Note: Descriptions are shown in the official language in which they were submitted.
CA 02347482 2004-06-09
INFRARED ATR GLUCOSE MEASUREMENT SYSTEM
Field of the Invention
This invention involves a non-invasive glucose measurement device and a
process
for determining blood glucose level in the human body using the device. In
typical
operation, the glucose measurement device is self normalizing in that it does
not employ an
independent reference sample in its operation. The inventive device uses
attenuated total
reflection (ATR) infrared spectroscopy. Preferably, the device is used on a
fingertip and
compares two specific regions of a measured infrared spectrum to determine the
blood
glucose level of the user. Clearly, this device is especially suitable for
monitoring glucose
levels in the human body, and is especially beneficial to users having
diabetes mellitus.
The device and procedure may be used for other maxerials which exhibit unique
mid-IR
signatures of the type described below and and that are found in appropriate
regions of the
outer skin. A cleaning kit for preparation of the skin surface is also
included.
Background of the Invention
The American Diabetes Association reports that nearly 6% of the population in
the
United States, a group of 16 million people, has diabetes. The Association
further reports
that diabetes is the seventh leading cause of death in the United States,
contributing to
nearly 200,000 deaths per year. Diabetes is a chronic disease having no cure.
The
complications of the disease include blindness, kidney disease, nerve disease,
and heart
disease, perhaps with stroke. Diabetes is said to be the leading cause of new
cases of
blindness in individuals in the range of ages between 20 and 74; from 12,000-
24,000
people per year lose their sight because of diabetes. Diabetes is the leading
cause of end-
stage renal disease, accounting for nearly 40% of new cases. Nearly 60-70% of
people
with diabetes have mild to severe forms of diabetic nerve damage which, in
severe forms,
CA 02347482 2001-04-12
WO 00/21437 PCT/US99/23823
can lead to lower limb amputations. People with diabetes are 2-4 times more
likely to have
heart disease and to suffer strokes.
Diabetes is a disease in which the body does not produce or properly use
insulin, a
hormone needed to convert sugar, starches, and the like into energy. Although
the cause of
diabetes is not completely understood, genetics, environmental factors, and
viral causes
have been partially identified.
There are two major types of diabetes: Type I and Type II. Type I diabetes
(formerly known as juvenile diabetes) is an autoimmune disease in which the
body does not
produce any insulin and most often occurs in young adults and children. People
with Type
I diabetes must take daily insulin injections to stay alive.
Type II diabetes is a metabolic disorder resulting from the body's inability
to make
enough, or properly to use, insulin. Type II diabetes accounts for 90-95% of
diabetes. In
the United States, Type II diabetes is nearing epidemic proportions,
principally due to an
increased number of older Americans and a greater prevalence of obesity and a
sedentary
lifestyle.
Insulin, in simple terms, is the hormone that unlocks the cells of the body,
allowing
glucose to enter those cells and feed them. Since, in diabetics, glucose
cannot enter the
cells, the glucose builds up in the blood and the body's cells literally
starve to death.
Diabetics having Type I diabetes typically are required to self administer
insulin
using, e.g., a syringe or a pin with needle and cartridge. Continuous
subcutaneous insulin
infusion via implanted pumps is also available. Insulin itself is typically
obtained from
pork pancreas or is made chemically identical to human insulin by recombinant
DNA
technology or by chemical modification of pork insulin. Although there are a
variety of
different insulins for rapid-, short-, intermediate-, and long-acting forms
that may be used
variously, separately or mixed in the same syringe, use of insulin for
treatment of diabetes
is not to be ignored.
It is highly recommended by the medical profession that insulin-using patients
practice self monitoring of blood glucose (SMBG). Based upon the level of
glucose in the
blood, individuals may make insulin dosage adjustments before injection.
Adjustments are
necessary since blood glucose levels vary day to day for a variety of reasons,
e.g., exercise,
stress, rates of food absorption, types of food, hormonal changes (pregnancy,
puberty, etc.)
and the like. Despite the importance of SMBG, several studies have found that
the
2
CA 02347482 2001-04-12
WO 00/21437 PCTNS99/23823
proportion of individuals who self monitor at least once a day significantly
declines with
age. This decrease is likely due simply to the fact that the typical, most
widely used,
method of SMBG involves obtaining blood from a finger stick. Many patients
consider
obtaining blood to be significantly more painful than the self administration
of insulin.
There is a desire for a less invasive method of glucose measurement. Methods
exist
or are being developed for a minimally invasive glucose monitoring, which use
body fluids
other than blood (e.g., sweat or saliva), subcutaneous tissue, or blood
measured less
invasively. Sweat and saliva are relatively easy to obtain, but their glucose
concentration
appears to lag in time significantly behind that of blood glucose. Measures to
increase
sweating have been developed and seem to increase the timeliness of the sweat
glucose
measurement, however.
Subcutaneous glucose measurements seem to lag only a few minutes behind
directly measured blood glucose and may actually be a better measurement of
the critical
values of glucose concentrations in the brain, muscle, and in other tissue.
Glucose may be
measured by non-invasive or minimally-invasive techniques, such as those
making the skin
or mucous membranes permeable to glucose or those placing a reporter molecule
in the
subcutaneous tissue. Needle-type sensors have been improved in accuracy, size,
and
stability and may be placed in the subcutaneous tissue or peripheral veins to
monitor blood
glucose with small instruments. See, "An Overview of Minimally Invasive
Technologies ",
Clin. Chem. 1992 Sep.; 38(9):1596-1600.
Truly simple, non-invasive methods of measuring glucose are not commercially
available.
U.S. Patent No. 4,169,676 to Kaiser, shows a method for the use of ATR glucose
measurement by placing the ATR plate directly against the skin and especially
against the
tongue. The procedure and device shown there uses a laser and determines the
content of
glucose in a specific living tissue sample by comparing the IR absorption of
the measured
material against the absorption of IR in a control solution by use of a
reference prism. See,
column 5, lines 31 et seq.
None of the cited prior art suggests the device and method of using this
device
described and claimed below.
3
CA 02347482 2001-04-12
WO 00/21437 PCT/US99/23823
SUMMARY OF THE INVENTION
This invention is a glucose level measurement device utilizing IR-ATR
spectroscopy and a method of using the device. The inventive device itself
preferably made
up of four parts:
a.) an IR source for emitting an IR beam into the ATR plate,
b.) the ATR plate against which the sampled human skin surface is pressed,
and
c.) at least two IR sensors.for simultaneously measuring absorbance of two
specific regions of the IR spectrum, i.e., a "referencing wavelength" and a
"measuring
wavelength." The IR source must emit IR radiation at least in the region of
the referencing
wavelength and the measuring wavelength. For glucose, the referencing
wavelength is
between about 8.25 micrometers and about 8.75 micrometers and the measuring
wavelength is between about 9.50 micrometers and about 10. 00 micrometers. The
IR
sources may be broadband IR sources, non-laser sources, or two or more
selected
wavelength lasers.
Other analyte materials which have both referencing wavelengths and measuring
wavelengths as are described in more detail below and that are found in the
outer regions of
the skin may be measured using the inventive devices and procedures described
herein.
The ATR plate is configured to permit multiple internal reflections, perhaps 3-
15
internal reflections, against said measurement surface prior to measurement by
the IR
sensors. Typically the IR beam emitted from the ATR plate is split for the IR
sensors using
a beam splitter or equivalent optical device. Once the split beams are
measured by the IR
sensors, the resulting signals are then transformed using analog comparators
or digital
computers into readable or displayable values.
It is usually important that the device have some accommodation for holding
the
body part against the ATR plate, preferably at some value which is constant
and above a
selected minimum pressure.
The method for determining the blood glucose level, using the glucose
measurement device, comprises the steps of.
a.) contacting a selected skin surface with the ATR plate,
4
CA 02347482 2001-04-12
WO 00/21437 PCT/US99/23823
b.) irradiating that human skin surface with an IR beam having components
at least in the region of the referencing wavelength and the measuring
wavelength, and
c.) detecting and quantifying those referencing and said measuring
wavelength components in that reflected IR beam.
The procedure ideally includes the further steps of maintaining the skin
surface on
said ATR plate at an adequate pressure which is both constant and above a
selected
minimum pressure and, desirably cleaning the skin surface before measurement.
A step of
actually measuring the pressure may also be included.
A normalizing step practiced by simultaneously detecting and quantifying the
referencing and measuring wavelength components prior to contacting the skin
surface is
also desirable.
A final portion of this invention is a cleaning kit used for cleaning the
object skin
prior to testing. The kit usually is made up of sealed packets, preferably
containing
absorbent pads, of each of.-
a.) a glucose solvent, e.g., water or other highly polar solvent,
b.) a solvent for removing the glucose solvent, e.g., isopropanol, and
c.) a skin softener or pliability enhancer, e.g., various mineral oils such as
"Nujol",
not having significant IR wavelength peaks between about 8.25 micrometers and
about
8.75 micrometers or between about 9.50 micrometers and about 10.00
micrometers. The
solvent for removing the glucose solvent similarly should not have an
interfering IR signal
which persists after several minutes.
BRIEF DESCRIPTION OF THE DRAWINGS
Figures 1 A, 1 B, 1 C, and 1 D show a side view of various ATR plates and
their
general operation.
Figure 2 shows an IR spectrum of d-glucose.
Figure 3 shows a schematicized layout of the optics of the inventive device.
Figure 4 shows a packaged variation of the inventive glucose measuring device.
Figure 5 shows a graph correlating glucose levels measured using a specific
variation of the device with glucose levels in the blood determined using a
commercial
device.
5
CA 02347482 2001-04-12
WO 00/21437 PCT/US99/23823
Figure 6 shows a pair of glucose IR curves (taken before and after eating) for
an
individual having diabetes made using the inventive glucose measuring device.
Figure 7 shows a graph comparing glucose levels in a non-diabetic individual
(taken
before and after eating) made using the inventive glucose measuring device and
direct
blood measurement. This graph shows that the inventive procedure tracks blood
glucose
levels with minimum time lag.
DESCRIPTION OF THE INVENTION
The device in this invention uses infrared ("IR") attenuated total internal
reflectance
("ATR") spectroscopy to detect and ultimately to determine the level of blood
glucose in
the human body. Preferably, the inventive device uses an ATR procedure in
which the size
and configuration of the crystal permits a number of internal reflections
before the beam is
allowed to exit the crystal with its measured information. In general, as
shown in Figures
1 A and 1 B, when an infrared beam { 102) is incident on the upper surface of
the ATR
crystal (104) -- or ATR plate -- at an angle which exceeds a critical angle
O~, the beam
(102) will be completely totally reflected within crystal (104). Each
reflection of the beam
within the ATR plate, and specifically against the upper surface (114),
provides a bit more
information about the composition of the sample (112) resting against that
upper surface
(114). The more numerous the reflections, and the greater the penetration
depth of the
reflection, the higher is the quality of the information. The incident beam (
102) becomes
reflected beam ( 106) as it exits crystal ( 104) as shown in Figure 1 A.
Higher refractive
index materials are typically chosen for the ATR crystal to minimize the
critical angle. The
critical angle is a function of the refractive indices of both the sample and
the ATR crystal
and is defined as:
n
O~, = sin-'
n,
Here, ni is the refractive index of the ATR crystal and n2 is the refractive
index of
the sample.
As shown in Figure 1 B, the internally reflected beam ( 108) includes an
evanescent
wave ( 110) which penetrates a short distance into sample { 112) over a wide
wavelength
6
CA 02347482 2001-04-12
WO 00/21437 PCT/US99/23823
range. In those regions of the IR spectrum in which the sample absorbs IR,
some portion of
the light does not return to the sensor. It is these regions of IR absorbance
which provide
information, in this inventive device, for quantification of the glucose
level.
We prefer the use of higher refractive crystals such as zinc selenide, zinc
sulfide,
diamond, germanium, and silicon as the ATR plate. The index of refraction of
the ATR
plate (104) should be significantly higher than that of the sample (112).
Further, the ATR crystal (104) shown in Figure lA is shown to be trapezoidal
and
having an upper surface (114) for contact with the sample, which sample, in
this case, is
skin from a living human body. However, this shape is only for the purposes of
mechanical
convenience and ease of application into a working commercial device. Other
shapes, in
particular, a parallelogram ( 111 ) such as shown in Figure 1 C and the
reflective crystal
( 113) shown in Figure 1 D having mirrored end ( 115), are also quite suitable
for this
inventive device should the designer so require. The mirrored reflective
crystal (113) has
the advantage of, and perhaps the detriment of having both an IR source and
the IR sensors
1 S at the same end of the crystal.
It is generally essential that the ATR crystal or plate (104) have a sample or
upper
surface (114) which is essentially parallel to the lower surface (116). In
general, the ATR
plate (104) is preferably configured and utilized so that the product of the
practical number
of internal reflections of internal reflected beam (108) and the skin
penetration per
reflection of this product is maximized. When maximizing this product, called
the
effective pathlength (EPL), the information level in beam (106) as it leaves
ATR plate
(104) is significantly higher. Further, the higher the value of the index of
refraction, n2 , of
the ATR plate (104), the higher is the number of internal reflections. The
sensitivity of the
IR sensors also need not be as high when the EPL is maximized. We consider the
number
of total reflections within the crystal to be preferably from 3-15 or more for
adequate
results.
We have surprisingly found that a glucose measuring device made according to
this
invention is quite effective on the human skin of the hands and fingers. We
have found that
the glucose concentration as measured by the inventive devices correlates very
closely with
the glucose concentration determined by a direct determination from a blood
sample. As
will be discussed below, the glucose level as measured by the inventive device
also is
surprisingly found closely to track the glucose level of blood in time as
well. This is
7
CA 02347482 2001-04-12
WO 00/21437 PCT/US99/23823
surprising in that the IR beam likely passes into the skin, i.e., the stratum
corneum, for
only a few microns. It is unlikely in a fingertip that any blood is crossed by
that light path.
The stratum corneum is the outer layer of skin and is substantially
unvascularized. The
stratum corneum is the final outer product of epidermal differentiation or
keratinization. It
is made up of a number of closely packed layers of flattened polyhedral
corneocytes (also
known as squames). These cells overlap and interlock with neighboring cells by
ridges and
grooves. In the thin skin of the human body, this layer may be only a few
cells deep, but in
thicker skin, such as may be found on the toes and feet, it may be more than
50 cells deep.
The plasma membrane of the corneocyte appears thickened compared with that of
keratinocytes in the lower layers of the skin, but this apparent deposition of
a dense
marginal band formed by stabilization of a soluble precursor, involucrin, just
below the
stratum corneum.
It is sometimes necessary to clean the skin exterior prior to taking a sample
to
remove extraneous glucose from the skin surface. When doing so, it is
important to select
1 S cleaning materials which have IR spectra that do not interfere with the IR
spectra of
glucose. We consider a kit of the following to be suitable for preparation of
the sample
skin for the testing. The three components are: a.) a glucose solvent, e.g.,
water or other
highly polar solvent; b.) a solvent for removing the water, e.g., isopropanol,
and c.) a skin
softener or pliability enhancer not having significant IR peaks in the noted
IR regions, e.g.,
mineral oils such as those sold as "Nujol". Certain mixtures of the first two
components
may be acceptable, but only if the sampling situation is such that the
solvents evaporate
without spectrographically significant residue. The inventive kit contains
sealed packets of
each of the three components, preferably each within an absorbent pad in the
sealed
packets.
Additionally, the inventive device can be highly simplified compared to other
known devices in that the device can be "self normalizing" due to the
specifics of the IR
signature of glucose. Figure 2 shows the IR absorbance spectra of d-glucose.
The family
of curves there shows that in certain regions of the IR spectrum, there is a
correlation
between absorbance and the concentration of glucose. Further, there is a
region in which
the absorbance is not at all dependent upon the concentration of glucose. Our
device, in its
preferable method of use, uses these two regions of the IR spectra. These
regions are in the
so-called mid-IR range, between 2.5 and 14 micrometers. In particular, the
"referencing
8
CA 02347482 2001-04-12
WO 00/21437 PCT/US99/23823
wavelength" point is just above 8 micrometers (150), e.g., 8.25 to 8.75
micrometers, and
the pronounced peaks (152) at the region between about 9.50 and 10.00
micrometers is
used as a "measuring wavelength". The family of peaks ( 152) may be used to
determine
the desired glucose concentration.
Use of the two noted IR regions is also particularly suitable since other
components
typically found in the skin, e.g., water, cholesterol, etc., do not cause
significant
measurement error when using the method described herein.
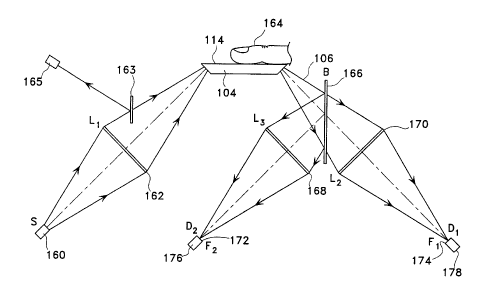
Figure 3 shows an optical schematic of a desired variation of the inventive
device.
ATR crystal ( 104) with sample side ( 114) is shown and IR source ( 160) is
provided. IR
source ( 160) may be any of a variety of different kinds of sources. It may be
a broadband
IR source, one having radiant temperatures of 300°C to 800°C, or
a pair of IR lasers
selected for the two regions of measurement discussed above, or other suitably
emitted or
filtered IR light sources. A single laser is usually not an appropriate light
source in that a
laser is a single wavelength source and the preferred operation of this device
requires light
sources simultaneously emitting two IR wavelengths. Lens (162), for focusing
light from
IR source (160) into ATR plate (104), is also shown. It may be desirable to
include an
additional mirror (163) to intercept a portion of the beam before it enters
the ATR plate
(104) and then to measure the strength of that beam in IR sensor (165).
Measurement of
that incident light strength (during normalization and during the sample
measurement)
assures that any changes in that value can be compensated for.
The light then passes into ATR plate (104) for contact with body part (164),
shown
in this instance to be the desired forger. The reflected beam (106) exits ATR
plate (104)
and is then desirably spit using beam splitter (166). Beam splitter (166)
simply transmits
some portion of the light through the splitter and reflects the remainder. The
two beams
may then be passed through, respectively, lenses (168) and (170). The so-
focussed beams
are then passed to a pair of sensors which are specifically selected for
detecting and
measuring the magnitude of the two beams in the selected IR regions.
Generally, the
sensors will be made up of filter ( 172) and ( 174) with light sensors ( 176}
and ( I 78) behind.
Generally, one filter (172), (174) will be in the region of the referencing
wavelength and
the other will be in that of the measuring wavelength.
Figure 4 shows perhaps a variation of this device (200) showing the finger of
the
user (202) aver the ATR plate (204) with a display (206). Further shown in
this desirable
9
CA 02347482 2001-04-12
WO 00/21437 PCT/US99/23823
variation (200) is a pressure maintaining component (208). We have found that
is very
highly desirable to maintain a minimum threshold pressure on the body part
which is to be
used as the area to be measured. Generally, a variance in the pressure does
not shift the
position of the detected IR spectra, but it may affect the sensitivity of the
overall device.
Although it is possible to teach the user to press hard enough on the device
to reach the
minimum threshold pressure, we have determined for each design of the device
it is much
more appropriate that the design of a particular variation of the inventive
device be
designed with a specific sample pressure in mind. The appropriate pressure
will vary with,
e.g., the size of the ATR plate and the like. A constant pressure above that
minimum
threshold value is most desired.
The variation shown in Figure 4 uses a simple component arm (208) to maintain
pressure of the finger (202) on ATR plate (204). Other variations within the
scope of this
invention may include clamps and the like.
It should be apparent that once an appropriate pressure is determined for a
specific
design, the inventive device may include a pressure sensor, e.g., (210) as is
shown in Figure
4, to measure adherence to that minimum pressure. Pressure sensor (210) may
alternatively
be placed beneath ATR plate (204). It is envisioned that normally a pressure
sensor such as
(210) would provide an output signal which would provide a "no-go/go" type of
signal to
the user.
Method of Use
In general, the inventive device described above is used in the following
manner: a
skin surface on a human being, for instance, on the skin of the finger, is
placed on the ATR
plate. The skin surface is radiated with an IR beam having components at least
in the two
IR regions we describe above as the "referencing wavelength" and the
"measuring
wavelength." The beam which ultimately is reflected out of the ATR plate then
contains
information indicative of the blood glucose level in the user. As noted above,
it is also
desirable to maintain that skin surface on the ATR plate at a relatively
constant pressure
that is typically above a selected minimum pressure. This may be done either
manually or
by measuring and maintaining the pressure.
Typically, the beam leaving the ATR plate is split using an optical beam
splitter
into at least two beams. Each of the two beams may be then focussed onto its
own IR
CA 02347482 2001-04-12
WO 00/21437 PCT/US99/23823
sensor. Each such IR sensor has a specific filter. This is to say that, for
instance, one IR
sensor may have a filter which removes all light which is not in the region of
the
referencing wavelength and the other IR sensor would have a filter which
remove all
wavelengths other than those in the region of the measuring wavelength. As
noted above,
for glucose, the referencing wavelength is typically in the range of about
8.25 to 8.75
micrometers. For glucose, the measuring wavelength is typically between about
9.5 and
10.0 micrometers.
Other analyte materials which have both referencing wavelengths and measuring
wavelengths in the mid-IR range and that are found in the outer regions of the
skin may
also be measured using the inventive devices and procedures described herein.
Respective signals may be compared using analog or digital computer devices.
The
signals are then used to calculate blood glucose concentration using various
stored
calibration values, typically those which are discussed below. The resulting
calculated
values may then be displayed.
1 S As noted above, it is also desirable both to clean the plate before use
and to clean
the exterior surface of the skin to be sampled. Again, we have found, for
instance in the
early morning that the exterior skin is highly loaded with glucose which is
easily removed
by washing the hands. Reproducible and accurate glucose measurements may then
be had
in a period as short as ten minutes after cleaning the area of the skin to be
measured.
We also note that, depending upon the design of a specific variation of a
device
made according to the invention, periodic at least an initial calibration of
the device, using
typical blood sample glucose determinations, may be necessary or desirable.
Determination of blood glucose level from the information provided in the IR
spectra is straightforward. A baseline is first determined by measuring the
level of infrared
absorbance at the measuring and referencing wavelengths, without a sample
being present
on the sample plate. The skin is then placed in contact with the ATR plate and
the two
specified absorbance values are again measured. Using these four values, the
following
calculation is then made.
Toy
A~ - ~n T~ = A~, + Ah, (Absorbance at referencing spectral band.)
11
CA 02347482 2001-04-12
WO 00121437 PCT/US99123823
AZ = .fin ~2 = ARZ + An2 (Absorbance at measuring spectral band.)
z
where: To~ = measured value at reference spectral band w/o sample
To2 = measured value at measuring spectral band w/o sample
S Tl = measured value at reference spectral band w/ sample
T2 = measured value at measuring spectral band w/ sample
Age = absorbance of glucose at reference spectral band
Ag2 = absorbance of glucose at measuring spectral band
Ab, = absorbance of background at reference spectral band
Ab2 = absorbance of background at measuring spectral band
d = effective path length through the sample.
a2 = specific absorptivity at measuring spectral band
k = calibration constant for the device
Cg = measured concentration of glucose
Since the background base values are approximately equal (i.e., Ab,
= Ab2) and Age = 0, then:
A2 - A~ = A~ = a2dcg = kCg
The value of Cg is the desired result of this procedure.
1'iYAMPTTiC
Example 1
Using a commercially available IR spectrometer (Nicolet 510) having a ZnSe
crystal ATR plate (SSmm long, l Omm wide, and 4mm thick) we tested the
inventive
12
CA 02347482 2001-04-12
WO 00/21437 PCT/US99/23823
procedure. We calibrated the output of the spectrometer by comparing the IR
signal to the
values actually measured using one of the inventor's blood samples. The
inventor used a
blood stick known as "Whisper Soft" sold by Amira Medical Co. and "Glucometer
Elite"
blood glucose test strips sold by Bayer Corp. of Elkhart, Ind. On each of the
various test
S days, the inventor took several test sticks and measured the glucose value
of the resulting
blood; the IR test was made at the same approximate time.
As shown in the calibration curve of Figure 5, the data are quite consistent.
So,
where the blood glucose concentration "B" is in (mg/dl) and "S" is the
difference between
the absorbance at the referencing region and the measuring region as measured
by the
spectrometer:
B=[(1950)~S]-(17).
Example 2
In accordance with a clinical protocol, a diabetic was then tested. Curve 1 in
Figure
6 shows the IR absorbance spectrum of the test subject's finger before eating
(and after
fasting overnight) and curve 2 shows IR absorbance spectrum of the same
individual after
having eaten. Incidentally, insulin was administered shortly after the
measurement of curve
2.
In any event, the significant difference in the two peak heights at the 9.75
micrometer wavelength and the equality of the two IR absorbance values at the
8.50
micrometer value shows the effectiveness of the procedure in measuring glucose
level.
Example 3
That the inventive glucose monitoring device non-invasively determines blood
glucose level and quickly follows changes in that blood glucose level is shown
in Figure 7.
Using both the inventive procedure and a commercial glucose device, one of the
inventors
followed his glucose level for a single day. The blood sticks are considered
to be accurate
within 15% of the actual reading.
The results are shown in Figure 7. Of particular interest is the measurement
just
before 4:40 wherein the two values are essentially the same. A high sugar
candy bar was
eaten at about 4:45 and measurements of glucose level were taken using the
inventive
13
CA 02347482 2001-04-12
WO 00/21437 PCT/US99/23823
procedure at about 5:03, 5:18, 5:35 and 5:50. A blood sample was taken at 5:35
and
reflected almost the same value as that measured using the inventive
procedure.
Consequently, the procedure tracks that measured by the blood very quickly.
This invention has been described and specific examples of the invention have
been
portrayed. The use of those specifics is not intended to limit the invention
in any way.
Additionally, to the extent there are variations of the invention with are
within the spirit of
the disclosure and yet are equivalent to the inventions found in the claims,
it is our intent
that this patent will cover those variations as well.
14