Note: Descriptions are shown in the official language in which they were submitted.
CA 02348733 2001-12-04
WO 00/26241 PCT/US99/25333
NOVEL COMPOSITIONS AND METHODS OF SCREENING FOR T.CELL
AND B-CELL ACTNATION MODULATORS
The invention relates to proteins useful in T-cell and B-cell activation, and
more particularly to a human
SWAP70 homolog found in a T-cell library and a human RasGRP homolog, and their
use in methods
for identifying candidate agents which modulate these activities.
BACI~'~,Q~ND OF THE INV~~(IION
Lymphocytes are the white blood cells responsible for the immune response.
Their characteristics
account for the immune system's attributes of diversity, specificity, memory,
and.setf/nonself
recognition. Lymphocytes, which constitute 20-40% of the body's white blood
cells, circulate in the
blood and lymph and are capable of migrating into the tissue spaces and
lymphoid organs. The
lymphocytes can be broadly subdivided on the basis of function and cell-
membrane components into
three populations: B cells, T cells, and NK cells. Of particular interest are
B cells and T cells, and
more particularly of interest is the activation of lymphocytes. As used
herein, lymphocytes refer to B
cells and T cells.
Activators of B cells have been identified. For example, SWAP70 was originally
identified as a B cell
specific protein involved in B cell isotype switching (Borggrefe et al., J.
Biol., Chem., 17025-17035
(199$), incorporated herein in its entirety).
Since activation of specific signaling pathways in lymphocytes determines the
quality, magnitude and
duration of immune responses, it is desirable to identify more activation
proteins and modulators
thereof. These proteins and modulators will find use in transplantation, acute
and chronic
CA 02348733 2001-12-04
WO i)4I1b241 PCT/US99/25333
-2-
SUM~~Y I~ THE INVENTION
The present invention provides proteins involved in activation of T and B
cells. More particularly,
JEST (also sometimes called T-SWAP) has teen identified. JEST has homology to
SWAP70,
originatty identified as a B cell specific protein. However, it is provided
herein that JEST is involved in
T cell signaling. Moreover, also provided herein is that RasGRP binds to JEST
and SWAP70 (also
sometimes called SWAP herein). Thus, RasGRP is provided herein as involved in
B and T cell
activation signaling. Also provided herein are methods for screening for
modulators of B and T cell
activation.
In one aspect, a recombinant nucleic acid encoding a JEST protein that is at
least about 85% identical
to the amino acid sequence depicted in Figure 2 is provided. Also provided is
a recombinant nucleic
encoding the amino acid sequence depicted in Figure 2. Further provided herein
is a recombinant
nucleic acid which will hybridize under high stringency conditions to the
nucleic acid sequence
depicted in Figure 2 or its complement. Moreover, a recombinant nucleic acid
that is at least about
90°~ identical to the nucleic acid sequence depicted in Figure 2 is
provided. Also provided is a
recombinant nucleic acid having the nucleic acid sequence depicted in Figure
2.
In another aspect of the invention, a n3combinant JEST protein is provided
that is at least about 8590
identical to the amino acid sequence depicted in Figure 2. Also provided is a
JEST protein according
comprising the amino acid sequence of Figure 2. Further provided herein is a
JEST protein according
encoded by a nucleic acid at least about 85% identical to the nucleic acid
sequence depicted in Figure
2. Moreover, a JEST protein is provided herein which is encoded by a nucleic
acid that will hybridize
under high stringency conditions to the nucleic acid sequence of Figure 2 or
its complement.
In yet another aspect of the invention, an isolated polypeptide which
specifically binds to a JEST
protein is provided. Preferably, the polypeptide is an antibody, more
preferably, a monoclonal
antibody. In one embodiment, a monoclonal antibody is provided that reduces or
eliminates the
biological function of JEST protein encoded by a nucleic acid that will
hybridize under high stringency
conditions to the nucleic acid of Figure 2 or its complement.
The present invention further provides a recombinant nucleic acid encoding a
human RasGRP protein
that is at least about 85% identical to the amino acid sequence depicted in
Figure 7B. Moreover, a
recombinant nucleic acid is provided which encodes the amino acid sequence
depicted in Figure 7B.
Also provided herein is a recombinant nucleic acid which will hybridize under
high stringency
CA 02348733 2001-12-04
WO 00/26241 PCTNS99/Z5333
-3-
recombinant nucleic acid that is at least about 90~o identical to the nucleic
acid sequence depicted in
Figure 7A. In another embodiment, a recombinant nucleic acid having the
nucleic acid sequence
depicted in Figure 7A is provided. Also provided are the sequences depicted in
Figures 6A, 6B, 6C
and 6D, which each bind to SWAP70. In one embodiment, the sequences of Figures
6A-6D bind to
JEST.
Further provided herein is a recombinant human RasGRP protein that is at least
about 95% identical
to the amino acid sequence depicted in Figure 7B. In one embodiment, a human
RasGRP protein is
provided which comprises the amino acid sequence of Figure 7B. In another
embodiment, a human
RasGRP protein is provided which is encoded lay a nucleic acid at least about
85°~ identical to the
nucleic acid sequence depicted in Figure 7A. Also provided herein is a human
RasGRP protein
encoded by a nucleic acid that will hybridize under high stringency conditions
to the nuGeic acid
sequence of Figure 7A or its complement.
In another aspect of the invention, an isolated polypeptide which specfically
binds to human RasGRP
protein is provided. In one embodiment, such a polypeptide is an antibody,
preferably a monoclonal
antibody. In a preferred embodiment, a monoclonal antibody that reduces or
eliminates the biological
function of RasGRP protein encoded by a nucleic acid that will hybridize under
high stringency
conditions to the nucleic acid of Figure 7A or its complement is provided.
Also provided herein are expression vectors comprising the nuGeic acids
described herein. Further
provided herein are the host cells comprising the nucleic acids and/or vectors
described herein.
in a further aspect of the invention, a process for producing a human RasGRP
protein or JEST is
provided. The methods comprise culturing the host cells provided herein under
conditions suitable for
expression of a human RasGRP protein or JEST.
In yet another aspect of the invention, a method for screening for a bioactive
agent capable of binding
to a JEST protein is provided. In one embodiment, said method comprises
combining a JEST protein
and a candidate bioactive agent, and determining the binding of said candidate
agent to said JEST
protein.
Also provided herein is a method for screening for a bioactive agent capable
of binding to a human
RasGRP protein. In one embodiment, said method comprises combining a human
RasGRP protein
and a candidate bioactive agent, and determining the binding of said candidate
agent to said human
CA 02348733 2001-12-04
WO OOI26241 PCTIUS99/25333
In yet another aspect of the invention, a method for screening for agents
capable of interfering with the
binding of a SWAP70 protein and RasGRP is provided. In one embodiment, said
method comprises
combining a SWAP70 protein, a candidate bioactive agent and a RasGRP protein
and determining the
binding of said SWAP70 protein and said RasGRP protein.
Also provided herein is a method for screening for agents capable of
interfering with the binding of a
JEST protein and RasGRP. In one embodiment, said method comprises combining a
JEST protein, a
candidate bioactive agent and a RasGRP protein and determining the binding of
said JEST protein
and said RasGRP protein.
In yet another aspect of the invention, a' method for screening for an
bioactive agent capable of
modulating the activity of JEST protein is provided. In one embodiment, said
method comprises
adding a candidate bioactive agent to a cell comprising a recombinant nucleic
acid encoding a JEST
protein and determining the effect of the candidate bioactive agent JEST
bioactivity including
lymphocyte activation.
Also provided herein is a method for screening for an bioactive agent capable
of modulating the
activity of human RasGRP protein, said method comprising the steps of adding a
candidate bioactive
agent to a cell comprising a recombinant nucleic acid encoding a human RasGRP
protein and
determining the effect of the candidate bioactive agent on. RasGRP bioactivity
including T-cell and B-
cell activation. The methods provided herein can be performed wherein a
library of candidate
bioactive agents are added to a plurality of cells comprising said recombinant
nucleic acid.
In yet another embodiment, a method for screening for a candidate protein
capable of binding to
SWAP70, JEST or RasGRP, is provided. It is understood that any one of these
proteins, can be used.
Said method comprises combining a nucleic acid encoding SWAP70, JEST or RasGRP
and a nucleic
acid encoding a candidate protein, wherein an identifiable marker is expressed
wherein said candidate
protein binds to said SWAP70, JEST or RasGRP.
Also provided herein is a complex consisting essentially of JEST or SWAP70 and
RasGRP. Other
aspects of the invention will be appreciated by the description that follows.
~iRIEF DESCRIPTION OF THE DRAWINGS
CA 02348733 2001-12-04
WO 00/26241 PCT/US99/25333
-5-
Figure 1 depicts a nucleic acid sequence (cDNA) which includes an embodiment
of the coding
sequence of human JEST. The start colon begins at nucleotide 541 and the stop
colon begins at
nucteotade 2434. The start and stop colons are circled.
Figures 2A-2B depict an embodiment of the coding sequence of human JEST
wherein the amino acid
sequence translation is shoum below the coding sequence.
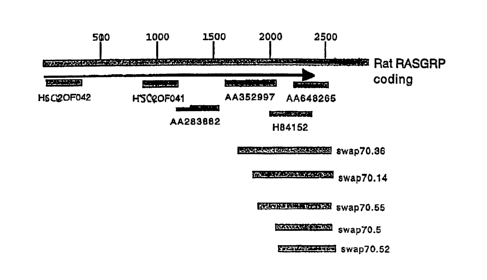
Figure 3 depicts a schematic of where human ESTs and human RasGRP fragments
provided herein
alive with rat RasGRP.
Figures 4A-4B depict a nucleic acid sequence and amino acid sequence,
respectively, for SWAP70.
Figures 5A-5B depict an amino acid alignment of SWAP70 and JEST, wherein the
SWAP70 is shown
above JEST such that SWAP70 is the query and JEST is the subject. Specific
parameters utilized for
the generation of the alignment are also shown.
Figures 6A-6D depict nucleic acid sequences of human RasGRP, wherein each of
these nucleic acids
encoded a product which binds with SWAP70. Figure 6A is shown as "swap70.14"
in Figure 3. Figure
6B is shown as "swap70.36" in Figure 3. Figure 6C is shown as "swap70.52" in
Figure 3. Figure 6D is
shown as "swap70.55" in Figure 3.
Figures 7A-7B depict a nucleic acid and amino acid sequence, respectively, of
a consensus sequence
for human RasGRP which binds to SWAP70.
The present invention provides novel lymphocyte activation prateins and
nucleic acids. In a preferred
embodiment, the lymphocyte activation proteins are from vertebrates and more
preferably from
mammals, including rodents (rats, mice, hamsters, guinea pigs, etc.),
primates, farm animals
(including sheep, goats, pigs, cows, horses, etc) and in the most preferred
embodiment, from humans.
A lymphocyte activation protein of the present invention may be identified in
several ways. "Protein" in
this sense includes proteins, polypeptides, and peptides. A lymphocyte
activation protein may be
initially identified by its association with a protein known to be involved in
T-cell and B-cell activation.
CA 02348733 2001-12-04
WO 00/2bI41 PCT/US99/25333
Lymphocyte activation proteins may be novel or may have been known in the art
to exist, but not
known to bind to SWAP or JEST or related to lymphocyte activation or
lymphocyte activation proteins.
Novel lymphocyte activation nucleic acids or lymphocyte activation proteins
are initially identified by
substantial nucleic acid and/or amino acid sequence identity or similarity to
the respective sequences
shown in the figures, Such sequence identity or similarity can be based upon
the overall nucleic acid
or amino acid sequence.
In an additional aspect, the invention provides nucleic acids encoding
lymphocyte activation proteins,
namely, those shown in the figures, and their homologues. Preferred
embodiments include JEST and
the human homologue of RasGRP.
RasGRP, guanyl nucleotide-releasing protein for the small guanosine
triphosphatase Ras, has been
shown to activate Ras and cause transformation in fibroblasts (Ebinu et al.,
Science, 280:1082-1086
(1998), incorporated herein in its entirety). Signaling of RasGRP was
associated with its partitioning in
the membrane fraction. Based on its expression in neurons and ability to
activate Ras, it may function
to promote neuron differentiation, axonal growth and synaptic plasticity. It
is now appreciated as
shown herein that RasGRP message is induced in activated T and B cells.
Moreover, the relationship
between SWAP and RasGRP shown herein indicates a novel cell activation pathway
having numerous
homologs in different cell lineages.
JEST has sequence homology to a human EST (AA306449) from a Jurkat T cell
library. The full
length sequence was derived from an activated human peripheral blood T-B cell
switching library and
from a Jurkat cDNA library. JEST has different expression pattern by Northern
than SWAP, being
most prominent in thymus, lymphoid organs, peripheral bload leukocytes and
testis. As provided
herein, JEST is involved in T cell signaling as well as mediating those
signaling events in part by
binding to RasGRP.
As discussed above, lymphocyte activation proteins include proteins which bind
to SWAP or JEST,
which are themselves considered lymphocyte activation proteins. Herein is
provided RasGRP as a
lymphocyte activation protein. In a preferred embodiment, human RasGRP is
provided.
In a preferred embodiment, a protein is a "lymphocyte activation protein" it
the overall sequence
identity of the protein sequence to any one of the amino acid sequences shown
in the figures is about
or greater than about 75°~, more preferably greater than about
80°~, even more preferably greater
CA 02348733 2001-12-04
wo oortsumcrms99ns333
will be as high as about 93 to 95 or 98%. It is understood that each sequence
identification number
provides an individual embodiment which can be selected individually or with
any combination of
members of the group. Sequence identity will be determined using standard
techniques known in the
art, including, but not limited to, the kacal sequence identity algorithm of
Smith & Waterman, Adv. Appl.
Math. 2:482 (1981), by the sequence identity alignment algorithm of Needleman
& Wunsch, J. Mol.
Biol. 48:443 (1970), by the search for similarity method of Pearson & Lipman,
PNAS USA 85:2444
(1988), by computerized implementations of these algorithms (GAP, BESTFIT,
FASTA, and TFASTA
in the Wisconsin Genetics Software Package, Genetics Computer Group, 575
Science Drive,
Madison, WI), the Best Fit sequence program described by Devereux et al.,
Nucl. Acid Res. 12:387-
395 (1984), preferably using the default settings, or by inspection.
One example of a useful algorithm is PILEUP. PILEUP creates a multiple
sequence alignment from a
group of related sequences using progressive, pairvvise alignments. It can
also plot a tree showing the
clustering relationships used to create the alignment. PILEUP uses a
simpli5cation of the progressive
alignment method of Feng & Doolittle, J. Mol. Evol. 35:351-360 (1987); ~e
method is similar to that
described by Higgins & Sharp CABIOS 5:151-153 (1989). Useful PILEUP parameters
including a
default gap weight of 3.00, a default gap length weight of 0.10, and weighted
end gaps.
Another example of a useful algorithm is the BLAST algorithm, described in
Altschul et al., J. Mol. Biol.
215, 403-410, (1990) and Karlin et al., PNAS USA 90:5873-5787 (1993). A
particularly useful BLAST
program is the WU-BLAST'-2 program which was obtained from Altschul et al.,
Methods in
logy, ~ø: 460-480 (1996); http:llblast.wustl/edu/blast/ README.html]. WU-BLAST-
2 uses
several search parameters, most of which are set to the default values. The
adjustable parameters
are set with the following values: overlap span =1, overlap fraction = 0.125,
word threshold (T) = 11,
The HSP S and HSP S2 parameters are dynamic values and are established by the
program itself
depending upon the composition of the particular sequence and composition of
the particular database
against which the sequence of interest is being searched; however, the values
may be adjusted to
increase sensitivity. A 9~° amino acid sequence identity value is
determined by the number of matching
identical residues divided by the total number of residues of the '9onger"
sequence in the aligned
region. The "longer' sequence is the one having the most actual residues in
the aligned region (gaps
introduced by WU-Blast-2 to maximize the alignment score are ignored).
In a similar manner, "percent (9~6) nucleic acid sequence identity" with
respect to the coding sequence
of the polypeptides identified herein is defined as the percentage of
nucleotide residues in a candidate
sequence that are identical with the nucleotide residues in the coding
sequence of the lymphocyte
CA 02348733 2001-12-04
WO OOIZb?,4i1 PGT/US991Z5333
_g_
activation protein. A preferred method utilizes the BLASTN module of WU-BLAST-
2 set to the default
parameters, with overlap span and overlap fraction set to 1 and 0.125,
respectively.
The alignment may include the introduction of gaps in the sequences to be
aligned. In addition, for
sequences which contain either more or fewer amino acids than the protein
shown in the Figures, it is
understood that the percentage of sequence identity will be determined based
on the number of
identical amino acids in relation to the total number of amino acids. Thus, in
one embodiment,
sequence identity of sequences shorter than that shown in the Figures, as
discussed below, will be
determined using the number of amino acids in the shorter sequence.
Lymphocyte activation proteins of the present invention may be shorter or
longer than the amino acid
sequences shown in the Figures. Thus, in a preferred embodiment, included
within the definition of
lymphocyte activation proteins are portions or fragments of the sequences
provided herein. In one
embodiment herein, fragments of lymphocyte activation proteins are considered
lymphocyte activation
proteins if a) they share at least one antigenic epitope; b) have at least the
indicated sequence identity;
c) and preferably have lymphocyte activation biological activity, including
binding to SWAP. In some
I S cases, where the sequence is used diagnostically, that is, when the
presence or absence of
lymphocyte activation protein nucleic acid is determined, only the indicated
sequence identity is
required. The nucleic acids of the present invention may also be shorter or
longer than the sequences
in the Figures. The nucleic acid fragments include any portion of the nucleic
acids provided herein
which have a sequence not exactly previously identified; fragments having
sequences with the
indicated sequence identity to that portion not previously identified are
provided in an embodiment
herein.
in addition, as is more fully outlined below, lymphocyte activation proteins
can be made that are longer
than those depicted in the Figures for example, by the addition of epitope or
purification tags, the
addition of other fusion sequences, etc.
Lymphocyte activation proteins may also be identified as being encoded by
lymphocyte activation
nucleic acids. Thus, in one embodiment, lymphocyte activation proteins are
encoded by nucleic acids
that will hybridize to any one of the complementary sequences to the sequences
depicted in the
figures. In another embodiment, any of the nucleic acid sequences in the
Figures can be utilized.
Hybridization conditions are further described below.
In a preferred embodiment, when the lymphocyte activation protein is to be
used to generate
CA 02348733 2001-12-04
WO OOn6241 iPCT/US99/Z5333
-9-
full length protein shown in the figures. By "epitope" or "determinant" herein
is meant a portion of a
protein which will generate andlor bind an antibody. Thus, in most instances,
antibodies made to a
smaller lymphocyte activation protein will be able to bind to the full length
protein. In a preferred
embodiment, the epitope is unique; that is, antibodies generated to a unique
epitope show little or no
cross-reactivity. The term "antibody" includes antibody fragments, as are
known in the art, including
Fab, Fab2, single chain antibodies (Fv for example), chimeric antibodies,
etc., either produced by the
modification of whole antibodies or those synthesized de novo using
recombinant DNA technologies.
In a preferred embodiment, the antibodies to lymphocyte activation are capable
of reducing or
eliminating the biological function of lymphocyte acfrvation proteins, as is
described below. That is, the
addition of anti-lymphocyte activation antibodies (either polyclonal or
preferably monoclonal) to
lymphocyte activation (or cells containing these proteins) may reduce or
eliminate the lymphocyte
activation activity. Generally, at feast a 25°~6 decrease in activity
is preferred, with at least about 50%
being particularly preferred and about a 95-900°~ decrease being
especially preferred.
The lymphocyte activation antibodies of the invention bind to lymphocyte
activation proteins. In a
preferred embodiment, the antibodies specifically bind to lymphocyte
activation proteins. By
"specifically bind" herein is meant that the antibodies bind to the protein
with a binding constant in the
range of at least 10''- 10$ M~', with a preferred range being 10'' -
10'° M-'. Antibodies are further
described below.
In the case of the nucleic acid, the overall sequence identity of the nucleic
acid sequence is
commensurate with amino acid sequence identity but takes into account the
degeneracy in the genetic
code and colon bias of different organisms. Accordingly, the nucleic acid
sequence identity may be
either lower or higher than that of the protein sequence. Thus the sequence
identity of the nucleic acid
sequence as compared to the nucleic acid sequences of the figures is
preferably greater than 85°~,
and preferably greater than 75%, more preferably greater than about
80°~, particularly greater than
about 85% and most preferably greater than 90%. In some embodiments the
sequence identity will be
as high as about 93 to 95 or 98%.
in a preferred embodiment, a lymphocyte activation nucleic acid encodes a
lymphocyte activation
protein. As will be appreciated by those in the art, due to the degeneracy of
the genetic code, an
extremely large number of nucleic acids may be made, all of which encode the
lymphocyte activation
proteins of the present invention. Thus, having identified a particular amino
acid sequence, those
skilled in the art could make any number of different nucleic acids, by simply
modifying the sequence
CA 02348733 2001-12-04
WO 00/Z6Z41 PCTIUS99/25333
-10-
of one or more colons in a way which does not change the amino acid sequence
of the lymphocyte
activation protein.
In one embodiment, the nucleic acid is determined through hybridization
studies. Thus, for example,
nucleic acids which hybridize under high stringency to the nucleic acid
sequences which complements
are shown in the figures and is considered a lymphocyte activation gene. High
stringency conditions
are known in the art; see for example Maniatis et al., Molecular Cloning: A
Laboratory Manual, 2d
Edition, 1989, and Short Protocols in Molecular Biology, ed. Ausubel, et al.,
both of which are hereby
incorporated by reference. Stringent conditions are sequence-dependent and
will be different in
different circumstances. Longer sequences hybridize speci0cally at higher
temperatures. An
extensive guide to the hybridization of nucleic acids is found in Tijssen,
Techniques in Biochemistry
and Molecular Biology-Hybridization with Nucleic Acid Probes, "Overview of
principles of hybridization
and the strategy of nucleic acid assays" (1993). Generally, stringent
conditions are selected to be
about 5-10°C lower than the thermal melting point (Tm) for the specific
sequence at a defined ionic
strength pH. The Tm is the temperature (under defined ionic strength, pH and
nucleic acid
concentration) at which 50% of the probes complementary to the target
hybridize to the target
sequence at equilibrium (as the target sequences are present in excess, at Tm,
50% of the probes are
occupied at equilibrium). Stringent conditions will be those in which the salt
concentration is less than
about 1.0 M sodium ion, typically abou! 0.01 to 1.0 M sodium ion concentration
(or other salts) at pH
7.0 to 8.3 and the temperature is at least about 30°C for short probes
(e.g. 10 to 50 nucleotides) and
at least about 60°C for long probes (e.g. greater than 50 nucleotides).
Stringent conditions may also
be achieved with the addition of destabilizing agents such as formamide.
In another embodiment, less stringent hybridization conditions are used; for
example, moderate or low
stringency cond'ttions may be used, as are known in the art; see Maniatis and
Ausubel, supra, and
Tijssen, supra.
The lymphocyte activation proteins and nucleic acids of the present invention
are preferably
recombinant. As used herein, "nucleic acid" may refer to either DNA or RNA, or
molecules which
contain both deoxy- and ribonucleotides. The nucleic acids include genomic
DNA, cDNA and
oligonucleotides including sense and anti-sense nucleic acids. Such nucleic
acids may also contain
modifications in the ribose-phosphate backbone to increase stability and half
life of such molecules in
physiological environments.
The nucleic acid may be double stranded, single stranded, or contain portions
of both double stranded
CA 02348733 2001-12-04
WO 00/26241 PCT/US99IZ5333
-11-
strand ("Watson') also defines the sequence of the other strand ("Crick');
thus the sequences depicted
in the Figures also include the complement of the sequence. By the term
"recombinant nucleic acid"
herein is meant nucleic acid, originally formed in vitro, in general, by the
manipulation of nucleic acid
by endonucleases, in a form not normally found in nature. Thus an isolated
lymphocyte activation
nucleic acid, in a linear form, or an expression vector formed in vitrr~ by
ligating DNA molecules that
are not normally joined, are both considered recombinant for the purposes of
this invention. It is
understood that once a recombinant nuGeic acid is made and reintroduced into a
host cell or
organism, it will replicate non-recombinantfy, i.e. using the '~~y'~ cellular
machinery of the host cell
rather than in vitro manipulations; however, such nucleic acids, once produced
recombinantly,
although subsequently replicated non-recombinantly, are still considered
recombinant for the purposes
of the invention.
Similarly, a "recombinant protein" is a protein made using recombinant
techniques, i.e. through the
expression of a recombinant nuGeic acid a5 depicted above. A recombinant
protein is distinguished
from naturally occurring protein by at least one or more characteristics. For
example, the protein may
be isolated or purified away from some or all of the proteins and compounds
with which it is nom~ally
associated in its wild type host, and thus may be substantially pure. For
example, an isolated protein
is unaccompanied by at least some of the material with which it is normally
associated in its natural
state, preferably constituting at least about 0.5%, more preferably at least
about 5°~ by weight of the
total protein in a given sample. A substantially pure protein comprises at
least about 75% by weight of
the total protein, with at least about 80% being preferred, and at least about
90°~ being particularly
preferred. The definition includes the production of a lymphocyte activation
protein from one organism
in a different organism or host cell. Alternatively, the protein may be made
at a significantly higher
concentration than is normally seen, through the use of a inducible promoter
or high expression
promoter, such that the protein is made at increased concentration levels.
Alternatively, the protein
may be in a form not normally found in nature, as in the addition of an
epitope tag or amino acid
substitutions, insertions and deletions, as discussed below.
Also included within the definition of lymphocyte activation proteins of the
present invention are amino
acid sequence variants. These variants fall into one or more of three classes:
substitutional,
insertional or deletional variants. These variants ordinarily are prepared by
site specific mutagenesis
of nucleotides in the DNA encoding the lymphocyte activation protein, using
cassette or PCR
mutagenesis or other techniques well known in the art, to produce DNA encoding
the variant, and
thereafter expressing the DNA in recombinant cell culture as outlined above.
However, variant
lymphocyte activation protein fragments having up to about 100-150 residues
may be prepared by in
CA 02348733 2001-12-04
WO OOIZ6241 PCT/US99125333
-12-
predetermined nature of the variation, a feature that sets them apart from
naturally occurring allelic or
interspecies variation of the lymphocyte activation protein amino acid
sequence. The variants typically
exhibit the same qualitative biological activity as the naturally occurring
analogue, although variants
can also be selected which have modified characteristics as will be more fully
outlined below.
While the site or region for introducing an amino acid sequence variation is
predetermined, the
mutation per se need not be predetermined. For example, in order to optimize
the performance of a
mutation at a given site, random mutagenesis may be conducted at the target
codon or region and the
expressed lymphocyte activation variants screened for the optimal combination
of desired activity.
Techniques for making substitution mutations at predetermined sites in DNA
having a known
sequence are well known, for example, M13 primer mutagenesis and PCR
mutagenesis. Screening of
the mutants is done using assays of lymphocyte activation protein activities.
Amino acid substitutions are typically of single residues; insertions usually
will be on the order of from
about 1 to 20 amino acids, although considerably larger insertions may be
tolerated. Deletions range
from about 1 to about 20 residues, although in some cases deletions may be
much larger.
Substitutions, deletions, insertions or any combination thereof may be used to
arrive at a final
derivative. Generally these changes are done on a few amino acids to minimize
the alteration of the
molecule. However, larger changes may be tolerated in certain circumstances.
When small
alterations in the characteristics of the lymphocyte activation protein are
desired, substitutions are
generally made in accordance with the following chart:
Chart I
Original Residue E~cemRlary Substitutions
Ala Ser
Arg Lys
Asn Gln, His
Asp Glu
Cys Ser
Gln ,qsn
Glu ~p
Gly Pro
His Asn, Gln
CA 02348733 2001-12-04
wo oon6m Pcrrus99ns~
-13-
Leu Ile, Val
Lys Arg, Gln, Glu
Met Leu, Ile
Phe Met, Leu, Tyr
Ser Thr
Thr Ser
Trp Tyr
Tyr Trp, Phe
Val lie, Leu
Substantial changes in function or immunological identity are made by
selecting substitutions that are
less conservative than those shown in Chart I. For example, substitutions may
be made which more
significantly affect: the structure of the polypeptide backbone in the area of
the alteration, for example
the alpha-helical or beta-sheet structun:; the charge or hydrophobicity of the
molecule at the target
S site; or the bulk of the side chain. The substitutions which in general ace
expected to produce the
greatest changes in the polypeptide's properties are those in which (a) a
hydrophilic residue, e.g. seryl
or threonyl, is substituted for (or by) a hydrophobic residue, e.g. leucyl,
isoleucyl, phenylalanyl, valyl or
alanyi; (b) a cysteine or proline is substituted for (or by) any other
residue; (c) a residue having an
electropositive side chain, e.g. lysyl, arginyt, or histidyl, is substituted
for (or by) an electronegative
residue, e.g. glutamyl or aspartyl; or (d) a residue having a bulky side
chain, e.g. phenylalanine, is
substituted for (or by) one not having a side chain, e.g. glycine.
The variants typically exhibit the same qualitative biological activity and
will elicit the same immune
response as the naturally-occurring analogue, although variants also are
selected to modify the
characteristics of the lymphocyte activation proteins as needed. Aftematively,
the variant may be
designed such that the biological activity of the lymphocyte activation
protein is altered. For example,
glycosylation sites may be altered or removed. In an embodiment provided
herein, mutations in the
JEST binding domain are made so as to modulate binding characteristics.
Covalent modifications of lymphocyte activation polypeptides are included
within the scope of this
invention. One type of covalent modification includes reacting targeted amino
acid residues of a
lymphocyte activation polypeptide with an organic derivatizing agent that is
capable of reacting with
selected side chains or the N-or C-terminal residues of a lymphocyte
activation polypeptide.
Derivatization with bifunctional agents is useful, for instance, for
crosslinking lymphocyte activation to
a water-insoluble support matrix or surface for use in the method for
purifying anti-lymphocyte
CA 02348733 2001-12-04
WO OO/Z6Z41 PCT/US99/25333
-14-
crosslinking agents include, e.g., 1,1-bis(diazoacetyl)-2-phenylethane,
glutaraldehyde, N-hydroxy-
succinimide esters, for example, esters with 4-azidosalicylic acid,
homobifunctional imidoesters,
including disuccinimidyl esters such as 3,3'-
dithiobis(succinimidylpropionate), bifunctional maleimides
such as bis-N-maleimido-1,8-octane and agents such as methyl-3-((p-
azidophenyl)dithiojpropioimi-
date.
Other modifications include deamidation of glutaminyl and asparaginyl residues
to the corresponding
glutamyl and aspartyl residues, respectively, hydroxylation of praline and
lysine, phosphorylation of
hydroxyl groups of seryl or threonyl residues, methylation of the "-amino
groups of lysine, arginine, and
histidine side chains [T.E. Greighton, Proteins: Structure and Molerw,ular
Properties, W.H. Freeman 8
Co., San Francisco, pp. 79-86 (1983)], acetylation of the N-terminal amine,
and amidat'ron of any C-
terminal carboxyl group.
Another type of covalent modification of a lymphocyte activation polypeptide
included within the scope
of this invention comprises altering the native glycosylation pattern of the
polypeptide. "Altering the
native glycosylation pattern" is intended for purposes herein to mean deleting
one or more
carbohydrate moieties found in native sequence lymphocyte activation
polypeptide, and/or adding one
or more glycosylation sites that are not present in the native sequence
lymphocyte activation
polypeptide.
Addition of glycosylation sites to lymphocyte activation polypeptides may be
accomplished by altering
the amino acid sequence thereof. The alteration may be made, for example, by
the addition of, or
substitution by, one or more serine or threonine residues to the native
sequence lymphocyte activation
polypeptide (for O-linked glycosylation sites). A lymphocyte activation amino
acid sequence may
optionally be altered through changes at the DNA level, particularly by
mutating the DNA encoding a
lymphocyte activation polypeptide at preselected bases such that codons are
generated that will
translate into the desired amino acids.
Another means of increasing the number of carbohydrate moieties on a
lymphocyte activation
polypeptide is by chemical or enzymatic coupling of glycosides to the
polypeptide. Such methods are
described in the art, e.g., in WO 87/05330 published 11 September 1987, and in
Aplin and Wriston,
CRC Crit. Rey B~f~em., pp. 259-306 (1987).
Removal of carbohydrate moieties present on a lymphocyte activation
polypeptide may be
accomplished chemically or enzymatically or by mutational subsfttution of
codons encoding for amino
CA 02348733 2001-12-04
WO 00lZ6241 PCT/US99l15333
-15-
in the art and described, for instance, by Hakimuddin, et al., Arch. Biochem.
Bioohvs., x:52 (1987)
and by Edge et al., (ipal. Bioghem., u$:131 (1981). Enzymatic cleavage of
carbohydrate moieties on
polypeptides can be achieved by the use of a variety of endo-and exo-
glycosidases as described by
Thotakura et al., Seth. Enzvmol., y~$:350 (1987).
Another type of covalent modahcation of lymphocyte activation comprises
linking a lymphocyte
activation polypeptide to one of a variety of nonproteina~ceous polymers,
e.g., polyethylene glycol,
polypropylene glycol, or polyoxyalkylenes, in the manner set forth in U.S.
Patent Nos. 4,640,835;
4,496,689; 4,301,144; 4,670,417; 4,791,192 or 4,179,337.
Lymphocyte activation polypeptldes of the present invention may also be
modified in a way to form
chimeric molecules comprising a lymphocyte activation polypeptide fused to
another, heterologous
polypeptide or amino acid sequence. In one embodiment, such a chimeric
molecule comprises a
fusion of a lymphocyte activation polypeptide with a tag polypeptide which
provides an epitope to
which an anti-tag antibody can selectively bind. The epitope tag is generally
placed at the amino-or
carboxyhterminus of a lymphocyte activation polypeptide. The presence of such
epitope-tagged forms
of a lymphocyte activation polypeptide can be detected using an antibody
against the tag polypeptide.
Also, provision of the epitope tag enables a lymphocyte activation polypeptide
to be readily purified by
affinity purification using an anti-tag antibody or another type of affinity
matrix that binds to the epitope
tag. In an alternative, embodiment, the chimeric molecule may comprise a
fusion of a lymphocyte
activation polypeptide with an immunoglobulin or a particular region of an
immunoglobulin. For a
bivalent form of the chimeric molecule, such a fusion could be to the Fc
region of an IgG molecule as
discussed further below.
Various tag polypeptides and their respective antibodies are well known in the
art. Examples include
poly-histidine (poly-his) or poly-histidine-glycine (poly-his-gly) tags; the
flu HA tag poiypeptide and its
antibody 12CA5 [Field et al., MoI. Cell. 8iol., $:2159-2165 (1988)]; the c-myc
tag and the 8F9, 3C7,
6E10, G4, B7 and 9E10 antibodies thereto [Evan et al., Molecular and Cellular
Bioloav, $:3610-3616
(1985)]; and the Herpes Simplex virus glycoprotein D (gD) tag and its antibody
[Paborsky et al.,
Protein Engineering, $(6):547-553 (1990)]. Other tag polypeptides include the
Fiag-peptide [Hopp et
al., BioTechnoloav, $:1204-1210 (1988)]; the KT3 epitope peptide [Martin et
al., ~jg~~g, x:192-194
(1992)]; tubulin epitope peptide [Skinner et al., ,I. Bi2. Chem., 2$ø:15163-
15166 (1991 )]; and the T7
gene 10 protein peptide tag [Lutz-Freyermuth et al., Proc. Natl. Acad. Sci.
USA, ~:6393~397 (1990)].
In an embodiment herein, lymphocyte activation proteins of the lymphocyte
activation family and
CA 02348733 2001-12-04
wo oon6m pcrms~ns333
-16-
Thus, probe or degenerate poiymerase chain n~action (PCR) primer sequences may
be used to end
other related lymphocyte activation proteins from humans or other organisms.
As will be appreciated
by those in the art, particularly useful probe andlor PCR primer sequences
include the unique areas of
a lymphocyte activation nucleic acid sequence. As is generally known in the
art, preferred PCR
primers are from about 15 to about 35 nucleotides in length, with from about
20 to about 30 being
preferred, and may contain inosine as needed. The conditions for the PCR
reaction are well known in
the art. It is therefore also understood that provided along with the
sequences listed herein are
portions of those sequences, wherein unique portions of 15 nucleotides or more
are particularly
preferred. The skilled artisan can routinely synthesize or cut a nucleotide
sequence to the desired
length.
Once a lymphocyte activation nucleic acid is identified, it can be cloned and,
if necessary, its
constituent parts recombined to form an entire full length or mature
lymphocyte activation nucleic acid.
Wherein the full length nucleic acid has a signal peptide and/or transmembrane
region(s), it can be
modified to exclude one or more of these regrons so as to encode a peptide in
its mature soluble form.
Once isolated from its natural source, e.g., contained within a piasmid or
other vector or excised
therefrom as a linear nucleic acid segment, the recombinant lymphocyte
activation nucleic acid can be
further-used as a probe to identify and isolate other lymphocyte activatron
nucleic acids. It can also be
used as a "precursor" nucleic acid to make modified or variant lymphocyte
activation nucleic acids and
proteins. The skilled artisan understands that wherein two or more nucleic
acids overlap, the
overlapping portions) of one of the overlapping nucleic acids can be omitted
and the nucleic acids
combined for example by ligation to form a longer linear lymphocyte activation
nucleic acid so as to,
for example, encode the full length or mature peptide. The same applies to the
amino acid sequences
of lymphocyte activation polypeptides in that they can be combined so as to
form one contiguous
peptide.
Using the nucleic acids of the present invention which encode a lymphocyte
activation protein, a
variety of expression vectors are made. The expression vectors may be either
self-replicating
extrachromosomal vectors or vectors which integrate into a host genome.
Generally, these
expression vectors include transcriptional and translational regulatory
nucleic acid operably (inked to
the nucleic acid encoding a lymphocyte activation protein. The term "control
sequences" refers to
DNA sequences necessary for the expression of an operably linked coding
sequence in a particular
host organism. The control sequences that are suitable for prokaryotes, for
example, include a
promoter, optionally an operator sequence, and a ribosome binding site.
Eukaryotic cells are known to
utilize promoters, polyadenylation signals, and enhancers.
CA 02348733 2001-12-04
WO 00/26241 PGT/US99/25333
-17-
Nucleic acid is "operably linked" when it is placed into a functional
relationship with another nucleic
acid sequence. For example, DNA for a presequence or secretory leader is
operably linked to DNA
for a polypeptide if it is expressed as a pneprotein that participates in the
secretion of the polypeptide;
a promoter or enhancer is operably linked to a coding sequence if it affects
the transcription of the
sequence; or a ribosome binding site is operably linked to a coding sequence
if it is positioned so as to
facilitate translation. Generally, "operably linked" means that the DNA
sequences being linked are
contiguous, and, in the case of a secretory leader, contiguous and in reading
phase. However,
enhancers do not have to be contiguous. Linking is accomplished by ligation at
convenient restriction
sites. If such sites do not exist, the synthetic oligonucleotide adaptors or
linkers are used in
accordance with conventional practice. The transcriptionai and translational
regulatory nucleic acid
will generally be appropriate to the host cell used to express a lymphocyte
activation protein; for
example, transcriptional and translational regulatory nucleic acid sequences
from Bacillus are
preferably used to express a lymphocyte activation protein in Bacillus.
Numerous types of appropriate
expression vectors, and suitable regulatory sequences are known in the art for
a variety of host cells.
In general, the transcriptionai and translational regulatory sequences may
include, but are not limited
to, promoter sequences, ribosomal binding sites, transcriptional start and
stop sequences,
translational start and stop sequences, and enhancer or activator sequences.
In a preferred
embodiment, the regulatory sequences include a promoter and transcriptional
start and stop
sequences.
Promoter sequences encode either constitutive or inducible promoters. The
promoters may be either
naturally occurring promoters or hybrid promoters, Hybrid promoters, which
combine elements of
more than one promoter, are also known in the art, and are useful in the
present invention.
In addition, the expression vector may comprise additional elements. For
example, the expression
vector may have two replication systems, thus allowing it to be maintained in
two organisms, for
example in mammalian or insect cells for expression anti in a procaryotic host
for cloning and
amplification. Furthermore, for integrating expression vectors, the expression
vector contains at least
one sequence homologous to the host cell genome, and preferably two homologous
sequences which
flank the expression construct. The integrating vector may be directed to a
specfic locus in the host
cell by selecting the appropriate homologous sequence for inclusion in the
vector. Constructs for
integrating vectors are well known in the art. Preferred methods to effect
homologous recombination
are described in PCT US93103868 and PCT US98105223, hereby incorporated by
reference.
CA 02348733 2001-12-04
WO 110n6241 IPGT/US99l25333
-18-
In addition, in a preferred embodiment, the expression vector contains a
selectable marker gene to
allow the selection of transformed host cells. Selection genes are well known
in the art and will vary
with the host cell used.
A preferred expression vector system is a retroviral vector system such as is
generally described in
PCT/US97I01019 and PCTIUS97/01048, both of which are hereby expressly
incorporated by
reference.
The lymphocyte activation proteins of the present invention are produced by
culturing a host cell
transformed with an expression vector containing nucleic acid encx~ding a
lymphocyte activation
protein, under the appropriate conditions to induce or cause expression of a
lymphocyte activation
protein. The conditions appropriate for lymphocyte activation protein
express'ron will vary with the
choice of the expression vector and the host cell, and will be easily
ascertained by one skilled in the
art through routine experimentation. For example, the use of constitutive
promoters in the expression
vector will require optimizing the growth and proliferation of the host cell,
while the use of an inducible
promoter requires the appropriate growth conditions for induction. In
addition, in some embodiments,
the timing of the harvest is important. For example, the baculoviral systems
used in insect cell
expression are lytic viruses, and thus harvest time selection can be crucial
for product yield.
Appropriate host cells include yeast, bacteria, archebacteria, fungi, and
insect and animal cells,
including mammalian cells. Of particular interest are Drosophila melangasfer
cells, Saccharomyces
cerevisiae and other yeasts, E. coli, Bacillus subtilis, SF9 cells, C129
cells, 293 cells, Neurospora,
BHK, CHO, COS, and HeLa cells, fibroblasts, Schwanoma cell lines, immortalized
mammalian myeloid
and lymphoid cell lines, Jurkat cells, neuronal cells, and those from the
spleen, thymus, prostate,
testes, uterus, colon, small intestine, PBL, lymph nodes, bone marrow and
liver, and all cells involved
in the immune system.
in a preferred embodiment, the lymphocyte activation proteins are expressed in
mammalian cells.
Mammalian expression systems are also known in the art, and include retroviral
systems. A
mammalian promoter is any ONA sequence capable of binding mammalian RNA
polymerise and
initiating the downstream (3') transcription of a coding sequence for
lymphocyte activation protein into
mRNA. A promoter will have a transcription initiating region, which is usually
placed proximal to the 5'
end of the coding sequence, and a TATA box, using a located 25-30 base pairs
upstream of the
transcription initiation site. The TATA box is thought to direct RNA
polymerise II to begin RNA
synthesis at the correct site. A mammalian promoter will also contain an
upstream promoter element
CA 02348733 2001-12-04
WO 00/26141 PCTIUS99/Z5333
-19-
upstream promoter element determines the rate at which transcription is
initiated and can act in either
orientation. Of particular use as mammalian promoters are the promoters from
mammalian viral
genes, since the viral genes are often highly expressed and have a broad host
range. Examples
include the SV40 early promoter, mouse mammary tumor virus l_TR promoter,
adenovirus major late
promoter, herpes simplex virus promoter, and the CMV promoter.
Typically, transcription termination and polyadenylation sequences recognized
by mammalian cells are
regulatory regions located 3' to the translat'ron stop colon and thus,
together with the promoter
elements, flank the coding sequence. The 3' terminus of the mature mRNA is
formed by site-specific
post-translational cleavage and polyadenytation. Examples of transcription
terminator and
polyadenlytion signals include those derived form SV40.
The methods of introducing exogenous nucleic acid into mammalian hosts, as
weft as other hosts, is
well known in the art, and will vary with the host cell used. Techniques
include dextran-mediated
transfection, calcium phosphate precipitation, polybrene mediated
transfection, protoplast fusion,
electroporation, viral infection, encapsulation of the poiynucleotide(s) in
liposomes, and direct
microinjection of the DNA into nuclei.
In a preferred embodiment, lymphocyte activation proteins are expressed in
bacterial systems.
Bacterial expression systems are well known in the art.
A suitable bacterial promoter is any nucleic acid sequence capable of binding
bacterial RNA
polymerise and initiating the downstream (3') transcription of the coding
sequence of lymphocyte
activation protein into mRNA. A bacterial promoter has a transcription
initiation region which is usually
placed proximal to the 5' end of the coding sequence. This transcription
initiation region typically
includes an RNA polymerise binding site and a transcription initiation site.
Sequences encoding
metabolic pathway enzymes provide particularly useful promoter sequences.
Examples include
promoter sequences derived from sugar metabolizing enzymes, such as galactose,
lactose and
maltose, and sequences derived from biosynthetic enzymes such as tryptophan.
Promoters from
bacteriophage may also be used and are known in the art. In addition,
synthetic promoters and hybrid
promoters are also useful; for example, the tic promoter is a hybrid of the
trp and lac promoter
sequences. Furthermore, a bacterial promoter can include naturally occurring
promoters of non-
bacterial origin that have the ability to bind bacterial RNA polymerise and
initiate transcription.
In addition to a functioning promoter sequence, an efficient ribosome binding
site is desirable. In E.
CA 02348733 2001-12-04
WO 00/26241 PCTNS99/ZS333
-20-
colon and a sequence 3-9 nuGeotides in length located 3 -11 nucleotides
upstream of the initiation
colon.
The expression vector may also include a signal peptide sequence that provides
for secretion of a
lymphocyte activation protein in bacteria. The signal sequence typically
encodes a signal peptide
comprised of hydrophobic amino acids which direct the secretion of the protein
from the cell, as is well
known in the art. The protein is either secreted into the growth media (gram-
positive bacteria) or into
the periplasmic space, located between the inner and outer membrane of the
cell (gram-negative
bacteria).
The bacterial expression vector may also include a selectable marker gene to
allow for the selection of
bacterial strains that have been transformed. Suitable selection genes include
genes which render the
bacteria resistant to drugs such as ampicillin, chloramphenicol, erythromycin,
kanamycin, neomycin
and tetracycline. Selectable markers also include biosynthetic genes, such as
those in the histidine,
tryptophan and leucine biosynthetic pathways.
These components are assembled into expression vectors. Expression vectors for
bacteria are well
known in the art, and include vectors for Bacillus subtilis, E. coli,
Streptococcus cremoris, and
Stnsptococcus lividans, among others.
The bacterial expression vectors are transformed into bacterial host cells
using techniques well known
in the art, such as calcium chloride treatment, electroporation, and others.
In one embodiment, lymphocyte activation proteins are produced in insect
cells. Expression vectors
for the transformation of insect cells, and in particular, baculovirus-based
expression vectors, are well
known in the art.
In a preferred embodiment, lymphocyte activation proteins are produced in
yeast cells. Yeast
expression systems are well known in the art, and include expression vectors
for Saccharomyces
cerevisiae, Candida albicans and C. maltosa, Hansenula polymoipha,
Kluyveromyces fragilis and K.
lactis, Pichia guillerimondii and P. pastoris, Schizosaccharomyces pombe, and
Yarrowia lipolytica.
Preferred promoter sequences for expression in yeast include the inducibte
GAI_1,10 promoter, the
promoters from alcohol dehydrogenase, enolase, glucokinase, glucose-6-
phosphate isomerase,
glyceraldehyde-3-phosphate-dehydrogenase, hexokinase, phosphofructokinase, 3-
phosphoglycerale
mutase, pyruvate kinase, and the acid phosphatase gene. Yeast selectable
markers include ADE2,
CA 02348733 2001-12-04
WO 011/26241 PCT/US99/Z5333
-21-
phosphotransferase gene, which confers resistance to 6418; and the CUP1 gene,
which allows yeast
to grow in the presence of copper ions.
A lymphocyte activation protein may also be made as a fusion protein, using
techniques well known in
the art. Thus, for example, for the creation of monoclonal antibodies, if the
desired epitope is small,
the lymphocyte activation protein may be fused to a carrier protein to form an
immunogen.
Alternatively, a lymphocyte activation protein may be made as a fusion protein
to increase expression,
or for other masons. For example, when a lymphocyte activation protein is a
lymphocyte activation
peptide, the nucleic acid encoding the peptide may be linked to other nucleic
acid for expression
purposes. Similarly, lymphocyte activation proteins of the invention an be
linked to protein labels, such
as green fluorescent protein (GFP), red fluorescent protein (RFP), yellow
fluorescent protein (YFP),
blue fluorescent protein (BFP), etc.
In one embodiment, the lymphocyte activation nucleic acids, proteins and
antibodies of the invention
are labeled. By "labeled" herein is meant that a compound has at least one
element, isotope or
chemical compound attached to enable the detection of the compound. In
general, labels fall into
three classes: a) isotopic labels, which may be radioactive or heavy isotopes;
b) immune labels, which
may be antibodies yr antigens; and c) colored or fluorescent dyes. The labels
may be incorporated
into the compound at any position.
In a preferred embodiment, a lymphocyte activation protein is purified or
isolated after expression.
lymphocyte activation proteins may be isolated or purified in a variety of
ways known to those skilled in
the art depending on what other components are present in the sample. Standard
purification
methods include electrophoretic, molecular, immunological and chromatographic
techniques, including
ion exchange, hydrophobic, affinity, and reverse-phase HPLC chromatography,
and
chromatofocusing. For example, a lymphocyte activation protein may be purified
using a standard
anti-lymphocyte activation antibody column. Ultrafiltration and diaflltration
techniques, in conjunction
with protein concentration, are also useful. For general guidance in suitable
purification techniques,
see Scopes, R., Protein Pur~cation, Springer-Verlag, NY (1982). The degree of
purification
necessary will vary depending on the use of the lymphocyte activation protein.
In some instances no
purification will be necessary.
Once expressed and purified if necessary, the lymphocyte activation proteins
and nucleic acids are
useful in a number of applications.
CA 02348733 2001-12-04
WO 00!'16241 PGT/US99/25333
-22-
The nucleotide sequences (or their complement) encoding lymphocyte activation
proteins have
various applications in the art of molecular biology, including uses as
hybridization probes, in
chromosome and gene mapping and in the generation of anti-sense RNA and DNA.
lymphocyte
activation protein nucleic acids wilt also be useful for the preparation of
lymphocyte activation protein
polypeptides by the recombinant techniques described herein.
A full-length native sequence lymphocyte activation protein gene, or portions
thereof, may be used as
hybridization probes for a cDNA library to isolate a full-length lymphocyte
activation protein gene or to
isolate still other genes (for instance, those encoding naturally-occurring
variants of a lymphocyte
activation protein or a lymphocyte activation protein from other species)
which have a desired
sequence identity to a lymphocyte activation protein coding sequence.
Optionally, the length of the
probes will be about 20 to about 50 bases. The hybridization probes may be
derived hom the
nucleotide sequences herein or from genomic sequences including promoters,
enhancer elements and
introns of native sequences as provided herein. By way of example, a screening
method will comprise
isolating the coding region of a lymphocyte activation protein gene using the
known DNA sequence to
synthesize a selected probe of about 40 bases. Hybridization probes may be
labeled by a variety of
labels, including radionucleotides such as ~P or ~S, or enzymatic labels such
as alkaline phosphatase
coupled to the probe via avidin/biotin coupling systems. Labeled probes having
a sequence
complementary to that of a lymphocyte activation protein gene of the present
invention can be used to
screen libraries of human cDNA, genomic DNA or rnRNA to determine to which
members of such
libraries the probe hybridizes.
The probes may also be employed in PCR techniques to generate a pool of
sequences for
identification of closely related lymphocyte activation protein coding
sequences.
Nucleotide sequences encoding a lymphocyte activation protein can also be used
to construct
hybridization probes for mapping the gene which encodes that lymphocyte
activation protein and for
the genetic analysis of individuals with genetic disorders. The nuGeotide
sequences provided herein
may be mapped to a chromosome and specific regions of a chromosome using known
techniques,
such as in situ hybridization, linkage analysis against known chromosomal
markers, and hybridization
screening with libraries.
Nucleic acids which encode lymphocyte activation proteins or their modified
forms can also be used to
generate either transgenic animals or "knock out' animals which, in turn, are
useful in the development
and screening of therapeutically useful reagents. A non-human transgenic
animal (e.g., a mouse or
CA 02348733 2001-12-04
WO 00/261A1 PCT/US99/25333
-23-
or an ancestor of the animal at a prenatal, e.g., an embryonic stage. A
transgene is a DNA which is
integrated into the genome of a cell from which a transgenic animal develops.
In one embodiment,
cDNA encoding a lymphocyte activation protein can be used to clone genomic DNA
encoding a
lymphocyte activation protein in accordance with established techniques and
the genomic sequences
used to generate transgenic animals that contain cells which express the
desired DNA. Methods for
generating transgenic animals, particularly animals such as mice or rats, have
become conventional in
the art and are described, for example, in U.S. Patent Nos. 4,736,866 and
4,870,009. Typically,
particular cells would be targeted for a lymphocyte activation protein
transgene incorporation with
tissue-specific enhancers. Transgenic animals that include a copy of a
transgene encoding a
lymphocyte activation protein introduced into the germ fine of the anima! at
an embryonic stage can be
used to examine the effect of increased expression of the desired nucleic
acid. Such animals can be
used as tester animals for reagents thought to confer protection from, for
example, pathological
conditions associated with its overexpression. fn accordance with this facet
of the invention, an animal
is treated with the reagent and a reduced incidence of the pathological
condition, compared to
untreated animals bearing the transgene, would indicate a potential
therapeutic intervention for the
pathological condition.
Alternatively, non-human homologues of a lymphocyte activation protein can be
used to construct a
lymphocyte activation protein "knock out" animal which has a defective or
altered gene encoding a
lymphocyte act'rvatron protein as a result of homologous recombination between
the endogenous gene
encoding a lymphocyte activation protein and altered genomic DNA encoding a
lymphocyte activation
protein introduced into an embryonic cell of the animal. For example, cDNA
encoding a lymphocyte
activation protein can be used to clone genomic DNA encoding a lymphocyte
activation protein in
accordance with established techniques. A portion of the genomic DNA encoding
a lymphocyte
activation protein can be deleted or replaced with another gene, such as a
gene encoding a selectable
marker which can be used to monitor integration. Typically, several kilobases
of unaltered flanking
DNA (both at the 5' and 3' ends) are included in the vector [see e.g., Thomas
and Capecchi, fig[,
x:503 (1987) for a description of homologous recombination vectorsj. The
vector is introduced into
an embryonic stem cell line (e.g., by electroporation) and cells in which the
introduced DNA has
homologously recombined with the endogenous DNA are selected [see e.g., Li et
al., II, X9:915
(1992)). The selected cells are then injected into a blastocyst of an animal
(e.g., a mouse or rat) to
farm aggregation chimeras [see e.g., Bradley, in Terafocan:inomas and
Embryonic Stem Cells: A
Practical Approach, ~. J. Robertson, ed. (IRL, Oxford, 1987), pp. 113-152). A
chimeric embryo can
then be implanted into a suitable pseudopregnant female foster animal and the
embryo brought to
term to create a "knock out" animal. Progeny harboring the homologously
recombined DNA in their
CA 02348733 2001-12-04
WO OOI26241 PCT/US99i25333
-24-
animal contain the homologously recombined DNA. Knockout animals can be
characterized for
instance, for their ability to defend against certain pathological conditions
and for their development of
pathological conditions due to absence of a lymphocyte activation protein
polypeptide.
Nucleic acids encoding lymphocyte activation polypeptides, antagonists or
agonists may also be used
in gene therapy. In gene therapy applications, genes are introduced into cells
in order to achieve in
vivo synthesis of a therapeutically effective genetic product, for example for
replacement of a defective
gene. 'Gene therapy' includes both conventional gene therapy where a lasting
effect is achieved by a
single treatment, and the administration of gene therapeutic agents, which
involves the one time or
repeated administration of a therapeutically effective DNA or mRNA. Antisense
RNAs and DNAs can
be used as therapeutic agents for blocking the expression of certain genes in
vivo. it has already
been shown that short antisense oligonucleotides can be imported into cells
where they act as
inhibitors, despite their low intracellular concentrations caused by their
restricted uptake by the cell
membrane. (Zamecnik et al., eroc. ~I~,O1~[,_Acad. Sci. USA ~, 4143-4146
[1986]). The oligonucleotides
can be modified to enhance their uptake, e.g. by substituting their negatively
charged phosphodiester
groups by uncharged groups.
There are a variety of techniques available for introducing nucleic acids into
viable cells. The
techniques vary depending upon whether the nucleic acid is transferred into
cultured cells in vitro, or in
vivo in the cells of the intended host. Techniques suitable for the transfer
of nucleic acid into
mammalian cells in vitro include the use of liposomes, electroporation,
microinjection, cell fusion,
DEAE-dextran, the catcium phosphate precipitation method, etc. The currently
preferred in vivo gene
transfer techniques include transfection with viral (typically retroviral)
vectors and viral coat protein-
liposome mediated transfection (Dzau ef al., Trends in Biotechnoloav y1, 205-
210 [1993]). In some
situations it is desirable to provide the nucleic acid source with an agent
that targets the target cells,
such as an antibody specific for a cell surface membrane protein or the target
cell, a ligand for a
receptor on the target cell, etc. Where iiposomes are employed, proteins which
bind to a cell surface
membrane protein associated with endocytosis may be used for targeting andlor
to facilitate uptake,
e.g. capsid proteins or fragments thereof tropic for a particular cell type,
antibodies for proteins which
undergo internalization in cycling, proteins that target intracellular
localization and enhance
intracellular half-life. The technique of receptor-mediated endocytosis is
described, for example, by
Wu et al., J. Biol. Chem. ~. 4429-4432 (1987); and Wagner ef al., Proc. Natl.
Acad. Sci. USA ~7,
3410-3414 (1990). For review of gene marking and gene therapy protocols see
Anderson et al.,
Science Z~ø, 808-813 (1992).
CA 02348733 2001-12-04
WO 00126241 PCT/US99/25333
-25-
In a preferred embodiment, the lymphocyte activation proteins, nucleic acids,
modified proteins and
cells containing the native or modified lymphocyte activation proteins are
used in screening assays.
Identification of this important T-cell and B-cell activation protein permits
the design of drug screening
assays for compounds that modulate lymphocyte activation activity.
Screens may be designed to first find candidate agents that can bind to
lymphocyte activation
proteins, and then these agents may be used in assays that evaluate the
ability of the candidate agent
to modulate lymphocyte activation activity. Thus, as will be appreciated by
those in the art, there are a
number of different assays which may be run; binding assays and activity
assays.
Thus, in a preferred embodiment, the methods comprise combining a lymphocyte
activation protein
and a candidate bioactive agent, and determining the binding of the candidate
agent to the lymphocyte
activation protein. Preferred embodiments utilize a human lymphocyte
activation protein, although
other mammalian proteins may also be used, including rodents (mice, rats,
hamsters, guinea pigs,
etc.), farm animals (cows, sheep, pigs, horses, etc.) and primates. These
latter embodiments may be
preferred in the development of animal models of human disease. In some
embodiments, as outlined
herein, variant or derivative Lymphocyte activation proteins may be used,
including deletion lymphocyte
activation proteins as outlined above.
The term "candidate bioactive agent" or "exogeneous compound" as used herein
describes any
molecule, e.g., protein, oligopeptide, small organic molecule, polysaccharide,
polynucleotide, etc., with
the capability of directly or indirectly altering the bioactivity of
Lymphocyte activation protein.
Generally a plurality of assay mixtures are run in parallel with different
agent concentrations to obtain a
differential response to the various concentrations. Typically, one of these
concentrations serves as a
negative control, i.e., at zero concentration or below the level of detection.
Candidate agents encompass numerous chemical classes, though typically they
are organic
molecules, preferably small organic compounds having a molecular weight of
more than 100 and less
than about 2,500 daltons. Candidate agents comprise functional groups
necessary for structural
interaction with proteins, particularly hydrogen bonding, and typically
include at least an amine,
carbonyl, hydroxyl or carboxyl group, preferably at least two of the
functional chemical groups. The
candidate agents often comprise cyclical carbon or heterocyclic structures
andlor aromatic or
poiyaromatic structures substituted with one or more of the above functional
groups. Candidate
agents are also found among biomolecules including peptides, saccharides,
fatty acids, steroids,
purines, pyrimidines, derivatives, structural analogs or combinations thereof.
Particularly preferred are
CA 02348733 2001-12-04
WO 00l2624I PCT/US99lZ5333
-26-
Candidate agents are obtained from a wide variety of sources including
libraries of synthetic or natural
compounds. For example, numerous means are available for random and directed
synthesis of a
wide variety of organic compounds and biomolecules, including expression of
randomized
oligonucleotides. Aftematively, libraries of natural compounds in the form of
bacterial, fungal, plant
and animal extracts are available or readily produced. Additionally, natural
or synthetically produced
libraries and compounds are readily modfied through conventional chemical,
physical and biochemical
means. Known pharmacological agents may be subjected to directed or random
chemical
mod~cations, such as acylation, alkylation, esterification, amidification to
produce structural analogs.
In a preferred embodiment, the candidate bioactive agents are proteins. By
"protein" herein is meant
at least two covalently attached amino acids, which includes proteins,
polypeptides, oligopeptides and
peptides. The protein may be made up of naturally occurring amino acids and
peptide bonds, or
synthetic peptidomimetic structures. Thus "amino acid", or "peptide residue",
as used herein means
both naturally occurring and synthetic amino acids. For example, homo-
phenylalanine, citrulline and
noreleucine are considered amino acids for the purposes of the invention.
"Amino acid" also includes
imino acid residues such as praline and hydroxyproline. The side chains may be
in either the (R} or
the (S} configuration. In the preferred embodiment, the amino acids are in the
(S) or L-configuration.
If non-naturally occurring side chains are used, non-amino acid substituents
may be used, for example
to prevent or retard in vivo degradations.
In a preferred embodiment, the candidate bioactive agents are naturally
occuring proteins or
fragments of naturally occuring proteins. Thus, for example, cellular extracts
containing proteins, or
random or directed digests of proteinaceous cellular extracts, may be used. In
this way libraries of
procaryotic and eucaryotic proteins may be made for screening against
Lymphocyte activation protein.
Particularly preferred in this embodiment are libraries of bacterial, fungal,
viral, and mammalian
proteins, with the latter being preferred, and human proteins being especially
preferred.
In a preferred embodiment, the candidate bioactive agents are peptides of from
about 5 to about 30
amino acids, with from about 5 to about 20 amino acids being preferred, and
from about 7 to about 15
being particularly preferred. The peptides may be digests of naturally
occuring proteins as is outlined
above, random peptides, or "biased" random peptides. By "randomized" or
grammatical equivalents
herein is meant that each nucleic acid and peptide consists of essentially
random nucleotides and
amino acids, respectively. Since generally these random peptides (or nucleic
acids, discussed below)
are chemically synthesized, they may incorporate any nucleotide or amino acid
at any position. The
synthetic process can be designed to generate randomized proteins or nucleic
acids, to allow the
CA 02348733 2001-12-04
WO 00/26241 )pGT/US991Z5333
_27_
formation of all or most of the possible combinations over tkte length of the
sequence, thus forming a
library of randomized candidate bioactive proteinaceous agents.
In one embodiment, the library is fully randomized, with no sequence
preferences or constants at any
position. In a preferred embodiment, the library is biased. That is, some
positions within the sequence
are either held constant, or are selected from a limited number of
possibilities. For example, in a
preferred embodiment, the nucleotides or amino acid residues are randomized
within a defined class,
for example, of hydrophobic amino acids, hydrophilic residues, sterically
biased (either small or large)
residues, towards the creation of cysteines; for cross-linking, pralines for
SH-3 domains, serines,
threonines, tyrosines or histidines for phosphorylation sites, etc., or to
purines, etc.
In a preferred embodiment, the candidate bioactive agents are nucleic acids.
By "nucleic acid" or
"oligonucleotide" or grammatical equivalents herein means at least two
nucleotides cavalently linked
together. A nucleic acid of the present invention will generally contain
phosphodiester bonds, although
in some cases, as outlined below, nucleic acid analogs are included that may
have alternate
backbones, comprising, for example, phosphoramide (Beaucage et al.,
Tetrahedron 49(10):1925
(1993) and references therein; Letsinger, J. Org. Chem. 35:3800 (1970);
Sprinzl et al., Eur. J.
Biochem. 81:579 (1977); Letsinger et al., Nucl. Acids Res. 14:3487 (1986);
Sawai et al, Chem. Lett.
805 (1984), Letsinger et al., J. Am. Chem. Soc_ 110:4470 (1988); and Pauwels
et al., Chemica Scripts
26:141 91986)), phosphorothioate (Mag et al., Nucleic Acids Res. 19:1437 (1991
); and U.S. Patent
No. 5,644,048), phosphorodithioate (Briu et al., J. Am. Chem. Soc. 111:2321
(1989), O-
methyiphophoroamidite linkages (see Eckstein, Oligonucleotides and Analogues:
A Practical
Approach, Oxford University Press), and peptide nucleic acid backbones and
linkages (see Egholm, J.
Am. Chem. Soc. 114:1895 (1992); Meier et al., Chem. Int. Ed. Engl. 31:1008
(1992); Nielsen, Nature,
365:566 (1993); Carlsson et al., Nature 380:207 (1996), all of which are
incorporated by reference).
Other analog nucleic acids include those with positive backbones (Denpcy et
al., Proc. Natl. Acad. Sci.
USA 92:6097 (1995); non-ionic backbones (U.S. Patent Nos. 5.386.023,
5,637,684, 5,602,240,
5,216,141 and 4,469,863; Kiedrowshi et al., Angew. Chem. Intl. Ed. English
30:423 (1991); Letsinger
et al., J. Am. Chem. Soc. 110:4470 (1988); Letsinger et al., Nucleoside &
Nucleotide 13:1597 (1994);
Chapters 2 and 3, ASC Symposium Series 580, "Carbohydrate Modifications in
Antisense Research",
Ed. Y.S. Sanghui and P. Dan Cook; Mesmaeker et al., Bioorganic 8 Medicinal
Chem. Lett. 4:395
(1994); Jeffs et al., J. Biomolecular NMR 34:17 (1994); Tetrahedron Lett.
37:743 (1996)) and non-
ribose backbones, including those described in U.S. Patent Nos. 5,235,033 and
5,034,506, and
Chapters 6 and 7, ASC Symposium Series 580, "Carbohydrate Modfications in
Antisense Research',
Ed. Y.S. Sanghui and P. Dan Cook. Nucleic acids containing one or more
carbocyclic sugars are also
CA 02348733 2001-12-04
WO 00!26241 PCT/US99/x5333
-28-
176). Several nucleic acid analogs are described in Rawls, C 8~ E News June 2,
1997 page 35. All of
these references are hereby expressly incorporated by reference. These
modifications of the ribose-
phosphate backbone may be done to facilitate the addition of additional
moieties such as labels, or to
increase the stability and half-life of such molecules in physiological
environments. In addition,
mixtures of naturally occurring nucleic acids and analogs can be made.
Alternatively, mixtures of
different nucleic acid analogs, and mixtures of naturally occuring nucleic
acids and analogs may be
made. The nucleic acids may be single stranded or double stranded, as
specified, or contain portions
of both double stranded or single stranded sequence. The nucleic acid may be
DNA, both genomic
and cONA, RNA or a hybrid, where the nucleic acid contains any combination of
deoxyribo- and ribo-
nucleotides, and any combination of bases, including uracil, adenine, thymine,
cytosine, guanine,
inosine, xathanine hypoxathanine, isocytosine, isoguanine, etc.
As described above generally for proteins, nucleic acid candidate bioactive
agents may be naturally
occuring nucleic acids, random nucleic acids, or "biased" random nucleic
acids. For example, digests
of procaryotic or eucaryotic genomes may be used as is outlined above for
proteins.
In a preferred embodiment, the candidate bioactive agents are organic chemical
moieties, a wide
variety of which are available in the literature.
The assays herein utilize lymphocyte activation proteins as defined herein. In
one embodiment,
portions of lymphocyte activation proteins are utilized, in a preferred
embodiment, portions having
lymphocyte activation activity are used. In addition, the assays described
herein may utilize either
isolated lymphocyte activation proteins or cells comprising the lymphocyte
activation proteins.
Generally, in a preferred embodiment of the methods herein, the lymphocyte
activation protein or the
candidate agent is non-diffusably bound to an insoluble support having
isolated sample receiving
areas (e.g. a microtiter plate, an array, etc.). It is understood that soluble
assays can also be used,
for example those which can be detected by fluorescent changes with binding,
etc. The insoluble
supports may be made of any composition to which the compositions can be
bound, is readily
separated from soluble material, and is otherwise compatible with the overall
method of screening.
The surface of such supports may be solid or porous and of any convenient
shape. Examples of
suitable insoluble supports include microtiter plates, arrays, membranes and
beads. These are
typically made of glass, plastic (e.g., polystyrene), polysaccharides, nylon
or nitrocellulose, teOonTM,
etc. Microtiter plates and arrays are especially convenient because a large
number of assays can be
carried out simultaneously, using small amounts of reagents and samples. In
some cases magnetic
CA 02348733 2001-12-04
WO 00/Z6241 t'CT/US99lZ5333
-29-
long as it is compatible with the reagents and overall methods of the
invention, maintains the activity of
the composttion and is nondiffusable. Preferred methods of binding include the
use of antibodies
(which do not sterically block either the ligand binding site or activation
sequence when the protein is
bound to the support), direct binding to °sticky" or ionic supports,
chemical crosslinking, the synthesis
of the protein or agent on the surface, etc. In some embodiments, SWAP or JEST
can be used.
Following binding of the protein or agent, excess unbound material is removed
by washing. The
sample receiving areas may then be blocked through incubation with bovine
serum albumin (BSA),
casein or other innocuous protein or other moiety. Also included in this
invention are screening
assays wherein solid supports are not used.
In a preferred embodiment, the lymphocyte activation protein is bound to the
support, and a candidate
bioactive agent is added to the assay. Alternatively, the candidate agent is
bound to the support and
the lymphocyte activation protein is added. Novel binding agents include
specific antibodies,
non-natural binding agents identified in screens of chemical libraries,
peptide analogs, etc. Of
particular interest are screening assays for agents that have a low toxicity
for human cells. A wide
variety of assays may be used for this purpose, including labeled in vitro
protein-protein binding
assays, electrophoretic mobility shift assays, immunoassays for protein
binding, functional assays
(phosphorylation assays, etc.) and the like.
The determination of the binding of the candidate bioactive agent to a
lymphocyte activation protein
may be done in a number of ways. In a preferred embodiment, the candidate
bioactive agent is
labelled, and binding determined directly. For example, this may be done by
attaching all or a portion
of a lymphocyte activation protein to a solid support, adding a labelled
candidate agent (for example a
fluorescent label), washing off excess reagent, and determining whether the
label is present on the
solid support. Various blocking and washing steps may be utilized as is known
in the art.
By "labeled" herein is meant that the compound is either directly or
indirectly labeled with a label which
provides a detectable signal, e.g. radioisotope, fluorescers, enzyme,
antibodies, particles such as
magnetic particles, chemiluminescers, or specific binding molecules, etc.
Specfic binding molecules
include pairs, such as biotin and streptavidin, digoxin and antidigoxin etc.
For the specific binding
members, the complementary member would normally be labeled with a molecule
which provides for
detection, in accordance with known procedures, as outlined above. The label
can directly or indirectly
provide a detectable signal.
In some embodiments, only one of the components is labeled. For example, the
proteins (or
CA 02348733 2001-12-04
WO 00/26241 PCTNS99I25333
-30-
Alternatively, more than one component may be labeled with different labels;
using'2~1 for the proteins,
for example, and a fluorophor for the candidate agents.
In a preferred embodiment, the binding of the candidate bioactive agent is
determined through the use
of competitive binding assays. In this embodiment, the competitor is a binding
moiety known to bind to
the target molecule (lymphocyte activation molecule), such as an antibody,
peptide, binding partner,
ligand, etc. In a preferred embodiment, the competitor is SWAP or JEST. Under
certain
circumstances, there may be competitive binding as between the bioactive agent
and the binding
moiety, with the binding moiety displacing the bioactive agent. This assay can
be used to determine
candidate agents which interfere with binding between lymphocyte activation
proteins and SWAP or
JEST.
In one embodiment, the candidate bioactive agent is labeled. Either the
candidate bioactive agent, or
the competitor, or both, is added first to the protein for a time sufficient
to allow binding, if present.
Incubations may be pertormed at any temperature which facilitates optimal
activity, typically between 4
and 40°C. Incubation periods are selected for optimum activity, but may
also be optimized to facilitate
rapid high through put screening. Typically between 0.1 and 1 hour will be
sufficient. Excess reagent
is generally removed or washed away. The second component is then added, and
the presence or
absence of the labeled component is followed, to indicate binding.
In a preferred embodiment, the competitor is added first, folknNed by the
candidate bioactive agent.
Displacement of the competitor is an indication that the candidate bioactive
agent is binding to the
lymphocyte activation protein and thus is capable of binding to, and
potentially modulating, the activity
of the lymphocyte activation protein. In this embodiment, either component can
be labeled. Thus, for
example, if the competitor is labeled, the presence of label in the wash
solution indicates displacement
by the agent. Aftemat'rvely, if the candidate bioactive agent is labeled, the
presence of the label on the
support indicates displacement.
In an alternative embodiment, the candidate bioactive agent is added first,
wish incubation and
washing, followed by the competitor. The absence of binding by the competitor
may indicate that the
bioactive agent is bound to the lymphocyte activation protein with a higher
affinity. Thus, if the
candidate bioactive agent is labeled, the presence of the label on the
support, coupled with a lack of
competitor binding, may indicate that the candidate agent is capable of
binding to the lymphocyte
activation protein.
CA 02348733 2001-12-04
wo oon6a,4>t PcrNS~ns~33
-31-
In a preferred embodiment, the methods comprise differential screening to
identity bioactive agents
that are capable of modulating the activity of the lymphocyte activation
proteins. In this embodiment,
the methods comprise combining a lymphocyte activation protein and a
competitor in a first sample. A
second sample comprises a candidate bioactive agent, a lymphocyte activation
protein and a
competitor. The binding of the competitor is determined for both samples, and
a change, or difference
in binding between the two samples indicates the presence of an agent capable
of binding to the
lymphocyte activation protein and potentially modulating its activity. That
is, if the binding of the
competitor is different in the second sample relative to the first sample, the
agent is capable of binding
to the lymphocyte activation protein.
Alternatively, a preferred embodiment utilizes differential screening to
identify drug candidates that
bind to the native lymphocyte activation protein, but cannot bind to modified
lymphocyte activation
proteins. The structure of the lymphocyte activation protein may be modeled,
and used in rational drug
design to synthesize agents that interact with that s'tte. Drug candidates
that affect lymphocyte
activation bioactivity are also identified by screening drugs for the ability
tv either enhance or reduce
the activity of the protein.
Positive controls and negative controls may be used in the assays. Preferably
all control and test
samples are performed in at least triplicate to obtain statistically
significant results. Incubation of all
samples is for a time sufficient for the binding of the agent to the protein.
Following incubation, all
samples are washed free of non-spec~cally bound material and the amount of
bound, generally
Labeled agent determined. For example, where a radiolabel is employed, the
samples may be counted
in a scintillation counter to determine the amount of bound compound.
A variety of other reagents may be included in the screening assays. These
include reagents like
salts, neutral proteins, e.g. albumin, detergents, etc which may be used to
facilitate optimal
protein-protein binding and/or reduce non-specific or background interactions.
Also reagents that
otherwise improve the efficiency of the assay, such as protease inhibitors,
nuclease inhibitors,
anti-microbial agents, etc., may be used. The mixture of components may be
added in any order that
provides for the requisite binding.
The components provided herein for the assays provided herein may also be
combined to form kits.
The kits can be based on the use of the protein and/or the nucleic acid
encoding the lymphocyte
activation proteins. Assays regarding the use of nucleic acids are further
described below.
CA 02348733 2001-12-04
WO 00/26241 PCTNS99IZS333
-32-
Screening for agents that modulate the activity of lymphocyte activation may
also be done. In a
preferred embodiment, methods for screening for a bioactive agent capable of
modulating the activity
of lymphocyte activation comprise the steps of adding a candidate bioact'rve
agent to a sample of
lymphocyte activation protein, as above, and determining an alteration in the
biological activity of
lymphocyte activation protein. "Modulating the activity of Lymphocyte
activation protein" includes an
increase in activity, a decrease in activit)r, or a change in the type or kind
of activity present. Thus, in
this embodiment, the candidate agent should both bind to lymphocyte activation
(although this may not
be necessary), and alter its biological or biochemical activitlr as defined
herein. The methods include
both in vitro screening methods, as are generally outlined above, and in vivo
screening of cells for
alterations in the presence, distribution, activvity or amount of lymphocyte
activation protein.
Thus, in this embodiment, the methods comprise combining a lymphocyte
activation sample and a
candidate bioactive agent, and evaluating the effect on T-cell and B-cell
activafron. By "Lymphocyte
activation activity" or grammatical equivalents herein is meant one of
lymphocyte activation protein's
biological activities, including, but not limited to, its ability to affect T-
cell and B-cell activation. One
activity herein is the capability to bind to SWAP or JEST.
In a preferred embodiment, the activity of the lymphocyte activation protein
is increased; in another
preferred embodiment, the activity of the lymphocyte activation protein is
decreased. Thus, bioactive
agents that are antagonists are preferred in some embodiments, and bioactive
agents that are
agonists may be preferred in other embodiments.
fn a preferred embodiment, the invention provides methods for screening for
bioactive agents capable
of modulating the activity of a lymphocyte activation protein. The methods
comprise adding a
candidate bioactive agent, as defined above, to a cell comprising lymphocyte
activation proteins.
Preferred cell types include almost any cell. The cells contain a recombinant
nucleic acid that encodes
a lymphacyte activation protein. In a preferred embodiment, a library of
candidate agents are tested on
a plurality of cells.
In some embodiments, the assays include exposing the cells to an T-cell and B-
cell activation agent
that will induce T-cell and B-cell activation in control cells, i.e. cells of
the same type but that do not
contain the exogeneous nucleic acid encoding an activation protein.
Alternatively, the cells may be
exposed ~ conditions that normally result in T-cell and B-cell activation, and
changes in the normal T-
cell and B-cell activation progression are determined. Alternatively, the
cells into which the
lymphocyte activation nucleic acids are introduced normally under T-cell and B-
cell activation, and
thus ChannPS lfnr pYamnlP inhihitinn of T-npll anri R-rpll aetivatinn~ ara
eiotcrminart rlnt~r,r,~lt.. rho
CA 02348733 2001-12-04
WO 00/26241 P~CTNS99~
-33-
cells normally do not undergo T-cell and B-cell activation, and the
introduction of a candidate agent
causes T-cell and B-cell activation.
Thus, the effect of the candidate agent on T-cell and B~eil activation is then
evaluated.
Detection of T-cell and B-cell activation may be done as will be appreciated
by those in the art. fn one
embodiment, indicators of T-cell and B-cell activation are used. Accordingly,
these agents can be
used as an affinity ligand, and attached to a solid support such as a bead, a
surface, etc. and used to
pull out cells that are undergoing T-cell and B-cell activation. Similarly,
these agents can be coupled
to a fluorescent dye such as PerCP, and then used as the basis of a
fluorescent-activated cell sorting
(FACS} separation.
In this way, bioacfrve agents are identified. Compounds with pharmacological
activity are able to
enhance or interfere with the activity of the lymphocyte activation protein.
The compounds having the
desired pharmacological activity may be administered in a physiologically
acceptable carrier to a host,
as previously described. The agents may be administered in a variety of ways,
orally, parenterally
e.g., subcutaneously, intraperitoneally, intravascularly, etc. Depending upon
the manner of
1 S introduction, the compounds may be formulated in a variety of ways. The
concentration of
therapeutically active compound in the formulation may vary from about 0.1-100
wt.%.
The pharmaceutical compositions can be prepared in various forms, such as
granules, tablets, pills,
suppositories, capsules, suspensions, salves, lotions and the like.
Pharmaceutical grade organic or
inorganic carriers andlor diluents suitable for oral and topical use can be
used to make up
compositions containing the therapeutically-active compounds. Diluents known
to the art include
aqueous media, vegetable and animal oils and fats. Stabilizing agents, wetting
and emulsifying
agents, salts for varying the osmotic pressure or buffers for securing an
adequate pH value, and skin
penetration enhancers can be used as auxiliary agents.
Without being bound by theory, it appears that a lymphocyte activation protein
is an important protein
in T-cell and B-cell activation. Accordingly, disorders based on mutant or
variant lymphocyte
activation genes may be determined. In one embodiment, the invention provides
methods for
identifying cells containing variant lymphocyte activation genes comprising
determining all or part of
the sequence of at least one endogeneous lymphocyte activation genes in a
cell. As wiH be
appreciated by those in the art, this may be done using any number of
sequencing techniques. in a
preferred embodiment, the invention provides methods of identifying the
lymphocyte activation
nennhm~n of .fin indiuidmnl nnrHnrie.inn d~~nrr~inin~ .,II i,r r....r~ ~~
~lvrv r.,"-,.~......v ni..~ 1..x..1 ...... L......L__..a_
CA 02348733 2001-12-04
wo oon6a,41 rc~rrtrs~ns333
-34-
activation gene of the individual. This is generally done in at least one
tissue of the individual, and
may include the evaluation of a number of tissues or different samples of the
same tissue. The
method may include comparing the sequence of the sequenced lymphocyte
activation gene to a
known lymphocyte activation gene, i.e. a wild-type gene.
The sequence of all or part of the lymphocyte activation gene can then be
compared to the sequence
of a known lymphocyte activation gene to determine if any differences exist.
This can be done using
any number of known sequence identity programs, such as Bestfit, etc. and
others outlined herein. In
a preferred embodiment, the presence of a difference in the sequence between
the lymphocyte
activation gene of the patient and the known lymphocyte activation gene is
indicative of a disease
state or a propensity for a disease state, as outlined herein.
The present discovery relating to the role of lymphocyte activation in T-cell
and B-cell activation thus
provides methods for inducing or preventing T-cell and B-cell activation in
cells. In a preferred
embodiment, the lymphocyte activation proteins, and particularly lymphocyte
activation fragments, are
useful in the study or treatment of conditions which are mediated by T-cell
and B-cell activation, i.e. to
diagnose, treat or prevent T-cell and B-cell activation-mediated disorders.
Thus, "T-cell and B-cell
activation mediated disorders" or "disease state" include conditions involving
both insufficient or
excessive T-cell and B-cell activation. immunological disorders are numerous
and known in the art.
Thus, in one embodiment, methods of modulating T-cell and B-cell activation in
cells or organisms are
provided. In one embodiment, the methods comprise administering to a cell an
anti-lymphocyte
activation antibody or other agent identified herein or by the methods
provided herein, that reduces or
eliminates the biological activity of the endogeneous lymphocyte activation
protein. Alternatively, the
methods comprise administering to a cell or organism a recombinant nucleic
acid encoding a
lymphocyte activation protein or modulator including anti-sense nucleic acids.
As will be appreciated
by those in the art, this may be accomplished in any number of ways. In a
preferred embodiment, the
activity of lymphocyte activation is increased by increasing the amount of
lymphocyte activation in the
cell, for example by overexpressing the endogeneous lymphocyte activation or
by administering a
gene encoding an Lymphocyte activation protein, using known gene-therapy
techniques, for example.
In a preferred embodiment, the gene therapy techniques include the
incorporation of the exogeneous
gene using enhanced homologous recombination (EHR), for example as described
in
PCTIUS93/03868, hereby incorporated by reference in its entireity.
In one embodiment, the invention provides methods for diagnosing an T-cell and
B-cell activation
--1-a_J _.--J:L:-.~ :_ -- :_J:..:J.....1 1'4~ ~..LL.-J.- ...-...-.--...~ ~~~-
.....-.~ LL- .-..L...a.. w~ 1.....~6......L~
CA 02348733 2001-12-04
WO OOIZ6241 PGT/US99/25333
-35-
activation in a tissue from the individual or patient, which may include a
measurement of the amount or
specific activity of Lymphocyte activation protein. This activity is compared
to the acfrvity of
lymphocyte activation from either a unaffected second individual or from an
unaffected tissue from the
first individual. When these activities are different, the first individual
may be at risk for an T-cell and
B-cell activation mediated disorder.
The proteins and nucleic acids provided herein can also be used for screening
purposes wherein the
protein-protein interacctieons of the lymphocyte activation proteins can be
idenfdred. Genetic systems
have been described to detect protein-protein interactions. The first work was
done in yeast systems,
namely the "yeast two-hybrid" system. The basic system requires a protein-
protein interaction in order
to turn on transcription of a reporter gene. Subsequent work was done in
mammalian cells. See
Fields et al., Nature 340:245 (1989); Vasavada et al., PNAS USA 88:10686 (1991
); Fearon et al.,
PNAS USA 89:7958 (1992); Dang et al., Mol. Cell. Biol. 11:954 (1991); Chien et
aL, PNAS USA
88:9578 (1991); and U.S. Patent Nos. 5,283,173, 5,667,973, 5,468,614,
5,525,490, and 5,637,463. A
preferred system is described in Serial No. 091050,863, filed March 30, 1998,
entitled "Mammalian
Protein Interaction Cloning System'. For use in conjunction with these
systems, a particularly useful
shuttle vector is described in Serial No. 091133,944, filed August 14, 1998,
entitled "Shuttle Vectors".
In general, two nucleic acids are transformed into a cell, where one is a
"bait" such as the gene
encoding SWAP, JEST or a portion thereof, and the other encodes a test
candidate. Only if the Nuo
expression products bind to one another will an indicator, such as a
fluorescent protein, be expressed.
Expression of the indicator indicates when a test candidate binds to SWAP or
JEST and can be
identified as a lymphocyte activation protein. Using the same system and the
identified lymphocyte
activation proteins the reverse can be performed. Namely, the lymphocyte
activation proteins
provided herein can be used to identify new baits, or agents which interact
with lymphocyte activation
proteins. Additionally, the two-hybrid system can be used wherein a test
candidate is added in
addition to SWAP or JEST and the lymphocyte activation protein encoding
nucleic aads to determine
agents which interfere with the bait, such as SWAP or JEST, and the lymphocyte
activation protein.
RasGRP can also be used.
In one embodiment, a mammalian two-hybrid system is preferred. Mammalian
systems provide post-
translational modifications of proteins which may contribute significantly to
their ability to interact. In
addition, a mammalian two-hybrid system can be used in a wide variety of
mammalian cell types to
mimic the regulation, induction, processing, etc. of specific proteins within
a particular cell type. For
example, proteins involved in a disease state such as those described above
could be tested in the
--1-..~~a J:..... __11_ fv:_.:1~_L. t...~-..t:.... ~1.,.--J_.._ .__-~_.~-
_.___...__ aL _.._. ..._ W _ . a1. . n
CA 02348733 2001-12-04
wo oorw~>I pc~r~us~ns333
-36-
cellular conditions will give the highest positive results. Furthermore, the
mammalian cells can be
tested under a variety of experimental conditions that may affect
intracellular protein-protein
interactions, such as in the presence of hortranes, drugs, growth factors and
cytokines, cellular and
chemical stimuli, etc., that may contribute to conditions which can effect
protein-protein interactions,
particuiarfy those involved in T-cell and B-cell activation.
Expression in various cell types, and assays for lymphocyte activation
activity are described above.
The activity assays, such as having an effect an T-cell and B-cell activation
can be performed to
confirm the acitivity of lymphocyte activation proteins which have already
been identified by their
sequence identitylsimilarity or binding to SWAP, JEST or RasGRP as well as to
further confirm the
activity of lead compounds identified as modulators of T-cell and B-cell
activation.
Assays involving binding such as the two-hybrid system may take into account
non-specific binding
proteins (NSB).
In one embodiment, the lymphocyte activation proteins of the present invention
may be used to
generate polyclonal and monoclonal antibodies to lymphocyte activation
proteins, which are useful as
described herein. Similarly, the lymphocyte activation proteins can be
coupled, using standard
technology, to affinity chromatography columns. These columns may then be used
to purify
lymphocyte activation antibodies. In a preferred embodiment, the antibodies
are generated to
epitopes unique to the lymphocyte activation protein; that is, the antibodies
show little or no cross-
reactivity to other proteins. These antibodies find use in a number of
applications. For example, the
lymphocyte activation antibodies may be coupled to standard affinity
chromatography columns and
used to purify lymphocyte activation proteins as further described below. The
antibodies may also be
used as blocking polypeptides, as outlined above, since they will specthcally
bind to the lymphocyte
activation protein.
The anti-lymphocyte acfrvation protein antibodies may comprise polyclonal
antibodies. Methods of
preparing polyclonal antibodies are known to the skilled artisan. Polyclonal
antibodies can be raised in
a mammal, for example, by one or more injections of an immunizing agent and,
if desired, an adjuvant.
Typically, the immunizing agent andlor adjuvant will be injected in the mammal
by multiple
subcutaneous or intraperitoneal injections. The immunizing agent may include
the lymphocyte
activation protein polypeptide or a fusion protein thereof. It may be useful
to conjugate the immunizing
agent to a protein known to be immunogenic in the mammal being immunized.
Examples of such
immunogenic proteins include but are not limited to keyhole limpet hemocyanin,
serum albumin,
1_....:~~ ~1-....~-.W 6..c.~ -__1 __..L__~ a._.~_c_ :.-Ltu:u.._ r_.__._n__ _r
_ m .. . .
CA 02348733 2001-12-04
WO 00126241 PCT/US99/25333
-37-
include Freund's complete adjuvant and MPL-TDM adjuvant (monophosphoryl Lipid
A, synthetic
trehalose dicorynomycolate). The immunization protocol may be selected by one
skilled in the art
without undue experimentation.
The anti-lymphocyte activation protein antibodies may, alternatively, be
monoclonal antibodies.
Monoclonal antibodies may be prepared using hybridoma methods, such as those
described by Kohler
and Milstein, Nature, xø:495 (1975). In a hybridoma method, a mouse, hamster,
or other appropriate
host animal, is typically immunized with an immunizing agent to elicit
lymphocytes that produce or are
capable of producing antibodies that will specficaliy bind to the immunizing
agent. AltemaCrvely, the
lymphocytes may be immunized in vitro.
The immunizing agent will typically include the lymphocyte activation protein
polypeptide or a fusion
protein thereof. Generally, either peripheral blood lymphocytes ("PBLs") are
used if cells of human
origin are desired, or spleen cells or lymph node cells are used if non-human
mammalian sources are
desired. The lymphocytes are then fused with an immortalized cell line using a
suitable fusing agent,
such as polyethylene glycol, to form a hybridoma cel! [coding, Monoclonal
Antibodies: Principles and
practice, Academic Press, (1986) pp. 59-103]. Immortalized cell lines are
usually transformed
mammalian cells, particularly myeloma cells of rodent, bovine and human
origin. Usually, rat or
mouse myeloma cell lines are employed. The hybridoma cells may be cultured in
a suitable culture
medium that preferably contains one or more substances that inhibit the growth
or survival of the
unfused, immortalized cells. For example, if the parental cells lack the
enzyme hypaxanthine guanine
phosphoribosyl transferase (HGPRT or HPRT), the culture medium for the
hybridomas typically will
include hypoxanthine, aminopterin, and thymidine ("HAT medium"), which
substances prevent the
growth of HGPRT-deficient cells.
Preferred immortalized cell lines are those that fuse efficiently, support
stable high level expression of
antibody by the selected antibody-producing cells, and are sensitive to a
medium such as HAT
medium. More preferred immortalized cell lines are murine myeloma lines, which
can be obtained, for
instance, from the Salk Institute Cell Distribution Center, San Diego,
California and the American Type
Culture Collection, Rockville, Maryland. Human myeloma and mouse-human
heteromyeloma cell lines
also have been described for the production of human monoclonal antibodies
[Kozbor, J. mmunol.,
x:3001 (1984); Brodeur et al., Monoclonal Antibody Prod~lction TechniauP,~ and
Apalications, Marcel
Dekker, Inc., New York, (1987) pp. 51-63].
The culture medium in which the hybridoma cells are cultured can then be
assayed for the presence of
Mnnnrlnns) ~n~ihnrline rliren~nil ~n~ine~ Iv.rnhnr...~a .".~;....~:.... ..-
..v...:.. n..,s..-..m,. ~~_ w:_~:__
CA 02348733 2001-12-04
WO 00/26241 PCT/US99/Z5333
-38-
specificity of monoclonal antibodies produced by the hybridoma cells is
determined by
immunoprecipitation or by an in vitro binding assay, such as radioimmunoassay
(RIA) or enzyme-
linked immunosorbent assay (ELiSA). Such techniques and assays are known in
the art. The binding
affinity of the monoclonal antibody can, for example, be determined by the
Scatchard analysis of
Munson and Pollard, 6 anhBi, ,~"~7:220 (1980).
After the desired hybridoma cells are identfied, the clones may be subdoned by
limiting dilution
procedures and grown by standard methods [coding, s_yral. Suitable culture
media for this purpose
include, for example, Dulbecco's Modified Eagle's Medium and RPMI-1640 medium.
Aftematively, the
hybridoma cells may be grown in vivo as ascites in a mammal.
The monoclonal antibodies secreted by the sutxtones may be isolated or
purified from the culture
medium or ascites fluid by conventional immunoglobulin purification procedures
such as, for example,
protein A-Sepharose, hydroxylapatite chromatography, gel electrophoresis,
dialysis, or affinity
chromatography.
The monoclonal antibodies may also be made by recombinant DNA methods, such as
those described
in U.S. Patent No. 4,816,567. DNA encoding the monoclonal antibodies of the
invention can be
readily isolated and sequenced using conventional procedures (e.g., by using
oligonucleotide probes
that are capable of binding specifically to genes encoding the heavy and light
chains of murine
antibodies). The hybridoma cells of the invention serve as a preferred source
of such DNA. Once
isolated, the DNA may be placed into expression vectors, which are then
transfected into host cells
such as simian COS cells, Chinese hamster ovary (CHO) cells, or myeloma cells
that do not otherwise
produce immunoglobulin protein, to obtain the synthesis of monoclonal
antibodies in the recombinant
host cells. The DNA also may be modified, for example, by substituting the
coding sequence for
human heavy and light chain constant domains in place of the homologous murine
sequences [U.S.
Patent No. 4,816,567; Morrison et al., suorai or by covalently joining to the
immunoglobulin coding
sequence all or part of the coding sequence for a non-immunoglobutin
polypeptide. Such a non-
immunoglobulin polypeptide can be substituted for the constant domains of an
antibody of the
invention, or can be substituted for the variable domains of one antigen-
combining site of an antibody
of the invention to create a chimeric bivalent antibody.
The antibodies may be monovalent antibodies. Methods for preparing monovalent
antibodies are well
known in the art. For example, one method involves recombinant expression of
immunoglobulin light
chain and modified heavy chain. The heavy chain is truncated generally at any
point in the Fc region
CA 02348733 2001-12-04
WO 00/26241 PCT/US99I25333
-39-
so as to prevent heavy chain crosslinking. Alternatively, the relevant
cysteine residues are substituted
with another amino acid residue or are deleted so as to prevent crosslinking.
In vitro methods are also suitable for preparing monovalent antibodies.
Digestion of antibodies to
produce fragments thereof, particularly, Fab fragments, can be accomplished
using routine techniques
known in the art.
The anti-lymphocyte activation protein antibodies of the invention may further
comprise humanized
antibodies or human antibodies. Humanized forms of non-human (e.g., murine)
antibodies are
chimeric immunoglobulins, immunoglobulin chains or fragments thereof {such as
Fv, Fab, Fab', F{ab')2
or other antigen-binding subsequences of antibodies) which contain minimal
sequence derived from
non-human immunoglobulin. Humanized antibodies include human immunoglobulins
{recipient
antibody) in which residues from a complementary determining region (CDR) of
the recipient are
replaced by residues from a CDR of a non-human species (donor antibody) such
as mouse, rat or
rabbit having the desired specificity, affinity and capacity. In some
instances, Fv framework residues
of the human immunoglobulin are replaced by corresponding non-human residues.
Humanized
antibodies may also comprise residues which are found neither in the recipient
antibody nor in the
imported CDR or framework sequences. In general, the humanized antibody will
comprise
substantially all of at least one, and typically two, variable domains, in
which all or substantially all of
the CDR regions correspond to those of a non-human immunoglobulin and all or
substantially all of the
FR regions are those of a human immunoglobulin consensus sequence. The
humanized antibody
optimally also will comprise at least a portion of an immunoglobulin constant
region (Fc), typically that
of a human immunoglobulin [Jones et al., , x:522-525 (1986); Riechmann et al.,
Nature,
x:323-329 {1988); and Presta, Curr. O . Struct. Biol., x:593-596 (1992)].
Methods for humanizing non-human antibodies are well known in the art.
Generally, a humanized
antibody has one or rrwre amino acid residues introduced into it from a source
which is non-human.
These non-human amino acid residues are often referred to as "import"
residues, which are typically
taken from an "import" variable domain. Humanization can be essentially
performed following the
method of Winter and co-workers [Jones et al., N~~re, ,x:522-525 (1986);
Riechmann et al., Nature,
,x:323-327 (1988); Verhoeyen et al., , cience, ~Q:1534-1536 (1988)], by
substituting rodent CDRs
or CDR sequences for the corresponding sequences of a human antibody.
Accordingly, such
"humanized" antibodies are chimeric antibodies {U.S. Patent No. 4,816,567),
wherein substantially
less than an intact human variable domain has been substituted by the
corresponding sequence from
a non-human species. In practice, humanized antibodies are typically human
antibodies in which
CA 02348733 2001-12-04
wo oon6za» pcrn~s~n3333
-ao-
some CDR residues and possibly some FR residues are substituted by residues
from analogous sites
in rodent antibodies.
Human antibodies can also be produced using various techniques known in the
art, including phage
display libraries (Hoogenboom and Winter, ~ ol. Biol., ~j:381 (1991 ); Marks
et al., ~. MQI. Biol.,
x:581 (1991)]. The techniques of Cole et al. and Boemer et al. are also
available for the preparation
of human monoclonal antibodies (Cole et al., I~OggJl~nal Anti iodise and
Cancer Theraov, Alan R.
Lies, p. 77 (1985) and Boemer et al., J. Immunol., 147(11:86-95 (1991 )].
Similarly, human antibodies
can be made by introducing of human immunoglobulin loci into transgenic
animals, e.g., mice in which
the endogenous immunoglobulin genes have been partially or completely
inactivated. Upon
challenge, human antibody production is observed, which closely resembles that
seen in humans in all
respects, including gene rearrangement, assembly, and antibody repertoire.
This approach is
described, for example, in U,S. Patent Nos. 5,545,807; 5,545,806; 5,569,825;
5,625,126; 5,633,425;
5,661,016, and in the following scientific publications: Marks et al., BioITec
r~oloav 1Q, 779-783
(1992); lonberg ef al., Nature ~$ 856-859 (1994); Morrison, Nature ,~, 812-13
(1994); Fishwild et
al., Nature Biotechnoloav ~, 845-51 (1996); Neuberger, Nature Biotechnoloav ~,
826 (1996);
Lonberg and Huszar, Int~ev._I~munol. ~ 65-93 (1995).
Bispecific antibodies are monoclonal, preferably human or humanized,
antibodies that have binding
specificities for at least two different antigens. In the present case, one of
the binding specificities is
for the lymphocyte activation protein, the other one is for any other antigen,
and preferably for a cell-
surface protein or receptor or receptor subunit.
Methods for making bispecific antibodies are known in the art. Traditionally,
the recombinant
production of bispecific antibodies is based on the co-expression of two
immunoglobulin heavy-
chain/light-chain pairs, where the two heavy chains have different
specificities (Milstein and Cuello,
Nature, x:537-539 {1983)]. Because of the random assortment of immunoglobulin
heavy and light
chains, these hybridomas (quadromas) produce a potential mixture of ten
different antibody
molecules, of which only one has the correct bispecific structure. The
purification of the correct
molecule is usually accomplished by affinity chromatography steps. Similar
procedures are disclosed
in WO 93108829, published 13 May 1893, and in Traunecker et al., EMBO
J.,1Q:3655-3659 (1991 ).
Antibody variable domains with the desired binding speci~cities (antibody-
antigen combining sites) can
be fused to immunoglobuNn constant domain sequences. The fusion preferably is
with an
immunoglobulin heavy-chain constant domain, comprising at least part of the
hinge, CH2, and CH3
CA 02348733 2001-12-04
WO 00/26241 PCT/US99lZ5333
-41-
necessary for light-chain binding present in at least one of the fusions. DNAs
encoding the
immunoglobulin heavy-chain fusions and, if desired, the immunoglobulin light
chain, are inserted into
separate expression vectors, and are co-transfected into a suitable host
organism. For further details
of generating bispecific antibodies see, for example, Suresh et al., Methods
in EnzXmoloav, 1:210
(1986).
Heteroconjugate antibodies are also within the scope of the present invention.
Heteroconjugate
antibodies are composed of two cavalenfly joined antibodies. Such antibodies
have, for example,
been proposed to target immune system cells to unwanted cells [U.S. Patent No.
4,676,980], and for
treatment of HIV infection (VllO 91/00360; WO 92/200373; EP 03089]. It is
contemplated that the
antibodies may be prepared in vitro using known methods in synthetic protein
chemistry, including
those involving crosslinking agents. For example, immunotoxins may be
constructed using a disulfide
exchange reaction or by forming a thioether bond. Examples of suitable
reagents for this purpose
include iminothiolate and methyl-4-mercaptobutyrimidate and those disclosed,
for example, in U.S.
Patent No. 4,676,980.
The anti-lymphocyte activation protein antibodies of the invention have
various utilities. For example,
anti-lymphocyte activation protein antibodies may be used in diagnostic assays
for a lymphocyte
activation protein, e.g., detecting its expression in specific cells, tissues,
or serum. Various diagnostic
assay techniques known in the art may be used, such as competitive binding
assays, direct or indirect
sandwich assays and immunoprecipitation assays conducted in either
heterogeneous or
homogeneous phases [Zola, Monoclonal Antibodies: A Manual r,~Teehniq~, CRC
Press, Inc. (1987)
pp. 147-158]. The antibodies used in the diagnostic assays can be labeled with
a detectable moiety.
The detectable moiety should be capable of producing, either directly or
indirectly, a detectable signal.
For example, the detectable moiety may be a radioisotope, such as'H,
"C,'ZP,'~S, or'zsl, a
fluorescent or chemiluminescent compound, such as fluorescein isothiocyanate,
rhodamine, or
luciferin, or an enzyme, such as alkaline phosphatase, beta-galactosidase or
horseradish peroxidase.
Any method known in the art for conjugating the antibody to the detectable
moiety may be employed,
including those methods described by Hunter et al., Nature, ~g:945 (1962);
David et al.,
Biochemistry, x:1014 (1974); Pain et al., J. Immunol. Meth., x:219 (1981 );
and Nygren, ,~
Histochem. and C, ochem., ~Q:407 (1982).
Anti-Lymphocyte activation protein antibodies also are useful for the affinity
purification of lymphocyte
activation protein from recombinant cell culture or natural sources. In this
process, the antibodies
against lymphocyte activation protein are immobilized on a suitable support,
such a Sephadex resin or
.... . .. .. . . .. . _. . . ... . ..
CA 02348733 2001-12-04
WO OOIZ6141 PCTNS99n5333
-42-
sample containing the lymphocyte activation protein to be purified, and
thereafter the support is
washed with a suitable solvent that will remove substantially all the material
in the sample except the
lymphocyte activation protein, which is bound to the immobilized antibody.
Finally, the support is
washed with another suitable solvent that will release the lymphocyte
activation protein from the
antibody.
The anti-lymphocyte activation protein antibodies may also be used in
treatment. In one embodiment,
the genes encoding the antibodies are provided, such that the antibodies bind
to and modulate the
lymphocyte activation protein within the cell.
In one embodiment, a therapeutically effective dose of a lymphocyte activation
protein, agonist or
antagonist is administered to a patient. By "therapeutically effective dose"
herein is meant a dose that
produces the effects for which it is administered. The exact dose will depend
on the purpose of the
treatment, and will be ascertainable by one skilled in the art using known
techniques. As is known in
the art, adjustments for lymphocyte activation degradation, systemic versus
localized delivery, and
rate of new protease synthesis, as well as the age, body weight, general
health, sex, diet, time of
administration, drug interaction and the severity of the condition may be
necessary, and will be
ascertainable with routine experimentation by those skilled in the art.
A "patient' for the purposes of the present invention includes both humans and
other animals,
particularly mammals, and organisms. Thus the methods are applicable to both
human therapy and
veterinary applications. In the preferred embodiment the patient is a mammal,
and in the most
preferred embodiment the patient is human.
The administration of the lymphocyte activation protein, agonist or antagonist
of the present invention
can be done in a variety of ways, including, but not limited to, orally,
subcutaneousty, intravenously,
intranasally, transdermally, intraperitoneally, intramuscularly,
intrapulmonary, vaginally, rectally, or
intraocularly. In some instances, for example, in the treatment of wounds and
inflammation, the
lymphocyte activation may be directly applied as a solution or spray.
The pharmaceutical compositions of the present invention comprise a lymphocyte
activation protein,
agonist or antagonist in a form suitable for administration to a patient. In
the preferred embodiment,
the pharmaceutical compositions are in a water soluble form, such as being
present as
pharmaceutically acceptable salts, which is meant to include both acid and
base addition salts.
"Pharmaceutically acceptable acid addition salt" refers to those salts that
retain the biological
~lf~.wiim..wwww wJ<IL.w A..w L..www wwd ~L. w~ wnw ww~ L.i..lw..iwwll.. .~~
w~L.ww..lww ..~J-~:~~LI- L-_~-J __.:aL
CA 02348733 2001-12-04
WO 00/26241 PCTNS99/Z5333
-43-
inorganic acids such as hydrochloric acid, hydrobromic acid, sulfuric acid,
nific acid, phosphoric acid
and the like, and organic acids such as acetic acid, propionic acid, glycolic
acid, pyruvic acid, oxalic
acid, malefic acid, malonic acid, succinic acid, fumaric acid, tartaric acid,
citric acid, benzoic acid,
cinnamic acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid, p-
toluenesulfonic acid,
salicylic acid and the like. "Pharmaceutically acceptable base addition salts"
include those derived
from inorganic bases such as sodium, potassium, lithium, ammonium, cak:ium,
magnesium, iron, zinc,
copper, manganese, aluminum salts and the like. Particularly preferred are the
ammonium,
potassium, sodium, calaum, and magnesium salts. Salts derived from
pharmaceutically acceptable
organic non-toxic bases include salts of primary, secondary, and tertiary
amines, substituted amines
including naturally occurring substituted amines, cyclic amines and basic ion
exchange resins, such as
isopropylamine, trimethylamine, diethylamine, triethylamine, tripropylamine,
and ethanolamine.
The pharmaceutical compositions may also include one or more of the following:
carrier proteins such
as serum albumin; buffers; fillers such as microcrystalline cellulose,
lactose, corn and other starches;
binding agents; sweeteners and other flavoring agents; coloring agents; and
polyethylene glycol.
Additives are well known in the art, and are used in a variety of
formulations.
Ail references and sequences of accession numbers cited herein are
incorporated by reference in their
entireity.