Note: Descriptions are shown in the official language in which they were submitted.
CA 02350145 2003-O1-21 .. .
WO #"!cTNS99/165Z1
IiEALTIi MONITORING AND DIAGNOSTIC DEVICE AND
NE~'WORK-BASED HEALTH ASSESSMI~NT AND MEDICAL
RECORDS MAINTENANCE SYSTEM
TECHI~TiCAL FIELD
This invention relates to health monitoring and diagnostic devices and, more
particularly, relates to a hand held device operable for determining blood
lipid
levels from test-strip analyses, obtaining additional diagnostic information
from a
user, displaying corresponding diagnostic results, and storing this data on a
secure
patient held data carrier, such as a smartcard. The invention also relates ~
to a
secure network based health assessment and medical records maintenance system
that receives medical information from the health monitoring and diagnostic
devices, produces health assessments based on the received medical
information,
and stores the received medical information in a secure xx~ical records
maintenance system.
BACKGROUND OF THE INVEI~tTJON
American health care is undergoing a revolution. By the year 2000, more
than two-thirds of all American workers with health insurance wall be enrolled
in
1
CA 02350145 2001-05-09
PCTNS99/26521
W O 00/28460
kind of managed care plan, where the emphasis is on early detection of
some
disease and preventive care.
Fuelin this revolution is the skyrocketing cost of health care, combined
g
new medical research showing lifestyle is important to good health. In fact,
with
its 1982 report on "Health and Behavior," the National Academy of Sciences
in
concluded that half of the ten leading causes of death in the United States
are
'maril related to lifestyle. Dietary patterns are identified as key lifestyle
choices.
pn Y
Cholesterol levels are particularly important in the United States. For this
n the American Heart Association, the Amencan Medical Association and
reaso ,
ealth related agencies of the U.S. government have embarked on national
the h
cation campaigns to inform the public about the importance of making lifestyle
edu
es to lower blood cholesterol and prevent heart disease. Although the
chang
ers of hi h cholesterol have been widely publicized, many people fail to make
dang g
ive use of this information because they do not know their own blood
effect
esterol levels. In other words, a great many of the people with high
chol
esterol levels fail to heed the advice to lower their cholesterol levels
simply
chol
because they are unaware of their own cholesterol levels.
This situation persists because of the high cost and inconvenience presently
involved in obtaining cholesterol information. To obtain this information,
most
le o to a hysician's office, have blood drawn, and wait for the return of the
peop g P
d chemistry analysis. Often, obtaining the results involves a second trip to
the
bloo
ian's office. This is expensive and time consuming; the average cost is
physic
t 83 for each office cholesterol consultation, and the average wait for the
abou $
results is several days.
The cost and inconvenience involved in obtaining cholesterol tests inhibits
eo le from testing their cholesterol frequently enough to provide effective
many p P
ositive feedback. As a result, many people who begin corrective exercise,
diet, or
P
thera rograms in response to high cholesterol tests often give up their
drug PY P
ective rograms because they do not monitor their cholesterol frequently
corn P
h to remain aware of the benefits of their programs. Moreover, blood
enoug
sterol numbers by themselves are often poor motivators for patients who feel
chole
d look fine, and do not immediately feel or look differently when they take
their
an
cri tions. In fact, studies have shown that 80% of the patients prescribed
pres p
2
CA 02350145 2003-O1-21
cholesterol-lowering drug therapies stop taking their prescriptions within a
few
months. And the attrition rates .for exercise and diet programs may be even
higher.
In addition, there is a need for a medical records maintenance system, not
only for blood cholesterol tests.but for many types of medical information
that
can be obtained outside of the hospital environment. "This need will increase
with
increases in the availability of remote health monitoring devices in the
future,
such as blood pressure measuring devices, blood sugar testing devices, blood
cholesterol testing devices, AIDS testing devices, heart monitoring devices,
sleep
respiration monitoring devices, reproductive cycle and pregnancy monitoring
devices, epileptic and other types of seizure monitoring devices, and a wide
range
of other remote health monitoring devices that may be developed in the future.
As the availability of the remote health monitoring devices increases, users
will
have an increasing need for securely storing the tests results in electronic
format.
~ 5. The cur-rent s3rstem of hard-copy and electronic medical recur-ds
maintained in
doctors' offices will become increasingly obsolete and inconvenient as the
availability of electronically-stored medical data increases. Because a
patient's
medical records are highly confidential, there is a need for a highly secure
and
permanent medical records maintenance system under the control of individual
patients and their doctors.
Downer et. al., U.S. Patent No. 4,975,647 describes an apparatus for
controlling the use of consumable accessory units with machines in which a
memory device associated with each accessory unit holds information concerning
the associated accessory unit. The memory device for an associated accessory
2~ unit stores information indicative of a pre-determined class of machines
with
which the accessory unit is intended to be used. The machine circuitry issues
a
signal when stored information indicates that the machine is not within a
class
intended for use with a particular accessory unit.
A document entitled "Clinical Instrument Systems" by Nelson L. Alpert,
describes the i-stat point-of care testing system. This system includes a
3
CA 02350145 2003-O1-21
removable cartridge that performs an analytical process on an analyte, such as
human blood. The cartridge is insertable into a hand-held unit which converts
electrical signals from the cartridge into reportable results, which are
displayed
on a display device located on the handheld unit. A removable printer may be
attached to the hand-held device for printing a report of the test results
computed
by the cartridge.
Worthington et. al., U.S. Patent No. 5,822,715, describes a diabetes
management system and method for controlling blood glucose. The system
includes a blood sugar meter including a user input device for entering into
the
meter insulin doses administered to a patient. The blood sugar test results
and
insulin dose information may be downloaded to a computer which computes
future blood glucose concentration estimates for the patient. This predicted
blood sugar information is then transmitted back to the meter for display to
the
patient.
15. Fenson et. al., International Publication No. W0 x$/243.58, describes a
system for downloading and reporting medical information. This system
describes an Internet transfer methodology for medical records and a system
for
viewing the records-with a standard Internet browser.
Johnson et. al., International Publication No. WO 96141288 describes an
apparatus and method for centralized storage of heterogeneous medical records
in
a managed healthcare organization, The system describes a central medical
record repository for a managed healthcare organization that accepts and
stores
medical records in different formats from various medical service providers.
The
system describes a methodology for automatically extracting certain
information
from the medical records and storing the extracted data in a document
database.
A document entitled "Trusted Third Party Services for Healthcare in
Europe" by Despina Polemi describes a secure session layer (SSL) protocol for
establishing secure communications among healthcare providers using the
Internet to exchange medical data. The SSL protocol supports certificate
validation and authentication services to provide security for system users.
The
3A
CA 02350145 2003-O1-21
SSL protocol is embedded in the web tools used and operates on the TCP layer
of
the TCP/IP Internet protocol suite.
However, none of these technologies address the general need in the art
for a less expensive and more convenient approach to providing cholesterol
tests.
There is a further need for making motivational information regarding
cholesterol
levels more readily available and more effective. .And there is yet another
need
for a highly secure and permanent medical records maintenance system under the
control of individual patients and their doctors.
SLfll~a~lARY OF THE INVENTION
The present invention meets the needs described above ui a health
monitoring and diagnostic device referred to as a LIFESTREAM cholesterol
meter. This meter is configured as a self contained testing and diagnostic
unit in
a clam-shell type case. One side of the case includes a biological sample
gathering d~vic-~; such as spring-loaded f roger ~ek, and a e~n3~ar~ent f$r
carrying one or
(The remainder of this page is intentionally left blank)
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
more packages of disposable items, typically including a test strip, a needle
for the
finger stick, and an alcohol swipe. The other half of the case includes a test
strip
reader, a user input device such as a key pad, and a display device such as a
liquid
crystal display. The meter reads a test strip carrying a biological sample,
such as a
droplet of blood, and within minutes displays test results, such as total
cholesterol
levels, on the meter's display.
The hand-held LIFESTREAM cholesterol meter drastically reduces the
costs and inconvenience associated with obtaining cholesterol tests by
performing
total cholesterol tests in virtually any location, including a physician's
office, a
l0 pharmacy, a clinic, or in the privacy of the patient's home. The meter
produces
the test results within minutes using on-board circuitry and programming. The
meter also includes an on-board diagnostic program that prompts for additional
diagnostic information, such as the patient's age, gender, weight, family
history of
heart disease, blood pressure, and so forth.
The meter then translates this diagnostic information, along with the test
results, into diagnostic results that may be more meaningful to the user than
the
test results alone. For example, the meter may use a well-known methodology,
such as the Framingham Medical Study, to produce diagnostic results including
the user's cardiac age (as compared to chronological age), recommended weight
loss, 5-year risk of heart attack, 10-year risk of heart attack, an assessment
of
stroke risk, and other results that will be easily and immediately understood
by the
patient. Like the test results themselves, these more meaningful diagnostic
results
are displayed on the meter within minutes.
Producing diagnostic results like "cardiac age" and "5-year risk of heart
attack" rather than total cholesterol levels alone may motivate more people to
change their lifestyles and reduce their cholesterol levels. Moreover,
producing
these diagnostic results instantaneously, inexpensively, and in a convenient
location
encourages frequent testing and provides patients with the positive feedback
necessary to encourage continued compliance with drug therapies and lifestyle
changes. Ultimately, widespread use of the LIFESTREAM cholesterol meter can
be expected to improve cardiac health nationwide, shift the focus of cardiac
treatment from corrective to preventative, improve the cardiac health of the
population in general, and reduce medical costs and health insurance rates.
4
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
The benefits of the LIFESTREAM cholesterol meter may be improved
over time and extended to other health problems because the meter is
programmable and configured to perform multiple types of tests. That is,
although the meter will be initially configured to perform total cholesterol
tests
using test strips and human blood samples, it is also configured to perform
multiple types of tests using different types of test strips or other test
media
carrying other types of biological fluid or tissue samples. For example, the
meter
may also produce other types of blood lipid test results, such as HDL
cholesterol,
triglycerides, LDL cholesterol, etc. The meter may also perform other types of
l0 tests, such as blood glucose tests, AIDS tests, cancer tests, and virtually
any other
type of test that can be performed using a test strip or another suitable test
medium carrying a sample of biological fluid or tissue. To accommodate
multiple
tests, the meter typically includes four ronnkey sockets that allow the meter
to
carry and read four different romkeys.
The LIFESTREAM cholesterol meter also works in connection with a
network-based comprehensive health analysis and reporting system. The meter
includes a data drive that writes patient data stored within the meter to a
patient-
held data storage device, such as a smartcard. This patient data typically
includes
patient identification information, the test results, the diagnostic
information, and
the diagnostic results. A computer station, such as a typical desktop or
laptop
personal computer, can then read the smartcard and establish a network
connection with a health report server, typically over the Internet. The
computer
then downloads the patient data to the health report server, which prepares a
comprehensive health report. This report is then transmitted back to the
computer station, where it is printed out and delivered to the patient.
The health report server typically works in concert with the patient's
physician or pharmacist, who may provide additional diagnostic information to
the
server, such as a newly-prescribed drug therapy, other currently-prescribed
drugs
for the patient, exercise and dietary recommendations, and so forth. Within
minutes, the health report server assembles a comprehensive health report
including a data sheet for the newly-prescribed drug, cross-reaction
information
for the newly-prescribed drug and the other currently-prescribed drugs, weight
and total cholesterol goals, exercise and dietary recommendations, any food or
5
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
activity warnings associated with the overall therapy package, and
recommendations for on-going monitoring using the meter. This provides a
complete written record of the patient's current condition, the therapy
prescribed
by the physician and filled by the pharmacist, and a roadmap for monitoring
the
patient's progress during the ensuing therapy.
The comprehensive health report may also include additional patient-specific
information, such as the diagnostic information and results compiled by the
meter,
and additional diagnostic and health assessment information compiled by the
server. For example, the report may include a trend analysis showing how
cholesterol, blood glucose, and weight levels have changed over multiple
readings.
The report may also include generally-applicable educational information, such
as
coronary risk factors, dietary guidelines for reducing cholesterol levels,
diabetes
information, cancer information, and the like. At present, a patient may have
to
undergo a physical examination, pay thousands of dollars, and wait weeks to
obtain a similar comprehensive health report. The network-based comprehensive
health analysis and reporting system, working in concert with the LIFESTREAM
cholesterol meter, allows the patient to obtain the report within minutes at a
fraction of the cost.
The meter also includes a number of advantageous security features. For
example, the meter cannot be activated until a user enters a proper activation
code. This typically requires that the user call the manufacturer, which
provides
an opportunity to verify the meter's authenticity, set up a data file for the
meter in
the health report server, and tell the user how to update the meter software,
necessary. If a software update is indicated, the user may be instructed to
activate
the meter, initialize a smartcard, load the smartcard into a computer station,
and
establish a network connection with the health report server. The server can
then
download the new software (e.g., new version of an existing software module or
a
new software module) to the smartcard, which, in turn, can be placed back in
the
meter. The new software can then be uploaded to the meter.
The meter may also require validation of all test strips. Validation is
important for some types of tests because readings obtained from each test
strip
will have to be interpreted correctly to obtain correct test results, and the
calibration data used to interpret the readings from different lots of test
strips may
6
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
vary significantly. To allow proper calibration, each lot of test strips has a
corresponding memory device, such as a romkey, that must be placed into the
meter. The romkey includes a code number, an expiration date, and the
calibration data for interpreting readings from the corresponding test strips.
A test
strip identification number that is mathematically derived from the code
number is
printed on the test strips or their packaging. The user must enter the proper
test
strip identification number into the meter, which the meter verifies with
reference
to the code number and the expiration date read from the romkey. This allows
the meter to prevent the use of expired test strips and to also prevent test
strips
l0 from being used in combination with incorrect romkeys.
Test strip validation is also an important aspect of one business model for
deploying the meters. That is, the meters themselves may be provided for use
at
little or no charge to individual patients, whereas proprietary test strips
will be sold
to generate revenue from use of the meter. This may be a desirable business
model for deploying the devices because it minimizes the initial cost that an
individual patient must pay to begin using the device. Having to sell each
device
at its full cost, on the other hand, would undermine the economic feasibility
of
using the device in many contexts. For this business model, the meter should
only
activate for use with proprietary test strips after validation of the test
strips.
The meter may also require each smartcard to be initialized with a personal
identification number (PIN}. Patient-specific PINS allow multiple patients to
use
the same meter, and also allows each patient's data to be secure to that
patient.
That is, only the patient or someone authorized by the patient (i.e., knowing
the
patient's PIN) can read the medical data stored on the smartcard. In this
manner,
each patient controls his or her own medical data, which can be a particularly
important attribute for highly sensitive medical data, such as AIDS tests,
cancer
tests, and the like.
Generally described, the invention provides a test strip for use with a health
monitoring device or meter. The test strip, when carrying a sample of
biological
fluid or tissue, may be read by the meter to obtain test results based on the
sample
and calibration data specific to the test strip. The test strip also
corresponds to a
memory device that stores a code number and the calibration data, which may
also be read by the meter. The test strip has an associated test strip
identification
7
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/Z6521
number that is mathematically derived from the code number and printed on the
test strips, the packaging for the test strips, or a tag packaged with the
test strips.
To verify test strips, the meter reads the code number from the memory
device, mathematically derives a test strip identification number
corresponding to
the code number, compares the received test strip identification number to the
derived test strip identification number, and activates the meter for use with
the
test strip only if the received test strip identification number corresponds
to the
derived test strip identification number.
The memory device may also store an expiration date for the test strip,
l0 which may be read by the meter. In this case, the meter rnay activate for
use with
the test strip only if the expiration date is prior to a current date read by
the meter
from an internal clock. The memory device may be a romkey that is inserted
into
a socket housed within the meter. The romkey is typically packaged with an
associated group of the test strips, and the test strip identification number
is
typically printed on the test strips, printed on packaging for the test
strips, or
printed on a tag packaged with the test strip.
The invention also provides a hand-held health monitoring device or meter
that includes an enclosure for housing a disposable test strip for use with
the
meter. The meter also includes a holder for removably supporting a device for
gathering a sample of biological fluid or tissue, such as a finger stick. The
meter
also includes a test strip reader operable for reading the test strip carrying
the
sample of biological fluid or tissue and obtaining test results based on the
sample
and calibration data specific to the test strip. A memory reading device
(e.g.,
romkey socket) functionally connected to the test strip reader reads the
calibration
data from a memory device (e.g., romkey). A user input device, such as a key
pad, receives user input commands and a display device, such as a liquid
crystal
display, displays information on the meter.
The meter also includes a processor that is functionally connected to the test
strip reader, the user input device, and the display device. The processor
contains
a program module that obtains the test results from the test strip reader and
causes the display device to display the test results. A data drive
functionally
connected to the processor writes the test results to a removable memory
storage
device, such as a smartcard. The meter may be packaged in a clam-shell case
that
8
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
opens to reveal first and second compartments. The first compartment may
contain the enclosure for housing the disposable test strip and the holder for
removably supporting the biological fluid or tissue gathering device, and the
second compartment may contain the test strip reader, the memory reading
device, the display device, the processor, and the data drive.
To provide activation verification, the meter may receive an activation code
through the user input device, compute an activation code based on the current
date and instructions contained in an activation routine stored within the
meter,
and activate the meter only if the computed activation code corresponds to the
to received activation code. In addition, to provide security to a patient's
medical
data, the meter may determine whether a PIN has been previously stored on the
removable memory storage device. If a PIN has not been previously stored on
the removable memory storage device, the meter prompts the user to enter a PIN
and stores the received PIN on the removable memory storage device.
Alternatively, if a PIN has been previously stored on the removable memory
storage device, the meter prompts the user to enter a PIN, compares the stored
PIN to the received PIN, and writes the test results to the removable memory
storage device only if the stored PIN corresponds to the received PIN.
The test strip reader may also be operable for reading a second type of test
strip carrying a second sample of biological fluid or tissue and obtaining
health
related test results based on the second sample of biological tissue or fluid
and
calibration data specific to the second type of test strip. In this case, the
meter
may include a second memory reading device (e.g., romkey socket) functionally
connected to the test strip reader and operable for reading calibration data
from a
second memory device (e.g., romkey) corresponding to the second type of test
strip. For example, the meter may read both blood lipid test strips and blood
glucose test strips. As noted previously, the meter typically includes four
romkey
sockets that allow the meter to carry and read four different romkeys.
The meter may also prompt the user to enter diagnostic information using
the user input device, such as gender, ethnicity, family history of heart
disease,
personal history of heart disease, personal history of diabetes, personal
history of
smoking, height, weight, age, blood pressure, and fitness level. The meter may
then perform a diagnostic analysis and produce diagnostic results based on the
test
9
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/Z6521
results and diagnostic information, and display diagnostic results. For
example,
the diagnostic results may include a medical risk index, a recommended weight
loss, a five-year risk of heart attack, a ten-year risk of heart attack, a
cardiac age,
an extended age, and a risk of stroke.
The invention also provides a system for remotely producing health reports.
This system includes a health monitoring device or meter, as described above,
a
computer station, and a health report server connected with the computer
station
through a network, such as the Internet. The meter writes health-related test
results to a memory storage device. The computer station reads the test
results
from the memory storage device, establishes a network connection with the
health
report server, receives additional diagnostic information from a user, and
transmits
the test results and the additional diagnostic information to the health
report
server. The server, in turn, compiles a health report based on the test
results and
the additional diagnostic information and transmits the health report to the
computer station, where the report may be printed and delivered to the
patient.
The health report may include a trend analysis with test results compiled for
a number of samples, such as total cholesterol level and blood glucose level
trend
reports. The additional diagnostic information may include a newly-prescribed
drug and other currently-prescribed drugs, and the health report may include a
data sheet for the newly-prescribed drug and information relating to cross
reactions between the newly-prescribed drug and the other currently-prescribed
drugs. The health report may also include a target weight and total
cholesterol
levels, a schedule for future testing using the meter, health assessment
summary, a
coronary risk assessment, dietary guidelines to lower cholesterol, and other
educational information.
The business model described above is largely dependent on the sale of
proprietary test strips for the collection of revenue from end users. That is,
the
health monitoring device itself may be made available to individual patients
at little
or no cost, with the sale of proprietary test strips providing a major source
of
revenue for the proprietor of the health monitoring device. As noted
previously,
this may be a desirable business model for deploying the devices because it
minimizes the initial cost that an individual patient must pay to begin using
the
CA 02350145 2001-05-09
WO 00/Z8460 PCT/US99/26521
device. Having to sell each device at its full cost, on the other hand, would
undermine the economic feasibility of using the device in many contexts.
Nevertheless, it may also be desirable to provide a health monitoring device
that does not rely on the sale of proprietary test strips as a major source of
revenue. For example, the health monitoring device may be adapted to read non
proprietary test strips, or may incorporate a reusable and/or non-invasive
testing
device, such as an electrode, blood pressure monitoring device, sonic testing
device, thermometer, saliva testing device, optical testing device, and the
like. Of
course, a non-invasive multi-use testing device may be used many times without
l0 affording the proprietor of the health monitoring device an opportunity
collect
revenue associated with each use of the device.
To provide an opportunity for the proprietor of the health monitoring
device to collect revenue based on use of the device, the removable memory
storage device may be utilized as a type of "debit card" or payment source for
use with the health monitoring device. That is, the removable memory storage
device may be purchased with a monetary value, or it may have a monetary value
that is replenishable over the Internet using a bank credit or debit card or
other
conventional payment source. The health monitoring device may then deduct the
cost of performing particular services from the monetary value represented by
the
monetary balance stored on the removable memory storage device. In other
words, the health monitoring device may be configured to activate for the
performance of a service upon deducting a charge for the service from a
monetary value stored on a removable memory storage device inserted into the
device.
This business model includes a health monitoring device operable for
obtaining medical data associated with a patient and reading an initial
monetary
balance stored on a removable memory storage device. The health monitoring
device determines whether the initial monetary balance is sufficient to pay a
monetary value assigned to performance of a test involving the medical data to
be
performed by the testing device. If the initial monetary balance is sufficient
to pay
for the test, the health monitoring device computes a revised monetary balance
by
deducting the monetary value assigned to performance of the test from the
initial
monetary balance, replaces the initial monetary balance with the revised
monetary
11
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
balance on the removable memory storage device, and activates the health
monitoring device for performance of the specified service.
The business model also includes a system that includes one or more of the
health monitoring devices described above, one or more removable memory
storage devices, and a network-based server operable for remotely charging a
cost
to a payment source and crediting the cost to an initial balance stored on the
removable memory storage device. The network-based server may also remotely
store the monetary value assigned to performance of the test on the removable
memory storage device. In this case, the health monitoring device reads the
to monetary value assigned to performance of the test from the removable
memory
storage device. Thus, rate schedules for various services to be performed by
the
health monitoring device may be changed from time to time, based on quantity
discounts or other considerations.
The invention also includes a secure medical records maintenance system.
Although this system is specifically adapted for use with the health
monitoring
device described above, it may be used to store any type of electronic data
including a wide variety of medical records, and is particularly convenient
for
storing a wide range of electronic medical data generated remotely from the
hospital or doctor's office environment. The secure medical records
maintenance
system includes a number of removable memory storage devices, which are each
operable for storing medical data for an associated patient. Each removable
memory storage device also stores a patient-specified personal identification
number (PIN), a medical records identification number secured by the PIN, and
a
patient identification number secured by the PIN.
The data stored on the removable memory storage device is downloadable
to a two-server system including a first remote server that stores patient
identification information indexed by patient identification numbers, and a
second
remote server that stores patient medical data indexed by the medical records
identification number. For security purposes, the medical data maintained in
the
second remote server cannot be correlated to the associated patient
identification
information maintained in the first remote server based on the information
contained in the first and second remote servers.
12
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/Z6521
To allow correlation of the data stored in the two servers, the secure
medical records maintenance system includes a correlation table uniquely
associating each medical records identification number with a particular one
of the
patient identification numbers. The correlation table for a particular patient
typically resides on the patient's removable memory storage device. The
correlation table for a practitioner's patients may also reside on the
practitioner's
computer, such as a doctor's or pharmacist's computer, that is associated with
a
licensed medical practitioner having an assigned professional registration
number.
For further security, the first and second remote servers are accessed by the
practitioner's computer through encrypted communications secured by an
application procedure that includes validation of the practitioner's
registration
number. The application procedure may be further secured by receipt and
validation of a practitioner-supplied PIN. Moreover, the application procedure
typically includes issuance of a client certificate insuring that access to
the first and
second remote servers occurs from the same practitioner's computer and browser
that initiated the application procedure.
Because the data on the servers is separate and secure from each other,
access may be granted to either server without identifying any particular
patient's
medical data. For example, access may be granted to the first remote server,
but
not to the second server, for the purpose of generating a mailing list of
patients
without divulging any medical data associated with the patients. Similarly,
access
may be granted to the second remote server, but not to the first server, for
the
purpose of conducting investigative analyses involving the medical data
without
divulging any patient identification information associated with the patients.
For further data security and because each removable memory storage
device only has a limited data storage capability, the medical data stored on
each
removable memory storage device may be automatically erased from the memory
storage device after the data is entered into the second remote server. To
obtain
the medical data, the removable memory storage device is receivable within a
hand-held health monitoring device operable for storing the medical data on
the
removable memory storage device. And to download the medical data to the
medical records maintenance system, the removable memory storage device is
13
CA 02350145 2001-05-09
WO 00/28460 PCTNS99/26521
receivable within a computer operable for reading the medical data and
transmitting it to the second remote server over the Internet.
That the invention improves over the drawbacks of health monitoring and
diagnostic systems and accomplishes the advantages described above will become
apparent from the following detailed description of the exemplary embodiments
and the appended drawings and claims.
BRIEF DESCRIPTION OF THE DRAWINGS
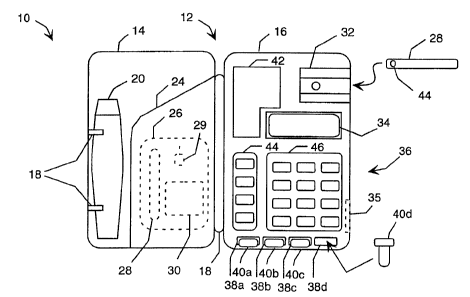
FIG. 1 A is a front view of a hand-held health monitoring and diagnostic
device in an open position.
FIG. 1 B is a rear view of the hand-held health monitoring and diagnostic
device of FIG. 1 in an open position.
FIG. 2 is a block diagram illustrating a system for remotely producing
health reports.
FIG. 3 is a functional block diagram of a health monitoring and diagnostic
device.
FIG. 4 is a logic flow diagram illustrating a routine for activating a health
monitoring and diagnostic device.
FIG. 5 is a logic flow diagram illustrating a routine for computing an
activation code for a health monitoring and diagnostic device.
FIG. 6 is a logic flow diagram illustrating a routine for verifying a test
strip
for a health monitoring and diagnostic device.
FIG. 7 is a logic flow diagram illustrating a routine for computing a test
strip identification number for a health monitoring and diagnostic device.
FIG. 8 is a logic flow diagram illustrating a routine for entering diagnostic
program modules into a health monitoring and diagnostic device.
FIG. 9 is a logic flow diagram illustrating a routine for computing
immediate diagnostic results in a health monitoring and diagnostic device, and
for
remotely producing health reports.
FIG. 10 is a logic flow diagram illustrating a routine for obtaining
cholesterol-related diagnostic information for a health monitoring and
diagnostic
device.
14
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
FIG. 11 is a logic flow diagram illustrating a routine for computing
immediate cholesterol-related diagnostic results for a health monitoring and
diagnostic device.
FIG. 12 is a logic flow diagram illustrating a routine for remotely producing
health reports.
FIG. 13 is a logic flow diagram illustrating a routine for saving medical data
to a PIN-secured removable memory storage device for a health monitoring and
diagnostic device.
FIG. 14 is a functional block diagram of a system for using a health
l0 monitoring and diagnostic device in connection with a secure medical
records
maintenance system.
FIG. 15 is software architecture diagram illustrating a system for conducting
secure communications between a health monitoring and diagnostic device and a
secure medical records maintenance system.
FIG. 16 a functional block diagram illustrating security aspects of a secure
medical records maintenance system.
FIG. 17 is a logic flow diagram illustrating a process for a communicating
with a secure medical records maintenance system.
FIG. 18 is a logic flow diagram illustrating a process for applying fox access
2o to a secure medical records maintenance system.
FIG. 19 is a logic flow diagram illustrating a process for logging into a
secure medical records maintenance system.
FIG. 20 is an illustration of a "switchboard" user interface in a secure
medical records maintenance system.
FIG. 21 is an illustration of an "address" user interface in a secure medical
records maintenance system.
FIG. 22 is an illustration of a "billing information" user interface in a
secure
medical records maintenance system.
FIG. 23 is an illustration of a "cover letter" user interface in a secure
medical records maintenance system.
FIG. 24 is an illustration of a "patient selection" user interface in a secure
medical records maintenance system.
CA 02350145 2001-05-09
WO 00/28460 PCTNS99/26521
FIG. 25 is an illustration of a "patient information" user interface in a
secure medical records maintenance system.
FIG. 26 is an illustration of a "questionnaire data" user interface in a
secure
medical records maintenance system.
FIG. 27 is an illustration of a "generate reports" user interface in a secure
medical records maintenance system.
FIG. 28 is an illustration of typical health assessment charts generated by a
secure medical records maintenance system.
FIG. 29 is an illustration of an additional health assessment chart generated
by a secure medical records maintenance system.
FIG. 30 is a logic flow diagram illustrating a routine for adding a monetary
value to a smartcard for use with a health monitoring device.
FIG. 31 is a logic flow diagram illustrating a routine for using a smartcard
to pay for a service provided by a health monitoring device.
DETAILED DESCRIPTION OF EMBODIMENTS OF THE INVENTION
Hand-Held Health Monitoring and Diagnostic Device
Turning now to the figures, in which like numerals refer to like elements
through the several figures, FIG. 1 A is a front view of a hand-held health
monitoring and diagnostic device 10, which is also referred to as a meter or a
LIFESTREAM cholesterol meter. The meter 10 is housed in a clam-shell case 12
including a first compartment 14 and a second compartment 16. The case 12
may be opened, as shown in FIG. 1 A, or closed about a hinge 18. This allows a
patient to close the meter 10 for transportation or storage, and then easily
open it
for use. When in use, the patient may place the meter 10 in the open position
on
a flat surface, such as a table or seat, or hold the meter by hand.
Although the meter 10 is shown in a hinged clam-shell, hand-held
configuration, it could alternatively be embodied in other configurations,
such
wall-mounted, built into a movable cart, built into a desktop computer, built
into a
fixed podium, and so forth. In addition, the hinged clam-shell case could be
replaced by a non-hinged case, a separable multi-piece case, a case with a
pull-out
drawer, a case with a flat cover, a meter that fits into a separate zippered
case, and
16
CA 02350145 2001-05-09
WO 00/28460 PCTNS99/26521
other types of single- or multi-piece configurations. Many other variations of
the
meter case configuration will be apparent to those skilled in the art.
The first compartment 14 includes a holder 18 for removably supporting a
biological sample gathering device, in this instance a conventional spring-
loaded
finger stick 20. Although the holder 18 is shown as a clip with two arms that
fit
snugly against the finger stick 20, the holder may have any other
configuration
suitable for removably supporting the finger stick, such as a channel into
which a
pencil-like finger stick is inserted, an openable enclosure, snaps, a VELCRO
fastener, and the like. If samples other than blood are to be gathered, the
first
compartment 14 could alternatively house other types of biological sample
gathering devices, such as a skin sample collector, a saliva collector, a
stool sample
collector, and so forth. In addition, the meter 10 may include other types of
instruments for gathering test data, for example the meter may be adapted to
read
non-proprietary test strips, or may incorporate a reusable and/or non-invasive
testing device, such as an electrode, blood pressure monitoring device, sonic
testing device, thermometer, saliva testing device, optical testing device,
and the
like.
The first compartment 14 also includes an openable enclosure 24 for storing
one or more packages 26 of disposable items. Specifically, each package may
contain a test strip 28, a needle 29 for the finger stick 20, and an alcohol
swipe 30.
These disposable items are tailored for one-time use with the finger stick 20.
If the
meter 10 includes biological sample gathering devices other than the finger
stick
20, other types of disposable items may be stored in the enclosure 24. In
addition,
the enclosure 24 may have other configurations suitable for storing or holding
disposable items, such as a drawer, a tilting channel, a clip, and so forth.
The second compartment 16 houses the electronic components of the meter
10, including a test strip reader 32, a display device 34, a user input device
36, and
one or more memory reading devices 38a-d. Each of these memory reading
devices is configured to receive a corresponding memory device 40a-d. The
second compartment 16 also includes an instructional label 42 located adjacent
to
the display device 34. Internally, the second compartment 16 houses a
motherboard, an analyzer board, and a data drive that control the
functionality of
the meter 10. These internal components are described with reference to FIG.
3,
17
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
and the functionality of the meter 10 is described with reference to FIGS. 4-
13.
Additional functionality of the meter 10 for use with a debit-card type
payment
system is described with reference to FIGS. 30-31.
The test strip reader 32 may be a GLUCOTREND Basic TIM (test
instrument module) assembly No. 1739905-741 manufactured by Boehringer
Mannheim, Roche Diagnostics GmbH. This is a commercially-available optical
test strip reader suitable for reading chemical test strips carrying human
blood
samples and producing either blood glucose readings, total cholesterol
readings, or
both. Alternatively, other suitable types of test strip readers may be
included in
the meter 10, and multiple test strip readers may be included in the meter,
appropriate. This may be desirable, for instance, if biological samples other
than
blood are to be analyzed by the meter. The meter 10 may be configured with
additional reusable and/or non-invasive testing devices, such as an electrode,
blood
pressure monitoring device, sonic testing device, thermometer, saliva testing
device, optical testing device, and the like.
The display device 34 may be a conventional liquid crystal display {LCD)
configured to display at least two lines of text including at least 14
characters per
line. This visual display works in concert with a speaker 35 that beeps to
convey
audible messages. The speaker may also produce other types of audible
messages,
such as tones, recorded messages, a simulated human voice, and the like. The
meter 10 may also include other types of visual display devices, such as an
electronic capacitive matrix, a small video display, or other types of
suitable visual
display devices. The meter 10 may also include a jack for connecting the meter
to
external display devices, such as a computer monitor or video display.
The user input device 36 may be a keypad with a first key section 44 and a
second key section 46. The first key section 44 includes four keys, a "scroll"
key,
a "yes" key, a "no" key and an "enter" key. The second key section 46
includes twelve keys, including ten numerical keys, a "clear" key, and an
"on/off'
key. The user input device 36 may include other key patterns and other types
of
user input devices, such as a touch-sensitive screen, a voice-recognition
device, or
other input devices. The meter 10 may also include a jack for connecting the
meter to external input devices, such as a keyboard or joystick.
18
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
The memory reading devices 38a-d may be romkey sockets, and the
memory devices 40a-d may be romkeys that removably insert into the sockets.
As shown in FIG. 1 A, the meter 10 preferably includes four romkey sockets.
Nevertheless, the meter 10 could also be configured with only one socket
because
the romkeys themselves are removable. The romkeys, which store identification,
expiration, and calibration data for a corresponding lot of test strips 28,
are
desirable because they are small, may be easily packaged with the
corresponding
test strips, and have an adequate amount of computer-readable memory. But the
romkey sockets may be replaced by a magnetic card reader, an optical reader,
or
another reader suitable for use with a memory storage device that can be
easily
shipped with a corresponding lot of test strips 28 and has an adequate amount
of
computer-readable memory.
The instructional label 42 located adjacent to the display device 34 typically
includes instructions for entering diagnostic information into the meter 10.
This
label may also include instructions for using the meter 10 in concert with a
remote
health report system, which is described with reference to FIG. 2. As this
type of
information may be understood best when explained by a physician or
pharmacist,
the instructional label 42 may be included on meters provided to physicians
and
pharmacists, but may be excluded from meters provided for home use by
individual patients. The programmed functionality of the meter may also be
adjusted accordingly.
To use the meter 10, a patient first opens the meter and removes the finger
stick 20 and the package of disposable items 26. The patient then opens the
package, installs the needle 29 in the finger stick 20, and wipes the alcohol
swipe
30 on the area of the finger to be stuck with the needle. The patient also
inserts
the correct romkey for the test strip 28, represented by the romkey 40d, into
a
corresponding romkey socket 38d and manipulates the keypad 44, 46 to indicate
to the meter 10 which romkey socket contains the correct romkey. The patient
then sticks the selected finger with the finger stick 20, places a droplet of
blood 44
on an indicated area of the test strip 28, and inserts the test strip 28 into
the test
strip reader 32.
The user then manipulates the input device 36 by following prompts
displayed on the display device 34 to complete the test. Within minutes, the
meter
19
CA 02350145 2001-05-09
WO 00!28460 PCT/US99/26521
completes the test and displays the test results, such as total cholesterol
levels,
blood glucose levels or another testing service provided by the meter, on the
display device 34. If appropriate, the user may also manipulate the input
device
36 to enter additional diagnostic information into the meter, such as gender,
5 ethnicity, family history of heart disease, personal history of heart
disease, personal
history of diabetes, personal history of smoking, height, weight, age, blood
pressure, and fitness level. Within minutes, the meter 10 performs a
diagnostic
analysis and produces diagnostic results, such as a medical risk index, a
recommended weight loss, a five-year risk of heart attack, a ten-year risk of
heart
10 attack, a cardiac age, an extended age, and a risk of stroke. These
diagnostic
results are also immediately displayed on the display device 34.
For a blood lipid or cholesterol test, the well-known Framingham Medical
Study may provide the methodology used by the meter 10 to produce the
diagnostic results from the test results and the diagnostic information. Other
methodologies, such as those sanctioned by the National Cholesterol Education
Program, the American Heart Association, the American Medical Association, or
another appropriate organization may also be used. In fact, the meter 10 may
allow the user to select among several alternative diagnostic program modules
stored within the meter. These diagnostic program modules may be updated from
time to time, and new diagnostic program modules may be added to the meter 10
through the data drive, which is described below.
FIG. 1 B is a rear view of the meter 10, which shows the outside of the
meter. The outside of the first compartment 14 includes the manufacturer's
name, Lifestream Technologies, Inc., and the meter's trademark, LIFESTREAM.
The outside of the second compartment 16 includes a data drive 50, which
includes an opening 52 for receiving a removable memory storage device 54. For
example, the data drive 50 may be a smartcard drive, such as an STM IC:
MC33560ADW manufactured by Motorola, and the removable memory storage
device 54 may be a smartcard usable with this drive. This smartcard typically
includes an electrical contact 56 for reading and writing data and a small
microprocessor 58, which typically controls security aspects of the smartcard.
Specifically, the smartcard includes a PIN-protected secure memory and an
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
unsecure memory. The microprocessor 58 controls the PIN and any other
functionality resident on the smartcard.
The smartcard is an advantageous memory storage device because of its
small size, its on-card PIN security feature, and its on-board programmable
processing unit. Moreover, it is expected that smartcards will become
increasingly
popular in the near future, and most personal computers will come with factory-
installed smartcard drives. Nevertheless, the meter 10 could include other
types of
data drives, such as a floppy disk drive, an optical disk drive, a removable
RAM
chip, or any other suitable type of removable memory storage device.
Furthermore, the meter 10 could also include a wire-line data port for
connecting
a cable or another computer station in addition to or as an alternative to the
data
drive. Similarly, the wire-line data port could be replaced by a wireless
communication device, such as a radio-frequency link, a laser link, an infra-
red
link, and so forth.
The second compartment 16 also includes a battery enclosure 60 housing a
removable battery 62 for powering the meter 10. The battery 60, which may be
a disposable 9 Volt battery, may be replaced or augmented by an A/C power cord
and an appropriate power inverter. The battery 60 also be replaced by a
rechargeable battery augmented with a battery charger that may be located
within
the meter 10 or in a separate enclosure, such as a storage container for
housing
the meter when it is not in use.
Remote Health Report SXstem
FIG. 2 is a block diagram illustrating a system 100 for remotely producing
health reports. The PIN-securable smartcard described above allows multiple
patients to use the same meter, and also allows each patient to control access
to
his or her medical data. The smartcard is typically used as a temporary
storage
location for one or several test results. From time to time, each patient is
expected to download the medical data contained on his or her smartcard to a
health report server 102 for permanent storage. The smartcard is then erased
(except for the PIN and any other security information contained in the secure
memory), which frees the memory space for new medical data. The smartcard
may also be used to receive new program modules or new versions of program
21
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
modules from the health report server 102 and upload these program modules to
the meter 10.
The system I00 includes the meter 10, which downloads medical data to
the removable memory storage device 54. This medical data typically includes
patient identification information, test results generated by the meter,
diagnostic
information entered into the meter 10 by the patient, and the diagnostic
results
104 computed by the meter and immediately displayed on the meter at the time
of the test. If the removable memory storage device 54 is a smartcard, it may
then be placed in a converter, such as a conventional smartcard-to-floppy-disk
converter 106, which can be directly inserted into a computer station 108. The
converter 106 will be unnecessary, of course, if the computer station 108
includes
a smartcard drive, or if another communication mechanism is employed to
transmit information between the computer station 108 and the meter 10.
Once the medical data arrives at the computer station 108, it establishes a
network connection with the health report server 102, typically over the
Internet
110. The medical data is then transmitted to the health report server 102,
which
may also prompt the user for additional diagnostic and health report
information.
Specifically, the health report server 102 typically works in concert with the
patient's physician or pharmacist, who may provide additional diagnostic
information to the server, such as a newly-prescribed drug therapy, other
currently-prescribed drugs for the patient, exercise and dietary
recommendations,
and so forth. Within minutes, the health report server 102 assembles a
comprehensive health report 112 that is transmitted back to the computer
station
108, where it may be printed on a local printer 114. User access procedures
and
a menu-driven user interface system for generating the health reports is
described
with reference to FIGS. 17-29.
The comprehensive health report 112 typically includes a data sheet for the
newly-prescribed drug, cross-reaction information for the newly-prescribed
drug
and the other currently-prescribed drugs, weight and total cholesterol goals,
exercise and dietary recommendations, any food or activity warnings associated
with the overall therapy package, and recommendations for on-going monitoring
using the meter. This provides a complete written record of the patient's
current
22
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
condition, the therapy prescribed by the physician and filled by the
pharmacist,
and a roadmap for monitoring the patient's progress during the ensuing
therapy.
The comprehensive health report may also include additional patient-specific
information, such as the diagnostic information and results compiled by the
meter,
and additional diagnostic and health assessment information compiled by the
server. For example, the report may include a trend analysis showing how blood
lipid, blood glucose, and weight levels have changed over multiple readings.
The
report may also include generally-applicable educational information, such as
coronary risk factors, dietary guidelines for reducing cholesterol levels,
diabeties
information, cancer information, and the like. At present, a patient may have
to
undergo a physical examination, pay thousands of dollars, and wait weeks to
obtain a similar comprehensive health report. The network-based comprehensive
health analysis and reporting system, working in concert with the LIFESTREAM
cholesterol meter, allows the patient to obtain the report within minutes at a
fraction of the cost.
The Internet 10 allows a wide variety of users to access the health report
server 102, which allows meters to be deployed in a variety of settings. For
example, the accessing computer stations may include a physician's computer
station 116, a pharmacist's computer station 118, an individual's computer
station
120, and many others. This will allow meters to be effectively deployed for
multi-
patient use in clinics, physicians' offices and pharmacies, as well as for
individual
patient or family use in the privacy of their own homes.
Functional Operation of the Meter
FIG. 3 is a functional block diagram of the health monitoring and diagnostic
device 10, which is also referred to as a meter. The meter includes an
analyzer
board 150, a mother board 152, a memory reading device 154 including the
romkey sockets 38a-d and corresponding romkeys 40a-d, and a user interface
156 including the display device 34, the speaker 35, and the input device 36.
The
memory reading device 154 and the user interface 156 were described with
reference to FIG. lA. Although the analyzer board 150 and the mother board
152 may be configured as two separate integrated circuit boards, alternatively
23
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
they may be combined into a single integrated circuit board, or deployed on
more
than two integrated circuit boards.
The analyzer board 150 may be part of the GLUCOTREND Basic TIM
(test instrument module) assembly No. 1739905-741 manufactured by Boehringer
Mannheim, Roche Diagnostics GmbH. This board includes the test strip reader
32, which was described with reference to FIG. 1 A, a test instrument module
158,
and a romkey driver 160. The test strip reader 32 reads the test strip 28
carrying
the blood sample 44. The romkey driver 160 reads the calibration data for the
test strip 28 from a corresponding romkey, such as the romkey 40d, and the
test
l0 instrument module 158 computes the test results from the test strip reading
and
the calibration data. These test results are then passed to the motherboard
152.
The motherboard 152 includes a non-interruptible battery 162, such as a
lithium battery. The non-intemuptible battery 162, which powers the on-board
clock 164, is distinct from the power supply battery 62 (shown on FIG. 1B).
The
additional non-intermptible battery 162 allows the clock to continue
functioning
even when the power supply battery 62 runs down or is removed from the meter
10. The motherboard 152 also includes a processor 166 and a memory 168 that
control the functionality of the meter 10. Specifically, the memory 168 stores
and
the processor 166 implements a meter activation routine, test strip
verification
routine, diagnostic routines, and a read/write security routine. These program
modules are described with reference to FIGS. 4-13. The motherboard 152 also
includes a data drive 170 that reads data from and writes data to the
removable
data storage device 54, such as a smartcard.
The mother board processor 166 may be a 40-pin DIP CPU Model No.
MC68HC705C9ACP manufactured by Motorola. The data drive 170 may be a
STM IC Model No. MC33560ADW ISO read/control card manufactured by
Motorola, supported by an ISO Card Socket, Model No. 145206-3 physical
interface manufactured by AMP. However, any of these specific devices may be
replaced by equivalent devices capable of performing the functionality of the
3o meter 10 described in this specification.
To verify test strips, the romkey driver 160 reads a code number from a
romkey, such as the romkey 40d, installed in the meter 10. The code number
typically includes the lot number for the corresponding test strips and a test
type
24
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
ID stored on the romkey by the manufacturer of the meter 10. The test strip 28
has an associated test strip identification number 172 that is mathematically
derived from the code number and printed on the test strip itself, the
packaging
for the test strip, or a tag packaged with the test strip. The meter 10
prompts the
user to enter the test strip identification number 172 into the meter using
the
keypad 46.
The meter 10 also reads the code number from the memory device,
mathematically derives a test strip identification number corresponding to the
code
number, compares the received test strip identification number to the derived
test
l0 strip identification number, and activates the meter for use with the test
strip only
if the received test strip identification number corresponds to the derived
test strip
identification number. The romkey 40d also stores an expiration date for the
corresponding test strip 28. The meter 10 reads the expiration date and
activates
for use with the test strip 28 and the romkey 40d only if the expiration date
is
prior to a current date read by the meter from the internal clock 164.
FIGS. 4-14 are logic flow diagrams illustrating examples of the functionality
that may be implemented by the meter 10 and the system 100 for remotely
generating health reports. The following description of these logic flow
diagrams
will also refer to the elements shown on FIGS. 2 and 3. It should be
understood
that these examples illustrate the use of these components in producing total
cholesterol tests and related health reports. In particular, the specific
diagnostic
information gathered and the specific diagnostic results described are those
associated with the well-known Framingham Medical Study, which is incorporated
herein by reference. Although this particular program module illustrates the
operation of an illustrative embodiment of the invention, those skilled in the
art
will understand that the meter 10 and the system 100 could be programmed with
additional and different program modules.
In addition, as the meter 10 is configured with multiple romkey sockets and
programmed to accept additional program modules in the future, it will be
appreciated that similar functionality may be implemented in the future for
blood
glucose tests and diabetes-related health reports, AIDS tests and related
health
reports, cancer tests and related health reports, and so forth. Additional
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
functionality of the meter 10 for use with a debit-card type payment system is
described with reference to FIGS. 30-31.
Meter Activation
FIG. 4 is a logic flow diagram illustrating a routine 400 for activating the
meter 10. This routine requires that a user enter a proper activation code to
activate the meter 10. This typically requires that the user call the
manufacturer,
which provides an opportunity to verify the meter's authenticity, set up a
data file
for the meter in the health report server, and tell the user how to update the
meter
l0 software, if necessary. If a software update is indicated, the user may be
instructed to activate the meter, initialize a smartcard, load the smartcard
into a
computer station, and establish a network connection with the health report
server. The server can then download the new software (e.g., new version of an
existing software module or a new software module) to the smartcard 54, which,
in turn, can be placed back in the meter 10. The new software can then be
uploaded to the meter.
In step 402, the meter 10 is programmed at the factory by setting the
internal clock 164 to the correct date and storing an activation code
algorithm
within the meter. Step 402 is followed by routine 404, in which the activation
site, typically the manufacturer, computes an activation code for the current
day.
That is, each day the activation site computes a new activation code that is
valid
only for that day. The activation code is computed using the same algorithm
that
was stored in the meter 10 in step 402. This algorithm is described below with
reference to FIG. 5.
Step 404 is followed by routine 406, in which the activation site receives an
activation communication for the purpose of activating a meter 10. The user of
the meter typically places this communication by placing a telephone call to a
telephone number (e.g., a "1-800" or other toll-free telephone number) on the
meter or on the packaging or documentation provided with the meter. Step 406
is followed by routine 408, in which the activation site delivers the
activation code
for the current date to the calling user. Step 408 is followed by routine 410,
in
which the calling user enters the activation code into the meter 10 by
manipulating the user input device 36.
26
CA 02350145 2001-05-09
WO OO/Z8460 PCT/US99/26521
Step 410 is followed by step 412, in which the meter 10 verifies the
received activation code by computing an activation code using its on-board
activation code algorithm and the current date read from its internal clock
164. In
doing so, the meter 10 uses the same algorithm that was used by the activation
site to compute the activation code that was delivered to the user and entered
into
the meter (i.e., the algorithm described with reference to FIG. 5). Step 412
is
followed by routine 414, in which the meter 10 compares the received
activation
code to the computed activation code. Step 414 is followed by routine 416, in
which the meter 10 determines whether the activation is verified by
determining
l0 whether the received activation corresponds to the computed activation
code.
If the activation is not verified, the "NO" branch is followed from step 416
to step 418, in which the meter 10 determines whether a timeout condition or
an
allowed number of tries has been reached. If a timeout condition or an allowed
number of tries has not been reached, the "NO" branch loops from step 418 to
step 410, and the meter 10 displays an error and prompts the user to reenter
the
activation code. If a timeout condition or an allowed number of tries has been
reached, the "YES" branch is followed from step 418 to the "END" step, and
the meter 10 is not activated.
Referring again to step 416, if the activation is verified, the "YES" branch
is followed from step 416 to step 420, in which the meter 10 is activated.
Step
4I2 is followed by the "END" step, in this case with the meter 10 activated.
FIG. 5 is a logic flow diagram illustrating a routine 404 for computing an
activation code for the meter 10 or the activation site, referred to
collectively
below as the "computing entity." Routine 404 begins following step 402 shown
on FIG. 4. In step 502, the computing entity gets the day, month, and year
(six
digit decimal) from the internal clock 164. Step 502 is followed by step 504,
in
which the computing entity combines the six digits defining the date into a
hex
value (six digit hex). Step 504 is followed by step 506, in which the
computing
entity applies a predetermined mathematical operation to this hex value to
compute a new hex value (new six digit hex).
Step 506 is followed by step 508, in which the computing entity applies a
mask to the new six digit hex value to obtain a four digit hex value (new four
digit
hex). In other words, the computing entity selects a predetermined four of the
six
27
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
digits for further processing. Step 508 is followed by step 510, in which the
computing entity converts this new four digit hex value to a decimal value
(four
digit decimal value). Step 510 is followed by step 512, in which the computing
entity relocates one or more of the digits and adds one or more null numbers
at
predefined locations (new four digit decimal value). Step 512 is followed by
step
514, in which the computing entity uses the resulting new four digit decimal
number as the activation code. Step 514 is followed by the "RETURN" step,
which goes to step 406 on FIG. 4.
The mathematical operation applied in step 506 may be any of a variety of
l0 algorithms designed to quasi-randomize the result. For example, the digits
may be
grouped into subsets that, in turn, are used in one or more linear
mathematical
operations, such as addition, subtraction, multiplication, division, raising
to a
power, raising to a fraction, and the like. For example, a polynomial formula
may
be applied to the digits or subsets of the digits. The digit shuffling
operation
applied in step 512 is also applied to quasi-randomize the result. Many other
types
of quasi-randomizing methodologies will be apparent to those skilled in the
art.
Test Strip V alidation
FIG. 6 is a logic flow diagram illustrating a routine 600 for verifying a test
strip for the meter 10. In step 602, the meter 10 is programmed at the factory
with a test strip validation algorithm. Step 602 is followed by step 604, in
which a
romkey with a corresponding lot of test strips is programmed with a test type
ID.
This test type ID, together with a lot number for the test strips placed on
the
romkey by the test strip manufacturer, forms a four-digit code number that is
resident on the romkey when it leaves the factory.
Step 604 is followed by routine 606, in which a test strip ID is
mathematically derived from the romkey code number. Routine 606 is described
below with reference to FIG. 7. Routine 606 is followed by step 608, in which
the
test strip ID is printed on the lot of test strips corresponding to the
romkey, on the
packaging for the test strips, or tags that are packaged with the test strips.
The
test strips are then packaged with corresponding romkeys and distributed for
use
with various meters. It should be understood that many identical romkeys may
be
produced for each lot of test strips because one romkey is included in each
28
CA 02350145 2001-05-09
WO 00/28460 PCT/US99I26521
distribution-sized package, and a production-sized lot of test strips may be
many
times larger than a distribution-sized package.
Step 608 is followed by step 610, in which the user of a meter 10 puts a
romkey into the meter and enters the corresponding test strip ID into the
meter
using the user input device 36. This is the test strip ID that was printed on
the test
strip, its packaging, or a tag packaged with the test strip in step 608. Step
610 is
followed by step 612, in which the meter 10 gets the current date from the
internal clock 164, reads the code number and expiration date from the romkey,
and computes a test strip ID number based on the code number. The test strip
ID
number is derived from the code number using the same algorithm that was used
to compute the test strip ID number at the factory in routine 606.
Step 612 is followed by step 614, in which the meter 10 determines
whether the current date is prior to the expiration date read from the romkey.
If
the current date is not prior to the expiration date read from the romkey, the
"NO" branch is followed to the "END" step, and the meter is not activated for
use with the instant romkey. If the current date is prior to the expiration
date
read from the romkey, the "YES" branch is followed to step 616, in which the
meter 10 compares the received test strip (input by the user) to the computed
test
strip ID (derived from the code number read from the romkey). Step 616 is
followed by step 618, in which the meter 10 verifies the test strip if the
received
test strip corresponds to the computed test strip ID.
If the meter 10 verifies the test strip, the "YES" branch is followed from
step 618 to step 622, in which the meter 10 activates for use with the instant
test
strip and romkey. Step 622 is followed by the "END" step. If the meter 10 does
not verify the test strip, the "NO" branch is followed from step 618 to step
620,
in which the meter 10 determines whether a timeout condition or an allowed
number of tries has been reached. If a timeout condition or an allowed number
of
tries has not been reached, the "NO" branch loops from step 620 to step 610,
and the meter 10 displays an error and prompts the user to reenter the test
strip
ID and/or insert the correct romkey. If a timeout condition or an allowed
number
of tries has been reached, the "YES" branch is followed from step 620 to the
"END" step, and the meter 10 is not activated for the instant test strip and
romkey.
29
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
Test Strip ID Assignment
FIG. 7 is a logic flow diagram illustrating routine 606 for computing a test
strip ID for the meter 10. Routine 606 begins following step 604 shown on FIG.
6. In step 702, the meter 10 reads the code number (i.e., lot number plus test
type
ID) from the romkey. Step 702 is followed by step 704, in which the meter 10
converts this four digit decimal value to 16-bit binary value. Step 704 is
followed
by step 706, in which the meter 10 applies a predetermined mathematical
operation to this 16-bit binary value.
l0 Step 70fi is followed by step 708, in which the meter 10 checks a value at
a
predetermined bit of the 16-bit binary value. Step 708 is followed by step
710, in
which the meter 10 performs a conditional bit swap. That is, a first type of
bit
swap is performed if the value of the checked bit is a "1" and a second type
of bit
swap is performed if the value of the checked bit is a "0." Step 710 is
followed
by step 712, in which the meter 10 converts the resulting number to a four
digit
decimal value. Step 712 is followed by step 714, in which the meter 10 uses
the
resulting four digit decimal number as the test strip ID. Step 714 is followed
by
the "RETURN" step, which goes to step 608 on FIG. 6.
The mathematical operation applied in step 706 may be any of a variety of
algorithms designed to quasi-randomize the result. For example, the digits may
be
grouped into subsets that, in turn, are used in one or more linear
mathematical
operations, such as addition, subtraction, multiplication, division, raising
to a
power, raising to a fraction, and the like. For example, a polynomial formula
may
be applied to the digits or subsets of the digits. The digit shuffling
operation
applied in steps 708 and 710 is also applied to quasi-randomize the result.
Many
other types of quasi-randomizing methodologies will be apparent to those
skilled
in the art.
Diagnostic Data Entrv
FIG. 8 is a logic flow diagram illustrating a routine 800 for entering
diagnostic program modules into the meter 10. In step 802, the meter 10 is
programmed at the factory with one or more initial diagnostic algorithms. In
addition, a computer maintained at the programming site, typically the
CA 02350145 2001-05-09
WO 00/28460 PCTNS99/26521
manufacturer, is programmed with a cross reference table indicating the serial
number for the meter and the version number of each diagnostic program module
installed in the meter 10.
At some later point, a user downloads medical data from the meter 10 to a
smartcard in the course of normal meter use. At this time, the serial number
for
the meter is also stored on the smartcard. The smartcard is then read by a
computer station, which establishes a network connection with a programming
server, which may be the same as, or coordinated with, the health report
server
102 shown on FIG. 2. At step 804, the programming server receives the data
that was stored on the smartcard. Step 804 is followed by step 806, in which
the
programming server instructs the computer station to erase the unsecured data
stored on the smartcard. That is, the computer station erases the medical data
stored in the unsecure memory but does not erase the PIN or any data stored in
the secure memory.
Step 806 is followed by step 808, in which the programming server gets the
serial number for the meter 10 from the received data and looks up the version
numbers for the diagnostic program modules installed on the meter. Step 808 is
followed by step 810, in which the programming server determines whether the
meter 10 includes the latest version of all of the program modules that should
be
installed on the meter. If the meter 10 includes the latest version of all of
the
program modules that should be installed on the meter, the "YES" branch is
followed to the "END" step, and the meter software is not updated.
If, on the other hand, the meter 10 does not include the latest version of all
of the program modules that should be installed on the meter, the "NO" branch
is
followed to step 812, in which the programming server loads new diagnostic
program modules, new versions of diagnostic program modules, or updates for
existing program modules on the smartcard. At some later point in step 814,
the
smartcard is placed back in the meter 10. At this point, step 814 is followed
by
step 816, in which the new diagnostic program modules, new versions of
diagnostic program modules, or updates for existing program modules stored on
the smartcard are uploaded to the meter 10. Step 816 is followed by the "END"
step.
31
CA 02350145 2001-05-09
WO 00/28460 PCTNS99/26521
Diagnostic Analysis
FIG. 9 is a logic flow diagram illustrating a routine 900 for computing
immediate diagnostic results in the meter 10, and for remotely producing
health
reports. In step 902, the meter 10 prompts the user to select a romkey and
insert
a corresponding test strip, with a blood sample, into the meter. Step 902 is
followed by step 904, in which the meter 10 prompts the user to select a
desired
diagnostic program module corresponding to the type of test strip inserted
into
the meter. Step 904 is followed by step 906, in which the meter 10 performs
the
test strip verification algorithm shown on FIG. 6 and, if the test strip is
verified,
obtains test results.
Step 906 is followed by routine 908, in which the meter 10 prompts the
user for additional diagnostic information. Routine 908 is described below
with
reference to FIG. 10. Routine 908 is followed by routine 910, in which the
meter
10 computes immediate diagnostic results. Routine 910 is described below with
reference to FIG. 11. Routine 910 is followed by step 912, in which the meter
10
displays the diagnostic results on the display device 34. Step 912 is followed
by
step 914, in which the meter 10 stores the test results, the diagnostic
information,
and the diagnostic results (and also stores the meter's serial number) on the
smartcard.
At some later point, in step 916 the user reads the smartcard with a
computer station. Step 916 is followed by step 918, in which the computer
station establishes a network connection with the health report server 102.
Step
918 is followed by step 920, in which the computer station downloads the data
from the smartcard to the health report server 102. Step 920 is followed by
routine 922, in which health report server 102 receives additional diagnostic
and
health report information from the user of the computer station and compiles a
personal health report based on the data received from the computer station.
Routine 922 is described below with reference to FIG. 12. Routine 922 is
followed by step 924, in which the health report server 102 transmits the
health
report to the computer station, where the health report is printed and
delivered to
the patient. Step 924 is followed by the "END" step.
FIG. 10 is a logic flow diagram illustrating routine 908 for obtaining
cholesterol-related diagnostic information for the meter 10. Routine 908
begins
32
CA 02350145 2001-05-09
WO 00/Z8460 PCT/US99/26521
following step 906 shown on FIG. 9. In step 1002, the meter 10 prompts for and
receives the patient's gender. Step 1002 is followed by step 1004, in which
the
meter 10 prompts for and receives the patient's ethnicity. Step 1004 is
followed
by step 1006, in which the meter 10 prompts for and receives an indication of
the
patient's family history of heart disease. Step 1006 is followed by step 1008,
in
which the meter 10 prompts for and receives an indication of the patient's
personal history of heart disease.
Step 1008 is followed by step 1010, in which the meter 10 prompts for and
receives an indication of whether the patient is a type-1 diabetic. Step 1010
is
followed by step 1012, in which the meter 10 prompts for and receives an
indication of whether the patient is a type-2 diabetic. Step 1012 is followed
by
step 1014, in which the meter 10 prompts for and receives an indication of
whether the patient is a smoker.
Step 1014 is followed by step 1016, in which the meter 10 prompts for and
receives an indication of the patient's height. Step 1016 is followed by step
1018,
in which the meter 10 prompts for and receives an indication of the patient's
weight. Step 1018 is followed by step 1020, in which the meter 10 prompts for
and receives an indication of the patient's age. Step 1020 is followed by step
1022, in which the meter 10 prompts for and receives an indication of the
patient's blood pressure. Step 1022 is followed by step 1024, in which the
meter
10 prompts for and receives an indication of the patient's fitness. Step 1024
is
followed by the "RETURN" step, which goes to routine 910 shown on FIG 9. It
will be appreciated that the preceding list of diagnostic information is only
illustrative of the type of information that may be gathered, and that less
data,
different data, or more data could be gathered, if desired.
FIG. 11 is a logic flow diagram illustrating routine 910 for computing
immediate cholesterol-related diagnostic results for the meter 10. Routine 910
begins following routine 908 shown on FIG. 9. In step 1102, the meter 10
prompts for and receives a selection of a diagnostic algorithm resident on the
meter. Step 1102 is followed by step 1104, in which the meter 10 gets total
cholesterol test results from the test instrument module 158 or, if they are
not
available, prompts the user to input the test results manually.
33
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
Step 1104 is followed by step 1106, in which the meter 10 calculates and
displays a medical risk index associated with heart disease or heart attack,
such as
"very high," "high," "moderate," "low," or "very low." Step 1106 is followed
by step 1108, in which the meter 10 calculates and displays a recommended
weight loss, if appropriate. Step 1108 is followed by step 1110, in which the
meter 10 calculates and displays a 5-year cardiac risk (e.g., risk of cardiac
arrest in
five years is 10%). Step 1110 is followed by step 1112, in which the meter 10
calculates and displays a 10-year cardiac risk (e.g., risk of cardiac arrest
in ten
years is 20%).
Step 1112 is followed by step 1114, in which the meter 10 calculates and
displays a cardiac age (to compare against the patient's chronological age).
Step
1112 is followed by step 1114, in which the meter 10 calculates and displays
an
extended cardiac age (e.g., cardiac age compared to chronological age for
five,
ten, fifteen, etc. years into the future). Step 1116 is followed by step 1118,
in
which the meter 10 calculates and displays a medical risk index associated
with
stroke, such as "very high," "high," "moderate," "low," or "very low." Step
1118 is followed by the "RETURN" step, which goes to step 912 on FIG. 9. It
will be appreciated that the preceding list of diagnostic results is only
illustrative of
the type of information that may be generated, and that less data, different
data, or
more data could be generated, if desired.
Remote Health Report Generation
FIG. 12 is a logic flow diagram illustrating routine 922 for remotely
producing health reports. Routine 922 begins following step 920 shown on FIG.
9. In step 1202, in which the health report server 102 prompts for and
receives a
new drug therapy, such as a cholesterol-lowering prescription. Step 1202 is
followed by step 1204, in which the health report server 102 gets a
prescription
data sheet for the drug therapy. This data sheet typically includes
instructions for
taking the prescription, such as dosage, times to take each dose, whether to
take
with food or liquid, whether to avoid driving, pregnancy-related instructions,
foods
to avoid, and so forth.
Step 1204 is followed by step 1206, in which the health report server 102
prompts for and receives information regarding any other prescription drugs
that
34
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
the patient is currently taking. Step 1206 is followed by step 1208, in which
the
health report server 102 gets cross-reaction information regarding the new
drug
therapy and the other current drug prescriptions. Step 1208 is followed by
step
1210, in which the health report server 102 prompts for and receives a
specific
description of exercise therapy for the patient, or a selection of standard
exercise
sections for inclusion in the health report. Step 1210 is followed by step
1212, in
which the health report server 102 prompts for and receives diet therapy for
the
patient, or a selection of a standard diet section for inclusion in the health
report.
Step 1212 is followed by step 1214, in which the health report server 102
1o prompts for and receives indications of additional standard sections for
inclusion in
the health report.
Step 1214 is followed by step 1216, in which the health report server 102
assembles the preceding information for inclusion in a health report. Step
1216 is
followed by step 1218, in which the health report server 102 prompts for and
receives trend analysis selections. Step 1218 is followed by step 1220, in
which
the health report server 102 prepares the selected trend analysis, such as
total
cholesterol and blood glucose levels over a series of tests. The trend
analysis may
be provided alone or as part of the health report. Step 1220 is followed by
step
1222, in which the health report server 102 assembles the preceding
information
into a health report and/or trend analysis report. Step 1222 is followed by
the
"RETURN" step, which goes to step 924 shown on FIG. 9. It will be appreciated
that the preceding list of health report information is only illustrative of
the type of
information that may be compiled, and that less data, different data, or more
data
could be compiled, if desired.
Smartcard PIN Securitv
FIG. 13 is a logic flow diagram illustrating a routine 1300 for saving
medical data to the PIN-secured removable memory storage device 54,
represented by a smartcard. In step 1302, a user places the smartcard in the
meter 10. Step 1302 is followed by step 1304, in which the meter 10 prompts
for
and receives a PIN from the user. Step 1304 is followed by step 1306, in which
the meter 10 reads the PIN field on the smartcard. Step 1306 is followed by
step
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
1308, in which the meter 10 determines whether the PIN field is occupied
(i.e.,
whether a PIN has been previously stored on the smartcard).
If the PIN field is not occupied, the "NO" branch is followed from step
1308 to step 1310, in which the meter 10 stores the PIN received from the user
in
the PIN field on the smartcard. Step 1310 is followed by step 1314, in which
the
meter 10 activates for use with the current smartcard. Step 1314 is followed
by
the "END" step.
Referring again to step 1308, if the PIN field is occupied, the "YES"
branch is followed to step 1312, in which the meter 10 determines whether the
l0 PIN received from the user matches the PIN stored in the PIN field on the
smartcard. If the PIN received from the user matches the PIN stored in the PIN
field on the smartcard, the "YES" branch is followed to step 1314, in which
the
meter 10 activates for use with the current smartcard. Step 1314 is followed
by
the "END" step.
Referring again to step 1312, if the PIN received from the user does not
match the PIN stored in the PIN field on the smartcard, the "NO" branch is
followed to step 1314, in which the meter 10 determines whether a timeout
condition or an allowed number of tries has been reached. If a timeout
condition
or an allowed number of tries has not been reached, the "NO" branch is
followed
to step 1318, in which the meter 10 displays an error and prompts the user to
reenter the activation code. From step 1318, routine 1300 loops to step 1312.
If
a timeout condition or an allowed number of tries has been reached, the "YES"
branch is followed from step 1316 to the "END" step, and the meter 10 is not
activated for use with the instant smartcard.
Secure Medical Records Maintenance System
FIG. 14 is a functional block diagram of a system for using the meter 10 in
connection with a secure medical records maintenance system 1400. The meter
10 stores a patient's test results and diagnostic information on a removable
memory storage device, such as the smartcard 54. A conventional interface 106
may then be used to interface the smartcard 54 with a conventional desktop,
laptop or other type of computer 108, which in turn communicates with other
computers over a network-based computer system, such as the Internet 110. As
36
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
discussed previously, this Internet link may be used to produce a printed
health
report booklet 112 including a health assessment based on a patient's test
results
and diagnostic information, which a health-care provider typically provides to
the
patient.
Although this secure medical records maintenance system 1400 is
specifically adapted for use with the meter 10, it may be used to store any
type of
electronic medical records, and is particularly convenient for storing a wide
range
of electronic medical data generated remotely from the hospital or doctor's
office
environment. In addition, the secure medical records maintenance system 1400
may read medical data stored on memory storage devices other than the
smartcard 54, and may operate over computer networks other than the Internet
100.
The secure medical records maintenance system 1400, which is presently
known as the "PRIVALINK" system, operates in connection with a software
module 1402 installed on the accessing computer 108. This software is
presently
known as the "PRIVALINK" user site software. The server-side "PRIVALINK"
system 1400 includes a first remote server 1404 that stores patient
identification
information, a second remote server 1406 that stores patient medical data, an
encryption/decryption module 1408 that implements encryption and other
security-related functions, and a booklet generation module 1410, which
produces
printed health report booklets 112 based on the information stored in the
servers
1404, 1406. The patient identification information and medical data are
maintained in separate, secure servers 1404, 1406 to prevent correlation of a
specific patient's medical data with the associated patient identification
information.
Because the data on the servers 1404, 1406 is separate and secure from
each other, access may be granted to either server without identifying any
particular patient's medical data. For example, access may be granted to the
first
remote server 1404, but not to the second server 1406, for the purpose of
generating a mailing list of patients without divulging any medical data
associated
with the patients. Similarly, access may be granted to the second remote
server
1406, but not to the first server 1404, for the purpose of conducting
investigative
37
CA 02350145 2001-05-09
WO 00/28460 PCTNS99/26521
analyses involving patient medical data without divulging any patient
identification
information associated with the medical data.
For further data security and because each smartcard 54 only has a limited
data storage capability, the medical data stored on each smartcard may be
automatically erased from the smartcard after the data is entered into the
second
remote server 1406. To obtain the medical data, the smartcard 54 is received
within the meter 10, which stores the medical data on the smartcard. And to
download the medical data to the medical records maintenance system 1400, the
removable memory storage device is receivable within the interface 106 for
l0 communication with the computer 108, which is operable for transmitting the
medical data to the second remote server 1406 over the Internet 110.
FIG. 15 is software architecture diagram illustrating a system for conducting
secure communications between the computer 108 and the secure medical records
maintenance system 1400. The "PRIVALINK" user site software 1402 includes
an encryption/decryption module 1412 that implements encryption/decryption
services on the client computer 108. A corresponding encryption/decryption
module 1408 on the secure medical records maintenance system 1400 implements
complimentary encryption/decryption services on the server side, typically
using a
well-known encryption/decryption package, such as that known as "BLOWFISH"
or "DES." The server-side encryption/decryption module 1408 also maintains
access to a table of valid professional registration numbers 1411, typically
DEA
numbers, received from the registering agency. This table is used to validate
the
professional registration numbers of practitioners attempting to access the
secure
medical records maintenance system 1400.
The server-side encryption/decryption module 1408 also maintains a record
of all transactions with accessing computers for future analysis. In addition,
all
data transfers from the secure medical records maintenance system 1400 to the
user site 108, such as health report booklets 112, are encrypted/decrypted
through
an Internet secure sockets layer connection 1500, which is well known to those
skilled in the art. These encryption/decryption services prevent theft or
inadvertent
loss of patient medical data through unintended transmission or extraction of
communications occurring on the Internet 110.
38
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
The "PRIVALINK" user site software 1402 also implements client
application, certification and login processes for accessing the secure
medical
records maintenance system 1400. The application, certification and login
process
is described below with reference to FIGS. 17-19. Upon successful login to the
secure medical records maintenance system 1400, the "PRIVALINK" user site
software 1402 implements a menu-driven user interface system 1414 for
conducting communications between the practitioner computer 108 and the
secure medical records maintenance system 1400. This interface system includes
a health care provider data entry screen 2100 shown in FIG. 21, a patient
address
l0 data entry screen 2500 shown in FIG. 25, a patient test data entry screen
2600
shown in FIG. 26, a booklet selection screen 2700 shown in FIG. 27, and a
booklet preview/printing screen 2800. The menu-driven user interface system
1412 also includes other related user interface screens. The operation of the
menu-driven user interface system 1412 is described below with reference to
FIG.
17.
FIG. 16 a functional block diagram illustrating security aspects of the secure
medical records maintenance system 1400. The secure medical records
maintenance system 1400 preferably includes a large number of smartcards 54a-
n, which operate in concert with a large number of meters l0a-n. Although each
smartcard 54a-n is preferably used to store medical data for an associated
patient,
each card could be used to store medical data for multiple patients. Each
smartcard 54 also stores a patient-specified personal identification number
(PIN),
and may store PINS for multiple patients if the smartcard is configured to
store
medical data for multiple patients. Each PIN is used to gain access to a
secure
storage area on the smartcard 54, which stores an associated patient
identification
number and medical records identification number, which are assigned by the
secure medical records maintenance system 1400.
The first remote server 1404 of the secure medical records maintenance
system 1400 stores patient identification information indexed by patient
identification numbers, and the second remote server 1406 stores patient
medical
data indexed by the medical records identification numbers. Typically, each
patient identification number and medical identification number is a unique
number assigned by the operator of the secure medical records maintenance
39
CA 02350145 2001-05-09
WO 00/28460 PCT/t?S99/26521
system 1400 upon entry of each patient into the system. For example, the
patient
identification numbers and medical identification numbers may be social
security
numbers or sequential registration numbers. Alternatively, the patient
identification numbers and medical identification numbers may be unique
numbers
computed by a non-repeating pseudo-random number generator. The patient
identification number and the medical records identification number may be 16-
bit
hexadecimal or another suitable value. In addition, either or both of the
patient
identification number and the medical records identification number may be a
global user identification number (GUID), which is a secure communication key
generated by well-known secure encryption systems, such as currently available
private-key and public-key encryption systems including "BLOWFISH," "DES"
and others. Many other schemes for assigning unique patient identification
numbers will become evident to those skilled in the art.
For security purposes, the medical data maintained in the second remote
server 1406 cannot be correlated to the associated patient identification
information maintained in the first remote server 1408 based on the
information
contained in the first and second remote servers. To allow correlation of the
data
stored in the two servers 1404, 1406 the secure medical records maintenance
system 1400 includes a correlation table 1600 uniquely associating each
medical
records identification number with a particular one of the patient
identification
numbers. The correlation table 1600 for a particular patient typically resides
in
the PIN-secured storage area on the patient's smartcard 54. The correlation
table
1600 for a practitioner's patients may also reside on the practitioner's
computer
108, such as a doctor's or pharmacist's computer, that is associated with a
licensed medical practitioner having an assigned professional registration
number
(DEA number). Each practitioner's correlation table 1600 is preferably
encrypted and maintained in a secure file. The proprietor of the secure
medical
records maintenance system 1400 may also maintain a complete back-up
correlation table 1600, typically in a secure encrypted file located on a
separate file
server.
For further security, the first and second remote servers 1404, 1406 are
accessed by the practitioners' computers 108a-n through encrypted
communications secured by an application procedure that includes validation of
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
the practitioner's registration number (DEA number). This access will be
limited
to medical records and patient identification information associated with the
accessing practitioner. In other words, each practitioner will only have
access to
his or her patients' medical records and patient identification information.
Similar access procedure may be implemented for indsvidual patients,
except that access will be limited to that particular patient's medical
records and
patient identification information. For example, individual patients may
register in
advance with the proprietor of the system 1400, which will issue each patient
a
unique registration number. In this case, the first and second remote servers
1404, 1406 may accessed by computers operated by the individual patients
through encrypted communications secured by an application procedure that
includes validation of the patient's registration number.
For both practitioners and individual patients, the application procedure
may be further secured by receipt and validation of a client-supplied PIN.
Moreover, the application procedure typically includes issuance of a client
certificate insuring that access to the first and second remote servers occurs
from
the client computer that initiated the application and certification process.
Those skilled in the art will appreciate that the data distribution system
implemented by the secure medical records maintenance system 1400 includes
many aspects of data security. For example, a patient's medical records
identification number cannot be obtained from the patient's smartcard 54
without
access to the patient-assigned PIN. In addition, while the patient's medical
data is
indexed by the patient's medical records identification number in the second
server 1406, the patient's name and other identification information cannot be
retrieved from the first server 1404 using this data. Similarly, a hacker
obtaining
assess to one or both of the servers 1404, 1406 cannot correlate patient
identification information with patient medical data. In addition, the
correlation
data for the entire secure medical records maintenance system 1400 is
distributed
among the various smartcards 54a-n and practitioner computers 108a-n
registered for use with the system. Thus, a hacker cannot obtain the
correlation
data for any single patient, much less the entire database, through access to
the
centrally-maintained data servers 1404, 1406.
41
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
For further security, the secure medical records maintenance system 1400
cannot be accessed from the a medical practitioner's computer 108 without
knowledge of the proper practitioner-assigned PIN. And all communication
between the practitioner's computer 108 and the secure medical records
maintenance system 1400 are encrypted for transmission security. Furthermore,
each correlation table 1600, which provides the link between patient
identification
numbers and medical records identification numbers for a particular
practitioner,
may itself be encrypted, with the key to this encryption stored in a separate
location. For example, this encryption key may be a practitioner PIN or GUID
to stored on a PIN-secured area the practitioner's computer 108 or on a
smartcard
54 assigned to the practitioner.
FIG. 17 is a logic flow diagram illustrating an illustrative process 1700 for
communicating with the secure medical records maintenance system 1400. In
routine 1702, a medical practitioner conducts a client application procedure
to
obtain a secure client certificate, which is also known as a "CA" or
certificate-
authentication. Routine 1702 is described in greater detail with reference to
FIG.
18. Routine 1702 is followed by a client login procedure 1704, which is
described
in greater detail with reference to FIG. 19. These procedures ensure that
access to
the secure medical records maintenance system 1400 is limited to registered
medical practitioners using the same computer and browser that the
practitioner
used to obtain a secure client certificate through the application procedure.
That
is, any attempt to access the secure medical records maintenance system 1400
by
a person without a valid DEA number, or from a non-certified computer or
browser, will be rejected. As noted previously, similar procedures may be
implemented for allowing individuaol patients to access their records on the
secure
medical records maintenance system 1400.
FIG. 18 is a logic flow diagram illustrating routine 1702 used by a medical
practitioner to apply for access to the secure medical records maintenance
system
1400. Routine 1702 begins at the start of FIG 17. In step 1802, the client-
side
PRIVALINK software 1402 issues a client application request to the server-side
encryption/decryption module 1408, which initiates a transaction document
record
for the communication. That is, all communications between the client-side
PRIVALINK software 1402 and the server-side encryption/decryption module
42
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
1408 are recorded for future analysis by the server-side encryption/decryption
module 1408. The client application request is typically received by an
Internet
link to a specified web page associated with the server-side
encryption/decryption
module 1408. The medical practitioner can obtain the proper Internet address
by
placing a telephone call to the operator of the system 1400. The phone number,
and possibly the Internet address, will be printed on each meter 10 and may
also
be available through other forms of notification, such as advertisement and
direct-
mail notification to licensed practitioners (e.g., physicians and
pharmacists).
Step 1802 is followed by step 1804, in which the PRIVALINK software
1402 displays an application screen and receives client input including the
practitioner's professional registration number, typically a DEA number. Step
1804 is followed by step 1806, in which the PRIVALINK software 1402 receives
a certification request including selection of an encryption type. This is
typically
associated with completion of the data-entry fields of the application screen
and
selection of a "submit" control item. Step 1806 is followed by step 1808, in
which the PRIVALINK software 1402 downloads an encryption program module
from the server-side encryption/decryption module 1408, such as an "ACTIVE
X" control or applet, to the client's computer 108. This encryption program
module typically includes a "key" or nugget of information to be stored in the
accessing browser. The server-side encryption/decryption module 1408 can later
check an accessing computer for the presence of the "key" to ensure that the
accessing computer and browser is the same as the one going through the
application procedure.
Step 1808 is followed by step 1810, in which the server-side
encryption/decryption module 1408 validates the registration number (e.g., DEA
number) entered by the applicant, typically by comparing the received
registration
number to the table of valid registration numbers 1411 received from the
registration authority (e.g., table of valid DEA numbers). If the registration
number is properly validated, step 1810 is followed by step 1812, in which the
server-side encryption/decryption module 1408 transmits an e-mail message to
the
PRIVALINK software 1402 including a URL for accessing the secure servers
1404, 1406. Step 1812 is followed by step 1814, in which the PRIVALINK
software 1402 transmits a client link to the designated URL. Upon receipt of
the
43
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
link, the server-side encryption/decryption module 1408 checks for the
presence
of the "key" to ensure that the accessing computer and browser is the same as
the one that went through the application procedure.
If the "key" is properly validated, step 1814 is followed by step 1816, in
which the server-side encryption/decryption module 1408 transmits a "server
root
CA" and a "client certificate" to the PRIVALINK software 1402 on the client's
computer 108. Step 1816 is followed by step 1818, in which the PRIVALINK
software 1402 links the applicant to a secure area of the
encryption/decryption
module 1408, which validates the presence of the server root CA. If the server
root CA is properly validated, step 1818 is followed by step 1820, in which
the
PRIVALINK software 1402 prompts the user to identify the client certificate
for
use in the transaction. If the client certificate is properly validated, step
1820 is
followed by step 1822, in which the PRIVALINK software 1402 links the
applicant to a login screen. Step 1822 is followed by step 1824, in which the
encryption/decryption module 1408 saves the transaction documentation for the
client's application procedure. Step 1824 is followed by the "CONTINUE" step,
which returns to routine 1704 shown in FIG. 7.
FIG. 19 is a logic flow diagram illustrating a routine 1704 for logging into
the secure medical records maintenance system 1400. Routine 1704 begins
following routine 1702 shown in FIG. 7. In step 1902, the PRIVALINK software
1402 displays a login screen on the client's computer 108. Step 1902 is
followed
by step 1904, in which the PRIVALINK software 1402 loads the client's
registration number, which was obtained during the application procedure
described with reference to FIG. 18, into the login screen. That is, an
accessing
client does not have an opportunity to enter the login procedure without
having
first having gone through the application procedure described with reference
to
FIG. 18, which requires the applicant to provide a valid registration number,
which typically may be a DEA number for a licensed medical practitioner or a
registration number issued by the proprietor of the secure medical records
maintenance system 1400 for an individual patient.
Step 1904 is followed by step 1906, in which the PRIVALINK software
1402 receives the practitioner's personal identification number (PIN). Step
1906
is followed by step 1908, in which the encryption/decryption module 1408
44
CA 02350145 2001-05-09
WO 00/28460 PCT/US99126521
validates the practitioner's personal identification number (PIN) for use with
the
received professional registration number. Step 1908 is followed by step 1910,
in
which the encryption/decryption module 1408 determines whether the received
PIN is a new PIN for use in connection with the received professional
registration
number. If the received PIN is a new PIN for use in connection with the
received
professional registration number, the "YES" branch is followed from step 1910
to
step 1912, in which the PRIVALINK software 1402 prompts the user to complete
the interface screens 2000-2007 (FIGS. 20-27). Step 1912 and the "NO" branch
from step 1910 are followed by step 1914, in which the client is linked to the
interface screens 2000-2007 (FIGS. 20-27). Step 1914 is followed by
"CONTINUE" step, which returns to step 1706 shown on FIG. 7.
User Interface Design
Referring again to FIG. 17, once the medical practitioner has successfully
I S logged into the secure medical records maintenance system 1400, routine
1704 is
followed by step 1706, in which the PRIVALINK software 1402 displays a
"switchboard" user interface 2000. FIG. 20 is an illustration of a typical
"switchboard" user interface 2000, which includes a number of selection items
corresponding to functions available on the server. For example, the interface
2000 typically includes a "provider" selection item 2002 and a "patients"
selection item 2004. To illustrate the operation of the user interface it is
assumed
that the practitioner initially selects the "provider" selection item 2002.
Thus, step
1706 is followed by step 1708, in which the PRIVALINK software 1402 receives
a "provider" selection from the "switchboard" user interface 2000.
Step 1708 is followed by step 1710, in which the PRIVALINK software
1402 displays an "address" user interface 2100. FIG. 21 is an illustration of
a
typical "address" user interface 2100. The interface 2100 includes a first
field
2102 for entering a practitioner registration number, typically a DEA number,
and
a second field 2104 for entering a practitioner-assigned PIN. The interface
2100
also includes a number of other fields for entering the practitioner's contact
information, such as address, phone number, and so forth. The interface 2100
also includes an "address" tab 2106, a "billing info" tab 2108, and a "cover
letter" tab 2110 displayed adjacent to the user interface 2100. These tabs
allow
CA 02350145 2001-05-09
WO 00/Z8460 PCT/US99/26521
the user to toggle among corresponding user interface screens. As noted above,
the interface 2100 initially appears in a "default" mode with the "address"
tab
2106 selected.
For example, step 1710 may be followed by step 1712, in which the
PRIVALINK software 1402 displays a "billing info" user interface in response
to
user selection of the "billing info" tab 2108. FIG. 22 is an illustration of a
typical
"billing information" user interface 2108. The interface 2100 includes a
number
of fields 2202 for entering payment authorization, such as a bank credit or
debit
card number. The interface 2100 may include other types of payment options,
l0 such as a bank account number for wire transfers, authorization to include
the
charges on a telephone bill or Internet service provider bill associated with
the
Internet link to the server-based system 1400, reference to an authorized
account
for billing at a later date, and the like.
Similarly, step 1712 may be followed by step 1714, in which the
PRIVALINK software 1402 displays a "cover letter" user interface 2300 in
response to user selection of the "cover letter" tab 2110. FIG. 23 is an
illustration
of a typical "cover letter" user interface 2300. This interface allows the
practitioner to author a cover letter that will be included with a health
report
booklet to be produced by the booklet generation module 1410 on the server-
based system 1400, as described previously with reference to FIG. 9. This
allows
the practitioner to customize the cover letter for each health report booklet
produced by the system.
To further illustrate the operation of the user interface, it is assumed that
the
practitioner next selects the "patients" selection item 2004 on the
"switchboard"
user interface 2000. Thus, step 1714 is followed by step 1716, in which the
PRIVALINK software 1402 displays a "patients" user interface 2400. FIG. 24 is
an illustration of a typical "patient selection" user interface 2400. This
user
interface allows the practitioner to select a preexisting patient and to enter
new
patients. Upon entry of a new patient, the PRIVALINK software 1402 typically
assigns a unique patient identification number and a unique medical records
identification number to the patient, which the user site "PRIVALINK" software
1402 stores in a correlation table 1600, as described previously with
reference to
FIG. 16.
46
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
Step 1716 is followed by step 1718, in which the PRIVALINK software
1402 displays a "patient information" user interface 2500. FIG. 25 is an
illustration of a typical "patient information" user interface 2500. This
interface
includes a number of fields for entering patient identification informarion,
such as
address, phone number, and so forth. As described previously with reference to
FIG. 16, this patient identification information is typically indexed by the
patient
identificarion number and stored in the first server 1404, whereas the
patient's
medical data is typically indexed by the patient's medical records
identification
number and stored in the second server 1406.
Step 1718 is followed by step 1720, in which the PRIVALINK software
1402 displays a "questionnaire data" user interface 2600. FIG. 26 is an
illustration of a typical "questionnaire data" user interface 2600. This
interface
includes a number of fields for entering patient diagnostic information, such
as
family history, age, weight, body fat, and so forth. This interface may be
used to
enter patient diagnostic information in addition to that received through the
meter
10. For example, the interface 2600 may be used to enter diagnostic data that
the
meter would have recorded, but the patient failed to enter the data into the
meter
10. In addition, the interface 2600 may be automatically filled in, either
partially
or completely, with the appropriate data stored on a corresponding smartcard
54.
This step may require entry of an appropriate patient PIN for the
corresponding
smartcard 54 into the interface 2600. The interface 2600 may also be used to
change or supplement the data read from the smartcard 54, or enter additional
diagnostic information that the meter 10 is not configured to collect from the
patient.
Step 1720 is followed by step 1722, in which the PRIVALINK software
1402 displays a "generate reports" user interface 2700. FIG. 27 is an
illustration
of a typical "generate reports" user interface 2700. This interface includes a
number of fields for selecting items to be included in a patient's health
report
booklet, such as cover letter, summary, evaluation, and so forth. The
interface
includes a number of fields for selecting therapy items to be prescribed for
the
patient and reflected in the patient's health report booklet, such as
lifestyle
therapy, lipid drug prescription, blood pressure drug prescription, and so
forth.
47
CA 02350145 2001-05-09
WO 00/28460 PCTIUS99/26521
FIG. 28 is an illustration of typical health report charts generated by the
secure medical records maintenance system 1400 for inclusion in a patient's
health
report booklet. These charts include a "coronary risk factors" chart 2802, a
"personal health consequences" chart 2804, and an "extended health assessment"
chart 2806. The "coronary risk factors" chart 2802 includes test results and
diagnostic information along with ideal ranges and patient goals for these
items.
The "personal health consequences" chart 2804 includes interpretive data, such
as
pounds overweight, cardiac age, and stroke risk. The "extended health
assessment" chart 2806 includes a projection of future interpretive data, such
as a
projected comparison of the patient's chronological age and cardiac age.
FIG. 29 is an illustration of an additional health assessment chart 2900
generated by the secure medical records maintenance system 1400 for inclusion
in
a patient's health report booklet. This chart includes a pictorial
representation of
cardiac risk factors, such as gender, smoker, personal history, and so forth.
The
health assessment chart 2900 typically presents a pictorial assessment of the
"coronary risk factors" shown in chart 2802.
Smartcard Payment System
The system described above is largely dependent on the sale of proprietary
test strips 28 for the collection of revenue from end users. That is, the
meter 10
may be made available to individual patients at little or no cost, with the
sale of
proprietary test strips 28 providing a major source of revenue for the
proprietor
of the health monitoring meter. This may be a desirable business model for
deploying the meters 10 because it minimizes the initial cost that an
individual
patient must pay to begin using the device. Having to sell each meter 10 at
its full
cost, on the other hand, would undermine the economic feasibility of using the
meter in many contexts.
Nevertheless, it may also be desirable to provide a meter 10 that does not
rely on the sale of proprietary test strips 28 as a major source of revenue.
For
example, the meter may be adapted to read non-proprietary test strips, or may
incorporate a reusable and/or non-invasive testing device, such as an
electrode,
blood pressure monitoring device, sonic testing device, thermometer, saliva
testing
device, optical testing device, and the like. Of course, a non-invasive multi-
use
48
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
testing device may be used many times without affording the proprietor of the
health monitoring device an opportunity collect revenue associated with use of
the
device.
To solve this problem, the smartcard 54 may be utilized as a type of "debit
card" for use with the meter 10. That is, the smartcard 54 device may be
purchased with a monetary value, or it may have a monetary value that is
replenishable over the Internet using a bank credit or debit card or other
conventional payment source. The meter 10 may then deduct the cost of
performing particular services (e.g., blood cholesterol test, health
assessment,
blood sugar test, AIDS test, etc.) from the monetary value represented by the
monetary balance stored on the smartcard 54. In other words, the meter 10 may
be configured to activate for the performance of a variety of services upon
deducting a charge for the selected service from a monetary value stored on a
smartcard 54 inserted into the device.
FIG. 30 is a logic flow diagram illustrating a routine 3000 for adding a
monetary value to a smartcard 54 for use with the meter 10. Although single-
use
smartcard could be sold with a monetary value for use with the meter 10, the
cost
of the smartcards makes a replenishable debit card system preferable. In step
3002, the proprietor of the system loads an illustrative smartcard 54 with
debit
card software and a zero balance. Step 3002 is followed by step 3004, in which
the smartcard 54 is issued for use with the meter 10. For example, the
smartcard
54 may be sold or given away separately or in connection with a meter 10.
Typically, the proprietor of the system will be motivated to make both the
meter
10 and the smartcard 54 available for little or no acquisition cost because
the cost
of these assets will be recovered through use of the debit-card type smartcard
54
with meter 10.
Step 3004 is followed by step 3006, in which the user establishes a PIN
and activates the smartcard 54, which can be performed using the meter 10 or a
conventional computer 108 having a smartcard interface 106, as shown in FIG.
2.
3o Step 3006 is followed by step 3008, in which the user loads the smartcard
S4 into
a conventional computer 108 having a smartcard interface 106 (if the smartcard
in
not already loaded) and accesses a predefined debit card Internet site by
linking to
an associated URL, which may be printed on the smartcard or made available
49
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
through advertisement, direct mail, or other communication media. The debit
card Internet site may typically be a separate server operated by the
proprietor of
the secure medical records maintenance system 1400, or it may be another site
operated by a separate proprietor. Step 3008 is followed by step 3010, in
which
the debit card Internet site establishes communications with the smartcard 54
and
verifies the authenticity of the smartcard.
Step 3010 is followed by step 3012, in which the debit card Internet site
prompts the user for input payment authorization for a monetary value to be
added to the smartcard. The debit card Internet site may accept a variety of
payment options, such as a bank credit or debit card number, a bank account
number for wire transfers, authorization to include the charges on a telephone
bill
or Internet service provider bill associated with the Internet link, and the
like.
Step 3012 is followed by step 3014, in which the debit card Internet site
validates
the received payment authorization. Step 3014 is followed by step 3016, in
which
the debit card Internet site determines whether the received authorized
payment
source has a sufficient balance or credit limit for the monetary value that
the user
has requested for addition to the smartcard 54.
If the authorized payment source has a sufficient balance or credit limit, the
"YES" branch is followed to step 3018, in which the debit card Internet site
adds
the requested monetary value to the smartcard 54 and charges the cost to the
authorized payment source. Optionally, the debit card Internet site may also
load
a rate schedule for services to be provided by the meter 10 onto the smartcard
54.
In this case, the meter 10 reads the monetary value assigned to performance of
the test from the smartcard 54. Thus, rate schedules for various services to
be
performed by the meter 10 may be changed from time to time, based on quantity
discounts or other considerations. Step 3020 is followed by the "END" step,
which concludes routine 3000.
Referring again to step 3016, if the payment source is invalid or does not
have a sufficient balance or credit limit, the "NO" branch is followed to step
3017, in which the user is prompted for another payment source. If the user
enters another payment source, the "YES" branch loops to step 3014 for
validation of the alternate payment source. If the user does not enter another
CA 02350145 2001-05-09
WO 00/28460 PCT/US99/26521
payment source, the "NO" branch is followed by the "END" step, which
concludes routine 3000.
FIG. 31 is a logic flow diagram illustrating a routine 3100 for using a
smartcard 54 to pay for a service provided by the meter 10. In step 3102, a
user
obtains a smartcard 54 with a usable monetary value, typically by purchasing a
smartcard having a usable monetary value or by adding a monetary value to a
smartcard as described with reference to FIG. 30. Step 3102 is followed by
step
3104, in which the meter 10 receives a request to provide a service, such as a
blood cholesterol test and/or production of a health report, with the
associated cost
to be charged against a monetary value stored on the smartcard 54. Step 3104
is
followed by step 3106, in which the meter 10 prompts the user to enter a
smartcard 54 with a usable monetary value into the meter. Step 3106 is
followed
by step 3108, in which the meter 10 receives the smartcard 54 in the reader
50.
It should be understood that at this point the meter is inactive for
performing
services that require payment. Note also that the meter 10 may be activated
alternatively through proprietary test strip validation, as described with
reference
to FIG. 6.
Step 3110 is followed by step 3112, in which the meter 10 prompts the
user for a PIN for use with the smartcard 54 and validates the PIN (i.e.,
checks the
received PIN against the PIN stored on the smartcard). If the PIN is
validated,
step 3112 is followed by step 3114, in which the meter 10 determines whether
the
monetary value stored on the smartcard 54 is sufficient to pay for the
requested
service. As noted previously, the meter 10 may also read the rate schedule
from
the smartcard 54. Alternatively, if no rate schedule is present on the
smartcard
54, the meter 10 may use a predefined "default" rate schedule for the
requested
service.
If the monetary value stored on the smartcard 54 is sufficient to pay for the
requested service, the "YES" branch is followed to step 3116, in which the
meter
10 deducts the cost for the requested service from the monetary value stored
on
the smartcard 54. That is, the meter 10 computes a revised monetary balance
equal to the initial monetary value stored on the smartcard less the cost for
the
requested service, and replaces the initial monetary value stored on the
smartcard
with the revised monetary balance. Step 3116 is followed by step 3118, in
which
51
CA 02350145 2003-O1-21
the meter 10 activates for performance of the requested service. Step 3118 is
followed by step 3120, in ~ which the meter 10 completes the requested service
and returns to the inactive state. Referring again to step 3114, if the
monetary
value stored on the smartcard 54 is insufficient to pay for the requested
service,
the "N4" branch is followed to step 3122, in which the meter displays a
"balance
insufficient" message. Steps 3120 and 3122 are followed by the "END" step,
which concludes routine 3100.
The present invention thus provides a health monitoring and diagnostic
device (LIFESTREAM cholesterol meter) that works in connection with a
network-based comprehensive health analysis and reporting system. The
invention also provides a secure medical records maintenance system that works
in conjunction with the health monitoring and diagnostic device, and
alternative
business models for deploying the health monitoring and diagnostic devices.
1~
(The remainder of this page is intentionally left blank)
57