Note: Descriptions are shown in the official language in which they were submitted.
CA 02349733 2001-06-21
TITLE OF THE INVENTION
METHOD FOR INHIBITING BONE RESORPTION
CROSS-REFERENCE TO RELATED APPLICATIONS
The present invention is related to U.S. application Serial
No. 09/060,419, filed April 15, 1998, and U.S. provisional applications
Serial Nos. 60/053,535, filed July 23, 1997, and 60/053,351, filed July 22,
1997, the contents of which are hereby incorporated by reference.
This Application is a Divisional of Canadian Patent
Application Serial No. 2,294,595, filed July 17, 1998.
FIELD OF THE INVENTION
The present invention relates to oral methods for inhibiting
bane resorption in a mammal while minimizing the occurrence of or
potential for adverse gastrointestinal effects. These methods comprise
orally administering to a mammal in need thereof of a pharmaceutically
effective amount of a bisphosphonate as a unit dosage according to a
continuous schedule having a dosing interval selected from the group
consisting of once-weekly dosing, twice-weekly dosing, biweekly dosing,
and twice-monthly dosing. The present invention also relates to
pharmaceutical compositions and kits useful for carrying out these
methods.
BACKGROUND OF THE INVENTION
A variety of disorders in humans and other mammals
involve or are associated with abnormal bone resorption. Such disorders
include, but are not limited to, osteoporosis, Paget's disease,
periprosthetic bone loss or osteolysis, and hypercalcemia of malignancy.
The most common of these disorders is osteoporosis, which in its most
frequent manifestation occurs in postmenopausal women. Osteoporosis
is a systemic skeletal disease characterized by a low bone mass and
microarchitectural deterioration of bone tissue, with a consequent
increase in bone fragility and susceptibility to fracture. Because
osteoporosis, as well as other disorders associated with bone loss, are
chronic conditions, it is believed that appropriate therapy will generally
require chronic treatment.
-1-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
Multinucleated cells called osteoclasts are responsible for
causing bone loss through a process known as bone resorption. It is
well known that bisphosphonates are selective inhibitors of osteoclastic
bone resorption, making these compounds important therapeutic agents
in the treatment or prevention of a variety of generalized or localized
bone disorders caused by ar associated with abnormal bone resorption.
See H. Fleisch, Bisphosphonates In Bone Disease, From The Laboratory
To The Patient, 2nd Edition, Parthenon Publishing (1995), which is
incorporated by reference herein in its entirety.
At present, a great amount of preclinical and clinical data
exists for the potent bisphosphonate compound alendronate. Evidence
suggests that other bisphosphonates such as risedronate, tiludronate,
ibandronate and zolendronate, have many properties in common with
alendronate, including high potency as inhibitors of osteoclastic bone
resorption. An older bisphosphonate compound, etidronate, also inhibits
bone resorption. However, unlike the more potent bisphosphonates,
etidronate impairs mineralization at doses used clinically, and may give
rise to osteomalacia, a condition resulting in an undesirable decrease in
bone mineralization. See :Boyce, B. F., Fogelman, L, Ralston, S. et al.
(1984) Lancet 1(8381), pp. 821-824 (1984), and Gibbs, C. J., Aaron, J. E.;
Peacock, M. (1986) Br. Med. J. 292, pp. 1227-1229 (1986), both of which are
incorporated by reference herein in their entirety.
Despite their therapeutic benefits, bisphosphonates are
poorly absorbed from the gastrointestinal tract. See B.J. Gertz et al.,
Clinical Pharmacology of Alendronate Sodium, Osteoporosis Int., Suppl.
3: S13-16 (1993) and B.J. Gertz et al., Studies of the oral bioauailability of
alendronate, Clinical Pharmacology & Therapeutics, vol. 58, number 3,
pp. 288-298 (September 1995), which are incorporated by reference herein
in their entirety. Intravenous administration has been used to overcome
this bioavailability problem. However, intravenous administration is
costly and inconvenient, especially when the patient must be given an
intravenous infusion lasting several hours on repeated occasions.
If oral administration of the bisphosphonate is desired,
relatively high doses must be administered to compensate for the low
bioavailability from the gastrointestinal tract. To offset this low
-2-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98I14796
bioavailability, it is generally recommended that the patient take the
bisphosphonate on an empty stomach and fast for at least 30 minutes
afterwards. However, many patients find the need for such fasting on a
daily basis to be inconvenient. Moreover, oral administration has been
associated with adverse gastrointestinal effects, especially those relating
to the esophagus. See Fleisch, Id. These effects appear to be related to
the irritant potential of the bisphosphonate in the esophagus, a problem
which is exacerbated by the presence of refluxed gastric acid. For
example, the bisphosphonate, pamidronate has been associated with
esophageal ulcers. See E.G. Lufkin et al., Pamidronate: An
Unrecognized Problem in Gastrointestinal Tolerability, Osteoporosis
International, 4: 320-322 (1994), which is incorporated by reference
herein in its entirety. Although not as common, the use of alendronate
has been associated with esophagitis and/or esophageal ulcers. See P.C.
De Groen, et al., Esophagitis Associated With The Use Of Alendronate,
New England Journal of Medicine, vol. 335, no. 124, pp. 1016-1021 (1996),
D.O. Castell, Pill Esophagitis -- The Case of Alendronate, New England
Journal of Medicine, vol. 335, no. 124, pp. 1058-1059 (1996), and U.A.
Liberman et al., Esophagitis and Alendronate, New England Journal of
Medicine, vol. 335, no. 124, pp. 1069-1070 (1996), which are incorporated
by reference herein in their entirety. The degree of adverse
gastrointestinal effects of bisphosphonates has been shown to increase
with increasing dose. See C.H. Chestnut et al., Alendronate Treatment
of the Postmenopausczl Osteoporotic Woman: Effect of Multiple Dosages
on Bone Mass ~znd Bone Remodeling, The American Journal of
Medicine, vol. 99, pp. 144-152, {August 1995 ), which is incorporated by
reference herein in its entirety. Also, these adverse esophageal effects
appear to be more prevalent in patients who do not take the
bisphosphonate with an adequate amount of liquid or who lie down
shortly after dosing, thereby increasing the chance for esophageal
reflux.
Current oral bisphosphonate therapies generally fall into
two categories: (1) those therapies utilizing continuous daily treatment,
and (2) those therapies utilizing a cyclic regimen of treatment and rest
periods.
-3-
CA 02349733 2001-06-21
WO 99!04773 PCT/US98/14796
The continuous daily treatment regimens normally involve
the chronic administration of relatively low doses of the bisphosphonate
compound, with the objective of delivering the desired cumulative
therapeutic dose over the course of the treatment period. However,
continuous daily dosing has the potential disadvantage of causing
adverse gastrointestinal effects due to the repetitive, continuous, and
additive irritation to the gastrointestinal tract. Also, because
bisphosphonates should be taken on an empty stomach followed by
fasting and maintenance of an upright posture for at least 30 minutes,
many patients find daily dosing to be burdensome. These factors can
therefore interfere with patient compliance, and in severe cases even
require cessation of treatment.
Cyclic treatment regimens were developed because some
bisphosphonates, such as etidronate, when given daily for more than
several days, have the disadvantage of actually causing a decline in bone
mineralization, i.e. osteomalacia. U.S. Patent No. 4,761,406, to Flora et
al, issued August 2, 1988, which is incorporated by reference herein in
its entirety, describes a cyclic regimen developed in an attempt to
minimize the decline in bone mineralization while still providing a
therapeutic anti-resorptive effect. Generally, cyclic regimens are
characterized as being intermittent, as opposed to continuous treatment
regimens, and have both treatment periods during which the
bisphosphonate is administered and nontreatment periods to permit the
systemic level of the bisphosphonate to return to baseline. However, the
cyclic regimens, relative to continuous dosing, appear to result in a
decreased therapeutic antiresorptive efficacy. Data on risedronate
suggests that cyclic dosing is actually less effective than continuous
daily dosing for maximizing antiresorptive bone effects. See L.
Mortensen, et al., Prevention Of Eczrly Postmenopausal Bone Loss By
Risedronate, Journal of Bone and Mineral Research, vol. 10, supp. 1, p.
s140 (1995), which is incorporated by reference herein in its entirety.
Furthermore, these cyclic regimens do not eliminate or minimize
adverse gastrointestinal effects, because such regimens typically utilize
periods of multiple daily dosing. Also, the cyclic regimens are
cumbersome to administer and have the disadvantage of low patient
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
compliance, and consequently compromised therapeutic efficacy. U.S.
Patent No. 5,366,965, to Strein, issued November 22, 1994, which is
incorpoated by reference herein in its entirety,. attempts to address the
problem of adverse gastrointestinal effects by administering a
polyphosphonate compound, either orally, subcutaneously, or
intravenously, according to an intermittent dosing schedule having both
a bone resorption inhibition period and a no-treatment rest period.
However, the regimen has the disadvantage of not being continuous and
regular, and requires nontreatment periods ranging from 20 to 120 days.
PCT Application No. WO 95/30421, to Goodship et al, published
November 16, 1995, which is incorporated by reference herein in its
entirety, discloses methods for preventing prosthetic loosening and
migration using various bisphosphonate compounds. Administration of
a once weekly partial dose of the bisphosphonate is disclosed. However,
the reference specifically fails to address the issue of adverse
gastrointestinal effects or to disclose administration of larger or multiple
dosages.
It is seen from current teachings that both daily and cyclic
treatment regimens have shortcomings, and that there is a need for
development of a dosing regimen to overcome these shortcomings.
In the present invention, it is found that the adverse
gastrointestinal effects that can be associated with daily or cyclic dosing
regimens can be minimized by administering the bisphosphonate at a
relatively high unit dosage according to a continuous schedule having a
dosing interval selected from the group consisting of once-weekly dosing,
twice-weekly dosing, biweekly dosing, and twice-monthly dosing. In
other words, it is found that the administration of a bisphosphonate at a
high relative dosage at a low relative dosing frequency causes less
adverse gastrointestinal effects, particularly esophageal effects,
compared to the administration of a low relative dosage at a high relative
dosing frequency. This result is surprising in view of the teachings
suggesting that adverse gastrointestinal effects would be expected to
increase as a function of increasing bisphosphonate dosage. Such
administration methods of the present invention would be especially
beneficial in treating patients that have been identified as suffering from
-5-
CA 02349733 2001-06-21
WO 99/04773 ~ PCT/LTS98/I4796
or are susceptible to upper gastrointestinal disorders, e.g.
gastrointestinal reflux disease (i.e. "GERD"), esophagitis, dyspepsia (i.e.
heatburn), ulcers, and other related disorders. In such patients
conventional bisphosphonate therapy could potentially exacerbate or
induce such upper gastrointestinal disorders.
From a patient lifestyle standpoint, the methods of the
present invention would also be more convenient than daily or cyclic
dosing regimens. Patients would be subjected less frequently to the
inconvenience of having to take the drug on an empty stomach and
having to fast for at least BO minutes after dosing. Also, patients would
not need to keep track of a complex dosing regimen. The methods of the
present invention are likely to have the advantage of promoting better
patient compliance, which in turn can translate into better therapeutic
efficacy.
It is an object of the present invention to provide methods for
inhibiting bone resorption and the conditions associated therewith.
It is another object of the present invention to provide
methods for treating abnormal bone resorption and the conditions
associated therewith
It is another object of the present invention to provide
methods for preventing abnormal bone resorption and the conditions
associated therewith.
It is another object of the present invention to provide
methods which are oral methods.
It is another object of the present invention to provide such
methods in humans.
It is another object of the present invention to provide such
methods in patients that have been identified as suffering from or are
susceptible to upper gastrointestinal disorders, e.g. gastrointestinal
reflux disease (i.e. "GERD"), esophagitis, dyspepsia (i.e. heatburn),
ulcers, and other related disorders.
It is another object of the present invention to provide such
methods while minimizing the occurrence of or potential for adverse
gastronintestinal effects.
-6-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
It is another object of the present invention to provide such
methods comprising a continuous dosing schedule having a dosing
interval selected from the group consisting of weekly dosing, twice-
weekly dosing, biweekly dosing, and twice-monthly dosing.
It is another object of the present invention to provide such
methods comprising a continuous dosing schedule having a dosing
periodicity ranging from about once every 3 days to about once every 16
days.
It is another object of the present invention to provide such
methods wherein the continuous dosing schedule is maintained until
the desired therapeutic effect is achieved.
It is another object of the present invention to treat or
prevent abnormal bone resorption in an osteoporotic mammal,
preferably an osteoporotic human.
It is another object of the present invention to provide
pharmaceutical compositions and kits useful in the methods herein.
These and other objects will become readily apparent from
the detailed description which follows. -
SUMMARY OF THE INVENTION
' The present invention relates to methods for inhibiting bone
resorption in a mammal in need thereof, while minimizing the
occurrence of or potential for adverse gastrointestinal effects, said
method comprising orally administering to said mammal a
pharmaceutically effective amount of a bisphosphonate as a unit dosage
according to a continuous schedule having a dosing interval selected
from the group consisting of once-weekly dosing, twice-weekly dosing,
biweekly dosing, and twice-monthly dosing, wherein said continuous
schedule is maintained until the desired therapeutic effect is achieved
for said mammal.
In other embodiments, the present invention relates to
methods comprising a continuous dosing schedule having a dosing
periodicity ranging from about once every 3 days to about once every 16
days.
-7-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14'196
In other embodiments, the present invention relates to
methods for treating abnormal bone resorption in a mammal in need of
such treatment.
In other embodiments, the present invention relates to
methods for preventing abnormal bone resorption in a mammal in need
of such prevention.
In other embodiments, the present invention relates to such
methods useful in humans.
In other embodiments, the present invention relates to such
methods useful in humans indentified as having or being susceptible to
upper gastrointestinal disorders.
In other embodiments, the present invention relates to
methods for treating or preventing osteoporosis in a mammal.
In other embodiments, the present invention relates to
methods for treating or preventing osteoporosis in a human.
In other embodiments, the present invention relates to
methods for inhibiting bone resorption, or treating or preventing
abnormal bone resorption in a human comprising administering to said
human from about 8.75 mg to about 140 mg, on an alendronic acid active
basis, of a bisphosphonate selected from the group consisting of
alendronate, pharmaceutically acceptable salts thereof, and mixtures
thereof.
In other embodiments the present invention relates to a
pharmaceutical composition comprising from about 8.75 mg to about 140
mg, on an alendronic acid active basis, of a bisphosphonate selected
from the group consisting of alendronate, pharmaceutically acceptable
salts thereof, and mixtures thereof.
All percentages and ratios used herein, unless otherwise
indicated, are by weight. The invention hereof can comprise, consist of,
or consist essentially of the essential as well as optional ingredients,
components, and methods described herein.
BRIEF DESCRIPTION OF THE FIGURES
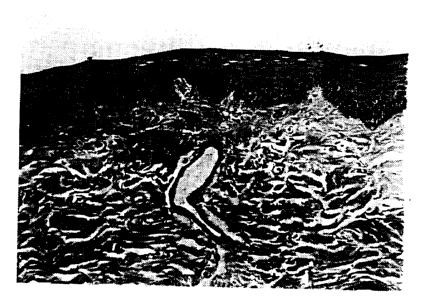
FIG. 1 is a photomicrograph (total magnification 270X) of canine
esophagus tissue (paraffin embedded and stained with hematoxylin and
_g_
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
eosin) from an animal sacrificed immediately after infusion of the last of
five separate dosages of 50 mL of simulated gastric juice administered
on five consecutive days.
FIG. 2 is a photomicrograph {total magnification 270X) of canine
S esophagus tissue (paraffin embedded and stained with hematoxylin and
eosin) from an animal sacrificed immediately after infusion of the last of
five separate dosages of 50 mL of 0.20 mg/mL alendronate in simulated
gastric juice administered on five consecutive days.
FIG. 3 is a photomicrograph (total magnification 270X) of canine
esophagus tissue (paraffin embedded and stained with hematoxylin and
eosin) from an animal sacrificed 24 hours after infusion with a single
dosage of 50 mL of 0.80 mg/mL alendronate in simulated gastric juice.
FIG. 4 is a photomicrograph {total magnification 270X) of canine
esophagus tissue (paraffin embedded and stained with hematoxylin and
eosin) from an animal sacrificed 7 days after infusion with a single
dosage of 50 mL of 0.80 mg/mL alendronate in simulated gastric juice.
FIG. 5 is a photomicrograph (total magnification 2?OX) of canine
esophagus tissue (paraffin embedded and stained with hematoxylin and
eosin) from an animal sacxified 7 days after infusion of the last of 4
separate dosages of 50 mL of 0.80 mg/mL alendronate in simulated
gastric juice administered once per week, i.e. once every 7 days.
FIG. 6 is a photomicrograph (total magnification 270X) of canine
esophagus tissue (paraffin embedded and stained with hematoxylin and
eosin) from an animal sacrified 4 days after infusion of the last of 8
separate dosages of 50 mL of 0.40 mg/mL alendronate in simulated
gastric juice administered twice per week, i.e. once every 3-4 days.
FIG. 7 is a photomicrograph (total magnification 270X) of canine
esophagus tissue (paraffin embedded and stained with hematoxylin and
eosin) from an animal sacrificed immediately after infusion of the last of
five separate dosages of 50 mL of 0.20 mg/mL risedranate in simulated
gastric juice administered an five consecutive days.
FIG. 8 is a photomicrograph (total magnification 270X) of canine
esophagus tissue (paraffin embedded and stained with hematoxyiin and
eosin) from an animal sacrificed immediately after infusion of the last of
_g_
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
five separate dosages of 50 mL of 4.0 mg/mL tiludronate in simulated
gastric juice administered on five consecutive days.
DESCRIPTION OF THE INVENTION
The present invention relates to a method, preferably an
oral method, for inhibiting bone resorption in a mammal in need
thereof, while minimizing the occurrence of or potential for adverse
gastrointestinal effects. The present invention relates to methods of
treating or preventing abnormal bone resorption in a mammal in need
of such treatment or prevention. The methods of the present invention
comprise orally administering to a mammal a pharmaceutically
effective amount of a bisphosphonate as a unit dosage, wherein said
dosage is administered according to a continuous schedule having a
dosing interval selected from the group consisting of once-weekly dosing,
1 S twice-weekly dosing, biweekly dosing, and twice-monthly dosing. In
other embodiments, the present invention relates to methods comprising
a continuous dosing schedule having a dosing periodicity ranging from
about once every 3 days to about once every 16 days. Typically, the
continuous dosing schedule is maintained until the desired therapeutic
effect is achieved for the mammal. .
The present invention utilizes higher unit dosages of the
bisphosphonate at each dosing point than has heretofore been typically
administered, yet because of the dosing schedule chosen, the potential
for adverse gastrointestinal effects are minimized. Moreover, the
method is more convenient because the disadvantages associated with
daily dosing are minimized.
The methods of the present invention are generally
administered to mammals in need of bisphosphonate therapy.
Preferably the mammals are human patients, particularly human
patients in need of inhibiting bone resorption, such as patients in need of
treating or preventing abnormal bone resorption.
The administration methods of the present invention are
especially useful in administering bisphosphonate therapy to human
patients that have been identified as suffering from or are susceptible to
upper gastrointestinal disorders, e.g. GERD, esophagitis, dyspepsia,
-10-
CA 02349733 2001-06-21
WO 99/04773 PCTlUS98/14796
ulcers, etc. In such patients conventional bisphosphonate therapy could
potentially exacerbate or induce such upper gastrointestinal disorders.
The term "pharmaceutically effective amount", as used
herein, means that amount of the bisphosphonate compound, that will
elicit the desired therapeutic effect or response when administered in
accordance with the desired treatment regimen. A preferred
pharmaceutically effective amount of the bisphosphonate is a bone
resorption inhibiting amount.
The term "minimize the occurrence of or potential for
adverse gastrointestinal effects", as used herein, means reducing,
preventing, decreasing, or lessening the occurrence of or the potential
for incurring unwanted side effects in the gastrointestinal tract, i.e. the
esophagus, stomach, intestines, and rectum, particularly the upper
gastrointestinal tract, i.e. the esophagus and stomach. Nonlimiting
adverse gastrointestinal effects include, but are not limited to GERD,
esophagitis, dyspepsia, ulcers, esophageal irritation, esophageal
perforation, abdominal pain, and constipation.
The term "abnormal bone resorption", as used herein
means a degree of bone resorption that exceeds the degree of bone
formation, either locally, or in the skeleton as a whole. Alternatively,
"abnormal bone resorption" can be associated with the formation of bone
having an abnormal structure.
The term "bone resorption inhibiting", as used herein,
means treating or preventing bone resorption by the direct or indirect
alteration of osteoclast formation or activity. Inhibition of bone
resorption refers to treatment or prevention of bone loss, especially the
inhibition of removal of existing bone either from the mineral phase
and/or the organic matrix phase, through direct or indirect alteration of
osteoclast formation or activity.
The terms '"continuous schedule" or "continuous dosing
schedule", as used herein, mean that the dosing regimen is repeated
until the desired therapeutic effect is achieved. The continuous schedule
or continuous dosing schedule is distinguished from cyclical or
intermittent administration.
-11-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
The term "until the desired therapeutic effect is achieved",
as used herein, means that the bisphosphonate compound is
continuously administered, according to the dosing schedule chosen, up
to the time that the clinical or medical effect sought for the disease or
condition is observed by the clinician or researcher. For methods of
treatment of the present invention, the bisphosphonate compound is
continuously administered until the desired change in bone mass or
structure is observed. In such instances, achieving an increase in bone
mass or a replacement of abnormal bone structure with more normal
bone structure are the desired objectives. For methods of prevention of
the present invention, the bisphosphonate compound is continuously
administered for as long as necessary to prevent the undesired
condition. In such instances, maintenance of bone mass density is often
the objective. Nonlimiting examples of administration periods can
1 S range from about 2 weeks to the remaining. Iifespan of the mammal. For
humans, administration periods can range from about 2 weeks to the
remaining lifespan of the human, preferably from about 2 weeks to about
years, more preferably from about 1 month to about 20 years, more
preferably from about 6 months to about 10 years, and most preferably
20 from about 1 year to about 10 years.
lVlethods of the Present IIIVPntinn
The present invention comprises methods for inhibiting
bone resorption in mammals. The present invention also comprises
treating abnormal bone resorption in mammals. The present invention
also comprises methods for preventing abnormal bone resorption in
mammals. In preferred embodiments of the present invention, the
mammal is a human.
The methods of the present invention do not have the
disadvantages of current methods of treatment which can cause or
increase the potential for adverse gastrointestinal effects or which
require cumbersome, irregular, or complicated dosing regimens.
The present invention comprises a continuous dosing
schedule whereby a unit dosage of the bisphosphonate is regularly
administered according to a dosing interval selected from the group
-12-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
consisting of once-weekly dosing, twice-weekly dosing, biweekly dosing,
and twice-monthly dosing.
By once-weekly dosing is meant that a unit dosage of the
bisphosphonate is administered once a week, i.e. one time during a
seven day period, preferably on the same day of each week. In the once-
weekly dosing regimen, the unit dosage is generally administered about
every seven days. A nonlimiting example of a once-weekly dosing
regimen would entail the administration of a unit dosage of the
bisphosphonate every Sunday. It is preferred that the unit dosage is not
administered on consecutive days, but the once-weekly dosing regimen
can include a dosing regimen in which unit dosages are administered
on two consecutive days falling within two different weekly periods.
By twice-weekly dosing is meant that a unit dosage of the
bisphosphonate is administered twice a week, i.e. two times during a
seven day period, preferably on the same two days of each weekly period.
In the twice-weekly dosing regimen, each unit dosage is generally
administered about every three to four days. A nonlimiting example of a
twice-weekly dosing regimen would entail the administration of a unit
dosage of the bisphosphonate every Sunday and Wednesday. It is
preferred that the unit dosages are not administered on the same or
consecutive days, but the twice-weekly dosing regimen can include a
dosing regimen in which unit dosages are administered on two
consecutive days within a weekly period or different weekly periods.
By biweekly dosing is meant that a unit dosage of the
bisphosphonate is administered once during a two week period, i.e. one
time during a fourteen day period, preferably on the same day during
each two week period. In the twice-weekly dosing regimen, each unit
dosage is generally administered about every fourteen days. A
nonlimiting example of a biweekly dosing regimen would entail the
administration of a unit dosage of the bisphosphonate every other
Sunday. It is preferred that the unit dosage is not administered on
consecutive days, but the biweekly dosing regimen can include a dosing
regimen in which the unit dosage is administered on two consecutive
days within two different biweekly periods.
-13-
CA 02349733 2001-06-21
WO 99/04773 PCTIUS98/14796
By twice-monthly dosing is meant that a unit dosage of the
bisphosphonate is administered twice, i.e. two times, during a monthly
calendar period. With the twice-monthly regimen, the doses are
preferably given on the same two dates of each month. In the twice-
monthly dosing regimen, each unit dosage is generally administered
about every fourteen to sixteen days. A nonlimiting example of a
biweekly dosing regimen would entail dosing on or about the first of the
month and on or about the fifteenth, i.e. the midway point, of the month.
It is preferred that the unit dosages are not administered on the same or
consecutive days but the twice-monthly dosing regimen can include a
dosing regimen in which the unit dosages are administered on two
consecutive days within a monthly period, or different monthly periods.
The twice-monthly regimen is defined herein as being distinct from, and
not encompassing, the biweekly dosing regimen because the two
regimens have a di~'erent periodicity and result in the administration of
different numbers of dosages over long periods of time. For example,
over a one year period, a total of about twenty four dosages would be
administered according to the twice-monthly regimen (because there are
twelve calendar months in a year), whereas a total of about twenty six
dosages would be administered according to the biweekly dosing
regimen (because there are about fifty-two weeks in a year).
In further embodiments or descriptions of the present
invention, the unit dosage is given with a periodicity ranging from about
once every 3 days to about once every 16 days.
The methods and compositions of the present invention are
useful for inhibiting bone resorption and for treating and preventing
abnormal bone resorption and conditions associated therewith. Such
conditions include both generalized and localized bone loss. Also, the
creation of bone having an abnormal structure, as in Paget's disease,
can be associated with abnormal bone resorption. The term
"generalized bone loss" means bone loss at multiple skeletal sites or
throughout the skeletal system. The term "localized bone loss" means
bone loss at one or more specific, defined skeletal sites.
Generalized boss loss is often associated with osteoporosis.
Osteoporosis is most common in post-menopausal women, wherein
-14-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
estrogen production has been greatly diminished. However, osteoporosis
can also be steroid-induced and has been observed in males due to age.
Osteoporosis can be induced by disease, e.g. rheumatoid arthritis, it can
be induced by secondary causes, e.g., glucocorticoid therapy, or it can
5 come about with no identifiable cause, i.e. idiopathic osteoporosis. In the
present invention, preferred methods include the treatment or
prevention of abnormal bone resorption in osteoporotic humans.
Localized bone loss has been associated with periodontal
disease, with bone fractures, and with periprosthetic osteolysis (in other
10 words where bone resorption has occured in proximity to a prosthetic
implant).
Generalized or localized bone loss can occur from disuse,
which is often a problem for those confined to a bed or a wheelchair, or
for those who have an immobilized limb set in a cast or in traction.
I S The methods and compositions of the present invention are
useful for treating and or preventing the following conditions or disease
states: osteoporosis, which can include post-menopausal osteoporosis,
steroid-induced osteoporosis, male osteoporosis, disease-induced
osteoporosis, idiopathic osteoporosis; Paget's disease; abnormally
20 increased bone turnover; periodontal disease; localized bone loss
associated with periprosthetic osteolysis; and bone fractures.
The methods of the present invention are intended to
specifically exclude methods for the treatment and/or prevention of
prosthesis loosening and prosthesis migration in mammals as
25 described in PCT application WO 95/30421, to Goodship et al, published
November 16, 1995, which is incorporated by reference herein in its
entirety.
Bisnhosuhonat~
30 The methods and compositions of the present invention
comprise a bisphosphonate. The bisphosphonates of the present
invention correspond to the chemical formula
-15-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
P03H2
A-C-X
P03H2
wherein
A and X are independently selected from the group
consisting of H, OH, halogen, NH2, SH, phenyl, C1-C30 alkyl, C1-C30
substituted alkyl, C1-C10 alkyl or dialkyl substituted NH2, C1-C10 alkoxy,
Cl-C10 alkyl or phenyl substituted thio, C1-C10 alkyl substituted phenyl,
pyridyl, furanyl, pyrrolidinyl, imidazonyl, and benzyl.
In the foregoing chemical formula, the alkyl groups can be
IO straight, branched, or cyclic, provided sufficient atoms are selected for
the chemical formula. The C1-C30 substituted alkyl can include a wide
variety of substituents, nonlimiting examples which include those
selected from the group consisting of phenyl, pyridyl, furanyl,
pyrrolidinyl, imidazonyl, NH2, C1-C10 alkyl or dialkyl substituted NH2,
I 5 OH, SH, and C 1-C 10 alkoxy.
In the foregoing chemical formula, A can include X and X
can include A such that the two moieties can form part of the same
cyclic structure.
The foregoing chemical formula is also intended to
20 encompass complex carbocyclic, aromatic and hetero atom structures
for the A and/or X substituents, nonlimiting examples of which include
naphthyl, quinolyi, isoquinolyl, adamantyl, and chlorophenylthio.
Preferred structures are those in which A is selected from
the group consisting of H, OH, and halogen, and X is selected from the
25 group consisting of C1-C30 alkyl, C1-C30 substituted alkyl, halogen, and
C 1-C 10 alkyl or phenyl substituted thio.
More preferred structures are those in which A is selected
from the group consisting of H, OH, and Cl, and X is selected from the
group consisting of C1-C30 alkyl, C1-C30 substituted alkyl, Cl, and
30 chlorophenylthio.
-16-
CA 02349733 2001-06-21
WO 99/04773 PCT/LTS98/14796
Most preferred is when A is OH and X is a 3-aminopropyl
moiety, so that the resulting compound is a 4-amino-1,-
hydroxybutylidene-1,1-bisphosphonate, i.e. alendronate.
Pharmaceutically acceptable salts and derivatives of the
bisphosphonates are also useful herein. Nonlimiting examples of salts
include those selected from the group consisting alkali metal, alkaline
metal, ammonium, and mono-, di, tri-, or tetra-C 1-C30-alkyl-substituted
ammonium. Preferred salts are those selected from the group
consisting of sodium, potassium, calcium, magnesium, and
ammonium salts. Nonlimiting examples of derivatives include those
selected from the group consisting of esters, hydrates, and amides.
"Pharmaceutically acceptable" as used herein means that
the salts and derivatives of the bisphosphonates have the same general
pharmacological properties as the free acid form from which they are
derived and are acceptable from a toxicity viewpoint.
It should be noted that the terms "bisphosphonate" and
"bisphosphonates", as used herein in referring to the therapeutic agents
of the present invention are meant to also encompass diphosphonates,
biphasphonic acids, and diphosphonic acids, as well as salts and
derivatives of these materials. The use of a specific nomenclature in
referring to the bisphosphonate or bisphosphonates is not meant to limit
the scope of the present invention, unless specifically indicated. Because
of the mixed nomenclature currently in use by those or ordinary skill in
the art, reference to a specific weight or percentage of a bisphosphonate
compound in the present invention is on an acid active weight basis,
unless indicated otherwise herein. For example, the phrase "about 70
mg of a bone resorption inhibiting bisphosphonate selected from the
group consisting of alendronate, pharmaceutically acceptable salts
thereof, and mixtures thereof, on an alendronic acid active weight basis"
means that the amount of the bisphosphonate compound selected is
calculated based on 70 mg of alendronic acid.
Nonlimiting examples of bisphosphonates useful herein
include the following:
Alendronic acid, 4-amino-1-hydroxybutylidene-1,1-
bisphosphonic acid.
-17-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
Alendronate (also known as alendronate sodium or
monosodium trihydrate), 4-amino-1-hydroxybutylidene-1,1-
bisphosphonic acid monosodium trihydrate.
Alendronic acid and alendronate are described in U.S.
Patents 4,922,007, to Kieczykowski et al., issued May 1, 1990, and
5,019,651, to Kieczykowski, issued May 28, 1991, both of which are
incorporated by reference herein in their entirety.
Cycloheptylaminomethylene-1,1-bisphosphonic acid, YM
175, Yamanouchi (cimadronate), as described in U.S. Patent
4,970,335, to Isomura et al., issued November 13, 1990, which is
incorporated by reference herein in its entirety.
1,1-dichloromethylene-1,1-diphosphonic acid (clodronic
acid), and the disodium salt (clodronate, Procter and Gamble), are
described in Belgium Patent 672,205 ( 1966) and J. Org. Chem 32, 4111
I S ( 1967 ), both of which are incorporated by reference herein in their
entirety.
1-hydroxy-3-( 1-pyrrolidinyl)-propylidene-1,1-
bisphosphonic acid (EB-1053).
1-hydroxyethane-1,1-diphosphonic acid (etidronic acid).
1-hydroxy-3-(N-methyl-N-pentylamino )propylidene-1,1-
bisphosphonic acid, also known as BM-210955, Boehringer-
Mannheim (ibandronatel, is described in U.S. Patent No. 4,927,814,
issued May 22, 1990, which is incorporated by reference herein in its
entirety.
6-amino-1-hydroxyhexylidene-1,1-bisphosphonic acid
(neridronate).
3-( dimethylamino )-1-hydroxypropylidene-1,1-
bisphosphonic acid (olpadronate).
3-amino-1-hydroxypropylidene-1,1-bisphosphonic acid
(pamidronate).
[2-(2-pyridinyl)ethylidene]-1,1-bisphosphonic acid
(piridronate) is described in U.S. Patent No. 4,761,406, which is
incorporated by reference in its entirety.
1-hydroxy-2-(3-pyridinyl )-ethylidene-1,1-bisphosphonic
acid (risedronate).
-18-
CA 02349733 2001-06-21
WO 99/04773 ~ PGT/US98/14796
(4-chlorophenyl)thiomethane-1,1-disphosphonic acid
(tiludronate) as described in U.S. Patent 4,876,248, to Breliere et al.,
October 24, 1989, which is incorporated by reference herein in its
entirety.
1-hydroxy-2-( 1H-imidazol-1-yl )ethylidene-1,1-
bisphosphonic acid (zolendronate).
Preferred are bisphosphonates selected from the group
consisting of alendronate, cimadronate, clodronate, tiludronate,
etidronate, ibandronate, risedronate, piridronate, pamidronate,
zolendronate, pharmaceutically acceptable salts thereof, and mixtures
thereof.
More preferred is alendronate, pharmaceutically acceptable
salts thereof, and mixtures thereof.
Most preferred is alendronate monosodium trihydrate.
Pharmace ~t;~at omooRst;o~
Compositions useful in the present invention comprise a
pharmaceutically effective amount of a bisphosphonate. The
bisphosphonate is typically administered in admixture with suitable
pharmaceutical diluents, excipients, or carriers, collectively referred to
herein as "carrier materials", suitably selected with respect to oral
administration, i.e. tablets, capsules, elixirs, syrups, effervescent
compositions, powders, and the like, and consistent with conventional
pharmaceutical practices. For example, for oral administration in the
form of a tablet, capsule, or powder, the active ingredient can be
combined with an oral, non-toxic, pharmaceutically acceptable inert
carrier such as lactose, starch, sucrose, glucose, methyl cellulose,
magnesium stearate, mannitol, sorbitol, croscarmellose sodium and the
like; for oral administration in liquid form, e.g., elixirs and syrups,
effervescent compositions, the oral drug components can be combined
with any oral, non-toxic, pharmaceutically acceptable inert carrier such
as ethanol, glycerol, water and the like. Moreover, when desired or
necessary, suitable binders, lubricants, disintegrating agents, buffers,
coatings, and coloring agents can also be incorporated. Suitable binders
can include starch, gelatin, natural sugars such a glucose, anhydrous
-19-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
lactose, free-flow lactose, beta-lactose, and corn sweeteners, natural and
synthetic gums, such as acacia, guar, tragacanth or sodium alginate,
carboxymethyl cellulose, polyethylene glycol, waxes, and the like.
Lubricants used in these dosage forms include sodium oleate, sodium
stearate, magnesium stearate, sodium benzoate, sodium acetate,
sodium chloride and the like. A particularly preferred tablet
formulation for alendronate monosodium trihydrate is that described in
U.S. Patent No. 5,358,941, to Bechard et al, issued October 25, 1994, which
is incorporated by reference herein in its entirety. The compounds used
in the present method can also be coupled with soluble polymers as
targetable drug carriers. Such polymers can include
polyvinylpyrrolidone, pyran copolymer, polyhydroxylpropyl-
methacrylamide, and the like.
The precise dosage of the bisphonate will vary with the
dosing schedule, the oral potency of the particular bisphosphonate
chosen, the age, size, sex and condition of the mammal or human, the
nature and severity of the disorder to be treated, and other relevant
medical and physical factors. Thus, a precise pharmaceutically
effective amount cannot be specified in advance and can be readily
determined by the caregiver or clinician. Appropriate amounts can be
determined by routine experimentation from animal models and human
clinical studies. Generally, an appropriate amount of bisphosphonate is
chosen to obtain a bone resorption inhibiting effect, i.e. a bone resorption
inhibiting amount of the bisphosphonate is administered. For humans,
an effective oral dose of bisphosphonate is typically from about 1.5 to
about 6000 ~.g/kg body weight and preferably about 10 to about 2000 ~.g/kg
of body weight.
For human oral compositions comprising alendronate,
pharmaceutically acceptable salts thereof, or pharmaceutically
acceptable derivatives thereof, a unit dosage typically comprises from
about 8.75 mg to about 140 mg of the alendronate compound, on an
alendronic acid actave weight basis.
For once-weekly dosing, an oral unit dosage comprises from
about 17.5 mg to about 70 mg of the alendronate compound, on an
alendronic acid active weight basis. Examples of weekly oral dosages
-20-
CA 02349733 2001-06-21
WO 99/04773 - PCT/US98/14796
include a unit dosage which is useful for osteoporosis prevention
comprising about 35 mg of the alendronate compound, and a unit dosage
which is useful for treating osteoporosis comprising about 70 mg of the
alendronate compound.
For twice-weekly dosing, an oral unit dosage comprises
from about 8.75 mg to about 35 mg of the alendronate compound, on an
alendronic acid active weight basis. Examples of twice-weekly oral
dosages include a unit dosage which is useful for osteoporosis
prevention comprising about 17.5 mg of the alendronate compound, and
a unit dosage which is useful for osteoporosis treatment, comprising
about 35 mg of the alendronate compound.
For biweekly or twice-monthly dosing, an oral unit dosage
comprises from about 35 mg to about 140 mg of the alendronate
compound, on an alendronic acid active weight basis. Examples of
I S biweekly or twice-monthly oral dosages include a unit dosage which is
useful for osteoporosis prevention comprising about ?0 mg of the
alendronate compound, and a unit dosage which is useful for
osteoporosis treatment, comprising about 140 mg of the alendronate
compound.
Nonlimiting examples of oral compositions comprising
alendronate, as well as other bisphosphonates, are illustrated in the
Examples, below.
enti 1 A i i r min H2 or 1 d/ r
Proton Pump Inhihitr,rc W;+~ R~SDhoQnhonatPc
In further embodiments, the methods and compositions of
the present invention can also comprise a histamine H2 receptor blocker
(i.e. antagonist) and/or a proton pump inhibitor. Histamine H2 receptor
blockers and proton pump inhibitors are well known therapeutic agents
for increasing gastric pH. See L.J. Hixson, et al., Current Trends in the
Pharmacotheracpy for Peptic Ulcer Disecxse, Arch. Intern. Med., vol. 152,
pp. 726-732 (April 1992), which is incorporated by reference herein in its
entirety. It is found in the present invention that the sequential oral
administration of a histamine H2 receptor Mocker and/or a proton pump
inhibitor, followed by a bisphosphonate can help to further minimize
-21-
CA 02349733 2001-06-21
WO 99/04773 PCTJUS98/14796
adverse gastrointestinal effects. In these embodiments, the histamine
H2 receptor blocker and/or proton pump inhibitor is administered from
about 30 minutes to about 24 hours prior to the administration of the
bisphosphonate. In more preferred embodiments, the histamine H2
receptor blocker and/or proton pump inhibitor is administered from
about 30 minutes to about 12 hours prior to the administration of the
bisphonate.
The dosage of the histamine H2 receptor blocker and/or
proton pump inhibitor will depend upon the particular compound
selected and factors associated with the mammal to be treated, i.e. size,
health, etc.
Nonlimiting examples of histamine H2 receptor blockers
and/or proton pump inhibitors include those selected from the group
consisting of cimetidine, famotidine, nizatidine, ranitidine, omprazole,
and lansoprazole.
Treatment 'ts
In further embodiments, the present invention relates to a
kit for conveniently and effectively carrying out the methods in
accordance with the present invention. Such kits are especially suited
for the delivery of solid oral forms such as tablets or capsules. Such a kit
preferably includes a number of unit dosages. Such kits can include a
card having the dosages oriented in the order of their intended use. An
example of such a kit is a "blister pack". Blister packs are well known in
the packaging industry and are widely used for packaging
pharmaceutical unit dosage forms. If desired, a memory aid can be
provided, for example in the form of numbers, letters, or other markings
or with a calendar insert, designating the days in the treatment
schedule in which the dosages can be administered. Alternatively,
placebo dosages, or calcium or dietary supplements, either in a form
similar to or distinct from the bisphosphonate dosages, can be included
to provide a kit in which a dosage is taken every day. In those
embodiments including a histamine H2 receptor and/or proton pump
inhibitor, these agents can be included as part of the kit.
-22-
CA 02349733 2001-06-21
WO 99/04773 PCT/L1S98/14'796
F_X_AMpLE,~S
The following examples further describe and demonstrate
embodiments within the scope of the present invention. The examples
are given solely for the purpose of illustration and are not to be construed
as limitations of the present invention as many variations thereof are
possible without departing from the spirit and scope of the invention.
Esophageal Irritation Potential
The esophageal irritation potential of the bisphosphonates is
evaluated using a dog model.
The experiments demonstrate the relative irritation
potential of the following dosing regimens: placebo (Group 1), a single
high concentration dosage of alendronate monosodium trihydrate
(Group 2), a low concentration dosage of alendronate monosodium
trihydrate administered for five consecutive days (Groups 3 and 4), a
high concentration dosage of alendronate monosodium trihydrate
administered once per week for four weeks (Group 5), a mid-range
concentration dosage of alendronate monosodium trihydrate
administered twice per week for four weeks (Group 6), a low dosage of
risedronate sodium administered for five consecutive days (Group ?),
and a low dosage of tiludronate disodium administered for five
consecutive days (Group 8 ).
The following solutions are prepared:
(1) simulated gastric juice (pH about 2), i.e. the control
solution.
(2) simulated gastric juice (pH about 2) containing about 0.20
mg/mL of alendronate monosodium trihydrate on an
alendronic acid active basis.
(3) simulated gastric juice (pH about 2) containing about 0.80
mg/mL of alendronate monosodium trihydrate on an
alendronic acid active basis.
-23-
CA 02349733 2001-06-21
WO 99104773 ~ PCT/US98/14796
(4) simulated gastric juice (pH about 2) containing about 0.40
mg/mL of alendronate monosodium trihydrate on an
alendronic acid active basis.
(5) simulated gastric juice (pH about 2) containing about 0.20
mg/mL of risedronate sodium on a risedronic acid active
basis.
(6) simulated gastric juice (pH about 2) containing about 4.0
mg/mL of tiludronate disodium on a tiludronic acid active
basis.
The simulated gastric juice is prepared by dissolving about 960 mg of
pepsin (L-585,2280008003, Fisher Chemical) in about 147 mL of 0.90 (wt
%) NaCl (aqueous), adding about 3mL of 1.0 M HCl (aqueous), and
adjusting the volume to about 300 mL with deionized water. The pH of
the resulting solution is measured and if necessary is adjusted to about 2
using 1.0 M HCl (aqueous) or 1.0 M NaOH (aqueous).
The animals used in the experiments are anesthetized and
administered about 50 mL of the appropriate solution over about 30
minutes by infusion into the esophagus using an infusion pump and a
rubber catheter. The following treatment experiments are run:
Group 1: This control group contains four animals: Each animal
is administered a dosage of about 50 mL of simulated gastric juice
[solution (1)] on each of five consecutive days. The animals are
sacrificed immediately after the last dose is administered.
Group 2: This group contains four animals. Each animal is
administered a dosage of about 50 mL of simulated gastric juice
containing about 0.20 mg/mL of alendronate [solution (2)] on each
of five consecutive days. The animals are sacrificed immediately
after the last dose is administered.
Group 3: This group contains five animals. Each animal is
administered a dosage of about 50 mL of simulated gastric juice
containing about 0.80 mg/mL of alendronate [solution (3)] on a
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
single treatment day. The animals are sacrificed about 24 hours
after the dose is administered.
Group 4: This group contains five animals. Each animal is
administered a dosage of about 50 mL of simulated gastric juice
containing about 0.80 mg/mL of alendronate [solution (3)] on a
single treatment day. The animals are sacrificed about 7 days
after the dose is administered.
Group 5: This group contains six animals. Each animal is
administered a dosage of about 50 mL of simulated gastric juice
containing about 0.80 mg/mL of alendronate [solution (3)] once per
week, i.e. every seven days, for four weeks. The animals are
administered a total of four dosages. The animals are sacrificed
about 7 days after the last dose is administered.
Group 6: This group contains six animals. Each animal is
administered a dosage of about 50 mL of simulated gastric juice
containing about 0.40 mg/mL of alendronate [solution (4)] twice
per week, i.e. every three to four days, for four weeks. The
animals are administered a total of eight dosages. The animals
are sacrificed about four days after the last dose is administered.
Group 7: This group contains eight animals. Each animal is
administered a dosage of about 50 mL of simulated gastric juice
containing about 0.20 mg/mL of risedronate [solution (5)] on each
of five consecutive days. The animals are sacrificed immediately
after the last dose is administered.
Group 8: This group contains four animals. Each animal is
administered a dosage of about 50 mL of simulated gastric juice
containing about 4.0 mg/mL of tiludronate [solution (6)] on each of
five consecutive days. The animals are sacrificed immediately
after the last dose is administered.
-25-
CA 02349733 2001-06-21
WO 99104773 PCT/US98/14796
The esophagus from each sacrificed animal is removed and
prepared for histopathology using standard techniques by embedding the
tissue in para~n, staining with hematoxylin and eosin. The sections
are examined microscopically. The histopathology results are
summarized in Table 1.
For the Group 1 animals (control group), the
photomicrographs show that the esophagus is normal with an intact
epithelium and absence of inflammatory cells in the submucosa. FIG. 1
is a representative photomicrograph from a Group 1 animal.
For the Group 2 animals, the photomicrographs show that
the esophagus exhibits deep ulceration of the epithelial surface and
marked submucosal inflammation and vacuolation. FIG. 2 is a
representative photomicrograph from a Group 2 animal.
For the Group 3 animals, the photomicrographs show that
the esophagus has an intact epithelial surface with very slight
submucosal inflammation and vacuolation. FIG. 3 is a representative
photomicrograph from a Group 3 animal.
For the Group 4 animals, the photomicrographs show that
the esosphagus has an intact epithelium with either minimal
inflammation (two of the five animals) or no inflammation (three of the
five animals) and no vacuolation. FIG. 4 is a representative
photomicrograph from a Group 4 animal exhibiting minimal
inflammation.
For the Group 5 animals, the photomicrographs show that
the esophagus is normal with an intact epithelium and absence of
inflammatory cells in the submucosa. FIG. 5 is a representative
photomicrograph from a Group 5 animal.
For the Group 6 animals, the photomicrographs show that
the esophagus exhibits deep ulceration of the epithelial surface and
marked submucosal inflammation and vacuolation. FIG. 6 is a
representative photomicrograph from a Group 6 animal.
For the Group 7 animals, the photomicrographs show that
the esophagus exhibits deep ulceration of the epithelial surface and
marked submucosal inflammation and vacuolation. FIG. 7 is a
representative photomicrograph from a Group 7 animal.
-26-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/i4796
For the Group 8 animals, the photomicrographs show that
the esophagus exhibits slight ulceration of the epithelial surface and
slight submucosal inflammation and vacuolation. FIG. 8 is a
representative photomicrograph from a Group 8 animal.
These experiments demonstrate that considerably less esophageal
irritation (comparable to control Group 1)is observed from the
administration of a single high concentration dosage of alendronate
(Groups 3 and 4) versus administration of low concentration dosages on
consecutive days (Group 2). These experiments also demonstrate
consideraly less esophageal irritation is observed from the
administration of a single high concentration of alendronate on a weekly
basis (Group 5) or twice-weekly basis (Group 6) versus administration of
low concentration dosages on consecutive days (Group 2). These
experiments also demonstrate that when other bisphosphonates such as
risedronate (Group 7) or tiludronate (Group 8) are administered at low
dosages on consecutive days that the esophageal irritation potential is
high.
-27-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
Table 1.
---
Esopha,~eal
Irritation
Potential
Studies
Group Active Dosing SacrificeHisto-pathology
Agent Schedule Time
m /mL
1 0 I X dailyimmediateNormal. Intact
(n=4) for 5 ly afterepithelium and
days
' last absence of
dosing inflammatory cells
in the submucosa.
2 Alendronate IX daily immediateDeep ulceration
of
(n=4) 0.20 for 5 ly afterepithelial surface.
days
last Marked submucosal
dosing inflammation and
vacuolation.
3 Alendronate 1X 24 hoursIntact epithelial
(n=5) 0.80 after surface with very
dosing slight submucosal
inflammation and
vacuolation.
4 Alendronate 1X _ Intact epithelium
7 days
(n=5) 0.80 after with either minimal
dosing inflammation (2
of
5 animals) or
no
inflammation (3
of
5 animals) and
no
vacuolation.
5 Alendronate IX 7 days Intact epithelium
(n=6) 0.80 weekly after with no
last
for a dosing inflammation and
total no
of 4 doses vacuolation.
-28-
CA 02349733 2001-06-21
WO 99/04773 PGT/C1S98/14796
6 Alendronate 2X immediateDeep ulceration
(n=6) 0.40 weekly ly after of
for 4 last epithelial surface.
weeks dosing Marked submucosal
inflammation
and
vacuolation.
Risedronate 1 X dailyimmediateDeep ulceration
of
(n=8) 0.20 for 5 ly after epithelial surface
days (4
last of 8 animals).
dosing Marked submucosal
inflammation
and
vacuolation.
8 Tiludronate 1X daily 24 hours Slight submucosal
(n=4) 4.0 for 5 after inflammation
days last and
dosing vacuolation (3
of 4
animals, including
1
of these animals
with slight
ulceration).
Once-weekly dosing regimen.
10
Treatment of osteoporosis.
Alendronate tablets or liquid formulations containing about
70 mg of alendronate, on an alendronic acid active basis, are prepared
(see EXAMPLES 7 and 8). The tablets or liquid formulations are orally
administered to a human patient once-weekly, i.e. preferably about once
every seven days (for example, every Sunday), for a period of at least one
year. This method of administration is useful and convenient for
treating osteoporosis and for minimizing adverse gastrointestinal
effects, particularly adverse esophageal effects. This method is also
useful for improving patient acceptance and compliance.
-29-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
Prevention of osteoporosis.
Alendronate tablets or liquid formulations containing about
35 mg of alendronate, on an alendronic acid active basis, are prepared
(see EXAMPLES 7 and8 ). The tablets or liquid formulations are orally
administered to a human patient once-weekly, i.e. preferably about once
every seven days (for example, every Sunday), for a period of at least one
year. This method of administration is useful and convenient for
preventing osteoporosis and for minimizing adverse gastrointestinal
effects, particularly adverse esophageal effects. This method is also
useful for improving patient acceptance and compliance.
Twice-weekly dosing regimen.
IS
Treatment of osteoporosis.
Alendronate tablets or liquid formulations containing about
35 mg of alendronate, on an alendronic acid active basis, are prepared
(see EXAMPLES 7 and 8). The tablets or liquid formulations are orally
administered to a human patient twice-weekly, preferably about once
every three or four days (for example, every Sunday and Wednesday), for
a period of at least one year. This method of administration is useful
and convenient for treating osteoporosis and for minimizing adverse
gastrointestinal effects, particularly adverse esophageal effects. This
method is also useful for improving patient acceptance and compliance.
Prevention of osteoporosis.
Alendronate tablets or liquid formulations containing about
17.5 mg of alendronate, on an alendronic acid active basis, are prepared
(see EXAMPLES ? and8). The tablets or liquid formulations are orally
administered to a human patient twice-weekly, preferably about once
every three or four days (for example, every Sunday and Wednesday), for
a period of at least one year. This method of administration is useful
and convenient for preventing osteoporosis and for minimizing adverse
-30-
CA 02349733 2001-06-21
WO 99/04773 ~ PCT/U598/14796
gastrointestinal effects, particularly adverse esophageal effects. This
method is also useful for improving patient acceptance and compliance.
ALE 4
Biweekly dosing regimen
Treatment of osteoporosis.
Alendronate tablets or liquid formulations containing about
140 mg of alendronate, on an alendronic acid active basis, are prepared
(see EXAMPLES 7 and 8). The tablets or liauir~ fnrmmlat;n"~ a,.o ,...~11,~
administered to a human patient biweekly, i.e. preferably about once
every fourteen days (for example, on alternate Sundays), for a period of
at least one year. This method of administration is useful and
I S convenient for treating osteoporosis and for minimizing adverse
gastrointestinal effects, particularly adverse esophageal effects. This
method is also useful for improving patient acceptance and compliance.
Prevention of osteoporosis.
Alendronate tablets or liquid formulations containing about
70 mg of alendronate, on an alendronic acid active basis, are prepared
(see EXAMPLES 7 and 8). The tablets or liquid formulations are orally
administered to a human patient biweekly, i.e. preferably about once
every fourteen days (for example, on alternate Sundays), for a period of
at least one year. This method of administration is useful and
convenient for preventing osteoporosis and for minimizing adverse
gastrointestinal effects, particularly adverse esophageal effects. This
method is also useful for improving patient acceptance and compliance.
EXAMPLE 5
Twice-monthly dosing regimen.
Treatment of osteoporosis.
-31-
CA 02349733 2001-06-21
WO 99/04773 PCT/US98/14796
Alendronate tablets or liquid formulations containing about
140 mg of alendronate, on an alendronic acid active basis, are prepared
(see EXAMPLES 7 and 8). The tablets or liquid formulations are orally
administered to a human twice-monthly, i.e. preferably about once every
S fourteen to sixteen days (for example, on about the first and fifteenth of
each month), for a period of at least one year. This method of
administration is useful and convenient for treating osteoporosis and for
minimizing adverse gastrointestinal effects, particularly adverse
esophageal effects. This method is also useful for improving patient
acceptance and compliance.
Prevention of osteoporosis.
Alendronate tablets or liquid formulations containing about
?0 mg of alendronate, on an alendronic acid active basis, are prepared
(see EXAMPLES 7 and 8). The tablets or liquid formulations are orally
administered to a human patient biweekly, i.e. preferably once every
fourteen to sixteen days (for example, on about the first and fifteenth of
each month), for a period of at least one year. This method of
administration is useful and convenient for preventing osteoporosis and
for minimizing adverse gastrointestinal effects, particularly adverse
esophageal effects. This method is also useful for improving patient
acceptance and compliance.
EXAMPLE 6
In further embodiments, alendronate tablets or liquid
formulations are orally dosed, at the desired dosage, according to the
dosing schedules of EXAMPLES 2-5, for treating or preventing other
disorders associated with abnormal bone resorption.
In yet further embodiments, other bisphosphonate
compounds are orally dosed, at the desired dosage, according to the
dosing schedules of EXAMPLES 2-5, for treating or preventing
osteoporosis or for treating or preventing other conditions associated
with abnormal bone resorption.
-32-
CA 02349733 2001-06-21
WO 99104773 PCT/US98/14796
EXAMPLE 7
Bisphosphonate tablets.
Bisphosphonate containing tablets are prepared using
standard mixing and formation techniques as described in U.S. Patent
Na. 5,358,941, to Bechard et al., issued October 25, 1994, which is
incorporated by reference herein in its entirety.
Tablets containing about 35 mg of alendronate, on an
alendronic acid active basis, are prepared using the following relative
weights of ingredients.
ln~redient Per Tablet Per 4000 Tablets
Alendronate Monosodium 45.68 mg 182.72
g
Trihydrate
Anhydrous Lactose, NF 71.32 mg 285.28
g
Microcrystalline Cellulose, 80.0 mg 320.0
g
NF
Magnesium Stearate, NF 1.0 mg 4.0 g
Croscarmellose Sodium, NF 2.0 mg 8.0 g
The resulting tablets are useful for administration in
accordance with the methods of the present invention for inhibiting bone
resorption.
Similarly, tablets comprising other relative weights of
alendronate, on an alendronic acid active basis are prepared: e.g., about
8.75, 17.5, 70, and 140 mg per tablet. Also, tablets containing other
bisphosphonates at appropriate active levels are similarly prepared:
e.g., cimadronate, clodronate, tiludronate, etidronate, ibandronate,
risedronate, piridronate, pamidronate, zolendronate, and
pharmaceutically acceptable salts thereof. Also, tablets containing
combinations of bisphosphonates are similarly prepared.
-33-
CA 02349733 2001-06-21
WO 99/04773 ~ PCT/US98/14796
Liquid Bisphosphonate Formulation.
Liquid bisphosphonate formulations are prepared using
standard mixing techniques.
A liquid formulation containing about 70 mg of alendronate
monosodium trihydrate, on an alendronic acid active basis, per about 75
mL of liquid is prepared using the following relative weights of
ingredients.
Ingredient Weight
Alendronate Monosodium 91.35 mg
Trihydrate
Sodium Propylparaben 22.5 mg
Sodium Butylparaben 7.5 mg
Sodium Citrate Dehydrate 1500 mg
Citric Acid Anhydrous 56.25 mg
Sodium Saccharin 7.5 mg
Water qs 75 mL
1 N Sodium Hydroxide (aq) qs pH 6.75
25 The resulting liquid formulation is useful for
administration as a unit dosage in accordance weth the methods of the
present invention for inhibiting bone resorption.
Similarly, liquid formulations comprising other relative
weights of alendronate, on an alendronic acid active basis, per unit
dosage are prepared: e.g., about 8.75, 17.5, 35, and 140 mg per 75 mL
volume. Also, the liquid formulations are prepared to provide other
volumes for the unit dosage, e.g. about 135 mL. Also, the liquid
formulations are prepared containing other bisphosphonates at
appropriate active levels: e.g., cimadronate, clodronate, tiludronate,
etidronate, ibandronate, risedronate, piridronate, pamidronate,
-34-
CA 02349733 2001-06-21
WO 99104773 PCT/US98/14796
zolendronate, and pharmaceutically acceptable salts thereof. Also,
liquid formulations containing combinations of bisphosphonates are
similarly prepared.
-35-