Note: Descriptions are shown in the official language in which they were submitted.
CA 02347106 2001-05-15
Theory and design of a
GARBAGE PROCESSING PLANT
I have invented a garbage processing plant for the production of high purity
CO, H2
gases, fertilizer gases (C02, N2, H20), dry ice, industrial or cryogenic grade
hydrogen,
urea, high quality steel scrap, micro-nutrient fertilizer. The process and
plant can produce
all these products or only selected products out of these depending on the
quality and
quantity of the garbage material available and sale potentials of these
products.
1o The raw materials used for this process and plant is the household and city
garbage,
which may consist of various carbohydrates, hydrocarbons, plastics and
rubbers, metal
and non- metal, ceramics and glass, lathers and protein materials and all
sorts of human
and animal wastes.
The oxidizing gases used may be oxygen or air or any mixture of these two.
Garbage disposal has become a great problem as environmental conscious public
would not allow any disposal site in their neighbor hood or even allow the
garbage
transportation through their localities. Garbage is a disposal problem but
also a costly
2o problem from transport and disposal view point.
INGREDIENTS OF GARBAGE.
Metro garbage consists of the following type of materials:
Category 1. Household garbage, which consists of leftover foods, a mixture of
starch and
proteins.
Wood based products namely paper and board. Cotton and polyester type mixed
clothing,
wool and other plastic mixes. Glass and plastic bottles.
Category 2. Thrown away electronics, which consist of plastic and metals. The
metals
may be copper wiring and large numbers of other metals.
3o Category 3.Rubber and plastic materials, which also include tires and
variety of, mixed
natural rubber and plastics.
Category 4. Leather and wool articles which consist of mainly proteins.
Category S. Metal articles, which may contain iron and steel and variety of
other metals.
Category 6. Paints and fillers in the paints. Paints are plastics, which
contain calcium
carbonate type of fillers and inorganic or organic colors.
Category 7. Human, animal excretions and decayed organic products.
It can be realized that most of the material is cellulose and starch type
which falls into CX
4o Hy OZ type of compounds. Other materials like lignin in wood and
constituents in plastics
and rubber are hydrocarbons, which are mainly -(CH")- type of structural
blocks.
Proteins contain carbohydrates with small amount of phosphorus, sulfur and
many other
elements.
Ash from wood will provide large number of metal ingredients.
Eventually all these will boil down to C, H, O iron and steel, non iron metals
which melt
before steel forming temperature, glass and ceramic which will melt before
steel forming
temperature forming slag.
5o In actual practice a batch of garbage will be analyzed in plant laboratory
and process
parameters will be adjusted accordingly.
Here for the sake of calculations we adopt a mix as given in the following.
Our result will
not very to any appreciable extent because Gases mixtures CO-COZ and H2- H20
will
adjust itself according water gas sift equilibrium. Thermocouples reading the
temperature
CA 02347106 2001-05-15
of gases will adjust the amount of oxygen over the oxygen already present in
the garbage
material.
The estimated percentage mixture of various ingredient is given as in the
following.
C6H~oOs type of compounds (building blocks in starch and cellulose) = 60
(CH32)- the building blocks in polyphone plastics and rubbers =30
Steel material = 6
Non steel metals =1.5%
Metals from ashes =0.5
1o Others like CaO, MgO, Na,K oxides, silica, P, S, Cl etc. =2.0
= 100
Based on this categorization it is estimated that metal component are in the
following
proportion.
C =51
H2 = 8 "
20 02 =24 "
Ca Mg, S = 3. "
Fe, Cu, Zn, etc = 4.25 "
p = 1.5 "
N =2. "
K,Na =1. "
Slag =5. "
Table 1.
METALS NOT REQUIRED IN STEEL THEIR RECOVERY AND SEPARATION.
3o Metals not required in steel their melting and boiling point is given in
the following.
Metal melting point C boiling point C
Hg -38 356
Se 217 685
As 817 613
Te 447 989
Cd 320 765
Sn 321 2620
4o Cra 298 2245
Sb 630 1585
In 156 2067
Bi 276 1585
Pb 323 1754
For more details see supplement A.
Thus heating and reducing the charge upto 1000 C separates these metals.
These may be separated as soon as a metal is melted and then filtering it out.
The
5o situation is bit more diW cult because these metals are mutually soluble in
all
compositions and at all temperatures. Some metal may become volatile and
melted
simultaneously. The metal may be recovered in suitable groups and then
separated into
individual metal as shown in figures 5 andl0. The volatile metals are
fractionally
condensed and separated. The liquid metals are separated by phase inversion
method. The
metal mixture is heated to the highest point and then gradually cooled with
2
CA 02347106 2001-05-15
simultaneously blowing oxygen through the melt. Oxide of the highest melting
metal will
be formed first which will be floating on the surface and is skimmed off. The
next metal
is then skimmed in a similar way.
The next group of metals is Cu and noble metals and platinum group metals.
These all are
soluble in copper and are separated jointly. Copper is cast into anode and
then these
metals are separated in conventional manner. After this all metals will go
with steel
forming scrap or slag.
The melting and volatizing sequence is different for metal oxides than for
metals. Some
to oxides are more volatile then metals. The melting and separating operation
should be
performed under appropriate chemical potentials for the metal or metals, which
are
separated. Copper will be melted and separated where the chemical potential is
reducing
or oxidizing because at 1200 C the oxide of copper is not stable.
RAW MATERIAL PREPARATION.
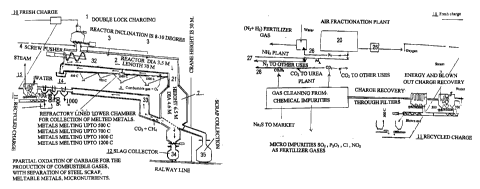
The process starts with the delivery of garbage material by railway wagons and
trucks.
When railways deliver material, wagon tipplers discharge the wagons. There
being two
such wagon tipplers discharging the wagons. The material discharged by a wagon
or
2o tick falls into under ground hoppers from where two 14 inches conveyors
takes these
either directly to the process line or temporary storage for 4-5 days. The
processing line
consists of first removing the large steel pieces manually. Cans and
magnetically
separable steel material are separated and sent on a different line.
These materials are sized to small pieces not larger than 2.5x2.5 inches by
automated
means using nitrogen plasma cutting torches as shown in figure 2. The sized
material
along with sized tires and sized automobile batteries is recharge on the main
conveyor.
Making it pass through heavy crushing rolls then crushes the material. After
crushing
rolls the size of the material is not larger than 2.5 x2.5 inches. Conveyors
takes this
3o material to stocking reclaiming facilities for temporary storage or
directly to the storage
bins (day bins).
Trucks in this area deliver tires, automobile batteries and coal used in this
process. The
sizing process for tires and other materials is similar to cutting of steel
pieces or cans; the
power of the cutting torches may be matched to the requirements of the
materials.
Cans are separated and sheared on a continuous line to make their inside open
so that tin
coating can be melted and filtered out. A closed steel can when crushed,
internal coating
will not be separated.
4o Material is discharges from day bins through weighing conveyors on a
carrying
conveyor, that discharge the material on an elevating conveyor. This high
inclination
conveyor discharges the material to the receiving hopper of an inclined
reactor.
PROCESSING OF PREPARED MATERIALS.
Garbage material is changed small size so that it can be charged by conveyor
means
under double lock devices to an inclined cylindrical reactor. The inclination
of this
reactor is near about 10-12 degree, at this inclination the sized garbage
material will not
50 move by itself but it will move by a small pushing force exerted by a
pushing screw
mechanism. The lower surface of this reactor is lined with porous refractory
through
which liquid metal can filter down ward and heating reducing gases can pass up
ward.
The hot gases raising counter current to liquid metal will keep the channels
open. Group
of metals will be collected separately and then separated out side the system
by phase
inversion method. The volatile metals will be volatilized and then collected
in particles
CA 02347106 2001-05-15
collection portions. All metals with melting point upto 1300 C will be melted
out at
separated temperature regions in this reactor whose temperature is increased
gradually
along the length of the reactor and metal with higher and higher melting
temperature and
volatizing temperature will be eliminated.
The material falling in the vertical reactor will be slag forming ingredients
and steel
scrap. Slag forming material will be melted and metals in the ashes of wood
and metal
not separated in the inclined reactor will be mixed with slag and will be
discharged at the
bottom of the reactor. The metal melting at higher temperature than 1300 C,
whose lower
1o melting ingredient have melted will be retained in the reactor and
discharged through
separate double lock mechanism. The mixed slag phase will be changed into
micro
particles by rapid quenching. Non melted material is high quality scrap which
will be
with drawn through a separate arrangement.
Material is reacted in an inclined reactor and its connected high temperature
reactor. The purpose is to attain two major aims. To gradually heat the
material in
reducing atmosphere so that melting temperature and vaporizing temperature of
some
metal is achieved ( Table -1 ). These metals are filtered out and received in
collecting
arrangement. The material is reacted so that maximum amount of reducing gases
(CO,
20 H2~ is produced.
These Energy carrying gases CO, HZ can be used for heating purposes.
This CO, HZ gases mixture is obtained when combustion gas used is 02 or CO,
H2, NZ
gas mixture is obtained when air or enriched air is used for combustion..
These can be
changed by shift reaction to HZ and C02 or H2, NZ and COa. H2 gas can be used
as
combustion gases.
COZ so obtained and N2 obtained from air fractionation plant, can be used as
fertilizer
gases. These gases can be mixed with required quantity of steam and heated by
the excess
3o energy sources of the plant.
H2, N2 are combined to form ammonia and then combined withC02 to form urea by
some modification of conventional technology.
High purity H2 and N2 are passed over iron based catalyst under high pressure
15-60 MPa
and temperature 400-600 C. The ammonia which forms is condensed by cooling
with
cold nitrogen. For the production of urea, ammonia and COZ are fed to a high-
pressure
reactor upto 30 Mpa and temperature about 200 C. Ammonium carbamate CH6N202,
urea
and water are formed.
40 2NH3+ C02 = NHZCOONH4 + heat
NHZCOONH4+ heat = NHzCONH2 + H20
Excess ammonia is fed to the reactor; NH3 and COz exit the reactor along with
urea.
Efficiencies differ with various technologies. Urea production step is
followed with
granulation and conditioning. Because it is a continuation process from
ammonia to urea
Hot ammonia stream and hot C02 streams are used, a modification of
conventional
technology.
The production quantity of the plant depends upon the hydrogen produced in the
so above steps. Usually a plant based upon garbage specified in the above as
raw material
will have excess COZ and fractionation plant will have excess N2 than required
by the
urea plant. These excess gases will be used as fertilizer gases, and other
needs of the
plant. The ingredients in the garbage, which will be producing, sulfur,
phosphorus,
nitrogen and chlorine gases will be separated before C02 separation and these
gases will
CA 02347106 2001-05-15
be mixed with NZ gas as sub soil micronutrients or with COZ as micronutrients
in the
gases phase.
Gases leaving the high temperature reactor are made to exit from a side
arrangement.
These gases are lowered in temperature from 1300 C to 1100 C by introducing
endothermically reacting gases, which will absorb heat from the high
temperature gases.
(see supplement A). The heat is then recovered from 1100 or even 1300 C to 300
C by a
boiler, where blown out particles are settled. Gases being passed through bag
filters to
further recover the blown out particles.
1o The heat recovery particles recovery system is a gradually cooling system
separated
into various temperature regions where volatile metal may condense according
to their
solidification temperature. The is no fuel particle left and metal obtained
may be
relatively pure.
There being two-bag houses in parallel, one on repair and other on stand by.
These gases are then freed of water-soluble impure gases and further purified
by passing
through NaOH solution. The gases are then freed of COZ contents and pure CO,
H2 gases
are obtained. The heat required in the desorption of adsorbed COZ is used from
the gases
stream itself which is coming at 500-300 C. The energy spend in the
compression of C02
2o is recovered by expansion of gases after the removal of COZ. Similarly
energy spend in
compression of absorption solution is regained by expansion of these solution
before C02
removal.
When CO, HZ ( CO, HZ, N2) mixture is changed to CO2, HZ by shift reaction
about
2.7 times steam is used then the stichometric requirement of CO. The reaction
is
performed at 200-250 C and the reaction is exothermic, the product gases are
cooled and
energy is recovered. The COZ containing solutions are cooled by adiabatic
expansion
before going to desorption process for the removal of CO2. Two flow lines are
shown one
3o when the end product is CO, H2 and the second when the end product gas is
H2.
With HZ gas COZ is also recovered. It is this COZ which will be used in urea
production
and as fertilizer gas in the green houses. Because the COZ is free of
impurities it can be
used to prepare food grade gas after deodorizing over activated charcoal.
Absolute pure hydrogen can be produced by cryogenic separation of CO, HZ
mixture
using low temperature nitrogen from the air fractionation plant. The hydrogen
so
produced will be about 98.5 % pure; CO still remaining will be separated by
absorption
through ammonical cupric chloride solution. This hydrogen can be liquefied for
space
4o industry.
MATERIAL AND ENERGY BALANCE OF THE PLANT.
The elemental contents per 100 kg of the charge are given previously. In the
following
the calculation basis is taken per m.ton of the garbage charge. Various
formalism and
theoretical basis of the calculation are given in supplement A.
The final temperature of the heated materials is 1300 C.
T1 =1275
T2 =1192
5o T3 = 282
510/12 * C* (1175* 4.003 + 1192* 1.14 + 282* 2.04) = 300,517
80/2*H * ( 1175 * 6.5 + 1192* .78 + 282*.12) = 37,000
240/32* 02 * ( 1175 * 7.16 + 1192* 1.0 + 282 * 0.4) = 78,353
Ca,Mg,S 30/40*(1175* 5.25 + 1192* 3.44) - 8.094
CA 02347106 2001-05-15
Fe,Cu,Pb 42.5/56* (1175* 3.04+ 1192*7.58 + 282* -0.6) - 9,670
P* 15/31 * ( 1275 * 4.74 + 1192* 3.9) - 5,007
N* 20/28 * (1275* 6.83 + 1192 * 0.9 = 282* .12) - 7,010
K,Na 10/39 * (1275*6.83 + 1192* 1.08) - 2,563
Slag 50/60 * ( 1275* .3 + 100 kcal/kg ) - 376
Slag is assumed as silica. --------------------
448,590
Heats taken out by melted out metals. - 74000
Io Heats taken out by slag forming ingredients - 16,565
Heat taken out by steel forming scrap - 12,000
Zinc oxide will not be reduced and will remain with steel forming metals. It
will be
separated in high temperature melting operation above 1650 C, where it will be
volatilized forming dust material.
HEATS GENERATED BY COMBUSTION REACTIONS.
These calculations are done with the assumptions that C and H will react with
Oxygen in
the ratio in which they are present in the reacting material. The amount of C
reacting to
CO and COZ is found by hit and trial at which the energy balance is obtained.
42.5 * .925 (C+'/2 02) = 26,400 * 39.3 CO = 39.3 Kg.mole
= 1,037,836
42.5 * .725 (C + OZ) - 94,050 * 3.08 - C02= 3.08 Kg.mole
289,792
40* .925 HZ H2 = 37 kg.mole
40* .0725 (H2 + 1/202) = 57,800 * 2.9 = H20 = 2.9 Kg.mole
167,620
Ca,Mg, S * 1. S * (Ca +'/z 02)=1 S 1,500* Ca,Mg O, SOZ = 1.5
1.5 = 227,250 kg mole
2P +3/2 02* 15/30.9 (P+3/20)= 356600* .242P2 03= .24 Kg. mole
= 43148
K * 10/39 ( 2K +'/z 02) =107,700 *.128 K20 = .128 Kg mole
= 13785
__ ______________________________
Total heats = 1,703,681Kca1
Heats brought from lower chamber CH4 +'/z = 104,525 Kcal.
OZ
Gases composition going out
CO = 39.3 kg mole
COZ = 3.08 "
HZ = 37 "
H20 = 2.9 "
P2O3 .12 "
Other gases = .6 "
Total = 83. kg.mole
Heat taken out by exit gases at 1300 C
83 * 8.5 Kcal/kg.mole-C * 1300 C = 917,150 Kcal
Heat absorbed by external oxygen input = 24.68 -7.2 =17.48 kg.mole
17.48* 7.5 * 1300 =160,466 Kcal
5o Heat taken by dissociation of hydrocarbons and polythenes = 45000 *0
.85=38,240 Kcal
ENERGY BALANCE
6
CA 02347106 2001-05-15
Inputs Kcal Outputs Kcal
Heat taken up by the charge = 448,590 Heat taken out by gases = 917,150
Taken by oxygen - 160,466 Heat taken out by steel scrap=12,000
Heat taken up for dissociation=38,240 Heat taken out by slag = 16562
Heat of reactions - -1,703,680 Heat taken out by melted metal=74,000
Heat from lower chamber - - 104,525
Conduction and other losses
to @6% of input heats =69,654
Net inputs = 1,091,255 Net out puts = 1,019,712
GASES COMPOSITION GOING OUT FROM HIGH TEMPERATURE REACTOR.:
CO = 39.3 kg mole
C02 =3.08
H2 =37
2o H2 O =2.9
Others ( SO2, HZS, N02,N2 O, PZOs, PH3, OC12, HCl ) = 1.5
83.45 kg mole
Gases after purification
CO = 39.3 kg. mole
HZ = 3 7 "
76.3 kg.mole
30 CO = 51.5% Hz =48.5
Calorific value of product gas
0.515*(CO +1/2 OZ = COZ OH = - 67650 Kcal) =34840 Kcal
0.485*( HZ +'/z OZ = H20 " -57800 " ) = 28033 "
one cu meter = 62873 /22 =2859 kcal
one cu meter of natural gas =7800 kcal
Caloric value of CO, H2 = .365 of natural gas.
Total annual production of CO, H2 =76.3 * 500,000 = 38.15 x 106 kg. mole
40 = 839.3x 106 cu meters
PRODUCTION OF HZ:
Shift conversion takes place according to the following reaction
CO+HZ+H20=HZ+COZ
In a two step process approximately 95 % CO changes to C02. We assume that 100
conversion takes place, so the volume of hydrogen produced is equal to the
volume of
CO,HZ gases.
HZ = 38.x 106 K~.mole 839x 106 cu.meters.
5o COZ = 19.65 x 10 kg. mole 431x106 cu. meters.
Because of economic reasons some 20 % of CO, Hz of the volume of these gases
will be
used for green house heating, which it is assumed it is an associated concern
of the
garbage processing plant.
7
CA 02347106 2001-05-15
The rest of the hydrogen is either marketed as heating gas as a clean heating
fuel for the
households, because when it burns it will produce water vapors only. If
properly
promoted this can bring better revenue than the natural gas heating. For the
present it can
be marketed as a combustible fuel bring revenue according its calorific value.
Both CO,H2 and HZ when marketed will be treated as combustible fuel brings
revenue
0.36 % of the natural gas.
UREA PRODUCTION FROM HYDROGEN.
A more economical proposition is to produce urea from the hydrogen, which is a
more
to accepted market commodity.
It goes through the following steps.
3H2+N2=2NH3
2NH3 + COZ = CO ( NH2)2 + Hz0
3-kg mole of hydrogen will produce 60-kg urea.
Total urea produced = 60 x 38x106/3 * 1000 = 760,000 m.ton per annum
C02 excess after using in urea formation = 7 x 10'° kg mole per
annum.
Nitrogen consumed in urea formation 12.6 x 106
20 Remaining nitrogen = 37.5 x 106 kg. mole per annum.
These gases heated to a desired temperature, having required moisture and
fertilizer
potential may be used as green house gases in cold and hot surroundings.
C02 has lowest coefficient of thermal conductivity as compared to other gases
and
construction material. It will be employed as insulation material between the
double glass
wall of Green house enclosures.
PLANT CAPACITY.
The plant is designed to process 500,000 ton of garbage per annum. This
includes about
50,000 tons of tires. Any deficiency in the availability of garbage will be
met by
3o inclusion of steam coal of the same tonnage.
This plant will use about 700-kg industrial oxygen (98.5%) per ton of the
charge. In stead
of using oxygen the plant can be design on oxygen enriched air, particularly
when a
mixture of H2, NZ is used for production of ammonia. For the present design
industrial
oxygen will be used.
It will produce about 42.5 * 106 kg. mole of CO, HZ per annum.
Alternatively it wil l produce about 41. 5 * 106 kg. mole of HZ per annum and
about 19, 5
106 kg.mole C02 gas per annum and 50x 106 kg.mole of NZ per annum. Nitrogen is
used
4o for cooling, for ammonia production, for plasma cutting, as form gas and
some
miscellaneous uses.
As a third alternate the plant will produce 750000 m.ton of urea per annum,
quite a large
amount of COZ and NZ for 4-5 industrial forms and other miscellaneous uses.
FERTILIZERS.
A fertilizer may be any substance which when applied to the soil will
contribute to the
cultivation of the plant by supplying nutrients to it. The optimum input level
of
fertilization is reached when a marginal monetary yield (marginal return) and
marginal
5o cost are equal. Along with fertilizers other beneficial chemical such as
pesticides,
insecticides, herbicides and stem shortening chemicals are also applied.
Consumption of fertilizers are usually in the ratio N: Pz05: K20 :1: 0.5: 0.4.
Western
Europe applies highest fertilizer per acre in the world, about 130 kg/hectare.
In North
America this figure is about 44 kg/hectare.
s
CA 02347106 2001-05-15
Table 1 Nutrients application kg/ha
N P205 K20 Total
North America 22.5 10.3 11.3 44.1
W. Europe 62.5 32.7 32.4 128.7
Eastern Europe 22.7 14.4 14.0 51.0
World average 14.5 7.1 5.5 27.0
Crrain crops use about 60% NZ
Vegetable crops use about 30 % N2
1o Division of nitrogen fertilizers.
Among the nitrogen compounds urea is applied in the highest about 33 %,
ammonia 14
%, nitrates and mixed ammonia compound 29%, multi-nutrients 19%, all-others
15%.
Plants dried material consist of 44 % C which enters the plant via leaves and
through C02
in the air.
Although air contain 79 % nitrogen by volume, but this is of little use for
the plants. Plant
is considered to consume nitrogen through its roots and via the formation of
water-
soluble nitrates. Ammonia is supplied to the soil via submerged soil injection
techniques,
2o it decomposes and then forms nitrates by reaction with soil moisture, which
is then taken
up by the plant. It is considered that only a fraction of ammonia is consumed
by the
plants, rest of the ammonia being volatile leaves the soils and goes to air.
Urea when
supplied to the soil in water decomposes to ammonia and carbon dioxide. Urea
is often
washed away with water. Plant take up nitrogen from urea is similar to ammonia
by the
roots and similar to COZ from the air.
Before the large scale production of nitrogen fertilizer by modern industry
the plant
intake of nitrogen was promoted by crop rotation means, i.e. certain plant
varieties
particularly legumes of guawara and sun crop and peas were grown and then
ploughed
3o into ground when the crop is tender and easily decomposed. This bacterial
soil so
produced has the ability to use atmospheric nitrogen. The regular crop, which
is grown
over this decayed soil, has the ability to use the atmospheric nitrogen. About
a month and
half is taken by this rotation process, effectively delaying the period of
regular crop
sowing. Instead of sowing and decaying of crop already decayed soil or
material may be
used. This consists of animal and human excretions, decayed vegetation, guano,
ground
fish meal and slaughterhouse waste. Nitrogen gas containing micro nutrient
gases is
blown under the layer of this material. A mechanical method of this system is
shown in
4o figure(13). Nitrogen blown is not pure nitrogen; it is air enriched with
nitrogen. The
nitrogen contents and humidity level will differ for particular crop. Other
two fertilizers
namely phosphates and potash has to be applied along with decayed soils. Human
and
animal excretion has many micronutrients contained in these. Solid
micronutrients should
be added with soil analysis a8er suitable periods of time.
Plants obtain C,O,H through atmosphere. N, P,K and micro constituents from
soil. A
regular supply of all these have to be maintained.
5o Methods and approach of directly supply of nitrogen from air fractionation
system has
not been developed because nitrogen production is not economical by this
route.
However by the development of large tonnage oxygen plant for metal industry
this
approach has become viable. Nitrogen available from fractionation plant has
large
amount of cold value. This cold can be used in certain operation as
replacement of
conventional refrigeration and cooling system.
CA 02347106 2001-05-15
This nitrogen may be used for temperature control in green houses of those
regions where
temperature is high, and plant growth is not possible.
USING CARBON DIOXIDE AND WATER VAPOR TO SUPPLY C, H,O to the plant.
C02 is also a necessary ingredient in life cycle of animal and plant. In
animal
metabolism oxygen from atmosphere reacts with sugars to produce energy.
C6 HI2 06 + 6 02 = 6 C02 + 6 H2 0 + energy
In plant metabolism C02 is taken by the leaves using energy from light and
enzymes as
catalyst producing sugar
to C02 + H2 O = C6 Hi2 06 (sugar)
Sugar ~ Cellulose
Thus for plant growth N, P, K in the roots and carbon dioxide and water vapors
in the
leaves are essential ingredients.
When the plant is small during initial growth both N2 and C02 can be given in
the
pipes) under the plant roots, where flow rate can be controlled.
When S02 is also available it can be with N2; C02 in small percentage or alone
where
soil is deficient in sulfur.
2o The end products of any combustion process is a mixture of gases containing
C02, H2 O,
N2 and some minor amount of S02,C0, H2, 02 and others. These at appropriate
temperature and saturated with water vapors can be supplied as plant nutrient
(fertilizer)
All combustion products saturated with water vapors can be used as
fertilizers. In case
the soil is already wet non-saturated combustion products can be used.
Plant need for their healthy growth the following structural and nutrients.
Structural
elements
O 45
C 44
3o H 6
Primary nutrients
N 2
P 0.5
K 1.0
Secondary
nutrients
Ca .6
Mg .3
40 S .4
Micro-nutrients
B .005
Cl .015
Cu .001
Fe .02
Mn .OS
Mo .0001
50 Zn .O1
99.90
BACK GROUND INFORMATION ABOUT GREEN HOUSE INDUSTRY.
CA 02347106 2001-05-15
Green house industry is a new growing industry where crops, vegetables, fruit
and flower
can be produced in any climate cold or hot though out the year.
C02 along with H20 is required for growth atmosphere. An appropriate quantity
of C02
for a particular crop may increase to growth rate to double as compared to
normal
atmosphere.
In normal atmosphere the concentration of C02 is 300 PPM. For flowers crop (
roses) it
may be 2000 PPM. For vegetables it is higher than flowers and for grain crops
higher
than vegetables. The human toxicity level is round about 50,000 PPM. ( 5%)
FACTORS EFFECTING COZ UPTAKE.
The following are the factors influencing the up take of CO2.
Plant species and variety.
Radiation intensity.
Wind velocity
Water stress.
C02 concentration in the air.
Temperature of the atmosphere.
When out side temperature is low, no COZ injection and no ventilation, inside
2o concentration of COz decreases plant growth decreases.
Light and water level has also large effect and depends upon plant to plant.
Due to boundary layer effect increase in flow rate will increase the rate of
photosynthesis.
ESTIMATED COz PER 1000 FT2.
The following figures are reported for Carnation.
Month cu.ft. 1b C02
Sept. S00 58
Oct. 520 61
3o Nov. 800 90
Dec. 720 85
Jan. 660: 78
Feb. 560 66
For the estimation given below the consumption for cucumber is taken as 1000
cu.feet
per month.
After urea production the amount of COZ available = 7 x 106 kg.mole per annum
Assuming the average consumption of 1000 cu m. per month for 1000 sq.ft this
C02 is
sufficient for = 280 forms of 150,000 sq.m.
At this stage only 4-5 such form are established each consuming about 25,000
kg.mole
per annum. Thus about 900 m.ton per day high purity C02 is available for dry
ice or food
grade COz.
AVAILABILTY OF NITROGEN.
Assuming nitrogen consumption is equal to C02 consumption ,the remaing
nitrogen is
so = 37.5x106 kg.mole
This nitrogen will be used for cooling purpose in the plant.
The hot water produced in the plant is used to provide moisture in the
fertilizer gases.
SOME SALIENT FEATURE .
A design of 1 s0,000 square meter floor area form is provided in figure 4-8
attached with
this document. This design is specifically suitable for very cold and very hot
areas.
11
CA 02347106 2001-05-15
The enclosure is double layer glass, the heating and cooling passage is
between the glass
sheets. This way the internal atmosphere of the form is not disturbed. COZ
with moisture
is blown from the ceiling down and nitrogen from the under ground embedded
pipes, the
exit is few inches above the ground level.
The heating and cooling gases may be supplied from the plant, with great
saving for the
form. In USA where heating and atmosphere control gases are to be supplied
from
natural gas the heating cost for 1000 sq.meter may be about $250 per month.
Natural gas
produces more water than require d for the form so the atmosphere for the crop
has to be
1o changed more often.
When fertilizer and heating gases are provided from the main plant the
estimated cost for
a double wall glass house may be US $ 150 per Sq. m.
Financial analysis of a green house set up is provided in the financial
section of this
report. In a normal green house the highest cost is manpower, after that the
second
highest cost is gaseous atmosphere and heating expenditure, and third large
cost is
fertilizer and herbicides. In the present situation the cost of gases and heat
is drastically
reduced, so is the cost of fertilizers. The form size is reasonable which can
be
2o mechanized and labor cost is reduced. These combined factors will make the
production
cost much cheaper than a similar operation on international level.
SUPPLEMENT A
(Theoretical basis used in calculation of energy balance of
the plant. Melting and separation of metals under controlled
chemical potentials, porous refractories, endothermic cooling
by gaseous reactions)
The enthalpy of a substance is described between the temperature limits To (=0
K°) and T
3o by the following:
~T = ~~ ~o + ~To 0 Cp dT ~ LT + J TtOCpdT ( 1
where Tt is transformation temperature and LT is the latent heat of
transformation.
For most substances the heat capacity may be expressed as a function of
temperature by a
power series:
CP a+bT+cT2+dT'Z+eT-vz
Where a,b,c etc; are constants derived from experimental heat capacity data.
In the calculations performed in this document the following relationship will
be assumed
4o CP = a + bT - cT'2 for most of the gases used.
The entropy change of a substance is a measure of the amount of energy, which
can not
be converted into useful work. It is a measure of unavailable energy.
The entropy change of a process is given by the following equation
OS = ST2- STl= ~To C~/T dT ~ LT/ TT + ,~zTr C~ T dT where T~ = T= temperature
for
transformation (3)
FREE ENERGY CHANGE:
so The free energy change of the system is net change in the energy contents
of the system
when contribution from enthalpy and entropy are jointly considered.
OG=~H-TOS
For a reaction at constant temperature and pressure for a spontaneous process
DG<-0
12
CA 02347106 2001-05-15
At equilibrium the free energy change must be minimum. A negative free energy
change
does not mean that the reaction will proceed at a measurable rate under a
given set of
conditions, but indicate only that a reaction is possible. It will proceed
depending upon
the kinetics of the system. That why most of the cases external energy has to
be supplied
for start of the process. The process may be stopped or it may continue
depending upon
evolution or absorption of energy.
VARIATION OF FREE ENERGY WITH TEMPERATURE may be eXpreSSed by the following
equation
OGT = OH298 + ! 298 ~CpdT ~ )_.t + J Tt OCp dT - T ~ OS298 + 298 Cp dT~T ~
L,T~T +
~TtCp/T dT ]
(5)
The temperature dependent terms in equation 5 are of opposite sign and to
cancel in the
summation process. The temperature dependence of free energy on temperature
may be
defined by the following equation.
OGT= OH + bT log T - TdS (6)
2o At high temperature were the accuracy of experimental determination has
certain degree
of limitation this relationship is generally described as
OGT = OH- T OS
where ~H and OS are average values in a particular temperature range.
OGT values are determined on both sides of transformation where OGT becomes
zero.
Thus there is slope change in the OG and Temperature plot at allotropic
transformations,
fusion and evaporation.
DETERMINATION OF AN EQUILIBRIUM RATIO FOR TTY REDUCTION OF AN METAL OXmE:
3o Reduction Potentials.
At a particular temperature metal gets oxidized or its oxide get changed to
metallic form
if there are more oxidizing gases then an equilibrium value or conversely more
reducing
gases.
This equilibrium value is a ratio between reducing and oxidizing gases where
neither
reduction nor oxidation of the metal takes place. The equilibrium ratio can be
determined
from free energy
Considerations.
4o Consider the following type of reaction
2 M (s) + 02 (g) = 2 MO (g) (7)
2 CO (g) + O2 (g) = 2C02 (g) (8)
A reaction between metal and oxygen and a reaction between gas in which oxygen
is one
of the participants
Subtracting equation (8) from (7)
M (s) + C02 (g) = MO (s) + CO (g)
Ke =p~° a Mo ~ p cot aM
5o Ke
= pcdpco2 where Ke is related to the free energy change by the following
relationship.
0G° + -RT In Ke or Ke = a -~c'°/RT
a 4Go/RT
= pco/ pco2
13
CA 02347106 2001-05-15
in place of CO/COz it may be Hz/HzO
In industrial systems usually there are mixers of CO, Hz and COz and H20, the
potentials
values are some approximations of the values of CO/COz and Hz /H20 potentials.
The values of OG° adopted can lead to somewhat different numerical
figures in above
equation, but do not effect the industrial results.
The fee energy value OG°= -RT In [ azMO/ azMpoz ]
When metals, metal oxides, and gases in equilibrium with them are not at
standard state.
1o Lighter metallic ores suspended in liquid metal through which reducing
gases are passed.
In this situation hydrogen water vapor mixer may reduce the oxide, which
otherwise in
standard conditions these gases are not able to reduce these metal oxides even
at their
highest reduction potentials. We will use these specific situations to prepare
some metal
by new routes.
Some useful gaseous equilibrium is given in the following~'~.
Reaction Kp Ln KP Range of validity C°
Fe~l_y~ O + CO Pcoz~co 2075/T -2.5 500- 1370
20 =~l-Y) Fe+ COz
Fe~l_y~ O + Hz PH20~H2 -1953/T -1.02 25 - 13?0
_ ~1-Y) + Ha0
COz + C = 2 CO Pzco/ Pcoz -20045/T + 20. S 500 - 2000
CO + H20 PcozPHZ 4028/T -353 500-- 2000
= COz + Hz / PCO PH2 O
1-y=0.947 T=K°
3o Table 2.1
Equilibrium
chemical
potentials
for some
metal oxides.
Fe O+CO =Fe Fe0+Hz=Fe +H20
+COz
Temperature PCOz lPCO Temperature PH20/ PHz
1070 K .56 1070 K .45
1250 K .41 1250 K .59
1500 K .34 1500 K .78
Pb0 +C0 =Pb +COz CuzO +C0 =2Cu +COz
Temperature PCOz/ PCO Temperature PCOz/ PCO
4o 8.4/ 10g 500 K 3.8/101o
500 K
1000 K 1.9/ 104 1000 K 5.8/104
1500 K 1.8/103 1500 K 5.8/10z
Zn + COz = Zn0 +C0
Temperature PCO/PCOz
500 K 3x106
1000 K 1.5x103
50 1500 K 5.9
2000 K .2
Zinc Oxide sublimates at 1800 C°.
It can be seen that iron is reducible at chemical potentials easily achievable
in practice.
While copper oxide will change to metallic copper at very low chemical
potentials not
easily achievable. It will change to copper when heated in air at high
temperature.
14
CA 02347106 2001-05-15
Zinc is reducible at very high temperature, at achievable chemical potentials
but it will
change back to oxide form as soon as temperature is lowered because
corresponding
reduction potential are not practically achievable.
Table 2.2
Sequence of reduction Potentials of various metal oxides.
Cu20
Fe203_______ > Fe304
Ni0
1o Sn02
Fe304-----~ Fe0
P2 03
Zn0
Cr2 03
Mn O
A12 03
Table 2.3
2o Densities of some important metals and their oxides.
Metal density of metal density of metal oxide
Pb 11.3 9.5
Ni 8.9 6.67
Fe 7.86 5.7
Cu 8.92 6.
Sn 7.28 6.95
These values of oxide densities are theoretical densities, the actual valued
of industrial
products will be some what lower because material formed will be porous.(
density
30 =Specific gravity)
Table 2. 4
Melting points , boiling points , specific gravity of some easily meltable and
volatilizable
metals and their oxides up to temperature of 1000 C° Metal
Specific gravity m.pt b.pt metal oxide m.pt b.pt.
Hg 13.5 -38 356 Hg0 unstable
Se 4.79 217 685 Se02 340 315
4o As 5.73 817 613 As203 313 465
Te 6.24 447 989 Te02 773 790
Cd 8.65 320 765 Cd0 1540 decomposes
WOZ will
vaporize
at 800
C
Non volatile 00C but
up to easily
meltable
metals.
Sn 5.74 321 2620 Sn0 1630 1900 sublimates
Ga 5.9 29.8 2245 Ga OZ 580 sublimates
Sb 6.69 630 1585 Sb2 03 655 1482
5o In 7.31 156 2067 In OZ 562 sublimates
Bi 9.74 271 1582 Bi203 825 1890
Pb 11.35 323 1754 Pb O 886 1472
TI 11.85 303 1485 Tl 2 03 717 ----
Ge 5.3 937 Ge02 1082 density 6.23
CA 02347106 2001-05-15
EXPLOSIVENESS OF CO, H2 COMBUSTION WITH OXYGEN
The combustion of CO, HZ with 02 is generally considered as hazardous The
explosive
situations described in literature do not apply in the present set up. In this
situations , the
number of moles of the product gases are less than the reacting gases and
materials are
present to absorb the shock waves. As the CO, HZ gaseous mixture is preheated
volume
increase of the product gases due to temperature effect is small. It is less
than double
when temperature of the product gases is doubled. The systems are capable to
absorb the
to expansion effects.
There are proper precautions as relief valves and rapture disks at appropriate
locations.
Also pressure in CO, H2 and O z lines should be positive so that these do not
diffuse in to
each other's lines~2~.
A COMPARISON OF COOLING BY PERFORMING AN ENDOTHERMIC
REACTION AND COOLING BY WATER.
A reaction between a hydrocarbon and an oxidant producing elementary gases is
given as
following.
20 1 ~ C" Hm + n C02 = 2nC0 + m/2 HZ
2. C" H", + n H20 = n CO +(2n +m)/2 HZ
Representative example is given in the following:
CH4 + COZ = 2C0 +2HZ ~H =+ 60,000 Kcal
Added to this is heat absorbed by the reacting gases to reach the reaction
temperature
(assumed here as 1000 C° ) =approx. 18000 Kcal
Total heat absorbed is 88,000 K. cal /kg. mole of CH4
If water is used to cool steel from 1000 C° to room temperature it will
absorb about
15,000 Kcal/ Kg. mole of water. Add to this exothermic heat of the reaction
3o Fe + HZO = Fe O+ HZ AH= -5,000 K.cal/kg. mole of wustite
The net cooling effect is 10,000 /Kg. mole of H20. The thermal resistance of
the oxide
scale has been neglected.
It can be seen that heat removed by water-cooling is about one eight of that
removed by
above given endothermic reaction.
The mixtures of gases, which absorb heat during chemical reaction, are termed
as Endo
gases.
ENERGY RECOVERED BY ENDOTHERMIC REACTION COZ + CH4
4o When energy is recovered by performing endothermic reaction of the type C02
+ CH4,
The following two step reaction as given below may take place.
(i) CH4+ COZ =2C0+ 2H2 endothermic
(ii) 2 CO + 2 H2 + 02= 2C02 + 2 H20 exothermic
This is the energy absorbed in step (i), which is saved.
This saving is approximately 45 % of natural gas saving if same energy
generation was
required by straight combustion of CH4.
so In this write up mainly CH4+C02 mixture is mentioned for endothermic
reactions, but it
may be CH4+H20 as well.
THE REFORMING REACTION:
The reforming reaction CH4 + C02 will adequately take place if temperature is
about
1000 C° or higher. If the temperature is lower than 1000 C catalyst is
required. The
catalyst has to be contained in a closed vessel and heat transfer will take
place through
16
CA 02347106 2001-05-15
the walls of the container. At limiting temperatures of about 700 C°,
and pressure is also
required. The term reforming gases is used to describe endothermic gases or
Endo gases.
OTHER ENDOTHERMIC REACTIONS. Decomposition of CH30H and NH3 are also
endothermic reaction and can be employed when lower quantity of heat is to be
absorbed
at relatively lower temperatures.
Nitrogen starts forming stable compounds with iron at about 700C with
exothermic
heats.
Purified natural gas is used for calcination of lime. The clean gases from
this system will
to have more H2 than CO. This gas can be used in annealing of cold rolled
steel after
performing shift reaction CO + HZ + H20= COZ + 2 HZ over a catalyst. C Oz is
removed
by absorption.
used where metal becomes volatile under certain chemical potentials.
HEATING AND COOLING BY SHIFT REACTIONS.
Heating and cooling is achieved by performing exothermic or endothermic
reaction in
direct contact with the processed material.
When it is not appropriate to perform these reaction in direct contact with
the materials
2o these reaction are performed in close vessels with or without catalyst. The
energy transfer
then takes place through the walls of the container.
The product gases from such system are energy carriers to a recovery system,
HZ or CO can be produced by carrying the following reaction by excess COZ or
by
excess H20 over the catalyst surface.
CO + HZ+ COZ = ZCO + H20 OH endothermic
CO+ H2 + H20 = 2 HZ + COZ 0H exothermic
COZ and H20 can be removed to get CO or H2, These steps can be repeated to get
the
gases of required purity.
3o The direct contact energy transfer reaction is performed when this has no
effect on the
material in contact with the reaction is performed. When temperature of the
system is low
and energy transfer by reaction system is not appropriate energy is
transferred through
water heat exchanger.
No direct cooling water is used. This eliminates the transfer of harmful
pollutant from
gases to water system from which it more diffcult to remove. Mud recycling and
recycling of scales is eliminated.
SULFUR RECOVERY FROM HIGH CONTENTS SOZ CONTA1NG GASES. Sulfur
4o recovery as elemental sulfur can be done in a more direct way as given in
the following
than the conventional Claus process:
2CH4 + 3 S02 = 2 COZ +2 H20+ HZS +S
2H2S+ SOZ = 2 H20+ 3S
2CH4 + 4 SOZ = 4 H20 + 2 COZ+ 4 S OH = - 58,000 K. cal. / Kg. mole of CH4
The S is cooled and condensed and product gases are send for further
purification from
5o particles and chemical impurities alone or joined with other gaseous
streams.
USE OF OXYGEN FOR EXOHERMIC REACTIONS.
All energy generation reaction during steel making and calcining of flux are
performed
with the combustion of O z..
By using 02 in close systems all CO, H2 gases are recycled for energy
generation. The
energy ei~iciency of the fuels is 100 % as compared to present systems where
energy
17
CA 02347106 2001-05-15
recovery is hardly more than 55. %. This recycling compensate expenditure on
oxygen
generation. As the fuel expenditure is decreased so the emission of COz is
decreased.
Some of the C02 is recycled within the plant for energy recovery purpose, thus
emission
of COZ is further decreased.
The flux calcination system is in parallel configuration with direct steel
making from
oxide ores with inclined rotary reactors connected to vertical steel making
reactor. In
calcining system instead of steel making reactor there are two discharge
receiving
vessels where one is receiving the hot calcined material which is being cooled
with heat
1o recovery from it, the other is discharging the cooled material to a
receiving bin.
Recycle of blown out calcined material is done directly to receiving hopper of
the
calcined material.
Porous filters.
Porous refractory filters are used for the separation of liquid metal from non-
melted
materials. These are made from aluminum oxide and stabilized with zirconium
oxide. An
2o example is the use of this type of filters in the separation of inclusions
from liquid steel
during continuous casting~3~. These may be further standardized for specific
requirements
of liquid filtration and counter current gas liquid filtration.
SENSIBLE HEATS.
If the specific heat of a material is defined as
CP = a + b * T + c/ TZ K cal/ K°/ kg mole then the heat capacity of the
material at
temperature T K° with respect line temperature of 298 K° is
J 298 Cp*dT = a * (T-298) + b* ( TZ - 2982) / 2 - c* ( 1/T- 1/298 ) Kcals/kg
mole
If Tl = T- 298; T2 = ( TZ - 2982)* 0.5/1000; T3 = -(1/T -1/298)* 100000
The sensible heats of multicomponent gas is
0.001*4.184* volume* ( %CH4*(8.147 * T1 + 8.9* T2 + 1.965* T3)
+ % CO* ( 6.79*T1 + 0.98*T2+0.11 *T3)
+ % COz*( 10.55*T1+2.16*T2+2.04*T3)
+%H2 * ( 6.52* T1+0.78*T2 -0.12*T3)
+% Na *( 6.66 * Tl +1.022*T2))/100*22.414) KJ/ton product.
Where volume is volume of gas stream in question in Nm3/ton product
SENSIBLE HEAT OF MULTI COMPONENT SOLIDS IS
0.001* 4.184* WEIGHT* ( % Fe203* (23.49*T1+18.2* T2+ 3.55* T3)/159.7
+ % Fe304*(21.88*T1+48.2*T2)/231.55
+%Fe0 (12.38 Tl+1.62*T2+ o,38*T3)/71.85
+% Fe",~~~;°* ( 4.18* T1+5.92*T2+H)/55.85
+%C (4.1 *T1+1.02*T2+2.1 * T3)/12
+ % gangue * (11.22*T1+6.2*T2+2.7*T3)/60)/100 . MJ/ton product.
Where weight of the solid stream is kg/ton product.
H is the heat of transformation of solid iron at 760, 910,1392 C and heat of
fusion at1537
C°.
CALCULATION OF HEAT OF CHEMICAL REACTION.
18
CA 02347106 2001-05-15
Heat of reaction depends upon the temperature at which the reaction occurs. It
is
difference between the enthalpies of the reactant and the products, which
depend upon
temperature.
The enthalpies may be related to the temperature by the equation H = N Cp ~T.
Therefore
it is possible the heats of reaction involved at any temperature from the heat
capacity data
of reactants and products. 4HR is known from tabulated data at standard
pressure and
temperature, it can be calculated at any other temperature from heat capacity
data without
additional experiments. Since enthalpy changes are independent of the path of
the
Io process, the following three-step process can accomplish the same changes
as the
reaction at T.
1. Cool the reactant from T K° to 25 C°.
OH = -(~ N Cp~a~erage) )R (T 298)
Where the summation terms represents the sum of the products of the number of
moles of
each reactant times its average molar heat capacity.
2. The change in reaction enthalpy is calculated at 298 K°
20 ~H ~HR298 Ko
3. Heat the product from 298 to T° K
The sum of these three OH values is the desired heat of reaction at
T° K
~RT = ~HR 298 + ~ (~ N CpV)p - (~ N Cp)R ~ ( T-298)
SOME IMPOTANT CHEMICAL REACTIONS AND THEIR ENTHALPIES.
Reactions with molecular oxygen (combustion)
C +'/2 02 = CO OH =-110.62 Kj /mol (1)
3o CO +'/2 02 = C02 DII =- 283.15 Kj/mol (2)
H2 +'/2 02 = H2 O 0H = -242.00 Kj/mol (3)
C" Hm + (ri+m/4)02 = riC02 +m/2H20 (4)
CH4 + 202 = C02 + 2H20 DH = - 802.26 Kj/mol (4a)
PARTIAL COMBUSTION
C" H",+ (n/2 + m/4) 02= riC0 + m/2 H20 (5)
REACTIONS WITH STEAM
C + H20 = CO + H2 ~H = + 131.31 Kj/mol (6)
CO + H20 = C02 + H2 OH = -41.16 Kj/mol (7)
4o C~ + H20 =CO + 3H2 DH = + 206.78 Kj/mol (8)
C"H", + 2n H20 = nC02 + (m/2+2n ) H2 (9)
REACTIONS WITH C02
C+C02 = 2C0 DH = + 172.54 Kj/mol ( 10)
(Boudourd reaction)
C"H",+ nC02 = 2nC0 +2m H2 (11)
Examples.
5o CH4+ C02 = 2C0 + 2H2 0H =+ 247.45 Kj/mol (12)
C2 I-I~ + 2C02 = 4C0 + 3H2 OH =+ 429.82 Kj/mol (13)
C3Hg + 3C02 = 6C0 + 4H2 OH = + 621. 53 Kj/mol (
14)
19
CA 02347106 2001-05-15
Equations 4 (to generate heat), 7 (CO shift reaction), 10 protective cooling
in CO/C02
atmosphere) and 12,14 (endothermic cooling) are of special significance to us
as
considerable use will be made of these in industrial processing
Temperature dependence of these reaction is particular important. In carbon
monoxide
shift reaction is performed at low temperature to obtain high contents of
hydrogen and
eventually to obtain pure hydrogen. At high temperature the hydrogen is
eliminated and
more and more CO is obtained. Reactions 12-14 increase with temperature, as
more heat
is available for absorption.
to MATERIALS SEPARATION BY PHASE SEPARATION.
We have described the chemical potential or potentials in which a metal o a
group of
metals can be oxidized or reduced or partially oxidized or partially reduced.
A metal can
be made to volatile, or melt at much lower temperature than when it is in
oxidized form.
Similarly certain metals can be made to volatile or melt at lower temperature
in their
oxidized form. Certain metals are very higher melting and are not volatile
when present
as completely reduced or completely oxidized form, but their partially
oxidized or
partially reduced form is volatile and in these chemical potentials these can
be separated.
2o Mo (iv) oxide is volatile at 1151 C° at one atmosphere; Mo metal is
volatile at 4606 C°
and one atmosphere.
Cd is volatile at 767 C°; Cd0 is volatile at 1558 C°.
We will use these concept in separation of mixture of metals as in preparation
of steel
from scrap, and separation of mixture of oxides.
In this supplement certain guiding principals are described which could be
foundation for
efficient, pollution free, energy saving method for upto now not process-able
materials:
1. Controlled chemical potential techniques for separation of metal by melting
and
volatizing.
3o 2. Changing from standard activity to nonstandard activities for reduction
of metal
oxides, which are not reducible in standard states.
3.Use of endothermic reactions for energy recovery and cooling of metals, slag
phases,
and high temperature gaseous streams. Using of endothermic reactions in
gaseous
insulation of electric system, and cooling of high temperature refractory.
4 Use of shift reaction for elimination of gaseous content not required in a
particular
mixture or material.
4o Supplement B
Meanings of some words and certain words used synonymously.
-Micronutrients fertilizer is a solid or gas fertilizer where solid
micronutrients is a
mixture formed by slag, metal from certain ashes, metals which were not
separated
during melt separation.
-Gaseous micronutrients are certain impurity gases { S02 , P203, N02, Cl,
etc.) small in
volume but are ingredient of plant growth system. The impurity gases are mixed
with
so either nitrogen feed or COZ
feed of the plants.
-Reducing gases and reduction gases are used synonymously.
-Greenhouse and controlled temperature, controlled potential form represents
the same
thing.
-Volatile and volatilizing (when some thing is made volatile by other means)
CA 02347106 2001-05-15
-Industrial hydrogen is hydrogen obtained after shift conversion and
methanation process,
may contain methane and other small impurities.
-Cryogenic hydrogen is high purity hydrogen obtained through fractionation and
absorption process.
-Sizing is a term used to cut the large size material to a small size which
can be handled
by automated charging system,
In brief I have invented a new method and plant for changing garbage to
valuable energy
containing gases for combustion and form uses. The solid materials in garbage
are
changed to micronutrient fertilizer and quality steel scrap. Metals not
required in steel are
separated and recovered. When reason quantity of garbage is available urea can
be
produced with better economic returns. The attached green house can get green
house
gases and micronutrients gas at cheaper rate and can work around the year in
hot and cold
regions.
Information
Some technical inventions of this project have been covered by U.S.A
2o Patent application, Serial no 09/546,014 Cnfrm No 2942, filing date 10-
april-
00.
Title. Making shaping and treating of steels in a continuous process steel
mill.
Inventor Ghulam Nabi.
40
21