
ketone 57 which may undergo nucleophilic 1,2-addition with
organometallic reagents such as alkyl- or aryllithiums,
84
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Grignards, or zinc reagents, with or without CeCl3 (T.
Imamoto, et al., Tet. I~ett. 1985, 26, 4763-4766; T.
Imamoto, et al., Tet. Lett. 1984, 25, 4233-4236) in aprotic
solvents s,ich as ether, dioxane, or THF to yield alcohol
58. The hydroxyl group can be optionally protected by a
variety of protecting groups familiar to one skilled in the
art. Deprotection of the nitrogen yields 56 which can be
finally el<~borated to the compounds of this invention as
previously discussed. Epoxides disclosed by structure _54
may be synt=hesized enantio-selectively from amino acid
starting materials by the methods of Dellaria, et al. J Med
Chem 1987, 30 (11), 2:L37, and Luly, et al. J Org Chem
1987, 52 (fi) , 1487.
The carbonyl group of ketone 57 in Scheme 8 may
:L5 undergo Wit:tig reactions followed by reduction of the
double bond to yield alkyl, arylalkyl, heterocyclic-alkyl,
cycloalkyl, cycloalky7Lalkyl, etc. substitution at that
position, reactions that are familiar to one skilled in the
art. Wittig reagents can also contain functional groups
:?0 which after reduction of the double bond yield the
following functionality: esters (Buddrus, J. Angew Chem.,
1968, 80), nitriles (C:ativiela, C.et al., Tetrahedron
1996, 52 (16), 5881-5E~88.), ketone (Stork, G.et al., J Am
Chem Soc 1996, 118 (43), 10660-10661), aldehyde and
~;5 methoxymethyl (Bertram, G.et al., Tetrahedron Lett 1996, 37
(44), 7955-7958.), gamma-butyrolactone Vidari, G.et
al.,Tetrahedron: Asymmetry 1996, 7 (10), 3009-3020.),
carboxylic acids (Svoboda, J.et al., Collect Czech Chem
Commun 1996, 61 (10), 1509-1519), ethers (Hamada, Y.et
30 al., Tetrahedron Lett 1984, 25 (47), 5413), alcohols (after
hydrogenation and deprotection--Schonauer, K.; Zbiral, E.;
Tetrahedron Lett 1983, 24 (6), 573), amines (Marxer, A.;
Leutert, T. Helv Chim Acta, 1978, 61) etc., all of which
may further undergo transformations familiar to one skilled
35 in the art 'to form a wide variety of functionality at this
position.
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Scheme 9 summarizes the displacement chemistry and
subsequent elaborations that can be used to synthesize the
R9 groups. In Scheme 9 we see that alcohol 55 or 58 may be
tosylated, mesylated, triflated, or converted to a halogen
by methods familiar to one skilled in the art to produce
compound 59. (Note that all of the following reactions in
this paragraph can be also performed on the compounds,
henceforth called carbon homologs of 55 or 58 where OH can
be (CH2)rOH and it is also understood that these carbon
homologs may have substituents on the methylene groups as
well). For example, a hydroxyl group may be converted to a
bromide by CBr4 and Ph3P (Takano, S. Heterocycles 1991, 32,
1587). For other methods of converting an alcohol to a
bromide or to a chloride or to an iodide see R.C. Larock,
Comprehensive Organic Transformations, VCH Publishers, New
York, 1989, pp. 354-360. Compound 59 in turn may be
displaced by a wide variety of nucieophiles as shown in
Scheme 9 including but not limited to azide, cyano,
malonate, cuprates, potassium thioacetate, thiols, amines,
etc., all nucleophilic displacement reactions being
familiar to one skilled in the art. Displacement by
nitrile yields a one-carbon homologation product. Nitrile
60 can be reduced with DIBAL to yield aldehyde 61. This
aldehyde can undergo reduction to alcohol 62 with, for
example, NaBH4 which in turn can undergo all of the SN2
displacement reactions mentioned for alcohol 55 or 58.
Alcohol 62 is a one carbon homolog of alcohol 55 or 58.
Thus one can envision taking alcohol 62, converting it to a
leaving group X as discussed above for compound 55 or 58,
and reacting it with NaCN or KCN to form a nitrile,
subsequent DIBAL reduction to the aldehyde and subsequent
NaBH4 reduction to the alcohol resulting in a two carbon
homologation product. This alcohol can undergo activation
followed by the same SN2 displacement reactions discussed
previously, ad infinitum, to result in 3,4,5...etc. carbon
homologation products. Aldehyde 61 can also be reacted
with a lithium or Grignard reagent to form an alcohol 61a
86
CA 02346933 2001-04-18
V~10 00/35449 PCT/US99/30292
which can also undergo the above displacement reactions.
Oxidation f>y methods famili~.r to one skilled in the art
yields ketone 61b. Displacement by malonate yields malonic
ester 63 which can be saponified and decarboxylated to
yield carboxylic acid 64, a two carbon homologation
product. Conversion to ester 65 (A. Hassner and V.
Alexanian, Tet. Lett., 1978, 46, 4475-8) and reduction with
LAH yields alcohol 68 which can undergo all of the
displacemer_t reactions discussed for alcohol 55 or 58.
:LO Alcohols may be converted to the corresponding fluoride 70
by DAST (diethylaminosulfur trifluoride) (Middleton, W. J.;
Bingham, E. M.; Org. Synth. 1988, VI, pg. 835). Sulfides
71 can be converted to the corresponding sulfoxides 72
(p=1) by sodium metaperiodate oxidation (N. J. Leonard, C.
.L5 R. Johnson J. Org. Chem. 1962, 27, 282-4) and to sulfones
72 (p=2) by Oxone~ (A. Castro, T.A. Spencer J. Org. Chem.
1992, 57, 3496-9). Sulfones 72 can be converted to the
corresponding sulfonamides 73 by the method of H.-C. Huang,
E. et al., Tet. Lett.. (1994) 35, 7201-7204 which involves
:?0 first, treatment with base followed by reaction with a
trialkylborane yielding a sulfinic acid salt which can be
reacted with hydroxyl,amine-O-sulfonic acid to yield a
sulfonamide. Another mute to sulfonamides involves
reaction of amines with a sulfonyl chloride (G. Hilgetag
25 and A. Martini, Preparative Organic Chemistry, New York:
John Wiley and Sons, 1972, p.679). This sulfonyl chloride
(not shown in Scheme 9) can be obtained from the
corresponding sulfide (71, where R9d=H in Scheme 9, the
hydrolysis product after thioacetate displacement),
_s0 disulfide, or isothiouronium salt by simply reacting with
chlorine in water. The isothiouronium salt may be
synthesized from the corresponding halide, mesylate or
tosylate 5~ via reaction with thiourea (for a discussion on
the synthesis of sulfonyl chlorides see G. Hilgetag and A.
a5 Martini, ibid., p. 6'70). Carbaxylic acid 64 can be
converted to amides 6~6_ by standard coupling procedures or
via an acid chloride by Schotten-Baumann chemistry or to a
87
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Weinreb amide (66: R9a=OMe, R9a'= Me in Scheme 9) (S. Nahm
and S. M. Weinreb, Tet. Lett., 1981, 22, 3815-3818) which
can undergo reduction to an aldehyde 67 (R9b=H in Scheme 9)
with LAH (S. Nahm and S. M. Weinreb, ibid.) or reactions
with Grignard reagents to form ketones 67 (S. Nahm and S.
M. Weinreb, ibid.). The aldehyde 67 obtained from the
Weinreb amide reduction can be reduced to the alcohol with
NaBH4. The aldehyde or ketone 67 (or 61 or 61b for that
matter) can undergo Wittig reactions as discussed
previously followed by optional catalytic hydrogenation of
the olefin. This Wittig sequence is one method for
synthesizing the carbocyclic and heterocyclic substituted
systems at R9 employing the appropriate carbocyclic or
heterocyclic Wittig (or Horner-Emmons) reagents. Of
course, the Wittig reaction may also be used to synthesize
alkenes at R9 and other functionality as well. Ester 65 can
also form amides 66 by the method of Weinreb (A. Basha, M.
Lipton, and S.M. Weinreb, Tet. Lett. 1977, 48, 4171-74) (J.
I. Levin, E. Turos, S. M. Weinreb, Syn. Comm. 1982, 12,
989-993). Alcohol 68 can be converted to ether 69 by
procedures familiar to one skilled in the art, for example,
NaH, followed by an alkyliodide or by Mitsunobu chemistry
(Mitsunobu, O. Synthesis, 1981, 1-28). Alcohol 55 or 58,
62, or 68, can be acylated by procedures familiar to one
skilled in the art, for example, by Schotten-Baumann
conditions with an acid chloride or by an anhydride with a
base such as pyridine to yield 78. Halide, mesylate,
tosylate or triflate 59 can undergo displacement with azide
followed by reduction to yield amine 74 a procedure
familiar to one skilled in the art. This amine can undergo
optional reductive amination and acylation to yield 75 or
reaction with ethyl formate (usually refluxing ethyl
formate) to yield formamide 75. Amine 74 can again undergo
optional reductive amination followed by reaction with a
sulfonyl chloride to yield 76, for example under Schotten-
Baumann conditions as discussed previously. This same
sequence may be employed for amine 60a, the reduction
88
CA 02346933 2001-04-18
WO OOI35449 PCT/US99/30292
product of nitrile 60. Tosylate 59 can undergo
displacement with cuprates to yield 77 (Hanessian, S.;
Thavonekham, B.; DeHoff, B.; J Org. Chem. 1989, 54, 5831).
Aldehyde 61 or its homologous extensions can be reacted
with a carbon anion of an aryl (phenyl, naphthalene, etc.)
or heterocyclic group to yield an aryl alcohol or a
heterocyclic alcohol. If necessary, CeCl3 may be added (T.
Imamoto, et al., Tet. Lett. 1985, 26, 4763-4766; T.
Imamoto, et al., Tet. Lett. 1984, 25, 4233-4236). This
alcohol may be reduced with Et3SiH and TFA (J. Org. Chem.
1969, 34, 4; J. Org. Chem. 1987, 52, 2226) (see discussion
of aryl and heterocyclic anions for Schemes 20-22). These
aryl and heterocyclic anions may also be alkylated by 59
(or its carbon homology to yield compounds where R9
contains an aryl or laeterocyclic group. Compound 59 or its
carbon homologs may be alkylated by an alkyne anion to
produce alkynes at R'-3 (see R.C. Larock, Comprehensive
Organic Transformations, New York, 1989, VCH Publishers, p
297). In addition, carboxaldehyde 61 or its carbon
homologs can undergo 1,2-addition by an alkyne anion
(Johnson, A.W. The Chemistry of Acetylenic Compounds. V.
1. "Acetylenic Alcohols," Edward Arnold and Co., London
(1946)). Nitro groups can be introduced by displacing
bromide 59 (or its carbon homologs) with sodium nitrite in
DMF (J.K. Stille and E.D. Vessel J. Org. Chem. 1960, 25,
478-490) or by the action of silver nitrite on iodide 59 or
its carbon homologs (Org. Syntheses 34, 37-39).
89
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 9
ZO
10 ~ 1 9d
R ~O~ ~ (O)pR9d
O(CO)R9b
7 8 ,,~,,. 7 0 ~ 71 7 2
1 . KSAc 10
2.-OH ~ (O)2~9a
DAST
3 . R9dX ~,, R9a
or KSR9a /~ ~ 73
OH ~ a to
NH _ P
R5 n R11 R12 ~ 59 NH-P
55 or 58 R5 n Rll R12
If an anion is made of the pyrrolidine/piperidine 1
5 with LDA or n-BuLi, etc., then that anion in a suitable
nonhydroxylic solvent such as THF, ether, dioxane, etc.,
can react in a Michael-type fashion (1,4-addition) with an
alpha, beta-unsaturated ester to yield an intermediate
enolate which can be quenched with an electrophile (R9X)
10 (where X is as described in Scheme 1) (Uyehara, T.; Asao,
N.; Yamamoto, Y.; J Chem Soc, Chem Commun 1987, 1410) as
shown in Scheme 10.
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 9 (con~t)
-P ~ -P
1, 1,
55 or 58 , 59
X=O~cs ; ( R9 ) 2cuLi ~ KCN , Et OEt
- Na
9 to
Rlo ~ CN OE
1 . N ~ g to
\ 2 . [H 60 '~''
~~C OEt
_lo
to
l0 63
CH2 OH
t CHO
74 ~
62 ~ LAH
1 . R 9'CHO
Na (Ac0) gBH 1 .R9aCH0, 61 1 . -OH
2 . R9bSO2C1 Na (Ac0) gBH 2 -H+.
i0
2 . R9aC:OC.1 or R 10 CO
Et0(C=C>)H,0 ~OH
i~ R CHZNH2
'NRaS02Rb
61a
76 l0 60a
NHR9aC ( O) R9a g H
( or -NHCHO ) ~ Rlo
..w~- 7 cp
to
7 g b R:LO _ reductive
~'OR9d ~ Cj O ainination + v
R acylation 64
i 69 ~ if desired
61b
1
g OH Rgb R9aR9a' R9b
1(1 /
// 7 g 10 7 g 10 7 g 10
LAH ~
1
6g ~ ~ ~ ''~ 65 ~
66 ,
91
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
It is to be understood that R9 is either in its final form
or in a suitable protected precursor form. This
electrophile can be a carbon-based electrophile, some
examples being formaldehyde to introduce a CH20H group, an
aldehyde or a ketone which also introduces a one-carbon
homologated alcohol, ethylene oxide (or other epoxides)
which introduces a -CH2CHZOH group (a two-carbon homologated
alcohol), an alkyl halide, etc., all of which can be later
elaborated into R9. It can also be an oxygen-based
electrophile such as MCPBA, Davis' reagent (Davis, F. A.;
Haque, M. S.; J Org Chem 1986, 51 (21),4083; Davis, F. A.;
Vishwaskarma, L. C.; Billmers, J. M.; Finn, J.; J Org Chem
1984, 49, 3241) or MoO~ (Martin, T. et al., J Org Chem
1996, 61 (18), 6450-6453) which introduces an OH group.
These OH groups can undergo the displacement reactions
discussed previously in Scheme 9 or protected by suitable
protecting groups and deprotected at a later stage when the
displacement reactions decribed in Scheme 9 can be
performed. In addition, these OH groups can also undergo
displacement reactions with heterocycles as described for
Schemes 19-22 to introduce N- or C-substituted heterocycles
at this position. Ester 80 can be converted into its
Weinreb amide 82 (S. Nahm and S. M. Weinreb, Tet. Lett.,
1981, 22, 3815-3818) or Weinreb amide 82 can be synthesized
via Michael-type addition of 1 to alpha, beta-unsaturated
Weinreb amide 83. Subsequent reaction with a Grignard
reagent forms ketone 85. This ketone can also be
synthesized in one step directly from 1 and alpha,beta-
unsaturated ketone 84 using the same procedure. This
ketone may be reduced with LAH, NaBH4 or other reducing
agents to form alcohol 86. Or else, ketone 85 can be
reacted with an organolithium or Grignard reagents to form
tertiary alcohol 87 . Or else, ester 80 can be directly
reduced with LiBH4 or LAH to yield primary alcohol 88.
92
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME: 10
R~ Ra
H ~ 1c
R ~ 1. BuLi
0 'OMe
R s~ w --;- ~
n 2 . R9X ) R' y -Rlo
R
OMe 5 'n
1 79 R 80
n:=0,1 1.
R ~ Rio R7 s
R~ io 84
O p ~ 'OH
R$ Rllorl2 R'9 'R10
N (CH3 ) OMe 2 . R9X R5 'n
l.BuLi 81
2 . R9X ~ 83
R~ s
R a
~ ~N(CH,3)OMe ~ Rllorl2
R9 'R10 R11 9 10
' MgBr or ~ R R
RS 'n 82 Ri2MgBr R5 n
R~ a _ p
Rll or12 R11or12MgBr
R9 R1o R~ s H
R5 n 89 R~ s H
R12
'j ~Rllorl2
Rllor 12M Br ~j~10 Rll
9 R~~~o
R5 rin )
Rs /n
1 -p ss ~ 87
R 1
R12 ~1 r
9 'R1~ -~ OH -~ NH2 R~ s
' w
9 0 to compounds .~ ~ OH
by ;methods ~ ' R9 'R10
prewioiusly ~ R5 'ri
des~~ribed
Alcohols 85, 87 , arid $8 can all be tosylated, mesylated,
triflated, or converted to a halogen by methods discussed
5 previously and displaced with an amine nucleophile such as
azide, diphenylphosphoryl azide (with or without DEAD and
93
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Ph3P), phthalimide, etc. as discussed previously (and which
are familiar to one skilled in the art) and after reduction
(azide) or deprotection with hydrazine (phthalimide), for
example, yield the corresponding amines. These can then be
elaborated into the compounds of this invention as
discussed previously. Ketone 85 can also be converted into
imine 89 which can be reacted with a Grignard reagent or
lithium reagent, etc., to form a protected amine 90 which
can be deprotected and elaborated into the compounds of
this invention as discussed previously. Some protecting
groups include benzyl and substituted benzyl which can be
removed by hydrogenation, and cyanoethyl, which can be
removed with aqueous base, etc. It is to be understood
that R~-12 in Scheme 10 can be in their final form or in
precursor form which can be elaborated into final form by
procedures familiar to one skilled in the art.
Magnesium amides of amines have been used to add in a
Michael-type manner to alpha, beta-unsaturated esters where
the substituents at the beta position of the unsaturated
ester are tied together to form a cyclopentane ring (for
example, compound 79 where R~ and Rs are taken together to
be -(CH2)4-) (Kobayashi, K. et al., Bull Chem Soc Jpn, 1997,
70 (7), 1697-1699). Thus reaction of pyrrolidine or
piperidine -1 with cycloalkylidine esters 79 as in Scheme 10
yields esters 80 where R~ and Ra are taken together to form
a cycloalkyl ring. Subsequent elaboration yields compounds
of this invention where R~ and R8 are taken together to form
a cycloalkyl ring.
Compounds of structure 95a may also be synthesized
from epoxyalcohols which are shown in Scheme 11. Allylic
alcohol 91 can be epoxidized either stereoselectively
using VO(acac)2 catalyst (for a review, see Evans: Chem.
Rev. 1993, 93, 1307) or enantioselectively (Sharpless: J.
Am. Chem. Soc. 1987, 109, 5765) to epoxyalcohol 92. SN2
displacement of the alcohol using zinc azide and
triphenylphosphine (Yoshida, A. J. Org. Chem. 57, 1992,
1321-1322) or diphenylphosphoryl azide, DEAD, and
94
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
triphenylphosphine (;3aito, A. et al., Tet. Lett. 1997, 38
(22), 3955-3958) yie:Lds azidoalcohol 93. Hydrogen~3tion over
a Pd catalyst yields aminoalcohol 94. This can be
protected in situ or :in a subsequent step with BOC20 to put
on a BOC protecting croup, or with CBZ-C1 and base to put
on a CBZ-group or other protecting groups. Alternatively,
the amino group can be reacted with an isocyanate, an
isothiocyanate, a carbamoyl chloride, or any reagent
depicted in Scheme 1 to form 95 which can be alkylated with
1 to form the compounds of this invention.
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 11
7 9 7 9
R R
s OH Rs OH
R
R1 1
R12 R R12
91 92
R7 9 ~ 9
R
s
Rg 2 Rs N3
1
R R12 R1 Ri2
93
94
R9
R R
RB NH- P Rs NH- ( C=Z ) NR2R3
1
R R12 R1 R12
54 95
H
as in Scheme 8 R~ 'n
1
n=0,1
_ ( C=Z ) NR2R3
95a
Sometimes amine 1 might have to be activated with
Lewis acids in order to open the epoxide ring (Fujiwara,
M.; Imada, M.; Baba, A.; Matsuda, H.;Tetrahedron Lett 1989,
30, 739; Caron, M.; Sharpless, K. B.; J Org Chem 1985, 50,
1557) or 1 has to be deprotonated and used as a metal
amide, for example the lithium amide (Gorzynski-Smith, J.;
Synthesis 1984 (8), 629) or MgBr amide (Carre, M. C.;
96
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Houmounou, J. P.; Cau:bere, P.; Tetrahedron Lett 1985, 26,
3107) or aluminum amide (Overman, L. E.; Flippin, L. A.;
Tetrahedron Lett 1981, 22, 195).
The quaternary salts (where R4 is present as a
substituent) of pyrro:Lidines and piperidines can be
synthesized by simply reacting the amine with an alkylating
agent, such as methyl iodide, methyl bromide, ethyl iodide,
ethyl bromide, ethyl or methyl bromoacetate,
bromoacetonitrile, allyl iodide, allylbromide, benzyl
7.0 bromide, et~~. in a suitable solvent such as THF, DMF, DMSO,
etc. at room temperature to the reflux temperature of the
solvent. Spiroquaternary salts can be synthesized in a
similar manner, the orally difference being that the
alkylating agent is located intramolecularly as shown in
Scheme 12. It is understood by one skilled in the art that
functional groups might: not be in their final form to
permit cycl~_zation to the quaternary ammonium salt and
might have t:o be in precursor form or in protected form to
be elaborated to their final form at a later stage. For
example, the' NR1(C=Z)NR2R3 group on the rightmost phenyl
ring of compound 104 might exist as a nitro group precursor
for ease of manipulation during quaternary salt formation.
Subsequent reduction a:nd NR1(C=Z)NR2R3 group formation
yields product 105. T:he leaving groups represented by X in
2!i Scheme 12 may equal those represented in Scheme 1, but are
not limited thereto. lV-oxides of pyrrolidines and
piperidines can be made by the procedure of L. W. Deady
(Syn. Comm. 1977, 7, 509-514). This simply entails
reacting the pyrrolidine or piperidine with MCPBA, for
3Cl example, in an inert solvent such as methylene chloride.
97
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 12
8
11812 ~ a 11812
NRl(C=Z)NR2R3 X 1(C=Z)NR2R3
R9 Rlo X=leaving ~ 9 to
) R
R5 n group R~ 'n
n=0,1 96 97
R7 fi 11 812 ~ Rg 11 812
X- R
NR1 ( C=Z ) NR2R3 NR1 ( C=Z ) NR2R3
/9 ~Rlo ~ ~ to
R
Rs n ~ 4_X ~~ R~R9
Rs n
n=0,1 99
98
4-X
12
11
R~ 8 ~11 12 4/R 1 ( C=Z ) NR2R 3
X_ R _._ _
NR1 ( C=Z ) NR2R 3 ' + R1o
R9 R1o ~ ~~~~R9
R5 n 10 0 Rs n R~ Rg 1 O l
12
R~ 8 g 10 ~ 11 1 (C=z) ~2R3
RI 10
1 (C=Z) NR2R3 X R
w +
R ~ lo~ _ R
11 812 ~ 810
Rs n ~4_X Rs n
R~ R8
102 103
1 (C=Z) NR2R3
X- R4-R1 ~ ~ ~1 ( C=Z ) NR2R 3
R4~R1
'+
Rlo ~ to
R5 n 104 R~ ~n R9 R 105
Multisubstituted pyrrolidines and piperidines may be
synthesized by the methods outlined in Scheme 13.
Monoalkylation of 106 via an enolate using LDA or potassium
98
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
hexamethyldisilazane, or converting 106 first to an
enamine, or by using other bases, all of which can be done
in THF, ether, dioxane, benzene, or an appropriate non-
hydroxylic solvent at -78 oC to room temperature with an
alkylating agent such as methyl iodide, benzyl bromide,
etc. where :X is as defined in Scheme 1, yields product 107.
This product can subsequently undergo alkylation again
under thermodynamic or kinetic conditions and afterwards,
if need be, can underg~a two more alkylations to produce
tri- and tetrasubstitu.ted analogs of 107. The
thermodynamic or kinetic conditions yield regioselectively
alkylated products (for a discussion on thermodynamic vs.
kinetic alk:~rlations see H. House Modern Synthetic
Reactions, Via. A. Benjamin, Inc. (Menlo Park, CA: 1972)
chapter 9).
SCHEME 13
~Ph 1. Base R R
~Ph ~Ph
--
n 2. R5-X
206 107n RSP 108
n=0,1 X = leaving group R5p=precursor to R5
HZ / Pd or
Pd(OH)z
R H
to compounds by
methods previously
described s
R5 n
109
cis and trans
99
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 14
C02Et COZEt C02Et
1 . Base ~Rb
-> --
2 . R 6X
H BOC BOC
110 111 X=leaving
112
group as
defined in 1_[H]
Scheme 1 2.Swern
5x
CHzR CH ( OH) R5* RS*MgBr or CHO
~R6 ~ R6 ~ Rs
RSxLi
BOC BOC BOC 113
119 128
Wittig
1
CH2 R5 * CH2 CH2R 5 CH= CHRSx
R6 Rs H2 Pd/C
R
H BOC 116 OC 114
120
H+ ~ * ~ H+ 5
CHz CH2R 5 CH= CHR
R6 ~Rs
1
H 11~ H 115
x
RS =R5 or a
precursor
thereof to products by methods
previously described
Subsequent Wittig olefination yields compound 108.
Hydrogenation (asymmetric hydrogenation is an option here:
Parshall, G.W. Homogeneous Catalysis, John Wiley and Sons,
New York: 1980, pp. 43-45; Collman, J.P., Hegedus, L.S.
Principles and Applications of Organotransition Metal
Chemistry, University Science Books, Mill Valley, CA, 1980,
pp. 341-348) yields pyrrolidine or piperidine 109 which can
100
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
be resolved :into its relative and/or absolute isomers at
this stage or later on in the synthesis either by
crystallizat_~on, chromatographic techniques, or other
methods familiar to one skilled in the art. The amine 109
an then be ei.aborated into the compounds of this invention
by methods discussed pr.=viousl.y (Scheme 1). The carbonyl-
containing intermediate 107 in Scheme 13 can also be
reduced to the methylene analog via a Wolff-Kishner
reduction and. modifications thereof, or by other methods
familiar to one skilled in the art. The carbonyl group can
also be reduced to an OH group, which can undergo all of
the reactions described in Scheme 9 to synthesize the R6
groups. This piperidine~ or pyrrolidine can be deprotected
and elaborated to the compounds of this invention by
methods discussed earlier. Thus, mono-, di-, tri-, or
tetraalkylated carbonyl-containing pyrrolidines or
piperidines can be synthesized, which in turn can be
reduced to thES corresponding -CH2- analogs employing the
Wolff-Kishner reduction or other methods.
Another method for synthesizing gem-substituted
pyrrolidines and piperidines is shown in Scheme 14. It is
understood by one skilled in the art that some of the steps
in this scheme can be rearranged. It is also understood
that gem-disubstitution :is only shown at only one position
on the piperidine ring and that similar transformations may
be performed on other carbon atoms as well, both for
piperidine and pyrrolidine. Thus, 3-carboethoxypiperidi.ne
110 may be BOC-protected and alkylated employing a base
such as LDA, KHMDS, LHDM:>, etc., in THF, ether, dioxane,
etc. at -78 °C to room temperature, and an alkylating agent
R6X where X is a halide (halide = C1, Br, I), mesylate,
tosylate or triflate, to yield 1~. Reduction using DIBAL,
for example, and if necessary followed by oxidation such as
a Swern oxidation (S. L. Huang, K. Omura, D. Swern J. Org.
Chem. 1976, 41, 3329-32) yields aldehyde 113. Wittig
olefination (114) followed by deprotection yields 115 which
may be elaborated as described previously into the
101
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
compounds of this invention. Reduction of the Wittig
adduct 114 yields 116 which may be deprotected to yield 117
which may be in turn elaborated as described previously
into the compounds of this invention. Reaction of
S aldehyde 113 with an alkyllithium or Grignard reagent
yields alcohol 118 which may be reduced catalytically or
with Et3SiH/TFA (J. Org. Chem. 1969, 34, 4; J. Org. Chem.
1987, 52, 2226) if R5* (R5* - R5 or a precursor thereof) is
aromatic to yield 119. If R5* is not aromatic, then the OH
may be reduced by the method of Barton (Barton, D. H. R.;
Jaszberenyi, J. C. Tet. Lett. 1989, 30, 2619 and other
references therein). Once tosylated, the alcohol can also
be displaced with dialkyllithium cuprates (not shown)
(Hanessian, S.; Thavonekham, B.; DeHoff, B.; J Org. Chem.
1989, 54, 5831). Deprotection if necessary yields 120
which may be elaborated as described previously into the
compounds of this invention.
102
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 15
R R
1. s-BuLi )n
n
RSOrRI3
TMEDA
BOC
BOC 2. vR5_ or R13-X 122
121
X-as defined
n=0, 1 i~z Scheme 1
1. s-BuLi
TMEDA
2. R~- or R13-X
R~ ~ n R
1. s-BuLi
R50 r R13 s
RSOrRl~ R5orR13 TMEDA
BOC 5_ 5~ 5 13
2. R R -R orR
124 or R13-X or R13 BOC
123
1. s-BuLi
TMEDA
2. R.5- or R13-X
R
a ~ /n
13
R r- o )Z5o rRl3
RSOr:Rl3 ~F;~orRl3
BOC
125
A method for the alkylation of alkyl groups, arylalkyl
groups, all:ylic groups, propargylic groups, etc., and a
variety of other electrophiles onto the pyrrolidinyl and/or
piperidinyl alpha-carbons (alpha to the ring nitrogen atom)
is represented by the work of Peter Beak, et al. as shown
in Scheme 1':5. It is understood by one skilled in the art
that the R5 and R13 groups are either in their precursor,
protected, or final form. Only one RS group is shown to
be substituted on piperidine/pyrrolidine 121. However it
is understood by one skilled in the art that additional
functionality may be present on the ring in either
103
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30293
precursor, protected, or final form. Thus lithiation with
an alkyllithium reagent such as n-BuLi or s-BuLi as shown,
followed by quenching with an electrophilic species such as
R5X or R13X where X is as defined in Scheme 1 and RS and R13
are in their precursor, protected, or final form, yields
monoalkylated piperidine/pyrrolidine 122. This alkylation
may occur either stereoselectively (P. Beak and W.K. Lee
J. Org. Chem. 1990, 55, 2578-2580) or enantioselectively if
sparteine is included as a source of chirality (P. Beak, et
al., J. Am. Chem. Soc. 1994, 116, 3231-3239). The
alkylation process may be repeated up to three more times
as shown in Scheme 15 to result in di-, tri-, and
tetrasubstitution at the alpha-positions.
Compounds where R9 and R1~ form a cyclic 3,4,5,6, or 7-
membered ring can be synthesized by the methods disclosed
in Scheme 16. These same methods may also be used to
synthesize gem-disubstituted compounds in which R9 can be
different from R1~ by step-wise alkylation of the malonate
derivative. Of course, this scheme may be used to
synthesize compounds where Rla=H also. For example, a
cyclohexyl-fused malonate may be synthesized by Michael
addition and alkylation of I(CH2)4CH=CC02Me with dimethyl
malonate employing NaH/DMF (Desmaele, D.; Louvet, J.-M.;
Tet Lett 1994, 35 (16), 2549-2552) or by a double Michael
addition (Reddy, D. B., et al., Org. Prep. Proced. Int. 24
(1992) 1, 21 -26) (Downes, A. M.; Gill, N. S.; Lions, F.; J
Am Chem or by an alkylation followed by a second
intromolecular alkylation employing an iodoaldehyde (Suami,
T.; Tadano, K.; Kameda, Y.; Iimura, Y.; Chem Lett 1984,
1919), or by an alkylation followed by a second
intramolecular alkylation employing an alkyl dihalide
(Kohnz, H.; Dull, B.; Mullen, K.; Angew Chem 1989, 101
(10), 1375), etc.
104
CA 02346933 2001-04-18
WO 00/35449 PCTNS99/30292
SCHEME. 16
R9 Rlo R9 Rlo
diethyl _~ OF,t OEt > H OEt
malonate
O
126 127
H
R5 n
1
n=0,1
OEt ~H~
h
129 128
R9 Rlp
~-OH
.\
R5 n 13 0
to compounds by methods
previous;ly described
Subsequent monosa.ponification (Pallai, P.V., Richman,
S., Struthe:rs, R.S., G'~c>odman, M. Int. J. Peptide Protein
Res. 1983, 21, 84-92; M. Goodman Int. J. Peptide Protein
Res. 19831, 17, 72-88), standard coupling with pyrrolidine/
piperidine L yields 12g. Reduction with borane yields 129
followed by reduction with LAH yields 130 which can be then
converted to amine X31. and then to the compounds of this
invention by procedures as discussed previously. Ester 1~
can also be converted to a Weinreb amide and elaborated to
the compounds of this invention as described in Scheme 10
for ester 80 which would introduce substituents R11 and R12.
105
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Scheme 17 describes another method for the synthesis
of compounds where R9 and R1~ are taken together to form
cycloalkyl groups. Aminoalcohols 132 are found in the
literature (CAS Registry Nos. for n = 0,1,2,3,
respectively: 45434-02-4, 2041-56-7, 2239-31-8, 2041-57-8).
They can easily be protected, as with a BOC group (or CBZ,
or any other compatible protecting group) by known
procedures familiar to one skilled in the art to yield
alcohols 133. The alcohols can then be activated either by
conversion to a halide or to a mesylate, tosylate or
triflate by methods familiar to one skilled in the art and
as discussed previously, and then alkylated with
pyrrolidine/piperidine 1 by the conditions described in
Scheme 1 to yield 135. Subsequent deprotection yields
amine 136 which can be elaborated to the compounds of this
invention as described previously. Of course, alcohol 133
can be oxidized to the aldehyde and then reacted with
R~°r8MgBr or R~°rBLi with or without CeCl3 to yield the
corresponding alcohol 133 where instead of -CHzOH, we would
have -CHR~°rgOH. This oxidation-1,2-addition sequence may
be repeated to yield a tertiary alcohol. The alcohol may
then be tosylated, mesylated, triflated, or converted to
C1, Br, or I by procedures familiar to one skilled in the
art to yield 134 and then displaced with
pyrrolidine/piperidine 1 to yield 135. Subsequent
deprotection yields 136 which may undergo elaboration to
the compounds of this invention as discussed previously.
106
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 17
(CH2)n (CH2)n (CH2)n
H N-CH ~ -'' BOC--NH-CH2 -~BOC-NH-CH2
2 2
BOC20
OH OH X
n=0,1,2,3 133 H 134
132
R5 n
1
n=0,1
.,...
- BOC
CH+)
136
135
to compounds by
previously described
A method to introduce cycloalkyl groups at R11R12 is
shown in Scheme 18. Protection of the nitrogen of
compounds 13'7 which are commercially available yields 138
(the protecting group m.ay be BOC, CBZ, or any other
compatible p~.~otecting group) by procedures familiar to one
skilled in the art. Esterification by any one of a number
procedures familiar to one skilled in the art (for example
A. Hassner and V. Alexar~ian, Tet. Lett, 1978, 46, 4475-8)
followed by :reduction with DIBAL (or alternatively
reduction to the alcohol. with, for example, LiBH4, followed
by Swern oxidation (op. cit.)) yields aldehyde 139. One
carbon homologation via the Wittig reaction followed by
hydrolysis o:E the vinyl ether yields aldehyde 141.
Reductive am:ination (Ab~del-Magid, A. F., et al. Tet. Lett.
1990, 31, (39) 5595-5598) yields 142 followed by
107
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
deprotection yields amine 143 which can be elaborated to
the compounds of this invention by the methods previously
discussed. Of course, aldehyde 139 can be reacted with
R9orloMggr or R9orloLi with or without CeCl3 to yield an
alcohol which can be oxidized to a ketone. Wittig one-
carbon homologation on this ketone as described above
followed by hydrolysis yields 141 where the -CHZCHO is
substituted with one R9orlo group (-CHR9orlo CHO) .
SCHEME 18
(CH2)n BOC20 (CHZ)n (CHZ)n
H 2 '' BOC ---1 BOC
2.ROH C02R CHO
COO H DCC
n=0,1,2,3 DAP 138 139
137
(CH2)n H+ (CHz)n
~-.-- BOC
BOC CH2CHO H=CHOMe
1' 140
Na(Ac0)3BH 141
~ ~u_ v
-BOC to compounds
~ H+ ~ -~ by methods
' ~. described
previously
RJ 11
RJ 11
142 143
Aldehyde 141 (-CH2CH0) or its monosubstituted analog
synthesized above (-CHR9or1oCH0) can undergo alkylation with
R9orloX where X is as defined in Scheme 1 to yield compound
141 containing one or both of the R9 and Rlo substituents
alpha to the aldehyde group. Alkylation can be.performed
using LDA or lithium bistrimethylsilyl amide amongst other
bases in an inert solvent such as ether, THF, etc., at -78
108
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
°C to room temperatur~L. Aldehyde 141 (-CH2CH0)or its
substituted analogs synthesized above (i.e., -CHR9R1~CH0)
can undergo reductive amination with 1 and subsequent
elaboration to the compounds of this invention. Aldehyde
141 (-CH2CH0)or its substituted analogs synthesized above
(i.e., -CHR.9R1~CH0) ca.n also undergo 2,2-addition with
R~°rBMgBr or. R~°reLi to yield the corresponding alcohol -
CH2CHR~°r80H or -CHR9R~LOCHR~°r80H . The alcohol may then
be
tosylated, mesylated, triflated, or converted to Cl, Br, or
1.0 I by procedures familiar to one skilled in the art and
displaced with pyrrol:ldine/piperidine 2 to yield, after
subsequent deprotection and elaboration, the compounds of
this invention. Or else alcohol -CHZCHR~°r80H or -
CR9R1~CHR~°r80H can be oxidized (i.e., Swern, op. cit.) to
the ketone and reductively aminated with 1 and subsequently
elaborated to the compounds of this invention. Or else
alcohol -CH2CHR~°r80H or -CR9R1~CHR~°rgOH can be oxidized
(i.e., Swer:n, op. cit.) to the ketone and reacted once more
with R~°r8MgBr or R~°~8Li to yield the corresponding alcohol
-CH2CR~R80H or -CR9R1~C'.R~R80H. If the ketone enolizes easily,
CeCl3 may beg used together with the Grignard or lithium
reagent. The alcohol can again be tosylated, mesylated,
triflated, or converted to C1, Br, or I by procedures
familiar to one skilled in the art and displaced with
2:~ pyrrolidine! piperidin.e 1 to yield, after subsequent
deprotection and elaboration, the compounds of this
invention. Thus each one of the R~, Rg, R9, and R1~ groups
may be introduced into compounds 141, ~ and 143 and and,
of course, un the compounds of this invention, by the
methods discussed above.
A method for the synthesis of N-substituted
heterocycles at RS is .shown in Scheme 19. The heterocycle
can be deprc>tonated with NaH or by other bases familiar to
one skilled in the art, in a solvent such as DMF, THF, or
3'. another appropriate non-hydroxylic solvent and reacted with
piperidine or pyrrolidine 143 at room temperature to the
reflux temperature of the solvent. Deprotection and
109
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
elaboration as described before yields compounds where R5
contains an N-substituted heterocycle. If the nitrogen
atom of the heterocycle is sufficiently nucleophilic, then
an acid scavenger, such as K2C03, KHC03, Na2C03, NaHC03,
amongst others, can be used in place of NaH, employing THF,
DMF, or methyl ethyl ketone as solvents. In this case
hydroxylic solvents may be used as well, such as methanol,
ethanol, etc. from room temperature to the reflux
temperature of the solvent. Compound 143 as well as its
other positional isomers are available, for example, from
commercially available 4-hydroxymethylpiperidine, 2-, 3-,
and 4-carboethoxypiperidine, L- or D-proline ethyl ester,
or from methyl 1-benzyl-5-oxo-3-pyrrolidinecarboxylate by
methods familiar to one skilled in the art and as discussed
previously in this application.
SCHEME 19
heterocycle heterocycle
BOC
H
n NaH or KzC03
X
143
1~~ 11
n=0, 1
deprotect
X = leaving group to compounds by
methods
described
previously
A method for the synthesis of C-substituted
heterocycles at R5 is shown in Scheme 20. Many
heterocycles such as the ones shown in Scheme 20, but not
limited thereto, can be metallated with strong bases such
as LDA, n-BuLi, sec-BuLi, t-BuLi, etc. to yield the
corresponding anionic species. These anions may also be
generated via halogen-metal exchange employing n-BuLi, or
other alkyllithium reagents. These reactions may be
110
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
performed in THF, ether, dioxane, DME, benzene, etc. at -?8
°C to room temperature.
SCHEME 20
\ (-)
(_.)
R
(_) (_) ~ (-) heterocycle
,BO(. C
N C \,
N N
(CH )m
In ~ \ ( - ) \
X
I 4 3 ~ ( ) ( - ) ~'BOC
1~N 14 5 j n
n= 0 , 1
X = leaving m=1,2
\
descpibed ~ / \, (-)
in Scheme 1 (_)
C02Li C02Li
to compounds
\ by methods
described
,(-) (-) previously
R=suitable protecting
(-) I (_) group or functional
C
N N group
etc.
For reviews of these metallations and halogen-metal
exchange reactions see Organometallics in Organic
Synthesis, FMC Corp., Lithium Division, 1993, pp. 17-39;
Lithium Link,. FMC Corp., Spring 1993, pp. 2-17; n-
Butyllithium in Organic Synthesis, Lithium Corp. of
America, 1982, pp. 8-16; G. Heinisch, T. Langer, P.
Lukavsky, J. Het. Chem. 1997, 34, 17-19. The anions can
then be quenched with e:lectrophile 143 or its positional
isomers to yield the co=rresponding C-alkylated heterocyclic
pyrrolidine or piperidine 145.
111
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 21
\ (-)
(-) (
i
heterocycle
BOC I ~( ) (-) (-)
C
N N H
s
''n ~ ( _ ) \ \ BOC
H I _
146 ~( ) / ( ) ..
n=0, 1
NON
\ \ \ CH)
I \ (_) I
C02Li )
C02 L i
ecc . net
R=suitable protecting
group or functional
group
to compounds
by methods described ,.
previously
Another method for the synthesis of C-substituted
heterocyclic-methylpyrrolidines or piperidines is shown in
Scheme 21. The protected aldehyde 146 is reacted with the
anion of the heterocycle (its generation as described
previously) at -78 °C to room temperature with or without
CeCl3 in an inert solvent such as THF, ether, dioxane, DME,
benzene, etc. to yield carbinol 147. Catalytic
hydrogenation of the alcohol yields the corresponding
methylene compound 145. Other reduction methods include
Et3SiH/TFA (J. Org. Chem. 1969, 34, 4; J. Org. Chem. 1987,
52, 2226} amongst others familiar to one skilled in the
art. It is understood by one skilled in the art that the
aldehyde group can be located in other positions instead
of, for example, the 4-position of piperidine in compound
112
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
146 as depicted in Scheme 21. It is to be understood that
other heterocycles may also be used besides the ones shown
in Scheme 20 and 21.
The anions of the methyl-substituted heterocycles may
also be reacted with a BOC-protected piperidone or
pyrrolidone (148) to yield alcohols 149 as shown in Scheme
22 (see above reviews an metallations for references).
These alcohols may be reduced using Pt02 and TFA (P. E.
Peterson and C. Casey, J. Org. Chem. 1964, 29, 2325-9) to
yield piper.idines and pyrrolidines 150. These can
subsequentl:~ be taken an to the compounds of this invention
as described previously. It is understood by one skilled
in the art that the carbonyl group can be located in other
positions instead of, for example, the 4-position of
piperidine _-in compound 148, as depicted in Scheme 22. It is
to be understood that ather heterocycles may also be used
besides the ones shown in Scheme 22.
SCHEME 22
heterocycle
Boc~ ~ ~ ( _ ) ~ ~.-- ( - )
C
N
d~ n
(_)
148 - (-)
n=0,1
et_c. TFA, Et3SiH
R=suitable protecting
group o:r functional het \ ~ycle
group
to compounds of by
methods described ' N.
previously
150 n
One may also react: aryl (phenyl, naphthyl, etc.)
anions, generated either by halogen-metal exchange or by
ortho-directed metallat:ion (Snieckus, V. Chem. Rev. 1990,
113
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
90, 879-933) using n- or s- or t-BuLi in a non-hydroxylic
solvent such as THF, ether, etc., with or without TMEDA and
allow them to react with compounds 143, 146, and 148 with
subsequent elaboration to yield the compounds of this
invention by the methods depicted in Schemes 19-22.
Another method for the preparation of C-substituted
heterocycles is shown in Scheme 23. Protected piperidone
148 undergoes a Wittig reaction with heterocyclic
phosphorous ylides to yield 151. Hydrogenation over a
noble metal catalyst such as Pd in an alcoholic solvent or
with an optically active transition metal catalyst (see
asymmetric hydrogenation references of Parshall and
Coleman, op. cit.) yields 152 which can be further
elaborated into the compounds of this invention by the
procedures described previously. It will be appreciated by
one skilled in the art that the carbonyl group can be
located in other positions instead of, for example, the 4-
position of piperidine in compound 148 as depicted in
Scheme 23. It is to be understood that other heterocycles
may also be used besides the ones shown in Scheme 23.
114
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Scheme 23
Ph3
heterocycle
Boc ~ ~, ~Ph3
N
C~ n
148 PPh3
n=0,1 \
N PPh3
etc.
R=suitable protecting
group or functional
group
nets
to compounds
by methods
described
previously
Syntha_ses of amines _9, 10, and the amines which are
precursors to isocyanates or isothiocyanates 5 will now be
discussed. For example, 3-nitrobenzeneboronic acid (153:
Scheme 24) is commerically available and can undergo Suzuki
couplings (Suzuki, A. Pure Appl. Chem. 1991, 63, 419) with
a wide variety of substituted iodo- or bromo aryls (aryls
such as phenyl, naphthalene, etc.), heterocycles, alkyls,
akenyls (Moreno-manas, M., et al., J. Org. Chem., 1995, 60,
2396), or alkynes. It can also undergo coupling with
triflates of aryls, h~eterocycles, etc. (Fu, J.-m, Snieckus,
V. Tet. Lett. 1990, 31, 1665-1668). Both of the above
reactions can also undergo carbonyl insertion in the
presence o:F an atmosphere of carbon monoxide (Ishiyama, et
al., Tet. Lett. 1993, 34, 7595). These nitro-containing
compounds (155 and X5'7) can then be reduced to the
corresponding amines ,either via catalytic hydrogenation, or
via a number of chemical methods such as ~n/CaCl2 (Sawicki,
E. J Org Chem 1956, 21). The carbonyl insertion compounds
115
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
(158) can also undergo reduction of the carbonyl group to
either the CHOH or CH2 linkages by methods already
discussed (NaBHq or Et3SiH, TFA, etc.). These amines can
then be converted to isocyanate 5 via the following methods
(Nowakowski, J. J Prakt Chem/Chem-Ztg 1996, 338 (7), 667-
671; Knoelker, H.-J. et al., Angew Chem 1995, 107 (22),
2746-2749; Nowick, J. S.et al., J Org Chem 1996, 61
(11), 3929-3934; Staab, H. A.; Benz, W.; Angew Chem 1961,
73); to isothiocyanate 5 via the following methods
(Strekowski L.et al., J Heterocycl Chem 1996, 33 (6), 1685-
1688; Kutschy, Pet al., Synlett 1997, (3), 289-290); to
carbamoyl chloride 11 (after 156 or 158 is reductively
aminated with an R2 group) (Hintze, F.; Hoppe, D.;
Synthesis (1992) 12, 1216-1218); to thiocarbamoyl chloride
11 (after 156 or 158 is reductively- aminated with an R2
group) (Ried, W.; Hillenbrand, H.; Oertel, G.; Justus
Liebigs Ann Chem 1954, 590); or just used as 9, or 10
(after 156 or 158 is reductively aminated with an R2
group), in synthesizing the compounds of this invention by
the methods depicted in Scheme 1.
116
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 24
02
Suzuki-type O2
\ X--O coupling
/ ~B
(OH) 2 X=Br, I, OTf
1~~3 154 155
Suzuki -type
coupling, CO (g) ~H~
~n2 p
n2
~H) ~ / \
O /
158
157 156
make isocyanate or
isothiocyanate 5,
or carbamoyl chlorides 11,
or used as 9_ or 10 to make
the compounds of this
invention as described for
the compounds of Scheme 1
Likewise, pro tected aminobromobenzenes or triflates or
protected. aminobromoheterocycles or triflates 159 (Scheme
25) may undergo Suzuki-type couplings with arylboronic
acids or heterocycl.ic boronic acids (160) These same
bromides or triflates 159 may also undergo Stifle-type
coupling (Echavarren, A. M., Stifle, J.K. J. Am. Chem.
Soc., 1987, 109, 54'78-5486) with aryl, vinyl, or
heterocyclic stannanes 163. Bromides or triflates 159 may
also undergo Negish:L-type coupling with other aryl or
heterocyclic bromides 164 (Negishi E. Accts. Chem. Res.
1982, 15, 340; M. Sletzinger, et al., Tet. Lett. 1985, 26,
2951). Deprotection of the amino group yields an amine
with can ;be coupled to make a urea and other linkers
117
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
containing Z as described above and for Scheme ~. Amino
protecting groups include phthalimide, 2,4-dimethyl pyrrole
(S. P. Breukelman, et al. J. Chem. Soc. Perkin Trans. I,
1984, 2801); N-1,1,4,4-Tetramethyldisilyl-azacyclopentane
(STABASE) (S. Djuric, J. Venit, and P. Magnus Tet. Lett
1981, 22, 1787) and others familiar to one skilled in the
art.
SCHEME 25
_P
Suzuki-type -P
coupling
+ (HO) 2
Br,I,OTf 160
159
Stille-type 161
coupliing
159 + Bu3 Sn~-
163 Negishi-type P
coupling
159 + Br or
vn2
164
make isocyanate or
isothiocyanate ~,
or carbamoyl chlorides
or used as 9 or IO to make 1 62
the compounds of this
invention as described for
the compounds of Scheme 1
Compounds where R7 and R8 are taken together to form
=NRBb can be synthesized by the methods in Scheme 25a.
Reacting _1 with nitrite a with CuCl catalysis forms amidine
b where R8b is H (Rousselet, G.; Capdevielle, P.; Maumy,
M.; Tetrahedron Lett. 1993, 34 (40), 6395-6398). Note that
the urea portion may be in final form or in precursor form
(for example, a protected nitrogen atom; P = protecting
group such as STABASE, bis-BOC, etc., as was discussed
previously) which may be subsequently elaborated into the
compounds of this invention. Compounds b may be also
synthesized by reacting iminoyl chloride c with
118
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
pyrrolidine/piperidine 1 to yield b where R8b is not H
(Povazanec, F., et al., J. J. Heterocycl. Chem., 1992,
29, 6, 1507-1512). Iminoyi chlorides are readily
available from the corresponding amide via PC15 or
S CC14/PPh3 (l~uncia, J.V. et al., J. Org. Chem., 1991, 56,
2395-2400). Again, the urea portion may be in final form
or in precuwsor form.
Scheme 25a
H 1~~ R1o
R~ NRl ( C=Z ) NR2R 3 or N-P
R5 n ~ ~R12 --1
NC' R° 1i -----!~
1 R
n=0,1 a
R 1(C=Z)NR2R3
2
JR1 ( C=Z ) NR2R 3 or N-P
RS Jn C ~ 1 b
1
n=0.1 c
Many amines are commercially available and can be used
as 9_, 10, or used as precursors to isocyanates or
isothiocyanat:es 5. There are numerous methods for the
synthesis of non-commercially available amines familiar to
one skilled in the art. For example, aldehydes and ketones
may be converted to their O-benzyl oximes and then reduced
with LAH to form an amine (Yamazaki, S.; Ukaji, Y.;
Navasaka, K.; Bull Chem Soc Jpn 19$6, 59, 525). Ketones
and trifluoromethylketones undergo reductive amination in
119
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
the presence of TiCl4 followed by NaCNBH4 to yield amines
(Barney, C.L., Huber, E.W., McCarthy, J.R. Tet. Lett. 1990,
31, 5547-5550). Aldehydes and ketones undergo reductive
amination with Na(Ac0)3BH as mentioned previously to yield
amines (Abdel-Magid, A. F., et al. Tet. Lett. 1990, 31,
(39) 5595-5598). Amines may also be synthesized from
aromatic and heterocyclic OH groups (for example, phenols)
via the Smiles rearrangement (Weidner, J.J., Peet, N.P. J.
Het. Chem., 1997, 34, 1857-1860). Azide and nitrile
displacements of halides, tosylates, mesylates, triflates,
etc. followed by LAH or other types or reduction methods
yield amines. Sodium diformyl amide (Yinglin, H., Hongwen,
H. Synthesis 2989 122), potassium phthalimide, and bis-BOC-
amine anion can all displace halides, tosylates, mesylates,
etc., followed by standard deprotection methods to yield
amines, procedures which are familiar to one skilled in the
art. Other methods to synthesize more elaborate amines
involve the Pictet-Spengler reaction, imine/immonium ion
Diels-Alder reaction (Larsen, S.D.; Grieco, P.A. J. Am.
Chem. Soc. 1985, 107, 1768-69; Grieco, P.A., et al., J.
Org. Chem. 1988, 53, 3658-3662; Cabral, J. Laszlo, P. Tet.
Lett. 1989, 30, 7237-7238; amide reduction (with LAH or
diborane, for example), organometallic addition to imines
(Bocoum, A. et al., J. Chem. Soc. Chem. Comm. 1993, 1542-4)
and others all of which are familiar to one skilled in the
art.
Compounds containing an alcohol side-chain alpha to
the nitrogen of the piperidine/pyrrolidine ring can be
synthesized as shown in Scheme 25b. Only the piperidine
case is exemplified, and it is to be understood by one
skilled in the art that the alpha-substituted pyrrolidines
may be synthesized by a similar route. It is also
understood that appropriate substituents may be present on
the piperidine/pyrrolidine ring. A 4-benzylpiperidine 196
is protected with a BOC group. The BOC-piperidine 197 is
then metallated under conditions similar to those Beak, et
al. (P. Beak and W.-K. Lee, J. Org. Chem. 1990, S5, 2578-
120
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
2580, and references therein) and quenched with an aldehyde
to yield alcohol 198. The metallation may also be done
enantioselectively using sparteine (P. Beak, S.T. Kerrick,
S. Wu, J. Chu J. Am. Chem. Soc. 1994, 116, 3231-3239).
This alcohol can be deprotonated with NaH and cyclized to
carbamate 198a which permits structural assignments of the
erythro and threo isomers. Deprotection with base yields
aminoalcohol 199. Subsequent N-alkylation yields
phthalimidoa.lkylpiperidine 201. It is to be understood
14 that the alkyl chain does not necessarily have to be n-
propyl, but that n-propyl was chosen for demonstration
purposes only. Deprote:ction of the phthalimido group with
hydrazine yields amine 202. Finally, reaction with an
isocyanate or via any of the previously described
1'i conditions described in Scheme 1 yields urea 203. If an
isocyanate is used, the isocyanate can add twice to yield
urea-carbamate 204.
l21
i
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Scheme 25b
0
N.H N~O
Di-t-butyl Bicarbonate
I T~', 0 C to 25 C I
197
19 6 O 1 ) Et2o, ~, -~o °c
2) sec-BuLi,
N O -70 °C to -30 °C ~
OH
1' again to -70 °C
I R N 3 ) RCFiO,
1 9 8 ~ O -70 °C to -30 °C then
v=
H R quench with water
NaOH, EtOH, /
reflex, 3h ~ 195a + threo
erythro
NH o
OH K2Cpj, ~, 2_butanone N~~N
R OH
o R o
19 9 Br~N ~ ~ ~ I 2 O 1
0
2 0 0 N2H4 , EtOH
O
N~N~NHR3
OH H R3NC0
i R 203
y I + O THF', 25 °C N ~2
OH
N~N~NHR3
O~ H ~ R
O~/'NHR3
R 202
204
Compounds where Z = N-CN, CHN02, and C(CN)2 can be
synthesized by the methods shown in Scheme 25c. Thus amine
208 reacts with malononitrile 207 neat or in an inert
solvent at room temperature to the reflex temperature of
the solvent, or at the melting point of the solidJsolid
mixture, to yield malononitrile 206. This in turn can
undergo reaction with amine 205 under similar conditions
stated just above to yield molononitrile 209. Likewise, a
similar reaction sequence may be used to make 212 and 21S
122
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
[for Z = C(CN) 2], see for example P. Traxler, et al., J.
Med. Chem. {1997), 40, 3601-3616; for Z = N-CN, see K. S.
Atwal, J. Med. Chem. (1998) 41, 271; for Z = CHN02, see J.
M. Hoffman, et al., J. Med. Chem. (1983) 26, 140-144).
Scheme 25c.
NC CN
+ R2R3NH
\S S/
207 208
1 ~7
NC CN
NC CN
/F~~ . /E~ ( /R3
~N ~ ~N N N
R ~ Rl + ~S N'/R2 ~ R5 ~ Rl RZ
i3
205 206 R 209
OZN
N02
/Ew /Ew ~ iR3
RS ~N ~ + 3 5 ~N N N
Rl \;S I N/R ~ R ~ Rl
12
205 210 R 212
NO2
+ RzR3~
\S S ~~
211 208
1 ~~
123
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
f.rCN
N
E
R5 ~Ni y + ~ N,,.rCN N/E\ ~ /R3
R3 ~ R5 ~ i N
1 ~2
0 N ~ R R
205 214 R2 2I5
RCN
I N
+ R2R3NH
O O
213 208
EXAMPLES
The compounds of this invention and their preparation
can be understood further by the following working
examples. These examples are meant to be illustrative of
the present invention, and are not to be taken as limiting
thereof.
EXAMPLE 1
Part A: Preparation of 4-benzyl-1-(3-N-phthalimido-n-prop-
1-yl)piperidine
~~N
4-benzylpiperidine (8.0 g , 45.6 mmol, leq), N-(3-
bromopropyl)-phthalimide (13.5 g, 50.2 mmol, 1.1 eq),
potassium iodide (7.6 g, 45.6 mmol, 1 eq) and potassium
carbonate (2.6 g, 91.3 mmol, 2 eq) were refluxed in 125 mL
of 2-butanone. The reaction was worked up after 5 hours by
filtering off the inorganic solids then adding EtOAc and
rinsing the organic layer 2X with water. The organic layer
124
CA 02346933 2001-04-18
WO 00135449
PCT/US99I30292
was dried over magnesium sulfate then the solvent removed
in vacuo to obtain an amber oil. The oil was purified bar
flash chromatography in 100 EtOAc to remove impurities
then 8:2 chloroformJmethanol to isolate 3.67 g of the
product as a light amber oil. NMR(300 MHz. CDC13) 8 8.00-
7.80 (m, 2H); 7.80-7.60 (m, 2H);7.35-7.10 (m, 3H); 7.08 (d,
2H, J=7 Hz.); 3.76 (t, 2H, J = 7 Hz); 2.83 (d, 2H, J=10 Hz);
2.45-2.30 (m, 4H); 1.95-1.30 (m, 7H); 1.20-0.90 (m, 2H)-
Part B: Preparaton of 4-benzyl-1-(3-wino-n-prop-1-
yl)piperidine
rf " Nf'I2
4-benzyl-1-(3-r~-phthalimido-n-prop-1-yl)piperidine
(13.72 g, 37.9 mmo:l, 1 eq.) was dissoved in 200 mL of EtOH
at 25 oC under N2, t:he anhydrous hydrazine (2.38 mL, 75.7
mmol, 2 eq.) was added. The solution was then refluxed
during which time a white precipitate formed. The reaction
was worked up after refluxing 4 hours by filtering off the
solids. The solvent was removed in vacuo to obtain an oil
which was re-rotovapped from toluene to remove excess
hydrazine. Obtained an oil which was stirred in Et20.
Insoluble material was filtered then the solvent removed in
vacuo to obtain 5.55g of an amber oil as product. NMR
(300 MHz, CDC13) b 7.40-7.21 (m, 2H); 7.21-7.05 (m, 3H):
2 .92 (d, 2H, J=10 F(z) ; 2 .73 (t, 2H, J=7 Hz) ; 2 .53 (d, 2H,
J=7 Hz); 2.40-2.20 (m, 2H); 1.84 (t of t, 2H, J=7,7 Hz);
1.75-1.10 (m, 9H).
Part C: N-(3-cyanophenyl)-N'-[3-[4-(phenylmethyl)-1-
piperidinyl]propyl]'urea
125
WO 00/35449
CA 02346933 2001-04-18
PCT/US99/3029~
N~
/ H H ~N
4-benzyl-1-(3-amino-n-prop_1_yl)piperidine (300 mg,
1.29 mmol, 1 eq) was dissoved in THF at 25 oC under N2 then
3-cyanophenyl isocyanate (186 mg, 1.29 mmol, 1 eq) was
added. TLC after 30 minutes shows the reaction complete.
The solvent was removed in vacuo then the residue was
purified over silica gel in 100$ EtOAc to 8:2
chloroform/MeOHto yield 437 mg of an amber oil as product.
1VI~ (300 MHz, DMSO-d6) $ 9.90-9.50 (m, 1H) ; 9.32 (s
1H) ;
7.93 (s, 1H) ; 7.59 (d, 1H, J= 7Hz) ; 7.43 (t, 1H, J= 7Hz) .
7.40-7.24 (m, 3H); 7.24-7.10 (m, 3H
6 . 68 ( t , IH, J=7 Hz ) ;
3.50-3.25 (m, 2H); 3.25-3.07 (m, 2H); 3.07-2.90 (m, 2H);
2.90-2.60 (m, 2H); 2.60-2.40 (m, 2H); 2.00-1.60 (m, SH);
1.60-1.30 (m, 2H).
EXAMPLE 2
Part A: Preparation of 4-benzyl-1-carbomethoxymethyl-1-
[3-(3-cyanophenylaminocarbonylamino)prop-1_yl]piperidinium
bromide
Br~
4-benzyl-1-[3-(3-cyanophenylaminocarbonylamino)prop-1-
yl]piperidine (50mg, 0.133 mmol, I a
q), was dissoved in
acetone at 25 oC under N2 then methyl bromoacetate (l3uL,
0.133 mmol, 1 eq),was added. After 16 hours, the solven
t '
was removed in vacuo and the residue was purified over
silica gel in 100$ EtOAc to 8:2 chloroform/MeOH to yield
m 50
g of white solids as product. NMR (300MHz, CD30D) $ 8.00-
7.80 (m, 1H) ; 7.65-7.45 (m, IH); 7.45-7.33 (m, 1H); 7.33-
126
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
7.05 (m, 6H); 4.50--4.25 (m, 2H); 4.00-3.60 (m, 5H); 3.50-
3.20 (m, 6H); 2.70--2.50 (m, 2H); 2.10-1.60 (m, 7H).
EXAMPLE 3
Part A: Preparation of 1-(t-Butoxycarbonyl)-3-piperidone
i. H2, Pd/C, CH30H,
- / 23 °C
C1
ii. (Boc)20, NaHC03,
THF, 23 °C
86~
To a deep yellow solution of 1-benzyl-3-piperidone
hydrochloride (3.0() g, 1.33 mmol, 1 equiv) in methanol (100
mL) was added 10 wt.. ~ (dry basis) palladium on activated
carbon (600 mg) under a stream of nitrogen. The resulting
black suspension was deoxygenated by alternate evacuation
and flushing with nitrogen (3x) followed by alternate
evacuation and flu:~hing with hydrogen (3x). The reaction
suspension was then shaken vigorously under a hydrogen
atmosphere of 55 p:~i. After 12 hours, gravity filtration
of the supsension and concentration of the resulting
filtrate in vacuo ~r:ielded crude 3-piperidone as a viscous
light green oil. '.Che oil was immediately treated with
tetrahydrofuran (150 mL) and di-t-butyldicarbonate (4.73 g,
21.7 mmol, 0.98 equiv). Upon addition of saturated aqueous
sodium bicarbonate (25 mL), the oil completely dissolved to
give a light yellow suspension. After stirring the
suspension vigorou:~ly for 2 hours, the now white suspension
was poured into aqueous hydrogen chloride (1N, 100 mL), and
the layers were separated. The aqueous layer was extracted
with ethyl acetate (3 x 70 mL), and the combined organic
layers were washed with saturated aqueous sodium chloride
(50 mL), dried over sodium sulfate, and filtered.
Concentration of the resulting filtrate in vacuo yielded 1-
(t-butoxycarbonyl)--3-piperidone (3.79 g, 86~) as a white
oily solid. 1H NM~Z (300 MHz, CDC13), 8:3.94 (s, 2H), 3.53
127
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
(t, 2H, J = 6 Hz), 2.41 (t, 2H, J = 7 Hz), 1.92 (m, 2H),
1.41 (s, 9H)
Part B: Preparation of 1',3-(2H)-Dehydro-3-benzyl-1-(t-
butoxycarbonyl)piperidine
NaH, Bn(0)P(OEt)2
DME, 23-80 °C
O
23~
To a flame-dried 100-mL flask charged with sodium
hydride (60~ wt. dispersion in mineral oil; 601 mg, 15.0
mmol, 2.3 equiv)) and 1,2-dimethoxyethane (20 mL) was added
benzyl diethylphosphite (3.42 g, 3.13 mL, 15.0 mmol, 2.3
equiv) dropwise over a period of 5 min. After 10 min, 1-
(t-butoxycarbonyl)-3-piperidone was added in one portion to
the pale yellow suspension. The flask was fitted with a
relfux condensor, and the resulting yellow-gray suspension
at heated under reflux conditions for 2 hrs. Upon cooling
to 23 °C, the reaction was poured into aqueous hydrogen
chloride (0.20 N, 100 mL) and diethyl ether (75 mL). The
layers were separated and the aqueous layer was basified
with saturated aqueous sodium bicarbonate to pH 9. The
aqueous layer was extracted with diethyl ether (4 x 75 mL),
and the combined organic layers were dried over sodium
sulfate. Filtration, concentration in vacuo, and
purification of the resulting residue by flash column
chromatography (5~ ethyl acetate in hexanes) afforded a
mixture of the desired olefin (410 mg, 23~) and the
corresponding ethoxycarbamate (550 mg, 34~) as a clear oil.
The ethoxycarbamate was removed in the subsequent step by
flash column chromatography. 1H NMR (300 MHz, CDC13), $:
7.30 (m, 2H), 7.18 (m, 3H), 6.42 (s, 1H), 4.02 (s, 2H),
3.50 (t, 2H, J = 6 Hz), 2.51 (t, 2H, J = 5 Hz), 1.61 (m,
128
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
2H), 1.49 (s, 9H). MS (CI), m+/z: (M+H)+ = 274, [(M+H)+ -
(-C(0)OC(CH3)3)] 174.
Part C: Preparation of 1-(t-Butoxycarbonyl)-3-
benzylpi~peridine
H2, Pd/C
CH30H, 23 °C
99~
To a solution of impure product (410 mg, 1.50 mmol)
obtained in the previous step in methanol (100 mL) was
added 10 wt. ~ (dry basis) palladium on activated carbon
(200 mg) under a stream of nitrogen. The resulting black
suspension was deoxygenated by alternate evacuation and
flushing with nitrogen (3x) followed by alternate
evacuation and flu~~hing with hydrogen (3x). The reaction
suspension was then shaken vigorously under a hydrogen
atmosphere of 55 ps~i . After 12 hours, gravity filtration
of the supsension and concentration of the resulting
filtrate in vacuo resulted in a pale yellow residue.
Purification of this residue by flash column chromatography
afforded 1-(t-butoacycarbonyl)-3-benzyl-piperidine (407 mg,
99~) as .a clear oi7_. 1H NMR (300 MHz, CDC13), 8: 7.23 (m,
2H), 7.14 (m, 3H), 3.86 (m, 2H), 2.75 (br m, 1H), 2.51 (m,
3H), 1.70 (br. m, 2H), 1.64 (br. m, 1H), 1.41 (s, 9H), 1.34
(br. m, 1H), 1.09 (br. m, 1H). MS (CI), m+/z: (M+ + 1)
276, I(M+H)+ - (-C'(0)OC(CH3)3)] - 176.
Part D: 3-Benzylp~_peridine hydrochloride
129
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
HC1
~ HC 1
To a solution of 1-(t-butoxycarbonyl)-3-
benzylpiperidine (400 mg, 1.45 mmol) in methanol (5 mL) was
added hydrogen chloride in dioxane (4M, 15 mL). The
resulting yellow solution was stirred for 1 hr, at which
time the reaction was concentrated in vacuo to provide 3-
benzylpiperidine hydrochloride (308 mg, 1000 as an
amorphous solid. 1H NMR (300 MHz, CD30D), b: 7.27 (m,
2H,), 7.19 (m, 3H), 3.29 (br. d, 1H, J = l2Hz), 3.20 (br.
d, 1H, J = 12 Hz), 2.87 (br. t, 1H, J = 12 Hz), 2.67 (m,
IH), 2.60 (d, 2H, J = 7Hz), 2.08 (m, 1H) 1.70-1.87 (m, 3H),
1.26 (m, 1H). MS (CI), m+/z: (M+H)+ = 176.
Part E: Preparation of N-(3-methoxyphenyl)-N'-[3-[3-
[(phenyl)methyl]-1-piperidinyl]propyl]urea
OCH3
The above compound was prepared by the methods similar
to the ones employed in Example 1, part C.
1H NMR (300 MHz, CD30D), 8:7.29-7.13 (m, 4H); 7/07 (d, 1H,
J=9 Hz); 7.02 (m, 1H); 6.78 (d, 1H, J = 9 Hz); 6.60 (d,
1H, J = 9 Hz); 3.77 (s, 3H); 3.30 (m, 2H); 2.80 (m, 2H);
2.53-2.32 (m, 4H); 1.85-1.55 (m, 7H); 1.44-0.78 (m, 2H).
MS (ESI), m+/z: (M+H)+ - 382.
130
CA 02346933 2001-04-18
WO 00/35449 PCT/U599/30292
EXAMPLE 4
Part A: 3?reparation of a,a'-Dibromo-3-nitro-o-xylene
B _
p+
B ~ o
3-Nitro-o-xylene (lO.Og, 66.14 mmol, 1.00 eq), N-
bromosuccinimide (24.14 g, 135.6 mmol, 2.05 eq), and
benzoyl peroxide (0.8 g, 3.30 mmol, 0.5 eq) were refluxed
under N2 .in 200 ml of carbon tetrachloride. The reaction
was worked up after two days by washing with 3 x 100 ml of
water. The organic phase was dried over sodium sulfate,
then the :solvent was removed in vacuo to obtain an amber
oil. The oil was purified by flash chromatography on a 8
cm x 20 crn quartz column, eluting with 7.5'k EtOAc/Hexanes
to yield 4.46 g of product as a sticky solid. NMR (300
MHz, CDC1;; ) 8 7 . 88 (d, 1H, J=7 Hz) , 7 .64 (d, 1H, J=7 Hz) ,
7.48 dd, :LH, J=8 Hz), 4.86 (s, 2H), 4.69(s, 2H).
Part B: Preparation of 1,3-Dihydro-4'-[4-
fluorophenylmethyl]-4-nitro-spiro[2H-isoindole-2,1'-
piperidinium] bromide
+ ~+ O
1
Br-~--~~
4-Fluorobenzylp~iperidine (0.94 g, 4.86 mmol, 1.0 eq),
a,a'-dibromo-3-nitro~-o-xylene (1.50 g, 4.86 mmol, 1.0 eq),
and sodium carbonate: (2.57 g, 24.3 mmol, 5.0 eq) were
combined :in 20 ml TH:F and stirred at 25~ C under N2, during
which time a white solid precipitated from the reaction
mixture. The reaction was worked up after 22 hours by
filtering the solids and rinsing with THF. The solids were
131
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
dissolved in methanol and applied to a 3.5 cm x 5 cm quartz
column via silica plug. The product was eluted with 200
MeOH/CHC13 to yield 1.04 g of a white foam. NMR (300 MHz,
CD30D) 8 8.27 (d, 1H, J=8 Hz), 7.84 -7.80 (m, 1H), 7.75-
7.69 (m, 1H), 7.23 (m, 2H), 7.01 (dd, 2H, J=8 Hz, 8 Hz),
5.38-5.37 (m, 2H), 5.09 (s, IH), 5.04 (s, 1H), 3.80-3.72
(m, 2H), 3.65-3.54 (m, 2H), 2.71-2.68 (m, 2H), 2.05-1.75
(m, 5H) .
Part C: Preparation of 4-Amino-1, 3-dihydro-4'-[4-
fluorophenylmethyl]-spiro[2H-isoindole-2,1'-piperidinium]
bromide
I ~l-~- 2
1,3-Dihydro-4'-[4-fluorophenylmethyl]-4-nitro-
spiro[2H-isoindole-2,1'-piperidinium] bromide (1.03 g, 2_46
mmol, 1.0 eq), zinc (5.32 g, 81.5 mmol, 33.0 eq), and
calcium chloride (0.18 g, 1.60 mmol, 0.65 eq) were refluxed
under N2 in 25 ml of a 78~ ethanol/water solution. The
reaction was worked up after 5 hours by filtering through
Celite~ and rinsing the cake with methanol. The filtrate
was concentrated in vacuo to a mixture of water and an
amber oil. The mixture was dissolved in 50 ml of 2-
propanol, and concentrated in vacuo to remove excess water.
The resulting yellow foam was dissolved in methanol and
applied to a 3.5 cm x 5 cm quartz column via silica plug.
The product was eluted with 20~ MeOH/CHC13 to yield 0.81g
of a yellow foam. NMR (300 MHz, DMSO) 8 7.27-7.05 (m, 5H),
6.61-6.53 (m, 2H}, 5.43-5.42 (m, 2H), 4.80 (bs, 1H), 4.74
(bs, 2H}, 4.63 (bs, 1H), 3.62-3.43 (m, 4H), 2.60 (bd, 2H,
J=7 Hz), 1.98-1.59 (m, 5H).
132
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Part D: Preparation of N-[1,3-Dihydro-4'-[4-fluorophenyl-
methyl]spiro(2H-isoindole-2,1'-piperdinium-4-yl]-N'-4-
f luorophen~~lurea bromide
4-Amino-1, 3-dihydro-4'-[4-fluorophenylmethyl]-
spiro(2H-isoindole-2, 1'-piperidinium] bromide (0.33 g,
0.84 mmol, 1.0 eg), and 4-fluorophenyl isocyanate (0.23 g,
J.0 1.69 mmol, 2.0 eq) were combined in 3 ml DMF and stirred at
25~ C under N2 . They reaction was worked up after 22
hours by removing the solvent in vacuo, dissolving the
residue in methanol, and applying the mixture to a 3.5 cm x
cm quartz column via silica plug. The product was
15 eluted with 10~ MeOH/CHC13 to yield 65 mg of a yellow foam.
NMR (300 MHz, DMSO) b 9.18 (s, 1H), 9.00 (s, 1H), 7.49-7.43
(m, 2H), 7.41-7.34 (m, 2H), 7.26-7.21 (m, 2H), 7.17-7.10
(m, 5H), 4.94 (s, 2H), 4.80 (s, 2H), 3.63-3.45 (m, 4H),
2.61 (bd, j=7 Hz), 1.91-1.62 (m, 5H)
EXAMPLE 5
Part A. Preparation c>f 4-benzyl-1-(3-hydroxy-3-phenylprop-
1-yl)piperidine
, 25
To a flame-dried 3-neck flask under a N2 atmosphere
with a magnetic stirring bar, 4-benzylpiperidine (5.00 mL,
133
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
28 mmol, 1 eq), DBU (42 uL, 0.28 mmol, 0.01 eq), and THF
(100 mL) were added, mixed, and cooled to -15 °C using a
CC14/C02(s) bath. Acrolein (1.87 mL, 28 mmol, 1 eq) was
then syringed in slowly during 10 minutes maintaining the
temp. at -15 °C. After 0.5 hours at -15 °C, phenylmagnesium
chloride (2.0 M, 14.0 mL, 28 mmol, 1 eq) was syringed in
slowly and the contents allowed to slowly warm to room
temperature and then stirred for 48 h. The reaction was
worked up by adding 0.1 N NaOH and EtOAc (200 mL each).
1D The viscous magnesium salts were suction filtered through
fiberglass filter paper. The layers were separated and the
aqueous layer was extracted again with ethyl acetate (2 x
200 mL). The organic layers were combined, washed with
brine (1 x 200 mL), dried (MgS04) and the solvent removed
in vacuo to yield 7.39 g of an amber oil. Flash
chromatography in 100 ethyl actetate yielded 2.48 g of an
orange oil. NMR (CDC13) 8 7.40-7.10 (m, lOH); 4.93 (d of
d, 1H, J=3,7 Hz); 3.12-2.96 (m, 2H); 2.68-2.46 (m, 4H);
2.01 (t of d, 1H, J=2, 10 Hz); 1.86-1.26 (m, 8H). ESI MS
detects (M+H)+ = 310.
Part B: Preparation of 4-benzyl-1-(3-azido-3-phenylprop-1-
yl)piperidine
3
The product from part A (209 mg, 0.675 mmol, 1 eq),
DBU (123 mg, 0.810 mmol, 1.2 eq), diphenylphosphoryl azide
(0.175 mL, 0.810 mmol, 1.2 eq), and toluene (1.0 mL) were
mixed and stirred overnight at room temperature under a N2
atmosphere. The reaction was then worked up by adding
ethyl acetate (50 mL), washing with water (3 x 25 mL),
followed by washing with brine (1 x 25 mL), drying (MgS04)
and removing the solvent in vacuo to yield 277 mg of an
134
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/3029.".".
amber oil. Flash chromatography in 1:I hexane/ethyl
acetate yiE:lded 84 mg of product as an oil. NMR (CDC13) 8
7.41-7.09 (m, 10 H); 4.56 (t, 1H, J=7 Hz); 3.83 (m, 2H);
2.52 (d, 2Fi, J=7 Hz) ; 2.32 (t, 2H, J=7 Hz) ; 2.30-1.77 (m,
5H); 2.59 (m, 2H); 1.98 (m, 1H); 1.39-1.26 (m, 4H). IR
(neat) 2095 cm'1.
Part C: Preparation of 4-benzyl-1-(3-amino-3-phenylprop-1-
yl ) piperid:ine
The compound from part B (100 mg), 10~ Pd on carbon
(120 mg), and methanol (100 mL) were carefully combined in
a flask under a N2 atmosphere. The contents were then
submitted to 1 atm of H2 being delivered via a sparge tube
for 0.5 h at room temperature. Filtration of the contents
through Celite~ and removal of the solvent in vacuo yielded
70 mg of product. NriR (CDC13) (key peak only) 53.94 (t, 1,
J = 7 Hz) . NH4-CI M~; detects (M+H) + = 309 .
Part D: N-(3-cyanophenyl)-N'-[3-[4-(phenylmethyl)-1-
piperidinyl]-1-pheny7_propyl]urea
The compound from Part C (57 mg, 0.185 mmol, 1 eq) was
mixed and stirred with 3-cyanophenylisocyanate 26.6 mg,
0.185 mmol, 1 eq) in T'HF (1 mL) overnight at room
135
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
temperature under a N2 atmosphere. The solvent was removed
in vacuo and the residue flash chromatographed on silica
gel in 3:1 to 1:1 hexane/ethyl acetate to 100 ethyl
acetate to yield 44.3 mg of a yellow oil. NMR (CDC13)
87.58 (s, 1H); 7.52 (d, 1H, J = 9 Hz); 7.42 (s, 1H);
7.30-7.27 9m, 8H); 7.12 (m, 3H); 4.82 (m, 1H); 2.97-2.80
(m, 3H); 2.52 (d, 2H, J=7 Hz); 2.35 (m, 2H); 2.05-1.85
(m, 4H); 1.81-1.60 (m, 2H); 1.54 (m, 1H); 1.25 (m, 1H).
ESI MS detects (M+H)+ = 453.
EXAMPLE 6
Part A: Preparation of 2-benzyloxycarbonylamino-1-phenyl-
3-butene.
To a stirred suspension of methyltriphenylphosphonium
bromide (10.72 g, 0.03 moles) in 100 mL of dry
tetrahydofuran at -78°C was added dropwise 1.6M n-butyl
lithium (17.5 mL, 0.028 moles), and the mixture was stirred
for 0.5 hrs at -78 ~ -20°C. Then was added a solution of N-
Cbz-phenylalaninal (5.67 g, 0.02 moles) in 50 rnL of dry
tetrahydrofuran, and the mixture was stirred for 16 hrs at
room temperature. After addition of saturated NH4C1 (50 mL)
the mixture was extracted with EtOAc, and the extract was
washed with water and brine. It was dried over Na2S04 and
evaporated to give an oily residue. The crude product was
purified by column chrornatograpy on silica gel with elution
by 5:95 EtOAc-hexane to give pure 2-benzyloxycarbonylamino-
1-phenyl-3-butene. ,
Part B: Preparation of 2-benzyloxycarbonylamino-1-phenyl-
3,4-epoxy-butane.
136
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
H
To a stirred solution of 2-benzyloxycarbonylamino-1-
phenyl-3-butene (1.43 g, 5.08 mmoles) in 20 mL of CH2C12
was added 3-chloroperoxybenzoic acid (2.19 g, 60~, 7.62
mmoles) in ;several portions, and the mixture was stirred at
room temperature for 30 hrs. After addition of EtOAc (60
mL), the mixture was washed with saturated NaHC03 and
brine, and ~he organic layer was dried over Na2S04.
Evaporation of the solvent afforded an oily residue. The
crude product was purified by column chromatography on
silica gel with elution by 2:8 EtOAc-hexane to give pure 2-
benzyloxyca~_bonylamino-1-phenyl-3,4-epoxy-butane.
1'S Part C: Preparation of 2-benzyloxycarbonylamino-4-[4-(4-
fluoropheny:L)methyl-1-piperidinyl]-1-phenyl-butan-3-ol.
F~'~U ~ "~°~
21) A solution of 4-(4-fluorophenyl)methyl-piperidine
(0.515 g, 2..314 mmoles) and 2-benzyloxycarbonylamino-1-
phenyl-3,4-epoxy-butane (0.688 g, 2.314 mmoles) in 5 mL of
DMF was stirred for 4 hours at 100°C and cooled to room
temperature.. After addition of EtOAc (30 mL), the mixture
2!i was washed with water (2x) and brine. The oranic solution
was dried over Na2S04, and evaporated to give an oily
residue. It was then purified by passing through a plug of
silica gel with elution by EtOAc to give pure product.
137
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Part D: Preparation of 2-amino-4-[4-(4-fluorophenyl)methyl-
1-piperidinyl]-1-phenyl-butan-3-ol.
~z
The above product was dissolved in 10 mL of ethanol,
and was added 0.1 g of 10~ Pd on carbon. The mixture was
stirred under hydrogen (1 atm) for 8 hours, and filtered
through Celite. Evaporation of the solvent gave the titled
product as solid (0.662 g).
Part E: Preparation of N-(3-cyanophenyl)-N'-[1-benzyl-2-
hydroxy-3-[4-(4-fluorophenylmethyl)-1-
piperidinyl]propyl]urea
H H ~N
~H
To a solution of 2-amino-4-[4-(4-fluorophenyl)methyl-
1-piperidinyl]-1-phenyl-butan-3-of (50 mg, 0.14 mmoles) in
2.5 mL of dry THF was added 3-cyanophenyl isocyanate (20.2
mg, 0.14 mmoles) and the mixture was stirred for 15 minutes
at room temperature. Then the solvent was evaporated off to
give an oily residue. It was purified by column
chromatography on silica gel with elution by EtOAc to give
pure titled compound as an amorphous solid.
MS (ES+) for C3pH33FN4O2 - 501.
The following examples were prepared by the procedures
previously described in Schemes 1-25 , Examples 1-6 and/or
by procedures familiar to one skilled in the art.
138
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
TABLE 1*
H H
C/~~ N~ NR3 ~~ ~ H
R~ ~~~~ R
H. H
a b C 0
Q H H
C~~~ N~ NR3 ~N\/~,~ ~ G H H N.
G R3 /~\~~ R
H H
0
f
H H H H H H
Ks i~ N.R G~~ IS
3 ~ R3
0 Ph 0 Ph
h i
G H H ~..~ ~ H H H G H H
~N ~ N N.R G N N N. N N N.
3 Y R3 ~ R
~ ~ 3
P~ O PYl 0 ~ 0
j k 1
Ex Core_ G ' M+1
# R3
7 a Ph _ 410
3-C02Et-Ph
8 a Ph 3_.I_p~- 464
9 a Ph 1-adamant 1 396
a Ph 3-OCH3-Ph 368
11 a Ph Ph 338
12 a Ph 4-F-Ph 356
13 a Ph 4-C02Et-Ph 410
14 a Ph 4-CN-Ph 363
b Ph 1-adamant 1 410
_16 b Ph 2-F-5-CF3-Ph 438
17 b Ph 2-na hth 1 402
~
18 b Ph 2-F-5-N02-Ph 415
19 b Ph 4-N(CH3)2-Ph 395
_20 b Ph 2-N02-Ph 397
21 b Ph 2-C2H5-Ph 380
22 b Ph 4-CF4-Ph 420
23 b Ph 3,5-diCF3-Ph 488
--
_24 b Ph 3-C02Et-Ph 424
b Ph 3-CN-Ph 377
26 b Ph 4-OBn-Ph 458
27 b Ph 2-Ph-Ph 428
28 b Ph 2-BrPh 431
29 b Ph 4-I-Ph 478
b Ph 3-I-Ph 478
31 b Ph 4-OEt-Ph 396
139
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
32 b Ph 4-nBu-Ph 408
33 b Ph 4-nBuO-Ph 424
34 b Ph CH(Bn)C02Et 452
35 b Ph CH(iPr)C02Et 404
36 b Ph nC8H17 3gg
37 b Ph 3-OCH3-Ph 382
38 b Ph Ph 352
39 b Ph 4-C02Et-Ph 424
40 b Ph 4-F-Ph 370
41 b Ph 2-Phenyl- 392
cyclo ro 1
42 b Ph 2-OCH3-Ph 382
43 b Ph 4-OCH3-Ph 382
44 b 4-F-Ph 3-CN-Ph 395
45 b 4-F-Ph 4-F-Ph 3gg
46 b 4-F-Ph 4-C02Et-Ph 442
47 b 3,4-OCH20-Ph 3-CN-Ph 421
48 b 4-F-Ph 3-OCH3-Ph 400
49 b 3,4-OCH20-Ph 3-C02Et-Ph 468
50 b 3,4-OCH20-Ph 3-OCH3-Ph 426
51 b 4-OCH3-Ph 3-OCH3-Ph 412
52 b 4-OCH3-Ph 4-F-Ph 400
53 b Ph 4-CN-Ph 377
54 b 3,4-OCH20-Ph 4-F-Ph 414
55 b 4-OCH3-Ph 4-CN-Ph 407
56 b 2,4-diF-Ph 4-F-Ph 406
57 b 2,4-diF-Ph 3-OCH3-Ph 418
58 b 2,4-diF-Ph 3-CN-Ph 413
59 b 3-CF3-Ph 4-F-Ph 438
60 b 3-CF3-Ph 3-OCH3-Ph 450
61 b 4-F-Ph CH2Ph 384
62 b 4-F-Ph CH2CH2Ph 398
63 b 4-F-Ph 2-F-Ph 388
64 b 4-F-Ph 3-F-ph 3gg
65 b 4-F-Ph c clohexyl 376
66 b 4-F-Ph iPr 336
67 b 4-F-Ph 2-phenyl- 410
c clo ro 1
68 b 4-CF3-Ph 3-CN-Ph 445
69 b 3-CF3-Ph 3-CN-Ph 445
70 b 4-CH3-Ph 3-OCH3-Ph 396
71 b 4-CH3-Ph 3-CN-Ph 391
72 b 4-C1-Ph 3-CN-Ph 411
73 b 4-CF3-Ph 4-C02Et-Ph 492
74 b 3-OCH3-Ph 3-OCH3-Ph 412
75 b 3-OCH3-Ph 3-CN-Ph 407
76 b 4-C02CH3-Ph 3-OCH3-Ph 440
77 b 4-C02CH3-Ph 3-CN-Ph 435
78 b 4-C02CH3-Ph 4-F-Ph 428
79 b 4-C02CH3-Ph 4-C02CH3-Ph 482
80 b 4-CF3-Ph 4-F-Ph 438
81 b 4-CF3-Ph 3-OCH3-Ph 450
82 b 3-OCH3-Ph 4-F-Ph 400
140
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
83 b 3-OCH3-Ph 4-C02Et-Ph _ 454
84 b 2-F-Ph 3-CN-Ph 395
85 b 3-OCH3-Ph 3-F-Ph _ 400
86 b 2-F-~?h 3-OCH3-Ph 400_
87 b 3-OCH3-Ph 3-C02Et-Ph 454
88 b 2-F-~?h 3-F-Ph 388_
89 b 2-F-1?h 4-F-Ph 388
90 b 2-F-1?h 3-C02Et-Ph __442_
91 b 3-F-1?h 3-CN-Ph 395
92 b 3 , 4-diF~-Ph 3-CN-Ph 413
93 b 3,4-diF-Ph 3-OCH3-Ph 418
94 b 4-C1-Ph 4-F-Ph 409
95 b 4-Cl.-Ph 3-OCH3-Ph 416
96 b 2-F-lPh 4-C02Et-Ph 442
97 b 3-F-1Ph 3-OCH3-Ph 400
98 b 3-F-lPh 4-F-Ph 388
99 b 3-F-1Ph 4-C02Et-Ph 442
100 b 3 , 4-di1?-Ph 4-F-Ph 406
101 b 3-Cl-Ph 3-CN-Ph 411
102 b 4-F-lPh 3-COCH3-Ph 412
103 b 3,5-dih-Ph 3-CN-Ph 413
104 b 3,5-dil=-Ph 3-OCH3-Ph 418
105 b 4-F-:Ph 4-COCH3-Ph 412
106 b 1-na h~th 1 3-CN-Ph 427
107 b 1-na hth 1 4-F-Ph 420
108 _ 1-na ht:h 1 3-OCH3-Ph 432
b
109 b 3-CH3-Ph 3-CN-Ph 391
110 b 3-CH3-Ph 4-F-Ph 384_
111 b 3-CH3-Ph 3-OCH3-Ph 396
112 b 4-F-Ph 2-iPr-Ph 412
113 b 4-F-Ph 2-CF3-Ph 438
114 b 4-F-P 3-C1-Ph 404
h
115 b _ 3-CF3-Ph 438
4-F-Ph
116 b 4-F-Ph 4-Ph-Ph 446
117 b 4-F-Ph 2-C1-Ph 404
118 b 4-F-Ph 2,4-diF-Ph 406
119 c Ph_ 3-C02Et-Ph 424
120 c Ph 3-CN-Ph 377
121 c Ph 4-F-Ph _ _370 _
122 c Ph Ph _352
123 c Ph 1-adamant 1 410
124 c- Ph 4-C02Et-Ph 424
125 c 4-F-Ph Ph 370
126 c 4-F-Ph 3-CN-Ph 39_5
127 c 4-F-Ph 1-adamant 1 428
128 c 4-F-Ph 3-OCH3-Ph 400
129 c 4-F-Ph 3-C02Et-Ph 442
130 c 4-F-Ph 4-F-Ph 388
130a c 4-F-Ph 3-COCH3-Ph 412
131 c 2-F-Ph Ph 370
132 c 2-F-Ph 3-CN-Ph 395 _
133 c 2-F-Ph 3-OCH3-Ph 400
134 c 2-F-Ph 4-F-Ph 388
141
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
135 c 3-F-Ph 3-OCH3-Ph 400
136 c 3-F-Ph 3-CN-Ph 395
137 c 2,4-diF-Ph 3-CN-Ph 413
138 c 2,4-diF-Ph 3-OCH3-Ph 418
139 c 2,4-diF-Ph Ph 388
140 c 2,4-diF-Ph 4-F-Ph 406
141 c 2,4-diF-Ph 3-COCH3-Ph 430
142 d Ph 3-CN-Ph 391
143 d Ph 3-C02Et-Ph 438
144 d Ph 3-I-Ph 492
145 d Ph 4-OCH2Ph-Ph 472
146 d Ph 1-adamant 1 424
147 d Ph 3-OCH3-Ph 396
148 d Ph Ph 366
149 d Ph 4-F-Ph 384
150 d Ph 4-C02Et-Ph 438
151 d Ph 4-CN-Ph 391
152 a 4-F-Ph Ph 356
153 a 4-F-Ph 3-CN-Ph 381
154 a 4-F-Ph 3-OCH3-Ph 386
155 a 4-F-Ph 4-F-Ph 374
156 a 4-F-Ph 3-C02Et-Ph 428
157 a 4-F-Ph 4-C02Et-Ph 428
158 a 4-F-Ph 1-adamant 1 414
159 f 4-F-Ph 3-CN-Ph 411
160 f 4-F-Ph 3-OCH3-Ph 416
161 j Ph Ph 458
162 j Ph 3-CN-Ph 483
163 Ph 3-OCH3-Ph 488
_164 j 4-F-Ph 3-OCH3-Ph 506
165 j 4-F-Ph 4-F-Ph 494
166 j 4-F-Ph 1-adamant 1 534
167 1 Ph 3-OCH3-Ph 458
168 1 Ph 1-adamant 1 486
169 c imidazol-1- 1 3-OCH3-Ph 372
* All stereocenters are (+/-) unless otherwise indicated
TABLE 2**
RS a Sb
R5C ~1 ~2
R~ N O ~~ Z
4
X
m
Ex Y Z R4 X R5a R5b R5c R1 R2
#
170 H H - - H H H H Ph
171 H H - - H H H H CH3
172 H 3-OCH3 CH2Ph Br H H H H H
142
CA 02346933 2001-04-18
WO 00/35449 PCTlUS99/30292
173 H 3-CN - - C02E H H H H
t
174 H 3-OCH3 CH3 I H H H H H
175 H 3-CN CH3 I H H H H H
176 H 3-CN CH2Ph Br H H H H H
177 H 3-CN - - H H H CH2P H
h
178 H 3-CN - - H H H Et H
179 H 4-F CH3 I H H H H H
180 H 4-F CH2Ph Br H H H H H
181 H 4-F CH2C02CH Br H H H H H
3
182 H 3-CN CH2CN Br H H H H H
183 H 3-CN CH2COPh Br H H H H H
184 H 2-OCH3 CH3 I H H H H H
185 H 4-OCH3 CH3 I H H H H H
186 F 3-CN CH3 I H H H H H
187 H 3-CN - - H H H
188 H 3-OCH3 _ - H H H H H
O
189 H 3-OCH3 - - CH2P
h
190 F 3-CN CH3 I H H H H H
191 F 3- _ - H CH2P H H H
COCH3
_
192 F 4-F-Ph - - H CH2P H H H
h
193 F 3-OCH3 - - H CH2P H H H
h
194 H 3-OCH3 - - H H H CH2P H
h
195 H 3-CN - - H H H CH2P H
h
**All compounds are amorphous unless otherwise indicted.
TABLE 3**
X ~~ Z Y + X ~H~~ Z
Y~ + L ~O N O
n o
Ex # Core Y Z X
196 n H 3-CN Br
197 n -- H 3-~ Br
198 n H _ 4-F Br
199 n H 4-F Br
_ __
2 0 0 n F 3 -~ Br
2 01 n F 3 -~ $r
202 n F 3-OCH3 Br
203 n F 3-OCH3 Br
143
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
204 o F 4-F gr
205 o F 4-F Br
206 o F 3-OCH3 Br
207 o F 3-OCH3 Br
208 o F 3-CN Br
209 o F 3-CN Br
r~~l compounas are amorphous unless otherwise indicted.
The compounds of the present invention in which E
contains ring A can be prepared in a number of ways well
known to one skilled in the art of organic synthesis. As
shown in Scheme 26, 4-benzyl piperidine is N-alkylated with
an alkylating agent, such as 165 (2-vitro-benzyl bromide (X
- Br, R14 = H), Scheme 26) to give the N-benzyl compound
166. The vitro group of 166 is then reduced using
catalytic hydrogenation to give the corresponding aniline
167. The aniline can be converted to the carbamate 168
using chloro-phenyl formate. The carbamate 168 can then be
reacted with various amines to give the urea 169.
Alternatively, the aniline 167 can be reacted with the
appropriate isocyanates to give the urea 169 directly. The
saturated ring analogs can also be used. For example, 4-
benzyl piperidine can be alkylated with the urea mesylate
185 (Scheme 30) to give corresponding cyclohexyl derivative
186.
As shown in Scheme 27, 4-benzyl piperidine can also be
N-alkylated with the phenacyl bromide 170 to give the vitro
ketone 171. The vitro group of 171 is then reduced using
catalytic hydrogenation to give the corresponding aniline
172. The aniline 172 can be reacted with the appropriate
isocyanates to give the ketone urea 173. The ketone of 173
can be reduced with NaBH4 to give the alcohol 1?4.
19
Alternatively, the epoxide ,~5 (R - H) can be opened
with the 4-benzyl piperidine to give the corresponding
vitro benzyl alcohol which is hydrogenated to give the
aniline alcohol 176. The aniline 176 may be treated with
various isocyanates to give the urea alcohols 174.
144
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
The 4-benzyl piperidine can also be N-alkylated with
3-cyanobenzyl bramide (177, Scheme 28) to give the cyano
analog 178. The cyano group is reduced using Raney nickel
to give the <:orresponding benzyl amine 179. Treatment of
179 with isoc:yanates gives the urea 180.
As shown in Scheme 29, treatment of 3-cyano aniline
with phenylisocyanate gives the urea 182. The cyano group
of 182 is converted to the imidate ~ by HC1/ethanol.
Reaction with 4-benzyl piperidine in ethanol then gives the
amidine 184.
The saturated ring analogs can also be synthesized
using analogous procedures as outlined in Schemes 30 and
31. For example, 4-benzyl piperidine can be alkylated with
the urea mesylate 185 (Scheme 29) to give corresponding
cyclohexyl derivative 186. Alternatively, starting with the
enantiomerically pure amino alcohol 187 [J. Am. Chem. Soc.
1996, 118, 5502-5503 and references therein) one can
protect the nitrogen to give the N-Cbz alcohol ~. Swern
oxidation of the alcohol gives the aldehyde 18~. Reductive
amination with piperidine analogs gives the cyclohexyl
methyl-1-piperidinyl analogue ~. The Cbz group is removed
by catalytic hydrogenation to give the free amine 191,
which is treated with a phenylisocyanate to give the
desired urea analogue 192. Several examples using these
synthetic methods are listed in Table 3a and Table 3.1.
145
CA 02346933 2001-04-18
WO 00/35449
PCT/US99/30292
SCHEME 26
X
R14 i / + w
NOa A .-.~ R1~ \
165 /
NOZ
X = C1, Br, MsO, etc. 166
1B
R1~ \ _ / \
I/
H ~ ~ Ri4
I /
NHz
168 D E
167
R14= \
I/
~~R
169
A: DMF/KzC03/RT or THF/RT. H:10%Pd/C, H z 50 psi.
C: THF/Et3N/chlorophenylformate. D:NHR/DMF/50°C.
E: R-N=C=O/THF
146
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 27
r
\ ~ /
v
R14 i + Rla
NO.
/ '~ ' / NOZ 171
i
170
B
R1
C Ri4.
172
173
D D
1
\ H (~.~/ ~/
R1~ C R1~. \ ~/
~~~R .,.-_~ / NHy 176
fIO
A, B
174
R +
/ NOZ
r
175
A: DMF/KzCO~/RT or DMF/50°C. B:10$Pd/C, ~I50
psi. C: R-~N=C=O/THF. D:NaBF~/MeOH/RT
sct~a~ z a
Br ~ A
178
CN CN
177 , 1 B
W
N.-I~ 17 s
N- R
NHZ
180
A: DMF/K 2C0~/RT B:Raney nickel, H 2 50 psi.
C: R-N=C=0/THF.
147
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 29
VH
CN A' ~ \ CN B' ~ \ pEt C ~ ~ \
/ / /
~2
o -.~~ '~' ~ \ '~' , \
/ o / o
182 183 184
A: R-N=C=O/THF. B:EtOH/HC1/RT
C: 4-benzylpiperidine/EtOH/RT
SCHEME 30
OH OMs
A,B
C
~~ N~ \ OMe ' y
N~ ~ ~ OMe
O
O
185
186
A: R-N=C=O/DMF. B:Ms-C1/THF
C:4-benzylpiperidine/DMF/RT
148
CA 02346933 2001-04-18
.WO 00/35449 PCT/US99/30292
SCHEME 31
OH
a OH
----
2
..
NH-CB Z
'187 188
0
H N
__ I
.'~NH-CBZ ~ ---~..
C 1V -C: t3G
189 H
190
B
..~~nNH2 , ----.-
____
191 0
192
a:Benzyl chloroformate:/Na2C03/CH2C12. b.Swern Ox.
c:NaBH(OAc)3 d:Hz/10~ F?d/C e:R-N=C=O/THF.
149
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
SCHEME 31a
OH
a OH
b
~2
~NH-CB Z
187 188
/ \ F / \
O F
H N
N
d
NH-CBZ -
~~~~'N-CBz
189 H
193
/ \ F / \
F
N- )
N-
~/a
...~~~NH2 -,-~. ...,.~N N O
194 C
195
a:Benzyl chloroformate/Na2C03/CHzCl2. b.Swern Ox.
c:NaBH(OAc)3 d:Hz/10$ Pd/C e:R-N=C=O/THF.
The following examples were synthesized using the
methods outlined in Schemes 26-31a. These examples are
meant to be illustrative of the present invention, and are
not to be limiting thereof.
150
CA 02346933 2001-04-18
WO 00135449 PCTNS99/30292
N-[1-(phenylmethyl)4-piperidinyl]-N'-[2-[[4-
(phenylmethyl)-1-piperidinyl]-methyl]phenyl]urea.
A solution of 4--benzylpiperidine (1.75 g, 10 mmol) in
25 mL of DMF was treat=ed with 2-nitrobenzyl bromide (2.16
g, 10 mmol ) and KZC03 ( 1 . 3 8 g, 10 mmol ) and the reaction
mixture stirred at room temperature for 2 h. The mixture
was diluted with water and extracted into ethyl acetate.
The organic extracts were washed successively with water
and brine, and the organic solvent removed und4r vacuum on
a rotary evaporator t:o give 1 6 (Scheme 26, R - H) as a
yellow oil.
The oil was re-dissolved in ethyl acetate (50 ml) and
treated with 10~ Pd/C: and hydrogenated at 50 psi hydrogen
at room temperature f:or 40 min. The solution was then
filtered and the solvent removed under vacuum to give the
aniline 167 as a white solid. The aniline was purified by
chromatography (MPLC, 40~ ethyl acetate/ hexane; silica
gel) to give 2.0 g oi_ aniline X67 as a white solid.
A solution of aniline ~ (1.2 g, 4.3 mmol) in THF was
treated with Et3N (1.0 g, 10 mmol) and cooled in an ice
bath to °0 C. Chlorophenyl formate (0.71 g, 4.5 mmol) was
added to the mixture and stirred for 1 h. The mixture was
diluted with water and extracted into ethyl acetate. The
extracts were washed with water and brine, and the solvent
removed under vacuum to give the phenyl carbamate 168 as an
off-white solid. ThE_ crude product was used without
further purification.
A solution of phenylcarbamate ~ (0.2 g, 0.5 mmol) in
DMF is treated with 4-amino-1-benzylpiperidine (95 mg, 0.5
mmol) and K2C03 (138 mg, 1 mmol) and the mixture was heated
at 50 °C for 2 h. The mixture was diluted with water and
extracted into ethyl acetate. The extracts were washed
with water' and brine, and the solvent removed under vacuum.
The residue was puri:Eied by chromatography (MPLC, 0-25 $
MeOH/ethyl acetate; ailica gel) to give 200 mg of the
target compound as a white solid. esi mss (M+H)' - 497.
151
CA 02346933 2001-04-18
WO OOI35449 PCT/US99/30292
EXAMPLE 219
N-(2,5-difluorophenyl)-N'-[2-[[4-(phenylmethyl)-1
piperidinyl]-methyl]phenyl]urea.
14
A solution of aniline 167 (Scheme 26; (R - H)) (140
mg, 0.5 mmol) in THF is treated with 2,5-difluoro-
isocyanate (80 mg, 0.5 mmol) at room temperature for 1 h.
The solvent is removed under vacuum and the residue was
purified by chromatography (MPLC, 20~ EtOAc/Hexane, silica
gel) to give the desired urea as a white solid. esi ms:
(M+H)~ - 436.
EXAMPLE 220
N-(2,5-difluorophenyl)-N'-[[3-[[4-(phenylmethyl)-1-
piperidinyl]methyl)phenyl]methyl]urea.
A solution of 4-benzylpiperidine (1.75 g, 10 mmol) in
mL of DMF was treated with 3-cyanobenzyl bromide 277
20 (1.96 g, 10 mmol) and KzC03 (2.76 g, 20 mmol) and the
reaction mixture stirred at room temperature for 2 h. The
mixture was diluted with water and extracted into ethyl
acetate. The organic extracts were washed successively with
water and brine, and the organic solvent removed under
25 vacuum on a rotary evaporator to give 178 (Scheme 28) as a
. yellow oil.
To a suspension of Raney nickel (2.0 g) in EtOH
(saturated with NH3~gas) ) was added crude 178 (Scheme 28)
(1.45 g, 5 mmol) and hydrogenated at 50 psi for 3 days.
The solution was then filtered and the solvent removed
under vacuum to give the amine 179 as a yellow oil. A
solution of amine 179 (200 mg, 0.68 mmol) in THF is treated
with 2,5-difluoroisocyanate (I15 mg, 0.74 mmol) at room
temperature for 1 hour. The solvent is removed under vacuum
and the residue is washed with 1 NaOH and water to give the
desired urea as a white solid. esi ms: (M+H)+ - 450.
152
CA 02346933 2001-04-18
. WO 00/35449 PCTNS99/30292
EXAMPLE 221
N-(2,5-difluoroph.enyl)-N'-[2-~[[4-(phenylmethyl)-1-
pigeridinyl)acetylJphenyl]urea
To an ice cold solution of 2-bromo-2'-nitro-
acetophenone 170 (2.4 g, 10 mmol) in DMF is added 4-
benzylpiperidine (1.75 g, 10 mmol) and stirred for 30 min.
The solution was poured into a mixture of K2C03 (1.38 g, 10
mmol) in water/ice and. extracted into ethyl acetate. The
.LO ethyl acetate extract was washed several times with water.
The resultant ethyl acetate solution of crude nitroketone
171 is treated with 10~ Pd/C and hydrogenated at 50 psi
hydrogen at room temperature for 40 min. The solution was
then filter, the solvent removed under vacuum, and the
J.5 residue purified by chromatography (MPLC, 30~ ethyl
acetate/hexane; silica. gel) to give 1.8 g of aniline 172 as
a tan/brown solid.
A solution of aniline 172 (Scheme 27) (310 mg, 1.0
mmol) in THF is treated with 2,5-difluoroisocyanate (160
~:0 mg, 1.0 mmol) at room temperature for 1 h. The solvent is
removed under vacuum a.nd the residue is purified by
chromatography (MPLC, 20~ EtOAc/Hexane, silica gel) to give
420 mg of the desired urea-ketone 173 as a white solid. esi
ms: (M+H)' - 464.
~! 5
EX~MgLE 222
N-(2,5-difluorophenyl)-N~-[2-[2-[4-(phenylmethyl)-1-
piperidinyl]-1-hydroxyethyl)phenyl]urea
.!0 A solution of the urea-ketone 17 (260 mg, 0.56 mmol)
in MeOH is treated with NaBHg (400 mg, 11 mmol) at room temp
for 1 hour. The solvent is removed under vacuum and the
residue is treated with 1 N NaOH and extracted into EtOAc.
The extracts are washed with water, brine and the solvent
.15 removed under vacuum t:o give the desired alcohol 174 as a
white solid. esi ms: LM+H)+ - 466.
153
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
EXAMPLE 223
N-[3-[imino-[4-(phenylmethyl)-1-piperidinyl]methyl]
phenyl]-N'-phenylurea
A solution of 3-cyanoaniline (3.54 g, 30 mmol) in THF
is treated with phenylisocyanate (3.58 g, 30 mmol) at room
temperature for 1 h. The solvent is removed under vacuum
and the residue is titurated with hexane to give 7 grams of
urea 182 (Scheme 29) as a white solid. Urea 182 (1.0 g,
4.2 mmol) is dissolved in EtOH, cooled in an ice bath while
HC1 is bubbled-in for 20 min. The solution is left
standing at room temperature for 24 h. The solvent is
removed under vacuum to give 1.1 g of the imidate 183 as a
white solid. The crude imidate (0.5 g, 1.8 mmol) was
dissolved in EtOH and treated with 4-benzyl-piperidine (1.8
g, 10 mmol) at room temperature for 2 days. The solvent was
removed under vacuum and the residue was purified by
chromatography (MPLC, 0 to 30~ MeOH/EtOAc, silica gel) to
give 200 mg of the desired amidine 184 (Scheme 29) as a
white solid. esi ms: (M+H)' - 413.
EXAMPLE 416
N-(3-methoxyphenyl)-N'-[(1R,2S)-2-[[(4-phenylmethyl)
piperidinyl]methyl]cyclohexyl]urea.
Step a: To a solution of (R,R) amino alcohol 187 [~T. Am.
Chem. Soc. 1996, 118, 5502-5503 and references therein]
( 1 . 9 g, 14 . 7 mmol ) in CHzCl~ ( 50 mL) is added 50 ml of an
aqueous solution of Na2C03 (2.4 g, 28.9 mmol)_ While
stirring, benzyl chloroformate (2.51 g, 14.7 mmol) is added
and the mixture is stirred at room temperature for 1 h. The
organic layer is separated and washed with water and brine.
The solution is concentrated on a rotary evaporator and the
residue is chromatographed on silica gel (30~ ethyl
acetate/hexane) to give 3.1 g (12 mmol) of 188 as a white
solid. 1H NMR (300 MHz, CDC13) 8 7.40-7.29 (m, 5 H) , 5.11
(s, 2 H) , 4.71 (bd, 1 H) , 3 . 76-3 .71 (m, 1 H) , 3 . 53-3 .28 (m,
154
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
3 H) , 2 . 00-1 .95 (m, 1 H) , 1.90-1.09 (m, 8 H) . MS AP' (M+H)'
- 264.3 (100
Step b: A solution of DMSO (2.52 g, 30 mmol) in CHZC12
(50 mL) is cooled to -'78°C. To this solution is added drop-
wise oxalyl chloride (:L.81 g, 14 mmol) and the resulting
solution is stirred fo:r an additional 10 min. Then a
solution of alcohol 18.3 ( 2 . 5 g, 9 . 5 mmol ) in CH~C1~ ( 7 0 ml )
is added via an addition funnel and stirred for 10 min.
Then Et3N (_'>.0 g, 50 rcumol) is added and the solution is
allowed to warm to room temperature. The solution is
diluted with water and the organic layer washed with water,
1 N HC1, and brine. The organic layer is dried over Na2S0,,
filtered, and concentrated to give 2.5 g (9.5 mmol) of the
aldehyde 183 as a white solid. 1H NMR (300 MHz, CDC1,) b
9.59 (d, 3.6 Hz, 1 H), 7.38-7.28 (m, 5 H), 5.07 (m, 2 H),
4.69 (m, 1 H), 3.84 (m, 21 H), 2.19-2.11 (m,l H), 2.09-2.01
( m, 1 H) , :L . 86-1. 75 (m, 3 H) , 1. 54-1.17 (m, 4 H) .
2 iJ
Step c: A solution of aldehyde ,~89 (2.0 g, 7.7 mmol),
4-(4-fluorophenylmethyl)piperidine hydrochloride (1.8 g,
7.8 mmol) in dichloroe~thane (80 ml) was treated with
Na(OAc),BH (3.23 g, 15 mmol) and 1 ml AcOH and stirred
overnight at room temperature. The resulting solution was
diluted with methylene chloride and washed with 1 n NaOH,
water, andbrine. The organic solvents were removed under
vacuum and the residue' chromatographed on silica gel (50$
EtOAc/hex - 100 EtOAc:) to give 3.0 g (6.8 mmol) of ~ as
an oil.
Step d: A solution of 190 (3.0 g, 6.8 mmol) in MeOH
was treated with 1.5 g of 10~ Pd/C and hydrogenated at 50
psi overnight in a Parr apparatus. The mixture was filtered
and the filtrate concentrated on a rotary evaporator to
give 1.8 g (5.9 mmol) of the amine 191 as an oil.
155
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Step e: A solution of amine 191 (200 mg, 0.67 mmol) in
THF is treated with 3-methoxyphenyl isocyanate (110 mg,
0.75 mmol) and the mixture is stirred for 30 min. The
solvent is removed on a rotary evaporator and the residue
is chromatographed on silica gel (50~ EtOAc/hex - 100
EtOAc) to give 250 mg of urea 192 as a solid. MS esi:
(M+H)' - 454.4 (1000 , HRMS (M+H)' - 454.2875.
EXAMPLE 415
N-(3-acetvlphenyl)-N'-f(1R 2S)-2 ff(3S) 3 (4
fluorophenvl)methvlloiraeridinyllmethyllcyclohexyllurea
Step a: To a solution of (R, R) amino alcohol 187 [J.Org.
Chem. 1996, 51, 5557-5563; J. Am. Chem. Soc. 1996, 118,
1S 5502-5503] (9.5 g, 73.8 mmol) in CH=C12 (200 mL) is added
200 ml of an aqueous solution of Na~CO, (15 g, 141 mmol).
While stirring, benzyl chloroformate (12.6 g, 73.8 mmol) is
added slowly and the mixture is stirred at room temperature
for 1 h. The organic layer is separated and washed with
2D water and brine. The organic solvent is removed on a rotary
evaporator to give a white solid. The solid is
recrystallized from hexane to give 16.3 g (62 mmol) of the
alcohol 188 (Scheme 31a)as a white solid. 1H NMR (300 MHz,
CDCI~} $ 7.40-7.29 (m, 5 H), 5.11 (s, 2 H), 4.71 (bd, 1 H),
25 3.76-3.71 (m, 1 H), 3.53-3.28 (m, 3 H), 2.00-1.95 (m, 1 H),
1.90-1.09 (m, 8 H) . MS AP' (M+H)' - 264.3 (100
Step b: A solution of DMSO (36 g, 430 mmol) in CHzClz (200
mL) is cooled to -78°C. To this solution is added drop-wise
30 oxalyl chloride (27.41 g, 216 mmol) and the resulting
solution is stirred for an additional 10 min. A solution of
alcohol 188 (38 g, 144 mmol) in CH,C12 (150 ml) is added via
an addition funnel and stirred for 10 min. Then, Et~N (58
g, 570 mmol) is added and the solution is stirred for 20
35 min and the ice bath removed and stirred for an additional
30 min. The solution is diluted with water and the organic
layer separated and washed with water, 1 N HC1, and brine.
The organic layer is dried over NazSO', filtered, and
156
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
concentrated to give ?.8 g of aldehyde 189 as a white solid.
The solid is recrystal.lized from hexane to dive 19.7 grams
of a first crop of alc~ehyde 189 as white needles. A second
crop gave an additional 11 grams. 1H NMR (300 MHz, CDClj) b
9.59 (d, 3.6 Hz, 1 H), 7.38-7.28 (m, 5 H), 5.07 (m, 2 H),
4.69 (m, 1 H), 3.84 (m, 21 H), 2.19-2.11 (m,l H), 2.09-2.01
( m, 1 H), 1.86-1.75 (m, 3 H), 1.54-1.17 (m, 4 H).
Step c: A solution of aldehyde 189 (19.6 g, 75 mmol) and
J.0 ( 3 S) -3 - ( 4- f luorophenyl.methyl ) piperidine ( 14 . 5 g, 75 mmol )
in dichloroethane (40Cr ml) was treated with Na(OAc)3BH (32
g, 152 mmol) and stirred overnight at room temperature. The
resulting solution was. poured slowly into a stirred mixture
of ice/water/1 N NaOH and stirred for 20 min. The organic
1.5 layer was separated and washed water, and brine. The
solution was dried over MgSOa and the organic solvent was
removed under vacuum a.nd the residue chromatographed on
basic alumina (50~ EtC>Ac/hexane) to give 32.1 g (73 mmol)
of amine ~3- as mixture of (15~)cis and traps isomers. 1H
f.0 NMR (300 MHz, CDC1,) b '7 .79 (bs, 1 H) , 7.38-7.29 (m, 5 H) ,
6.95-6.84 (m, 4 H), 5.08 (m, 2 H), 3.71 (m, 1 H, cis isomer
), 3.06 (m, 1 H, trans~ isomer), 2.80 (m, 1 H), 2.55-2.36
(m, 2 H), 2.30 (dd, J ~ 9 Hz, J = 13 Hz, 1 H, traps
isomer), 2.05 (dd, J = 2 Hz, J = 13 Hz , 1 H, traps
25 isomer), 1.81-0.90 (m, 16 H).
Step d: A solution of 193 (32 g, 73 mmol) in MeOH was
treated with 8 g of 10~~ Pd/C and hydrogenated at 50 psi
overnight in a Parr aF~paratus. The mixture was filtered and
30 the filtrate concentrated on a rotary evaporator to give 20
g (65 mmol) of the amine 1~4, which was used without
further purification.
Step e: A solution of amine 1_~ (10 g, 32.8 mmol) in THF is
35 treated with 3-acetylyphenyl isocyanate (5.3 g, 32.8 mmol)
and the mixture is stirred for 30 min. The solvent is
removed on a rotary evaporator and the residue is
157
CA 02346933 2001-04-18
WO 00!35449 PCT/US99/30292
chromatographed on silica gel (0.5:4.5:95 NHaOH/MeOH/CH2Clz)
to give 11 g of urea 295 (Example 415) as a solid. Also
obtained 2 g of cis isomer (Example 416a). The urea Example
415 was further purified by a second chromatography on
silica gel (40:60:1 EtAc/Hex/TEA) and final
recrystallization from ether to give crystalline solid. mp
115-117 °C, [a]DZS - +16.8° (CH30H, c = 0.23 g/dL) . 1H NMR
(300 MHz, CDC1~) 87.86 (m, 1 H), 7.78 (bs, 1 H), 7.68-7.64
(m, 1 H), 7.62-7.59(m, 1 H), 7.38 (t, J = 8 Hz, 1 H), 6.95
6_90 (m, 2 H), 6.79-6.72 (m, 2 H), 6.25 (s, 1 H), 3.21 (dt,
J = 3 Hz, 11 Hz, 1 H), 3.00-2.97 (m, 1 H), 2.66-2.56 (m, 1
H), 2.61 (s, 3 H), 2.44-2.32 (m, 4 H), 2.06 (dd, J = 2 Hz,
J = 13 Hz, 1 H), 1.80-0.86 (m, 15 H). MS esi: (M+H)' - 466.3
(1000 . Anal. Calcd for C28H~6N~OZF: C, 72.23; H 7.70; N,
9.02. Found: C, 72.33; H, 7.91; N, 9.00.
EXAMPLE 415a
N-(3-acetylphenvl)-N~-f(1R,2S)-2-ft(3S)-3-(4
fluorophenyl)methvllpiperidinvllmethyllcyclohexvllurea
Hydrochloride.
A solution of example 415 (15 g, 32 mmol) in 300 ml of THF
was cooled in an ice bath and treated drop-wise with 36 ml
of a 1 M HC1/ether solution. The resulting solution was
stirred for 30 min and concentrated in vacuo. The resulting
solid was titurated with ether and the resulting white
solid dried under high vacuum overnight to give 16 g of the
hydrochloride salt. mp 58-60 °C. [a]DZ5 - +20.0 ° (CH,OH, c =
0.23 g/dL). 1H NMR (400 MHz, DMSO-D6) 8 9.61 (s, 1 H), 9.15
(s, 1 H), 8.00 (m, 1 H), 7.63-7.61 (m, 1 H), 7.51-7.49(m, 1
H), 7.39-7.34 (m, 1 H), 7.22-7.17 (m, 2 H), 7.09-7.04 (m, 2
H), 6.86 (d, J = 8 Hz, 1 H), 3.47-3.31 (m, 4 H), 3.11 (m, 1
H), 2.98-2.82 (m, 2 H), 2.67-2.62 (dd, J = 5 Hz, J = 13 Hz,
1 H), 2.58-2.50 (m, 2 H), 2.52 (s, 3 H), 2.39 (dd, J = 8
Hz, J = 13 Hz, 1 H), 2.16-2.06 (m, 2 H), 1.84-1.556 (m, 7
H), 1.30-1.00 (m, 4 H)_ Anal. Calcd for
158
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
CZBH~7N;02FC1 ~H20 ~THFo.25 : C, 64 .73 ; H 7 . 68 ; N, 7 . 81 . Found: C,
64.89; H, 7.41; N, 7.81.
EXAMPLE 415b
N~(3-acetylphenyl)-N'-f (1R.2S)-. 2-f f (3S)-3-(4-
fluorophenyl)methv7.lpiperidinvllmethyllcvclohexyllurea
Benzenesulfonate.
1.0 Bezenesulfonic acid monohydrate (1.06 g, 6 mmol) was dried
by azeotroping off they water of a benzene solution (twice)
and adding 'the dried acid solution to a solution of example
(2.81 g, 6 mmol) in toluene (40 ml). The solvents were
removed in vacuo (twice) and the resulting residue
recrystallized twice from toluene and dried under high
vacuum overnight give 'x.77 g of benzenesulfonic acid salt
as a white aolid. mp 157-159 °C. [oc]°zs - +16.9 °
(CHlOH, c =
0.23 g/dL) . Anal. Ca7.cd for C~aH4~N~O5FS: C, 65.47; 'H 6.80;
N, 6.75; S, 5.14. Found: C, 65.48; H, 6.80; N, 6.70; S,
5.35.
The compounds of Table 3a and Table 3.1 were prepared
by procedures described in Schemes 26-31A, other examples
and methods taught herein, and procedures familiar to one
skilled in the~art.
TABLE 3a
159
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
R~s w Ras w
i N.E i N.E
0 5~ 1 0
Rya ~ 2 ~N-R3 Rya ~,/IZ~CN-Rs
H 4~ 2 H
P
R~s ~ ~ ~s ~ H
N.E R -~ +N E s
i ~ Z 0 5f li 0
Rya 4~ Z ~N.R3 R'4 /ZEN-Rs
a H 4~ 2 H
r
s
Ex Core RI6 E Z R14 R3 S
#
M+H~
218 1-
p H CHZ (1) (phenylmethyl) 497
NH H _4-
i eridin 1)
2,5-
219 p H CHZ (1) difluorophenyl 436
NH H
2,5-
220 p H CHZ (2) difluorophenyl 450
CHZNH H
2, 5-
221 p H .~ (1) difluorophenyl 464
~,
~ NH H
O
2,5-
222 p H ,~ (1) difluorophenyl 466
~
~ NH H
OH
2 2 p H C=~ ( 2 phenyl
3 )
413
NH H
1-
224 p H CH2 (2) (phenylmethyl) 497
NH H -4_
i eridin 1]
2_(4_
225 p H CHZ (1) fluorophenyl)- 446
H ethyl
160
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
3-
226 p H CHl (1) H hydroxypropyl 382
NH
_ 2-{1-
227 p H CHI {1) H piperidinyl)- 435
NH eth 1
228 p H CHa (1) H (dimethylamino 395
j eth 1
229 p H CH2 (1) H (phenylmethyl) 483
NH -1-piperazine
4-
230 p H CH2 (1) H (phenylmethyl) 482
NH -1-piperidine
(l, 3-
231 p H CH2 (1) H benzodioxol-5- 458
NH ylmethyl)
2,2-
232 p H CH2 (1) H (diphenyl)ethy 504
NH 1
4-(4-
233 p H CHz (1) H chlorophenyl)- 518
NH 4-hydroxy-1-
piperidine
4-phenyl-4-
234 p H CHZ (1) H hydroxy-1- 484
NH piperidine
4-phenyl-1-
235 p H CH2 (1) H piperidine 468
NH
(1H)-indazol-
236 p H CH2 (1) H 5-yl 440
NH
(1H)-indazol-
237 p H CH2 (1) H 6-yl 440
NH
phenylmethyl
238 p H CH2 (1) H 414
NH
_ 1, 3-
239 p H CH2 (1) H benzodioxol-5- 444
i
1
161
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
240 (3 4) 1-
P H CH (1)
z (phenylmethyl)
541
NH o _4_
~ piperidinyl]
(3-4) 2-(4-
241 H
p CHZ (1) fluorophenyl)- 490
o ethyl
'O
242 (3-4) 4- ( (2-
H
P CH2 (1) phenyl)ethyl) 541
NH
'O -1-piperazine
243 (3-4) (1H)-indazol-
H
P CH2 (1) 5-yl 484
NH
~O
244 (3-4) (1H)-indazol-
H
p CH2 ( 1 6-yl 484
)
NH
'O
245 (3-4) benzothiazol-
P H CH2 (1) 6-yl 501
NH
'O
246 f2- (4-
p H CHz (1) (4) fluorophenyl)- 462
OH ethyl
1-
247 p H CH2 (1) (4) (phenylmethyl) 513
NH OH _4-
i eridin 1]
(3-4) 3-phenylpropyl
248
p H CH2 (1) 486
NH
'O
249 (1H)-indazol-
P H CH 2
Z ( H 5-yl 440
)
NH
250 f 2- (4-
p H CH (2)
z H fluorophenyl)- 446
ethyl
162
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
2 ~ 5 - _
251 p H bond (1) H difluorophenyl 422
NH
Phenyl
252 p H CHI (1) H 400
NH
_ 4-
253 p H CHz (1) H methoxyphenyl 430
3_
254 p H CH2 (1) H methoxyphenyl 430
NH
3_
255 q 4-F CH2 (2) H methoxyphenyl 454
NH
3-acetylphenyl
256 q 4-F CHz (2) H 466
NH
257 r H CHZ (1) H methoxyphenyl 430
NH
3-cyanophenyl
258 p H CHz (2) H 425
NH
3-cyanophenyl
259 p H CH2 (3) H 425
NH
260 p H CH2 (3) H methoxyphenyl 430
NH
2-phenylethyl
261 p H CHz (3) H 428
NH
3-carboethoxy-
2 62 p H CHZ ( 1 H phenyl 472
)
NH
3-cyanophenyl
263 p H CH2 (1) H 425
NH
phenyl
264 p 4-F CHZ (1) H 418
NH
phenyl
265 p H CHz (1) H 490
N-
Benzyl
3-cyanophenyl
266 p CHz (1) H 515
H
N_
Benzyl
163
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
267 2-Phenylethyl
p H CHz (1) H 428
NH
(3-4) 3-cyanophenyl
268 p H CH2 (1) 469
NH
O
(3-4) 3-carboethoxy-
269 p H CH2 (1) phenyl 516
NH
'O
270 ~ (3-4) 4-carboethoxy-
P H CHz (1) phenyl 516
NH
'O
271 phenyl
p H CHz (1) (4)
416
NH OH
3-cyanophenyl
272
p H CH2 (1) (4)
441
NH OH
273 (4 ) 3-
P H CHz (1) methoxyphenyl 524
W CH3
274 (4) Trans-2-
P H CH2 (1) phenyl- 534
cyclopropyl
-O CH3
275 (3) 3-cyanophenyl
P H CH2 ( 1 483
)
NH COZMe
276 (3 ) 3-
p H CH2 (1) methoxyphenyl 488
NH COZMe
164
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
(4) 3-cyanophenyl
277 p H CHz (1) 519
NH O~~
O
S
-O~ CHa
(3) 3-
278 p H CHz (1) methoxyphenyl 460
NH ~H
(3) 3-cyanophenyl
~
279 p H CHz (1) 455
NH ~H
(4) 3-cyanophenyl
280 p 4-F CHZ (1) 501
NH CO~Me
(5) 3-cyanophenyl
280a p 4-F CHz (1) 501
NH COzMe
(5) 3-cyanophenyl
280b p 4-F CH2 (1)
500
NH CONMe
(5) 3-cyanophenyl
280c p 4-F CHI (1) 486
NH CONHz
(5) 3- (1-
280d P 4-F CH2 (1) hydroxyethyl)- 520
NH COzMe phenyl
(5) phenyl
280e r H CHz (1) 458
NH C02Me
( 5 ) phenyl
280f P 4-F CHl (1) 462
NH COZH
(5) 3-cyanophenyl
280g r H ~H2 (1) 483
NH CO~Me
(5) ~ 3_
280h r H CHz (1) methoxyphenyl 488
NH COzMe
f
165
CA 02346933 2001-04-18
WO 00135449 PCT/US99130292
(5) 3-acetylphenyl
2801 r H CHZ (1) 500
NH COzMe
(5) 3-acetylphenyl
280j p 4-F CH2 (1) 518
HC1 NH COzMe
( sa
lt)
(5) 3-cyanophenyl
280k p 4-F CHZ (1) 501
HC1 NH CO.Me
( sa '
1 r.
)
(4) phenyl
281 p 4-F CHz (1) 476
NH COZMe
(5) phenyl
281a p 4-F CHZ (1) 476
NH COzMe
(5) phenyl
281b p 4-F CHz (1) 475
NH CONMe
(5) phenyl
281c p 4-F CH2 (1) 461
NH CONHz
3-
282 p 4-F CHz (1) (4) metho
xyphenyl 506
NH
CO Me
282a
p 4-F CH2 (1) (5) methoxyphenyl 506
NH
CO Me
(5) 3-
282b p 4-F CH2 (1) methoxyphenyl 505
NH CONMe
(5) 3-acetylphenyl
282c p 4-F CHz (1) 518
NH COzMe
(5) 3-acetylphenyl
282d p 4-F CHz (1) 517
NH CONMe
(5) 3-acetylphenyl
282e p 4-F CHZ (1) 503
NH CONHZ
166
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
(4) 3-cyanophenyl
283 p 4-F CHz (1) 473
NH OOH
3-cyanophenyl
284 p 4-F CHz (1) (3-4) 493
fused
Phenyl
3-
285 p 4-F CHz (1) (3-4) methoxyphenyl 498
fused
Phenyl
3-cyanophenyl
286 p 4-F CHz (1) (4) 562
NH
-CONPh
3-cyanophenyl
286a p 4-F CHz (1) (5) 562
NH
-CONPh
3-acetylphenyl
286b p 4-F CHz (1) (5) 579
NH
-CONPh
(4) 3-
287 p 4-F CHz (1) methoxyphenyl 478
NH w,\,~OH
(4) 3-cyanophenyl
288 p 4-F CHz (1)
500
NH CONMe
(4) 3-cyanophenyl
288a p 4-F CHz (1) 500
HC 1 NH CONMe
( s.
a
lt)
(5) 3-acetylphenyl
288b p 4-F CHz (1)
517
HC1 ( NH CONMe
s,a
lt)
(5) 3-acetylphenyl
288c p 4-F CHz (1) 574
NH CON
( CHz
) z
NMez
(5) 3-acetylphenyl
288d p 4-F CHz (1) 557
NH CON
(CHz)z
NMez
167
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
(5) 3-acetylphenyl
288e p 4-F CHz (1) 453
NH CON
C3Hs
(5) 3-acetylphenyl
288f p 4-F CH2 (1)
531
NH CON
C3Hs
(5) 3
2888 p 4-F CH2 ~ (1) metho 519
xyphenyl
NH CONMe2
(5) 3-acetylphenyl
288h p 4-F CH2 (1}
531
NH CONMe2
(5) 3-acetylphenyl
2881 p 4-F CHZ (1)
580
NH CON (
2 -
pyridi
nyl)
(5) 3-
288j p 4-F CHz (1) methoxyphenyl 568
NH CONMez
2,5-
289 p H CHz (1) H difluorophenyl 450
CH2NH
3-cyanophenyl
290 p H CH2 (1) H 439
CH2NH
3-carboethoxy-
291 p H CHz (I) H phenyl 486
CH2NH
3-
292 p H CHz (1) H metho
xyphenyl 444
CHzNH
4-
293 p H CH2 (1} methoxyphenyl 444
CH2NH H
3-
294 p H ~,~ (1) H methoxyphenyl 460
~
~ NH
OH
3-
295 r H ,~ (1) H methoxyphenyl 460
~
~ NH
OH
168
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
3-cyanophenyl
296 p H ,~ (1) H 455
~~ NH
Ohl
3-carboethoxy-
297 p H ,~ (1) H phenyl 502
~
~ NH
Oh-I
phenyl
298 p H .~ (1) H 430
~
~ NH
C~h-I
(5) phenyl
299 p 4-F CHz (1) 448
LVH OOH
S 3 phenyl
~~
r
3 00 p H ~- ( 1 443
)
NOH NH H
phenyl
301 p H .~ (2) H 428
~.
~ NH
O
phenyl
302 p H ,~ (2) H 430
~.~ NH
OH
phenyl
3 03 p 4-F .~ ( 1 H 448
)
~.~ NH
Oti
3-
3 04 p 4-F ,~ ( 1 H methoxyphenyl 478
~ )
~I NH
S ~'-
a-~
3-cyanophenyl
3 05 p 4-F ,~ ( 1 H 473
)
~r~ NH
a-~
( 3-4 3 -cyanophenyl
)
306 p H ,~ (1) O 499
~,~ NH
OIH p
169
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
3-cyanophenyl
307 p H CHz- (1) H 439
CH2 NH
3-cyanophenyl
308 p 4-F CH2- (1) H 457
CHZ NH
3-
309 p H CHZ- (1) H methoxyphenyl 444
CHZ NH
3-
310 p 4-F CH2CH~ ~ ( H methoxyphenyl 462
1
)
NH
3-
311 r H CH2- (1) H methoxyphenyl 444
CHz NH
3-acetylphenyl
312 p 4-F CH2- (1) H
474
CH2 NH
4-fluorophenyl
313 p 4-F CH2- (1) H 450
CH2 NH
1-adamantyl
314 p 4-F CHZ- (1) H 490
CHz NH
( 3-4 3 -cyanophenyl
)
315 s H CHZ (1) ~ 483
NH
(M+)
3-cyanophenyl
316 s H CH2 (1) (4) 455
NH OH (M+)
3-cyanophenyl
317 s H CH2 (1) (4) 539
o- ( M+
)
(2-THP)
170
CA 02346933 2001-04-18
WO 00/35449 PCTNS99/30292
TABLE 3.1
R~s Rts
/ %~ / /
2 ~N Z N
a
HN~NH R3 HN-~-NH R3
0 0
R~s
-,.
y
--N
HN~-NH R3
0
b
Ex Core R16 Stereo- Salt MS
# 3 f
R M+H
chemistry Form
400 a H 1,2 traps - 3-methoxylphenyl 436
racemic
401 a 4-F 1,2 traps - 3-methoxylphenyl 454
racemic
402 a H 1,2 cis - 3-methoxylphenyl 436
racemic
403 a 4-F 1,2 traps - 3-cyanophenyl 449
rac emi
c
403a a 4-F 1,2 traps - 3-acetylphenyl 466
racemic
403b a 4-F _ - 3-nitrophenyl 469
1,2 traps
racemic
403c a 4-F 1,2 traps - 4-nitrophenyl 469
racemic
403d a 4-F 1,2 t.rans - 4-pyridinyl 425
racemic
403e a 4-F 1,2 t.rans HC1 ~ 3-acetylphenyl 466
racemic
403f a 4-F 2,2 t,rans - (1H)-indazol-5-yl 464
racemic
404 a 4-F _ - 3-acetylphenyl 466
1S,2R
405 a 4-F 1.S,2R - 3-cyanophenyl 449
406 a 4-F 1S,2R - 3-methoxylphenyl 454
171
CA 02346933 2001-04-18
WO OOI35449 PCT/US99/30292
407 a 4-F 1S,2R - phenyl 424
408 a 4-F 1R,2S - 3-acetylphenyl 466
409 a 4-F 1R,2S - 3-cyanophenyl 449
410 a 4-F 1R,2S - 3-methoxyphenyl 454
411 a 4-F 1R,2S - phenyl 424
412 a 4-F 1R,2S - phenylmethyl 438
413 a 4-F 1R,2S - (1H)-indazol-5-yl 464
414 a 4-F 1R,2S - (1H)-indol-5-yl 463
414a b H 1,2 traps - 3-methoxyphenyl 464
(3RS)
racemic
414b b H 1,2 traps - 3-cyanophenyl 431
(3RS)
racemic
414c b H 1,2 traps - 3-acetylphenyl 448
(3RS)
racemic
414d b 4-F 1,2 traps - 3-acetylphenyl 466
(3RS)
racemic
414e b 4-F 1,2 traps - 3-cyanophenyl 449
(3RS)
racemic
414f b 4-F 1,2 traps - 3-methoxyphenyl 454
(3RS)
racemic
414g b 4-F 1,2 traps - 3-nitrophenyl 469
(3RS)
racemic
415 b 4-F 1R,2S,3S - 3-acetylphenyl 466
415a b 4-F 1R,2S,3S HC1 3-acetylphenyl 466
415b b 4-F 1R,2S,3S Besyl 3-acetylphenyl 466
416 b 4-F 1R,2S,3R - 3-acetylphenyl 466
416a b 4-F 1R,2R,3S - 3-acetylphenyl 466
416b b 4-F 1R,2S,3R HC1 3-acetylphenyl 466
417 b 4-F 1R,2S,3S - 3-cyanophenyl 449
418 b 1 4-FI1R,2S,3R - 3-cyanophenyl 449
~
172
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
419 b 4-F 1R,2S,3S - 3-methoxylphenyl 454
420 b 4-F 1R,25,3R - 3-methoxylphenyl 454
421 b 4-F 1R,2S,3S - 4-fluorohenyl 442
422 b 4-F 1R,2S,3R - 4-fluorohenyl 442
423 b 4-F 1R,2S,3S - phenyl 424
424 b 4-F 1R,2S,3S - (1H)-indazol-5-yl 464
425 b 4-F 1R,2S,3S - (1H)-indazol-6-yl 464
426 b 4-F 1R,2S,3S - benzthiazol-6-yl 481
427 b 4-F 1R,2S,3S - (1H)-indol-5-yl 463
428 b 4-F 1R,2S,3S - (1H)-indol-6-yl 463
429 b 4-F 1R,2S,3S - (1H)-2,3- 491
dimethylindol-5-1
430 b 4-F 1R,2S,3S - benzimidazol-5-yl 464
431 b 4.-F 1R,2S,3S - indolin-S-yl 465
432 b 4-F 1R,2S,3S - 3-cyano-4- 467
fluoro hen 1
433 b 9:-F 1R,2S,3S - 3-acetyl-4- 484
fluoro hen 1
434 b 9:-F 1R,2S,3S - 3,5-diacetylphenyl 508
435 b 4-F 1R,2S,3S - 3-(1- 468
hydroxyethyl)-
hen 1
436 b 4.-F 1R,2S,3S - 4-methyl-thiazol- 445
2- 1
437 b 4-F 1R,2S,3S - 4-methyl-5-acetyl- 487
thiazol-2-yl
438 b 9:-F 1R,2S,3S - 1,3,4-thiadiazol- 432
2- 1
439 b 4-F 1R,2S,3S - 4-chlorol- 515
benzthiazol-2- 1
440 b 4-F 1R,2S,3S - thiazol-2-yl 431
441 b 4:-F 1R,2S,3S - 5-methyl-isoxazol- 429
3-yl
442 b 4-F 1R,2S,3S - 1-methyl-pyrazol- 428
3- 1
443 b 4-F 1R,2S,3S - 4-(1,2,4-triazol- 491
1- 1) hen 1
443a b 9-F 1R,2R,3S - 4-(1,2,4-triazol- 491
1- 1) hen 1
444 b 4-F 1R,2S,3S - (1H)-3-chloro- 499
indazol-5- 1
173
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
445 b 4-F 1R,2S,3S - 4-fluorophenyl 492
446 b 4-F 1R,2S,3S - 4-chlorophenyl 458
447 b 4-F 1R,2S,3S - 4-bromophenyl 502
448 b 4-F 1R,2S,3S - 3-bromophenyl 502
449 b 4-F 1R,2S,3S - 3-fluorophenyl 442
450 b 4-F 1R,2S,3S - 3,4-difluorophenyl 460
451 b. 4-F 1R,2S,3S - 3-chloro-4- 476
fluoro hen 1
452 b 4-F 1R,2S,3S - 3,5-dichiorophenyl 492
453 c 4-F 1R,2S,3S - 3-acetylphenyl 452
454 c 4-F 1R,2S,3R - 3-acetylphenyl 452
455 c 4-F 1R,2R,3S - 3-acetylphenyl 452
456 c 4-F 1R,2S,3S - 3-cyanophenyl 435
457 c 4-F 1R,2S,3R - 3-cyanophenyl 435
458 c 4-F 1R,2R,3S - 3-cyanophenyl 435
458a c 4-F 1R,2R,3R - 3-cyanophenyl 435
459 c 4-F 1R,2S,3S - phenyl 410
460 c 4-F 1R,2S,3R - phenyl 410
461 c 4-F 1R,2R,3S - phenyl 4I0
462 b 4-F 1R,2S,3S - (1H)-5-amino- 464
indazol-1- 1
463 b 4-F 1R,2S,3S - 3-chlorophenyl 458
464 b 4-F 1R,2S,3S - 3-fluoro-4- 456
meth 1 hen 1
465 b 4-F 1R,2S,3S - 3-cyano-4-(1- 515
razol 1) hen 1
466 b 4-F 1R,2S,3S - 2-methylphenyl 454
467 b 4-F 1R,2S,3S - 2-methylphenyl 438
468 b 4-F 1R,2S,3S - 2,4-dimethylphenyl 452
469 b 4-F ~R,2S,3S - 2,4- 484
dimetho hen 1
470 b 4-F 1R,2S,3S - 2,5- 484
dimetho henyl
174
CA 02346933 2001-04-18
PCTNS99l30292
~WO 00/35449
X71 b 4-F 1R,2S,3S - 2-methoxy-5- 468
me thy lphen. 1
472 b 4-F 1R,2S,3S - fluoro hen 1 456
473 b 4-F 1R,2S,3S~ - 3,5-bis((1H)-1- 588
methyltetrazol-5
yl ) phen 1
474 b 4-F 1R,2S,3S - (3-((1H)-1- 506
methyltetrazol-5-
yl ) hen 1
475 b 4-F 1R,2S,3S - (4- 517
(carboethoxymethYl
)thiazol-2- 1
476 b 4--F 1R,2S,3S - 5-bromothiazol-2- 509
1
477 b 4--F 1R, 2S , 3 S -- 4 , 5-di ( 4- 619
fluorophenyl)thiaz
0l-2- 1
478 b 4-F 1R,2S,3S - 2-fluorophenyl 442
479 b 4-F 1R.2S, 3S - 2-chloroplzenyl 458
480 b 4-F 1R, 2S, 3S CF.,CO,H indanon-6-Y1 478
i
481 b 4-F 1R,2S,3S CF,CO,H indanon-4-yl 478
482 b 4-F 1R, 2S, 3S CF,CO,H 4- 466
(iso ro 1) hen 1
483 b 4-F 1R, 25, 3S CF,CO,H 3-vitro-4- 483
~eth 1 hen 1
484 b 4-F 1R, 2S, 3S CF,CO,H trans-2- - ~ 464
phenylcycioprop-1
485 b 4-F 1R,2S,3S CF,CO,H 2,4-difluorophenyl 460
486 b 4-F 1R,2S,3S CF,CO,H 2,5-difluorophenyl 460
487 b 4-F 1R.2S,3S CF,CO,H 2,4-dichlorophenyl 492
488 b 4-F 1R. 2S, 3S CF,CO,H 2, 5-dichlorophenyl 492
489 b 4-F 1R, 2S, 3S CF,CO,H 2-methoxyphenyl 454
490 b 4-F 1R.2S,3S CF,CO,H 2,4-dimethoxy- 484
her~~~
491 b 4-F 1R,2S.3S CF,CO,H 2,5- 484
dimetho henyl
492 b 4-F 1R, 2S, 3S CF,CO,H 2- 492
trifluoromethylyph
en 1
493 b 4-F 1R,2S,3S CF,CO,H 2-methylphenyl 438
494 b 4-F 1R, 2S, 3S CF,CO,H trifluoromethyly- 492
I I 1 I r,henvl
175
i
CA 02346933 2001-04-18
WO 00/35449
PCT/US99/30292
495 b 4-F 1R,2S,3S CF,CO,H 3-methylphenyl 43
8
496 b 4-F 1R, 2S, 3S CF CO H 4-methoxyphenyl 4
54
497 b 4-F 1R, 2S, 3S CF~CO,H 4-carboethoxy-
496
henyl
498 b 4-F 1R, 2S, 3S CF,CO,H 4- 4
92
trifluoromethyly-
hen 1
499 b 4-F 1R,2S,3S CF CO H 4-methylphenyl
38
S00 b 4-F 1R,2S,3S CF CO H 2-fluorophenyl
442
SO1 b 4-F 1R, 2S, 3S CFzCO,H 2-chloropheny 4
58
502 b 4-F 1R, 2S, 3S CF,CO,H 2-nitrophen 1
Y 469
503 b 4-F 1R, 2S, 3S CF~CO,H 2, 4-dichlorophenyl 5
63
504 b 4-F 1R, 2S, 3S CFtCO,H 3-nitrophenyl
469
505 b 4-F 1R, 2S, 3S CF~CO,H 3 , 5-di
S60
ltrifluoromethyly)
506 -phenyl
b 4-F 1R, 2S, 3S CF~CO,H 2, 4- 452
dimeth 1 henyl
507 b 4-F 1R,2S,3S CF~CO,H 2,4-dimethoxy-5_
518
chloro hen 1
508 b 4-F 1R, 2S, 3S CF CO H 3, 4, 5-
514
trimetho hen 1
509 b 4-F 1R,2S,3S CF CO H 3,5-dimethylphen 1
Y 452
510 b 4-F 1R,2S,3S CF,CO,H 3-trifluoromethyl-
52 6
4-chloro hen 1
511 b 4-F 1R, 2S, 3S CF,CO,H 4-phenoxyphen 1
Y 516
512 b 4-F 1R,2S,3S CF~CO,H 4-ethoxyphen 1
Y 468
513 b 4-F 1R, 2S, 3S CF,CO,H 4-thiomethylphen 1
Y 470
5I4 b 4-F 1R, 2S, 3S CF~CO,H 2-naphthyl
474
515 b 4-F 1R, 2S, 3S CF~CO,fi 4-acetylphenyl
466
516 b 4-F 1R,2S,3S CF CO H 2,6-dichloro-
493
ridin-4-yl
517 b 4-F 1R,2S,3S CF CO H 5-indan-4-yl
464
518 b 4-F 1R, 2S, 3S CF1CO,H 4-chloronaphth-1-
508
1
519 b 4-F 1R, 2S, 3S CF,CO,H 3-fluoro-4-
472
methoxvwhenyl
520 b 4-F 1R, 2S, 3S CF,CO,H 4-
502
fmethylsulfonyl)-
phenvl)
176
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
521 b 4-F 1R, 2 S, CF,CO,H 3- 502
3S
(methylsulfonyl)-
hen 1
522 b 4-F 1R, 2S, CF,CO,H 2- ( ( 1H) -pyrrol-1-489
3S
)
523 b 4-F 1R, 2S, CF,CO,H 1, 3-ben 468
3S zodioxol-5-
1
524 b 4-F 1R,2S,3S CF,CO,H 1-acetylindolin-6- 507
1
525 b 4-F 1R, 2S, CF,CO,H 4- (6- 571
3S
rnethylbenzothiazol
-2- 1) hen 1
526 b 4-F 1R, 2S, CF,CO,H 4- ( (2, 2- 523
3S
dimethylpropanoyl)
amino) hen 1
527 b 4-F 1R, 2S, CF,CO,H 4- (1- 506
3S
methyltetrazol-5-
1)phen 1
528 b 4-F 1R, 2S, CF.,CO,H4- ( 1- 509
3S
mo holino) hen 1
529 b 4-F 1R, 2S, CF.,CO,Hquinolin-8-yl 475
3S c
530 b 4-F 1R, 2S, CF.,CO,H3-hydroxyphenyl 440
3S I
531 b 4-F 1R, 2S, CF.,CO,H4- (acetylamino) 481
3S -
hen 1
532 b 4-F 1R, 2S, CF.,CO,H4-hydroxyphenyl 440
3S
533 b 4-F 1R,2S,3S CF,CO,H 3-hydroxy-4- 470
metho hen 1
534 b 4-F 1R, 2S, CF,CO,H 3- (acetylamino) 48I
3S -
hen 1
535 b 4-F 1R,2S,:3S CF,CO,H 4-fluoro-3- 456
meth 1 hen 1
536 b 4-F 1R,25,:3S CF,CO,H 3-methoxy-4- 468
meth 1 hen 1
537 b 4-F 1R, 2S, CF,CO,H 4-chloro-3- 472
:3S
meth 1 hen 1
538 b 4-F 1R, 2S, CF,CO,H 4- (N- 4g1
3S
methylcarboxamide)
hen 1
539 b 9-F 1R,2S,:3S CF,CO,H 1-adamantyl 482
540 b 4-F 1R, 2S, CF,CO,H quinolin-5-yl 475
3S
541 b 4-F 1R, 25, CF,CO,H quinolin-6-yl 475
3S
542 b 4-F 1R, 2S, CF,CO,H 1, 4-benzodioxan-6- 482
3S
1
_
543 b 4-F 1R,2S,3S CF,CO,H isoquinolin-5-yl 475
544 b 4-F 1R, 25, CF,CO,H 4- (sulfonamide) 503
~S -
hen 1
545 b 4-F 1R, 2S, CF,CO,H benzotriazol-5-yl 465
3S
177
CA 02346933 2001-04-18
WO 00!35449 PCT/US99/30292
546 b 4-F 1R,2S,3S CF.~CO~H2-hydroxy-4- 454
methylphenyl
547 b 4-F 1R,2S,3S CF.~CO~H3-hydroxy-4- 454
methyl henyl
548 b 4-F 1R,2S,3S CF~CO~H 2-methyl- 495
benzothiazol-5-yl
549 b 4-F 1R, 2S, CF.~CO~H(4- 468
3S
methoxylphenyl)-
methyl
550 b 4-F 1R,2S,3S CF.~CO~H(4-fluorophenyl)- 456
methyl
551 b 4-F 18,25,35 CF.~CO~H(4-methylphenyl)- 452
meth 1
552 b 4-F 1R,2S,3S (1R)-1- 452
CF~CO~H
( henyl)eth 1
553 b 4-F 1R,2S,3S CF~CO~H 1-acetylindolin-5- 507
1
554 b 4-F 1R,2S,3S CF~CO~H 5,6,7,8- 478
tetrahydronaphth-
1- 1
555 b 4-F 1R,2S,3S CF~CO~H 3-acetyl-4- 482
h dro hen 1
556 b 4-F 1R,2S,3S CF~CO~H 4-(PiPeridin-1- 507
1) henyl
557 b 4-F 1R,2S,3S CF~CO~H cyclohexyl 430
558 b 4-F 1R,2S,3S CF.~CO~H2-methoxyphenyl 468
559 b 4-F 1R,2S,3S CF.~CO~H2.6-dimethylphenyl 452
560 b 4-F 1R,2S,3S CF~CO~H 2-ethylphenyl 452
561 b 4-F 1R, 2S, CF,~CO~H2, 4, 6- 466
3S
trimeth 1 hen 1
562 b 4-F 1R, 2S, CF.~CO~H, 2, 5- 484
3S
dimetho hen 1
563 b 4-F 1R, 2S, CF.~CO~Ht-butyl 404
3S
564 b 4-F 1R,2S,3S CF~CO~H i-Propyl 390
565 b 4-F 1R,2S,3S CF.~CO~HEthoxycarbonyl- 434
meth 1 )
566 b 4-F 1R,2S,3S CF~CO~H ~ 2- 508
trifluoromethoxy-
henyl
567 b 4-F 1R,2S,3S CF~CO~H (1R,S)-1-- 462
(methoxycarbonyl)-
2-meth 1- ro 1
568 b 4-F 1R,2S,3S [(1S)-1- 510
CF.~CO~H
(methoxycarbonyl)-
2-phen leth 1
569 b 4-F 1R,2S,3S CF~CO~H 24,4-trimethyl-2- 460
ent 1
570 b 4-F 1R,2S,3S CF~CO~H 2-Phenylethyl 452
178
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
571 b 4-F 1R,2S,3S CF~CO~H 3-acetylphenyl 466
572 b 4-F 1R,2S,3S CF.~CO~H2-carbomethoxy- 482
hen 1
573 b 4-F _ (1S)-1- 452
1R,2S,3S CF.~CO~H
(rhenyl)ethyl
574 b 4-F 1R,2S,3S CFiCO~H 4-(Phenyl)phenyl 500
575 b 4-F 1R,2S,3S CF~CO~H 1-naphthyl 474
576 b 4-F 1R,2S,3S CF.~CO~H2-(Phenyl)phenyl 500
577 b 4-F 1R,2S,3S CF.~CO~HPhenylmethoxy 454
578 b 4-F 1R, 2S, CF~CO.,H3. 4- 484
3S
dimethoxyphenyl
579 b 4-F 1R, 2S, CF (3H) -2- 520
3S CO~H
~ ethylquinazolin-4-
on-3- 1
580 b 4-F 1R,2S,3S CF~CO,,H3-PYridinyl 425
581 b 4-F 1R,2S,3S CF 6-methoxy-3- 455
CO~H
~ ridin 1
582 b 4-F 1R,2S,3S CF~C07H 2-methylquinolin- 489
8-yl
583 b 4-F 1R,2S,3S CF.~CO?H2-methylnaphth-1- 488
1
584 b 4-F 1R,2S,3S CF. 4-((1H)-1-propyl- 534
CO~H
~ tetrazol-5-
1) hen 1
585 b 4-F 1R,2S,3S CF~CO~H 3-aminophenyl 439
586 b 4-F 1R,2S,3S - 3-(acetylamino)- 481
phenyl
587 b 4-F 1R, 2S, CF.~CO~H3- (N- 481
3S
methylcarbamoyl)-
hen 1
588 b 4-F 1R,2S,3S CF, 2-nitro-4- 499
CO~H
~ metho hen 1
589 b 4-F 1R,2S,3S CF.~CO~H8-hydroxyquinolin- 491
5- 1
590 b 4-F 1R,2S,3S CFzCO~H 3-methylpyridin-2- 439
1
591 b 4-F ~ 1R, 2S, ~ CF.~CO~H~ isoquinolin-1-yl ~ 475
3S
Ex mple 318
179
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
O
N ~0~
~1
Part A: Preparation of 1-t-butvloxycarbonvl 4
benzvlpiperidine
4-benzylpiperidine (10.0 g, 57.1 mmol, 1.0 eq.) was
dissolved in 100 mL of THF under Nz and subsequently cooled
to 0 °C. Di-tert-butyl dicarbonate (11.21 g, 51.3 mmol, 0.9
eq.) dissolved in 50 mL of THF, was added dropwise. Gas
20 evolution was observed. Once gas evolution ceased, the ice
bath was removed. After 20 hours, the THF was removed in
vacuo then the residue was dissolved in EtOAc and rinsed 3X
with 1N citric acid, IX with brine. The organic was dried
over magnesium sulfate and stripped to yield 15.4 g of
colorless oil as
product. Yield = 97.9. NMR (300 MHz, CDC13)8 7.35-7.17
(m,3H); 7.14 (d, 2H, J = 7 Hz); 4.20-3.90 (m, 2H); 2.75-
2.55 (m, 2H); 2.54 (d, 2H, J = 7 Hz); 1.70-1.50 (m, 3H);
1_46 (s, 9H); 1.20-1.00 (m, 2H).
180
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
0 O
II Ij
N~O
+ ~~,OH
er_ythro threo
Part B: Preparation of erythro-and threo-cis-4-benzyl-
1-t-butoxycarbonyl-a-ethylpiperidinemethanol
1-t-butyloxycarbonyl-4-benzylpiperidine (5.0 g, 18.2
mmol; 1.0 eq. ) was dissolved in EtzO at 25 °C under N, and
cooled to -'78 °C. N,N,Td',N'-Tetramethylethylenediamine
(TMEDA) (3.29 mL, 21.8 mmol, 1.2 eq.) was added followed by
the dropwise addition of sec--butyllithium (16.?6 mL, 2;1.8
mmol, 1.2 eq.). The reaction was allowed to warm and stir
1.5 at -30 °C for 30 minutes then again cooled to -78 °C. Once
cool, propionaldehyde (1.31 mL, 20.0 mmol, 1.1 eq.) was
added neat. The reaction was allowed warmed to warm t=o -30
°C then imme:diateiy quenched with 10 mL of water and the
organic layer was separated. The aqueous layer was
extracted 2X more with Et~O. The organic layers were
combined, dried over magnesium sulfate and the solvenr_
removed in vacuo to y.i_eld a colorless oil which was
purified by flash chromatography in 4 . 1 to 1 . 1 hexane/
EtOAc: Obtained 0.68 g of a colorless oil as isomer A,
yield = 11.2% and -0.91 g of a colorless oil as isomer B,
yield = lS.Oo.
Isomer A NMR (300 MHz,, CDC13)b7.40-7.25 (m, 2H); 7.21 (d,
1H, J = 7 Hz); 7.16 (d, 2H, J = 7 Hz); 3.60-3.30 (m, 2H);
2.56 (d, 2H J = 7 Hz); 1.90-1.00 (m, 7H); 1.46 (s, 9H);
1.00-0.70 (m, 5H).
Isomer B NMR (300 MHz, CDC13)8 7.30-7.23 (m, 2H); 7.20 (d,
1H, J = 7 Hz); 7.14 (d, 2H, J = 7 Hz.); 3.60-3.20 (m, 2H);
181
CA 02346933 2001-04-18
WO 00/35449 PCT/US99I30292
2.60-2.40 (m, 2H); 1.90-1.00 (m, 9H); 1.44 (s, 9H); 0.96
(t, 3H, J = 7 Hz) .
O
N-
O
H 1-
erythro
Part C: Structure determination of Isomer B via cyclization
to 4a,6a,7a-4-benzyl-7-ethyl-8-oxa-1-
azabicyclo[4.3.0]nonane-9-one
Isomer B (60 mg, 0.18 mmol, 1 eq.) was dissolved in DMF at
2 5 °C under N2 then NaH ( 7 . 9 mg , 0 .19 8 mmal , 1 eq _ ) was
added. After 20 hours, 2 mL of water was added followed by
EtOAc. The layers were separated. The aqueous layer was
extracted 2X more with EtOAc. The organic layers were
combined, dried over magnesium sulfate, and the solvent
removed in vacuo to yield an oil which was purified over
silica gel in 9:1 to 1:1 hexane/EtOAc. Obtained 30 mg.
Yield = 64~. Product structure confirmed by N.O.E. NMR
(300 MHz, CDCl~) 8 7.40-7.20 (m, 3H); 7.16 (d, 2H, J = 7
Hz); 4.45-4.25 (m, 1H); 4.00-3.80 (m, 1H); 3.65-3.45 (m,
1H); 2.95-2.70 (m, 1H); 2.65-2.45 (m, 2H); 1.85-1.40 (m,
4H) ; 1.40-1 . 00 (m, 6H) .
182
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Part D: Preparation of erythro-cis-4-benzyl-a-
ethylpiperidinemethanol
Erythro-cis-4-benzyl-1-t-butoxycarbonyl-a-
ethylpiperidinemethano:l(isomer B from part B)(815 mg, 2.44
mmol, 1 eq.) was dissolved in 8 mL of ethanol at 25 °C under
N2. NaOH (391 mg, 9.78 mmol, 4 eq.) was added and the
mixture ref:luxed for 4 hours. The solvent was removed .in
vacuo to yield an oil. Water was added followed by EtOAc.
The layers were separated. The aqueous layer was extracted
2X more with EtOAc. The organic layers were combined dried
over magnesium sulfate, and the solvent removed in vacuo to
yield 390 mg of an oil. Yield = 68~. NMR (300 MHz, CDC13)
S 7 . 35-7 .20 (m, 2H) ; 7 .23-7 . 00 (m, 3H) ; 3 .75-3 . 65 (m, l.H) ;
3.20-3.00 (m, 1H); 2.90-2.40 (m, 4H); 1.70-1.50 (m, 2H);
1.50-1.30 (m, 1H); 1.20-0.80 (m, 5H).
2 ~D
O . ,
'_ /~'N ~ \
i ~a
)H O
Part E: Preparation of erythro-cis-4-benzyl-ot-ethyl-1-
(3-N-phthalimido-n-prop-1-yl)piperidinemethanol
2 ~i
Erythro-cis-4-benzyl-ot-ethylpiperidinemethanol
183
CA 02346933 2001-04-18
WO OOI35449 PCT/US99/30292
(195 mg, 0.84 mmol, 1 eq.), N-(3-bromopropyl)phthalimide
(224 mg, 0.84 mmol, 1 eq.), potassium iodide (139 mg, 0.84
mmol, 1 eq.), and potassium carbonate (231 mg, 0.84 mmol, 1
eq.) were refluxed in 10 mL of 2-butanone for 3 hours. The
reaction was worked up by filtering off the inorganic
solids. The filtrate solvent was removed in vacuo to yield
an oil. Purified by flash chromatography in 100 EtOAc
then 4:1 chloroform/MeOH. Obtained 200 mg. Yield = 57$.
NMR (300 MHz, CDClj) 8 7.95-7.80 (m, 2H); 7.80-7.65 (m,
2H); 7.35-7.00 (m, 5H); 3.90-3.60 (m, 3H); 3.20-2.90 (m,
2H); 2.65-2.30 (m, 3H); 2.20-2.00 (m, 2H); 2.00-1.75 (m,
2H); 1.70-1.40 (m, 4H); 1.35-0.90 (m, 3H); 0.96 (t, 3H, J =
7 Hz).
.~ ~NH.,
)H
Part F. Preparation of erythro-cis-1-(3-amino-n-prop-
1-yl)-4-benzyl-a-ethylpiperidinemethanol
Erythro-cis-4-benzyl-a-ethyl-1-(3-N-phthalimido-n-
prop-1-yl)piperidinemethanol(200 mg, 0.48 mmol, 1 eq.) was
dissolved in 5 mL of ethanol at 25 °C under N2. Anhydrous
hydrazine (0.03mL, 0.95 mmol, 2 eq.) was added and the
reaction refluxed for 3 hours during which time a white
precipitate (phthalhydrazide) formed. Once cool, The
solids were filtered. The filtrate solvent was removed in
vacuo to yield an oil which was stirred in EtzO. The
triturated solids were filtered and the filtrate solvent
was removed in vacuo to yield 120 rng of an oil. Yield =
87~. NMR (300 MHz, CDC1,) ~ 7 (t, 2H, J = 7 Hz) ; 7.17
.27 (d,
1H, J Hz); 7.13 (d, 2H, J 7 Hz); 3.70-3.30 (m, 2H);
= 7 =
3.20-3.00(m, 2H); 3.00-2.70 2H); 2.70-2.40 (m, 2H);
(m,
184
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
2.30-2.10 (m., 1H); 2.10-1.90 (m, 2H); 1.90-1.40 (m, 5H);
1.40-1.00 (m, 3H); 0.96 (t, 3H, J = 7 Hz).
O ~ , O
~N ~,~ + .~/~.N~N O
.~ ~ H H H\ H H
)H ~~N I
COI /
O
Part G: preparation of erythro-cis-1-[3-(3-
acetylphenylaminocarbonylamino)-n-prop-1-yl]-4-benzyl-a-
ethylpiperidinemethano:L and erythro-cis-1-[3-(3-
acetylphenylaminocarbonylamino)-n-prop-1-yl]-2-(1-(3-
acetylphenylaminocarbonyloxy)-n-prop-1-yl)-4-
benzylpiperidine
Erythro-cis-1-(3-amino-n-prop-1-yl)-4-benzyl-oc-
ethylpiperidinemethanol (120 mg, 0.41 mmol, 1 eq.) was
dissolved in 5 mL of THF at 25 °C under N2 then 3-
acetylphenyl isocyanate added neat. After 1 hour the
solvent was removed in vacuo to yield an oil. Purified by
flash chromatography in 100$ EtOAc to 4:1 chloroform/MeOH.
Isolated mono-addition product (product A) along with an
additional bis-addition product (product B). Prouct A
yielded 81 mg of an oil. Yield = 43~. Product B yielded
43 mg of an oil.
Product A NMR (300 MHz, CDC1,) 8 7.86 (bs, 1H) ; 7.73 (d, 1H,
J = 7 Hz); 7.60 (s, 1H); 7.56 (d, 1H, J = 7 Hz); 7.40-7.15
(m, 4H) ; 7.12 (d, 2H, ;7 = 7 Hz) ; 6.30-6.05 (m, 1H) ; 4.00-
3.80 (m, 1H); 3.50-3.30 (m, 1H); 3.30-2.90 (m, 5H); 2.60-
2.40 (m, 2H); 2.57 (s, 3H); 2.30-2.10 (m, 1H); 2.10-1.90
185
CA 02346933 2001-04-18
WO 00/35449
PCT/US99/30292
(m. 2H); 1.80-1.40 (m, 5H); 1.30-1.05 (m, 2H); 0.94 (t, 3H,
J = 7 Hz) .
Product B NMR (300 MHz,CDC13) $ 10.80-10.60 (m, 1H);8.20-
8.00 (m, 1H); 7.9I (bs,1H); 7.80-7.18 (m, 9H); 7.11 (d,
2H, J = 7 Hz); 6.20 -6.00(m, 1H); 5.20-5.00 (m, 1H); 3.50-
3.00 (m, 4H); 2.57 (s, H); 2.56 (s, 3H); 2.55-2.00 (m,
3
5H); 2.00-1.00 (m, lOH);1.00-0.80 (m, 3H)
Product A was separated into its enantiomers employing a
Daicel Chiral Pack AD column, eluting with 0.1~
diethylamine in methanol. (-)-isomer [a]p5 (c - 0.300 g/dL,
MeOH) - -I4.9°. (+)-isomer [a]p5 (c = 0.290 g/dL, MeOH) -
+20.2°.
The following compounds can be synthesized by the methods
discussed previously:
186
CA 02346933 2001-04-18
WO 00/35449 PCT/IJS99/30292
TABLE 3b.
4 R3 5
6 / ~ 6 / . 4 R3
3 O 1 ~3
N
~ ~ ~N 2
N~ H H ~D' H
OH H
OH
R1 _~ Ri
a b
5 ~ R 6 /
C' R3
/~3 3 O 1 a ~ 3
1~
~N 2
~N~N 2 ._ f'N' Ii
N/'~ H H H
OH O ~ NH
1
/ v R R2b O ( / R3
Ri
RZ m
c
d
5 4
6 / ~ R3
O 1 ~ ~ 3
~N~N 2
H
H
O~~
O ~ R3
R~ /
a
Cores R1 R2 R2a, R2b R3
M+1
319 a,b H CH3 -- 3-COCH3 438
320 a,b H CH3 --- 4-N02 441
321 a,b H CH3CH2 --- 3-COCH3 452
322 c H -- CH3, CH3 3-COCH3 452
323 a,b H CH3CH2CH2 ~ --- ~ 3-COCH3 466
187
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
324 ' a,b H (CH3)2CH --- 3-COCH3 466
325 a,b H CH3CH2CH2CH2 --- 3-COCH3 480
326 a,b H (CH3)2CHCH2 --- 3-COCH3 480
327 d,e H CH3CH2 --- 3-COCH3 613
328 d,e H CH3CH2CH2 --- 3-COCH3 627
329 d,e H (CH3)2CH --- 3-COCH3 627
330 d,e H CH3CH2CH2CH2 --- 3-COCH3 641
331 d,e H (CH3)2CHCH2 --- 3-COCH3 641
Example 332
Part A Prebaration of N-cvano-N'-3-
methoxvnhenylcarbamimidic acid phenyl ester
/N
N/
\ ~ \
O N O
m-Anisidine (4.56 mL, 4.06 mmol, 1 eq.), and
diphenylcyanocarbonimidate (967 mg, 4.06 mmol, 1 eq.) were
mixed and refluxed in acetonitrile under N2 for 1 hour.
Solids precipitated. The reaction was worked up by
filtering off the solids. Obtained 580 mg as product.
M.P. - 170.0 - I71.0 °C. NMR (300 MHz, DMSO-d6) 8 8.70 -
8.50 (m, 1H); 7.43 (t, 2H, J = 7 Hz); 7.40 - 7.20 (m, 2H);
7.14 (d, 2H, J = 7 Hz); 7.00 - 6.80 (m, 2H); 6.80 - 6.70
(m, 1H); 3.80 (s, 3H).
Part B Preparation of N " -cyano-N'-(3-(4-(4
2 0 f_luorobenzvl ) piperidine 1 propyl-N- ( 3 -methoxvt~henyl ) cruanidine
188
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
~N
F
/ I NON N 0
3-(4-(4-fluorophenylmethyl)piperidin-1-yl)propylamine,
(synthesized in a similar fashion to the previously
described des-fluoro compound} (53 mg, 0.20 mmol, 1 eq.)
and the product from Part A (50 mg, 0.20 mmol, 1 eq..) were
mixed and refluxed :in 2-propanol under N2 for 1 hour. The
reaction was stripped and the residue then purified over
silica gel in 100 ~ ethyl acetate followed by 8:2
chloroform/methanol. Obtained 55 mg of off-white solids as
product. NMR (300 MHz, CDC1~) 8 7.33 (t, 1H, J = 7 Hz);
7.10 - 6.90 (m, 4H); 6.90 - 6.80 (m, 3H); 3.83 (s, :3H);
3.50 - 3.35 (m, 2H); 2.90 - 2.70 (m, 2H); 1.50 - 1.20 (m,
3H). Mass Spec detects 424 (M+H).
Example 334
art A Preparation of ((Methvlthiol(3-acetylnhenvl
amino)lmethylenebroganedinitrile
N~~ ~ N
~S N
O
[Bis(methylthio)methylene]propanedinitrile 3.00 g,
17.6 mmol, 1 eq.), and 3'amino-acetophenone (2.38 g, 17.6
mmol, 1 eq.), were mixed and refluxed under Nz in ethanol
for 16 hours. Solids precipitated while cooling to 25 °C.
The solids were filtered. Obtained 1.86 g of tan solids.
M.P. - 165.0 - 166.5 °C. NMR (300 MHz, DMSO-db) S 10.66
(m, 1H); 7.90 - 7.80 (m, 2H); 7.60 - 7.50 (m, 2H); 2.60 (s,
3H) ; 2 . 54 (s, 3H) .
189
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Part B: Preparation of 2-[(3-acetylanilino)({3-[4-(4-
fluorobenzyl)-1-piperidinyl]propyl~
amino)methylene]malononitrile
N\ / N
F ~ \
NON N
O
3-(4-(4-fluorophenylmethyl)piperidin-1-yl)propylamine,
49 mg, 0_194 mmol, 1 eq.) and the product from Part A (50
mg, 0.194 mmol, 1 eq.) were mixed then stirred under N2
overnight. The reaction was stripped and the residue
purified over chloroform/methanol. Obtained 17 mg of a
white amphorphous solid. NMR (300 MHz, CDC1,) S 7.82 (d,
1H, J = 7 Hz); 7.73(s, 1H); 7.51 (t, 1H, J= 7 Hz); 7.34 (d,
1H, J = 7Hz); 7.10-6.80 (m, 4H); 3.28 (m, 2H); 2.62 (s,
3H); 2.64-2.40 (m,2H); 2.40-2.25 (m, 2H); 2.05-1.70 (m,
2H); 1.70-1.35 (m, 3H); 1.20-0.80 (m, 2H).
Mass Spec detects 460 (M+H).
Example 335
Part A: Preparation of N-[1-(methylthio)-2-
nitroethenyl]-3-acetylbenzenamine
O
H
S N
OZN
A neat mixture of 1,1-bismethylthio-2-nitroethylene
(6.5 g, 38.5 mmol, 10 eq) and 3-aminoacetophenone (0.5 g,
3.85 mmol, leq) was melted together and heated at 140° C for
four hours. The mixture was cooled to room temperature,
190
CA 02346933 2001-04-18
WO 00/35449 PCT~'US99/30292
then subjected to flash chromatography, eluting with 50°s
ethyl acetate/hexanes, to yield 0.63 g of a yellow powder
as product. Yiel~3 = 65~. NMR (300 MHz, CDCl~) b 12_82 (bs,
1H), 7.95-7.91 (m, 2H), 7..59-7.48 (m, 2H), 6.73 (s, 1H),
2.65 (s, 3H), 2.41 (s, 3H).
Part B: Preparation of 1-(3-{[(E)-1-(t-[4-(4-
fluorobenzyl)-1-piperidinyl]propyl}amino)-2-
nitroethylenyl)amino}phenyl)ethanone
O
H H
F ~ ~\/N\.~\,/N N ~ _
O2N
To a suspension of N-[1-(methylthio)-2-nitroethenyl]-
3-acetylbenzenamine (0.30 g, 1.19 mmol, 1.00 eq) in 20 ml
of methanol was added 3-(4-fluorobenzyl)piperidin-1--
yl)propyl.amine (0.3:L g, 1.25 mmol, 1.05 eq), and the
mixture was stirred at room temperature. After three days,
a colorless solution was observed. The solvent was removed
in-vacuo, and the re=sidue was subjected to flash
chromatography, eluting with 10~ methanol/chloroform, to
yield 0.38 g of an orange glass as product. Yield = 70~.
NMR (300 MHz, CDCla) 8 10.51 (bs, 1H) , 7.92 (d, 1H, .j - 8
Hz), 7.72 (bs, 1H), 7.54 (dd, 1H, j - 8 Hz, 8 Hz), 7.35
(bd, 1H), 6.90-6.88 (m, 5H), 6.17 (s, 1H), 3.54 (bs, 2H),
2.92-2.84 (m, 2H), 2.63 (s. 3H), 2.51 (m, 2H), 1.99--1.91
(m, 4H), 1.55-1.50 (m, 3H), 0.88-0.85 (m, 2H). MS (ESI)
detects (M+H)' - 455.
The following compounds can be prepared by procedures
described previously:
191
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
Table 3c
H H ,..~ H H
F i N~N~N~R F i N~.N~N~R3
Z 3 Z
F ~ ~ Z
~,.~-rN H F~,.~'~N..~N~N~Rs
H H H
~'N Z N~R3 d
F i ,~. ~N
H H
i I..~N Z N~R3
Core Z R3 Mass
Spec
M+1
332 a N-CN 3-methoxyphenyl 424
333 a N-CN 3-acetylphenyl 460
334 a C(CN)2 3-acetylphenyl 460
335 a CHN02 3-acetylphenyl 455
336 b N-CN 3-acetylphenyl 436
337 b C(CN)2 3-acetylphenyl 460
338 b NCONH2 3-acetylphenyl 454
339 b CHN02 3-acetylphenyl 455
340 b N-CN 3,5-diacetylphenyl 478
341 b NCONH2 3,5-diacetylphenyl 496
342 b NC02CH3 3,5-diacetylphenyl 511
343 b C(CN)2 3,5-diacetylphenyl
344 b N-CN 3-(1-methyl-1H- 476
tetrazol-5-yl)phenyl
345 b C(CN)2 3-(1-methyl-1H- 500
tetrazol-5-yl)phenyl
346 b NCONH2 3-(1-methyl-1H- 494
tetrazol-5-yl)phenyl
347 b N-CN 2,4-dimethoxy-phenyl 454
192
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
348 b N-CN 5-acetyl-2-methoxy- 466
phenyl
349 d N-CN 3-(1-methyl-1H- 488
tetrazol-5-yl)phenyl
350 c N-CN phenyl 448
351 c N-CN :3-acetylphenyl 490
352 c N-CN 3-cyanopneyl 473
353 c N-CN 2,4-dimethoxyphenyl 508
354 c N-CN 2,5-dimethoxyphenyl 508
355 c N-CN 5-acetyl-2-methoxy- 520
phenyl
356 c N-CN 2,4-dimethylphenyl 476
357 c N-CN 4-(1-methyl-1H- 530
tetrazol-5-yl)phenyl
358 c N-CN 4-(1-propyl-1H- 558
tetrazol-5-yl)phenyl
359 c N-CN 5,6,?,8-tetrahydro- 502
naphthy-2-yl-phenyl
360 c N-CN 4-(4-morpholinyl)- 533
phenyl
361 C N-CN 2,5-dimethylphenyl
362 c N-CN 4-hydroxy-2-
methylphenyl
363 c N-CN 2-methylphenyl
364 c N-CN 2-phenylethyl
365 c N-CN 1-adamantyl
366 c N-CN 2-adamantyl
367 c C(CN)2 3-acetylphenyl 514
368 c C(CN)2 5-acetyl-2-methoxy- 544
phenyl
369 c CHN02 3-acetylphenyl 509
370 a CHN02 3-acetylphenyl 560
371 a N-CN 3,5-diacetylphenyl 583
372 a N-CN 3-acetylphenyl 541
373 a N-CN 4-(1-propyl-1H- 581
tetrazol-5-yl)phenyl
193
CA 02346933 2001-04-18
WO 00/35449 PCT/US99I30292
The following tables contain representative examples
of the present invention, and may be prepared by procedures
described above, or methods familiar to one skilled in the
art. Each entry in each table is intended to be paired
with each formulae at the start of the table. For example,
Entry 1 in Table 4 is intended to be paired with each of
formulae la-44.
I94
CA 02346933 2001-04-18
WO 00!35449 PCT/US99/30292
SABLE 4*
G/~N~ ~ R3 G/~J~N N.R G~N~N~N,R3
H H p 3 H H
la 2a 3a
~;~~N~ ,~ Rz G~,~N~N N.R ~N~/~N~N~R3
H H p 3 H H
lb 2b 3b
H H ~
G~N~'~N~NR3 G~N~'N~N R3 G~N~N~N'R3
H H p H H
G~~N~ G~N G~N
1 H a H
~N.R3 ~'~R3 Hl~'/'N.R3
8a a 9a p 10
G~,~N~ ~~N G~N
a H ~ H H
~~R3 ~N.R3 ~'N:R3
8b p 9b p 11 p
1 H ~ H
HNYN,R3 HNY~R3
12a p 13a O
H ~ H
HN~I~L R3 HN~N'R3
12b p 13b p
G~N.~ G~N
H ~ H
HNYN: R3 HNY1~I. R3
14 p 15 p
195
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
G'~~ G
HN N- HN N-
R3 ~ R3
16a O 17a O
. H ~ H
HN N. HN N_
R3 ~ R3
16b O 17b O
N N
G~~ H R~ _ 1 H
~~R3 ~N.R3
18 O 19 O
G~N~ ~ R3 G~N~'r7~NR3
H H H H
20a 21a
~~I~l~ N~. NR3 ~~1~ N~ NR3
H H H H
20b 21b
~n!\~/~N~N_RZ R~N~/~N~N.R2
R1 H H 1 H H
22 23
G~N~N N.R3 ~;~~N~N N_R ~N~N N.R
G Y 3
O O O
24 25 26
G~N H N N_ R G~.,~N H N N_ R3 G~N~ NY N-R 3
3
Me O Me O Me O
27 28 29
H
H H
G~N H N N. Nw/~.NYN-R3 G N N~N_R3
Y R3
iPrO iPrO iPrO
30 31 32
G~'~ H H H C~ n H H H ~ H H H
N~N~N_R3 ,,/~N~N~N-R3 G N~NYN R3
iBuO iBuO iBuO
33 34 35
G~N H N N. R G~.~N H N N_ R ~N H N N_
3 G R3
Ph O Ph O Ph O
36 37 38
196
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
H H N, G~ H H ~ /""~N H H N.
R3 ~ R3 G ~ R3
P O Ph 0 P O
39 40 41
H ~ G'~ H H N, R ~~N H H ~ R
R ~ 3 G 3
3
Ph
42 43 44
Entry G R3
1 4-F-Ph Ph
2 4-F-Ph 3-CN-Ph
3 4-F-Ph 3-COCH3-Ph
4 _ 4-F-Ph 3-C02Me_-Ph
~~ ~
4-F-Ph 3-C02Et-Ph
6 4-F-Ph 3-C02H-Ph
7 4-F-Ph 3-CONH2-Ph
8 4 -F-Ph 3 -CONHMe-~?h
9 4-F-Ph 3-F-Ph
4-F-Ph 3-C1-Ph
11 4-F-Ph 3-Br-Ph
12 4-F-Ph 3-N02-Ph
13 4-F-Ph 3-NH2-Ph
14 4-F-Ph 3-NHMe-Ph
4-F-Ph 3-NMe2-Ph
16 4-F-Ph 3-NHCOCH3-Ph
1? 4-F-Ph 3-S02NH2-Ph
18 4-F-Ph 3-S02NHMe-Ph
19 4-F-Ph 3-CF3-Ph.
~ 4-F-Ph 3-OCH3-Ph
21 4-F-Ph 3-OPh-Ph
22 4-F-Ph 3-OCF3-Ph
23 4-F-Ph 3-SCH3-Ph
24 4-F-Ph 3-SOCH3-Ph
4-F-Ph 3-S02CH3-Ph
26 4-F-Ph 3-OH-Ph
27 4-F-Ph 3-CH20H-Ph
28 4-F-Ph 3-CHOHCH3-Ph
29 4-F-P:h 3-COH(CH3)2-Ph
4-F-Ph 3-CHOHPh-Ph
31 4-F-Ph 3-CH3-Ph
32 4-F-Ph 3-C2H5-Ph
33 4-F-Ph 3-iPr-Pra
34 4-F-Ph 3-tBu-Ph
4-F-Ph 3-Ph-Ph
36 4-F-Ph 3-CH2Ph-Ph
37 4-F-Ph 3-CH2C02Me-Ph
38 4-F-Ph 3-(1- i eridin 1)-Ph
39 4-F-Ph 3-(1- rrolidin 1)-Ph
197
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
40 4-F-Ph 3-(2-imidazolyl)-Ph
41 4-F-Ph 3-(1-imidazol 1)-Ph
42 4-F-Ph 3-t2-thiazolyl)-Ph
43 4-F-Ph 3-(3- yrazol 1)-Ph
44 4-F-Ph 3-(1- razolyl)-Ph
45 4-F-Ph 3-(1-tetrazol 1)-Ph
46 4-F-Ph 3-(5-tetrazolyl)-~Ph
47 4-F-Ph 3-(2- ridyl)-Ph
48 4-F-Ph 3-(2-thien 1)-Ph
49 4-F-Ph 3-(2-furan 1)-Ph
50 4-F-Ph 4-CN-Ph
51 4-F-Ph 4-COCH3-Ph
52 4-F-Ph 4-C02Me-Ph
53 4-F-Ph 4-C02Et-Ph
54 4-F-Ph 4-C02H-Ph
55 4-F-Ph 4-CONH2-Ph
56 4-F-Ph 4-CONHMe-Ph
57 4-F-Ph 4-CONHPh-Ph
58 4-F-Ph 4-NHCONH2-Ph
59 4-F-Ph 4-F-Ph
60 4-F-Ph 4-Cl-Ph
61 4-F-Ph 4-Br-Ph
62 4-F-Ph 4-N02-Ph
63 4-F-Ph 4-NH2-Ph
64 4-F-Ph 4-NHMe-Ph
65 4-F-Ph 4-NMe2-Ph
66 4-F-Ph 4-NHCOCH3-Ph
67 4-F-Ph 4-S02NH2-Ph
68 4-F-Ph 4-S02NHMe-Ph
69 4-F-Ph 4-CF3-Ph
70 4-F-Ph 4-OCH3-Ph
71 4-F-Ph 4-OPh-Ph
72 4-F-Ph 4-OCF3-Ph
73 4-F-Ph 4-SCH3-Ph
74 4-F-Ph 4-SOCH3-Ph
75 4-F-Ph 4-S02CH3-Ph
76 4-F-Ph 4-OH-Ph
77 4-F-Ph 4-CH20H-Ph
78 4-F-Ph 4-CHOHCH3-Ph
79 4-F-Ph 4-COH(CH3)2-Ph
80 4-F-Ph 4-CH3-Ph
81 4-F-Ph 4-C2H5-Ph
82 4-F-Ph 4-iPr-Ph
83 4-F-Ph 4-tBu-Ph
84 4-F-Ph 4-Ph-Ph
85 4-F-Ph 4-CH2Ph-Ph
86 4-F-Ph 4-CH2C02Me-Ph
87 4-F-Ph 4-(1- i eridin 1)-Ph
88 4-F-Ph 4-(1- rrolidin 1)-Ph
89 4-F-Ph 4-(2-imidazol 1)-Ph
90 4-F-Ph 4-(1-imidazolyl)-Ph
91 4-F-Ph 4-(2-thiazol 1)-Ph
92 4-F-Ph 4-(3-pyrazol 1)-Ph
198
CA 02346933 2001-04-18
WO 00/35449 PCTlUS99/30292
93 4-F--Ph 4- (1-pyrazolyl) -Ph
94 4 -F--Ph 4- ( 1-_tetrazolyl )
-Ph
95 4 -F--Ph 4- ( 5-tetrazol 1 ) -Ph
96 4-F--Ph 4-(2- rid 1)-Ph
97 4-F-Ph 4-(2-thien 1)-Ph
98 _ 4-(2-furanyl)-Ph
4-F--Ph
99 4-F--Ph 2-CN-Ph
0 4 - F--Ph 2 -COCH3 --Ph
101 4-F-Ph 2-C02Me-Ph
102 4-F-Ph 2-C02Et-Ph
103 4-F-Ph 2-C02H-Ph
104 _ 2-CONH2-Ph
4-F--Ph
105 4-F--Ph 2-CONHMe-Ph
106 4-F--Ph 2-F-Ph
107 4-F--Ph 2-C1-Ph
108 4 -F--Ph 2-Br-Ph
109 4-F-Ph 2-N02-Ph
110 4-F-Ph 2-NH2-Ph
111 4-F--Ph 2-NHMe-Ph
212 4-F-Ph 2-NMe2-Ph
113 ~ 4-F-Ph 2-NHCOCH:3-Ph
114 4-F--Ph 2-S02NH2-Ph
115 4-F-Ph 2-S02NHMe-Ph
116 4-F--Ph 2-CF3-Ph
117 4-F--Ph 2-OCH3_-Ph
118 _ 2-OPh-Ph
- 4-F--Ph
119 4-F--Ph 2-OCF3-Ph
120 4-F--Ph 2-SCH3-Ph
121 4 -F-- Ph 2 - SOCH3 - Ph
122 4-F-Ph 2-S02CH3-Ph
123 h 2-OH-F'h
4-F-
P
124 _ 2-CH20H-Ph
_
4-F--Ph
125 4-F-Ph 2-CHOHCH3-Ph
126 ' 4-F-Ph 2-COH(CH3)2-Ph
127 4-F-Ph 2-CHOHPh-Ph
128 4-F~-Ph 2-CH3-Ph
129 4-F-Ph 2-C2H5--Ph
130 4-F-Ph 2-iPr-Ph
131 4-F-Ph 2-tBu-Ph
132 4-F-Ph 2-Ph-Ph
133 4-F-Ph 2-CH2Ph-Ph
134 4-F-Ph 2-CH2C02Me-Ph
135 4-F-Ph 2-(1-piperidin 1)-Ph
136 4-F-Ph 2-(1- rrolidin 1)-Ph
137 4-F-Ph 2-(2-imidazol 1)-Ph
138 4-F-Ph 2- (1-imidazal 1) -Ph
_ 4-F-Ph 2-(2-thiazol 1)-Ph
139
140 4-F-Ph 2-(3-_ razol 1)-Ph
141 _ 2-(1- razo~l 1)-Ph
4-F-Ph
142 4-F-Ph 2-(1-tetrazol 1)-Ph
143 4-F-Ph 2-(5-tetrazo_lyl)-Ph
144 4-F-Ph 2-(2- rid 1)-Ph
145 4-F-Ph 2- (2-thien 1) -Ph
199
CA 02346933 2001-04-18
WO 00/35449 PCT/US99130292
146 4-F-Ph ~~ 2-(2-furanyl)-Ph
147 4-F-Ph 2,4-diF-Ph
148 4-F-Ph 2,5-diF-Ph
149 4-F-Ph 2,6-diF-Ph
150 4-F-Ph 3,4-diF-Ph
151 4-F-Ph 3,5-diF-Ph
152 4-F-Ph 2,4-diCl-Ph
153 4-F-Ph 2,5-diCl-Ph
154 4-F-Ph 2,6-diCl-Ph
155 4-F-Ph 3,4-diCl-Ph
156 4-F-Ph 3,5-diCl-Ph
157 4-F-Ph 3,4-diCF3-Ph
158 4-F-Ph 3,5-diCF3-Ph
159 4-F-Ph 5-C1-2-Me0-Ph
160 4-F-Ph 5-C1-2-Me-Ph
161 4-F-Ph 2-F-5-Me-Ph
162 4-F-Ph 2-F-5-N02-Ph
163 4-F-Ph 3,4-OCH20-Ph
164 4-F-Ph 3,4-OCH2CH20-Ph
165 4-F-Ph 2-Me0-4-Me-Ph
166 4-F-Ph 2-Me0-5-Me-Ph
167 4-F-Ph 1-na hth 1
168 4-F-Ph 2-na hth 1
169 4-F-Ph 2-thienyl
1?0 4-F-Ph 3-thien 1
171 4-F-Ph 2-furanyl
172 4-F-Ph 3-furanyl
173 4-F-Ph 2- rid 1
174 4-F-Ph 3- rid 1
175 4-F-Ph 4- ridyl
176 4-F-Ph 2-indolyl
177 4-F-Ph 3-indol 1
178 4-F-Ph 5-indol 1
179 4-F-Ph 6-indol 1
180 4-F-Ph 3-indazolyl
181 4-F-Ph 5-indazol 1
182 4-F-Ph 6-indazol 1
183 4-F-Ph 2-imidazol 1
184 4-F-Ph 3- yrazolyl
185 4-F-Ph 2-thiazol 1
186 4-F-Ph 5-tetrazol 1
187 4-F-Ph 2-benzimidazolyl
188 4-F-Ph 5-benzimidazolyl
189 4-F-Ph 2-benzothiazol 1
190 4-F-Ph 5-benzothiazol 1
191 4-F-Ph 2-benzoxazol 1
192 4-F-Ph 5-benzoxazolyl
193 4-F-Ph 1-adamant 1
194 4-F-Ph 2-adamant 1
195 4-F-Ph t-Bu
196 2-F-Ph 3-CN-Ph
197 2-F-Ph 3-COCH3-Ph
198 2-F-Ph 3-C02Me-Ph
200
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
199 2-F-Ph 3-C02Et-Ph
200 2-F-Ph 3-C02H-Ph
201 _ 3-CONH2-Ph
2-F-Ph
202 2-F-Ph 3-F-Ph
203 _ 3-C1-Ph
2-F-Ph
204 2-F-Ph 3-NH2-Ph
205 2-F-Ph 3-S02NH2-Ph
2 0 6 2 -F- Ph 3 -CF3 - Ph
207 2-F-Ph 3-OCH3-Ph
208 2-F-Ph 3-OEt-Ph
209 2-F-Ph 3-OCF3-Ph
210 2-F-Ph 3-S02CH3--Ph
211 2-F-Ph 3-OH-Ph
212 2-F-Ph 3-CH3-Ph
213 _ 3-C2H5-Ph
2-F-:Ph
214 2 -F-:Ph 4 -CN- Ph
215 2-F-Ph 4-COCH3-Ph
216 2-F-Ph 4-C02Me-Ph
217 2-F-Ph 4-C02Et-Ph
218 2-F-Ph 4-C02H-Ph
219 2 -F-l?h 4 -CONH2 -Ph
220 2-F-Ph 4-F-Ph
221 2-F-Ph ~ 4-C1-Ph
222 2-F-Ph 4-NH2-Ph
223 2-F-Ph 4-S02NH2--
Ph
224 2-F-Ph ~ _
_
4-CF3-Ph
225 2-F-Ph 4-OCH3-Ph
226 2-F-Ph 4-OEt-Ph
227 2-F-Ph _
4-OCF3-P
h
228 2-F-Ph _
4-S02CH3-Ph
229 2-F-Ph _
4-OH-Ph
_
230 2-F-Ph 4-CH3-Ph
231 2-F-F>h 4-C2H5-Ph
232 _ 2,4-diF-Ph
2-F-Ph
233 2-F-Ph 2,5-diF-Ph
234 2-F-Ph 3,4-diF-Ph
235 2-F-Ph 3,5-diF-Ph
236 2-F-Ph 2,4-diCl-Ph
237 2-F-Ph 2,5-diCl-Ph
238 2-F-Ph 3,4-diCl-Ph
239 2-F-Ph 3,5-diCl-Ph
240 2-F-Ph 3,4-OCH20-Ph
241 2-F-Ph 3,4-OCH2CH20-Ph
242 2-F-Ph 2-thien '1
243 2-F-Ph 2-furan l
244 2-F-Ph 2- rid 1
245 2-F-Ph 4- rid
1
246 2-F-Ph _
2-imidazol 1
247 2-F-Ph 3- yrazolLrl
248 2-F-Ph 2-thiazol 1
249 2-F-Ph 5-tetrazol 1
250 2-F-Ph 1-adamant 1
251 2,4-diF-Ph 3-CN-Ph
201
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
252 _ 2,4-diF-Ph 3-COCH3-Ph
~ ~
253 2, 4-_di_F-Ph 3-C02Me-Ph
.
254 2,4- 3-C02Et-Ph
diF-Ph
255 2,4-diF-Ph 3-C02H-Ph
256 2,4-diF-Ph 3-CONH2-Ph
257 2,4-diF-Ph 3-F-Ph
258 2,4-diF-Ph 3-Cl-Ph
259 2,4-diF-Ph 3-NH2-Ph
260 2,4-diF-Ph 3-S02NH2-Ph
261 2,4-diF-Ph 3-CF3-Ph
262 2,4-diF-Ph 3-OCH3-Ph
263 2,4-diF-Ph 3-OEt-Ph
264 2,4-diF-Ph 3-OCF3-Ph
265 2,4-diF-Ph 3-S02CH3-Ph
266 2,4-diF-Ph 3-OH-Ph
267 2,4-diF-Ph 3-CH3-Ph
268 2,4-diF-Ph 3-C2H5-Ph
269 2,4-diF-Ph 4-CN-Ph
270 2,4-diF-Ph 4-COCH3-Ph
271 2,4-diF-Ph 4-C02Me-Ph
272 2,4-diF-Ph 4-C02Et-Ph
273 2,4-diF-Ph 4-C02H-Ph
274 2,4-diF-Ph 4-CONH2-Ph
275 2,4-diF-Ph 4-F-Ph
276 2,4-diF-Ph 4-Cl-Ph
277 2,4-diF-Ph 4-NH2-Ph
2?8 2,4-diF-Ph 4-S02NH2-Ph
279 2,4-diF-Ph 4-CF3-Ph
280 2,4-diF-Ph 4-OCH3-Ph
281 2,4-diF-Ph 4-OEt-Ph
282 2,4-diF-Ph 4-OCF3-Ph
283 2,4-diF-Ph 4-S02CH3-Ph
284 2,4-diF-Ph 4-OH-Ph
285 2,4-diF-Ph 4-CH3-Ph
286 2,4-diF-Ph 4-C2H5-Ph
287 2,4-diF-Ph 2,4-diF-Ph
288 2,4-diF-Ph 2,5-diF-Ph
289 2,4-diF-Ph 3,4-diF-Ph
290 2,4-diF-Ph 3,5-diF-Ph
291 2,4-diF-Ph 2,4-diCl-Ph .
292 2,4-diF-Ph 2,5-diCl-Ph
293 2,4-diF-Ph 3,4-diCl-Ph
294 2,4-diF-Ph 3,5-diCl-Ph
295 2,4-diF-Ph 3,4-OCH20-Ph
296 2,4-diF-Ph 3,4-OCH2CH20-Ph
297 2,4-diF-Ph 2-thien 1
298 2,4-diF-Ph 2-furan 1
299 2,4-diF-Ph 2- rid 1
300 2,4-diF-Ph 4- rid 1
301 2,4-diF-Ph 2-imidazolyl
302 2,4-diF-Ph 3- razolyl
303 2,4-diF-Ph 2-thiazolyl
304 2,4-diF-Ph 5-tetrazolyl
202
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
305 2,4-diF-Ph 1-adamant 1
306 _ 4-Cl-Ph Ph
307 4-Cl-Ph 3-CN-Ph
.__
308 4-Cl-Ph 3-COCH3-P
h
309 4-C1-Ph _
3-C02Me-Ph
310 4-C1-Ph 3-C02Et-Ph
311 4-C1-Ph 3-C02H-Fh
312 4-Cl-Ph 3-CONH2-Ph
313 4-C1-:Ph _
3-CONHMe-Ph
314 4 -C1-:Ph 3 -F-Ph
315 4- 3-C1-Ph
Cl-Ph
316 _ 3-Br-Ph
4-Cl-Ph
317 4-C1-Ph 3-N02-Ph
318 4-C1-Ph 3-NH2-Ph
319 4-C1-Ph 3-NHMe-Ph
320 4-C1-Ph 3-NMe2-Ph
321 4-C1-Ph 3-NHCOCH3--Ph
322 4-Cl-Ph _
3-S02NH2-Ph
323 4-C1-Ph 3-S02NHMe-Ph
324 4~C1-P_h 3-CF3-Pry
325 4-Cl-Ph 3-OCH3-Ph
326 4-C1-Ph 3-OPh-Ph
327 4-C1-Ph 3-OCF3-Ph
_
328 4-C1-Ph 3-SCH3-Ph
329 4-C1-Ph 3-SOCH3-Fh
330 4-C1-Ph 3-S02CH3-Ph
331 4- 3-OH-Ph
C1-Ph
332 _ 3-CH20H-Ph
4-C1-Ph
333 4-C1-Ph _
~ 3-CHOHCH3-Ph
334 4-C1-F'h 3-COH(CH3)2-Ph
335 4-C1-Ph 3-CHOHPh-Ph
336 4-C:L-Ph 3-CH3-Ph.
337 4-C1-Ph 3-C2H5-Ph
338 4-Cl-Ph 3-iPr-Ph
339 4-Cl 3-tBu-Ph
-Ph
340 _ 3-Ph-Ph
4-C1-Ph
341 4-C1-Ph 3-CH2Ph-Ph
342 4-Cl-Ph 3-CH2C02Me-Ph
343 4-Cl-Ph 3-(1- i eridin 1)-Ph
344 4-Cl-Ph 3-(1- rrolidin I)-Ph
345 4-C1-Ph _3-(2-imidazolrl)-Ph
346 4-C1-Ph 3-(1-imidazolyl)-Ph
347 4-C1-Ph 3-(2-thiazolyl)-Ph
348 4-C1-Ph 3-(3-pyrazolyl}-Ph
349 4-Cl-Ph 3-(1-pyrazolyl}-Ph
350 4-C1-Ph 3-(1-tetrazol 1)-Ph
351 4-C1-Ph 3-(5-tetrazol 1)-Ph
352 4-C1-Ph 3-(2- rid 1)-Ph
353 4-C1-Ph 3-(2-thien 1)-Ph
354 4-Cl-Ph 3-(2-furanyl)-Ph
355 4-C:1-Ph 4-CN-Ph
356 4-C:1-P:h 4-COCH3-Ph
357 4-Cl-Ph 4-C02Me-Ph
203
CA 02346933 2001-04-18
WO 00/35449 PCT/iJS99I30292
358 4-C1-Ph _ 4-C02Et-Ph
~
359 __ 4-C02H-Ph
4-CI-Ph
360 4-CI-Ph 4-CONH2-Ph
361 4-CI-Ph 4-CONHMe-Ph
362 4-Cl-Ph 4-CONHPh-Ph
363 4-C1-Ph 4-NHCONH2-Ph
364 4-C1-Ph 4-F-Ph
365 4-C1-Ph 4-C1-Ph
366 4-CI-Ph 4-Br-Ph
367 4-C1-Ph 4-N02-Ph
368 4-C1-Ph 4-NH2-Ph
369 4-C1-Ph 4-NHMe-Ph
370 4-C1-Ph 4-NMe2-Ph
371 4-C1-Ph 4-NHCOCH3-Ph
372 4-C1-Ph 4-S02NH2-Ph
373 4-C1-Ph 4-S02NHMe-Ph
374 4-C1-Ph 4-CF3-Ph
375 4-Cl-Ph 4-OCH3-Ph
376 4-Cl-Ph 4-OPh-Ph
377 4-Cl-Ph 4-OCF3-Ph
378 4-C1-Ph 4-SCH3-Ph
379 4-C1-Ph 4-SOCH3-Ph
380 4-Cl-Ph 4-S02CH3-Ph
381 4-C1-Ph 4-OH-Ph
382 4-C1-Ph 4-CH20H-Ph
383 4-C1-Ph 4-CHOHCH3-Ph
384 4-Cl-Ph 4-COH(CH3)2-Ph
385 4-C1-Ph 4-CH3-Ph
386 4-C1-Ph 4-C2H5-Ph
387 4-C1-Ph 4-iPr-Ph
388 4-C1-Ph 4-tBu-Ph
389 4-C1-Ph 4-Ph-Ph
390 4-C1-Ph 4-CH2Ph-Ph
391 4-C1-Ph 4-CH2C02Me-Ph
392 4-Cl-Ph 4-(1-piperidin 1)-Ph
393 4-C1-Ph 4-(1- rrolidinyl)-Ph
394 4-C1-Ph 4-(2-imidazol 1)-Ph
395 4-C1-Ph 4-(1-imidazol 1)-Ph
396 4-C1-Ph 4-(2-thiazol 1)-Ph
397 4-C1-Ph 4-(3- razol 1)-Ph
398 4-CI-Ph 4-(1- razol 1)-Ph
399 4-CI-Ph 4-(1-tetrazol 1)-Ph
400 4-C1-Ph 4-(5-tetrazol 1)-Ph
401 4-C1-Ph 4-(2- rid 1)-Ph
402 4-C1-Ph 4-(2-thien I)-Ph
403 4-C1-Ph 4-(2-furan 1)-Ph
404 4-Cl-Ph 2-CN-Ph
405 4-C1-Ph 2-COCH3-Ph
406 4-C1-Ph 2-C02Me-Ph
407 4-C1-Ph 2-C02Et-Ph
408 4-Ci-Ph 2-C02H-Ph
409 4-C1-Ph 2-CONH2-Ph
410 4-Cl-Ph 2-CONHMe-Ph
204
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
411 4-C1--Ph 2-F-Ph
412 4-~C1--Ph 2-C1-Ph
413_ 4-C1-Ph 2-Br-Ph
414 4-C1-Ph 2-N02-Ph
415 4-CI-Ph 2-NH2-Ph
416 4-C1-~Ph 2-NHMe-Ph
417 4-C1--Ph 2-NMe2-P
h
418 4-C1-Ph _
2-NHCOCH3-
Ph
419 4-C1-Ph _
2-S02NH2-~Ph
420 4-C1-Ph 2-S02NHMe-Ph
421 4-C1-Ph 2-CF3-Ph
422 4-C1-Ph 2-OCH3-Ph
423 4-C1-Ph 2-OPh-Ph
424 4-C1-Ph _
2-OCF3-Ph
425 4-Cl-Ph 2-SCH3-Ph
426 4-C1-Ph 2-SOCH3-Ph
427 ~ 4-C1-Ph 2-S02CH3-Ph
428 4~C1-Ph 2-OH-Ph
429 4-C1-Ph 2-CH20H-Ph
430 4-C1-Ph 2-CHOHCH3~-Ph
431 4-C1-Ph 2-COH(CH3)2-Ph
432 4-Cl-Ph 2-CHOHPh-Ph
433 4-C1-Ph _
2-CH3-Ph
434 4-C1-Ph 2-C2H5-Ph
435 4-Cl-Ph 2-iPr-Ph
436 4-C1-Ph 2-tBu-Ph
437 4-C1-Ph 2-Ph-Ph
-
438 4-C1-Ph 2-CH2Ph-Ph
439 4-C1-ph 2-CH2C02Me-Ph
440 4-C1-:Ph _
441 4-C1-Ph ~ 2- ( 1- i eridiri 1
442 4-C1-:Ph ) -Ph
443 4-C1-Ph 2-(1-pyrrolidin 1)-Ph
444 4-C1-Ph 2- (2-imidazoh I) -Ph
445 4-C1-P_h _2-(1-imidazol:yl)-Ph
446 4-Cl-Ph 2-(2-thiazol1)-Ph
447 4-Cl-Ph 2- (3-pyrazol~I ) -Ph
448 4-C1-Ph 2-(1- razol~1)-Ph
449 4-C1-Ph _2-(1-tetrazoh l)-Ph
450 4-C1-Ph ~2-(5-tetrazol~l)-Ph
451 4-Cl-Ph _2-(2-pyridyl)-Ph
2-(2-thien 1)-Ph
2-(2-furanyl)-Ph
452 4-C1-1?h 2, 4-diF-Ph
453 4-C1-Ph 2,5-diF-Ph
454 4-C1-Ph 2,6-diF-Ph
455 4-C1-~?h 3 , 4-diF-
Ph
456 4 _
-C1-F?h 3, 5-diF-Ph
457 _ 2 , 4-diCl-Ph
4-C1-F'h
458 4-C1-Ph 2,5-diCl-Ph
459 _4-C1-Ph 2,6-diCl-Ph
460 4-CI-Ph 3 , 4-diCl-Ph
461 4-C1-Ph _
3,5-diCl-Ph
462 _ 3,4-
4-~1-Ph T iCF3-Ph
d
463 4-C1-F'h 3, 5-diCF3-Ph
205
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
464 4-C1-Ph 5-C1-2-Me0-Ph
465 4-C1-Ph 5-C1-2-Me-Ph
466 _ 2-F-5-Me-Ph
4-C1-Ph
467 4-C1-Ph 2-F-5-N02-Ph
468 4-C1-Ph 3,4-OCH20-Ph
469 4-C1-Ph 3,4-OCH2CH20-Ph
470 4-C1-Ph 2-Me0-4-Me-Ph
471 4-C1-Ph 2-Me0-5-Me-Ph
472 4-C1-Ph 1-na hth 1
473 4-C1-Ph 2-na hthyl
474 4-C1-Ph 2-thienyl
475 4-C1-Ph 3-thien 1
476 4-Cl-Ph 2-furan 1
477 4-C1-Ph 3-furan 1
478 4-C1-Ph 2- rid 1
479 4-C1-Ph 3- rid 1
480 4-C1-Ph 4- ridyl
481 4-C1-Ph 2-indol 1
482 4-C1-Ph 3-indol 1
483 4-Cl-Ph 5-indol 1
484 4-Cl-Ph 6-indol 1
485 4-C1-Ph 3-indazol 1
486 4-Cl-Ph 5-indazol 1
487 4-C1-Ph 6-indazol 1
488 4-Cl-Ph 2-imidazolyl
489 4-Cl-Ph 3- razol 1
490 4-Cl-Ph 2-thiazol 1
491 4-C1-Ph 5-tetrazolyl
492 4-Cl-Ph 2-benzimidazolyl
493 4-C1-Ph 5-benzimidazol 1
494 4-Cl-Ph 2-benzothiazolyl
495 4-C1-Ph 5-benzothiazolyl
496 4-Cl-Ph 2-benzoxazol 1
497 4-C1-Ph 5-benzoxazolyl
498 4-C1-Ph 1-adamant 1
499 4-C1-Ph 2-adamant 1
500 4-Cl-Ph t-Bu
501 2-C1-Ph 3-CN-Ph
502 2-C1-Ph 3-COCH3-Ph
503 2-Cl-Ph 3-C02Me-Ph
504 2-C1-Ph 3-C02Et-Ph
505 2-C1-Ph 3-C02H-Ph
506 2-C1-Ph 3-CONH2-Ph
__5_07_ 2-C1-Ph 3-F-Ph
2-C1-Ph 3-C1-Ph
08__
_ 2-C1-Ph 3-NH2-Ph
509
510 2-C1-Ph 3-S02NH2-Ph
51 2-C1-Ph 3-CF3-Ph
1
_ 2-C1-Ph 3-OCH3-Ph
_
512
513 2-C1-Ph 3-OEt-Ph
514 2-C1-Ph 3-OCF3-Ph
515 2-C1-Ph 3-S02CH3-Ph
516 2-Cl-Ph 3-OH-Ph
206
CA 02346933 2001-04-18
WO 00/35449
PCT/US99/30292
517 2~C1-Ph 3-CH3-Ph
518 2-C1-Ph __
3-C2H5-Ph
519 2-C1-Ph 4-CN-Ph
520 __ 2-C1-Ph 4-COCH3-Ph
521 2-C1-Ph 4-C02Me-Ph
522 _ 2-C1-Ph 4-C02Et-Ph
523 2-C1-Ph 4-C02H-Ph
524 2-C1-Ph 4-CONH2-Ph
525 __ 2-C1-Ph _
4-F-Ph
526 2-C1-Ph 4-C1-Ph
-
527 2-C1-Ph 4-NH2-Ph
528 2-CI-Ph 4-S02NH2-Ph
529 _ 2-C1-:Ph 4-CF3-Ph
~
530 2-C1-Ph 4-OCH3-Ph
531 2-C1-:Ph 4-OEt-Ph
532 __ 2-C1-Ph 4-OCF3-Ph
533 _ _ 2-C1-Ph 4-S02CH3-Ph
534 2-C1-Ph 4-OH-Ph
535 _ 2-C1-Ph 4-CH3-Ph
536 2-C1-Ph 4-C2H5-Ph
537 _ 2-Cl-Ph 2, 4-diF-Ph
538 2-C1-Ph 2,5-diF-Ph
539 2-C1-I?h 3, 4-diF-Ph
540 2-C1-Ph 3,5-diF-Ph
541 __ 2-C1-Ph 2, 4-diCl-Ph
542 2-CI-Ph 2,5-diCl-Ph
543 2 -C1-Ph 3 , 4 -diCl-:Ph
544 2-C1-Ph 3, 5-diCl-:Ph
545 2-C1-Ph 3,4-OCH20-~Ph
~
546 _ 2-C1-Ph 3 , 4-OCH2CH20-Ph
547 2-C1-Ph 2-thien I
548 2-C1-Ph 2-furan :1
__-
549 2-C1-Ph 2- rid 1
550 _
l
551 _ 2-C1-Ph 2 irnidazol
~ l
552 2-C1-Ph 3- razor 1
553 _ 2-C1-Ph 2-thiazol 1
~
554 2-C1-Fh 5-tetrazol 1
555 2-C1-Ph 1-adamant 1
556 2,4-diCl-Ph 3-CN-Ph
557 2,4-diCl-Ph 3-COCH3-Ph
558 2,4-diCl-Ph 3-C02Me-Ph
559 2,4-diCl-Ph 3-C02Et-Ph
560 2_, 4-diCl-Ph 3-C02H-Ph
561 2,4-diCl-Ph 3-CONH2-Ph
562 _ 3-F-Ph
2,4-diCl-Ph
563 2,4-diCl-Ph 3-C1-Ph
564 2,4-diCl-Ph 3-NH2-Ph
565 2,4-diCl-Ph 3-S02NH2-Ph
566 2,4-diCl-Ph 3-CF3-Ph
567 2,4-diCl-Ph 3-OCH3-Ph
568 _ 3-OEt-Ph
2,4-diCl-Ph
569 2,4-diCl-Ph ~ 3-OCF3-Ph
207
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
570 2,4-diCl-Ph 3-S02CH3-Ph
5?1 2,4-diCl-Ph 3-OH-Ph
572 2,4-diCl-Ph 3-CH3-Ph
573 2,4-diCl-Ph 3-C2H5-Ph
574 2,4-diCl-Ph 4-CN-Ph
575 2,4-diCl-Ph 4-COCH3-Ph
576 2,4-diCl-Ph 4-C02Me-Ph
577 2,4-diCl-Ph 4-C02Et-Ph
578 2,4-diCl-Ph 4-C02H-Ph
579 2,4-diCl-Ph 4-CONH2-Ph
580 2,4-diCl-Ph 4-F-Ph
581 2,4-diCl-Ph 4-C1-Ph
582 2,4-diCl-Ph 4-NH2-Ph
583 2,4-diCl-Ph 4-S02NH2-Ph
584 2,4-diCl-Ph 4-CF3-Ph
585 2,4-diCl-Ph 4-OCH3-Ph
586 2,4-diCl-Ph 4-OEt-Ph
587 2,4-diCl-Ph 4-OCF3-Ph
588 2,4-diCl-Ph 4-S02CH3-Ph
589 2,4-diCl-Ph 4-OH-Ph
590 2,4-diCl-Ph 4-CH3-Ph
591 2,4-diCl-Ph 4-C2H5-Ph
592 2,4-diCl-Ph 2,4-diF-Ph
593 2,4-diCl-Ph 2,5-diF-Ph
594 2,4-diCl-Ph 3,4-diF-Ph
595 2,4-diCl-Ph 3,5-diF-Ph
596 2,4-diCl-Ph 2,4-diCl-Ph
597 2,4-diCl-Ph 2,5-diCl-Ph
598 2,4-diCl-Ph 3,4-diCl-Ph
599 2,4-diCl-Ph 3,5-diCl-Ph
600 2,4-diCl-Ph 3,4-OCH20-Ph
601 2,4-diCl-Ph 3,4-OCH2CH20-Ph
602 2,4-diCl-Ph 2-thien 1
603 2,4-diCl-Ph 2-furanyl
604 2,4-diCl-Ph 2-p ridyl
605 2,4-diCl-Ph 4- rid 1
606 2,4-diCl-Ph 2-imidazol 1
607 2,4-diCl-Ph 3- razolyl
608 2,4-diCl-Ph 2-thiazolyl
609 2,4-diCl-Ph 5-tetrazol 1
610 2,4-diCl-Ph 1-adamant 1
611 3-OCH3-Ph 3-CN-Ph
612 3-OCH3-Ph 3-COCH3-Ph
613 3-OCH3-Ph 3-C02Me-Ph
614 3-OCH3-Ph 3-C02Et-Ph
615 3-OCH3-Ph 3-C02H-Ph
616 3-OCH3-Ph 3-CONH2-Ph
617 3-OCH3-Ph 3-F-Ph
618 3-OCH3-Ph 3-C1-Ph
619 3-OCH3-Ph 3-NH2-Ph
620 3-OCH3-Ph 3-S02NH2-Ph
621 3-OCH3-Ph 3-CF3-Ph
622 3-OCH3-Ph 3-OCH3-Ph
208
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
623 3-OCH3--Ph 3-OEt-Ph
624 _3 -OCH3--Ph 3 -OCF3-Ph
~
625 3-OCH3--Ph 3-S02CH3-Ph
626 3-OCH3-Ph 3-OH-Ph
627 3-OCH3-Ph 3-CH3-Ph
628 3-OCH3-Ph 3-C2H5-Ph
629 3-OCH3-Ph 4-CN-Ph
630 3-OCH3--Ph 4-COCH3-Ph
631 3-OCH3-Ph 4-C02Me-Ph
632 3-OCH3-Ph 4-C02Et-Ph
633 3-OCH3-Ph 4-C02H-Ph
634 3-OCH3--Ph 4-CONH2-Ph
635 3-OCH3--Ph 4-F-Ph
636 3-OCH3-Ph 4-C1-Ph
637 3-OCH3-Ph 4-NH2-Ph
638 3-OCH3-Ph 4-S02NH2-Ph
639 3-OCH3-Ph 4-CF3-Ph
64 0 3 -OCH3 --Ph 4 -OCH3 -Ph
641 3-OCH3-Ph 4-OEt-Ph
642 3-OCH3-Ph 4-OCF3-Ph
643 3-OCH3-Ph 4-S02CH3-Ph
~
644 3-OCH3-Ph 4-OH-Ph
645 3-OCH3-Ph 4-CH3-Ph
646 3-OCH3-Ph 4-C2H5-Ph
647 3-OCH3--Ph 2, 4-diF-Ph
648 3-OCH3-Ph 2,5-diF-Ph
649 3-OCH3-Ph 3,4-diF-Ph
650 3-OCH3-Ph 3,5-diF-Ph
651 3-OCH3-Ph 2, 4-diCl-1?h
652 3-OCH3--Ph 2, 5-diCl-~?h
653 3-OCH3-Ph 3,4-diCl-Ph
~
654 3-OCH3-Ph 3 , 5-diCl-l?h
655 3-OCH3-Ph 3,4-OCH20-Ph
656 3-OCH3--Ph 3, 4-OCH2CH20-Ph
657 3-OCH3--Ph 2-thien :L
658 3-OCH3--Ph 2-furan :L
659 3-OCH3-Ph 2- rid :L
660 3-OCH3-Ph 4- rid :L
661 3-OCH3-Ph 2-imidazolyl
662 3-OCH3-Ph 3- razol 1
663 _ 2-thiazolyl
3-OCH3--Ph
664 3-OCH3--Ph 5-tetrazol 1
665 3-OCH3-Ph 1-adamantyl
666 2-thien~l -_ 3-CN-Ph
667 2-thien l1 3-COCH3-Ph
668 2-thien 1 3-F-Ph
669 2-thien 1 3-C1-Ph
670 2-thienyl -, 3-NH2-Ph
671 2-thienyl 3-OCH3-Ph
672 2-thien 1 3-OH-Ph
673 2-thien 1 ~ 4-CN-Ph
674 2-thien 1 4-COCH3-Ph
675 2-thien 1 4-F-Ph
209
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
_676 2-thien 1 ~ 4-C1-Ph
677 2-thien 1 _
4-NH2-Ph
678 2-thienyl 4-OCH3-Ph
679 2-thien 1 4-OH-Ph
680 2-thien 1 3,4-diF-Ph
681 2-thienyl 3,5-diF-Ph
682 2-thien 1 3,4-diCl-Ph
683 2-thien 1 3,5-diCl-Ph
684 2-thien 1 3,4-OCH20-Ph
685 2-thien 1 3,4-OCH2CH20-Ph
686 3-thien 1 3-CN-Ph
687 3-thien 1 3-COCH3-Ph
688 3-thien 1 3-F-Ph
689 3-thien 1 3-C1-Ph
690 3-thien 1 3-NH2-Ph
691 3-thien 1 3-OCH3-Ph
692 3-thien 1 3-OH-Ph
693 3-thien 1 4-CN-Ph
694 3-thienyl 4-COCH3-Ph
695 3-thien 1 4-F-Ph
696 3-thien 1 4-C1-Ph
697 3-thien 1 4-NH2-Ph
698 3-thien 1 4-OCH3-Ph
699 3-thien 1 4-OH-Ph
700 3-thien 1 3,4-diF-Ph
701 3-thien 1 3,5-diF-Ph
702 3-thien 1 3,4-diCl-Ph
703 3-thien 1 3,5-diCl-Ph
704 3-thien 1 3,4-OCH20-Ph
705 3-thien 1 3,4-OCH2CH20-Ph
706 2-furanyl 3-CN-Ph
707 2-furan 1 3-COCH3-Ph
708 2-furan 1 3-F-Ph
709 2-furan 1 3-C1-Ph
710 2-furan 1 3-NH2-Ph
711 2-furan 1 3-OCH3-Ph
712 2-furan 1 3-OH-Ph
713 2-furan 1 4-CN-Ph
714 2-furan 1 4-COCH3-Ph
715 2-furan 1 4-F-Ph
716 2-furan 1 4-C1-Ph
717 2-furan 1 4-NH2-Ph
718 2-furan 1 4-OCH3-Ph
719 2-furan 1 4-OH-Ph
720 2-furan 1 3,4-diF-Ph
721 2-furan 1 3,5-diF-Ph
722 2-furan 1 3,4-diCl-Ph
723 2-furan 1 3,5-diCl-Ph
?24 2-furan 1 3,4-OCH20-Ph
725 2-furan 1 3,4-OCH2CH20-Ph
726 3-furan 1 3-CN-Ph
727 3-furan 1 3-COCH3-Ph
728 3-furan 1 3-F-Ph
210
CA 02346933 2001-04-18
WO 00/35449 PCT/1JS99/30292
729 =i-furan 1 3-C1-Ph
730 3-furanyl _
~ ~
3-NH2-Ph
731 3-furan 1 _
3-OCH3-Ph
732 3-furan 1 3-OH-Ph
733 :3-furanyl 4-CN-Ph
734 3-furanyl 4-COCH3-Ph
735 _ 4-F-Ph
3-furan 1
736 3-furan 1 4-C1-Ph
737 3-furan 1 4-NH2-Ph
738 ~~-furan 1 4-OCH3-Ph.
739 3-furan 1 4-OH-Ph
740 3-furanyl 3,4-diF-Ph
741 =~-furanyl 3,5-diF-Ph
_742 _ 3,4-diCl-Ph
3-furan 1 ~
743 _ ?I-furan 1 3,5-diCl-Ph
744 ~-furan 1 3,4-OCH20-Ph
745 ?.-furan 1 3,4-OCH2CH20-Ph
?46 2.-pyrid 1 3-CN-Ph
747 2-pyridyl 3-COCH3-Ph
?48 ~ - rid' 1 3-F-Ph
749 2- rid 1 3-Cl-Ph
750 2- rid 1 3-NH2-Ph
751 ~,- .rid 1 3-OCH3-Ph
_
752 2- rid 1 3-OH-Ph
753 2 - .rid 1 4-CN-Ph
754 2- rid I 4-COCH3-P h
_
755 2- rid 1 4-F-Ph
_
756 e:- rid 1 4-C1-Ph
757 __ 2- rid 1 4-NH2-Ph -
758 _2;-pyridyl 4-OCH3-Ph
759 2- id 1 _
4-OH-Ph
760 2- rid 1 3,4-diF-Ph
761 2-pyrid 1 3,5-diF-Ph
762 2-pyridyl 3,4-diCl-Ph
763 2;- rid 1 3,5-diCl-Ph
764 ~- rid I 3,4-OCH20-Ph
765 2- rid I 3,4-OCH2CH20-Ph
766 3.- rid 1 3-CN-Ph
7 67 _3. -pyridyl 3 -COCH3 -Ph
768 3- rid' 1 3-F-Ph
769 3- rid 1 3-C1-Ph
770 _3.- rid 1 3-NH2-Ph
771 3- rid 1 3-OCH3-Ph
772 3- rid 1 3-OH-Ph
_773 3- rid 1 4-CN-Ph
774 3-pyrid 1 4-COCH3-Ph
775 3.- rid 1 4-F-Ph
776 3- rid 1 4-C1-Ph
777 3-pyridyl 4-NH2-Ph
778 3-pyridyl 4-OCH3-Ph
779 3- rid 1 4-OH-Ph
780 3~- rid 1 3,4-diF-Ph
781 3-pyridyl 3,5-diF-Ph
211
CA 02346933 2001-04-18
WO 00135449 PCT/US99/30292
782 3- ridyl 3,4-diCl-Ph
783 3-p rid 1 3,5-diCl-Ph
784 3-pyridyl 3,4-OCH20-Ph
785 3- rid 1 3,4-OCH2CH20-Ph
786 4- rid 1 3-CN-Ph
787 4-pyrid 1 3-COCH3-Ph
788 4- rid 1 3-F-Ph
789 4- rid 1 3-C1-Ph
790 4- rid 1 3-NH2-Ph
791 4- yrid 1 3-OCH3-Ph
792 4- yrid 1 3-OH-Ph
793 4- rid 1 4-CN-Ph
794 4- rid 1 4-COCH3-Ph
795 4- yrid 1 4-F-Ph
4-pyrid 1 4-C1-Ph
4- rid 1 4-NH2-Ph
798 4- rid 1 4-OCH3-Ph
799 4- rid 1 4-OH-Ph
800 4- rid 1 3,4-diF-Ph
801 4- rid 1 3,5-diF-Ph
802 4- rid 1 3,4-diCl-Ph
803 4-p rid 1 3,5-diCl-Ph
804 4- rid 1 3,4-OCH20-Ph
805 4-p rid 1 3,4-OCH2CH20-Ph
806 3-indol 1 3-CN-Ph
807 3-indolyl 3-COCH3-Ph
808 3-indol 1 3-F-Ph
809 3-indol 1 3-C1-Ph
810 3-indol 1 3-NH2-Ph
811 3-indol 1 3-OCH3-Ph
812 3-indol 1 3-OH-Ph
813 3-indol 1 4-CN-Ph
814 3-indol 1 4-COCH3-Ph
815 3-indolyl 4-F-Ph
816 3-indolyl 4-C1-Ph
817 3-indol 1 4-NH2-Ph
818 3-indol 1 4-OCH3-Ph
819 3-indol 1 4-OH-Ph
820 3-indol 1 3,4-diF-Ph
821 3-indol 1 3,5-diF-Ph
822 3-indol 1 3,4-diCl-Ph
823 3-indol 1 3,5-diCl-Ph
824 3-indol 1 3,4-OCH20-Ph
825 3-indol l 3,4-OCH2CH20-Ph
826 5-indolyl 3-CN-Ph
827 5-indol 1 3-COCH3-Ph
828 5-indolyl 3-F-Ph
829 5-indol 1 3-C1-Ph
830 5-indol 1 3-NH2-Ph
831 5-indol 1 3-OCH3-Ph
832 5-indol 1 3-OH-Ph
833 5-indol 1 4-CN-Ph
834 5-indol 1 4-COCH3-Ph
212
CA 02346933 2001-04-18
WO 00;35449 PCT/US99/30292
835 5-indol 1 4-F-Ph
836 5-indol 1 4-Cl-Ph
837 5-indol 4-NH2-Ph
1
838 _ 4-OCH3-Ph
5-indol 1
839 5-indol 1 _
4-OH-Ph
840 5-indol 1 3,4-diF-Ph
841 __ 5-indol 1 3,5-diF-Ph
_
842 5-indo~ 3,4-diCl-P
h
843 5-indolyl _
3,5-diCl-Ph
8_44 5-indol 1 3,4-OCH20-Ph
845 5-indolyl 3,4-OCH2CH20-Ph
846 5-indazol 1 3-CN-Ph
.
847 5-indazol 3-COCH3-Ph
l
848 _5-indazolyl _ 3-F-Ph
849 5-indazolyl 3-C1-Ph
850 5-indazol 1 _
3-NH2-Ph
851 5-indazol 1 3-OCH3-Ph.
852 5-indazol 1 3-OH-Ph
853 5~-indazol 1 4-CN-Ph
854 5-indazol 1 4-COCH3-Ph
855 5-indazol 1 4-F-Ph
856 _5-indazol 1 4-C1-Ph
857 5-indazol 1 _
~ 4-NH2-Ph
858 5-indazol 1 4-OCH3-Ph.
859 5-indazol 1 4-OH-Ph
860 5-indazolyl 3,4-diF-Ph
861 _ 5-indazol 1 3,5-diF-Ph
862 5-indazol 1 _
3,4-diCl-Ph
863 5-indazol 1 3,5-diCl-Ph
864 5-indazol 1 3,4-OCH20-Ph
865 5-indazolyl 3,4-OCH2CH20-Ph
866 5-benzimidazol 1 3-CN-Ph
867 5-benzimidazol 1 ___
- ~ 3-COCH3-Ph
868 5-benzimidazol 1 3-F-Ph
869 5-benzimidazolyl 3-C1-Ph
870 5-benzimidazol 1 3-NH2-Ph
8_7_1 5-benzimidazolyl 3-OCH3-Ph
872 _ _
5-be_nzimidazol 1 3-OH-Ph
873 5-benzimidazolyl 4-CN-Ph
874 5-benzimidazol 1 4-COCH3-Ph
875 5-benzimidazol~ 4-F-Ph
876 5-benzimidazolyl 4-C1-Ph
877 5-benzimidazolyl 4-NH2-Ph
878 5-benzimidazol 1 4-OCH3-Ph
879 5-benzimidazol 1 4-OH-Ph
880 5-benzimidazol 1 3,4-diF-Ph
881 5-benzimidazolyl 3,5-diF-Ph
882 5-benzimidazolyl 3,4-diCl-Ph
883 5-benzimidazol 1 3,5-diCl-Ph
884 5-benzimidazol l 3,4-OCH20-Ph
885 5-benzimidazolyl 3,4-OCH2CH20-Ph
886 5-benzothiazol l _
3-CN-Ph
887 5-benzothiazolyl 3-COCH3-Ph
213
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
888 5-benzothiazolyl 3-F-Ph
889 5-benzothiazolyl 3-C1-Ph
890 5-benzothiazol 1 3-NH2-Ph
891 5-benzothiazolyl 3-OCH3-Ph
892 5-benzothiazol 1 3-OH-Ph
893 5-benzothiazol 1 4-CN-Ph
894 5-benzothiazolyl 4-COCH3-Ph
895 5-benzothiazol 1 4-F-Ph
896 5-benzothiazol 1 4-C1-Ph
897 5-benzothiazol 1 4-NH2-Ph
898 5-benzothiazolyl 4-OCH3-Ph
899 5-benzothiazol 1 4-OH-Ph
900 5-benzothiazol 1 3,4-diF-Ph
901 5-benzothiazol 1 3,5-diF-Ph
902 5-benzothiazol 1 3,4-diCl-Ph
903 5-benzothiazol 1 3,5-diCl-Ph
904 5-benzothiazol 1 3,4-OCH20-Ph
905 5-benzothiazol 1 3,4-OCH2CH20-Ph
906 5-benzoxazolyl 3-CN-Ph
5-benzoxazolyl 3-COCH3-Ph
908 5-benzoxazol 1 3-F-Ph
909 5-benzoxazal 1 3-C1-Ph
910 5-benzoxazol 1 3-NH2-Ph
911 5-benzoxazol 1 3-OCH3-Ph
912 5-benzoxazol I 3-OH-Ph
913 5 -benzoxazol 1 4 -CN-Ph
914 5-benzoxazol 1 4-COCH3-Ph
915 5-benzoxazol 1 4-F-Ph
916 5-benzoxazol 1 4-C1-Ph
91~ 5-benzoxazol 1 4-NH2-Ph
918 5-benzoxazolyl 4-OCH3-Ph
919 5-benzoxazolyl 4-OH-Ph
920 5-benzoxazol 1 3,4-diF-Ph
921 5-benzoxazol 1 3
5-diF-Ph
922 S-benzoxazol 1 ,
3,4-diCl-Ph
923 5-benzoxazol 1 3,5-diCl-Ph
924 5-benzoxazol 1 3,4-OCH20-Ph
925 5-benzoxazol 1 3,4-OCH2CH20-Ph
214
CA 02346933 2001-04-18
WO 00/35449 PCT/IJS99/30292
'TABLE 6
~~N'R ~~N'R ~ _R3
H F J~ 'v H Iv HN H
R a R a R a
O
3 O O
C HN~H.R HN~H_R3 HN~N.R3
H
F
1a
R R 4
0
C1 ~ HN~N~R~
H
1
O O
F ~ HN~N~R~ a HN~L "Rs
H 8 ~.. H
C1
1
is O is
HN~N~R3 9
~~..// ~~'~~'' H
O
~~ R3 0
HN'°'H~ a HN~ ~R3
F 1:1 H
a
4
1~
12
Q 3
~~N.R
F H
F
3
N N.R ~ R3
F~~~t~ H R ~~~/~ ~ H
F 14 F
R a
8 Ria
13 Cl ~~N.R3 1 S
C1 ~ H C
~ 17 ~ 3
N H~N'R3 HN N-R
CI~~~f~ H R C H
C1
Rla R a
16 lg
215
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
1 Q
~~N_R
H
C1
F O
3 Rla k .R3
_R
HN N
F HN H 20 H
F
Rla Rla
19 21
~ 3
HN" H R
F
13 Q 3
HN I3'R Rla ~~N_R
H 23 C1 ~ H
Rla 24 Rla
22
216
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
H ~ _R'; H ~ .R3
HIV H HN N
H H p N 3 C1 H
~j_ R
Rla H Rla
F
25 27
Rla
~~H.R3 26 CI R~H R3
F' HN~NR3 30
la la
R ~./~ H R
28
Rla
N\ H ~~N.R-~ 29 C1 Fi 1NR3
H HIS ~H
R1a F H ~ 3 Rla
HN_ 'NR 3 3
31 H
Q R1 Q
~~N.R-~ 32 C1 1 NR3
H FiN~ ~H
36
Rla Rla
NR
34 H
H ~ .R3
H Rla CI H ~ R3
35 ~ ~ H
Rla
F H~ NR3 R1a
37 ~H 39
R'"
38
HN_ _N'R3 HN~NR3 Cl.~~\~~~ HN~NR3
H H I~/~ H
Ria Hla Rla
40 4~ 42
217
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
p O
11 R3 F ~.
t ~w . N ~- HN N
H ~;, N .. ~ . .. N i~. HN N
Ri 4 ; ~_ ' ~' 1~=y H =- , H
F 1a is
i R R -;:
__
43 45
44
_ "~:~ O
O
/ ~,.N HN N-R3 C1 ~~~"-1'y, ~ R3 F ~\~ ~,~ O
~.N~ HN N ~ i
C 1 14 ~ v H ''~ ~ ~ I-I 1' ' ~ N ~ ~ N
1
R ~~~ Rl a--~'' J/i F R14~!-'~ H
\~
46
47 48
/~,/~ ~, O 3
F ~~, ~- N 1 HN ~ N R C 1 %~ ~'~, ~4 ' ~ 3 ~~-~i O
v H ~; '
14 ~-.- 'w~ . N'~~ HN y\N R C1 '~~ ' N~., HN!'~1V R
F R ~;s /' \' 1a j=~ H ~ ~-_. 1
C I R -~~~~~~ C 1 R i a~ ~~~ , H
4 9 _,%
F ~ O 51
J~ R3 ~ ,,~-''~, O
F ~~ ~~ ~,. N ~ HN N C 1 , ~, , 3 ~-~ =- O .
H ~,i, ' N \ ~'~. N R C 1 ~(- Y ~ a R.
i4 '-=- ~ ~L~ wN~ HN~ ~N
R ~~~ Ria H F ~% J
Ria_~% H
5 2 \'''~/,
53 54
_ ~ O
N, J:- R3 _..I O
HN N F , ~ JL R3 ~ r'~ O
3
i 4 ~~ H ~ ~ ~ ~ N 1 ~ H ~ , _ ~ ~-- '~~.. N __ ~ J~ N R
R ~,~ / R i a~ F ~' l a ~-. H
R -
56
57
F ~ y ~ 3 F ~~ ~, W O 3
~N1 ~hN R ~~ iw.~'"N1 ~~N R /~~~ ; W.
CI~ 14 ~ H F 14 ~ H ~~ /~-- ,~,- N ~ ~ N R
R ~~ R ~/, F 1 a ~r---1, H
F R --!;
5g 59
~~~N HN N R ~I ,~ -''~~N1 HN N R C1 ~ ~~\, ~ R3
R14 \- H CI~ R14 H ~~ ~''~'Nw HN N
C1 ~ la ~-~ H
R --~ /ri
61 62
63
Entry R3 R14
CN
218
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
2 - Ph - F
_
Ph Cl -
4 Ph CH2OH
Ph OH
6 Ph _. _ NH2
7 Ph C02Me
8 Ph C02Et
_
9 Ph CONH2
__.
Ph NHPh
11 Ph NHMe
12 Ph OMe
13 Ph C(O)(2-imidazolyl)
14 Ph C(0)(4-imidazolyl)
Ph ~ C(0)(2-thiazolyl)
16 Ph C(O)(4-thiazolyl)
17 Ph C(O)(2-oxazolyl)
18 Ph C(O)(4-oxazolyl)
19 Ph C(O)C3-pyrazolyl)
Ph C(O)(4-pyrazolyl)
21 Ph C(O)(5-tetrazolyl)
22 Ph C(O)(2-pyridyl)
23 Ph C(O)(3-pyridyl)
24 Ph _
C(O)(4-pyridyl)
Ph C(O)(2-thienyl)
26 Ph C(O)(3-thienyl)
27 Ph C(O)(2-furanyl)
28 Ph C(O)(3-furanyl)
29 Ph 2-thienyl
Ph 3-thienyl
31 Ph 2-furanyl
32 Ph 3-furanyl
33 Ph 2-pyridyl
_
34 Ph 3-pyridyl
Ph 4-pyridyl
_
36 Ph 1-imidazolyl
37 Ph 2-imidazolyl
38 Ph 4-imidazolyl
39 Ph 1-pyrazolyl
Ph 3-pyrazolyl
41 Ph 4-pyrazolyl
42 Ph 2-thiazolyl
43 Ph 4-thi,azolyl
44 Ph 5-tetrazolyl
Ph 2-oxazolyl
46 Ph 4-oxazolyl
47 Ph C(O)N(2-imidazolyl)
48 Ph C(O)N(4-imidazolyl)
49 Ph C(O)N(2-thiazolyl)
~ Ph ~ C(O)N(4-thiazolyl)
219
CA 02346933 2001-04-18
WO 00/35449 PCT/US99130292
51 Ph C (O) N (2-oxazolyl)
52 ___ C(O)N(4-oxazolyl)
Ph
53 _ C(O)N(3-pyrazolyl)
Ph
54 Ph C(O)N(4-pyrazolyl)
55 Ph C(O)N(2-pyridyl)
56 Ph C(O)N(3-pyridyl)
57 Ph C(O)N(4-pyridyl)
58 Ph C(O)N(2-thienyl)
59 Ph C(O)N(3-thienyl)
60 Ph C (O)N(2-furanyl)
61 Ph C(O)N(3-furanyl)
62 Ph C ( O ) N ( 2 -pyrro lyl )
63 Ph C(O)N(3-pyrrolyl)
64 Ph CH2(1-imidazolyl)
65 Ph CH2(1-(1,2,3-triazolyl))
66 Ph CH2(2-(1,2,3-triazolyl))
67 Ph CH2 (1- ( 1, 2, 4-triazolyl)
)
68 Ph CH2(1-pyrazolyl)
69 3-CN-Ph CN
70 3-CN-Ph F
71 3-CN-Ph C1
72 3-CN-Ph CH20H
73 3-CN-Ph OH
74 3-CN-Ph NH2
75 3-CN-Ph C02Me
76 3-CN-Ph C02Et
77 3-CN-Ph CONH2
78 3-CN-Ph NHPh
79 3-CN-Ph NHMe
80 3-CN-Ph OMe
81 3-CN-Ph C(O)(2-imidazolyl)
82 3-CN-Ph C(O)(4-imidazolyl)
83 3-CN-Ph C(O)(2-thiazolyl)
84 3-CN-Ph C(O)(4-thiazolyl)
85 3-CN-Ph C(O)(2-oxazolyl)
86 3-CN-Ph C(O)(4-oxazolyl)
87 3-CN-Ph C(O)(3-pyrazolyl)
88 3-CN-Ph C(O)(4-pyrazolyl)
89 3-CN-Ph C(O)(5-tetrazolyl)
90 3-CN-Ph C(O)(2-pyridyl)
91 3-CN-Ph C(O)(3-pyridyl)
92 3-CN-Ph C(O)(4-pyridyl)
93 3-CN-Ph C(O)(2-thienyl)
94 3-CN-Ph C(O)(3-thienyl)
95 3-CN-Ph C(O)(2-furanyl)
96 3-CN-Ph C(O)(3-furanyl)
97 3-CN-Ph 2-thienyl
98 3-CN-Ph 3-thienyl
99 3-CN-Ph 2-furanyl
220
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
100 3-CN-Ph 3-furanyl
101 3-CN-Ph 2-pyridyl
102 3-CN-Ph 3-pyridyl
103 3-CN-Ph 4-pyridyl
104 3-CN-Ph 1-imidazolyl
105 3-CN-Ph 2-imidazolyl
106 3-CN-Ph 4-imidazolyl
107 3-CN-Ph 1-pyrazolyl
108 3-CN-Ph 3-pyrazolyl
109 3-CN-Ph 4-pyrazolyl
110 3-CN-Ph 2-thiazolyl
111 3-CN-Ph 4-thiazolyl
112 3-CN-Ph 5-tetrazolyl
113 3-CN-Ph 2-oxazolyl
114 3-CN-Ph 4-oxazolyl
115 3-CN-Ph C(O)N(2-imidazolyl)
116 3-CN-Ph C(O)N(4-imidazolyl)
117 3-CN-Ph C(O)N(2-thiazolyl)
118 3-CN-Ph C(O)N(4-thiazolyl)
119 3-CN-Ph C(O)N(2-oxazolyl)
120 3-CN-Ph C(O)N(4-oxazolyl)
121 3-CN-Ph C(O)N(3-pyrazolyl)
122 3-CN-Ph C(O)N(4-pyrazolyl)
123 3-CN-Ph C(O)N(2-pyridyl)
124 3-CN-Ph C(O)N(3-pyridyl)
125 3-CN-Ph C(O)N(4-pyridyl)
126 3-CN-Ph C(O)N(2-thienyl)
127 3-CN-Ph C(O)N(3-thienyl)
128 3-CN-Ph C(O)N(2-furanyl)
129 3-CN-Ph C(O)N(3-furanyl)
130 3-CN-Ph C(O)N(2-pyrrolyl)
131 3-CN-Ph C(O)N(3-pyrrolyl)
132 3-CN-Ph CH2(1-imidazolyl)
133 3-CN-Ph CH2(1-(1,2,3-triazolyl.))
134 3-CN-Ph CH2(2-(1,2,3-triazolyl))
135 3-CN-Ph CH2(1-(1,2,4-triazolyl.))
136 3-CN-Ph CH2(:1-pyrazolyl)
137 3-OMe-Ph ~ CN
138 3-OMe-Ph ' F
139 3-OMe-Ph C1
1 ~'= 3 -OMe- Ph CH2
0 OH
141 3-OMe-Ph OH
142 3-OMe-Ph NH2
143 3-OMe-Ph C02Me
144 3-OMe-Ph ~ C02Et
145 3-OMe-Ph ) CONH2
146 3-OMe-Ph NHPh
147 3-OMe-Ph ~e
148 3-OMe-Ph OMe
221
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
_149 3-OMe-Ph C(O)(2-imidazolyl)
150 3-OMe-Ph ~~~~ C(0)(4-imidazolyl)
151 3-OMe-Ph C(O)(2-thiazolyl)
152 3-OMe-Ph C(O)(4-thiazolyl)
153 3-OMe-Ph C(0)(2-oxazolyl)
154 3-OMe-Ph C(0)(4-oxazolyl)
155 3-OMe-Ph C{O)(3-pyrazolyl)
156 3-OMe-Ph C(O)(4-pyrazolyl)
157 3-OMe-Ph C(O)(5-tetrazolyl)
158 3-OMe-Ph C(0)(2-pyridyl)
159 3-OMe-Ph C(O)(3-pyridyl)
160 3-OMe-Ph C(O)(4-pyridyl)
161 3-OMe-Ph C(0)(2-thienyl)
162 3-OMe-Ph C(O)(3-thienyl)
163 3-OMe-Ph C(O)(2-furanyl)
164 3-OMe-Ph C(0)(3-furanyl)
165 3-OMe-Ph 2-thienyl
166 3-OMe-Ph 3-thienyl
167 3-OMe-Ph 2-furanyl
168 3-OMe-Ph 3-furanyl
169 3-OMe-Ph
2-PYridyl
170 3-OMe-Ph 3-PYridyl
171 3-OMe-Ph 4-pYridyl
172 3-OMe-Ph 1-imidazolyl
173 3-OMe-Ph 2-imidazolyl
174 3-OMe-Ph 4-imidazolyl
175 3-OMe-Ph
1-pYrazolyl
176 3-OMe-Ph 3-PYrazolyl
177 3-OMe-Ph 4-PYrazolyl
178 3-OMe-Ph 2-thiazolyl
179 3-OMe-Ph 4-thiazolyl
180 3-OMe-Ph 5-tetrazolyl
181 3-OMe-Ph 2-oxazolyl
182 3-OMe-Ph 4-oxazolyl
183 3-OMe-Ph C(O)N(2-imidazolyl)
184 3-OMe-Ph C(O)N(4-imidazolyl)
185 3-OMe-Ph C(O)N(2-thiazolyl)
186 3-OMe-Ph C(O)N(4-thiazolyl)
187 3-OMe-Ph C(O)N(2-oxazolyl)
188 3-OMe-Ph C(0)N(4-oxazolyl)
189 3-OMe-Ph C(O)N(3-pyrazolyl)
190 3-OMe-Ph C(O)N(4-pyrazolyl)
191 3-OMe-Ph C(0)N(2-pyridyl)
192 3-OMe-Ph
C(0)N(3-pyridyl)
193 3-OMe-Ph C(0)N(4-pyridyl)
194 3-OMe-Ph C(O)N(2-thienyl)
195 3-OMe-Ph C(O)N(3-thienyl)
196 3-OMe-Ph C(0)N(2-furanyl)
197 3-OMe-Ph C(0)N(3-furanyl)
222
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
19 8 3 -OMe-~ Ph C ( O ) N ( 2 -pyrro lyl )
199 3 -OMe-~Ph C ( O ) N ( 3 -pyrrolyl )
200 3-OMe-Ph CH2(1-imidazolyl)
201 3-OMe-Ph CH2(1-(1,2,3-triazolyl))
202 3-OMe-Ph CH2(2-(1,2,3-triazolyl))
203 3-OMe-Ph CH_2(1-(1,2,4-triazolyl))
204 3-OMe-_Ph ~ CH2(1-pyrazolyl)
205 3-C(O)Me-Ph CN
206 3-C(O)MES-Ph g
207 3-C(O)Me-Ph C1
208 3-C(OjMe-Ph CH20H
209 3-C(O)Me-Ph OH
210 3-C(0)ME~-Ph ~2
211 _ C02Me
3-C(0)Me-Ph
212 3-C(O)Me-Ph C02Et
213 3~-C(O)Me-Ph CONH2
214 3 -C ( O) Me-Ph kph
215 3-C(O)M
e-Ph
216 _ OMe
3-~C(O)Me-Ph
217 3-~C(O)Me-Ph C(O)(2-imidazolyl)
218 3-C(O)Me-Ph C(O)(4-imidazolyl)
219 3-C (O)Me-Ph C (O) (2-thiazolyl)
220 3-C(O)Me-Ph C(O)(4-thiazolyl)
221 3-C(O)Me~-Ph C(O) (2-oxazolyl)
222 3-C(O)Nte-ph C(0) (4-oxazolyl)
223 3-C(O)Me-Ph C(O)(3-pyrazolyl)
224 3-C(O)Me-Ph C(O)(4-pyrazolyl)
225 3-C(O)Me-Ph C(O)(5-tetrazolyl)
226 3-C(O)Me-Ph C(O)(2-pyridyl)
227 3-C(O)Me-Ph C(O)(3-pyridyl)
228 3-C(O)Me-Ph C(O)(4-pyridyl)
229 3-C(O)Me-Ph C(O)(2-thienyl)
230 3-C(O)Me-Ph C(O)(3-thienyl)
231 3-C(O)Me-Ph C(0)(2-furanyl)
232 3-C(O)Me-Ph C(O) (3-furanyl)
233 3-C(O)Me-Ph 2-thienyl
234 3-C(O)Me-Ph 3-thienyl
235 3-C(O)Me-Ph 2-furanyl
236 3-C(O)Me-Ph 3-furanyl
237 3-C(O)Me-Ph
2-PYridyl
238 3-C(O)Me-Ph 3-pyridyl
239 _ 4-pyridyl
3-C(O)Me-Ph
240 3-C(O)Me~-Ph 1-imidazolyl
241 3-C(O)Me~-Ph 2-imidazolyl
242 3-C(O)Me-Ph 4-imidazolyl
243 3-C(O)Me~-Ph 1-pyrazolyl
244 3-C(O)Me--Ph 3-pyrazolyl
245 3-~ (O)Me--Ph
4-PYrazolyl
246 3-C(O)Me--Ph 2-thiazolyl
223
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
247 3-C(O)Me-Ph 4-thiazolyl
248 3-C(O)Me-Ph 5-tetrazolyl
249 3-C(0)Me-Ph 2-oxazolyl
250 3-C(O)Me-Ph 4-oxazolyl
252 3-C(O)Me-Ph C(O)N(2-imidazolyl)
252 3-C(O)Me-Ph C(O)N(4-imidazolyl)
253 3-C(O)Me-Ph C(O)N(2-thiazolyl)
254 3-C(0)Me-Ph C(O)N(4-thiazolyl)
255 3-C(0)Me-Ph C(O)N(2-oxazolyl)
256 3-C(O)Me-Ph C(O)N(4-oxazolyl)
257 3-C(O)Me-Ph C(O)N(3-pyrazolyl)
258 3-C(O)Me-Ph C(O)N(4-pyrazolyl)
259 3-C(O)Me-Ph C(O)N(2-pyridyl)
260 3-C(O)Me-Ph C(0)N(3-pyridyl)
261 3-C(O)Me-Ph C(O)N(4-pyridyl)
262 3-C(O)Me-Ph C(O)N(2-thienyl)
263 3-C(O)Me-Ph C(O)N(3-thienyl)
264 3-C(O)Me-Ph C(O)N(2-furanyl)
265 3-C(O)Me-Ph C(0)N(3-furanyl}
266 3-C(O)Me-Ph C(O)N(2-pyrrolyl)
267 3-C(O)Me-Ph C(O)N(3-pyrrolyl)
268 3-C(O)Me-Ph CH2(I-imidazolyl)
269 3-C(O)Me-Ph CH2(1-(1,2,3-triazolyl))
270 3-C(O)Me-Ph CH2(2-(1,2,3-triazolyl))
271 3-C(O)Me-Ph CH2(1-(1,2,4-triazolyl))
272 3-C(O)Me-Ph CH2(1-pyrazolyl)
273 4-F-Ph CN
274 4-F-Ph F
275 4-F-Ph C1
276 4-F-Ph CH20H
277 4-F-Ph OH
278 4-F-Ph ~2
279 4-F-Ph C02Me
280 4-F-Ph C02Et
281 4-F-Ph CONH2
282 4-F-Ph NHPh
283 4-F-Ph
284 4-F-Ph OMe
285 4-F-Ph C(O)(2-imidazolyl)
286 4-F-Ph C(O)(4-imidazolyl)
287 4-F-Ph C(O)(2-thiazolyl)
288 4-F-Ph C(O)(4-thiazolyl)
289 4-F-Ph C(0)(2-oxazolyl)
290 4-F-Ph C(O)(4-oxazolyl)
291 4-F-Ph C(O)(3-pyrazolyl)
292 4-F-Ph C(O)(4-pyrazolyl)
293 4-F-Ph C(O)(5-tetrazolyl)
294 4-F-Ph C(O)(2-pyridyl)
295 4-F-Ph C(O)(3-pyridyl)
224
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
296 4-F-Ph C(0)(4-pyridyl)
297 4-F-Ph C(0)(2-thienyl)
298 4-F-Ph C(0) (3-thienyl)
299 4-F-Ph C (0) (2-furanyl )
300 4-F-Ph C(O) (3-furanyl)
301 4-F-Ph 2-thienyl
302 4-F-Ph 3-thienyl
303 4-F-Ph 2-furanyl
304 4-F--Ph 3-furanyl
305 4-F-Ph 2-pyridyl
306 4-F-Pla 3-pyridyl
307 4-F-Plz 4-pyridyl
308 4-F-Ph 1-imidazolyl
309 4-F-Plz 2-imidazolyl
310 4-F-Plz 4-imidazolyl
311 4-F-Ph 1-pyrazolyl
312 4-F-Ph 3-pyrazolyl
313 4-F-Ph 4-pyrazolyl
314 4-F-Ph 2-thiazolyl
315 4-F-Ph 4-thiazolyl
316 4-F-Ph 5-tetrazolyl
317 4-F-_Ph 2-oxazolyl
318 4-F-Ph 4-oxazolyl
319 4-F-Ph C(O)N(2-imidazolyl)
320 4-F-Ph C(O)N(4-imidazolyl)
321 4-F-Ph C(O)N(2-thiazolyl)
322 4-F-Ph C(O)N(4-thiazolyl)
323 4-F-Ph C(O)N(2-oxazolyl)
324 4-F-Ph C(O)N(4-oxazolyl)
325 4-F-Ph C(O)N(3-pyrazolyl)
326 4-F-Ph C(O)N(4-pyrazolyl)
327 4-F-Ph_ (O)N(2-pyridyl)
C
328 4-F-Ph _
C(O~)N(3-pyridyl)
_
329 4-F-Ph_ C(0)N(4-pyridyl)
330 4-F-Ph C(O)N(2-thienyl)
331 4-F-Ph C (O) N (3-thienyl )
332 4-F-Ph C(O)N(2-furanyl)
333 4-F-Ph C(O)N(3-furanyl)
334 4-F-Ph C(0)N(2-pyrrolyl)
335 4-F-Ph C(0)N(3-pyrrolyl)
336 4'-F-Ph CH2(1-imidazolyl)
337 4-F-Ph CH2(1-(1,2,3-triazolyl))
338 4-F-Ph CH2(2-(1,2,3-triazolyl))
339 4-F-Ph CH2(1-(1,2,4-triazolyl))
340 4-F-Ph CH2(1-pyrazolyl)
Table 7.
225
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
G'~;~ 0 G~ ~ ~~ H H G"~~~'~~. O
I
w-Y-N,.- ~ N N R ~,~N v~-y, N=~ N R
N-, N N R _If 3 3
Ri OH H H Ri \~OH 0 R1 '\OH H H
2a 3a
la '~i H H 0
0 N ~
G ~
%'
~~.~ N N
~ N. _N
' ~,~
N '~~ J~ R G ~,, ~ N ~~
~ R3 R3
Y N N z
Ri OH H H _~ Ri OOH H H
Rl ~OH O
lb 2b 3b
O .;~: H H /~ 0
~ .-
~, G ~~ N '.;~,.~ N ~ G i N ~~~J N N R3
R3 N R
~'.,~ N ~~
N 3
N Ri ~A.\OH O Ri OH H H
G
Ri ~
H H
OH 6 7
~ ~ ~'\
G N~ ~
~l I N~
/
Y _~ H
N (~ G
H H ~
i ' ~~
R ~
R
HN
N
OH HN,~N R OH HN~N R i
R 1 OH
~
R3
8a O 3
0 10 0
9a
/~1 y, /,~ ~~ ~ ~I
~
J
.~ ~N~J G~. G YN~~ H
-~N~
H
~
H _ RnOH ~ ~N R3
R ~OH HN.-N R R1 OH HN ~N R3
3
8b O 9b O 11 O
-,
'~ N .~ % H : ~
, ~'
~ N ~ ~ N
G
, ~ ,
Ri OH HN H ~.
N 1 '/
Ri j.~OH HN H
N ~
~ ~ Ri
R3 R3 OH HN~N R
3
12a O 13a O 12b O
G ~~~N'~ H
Ri ~OH H,N ~N R3
13b O
226
CA 02346933 2001-04-18
WO 00/35449 PCT/LIS99/30292
v
--1
G ,r N .~ : H G , .~ N ~: H
Ri ~~OH ~T~N R3 Ri OH ~~N R3
14 0 15 0
G '~ ~
N ~ H G '~ N
~~ H
R1 \OH ~~N R3 R1 OH ~yN R3
16a O 17a O
G~~N./~ H G~N~ H
1/
Rl 'OH ~ j~N R3 Ri OH ~~~N R3
16b O 17b O
~1 ~~1
~~N ~/'~ H ~ '~ , N .~ H
R ~OH ~~N R~ Ri OH ~ N R
3
18 O 19 O
G ~~I ~ O G ~~ ~.,, O
N,/ N~ N R3 N ,N.~N R3
R1 OH 20a H H Ri \OH 21a H H
I ~'\ O ~\1 O
N~~N~N R3 G. ~ N~w~N~N R3
R1 OH H H R~ H H H
20b 21b
~N~N~IN R2, ~~ N~",N~N.R2
R1 ~ G ~
R H H Ri 0H H H
1 OH 22 23
227
CA 02346933 2001-04-18
WO 00/35449 PCT/US99130292
G ~'~~~ OH H H OH H H OH H H
N,~~~,N,~~N G : N _ - _~N, : N ,N _. ',N, ,N
R3 - ~ R3 G~ ~ R3
0
R1 OH 24 Ri ~OH 25 0 Ri -v_OH 26 0
G ~~~~ OH H H '~ 1 -; OH H H ~~, OH H H
N~~wN N G~,~~ ,N'.-~ ,N N ' N~ N N
R3 ~' ~ Y R3 G \~ Y ~ R3
Ri OH Me O Ri ~~ OH Me 0 R ~~OH Me 0
27 28 29
G ~~~ OH H H ~ ~\; OH H H ~ OH H H
G,~yN,~~, ,N ,N '~'YN~1~N~N 3
N ~~ N , N ~l ~1 R 3 G ~ ~ R
' 1i R3
iPrO
R1~OH 1P~ Ri OH ipr0 R1 OH
1 30 31 32
G ~~i OH H H ~ ~~ ~'; OH H H ~ '~, OH H H
N~~,~N,~N G "r~,N_ ;~~ N N ~ v,,N_ -~, N N
~~ R3 _ ~~ Y R3 G _. i ~! R3
Ri OH iBuO Ri~~~OH iBuO Ri OH iBuO
33 34 35
Gy OH H H ~-~~ ~ OH H H /y OH H H
~N~~N~N R3 G~-'y~N_v.-~,~N~,N R3 G ,~N~N~N
' R
Ri OH Ph O Ri ~~OH Ph 0 Rig OH Ph O
36 37 38
G~ "~~ OH H H ~'; OH H H /~~ OH H H
N~-NON 3 G ~-~N~~~-NON R3 G~' N ~~N,i N R3
R
Rl 'OH ph ~ O Ri~~ OH phi 0 Ri OHphJ O
39 40 41
G H H i ,~ OH H H /'~ OH H H
~N~- N NR G~'~yNJ~N~NR3 ~ ,1V~N NR
3 ~ J ' G ~ ~ ~i 3
R OH ph O Ri OH ph O R1 OH ph O
1
42 43 44
G N y H G~N w ~ H ~ N
H
R ~ HN N G
R1 OH ~ ~ N R3 1 \OH ~! R3 Ri ' OH ~ ~ N R3
45 O 46 O 47 O
228
CA 02346933 2001-04-18
WO 00/35449 PCT/US99I30292
R1 = a) H, b) methyl, c) ethyl, d) n-propyl, e) allyl., f)
n-butyl, g) n-penty:l, and h) n-hexyl.
Entry G R3
1 4-F-Ph Ph
2 4-F-Ph 3-CN-Ph
3 4-F-Ph 3-COCH3-Ph
4 4-F-Ph 3-C02Me-Ph
4-F-Ph 3-C02Et-Ph
6 4-_F-Ph 3-C02H-Ph
7 4-F-Ph 3-CONH2-Ph
8 4-F-Ph 3-CONHMe-Ph
9 4-F-Ph 3-F-Ph
4-F-Ph 3-C1-Ph
11 4-F-Ph 3-Br-Ph
12 4-F-Ph 3-N02-Ph
13 4-F-Ph 3-NH2-Ph
14 4--F-Ph 3-NHMe-Ph
4--F-Ph 3-NMe2-Ph
16 4--F-Ph _ 3-NHCOCH3-Ph
17 4-F-Ph 3-S02NH2-Ph
18 4-F-Ph 3-S02NHMe-Ph
19 4-F-Ph 3-CF3-Ph
4-F-Ph 3-OCH3-Ph
21 4--F-Ph _ 3-OPh-Ph
22 4-F-Ph 3-OCF3-Ph
23 4-F-Ph 3-SCH3-Ph
24 4-F-Ph 3-SOCH3-Ph
4-F-Ph 3-S02CH3-Ph
26 4-F-Ph 3-OH-Ph
27 4-F-Ph 3-CH20H-Ph
28 4-F-Ph 3-CHOHCH3-Ph
29 4-F-Ph 3-COH(CH3)2-Ph
3 0 4 --F-Ph 3 -CHOHPh-Ph
31 4-F-Ph 3-CH3-Ph
32 4-F-Ph 3-C2H5-Ph
33 4-F-Ph 3-iPr-Ph
34 4-F-Ph 3-tBu-Ph
4-F-Ph 3-Ph-Ph
3 6 4 -F'- Ph 3 -CH2 Ph-Ph
37 4~-F'-Ph 3-CH2C02Me-Ph
38 4-F-Ph 3-(1- i eridin 1)-Ph
39 4~-F-Ph 3-(1- rrolidinyl)-Ph
4-F'-Ph 3-(2-imidazolyl)-Ph
41 4-F-Ph 3-(1-imidazol 1)-Ph
42 4-F'-Ph 3-(2-thiazol 1)-Ph
43 4-F'-Ph 3- (3- razol 1) -Ph
44 4-F'-Ph 3- ( 1-pyrazol 1 ) -Ph
4-F'-Ph 3- ( 1-tetrazol 1 ) -Ph
46 4-F-Ph 3- ( 5-tetrazol 1 ) -Ph
47- 4-F-Ph 3-(2- rid 1)-Ph
48 4-F'-Ph 3-(2-thien 1)-Ph
229
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
49 4-F-Ph 3-(2-furanyl)-Ph
50 4-F-Ph 4-CN-Ph
51 4-F-Ph _
4-COCH3-Ph
52 4-F-Ph 4-C02Me-Ph
53 4-F-Ph 4-C02Et-Ph
54 4-F-Ph 4-C02H-Ph
55 4-F-Ph 4-CONH2-Ph
56 4-F-Ph 4-CONHMe-Ph
57 4-F-Ph 4-CONHPh-Ph
58 4-F-Ph 4-NHCONH2-Ph
59 4-F-Ph 4-F-Ph
60 4-F-Ph 4-C1-Ph
61 4-F-Ph 4-Br-Ph
62 4-F-Ph 4-N02-Ph
63 4-F-Ph 4-NH2-Ph
64 4-F-Ph 4-NHMe-Ph
65 4-F-Ph 4-NMe2-Ph
66 4-F-Ph 4-NHCOCH3-Ph
67 4-F-Ph 4-S02NH2-Ph
68 4-F-Ph 4-S02NHMe-Ph
69 4-F-Ph 4-CF3-Ph
70 4-F-Ph 4-OCH3-Ph
71 4-F-Ph 4-OPh-Ph
72 4-F-Ph 4-OCF3-Ph
73 4-F-Ph 4-SCH3-Ph
74 4-F-Ph 4-SOCH3-Ph
75 4-F-Ph 4-S02CH3-Ph
76 4-F-Ph 4-OH-Ph
77 4-F-Ph 4-CH20H-Ph
78 4-F-Ph 4-CHOHCH3-Ph
79 4-F-Ph 4-COH(CH3)2-Ph
80 4-F-Ph 4-CH3-Ph
81 4-F-Ph 4-C2H5-Ph
82 4-F-Ph 4-iPr-Ph
83 4-F-Ph 4-tBu-Ph
84 4-F-Ph 4-Ph-Ph
85 4-F-Ph 4-CH2Ph-Ph
86 4-F-Ph 4-CH2C02Me-Ph
87 4-F-Ph 4-(1- i eridin 1)-Ph
88 4-F-Ph 4-(1- rrolidin 1)-Ph
89 4-F-Ph 4-(2-imidazol 1)-Ph
90 4-F-Ph 4-(1-imidazol 1)-Ph
91 4-F-Ph 4-(2-thiazolyl)-Ph
92 4-F-Ph 4-(3- razolyl)-Ph
93 4-F-Ph 4-(1- razol 1)-Ph
94 4-F-Ph 4-(1-tetrazol 1)-Ph
95 4-F-Ph 4-(5-tetrazolyl)-Ph
96 4-F-Ph 4-(2- rid 1)-Ph
97 4-F-Ph 4-(2-thienyl)-Ph
98 4-F-Ph 4-(2-furan 1)-Ph
99 4-F-Ph 2-CN-Ph
100 4-F-Ph 2-COCH3-Ph
101 4-F-Ph 2-C02Me-Ph
230
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
102 4-F-Ph 2-C02Et-Ph
103 4-F--Ph 2-C02H-Ph
104 4-F--Ph 2-CONH2-Ph
105 4-F--Ph 2-CONHMe-Ph
106 4-F--Ph 2-F-Ph
107 4-F--Ph 2-Cl-Ph
108 4-F--Ph 2-Br-Ph
109 4-F~-Ph 2-N02-Ph
110 4 -F ~- Ph 2 -NH2 - Ph
111 4 - F ~-Ph 2 -NF~ie-Ph
112 4-F~-Ph 2-NMe2-Ph
113 4-F-Ph 2-NHCOCH3-Ph
114 4-_F-Ph 2-SO2NH2-Ph
I15 4-F-Ph 2-S02NHMe-Ph
116 4-F-Ph 2-CF3-Ph
117 4-F-Ph 2-OCH3-Ph
118 4-F-Ph 2-OPh-Ph
119 4-F-Ph 2-OCF3-Ph
120 4-F-Ph 2-SCH3-Ph
121 4-F-Ph 2-SOCH3-Ph
122 4-F-Ph 2-S02CH3-Ph
123 4-F-Ph 2-OH-Ph
124 4-F-Ph 2-CH20H-Ph
125 4-F-Ph 2-CHOHCH3-Ph
126 4-F-Ph 2-COH(CH3)2-Ph
127 4-F-Ph 2-CHOHPh-Ph
128 4-F-Ph 2-CH3-Ph
129 F-Ph 2-C2H5-Ph
4-
130 _ 2-iPr-Ph
4-F-Ph
131 F-Ph 2-tBu-Ph
4-
I32 _ 2-Ph-Ph_
4-F-Ph
133 4-F-Ph 2-CH2Ph-Ph
134 4-F-Ph 2-CH2C02Me-Ph
135 4--F-Ph 2- ( 1- i eridin 1) -Ph
136 4-F-Ph 2-(1-p rrolidinyl)-Ph
137 4--F-Ph 2- (2-imidazol 1) -Ph
138 4-F-Ph 2-(1-imidazol 1)-Ph
139 4-F-Ph 2-(2-thiazol 1)-Ph
140 4-F-Ph 2-(3-pyrazolyl)-Ph
141 4--F-Ph 2- ( 1- razol 1 ) -Ph
142 4-F-Ph 2-(1-tetrazol 1)-Ph
143 4-F-Ph 2-(5-tetrazolyl)-Ph
144 4-F-Ph 2-pyrid 1)-Ph
2-(
145 4-F-Ph -
~-~(2-thien 1)-Ph
146 4--F-Ph 2- (2-furan 1) -Ph
147 4-F-Ph 2,4-diF-Ph
148 4-F-Ph _ 2,5-diF-Ph
149 4-F-Ph 2,6-diF-Ph
150 4-F-Ph ___ 3,4-diF_-Ph
~~
151 4-F-Ph 3,5-diF-Ph
152 4-F-Ph 2,4-diCl-Ph
153 4-~F-Ph 2,5-diCl-Ph
154 4-F-Ph 2,6-diCl-Ph
231
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
155 4-F-Ph 3,4-diCl-Ph
156 4_-F-Ph 3,5-diCl-Ph
157 4-F-Ph 3,4-diCF3-Ph
158 4-F-Ph 3,5-diCF3-Ph
159 4-F-Ph 5-C1-2-Me0-Ph
160 4-F-Ph 5-C1-2-Me-Ph
161 4-F-Ph 2-F-5-Me-Ph
162 4-F-Ph 2-F-5-N02-Ph
163 4-F-Ph 3,4-OCH20-Ph
164 4-F-Ph 3,4-OCH2CH20-Ph
165 4-F-Ph 2-Me0-4-Me-Ph
166 4-F-Ph 2-Me0-5-Me-Ph
167 4-F-Ph 1-na hth 1
168 4-F-Ph 2-na hthyl
169 4-F-Ph 2-thien 1
170 4-F-Ph 3-thien 1
171 4-F-Ph 2-furan 1
172 4-F-Ph 3-furan 1
173 4-F-Ph 2- ridyl
174 4-F-Ph 3- rid 1
175 4-F-Ph 4- rid 1
176 4-F-Ph 2-indol 1
177 4-F-Ph 3-indol 1
178 4-F-Ph 5-indol 1
179 4-F-Ph 6-indol 1
180 4-F-Ph 3-indazol 1
181 4-F-Ph 5-indazol 1
182 4-F-Ph 6-indazolyl
183 4-F-Ph 2-imidazol 1
184 4-F-Ph
3-p razol 1
185 4-F-Ph 2-thiazol 1
186 4-F-Ph 5-tetrazol 1
187 4-F-Ph 2-benzimidazol 1
188 4-F-Ph 5-benzimidazol 1
189 4-F-Ph 2-benzothiazolyl
190 4-F-Ph 5-benzothiazol 1
191 4-F-Ph 2-benzoxazal 1
192 4-F-Ph 5-benzoxazol 1
193 4-F-Ph 1-adamantyl
194 4-F-Ph 2-adamant 1
295 4-F-Ph t-Bu
196 2-F-Ph 3-CN-Ph
197 2-F-Ph 3-COCH3-Ph
198 2-F-Ph 3-C02Me-Ph
199 2-F-Ph 3-C02Et-Ph
200 2-F-Ph 3-C02H-Ph
201 2-F-Ph 3-CONH2-Ph
202 2-F-Ph 3-F-Ph
203 2-F-Ph 3-C1-Ph
204 2-F-Ph 3-NH2-Ph
205 2-F-Ph 3-S02NH2-Ph
206 2-F-Ph 3-CF3-Ph
207 2-F-Ph 3-OCH3-Ph
232
CA 02346933 2001-04-18
WO 00/35449 PCT/1JS99/30292
208 2-F-Ph 3-OEt-Ph
209 2-F-Ph 3-OCF3-Ph
210 2-F-Ph 3-S02CH3-Ph
211 2-F-Ph 3-OH-Ph
212 2-F-Ph 3-CH3-Ph
213 2-F-Ph 3-C2H5-Ph
214 2-F-Ph 4-CN-Ph
215 2-F-Ph 4-COCH3-Ph
216 2-F-Ph 4-C02Me-Ph
217 2-F-Ph 4-C02Et-Ph
218 2-F-Ph 4-C02H-Ph
219 2-F-Ph 4-CONH2-Ph
220 2-F-Ph 4-F-Ph
221 2-F-Ph 4-C1-Ph
222 2-F-Ph 4-NH2-Ph
223 _ 4-S02NH2-Ph
2--F-Ph
224 2--F-Ph _ _
~~ 4-CF3-Ph
225 2-F-Ph 4-OCH3-Ph
226 2-F-Ph 4-OEt-Ph
227 2-F-Ph 4-OCF3-Ph
228 2-F-Ph 4-S02CH3-Ph
229 2 --F'-Ph 4-OH-Ph
230 2-F-Ph 4-CH3-Ph
231 2-F-Ph 4-C2H5-Ph
232 _ 2,4-diF-Ph
2-F-Ph
233 2--F'-Ph 2, 5-diF-Ph
234 2-F-Ph 3,4-diF-Ph
235 2-F-Ph 3,5-diF-Ph
236 2-F-Ph 2,4-diCl-Ph
237 2-F-Ph 2,5-diCl-Ph
238 2-F-Ph 3,4-diCl-Ph
239 2-F-Ph 3,5-diCl-Ph
240 2-F-Ph 3,4-OCH20-Ph
241 2-F-Ph 3,4-OCH2CH20-Ph
242 _ 2-thien 1
2--F-Ph
243 2-F-Ph 2-furan 1
244 2-F-Ph _
2-p~yridyl
245 _ 4- rid 1
2-F-Ph
246 2--F-Ph 2-imidazol 1
247 2--F-Ph 3- razol 1
248 2-F-Ph 2-thiazol 1
249 2-F-Ph 5-tetrazol 1
250 2-F-Ph 1-adamant 1
251 2 , 4 --diF-Ph 3 -CN-Ph
252 2,4-diF-Ph 3-COCH3-Ph
253 2,4-diF-Ph 3-C02Me-Ph
254 2,4-diF-Ph 3-C02Et-Ph
255 2,4-diF-Ph 3-C02H-Ph
256 2,4-d_iF-Ph 3-C
ONH2-Ph
257 2,4-d.iF-Ph _
3-
F-Ph
258 2, 4-d:iF-Ph _
3-C1-Ph
259 2,4-diF-Ph _
3-NH2-Ph
260 2,4-d:iF-Ph 3-S02NH2-Ph
233
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
261 2,4-diF-Ph 3-CF3-Ph
262 2,4-diF-Ph 3-OCH3-Ph
263 2,4-diF-Ph 3-OEt-Ph
264 2,4-diF-Ph 3-OCF3-Ph
265 2,4-diF-Ph 3-S02CH3-Ph
266 2,4-diF-Ph 3-OH-Ph
267 2,4-diF-Ph 3-CH3-Ph
268 2,4-diF-Ph 3-C2H5-Ph
269 2,4-diF-Ph 4-CN-Ph
270 2,4-diF-Ph 4-COCH3-Ph
271 2,4-diF-Ph 4-C02Me-Ph
272 2,4-diF-Ph 4-C02Et-Ph
273 2,4-diF-Ph 4-C02H-Ph
274 2 , 4-diF-Ph 4-CONFi2-Ph
275 2,4-diF-Ph 4-F-Ph
276 2,4-diF-Ph 4-C1-Ph
277 2,4-diF-Ph 4-NH2-Ph
278 2,4-diF-Ph 4-S02NH2-Ph
279 2,4-diF-Ph 4-CF3-Ph
280 2,4-diF-Ph 4-OCH3-Ph
281 2,4-diF-Ph 4-OEt-Ph
282 2,4-diF-Ph 4-OCF3-Ph
283 2,4-diF-Ph 4-S02CH3-Ph
284 2,4-diF-Ph 4-OH-Ph
285 2,4-diF-Ph 4-CH3-Ph
286 2,4-diF-Ph 4-C2H5-Ph
287 2,4-diF-Ph 2,4-diF-Ph
288 2,4-diF-Ph 2,5-diF-Ph
289 2,4-diF-Ph 3,4-diF-Ph
290 2,4-diF-Ph 3,5-diF-Ph
291 2,4-diF-Ph 2,4-diCl-Ph
292 2,4-diF-Ph 2,5-diCl-Ph
293 2,4-diF-Ph 3,4-diCl-Ph
294 2,4-diF-Ph 3,5-diCl-Ph
295 2,4-diF-Ph 3,4-OCH20-Ph
296 2,4-diF-Ph 3,4-OCH2CH20-Ph
297 2,4-diF-Ph 2-thien 1
298 2,4-diF-Ph 2-furan 1
299 2,4-diF-Ph 2- rid 1
300 2,4-diF-Ph 4- rid 1
301 2,4-diF-Ph 2-imidazol 1
302 2,4-diF-Ph 3- razol 1
303 2,4-diF-Ph 2-thiazolyl
304 2,4-diF-Ph 5-tetrazol 1
305 2,4-diF-Ph 1-adamant 1
306 4-C1-Ph Ph
307 4-Cl-Ph 3-CN-Ph
308 4-C1-Ph 3-COCH3-Ph
309 4-C1-Ph 3-C02Me-Ph
310 4-C1-Ph 3-C02Et-Ph
311 4-C1-Ph 3-C02H-Ph
312 4-C1-Ph 3-CONH2-Ph
313 4-Cl-Ph 3-CONHMe-Ph
234
CA 02346933 2001-04-18
WO OOI35449 PCT/US99/30292
314 4-C1-Ph 3-F-Ph
315 4-C1-Ph 3-Cl-Ph
316 4-C1-Ph 3-Hr-Ph
317 4-C1-Ph 3-N02-Ph
318 4-C1-Ph 3-NH2-Ph
319 4-C1-Ph 3-NHMe-Ph
320 4-C1-Ph 3-NMe2-Ph
321 4-C1-Ph _
3-NHCOCH3-Ph
322 4-C1-Ph 3-S02NH2-Ph
323 4-C1-Ph 3-S02NHMe-Ph
324 4-C1-Ph 3-CF3-Ph
325 4-C1-Ph 3-OCH3-Ph
326 4-C1-Ph 3-OPh-Ph
327 4-C1-Ph 3-OCF3-Ph
328 4-C1-Ph 3-SCH3-Ph
329 4-C1-Ph 3-SOCH3-Ph
330 4-C1-Ph 3-S02CH3-Ph
331 4-C1-Ph 3-OH-Ph
332 4-C1-Ph 3-CH20H-Ph
333 4-C1-Ph 3-CHOHCH3-Ph
334 4-C1-Ph 3-COH(CH3)2-Ph
335 4-C1-Ph 3-CHOHPh-Ph
336 4-C1-Ph 3-CH3-Ph
337 4-C1-Ph 3-C2H5-Ph
338 4-C1-Ph 3-iPr-Ph
339 4-C1-Ph 3-tBu-Ph
340 4-C1-Ph 3-Ph-Ph
341 4-C1-Ph 3-CH2Ph-Ph
342 4-C1-Ph 3-CH2C02Me-Ph
343 4-C1-Ph 3-(1- i eridin 1)-Ph
344 4-C1-Ph 3-(1- yrrolidin 1)-Ph
345 4-Cl-Ph 3-(2-imidazol 1)-Ph
346 4-C1-Ph 3-(1-imidazol 1)-Ph
347 4-Cl-Ph 3-(2-thiazol 1)-Ph
348 4-C1-Ph 3-(3- razolyl)-Ph
349 4-C1-Ph 3-(1-pyrazol 1)-Ph
350 4-C1-Ph 3-(1-tetrazol 1)-Ph
351 4-C1-Ph 3-(5-tetrazol 1)-Ph
352 4-C1-Ph 3-(2- rid 1)-Ph
353 4-C1-Ph 3-(2-thien 1)-Ph
354 4-C1-Ph 3-(2-furan 1)-Ph
355 4-C1-Ph 4-CN-Ph
356 4-Cl-Ph 4-COCH3-Ph
357 4-C1-Ph 4-C02Me-i~h
358 4-Cl-Ph 4-C02Et-Ph
359 4-C1-Ph 4-C02H-Ph
360 4-C1.-Ph 4-CONH2-Ph
361 4-C1.-Ph 4-CONHMe-Ph
362 4-C1.-Ph 4-CONHPh-Ph
363 4-C~.-Ph 4-NHCONH2-Ph
3 64 4-C1.-Ph 4-F-Ph
365 4-C~.-Ph 4-Cl-Ph
366 4-C1.-Ph 4-Br-Ph
235
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
367 4-Cl-Ph 4-N02-Ph
368 4-Cl-Ph 4-NH2-Ph
~
369 4-Cl-Ph 4-NHMe-Ph
370 4-Cl-Ph 4-NMe2-Ph
371 4-CI-Ph 4-NHCOCH3-Ph
372 4-CI-Ph 4-S02NH2-Ph
373 4-Cl-Ph 4-S02NHMe-Ph
374 4-C1-Ph 4-CF3-Ph
375 4-Cl-Ph 4-OCH3-Ph
376 4-C1-Ph 4-OPh-Ph
377 4-C1-Ph 4-OCF3-Ph
378 4-Cl-Ph 4-SCH3-Ph
379 4-CI-Ph 4-SOCH3-Ph
380 4-C1-Ph 4-S02CH3-Ph
381 4-CI-Ph 4-OH-Ph
382 4-Cl-Ph 4-CH20H-Ph
383 4-Cl-Ph 4-CHOHCH3-Ph
384 4-C1-Ph 4-COH(CH3)2-Ph
385 4-C1-Ph 4-CH3-Ph
386 4-C1-Ph 4-C2H5-Ph
387 4-Cl-Ph 4-iPr-Ph
388 4-C1-Ph 4-tBu-Ph
389 4-C1-Ph 4-Ph-Ph
390 4-C1-Ph 4-CH2Ph-Ph
391 4-Cl-Ph 4-CH2C02Me-Ph
392 4-Cl-Ph 4-(1- i eridin 1)-Ph
393 4-C1-Ph 4-(1-p rrolidinyl)-Ph
394 4-C1-Ph 4-(2-imidazol 1)-Ph
395 4-Cl-Ph 4-(1-imidazolyl)-Ph
396 4-Cl-Ph 4-(2-thiazol 1)-Ph
397 4-Cl-Ph 4-(3-p azol 1)-Ph
398 4-Cl-Ph 4-(1- razol 1)-Ph
399 4-C1-Ph 4-(1-tetrazol 1)-Ph
400 4-C1-Ph 4-(5-tetrazol 1)-Ph
401 4-Cl-Ph 4-(2- rid 1)-Ph
402 4-Cl-Ph 4-(2-thien 1)-Ph
403 4-Cl-Ph 4-(2-furan 1)-Ph
404 4-CI-Ph 2-CN-Ph
405 4-Cl-Ph 2-COCH3-Ph
406 4-C1-Ph 2-C02Me-Ph
407 4-C1-Ph 2-C02Et-Ph
408 4-Cl-Ph 2-C02H-Ph
409 4-CI-Ph 2-CONH2-Ph
410 4-C1-Ph 2-CONHMe-Ph
411 4-Cl-Ph 2-F-Ph
412 4-C1-Ph 2-C1-Ph
413 4-CI-Ph 2-Br-Ph
414 4-C1-Ph 2-N02-Ph
415 4-Cl-Ph 2-NH2-Ph
416 4-C1-Ph 2-NHMe-Ph
417 4-C1-Ph 2-NMe2-Ph
418 4-Cl-Ph 2-NHCOCH3-Ph
419 4-CI-Ph 2-S02NH2-Ph
236
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
420 4-C1-Ph 2-S02NHMe-Ph
421 4-C~-Ph 2-CF3-Ph
422 4-Cl-Ph 2-OCH3-Ph
423 4-Cl-Ph 2-OPh-Ph
424 4-C1-Ph 2-OCF3-Ph
425 4-Cl-Ph 2-SCH3-Ph
426 4-C1-Ph 2-SOCH3-Ph
427 _ 4-C1-Ph 2-S02CH3-Ph
428 4-C1-Ph 2-OH-Ph
429 4-C1-Ph 2-CH20H-Ph
430 4-C1-Ph 2-CHOHCH3-Ph
431_ 4-C1-Ph 2-COH(CH3)2-Ph
432 ~ 4-C1-Ph 2-CHOHPh-Ph
433 4-C1-Ph _
2-CH3-Ph
434 4-C1-Ph 2-C2H5-Ph
~
435 4-C1-Ph 2-iPr-Ph
436 4-C1-Ph 2-tBu-Ph
_ _
_
437 4-C1-Ph _
2-Ph-Ph
438 4-C1-Ph 2-CH2Ph-Ph
439 4-C1-Ph 2-CH2C02Me-Ph
440 4-C1-Ph 2-(1- i eridin 1)-Ph
441 4-C1-Ph 2-(1- rrolidin 1)-Ph
442 4-C1-Ph 2-(2-imidazol 1)-Ph
443 4-C1-Ph 2-(1-imidazol 1)-Ph
444 4-C1-Ph 2-(2-thiazol 1)-Ph
445 4-C1-Ph 2-(3-p razol 1)-Ph
446 4-C1-Ph 2-(1- azol I)-Ph
447 4-C1-Ph 2-(1-tetraaol 1)-Ph
448 4-C1-Ph 2-(5-tetrazol 1)-Ph
449 4-C1-Ph 2-(2- rid 1)-Ph
450 4-C1-Ph 2-(2-thien 1)-Ph
451 4-C1-Ph 2-t2-furan 1)-Ph
452 4-C1-Ph 2,4-diF-Ph
453 4-C1-Ph 2,5-diF-Ph
454 4-C1-Ph 2,6-diF-Ph
455 4-Cl-Ph 3,4-diF-Ph
456 4-Cl-Ph 3,5-diF-Ph
457 4-C1-Ph 2,4-diCl-Ph
458 _ _
4-C1-Ph 2,5-diCl-Ph
459 4-C1-Ph 2,6-diCl-Ph
460 4-C1-Ph 3,4-diCl-Ph
461 4-C1-Ph 3,5-diCl-Ph
462 4-C1-Ph 3,4-diCF3=Ph
463 4-C1-Ph 3,5-diCF3-Ph
464 4-C1-Ph 5-C1-2-Me0-Ph
465 4-C1-Ph 5-C1-2-Me-Ph
466 4-C1-Ph 2-F-5-Me-Ph
467 4-C1-Ph 2-F-5-N02-Ph
468 4-C1-Ph 3,4-OCH20-Ph
469 4-C1-Ph 3,4-OCH2CH20-Ph
470 4-C1-Ph 2-Me0-4-Me-Ph
471 4-C1-Ph 2-Me0-5-Me-Ph
472 4-C1-Ph 1-na hth 1
237
CA 02346933 2001-04-18
WO 00!35449 PCT/US99/30292
473 4-C1-Ph 2-naphth 1
474 4-Cl-Ph 2-thien 1
475 4-C1-Ph 3-thienyl
476 4-C1-Ph 2-furan 1
477 4-C1-Ph 3-furanyl
478 4-Cl-Ph 2- rid 1
479 4-Cl-Ph 3- ridyl
480 4-C1-Ph 4- ridyl
481 4-C1-Ph 2-indol 1
482 4-Cl-Ph 3-indol 1
483 4-C1-Ph 5-indol 1
484 4-C1-Ph 6-indol 1
485 4-C1-Ph 3-indazol 1
486 4-C1-Ph 5-indazol 1
487 4-C1-Ph 6-indazol 1
488 4-C1-Ph 2-imidazol 1
489 4-C1-Ph 3- yrazolyl
490 4-Cl-Ph 2-thiazol 1
491 4-C1-Ph 5-tetrazol 1
492 4-C1-Ph 2-benzimidazol 1
493 4-C1-Ph 5-benzimidazol 1
494 4-C1-Ph 2-benzothiazol 1
495 4-C1-Ph 5-benzothiazolyl
496 4-C1-Ph 2-benzoxazol 1
497 4-Cl-Ph 5-benzoxazol 1
498 4-C1-Ph 1-adamant 1
499 4-C1-Ph 2-adamant 1
500 4-C1-Ph t-Bu
501 2-C1-Ph 3-CN-Ph
502 2-C1-Ph 3-COCH3-Ph
503 2-C1-Ph 3-C02Me-Ph
504 2-C1-Ph 3-C02Et-Ph
505 2-C1-Ph 3-C02H-Ph
506 2-C1-Ph 3-CONH2-Ph
507 2-C1-Ph 3-F-Ph
508 2-C1-Ph 3-C1-Ph
509 2-C1-Ph 3-NH2-Ph
510 2-C1-Ph 3-S02NH2-Ph
511 2-C1-Ph 3-CF3-Ph
512 2-C1-Ph 3-OCH3-Ph
513 2-C1-Ph 3-OEt-Ph
514 2-C1-Ph 3-OCF3-Ph
515 2-Cl-Ph 3-S02CH3-Ph
516 2-C1-Ph 3-OH-Ph
527 2-C1-Ph 3-CH3-Ph
518 2-C1-Ph 3-C2H5-Ph
519 2-Cl-Ph 4-CN-Ph
520 2-C1-Ph 4-COCH3-Ph
521 2-Cl-Ph 4-C02Me-Ph
522 2-C1-Ph 4-C02Et-Ph
523 2-C1-Ph 4-C02H-Ph
524 2-C1-Ph 4-CONH2-Ph
525 2-Cl-Ph 4-F-Ph
238
CA 02346933 2001-04-18
. WO 00/35449 PCT/US99/30292
526 2-C1-Ph 4-C1-Ph
527 2-Cl-Ph 4-NH2-Ph
528 2-C1-Ph 4-S02NH2-Ph
529 2-C3.-Ph 4-CF3-Ph
530 2-C1-Ph 4-OCH3-Ph
531 2-C1-Ph 4-OEt-Ph
532 2-C1-Ph 4-OCF3-Ph
533 2-C1-Ph 4-S02CH3-Ph
534 2-C1-Ph 4-OH-Ph
535 2-C1-Ph 4-CH3-Ph
536 2-C1-Ph 4-C2H5-Ph
537 2-C1-Ph 2,4-diF-Ph
538 2-C1-Ph 2,5-diF-Ph
539 2-Cl-Ph 3,4-diF-Ph
540 2-C1-Ph 3,5-diF-Ph
541 2-C1-Ph 2,4-diCl-Ph
542 2-C1-Ph 2,5-diCl-Ph
543 2-Cl-Ph 3,4-diCl-Ph
544 2-C1-Ph 3,5-diCl-Ph
545 2-C1-Ph 3,4-OCH20-Ph
546 2-C1-Ph 3,4-OCH2CH20-Ph
547 2-C1-Ph 2-thien 1
548 2-C1-Ph 2-furan 1
549 2-C1-Ph 2- id 1
550 2-C1-Ph 4- rid 1
551 2-C_1-Ph 2-imidazol 1
552 2-C1-Ph 3-p razol 1
553 2-C1-Ph 2-thiazol 1
554 2-C1-Ph 5-tetrazol 1
555 2-C1-Ph 1-adamant 1
556 2,4-diCl-Ph 3-CN-Ph
557 2,4-diCl-Ph 3-COCH3-Ph
558 2,4-diCl-Ph 3-C02Me-Ph
559 2,4-diCl-Ph 3-C02Et-Ph
560 2,4-diCl-Ph 3-C02H-Ph
561 2,4-diCl-Ph 3-CONH2-Ph
562 2,4-diCl-Ph 3-F-Ph
563 2,4-diCl-Ph 3-C1-Ph
564 2,4-diCl-Ph 3-NH2-Ph
565 2,4-diCl-Ph 3-S02NH2-Ph
566 2,4-diCl-Ph 3-CF3-Ph
567 2,4-diCl-Ph 3-OCH3-Ph
568 2,4-diCl-Ph 3-OEt-Ph
569 2,4-diCl-Ph 3-OCF3-Ph
570 2,4-diCl-Ph 3-S02CH3-Ph
571 2,4-diCl-Ph 3-OH-Ph
572 2,4-diC1-Ph 3-CH3-Ph
573 2,4-di.Cl-Ph 3-C2H5-Ph
574 2,4-diCl-Ph 4-CN-Ph
575 2,4-diCl-Ph 4-COCH3-Ph
576 2,4-diCl-Ph 4-C02Me-Ph
577 2,4-diCl-Ph 4-C02Et-Ph
578 2,4-diCl-Ph ~
4-C02H-Ph
239
CA 02346933 2001-04-18
WO OOI35449 PCT/US99/3~7292
579 2,4-diCl-Ph 4-CONH2-Ph
580 2,4-diCl-Ph _
4-F-Ph
581 2,4-diCl-Ph 4-C1-Ph
582 2,4-diCl-Ph 4-NH2-Ph
583 2,4-diCl-Ph 4-S02NH2-Ph
584 2,4-diCl-Ph 4-CF3-Ph
585 2,4-diCl-Ph 4-OCH3-Ph
586 2,4-diCl-Ph 4-OEt-Ph
587 2,4-diCl-Ph 4-OCF3-Ph
588 2,4-diCl-Ph 4-S02CH3-Ph
589 2,4-diCl-Ph 4-OH-Ph
590 2,4-diCl-Ph 4-CH3-Ph
591 2,4-diCl-Ph 4-C2H5-Ph
592 2,4-diCl-Ph 2,4-diF-Ph
593 2,4-diCl-Ph 2,5-diF-Ph
594 2,4-diCl-Ph 3,4-diF-Ph
595 2,4-diCl-Ph 3,5-diF-Ph
596 2,4-diCl-Ph 2,4-diCl-Ph
597 2,4-diCl-Ph 2,5-diCl-Ph
598 2,4-diCl-Ph 3,4-diCl-Ph
599 2,4-diCl-Ph 3,5-diCl-Ph
600 2,4-diCl-Ph 3,4-OCH20-Ph
601 2,4-diCl-Ph 3,4-OCH2CH20-Ph
602 2,4-diCl-Ph 2-thien 1
603 2,4-diCl-Ph 2-furan 1
604 2,4-diCl-Ph 2- rid 1
605 2,4-diCl-Ph 4- ridyl
606 2,4-diCl-Ph 2-imidazol 1
607 2,4-diCl-Ph 3- razal 1
608 2,4-diCl-Ph 2-thiazol 1
609 2,4-diCl-Ph 5-tetrazolyl
610 2,4-diCl-Ph 1-adamant 1
611 3-OCH3-Ph 3-CN-Ph
612 3-OCH3-Ph 3-COCH3-Ph
613 3-OCH3-Ph 3-C02Me-Ph
614 3-OCH3-Ph 3-C02Et-Ph
615 3-OCH3-Ph 3-C02H-Ph
616 3-OCH3-Ph 3-CONH2-Ph
617 3-OCH3-Ph 3-F-Ph
618 3-OCH3-Ph 3-C1-Ph
619 3-OCH3-Ph 3-NH2-Ph
620 3-OCH3-Ph 3-S02NH2-Ph
621 3-OCH3-Ph 3-CF3-Ph
622 3-OCH3-Ph 3-OCH3-Ph
623 3-OCH3-Ph 3-OEt-Ph
624 3-OCH3-Ph 3-OCF3-Ph
625 3-OCH3-Ph 3-S02CH3-Ph
626 3-OCH3-Ph 3-OH-Ph
627 3-OCH3-Ph 3-CH3-Ph
628 3-OCH3-Ph 3-C2H5-Ph
629 3-OCH3-Ph 4-CN-Ph
630 3-OCH3-Ph 4-COCH3-Ph
631 3-OCH3-Ph 4-C02Me-Ph
240
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
632 3-OCH3-Ph 4-C02Et-Ph
633 3-OCH3-Ph 4-C02H-Ph
634 3-OCH3-Ph 4-CONH2-Ph
635 3-OCH.3-ph 4-F-Ph
636 3-OCH3-Ph 4-C1-Ph
637 3-OCH3-Ph 4-NH2-Ph
638 3-OCH3-Ph 4-S02NH2-Ph
639 3-OCH3-Ph 4-CF3-Ph
640 3-OCH3-Ph 4-OCH3-Ph
641 3-OCH3-Ph 4-OEt-Ph
642 3-OCH3-Ph 4-OCF3-Ph
643 3-OCH3-Ph 4-S02CH3-Ph
644 3-OCH3-P 4-OH-Ph
h
645 _ 4-CH3-Ph
3-OCH3-Ph
646 3-OCH3-Ph- 4-C2H5-Ph
647 3-OCH3-Ph 2,4-diF-Ph
648 3-OCH3-Ph 2,5-diF-Ph
649 3-OCH3-Ph 3,4-diF-Ph
650 3-OCH3-Ph 3,5-diF-Ph
651 3-OCH3-Ph 2,4-diCl-Ph
652 3-OCH3-Ph 2,5-diCl-Ph
653 _3-OCH3-Ph 3,4-diCl-Ph
654 3-OCH3-Ph _
~ 3,5-diCl-Ph
655 3-OCH3-Ph 3,4-OCH20-Ph
656 3-OCH3-Ph 3,4-OCH2CH20-Ph
657 3-OCH3-Ph 2-thien 1
658 3-OCH3-Ph 2-furanyl
659 3-OCH3-Ph 2- rid 1
660 3-OCH3-Ph 4- rid 1
661 3-OCH3-Ph 2-imidazol 1
662 3-OCH3-Ph 3- razol 1
663 3-OCH3-Ph 2-thiazol 1
664 3-OCH3-Ph 5-tetrazol 1
665 3-OCH3-Ph 1-adamant 1
666 2-thien 1 3-CN-Ph
667 2-thien 1 3-COCH3-Ph
668 2-thien 1 3-F-Ph
669 2-thien 1 3-C1-Ph
670 2-thienyl 3-NH2-Ph
671 2-thien 1 3-OCH3-Ph
672 2-thien 1 3-OH-Ph
673 2-thien1 4-CN-Ph
674 2-thien 1 4-COCH3-Ph
675 2-thien 1 4-F-Ph
676 2-thien l 4-C1-Ph
677 2-thien 1 4-NH2-Ph
678 2-thien1 4-OCH3-Ph
679 2-thien 1 4-OH-Ph
680 2-thienyl 3,4-diF-Ph
681 2-thien 1 3,5-diF-Ph
682 2-thienyl.- 3,4-diCl-Ph
683 2-thienyl ~ 3,5-diCl-Ph
684 2-thien 1 3,4-OCH20-Ph
241
CA 02346933 2001-04-18
WO 00135449 PCT/US99C,i0292
685 2-thien 1 3,4-OCH2CH20-Ph
686 3-thien 1 3-CN-Ph
687 3-thien 1 ~ 3-COCH3-Ph
688 3-thienyl 3-F-Ph
689 3-thien 1 3-C1-Ph
690 3-thienyl 3-NH2-Ph
691 3-thienyl 3-OCH3-Ph
692 3-thien I 3-OH-Ph
693 3-thien 1 4-CN-Ph
694 3-thien 1 4-COCH3-Ph
695 3-thien 1 4-F-Ph
696 3-thienyl 4-CI-Ph
697 3-thien 1 4-NH2-Ph
698 3-thien 1 4-OCH3-Ph
699 3-thien 1 4-OH-Ph
700 3-thien 1 3,4-diF-Ph
701 3-thien 1 3,5-diF-Ph
702 3-thien 1 3,4-diCl-Ph
703 3-thienyl 3,5-diCl-Ph
704 3-thien 1 3,4-OCH20-Ph
705 3-thien 1 3,4-OCH2CH20-Ph
706 2-furan 1 3-CN-Ph
707 2-furan 1 3-COCH3-Ph
708 2-furan 1 3-F-Ph
709 2-furan 1 3-C1-Ph
710 2-furan 1 3-NH2-Ph
711 2-furanyl 3-OCH3-Ph
712 2-furan 1 3-OH-Ph
713 2-furan 1 4-CN-Ph
714 2-furan 1 4-COCH3-Ph
715 2-furanyl 4-F-Ph
716 2-furan 1 4-Cl-Ph
717 2-furanyl 4-NH2-Ph
718 2-furan 1 4-OCH3-Ph
719 2-furanyl 4-OH-Ph
720 2-furan 1 3,4-diF-Ph
721 2-furan 1 3,5-diF-Ph
722 2-furan 1 3,4-diCl-Ph
723 2-furan 1 3,5-diCl-Ph
724 2-furan 1 3,4-OCH20-Ph
725 2-furan 1 3,4-OCH2CH20-Ph
726 3-furan 1 3-CN-Ph
727 3-furan 1 3-COCH3-Ph
728 3-furan 1 3-F-Ph
729 3-furan 1 3-CI-Ph
730 3-furan 1 3-NH2-Ph
731 3-furan 1 3-OCH3-Ph
732 3-furan 1 3-OH-Ph
733 3-furan 1 4-CN-Ph
734 3-furan 1 4-COCH3-Ph
735 3-furan 1 4-F-Ph
736 3-furan 1 4-C1-Ph
737 3-furanyl 4-NH2-Ph
242
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
738 3-furan l 4-OCH3-Ph
739 3-furanyl 4-OH-Ph
740_ 3-furan 1 3,4-diF-Ph
741 3-furan 1 3,5-diF-Ph
742 3-furan 1 3,4-diCl-Ph
743 3-furan 1 3,5-diCl-Ph
744 3-furan 1 3,4-OCH20-Ph
745 3-furan 1 3,4-OCH2CH20-Ph
746 2- rid 1 3-CN-Ph
747 2- rid 1 3-COCH3-Ph
748 2- rid 1 3-F-Ph
749 2- ~ridyl 3-C1-Ph
750 2- rid 1 3-NH2-Ph
751 2- rid 1 3-OCH3-Ph
752 2- ridyl 3-OH-ph
753 2- ridyl _
- _ 4-CN-Ph
754 2-p rid 4-COCH3-Ph
1
755 2-pyrid 1 4-F-Ph
756 2- rid 1 4-C1-Ph
757 2- ~ridyl 4-NH2-Ph
758 2- yridyl 4-OCH3-Ph
759 2 =
_ridyl 4-OH-Ph
760 2-p 'rid 1 3, 4--diF-Ph
761 2- rid 1 3,5-diF-Ph
762 2- rid 1 3,4-diCl-Ph
763 2- rid 1 3,5-diCl-Ph
1
764 2- ridyl 3,4-OCH20-Ph
765 2- ridyl 3,4-OCH2CH20-Ph
766 3- ridyl _ 3-CN-Ph
767 _ 3- ridyl 3-COCH3-Ph
768 3- yridyl 3--F-Ph
769_ 3- rid l 3-C1-Ph
770 3- grid 1 3-NH2-Ph
771 3- rid 1 3-OCH3-Ph
772_ 3- ridyl _
3-OH-Ph
773 3- rid 1 4-CN-Ph
774 3- rid 1 4-COCH3-Ph
775 3- _rid 1 4-
F-Ph
776 3-pyridyl _
4-C1-Ph
777 3- y:ridyl _ '4-NH2-Ph
778 3- rid 1 4-OCH3-Ph
779 3- ridyl 4-OH-Ph
780 3- y:rid 1 3,4-diF-Ph
781 3- rid 1 3,5-diF-Ph
782 3- riayl 3,4-di
C1-Ph
783 3- ridyl _
3,5-diCl-Ph
784 3- rid 1 3,4-OCH20-Ph
785 3- rid l 3,4-OCH2CH20-Ph
786 4- xidyl 3-CN-Ph
787 4- rid 1 3-COCH3-Ph
788 4- ridyl 3-F-Ph
789 4- ridyl 3-C1-Ph
790 4-p~rridyl 3-NH2-Ph
243
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/i0292
791 4- ridyl 3-OCH3-Ph
792 4- yrid 1 3-OH-Ph
793 4- yridyl _ __
~~ 4-CN-Ph
794 4- rid 1 4-COCH3-Ph
795 4- rid 1 4-F-Ph
796 4- rid 1 4-C1-Ph
797 4- rid 1 4-NH2-Ph
798 4- ridyl 4-OCH3-Ph
799 4- rid 1 4-OH-Ph
800 4- yrid 1 3,4-diF-Ph
80I 4- grid 1 3,5-diF-Ph
802 4- rid 1 3,4-diCl-Ph
803 4- yrid 1 3,5-diCl-Ph
804 4- yridyl 3,4-OCH20-Ph
805 4- yrid 1 3,4-OCH2CH20-Ph
806 3-indol 1 3-CN-Ph
807 3-indol 1 3-COCH3-Ph
808 3-indol 1 3-F-Ph
809 3-indolyl 3-C1-Ph
810 3-indol 1 3-NH2-Ph
811 3-indol 1 3-OCH3-Ph
812 3-indol 1 3-OH-Ph
813 3-indol 1 4-CN-Ph
814 3-indolyl 4-COCH3-Ph
815 3-indol 1 4-F-Ph
816 3-indolyl 4-C1-Ph
817 3-indol 1 4-NH2-Ph
818 3-indolyl 4-OCH3-Ph
819 3-indol 1 4-OH-Ph
820 3-indol 1 3,4-diF-Ph
821 3-indol 1 3,5-diF-Ph
822 3-indol 1 3,4-diCl-Ph
823 3-indol 1 3,5-diCl-Ph
824 3-indol 1 3,4-OCH20-Ph
825 3-indol 1 3,4-OCH2CH20-Ph
826 5-indol 1 3-CN-Ph
827 5-indol 1 3-COCH3-Ph
828 5-indol 1 3-F-Ph
829 5-indolyl 3-C1-Ph
830 5-indol 1 3-NH2-Ph
831 5-indol 1 3-OCH3-Ph
832 5-indol 1 3-OH-Ph
833 5-indolyl 4-CN-Ph
834 5-indol 1 4-COCH3-Ph
835 5-indol 1 4-F-Ph
836 5-indol 1 4-C1-Ph
837 5-indol 1 4-NH2-Ph
838 5-indol 1 4-OCH3-Ph
839 5-indol 1 4-OH-Ph
840 5-indol 1 3,4-diF-Ph
841 5-indol 1 3,5-diF-Ph
842 5-indol 1 3,4-diCl-Ph
843 5-indol 1 3,5-diCl-Ph
244
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
844 5-indol 1 3,4-OCH20-Ph
845 5-indol 1 3,4-OCH2CH20-Ph
846 5-indazol 1 3-CN-Ph
847 5-indazol 1 3-COCH3-Ph
848 5-indazolyl 3-F-Ph
849 5-indazol 1 3-C1-Ph
850 5-indazol 1 3-NH2-Ph
851 5-indazol 1 3-OCH3-Ph
852 5-indazol 1 3-OH-Ph
853 5-indazol 1 4-CN-Ph
854 5-indazolyl 4-COCH3-Ph
855 5-indazol 1 4-F-Ph
856 5-indazolyl 4-C1-Ph
857 5-indazol 1 4-NH2-Ph
858 5-indazolyl 4-OCH3-Ph
859 5-indazol 1 4-OH-Ph
860 S-indazol 1 3,4-diF-Ph
861 5-indazol 1 3,5-diF-Ph
862 5-indazol I. 3,4-diCl-Ph
863 5-indazol 1 3,5-diCl-Ph
864 5-indazol 1 3,4-OCH20-Ph
865 5-indazol 1 _
,4
-OCH2CH20-Ph
3
866 5- _
benzimidazol _
1 3-CN-Ph
867 5- 3-COCH3-Ph
benzimidazolyl
868 S- 3-F-Ph
benzimidazol
1
869 5~- 3-C1-Ph
benzimidazol
1
87 0 5 ~- 3 -NH2 -Ph
benzimidazolyl
871 5-- 3 -OCH3 -Ph
benzimidazol
1
872 5-- 3-OH-Ph
benzimidazol
1
87 3 5 ~- 4 -CN-Ph
benzimidazol
1
874 5~- 4-COCH3-Ph
benzimidazolyl
8?5 5~- 4-F-Ph
benzimidazol
1
876 5~- 4-C1-Ph
benzimid_azolyl
877 5~- 4-NH2-Ph
benzimidazol
1
878 5- 4-OCH3-Ph
benzimidazolyl
879 5 ~- 4 -OH-Ph
benzimidazol
1
880 5~- 3, 4-diF-Ph
benzimidazol
1
881 5~- 3, 5-diF-Ph
benzimidazol
1
245
CA 02346933 2001-04-18
WO 00/35449 PCT/US99/30292
882 5- 3,4-diCl-Ph i
benzimidazolyl
883 5- 3,5-diCl-Ph
benzimidazol
1
884 5- 3,4-OCH20-Ph
benzimidazolyl
885 5- 3,4-OCH2CH20-Ph
benzimidazol
1
886 5- 3-CN-Ph
benzothiazol
1
887 5- 3-COCH3-Ph
benzothiazol
1
888 5- 3-F-Ph
benzothiazolyl
889 5- 3-C1-Ph
benzothiazolyl
890 5- 3-~2-Ph
benzothiazolyl
891 5- 3-OCH3-Ph
benzothiazolyl
892 5- 3-OH-Ph
benzothiazolyl
893 5- 4-CN-Ph
benzothiazol
1
894 5- 4-COCH3-Ph
benzothiazol
1
895 5- 4-F-Ph
benzothiazol
1
896 5- 4-Cl-Ph
benzothiazol
1
897 5- 4_~2_Ph
benzothiazolyl
898 5- 4-OCH3-Ph
benzothiazol
1
899 5- 4-OH-Ph
benzothiazolyl
900 5- 3,4-diF-Ph
benzothiazol
1
901 5- 3,5-diF-Ph
benzothiazol1
902 5- 3,4-diCl-Ph
benzothiazolyl
903 5- 3,5-diCl-Ph
benzothiazol
1
904 5- 3,4-OCH20-Ph
benzothiazolyl
905 5- 3,4-OCH2CH20-Ph
benzothiazol
1
906 5-benzoxazol 3-CN-Ph
1
907 5-benzoxazol 3-COCH3-Ph
1
908 5-benzoxazol 3-F-Ph
1
909 5-benzoxazol 3-C1-Ph
1
910 5-benzoxazol 3-NH2-Ph
1
921 5-benzoxazolyl 3-OCH3-Ph
246
CA 02346933 2001-04-18
,WO 00/35449 PCT/US99/30292
912 5-benzoxazol 3-OH-Ph
1
913 5-benzoxazol~rl 4-CN-Ph
914 5-benzoxazolyl 4-COCH3-Ph
915 5-benzoxazol ~ 4-F-Ph
1
916 5-benzoxazol _
1 4-C1-Ph
917 5-benzoxazo1 4-NH2-Ph
1
918 5-benzoxazo1 4-OCH3-Ph
1
919 5-ben~:oxazol 4-OH-Ph
1
920 5-benzoxazol 3,4-diF-Ph
1
921 5-benzoxazol 3,5-diF-Ph
1
922 5-ben~:oxazol 3,4-diCl-Ph
1
923 5-benzaxazoh 3,5-diCl
1 -Ph
924 5-benz;oxazol;yl_
3 , 4-OCH20-Ph
925 5-benzoxazolyl~ 3,4-OCH2CH20-Ph
Utilitv
The utility of the compounds in accordance with
the present invention as modulators of chemokine
receptor activity may be demonstrated by methodology
known in the art, such as the assays for CCR-2 and
CCR-3 ligand binding, as disclosed by Ponath et al.,
J. Exp. Med., 183, 2437-2448 (1996) and Uguccioni et
al., J. Clin. :Invest., 100, 1137-1143 (1997). Cell
lines for expressing the receptor of interest include
those naturally expressing the chemokine receptor,
such as EOL-3 or THP-1, those induced to express the
chemokine receptor by the addition of chemical or
protein agents, such as HL-60 or AML14.3D10 cells
treated with, for example, butyric acid with
interleukin-5 present, or a cell engineered to express
a recombinant chemokine receptor, such as CHO ~or HEK-
293. Finally, blood or tissue cells, for example
human peripheral blood eosinophils, isolated using
methods as described by Hansel et al., J. Immunol.
Methods, 145, 105- 110 (1991), can be utilized in such
assays. In particular, the compound of the present
invention have activity in binding to the CCR-3
receptor in then aforementioned assays. As used
herein, "activi.ty" is intended to mean a compound
demonstrating an IC50 of 10 ).1M or lower in
concentration when measured in the aforementioned
247
CA 02346933 2001-04-18
WO 00/35449 PCT/US99I30292
assays. Such a result is indicative of the intrinsic
activity of the compounds as modulators of chemokine
receptor activity. A general binding protocol is
described below.
CCR3-Receptor Binding Protocol
Millipore filter plates (#MABVN1250) are treated with
5 ~lg/ml protamine in phosphate buffered saline, pH 7.2, for
ten minutes at room temperature. Plates are washed three
times with phosphate buffered saline and incubated with
phosphate buffered saline for thirty minutes at room
temperature. For binding, 50 X11 of binding buffer (0.5~
bovine serum albumen, 20 mM HEPES buffer and 5 mM magnesium
chloride in RPMI 1640 media) with or without a test
concentration of a compound present at a known
concentration is combined with 50 X11 of I25-I labeled human
eotaxin (to give a final concentration of 150 pM
radioligand) and 50 ~1 of cell suspension in binding buffer
containing 5x105 total cells. Cells used for such binding
assays can include cell lines transfected with a gene
expressing CCR3 such as that described by Daugherty et al.
(1996), isolated human eosinophils such as described by
Hansel et al. (1991) or the AML14.3D10 cell line after
differentiation with butyric acid as described by Tiffany
et al. (1998). The mixture of compound, cells and
radioligand are incubated at room temperature for thirty
minutes. Plates are placed onto a vacuum manifold, vacuum
applied, and plates washed three times with binding buffer
with 0.5M NaCl added. The plastic skirt is removed from
the plate, the plate allowed to air dry, the wells punch
out and CPM counted. The percent inhibition of binding is
calculated using the total count obtained in the absence of
any competing compound or chemokine ligand and the
background binding determined by addition of 100 nM eotaxin
in place of the test compound.
248
CA 02346933 2001-04-18
DEMANDES OU BREVETS VOLUMlNEUX
L.~4 PftESENTE PARTIE DE CETTE DEMANDE OU CE BREVET
COMPftEND PLUS D'UN TONIE.
CECi EST LE TOME ( DE
NOTi=: Pour les tomes ;additionels, wauiltez contacter le Bureau canadien des
brevets
JUMBO APPL1CATIONS/PATENTS
TrliS SECTION OF THE APPLICAT10N/PATENT CONTAINS MORE
THAN ONE VOLUME
THIS IS VOLUME ~ OF
' NOTE= For additional volumes-phase contact the Canadian Patent Office