Note: Descriptions are shown in the official language in which they were submitted.
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
IMAGING WITH TC-99M LABELED FIBRIN-c~-CHAIN PEPTIDE
GOVERNMENT RIGHTS IN THE INVENTION
This invention was made with government support under grant R41 HL
59769-O1 (MLT) awarded by the National Institutes of Health. The -government
has
certain rights in the invention.
CROSS REFERENCE TO RELATED APPLICATIONS
15 This application claims priority under 35 U.S.C. ~ 119 based upon U.S.
Provisional Patent Application No. 60/096,803 filed August 17, 1998.
FIELD OF THE INVENTION
The present invention generally relates to the field of nuclear medicine and,
more
particularly, to compositions for radivlabeled agents for imaging mammalian
tissue or cells,
compositions for radiolabeling agents that bind to mammalian tissue or cells,
compositions
for radiolabeling agents that bind to fibrin, and methods of using said
compositions.
BACKGROUND OF THE INVENTION
Development of radioactive agents for "hot spot" imaging of deep venous
thrombosis (DVT) and pulmonary embolism (PE) has been the subject of many
investigations for more than two decades. (Thakur ML, Coleman RE, Hoist JH,
Welch M. Indium-111 labeled platelets: Studies on preparation and in vivo and
in
vitro functions. Thrombosis Research 9:345-354, 1976; Knight LC.
1
CA 02348617 2001-04-17
WO 00/09076 PCTNS99/19011
Radiopharmaceuticals for thrombus detection. Seminars in Nucl Med XX:52-67,
1990; Koblik PD, DeNardo GL, Berger HJ. Current status of Immunoscintigraphy
in the detection of thrombosis and thromboembolism. Seminars in Nucl Med
XIX:221-231, 1989; Thakur ML. Potential of radiolabeled antiplatelet
antibodies in
the detection of vascular thrombi. In: S.C. Srivastava, ed. Radiolabeled
monoclonal
antibodies for imaging and therapy. Plenum Publishing Co., NATO ASI, series
152, 1988; Thakur ML. Scintigraphic imaging of venous thrombosis: A state of
the
art. Thrombotic and Hematologic Disorders 5:29-36, 1992). One approach to "hot
spot" imaging has been to radiolabel platelets, which form a major
biochemically
active constituent of a thrombus. A large number of agents, therefore, have
been
evaluated that would target platelets on the assumption that radiolabeled
platelets
will acreed on an occult thrombus and thereby facilitate its detection by
external
scintigraphy. Platelets have been labeled in vitro using such agents as In-111-
oxine
which internalizes and binds to platelet cytoplasmic components. (Thakur ML et
al.,
1976). Platelets have also been labeled in vivo using radiolabeled proteins or
peptides that are specific for platelet surface glycoprotein complex IIb-IIIa
(Knight
LC, 1990; Koblik PD et al., 1989; Thakur ML, 1988; Thakur ML, 1992; Knight
LC, Radcliffe R, Maurer AH, Rodwell JD, Alvarez VL. Thrombus imaging with
Tc-99m synthetic peptides based upon the binding domain of a monoclonal
antibody to activated platelets. J Nucl Med 35:282-288, 1994; Knight LC,
Maurer AH, Romano JE. Comparison of Iodine-123-Disintegrins for Imaging
Thrombi and Emboli in a Canine Model. J Nucl Med 37:476-482, 1996; Pearson
DA, Lister-James J, McBride WJ, Wilson DM, Martel LJ, Civitello ER, Dean
RT. Thrombus imaging using Tc-99m labeled high potency GPIIb./IIIa receptor
antagonist. Chemistry and initial biological studies. J Med Chem 39:1372-1382,
1996; Lister-James J, Vallabhajosula S, Moyer BR, Pearson DA, McBride BJ, De
Rosch MA. Bush LR, Machac J, Dean RT. Pre-Clinical Evaluation of
Technetium-99m platelet receptor-binding platelet. J Nucl Med 38:105-111,
1997; Line BR, Crane P, Lazewatsky J, Barrett JA, Cloutier D, Kagan M,
Lukasiewicz R, Holmes RA. Phase I trial of DMP444, a new thrombus imaging
agent. J Nucl Med 37:117P, 1996; Barrett JA, Crocker AC, Damphousse DJ,
Heminway SJ, Liu S, Edwards DS, Lazewatsky JL, Kagan M, Mazaika TJ,
Carroll TR. Biological evaluation of 99mTc cyclic glycoprotein IIb/IIIa
receptor
2
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
antagonists in the canine arteriovenous shunt and deep vein thrombosis models:
Effects of chelators on biological properties of [99mTc]chelator-peptide
conjugates. Bioconjugate Cftem 7:203-208, 1996). Despite the success achieved
with these agents in experimental animals and in limited human subjects, only
one
5 agent, AcuTect, the Tc-99m labeled peptide P-280, has recently been approved
for
clinical use. AcuTect is expected to detect acute but not chronic venous
thrombosis
(AcuTect. Diatide, Inc. J Nucl Med 39(10):19N, 1998) or pulmonary embolism,
which may harbor activated platelets only sparingly.
A second approach to "hot spot" imaging has been to radiolabel proteins
10 involved in clot formation. During the vessel wall injury, coagulation
proteins are
activated sequentially and generate the enzyme thrombin. Thrombin cleaves
plasma fibrinogen into fibrin monomers, which then polymerize around the
platelets and hold them in place as a clot. Fibrin therefore remains an
integral part
of DVT, fresh or old, and embolized in the lungs or elsewhere in the body. it
is
15 primarily for these reasons that I-125-fibrinogen enjoyed popularity for
external
detection of DVT for a long time (Knight LC, 1990; Koblik PD et al., 1989;
Thakur
ML, 1992). However, it is no longer available commercially. Iodine-123-
fibrinogen and many antifibrin monoclonal antibodies labeled with various
radionuclides have also been evaluated. (Koblik PD et al., 1989). However, for
20 many reasons such as the long circulation times or poor image quality,
agents
other than I-125-fibrinogen did not make it into common nuclear medicine
practice.
A third approach to "hot spot" imaging of DVT and PE is to radiolabel
antifibrin peptides. The feasibility of this approach has not been previously
25 investigated. One peptide of particular interest is the N-terminus
tripeptide, H-Gly
Pro-Arg-OH, of fibrin-a-chain, which was reported by Laudano and Doolittle to
be
an inhibitor of fibrinogen/thrombin clotting. (Laudano AP, Doolittle RF.
Synthetic
peptide derivatives that bind to fibrinogen and prevent the polymerization of
fibrin
monomers. Proc Natl Acad Sci 75:3085-3089, 1978). The investigators observed
30 that H-Gly-Pro-Arg-Pro-OH analog of the tripeptide was an even more potent
inhibitor of fibrinogen/thrombin clot by binding to C-terminus portion of the
y-chain
of fibrin and preventing fibrin polymerization. More recently, Kawasaki et al
prepared several more analogs and found that a pentapeptide, H-Gly-Pro-Arg-Pro-
3
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
Pro-OH had the highest fibrinogen/thrombin clotting inhibiting activity.
(Kawasaki
K, Miyano M, Hirase K, Iwamoto M. Amino acids and peptides. XVIII. Synthetic
peptides related to N-terminus portion of fibrin a-chain and their inhibitory
effect on
firbrinogen/thrombin clotting. Chem Pharm Bull 41:975-977, 1993).
The present invention comprises composition for diagnostic imaging of
mammalian cells and tissue. The composition comprises amino acids joined to a
linker, which is bound to a moiety that is chelated to a radionuclide. In one
of the
embodiments, the present invention is a pentapeptide labeled with Tc-99m, that
facilitates imaging of DVT and PE.
DEFINITIONS
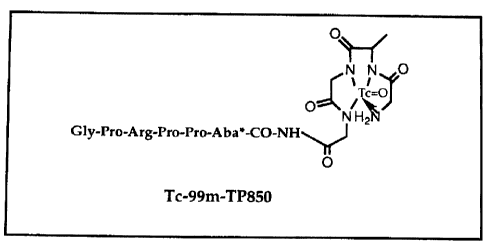
"TP 850" means the decapeptide, Gly-Pro-Arg-Pro-Pro-Ana-Gly-Gly-{D)-
Ala-Gly. (SEQ ID NO:1).
SUMMARY OF THE INVENTION
The present invention comprises a composition for imaging mammalian cells
and tissue and method of using said composition.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1. The amino acid sequence and the proposed structure of Tc-99m-
TP850.
Fig. 2. A composite of two HPLC elution spectra obtained under identical
conditions of solvent composition, flow rate, and column. The x axis in both
panels is time in minutes and the y axis is radioactivity peak height in pV.
The
diagonal line is the percent solvent composition. The upper panel is the
elution
profile of Tc-99m-TP 850 that was injected into the rabbit, and the lower
panel is
4
CA 02348617 2001-04-17
WO 00/09076 PC'T/US99/19011
that of the urine sample collected from the rabbit 3 hrs later. Note that the
major
proportion of the radioactivity eluted in the urine has the retention time
(Rt)
similar to that in the sample injected. The radioactivity at Rt 4 is unbound
Tc-
99m. The small radioactivity peaks at Rt 6.2 and 9.08 are considered as
impurities in the sample. The quantity of the peptide injected was small and
was
not detectable at 280 nm.
Fig. 3. An anterior image of a rabbit obtained at 3 hr post-injection. A
small thrombus induced by stimulating electrode in the right arm (arrowhead)
and
PE in both upper lobes of the lungs (long arrows) are detectable. Also seen in
the
right side of the neck (short arrows) is radioactivity accumulated in the
incision.
The radioactivity in the heart and sinus can be seen.
Fig. 4. An anterior image of a rabbit obtained at lhr 20 min post-injection.
A clot induced by stimulating electrode (clot/blood = 6.5) and the one by
thrombine-soaked suture (clot/blood - 3.7) are detectable. In addition,
radioactivity in the heart, thyroid, and paranasal sinuses can be seen. Free
Tc-
99m in preparation was approximately 3 % .
Fig. 5. Anterior gamma camera images of a rabbit which was injected
with 2 mCi Tc-99m-TP850 2 hr and 30 min previously. A clot due to thrombine-
soaked suture in the right (arrow) jugular vein and due to stimulating
electrode in
the left (arrow) are detectable. The activity due to some free Tc-99m in the
thyroid can also be seen. As stated in the text, the electrode clot had 7.1
times
more Tc-99m than that in the equal weight of blood and the thrombin clot had
3.6
times more Tc-99m than in the blood. The lower part of the radioactivity is in
the
heart.
Fig. 6. A composite of three images from one rabbit, in which PE was
induced 24 hr prior to the i.v. administration of 2.4 mCi of Tc-99m-TP 850.
The
scintiphoto in the left panel of the figure was obtained at 1 hr 15 min post-
injection
in the anterior position. It shows abnormal accumulation of radioactivity in
both
lungs (arrow). A clot formed spontaneously in the left neck where the incision
was
made for the placement of the PE introducer sheath is also seen in the
scintiphoto
given in the left panel of the figure. At the conclusion of in vivo
scintigraphy, the
heart and lungs were excised, spread for clarity, and then imaged under a
gamma
camera, as well as x-rayed. The x-ray image (center panel) shows a tantalum
5
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
mixed clot in the left lung which corresponds to the shape of a clot seen in
the left
lung (anterior scintiphoto in the left panel of the figure), as well as to the
left lung
clot seen in the gamma camera image of the excised lungs and heart given in
the
right panel of the figure. The clot seen in the right lung, in both in vivo
(arrow,
5 left panel) and ex vivo (arrow, right panel) images is not seen by x-ray
(center
panel) because it is free of tantalum. This indicates that this piece of clot
may
have formed without tantalum in it and lodged in the right lung. Both lung
clots
were separated, weighed, and counted for radioactivity. The clot in the left
lung
had three times more and the one in the right lung had 6.1 times more activity
than
in the unit weight of blood. This clot in the neck had 3.2 times more activity
than
in the unit weight of blood. Residual blood radioactivity in the heart (H) can
also
be seen in the right panel of the figure.
DETAILED DESCRIPTION
Materials and Methods
i) Preparation of peptide
For this study, a group of four amino acids, Gly-(D)-Ala-Gly-Gly
20 (GAGG)was chosen as a chelating moiety. Through their NHz groups these
peptides provide an N4 configuration for a strong chelation of Tc-99m. Rather
than the conventional post-synthesis conjugation, the tetrapeptide chelating
moiety
permitted the modification of the primary peptide at the C terminus during the
synthesis. Furthermore, during the synthesis, an additional amino acid, Aba (4
25 aminobutyric acid), was inserted as a spacer between the chelating moiety
and the
primary peptide. The purpose of inserting Aba as a spacer was to minimize any
possible steric hindrance resulting from the Tc-99m complex. The synthesis of
this modified peptide was one hybrid process which eliminated the mufti-step,
lengthy, and frequently inefficient conjugation procedure, yet provided a
chelating
30 group for a strong chelation of Tc-99m. The resultant decapeptide, Gly-Pro-
Arg-
Pro-Pro-Aba-Gly-Gly-(D) Ala-Gly which has an expected M.W. of 850, is
hereafter referred to as TP 850.
6
CA 02348617 2001-04-17
WO 00/09076 PCTNS99/19011
The peptide was custom synthesized (PeptidoGenic Research Co., Inc.
Livermore, CA) using a Shimadzu solid phase synthesizer (Shimadzu, Columbia,
MD) and separated using HFIsiI, C-18, 5 micron preparative HPLC column. Ion
spray mass analysis was performed using Perkin Elmer's Sciex APZ I mass
5 spectrometer (Norwalk, CT). Using this chelating moiety and facility several
peptides have previously been prepared and labeled with Tc-99m in our
laboratory.
(Thakur ML, Pallela VR, Consigny PM. Tc-99m-TP 1201 for imaging
thromboembolism. Radiology 205:267P, 1997; Pallela VR, Consigny PM, Shi R,
Thakur ML. Imaging vascular thrombosis with Tc-99m-TP 1300 peptide derived
10 from active domain of thrombospondin. J Nucl Med 39:64P, 1998; Pallela VR,
Consigny PM, Shi R, Thakur ML. Tc-99m-labeled Fibrin-a-chain nentide analog
for imaging vascular thrombosis. Eur J Nucl Med 25:878, 1998; Pallela VR,
Thakur ML, Consigny PM, Rao PS, Vassileva-Belnikolovska D, Shi R. Imaging
thromboembolism with Tc-99m labeled thrombospondin receptor analogs TP-1201
15 and TP-1300. Thrombosis Research 93:191-202, 1999; Pallela VR, Thakur ML,
Chakder S, and Rattan S. 99mTc-labeled vasoactive intestinal peptide receptor
agonist: Functional studies. J Nucl Med 40:352-360, 1999).
20 ii) RadiolabelinR and guality control:
Fifty p,g of TP 850 was dissolved in 10 pl 10% acetonitrile in water, then
200 pl of 0.1 M Na3P04 were added, followed by 10-30 mCi Tc-99m in 200 pl
isotonic saline previously reduced with 100 pg SnClz in 10 p,l of 0.05 M HCI.
25 Lately, with a new batch of high purity SnCl2 (Sigma Chemicals, St. Louis,
MO)
we have been able to reduce the SnCl2 to 10 ~,g. The reaction mixture was then
incubated for 30 min in a boiling water bath. The product was examined by
HPLC (Rainin, Emeryville,CA) using a reverse phase C-18 column and gradient
solvents of 0.1 % TFA in water (A) and 0.1 % TFA in acetonitrile (B). The
30 gradient was such that at zero minutes solvent A was 90%, and at 30 min
solvent
B was 100% . The flow rate was 1 ml/min. The HPLC was equipped with a u.v.
detector set at 278 nm, a 2" NaI (Tl) gamma counter, and a rate meter.
7
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
iii) Stability of Tc-99m-TP 850:
The stability of the radiolabeled peptide at 22° C was examined by
HPLC
for up to 24 hrs as determined by the characteristic retention time of the
radioactivity peak. The in vivo stability was examined by injecting
approximately
2 mCi Tc-99m-TP 850 preparation, collecting urine 3 hrs later, and analyzing a
20
pl portion of the urine by HPLC.
iv) Fibrin binding:
The ability of Tc-99m-TP 850 to bind to rabbit, dog, and human fibrin was
examined in vitro. Institutional approval was obtained to draw human blood and
to perform all animal experiments. Approximately 10 ml of venous blood was
obtained from a healthy human volunteer and from a normal young adult dog and
a
rabbit. No anticoagulating agent was added to the blood. After the blood was
clotted, from each blood sample, one ml serum samples were dispensed in four
separate test tubes and approximately 25 ~Ci of Tc-99m-TP 850 (specific
activity
approximately 340 Ci/m mol) were added to each tube and the reagents were
gently mixed. Thrombin (six i.u.) was then added to the first two test tubes
and an
equal volume of saline to the other two. The contents were gently mixed and
allowed to incubate for 10 min at 37°C. The test tubes were then
centrifuged
(2000 g x 10 min), the supernatant carefully removed, and the fibrin clots in
the
first two test tubes were washed twice with 2 ml 0.9 % NaCI. Following
centrifugation, the washing liquid was combined with the supernatant.
Radioactivity associated with the clot and the supernatant were measured and
calculated as the percent of total activity found in the compact fibrin clot.
8
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
v) Inhibition of platelet aR~re~ation:
Seventeen ml of venous blood from a rabbit and a dog were
collected in 3 ml Acid Citrate Dextrose A (ACD A), centrifuged at 180 g for 10
min
5 and platelet rich plasma (PRP) was separated. (Thakur ML, Coleman RE, Hoist
JH,
Welch M. Indium-111 labeled platelets: Studies on preparation and in vivo and
in
vitro functions. Thrombosis Research 9:345-354, 1976). Aggregation studies
were
performed using a Chronolog (Havertown, PA) aggregometer. For each study,
increasing quantities of TP 850 and 4 p,M ADP were added to 500 pl PRP
10 containing approximately 1.5 x 10$ platelets, stirring at 37°C.
Aggregation in the
absence of TP 850 was considered 100% and ICso values were determined using
the
quantity of TP 850 that inhibited aggregation by 50%.
15 vi) Blood clearance:
All animal protocols were approved by the Institutional Animal Care and
Use Committee and were strictly followed. Blood clearance of the agent was
20 examined in adult New Zealand white rabbits weighing between 3 to 3.5 kg.
Each
rabbit was anesthetized by an i.m. injection of ketamine (30 mg/kg) and
zylaxine
(5 mg/kg). Thereafter a 23 gauge catheter was inserted in the right ear artery
and
connected to a leuer lock (Burron Med. Inc., Bethlehem, PA). The patency of
the
catheter was maintained by the administration of 6 i.u. heparin per ml of
sterile
25 0.9 % NaCI administered through the leuer lock. This catheter was used for
drawing 0.5 ml blood samples in duplicate at 1,5,10,15, and 30 min, and then
at
1, 2, and 3 hrs after radionuclide injection. Before each sample collection
enough
blood was withdrawn to replace saline, which avoided the dilution of each
blood
sample collected.
30 The marginal vein of the contralateral ear was used for injecting
radioactive
agents. The radioactivity in the syringe was measured before and after
injection to
determine the dose injected, and a suitable Tc-99m standard solution was
prepared.
Blood samples were then weighed, radioactivity counted, and results were
9
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
expressed as percent injected dose per gram (% LD./g) of blood and plotted as
a
function of time.
vii) Tissue distribution studies:
Tissue samples were harvested from three rabbits three hrs after the
administration of Tc-99m-TP 850. Tissues were weighed, radioactivity
associated
with each tissue, and a reference standard solution of Tc-99m prepared at the
time
10 of injection was determined. Radioactivity was expressed as % injected
dose/g (%
LD./g) of tissue. Results were averaged and standard deviation was determined.
viii) Inducing DVT:
Each of the eight adult (male or female) New Zealand white rabbits, weighing
between 3-3.5 kg was anesthetized as described above, the right cubital vein
and/or jugular vein was exposed, and a stimulating electrode was inserted
(Leadley
RJ, Humphrey WR, Erickson LA, Shebuski RJ. Inhibition of thrombus formation
by Endothelin-1 in canine models of arterial thrombosis. Thrombosis and
Haemostasis 74:1583-1590, 1995). The electrode was constructed from a 26-
gauge stainless steel hypodermic needle bent at a 90° angle and
attached to a 30-
gauge, Teflon insulated silver coated copper wire. The needle was inserted
into
the vessel and then gently pulled so that it was in contact with the
endothelial
lining of the vessel and secured in place with a flared sleeve inserted over
the
copper wire. The second electrode was applied to the tongue of-the rabbit. The
stimulating electrode was attached to the anode and the other electrode to the
cathode of a power supply. A 450 p,A current was then applied and 10 min later
2 mCi of Tc-99m TP 850 (specific activity approximately 510 Ci/m mol) in 2 ml
30 0.9 % solution was injected through a marginal ear vein. Radioactivity in
each
dose was measured before and after administration and recorded. A suitable
reference solution with a known quantity of Tc-99m was also prepared. In two
additional rabbits thrombus was induced by inserting a thrombin-soaked suture
into
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
a jugular vein 10 min prior to the administration of Tc-99m-TP 850. Serial
gamma camera images of the rabbit, in the supine position, were then obtained
for
up to four hours, using a GE Starcam gamma camera (GE, Milwaukee, WI)
coupled to a low energy parallel hole collimator. For each image a total of
5 350,000 counts were collected.
ix) Inducing pulmonary embolism:
Pulmonary emboli were induced in six additional rabbits. Radio opaque
pulmonary emboli were prepared by drawing 0.5 to .75 ml blood, through a 23G
butterfly needle inserted in the marginal ear vein, into a one ml syringe
containing
mg tantalum powder and 6 i.u. of thrombin. The contents of the syringe were
15 then mixed gently and a clot was allowed to form and harden for 20 min. The
clot
was removed from the syringe and a one cm long piece of the clot was drawn in
an
introducer sheath (6Fr, Pinnacle, MediTech, Watertown, MA), which was then
inserted into a previously isolated jugular vein and advanced into the right
atrium.
The clot was then flushed from the sheath with isotonic saline. The position
of the
20 tantalum containing clots was confirmed by recording a chest x-ray of the
animals
before the administration of Tc-99m-TP 850 and an x-ray of the excised lungs
after
sacrifice. Following clot administration and the confirmation of its
localization by
x-ray, Tc-99m-TP 850 was injected and the rabbits were imaged as described in
the previous section. Four animals with PE were allowed to recover from the
surgery. Two rabbits were injected with Tc-99m-TP 850 24 hrs later and the
other
two were injected 48 hours later.
Upon the conclusion of imaging for PE or DVT, each rabbit was given an
intravenous injection of heparin (1000 i.u.) and was then euthanized with
sodium
pentobarbital (100 mg/kg). A blood sample was drawn, and then the lungs and
heart were excised, radiographed, and the clots were harvested. The clots and
blood were weighed, radioactivity associated with them was counted, and
clot/blood ratios were determined.
11
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
Results
i) Peptide radiolabelinR, quality control, and stability:
The purity of the peptide as determined by HPLC analysis was > 90 % .
The expected M.W. of the peptide was 850, and the one observed by mass
spectroscopic analysis was 849.4. The proposed structure of Tc-99m labeled TP
850 is given in Fig. 1, which shows that Tc-99m is bound to the chelating
moiety
10 with N4 configuration. The Tc-99m labeling consistently produced > 95 %
yield.
HPLC analysis indicated that > 90 % of that activity was eluted in a single
peak at
retention time (Rt) of seven min. A small quantity ( < 5 % ) of radioactivity
was
eluted at a Rt of 6.2 min and any unbound Tc-99m at a Rt of 3.5 min. An
elution
profile is given in Fig. 2.
The preparations of Tc-99m-TP 850 were stable at 22°C for 24 hrs.
HPLC
analysis of a urine sample (Fig. 2) collected at three hrs after an injection
of Tc-
99m-TP 850 showed that the radioactivity elution profile was similar to that
of the
preparation injected, and that the retention time of the radioactivity peak in
the
urine sample was similar to that of the radioactivity sample injected. This
suggested that the small peptide was not susceptible to a rapid in vivo
proteolysis.
ii) Blood clearance and tissue distribution:
The blood clearance was biphasic with a t'h-a being approximately four
min (20 % ) and t'h - ~i being approximately 13 min (80 % ). Examination of
three
hr tissue distribution of Tc-99m-TP 850 indicated that the highest
radioactivity was
in the kidneys (0.10 ~ 0.086 % I.D./g), suggesting that the kidneys were the
primary route of excretion. The liver uptake was 0.016 ~ O.OI4 % I. D. /g and
intestine 0.01 t 0.009% LD./g. The blood uptake at this time was only 0.007 ~
30 0.004% LD./g. This small proportion of radioactivity in circulating blood
facilitated the imaging of vascular thrombi. Radioactivity in all other
tissues was
unremarkable.
12
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
ii) Fibrin binding and inhibition~platelet a~Rregation:
TP 850 radioactivity associated with human, dog, and rabbit fibrin was 42
t 2 % , 60 t 39 % , and 56 ~ 2.5 % , respectively. The ICso values for the dog
and
rabbit platelet aggregation inhibition were 236 pm and 167 p,m, respectively.
These data justified the use of rabbit as a model for studies with Tc-99m-TP
850.
iii) Imaging DVT' and PE:
Although Tc-99m-TP 850 cleared rapidly from the blood, cardiac blood pool
activity was detectable in all animals at all imaging times. Radioactivity in
the sinus
was also detectable in all animals studied. This was consistent with the
results of
15 Tc-99m-TP1201 and Tc-99m-TP1300, the activated platelet receptor specific
thrombospondin analogs studied previously in our laboratory. (Pallela VR,
Thakur
ML, Consigny PM, Rao PS, Vassileva-Belnikolovska D, Shi R. Imaging
thrornboembolism with Tc-99m labeled thrombospondin receptor analogs TP-1201
and TP-1300. Thrombosis Research 93:191-202, 1999). Unlike these two agents,
however, no Tc-99nn-TP 850 radioactivity was seen either in the bone or in the
bone
cartilage. All fresh DVT and PE were detectable by Tc-99m-TP 850 generally
within 90-120 min post-injection. Clots that formed spontaneously in surgical
incision or ligated vessels were also detectable. Similarly, PE that were
formed by a
piece of a clot broken off or separated from a clot that was injected into the
right
25 atrium were imageable. An example is given in Fig. 3, in which an electrode
induced clot in the right forearm, two PE, one in each upper lobe of the
lungs, and
radioactivity accumulated in the incision was seen. In this animal, the
forearm clot
to blood radioactivity ratio was 12, and the PE to blood 1.3 (L), and 2.1 (R).
The
radioactivity associated with the clots was 0.087% LD./g, 0.006 % LD./g and
30 0.007% LD./g, respectively.
The clot/blood ratios in the rabbits studied ranged from 1.2 to 12. Many
times these clots were small and could not be easily separated without the
vessel
wall or adjoining fatty tissue. Similarly, tantalum was embedded in many PE.
13
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
Consequently, the weight contributed by the additional tissue or tantalum,
resulted
in the low and variable radioactivity per unit weight of clots, PE, or DVT.
Fig. 4 shows an anterior image of a rabbit given Tc-99m-TP 850 one hr
and 20 min previously. A clot in the right jugular vein induced by stimulating
5 electrode and the one in the left jugular vein induced by thrombin-soaked
suture
were detectable. The clot to blood ratios were 6.5 and 3.7, respectively. The
clot
radioactivity was 0.035% LD./g and 0.02% LD./g. In this animal, the
radioactivity was also seen in the thyroid due to 3.5 % unbound free Tc-99m
that
was injected.
10 Fig. 5 shows an anterior image of a rabbit obtained at 150 min after the
injection of Tc-99m-TP 850 into which thrombin-soaked suture was placed in the
right jugular vein and a stimulating electrode clot was formed in the left
jugular
vein. Both clots were detectable with the electrode clot to blood ratio of 7.1
and
the suture soaked thrombin clot/blood ratio of 3.6. Included in each suture
clot
15 was the weight of the suture itself which artificially decreased the
clot/blood
radioactivity ratios. The radioactivity incorporated into these 'clots
measured
0.046 % I. D. /g and 0.024 % I. D . /g of the weight of the clot.
Fig. 6 is an anterior image of a rabbit with PE in both lungs induced 24 hrs
previously. The image was positive at one hr and 15 min post-injection of Tc
20 99m-TP 850. Lungs were excised, imaged, and x-rayed. The location of the
clots
was corroborated. The clots were then retrieved, weighed, and associated
radioactivity was measured. The clot to blood ratios were 6.1 for the right
clot
and 3.0 for the one in the left clot. The radioactivity in the clot was 0.021
% LD./
and 0.01 % LD./g.
25 In contrast, 48 hr old clots were neither detectable by scintigraphy nor by
x-
ray. This suggested that they were lysed and had disappeared. This is
consistent
with the high fibrinolytic activity in rabbits. (Didisheim P. Animal models
useful
in the study of thrombosis and antithrombotic agnents. Prog in Hemostasis and
Thrombosis Spaet TH, ed. Grune and Stratton: New York, pp. 165-197, 1976;
30 Doolittle RF, Omcley JL, and Surgenor DM. Species differences in the
interaction of thrombin and fibrinogen. J Biol Chem 237:3123, 1962; Gallimore
MJ, Nulkar MV, and Shaw JTB. A comparative study of the inhibitors of
fibrinolysis in human, dog, and rabbit blood. Thromb Diath Haemorrh 14:145-
14
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
158, 1965; Hawkey CM, Fibrinolysis in animals. In The Haemotstatic .
Mechanism in Man and Other Animals, MacFariane RG, ed. Academic Press:
London, pp. 143-150, 1979; Mason RG and Read MS. Some species differences
in firbrinolysis blood coagulation. J Biomed Mater Res 5:121-128, 1971; Craig
5 IH, Bell FP, and Schwartz CJ. Thrombosis and atherosclerosis: The
organization
of pulmonary thromboemboli in the pig. Exper Mol Path 18:290-301, 1973). The
influence of any anticoagulant therapeutic intervention has not yet been
studied.
Discussion
In the USA, more than 378,000 patients are hospitalized annually for DVT,
and more than 103,000 for PE. (Vital and Health Statistics. Series 13: Data
from
National Health Survey Ditts Publication No. (PHS)95-1783, 1993). These
15 conditions, despite modern techniques, contribute to more than 200,000
deaths every
year. The clinical diagnosis of DVT and PE is unreliable (Burke B, Sostman D,
Carroll B, and Witty LA. The diagnostic approach to deep venous thrombosis.
Clinics in Chest Medicine 16:253-1568, 1995;Worsley DF, Alavi A, Palevsky,
HI. Role of radionuclide imaging in patients with suspected pulmonary
embolism.
Radiologic Clinics of North America 31:849-859, 1993), and PE is an often
underestimated, underdiagnosed, and undertreated disease. {Janata-Schwatzek K,
Weiss K, Riezinger I, Bankier A, Domanovits H, Seidler, D. Pulmonary
Embolism: Diagnosis and treatment. Seminars in Thrombosis and Hemostasis
22:33-52, 1996).
25 Venography is invasive and other modalities have limitations. Spiral CT,
MRI, and ventilation-perfusion (VQ) scans remain the leading diagnostic tools
for
its diagnosis. In spiral CT, a number of interpretive pitfalls exist in
assessing
images of PE and MRI is not likely to replace CT. Although CT has better
resolution and less sensitivity to moving lung artifacts, its pitfalls, and
use of
30 frequently allergic contrast agents have led investigators to rely upon VQ
scanning.
VQ scanning itself is a cold spot imaging techniques and can only predict low
or
high probability of PE. For many clinicians this type of diagnosis is
inadequate.
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
In principle, external scintigraphic techniques aided by the use of a suitable
radiopharmaceutical can provide hot spot images and can fulfill the need since
scintigraphic techniques are non-invasive, and can scan the entire body of a
patient
without unreasonable inconvenience or added morbidity to the patient.
5 During the past few years, a large number of radiopharmaceuticals have
been investigated as potential agents to localize DVT or PE. Since thrombi are
largely composed of fibrin, platelets and other cells entrapped in the fibrin
network, much attention was drawn to the use of radioiodine labeled fibrinogen
and In-111 labeled platelets.
10 In many ways, radiolabeled platelets should be a simple and ideal agent,
for
they form a major and the most biologically active constituent of a thrombus.
However, radiolabeled platelets have been less attractive largely due to their
long
life span (8 days) that elevated background radioactivity for several days
after their
administration. The slow clearance of radioactivity causes delay in diagnosis
due
15 to suboptimal lesion to background radioactivity ratios. The platelets must
also be
labeled in vitro which requires skilled personnel. Furthermore, in the
presence of
anticoagulant therapy, heparin in particular, when accretion of fresh
platelets is
impeded, In-111 platelet scintigraphy is less successful. An array of
antibodies, the
majority of them specific for IIb and IIIa glycoprotein complex on the
platelet
20 surface, have also been investigated. Success with these has been limited
for a
variety of reasons including the lack of specificity, unfavorable
pharmacokinetics
or cumbersome preparation of the agent. The pros and cons of these and other
agents have been described by Knight, Thakur, and Koblik et al.
Prompted by the advancements in science and technology of molecular
25 biology, recent development of radioactive agents for non-invasive
diagnosis of
thromboembolism are centered around the use of Tc-99m labeled peptides
specific
for resting or activated platelets. Peptides are smaller in size and easier to
produce
than monoclonal antibodies. They are expected to clear more rapidly from
circulation than radiolabeled proteins, less likely to induce any
immunological
30 reaction, yet in most cases they enjoy as high a receptor specificity and
binding
constants as the monoclonal antibodies. Because of the physical characteristic
of
Tc-99m, the Tc-99m labeled peptides have become even more attractive
biomolecules for diagnostic imaging than antibodies labeled with In-111.
16
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
Technetium-99m is easy to obtain worldwide, inexpensive, and decays with
gamma ray energy ( 140 KeV , 90 % ) that can be efficiently detected by gamma
cameras, planar or tomographic. It has a half life (6 hr) that is long enough
to
perform examinations before excessive radioactive decay has occurred, yet not
too
5 long to persist in the body long after the examinations have been carried
out and to
give excessive radiation dose to the subject.
All of the peptides evaluated thus far are specific for platelet glycoprotein
receptor complex IIb IIIa. Among them, one peptide, Tc-99m-P2$0 was recently
approved by the FDA under the trade name AcuTect. As per the manufacturer's
10 description, the peptide is expected to detect only acute thrombi but not
old clots
or PE. A primary reason for this is physiologic, in that fresh platelets, to
which
AcuTect may bind in vivo, seldom accumulate in chronic clots or PE. A
different
approach to the problem is therefore necessary that will permit imaging of DVT
as
well as PE.
15 The coagulation process described earlier generates fibrin monomers that
form a substantial part of a clot. The actual quantity of fibrin content may
vary
from clot to clot, but generally it is expected to be the same as the
fibrinogen of
the blood which in most adults is as high as five grams per 100 grams of
plasma
proteins. Since fibrin exists on both the surface and within clots that are
forming
20 or dissolving, the development of Tc-99m labeled peptide, specific for
fibrin is
appealing. Such agents, in principle, can target fibrin at any stage or state
of a
clot and reliably image it. For this purpose, one peptide of particular
interest is
the N-terminus fibrin a-chain peptide, H-Gly-Pro-Arg-OH, which was reported by
Laudano and Doolittle to be an inhibitor of fibrinogen/ thrombin clotting
(13).
25 The a-chain of fibrin begins with the same tripeptide sequence in many
animal
species as well as in humans. These investigators observed that H-Gly-Pro-Arg-
Pro-OH analog of the tripeptide was an even more potent inhibitor of
fibrinogen/thrombin clotting than the tripeptide itself since it also bound to
C-
terminus portion of the y-chain of fibrin and prevented fibrin polymerization.
30 More recently, Kawasaki, et al, prepared several more analogs and found
that a
pentapeptide, H -Gly-Pro-Arg-Pro-Pro-OH had the highest fibrinogen/thrombin
clot inhibiting activity.
17
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
Peptides chosen for scintigraphic imaging are modified before they are
labeled with a radionuclide of choice. In order to accomplish efficient
radiolabeling, most commonly, the presynthesized peptides are conjugated with
a
metal chelating agent. This is a multi-step process in which peptide
functional
5 groups are first blocked, chelating agents are conjugated, and excess of
reagents
are eliminated. The functional groups from the resultant product are then
deblocked, the product is separated using preparative HPLC, and the required
product is identified by mass spectroscopic analysis. Not only is the
procedure
time-consuming, but it can also be frequently inefficient.
10 The hybrid peptide technique which we developed to label the peptide with
Tc-99m, is simple, efficient, and eliminates the drawbacks stated above. The
results of our fibrin clot binding and platelet aggregation inhibition studies
support
the notion that these modifications did not compromise the biological activity
of
the peptide. These results are consistent with previous findings using
biologically
15 active peptides.
The binding of Tc-99m-TP 850 to rabbit fibrin and its ICso value for
inhibition of rabbit platelet aggregation observed in this study were high
enough to
justify the use of the rabbit as a model for imaging experimental clots and
PE. All
clots, formed by vessel wall injury, by stimulating electrode or by thrombin-
20 soaked sutures implanted in the jugular vein, were detectable by gamma
scintigraphy. In general, the clots were small and the radioactivity
incorporated
into them varied from 0.01 % LD./g to 0.087% LD./g. This variability was
probably due to the presence of non-radioactive tissues or tantalum that were
not
separated but contributed to the weight of the clots. However, neither the
25 proportion of radioactivity incorporated into the clots, nor the variation
of this
proportion is uncommon in such animal experiments. Despite the relatively
small
proportion of radioactivity, the clots were detectable in approximately 90 min
after
injection.
In experiments, Tc-99m-TP 850 had considerably higher radioactivity
30 uptake on PE than at least two activated platelet specific Tc-99m labeled
peptides
we had evaluated previously. With Tc-99m-TP 850, all PE were detectable except
those that had lysed spontaneously at 48 hr post-placement. The disappearance
of
the 48 hr old clots was confirmed by the loss of x-ray opacity of these clots
which
18
CA 02348617 2001-04-17
WO 00/09076 PCT/US99/19011
had been impregnated with tantalum at the time of preparation. The choice of
using the rabbit as a model was based upon our supportive in vitro data
described
previously. However, the plasminogen concentration in rabbits is greater than
twice as high as in humans. The fibrinolytic activity in rabbits, therefore,
is much
higher and leads to a rapid dissolution of these clots.
In principle, an antifibrin agent should be more successful in imaging aged
thrombi and may be less susceptible to interference by anticoagulant therapy
because in such circumstances more fibrin may be exposed on the clot surface
and
blood flow around the clot may be greater.
15
25
35
19