Note: Descriptions are shown in the official language in which they were submitted.
CA 02348236 2001-04-24
WO 00/41994 PCT/US99130418 -
4-ARYLAMINO, 4-ARYLOXY, AND 4-ARYLTHIO DIARYLAMINES AND DERIVATIVES THEREOF AS
SELECTIVE MEK INHIBITORS
This invention relates to 4-arylamino, 4-aryloxy, and 4-arylthio
diarylamines and derivatives thereof.
BACKGROUND
MEK enzymes are dual spec~city kinases involved in, for example,
immunomodulation, inflammation, and proliferative diseases such as cancer and
restenosis.
Proliferative diseases are caused by a defect in the intracellular signaling
system, or the signal transduction mechanism of certain proteins. Defects
include a change either in the intrinsic activity or in the cellular
concentration of
one or more signaling proteins in the signaling cascade . The cell may produce
a
growth factor that binds to its own receptors, resulting in an autocrine loop,
which
continually stimulates proliferation. Mutations or overexpression of
intracellular
signaling proteins can lead to spurious mitogenic signals within the cell.
Some of
the most common mutations occur in genes encoding the protein known as Ras,
a G-protein that is activated when bound to GTP, and inactivated when bound to
GDP. The above-mentioned growth factor receptors, and many other mitogenic
receptors, when activated, lead to Ras being converted from the GDP-bound
state to the GTP-bound state. This signal is an absolute prerequisite for
proliferation in most cell types. Defects in this signaling system, especially
in the
deactivation of the Ras-GTP complex, are common in cancers, and lead to the
signaling cascade below Ras being chronically activated.
Activated Ras leads in tum to the activation of a cascade of serine/threonine
kinases. One of the groups of kinases known to require an active Ras-GTP for
its
own activation is the Raf family. These in tum activate MEK (e.g., MEK1 and
MEK2) which then activates MAP kinase, ERK (ERK~ and ERKZ). Activation of
MAP kinase by mitogens appears to be essential for proliferation; constitutive
activation of this kinase is sufficient to induce cellular transformation.
Blockade of
downstream Ras signaling, for example by use of a dominant negative Raf
1 protein, can completely inhibit mitogenesis, whether induced from cell
surface
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
receptors or from oncogenic Ras mutants. Although Ras is not itself a protein
kinase, it participates in the activation of Raf and other kinases, most
likely
through a phosphorylation mechanism. Once activated, Raf and other kinases
phosphorylate MEK on two closely adjacent serine residues, S218 and S222 in
the case of MEK-1, which are the prerequisite for activation of MEK as a
kinase.
MEK in turn phosphorylates MAP kinase on both a tyrosine, Y185, and a
threonine residue, T183, separated by a single amino acid. This double
phosphorylation activates MAP kinase at feast 100-fold. Activated MAP kinase
can then catalyze the phosphorylation of a large number of proteins, including
several transcription factors and other kinases. Many of these MAP kinase
phosphorylations are mitogenically activating for the target protein, such as
a
kinase, a transcription factor, or another cellular protein. In addition to
Raf 1 and
MEKK, other kinases activate MEK, and MEK itself appears to be a signal
integrating kinase. Current understanding is that MEK is highly speck for the
phosphoryiation of MAP kinase. In fact, no substrate for MEK other than the
MAP kinase , ERK, has been demonstrated to date and MEK does not
phosphorylate peptides based on the MAP kinase phosphorylation sequence, or
even phosphorylate denatured MAP kinase. MEK also appears to associate
strongly with MAP kinase prior to phosphorylating it, suggesting that
phosphorylation of MAP kinase by MEK may require a prior strong interaction
between the two proteins. Both this requirement and the unusual spec~city of
MEK are suggestive that it may have enough difference in its mechanism of
action to other protein kinases that selective inhibitors of MEK, possibly
operating
through allosteric mechanisms rather than through the usual blockade of the
ATP
binding site, may be found.
2
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
SUMMARY
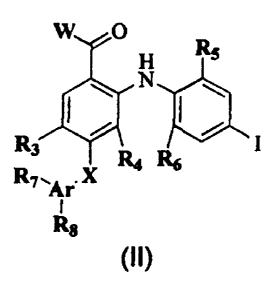
The invention features compounds of formulae (II) below, such as
formula(I):
w o
H
R3 ~ R6 I
R~.Ar X
I
Rg
W ~O R
s
\ ~ \
~ R I ~I
°R
6
i
In formulae (I) and (II), W is ORS, NR20R~, NRARB, NR2NRARB, or NR2(CH2)z-~
NRARe. R~ is H, C ,.~ alkyl, C ~.s alkenyl, C ~ alkynyl, C ~e cycloalkyl,
phenyl,
(phenyl)C » alkyl, (phenyl)C ~ alkenyl, (phenyl)C ~ alkynyl, (C ~ cycloalkyl)-
C ~~ alkyl, (C ~ cycloalkyl)C ~ alkenyl, (C ~e cycloalkyl)C ~ alkynyl, C ~
heterocyclic radical, (C ~ heterocyclic radical)C » alkyl, (C ~ heterocyclic
radical)C ~ alkenyl, (C ~ heterocyclic radical)C ~ alkynyl, or (CH2)2.~NRARB.
R2
3
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
is H, phenyl, C ~.~ alkyl, C~ alkenyl C ~ alkynyl, C ~.8 cycloalkyl, or (C ~
cycloalkyl)C ,.~ alkyl. RA is H, C ,~ alkyl, C ~ alkenyl, C ~8 alkynyl, C ~
cycloalkyl, phenyl, (C ~ cycloalkyl)C ,.~ alkyl, (C ~ cycloalkyl)C ~ alkenyl,
(C ~
cycloalkyl)C 3.~ alkynyl, C ~ heterocyclic radical, (C ~ heterocyclic
radical)C ~~
alkyl, (aminosulfonyl)phenyl, [(aminosulfonyl)phenyl]C ,~ alkyl,
(aminosuffonyl)C
~.~ alkyl, (aminosulfonyl)C ~ cycloalkyl, or [(aminosulfonyl)C ~ cycloalkyljC
~.~
alkyl. RB is H, C » alkyl, C ~ alkenyl, C 3~ alkynyl, C 3~ cycloalkyl, or C ~
aryl.
R3 is halo, N02, S02NR, (CH2)2.4NRERF, S02NR, RK or (CO)T. T is C ,.~ alkyl, C
~
a cycloalkyl, (NRERF)C ~.~ alkyl, ORF, NR,(CH2)2.~NRERF, or NRERF. R4 is H or
F;
R5 is H, methyl, halo, or N02; and R6 is H, methyl, halo, or N02. In formula
(II), Ar
is phenyl, 2-pyridyl, 3-pyridyl, or 4-pyridyl. Each of R~ and R$ is
independently
selected from H, halo, C ,.~ alkyl, S02NR~ (CH2)2~NRcRH, (CO)(CH2)2.~NRoRH,
(CO)NR~(CH2)2~NRoRH, (CO)O(CH2)2.~NRcRN, S02NRcRH, and (CO)NRoRH.
However, where Ar is a pyridyl, each of R~ and R8 is H. Each of Rc, Rp, RE,
RF,
Rc, and RH is independently selected from H, C ~.~ alkyl, C ~ alkenyl, C a.,,
alkynyl, C ~ cycloalkyl, and phenyl. Each of NRcRo, NRERF, and NRoRH can
also be independently morpholinyl, piperazinyl, pyrrolidinyl, or piperadinyl.
Each
of R, and R,~ is independently H, methyl, or ethyl. RK is C ~.~ alkyl, C 3..,
alkenyl, C
3.,4 alkynyf, C ~ cycloalkyl, or phenyl. X is O, S, or NH. Finally, each
hydrocarbon radical or heterocyclic radical above is optionally substituted
with
between 1 and 3 substituents independently selected from halo, C ~.~ alkyl, C
~
cycloalkyl, C ~.~ alkenyl, C ,.~ alkynyl, phenyl, hydroxyl, amino,
(amino)sutfonyl,
and N02, wherein each substituent alkyl, cycloalkyl, alkenyl, alkynyl or
phenyl is
in turn optionally substituted with between 1 and 3 substituents independently
selected from halo, C ,_2 alkyl, hydroxyl, amino, and N02. In addition to the
above
compounds, the invention also provides a pharmaceutically acceptable salt or
C ~_~ ester thereof.
The invention also relates to a pharmaceutical composition including (a) a
diarylamine (e.g., of formula I) and (b) a pharmaceutically acceptable
carrier.
The invention further relates to a method for treating proliferative diseases,
such as cancer, restenosis, psoriasis, autoimmune disease, and
atherosclerosis.
Other aspects of the invention include methods for treating MEK-related
cancer,
4
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
tumors of the breast, lung, colorectal, pancreas, prostate, brain, kidney, or
ovary,
and other solid or hematopoietic cancers. Further aspects of the invention
include methods for treating or reducing the symptoms of xenograft (organ,
cell(s), limb, skin, or bone marrow transplant) rejection, osteoarthritis,
rheumatoid
arthritis, cystic frbrosis, hepatomegaly, cardiomegaly, complications of
diabetes
(including diabetic nephropathy and diabetic retinopathy), stroke, heart
failure,
septic shock, asthma, and Alzheimer's disease. Compounds of the invention are
also useful as antiviral agents for treating viral infections such as HIV,
hepatitis
(B) virus (HBV), human papilloma virus (HPV), cytomegalovirus (CMV), and
Epstein-Barr virus (EBV). The methods include administering to a patient in
need
of such treatment, or suffering from such a disease or condition, a
pharmaceutically effective amount of a disclosed compound or pharmaceutical
composition thereof.
The invention also features methods of combination therapy, such as a
method for treating cancer, wherein the method further includes providing
radiation therapy or chemotherapy, for example, with mitotic inhibitors such
as a
taxane or a vinca alkaloid. Examples of mitotic inhibitors include paclitaxel,
docetaxel, vincristine, vinblastine, vinorelbine, and vinflunine. Other
therapeutic
combinations include a MEK inhibitor of the invention and an anticancer agent
such as cisplatin, 5-fluorouracil or 5-fluoro-2-4(1 H,3H)-pyrimidinedione
(5FU),
flutamide, and gemcitabine.
The chemotherapy or radiation therapy may be administered before,
concurrently, or after the administration of a disclosed compound according to
the
needs of the patient.
The invention also features synthetic intermediates and methods disclosed
herein.
Other aspects of the invention are provided in the description, the
examples, and the claims below.
5
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
DETAILED DESCRIPTION
The invention features diarylamine compounds of formula (I),
pharmaceutical compositions thereof, and methods of using such compounds
and compositions.
According to one aspect of the invention, the compounds are MEK
inhibitors. MEK inhibition assays include the cascade assay for inhibitors of
MAP
kinase pathway described at column 6, line 36 to column 7, line 4 of U.S.
Patent
Number 5,525,625 and the in vitro MEK assay at column 7, lines 4-27 of the
same patent, the entire disclosure of which is incorporated by reference (see
also
Examples 2-5 below).
A. Terms
Certain terms are defined below and by their usage throughout this
disclosure.
Alkyl groups include aliphatic {i.e., the subset of hydrocarbyl or
hydrocarbon radical structures containing hydrogen and carbon atoms, but not
containing heteroatoms in the skeleton, or unsaturated carbon-carbon bonds)
with a free valence. Alkyl groups are understood to include straight chain and
branched structures. Examples include methyl, ethyl, propyl, isopropyl, butyl,
n-
butyl, isobutyl, t-butyl, pentyl, isopentyl, 2,3-dimethylpropyl, hexyl, 2,3-
dimethyl
hexyl, 1,1-dimethylpentyl, heptyl, and octyl. Cycloalkyl groups include
cyclopropyl, cyclobutyl, cyc)opentyl, cyclohexyl, and cyclooctyl.
Alkyl groups can be substituted with 1, 2, 3 or more substituents which are
independently selected from halo (fluoro, chloro, bromo, or iodo), hydroxy,
amino,
alkoxy, alkylamino, dialkylamino, cycloalkyl, aryl, aryloxy, arylalkyloxy,
heterocyclic radical, and (heterocyclic radical)oxy. Specific examples include
fluoromethyl, hydroxyethyl, 2,3-dihydroxyethyl, {2- or 3-furanyl)methyl,
cyclopropylmethyl, benryloxyethyl, (3-pyridinyl)methyl, (2- or 3-
furanyl)methyl, (2-
thienyl)ethyl, hydroxypropyl, aminocyclohexyl, 2-dimethylaminobutyl,
methoxymethyl, N pyridinylethyl, diethylaminoethyl, and cyclobutylmethyl.
Alkenyl groups are analogous to alkyl groups, but have at least one double
bond (two adjacent sp2 carbon atoms). Depending on the ptacement of a double
6
CA 02348236 2001-04-24
WO 00/41994 PCTNS99/30418
bond and substituents, if any, the geometry of the double bond may be entgegen
(E), zusammen (Z), cis, or traps. Similarly, alkynyl groups have at least one
triple
bond (two adjacent sp carbon atoms). Unsaturated alkenyl or alkynyl groups may
have one or more double or triple bonds, respectively, or a mixture thereof;
tike
alkyl groups, they may be straight chain or branched, and they may be
substituted as described above and as exemplified throughout the disclosure.
Examples of alkenyls, alkynyls, and substituted fom~s include cis-2-butenyl,
trans-
2-butenyl, 3-butynyl, 3-phenyl-2-propynyl, 3-(2'-fluorophenyl)-2-propynyl, 3-
methyl(5-phenyl)-4-pentynyl, 2-hydroxy-2-propynyl, 2-methyl-2-
propynyl, 2-propenyl, 4-hydroxy-3-butynyl, 3-(3-fluorophenyl)-2-propynyl, and
2-
methyl-2-propenyl. In formulae (I) and (II) alkenyls can be C 2~ or C 2$, and
are
preferably C ~ or C ~.
More general forms of substituted hydrocarbon radicals include
hydroxyalkyl, hydroxyalkenyl, hydroxyalkynyl, hydroxycycloatkyl, hydroxyaryl,
and
corresponding forms for the prefixes amino-, halo- (e.g., fluoro-, chloro-, or
bromo-), vitro-, alkyl-, phenyl-, cycloalkyl- and so on, or combinations of
substituents. According to formula (I), therefore, substituted alkyls include
hydroxyalkyl, aminoalkyl, nitroalkyl, haloalkyl, alkylalkyl (branched alkyls,
such as
methylpentyl), (cycloalkyl)alkyl, phenylalkyl, alkoxy, alkylaminoalkyl,
dialkylaminoalkyl, arylalkyl, aryloxyalkyl, arylalkyloxyalkyl, (heterocyclic
radical)alkyl, and (heterocyclic radical)oxyalkyl. R~ thus includes
hydroxyalkyl,
hydroxyalkenyl, hydroxyalkynyl, hydroxycycloalkyl, hydroxyaryl, aminoalkyl,
aminoalkenyl, aminoalkynyt, aminocyctoalkyl, aminoaryl, alkytalkenyl,
(alkylaryl)alkyl, (haloaryl)alkyl, (hydroxyaryl)alkynyl,.and so forth.
Similarly, RA
includes hydroxyalkyl and aminoaryl, and RB includes hydroxyalkyl, aminoalkyl,
and hydroxyalkyl(heterocyclic radical)alkyl.
Heterocyclic radicals, which include but are not limited to heteroaryls,
include: furyl, oxazolyl, isoxazolyl, thiophenyl, thiazolyl, pyrrolyl,
imidazolyl, 1,3,4-
triazolyl, tetrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, indolyl, and their
nonaromatic counterparts. Further examples of heterocyclic radicals include
pipertdyl, quinolyl, isothiazolyl, piperidinyl, morpholinyl, piperazinyl,
7
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
tetrahydrofuryl, tetrahydropyrrolyl, pyrrolidinyl, octahydroindolyl,
octahydrobenzothiofuranyl, and octahydrobenzofuranyl.
Selective MEK 1 or MEK 2 inhibitors are those compounds which
inhibit the MEK 1 or MEK 2 enzymes, respectively, without substantially
inhibiting
ocher enzymes such as MKK3, PKC, Cdk2A, phosphorylase kinase, EGF, and
PDGF receptor kinases, and C-src. In general, a selective MEK 1 or MEK 2
inhibitor has an IC5o for MEK 1 or MEK 2 that is at least one-fiftieth (1/50)
that of
its IC~fl for one of the above-named other enzymes. Preferably, a selective
inhibitor has an ICSO that is at least 1/100, more preferably 1/500, and even
more
preferably 1/1000, 1/5000, or less than that of its IC5o or one or more of the
above-named enzymes.
B. Compounds
One aspect of the invention features disclosed compounds shown in
formulae (I) and (II) in the Summary section. Embodiments of the invention
includes compounds of formula (I) wherein: (a) R~ is N02; (b) R4 is fluoro;
(c) each of R3 and R4 is independently selected from H and fluoro; (d) R5 is
methyl, fluoro, or chloro; (e) R6 is methyl, chloro, fluoro, vitro, or
hydrogen; {f) R6
is H; (g) R6 is fluoro; (h) RK is methyl or ethyl; (i) R~ is H, methyl, ethyl,
propyl,
isopropyl, isobutyl, benzyl, phenyl, phenethyl, allyl, C ~.5 alkenyl,
C ~ cycloalkyl, (C ~.5 cycloalkyl)C ~_2 alkyl, (C ~5 heterocyclic radical)C
,_2 alkyl,
or (CH2)2~, NR~Rp; (j) R~ is H or (C ~ cycloalkyl)C ,_2 alkyl; (k) R2 is H or
methyl;
(I) R~, has at least one hydroxyl substituent; (m) RA is H, methyl, ethyl,
isobutyl,
hydroxyethyl, phenyl, 2-piperidin-1-yl-ethyl, 2,3-dihydroxy-propyl, 3-[4-(2-
hydroxyethyl)-piperazin-1-ylJ-propyl, 2-pyrrolidin-1-yl-ethyl, or 2-
diethylamino-
ethyl; and RB is H; or where RB is methyl and RA is phenyl; (n) W is NRnRB or
NR2NRARB; (o) W is NRZ(CH2)2.~ NRARB or O(CH2)2-3 NRaRB; (p) W is NR20R,;
(q) W is ORB; (r) R~ is in the para position relative to X; (s) R~ is iodo;
(t) Re is in
the ortho position relative to X; (u) or combinations thereof.
In additional embodiments, if R6 is H, then R5 is vitro; or Rs is methyl,
halo,
or vitro; or R3 is S02NR, (CH2)2.~NRERF, S02NR, R,~ or (CO)T. fn some
8
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
embodiments, Ar is phenyl (e.g., formula (I)), and in other embodiments, Ar is
2-
pyridyl, 3-pyridyl, or 4-pyridyl. Preferably, where one of R~, RZ, RA, RB, R~,
and Rp
is an alkenyl or alkynyl group, the double or triple bond, respectively, is
not
adjacent the point of attachment. For example, where W is NRZOR~, R2 is
preferably prop-2-ynyl, or but-2 or 3-enyl, and less preferably prop-1-ynyl or
but-
1-enyl. Some embodiments include the formula 2,4-bis-(2-chloro-4-iodo-
phenylamino)-3-fluoro-5-nitro-benzoic acid, the compounds in the following
list,
and 2-methyl (instead of 2-chloro) analogs thereof.
1. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-5-vitro-4-(4-sulfamoyl-
phenylamino)-benzoic acid
2. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-5-vitro-4-phenylamino-benzoic
acid
3. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-5-vitro-4-phenoxy-benzoic acid
4. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-5-vitro-4-phenylsulfanyl-benzoic
acid
5. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-4-(methyl-phenyl-amino)-5-nitro-
benzoic acid
6. 2-((2-chloro-4-iodophenyl)amino]-3-fluoro-4-([4-[[(2-hydroxyethyl)amino]-
carbonyl]phenyl]amino]-5-vitro-benzoic acid;
7. 2-[(2-chloro-4-iodophenyl)amino]-4-[[4-j(dimethylamino)carbonyl]
phenyl]amino]-3-fluoro-5-vitro-benzoic acid;
8. 2-(2-Chloro-4-iodo-phenylamino)-3,5-difluoro-4-phenylamino-benzoic acid
9. 2-(2-Chioro-4-iodo-phenylamino)-3-fluoro-5-vitro-4-(3-sulfamoyl-
phenylamino)-benzoic acid;
10. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-5-vitro-4-(2-sulfamoyl-
phenylamino)-benzoic acid;
11. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-N-hydroxy-5-vitro-4-(4-
sulfamoyl-phenylamino)-benzamide;
12. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-N-hydroxy-5-vitro-4-
phenylamino-benzamide;
9
CA 02348236 2001-04-24
WO 00/41994 PC1'/US99f30418
13. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-N=hydroxy-5-vitro-4-phenoxy-
benzamide;
14. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-N-hydroxy-5-vitro-4-
phenylsulfanyl-benzamide;
15. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-N-hydroxy-4-(methyl-phenyl-
amino)-5-vitro-benzamide;
16. 2-[(2-chloro-4-iodophenyl)amino]-3-fluoro-N-hydroxy-4-[[4-[[(2-
hydroxyethyl)aminoj-carbonyl]phenyl]aminoj-5-vitro-benzamide;
17. 2-[(2-chloro-4-iodophenyl)amino]-4-[[4-[(dimethylamino)carbonyl]
phenyl]amino]-3-fluoro-N-hydroxy-5-vitro-benzamide;
18. 2-(2-Chloro-4-iodo-phenylamino)-3,5-difluoro-N-hydroxy-4-phenylamino-
benzamide;
19. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-N-hydroxy-5-vitro-4-(3-
sulfamoyl-phenyiamino)-benzamide;
20. 2-(2-Chloro-4-iodo-phenylamino)-3-fluoro-N-hydroxy-5-vitro-4-(2-
sulfamoyl-phenylamino)-benzamide;
21. 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3-fluoro-5-vitro-.4-
(4-sulfamoyl-phenylamino)-benzamide;
22. 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3-fluoro-5-vitro-4-
phenylamino-benzamide;
23. 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3-fluoro-5-vitro-4-
phenoxy-benzamide;
24. 2-(2-Chioro-4.-iodo-phenylamino)-N-cyclopropyimethoxy-3-fluoro-5-vitro-4-
phenylsuifanyl-benzamide;
25. 2-(2-Chloro~-iodo-phenylamino)-N-cyclopropylmethoxy-3-fluoro-4-
(methyl-phenyl-amino)-5-vitro-benzamide;
26. 2-[(2-chloro-4.-iodophenyl)aminoj-3-fluoro-N-cyclopropylmethoxy-4-[[4-[[(2-
hydroxyethyl)aminoj-carbonyljphenyl]amino]-5-vitro-benzamide;
27. 2-[(2-chloro-4-iodophenyl)amino]~-[[4-[(dimethylamino)carbonyljphenyl]-
amino]-3-fluoro-N-cyclopropylmethoxy-5-vitro-benzamide;
28. 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,5-difluoro-4-
phenylamino-benzamide;
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
29. 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmetho~cy-3-fluoro-5-vitro-4-
(3-sulfamoyl-phenylamino)-benzamide; and
30. 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3-fluoro-5-vitro-4-
(2-sulfamoyl-phenylamino)-benzamide.
In the scheme below, W can also be any of the values described herein for
formula (I) or (ll) in the section describing preferred values for W. The
compound
numbers provided in the scheme correspond to the numbers provided in the
above list; these compounds are illustrative, not limitative, of the
invention.
11
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
W O H CI W o H CI
I \ N I \ Cmod * IW) s I \ N I \ Cmnd * (Vh
OzN / F / I 11 (NHOH) OzN / F / I 1(>OH)
HN I \ 21 (NHOCHz'pr) HN I \ H C 26 (NHOCHz'Pr)
31
/ N'CH
3
O
W O CI ' W o Cl
I \ cmod * (VV~ I I \ Cmod * (Wl
OzN / F / I 12 ~p~ OzN, / F / I 170H)
(NHOH)
I \ O 22 (NHOCHz'Pr) ~ I \ 27 (NHOCHz'Pr)
/ ~i /
S, NHz OH
O O
W O CI if W O Cl
I I \ 3 C ~* n'v) I w I \ g ~o~* cvh
/ / 13 (NHOH) / ~ 18 (NHOH)
OZN O F I 23 (NHOCHz'Pr) F ~ F I 28 (NHOCHz'Pr)
\
I/ I/
4
w o cl W ° cl
I \ I \ Cmnd*lWl I \ ~ \ Cmod*lwl
02N / F / I 14 (NHOH) OzN ~ ~ F / I 99 (NHOH)
S~ 24 (NHOCHz'Pr) HN \ 29 (NHOCHz'Pr)
I/ I/
o=S=o
NHz
W O Cl W O
ca
( I \ Cmod * lW1 \ \ Cmud * (1~
5 (OH) I I 10 (OH)
/ / 15 (NHOH) O N ~ F / 1 20 (NHOH)
Oz H C ~ N F I 25 {NHOCHz'Pr) z HN 30 (NHOCHz'Pr)
3
/ HzNOS~
O
12
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
C. Synthesis
The disclosed compounds can be synthesized according to the following
two Schemes, or variants thereof (see also Example 1 ).
Regarding the first step of synthetic Scheme 1, the reaction of the aniline
and the benzoic acid derivative generally is accomplished by mixing the
benzoic
acid with an equimolar quantity or excess of the aniline in an unreactive
organic
solvent such as tetrahydrofuran, or toluene, in the presence of a base such as
lithium diisopropylamide, lithium hexamethyldisilazide, n-butyl lithium,
sodium
hydride, or sodium amide. The reaction generally is carried out at a
temperature
90 of about -78 °C to about 25 °C, and normally is complete
within 2 hours to about
4 days. The product can be isolated by removing the solvent, for example by
evaporation under reduced pressure, and further purified, if desired, by
standard
methods such as chromatography, crystallization, or distillation.
Turning to the second step, the 2-phenylamino benzoic acid derivative is
next reacted with an equimolar quantity or excess of a nucleophile such as an
aniline, a phenol, or a thiophenol by mixing in an unreactive organic solvent
such
as tetrahydrofuran, or toluene, in the presence of a base such as lithium
diisopropylamide, lithium hexamethyldisilazide, n-butyl lithium, sodium
hydride, or
sodium amide. The reaction generally is carried out at a temperature of about -
78 °C to boiling, and normally is complete within 2 hours to about 4
days. The
product can be isolated by removing the solvent, for example by evaporation
under reduced pressure, and further purified, if desired, by standard methods
such as chromatography, crystallization, or distillation.
Finally, regarding step 3, the 4-arylheteroatom-2-phenylamino benzoic
acid derivative next is reacted with a nucleophile such as ammonia, an amine,
an
alcohol, hydrazine, a hydrazine derivative, or a hydroxylamine derivative in
the
presence of a peptide coupling reagent. Amines that can be employed include
monomethylamine and aniline. Alcohols that can be employed include
cyclobutylmethanol and phenol. Hydrazine derivatives that can be employed
include N,N-dimethylhydrazine and 1-aminopiperidine. Hydroxylamine
derivatives that can be employed include methoxylamine, N-ethyl-isopropoxy
amine, and tetrahydrooxazine. Typical coupling reagents include 2-ethoxy-1-
13
CA 02348236 2001-04-24
WO 00/41994 PCTNS99/30418
ethoxycarbonyl-1,2-dihydroquinoline (EEDQ), 1,3-dicyclohexylcarbodiimide
(DCC), bromo-tris-(pyrrolidino)-phosphonium hexafluorophosphate (PyBrOP) and
(benzotriazolyioxy) tripyrrolidino phosphonium hexafluorophosphate (PyBOP).
The 4-arylheteroatom-2-phenylamino benzoic acid derivative and the nucleophile
normally are mixed in approximately equimolar quantities in an unreactive
solvent
such as dichloromethane, tetrahydrofuran, chloroform, or xylene, and an
equimolar quantity of the coupling reagent is added. A base such as
triethylamine or diisopropylethylamine can be added to act as an acid
scavenger
if desired. The coupling reaction generally is complete after about 10 minutes
to
2 hours, and the product is readily isolated by removing the reaction solvent,
for
instance by evaporation under reduced pressure, and purifying the product by
standard methods such as chromatography or crystallizations from solvents such
as acetone diethyl ether or ethanol.
Referring to synthetic Scheme 2, an alternative method for making the
compounds of the invention involves first coupling the benzoic acid derivative
with
the arylheteroatom nucleophile, and then reacting this 4-arylheteroatom
benzoic
acid derivative with an aniline. The final step involves the coupling of the
4-arylheteroatom-2-phenylamino benzoic acid derivative with the ammonia,
amine, alcohol, hydrazine, hydrazine derivative, or hydroxylamine derivative
with
a peptide coupling reagent. The genera! reaction conditions for all of the
steps in
Scheme 2 are similar to those described above for synthetic Scheme 1.
30
14
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
Scheme 1
HO O HO O R
R5 H 5 R
H2N ~ Base I ~~ N ( w + ArXH
R3 I , R4 R6 I / I ~ R3 ~ R4 R6 ~ I R8
X N,O,S
HO O R2
H R5 H Rt.O.N O R
---. I ~ N I ~ R2_N_O_Rt ~ N
i i
R7 Ar. X Ra R6 I R~. . X Ra R6 I
i Ar
Rs Rs
Scheme 2
HO O
HO O
R5
I,t R~ Base I H2N w
I + ArXH R i R4 +
3
R3 ~ Rt Rs R~.~,.X R5 I
X=N,O,S Rs
HO O R2
H R5 H Rt.O.N O R
Base I ~ N I ~ R2-N-O_Rt H 5
N
i i I ~ I ,
R~ Ar'X Re R6 I R7' .X ~ R6 I
R
s Re
HO O CI
H
N
O2N I ~ F I ~ I PD 195928
APK IC~30~8 nM
~I
C1~1
CA 02348236 2001-04-24
WO 00/41994 PCT/US99130418
~. USe8
The disclosed compositions are useful as both prophylactic and
therapeutic treatments for diseases or conditions as provided in the Summary
section, as well as diseases or conditions modulated by the MEK cascade.
Examples include stroke, heart failure, osteoarthritis, rheumatoid arthritis,
organ
transplant rejection, and a variety of tumors such as ovarian, lung,
pancreatic,
and colon.
1. Dosages
Those skilled in the art will be able to determine, according to known
methods, the appropriate dosage for a patient, taking into account factors
such as
age, weight, general health, the type of pain requiring treatment, and the
presence of other medications. In general, an effective amount will be between
0.1 and 1000 mg/kg per day, preferably between 1 and 300 mg/kg body weight,
and daily dosages will be between 10 and 5000 mg for an adult subject of
normal
weight. Commercially available capsules or other formulations (such as liquids
and film-coated tablets) of 100 mg, 200 mg, 300 mg, or 400 mg can be
administered according to the disclosed methods.
2. Formulations
Dosage unit forms include tablets, capsules, pills, powders, granules,
aqueous and nonaqueous oral solutions and suspensions, and parenteral
solutions packaged in containers adapted for subdivision into individual
doses.
Dosage unit forms can also be adapted for various methods of administration,
including controlled release formulations, such as subcutaneous implants.
Administration methods include oral, rectal, parenteral (intravenous,
intramuscular, subcutaneous), intracistemal, intravaginal, intraperitoneal,
intravesical, local (drops, powders, ointments, gels, or cream), and by
inhalation
{a buccal or nasal spray).
Parenteral formulations include pharmaceutically acceptable aqueous or
nonaqueous solutions, dispersion, suspensions, emulsions, and sterile powders
for the preparation thereof. Examples of carriers include water, ethanol,
polyols
(propylene glycol, polyethylene glycol), vegetable oils, and injectable
organic
16
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
esters such as ethyl oleate. Fluidity can be maintained by the use of a
coating
such as lecithin, a surfactant, or maintaining appropriate particle size.
Carriers
for solid dosage forms include (a) fillers or extenders, (b) binders, (c)
humectants,
(d) disintegrating agents, (e) solution retarders, (f) absorption
acccelerators, (g)
adsorbents, (h) lubricants, (i) buffering agents, and (j) propellants.
Compositions may also contain adjuvants such as preserving, wetting,
emulsifying, and dispensing agents; antimicrobial agents such as parabens,
chlorobutanol, phenol, and sorbic acid; isotonic agents such as a sugar or
sodium
chloride; absorption-prolonging agents such as aluminum monostearate and
gelatin; and absorption-enhancing agents.
3. Related compounds
The invention provides the disclosed compounds and closely related,
pharmaceutically acceptable forms of the disclosed compounds, such as salts,
esters, amides, hydrates or solvated forms thereof; masked or protected forms;
and racemic mixtures, or enantiomerically or optically pure forms.
Pharmaceutically acceptable salts, esters, and amides include carboxylate
salts (e.g., C ,.~ alkyl, cycloalkyl, aryl, heteroaryl, or non-aromatic
heterocyclic),
amino acid addition salts, esters, and amides which are within a reasonable
benefit/risk ratio, pharmacologically effective, and suitable for contact with
the
tissues of patients without undue toxicity, irritation, or allergic response.
Representative salts include hydrobromide, hydrochloride, sulfate, bisulfate,
nitrate, acetate, oxalate, valerate, oleate, palmitate, stearate, laurate,
borate,
benzoate, lactate, phosphate, tosylate, citrate, maleate, fumarate, succinate,
tartrate, naphthylate, mesylate, glucoheptonate, lactiobionate, and
laurylsulfonate. These may include alkali metal and alkali earth rations such
as
sodium, potassium, calcium, and magnesium, as well as non-toxic ammonium,
quaternary ammonium, and amine rations such as tetramethyl ammonium,
methylamine, trimethylamine, and ethylamine. See, for example, S.M. Berge, et
al., "Pharmaceutical Salts," J. Pharm. Sci., 1977, 66:1-19 which is
incorporated
herein by reference. Representative pharmaceutically acceptable amides of the
invention include those derived from ammonia, primary C ~.~ alkyl amines and
secondary di (C ~.~ alkyl) amines. Secondary amines include 5- or 6-membered
17
CA 02348236 2001-04-24
WO 00141994 PCT/US99I30418
heterocyclic or heteroaromatic ring moieties containing at least one nitrogen
atom
and optionally between 1 and 2 additional heteroatoms. Preferred amides are
derived from ammonia, C ~_3 alkyl primary amines, and di (C ~_2 alkyl)amines.
Representative pham~aceutically acceptable esters of the invention include C
~_~
alkyl, C ~.~ cycloalkyl, phenyl, and phenyl(C »)alkyl esters. Preferred esters
include methyl esters.
The invention also includes disclosed compounds having one or more
functional groups {e.g., hydroxyl, amino, or carboxyl) masked by a protecting
group. Some of these masked or protected compounds are pharmaceutically
acceptable; others will be useful as intermediates. Synthetic intermediates
and
processes disclosed herein, and minor modifications thereof, are also within
the
scope of the invention.
HYDROXYL PROTECTING GROUPS
Hydroxyl protecting groups include: ethers, esters, and protection for 1,2-
and
1,3-diols. The ether protecting groups include: methyl, substituted methyl
ethers, substituted ethyl ethers, substituted benzyl ethers, silyl ethers and
conversion of silyl ethers to other functional groups.
Substituted Methyl Ethers
Substituted methyl ethers include: methoxymethyl, methylthiomethyl, t
utylthiomethyl, (phenyldimethylsilyl) methoxymethyl, benzyloxymethyl, p-
ethoxybenzyloxymethyl, (4-methoxyphenoxy) methyl, guaiacolmethyl, t
butoxymethyl, 4-pentenyloxymethyl, siloxymethyl, 2-methoxyethoxymethyl, 2,2,2-
trichloroethoxymethyl, bis(2-chloro- ethoxy)methyl, 2-
(trimethylsilyl)ethoxymethyl,
tetrahydropyranyl, 3-bromotetrahydro-pyranyl, tetrahydrothiopyranyl, 1-
methoxycyclohexyl, 4-methoxytetrahydropyranyl, 4-methoxytetrahydrothio-
pyranyl, 4-methoxytetrahydrothiopyranyl S,S-dioxido, 1-[(2-chioro-4-
methyl)phenyl]-4-methoxypiperidin-4-yl, 1,4-dioxan-2-yl, tetrahydrofuranyl,
tetrahydrothiofuranyl, and 2,3,3a,4,5,6,7,7a-octahydro-7,8,8-trimethyt-4,7-
ethanobenzofuran-2-yl.
Substituted Ethyi Ethers
Substituted ethyl ethers include: 1-ethoxyethyl, 1-{2,chloroethoxy)ethyl,
18
CA 02348236 2001-04-24
WO 00/41994 PCTNS99/30418
1-methyl-1-methoxyethyl, 1-methyl-1-benzyloxyethyl, 1-methyl-1-benzyloxy-2-
fluoroethyl, 2,2,2-trichloroethyl, 2-trimethylsilyethyl, 2-
(phenylselenyl)ethyl, t butyl,
allyl, p-chlorophenyl, p-methoxyphenyl, 2,4-dinitrophenyl, and benzyl.
Substituted Benzyl Ethers
Substituted benzyl ethers include: p-methoxybenzyl, 3,4-dimethoxyben2yl,
o-nitrobenzyl, p-nitrobenzyl, p-halobenzyl, 2,6-dichlorobenzyl, p-cyanobenzyl,
p-phenylbenzyl, 2- and 4-picolyl, 3-methyl-2-picolyl N-oxido, diphenylmethyl,
p, p'-dinitrobenzhydryl, 5-dibenzosuberyl, triphenylmethyl, a-
naphthyldiphenylmethyl, p-methoxyphenyldiphenylmethyl, di(p-
methoxyphenyl)phenylmethyl, tri-(p-methoxyphenyl)methyl, 4-(4'-
bromophenacyloxy)phenyldiphenylmethyl, 4,4',4"-tris(4,5-dichiorophthalimido-
phenyl)methyl, 4,4',4"-tris(levulinoyloxyphenyl) methyl, 4,4',4"tris(benzoylox-
phenyl)methyl, 3-(imidazol-1-ylmethyl)bis(4',4"-dimethoxyphenyl)-methyl, 1,1-
bis(4-methoxyphenyl)-1'-pyrenylmethyl, 9-anthryl, 9-(9-phenyl) xanthenyl, 9-(9-
phenyl-10-oxo) anthryl, 1,3-benzodithiolan-2-yl, and benzisothiazolyl S,S
dioxido.
Si~l Ethers
Silyl ethers include: trimethylsilyl, triethylsilyl, triisopropylsilyl,
dimethylisopropylsilyl, diethylisopropylsilyl, dimethylthexylsilyl, t
butyldimethylsilyl,
t butyldiphenylsilyl, tribenzylsilyl, tri p-xylylsilyl, triphenylsilyl,
diphenylmethylsilyl,
and t butylmethoxyphenylsilyl.
ESTERS
Esters protecting groups include: esters, carbonates, assisted cleavage,
miscellaneous esters, and sutfonates.
Esters
Examples of protective esters include: formate, benzoylformate, acetate,
chloroacetate, dichloroacetate, trichloroacetate, trifluoroacetate,
methoxyacetate,
triphenylmethoxyacetate, phenoxyacetate, p-chlorophenoxyacetate, p-P-
phenylacetate, 3-phenylpropionate, 4-oxopentanoate (levulinate), 4,4-
(ethylenedithio) pentanoate, pivaloate, adamantoate,crotonate,4-
19
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
methoxycrotonate, benzoate, p-phenylbenzoate, and 2,4,6-trimethylbenzoate
(mesitoate).
Carbonates
Carbonates include: methyl, 9-fluorenylmethyl, ethyl, 2,2,2-trichloroethyl,
2-(trimethylsilyl) ethyl, 2-(phenylsulfonyl) ethyl, 2-(triphenylphosphonio)
ethyl,
isobutyl, vinyl, allyl, p-nitrophenyl, benzyl, p-methoxybenzyt, 3,4-
dimethoxybenzyl,
o-nitrobenzyl, p-nitrobenzyl, S-benzyl thiocarbonate, 4-ethoxy-1-naphthyl, and
methyl dithiocarbonate.
Assisted Cleavage
Examples of assisted cleavage protecting groups include: 2-iodobenzoate, 4-
azido-butyrate, 4-nitro-4-methylpentanoate, o-(dibromomethyl) benzoate, 2-
formylbenzene-sulfonate, 2-(methylthiomethoxy) ethyl carbonate, 4-(methylthio-
methoxymethyl) benzoate, and 2-(methylthiomethoxymethyl) benzoate.
Miscellaneous Esters
In addition to the above classes, miscellaneous esters include: 2,6-dichloro-4-
methylphenoxyacetate, 2,6-dichloro-4-(1,1,3,3-tetramethylbutyl)
phenoxyacetate,
2,4-bis(1,1-dimethylpropyl) phenoxyacetate, chtorodiphenytacetate,
isobutyrate,
monosuccinoate, (E~-2-methyl-2-butenoate (tigloate), o-(methoxycarbonyl)
benzoate, p-P-benzoate, a-naphthoate, nitrate, alkyl N,N,N' N'-
tetramethylphosphorodiamidate, N-phenylcarbamate, borate,
dimethylphosphinothioyl, and 2,4-dinitrophenylsulfenate.
Sulfonates
Protective sulfates includes: sulfate, methanesulfonate(mesylate),
benzylsulfonate, and tosylate.
PROTECTION FOR 1.2- AND 1.3-DIOLS
The protection for 1,2 and 1,3-diols group includes: cyclic acetats and
ketals,
cyclic ortho esters, and silyl derivatives.
Cvclic Acetals and Ketals
Cyclic acetals and ketals include: methylene, ethytidene, 1-t butylethytidene,
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
1-phenylethylidene, (4-methoxyphenyl) ethylidene, 2,2,2-trichloroethylidene,
acetonide (isopropylidene), cyclopentylidene, cyclohexyiidene,
cycloheptylidene,
benzylidene, p-methoxybenzylidene, 2,4-dimethoxybenzylidene, 3,4-
dimethoxybenzylidene, and 2-nitrobenzylidene.
Cyclic Ortho Esters
Cyclic ortho esters include: methoxymethylene, ethoxymethylene, dimethoxy-
methylene, 1-methoxyethylidene, 1-ethoxyethylidine, 1,2-dimethoxyethylidene,
a-methoxybenzylidene, 1-(N,N-dimethylamino)ethylidene derivative, a-(N,N-
dimethylamino) benzylidene derivative, and 2-oxacyclopentylidene.
PROTECTION FOR THE CARBOXYL GROUP
ESTERS
Ester protecting groups include: esters, substituted methyl esters, 2-
substituted
ethyl esters, substituted benzyl esters, silyl esters, activated esters,
miscellaneous derivatives, and stannyi esters.
Substituted Methyl Esters
Substituted methyl esters include: 9-fluorenylmethyl, methoxymethyl,
methylthiomethyl, tetrahydropyranyl, tetrahydrofuranyl, methoxyethoxymethyl, 2-
(trimethylsilyl)ethoxy-methyl, benzyloxymethyl, phenacyl, p-bromophenacyl, a-
methylphenacyl, p-methoxyphenacyl, carboxamidomethyl, and N
phthalimidomethyl.
2-Substituted Ethyl Esters
2-Substituted ethyl esters include: 2,2,2-trichloroethyl, 2-haloethyl, ~-
chloroalkyl,
2-(trimethylsiiy)ethyl, 2-methylthioethyl, 1,3-dithianyl-2-methyl, 2(p-
nitrophenylsulfenyl)-ethyl, 2-(p-toluenesulfonyl)ethyl, 2-(2'-pyridyl)ethyl, 2-
(diphenylphosphino)ethyl, 1-methyl-1-phenylethyl, t butyl, cyclopentyl,
cyclohexyl,
allyl, 3-buten-1-yl, 4-(trimethylsily)-2-buten-1-yl, cinnamyl, a-
methylcinnamyl,
phenyl, p-(methylmercapto)-phenyl, and benzyl.
Substituted Benzyl Esters
Substituted benzyl esters include: triphenylmethyl, diphenylmethyl,
21
CA 02348236 2001-04-24
WO 00/41994 PCTNS99/30418
bis(o-nitrophenyl)methyl, 9-anthrylmethyl, 2-(9,10-dioxo)anthrylmethyl, 5-
dibenzo-
suberyl, 1-pyrenylmethyl,2-(trifluoromethyl)-6-chromylmethyl, 2,4,6-
trimethylbenzyl, p-bromobenzyl, o-nitrobenzyl, p-nitrobenzyl, p-methoxybenzyl,
2,6-dimethoxybenzyl, 4-(methylsulfinyl)benzyl, 4-sulfobenzyl, piperonyl, and 4-
P-
benzyl.
Silvl Esters
Silyl esters include: trimethylsilyl, triethylsilyl, t-butyidimethylsilyl, i-
propyldimethylsilyl, phenyldimethylsilyl, and di- t butylmethylsilyl.
Miscellaneous Derivatives
Miscellaneous derivatives includes: oxazoles, 2-alkyl-1,3-oxazolines, 4-alkyl-
5-
oxo-1,3-oxazolidines, 5-alkyl-4-oxo-1,3-dioxolanes, ortho esters, phenyl
group,
and pentaaminocobalt(111) complex.
Stannvl Esters
Examples of stannyl esters include: triethyistannyi and tri-n-butylstannyi.
AM1DES AND HYDRAZIDES
Amides include: N,N-dimethyl, pyrrolidinyl, piperidinyl, 5,6-dihydrophen-
anthridinyl, o-nitroanilides, N-7-nitroindolyl, N-8-vitro-1,2,3,4-
tetrahydroquinolyl,
and p-P-benzenesulfonamides. Hydrazides include: N phenyl, N,N'-diisopropyl
and other dialkyl hydrazides.
PROTECTION FOR THE AMINO GROUP
CARBAMATES
Carbamates include: carbamates, substituted ethyl, assisted cleavage,
photolytic
cleavage, urea-type derivatives, and miscellaneous carbamates.
Carbamates
Carbamates include: methyl and ethyl, 9-fluorenylmethyl, 9-(2-sulfo)fluorenyl-
methyl, 9-(2,7-dibromo)fluorenylmethyl, 2,7-di-t butyl-[9-(10,10-dioxo-
10,10,10,10-tetrahydro- thioxanthyl)Jmethyl, and 4-methoxyphenacyl.
22
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
Substituted Ethyl
Substituted ethyl protective groups include: 2,2,2-trichloroethyl, 2-
trimethylsilylethyl, 2-phenylethyl, 1-(1-adamantyt)-1-methylethyl, 1,1-
dimethyl-2-
haloethyl, 1,1dimethyl-2,2-dibromoethyl, 1,1-dimethyl-2,2,2-trichloroethyl, 1-
methyl-1-(4-biphenylyl)ethyl, 1-(3,5-di-f butylphenyl)-1-methylethyl, 2-(2'-
and 4'-
pyridyl)ethyt, 2-(N,N icyclohexylcarboxamido)- ethyl, t butyl, 1-adamantyl,
vinyl,
allyl, 1-isopropylallyl, connamyl, 4-nitrocinnamyl, quinolyl, N
hydroxypiperidinyl,
alkyldithio, benzyl, p-methoxybenzyl, p-nitrobenzyl, p-bromobenzyl, p-
chlorobenzyl, 2,4dichlorobenzyl, 4-methylsulfinylbenzyl, 9-anthrylmethyl, and
diphenylmethyl.
Assisted Cleavage
Protection via assisted cleavage includes: 2-methylthioethyl, 2-
methylsulfonylethyl, 2-(p-toluenesulfonyl)ethyl, [2-(1,3-dithianyl)]methyl, 4-
methylthiophenyl, 2,4-dimethyl-thiophenyl, 2-phosphonioethyl, 2-
triphenylphosphonioisopropyl, 1,1-dimethyl-2cyanoethyl, m-chloro p-
acyloxybenzyl, p-(dihydroxyboryl)benzyl, 5-benzisoxazolyl-methyl, and 2-
(trifluoromethyl)-6-chromonylmethyl.
Photortic Cleavage
Photolytic cleavage methods use groups such as: m-nitrophenyl, 3,5-
dimethoxybenzyt, o-nitrobenzyl, 3,4-dimethoxy-6-nitrobenzyl, and phenyl(o-
nitrophenyl)methyl.
Urea-Type Derivatives
Examples of of urea-type derivatives include: phenothiazinyl-(10)-carbonyl
derivative,N'-p-toluenesulfonylaminocarbonyl, and N'-phenylaminothiocarbonyl.
Miscellaneous Carbamates
In addition to the above, miscellaneous carbamates include: t amyl, S-benzyl
thiocarbamate, p-cyanobenzyl, cyclobutyl, cyclohexyt, cyclopentyf,
cyclopropylmethyl, p-decyloxy-benzyl, diisopropylmethyl, 2,2-
dimethoxycarbonylvinyl, o-(N,N dimethyl-carboxamido)-benzyl, 1,1-dimethyl-
3(N,N dimethylcarboxamido)propyl, 1,1-dimethyl-propynyl, di(2-pyridyl)methyl,
2-
23
CA 02348236 2001-04-24
WO 00/41994 PCTNS99/30418
furanylmethyl, 2-iodoethyl, isobornyl, isobutyl, isonicotinyl, p(p =
methoxyphenylazo)benzyl, 1-methylcyclobutyl, 1-methylcyclohexyl,
1-methyl-1-cyclopropylmethyl, 1-methyl-(3,5-dimethoxyphenyl)ethyl, 1-methyl-
1(p-henylazophenyl)- ethyl, 1-methyl-1-phenylethyl, 1-methyl-1-(4-
pyridyl)ethyl,
phenyl, p-(phenylazo)benzyl, 2,4,6-tri-t butylphenyl, 4-
(trimethylammonium)benzyl, and 2,4,6-trimethylbenzyl.
AMIDES
Amides
Amides includes: N-formyl, N acetyl, N-chloroacetyl, N trichloroacetyl,
N trifluoroacetyl, N-phenylacetyl, N 3-phenylpropionyl, N picolinoyl, N 3-
pyridyi-
carboxamide, N benzoylphenylaianyi derivative, N benzoyl, and N p-
phenylbenzoyl.
Assisted Cleavaye
Assisted cleavage groups include: N o-nitrophenylacetyl, N o-
nitrophenoxyacetyl, N acetoacetyl, (N'-dithiobenzyloxycarbonylamino)acetyl, N
3-
(p-hydroxphenyl) propionyl, N 3-(o-nitrophenyl)propionyl, N 2-methyl-2-(o-
nitrophenoxy)propionyl, N 2-methyl-2-(o-phenylazophenoxy)propionyl, N-4-
chlorobutyryl, N 3-methyl-3-nitrobutyryl, N o-nitrocinnamoyl, N
acetylmethionine
derivative, N o-nitrobenzoyl, N o-(benzoyloxymethyl)benzoyl, and 4,5-Biphenyl-
3-
oxazolin-2-one.
Cyclic Imide Derivatives
Cyclic imide derivatives include: N phthalimide, N dithiasuccinoyl,
N 2,3-Biphenyl-maleoyl, N 2,5-dimethylpyrrolyl,
N 1,1,4,4-tetramethyldisilylazacyclopentane adduct, 5-substituted
1,3-dimethyl-1,3,5-triazacyclohexan-2-one, 5-substituted 1,3-dibenzyl-
1,3,5-triazacyclohexan-2-one, and 1-substituted 3,5-dinitro-4-pyridonyl.
24
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
SPECIAL -NH PROTECTIVE GROUPS
Protective groups for - NH include: N alkyl and N aryl amines, imine
derivatives,
enamine derivatives, and N hetero atom derivatives (such as N-metal, N-N, N-P,
N-Si, and N-S), N sulfenyl, and N sulfonyl.
N-Alkvl and N Arvl Amines
N alkyl and N aryl amines include: N methyl, N allyl, N [2-
(trimethylsilyl)ethoxyl]-
methyl, N 3-acetoxypropyl, N (1-isopropyl-4-vitro-2-oxo-3-pyrrolin-3-yl),
quaternary ammonium salts, N benzyl, N di(4-methoxyphenyl)methyl,
N 5-dibenzosuberyl, N triphenylmethyl, N (4-methoxyphenyl)diphenylmethyl,
N 9-phenylfluorenyl, N 2,?-dichloro-9-fluorenylmethylene, N-ferrocenylmethyl,
and N 2-picolylamine N'-oxide.
Imine Derivatives
(mine derivatives include: N 1,1-dimethylthiomethylene, N benzylidene,
N p-methoxybenzylidene, N-diphenylmethylene, N [(2-pyridyl)mesityl]methylene,
N (N',N'-dimethylaminomethylene), N,N'-isopropylidene, N p-nitrobenzylidene,
N salicylidene, N 5-chlorosalicylidene, N (5-chloro-2-hydroxyphenyl)phenyl-
methylene, and N cyclohexylidene.
Enamine Derivatiye
An example of an enamine derivative is N (5,5-dimethyl-3-oxo-1-cyclohexenyl).
N Hetero Atom Derivatives
N metal derivatives include: N borane derivatives, N diphenylborinic acid
derivative, N [phenyl(pentacarbonylchromium- or -tungsten)]carbenyl, and
N copper or N zinc chelate. Examples of N N derivatives include: N vitro,
N nitroso, and N oxide. Examples of N P derivatives include:
N-diphenylphosphinyl, N-dimethylthiophosphinyl, N dipheny)thiophosphinyl,
N-dialkyl phosphoryl, N-dibenzyl phosphoryl, and N Biphenyl phosphoryl.
Examples of N suffenyl derivatives include: N benzenesutfenyl,
N o-nitrobenzenesulfenyl, N 2,4-dinitrobenzenesulfenyl,
N pentachlorobenzenesulfenyl, N 2-vitro-4-methoxy-benzenesulfenyl, N
triphenylmethylsulfenyl, and N 3-nitropyridinesutfenyl. N sutfonyl derivatives
include: N p-toluenesulfonyl, N-benzenesulfonyl, N 2,3,6-trimethyl-
CA 02348236 2001-04-24
WO 00/41994 PCTNS99/30418
4-methoxybenzenesulfonyl, N-2,4,6-trimethoxybenzenesulfonyl, N
2,6-dimethyl-4-methoxy-benzenesulfonyl, N-pentamethylbenzenesulfonyl, N
2,3,5,6-tetramethyl-4-methoxybenzene- sulfonyl, N-4-methoxybenzenesutfonyl,
N 2,4,6-trimethylbenzenesulfonyl, N-2,6-dimethoxy- 4-methylbenzenesulfonyl, N
2,2,5,7,8-pentamethylchroman-6-suifonyl, N-methanesulfonyl,
N ~3-trimethylsilylethanesulfonyl, N-9-anthracenesulfonyl, N-
4-(4',8'-dimethoxynaphthylmethyl)-benzenesulfonyl, N benzylsulfonyl, N-
trifluoromethylsulfonyl, and N phenacylsulfonyl.
Disclosed compounds which are masked or protected may be prodrugs,
compounds metabolized or otherwise transformed in vivo to yield a disclosed
compounc
e.g., transiently during metabolism. This transformation may be a hydrolysis
or oxidatio
which results from contact with a bodily fluid such as blood, or the action of
acids, or
liver, gastrointestinal, or other enzymes.
Features of the invention are further described in the examples below.
26
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
E. Examples
EXAMPLE 1
Preparation of 2.4-bis-(2-chloro-4-iodo-phenylamino)-3-fluoro-5-nitrobenzoic
acid
Step a: Preparation of 5-vitro-2.3,4-tri8uorobenzoic acid
To gently stirring concentrated sulfuric acid (50 ml) was added fuming
nitric acid (3.4 mi, 0.076 mol). Solid 2,3,4-trifluorobenzoic acid (10.00 g,
0.05565
mol) was added directly in increments. After stirring 45 minutes, the reaction
mixture had become an orange homogeneous solution which was then poured
over chilled water (400 ml). The resulting aqueous suspension was extracted
with diethyl ether (3 x 200 ml). The combined extracts were dried with
anhydrous
magnesium sulfate and concentrated in vacuo to yield 12.30 g of a dull, light-
yellow solid. Recrystallization from chloroform (50 ml) afforded 9.54 g of the
pale
yellow microcrystalline product; 78 % yield; m.p. ; 'H-NMR (400 MHz; DMSO) 8
14.29 (broad s, 1H), 8.43-8.38 (m, 1H);'3C-NMR (100 MHz; DMSO) 8162.41,
154.24 (dd, Jc.~=270.1, 10.7 Hz), 148.35 (dd, JGF=267.0, 9.2 Hz), 141.23 (dt,
Jc.
F=253.4 Hz), 133.95, 123.30 (d, Jc~=2.2 Hz), 116.92 (dd, Jc_F=18.2, 3.8
Hz);'9F-
NMR (376 MHz; DMSO) 8 -120.50 to -120.63 (m), -131.133 to -131.27 (m), -
153.63 to -153.74 (m).
Step b: Preparation of 2.4-bis-(2-chloro-4-iodo-nhenvlamino~-3-fluoro-5-
nitrobenzoic acid
To a stirring solution comprised of 2-chloro-4-iodoanitine (Lancaster, 98 %,
12.33 g, 0.04864 mol) in tetrahydrofuran (20 ml) at -78 °C under
nitrogen was
added a 2.0 M lithium diisopropylamide solution in tetrahydrofuran-heptane-
ethylbenzene (Aldrich, 35 ml, 0.070 mol) with a syringe. The addition formed a
thick suspension. After five minutes of stirring, a solution comprised of 5-
nitro-
2,3,4-triftuorobenzoic acid (5.00 g, 0.0226 mol) in tetrahydrofuran (30 ml)
was
added with a syringe to give a dark reaction mixture. The cold bath was
removed
27
CA 02348236 2001-04-24
WO 00/41994 PCTIUS99/30418
and the reaction mixture stirred for 20 minutes. The cool reaction mixture was
poured into ether (600 ml) containing an excess of hydrogen chloride. The red
solution instantly turned to a yellow suspension as a precipitate formed. This
precipitate was removed by vacuum filtration. The filtrate was concentrated in
vacuo to a red powder {10.5 g). The red powder was triturated with boiling
chloroform (800 ml). The triturated solids were collected by vacuum filtration
to
give an orange powder (2.42 g). The mother liquor from the trituration was
concentrated in vacuo to give a red-orange solid (ca. 10 g undried). This
solid
was loaded onto a flash silica column. Elution with dichloromethane removed
some impurities. Continuing elution with 1 % methanol in dichloromethane
afforede ca. 4 g of a red solid. This red solid was dissolved in hot absolute
ethanol (100 ml). The solution was boiled down to 50 ml before dilution to 300
ml
with hexanes. This solution was boiled to 150 ml and rediluted to 300 ml with
hexanes to produce slight turbidity. The mixture was cooled in the
refrigerator for
three days, affording a yellow precipitate. The precipitate was collected by
vacuum filtration and was dried with suction to afford 0.15 g of a yellow
solid; 1
yield; 'H-NMR (400 MHz; DMSO) b 8.94 (s, 1 H), 8.55 (s, 1 H), 7.79 (d, 2H,
J=2.0
Hz), 7.61-7.57 (m, 2H), 6.90 (dd, 1 H, J=8.5, 3.9 Hz), 6.84 (dd, 1 H, J=8.3,
6.6 Hz);
'9F-NMR {376 MHz; DMSO) b -122.62 (s); MS (APCI+) 692 (6), 691 {8), 690 (31 ),
689 (10), 688 (55), 171 (47), 130 (100); (APCi-) 691 {4), 690 (12), 689 (14),
688
{70), 687 (32), 686 (100), 506 (50), 453 (97); IR (KBr) 1523 cni'; Anal.
calcdffound for: C~9H~oCIzFI2N30,, C, 33.17/33.32; H, 1.47/1.73; N, 6.11/5.73;
CI,
10.31/10.04; F, 2.76/3.70; I, 36.89/34.32.
The APK ICS for 2,4-bis-(2-chloro-4-iodo-phenylamino)-3-fluoro-5-
nitrobenzoic acid is 29.6 nM.
EXAMPLE 2
Cascade assay for inhibitors of the MAP kinase pathway
Incorporation of 32P into myelin basic protein (MBP) is assayed in the
presence of a glutathione S-transferase fusion protein containing p44MAP
kinase
(GST-MAPK) and a glutathione S-transferase fusion protein containing p45MEK
28
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
(GST-MEK). The assay solution contains 20 mM HEPES, pH 7.4, 10 mM MgC12,
1 mM MnC12, 1 mM EGTA, 50,~M [y-32PjATP, 10 Izg GST-MEK, 0.5 ~g
GST-MAPK and 40 ~.g MBP in a final volume of 100 ~L. Reactions are stopped
after 20 minutes by addition of trichloroacetic acid and filtered through a
GF/C
filter mat. 32P retained on the filter mat is determined using a 120S
Betaplate.
Compounds are assessed at 10 pM for ability to inhibit incorporation of 32P.
To ascertain whether compounds are inhibiting GST-MEK or GST MAPK,
two additional protocols are employed. In the first protocol, compounds are
added to tubes containing GST-MEK, followed by addition of GST-MAPK, MBP
and [y-32P]ATP. In the second protocol, compounds are added to tubes
containing both GST-MEK and GST-MAPK, followed by MBP and [y-~PJATP.
Compounds that show activity in both protocols are scored as MAPK
inhibitors, while compounds showing activity in only the first protocol are
scored
as MEK inhibitors.
EXAMPLE 3
In vitro MAP kinase assay
Inhibitory activity can be confirmed in direct assays. For MAP kinase, 1 pg
GST-MAPK is incubated with 40 ~g MBP for 15 minutes at 30°C in a
final
volume of 50 ~L containing 50 mM Tris (pH 7.5), 10 ~.M MgC12, 2 ~M EGTA, and
10 teM [Y s2PjATP. The reaction is stopped by addition of Laemmli SDS sample
buffer and phosphorylated MBP resolved by electrophoresis on a 10%
polyacrylamide gel. Radioactivity incorporated into MBP is determined by both
autoradiography, and scintillation counting of excised bands.
EXAMPLE 4
In vitro MEK assay
For evaluation of direct MEK activity, 10 pg GST-MEK~ is incubated with 5
pg of a glutathione S-transferase fusion protein containing p44MAP kinase with
a
lysine to alanine mutation at position 71 (GST-MAPK-KA). This mutation
eliminates kinase activity of MAPK, so only kinase activity attributed to the
added
29
CA 02348236 2001-04-24
WO 00/41994 PCTNS99/30418
MEK remains. Incubations are 15 minutes at 30°C in a final volume of
50 ~.L
containing 50 mM Tris (pH 7.5), 10 pM MgC12, 2 " ~M EGTA, and 10 ~M
[y-32P]ATP. The reaction is stopped by addition of Laemrnli SDS sample buffer.
Phosphorylated GST-MAPK-KA is resolved by electrophoresis on a 10%
polyacrylamide gel. Radioactivity incorporated into GST-MAPK-KA is determined
by autoradiography, and subsequent scintillation counting of excised bands.
Additionally, an artificially activated MEK containing serine to glutamate
mutations at positions 218 and 222 (GST-MEK-2E) is used. When these two
sites are phosphorylated, MEK activity is increased. Phosphorylation of these
sites can be mimicked by mutation of the serine residues to glutamate. For
this
assay, 5 pg GST-MEK-2E is incubated with 5 ~g GST-MAPK-KA for 15 minutes
at 30°C in the same reaction buffer as described above. Reactions are
terminated and analyzed as above.
EXAMPLE 5
Whole cell MAP kinase assay
To determine if compounds block activation of MAP kinase in whole cells,
the following protocol is used. Cells are plated in multi-well plates and
grown to
confluence. Cells are serum-deprived overnight. Cells are exposed to the
desired concentrations of compound or vehicle (DMSO) for 30 minutes, followed
by addition of a growth factor, for example, PDGF (100 nglmL). After a 5-
minute
treatment with the growth factor, cells are washed with PBS, and lysed in a
buffer
consisting of 70 mM NaCI, 10 mM HEPES (pH 7.4), 50 mM glycerol phosphate,
and 1 °~ Triton X-100. Lysates are clarified by centrifugation at
13,000 x g for 10
minutes. Five micrograms of the resulting supernatants are incubated with 10
~,g
microtubule associated protein-2 (Map2) for 15 minutes at 30°C in a
final volume
of 25 ~L containing 50 mM Tris (pH 7.4), 10 mM MgCl2, 2 mM EGTA and 30 ~M
(y-32P]ATP. Reactions are terminated by addition of Laermmli sample buffer.
Phosphorylated Map2 is resolved on 7.5% acrylamide gels and incorporated
radioactivity is determined by scintillation counting of excised bands.
CA 02348236 2001-04-24
WO 00/41994 PCTNS99/30418
EXAMPLE 6
Monolayer rq~ owth
Cells are plated into multi-well plates at 10 to 20,000 ceIIs/mL. Forty-eight
hours after seeding, test compounds are added to the cell growth medium and
incubation is continued for 2 additional days. Cells are then removed from the
wells by incubation with trypsin and enumerated with a Coulter counter.
EXAMPLE 7
Growth in soft-agar
Celis are seeded into 35-mm dishes at 5 to 10,000 cells/dish using growth
medium containing 0.3% agar. After chilling to solidify the agar, cells are
transferred to a 37°C incubator. After 7 to 10 days' growth. visible
colonies are
manually enumerated with the aid of a dissecting microscope.
EXAMPLE 8
Collaclen-Induced Arthritis in Mice
Type II collagen-induced arthritis (CIA) in mice is an experimental model of
arthritis that has a number of pathologic, immunologic, and genetic features
in
common with rheumatoid arthritis. The disease is induced by immunization of
DBA/1 mice with 100 Ng type II collagen, which is a major component of joint
cartilage, delivered intradermally in Freund's complete adjuvant. The disease
susceptibility is regulated by the class II MHC gene locus, which is analogous
to
the association of rheumatoid arthritis with HLA-DR4.
A progressive and inflammatory arthritis develops in the majority of mice
immunized, characterized by paw width increases of up to 100%. A test
compound is administered to mice in a range of amounts; such as 20, 60, 100,
and 200 mg/kg body weight/day. The duration of the test can be several weeks
to a few months, such as 40, 60, or 80 days. A clinical scoring index is used
to
assess disease progression from erythema and edema (stage 1), joint distortion
(stage 2), to joint ankylosis (stage 3). The disease is variable in that it
can affect
one or all paws in an animal, resulting in a total possible score of 12 for
each
mouse. Histopathology of an arthritic joint reveals synovitis, pannus
formation,
31
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
and cartilage and bone erosions. All mouse strains that are susceptible to CIA
are high antibody responders to type II collagen, and there is a marked
cellular
response to CII.
EXAMPLE 9
SCW-induced monoarticular arthritis
Arthritis is induced as described by Schwab, et al. ,Infection and Immunity
59:4436-4442 (1991)) with minor modifications. Rats receive 6 p.g sonicated
SCW [in 10 ~I Dulbecco's PBS (DPBS)] by an intraarticular injection into the
right
tibiotalar joint on day 0. On day 21, the DTH is initiated with 100 pg of SCW
(250
p,l) administered i.v. For oral compound studies, compounds are suspended in
vehicle (0.5% hydroxypropyl-methylcellulose/0.2% Tween 80), sonicated, and
administered twice daily (10 ml/kg volume) beginning 1 hr prior to
reactivation
with SCW. Compounds are administered in amounts between 10 and 500 mg/kg
body weight/day, such as 20, 30, 60, 100, 200, and 300 mg/kg/day. Edema
measurements are obtained by determining the baseline volumes of the
sensitized hindpaw before reactivation on day 21, and comparing them with
volumes at subsequent time points such as day 22, 23, 24, and 25. Paw volume
is determined by mercury plethysmography.
EXAMPLE 10
Mouse ear-heart transplant model
Fey, T.A. ef aG describe methods for transplanting split-heart neonatal
cardiac grafts into the ear pinna of mice and rats (J. Pharm. and Toxic. Mefh.
39:9-17 (1998)). Compounds are dissolved in solutions containing combinations
of absolute ethanol, 0.2% hydroxypropyl methylcellulose in water, propylene
glycol, cremophor, and dextrose, or other solvent or suspending vehicle. Mice
are dosed orally or intraperitoneally once, twice or three times daily from
the day
of transplant (day 0) through day 13 or until grafts have been rejected. Rats
are
dosed once, twice, or three times daily from day 0 through day 13. Each animal
is anesthetized and an incision is made at the base of the recipient ear,
cutting
32
CA 02348236 2001-04-24
WO 00/41994 PCT/US99/30418
only the dorsal epidermis and dermis. The incision is spread open and down to
the cartilage parallel to the head, and sufficiently wide to accommodate the
appropriate tunneling for a rat or insertion tool for a mouse. A neonatal
mouse or
rat pup less than 60 hours old is anesthetized and cervically dislocated. The
heart is removed from the chest, rinsed with saline, bisected longitudinally
with a
scalpel, and rinsed with sterile saline. The donor heart fragment is placed
into
the preformed tunnel with the insertion tool and air or residual fluid is
gently
expressed from the tunnel with light pressure. No suturing, adhesive bonding,
bandaging, or treatment with antibiotics is required.
Implants are examined at 10-20-fold magnification with a stereoscopic
dissecting microscope without anesthesia. Recipients whose grafts are not
visibly beating may be anesthetized and evaluated for the presence of
electrical
activity using Grass E-2 platinum subdermal pin microelectodes placed either
in
the pinna or directly into the graft and a tachograph. Implants can be
examined
1-4 times a day for 10, 20, 30 or more days. The ability of a test compound to
ameliorate symptoms of transplant rejection can be compared with a control
compound such as cyclosporine, tacrolimus, or orally-administered
lefluonomide.
EXAMPLE 11
Murine ovalbumin-induced eosinophilia
Female C57BIJ6 mice are obtained from the Jackson Laboratory (Bar
Harbor, ME). All animals are given food and water ad libitum. Mice are
sensitized
w'tth a single i.p. injection of OVA (grade V, Sigma Chemical Company, St.
Louis,
MO) adsorbed to alum, (10 ~g OVA + 9 mg alum in 200 p,l saline) or vehicle
control, (9 mg alum in 200 pl saline) on day 0. On day 14, the mice are
challenged with a 12-minute inhalation of an aerosol consisting of 1.5% OVA
(weight/volume) in saline produced by a nebulizer (small particle generator,
model SPAG-2; ICN Pharmaceuticals, Costa Mesa, CA). Groups of eight mice
are dosed with oral vehicle (0.5% hydroxypropylmethylcellulose / 0.25% TWEEN-
80), or a test compound at 10, 30, or 100 mg/kg in oral vehicle, 200 p.l per
mouse
33
CA 02348236 2001-04-24
WO 00/41994 PCTNS99/30418
p.o. Dosing is performed once per day starting on day 7 or day, and extending
through day 16.
For determination of pulmonary eosinophilia, three days after the first
OVA aerosol challenge (day 17), the mice are anesthetized with an i.p.
injection
of anesthetic (Ketamine/Acepromazine/Xylazine), and the tracheae is exposed
and cannulated. The lungs and upper airways are lavaged twice with 0.5 ml of
cold PBS. A portion (200 NI) of the bronchoalveolar lavage (BAL) fluid is
enumerated using a Coulter counter Model ZB1 (Coulter Electronics, Hialeah,
FL). The remaining BAL fluid is then centrifuged at 300 x g for five minutes,
and
the cells are resuspended in 1 ml of HESS (Gibca BRL) containing 0.5% fetal
calf serum (HyClone) and 10 mM HEPES (Gibco BRL). The cell suspension is
centrifuged in a cytospin (Shandon Southern Instruments, Sewickley, PA) and
stained by Diff Quick (American Scient~c Products, McGraw Park, IL) to
differentiate BAL leukocytes into neutrophil, eosinophil, monocyte or
lymphocyte
subsets. The number of eosinophils in the BAL fluid is determined by
multiplying the percentage of eosinophils by the total cell count.
F. Other Embodiments
From the above disclosure and examples, and from the claims below, the
essential features of the invention are readily apparent. The scope of the
invention also encompasses various modifications and adaptations within the
knowledge of a person of ordinary skill. Examples include a disclosed compound
modified by addition or removal of a protecting group, or an ester,
pharmaceutical
salt, hydrate, acid, or amide of a disclosed compound. Publications cited
herein
are hereby incorporated by reference in their entirety.
What is claimed is:
34